Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Audentes Therapeutics, Inc. | bold-8k_20181005.htm |
ASPIRO Phase 1/2 Gene Therapy Trial in X-Linked Myotubular Myopathy (XLMTM): Preliminary Safety and Efficacy Findings Exhibit 99.1

Safe Harbor Except for statements of historical fact, any information contained in this presentation may be a forward-looking statement that reflects the Company’s current views about future events and are subject to risks, uncertainties, assumptions and changes in circumstances that may cause events or the Company’s actual activities or results to differ significantly from those expressed in any forward-looking statement. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “plan,” “expect,” “estimate,” “anticipate,” “intend,” “goal,” “strategy,” “believe” and similar expressions and variations thereof. Forward-looking statements include statements regarding the Company’s business strategy; potential growth opportunities; clinical development activities; the timing and results of Investigational New Drug application and Clinical Trial Authorisation submissions; the timing and results of preclinical studies and clinical trials; the nature and timing of potential regulatory approvals; and the likelihood of future commercialization of product candidates. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, the Company cannot guarantee future events, results, actions, levels of activity, performance or achievements, and the timing and results of biotechnology development and potential regulatory approval is inherently uncertain. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under the heading “Risk Factors” in documents the Company has filed with the SEC. These forward-looking statements speak only as of the date of this presentation, and the Company undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof. Certain information contained in this presentation may be derived from information provided by industry sources. We believe such information is accurate and that the sources from which it has been obtained are reliable. However, we cannot guarantee the accuracy of, and have not independently verified, such information.
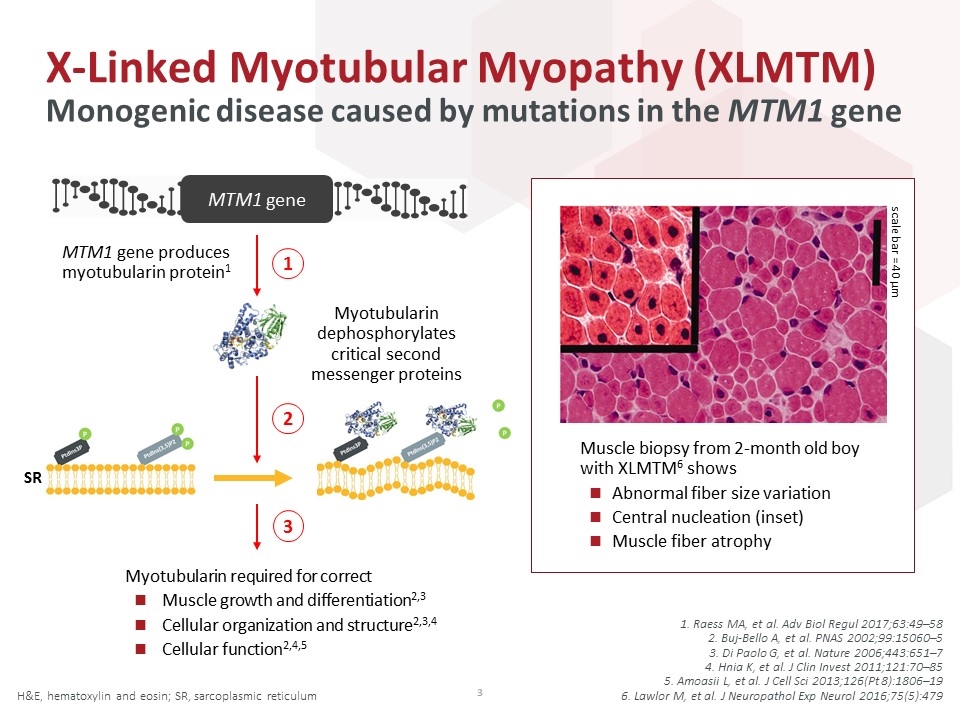
1. Raess MA, et al. Adv Biol Regul 2017;63:49–58 2. Buj-Bello A, et al. PNAS 2002;99:15060–5 3. Di Paolo G, et al. Nature 2006;443:651–7 4. Hnia K, et al. J Clin Invest 2011;121:70–85 5. Amoasii L, et al. J Cell Sci 2013;126(Pt 8):1806–19 6. Lawlor M, et al. J Neuropathol Exp Neurol 2016;75(5):479 X-Linked Myotubular Myopathy (XLMTM) H&E, hematoxylin and eosin; SR, sarcoplasmic reticulum MTM1 gene produces myotubularin protein1 Myotubularin dephosphorylates critical second messenger proteins 1 2 3 SR scale bar = 40 µm Muscle biopsy from 2-month old boy with XLMTM6 shows Abnormal fiber size variation Central nucleation (inset) Muscle fiber atrophy MTM1 gene Monogenic disease caused by mutations in the MTM1 gene Myotubularin required for correct Muscle growth and differentiation2,3 Cellular organization and structure2,3,4 Cellular function2,4,5
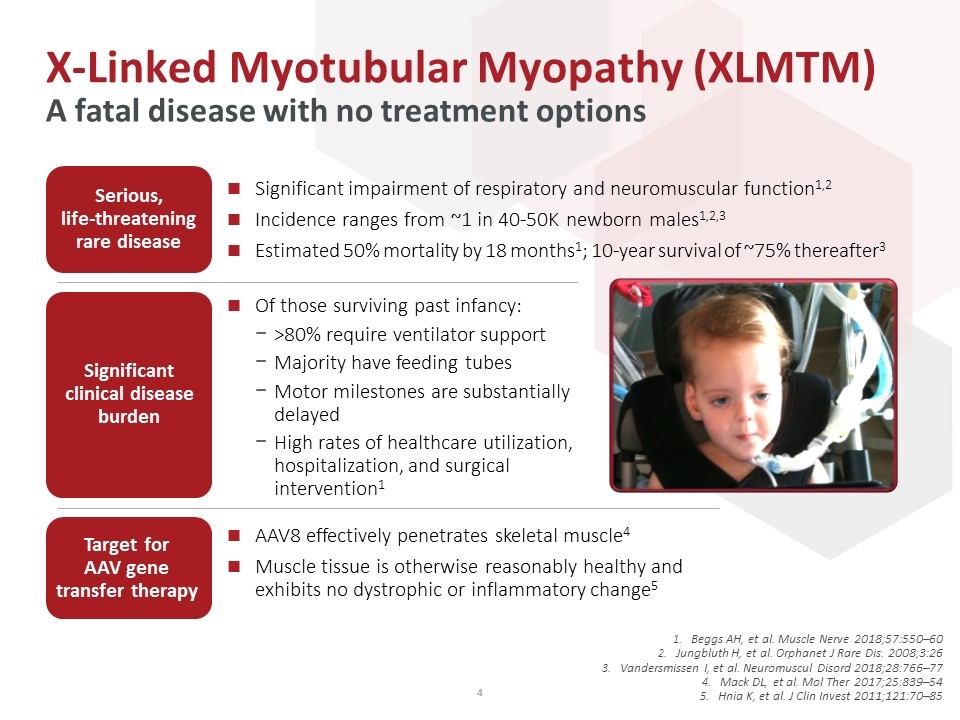
