Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AMICUS THERAPEUTICS, INC. | a18-13092_1ex99d1.htm |
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a18-13092_18k.htm |
Safe Harbor This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 relating to preclinical and clinical development of our product candidates, the timing and reporting of results from preclinical studies and clinical trials, the prospects and timing of the potential regulatory approval of our product candidates, commercialization plans, manufacturing and supply plans, financing plans, and the projected revenues and cash position for the Company. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Any or all of the forward-looking statements in this presentation may turn out to be wrong and can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. For example, with respect to statements regarding the goals, progress, timing, and outcomes of discussions with regulatory authorities, and in particular the potential goals, progress, timing, and results of preclinical studies and clinical trials, actual results may differ materially from those set forth in this release due to the risks and uncertainties inherent in our business, including, without limitation: the potential that results of clinical or preclinical studies indicate that the product candidates are unsafe or ineffective; the potential that it may be difficult to enroll patients in our clinical trials; the potential that regulatory authorities, including the FDA, EMA, and PMDA, may not grant or may delay approval for our product candidates; the potential that we may not be successful in commercializing Galafold in Europe and other geographies or our other product candidates if and when approved; the potential that preclinical and clinical studies could be delayed because we identify serious side effects or other safety issues; the potential that we may not be able to manufacture or supply sufficient clinical or commercial products; and the potential that we will need additional funding to complete all of our studies and manufacturing. Further, the results of earlier preclinical studies and/or clinical trials may not be predictive of future results. With respect to statements regarding projections of the Company's revenue and cash position, actual results may differ based on market factors and the Company's ability to execute its operational and budget plans. In addition, all forward-looking statements are subject to other risks detailed in our Annual Report on Form 10-K for the year ended December 31, 2017 as well as our Quarterly Report on Form 10-Q for the quarter ended March 31, 2018 to be filed May 9, 2018 with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and we undertake no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. Introduction

2018 Key Strategic Priorities Introduction Focused on FIVE Key Strategic Priorities in 2018 1 2 3 4 5 Double Galafold (migalastat) revenue to $75-$85M Secure approvals for migalastat in Japan and the U.S. Achieve clinical, manufacturing and regulatory milestones to advance AT-GAA* toward global regulatory submissions and approvals Develop and expand preclinical pipeline to ensure at least one new clinical program in 2019 Maintain financial strength *Advanced and Targeted GAA (AT-GAA, also known as ATB200/AT2221)

