Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Sio Gene Therapies Inc. | a18-14885_1ex99d1.htm |
| 8-K - 8-K - Sio Gene Therapies Inc. | a18-14885_18k.htm |
FORWARD LOOKING STATEMENTS Statements made in this presentation contain forward-looking statements, including statements regarding Axovant’s expectations about timing of the results for the Phase 1/2 clinical study for AXO-Lenti-PD in Parkinson’s disease, expected outcomes of planned clinical development for AXO-Lenti-PD, Axovant’s license arrangement with Oxford BioMedica, the expected equity investment from Roivant, and other elements of Axovant’s clinical development and regulatory strategy. Forward-looking statements can be identified by the words “believe,” “anticipate,” “continue,” “estimate,” “project,” “expect,” “plan,” “potential,” “intend,” “will,” “would,” “could,” “should” or the negative or plural of these words or other similar expressions that are predictions or indicate future events, trends or prospects. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially and reported results should not be considered as an indication of future performance. These risks and uncertainties include, but are not limited to: risks associated with the success, cost and timing of our product development activities and clinical trials; the approval and commercialization of Axovant’s product candidates, including AXO-Lenti-PD; and increased regulatory requirements. These statements are subject to the risk that clinical trial data are subject to differing interpretations, and regulatory agencies, medical and scientific experts and others may not share Axovant’s views of the clinical study data. In addition, promising interim results or other preliminary analyses do not in any way ensure that later or final results in a clinical trial or in related or similar clinical trials will replicate those interim results. The product candidates discussed are investigational and not approved and there can be no assurance that the clinical programs including the program for AXO-Lenti-PD will be successful in demonstrating safety and/or efficacy, that Axovant will not encounter problems or delays in clinical development, or that any of Axovant’s product candidates will ever receive regulatory approval or be successfully commercialized. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Axovant’s business in general, see the “Risk Factors” section of Axovant’s quarterly report on Form 10-Q filed with the Securities and Exchange Commission on February 9, 2018, and other filings that Axovant makes with the SEC from time to time. These forward-looking statements are based on information available to Axovant as of the date of this presentation and speak only as of the date of this presentation. Axovant disclaims any obligation to update these forward-looking statements, except as may be required by law.
Building a leader in innovative CNS therapies AXO-Lenti-PD: potential best-in-class gene therapy for Parkinson’s disease Delivery of three genes that encode critical enzymes necessary for endogenous dopamine synthesis in the brain (AADC, TH, and CH1) using lentiviral vector Plan to initiate Phase 1/2 study in advanced Parkinson’s disease by the end of 2018 Oxford BioMedica will be the clinical & commercial supplier of AXO-Lenti-PD, if approved World leader in lentiviral vector product development and manufacturing Commercial supplier of lentiviral vector for KYMRIAH® (Novartis), first FDA-approved CAR-T therapy in US New additions to leadership to bolster gene therapy and CNS expertise Fraser Wright, Chief Technology Officer (Co-Founder and former CTO of Spark Therapeutics) Michael Hayden, Senior Scientific Advisor and Chairman, Axovant Scientific Advisory Board (former President of Global R&D and Chief Scientific Officer of Teva) Gavin Corcoran, EVP Research & Development (CMO of Allergan) Roivant strategic financing enables continued pipeline expansion Roivant committed to invest $25 million in Axovant Funds will be used to support clinical development of AXO-Lenti-PD and additional business development CAR-T = chimeric antigen receptor T-cell TH = Tyrosine hydroxylase; CH1 = Cyclohydrolase 1; AADC = Aromatic L-amino acid decarboxylase

