Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Sesen Bio, Inc. | ebio-form8xkauapresentatio.htm |

PRELIMINARY RESULTS FROM VISTA PHASE 3 PIVOTAL STUDY OF THE NOVEL AGENT VICINIUM IN BCG-UNRESPONSIVE NON-MUSCLE INVASIVE BLADDER CANCER RIAN DICKSTEIN, N Wu, B Cowan, C Dunshee, M Franks, F Wolk, L Belkoff, S Castellucci, J Holzbeierlein, G Kulkarni, A Weizer, D Lamm, S Ali, J Epstein and W Kassouf AMERICAN UROLOGICAL ASSOCIATION 2018 ANNUAL MEETING May 21, 2018 Phase 3 Study of Vicinium in BCG-unresponsive Non-muscle Invasive Bladder Cancer: Initial Results (Abstract ID: 18-9707)

DISCLOSURES No industry or financial disclosures or conflict of interests to report 2

LACK OF TREATMENT INNOVATION FOR NMIBC • Bacillus Calmette-Guérin (BCG): primary treatment for all high-grade NMIBC since 1980s - Potential for shortages in BCG supply based on single-source provider • Radical cystectomy remains standard-of-care for BCG-unresponsive NMIBC - Significant morbidity and mortality rates • Last U.S. approved treatment in 1998 limited to BCG-refractory carcinoma in situ (CIS) in subjects for whom immediate cystectomy would be associated with unacceptable morbidity or mortality1 - Associated with challenging toxicities and limited use • Significant need for new treatment for BCG-unresponsive NMIBC 3 1 Valstar® (valrubicin) Sterile Solution for Intravesical Instillation package insert
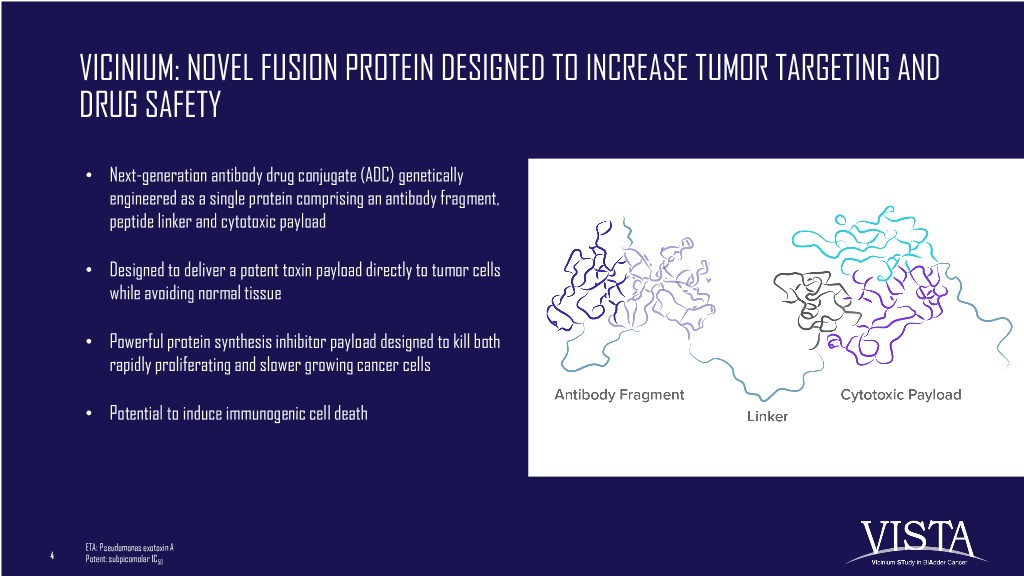
VICINIUM: NOVEL FUSION PROTEIN DESIGNED TO INCREASE TUMOR TARGETING AND DRUG SAFETY • Next-generation antibody drug conjugate (ADC) genetically engineered as a single protein comprising an antibody fragment, peptide linker and cytotoxic payload • Designed to deliver a potent toxin payload directly to tumor cells while avoiding normal tissue • Powerful protein synthesis inhibitor payload designed to kill both rapidly proliferating and slower growing cancer cells • Potential to induce immunogenic cell death ETA: Pseudomonas exotoxin A 4 Potent: subpicomolar IC50

