Attached files
| file | filename |
|---|---|
| EX-23.1 - CONSENT OF WITHUMSMITH+BROWN, PC, INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM - MedAvail Holdings, Inc. | f10k2017ex23-1_myosrenstech.htm |
| EX-32.1 - CERTIFICATION - MedAvail Holdings, Inc. | f10k2017ex32-1_myosrenstech.htm |
| EX-31.1 - CERTIFICATION - MedAvail Holdings, Inc. | f10k2017ex31-1_myosrenstech.htm |
| EX-10.10 - 2012 EQUITY INCENTIVE PLAN, AS AMENDED - MedAvail Holdings, Inc. | f10k2017ex10-10_myosrens.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended: December 31, 2017
Or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission File No. 000-53298
| MYOS RENS TECHNOLOGY INC. |
| (Exact name of small business issuer as specified in its charter) |
| Nevada | 90-0772394 | |
| (State
or other jurisdiction of incorporation or organization) |
(I.R.S.
Employer Identification No.) |
45 Horsehill Road, Suite 106
Cedar Knolls, New Jersey 07927
(Address of Principal Executive Offices)
(973) 509-0444
(Issuer’s telephone number)
Securities registered under Section 12(b) of the Exchange Act:
Common Stock, $0.001 par value
Series A Preferred Stock Purchase Rights, $0.001 par value
(Title of class)
Securities registered under Section 12(g) of the Exchange Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the past 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers in response to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☐ (Do not check if a smaller reporting company) | Smaller reporting company | ☒ |
| Emerging growth company | ☐ | ||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the outstanding common stock, other than shares held by persons who may be deemed affiliates of the registrant, computed by reference to the closing sales price of $1.82 for the registrant’s common shares on June 30, 2017, as reported on the Nasdaq Capital Market, was approximately $10.6 million.
As of March 27, 2018, there were 6,480,899 shares of the registrant’s common stock outstanding.
Table of Contents
| i |
CAUTIONARY NOTE REGARDING FORWARD LOOKING STATEMENTS
This Annual Report on Form 10-K (the “Report”) includes certain “forward-looking statements” relating to such matters as anticipated financial performance, future revenues or earnings, business prospects, projected ventures, new products and services, anticipated market performance and similar matters. The words “may,” “will,” expect,” anticipate,” “continue,” “estimate,” “project,” “intend,” and similar expressions are intended to identify forward-looking statements regarding events, conditions, and financial trends that may affect future plans of operations, business strategy, operating results, and financial position.
We caution readers that a variety of factors could cause actual results to differ materially from anticipated results or other matters expressed in forward-looking statements. These risks and uncertainties, many of which are beyond our control, include:
| ● | our ability to market and generate sales of our products, including Fortetropin®, Qurr, and other products;
| |
| ● | our ability to successfully expand into new market categories, as well as geographic markets;
| |
| ● | our ability to adequately protect our intellectual property; | |
| ● | our ability to develop and introduce new products and mitigate competitive threats from other providers and products; | |
| ● | our ability to generate future sales and achieve profitability; | |
| ● | our ability to attract and retain key members of our management team; | |
| ● | our ability to collect our accounts receivable from our customers; | |
| ● | our reliance on third-party processors; | |
| ● | our ability to maintain and expand our manufacturing capabilities and reduce the cost of our products; | |
| ● | shortages in the supply of, or increases in the prices of, raw materials or shelf life limits on ingredients or finished product; | |
| ● | our ability to conduct research and development activities and the success of such activities to create new products and further validate our existing ones, including continued research on Fortetropin® and its impact on muscular disorders; | |
| ● | our ability to maintain raw material import permits, obtain regulatory approvals in countries of interest and comply with government regulations; | |
| ● | future financing plans; | |
| ● | our ability to attract additional investors, increase shareholder value and continue to comply with NASDAQ’s continuing listing standards; | |
| ● | anticipated needs for working capital; | |
| ● | anticipated trends in our industry; | |
| ● | the effect of economic conditions; and | |
| ● | competition existing today or that will likely arise in the future. |
Although management believes the expectations reflected in these forward-looking statements are reasonable, such expectations cannot guarantee future results, levels of activity, performance or achievements.
| ii |
| Item 1. | Business. |
Overview
We are an emerging bionutrition and biotherapeutics company focused on the discovery, development and commercialization of products that improve muscle health and function essential to the management of sarcopenia, cachexia and degenerative muscle diseases, and as an adjunct to the treatment of obesity. As used in this report, the “Company”, “MYOS”, “our”, or “we” refers to MYOS RENS Technology Inc. and its wholly-owned subsidiary, unless the context indicates otherwise.
We were incorporated under the laws of the State of Nevada on April 11, 2007. On March 17, 2016, we merged with our wholly-owned subsidiary and changed our name from MYOS Corporation to MYOS RENS Technology Inc. Prior to February 2011, we did not have any operations and did not generate revenues. In February 2011, we entered into an intellectual property purchase agreement pursuant to which our subsidiary purchased from Peak Wellness, Inc., or Peak, the intellectual property pertaining to Fortetropin®, a dietary supplement that has been shown in clinical studies to temporarily decrease the levels of serum myostatin, MYO-T12, a proprietary formulation containing Fortetropin®, certain trademarks, trade secrets, patent applications and certain domain names.
Since February 2011, our principal business activities have been to: (i) deepen our scientific understanding of the activity of Fortetropin®, which refers to a proprietary proteo-lipid composition derived from fertilized eggs of specific chicken species processed using a patented methodology which preserves the bioactivity of the constituent proteins and lipids, specifically as a natural, reversible, temporary reducing agent of myostatin, and to leverage this knowledge to strengthen and build our intellectual property; (ii) conduct research and development activities to evaluate myostatin modulation in a range of both wellness and disease states; (iii) identify other products and technologies which may broaden our portfolio and define a business development strategy to protect, enhance and accelerate the growth of our products; (iv) reduce the cost of manufacturing through process improvement; (v) identify contract manufacturing organizations that can fully meet our future growth requirements; (vi) develop a differentiated and advantaged consumer positioning, brand name and iconography; and, (vii) create sales and marketing capabilities to maximize near-term and future revenues.
We believe that existing wellness and therapeutic targets, such as myostatin, represent a rational entry point for additional drug discovery efforts and are evaluating a separate, concurrent objective in this area. We continue to pursue additional distribution and branded sales opportunities. We expect to continue developing our own core branded products in markets such as functional foods, sports and fitness nutrition and rehabilitation and restorative health and to pursue international sales opportunities. There can be no assurance that we will be able to secure distribution arrangements on terms acceptable to us, or that we will be able to generate significant sales of our current and future branded products.
Our executive offices are currently located at 45 Horsehill Road, Suite 106, Cedar Knolls, New Jersey 07927 and our telephone number is (973) 509-0444. Our corporate website address is http://www.myosrens.com and our new muscle health education and product website is http://www.qurr.com. Neither the information on our current or future website is, nor shall such information be deemed to be, a part of this Report or incorporated in filings we make with the Securities and Exchange Commission.
1
General
Following our purchase of Fortetropin® in February 2011, we have been focusing on the discovery, development, and commercialization of nutritional ingredients, functional foods, therapeutic products, and other technologies aimed at maintaining or improving the health and performance of muscle tissue. Our officers, directors and members of our Scientific Advisory Board, including Dr. Robert Hariri, Dr. Louis Aronne, Dr. Neilank Jha and Dr. Caroline Apovian, have significant research and development experience.
Fortetropin® is the Company’s proprietary all-natural food ingredient clinically shown to increase muscle size, lean body mass and strength as part of resistance training. Fortetropin® is made from fertilized chicken egg yolks using a proprietary process that retains the biological integrity and bioactivity of the product. In an animal study, Fortetropin® was shown to up-regulate muscle building pathways and down-regulate muscle degrading pathways. While Fortetropin® is our first proprietary ingredient, we plan to discover, develop, formulate and/or acquire additional products in the future.
We are developing nutritional and therapeutic products aimed at maintaining and improving the health and performance of muscle tissue. Our research is focused on developing strategies and therapeutic interventions to address muscle related conditions including sarcopenia, cachexia, and inherited and acquired muscle diseases as described in more detail below.
| ● | Sarcopenia is a degenerative process characterized by the progressive loss of muscle mass with advancing age. The loss of muscle affects all individuals regardless of ethnicity or gender although the rate and degree of muscle loss varies between individuals and is affected by many factors. Those individuals who have lost significant amounts of muscle mass and strength often require assistance for accomplishing daily living activities, which has a significant economic burden on a nation’s healthcare system and impacts the overall economy. In addition to the many direct costs, sarcopenia adversely affects the overall quality of life. |
| ● | Cachexia is a syndrome that occurs in many diseases such as cancer, chronic heart failure, chronic kidney failure and AIDS. It is characterized by a significant loss of body weight as a consequence of pathological changes in different metabolic pathways, with the loss of muscle mass as the core component of the syndrome. Cachexia leads to a poor quality of life and increased mortality. As skeletal muscle is diminished, individuals experience a reduced ability to move, a loss of strength, and an increase in conditions associated with immobility such as thrombosis, pneumonia, respiratory failure and ultimately death. Weight loss is an important prognosticator in cancer therapy with the greater the weight loss, generally the shorter the survival time. Weight loss in cancer patients due to cachexia arises from the loss of both adipose tissue and skeletal muscle. | |
| ● | Inherited and acquired muscle diseases, such as muscular dystrophy and muscle dysfunction that occur as a consequence of denervation such as seen in amyotrophic lateral sclerosis (ALS), are conditions marked by the progressive deterioration of muscle tissue that results in weakness and impairs normal function. These diseases are typified by difficulty with walking, balance, and coordination with many such diseases affecting speech, swallowing, and breathing. There are currently very few treatment options for most degenerative muscle diseases. |
2
Myostatin
Myostatin, which is a natural regulatory protein, plays a central role in skeletal muscle health. Interest in myostatin continues to grow within the medical community. Research on animals and humans with genetic deficiency for producing myostatin have shown an increased muscle mass, suggesting that myostatin is responsible for down-regulating muscle growth and development.
A 1997 article in the journal Nature first described the discovery of a novel member of the transforming growth factor-β (TGF-β) superfamily of growth and differentiation factors. This factor was expressed specifically in adult skeletal muscle and referred to as growth/differentiation factor-8 (GDF-8) (McPherron et al., 1997). The researchers created “knockout” mice, whereby they disrupted the expression of GDF-8 throughout the organism, with the resulting mice showing a large and widespread increase in skeletal muscle mass. Individual muscles of mutant animals weighted 2-3 times more than those of wild-type animals, with the increase a result of both muscle cell hypertrophy and hyperplasia. The newly created mice were subsequently named “mighty mice”. Based on the phenotype, the researchers dubbed the newly discovered protein myostatin.
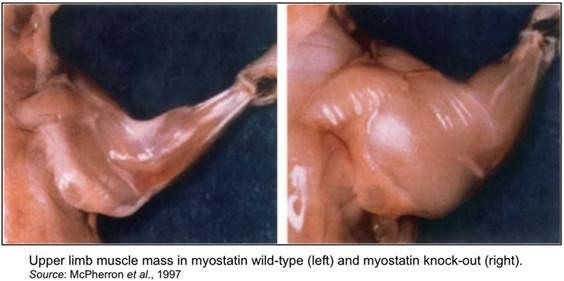
This work suggests myostatin exerts an effect on both muscle hypertrophy and hyperplasia, as myostatin knock-out “mighty mice” were shown to have an increase in both the number of muscle fibers and in fiber sizes. Hypertrophy refers to the enlargement of a tissue or organ due to the enlargement of its component cells. In contrast, hyperplasia refers to an increase in the number of cells or a proliferation of cells. Both of these processes can lead to enlargement of an organ.
Skeletal muscle is the primary producer of myostatin, where it is secreted into the blood stream and acts as a negative regulator of muscle differentiation and growth. The protein begins as a 375 amino acid dimer that is cleaved by proteases to a 109 amino acid active domain. The active form of the protein binds to activin type II receptors, ActRIIA and ActRIIB (Lee et al., 2001). Binding to the receptors initiates a signaling cascade that results in an increase in protein breakdown and subsequent inhibition of protein synthesis.
3
Clinical Research to Evaluate Effects of Fortetropin®
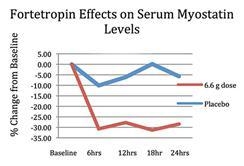
In March 2013, we completed a human clinical trial which demonstrated the beneficial effects of Fortetropin® in suppressing free serum myostatin levels. In this double blind, randomized, placebo-controlled, parallel, single dose study involving 12 healthy adult male subjects per arm, test subjects in the active arm were administered a 6.6 gram dose of Fortetropin® mixed with vanilla fat free/sugar free pudding. An equal amount of vanilla fat free/sugar free pudding alone was given to the placebo arm. Blood samples were collected at baseline (before dosing) and at 6, 12, 18, and 24 hours post dose intervals for measurement of myostatin blood concentration. Results demonstrated greater than 30% decrease in serum myostatin levels compared to baseline during the 24 hour period. No study related adverse events were reported during this study.
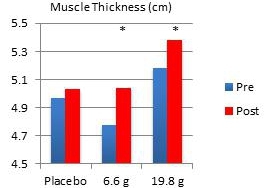
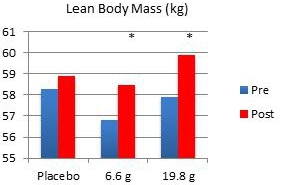
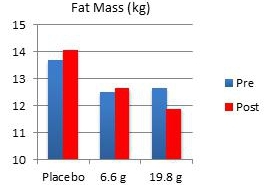
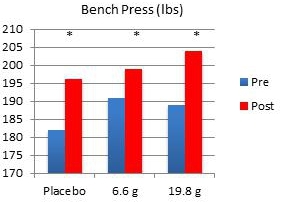
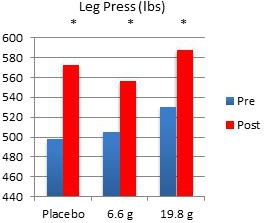
In another study performed on our behalf at the University of Tampa, a randomized, double-blind, placebo-controlled trial examined the effects of Fortetropin® on skeletal muscle growth, lean body mass, strength, and power in recreationally trained individuals who rely heavily on satellite cell activation. Forty-five subjects were divided into placebo, 6.6 gram and 19.8 gram dosing arms of Fortetropin® daily for a period of 12 weeks. All exercise sessions were conducted and monitored by trained personnel. Standardized diets consisted of roughly 54% carbohydrates, 22% fat and 24% protein. There were no differences in total calories and macronutrients between groups. Dual emission X-ray absorptiometry (DEXA) was utilized to measure lean body mass and fat mass. Direct ultrasound measurements determined muscle thickness of the quadriceps.
Results demonstrated a statistically significant increase in both muscle thickness and lean body mass in subjects taking Fortetropin® but not in subjects taking a placebo. Strength and power endpoints, as measured by bench press, leg press and Wingate power, significantly increased from baseline in all study groups. No study related adverse events were reported during the study.
 |
 |
4
 |
 |
 |
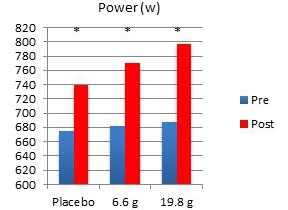 |
* p <0.05 post measurement compared to pre
Association between Muscular Strength and Mortality
In a clinical study at the Karolinska Institutet’s Department of Biosciences and Nutrition at NOVUM, Unit for Preventive Nutrition, in Huddinge, Sweden, 8,762 men aged 20-80 were evaluated over an average period of 18.9 years in a prospective cohort study to measure the association between muscular strength and mortality in men. After adjusting for age, physical activity, smoking, alcohol intake, body mass index, baseline medical conditions, and family history of cardiovascular disease, the study found that muscular strength is inversely and independently associated with deaths from all causes and cancer in men. The findings were valid for men of normal weight, those who were overweight, and younger or older men, and were valid even after adjusting for several potential confounders, including cardiorespiratory fitness. This study extends previous studies that showed the importance of muscular strength as a predictor of death from all causes, cardiovascular disease, and cancer in a large cohort of men. Several prospective studies have also shown that muscular strength is inversely associated with all-cause mortality. These data suggests that muscular strength adds to the protective effect of cardiorespiratory fitness against the risk of death in men. Moreover, it might be possible to reduce all-cause mortality among men by promoting regular resistance training.
5
WADA Compliance
Fortetropin® has received Certified Drug Free® certification from the Banned Substances Control Group (BSCG). The BSCG Certified Drug Free® program is a comprehensive certification program for the dietary supplement industry and includes screening for substances prohibited by the World Anti-Doping Agency (WADA) along with most U.S. professional sports leagues. WADA is a foundation created through a collective initiative led by the International Olympic Committee to promote, coordinate and monitor the fight against drugs in sports.
Research and Development
As an advanced nutrition and biotherapeutics company, we are dedicated to basic and clinical research that supports our existing and future product portfolio. We are focused on the following areas of research:
Basic Research
| ● | Biochemical characterization of Fortetropin®, including cutting edge proteomic and lipidomic approaches | |
| ● | Novel biotherapeutics products | |
| ● | Computational design of novel peptide inhibitors of myostatin | |
| ● | Identifying proteins, peptides, and lipids responsible for pro-myogenic activity | |
| ● | Pro-myogenic activity of novel bioactive molecules and formulations |
Pre-Clinical Research
| ● | Effect of Fortetropin® to reverse disuse atrophy in dogs after orthopedic surgery to repair the cranial cruciate ligament (CCL) | |
| ● | PK/PD studies of novel bioactive molecules with pro-myogenic activity |
Clinical Research
| ● | Effect of Fortetropin® on lean muscle mass, thickness and strength in older adults | |
| ● | Effect of Fortetropin® on muscle function and recovery after orthopedic procedures | |
| ● | We expect our investment in research and development to continue in the future |
Our research program is actively evaluating the many active proteins, lipids and peptides in Fortetropin®. We believe our research programs will establish a basis for the continued prosecution of patent applications in order to protect and augment our intellectual property assets. We are dedicated to protecting our innovative technology.
6
Clinical and Basic Research Programs
We invest in research and development activities externally through academic and industry collaborations aimed at enhancing our products, optimizing manufacturing and broadening the product portfolio. We have developed the following collaborations with various academic centers:
| ● | In March 2018, we entered into a research agreement with Rutgers University, The State University of New Jersey, to work with Rutgers researchers in a program focused on discovering compounds and products for improving muscle health and performance. |
| ● | In December 2017, we entered into an agreement with the University of California, Berkeley’s Department of Nutritional Sciences & Toxicology. The research project will study the effects of Fortetropin® on increasing the fractional rate of skeletal muscle protein synthesis in men and women between 60 and 75 years old. The Principal Investigator for this clinical study is William J. Evans, PhD, Adjunct Professor of Human Nutrition at the Department of Nutritional Sciences & Toxicology at the University of California, Berkeley campus. Professor Evans, a leading authority in muscle health research, will coordinate the activities of a multi-disciplinary team of scientists and physicians. In this randomized, double-blind, placebo-controlled clinical study, 20 subjects, men and women 60 – 75 years of age, will consume either Fortetropin® or a placebo for 21 days along with daily doses of a heavy water tracer. After 21 days, a micro-biopsy will be collected from each subject to determine the fractional rate of muscle protein synthesis. MYOS anticipates the clinical study will be completed and its results announced in the second half of 2018. |
| ● | In April 2017, we entered into an agreement with the College of Veterinary Medicine at Kansas State University to study the impact of Fortetropin® on reducing muscle atrophy in dogs after tibial-plateau-leveling osteotomy (TPLO) surgery to repair the cranial cruciate ligament (CCL). The study is expected to be completed by the end of the second quarter of 2018. |
| ● | In May 2015, we initiated a dose response clinical study led by Jacob Wilson, Ph.D., CSCS*D, Professor of Health Sciences and Human Performance at the University of Tampa, to examine the effects of Fortetropin® supplementation on plasma myostatin levels at various dosing levels in young adult males and females. This study is intended to help us better define the dose response curve, the minimal effective dose and effects of Fortetropin® on serum myostatin. In this double blind placebo controlled clinical study, 80 male and female subjects ranging in ages between 18 and 22 were randomized into four groups such that no significant differences in serum myostatin concentration existed between groups. Following assignment to one of the four groups, blood samples were collected to establish baseline values. Subjects were subsequently supplemented with three different doses of Fortetropin® (2.0g, 4.0g and 6.6g) and a matching placebo for one week. Following one week of supplementation, blood samples were collected and serum myostatin levels were assayed. Results demonstrated that Fortetropin® is effective as a myostatin reducing agent at daily doses of 4.0g and 6.6g. This research, which continues to build upon our current understanding of Fortetropin®, may result in the formulation of new products. An abstract of this study was presented at the 2016 International Conference on Frailty & Sarcopenia Research (Philadelphia, PA) in April 2016. |
| ● | In August 2014, we entered into a research agreement with Human Metabolome Technologies America, Inc., (“HMT”), to apply their proprietary, state-of-the-art capillary electrophoresis-mass spectrometry (CE-MS) technologies to characterize the metabolomic profiles of plasma samples obtained from healthy male subjects who used either Fortetropin® or placebo with the goal of identifying metabolites with pro-myogenic activity in the plasma samples of subjects who took Fortetropin® as well as examining the effect on glucose and fat metabolism. HMT used a metabolite database of over 290 lipids and over 900 metabolites to identify potential plasma biomarkers of muscle growth. The study was completed during the fourth quarter of 2014. Initial data from this study indicated that subjects who received Fortetropin® displayed differential metabolomic profiles relative to subjects who received placebo. The results of this study enhance our understanding of the mechanism of action of Fortetropin® and provides guidance for the development of biotherapeutics based on Fortetropin®. Additionally, the early indications of plasma biomarkers may guide future study design for Fortetropin® clinical trials by identifying clinically-relevant endpoints and potential stratification of patient populations. The results from this study were presented at the Sarcopenia, Cachexia and Wasting Disorders Conference (Berlin, Germany) in December 2016. |
7
| ● | In May 2014, we entered into an agreement with the University of Tampa to study the effects of Fortetropin® supplementation in conjunction with modest resistance training in18-21 year old males. The study was a double-blind, placebo-controlled trial which examined the effects of Fortetropin® on skeletal muscle growth, lean body mass, strength, and power in recreationally trained males. Forty-five subjects were divided into placebo, 6.6g and 19.8g dosing arms of Fortetropin® daily for a period of 12 weeks. Results demonstrated a statistically significant increase in both muscle thickness and lean body mass in subjects taking Fortetropin® but not in subjects taking placebo. The clinical study also analyzed blood myostatin and cytokines levels via high-sensitivity enzyme-linked immunosorbent assay (“ELISA”) based analysis. Serum was analyzed for a plethora of relative cytokine levels via high-sensitivity enhanced chemiluminescent-based methods. The Interferon-Gamma (“IFN-γ”) inflammatory cytokine protocol screening showed no statistically significant changes in serum levels of IFN-γ for subjects in the placebo group. However, subjects in both Fortetropin® daily dosing arms experienced statistically significant decreases (p < 0.05) in serum levels of the IFN-γ inflammatory cytokine. IFN-γ is recognized as a signature pro-inflammatory cytokine protein that plays a central role in inflammation and autoimmune diseases. Excess levels of inflammatory cytokines are associated with muscle-wasting diseases such as sarcopenia and cachexia. The lipid serum safety protocol demonstrated that daily use of Fortetropin® at recommended and three times the recommended dose had no adverse lipid effect and did not adversely affect cholesterol, HDL or triglyceride levels. Data from the study was presented at the American College of Nutrition’s 55th annual conference. A separate mechanism of action study at the University of Tampa demonstrated that in addition to reducing serum myostatin levels, Fortetropin® showed activity in mTOR and Ubiquitin pathways, two other crucial signaling pathways in the growth and maintenance of healthy muscle. Specifically, the preclinical data showed that Fortetropin® up-regulates the mTOR regulatory pathway. The mTOR pathway is responsible for production of a protein kinase related to cell growth and proliferation that increases skeletal muscle mass. Up-regulation of the mTOR pathway is important in preventing muscle atrophy. We believe Fortetropin®’s ability to affect the mTOR pathway may have a significant impact in treating patients suffering from degenerative muscle diseases and suggests that Fortetropin®-based products may help slow muscle loss secondary to immobility and denervation. The preclinical data also demonstrated that Fortetropin® acts to reduce the synthesis of proteins in the Ubiquitin Proteasome Pathway, a highly selective, tightly regulated system that serves to activate muscle breakdown. Over-expression of the Ubiquitin Proteasome Pathway is responsible for muscle degradation. We believe Fortetropin®’s ability to regulate production in the Ubiquitin Proteasome Pathway may have significant implications for repairing age-related muscle loss and for patients suffering from chronic diseases such as cachexia. |
| ● | In May 2014, we entered into a three-year master service agreement with Rutgers University. The initial phase under the agreement was to develop cell-based assays for high-throughput screening studies of next generation myostatin inhibitors. Additionally, we initiated a second phase of the agreement to develop a secondary assay for measuring myostatin activity using a genetically engineered muscle cell line that fluoresce in the presence of myostatin. Phase I and II were completed in 2015. We believe the assays developed will enable us to elucidate the specific molecules in Fortetropin® that impart activity as it relates to the development of muscle tissue. |
The foregoing agreements are an integral part of our business strategy and we believe they will provide a clear scientific rationale for Fortetropin®’s role as an advanced nutritional product and support its use in different medical and health applications in the future.
We are also building a small molecule and biologics discovery program aimed at regulators of myostatin synthesis and activation and the different pathways that act upon muscle development. In July 2014, we entered into a research and development agreement with Cloud Pharmaceuticals, Inc., (“Cloud”), to discover product candidates related to the inhibition of targets in the myostatin regulatory pathway as well as inflammatory mediators associated with sarcopenia and cachexia. Cloud utilizes cloud computing technology to identify and design small molecule drug candidates based on their proprietary Inverse Design drug discovery platform. The research is focusing on the development of product candidates related to the myostatin pathway. Cloud has identified several peptides that may have myostatin inhibition properties based on computational modeling. We intend to evaluate the physiological activity of these peptides on myostatin.
We intend to pursue additional clinical studies and medical research to support differentiated and advantaged marketing claims, to build and enhance our competitive insulation through an aggressive intellectual property strategy, to develop product improvements and new products in consumer preferred dosage forms, to enhance overall marketing, to establish a scientific foundation for therapeutic applications for our technology, and to pursue best in class personnel.
Market Overview
According to the Natural Marketing Institute, the Dietary Supplement, Functional Food and Beverage, and Natural Personal Care markets represent more than $250 billion in annual worldwide sales. The global market for functional foods alone in 2017 was worth an estimated $54 billion. In 2018, it is expected to continue to grow and the United States is expected to be the fastest growing market for functional foods. The global sports nutrition market was valued at $30.7 billion in 2017, and is expected to grow at a compounded annual growth rate of 8.1% during the period from 2018 to 2022 up to $45 billion. We believe our proprietary ingredient, Fortetropin®, which is the only clinically proven natural supplement available in the market that temporarily reduces free serum myostatin level, is well-positioned to market to a wide base of consumers looking for nutritional and performance maximization as well as for wellness and maintenance products as they age. Additionally, the medical community has increased its focus on muscle health, specifically focusing on the aging U.S. population that can benefit most from myostatin modulation.
We believe the combination of the foregoing marketplace characteristics, combined with the experience of our directors and our management team and our current and future products, will enable our business model to succeed.
8
Strategy
Our strategy is to understand the complex genetic and molecular pathways regulating muscle mass and function as well as other disease mechanisms. Understanding the impact of complex regulatory pathways which act to build and maintain healthy lean muscle is central to our biotherapeutic research. We are developing nutritional products that target specific mechanisms to promote muscle health in ways that cannot be met by other diets or lifestyle changes.
We will seek to gain market share for our core branded products in functional foods, sports and fitness nutrition and rehabilitation and restorative health verticals by (i) formulating and developing new and complementary product lines, (ii) expanding U.S. distribution by increasing the channels of sale, (iii) expanding distribution geography beyond the U.S. and (iv) seeking strategic relationships with other distributors. Our strategy is to utilize the revenue and awareness generated by the sales and marketing of Fortetropin® to further advance our research and development of therapeutic treatments for muscular disorders, including sarcopenia.
Marketing, Sales and Distribution
Our commercial focus is to leverage our clinical data to develop multiple products to target the large, but currently underserved markets focused on muscle health. The sales channels through which we sell our products are evolving. The first product we introduced was MYO-T12, which was sold in the sports nutrition market. MYO T-12 is a proprietary formula containing Fortetropin® and other ingredients. The formula was sold under the brand name MYO-T12 and later as MYO-X through an exclusive distribution agreement with Maximum Human Performance (“MHP”). The exclusive distribution agreement with MHP terminated in March 2015 and there were no subsequent sales to MHP.
