Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Zymeworks Inc. | d596080d8k.htm |
Exhibit 99.1
FORM 51-102F3
MATERIAL CHANGE REPORT
| Item 1: | Name and Address of Company |
Zymeworks Inc. (Zymeworks or the Company)
1385 West 8th Avenue, Suite 540
Vancouver, BC, Canada
V6H 3V9
| Item 2: | Date of Material Change |
June 1, 2018
| Item 3: | News Release |
A news release announcing the material change was disseminated through the facilities of Business Wire on June 1, 2018, and a copy was filed on the Company’s profile at www.sedar.com.
| Item 4: | Summary of Material Change |
On June 1, 2018, Zymeworks announced the presentation of ZW25 clinical data by Funda Meric-Bernstam, MD, Principal Investigator for the ZW25 study at the University of Texas MD Anderson Cancer Center. Data from the ongoing multi-center Phase 1 study showed single agent ZW25 induced anti-tumor activity and was well tolerated in heavily pretreated patients across a range of HER2-expressing cancers.
| Item 5: | Full Description of Material Change |
5.1 Full Description of Material Change
On June 1, 2018, Zymeworks announced the presentation of ZW25 clinical data by Funda Meric-Bernstam, MD, Principal Investigator for the ZW25 study at the University of Texas MD Anderson Cancer Center. Data from the ongoing multi-center Phase 1 study showed single agent ZW25 induced anti-tumor activity and was well tolerated in heavily pretreated patients across a range of HER2-expressing cancers.
ZW25 Clinical Results Presented on June 1, 2018
To the date of the news release, a total of 50 patients have been enrolled in the study; data from 42 patients were available as of the data cut-off date of April 18, 2018 for ASCO and presented June 1, 2018. Durable cytotoxin-free single agent activity was observed in patients with heavily pretreated HER2 expressing cancers across a range of tumor types.
The best overall response observed with ZW25 as a single agent therapy in 33 response-evaluable patients (defined as having measurable disease and at least one tumor restaging or clinical progression) was 12 partial responses (36%), six stable disease (18%) and 15 progressive disease (45%). Overall, 68% (21/31) of all patients with measurable disease (at least one restaging scan) had a decrease in target lesions.
In 18 breast cancer patients, with a median of six prior systemic regimens, including trastuzumab, pertuzumab, T-DM1, and lapatinib in the majority of patients, the disease control rate (DCR, percentage of patients with either a partial response or stable disease) was 50%. In nine gastroesophageal cancer patients, with a median of four prior systemic regimens, including trastuzumab in all cases, the DCR was 56%, and in six other HER2-expressing cancer patients, including colorectal cancer, the DCR was 67%. Anti-tumor activity was assessed per RECIST every eight weeks.
In the study, ZW25 was well tolerated at all dose levels and schedules and there were no dose-limiting toxicities observed at any dose (n=42). Treatment-related adverse events were primarily Grade 1 or 2, and no treatment-related serious adverse events or discontinuations were seen.
Please see Schedule A to this material change report for further information on ZW25.
Clinical Development Plans
Based on the clinical data generated to date, Zymeworks plans to focus development of ZW25 in three areas:
| • | First, as a single agent treatment for advanced HER2 high gastroesophageal cancer in patients who have received prior trastuzumab therapy, as well as in other HER2 high cancers, such as colorectal, where a HER2-targeted agent has not yet been approved; |
| • | Second, in combination with chemotherapeutics in earlier lines of therapy for HER2 high gastroesophageal and breast cancers; and |
| • | Third, in combination with other anti-cancer agents in patients with lower HER2 expressing cancers. |
Zymeworks’ top priority is to focus on advanced gastroesophageal cancer. A potential Phase 2/3 study could begin as early as the second half of 2019 pending discussion with the US Food and Drug Administration (FDA). In addition, new studies to evaluate combinations beyond those ongoing in the current Phase 1 study are planned to start later this year.
