Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - LIGAND PHARMACEUTICALS INC | lgnd_63018exhibit321.htm |
| EX-31.2 - EXHIBIT 31.2 - LIGAND PHARMACEUTICALS INC | lgnd_63018exhibit312.htm |
| EX-31.1 - EXHIBIT 31.1 - LIGAND PHARMACEUTICALS INC | lgnd_63018exhibit311.htm |
| EX-10.14 - EXHIBIT 10.14 - LIGAND PHARMACEUTICALS INC | a1014redlineamendmentnum.htm |
| EX-10.13 - EXHIBIT 10.13 - LIGAND PHARMACEUTICALS INC | a101redlineplatformlicen.htm |
| 10-Q - 10-Q - LIGAND PHARMACEUTICALS INC | lgnd-6301810q.htm |

Exhibit 10.15 ***Text Omitted and Filed Separately with the Securities and Exchange Commission. Confidential Treatment Requested Under 17 C.F.R. Sections 200.80(b)(4) and 240.24b-2. AMENDMENT NUMBER TWO TO PLATFORM LICENSE AGREEMENT This Amendment Number Two to Platform License Agreement (this “ Amendment No. 2”) is entered into effective as of June 25, 2018 (“Amendment No. 2 Effective Date ”) and made by and between OMT, Inc. (“ OMT ”), which has its principal place of business at 3911 Sorrento Valley Boulevard, Suite 110, San Diego, California 92121, U.S.A., and Wuxi Biologics Ireland Limited (“ Licensee ”), which has its principal place of business at One Spencer Dock, North Wall Quay, Dublin 1, Ireland. OMT and Licensee may each be referred to herein as a “ Party ” and collectively as the “ Parties .” WHEREAS , OMT and WuXi AppTec Biopharmaceuticals Co., Ltd., which has its principal place of business at 108 Meiliang Rd., Mashan, Wuxi, P.R. China, entered into the Platform License Agreement, effective on March 23, 2015 (the “ Original Agreement ”); WHEREAS , pursuant to the Assignment and Assumption Agreement entered by and among OMT, WuXi AppTec Biopharmaceuticals Co., Ltd., and Wuxi Biologics (Hong Kong) Limited as of July 19, 2016, WuXi AppTec Biopharmaceuticals Co., Ltd. assigned the Original Agreement to Wuxi Biologics (Hong Kong) Limited; WHEREAS , OMT and Wuxi Biologics (Hong Kong) Limited amended the Original Agreement by Amendment Number One to Platform License Agreement, effective as of June 11, 2017 (together with the Original Agreement, the “ Agreement ”); WHEREAS , pursuant to the Assignment and Assumption Agreement entered by and among the Parties and Wuxi Biologics (Hong Kong) Limited as of June 25, 2018, Wuxi Biologics (Hong Kong) Limited assigned the Agreement to Licensee; and WHEREAS , the Parties desire to amend certain terms of the Agreement in accordance with this Amendment No. 2. NOW T HEREFORE , for good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged by the Parties, the Parties, intending to be legally bound, agree as follows: 1. All capitalized terms used in this Amendment No. 2 and not otherwise defined in this Amendment No. 2 shall have the meanings assigned to such terms in the Agreement. 2. Within ten (10) days of the Amendment No. 2 Effective Date, Licensee shall pay OMT fifty-one million U.S. dollars (US $51 million), which is in lieu of any milestone payments that will become due or payable arising under the Agreement after the Amendment No. 2 Effective Date [***] . Upon receipt of the payment described in this [***] Certain information on this page has been omitted and filed separately with the 4840-7056-1386 v.6 1 US-DOCS\102805239.1102810859.2