VICINIUM: A UNIQUE MECHANISM OF ACTION FOR TREATING HIGH-GRADE NMIBC • EpCAM (the target) is overexpressed in >98% of high grade NMIBCs*, minimal expression on healthy bladder tissue • Stable, genetically engineered peptide linker allows fusion protein to remain intact until internalized by cancer cell • Anti-EpCAM Ab fragment delivers toxin that kills tumor cells by blocking protein synthesis 5 * Data generated in prior EBIO studies using internal antibody

VISTA TRIAL: PHASE 3 STUDY OF VICINIUM FOR NMIBC • Single-arm, open label, multi-center phase 3 study of subjects with BCG-unresponsive NMIBC – BCG-unresponsive defined as having completed 2+ courses (≥ 5 and ≥ 2 treatments resp.) of full dose BCG starting within 13 months of each other • 3 cohorts by histology and time to recurrence after adequate BCG - Cohort 1: CIS with or without papillary tumors that recurred within 6 months of BCG - Cohort 2: CIS with or without papillary tumors that recurred >6 months but ≤11 months of BCG - Cohort 3: Papillary tumors (without CIS) that recurred within 6 months of BCG • Dosing: intravesical instillation of 30mg Vicinium in 50 ml buffered saline held for 2 hours - Induction: biweekly for 6 weeks → weekly for 6 weeks - Subjects with CR proceed to maintenance of every other week for 2 years total 6

VISTA TRIAL: ASSESSMENT OF RESPONSE • Primary Endpoint: Complete response rate and duration of response in subjects enrolled in Cohort 1 • Complete response defined as negative central urine cytology or pathology, or local cystoscopy • Key Secondary Endpoints – Event-free survival (EFS) in all subjects, time to disease recurrence, time to cystectomy, progression-free survival, overall survival, safety and tolerability 7

VISTA TRIAL PATIENT DEMOGRAPHICS COHORT 1 COHORT 2 COHORT 3 CHARACTERISTICS CIS that recurred within 6 months of CIS that recurred >6 months but ≤11 Papillary (without CIS) that recurred BCG months of BCG within 6 months of BCG Total subjects enrolled 87 6 40 Evaluable subjects at 3-months 72 5 34 Median age (current) 75 71 77 Males/Females 54/18 4/1 29/5 Median prior treatment for NMIBC 4 (range 2-14) BCG cycles 1 (range 0-23) Intravesical chemotherapy 4 (range 0-11) TURBT 8 Data as of 20 April 2018 cut off

VICINIUM DEMONSTRATES 42% COMPLETE RESPONSE RATE AT 3-MONTHS IN CIS PATIENTS 90% 80% 80% BCG refractory within 12 months of last BCG per FDA 70% Final Guidance* 60% 50% 42% 39% 40% 30% 20% month complete responserate complete month 10% - 3 0% Cohort 1 (VISTA): Cohort 2 (VISTA): All CIS Subjects (VISTA) recurrent CIS w/in 6 months of BCG recurrent CIS >6 months but ≤11 months of BCG n=72 n=5 n=77 Data as of 20 April 2018 cut off Efficacy assessment based on cystoscopy as well as centrally read cytology and, where suspicious lesions found on cysto, central pathology 9 * BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment Guidance for Industry, February 2018

CIS PATIENT JOURNEY TO COMPLETE RESPONSE • 76 year old female • CIS diagnosed in March 2016 • Post TURBT treated BCG per AUA guidelines Subject remains on treatment with (induction course of 6 doses over 6 weeks followed by maintenance course of 3 doses complete response at last observation out over 3 weeks) to • CIS remained confirmed by local and central pathology at September 2016 19 MONTHS • Enrolled into VISTA Trial cohort 1 in October 2016; treated with Vicinium • Confirmed complete response observed January 2017 10 Data as of 20 April 2018 cut off

