Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Alkermes plc. | alks-20180726ex991023092.htm |
| 8-K - 8-K - Alkermes plc. | alks-20180726x8k.htm |
Exhibit 99.2
|
Alkermes Patient inspired Second Quarter 2018 Financial Results & Update July 26, 2018 2018 Alkermes. All rights reserved |
|
Forward-Looking Statements and Non-GAAP Financial Information Certain statements set forth in this presentation constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: the future financial and operating performance, business plans or prospects of the company; the continued growth of the long-acting injectable antipsychotic market and revenue from the company’s commercial products, including VIVITROL®, ARISTADA® and ARISTADA INITIO™; improvements to and modernization of the treatment ecosystem for opioid dependence; the timing, funding, results and feasibility of clinical development activities, including the timing of the phase 3 data readout for ALKS 3831, the timing of the initial phase 1 data readout, the expansion of the phase 1 study and other development activities for ALKS 4230, and the timing of completion of the registration packages and submission of the new drug applications (“NDAs”) for each of BIIB098 and ALKS 3831; whether the studies conducted for ALKS 5461, ALKS 3831 and BIIB098 will meet the U.S. Food and Drug Administration’s (“FDA”) requirements for approval; the company’s expectations and timelines for regulatory interactions with the FDA, and actions by the FDA, relating to its review of the NDA submission for ALKS 5461; expectations concerning the timing and results and nature of commercial activities, including preparations for the anticipated launch of ALKS 5461; the potential financial benefits that may be achieved under the license and collaboration agreement between the company and Biogen for BIIB098; the therapeutic value and commercial potential of the company’s commercial products and development candidates; and funding for, and patient access to, the company’s commercial products and development candidates and other related services. Although the company believes that such forward-looking statements are