Paragraph 2, above, OMT acknowledges and agrees that Licensee shall have no further obligation to pay any milestone payments under the Agreement. 3. The following shall be added to the end of Section 3.1 of the Agreement: “If, OMT intends to or does cease supplying Animals directly or through authorized third parties in accordance with this Section 3.1 for more than five (5) consecutive months for any reason including, but not limited to, bankruptcy of OMT, voluntary or involuntary cessation of OMT’s Animal supply business, or change of control of OMT, OMT shall immediately notify Licensee in writing of such intent or cessation, and, to the extent permitted under applicable law, shall grant to Licensee the right to breed the Animals for use consistent with this Agreement, under any and all intellectual property rights, including but not limited to any and all patents and patent applications, know-how, and trade secrets, owned or otherwise controlled by OMT that are necessary for Licensee to breed the Animals for use consistent with this Agreement, and shall transfer to Licensee the technologies that are necessary for Licensee to breed the Animals for use consistent with this Agreement (“Animal Technology Transfer ”). OMT shall provide reasonable technical assistance requested by Licensee to enable the Animal Technology Transfer and shall execute any documentation reasonably necessary to transfer such technology and any regulatory or other governmental consents or approvals regarding the same, and will novate any contracts with any third party that relate solely to the provision of such Animals to the benefit of Licensee. For the avoidance of doubt, the royalty obligations provided in Sections 5.2(a), (b) and (c) will remain unchanged.” 4. Section 4.2(a) of the Agreement shall be deleted in its entirety and replaced with the following: “Relative to OMT, Licensee will exclusively own the Antibodies and any Products (including Products developed by any Outlicensee) and all intellectual property rights therein, including any patent rights, developed by Licensee or any such Outlicensee at any time under this Agreement (collectively, “IP Rights ”), and each hereby makes all assignments necessary to achieve the foregoing. Subject to compliance with all terms and conditions of this Agreement, Licensee hereby grants OMT a non- exclusive, irrevocable, perpetual, worldwide, royalty-free, non- sublicenseable, non-transferable right and license in and to the IP Rights, to the extent necessary to allow OMT to exercise its rights hereunder Commission. Confidential treatment has been requested with respect to the omitted portions. 4840-7056-1386 v.6 2 US-DOCS\102805239.1102810859.2

and/or to allow OMT to exploit such IP Rights for non-commercial purposes including research, provided that OMT will not distribute, sell, offer for sale, use, or otherwise transfer any such Antibodies and Products.” 5. Section 4.2(b) of the Agreement shall be deleted in its entirety and replaced with the following: “No less frequently than once per each calendar quarter, Licensee shall provide to OMT a report summarizing Licensee’s and Outlicensees’ prosecution and maintenance activities regarding any patent rights with respect to the IP Rights, including, if requested by OMT, copies of any significant office actions, communications, and correspondence relating to such patent rights. OMT shall have the right to comment on and to discuss such activities, and Licensee shall consider (and cause the applicable Outlicensees to consider) OMT’s comments in good faith.” 