Attached files
| file | filename |
|---|---|
| EX-32.1 - CERTIFICATION - Home Bistro, Inc. /NV/ | f10k2017ex32-1_gratitude.htm |
| EX-31.1 - CERTIFICATION - Home Bistro, Inc. /NV/ | f10k2017ex31-1_gratitude.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2017
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission File Number: 333-185083
GRATITUDE HEALTH, INC.
(Exact Name of Registrant as Specified in Charter)
| Nevada | 333-185083 | 27-1517938 | ||
| (State
or Other Jurisdiction of Incorporation) |
(Commission File Number) | (IRS
Employer Identification Number) |
11231 US Highway One
Suite 200
North Palm Beach, Fl. 33408
(Address of Principal Executive Offices, Zip Code)
Registrant’s telephone number, including area code: (561) 227-2727
Vapir Enterprises, Inc.
3511 Ryder Street, Santa Clara, California 95051
(Former Name or Former Address, if Changed Since Last Report)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☐ No ☒
Indicate by check mark if disclosure of delinquent filers in response to Item 405 of Regulation S-K (§ 229.405) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check One)
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☐ (Do not check if a smaller reporting company) | Smaller reporting company | ☒ |
| Emerging growth company | ☐ | ||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Section 12, 13 or 15(d) of the Exchange Act subsequent to the distribution of securities under a plan confirmed by a court. Yes ☒ No ☐
As of March 29, 2018, the Company had 53,141,833 shares of its common stock, $0.001 par value per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
| Item 1. | Business |
Vapir, Inc., the wholly owned subsidiary of Vapir Enterprises, Inc., a Nevada corporation, was incorporated on October 26, 2006 in the State of California.
On March 26, 2018, Gratitude Health, Inc. f/ka Vapir Enterprises, Inc., a corporation organized under the laws of Nevada (the “Acquiror” or “Company”), Hamid Emarlou, the principal shareholder of the Acquiror (the “Acquiror Principal Shareholder”), Gratitude Health, Inc. (FL), a corporation organized under the laws of Florida (the “Acquiree”), and each of the Persons who are shareholders of the Acquiree (collectively, the “Acquiree Shareholders,” and individually an “Acquiree Shareholder”) entered into a Share Exchange Agreement (the “Agreement”) pursuant to which the Acquiree Shareholders (who are the holders of all of the issued and outstanding shares of common stock of the Acquiree (the “Acquiree Interests”)) have agreed to transfer to the Acquiror, and the Acquiror has agreed to acquire from the Acquiree Shareholders, all of the Acquiree Interests, in exchange for the issuance of 520,000 shares of Series A Preferred Stock and 500,000 shares of Series B Preferred Stock, to the Acquiree Shareholders the “Acquiror Shares”), which Acquiror Shares shall, upon conversion into 102,000,000 shares of common stock of the Acquiror, constitute approximately 85.84% on a fully diluted basis of the issued and outstanding shares of Acquiror Common Stock immediately after the closing of the transactions contemplated herein, in each case, on the terms and conditions as set forth in the Agreement. For accounting purposes, the Share Exchange was treated as an acquisition of Acquiror and a recapitalization of Acquiree. Acquiree is the accounting acquirer, and the result is of its operations carryover. On the Closing Date, Acquiror Principal Shareholder entered into a Spin Off Agreement with Acquiror for the sale of the existing wholly owned Vapir, Inc. subsidiary of the Acquiror in exchange for Acquiror Principal Shareholder’s shares of Common Stock of Acquiror. The Spin Off Agreement shall not close less than five (5) days from the closing of the Agreement. As of the date of filing this Annual Report on Form 10-K, the disposition of the Company’s Vapir business has not been consummated. This Business description described both the traditional business of the Company as it existed on December 31, 2017, the end of the fiscal year for which this Form 10-K is filed, and the new business of Gratitude Health, Inc. after the March 26, 2018 share exchange.
Business of Vapir, Inc.
Vapir, Inc. specializes in the revolutionary technology of digital aromatherapy which is the art and science of utilizing naturally extracted aromatic essences from plants to balance and harmonize while freshening the environment with pleasant and distinctive fragrances. We invent, develop and produce revolutionary and easy to use digital aromatherapy devices. The unique value proposition of the Company’s proprietary technology (US Patent 6,095,153) is to prevent the creation of toxic by-products whenever plant materials are inhaled. This is accomplished by using convection heat that induces the safe release of plant essences without burning the source material. Our devices are designed in San Jose, California and manufactured in Guang Dong, China.
Our Industry
In general, “Vaporizers” are battery-powered products that enable users to inhale vapor without smoke, Vaporizers are not like traditional cigarettes, and their construction is comprised of three functional components:
| ● | Digital temperature control, means you have the ability to choose the warmth of your vaporizer’s heating element. The heating element that vaporizes liquid and/or botanicals so that it can be inhaled; | |
| ● | Vaporizers either heat the liquid or botanicals with direct contact with a heating element (conduction) or by exposing the herb to hot air (convection); and | |
| ● | Vaporizers require energy when hot. Depending on the intention behind the vaporizer’s design - the energy source will match the portable or stationary model paradigm. |
| 1 |
Our Products
Vaporizers
As of December 31, 2017, we marketed 5 vaporizers, the Prima, VapirRise 2.0 ultimate, VAPIR NO2 Portable Digital Vaporizer and VAPIR Oxygen Mini Corded Vaporizer, and the New Vapir Pen.
Prima

Prima is a digital vaporizer that supports extracts and botanicals. The unit is equipped with a removable/rechargeable lithium battery, a removable stainless steel vapor path and removable mouth piece which allows for easy cleaning, and four (4) pre-set temperatures which maintains pre-set heat levels by turning the heating element on and off as needed. Once the optimal temperature is reached, a green light on the casing will indicate that the device is now ready. This relatively simple technology enables the vaporizers to maintain heat levels.
| ● | Prima measures in at just 4.7 inches, and weighs 5.7 oz, and comes in four (4) different colors (Blue, Black, Silver, and Orange). |
VapirRise 2.0 Ultimate

| 2 |
The VapirRise 2.0 Ultimate is designed for loose-leaf herbs and essential oils. It supports both balloon inflation and direct inhalation. It can serve up to 4 users simultaneously. It has touch pad controls, an LCD temperature display and medical grade stainless steel vapir path and a ceramic heating element.
As the ceramic heating element of the device reaches the pre-set temperature, a fan blows air through the heating element. A sensor, which is located in the chamber of the unit, will constantly monitor the air temperature and maintain pre-set heat levels by turning the heating element on and off as needed. Once the optimal temperature is reached, a green light on the casing will indicate that the device is now ready. This relatively simple technology enables the vaporizers to maintain heat levels.
This convection vaporizer is a stationary desktop model - which means it isn’t intended for on-the-go consumption. The VapirRise offers an exceptional approach to the at-home vaporization experience.
Users can control the temperature (in both degrees Celsius & Fahrenheit); control the Fan Speed (ten options including a fanless setting); pick between a balloon or hose inhalation methods; and choose to serve up to four people at once with the exclusive hookah adapter.
VAPIR NO2 Portable Digital Vaporizer

The VAPIR NO2 is designed for loose-leaf herbs and direct inhalation. It is compact, portable and rechargeable. It has a medical grade pure brass element, an LCD temperature display and a silent operation. The VAPIR NO2 will be your number one portable vaporizer.
This compact portable vaporizer features touch-to-heat digital controls, an LCD thermostat, an internal rechargeable battery, and 100% silent operation. The NO2 vaporizer is designed for use with raw herbs and heats up in less than a minute. You can even vaporize while it’s charging.
The NO2 requires little to no maintenance for optimal operation and it even remembers your favorite temperature settings for quick and consistent vapor at the touch of a button.
| 3 |
VAPIR OXYGEN MINI CORDED

The VAPIR Oxygen Mini Corded is designed for loose-leaf herbs and direct inhalation. It is small and lightweight and is a corded vaporizer. It has an LCD temperature display and silent operation. Every portable vaporizer needs to produce clouds, not a mere mist. The Vapir Oxygen lets you live and breathe premium vapor. This herbal vaporizer harnesses premium materials and innovative design to deliver everything you’d expect from a premium vaporizer.
Our Oxygen Vaporizer features digital controls, portable design, and consistent vapor sessions with little maintenance.
VAPIR PEN

| 4 |
The Vapir Pen is a sleek and portable device that may be used with both concentrate and wax substances. It features an ergonomic mouthpiece that leads to a deep chamber. The Vapir Pen comes equipped with two different type of atomizers; the Coil-less Ceramic Atomizer and the Quartz Double-Rod Titanium Atomizer, both with Ceramic Chamber. The temperature controlled battery allows for a range of 3 pre-set temperatures. The Vapir Pen is directly charged through a Micro USB charging port at the bottom of the battery. This feature allows the device to be charged with any regular USB charger anywhere, whether at home or on the go. It is also included a USB charging cord in the box that can be connected directly to the device. The Vapir Pen has an elevated air flow system which reduces the likelihood of clogging or leakage.
Our Vaporizers and Accessories
Our vaporizers are sold with all the essentials that are needed to begin the vaporizing experience. In addition to the vaporizers, we also sell approximately 100 different accessories and spare parts that ranges from replacement batteries, replacement mouthpieces, recharging pieces, and all other essential accessories and spare parts.
Seasonality of our Business
We do not consider our business to be seasonal.
Marketing
We offer our vaporizers and related products through our website, distributors, online stores and retail stores. Retailers of our products include small-box smoke shops, vape stores, and online retailers throughout the United States and the world.
Competition
Competition in the vaporizer industry is intense and we anticipate that competition will likely remain intense for the foreseeable future. We compete with other sellers of vaporizers that are similar to our products and our competitors use the same sales practices and marketing strategies as we use and as a result, we face a continuing challenge in attempting to differentiate our products.
The nature of our competitors is varied as the market is highly fragmented and the barriers to entry into the business are low. Our direct competitors sell products that are substantially similar to ours and through the same channels through which we sell our vaporizers. We compete with these direct competitors for sales through distributors, wholesalers and retailers and we cannot assure you that we will be successful in meeting the competitive challenges that we face that will allow us to achieve unit sales volumes at levels that will allow us to achieve profitability and positive cash flow or if we achieve either or both of these objectives, that we can sustain either or both thereafter.
Our current competitive position in the vaporizer industry is difficult to gauge as most of our competition are also smaller companies or are privately held and do not publicly report their earnings. We do know of several competitors, but, like us, many are in their initial stages of development and are focusing on different areas of this industry. We also believe that the industry likely will attract other larger companies that possess greater financial and managerial resources with the result that we are likely to face greater competitive pressures that will adversely affect our sales volume, profits, and cash flow.
As a general matter, we have access to and market and sell similar products as our competitors, and since we sell our products at substantially similar prices as our competitors; accordingly, the key competitive factors for our success is the quality of service and design we offer our customers, the scope and effectiveness of our marketing efforts, including media advertising campaigns and, increasingly, the ability to identify and develop new sources of customers by attending trade shows and word of mouth. We cannot assure you that we will be successful in these efforts or, if we are successful, that we can maintain any competitive advantages that we may currently have.
| 5 |
Regulatory Matters/Compliance
The United States Food and Drug Administration (the “FDA”) regulates electronic cigarettes as “tobacco products” under the Family Smoking Prevention and Tobacco Control Act of 2009 (the “Tobacco Control Act”). The FDA is not permitted to regulate electronic cigarettes as “drugs” or “devices” or a “combination product” under the Federal Food, Drug and Cosmetic Act unless they are marketed for therapeutic purposes.
The Tobacco Control Act imposes significant new restrictions on the advertising and promotion of tobacco products. The law also requires the FDA to issue future regulations regarding the promotion and marketing of tobacco products sold or distributed over the internet, by mail order or through other non-face-to-face transactions in order to prevent the sale of tobacco products to minors.
It is likely that the Tobacco Control Act could result in a decrease in tobacco product sales in the United States, including sales of our electronic cigarettes and vaporizers.
The Tobacco industry expects significant regulatory developments to take place over the next few years, driven principally by the World Health Organization’s Framework Convention on Tobacco Control (“FCTC”). The FCTC is the first international public health treaty on tobacco, and its objective is to establish a global agenda for tobacco regulation with the purpose of reducing initiation of tobacco use and encouraging cessation. Regulatory initiatives that have been proposed, introduced or enacted include:
| ● | the levying of substantial and increasing tax and duty charges; | |
| ● | restrictions or bans on advertising, marketing and sponsorship; | |
| ● | the display of larger health warnings, graphic health warnings and other labeling requirements; | |
| ● | restrictions on packaging design, including the use of colors and generic packaging; | |
| ● | restrictions or bans on the display of tobacco product packaging at the point of sale, and restrictions or bans on cigarette vending machines; | |
| ● | requirements regarding testing, disclosure and performance standards for tar, nicotine, carbon monoxide and other smoke constituents levels; | |
| ● | requirements regarding testing, disclosure and use of tobacco product ingredients; | |
| ● | increased restrictions on smoking in public and work places and, in some instances, in private places and outdoors; | |
| ● | elimination of duty free allowances for travelers; and | |
| ● | encouraging litigation against tobacco companies. |
If electronic cigarettes or vaporizers are subject to one or more significant regulatory initiatives, our business, results of operations and financial condition could be materially and adversely affected.
Intellectual Property
Patents
We currently own four domestic utility patents and two design patents relating to vaporizers, as well as two utility patent applications and one design application pending in the United States as described below. There is no assurance that we will be awarded patents for of any of these pending patent applications. Further, we have not obtained an independent evaluation of our patent tights or any of our intellectual property rights. As a result, we cannot assure you that our patents and all of our intellectual property rights do not infringe upon those rights claimed by others. In that event we may be exposed to superior claims asserted by others and thereby we may be liable for significant damages arising out of any such infringement claims.
| 6 |
US Patent # 9,155,848 Method and System for Vaporization of a Substance
We have a utility patent for an apparatus for the vaporization of materials that releases active constituents for inhalation without the creation of harmful byproducts such as carcinogens associated with combustion and inhalation of substances. This patent expires on October 13, 2035.
U.S. Patent # 6,095,153 - Vaporization of volatile materials
We have a utility patent for the vaporization of volatile materials while avoiding combustion and denaturation of such material provide an alternative to combustion as means of volatilizing bioactive and flavor compounds to make such compounds available for inhalation without generating toxic or carcinogenic substances that are by-products of combustion and pyrolysis. This patent expires on June 19, 2018.
U.S. Patent # 6,772,756 - Method and System for Vaporization of a Substance
We have a utility patent for an apparatus for the vaporization of materials that releases active constituents for inhalation without the creation of harmful byproducts such as carcinogens associated with combustion and inhalation of substances. This patent expires on February 9, 2022.
U.S. Patent # 6,990,978 - Method and System for Vaporization of a Substance
We have a utility patent for an apparatus for the vaporization of materials that releases active constituents for inhalation without the creation of harmful byproducts such as carcinogens associated with combustion and inhalation of substances. This patent expires on January 31, 2026.
U.S. Design Patent # 489,448 - Vaporization Apparatus
We have a design patent for the ornamental design for the vaporization apparatus. This patent expires on May 4, 2018.