based on reasonable assumptions within the bounds of its knowledge of its business and operations, the forward-looking statements are neither promises nor guarantees and they are necessarily subject to a high degree of uncertainty and risk. Actual performance and results may differ materially from those expressed or implied in the forward-looking statements due to various risks, assumptions and uncertainties. These risks, assumptions and uncertainties include, among others: the unfavorable outcome of litigation, including so-called “Paragraph IV” litigation and other patent litigation, related to any of our products or partnered products, which may lead to competition from generic drug manufacturers; data from clinical trials may be interpreted by the FDA in different ways than we interpret it; the FDA may not agree with our regulatory approval strategies or components of our filings for our products, including our clinical trial designs, conduct and methodologies and, for ALKS 5461, evidence of efficacy and adequacy of bridging to buprenorphine; clinical development activities may not be completed on time or at all; the results of our clinical development activities may not be positive, or predictive of real-world results or of results in subsequent clinical trials; regulatory submissions may not occur or be submitted in a timely manner; the company and its licensees may not be able to continue to successfully commercialize their products; there may be a reduction in payment rate or reimbursement for the company’s products or an increase in the company’s financial obligations to governmental payers; the FDA or regulatory authorities outside the U.S. may make adverse decisions regarding the company’s products; the company’s products may prove difficult to manufacture, be precluded from commercialization by the proprietary rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; and those risks, assumptions and uncertainties described under the heading “Risk Factors” in the company’s most recent Annual Report on Form 10-K and in subsequent filings made by the company with the U.S. Securities and Exchange Commission (“SEC”), which are available on the SEC’s website at www.sec.gov and on the company’s website at www.alkermes.com in the “Investors—SEC filings” section. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the company disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation. Non-GAAP Financial Measures: This presentation includes information about certain financial measures that are not prepared in accordance with generally accepted accounting principles in the U.S. (GAAP), including non-GAAP net income/(loss) and non-GAAP earnings/(loss) per share. These non-GAAP measures are not based on any standardized methodology prescribed by GAAP and are not necessarily comparable to similar measures presented by other companies. Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP financial measures can be found in the Alkermes plc Current Report on Form 8-K filed with the SEC on July 26, 2018. Note Regarding Trademarks: The company is the owner of various U.S. federal trademark registrations (®) and other trademarks (TM), including ARISTADA®, VIVITROL® and ARISTADA INITIO™. Any other trademarks referred to in this presentation are the property of their respective owners. Appearances of such other trademarks herein should not be construed as any indicator that their respective owners will not assert their rights thereto. Alkermes 2018 Alkermes. All rights reserved. |
|
Q2 Earnings Call Agenda Q2 Financial Results Jim Frates Chief Financial Officer Commercial update Jim Robinson President & Chief Operating Officer R&D Update Richard Pops Chief Executive Officer Alkermes 2018 Alkermes. All rights reserved. |
|
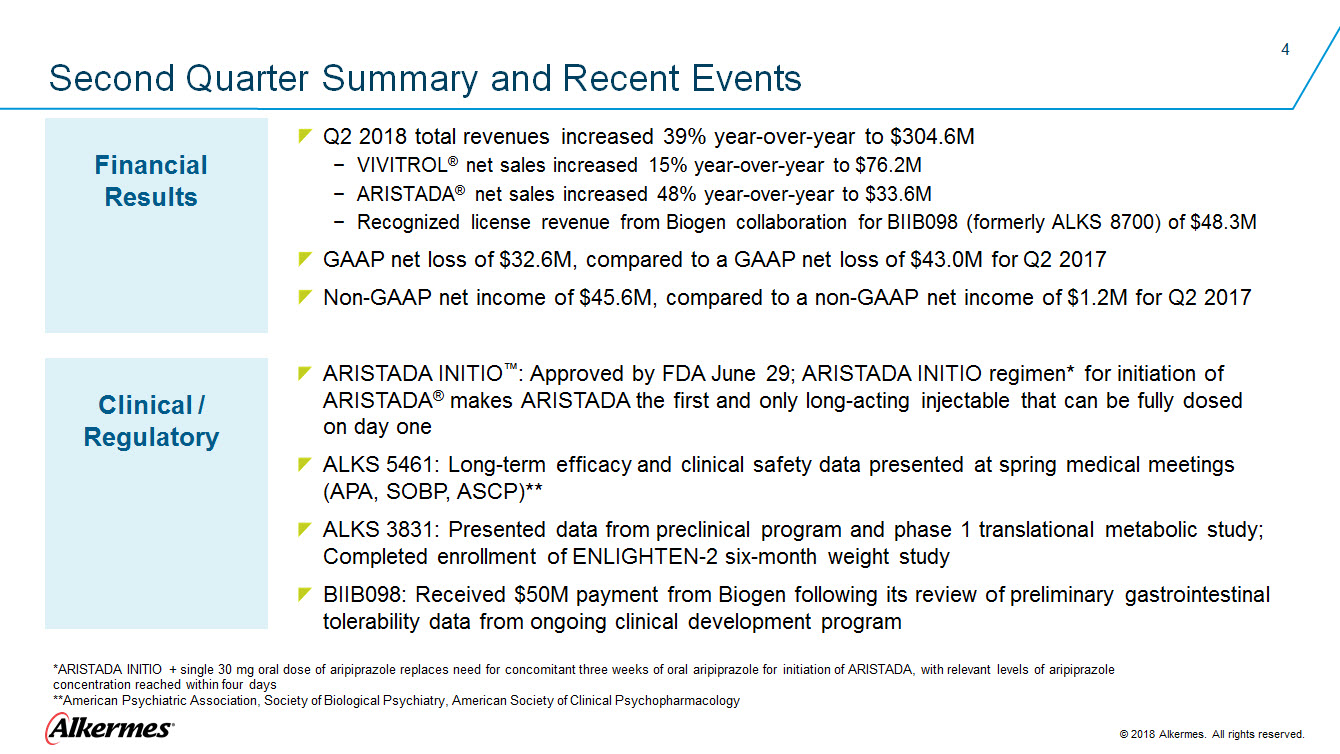
Second Quarter Summary and Recent Events Financial Results Q2 2018 total revenues increased 39% year-over-year to $304.6M VIVITROL® net sales increased 15% year-over-year to $76.2M ARISTADA® net sales increased 48% year-over-year to $33.6M Recognized license revenue from Biogen collaboration for BIIB098 (formerly ALKS 8700) of $48.3M GAAP net loss of $32.6M, compared to a GAAP net loss of $43.0M for Q2 2017 Non-GAAP net income of $45.6M, compared to a non-GAAP net income of $1.2M for Q2 2017 Clinical / Regulatory
