Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - Option Care Health, Inc. | bios-ex322xx201712x31x10k.htm |
| EX-32.1 - EXHIBIT 32.1 - Option Care Health, Inc. | bios-ex321xx20171231x10k.htm |
| EX-31.2 - EXHIBIT 31.2 - Option Care Health, Inc. | bios-ex312xx20171231x10k.htm |
| EX-31.1 - EXHIBIT 31.1 - Option Care Health, Inc. | bios-ex311xx20171231x10k.htm |
| EX-23.1 - EXHIBIT 23.1 - Option Care Health, Inc. | bios-ex231xx20171231x10k.htm |
| EX-21.1 - EXHIBIT 21.1 - Option Care Health, Inc. | bios-ex211xx20171231x10k.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One) | |
þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2017 | |
OR | |
o | PERIODIC REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to | |
Commission file number: 001-11993

BioScrip, Inc.
(Exact name of registrant as specified in its charter)
Delaware | 05-0489664 |
(State of incorporation) | (I.R.S. Employer Identification No.) |
1600 Broadway, Suite 700, Denver, Colorado | 80202 |
(Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code:
720-697-5200
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of each exchange on which registered |
Common Stock, $0.0001 par value per share | Nasdaq Global Market |
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted to its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No £
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definition of “large accelerated filer”, “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer o Accelerated filer þ Non-accelerated filer o Smaller reporting company o Emerging growth company o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No þ
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
The aggregate market value of the registrant’s Common Stock held by non-affiliates of the registrant as of June 30, 2017, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $346,641,944 based on the closing price of the Common Stock on the Nasdaq Global Market on such date.
On March 21, 2018, there were 127,697,318 shares of the registrant’s Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its 2018 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission (the “SEC”) within 120 days after the close of the registrant’s fiscal year are incorporated by reference into Part III of this Annual Report.
TABLE OF CONTENTS
Page Number | ||
PART I | ||
PART II | ||
PART III | ||
PART IV | ||
Item 16. | Summary | |
2
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K (“Annual Report”) contains statements that are not purely historical and which may be considered “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), including statements regarding our expectations, beliefs, future plans and strategies, anticipated events or trends concerning matters that are not historical facts or that necessarily depend upon future events. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “predict,” “potential” and similar expressions. Specifically, this Annual Report contains, among others, forward-looking statements about:
• | our ability to make principal and interest payments on our debt and unsecured notes and satisfy the other covenants contained in our Notes Facilities (as defined below); |
• | our high level of indebtedness; |
• | our expectations regarding financial condition or results of operations in future periods; |
• | our future sources of, and needs for, liquidity and capital resources; |
• | our expectations regarding economic and business conditions; |
• | our expectations regarding legislative and regulatory changes impacting the level of reimbursement received from the Medicare and state Medicaid programs; |
• | periodic reviews and billing audits of payments from governmental reimbursement programs and private payors; |
• | our expectations regarding the size and growth of the market for our products and services; |
• | our business strategies and our ability to grow our business; |
• | the implementation or interpretation of current or future regulations and legislation, particularly governmental oversight of our business; |
• | our expectations regarding the outcome of litigation; |
• | our ability to maintain contracts and relationships with our customers; |
• | our ability to avoid delays in payment from our customers; |
• | sales and marketing efforts; |
• | status of material contractual arrangements, including the negotiation or re-negotiation of such arrangements; |
• | future capital expenditures; |
• | our ability to hire and retain key employees; |
• | our ability to execute our strategy; |
• | our ability to successfully integrate businesses we may acquire. |
Investors are cautioned that any such forward-looking statements are not guarantees of future performance, involve risks and uncertainties and that actual results may differ materially from those possible results discussed in the forward-looking statements as a result of various factors. Important factors that could cause such differences include, among other things:
• | risks associated with increased and complex government regulation related to the health care and insurance industries in general, and more specifically, home infusion providers; |
• | our ability to comply with debt covenants in our Notes Facilities and unsecured notes indenture; |
• | risks associated with our issuance of Preferred Stock and PIPE Warrants to the PIPE Investors and the 2017 Warrants (as defined below); |
• | risks associated with the retention or transition of executive officers and key employees; |
• | our expectation regarding the interim and ultimate outcome of commercial disputes, including litigation; |
• | unfavorable economic and market conditions; |
• | disruptions in supplies and services resulting from force majeure events such as war, strike, riot, crime, or “acts of God” such as hurricanes, flooding, blizzards or earthquakes; |
• | delays or suspensions of Federal and state payments for services provided; |
• | efforts to reduce healthcare costs and alter health care financing, which may involve reductions in reimbursement for our products and services; |
• | effects of the 21st Century Act (the “Cures Act”); |