U.S. Design Patent # 508,119 - Mesh Filter with Glass Insert
We have a design patent for the ornamental design for a component for a vaporizer. This patent expires on August 2, 2019.
U.S. Patent Application # 11/872,040 - Method and System for Vaporization of a Substance
We have a utility patent (filed on October 15, 2007) pending for an apparatus for the vaporization of materials that releases active constituents for inhalation without the creation of harmful byproducts such as carcinogens associated with combustion and inhalation of substances.
U.S. Patent # 14/254,723 - Multi-User Inhalation Adaptor
We have a utility patent (filed on April 16, 2014) pending for a component of a vaporizer that allows multiple users to inhale the vapors of materials.
U.S. Design Patent # 29/473,910 - Vaporizer
We have a design patent (filed on November 26, 2013) for the ornamental design for the vaporization apparatus.
Trademarks
We own trademarks on certain of our products, including: Digital Air®, Nicohale®, and Vapir®.
| 7 |
Employees
As of December 31, 2017, we have three full-time and two part-time employees. None of these employees are represented by collective bargaining agreements and the Company considers it relations with its employees to be good.
Business of Gratitude Health, Inc.
Effective March 26, 2018, we acquired all the issued and outstanding shares of Acquiree pursuant to the Exchange Agreement and Acquiree became our wholly-owned subsidiary. The acquisition was accounted for as a recapitalization effected by a share exchange, wherein Acquiree is considered the acquirer for accounting and financial reporting purposes. The assets and liabilities of Acquiree have been brought forward at their book value and no goodwill has been recognized.
At the time of the acquisition, the Company was engaged in the business of engaged in inventing, developing and producing aromatherapy devices and vaporizers. As a result of the acquisition of all the issued and outstanding shares of common stock of Acquiree, we have now assumed Acquiree’s business operations as our own. The acquisition of Acquiree is treated as a reverse acquisition, and the business of Acquiree became the business of the Company.
Business Plan
The Company manufactures, sells and markets functional RTD (Ready to Drink) beverages sold under the Gratitude trademark. The Company’s first five drinks are Chinese Dragon Well Green Teas. Second and third functional-drink lines are now in development. The Gratitude mission is to disrupt this beverage market through our manufacturing and marketing of functional beverages that specifically promote healthy aging and to never produce a product that in any way will adversely affect the health of our customer. The Gratitude vision is to work with research partners to develop functional drinks that can easily be incorporated into one’s daily lifestyle and fit their nutritional goals.
THE PRODUCTS AND PACKAGING:
Gratitude Health, Inc. is building the “Gratitude” brand with the launch of unique, naturally flavored and unsweetened RTD (Ready To Drink) teas.
Today, tea is the second largest drink category in America and is an obvious driver and mainstay in delivery systems across food and beverage categories. Americans and, especially, Millennial Americans are increasingly becoming more and more aware of the generous levels of cancer-fighting antioxidants found in tea.
While Green, White and Black teas are the most common types consumed in America, research shows that their growth is solid year-over-year but barely above flat. We believe this is due to two factors:
| ● | The category is staid and boring and, | |
| ● | RTD teas are not delivering on their health promises. |
We will address these issues with unique varieties of tea that not only have interesting and curious names but also health research that supports their benefits. Naturally, we will be calorie and carb free and always strive for maximum antioxidant delivery. Our teas are sourced from Dragon Well in China. Our initial five flavors are:
| ● | Wildberry | |
| ● | Blood Orange | |
| ● | Mint | |
| ● | Original | |
| ● | Peach |
| 8 |
Supporting these unique flavors and key to our brand-building presentation will be our package, a first-to- RTD-market “mason jar” like 16-oz bottle featuring artistic, graphic designs actually etched onto the glass-container.
We will promote this unique presentation as more than eco-friendly (“eco” being of great importance to millennials) because they will be collectibles and advertised as such thus making their collectability and reuse arguably the eco-friendliest mass-market beverage bottle in the world. To even further support this unique presentation, artistic and colorful labels (logo, art, nutritional and redemption information) will cover the etchings driving the desires for those that keep them to buy their “missing” collectible.
Interestingly, the 2017 Natural Beverage Guide (Bevnet, Inc) displays hundreds of Natural-Drink companies and not one has a “Mason” jar configuration. We believe this packaging will be widely preferred to the predominantly plastic bottles in the market today, we further expect modern consumers to understand and appreciate the flavor profile and obvious nutritional benefits of the teas.

Having identified the opportunity and clearly focused upon the healthy features and benefits available in Gratitude’s tea offerings, Gratitude will shortly introduce its first five flavors of tea. This will kick off sales efforts in earnest for the larger chained retail accounts across the Country. These accounts generally review new products for “planogram” resets during calendar Q-1 with a view to begin new product offering and new sets in April each year.
This gives Gratitude three months to sell and arrange for new product authorizations in regional and national accounts while managing our initial placements in New York City and smaller accounts with whom we have relationships in the Northeastern United States.
| 9 |
MARKET INFORMATION AND THE VALUE CHAIN AND ROUTES TO MARKET
According to Beverage Marketing Corporation’s 2017 “DrinkTell” database, RTD tea sales totaled $10.31 billion in 2017 up four percent. Importantly, Gratitude’s market analyses and product development perfectly targets the main attributes fueling this growth. According to this database, “Ready-to-drink tea is leading the growth in its category with new forms and formulations that offer both function and flavor. Consumers are willing to trade up to products that offer them better quality or benefits.” Further according to BMC, “Retailers say refrigerated RTD teas are the leading segment in this category, and new varieties are emerging. In the RTD tea category, reduced-sugar formulations and local and artisanal brands are poised for continued growth.”
We are the first company to introduce Chinese Dragon Well Tea to the mass RTD American market. Dragon Well is subtle in taste with a hint of chestnut and is the most popular tea in China being granted “Imperial” status. Because it is “Green”, our tea will be well known to the US consumer. Because it is “DragonWell”, it will be a welcome new experience to the US RTD market. All our teas are either low calorie--45 per 16z—or totally unsweetened. Our “small batch * hand made” brand positioning features totally unique and first-to-market packaging that meets artisanal characteristics influencing today’s consumer purchase.
According to the Tea Association of the USA, in its 2017-2018 Tea Market Review and Forecast, Ready-to-drink (RTD) tea accounted for some 45.7% of the tea market share in 2017 and will exceed 1.7 billion gallons in 2017. The Review states “Specialty Tea is still driving interest and consumption in the category with consumers grazing for new and different options and flavors and origins. Sustainability of these high quality, higher priced teas confirms that the analogy to wine is stronger than ever.” Furthermore, according to the Tea Association: “Naturalness continues to drive consumers who demand foods that are closer to their unrefined or pure state, seeking “less processed” drinks, incentivizing companies to remove artificial ingredients. This trend will also encourage consumers to reach for foods in their most natural, original form, such as true teas, for health benefits, instead of supplements and nutraceuticals. Tea is a natural, simple and whole food.”
By marrying a famous and revered green tea in China with such organic flavors as Peach, Mint, Wildberry and Blood Orange and putting those complementary flavors in a totally unique glass package, Gratitude is well positioned for our targeted consumer audience.
Gratitude’s retail shelf price is between $2.99 to $3.49 per bottle. We have established cost for finished product per bottle and per 12-count case. We will approach and engage the same retail systems and value chain structure relative to all RTD beverage brands in the industry.
| 10 |
There will be three routes to market:
| 1. | Direct-to-Retail sales (grocery, big box, and drug chains). | |
| 2. | Direct Store Delivery (DSD) with shelf management for C-stores, specialty accounts, and such institutions as colleges and universities. | |
| 3. | Direct-to-Consumer sales via the internet. We will partner with Amazon for fulfillment. |
| 1. | Value chain for channel one includes delivered pricing from Gratitude direct to the retailer. We will target a suggested retail price in these accounts. Knowing that these accounts demand net delivery to their warehouses and a SET gross margin, Gratitude will price cases at wholesale to this channel of trade at a set price per bottle per cost of goods sold as calculated so that the Company has already calculated its margin less cost of freight for delivery. |
| 2. | Sales to DSD delivery systems require a more competitive wholesale price and higher retail price, which enables a structure where a distributor can earn a meaningful margin in an extremely competitive environment. The DSD retail customer is often a C-Store or Specialty store that requires regular deliveries and in-store management of inventory in “reaches”, meaning that often the DSD merchandises the shelf for his customer. This service cost is passed to the wholesaler. Therefore, to accommodate this pricing model, Gratitude must be more competitively priced to the DSD. The retailer also needs room to mark up the brand to accommodate their gross margin goals. So, Gratitude will reduce its gross margin in this channel and the DSD distributor will expect a set gross margin. The C-Store retailer usually expects a minimum set gross margin at his level. |
| 3. | The third sales model is direct consumer sales through the internet. These sales are always made by the case with an added delivery fee to the individual. We will partner with Amazon to handle orders and fulfillment and simply deliver to their regional warehouses. These sales provide great opportunity for gross margin having eliminated the distributor and retailer margin, but require significant digital marketing programs and advertising. We will use this channel immediately to service consumers that have heard of the brand but can’t find it at their local store yet. We know this channel will not be a large business initially but, as the brand grows, we plan to build this segment aggressively. |
COMPETITION
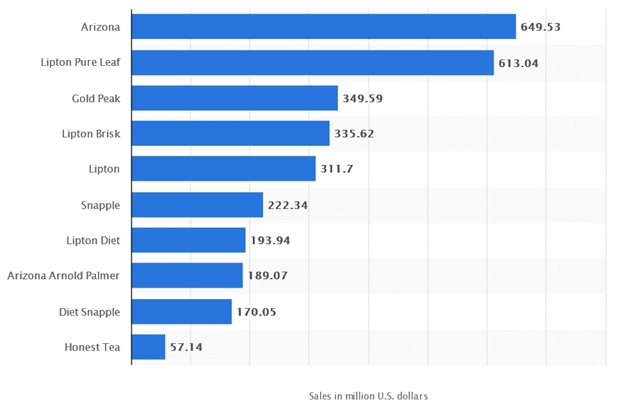
Source: Statista 2018
| 11 |
RESEARCH, DEVELOPMENT AND PHILANTHROPY
Gratitude’s RD&P (Research, Development and Philanthropy) is working with a number of institutions to develop, license and/or acquire valid and proven intellectual property that fights cancer and promotes healthy aging. To that end, Gratitude has solely licensed patented technology from a prominent U.S. university research foundation for their patent (U.S. Patent #: 6,713,605) for the use of tea polyphenol esters for cancer prevention and treatment. The patented invention relates to novel polyphenol esters derived from green teas, which are potent inhibitors of the growth of cancerous cells, and their use in the prevention and treatment of conditions characterized by abnormal cellular proliferation. Gratitude will fund our charities from our sales and, in addition to giving away product to those who cannot afford them, we will contribute to university and institutional research studies that focus on chemo-protection, healthy aging, phytochemical superfoods and clean nutrition.
Corporation Information
Our principal executive offices are now located at 11231 US Highway One, Suite 200, North Palm Beach, Fl. 33408
. Our phone number is (561) 227-2727
. Our website is www.gratitudehealth.com.
| Item 1A. | Risk Factors |
We are a small public company and we face ever-increasing challenges in fulfilling the ever-rising costs of regulatory compliance under our federal, state, and other laws.
As a small company we have limited financial resources and we face compliance and regulatory costs that are increasing yearly. While we believe that our business strategies are sound, we cannot assure you that we will not incur such costs and have the ability to pass these increased costs onto our customers. As a result, we may incur significant operating losses and negative cash flow for the foreseeable future with resulting adverse impact on our continued existence as a corporation.
Our Total Current Liabilities were greater than our Total Current Assets as of December 31, 2017.
As our Total Current Liabilities as of December 31, 2017 were $1,889,243 and our Total Current Assets were only $203,889, we are and remain insolvent in that as of December 31, 2017 our Current Ratio (defined as Total Current Assets divided by Total Current Liabilities) was only 0.11. As a result, we do not have sufficient cash or other liquid current assets to meet our current financial obligations that become due within the twelve-months.
We incurred significant losses in 2017 and our cash flow is limited and volatile with the result that we face constant financial challenges in meeting our monthly operating and other financial obligations.
We incurred significant losses in 2017 and we cannot assure you that we will achieve profitability or if we do achieve it that we can sustain any profitability in the future. Further and as a small company, our cash flow is limited and we do not have a diversified customer base with diversified customers and markets compared to larger companies. As a result, our monthly cash flow is limited and more volatile than other larger companies. For this reason we are exposed to greater financial risks and persons who acquire our common stock could lose all or substantially all of their investment.
Continuing and increasing losses may directly impact our ability to remain in business.
We incurred approximately $365,000 in net losses for the year ended December 31, 2017. While we believe that if circumstances and market conditions allow, we may be able to achieve profitability, there can be no assurance that we will be successful in these efforts or if we are successful, that we can sustain any profitability. If are not successful in achieving and sustaining profitability and positive cash flow, then persons who acquire our common stock could lose all or substantially all of their investment.
Limited trading market and limited and sporadic trading of our Common Stock.
The trading market for our Common Stock is limited and any holder of our Common Stock will likely find it difficult to sell their shares in any large amount without incurring protracted delays and difficulty and without a significant reduction in the market price of the overall trading market for our Common Stock. The trading market for our Common Stock is limited and sporadic and there can be no assurance that any liquid trading market will develop or if it does develop that it can be sustained. As a result, any purchase of our Common Stock or any conversion of any debt instrument that is convertible into our Common Stock should only be considered by those who can afford to own a relatively illiquid investment and the likelihood that they may incur the total loss of their investment.
| 12 |
| Item 2. | Properties |
The Company’s corporate headquarters is located in Florida at 11231 US Highway One, Suite 200, North Palm Beach, Fl. 33408. Our telephone number, including area code, is (561) 227-2727
| Item 3. | Legal Proceedings. |
From time to time, the Company is involved in litigation matters relating to claims arising from the ordinary course of business. While the results of such claims and legal actions cannot be predicted with certainty, the Company’s management does not believe that there are claims or actions, pending or threatened against the Company, the ultimate disposition of which would have a material adverse effect on our business, results of operations, financial condition or cash flows.
| Item 4. | Mine Safety Disclosure |
Not Applicable
| 13 |
PART II
| Item 5. | Market For Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
Our common stock is currently approved for quotation on the OTC Bulletin Board (OTCQB) maintained by the Financial Industry Regulatory Authority, Inc. under the symbol “GRTD”. There were no trades of our stock prior to April 6, 2015. The table below sets forth the high and low closing price per share of our common stock for each quarter during 2016 and 2017. These prices represent inter-dealer quotations without retail markup, markdown, or commission and may not necessarily represent actual transactions.
| Fiscal Quarter Ended | High | Low | ||||||
| 31-Mar-16 | $ | 0.35 | $ | 0.06 | ||||
| 30-Jun-16 | $ | 0.16 | $ | 0.05 | ||||
| 30-Sep-16 | $ | 0.16 | $ | 0.08 | ||||
| 31-Dec-16 | $ | 0.22 | $ | 0.08 | ||||
| 31-Mar-17 | $ | 0.12 | $ | 0.01 | ||||
| 31-June-17 | $ | 0.07 | $ | 0.0151 | ||||
| 31-Sep-17 | $ | 0.035 | $ | 0.012 | ||||
| 31-Dec-17 | $ | 0.11 | $ | 0.021 | ||||
Holders
As of March 29, 2018, there were approximately 64 holders of record of our common stock, and an indeterminate number of holders of unrestricted shares.