In February 2014, we expanded our commercial operations into the age management market through a distribution agreement with Cenegenics Product and Lab Services, LLC (“Cenegenics”), under which Cenegenics distributed and promoted a proprietary formulation containing Fortetropin® through its age management centers and its community of physicians focused on treating a growing population of patients focused on proactively addressing age-related health and wellness concerns. The distribution agreement with Cenegenics expired in December 2016. As of December 31, 2016 we recognized all of the deferred revenue. In 2017, we recorded $200 of sales to Cenegenics.
During the second quarter of 2015 we launched Rē Muscle HealthTM, our own direct-to-consumer brand with a portfolio of muscle health bars, meal replacement shakes and daily supplement powders each powered by a full 6.6 gram single serving dose of Fortetropin®. Our Rē Muscle Health products were sold through our e-commerce website, remusclehealth.com, and amazon.com until March 2017 when we introduced our new Qurr line of products.
On March 13, 2017 we launched Qurr, a Fortetropin®-powered product line formulated to support the vital role of muscle in overall well-being as well as in fitness. Qurr is a line of flavored puddings, powders, and shakes for daily use. Our Qurr line of muscle-focused over-the-counter products are available through a convenient, direct-to-consumer e-commerce platform. All Qurr products contain Fortetropin®, our proprietary ingredient which has been clinically demonstrated to reduce serum myostatin levels which helps increase muscle size and lean body mass in conjunction with resistance training.
We expect to launch our Fortetropin based pet product in the near future. Two veterinarian hospitals, which performed some informal observational studies with older dogs experiencing muscle atrophy and saw positive results after taking our pet product, are seeking to purchase our product. We believe that the positive feedback we are receiving from these two hospitals, together with the potential results from our Kansas State University study, will enable us to launch and grow our pet business product line.
We continue to pursue additional distribution and branded sales opportunities. There can be no assurance that we will be able to secure distribution arrangements on terms acceptable to us, or that we will be able to generate significant sales of our current and future branded products. We expect to continue developing our own core branded products in markets such as functional foods, sports and fitness nutrition and to pursue international sales opportunities. The growing awareness of the potential uses of myostatin reducing ingredients supports continued development of our own core products. We remain committed to continuing our focus on various clinical trials in support of enhancing our commercial strategy as well as enhancing our intellectual property assets, to develop product improvements and new products, and to reduce the cost of our products by finding more efficient manufacturing processes and contract manufacturers.
Intellectual Property
We have adopted a comprehensive intellectual property strategy, the implementation of which is ongoing. We are focusing our efforts on ensuring our current commercial products and processes, and those currently under development, are being protected to the maximum extent possible. We are in the process of filing multiple patent applications in the United States and abroad, and we are currently prosecuting pending patent applications in the United States, all of which are directed towards our compositions and methods of manufacturing the same. In addition to a proactive protection strategy, we are conducting defensive due diligence to ensure our products and processes do not encroach upon the rights of third parties. Moreover, we are also engaged in a survey of the intellectual property landscape of potential competitors, and are devising a proactive path to stay ahead of such potential competitors.
9
In August 2014, the U.S. Patent and Trademark Office, or USPTO, issued U.S. Patent No. 8,815,320 B2 to us covering our proprietary methods of manufacturing Fortetropin®. The patent entitled “Process for Producing a Composition Containing Active Follistatin,” provides intellectual property protection for manufacturing Fortetropin®, the key ingredient in our core commercial muscle health products, and carries a patent term through early 2033. Additionally, we are currently prosecuting a core patent application covering the basic science on which our business was built, which is currently undergoing examination at the USPTO. The scope of this application covers the various applications of avian follistatin products and the benefits thereof. In particular, this application is focused on the composition currently in our commercially available Fortetropin®-powered products and the known benefits thereof.
We intend to file as many applications as possible as continuation/divisional/continuation-in-part applications. Several additional pending patent applications that we are pursuing include:
| ● | Method of obtaining effective amounts of avian follistatin - covering a method of controlling the amount of avian follistatin and the concentrations thereof within a product by extracting the proteins from various parts of fertilized and unfertilized avian eggs. | |
| ● | Methods of treating degenerative muscle disease – covering methods of treating various degenerative muscle diseases, such as sarcopenia, with avian egg-based products and the compositions thereof. | |
| ● | Methods and products for increasing muscle mass – covering various combinations of proteins, lipids and other molecules, which are active in the natural form of our core commercial products, which may be combined in advantageous amounts to yield improved products and methods for increasing muscle mass. |
| ● | Egg-based product containing hydroxymethylbutyrate, or HMB, for the treatment of degenerative muscle disease – covering a line of products combining avian egg-based products with HMB for improved treatment of degenerative muscle diseases and the methods of treating the same. | |
| ● | Egg-based product containing leucine for treatment of degenerative muscle disease - covering a line of products combining avian egg-based products with leucine for improved treatment of degenerative muscle diseases and the methods of treating the same. | |
| ● | Methods of treatment of degenerative muscle disease using egg-based products and testosterone replacement therapy – covering methods of treating degenerative muscle disease in combination with testosterone replacement therapy for improved results. | |
| ● | Methods of treatment of cancer using avian egg powder. | |
| ● | Methods of treatment of insulin resistance and Type II diabetes using avian egg powder. | |
| ● | Methods of treatment of neurological diseases using avian egg powder. | |
| ● | Method of enhancing overall health and longevity using avian egg powder. |
In addition to patent protection, we are also engaged in protecting our brands, including corporate brands and product brands, and have sought trademark registrations in the United States for the same. We have implemented a clearance strategy for new brands that we intend to launch, to ensure any risk of encroaching on the rights of third parties is minimized.
We regard our trademarks and other proprietary rights as valuable assets and believe that protecting our key trademarks is crucial to our business strategy of building strong brand name recognition. These trademarks are crucial elements of our business, and have significant value in the marketing of our products. Federally registered trademarks have a perpetual life, provided that they are maintained and renewed on a timely basis and used correctly as trademarks, subject to the rights of third parties to attempt to cancel a trademark if priority is claimed or there is confusion of usage. We rely on common law trademark rights to protect our unregistered trademarks. Common law trademark rights generally are limited to the geographic area in which the trademark is actually used, while a United States federal registration of a trademark enables the registrant to stop the unauthorized use of the trademark by third parties in the United States. Much of our ongoing work, including our research and development, is kept highly confidential. As such, we have adopted corporate confidentiality policies that comply with the Uniform Trade Secrets Act and the New Jersey Trade Secret Act to protect our most valuable intellectual property assets.
10
Regulatory Environment
The importing, manufacturing, processing, formulating, packaging, labeling, distributing, selling and advertising of our current and future products may be subject to regulation by one or more federal or state agencies. The Food and Drug Administration, or the FDA, has primary jurisdiction over our products pursuant to the Federal Food, Drug and Cosmetic Act, as amended by the Dietary Supplement and Health Education Act, or the FDCA, and the regulations promulgated thereunder. The FDCA provides the regulatory framework for the safety and labeling of dietary supplements, foods and medical foods. In particular, the FDA regulates the safety, manufacturing, labeling and distribution of dietary supplements. In addition, the Animal Plant Health and Inspection Service, or APHIS, regulates the importation of our primary product from Germany. The Federal Trade Commission, or the FTC, and the FDA share jurisdiction over the promotion and advertising of dietary supplements. Pursuant to a memorandum of understanding between the two agencies, the FDA has primary jurisdiction over claims that appear on product labels and labeling and the FTC has primary jurisdiction of product advertising.
The term “medical foods” does not pertain to all foods fed to sick patients. Medical foods are prescription foods specially formulated and intended for the dietary management of a disease that has distinctive nutritional needs that cannot be met by normal diet alone. They were defined in the FDA’s 1988 Orphan Drug Act Amendments and are subject to the general food safety and labeling requirements of the FDCA but are exempt from the labeling requirements for health claims and nutrient content claims under the Nutrition Labeling and Education Act of 1990. Medical foods are distinct from the broader category of foods for special dietary use and from traditional foods that bear a health claim. In order to be considered a medical food, a product must, at a minimum, be a specially formulated and processed product (as opposed to a naturally occurring food in its natural state) for oral ingestion or tube feeding (nasogastric tube), be labeled for the dietary management of a specific medical disorder, disease or condition for which there are distinctive nutritional requirements and be intended to be used under medical supervision.
Compliance with applicable federal, state, and local laws and regulations is a critical part of our business. We endeavor to comply with all applicable laws and regulations. However, as with any regulated industry, the laws and regulations are subject to interpretation and there can be no assurances that a government agency would necessarily agree with our interpretation of the governing laws and regulations. Moreover, we are unable to predict the nature of such future laws, regulations, interpretations or applications, nor can we predict what effect additional governmental regulations or administrative orders, when and if promulgated, would have on our business in the future. These regulations could, however, require the reformulation of our products to meet new standards, market withdrawal or discontinuation of certain products not able to be reformulated. The risk of a product recall exists within the industry although we endeavor to minimize the risk of recalls by distributing products that are not adulterated or misbranded. However, the decision to initiate a recall is often made for business reasons in order to avoid confrontation with the FDA.
Our products are required to be prepared in compliance with the FDA’s Good Manufacturing Practices, or GMPs, as set forth in 21 CFR Part 111. Fortetropin®, the active ingredient in our products, must be imported into the United States in conformance with USDA-APHIS’s requirements for egg products. Other statutory obligations include reporting all serious adverse events on a Medwatch Form 3500A. To date, we have not filed a Medwatch Form 3500A with the FDA nor have we been placed on notice regarding any serious adverse events related to any of our products. Since eggs are considered a major food allergen under the Food Allergen Labeling and Consumer Protection Act of 2004, we are required to label all our products containing Fortetropin® to note that they contain egg product.
Advertising of dietary supplement products is subject to regulation by the FTC under the Federal Trade Commission Act, or FTCA, which prohibits unfair methods of competition and unfair or deceptive trade acts or practices in or affecting commerce. The FTCA provides that the dissemination of any false advertising pertaining to foods, including dietary supplements, is an unfair or deceptive act or practice. Under the FTC’s substantiation doctrine, an advertiser is required to have a reasonable basis for all objective product claims before the claims are made. All advertising is required to be truthful and not misleading. All testimonials are required to be typical of the results the consumer may expect when using the product as directed. Accordingly, we are required to have adequate substantiation of all material advertising claims made for our products. Failure to adequately substantiate claims may be considered either deceptive or unfair practices.
11
In addition, medical foods must comply with all applicable requirements for the manufacturing of foods, including food Current Good Manufacturing Practices (“cGMP”), registration of food facility requirements and, if applicable, FDA regulations for low acid canned food and emergency permit controls. The FDA considers the statutory definition of medical foods to narrowly constrain the types of products that fit within this category of food. The FDA inspects medical food manufacturers annually to assure the safety and integrity of the products. Failure of our contract manufacturers to comply with applicable requirements could lead to sanctions that could adversely affect our business.
We cannot predict what effect additional domestic or international governmental legislation, regulations, or administrative orders, when and if promulgated, would have on our business in the future. New legislation or regulations may require the reformulation of certain products to meet new standards, require the recall or discontinuance of certain products not capable of reformulation, impose additional record keeping or require expanded documentation of the properties of certain products, expanded or different labeling or scientific substantiation.
Manufacturing; Raw Materials and Suppliers
We are committed to producing and selling highly efficacious products that are trusted for their quality and safety. To date, our products have been outsourced to third party manufacturers where the products are manufactured in full compliance with cGMP standards set by the FDA. All of the raw materials for our current products are currently sourced from third-party suppliers. Any shortages in our raw materials could result in materially higher raw material prices and adversely affect our ability to source our product. Since the beginning of 2012, we have been focusing on the efficiency and economics of manufacturing Fortetropin®. Our management has examined the production cost and is working to achieve cost savings in production.
We currently have an agreement with only one third-party manufacturer of Fortetropin®, who will manufacture the formula exclusively for us in perpetuity, and may not manufacture the formula for other entities. We have multiple vendors for blending, packaging and labeling our products.
Competition
Given the large populations that could potentially benefit from myostatin modulation, a number of pharmaceutical companies are currently developing various types of myostatin inhibitors. Eli Lilly and Co., Novartis AG, Pfizer Inc., Scholar Rock and Acceleron Pharma Inc, are among the companies that we are aware of that are testing new compounds in the field of myostatin inhibition. The market for nutritional supplements is highly competitive. Companies operating in the space include PepsiCo Inc., Glanbia Plc. GNC Holdings, The Coca-Cola Company, GlaxoSmithKline, Abbott Laboratories, Nestle S.A. and Universal Nutrition. Competition is based on price, quality, customer service, marketing and product effectiveness. Our competition includes numerous nutritional supplement companies that are highly fragmented in terms of geographic market coverage, distribution channels and product categories. In addition, large pharmaceutical companies and packaged food and beverage companies compete with us in the nutritional supplement market. These companies and certain nutritional supplement companies have broader product lines and/or larger sales volumes than us and have greater financial and other resources available to them and possess extensive manufacturing, distribution and marketing capabilities. Other companies are able to compete more effectively due to a greater extent of vertical integration. Private label products of our competitors, which in recent years have significantly increased in certain nutrition categories, compete directly with our products. In several product categories, private label items are the market share leaders. Increased competition from such companies, including private label pressures, could have a material adverse effect on our results of operations and financial condition. Many companies within our industry are privately-held and therefore, we are unable to assess the size of all of our competitors or where we rank in comparison to such privately-held competitors with respect to sales.
Insurance
We maintain commercial liability, including product liability coverage, and property insurance. Our policy provides for a general liability of $5.0 million per occurrence, and $10.0 million annual aggregate coverage. We carry property coverage on our main office facility to cover our legal liability, tenant’s improvements, business property, and inventory. We maintain commercial general liability and products liability insurance with coverage of up to $5.0 million.
Employees
We currently have ten full-time employees (including one executive officer). We also employ several consultants. None of our employees are represented by a labor union and we consider our employee relations to be good.
12
| Item 1A. | Risk Factors. |
Investing in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should carefully consider the risk factors set forth below, and other information contained in this Report including our financial statements and the related notes thereto. The risks and uncertainties set forth below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently consider immaterial may also adversely affect us. If any of the described risks occur, our business, financial condition or results of operations could be materially harmed. In such case, the value of our securities could decline and you may lose all or part of your investment. Amounts in this section are in thousands, unless otherwise indicated.
RISKS RELATING TO OUR BUSINESS
Our limited operating history makes it difficult to evaluate our future prospects and results of operations.
We are an early stage company and have a limited operating history. Our future prospects should be considered in light of the risks and uncertainties experienced by early stage companies in evolving markets such as the market for our current and future products, if any, in the United States. We will continue to encounter risks and difficulties that companies at a similar stage of development frequently experience, including the potential failure to:
| ● | build a strong and compelling consumer brand; | |
| ● | adequately protect and build our intellectual property; | |
| ● | develop new products; | |
| ● | conduct successful research and development activities; | |
| ● | increase awareness of our products and develop customer loyalty; | |
| ● | respond to competitive market conditions; | |
| ● | respond to requirements and changes in our regulatory environment; | |
| ● | maintain effective control of our costs and expenses; | |
| ● | availability of sufficient capital resources to adequately promote and market our products; and | |
| ● | attract, retain and motivate qualified personnel. |
If we are unable to address any or all of the foregoing risks, our business may be materially and adversely affected.
If we are unable to successfully market and promote our own core branded products, we will not be able to increase our sales and our business and results of operations would be adversely affected.
In March 2017, we launched Qurr, our proprietary branded products, using multiple delivery formats. Successfully marketing and promoting products is a complex and uncertain process, dependent on the efforts of management, outside consultants and general economic conditions, among other things. There is no assurance that we will successfully market and/or promote our own core branded products. Any factors that adversely impact the marketing or promotion of our products including, but not limited to, competition, acceptance in the marketplace, or delays related to production and distribution or regulatory issues, will likely have a negative impact on our cash flow and operating results. The commercial success of our products also depends upon various other factors including:
| ● | the quality and acceptance of other competing brands and products; | |
| ● | creating effective distribution channels and brand awareness; |
| ● | critical reviews; | |
| ● | the availability of alternatives; | |
| ● | general economic conditions; and | |
| ● | the availability of sufficient capital resources to adequately promote and market our products. |
Each of these factors is subject to change and cannot be predicted with certainty. We cannot assure you that we will be successful in marketing or promoting any of our own core branded products. If we are unable to successfully market and promote our own core branded products or any enhancements to our products which we may develop, we will not be able to increase our sales, and our results of operations would be adversely affected.
13
If distributors are unable or unwilling to purchase our products and we are unable to secure alternative distributors or customers, our operating results and financial condition will be adversely affected since historically this represents a large percentage of our sales.
We have previously sold our products primarily through two distributors, MHP and Cenegenics. For the year ended December 31, 2017, our net sales were $526, of which 38% was attributable to Cenegenics. For the year ended December 31, 2016, our net sales were $327, of which 50% was attributable to Cenegenics. We did not sell any products through MHP during the years ended December 31, 2016 and 2017.
In March 2017 we launched a new product line Qurr which we sell direct to consumers. About 80% of our sales were purchased via our website www.qurr.com and the remainder were purchased via our amazon.com site.
If we decide to continue selling our products to distributors and our prior distributors are unable or unwilling to purchase our products and we are unable to secure alternative distributors or customers, our operating results and financial condition will be adversely affected.
We have a history of losses and cash flow deficits, and we expect to continue to operate at a loss and to have negative cash flow for the foreseeable future, which could cause the price of our stock to decline.
At December 31, 2017, we had cumulative net losses from inception of $31,844. Our net loss for the years ended December 31, 2017 and 2016 were $4,058 and $4,341, respectively. We also had negative cash flow from operating activities. Historically, we have funded our operations from the proceeds from the sale of equity securities, debt issuances, and to a lesser extent, internally generated funds. Our strategic business plan is likely to result in additional losses and negative cash flow for the foreseeable future. We cannot give assurances that we will ever become profitable.
There is no assurance that we will be able to increase our sales.
Our sales for the year ended December 31, 2017 were $526 and our sales for the year ended December 31, 2016 were $327. We cannot give assurances that our current business model will enable us to increase our sales.
The report of our independent registered public accounting firm expresses substantial doubt about our ability to continue as a going concern.
Our auditors have indicated in their report on our financial statements for the years ended December 31, 2017 and December 31, 2016 that conditions exist that raise substantial doubt about our ability to continue as a going concern since we may not have sufficient capital resources from operations and existing financing arrangements to meet our operating expenses and working capital requirements. A “going concern” opinion could impair our ability to finance our operations through the sale of equity, incurring debt, or other financing alternatives. There can be no assurance that we will be able to generate the level of operating revenues projected in our business plan, or if additional sources of financing will be available on acceptable terms, if at all. If no additional sources of financing become available, our future operating prospects may be adversely affected and investors may lose all or a part of their investment.
Our intangible assets, which represent a significant amount of our total assets, are subject to impairment testing and may result in impairment charges, which would adversely affect our results of operations and financial condition.
At December 31, 2017, our total assets were $4,795, of which $1,640, or approximately 34%, represents intangible assets, net of accumulated amortization. Our intangible assets primarily relate to intellectual property pertaining to Fortetropin®, including the MYO-T12 formula, trademarks, trade secrets, patent application and domain names acquired from Peak Wellness, Inc. in February 2011. The intellectual property asset was initially recorded as an indefinite-lived intangible asset and tested annually for impairment or more frequently if events or circumstances changed that could potentially reduce the fair value of the asset below its carrying value. Impairment testing requires the development of significant estimates and assumptions involving the determination of estimated net cash flows, selection of the appropriate discount rate to measure the risk inherent in future cash flow streams, assessment of an asset’s life cycle, competitive trends impacting the asset as well as other factors. Our forecasted future results and related net cash flows contemplate the direct offering of product and successfully establishing future sales channels among other factors. Changes in these underlying assumptions could significantly impact the asset’s estimated fair value.
In 2011, based on (i) assessment of current and expected future economic conditions, (ii) trends, strategies and projected revenues and (iii) assumptions similar to those that market participants would make in valuing our intangible assets, management determined that the carrying values of the intellectual property asset exceeded its fair value. Accordingly, we recorded noncash impairment charges totaling $2,662 and reduced the intellectual property asset to its fair value of $2,000. During the second quarter of 2015, management made an assessment and based on expansion into new markets and introduction of new formulas determined that the intellectual property had a finite useful life of ten (10) years and began amortizing the carrying value of the intellectual property asset over its estimated useful life. Management made a separate determination that no further impairment existed at that time. Based on fourteen consecutive quarters of minimal revenues combined with changes in the sales channels through which we sell our products and our inability to predict future orders, if any, from MHP or Cenegenics or to what extent we will be able to secure new distribution arrangements, we tested the intellectual property for impairment in the fourth quarter of 2017 and 2016 and determined that the asset value was recoverable and therefore no impairment was recognized. Nevertheless, a significant amount of our total assets are subject to impairment testing and may result in noncash impairment charges, which would adversely affect our results of operations and financial condition.
14
We will need to raise additional funds in the future to continue our operations. If we are unable to raise funds as needed, we may not be able to maintain our business.
We expect that our current funds will not be sufficient to fund our projected operations through December 2018. We require substantial funds for operating expenses, research and development activities, to establish manufacturing capability, to develop consumer marketing and retail selling capability, and to cover public company costs. In addition, we have incurred substantial costs in connection with our litigation with Mr. Ren and RENS Technology Inc., or the RENS litigation See “Part 1 Item 3 – Legal Proceedings” for additional information regarding the RENS litigation. The extent of our capital needs will depend on numerous factors, including (i) our profitability, (ii) the release of competitive products, (iii) the level of investment in research and development, (iv) the amount of our capital expenditures, (v) the amount of our working capital including collections on accounts receivable, (vi) the sales, marketing and distribution investment needed to develop and launch our own core branded products, (vii) cash generated by sales of those products and (viii) the status of the RENS litigation. We expect that we will need to seek additional funding in 2018 through public or private financing or through collaborative arrangements with strategic partners.
We cannot assure you that we will be able to obtain additional financing or that such financing would be sufficient to meet our needs. If we cannot obtain additional funding, we may be required to limit our marketing efforts, decrease or eliminate capital expenditures or cease all or a portion of our operations, including any research and development activities. Any available additional financing may not be adequate to meet our goals.
Even if we are able to locate a source of additional capital, we may not be able to negotiate terms and conditions for receiving the additional capital that are acceptable to us.
Any future capital investments could dilute or otherwise materially adversely affect the holdings or rights of our existing stockholders. In addition, new equity or convertible debt securities issued by us to obtain financing could have rights, preferences and privileges senior to our common stock. There is no assurance that any additional financing will be available, or if available, will be on terms favorable to us. In addition, any equity financing would result in dilution to stockholders.
Since our revenues are generated in U.S. dollars but a portion of our expenses are incurred in foreign currencies, our earnings may be reduced due to currency exchange rate fluctuations.
Our revenues are generated in U.S. dollars, while a portion of our expenses related to our supply agreement are incurred in foreign currencies, principally the payments to our primary manufacturer that are paid in euros. The exchange rates between the U.S. dollar and other currencies fluctuate and are affected by, among other things, changes in political and economic conditions. Any significant fluctuation in the exchange rate for these currencies may materially and adversely affect our earnings, cash flows and financial condition.
If we are unable to manage our infrastructure growth, our business results may be materially and adversely affected.
We need to manage our infrastructure growth to support and maximize our potential revenue growth and achieve our expected business results. Engaging the full capacity of our limited staff may place a significant strain on our management, operations, and accounting and information systems. We expect that we will need to continue to improve our financial controls, operating procedures and management information systems. The failure to manage our infrastructure growth could adversely affect our business results.
If we are not able to implement our business objectives, our operations and financial performance may be adversely affected.
Our principal objectives are to: (i) create a sales platform through marketing products containing our proprietary ingredient Fortetropin® in established, growing, and new markets and strategic selection of partnerships and collaborations to maximize near-term and future revenues, (ii) deepen the scientific understanding of the activity of Fortetropin®, specifically as a natural, reversible, temporary modulator of the regulatory protein myostatin, and to leverage this knowledge to strengthen and build our intellectual property, (iii) conduct research and development activities to evaluate myostatin modulation in a range of both wellness and disease states, (iv) identify other products and technologies which may broaden our portfolio and define a business development strategy to protect, enhance and accelerate the growth of our products, (v) reduce the cost of manufacturing through process improvement, and (vi) identify contract manufacturing organizations that can fully meet our future growth requirements. Our business plan is based on circumstances currently prevailing and assumptions that certain circumstances will or will not occur as well as the inherent risk and uncertainties involved in various stages of development. However, there is no assurance that we will be successful in achieving our objectives. If we are not able to achieve our objectives, our business operations and financial performance may be adversely affected.
15
If we lose the services of our key personnel, we may be unable to replace them, and our business, financial condition and results of operations could be adversely affected.
Our success largely depends on the continued skills, experience, efforts and policies of our management, directors and other key personnel and our ability to continue to attract, motivate and retain highly qualified employees. In particular, certain of our directors, including Dr. Robert Hariri and Dr. Louis Aronne have significant research and development experience and are integral to the creation of our future products and the execution of our business strategy. In addition, our prospects depend substantially on the services of our executive management team.
If one or more of our key employees or directors leaves us, we will need to find a replacement with the combination of skills and attributes necessary to execute our strategy. Because competition for skilled personnel is intense, and the process of finding qualified individuals can be lengthy and expensive, we believe that the loss of the services of key personnel could adversely affect our business, financial condition and results of operations. We cannot assure you that we will continue to retain such personnel.
Our success depends on our ability to anticipate and respond in a timely manner to changing consumer demands.
Our success depends on the appeal of our current and future products to a broad range of consumers whose preferences cannot be predicted with certainty and are subject to change. If our current and future products do not meet consumer demands, our sales may decline. In addition, our growth depends upon our ability to develop new products through product line extensions and product modifications, which involve numerous risks. We may not be able to accurately identify consumer preferences, translate our knowledge into customer accepted products, establish the appropriate pricing for our products or successfully integrate these products with our existing product platform or operations. We may also experience increased expenses incurred in connection with product development, marketing and advertising that are not subsequently supported by a sufficient level of sales, which would negatively affect our margins. Furthermore, product development may divert management’s attention from other business concerns, which could cause sales of our existing products to suffer. We cannot assure you that newly developed products will contribute favorably to our operating results.
Products often have to be promoted heavily in stores or in the media to obtain visibility and consumer acceptance. Acquiring distribution for products is difficult and often expensive due to slotting and other promotional charges mandated by retailers. Products can take substantial periods of time to develop consumer awareness, consumer acceptance and sales volume. Accordingly, some products may fail to gain or maintain sufficient sales volume and as a result may have to be discontinued.
If our current or future products fail to properly perform, our business could suffer due to increased costs and reduced income. Failure of our current or future products to meet consumer expectations could result in decreased sales, delayed market acceptance of our products, increased accounts receivable, unsaleable inventory and customer returns, and divert our resources to reformulation or alternative products.
Intense competition from existing and new entities may adversely affect our revenues and profitability.
We face competitors that will attempt to create, or are already creating, products that are similar to our current and future products. Many of our current and potential competitors have significantly longer operating histories and significantly greater managerial, financial, marketing, technical and other competitive resources, as well as greater brand recognition, than we do. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers or adopt more aggressive pricing policies. We cannot assure you that we will be able to compete effectively with current or future competitors or that the competitive pressures we face will not harm our business.
Our business is dependent on continually developing or acquiring new and advanced products and processes and our failure to do so may cause us to lose our competitiveness and may adversely affect our operating results.
To remain competitive in our industry, we believe it is important to continually develop new and advanced products and processes. There is no assurance that competitive new products and processes will not render our existing or new products obsolete or non-competitive. Our competitiveness in the marketplace relies upon our ability to continuously enhance our current products, introduce new products, and develop and implement new technologies and processes. Our failure to evolve and/or develop new or enhanced products may cause us to lose our competitiveness in the marketplace and adversely affect our operating results.
Adverse publicity or consumer perception of our products and any similar products distributed by others could harm our reputation and adversely affect our sales and revenues.
We are highly dependent upon positive consumer perceptions of the safety, efficacy and quality of our products as well as similar products distributed by our competitors. Consumer perception of dietary supplements and our products in particular can be substantially influenced by scientific research or findings, national media attention including social media attention and other publicity about product use. Adverse publicity from such sources regarding the safety, efficacy or quality of dietary supplements, in general, and our products in particular, could harm our reputation and results of operations. The mere publication of reports asserting that such products may be harmful or questioning their efficacy could have a material adverse effect on our business, financial condition and results of operations, regardless of whether such reports are scientifically supported or whether the claimed harmful effects would be present at the dosages recommended for such products.
16
Marketing of our products through social media and other advertising methods could harm our business and reputation.
There are many considerations that can affect the marketing and advertising of our products through social media such as claims and concerns about safety, new discoveries, patent disputes and claims about adverse side effects. Further, claims and concerns about safety can result in a negative impact on product sales, product recalls or withdrawals, and/or consumer fraud, product liability and other litigation and claims. A video published online, a blog on the internet, or a post on a website, can be distributed rapidly and negatively harm our reputation.
Cyberattacks and other security breaches could compromise our proprietary and confidential information as well as our e-commerce infrastructure and customer database which could harm our business and reputation.