About the Trial
Zymeworks’ adaptive Phase 1 study has three parts. Enrollment in the first portion of the study (the dose-escalation phase) has been completed. The recommended single agent dose was determined to be 20 mg/kg once every two weeks. In the second part of the study (the cohort expansion phase) now underway, additional patients are being enrolled to further assess ZW25’s single agent tolerability and anti-tumor activity against a variety of cancer types in different settings. The third part of the study (the combination phase), which is also underway, is evaluating ZW25 in combination with selected chemotherapy agents in gastroesophageal and breast cancer patients with HER2 high or lower HER2 expression levels.
2
About ZW25
ZW25 is being evaluated in a Phase 1 clinical trial in the United States and Canada. It is a bispecific antibody, based on Zymeworks’ Azymetric™ platform, that can simultaneously bind two non-overlapping epitopes of HER2, known as biparatopic binding. This unique design results in multiple mechanisms of action including dual HER2 signal blockade, increased binding and removal of HER2 protein from the cell surface, and potent effector function and has led to encouraging anti-tumor activity in patients. Zymeworks is developing ZW25 as a HER2-targeted treatment option for patients with any solid tumor that expresses HER2. The FDA has granted Orphan Drug Designation to ZW25 for the treatment of both gastric and ovarian cancers.
About the Azymetric™ Platform
The Azymetric platform enables the transformation of monospecific antibodies into bispecific antibodies, giving them the ability to simultaneously bind two different targets. Azymetric bispecific technology enables the development of multifunctional biotherapeutics that can block multiple signaling pathways, recruit immune cells to tumors, enhance receptor clustering and degradation, and increase tumor-specific targeting. These features are intended to enhance efficacy while reducing toxicities and the potential for drug-resistance. Azymetric bispecifics have been engineered to retain the desirable drug-like qualities of naturally occurring antibodies, including low immunogenicity, long half-life, and high stability. In addition, they are compatible with standard manufacturing processes with high yields and purity with the potential to significantly reduce drug development costs and timelines.
5.2 Disclosure of Restructuring Transactions
Not applicable.
| Item 6: | Reliance on subsection 7.1(2) of National Instrument 51-102 |
Not applicable.
| Item 7: | Omitted Information |
Not applicable.
| Item 8: | Executive Officer |
For further information, please contact Neil Klompas, Chief Financial Officer of the Company at (604) 678-1388.
| Item 9: | Date of Report |
June 6, 2018
3
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This material change report includes “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995 and “forward-looking information” within the meaning of Canadian securities laws, or collectively, forward-looking statements. Forward-looking statements in this material change report include statements that relate to ZW25 and its potential as a single agent therapy or in combination with other approved anti-cancer treatments, Zymeworks’ clinical plans and future results, Zymeworks’ technology platform, and other information that is not historical information. When used herein, words such as “believe”, “may”, “plan”, “will”, “estimate”, “continue”, “anticipate”, “intend”, “expect”, and similar expressions are intended to identify forward-looking statements. In addition, any statements or information that refer to expectations, beliefs, plans, projections, objectives, performance or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking. All forward-looking statements are based upon Zymeworks’ current expectations and various assumptions. Zymeworks believes there is a reasonable basis for its expectations and beliefs, but they are inherently uncertain. Zymeworks may not realize its expectations, and its beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements as a result of various factors, including, without limitation, market conditions and the factors described under “Risk Factors” in Zymeworks’ Annual Report on Form 10-K for its fiscal year ended December 31, 2017 (a copy of which may be obtained at www.sec.gov and www.sedar.com). Consequently, forward-looking statements should be regarded solely as Zymeworks’ current plans, estimates and beliefs. You should not place undue reliance on forward-looking statements. Zymeworks cannot guarantee future results, events, levels of activity, performance, or achievements. Zymeworks does not undertake and specifically declines any obligation to update, republish, or revise any forward-looking statements to reflect new information, future events or circumstances, or to reflect the occurrences of unanticipated events, except as may be required by law.
4
SCHEDULE A
[Please see attached.]
