Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Boston Therapeutics, Inc. | s110558_8k.htm |
Exhibit 99.1

TRANSFORMING METABOLIC DISEASES CARL W. RAUSCH, CEO | LORAINE V. UPHAM, COO June 2018 Confidential and Proprietary 1 OTCQB:BTHE

Boston Therapeutics We are a BioPharmaceutical company with technology and treatments to prevent or reverse diabetes and its complications. WHO WE ARE Confidential and Proprietary 2 GLYCO CHEMISTRY BTI - 320 SUGARDOWN ® PEPTIDE CHEMISTRY BTI - 410

Safe Harbor Statement Confidential and Proprietary 3 This presentation includes forward - looking statements . These statements may be identified by words such as "feel," "believes," expects," "estimates," "projects," "intends," "should," "is to be," or the negative of such terms, or other comparable terminology . Forward - looking statements are statements that are not historical facts . Such forward - looking statements are subject to risks and uncertainties, which could cause actual results to differ materially from the forward - looking statements contained herein . Factors that could cause actual results to differ materially include, but are not limited to, our limited operations and need to expand in the near future ; risks associated with obtaining regulatory approval of our products ; the ability to protect our intellectual property ; the potential lack of market acceptance of our products ; potential competition ; our inability to retain key members of our management team ; our inability to raise additional capital to fund our operations and business plan ; our ability to continue as a going concern ; our inability to close and integrate acquisitions ; our liquidity and other risks and uncertainties and other factors discussed from time to time in our filings with the Securities and Exchange Commission ("SEC"), including our annual report on Form 10 - K filed with the SEC . Boston Therapeutics expressly disclaims any obligation to publicly update any forward - looking statements contained herein, whether as a result of new information, future events or otherwise, except as required by law .
A Global Presence Confidential and Proprietary 4 WHO WE ARE We have operations in both North America and Asia. 2007 BTI Founded 2011 - 2014 ▪ BTI - 320 Phase I Safety Confirmed ▪ HIP2B Phase I Safety Confirmed 2015 - 2017 ▪ BTI - 320 POC Study Completed in Prediabetes ▪ HIP2B Phase Ib Study Efficacy Confirmed in T2DM 2018 ▪ BTI Acquires CureDM ▪ BTI - 320 Phase II initiated ▪ HIP2B becomes BTI - 410 ▪ BTI - 410 Phase II Manufacturing initiated ▪ BTI - 320 in combination with existing treatments 2019 ▪ BTI - 320 Phase II in T2DM Completed ▪ BTI - 410 Phase II in T1DM completed 2020 ▪ BTI - 320 Phase III in T2DM Initiated ▪ BTI - 410 Phase II in T2DM completed ▪ Commercial Partnership(s)