Michael Hayden, MB ChB, Ph.D. Senior Scientific Advisor and Chairman, Axovant Scientific Advisory Board Former President, Global R&D, and Chief Scientific Officer, Teva GLYBERA®, first-ever approved gene therapy, was initially conceived in Hayden’s lab Killam Professor of Medical Genetics, University of British Columbia; Canada Research Chair in Human Genetics and Molecular Medicine Program Director, Translational Laboratory in Genetic Medicine, National University of Singapore Fraser Wright, Ph.D. Chief Technology Officer, Gene Therapies Co-Founder & Former Chief Technology Officer, Spark Therapeutics More than 20 years of experience in gene therapy product development, manufacturing, and quality control testing, including LUXTURNA™ and KYMRIAH® Director, Clinical Vector Core Laboratory, Children’s Hospital of Philadelphia Faculty, University of Pennsylvania Perelman School of Medicine Director, Development and Clinical Manufacturing, Avigen New additions to leadership team bring decades of gene therapy and CNS expertise Neurovir Gavin Corcoran, MB ChB, FACP Executive Vice President, Research and Development Currently Chief Medical Officer at Allergan plc Previously served as Chief Medical Officer of Actavis and Executive Vice President for Global Medicines Development at Forest Laboratories Head of Late Stage Clinical Development for Inflammation and Immunology at Celgene

Track record of rapid clinical execution in neurology Global leader in gene therapy with 20+ years of experience Demonstrated manufacturing capabilities for commercially available gene therapy products Extensive drug development experience in neurodegeneration
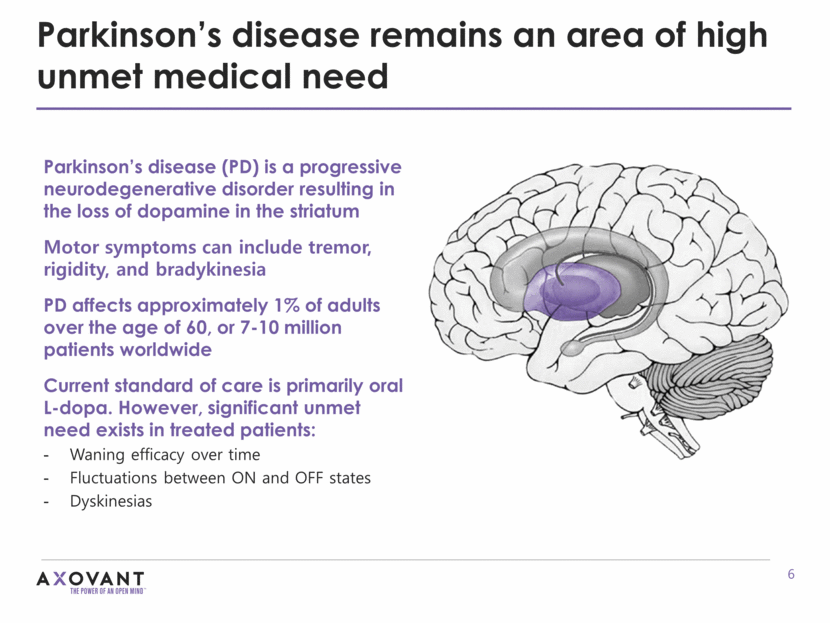