6. Section 4.3(a) of the Agreement shall be deleted in its entirety and replaced with the following: “China Outlicensing Agreement . Licensee may authorize, including without limitation under an assignment, a license or option grant and/or an appointment as distributor, a third party to use, develop, market, distribute, offer for sale, and/or sell an Antibody in China (and, as applicable with respect to Existing Antibodies, the China Plus Territory) solely pursuant to an agreement that is consistent with the terms and conditions of this Agreement, including without limitation this Section 4.3(a) (each such third party agreement, a “China Outlicensing Agreement ”). Pursuant to a China Outlicensing Agreement, Licensee may distribute or otherwise transfer any Antibodies generated by or on behalf of Licensee to the applicable China Outlicensee using antigens selected by Licensee or such China Outlicensee. Licensee shall be responsible and liable for all actions and omissions of China Outlicensees in connection with any such Antibodies. Licensee may provide (i) any such resulting Antibodies to a China Outlicensee that is bound by a China Outlicensing Agreement; and (ii) Existing Antibodies to a China Outlicensee that is bound by a written addendum to such China Outlicensing Agreement for use, development, and marketing in the China Plus Territory (a “China Plus Addendum ”, which shall be deemed a part of the applicable China Outlicensing Agreement). Each China Outlicensing Agreement will include payment provisions that enable Licensee to comply with its obligations to make payments to OMT under this Agreement, and will prohibit the licensing, distribution, sale, offer for sale, use, or other transfer of any Antibody or Product outside of China (and as applicable with respect to Existing Antibodies, the China Plus Territory) by or on 4840-7056-1386 v.6 3 US-DOCS\102805239.1102810859.2

behalf of the applicable China Outlicensee, where “use” includes development of and distribution of Products.” 7. The last sentence of Section 4.3(b) of the Agreement shall be deleted in its entirety and replaced with the following sentence: “Each Ex-China Outlicensing Agreement will prohibit the licensing, distribution, sale, offer for sale, use, or other transfer of any Antibody or Product in and/or into China (and, as applicable, with respect to Existing Antibodies, the China Plus Territory) by or on behalf of the applicable Ex- China Outlicensee, where “use” includes development of and distribution of Products.” 8. Section 4.3(d) of the Agreement shall be deleted in its entirety and replaced with the following: “Licensee may not outlicense or otherwise provide rights in any Antibodies to any third party except pursuant to Section 4.3(a), (b), and/or (c).” 9. The first sentence of Section 4.3(f) of the Agreement shall be deleted and replaced with the following sentence: “Each Outlicensing Agreement shall contain terms no less protective of OMT (including without limitation OMT’s intellectual property rights in the Animals and Animal Improvements) than those set forth in this Agreement.” 10. The last sentence of Section 4.3(f) of the Agreement shall be deleted and replaced with the following sentence: “Licensee will keep OMT reasonably informed of the status of any Outlicensing Agreements, and promptly after execution of the Outlicensing Agreement, Licensee shall provide to OMT a full copy of such Outlicensing Agreement (with any confidential or financial terms redacted), translated into English if applicable. ” 11. Section 4.4 of the Agreement shall be deleted in its entirety and replaced with the following: “As between the parties, and without limiting any other available remedy, OMT owns (i) the Animals and (ii) any modification, improvement, enhancement, or progeny of the Animals ( “Animal Improvements ”) created in violation of Section 4.1(a) above, and all intellectual property rights in any of the foregoing. Licensee hereby makes (and will cause its Approved Affiliates and its and their Approved Subcontractors, and any Outlicensee, as applicable, to make) all assignments necessary to 4840-7056-1386 v.6 4 US-DOCS\102805239.1102810859.2