• | the effect of health reform efforts including the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (together the “Affordable Care Act”), and value-based payment initiatives, including accountable care organizations; |
• | existence of complex laws and regulations relating to our business; |
• | availability of financing sources; |
• | declines and other changes in revenue due to the expiration of short-term contracts; |
• | network lockouts and decisions to in-source by health insurers including lockouts with respect to acquired entities; |
• | unforeseen contract terminations; |
3
• | difficulties in the implementation and ongoing evolution of our operating systems; |
• | difficulties with the implementation of our growth strategy and integrating businesses we have acquired or will acquire; |
• | increases or other changes in our acquisition cost for our products; |
• | increased competition from competitors having greater financial, technical, reimbursement, marketing and other resources could have the effect of reducing prices and margins; |
• | disruptions in our relationship with our primary supplier of prescription products; |
• | the level of our indebtedness and its effect on our ability to execute our business strategy and increased risk of default under our debt obligations; |
• | introduction of new drugs, which can cause prescribers to adopt therapies for patients that are less profitable to us; |
• | changes in industry pricing benchmarks, which could have the effect of reducing prices and margins; and |
• | other risks and uncertainties described from time to time in our filings with the SEC. |
We make these forward-looking statements in reliance on the safe harbor protections provided under the Private Securities Litigation Reform Act of 1995. You should not place undue reliance on such forward-looking statements as they speak only as of the date they are made. Except as required by law, we assume no obligation to publicly update or revise any forward-looking statement even if experience or future changes make it clear that any projected results expressed or implied therein will not be realized.
4
PART I
Item 1. | Business |
Overview
BioScrip, Inc. (“BioScrip”, “we”, “us”, “our” or the “Company”) is a national provider of infusion solutions. We work with physicians, hospital systems, skilled nursing facilities, and healthcare payors to provide patients access to post-acute care services. We operate with a commitment to bring customer-focused healthcare infusion therapy services into the home or alternate-site setting. By collaborating with the full spectrum of healthcare professionals and the patient, we aim to provide cost-effective care that is driven by clinical excellence, customer service and values that promote positive outcomes and an enhanced quality of life for those whom we serve.
Our platform provides nationwide service capabilities and the ability to deliver clinical management services that offer patients a high-touch, community-based and home-based care environment. Our core services are provided in coordination with, and under the direction of, the patient’s physician. Our multidisciplinary team of clinicians, including pharmacists, nurses, dietitians and respiratory therapists, work with the physician to develop a plan of care suited to each patient’s specific needs. Whether in the home, physician office, ambulatory infusion center, skilled nursing facility or other alternate sites of care, we provide products, services and condition-specific clinical management programs tailored to improve the care of individuals with complex health conditions such as gastrointestinal abnormalities, infectious diseases, cancer, multiple sclerosis, organ and blood cell transplants, bleeding disorders, immune deficiencies and heart failure.
We were incorporated in Delaware in 1996 as MIM Corporation, with our primary business and operations consisting of pharmacy benefit management services at the time.
Strategic Assessment and Transactions
We continually perform strategic assessments of our business and operations. The assessments examine our market strengths and opportunities and compare our position to that of our competitors. As a result of these ongoing assessments, we have focused our growth on investments in the Infusion Services business, which remains the primary driver of our growth strategy. Recent transactions which represent execution of the strategic assessments include:
• | On August 27, 2015, we completed the sale of substantially all of our pharmacy benefit management services segment (the “PBM Business”) pursuant to an Asset Purchase Agreement dated as of August 9, 2015 (the “PBM Asset Purchase Agreement”), by and among the Company, BioScrip PBM Services, LLC and ProCare Pharmacy Benefit Manager Inc. |
• | On September 9, 2016, we acquired substantially all of the assets and assumed certain liabilities of HS Infusion Holdings, Inc. and its subsidiaries pursuant to an Asset Purchase Agreement dated June 11, 2016, by and among Home Solutions, a Delaware corporation, certain subsidiaries of Home Solutions, the Company and HomeChoice Partners, Inc., a Delaware corporation. Home Solutions, a privately held company, provided home infusion and home nursing products and services to patients suffering from chronic and acute medical conditions. |
Business Outlook
As a result of the strategic assessments discussed above, we have focused on expanding revenue opportunities and reducing corporate overhead as well as strategically redeploying our resources. Restructuring, acquisition, integration and other expenses include non-operating costs associated with restructuring, acquisition and integration initiatives such as employee severance costs, certain legal and professional fees, training costs, redundant wage costs, impacts recorded from the change in contingent consideration obligations, and other costs related to contract terminations and closed branches/offices. The redeployment of resources following the strategic transactions has better positioned us for growth in our strategic areas of operation; however, the impact of these actions on our future consolidated financial statements cannot be estimated.