We generate, collect and store proprietary information, including intellectual property and business information. The secure storage, maintenance, and transmission of and access to this information is important to our operations and reputation. Computer hackers may attempt to penetrate our computer systems and, if successful, misappropriate our proprietary and confidential information including e-mails and other electronic communications. In addition, an employee, contractor, or other third-party with whom we do business may attempt to obtain such information, and may willfully or inadvertently cause a breach involving such information. While we have certain safeguards in place to reduce the risk of and detect cyber-attacks, our information technology networks and infrastructure may be vulnerable to unpermitted access by hackers or other breaches, or employee error or malfeasance. Any such compromise of our data security and access to, or public disclosure or loss of, confidential business or proprietary information could disrupt our operations, damage our reputation, provide our competitors with valuable information, and subject us to additional costs which could adversely affect our business.
The scientific support for Fortetropin® is subject to uncertainty.
Our research, scientific knowledge and clinical testing supporting the benefits of our products are an essential element of our ability to legally market our products. There is, however, the risk that new or undiscovered information may become available that may undermine or refute our scientific support. In addition, our clinical studies of Fortetropin® have been limited in scope and additional testing may reveal deficiencies and side effects that we are currently unaware of. A reduction in the credibility of our scientific support for the effectiveness of Fortetropin® could have a material adverse effect on our operations and financial condition.
If we are required to withdraw our products from the market, change the labeling of our products and/or are subject to product liability claims, our operations and financial performance may be adversely affected.
There is a potential for any ingested product to result in side effects in certain consumers. Although we are not aware of any adverse effects of our products on the health of consumers, if any such side effects are identified after marketing and sale of the product, we may be required to withdraw our products from the market or change its labeling. We may also be required to withdraw our products from the market as a result of regulatory issues. If we are required to withdraw our products from the market, our business operations and financial performance may be adversely affected. Furthermore, if a product liability claim is brought against us, it may, regardless of merit or eventual outcome, result in damage to our reputation, decreased demand for our products, costly litigation and loss of revenue.
An increase in product returns could negatively impact our operating results and profitability.
Historically, sales allowances for product returns have not been provided, since under our existing arrangements, customers are not permitted to return product except for non-conforming product. In certain instances we may permit the return of damaged or defective products and accept limited amounts of product returns. While such returns have historically been nominal and within management’s expectations and the provisions established, future return rates may differ from those experienced in the past. Any significant increase in damaged or defective products or expected returns could have a material adverse effect on our operating results for the period or periods in which such returns materialize. With respect to future sales, we may need to offer retail customers sales incentives, including the right to return product. If those customers are not able to sell our products to end-consumers, significant product returns may materialize, which could have a material adverse effect on our operating results.
17
We are dependent on third-party manufacturers, suppliers and processors to produce our products.
We currently rely on third-party manufacturers, suppliers and processors to produce our products. If our manufacturers, suppliers or processors are unable to provide us with the required finished products or raw materials or are unable or unwilling to produce sufficient quantities of our products, our business and revenues will be adversely affected.
A shortage in the supply of, or a price increase in, raw materials could increase our costs or adversely affect our sales and revenues.
All of the raw materials for our products are sourced from third-party suppliers. Currently, we have one primary third-party manufacturer to produce Fortetropin® under a fixed price agreement that runs through December 2018. If we are unable to renew the agreement, any shortages in our raw materials could adversely affect operations. Price increases from a supplier will affect our profitability if we are not able to pass price increases on to customers. The inability to obtain adequate supplies of raw materials in a timely manner of our raw materials could have a material adverse effect on our business, financial condition and results of operations.
While our raw material inventories generally have a long shelf life, we may be required to write-off or reserve for inventories that are slow-moving, off-grade, damaged or otherwise not saleable. Such write-offs and/or reserves could have a material adverse effect on our business, financial condition and results of operations.
Our raw material inventories are comprised of dried powder derived from egg-yolk, and despite generally having a long shelf life, we may be required to write-off or reserve for inventories that are slow-moving, off-grade, damaged or otherwise not saleable. Cost of sales for the year ended December 31, 2017 and 2016 included slow moving obsolete/damaged goods inventory charges of $-2- and $107, respectively. Future required write-offs or reserves could have a material adverse effect on our business, financial condition and results of operations.
We have no manufacturing capacity and anticipate continued reliance on third-party manufacturers for the development and commercialization of our products.
We do not currently operate manufacturing facilities for production of our product. We lack the resources and the capabilities to manufacture our products on a commercial scale. We do not intend to develop facilities for manufacturing our products in the foreseeable future. We rely on third-party manufacturers to produce bulk products required to meet our sales needs. We plan to continue to rely upon contract manufacturers to manufacture commercial quantities of our products.
Our contract manufacturers’ failure to achieve and maintain high manufacturing standards, in accordance with the FDA’s GMP’s as set forth in 21 CFR Part 111 and/or applicable regulatory requirements, or the incidence of manufacturing errors, could result in consumer injury or death, product shortages, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could seriously harm our business. Contract manufacturing organizations often encounter difficulties involving production yields, quality control and quality assurance, as well as shortages of qualified personnel. Our existing manufacturers and any future contract manufacturing organizations may not perform as agreed upon or may not remain in the contract manufacturing business. In the event of a natural disaster, business failure, strike or other difficulty, we may be unable to replace a third-party manufacturer in a timely manner and the production of our products would be interrupted, resulting in delays, additional costs and reduced revenues.
Our research and development activities may be costly and/or untimely, and there are no assurances that our research and development activities will either be successful or completed within the anticipated timeframe, if ever at all.
Research and development activities may be costly and/or untimely, and there are no assurances that our research and development activities will either be successful or completed within the anticipated timeframe, if at all. The continued research and development relating to Fortetropin® and our future products is important to our success. In addition, the development of new products requires significant research, development and testing all of which require significant investment and resources. At this time, our resources are limited and our research and development activities are dependent upon our ability to fund our activities and to raise capital which may not be possible. We may enter into agreements with third party contract research organizations (CROs), academic institutes or non-profit research institutes to engage in research and development for us. However, the failure of the third-party researcher to perform under agreements entered into with us, or our failure to renew important research agreements with a third party, may delay or curtail our research and development efforts. The research and development of new products is costly and time consuming, and there are no assurances that our research and development activities will be successful. Even if a new product is developed, there is no assurance that it will be commercialized or result in sales.
We may not be able to protect our intellectual property rights which could cause our assets to lose value.
Our business depends on and will continue to depend on our intellectual property, including our valuable brands and internally-developed products. We believe our intellectual property rights are important to our continued success and our competitive position. However, we may be unable or unwilling to strictly enforce our intellectual property rights, including our patents and trademarks, from infringement due to the substantial costs of such enforcement. In addition, while there are patent applications pending for our core product, there is no assurance that such applications will issue as patents. Our failure to enforce our intellectual property rights could diminish the value of our brands and product offerings and harm our business and future growth prospects.
18
In addition, unauthorized parties may attempt to copy or otherwise obtain and use our services, technology and other intellectual property, and we cannot be certain that the steps we have taken to protect our proprietary rights will prevent any misappropriation or confusion among consumers and merchants, or unauthorized use of these rights. Advancements in technology have exacerbated the risk by making it easier to duplicate and disseminate intellectual property. In addition, as our business becomes more global in scope, we may not be able to protect our proprietary rights in a cost-effective manner in a multitude of jurisdictions with varying laws. If we are unable to procure, protect and enforce our intellectual property rights, we may not realize the full value of these assets, and our business may suffer. If we need to commence litigation to enforce our intellectual property rights or determine the validity and scope of the proprietary rights of others, such litigation may be costly and divert the attention of our management.
We may be subject to intellectual property rights claims, which are costly to defend, could require us to pay damages and could limit our ability to sell some of our products.
We may become subject to intellectual property litigation or infringement claims, which could cause us to incur significant expenses to defend such claims, divert management’s attention or prevent us from manufacturing, importing, selling or using some aspect of our current or future products. If we choose or are forced to settle such claims, we may be required to pay for a license to certain rights, pay royalties on both a retrospective and prospective basis, and/or cease manufacturing importing and selling certain infringing products. Future infringement claims against us by third parties may adversely impact our business, financial condition and results of operations.
In addition, our primary third-party manufacturer assigned its United States patent application for making Fortetropin®, the key ingredient in our products, to us in exchange for royalty payments for each kilogram of Fortetropin® that we produce, for a period of seven years from the expiration date of the supply agreement on December 31, 2016. Subsequent to the assignment of the patent application, in August 2014, the USPTO issued to us U.S. Patent No. 8,815,320 B2 covering the proprietary methods of manufacturing Fortetropin®.
Our advertising and marketing efforts may be costly and may not achieve desired results.
We intend to incur substantial expenses in connection with our advertising and marketing efforts for our products. Although we intend to target our advertising and marketing efforts on current and potential customers who we believe are likely to be in the market for the products we sell, we cannot assure you that our advertising and marketing efforts will achieve our desired results. We will periodically adjust our advertising expenditures in an effort to optimize the return on such expenditures knowing that any such decrease we make to optimize such return could adversely affect our sales.
We rely on independent shipping companies to deliver the products we sell.
We rely upon third party carriers, especially FedEx and UPS, for timely delivery of our product shipments. As a result, we are subject to carrier disruptions and increased costs due to factors that are beyond our control, including employee strikes, inclement weather and increased fuel costs. Any failure to deliver products to our customers in a timely and accurate manner may damage our reputation and brand and could cause us to lose customers. We do not have a written long-term agreement with any of these third party carriers, and we cannot be sure that these relationships will continue on terms favorable to us, if at all. If our relationship with any of these third party carriers is terminated or impaired, or if any of these third parties are unable to deliver products for us, we would be required to use alternatives for shipment of products to our customers. We may be unable to engage alternative carriers on a timely basis or on terms favorable to us, if at all. Potential adverse consequences include:
| ● | reduced visibility of order status and package tracking; | |
| ● | delays in order processing and product delivery; | |
| ● | increased cost of delivery, resulting in reduced margins; and | |
| ● | reduced shipment quality, which may result in damaged products and customer dissatisfaction. |
Furthermore, shipping costs represent a significant operational expense for us. Any future increases in shipping rates could have a material adverse effect on our business, financial condition and results of operations.
19
We rely on fulfillment centers to package and deliver our product to customers who place orders online
We have an agreement with one fulfillment center to box and ship our products to customers once an order has been placed. We cannot be sure that our relationship with the fulfillment center will continue on terms favorable to us, if at all. If our relationship with them is terminated or impaired, or if they are unable to deliver products for us, we would be required to use alternatives for shipment of products to our customers.
We face significant inventory risk.
We are exposed to significant inventory risks that may adversely affect our operating results as a result of new product launches, rapid changes in product cycles and pricing, defective merchandise, changes in consumer demand and consumer spending patterns, changes in consumer tastes with respect to our products, and other factors. We endeavor to accurately predict these trends and avoid overstocking or understocking our products. Demand for products, however, can change significantly between the time inventory is ordered and the date of sale. In addition, when we begin selling or manufacturing a new product, it may be difficult to determine appropriate product selection, and accurately forecast demand. The acquisition of inventory may require significant lead-time and prepayment and we may be unable to sell products in sufficient quantities or during the relevant selling seasons. Any one of these risks may adversely affect our operating results.
Our failure to respond appropriately to competitive challenges, changing consumer preferences and demand for new products could significantly harm our customer relationships and product sales.
The nutritional supplement industry is characterized by intense competition for product offerings and rapid and frequent changes in consumer demand. Our failure to predict accurately product trends could negatively impact our products and cause our revenues to decline.
Our success with any particular product offering (whether new or existing) depends upon a number of factors, including our ability to:
| ● | deliver quality products in a timely manner in sufficient volumes; | |
| ● | accurately anticipate customer needs and forecast accurately to our manufacturers; | |
| ● | differentiate our product offerings from those of our competitors; | |
| ● | competitively price our products; and | |
| ● | develop new products. |
Furthermore, products often have to be promoted heavily in stores or in the media to obtain visibility and consumer acceptance. Acquiring distribution for products is difficult and often expensive due to slotting and other promotional charges mandated by retailers. Products can take substantial periods of time to develop consumer awareness, consumer acceptance and sales volume. Accordingly, some products may fail to gain or maintain sufficient sales volume and as a result may have to be discontinued.
Our industry is highly competitive, and our failure to compete effectively could adversely affect our market share, financial condition and future growth.
The nutritional supplement industry is highly competitive with respect to:
| ● | price; | |
| ● | shelf space and store placement; | |
| ● | brand and product recognition; | |
| ● | product introductions; and | |
| ● | raw materials. |
Most of our competitors are larger, more established companies and possess greater financial strength, personnel, distribution and other resources than we have. We face competition in the supplement market from a number of large nationally known manufacturers, private label brands and many smaller manufacturers.
Adverse publicity or consumer perception of our products and any similar products distributed by others could harm our reputation and adversely affect our sales.
We believe we are highly dependent upon positive consumer perceptions of the safety and quality of our products as well as similar products distributed by other nutritional supplement companies. Consumer perception of nutritional supplements and our products in particular can be substantially influenced by scientific research or findings, national media attention and other publicity about product use. Adverse publicity from these sources regarding the safety, quality or efficacy of nutritional supplements and our products could harm our reputation and results of operations. The mere publication of news articles or reports asserting that such products may be harmful or questioning their efficacy could have a material adverse effect on our business, financial condition and results of operations, regardless of whether such news articles or reports are scientifically supported or whether the claimed harmful effects would be present at the dosages recommended for such products.
20
Changes in the economies of the markets in which we do business may affect consumer demand for our products.
Consumer spending habits, including spending for our products, are affected by, among other things, prevailing economic conditions, levels of employment, fuel prices, changes in exchange rates, salaries and wages, the availability of consumer credit, consumer confidence and consumer perception of economic conditions. Economic slowdowns in the markets in which we do business and an uncertain economic outlook may adversely affect consumer spending habits, which may result in lower sales of our products in future periods. A prolonged global or regional economic downturn could have a material negative impact on our financial position, results of operation or cash flows.
Our insurance coverage may be insufficient to cover our legal claims or other losses that we may incur in the future.
We maintain insurance, including property, general and product liability and other forms of insurance to protect ourselves against potential loss exposures. In the future, insurance coverage may not be available at adequate levels or on adequate terms to cover potential losses. If insurance coverage is inadequate or unavailable, we may face claims that exceed coverage limits or that are not covered, which could increase our costs and adversely affect our operating results.
We may be subject to uncertain and costly compliance with government regulations.
The importing, manufacturing, processing, formulating, packaging, labeling, distributing, selling and advertising of our current and future products may be subject to regulation by one or more federal or state agencies. The Food and Drug Administration, or the FDA, has primary jurisdiction over our products pursuant to the Federal Food, Drug and Cosmetic Act, as amended by the Dietary Supplement and Health Education Act, or the FDCA, and regulations promulgated thereunder. The FDCA provides the regulatory framework for the safety and labeling of dietary supplements, foods and medical foods. In particular, the FDA regulates the safety, manufacturing, labeling and distribution of dietary supplements. In addition, the Animal Plant Health and Inspection Service, or APHIS, regulates the importation of our primary product from Germany. The Federal Trade Commission, or the FTC, and the FDA share jurisdiction over the promotion and advertising of dietary supplements. Pursuant to a memorandum of understanding between the two agencies, the FDA has primary jurisdiction over claims that appear on product labels and labeling and the FTC has primary jurisdiction over product advertising.
Compliance with applicable federal, state, and local laws and regulations is a critical part of our business. We endeavor to comply with all applicable laws and regulations. However, as with any regulated industry, the laws and regulations are subject to interpretation and there can be no assurances that a government agency would necessarily agree with our interpretation of the governing laws and regulations. Moreover, we are unable to predict the nature of such future laws, regulations, interpretations or applications, nor can we predict what effect additional governmental regulations or administrative orders, when and if promulgated, would have on our business in the future. These regulations could, however, require the reformulation of our products to meet new standards, market withdrawal or discontinuation of certain products not able to be reformulated. The risk of a product recall exists within the industry although we endeavor to minimize the risk of recalls by distributing products that are not adulterated or misbranded. However, the decision to initiate a recall is often made for business reasons in order to avoid confrontation with the FDA.
Our products are required to be prepared in compliance with cGMPs and 21 CFR Part 111 (also known as the FDA’s “Dietary Supplement Rule”). Fortetropin®, the main ingredient in our products, is also required to be imported into the United States in conformance with APHIS’s requirements for egg products. In the event it is determined that we have not complied with the foregoing requirements, we may be required to initiate a product recall and/or be subject to financial or other penalties. We are continuously monitoring and reviewing our processes to ensure compliance with APHIS and limit the likelihood of potential recalls.
Other statutory obligations include reporting all serious adverse events on a Medwatch Form 3500A. To date, we have not filed a Medwatch Form 3500A with the FDA nor have we been placed on notice regarding any serious adverse events related to any of our products. Since eggs are considered a major food allergen under the Food Allergen Labeling and Consumer Protection Act of 2004, the labeling of all our products must note that they contain an egg product.
Advertising of dietary supplement products is subject to regulation by the FTC under the Federal Trade Commission Act, or FTCA, which prohibits unfair methods of competition and unfair or deceptive trade acts or practices in or affecting commerce. The FTCA provides that the dissemination of any false advertising pertaining to foods, including dietary supplements, is an unfair or deceptive act or practice. Under the FTC’s substantiation doctrine, an advertiser is required to have a reasonable basis for all objective product claims before the claims are made. All advertising is required to be truthful and not misleading. All testimonials are required to be typical of the results the consumer may expect when using the product as directed. Accordingly, we are required to have adequate substantiation of all material advertising claims made for our products. Failure to adequately substantiate claims may be considered either deceptive or unfair practices.
We cannot predict what effect additional domestic or international governmental legislation, regulations, or administrative orders, when and if promulgated, would have on our business in the future. New legislation or regulations may require the reformulation of certain products to meet new standards, require the recall or discontinuance of certain products not capable of reformulation, impose additional record keeping or require expanded documentation of the properties of certain products, expanded or different labeling or scientific substantiation.
21
RISKS RELATED TO OUR COMMON STOCK
Trading in our common stock over the last 12 months has fluctuated, so investors may not be able to sell as many of their shares as they want at prevailing prices.
Our common stock is listed on the Nasdaq Capital Market. There has been a fluctuation in trading of our shares over the last 12 months, but it still may be difficult for investors to sell such shares in the public market at any given time.
Our common stock may be delisted from the Nasdaq Capital Market if we cannot satisfy its continued listing requirements.
Among the conditions required for continued listing on the Nasdaq Capital Market is that we maintain at least $2.5 million in stockholders’ equity. There can be no assurance that our stockholders’ equity will remain above the $2.5 million minimum. If we fail to timely comply with the stockholders’ equity requirement, our common stock may be delisted from the Nasdaq Capital Market. In addition, even if we demonstrate compliance with the stockholders’ equity requirement, we will need to continue to meet other objective and subjective listing requirements to continue to be listed on the Nasdaq Capital Market. Delisting from the Nasdaq Capital Market could make trading our common stock more difficult for investors, potentially leading to declines in our share price and liquidity. Without a Nasdaq Capital Market listing, stockholders may have a difficult time getting a quote for the sale or purchase of our common stock, the sale or purchase of our common stock would likely be made more difficult and the trading volume and liquidity of our stock could decline. Delisting from the Nasdaq Capital Market could also result in negative publicity and could also make it more difficult for us to raise additional capital. The absence of such a listing may adversely affect the acceptance of our common stock as currency or the value accorded by other parties. Further, if we are delisted, we would be required to incur additional costs under state blue sky laws in connection with any sale of our securities. These requirements could severely limit the market liquidity of our common stock and the ability of our stockholders to sell our common stock in the secondary market. If our common stock is delisted from the Nasdaq Capital Market, our common stock may be eligible to trade on an over-the-counter quotation system, such as the OTCQB market, where an investor may find it more difficult to sell our stock or obtain accurate quotations as to the market value of our common stock. We cannot assure you that our common stock, if delisted from the Nasdaq Capital Market, will be listed on another national securities exchange or quoted on an over-the-counter quotation system.
If the Nasdaq Capital Market delists our shares of common stock from trading on its exchange and we are not able to list our securities on another national securities exchange, we expect our securities could be quoted on an over-the-counter market. If this were to occur, we could face significant material adverse consequences, including:
| ● | a limited availability of market quotations for our securities; | |
| ● | reduced liquidity for our shares; | |
| ● | a determination that our common stock is a “penny stock” which will require brokers trading in our common stock to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our shares; | |
| ● | a limited amount of news and analyst coverage; and | |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future. |
An active and visible trading market for our common stock may not develop.
We cannot predict whether an active market for our common stock will develop in the future. In the absence of an active trading market:
| ● | investors may have difficulty buying and selling our common stock or obtaining market quotations; | |
| ● | market visibility for our common stock may be limited; and | |
| ● | a lack of visibility for our common stock may have a depressive effect on the market price for our common stock. |
The trading price of our common stock is expected to be subject to significant fluctuations in response to variations in quarterly operating results, changes in analysts’ earnings estimates, announcements of innovations by us or our competitors, general conditions in the industry in which we operate and other factors. These fluctuations, as well as general economic and market conditions, may have a material or adverse effect on the market price of our common stock.
The market price for our stock may be volatile.
The market price for our stock may be volatile and subject to wide fluctuations in response to factors including the following:
| ● | actual or anticipated fluctuations in our quarterly operating results; | |
| ● | changes in financial estimates by securities research analysts; |
22
| ● | conditions in nutritional supplement markets; | |
| ● | changes in the economic performance or market valuations of other nutritional supplement companies; | |
| ● | announcements by us or our competitors of new products, acquisitions, strategic partnerships, joint ventures or capital commitments; | |
| ● | addition or departure of key personnel; | |
| ● | intellectual property prosecution or other litigation; and | |
| ● | general economic or political conditions. |
In addition, the securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our stock.
Our stockholders may experience significant dilution if future equity offerings are used to fund operations or acquire complementary businesses or as a result of the issuance of a substantial number of shares of common stock upon the exercise of outstanding options and warrants.
If our future operations or acquisitions are financed through the issuance of equity securities, our stockholders could experience significant dilution. In addition, securities issued in connection with future financing activities or potential acquisitions may have rights and preferences senior to the rights and preferences of our common stock. We have also reserved 850,000 shares of our common stock under an equity incentive plan for our directors, officers, employees, consultants and advisors and granted options to purchase shares of our common stock under the plan. The issuance of shares of our common stock upon the exercise of these options as well as upon the exercise of outstanding warrants to purchase up to 821,202 shares of our common stock, which includes a warrant to purchase 375,000 shares of common stock previously issued to RENS Technology Inc., may result in significant dilution to our stockholders.
Mr. Ren can exert significant influence over us and make decisions that are not in the best interests of all stockholders.
Mr. Ren and his affiliates currently beneficially own approximately 27% of our outstanding shares of common stock. As a result, he is able to assert significant influence over all matters requiring stockholder approval, including the election and removal of directors and any change in control. In particular, this concentration of ownership of our outstanding shares of common stock could have the effect of delaying or preventing a change in control, or otherwise discouraging or preventing a potential acquirer from attempting to obtain control. This, in turn, could have a negative effect on the market price of our common stock. It could also prevent our stockholders from realizing a premium over the market prices for their shares of common stock. In addition, we are currently involved in litigation with Mr. Ren and RENS Technology Inc. See “Business – Legal Proceedings” for additional information regarding the litigation. Moreover, the interests of the owners of this concentration of ownership may not always coincide with our interests or the interests of other stockholders and, accordingly, could cause us to enter into transactions or agreements that we would not otherwise consider.
Compliance with changing corporate governance regulations and public disclosure, and our management’s inexperience with such regulations, will result in additional expenses and creates a risk of non-compliance.
Our reporting obligations as a public company will place a significant strain on our management, operational and financial resources and systems for the foreseeable future. Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 and related SEC regulations, have created uncertainty for public companies and significantly increased the costs and risks associated with accessing the public markets and public reporting. Our management team will need to invest significant time and financial resources to comply with both existing and evolving standards for public companies, which will lead to increased general and administrative expenses and a diversion of management time and attention from revenue generating activities to compliance activities.
We do not foresee paying cash dividends in the foreseeable future and, as a result, our investors’ sole source of gain, if any, will depend on capital appreciation, if any.
We do not plan to declare or pay any cash dividends on our shares of common stock in the foreseeable future and currently intend to retain any future earnings for funding growth. As a result, investors should not rely on an investment in our securities if they require the investment to produce dividend income. Capital appreciation, if any, of our shares may be investors’ sole source of gain for the foreseeable future. Moreover, investors may not be able to resell their common stock at or above the price they paid for them.
23
Provisions in our charter documents, the shareholder rights plan we have adopted, and under Nevada law could discourage a takeover that stockholders may consider favorable.
Our articles of incorporation provides for the authorization to issue up to 500,000 shares of blank check preferred stock with designations, rights and preferences as may be determined from time to time by our board of directors. Our board of directors is empowered, without stockholder approval, to issue a series of preferred stock with dividend, liquidation, conversion, voting or other rights which could dilute the interest of, or impair the voting power of, our common stockholders. The issuance of a series of preferred stock could be used as a method of discouraging, delaying or preventing a change in control. For example, it would be possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of our company. In addition, we have a classified board of directors that consists of three groups, which may increase the length of time necessary for an acquirer to change the composition of a majority of directors to gain control of our board of directors.
We have also adopted a shareholder rights plan that could make it more difficult for a third party to acquire, or could discourage a third party from acquiring, us or a large block of our common stock. A third party that acquires 10% or more of our common stock could suffer substantial dilution of its ownership interest under the terms of the shareholder rights plan through the issuance of our shares to all stockholders other than the acquiring person. These and other provisions in our articles of incorporation and bylaws could make it more difficult for stockholders or potential acquirers to obtain control of our board of directors or initiate actions that are opposed by our then-current board of directors, including a merger, tender offer, or proxy contest involving our company. Any delay or prevention of a change of control transaction or changes in our board of directors could cause the market price of our common stock to decline.
Provisions of Nevada corporate law limit the personal liability of corporate directors and officers and require indemnification under certain circumstances.
Section 78.138(7) of the Nevada Revised Statutes provides that, subject to certain very limited statutory exceptions or unless the articles of incorporation provide for greater individual liability, a director or officer of a Nevada corporation is not individually liable to the corporation or its stockholders for any damages as a result of any act or failure to act in his or her capacity as a director or officer, unless it is proven that the act or failure to act constituted a breach of his or her fiduciary duties as a director or officer and such breach involved intentional misconduct, fraud or a knowing violation of law. We have not included in our articles of incorporation any provision intended to provide for greater liability as contemplated by this statutory provision.
In addition, Section 78.7502(3) of the Nevada Revised Statutes provides that to the extent a director or officer of a Nevada corporation has been successful on the merits or otherwise in the defense of certain actions, suits or proceedings (which may include certain stockholder derivative actions), the corporation shall indemnify such director or officer against expenses (including attorneys’ fees) actually and reasonably incurred by such director or officer in connection therewith.
If securities or industry analysts do not publish research or reports about our business, or if they change their recommendations regarding our stock adversely, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. We do not currently have and may never obtain significant research coverage by industry or financial analysts. If few analysts commence coverage of us, the trading price of our stock would likely decrease. Even if we do obtain significant analyst coverage, if one or more of the analysts who cover us downgrade our stock, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
A failure of our internal control over financial reporting could materially impact our business or share price.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. An internal control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all internal control systems, internal control over financial reporting may not prevent or detect misstatements. Any failure to maintain an effective system of internal control over financial reporting could limit our ability to report our financial results accurately and timely or to detect and prevent fraud, and could expose us to litigation or adversely affect the market price of our common stock.
24
RISKS RELATED TO OUR FUTURE PRODUCTS
The research and development of pharmaceutical products, which is separate from nutritional supplements, entails special considerations and risks. If we are successful in developing pharmaceutical products for muscular disorders, we will be subject to, and possibly adversely affected by, the following risks:
Our failure to obtain costly government approvals, including required FDA approvals, or to comply with ongoing governmental regulations relating to our technologies and proposed products and formulations could delay or limit introduction of our proposed formulations and products and result in failure to achieve revenues or maintain our ongoing business.
Our research and development activities for our products and product candidates are currently at an early development stage and are subject to extensive regulation for safety, efficacy and quality by numerous government authorities in the United States and abroad. Before receiving FDA regulatory clearance to market our future proposed formulations and products, we will have to demonstrate that our formulations and products are safe and effective in the patient population and for the indicated diseases that are to be treated. Clinical trials, manufacturing and marketing of drugs are subject to the rigorous testing and approval process of the FDA and equivalent foreign regulatory authorities such as the European Medicines Agency (EMA). The Federal Food, Drug and Cosmetic Act and other federal, state and foreign statutes and regulations govern and influence the testing, manufacturing, labeling, advertising, distribution and promotion of drugs and medical devices. As a result, regulatory approvals can take a number of years or longer to accomplish and require the expenditure of substantial financial, managerial and other resources.