Beggs AH, et al. Muscle Nerve 2018;57:550–60 Jungbluth H, et al. Orphanet J Rare Dis. 2008;3:26 Vandersmissen I, et al. Neuromuscul Disord 2018;28:766–77 Mack DL, et al. Mol Ther 2017;25:839–54 Hnia K, et al. J Clin Invest 2011;121:70–85 X-Linked Myotubular Myopathy (XLMTM) A fatal disease with no treatment options Significant impairment of respiratory and neuromuscular function1,2 Incidence ranges from ~1 in 40-50K newborn males1,2,3 Estimated 50% mortality by 18 months1; 10-year survival of ~75% thereafter3 AAV8 effectively penetrates skeletal muscle4 Muscle tissue is otherwise reasonably healthy and exhibits no dystrophic or inflammatory change5 Of those surviving past infancy: >80% require ventilator support Majority have feeding tubes Motor milestones are substantially delayed High rates of healthcare utilization, hospitalization, and surgical intervention1 Target for AAV gene transfer therapy Significant clinical disease burden Serious, life-threatening rare disease
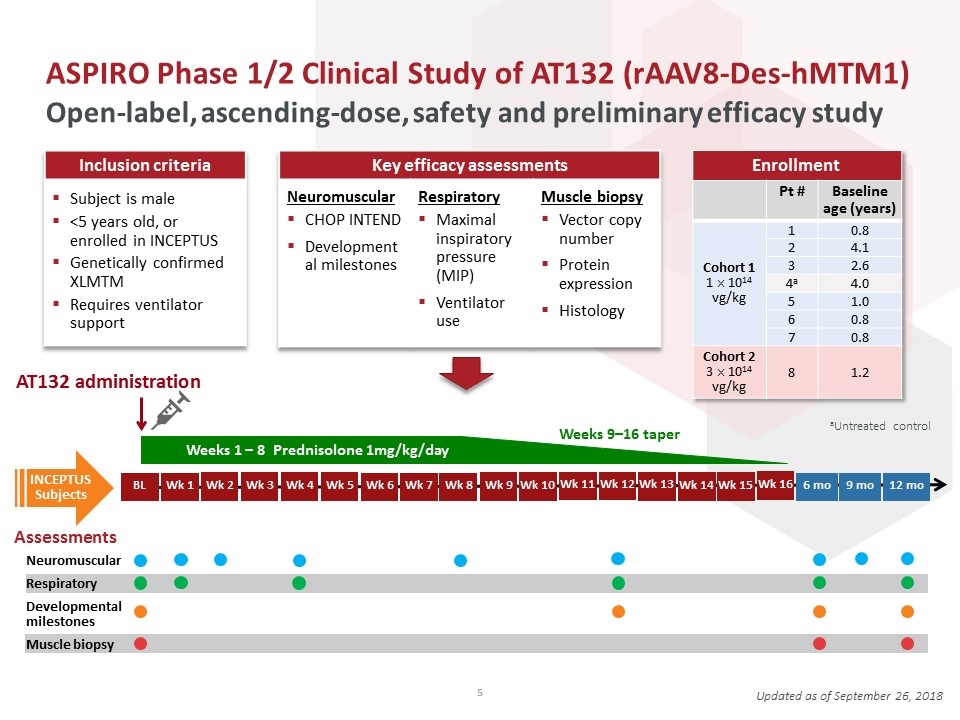
ASPIRO Phase 1/2 Clinical Study of AT132 (rAAV8-Des-hMTM1) Open-label, ascending-dose, safety and preliminary efficacy study Updated as of September 26, 2018 Inclusion criteria Subject is male <5 years old, or enrolled in INCEPTUS Genetically confirmed XLMTM Requires ventilator support Key efficacy assessments Neuromuscular CHOP INTEND Developmental milestones Respiratory Maximal inspiratory pressure (MIP) Ventilator use Muscle biopsy Vector copy number Protein expression Histology Enrollment Pt # Baseline age (years) Cohort 1 1 ´ 1014 vg/kg 1 0.8 2 4.1 3 2.6 4a 4.0 5 1.0 6 0.8 7 0.8 Cohort 2 3 ´ 1014 vg/kg 8 1.2 aUntreated control AT132 administration Weeks 9–16 taper Wk 12 Wk 11 Wk 10 Wk 1 Wk 2 Wk 3 Wk 4 Wk 5 Wk 6 Wk 8 Wk 9 Wk 7 Assessments Neuromuscular Respiratory Developmental milestones Muscle biopsy INCEPTUS Subjects Wk 13 Wk 14 Weeks 1 – 8 Prednisolone 1mg/kg/day BL Wk 15 6 mo 12 mo Wk 16 9 mo

Safety and Tolerability
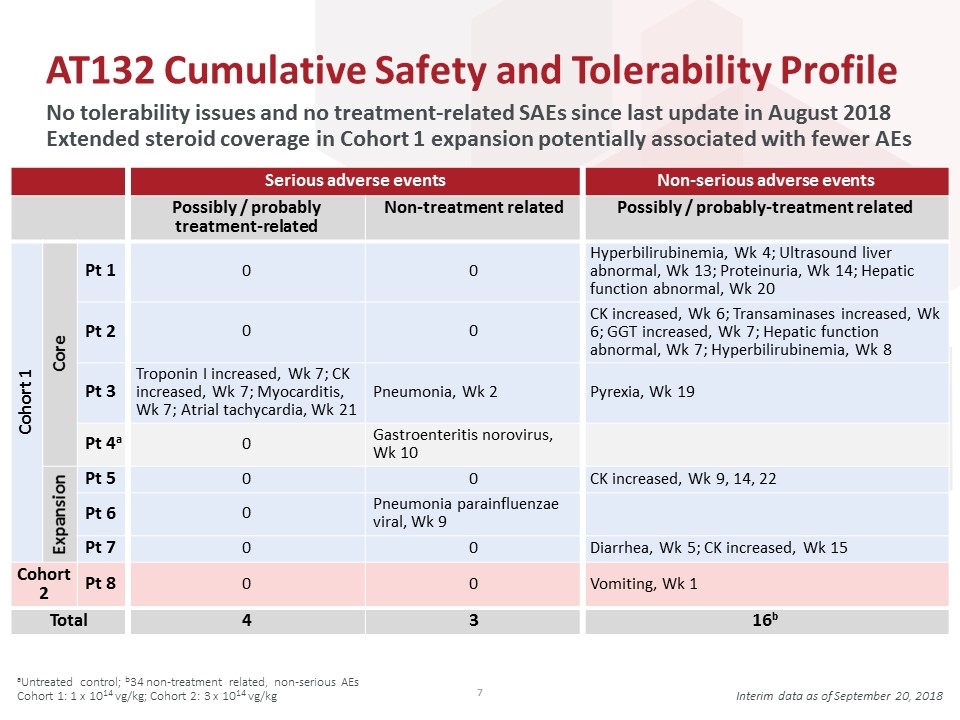
AT132 Cumulative Safety and Tolerability Profile No tolerability issues and no treatment-related SAEs since last update in August 2018 Extended steroid coverage in Cohort 1 expansion potentially associated with fewer AEs aUntreated control; b34 non-treatment related, non-serious AEs Cohort 1: 1 x 1014 vg/kg; Cohort 2: 3 x 1014 vg/kg Interim data as of September 20, 2018 Serious adverse events Non-serious adverse events Possibly / probably treatment-related Non-treatment related Possibly / probably-treatment related Cohort 1 Core Pt 1 0 0 Hyperbilirubinemia, Wk 4; Ultrasound liver abnormal, Wk 13; Proteinuria, Wk 14; Hepatic function abnormal, Wk 20 Pt 2 0 0 CK increased, Wk 6; Transaminases increased, Wk 6; GGT increased, Wk 7; Hepatic function abnormal, Wk 7; Hyperbilirubinemia, Wk 8 Pt 3 Troponin I increased, Wk 7; CK increased, Wk 7; Myocarditis, Wk 7; Atrial tachycardia, Wk 21 Pneumonia, Wk 2 Pyrexia, Wk 19 Pt 4a 0 Gastroenteritis norovirus, Wk 10 Expansion Pt 5 0 0 CK increased, Wk 9, 14, 22 Pt 6 0 Pneumonia parainfluenzae viral, Wk 9 Pt 7 0 0 Diarrhea, Wk 5; CK increased, Wk 15 Cohort 2 Pt 8 0 0 Vomiting, Wk 1 Total 4 3 16b

