Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Emergent BioSolutions Inc. | a18-18368_1ex99d1.htm |
| 8-K - 8-K - Emergent BioSolutions Inc. | a18-18368_18k.htm |
Emergent BioSolutions Planned Acquisition of Specialty Vaccines Company PaxVax August 9, 2018 Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. Advancing Emergent’s Leadership in the Public Health Threats Market

Emergent Acquisition of PaxVax Conference Call Speakers EMERGENT BIOSOLUTIONS Robert Burrows Vice President, Investor Relations Robert Kramer President and Chief Operating Officer Adam Havey Executive Vice President, Business Operations Richard Lindahl Executive Vice President and Chief Financial Officer Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances.
Emergent Acquisition of PaxVax Conference Call Agenda Benefits of the Transaction Kramer PaxVax Overview Havey Financial and Other Considerations Lindahl Key Takeaways Kramer Q&A Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances.
Emergent Acquisition of PaxVax Forward-Looking Statements / Non-GAAP Financial Measures / Trademarks At a Glance Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. Safe-Harbor Statement This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact, including statements regarding the expected closing of the transaction, the potential opportunities and financial impact of the transaction, and any other statements containing the words “believes,” “expects,” “anticipates,” “intends,” “plans,” “targets,” “forecasts,” “estimates” and similar expressions in conjunction with, among other things, discussions of the amount and timing of additional revenue expected to be generated by PaxVax’s products and the timing of accretion by such products, the company's outlook, financial performance or financial condition, growth strategy, product sales, government development or procurement contracts or awards, manufacturing capabilities, product development regulatory approvals or expenditures are forward-looking statements. These forward-looking statements are based on our current intentions, beliefs and expectations regarding future events. We cannot guarantee that any forward-looking statement will be accurate. Investors should realize that if underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Investors are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statement speaks only as of the date of this presentation, and, except as required by law, we do not undertake to update any forward-looking statement to reflect new information, events or circumstances. There are a number of important factors that could cause the company’s actual results to differ materially from those indicated by such forward-looking statements, including uncertainties as to the satisfaction of closing conditions with respect to the transaction, including the timing and receipt of third party and regulatory approvals related to the transaction; our ability to successfully integrate the business and realize the benefits of the transaction, including our ability to continue the momentum of the sales of PaxVax’s products; availability of funding for government grants and contracts; whether anticipated synergies and benefits from the acquisition are realized within expected time periods, if at all; our ability to utilize our manufacturing facilities; our ability and the ability of our contractors and suppliers to maintain compliance with current good manufacturing practices and other regulatory obligations; general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations as we continue to expand internationally; the impact of pharmaceutical industry regulation in the United States and internationally; new products and patents attained by competitors; the company’s ability to accurately predict future market conditions; and financial instability of international economies and sovereign risk. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Investors should consider this cautionary statement, as well as the risk factors identified in our periodic reports filed with the SEC, when evaluating our forward-looking statements. Trademarks BioThrax® (Anthrax Vaccine Adsorbed), RSDL® (Reactive Skin Decontamination Lotion Kit), BAT® [Botulism Antitoxin Heptavalent (A,B,C,D,E,F,G)-(Equine)], Anthrasil® (Anthrax Immune Globulin Intravenous [human]), NuThrax™ (anthrax vaccine adsorbed with CPG 7909 adjuvant), VIGIV [Vaccinia Immune Globulin Intravenous (Human)], Trobigard™ (atropine sulfate, obidoxime chloride), ACAM2000®, (Smallpox (Vaccinia) Vaccine, Live), Raxibacumab (Anthrax Monoclonal), and any and all Emergent BioSolutions Inc. brands, products, services and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries. All other brands, products, services and feature names or trademarks are the property of their respective owners.

