Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - MESA LABORATORIES INC /CO/ | ex_115263.htm |
| EX-32.1 - EXHIBIT 32.1 - MESA LABORATORIES INC /CO/ | ex_115262.htm |
| EX-31.2 - EXHIBIT 31.2 - MESA LABORATORIES INC /CO/ | ex_115261.htm |
| EX-31.1 - EXHIBIT 31.1 - MESA LABORATORIES INC /CO/ | ex_115260.htm |
| EX-23.1 - EXHIBIT 23.1 - MESA LABORATORIES INC /CO/ | ex_115259.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark one)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITES EXCHANGE ACT OF 1934
For the fiscal year ended March 31, 2018
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITES EXCHANGE ACT OF 1934
For the transition period from ____ to ____
Commission File No: 0-11740
MESA LABORATORIES, INC.
(Exact name of registrant as specified in its charter)
|
Colorado |
84-0872291 |
|
(State or other jurisdiction of |
(I.R.S. Employer |
|
Incorporation or organization) |
Identification number) |
|
12100 West Sixth Avenue |
|
|
Lakewood, Colorado |
80228 |
|
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (303) 987-8000
Securities registered under Section 12(b) of the Act:
|
Title of each class |
Name of each exchange on which registered |
|
|
Common Stock, no par value |
Nasdaq |
Securities registered under Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
YES ☐ NO ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
YES ☐ NO ☒
Indicate by check mark whether the registrant (1) has filed all reports to be filed by Section13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
YES ☒ NO ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (Section 232.405 of the chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES ☒ NO ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of the Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (check one):
|
Large accelerated filer ☐ |
Accelerated filer ☒ |
Non-accelerated filer ☐ |
Smaller reporting company ☐ |
Emerging growth company ☐ |
|
(Do not check if a smaller reporting company) |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
YES ☐ NO ☒
The aggregate market value as of September 30, 2017 (the last business day of the registrant's most recently completed second fiscal quarter), of the voting and non-voting common equity of Mesa Laboratories Inc. held by non-affiliates (assuming, for this purpose, that all directors, officers and owners of 5% or more of the registrant’s common stock are deemed affiliates) computed by reference to the price at which the common equity was last sold ($149.32 per share) was $384,259,000.
The number of outstanding shares of the Issuer’s common stock as of May 31, 2018 was 3,808,436.
Table of Contents
Forward Looking Statements
|
Part I |
||
|
Item 1. |
Business |
1 |
|
Item 1A. |
Risk Factors |
8 |
|
Item 1B. |
Unresolved Staff Comments |
16 |
|
Item 2. |
Properties |
16 |
|
Item 3. |
Legal Proceedings |
16 |
|
Item 4. |
Mine Safety Disclosures |
16 |
|
Part II |
||
|
Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
17 |
|
Item 6. |
Selected Financial Data |
19 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
21 |
|
Item 7A. |
Quantitative and Qualitative Disclosures About Market Risk |
35 |
|
Item 8. |
Financial Statements and Supplementary Data |
35 |
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
62 |
|
Item 9A. |
Controls and Procedures |
62 |
|
Item 9B. |
Other Information |
63 |
|
Part III |
||
|
Item 10. |
Directors, Executive Officers and Corporate Governance |
64 |
|
Item 11. |
Executive Compensation |
69 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
77 |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
78 |
|
Item 14. |
Principal Accountant Fees and Services |
78 |
|
Part IV |
||
|
Item 15. |
Exhibits and Consolidated Financial Statement Schedules |
79 |
|
Signatures |
Forward-Looking Statements
This report contains information that may constitute "forward-looking statements.” Generally, the words "believe," “estimate,” “will,” "expect," "project," "anticipate," "intend," and similar expressions identify forward-looking statements, which generally are not historical in nature. However, the absence of these words or similar expressions does not mean that a statement is not forward-looking. All statements that address operating performance, events or developments that we expect or anticipate will occur in the future — including statements relating to revenues growth and statements expressing general views about future operating results — are forward-looking statements. Management believes that these forward-looking statements are reasonable as and when made. However, caution should be taken not to place undue reliance on any such forward-looking statements because such statements speak only as of the date when made. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. In addition, forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from our historical experience and our present expectations or projections. These risks and uncertainties include, but are not limited to, those described in Part I, "Item 1A. Risk Factors" and elsewhere in this report and those described from time to time in our future reports to be filed with the Securities and Exchange Commission.
Part I
Item 1. Business
Introduction
Mesa Laboratories, Inc. was incorporated under the laws of the State of Colorado on March 26, 1982. The terms “we,” “us,” “our,” the “Company” or “Mesa” are used in this report to refer collectively to the parent company and the subsidiaries through which our various businesses are conducted. We pursue a strategy of focusing primarily on quality control products and services, which are sold into niche markets that are driven by regulatory requirements. We prefer markets where we can establish a strong presence and achieve high gross margins. We are organized into four divisions across nine physical locations. Our Sterilization and Disinfection Control Division (formerly named the Biological Indicators Division) provides testing services, along with the manufacturing and marketing of biological, chemical and cleaning indicators used to assess the effectiveness of sterilization and disinfection processes in the hospital, dental, medical device and pharmaceutical industries. Our Instruments Division designs, manufactures and markets quality control instruments and disposable products utilized in the healthcare, pharmaceutical, food and beverage, medical device, industrial hygiene and environmental air sampling industries. Our Cold Chain Monitoring Division designs, develops and markets systems which are used to monitor various environmental parameters such as temperature, humidity and differential pressure to ensure that critical storage and processing conditions are maintained in hospitals, pharmaceutical and medical device manufacturers, blood banks, pharmacies and laboratory environments. Our Cold Chain Packaging Division provides packaging development consulting services and thermal packaging products such as coolers, boxes, insulation materials and phase-change products to control temperature during transport.
Our Bozeman, Montana and Munich, Germany locations manufacture our Sterilization and Disinfection Control Division products which include the EZTest®, ProSpore®, PCD®, Apex® and Simicon biological and cleaning indicators, while our Bozeman, Montana, facility also provides sterility assurance testing services to dental offices in the United States and Canada. Our Lakewood, Colorado, and Butler, New Jersey, facilities manufacture our Instruments Division products which include the DataTrace®, DialyGuard®, DryCal®, Torqo®, SureTorque® and BGI brands. Our Lakewood, Colorado, facility also manufactures our Cold Chain Monitoring Division products which include CheckPoint®, AmegaView, ViewPoint® and FreshLoc brands. Our Markham, Ontario, facility manufactures our Mesa brand real time monitoring solutions and outsources the manufacture of our TempTrust® brand of packaging materials.
Our philosophy is to manufacture products of exceptional quality and provide a high level of on-going service for those products. Our revenues come from two main sources – product sales and services. Our strategic goals involve continuing to grow revenues and profits through three key strategies – a) improving our commercial channels, b) introducing new products to the market, and c) seeking out companies or product lines to acquire.
Acquisitions
Year Ended March 31, 2018 Acquisitions
During the year ended March 31, 2018, we completed the following three acquisitions:
In November 2017, we completed a business combination (the “BAG Acquisition”) whereby we acquired substantially all of the assets and certain liabilities of BAG Health Care GmbH’s (“BAG”) Hygiene Monitoring business which is comprised of the distribution of biological, chemical and cleaning indicator products;
In October 2017, we completed a business combination (the “Simicon Acquisition”) whereby we acquired the common stock of SIMICON GmbH (“Simicon”), a company whose business manufactures both biological and cleaning indicators; and
In May 2017, we completed a business combination (the “Hucker Acquisition”) whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of Hucker & Hucker GmbH’s (“Hucker”) business segment associated with the distribution of our biological indicator products.
Year Ended March 31, 2017 Acquisitions
During the year ended March 31, 2017, we completed the following six acquisitions:
In November 2016, we completed a business combination (the “Mydent Acquisition”) whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of Mydent International Corp’s business segment associated with biological indicator mail-in testing services to the dental market in the United States;
In November 2016, we completed a business combination (the “FreshLoc Acquisition”) whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of the cold chain monitoring business of FreshLoc Technologies, Inc.;
In August 2016, we completed a business combination (the “Rapid Aid Acquisition”) whereby we acquired certain assets (consisting primarily of fixed assets) and certain liabilities of Rapid Aid Corp’s (“Rapid Aid”) business segment associated with the manufacture and sale of cold chain packaging gel products;
In July 2016, we completed a business combination (the “HANSAmed Acquisition”) whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of HANSAmed Limited’s (“HANSAmed”) business segment associated with the distribution of our biological indicator products and mail-in testing services to the dental market in Canada;
In April 2016, we completed a business combination (the “ATS Acquisition”) whereby we acquired substantially all the assets (other than cash and certain inventories and fixed assets) and certain liabilities of Autoclave Testing Services, Inc. and Autoclave Testing Supplies, Inc., (collectively, “ATS”). ATS was in the business of supplying products and services for dental sterilizer testing in both the U.S. and Canada; and
In April 2016, we completed a business combination (the “Pulse Acquisition”) whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of Pulse Scientific, Inc.’s (“Pulse”) business segment associated with the distribution of our biological indicator products.
Year Ended March 31, 2016 Acquisitions
During the year ended March 31, 2016, we completed the following ten acquisitions:
In January 2016, we completed two business combinations (the “January 2016 European BI Distributor Acquisitions”) whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of the business segment associated with the distribution of our biological indicator products from CoaChrom Diagnostica GmbH of Austria and bioTRADING Benelux B.V of the Netherlands;
In October 2015, we completed six business combinations (the “October 2015 European BI Distributor Acquisitions”) whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of the business segment associated with the distribution of our biological indicator products from BIOLOGIK S.R.L.(Italy), VWR International PBI S.R.L.(Italy), Cruinn Diagnostics Ltd.(Ireland), Mecolab AG (Switzerland), Miclev Medical Products AB (Sweden) and Tiselab S.L.(Spain);
In August 2015, we completed a business combination (the “North Bay Acquisition”) whereby we acquired substantially all of the assets (other than certain fixed assets) and certain liabilities of the dental sterilizer testing business of North Bay Bioscience, LLC (“North Bay”); and
In July 2015, we completed a business combination (the “Infitrak Acquisition”) whereby we acquired all of the common stock of 2396081 Ontario Inc. and its wholly owned operating subsidiary, Infitrak Inc. (collectively, “Infitrak”), a company whose business provides consulting, packaging and measuring solutions for cold chain applications.
Our principal executive offices and corporate headquarters are located at 12100 West Sixth Ave., Lakewood, Colorado 80228, and our telephone number is 303-987-8000. Our website is www.mesalabs.com. The information contained or connected to our website is not incorporated by reference into this Annual Report on Form 10-K and should not be considered part of this report.
Sterilization and Disinfection Control Division
Our Sterilization and Disinfection Control Division (formerly named the Biological Indicators Division) provides testing services, along with the manufacture and marketing of biological, chemical and cleaning indicators used to assess the effectiveness of sterilization processes, including steam, gas, hydrogen peroxide, ethylene oxide and radiation, in the hospital, dental, medical device and pharmaceutical industries. Our biological indicators are developed and manufactured according to International Standards Organization (“ISO”) 11138 under a quality system that complies with ISO 13485 and 21 CFR 820.
Biological indicators consist of resistant spores of certain microorganisms that are applied on a convenient substrate, such as a small piece of filter paper. The spores are well characterized in terms of purity, numbers and resistance to sterilization. In use, the biological indicator is exposed to a sterilization process and then tested to determine the presence of surviving organisms. Our biological indicators include a) spore strips, which require post-processing transfer to a growth media, b) self-contained products, which have the growth media already pre-packaged in crushable ampoules, c) culture media, and d) process challenge devices (“PCD’s”) which increase the resistance of biological indicators, mimicking the packaging or other unique characteristics of a product being sterilized. Biological indicators are used to validate equipment and monitor the effectiveness of a process in any industrial or healthcare setting which uses sterilization. Key markets include healthcare, such as dental offices and hospitals, and industrial, such as medical device and pharmaceutical manufacturers.
Our biological indicators are distinguished in the marketplace by their high level of quality, consistency and flexibility. A variety of different formats allows our biological indicators to be used in many different types of processes and products. For example, the simple spore strips are used most often in the small table-top steam sterilizers in dental offices, while a more complex self-contained biological indicator, either with or without a PCD, may be used by a medical device manufacturer to assure the sterility in a complex ethylene oxide sterilization process. In either case, the number of spores contained on the carrier and the resistance of the spores to the sterilization process must be well characterized in order to accurately assess the effectiveness of sterilization. During manufacturing, extensive quality control steps are used to ensure that the microorganism spores are well-characterized and their resistance is known following placement on the target carrier.
Chemical indicators use a chemical change (generally determined by color) to assess the exposure to sterilization conditions. Biological indicators and chemical indicators are often used together to monitor processes.
Cleaning indicators are used to assess the effectiveness of cleaning processes, including washer-disinfectors and ultrasonic cleaners in healthcare settings. Cleaning is the critical first step performed prior to disinfection and sterilization. Debris left on an instrument may interfere with microbial inactivation and can compromise the disinfection or sterilization process. Cleaning indicators compliment sterilization and disinfection processes within central sterile supply departments in hospitals.
Instruments Division
Our Instruments Division designs, manufactures and markets quality control instruments and disposable products utilized in the healthcare, pharmaceutical, medical device, food and beverage, industrial hygiene, and environmental air sampling industries. Generally, our instrument products are used for testing, quality control, safety, validation and regulatory compliance. Our Instruments Division products include: 1) Data loggers, which are used in critical manufacturing and quality control processes in the pharmaceutical, medical device, food and tool industries; 2) Medical meters and calibration solutions, which are used for quality control in dialysis clinics and dialysis machine manufacturing operations; 3) Gas flow calibration and air sampling equipment, which are used for industrial hygiene monitoring, calibration of gas metering equipment and environmental air assessments by a variety of organizations, including metrology labs, manufacturing companies and government agencies; and 4) Torque testing systems, which are used to measure bottle cap tightness in the pharmaceutical and beverage industries.
Data Loggers
Our data logger products are self-contained, wireless, high precision instruments that are used in critical manufacturing, quality control and validation applications. They are used to measure temperature, humidity and pressure inside a process or a product during manufacturing. In addition, data loggers can be used to validate the proper operation of laboratory or manufacturing equipment, either during its installation or for annual re-certifications. The products consist of individual data loggers, a personal computer (“PC”) interface, software and various accessories. A customer typically purchases a large number of data loggers along with a single PC interface and the software package. In practice, using the PC interface, the user programs the loggers to collect environmental data at a pre-determined interval, places the data loggers in the product or process, and then collects stored process data from the data logger either through the PC interface or wirelessly via a radio link. The user can then prepare tabular and graphical reports using the software. Unique aspects of our data loggers are their ability to operate at elevated temperatures and in explosive environments – important differentiating factors in the marketplace and, consequently, they are used by companies to control their most critical processes, such as sterilization. Industries utilizing the data loggers include pharmaceutical and medical device manufacturers, and food processors.
Medical Meters and Calibration Solutions
Our medical meters are used to test various parameters of the dialysis fluid (dialysate), and the proper calibration and operation of the dialysis machine. Each meter measures some combination of temperature, pressure, pH and conductivity to ensure that the dialysate has the proper composition to promote the transfer of waste products from the blood to the dialysate. The meters provide a digital readout that the patient, physician or technician uses to verify that the dialysis machine is working within prescribed limits and delivering properly prepared dialysate. We manufacture two styles of medical meters; those designed for use by dialysis machine manufacturers and biomedical technicians, and those used primarily by dialysis nurses. The meters for technicians are characterized by exceptional accuracy, stability and flexibility, and are used by the industry as the primary standard for the calibration of dialysis machines. The meters designed for use by dialysis nurses are known primarily for their ease of use and incorporate a previously patented, built-in syringe sampling system. These meters are used as the final quality control check on the dialysate just prior to starting a treatment. In addition to the dialysate meters, we market a line of standard solutions for use in dialysis clinics for calibration of our meters. These standard solutions are regularly consumed by the dialysis clinics; thus, along with calibration services, are less impacted by general economic conditions than instrument sales. Customers that utilize these products include dialysis facilities, medical device manufacturers and biomedical service companies.
Gas Flow Calibration and Air Sampling Equipment
We manufacture a variety of instruments and equipment for gas flow calibration and environmental air sampling. In the air sampling area, our technology is used primarily for the determination of particulate concentrations in air as a measure of urban or industrial air pollution, and for industrial hygiene assessments. The primary products include air samplers, particle separators and pumps. In the environmental area, our particle samplers were some of the first on the market and they were recognized early-on as “reference samplers” by the U.S. Environmental Protection Agency.
We also manufacture gas flow calibration instruments to support the use of our air sampling equipment, and for broader industrial applications. Our gas flow calibration instruments provide the precise standards required by laboratories and industry in the design, development, manufacture, installation and calibration of various gas flow meters and air sampling devices. Our flow calibrators are used in many industries where professionals require the superior accuracy, reliability and ease of operation that they provide, including 1) industrial hygienists, 2) calibration and research laboratories, 3) manufacturers who design, develop and manufacture gas flow metering devices, and 4) industrial engineering and manufacturing companies that utilize gas flow metering devices.
Torque Testing Systems
Our automated torque testing systems are durable and reliable motorized cap torque analyzers used throughout the packaging industry. The primary advantages of our torque instruments are their high accuracy and long-term consistency of measurement. Unlike manual torque testing instruments, our motorized torque systems eliminate the effects on the measurement results of different operators and different cap removal speeds. With a motorized torque testing system, the force applied to a cap is precisely the same in each testing cycle, regardless of who may be operating the machine, or how strong they may be. Our torque systems provide the information that helps the packaging operation track events, and potential problems during the manufacturing process so that corrections can be performed in a timely fashion. Industries utilizing these instruments include beverage, pharmaceutical, and food processing companies.
Cold Chain Monitoring Division
Our Cold Chain Monitoring Division designs, develops and markets systems which are used to monitor various environmental parameters such as temperature, humidity and differential pressure to ensure that critical storage and processing conditions are maintained. Cold chain monitoring systems are used in controlled environments such as refrigerators, freezers, warehouses, laboratory incubators, clean rooms and a number of other settings. The cold chain monitoring systems consist of wireless sensors that are placed in controlled environments, hardware modules to receive the wireless data, and various software programs to collect, store and process the data. Our systems are designed to operate continuously, providing data around the clock, 365 days per year. A critical function of our systems is the ability to provide local alarms and notifications via e-mail, text or telephone, in the case where established environmental conditions are exceeded. Key markets for our cold chain monitoring systems are hospitals, pharmaceutical and medical device manufacturers, blood banks, pharmacies and laboratory environments.
Among the important competitive differentiators for our cold chain monitoring systems are 1) their high degree of reliability and up-time; 2) a large variety of sensor types to meet the needs of most applications; 3) a skilled, distributed installation and service team; and 4) a full-featured and 21 CFR Part 11 validated software program, providing extensive reporting and alarm capability. An important aspect of our cold chain monitoring business is the ability to provide post-installation service and support. For most systems, annual re-calibration of each sensor is required, and we provide this service through our dedicated service organization.
Our Cold Chain Monitoring Division also provides parameter (primarily temperature) monitoring of products during transport in a cold chain and consulting services such as compliance monitoring and validation or mapping of transport and storage containers. Our compliance services help customers validate the effectiveness of their cold chain and our monitoring systems record temperature during shipment and provide alarms in case of temperature excursions throughout a cold chain, from point of manufacture or collection, all the way to point of use.
Cold Chain Packaging Division
Our Cold Chain Packaging Division provides packaging development consulting services and thermal packaging products such as coolers, boxes, insulation materials and phase-change products to control temperature during transport. We provide a full suite of products and services to help our customers meet the requirements of their Good Distribution Practices (“GDP”) regulations.
The competitive advantages of our Cold Chain Packaging Division include 1) our in-depth knowledge of cold chain characteristics and requirements, 2) packaging materials that are very durable and can control temperatures for up to 168 hours during transport, and 3) extensive package development and testing capability to help in the design and validation of custom packaging solutions.
Market Factors
Product sales are dependent on several factors, including general economic conditions, both domestic and international, customer capital spending trends, competition, introduction of new products and acquisitions. SDC products and most products in our Cold Chain Packaging Division are disposable and are used on a routine basis, thus product sales are less sensitive to general economic conditions. Instrument products and Cold Chain Monitoring products and systems have a longer life, and their purchase by our customers is somewhat discretionary, so sales are more sensitive to general economic conditions. Service demand is driven by our customers’ quality control and regulatory environments, which require periodic repair and recalibration or certification of our instrument products and cold chain monitoring systems. We typically evaluate costs and pricing annually. Our policy is to price our products competitively and, where possible, we pass along cost increases in order to maintain our margins.
Manufacturing
We conduct product development, manufacturing and support of our Instruments Division products from our facilities in Lakewood, Colorado and Butler, New Jersey. Our instrument products are manufactured primarily by assembling the products from purchased components and calibrating the final products prior to release. The manufacture and support of our Cold Chain Monitoring Division systems are conducted from our facility in Lakewood, Colorado and primarily involve assembling the systems from purchased components and calibrating the sensors, either at the factory or at the point of installation at the customer’s facility. Facilities in Bozeman, Montana, and Munich, Germany are used for the Sterilization and Disinfection Control Division. Our biological indicator products are manufactured by growing microbiological spores from raw materials, forming the finished products and testing the finished biological indicators using established quality control tests. Our dental sterilizer testing products are assembled into kits containing biological indicator spore strips and our microbiological laboratory tests these kits when they are returned to us to determine the effectiveness of our customer’s sterilization process. Our cleaning indicator products are manufactured by inoculating a test soil onto a stainless-steel coupon. The test solid is designed to mimic the challenge of removing blood and tissue from surgical instruments and evaluates the effectiveness of our customer’s cleaning process. Our Cold Packaging products are manufactured by third party suppliers.
Most of the materials and components used in our product lines are available from a number of different suppliers. We generally maintain multiple sources of supply, but we are dependent on a single source for certain items. We believe that in most cases, alternative sources could be developed, if required, for present single supply sources. Although our dependence on these single supply sources may involve a degree of risk, to date we have been able to source sufficient stock to meet our production requirements.
Marketing and Distribution
Domestically, we generate sales to end users through our sales and marketing staff and distributors. We use approximately 220 distributors throughout Europe, Africa, Asia, South America, Australia, Canada and Central America for international sales and distribution. Sales promotions include trade shows, direct mail campaigns, internet and other digital forms of advertising.
Our Sterilization and Disinfection Control Division commercial efforts focus on providing quality test products in a variety of different formats, which minimize incubation and test result time and provide the highest levels of sterility assurance. Customers include hospitals, dental offices, contract sterilization providers and various industrial users involved in pharmaceutical and medical device manufacturing.
Our Instruments Division commercial efforts focus on offering quality products to our customers that will aid them in containing cost, improving the quality of their products and services, and helping them meet their regulatory requirements. Customers include dialysis clinics, pharmaceutical, medical device and food and beverage manufacturers, contract sterilizing services, governmental agencies and environmental testing labs.
Our Cold Chain Monitoring Division commercial efforts focus on providing quality systems to our customers that monitor various environmental parameters such as temperature, humidity and differential pressure to ensure that critical storage and processing conditions are maintained. Customers include hospitals, pharmaceutical and medical device manufacturers, blood banks, pharmacies and laboratory environments.
Our Cold Chain Packaging Division commercial efforts focus on providing packaging development consulting services and thermal packaging products such as coolers, boxes, insulation materials and phase-change products to control temperature during transport. Customers primarily include pharmaceutical manufacturers and distribution companies.
As of and for the years ended March 31, 2018, 2017 and 2016, no individual customer represented more than 10% of our accounts receivable or revenues.
Competition
Our products compete across several industries with a variety of companies, many of which are well established, with substantially greater capital resources and sales forces and larger research and development capabilities. Furthermore, many of these companies have established product lines and a significant operating history. Accordingly, we may be at a competitive disadvantage with some competitors due to their respective size and market presence.
Companies with which our Instruments Division products compete include the Myron L Company, IBP Medical GmbH, Amphenol Corporation, Ellab, TMI Orion, Fortive Corporation, Thermo Fisher Scientific, Inc., Mecmesin, Steinfurth, Met One Instruments, Inc. and Tisch Environmental. Our Sterilization and Disinfection Control Division products compete with 3M, Crostex, Terragene, and Steris, among others. Our Cold Chain Monitoring Division systems compete with Rees Scientific Corporation, Amphenol Corporation and Cooper-Atkins/Emmerson, among others. Our Cold Chain Packaging Division products compete with Sonoco Thermosafe, Cold Chain Technologies, Inc., Pelican Biothermal LLC and Cryopak.
Research and Development
We are committed to an active research and development program dedicated to innovating new products and improving the quality and performance of our existing products. We spent $3,539,000, $4,157,000 and $4,976,000 for the years ended March 31, 2018, 2017 and 2016, respectively, on research and development activities, including amounts capitalized as intangible assets and construction-in-progress. Amounts capitalized, which relate primarily to the development of Cold Chain Monitoring products, were $0, $0 and $1,004,000 for the years ended March 31, 2018, 2017 and 2016, respectively.
Government Regulation
While our quality system and manufacturing processes are generally the same throughout the Instruments Division, specific products are compliant under ISO 13485, ISO 17025, ISO 9001 and certain U.S. Federal regulations. Compliance requires us to obtain third party certification for certain products.
Several products in both the Instruments and Sterilization and Disinfection Control Divisions are medical devices subject to the provisions of the Federal Food, Drug and Cosmetic Act, as amended by the Medical Device Amendments of 1976 (hereinafter referred to as the "Act"). The Act requires any company proposing to market a medical device to notify the Food and Drug Administration (“FDA”) of its intention at least ninety days before doing so and in such notification must advise the FDA as to whether the device is substantially equivalent to a device marketed prior to May 28, 1976. We have received permission from the FDA to market all of our products requiring such permission.
Some of our facilities are subject to FDA regulations and inspections, which may be time-consuming and costly. This includes on-going compliance with the FDA's current Good Manufacturing Practices regulations that require, among other things, the systematic control of manufacture, packaging and storage of products intended for human use. Failure to comply with these practices renders the product adulterated and could subject us to an interruption of manufacturing and selling these products, and possible regulatory action by the FDA.
The manufacture and sale of medical devices is also regulated by some states. Although there is substantial overlap between state regulations and the regulations of the FDA, some state laws may apply. We do not anticipate that complying with state regulations, however, will create any significant problems. Foreign countries also have laws regulating medical devices sold in those countries, which may cause us to expend additional resources on compliance.
Employees
On March 31, 2018, we had 366 employees, of which 173 are employed for manufacturing and quality assurance, 25 for research and development and engineering, 110 for sales and marketing, and 58 for administration.
Item 1A. Risk Factors
In addition to the other information set forth in this Annual Report on Form 10-K and other documents we filed with the SEC, you should carefully consider the following factors, which could materially affect our business, financial condition or results of operations in future periods. The risks and uncertainties described below are those that we have identified as material, but these are not the only risks and uncertainties facing us. Our business is also subject to general risks and uncertainties that affect many other companies, such as market conditions, economic conditions, geopolitical events, changes in laws, regulations or accounting rules, fluctuations in interest rates, terrorism, wars or conflicts, major health concerns, natural disasters or other disruptions of expected business conditions. Additional risks and uncertainties not currently known to us or that we currently believe are immaterial also may impair our business, including our results of operations, liquidity and financial condition.
Conditions in the global economy, the markets we serve, and the financial markets may adversely affect our business and results of operations.
Our business is sensitive to general economic conditions. Slower global economic growth, actual or anticipated default on sovereign debt, volatility in the currency and credit markets, high levels of unemployment or underemployment, reduced levels of capital expenditures, changes or anticipation of potential changes in government fiscal, tax, trade and monetary policies, changes in capital requirements for financial institutions, government deficit reduction and budget negotiation dynamics, sequestration, austerity measures and other challenges that affect the global economy adversely could affect us and our distributors, customers and suppliers, including having the effect of:
|
● |
reducing demand for our products and services, limiting the financing available to our customers and suppliers, increasing order cancellations and resulting in longer sales cycles; |
|
● |
increasing the difficulty in collecting accounts receivable and the risk of excess and obsolete inventories; |
|
● |
increasing price competition in our served markets; |
|
● |
supply interruptions, which could disrupt our ability to produce our products; and |
|
● |
increasing the risk that counterparties to our contractual arrangements will become insolvent or otherwise unable to fulfill their contractual obligations, which could increase the risks identified above. |
If growth in the global economy or in any of the markets we serve slows for a significant period, if there is significant deterioration in the global economy or such markets or if improvements in the global economy do not benefit the markets we serve, our business and results of operations could be adversely affected.
Our growth could suffer if the markets into which we sell our products and services decline, do not grow as anticipated or experience cyclicality.
Our growth depends in part on the growth of the markets which we serve, and visibility into our markets is limited (particularly for markets into which we sell through distributors). Our quarterly results of operations depend substantially on the volume and timing of orders received during the quarter, which are difficult to forecast. Any decline or lower than expected growth in our served markets could diminish demand for our products and services, which could adversely affect our results of operations and consolidated financial statements. Certain of our businesses operate in industries that may experience periodic, cyclical downturns. In addition, in certain of our businesses, demand depends on customers’ capital spending budgets as well as government funding policies, and matters of public policy and government budget dynamics, as well as product and economic cycles which can affect the spending decisions of these entities. Demand for our products and services is also sensitive to changes in customer order patterns, which may be affected by announced price changes, new product introductions, competition and customer inventory levels. Any of these factors could adversely affect our growth and results of operations in any given period.