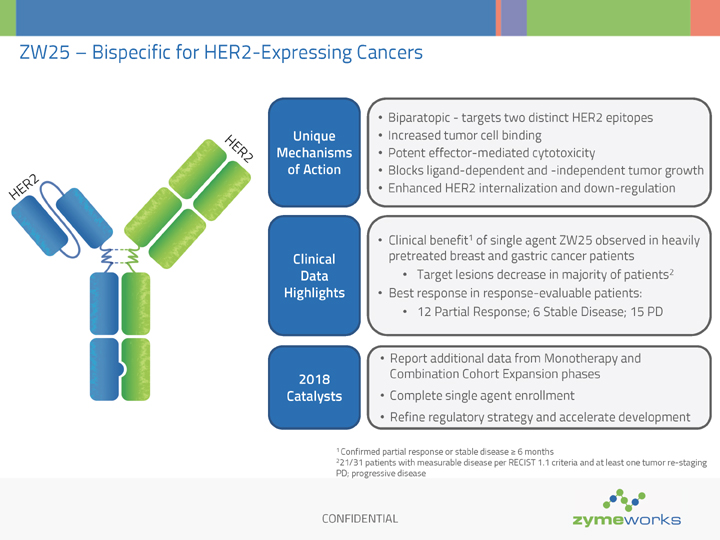
CONFIDENTIAL ZW25 – Bispecific for HER2-Expressing Cancers HER2 HER2 Unique Mechanisms of Action Biparatopic - targets two distinct HER2 epitopes Increased tumor cell binding Potent effector-mediated cytotoxicity Blocks ligand-dependent and -independent tumor growth Enhanced HER2 internalization and down-regulation 2018 Catalysts Report additional data from Monotherapy and Combination Cohort Expansion phases Complete single agent enrollment Refine regulatory strategy and accelerate development 1 Confirmed partial response or stable disease ³ 6 months 221/31 patients with measurable disease per RECIST 1.1 criteria and at least one tumor re-staging PD; progressive disease Clinical Data Highlights Clinical benefit1 of single agent ZW25 observed in heavily pretreated breast and gastric cancer patients Target lesions decrease in majority of patients2 Best response in response-evaluable patients: 12 Partial Response; 6 Stable Disease; 15 PD
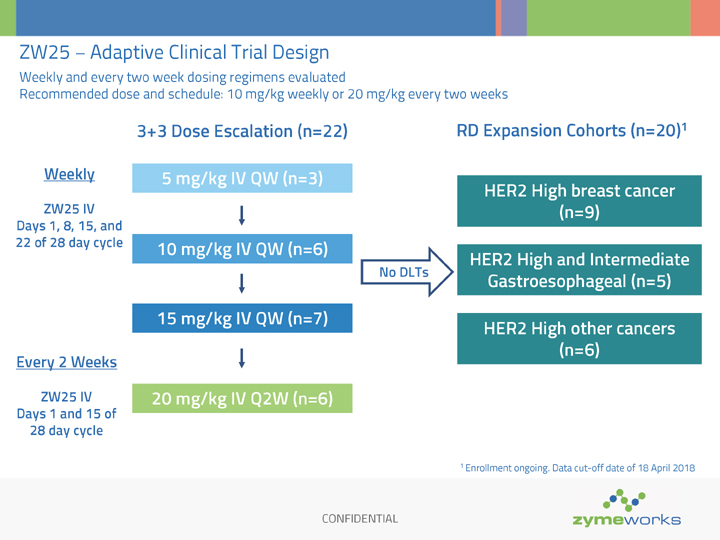
CONFIDENTIAL ZW25 – Adaptive Clinical Trial Design Weekly and every two week dosing regimens evaluated Recommended dose and schedule: 10 mg/kg weekly or 20 mg/kg every two weeks Weekly ZW25 IV Days 1, 8, 15, and 22 of 28 day cycle 3+3 Dose Escalation (n=22) Every 2 Weeks ZW25 IV Days 1 and 15 of 28 day cycle RD Expansion