Conducting and completing the clinical trials necessary for FDA approval is costly and subject to intense regulatory scrutiny as well as the risk of failing to meet the primary endpoint of such trials. We will not be able to commercialize and sell our future products and formulations without successfully completing such trials.
In order to conduct clinical trials that are necessary to obtain approval by the FDA to market a formulation or product, it is necessary to receive clearance from the FDA to conduct such clinical trials. The FDA can halt clinical trials at any time for safety reasons or because we or our clinical investigators did not follow the FDA’s requirements for conducting clinical trials. If we are unable to receive clearance to conduct clinical trials or the trials are permanently halted by the FDA, we would not be able to achieve any revenue from such product as it is illegal to sell any drug or medical device for human consumption or use without FDA approval.
Data obtained from clinical trials are susceptible to varying interpretations, which could delay, limit or prevent regulatory clearances.
Data we may obtain in the future, from non-clinical studies and clinical trials do not necessarily predict the results that will be obtained from later-stage non-clinical studies and clinical trials. Moreover, non-clinical and clinical data are susceptible to multiple and varying interpretations, which could delay, limit or prevent regulatory approval. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials, even after promising results in earlier trials. The failure to adequately demonstrate the safety and effectiveness of a proposed formulation or product under development could delay or prevent regulatory clearance of the product candidate, resulting in delays to commercialization, and could materially harm our business. In addition, our clinical trials may not demonstrate sufficient levels of safety and efficacy necessary to obtain the requisite regulatory approvals for our drugs, and thus our proposed drugs may not be approved for marketing. Finally, if any of our clinical trials do not meet their primary endpoints, we would need to repeat such clinical trials in order to progress the development of the investigational drug candidate. These additional trials would be costly and divert resources from other projects.
Competitors may develop competing technologies or products which outperform or supplant our technologies or products.
Drug companies and/or other technology companies may in the future seek to develop and market pharmaceutical products which may compete with our future technologies and products. Competitors may in the future develop similar or different technologies or products which may become more accepted by the marketplace or which may supplant our technology entirely. In addition, many of our future competitors may be significantly larger and better financed than we are, thus giving them a significant advantage over us.
We may be unable to respond to competitive forces presently in the marketplace (including competition from larger companies), which would severely impact our business. Moreover, should competing or dominating technologies or products come into existence and the owners thereof patent the applicable technological advances, we could also be required to license such technologies in order to continue to manufacture, market and sell our products. We may be unable to secure such licenses on commercially acceptable terms, or at all, and our resulting inability to manufacture, market and sell the affected products could have a material adverse effect on us.
The market for our product candidates is rapidly changing and competitive, and new drug delivery mechanisms, drug delivery technologies, new drugs and new treatments which may be developed by others could impair our ability to maintain and grow our business and remain competitive.
Even if successfully developed, our product candidates may not gain market acceptance among physicians, patients and healthcare payers, which may not utilize our products. If our product candidates do not achieve market acceptance, our business and financial condition will be materially adversely affected. The pharmaceutical industry is subject to rapid and substantial technological change. Developments by others may render our technologies and our product candidates noncompetitive or obsolete, or we may be unable to keep pace with technological developments or other market factors. Technological competition from pharmaceutical and biotechnology companies, universities, government entities and others now existing or diversifying into the field is intense and is expected to increase. Many of these entities have significantly greater research and development capabilities, human resources and budgets than we do, as well as substantially more marketing, manufacturing, financial and managerial resources. These entities represent significant competition for us. Acquisitions of, or investments in, competing pharmaceutical or biotechnology companies by large corporations could increase such competitors’ financial, marketing, manufacturing and other resources.
25
The market for our future products is rapidly changing and competitive, and new drug delivery mechanisms, drug delivery technologies, new drugs and new treatments which may be developed by others could impair our ability to maintain and grow our business and remain competitive.
Even if successfully developed, our future products may not gain market acceptance among physicians, patients and healthcare payers, which may not utilize our products. If our future products do not gain market acceptance, our business and financial condition will be materially adversely affected. The pharmaceutical industry is subject to rapid and substantial technological change. Developments by others may render our technologies and our product candidates noncompetitive or obsolete, or we may be unable to keep pace with technological developments or other market factors. Technological competition from pharmaceutical and biotechnology companies, universities, governmental entities and other entities now existing or diversifying into the field is intense and is expected to increase. Many of these entities have significantly greater research and development capabilities, human resources and budgets than we do, as well as substantially more marketing, manufacturing, financial and managerial resources. These entities represent significant competition for us. Acquisitions of, or investments in, competing pharmaceutical or biotechnology companies by large corporations could increase such competitors’ financial, marketing, manufacturing and other resources.
| Item 1B. | Unresolved Staff Comments. |
Not applicable.
| Item 2. | Properties. |
We do not own any real estate or other physical properties materially important to our operation. Our executive office is located at 45 Horsehill Road, Suite 106, Cedar Knolls, New Jersey 07927. Our office space consists of 5,225 square feet. The lease expires on December 31, 2019. We have two options to renew our lease for an additional three years each. We consider our current office space adequate for our current operations. For additional information refer to Part IV, Item 15, “Notes to Consolidated Financial Statements: Note 12 – Commitments and Contingencies.”
| Item 3. | Legal Proceedings. |
On October 27, 2016, Cutler Holdings, L.L.C. (“Cutler”) filed a complaint in the Superior Court of New Jersey alleging that the Company failed to make certain rental payments. On March 30, 2017, the Company entered into a settlement agreement with Cutler, pursuant to which Cutler released the Company from any liability for the claims asserted in the complaint.
On January 6, 2017, in connection with the financing contemplated by a securities purchase agreement with RENS Technology Inc. (the “Purchaser”), we commenced an action in the Supreme Court of New York, County of New York (the “Court”), against the Purchaser, RENS Agriculture, the parent company of the Purchaser, and Ren Ren, a principal in both entities and one of our directors, arising from the Purchaser’s breach of the agreement under which the Purchaser agreed to invest an aggregate of $20.25 million in our company in exchange for an aggregate of 3,537,037 shares of our common stock and warrants to purchase an aggregate of 884,259 shares of common stock.
On April 11, 2017, the Court noted that we had demonstrated a likelihood of success on the merits of the breach of contract claim. Thereafter, a hearing was scheduled on the application by the Purchaser to dismiss the complaint and various pre-trial discovery applications by both parties.
In August 2017, the Company amended its complaint repeating most of the initial claims but adding several additional claims against RENS Agriculture, Mr. Ren and two additional Chinese defendants, including a claim against RENS Agriculture for breaching the exclusive distribution agreement, as well as claims against all defendants for theft and misappropriation of our confidential proprietary information and trade secrets, breach of fiduciary duty and duty of loyalty, misappropriation of corporate opportunity, unfair competition and a number of other torts. We are seeking damages and injunctive relief. The Purchaser has filed a motion to dismiss the amended complaint, which is still pending and scheduled for oral argument in April 2018.
On August 16, 2017, the Purchaser commenced an action in the District Court of Clark County in the State of Nevada against us and Joseph Mannello, our then interim Chief Executive Officer, alleging that Mr. Mannello had breached his fiduciary duties and was grossly negligent in managing our company. The action seeks monetary damages and injunctive relief from Mr. Mannello as well as the appointment of a receiver over us. Subsequently, the Purchaser submitted a petition to appoint a receiver and we and Mr. Mannello submitted a motion to dismiss the action, both of which are currently pending and are due to be heard in April 2018. An application on consent to adjourn the hearing date on the receiver application and motion to dismiss is pending.
| Item 4. | Mine Safety Disclosures. |
None.
26
| Item 5. | Market for Registrant’s Common Equity and Related Stockholder Matters and Issuer Purchases of Equity Securities. |
(a) Market Information
Our common stock is listed on the Nasdaq Capital Market under the symbol “MYOS.” The following table sets forth, for the periods indicated, the high and low bid prices for shares of our common stock as reported on the Nasdaq Capital Market:
| Period | High | Low | ||||||
| October 1, 2017 through December 31, 2017 | $ | 1.49 | $ | 1.15 | ||||
| July 1, 2017 through September 30, 2017 | $ | 2.37 | $ | 1.25 | ||||
| April 1, 2017 through June 30, 2017 | $ | 2.83 | $ | 1.77 | ||||
| January 1, 2017 through March 31, 2017 | $ | 6.82 | $ | 1.14 | ||||
| October 1, 2016 through December 31, 2016 | $ | 1.80 | $ | 1.12 | ||||
| July 1, 2016 through September 30, 2016 | $ | 2.28 | $ | 1.35 | ||||
| April 1, 2016 through June 30, 2016 | $ | 2.94 | $ | 1.23 | ||||
| January 1, 2016 through March 31, 2016 | $ | 2.40 | $ | 1.23 | ||||
These bid prices were obtained from the Nasdaq Capital Market and do not necessarily reflect actual transactions, retail markups, mark downs or commissions.
As of March 26, 2018, the closing price of our shares on the NASDAQ Capital Market was $1.29.
(b) Holders
The Company had approximately 324 record holders of the common stock as of March 26, 2018. This does not include an indeterminate number of stockholders whose shares may be held by brokers in street name. The holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. Holders of the common stock have no preemptive rights and no right to convert their common stock into any other securities. There are no redemption or sinking fund provisions applicable to the common stock.
Our independent stock transfer agent is Island Stock Transfer which is located at 15500 Roosevelt Boulevard, Suite 301, Clearwater, Florida 33760.
(c) Dividends
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain future earnings, if any, for development of our business and therefore do not anticipate that we will declare or pay cash dividends on our capital stock in the foreseeable future.
27
(d) Securities Authorized for Issuance under Equity Compensation Plans
The following table indicates shares of common stock authorized for issuance under equity incentive plans as of December 31, 2017:
| Number of securities to be issued upon exercise of outstanding options | Weighted-average exercise price of outstanding options |
Number of securities remaining available for future issuance |
||||||||||
| Plan category | (a) | (b) | (c) | |||||||||
| Equity compensation plans approved by security holders | 531,740 | (1) | $ | 9.46 | 288,260 | |||||||
| Equity compensation plans not approved by security holders | 30,000 | (2) | $ | 32.00 | — | |||||||
| Total | 561,740 | $ | 7.32 | 288,260 | ||||||||
| (1) | Includes 300,000, 59,425, and 87,000 shares of common stock underlying options granted in 2017, 2016 and 2016, respectively, under our 2012 Equity Incentive Plan, which plan was approved by our stockholders on November 20, 2012 and amended on December 18, 2014 and December 21, 2016. |
| (2) | Includes option awards issued to certain current and former directors during 2011-2012 prior to the adoption of the 2012 Equity Incentive Plan. The options provide for annual vesting over three or four year and expire ten years from the respective issuance dates. |
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
Recent Sales of Unregistered Securities
None.
| Item 6. | Selected Financial Data. |
We are a smaller reporting company and therefore, we are not required to provide information required by this Item 6.
28
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
The following discussion and analysis of our results of operations and financial condition should be read in conjunction with our financial statements and related notes appearing elsewhere in this report. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. The actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including but not limited to, those factors which are not within our control. Amounts in this section are in thousands, unless otherwise indicated.
Overview
We were incorporated in the State of Nevada on April 11, 2007. On March 17, 2016, we merged with our wholly-owned subsidiary and changed our name from MYOS Corporation to MYOS RENS Technology Inc. Prior to February 2011, we did not have any operations and did not generate any revenues. In February 2011, we acquired our proprietary active ingredient called Fortetropin®, the first clinically demonstrated natural myostatin reducing agent. Since February 2011, our principal business activities have been focused on deepening our scientific understanding relating to the activity of Fortetropin®, and to leverage this knowledge to strengthen and build our intellectual property estate; developing sales and marketing strategies aimed at expanding our commercial presence; evaluating the value of Fortetropin® in therapeutic markets, including the treatment of sarcopenia, cachexia, anorexia, obesity and muscular disorders; and, conducting research and development focused on the discovery, development and commercialization of other products and technologies aimed at maintaining or improving the health and performance of muscle tissue. Since our inception in April 2007, we have recognized cumulative revenues of approximately $8.2 million.
Plan of Operation
We are focused on the discovery, development and commercialization of nutritional supplements, functional foods, therapeutic products and other technologies aimed at maintaining or improving the health and performance of muscle tissue. Our initial core ingredient is Fortetropin®, a natural, reversible, temporary myostatin reducing agent. Our plan of action is to: (i) create a sales platform through marketing products containing our proprietary ingredient Fortetropin® in established, growing, and new markets and strategic selection of partnerships and collaborations to maximize near-term and future revenues, (ii) deepen the scientific understanding of the activity of Fortetropin®, specifically as a natural, reversible, temporary modulator of the regulatory protein myostatin, and to leverage this knowledge to strengthen and build our intellectual property estate, (iii) conduct research and development activities to evaluate myostatin modulation in a range of both wellness and disease states, (iv) identify other products and technologies which may broaden our portfolio and define a business development strategy to protect, enhance and accelerate the growth of our products, (v) reduce the cost of manufacturing through process improvement, and (vi) identify contract manufacturing organizations that can fully meet our future growth requirements. We believe that myostatin regulation represent a rational entry point for our drug discovery efforts and are evaluating therapeutic targets in this area.
Our commercial focus is to leverage our clinical data to develop multiple products to target the large, but currently underserved, markets focused on muscle health. The sales channels through which we sell our products are evolving. The first product we introduced was MYO-T12, which was sold in the sports nutrition market. MYO T-12 is a proprietary formula containing Fortetropin® and other ingredients. The formula was sold under the brand name MYO T-12 and later as MYO-X through an exclusive distribution agreement with Maximum Human Performance (“MHP”). While the exclusive distribution agreement with MHP terminated in March 2015, MHP continues to distribute its remaining MYO-X inventories on popular retailer websites and in specialty retailers principally in the U.S. There were no sales to MHP in 2016 and 2017. We do not expect any orders from MHP in 2018.
29
In February 2014, we expanded our commercial operations into the age management market through a distribution agreement with Cenegenics Product and Lab Services, LLC (“Cenegenics”), under which Cenegenics distributes a proprietary formulation containing Fortetropin® which it previously purchased from the Company through its age management centers and its community of physicians focused on treating a growing population of patients focused on proactively addressing age-related health and wellness concerns. On November 28, 2014, we entered into a settlement agreement with Cenegenics wherein we agreed to accept $1,900 by April 2016, in full satisfaction of Cenegenics’ outstanding obligations with respect to units of product produced by the Company, including units that had not yet been shipped to Cenegenics at the time of the settlement agreement. During the second quarter of 2015, Cenegenics accepted delivery of the remaining units that we were storing on its behalf. Given the settlement agreement’s extended payment schedule, the Company deferred the revenue and related costs and recorded the revenue and cost of sales as the payments were received in full through April 2016. The distribution agreement with Cenegenics expired in December 2016. In 2017, we recorded sales of $200 to Cenegenics.
During the second quarter of 2015 we launched Rē Muscle HealthTM, our own direct-to-consumer portfolio of muscle health bars, meal replacement shakes and daily supplement powders each powered by a full 6.6 gram single serving dose of Fortetropin®. Our Rē Muscle Health products were sold through our e-commerce website, remusclehealth.com, and amazon.com. As of March 2017 we discontinued actively selling this line of products.
On March 1, 2017 the Company announced the launch of Qurr, its Fortetropin® powered product line formulated to support the vital role of muscle in overall well-being as well as in fitness. The muscle focused over-the-counter (OTC) products will be made available through a direct-to-consumer e-commerce platform. All Qurr products are blended with Fortetropin®, MYOS’ proprietary ingredient which has been clinically demonstrated to reduce serum myostatin levels, which helps increase muscle size and lean body mass. MYOS’ earlier product formulations featuring Fortetropin® have become part of the daily routine of many athletes and fit-conscious people.
Qurr is a line of flavored puddings, powders, and shakes all shown to be safe for daily use. While pharmaceutical companies are working on drugs to accomplish what Fortetropin® already does, there is no approved pharmaceutical drug that can reduce myostatin, safely. Fortetropin® has been shown in clinical trials to reduce serum myostatin levels and increase lean muscle mass and thickness when taken in conjunction with resistance training.
We expect to launch our Fortetropin based pet product in the near future. Two veterinarian hospitals, which performed some informal observational studies with older dogs experiencing muscle atrophy and saw positive results after taking our pet product, are seeking to purchase our product. We believe that the positive feedback we are receiving from these two hospitals, together with the potential results from our Kansas State University study, will enable us to launch and grow our pet business product line.
We continue to pursue additional distribution and branded sales opportunities. There can be no assurance that we will be able to secure distribution arrangements on terms acceptable to the Company, or that we will be able to generate significant sales of our current and future branded products. We expect to continue developing our own core branded products in markets such as functional foods, sports and fitness nutrition and rehabilitation and restorative health and to pursue international sales opportunities.
The Company currently relies on one third-party manufacturer to produce Fortetropin®. This manufacturer purchases all the necessary raw materials from suppliers and coordinates any additional production steps with third-parties. We have multiple vendors for blending, packaging and labeling our products. The Company is pursuing other alternatives in order to build a more robust supply chain going forward. See Risk Factors – “We are dependent on third-party manufacturers, suppliers and processors” for additional information regarding our relationship with our third-party manufacturers.
As an early-stage bionutritional and biotherapeutics company, we are dedicated to basic and clinical research that supports our existing and future product portfolio. Our research program is actively evaluating the many active proteins, lipids and peptides in Fortetropin®, specifically as a natural, reversible, temporary modulator of the regulatory protein myostatin, and to leverage this knowledge to strengthen and build our intellectual property. We are dedicated to protecting our innovative technology and believe that our research programs will establish a basis for the continued prosecution of patent applications in order to protect and augment the Company’s intellectual property estate. We expect our investment in research and development to continue to grow in the future.
30
Results of Operations
Year Ended December 31, 2017 Compared to Year Ended December 31, 2016
| (In thousand $) | Years Ended December 31, | Change | ||||||||||||||
| 2017 | 2016 | Dollars | % | |||||||||||||
| Net sales | $ | 526 | $ | 327 | $ | 199 | 61 | % | ||||||||
| Cost of sales | 308 | 319 | (11 | ) | (3 | )% | ||||||||||
| Gross profit | 218 | 8 | 210 | 2,625 | % | |||||||||||
| Operating expenses: | ||||||||||||||||
| Sales and marketing | 822 | 846 | (24 | ) | (3 | )% | ||||||||||
| Personnel and benefits | 1,450 | 1,853 | (403 | ) | (22 | )% | ||||||||||
| General and administrative | 2,014 | 1,616 | 398 | 25 | % | |||||||||||
| Total operating expenses | 4,286 | 4,315 | (29 | ) | 1 | % | ||||||||||
| Operating loss | (4,068 | ) | (4,307 | ) | 239 | (6 | )% | |||||||||
| Other income (expense), net | 10 | (34 | ) | 44 | (129 | )% | ||||||||||
| Loss before income taxes | (4,058 | ) | (4,341 | ) | 283 | (7 | )% | |||||||||
| Income tax expense | - | - | - | - | ||||||||||||
| Net loss | $ | (4,058 | ) | $ | (4,341 | ) | $ | 283 | (7 | )% | ||||||
Net sales
Net sales for the year ended December 31, 2017 increased $199, or 61%, compared to net sales for the year ended December 31, 2016 primarily due to sale to Cenegencis for $200.
31
Cost of sales and gross profit
Cost of sales for the year ended December 31, 2017 decreased $11, or 3%, compared to cost of sales for the year ended December 31, 2016. The decrease in cost of sales was primarily due to lower costs associated with direct sales to consumers.
Operating expenses
Sales and marketing expenses for the year ended December 31, 2017 decreased $24 or 3%, compared to the year ended December 31, 2016. This was primarily due to the decrease in marketing and promotional costs associated with the development and launch of the Qurr website that was expensed for the year ended December 31, 2016.
Personnel and benefits costs for the year ended December 31, 2017 decreased $403 or 22% primarily due to the departures of certain of our officers in 2016 as well as our Chief Executive Officer accepting payment in stock instead of cash commencing in September 2017.
General and administrative expenses for the year ended December 31, 2017 increased $398, or 25%, compared to the year ended December 31, 2016 primarily due to costs associated with legal proceedings (See Item 3 - Legal Proceedings).
Income tax expense
Income tax expense for the years ended December 31, 2017 and December 31, 2016 was zero, which reflects minimum state corporate taxes.
Liquidity and Capital Resources
Working capital at December 31, 2017 and December 31, 2016 is summarized as follows:
| (In thousand $) | December 31, | December 31, | Increase | |||||||||
| 2017 | 2016 | (Decrease) | ||||||||||
| Current Assets: | ||||||||||||
| Cash | $ | 923 | $ | 1,866 | $ | (943 | ) | |||||
| Accounts receivable, net | 4 | 8 | (4 | ) | ||||||||
| Inventories, net | 1,779 | 1,862 | (83 | ) | ||||||||
| Prepaid expenses and other current assets | 163 | 85 | 78 | |||||||||
| Total current assets | 2,869 | 3,821 | (952 | ) | ||||||||
| Current liabilities: | ||||||||||||
| Accounts payable | 176 | 226 | (50 | ) | ||||||||
| Accrued expenses and other current liabilities | 255 | 417 | (162 | ) | ||||||||
| Total current liabilities | 431 | 643 | (212 | ) | ||||||||
| Working Capital | $ | 2,438 | $ | 3,178 | $ | (740 | ) | |||||
Working capital decreased $740 to $2,438 at December 31, 2017 compared to $3,178 at December 31, 2016.
Changes in working capital components were as follows:
| ● | Cash decreased $943 primarily due to $2,819 of net proceeds received from financing activities, offset by $3,760 used in operating activities and $2 used in investing activities. |
| ● | Accounts receivable, net decreased $4 primarily due to timing of sales collections. |
| ● | Inventories, net decreased $83 primarily due to raw inventory placed into production in 2017 in order to manufacture Qurr and the products sold to Cenegenics. |
| ● | Prepaid expenses and other current assets increased $78, primarily due to an increase in prepaid insurance. |
| ● | Accounts payable decreased $50 primarily due to the timing of payments to vendors. |
| ● | Accrued expenses and other current liabilities decreased $162, primarily due to a decrease of $171 in accrued marketing expense and deferred revenue of $56 offset by an increase of $65 in accrued insurance expense. |
32
At December 31, 2017, we had cash of $923 and total assets of $4,795 (which includes $1,640 of intangible assets, net).
Summarized cash flows for the years ended December 31, 2017 and 2016 are as follows:
| Years Ended December 31, | ||||||||||||
| (In thousand $) | 2017 | 2016 | Change | |||||||||
| Net cash used in operating activities | $ | (3,783 | ) | $ | (3,673 | ) | $ | (110 | ) | |||
| Net cash used in investing activities | (2 | ) | (381 | ) | 379 | |||||||
| Net cash provided by financing activities | 2,842 | 5,041 | (2,199 | ) | ||||||||
| Net increase (decrease) in cash | $ | (943 | ) | $ | 987 | $ | (1,930 | ) | ||||
Net cash used in operating activities represents net loss adjusted for certain non-cash items and changes in operating assets and liabilities. Net cash used in operating activities for the year ended December 31, 2017 increased $110 compared to the year ended December 31, 2016. For additional information about the changes in operating assets and liabilities, refer to the above discussion on working capital and the consolidated statements of cash flow.
Net cash used in investing activities includes cash used to purchase capital assets. Net cash used in investing activities for the year ended December 31, 2017 decreased $379 compared to the year ended December 31, 2016 primarily due to capitalized software costs of $380 in 2016 from the development of the new website launched in 2017.
Net cash provided by financing activities primarily includes proceeds from issuance of common stock. Net cash provided by financing activities for the year ended December 31, 2017 decreased $2,199 compared to the year ended December 31, 2016. For additional information refer to the consolidated statements of cash flows.
Convertible Note
On December 17, 2015, concurrent with the execution of the Purchase Agreement with RENS Technology Inc., the Company issued an unsecured promissory note in the principal amount of $575 (the “Note”) to Gan Ren, a related party of RENS Agriculture. The Note accrued interest at a rate of 8% per annum and matured (the “Maturity Date”) on December 17, 2016. On the Maturity Date, the Note and the accrued interest of $46 were automatically converted into 225,864 shares of Common Stock at $2.75 per share.
Term Note
On September 10, 2015, the Company converted its outstanding revolving note with City National Bank, which had a termination date of August 31, 2015, into a term note (the “Term Note”). At December 31, 2015, the balance under the Term Note was $100, which was subsequently paid in full in January 2016.
Additional Financings
We may seek to raise additional capital through the issuance of debt or equity securities. Should the Company seek additional debt and/or equity financing, it cannot assure that such financing will be available on acceptable terms, if at all.
Going Concern Uncertainty
As of the filing date of this Report, management believes that there may not be sufficient capital resources from operations and existing financing arrangements in order to meet operating expenses and working capital requirements for the next twelve months, primarily due to the failure of RENS Technology Inc. to fund the required amounts. These facts raise substantial doubt about the Company’s ability to continue as a going concern. Accordingly, we are evaluating various alternatives, including reducing operating expenses, securing additional financing for future business activities and other strategic alternatives. There can be no assurance that the Company will be able to generate the level of operating revenues in its business plan, or if additional sources of financing will be available on acceptable terms, if at all. If no additional sources of financing are available, our future operating prospects may be adversely affected.
33
Registered Direct Offering
On February 3, 2017, the Company entered into a securities purchase agreement with an institutional investor providing for the issuance and sale by the Company of 500,000 shares of common stock, par value in a registered direct offering at a purchase price of $4.25 per share, for gross proceeds of $2.125 million. The offering closed on February 8, 2017.
Preferred Stock Purchase Rights
Effective February 14, 2017, the Board of Directors declared a dividend of one right for each of the Company’s issued and outstanding shares of common stock. The dividend was paid to the stockholders of record at the close of business on February 24, 2017. Each Right entitles the registered holder, subject to the terms of the Rights Agreement to purchase from the Company one one-thousandth of a share of the Company’s Series A Preferred Stock at a price of $7.00), subject to certain adjustments. The description and terms of the Rights are set forth in the Rights Agreement dated as of February 14, 2017 between the Company and Island Stock Transfer, as Rights Agent.
At-the-market Offering
On February 21, 2017, the Company entered into a sales agreement with H.C. Wainwright & Co., LLC establishing an at-the-market equity program pursuant to which we may offer and sell up to $6.0 million of our shares of common stock from time to time through H.C. Wainwright. The sales agreement terminates upon the sale of all the shares unless terminated earlier by one of the parties.
On October 26, 2017, the Company sold 500,000 shares of common stock for $2.144 per share for gross proceeds of $1,070 in an at-the-market offering.
Subsequent to year end, on January 19, 2018, the Company sold 140,295 shares of common stock for $2.111 per share for gross proceeds of $287 in an at-the-market offering.
Long-term Contractual Obligations
As of December 31, 2017, the Company’s enforceable and legally binding contractual obligations include future minimum lease payments under a non-cancellable operating lease and purchase obligations under a long-term supply agreement.
At December 31, 2017, the future minimum lease payments under the non-cancellable operating lease in excess of one year were as follows:
| (In thousand $) | ||||
| Years Ended December 31, | Amount | |||
| 2018 | $ | 71 | ||
| 2019 | 72 | |||
| Total | $ | 143 | ||
For additional information about the operating lease refer to PART IV, Item 15, “Notes to Consolidated Financial Statements: Note 12 – Commitments and Contingencies – Operating Lease.”
On November 18, 2016, the Company entered into an Amended Supply Agreement with DIL Technologie GmbH (“DIL”). Pursuant to the agreement (and so long as the agreement is effective), DIL will manufacture and supply the Company with Fortetropin®, the active ingredient for its products, and the Company will purchase quantities of Fortetropin® from DIL at its discretion. DIL will manufacture the formula exclusively for the Company in perpetuity, and may not manufacture the formula for other entities (but may manufacture it for its own non-commercial research).
The Company agreed, commencing January 2017, to pay DIL €10 (approximately USD $12) per month for collaborative research. For the year ended December 31, 2017 we paid DIL USD $194 which is recorded as sales and marketing expense on the consolidated statement of operations. The monthly payments terminate upon the earlier of: (a) the date that the Company orders additional product in accordance with the terms of the agreement and (b) December 31, 2018, and the Company has no further financial obligations to DIL thereafter.
The Company also agreed to pay DIL €400 (approximately USD $480) in satisfaction of all prior liabilities and obligations under its prior agreements with DIL. The agreement expires on December 31, 2018, and the Company has the unilateral right to renew the agreement for subsequent one-year terms.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
34
Recent Accounting Pronouncements
In September 2017, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2017-13, Revenue from Contracts with Customers which amended FASB Accounting Standards Codification® (ASC) by creating Topic 606, Revenue from Contracts with Customers. In May 2014, the FASB issued ASU No. 2014-09, “Revenue from Contracts with Customers” (“ASU 2014-09”). ASU 2014-09 supersedes nearly all existing revenue recognition guidance under U.S. GAAP and requires revenue to be recognized when promised goods or services are transferred to customers in an amount that reflects the consideration that is expected to be received for those goods or services. Additionally, qualitative and quantitative disclosures are required about customer contracts, significant judgments and changes in judgments, and assets recognized from the costs to obtain or fulfill a contract.