Dividends
We have not declared cash dividends on our common stock since our inception and we do not anticipate paying any cash dividends in the foreseeable future. Our current policy is to retain earnings, if any, for use in our operations and in the development of our business. Our future dividend policy will be determined from time to time by our board of directors.
| Item 6. | Selected Financial Data |
Not Applicable as we are a smaller reporting company.
| 14 |
| Item 7 | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
Except for the historical information, the following discussion contains forward-looking statements that are subject to risks and uncertainties. We caution you not to put undue reliance on any forward-looking statements, which speak only as of the date of this report. Our actual results or actions may differ materially from these forward-looking statements for many reasons. Our discussion and analysis of our financial condition and results of operations should be read in conjunction with the financial statements and related notes and with the understanding that our actual future results may be materially different from what we currently expect.
Forward-Looking Statements
Certain information contained in this Annual Report on Form 10-K, as well as other written and oral statements made or incorporated by reference from time to time by the Company and its representatives in other reports, filings with the Securities and Exchange Commission, press releases, conferences or otherwise, may be deemed to be forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934. This information includes, without limitation, statements concerning the Company's future financial position and results of operations, planned expenditures, business strategy and other plans for future operations, the future mix of revenues and business, customer retention, project reversals, commitments and contingent liabilities, future demand and industry conditions. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, it can give no assurance that such expectations will prove to have been correct. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Generally, the words “anticipate,” “believe,” “estimate,” “expect,” “may” and similar expressions, identify forward-looking statements, which generally are not historical in nature. Actual results could differ materially from the results described in the forward-looking statements due to the risks and uncertainties set forth in this Annual Report on Form 10-K, and those described from time to time in our future reports filed with the Securities and Exchange Commission.
The following discussion is qualified in its entirety by, and should be read in conjunction with, the Company's financial statements, including the notes thereto, included in this Annual Report on Form 10-K.
As used herein, the terms “we,” “us,” and “the Company” refers to Gratitude Health, Inc. (f/k/a Vapir Enterprises, Inc.), a Nevada corporation and its subsidiaries.
On March 26, 2018, Gratitude Health, Inc. f/ka Vapir Enterprises, Inc., a corporation organized under the laws of Nevada (the “Acquiror” or “Company”), Hamid Emarlou, the principal shareholder of the Acquiror (the “Acquiror Principal Shareholder”), Gratitude Health, Inc. (FL), a corporation organized under the laws of Florida (the “Acquiree”), and each of the Persons who are shareholders of the Acquiree (collectively, the “Acquiree Shareholders,” and individually an “Acquiree Shareholder”) entered into a Share Exchange Agreement (the “Agreement”) pursuant to which the Acquiree Shareholders (who are the holders of all of the issued and outstanding shares of common stock of the Acquiree (the “Acquiree Interests”)) have agreed to transfer to the Acquiror, and the Acquiror has agreed to acquire from the Acquiree Shareholders, all of the Acquiree Interests, in exchange for the issuance of 520,000 shares of Series A Preferred Stock and 500,000 shares of Series B Preferred Stock, to the Acquiree Shareholders the “Acquiror Shares”), which Acquiror Shares shall, upon conversion into 102,000,000 shares of common stock of the Acquiror, constitute approximately 85.84% on a fully diluted basis of the issued and outstanding shares of Acquiror Common Stock immediately after the closing of the transactions contemplated herein, in each case, on the terms and conditions as set forth in the Agreement. For accounting purposes, the Share Exchange was treated as an acquisition of Acquiror and a recapitalization of Acquiree. Acquiree is the accounting acquirer, and the result is of its operations carryover. On the Closing Date, Acquiror Principal Shareholder entered into a Spin Off Agreement with Acquiror for the sale of the existing wholly owned Vapir, Inc. subsidiary of the Acquiror in exchange for Acquiror Principal Shareholder’s shares of Common Stock of Acquiror. The Spin Off Agreement shall not close less than five (5) days from the closing of the Agreement. As of the date of filing this Annual Report on Form 10-K, the disposition of the Company’s Vapir business has not been consummated. This MD&A, which addresses the Company as of December 31, 2017, describes the traditional business of the Company as it existed on December 31, 2017, the end of the fiscal year for which this Form 10-K is filed. For information on the new business of Gratitude Health, Inc. after the March 26, 2018 share exchange, please refer to Section 1 of this Annual Report on Form 10-K.
| 15 |
Overview
Vapir Enterprises, Inc. was originally incorporated under the laws of the State of Nevada on December 17, 2009 under the name Apps Genius Corp and changed its name to Gratitude Health, Inc. on March 22, 2018. Our original business was to develop, market, publish and distribute social games and software applications that consumers could use on a variety of platforms, including social networks, wireless devices and stand-alone websites. We were unsuccessful in operating our business and on October 7, 2013 we entered into a Membership Interest Purchase Agreement with FAL Minerals LLC and we changed our name to FAL Exploration Corp. The agreement with FAL Minerals LLC has since been terminated and we have now entered into an Exchange Agreement with Vapir, Inc. and its shareholders. In addition, we changed our name to Vapir Enterprises, Inc. to better represent our new business operations.
On December 30, 2014, Vapir, Inc., a private California corporation (“Vapir”), which is the historical business of the Company’s wholly-owned subsidiary, entered into a Share Exchange Agreement with the Company, all of the stockholders of Vapir (the “Vapir Shareholders”), and the Company’s controlling stockholders whereby the Company agreed to acquire all of the issued and outstanding capital stock of Vapir in exchange for 38,624,768 shares of the Company’s common stock. On December 30, 2014, the transaction closed and Vapir is now a wholly-owned subsidiary of the Company. The number of shares issued represented approximately 80.0% of the issued and outstanding common stock immediately after the consummation of the Share Exchange Agreement. In addition, Vapir’s board of directors and management obtained the board and management control of the combined entity stock immediately after the consummation of the Share Exchange Agreement.
Vapir, Inc., our wholly-owned subsidiary, was incorporated on October 26, 2006 in the State of California.
Vapir, Inc. specializes in the revolutionary technology of digital aromatherapy which is the art and science of utilizing naturally extracted aromatic essences from plants to balance and harmonize while freshening the environment with pleasant and distinctive fragrances. We invent, develop and produce revolutionary and easy to use digital aromatherapy devices by utilizing heat and convection air.
Results of Operations
Year Ended December 31, 2017 Compared to the Year Ended December 31, 2016
Net Revenues
Net revenues for the years ended December 31, 2017 and 2016 were $761,102 and $1,086,971 respectively, a decrease of $325,869 or approximately 30%. The decrease in sales during the year ended December 31, 2017 was primarily attributable to a decrease in sales of our Prima vaporizer product as a result of a decline in demand.
Management views future sales level with a fair degree of uncertainty in that management has not been able to identify whether our sales level are trending up or down over the near term. As a result, we believed our sales level are subject to high level of uncertainty and unless market conditions and competitive conditions dramatically improve, we may not achieve or maintain sufficient sales volumes at levels that will allow us to achieve or maintain any profitability or positive cash flow. Our Total Liabilities as of December 31, 2017 far exceed our Total Assets. As a result we are insolvent and face a clear, existential risk of potential bankruptcy or other adverse actions by our creditors that could result in stockholders losing all of their investment.
Cost of Revenues
Cost of revenues for the year ended December 31, 2017 and 2016 were $366,075 and $678,056, respectively, a decrease of $311,981 or approximately 46%. The decrease is primarily due to the decrease in sales of our vaporizer products.
| 16 |
Operating Expenses
Total operating expenses for the year ended December 31, 2017 and 2016 were $661,179 and $1,944,309, respectively, a decrease of $1,283,130 or approximately 66%. The decrease in operating expenses during the year ended December 31, 2017 is primarily due to decrease in stock based consulting and stock based compensation expense of approximately $579,016, decrease in impairment of intangible assets of $126,426, and decrease in compensation of $237,191 as a result of decrease in number of employees. Additionally, an overall decrease in Selling, General and Administrative costs of approximately $281,141 as a result of cost cutting measures. While we implemented these cost cutting measures, we cannot assure you that these measures can be sustained or, if sustained that we will not incur other costs that far exceed the benefits obtained from these cost cutting measures. These and other factors would likely cause us to continue to incur significant and protracted losses in the future with the result that we may be facing claims by our creditors that we cannot satisfy since we are insolvent. As a result, there can be no guarantee that we will be successful in reducing our operating costs to a level that would allow us to achieve profitability, positive cash flow or both of them or if we do achieve these objectives that we can sustain profitability, positive cash flow or both of them. As of December 31, 2017, our Total Liabilities were $1,893,764 and our Total Assets as of that date were $387,655. As a result we are insolvent and any person who acquires our Common Stock or any other instrument that we have or will issue faces a high likelihood that they will lose their entire investment as we face a clear prospect of bankruptcy.
We continue to evaluate our operating cost with an aim of reducing our operating expenses in the future. However, some of our costs are fixed and we face intense competition from others who have more favorable operating cost structures and greater unit volumes that allows them to have an ability to compete aggressively on pricing. These and other factors would likely cause us to continue to incur significant and protracted losses in the future. As a result, there can be no guarantee that we will be successful in reducing our operating costs to a level that would allow us to achieve profitability, positive cash flow or both of them or if we do achieve these objectives that we can sustain profitability, positive cash flow or both of them.
Other Income (Expense), net
Total other expense, net, for the year ended December 31, 2017 and 2016 were $99,145 and $348,176, respectively, a decrease of $249,031 or %72%. The decrease in other expense is the primary result of the decreased amortization of debt discount of approximately $252,000 in connection with the issuance of convertible debentures.
Net loss
Net loss for the year ended December 31, 2017 and 2016 was $365,297 and $1,883,570, respectively, as a result of the items discussed above.
Liquidity and Capital Resources
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations, and otherwise operate on an ongoing basis. As of December 31, 2017, our total current liabilities exceeded our total current assets and, as a result, we had a working capital deficit and a further deterioration in our liquidity over the past 12 months.
We are not aware of any known demands, commitments or events that will result in our working capital liquidity increasing or decreasing in any material way. We are not aware of any matters that would have a positive impact on future operations. But over the past twelve months we have had increasing losses that is likely to continue if current market conditions continue.
| 17 |
Our net revenues are not sufficient to fund our operating expenses. At December 31, 2017, we had a cash balance of approximately $5,200 and working capital deficit of $1,685,000. Our cash decreased during the year ended December 31, 2017 by approximately $6,800 from our cash balance at December 31, 2016 of $12,000.
During the year ended December 31, 2017, we received related party advances for a total of $57,000 to fund our operating expenses, pay our obligations, and grow our company. We currently have no material commitments for capital expenditures.
During the year ended December 31, 2016, we borrowed $50,000 by issuing a note payable which will mature in February 2019 and we also received related party advances for a total of $430,000 to fund our operating expenses, pay our obligations, and grow our company. We currently have no material commitments for capital expenditures.
We are facing increasing demands that will likely require that we raise additional funds. If circumstances and market conditions allow, we may be able to raise additional capital but it may be under market conditions that are not favorable with the result that we may incur dilution or be required to accept debt covenants or other conditions that are onerous or which otherwise limit our ability to gain or attract additional financing in the future. Further, there can be no assurance that we will be successful in raising any additional funds or if we are successful, that we will be able to do so on terms that are reasonable in light of our current circumstances. As a small company with a limited product line and limited customer base, we face continuing risks and uncertainties that serve to make our company and an investment in our common stock subject to risks that are beyond our control. We estimate that based on current plans and assumptions, that our cash is not sufficient to satisfy our cash requirements under our present operating expectations, without further financing, for the next 12 months.
Our ability to generate and maintain a positive cash flow from our operations cannot be assured. Based solely on our own internal estimates without the benefit of any independent third party evaluation, we anticipate that our cash and cash flow will not be sufficient to satisfy our cash requirements and we will likely require significant additional external financing. The magnitude of the additional financing and its timing is not yet precisely known and depending on the level of our sales revenues and other operating needs we may be facing a prolonged multi-period scenario of negative cash flows with increasing losses. Further and since our Total Current Liabilities as of December 31, 2017 were $1,889,243 and our Total Current Assets were only $203,889, we are and remain insolvent in that as of December 31, 2017 our Current Ratio (defined as Total Current Assets divided by Total Current Liabilities) was only 0.11. That is, it was far below one (1). Overall, there can be no assurance that we will be successful in raising additional capital on a timely basis or if we are able to raise additional capital that we can raise it on terms that are reasonable in light of our current circumstances. As a result, we face increasing risks and persons who acquire our common stock may incur the loss of all or substantially all of their investment.
On April 3, 2015, we closed a financing transaction by entering into a Securities Purchase Agreement dated April 3, 2015 (the “Securities Purchase Agreement”) with two accredited investors (the “Purchasers”) for an aggregate subscription amount of $500,000 (the “Purchase Price”). Pursuant to the Securities Purchase Agreement, we issued a 6% Convertible Debenture (the “Debenture”) to each investor and warrants exercisable into an aggregate of 500,000 shares of common stock at an exercise price of $0.60 per share (the “Warrants”).
Each Debenture accrues interest at a rate equal to 6% per annum and the Debenture has an extended maturity date of July 26, 2018.
Each Debenture is convertible at any time and from time to time after its issuance date. Each Purchaser has the right to convert the Debenture that they hold into shares of the Company’s common stock at a conversion price equal to ten cents ($0.10). The conversion price, however, is subject to full ratchet anti-dilution in the event that Company issue any securities at a price lower than the conversion price then in effect. We have relied upon the use of debt financing to raise significant amounts of capital and we may continue to do so in the future. Under these circumstances and given our financial condition, any holder of our Common Stock is faced with the risk of the total loss of their investment.
Pursuant to the Securities Purchase Agreement, the Company issued warrants to acquire 500,000 shares of our common stock. The Warrants issued in this transaction are immediately exercisable and currently at an exercise price of ten cents ($0.10) per share, subject to applicable adjustments including full ratchet anti-dilution in the event that the Company issues any securities at a price lower than the exercise price then in effect. The Warrants have an expiration period of five years from the original issue date.
| 18 |
In January 2018, the Company issued an aggregate of 3,375,000 shares of common stock to Purchasers upon the conversion of $327,500 principal amount of note and $10,000 accrued interest pursuant to the conversion terms of the Convertible Debenture.
Currently, we have no other known alternative source for any additional financing except those sources which we have previously used and we cannot be assured that our prior sources will have any willingness to provide us with additional capital or, if they do, that the terms of any such additional financing will be reasonable in light of our current conditions. Further, we cannot assure you that we can continue to rely upon those existing financing sources in the future. We may not have sufficient working capital and funds from the collection of revenues that may allow us to maintain or expand our existing operations, to provide sufficient working capital to meet our operating needs and our outstanding financial obligations. For this reason, we anticipate that, based on current market conditions and our existing financial condition, we will likely need to obtain significant additional capital. In this sense we currently anticipate that we will remain dependent on our ability to secure additional financing.