We face competition and if we are unable to compete effectively, we may experience decreased demand and decreased market share. Even if we compete effectively, we may be required to reduce prices for our products and services.
The markets for our current and potential products are competitive. Because of the range of products and services we sell and the variety of markets we serve, we encounter a wide variety of competitors, including several that possess both larger sales forces and greater capital resources. In order to compete effectively, we must maintain longstanding relationships with major customers, continue to grow our business by establishing relationships with new customers, develop new products and services to maintain and expand our brand recognition and leadership position in various product and service categories, and penetrate new markets, including in developing countries and high growth markets. In addition, significant shifts in industry market share can occur in connection with product problems, safety alerts and publications about products, reflecting the competitive significance of product quality, product efficacy and quality systems in our industries. Our failure to compete effectively and/or pricing pressures resulting from competition may adversely impact our results of operations, and our expansion into new markets may result in greater-than-expected risks, liabilities and expenses.
Changing industry trends may affect our results of operations.
Various changes within the industries we serve may limit future demand for our products and may include the following:
|
● |
changes in dialysis reimbursements; |
|
|
● |
mergers within the dialysis provider industry, concentrating our medical meter and solutions sales with a few, large customers; |
|
|
● |
mergers within other industries we serve, making us more dependent upon fewer, larger customers for our sales; |
|
|
● |
decreased product demand, driven by changes in our customers’ regulatory environments or standard industry practices; and |
|
|
● |
price competition for key products. |
Our growth depends in part on the timely development and commercialization, and customer acceptance, of new and enhanced products and services and the efforts of third party distributors.
Our growth depends on the acceptance of our products and services in the marketplace, the penetration achieved by the companies which we sell to, and rely on, to distribute and represent our products, and our ability to introduce new and innovative products that meet the needs of the various markets we serve. We can offer no assurance that we will be able to continue to introduce new and enhanced products, that the products we introduce, or have introduced, will be widely accepted by the marketplace, or that the companies that we contract with to distribute and represent our products will continue to successfully penetrate our various markets. Our failure to continue to introduce new and enhanced products or gain widespread acceptance of our products and services could adversely affect our results of operations. In order to successfully commercialize our products and services in new markets, we will need to enter into distribution arrangements with companies that can successfully distribute and represent our products and services into various markets.
Our reputation, ability to do business and consolidated financial statements may be impaired by improper conduct by any of our employees, agents or business partners.
We cannot provide assurance that our internal controls and compliance systems will always protect us from acts committed by employees, agents or business partners of ours (or of businesses we acquire or partner with) that would violate U.S. and/or non-U.S. laws, including the laws governing payments to government officials, bribery, fraud, kickbacks and false claims, pricing, sales and marketing practices, conflicts of interest, competition, export and import compliance, money laundering and data privacy. In particular, the U.S. Foreign Corrupt Practices Act and similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business. Any such improper actions or allegations of such acts could damage our reputation and subject us to civil or criminal investigations in the United States and in other jurisdictions and related shareholder lawsuits, could lead to substantial civil and criminal, monetary and non-monetary penalties and could cause us to incur significant legal and investigatory fees. We also rely on our suppliers to adhere to our supplier standards of conduct and material violations of such standards of conduct could occur that could have a material effect on our business, reputation and consolidated financial statements.
Certain of our businesses are subject to extensive regulation by the U.S. Food and Drug Administration (“FDA”) and by comparable agencies of other countries. Failure to comply with those regulations would likely adversely affect our reputation and consolidated financial statements
Certain of our products are medical devices and other products that are subject to regulation by the U.S. FDA, by other federal and state governmental agencies, by comparable agencies of other countries and regions and by regulations governing radioactive or other hazardous materials. We cannot guarantee that we will be able to obtain regulatory clearance (such as 510(k) clearance) or approvals for our new products or modifications to (or additional indications or uses of) existing products within our anticipated timeframe or at all, and if we do obtain such clearance or approval it may be time-consuming, costly and subject to restrictions. Our ability to obtain such regulatory clearances or approvals will depend on many factors and the process for obtaining such clearances or approvals could change over time and may require the withdrawal of products from the market until such clearances are obtained. Failure to comply with applicable regulations would likely adversely impact our results of operations.
Any inability to consummate acquisitions at our historical rate and at appropriate prices could negatively impact our growth rate and stock price.
Our ability to grow revenues, earnings and cash flows at or above our historic rates depends in part upon our ability to identify and successfully acquire and integrate businesses at appropriate prices and realize anticipated synergies. We may not be able to consummate acquisitions at rates similar to the past, which could adversely impact our growth rate and our stock price. Promising acquisitions are difficult to identify and complete for a number of reasons, including high valuations, competition among prospective buyers, the availability of affordable funding in the capital markets and the need to satisfy applicable closing conditions. In addition, competition for acquisitions may result in higher purchase prices. Changes in accounting or regulatory requirements, or instability in the credit markets, could also adversely impact our ability to consummate acquisitions.
Our acquisition of businesses could negatively impact our results of operations.
As an important part of our business strategy, we acquire businesses, some of which may be material. Please see “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” for additional details. These acquisitions involve a number of financial, accounting, managerial, operational, legal, compliance and other risks and challenges, including the following, any of which could adversely affect our business and our results of operations:
|
● |
any business, technology, service or product that we acquire could under-perform relative to our expectations and the price that we paid for it, or not perform in accordance with our anticipated timetable, or we could fail to make such business profitable; |
|
● |
we may incur or assume significant debt in connection with our acquisitions; |
|
● |
acquisitions could cause our results of operations to differ from our own or the investment community’s expectations in any given period, or over the long-term; |
|
● |
pre-closing and post-closing acquisition-related earnings charges could adversely impact our results of operations in any given period, and the impact may be substantially different from period to period; |
|
● |
acquisitions could create demands on our management, operational resources and financial and internal control systems that we are unable to effectively address, or for which we may incur additional costs; |
|
● |
we could experience difficulty in integrating personnel, operations, financial and other systems, and in retaining key employees and customers; |
|
● |
we may be unable to achieve cost savings or other synergies anticipated in connection with an acquisition; |
|
● |
we may assume by acquisition unknown liabilities, known contingent liabilities that become realized, known liabilities that prove greater than anticipated, internal control deficiencies or exposure to regulatory sanctions resulting from the acquired company’s activities. The realization of any of these liabilities or deficiencies may increase our expenses, adversely affect our financial position or cause us to fail to meet our public financial reporting obligations; |
|
● |
in connection with acquisitions, we often enter into post-closing financial arrangements such as purchase price adjustments, earn-out obligations and indemnification obligations, which may have unpredictable financial results; and |
|
● |
as a result of our acquisitions, we have recorded significant goodwill and intangible assets on our consolidated balance sheet. If we are not able to realize the value of these assets, we may be required to incur charges relating to the impairment of these assets, which could materially impact our results of operations. |
The indemnification provisions of acquisition agreements by which we have acquired companies may not fully protect us and as a result we may face unexpected liabilities.
Certain of the acquisition agreements by which we have acquired companies require the former owners to indemnify us against certain liabilities related to the operation of the company before we acquired it. In most of these agreements, however, the liability of the former owners is limited, and certain former owners may be unable to meet their indemnification responsibilities. We cannot guarantee that these indemnification provisions will protect us fully or at all, and as a result we may face unexpected liabilities that could adversely impact our results of operations.
Divestitures or other dispositions could negatively impact our business.
We continually assess the strategic fit of our existing businesses and may divest or otherwise dispose of businesses that are deemed not to fit with our strategic plan or are not achieving the desired return on investment. Transactions such as these pose risks and challenges that could negatively impact our business and our results of operations. For example, when we decide to sell or otherwise dispose of a business or assets, we may be unable to do so on satisfactory terms within our anticipated timeframe or at all, and even after reaching a definitive agreement to sell or dispose a business the sale may be subject to satisfaction of pre-closing conditions which may not become satisfied. In addition, divestitures or other dispositions may dilute our earnings per share, have other adverse financial and accounting impacts and distract management, and disputes may arise with buyers.
The contingent consideration associated with certain of our acquisitions may negatively impact our available cash and results from operations.
As part of certain of our acquisitions, we are required to make contingent consideration payments based on defined growth metrics over a specified earn-out period. The ultimate amount we pay may differ significantly from the liability we recorded at the time of the acquisition. If we are required to pay more than the amount initially recorded, the difference is recorded as expense in our consolidated statements of operations, which could materially impact our results of operations.
If we do not or cannot adequately protect our intellectual property, or if third parties infringe our intellectual property rights, we may suffer competitive injury or expend significant resources enforcing our rights.
We own patents, trademarks, copyrights, trade secrets and other intellectual property and licenses to intellectual property owned by others, which in the aggregate are important to our business. The intellectual property rights that we obtain, however, may not be sufficiently broad or otherwise may not provide us a significant competitive advantage, and patents may not be issued for pending or future patent applications owned by or licensed to us. In addition, the steps that we and our licensors have taken to maintain and protect our intellectual property may not prevent it from being challenged, invalidated, circumvented or designed-around, particularly in countries where intellectual property rights are not highly developed or protected. In some circumstances, enforcement may not be available to us because an infringer has a dominant intellectual property position or for other business reasons, or countries may require compulsory licensing of our intellectual property. We also rely on nondisclosure and noncompetition agreements with employees, consultants and other parties to protect, in part, trade secrets and other proprietary rights. There can be no assurance that these agreements will adequately protect our trade secrets and other proprietary rights and will not be breached, that we will have adequate remedies for any breach, that others will not independently develop substantially equivalent proprietary information or that third parties will not otherwise gain access to our trade secrets or other proprietary rights. Our failure to obtain or maintain intellectual property rights that convey competitive advantage, adequately protect our intellectual property, detect or prevent circumvention or unauthorized use of such property, and the cost of enforcing our intellectual property rights, could adversely impact our competitive position and results of operations.
Several of our products are extensively regulated, which could delay product introduction or halt sales.
The process of obtaining and maintaining required regulatory approvals is lengthy, expensive and uncertain. Although we have not experienced any substantial regulatory delays to date, we can offer no assurance that delays will not occur in the future, which could have a significant adverse effect on our ability to introduce new products on a timely basis. Regulatory agencies periodically inspect our manufacturing facilities to ascertain compliance with “good manufacturing practices” and can subject approved products to additional testing and surveillance programs. Failure to comply with applicable regulatory requirements can, among other things, result in fines, suspension of regulatory approvals, product recalls, operating restrictions and criminal penalties. While we believe that we are currently in compliance, if we fail to comply with regulatory requirements it could have an adverse effect on our results of operations and financial condition.
Product defects and unanticipated use or inadequate disclosure with respect to our products or services could adversely affect our business, reputation and our results of operations.
Manufacturing or design defects in, unanticipated use of, safety or quality issues (or the perception of such issues) with respect to, or inadequate disclosure of risks relating to the use of products and services that we make or sell (including items that we source from third parties) can lead to personal injury, property damage or other liability. These events could lead to recalls or safety alerts, result in the removal of a product or service from the market and result in product liability or similar claims being brought against us. Recalls, removals and product liability and similar claims (regardless of their validity or ultimate outcome) can results in significant costs, as well as negative publicity and damage to our reputation that could reduce demand for our products and services.
Catastrophic events or environmental conditions may disrupt our business.
A disruption or failure of our systems or operations because of a major weather event, cyber-attack, terrorist attack, or other catastrophic event could cause delays in completing sales, providing services or performing other mission-critical functions. A catastrophic event that results in the destruction or disruption of any of our critical business or IT systems could harm our ability to conduct normal business operations. Abrupt political change, terrorist activity, and armed conflict pose a risk of general economic disruption in affected countries, which may increase our operating costs or adversely affect our revenues. These conditions also may add uncertainty to the timing and budget for purchase/investment decisions by our customers and may result in supply chain disruptions for hardware manufacturers, either of which may adversely affect our revenues. The long-term effects of climate change on the global economy in general are unclear. Environmental regulations or changes in the supply, demand or available sources of energy may affect the availability or cost of goods and services, including natural resources, necessary to run our business. Changes in weather where we operate may increase the costs of powering and maintaining the equipment we need to produce our product lines.
Significant developments stemming from the current U.S. administration or the United Kingdom’s referendum on membership in the EU could have an adverse effect on us.
Changes, potential changes or uncertainties in U.S. social, political, regulatory and economic conditions or laws and policies governing the health care system and drug prices, foreign trade, manufacturing, and development and investment in the territories and countries where we or our customers operate, stemming from the current U.S. administration, could adversely affect our business and consolidated financial statements. For example, the current U.S. administration has called for substantial changes to trade agreements, such as the North American Free Trade Agreement (“NAFTA”), has increased tariffs on certain goods imported into the United States and has raised the possibility of imposing significant, additional tariff increases. Additionally, on June 23, 2016, the United Kingdom held a referendum and voted in favor of leaving the EU. This referendum has caused and may continue to cause political and economic uncertainty, including significant volatility in global stock markets and currency exchange rate fluctuations. Although it is unknown what the full terms of the United Kingdom’s future relationship with the EU will be, it is possible that there will be greater restrictions on imports and exports between the United Kingdom and other countries, including the United States, and increased regulatory complexities. Any of these factors could adversely affect customer demand, our relationships with customers and suppliers and our business and financial statements.
We may be required to recognize impairment charges that could materially affect our results of operations.
We assess our goodwill and other intangible assets, and our other long-lived assets as and when required by accounting principles generally accepted in the United States (“GAAP”) to determine whether they are impaired. If they are impaired, we would record appropriate impairment charges. It is possible that we may be required to record significant impairment charges in the future and, if we do so, our results of operations could be materially adversely affected.
Changes in accounting standards could affect our reported financial results.
New accounting standards or pronouncements that may become applicable to our Company from time to time, or changes in the interpretation of existing standards and pronouncements, could have a significant effect on our reported results of operations for the affected periods.
Foreign currency exchange rates may adversely affect our consolidated financial statements.
Sales and purchases in currencies other than the U.S. dollar expose us to fluctuations in the exchange rates of foreign currencies relative to the U.S. dollar and may adversely affect our consolidated financial statements. Increased strength of the U.S. dollar increases the effective price of our products sold in U.S. dollars into other countries, which may require us to lower our prices or adversely affect sales to the extent we do not increase local currency prices. Decreased strength of the U.S. dollar could adversely affect the cost of materials, products and services we purchase overseas. Revenues and expenses of our non-U.S. businesses are also translated into U.S. dollars for reporting purposes and the strengthening or weakening of the U.S. dollar could result in unfavorable translation effects. In addition, we face exchange rate risk from our investment in subsidiaries owned and operated in foreign countries.
Changes in our tax rates or exposure to additional income tax liabilities or assessments could affect our profitability. In addition, audits by tax authorities could result in additional tax payments for prior periods.
We are subject to income taxes in the U.S. and in various non-U.S. jurisdictions. On December 22, 2017, the Tax Cuts and Jobs Act (“TCJA”) was enacted. The TCJA significantly revises the U.S. federal corporate income tax law by, among other things, lowering the corporate income tax rate to 21% (beginning in calendar 2018), implementing a territorial tax system, and imposing a one-time tax on unremitted cumulative non-U.S. earnings of foreign subsidiaries (“Transition Tax”). The U.S. Treasury Department and IRS have not yet issued regulations with respect to the TCJA.
Due to the potential for changes to tax laws and regulations or changes to the interpretation thereof (including regulations and interpretations pertaining to the TCJA), the ambiguity of tax laws and regulations, the subjectivity of factual interpretations, the complexity of our intercompany arrangements, uncertainties regarding the geographic mix of earnings in any particular period, and other factors, our estimates of effective tax rate and income tax assets and liabilities may be incorrect and our consolidated financial statements could be adversely affected. For example, our estimate of the net one-time charge we have incurred related to the TCJA could differ materially from our actual liability, due to, among other things, further refinement of our calculations, changes in interpretations and assumptions that we have made, additional guidance that may be issued by the U.S. Treasury Department and IRS, and actions we may take as a result of the TCJA. The impact of the factors referenced in the first sentence of this paragraph may be substantially different from period-to-period.
In addition, the amount of income taxes we pay is subject to ongoing audits by U.S. federal, state and local tax authorities and by non-U.S. tax authorities. If audits result in payments or assessments different from our reserves, our future results may include unfavorable adjustments to our tax liabilities and our consolidated financial statements could be adversely affected. Any further significant changes to the tax system in the United States or in other jurisdictions (including changes in the taxation of international income as further described below) could adversely affect our consolidated financial statements.
Changes in tax law relating to multinational corporations could adversely affect our tax position.
The U.S. Congress, government agencies in non-U.S. jurisdictions where we and our affiliates do business, and the Organisation for Economic Co-operation and Development (“OECD”) have recently focused on issues related to the taxation of multinational corporations. One example is in the area of “base erosion and profit shifting,” where profits are claimed to be earned for tax purposes in low-tax jurisdictions, or payments are made between affiliates from a jurisdiction with high tax rates to a jurisdiction with lower tax rates. The OECD has released several components of its comprehensive plan to create an agreed set of international rules for addressing base erosion and profit shifting. As a result, the tax laws in the United States and other countries in which we do business could change on a prospective or retroactive basis, and any such changes could adversely affect our business and financial statements.
Our business is subject to sales tax in numerous states.
The application of indirect taxes, such as sales tax, is a complex and evolving issue. A company is required to collect and remit state sales tax from certain of its customers if that company is determined to have “nexus” in a particular state. The determination of nexus varies by state and often requires knowledge of each jurisdiction’s tax case law. The application and implementation of existing, new or future laws could change the states in which we are required to collect and remit sales taxes. If any jurisdiction determines that we have “nexus” in additional locations that we have not contemplated, it could have an adverse effect on our results of operations and financial condition.
We are subject to the possibility of a variety of litigation and other legal and regulatory proceedings in the course of our business that could adversely affect our consolidated financial statements.
We are subject to the possibility of a variety of litigation and other legal and regulatory proceedings incidental to our business, including claims for damages arising out of the use of products or services and claims relating to intellectual property matters, employment matters, tax matters, commercial disputes, marketing matters, competition and sales and trading practices, environmental matters, personal injury, insurance coverage and acquisition or divestiture-related matters, as well as regulatory investigations or enforcement. We may also become subject to lawsuits as a result of past or future acquisitions or as a result of liabilities retained from, or representations, warranties or indemnities provided in connection with, divested businesses. Any of these lawsuits may include claims for compensatory damages, punitive and consequential damages and/or injunctive relief. The defense of these lawsuits may divert our management’s attention, we may incur significant expenses in defending these lawsuits, and we may be required to pay damage awards or settlements or become subject to equitable remedies that could adversely affect our operations and consolidated financial statements. Moreover, any insurance or indemnification rights that we may have may be insufficient or unavailable to protect us against such losses. In addition, developments in proceedings in any given period may require us to adjust the loss contingency estimates that we have recorded in our consolidated financial statements, record estimates for liabilities or assets previously not susceptible of reasonable estimates or pay cash settlements or judgments. Any of these developments could adversely affect our consolidated financial statements in any given period. We cannot make assurances that our liabilities in connection with litigation and other legal regulatory proceedings will not exceed our estimates or adversely affect our consolidated financial statements and business. Please see Note 13 of Notes to Consolidated Financial Statements contained in “Item 8. Financial Statements and Supplementary Data” for additional discussion.
We are utilizing variable rate financing.
As of March 31, 2018, we had $46,625,000 in outstanding indebtedness which bears interest at either: (1) LIBOR, as defined, plus an applicable margin ranging from 1.5% to 2.50%; or (2) the alternate base rate (“ABR”), which is the greater of JPMorgan’s prime rate or the federal funds effective rate or the overnight bank funding rate plus 0.5%. A change in interest rate market conditions could increase our interest costs in the future and may have an adverse effect on our results of operations.
Our indebtedness may limit our operations and our use of our cash flow, and any failure to comply with the covenants that apply to our indebtedness could adversely affect our liquidity and consolidated financial statements.
As of March 31, 2018, we had $46,625,000 in outstanding indebtedness and, based on the remaining availability under our Credit Facility, we have the ability to incur an additional $52,000,000 of indebtedness. Our debt level and related debt service obligations can have negative consequences, including (1) requiring us to dedicate significant cash flow from operations to the payment of principal and interest on our debt, which would reduce the funds we would have available for other purposes such as acquisitions and capital investment; (2) reducing our flexibility in planning for or reacting to changes in our business and market conditions; and (3) exposing us to interest rate risk since our debt obligations are at variable rates. We may incur significantly more debt in the future, particularly to finance acquisitions.
If global credit market conditions deteriorate, our financial performance could be adversely affected.
The cost and availability of credit are subject to changes in the global economic environment. If conditions in major credit markets deteriorate, our ability to obtain debt financing or the terms associated with that debt financing may be negatively affected, which could affect our results of operations.
If we suffer loss to our facilities, supply chains, distribution systems or information technology systems due to catastrophe or other events, our operations could be seriously harmed.
Our facilities, supply chains, distribution systems and information technology systems are subject to catastrophic loss due to fire, flood, earthquake, hurricane, public health crisis, war, terrorism or other natural or man-made disasters. If any of these facilities, supply chains or systems were to experience catastrophic loss, it could disrupt our operations, delay production and shipments, result in defective products or services, damage customer relationships and our reputation and result in legal exposure and large repair or replacement expenses. The third-party insurance coverage that we maintain will vary from time to time in both type and amount depending on cost, availability and our decisions regarding risk retention, and may be unavailable or insufficient to protect us fully against losses.
Adverse changes in our relationships with, or the financial condition, performance, purchasing patterns or inventory levels of, key distributors and other channel partners could adversely affect our consolidated financial statements.
Certain of our businesses sell a significant amount of their products to key distributors and other channel partners that have valuable relationships with customers and end-users. Some of these distributors and other partners also sell our competitors’ products or compete with us directly, and if they favor competing products for any reason they may fail to market our products effectively. Adverse changes in our relationships with these distributors and other partners, or adverse developments in their financial condition, performance or purchasing patterns, could adversely affect our business and consolidated financial statements. The levels of inventory maintained by our distributors and other channel partners, and changes in those levels, can also negatively impact our results of operations in any given period.
A significant disruption in, or breach in security of, our information technology systems or violation of data privacy laws could adversely affect our business, reputation and consolidated financial statements.
We rely on information technology systems, some of which are managed by third parties, to process, transmit and store electronic information (including sensitive data such as confidential business information and personally identifiable data relating to employees, customers and other business partners), and to manage or support a variety of critical business processes and activities. These systems may be damaged, disrupted or shut down due to attacks by computer hackers, computer viruses, employee error or malfeasance, power outages, hardware failures, telecommunication or utility failures, catastrophes or other unforeseen events, and in any such circumstances our system redundancy and other disaster recovery planning may be ineffective or inadequate. Attacks may also target hardware, software and information installed, stored or transmitted in our products after such products have been purchased and incorporated into third-party products, facilities or infrastructure. Security breaches of systems provided or enabled by us, regardless of whether the breach is attributable to a vulnerability in our products or services, could result in the misappropriation, destruction or unauthorized disclosure of confidential information or personal data belonging to us or to our employees, partners, customers or suppliers. Like most multinational corporations, our information technology systems have been subject to computer viruses, malicious codes, unauthorized access and other cyber-attacks and we expect the sophistication and frequency of such attacks to continue to increase. Any of the attacks, breaches or other disruptions or damage described above could interrupt our operations or the operations of our customers and partners, delay production and shipments, result in theft of our and our customers’ intellectual property and trade secrets, damage customer and business partner relationships and our reputation or result in defective products or services, legal claims and proceedings, liability and penalties under privacy laws and increased costs for security and remediation, each of which could adversely affect our business and consolidated financial statements.
While we select our third-party vendors carefully (including the provider of our ERP system), we don’t control their actions. Any problems caused by these third parties, including those resulting from breakdowns or other disruptions in communication services provided by a vendor, failure of a vendor to handle current or higher volumes or cyber-attacks and security breaches at a vendor could adversely affect our ability to deliver products and services to our customers and otherwise conduct our business.
We may face continuing challenges in complying with certain sections of the Sarbanes-Oxley Act.
Like many public companies, we face challenges in complying with the internal control requirements of the Sarbanes-Oxley Act (Section 404). Under current frameworks, compliance in areas such as separation of duties, information system controls, etc. may prove problematic for a smaller company with limited human resources. We may also be forced to incur on-going expense in order to comply with the law under current control frameworks or if the framework changes. These expenses may have a material adverse effect on our results of operations.
Item 1B. Unresolved Staff Comments
None
Item 2. Properties
Set forth below is a listing of our facilities. The Lakewood, Butler, Bozeman, Munich and Markham facilities all have manufacturing, research and development, marketing and administrative functions. The Berlin, Traverse City, Addison and Chassieu facilities have marketing and administrative functions.
|
Location |
Operations |
Square Feet |
||
|
Lakewood, Colorado |
Instruments, Cold Chain Monitoring and Corporate Headquarters |
44,000 |
Owned |
|
|
Lakewood, Colorado |
Corporate administration |
9,000 |
Leased |
|
|
Butler, New Jersey |
Instruments |
20,000 |
Leased |
|
|
Bozeman, Montana |
Sterilization and Disinfection Control |
129,000 |
Owned |
|
|
Berlin, New Jersey |
Cold Chain Monitoring |
2,000 |
Leased |
|
|
Traverse City, Michigan |
Sterilization and Disinfection Control |
3,800 |
Leased |
|
|
Addison, Texas |
Cold Chain Monitoring |
2,000 |
Leased |
|
|
Chassieu, France |
Sterilization and Disinfection Control |
6,000 |
Leased |
|
|
Markham, Canada |
Cold Chain Packaging and Sterilization and Disinfection Control |
8,000 |
Leased |
|
|
Munich, Germany |
Sterilization and Disinfection Control |
5,000 |
Leased |
Item 3. Legal Proceedings
In February 2018, we were sued in a putative civil class action in the United States District Court for the Northern District of Illinois, Eastern Division whereby it was alleged that we sent unsolicited advertisements to telephone facsimile machines in violation of the Telephone Consumer Protection Act (“TCPA”), as well as analogous state statutes and state consumer protection laws. The plaintiff in this lawsuit is seeking various forms of relief, including statutory damages of $500 for each violation of the TCPA or, in the alternative, treble damages of up to $1,500 for each knowing and willful violation of the TCPA, as well as payment of interest, attorneys’ fees and costs, and certain injunctive relief prohibiting the further transmission of unsolicited fax advertising in the future. We intend to vigorously defend this case however we cannot predict with any degree of certainty the outcome of the lawsuit or determine the extent of any potential liability or damages.
Item 4. Mine Safety Disclosures
Not applicable
Part II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is traded on the Nasdaq Global Market (“Nasdaq”) under the symbol "MLAB.”
The following table sets forth the high and low market prices per share for our common stock, as reported by Nasdaq, and dividend per share information:
|
Quarter Ended |
High |
Low |
Dividends Per Share |
|||||||||
|
June 30, 2017 |
$ | 166.41 | $ | 122.10 | $ | 0.16 | ||||||
|
September 30, 2017 |
153.66 | 128.19 | 0.16 | |||||||||
|
December 31, 2017 |
160.45 | 124.16 | 0.16 | |||||||||
|
March 31, 2018 |
152.68 | 124.62 | 0.16 | |||||||||
|
Quarter Ended |
High |
Low |
Dividends Per Share |
|||||||||
|
June 30, 2016 |
$ | 130.03 | $ | 92.83 | $ | 0.16 | ||||||
|
September 30, 2016 |
126.48 | 102.54 | 0.16 | |||||||||
|
December 31, 2016 |
135.24 | 115.02 | 0.16 | |||||||||
|
March 31, 2017 |
126.99 | 116.41 | 0.16 | |||||||||
While we have paid dividends to holders of our common stock on a quarterly basis since 2003, the declaration and payment of future dividends will depend on many factors, including, but not limited to, our earnings, financial condition, business development needs and regulatory considerations, and is at the sole discretion of our Board of Directors.
The Nasdaq Global Market quotations set forth herein reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions.
As of March 31, 2018, there were 101 record holders of our common stock. This amount does not include “street name” holders or beneficial holders of our common stock, whose holders of record are banks, brokers and other financial institutions.
During the year ended March 31, 2018, we did not sell any equity securities that were not registered under the Securities Act of 1933, as amended.
We made the following repurchases of our common stock, by month, within the fourth quarter of the year covered by this report:
|
Shares Purchased |
Average Price Paid |
Total Shares Purchased as Part of Publicly Announced Plan |
Remaining Shares Able to Purchase Under Plan |
|||||||||||||
|
January 1 – 31, 2018 |
-- | -- | 162,486 | 137,514 | ||||||||||||
|
February 1 – 28, 2018 |
-- | -- | 162,486 | 137,514 | ||||||||||||
|
March 1 – 31, 2018 |
-- | -- | 162,486 | 137,514 | ||||||||||||
|
Total |
-- | -- | ||||||||||||||
On November 7, 2005, our Board of Directors adopted a share repurchase plan which allows for the repurchase of up to 300,000 of our common shares. This plan will continue until the maximum is reached or the plan is terminated by further action of the Board of Directors.
