Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Moleculin Biotech, Inc. | form8-kinvestorpresentation.htm |

NASDAQ: MBRX Public Information- September 18, 2018

DISCLAIMER All statements contained herein other than statements of historical fact, including statements regarding our future results of operations and financial position, our business strategy and plans, and our objectives for future operations, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives, and financial needs. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under Item 1A. "Risk Factors" in our most recently filed Form 10-K filed with the Securities and Exchange Commission ("SEC") and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward looking statements. More detailed information about Moleculin is set forth in our filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov.

Robust pipeline 3 distinctly different technologies - six potential drug candidates, all with blockbuster potential World-leading collaboration MD Anderson Cancer Center/Mayo Clinic/Emory University Breakthrough disruptive technologies Annamycin for AML: designed to be non-cardiotoxic, avoids MDR1 WP1066 Portfolio: STAT3 inhibitor that also stimulates immune response WP1122 Portfolio: metabolic inhibitor with improved BBB transmission and animal model activity against pancreatic cancer Highly experienced leadership Veteran pharma/biotech, life science micro-cap managers Proprietary positioning Orphan drug and/or patents, exclusive licenses (applied and received) 3

Experienced Management Team Selected Prior Experience Walter Klemp Chairman, President & CEO Don Picker, PhD Chief Science Officer Jonathan P. Foster. CPA, CGMA EVP & CFO Robert Shepard, MD, FACP Chief Medical Officer Sandra Silberman, MD & PhD Chief Medical Officer – New Products 4

Science Advisory Board Members Selected Prior Experience/Publications Waldemar Priebe, PhD John Paul Waymack, MD, SCD Jorge Cortes, MD Elihu Estey, MD 5

Development Pipeline Preclinical Clinical Preparation Phase I/II Collaboration Annamycin AML Annamycin + WP1066 WP1066 - Glioblastoma, melanoma metastasized to the brain WP1066 - Diffuse intrinsic pontine gliomas (children) WP1122 - Glioblastoma Brain Tumors Brain WP1122 + Avastin - Glioblastoma WP1066/WP1732 Cancer WP1122/1234 - Pancreatic cancer Pancreatic Pancreatic WP1220 - Cutaneous T-Cell Lymphoma Medical University of Gdansk Other Cancers WP1066 - Ocular tumors DNA Targeting Cell Signaling Tumor Metabolism Combination

Development Roadmap WP1066 WP1122 Annamycin IND MDA WP1220 Mouse Study Avastin POC IND/Clinic (Clinic; adult Mouse Study (CTCL Poland) (Norway) GBM) (Norway; GBM) (AML) Potential for Mayo CTA/Clinic (grant; pediatric MDA SRA Pancreatic WP1732 (AML; Poland) DIPG) Cancer WP1234 Other Potential for Institutions other indications 7

Value Inflection Points Points we consider to be key development milestones that may influence big pharma’s decision to license or acquire: • Annamycin – generation of Phase I/II data consistent with the clinical trials already completed • WP1066 and WP1122 – Proof of concept (showing activity against the targeted indication) in planned Phase I/II trials We believe we have the potential to hit one or more of these value inflection points within 2-3 years 8

Development Strategy • Orphan Drug targets with high revenue potential • Focus on game-changing breakthroughs • Collaborate closely with MD Anderson Cancer Center • Minimize cash burn through virtual structure • Worldwide exclusive licenses and regulatory exclusivity create proprietary positioning for all projects 9

Industry Opportunity • Orphan Drug sales represented approximately $29 billion in revenue in 2013 • There are no approved second-line therapies for AML • CPXX sold for $1.5 billion based on improving OS by 3.5 months, yet still leaves most AML patients without hope • Similar opportunities exist in brain tumors (WP1066), pancreatic cancer (WP1122) and other rare diseases we are pursuing 10

Most of our technologies were developed at MD Anderson Cancer Center, the world’s largest cancer research facility centered within the world’s largest medical center With over 1,500 cancer researchers, our sponsored research has been supported by truly state of the art resources 11

Delivering Solutions 12

Annamycin Critical Leading drugs are cardiotoxic and lose efficacy due to multidrug Advantages resistance over Leading Annamycin has little to no cardiotoxicity, avoids multidrug Annamycin Process Drug resistance, has been shown to be more potent in AML cell lines and has shown activity in patients who failed standard of care Potential to Annamycin has shown the potential to significantly improve health Significantly in a Phase I/II acute myeloid leukemia (AML) trial Improve Health Orphan Drug status as single agent for relapsed or refractory AML Positioned for Annamycin appears to be well suited for an accelerated approval Accelerated pathway here in the US, and in Europe Approval Absence of any approved second-line drug for most AML patients represents a significant unmet need Potentially shorter time scale for saving lives than with typical cancer drugs 13