In the event that we are able to secure a sufficient amount additional financing on a timely basis, it may include the issuance of equity or debt securities, obtaining credit facilities, or entering into other financing arrangements on such terms as then existing market conditions require. The capital market for small or micro-cap companies has been and likely will remain very difficult in the near future. As a result our ability to obtain additional capital on terms that are reasonable in light of current market conditions cannot be assured. We may be forced to obtain additional capital on terms that could limit our long-term ability to remain in business or otherwise materially restrict our operations. Further, our current financial structure and the demands of our existing creditors is such that we face a clear risk of not being able to meet the obligations to our creditors. In that event, we face a clear risk of insolvency with the result that persons who acquire our common stock may lose all or substantially all of their investment.
Further, the market price of our common stock and the uncertainties of the U.S. economy and other factors will likely negatively impact us and the financing options that we may have. Any downturn in the U.S. equity and debt markets could also make it more difficult for us to obtain additional financing.
Even if we are able to raise the additional financing, it is possible that we could incur unexpected costs and expenses, fail to collect amounts owed to us, or experience significant and protracted unexpected cash requirements that would force us to seek other, less-attractive alternative financing on terms that could result in significant dilution and with other terms that are not reasonable in light of our current circumstances.
Currently we do not have any commitment from any outside financing source to meet our anticipated financing needs and we have no basis to believe that any such commitment is forthcoming. Furthermore, in the event that we were to issue additional equity or debt securities, stockholders may experience significant additional dilution or the new equity securities may have rights, preferences or privileges senior to those of existing holders of our common stock. And in the case of any issuance of one or more debt securities, the debt covenants may restrict our operating ability and our ability to raise additional financing from debt.
Overall if we are unable to raise additional capital on terms that are reasonable in light of current market conditions we will likely restrict our ability to grow and may reduce our ability to continue to conduct business operations. We face a clear risk of insolvency unless we are able to successfully raise significant additional capital on terms that will allow us to reduce our financial obligations and improve our profitability and cash flow. If we are unable to obtain significant additional financing, we will likely be required to curtail our marketing and development plans and possibly cease our operations with the clear risk of insolvency.
We anticipate that depending on market conditions and our plan of operations, we may incur operating losses in the foreseeable future. Therefore, there is substantial doubt about our ability to continue as a going concern and persons who acquire our common stock face the risk of losing all or substantially all of their investment.
| 19 |
Inflation and Changing Prices
Neither inflation nor changing prices for the year ended December 31, 2017 had a material impact on our operations.
Off-Balance Sheet Arrangements
None.
Quantitative and Qualitative Disclosures About Market Risk
Not applicable.
Critical Accounting Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America (“US GAAP”) requires our management to make assumptions, estimates, and judgments that affect the amounts reported, including the notes thereto, and related disclosures of commitments and contingencies, if any. We have identified certain accounting policies that are significant to the preparation of our financial statements. These accounting policies are important for an understanding of our financial condition and results of operations. Critical accounting policies are those that are most important to the portrayal of our financial condition and results of operations and require management’s difficult, subjective, or complex judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Certain accounting estimates are particularly sensitive because of their significance to financial statements and because of the possibility that future events affecting the estimate may differ significantly from management’s current judgments.
We believe the following critical accounting policies involve the most significant estimates and judgments used in the preparation of our financial statements. We believe the critical accounting policies in Note 2 to the consolidated financial statements appearing in the Annual Report, Form 10-K for the year ended December 31, 2017, affect our more significant judgments and estimates used in the preparation of our consolidated financial statements.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities, and the reported amounts of revenues and expenses during the period. Actual results could differ from those estimates. Significant matters requiring the use of estimates and assumptions include, but are not limited to allowance for doubtful accounts, inventory obsolescence and markdowns, the useful life of property and equipment, the valuation of deferred tax assets and liabilities, valuation of intangible assets, the assumptions used to calculate fair value of stock options and warrants granted, stock-based compensation and the fair value of common stock issued.
| 20 |
| Item 8. | Financial Statements and Supplementary Data |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
DECEMBER 31, 2017 and 2016
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
CONTENTS
| F-1 |
 |
D. Brooks and Associates CPA’s, P.A. Certified Public Accountants ● Certified Valuation Analysts |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Gratitude Health, Inc, formerly known as Vapir Enterprises, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Gratitude Health, Inc, formerly known as Vapir Enterprises, Inc. (the “Company”) as of December 31, 2017 and 2016, and the related consolidated statements of operations, stockholders’ deficit, and cash flows for each of the years in the two-year period ended December 31, 2017, and the related notes to the consolidated financial statements (collectively referred to as the financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the two year period ended December 31, 2017, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the consolidated financial statements, the Company has incurred operating losses, has incurred negative cash flows from operations and has a working capital deficit. These and other factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plan regarding these matters is also described in Note 3 to the financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ D. Brooks and Associates CPA’s, P.A | |
| D. Brooks and Associates CPA’s, P.A |
We have served as the Company’s auditor since 2016.
|
Palm Beach Gardens, Florida |
|
| March 30, 2018 |
| F-2 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
CONSOLIDATED BALANCE SHEETS
| As of | As of | |||||||
| December 31, 2017 | December 31, 2016 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 5,203 | $ | 12,022 | ||||
| Accounts receivable, net | 1,469 | 4,773 | ||||||
| Inventory, net | 112,399 | 155,938 | ||||||
| Prepaid expense and other current assets | 14,078 | 12,486 | ||||||
| Advances to suppliers | 70,740 | 103,274 | ||||||
| Total Current Assets | 203,889 | 288,493 | ||||||
| OTHER ASSETS: | ||||||||
| Property and equipment, net | 38,740 | 64,562 | ||||||
| Intangible assets, net | 142,213 | 181,574 | ||||||
| Deposit | 2,813 | 2,813 | ||||||
| Total Other Assets | 183,766 | 248,949 | ||||||
| TOTAL ASSETS: | $ | 387,655 | $ | 537,442 | ||||
| LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable and accrued expenses | $ | 266,478 | $ | 299,356 | ||||
| Convertible notes payable | 500,000 | 500,000 | ||||||
| Loan payable | 197,000 | 197,000 | ||||||
| Notes payable - current maturities | 15,858 | 21,722 | ||||||
| Customer deposits | 3,851 | 27,633 | ||||||
| Advances from related party | 892,500 | 795,984 | ||||||
| Deferred rent | 13,556 | 14,191 | ||||||
| Total Current Liabilities | 1,889,243 | 1,855,886 | ||||||
| LONG-TERM LIABILITIES: | ||||||||
| Notes payable, net of current maturities | 4,521 | 20,410 | ||||||
| Total Long-term Liabilities | 4,521 | 20,410 | ||||||
| Total Liabilities | 1,893,764 | 1,876,296 | ||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||
| STOCKHOLDERS' DEFICIT: | ||||||||
| Preferred stock $0.001 par value: 20,000,000 shares authorized; | ||||||||
| none issued and outstanding | - | - | ||||||
| Common stock $0.001 par value: 300,000,000 shares authorized; | ||||||||
| 49,766,819 shares issued and outstanding. | 49,767 | 49,767 | ||||||
| Additional paid in capital | 1,893,349 | 1,501,220 | ||||||
| Accumulated deficit | (3,449,225 | ) | (2,889,841 | ) | ||||
| Total Stockholders' Deficit | (1,506,109 | ) | (1,338,854 | ) | ||||
| Total Liabilities and Stockholders' Deficit | $ | 387,655 | $ | 537,442 | ||||
See accompanying notes to the consolidated financial statements.
| F-3 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
CONSOLIDATED STATEMENTS OF OPERATIONS
| For the Years Ended | ||||||||
| December 31, 2017 | December 31, 2016 | |||||||
| Revenues, net | $ | 761,102 | $ | 1,086,971 | ||||
| Cost of revenues | 366,075 | 678,056 | ||||||
| Gross profit | 395,027 | 408,915 | ||||||
| OPERATING EXPENSES: | ||||||||
| Selling expenses | 38,678 | 183,382 | ||||||
| Compensation | 387,698 | 839,652 | ||||||
| Impairment of intangible assets | - | 126,426 | ||||||
| Professional and consulting fees | 72,813 | 496,422 | ||||||
| General and administrative | 161,990 | 298,427 | ||||||
| Total Operating Expenses | 661,179 | 1,944,309 | ||||||
| LOSS FROM OPERATIONS | (266,152 | ) | (1,535,394 | ) | ||||
| OTHER EXPENSE: | ||||||||
| Interest expense, net | (99,145 | ) | (348,176 | ) | ||||
| Other expense, net | (99,145 | ) | (348,176 | ) | ||||
| LOSS BEFORE INCOME TAX PROVISION | (365,297 | ) | (1,883,570 | ) | ||||
| INCOME TAX PROVISION | - | - | ||||||
| NET LOSS | $ | (365,297 | ) | $ | (1,883,570 | ) | ||
| LOSS PER SHARE: | ||||||||
| Basic and diluted | $ | (0.007 | ) | $ | (0.04 | ) | ||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: | ||||||||
| Basic and diluted | 49,766,819 | 49,719,009 | ||||||
See accompanying notes to the consolidated financial statements.
| F-4 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS' DEFICIT
For the Years Ended December 31, 2017 and 2016
| Common Stock $0.001 Par Value | Additional | Total | ||||||||||||||||||
| Number of | Paid-in | Accumulated | Stockholders' | |||||||||||||||||
| Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||
| Balance, December 31, 2015 | 48,466,819 | $ | 48,467 | $ | 31,374 | $ | (514,806 | ) | $ | (434,965 | ) | |||||||||
| Common stock issued for services | 1,300,000 | 1,300 | 338,700 | - | 340,000 | |||||||||||||||
| Stock-based compensation in connection with options granted | - | - | 437,058 | - | 437,058 | |||||||||||||||
| Cumulative effect adjustment upon adoption of ASU 2017-11 | - | - | 694,088 | (491,465 | ) | 202,623 | ||||||||||||||
| Net loss | - | - | - | (1,883,570 | ) | (1,883,570 | ) | |||||||||||||
| Balance, December 31, 2016 | 49,766,819 | 49,767 | 1,501,220 | (2,889,841 | ) | (1,338,854 | ) | |||||||||||||
| Cumulative effect adjustment upon adoption of ASU 2017-11 | - | - | 194,087 | (194,087 | ) | - | ||||||||||||||
| Stock-based compensation in connection with options granted | - | - | 198,042 | - | 198,042 | |||||||||||||||
| Net loss | - | - | - | (365,297 | ) | (365,297 | ) | |||||||||||||
| Balance, December 31, 2017 | 49,766,819 | $ | 49,767 | $ | 1,893,349 | $ | (3,449,225 | ) | $ | (1,506,109 | ) | |||||||||
See accompanying notes to the consolidated financial statements.
| F-5 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended | ||||||||
| December 31, 2017 | December 31, 2016 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (365,297 | ) | $ | (1,883,570 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities | ||||||||
| Bad debt expense (recovery) | (3,013 | ) | 1,619 | |||||
| Depreciation | 25,822 | 25,906 | ||||||
| Inventory markdown | - | 39,734 | ||||||
| Amortization of intangible assets | 39,361 | 67,176 | ||||||
| Amortization of deferred financing cost | - | 11,352 | ||||||
| Amortization of debt discount | - | 252,276 | ||||||
| Impairment of intangible assets | - | 126,426 | ||||||
| Stock based compensation | 198,042 | 777,058 | ||||||
| Changes in assets and liabilities: | ||||||||
| Accounts receivable | 6,317 | 18,126 | ||||||
| Prepaid expense and other current assets | (1,592 | ) | (5,414 | ) | ||||
| Advances to suppliers | 32,534 | 33,153 | ||||||
| Inventory | 43,539 | 16,739 | ||||||
| Accounts payable and accrued expenses | 6,638 | 73,444 | ||||||
| Deferred rent | (635 | ) | 3,463 | |||||
| Customer deposits | (23,782 | ) | 7,024 | |||||
| NET CASH USED IN OPERATING ACTIVITIES | (42,066 | ) | (435,488 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of property and equipment | - | (2,800 | ) | |||||
| NET CASH USED IN OPERATING ACTIVITIES | - | (2,800 | ) | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Advances from related party | 57,000 | 430,000 | ||||||
| Repayments to related party for advances | - | (4,630 | ) | |||||
| Proceeds received from notes payable | - | 50,000 | ||||||
| Repayments of notes payable | (21,753 | ) | (32,918 | ) | ||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 35,247 | 442,452 | ||||||
| NET CHANGE IN CASH | (6,819 | ) | 4,164 | |||||
| CASH - beginning of year | 12,022 | 7,858 | ||||||
| CASH - end of year | $ | 5,203 | $ | 12,022 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Interest paid | $ | 12,709 | $ | 18,875 | ||||
| Income taxes paid | $ | - | $ | - | ||||
See accompanying notes to the consolidated financial statements.
| F-6 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
DECEMBER 31, 2017 and 2016
Note 1 - Organization and Operations
Gratitude Health, Inc., (formerly known as Vapir Enterprises Inc.) ( the “Company”) was incorporated in the State of Nevada on December 17, 2009. Effective March 23, 2018, the Company changed its name to Gratitude Health, Inc. The Company’s principal business was focused on inventing, developing and producing aromatherapy devices and vaporizers. The Company’s aromatherapy devices utilize heat and convection air and thereby extract natural essences and produce fresh fragrances. Vapir, Inc. (“Vapir”) is a wholly owned subsidiary of the Company and was incorporated in the State of California in October 2006.
Note 2 - Significant and Critical Accounting Policies and Practices
The Management of the Company is responsible for the selection and use of appropriate accounting policies and the appropriateness of accounting policies and their application. Critical accounting policies and practices are those that are both most important to the portrayal of the Company’s financial condition and results and require management’s most difficult, subjective, or complex judgments, often as a result of the need to make estimates about the effects of matters that are inherently uncertain. The Company’s significant and critical accounting policies and practices are disclosed below as required by generally accepted accounting principles.
Basis of Presentation
The Company’s consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”).
Use of Estimates and Assumptions and Critical Accounting Estimates and Assumptions
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. In preparing the unaudited condensed consolidated financial statements, management is required to make estimates and assumptions that affect the reported amounts of assets and liabilities as of the date of the consolidated balance sheet, and revenues and expenses for the period then ended. Actual results may differ significantly from those estimates. Significant estimates made by management include, but are not limited to allowance for doubtful accounts, inventory obsolescence and markdowns, the useful life of property and equipment, the valuation of deferred tax assets and liabilities, valuation of intangible assets, the assumptions used to calculate fair value of stock options and warrants granted, stock-based compensation and the fair value of common stock issued.
Principles of Consolidation
The Company applies the guidance of Topic 810 “Consolidation” of the Financial Accounting Standard Board’s (“FASB”) Accounting Standards Codification (“ASC”) to determine whether and how to consolidate another entity. The consolidated financial statements include all accounts of Gratitude Health, Inc., (formerly known as Vapir Enterprises, Inc.) and Vapir, Inc. All inter-company balances and transactions have been eliminated.