Neuromuscular Function CHOP INTEND and Developmental Milestones
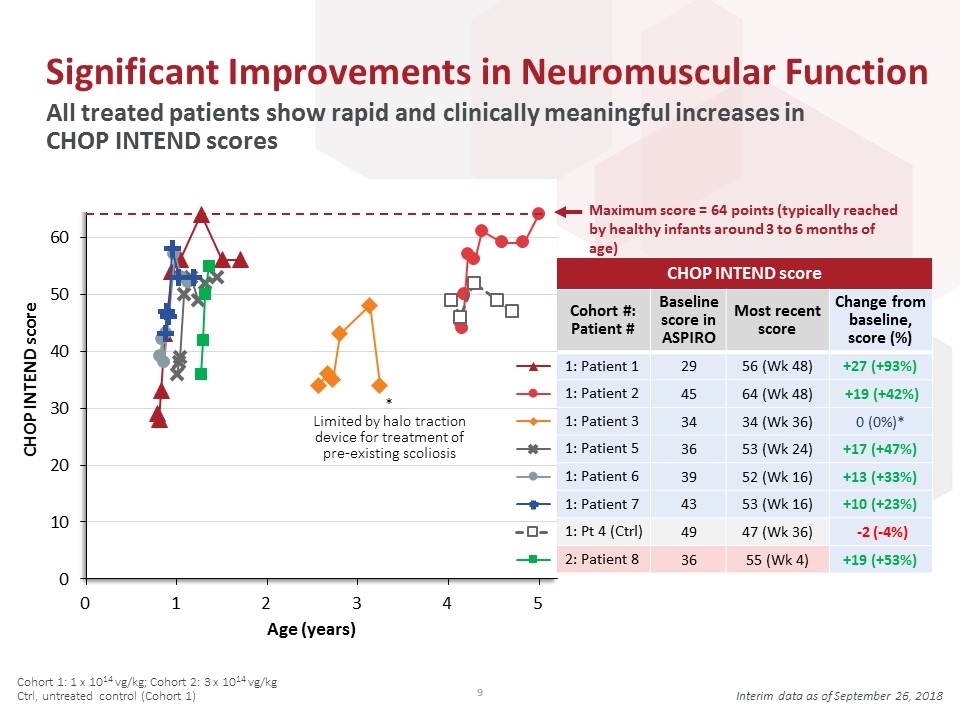
CHOP INTEND score Cohort #: Patient # Baseline score in ASPIRO Most recent score Change from baseline, score (%) 29 56 (Wk 48) +27 (+93%) 45 64 (Wk 48) +19 (+42%) 34 34 (Wk 36) 0 (0%)* 36 53 (Wk 24) +17 (+47%) 39 52 (Wk 16) +13 (+33%) 43 53 (Wk 16) +10 (+23%) 49 47 (Wk 36) -2 (-4%) 36 55 (Wk 4) +19 (+53%) Significant Improvements in Neuromuscular Function All treated patients show rapid and clinically meaningful increases in CHOP INTEND scores Cohort 1: 1 x 1014 vg/kg; Cohort 2: 3 x 1014 vg/kg Ctrl, untreated control (Cohort 1) Interim data as of September 26, 2018 Maximum score = 64 points (typically reached by healthy infants around 3 to 6 months of age) * Limited by halo traction device for treatment of pre-existing scoliosis
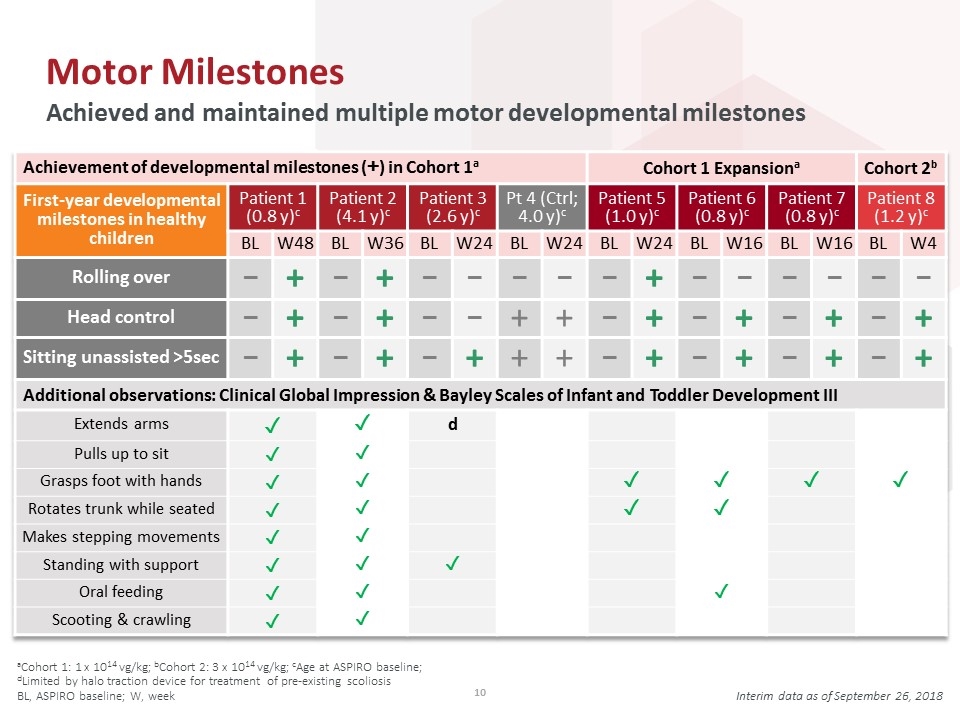
Motor Milestones Achieved and maintained multiple motor developmental milestones aCohort 1: 1 x 1014 vg/kg; bCohort 2: 3 x 1014 vg/kg; cAge at ASPIRO baseline; dLimited by halo traction device for treatment of pre-existing scoliosis BL, ASPIRO baseline; W, week Interim data as of September 26, 2018 Achievement of developmental milestones (+) in Cohort 1a Cohort 1 Expansiona Cohort 2b First-year developmental milestones in healthy children Patient 1 (0.8 y)c Patient 2 (4.1 y)c Patient 3 (2.6 y)c Pt 4 (Ctrl; 4.0 y)c Patient 5 (1.0 y)c Patient 6 (0.8 y)c Patient 7 (0.8 y)c Patient 8 (1.2 y)c BL W48 BL W36 BL W24 BL W24 BL