Benefits of the Transaction Robert Kramer President and Chief Operating Officer

Benefits of the Transaction PaxVax Acquisition is Strategically, Operationally and Financially Attractive STRATEGIC Solidifies leadership position in public health threats market with addition of two revenue-generating FDA-licensed vaccines – Vivotif® for typhoid and Vaxchora® for cholera – both with dual-market potential Broadens development pipeline with clinical stage programs addressing EIDs; one funded by US DoD Expands sales capabilities with addition of global commercial infrastructure focused on travelers’ market OPERATIONAL Establishes European-based cGMP manufacturing footprint; potential for contribution to CDMO BU Adds established R&D capabilities focused on novel specialty vaccines Provides opportunities for synergies through integration of both operations Strengthens the Emergent team with PaxVax’s skilled and talented employees FINANCIAL Contributes to growth in Emergent’s financial performance Structured as all-cash transaction for $270M, on a cash-free, debt-free basis Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances.
PaxVax Overview Adam Havey Executive Vice President, Business Operations

PaxVax Overview PaxVax is a Specialty Vaccines Company Focused on the Development and Commercialization of Travelers’ Vaccines Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. Company Overview Founded in 2006; privately held Sites: Redwood City, CA [HEADQUARTERS] San Diego, CA [R&D; MANUFACTURING] Bern, Switzerland [MANUFACTURING] Employees: ~250
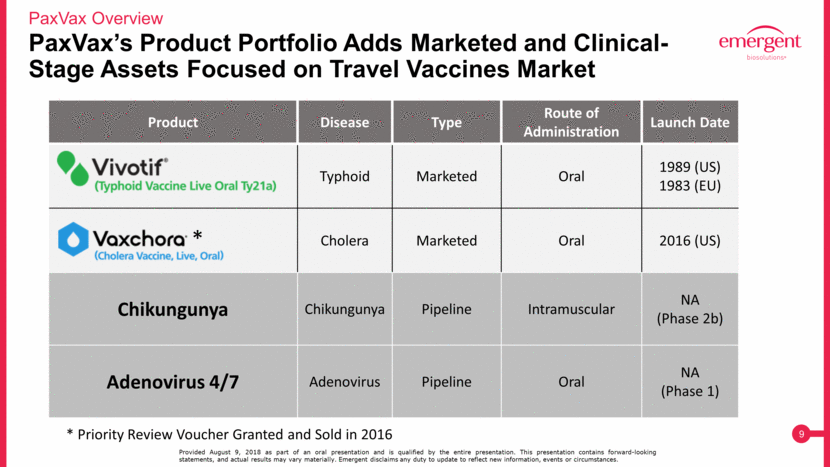
PaxVax’s Product Portfolio Adds Marketed and Clinical-Stage Assets Focused on Travel Vaccines Market PaxVax Overview Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. Product Disease Type Route of Administration Launch Date Typhoid Marketed Oral 1989 (US) 1983 (EU) Cholera Marketed Oral 2016 (US) Chikungunya Chikungunya Pipeline Intramuscular NA (Phase 2b) Adenovirus 4/7 Adenovirus Pipeline Oral NA (Phase 1) * Priority Review Voucher Granted and Sold in 2016 *
Overview of Typhoid Fever and Cholera PaxVax Overview Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. Prevalence: Over 21 million people contract typhoid fever annually Three to five million cases of cholera each year Most prevalent in areas with poor sanitation and limited access to clean water Disease: Both infectious diseases are spread through the fecal-oral route, most often transmitted through food and water Typhoid is commonly characterized by fever, headaches, rashes, abdominal and muscle pain, and diarrhea Complications can lead to intestinal bleeding, shock, and death Diagnosis is made by a blood or stool culture and with