Annamycin Delivers Relapsed/Refractory Acute Leukemia Patients Remarkable In a proof-of-concept Phase I/II clinical trial, 63% Performance Annamycin was given to patients who had failed an average of five previous induction Efficacy therapy attempts Signal 37% of those patients cleared enough of their leukemic cells to qualify for a bone marrow transplant 37% Cleared Bone Marrow We believe repeating this performance in a Blasts larger clinical trial, could warrant new drug approval Annamycin gives new hope to patients who have run out of options 14

Annamycin has the potential to qualify more people for induction therapy Leukemia is a cancer of the white blood cells Induction Therapy BMT and the acute forms of leukemia can manifest quickly and leave patients with limited (Qualify for BMT) (Results in treatment options Cure) The only viable option for acute leukemia patients is a bone marrow transplant (BMT), and those BMTs are successful about 80% of 20 the time % The problem is that in order to qualify for a 80% BMT, patients have to first eliminate most of 20% their cancerous white blood cells through 80 what is called “induction therapy” % However, induction therapy is currently only successful in about 20% of patients Success Failure 15
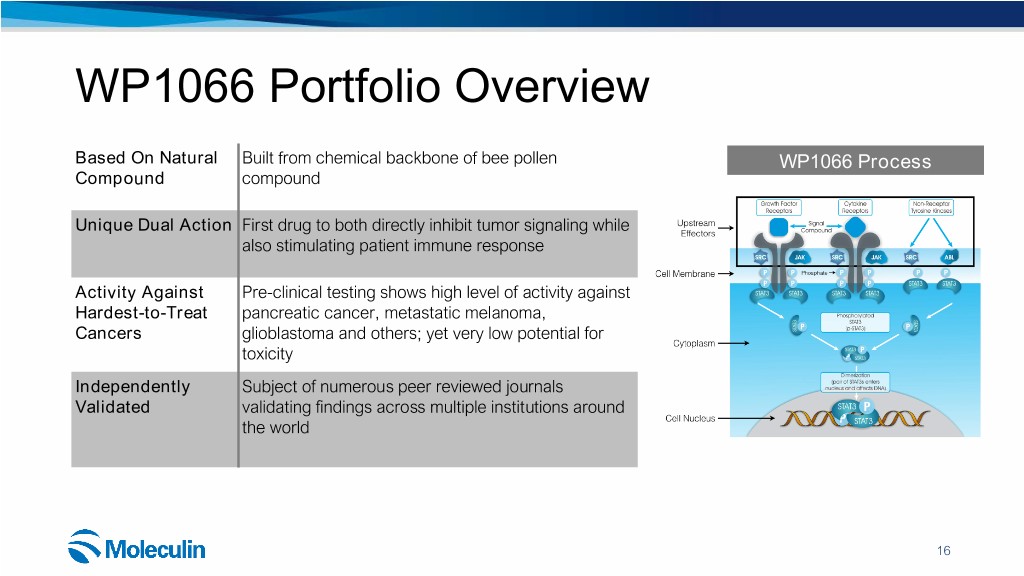
WP1066 Portfolio Overview Based On Natural Built from chemical backbone of bee pollen WP1066 Process Compound compound Unique Dual Action First drug to both directly inhibit tumor signaling while also stimulating patient immune response Activity Against Pre-clinical testing shows high level of activity against Hardest-to-Treat pancreatic cancer, metastatic melanoma, Cancers glioblastoma and others; yet very low potential for toxicity Independently Subject of numerous peer reviewed journals Validated validating findings across multiple institutions around the world 16

Proposed MOA of WP1066: Deubiquitinase-Mediated Control of p-STAT3 Aberrant deubiquitination can lead to constitutive WP1066 activation of STAT3 p-STAT3, an blockade activated form WP1066 blocking of DUBs of STAT3 shifts the equilibrium Ubiquitinatedtowards ubiquitinated p-STAT3 STAT3 Activated STAT3 (p-STAT3) relies on deubiquitinating enzymes (DUBs) to reverse ubiquitination and decrease proteasomal hydrolysis Proteasomal WP1066 inhibition of DUBs Degradation increases ubiquitination of p-STAT3 and leads to the depletion of this Proteasome oncogenic protein in the tumor cell 17