The FASB also issued the following amendments to ASU No. 2014-09 to provide clarification on the guidance:
| ● | ASU No. 2015-14, Revenue from Contracts with Customers (Topic 606) – Deferral of the Effective Date | |
| ● | ASU No. 2016-08, Revenue from Contracts with Customers (Topic 606) – Principal versus Agent (Reporting Revenue Gross vs. Net) | |
| ● | ASU No. 2016-10, Revenue from Contracts with Customers (Topic 606) – Identifying Performance Obligations and Licensing | |
| ● | ASU No. 2016-12, Revenue from Contracts with Customers (Topic 606) – Narrow-Scope Improvements and Practical Expedients |
The adoption of Topic 606 is required for public entities for reporting periods beginning after December 15, 2017. This accounting guidance is effective for us beginning January 1, 2018 using one of two prescribed transition methods. The Company has evaluated the impact of the updated guidance and has determined that the adoption of ASU 2017-09 is not expected to have a significant impact on its consolidated financial statements for 2016 and 2017 and related disclosure.
The Company will adopt the provisions of this ASU for its fiscal year beginning January 1, 2018 using the modified retrospective transition method. This method involves application of the new guidance to either: (a) all contracts at the date of initial application or (b) only contracts that are not completed at the date of initial application. Under this method, a cumulative effect adjustment is recognized as of the date of initial application.
In May 2017, the FASB issued ASU No. 2017-09, Compensation – Stock Compensation (Topic 718). The amendments in this Update provide guidance about which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting in Topic 718. This update is effective for all entities for annual periods, and interim periods within those annual periods, beginning after December 15, 2017. Early adoption is permitted, including adoption in any interim period, for (1) public business entities for reporting periods for which financial statements have not yet been issued and (2) all other entities for reporting periods for which financial statements have not yet been made available for issuance. The amendments in this update should be applied prospectively to an award modified on or after the adoption date. The Company has evaluated the impact of the updated guidance and has determined that the adoption of ASU 2017-09 is not expected to have a significant impact on its consolidated financial statements.
In January 2017, the FASB issued ASU No. 2017-04, “Simplifying the Test for Goodwill”, which accomplishes exactly what its title indicates by eliminating the second step in the current goodwill impairment calculation. Currently there is a two-step process for determining the amount of any goodwill impairment. In Step 1, an entity determines if the carrying value of the reporting unit (for which goodwill has been recorded) exceeds the fair value of the reporting unit. If the calculation in Step 1 indicates that the carrying value of a reporting unit for which goodwill has been recorded exceeds the fair value, the entity would have to determine the implied fair value of the reporting unit’s goodwill. An impairment would be recorded to the extent that the goodwill carrying value exceeded the implied fair value of goodwill at the reporting date. The amount of any goodwill impairment must take into consideration the effects of income taxes for any tax deductible goodwill. The effective date to adopt the ASU is for fiscal years beginning after December 15, 2019. The ASU is to be applied prospectively. Early adoption is permitted. The Company has evaluated the impact of the updated guidance and has determined that the adoption of ASU 2017-04 is not expected to have a significant impact on its consolidated financial statements.
In August 2016, the FASB issued ASU 2016-15, “Classification of Certain Cash Receipts and Cash Payments (a consensus of the Emerging Issues Task Force).” The amendments in this Update relate to eight specific types of cash receipts and cash payments which current U.S. GAAP either is unclear or does not include specific guidance on the cash flow classification issues. The amendments in this Update are effective for public business entities for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. The Company has adopted the provisions of this ASU for its fiscal year beginning January 1, 2018. The adoption of ASU 2016-15 did not have a significant impact on its consolidated financial statements.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842) (“ASU 2016-02”), which requires lessees to recognize on the balance sheet the assets and liabilities for the rights and obligations created by leases with lease terms of more than 12 months. The recognition, measurement, and presentation of expenses and cash flows arising from a lease by a lessee will continue to primarily depend on its classification as a finance or operating lease. However, unlike U.S. GAAP, which requires only capital leases to be recognized on the balance sheet, ASU 2016-02 will require both types of leases to be recognized on the balance sheet. ASU 2016-02 also requires disclosures about the amount, timing, and uncertainty of cash flows arising from leases. These disclosures include qualitative and quantitative requirements, providing additional information about the amounts recorded in the financial statements. ASU 2016-02 is effective beginning January 1, 2019, with early application permitted. We have evaluated the adoption of ASU 2016-12 and determined that the standard will not have a significant impact on our consolidated financial statements.
35
In July 2015, the FASB issued ASU No. 2015-11, Inventory (Topic 330), “Simplifying the Measurement of Inventory” (“ASU 2015-11”), which changes the measurement principle for inventory from the lower of cost or market to the lower of cost and net realizable value. ASU 2015-11 defines net realizable value as estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The new guidance must be applied on a prospective basis by us beginning January 1, 2017, with early adoption permitted. The adoption of ASU 2015-17 did not have a significant impact on the consolidated financial statements.
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements – Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern” (“ASU 2014-15”). The amendments in this update define management’s responsibility to evaluate whether there is substantial doubt about an organization’s ability to continue as a going concern and provides related footnote disclosure requirements. Under U.S. GAAP, financial statements are prepared under the presumption that the reporting organization will continue to operate as a going concern, except in limited circumstances. Financial reporting under this presumption is commonly referred to as the going concern basis of accounting. The going concern basis of accounting establishes the fundamental basis for measuring and classifying assets and liabilities. This update provides guidance on when there is substantial doubt about an organization’s ability to continue as a going concern and how the underlying conditions and events should be disclosed in the footnotes. It is intended to reduce diversity that existed in footnote disclosures because of the lack of guidance about when substantial doubt existed. The amendments in this update are effective for us beginning December 31, 2016. The Company has evaluated the impact of the updated guidance and has disclosed the impact in the footnotes on its consolidated financial statements.
Critical Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, equity and the disclosures of contingent assets and liabilities at the date of the financial statement and the reported amounts of revenues and expenses during the reporting period. Making estimates requires management to exercise significant judgment. It is possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future non-conforming events. Accordingly, the actual results could differ significantly from estimates. Significant items subject to such estimates include but are not limited to the valuation of stock-based awards, measurement of allowances for doubtful accounts and inventory reserves, the deferred tax asset valuation, the selection of asset useful lives, fair value estimations used to test long-lived assets, including intangibles, impairments and provisions necessary for assets and liabilities.
The Company has recorded minimal sales to its distributors during the past fourteen consecutive quarters, and launched its Qurr branded products in March 2017. Management’s estimates, including evaluation of impairment of long-lived assets and inventory reserves are based in part on forecasted future results. A variety of factors could cause actual results to differ from forecasted results and these differences could have a significant effect on asset carrying amounts.
Concentrations of Credit Risk
Management regularly reviews accounts receivable, and if necessary, establishes an allowance for doubtful accounts that reflects management’s best estimate of amounts that may not be collectible based on historical collection experience and specific customer information. Expense recognized as a result of an allowance for doubtful accounts is classified under selling, general and administrative expenses in the statements of operations.
As part of our ongoing liquidity assessments, management evaluates our cash and cash equivalents. The amount of funds held in the bank can fluctuate due to the timing of receipts and payments during the ordinary course of business and other reasons, such as business-development activities. As a result, the Company may have exposure to cash in excess of FDIC insured limits.
Fair Value of Long-Lived Assets
We test long-lived assets, including fixed assets and intangibles with finite lives, for recoverability when events or changes in circumstances indicate that the net carrying amount is greater than its fair value. Assets are grouped and evaluated at the lowest level for their identifiable cash flows that are largely independent of the cash flows of other groups of assets. We consider historical performance and future estimated results in our evaluation of potential impairment and then compare the carrying amount of the asset to the future estimated cash flows expected to result from the use of the asset. If the carrying amount of the asset exceeds estimated expected undiscounted future cash flows, we measure the amount of impairment by comparing the carrying amount of the asset to its fair value. The estimation of fair value is generally measured by discounting expected future cash flows at the rate we utilize to evaluate potential investments. We estimate fair value based on the information available in making the necessary estimates, judgments and projections.
36
Impairment testing of intangible assets subject to amortization involves comparing the carrying amount of the asset to the forecasted, undiscounted future cash flows whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. In the event the carrying value of the asset exceeds the undiscounted future cash flows, the carrying value is considered not recoverable and an impairment exists. An impairment loss is measured as the excess of the asset’s carrying value over its fair value, calculated using a discounted future cash flow method. The computed impairment loss is recognized in the period that the impairment occurs. Assets which are not impaired may require an adjustment to the remaining useful lives for which to amortize the asset. Impairment testing requires the development of significant estimates and assumptions involving the determination of estimated net cash flows, selection of the appropriate discount rate to measure the risk inherent in future cash flow streams, assessment of an asset’s life cycle, competitive trends impacting the asset as well as other factors. Changes in these underlying assumptions could significantly impact the asset’s estimated fair value.
Share-based Compensation
Generally, share-based payments are measured at their estimated fair value on the date of grant. Stock-based awards to non-employees are re-measured at fair value each financial reporting date until performance is completed. Share-based compensation expense recognized during a period is based on the estimated number of awards that are ultimately expected to vest. For stock options and restricted stock that do not vest immediately but which contain only a service vesting feature, we recognize compensation cost on the unvested shares and options on a straight-line basis over the remaining vesting period.
The Company uses the Black-Scholes option-pricing model to estimate the fair value of options and the market price of our common stock on the date of grant for the fair value of restricted stock issued. Our determination of the fair value of stock-based awards is affected by our stock price as well as assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to our expected stock price volatility over the term of the awards, and certain other market variables such as the risk-free interest rate.
Income Taxes
Income taxes are accounted for under the asset and liability method in accordance with ASC 740, Accounting for Income Taxes (“ASC 740”). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial carrying amounts of existing assets and liabilities and their respective tax bases as well as operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the periods in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Deferred tax assets are reduced by a valuation allowance to the extent that the recoverability of the asset is unlikely to be recognized. The Company follows ASC 740 rules governing uncertain tax positions, which provides guidance for recognition and measurement. This prescribes a threshold condition that a tax position must meet for any of the benefits of the uncertain tax position to be recognized in the financial statements. It also provides accounting guidance on recognition, classification and disclosure of these uncertain tax positions. The Company has no uncertain income tax positions.
The Tax Cut and Jobs Act (the “Tax Act”) was enacted on December 22, 2017. The Tax Act contains several key provisions including, among other things, reducing the U.S. federal corporate tax rate from thirty-five percent to twenty-one percent. Changes in tax law are accounted for in the period of enactment. In addition, Federal net operating losses (“NOL”) generated during future periods will be carried forward indefinitely, but will be subject to an eighty percent utilization against taxable income. The carryback provision has been revoked for NOL after January 1, 2018.
The Company continues to evaluate the impact of the Tax Act and analyze additional guidance.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk. |
We are a smaller reporting company, and therefore, we are not required to provide information required by this Item 7A.
| Item 8. | Financial Statements and Supplemental Data. |
The Company’s financial statements for the fiscal years ended December 31, 2017, and 2016 have been examined to the extent indicated in their reports by our independent registered accountants and have been prepared in accordance with U.S. GAAP pursuant to regulations promulgated by the SEC. The aforementioned financial statements are included herein under Item 15.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure. |
On May 19, 2016, the audit committee and the board of directors approved the engagement of WithumSmith+Brown, PC (“Withum”) as the Company’s new independent registered public accounting firm, effective immediately. During the fiscal years ended December 31, 2015 and 2014 and through May 19, 2016, neither the Company nor anyone acting on its behalf consulted Withum with respect to (ii) the application of accounting principles to a specified transaction, either completed or proposed, nor the type of audit opinion that might be rendered on the Company’s financial statements, and neither a written report was provided to the Company nor oral advice provided that Withum concluded was an important factor considered by the Company in reaching a decision as to any accounting, auditing or financial reporting issue; or (ii) any matter that was the subject of a disagreement or a “reportable event” as described in Items 304(a)(1)(iv) and (v), respectively, of Regulation S-K.
37
| Item 9A. | Controls and Procedures. |
Evaluation of Disclosure Controls and Procedures
Our management is responsible for establishing and maintaining a system of disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) that is designed to provide reasonable assurance that information we are required to disclose in the reports that we file or submit under the Exchange Act are recorded, processed, summarized and reported, within the time periods specified in the Commission’s rules and forms. Disclosure controls and procedure include, without limitations, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is accumulated and communicated to the issuer’s management, including its principal executive officer or officers and principal financial officer or officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
In accordance with Exchange Act Rules 13a-15 and 15d-15, an evaluation was completed by our Principal Executive Officer and Principal Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered by this Annual Report. Based on that evaluation, management concluded that, due to a material weakness in our internal control over financial reporting as noted below, our disclosure controls and procedures were not effective. We intend to implement remedial measures designed to address the material weakness.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) promulgated under the Securities Exchange Act of 1934 as a process designed by, or under the supervision of, our principal executive officer and principal financial officer and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America and includes those policies and procedures that:
| ● | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
| ● | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America and that our receipts and expenditures are being made only in accordance with authorizations of our management and board of directors; and |
| ● | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Because of the inherent limitations of internal control, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
Our management assessed the effectiveness of our internal control over financial reporting based on the criteria for effective internal control over financial reporting established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and SEC guidance on conducting such assessments. Based on management’s assessment using this framework, management concluded that, as of December 31, 2017, our internal control over financial reporting was not effective. This is due to the lack of segregation of duties within our accounting and finance group as a result of our limited financial resources, which may be considered a material weakness. We plan to remediate this weakness, primarily through supplementing our accounting and finance staff with an outside financial expert to review our financial statements and periodic reports.
This Report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to rules of the SEC that permit us to provide only the management’s report in this annual report.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) during the most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other Information. |
None.
38
| Item 10. | Directors and Executive Officers and Corporate Governance. |
Our directors and executive officers are as follows:
| Name | Age | Position | Class | |||
| Dr. Robert J. Hariri | 59 | Chairman of the Board of Directors | I | |||
| Ren Ren | 56 | Director (Global Chairman) | I | |||
| Joseph Mannello | 59 | Chief Executive Officer and Director | III | |||
| Dr. Louis J. Aronne | 62 | Director | II | |||
| Christopher Pechock | 53 | Director | II | |||
| Victor Mandel | 53 | Director | III | |||
| John Nosta | 58 | Director | III | |||
| Bin Zhou | 39 | Director | I |
Our Board is classified into three separate classes, as nearly equal in number as possible, with one class to be elected annually for staggered three-year term or until their respective successors are duly elected and qualified, or until their earlier resignation, removal or death.
The term of our current Class III directors will expire at the 2019 Annual Meeting of Stockholders, the term of our current Class II directors will expire at the 2020 Annual Meeting of Stockholders and the term of our current Class I directors will expire at the 2018 Annual Meeting of Stockholders. Any director chosen as a result of a newly created directorship or to fill a vacancy on the Board would hold office for a term expiring at the next Annual Meeting of Stockholders for the class identified. This does not change the present number of directors or the Board’s authority to change that number and to fill any vacancies or newly created directorships.
The experience of each or our directors and executive officers is as follows:
Dr. Robert J. Hariri joined us as a Director in July 2011 and was elected Chairman of the Board in April 2012. Dr. Hariri is currently the CEO and Founder of Celularity, Inc., a private company based in Warren, NJ. Dr. Hariri has served as the chairman and chief scientific officer of Celgene Cellular Therapeutics, a division of Celgene Corporation (NASDAQ: CELG), since 2014. From 2002 to 2014, he served in various positions at Celgene Cellular Therapeutics, including chief executive officer and president. Prior to joining Celgene Cellular Therapeutics, Dr. Hariri was founder, chairman and chief scientific officer at Anthrogenesis Corporation/LIFEBANK, Inc., a privately held biomedical technology and service corporation involved in the area of human stem cell therapeutics, which was acquired by Celgene Corporation in 2002. Dr. Hariri also serves as president of Human Longevity Cellular Therapeutics, Inc., a privately-held genomics and cell therapy-based diagnostic and therapeutic company focused on extending the healthy, high performance human life span, which he co-founded in 2013. He has also served as co-founder, vice chairman and chief scientific officer of Neurodynamics, a privately held medical device and technology corporation. Dr. Hariri is an adjunct associate professor of pathology at the Mount Sinai School of Medicine and has also held key academic positions at Weill Medical College of Cornell University and the Cornell University Graduate School of Medical Science, including serving as the director of the Center for Trauma Research. Dr. Hariri is also a director of Cryoport, Inc. (NASDAQ: CYRX), Bionik Laboratories Corp. (OTCQX: BNKL), Provista Diagnostics and Rocket Racing, Inc. Dr. Hariri is a member of the scientific advisory board for the Archon X Prize for Genomics, which is awarded by the X Prize Foundation. Dr. Hariri serves as a trustee of the J. Craig Venter Institute, a trustee of the Liberty Science Center and a commissioner of the New Jersey Commission for Cancer Research. Dr. Hariri received the Thomas Alva Edison Award in 2007 and 2011, The Fred J. Epstein Lifetime Achievement Award in 2012 and numerous other honors for his contributions to biomedicine and aviation. He has served as a member of the board of visitors at Columbia University School of Engineering & Applied Sciences and the Science & Technology Council of the College of Physicians and Surgeons. Dr. Hariri received his undergraduate training at Columbia College and Columbia University School of Engineering and Applied Sciences and was awarded his M.D. and Ph.D. degrees from Cornell University Medical College. Dr. Hariri received his surgical training at The New York Hospital-Cornell Medical Center and directed the Aitken Neurosurgery Laboratory and the Center for Trauma Research. We believe Dr. Hariri’s training as a scientist, his knowledge and experience with respect to the biomedical and pharmaceutical industries and his extensive research and experience qualifies him to serve on our Board of Directors.
39
Ren Ren joined us as a Director (Global Chairman) in March 2016. Mr. Ren has more than 28 years of experiences in China’s food and agricultural business. Since 2001, he formed and operated Beijing Seasons Investment Group Co, Ltd and RENS Agriculture Science and Technology Co, Ltd. Mr. Ren is also chairman of China’s Nutrition and Health Guidance Committee, Editor in Chief of The Capital Food Safety Weekly, chairman of Beijing Seasons Investment Group Co., Ltd, chairman of Anhui Woyang Huadu Properties Co., Ltd., chairman of Xingguo Hongtianxia Camellia Oil Co., Ltd, and chairman of Nanjing Xingfeng Ecological Agriculture Co., Ltd. From 1993 to 2001, he formed and operated multiple companies in Nanchang, Jiangxi Province, mainly engaged in agricultural products operation and management. From 1987 to 1992, he was a department director at Sheyang Food Bureau, responsible for grain purchasing and management. We believe Mr. Ren’s extensive knowledge and experience with respect to health and nutrition products and his extensive food product industry background qualifies him to serve on our Board of Directors.
Joseph Mannello joined us as a Director in December 2015. He has served as our Chief Executive Officer since August 2017, and as our interim chief executive officer from September 2016 until August 2017. From May 2015 to September 2016, he served as a consultant at Brean Capital LLC, an independent investment bank and asset management firm, where he also served as a member of the firm’s operating committee and from March 2013 to May 2015, he served as their executive managing director. From March 2008 to March 2012, Mr. Mannello was the head of corporate credit for Gleacher & Company, Inc. (OTC:GLCH), a publicly-traded investment bank. Prior to that, he was the head of the fixed income division of BNY Capital Markets, Inc., a subsidiary of The Bank of New York Mellon Corp. (NYSE:BK). We believe that Mr. Mannello’s extensive financial markets and management background qualifies him to serve on our Board of Directors.
Dr. Louis Aronne joined us as a Director and a member of our Scientific Advisory Board in July 2011. Dr. Aronne is the Weill Professor of Metabolic Research and Director of the Comprehensive Weight Control Center which he founded in 1986 at Weill-Cornell Medical College. He is the Charirman of the American Board of Obesity Medicine and an Adjunct Clinical Associate Professor of Medicine at Columbia University College of Physicians and Surgeons. Dr. Aronne is former president of the Obesity Society and a fellow of the American College of Physicians. He has been an investigator on more than 40 trials, authored more than 90 papers and book chapters on obesity and edited the National Institutes of Health Practical Guide to the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Dr. Aronne has won several awards for teaching, including the Leo M. Davidoff Society Prize from Albert Einstein College of Medicine in 1983 and Eliot Hochstein Teaching Award from Cornell University in 1990. Dr. Aronne graduated Phi Beta Kappa from Trinity College with a BS in biochemistry and from Johns Hopkins University School of Medicine. We believe Dr. Aronne’s skills as a physician and his knowledge and experience with respect to obesity and related metabolic diseases qualifies him to serve on our Board of Directors.
Christopher Pechock joined us as a Director in February 2014. Mr. Pechock was a partner at Matlin Patterson Global Advisers, a global alternative asset manager, since its inception in July 2002 through September 2017. From November 1998 to July 2002, Mr. Pechock served as a member of the Global Distressed Securities Group Credit Suisse (NYSE:CS). From January 1997 to October 1998, Mr. Pechock served as a Portfolio Manager and Research Analyst at Turnberry Capital Management, L.P. Prior to that, Mr. Pechock served as a Portfolio Manager at Eos Partners, L.P. (February 1996 to December 1996), a Vice President and high yield analyst at PaineWebber Inc. (May 1993 to January 1996) and an analyst in risk arbitrage at Wertheim Schroder & Co., Incorporated (August 1987 to April 1991). He serves on the board of directors of Gleacher & Company, Inc. (NASDAQ: GLCH). Mr. Pechock received a BA in Economics from the University of Pennsylvania and an MBA from the Columbia University Graduate School of Business. We believe Mr. Pechock’s extensive financial background qualifies him to serve on our Board of Directors.
Victor Mandel joined us as a director in August 2016 and previously served as a director of the Company from December 2015 until March 2016. He has over twenty-five years of experience in investments, corporate strategy and corporate governance. Mr. Mandel previously served as Co-Chairman of Ambac Financial Group, Inc. (NASDAQ: AMBC) from May 2013 through December 2014 and as a director, chair of its Governance and Nominating Committee and member of its Audit and Strategy and Risk Policy Committees from May 2013 until May 2016. Additionally, he has previously served as a member of the board of directors and on the audit committees of Comsys IT Partners, Inc. (now a Manpower company), Broadpoint Gleacher Securities Group, Inc. (now Gleacher & Co., Inc.), and XLHealth Corp. (now a United Healthcare company). He previously served as the Chief Financial Officer of Circle.com (NASDAQ:CIRC) and served as Executive Vice President, Finance and Development of Snyder Communications, Inc. (NYSE:SNC) from 1999 to 2000. From 1991 to 1999, Mr. Mandel served as vice president in the Investment Research department at Goldman Sachs & Co. (NYSE:GS). Mr. Mandel holds an MBA in Finance from the Wharton School of Business at the University of Pennsylvania, an A.B. in Computer Science from Harvard University, and is a Chartered Financial Analyst. We believe Mr. Mandel’s extensive financial background qualifies him to serve on our Board of Directors.
40
John Nosta has served as the founder and president of NOSTALAB, a digital health think tank, since June 2013. He is generally regarded as a leading global strategic and creative thinker in the digital health area. A leading voice in the convergence of technology and health, Mr. Nosta helps define, dissect and deliberate global trends in digital health. He has also served as a member of the Google Health Advisory Board since October 2014 and has penned HEALTH CRITICAL for Forbes, a top global blog on health and technology. For over 20 years, Mr. Nosta was part of the leadership of Omnicom and WPP, leading healthcare communication companies. Prior to founding NOSTALAB, Mr. Nosta was employed by Ogilvy CommonHealth, a leading healthcare communication company, from April 2003 to June 2013, where he held a series of positions including Chief Creative Officer, Chief Strategic Officer and unit President. From 1990 to 1997, he held various senior-level positions at LLNS, a division of Omnicom Group Inc. (NYSE:OMC), a leading healthcare communication company. Mr. Nosta previously served as a director of the Company from December 2015 until March 2016. Mr. Nosta served as a research associate at Harvard University Medical School from 1980 to 1981 and has co-authored several papers with global thought-leaders in the field of cardiovascular physiology, with a focus on acute myocardial infarction, ventricular arrhythmias and sudden cardiac death. He received a Bachelor of Arts degree from Boston University in 1981. We believe Mr. Nosta’s scientific and pharmaceuticals industry background qualifies him to serve on our Board of Directors.
Bin Zhou joined us as a Director in March 2016. Mr. Zhou is an attorney licensed in the State of New Jersey. Since November 2007, he has been an attorney and a partner at Bernard & Yam, LLP, a New York law firm. He has advised companies on their public listings on U.S. stock exchanges including NASDAQ, NYSE and OTC markets, as well as on their private and public offering of securities. He received a bachelor’s degree in Economic Laws from Nanjing University, China, in 2001. He received a Master of Social Work from University of Georgia in 2003 and a Juris Doctor’s degree from Rutgers University School of Law in 2006. We believe Mr. Zhou’s extensive background in corporate compliance and international law qualifies him to serve on our Board of Directors.
Members of the Scientific Advisory Board
In addition to our Board of Directors, we maintain a Scientific Advisory Board, comprised of scientists and medical professionals who advise us on science and medical health issues, medical conditions and health care trends as they relate to our current and future products. Members of the Scientific Advisory Board provide us with advice, insights, contacts and other assistance based on their extensive knowledge and experience. Specifically, they advise us on: (a) the use of myostatin modulators in the treatment of various disorders including sarcopenia, obesity, muscle repair, anti-aging and longevity therapy, (b) the biological activities of our products and (c) the development of clinical research programs relating to the biomedical activities and benefits of our products. We enter into advisory board agreements with members of the Scientific Advisory Board pursuant to which they are entitled to receive a fixed number of shares of common stock (which may vary as determined by the Board of Directors), which generally vest over a number of years. The Scientific Advisory Board is currently comprised of the following members: Dr. Robert J. Hariri, Dr. Louis Aronne, Dr. Caroline Apovian and Dr. Neilank Jha.
The experience of each of the members of the Scientific Advisory Board (other than members who are our current directors, whose experience is set forth above) is as follows:
Dr. Caroline Apovian joined the Scientific Advisory Board in February 2013. Since November 2010, Dr. Apovian has served as Professor of Medicine and Pediatrics, in the Section of Endocrinology, Diabetes, and Nutrition at Boston University School of Medicine. She has also served as Director of the Center for Nutrition and Weight Management at Boston Medical Center since January 2000. Dr. Apovian is a nationally and internationally recognized authority on nutrition and has been in the field of obesity and nutrition since 1990. Dr. Apovian was a recipient of the Physician Nutrition Specialist Award given by the American Society of Clinical Nutrition for her work on developing and providing nutrition education, to medical students and physicians in training at Boston University School of Medicine. She has published over 200 articles, chapters, and reviews on the topics of obesity, nutrition, and the relationship between adipose tissue and risk of developing cardiovascular disease. Dr. Apovian has recently published a new book entitled The Age-Defying Diet and has also written two popular books called The Overnight Diet and The ALLI Diet Plan. Dr. Apovian has been a member of The Obesity Society since 1992, and has served on the Clinical Committee as well as Secretary/Treasurer and is currently serving as its President. Additionally, she serves as Associate Editor for the Society’s journal, Obesity. Dr. Apovian received her B.A. from Barnard College and her M.D. from the University of Medicine and Dentistry of New Jersey.
Dr. Neilank Jha joined the Scientific Advisory Board in December 2011. Since July 2010, Dr. Jha has served as a Clinical Fellow in the Spinal Program of Toronto Western Hospital. From 2004 to 2010, he was in the Neurosurgery Residency Program at McMaster University. Dr. Jha received his B.S. from the University of Toronto and his Doctor of Medicine from McMaster University.
Biographical information for Dr. Robert Hariri and Dr. Louis Aronne is set forth above in “Directors and Executive Officers.”
41
Board Meetings
During the fiscal year ended December 31, 2017, the Board held twelve formal meetings. We have no written policy regarding director attendance at annual meetings of stockholders. Our last annual meeting of stockholders was held on December 28, 2017 and five of our directors attended such meeting.
Director Independence
The Board evaluates the independence of each nominee for election as a director in accordance with the Nasdaq listing rules (the “Nasdaq Listing Rules”). Pursuant to these rules, a majority of our Board must be “independent directors” within the meaning of the Nasdaq Listing Rules, and all directors who sit on our Audit Committee and Compensation Committee must also be independent directors.
The Nasdaq definition of “independence” includes a series of objective tests, such as the director or director nominee is not, and was not during the last three years, our employee and has not received certain payments from, or engaged in various types of business dealings with, us. In addition, as further required by the Nasdaq Listing Rules, the Board has made a subjective determination as to each independent director that no relationships exist which, in the opinion of the Board, would interfere with such individual’s exercise of independent judgment in carrying out his or her responsibilities as a director. In making these determinations, the Board reviewed and discussed information provided by the directors with regard to each director’s business and personal activities as they may relate to us and our management.