Parkinson’s disease (PD) is a progressive neurodegenerative disorder resulting in the loss of dopamine in the striatum Motor symptoms can include tremor, rigidity, and bradykinesia PD affects approximately 1% of adults over the age of 60, or 7-10 million patients worldwide Current standard of care is primarily oral L-dopa. However, significant unmet need exists in treated patients: Waning efficacy over time Fluctuations between ON and OFF states Dyskinesias Parkinson’s disease remains an area of high unmet medical need
AXO-Lenti-PD: a novel gene therapy for Parkinson’s disease AXO-Lenti-PD contains three genes that encode the critical enzymes required for endogenous dopamine synthesis Tyrosine hydroxylase (TH): converts tyrosine to L-dopa Cyclohydrolase 1 (CH1): rate-limiting enzyme for synthesis of essential cofactor in TH activity Aromatic L-amino acid decarboxylase (AADC): converts L-dopa to dopamine One-time MRI-guided stereotactic delivery into the putamen Lentiviral vector system with large gene packaging capacity Permits delivery of multiple genes at once TH AADC Tyrosine L-dopa Dopamine CH1 AAV = adeno-associated virus
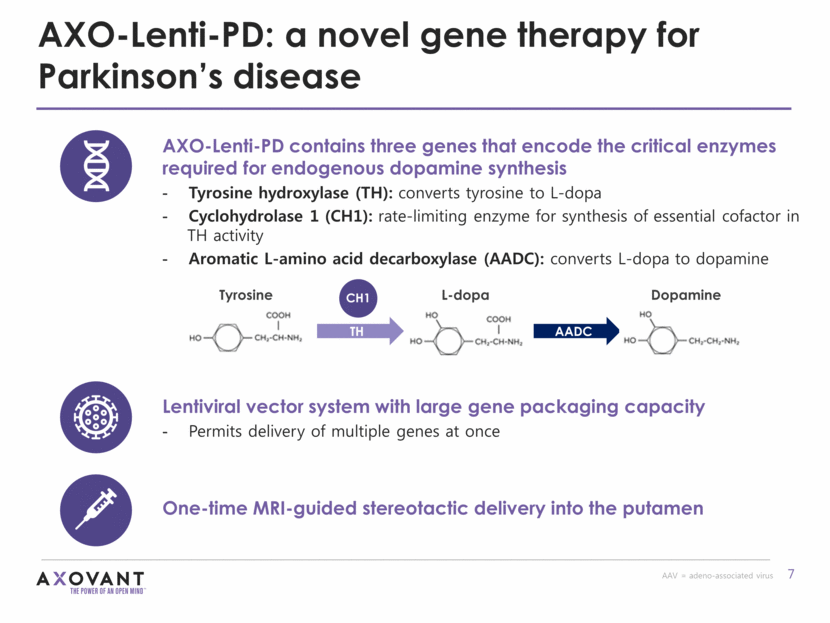
AXO-Lenti-PD: designed to reduce motor fluctuations in Parkinson’s disease AXO-Lenti-PD’s novel 3-gene therapy approach is designed to (1) increase basal dopamine production and (2) reduce dopamine variability GOALS OF THERAPY: Less troublesome dyskinesia Less OFF time More ON time Lower requirement for exogenous L-dopa * Theoretical benefits based on postulated mechanism of action (not data from investigational studies) Oral L-dopa [Dopamine] Endogenous production Oral L-dopa AXO-Lenti-PD*
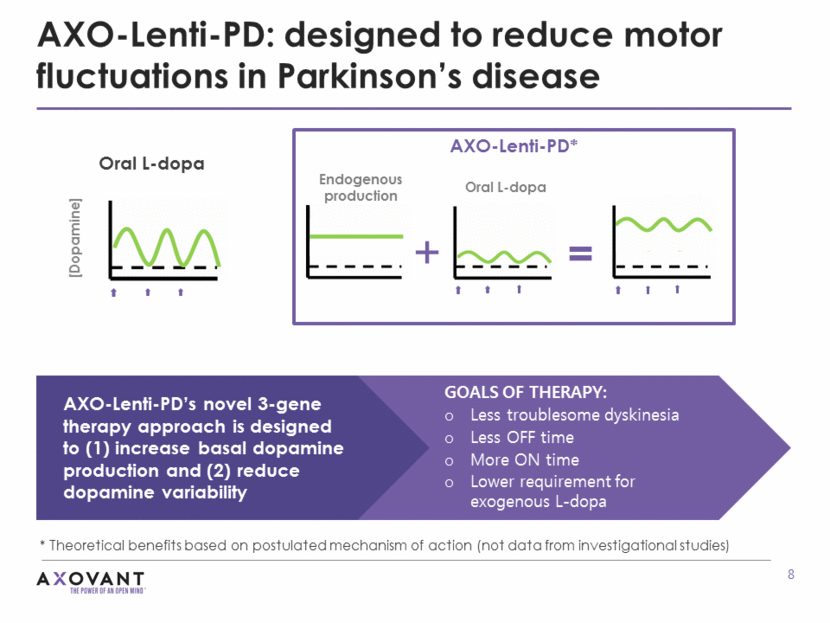
Why use lentiviral vector? Large vector packaging capacity enables efficient delivery of multiple genes in a single cassette Lentiviral vectors are capable of integrating into the host genome, which may help maintain long-term stable transgene expression To date, well-tolerated with limited treatment-related adverse events, based on clinical and commercial experience with lentiviral vectors Lentiviral vector approach may confer unique advantages: AAV = adeno-associated virus
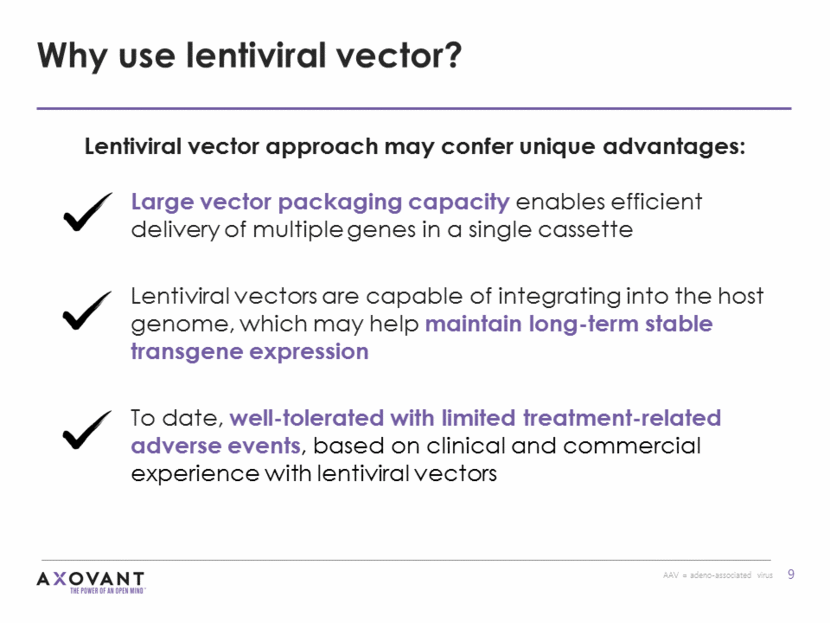