WP1122 Portfolio Overview Addicted to A brain tumor requires as much as 18 to 37 times as Tumors are hyper-consumers of glucose Sugar much glucose to survive as a healthy brain cell and starve to death without it Starving a Tumor This eventually led to the theory that, if we feed tumor to Death cells a glucose decoy (one that can’t convert into energy), we can kill the tumor This works well in a laboratory setting, but the problem Tumor cell is making these decoys drugable Breakthrough WP1122 has been combined with a pro-drug to design increase half-life and enable transmission across the blood brain barrier Potential to WP1122 (at suboptimal doses) performs as well or Change Standard better than temozolomide in live human brain tumors; Normal cell of Care even better performance by combining the two drugs was shown in trials 18

WP1122 is Effective In Vivo against Gliomas - July 2017 Control Temodar WP1122 10 8 6 4 # of Animals 2 Orthotopic 0 Glioblastoma 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 Days Model in Mice WP1122 used alone has at least the same or greater activity than temozolomide (Temodar®), a current standard of care in patients diagnosed with glioblastoma 19
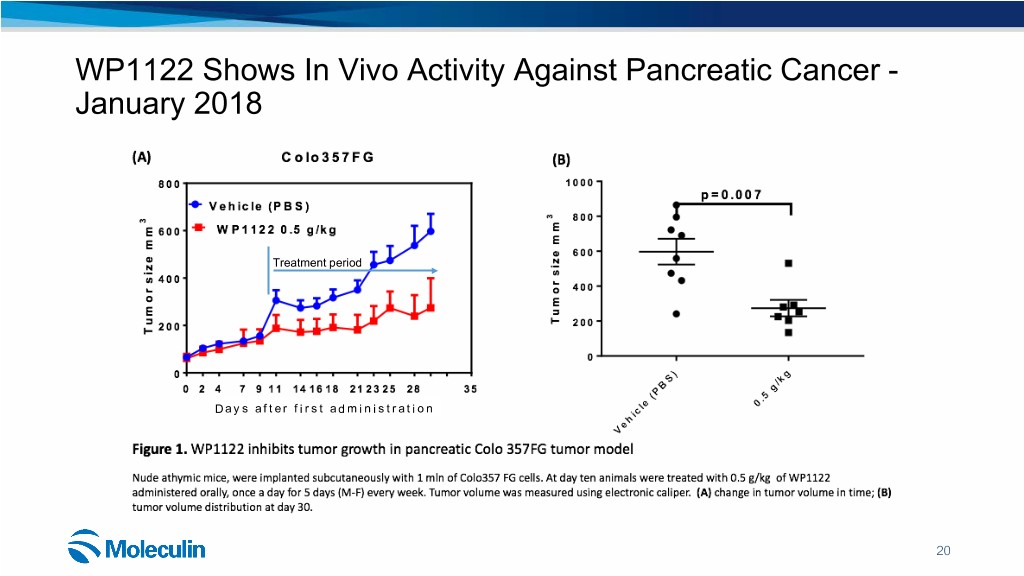
WP1122 Shows In Vivo Activity Against Pancreatic Cancer - January 2018 Treatment period Days after first administration 20

Recent Developments Since June 2018 • WP1066 Begins Patient Dosing at Clinical Trial Being Conducted at MD Anderson • Seeks Approval from Polish Regulatory Agency for Skin Cancer Clinical (WP1220 - WP1066 Portfolio) Trial • Enrollment Opens for Brain Tumor Trial of WP1066 • Expects to Meet FDA IND Filing Requirements by YE for its Pancreatic Cancer Drug Candidate with Development Work in Australia (filing in 1H 2019) • Expands Operations to Australia with preclinical work on WP1732; Taps R&D Incentive Program • Selected for the Russell Microcap Index • Announces $2.3 Million Registered Direct Offering Priced At-the-Market • Receives Approval for Annamycin AML Clinical Trial in Poland • Targets accelerated FDA approval of WP1732; Pursues Development for Ocular Tumors 21