Our Strengths
Our company has a number of competitive strengths, including:
5
Local Competitive Market Position within Our National Platform and Infrastructure
As of December 31, 2017, we had a total of 66 service locations in 27 states. Our model combines local presence with comprehensive clinical programs for multiple therapies and specific delivery technologies (infusible and injectable). We have the capabilities and payor relationships to dispense prescriptions to all 50 states. We have relationships with approximately 1,000 payors, including Managed Care Organizations (“MCOs”), government programs such as Medicare and Medicaid and commercial insurers (“Third Party Payors”). We believe payors generally favor fully integrated vendors that can provide high-touch pharmacy solutions to their patients. We believe we are one of a limited number of pharmacy providers that can offer a truly national, integrated and comprehensive approach to managing a patient’s chronic or acute conditions.
Diversified and Favorable Payor Base
We provide prescription drugs, infusion therapy and clinical management services for a broad range of commercial and governmental payors. Approximately 84% of our payor base is comprised of commercial payors that operate at a national, regional or local level. Four national commercial payors accounted for 18%, 9%, 7%, and 6% of consolidated revenue during the year ended December 31, 2017. No other commercial payor accounted for more than 5% of consolidated revenue during the year ended December 31, 2017. Government payors, including Medicare, state Medicaid and other government payors, accounted for 16% of consolidated revenue during the year ended December 31, 2017. For the year ended December 31, 2017, Medicare accounted for 7% of our consolidated revenue. No individual state Medicaid program accounted for more than 5% of consolidated revenue during the year ended December 31, 2017.
The costs savings realized by administering infusion therapies in the home versus hospitals, skilled nursing facilities or other post-acute care facilities positions our business to benefit from healthcare reform initiatives that focus on cost savings. Medicare currently offers limited reimbursement for home infusion therapy products and services. Although the Cures Act significantly reduced the level of reimbursement for certain of the therapies that we provide, we believe that home infusion and other low-cost in-home therapeutic alternatives will be impacted favorably by health reform initiatives focused on cost-reduction. Significant health plan cost savings per infusion can be achieved when therapy is provided at an alternative treatment site compared to other patient settings.
Effective Care Management Clinical Programs that are Designed to Produce Positive Clinical Outcomes and Reduce Readmissions
Our diversified and comprehensive clinical programs, which span numerous therapeutic areas, are designed to improve patient outcomes. Our home infusion business provides traditional infusion therapies for acute conditions with accompanying clinical management and home care. Our infusion product offerings and services are also designed to treat patients with chronic infusion needs. Chronic conditions require long-term treatment, ongoing caregiver and patient counseling and education, and ongoing monitoring and communication with physicians to encourage patients to follow therapies prescribed by their physicians.
Our Centers of Excellence focus on interdisciplinary teams to provide clinical excellence with outstanding personal service. Externally qualified by a panel of leading industry experts, these centers employ evidence-based standards of care, policies and procedures built on industry-recognized best practices. They are led by specialists with advanced certifications and training who are dedicated to developing, improving and sustaining clinical services to achieve optimal patient outcomes and exceed the expectations of patients and referral sources.
Our clinical management programs in multiple disease-state therapy provide us opportunities to cross-sell services. We believe we have earned a positive reputation among patients, physicians, payors and pharmaceutical manufacturers by providing quality service and favorable clinical outcomes. We believe our platform provides the necessary programs and services for better and more efficient clinical outcomes for our patients.
Segment Information
Following the sale of our PBM Business on August 27, 2015, Infusion Services is the only remaining operating segment. On an ongoing basis we will no longer report operating segments unless a change in the business necessitates the need to do so.
