Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Dova Pharmaceuticals Inc. | a18-36199_18k.htm |
Disclaimer Certain information contained in this presentation relates to or is based on studies, surveys and other data obtained from third-party sources and Dova’s own internal estimates and research. While Dova believes these sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. While Dova believes its internal research is reliable, such research has not been verified by any independent source. This presentation contains forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on Dova’s current beliefs, expectations and assumptions regarding the future of Dova’s business, future plans and strategies, including the potential approval of DOPTELET for the treatment of adult patients with ITP who have had an insufficient response to a previous treatment and the potential to expand the treatment applications for DOPTELET. All statements other than statements of historical facts contained in this presentation, including statements regarding business strategy, degree of market acceptance of approved product, timing for commercial launch, timing and likelihood of success, and plans and objectives of management for future operations, are forward-looking statements. The words ”may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The forward-looking statements in this presentation represent Dova’s views as of the date of this presentation. Although Dova believes the expectations reflected in such forward-looking statements are reasonable, Dova can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Except as required by applicable law, Dova does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. New risk factors and uncertainties may emerge from time to time, and it is not possible to predict all risk factors and uncertainties. For further information regarding these risks, uncertainties and other factors, you should read the “Risk Factors” section of Dova’s Annual Report on Form 10-K for the year ended December 31, 2017, filed with the U.S. Securities and Exchange Commission (“SEC”) on February 16, 2018, Dova's Quarterly Report on Form 10-Q filed with the SEC on August 9, 2018 and Dova’s other Periodic Reports filed with the SEC. 2