the Widal test (antibody) Cholera is commonly characterized by severe profuse water diarrhea, vomiting, dehydration, and a rapid heart rate Complications of cholera can include muscle cramps, seizures, acute renal failure, shock and death if left untreated Cholera is often diagnosed presumptively based on clinical suspicion, confirmed by isolating Vibrio cholerae in a patient sample Source: Halstead S 2015, Silva et al., 2016, WHO, CDC, Management information Typhoid virus Cholera Bacteria
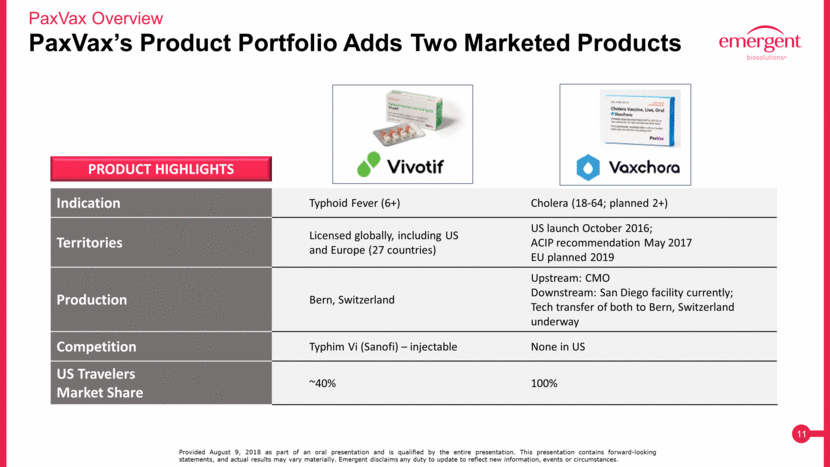
PaxVax’s Product Portfolio Adds Two Marketed Products PaxVax Overview Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. Indication Typhoid Fever (6+) Cholera (18-64; planned 2+) Territories Licensed globally, including US and Europe (27 countries) US launch October 2016; ACIP recommendation May 2017 EU planned 2019 Production Bern, Switzerland Upstream: CMO Downstream: San Diego facility currently; Tech transfer of both to Bern, Switzerland underway Competition Typhim Vi (Sanofi) – injectable None in US US Travelers Market Share ~40% 100% PRODUCT HIGHLIGHTS
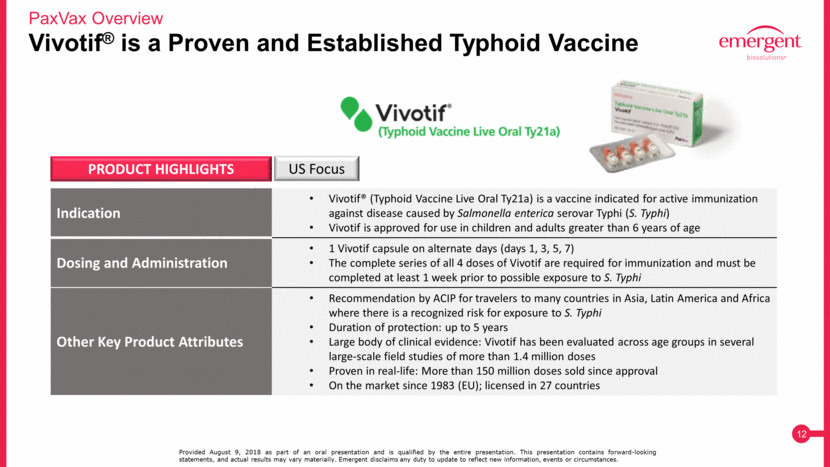
Vivotif® is a Proven and Established Typhoid Vaccine PaxVax Overview Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. Indication Vivotif® (Typhoid Vaccine Live Oral Ty21a) is a vaccine indicated for active immunization against disease caused by Salmonella enterica serovar Typhi (S. Typhi) Vivotif is approved for use in children and adults greater than 6 years of age Dosing and Administration 1 Vivotif capsule on alternate days (days 1, 3, 5, 7) The complete series of all 4 doses of Vivotif are required for immunization and must be completed at least 1 week prior to possible exposure to S. Typhi Other Key Product Attributes Recommendation by ACIP for travelers to many countries in Asia, Latin America and Africa where there is a recognized risk for exposure to S. Typhi Duration of protection: up to 5 years Large body of clinical evidence: Vivotif has been evaluated across age groups in several large-scale field studies of more than 1.4 million doses Proven in real-life: More than 150 million doses sold since approval On the market since 1983 (EU); licensed in 27 countries PRODUCT HIGHLIGHTS US Focus
Vaxchora® was Approved in the US in 2016 PaxVax Overview Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. Indication Vaxchora® (Cholera Vaccine, Live, Oral) is a vaccine indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 Vaxchora is approved for use in adults 18 through 64 years of age traveling to cholera-affected areas Dosing and Administration Single oral dose reconstituted in water Vaxchora should be administered at least 10 days before potential exposure to cholera Other Key Product Attributes Category A (all persons) recommendation by ACIP for travelers to areas of active cholera transmission (since June 2017) DoD policy of Defense Health Agency Immunization Healthcare Branch (DHA-IHB) for U.S. military to use Vaxchora as per ACIP recommendation (October 2017) Limited near-term cholera vaccine development in US and EU PRODUCT HIGHLIGHTS