W24 BL W16 BL W16 BL W4 Rolling over – + – + – – – – – + – – – – – – Head control – + – + – – + + – + – + – + – + Sitting unassisted >5sec – + – + – + + + – + – + – + – + Additional observations: Clinical Global Impression & Bayley Scales of Infant and Toddler Development III Extends arms ✓ ✓ d Pulls up to sit ✓ ✓ Grasps foot with hands ✓ ✓ ✓ ✓ ✓ ✓ Rotates trunk while seated ✓ ✓ ✓ ✓ Makes stepping movements ✓ ✓ Standing with support ✓ ✓ ✓ Oral feeding ✓ ✓ ✓ Scooting & crawling ✓ ✓

Respiratory Function Maximal Inspiratory Pressure and Ventilator Dependence
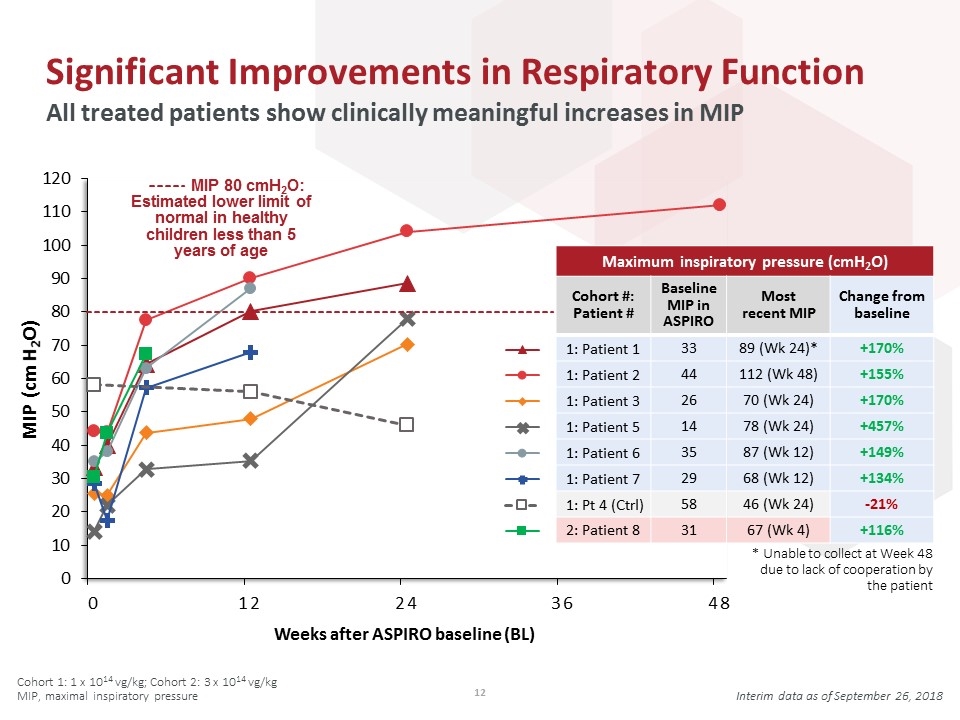
Maximum inspiratory pressure (cmH2O) Cohort #: Patient # Baseline MIP in ASPIRO Most recent MIP Change from baseline 33 89 (Wk 24)* +170% 44 112 (Wk 48) +155% 26 70 (Wk 24) +170% 14 78 (Wk 24) +457% 35 87 (Wk 12) +149% 29 68 (Wk 12) +134% 58 46 (Wk 24) -21% 31 67 (Wk 4) +116% Significant Improvements in Respiratory Function All treated patients show clinically meaningful increases in MIP MIP 80 cmH2O: Estimated lower limit of normal in healthy children less than 5 years of age Weeks after ASPIRO baseline (BL) * Unable to collect at Week 48 due to lack of cooperation by the patient Interim data as of September 26, 2018 Cohort 1: 1 x 1014 vg/kg; Cohort 2: 3 x 1014 vg/kg MIP, maximal inspiratory pressure
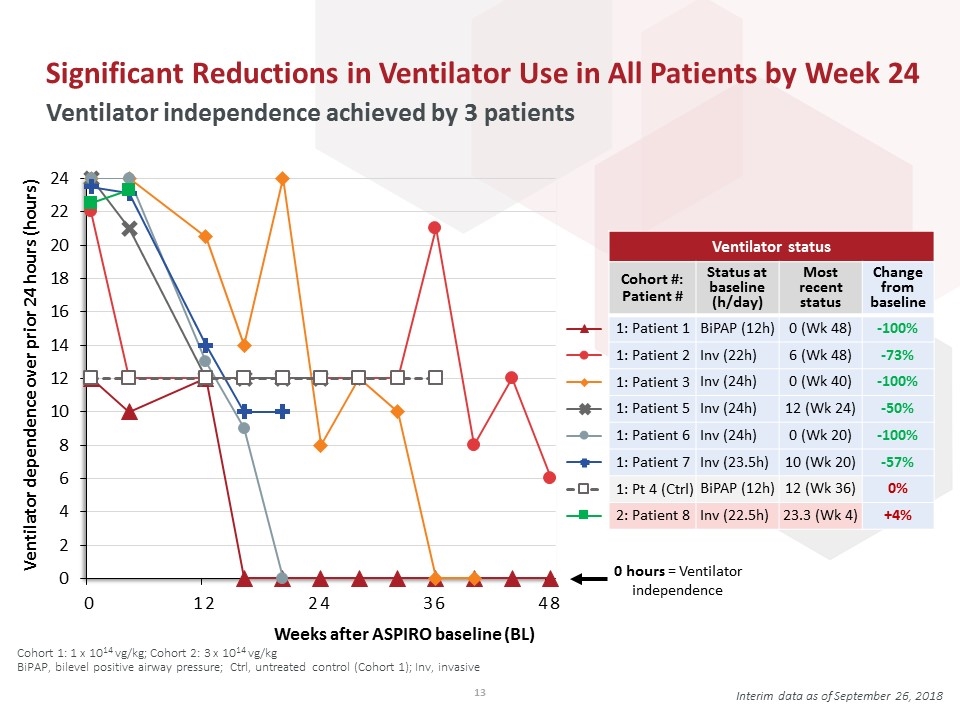