We have certain equity compensation plans, all of which were approved by our shareholders. As of March 31, 2018, we have issued 8,788 restricted stock awards, 458,358 shares of common stock may be issued upon exercise of outstanding options, with a weighted-average exercise price of $86.38 and 767,888 shares are available for future issuance under the plans. Please see notes contained in “Item 8. Financial Statements and Supplementary Data” of this report for additional details.
Set forth below is a line graph comparing, for the period March 31, 2013 through March 31, 2018, the cumulative total shareholder return on our common stock against the cumulative total return of (a) the S&P Composite Stock Index and (b) a self-selected peer group, comprised of the following companies: Danaher Corp., ARCA Biopharma, Inc., Steris Corp., Utah Medical Products, Inc., Cantel Medical Corp., Fortive Corporation, Merit Medical Systems, Inc., Transcat Inc., Electro-Sensors Inc., and Rudolph Technologies Inc. The graph shows the value at March 31 of each year, assuming an original investment of $100 in each and reinvestment of cash dividends.
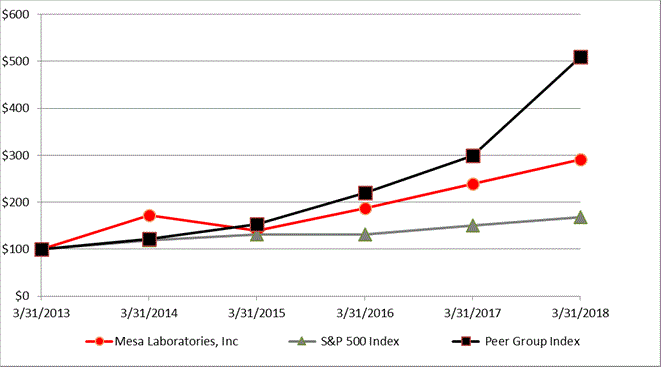
Item 6. Selected Financial Data
The following selected financial data should be read in conjunction with “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and financial statements and notes thereto contained in “Item 8. Financial Statements and Supplementary Data” of this report.
|
(In thousands, except per share data) |
As of and for The Year Ended March 31, |
|||||||||||||||||||
|
2018 |
2017 |
2016 |
2015 |
2014 |
||||||||||||||||
|
Cash and cash equivalents |
$ | 5,469 | $ | 5,820 | $ | 5,695 | $ | 2,034 | $ | 5,575 | ||||||||||
|
Working capital |
$ | 14,698 | $ | 19,218 | $ | 13,215 | $ | 14,965 | $ | 16,351 | ||||||||||
|
Average return on: |
||||||||||||||||||||
|
Stockholder investment (1) |
(3 | )% | 12 | % | 14 | % | 14 | % | 15 | % | ||||||||||
|
Assets |
(2 | )% | 7 | % | 8 | % | 9 | % | 11 | % | ||||||||||
|
Invested capital (2) |
(2 | )% | 8 | % | 10 | % | 11 | % | 13 | % | ||||||||||
|
Revenues |
$ | 96,179 | $ | 93,665 | $ | 84,659 | $ | 71,330 | $ | 52,724 | ||||||||||
|
Gross profit |
$ | 54,619 | $ | 53,239 | $ | 51,413 | $ | 43,392 | $ | 31,688 | ||||||||||
|
Gross profit margin |
57 | % | 57 | % | 61 | % | 61 | % | 60 | % | ||||||||||
|
Operating income |
$ | 2,183 | $ | 16,313 | $ | 16,323 | $ | 15,864 | $ | 11,785 | ||||||||||
|
Operating income margin |
2 | % | 17 | % | 19 | % | 22 | % | 22 | % | ||||||||||
|
Net (loss) income |
$ | (2,962 | ) | $ | 11,183 | $ | 11,169 | $ | 9,583 | $ | 9,000 | |||||||||
|
Net income margin |
(3 | )% | 12 | % | 13 | % | 13 | % | 17 | % | ||||||||||
|
Net (loss) income per diluted share |
$ | (0.79 | ) | $ | 2.91 | $ | 2.97 | $ | 2.63 | $ | 2.49 | |||||||||
|
Adjusted operating income (3) |
$ | 24,603 | $ | 24,174 | $ | 23,437 | $ | 21,532 | $ | 15,604 | ||||||||||
|
Adjusted operating income per diluted share |
$ | 6.53 | $ | 6.29 | $ | 6.24 | $ | 5.90 | $ | 4.32 | ||||||||||
|
Average return on: |
||||||||||||||||||||
|
Adjusted invested capital (4) |
17 | % | 17 | % | 20 | % | 24 | % | 22 | % | ||||||||||
|
(1) |
Average return on stockholder investment is calculated by dividing total net income by the average of end and beginning of year total stockholders’ equity. |
|
(2) |
Average return on invested capital (invested capital = total assets – current liabilities – cash and cash equivalents) is calculated by dividing total net income by the average of end and beginning of year invested capital. |
|
(3) |
Adjusted operating income is a non-GAAP measure and is defined to exclude the non-cash impact of amortization of intangible assets, stock-based compensation and impairment of goodwill. |
|
(4) |
Adjusted invested capital is a non-GAAP measure which substitutes adjusted operating income for net income in the average return on invested capital calculation (2). |
Reconciliation of Non-GAAP Measure
Adjusted operating income (which excludes the non-cash impact of amortization of intangible assets, stock-based compensation and impairment of goodwill) is used by management as a supplemental performance and liquidity measure, primarily to exclude the impact of acquisition-related intangible assets in order to compare current financial performance to historical performance, assess the ability of our assets to generate cash and the evaluation of potential acquisitions.
Adjusted operating income should not be considered an alternative to, or more meaningful than, net income, operating income, cash flow from operating activities or any other measure of financial performance presented in accordance with GAAP as measures of operating performance or liquidity.
The following table sets forth our reconciliation of adjusted operating income, a non-GAAP measure:
|
(In thousands) |
Year Ended March 31, | |||||||||||||||||||
|
2018 |
2017 |
2016 |
2015 |
2014 |
||||||||||||||||
|
Operating income |
$ | 2,183 | $ | 16,313 | $ | 16,323 | $ | 15,864 | $ | 11,785 | ||||||||||
|
Amortization of intangible assets |
6,929 | 6,450 | 5,787 | 4,675 | 2,979 | |||||||||||||||
|
Stock based compensation |
1,672 | 1,411 | 1,327 | 993 | 840 | |||||||||||||||
|
Impairment of goodwill |
13,819 | -- | -- | -- | -- | |||||||||||||||
|
Adjusted net income |
$ | 24,603 | $ | 24,174 | $ | 23,437 | $ | 21,532 | $ | 15,604 | ||||||||||
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Overview
We pursue a strategy of focusing primarily on quality control products and services, which are sold into niche markets that are driven by regulatory requirements. We prefer markets where we can establish a strong presence and achieve high gross margins. We are organized into four divisions across nine physical locations. Our Sterilization and Disinfection Control Division (formerly named the Biological Indicators Division) provides testing services, along with the manufacturing and marketing of biological, chemical and cleaning indicators used to assess the effectiveness of sterilization and disinfection processes in the hospital, dental, medical device and pharmaceutical industries. Our Instruments Division designs, manufactures and markets quality control instruments and disposable products utilized in the healthcare, pharmaceutical, food and beverage, medical device, industrial hygiene and environmental air sampling industries. Our Cold Chain Monitoring Division designs, develops and markets systems which are used to monitor various environmental parameters such as temperature, humidity and differential pressure to ensure that critical storage and processing conditions are maintained in hospitals, pharmaceutical and medical device manufacturers, blood banks, pharmacies and laboratory environments. Our Cold Chain Packaging Division provides packaging development consulting services and thermal packaging products such as coolers, boxes, insulation materials and phase-change products to control temperature during transport.
Our revenues come from two main sources – product sales and services. Product sales are dependent on several factors, including general economic conditions, both domestic and international, customer capital spending trends, competition, introduction of new products and acquisitions. Sterilization and Disinfection Control products and many of the packaging products of our Cold Chain Packaging Division are disposable and are used on a routine basis, thus product sales are less sensitive to general economic conditions. Instrument products and cold chain monitoring systems and products have a longer life, and their purchase by our customers is somewhat discretionary, so sales are more sensitive to general economic conditions. Service demand is driven by our customers’ quality control and regulatory environments, which require periodic repair and recalibration or certification of our instrument products and cold chain monitoring systems. We typically evaluate costs and pricing annually. Our policy is to price our products competitively and, where possible, we pass along cost increases in order to maintain our margins.
Gross profit is affected by our product mix, manufacturing efficiencies and price competition. Historically, as we have integrated our acquisitions and taken advantage of manufacturing efficiencies, our gross margin percentages for some products have improved. There are, however, differences in gross margin percentages between product lines, and ultimately the mix of sales will continue to impact our overall gross margin.
Selling expense is driven primarily by labor costs, including salaries and commissions. Accordingly, it may vary with sales levels and our commercial mix. Labor costs and amortization of intangible assets drive the substantial majority of general and administrative expense. Research and development expense is predominantly comprised of labor costs and third-party consultants.
Year Ended March 31, 2018 Acquisitions
During the year ended March 31, 2018, we completed the following three acquisitions (the “2018 Acquisitions”):
In November 2017, we completed the BAG Acquisition whereby we acquired substantially all of the assets and certain liabilities of BAG’s Hygiene Monitoring business which is comprised of the distribution of biological, chemical and cleaning indicator products;
In October 2017, we completed the Simicon Acquisition whereby we acquired the common stock of Simicon, a company whose business manufactures both biological and cleaning indicators; and
In May 2017, we completed the Hucker Acquisition whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of Hucker’s business segment associated with the distribution of our biological indicator products.
Year Ended March 31, 2017 Acquisitions
During the year ended March 31, 2017, we completed the following six acquisitions (the “2017 Acquisitions”):
In November 2016, we completed the Mydent Acquisition whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of Mydent International Corp’s business segment associated with biological indicator mail-in testing services to the dental market in the United States;
In November 2016, we completed the FreshLoc Acquisition whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of the cold chain monitoring business of FreshLoc Technologies, Inc.;
In August 2016, we completed the Rapid Aid Acquisition whereby we acquired certain assets (consisting primarily of fixed assets) and certain liabilities of Rapid Aid’s business segment associated with the manufacture and sale of cold chain packaging gel products;
In July 2016, we completed the HANSAmed Acquisition whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of HANSAmed’s business segment associated with the distribution of our biological indicator products and mail-in testing services to the dental market in Canada;
In April 2016, we completed the ATS Acquisition whereby we acquired substantially all the assets (other than cash and certain inventories and fixed assets) and certain liabilities of ATS. ATS was in the business of supplying products and services for dental sterilizer testing in both the U.S. and Canada; and
In April 2016, we completed the Pulse Acquisition whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of Pulse’s business segment associated with the distribution of our biological indicator products.
Year Ended March 31, 2016 Acquisitions
During the year ended March 31, 2016, we completed the following ten acquisitions (the “2016 Acquisitions”):
In January 2016, we completed the January 2016 European BI Distributor Acquisitions whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of the business segment associated with the distribution of our biological indicator products from CoaChrom Diagnostica GmbH of Austria and bioTRADING Benelux B.V of the Netherlands;
In October 2015, we completed the October 2015 European BI Distributor Acquisitions whereby we acquired substantially all of the assets (other than cash and accounts receivable) and certain liabilities of the business segment associated with the distribution of our biological indicator products from BIOLOGIK S.R.L.(Italy), VWR International PBI S.R.L.(Italy), Cruinn Diagnostics Ltd.(Ireland), Mecolab AG (Switzerland), Miclev Medical Products AB (Sweden) and Tiselab S.L.(Spain);
In August 2015, we completed the North Bay Acquisition whereby we acquired substantially all of the assets (other than certain fixed assets) and certain liabilities of the dental sterilizer testing business of North Bay; and
In July 2015, we completed the Infitrak Acquisition whereby we acquired all of the common stock of Infitrak, a company whose business provides consulting, packaging and measuring solutions for cold chain applications.
General Trends and Outlook
Our strategic objectives include growth both organically and through further acquisitions. During the year ended March 31, 2018, we continued to build our infrastructure to prepare for future growth, including the relocation of our Omaha manufacturing facilities into the new Bozeman building, the addition of key personnel to operations and sales and marketing functions, and the initiation of phase three of our ERP implementation project (European operations).
The markets for Sterilization and Disinfection Control products remain strong, as the disposable nature of these products makes them less sensitive to general economic conditions. The worldwide market for sterilization and disinfection control products is growing as more countries focus on verifying the effectiveness of sterilization and disinfection processes.
In general, our Instruments products and Cold Chain monitoring systems are more impacted by general economic conditions than our Sterilization and Disinfection Control and Cold Chain Packaging products. As a result, uncertainty about global economic conditions may cause businesses to postpone spending in response to tighter credit, unemployment, negative financial news and/or declines in income or asset values. Worldwide and regional economic conditions could also reduce the demand for our products and services, as our customers reduce or delay capital equipment and other types of purchases. However, demand for our instruments products, and cold chain services and monitoring systems remains solid and we strive to continue to grow revenues going forward.
Overall revenues increased by $2,514,000, while organic revenues declined one percent, for the year ended March 31, 2018 resulting from organic decreases of one, three and 27 percent from the Instruments, Cold Chain Monitoring and Cold Chain Packaging Divisions, respectively, partially offset by an increase of six percent for the Sterilization and Disinfection Control Division.
At the beginning of our year ended March 31, 2018, we elected to discontinue for sale certain products in our Instruments, Cold Chain Monitoring and Sterilization and Disinfection Control Divisions due to the recent introduction of new or modified products and the consolidation of other product sets. As part of this process, we analyzed the remaining inventories associated with these products to determine future usability and reserved against what we believed to be excess or obsolete, resulting in an increase in our inventory reserve of $406,000 (of which $216,000 related to the Cold Chain Monitoring Division). At this time, we also established a plan to liquidate certain Cold Chain Monitoring raw material components related to the above-mentioned discontinued products. During the second and third quarters, we subjected additional inventories to our liquidation program due to the discontinuance or winding-down of additional older product sets resulting from the release of our new ViewPoint operating platform. During the three months ended December 31, 2017, it became evident that our liquidation program was ineffective, and we determined that a significant amount of these inventories was not recoverable as previously planned. As such, we increased our Cold Chain Monitoring inventory reserve by $1,700,000. Company-wide gross margin percentage for the year ended March 31, 2018 was 57 percent, but would have been 59 percent, without the impact of these additional inventory reserves. Additional changes to our reserve for inventories were related to normal business operations (please see Note 3 of Notes to Consolidated Financial Statements contained in “Item. 8. Financial Statements and Supplementary Data”).
During the nine months ended December 31, 2017, revenues in our Cold Chain Packaging Division decreased significantly as compared to the same period in the prior year primarily due to a significant decrease in revenues from our largest customer. Due to this event, we believed that revenues for this segment would be approximately $2,250,000 to $2,750,000 lower for the year ending March 31, 2018 as compared to the year ended March 31, 2017. During the three months ended December 31, 2017 we completed a detailed review of the cold chain packaging business and concluded that long and difficult sales-cycles associated with this product set, when coupled with higher than previously contemplated costs for operating and expanding the necessary infrastructure to support revenues growth, have resulted in a forecast of lower than expected revenues, gross margin percentages and overall profitability as compared to our original model for this business. Based on these facts, we concluded that we had a triggering event requiring assessment of impairment for certain of our long-lived assets associated with the Cold Chain Packaging Division. As a result, we reviewed the long-lived assets associated with this reporting segment and recorded a $13,819,000 impairment charge related to goodwill, which is included in impairment charge on goodwill on the accompanying consolidated statements of operations for the year ended March 31, 2018.
We continue to monitor the operational results of our Cold Chain Packaging Division and if revenues, gross margin percentage and overall profitability fail to meet our revised projections, the remaining long-lived assets (including $1,401,000 of Goodwill and $4,081,000 of intangible assets as of March 31, 2018, respectively) could be subject to further impairment losses.
While we believe that the revenues from our largest customer will revert back to a normal run rate for our year ending March 31, 2019, we will continue to focus on growing the business in the future through new customer acquisition and implementing changes to optimize the underlying gross and operating margins. We believe that these steps will help drive the business to a level of profitability and performance in line with our expectations, however there is no guarantee that we will be successful. If, in the future, we determine that successfully trending toward our goals for this segment is not likely, we will, at that time, consider any and all available avenues including a potential disposition of this business segment.
Results of Operations
The following table sets forth, for the periods indicated, condensed consolidated statements of operations data. The table and the discussion below should be read in conjunction with the accompanying consolidated financial statements and the notes thereto appearing elsewhere in “Item 8. Financial Statements and Supplementary Data” (in thousands, except percent data):
|
Year Ended March 31, |
2018 vs 2017 |
2017 vs 2016 |
||||||||||||||||||||||||||
|
2018 |
2017 |
2016 |
Change |
Percent Change |
Change |
Percent Change |
||||||||||||||||||||||
|
Revenues |
$ | 96,179 | $ | 93,665 | $ | 84,659 | $ | 2,514 | 3 | % | $ | 9,006 | 11 | % | ||||||||||||||
|
Cost of revenues |
41,560 | 40,426 | 33,246 | 1,134 | 3 | % | 7,180 | 22 | % | |||||||||||||||||||
|
Gross profit |
$ | 54,619 | $ | 53,239 | $ | 51,413 | $ | 1,380 | 3 | % | $ | 1,826 | 4 | % | ||||||||||||||
|
Gross profit margin |
57 | % | 57 | % | 61 | % | -- | % | (4 | )% | ||||||||||||||||||
|
Operating Expenses: |
||||||||||||||||||||||||||||
|
Selling |
$ | 8,823 | $ | 9,955 | $ | 7,500 | $ | (1,132 | ) | (11 | )% | $ | 2,455 | 33 | % | |||||||||||||
|
General and administrative |
26,255 | 22,814 | 23,618 | 3,441 | 15 | % | (804 | ) | (3 | )% | ||||||||||||||||||
|
Research and development |
3,539 | 4,157 | 3,972 | (618 | ) | (15 | )% | 185 | 5 | % | ||||||||||||||||||
|
Impairment loss on goodwill |
13,819 | -- | -- | 13,819 | 100 | % | -- | -- | % | |||||||||||||||||||
|
Total operating expenses |
$ | 52,436 | $ | 36,926 | $ | 35,090 | $ | 15,510 | 42 | % | $ | 1,836 | 5 | % | ||||||||||||||
|
Operating income |
$ | 2,183 | $ | 16,313 | $ | 16,323 | $ | (14,130 | ) | (87 | )% | $ | (10 | ) | -- | % | ||||||||||||
|
Net (loss) income |
$ | (2,962 | ) | $ | 11,183 | $ | 11,169 | $ | (14,145 | ) | (126 | )% | $ | 14 | -- | % | ||||||||||||
|
Net (loss) income margin |
(3 | )% | 12 | % | 13 | % | (15 | )% | (1 | )% | ||||||||||||||||||
Revenues
The following table summarizes our revenues by source (in thousands, except percent data):
|
Year Ended March 31, |
2018 vs 2017 |
2017 vs 2016 |
||||||||||||||||||||||||||
|
2018 |
2017 |
2016 |
Change |
Percent Change |
Change |
Percent Change |
||||||||||||||||||||||
|
Sterilization and Disinfection Control |
||||||||||||||||||||||||||||
|
Product |
$ | 36,500 | $ | 32,195 | $ | 30,348 | $ | 4,305 | 13 | % | $ | 1,847 | 6 | % | ||||||||||||||
|
Service |
6,760 | 6,440 | 3,301 | 320 | 5 | % | 3,139 | 95 | % | |||||||||||||||||||
| 43,260 | 38,635 | 33,649 | 4,625 | 12 | % | 4,986 | 15 | % | ||||||||||||||||||||
|
Instruments |
||||||||||||||||||||||||||||
|
Product |
24,660 | 25,152 | 25,957 | (492 | ) | (2 | )% | (805 | ) | (3 | )% | |||||||||||||||||
|
Service |
9,444 | 9,253 | 9,735 | 191 | 2 | % | (482 | ) | (5 | )% | ||||||||||||||||||
| 34,104 | 34,405 | 35,692 | (301 | ) | (1 | )% | (1,287 | ) | (4 | )% | ||||||||||||||||||
|
Cold Chain Monitoring |
||||||||||||||||||||||||||||
|
Product |
5,948 | 6,916 | 6,508 | (968 | ) | (14 | )% | 408 | 6 | % | ||||||||||||||||||
|
Service |
7,030 | 5,668 | 5,058 | 1,362 | 24 | % | 610 | 12 | % | |||||||||||||||||||
| 12,978 | 12,584 | 11,566 | 394 | 3 | % | 1,018 | 9 | % | ||||||||||||||||||||
|
Cold Chain Packaging |
||||||||||||||||||||||||||||
|
Product |
5,064 | 6,792 | 3,461 | (1,728 | ) | (25 | )% | 3,331 | 96 | % | ||||||||||||||||||
|
Service |
773 | 1,249 | 291 | (476 | ) | (38 | )% | 958 | 329 | % | ||||||||||||||||||
| 5,837 | 8,041 | 3,752 | (2,204 | ) | (27 | )% | 4,289 | 114 | % | |||||||||||||||||||
|
Total |
$ | 96,179 | $ | 93,665 | $ | 84,659 | $ | 2,514 | 3 | % | $ | 9,006 | 11 | % | ||||||||||||||
Year ended March 31, 2018 versus March 31, 2017
Sterilization and Disinfection Control revenues increased 12 percent, primarily due to the 2018 Acquisitions and organic growth of six percent, which was achieved through existing customers, expansion into new markets, price increases and strengthening of the Euro.
Instruments revenues decreased by one percent, primarily due to the slower than expected adoption of an updated medical product, although we realized a normalization of the adoption rate of this product towards the end of the year.
Cold Chain Monitoring revenues increased three percent primarily due to the FreshLoc Acquisition, partially offset by organic decreases of three percent. Revenues in this division fluctuate quarter over quarter due to the timing of customer acceptance of certain installations and the nature and timing of orders within any given quarter.
Cold Chain Packaging revenues decreased by 27 percent primarily due to a lower order rate based on timing issues with our largest customer (which accounted for approximately half of division revenues for the year ended March 31, 2017) and longer than expected sales cycles. We noted that the order rate from our largest customer did begin to normalize during the three months ending March 31, 2018 and we expect it to continue to do so throughout our next fiscal year. See General Trends and Outlook above for additional discussion.
Year ended March 31, 2017 versus March 31, 2016
Sterilization and Disinfection Control revenues increased as a result of the North Bay, October 2015 European BI Distributor, January 2016 European BI Distributor, Pulse, ATS, HANSAmed and Mydent Acquisitions, and organic growth of seven percent which was achieved through existing customers, expansion into new markets and price increases.
Instruments revenues decreased by four percent. The decrease was due primarily to the impact of a large one-time order during the year ended March 31, 2016 that was not replicated during the year ended March 31, 2017 and the timing of other orders related to the same product being accelerated into the fourth quarter of the year ended March 31, 2016 which resulted in lower orders for this same product during the first quarter of the year ended March 31, 2017.
Cold Chain Monitoring revenues increased as a result of the FreshLoc Acquisition, partially offset by an organic decrease of two percent.
Cold Chain packaging revenues increased primarily due to organic growth of 85 percent which was achieved through existing and new customers.
Gross Profit
The following table summarizes our gross profit by operating segment (in thousands, except percent data):
|
Year Ended March 31, |
2018 vs 2017 |
2017 vs 2016 |
||||||||||||||||||||||||||
|
2018 |
2017 |
2016 |
Change |
Percent Change |
Change |
Percent Change |
||||||||||||||||||||||
|
Sterilization and Disinfection Control |
$ | 29,333 | $ | 25,674 | $ | 22,205 | $ | 3,659 | 14 | % | $ | 3,469 | 16 | % | ||||||||||||||
|
Gross profit margin |
68 | % | 66 | % | 66 | % | 2 | % | -- | % | ||||||||||||||||||
|
Instruments |
$ | 20,395 | $ | 21,037 | $ | 23,223 | $ | (642 | ) | (3 | )% | $ | (2,186 | ) | (9 | )% | ||||||||||||
|
Gross profit margin |
60 | % | 61 | % | 65 | % | (1 | )% | (4 | )% | ||||||||||||||||||
|
Cold Chain Monitoring |
$ | 3,854 | $ | 4,557 | $ | 4,201 | $ | (703 | ) | (15 | )% | $ | 356 | 8 | % | |||||||||||||
|
Gross profit margin |
30 | % | 36 | % | 36 | % | (6 | )% | -- | % | ||||||||||||||||||
|
Cold Chain Packaging |
$ | 1,037 | $ | 1,971 | $ | 1,784 | $ | (934 | ) | (47 | )% | $ | 187 | 10 | % | |||||||||||||
|
Gross profit margin |
18 | % | 25 | % | 48 | % | (7 | )% | (23 | )% | ||||||||||||||||||
|
Total gross profit |
$ | 54,619 | $ | 53,239 | $ | 51,413 | $ | 1,380 | 3 | % | $ | 1,826 | 4 | % | ||||||||||||||
|
Gross profit margin |
57 | % | 57 | % | 61 | % | -- | % | (4 | )% | ||||||||||||||||||
Year ended March 31, 2018 versus March 31, 2017
Sterilization and Disinfection Control gross profit margin percentage increased primarily due to volume-based efficiencies associated with increased revenues and the impact of using internally manufactured biological indicators for our dental sterilizer testing business as opposed to the prior year where we were contractually committed to purchase a significant portion of those biological indicators from an outside supplier at a significantly higher price. Included in gross profit margin percentage are $573,000 and $680,000 of Bozeman relocation costs for the years ended March 31, 2018 and 2017, respectively (see Liquidity and Capital Resources for additional discussion). Without these costs, gross margin percentages would have been 69 percent and 68 percent for the years ended March 31, 2018 and 2017, respectively.
Instruments gross margin percentage decreased by one percent, primarily due to product and service mix and the loss of certain volume-based efficiencies associated with a decrease in revenues and a $163,000 increase in the related inventory reserve due to the decision to discontinue for sale certain instruments products.
Cold Chain Monitoring gross profit margin percentage decreased primarily due to a $1,916,000 increase in the related inventory reserve (see General Trends and Outlook above for additional discussion), partially offset by product and service mix. Excluding the impact of these additional reserves for inventory, gross profit percentage would have been 45 percent for the year ended March 31, 2018.
Cold Chain Packaging gross profit margin percentage decreased primarily due to lower revenues. A certain portion of the cost of revenues are personnel and warehousing costs which are primarily fixed and as a result, fluctuations in revenues significantly impact the gross profit margin percentage for this division. See General Trends and Outlook above for additional discussion.
Year ended March 31, 2017 versus March 31, 2016
Sterilization and Disinfection Control gross profit margin percentage was flat as compared to the year ended March 31, 2016. Included in the gross profit margin are $680,000 of relocation costs (see Liquidity and Capital Resources for additional discussion) that decreased the gross margin percentage by two percentage points. In addition, after the completion of the North Bay Acquisition, we were contractually committed to purchase from a third party a significant portion of the BI’s that were used in the acquired North Bay dental sterilizer testing business which negatively impacted our gross margin percentage. The contractual commitment gradually decreased each quarter after the acquisition and was completed during the three months ended December 31, 2016. Each quarterly decrease in these purchases allowed for the additional use of internally produced BI’s which resulted in greater gross margin percentages.
Instruments gross margin percentage decreased as a result of product and services mix and the loss of certain volume-based efficiencies associated with the decrease in revenues in one product line (see Revenues for additional discussion).
Cold Chain Monitoring gross profit margin percentage was flat as compared to the year ended March 31, 2016. Gross profit margin percentage increased as a result of the product and service revenues mix along with the impact of the FreshLoc Acquisition but was offset by a $580,000 expense related to a reserve for slow moving inventory associated with a specific model of our cold chain monitoring sensors.
Cold Chain Packaging gross profit margin decreased primarily as a result of increased revenues from a large customer contract with higher than normal discount rates. We expect that our Cold Chain Packaging gross profit margin percentage will continue to be lower than the historical results of our other segments due to the nature of these products.
Operating Expenses
The following table summarizes the change in our operating expenses (in thousands):
|
Increase (Decrease) |
||||||||
|
Year Ended March 31, |
||||||||
|
2018 vs 2017 |
2017 vs 2016 |
|||||||
|
Selling |
$ | (1,132 | ) | $ | 2,455 | |||
|
General and administrative |
||||||||
|
ERP system implementation |
-- | (515 | ) | |||||
|
Legal costs and litigation settlement |
4 | (1,718 | ) | |||||
|
Amortization |
479 | 663 | ||||||
|
Personnel |
1,465 | 724 | ||||||
|
Professional services |
(244 | ) | 10 | |||||
|
Employee moving |
525 | -- | ||||||
|
Banking fees |
144 | 129 | ||||||
|
Depreciation |
116 | 279 | ||||||
|
Property taxes |
221 | -- | ||||||
|
Investor relations |
164 | -- | ||||||
|
Medical device excise tax |
-- | (245 | ) | |||||
|
Acquisition related |
176 | -- | ||||||
|
Other, net |
391 | (131 | ) | |||||
| 3,441 | (804 | ) | ||||||
|
Research and development |
(618 | ) | 185 | |||||
|
Impairment loss on goodwill |
13,819 | -- | ||||||
|
Operating expenses |
$ | 15,510 | $ | 1,836 | ||||
Selling
Year ended March 31, 2018 versus March 31, 2017
Selling expense decreased primarily due to reductions of selling personnel, trade show activities and outside commissions. As a percentage of revenues, selling expense was nine percent as compared to 11 percent in the prior year.