Key Business Highlights At-A-Glance treatment of thrombocytopenia (i.e., low platelet counts) patients with chronic liver disease scheduled to undergo a procedure Thrombocytopenic Purpura (ITP) 3 $134.7M cash and equivalents on hand (as of June 30, 2018) $12M used to fund Clinical and Commercial operations for Q2, 2018 Revenue generation commenced in Q2, 2018 following the launch of DOPTELET FINANCIALS Supplemental NDA submitted to FDA on August 30 for the treatment of Immune Well differentiated versus Promacta® (eltrombopag) and Nplate® (romiplostim) Chemotherapy Induced Thrombocytopenia (CIT) study remains on track for 2020 PIPELINE DOPTELET launched in June 2018 for the treatment of thrombocytopenia in adult Physician interest and Payer acceptance continues to be strong Partnership with Salix positions DOPTELET for significantly increased market presence LAUNCH DOPTELET®, a second generation thrombopoietin receptor agonist used in the DOPTELET has demonstrated robust efficacy in both the acute and the chronic setting Patent until May 2025; pending patent term ext. app. to extend patent until 10/2029 DOPTELET®
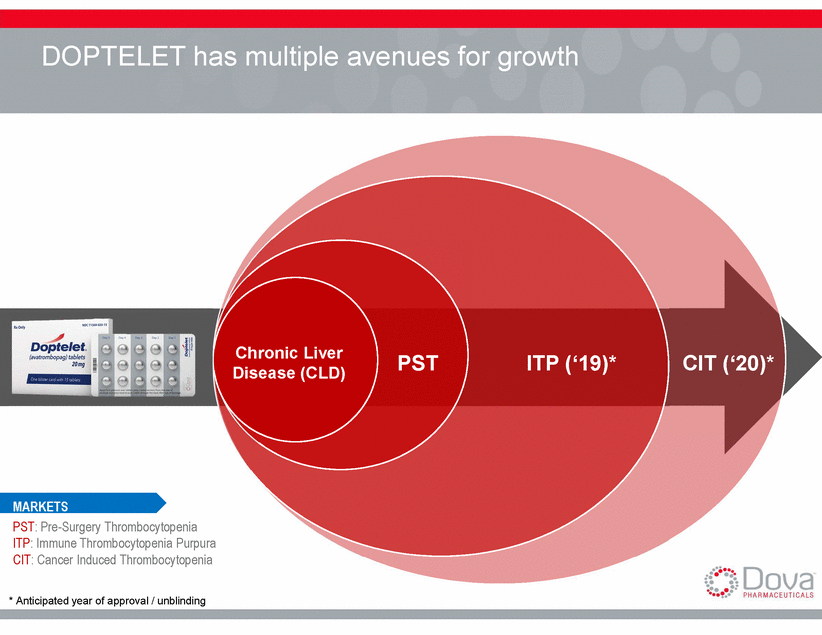
DOPTELET has multiple avenues for growth PST: Pre-Surgery Thrombocytopenia ITP: Immune Thrombocytopenia Purpura CIT: Cancer Induced Thrombocytopenia * Anticipated year of approval / unblinding
May 21st, 2018: DOPTELET receives FDA approval THE FIRST THERAPEUTIC AGENT FOR THE TREATMENT OF THROMBOCYTOPENIA IN ADULT PATIENTS WITH CHRONIC LIVER DISEASE PRIOR TO PLANNED PROCEDURES 5

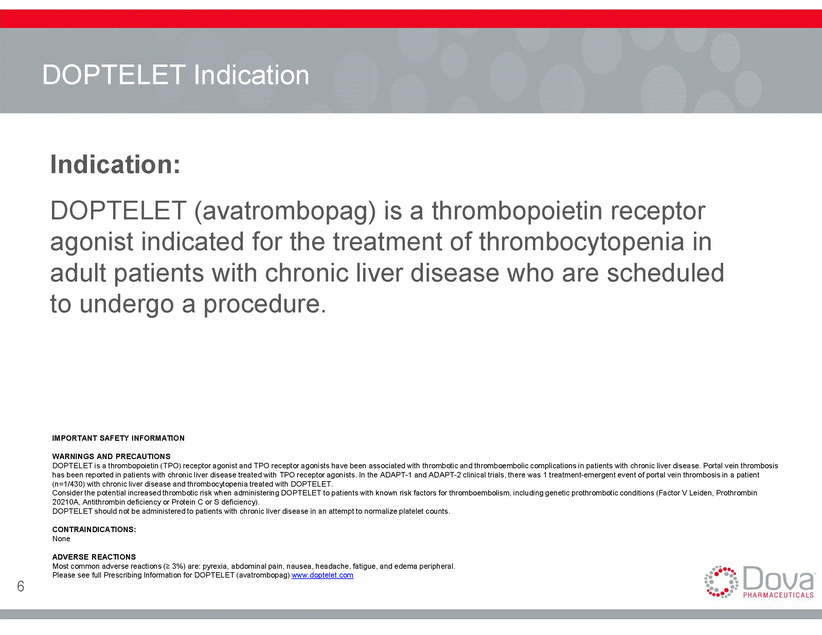
DOPTELET Indication Indication: DOPTELET (avatrombopag) is a thrombopoietin receptor agonist indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. IMPORTANT SAFETY INFORMATION WARNINGS AND PRECAUTIONS DOPTELET is a thrombopoietin (TPO) receptor agonist and TPO receptor agonists have been associated with thrombotic and thromboembolic complications in patients with chronic liver disease. Portal vein thrombosis has been reported in patients with chronic liver