ProSavin was the predecessor therapy to AXO-Lenti-PD Delivery of three genes in the same lentiviral vector as AXO-Lenti-PD, but in a different payload configuration One-time MRI-guided stereotactic delivery Phase 1/2 study of ProSavin in patients with advanced Parkinson’s disease (n=15) completed Mean UPDRS Part III (motor) “OFF” scores were significantly improved at 6 months and 12 months (p-value=0.0001 at both time points) Sustained improvement seen through 4 years of follow-up Favorable safety and tolerability profile No serious adverse events related to ProSavin or the surgical procedure Long-term follow-up ongoing ProSavin®: proof of concept from earlier vector construct Source: Palfi S, Gurruchaga JM, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. The Lancet. January 2014. http://dx.doi.org/10.1016/S0140-6736(13)61939-X UPDRS = Unified Parkinson’s Disease Rating Scale
Phase 1/2 study completed in patients with advanced Parkinson’s disease (n=15) Study was conducted in UK and France Results published in The Lancet (Palfi, 2014) Cohorts in dose-escalation paradigm: Cohort 1: Low dose (1.9 x 107 TU) Cohort 2a: Mid dose (4.0 x 107 TU) Cohort 2b: Mid dose (4.0 x 107 TU) with new delivery method Cohort 3: High dose (1.0 x 108 TU) Primary endpoints in Phase 1/2 study Number and severity of adverse events UPDRS Part III (motor) “OFF” scores at 6 months after vector administration ProSavin Phase 1/2 Study Design Source: Palfi S, Gurruchaga JM, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. The Lancet. January 2014. http://dx.doi.org/10.1016/S0140-6736(13)61939-X UPDRS = Unified Parkinson’s Disease Rating Scale ProSavin: completed Phase 1/2 study
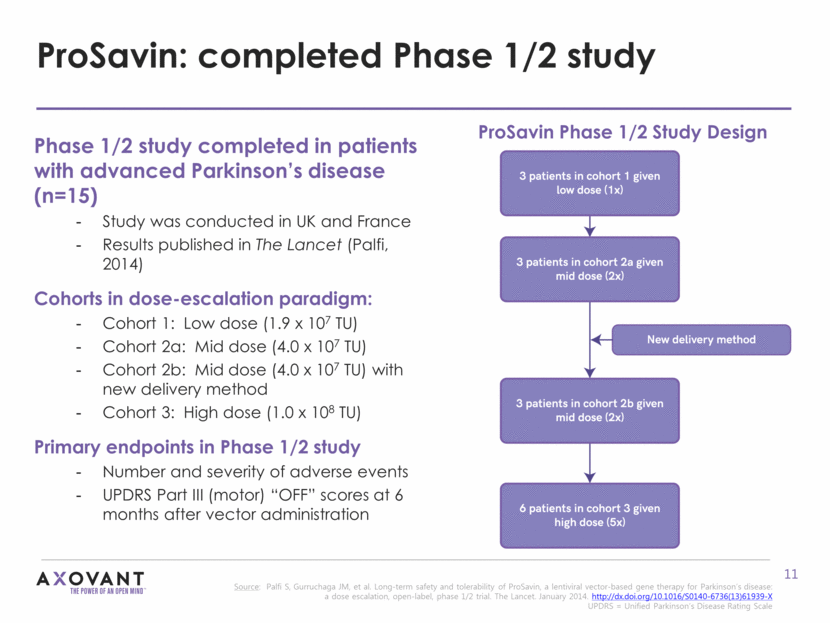
ProSavin: multiple doses evaluated in Phase 1/2 study Source: Palfi S, Gurruchaga JM, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. The Lancet. January 2014. http://dx.doi.org/10.1016/S0140-6736(13)61939-X Figure shows mean change in UPDRS III score OFF medication relative to baseline at 12 month for each cohort. Error bars show standard error. UPDRS = Unified Parkinson’s Disease Rating