Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Audentes Therapeutics, Inc. | bold-8k_20180516.htm |
| EX-99.1 - EX-99.1 - Audentes Therapeutics, Inc. | bold-ex991_7.htm |

X-Linked Myotubular Myopathy (XLMTM) ASPIRO Phase 1/2 Gene Therapy Trial In XLMTM: Interim Safety And Efficacy Findings Exhibit 99.2

Except for statements of historical fact, any information contained in this presentation may be a forward-looking statement that reflects the Company’s current views about future events and are subject to risks, uncertainties, assumptions and changes in circumstances that may cause events or the Company’s actual activities or results to differ significantly from those expressed in any forward-looking statement. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “plan,” “expect,” “estimate,” “anticipate,” “intend,” “goal,” “strategy,” “believe” and similar expressions and variations thereof. Forward-looking statements include statements regarding the Company’s business strategy; potential growth opportunities; clinical development activities; the timing and results of Investigational New Drug application and Clinical Trial Authorisation submissions; the timing and results of preclinical studies and clinical trials; the nature and timing of potential regulatory approvals; and the likelihood of future commercialization of product candidates. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, the Company cannot guarantee future events, results, actions, levels of activity, performance or achievements, and the timing and results of biotechnology development and potential regulatory approval is inherently uncertain. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under the heading “Risk Factors” in documents the Company has filed with the SEC. These forward-looking statements speak only as of the date of this presentation, and the Company undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof. Certain information contained in this presentation may be derived from information provided by industry sources. We believe such information is accurate and that the sources from which it has been obtained are reliable. However, we cannot guarantee the accuracy of, and have not independently verified, such information. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. Safe Harbor
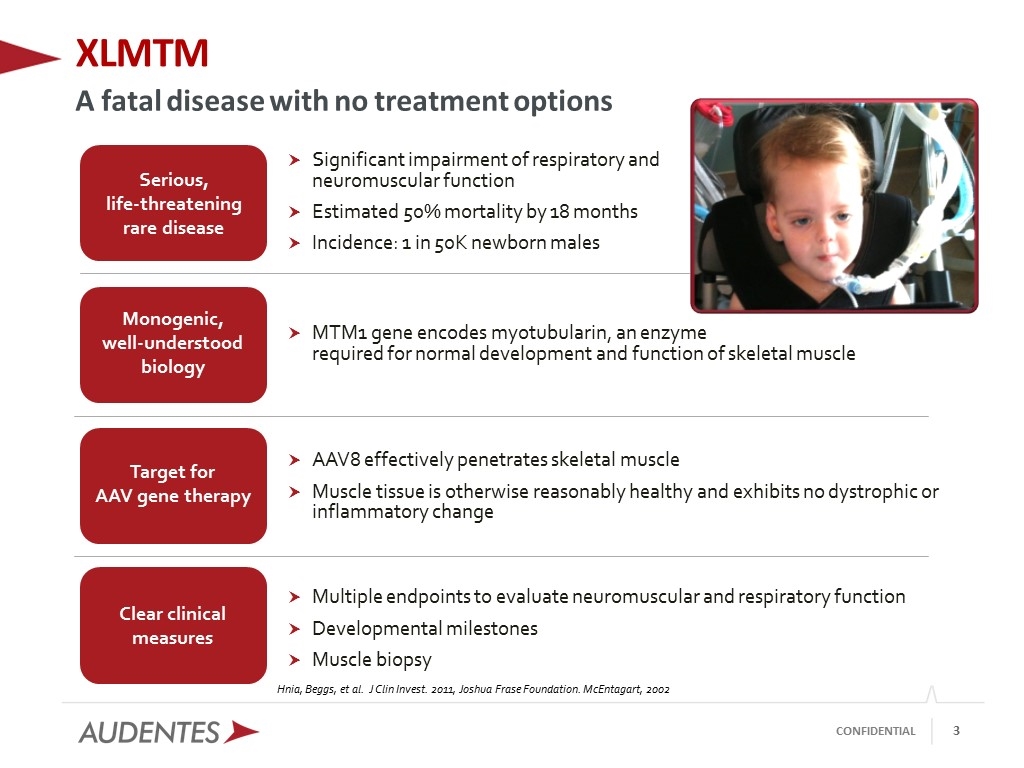
Significant impairment of respiratory and neuromuscular function Estimated 50% mortality by 18 months Incidence: 1 in 50K newborn males AAV8 effectively penetrates skeletal muscle Muscle tissue is otherwise reasonably healthy and exhibits no dystrophic or inflammatory change MTM1 gene encodes myotubularin, an enzyme required for normal development and function of skeletal muscle Multiple endpoints to evaluate neuromuscular and respiratory function Developmental milestones Muscle biopsy Hnia, Beggs, et al. J Clin Invest. 2011, Joshua Frase Foundation. McEntagart, 2002 Serious, life-threatening rare disease Target for AAV gene therapy Clear clinical measures Monogenic, well-understood biology XLMTM A fatal disease with no treatment options
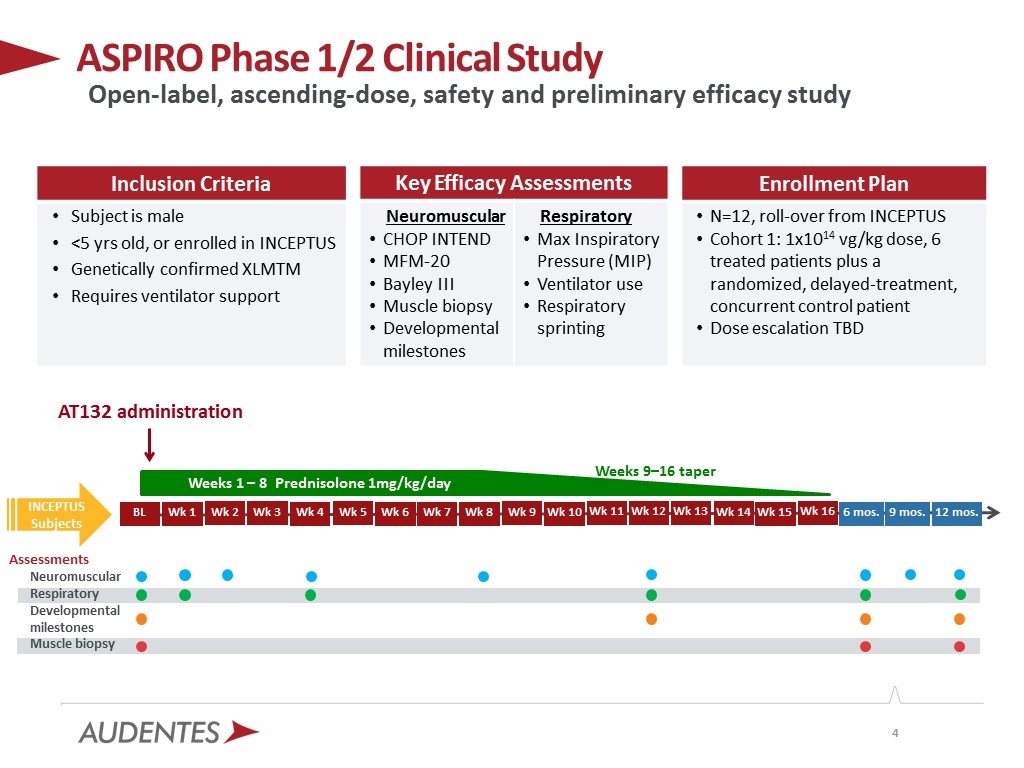
ASPIRO Phase 1/2 Clinical Study AT132 administration Weeks 9–16 taper Inclusion Criteria Subject is male <5 yrs old, or enrolled in INCEPTUS Genetically confirmed XLMTM Requires ventilator support Key Efficacy Assessments Neuromuscular CHOP INTEND MFM-20 Bayley III Muscle biopsy Developmental milestones Respiratory Max Inspiratory Pressure (MIP) Ventilator use Respiratory sprinting Enrollment Plan N=12, roll-over from INCEPTUS Cohort 1: 1x1014 vg/kg dose, 6 treated patients plus a randomized, delayed-treatment, concurrent control patient Dose escalation TBD Open-label, ascending-dose, safety and preliminary efficacy study Wk 12 Wk 11 Wk 10 Wk 1 Wk 2 Wk 3 Wk 4 Wk 5 Wk 6 Wk 8 Wk 9 Wk 7 Assessments Neuromuscular Respiratory Developmental milestones Muscle biopsy INCEPTUS Subjects Wk 13 Wk 14 Weeks 1 – 8 Prednisolone 1mg/kg/day BL Wk 15 6 mos. 12 mos. Wk 16 9 mos.