Galafold™ (Migalastat) Precision Medicine for Fabry Disease

Galafold Snapshot (as of March 31, 2018) Galafold Indicated for Long-Term Treatment of Adults and Adolescents Aged > 16 years with a Confirmed Diagnosis of Fabry Disease and Who Have an Amenable Mutation** Galafold: Precision Medicine for Fabry Disease 348 Amenable Mutations in EU Label 7 Regulatory Approvals* FIRST Oral Precision Medicine for Fabry Disease *EU, Australia, Canada, Japan, Israel, Switzerland, South Korea **For important safety information for Galafold visit www.ema.europa.eu. 18 Countries with Pricing & Reimbursement 2 Pending Regulatory Approvals (U.S., Taiwan) $75-$85M FY18 Galafold Revenue Guidance $16.7 1Q18 Galafold Revenue
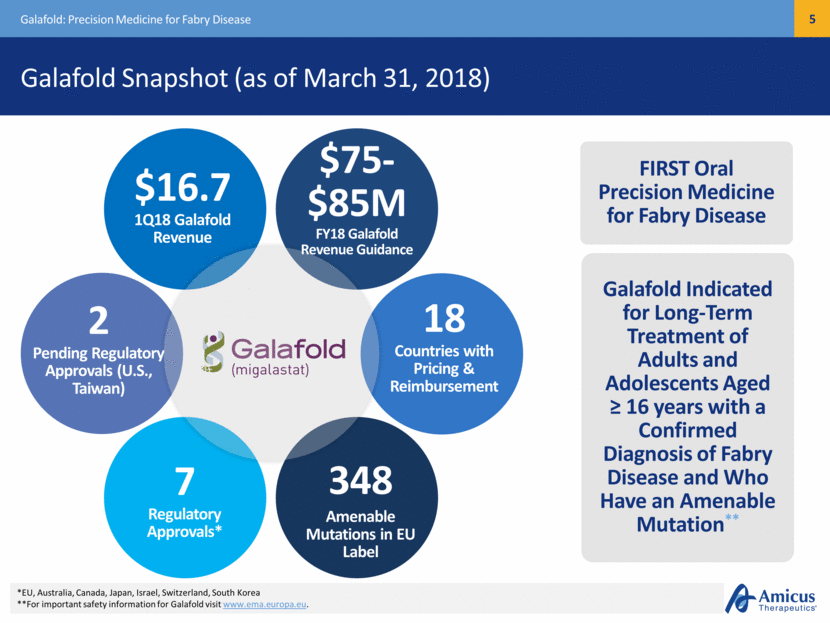
Migalastat Launch Progress (3/31/18) Precision Medicine for Fabry Disease Approved in Japan in 2Q18 – Now Approved in 7 Target Markets Canada = approved = pending approvals Israel Switzerland E.U. S. Korea Japan United States (Q3) Australia Taiwan
Additional Galafold Launch Dynamics Robust clinical data set1,2,3 Broad labels for amenable mutations (35%-50% of Fabry patients) Growth in patient and physician adoption from existing markets Expansion into new countries Very high rate of compliance and adherence Innovative precision medicine Compelling value proposition leading to rapid reimbursement Focus on hiring the best and brightest from a range of leading rare disease companies Compelling value proposition led to rapid reimbursement Key Success Factors Driving Strong Launch Across Geographies and Support $500M+ Global Peak Sales Potential Galafold: Precision Medicine for Fabry Disease Germain, DP et al., New England Journal of Medicine. 2. Hughes, et al., Journal of Medical Genetics. 3. For important safety information for Galafold visit www.ema.europa.eu.
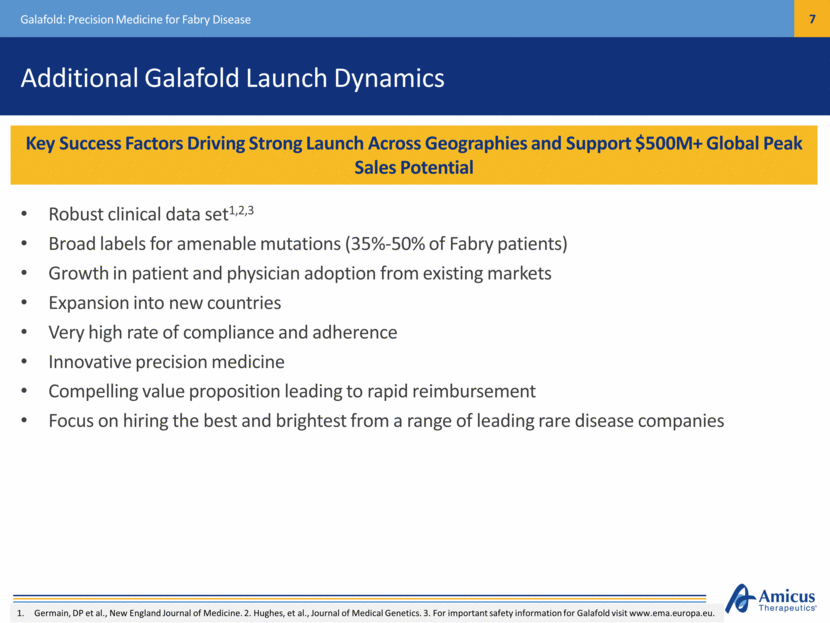
U.S. and Japan Demographics for Galafold U.S. NDA Filed Under Priority Review Galafold: Precision Medicine for Fabry Disease Japan Launch Anticipated in 2Q18 Japan Overview J-NDA Approved March 2018 Launch team hired and trained Launch anticipated 2Q18 ~850 patients diagnosed (>700 treated) No ERT home infusion currently available Physicians tend to initiate treatment early US Overview NDA Filed (Priority Review) August 13, 2018 PDUFA Leadership and majority of field team in place Appropriate stakeholder engagement underway ~3,000 diagnosed (1,500 treated), 35-50% amenable Orphan Drug and Fast Track designations
AT-GAA Novel ERT for Pompe Disease