disease treated with TPO receptor agonists. In the ADAPT-1 and ADAPT-2 clinical trials, there was 1 treatment-emergent event of portal vein thrombosis in a patient (n=1/430) with chronic liver disease and thrombocytopenia treated with DOPTELET. Consider the potential increased thrombotic risk when administering DOPTELET to patients with known risk factors for thromboembolism, including genetic prothrombotic conditions (Factor V Leiden, Prothrombin 20210A, Antithrombin deficiency or Protein C or S deficiency). DOPTELET should not be administered to patients with chronic liver disease in an attempt to normalize platelet counts. CONTRAINDICATIONS: None ADVERSE REACTIONS Most common adverse reactions ( 3%) are: pyrexia, abdominal pain, nausea, headache, fatigue, and edema peripheral. Please see full Prescribing Information for DOPTELET (avatrombopag) www.doptelet.com 6
There are ~1MM patients with Chronic Liver Disease (CLD) and associated thrombocytopenia Mild 170,000 Moderate 70,000 Severe 20,000 treme 7 Source: Poordad et. al., Aliment Pharmacol Ther, 26 (Suppl 1), 5-11, 2007, Giannini et al, Alimentary Pharm and Therapeutics, 24, 1055-1064, 2006 PATIENTS >75,000 / µL >50K, <75K / µL >25K, <50K / µL <25,000 / µL Ex
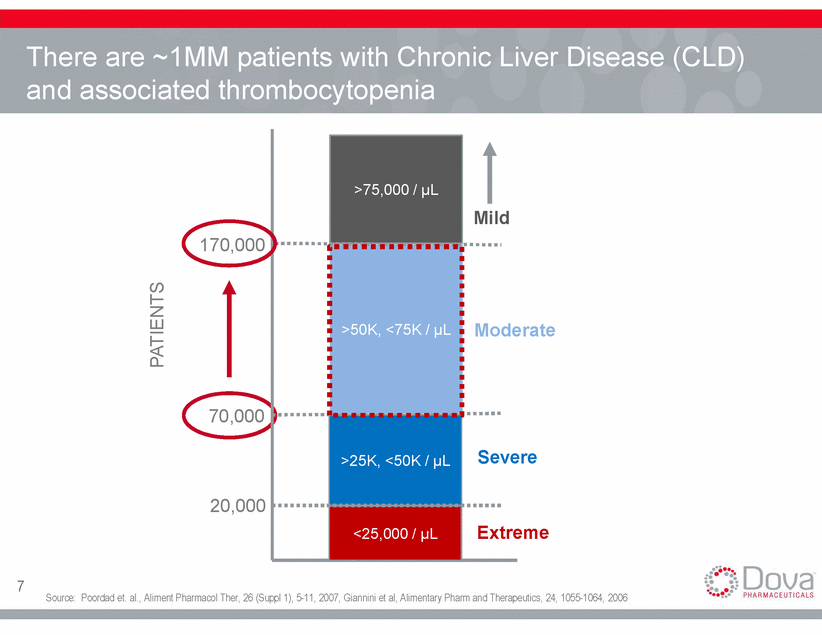
Thrombocytopenic CLD patients who had procedure-related bleeding Patients with Moderate or Severe Thrombocytopenia have a 30% risk of Procedure-Related Bleeding 50% 40% 30% 20% 10% 0% <75,000/µL >75,000/µL Platelet count Number and proportion of thrombocytopenic patients who had procedure-related bleeding subdivided according to the degree of thrombocytopenia. Source: Giannini et al, Clinical Gastroenterology and Hepatology, Vol. 8, No. 10, page 901 8 Proportion of bleeding patients 31% 10 / 32 P = .008 0% 0 / 18
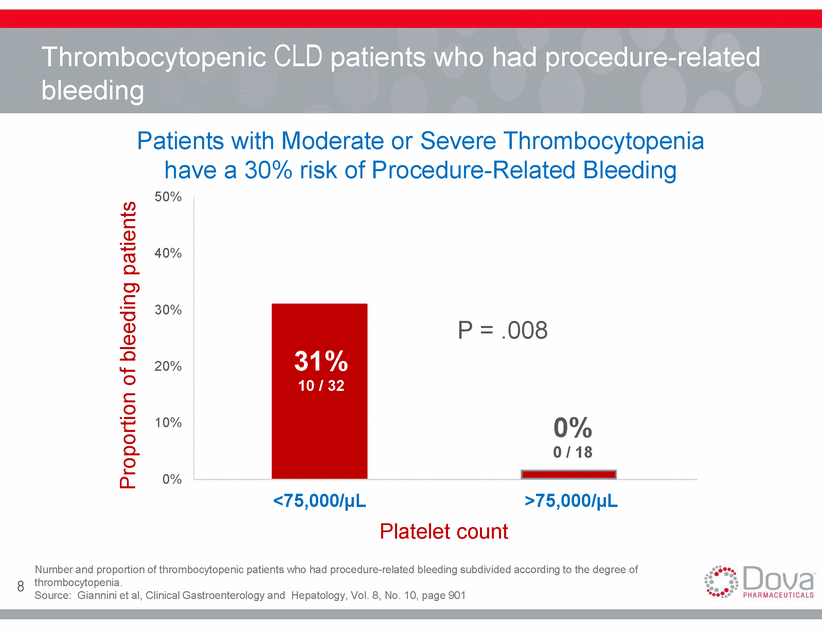
Platelet transfusions issues are associated with clinically significant RISK OF ANTIBODY DEVELOPMENT SHORT DURATION OF EFFECT AND LIMITED SUPPLY 3 1 • May lead to refractoriness in up to ~50% of patients • • Short shelf life of platelets (5 days) Transfusion must be given same day as procedure RISK OF INFECTION & ADVERSE REACTIONS INCONVENIENT ADMINISTRATION 2 4 • • • Immune reaction Febrile non-hemolytic reactions Sepsis • • Administration time Potential AE risk 9