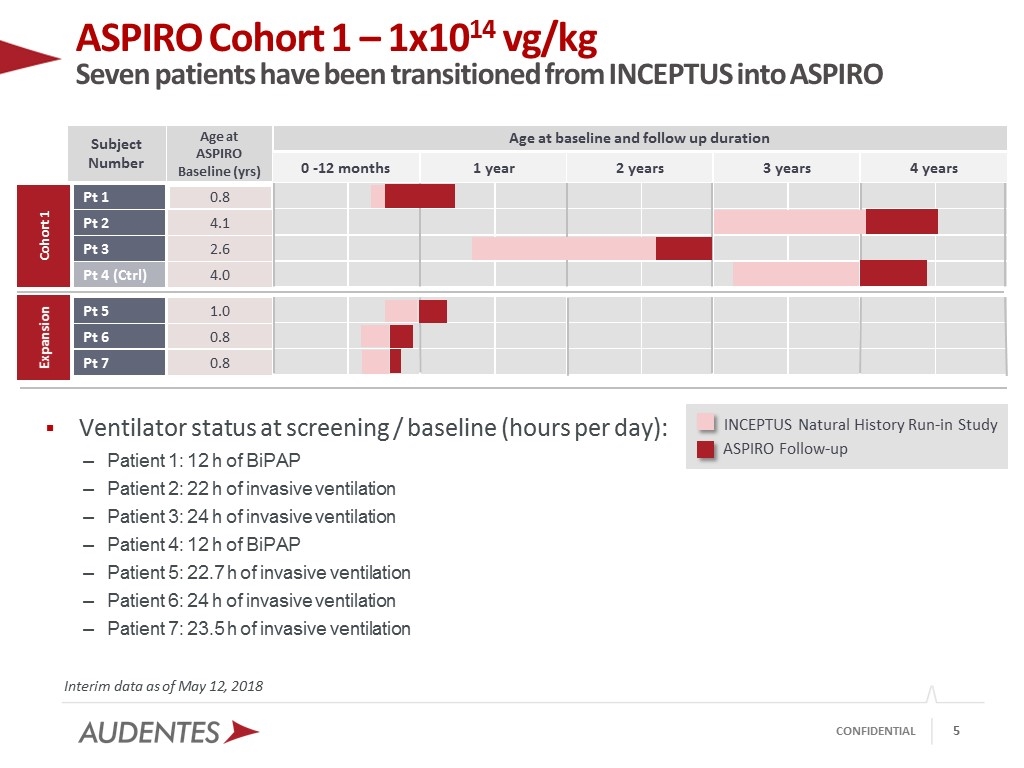
ASPIRO Cohort 1 – 1x1014 vg/kg Seven patients have been transitioned from INCEPTUS into ASPIRO Interim data as of May 12, 2018 Ventilator status at screening / baseline (hours per day): Patient 1: 12 h of BiPAP Patient 2: 22 h of invasive ventilation Patient 3: 24 h of invasive ventilation Patient 4: 12 h of BiPAP Patient 5: 22.7 h of invasive ventilation Patient 6: 24 h of invasive ventilation Patient 7: 23.5 h of invasive ventilation ASPIRO Follow-up INCEPTUS Natural History Run-in Study Age at baseline and follow up duration Age at ASPIRO Baseline (yrs) 0.8 2.6 4.1 4.0 Subject Number Pt 1 Pt 3 Pt 2 Pt 4 (Ctrl) 0 -12 months 1 year 2 years 4 years 3 years Cohort 1 1.0 0.8 0.8 Pt 5 Pt 7 Pt 6 Expansion

Safety and Tolerability Adverse Events and Laboratory Parameters

AT132 Safety and Tolerability Profile at 1 x 1014 vg/kg Interim data as of May 12, 2018 No tolerability issues during study drug administration Six serious adverse events (SAEs), five