ARISTADA INITIO™: Approved by FDA June 29; ARISTADA INITIO regimen* for initiation of ARISTADA® makes ARISTADA the first and only long-acting injectable that can be fully dosed ALKS 5461: Long-term efficacy and clinical safety data presented at spring medical meetings (APA, SOBP, ASCP)** ALKS 3831: Presented data from preclinical program and phase 1 translational metabolic study; Completed enrollment of ENLIGHTEN-2 six-month weight study BIIB098: Received $50M payment from Biogen following its review of preliminary gastrointestinal tolerability data from ongoing clinical development program *ARISTADA INITIO + single 30 mg oral dose of aripiprazole replaces need for concomitant three weeks of oral aripiprazole for initiation of ARISTADA, with relevant levels of aripiprazole concentration reached within four days **American Psychiatric Association, Society of Biological Psychiatry, American Society of Clinical Psychopharmacology Alkermes 2018 Alkermes. All rights reserved. |
|
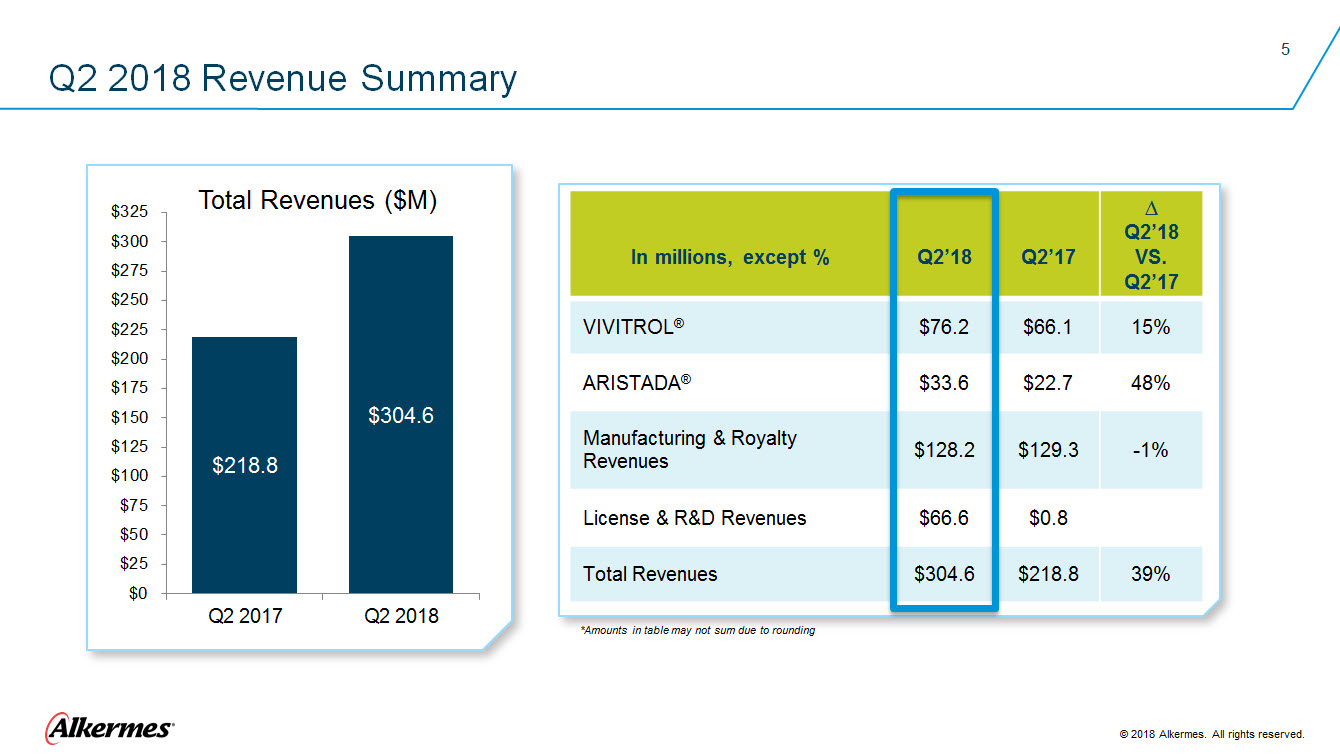
Q2 2018 Revenue Summary Total Revenues ($M) $218.8 Q22017 $304.6 Q22018 In millions, except % Q2’18 Q2’17 ∆ Q2’18 VS. Q2’17 VIVITROL® $76.2 $66.1 15% ARISTADA® $33.6 $22.7 48% Manufacturing & Royalty Revenues $128.2 $129.3 -1% License & R&D Revenues $66.6 $0.8 Total Revenues $304.6 $218.8 39% Alkermes 2018 Alkermes. All rights reserved. |
|
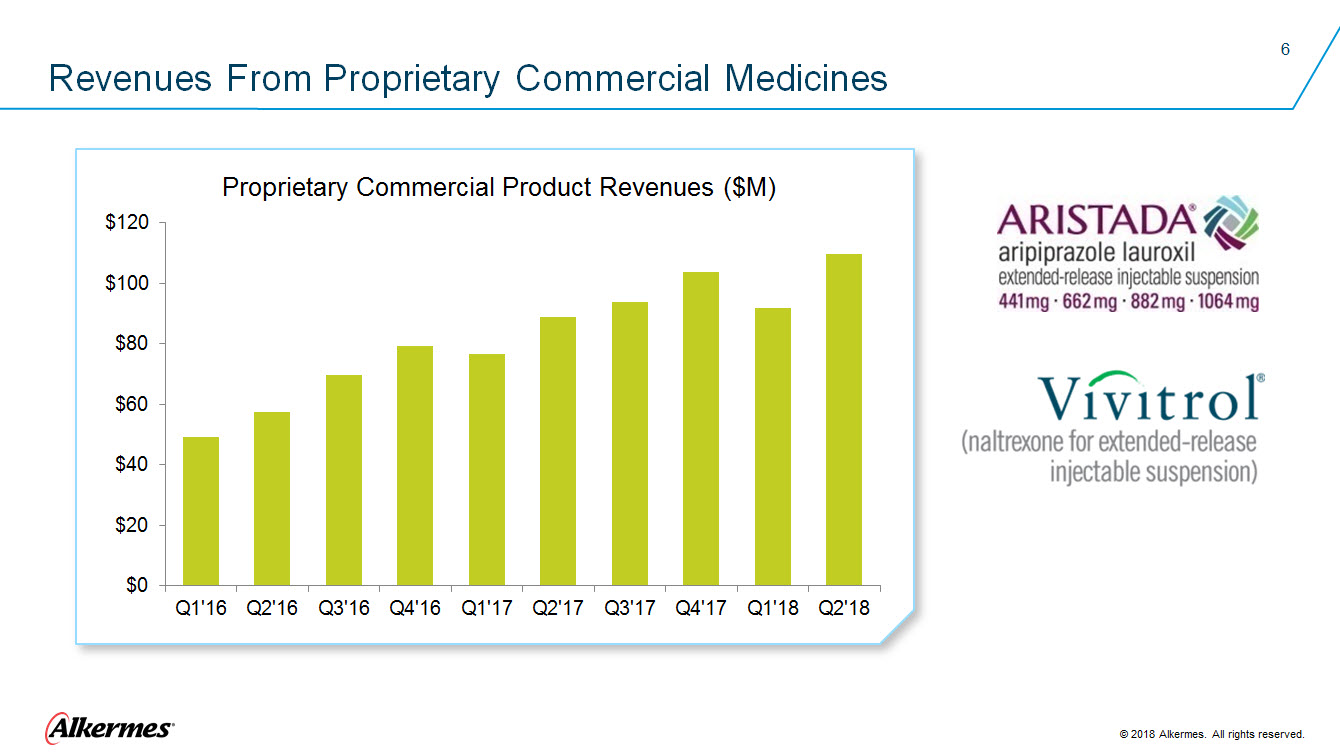
Revenues From Proprietary Commercial Medicines Proprietary Commercial Product Revenues ($M) $120 $100 $80 $60 $40 $20 $0 Q1’16 Q2’16 Q3’16 Q4’16 Q1’17 Q2’17 Q3’17 Q4’17 Q1’18 Q2’18 ARISTADA aripiprazole lauroxil extended-release injectable suspension 441mg 662 mg 882 mg 1064 mg VIVITROL (naltrexone for extended-release injectable suspension) Alkermes 2018 Alkermes. All rights reserved. |
|
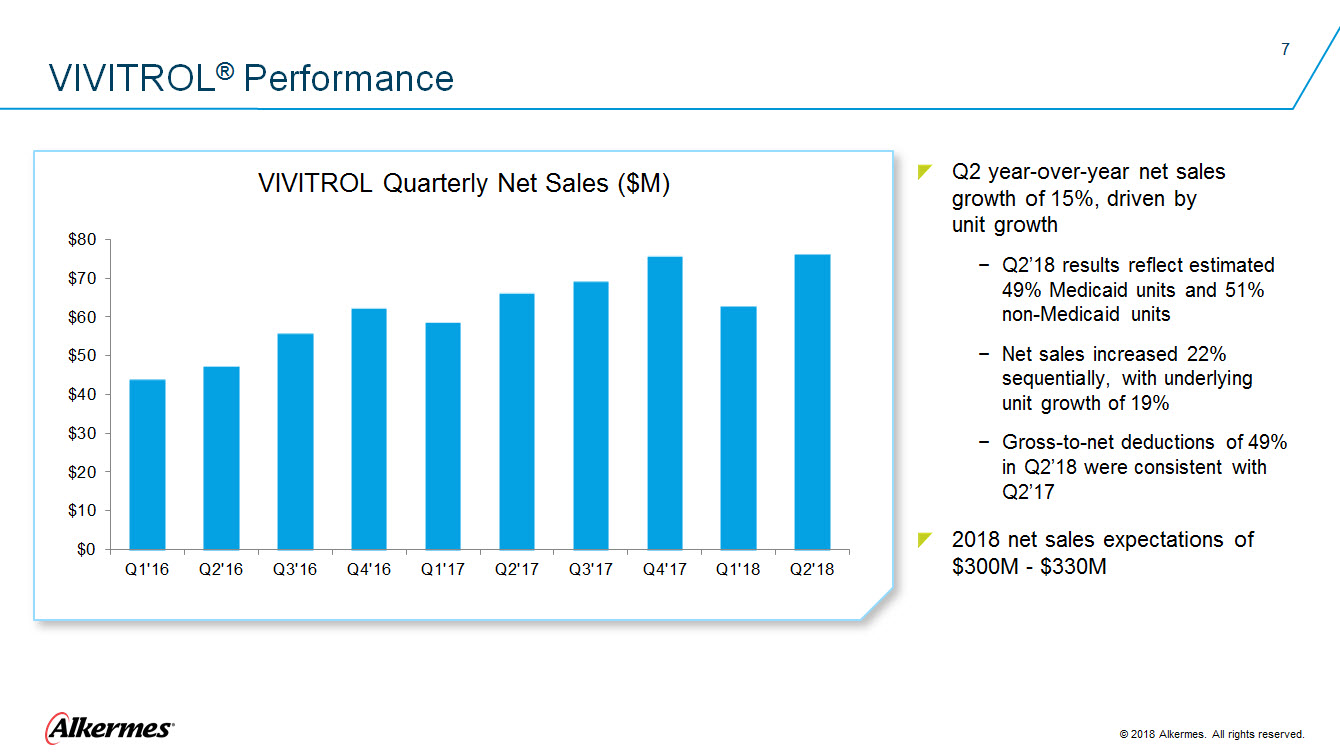
VIVITROL ®Performance VIVITROL Quarterly Net Sales ($M) $80 $70 $60 $50 $40 $30 $20 $10 $0 Q1’16 Q2’16 Q3’16 Q4’16 Q1’17 Q2’17 Q3’17 Q4’17 Q1’18 Q2’18
Q2 year-over-year net sales
Q2’18 results reflect estimated
Net sales increased 22% sequentially, with underlying Gross-to-net deductions of 49% in Q2’18 were consistent with Q2’17