Year ended March 31, 2017 versus March 31, 2016
Selling expense increased primarily due to additional personnel related to the 2017 and 2016 Acquisitions. As a percentage of revenues, selling expense was 11 percent as compared to nine percent in the prior year.
Included in the increase of selling expenses is $900,000 of U.S. Cold Chain Packaging sales personnel hired during the year ended March 31, 2017. We are continuing to make an investment to grow this division and are hopeful that increases in related revenues will continue to be realized during the year ending March 31, 2018.
General and Administrative
Year ended March 31, 2018 versus March 31, 2017
General and administrative expense increased primarily due to increases in personnel (including those associated with the 2017 Acquisitions), amortization and employee moving costs, partially offset by a decrease in professional services expenses.
Year ended March 31, 2017 versus March 31, 2016
General and administrative expenses decreased primarily due to the prior year $1,709,000 charge related to the Amato Settlement and a decrease in ERP system implementation charges for the year ended March 31, 2017, partially offset by increases in amortization, personnel and depreciation costs for the year ended March 31, 2017.
Research and Development
Year ended March 31, 2018 versus March 31, 2017
Research and development costs decreased primarily due to a streamlining of the necessary engineers and materials and supplies required to support existing businesses.
Year ended March 31, 2017 versus March 31, 2016
Research and development expenses were essentially flat.
Impairment Loss on Goodwill
Year ended March 31, 2018 versus March 31, 2017
Impairment loss on goodwill is associated with our Packaging Division. See General Trends and Outlook above for additional discussion.
Other Expense, net
Other expense, net for the year ended March 31, 2018 is comprised primarily of interest expense associated with our Credit Facility and $300,000 related to an additional accrual for the PCD earn-out (see Liquidity and Capital Resources for additional discussion), partially offset by a $116,000 gain from the sale of our Omaha facility. Other expense, net for the year ended March 31, 2017 is comprised primarily of interest expense associated with our Credit Facility and $450,000 related to an additional accrual for the PCD earn-out (see Liquidity and Capital Resources for additional discussion). Other expense, net for the year ended March 31, 2016 is comprised primarily of interest expense associated with our Credit Facility.
Net Income
Our income tax rate varies based upon many factors (please see Note 11 of Notes to Consolidated Financial Statements contained in “Item 8. Financial Statements and Supplementary Data”). Net income for the year ended March 31, 2018 was significantly impacted by a $13,819,000 impairment loss on goodwill (see General Trends and Outlook above for additional discussion), $842,000 of facility relocation costs (see Liquidity and Capital Resources), $300,000 in PCD earn-out accruals, $256,000 of employee moving expenses not related to the Bozeman facility relocation and a $2,106,000 expense related to a reserve for inventory due to operational decisions to end of life certain products and other slow moving inventory (see General Trends and Outlook above for additional discussion). Net income for the year ended March 31, 2017 was significantly impacted by $725,000 of relocation costs (see liquidity and capital resources), $450,000 in PCD earn-out accruals and a $580,000 expense related to a reserve for slow moving inventory in our Cold Chain Monitoring Division. Net income for the year ended March 31, 2016 was significantly impacted by the $1,709,000 Amato Settlement. Otherwise, net income for the years ended March 31, 2018, 2017 and 2016 varied with the changes in revenues, gross profit and operating expenses (which includes $6,929,000, $6,450,000 and $5,787,000 of non-cash amortization of intangible assets, respectively).
Liquidity and Capital Resources
Our sources of liquidity include cash generated from operations, working capital and capacity under our Credit Facility. In addition, we believe that we have the ability to access the public equity and/or debt markets to raise capital in order to further fund our acquisition program. If we continue to acquire companies at our recent historical rate, it is possible, if not likely that at some point in the future we will be required to access the above mentioned public equity and/or debt markets. Our more significant uses of resources include quarterly dividends to shareholders, payment of debt obligations, long-term capital equipment expenditures and potential acquisitions. In addition, we are at times also subject to outstanding legal proceedings which could ultimately require the use of cash (Please see “Item 3. Legal Proceedings” for additional discussion).
Due to continued organic and acquisition related growth, we outgrew the capacity of our current building in Bozeman, Montana and as a result, we built a new facility in the same general area. Construction began in July 2015 and was completed in September 2017. We spent $17,650,000 on the development of the building and the related land, which is included in property, plant and equipment, net on the accompanying condensed consolidated balance sheets.
In August 2016, we announced that we plan to shut down both our Omaha and Traverse City manufacturing facilities and relocate those operations to the new Bozeman building. The move of these two facilities, along with the current Bozeman operations, began in March 2017 and is estimated to be completed by June 30, 2018. We estimate that the total costs of the relocation will be $2,000,000, which is comprised primarily of facility moving expenses, retention bonuses for existing personnel and payroll costs for duplicative personnel during the transition period. We incurred $842,000 and $725,000 of these relocation expenses during the years ended March 31, 2018 and 2017, respectively which are reflected in cost of revenues in the accompanying consolidated statements of operations (other than $269,000 and $45,000, respectively which are included in general and administrative expenses).
In July 2017, we completed the move from the Omaha facility and subsequently sold that building for $1,116,000 (net of commission costs). After completing the move of the old Bozeman facility (which is anticipated to be completed by June 30, 2018), we expect to be able to sell that building for approximately $2,000,000.
Working capital is the amount by which current assets exceed current liabilities. We had working capital of $14,698,000 and $19,218,000, respectively, at March 31, 2018 and 2017.
On March 1, 2017, we entered into a five-year agreement (the “Credit Facility”) for a $80,000,000 revolving line of credit (“Line of Credit”), a $20,000,000 term loan (“Term Loan”) and up to $2,500,000 of letters of credit with a banking syndicate comprised of four banks. In addition, the Credit Facility provides a post-closing accordion feature which allows the Company to request to increase the Line of Credit or Term Loan up to an additional $100,000,000.
Line of Credit and Term Loan indebtedness bears interest at either: (1) LIBOR, as defined, plus an applicable margin ranging from 1.5% to 2.50%; or (2) the alternate base rate (“ABR”), which is the greater of JPMorgan’s prime rate or the federal funds effective rate or the overnight bank funding rate plus 0.5%. We elect the interest rate with each borrowing under the line of credit. In addition, there is an unused line fee of 0.15% to 0.35%. Letter of credit fees are based on the applicable LIBOR rate.
The Term Loan requires 20 quarterly principal payments (the first due date was March 31, 2017) in the amount of $250,000 (increasing by $125,000 each year up to $750,000 in the fifth year). The remaining balance of principal and accrued interest are due on March 1, 2022.
The Credit Facility is secured by all of our assets and requires us to maintain a ratio of funded debt to our trailing four quarters of EBIDTA (the “Leverage Ratio”), as defined, of less than 3.0 to 1.0, provided that, we may once during the term of the Credit Facility, in connection with a Permitted Acquisition for which the aggregate consideration paid or to be paid in respect thereof equals or exceeds $20,000,000, elect to increase the maximum Leverage Ratio permitted hereunder to (i) 3.50 to 1.00 for a period of four consecutive fiscal quarters commencing with the fiscal quarter in which such Permitted Acquisition occurs (the “Initial Holiday Period”) and (ii) 3.25 to 1.00 for the period of four consecutive fiscal quarters immediately following the Initial Holiday Period. The Credit Facility also requires us to maintain a minimum fixed charge coverage ratio of less than 1.25 to 1.0.
As of May 31, 2018, we had $43,625,000 in outstanding indebtedness and unused capacity under our Credit Facility of $55,000,000.
We routinely evaluate opportunities for strategic acquisitions. Future material acquisitions may require that we obtain additional capital, assume third party debt or incur other long-term obligations. We believe that we have the option to utilize both equity and debt instruments as vehicles for the long-term financing of our investment activities and acquisitions.
On November 7, 2005, our Board of Directors authorized a program to repurchase up to 300,000 shares of our outstanding common stock. Under the program, the shares may be purchased from time to time in the open market at prevailing prices or in negotiated transactions off the market. Shares purchased are canceled and repurchases are made with existing cash reserves. We do not maintain a set policy or schedule for our buyback program. We have purchased 162,486 shares of common stock under this program from inception through March 31, 2018.
We have paid regular quarterly dividends since 2003. Dividends per share paid by quarter were as follows:
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
First quarter |
$ | 0.16 | $ | 0.16 | $ | 0.16 | ||||||
|
Second quarter |
0.16 | 0.16 | 0.16 | |||||||||
|
Third quarter |
0.16 | 0.16 | 0.16 | |||||||||
|
Fourth quarter |
0.16 | 0.16 | 0.16 | |||||||||
In April 2018, our Board of Directors declared a quarterly cash dividend of $0.16 per share of common stock, payable on June 15, 2018, to shareholders of record at the close of business on May 31, 2018.
Cash Flow – Operating, investing and financing activities were as follows (in thousands):
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
Net cash provided by operating activities |
$ | 24,814 | $ | 7,750 | $ | 16,903 | ||||||
|
Net cash used in investing activities |
(17,184 | ) | (18,405 | ) | (31,840 | ) | ||||||
|
Net cash (used in) provided by financing activities |
(8,119 | ) | 10,708 | 18,620 | ||||||||
Net cash provided by operating activities increased for the year ended March 31, 2018 primarily due to a reduction in inventories of $2,286,000 (net of the impact of increases in the reserve for inventories) and the payment of $9,554,000 of contingent consideration in the prior year. Net cash provided by operating activities for the year ended March 31, 2017 decreased primarily due to the payment of $9,554,000 in contingent consideration and $3,066,000 in accrued salaries, taxes and various other accrued expenses, partially offset by increases in collections of accounts receivable of $994,000. Net cash provided by operating activities for the year ended March 31, 2016 increased primarily due to the efficient management of working capital.
Net cash used in investing activities for the year ended March 31, 2018 resulted from $15,518,000 associated with the 2018 Acquisitions and the purchase of $2,799,000 of property, plant and equipment, partially offset by $1,133,000 of proceeds associated with the sale of the Omaha facility. Net cash used in investing activities for the year ended March 31, 2017 resulted from $6,800,000 associated with the 2017 Acquisitions and the purchase of $11,605,000 of property, plant and equipment. Net cash used in investing activities for the year ended March 31, 2016 resulted from $24,111,000 associated with the 2016 Acquisitions and the purchase of $7,729,000 of property, plant and equipment.
Net cash used in financing activities for the year ended March 31, 2018 resulted from the repayment of debt of $19,625,000 and the payment of dividends of $2,413,000, partially offset by borrowings under our Credit Facility of $11,000,000 and proceeds from the exercise of stock options of $2,919,000. Net cash provided by financing activities for the year ended March 31, 2017 resulted from borrowings under our Credit Facility of $66,550,000 and proceeds from the exercise of stock options of $3,513,000, partially offset by the repayment of debt of $57,000,000 and the payment of dividends of $2,355,000. Net cash provided by financing activities for the year ended March 31, 2016 resulted from borrowings under our Credit Facility of $25,000,000 and proceeds from the exercise of stock options of $1,923,000, partially offset by the repayment of debt of $6,000,000 and the payment of dividends of $2,303,000.
At March 31, 2018, we had contractual obligations for open purchase orders of $439,000 for routine purchases of supplies and inventory, which are payable in less than one year.
Under the terms of the PCD Agreement, we were required to pay contingent consideration if the cumulative revenues for our process challenge device business for the three years subsequent to the acquisition met certain levels. The potential consideration payable ranged from $0 to $1,500,000 and was based upon a sliding scale of three-year cumulative revenues between $9,900,000 and $12,600,000, with payments made annually. Based upon both historical and projected growth rates, we initially recorded $300,000 of contingent consideration payable which represented our best estimate of the amount that would ultimately be paid. We paid $150,000 of the contingent consideration during the year ended March 31, 2016 (based upon the then current run rate projected over the entire three-year contingent consideration period).
Since the initial payment, the revenues for these products significantly increased and as a result, during the year ended March 31, 2017 we recorded an additional $450,000 accrual (which was paid in our third quarter ending December 31, 2016). During the year ended March 31, 2018, process challenge device (“PCD”) product revenues continued to increase which resulted in an additional $300,000 accrual, which is included in other income, net in the accompanying consolidated statement of operations for the year ended March 31, 2018. We paid the remaining contingent consideration due of $450,000 in November 2017.
Under the terms of the Infitrak Agreement, we were required to pay contingent consideration if the gross profit (as defined in the Infitrak Earn-Out Agreement) for our cold chain packaging business for the two years subsequent to the acquisition met certain levels. The potential undiscounted consideration payable ranged from $0 to $15,000,000 CDN and was based upon a sliding scale of growth in gross profit (as defined in the Infitrak Earn-Out Agreement) for year one and year two of 30 to 70 percent and 15 to 75 percent, respectively. Based upon both historical and projected growth rates, we recorded $9,271,000 of contingent consideration payable which represented our best estimate of the then current fair value of the amount that would ultimately be paid. Any changes to the contingent consideration ultimately paid would have resulted in additional income or expense in our consolidated statements of income.
In July 2016, we made the first Earn-Out payment in the amount of $6,000,000 CDN ($4,594,000). In March 2017, we agreed to settle the remaining earn-out obligation (which was originally due in the second quarter of our year ending March 31, 2018) early by making a payment of $6,000,000 CDN ($4,558,000).
Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States, which require management to make estimates, judgments, and assumptions that affect the amounts reported in our consolidated financial statements and accompanying notes. We believe that the following are the more critical judgment areas in the application of our accounting policies that currently affect our financial condition and results of operations. Management has discussed the development, selection, and disclosure of critical accounting policies and estimates with the Audit Committee of our Board of Directors. While our estimates and assumptions are based on our knowledge of current events and actions we may undertake in the future, actual results may ultimately differ from these estimates and assumptions. For a discussion of our significant accounting policies, please see Note 1 of Notes to Consolidated Financial Statements contained in “Item 8. Financial Statements and Supplementary Data.”
Accounts Receivable
We estimate an allowance for doubtful accounts based on overall historic write-offs, the age of our receivable balances, and the payment history and creditworthiness of the customer. If actual results are not consistent with our assumptions and judgments or our assumptions and estimates change due to new information, we may experience material changes in our allowance for doubtful accounts and bad debt expense.
Inventories
Inventories are stated at the lower of cost or market, using the weighted average method to determine cost. We evaluate labor and overhead costs annually, unless specific circumstances necessitate a mid-year evaluation for specific items. Our work in process and finished goods inventory includes raw materials, labor and overhead, which are estimated based on trailing twelve months of expense and standard labor hours for each product. Our biological indicator inventory is tracked by lot number thus labor is generally based on actual hours.
We monitor inventory cost compared to selling price in order to determine if a lower of cost or market reserve is necessary. Throughout the year, we perform various physical cycle count procedures on our inventories and we estimate and maintain an inventory reserve, as needed, for such matters as obsolete inventory, shrink and scrap. This reserve may fluctuate as our assumptions change due to new information, discrete events, or changes in our business, such as entering new markets or discontinuing a specific product.
Recoverability of Long-lived Assets
For property, plant and equipment, and intangible assets subject to amortization, recoverability and/or impairment tests are required only when conditions exist that indicate the carrying value may not be recoverable. We monitor the same conditions for our goodwill, but an annual evaluation is required.
Monitoring these conditions requires significant management judgment, including evaluating general economic conditions, industry and market considerations, changes in production costs, cash flow trends, and other relevant entity-specific events such as changes in management, key personnel, strategy or customers.
If conditions exist that indicate the carrying value may not be recoverable, we would be required to estimate the fair value of the asset, asset group, or reporting unit. We determine fair value using widely accepted valuation techniques, primarily discounted cash flow and market multiple analyses. These techniques are also used when initially allocating the purchase price to acquired assets and liabilities. These types of analyses require us to make assumptions and estimates regarding industry and economic factors, the profitability of future business strategies, and cash flow.
We recorded an impairment charge related to goodwill associated with the Cold Chain Packaging Division during the year ended March 31, 2018 (for additional discussion, please see Note 5 of Notes to Consolidated Financial Statements contained in “Item 8. Financial Statements and Supplementary Data”).
We did not record any impairment charges for the years ended March 31, 2017 or 2016. If actual results are not consistent with our assumptions and estimates, or our assumptions and estimates change due to new information, we may be exposed to an impairment charge in the future.
Purchase Accounting for Acquisitions
We apply the acquisition method of accounting for a business combination. In general, this methodology requires companies to record assets acquired and liabilities assumed at their respective fair values at the date of acquisition. Any amount of the purchase price paid that is in excess of the estimated fair value of the net assets acquired is recorded as goodwill. For certain acquisitions, we also record a liability for contingent consideration based on estimated future business performance. We monitor our assumptions surrounding these estimated future cash flows and, if there is a significant change, would record an adjustment to the contingent consideration liability and a corresponding adjustment to either income or expense.
We determine fair value using widely accepted valuation techniques, primarily discounted cash flow and market multiple analyses. These types of analyses require us to make assumptions and estimates regarding industry and economic factors, the profitability of future business strategies, discount rates and cash flow.
If actual results are not consistent with our assumptions and estimates, or our assumptions and estimates change due to new information, we may be exposed to an impairment charge in the future. If the contingent consideration paid for any of our acquisitions differs from the amount initially recorded, we would record either income or expense.
Stock-based Compensation – Stock Options
We estimate the fair value of option grants using the Black-Scholes model, which requires us to estimate the volatility and forfeiture rate. Under our current stock-based compensation plan, we recognize the expense on a straight-line basis over the service period.
Income Taxes
Income tax expense includes U.S., state, local and international income taxes, plus a provision for U.S. taxes on undistributed earnings of foreign subsidiaries and other prescribed foreign entities not deemed to be indefinitely reinvested. Deferred tax assets and liabilities are recognized for the tax consequences of temporary differences between the financial reporting basis and the tax basis of existing assets and liabilities. The tax rate used to determine the deferred tax assets and liabilities is the enacted tax rate for the year and manner in which the differences are expected to reverse. Valuation allowances are recorded to reduce deferred tax assets to the amount that will more likely than not be realized.
We are involved in various tax matters, with respect to some of which the outcome is uncertain. We establish reserves to remove some or all of the tax benefit of any of our tax positions at the time we determine that it becomes uncertain based upon one of the following conditions: (1) the tax position is not "more likely than not" to be sustained, (2) the tax position is "more likely than not" to be sustained, but for a lesser amount, or (3) the tax position is "more likely than not" to be sustained, but not in the financial period in which the tax position was originally taken. For purposes of evaluating whether or not a tax position is uncertain, (1) we presume the tax position will be examined by the relevant taxing authority that has full knowledge of all relevant information; (2) the technical merits of a tax position are derived from authorities such as legislation and statutes, legislative intent, regulations, rulings and case law and their applicability to the facts and circumstances of the tax position; and (3) each tax position is evaluated without consideration of the possibility of offset or aggregation with other tax positions taken. A number of years may elapse before a particular uncertain tax position is audited and finally resolved or when a tax assessment is raised. The number of years subject to tax assessments varies depending on the tax jurisdiction. The tax benefit that has been previously reserved because of a failure to meet the "more likely than not" recognition threshold would be recognized in income tax expense in the first interim period when the uncertainty disappears under any one of the following conditions: (1) the tax position is "more likely than not" to be sustained, (2) the tax position, amount, and/or timing is ultimately settled through negotiation or litigation, or (3) the statute of limitations for the tax position has expired. Please see Note 11 of Notes to Consolidated Financial Statements contained in “Item 8. Financial Statements and Supplementary Data” for additional discussion.
Contingent Liabilities
We accrue a loss for contingencies if it is probable that an asset has been impaired or a liability has been incurred, and when the amount of loss can be reasonably estimable. When no accrual is made because one or both of these conditions does not exist, we disclose the contingency if there is at least a reasonable possibility that a loss may be incurred. We estimate contingent liabilities based on the best information available at the time. If there is a range of possible outcomes, we accrue the low end of the range.
Recent Accounting Standards and Pronouncements
In May 2014, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2014-09, Revenue from Contracts with Customers (Topic 606), which will replace most existing revenue recognition guidance in U.S. GAAP and is intended to improve and converge with international standards the financial reporting requirements for revenue from contracts with customers. The core principle of ASU 2014-09 is that an entity should recognize revenue for the transfer of goods or services equal to the amount that it expects to be entitled to receive for those goods or services. ASU 2014-09 also requires additional disclosures about the nature, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments. ASU 2014-09 allows for adoption either on a full retrospective basis to each prior reporting period presented or on a modified retrospective basis with the cumulative effect of initially applying the new guidance recognized at the date of initial application, which will be effective for the Company beginning April 1, 2018.
We will adopt ASU 2014-09 and its amendments on a modified retrospective basis effective April 1, 2018. We have identified and implemented minor necessary changes to our accounting policies and practices, business processes, and systems and controls as well as designed and implemented specific controls over our evaluation of the impact of the new guidance on the Company, including the cumulative effect calculation, disclosure requirements and the collection of relevant data into the reporting process. We are substantially complete with the process of finalizing changes to our business processes and systems and controls to support recognition and new required disclosures under the new revenue standard. While we expect the impact of the new standard on the amount and timing of revenue recognized in the year ending March 31, 2019 to be insignificant, our assessment will be finalized during the first quarter of our year ending March 31, 2019.
In February 2016, the FASB issued ASU 2016-02, Leases, which requires lessees to recognize right-of-use assets, representing their right to use the underlying asset for the lease term, and lease liabilities on the consolidated balance sheet for all leases with terms greater than 12 months. The guidance also requires qualitative and quantitative disclosures designed to assess the amount, timing and uncertainty of cash flows arising from leases. We have initiated our plan for the adoption and implementation of this new accounting standard, including assessing our lease arrangements, evaluating practical expedient and accounting policy elections. The standard requires the use of a modified retrospective transition approach, which includes a number of optional practical expedients that entities may elect to apply. ASU 2016-02 is effective for the Company beginning April 1, 2019. We anticipate that the adoption of this new standard will not result in a significant increase in lease-related assets and liabilities on our consolidated balance sheets or significantly impact our consolidated statements of operations and cash flows.
In January 2017, the FASB issued ASU 2017-04, Intangibles – Goodwill and Other, which eliminates the requirement to calculate the implied fair value of goodwill to measure a goodwill impairment charge. ASU 2017-04 is required to be applied prospectively and we elected to early adopt ASU 2017-04 effective April 1, 2017.
Contractual Obligations, Commitments and Off-Balance Sheet Arrangements
Off-Balance Sheet Arrangements
In accordance with the definition under SEC rules, the following qualify as off-balance sheet arrangements:
|
• |
any obligation under certain guarantee contracts; |
|
• |
a retained or contingent interest in assets transferred to an unconsolidated entity or similar arrangement that serves as credit, liquidity or market risk support to that entity for such assets; |
|
• |
any obligation under certain derivative instruments; and |
|
• |
any obligation arising out of a material variable interest held by the registrant in an unconsolidated entity that provides financing, liquidity, market risk or credit risk support to the registrant, or engages in leasing, hedging or research and development services with the registrant. |
As of March 31, 2018, we have no obligations or interests which qualify as off-balance sheet arrangements.
Contractual Obligations
As of March 31, 2018, our contractual obligations, including payments due by period, are as follows (in thousands):
|
Payments Due For Years Ending March 31, |
||||||||||||||||||||
|
Total |
2019 |
2020-2021 | 2022-2023 |
Thereafter |
||||||||||||||||
|
Purchase Commitments |
$ | 439 | $ | 439 | $ | -- | $ | -- | $ | -- | ||||||||||
|
Line of Credit |
28,000 | -- | -- | 28,000 | -- | |||||||||||||||
|
Term loan |
18,625 | 1,625 | 4,750 | 12,250 | -- | |||||||||||||||
|
Other |
794 | 555 | 239 | -- | -- | |||||||||||||||
|
Total |
$ | 47,858 | $ | 2,619 | $ | 4,989 | $ | 40,250 | $ | -- | ||||||||||
Our purchase commitments consist primarily of open purchase orders, which we have established to take advantage of volume discounts for materials and to ensure a reliable supply of critical parts.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We have no derivative instruments and minimal exposure to commodity market risks. Approximately 15 percent of our revenues are exposed to foreign currency risk, of which all is within stable markets, minimizing our exposure to foreign currency fluctuations.
We are subject to interest rate volatility with regard to existing and future issuances of debt, as our current credit facility is variable-rate. Based on annualized variable-rate debt for the year ended March 31, 2018, a one percentage point increase in interest rates would have increased interest expense by $527,000.
Item 8. Financial Statements and Supplementary Data
|
Report of Independent Registered Public Accounting Firm |
36 |
|
Consolidated Balance Sheets |
38 |
|
Consolidated Statements of Operations |
39 |
|
Consolidated Statements of Comprehensive (Loss) Income |
40 |
|
Consolidated Statements of Stockholders’ Equity |
41 |
|
Consolidated Statements of Cash Flows |
42 |
|
Notes to Consolidated Financial Statements |
43 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Stockholders and Board of Directors
Mesa Laboratories, Inc.
Lakewood, Colorado
OPINIONS ON THE CONSOLIDATED FINANCIAL STATEMENTS AND INTERNAL CONTROL OVER FINANCIAL REPORTING
We have audited the accompanying consolidated balance sheets of Mesa Laboratories, Inc. (the "Company") as of March 31, 2018 and 2017, and the related consolidated statements of operations, comprehensive (loss) income, stockholders' equity, and cash flows, for each year in the three‑year period ended March 31, 2018, and the related notes and schedules (collectively referred to as the " consolidated financial statements"). We have also audited the Company's internal control over financial reporting as of March 31, 2018, based on the criteria established in Internal Control ‑ Integrated Framework: (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO").
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of March 31, 2018 and 2017, and the results of its operations and its cash flows for each year in the three‑year period ended March 31, 2018, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of March 31, 2018, based on criteria established in Internal Control ‑ Integrated Framework: (2013) issued by COSO.
BASIS FOR OPINIONS
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management's Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company's consolidated financial statements and an opinion on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As described in Management’s Report on Internal Control over Financial Reporting, management has excluded acquired entities from its assessment of internal control over financial reporting as of March 31, 2018 because they were acquired by the Company in purchase business combinations during 2018. We have also excluded these entities from our audit of internal control over financial reporting. The acquired entities represent 12% and 3% of consolidated total assets and revenues, respectively, for the year ended March 31, 2018.
DEFINITION AND LIMITATIONS OF INTERNAL CONTROL OVER FINANCIAL REPORTING
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ EKS&H LLLP
Denver, Colorado
June 5, 2018
We have served as the Company's auditor since 1986.
Mesa Laboratories, Inc.