Scale. Wilcoxon signed-rank paired test is used to compare the difference of UPDRS scores at 6 or 12 months versus baseline. All patients (n=15): Mean improvement from baseline of 11.8 points at 12 months (p=0.0001) Mean reduction in L-dopa equivalent daily dose (LEDD) of 19% at 12 months Cohort 1 (low dose): 1.9 x 107 TU Cohort 2a and 2b (mid dose): 4.0 x 107 TU Cohort 3 (high dose): 1.0 x 108 TU Mean Improvement in UPDRS Part III (motor) “OFF” score at 12 Months
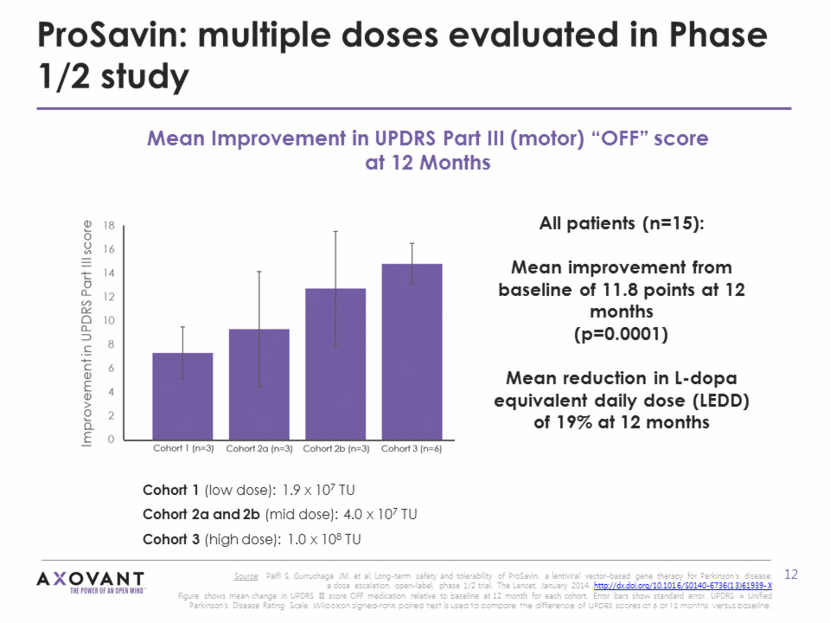
ProSavin: sustained response observed several years after administration Durable effects seen through 4 years after one-time administration of ProSavin UPDRS Part III (motor) “OFF” scores are typically expected to worsen by 3-4 points/year in this population* Mean UPDRS III (OFF) scores pooled across low, mid, and high dose cohorts. Number of subjects: 15 (baseline to 24 months), 14 (24 months), 11 (36 and 48 months), Assessments post-deep brain stimulation (DBS) are excluded (n=3) *Source: Palfi S, Gurruchaga JM, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. The Lancet. January 2014. http://dx.doi.org/10.1016/S0140-6736(13)61939-X. Mean UPDRS Part III (motor) “OFF” score n = 15 n = 15 n = 14 n = 11 n = 10 UPDRS Part III score
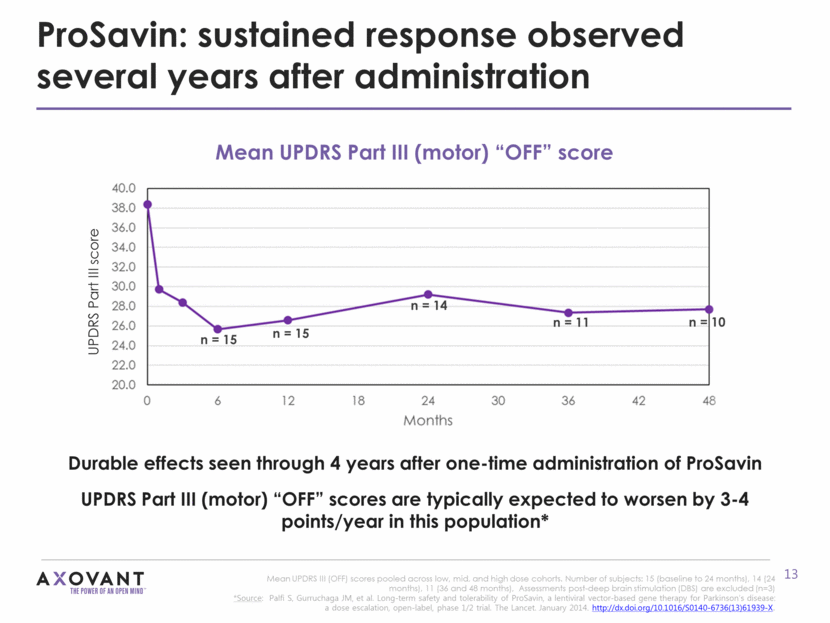
AXO-Lenti-PD: a re-engineered gene therapy product AXO-Lenti-PD was the product of multifactorial experimentation to modify the payload configuration to improve dopamine production Different ordering of genes Balanced stoichiometry of transgene expression to ensure consistent 1:1 production of TH and CH1 Fusion of TH and CH1 with flexible linker Vector Configuration ProSavin AXO-Lenti-PD AXO-Lenti-PD achieves up to 10-fold increases in dopamine + L-dopa production compared to ProSavin, without impacting infusion volume or rate of administration Source: Palfi S, Gurruchaga JM, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. The Lancet. January 2014. http://dx.doi.org/10.1016/S0140-6736(13)61939-X TH AADC CH1 IRES CMVp WPRE IRES SIN LTR SIN LTR TH AADC CH1 CMVp WPRE IRES SIN LTR SIN LTR