2018 net sales expectations of Alkermes 2018 Alkermes. All rights reserved. |
|
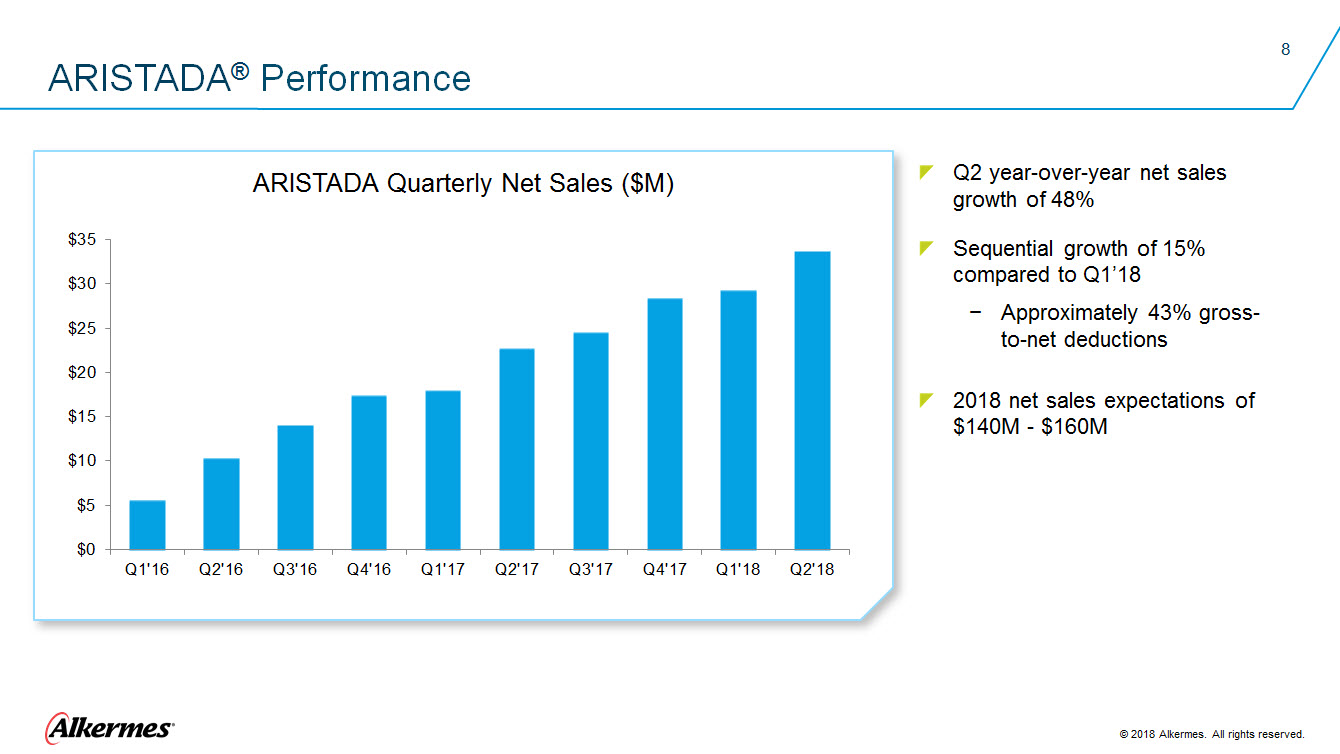
ARISTADA® Performance ARISTADA Quarterly Net Sales ($M) $35 $30 $25 $20 $15 $10 $5 $0 Q1’16 Q2’16 Q3’16 Q4’16 Q1’17 Q2’17 Q3’17 Q4’17 Q1’18 Q2’18
Q2 year-over-year net sales Sequential growth of 15% compared to Q1’18 Approximately 43% gross-to-net deductions 2018 net sales expectations of $140M - $160M |
|
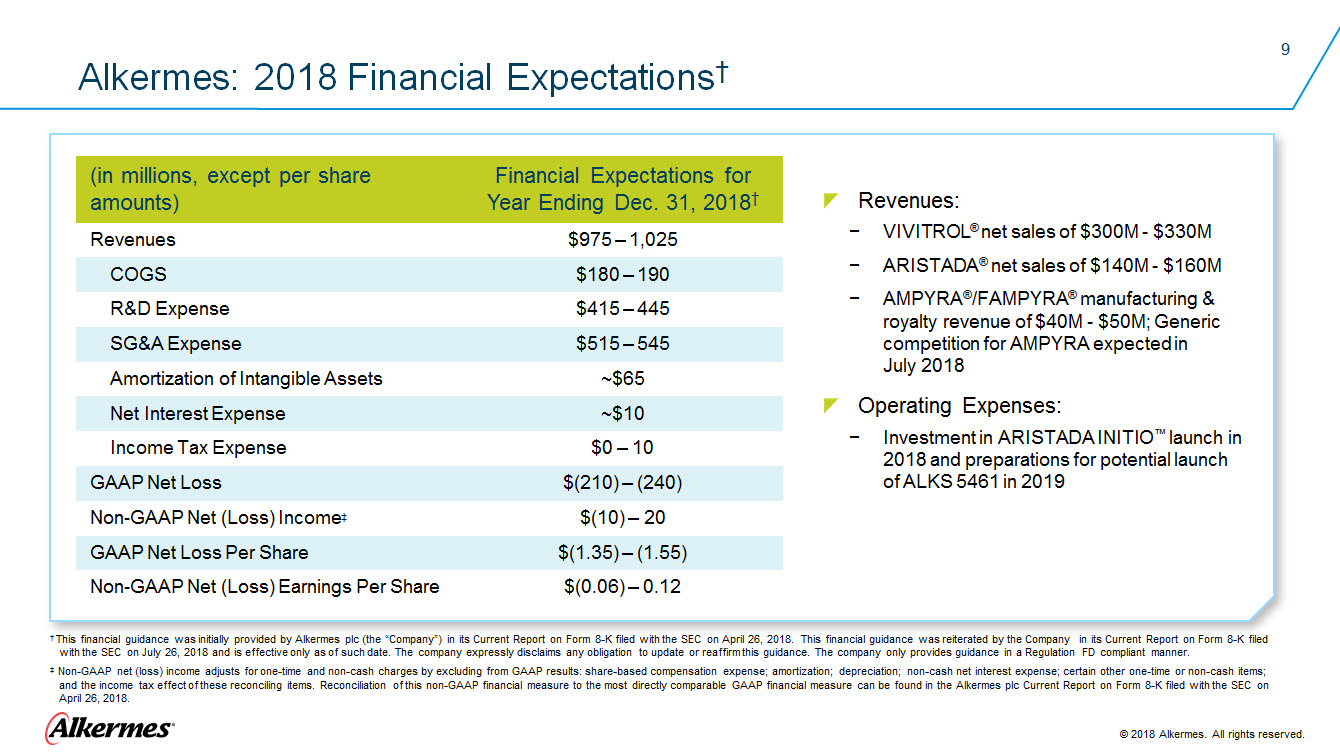
Alkermes: 2018 Financial Expectations † (in millions, except per share amounts) Financial Expectations for Year Ending Dec. 31, 2018 Revenues $975 – 1,025 COGS $180-180 R&D Expense $415 – 445 SG&A Expense $515 – 545 Amortization of Intangible Assets ~$65 Net Interest Expense ~$10 Income Tax Expense $0 -–10 GAAP Net Loss ($(210) – (240) Non-GAAP Net (Loss) Income ‡ $(10) – 20 GAAP Net Loss Per Share $(1.35) – (1.55) Non-GAAP Net (Loss) Earnings Per Share $(0.06) – 0.12 Revenues: VIVITROL® net sales of $300M - $330M ARISTADA® net sales of $140M - $160M
AMPYRA®/FAMPYRA® manufacturing & royalty revenue of $40M - $50M; Generic competition for AMPYRA expected in Operating Expenses: Investment in ARISTADA INITIO™ launch in 2018 and preparations for potential launch of ALKS 5461 in 2019 † This financial guidance was initially provided by Alkermes plc (the “Company”) in its Current Report on Form 8-K filed with the SEC on April 26, 2018. This financial guidance was reiterated by the Company in its Current Report on Form 8-K filed with the SEC on July 26, 2018 and is effective only as of such date. The company expressly disclaims any obligation to update or reaffirm this guidance. The company only provides guidance in a Regulation FD compliant manner. ‡ Non-GAAP net (loss) income adjusts for one-time and non-cash charges by excluding from GAAP results: share-based compensation expense; amortization; depreciation; non-cash net interest expense; certain other one-time or non-cash items; and the income tax effect of these reconciling items. Reconciliation of this non-GAAP financial measure to the most directly comparable GAAP financial measure can be found in the Alkermes plc Current Report on Form 8-K filed with the SEC on April 26, 2018. Alkermes 2018 Alkermes. All rights reserved. |