As a result, the Board has affirmatively determined that other than Mr. Ren and Mr. Mannello, none of our directors has a material relationship with the Company. The Board has also affirmatively determined that all members of our Audit Committee and Compensation Committee are independent directors.
Audit Committee and Audit Committee Financial Expert
In April 2014, we established a separately-designated standing Audit Committee in accordance with Section 3(a) (58) (A) of the Exchange Act and the Nasdaq Listing Rules. The Audit Committee is comprised of Victor Mandel (chair), Chris Pechock and Bin Zhou. Our Board has determined that Mr. Mandel qualifies as an audit committee financial expert as defined by the rules of the SEC, based on his education, experience and background. During the fiscal year ended December 31, 2017, the Audit Committee held four formal meetings.
The Audit Committee:
| ● | oversees the accounting and financial reporting processes of the Company and the audits of the financial statements of the Company; | |
| ● | meets at least once per fiscal year with the Company’s outside auditors with respect to matters relating to the Company’s accounting and financial reporting processes, the audits of the Company’s financial statements, the Company’s application of accounting principles and the Company’s internal controls, and advises the Board of Directors with respect thereto; | |
| ● | is responsible for ensuring its receipt from the outside auditors of a formal written statement delineating all relationships between the auditor and the Company, actively engaging in a dialogue with the auditor with respect to any disclosed relationships or services that may impact the objectivity and independence of the auditor and taking, or recommending that the full Board take, appropriate action to oversee the independence of the outside auditor; | |
| ● | is directly responsible for the appointment, compensation, retention, oversight of the work and, where appropriate, replacement of any registered public accounting firm engaged for the purpose of preparing or issuing an audit report or performing other audit, review or attest services for the Company, and each such registered public accounting firm must report directly to the Audit Committee; and | |
| ● | oversees procedures established for (i) the receipt, retention and treatment of complaints received by the Company regarding accounting, internal accounting controls or auditing matters; (ii) confidential, anonymous submissions by the Company’s employees of concerns regarding questionable accounting or auditing matters and compliance with the Company’s Code of Ethics; and (iii) the review and oversight of all related party transactions. |
42
Compensation Committee
In April 2014, we established a separately-designated standing Compensation Committee in accordance with the Nasdaq Listing Rules. The Compensation Committee is comprised of Christopher Pechock (chair) and Dr. Louis J. Aronne. During the fiscal year ended December 31, 2017, the Compensation Committee held two formal meetings.
The Compensation Committee:
| ● | oversees the compensation policies and their specific application to our executive officers; | |
| ● | prepares an annual report on executive compensation for inclusion in our Annual Report on Form 10-K and/or proxy statement; | |
| ● | negotiates and approves the compensation of our chief executive officer and our other executive officers; | |
| ● | selects a peer group of companies against which to compare our compensation of our executive officers, if it deems such comparison necessary; | |
| ● | monitors compensation trends and solicits independent advice when deemed appropriate; and | |
| ● | approves, rejects or modifies incentive bonus compensation plans for our senior management, as recommended by management. |
Director Nominations
Our Board of Directors does not maintain a separate nominating committee. Functions customarily performed by a nominating committee are performed by the independent members of our Board. In evaluating and determining whether to nominate a candidate for a position on the Board, the independent members of our Board utilize a variety of methods and considers criteria such as high professional ethics and values, experience on the policy-making level in business or scientific/medical research experience relevant to our product candidates and a commitment to enhancing stockholder value. Candidates may be brought to the attention of the independent members of the Board by current Board members, stockholders, officers or other persons. The independent members of the Board will review all candidates in the same manner regardless of the source of the recommendation.
We have no formal policy regarding diversity of our Board of Directors. The independent members of our Board may therefore consider a broad range of factors relating to the qualifications and background of nominees, which may include diversity, which is not only limited to race, gender or national origin. The priority of the independent members of our Board in selecting members of the Board of Directors is identifying persons who will further the interests of our stockholders through his or her established record of professional accomplishment, the ability to contribute positively to the collaborative culture among Board members and professional and personal experiences and expertise relevant to our growth strategy.
The independent members of the Board also consider stockholder recommendations for director nominees that are properly received in accordance with the applicable rules and regulations of the SEC. In order to validly nominate a candidate for election or reelection as a director, stockholders must give timely notice of such nomination in writing to our Corporate Secretary and include, as to each person whom the stockholder proposes to nominate, all information relating to such person that is required to be disclosed in solicitations of proxies for the election of directors in an election contest, or is otherwise required, in each case pursuant to Regulation 14A under the Exchange Act, and the rules and regulations thereunder (including such person’s written consent to being named in the proxy statement as a nominee and to serving as a director if elected).
Board Leadership Structure
Dr. Robert J. Hariri serves as Chairman of the Board of Directors and Mr. Ren serves as our Global Chairman. Mr. Mannello currently serves as our principal executive officer. The Board of Directors has chosen to separate the principal executive officer and chairman positions because it believes that (i) independent oversight of management is an important component of an effective board of directors and (ii) this structure benefits the interests of all stockholders. If the Board of Directors convenes for a special meeting, the non-management directors will meet in executive session if circumstances warrant. Given the composition of the Board of Directors with a strong slate of independent directors, the Board of Directors does not believe that it is necessary to formally designate a lead independent director at this time, although it may consider appointing a lead independent director if circumstances change. We believe that the structure described above is the best structure to lead us in the achievement of our goals and objectives and establishes an effective balance between management leadership and appropriate oversight by independent directors.
43
Board Role in Risk Oversight
Senior management is responsible for assessing and managing our various exposures to risk on a day-to-day basis, including the creation of appropriate risk management programs and policies. The Board is responsible for overseeing management in the execution of its responsibilities and for assessing our approach to risk management. In addition, an overall review of risk is inherent in the Board’s consideration of our long-term strategies and in the transactions and other matters presented to the Board, including capital expenditures, acquisitions and divestitures, and financial matters.
Code of Ethics
We have adopted a corporate Code of Ethics. The text of our Code of Ethics, which applies to our employees, officers and directors, is posted in the “Corporate Governance” section of our website, http://www.myosrens.com. A copy of our Code of Conduct and Ethics is also available in print, free of charge, upon written request to 45 Horsehill Road, Suite 106, Cedar Knolls, New Jersey 07927 Attention: Joseph Mannello.
Compliance with Section 16(a) of the Exchange Act
Section 16(a) of the Securities Exchange Act of 1934, as amended requires our directors and executive officers, and persons who beneficially own more than 10% of a registered class of our equity securities, to report their initial beneficial ownership and any subsequent changes in that beneficial ownership of our securities to the SEC. Based solely on a review of the copies of the reports furnished to us, we believe that all such reports for the year ended December 31, 2017 were filed on a timely basis.
| Item 11. | Executive Compensation. |
Summary Compensation Table
The table below sets forth the compensation earned for services rendered to us, for fiscal years indicated, by our executive officers.
| Name and Position | Fiscal Year | Salary ($) | Stock Awards ($) | Option Awards ($) (5) | All Other Compensation ($) (6) | Total ($) | ||||||||||||||||||
| Joseph Mannello (1) | 2017 | 167,400 | - | 258,000 | - | 425,400 | ||||||||||||||||||
| (Chief Executive Officer) | 2016 | 82,600 | 12,631 | - | 16,081 | 111,312 | ||||||||||||||||||
| K. Bryce Toussaint (2) | 2017 | - | - | - | - | |||||||||||||||||||
| (Former Chief Executive Officer) | 2016 | 173,569 | 1,755 | - | 4,123 | |||||||||||||||||||
| Joseph C. DosSantos (3) | 2017 | - | - | - | - | |||||||||||||||||||
| (Former Chief Financial Officer) | 2016 | 122,437 | 9,350 | 52,700 | 37,660 | |||||||||||||||||||
| Dr. Robert C. Ashton, Jr. (4) | 2017 | - | - | - | - | - | ||||||||||||||||||
| (Former Chief Medical Officer) | 2016 | 55,687 | - | - | - | |||||||||||||||||||
| (1) | On August 24, 2017, the board of directors appointed Joseph Mannello as the Company’s permanent Chief Executive Officer, |
| (2) | K.Bryce Toussaint was hired as Chief Executive Officer on December 17, 2015 and resigned on August 31, 2016. |
| (3) | On June 30, 2016, Joseph C. DosSantos resigned as the Chief Financial Officer. |
| (4) | Dr. Ashton resigned as Chief Medical Officer on January 31, 2016. |
| (5) | Amounts reflect the aggregate grant date fair value of stock option awards computed in accordance with Accounting Standards Codification (“ASC”) 718, “Compensation – Stock Compensation.” The assumptions used in determining the grant date fair value of these awards for their respective years are set forth in Part IV, Item 15, “Notes to Consolidated Financial Statements: Note 10 – Stock Compensation.” |
44
| (6) | The amounts in All Other Compensation column of the Summary Compensation Table reflect the following: |
| Name | Fiscal Year | Health Insurance Expenses | 401(k) Matching Contribution | Total Other Compensation | ||||||||||||
| Joseph Mannello | 2017 | 18,750 | - | $ | 18,750 | |||||||||||
| 2016 | 5,170 | - | $ | 5,170 | ||||||||||||
| K. Bryce Toussaint | 2017 | - | - | $ | - | |||||||||||
| 2016 | 14,590 | - | $ | 14,590 | ||||||||||||
| Joseph C. DosSantos | 2017 | - | - | $ | - | |||||||||||
| 2016 | 14,590 | 4,183 | $ | 18,830 | ||||||||||||
Employment Agreements
Joseph Mannello
On August 30, 2016, we entered into an offer letter with Joseph Mannello, pursuant to which Mr. Mannello agreed to serve as our interim Chief Executive Officer commencing September 1, 2016. Pursuant to the terms of the Offer Letter, Mr. Mannello will work a full-time basis as an at-will employee for an annual base salary of $240,000. Mr. Mannello will be entitled to an annual bonus of up to 100% of his annual base salary, as determined by the Board (or its compensation committee) in its sole discretion. Mr. Mannello also received a grant of 10,000 shares of common stock which vested upon the six-month anniversary of his start date.
On August 24, 2017, we entered into an employment agreement with Mr. Mannello to serve as the permanent Chief Executive Officer of the Company, effective immediately. Pursuant to the terms of the agreement, Mr. Mannello agreed to work for the Company on a full-time basis and receive a weekly base salary of $455. He may receive an annual bonus in cash or equity of the Company, as may be determined by the Board in its sole discretion. Mr. Mannello was also granted a stock option to purchase 300,000 shares of the Company’s common stock at an exercise price of $4.00 per share, which option will vest in eight equal annual installments on the last day of each fiscal quarter starting with September 30, 2017. The initial term of the agreement is two years, and the agreement will automatically renew for successive one-year periods, unless a notice of non-renewal is provided by either party at least sixty days prior to the expiration date of the term.
In the event Mr. Mannello’s employment is terminated by the Company for cause (as defined in the agreement) or as a result of death or disability, or if Mr. Mannello terminates his employment without good reason (as defined in the agreement), Mr. Mannello will be entitled to receive any accrued and unpaid base salary, any unreimbursed reasonable business expenses and employee benefits up to the date of termination as well as retain any portion of the stock option that has previously vested.
In the event Mr. Mannello’s employment is terminated by the Company for any reason other than cause, death or disability, or if Mr. Mannello terminates his employment for good reason, he will be entitled to receive any accrued and unpaid base salary and employee benefits up to the date of termination as well as the vested portion of the stock option. In addition, he will be entitled to receive accrued and unpaid base salary up to the date of the termination, full reimbursement of all business expenses prior to termination, all applicable COBRA-related health insurance continuation rights to the extent provided for under applicable law or based on the Company’s practice and an amount equal to 100% of the COBRA premiums for him and his family for twelve months following the date of termination.
In the event Mr. Mannello’s employment is terminated by the Company without cause and in connection with, or as a result of, a change of control (as defined in the agreement), or if Mr. Mannello terminates his employment for good reason following a change in control, he will also be entitled to retain the stock option and the unvested portion of the stock option will vest as of the date of the consummation of the change in control.
The agreement contains customary non-competition and non-solicitation provisions that extend to two years after termination of Mr. Mannello’s employment with the Company. Mr. Mannello also agreed to customary terms regarding confidentiality and ownership of product ideas.
45
Outstanding Equity Awards at 2017 Fiscal Year End
The following table presents, for each of the named executive officers, information regarding outstanding equity awards as of December 31, 2017.
| Outstanding Equity Awards | ||||||||||||||||||||||||||
| Option Awards | Stock Awards | |||||||||||||||||||||||||
| Name | Grant Date | Number of Securities Underlying Unexercised Options Exercisable | Number of Securities Underlying Unexercised Options Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units That Have Not Vested ($) | |||||||||||||||||||
| Joseph Mannello (1) | 8/24/2017 |
75,000 | 225,000 | 4.00 | 8/24/2027 |
- | - | |||||||||||||||||||
| (1) | Mr. Mannello was hired as the Company’s permanent Chief Executive Officer effective September 1, 2017 |
Stock Vested at 2017 Fiscal Year End
Director Compensation
The following table summarizes the compensation earned by our non-employee board of directors for the fiscal year ended December 31, 2017. All compensation paid to our employee directors is included under the summary compensation table above.
| Name | Share Awards(1) | Cash Paid ($) | ||||||
| Dr. Robert J. Hariri | 7,353 | $ | 10,000 | |||||
| Dr. Louis J. Aronne | 9,191 | 12,500 | ||||||
| Christopher Pechock | 11,949 | 16,250 | ||||||
| Victor Mandel | 10,110 | 13,750 | ||||||
| John Nosta | 9,191 | 12,500 | ||||||
40,441 | $ | 65,000 | ||||||
| (1) | The value of awards and stock options equals the aggregate grant date fair value of awards computed in accordance with ASC 718. The assumptions used in determining the grant date fair value of these awards for their respective years are set forth in Part IV, Item 15, “Notes to Consolidated Financial Statements: Note 10 - Stock Compensation.” These share awards and cash payments were made in January 2018. |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights.
The following table sets forth information known to us regarding the beneficial ownership of our common stock as of March 26, 2018 by:
| ● | each person known by us at that date to be the beneficial owner of more than 5% of the outstanding shares of our based solely on Schedule 13D/13G filings with the SEC; | |
| ● | each of our executive officers and directors at such date; and | |
| ● | all of our executive officers and directors at such date, as a group. |
46
Unless otherwise indicated, we believe that all persons named in the table below have sole voting and investment power with respect to all shares of common stock beneficially owned by them. As of March 26, 2018, there were 6,480,899 shares of our common stock outstanding.
| Name of Beneficial Owner (1) | Number of Shares Beneficially Owned | Percentage of Class | ||||||
| RENS Technology Inc. & Ren Ren (2) | 1,897,568 | 29.2 | % | |||||
| Joseph Mannello (6) | 616,811 | 9.6 | % | |||||
| Dr. Robert J. Hariri (3) | 429,250 | 6.6 | % | |||||
| Christopher Pechock (5) | 185,386 | 2.9 | % | |||||
| Victor Mandel | 62,021 | * | ||||||
| Dr. Louis J. Aronne (4) | 60,086 | * | ||||||
| Bin Zhou | 4,386 | * | ||||||
| John Nosta | - | - | ||||||
| Directors and officers as a group (8 persons) | 3,255,508 | 50.2 | % | |||||
* Less than 1%
| (1) | Unless otherwise indicated, the business address of each of the individuals is c/o MYOS RENS Technology Inc., 45 Horsehill Road, Suite 106, Cedar Knolls, New Jersey 07927. |
| (2) | Mr. Ren has 22,568 of his own shares and has sole voting and investment control over the 1,875,000 securities held by RENS Technology Inc. which includes 375,000 shares issuable upon a warrant. |
| (3) | Includes 166,000 shares held by Hariri Family Ltd. Partnership and 150,250 shares issuable upon exercise of vested stock options. |
| (4) | Includes 30,500 shares issuable upon exercise of vested stock options. |
| (5) | Includes 75,000 shares issuable upon exercise of warrants and 3,000 shares issuable upon exercise of vested stock options. |
| (6) | Includes 100,001 shares issuable upon exercise of warrants. |
| Item 13. | Certain Relationships and Related Transactions and Director Independence. |
The following is a description of the transactions we have engaged in during the year ended December 31, 2017 and through the date of this Report, with our directors and officers and beneficial owners of more than five percent of our voting securities and their affiliates.
On December 17, 2015, we issued an unsecured promissory note in the principal amount of $575,000 to Gan Ren, the son of Ren Ren, a current director and our largest stockholder. The note bears interest at a rate of 8% per annum and matures one year from the date of issuance. On December 17, 2016 the note and accrued interest of $46,000 was automatically converted into 225,864 shares of common stock at $2.75 per share.
On December 17, 2015, we entered into the Purchase Agreement with the Purchaser, an entity which is controlled by Ren Ren, a current director and our largest stockholder. Pursuant to terms of the Purchase Agreement, the Purchaser agreed to invest $20.25 million in the Company in exchange for (i) an aggregate of 3,537,037 shares of common stock and (ii) warrants to purchase an aggregate of 884,259 shares of common stock. In connection with the Financing, the Board agreed to issue Mr. Ren 18,182 shares of common stock following the closing of the Financing for his services to the Company as a member of the Board.
On March 3, 2016, we completed the first tranche of the Financing pursuant to which the Purchaser acquired 1,500,000 shares of common stock and a warrant to purchase 375,000 shares of the Company’s common stock for $5.25 million.
On August 19, 2016, the Purchaser notified the Company that it did not intend to fulfill its obligation to fund the second tranche of the Financing, notwithstanding its confirmation to the Company in June 2016 that the Purchaser would provide such funding in accordance with the terms of the Purchase Agreement.
In October 2016, the Company received a purchase order from RENS Agriculture to purchase $118 of our product. We received a 50% deposit in November 2016 in order to manufacture the product. The goods were shipped in January 2017 and received in China in March 2017. We have not received payment for the order to date. As a result of the ongoing litigation, the Company recorded an allowance for bad debt of $59 related to the receivable due from RENS Agriculture.
47
On January 6, 2017, in connection with the financing contemplated by a securities purchase agreement with RENS Technology Inc. (the “Purchaser”), the Company commenced an action in the Supreme Court of New York, County of New York (the “Court”), against the Purchaser, RENS Agriculture, the parent company of the Purchaser, and Ren Ren, a principal in both entities and one of our directors, arising from the Purchaser’s breach of the agreement under which the Purchaser agreed to invest an aggregate of $20.25 million in our company in exchange for an aggregate of 3,537,037 shares of the Company’s common stock and warrants to purchase an aggregate of 884,259 shares of common stock.
On April 11, 2017, the Court noted that we had demonstrated a likelihood of success on the merits of the breach of contract claim. Thereafter, a hearing was scheduled on the application by the Purchaser to dismiss the complaint and various pre-trial discovery applications by both parties.
In August 2017 the Company amended its complaint, repeating most of the initial claims but added several additional claims against RENS Agriculture, Mr. Ren and two additional Chinese defendants, including a claim against RENS Agriculture for breaching the exclusive distribution agreement, as well as claims against all defendants for theft and misappropriation of our confidential proprietary information and trade secrets, breach of fiduciary duty and duty of loyalty, misappropriation of corporate opportunity, unfair competition and a number of other torts. We are seeking damages and injunctive relief. The Purchaser has filed a motion to dismiss the amended complaint, which is still pending and scheduled for oral argument in April 2018.
On August 16, 2017, the Purchaser commenced an action in the District Court of Clark County in the State of Nevada against us and Joseph Mannello, our then interim Chief Executive Officer, alleging that Mr. Mannello had breached his fiduciary duties and was grossly negligent in managing our company. The action seeks monetary damages and injunctive relief from Mr. Mannello as well as the appointment of a receiver over us. Subsequently, the Purchaser submitted a petition to appoint a receiver and we and Mr. Mannello submitted a motion to dismiss the action, both of which are currently pending and are due to be heard in April 2018. An application on consent to adjourn the hearing date on the receiver application and motion to dismiss is pending.
Review, Approval or Ratification of Transactions with Related Persons.
Our Board of Directors has established an audit committee consisting of independent directors. This committee, among other duties, is charged to review, and if appropriate, ratify all agreements and transactions which had been entered into with related parties, as well as review and ratify all future related party transactions.
| Item 14. | Principal Account Fees and Services. |
For fiscal years ended December 31, 2017 and December 31, 2016, WithumSmith+Brown, PC, served as our principal accountant.
Audit Fees. Audit fees consist of fees for professional services rendered for the annual audits of our financial statements, quarterly reviews of financial statements and services that are normally provided in connection with statutory and regulatory filings or engagements. Audit fees billed by WithumSmith+ Brown, PC for the fiscal years ended December 31, 2017 and December 31, 2016 were approximately $108,000 and $84,000 respectively.
Audit-Related Fees. Audit-related services consist of fees for assurance and related services that are reasonably related to performance of the audit or review of our financial statements and are not reported under “Audit Fees.” These services include attest services that are not required by statute or regulation and consultations concerning financial accounting and reporting standards. There were no fees billed for audit-related services rendered during the last two fiscal years.
Tax Fees. Tax services consist of fees for the preparation of federal and state tax returns. Tax fees estimated to be billed by WithumSmith+Brown, PC for the fiscal year ended December 31, 2017 are $10,700. Tax fees paid to WithumSmith+Brown, PC in 2017 for the tax return related to the fiscal year ended December 31, 2016 were $9,500.
48
| Item 15. | Exhibits and Financial Statement Schedules. |
Financial Statements and Schedules
Exhibits
The following exhibits are filed herewith or are incorporated by reference to exhibits previously filed with the Securities and Exchange Commission.
49
| * | Filed herewith |
| ** | Furnished herewith |
| ^ | Certain portions have been omitted pursuant to a confidential treatment request. Omitted information has been filed separately with the SEC. |
| Item 16. | Form 10-K Summary |
Not applicable.
50
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| MYOS RENS Technology Inc. | ||
| Date: March 27, 2018 | By: | /s/ Joseph Mannello |
| Name: | Joseph Mannello | |
| Title: | Chief Executive Officer | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Name | Title(s) | Date | ||
| /s/ Dr. Robert J. Hariri | Chairman of the Board | March 27, 2018 | ||
| Dr. Robert J. Hariri | ||||
| /s/ Joseph Mannello | Chief Executive Officer and Director (Principal Executive | March 27, 2018 | ||
| Joseph Mannello | Officer, Principal Financial Officer and Principal Accounting Officer) | |||
| /s/ Dr. Louis Aronne | Director | March 27, 2018 | ||
| Dr. Louis Aronne | ||||
| /s/ Christopher Pechock | Director | March 27, 2018 | ||
|
Christopher Pechock
|
||||
| /s/ Victor Mandel | Director | March 27, 2018 | ||
| Victor Mandel | ||||
| /s/ John Nosta | Director | March 27, 2018 | ||
| John Nosta | ||||
| Global Chairman | March 27, 2018 | |||
|
Ren Ren |
||||
| Director | March 27, 2018 | |||
|
Bin Zhou |
51
CONTENTS
F-1
Report of Independent Registered Public Accounting Firm
To The Board of Directors and Stockholders of
MYOS RENS Technology Inc:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of MYOS RENS Technology Inc. (the ”Company”) as of December 31, 2017 and 2016, and the related consolidated statements of operations, changes in stockholders' equity, and cash flows for each of the two years in the period ended December 31, 2017 and the related notes (collectively referred to as the “financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2017 and 2016, and the consolidated results of its operations and its consolidated cash flows for each of the two years in the period ended December 31, 2017, in conformity with accounting principles generally accepted in the United States.
Substantial Doubt Regarding Going Concern
The accompanying consolidated financial statements have been prepared assuming that the entity will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the entity has suffered recurring losses from operations, and has an accumulated deficit, that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ WithumSmith+Brown, PC
WithumSmith+Brown, PC
We have served as the Company's auditor since 2016.
East Brunswick, New Jersey
March 27, 2018
F-2
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
Consolidated Balance Sheets
(in thousands, except share amounts)
| December 31, | December 31, | |||||||
| 2017 | 2016 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 923 | $ | 1,866 | ||||
| Accounts receivable, net | 4 | 8 | ||||||
| Inventories, net | 1,779 | 1,862 | ||||||
| Prepaid expenses and other current assets | 163 | 85 | ||||||
| Total current assets | 2,869 | 3,821 | ||||||
| Deferred offering costs | 102 | - | ||||||
| Fixed assets, net | 184 | 233 | ||||||
| Intangible assets, net | 1,640 | 1,907 | ||||||
| Total assets | $ | 4,795 | $ | 5,961 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 176 | $ | 226 | ||||
| Accrued expenses and other current liabilities | 255 | 361 | ||||||
| Deferred revenue | - | 56 | ||||||
| Total current liabilities | 431 | 643 | ||||||
| Total liabilities | 431 | 643 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ equity: | ||||||||
| Preferred stock, $.001 par value; 500,000 shares authorized; no shares issued and outstanding | - | - | ||||||
| Common stock, $.001 par value; 12,000,000 shares authorized at December 31, 2017 and 2016; 6,340,604 and 5,344,372 shares issued and outstanding at December 31, 2017 and 2016, respectively | 6 | 5 | ||||||
| Additional paid-in capital | 36,202 | 33,099 | ||||||
| Accumulated deficit | (31,844 | ) | (27,786 | ) | ||||
| Total stockholders’ equity | 4,364 | 5,318 | ||||||
| Total liabilities and stockholders’ equity | $ | 4,795 | $ | 5,961 | ||||
See accompanying Notes to Consolidated Financial Statements
F-3
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
Consolidated Statements of Operations
(in thousands, except per share amounts)
| Years Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Net revenues | $ | 526 | $ | 327 | ||||
| Cost of sales | 308 | 319 | ||||||
| Gross profit | 218 | 8 | ||||||
| Operating expenses | ||||||||
| Sales and marketing | 822 | 846 | ||||||
| Personnel and benefits | 1,450 | 1,853 | ||||||
| General and administrative | 2,014 | 1,616 | ||||||
| Total operating expenses | 4,286 | 4,315 | ||||||
| Operating loss | (4,068 | ) | (4,307 | ) | ||||
| Other income (expense): | ||||||||
| Other income | 12 | 1 | ||||||
| Interest expense | (2 | ) | (35 | ) | ||||
| Total other income (expense) | 10 | (34 | ) | |||||
| Loss before income taxes | (4,058 | ) | (4,341 | ) | ||||
| Income tax provision | - | - | ||||||
| Net loss | $ | (4,058 | ) | $ | (4,341 | ) | ||
| Net loss per share attributable to common shareholders: | ||||||||
| Basic and diluted | $ | (0.69 | ) | $ | (0.90 | ) | ||
| Weighted average number of common shares outstanding: | ||||||||
| Basic and diluted | 5,875 | 4,806 | ||||||
See accompanying Notes to Consolidated Financial Statements
F-4
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
Consolidated Statement of Changes in Stockholders’ Equity
(in thousands, except share amounts)
| Common Stock | Additional | Total | ||||||||||||||||||
| Shares | Amount $.001 par | paid-in capital | Accumulated deficit | stockholders’ equity | ||||||||||||||||
| Balance at January 1, 2016 | 3,552,873 | $ | 4 | $ | 26,946 | $ | (23,445 | ) | $ | 3,505 | ||||||||||
| Proceeds from issuance of common stock, net | 1,500,000 | 1 | 5,140 | - | 5,141 | |||||||||||||||
| Shares issued to employees and directors | 65,639 | - | 117 | - | 117 | |||||||||||||||
| Shares issued upon conversion of note | 225,860 | - | 621 | - | 621 | |||||||||||||||
| Share-based compensation expense | - | - | 275 | - | 275 | |||||||||||||||
| Net loss | - | - | - | (4,341 | ) | (4,341 | ) | |||||||||||||
| Balance at December 31, 2016 | 5,344,372 | 5 | 33,099 | (27,786 | ) | 5,318 | ||||||||||||||
| Proceeds from issuance of common stock, net | 1,000,000 | 1 | 2,943 | - | 2,944 | |||||||||||||||
| Shares forfeited by employees and directors | (3,768 | ) | - | - | - | - | ||||||||||||||
| Share-based compensation expense | - | - | 160 | - | 160 | |||||||||||||||
| Net loss | - | - | - | (4,058 | ) | (4,058 | ) | |||||||||||||
| Balance at December 31, 2017 | 6,340,604 | 6 | 36,202 | (31,844 | ) | 4,364 | ||||||||||||||
See accompanying Notes to Consolidated Financial Statements
F-5
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
Consolidated Statements of Cash Flows
(in thousands)
| Years Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Cash Flows From Operating Activities: | ||||||||
| Net loss | $ | (4,058 | ) | $ | (4,341 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 51 | 54 | ||||||
| Amortization | 267 | 210 | ||||||
| Change in contract liability | - | (117 | ) | |||||
| Provision for inventory reserve | 2 | 107 | ||||||
| Bad debt expense | 59 | - | ||||||
| Share-based compensation | 160 | 392 | ||||||
| Impairment charge | - | 44 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| (Increase) decrease in accounts receivable | (55 | ) | 398 | |||||
| Decrease (increase) in inventories | 81 | (501 | ) | |||||
| (Increase) decrease in prepaid expenses and other current assets | (78 | ) | 437 | |||||
| Decrease in deferred revenue | (56 | ) | - | |||||
| Decrease in accounts payable and accrued expenses | (156 | ) | (356 | ) | ||||
| Net cash used in operating activities | (3,783 | ) | (3,673 | ) | ||||
| Cash Flows From Investing Activities: | ||||||||
| Purchase of capitalized software | - | (380 | ) | |||||
| Purchases of fixed assets | (2 | ) | (1 | ) | ||||
| Net cash used in investing activities | (2 | ) | (381 | ) | ||||
| Cash Flows From Financing Activities: | ||||||||
| Proceeds from issuance of common stock, net | 2,944 | 5,141 | ||||||
| Deferred offering costs | (102 | ) | - | |||||
| Repayments of term note | - | (100 | ) | |||||
| Net cash provided by financing activities | 2,842 | 5,041 | ||||||
| Net (decrease) increase in cash | (943 | ) | 987 | |||||
| Cash at beginning of year | 1,866 | 879 | ||||||
| Cash at end of year | $ | 923 | $ | 1,866 | ||||
| Supplemental disclosure of cash flow information: | ||||||||
| Cash paid during the year for: | ||||||||
| Interest | $ | 2 | $ | 34 | ||||
| Income taxes | $ | - | $ | - | ||||
| Supplemental schedule of non-cash investing and financing activities: | ||||||||
| Issuance of common stock upon conversion of convertible note | $ | - | $ | 621 | ||||
See accompanying Notes to Consolidated Financial Statements
F-6
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
NOTE 1 – NATURE OF OPERATIONS, BASIS OF PRESENTATION AND LIQUIDITY
Nature of Operations
MYOS RENS Technology Inc. is an emerging bionutrition and biotherapeutics company focused on the discovery, development and commercialization of products that improve muscle health and function. The Company was incorporated under the laws of the State of Nevada on April 11, 2007. On March 17, 2016, the Company merged with its wholly-owned subsidiary and changed its name from MYOS Corporation to MYOS RENS Technology Inc. As used in these financial statements, the terms “the Company”, “MYOS”, “our”, or “we”, refers to MYOS RENS Technology Inc. and its subsidiary, unless the context indicates otherwise. On February 25, 2011, the Company entered into an agreement to acquire the intellectual property for Fortetropin®, our proprietary active ingredient from Peak Wellness, Inc. The Company’s activities are subject to significant risks and uncertainties.