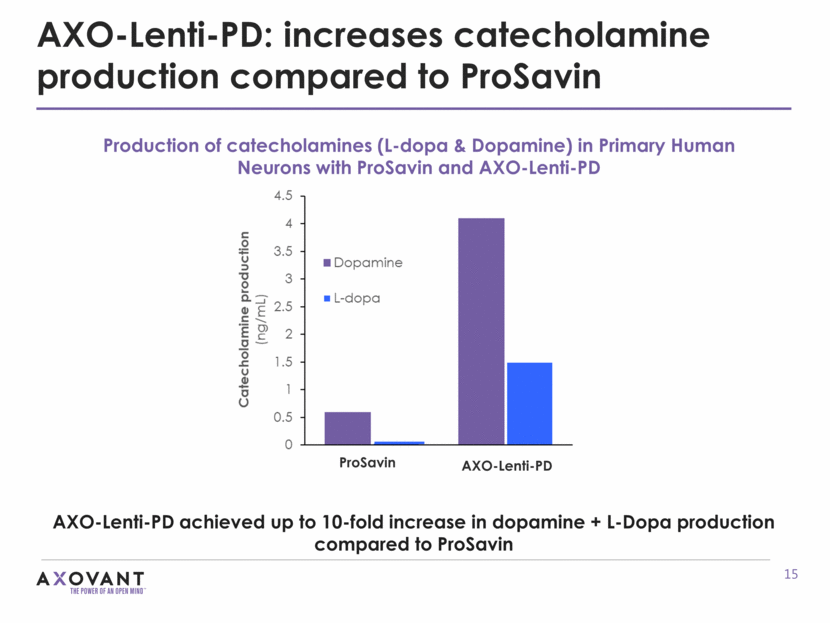
AXO-Lenti-PD: increases catecholamine production compared to ProSavin AXO-Lenti-PD achieved up to 10-fold increase in dopamine + L-Dopa production compared to ProSavin Production of catecholamines (L-dopa & Dopamine) in Primary Human Neurons with ProSavin and AXO-Lenti-PD AXO-Lenti-PD ProSavin
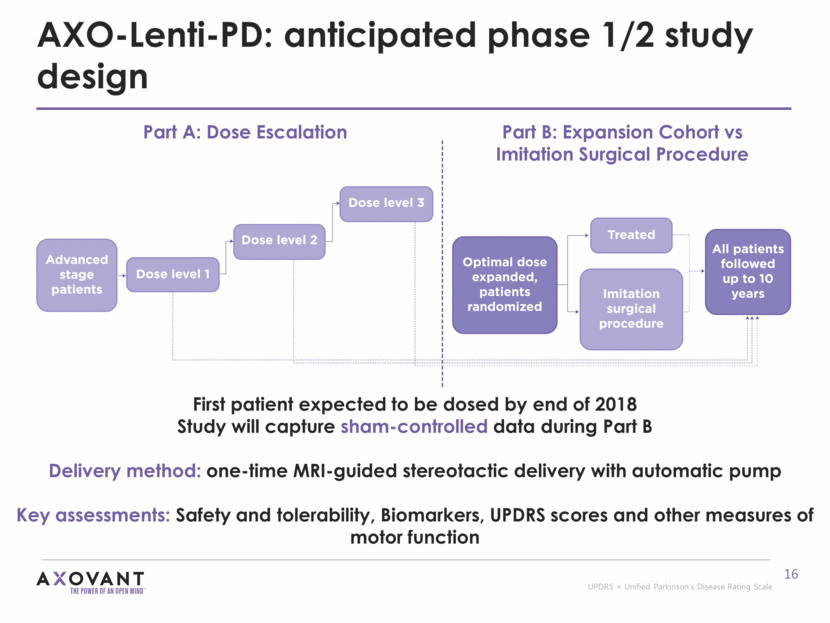
AXO-Lenti-PD: anticipated phase 1/2 study design First patient expected to be dosed by end of 2018 Study will capture sham-controlled data during Part B Delivery method: one-time MRI-guided stereotactic delivery with automatic pump Key assessments: Safety and tolerability, Biomarkers, UPDRS scores and other measures of motor function Part A: Dose Escalation Part B: Expansion Cohort vs Imitation Surgical Procedure UPDRS = Unified Parkinson’s Disease Rating Scale
Oxford BioMedica: our clinical and commercial manufacturing partner An industry leader in GMP lentiviral vector manufacturing and commercial production Commercial-grade vector manufacturing GMP cell banks for adherent and suspension processes Certified clean room capacity across three independent suites on two separate sites The first company to administer an in vivo lentiviral vector into patients Established strategic partnerships with Novartis (CAR-T), Sanofi/Bioverativ (ophthalmological, hemophilia), Orchard Therapeutics (stem cells) CAR-T = chimeric antigen receptor T-cell
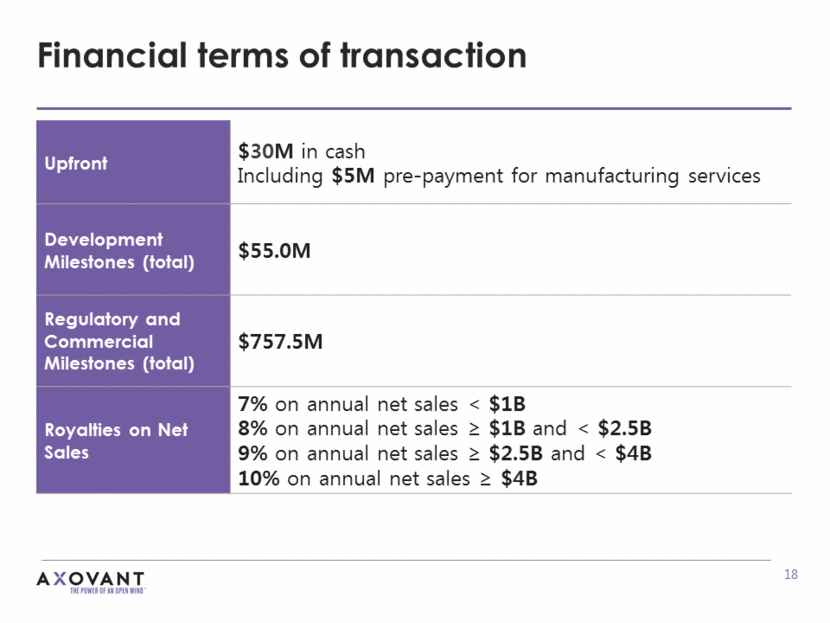
Financial terms of transaction Upfront $30M in cash Including $5M pre-payment for manufacturing services Development Milestones (total) $55.0M Regulatory and Commercial Milestones (total) $757.5M Royalties on Net Sales 7% on annual net sales < $1B 8% on annual net sales > $1B and < $2.5B 9% on annual net sales > $2.5B and < $4B 10% on annual net sales > $4B
Plans for continued pipeline expansion CNS-targeted gene therapies Parkinson’s disease and related neurodegenerative conditions AXO-Lenti-PD More pipeline expansion expected along these verticals in FY2018