Fair value measurements and fair value of financial instruments
The estimated fair value of certain financial instruments, including cash, accounts receivable, prepaid expenses and accounts payable are carried at historical cost basis, which approximates their fair values because of the short-term nature of these instruments.
Cash equivalents
The Company considers all highly liquid debt instruments and other short-term investments with maturities of three months or less, when purchased, to be cash equivalents. The Company maintains cash and cash equivalent balances at one financial institution that is insured by the Federal Deposit Insurance Corporation. The Company’s account at this institution is insured by the Federal Deposit Insurance Corporation (“FDIC”) up to $250,000. As of December 31, 2017 and 2016, the Company has not reached bank balances exceeding the FDIC insurance limit. To reduce its risk associated with the failure of such financial institution, the Company evaluates at least annually the rating of the financial institution in which it holds deposits.
| F-7 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2017 and 2016
Note 2 - Significant and Critical Accounting Policies and Practices (continued)
Accounts receivable and allowance for doubtful accounts
The Company has a policy of providing on allowance for doubtful accounts based on its best estimate of the amount of probable credit losses in its existing accounts receivable. The Company periodically reviews its accounts receivable to determine whether an allowance is necessary based on an analysis of past due accounts and other factors that may indicate that the realization of an account may be in doubt. Account balances deemed to be uncollectible are charged to bad debt expense and included in the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. As of December 31, 2017, the Company has included $1,932 and $2,009, respectively, in the allowance for doubtful accounts. The Company recorded bad debt recovery (expense) of $3,013 and $(1,619) during the years ended December 31, 2017 and 2016, respectively.
Inventory
Inventory Valuation
The Company values inventory, consisting of finished goods, at the lower of cost or market. Cost is determined on the first-in and first-out (“FIFO”) method. The Company reduces inventory for the diminution of value, resulting from product obsolescence, damage or other issues affecting marketability, equal to the difference between the cost of the inventory and its estimated market value. Factors utilized in the determination of estimated market value include (i) estimates of future demand, and (ii) competitive pricing pressures. As of December 31, 2017 and 2016, the Company had recorded a reserve for slow-moving inventory of $23,245 and $39,734, respectively.
Inventory Obsolescence and Markdowns
The Company evaluates its current level of inventory considering historical sales and other factors and, based on this evaluation, classifies inventory markdowns in the income statement as a component of cost of goods sold pursuant to ASC 420 – “Exit or Disposal Cost Obligations”, to adjust inventory to net realizable value. These markdowns are estimates, which could vary significantly from actual requirements if future economic conditions, customer demand or competition differ from expectations.
During the years ended December 31, 2017 and 2016, the Company wrote down slow moving inventory in the amount of $0 and $39,734, respectively. There was no lower of cost or market adjustments for the years ended December 31, 2017 or 2016.
Advances to suppliers
Advances to a supplier represents the cash paid in advance which is usually in three installment payments for the purchase of inventory. The advances to a supplier are interest free and unsecured. As of December 31, 2017 and 2016, advances to the Company’s major supplier amounted to $70,740 and $103,274, respectively. Upon shipment of the purchase inventory, the Company reclassifies or records such advances to the supplier into inventory.
Property and Equipment
Property and equipment are carried at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets from 3 to 5 years. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired, or disposed of, the cost and accumulated depreciation are removed, and any resulting gains or losses are included in the consolidated statement of operations.
Intangible assets
The Company records the purchase of intangible assets not purchased in a business combination in accordance with ASC 350-30-65 “Goodwill and Other Intangible Assets” and records intangible assets acquired in a business combination or pushed-down pursuant to acquisition by its parent in accordance with ASC 805 “Business Combinations”.
Customer Relationships are based upon the estimated percentage of annual or period projected cash flows generated by such relationships, to the total cash flows generated over the estimated life of the customer relationships.
| F-8 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2017 and 2016
Note 2 - Significant and Critical Accounting Policies and Practices (continued)
In accordance with ASC 350-30-65, “Intangibles - Goodwill and Others”, the Company assesses the impairment of identifiable intangibles whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Factors the Company considers to be important which could trigger an impairment review include the following:
| 1. | Significant underperformance relative to expected historical or projected future operating results; | |
| 2. | Significant changes in the manner of use of the acquired assets or the strategy for the overall business; and | |
| 3. | Significant negative industry or economic trends. |
When the Company determines that the carrying value of intangibles may not be recoverable based upon the existence of one or more of the above indicators of impairment and the carrying value of the asset cannot be recovered from projected undiscounted cash flows, the Company records an impairment charge. The Company measures any impairment based on a projected discounted cash flow method using a discount rate determined by management to be commensurate with the risk inherent in the current business model. Significant management judgment is required in determining whether an indicator of impairment exists and in projecting cash flows. The Company evaluates the recoverability of intangible assets annually or whenever events or changes in circumstances indicate that an intangible asset’s carrying amount may not be recoverable.
Impairment of long-lived assets
The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable, or at least annually. The Company recognizes an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value. The Company record impairment losses of $0 and $126,426 during the years ended December 31, 2017 and 2016, respectively.
Customer Deposits
Customer deposits consisted of prepayments from customers to the Company. The Company will recognize the prepayments as revenue upon delivery of the products, in compliance with its revenue recognition policy.
Revenue recognition
The Company follows ASC 605 – “Revenue Recognition” in accounting for revenue related transactions. The Company will recognize revenue when it is realized or realizable and earned. The Company considers revenue realized or realizable and earned when all of the following criteria are met: (i) persuasive evidence of an arrangement exists, (ii) the product has been shipped or the services have been rendered to the customer, (iii) the sales price is fixed or determinable, and (iv) collectability is reasonably assured.
Consideration paid to promote and sell the Company’s products to customers is typically recorded as marketing costs incurred by the Company. If the amount of consideration paid to customers exceeds the marketing costs, any excess is recorded as a reduction of revenue. The Company follows the requirements of ASC 605-50-45-2, Revenue Recognition—Customer Payments and Incentives.
The Company adopted ASU 2015-14 Revenue from Contracts with Customers for their fiscal year beginning January 1, 2018. The Company does not expect this ASU to have a material impact on its financial statements.
Cost of Sales
The primary components of cost of sales include the cost of the product and shipping fees.
Stock-based compensation
Stock-based compensation is accounted for based on the requirements of the Share-Based Payment Topic of ASC 718, “Compensation — Stock Compensation” (“ASC 718”), which requires recognition in the consolidated financial statements of the cost of employee and director services received in exchange for an award of equity instruments over the period the employee or director is required to perform the services in exchange for the award (presumptively, the vesting period). ASC 718 also requires measurement of the cost of employee and director services received in exchange for an award based on the grant-date fair value of the award.
| F-9 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2017 and 2016
Note 2 - Significant and Critical Accounting Policies and Practices (continued)
Pursuant to ASC Topic 505-50, “Equity Based Payments to Non-employees”, for share-based payments to consultants and other third-parties, compensation expense is determined at the measurement date. The expense is recognized over the vesting period of the award. Until the measurement date is reached, the total amount of compensation expense remains uncertain. The Company initially records compensation expense based on the fair value of the award at the reporting date.
Shipping and Handling Costs
The Company accounts for shipping and handling fees in accordance with ASC 605. While amounts charged to customers for shipping products are included in revenues, the related costs are classified in cost of goods sold as incurred. Shipping costs included in cost of goods sold were $3,518 and $43,151 for the years ended December 31, 2017 and 2016, respectively.
Advertising Costs
The Company applies ASC 720 “Other Expenses” to account for advertising related costs. Pursuant to ASC 720-35-25-1, the Company expenses the advertising costs when the first time the advertising takes place. Advertising costs were $7,102 and $9,876 for the years ended December 31, 2017 and 2016, respectively.
The amounts paid to customers for marketing expenses incurred on behalf of the Company are recorded as marketing cost and not as a reduction of revenue in accordance with ASC 605-50-45-2, Revenue Recognition—Customer Payments and Incentives. For the years ended December 31, 2017, the Company did not pay customers for marketing expenses. During the year ended December 31, 2016, the Company recorded expenses in the amount of $30,000.
Deferred Tax Assets and Income Tax Provision
The Company accounts for income taxes under ASC 740-10-30 “Income Taxes”. Deferred income tax assets and liabilities are determined based upon differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the statements of operations in the period that includes the enactment date.
The Company adopted ASC 740-10-25. ASC 740-10-25 addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under ASC 740-10-25, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than fifty percent (50%) likelihood of being realized upon ultimate settlement. ASC 740-10-25 also provides guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures.
The estimated future tax effects of temporary differences between the tax basis of assets and liabilities are reported in the accompanying consolidated balance sheets, as well as tax credit carry-backs and carry-forwards. The Company periodically reviews the recoverability of deferred tax assets recorded on its consolidated balance sheets and provides valuation allowances as management deems necessary.
Management makes judgments as to the interpretation of the tax laws that might be challenged upon an audit and cause changes to previous estimates of tax liability. In addition, the Company operates within multiple taxing jurisdictions and is subject to audit in these jurisdictions. In management’s opinion, adequate provisions for income taxes have been made for all years. If actual taxable income by tax jurisdiction varies from estimates, additional allowances or reversals of reserves may be necessary.
| F-10 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2017 and 2016
Note 2 - Significant and Critical Accounting Policies and Practices (continued)
Earnings per Share
Earnings per share (“EPS”) is the amount of earnings attributable to each share of common stock. For convenience, the term is used to refer to either earnings or loss per share. EPS is computed pursuant to ASC 260 – “Earnings per Share”. Pursuant to ASC 260-10-45-10 through 260-10-45-16, basic EPS shall be computed by dividing income available to common stockholders (the numerator) by the weighted-average number of common shares outstanding (the denominator) during the period. Income available to common stockholders shall be computed by deducting both the dividends declared in the period on preferred stock (whether or not paid) and the dividends accumulated for the period on cumulative preferred stock (whether or not earned) from income from continuing operations (if that amount appears in the income statement) and also from net income. The computation of diluted EPS is similar to the computation of basic EPS except that the denominator is increased to include the number of additional common shares that would have been outstanding if the dilutive potential common shares had been issued during the period to reflect the potential dilution that could occur from common shares issuable through contingent shares issuance arrangement, stock options or warrants.
The Company’s contingent shares issuance arrangement, stock options or warrants are as follows which were excluded from the computation of loss per share because their impact was antidilutive:
| For the Year Ended December 31, 2017 | For the Year Ended December 31, 2016 | |||||||
| Stock Options | 1,940,000 | 1,940,100 | ||||||
| Convertible Debt | 5,825,200 | 5,525,385 | ||||||
| Stock Warrants | 500,000 | 501,243 | ||||||
| Total contingent shares issuance arrangement, convertible debt, stock options or warrants | 8,265,200 | 7,969,728 | ||||||
Recently Issued Accounting Pronouncements
In August 2015, the FASB issued ASU 2015-14 Revenue from Contracts with Customers. The ASU defers the effective date of previously issued ASU 2014-09 (the new revenue recognition standard) by one year for both public and private companies. The ASU requires public entities to apply the new revenue recognition guidance for annual reporting periods beginning after December 15, 2017, and interim reporting periods within annual reporting periods beginning after December 15, 2017. Both public and nonpublic entities will be permitted to apply the new revenue recognition standard as of the original effective date for public entities (annual periods beginning after December 15, 2016). The Company adopted this standard for their fiscal year beginning January 1, 2018. The Company does not expect this ASU to have a material impact on its financial statements.
In January 2017, the FASB issued ASU 2017-04, “Intangibles – Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment”, which eliminates Step 2 from the goodwill impairment test. When an indication of impairment was identified after performing the first step of the goodwill impairment test, Step 2 required that an entity determine the fair value at the impairment testing date of its assets and liabilities (including unrecognized assets and liabilities) using the same procedure that would be required in determining the fair value of assets acquired and liabilities assumed in a business combination. Under the amendments in ASU No. 2017-04, an entity would perform its annual, or interim, goodwill impairment test by comparing the fair value of a reporting unit with its carrying value. An entity would recognize an impairment charge for the amount by which the carrying value exceeds the reporting unit’s fair value. In addition, an entity must consider income tax effects from any tax deductible goodwill on the carrying amount of the reporting unit when measuring the goodwill impairment loss, if applicable. A public business entity that is a SEC filer should adopt the amendments in ASU No. 2017-04 for its annual, or any interim, good will impairment tests in fiscal years beginning after December 15, 2019. The Company does not believe the guidance will have a material impact on its consolidated financial statements.
In May 2017, the FASB issued ASU 2017-09, “Compensation
- Stock Compensation”. The update provides guidance about which changes to the terms or conditions of a share-based payment
award require an entity to apply modification accounting in ASC Topic 718. An entity shall account for the effects of a modification
described in ASC paragraphs 718-20-35-3 through 35-9, unless all the following are met: (1) The fair value of the modified award
is the same as the fair value of the original award immediately before the original award is modified; (2) The vesting conditions
of the modified award are the same as the vesting conditions of the original award immediately before the original award is modified;
and (3) The classification of the modified award as an equity instrument or a liability instrument is the same as the classification
of the original award immediately before the original award is modified. The provisions of this update become effective for annual
periods and interim periods within those annual periods beginning after December 15, 2017. The Company does not believe the guidance
will have a material impact on its consolidated financial statements.
| F-11 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2017 and 2016
Note 2 - Significant and Critical Accounting Policies and Practices (continued)
In July 2017, the FASB issued ASU 2017-11 “Earnings Per Share (Topic 260)”. The amendments in the update change the classification of certain equity-linked financial instruments (or embedded features) with down round features. The amendments also clarify existing disclosure requirements for equity-classified instruments. For freestanding equity-classified financial instruments, the amendments require entities that present earnings per share (“EPS”) in accordance with Topic 260, Earnings Per Share, to recognize the effect of the down round feature when it is triggered. That effect is treated as a dividend and as a reduction of income available to common shareholders in basic EPS. Convertible instruments with embedded conversion options that have down round features would be subject to the specialized guidance for contingent beneficial conversion features (in Subtopic 470-20, Debt—Debt with Conversion and Other Options), including related EPS guidance (in Topic 260). For public business entities, the amendments in Part I of this update are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. The Company adopted this pronouncement as of fiscal 2017.
Other accounting standards that have been issued or proposed by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption. The Company does not discuss recent pronouncements that are not anticipated to have an impact on or are unrelated to its financial condition, results of operations, cash flows or disclosures.
Note 3 - Going Concern
The Company’s consolidated financial statements have been prepared assuming that it will continue as a going concern, which contemplates continuity of operations, realization of assets, and liquidation of liabilities in the normal course of business. As reflected in the consolidated financial statements, the Company has an accumulated deficit of approximately $3.45 million at December 31, 2017, a net loss of approximately $365,000 and net cash used in operating activities of approximately $42,000 for the year ended December 31, 2017. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
The Company is attempting to further implement its business plan and generate sufficient revenue; however, the Company’s cash position may not be sufficient to support its daily operations. Management intends to raise additional funds by way of a private or public offering. While the Company believes in the viability of its strategy to further implement its business plan and generate sufficient revenue and in its ability to raise additional funds, there can be no assurances to that effect. The ability of the Company to continue as a going concern is dependent upon its ability to further implement its business plan and generate sufficient revenue and its ability to raise additional funds by way of a public or private offering. The consolidated financial statements do not include any adjustments related to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company is unable to continue as a going concern.