Our commercial focus is to leverage our clinical data to develop multiple products to target the large, but currently underserved, markets focused on muscle health. The sales channels through which we sell our products are evolving. The first product we introduced was MYO-T12, which was sold in the sports nutrition market. MYO-T12 is a proprietary formula containing Fortetropin® and other ingredients. The formula was sold under the brand name MYO-T12 and later as MYO-X through an exclusive distribution agreement with Maximum Human Performance, or MHP. The exclusive distribution agreement was terminated in March 2015 and there were no subsequent sales to MHP.
In February 2014, we expanded our commercial operations into the age management market through a distribution agreement with Cenegenics Product and Lab Services, LLC (“Cenegenics”), under which Cenegenics distributed and promoted a proprietary formulation containing Fortetropin® through its age management centers and its community of physicians focused on treating a growing population of patients focused on proactively addressing age-related health and wellness concerns. The distribution agreement with Cenegenics expired in December 2016.
During the second quarter of 2015 we launched Rē Muscle HealthTM, our own direct-to-consumer portfolio of muscle health bars, meal replacement shakes and daily supplement powders each powered by a full 6.6 gram single serving dose of Fortetropin®. Our Rē Muscle HealthTM products were sold through our direct-to-consumer e-commerce platform, remusclehealth.com, and amazon.com until March 2017 when we introduced our new Qurr line of products.
In March 2017 the Company launched Qurr, its Fortetropin®-powered product line formulated to support the vital role of muscle in overall well-being as well as in fitness. Qurr is a line of flavored puddings, powders, and shakes proven to be safe for daily use, muscle-focused, natural, over-the-counter products which are available through convenient direct online ordering without a prescription. All Qurr products are blended with Fortetropin®, MYOS’ proprietary ingredient. MYOS’ earlier product formulations featuring Fortetropin® have become part of the daily routine of many athletes and fitness-conscious people.
We continue to pursue additional distribution and branded sales opportunities. We expect to continue developing our own core branded products in markets such as functional foods, sports and fitness nutrition and rehab and restorative health and to pursue international sales opportunities. There can be no assurance that we will be able to secure distribution arrangements on terms acceptable to the Company, or that we will be able to generate significant sales of our current and future branded products.
F-7
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
Strategic Investment Transaction
On December 17, 2015, the Company entered into a Securities Purchase Agreement (the “Purchase Agreement”) with RENS Technology Inc. (the “Purchaser”), pursuant to which the Purchaser agreed to invest $20.25 million in the Company in three tranches (the “Financing”) in exchange for an aggregate of 3,537,037 shares (the “Shares”) of the Company’s common stock, par value $0.001 per share (“Common Stock”).
In the first tranche which closed on March 3, 2016 the Purchaser acquired 1,500,000 Shares and a warrant to purchase 375,000 shares of Common Stock (the “Initial Warrant”) for $5.25 million.
On August 19, 2016, the Purchaser notified the Company that it did not intend to fulfill its obligation to fund the second tranche of the Financing, notwithstanding its confirmation to the Company in June 2016 that the Purchaser would provide such funding in accordance with the terms of the Purchase Agreement.
Pursuant to the terms of the Purchase Agreement, effective August 23, 2016, Guiying Zhao resigned as a director of the Company. In addition, the Purchaser’s Rights terminated, effective August 19, 2016.
On January 6, 2017, in connection with the financing contemplated by a securities purchase agreement with RENS Technology Inc. (the “Purchaser”), the Company commenced an action in the Supreme Court of New York, County of New York (the “Court”), against the Purchaser, RENS Agriculture, the parent company of the Purchaser, and Ren Ren, a principal in both entities and one of our directors, arising from the Purchaser’s breach of the aforementioned agreement. See Note 14 – legal PROCEEDINGS.
Going Concern
The accompanying consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”), which contemplates continuation of the Company as a going concern. The Company has suffered recurring losses from operations and incurred a net loss of approximately $4,058 for the year ended December 31, 2017 and $4,341 for the year ended December 31, 2016. As of the filing date of this Report, management believes that there may not be sufficient capital resources from operations and existing financing arrangements in order to meet operating expenses and working capital requirements for the next twelve months, primarily due to the failure of RENS Technology Inc. to fund the required amounts. These facts raise substantial doubt about the Company’s ability to continue as a going concern.
Accordingly, we are evaluating various alternatives, including reducing operating expenses, securing additional financing through debt or equity securities to fund future business activities and other strategic alternatives. There can be no assurance that the Company will be able to generate the level of operating revenues in its business plan, or if additional sources of financing will be available on acceptable terms, if at all. If no additional sources of financing are available, our future operating prospects may be adversely affected. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
F-8
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance to U.S. GAAP and the rules and regulations of the U.S. Securities and Exchange Commission (“SEC”). The consolidated financial information presented herein reflects all normal adjustments that are, in the opinion of management, necessary for a fair statement of the financial position, results of operations and cash flows for the periods presented. The Company is responsible for the consolidated financial statements included in this report.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of MYOS RENS Technology Inc. and its wholly-owned subsidiary, Atlas Acquisition Corp. All material intercompany balances and transactions have been eliminated in consolidation.
Reclassification of Prior Year Presentation
Certain prior year amounts have been reclassified for consistency with the current period presentation. These reclassifications did not have an impact on the reported results of operations.
Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, equity and the disclosures of contingent assets and liabilities at the date of the financial statement and the reported amounts of revenues and expenses during the reporting period. Making estimates requires management to exercise significant judgment. It is possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future non-conforming events. Accordingly, the actual results could differ significantly from estimates. Significant items subject to such estimates include but are not limited to the valuation of stock-based awards, measurement of allowances for doubtful accounts and inventory reserves, the selection of asset useful lives, fair value estimations used to test long-lived assets, including intangibles, impairments and provisions necessary for assets and liabilities.
The Company has recorded minimal sales to its distributors during the past fourteen consecutive quarters, and launched its Qurr portfolio of branded products in March 2017. Management’s estimates, including evaluation of impairment of long-lived assets and inventory reserves are based in part on forecasted future results. A variety of factors could cause actual results to differ from forecasted results and these differences could have a significant effect on asset carrying amounts.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with a maturity of three months or less and money market accounts to be cash equivalents. At December 31, 2017 and 2016, the Company had no cash equivalents.
The Company maintains its bank accounts with high credit quality financial institutions and has never experienced any losses related to these bank accounts. The Company minimizes its credit risk associated with cash by periodically evaluating the credit quality of its financial institutions.
As part of our ongoing liquidity assessments management evaluates our cash, cash equivalents. The amount of funds held in bank can fluctuate due to the timing of receipts and payments in the ordinary course of business and due to other reasons, such as business-development activities so the Company may at times have exposure to cash in excess of FDIC insured limits.
F-9
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
Concentrations of Credit Risk, Significant Customers and Significant Supplier
Management regularly reviews accounts receivable, and if necessary, establishes an allowance for doubtful accounts that reflects management’s best estimate of amounts that may not be collectible based on historical collection experience and specific customer information. Expense recognized as a result of an allowance for doubtful accounts is classified under general and administrative expenses in the Consolidated Statements of Operations. Based primarily on collections, during the year ended December 31, 2017, management determined that the allowance for doubtful accounts should be increased. Accordingly, an allowance for doubtful accounts of $59 was recorded for the year ended December 31, 2017. There was no such allowance recorded in 2016.
At December 31, 2017 and 2016, the Company had the following concentrations of net accounts receivable with customers:
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Egg Yolk Powder | $ | 59 | $ | - | ||||
| Direct-to-consumer | 4 | 8 | ||||||
| Subtotal | - | 8 | ||||||
| Allowance for doubtful accounts | (59 | ) | - | |||||
| Accounts receivable, net | $ | 4 | $ | 8 | ||||
For the years ended December 31, 2017 and 2016, the Company had the following concentrations of revenues with customers:
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Cenegenics | 38 | % | 50 | % | ||||
Inventories, net
Inventories are valued at the lower of cost or net realizable value, with cost determined on a first in, first-out basis. Each quarter the Company evaluates the need for a change in the inventory reserve based on sales and expiration dates of products.
Fixed Assets
Fixed assets are stated at cost and depreciated to their estimated residual value over their estimated useful lives of 3 to 7 years. Leasehold improvements are amortized over the lesser of the asset’s useful life or the contractual remaining lease term including expected renewals. When assets are retired or otherwise disposed of, the assets and related accumulated depreciation are reversed from the accounts and the resulting gains or losses are included in the Consolidated Statements of Operations. Repairs and maintenance are expensed as incurred.
Depreciation is provided using the straight-line method for all fixed assets.
We review our fixed assets for impairment when events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. We use an estimate of future undiscounted net cash flows of the related assets or groups of assets over their remaining lives in measuring whether the assets are recoverable. If the assets are determined to be unrecoverable, an impairment loss is calculated by determining the difference between the carrying values and the estimated fair value. We did not consider any of our fixed assets to be impaired during the years ended December 31, 2017 and 2016.
Intangible Assets
The Company’s intangible assets consist primarily of intellectual property pertaining to Fortetropin®, including its formula, trademarks, trade secrets, patent application and domain names, which were determined to have a fair value of $2,000 as of December 31, 2011. Based on expansion into new markets and introduction of new formulas, management determined that the intellectual property had a finite useful life of ten (10) years and began amortizing the asset over its estimated useful life beginning April 2014.
F-10
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
In July 2014, the Company acquired the United States patent application for the manufacture of Fortetropin® from Deutsches Institut fur Lebensmitteltechnik e.V. - the German Institute for Food Technologies (“DIL”). The cost of the patent application, which was capitalized as an intangible asset, was determined to be $101, based on the present value of the minimum guaranteed royalty payable to DIL using a discount rate of 10%. The intangible asset is being amortized over an estimated useful life of ten (10) years. The remaining contingent royalty payments will be recorded as the contingency is resolved and the royalty becomes payable under the arrangement. For additional information on the amended supply agreement with DIL refer to “NOTE 12 – Commitments and Contingencies - Supply Agreement.”
In March 2017, the Company launched a new product line QURR and a related website qurr.com. The Company capitalized $380 of the costs to build the website in accordance with U.S. GAAP and will amortize this asset over 60 month’s useful life.
Intangible assets at December 31, 2017 and December 31, 2016 consisted of the following:
| December 31, | December 31, | |||||||
| (In thousand $) | 2017 | 2016 | ||||||
| Intangibles with finite lives: | ||||||||
| Intellectual property | $ | 2,101 | $ | 2,101 | ||||
| Website - qurr.com | 380 | 380 | ||||||
| Less: accumulated amortization | (841 | ) | (574 | ) | ||||
| Total intangibles with finite lives: | 1,640 | 1,907 | ||||||
| Intangibles with indefinite lives: | ||||||||
| Patent costs | 44 | 44 | ||||||
| Less: impairment charge on patent costs | (44 | ) | (44 | ) | ||||
| Total intangibles with indefinite lives: | - | - | ||||||
| Total intangible assets, net | $ | 1,640 | $ | 1,907 | ||||
Amortization expense related to intangible assets for the years ended December 31, 2017 and 2016 was $267 and $210
Based on fourteen consecutive quarters of minimal revenues combined with changes in the sales channels through which the Company sells its products and an inability to predict future orders, if any, we tested the intellectual property for impairment in the fourth quarter of 2017 and determined that the asset value was recoverable and therefore no impairment was recognized.
We had impairment losses recorded during the years ended December 31, 2017 and 2016 of $-0- and $44, respectively. The impairment losses were related to the write-off of capitalized patent costs due to the unlikelihood of certain patents being issued.
Assuming no additions, disposals or adjustments are made to the carrying values and/or useful lives of the intangible assets, annual amortization expense for intangible assets is estimated to be $286 in each of the next five years.
Intangible assets also includes patent costs associated with applying for a patent and being issued a patent. Costs to defend a patent and costs to invalidate a competitor’s patent or patent application are expensed as incurred. Upon issuance of the patent, capitalized patent costs are reclassified from intangibles with indefinite lives to intangibles with finite lives and amortized on a straight-line basis over the shorter of the estimated economic life or the initial term of the patent, generally 20 years.
Impairment testing of intangible assets subject to amortization involves comparing the carrying amount of the asset to the forecasted, undiscounted future cash flows whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. In the event the carrying value of the asset exceeds the undiscounted future cash flows, the carrying value is considered not recoverable and an impairment exists. An impairment loss is measured as the excess of the asset’s carrying value over its fair value, calculated using a discounted future cash flow method. The computed impairment loss is recognized in the period that the impairment occurs. Assets which are not impaired may require an adjustment to the remaining useful lives for which to amortize the asset. Impairment testing requires the development of significant estimates and assumptions involving the determination of estimated net cash flows, selection of the appropriate discount rate to measure the risk inherent in future cash flow streams, assessment of an asset’s life cycle, competitive trends impacting the asset as well as other factors. Changes in these underlying assumptions could significantly impact the asset’s estimated fair value.
F-11
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
Revenue Recognition
The Company records revenue from product sales when persuasive evidence of an arrangement exists, product has been shipped or delivered, the sales price to the customer is fixed or determinable, and collectability is reasonably assured. Product sales represent revenue from the sale of products and related shipping amounts billed to customers, net of promotional discounts, rebates, and return allowances. Depending on individual customer agreements, sales are recognized either upon shipment of product to customers or upon delivery. With respect to direct-to-consumer sales, both title and risk of loss transfer to customers upon our delivery to the customer. The Company’s gross product sales may be subject to sales allowances and deductions in arriving at reported net product sales. For example, we may periodically offer discounts and sales incentives to customers to encourage purchases. Sales incentives are treated as a reduction to the purchase price of the related transaction. Reductions from gross sales for customer discounts and rebates have been minimal, and sales allowances for product returns have not been provided, since under our existing arrangements, customers are not permitted to return product except for non-conforming product.
The adoption of Topic 606 is required for public entities for reporting periods beginning after December 15, 2017. This accounting guidance is effective for us beginning January 1, 2018 using one of two prescribed transition methods. The Company will adopt the provisions of Topic 606 for its fiscal year beginning January 1, 2018 using the modified retrospective transition method. This method involves application of the new guidance to either: (a) all contracts at the date of initial application or (b) only contracts that are not completed at the date of initial application. Under this method, a cumulative effect adjustment is recognized as of the date of initial application. The Company has evaluated the impact of the updated guidance and has determined that the adoption is not expected to have a significant impact on its consolidated financial statements for 2016 and 2017 and related disclosure.
Advertising
The Company charges the costs of advertising to sales and marketing expenses as incurred. Advertising costs were $267 and $172 for the years ended December 31, 2017 and 2016, respectively. For the year ended December 31, 2017, advertising costs consisted primarily of marketing costs for our QURR products. For the year ended December 31, 2016, advertising costs consisted primarily of marketing costs for our Rē Muscle Health products.
Research and Development
Research and development expenses consist primarily of the cost of manufacturing our product for clinical study, the cost of conducting clinical studies and the cost of conducting preclinical and research activities. Nonrefundable advance payments for goods or services that will be used or rendered for future research and development activities are initially capitalized and are then recognized as an expense as the related goods are consumed or the services are performed. During the years ended December 31, 2017 and 2016, the Company incurred research and development expenses of $46 and $-0- respectively which are charged to sales and marketing expenses in the consolidated statement of operations.
Shipping and Handling Costs
The Company records costs for the shipping and handling of products to our customers in cost of sales. These expenses were $37 and $21 for the years ended December 31, 2017 and 2016, respectively.
Share-based Compensation
Share-based payments are measured at their estimated fair value on the date of grant. Share-based awards to non-employees are re-measured at fair value each financial reporting date until performance is completed. Share-based compensation expense recognized during a period is based on the estimated number of awards that are ultimately expected to vest. For stock options and restricted stock that do not vest immediately but which contain only a service vesting feature, we recognize compensation cost on the unvested shares and options on a straight-line basis over the remaining vesting period.
The Company uses the Black-Scholes option-pricing model to estimate the fair value of options and the market price of our common stock on the date of grant for the fair value of restricted stock issued. Our determination of the fair value of stock-based awards is affected by our stock price as well as assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, our expected stock price volatility over the term of the awards, and certain other market variables such as the risk-free interest rate.
F-12
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
Deferred Offering Costs
Upon the successful completion of issuance of our common stock the Company recognizes offering costs as a reduction of equity. In the event that an offering is aborted, such costs are recorded as an expense.
Segment Information
Accounting Standards Codification (“ASC”) 280, Disclosures about Segments of an Enterprise and Related Information, establishes standards for reporting information about operating segments and requires selected information for those segments to be presented in the financial statements. It also establishes standards for related disclosures about products and services and geographic areas. Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions how to allocate resources and assess performance. Management has determined that the Company operates in one segment.
Fair Value Measurement
Fair value is the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants. The authoritative guidance on fair value measurements establishes a consistent framework for measuring fair value on either a recurring or nonrecurring basis whereby observable and unobservable inputs, used in valuation techniques, are assigned a hierarchical level.
The following are the hierarchy levels of inputs to measure fair value:
| Level 1: | Inputs that utilize quoted prices (unadjusted) in active markets for identical assets or liabilities. | |
| Level 2: | Inputs that utilize observable quoted prices for similar assets and liabilities in active markets and observable quoted prices for identical or similar assets in markets that are not very active. | |
| Level 3: | Inputs that utilize unobservable inputs and include valuations of assets or liabilities for which there is little, if any, market activity. |
A financial asset or liability’s classification within the above hierarchy is determined based on the lowest level input that is significant to the fair value measurement. At December 31, 2017 and 2016, the Company’s financial instruments consist primarily of cash and cash equivalents, accounts receivable, prepaid expenses and other current assets, accounts payable and accrued expenses and other current liabilities. Due to their short-term nature, the carrying amounts of the Company’s financial instruments approximated their fair values.
Basic and Diluted Loss Per Share
Basic net loss per share is computed by dividing net loss available to common stockholders for the period by the weighted average number of common shares outstanding during the period. Diluted net loss per share is computed by dividing net loss for the period by the weighted average number of common shares outstanding during the period increased to include the number of additional shares of common stock that would have been outstanding if potential dilutive securities outstanding had been issued. The Company uses the “treasury stock” method to determine the dilutive effect of common stock equivalents such as options, warrants and restricted stock. For the years ended December 31, 2017 and 2016, the Company incurred a net loss. Accordingly, the Company’s common stock equivalents were anti-dilutive and excluded from the diluted net loss per share computation.
The aggregate number of potentially dilutive common stock equivalents outstanding at December 31, 2017 excluded from the diluted net loss per share computation because their inclusion would be anti-dilutive were 1,384,192, which includes warrants to purchase an aggregate 821,202 shares of common stock, options to purchase an aggregate of 561,740 shares of common stock, and unvested restricted stock awards of 1,250 shares of common stock.
The aggregate number of potentially dilutive common stock equivalents outstanding at December 31, 2016 excluded from the diluted net loss per share computation because their inclusion would be anti-dilutive were 1,491,075, which includes warrants to purchase an aggregate 1,136,878 shares of common stock, options to purchase an aggregate of 300,340 shares of common stock, and unvested restricted stock awards of 53,857 shares of common stock.
F-13
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
Income Taxes
Income taxes are accounted for under the asset and liability method in accordance with ASC 740, Accounting for Income Taxes (“ASC 740”). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial carrying amounts of existing assets and liabilities and their respective tax bases as well as operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the periods in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Deferred tax assets are reduced by a valuation allowance to the extent that the recoverability of the asset is unlikely to be recognized. The Company follows ASC 740 rules governing uncertain tax positions, which provides guidance for recognition and measurement. This prescribes a threshold condition that a tax position must meet for any of the benefits of the uncertain tax position to be recognized in the financial statements. It also provides accounting guidance on recognition, classification and disclosure of these uncertain tax positions. The Company has no uncertain income tax positions.
The Tax Cut and Jobs Act (the “Tax Act”) was enacted on December 22, 2017. The Tax Act contains several key provisions including, among other things, reducing the U.S. federal corporate tax rate from thirty-five percent to twenty-one percent. Changes in tax law are accounted for in the period of enactment. In addition, Federal net operating losses (“NOL”) generated during future periods will be carried forward indefinitely, but will be subject to an eighty percent utilization against taxable income. The carryback provision has been revoked for NOL after January 1, 2018.
The Company continues to evaluate the impact of the Tax Act and analyze additional guidance.
Interest costs and penalties related to income taxes are classified as interest expense and operating expenses, respectively, in the Company’s financial statements. For the years ended December 31, 2017 and 2016, the Company did not recognize any interest or penalty expense related to income taxes. The Company files income tax returns in the U.S. federal jurisdiction and states in which it does business.
NOTE 3 – RECENT ACCOUNTING PRONOUNCEMENTS
In September 2017, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2017-13, Revenue from Contracts with Customers which amended FASB Accounting Standards Codification® (ASC) by creating Topic 606, Revenue from Contracts with Customers. In May 2014, the FASB issued ASU No. 2014-09, “Revenue from Contracts with Customers” (“ASU 2014-09”). ASU 2014-09 supersedes nearly all existing revenue recognition guidance under U.S. GAAP and requires revenue to be recognized when promised goods or services are transferred to customers in an amount that reflects the consideration that is expected to be received for those goods or services. Additionally, qualitative and quantitative disclosures are required about customer contracts, significant judgments and changes in judgments, and assets recognized from the costs to obtain or fulfill a contract.
The FASB also issued the following amendments to ASU No. 2014-09 to provide clarification on the guidance:
| ● | ASU No. 2015-14, Revenue from Contracts with Customers (Topic 606) – Deferral of the Effective Date |
| ● | ASU No. 2016-08, Revenue from Contracts with Customers (Topic 606) – Principal versus Agent (Reporting Revenue Gross vs. Net) |
| ● | ASU No. 2016-10, Revenue from Contracts with Customers (Topic 606) – Identifying Performance Obligations and Licensing |
| ● | ASU No. 2016-12, Revenue from Contracts with Customers (Topic 606) – Narrow-Scope Improvements and Practical Expedients |
The adoption of Topic 606 is required for public entities for reporting periods beginning after December 15, 2017. This accounting guidance is effective for us beginning January 1, 2018 using one of two prescribed transition methods. The Company has evaluated the impact of the updated guidance and has determined that the adoption of ASU 2017-09 is not expected to have a significant impact on its consolidated financial statements for 2016 and 2017 and related disclosure.
F-14
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
The Company will adopt the provisions of this ASU for its fiscal year beginning January 1, 2018 using the modified retrospective transition method. This method involves application of the new guidance to either: (a) all contracts at the date of initial application or (b) only contracts that are not completed at the date of initial application. Under this method, a cumulative effect adjustment is recognized as of the date of initial application.
In May 2017, the FASB issued ASU No. 2017-09, Compensation – Stock Compensation (Topic 718). The amendments in this Update provide guidance about which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting in Topic 718. This update is effective for all entities for annual periods, and interim periods within those annual periods, beginning after December 15, 2017. Early adoption is permitted, including adoption in any interim period, for (1) public business entities for reporting periods for which financial statements have not yet been issued and (2) all other entities for reporting periods for which financial statements have not yet been made available for issuance. The amendments in this update should be applied prospectively to an award modified on or after the adoption date. The Company has evaluated the impact of the updated guidance and has determined that the adoption of ASU 2017-09 is not expected to have a significant impact on its consolidated financial statements.
In January 2017, the FASB issued ASU No. 2017-04, “Simplifying the Test for Goodwill”, which accomplishes exactly what its title indicates by eliminating the second step in the current goodwill impairment calculation. Currently there is a two-step process for determining the amount of any goodwill impairment. In Step 1 an entity determines if the carrying value of the reporting unit (for which goodwill has been recorded) exceeds the fair value of the reporting unit. If the calculation in Step 1 indicates that the carrying value of a reporting unit for which goodwill has been recorded exceeds the fair value, the entity would have to determine the implied fair value of the reporting unit’s goodwill. An impairment would be recorded to the extent that the goodwill carrying value exceeded the implied fair value of goodwill at the reporting date. The amount of any goodwill impairment must take into consideration the effects of income taxes for any tax deductible goodwill. The effective date to adopt the ASU is for fiscal years beginning after December 15, 2019. The ASU is to be applied prospectively. Early adoption is permitted. The Company has evaluated the impact of the updated guidance and has determined that the adoption of ASU 2017-04 is not expected to have a significant impact on its consolidated financial statements.
In August 2016, the FASB issued ASU 2016-15, “Classification of Certain Cash Receipts and Cash Payments (a consensus of the Emerging Issues Task Force).” The amendments in this Update relate to eight specific types of cash receipts and cash payments which current U.S. GAAP either is unclear or does not include specific guidance on the cash flow classification issues. The amendments in this Update are effective for public business entities for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. The Company has adopted the provisions of this ASU for its fiscal year beginning January 1, 2018. The adoption of ASU 2016-15 did not have a significant impact on its consolidated financial statements.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842) (“ASU 2016-02”), which requires lessees to recognize on the balance sheet the assets and liabilities for the rights and obligations created by leases with lease terms of more than 12 months. The recognition, measurement, and presentation of expenses and cash flows arising from a lease by a lessee will continue to primarily depend on its classification as a finance or operating lease. However, unlike U.S. GAAP, which requires only capital leases to be recognized on the balance sheet, ASU 2016-02 will require both types of leases to be recognized on the balance sheet. ASU 2016-02 also requires disclosures about the amount, timing, and uncertainty of cash flows arising from leases. These disclosures include qualitative and quantitative requirements, providing additional information about the amounts recorded in the financial statements. ASU 2016-02 is effective beginning January 1, 2019, with early application permitted. We have evaluated the adoption of ASU 2016-12 and determined that the standard will not have a significant impact on our consolidated financial statements.
In July 2015, the FASB issued ASU No. 2015-11, Inventory (Topic 330) “Simplifying the Measurement of Inventory” (“ASU 2015-11”), which changes the measurement principle for inventory from the lower of cost or market to the lower of cost and net realizable value. ASU 2015-11 defines net realizable value as estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The new guidance must be applied on a prospective basis by us beginning January 1, 2017, with early adoption permitted. The adoption of ASU 2015-17 did not have a significant impact on the consolidated financial statements.
F-15
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements – Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern” (“ASU 2014-15”). The amendments in this update define management’s responsibility to evaluate whether there is substantial doubt about an organization’s ability to continue as a going concern and provides related footnote disclosure requirements. Under U.S. GAAP, financial statements are prepared under the presumption that the reporting organization will continue to operate as a going concern, except in limited circumstances. Financial reporting under this presumption is commonly referred to as the going concern basis of accounting. The going concern basis of accounting establishes the fundamental basis for measuring and classifying assets and liabilities. This update provides guidance on when there is substantial doubt about an organization’s ability to continue as a going concern and how the underlying conditions and events should be disclosed in the footnotes. It is intended to reduce diversity that existed in footnote disclosures because of the lack of guidance about when substantial doubt existed. The amendments in this update are effective for us beginning December 31, 2016. The Company has evaluated the impact of the updated guidance and has disclosed the impact in the footnotes on its consolidated financial statements.