|
VIVITROL®: Opportunities to Increase Utilization and Drive Growth State and federal dollars are being allocated; Funding slowly flowing into fragmented treatment system ~$1B of funding provided by 21st Century Cures Act has been distributed to states via block grants Small percentage has flowed from the states into changing the treatment system Federal budget included $6B over the next two years to address the opioid epidemic and mental health programs $1B for new State Opioid Response Grant program Working with state authorities to encourage timely distribution of funds to local treatment systems Improvements in accessibility of VIVITROL and implementation of public policy initiatives driving strong growth in certain states California, Florida, Pennsylvania, Kentucky State programs expanded to ~690 at the end of Q2’18, primarily driven by criminal justice re-entry and drug court programs Alkermes 2018 Alkermes. All rights reserved |
|
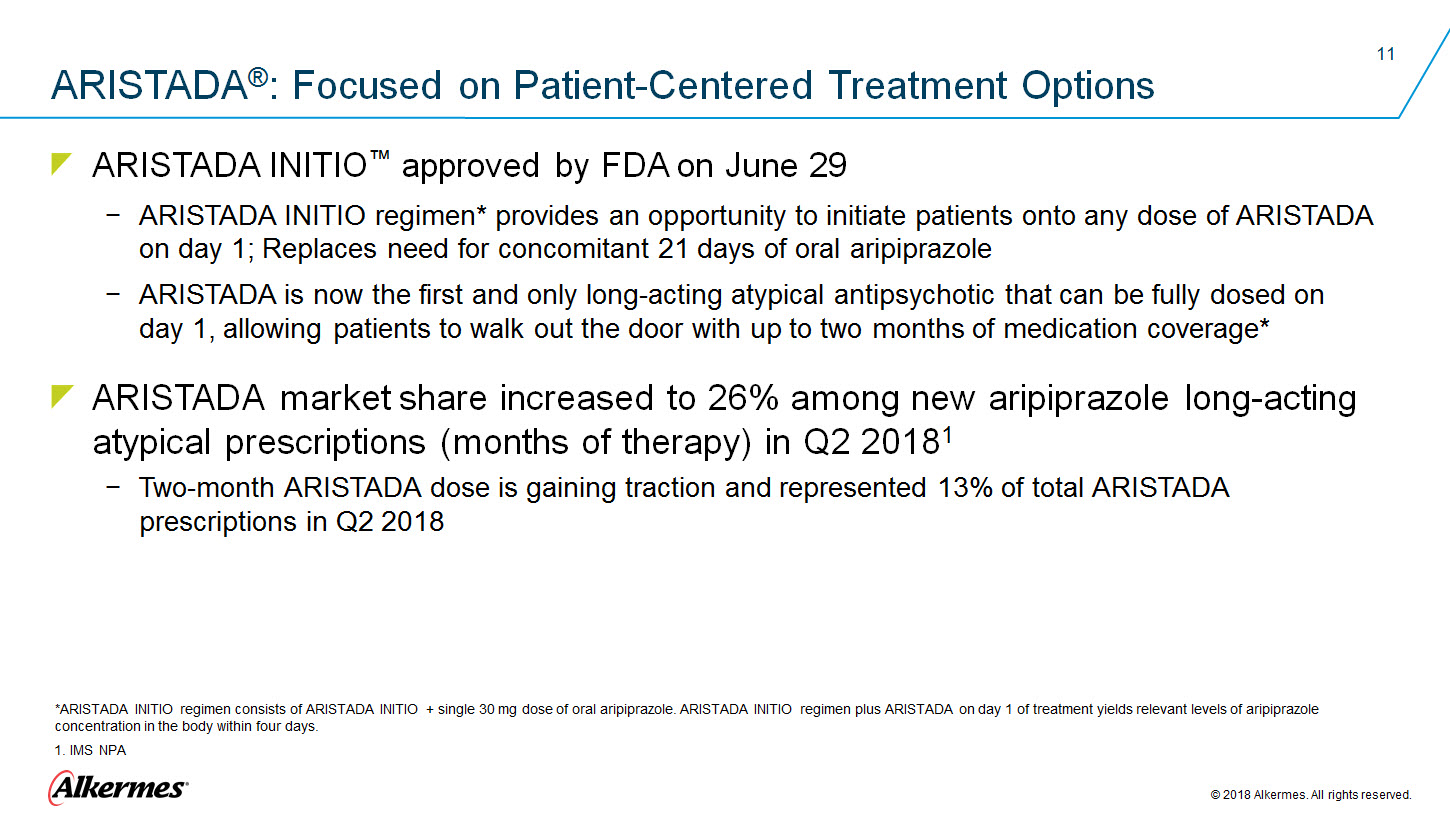
ARISTADA®: Focused on Patient-Centered Treatment Options ARISTADA INITIO™ approved by FDA on June 29 ARISTADA INITIO regimen* provides an opportunity to initiate patients onto any dose of ARISTADA on day 1; Replaces need for concomitant 21 days of oral aripiprazole ARISTADA is now the first and only long-acting atypical antipsychotic that can be fully dosed on day 1, allowing patients to walk out the door with up to two months of medication coverage* ARISTADA market share increased to 26% among new aripiprazole long-acting atypical prescriptions (months of therapy) in Q2 20181 Two-month ARISTADA dose is gaining traction and represented 13% of total ARISTADA prescriptions in Q2 2018 *ARISTADA INITIO regimen consists of ARISTADA INITIO + single 30 mg dose of oral aripiprazole. ARISTADA INITIO regimen plus ARISTADA on day 1 of treatment yields relevant levels of aripiprazole concentration in the body within four days. 1. IMS NPA Alkermes 2018 Alkermes. All rights reserved. |
|