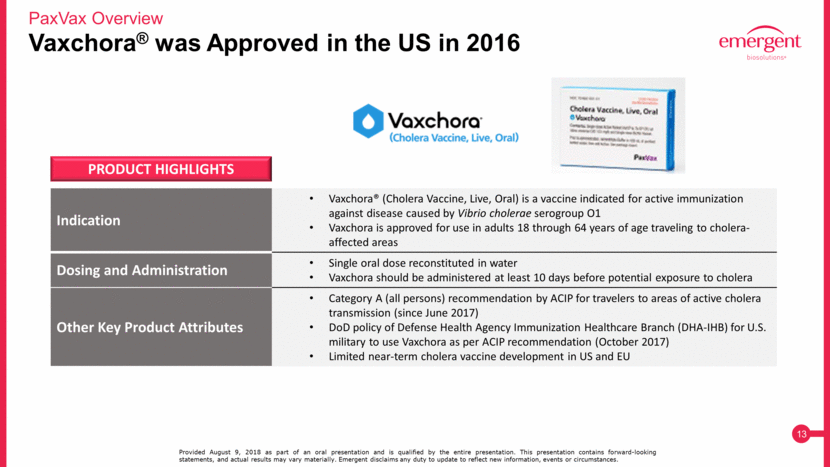
Commercial Operations Adds New Sales, Marketing and Distribution Capabilities Focused on Infectious Diseases PaxVax Overview Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. In-house sales reps domiciled in US and select European countries Sales also achieved through third-party partners Additional countries served by partners include: Australia; South Korea; Greece; Hong Kong; Singapore; Malaysia; Central Europe; Canada
PaxVax’s Product Portfolio Adds Two Key Clinical-Stage Candidates Focused on Infectious Diseases of High Interest to US Military PaxVax Overview Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. Indication Chikungunya Adenovirus Product Specification Chikungunya virus-like particle (VLP) licensed from Vaccine Research Center (VRC) at National Institutes of Health (NIH) Alum adjuvanted Purified Ad4 and Ad7 live viruses delivered orally as a single-dose in enteric-coated capsules Manufactured in serum-free suspension cells Advantages VLP size potentially enhances immunogenicity No denaturing from inactivation Noninfectious No potential for vector immunity High production yields Support from US DoD Improvement in formulation and presentation over licensed vaccine to remove all components that present a supply risk Potential for high production yields Currently funded through development grant from DoD PROGRAM HIGHLIGHTS
European-Based Manufacturing Site Broadens CDMO Capabilities and Establishes International Footprint PaxVax Overview Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. Fermentation Downstream Purification Analytical Development/QC Labs Lyophilization Capsulation Inspection and Packaging Warehouse Distribution Fill/Finish SITE CAPABILITIES Currently manufacturing Vivotif Transfer of manufacturing and increase in manufacturing scale of Vaxchora underway
Financial and Other Considerations Richard Lindahl Executive Vice President and Chief Financial Officer

Financial and Other Considerations All-Cash Consideration Reflects Efficient Use of Balance Sheet TRANSACTION Purchase price of $270M all cash Cash-free and debt-free acquisition of 100% of PaxVax FINANCING Committed credit facility of $200M in place Existing cash balance of $190M (at 6/30/2018) Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances.