Products and Services
We are one of the largest providers of home infusion services in the United States. Home infusion involves the preparation, delivery, administration and clinical monitoring of pharmaceutical treatments that are administered to a patient via intravenous (into the vein), subcutaneous (into the fatty layer under the skin), intramuscular (into the muscle), intra-spinal (into the membranes
6
around the spinal cord) and enteral (into the gastrointestinal tract) methods. These methods are employed when a physician determines that the best outcome can be achieved through utilization of one or more of the therapies provided through the routes of administration described above.
Our home infusion services primarily involve the intravenous administration of medications to treat a wide range of acute and chronic conditions, such as infections, nutritional deficiencies, various immunologic and neurologic disorders, cancer, pain and palliative care. Our services are usually provided in the patient’s home but may also be provided at outpatient clinics, skilled nursing facilities, physician offices or at one of our ambulatory infusion centers. We receive payment for our home infusion services and medications, pursuant to provider agreements with government sources, such as Medicare and Medicaid programs, MCOs and Third Party Payors.
We provide a wide array of home infusion products and services to meet the diverse needs and preferences of physicians, patients and payors. Diseases commonly requiring infusion therapy include infections that are unresponsive to oral antibiotics, cancer and cancer-related pain, dehydration and gastrointestinal diseases or disorders that require IV fluids, parenteral or enteral nutrition. Other conditions that may be treated with infusion therapies include chronic diseases such as heart failure, Crohn’s disease, hemophilia, immune deficiencies, multiple sclerosis, rheumatoid arthritis, growth disorders and genetic enzyme deficiencies, such as Gaucher’s or Pompe’s disease. The therapies and products most commonly provided are listed below:
Therapy Type | Description |
Parenteral Nutrition (PN) | Provide intravenous nutrition customized to the nutritional needs of the patient. PN is used in patients that cannot meet their nutritional needs via other means due to disease process or as a complication of a disease process, surgical procedure or congenital anomaly. PN may be used short term or chronically. |
Enteral Nutrition (EN) | Provide nutrition directly to the stomach or intestine in patients who cannot chew or swallow nutrients in the usual manner. EN may be delivered via a naso-gastric tube or a tube placed directly into the stomach or intestine. EN may be used short term or chronically. |
Antimicrobial Therapy (AT) | Provide intravenous antimicrobial medications used in the treatment of patients with various infectious processes such as: wound infections, pneumonia, osteomyelitis, cystic fibrosis, Lyme disease and cellulitis. AT may also be used in patients with disease processes or therapies that may lead to infections when oral antimicrobials are not effective. |
Chemotherapy | Provide injectable and/or infused medications in the home or the prescriber’s office for the treatment of cancer. Adjuvant medications may also be provided to minimize the side effects associated with chemotherapy. |
Immune Globulin (IG) Therapy | Provide immune globulins intravenously or subcutaneously on an as-needed basis in patients with immune deficiencies or auto-immune diseases. This therapy may be chronic based on the etiology of the immune deficiency. |
Pain Management | Provide analgesic medications intravenously, subcutaneously or epidurally. This therapy is generally administered as a continuous infusion via an internal or external infusion pump to treat severe pain associated with diseases such as COPD, cancer and severe injury. |
Blood Factor Therapies | Provide medications to patients with one of several inherited bleeding disorders in which a patient does not manufacture the clotting factors necessary or use the clotting factors their liver makes appropriately in order to halt an external or internal bleed in response to a physical injury or trauma. |
Inotropes Therapy | Provide intravenous inotropes in the home for the treatment of heart failure, either in anticipation of cardiac transplant or to provide palliation of heart failure symptoms. Inotropes increase the strength of weak heart muscles to pump blood. The therapy is only started in late phase heart failure when alternative therapies proved inadequate. |
Respiratory Therapy/Home Medical Equipment | Provide oxygen systems, continuous or bi-level positive airway pressure devices, nebulizers, home ventilators, respiratory devices, respiratory medications and other medical equipment. |
Patients generally are referred to us by physicians, hospital discharge planners, MCOs and other referral sources. Our medications are compounded and dispensed under the supervision of a registered pharmacist in a state licensed pharmacy that is
7
accredited by an independent accrediting organization. We compound pursuant to a patient specific prescription and do so consistently with U.S. Pharmacopeial Convention (“USP”) 797 standards. A national accrediting organization surveys our pharmacies for compliance with the USP 797 standards for sterile drug compounding pharmacies and has confirmed that we operate consistently with those standards. Therapies are typically administered in the patient’s home by a registered nurse or trained caregiver. Depending on the preferences of the patient or the payor, these services may also be provided at one of our ambulatory infusion centers, a physician's office or another alternate site of administration.