Type 2 Diabetes (T2DM) – A Worldwide Reality Source: IDF Diabetes Atlas 7 th Edition, 2015 Confidential and Proprietary 5 WHY DO WE EXIST? In addition to the 425 million adults who are estimated to currently have diabetes, there are 318 million adults with impaired glucose tolerance, which puts them at high risk of developing the disease in the future. “ “
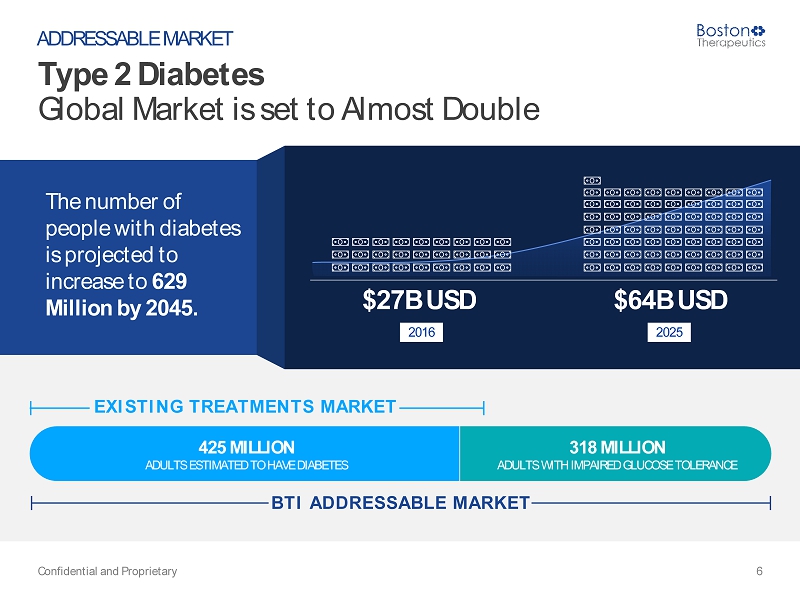
425 MILLION 318 MILLION ADULTS ESTIMATED TO HAVE DIABETES ADULTS WITH IMPAIRED GLUCOSE TOLERANCE The number of people with diabetes is projected to increase to 629 Million by 2045. Type 2 Diabetes Global Market is set to Almost Double Confidential and Proprietary 6 ADDRESSABLE MARKET $64B USD 2025 EXISTING TREATMENTS MARKE T BTI ADDRESSABLE MARKE T $27B USD 2016
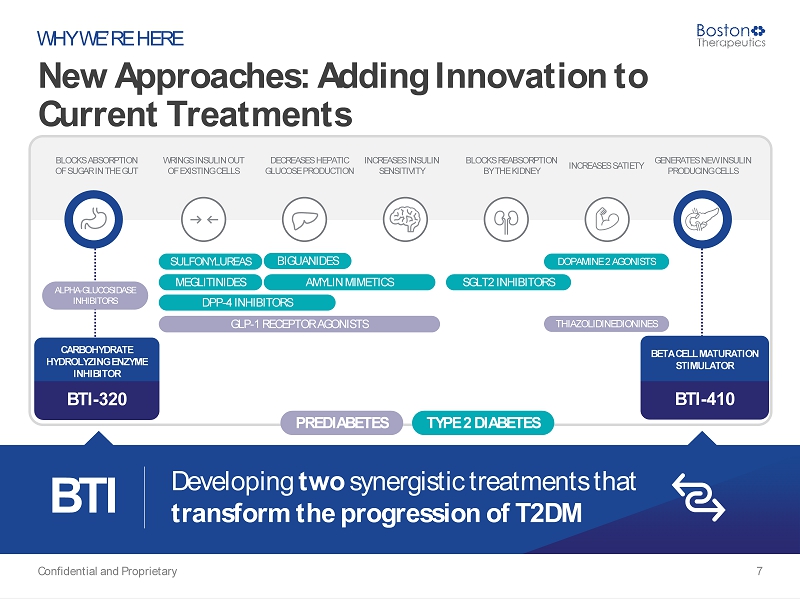
GENERATES NEW INSULIN PRODUCING CELLS WRINGS INSULIN OUT OF EXISTING CELLS DECREASES HEPATIC GLUCOSE PRODUCTION INCREASES INSULIN SENSITIVITY BLOCKS REABSORPTION BY THE KIDNEY INCREASES SATIETY BLOCKS ABSORPTION OF SUGAR IN THE GUT New Approaches: Adding Innovation to Current Treatments Confidential and Proprietary 7 WHY WE’RE HERE SULFONYLUREAS MEGLITINIDES DPP - 4 INHIBITORS BIGUANIDES DOPAMINE 2 AGONISTS SGLT2 INHIBITORS AMYLIN MIMETICS TYPE 2 DIABETES CARBOHYDRATE HYDROLYZING ENZYME INHIBITOR BTI - 320 BETA CELL MATURATION STIMULATOR BTI - 410 BTI Developing two synergistic treatments that transform the progression of T2DM GLP - 1 RECEPTOR AGONISTS THIAZOLIDINEDIONINES PREDIABETES ALPHA - GLUCOSIDASE INHIBITORS