RECURRENCE-FREE RATE AT 3-MONTHS IN COHORT 3 SUBJECTS WITH PAPILLARY TUMORS ONLY • Subjects deemed to have no visible evidence of 68% disease when starting Vicinium treatment recurrence-free rate • Disease recurrence remains appropriate response at 3-months in criteria papillary only subjects • Time to disease recurrence remains (n=34) standard clinical endpoint 11 Data as of 20 April 2018 cut off
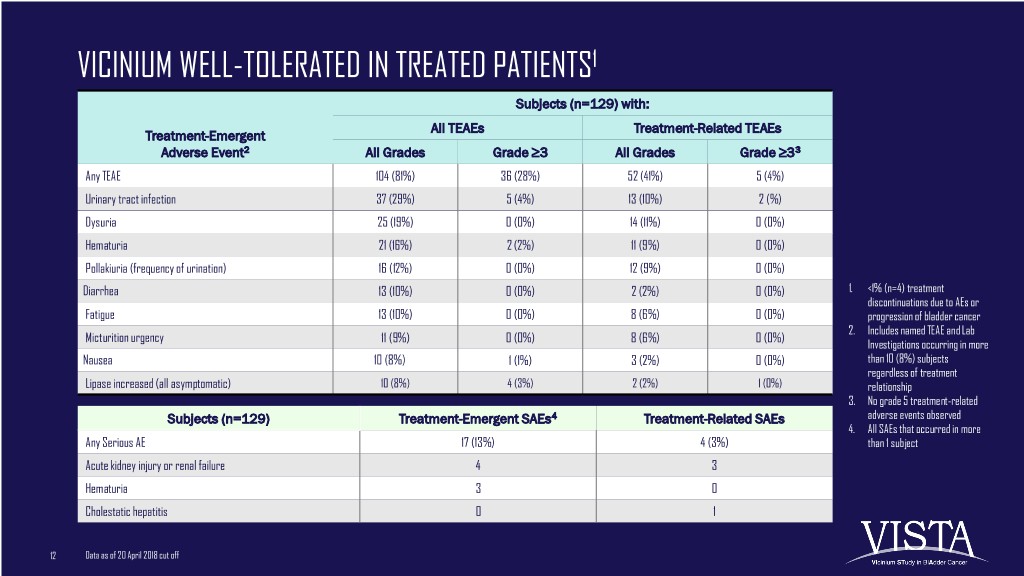
VICINIUM WELL-TOLERATED IN TREATED PATIENTS1 Subjects (n=129) with: All TEAEs Treatment-Related TEAEs Treatment-Emergent Adverse Event2 All Grades Grade ≥3 All Grades Grade ≥33 Any TEAE 104 (81%) 36 (28%) 52 (41%) 5 (4%) Urinary tract infection 37 (29%) 5 (4%) 13 (10%) 2 (%) Dysuria 25 (19%) 0 (0%) 14 (11%) 0 (0%) Hematuria 21 (16%) 2 (2%) 11 (9%) 0 (0%) Pollakiuria (frequency of urination) 16 (12%) 0 (0%) 12 (9%) 0 (0%) Diarrhea 13 (10%) 0 (0%) 2 (2%) 0 (0%) 1. <1% (n=4) treatment discontinuations due to AEs or Fatigue 13 (10%) 0 (0%) 8 (6%) 0 (0%) progression of bladder cancer 2. Includes named TEAE and Lab Micturition urgency 11 (9%) 0 (0%) 8 (6%) 0 (0%) Investigations occurring in more Nausea 10 (8%) 1 (1%) 3 (2%) 0 (0%) than 10 (8%) subjects regardless of treatment Lipase increased (all asymptomatic) 10 (8%) 4 (3%) 2 (2%) 1 (0%) relationship 3. No grade 5 treatment-related Subjects (n=129) Treatment-Emergent SAEs4 Treatment-Related SAEs adverse events observed 4. All SAEs that occurred in more Any Serious AE 17 (13%) 4 (3%) than 1 subject Acute kidney injury or renal failure 4 3 Hematuria 3 0 Cholestatic hepatitis 0 1 12 Data as of 20 April 2018 cut off

SUMMARY • 3-month data demonstrate Vicinium efficacy in subjects with NMIBC - 42% complete response rate at 3-months in cohort 1 and 2 subjects with recurrent CIS within 12 months of last BCG treatment, according to FDA Final Guidelines - 68% recurrence-free rate in subjects with papillary tumor • Vicinium safety profile was tolerable and manageable • Phase 3 findings consistent with Vicinium experience in Phase 1 and 2 clinical trials • Convenient BCG-like instillation • VISTA Trial enrollment complete; 12-month complete response data anticipated by mid-2019 • Unmet need in BCG-unresponsive NMIBC supports continued development of Vicinium 13 Data as of 20 April 2018 cut off

ACKNOWLEDGMENTS We wish to thank all of the physicians, nurses, study staffs, pathologists, scientists, advisors and most of all, the patients and families who contributed to the VISTA Trial. 14