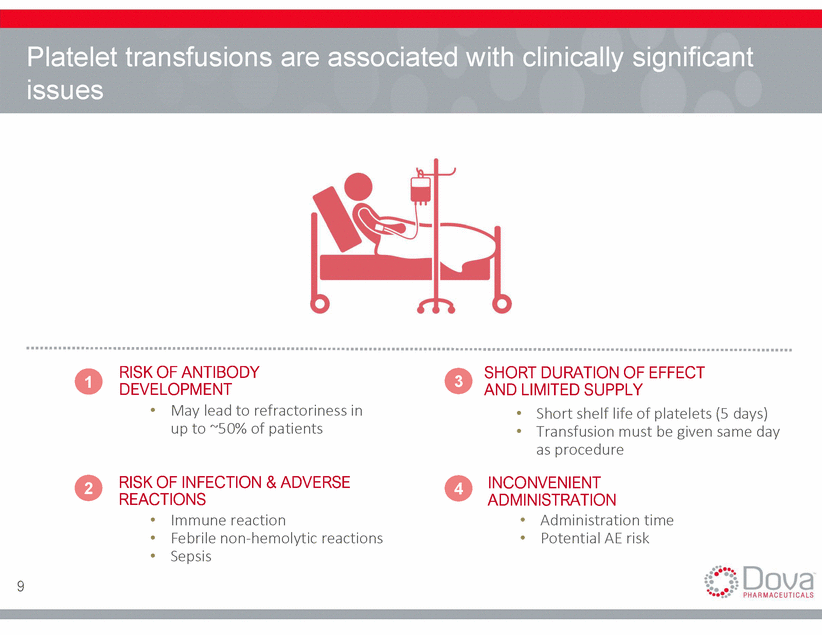
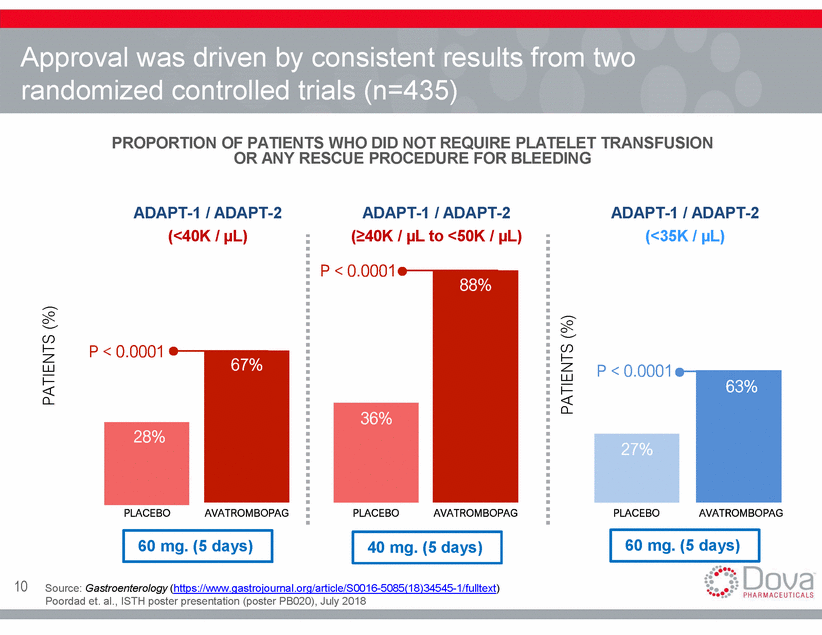
Approval was driven by consistent results from two randomized controlled trials (n=435) PROPORTION OF PATIENTS WHO DID NOT REQUIRE PLATELET TRANSFUSION OR ANY RESCUE PROCEDURE FOR BLEEDING ADAPT-1 / ADAPT-2 (<40K / µL) ADAPT-1 / ADAPT-2 (40K / µL to <50K / µL) ADAPT-1 / ADAPT-2 (<35K / µL) P < 0.0001 P < 0.0001 P < 0.0001 36% 28% 27% PLACEBO AVATROMBOPAG PLACEBO AVATROMBOPAG PLACEBO AVATROMBOPAG 60 mg. (5 days) 60 mg. (5 days) 40 mg. (5 days) 10 Source: Gastroenterology (https://www.gastrojournal.org/article/S0016-5085(18)34545-1/fulltext) Poordad et. al., ISTH poster presentation (poster PB020), July 2018 PATIENTS (%) PATIENTS (%) 63% 67% 88%
Encouraging early launch metrics Q2 Launch Metrics Q3 Launch Metrics Q4 Launch Metrics # of unique prescribers 148* % of adjudicated Rx’s approved by payers 81% Average time to payer approval 6.9 days % of target prescribers contacted 62%: average of 3.1 times Change in channel inventory levels N/A 11 * As of August 8, 2018
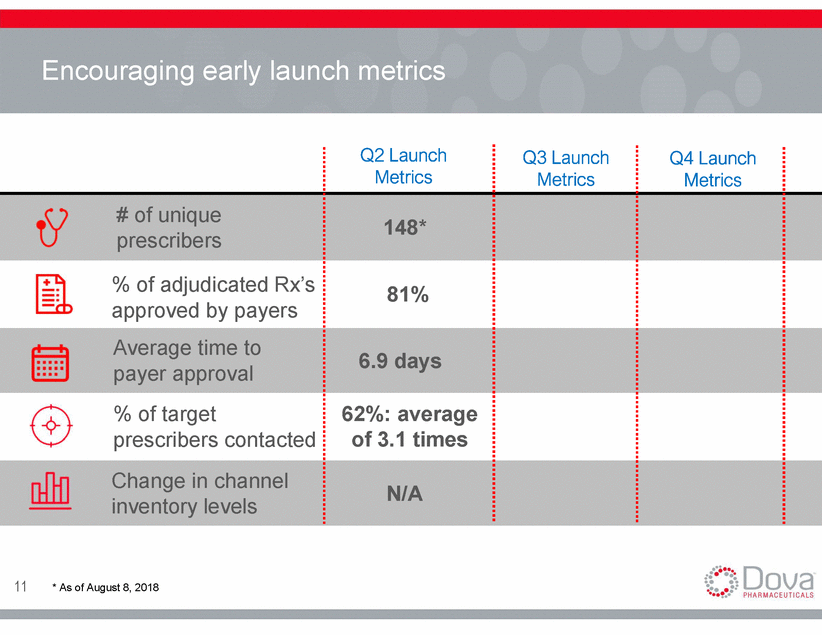
Unique prescribers of DOPTELET* Unique Prescribers of DOPTELET* 61% 39% Non-Gastroenterologist Gastroenterologist *number of Unique Prescribers that had prescribed DOPTELET to a patient as of August 8, 2018 = 148 12
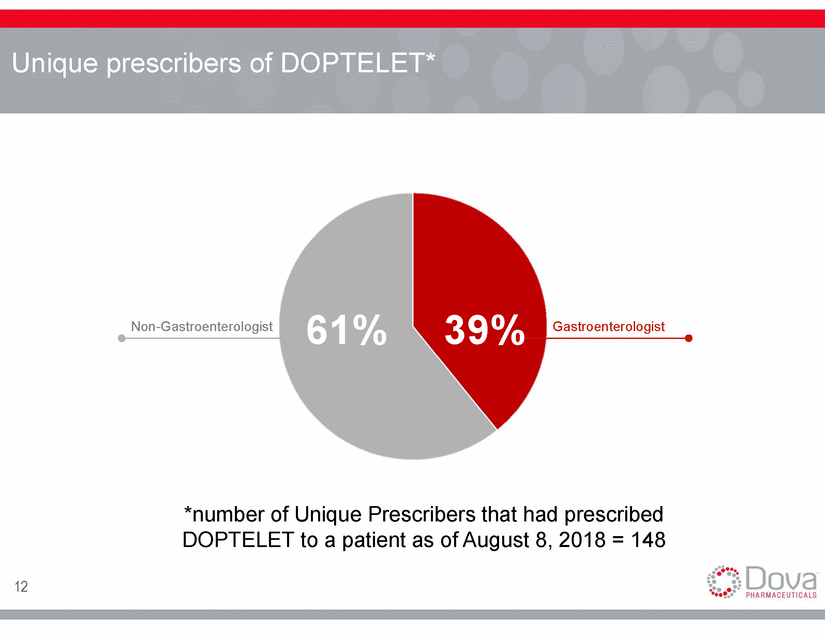
Market Segmentation Centers (LTC) w/o LTC Large Gastro. Group Independent Setting 13 Low Potential Medium Potential High Potential Academic Setting Academic Centers Liver Transplant Small GI PracticesPractices Community
September 27, 2018: Dova announces co-promotion agreement with Salix Pharmaceuticals Unique Prescribers of DOPTELET* 100 sales representatives focused on large GI Group Practices 4-year term Salix receives commission (mid 20% - mid 30%) on Net Sales generated by GI Training commences October 9th Selling commences October 15th 14

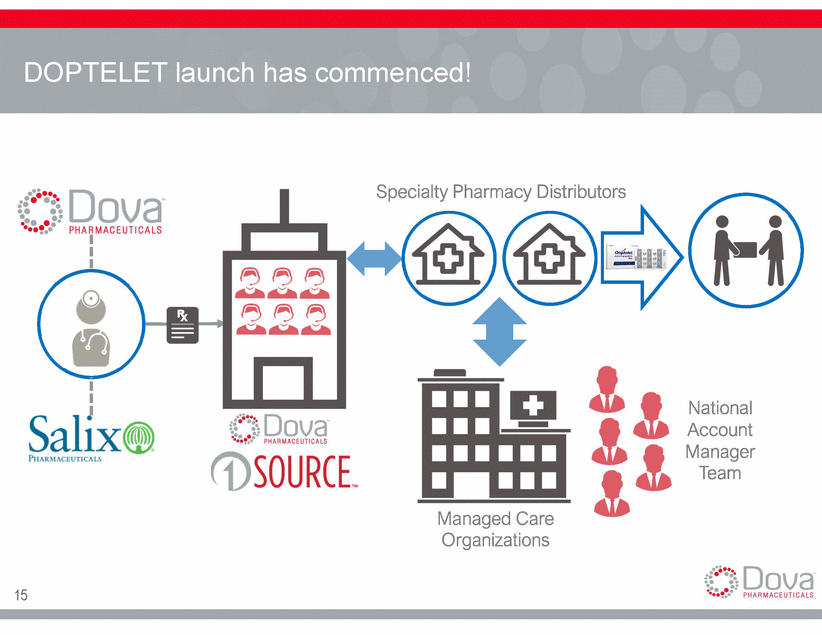
DOPTELET launch has commenced! Specialty Pharmacy Distributors National Account Manager Team Managed Care Organizations 15