NOTE 4 – INVENTORIES, NET
Inventories, net at December 31, 2017 and 2016 consisted of the following:
| (In thousands $) | December 31, 2017 | December 31, 2016 | ||||||
| Raw materials | $ | 2,223 | $ | 2,378 | ||||
| Work in process | 64 | 5 | ||||||
| Finished goods | 203 | 188 | ||||||
| 2,490 | 2,571 | |||||||
| Less: inventory reserves | (711 | ) | (709 | ) | ||||
| Inventories, net | $ | 1,779 | $ | 1,862 | ||||
NOTE 5 – FIXED ASSETS
Fixed assets at December 31, 2017 and 2016 consisted of the following:
| (In thousands $) | December 31, 2017 | December 31, 2016 | ||||||
| Furniture, fixtures and equipment | $ | 116 | $ | 116 | ||||
| Computers and software | 68 | 66 | ||||||
| Leasehold improvements | 239 | 239 | ||||||
| Other | 7 | 7 | ||||||
| Total fixed assets | 430 | 428 | ||||||
| Less: accumulated depreciation and amortization | (246 | ) | (195 | ) | ||||
| Net book value of fixed assets | $ | 184 | $ | 233 | ||||
Depreciation and amortization expense was $51 and $54 for the years ended December 31, 2017 and 2016, respectively. Repairs and maintenance costs are expensed as incurred.
NOTE 6 – DEBT
Convertible Note
On December 17, 2015, concurrent with the execution of a securities purchase agreement with RENS Technology Inc., the Company issued an unsecured promissory note in the principal amount of $575 (the “Note”) to Gan Ren, a related party of RENS Agriculture. The Note accrued interest at a rate of 8% per annum and matured on December 17, 2016. On December 17, 2016, the Note and accrued interest of $46 were automatically converted into 225,860 shares of common stock at $2.75 per share.
F-16
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
Term Note
On September 10, 2015, the Company converted its outstanding revolving note of $400 with City National Bank, which had a termination date of August 31, 2015, into a term note (the “Term Note”). At December 31, 2015, the balance under the Term Note was $100, which was subsequently paid in full in January 2016 (as reflected in cash flows from financing activities in our Consolidated Statements of Cash Flows as of December 31, 2016).
NOTE 7 – PREPAID EXPENSES, ACCRUED EXPENSES, OTHER CURRENT ASSETS AND LIABILITIES
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consist of various payments that the Company has made in advance for goods or services to be received in the future. Prepaid expenses and other current assets at December 31, 2017 and 2016 consisted of the following:
| (In thousands $) | December 31, 2017 | December 31, 2016 | ||||||
| Prepaid insurance | $ | 88 | $ | 27 | ||||
| Prepaid consulting and other | 75 | 58 | ||||||
| Total prepaid expenses and other current assets | $ | 163 | $ | 85 | ||||
Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of estimated future payments that relate to the current and prior accounting periods. Management reviews these estimates regularly to determine their reasonableness. Accrued expenses and other current liabilities at December 31, 2017 and 2016 consisted of the following:
| (In thousands $) | December 31, 2017 | December 31, 2016 | ||||||
| Marketing | $ | - | $ | 171 | ||||
| Audit and tax fees | 82 | 88 | ||||||
| Insurance premium financing | 66 | - | ||||||
| Deferred rent | 19 | 23 | ||||||
| Bonus | 17 | 15 | ||||||
| Legal fees | 69 | 47 | ||||||
| Other accrued expenses | 2 | 17 | ||||||
| Total accrued expenses | $ | 255 | $ | 361 | ||||
F-17
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
Note 8 – Stockholders’ Equity
Preferred Stock Rights
Effective February 14, 2017, the Board of Directors declared a dividend of one Right for each of the Company’s issued and outstanding shares of common stock. The Rights were granted to the stockholders of record at the close of business on February 24, 2017. Each Right entitles the registered holder, upon the occurrence of certain events specified in the Rights Agreement, to purchase from the Company one one-thousandth of a share of the Company’s Series A Preferred Stock at a price of $7.00), subject to certain adjustments. The Rights are not exercisable until the occurrence of certain events, including a person acquiring or obtaining the right to acquire beneficial ownership of 10% or more of the Company’s outstanding common stock. The Rights are evidenced by certificates for the common stock and automatically transfer with the common stock unless they become exercisable. If the Rights become exercisable, separate certificates evidencing the Rights will be distributed to each holder of common stock. Holders of the preferred stock will be entitled to certain dividend, liquidation and voting rights. The rights are redeemable by the Company at a fixed price as determined by the Board, after certain defined events.
As of December 31, 2017, the Rights have no dilutive effect on the earnings per common share calculation and no shares of preferred stock have been issued. The Company has determined that these rights have a de minimis fair value. The description and terms of the Rights are set forth in the Rights Agreement dated as of February 14, 2017 between the Company and Island Stock Transfer, as Rights Agent.
Increase in Number of Authorized Shares
On March 8, 2016, the Company filed a Certificate of Amendment to its Articles of Incorporation with the Secretary of State of the State of Nevada to increase the number of authorized shares of common stock. As a result of the amendment, the number of the Company’s authorized shares of common stock increased from 8,000,000 to 12,000,000.
Issuance of Common Stock
The Company has periodically issued common stock in connection with certain private and public offerings. For the years ended December 31, 2017 and 2016, the Company has received aggregate gross proceeds of $8,447 from these offerings as follows:
| (In thousand $) | Gross | |||||||
| Date | Shares | Proceeds | ||||||
| March 3, 2016 | 1,500,000 | (1) | $ | 5,250 | ||||
| February 8, 2017 | 500,000 | (2) | 2,125 | |||||
| October 31, 2017 | 500,000 | (3) | 1,072 | |||||
| 2,000,000 | $ | 8,447 | ||||||
| (1) | Shares issued pursuant to the closing of first tranche of the financing with RENS Technology Inc. |
| (2) | Shares issued pursuant to a registered direct offering at a purchase price of $4.25 per share. |
| (3) | Shares issued at $2.144 per share under the at the market program. |
Registered Direct Offering
On February 3, 2017, the Company entered into a securities purchase agreement with an institutional investor providing for the issuance and sale by the Company of 500,000 shares of common stock, in a registered direct offering at a purchase price of $4.25 per share, for gross proceeds of $2,125. The offering closed on February 8, 2017. Offering costs of $199 were recognized as an offset to additional paid in capital.
At-the-Market Offering
On February 21, 2017, the Company entered into a sales agreement with H.C. Wainwright & Co., LLC which established an at-the-market equity program pursuant to which the Company may offer and sell up to $6.0 million of its shares of common stock from time to time through H.C. Wainwright. The Company incurred $125 of deferred offering costs in connection with this program which it has recorded as a long term other asset on the accompanying balance sheet. In October 2017 the Company raised $1,072 through the sale of 500,000 shares of common stock at $2.144 per share under the program and recognized deferred costs of $54 with this offering.
Subsequent to year end on January 19, 2018 the Company sold 140,295 shares of common stock at $2.111 per share for gross proceeds of $296 less deferred costs of $9 in an at-the-market transaction.
F-18
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
Note 9 – Warrants
On March 3, 2016, the Company completed the first tranche of the financing, in which it issued a warrant to purchase 375,000 shares of common stock. The warrant is immediately exercisable upon issuance, will expire five years after issuance and has an exercise price of $7.00 per share. The warrant was determined to have an estimated aggregate fair value of $480 at issuance.
The following table summarizes information about outstanding and exercisable warrants at December 31, 2017:
| Shares | ||||||||||||||||||||||
| Underlying | ||||||||||||||||||||||
| Shares | Warrants | |||||||||||||||||||||
| Number of | Underlying | Outstanding | ||||||||||||||||||||
| Shares | Warrants | and | ||||||||||||||||||||
| Underlying | Exchanged, | Exercisable | ||||||||||||||||||||
| Warrants | Exercised | at | Expiration | |||||||||||||||||||
| Originally | or | December 31, | Exercise | Term | ||||||||||||||||||
| Description | Grant Date | Granted | Expired | 2017 | Price | in years | ||||||||||||||||
| Series A(1) | January 27, 2014 | 315,676 | (315,676 | ) | - | N/A | N/A | |||||||||||||||
| Series B(1) | January 27, 2014 | 157,846 | - | 157,846 | $ | 45.00 | 1.07 | |||||||||||||||
| Series C(2) | November 19, 2014 | 145,399 | (142,957 | ) | 2,442 | $ | 12.00 | 2.38 | ||||||||||||||
| 142,957 | 142,957 | $ | 9.00 | 2.38 | ||||||||||||||||||
| Series D(2) | November 19, 2014 | 193,865 | (193,865 | ) | - | N/A | N/A | |||||||||||||||
| Series E(2) | November 19, 2014 | 145,399 | (145,399 | ) | - | N/A | N/A | |||||||||||||||
| 142,957 | 142,957 | $ | 9.00 | 4.38 | ||||||||||||||||||
| Rens(3) | March 3, 2016 | 375,000 | - | 375,000 | $ | 7.00 | 3.17 | |||||||||||||||
| 1,333,185 | (511,983 | ) | 821,202 | |||||||||||||||||||
| (1) | Issued in connection with the January 27, 2014 private placement transaction. |
| (2) | Issued in connection with the November 19, 2014 registered-direct public offering, and subsequently revised pursuant to Warrant Exercise Agreements entered into on May 18, 2015. |
| (3) | Shares issued pursuant to the closing of the first tranche of the financing with RENS Technology Inc. |
F-19
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
The following table summarizes the activities in warrants for the years ended December 31, 2017 and 2016:
| Shares Underlying Warrants | Average Exercise Price | |||||||
| Balance at December 31, 2015 | 761,878 | $ | 18.95 | |||||
| Warrants granted | 375,000 | 7.00 | ||||||
| Balance at December 31, 2016 | 1,136,878 | $ | 15.01 | |||||
| Warrants expired | (315,676 | ) | 15.00 | |||||
| Balance at December 31, 2017 | 821,202 | $ | 11.68 | |||||
The following table summarizes the assumptions used to value the warrants at the issuance date using the Black-Scholes option pricing model:
| Number | ||||||||||||||||||||||||||||||
| of | ||||||||||||||||||||||||||||||
| Shares | Stock | |||||||||||||||||||||||||||||
| Grant / | Underlying | Price on | ||||||||||||||||||||||||||||
| Modification | Warrants | Measurement | Exercise | Expected | Expected | Dividend | Risk Free | |||||||||||||||||||||||
| Description | Date | Granted | Date | Price | Term | Volatility | Yield | Rate | ||||||||||||||||||||||
| Series B | 1/27/2014 | 157,846 | $ | 7.00 | $ | 45.00 | 5.00 | 150.00 | % | 0.00 | % | 1.61 | % | |||||||||||||||||
| Series C | 11/19/2014 | 2,442 | $ | 9.37 | $ | 12.00 | 5.50 | 94.60 | % | 0.00 | % | 1.64 | % | |||||||||||||||||
| Repricing Series C | 5/18/2015 | 142,957 | $ | 5.95 | $ | 9.00 | 5.00 | 96.34 | % | 0.00 | % | 1.46 | % | |||||||||||||||||
| Repricing Series E | 5/18/2015 | 142,957 | $ | 5.95 | $ | 9.00 | 7.00 | 96.34 | % | 0.00 | % | 1.87 | % | |||||||||||||||||
| Rens | 3/3/2016 | 375,000 | $ | 7.00 | $ | 7.00 | 4.00 | 96.34 | % | 0.00 | % | 1.87 | % | |||||||||||||||||
NOTE 10 – STOCK COMPENSATION
Equity Incentive Plan
The Company increased the number of shares available for issuance under its 2012 Equity Incentive Plan (as amended, the “Plan”) from 550,000 to 850,000 in November 2016, which was approved by the Company’s shareholders in December 2016. The plan provides for the issuance of up to 850,000 shares. The Plan provides for grants of stock options, stock appreciation rights, restricted stock, other stock-based awards and other cash-based awards. As of December 31, 2017, the remaining shares of common stock available for future issuances of awards was 288,260. The Company granted an aggregate of 30,000 options to purchase restricted common stock to certain directors prior to the adoption of the Plan.
Stock options generally vest and become exercisable with respect to 100% of the common stock subject to such stock option on the third (3rd) anniversary of the date of grant. Any unvested portion of a stock option shall expire upon termination of employment or service of the participant granted the stock option, and the vested portion shall remain exercisable in accordance with the provisions of the Plan.
F-20
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
Stock Options
The following table summarizes stock option activity for the years ended December 31, 2017 and 2016:
| Weighted | ||||||||||||
| Weighted | Average | |||||||||||
| Shares | Average | Remaining | ||||||||||
| Under | Exercise | Contractual | ||||||||||
| Options | Price | Term (Years) | ||||||||||
| Balance at December 31, 2015 | 400,545 | $ | 14.56 | 7.82 | ||||||||
| Options cancelled | (65,455 | ) | 13.14 | |||||||||
| Options forfeited | (34,750 | ) | 12.51 | |||||||||
| Balance at December 31, 2016 | 300,340 | $ | 15.09 | 6.71 | ||||||||
| Options granted | 300,000 | $ | 4.00 | |||||||||
| Options forfeited | (38,600 | ) | 7.15 | |||||||||
| Balance at December 31, 2017 | 561,740 | $ | 7.32 | 5.61 | ||||||||
The weighted average grant date fair value of stock options granted during 2017 was $0.86. The following table summarizes the assumptions used to value stock options granted in 2017 using a Black-Scholes model:
| 2017 | ||||
| Risk-free interest rate | 2.19 | % | ||
| Expected volatility | 100 | % | ||
| Expected forfeiture rate | 0 | % | ||
| Expected term (years) | 6.0 | |||
| Expected dividend yield | 0 | % | ||
The risk-free rate is based on the U.S. Treasury rate for a note with a similar term in effect at the time of the grant. The expected volatility is based on the volatility of the Company’s historical stock prices.
At December 31, 2017 and 2016, the exercisable options had no intrinsic value.
F-21
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
The following table summarizes information about options outstanding and exercisable at December 31, 2017 that were granted under the Plan:
| Options Outstanding | Options Exercisable | |||||||||||||||||||||
| Weighted Average | Weighted Average | |||||||||||||||||||||
| Exercise | Options | Remaining | Exercise | Options | Remaining | |||||||||||||||||
| Price | Outstanding | Contractual Life | Price | Exercisable | Contractual Life | |||||||||||||||||
| $ | 4.00 | 300,000 | 9.65 | $ | 4.00 | - | 9.65 | |||||||||||||||
| $ | 8.60 | 16,000 | 6.19 | $ | 8.60 | 16,000 | 6.19 | |||||||||||||||
| $ | 10.00 | 40 | 4.89 | $ | 10.00 | 40 | 4.89 | |||||||||||||||
| $ | 12.10 | 30,000 | 6.35 | $ | 12.10 | 30,000 | 6.35 | |||||||||||||||
| $ | 12.50 | 81,700 | 6.42 | $ | 12.50 | 67,278 | 5.94 | |||||||||||||||
| $ | 13.45 | 2,000 | 6.47 | $ | 13.45 | 1,000 | 6.47 | |||||||||||||||
| $ | 13.50 | 12,000 | 6.49 | $ | 13.50 | 10,562 | 6.49 | |||||||||||||||
| $ | 17.50 | 100,000 | 5.11 | $ | 17.50 | 100,000 | 5.11 | |||||||||||||||
| $ | 32.00 | 15,000 | 3.54 | $ | 32.00 | 15,000 | 3.54 | |||||||||||||||
| $ | 34.50 | 5,000 | 3.57 | $ | 34.50 | 5,000 | 3.57 | |||||||||||||||
| 561,740 | 244,880 | |||||||||||||||||||||
As of December 31, 2017, 244,880 options have vested and 316,860 options remain unvested. The vesting terms range from 4.5 to 9.7 years and the vested options have a weighted average remaining term of 5.7 years and a weighted average exercise price of $15.3 per share.
Restricted Stock
The following table summarizes restricted stock awards activity for the years ended December 31, 2017 and 2016:
| Weighted | ||||||||
| Average | ||||||||
| Grant Date | ||||||||
| Shares | Share Price | |||||||
| Restricted stock awards unvested at December 31, 2015 | 18,450 | $ | 9.09 | |||||
| Granted | 70,639 | 2.08 | ||||||
| Vested | (30,232 | ) | 5.40 | |||||
| Forfeited | (5,000 | ) | 2.27 | |||||
| Restricted stock awards unvested at December 31, 2016 | 53,857 | 2.74 | ||||||
| Vested | 46,607 | 5.17 | ||||||
| Forfeited | (6,000 | ) | 1.75 | |||||
| Restricted stock awards unvested at December 31, 2017 | 1,250 | $ | 1.02 | |||||
At December 31, 2017, the weighted-average vesting period of unvested restricted stock awards was 1.02 years.
F-22
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
Share-Based Compensation
Share-based compensation was $160 and $392 for the years ended December 31, 2017 and 2016, respectively. Share-based compensation consists of expenses related to the issuance of stock options and restricted stock.
The aggregate unrecognized compensation expense of stock options and restricted stock at December 31, 2017 was $154, which will be recognized through January 2019.
NOTE 11 – INCOME TAXES
Income tax expense for the years ended December 31, 2017 and 2016 is shown as follows:
| December 31, | December 31, | |||||||
| (In thousand $) | 2017 | 2016 | ||||||
| Current provision | $ | - | $ | - | ||||
| Deferred provision | - | - | ||||||
| Total tax provision (benefit) | $ | - | $ | - | ||||
The significant components of the Company’s deferred tax assets and liabilities at December 31, 2017 and 2016 are as follows:
| December 31, | December 31, | |||||||
| (In thousand $) | 2017 | 2016 | ||||||
| Federal net operating losses | $ | 5,031 | $ | 6,714 | ||||
| State net operating losses | 1,060 | 635 | ||||||
| Stock options | 733 | 1,043 | ||||||
| Federal tax credit | 190 | 190 | ||||||
| Amortization | 295 | 448 | ||||||
| Depreciation | (3 | ) | 11 | |||||
| Contributions | 15 | 21 | ||||||
| Other | 202 | 392 | ||||||
| Total gross deferred tax assets/(liabilities) | 7,523 | 9,454 | ||||||
| Less valuation allowance | (7,523 | ) | (9,454 | ) | ||||
| Net deferred tax assets/(liabilities) | $ | - | $ | - | ||||
F-23
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
On December 22, 2017, the United States enacted the Tax Cuts and Jobs Act ("Tax Reform Legislation"), which made significant changes to U.S. federal income tax law. The Company expects that certain aspects of the Tax Reform Legislation will positively impact the Company’s future after-tax earnings primarily due to the lower federal statutory tax rate. Beginning January 1, 2018, the Company’s U.S. income will be taxed at a 21 percent federal corporate rate. Further, we are required to recognize the effect of this rate change on our deferred tax assets and liabilities, and deferred tax asset valuation allowances in the period the tax rate change is enacted. We do not expect any material non-cash impact from this rate change, with adjustments to deferred tax balances offset by adjustments to deferred tax valuation allowances.
The income tax benefit for the year ended December 31, 2017 differed from the amounts computed by applying the U.S. federal income tax rate of 34% to loss before tax benefit as a result of nondeductible expenses, tax credits generated, utilization of net operating loss carryforwards, and increases in the Company’s valuation allowance.
| December 31, | December 31, | |||||||
| (In thousand $) | 2017 | 2016 | ||||||
| Federal statutory tax benefit | $ | (1,380 | ) | $ | (1,568 | ) | ||
| Permanent differences | 55 | 103 | ||||||
| Federal tax rate change | 3,696 | - | ||||||
| Valuation allowance | (2,371 | ) | 1,465 | |||||
| Income tax provision (benefit) | $ | - | $ | - | ||||
A valuation allowance is required to reduce the deferred tax assets reported if, based on the weight of the evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized.
At December 31, 2017, the Company had approximately $23.9 million of gross federal net operating loss carry-forwards. At December 31, 2017, the Company had approximately $14.9 million of gross state net operating loss carry-forwards. If not utilized, the federal and state net operating loss carry-forwards will begin to expire in 2027. The utilization of such net operating loss carry-forwards and realization of tax benefits in future years depends predominantly upon having taxable income. The Company also has $190 of federal research and development credits which will begin to expire in 2033 if not utilized.
The Company may be subject to the net operating loss provisions of Section 382 of the Internal Revenue Code. The Company has not calculated if an ownership change has occurred. The effect of an ownership change would be the imposition of an annual limitation on the use of NOL carryforwards attributable to periods before the change. The amount of the annual limitation depends upon the value of the Company immediately before the change, changes to the Company’s capital during a specified period, and the federal published interest rate.
Entities are also required to evaluate, measure, recognize and disclose any uncertain income tax provisions taken on their income tax returns. The Company has analyzed its tax positions and has concluded that as of December 31, 2017 there were no uncertain positions. The federal and state income tax returns of the Company for 2013, 2014, 2015 and 2016 are subject to examination by the IRS and state taxing authorities, generally for three years after they are filed. Interest and penalties, if any, as they relate to income taxes assessed, are included in the income tax provision. There was no income tax related interest and penalties included in the income tax provision for 2017 and 2016.
F-24
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
Note 12 – Commitments and Contingencies
Operating Lease
The Company leases its corporate offices under an operating lease. The term of the lease is five years commencing on January 1, 2015 and expiring on December 31, 2019. The Company has two options to renew its lease for an additional three years each.
At December 31, 2017, the future minimum lease payments under the non-cancellable operating lease in excess of one year is as follows:
| (In thousand $) | ||||
| Years Ended December 31, | Amount | |||
| 2018 | $ | 71 | ||
| 2019 | 72 | |||
| Total | $ | 143 | ||
Rent expense including common area maintenance charges and taxes for the years ended December 31, 2017 and 2016 was $68 and $72, respectively.
Defined Contribution Plan
The Company established a 401(K) Plan (the “401(K) Plan”) for eligible employees of the Company effective April 1, 2014. Generally, all employees of the Company who are at least twenty-one years of age and who have completed three months of service are eligible to participate in the 401(K) Plan. The 401(K) Plan is a defined contribution plan that provides that participants may make salary deferral contributions, of up to the statutory maximum allowed by law (subject to catch-up contributions) in the form of voluntary payroll deductions. The Company’s matching contribution is equal to 100 percent on the first four percent of a participant’s compensation which is deferred as an elective deferral. The Company’s aggregate matching contributions were $26 and $26 for the years ended December 31, 2017 and 2016, respectively.
Supply Agreement
On November 18, 2016, the Company entered into an Amended Supply Agreement with DIL Technologie GmbH (“DIL”). Pursuant to the agreement (and so long as the agreement is effective), DIL will manufacture and supply the Company with Fortetropin®, the active ingredient for its products, and the Company will purchase quantities of Fortetropin® from DIL at its discretion. DIL will manufacture the formula exclusively for the Company in perpetuity, and may not manufacture the formula for other entities (but may manufacture it for its own non-commercial research).
The Company agreed, commencing January 2017, to pay DIL €10 (approximately USD $12) per month for collaborative research. For the year ended December 31, 2017 the Company paid USD $194 to DIL for collaborative research. The monthly payments terminate upon the earlier of: (a) the date that the Company orders additional product in accordance with the terms of the agreement and (b) December 31, 2018, and the Company has no further financial obligations to DIL thereafter.
The Company also agreed to pay DIL €400 (approximately USD $480) in satisfaction of all prior liabilities and obligations under its prior agreements with DIL. The agreement expires on December 31, 2018, and the Company has the unilateral right to renew the agreement for subsequent one-year terms.
At December 31, 2017, the future minimum payments based on exchange rates under the supply agreement were as follows:
| (In thousand $) | ||||
| Years Ended December 31, | Amount | |||
| 2018 | 132 | |||
| Total | $ | 132 | ||
F-25
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
Product Liability
As a manufacturer of nutritional supplements that are ingested by consumers, the Company may be subject to various product liability claims. Although we have not had any claims to date, it is possible that future product liability claims could have a material adverse effect on our business or financial condition, results of operations or cash flows. The Company currently maintains products liability insurance of $5 million per-occurrence and a $10 million annual aggregate coverage. At December 31, 2017 and 2016, the Company had not recorded any accruals for product liability claims.
Note 13 – Related Party Transactions
The following is a description of the transactions we have engaged in with our directors, director nominees and officers and beneficial owners of more than five percent of our voting securities and their affiliates:
On December 17, 2015, concurrent with the execution of the Purchase Agreement with RENS Technology Inc., the Company issued an unsecured promissory note in the principal amount of $575 (the “Note”) to Gan Ren, a related party of RENS Agriculture. The Note accrued interest at a rate of 8% per annum and matured (the “Maturity Date”) on December 17, 2016. On the Maturity Date, the Note and accrued interest of $46 were automatically converted into 225,864 shares of Common Stock at $2.75 per share.
On December 17, 2015, we entered into the Purchase Agreement with Rens Technology Inc. (the “Purchaser”), an entity which is controlled by Ren Ren, who is currently a director of the Company and its largest stockholder. For additional information refer to Note 1 – Strategic Investment Transaction. The Board agreed to issue Mr. Ren 18,182 shares of the Company’s common stock upon completion of the first tranche of the Financing for his services to the Company as a member of the Board. (See Note 14 - Legal Proceedings)
In October 2016, the Company received a purchase order from RENS Agriculture to purchase $118 of our product. We received a 50% deposit in November 2016 in order to manufacture the product. The goods were shipped in January 2017 and received in China in March 2017. We have not received payment for the order to date. As a result of the ongoing litigation (See Note 14), the Company recorded an allowance for bad debt of $59 related to the receivable due from RENS Agriculture.
Note 14 – legal PROCEEDINGS
On October 27, 2016, Cutler Holdings, L.L.C. (“Cutler”) filed a complaint in the Superior Court of New Jersey alleging that the Company failed to make certain rental payments. On March 30, 2017, the Company entered into a settlement agreement with Cutler, pursuant to which Cutler released the Company from any liability for the claims asserted in the complaint.
On January 6, 2017, in connection with the financing contemplated by a securities purchase agreement with RENS Technology Inc. (the “Purchaser”), we commenced an action in the Supreme Court of New York, County of New York (the “Court”), against the Purchaser, RENS Agriculture, the parent company of the Purchaser, and Ren Ren, a principal in both entities and one of our directors, arising from the Purchaser’s breach of the agreement under which the Purchaser agreed to invest an aggregate of $20.25 million in our company in exchange for an aggregate of 3,537,037 shares of our common stock and warrants to purchase an aggregate of 884,259 shares of common stock.
F-26
MYOS RENS TECHNOLOGY INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
(amounts in thousands, except share and per share amounts, unless otherwise indicated)
On April 11, 2017, the Court noted that we had demonstrated a likelihood of success on the merits of the breach of contract claim. Thereafter, a hearing was scheduled on the application by the Purchaser to dismiss the complaint and various pre-trial discovery applications by both parties.
In August 2017, before the hearing occurred, the Company amended its complaint repeating most of the initial claims but adding several additional claims against RENS Agriculture, Mr. Ren and two additional Chinese defendants, including a claim against RENS Agriculture for breaching the exclusive distribution agreement, as well as claims against all defendants for theft and misappropriation of our confidential proprietary information and trade secrets, breach of fiduciary duty and duty of loyalty, misappropriation of corporate opportunity, unfair competition and a number of other torts. We are seeking damages and injunctive relief. The Purchaser has filed a motion to dismiss the amended complaint, which is still pending and scheduled for oral argument in April 2018.
On August 16, 2017, the Purchaser commenced an action in the District Court of Clark County in the State of Nevada against us and Joseph Mannello, our then interim Chief Executive Officer, alleging that Mr. Mannello had breached his fiduciary duties and was grossly negligent in managing our company. The action seeks monetary damages and injunctive relief from Mr. Mannello as well as the appointment of a receiver over us. Subsequently, the Purchaser submitted a petition to appoint a receiver and we and Mr. Mannello submitted a motion to dismiss the action, both of which are currently pending and are due to be heard in April 2018. An application on consent to adjourn the hearing date on the receiver application and motion to dismiss is pending.
The outcome of the aforementioned matters cannot be determined as of the date of these financial statements.
Note 15 – Subsequent Events
At-the-Market Offering
On January 19, 2018 the Company sold 140,295 shares of common stock at $2.111 per share for gross proceeds of $296 less deferred costs of $9 in an at-the-market transaction pursuant to the sales agreement with H.C. Wainwright & Co., LLC.
On March 27, 2018, we announced that we entered into a research agreement with Rutgers University, The State University of New Jersey, to work with Rutgers researchers in a program focused on discovering compounds and products for improving muscle health and performance.
F-27