We currently have relationships with a large number of MCOs and other Third Party Payors to provide home infusion services. These relationships are at a national, regional or local level. A key element of our business strategy is to leverage our relationships, geographic coverage, clinical expertise and reputation in order to gain contracts with payors. Our infusion service contracts typically provide for us to receive a fee for preparing and delivering medications and related equipment to patients in their homes or in Ambulatory Infusion Sites (“AIS”). Pricing for pharmaceutical products is typically negotiated in advance on the basis of Average Wholesale Price (“AWP”) minus some percentage of contractual discount, or Average Sales Price (“ASP”) plus some percentage. In addition, we typically receive a per diem payment for additional services and supplies provided to patients in connection with infusion services. An additional payment is made for nursing services when services are provided.
Sales and Marketing
We have over 264 sales and marketing representatives and approximately 1,000 payor relationships including MCOs, Medicare Part D pharmacy networks, other government programs such as Medicare and Medicaid and other Third Party Payors. Our sales and marketing efforts are focused on payors, healthcare systems and physician prescribers and are driven by dedicated managed care and physician sales teams as well as home health care consultants. Our sales and marketing strategies include the development of strong relationships with key referral sources, such as physicians, hospital discharge planners, case managers, long-term care facilities and other healthcare professionals, primarily through regular contact with the referral sources and by fulfilling the care and service expectations of our many customers. Contracts with Third Party Payors, including MCOs, are an integral component for sales success.
Intellectual Property
We own and use a variety of trademarks, trade names and service marks, including without limitation “BioScrip”, “BioScrip Infusion Services”, “BioScrip Nursing Services”, “BioScrip Pharmacy Services”, “CarePoint Partners”, “HomeChoice Partners”, “InfuScience”, “InfusionCare”, “Infusion Partners”, “Infusion Solutions”, “New England Home Therapies”, “Option Health”, “Professional Home Care Services”, “Wilcox Home Infusion”, and “Home Solutions”, each of which has either been registered at the state or federal level or is being used pursuant to common law rights. We are recognized in local markets by several of these trade names, but we do not consider the marks material to our business.
Competition
The home infusion services market is highly competitive and includes a limited number of national providers and numerous local and regional companies. Providers strive to differentiate their services based on their responsiveness to patient needs, quality of care, reputation, outcomes, and cost of service. Our Centers of Excellence offer a high touch, high service approach to care on a local basis, which we believe differentiates our service.
Our competitors within the home infusion market include Option Care, Coram CVS/specialty infusion services (a division of CVS Health), Accredo Health Group, Inc. (a subsidiary of Express Scripts Holding Company), Briova (a subsidiary of OptumRx, which is a unit of the UnitedHealthcare Group) and various regional and local providers of alternate site healthcare services such as hospitals and physician practices.
Government Regulation
The healthcare industry is subject to extensive regulation by a number of governmental entities at the federal, state and local level. Laws and regulations in the healthcare industry are extremely complex and, in many instances, the industry does not have the benefit of significant regulatory or judicial interpretation. Our business is impacted not only by those laws and regulations that are directly applicable to us but also by certain laws and regulations that are applicable to our payors, vendors and referral sources. While our management believes we are in substantial compliance with all of the existing laws and regulations applicable to us, such laws and regulations are subject to rapid change and often are uncertain in their application and enforcement. Further, to the extent we engage in new business initiatives, we must continue to evaluate whether new laws and regulations are applicable to us. There can be no assurance that we will not be subject to scrutiny or challenge under one or more of these laws or that any
8
enforcement actions would not be successful. Any such challenge, whether or not successful, could have a material adverse effect upon our business and consolidated financial statements.
Among the various federal and state laws and regulations that may govern or impact our current and planned operations are the following:
Medicare and Medicaid Reimbursement
Many of the products and services that we provide are reimbursed by Medicare and state Medicaid programs and are therefore subject to extensive government regulation.
Medicare is a federally funded program that provides health insurance coverage for qualified persons age 65 or older, some disabled persons, and persons with end-stage renal disease and persons with Lou Gehrig’s disease. Medicaid programs are jointly funded by the federal and state governments and are administered by states under approved plans.