Our campaign has evolved increase to focus on platelet count May 21, 2018 DOPTELET Receives FDA Approval Sept. 27, 2018 Co-Promote with Salix Announced TARGET A MEANINGFUL RESPONSE 16

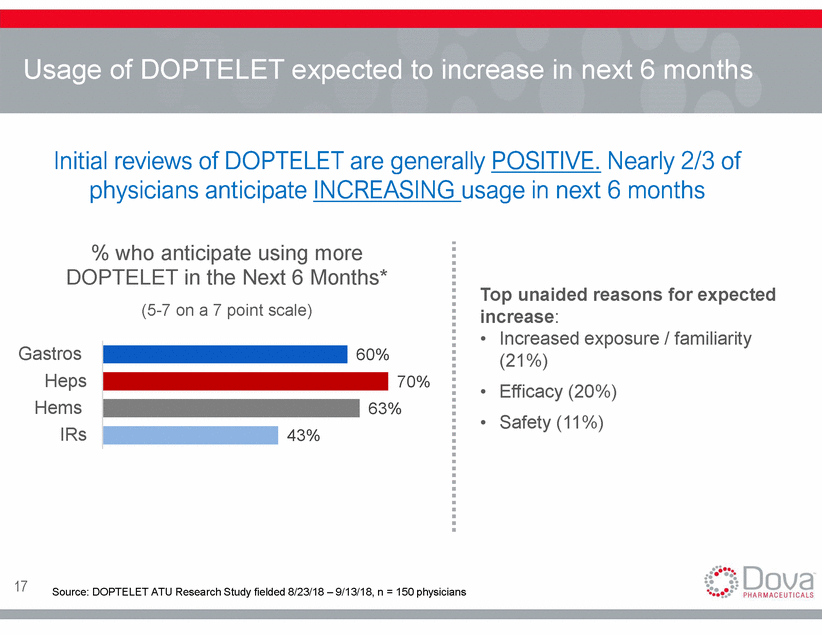
Usage of DOPTELET expected to increase in next 6 months Initial reviews of DOPTELET are generally POSITIVE. Nearly 2/3 of physicians anticipate INCREASING usage in next 6 months % who anticipate using more DOPTELET in the Next 6 Months* (5-7 on a 7 point scale) Top unaided reasons for expected increase: • Increased exposure / familiarity (21%) Efficacy (20%) Safety (11%) Gastros Heps Hems IRs 60% 70% • • % 17 Source: DOPTELET ATU Research Study fielded 8/23/18 – 9/13/18, n = 150 physicians 63 43%
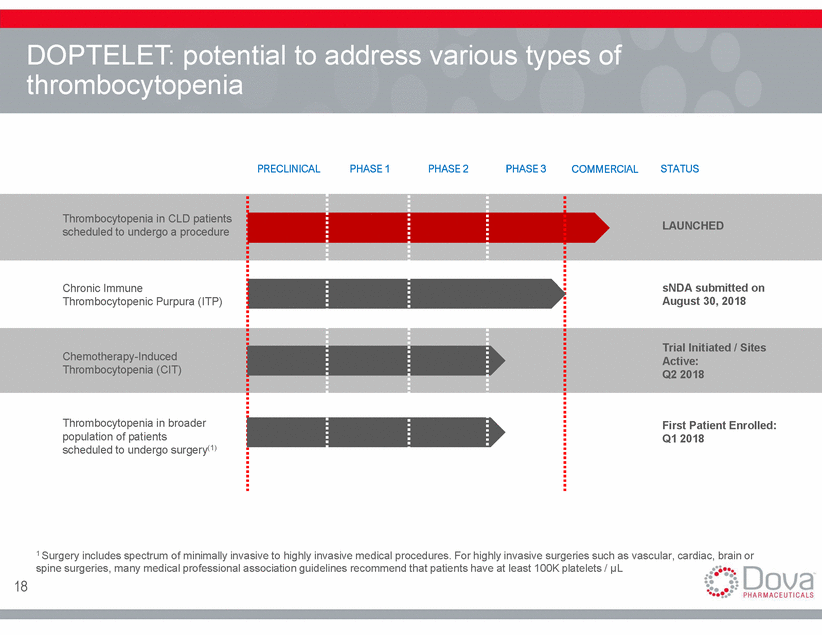
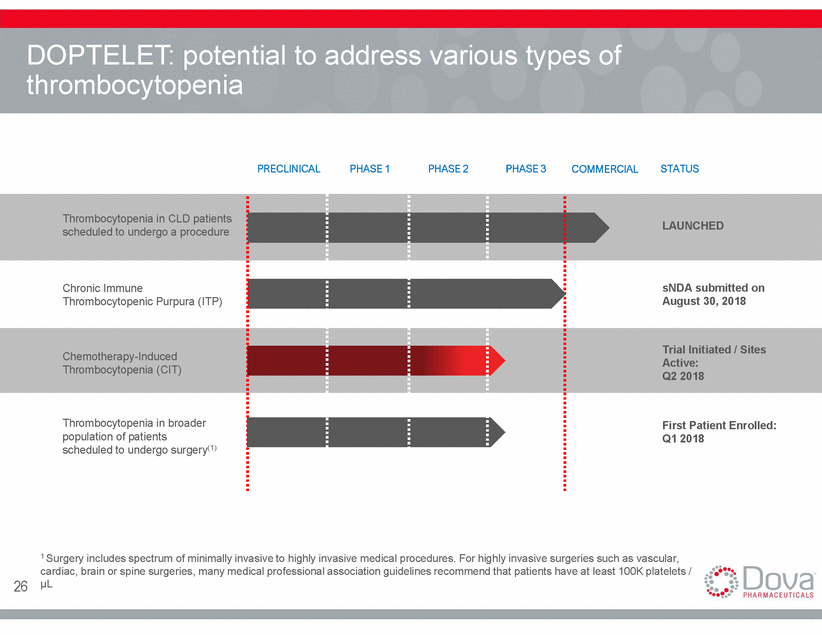
DOPTELET: potential to address thrombocytopenia various types of PRECLINICAL PHASE 1 PHASE 2 PHASE 3 COMMERCIAL STATUS Thrombocytopenia in CLD patients scheduled to undergo a procedure LAUNCHED Chronic Immune Thrombocytopenic Purpura (ITP) sNDA submitted on August 30, 2018 Trial Initiated / Sites Active: Q2 2018 Chemotherapy-Induced Thrombocytopenia (CIT) Thrombocytopenia in broader population of patients scheduled to undergo surgery(1) First Patient Enrolled: Q1 2018 1 Surgery includes spectrum of minimally invasive to highly invasive medical procedures. For highly invasive surgeries such as vascular, cardiac, brain or spine surgeries, many medical professional association guidelines recommend that patients have at least 100K platelets / µL 18
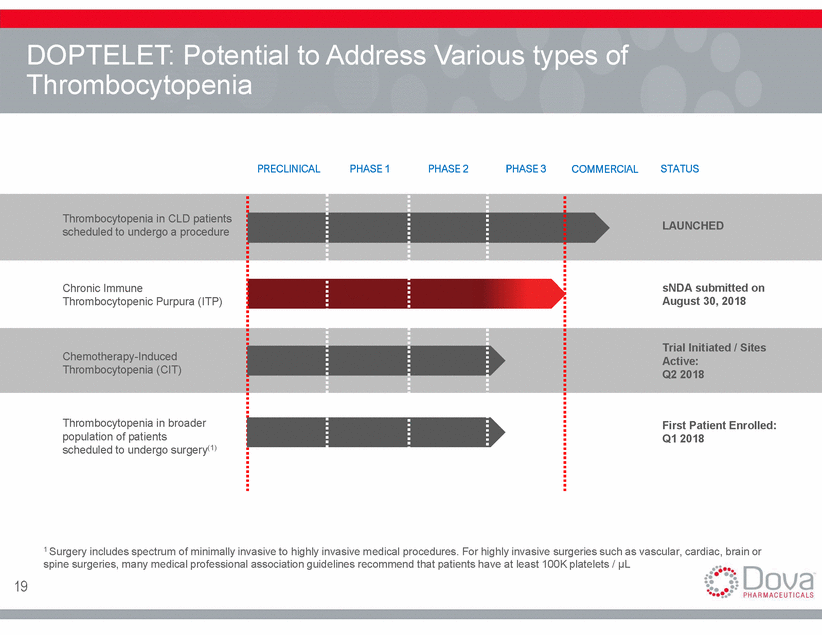
DOPTELET: Potential to Thrombocytopenia Address Various types of PRECLINICAL PHASE 1 PHASE 2 PHASE 3 COMMERCIAL STATUS Thrombocytopenia in CLD patients scheduled to undergo a procedure LAUNCHED Chronic Immune Thrombocytopenic Purpura (ITP) sNDA submitted on August 30, 2018 Trial Initiated / Sites Active: Q2 2018 Chemotherapy-Induced Thrombocytopenia (CIT) Thrombocytopenia in broader population of patients scheduled to undergo surgery(1) First Patient Enrolled: Q1 2018 1 Surgery includes spectrum of minimally invasive to highly invasive medical procedures. For highly invasive surgeries such as vascular, cardiac, brain or spine surgeries, many medical professional association guidelines recommend that patients have at least 100K platelets / µL 19