Financial and Other Considerations Transaction Contributes to Growth in Emergent’s Financial Performance Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. 2018 Guidance will be updated following transaction closing, anticipated Q4 2018 2019 Anticipate 2019 contribution of $70M-$90M in total revenue Anticipate transaction will be accretive by year-end 2019 Clear contributor to progress toward 2020 financial and operational goals pursuant to company growth strategy
Financial and Other Considerations Transaction Expected to Close in Q4 2018 Approved by Boards of Directors of both companies Structured as a merger agreement Subject to customary closing conditions Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances.
Key Takeaways Robert Kramer President and Chief Operating Officer

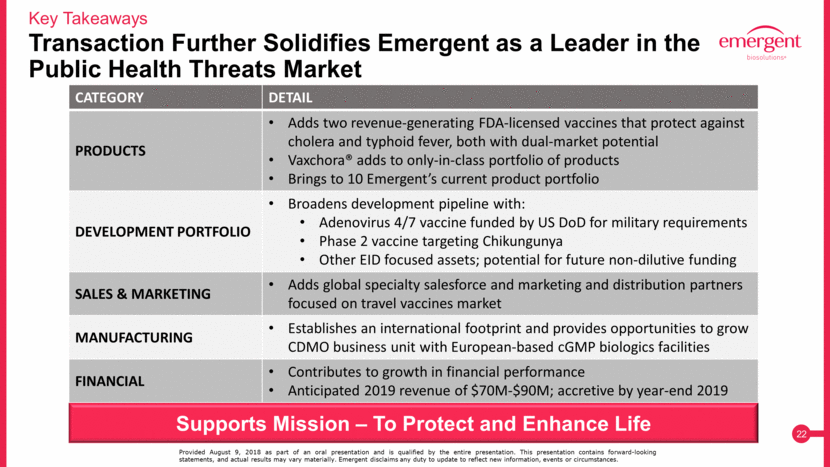
Key Takeaways Transaction Further Solidifies Emergent as a Leader in the Public Health Threats Market Provided August 9, 2018 as part of an oral presentation and is qualified by the entire presentation. This presentation contains forward-looking statements, and actual results may vary materially. Emergent disclaims any duty to update to reflect new information, events or circumstances. CATEGORY DETAIL PRODUCTS Adds two revenue-generating FDA-licensed vaccines that protect against cholera and typhoid fever, both with dual-market potential Vaxchora® adds to only-in-class portfolio of products Brings to 10 Emergent’s current product portfolio DEVELOPMENT PORTFOLIO Broadens development pipeline with: Adenovirus 4/7 vaccine funded by US DoD for military requirements Phase 2 vaccine targeting Chikungunya Other EID focused assets; potential for future non-dilutive funding SALES & MARKETING Adds global specialty salesforce and marketing and distribution partners focused on travel vaccines market MANUFACTURING Establishes an international footprint and provides opportunities to grow CDMO business unit with European-based cGMP biologics facilities FINANCIAL Contributes to growth in financial performance Anticipated 2019 revenue of $70M-$90M; accretive by year-end 2019 Supports Mission – To Protect and Enhance Life
Q&A Robert Kramer President and Chief Operating Officer Adam Havey Executive Vice President, Business Operations Richard Lindahl Executive Vice President and Chief Financial Officer Abigail Jenkins Senior Vice President and Vaccines & Anti-Infectives Business Unit Head Sean Kirk Senior Vice President, Manufacturing Operations and CDMO Business Unit Head