Medicaid provides medical benefits to eligible people with limited income and resources and people with disabilities, among others. Although the federal government establishes general guidelines for the Medicaid program, each state sets its own guidelines regarding eligibility and covered services. Some individuals, known as dual eligibles, may be eligible for benefits under both Medicare and a state Medicaid program. Reimbursement under the Medicare and Medicaid programs is contingent on the satisfaction of numerous rules and regulations, including those requiring certification and/or licensure. Congress often enacts legislation that affects the reimbursement rates under government healthcare programs.
Approximately 16% of our revenue for the year ended December 31, 2017 was derived directly from Medicare, Medicaid or other government-sponsored healthcare programs. Also, we indirectly provide services to beneficiaries of Medicare, Medicaid and other government-sponsored healthcare programs through managed care entities. Should there be material changes to federal or state reimbursement methodologies, regulations or policies, our direct reimbursements from government-sponsored healthcare programs, as well as service fees that relate indirectly to such reimbursements, could be adversely affected.
Medicare
We receive reimbursement for infusion therapy under the Medicare program, which has four parts. Medicare Part A generally covers inpatient hospital, skilled nursing facility, home nursing and hospice services; Medicare Part B covers physicians' services, outpatient services, items and services provided by medical suppliers and a limited number of prescription drugs; Medicare Part C allows beneficiaries to enroll in private healthcare plans (known as Medicare Advantage plans); and Medicare Part D provides for a voluntary prescription drug benefit.
Medicare fee-for-service programs, Part A and Part B, generally cover infusion therapy provided in hospitals and hospital outpatient departments, skilled nursing facilities, and physician offices. Part A covers infusion therapy services under the home health benefit if the services are rendered by a Medicare-certified home health agency and the beneficiary meets criteria for homebound status. Part B generally does not cover the full range of services for infusion therapies in a patient’s home but it covers a limited number of drugs administered using an external infusion pump under the durable medical equipment (“DME”) benefit. Although Medicare Part D covers payment for drugs (including many not covered under Part B) and a retail-based dispensing fee, Part D does not cover infusion-related services, equipment and supplies. For eligible Medicare beneficiaries, the cost of equipment and supplies associated with infused drugs covered under Medicare Part D may be reimbursed on a limited basis under Part A or Part B, and the cost of associated professional services may be reimbursed on a limited basis under Medicare Part A. CMS has attempted to clarify the relationship of Part B and Part D with regard to coverage of infused drugs. CMS has stated that coverage is generally determined by the diagnosis and the method of drug delivery.
The U.S. Department of Health and Human Services (“HHS”), Office of the Inspector General (“OIG”) and CMS continue to issue regulations and guidance with regard to the Medicare Part D program and compliance by Medicare Part D sponsors and their subcontractors. The receipt of funds made available through Medicare Part D is subject to compliance with government laws and regulations and provisions in contracts with prescription drug plans. There are many uncertainties about the financial and regulatory risks of participating in the Medicare Part D program, and these risks could negatively impact our business in future periods.
Medicare Part C - Medicare Advantage
Under Medicare Part C, also known as Medicare Advantage, beneficiaries can choose to enroll in a health insurance plan administered by an MCO. Medicare Advantage plans are required to offer the benefits covered under Medicare Part A and Part
9
B, with the exception of hospice care, and may include additional benefits. To serve Part C beneficiaries, a provider must contract with an MCO plan. Reimbursement and other requirements imposed on the provider are governed by the agreement with the MCO plan rather than by statute or regulation and as such vary from plan to plan. Medicare Advantage plans are permitted to cover certain services that fee-for-service Medicare does not cover. Home infusion therapy services are covered under many Medicare Advantage plans. We currently have contracts with a number of Medicare Advantage plans.
Legislative Changes to Medicare Reimbursement
In recent years, legislative and regulatory changes have resulted in limitations and reductions in reimbursement under government healthcare programs. For example, the Cures Act, which Congress passed in December of 2016, changed the payment methodology for certain infusion drugs under the Part B DME benefit. Significant reductions to the amount paid by Medicare for many infusion drugs took effect January 1, 2017. In addition, the Cures Act provides for the implementation of a clinical services payment under Part B for “qualified home infusion therapy suppliers.” Under this new payment system, Medicare will reimburse home infusion therapy suppliers based on a single, all-inclusive rate. The services payment provision does not take effect until January 1, 2021. However, the Bipartisan Budget Act of 2018 provides for temporary transitional benefit payments, starting January 1, 2019, for Medicare Part B home infusion services. This temporary benefit will continue until January 1, 2021 when the services payment in the Cures Act takes effect. We have taken steps to mitigate the impact of the Cures Act on our business, but the Act has had a material negative impact on our revenues and profitability.