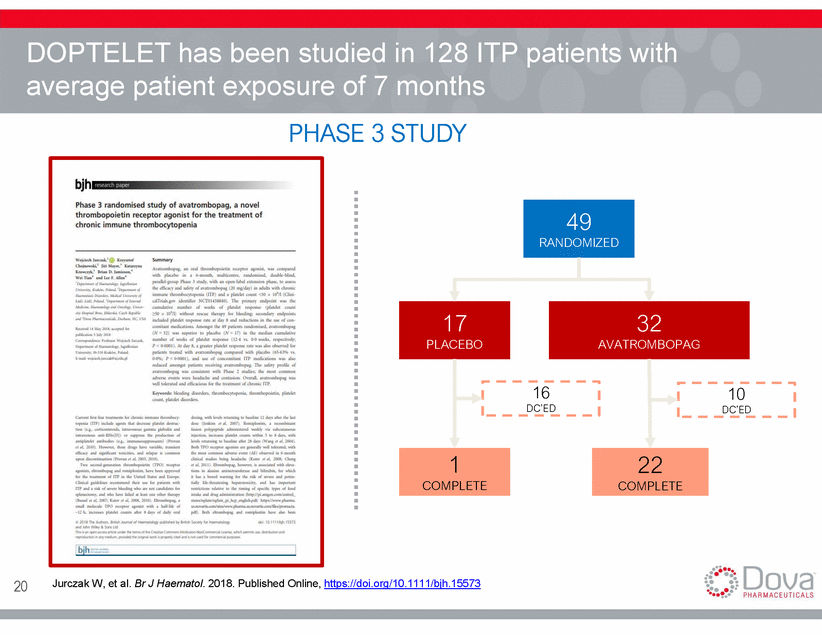
DOPTELET has been studied in 128 ITP patients with average patient exposure of PHASE 7 months 3 STUDY 16 DC’ED 10 DC’ED 20 Jurczak W, et al. Br J Haematol. 2018. Published Online, https://doi.org/10.1111/bjh.15573 22 COMPLETE 1 COMPLETE 32 AVATROMBOPAG 17 PLACEBO 49 RANDOMIZED
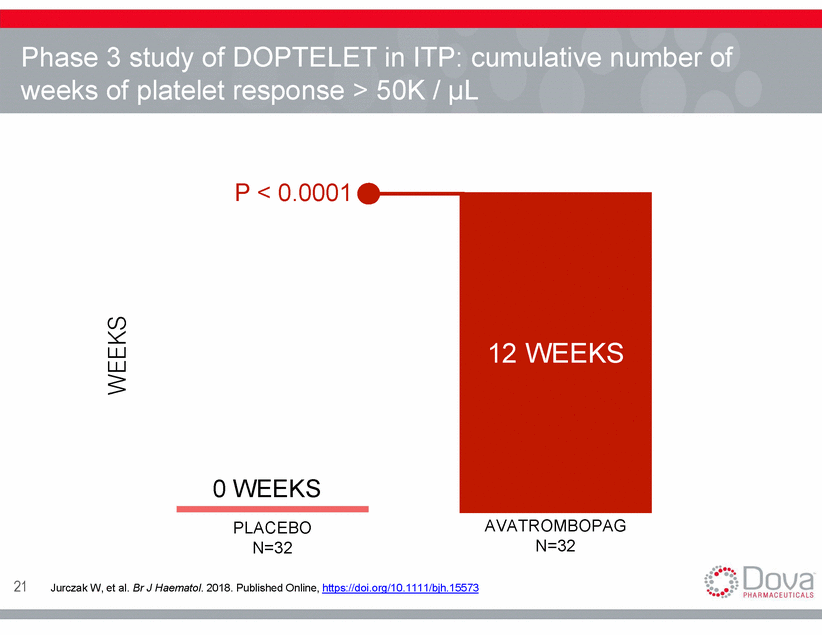
Phase weeks 3 study of DOPTELET in ITP: cumulative number of of platelet response > 50K / µL P < 0.0001 67% 0 WEEKS PLACEBO N=32 AVATROMBOPAG N=32 21 Jurczak W, et al. Br J Haematol. 2018. Published Online, https://doi.org/10.1111/bjh.15573 WEEKS 12 WEEKS
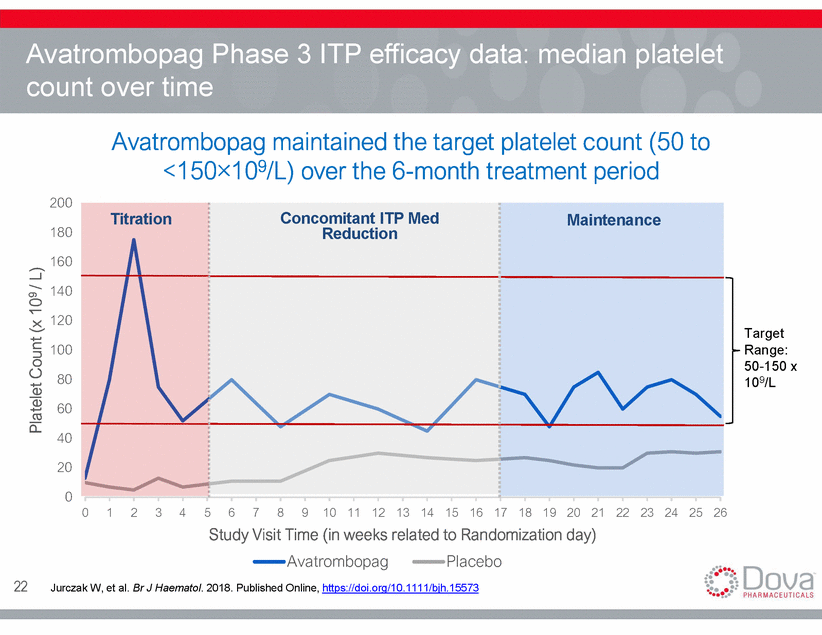
Avatrombopag Phase 3 ITP efficacy data: median platelet count over time Avatrombopag maintained the target platelet count (50 to <150×109/L) over the 6-month treatment period 200 Concomitant ITP Med Reduction Titration Maintenance 180 160 140 120 Target Range: 50-150 x 109/L 100 80 60 40 20 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 Study Visit Time (in weeks related to Randomization day) Avatrombopag Placebo 22 Jurczak W, et al. Br J Haematol. 2018. Published Online, https://doi.org/10.1111/bjh.15573 Platelet Count (x 109 / L)
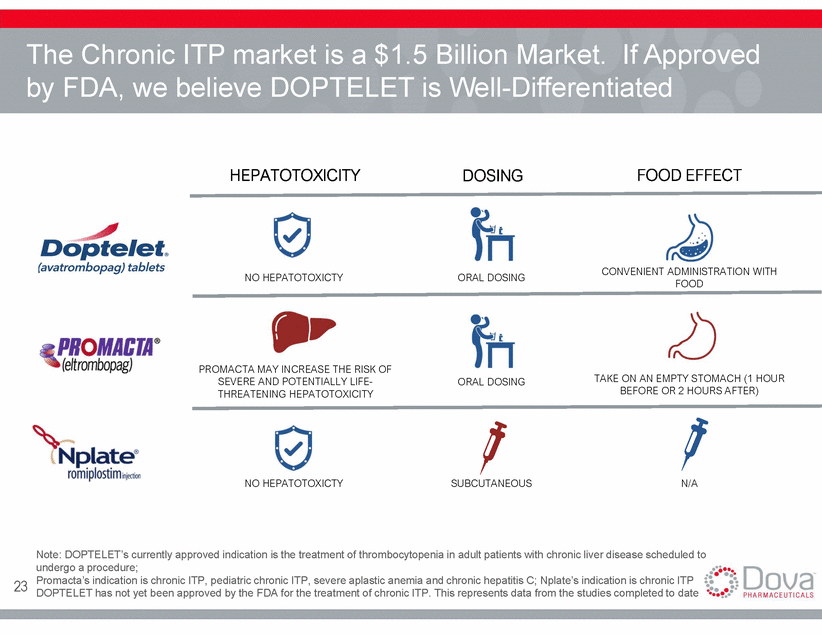
The Chronic ITP market is a $1.5 Billion Market. If Approved by FDA, we believe DOPTELET is Well-Differentiated HEPATOTOXICITY DOSING FOOD EFFECT CONVENIENT ADMINISTRATION WITH FOOD NO HEPATOTOXICTY ORAL DOSING PROMACTA MAY INCREASE THE RISK OF SEVERE AND POTENTIALLY LIFE-THREATENING HEPATOTOXICITY TAKE ON AN EMPTY STOMACH (1 HOUR BEFORE OR 2 HOURS AFTER) ORAL DOSING NO HEPATOTOXICTY SUBCUTANEOUS N/A Note: DOPTELET’s currently approved indication is the treatment of thrombocytopenia in adult patients with chronic liver disease scheduled to undergo a procedure; Promacta’s indication is chronic ITP, pediatric chronic ITP, severe aplastic anemia and chronic hepatitis C; Nplate’s indication is chronic ITP DOPTELET has not yet been approved by the FDA for the treatment of chronic ITP. This represents data from the studies completed to date 23