Cohorts (n=20)1 No DLTs 15 mg/kg IV QW (n=7) 10 mg/kg IV QW (n=6) 5 mg/kg IV QW (n=3) 20 mg/kg IV Q2W (n=6) HER2 High breast cancer (n=9) HER2 High and Intermediate Gastroesophageal (n=5) HER2 High other cancers (n=6) 1 Enrollment ongoing. Data cut-off date of 18 April 2018
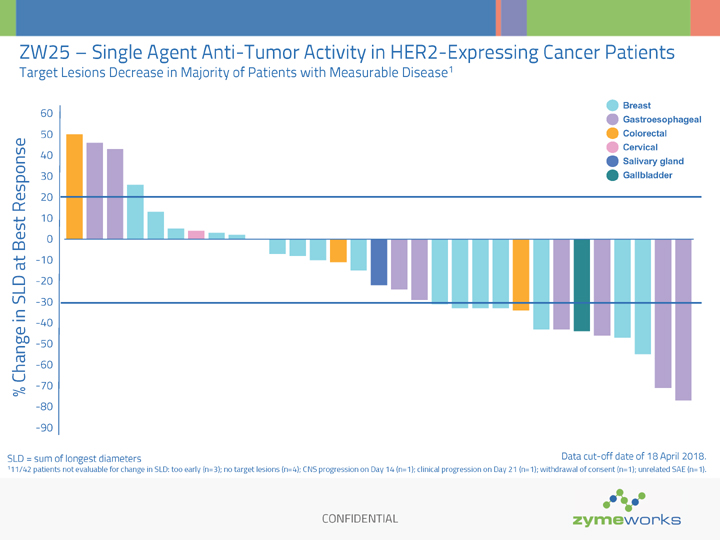
CONFIDENTIAL ZW25 – Single Agent Anti-Tumor Activity in HER2-Expressing Cancer Patients Target Lesions Decrease in Majority of Patients with Measurable Disease1 SLD = sum of longest diameters 111/42 patients not evaluable for change in SLD: too early (n=3); no target lesions (n=4); CNS progression on Day 14 (n=1); clinical progression on Day 21 (n=1); withdrawal of consent (n=1); unrelated SAE (n=1). % Change in SLD at Best Response -90 -80 -70 -60 -50 -40 -30 -20 -10 0 10 20 30 40 50 60 Breast Cervical Colorectal Gastroesophageal Salivary gland Gallbladder Data cut-off date of 18 April 2018.
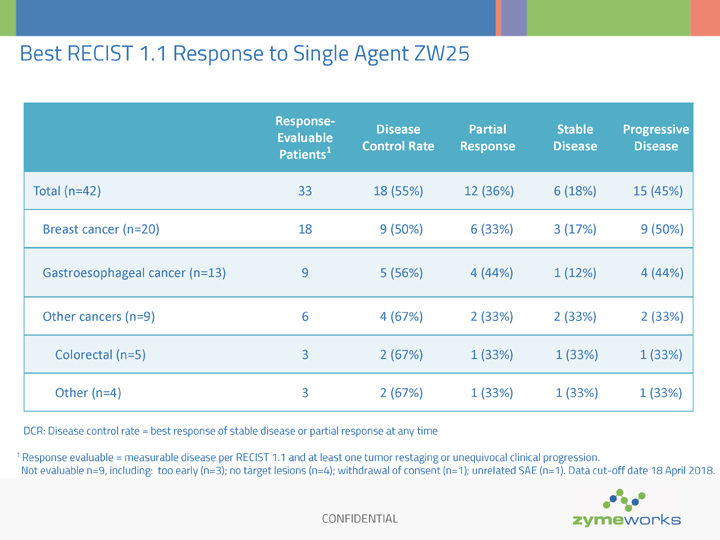
CONFIDENTIAL Best RECIST 1.1 Response to Single Agent ZW25 DCR: Disease control rate = best response of stable disease or partial response at any time 1 Response evaluable = measurable disease per RECIST 1.1 and at least one tumor restaging or unequivocal clinical progression. Not evaluable n=9, including: too early (n=3); no target lesions (n=4); withdrawal of consent (n=1); unrelated SAE (n=1). Data cut-off date 18 April 2018. Response- Evaluable Patients1 Disease Control Rate Partial Response Stable Disease Progressive Disease Total (n=42) 33 18 (55%) 12 (36%) 6 (18%) 15 (45%) Breast cancer (n=20) 18 9 (50%) 6 (33%) 3 (17%) 9 (50%) Gastroesophageal cancer (n=13) 9 5 (56%) 4 (44%) 1 (12%) 4 (44%) Other cancers (n=9) 6 4 (67%) 2 (33%) 2 (33%) 2 (33%) Colorectal (n=5) 3 2 (67%) 1 (33%) 1 (33%) 1 (33%) Other (n=4) 3 2 (67%) 1 (33%) 1 (33%) 1 (33%)
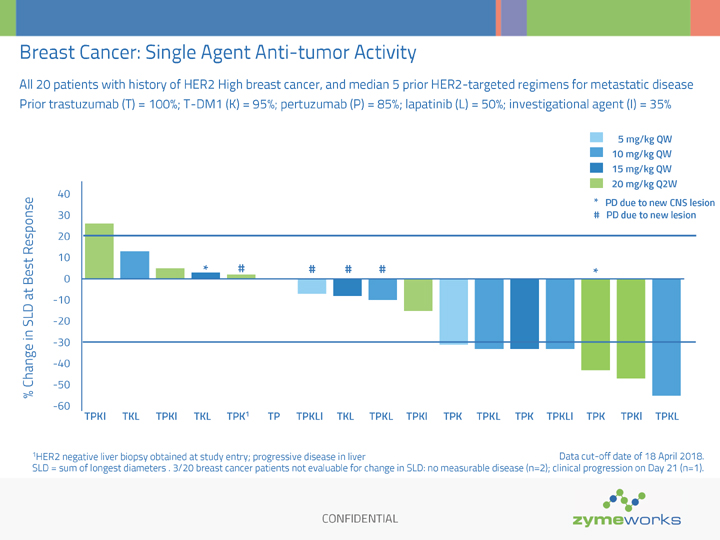
CONFIDENTIAL Breast Cancer: Single Agent Anti-tumor Activity SLD = sum of longest diameters . 3/20 breast cancer patients not evaluable for change in SLD: no measurable disease (n=2); clinical progression on Day 21 (n=1). 5 mg/kg QW 10 mg/kg QW 15 mg/kg QW 20 mg/kg Q2W % Change in SLD at Best Response -60 -50 -40 -30 -20 -10 0 10 20 30 40 TPKI TKL TPKI TKL TPK1 TP TPKLI TKL TPKL TPKI TPK TPKL TPK TPKLI TPK TPKl TPKL 1HER2 negative liver biopsy obtained at study entry; progressive disease in liver * # # # # * * PD due to new CNS lesion # PD due to new lesion All 20 patients with history of HER2 High breast cancer, and median 5 prior HER2-targeted regimens for metastatic disease Prior trastuzumab (T) = 100%; T-DM1 (K) = 95%; pertuzumab (P) = 85%; lapatinib (L) = 50%; investigational agent (I) = 35% Data cut-off date of 18 April 2018.