Prediabetes and Type 2 Diabetes – Eliminating Post - prandial spikes Source: CDC Data 2014 Confidential and Proprietary 8 ADDRESSABLE MARKET Prediabetes is defined as: ▪ HbA1c ≥ 5.7 ▪ BMI ▪ Age ▪ Cholesterol? BTI - 320 eliminates postprandial spikes that occur with almost every meal in prediabetes patients. DIABETES STARTS WITH POSTPRANDIAL GLUCOSE PEAKS Continuous Glucose Monitoring shows Postprandial spikes Postprandial spikes are the precursor to hyperinsulinemia which leads to type 2 diabetes. 22.2 16.7 11.1 5.6 0.0 Glucose – mmol/L Time of Day 03:00 06:00 09:00 12:00 15:00 18:00 21:00 Prof. Hanefeld Increase in fluctuation leads to increase in oxidative stress 61 year - old male patient with IGT, HbA1c 5.0%
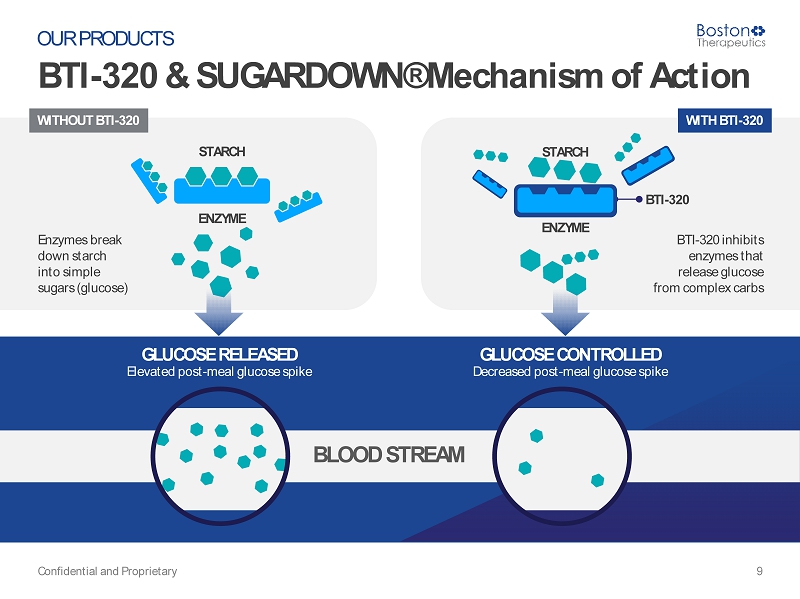
WITHOUT BTI - 320 STARCH ENZYME Enzymes break down starch into simple sugars (glucose) BTI - 320 WITH BTI - 320 BTI - 320 inhibits enzymes that release glucose from complex carbs STARCH ENZYME BLOOD STREAM BTI - 320 & SUGARDOWN ® Mechanism of Action Confidential and Proprietary 9 OUR PRODUCTS GLUCOSE RELEASED Elevated post - meal glucose spike GLUCOSE CONTROLLED Decreased post - meal glucose spike

WITHOUT BTI - 410 Existing islets are diminished in number and have little to no insulin in reserve ready to release. Existing islets are too few in number and have insufficient insulin response to glucose needs and blood sugar gets out of control. WITH BTI - 410 BTI - 410 triggers the maturation of beta cells within new islets directly in the pancreas. Stress on existing beta cells is alleviated and normal insulin response to glucose can be restored. BTI - 410 Mechanism of Action Confidential and Proprietary 10 OUR PRODUCTS INSULIN (UU/ML) Type 2DM Insulin (uU/mL) Normal Insulin (uU/mL) Normal insulin levels spike throughout the day in response to meal intake. Pancreas is able to produce insulin in response to glucose needs. Type 2DM insulin levels remain flat throughout the day. Pancreas has insufficient insulin response to glucose needs.