Ventilator status Cohort #: Patient # Status at baseline (h/day) Most recent status Change from baseline BiPAP (12h) 0 (Wk 48) -100% Inv (22h) 6 (Wk 48) -73% Inv (24h) 0 (Wk 40) -100% Inv (24h) 12 (Wk 24) -50% Inv (24h) 0 (Wk 20) -100% Inv (23.5h) 10 (Wk 20) -57% BiPAP (12h) 12 (Wk 36) 0% Inv (22.5h) 23.3 (Wk 4) +4% Significant Reductions in Ventilator Use in All Patients by Week 24 Ventilator independence achieved by 3 patients 0 hours = Ventilator independence Weeks after ASPIRO baseline (BL) Interim data as of September 26, 2018 Cohort 1: 1 x 1014 vg/kg; Cohort 2: 3 x 1014 vg/kg BiPAP, bilevel positive airway pressure; Ctrl, untreated control (Cohort 1); Inv, invasive

Muscle Biopsy
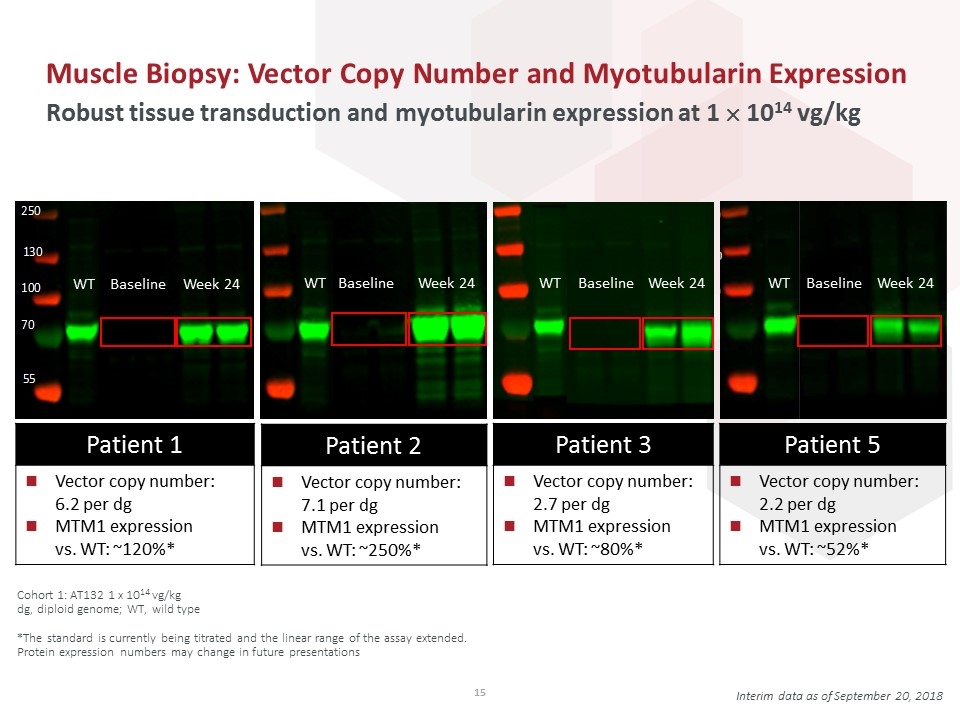
Muscle Biopsy: Vector Copy Number and Myotubularin Expression Robust tissue transduction and myotubularin expression at 1 ´ 1014 vg/kg Interim data as of September 20, 2018 Cohort 1: AT132 1 x 1014 vg/kg dg, diploid genome; WT, wild type *The standard is currently being titrated and the linear range of the assay extended. Protein expression numbers may change in future presentations Baseline WT Week 24 Baseline WT Week 24 Baseline WT Week 24 Patient 1 Vector copy number: 6.2 per dg MTM1 expression vs. WT: ~120%* Patient 2 Vector copy number: 7.1 per dg MTM1 expression vs. WT: ~250%* Patient 3 Vector copy number: 2.7 per dg MTM1 expression vs. WT: ~80%* Patient 5 Vector copy number: 2.2 per dg MTM1 expression vs. WT: ~52%* Baseline WT Week 24
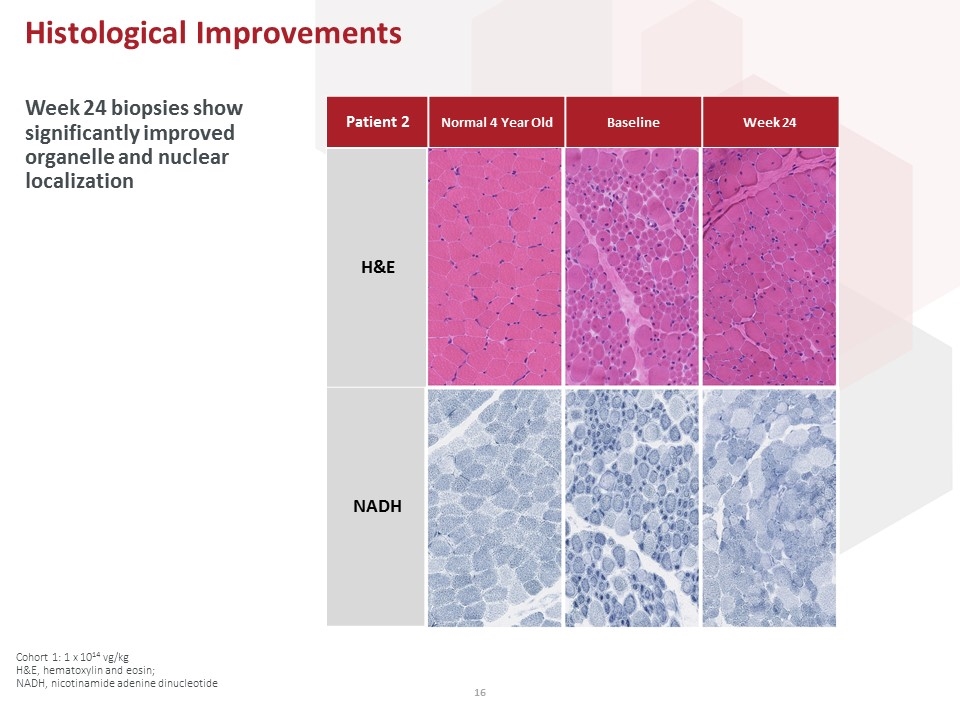
Cohort 1: 1 x 1014 vg/kg H&E, hematoxylin and eosin; NADH, nicotinamide adenine dinucleotide Histological Improvements Week 24 biopsies show significantly improved organelle and nuclear localization Patient 2 Normal 4 Year Old Baseline Week 24 H&E NADH