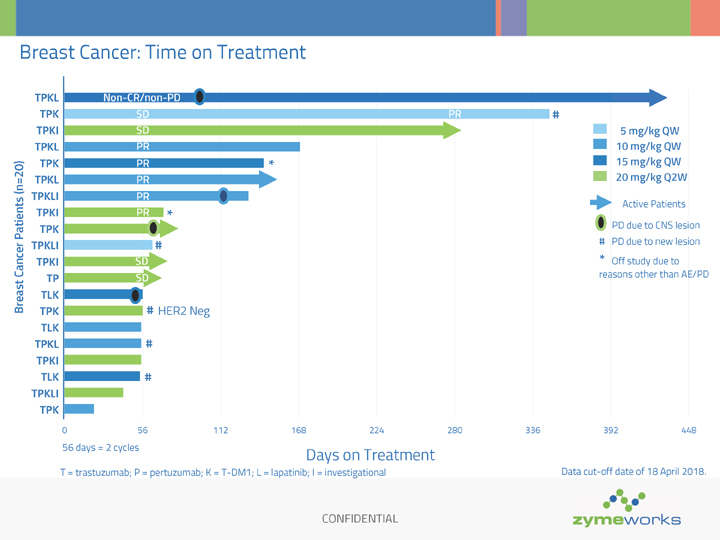
CONFIDENTIAL Breast Cancer: Time on Treatment TPKL TPK TPKI TPKL TPK TPKL TPKLI TPKI TPK TPKLI TPKI TP TLK TPK TLK TPKL TPKI TLK TPKLI TPK 56 days = 2 cycles Days on Treatment T = trastuzumab; P = pertuzumab; K = T-DM1; L = lapatinib; I = investigational Breast Cancer Patients (n=20) 0 56 112 168 224 280 336 392 448 HER2 Neg PR Non-CR/non-PD PR PR SD SD SD PR SD PR PR 5 mg/kg QW 10 mg/kg QW 15 mg/kg QW 20 mg/kg Q2W PD due to CNS lesion Active Patients # # # # Data cut-off date of 18 April 2018. # PD due to new lesion # * Off study due to reasons other than AE/PD * *
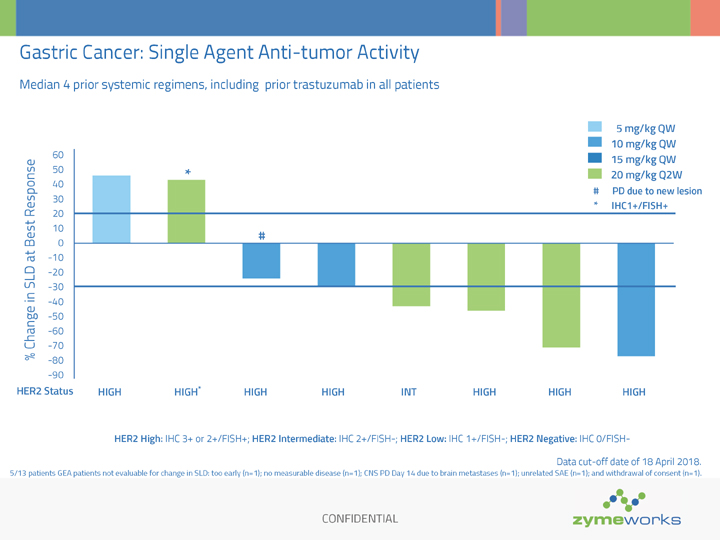
CONFIDENTIAL Gastric Cancer: Single Agent Anti-tumor Activity Median 4 prior systemic regimens, including prior trastuzumab in all patients % Change in SLD at Best Response 5 mg/kg QW 10 mg/kg QW 15 mg/kg QW 20 mg/kg Q2W 5/13 patients GEA patients not evaluable for change in SLD: too early (n=1); no measurable disease (n=1); CNS PD Day 14 due to brain metastases (n=1); unrelated SAE (n=1); and withdrawal of consent (n=1). HER2 High: IHC 3+ or 2+/FISH+; HER2 Intermediate: IHC 2+/FISH-; HER2 Low: IHC 1+/FISH-; HER2 Negative: IHC 0/FISH- -90 -80 -70 -60 -50 -40 -30 -20 -10 0 10 20 30 40 50 60 HER2 Status HIGH HIGH* HIGH HIGH INT HIGH HIGH HIGH # PD due to new lesion # Data cut-off date of 18 April 2018. * * IHC1+/FISH+
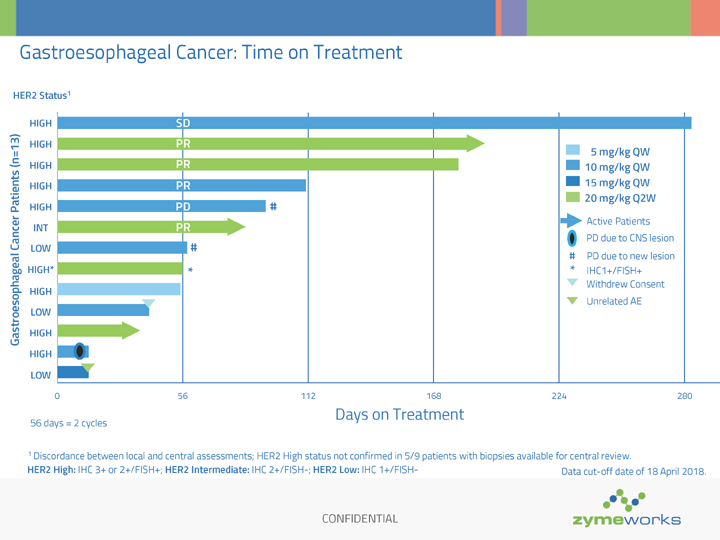