BTI - 410 REVERSES THE PROGRESSION OF T2DM Aids maturation of new insulin secreting cells to avoid ever needing insulin injections. No other treatments can reverse disease progression* Only BTI - 320 is being developed help to prevent diabetes. BTI - 320 STAVES OFF THE START OF T2DM Minimizes postprandial spikes that lead to hyperinsulinemia Can be used with Combination Products BTI - 320 and BTI - 410 *Besides diet and exercise and bariatric surgery! Confidential and Proprietary 11 OUR OPPORTUNITY
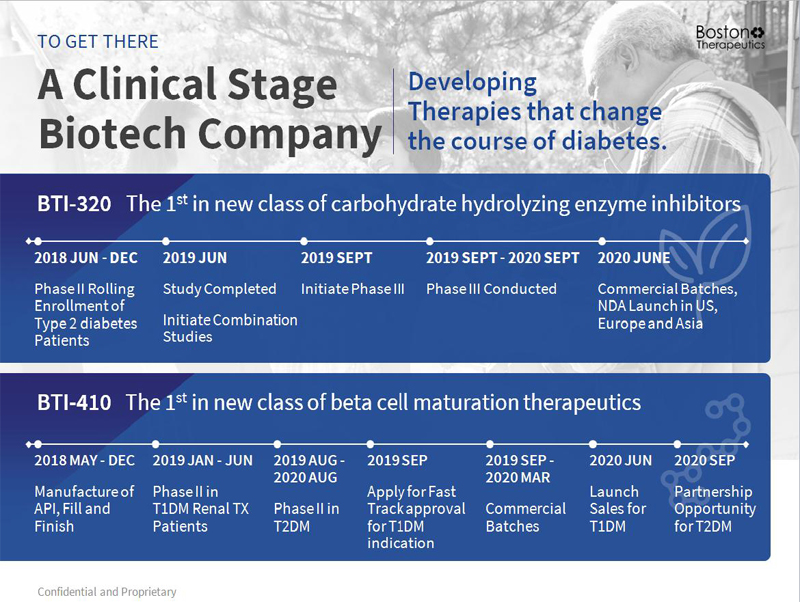
Confidential and Proprietary A Clinical Stage Biotech Company TO GET THERE Developing Therapies that change the course of diabetes. 12 BTI - 320 The 1 st in new class of carbohydrate hydrolyzing enzyme inhibitors 2018 JUN - DEC Phase II Rolling Enrollment of Type 2 diabetes Patients 2019 JUN Study Completed Initiate Combination Studies 2019 SEPT Initiate Phase III 2019 SEPT - 2020 SEPT Phase III Conducted 2020 JUNE Commercial Batches, NDA Launch in US, Europe and Asia BTI - 410 The 1 st in new class of beta cell maturation therapeutics 2018 MAY - DEC Manufacture of API, Fill and Finish 2019 JAN - JUN Phase II in T1DM Renal TX Patients 2019 AUG - 2020 AUG Phase II in T2DM 2019 SEP Apply for Fast Track approval for T1DM indication 2019 SEP - 2020 MAR Commercial Batches 2020 JUN Launch Sales for T1DM 2020 SEP Partnership Opportunity for T2DM
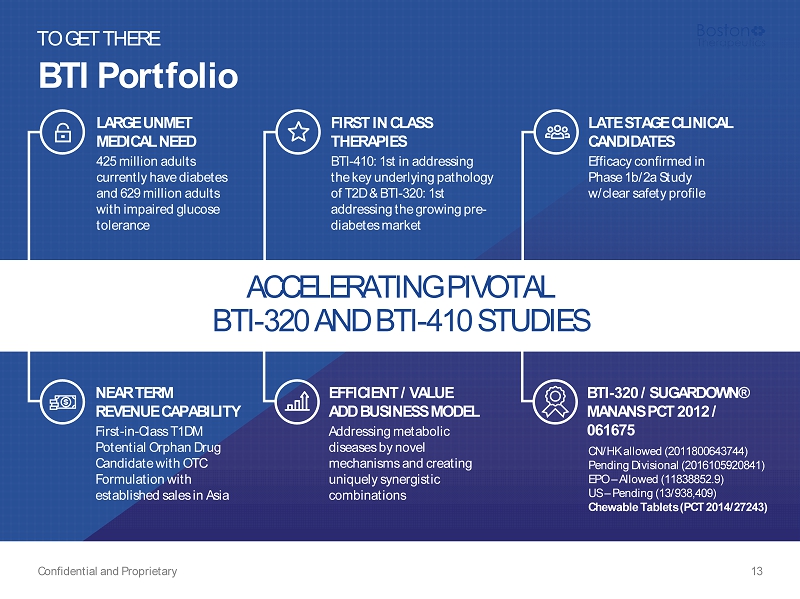
BTI Portfolio Confidential and Proprietary 13 TO GET THERE ACCELERATING PIVOTAL BTI - 320 AND BTI - 410 STUDIES LATE STAGE CLINICAL CANDIDATES Efficacy confirmed in Phase 1b/2a Study w/clear safety profile EFFICIENT / VALUE ADD BUSINESS MODEL Addressing metabolic diseases by novel mechanisms and creating uniquely synergistic combinations NEAR TERM REVENUE CAPABILITY First - in - Class T1DM Potential Orphan Drug Candidate with OTC Formulation with established sales in Asia LARGE UNMET MEDICAL NEED 425 million adults currently have diabetes and 629 million adults with impaired glucose tolerance FIRST IN CLASS THERAPIES BTI - 410: 1st in addressing the key underlying pathology of T2D & BTI - 320: 1st addressing the growing pre - diabetes market BTI - 320 / SUGARDOWN® MANANS PCT 2012 / 061675 CN/HK allowed (2011800643744) Pending Divisional (2016105920841) EPO – Allowed (11838852.9) US – Pending (13/938,409) Chewable Tablets (PCT 2014/27243)