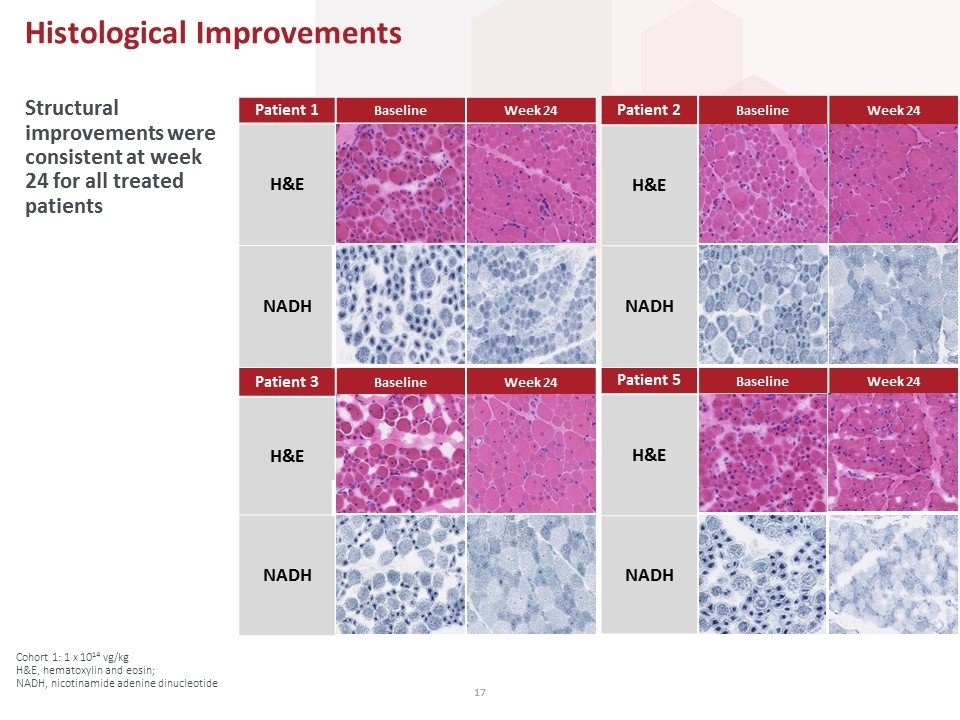
Patient 2 Baseline Week 24 H&E NADH Patient 3 Baseline Week 24 H&E NADH Patient 1 Baseline Week 24 H&E NADH Patient 5 Baseline Week 24 H&E NADH Structural improvements were consistent at week 24 for all treated patients Histological Improvements Cohort 1: 1 x 1014 vg/kg H&E, hematoxylin and eosin; NADH, nicotinamide adenine dinucleotide

Patient Videos Please: No photographic or video recordings of patient videos

ASPIRO Key Findings and Program Next Steps DMC, data monitoring committee AT132 has been well tolerated and has shown a manageable safety profile to date at doses up to 3 ´ 1014 vg/kg Rapid and significant improvements in neuromuscular and respiratory function Clinically meaningful improvements in CHOP INTEND scores and MIP in all treated patients Significant reductions in ventilator use in all patients by Week 24 in Cohort 1 and 3 patients reached ventilator independence Improvement continues through 48 weeks post study drug administration Muscle biopsy results consistent with marked functional improvements Transduction: unprecedented vector copy number in target tissue Expression: robust protein expression as evidenced by Western blots Histopathology: significant improvements in myofiber size, nuclear peripheralization, and organelle localization Assessed Cohort 2 sentinel patient data with DMC and rest of Cohort 2 expected to be enrolled imminently
ASPIRO Phase 1/2 Gene Therapy Trial in X-Linked Myotubular Myopathy (XLMTM): Preliminary Safety and Efficacy Findings