ALKS 5461 Program Investigational product for adjunctive treatment of major depressive disorder (MDD) in patients with inadequate response to standard antidepressant therapy Opioid system modulator represents a new mechanism of action for the treatment of MDD Status Regulatory review underway, PDUFA target action date Jan. 31, 2019 Long-term efficacy and clinical safety data presented at APA, SOBP, ASCP Continued scientific exchange with medical community on opioid system dysregulation; New manuscript published in Molecular Psychiatry Priorities FDA Advisory Committee meeting tentatively scheduled for Nov. 1 Preparations for anticipated launch Investment in manufacturing, senior leadership and necessary commercial infrastructure Alkermes 2018 Alkermes. All rights reserved. |
|
ALKS 3831 Program Investigational, novel, once-daily, oral atypical antipsychotic drug candidate for the treatment of schizophrenia Designed to provide antipsychotic efficacy of olanzapine and a differentiated safety profile with favorable weight and metabolic properties Status
Positive results from ENLIGHTEN-1 pivotal antipsychotic efficacy study Presented data from phase 1 translational medicine study evaluating metabolic profile of ALKS 3831 compared to olanzapine in May 2018 Priorities Complete ENLIGHTEN-2, a six-month phase 3 study assessing weight gain with olanzapine compared to ALKS 3831; Topline data expected Q4 2018 Enrollment of ENLIGHTEN-2 completed April 2018 Alkermes 2018 Alkermes. All rights reserved. |
|
BIIB098 (Formerly ALKS 8700) Program Investigational product for the treatment of relapsing forms of multiple sclerosis (MS)
License and collaboration agreement with Status Long-term safety study ongoing
Pharmacokinetic bridging studies and clinical
Received $50M payment from Biogen following Priorities Complete remaining clin/pharm studies for registration package Planned NDA submission in Q4 2018 Biogen License and Collaboration Agreement Granted Biogen exclusive, worldwide license to commercialize BIIB098 Mid-teens percentage royalty to Alkermes on worldwide net sales
$150M milestone upon regulatory approval by FDA Biogen responsible for development and commercial expenses (as of 1/1/18) Alkermes 2018 Alkermes. All rights reserved. |