Milestones Anticipated Milestone Potential Timeframe Announcement that our Investigational New Drug (IND) for Annamycin has become effective and that we Accomplished may begin clinical trials Initial IRB (Institutional Review Board) approvals and site initiations of various clinical sites participating in Accomplished and ongoing through Second Half of our Phase I/II clinical trial of Annamycin 2018 Establishment of a new Recommended Phase 2 Dose for Annamycin Second Half of 2018 A clinician sponsored IND for WP1066 for treatment of adult brain tumors moving forward Accomplished; Now open for enrollment Announcement of initial clinical data for Annamycin trial 2018 Accomplished and Ongoing Announcement of further benefits of our sponsored research agreement with MD Anderson into 2019 2018 Announce CTA for WP1220 for the treatment of cutaneous T-cell lymphoma (CTCL) (CTA Filed) Announce WP1122 move into preclinical work 2018 Announce WP1732 move into preclinical work Accomplished Announce IND for WP1732 submitted First Half of 2019 Announce the fourth drug approved for clinical trial 2019

Financial Overview 23
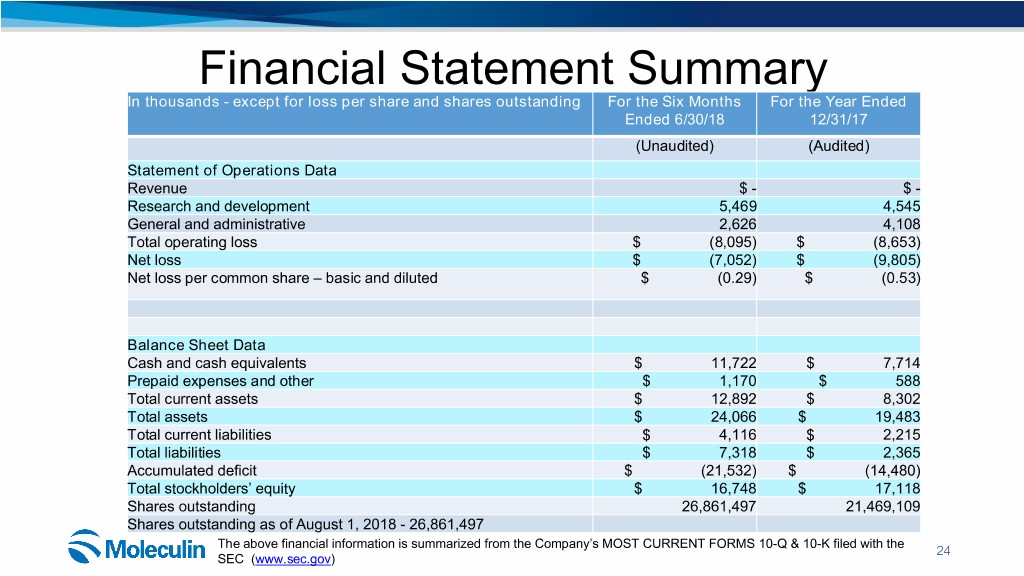
Financial Statement Summary In thousands - except for loss per share and shares outstanding For the Six Months For the Year Ended Ended 6/30/18 12/31/17 (Unaudited) (Audited) Statement of Operations Data Revenue $ - $ - Research and development 5,469 4,545 General and administrative 2,626 4,108 Total operating loss $ (8,095) $ (8,653) Net loss $ (7,052) $ (9,805) Net loss per common share – basic and diluted $ (0.29) $ (0.53) Balance Sheet Data Cash and cash equivalents $ 11,722 $ 7,714 Prepaid expenses and other $ 1,170 $ 588 Total current assets $ 12,892 $ 8,302 Total assets $ 24,066 $ 19,483 Total current liabilities $ 4,116 $ 2,215 Total liabilities $ 7,318 $ 2,365 Accumulated deficit $ (21,532) $ (14,480) Total stockholders’ equity $ 16,748 $ 17,118 Shares outstanding 26,861,497 21,469,109 Shares outstanding as of August 1, 2018 - 26,861,497 The above financial information is summarized from the Company’s MOST CURRENT FORMS 10-Q & 10-K filed with the 24 SEC (www.sec.gov)

Robust pipeline 3 distinctly different technologies - six potential drug candidates, all with blockbuster potential World-leading collaboration MD Anderson Cancer Center/Mayo Clinic/Emory University Breakthrough disruptive technologies Annamycin for AML: designed to be non-cardiotoxic, avoids MDR1 WP1066 Portfolio: STAT3 inhibitor that also stimulates immune response WP1122 Portfolio: metabolic inhibitor with improved BBB transmission and animal model activity against pancreatic cancer Highly experienced leadership Veteran pharma/biotech, life science micro-cap managers Proprietary positioning Orphan drug and/or patents, exclusive licenses (applied and received) 25