in Patient 3 Patient 3 Hospitalization for pneumonia (week 2); not treatment-related; history of 8 similar events during INCEPTUS run-in study Hospitalization for GI infection and elevated CK and troponin levels - suggestive of myocarditis (week 7); probably treatment-related; controlled with immunosuppressive treatment; no compromise of cardiac function Hospitalization for monitoring of atrial tachycardia (week 21), possibly treatment-related; patient was noted to have experienced an episode of tachycardia prior to enrollment in ASPIRO Patient 4 (Delayed-treatment concurrent control) Hospitalization for norovirus gastroenteritis (week 10), recovered, not treatment related Seven possibly/probably treatment-related adverse events (AEs) Patient 1 Mild, clinically asymptomatic exacerbation of preexisting hyperbilirubinemia; possibly treatment-related; resolved Mild unspecific abnormalities in liver ultrasound and proteinuria; possibly treatment-related; resolved Patient 2 Clinically asymptomatic hyperbilirubinemia; and liver enzyme and CK elevations which have been controlled by extended steroid coverage; probably treatment-related Eleven additional non-treatment related AEs
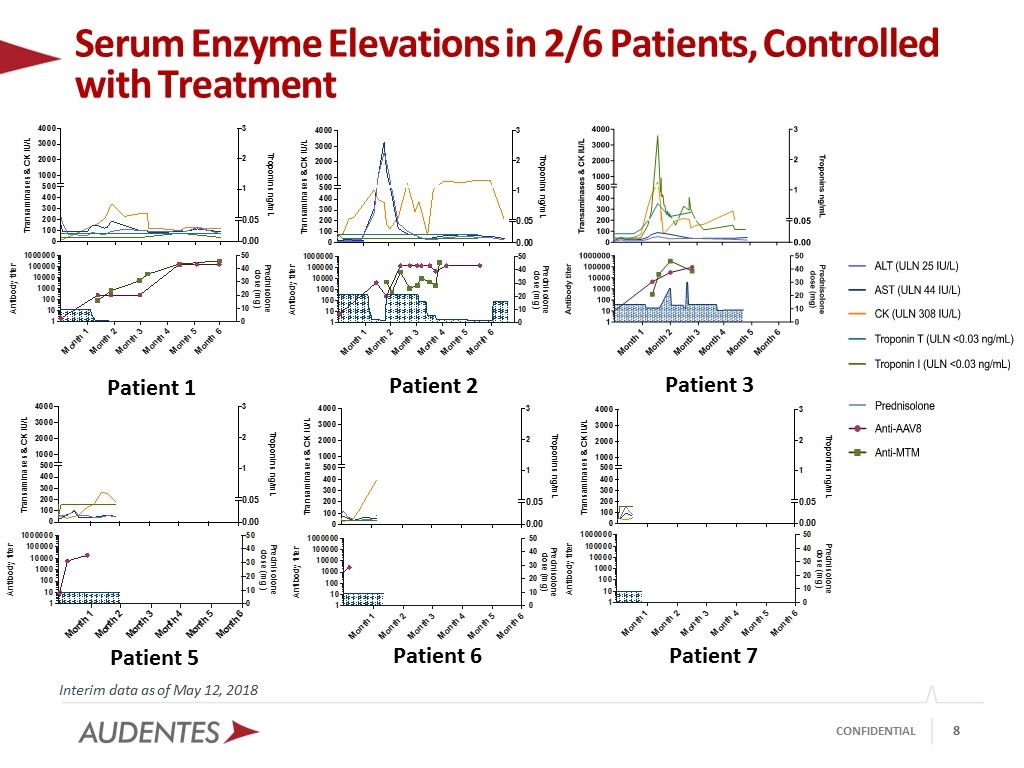
Serum Enzyme Elevations in 2/6 Patients, Controlled with Treatment Interim data as of May 12, 2018 Patient 1 Patient 2 Patient 3 Patient 5 Patient 6 Patient 7

NEUROMUSCULAR FUNCTION CHOP-INTEND and Developmental Milestones
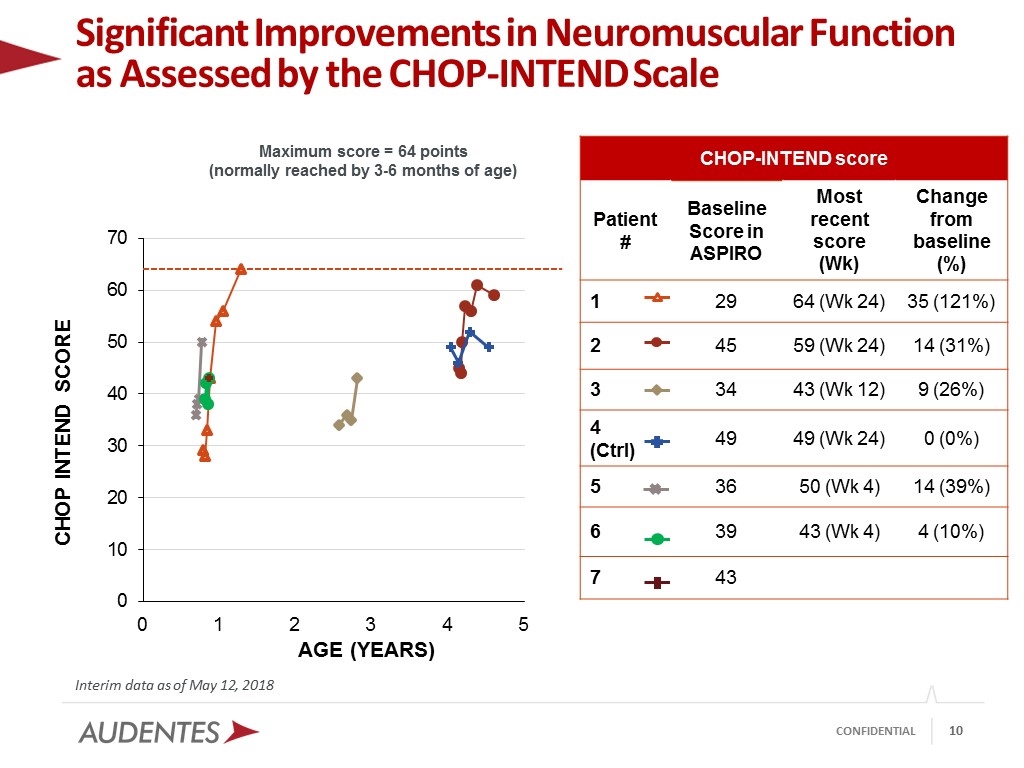
Significant Improvements in Neuromuscular Function as Assessed by the CHOP-INTEND Scale Interim data as of May 12, 2018 Maximum score = 64 points (normally reached by 3-6 months of age) CHOP-INTEND score Patient # Baseline Score in ASPIRO Most recent score (Wk) Change from baseline (%) 1 29 64 (Wk 24) 35 (121%) 2 45 59 (Wk 24) 14 (31%) 3 34 43 (Wk 12) 9 (26%) 4 (Ctrl) 49 49 (Wk 24) 0 (0%) 5 36 50 (Wk 4) 14 (39%) 6 39 43 (Wk 4) 4 (10%) 7 43
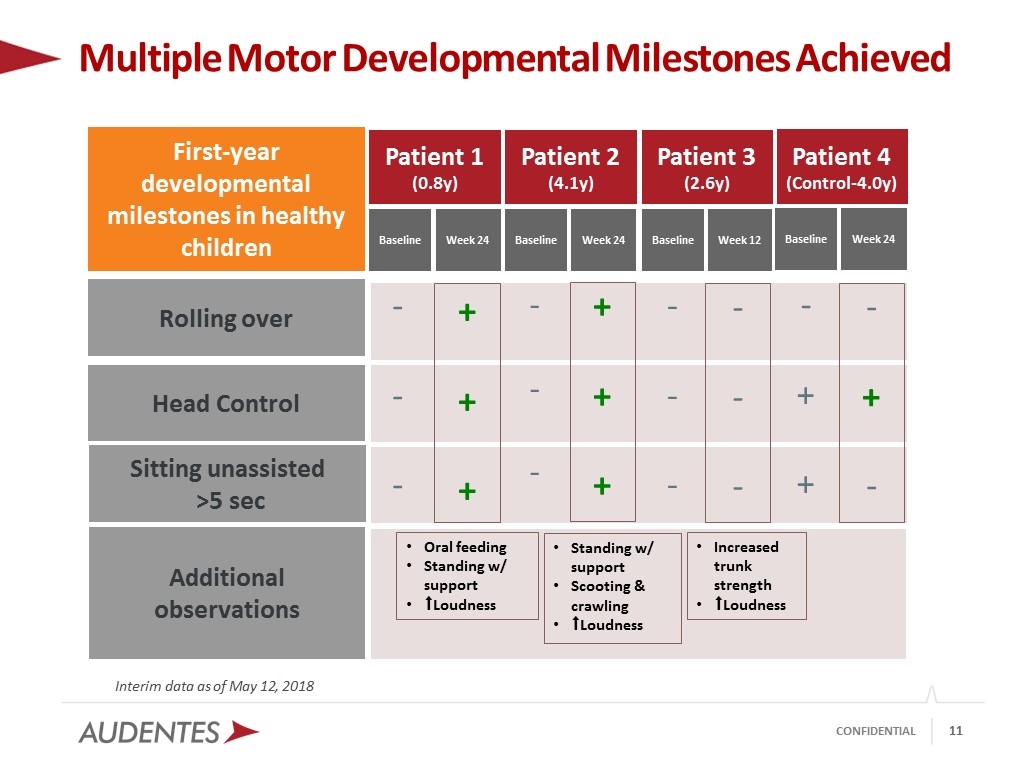