Medicaid
Medicaid coverage of infusion therapy varies by state. We are sensitive to possible changes in state Medicaid programs. Budgetary concerns in many states have resulted in, and may continue to result in, reductions to Medicaid reimbursement and delays in payment of outstanding claims. In addition, many states have implemented or are considering strategies to reduce coverage, restrict eligibility, or enroll Medicaid recipients in managed care programs. As of January 1, 2018, all pharmacies participating in a Medicaid managed care program must be registered with the state Medicaid agency. Any reductions to or delays in collecting amounts reimbursable by state Medicaid programs for our products or services, or changes in regulations governing such reimbursements, could cause our revenue and profitability to decline and increase our working capital requirements. Effective January 1, 2018, CMS limited Medicaid reimbursement for DME to no more than Medicare payment rates. For further discussion on state Medicaid reductions, refer to “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Part II, Item 7.
In addition, some Medicaid programs only allow for reimbursement to pharmacies residing in the state or in a border state. While we believe we can service our current Medicaid patients through our existing infusion pharmacies, there can be no assurance that additional states will not enact in-state dispensing requirements for their Medicaid programs. To the extent such requirements are enacted, certain therapeutic pharmaceutical reimbursements could be adversely affected.
State Legislation and Other Matters Affecting Drug Prices
Many states have adopted “most favored nation” legislation, which limits the amount a pharmacy participating in the state Medicaid program is paid based on the pharmacy’s prices applicable to third party plans, or in some instances, self-pay patients. Because of these limitations, we may not receive the full Medicaid fee schedule amounts in some instances. There is wide variation in drafting, interpretation and enforcement of state “most favored nation” legislation. Our management carefully considers these laws and believes that each of our respective companies is in material compliance with them; however, we cannot predict whether the regulators will disagree with our interpretation or change their interpretation of the laws or their enforcement priorities.
Pricing benchmarks in the pharmacy industry are published by third-parties such as First DataBank, Medi-Span, Micromedex, RJ Health, and CMS. The Average Wholesale Price (“AWP”) is one of the most commonly used benchmarks. Although various payors have discussed establishing a new benchmark and First DataBank ceased publication of the AWP, the industry has not yet developed a viable generally accepted alternative to the AWP benchmark. See “Risk Factors - Risks Related to Our Business - Changes in industry pricing benchmarks could adversely affect our financial performance.”
Healthcare Reform
In recent years, federal and state governments have considered and enacted policy changes designed to reform the healthcare industry. The most prominent of these healthcare reform efforts, the Affordable Care Act, has resulted in sweeping changes to the U.S. system for the delivery and financing of health care. As currently structured, the Affordable Care Act increases the number of persons covered under government programs and private insurance; furnishes economic incentives for measurable improvements in health care quality outcomes; promotes a more integrated health care delivery system and the creation of new health care delivery
10
models; revises payment for health care services under the Medicare and Medicaid programs; and increases government enforcement tools and sanctions for combating fraud and abuse. In addition, the Affordable Care Act reduced cost sharing for Medicare beneficiaries under the Part D prescription drug benefit program and expanded medication therapy management services for individuals with chronic conditions.
However, the future of the Affordable Care Act is uncertain. The presidential administration and certain members of Congress have expressed their intent to repeal or make significant changes to the Affordable Care Act, its implementation or interpretation. In 2017, Congress eliminated the financial penalty associated with the individual mandate, effective January 1, 2019, which may result in fewer individuals electing to purchase health insurance.
Regulation of the Pharmacy Industry
For each physical pharmacy location in a state, laws require maintenance of an in-state pharmacy license to dispense pharmaceuticals. Pharmacy and controlled substances laws often address the qualifications of personnel, the adequacy of prescription fulfillment and inventory control practices and the adequacy of facilities. We believe our pharmacy locations materially comply with all state licensing laws applicable to their practice. If our pharmacy locations become subject to additional licensure requirements, are unable or otherwise fail to maintain their required licenses or if states place overly burdensome restrictions or limitations on pharmacies, our ability to operate in some states would be limited, which could have an adverse impact on our business. We believe the impact of any such requirements would be mitigated by our ability to shift business among our numerous locations.