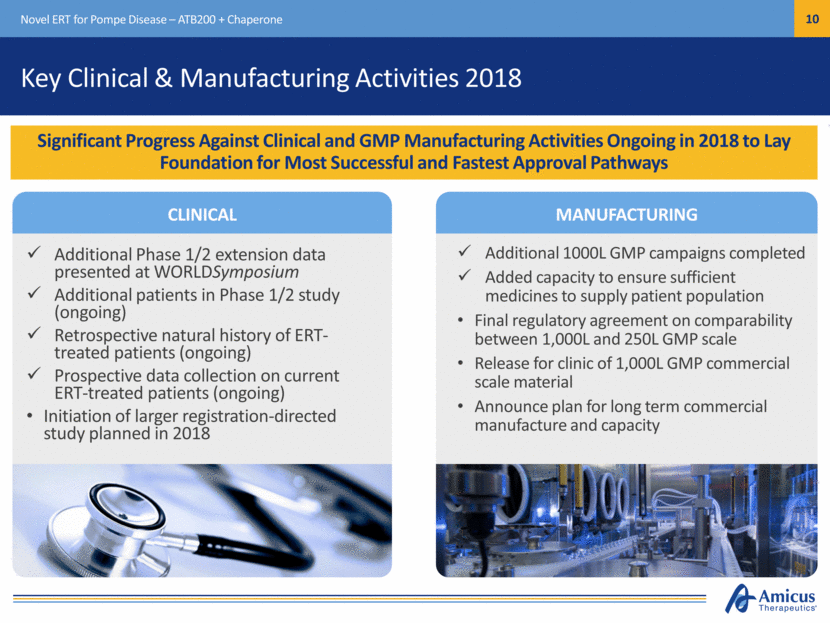
Key Clinical & Manufacturing Activities 2018 Additional Phase 1/2 extension data presented at WORLDSymposium Additional patients in Phase 1/2 study (ongoing) Retrospective natural history of ERT-treated patients (ongoing) Prospective data collection on current ERT-treated patients (ongoing) Initiation of larger registration-directed study planned in 2018 Significant Progress Against Clinical and GMP Manufacturing Activities Ongoing in 2018 to Lay Foundation for Most Successful and Fastest Approval Pathways Novel ERT for Pompe Disease – ATB200 + Chaperone CLINICAL MANUFACTURING Additional 1000L GMP campaigns completed Added capacity to ensure sufficient medicines to supply patient population Final regulatory agreement on comparability between 1,000L and 250L GMP scale Release for clinic of 1,000L GMP commercial scale material Announce plan for long term commercial manufacture and capacity
Pompe Regulatory Strategy Significant progress in collaborative discussions with EMA and FDA since 4Q17 Ongoing interactions include formal meetings scheduled with both agencies EMA - scientific advice meeting and EU update anticipated in 2Q18 U.S. FDA – type C meeting and US update anticipated in 3Q18 Goals of interactions and formal meetings Alignment on design of pivotal and supportive studies for full approval Discussion of potential conditional (EU) and accelerated (US) approval pathways A series of further iterative discussions with regulators and additional updates are expected as program advances, new data is collected, and registration-directed study initiated Goal continues to remain to determine the best and fastest pathway to approval A series of regulatory updates regarding a registration-directed study for full approval, manufacturing activities and the best and fastest path forward for AT-GAA AT-GAA Novel ERT + Chaperone for Pompe Disease

Key Clinical Activities 2018 Additional Phase 1/2 ATB200-02 extension data presented at WORLDSymposium Additional patients in Phase 1/2 ATB200-02 clinical study (ongoing) Retrospective natural history of ERT-treated patients (ongoing) Prospective data collection on current ERT-treated patients (ongoing) Initiation of larger registration-directed study (2H18) 18-month data from ATB200-02 clinical study (4Q18) Significant Progress In Clinical Activities in 2018 to Support Fastest and Best Approval Pathways AT-GAA Novel ERT + Chaperone for Pompe Disease

Biologics Manufacturing Progress & Upcoming Milestones AT-GAA Novel ERT + Chaperone for Pompe Disease Maintaining Identical Key Quality Attributes Throughout Scale Up to 1000L Analytical and in vivo comparability studies completed between 250L and 1000L engineering batches FDA agreement on comparability between 250L GMP scale and 1000L engineering batches; and testing strategy for demonstrating comparability between 250L scale and 1000L GMP batches GMP Manufacturing Campaigns of Drug Substance and Drug Product at 1000L GMP Scale Successfully Completed Release of 1,000L GMP material for initiation of registration-directed study Final regulatory agreement on comparability between 1,000L and 250L GMP scale

Long-Term Biologics Manufacturing Strategy AT-GAA Novel ERT + Chaperone for Pompe Disease Strategy for Added Capacity and Long-Term Commercial Manufacture Additional capacity at WuXi (China & Ireland) to ensure sufficient medicine to supply patient population Diligence ongoing for long-term U.S. commercial manufacture and capacity Project update anticipated 2H18