accomplish the foregoing ownership with respect to any Animal Improvements. Licensee acknowledges and agrees that the Animals and Animal Improvements, together with any related biological material or substance that is replicated, synthesized or in any way derived from the Animals (including progeny), except for Antibodies, include and constitute valuable trade secrets of OMT, and OMT has used diligent efforts to maintain such items as its trade secrets and Confidential Information .” 12. Section 4.5(a) of the Agreement shall be deleted in its entirety and replaced with the following: “Each of OMT and Licensee shall notify the other promptly in writing when each learns of or reasonably suspects infringement of any IP Rights by a third party (an “Infringement ”). Licensee, and its Outlicensees, shall have the first right to enforce any IP Right in the Territory, and control, defend, and settle such suit in a manner consistent with the terms and provisions of this Agreement, and recover any damages, awards, or settlements resulting therefrom, subject to Section 4.5(c). OMT agrees to use commercially reasonable efforts to reasonably cooperate in any such litigation, including participating in a suit if required to provide standing.” 13. Section 4.5(b) of the Agreement shall be deleted in its entirety and replaced with the following: “If Licensee or its Outlicensee does not bring suit to enforce the applicable IP Rights with respect to an Infringement within one hundred and eighty (180) days of becoming aware that such Infringement exists, then OMT may, in its sole judgment and at its own expense, take steps to enforce any such rights, including instituting suit against any such infringer or alleged infringer, and control, defend and settle such suit in a manner consistent with the terms and provisions hereof, and recover any damages, awards or settlements resulting therefrom, subject to Section 4.5(c). Licensee agrees to reasonably cooperate in any such litigation, including participating in a suit if required to provide standing.” 14. A new Section 4.8 shall be added to the Agreement as follows: “Non-Animal Improvements . Licensee, or Outlicensees, shall solely own any improvements developed by Licensee or any such Outlicensee to the Antibodies and Products that are not Animal Improvements (“Non-Animal Improvements ”). Non-Animal Improvements are derivatives of Antibodies, bispecific/multispecific antibodies, Antibody-drug conjugates, and any drugs or further products generated from the Antibodies, in each case to the extent that it is not an Animal Improvement. All intellectual property rights developed by Licensee or an Outlicensee in any Non- Animal Improvement at any time under this Agreement shall be exclusively 4840-7056-1386 v.6 5 US-DOCS\102805239.1102810859.2