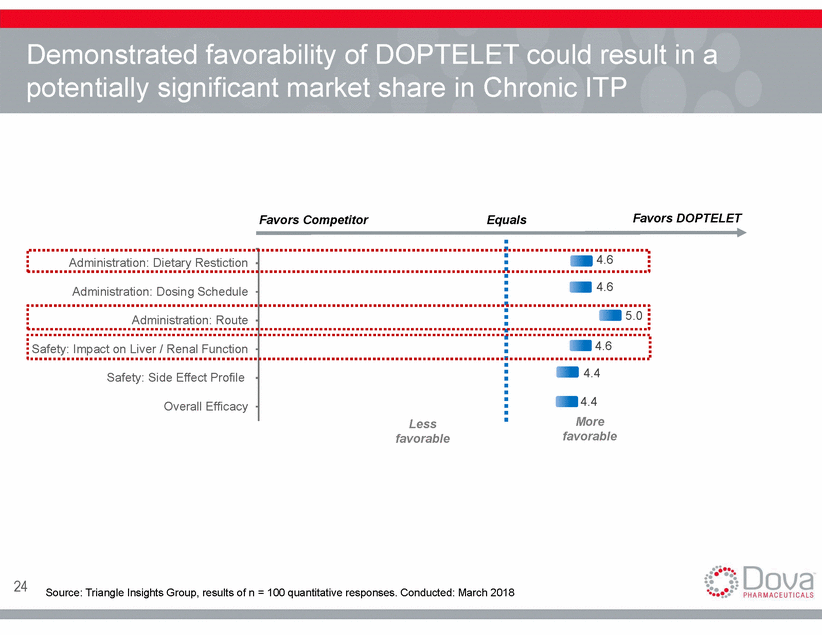
Demonstrated favorability of DOPTELET could result in a potentially significant market share in Chronic ITP Favors DOPTELET Favors Competitor Equals More favorable Less favorable 24 Source: Triangle Insights Group, results of n = 100 quantitative responses. Conducted: March 2018 Administration: Dietary Restiction 4.6 4.6 5.0 Administration: Dosing Schedule Administration: Route Safety: Impact on Liver / Renal Function 4.6 Safety: Side Effect Profile Overall Efficacy 4.4 4.4
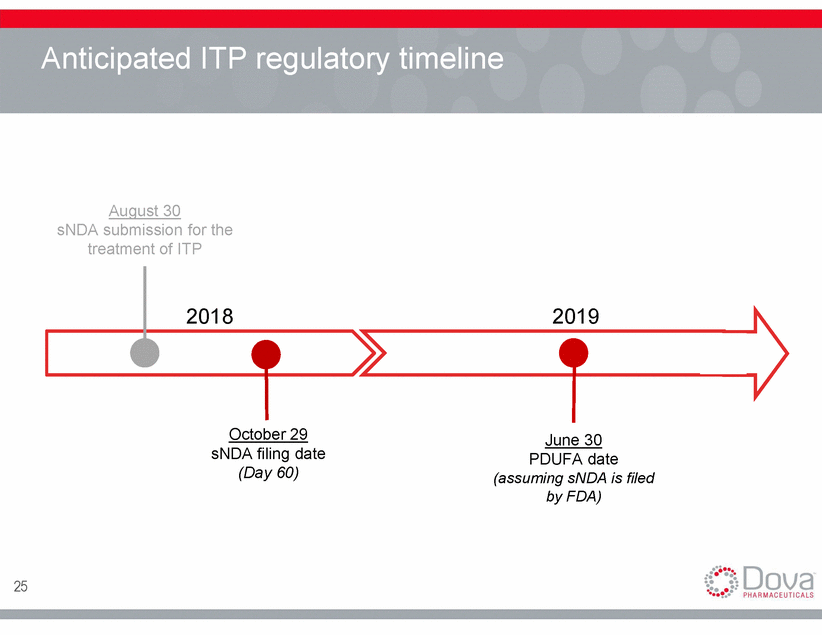
Anticipated ITP regulatory timeline August 30 sNDA submission for the treatment of ITP 2018 2019 October 29 June 30 sNDA filing date (Day 60) PDUFA date (assuming sNDA is filed by FDA) 25
DOPTELET: potential to address thrombocytopenia various types of PRECLINICAL PHASE 1 PHASE 2 PHASE 3 COMMERCIAL STATUS Thrombocytopenia in CLD patients scheduled to undergo a procedure LAUNCHED Chronic Immune Thrombocytopenic Purpura (ITP) sNDA submitted on August 30, 2018 Trial Initiated / Sites Active: Q2 2018 Chemotherapy-Induced Thrombocytopenia (CIT) Thrombocytopenia in broader population of patients scheduled to undergo surgery(1) First Patient Enrolled: Q1 2018 1 Surgery includes spectrum of minimally invasive to highly invasive medical procedures. For highly invasive surgeries such as vascular, cardiac, brain or spine surgeries, many medical professional association guidelines recommend that patients have at least 100K platelets / µL 26
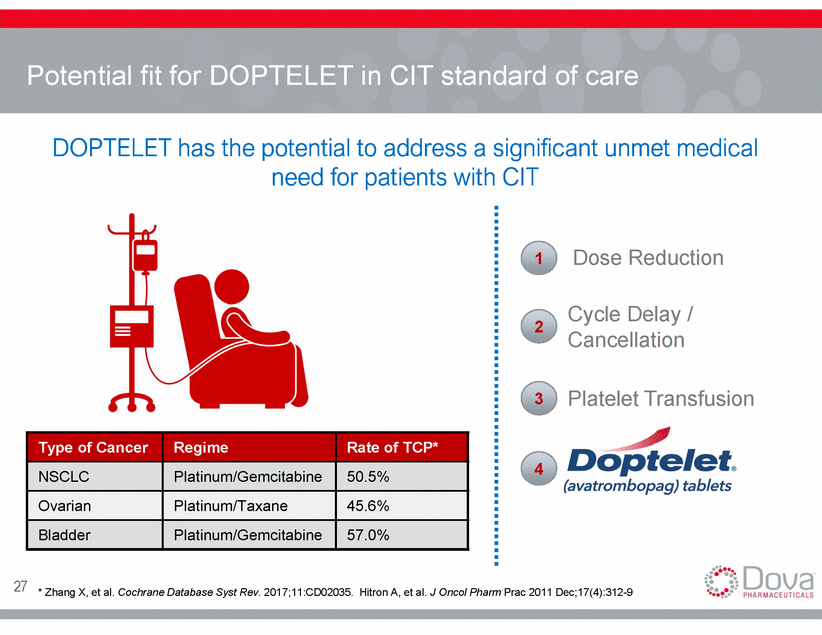
Potential fit for DOPTELET in CIT standard of care DOPTELET has the potential to address a significant unmet medical need for patients with CIT Dose Reduction 1 Cycle Delay / Cancellation 2 Platelet Transfusion 3 4 27 * Zhang X, et al. Cochrane Database Syst Rev. 2017;11:CD02035. Hitron A, et al. J Oncol Pharm Prac 2011 Dec;17(4):312-9 Type of Cancer Regime Rate of TCP* NSCLC Platinum/Gemcitabine 50.5% Ovarian Platinum/Taxane 45.6% Bladder Platinum/Gemcitabine 57.0%