Consolidated Balance Sheets
(In thousands, except share amounts)
|
March 31, |
||||||||
|
|
2018 |
2017 |
||||||
| ASSETS | ||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ | 5,469 | $ | 5,820 | ||||
|
Accounts receivable, less allowances of $179 and $252, respectively |
14,302 | 14,319 | ||||||
|
Inventories, net |
9,228 | 13,873 | ||||||
|
Prepaid income taxes |
273 | 587 | ||||||
|
Prepaid expenses and other |
782 | 1,186 | ||||||
|
Assets held for sale |
1,934 | -- | ||||||
|
Total current assets |
31,988 | 35,785 | ||||||
|
Deferred income taxes |
127 | -- | ||||||
|
Property, plant and equipment, net |
23,593 | 26,002 | ||||||
|
Intangibles, net |
42,850 | 37,790 | ||||||
|
Goodwill |
65,543 | 72,156 | ||||||
|
Total assets |
$ | 164,101 | $ | 171,733 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 2,380 | $ | 2,168 | ||||
|
Accrued salaries and payroll taxes |
4,284 | 4,350 | ||||||
|
Unearned revenues |
3,921 | 4,117 | ||||||
|
Current portion of contingent consideration |
709 | 1,294 | ||||||
|
Other accrued expenses |
3,363 | 2,999 | ||||||
|
Income taxes payable |
1,008 | 514 | ||||||
|
Current portion of long-term debt |
1,625 | 1,125 | ||||||
|
Total current liabilities |
17,290 | 16,567 | ||||||
|
Deferred income taxes |
2,621 | 3,554 | ||||||
|
Long-term debt, net of debt issuance costs and current portion |
44,635 | 53,675 | ||||||
|
Other long-term liabilities |
194 | 116 | ||||||
|
Total liabilities |
64,740 | 73,912 | ||||||
|
Commitments and Contingencies (Note 13) |
||||||||
|
Stockholders’ equity: |
||||||||
|
Common stock, no par value; authorized 25,000,000 shares; issued and outstanding, 3,801,439 shares (March 31, 2018) and 3,734,704 shares (March 31, 2017) |
30,516 | 25,925 | ||||||
|
Retained earnings |
68,281 | 73,656 | ||||||
|
Accumulated other comprehensive income (loss) |
564 | (1,760 | ) | |||||
|
Total stockholders’ equity |
99,361 | 97,821 | ||||||
|
Total liabilities and stockholders’ equity |
$ | 164,101 | $ | 171,733 | ||||
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Consolidated Statements of Operations
(In thousands, except per share data)
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
Revenues |
||||||||||||
|
Product |
$ | 72,172 | $ | 71,055 | $ | 66,274 | ||||||
|
Service |
24,007 | 22,610 | 18,385 | |||||||||
|
Total revenues |
96,179 | 93,665 | 84,659 | |||||||||
|
Cost of revenues |
||||||||||||
|
Cost of products |
28,608 | 26,548 | 26,957 | |||||||||
|
Cost of services |
12,952 | 13,878 | 6,289 | |||||||||
|
Total cost of revenues |
41,560 | 40,426 | 33,246 | |||||||||
|
Gross profit |
54,619 | 53,239 | 51,413 | |||||||||
|
Operating expenses |
||||||||||||
|
Selling |
8,823 | 9,955 | 7,500 | |||||||||
|
General and administrative |
26,255 | 22,814 | 23,618 | |||||||||
|
Research and development |
3,539 | 4,157 | 3,972 | |||||||||
|
Impairment loss on goodwill |
13,819 | -- | -- | |||||||||
|
Total operating expenses |
52,436 | 36,926 | 35,090 | |||||||||
|
Operating income |
2,183 | 16,313 | 16,323 | |||||||||
|
Other expense, net |
1,882 | 2,017 | 768 | |||||||||
|
Earnings before income taxes |
301 | 14,296 | 15,555 | |||||||||
|
Income taxes |
3,263 | 3,113 | 4,386 | |||||||||
|
Net (loss) income |
$ | (2,962 | ) | $ | 11,183 | $ | 11,169 | |||||
|
Net (loss) income per share: |
||||||||||||
|
Basic |
$ | (0.79 | ) | $ | 3.04 | $ | 3.10 | |||||
|
Diluted |
(0.79 | ) | 2.91 | 2.97 | ||||||||
|
Weighted average common shares outstanding: |
||||||||||||
|
Basic |
3,770 | 3,679 | 3,605 | |||||||||
|
Diluted |
3,770 | 3,844 | 3,757 | |||||||||
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Consolidated Statements of Comprehensive (Loss) Income
(In thousands except per share data)
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
Net (loss) income |
$ | (2,962 | ) | $ | 11,183 | $ | 11,169 | |||||
|
Other comprehensive income (loss), net of tax: |
||||||||||||
|
Foreign currency translation |
2,324 | (609 | ) | (917 | ) | |||||||
|
Total comprehensive (loss) income |
$ | (638 | ) | $ | 10,574 | $ | 10,252 | |||||
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Consolidated Statements of Stockholders’ Equity
(In thousands, except share amounts)
|
Common Stock |
||||||||||||||||||||
|
Number of Shares |
Amount |
Retained Earnings |
Accumulated Other Comprehensive Income (Loss) |
Total |
||||||||||||||||
|
March 31, 2015 |
3,561,540 | $ | 17,751 | $ | 55,962 | $ | (234 | ) | $ | 73,479 | ||||||||||
|
Common stock issued for conversion of stock options net of 13,491 shares returned as payment |
75,733 | 1,923 | -- | -- | 1,923 | |||||||||||||||
|
Dividends paid |
-- | -- | (2,303 | ) | -- | (2,303 | ) | |||||||||||||
| Stock-based compensation | -- | 1,327 | -- | -- | 1,327 | |||||||||||||||
|
Foreign currency translation |
-- | -- | -- | (917 | ) | (917 | ) | |||||||||||||
|
Net income |
-- | -- | 11,169 | -- | 11,169 | |||||||||||||||
|
March 31, 2016 |
3,637,273 | 21,001 | 64,828 | (1,151 | ) | 84,678 | ||||||||||||||
|
Common stock issued for conversion of stock options net of 13,964 shares returned as payment |
97,431 | 3,513 | -- | -- | 3,513 | |||||||||||||||
|
Dividends paid |
-- | -- | (2,355 | ) | -- | (2,355 | ) | |||||||||||||
|
Stock-based compensation |
-- | 1,411 | -- | -- | 1,411 | |||||||||||||||
|
Foreign currency translation |
-- | -- | -- | (609 | ) | (609 | ) | |||||||||||||
|
Net income |
-- | -- | 11,183 | -- | 11,183 | |||||||||||||||
|
March 31, 2017 |
3,734,704 | 25,925 | 73,656 | (1,760 | ) | 97,821 | ||||||||||||||
|
Common stock issued for conversion of stock options net of 8,562 shares returned as payment |
66,735 | 2,919 | -- | -- | 2,919 | |||||||||||||||
|
Dividends paid |
-- | -- | (2,413 | ) | -- | (2,413 | ) | |||||||||||||
|
Stock-based compensation |
-- | 1,672 | -- | -- | 1,672 | |||||||||||||||
|
Foreign currency translation |
-- | -- | -- | 2,324 | 2,324 | |||||||||||||||
|
Net loss |
-- | -- | (2,962 | ) | -- | (2,962 | ) | |||||||||||||
|
March 31, 2018 |
3,801,439 | $ | 30,516 | $ | 68,281 | $ | 564 | $ | 99,361 | |||||||||||
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Consolidated Statements of Cash Flows
(In thousands)
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
Cash flows from operating activities: |
||||||||||||
|
Net (loss) income |
$ | (2,962 | ) | $ | 11,183 | $ | 11,169 | |||||
|
Depreciation and amortization |
9,471 | 8,737 | 7,174 | |||||||||
|
Gain on sale of assets |
(116 | ) | -- | -- | ||||||||
|
Deferred income taxes |
(2,704 | ) | (630 | ) | (807 | ) | ||||||
|
Stock-based compensation |
1,672 | 1,411 | 1,327 | |||||||||
|
Foreign currency adjustments |
(490 | ) | 93 | 53 | ||||||||
|
Amortization of debt issuance costs |
110 | -- | -- | |||||||||
|
Change in inventory reserve |
2,474 | 194 | 305 | |||||||||
|
Impairment loss on goodwill |
13,819 | -- | -- | |||||||||
|
Adjustment to contingent consideration |
300 | -- | -- | |||||||||
|
Change in assets and liabilities, net of effects of acquisitions and dispositions |
||||||||||||
|
Accounts receivable, net |
680 | 994 | (1,958 | ) | ||||||||
|
Inventories, net |
2,286 | 101 | (1,507 | ) | ||||||||
|
Prepaid expenses and other |
755 | (830 | ) | 391 | ||||||||
|
Accounts payable |
212 | (655 | ) | (150 | ) | |||||||
|
Accrued liabilities and taxes payable |
408 | (3,066 | ) | 2,865 | ||||||||
|
Unearned revenues |
(196 | ) | (228 | ) | 99 | |||||||
|
Contingent consideration |
(905 | ) | (9,554 | ) | (2,058 | ) | ||||||
|
Net cash provided by operating activities |
24,814 | 7,750 | 16,903 | |||||||||
|
Cash flows from investing activities: |
||||||||||||
|
Acquisitions |
(15,518 | ) | (6,800 | ) | (24,111 | ) | ||||||
|
Proceeds from sale of assets |
1,133 | |||||||||||
|
Purchases of property, plant and equipment |
(2,799 | ) | (11,605 | ) | (7,729 | ) | ||||||
|
Net cash used in investing activities |
(17,184 | ) | (18,405 | ) | (31,840 | ) | ||||||
|
Cash flow from financing activities: |
||||||||||||
|
Proceeds from the issuance of debt |
11,000 | 66,550 | 25,000 | |||||||||
|
Payments on debt |
(19,625 | ) | (57,000 | ) | (6,000 | ) | ||||||
|
Dividends |
(2,413 | ) | (2,355 | ) | (2,303 | ) | ||||||
|
Proceeds from the exercise of stock options |
2,919 | 3,513 | 1,923 | |||||||||
|
Net cash (used in) provided by financing activities |
(8,119 | ) | 10,708 | 18,620 | ||||||||
|
Effect of exchange rate changes on cash and cash equivalents |
138 | 72 | (22 | ) | ||||||||
|
Net (decrease) increase in cash and cash equivalents |
(351 | ) | 125 | 3,661 | ||||||||
|
Cash and cash equivalents at beginning of year |
5,820 | 5,695 | 2,034 | |||||||||
|
Cash and cash equivalents at end of year |
$ | 5,469 | $ | 5,820 | $ | 5,695 | ||||||
|
Cash paid during the year for: |
||||||||||||
|
Income taxes |
$ | 4,551 | $ | 5,605 | $ | 3,951 | ||||||
|
Interest |
1,956 | 1,384 | 848 | |||||||||
|
Supplemental non-cash activity: |
||||||||||||
|
Contingent consideration as part of an acquisition |
-- | 1,822 | 9,271 | |||||||||
See accompanying notes to consolidated financial statements.
Mesa Laboratories, Inc.
Notes to Consolidated Financial Statements
Note 1. Description of Business and Summary of Significant Accounting Policies
Description of Business
Mesa Laboratories, Inc. was incorporated under the laws of the State of Colorado on March 26, 1982. The terms “we,” “us,” “our,” the “Company” or “Mesa” are used in this report to refer collectively to the parent company and the subsidiaries through which our various businesses are conducted. We pursue a strategy of focusing primarily on quality control products and services, which are sold into niche markets that are driven by regulatory requirements. We prefer markets where we can establish a strong presence and achieve high gross margins. We are organized into four divisions across nine physical locations. Our Sterilization and Disinfection Control Division (formerly named the Biological Indicators Division) provides testing services, along with the manufacturing and marketing of biological, chemical and cleaning indicators used to assess the effectiveness of sterilization and disinfection processes in the hospital, dental, medical device and pharmaceutical industries. Our Instruments Division designs, manufactures and markets quality control instruments and disposable products utilized in the healthcare, pharmaceutical, food and beverage, medical device, industrial hygiene and environmental air sampling industries. Our Cold Chain Monitoring Division designs, develops and markets systems which are used to monitor various environmental parameters such as temperature, humidity and differential pressure to ensure that critical storage and processing conditions are maintained in hospitals, pharmaceutical and medical device manufacturers, blood banks, pharmacies and laboratory environments. Our Cold Chain Packaging Division provides packaging development consulting services and thermal packaging products such as coolers, boxes, insulation materials and phase-change products to control temperature during transport.
Basis of Presentation
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). The consolidated financial statements include the accounts of Mesa Laboratories, Inc. and its subsidiaries. Intercompany transactions and balances have been eliminated. The preparation of our consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our consolidated financial statements and accompanying notes. Although these estimates are based on our knowledge of current events and actions we may undertake in the future, actual results may ultimately differ from these estimates and assumptions. Furthermore, when testing assets for impairment in future periods, if management uses different assumptions or if different conditions occur, impairment charges may result.
Summary of Significant Accounting Policies
Revenue Recognition
We recognize revenue when the four revenue recognition criteria are met, as follows:
Product sales: Revenue is recognized upon shipment of the product. Evidence of an arrangement is typically in the form of a customer purchase order. Custody is transferred upon shipment (FOB Shipping Point). Prices are fixed at the time of order and no price protections or variables are offered. Collectability is reasonably assured via our customer credit and review processes.
Services: Revenue is recognized upon completion of the work/services to be performed. Evidence of an arrangement is typically in the form of a contract and/or a customer purchase order. Custody is transferred upon completion and acceptance of the service or installation process. Prices are fixed at the time of order and no price protections or variables are offered. Collectability is reasonably assured via our customer credit and review processes.
Shipping and handling
Payments by customers to us for shipping and handling costs are included in revenues on the consolidated statements of operations, while our expense is included in cost of revenues. Shipping and handling for inventory and materials purchased by us is included as a component of inventory on the consolidated balance sheets, and in cost of revenues when the product is sold.
Unearned Revenues
Certain of our products have associated annual service contracts whereby we provide repair, technical support and various other analytical or maintenance services. In the event that these contracts are paid up front by the customer, the associated amounts are deferred and recognized ratably over the term of the service period, generally one year.
Accrued Warranty Expense
We provide limited product warranty on our products and, accordingly, accrue an estimate of the related warranty expense at the time of sale.
Cash Equivalents
We classify time deposits and other investments that are highly liquid and have maturities of three months or less at the date of purchase as cash equivalents.
Accounts Receivable
We record trade accounts receivable at net realizable value. This value includes an appropriate allowance for estimated uncollectible accounts to reflect any loss anticipated on the trade accounts receivable balances and is charged to the provision for doubtful accounts. We calculate this allowance based on our history of write-offs, the level of past-due accounts based on the contractual terms of the receivables, and our relationships with, and the economic status of, our customers.
Concentration of Credit Risk
Financial instruments that potentially subject us to concentrations of credit risk consist of accounts receivable. For the years ended March 31, 2018, 2017 and 2016, no individual customer represented more than 10 percent of our revenues or more than 10 percent of our accounts receivable balance. Approximately 59 percent and 41 percent of our sales for the year ended March 31, 2018 were to customers located in the United States and foreign countries, respectively.
Inventories
Inventories are stated at the lower of cost or market, using the weighted average method to determine cost. We evaluate labor and overhead costs annually, unless specific circumstances necessitate a mid-year evaluation. Our work in process and finished goods inventory includes raw materials, labor and overhead, which are estimated based on trailing twelve months of expense and standard labor hours for each product. Our biological indicator inventory is tracked by lot number, thus it is generally based on actual hours.
We monitor inventory cost compared to selling price in order to determine if a lower of cost or market reserve is necessary. Throughout the year, we perform various physical cycle count procedures on our inventories and we estimate and maintain an inventory reserve, as needed, for such matters as obsolete inventory, shrink and scrap.
Property, Plant and Equipment
Property, plant and equipment are stated at cost. Repair and maintenance costs that do not improve service potential or extend the economic life are expensed as incurred. Depreciation is recorded using the straight-line method over the estimated useful lives of our assets, which are reviewed periodically and generally have the following ranges: buildings: 40 years or less; manufacturing equipment: seven years or less; and computer equipment: three years or less. Land is not depreciated and construction in progress is not depreciated until placed in service.
Goodwill and Intangible Assets
We classify intangible assets into three categories: (1) intangible assets with definite lives subject to amortization, (2) intangible assets with indefinite lives not subject to amortization and (3) goodwill. We determine the useful lives of our identifiable intangible assets after considering the specific facts and circumstances related to each intangible asset. Factors we consider when determining useful lives include the contractual term of any agreement related to the asset, the historical performance of the asset, our long-term strategy for using the asset, any laws or other local regulations which could impact the useful life of the asset and other economic factors, including competition and specific market conditions. Intangible assets that are deemed to have definite lives are amortized, primarily on a straight-line basis, over their useful lives, generally ranging from three to sixteen years (See Note 5).
When facts and circumstances indicate that the carrying value of definite-lived intangible assets may not be recoverable, management assesses the recoverability of the carrying value by preparing estimates of revenues and the resulting gross profit and cash flows expected to result from the use of the asset group and its eventual disposition. These estimated future cash flows are consistent with those we use in our internal planning. If the sum of the expected future cash flows (undiscounted and without interest charges) is less than the carrying amount, we recognize an impairment loss. The impairment loss recognized is the amount by which the carrying amount of the asset or asset group exceeds the fair value. We use a variety of methodologies to determine the fair value of these assets, including discounted cash flow models, which are consistent with the assumptions we believe hypothetical marketplace participants would use.
We test intangible assets determined to have indefinite useful lives, including trademarks and goodwill, for impairment annually, or more frequently if events or circumstances indicate that assets might be impaired. We perform these annual impairment reviews as of the first day of our fourth fiscal quarter. We use a variety of methodologies in conducting impairment assessments of indefinite-lived intangible assets, including, but not limited to, discounted cash flow models, which are based on the assumptions we believe hypothetical marketplace participants would use. For indefinite-lived intangible assets, other than goodwill, if the carrying amount exceeds the fair value, an impairment charge is recognized in an amount equal to that excess.
We have the option to perform a qualitative assessment of indefinite-lived intangible assets, other than goodwill, rather than completing the impairment test. We must assess whether it is more likely than not that the fair value of the intangible asset is less than its carrying amount. If we conclude that this is the case, we must perform the testing described above. Otherwise, there is no requirement to perform any further assessment.
We perform impairment tests of goodwill at our reporting unit level, which is one level below our operating segments. Our operating segments consist of our Instruments, Sterilization and Disinfection Control, Cold Chain Monitoring and Cold Chain Packaging. These operating segments are consistent with the way management runs and analyzes our business. Our Instruments operating segment is subdivided into smaller business units. These business units are also our reporting units. Goodwill is assigned to the reporting unit or units that benefit from the synergies arising from each business combination.
In order to test for goodwill impairment, we compare the fair value of a reporting unit to its carrying value, including goodwill. If the fair value of the reporting unit is lower than its carrying amount, goodwill is written down for the amount by which the carrying amount exceeds the fair value. However, the loss recognized cannot exceed the carrying amount of the goodwill. We typically use discounted cash flow models to determine the fair value of a reporting unit. The assumptions used in these models are consistent with those we believe a hypothetical marketplace participant would use. We have the option to perform a qualitative assessment of goodwill in order to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount, including goodwill and other intangible assets. If we conclude that this is the case, we must perform the testing discussed above. Otherwise, there is no requirement to perform any further assessment.
Research & Development Costs
Internal costs related to research and development efforts on existing or potential products are expensed as incurred. The costs of intangible assets that are purchased from others for use in research and development activities, and also have alternative future benefit, are capitalized and amortized over their expected useful life.
Although rare, under certain agreements, we may receive advance payments from customers to perform research and development on their behalf. These payments are recovered by the customer through lower product prices and as such, are initially recorded as unearned revenues in the accompanying consolidated balance sheets. As product is sold, this liability is reduced through revenues on the consolidated statements of operations.
Stock-based Compensation
We sponsor equity plans that provide for the grant of awards including stock options and restricted stock. The fair value of our stock option grants is estimated on the date of grant, based on the closing stock price at grant date, using a Black-Scholes option-pricing model. The fair value of our restricted stock awards (“RSA’s”) is the quoted market value of our stock on the grant date. We recognize expense on a straight-line basis over the service period, net of an estimated forfeiture rate, resulting in a compensation cost for only those shares expected to vest. We do not have any liability classified stock-based compensation. We allocate stock-based compensation expense to cost of revenues and general and administrative expense in the accompanying consolidated statements of operations.
Income Taxes
Income tax expense includes U.S., state, local and international income taxes, plus a provision for U.S. taxes on undistributed earnings of foreign subsidiaries and other prescribed foreign entities not deemed to be indefinitely reinvested. Deferred tax assets and liabilities are recognized for the tax consequences of temporary differences between the financial reporting basis and the tax basis of existing assets and liabilities. The tax rate used to determine the deferred tax assets and liabilities is the enacted tax rate for the year and manner in which the differences are expected to reverse. Valuation allowances are recorded to reduce deferred tax assets to the amount that will more likely than not be realized.
We are involved in various tax matters, with respect to some of which the outcome is uncertain. We establish reserves to remove some or all of the tax benefit of any of our tax positions at the time we determine that it becomes uncertain based upon one of the following conditions: (1) the tax position is not "more likely than not" to be sustained, (2) the tax position is "more likely than not" to be sustained, but for a lesser amount, or (3) the tax position is "more likely than not" to be sustained, but not in the financial period in which the tax position was originally taken. For purposes of evaluating whether or not a tax position is uncertain, (1) we presume the tax position will be examined by the relevant taxing authority that has full knowledge of all relevant information; (2) the technical merits of a tax position are derived from authorities such as legislation and statutes, legislative intent, regulations, rulings and case law and their applicability to the facts and circumstances of the tax position; and (3) each tax position is evaluated without consideration of the possibility of offset or aggregation with other tax positions taken. A number of years may elapse before a particular uncertain tax position is audited and finally resolved or when a tax assessment is raised. The number of years subject to tax assessments varies depending on the tax jurisdiction. The tax benefit that has been previously reserved because of a failure to meet the "more likely than not" recognition threshold would be recognized in income tax expense in the first interim period when the uncertainty disappears under any one of the following conditions: (1) the tax position is "more likely than not" to be sustained, (2) the tax position, amount, and/or timing is ultimately settled through negotiation or litigation, or (3) the statute of limitations for the tax position has expired (See Note 11).
Acquisition Related Contingent Consideration Liability
The acquisition related contingent consideration liability consists of estimated amounts due under various acquisition agreements and is typically based upon either revenues growth or specified profitability growth metrics. At each reporting period, we evaluate the expected future payments and the associated discount rate to determine the fair value of the contingent consideration. These amounts represent our best estimate of the amounts which will ultimately be paid. The discount rate is based upon our estimated credit adjusted risk free rate or current market conditions which includes an estimate for risk premiums. Changes in the fair value of the acquisition related contingent consideration is included in other expense, net on the accompanying consolidated statements of operations.
Legal Contingencies
We are involved in various legal proceedings. Due to their nature, such legal proceedings involve inherent uncertainties including, but not limited to, court rulings and negotiations between affected parties. We assess the probability of loss for such contingencies and accrue a liability and/or disclose the relevant circumstances, as appropriate. (See Note 13).
Fair Value of Measurements
Our financial instruments include cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities and long-term debt. The carrying value of these financial instruments (other than acquisition related contingent consideration liabilities, see above) is considered to be representative of their fair value due to the short maturity of these instruments. Our debt has a variable interest rate, so the carrying amount approximates fair value because interest rates on these instruments approximate the interest rate on debt with similar terms available to us.
Recently Issued Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2014-09, Revenue from Contracts with Customers (Topic 606), which will replace most existing revenue recognition guidance in U.S. GAAP and is intended to improve and converge with international standards the financial reporting requirements for revenue from contracts with customers. The core principle of ASU 2014-09 is that an entity should recognize revenue for the transfer of goods or services equal to the amount that it expects to be entitled to receive for those goods or services. ASU 2014-09 also requires additional disclosures about the nature, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments. ASU 2014-09 allows for adoption either on a full retrospective basis to each prior reporting period presented or on a modified retrospective basis with the cumulative effect of initially applying the new guidance recognized at the date of initial application, which will be effective for the Company beginning April 1, 2018.
We will adopt ASU 2014-09 and its amendments on a modified retrospective basis effective April 1, 2018. We have identified and implemented minor necessary changes to our accounting policies and practices, business processes, and systems and controls as well as designed and implemented specific controls over our evaluation of the impact of the new guidance on the Company, including the cumulative effect calculation, disclosure requirements and the collection of relevant data into the reporting process. We are substantially complete with the process of finalizing changes to our business processes and systems and controls to support recognition and new required disclosures under the new revenue standard. While we expect the impact of the new standard on the amount and timing of revenue recognized in the year ending March 31, 2019 to be insignificant, our assessment will be finalized during the first quarter of our year ending March 31, 2019.
In February 2016, the FASB issued ASU 2016-02, Leases, which requires lessees to recognize right-of-use assets, representing their right to use the underlying asset for the lease term, and lease liabilities on the consolidated balance sheet for all leases with terms greater than 12 months. The guidance also requires qualitative and quantitative disclosures designed to assess the amount, timing and uncertainty of cash flows arising from leases. We have initiated our plan for the adoption and implementation of this new accounting standard, including assessing our lease arrangements, evaluating practical expedient and accounting policy elections. The standard requires the use of a modified retrospective transition approach, which includes a number of optional practical expedients that entities may elect to apply. ASU 2016-02 is effective for the Company beginning April 1, 2019. We anticipate that the adoption of this new standard will not result in a significant increase in lease-related assets and liabilities on our consolidated balance sheets or significantly impact our consolidated statements of operations and cash flows.
In January 2017, the FASB issued ASU 2017-04, Intangibles – Goodwill and Other, which eliminates the requirement to calculate the implied fair value of goodwill to measure a goodwill impairment charge. ASU 2017-04 is required to be applied prospectively and we elected to early adopt ASU 2017-04 effective April 1, 2017.
Note 2. Acquisitions and Dispositions
Acquisitions
For the years ended March 31, 2018 and 2017, our acquisitions of businesses (net of cash acquired) totaled $15,518,000 and $8,622,000, respectively, of which none were individually material in nature (see Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations).
For the year ended March 31, 2016, our acquisitions of businesses (net of cash acquired) totaled $33,382,000, which consisted primarily of the following material acquisitions:
Infitrak
On July 6, 2015, we completed a business combination (the “Infitrak Acquisition”) whereby we acquired all of the common stock of 2396081 Ontario Inc. and its wholly owned operating subsidiary, Infitrak Inc. (collectively “Infitrak”), a company whose business provides consulting, packaging and measuring solutions for cold chain applications. The stock purchase agreement (the “Infitrak Agreement”) includes provisions for both contingent consideration based upon the two- year growth in gross profit (as defined in the Earn-Out Agreement) of the packaging component of our cold chain business subsequent to the acquisition and for a holdback payment (subject to a post-closing adjustment), payable at the one year anniversary of the closing date.
Under the terms of the Infitrak Agreement, we were required to pay contingent consideration if the gross profit (as defined in the Infitrak Earn-Out Agreement) for our cold chain packaging business for the two years subsequent to the acquisition met certain levels. The potential undiscounted consideration payable ranged from $0 to $15,000,000 CDN and was based upon a sliding scale of growth in gross profit (as defined in the Infitrak Earn-Out Agreement) for year one and year two of 30 to 70 percent and 15 to 75 percent, respectively. Based upon both historical and projected growth rates, we recorded $9,271,000 of contingent consideration payable which represented our best estimate of the then current fair value of the amount that would ultimately be paid. Any changes to the contingent consideration ultimately paid would have resulted in additional income or expense in our consolidated statements of income.
In July 2016, we made the first Earn-Out payment in the amount of $6,000,000 CDN ($4,594,000). In March 2017, we agreed to settle the remaining earn-out obligation (which was originally due in the second quarter of our year ending March 31, 2018) early by making a payment of $6,000,000 CDN ($4,558,000).
We expected to achieve savings and generate growth as we integrated the Infitrak operations and sales and marketing functions. These factors, among others, contributed to a purchase price in excess of the estimated fair value of the net identifiable assets acquired and, as a result, we recorded goodwill in connection with this transaction. The goodwill is not deductible for tax purposes and it was assigned to our Cold Chain Packaging segment.
The Infitrak Acquisition constituted the acquisition of a business and was recognized at fair value. We determined the estimated fair values using discounted cash flow analyses and estimates made by management. The following reflected our allocation of the consideration, subject to customary purchase price adjustments in accordance with the Infitrak Agreement (in thousands):
|
Cash consideration |
$ | 8,747 | ||
|
Holdback payment liability |
637 | |||
|
Contingent consideration liability |
9,271 | |||
|
Aggregate consideration |
$ | 18,655 | ||
|
Accounts receivable |
$ | 925 | ||
|
Inventories |
310 | |||
|
Property, plant and equipment |
530 | |||
|
Intangibles |
5,869 | |||
|
Goodwill |
13,833 | |||
|
Accounts payable |
(470 | ) | ||
|
Accrued liabilities |
(767 | ) | ||
|
Deferred income taxes |
(1,575 | ) | ||
|
Total purchase price allocation |
$ | 18,655 |
The accompanying consolidated statements of operations include the results of the Infitrak Acquisition from the acquisition date of July 6, 2015. The pro forma effects of the acquisition on the results of operations as if the acquisition had been completed on April 1, 2015 and 2014, are as follows (in thousands, except per share data):
|
Year Ended March 31, |
||||||||
|
2016 |
2015 |
|||||||
|
Revenues |
$ | 86,499 | $ | 74,379 | ||||
|
Net income |
11,471 | 9,944 | ||||||
|
Net Income per common share: |
||||||||
|
Basic |
$ | 3.18 | $ | 2.82 | ||||
|
Diluted |
3.05 | 2.72 | ||||||
North Bay
On August 6, 2015, we completed a business combination (the “North Bay Acquisition”) whereby we acquired substantially all of the assets (other than certain fixed assets) and certain liabilities of the dental sterilizer testing business of North Bay Bioscience, LLC (“North Bay”). The asset purchase agreement (the “North Bay Agreement”) included a provision for a holdback payment (subject to a post-closing adjustment), payable at the one year anniversary of the closing date.
We expected to achieve savings and generate growth as we integrated the North Bay operations and sales and marketing functions. These factors, among others, contributed to a purchase price in excess of the estimated fair value of the net identifiable assets acquired and, as a result, we recorded goodwill in connection with this transaction. The goodwill is deductible for tax purposes and it was assigned to our Biological Indicators segment.