Interim data as of May 12, 2018 Multiple Motor Developmental Milestones Achieved First-year developmental milestones in healthy children Patient 1 (0.8y) Baseline Week 24 Rolling over Head Control Sitting unassisted >5 sec - - - Patient 2 (4.1y) Baseline Week 24 Baseline Week 12 Baseline Week 24 Patient 3 (2.6y) Patient 4 (Control-4.0y) - - - + + + - - - - - - - + + - + - + + + Additional observations Oral feeding Standing w/ support ⬆︎Loudness Standing w/ support Scooting & crawling ⬆︎Loudness Increased trunk strength ⬆︎Loudness

RESPIRATORY FUNCTION Maximal Inspiratory Pressure and Ventilator Dependence
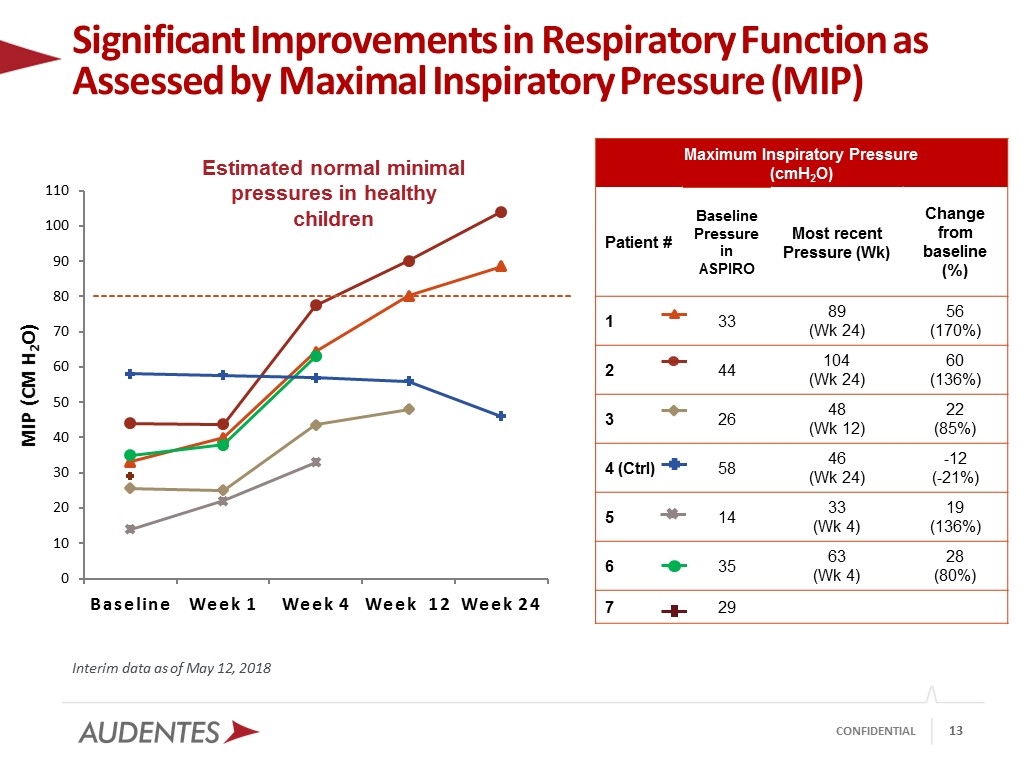
Interim data as of May 12, 2018 Significant Improvements in Respiratory Function as Assessed by Maximal Inspiratory Pressure (MIP) Estimated normal minimal pressures in healthy children Maximum Inspiratory Pressure (cmH2O) Patient # Baseline Pressure in ASPIRO Most recent Pressure (Wk) Change from baseline (%) 1 33 89 (Wk 24) 56 (170%) 2 44 104 (Wk 24) 60 (136%) 3 26 48 (Wk 12) 22 (85%) 4 (Ctrl) 58 46 (Wk 24) -12 (-21%) 5 14 33 (Wk 4) 19 (136%) 6 35 63 (Wk 4) 28 (80%) 7 29