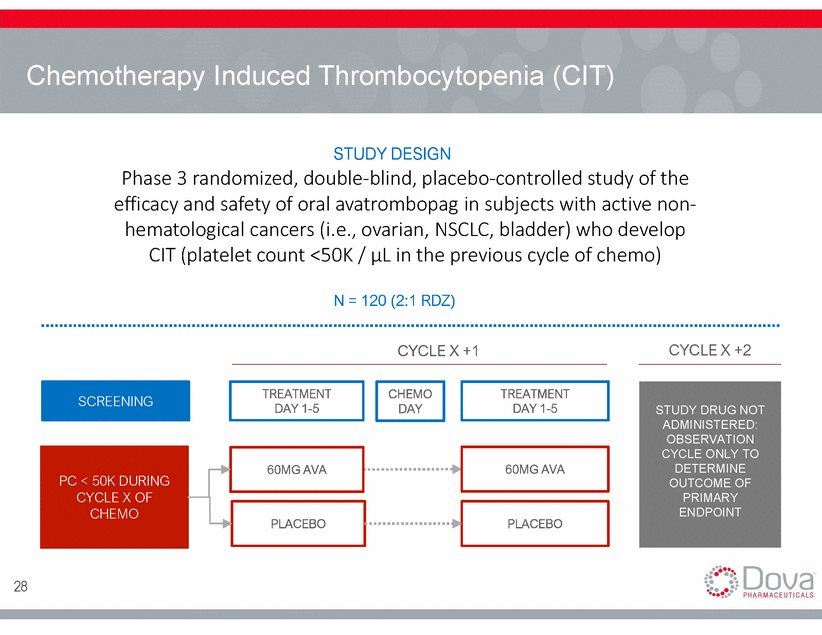
Chemotherapy Induced Thrombocytopenia (CIT) STUDY DESIGN Phase 3 randomized, double-blind, placebo-controlled study of the efficacy and safety of oral avatrombopag in subjects with active non-hematological cancers (i.e., ovarian, NSCLC, bladder) who develop CIT (platelet count <50K / µL in the previous cycle of chemo) N = 120 (2:1 RDZ) CYCLE X +1 CYCLE X +2 TREATMENT DAY 1-5 CHEMO DAY TREATMENT DAY 1-5 STUDY DRUG NOT ADMINISTERED: OBSERVATION CYCLE ONLY TO DETERMINE OUTCOME OF PRIMARY ENDPOINT 60MG AVA 60MG AVA PLACEBO PLACEBO 28 PC < 50K DURING CYCLE X OF CHEMO SCREENING
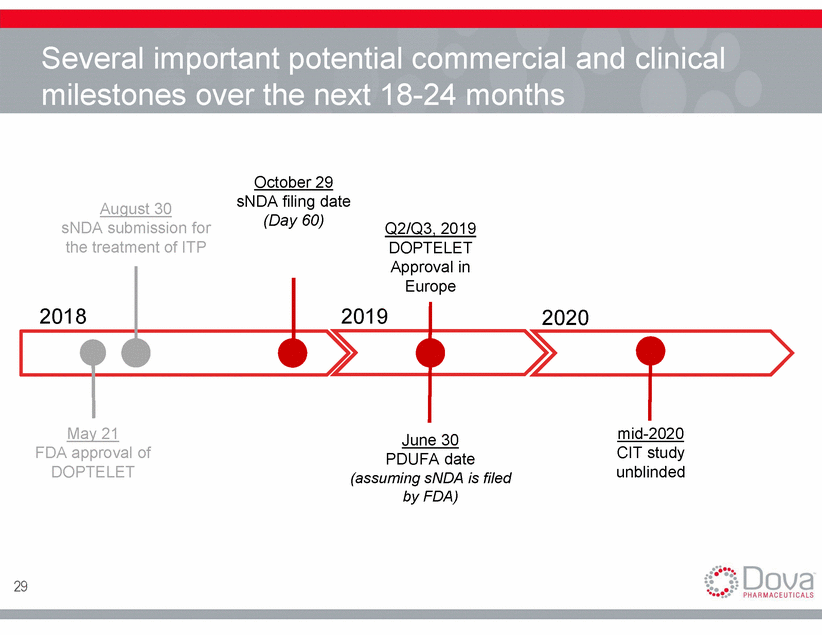
Several important potential commercial and clinical milestones over the next 18-24 months October 29 sNDA filing date (Day 60) August 30 sNDA submission for the treatment of ITP Q2/Q3, 2019 DOPTELET Approval in Europe 2018 2019 2020 May 21 mid-2020 CIT study unblinded June 30 FDA approval of DOPTELET PDUFA date (assuming sNDA is filed by FDA) 29
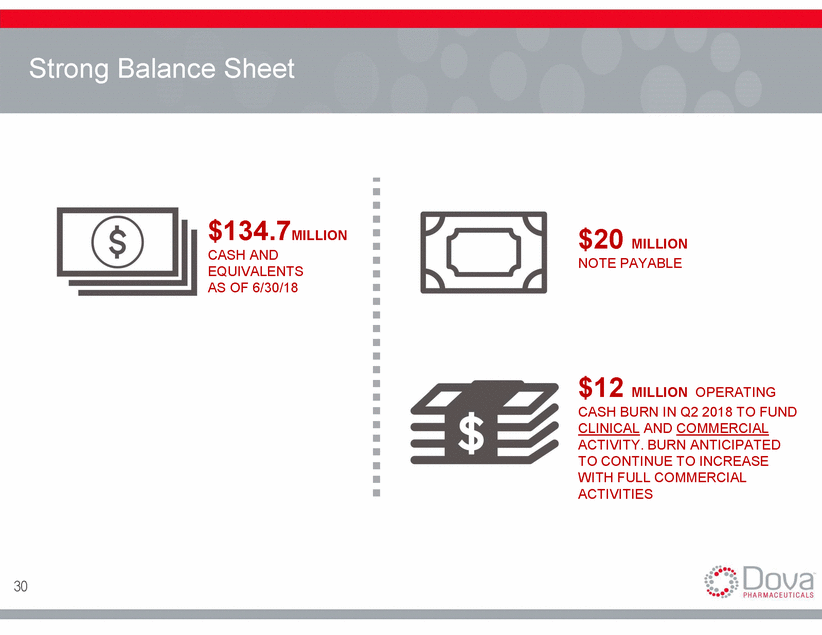
Strong Balance Sheet $134.7MILLION CASH AND EQUIVALENTS AS OF 6/30/18 $20 MILLION NOTE PAYABLE $12 MILLION OPERATING CASH BURN IN Q2 2018 TO FUND CLINICAL AND COMMERCIAL ACTIVITY. BURN ANTICIPATED TO CONTINUE TO INCREASE WITH FULL COMMERCIAL ACTIVITIES 30
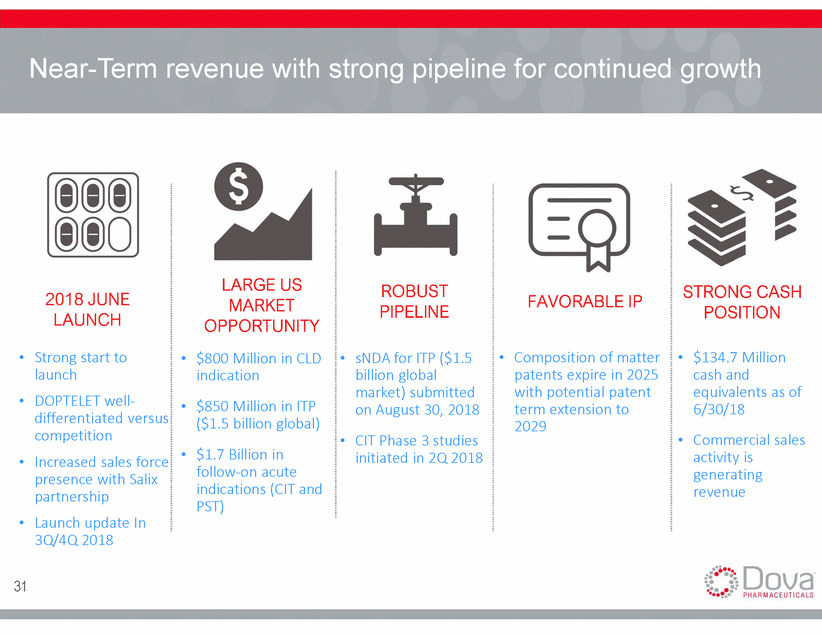
Near-Term revenue with strong pipeline for continued growth LARGE US MARKET OPPORTUNITY $800 Million in CLD indication $850 Million in ITP ($1.5 billion global) $1.7 Billion in follow-on acute indications (CIT and PST) ROBUST PIPELINE STRONG CASH POSITION 2018 JUNE LAUNCH FAVORABLE IP Strong start to launch DOPTELET well-differentiated versus competition Increased sales force presence with Salix partnership Launch update In 3Q/4Q 2018 sNDA for ITP ($1.5 billion global market) submitted on August 30, 2018 CIT Phase 3 studies initiated in 2Q 2018 Composition of matter patents expire in 2025 with potential patent term extension to 2029 $134.7 Million cash and equivalents as of 6/30/18 Commercial sales activity is generating revenue • • • • • • • • • • • • 31
Dova PHARMACEUTICALS