Development Plans Confidential and Proprietary 14 TO GET THERE Execute marketing plan for SUGARDOWN ® in Asia and US Complete BTI - 320 Phase II Study in Type 2 Diabetes in collaboration with the Joslin Diabetes Center Complete Phase II Clinical Trial in Renal Transplant Patients ▪ Enroll 36 patients ▪ Treat and analyze results ▪ Apply for orphan drug or fast track approval Complete Phase II Clinical Trial in T2DM ▪ Enroll 120 patients ▪ Treat and analyze results Develop and market vascular disease products Develop platform technology and define new mechanisms and compounds

Proposed BTI - 320 STEPS Study Confidential and Proprietary 15 TO GET THERE STEPS: SUGARDOWN ® TO ELIMINATE POSTPRANDIAL SPIKES Type 2 diabetes Adults 18 - 70 TID Chewable Tablets; 4g and 8g doses 27 Weeks Treatment Monitored 3 days with CGM, 5 times N = 60 2:2:1 design Blinded, Placebo Controlled

Proposed Phase 2 in T1DM TRIUMPH Study Confidential and Proprietary 16 TO GET THERE N = 36 2:1 design T1DM On multiple daily insulin dosages, tacrolimus and mycophenolate or <5mg / day Prednisone Adults 30 - 65 7.0 ≤HbA1c ≤10.0 200 mg BID daily subQ injections 90 days Treatment & Followed out to 180 Days Blinded, Placebo Controlled N = 120 1:1 design Not Blinded, Placebo Controlled T2DM Not on Metformin Adults 30 - 65 7.0 ≤HbA1c ≤10.0 200 mg BID daily subQ injections 90 days Treatment Followed out to 180 Days Proposed Phase 2 in T2DM TRINITY Study
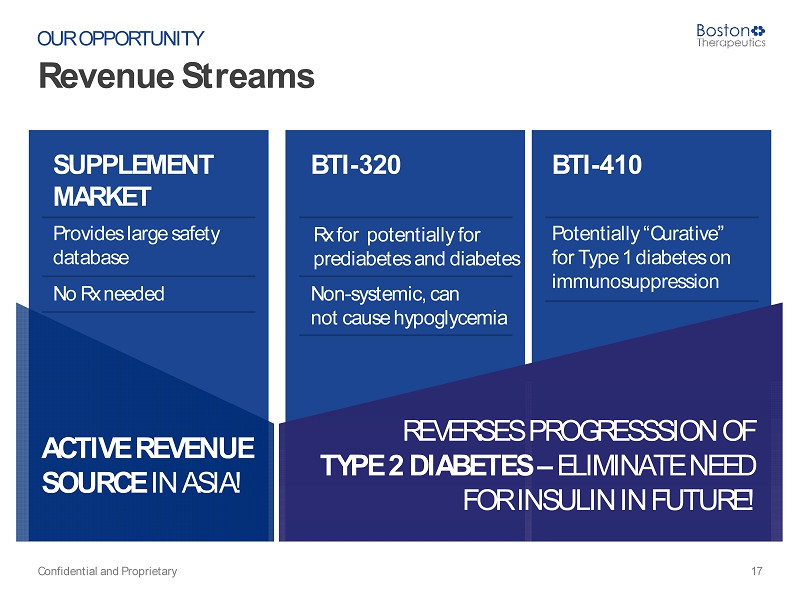
ACTIVE REVENUE SOURCE IN ASIA! Revenue Streams Confidential and Proprietary 17 OUR OPPORTUNITY SUPPLEMENT MARKET BTI - 320 BTI - 410 Provides large safety database Rx for potentially for prediabetes and diabetes Potentially “Curative” for Type 1 diabetes on immunosuppression No Rx needed Non - systemic, can not cause hypoglycemia REVERSES PROGRESSSION OF TYPE 2 DIABETES – ELIMINATE NEED FOR INSULIN IN FUTURE!

Questions? Confidential and Proprietary 18