Note 4 - Property and Equipment
Property and equipment, stated at cost, less accumulated depreciation consisted of the following:
| Estimated life | As of December 31, 2017 | As of December 31, 2016 | ||||||||
| Auto | 3 years | $ | 12,522 | $ | 12,522 | |||||
| Furniture and fixtures | 5 years | 23,743 | 23,743 | |||||||
| Tooling equipment | 4 years | 100,510 | 100,510 | |||||||
| Leasehold improvements | 5 years | 35,206 | 35,206 | |||||||
| Less: Accumulated depreciation | (133,241 | ) | (107,419 | ) | ||||||
| $ | 38,740 | $ | 64,562 | |||||||
Depreciation expense amounted to $25,822 and $25,906 for the years ended December 31, 2017 and 2016, respectively.
| F-12 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2017 and 2016
Note 5 - Intangible Assets
Intangible assets consist of the following:
| As of December 31, 2017 | As of December 31, 2016 | |||||||
| Customer relationships | $ | 1,001,212 | $ | 1,001,212 | ||||
| Trademarks | 6,430 | 6,430 | ||||||
| 1,007,642 | 1,007,642 | |||||||
| Accumulated amortization | (865,429 | ) | (826,646 | ) | ||||
| Intangible assets, net | $ | 142,213 | $ | 181,574 | ||||
Customer Relationships are amortized based upon the estimated percentage of annual or period projected cash flows generated by such relationships, to the total cash flows generated over the estimated fifteen-year life of the Customer Relationships.
The Company filed trademarks for its company logos with an estimated useful life of 15 years. The Company is amortizing the costs of trademarks over their estimated useful lives on a straight-line basis. Amortization of trademarks is included in general and administrative expenses as reflected in the accompanying consolidated statements of operations. The Company assesses fair value for any impairment to the carrying values. The Company record impairment of its intangible assets of $0 and $126,426 at December 31, 2017 and 2016, respectively.
Amortization expense was $39,361 and $67,176 for the years ended December 31, 2017 and 2016, respectively. Future amortization of intangible assets is as follows:
| 2018 | $ | 39,361 | ||
| 2019 | 39,361 | |||
| 2020 | 39,361 | |||
| 2021 | 24,130 | |||
| Total | $ | 142,213 |
Note 6 - Loan and Notes Payable
| As of December 31, 2017 | As of December 31, 2016 | |||||||
| The Company obtained a business loan in May 2011 from a financial institution with a credit line up to $200,000 and secured by all assets of the Company. This loan bears a variable interest rate based on changes in the Bank of the West Prime Rate and is due on demand. As of December 31, 2017 and 2016, the variable interest rate was 4.75%. | $ | 197,000 | $ | 197,000 | ||||
| F-13 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2017 and 2016
Note 6 - Loan and Notes Payable (continued)
Notes payable | As of December 31, 2017 | As of December 31, 2016 | ||||||
| The Company has a 4.75% Promissory note of $100,000 issued with the same financial institution on May 10, 2011, payable over 60 consecutive monthly installments with monthly principal payment of $1,650 and interest starting in June 2012. Amounts outstanding under this loan and note were personally guaranteed by the CEO of the Company and due in full by on April 23, 2017. This note has been repaid accordingly. | $ | - | $ | 5,250 | ||||
| Unsecured Promissory note of $50,000 bearing interest of 5.28%, issued in February 2016 payable over 36 consecutive monthly installments of $1,506 starting in March 2016 and is due on March 9, 2019. | 20,379 | 36,882 | ||||||
| Total | 20,379 | 42,132 | ||||||
| Less: Current maturities | (15,858 | ) | (21,722 | ) | ||||
| Note payable, net of current maturities | $ | 4,521 | $ | 20,410 | ||||
Future minimum Loan and Notes Payable principal payments are as follows:
| 2018 | $ | 15,858 | ||
| 2019 | 4,521 | |||
| Total Remaining Payments | $ | 20,379 |
Convertible Notes payable
On April 3, 2015, the Company closed a financing transaction by entering into a Securities Purchase Agreement with two accredited investors for an aggregate subscription amount of $500,000. Pursuant to the Securities Purchase Agreement, the Company issued 6% Convertible Debentures and warrants to acquire 500,000 shares of the Company’s common stock at an exercise price of $0.10 per share.
The terms of the Debenture and the Warrants are as follows:
6% Convertible Debenture
The total principal amount of the Debenture is $500,000. The Debenture accrues interest at 6% per annum and matured on October 3, 2016. The Debenture is convertible any time after its issuance date. The Purchaser has the right to convert the Debenture into shares of the Company’s common stock at $0.10 per share. The conversion price, however, is subject to full ratchet anti-dilution in the event that the Company issues any securities at a per share price lower than the conversion price then in effect. The Company paid financing costs of $22,500 in connection with this Debenture which was initially recorded as prepaid financing cost and was amortized over the term of the Debenture. The note was initially issued on April 3, 2015 at a discount of $500,000. The unpaid principal balance due as of December 31, 2017 and 2016 was $500,000. Debt discount was fully amortized during the year ended December 31, 2016. As of December 31, 2017 and 2016, accrued interest related to this Debenture amounted to $82,521 and $52,538, respectively.
On March 23, 2017, the Company completed the extension of its $500,000 6% Senior Convertible Debenture. The Company and the investors held on-going discussions prior to and post maturity to extend the original agreement. As a result of the extension, the new maturity date is amended to July 26, 2018.
Warrants
In April 2015, the Company issued warrants to acquire 500,000 shares of the Company’s common stock. The Warrants issued in this transaction are immediately exercisable at an exercise price of $0.10 per share, subject to applicable adjustments including full ratchet anti-dilution in the event that the Company issue any securities at a per share price lower than the exercise price then in effect. The Warrants have an expiration period of five years from the date of the original issuance.
| F-14 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2017 and 2016
Note 7 - Related Party Transactions
Advances from Executive Officer, Significant Stockholder
From time to time, the Company’s Chairman of the Board of Directors, former CEO and significant stockholder advances funds to the Company for working capital purposes. These advances are unsecured, due upon demand and bear interest at 5% per annum.
At December 31, 2017 and 2016, these advances amounted to $892,500 and $795,984, respectively. Included in the advances are accrued interest due to the Company’s former CEO totaling $77,100 and $37,583, at December 31, 2017 and 2016, respectively.
Note 8 - Derivative Liabilities
The Company applies the provisions of ASC Topic 815-40, Contracts in Entity’s Own Equity, under which convertible instruments and warrants, which contain terms that protect holders from declines in the stock price (reset provisions), may not be exempt from derivative accounting treatment. As a result, warrants and embedded conversion options are recorded as a liability and are revalued at fair value at each reporting date. The Company has 500,000 warrants with repricing options and $5,525,385 of convertible debt qualifying for derivative accounting at December 31, 2016. The Company calculates the estimated fair values of the liabilities for warrant and embedded conversion option derivative instruments at each quarter-end using the Black Scholes Model.
In July 2017, the FASB issued Accounting Standards Update No. 2017-11 Earnings Per Share (Topic 260) Distinguishing Liabilities from Equity (Topic 480) Derivatives and Hedging (Topic 815) (“ASU 2017-11”), which changes the classification analysis of certain equity-linked financial instruments (or embedded features) with down round features. When determining whether certain financial instruments should be classified as liabilities or equity instruments, a down round feature no longer precludes equity classification when assessing whether the instrument is indexed to an entity’s own stock. ASU 2017-11 also clarifies existing disclosure requirements for equity-classified instruments. As a result, a freestanding equity-linked financial instrument (or embedded conversion option) no longer would be accounted for as a derivative liability at fair value as a result of the existence of a down round feature. For freestanding equity classified financial instruments, ASU 2017-11 requires entities that present earnings per share (EPS) in accordance with ASC Topic 260 to recognize the effect of the down round feature when it is triggered. That effect is treated as a dividend and as a reduction of income available to common shareholders in basic EPS. For the Company, ASU 2017-11 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. Early adoption is permitted, including adoption in an interim period. If an entity early adopts ASU 2017-11 in an interim period, any adjustments should be reflected as of the beginning of the fiscal year that includes that interim period in either of the following ways:1. Retrospectively to outstanding financial instruments with a down round feature by means of a cumulative-effect adjustment to the statement of financial position as of the beginning of the first fiscal year and interim period(s) in which ASU 2017-11 is effective or 2. Retrospectively to outstanding financial instruments with a down round feature for each prior reporting period presented in accordance with the guidance on accounting changes in paragraphs 250-10-45-5 through 45-10. The Company has elected to adopt ASU 2017-11 during the third quarter of year 2017 by applying ASU 2017-11 retrospectively to outstanding financial instruments with a down round feature by means of a cumulative-effect adjustment to the Company’s beginning accumulated deficit as of January 1, 2017 as follows:
| As Reported | Cumulative Effect Adjustment | Adjusted | ||||||||||
| Derivative Liabilities | $ | 305,913 | $ | (305,913 | ) | $ | — | |||||
| Current Liabilities | $ | 1,661,799 | $ | (305,913 | ) | $ | 1,855,886 | |||||
| Total Liabilities | $ | 2,182,209 | $ | (305,913 | ) | $ | 1,876,296 | |||||
| Accumulated Deficit | $ | (2,501,666 | ) | $ | (947,559 | ) | $ | (3,449,225 | ) | |||
| F-15 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2017 and 2016
Note 8 - Derivative Liabilities (continued)
If the comparative prior period financial statements were prepared using the newly adopted standard, the derivative liabilities would be zero and the change in fair value of derivative instruments would be zero. The following is a roll forward for the year ended December 31, 2017 of the fair value liability of price adjustable derivative instruments:
| Fair Value of | ||||
| Liability for | ||||
Warrant and Conversion | ||||
| Derivative | ||||
| Instruments | ||||
| Balance at December 31, 2016 | $ | 305,913 | ||
| Cumulative effect adjustment (See Note 2) | (305,913 | ) | ||
| Balance at December 31, 2017 | $ | — | ||
Note 9 - Commitments and Contingencies
Operating lease
In June 2014, a lease agreement was signed for an office and warehousing space consisting of approximately 5,000 square feet located in San Jose, California with a term commencing in June 2014 and expiring in October 2015. In August 2015, the Company entered into an amendment agreement to extend the term of the lease which will expire on December 31, 2018. Pursuant to the amended agreement, the lease requires the Company to pay a monthly base rent of $5,050 plus a pro rata share of operating expenses beginning November 1, 2015. The base rent is subject to an annual increase beginning in November 2016 as defined in the amended lease agreement. This lease agreement is personally guaranteed by the President of the Company.
Effective September 15, 2016, the Company entered into a one year lease of space consisting of approximately 1,819 square feet located in San Jose, California, which expired in September 14, 2017. In September 2017, the Company entered into an amendment agreement to extend the term of the lease which will expire on September 30, 2018. The base rent for the new agreement was increased from $1,819 to $2,001 per month.
As a result, the Company entered into a sublease agreement (“Sub Lessee”) to sublease the previous office and warehousing space in San Jose, California with a term commencing on September 1, 2016 and expired on October 31, 2017. The Company entered into a renewal sublease agreement in July 2017 to extend the term of the sublease and will expire on December 31, 2018. The renewal sublease agreement requires the sub lessee to pay to the Company a base rent of $5,050 plus pro rata share of operating expenses beginning September 1, 2016. The base rent increased to $5,202 beginning in November 2016 and $5,353 beginning in November 2017 as defined in the amended lease agreement.
Future minimum rental payments required under this operating lease are as follows:
| Years ending December 31: | ||||
| 2018 | $ | 64,236 | ||
| Total | $ | 64,236 | ||
Rent expense was $24,452 and $57,028 for the years ended December 31, 2017 and 2016, respectively.
Litigation
From time to time, the Company is involved in litigation matters relating to claims arising from the ordinary course of business. While the results of such claims and legal actions cannot be predicted with certainty, the Company’s management does not believe that there are claims or actions, pending or threatened against the Company, the ultimate disposition of which would have a material adverse effect on the Company’s business, results of operations, financial condition or cash flows.
| F-16 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2017 and 2016
Note 10 - Stockholders’ Deficit
Shares Authorized
The authorized capital of the Company consists of 300,000,000 shares of common stock, par value $0.001 per share and 20,000,000 shares of preferred stock, par value $0.001 per share.
Common Stock
On January 7, 2016, the Company entered into a seven-month consulting agreement for strategic consulting and business advisory services. Pursuant to the consulting agreement, the Company issued 500,000 shares of the Company’s common stock. The Company revalued these common shares at the fair value of $60,000 or $0.12 per common share based on the quoted trading price at the end of the contract period, August 1, 2016. In connection with the issuance of these common shares, the Company recorded stock based compensation of $60,000 for the year ended December 31, 2016.
On January 12, 2016, the Company issued an aggregate of 200,000 shares of the Company’s common stock to two board of directors of the Company. These shares vested immediately on the date of issuance. The Company valued these common shares at the fair value of $70,000 or $0.35 per common share based on the quoted trading price on the date of grant. In connection with the issuance of these common shares, the Company recorded stock based compensation of $70,000 for the year ended December 31, 2016.
On January 12, 2016, the Company issued 100,000 shares of the Company’s common stock to a consultant and such consultant will also receive $5,600 per month in connection with a consulting agreement. These shares vested immediately on the date of issuance. The Company valued these common shares at the fair value of $35,000 or $0.35 per common share based on the quoted trading price on the date of grant. In connection with the issuance of these common shares, the Company recorded stock based compensation of $35,000 for the year ended December 31, 2016.
On January 12, 2016, the Company issued 500,000 shares of the Company’s common stock to a consultant. These shares vested immediately on the date of issuance. The Company valued these common shares at the fair value of $175,000 or $0.35 per common share based on the quoted trading price on the date of grant. In connection with the issuance of these common shares, the Company recorded stock based compensation of $175,000 for the year ended December 31, 2016.
Warrants
In April 2015, the Company issued a 6% Convertible Debenture (the “Debenture”) and warrants exercisable into 500,000 shares of common stock at an exercise price of $0.60 per share which was adjusted down to $0.10 as a result of the Company’s issuance of options with an exercise price of $0.10 in January 2016 (the “Warrants”) (see Note 6). Stock warrant activities for the years ended December 31, 2017 and 2016 are summarized as follows:
| Number of Warrants | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (Years) | Aggregate Intrinsic Value | |||||||||||||
| Balance at December 31, 2015 | 501,263 | 3.74 | 4.25 | - | ||||||||||||
| Forfeited | (20 | ) | 250 | - | - | |||||||||||
| Balance at December 31, 2016 | 501,243 | 3.73 | 3.25 | - | ||||||||||||
| Expired | (1,243 | ) | 1,264 | - | - | |||||||||||
| Balance at December 31, 2017 | 500,000 | 0.10 | 2.26 | - | ||||||||||||
| Warrants exercisable at December 31, 2017 | 500,000 | $ | 0.10 | 2.26 | $ | - | ||||||||||
| F-17 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2017 and 2016
Note 10 - Stockholders’ Deficit (continued)
Options
Stock option activities for the years ended December 31, 2017 and 2016 are summarized as follows:
| Number of Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (Years) | Aggregate Intrinsic Value | |||||||||||||
| Balance at December 31, 2015 | 100 | 700 | 1.17 | - | ||||||||||||
| Granted | 2,480,000 | 0.10 | 5.00 | - | ||||||||||||
| Forfeited | (540,000 | ) | 0.10 | 5.00 | - | |||||||||||
| Balance at December 31, 2016 | 1,940,100 | 0.14 | 4.04 | - | ||||||||||||
| Expired | (100 | ) | 700 | - | - | |||||||||||
| Balance at December 31, 2017 | 1,940,000 | 0.10 | 3.04 | - | ||||||||||||
| Options exercisable at December 31, 2017 | 1,293,333 | $ | 0.10 | 3.04 | $ | - | ||||||||||
As of the balance sheet date, total compensation cost related to unvested stock options not yet recognized equaled $83,303 and is expected to be recognized over a weighted-average period of 1.25 years.