The North Bay Acquisition constituted the acquisition of a business and was recognized at fair value. We determined the estimated fair values using discounted cash flow analyses and estimates made by management. The following reflected our allocation of the consideration, subject to customary purchase price adjustments in accordance with the North Bay Agreement (in thousands):
|
Cash consideration |
$ | 10,322 | ||
|
Holdback payment liability |
1,000 | |||
|
Aggregate consideration |
$ | 11,322 | ||
|
Cash |
$ | 20 | ||
|
Accounts receivable |
285 | |||
|
Inventories |
85 | |||
|
Property, plant and equipment |
229 | |||
|
Intangibles |
4,454 | |||
|
Goodwill |
7,962 | |||
|
Accrued liabilities |
(100 | ) | ||
|
Unearned revenues |
(1,613 | ) | ||
|
Total purchase price allocation |
$ | 11,322 |
The accompanying consolidated statements of operations include the results of the North Bay Acquisition from the acquisition date of August 6, 2015. The pro forma effects of the acquisition on the results of operations as if the acquisition had been completed on April 1, 2015 and 2014, are as follows (in thousands, except per share data):
|
Year Ended March 31, |
||||||||
|
2016 |
2015 |
|||||||
|
Revenues |
$ | 86,053 | $ | 75,649 | ||||
|
Net income |
11,463 | 10,182 | ||||||
|
Net Income per common share: |
||||||||
|
Basic |
$ | 3.18 | $ | 2.89 | ||||
|
Diluted |
3.05 | 2.79 | ||||||
Note 3. Inventories
Inventories consist of the following (in thousands):
|
March 31, |
||||||||
|
2018 |
2017 |
|||||||
|
Raw materials |
$ | 9,059 | $ | 10,815 | ||||
|
Work-in-process |
380 | 342 | ||||||
|
Finished goods |
3,152 | 3,604 | ||||||
|
Less reserve |
(3,363 | ) | (888 | ) | ||||
| $ | 9,228 | $ | 13,873 | |||||
Note 4. Property, Plant and Equipment
Property, plant and equipment consist of the following (in thousands):
|
March 31, |
||||||||
|
2018 |
2017 |
|||||||
|
Land |
$ | 889 | $ | 1,614 | ||||
|
Buildings |
18,543 | 4,726 | ||||||
|
Manufacturing equipment |
9,156 | 8,861 | ||||||
|
Computer equipment |
3,459 | 4,143 | ||||||
|
Construction in progress |
60 | 15,882 | ||||||
|
Other |
1,221 | 1,220 | ||||||
| 33,328 | 36,446 | |||||||
|
Less accumulated depreciation |
(9,735 | ) | (10,444 | ) | ||||
| $ | 23,593 | $ | 26,002 | |||||
Depreciation expense for the years ended March 31, 2018, 2017 and 2016 was $2,542,000, $2,287,000 and $1,387,000, respectively.
Note 5. Goodwill and Intangible Assets
During the year ended March 31, 2018, revenues in our Cold Chain Packaging reporting segment decreased significantly as compared to the prior year primarily due to a significant decrease in revenues from our largest customer. During the three months ended December 31, 2017 we completed a detailed review of the cold chain packaging business and concluded that long and difficult sales-cycles associated with this product set, when coupled with higher than previously contemplated costs for operating and expanding the necessary infrastructure to support revenues growth have resulted in a forecast of lower than expected revenues, gross margin percentages and overall profitability as compared to our original model for this business. Based on these facts, we concluded that we had a triggering event requiring assessment of impairment for certain of our long-lived assets associated with the Cold Chain Packaging reporting segment. As a result, we reviewed the long-lived assets associated with this reporting segment and recorded a $13,819,000 impairment charge related to goodwill, which is included in impairment loss on goodwill on the accompanying consolidated statements of operations for the year ended March 31, 2018. The impairment loss was measured using a market approach utilizing an EBITA multiple model. The remaining goodwill and intangible assets associated with this segment are $1,401,000 and $4,081,000, respectively as of March 31, 2018.
The change in the carrying amount of goodwill was as follows (in thousands):
|
Sterilization and Disinfection Control |
Instruments |
Cold Chain Monitoring |
Cold Chain Packaging |
Total |
||||||||||||||||
|
March 31, 2016 |
$ | 20,898 | $ | 18,235 | $ | 13,647 | $ | 13,357 | $ | 66,137 | ||||||||||
|
Effect of foreign currency translation |
(97 | ) | -- | -- | (374 | ) | (471 | ) | ||||||||||||
|
Acquisitions |
3,218 | -- | 1,757 | 1,515 | 6,490 | |||||||||||||||
|
March 31, 2017 |
24,019 | 18,235 | 15,404 | 14,498 | 72,156 | |||||||||||||||
|
Effect of foreign currency translation |
536 | -- | -- | 722 | 1,258 | |||||||||||||||
|
Acquisitions |
5,948 | -- | -- | 5,948 | ||||||||||||||||
|
Impairment |
-- | -- | -- | (13,819 | ) | (13,819 | ) | |||||||||||||
|
March 31, 2018 |
$ | 30,503 | $ | 18,235 | $ | 15,404 | $ | 1,401 | $ | 65,543 | ||||||||||
Other intangible assets are as follows:
|
(In thousands) |
March 31, 2018 |
||||||||||||||||
|
Carrying Amount |
Accumulated Amortization |
Net |
Useful Life (Years) |
||||||||||||||
|
Intellectual property |
$ | 7,210 | $ | (4,554 | ) | $ | 2,656 | 10 | - | 16 | |||||||
|
Trade names |
3,675 | (2,154 | ) | 1,521 | 3 | - | 10 | ||||||||||
|
Customer relationships |
64,363 | (26,128 | ) | 38,235 | 7 | - | 10 | ||||||||||
|
Non-compete agreements |
1,865 | (1,427 | ) | 438 | 3 | - | 10 | ||||||||||
| $ | 77,113 | $ | (34,263 | ) | $ | 42,850 | |||||||||||
|
March 31, 2017 |
|||||||||||||||||
|
Carrying Amount |
Accumulated Amortization |
Net |
Useful Life (Years) |
||||||||||||||
|
Intellectual property |
$ | 7,210 | $ | (3,824 | ) | $ | 3,386 | 10 | - | 16 | |||||||
|
Trade names |
3,663 | (1,727 | ) | 1,936 | 3 | - | 10 | ||||||||||
|
Customer relationships |
52,134 | (20,260 | ) | 31,874 | 7 | - | 10 | ||||||||||
|
Non-compete agreements |
1,845 | (1,251 | ) | 594 | 3 | - | 10 | ||||||||||
| $ | 64,852 | $ | (27,062 | ) | $ | 37,790 | |||||||||||
The following is estimated amortization expense for the years ending March 31:
|
(In thousands) |
||||
|
2019 |
$ | 7,249 | ||
|
2020 |
6,915 | |||
|
2021 |
5,870 | |||
|
2022 |
5,869 | |||
|
2023 |
5,235 |
Amortization expense for the years ended March 31, 2018, 2017 and 2016 was $6,929,000, $6,450,000 and $5,787,000, respectively.
Note 6. Facility Relocation
In August 2016, we announced that we plan to shut down both our Omaha and Traverse City manufacturing facilities and relocate those operations to the new Bozeman building. The move of these two facilities, along with the current Bozeman operations, began in March 2017 and is estimated to be completed by June 30, 2018. We estimate that the total costs of the relocation will be $2,000,000, which is comprised primarily of facility moving expenses, retention bonuses for existing personnel and payroll costs for duplicative personnel during the transition period. We incurred $842,000 and $725,000 of these relocation expenses during the years ended March 31, 2018 and 2017, respectively which are reflected in cost of revenues in the accompanying consolidated statements of operations (other than $269,000 and $45,000, respectively which are included in general and administrative expenses). Facility relocation costs, which are associated with our Sterilization and Disinfection Control segment, are as follows for the year ended March 31, 2018:
|
● |
Retention bonuses for existing personnel of $350,000 |
|
● |
Duplicative employment costs of $122,000 |
|
● |
Moving costs of $370,000 |
Facility relocation amounts accrued and paid for the year ended March 31, 2018 are as follows (in thousands):
|
March 31, 2018 |
||||
|
Beginning balance at March 31, 2017 |
$ | 673 | ||
|
Facility relocation expense |
842 | |||
|
Cash payments |
(1,107 | ) | ||
|
Ending balance at March 31, 2018 |
$ | 408 | ||
Note 7. Long-term Debt
Long-term debt consists of the following (in thousands):
|
March 31, 2018 |
March 31, 2017 |
|||||||
|
Line of credit (3.75% at March 31, 2018) |
$ | 28,000 | $ | 35,500 | ||||
|
Term loan (3.69% at March 31, 2018) |
18,625 | 19,750 | ||||||
|
Less: discount |
(365 | ) | (450 | ) | ||||
|
Less: current portion |
(1,625 | ) | (1,125 | ) | ||||
|
Long-term portion |
$ | 44,635 | $ | 53,675 | ||||
On March 1, 2017, we entered into a five-year agreement (the “Credit Facility”) for an $80,000,000 revolving line of credit (“Line of Credit”), a $20,000,000 term loan (“Term Loan”) and up to $2,500,000 of letters of credit with a banking syndicate of four banks. In addition, the Credit Facility provides a post-closing accordion feature which allows for the Company to request to increase the Line of Credit or Term Loan up to an additional $100,000,000. Funds from the Credit Facility may be used to pay down its previous credit facility, finance working capital needs and for general corporate purposes in the ordinary course of business (including, without limitation, permitted acquisitions).
Line of Credit and Term Loan indebtedness bears interest at either: (1) LIBOR, as defined, plus an applicable margin ranging from 1.5% to 2.50%; or (2) the alternate base rate (“ABR”), which is the greater of JPMorgan’s prime rate or the federal funds effective rate or the overnight bank funding rate plus 0.5%. We elect the interest rate with each borrowing under the line of credit. In addition, there is an unused line fee of 0.15% to 0.35%. Letter of credit fees are based on the applicable LIBOR rate.
The Term Loan requires 20 quarterly principal payments (the first due date was March 31, 2017) in the amount of $250,000 (increasing by $125,000 each year up to $750,000 in the fifth year). The remaining balance of principal and accrued interest are due on March 1, 2022.
The Credit Facility is secured by all of our assets and requires us to maintain a ratio of funded debt to our trailing four quarters of EBIDTA (the “Leverage Ratio”), as defined in the agreement, of less than 3.0 to 1.0, provided that, we may once during the term of the Credit Facility, in connection with a Permitted Acquisition for which the aggregate consideration paid or to be paid in respect thereof equals or exceeds $20,000,000, elect to increase the maximum Leverage Ratio permitted hereunder to (i) 3.50 to 1.00 for a period of four consecutive fiscal quarters commencing with the fiscal quarter in which such Permitted Acquisition occurs (the “Initial Holiday Period”) and (ii) 3.25 to 1.00 for the period of four consecutive fiscal quarters immediately following the Initial Holiday Period. The Credit Facility also requires us to maintain a minimum fixed charge coverage ratio of less than 1.25 to 1.0. We were in compliance with the required covenants at March 31, 2018.
We incurred origination and debt issuance costs of $460,000 which are treated as a debt discount and are netted against amounts outstanding on the consolidated balance sheets.
Future contractual maturities of debt as of March 31, 2018 are as follows (in thousands):
|
Year ending March 31, |
||||
|
2019 |
$ | 1,625,000 | ||
|
2020 |
2,125,000 | |||
|
2021 |
2,625,000 | |||
|
2022 |
40,250,000 | |||
| $ | 46,625,000 |
Subsequent to March 31, 2018, we made $3,000,000 in payments under the Line of Credit.
Note 8. Stockholders' Equity
Under applicable law, Colorado corporations are not permitted to retain treasury stock. The price paid for repurchased shares is allocated between common stock and retained earnings, based on management’s estimate of the original sales price of the underlying shares.
In November 2005, our Board of Directors approved a program to repurchase up to 300,000 shares of our outstanding common stock. Under the program, shares of common stock may be purchased from time to time in the open market at prevailing prices or in negotiated transactions off the market. Shares of common stock purchased will be cancelled and repurchases of shares of common stock will be funded through existing cash reserves. There were no repurchases of our shares of common stock under this plan during the years ended March 31, 2018, 2017 and 2016. As of March 31, 2018, we have purchased 162,486 shares under this plan.
Dividends per share paid by quarter were as follows:
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
First quarter |
$ | 0.16 | $ | 0.16 | $ | 0.16 | ||||||
|
Second quarter |
0.16 | 0.16 | 0.16 | |||||||||
|
Third quarter |
0.16 | 0.16 | 0.16 | |||||||||
|
Fourth quarter |
0.16 | 0.16 | 0.16 | |||||||||
Note 9. Employee Benefit Plans
We adopted our 401(k) plan effective January 1, 2000. Participation is voluntary, and employees are eligible the first day of the following month that an employee attains an age of 21 and one hour of service time. We match 100 percent of the employee’s contributions up to four percent of the employee’s salary and those contributions are vested immediately. Prior to January 1, 2017, we matched 50 percent of the employee’s contribution up to six percent of the employee’s salary and those contributions were vested immediately. We contributed $680,000, $501,000 and $387,000, respectively, to the plan for the years ended March 31, 2018, 2017 and 2016.
Note 10. Stock-Based Compensation
We adopted equity award plans, which are used to grant stock options and/or RSA’s for the benefit of our employees and outside directors. Under terms of the plans, stock options are granted at an amount not less than 100% of the quoted market price of the underlying shares at the date of grant. Stock options are exercisable for terms of five to ten years and vest ratably over terms of four to seven years. RSA’s vest ratably over terms of approximately five to seven years. All of our equity award plans have been approved by our shareholders.
On August 8, 2014, we adopted The Mesa Laboratories, Inc. 2014 Equity Plan (the “2014 Plan”), which was subsequently approved by our shareholders on October 2, 2014 at our 2014 Annual Meeting of Shareholders. The purpose of the 2014 Plan is to promote the success and enhance the value of the Company by linking the personal interests of our employees, officers and directors to those of our shareholders by providing such persons with an incentive for outstanding performance. A total of 1,100,000 shares of common stock were reserved for issuance under the 2014 Plan and are subject to terms as set by the Compensation Committee of the Board of Directors at the time of grant. As of March 31, 2018, we have 289,225 stock options outstanding and have issued 8,788 RSA’s under the 2014 Plan.
Under the December 8, 2006 plan (the “2006 Plan”), a total of 400,000 shares of common stock were reserved for issuance and were subject to terms as set by the Compensation Committee of the Board of Directors at the time of grant. On September 23, 2010, our shareholders approved an amendment to the 2006 Plan whereby the number of shares authorized for issuance was increased to 800,000. As a result of the approval of the 2014 Plan by our shareholders, no further awards will be made under the 2006 Plan and it will remain in effect only as long as awards previously made thereunder remain outstanding. As of March 31, 2018, we have 169,133 stock options outstanding under the 2006 Plan. On February 27, 2013, we filed a Registration Statement on Form S-8 whereby we registered the additional 400,000 shares of common stock underlying stock options issuable under the 2006 Plan.
Amounts recognized in the consolidated financial statements related to stock-based compensation are as follows (in thousands, except per share data):
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
Total cost of stock-based compensation charged against earnings before income taxes |
$ | 1,672 | $ | 1,411 | $ | 1,327 | ||||||
|
Amount of income tax benefit recognized in earnings |
1,194 | 1,737 | 794 | |||||||||
|
Amount charged (added) against (to) net (loss) income |
$ | 478 | $ | (326 | ) | $ | 533 | |||||
|
Impact on net (loss) income per common share: |
||||||||||||
|
Basic |
$ | 0.13 | $ | (0.09 | ) | $ | 0.15 | |||||
|
Diluted |
0.13 | (0.08 | ) | 0.14 | ||||||||
Stock-based compensation expense is included in cost of revenues, selling and general and administrative expense in the accompanying consolidated statements of operations.
The fair value of each option grant is estimated on the date of grant using the Black-Scholes option-pricing model (“Black-Scholes”) that uses assumptions noted in the following table. We use historical data to estimate the expected price volatility, the expected stock option life and expected forfeiture rate. The risk-free rate is based on the United States Treasury yield curve in effect at the time of grant for the estimated life of the stock option. The dividend yield is calculated based upon the dividend payments made during the prior four quarters as a percent of the average stock price for that period.
|
Year Ended March 31, |
|||||||||||||||
|
2018 |
2017 |
2016 |
|||||||||||||
|
Volatility |
32.85% | - | 35.44% | 32.05% | - | 33.76% | 27.1% | - | 30.2% | ||||||
|
Risk-free interest rate |
1.77% | 1.22% | 1.09% | ||||||||||||
|
Expected option life (years) |
6 | 5 | 8 | ||||||||||||
|
Dividend yield |
. 57% | .55% | .70% | ||||||||||||
A summary of the option activity as of and for the years ended March 31, 2018, 2017 and 2016 is as follows:
|
Number of |
Weighted- |
Weighted- |
Aggregate (000s) |
|||||||||||||
|
Outstanding at March 31, 2015 |
437,248 | $ | 55.81 | 4.9 | $ | 9,445 | ||||||||||
|
Granted |
184,030 | 72.89 | 6.7 | -- | ||||||||||||
|
Forfeited |
(16,334 | ) | 75.16 | 6.5 | -- | |||||||||||
|
Expired |
-- | -- | -- | -- | ||||||||||||
|
Exercised |
(89,224 | ) | 38.28 | -- | -- | |||||||||||
|
Outstanding at March 31, 2016 |
515,720 | 64.32 | 5.2 | 16,561 | ||||||||||||
|
Granted |
134,955 | 102.52 | 5.5 | -- | ||||||||||||
|
Forfeited |
(35,015 | ) | 87.5 | 4.5 | -- | |||||||||||
|
Expired |
(904 | ) | 41.51 | -- | -- | |||||||||||
|
Exercised |
(104,395 | ) | 50.12 | -- | -- | |||||||||||
|
Outstanding at March 31, 2017 |
510,361 | 75.78 | 5.0 | 23,956 | ||||||||||||
|
Granted |
96,330 | 123.13 | 5.0 | -- | ||||||||||||
|
Forfeited |
(72,166 | ) | 90.07 | 4.2 | -- | |||||||||||
|
Expired |
(1,024 | ) | 82.24 | -- | -- | |||||||||||
|
Exercised |
(75,143 | ) | 57.99 | -- | -- | |||||||||||
|
Outstanding at March 31, 2018 |
458,358 | 86.38 | 4.5 | 28,445 | ||||||||||||
|
Exercisable at March 31, |
||||||||||||||||
|
2018 |
143,836 | $ | 62.96 | 4.0 | $ | 12,295 | ||||||||||
|
2017 |
136,595 | 50.61 | 3.9 | 9,847 | ||||||||||||
|
2016 |
157,457 | 42.49 | 3.6 | 8,481 | ||||||||||||
A summary of the status of our unvested option shares as of and for the years ended March 31, 2018, 2017 and 2016 is as follows:
|
Unvested Shares |
Weighted-average |
|||||||
|
Unvested at March 31, 2015 |
274,038 | $ | 18.42 | |||||
|
Options granted |
184,030 | 18.78 | ||||||
|
Options forfeited |
(16,334 | ) | 19.07 | |||||
|
Options vested |
(83,471 | ) | 14.65 | |||||
|
Unvested at March 31, 2016 |
358,263 | 19.46 | ||||||
|
Options granted |
134,955 | 31.27 | ||||||
|
Options forfeited |
(35,015 | ) | 22.64 | |||||
|
Options vested |
(84,437 | ) | 16.96 | |||||
|
Unvested at March 31, 2017 |
373,766 | 22.49 | ||||||
|
Options granted |
96,330 | 39.06 | ||||||
|
Options forfeited |
(72,166 | ) | 28.43 | |||||
|
Options vested |
(83,408 | ) | 21.84 | |||||
|
Unvested at March 31, 2018 |
314,522 | 29.01 | ||||||
The total intrinsic value of options exercised was $6,309,000, $7,574,000 and $5,260,000 for the years ended March 31, 2018, 2017 and 2016, respectively. As of March 31, 2018, there was $5,958,000 and $963,000 of total unrecognized compensation expense related to unvested options and RSA’s, respectively. As of March 31, 2018, we have 767,888 shares available for future option or RSA grants.
Note 11. Income Taxes
Earnings before income taxes are as follows (in thousands):
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
Domestic |
$ | 12,708 | $ | 12,913 | $ | 14,427 | ||||||
|
Foreign |
(12,407 | ) | 1,383 | 1,128 | ||||||||
| Total earnings before income taxes | $ | 301 | $ | 14,296 | $ | 15,555 | ||||||
The components of our provision for income taxes are as follows (in thousands):
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
Current tax provision |
||||||||||||
|
Federal |
$ | 3,732 | $ | 2,282 | $ | 3,666 | ||||||
|
State |
715 | 510 | 627 | |||||||||
|
Foreign |
1,299 | 849 | 658 | |||||||||
| Total current tax expense | 5,746 | 3,641 | 4,951 | |||||||||
|
Deferred tax provision: |
||||||||||||
|
Federal |
(1,589 | ) | (126 | ) | (189 | ) | ||||||
|
State |
(216 | ) | (32 | ) | (138 | ) | ||||||
|
Foreign |
(678 | ) | (370 | ) | (238 | ) | ||||||
| Total deferred tax expense | (2,483 | ) | (528 | ) | (565 | ) | ||||||
| Total income tax expense | $ | 3,263 | $ | 3,113 | $ | 4,386 | ||||||
The components of net deferred tax assets and liabilities are as follows (in thousands):
|
March 31, |
||||||||
|
2018 |
2017 |
|||||||
|
Current deferred tax assets: |
||||||||
|
Accrued employee-related expenses |
$ | 277 | $ | 242 | ||||
|
Allowances and reserves |
101 | 132 | ||||||
|
Stock compensation deductible differences |
779 | 898 | ||||||
|
Inventories |
1,388 | 691 | ||||||
|
Currency translation adjustment |
51 | -- | ||||||
|
Net operating loss |
90 | -- | ||||||
|
Foreign tax credit |
100 | -- | ||||||
|
Other |
-- | 9 | ||||||
| Total current deferred tax assets | 2,786 | 1,972 | ||||||
|
Long-term deferred tax liabilities: |
||||||||
|
Property, plant and equipment |
(1,236 | ) | (1,635 | ) | ||||
|
Goodwill and intangible assets |
(3,940 | ) | (3,807 | ) | ||||
|
Currency translation adjustment |
-- | (3 | ) | |||||
|
Other |
(4 | ) | (81 | ) | ||||
| Total long-term deferred tax liabilities | (5,180 | ) | (5,526 | ) | ||||
|
Valuation allowance |
(100 | ) | -- | |||||
|
Net deferred tax liability |
$ | (2,494 | ) | $ | (3,554 | ) | ||
A reconciliation of our income tax provision and the amounts computed by applying statutory rates to income before income taxes is as follows (in thousands):
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
Federal income taxes at statutory rates |
$ | 93 | $ | 4,861 | $ | 5,445 | ||||||
|
State income taxes, net of federal benefit |
328 | 302 | 293 | |||||||||
|
Tax benefit of stock option exercises |
(1,087 | ) | (1,576 | ) | (751 | ) | ||||||
|
Section 199 manufacturing deduction |
(381 | ) | (304 | ) | (440 | ) | ||||||
|
Research and development credit |
(162 | ) | (385 | ) | (345 | ) | ||||||
|
Tax Cuts and Jobs Act |
(59 | ) | -- | -- | ||||||||
|
Impairment of non-deductible goodwill |
4,257 | -- | -- | |||||||||
|
Other |
274 | 215 | 184 | |||||||||
| Total income tax expense | $ | 3,263 | $ | 3,113 | $ | 4,386 | ||||||
On December 22, 2017, the Tax Cuts and Jobs Act ("TCJA") was enacted in the U.S., making significant changes to U.S. tax law. The TCJA reduces the U.S. federal corporate income tax rate from 34 percent to 21 percent, requires companies to pay a one-time transition tax on certain un-remitted earnings of foreign subsidiaries that were previously tax deferred, generally eliminates U.S. federal income tax on dividends from foreign subsidiaries, creates new taxes on certain foreign-sourced earnings, repeals the Section 199 deduction and imposes limitations on executive compensation under Section 162(m). During the year ended March 31, 2018, we revised our estimated annual effective tax rate to reflect the change in the federal statutory rate. The rate change results in the Company using a blended statutory rate for the annual period of 30.8 percent.
Shortly thereafter, the SEC staff issued SAB 118, which provides guidance on accounting for the tax effects of the TCJA for which the accounting under ASC 740 is incomplete. To the extent that a company's accounting for certain income tax effects of the TCJA is incomplete but it is able to determine a reasonable estimate, it must record a provisional estimate in the financial statements. If a company cannot determine a provisional estimate to be included in the financial statements, it should continue to apply ASC 740 on the basis of the provisions of the tax laws that were in effect immediately before enactment of the TCJA.
Accordingly, as of March 31, 2018, we have not completed our accounting for the tax effects of the TCJA. However, we made a reasonable estimate of the one-time transition tax and recognized a provisional tax liability of $220,000. We also re-measured the applicable deferred tax assets and liabilities based on the rates at which they are expected to reverse. However, we are still analyzing certain aspects of the TCJA and refining our calculations, which could potentially affect the measurement of these balances or potentially give rise to new deferred tax amounts. The provisional amount recorded related to the re-measurement of our deferred tax balance was a benefit of $279,000. Overall, the TCJA resulted in a net tax benefit of $59,000. Such amount was recorded as a tax benefit and is included as a component of income tax expense in the accompanying condensed consolidated statements of operations for the year ending March 31, 2018.
We or one of our subsidiaries files income tax returns in the U.S. federal jurisdiction and various state and foreign jurisdictions. Our federal tax returns for all years after 2014, state tax returns after 2013 and foreign tax returns after 2013 are subject to future examination by tax authorities for all our tax jurisdictions. Although the outcome of tax audits, if any, is always uncertain, we believe that we have adequately accrued for all amounts of tax, including interest and penalties and any adjustments that may result.
As of March 31, 2018, the gross amount of unrecognized tax benefits was $827,000. There would have been no material impact on our effective tax rate for the year ended March 31, 2018 had these benefits been recognized. We recognize interest and penalties related to unrecognized tax benefits in other expense and general and administrative expense, respectively. Accrued interest and penalties related to unrecognized tax benefits were $24,000, $17,000 and $3,000 as of March 31, 2018, 2017 and 2016, respectively.
A reconciliation of the changes in the gross balance of unrecognized tax benefit amounts is as follows (in thousands):
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
Beginning balance |
$ | 331 | $ | 221 | $ | -- | ||||||
|
Increases related to current period tax positions |
496 | 110 | 221 | |||||||||
|
Ending balance |
$ | 827 | $ | 331 | $ | 221 | ||||||
We expect that the amount of unrecognized tax benefits will change in the next 12 months; however, we do not expect the change to have a significant impact on our consolidated statements of operations or consolidated balance sheets. At this time, we expect resolution of the uncertain tax position within 12 months.
As of March 31, 2018, undistributed earnings of our foreign subsidiaries amounted to $3,724,450. Those earnings are considered indefinitely reinvested and, accordingly, no U.S. federal and state income taxes have been provided thereon. Upon distribution of those earnings in the form of dividends or otherwise, we would be subject to both U.S. income taxes (subject to an adjustment for foreign tax credits) and withholding taxes payable to the various foreign countries. Determination of the amount of unrecognized deferred U.S. income tax liability is not practicable because of the complexities associated with its hypothetical calculation; however, unrecognized foreign tax credits would be available to reduce a portion of the U.S. tax liability.
As of March 31, 2018, we had $272,000 of net operating losses for foreign tax purposes. The foreign net operating losses do not expire. In addition, we had $100,000 of foreign tax credit carryovers which will expire in the tax year 2028.
Note 12. Net (Loss) Income Per Share
Basic net (loss) income per share is computed by dividing net income by the weighted-average number of common shares outstanding during the reporting period. Diluted net (loss) income per share is computed similarly to basic net (loss) income per share, except that it includes the potential dilution that could occur if dilutive securities were exercised.
The following table presents a reconciliation of the denominators used in the computation of net (loss) income per share - basic and diluted (in thousands, except share data):
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
Net (loss) income available for shareholders |
$ | (2,962 | ) | $ | 11,183 | $ | 11,169 | |||||
|
Weighted average outstanding shares of common stock |
3,770 | 3,679 | 3,605 | |||||||||
|
Dilutive effect of stock options |
-- | 165 | 152 | |||||||||
|
Common stock and equivalents |
3,770 | 3,844 | 3,757 | |||||||||
|
Net (loss) income per share: |
||||||||||||
|
Basic |
$ | (0.79 | ) | $ | 3.04 | $ | 3.10 | |||||
|
Diluted |
(0.79 | ) | 2.91 | 2.97 | ||||||||
For the years ended March 31, 2018, 2017 and 2016, 106,000, 110,000 and 137,000 outstanding stock options, respectively, were excluded from the calculation of diluted earnings per share because the exercise prices of the stock options were greater than or equal to the average price of the common shares and, therefore, their inclusion would have been anti-dilutive. Additionally, as a result of generating a net loss for the year ended March 31, 2018, 352,358 additional outstanding stock options were excluded from the calculation of diluted earnings per share.
Note 13. Commitments and Contingencies
In February 2018, we were sued in a putative civil class action in the United States District Court for the Northern District of Illinois, Eastern Division whereby it was alleged that we sent unsolicited advertisements to telephone facsimile machines in violation of the Telephone Consumer Protection Act (“TCPA”), as well as analogous state statutes and state consumer protection laws. The plaintiff in this lawsuit is seeking various forms of relief, including statutory damages of $500 for each violation of the TCPA or, in the alternative, treble damages of up to $1,500 for each knowing and willful violation of the TCPA, as well as payment of interest, attorneys’ fees and costs, and certain injunctive relief prohibiting the further transmission of unsolicited fax advertising in the future. We intend to vigorously defend this case however we cannot predict with any degree of certainty the outcome of the lawsuit or determine the extent of any potential liability or damages.