|
ALKS 4230 Program Novel immuno-oncology candidate Designed to selectively activate intermediate-affinity IL-2 receptors to enhance tumor-killing immune cells Status Monotherapy dose-escalation stage of phase 1 study ongoing Plans to initiate evaluation of safety and anti-tumor activity of ALKS 4230 in combination with pembrolizumab in Q3 2018 Priorities
Complete dose-escalation stage; Present initial data from ongoing phase 1 study
Optimize dosing: Planning subcutaneous dosing phase 1 study and evaluation Alkermes 2018 Alkermes. All rights reserved. |
|
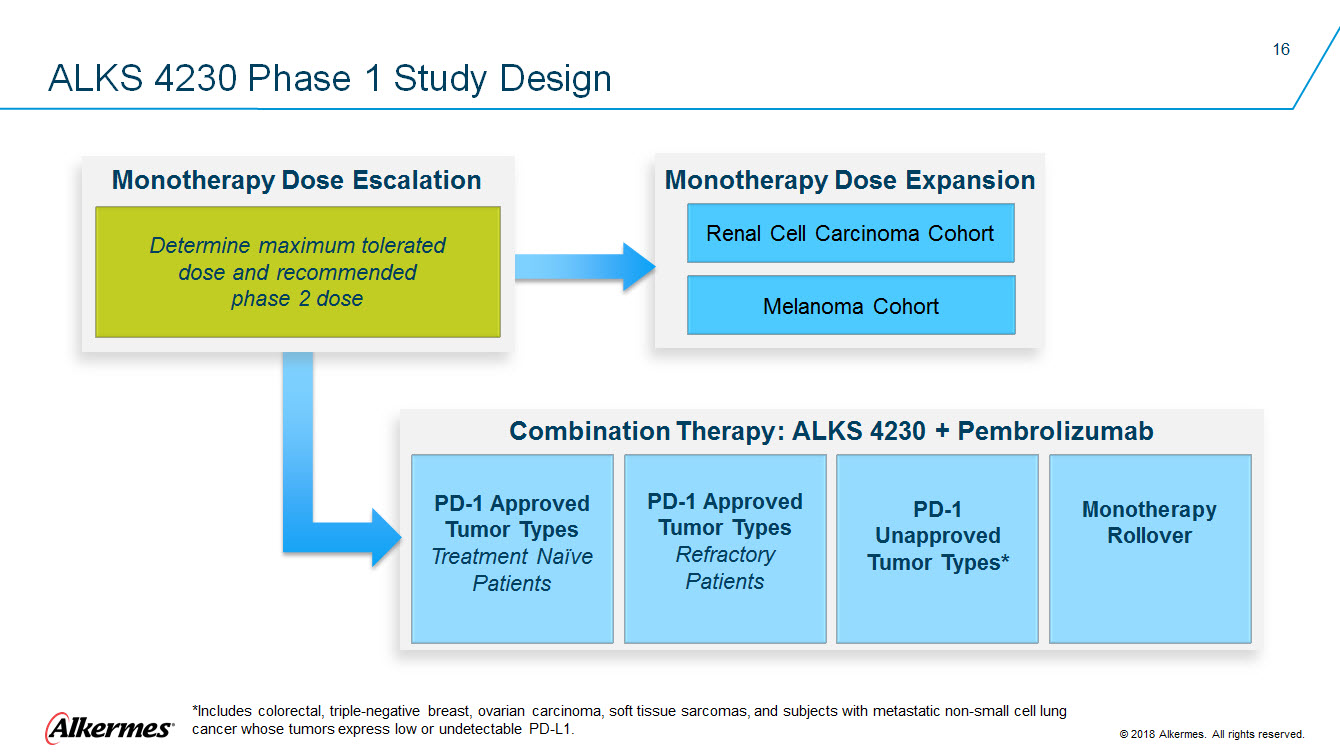
ALKS 4230 Phase 1 Study Design Monotherapy Dose Escalation Determine maximum tolerated dose and recommend phase 2 dose Monotherapy Dose Expansion Renal Cell Carcinoma Cohort Melanoma Cohort Combination Therapy: ALKS 4230 + Pembrolizumab PD-1 Approved Tumor Types Treatment Naïve Patients PD-1 Approved Tumor Types Refractory Patients PD-1 Unapproved Tumor Types* Monotherapy Rollover Alkermes *Includes colorectal, triple-negative breast, ovarian carcinoma, soft tissue sarcomas, and subjects with metastatic non-small cell lung cancer whose tumors express low or undetectable PD-L1. 2018 Alkermes. All rights reserved. |
|
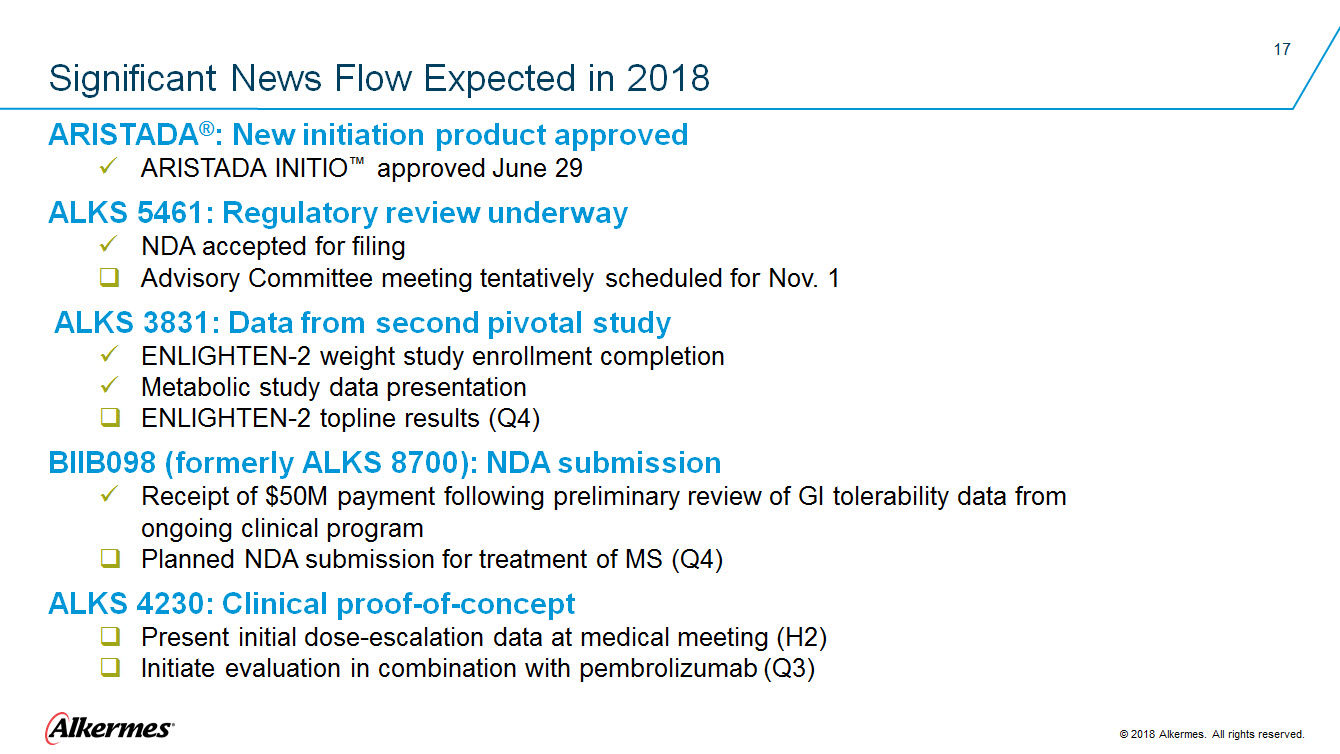
Significant News Flow Expected in 2018 ARISTADA®: New initiation product approved ARISTADA INITIO™ approved June 29 ALKS 5461: Regulatory review underway NDA accepted for filing Advisory Committee meeting tentatively scheduled for Nov. 1 ALKS 3831: Data from second pivotal study ENLIGHTEN-2 weight study enrollment completion Metabolic study data presentation ENLIGHTEN-2 topline results (Q4) BIIB098 (formerly ALKS 8700): NDA submission
Receipt of $50M payment following preliminary review of GI tolerability data from Planned NDA submission for treatment of MS (Q4) ALKS 4230: Clinical proof-of-concept Present initial dose-escalation data at medical meeting (H2) Initiate evaluation in combination with pembrolizumab (Q3) Alkermes 2018 Alkermes. All rights reserved |
|
www.alkermes.com 2018 Alkermes. All rights reserved. |
|