Note 11 - Concentration of Credit Risk
Concentration of Revenue and Supplier
During the years ended December 31, 2017, sales to one customer represented approximately 31% of the Company’s net sales and accounts receivable of $1,469 at December 31, 2017 relative to two customers representing 35% during the year ended December 31, 2016.
As of December 31, 2017, and 2016, the Company had one customer representing approximately 100% of accounts receivable and one customer representing approximately 29% of accounts receivable, respectively.
Additionally, the Company use electronic contract manufacturers (EMS) to make the Company’s products (primarily located in China). The Company specify the requirements and specification and the products are built based on the specification and design. The Company has been able to extend credit with the Company’s suppliers but there are always risk that suppliers reduce their credit limit or terms of credit.
Note 12 – Income Taxes
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. At December 31, 2017, the Company has a Net Operating Loss (“NOL”) carryforward of approximately $2,991,000. The NOL expires during the years 2034 to 2037. In the event that a significant change in ownership of the Company occurs as a result of the Company’s issuance of common stock, the utilization of the NOL carryforward will be subject to limitation under certain provisions of the Internal Revenue Code. Management does not presently believe that such a change has occurred. Realization of any portion of the $891,195 of net deferred tax assets at December 31, 2017 is not considered more likely than not by management; accordingly, a valuation allowance has been established for the full amount. The valuation allowance as of December 31, 2017 was $891,195. The change in the valuation allowance during the year ended December 31, 2017 amounted to $522,835. The Company does not have any uncertain tax positions or events leading to uncertainty in a tax position. The Company’s 2015, 2016 and 2017 Corporate Income Tax Returns are subject to Internal Revenue Service examination.
On December 22, 2017, the Tax Cuts and Jobs Act (the “Act”) was signed into law. The Act decreases the U.S. corporate federal income tax rate from a maximum of 35% to a flat 21% effective January 1, 2018. The impact of the re-measurement on the Corporation’s net deferred tax asset, as of December 31, 2017, was an approximately $388,776 decrease in deferred tax assets, with a corresponding decrease in the Company’s valuation allowance, and no impact on income tax expense. The Act also includes a number of other provisions including, among others, the elimination of net operating loss carrybacks and limitations on the use of future losses, the repeal of the Alternative Minimum Tax regime and the repeal of the domestic production activities deduction. These provisions are not expected to have a material effect on the Corporation.
Given the significant complexity of the Act and anticipated additional implementation guidance from the Internal Revenue Service, further implications of the Act may be identified in future periods.
| F-18 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
DECEMBER 31, 2017 and 2016
Note 12 – Income Taxes (continued)
Significant components of the Company’s deferred tax assets are as follows:
| Year ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Deferred tax assets: | ||||||||
| Organizational costs, accrued liabilities and other | $ | - | $ | 38,608 | ||||
| NOL carryforwards | 842,253 | 1,208,386 | ||||||
| Depreciation | - | 11,098 | ||||||
| Compensation related to equity instruments issued for services | 49,842 | 155,938 | ||||||
| Valuation allowance | (891,195 | ) | (1,414,030 | ) | ||||
| Net deferred tax assets | $ | - | $ | - | ||||
Reconciliation of the differences between income tax benefit computed at the federal and state statutory tax rates and the provision for income tax benefit for the years ended December 31, 2017 and 2016 is as follows:
| 2017 | 2016 | |||||||
| Income tax expense (benefit) at federal statutory rate | 34.0 | % | 34.0 | % | ||||
| State taxes, net of federal benefit | 8.8 | % | 8.8 | % | ||||
| Change in valuation allowance | -42.8 | % | -42.8 | % | ||||
| - | - | |||||||
Note 13 – Subsequent Events
In January 2018, the Company issued an aggregate of 3,375,000 shares of common stock to two note holders upon the conversion of $327,500 principal amount of note and $10,000 accrued interest pursuant to the conversion terms of the convertible notes (see Note 6).
On March 26, 2018, Gratitude Health, Inc. f/ka Vapir Enterprises, Inc., a corporation organized under the laws of Nevada (the “Acquiror” or “Company”), Hamid Emarlou, the principal shareholder of the Acquiror (the “Acquiror Principal Shareholder”), Gratitude Health, Inc. (FL), a corporation organized under the laws of Florida (the “Acquiree”), and each of the Persons who are shareholders of the Acquiree (collectively, the “Acquiree Shareholders,” and individually an “Acquiree Shareholder”) entered into a Share Exchange Agreement (the “Agreement”) pursuant to which the Acquiree Shareholders (who are the holders of all of the issued and outstanding shares of common stock of the Acquiree (the “Acquiree Interests”)) have agreed to transfer to the Acquiror, and the Acquiror has agreed to acquire from the Acquiree Shareholders, all of the Acquiree Interests, in exchange for the issuance of 520,000 shares of Series A Preferred Stock and 500,000 shares of Series B Preferred Stock, to the Acquiree Shareholders the “Acquiror Shares”), which Acquiror Shares shall, upon conversion into 102,000,000 shares of common stock of the Acquiror, constitute approximately 85.84% on a fully diluted basis of the issued and outstanding shares of Acquiror common stock immediately after the closing of the transactions on the terms and conditions as set forth in the Agreement.
Effective March 26, 2018, the Company acquired all the issued and outstanding shares of Acquiree pursuant to the Agreement and Acquiree became the Company’s wholly-owned subsidiary. The acquisition was accounted for as a recapitalization effected by a share exchange, wherein Acquiree is considered the acquirer for accounting and financial reporting purposes. The assets and liabilities of Acquiree have been brought forward at their book value and no goodwill has been recognized. At the time of the acquisition, the Company was engaged in the business of inventing, developing and producing aromatherapy devices and vaporizers. As a result of the acquisition of all the issued and outstanding shares of common stock of Acquiree, the Company has now assumed Acquiree’s business operations as our own. The acquisition of Acquiree is treated as a reverse acquisition, and the business of Acquiree became the business of the Company. The business of Acquiree is engaged in manufacturing, selling and marketing functional RTD (Ready to Drink) beverages sold under Acquiree’s trademark.
| F-19 |
GRATITUDE HEALTH, INC. AND SUBSIDIARY
(FORMERLY KNOWN AS VAPIR ENTERPRISES, INC.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2017 and 2016
Note 13 – Subsequent Events (continued)
In connection with the Agreement, on March 26, 2018, the current officers and directors of the Company resigned, and concurrently, the Company appointed new officers and a new board of directors of the Company.
On the Closing Date, Acquiror Principal Shareholder entered into a Spin Off Agreement with Acquiror for the sale of the existing wholly owned Vapir, Inc. subsidiary of the Acquiror in exchange for Acquiror Principal Shareholder’s shares of Common Stock of Acquiror. The Spin Off Agreement shall not close less than five (5) days from the closing of the Agreement.
Effective March 26, 2018, the Board of Directors of the Company approved and amended its Articles of Incorporation to include two series of preferred stock authorized, Series A and Series B Preferred Stock.
| F-20 |
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| Item 9A. | Controls and Procedures. |
Management’s Report on Internal Control over Financial Reporting
As of the end of the period covered by this Annual Report, our Chief Executive Officer and Chief Financial Officer performed an evaluation of the effectiveness of our disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act. Based on the evaluation and the identification of the material weaknesses in internal control over financial reporting described below, our Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2017, the Company’s disclosure controls and procedures were not effective.
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with US GAAP. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projection of any evaluation of effectiveness to future periods is subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our Chief Executive Officer and Chief Financial Officer have assessed our internal control over financial reporting as of December 31, 2017. Management’s assessment of internal control over financial reporting was conducted using the criteria in Internal Control over Financial Reporting (“ICFR”) – Guidance for Smaller Public Companies (2006) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) and Management has determined that the ICFR was not effective due to the material weaknesses.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. In connection with management’s assessment of our internal control over financial reporting as required under Section 404 of the Sarbanes-Oxley Act of 2002, we identified the following material weaknesses in our internal control over financial reporting as of December 31, 2017:
| (1) | Lack of an independent audit committee or audit committee financial expert. Although our board of directors serves as the audit committee it has no independent directors. These factors are counter to corporate governance practices as defined by the various stock exchanges and may lead to less supervision over management. | |
| (2) | We do not have sufficient experience from our accounting personnel with the requisite U.S. GAAP public company reporting experience that is necessary for adequate controls and procedures due to our limited resources with appropriate skills, training and experience to perform the review processes to ensure the complete and proper application of generally accepted accounting principles. | |
| (3) | Need for greater integration, oversight, communication and financial reporting of the books and records of our office. | |
| (4) | Lack of sufficient segregation of duties such that the design over these areas relies primarily on detective controls and could be strengthened by adding preventative controls to properly safeguard company assets. |
Remediation of Material Weaknesses in Internal Control over Financial Reporting
As a smaller reporting company, the Company does not have the resources to install a dedicated staff with deep expertise in all facets of SEC disclosure and US GAAP compliance. As is the case with many small businesses, the Company will continue to work with its external consultants and attorneys as it relates to new accounting principles and changes to SEC disclosure requirements. The Company has found that this approach worked well in the past and believes it to be the most cost effective solution available for the foreseeable future. We are seeking mitigating measures commencing with our recent appointment of a majority independent board of directors.
| 21 |
This Annual Report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to rules that permit the Company to provide only management’s report in this annual report.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the fourth quarter ended December 31, 2017 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| (1) | We do not have sufficient experience from our accounting personnel with the requisite U.S. GAAP public company reporting experience that is necessary for adequate controls and procedures due to our limited resources with appropriate skills, training and experience to perform the review processes to ensure the complete and proper application of generally accepted accounting principles. |
| (2) | Need for greater integration, oversight, communication and financial reporting of the books and records of our office. |
| (3) | Lack of sufficient segregation of duties such that the design over these areas relies primarily on detective controls and could be strengthened by adding preventative controls to properly safeguard company assets. |
Changes in Internal Controls Over Financial Reporting
There have been no changes in our internal controls over financial reporting or in other factors during the fourth fiscal quarter ended December 31, 2017, that materially affected, or is likely to materially affect, our internal control over financial reporting.
| 22 |
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Set forth below are our present directors and executive officers as of March 29, 2018. Note that there are no other persons who have been nominated or chosen to become directors nor are there any other persons who have been chosen to become executive officers. There are no arrangements or understandings between any of the directors, officers and other persons pursuant to which such person was selected as a director or an officer. Directors are elected to serve until the next annual meeting of stockholders and until their successors have been elected and have qualified. Officers serve at the discretion of the Board of Directors.
| Name | Age | Position | Commencement of Service As Officer/Director | |||
| Roy G. Warren | 63 | Chairman and CEO and Director | 2018 | |||
| Andy Schamisso | 55 | President and COO | 2018 | |||
| Jack Shea | 68 | Director | 2018 | |||
| Mike Edwards | 58 | Director | 2018 | |||
| Bruce Zanca | 57 | Director | 2018 |
Set forth below are brief accounts of the business experience during the past five years of each director and executive officer of the Company.
Roy G. Warren, Chairman and CEO
Roy Warren was founder and CEO of a public company called Bravo Brands from 1997 to 2007. There, he developed America’s first vitamin-fortified, branded consumer friendly flavored milk line. Having introduced shelf stable bottled flavored milk in 2003, Roy led the company to a market capitalization that increased from approximately $20mm to over $300mm. Roy left Bravo after 10 years and immediately, in late 2007, founded Attitude Drinks to develop functional protein delivery drinks made with ultra-filtered milk with which he was involved until 2015, when he temporarily stepped back from business pursuits for personal reasons. After this temporary hiatus, in 2017, Roy began his quest to develop drinks specifically intended to promote healthy aging and, thus, Gratitude Health, Inc. was born.
Andy Schamisso, President and CEO
Andy Schamisso was the President and Founder of Inko’s Tea from 2002 to 2015, creating that brand for the health-conscious consumer and focusing on low carb/calorie and totally unsweetened teas. He was the first to introduce RTD white tea to the beverage market and his drive to provide great-tasting, organic and healthy alternative drinks is evident in every offering Gratitude provides. Andy successfully commercialized the line of all natural RTD white teas and sold the company and drink brand to a private brand developer in 2015. He created the brand for the discerning consumer and the entire offerings (17 skus in all) were low carb/calorie and/or totally unsweetened. Andy was the first to introduce RTD white tea to the beverage market and white tea is now offered by all “Big Three” companies.
Jack Shea, Independent Director
Jack Shea has been involved in the sales and marketing of food and beverages for over 35 years. Mr. Shea has directed the sales and marketing efforts of soft drink bottlers, RTD tea brewers, beer, wine and spirits importers and shelf stable milk companies. He has also led the US divisions of companies from China, Israel and the United Kingdom. Since 2009, Mr. Shea has been Director of Parish Ministries of Religious education at Saints John and Paul Parish and School in Mamaroneck, NY. Mr. Shea was ordained a Deacon in the Catholic Church in 2010 and now devotes his talents and abilities to taking care of the poor and the sick and also teaching in a Catholic School. Deacon Shea is well suited to be our director as a result of his long experience in the food and beverage industry.
| 23 |
Mike Edwards, Independent Director
Mike Edwards, who is the current owner of Car Pro Auto Spa in Stuart, Florida, a position he has held since 2008, is a self-motivated, results-oriented entrepreneur and has extensive executive and marketing professional experience with over 26 years in business development, promotion, strategic planning, and finance. He previously worked as the Executive Vice President of International Sales and Marketing at the Bravo! Brands International Corporation, an international functional drink marketing firm operational in the Americas, Australia, Europe, and Asia. He also has more than two decades of experience in private financial banking.
Mr. Edwards also worked as an Executive for Peregrine Enterprises, a market research company that provided custom study design and implementation for a wide range of Fortune 500 clients. He holds a Bachelor of Science degree from Florida State University, and has completed training in market research, banking, sales management, commercial lending, and credit analysis. He also served in the United States Navy Reserve as a Lieutenant for five years. Mr. Edwards is suited to sit on the Company’s board due to his experience in private financial banking and his industry experience with Bravo! Brands.