owned by Licensee or the Outlicensee. For the avoidance of doubt, Non- Animal Improvements do not include Animals or Animal Improvements.” 15. A new Section 5.1(d) shall be added to the Agreement as follows: “Licensee shall be required to make each of the payments set forth in Sections 5.1(a), (b), and (c) only if such payment becomes payable in accordance with the terms of Section 5.1(a), (b), or (c) on or before the Amendment No. 2 Effective Date . For clarity, Licensee shall not be required to make any payment set forth in Sections 5.1(a), (b), and (c) if such payment becomes payable in accordance with the terms of Section 5.1(a), (b), or (c) after the Amendment No. 2 Effective Date. ” 16. All instances of the term “Joint Rights” in the Agreement that are not modified by the above provisions are hereby modified to the term “IP Rights”. The Parties agree that this Paragraph 16 retroactively applies to all Outlicensee Agreements entered into even before this Amendment No. 2 Effective Date. 17. In the event that OMT does not receive the fifty-one million U.S. dollars (US $51 million) [***] set forth in Paragraph 2 of this Amendment No. 2 within ten (10) days of the Amendment No. 2 Effective Date, OMT will have the right to terminate this Amendment No. 2 immediately upon written notice. If OMT terminates this Amendment No. 2 as set forth in this Paragraph 17, above, this Amendment No 2 shall have no force or effect, retroactive to the Amendment No 2 Effective Date, and the Agreement shall continue in full force and effect as it existed prior to the Amendment No. 2 Effective Date. 18. In the event of any discrepancies or conflicting terms between this Amendment No. 2 and the Agreement, the terms of this Amendment No. 2 shall control. 19. The Agreement and this Amendment No. 2 represent the complete and entire understanding between the Parties regarding the subject matter hereof and supersede all prior or contemporaneous negotiations, representations or agreements, either written or oral, regarding this subject matter. 20. This Amendment No. 2 and the rights and obligations of the Parties hereunder shall be governed by the laws of the State of California, without regard to the conflicts of law provisions thereof. [***] Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. 4840-7056-1386 v.6 6 US-DOCS\102805239.1102810859.2

21. This Amendment No. 2 may be executed in counterparts, each of which shall be deemed an original and, together, one and the same instrument. A facsimile, PDF or any other copy of this Amendment No. 2 signed by a Party is binding upon the signing Party to the same extent as the original of the signed Amendment No. 2, and may be delivered electronically. 22. Except for the matters set forth in this Amendment No. 2, all other terms of the Agreement shall remain unchanged and in full force and effect. [Signature page follows.] 4840-7056-1386 v.6 7 US-DOCS\102805239.1102810859.2
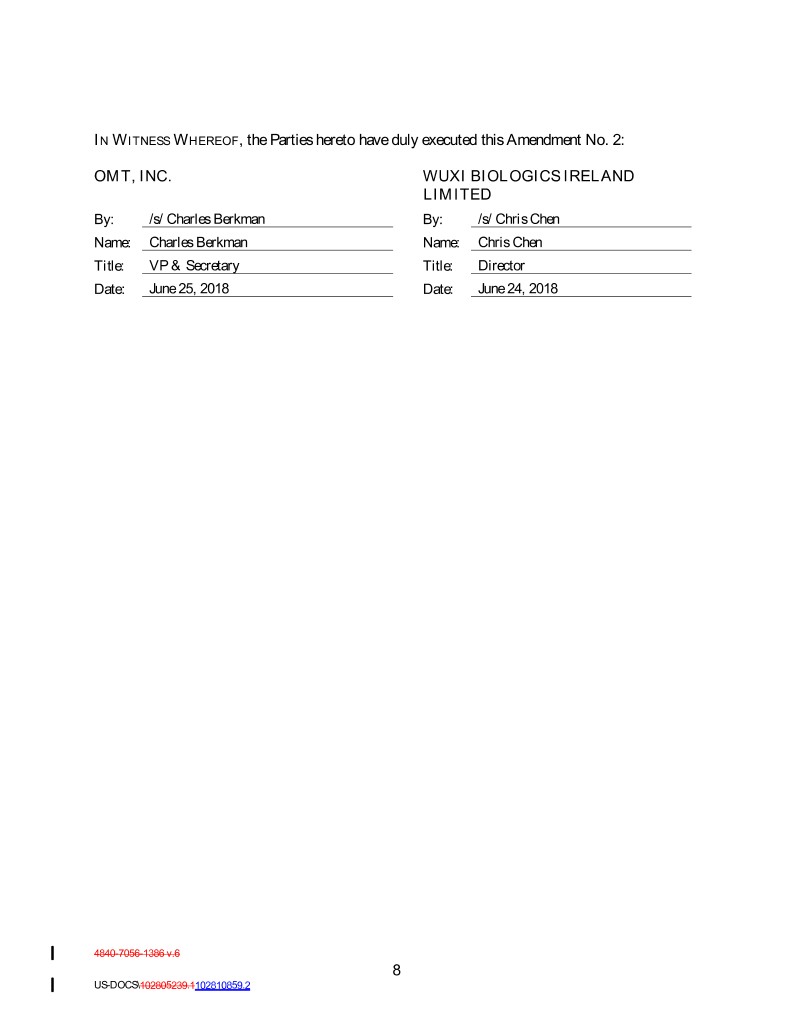
IN W ITNESS W HEREOF , the Parties hereto have duly executed this Amendment No. 2: OMT, INC. WUXI BIOLOGICS IRELAND LIMITED By: /s/ Charles Berkman By: /s/ Chris Chen Name: Charles Berkman Name: Chris Chen Title: VP & Secretary Title: Director Date: June 25, 2018 Date: June 24, 2018 4840-7056-1386 v.6 8 US-DOCS\102805239.1102810859.2
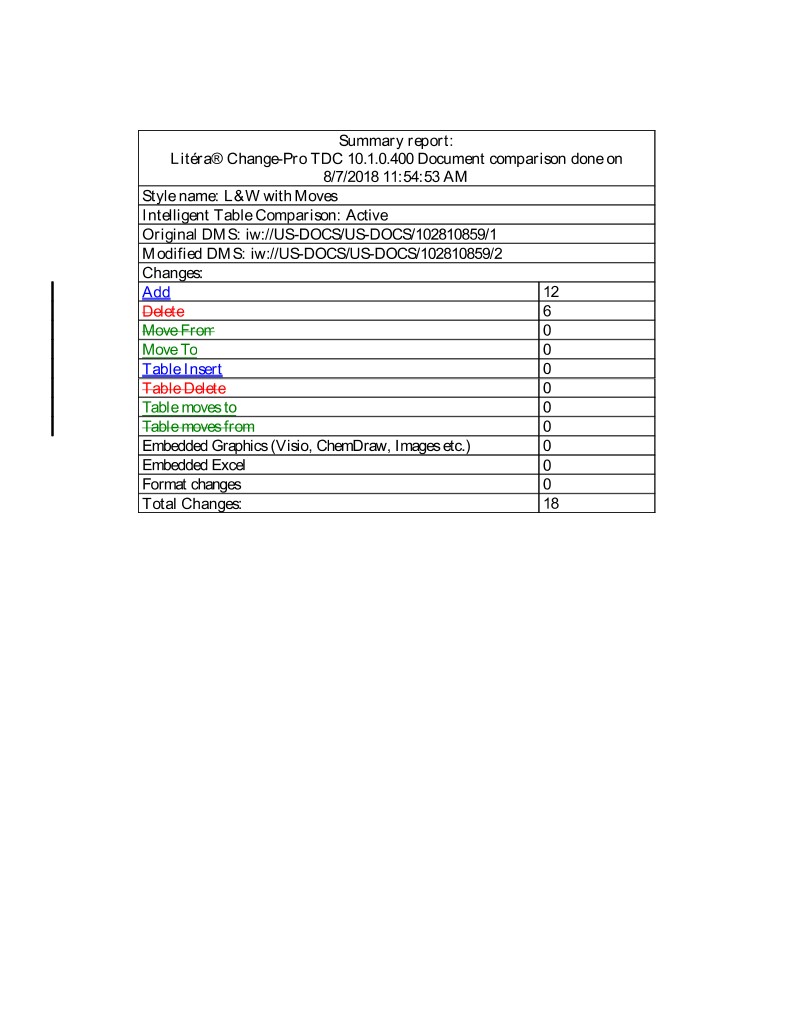
Summary report: Litéra® Change-Pro TDC 10.1.0.400 Document comparison done on 8/7/2018 11:54:53 AM Style name: L&W with Moves Intelligent Table Comparison: Active Original DMS: iw://US-DOCS/US-DOCS/102810859/1 Modified DMS: iw://US-DOCS/US-DOCS/102810859/2 Changes: Add 12 Delete 6 Move From 0 Move To 0 Table Insert 0 Table Delete 0 Table moves to 0 Table moves from 0 Embedded Graphics (Visio, ChemDraw, Images etc.) 0 Embedded Excel 0 Format changes 0 Total Changes: 18