Pipeline Strategy

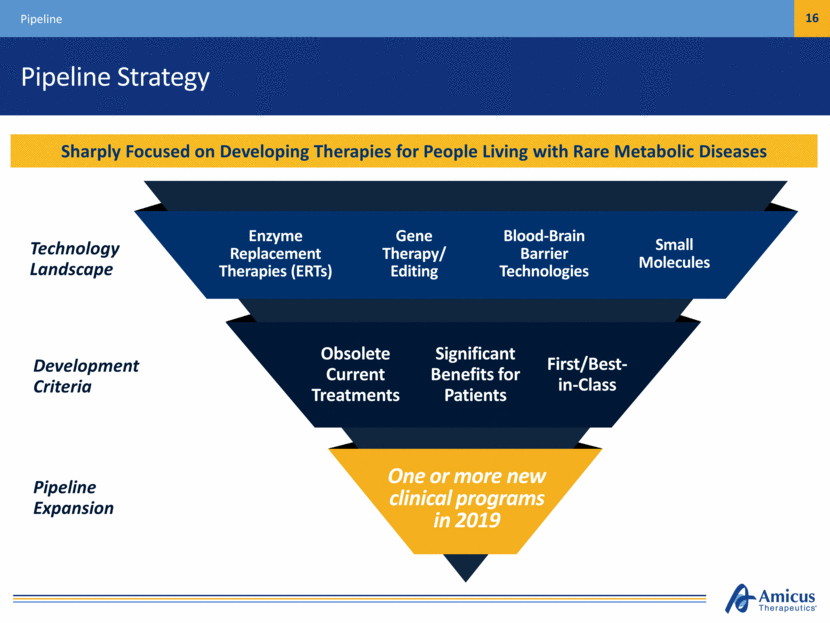
Pipeline Strategy Pipeline 16 Sharply Focused on Developing Therapies for People Living with Rare Metabolic Diseases Technology Landscape One or more new clinical programs in 2019 Significant Benefits for Patients First/Best- in-Class Obsolete Current Treatments Gene Therapy/ Editing Blood-Brain Barrier Technologies Small Molecules Enzyme Replacement Therapies (ERTs) Development Criteria Pipeline Expansion
Financial Summary and Upcoming Milestones

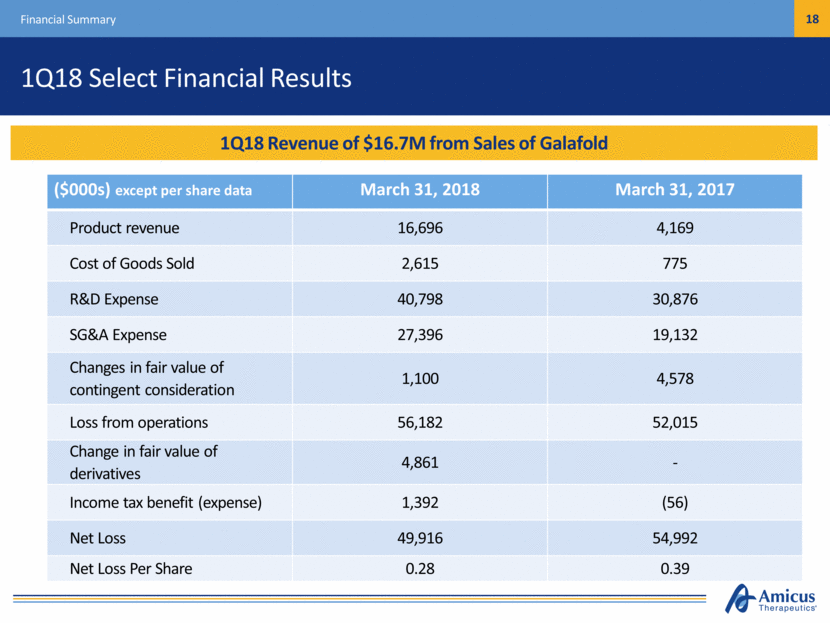
1Q18 Select Financial Results Financial Summary ($000s) except per share data March 31, 2018 March 31, 2017 Product revenue 16,696 4,169 Cost of Goods Sold 2,615 775 R&D Expense 40,798 30,876 SG&A Expense 27,396 19,132 Changes in fair value of contingent consideration 1,100 4,578 Loss from operations 56,182 52,015 Change in fair value of derivatives 4,861 - Income tax benefit (expense) 1,392 (56) Net Loss 49,916 54,992 Net Loss Per Share 0.28 0.39 1Q18 Revenue of $16.7M from Sales of Galafold
Financial Summary & Guidance Financial Summary Cash Position Provides Runway Under Current Operating Plan into mid-2017 FINANCIAL POSITION March 31, 2018 Cash $605M Debt $250M Cash Runway1 Into at least 2021 CAPITALIZATION Shares Outstanding2 187,972,218 FINANCIAL GUIDANCE FY18 Net Cash Spend Guidance $230-$260M Galafold Revenue Guidance $75-$85M 1Based on existing operating plan for Fabry and Pompe programs. 2Includes shares from the February 2018 equity offering

2018 Key Strategic Priorities Introduction Focused on FIVE Key Strategic Priorities in 2018 1 2 3 4 5 Double Galafold (migalastat) revenue to $75-$85M Secure approvals for migalastat in Japan and the U.S. Achieve clinical, manufacturing and regulatory milestones to advance AT-GAA* toward global regulatory submissions and approvals Develop and expand preclinical pipeline to ensure at least one new clinical program in 2019 Maintain financial strength *Advanced and Targeted GAA (AT-GAA, also known as ATB200/AT2221)

Thank You “Our passion for making a difference unites us” -Amicus Belief Statement