Bruce Zanca, Independent Director
Over the course of a career spanning more than 30 years, Bruce Zanca has developed a core competency in building award-winning “earned media campaigns” for companies to gain positive exposure in broadcast, print, and social media. He worked as a “C-Suite” communications executive at four publicly traded businesses; assisting three companies to complete initial public offerings and helping to bring one public company private. He served as a White House spokesperson and communications advisor for three presidential administrations. He has lead teams of PR experts to win numerous awards including four American Business Awards "Stevies" and a Telly award for video production. Bankrate, Inc (NASDAQ:RATE) in July 2004 Bruce Zanca joined Bankrate as Senior Vice President/Chief Marketing Communications Office. Among Zanca’s other responsibilities is managing the company’s communications and investor relations efforts. In July 2011 Bankrate once again became a public company by making an initial public offering New York Stock Exchange. (NYSE: RATE) Zanca continued to manage the company’s investor relations programs until he retired from the company as of December 31, 2014. Since he has retired, he has headed Zanca PR & Corporate Communications, a public relations and corporate communications firm, which has the ability to lead the public opinion for all aspects of external communications: Investor Relations, television including the major television networks and news shows (CNN, MSNBC, CSPAN, Fox News, etc.), internet, social media, and print. In 2011 Bruce J Zanca was recruited and elected as a board member/director to the charity Scenarios USA, (501(c)(3)). He was elected chairman of the board in 2013 and served in that position until his term ended at the end of 2015. Founded in 1999, scenarios usa is a national non-profit organization that uses writing and film to foster youth leadership, advocacy and self-expression in students across the country with a focus on marginalized populations and underserved communities. The Company believes that Mr. Zanca has the qualifications to act as a director for the Company due to his public relations and public company experience.
Involvement in Certain Legal Proceedings
To the best of our knowledge, neither of our sole director and executive officer or our promoter and control person (as identified under “Certain Relationships and Related Transactions”) has, during the past ten years:
| ● | been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| ● | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| ● | been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| 24 |
| ● | been found by a court of competent jurisdiction in a civil action or by the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| ● | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| ● | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Except as set forth in our discussion below in “Certain Relationships and Related Transactions,” none of our directors or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Committees; Audit Committee Financial Expert
Our board does not have an Audit Committee or other committees.
Changes in Nominating Procedures
None.
Significant Employees
We have no employees who are not executive officers, but who are expected to make a significant contribution to our business. We intend in the future to hire independent contractors on an as needed basis.
Family Relationships
None of the directors and officers is related to any other director or officer of the Company.
| ITEM 11. | EXECUTIVE COMPENSATION |
The following table sets forth information concerning the total compensation paid during the years ended December 31, 2017 and 2016 for our named executive officers of Vapir.
| Name and Principal Position | Year | Salary ($) | All Other Compensation ($) | Total ($) | ||||||||||
| Hamid Emarlou (1) | 2017 | $ | 14,183 | $ | 6,200 | (1) | $ | 20,383 | ||||||
| Former President and Chief Executive Officer | 2016 | 107,885 | 15,600 | (1) | 123,485 | |||||||||
| Mitra Sadeghzadeh (2) | 2017 | 27,953 | 4,520 | 32,473 | ||||||||||
| President and Chief Executive Officer | 2016 | - | - | - | ||||||||||
| Dr. Shadi Shayegan (3) | 2017 | - | - | - | ||||||||||
| Corporate Secretary | 2016 | $ | 14,173 | - | $ | 14,173 | ||||||||
| (1) | Mr. Emarlou resigned as President and Chief Executive Officer of Vapir in May 2017. Mr. Emarlou was provided a car allowance in 2017 and 2016 of $1,300 a month covering car payments and insurance expenses. Mr. Emarlou resigned as a director of Vapir as of March 26, 2018. |
| (2) | Ms. Sadeghzadeh was elected by the Board of Directors of Vapir as President and Chief Executive Officer of Vapir in May 2017 and resigned as of March 26, 2018. |
| (3) | Ms. Shayegan resigned as Corporate Secretary of Vapir in March 2016. |
| 25 |
Employment Agreement with Executives
We have not entered into employment agreements with our officers and directors. Additionally, we have not approved any retirement benefit plan, termination or severance provisions for any of our named executive officers. Mr. Emarlou was employed at will such that either the Company or Mr. Emarlou may terminate the employment relationship at any time, with or without cause. Mr. Emarlou received salaries and car allowance.
Outstanding Equity Awards at December 31, 2017.
On September 23, 2010, the Company’s board of directors adopted, and the Company’s stockholders approved the Apps Genius Corp Equity Incentive Plan (the “Plan”), which covers 5,000,000 shares of common stock. The purpose of the Plan is to advance the interests of the Company by enhancing the ability of the Company to (i) attract and retain employees and other persons or entities who are in a position to make significant contributions to the success of the Company and its subsidiaries; (ii) reward such persons for such contributions; and (iii) encourage such persons or entities to take into account the long-term interest of the Company through ownership of shares of the Company’s common stock, par value $0.0001 per share. The Plan became effective on September 23, 2010 and will terminate on September 23, 2020.
Subject to adjustment as provided in the Plan, the aggregate number of shares of common stock reserved for issuance pursuant to awards granted under the Plan shall be five million (5,000,000) shares; provided, however , that within sixty (60) days of the end of each fiscal year following the adoption of the Plan, the Board, in its discretion, may increase the aggregate number of shares of Common Stock available for issuance under the Plan by an amount not greater than the difference between (i) the number of shares of Common Stock available for issuance under the Plan on the last day of the immediately preceding fiscal year, and (ii) the number of shares of Common Stock equal to 15% of the shares of Common Stock outstanding on the last day of the immediately preceding fiscal year.
On January 12, 2016, the Company granted an aggregate of 2,480,000 five year options to purchase shares of common stock to the CEO of the Company and six employees of the Company. The options granted vest one third at the end of each of the first three years from the date of issuance and are exercisable at $0.10 per share. The 2,480,000 options were valued on the grant date at approximately $0.35 per option or a total of $728,000 using a Black-Scholes option pricing model with the following assumptions: stock price of $0.35 per share (based on the quoted trading prices on the date of grant), volatility of 286% (based from volatilities of similar companies), expected term of 5 years, and a risk-free interest rate of 1.55%. In March, August, and September of 2016, 40,000, 100,000, and 400,000, respectively, of these unvested options were forfeited due to the termination of employees. There remains 1,940,000 options which are fully vested upon the change of ownership.
Board of Directors
All directors hold office until the next annual meeting of shareholders and until their successors have been duly elected and qualified, or until their earlier death, resignation or removal. Officers are elected by and serve at the discretion of the board.
Our directors are reimbursed for expenses incurred by them in connection with attending board meetings, but they do not receive any other compensation for serving on the board.
| 26 |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The following table sets forth certain information regarding beneficial ownership of our common stock as of March 26, 2018 by (i) each person (or group of affiliated persons) who is known by us to own more than five percent of the outstanding shares of our common stock, (ii) each director, executive officer and director nominee, and (iii) all of our directors, executive officers and director nominees as a group.
Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. All share ownership figures include shares of our Common Stock issuable upon securities convertible or exchangeable into shares of our Common Stock within sixty (60) days of the share exchange, which are deemed outstanding and beneficially owned by such person for purposes of computing his or her percentage ownership, but not for purposes of computing the percentage ownership of any other person.
Combined Common Stock and Series A and Series B Preferred Stock on an as Converted Basis
| Name and Address | Beneficial Ownership | Percentage of Class (1) | ||||||
| Officers and Directors | ||||||||
| Roy G. Warren, 11231 US Highway One Suite 200, North Palm Beach, F1. 33408 (1) | 25,000,000 | 24.24 | % | |||||
| Andy Schamisso, 11231 US Highway One Suite 200, North Palm Beach, F1. 33408 (1) | 25,000,000 | 24.24 | % | |||||
| All officers/directors as a group (2 persons) | 50,000,000 | 48.48 | % | |||||
| Hamid Emarlou 18188 Wagner Road, Los Gatos, CA 95032(2) | 36,309,768 | 35.20 | % | |||||
Alpha Capital Anstalt, Lettstrasse 32, FL-9490 Vaduz, Furstentums, Liechtenstein (3) |
10,211,041 | 9.9 | % | |||||
(1) Based on shares of common stock outstanding of 103,141,833 shares as of March 26, 2018 assuming all shares of Series B preferred stock converted. Upon surrender by Mr. Emarlou of his shares as set forth in footnote (2) below, there shall be 66,832,065 shares of common stock outstanding of which Messrs. Warren and Schamisso shall each own 37.41% or collectively, 74.82%. All shares owned by Messrs. Warren and Schamisso are in the form of Series B Preferred Stock which is convertible into common stock in a ratio of 100 shares of common stock for each share of preferred stock converted. The Series B Preferred Stock votes with the common stock on a fully as converted basis.
(2) Mr. Emarlou resigned as of March 26, 2018 as an officer and director of the Company. Mr. Emarlou’s shares shall be surrendered to the Company on the date of the disposition of the stock and assets of Vapir (California) to him.
(3) This is a theoretical number because any conversion would take place after surrender as set forth in foonote (2) above. Based on footnote (1) below with regard to the 9.9% beneficial ownership limitation, after the share surrender in footnote (2) above, the number of shares issuable upon conversion would be limited to 6,616,374. Konrad Ackermann is the director of Alpha Capital Anstalt.
Series A Preferred Stock
| Name and Address | Beneficial Ownership | Percentage of Class (1) | ||||||
| Alpha Capital Anstalt, Lettstrasse 32, FL-9490 Vaduz, Furstentums, Liechtenstein | 490,000 | 94.23 | % | |||||
(1) Based on 520,000 shares of Series A Preferred Stock issued and outstanding as of March 26, 2018. The Series A Preferred Stock is nonvoting and contains blocker provisions such that the Preferred Shares held by a holder shall not be convertible by such Holder to the extent (but only to the extent) that such Holder or any of its affiliates would beneficially own in excess of 9.99% of the Common Stock. Konrad Ackermann is the director of Alpha Capital Anstalt.
Series B Preferred Stock
| Name and Address | Beneficial Ownership | Percentage of Class (1) | ||||||
| Roy G. Warren | 250,000 | 50 | % | |||||
| Andy Schamisso | 250,000 | 50 | % | |||||
Officers and directors as a group | 500,000 | 100 | % | |||||
Change in Control
There are no present arrangements known to the Company, including any pledge by any person of the Company’s securities, the operation of which may at a subsequent date result in a change in control of the Company, except as disclosed in the subsequent event footnote to the audited financial statements accompanying this Annual Report on Form 10-K.
| 27 |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Director Independence
Currently, we have three independent directors. Because our common stock is not currently listed on a national securities exchange, we have used the definition of “independence” of The NASDAQ Stock Market to make this determination. NASDAQ Listing Rule 5605(a)(2) provides that an “independent director” is a person other than an officer or employee of the company or any other individual having a relationship which, in the opinion of the company’s board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. The NASDAQ listing rules provide that a director cannot be considered independent if:
| ● | the director is, or at any time during the past three years was, an employee of the Company; |
| ● | the director or a family member of the director accepted any compensation from the Company in excess of $120,000 during any period of 12 consecutive months within the three years preceding the independence determination (subject to certain exclusions, including, among other things, compensation for board or board committee service); |
| ● | a family member of the director is, or at any time during the past three years was, an executive officer of the Company; |
| ● | the director or a family member of the director is a partner in, controlling stockholder of, or an executive officer of an entity to which the Company made, or from which the Company received, payments in the current or any of the past three fiscal years that exceed 5% of the recipient’s consolidated gross revenue for that year or $200,000, whichever is greater (subject to certain exclusions); |
| ● | the director or a family member of the director is employed as an executive officer of an entity where, at any time during the past three years, any of the executive officers of the Company served on the compensation committee of such other entity; or |
| ● | the director or a family member of the director is a current partner of the Company’s outside auditor, or at any time during the past three years was a partner or employee of the Company’s outside auditor, and who worked on the company’s audit. |
Related Party Transactions
From time to time, the Company’s Chairman of the Board of Directors, former CEO and significant stockholder advances funds to the Company for working capital purposes. These advances are unsecured, due upon demand and bear interest at 5% per annum. At December 31, 2017 and 2016, these advances amounted to $892,500 and $795,984, respectively. Included in the advances are accrued interest due to the Company’s former CEO totaling $77,100 and $37,583, at December 31, 2017 and 2016, respectively.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Fees
Aggregate fees for professional services rendered to us by our independent registered public accounting firm engaged to provide accounting services for the years ended December 31, 2017 and 2016 were:
| Year Ended December 31, 2017 | Year Ended December 31, 2016 | |||||||
| Audit fees | $ | 34,000 | $ | 36,700 | ||||
| Audit related fees | - | - | ||||||
| Tax fees | - | - | ||||||
| All other fees | - | - | ||||||
| Total | $ | 34,000 | $ | 36,700 | ||||
| 28 |
Policy on Pre-Approval of Audit and Permissible Non-audit Services of Independent Auditors
Consistent with the SEC policies regarding auditor independence, our Board of Directors has responsibility for appointing, setting compensation and overseeing the work of the independent auditor. In recognition of this responsibility, our Board of Directors has established a policy to pre-approve all audit and permissible non-audit services provided by the independent auditor.
Prior to engagement of the independent auditor for the next year’s audit, management will submit an aggregate of services expected to be rendered during that year for each of the following four categories of services to the Board of Directors for approval.
1. Audit services include audit work performed in the preparation of year-end financial statements, as well as work that generally only the independent auditor can reasonably be expected to provide, including comfort letters, statutory audits, and attest services, assistance reviewing our quarterly financial statements and SEC filings, and consultation regarding financial accounting and/or reporting standards.
2. Audit-Related services are for assurance and related services that are traditionally performed by the independent auditor, including due diligence related to mergers and acquisitions, employee benefit plan audits, and special procedures required to meet certain regulatory requirements.
3. Tax services include all services performed by the independent auditor’s tax personnel except those services specifically related to the audit of the financial statements, and includes fees in the areas of tax compliance, tax planning, and tax advice.
4. Other Fees are those associated with services not captured in the other categories.
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a) The following documents are filed as part of this Annual Report on Form 10-K.
| Exh. No. | Exhibit Description | |
| 31.1 | Section 302 Certification by the Registrant’s Principal Executive Officer and Principal Financial Officer | |
| 32.1 | Section 906 Certification by the Registrant’s Principal Executive Officer and Principal Financial Officer | |
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Taxonomy Extension Schema | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase | |
| 101.LAB | XBRL Taxonomy Extension Presentation Linkbase | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase |
| 29 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act, the Registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Gratitude Health, Inc. | ||
| Date: April 2, 2018 | By: | /s/ Roy G. Warren |
| Roy G. Waren | ||
| Chief Executive Officer | ||
| (Principal Executive Officer and | ||
| Principal Financial Officer) | ||
Pursuant to the requirements of the Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Roy G. Warren | ||||
| Roy G. Warren | CEO, Chairman and Director | April 2, 2018 | ||
| /s/ Andy Schamisso | ||||
| Andy Schamisso | President and COO | April 2, 2018 | ||
| /s/ Jack Shea | ||||
| Jack Shea | Director | April 2, 2018 | ||
| /s/ Mike Edwards | ||||
| Mike Edwards | Director | April 2, 2018 | ||
| /s/ Bruce Zanca | ||||
| Bruce Zanca | Director | April 2, 2018 |
30