CONFIDENTIAL Gastroesophageal Cancer: Time on Treatment 56 days = 2 cycles Days on Treatment 0 56 112 168 224 280 HIGH HIGH HIGH HIGH HIGH INT LOW HIGH* HIGH LOW LOW HIGH HIGH HER2 Status1 Gastroesophageal Cancer Patients (n=13) 1 Discordance between local and central assessments; HER2 High status not confirmed in 5/9 patients with biopsies available for central review. HER2 High: IHC 3+ or 2+/FISH+; HER2 Intermediate: IHC 2+/FISH-; HER2 Low: IHC 1+/FISHSD PR PR PR PR PD 5 mg/kg QW 10 mg/kg QW 15 mg/kg QW 20 mg/kg Q2W PD due to CNS lesion Active Patients Unrelated AE Withdrew Consent Data cut-off date of 18 April 2018. # # * # PD due to new lesion * IHC1+/FISH+
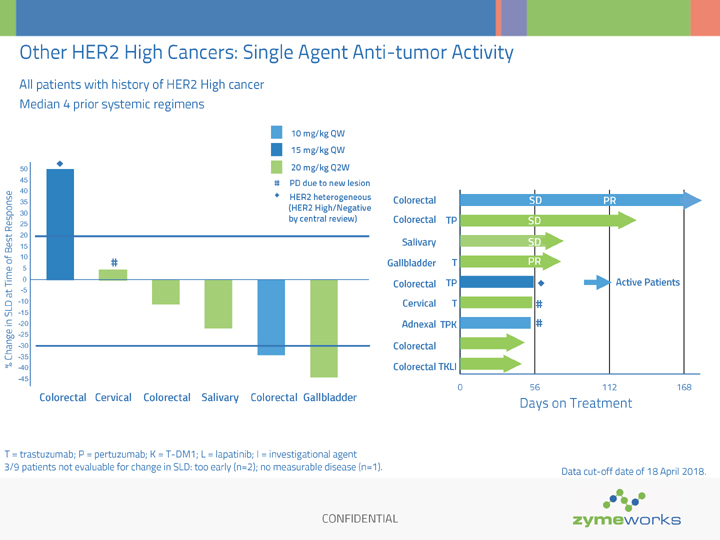
CONFIDENTIAL Other HER2 High Cancers: Single Agent Anti-tumor Activity All patients with history of HER2 High cancer Median 4 prior systemic regimens 0 56 112 168 Colorectal Colorectal Salivary Gallbladder Colorectal Cervical Adnexal Colorectal Colorectal Active Patients Days on Treatment 10 mg/kg QW 15 mg/kg QW 20 mg/kg Q2W % Change in SLD at Time of Best Response 50 45 40 35 30 25 20 15 10 5 0 -5 -10 -15 -20 -25 -30 -35 -40 -45 Colorectal Cervical Colorectal Salivary Colorectal Gallbladder 3/9 patients not evaluable for change in SLD: too early (n=2); no measurable disease (n=1). SD SD PR TP T TP T TKLI TPK SD PR # PD due to new lesion HER2 heterogeneous (HER2 High/Negative by central review) # T = trastuzumab; P = pertuzumab; K = T-DM1; L = lapatinib; I = investigational agent # Data cut-off date of 18 April 2018. #