Under the terms of the PCD Agreement, we were required to pay contingent consideration if the cumulative revenues for our process challenge device business for the three years subsequent to the acquisition met certain levels. The potential consideration payable ranged from $0 to $1,500,000 and was based upon a sliding scale of three-year cumulative revenues between $9,900,000 and $12,600,000, with payments made annually. Based upon both historical and projected growth rates, we initially recorded $300,000 of contingent consideration payable which represented our best estimate of the amount that would ultimately be paid. We paid $150,000 of the contingent consideration during the year ended March 31, 2016 (based upon the then current run rate projected over the entire three-year contingent consideration period).
Since the initial payment, the revenues for these products significantly increased and as a result, during the year ended March 31, 2017 we recorded an additional $450,000 accrual (which was paid in our third quarter ending December 31, 2016). During the year ended March 31, 2018, process challenge device (“PCD”) product revenues continued to increase which resulted in an additional $300,000 accrual, which is included in other income, net in the accompanying consolidated statement of operations for the year ended March 31, 2018. We paid the remaining contingent consideration due of $450,000 in November 2017.
On November 6, 2013, we completed a business combination (the “Amega Acquisition”) whereby we acquired substantially all of the assets and certain liabilities of Amega Scientific Corporation’s (“Amega”) business which provides continuous monitoring systems to regulated industries. Under the terms of the Acquisition Agreement (the “Amega Agreement”), we were required to pay contingent consideration (the “Amega Earn-Out”) if the cumulative revenues for our Cold Chain Monitoring Division for the three years subsequent to the acquisition met certain levels. The potential consideration payable ranged from $0 to $10,000,000 and was based upon a sliding scale of three-year cumulative revenues between $31,625,000 and $43,500,000. Based upon both historical and projected growth rates, we recorded $500,000 of contingent consideration payable which represented our best estimate of the amount that would ultimately be paid. Any changes to the contingent consideration ultimately paid would have resulted in additional income or expense in our consolidated statements of income. The contingent consideration would have been payable in the third quarter of our year ending March 31, 2017.
In November 2014, Amega and its owner Anthony Amato (“Amato”) filed a complaint (Anthony Amato and Amega Scientific Corporation v. Mesa Laboratories, Inc., Civil Action No. 1:14-cv-03228) in the United States District Court for the District of Colorado asserting, among other items, that our termination of Amato as an employee impacted his ability to maximize the potential consideration payable under the Amega Earn-Out and to exercise stock options that failed to vest. The plaintiff was seeking an immediate maximum payout of $10,000,000 under the Amega Earn-Out, the immediate acceleration of the 10,000 stock options granted Amato upon his initial employment along with other consequential damages in excess of $500,000, lost future earnings and punitive damages. In addition, Amato alleged that we improperly withheld $704,065.86 from the holdback consideration under the Amega Agreement. In January 2015, we filed a motion to dismiss the complaint with prejudice.
In October 2015, we entered into a settlement agreement (the “Amato Settlement”) whereby we paid Amato $3,165,000. In exchange, Amato agreed to dismiss the complaint, release Mesa of any and all claims by Amega and Amato and relieve us of any future payment obligation under the Amega Earn-Out. Insurance covered $415,000 of the settlement payment and we had $1,041,000 accrued on our consolidated balance sheet remaining from the original hold back and contingent consideration payable. The remaining $1,709,000 was recorded as general and administrative expense in the accompanying consolidated statements of operations for the year ended March 31, 2016.
Note 14. Accumulated Other Comprehensive Income (Loss)
The following table summarizes the changes in each component of accumulated other comprehensive income (loss) (“AOCI”), net of tax (in thousands):
|
Foreign Currency Translation |
AOCI |
|||||||
|
Balance at March 31, 2016 |
$ | (1,151 | ) | $ | (1,151 | ) | ||
|
Unrealized losses arising during the year |
(609 | ) | (609 | ) | ||||
|
Balance at March 31, 2017 |
(1,760 | ) | (1,760 | ) | ||||
|
Unrealized gains arising during the year |
2,324 | 2,324 | ||||||
|
Balance at March 31, 2018 |
$ | 564 | $ | 564 | ||||
Note 15. Segment Data
We have four operating segments: Sterilization and Disinfection Control (formerly named Biological Indicators), Instruments, Cold Chain Monitoring and Cold Chain Packaging. The following tables set forth our segment information (in thousands):
|
Year Ended March 31, 2018 |
||||||||||||||||||||
|
Sterilization and Disinfection Control |
Instruments |
Cold Chain Monitoring |
Cold Chain Packaging |
Total |
||||||||||||||||
|
Revenues |
$ | 43,260 | $ | 34,104 | $ | 12,978 | $ | 5,837 | $ | 96,179 | ||||||||||
|
Gross profit |
$ | 29,333 | $ | 20,395 | $ | 3,854 | $ | 1,037 | 54,619 | |||||||||||
|
Reconciling items (1) |
(54,318 | ) | ||||||||||||||||||
|
Earnings before income taxes |
$ | 301 | ||||||||||||||||||
|
Year Ended March 31, 2017 |
||||||||||||||||||||
|
Sterilization and Disinfection Control |
Instruments |
Cold Chain Monitoring |
Cold Chain Packaging |
Total |
||||||||||||||||
|
Revenues |
$ | 38,635 | $ | 34,405 | $ | 12,584 | $ | 8,041 | $ | 93,665 | ||||||||||
|
Gross profit |
$ | 25,674 | $ | 21,037 | $ | 4,557 | $ | 1,971 | 53,239 | |||||||||||
|
Reconciling items (1) |
(38,943 | ) | ||||||||||||||||||
|
Earnings before income taxes |
$ | 14,296 | ||||||||||||||||||
|
Year Ended March 31, 2016 |
||||||||||||||||||||
|
Sterilization and Disinfection Control |
Instruments |
Cold Chain Monitoring |
Cold Chain Packaging |
Total |
||||||||||||||||
|
Revenues |
$ | 33,649 | $ | 35,692 | $ | 11,566 | $ | 3,752 | $ | 84,659 | ||||||||||
|
Gross profit |
$ | 22,205 | $ | 23,223 | $ | 4,201 | $ | 1,784 | 51,413 | |||||||||||
|
Reconciling items (1) |
(35,858 | ) | ||||||||||||||||||
|
Earnings before income taxes |
$ | 15,555 | ||||||||||||||||||
(1) Reconciling items include selling, general and administrative, research and development, impairment of goodwill and other expenses.
Revenues from external customers are attributed to individual countries based upon locations to which the product is shipped or exported, as follows (in thousands):
|
Year Ended March 31, |
||||||||||||
|
2018 |
2017 |
2016 |
||||||||||
|
Revenues from unaffiliated customers |
||||||||||||
|
United States |
$ | 56,998 | $ | 52,989 | $ | 53,094 | ||||||
|
Foreign |
39,181 | 40,676 | 31,565 | |||||||||
| $ | 96,179 | $ | 93,665 | $ | 84,659 | |||||||
No foreign country exceeds ten percent of total revenues.
|
March 31, |
||||||||
|
2018 |
2017 |
|||||||
|
Total assets |
||||||||
|
Sterilization and Disinfection Control |
$ | 83,452 | $ | 67,233 | ||||
|
Instruments |
33,479 | 40,805 | ||||||
|
Cold Chain Monitoring |
30,796 | 35,789 | ||||||
|
Cold Chain Packaging |
7,091 | 20,313 | ||||||
|
Corporate and administrative |
9,283 | 7,593 | ||||||
| $ | 164,101 | $ | 171,733 | |||||
All long-lived assets are located in the United States except for $6,765,000, $7,079,000 and $17,068,000 which are associated with our French, Canadian and German subsidiaries, respectively.
Note 16. Quarterly Results (unaudited)
Quarterly financial information for the years ended March 31, 2017, 2016 and 2015 is summarized as follows (net income per share per quarter will not add up to reported annual earnings per share due to differences in average outstanding shares as reported on a quarterly basis) (in thousands, except per share data):
|
First |
Second |
Third |
Fourth |
|||||||||||||
|
2018 |
Quarter |
Quarter |
Quarter |
Quarter |
||||||||||||
|
Revenues |
$ | 22,673 | $ | 22,954 | $ | 23,671 | $ | 26,881 | ||||||||
|
Gross profit |
12,671 | 13,233 | 12,681 | 16,034 | ||||||||||||
|
Net income (loss) |
1,517 | 2,353 | (11,086 | ) | 4,254 | |||||||||||
|
Net income (loss) per share – basic |
$ | 0.41 | $ | 0.63 | $ | (2.93 | ) | $ | 1.12 | |||||||
|
Net income (loss) per share – diluted |
0.39 | 0.60 | (2.93 | ) | 1.08 | |||||||||||
|
First |
Second |
Third |
Fourth |
|||||||||||||
|
2017 |
Quarter |
Quarter |
Quarter |
Quarter |
||||||||||||
|
Revenues |
$ | 21,114 | $ | 24,409 | $ | 23,843 | $ | 24,299 | ||||||||
|
Gross profit |
12,014 | 13,724 | 13,537 | 13,964 | ||||||||||||
|
Net income |
1,930 | 2,358 | 3,252 | 3,643 | ||||||||||||
|
Net income per share – basic |
$ | 0.53 | $ | 0.64 | $ | 0.88 | $ | 0.98 | ||||||||
|
Net income per share – diluted |
0.51 | 0.62 | 0.84 | 0.94 | ||||||||||||
|
First |
Second |
Third |
Fourth |
|||||||||||||
|
2016 |
Quarter |
Quarter |
Quarter |
Quarter |
||||||||||||
|
Revenues |
$ | 18,158 | $ | 21,776 | $ | 19,913 | $ | 24,812 | ||||||||
|
Gross profit |
11,141 | 13,067 | 12,209 | 14,996 | ||||||||||||
|
Net income |
2,755 | 1,826 | 2,597 | 3,991 | ||||||||||||
|
Net income per share – basic |
$ | 0.77 | $ | 0.51 | $ | 0.72 | $ | 1.10 | ||||||||
|
Net income per share – diluted |
0.74 | 0.48 | 0.69 | 1.06 | ||||||||||||
Note 17. Subsequent Events
In April 2018, our Board of Directors declared a quarterly cash dividend of $0.16 per share of common stock, payable on June 15, 2018, to shareholders of record at the close of business on May 31, 2018.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended) that are designed to reasonably ensure that information required to be disclosed by us in the reports we file or submit under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms and that such information is accumulated and communicated to our management, including our principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Our management evaluated, with the participation of our Chief Executive Officer and Chief Financial Officer, the effectiveness of our disclosure controls and procedures as of March 31, 2018. Based on that evaluation, our management concluded that our disclosure controls and procedures were effective at March 31, 2018.
Our management, including our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with generally accepted accounting principles in the United States. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance of achieving their control objectives. Management evaluated the effectiveness of our internal control over financial reporting based on the framework in “Internal Control – Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in 2013.
Our management evaluated, with the participation of our Chief Executive Officer and Chief Financial Officer, the effectiveness of our internal control over financial reporting as of March 31, 2018. Based on that evaluation, our management concluded that our internal control over financial reporting was effective at March 31, 2018. As allowed, this evaluation excludes the operations of acquired entities during the year ended March 31, 2018 due to the timing of the acquisitions. Revenues related to these acquisitions were three percent of total revenues for the year ended March 31, 2018.
Our independent auditors, EKS&H LLLP, a registered public accounting firm, are appointed by the Audit Committee of our Board of Directors, subject to ratification by our shareholders. EKS&H LLLP has audited and reported on the consolidated financial statements of Mesa Laboratories, Inc. and our internal control over financial reporting as of March 31, 2018. The attestation report of our registered public accounting firm is contained in this annual report.
Changes in internal control over financial reporting
There were no significant changes in our internal control over financial reporting that occurred during the quarter ended March 31, 2018, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
Part III
Item 10. Directors, Executive Officers, and Corporate Governance
The Board of Directors and its Committees
Our business is managed through the oversight and direction of our Board of Directors. We have three standing committees: Audit, Compensation, and Nominating and Governance. Our Board of Directors currently consists of eight persons. Under applicable Nasdaq and SEC requirements, (a) we are required to have a majority of independent directors, and (b) all of the members of each committee, with the exception of the Nominating and Governance Committee, must be independent. The Board of Directors has affirmatively determined that each of H. Stuart Campbell, Michael T. Brooks, David M. Kelly, John B. Schmieder, Evan C. Guillemin and Robert V. Dwyer is an “independent director” as such term is defined in Nasdaq Listing Rule 5605. The Board of Directors has also affirmatively determined that each member of each committee of the Board of Directors satisfies the independence requirements applicable to committees as prescribed by the Nasdaq Listing Rules and the rules and regulations of the SEC. Dr. Sullivan and Mr. Owens are not an “independent director” because they are our employees.
The Board of Directors has the responsibility for establishing broad corporate policies and for our overall performance, although it is not involved in day-to-day operating details. The Board of Directors meets regularly throughout the year, including the annual organizational meeting following the Annual Meeting of Shareholders (“Annual Meeting”), to review significant developments affecting the Company and to act upon matters requiring Board of Director approval. It also holds special meetings as required from time to time when important matters arise, requiring Board of Director action between scheduled meetings.
Directors are elected at each Annual Meeting and serve a one-year term or until a successor is duly elected and qualified at an appropriate Annual Meeting. Vacancies may be filled by an affirmative vote of the majority of the remaining directors.
Each director is compensated separately (other than the President and Chief Executive Officer) for service on the Board of Directors and is reimbursed for expenses to attend Board of Director meetings. Chairs of the Audit, Compensation, and Nominating and Governance Committees are compensated separately for service on those committees.
Meeting Attendance and Preparation
The Board of Directors met eight times during the year ended March 31, 2018. Each director attended at least 75% of the Board of Director meetings, and at least 75% of the regular and special meetings of the committees on which they serve, either in person or telephonically. In addition, directors are required to prepare for each meeting by reviewing materials distributed in advance.
Directors and Executive Officers
The following table sets forth the names and ages of all of our directors and executive officers, and the positions held by each such person as of March 31, 2018. Each of the directors holds office until the next Annual Meeting or until his successor is elected and qualified or until his earlier death, resignation or removal. Each officer serves at the discretion of the Board of Directors.
|
Name |
Age |
Postion |
|
John J. Sullivan, Ph.D. (3) |
65 |
Chairman of the Board of Directors |
|
Gary M. Owens(3) |
50 |
Chief Executive Officer, President and Director |
|
John V. Sakys |
49 |
Chief Financial Officer and Treasurer |
|
Glenn E. Adriance(2) |
63 |
Chief Sales and Marketing Officer |
|
H. Stuart Campbell (1) |
88 |
Director, Lead Independent Director |
|
Michael T. Brooks (1) |
68 |
Director |
|
John B. Schmieder (1) |
49 |
Director |
|
Robert V. Dwyer (1) |
77 |
Director |
|
Evan C. Guillemin (1) |
52 |
Director |
|
David M. Kelly (1) |
76 |
Director |
(1) Member of the Audit, Compensation and Nominating and Governance Committees.
(2) Effective April 6, 2018 Mr. Adriance retired as Chief Sales and Marketing Officer.
(3) Effective September 1, 2017 John J. Sullivan retired as President and Chief Executive Officer and Gary M. Owens was named President and Chief Executive Officer.
John J. Sullivan, Ph.D. was appointed as the Chairman of the Board of Directors upon his retirement from his position as Chief Executive Officer and President in September 2017. Dr. Sullivan was previously promoted to the position of Chief Executive Officer and President, and appointed to the Board of Directors, in March 2009. Dr. Sullivan joined us in October 2004 in the role of Vice President of Sales and Marketing and was promoted to the positions of President and Chief Operating Officer in 2006. In 1988, Dr. Sullivan joined Varian, Inc. (a major analytical instrument manufacturer) and served in various capacities in Research and Development, Sales and Marketing Management, and Business Development until 2004. In 1982, Dr. Sullivan joined the U.S. Food and Drug Administration’s Seattle District Laboratory as a Senior Research Scientist and worked there until 1988. From 1976 until 1980, Dr. Sullivan was employed as an Analytical Chemist at BioMed Research Labs (an independent research and testing laboratory). Dr. Sullivan received his Bachelor of Science degree in Biology from Western Washington University in 1976 and a Ph.D. degree in Food Science from the University of Washington in 1982.
Gary M. Owens was promoted to the position of Chief Executive Officer and President, and appointed to the Board of Directors, in September 2017. Mr. Owens joined us in March 2017 as our Chief Operating Officer. From 2006 through March 2017, Mr. Owens held several positions with Danaher Corporation. From 2016 through March 2017, Mr. Owens served as Corporate Vice-President of Strategic Development for Danaher Corporation and was seconded to Pall Corporation, a wholly owned subsidiary of Danaher Corporation as Commercial Integration Lead. From 2012 to 2016, Mr. Owens served as Vice-President and General Manager of Beckman Coulter Life Sciences, a wholly owned subsidiary of Danaher Corporation. From 2009 to 2012, Mr. Owens was Corporate Vice-President of Strategic Development for Danaher Corporation. From 2006 to 2009, Mr. Owens served as the Group Vice-President of Business Development for Danaher Industrial. From 1998 to 2006, Mr. Owens served in various product management, sales and business development roles for Canon Incorporated and Trilogy Software. From 1994 to 1998, Mr. Owens was a team leader for Bain & Company. Mr. Owens received his BSE in Mechanical Engineering from Tulane University in 1990 and his Master’s degree in Business Administration from Columbia Business School in 1994.
John V. Sakys joined us in October 2012 as our Chief Financial Officer. From 2009 through October 2012, Mr. Sakys held several positions with The Berry Company, LLC, and its predecessor company, Local Insight Regatta Holdings, Inc., most recently as its Vice President and Chief Accounting Officer. From 2001 to 2009, Mr. Sakys was the Vice President and Chief Financial Officer of Isonics Corporation, a former Nasdaq listed company based in the Denver area. From September 2000 to April 2001, Mr. Sakys was Controller of AuraServ Communications. From July 1998 to September 2000, Mr. Sakys was Director of Financial Reporting for Media One, Inc. From December 1994 to July 1998, Mr. Sakys was an audit manager at Ernst and Young LLP. Mr. Sakys received his Bachelor of Arts degree in Business Economics with an emphasis in accounting from the University of California at Santa Barbara in 1990 and is a Certified Public Accountant.
Glenn E. Adriance joined us in October 2007 and retired in April 2018. From 2000 to 2007, Mr. Adriance was employed with two other software firms, Lakeview Technology and Scientific Technologies Corporation as Global Business Partner Director and VP/COO/Executive Officer, respectively. From 1983 until 2000, Mr. Adriance held various sales and marketing roles with increasing responsibility at IBM. From 1981 to 1983, Mr. Adriance was employed at Sandia National Laboratories as a senior Business Systems Analyst responsible for various business systems that were fundamental to Sandia’s operations. Mr. Adriance received his Bachelor of Science degree in Forestry from the University of Massachusetts in 1978 and his Master’s degree in Business Administration from Colorado State University in 1981.
H. Stuart Campbell has served as a director since May 1983 and was appointed as Lead Independent Director in September 2017 while previously serving as the Chairman of the Board of Directors from April 2015 until September 2017. Mr. Campbell owned and served as an officer of Highland Packaging Labs, Inc., Somerville, New Jersey (contract packaging business) until its sale in 2002. From 1977 through September 1982, he was a Company Group Chairman with Johnson & Johnson and served as Chief Executive Officer and Chairman of the Board of Directors of eight major corporate subsidiaries. From 1960 through September 1982, Mr. Campbell served in various capacities for Johnson & Johnson and Ethicon, Inc., a domestic subsidiary of Johnson & Johnson. Mr. Campbell received his Bachelor of Science degree from Cornell University in 1951.
Michael T. Brooks has served as a director since October 1998. Mr. Brooks was an independent manufacturer’s representative from 1982 to 1985, at which time he purchased an interest in Fiero Fluid Power, which he presently owns and operates. Fiero Fluid Power is a Rep/Distributor selling pneumatic and instrumentation equipment. While pursuing a career in fluid power, he received a Master’s degree in Business Administration from the University of Denver in 1983. Mr. Brooks received his Bachelor of Arts degree in History from Ohio Wesleyan University in 1971.
John B. Schmieder has served as a director since April 2015. Mr. Schmieder has been the co-owner and operator of Community Acupuncture of Saint Louis since 2005 and Holistic Fitness since 2010. From 2008 to 2010, Mr. Schmieder also served as the president of the Acupuncture Association of Missouri. From 1995 to 2002, Mr. Schmieder served as an equity and financial analyst at Macy’s Corporation, George K. Baum &Co. and Citizens Funds (now Sentinel Investments). From 1990 to 1993, Mr. Schmieder served in various positions, including senior auditor, at Arthur Andersen & Co. He received a Bachelor of Administration in Business degree from the University of San Diego in 1990, a Master’s degree in Business Administration with emphasis in finance and corporate strategy from the University of Michigan in 1995 and a Master’s of Oriental Medicine from the New England School of Acupuncture in 2005.
Robert V. Dwyer has served as a director since May 2006. Mr. Dwyer served as President of our Raven Biological Laboratories operation until November 2010. Mr. Dwyer currently serves on the Board of Directors of American National Bank, based in Omaha, Nebraska. In addition, Mr. Dwyer holds ownership positions in other small business entities. Mr. Dwyer served as President and was the majority owner of Raven Biological Laboratories, Inc. and is also an Attorney at Law. Mr. Dwyer received his Bachelor of Arts in Philosophy degree from Creighton University in 1962, and he received his J.D. from Creighton University in 1964.
Evan C. Guillemin has served as a director since February 2009. Mr. Guillemin has served as Chief Financial Officer (“CFO”) and Associate Portfolio Manager at Select Equity Group Inc., a registered investment adviser based in New York City, since 2004. Mr. Guillemin also currently serves on the Board of Directors of Shake Shack, Inc. (NYSE: SHAK), and a privately held company. Prior to joining Select Equity Group, Mr. Guillemin served as CFO of Alloy Merchandising Group Inc., the successor to Delias Inc. Mr. Guillemin was an executive and board member of Delias Inc., a Nasdaq-traded specialty retailing company. He served as CFO and then Chief Operating Officer of Delias from 1996 to 2003, when the company was acquired by Alloy Inc. He received his Bachelor of Arts degree from Yale University in 1987 and a Master's degree in Business Administration with distinction from Harvard Business School in 1996.
David M. Kelly has served as a director since October 2010. Mr. Kelly currently serves as a member of the Board of Directors of Federated Investors, Inc. (NYSE: FII), Mestek (OTC: MCCK), and a privately held company. In 1995, Mr. Kelly joined Matthews International Corporation, where he served as Chairman of the Board, Chief Executive Officer and President until his retirement in 2007. From 1972 to 1995, Mr. Kelly was with Carrier Corporation and held a variety of executive positions, in the United States and in Asia, in marketing, finance, manufacturing and operations, including President of North America operations. He received a Bachelor of Science degree in Physics from Boston College in 1964, a Master’s degree in Molecular Biophysics from Yale in 1966, and a Masters of Business Administration from Harvard Business School in 1968.
Unless otherwise indicated, no director has held any other directorships for the past five years.
Significant Employees and Family Relationships
There are no family relationships among the Named Executive Officers (“NEOs”), directors or any person chosen to become a director or executive officer.
Involvement in Certain Legal Proceedings
Based on information submitted by the directors and executive officers, none of the directors or executive officers is involved in, or has been involved in, legal proceedings during the past ten years that are material to an evaluation of the ability or integrity of any such director or executive officer.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934 (the “Exchange Act’) requires our directors, executive officers and persons who own more than five percent of a registered class of our equity securities to file with the SEC initial reports of ownership and reports of changes in ownership of our common stock. Officers, directors and greater than five percent shareholders are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file. To our knowledge, based upon a review of the copies of such reports furnished to us and based upon written representations that no other reports were required, all Section 16(a) filing requirements applicable to our officers, directors and greater than five percent beneficial shareholders were complied with during the fiscal year ended March 31, 2018.
Committees of the Board of Directors
The charter of each committee is available in print to any shareholder who requests it, or on our website at http://mesalabs.com/corporate. Each of the following directors is a member of all of our committees (Audit, Compensation, and Nominating and Governance):
Michael T. Brooks
H. Stuart Campbell, Nominating and Governance Committee Chairman
Robert V. Dwyer
Evan C. Guillemin, Audit Committee Chairman
David M. Kelly, Compensation Committee Chairman
John B. Schmieder
In addition to the standing committees mentioned above, the Board of Directors may convene special committees to consider various other matters as they arise. During the year ended March 31, 2018, the Board of Directors did not convene any special committees.
Audit Committee
Pursuant to its charter, the Audit Committee assists the Board of Directors in overseeing (i) the consolidated financial statements and audits of the Company, (ii) our compliance with financial reporting requirements, and (iii) the independence and performance of our internal and external auditors. The Audit Committee charter further requires the Audit Committee to, among other things:
|
● |
Review the annual audited consolidated financial statements with management and the independent auditors and determine whether to recommend to the Board of Directors that they be included in our Annual Report on Form 10-K; |
|
● |
Review proposed major changes to our auditing and accounting principles and practices; |
|
● |
Review and evaluate our system of internal control; |
|
● |
Review significant financial reporting issues raised by management or the independent auditors; |
|
● |
Approve the compensation of the independent auditors for annual audit and quarterly review work; and |
|
● |
Establish procedures for the receipt, retention and treatment of complaints regarding accounting, internal accounting controls or auditing matters as well as the confidential and anonymous submission by our employees of concerns regarding questionable accounting or auditing matters. |
The Audit Committee met four times during the year ended March 31, 2018. All members of the Audit Committee were present at each meeting. The Board of Directors has determined that Mr. Evan Guillemin is an “audit committee financial expert” as defined in the applicable rules and regulations of the Exchange Act and is “independent.” The SEC has indicated that the designation of a person as an “audit committee financial expert” does not (i) mean that such person is an expert for any purpose, including without limitation for purposes of Section 11 of the Securities Act of 1933, as amended (ii) impose on such person any duties, obligations, or liability that are greater than the duties, obligations, and liability imposed on such person as a member of the Audit Committee and the Board of Directors in the absence of such designation, or (iii) affect the duties, obligations, or liability of any other member of the Audit Committee or the Board of Directors.
As required by Nasdaq, our Board of Directors has reviewed the qualifications of our Audit Committee members and has determined that none of them has a relationship with us that may interfere with the exercise of their independence from management and the Company.
Compensation Committee
Pursuant to its charter, the Compensation Committee assists the Board of Directors in fulfilling its oversight responsibilities for compensation of executive officers and administration of our compensation and benefit plans. The Compensation Committee met three times during the year ended March 31, 2018, and all members of the Compensation Committee were present at each meeting.
During the year ended March 31, 2018, no members of our Compensation Committee were executive officers serving on the compensation committee of another entity whose executive officers served on our Board of Directors. No member of the Compensation Committee was an officer or employee of the Company or had a business relationship with or conducted any undisclosed transactions with the Company. Our Chief Executive Officer and/or other Officers, upon request, may attend selected meetings of the Compensation Committee.
Nominating and Governance Committee
The Nominating and Governance Committee assists the Board of Directors in identifying qualified individuals to become members of the Board of Directors, oversees, reviews and where appropriate, makes recommendations concerning the Company’s corporate governance guidelines and conducts an annual self-assessment of the Board of Directors performance. The Nominating and Governance Committee met two time during the year ended March 31, 2018, and all members of the Nominating and Governance Committee were present.
In evaluating potential director candidates, the Nominating and Governance Committee considers the appropriate balance of experience, skills and characteristics required of the Board of Directors, and seeks to ensure that at least a majority of the directors are independent under the applicable Listing Rules of Nasdaq. The Nominating and Governance Committee selects director nominees based on their personal and professional integrity, depth and breadth of experience, ability to make independent analytical inquiries, understanding of our business, willingness to devote adequate attention and time to duties of the Board of Directors, and such other criteria as are deemed relevant by the Nominating and Governance Committee. Consistent with the policy of the Company, the Nominating and Governance Committee will strive to develop a Board of Directors that is diverse and inclusive regarding gender, race and ethnicity. The Nominating and Governance Committee believes that the backgrounds and qualifications of the directors, considered as a group, should provide a diverse mix of experience, knowledge, and skills.
In identifying potential director candidates, the Nominating and Governance Committee relies on recommendations made by current directors and officers. In addition, the Nominating and Governance Committee may engage a third-party search firm to identify and recommend potential candidates. Finally, the Nominating and Governance Committee will consider candidates recommended by shareholders.
Risk Oversight
The Board of Directors takes a key role in overseeing our risks. The Board of Directors receives frequent timely reports of our financial performance, changes in and composition of consolidated balance sheet accounts, quality assurance program effectiveness, product liability risks and status of relationships with all business constituencies including customers, employees, suppliers and government entities. The Audit Committee receives regular reports on our compliance with securities laws and communications with the SEC and shareholders. The Audit Committee has established an independent whistleblower hot line to encourage early and anonymous reporting of accounting irregularities or other violations of our codes of ethics. The Board of Directors routinely reviews our litigation threats, product/market strategies and operational activities.