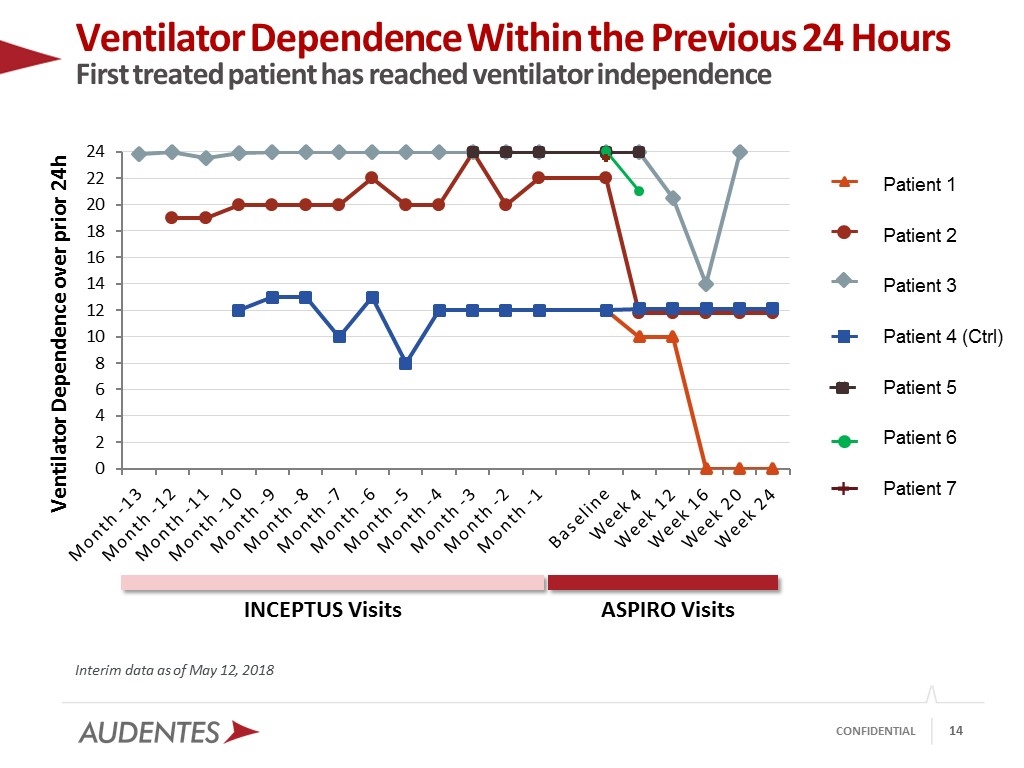
Ventilator Dependence Within the Previous 24 Hours First treated patient has reached ventilator independence Interim data as of May 12, 2018 INCEPTUS Visits ASPIRO Visits Patient 1 Patient 2 Patient 3 Patient 4 (Ctrl) Patient 5 Patient 6 Patient 7

summary

ASPIRO Key Findings and Program Next Steps Well tolerated during administration and manageable safety profile to date Significant improvements in neuromuscular and respiratory function at 24-week timepoint Encouraging initial efficacy results observed at the four-week timepoint in Cohort 1 expansion patients Patient 1 achieved ventilator independence and oral feeding Progressive qualitative improvements in disease severity observed in all treated patients Plan to review muscle biopsy data in conjunction with all safety and efficacy data to inform dose escalation decision