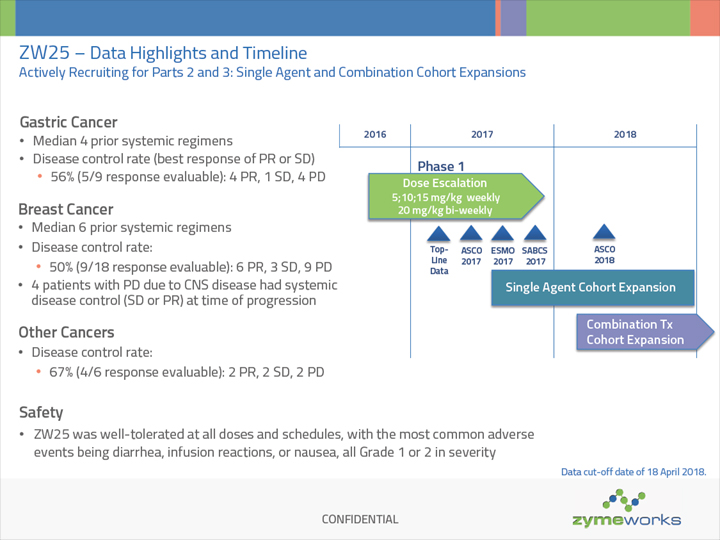
CONFIDENTIAL 2016 2017 2018 ZW25 – Data Highlights and Timeline Actively Recruiting for Parts 2 and 3: Single Agent and Combination Cohort Expansions Phase 1 Top- Line Data ASCO 2017 Dose Escalation 5;10;15 mg/kg weekly 20 mg/kg bi-weekly Single Agent Cohort Expansion Combination Tx Cohort Expansion SABCS 2017 ESMO 2017 Breast Cancer Median 6 prior systemic regimens Disease control rate: 50% (9/18 response evaluable): 6 PR, 3 SD, 9 PD 4 patients with PD due to CNS disease had systemic disease control (SD or PR) at time of progression Gastric Cancer Median 4 prior systemic regimens Disease control rate (best response of PR or SD) 56% (5/9 response evaluable): 4 PR, 1 SD, 4 PD ASCO 2018 Other Cancers Disease control rate: 67% (4/6 response evaluable): 2 PR, 2 SD, 2 PD Safety ZW25 was well-tolerated at all doses and schedules, with the most common adverse events being diarrhea, infusion reactions, or nausea, all Grade 1 or 2 in severity Data cut-off date of 18 April 2018.
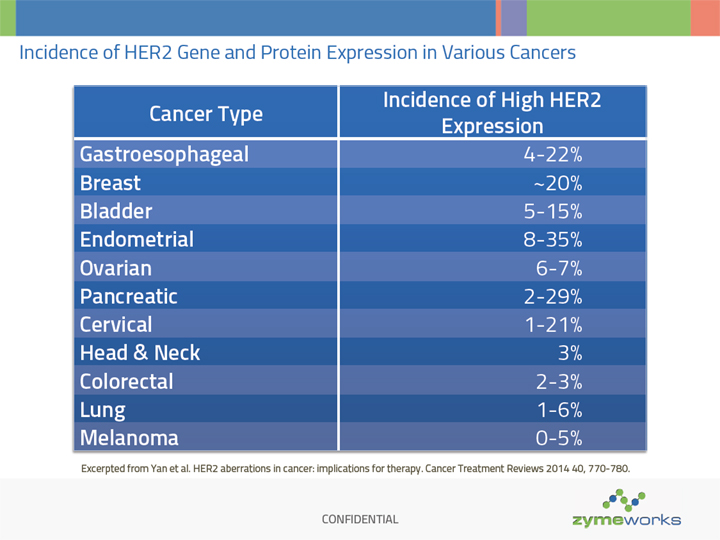
CONFIDENTIAL Incidence of HER2 Gene and Protein Expression in Various Cancers Cancer Type Incidence of High HER2 Expression Gastroesophageal 4-22% Breast ~20% Bladder 5-15% Endometrial 8-35% Ovarian 6-7% Pancreatic 2-29% Cervical 1-21% Head & Neck 3% Colorectal 2-3% Lung 1-6% Melanoma 0-5% Excerpted from Yan et al. HER2 aberrations in cancer: implications for therapy. Cancer Treatment Reviews 2014 40, 770-780.