Code of Ethics
We adopted a Code of Business Conduct and Ethics (the “Code of Ethics”) that applies to all of our employees, executive officers and directors, including our principal executive officer and principal financial officer. The Code of Ethics contains written standards that are reasonably designed to deter wrongdoing and includes provisions regarding ethical conduct, conflicts of interest, proper disclosure in all public communications, compliance with all applicable governmental laws, rules and regulations, and the prompt reporting of violations of the Code of Ethics and accountability for adherence to the Code of Ethics. A copy of the Code of Ethics is available on our website at http://mesalabs.com/corporate.
Shareholder Communications with the Board of Directors
Shareholders and other interested parties may communicate with one or more members of the Board of Directors by writing to all or any of the following: Audit Committee Chairman, Compensation Committee Chairman or Nominating and Governance Committee Chairman, c/o Corporate Secretary, Mesa Laboratories, Inc., 12100 West Sixth Avenue, Lakewood CO 80228.
Item 11. Executive Compensation
Compensation Philosophy
The Compensation Committee supervises (on a direct basis for our three executive officers and a non-direct basis for all other NEOs) our executive compensation program for NEOs. The Compensation Committee has designed a compensation program for NEOs to attract, retain and motivate talent in our competitive market environment while focusing the executive team and the Company on the creation of long-term value for our shareholders. The Compensation Committee has the authority to engage outside consultants or purchase compensation surveys, if needed, for evaluation of executive compensation levels.
NEO positions during the year ended March 31, 2018 included: Chief Executive Officer and President, Chief Executive Officer/Chairman of the Board, Chief Financial Officer, Chief Sales and Marketing Officer and Senior Vice President of Commercial Operations. Other positions may be added as business conditions warrant.
We have designed our compensation programs for our NEOs to:
|
● |
attract and retain high performing and experienced executives; |
|
● |
motivate and reward executives whose knowledge, skills and performance are critical to our success; |
|
● |
align the interests of our executives and shareholders by motivating executives to increase shareholder value; |
|
● |
foster a shared commitment among executives by coordinating their goals; and |
|
● |
motivate our executives to manage our business to meet our short-term and long-term objectives and reward them for meeting these objectives. |
Our Compensation Committee administers four elements for NEOs: base salary (cash), short-term incentives (cash), long-term incentives (equity), and benefits. The total compensation package reflects our “Pay for Performance” philosophy, which is to couple employee rewards with the interests of our shareholders. We believe strongly that retention and motivation of successful employees is in the long-term interest of our shareholders. The Compensation Committee targets the total compensation level to be competitive with comparable companies in terms of size (as measured by revenues and market capitalization), our industry segments and geographic locations.
Benchmarking
The Compensation Committee, with assistance from our executives if required, researches compensation levels by investigating comparable company records, purchasing third party compensation surveys or engaging compensation consultants. The acquired data is evaluated by the Compensation Committee and is one factor in establishing compensation plans for the NEOs.
To help establish competitive compensation levels, the Compensation Committee examined executive compensation survey data, including “base salaries”, “incentive compensation” and total cash compensation, from Economic Research Institute (“ERI”). The survey data was tailored to include only those U.S. public companies in the “Instrument Manufacturing” segment with revenues between $50 - $100 million per year. This included companies that produced both medical devices and general electronic instruments, along with consumable supplies. The data was further adjusted for the geographic location of each NEO. The data from this analysis was used by the Compensation Committee as one factor in determining compensation levels for base salary and total compensation.
Determination of Target Compensation
For the year ended March 31, 2018, the Compensation Committee determined that an appropriate starting point for total compensation of our NEOs was approximately between the 50th and 75 th percentile level, compared to the data obtained from the ERI survey. The Compensation Committee used not only the data from the ERI survey, but also considered individual and team executive performance, along with our financial performance, as criteria to establish target compensation levels for each NEO. From that analysis, and in consideration of our past and future expected financial performance, the Compensation Committee made adjustments to base salaries and target total compensation levels for each NEO that were implemented effective June 1, 2017, except for Gary Owens, for whom compensation levels were established when he joined the Company in March 2017 and further modified effective September 1, 2017 upon his transition to President and Chief Executive Officer.
Base Salary
Base salaries for NEOs are determined based upon job responsibilities, level of experience, individual performance and comparisons to the salaries of executives in similar positions from the ERI survey.
Short-term Incentive Plan
Each NEO participates in our Short-term Incentive Plan. The Compensation Committee believes that it is in the best interest of our shareholders to have a substantial component of total compensation “at-risk” and dependent upon our financial performance. For the purpose of the Short-term Incentive Plan, performance is measured by two variables: revenues growth and profit growth. These variables are considered by the Compensation Committee to be a reliable indicator for the creation of long-term shareholder value. Bonus payouts under the Short-term Incentive Plan are tied directly to achievement of these revenues and profit growth targets for the year. If both the revenues and profit growth targets are exceeded by a substantial margin, the maximum bonus payments are set at between 50 percent and 115 percent of the base salary for the various NEOs. Of course, if our financial performance is poor, bonus payments could be at or near $0. The Compensation Committee reserves the right to adjust payments under the Short-term Incentive Plan, in the case of unusual circumstances or events, or economic conditions in general.
We do not disclose the specific revenues and profit growth targets set forth in the Short-term Incentive Plan as we believe that the disclosure thereof would cause us competitive harm. Because we are not disclosing these target objectives, we are stating our assessment of how likely it will be for these targets to be achieved by our NEOs. Although achievement of our target objectives involves future performance and, therefore, is subject to uncertainties, the Compensation Committee believes it has established target objectives that are achievable with an appropriate amount of dedication and hard work and, therefore, it is more likely than not that each NEO will earn a bonus under the Short-term Incentive Plan.
Long-term Incentive Plan
The Compensation Committee believes that it is in the best interest of our shareholders to provide long-term incentive to each NEO through their ownership of our stock. Stock ownership by our NEOs directly ties their long-term compensation to the performance of our share price. To achieve this goal, we make stock option grants (and in certain cases grants of restricted stock) to each NEO at the time of hire. We also make stock option grants on an annual basis under our stock compensation plan. These grants are non-qualified stock options with a term of generally from six to eight years. The grant price is set at 100% of the market price on the day of the grant and the options vest ratably over five to seven years. The awards may or may not have performance conditions associated with the vesting of the stock options. The number of stock options awarded is at the discretion of the Compensation Committee.
Other Benefits
Our philosophy is to provide only those other benefits to our named executives that are consistent with those generally offered to all of our other employees. As such, the NEOs have available various health, welfare and retirement (401(k)) benefits.
Employment and Change-in-Control Agreements. We have provided our Executive NEOs with employment agreements. The employment agreements provide that in the case of an involuntary termination of the executive without cause or for good reason (as defined), salary continuation benefits of one year annual salary (payable over a 12 month period), a prorated payment under the then current cash incentive plan equal to the amount the executive would have earned based on the actual performance of the Company for the relevant fiscal year had the executive’s employment not terminated and continuation of Company-provided health benefits for one year. In the case of an involuntary termination without cause or for good reason that occurs immediately prior to or within two years following a change of control (as defined), the employment agreements provide salary continuation benefits, payments under the then current cash incentive plan equal to the greater of the executive’s annual maximum cash incentive for the fiscal year in which the executive’s termination occurs or the executive’s annual maximum cash incentive payment for the fiscal year immediately preceding the fiscal year in which the executive’s termination occurs and Company-provided health benefits shall continue for two years.
In the case of an involuntary termination of the executive without cause or for good reason, the vesting and other terms of all unvested equity awards will be at the discretion of the Board of Directors, while the terms, conditions and restriction of the original grant and respective equity plans for vest equity awards shall remain, provided however, that in the case of stock options, the executive may exercise vested options at any time; (1) within one month after such termination in the case of options granted prior to March 31, 2017; and (2) prior to the natural termination date as stated in the original grant in the case of options granted after March 31, 2017. In the case of an involuntary termination without cause or for good reason immediately prior to or within two years following a change of control, all unvested equity awards will immediately become 100 percent vested, while all other terms, conditions and restrictions of the original grant and the respective equity plan for both vested and unvested equity awards shall remain, including the period during which equity awards may be exercisable.
Nonqualified Deferred Compensation. We do not have any nonqualified defined contribution or deferred compensation plans.
Post-Employment Compensation. We do not have any defined benefit plans, supplemental executive retirement plans or actuarial plans.
Summary Compensation Table
The following table lists compensation awarded to or earned by the NEOs for the years ended March 31, 2018, 2017 and 2016.
|
Name and Principal Position |
Year |
Salary |
Stock Awards(1) |
Option Awards(2) |
Non-equity Incentive Plan Compensation(3) |
All Other Compensation(4) |
Total |
||||||||||||||||||
|
(a) |
(b) |
(c) |
(e) |
(f) |
(g) |
(i) |
(j) |
||||||||||||||||||
|
John J. Sullivan, Ph.D.(5) |
2018 |
$ | 12,000 | $ | -- | $ | 582,000 | $ | -- | $ | 480 | $ | 594,480 | ||||||||||||
| Chairman of the Board | 2017 | 412,637 | -- | 255,300 | 207,130 | 12,379 | 887,446 | ||||||||||||||||||
| 2016 | 373,976 | -- | 361,636 | 398,339 | 11,219 | 1,145,170 | |||||||||||||||||||
|
Gary M. Owens(5) |
2018 |
416,851 | -- | 92,480 | 172,875 | 323,674 | 1,005,880 | ||||||||||||||||||
| Chief Operating Officer | 2017 | 10,959 | 860,860 | 1,014,300 | 11,538 | 329 | 1,897,986 | ||||||||||||||||||
| 2016 | -- | -- | -- | -- | -- | ||||||||||||||||||||
|
John V. Sakys |
2018 |
263,596 | -- | 135,800 | 108,000 | 10,544 | 517,940 | ||||||||||||||||||
| Chief Financial Officer | 2017 | 254,552 | -- | 127,650 | 95,280 | 7,637 | 485,119 | ||||||||||||||||||
| 2016 | 235,995 | -- | 195,049 | 177,478 | 7,080 | 615,602 | |||||||||||||||||||
|
Glenn E. Adriance |
2018 |
222,901 | -- | 135,800 | 100,000 | 8,916 | 467,617 | ||||||||||||||||||
| Chief Sales and | 2017 | 255,449 | -- | 127,650 | 95,280 | 7,663 | 486,042 | ||||||||||||||||||
| Marketing Officer | 2016 | 240,153 | -- | 195,049 | 177,478 | 7,205 | 619,885 | ||||||||||||||||||
|
Gregory T. Dinoia(6) |
2018 |
117,400 | 193,550 | -- | 58,558 | 4,696 | 374,204 | ||||||||||||||||||
| Senior Vice President of | 2017 | -- | -- | -- | -- | -- | -- | ||||||||||||||||||
| Commercial Operations | 2016 | -- | -- | -- | -- | -- | -- | ||||||||||||||||||
|
(1) |
The amounts reported in the Stock Awards column is the grant date fair value of stock awards determined pursuant to ASC Topic 718. |
|
(2) |
The amounts reported in the Option Awards column represent the grant date fair value of stock option awards granted under our 2014 Equity Plan to each of the Named Executive Officers, calculated in accordance with ASC Topic 718. |
|
(3) |
The amounts reported in the Non-Equity Incentive Plan Compensation column reflect the amounts earned by each Named Executive Officer under the Company’s Short-Term Incentive Plan. |
|
(4) |
The amounts reported in the All Other Compensation column reflect 401(K) matching funds and for Gary M. Owens in 2018, reimbursed moving expenses. |
|
(5) |
Effective September 1, 2017 John J. Sullivan retired as President and Chief Executive Officer and Gary M. Owens was named President and Chief Executive Officer. |
|
(6) |
Gregory T. Dinoia joined the Company as its Senior Vice President of Commercial Operation on November 7, 2017. |
Grant of Plan-Based Awards
|
Estimated Future Payments Under Non-Equity Incentive Plan Awards (1) |
All Other Option Awards: Number of Securities Underlying Options |
Exercise or Base Price of Option Awards ($/Sh) |
Grant Date Fair Value of Stock and Option Awards |
||||||||||||||||||||||
|
Name |
Grant Date |
Threshold ($) |
Target ($) |
Maximum ($) |
|||||||||||||||||||||
|
(a) |
(b) |
(c) |
(d) |
(e) |
(j) |
(k) |
(l) |
||||||||||||||||||
|
John J. Sullivan, Ph.D. |
4/3/2017 |
$ | -- | $ | -- | $ | -- | 15,000 | $ | 122.66 | $ | 38.80 | |||||||||||||
|
Gary M. Owens |
9/1/2017 |
-- | -- | -- | 2,000 | 136.42 | 46.24 | ||||||||||||||||||
|
9/1/2017 |
19,200 | 288,125 | 384,000 | -- | -- | -- | |||||||||||||||||||
|
John J. Sakys |
4/3/2017 |
-- | -- | -- | 3,500 | 122.66 | 38.80 | ||||||||||||||||||
|
6/1/2017 |
14,400 | 180,000 | 240,000 | -- | -- | -- | |||||||||||||||||||
|
Glenn E. Adriance |
4/3/2017 |
-- | -- | -- | 3,500 | 122.66 | 38.80 | ||||||||||||||||||
|
6/1/2017 |
15,600 | 195,000 | 260,000 | -- | -- | -- | |||||||||||||||||||
|
Gregory T. Dinoia |
11/6/2017(2) |
-- | -- | -- | 1,400 | -- | 138.25 | ||||||||||||||||||
|
11/6/2017 |
58,558 | 58,558 | 58,558 | -- | -- | -- | |||||||||||||||||||
|
(1) |
This section represents compensation to NEOs under our Short-term Incentive Plan. These amounts are included for the year earned, not when paid. These awards are based on various combinations of total revenues and profit growth. |
|
(2) |
Amount is comprised of 1,400 shares of restricted stock which vest ratably over five years. |
Outstanding Equity Awards at March 31, 2018
|
Option Awards |
||||||||||||||||||||
|
Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
Option Exercise Price ($) |
Option Expiration Date |
|||||||||||||||
|
(a) |
(b) |
(c) |
(d) |
(e) |
(f) |
|||||||||||||||
|
John J. Sullivan Ph.D. |
3,200 | -- | -- | 25.56 |
4/1/2020 |
|||||||||||||||
| 8,800 | -- | -- | 29.20 |
4/6/2021 |
||||||||||||||||
| 4,527 | 8,573 | -- | 89.70 |
4/1/2022 |
||||||||||||||||
| 1,840 | 7,360 | -- | 97.78 |
4/1/2022 |
||||||||||||||||
| 6,000 | -- | -- | 50.50 |
4/2/2022 |
||||||||||||||||
| 3,800 | -- | -- | 51.85 |
4/1/2023 |
||||||||||||||||
| 4,284 | 10,716 | -- | 70.56 |
4/1/2023 |
||||||||||||||||
| -- | 15,000 | -- | 122.66 |
4/3/2023 |
||||||||||||||||
|
Gary M. Owens |
-- | 2,000 | -- | 136.42 |
9/1/2023 |
|||||||||||||||
| 3,000 | 18,000 | -- | 122.98 |
3/20/25 |
||||||||||||||||
|
John V. Sakys |
3,213 | 4,287 | -- | 89.70 |
4/1/2022 |
|||||||||||||||
| 920 | 3,680 | -- | 97.78 |
4/1/2022 |
||||||||||||||||
| 5,000 | -- | -- | 48.72 |
10/29/2022 |
||||||||||||||||
| 3,800 | -- | -- | 51.85 |
4/1/2023 |
||||||||||||||||
| 2,141 | 5,359 | -- | 70.56 |
4/1/2023 |
||||||||||||||||
| -- | 3,500 | -- | 122.66 |
4/3/2023 |
||||||||||||||||
|
Glenn E. Adriance |
1,071 | 4,287 | -- | 89.70 |
4/1/2022 |
|||||||||||||||
| 920 | 3,680 | -- | 97.78 |
4/1/2022 |
||||||||||||||||
| 950 | -- | -- | 51.85 |
4/1/2023 |
||||||||||||||||
| 1,071 | 5,359 | -- | 70.56 |
4/1/2023 |
||||||||||||||||
| -- | 3,500 | -- | 122.66 |
4/3/2023 |
||||||||||||||||
|
Gregory T. Dinoia |
-- | -- | -- | -- | -- | |||||||||||||||
Outstanding Equity Awards at March 31, 2018
|
STOCK AWARDS |
||||||||||||||||
|
Name |
Number of Shares or Units of Stock That Have Not Vested (#) |
Market Value of Shares or Units of Stock That Have Not Vested ($) |
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) |
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) |
||||||||||||
|
(a) |
(g) |
(h) |
(i) |
(j) |
||||||||||||
|
Gary M. Owens |
6,000 | $ | 890,640 | -- | -- | |||||||||||
|
Gregory T. Dinoia |
1,400 | 207,816 | -- | -- | ||||||||||||
Options Exercised During the Year Ended March 31, 2018
|
Name |
Number of Shares Acquired upon Exercise (#) |
Value Realized On Exercise (1) |
||||||
|
(a) |
(b) |
(c) |
||||||
|
John J. Sullivan, Ph.D. |
13,000 | $ | 1,571,373 | |||||
|
Gary M. Owens |
-- | -- | ||||||
|
John V. Sakys |
1,000 | 89,620 | ||||||
|
Glenn E. Adriance |
-- | -- | ||||||
|
Gregory T. Dinoia |
-- | -- | ||||||
|
(1) |
Determined by multiplying the number of options that were exercised during the year ended March 31, 2018 by the difference between the per share closing price of our common stock on the date of exercise and the exercise price of the options, but not including any tax impact incurred in connection with such exercise. |
Potential Payments upon Termination or Change-in-Control
|
Salary Continuation upon Termination (1) |
Salary Continuation upon Change in Control (1) |
Value of Equity Awards Received or to be Received (2) |
||||||||||
|
Gary M. Owens |
$ | 430,000 | $ | 860,000 | $ | 482,320 | ||||||
|
John V. Sakys |
265,000 | 530,000 | 945,836 | |||||||||
|
Glenn E. Adriance |
132,500 | 265,000 | 945,836 | |||||||||
|
(1) |
This amount is based on the NEO’s salary at March 31, 2018 |
|
(2) |
The value of accelerating these unvested stock options was calculated by multiplying the number of shares underlying the NEO’s unvested stock options that were in-the-money at March 31, 2018 by the difference between the weighted average exercise price for options in-the-money at March 31, 2018, and our closing price per share on March 31, 2018 (the last trading day of the period). |
Pay Ratio Disclosure
Under Section 953(b) of the Dodd-Frank Wall Street Reform and Consumer Protection Act and Item 402(u) of Regulation S-K, we are required to provide the ratio of the annual total compensation of Mr. Owens, who has served as our Chief Executive Officer since September 1, 2017, to the annual total compensation of the median employee of the Company (the “Pay Ratio Disclosure”).
For the year ended March 31, 2018, the median annual total compensation of all employees of the Company and its consolidated subsidiaries (other than the Chief Executive Officer) was $46,286. Based upon the total compensation for the year ended March 31, 2018 reported for Mr. Owens of $1,005,880, our ratio of CEO to median employee pay was 22 to 1. Our median employee is employed in New Jersey in our Instruments business.
The Pay Ratio Disclosure presented above is a reasonable estimate. Because the SEC rules for identifying the median employee and calculating the pay ratio allow companies to use different methodologies, exemptions, estimates and assumptions, the Pay Ratio Disclosure may not be comparable to the pay ratio reported by other companies.
HOW WE IDENTIFIED THE MEDIAN EMPLOYEE. To identify the median Mesa employee, we identified the total employee population that earned wages during the year ended March 31, 2018 and, in accordance with SEC rules, excluded the CEO to arrive at the median employee consideration pool. We then calculated each employee’s total compensation earned for the year in accordance with SEC rules to use as the basis for the pay ratio. We used this total calculated compensation data to then identify the mathematically median employee from the consideration pool. Foreign exchange rates were translated to the U.S. dollar equivalent based on the average rates for the year ended March 31, 2018. We compared the median employee’s compensation to Mr. Owens as he was our CEO as of the reporting date.
Director Compensation
|
Name |
Fees Earned or Paid in Cash ($) |
Option Awards(1) ($) |
Total ($) |
|||||||||
|
(a) |
(b) |
(d) |
(h) |
|||||||||
|
Michael T. Brooks |
$ | 50,000 | $ | 38,800 | $ | 88,800 | ||||||
|
H. Stuart Campbell |
52,000 | 38,800 | 90,800 | |||||||||
|
Robert V. Dwyer |
50,000 | 38,800 | 88,800 | |||||||||
|
Evan C. Guillemin |
55,000 | 38,800 | 93,800 | |||||||||
|
David M. Kelly |
53,000 | 38,800 | 91,800 | |||||||||
|
John B. Schmieder |
50,000 | 38,800 | 88,800 | |||||||||
|
(1) |
1,000 stock options were granted to each director on April 3, 2017. We calculated these amounts in accordance with the provisions of ASC Section 718 – Compensation – Stock Compensation, using the Black-Scholes option-pricing model. |
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth the number of shares of our common stock owned beneficially as of March 31, 2018 (unless otherwise noted), by each person known by the Company to have owned beneficially more than five percent of such shares then outstanding, by each of our executive officers and directors, and by all of our executive officers and directors as a group. This information gives effect to securities deemed outstanding pursuant to Rule 13d-3(d)(1) under the Securities Exchange Act of 1934, as amended. As far as is known, no person owns beneficially more than five percent of the outstanding shares of common stock as of March 31, 2018 except as set forth below.
|
Amount and Nature of Beneficial |
Percentage of Class- |
|||
|
Name of Beneficial Owner |
Owner |
Beneficially Owned |
||
|
John B. Schmieder (1) |
79,612 |
(4) |
2.1 |
|
|
John J. Sullivan, Ph.D. (1) |
112,723 |
(5) |
2.9 |
|
|
Glenn E. Adriance (1) |
17,774 |
(6) |
0.5 |
|
|
H. Stuart Campbell (1) |
43,968 |
(7) |
1.2 |
|
|
Michael T. Brooks (1) |
39,599 |
(8) |
1.0 |
|
|
Robert V. Dwyer (1) |
71,670 |
(9) |
1.9 |
|
|
Evan C. Guillemin (1) |
181,054 |
(10) (11) |
4.8 |
|
|
David M. Kelly (1) |
10,049 |
(12) |
0.3 |
|
|
John V. Sakys (1) |
20,836 |
(13) |
0.5 |
|
|
Gary M. Owens (1) |
10,000 |
(14) (15) |
0.3 |
|
|
FMR LLC (2) |
272,599 |
7.2 |
||
|
Conestoga Capital Advisors (3) |
423,804 |
11.1 |
||
|
All executive officers and directors as a group (10 in number) |
587,285 |
(16) |
15.0 |
|
(1) |
The business address is 12100 West Sixth Avenue, Lakewood, Colorado 80228. |
|
(2) |
The business address is 82 Devonshire Street, Boston, Massachusetts 02109. |
|
(3) |
The business address is 550 E. Swedesford Road, Suite 120, Wayne, Pennsylvania 19087. |
|
(4) |
Includes 1,743 shares which Mr. Schmieder has the right to acquire within 60 days by exercise of stock options. |
|
(5) |
Includes 41,576 shares which Dr. Sullivan has the right to acquire within 60 days by exercise of stock options. |
|
(6) |
Includes 7,774 shares which Mr. Adriance has the right to acquire within 60 days by exercise of stock options. |
|
(7) |
Includes 1,602 shares which Mr. Campbell has the right to acquire within 60 days by exercise of stock options. |
|
(8) |
Includes 9,299 shares which Mr. Brooks has the right to acquire within 60 days by exercise of stock options. |
|
(9) |
Includes 3,399 shares which Mr. Dwyer has the right to acquire within 60 days by exercise of stock options. |
|
(10) |
Includes 9,899 shares which Mr. Guillemin has the right to acquire within 60 days by exercise of stock options |
|
(11) |
Includes 171,155 shares beneficially owned by SEG Ventures, LLC, of which Mr. Guillemin is a partner. |
|
(12) |
Includes 6,299 shares which Mr. Kelly has the right to acquire within 60 days by exercise of stock options. |
|
(13) |
Includes 18,836 shares which Mr. Sakys has the right to acquire within 60 days by exercise of stock options. |
|
(14) |
Includes 6,000 shares of restricted stock that vest ratably over the next six years beginning March 20, 2019 |
|
(15) |
Includes 3,000 shares which Mr. Owens has the right to acquire within 60 days by exercise of stock options. |
|
(16) |
Includes 103,427 shares that our executive officers and directors as a group have the right to acquire within 60 days by exercise of stock options. |
For information regarding securities authorized for issuance under our equity compensation plans, please see Note 10 contained in “Item 8. Financial Statements and Supplementary Data” of this report.
Item 13. Certain Relationships and Related Transactions, and Director Independence
None
Item 14. Principal Accountant Fees and Services
The following table presents fees for professional services rendered by EKS&H LLLP, our principal accountant, for the audit of our financial statements, and the fees for other services:
|
Year Ended March 31, |
||||||||||||
|
Type of Fees |
2018 |
2017 |
2016 |
|||||||||
|
Annual audit and quarterly reviews |
$ | 357,712 | $ | 317,820 | $ | 284,720 | ||||||
|
Audit-related fees – acquisitions |
-- | 1,000 | 4,905 | |||||||||
|
Tax fees |
-- | -- | -- | |||||||||
|
All other fees |
9,035 | 10,000 | 10,500 | |||||||||
|
Total |
$ | 366,747 | $ | 328,820 | $ | 300,125 | ||||||
Part IV
Item 15. Exhibits and Consolidated Financial Statement Schedules
|
a) |
Consolidated Financial Statements |
The Financial Statements of the Registrant listed on the accompanying index (please see “Item 8. Financial Statements and Supplementary Data”) are filed as part of this Annual Report.
All financial statement schedules have been omitted either because they are not applicable or required, or the information that would be required to be included is disclosed in the notes to the financial statements.
|
b) |
Exhibits |
|
3.1 |
Articles of Incorporation and Articles of Amendment and Bylaws of Registrant -incorporated by reference to the Exhibits to the Registration Statement on Form S-18, file number 2-88647-D, filed December 21, 1983. |
|
3.2 |
Articles of Amendment of Registrant - incorporated by reference to the Exhibit to the Annual Report on Form 10-K for the year ended March 31, 1988. |
|
3.3 |
Articles of Amendment of Registrant dated October 4, 1990 - incorporated by reference to the Exhibit to the Annual Report on Form 10-K for the year ended March 31, 1991. |
|
3.4 |
Articles of Amendment of Registrant dated October 20, 1992 - incorporated by reference to the Exhibit to the Annual Report on Form 10-KSB for the year ended March 31, 1993. |
| 3.5 | Articles of Amendment of Registrant dated October 1, 2012 – incorporated by reference to the Exhibit to the Annual Report on Form 10-K for the year ended March 31, 2013. |
| 3.6 | Amended and Restated Bylaws of the Registrant – incorporated by reference to the Exhibit to the Current Report on Form 8-K dated October 1, 2014. |
| 3.7 | Amended and Restated Bylaws of the Registrant – incorporated by reference to the Exhibit to the Current Report on Form 8-K dated April 14, 2016 |
|
23.1 |
|
|
31.1 |
Certification of Chief Executive Officer Pursuant to Rule 13a-14(a). |
|
31.2 |
Certification of Chief Financial Officer Pursuant to Rule 13a-14(a). |
|
32.1 |
Certification of Chief Executive Officer Pursuant to Rule 13a-14(a) and 18 U.S.C. Section 1350. |
|
32.2 |
Certification of Chief Financial Officer Pursuant to Rule 13a-14(a) and 18 U.S.C. Section 1350. |
|
101 |
Financial statements for the Annual Report on Form 10-K of Mesa Laboratories, Inc. for the annual period ended March 31, 2018, formatted in XBRL: (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Operations, (iii) the Consolidated Statements of Comprehensive Income, (iv) the Consolidated Statements of Stockholders’ Equity, (v) the Consolidated Statements of Cash Flows, and (vi) the Notes to the Consolidated Financial Statements. |
Signatures
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
MESA LABORATORIES, INC. Registrant |
|
| Date: June 5, 2018 | By: |
/s/John J. Sullivan, Ph.D. |
|
John J. Sullivan, Ph.D. Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | Title | Date | ||
| /s/John J. Sullivan, Ph.D. | Chairman of the Board of Directors | June 5, 2018 | ||
| John J. Sullivan | ||||
| /s/Gary M. Owens | Chief Executive Officer, President, | June 5, 2018 | ||
| Gary M. Owens | and Director | |||
| /s/John V. Sakys | Chief Financial and Chief Accounting Officer | June 5, 2018 | ||
| John V. Sakys | Secretary and Treasurer | |||
| /s/H. Stuart Campbell | Director | June 5, 2018 | ||
| H. Stuart Campbell | ||||
|
/s/John B. Schmieder |
Director | June 5, 2018 | ||
| John B Schmieder | ||||
| /s/Michael T. Brooks | Director | June 5, 2018 | ||
| Michael T. Brooks | ||||
| /s/Robert V. Dwyer | Director | June 5, 2018 | ||
|
Robert V. Dwyer |
||||
| /s/Evan Guillemin | Director | June 5, 2018 | ||
|
Evan Guillemin |
||||
| /s/David M. Kelly | Director | June 5, 2018 | ||
| David M. Kelly |
Page 80