Many states, as well as the federal government, are considering imposing, or have already begun to impose, more stringent requirements on compounding pharmacies including the Drug Quality and Security Act (“DQSA”) (see Food, Drug, and Cosmetic Act below). We believe that our compounding is done in safe environments with clinically appropriate policies and procedures in place. Those compounding pharmacies adhere to rigorous safety and quality standards for compounded sterile preparations and only fill prescriptions for individually identified patients pursuant to a valid prescription from a prescriber. All compounding is done consistently with USP 797 standards.
Many of the states into which we deliver pharmaceuticals have laws and regulations that require out-of-state pharmacies to register with or be licensed by the boards of pharmacy or similar regulatory bodies in those states. These states generally permit the dispensing pharmacy to follow the laws of the state within which the dispensing pharmacy is located. However, various state pharmacy boards have enacted laws and/or adopted rules or regulations directed at restricting or prohibiting the operation of out-of-state pharmacies by, among other things, requiring compliance with all laws of the states into which the out-of-state pharmacy dispenses medications, whether or not those laws conflict with the laws of the state in which the pharmacy is located, or requiring the pharmacist-in-charge to be licensed in that state. To the extent that such laws or regulations are applicable to our operations, we believe we comply with them. To the extent that the foregoing laws or regulations prohibit or restrict the operation of out-of-state pharmacies and are found to be applicable to us, they could have an adverse effect on our operations.
Laws enforced by the U.S. Drug Enforcement Administration (“DEA”) require each of our pharmacy locations to register with the DEA in order to handle and dispense controlled substances. A separate registration is required at each principal place of business where we dispense controlled substances. Federal and state laws also require us to follow specific labeling, reporting and record-keeping requirements for controlled substances. We maintain federal and state controlled substance registrations for each of our facilities that require such registration and follow procedures intended to comply with all applicable federal and state requirements regarding controlled substances. These laws can change from time to time. We continuously review these changes to laws and believe we are in material compliance with the applicable federal and state controlled substances laws. If any of our pharmacy locations is deemed to be out of compliance, it could have an adverse impact on our business.
Many states in which we operate also require home infusion companies to be licensed as home health agencies. We believe we materially comply with these laws. If our infusion locations become subject to new licensure requirements, are unable to maintain required licenses or if states place burdensome restrictions or limitations on home health agencies or home nursing agencies, our infusion locations’ ability to provide nursing services in some states would be limited, which could have an adverse impact on our business.
Professional Licensure
Nurses, pharmacists and certain other professionals employed by us are required to be individually licensed and/or certified under applicable state law. We perform criminal and other background checks on employees to the extent allowed by state law and confirm that our employees possess all licenses and certifications required in order to provide healthcare-related services. We believe our employees comply with applicable licensure laws.
11
Food, Drug and Cosmetic Act
Pharmacy operations
Certain provisions of the Federal Food, Drug and Cosmetic Act (“FDCA”) govern the preparation, handling, storage, marketing and distribution of pharmaceutical products. Many of the pharmaceuticals and medical devices we dispense are exempt from certain federal requirements as long as they are not adulterated or misbranded and are dispensed in accordance with, and pursuant to, a valid prescription.
The FDA directly regulates outsourcing facilities, but does not directly regulate non-outsourcing facilities or pharmacies. Outsourcing facilities are pharmacies that are engaged in sterile compounding of drugs that are not for an individually identifiable patient. Outsourcing facilities are subject to standards relating to sterilization and the physical facility including the Current Good Manufacturing Practice (“cGMP”) regulations. Because our compounding activities are limited to products compounded pursuant to valid prescriptions for individually identifiable patients, we do not qualify as an outsourcing facility, and therefore, should not be required to comply with the cGMP standards. The FDA has been conducting inspections of pharmacies that engage in compounding, including ours, and has been attempting to apply the cGMP standards even though those pharmacies are not outsourcing facilities. While the FDA has issued reports following their surveys, to date, no enforcement action has been taken against us. We cannot predict what further actions the FDA may take. We believe our operations are in compliance with applicable laws and that the requirements for outsourcing facilities are not applicable to our operations. We cannot predict the impact of increased scrutiny on or new regulation of compounding pharmacies.
In addition, the FDCA governs pharmaceutical products’ movement in interstate commerce. The FDA has begun scrutinizing more closely compounding pharmacies’ operations and compounded pharmaceuticals’ movement in interstate commerce. Specifically, the FDA has proposed regulations that could have the effect of limiting our ability to ship prescriptions out of state by pharmacies that hold valid licenses but do not comply with cGMP standards. We do not know if these regulations, as proposed, will be adopted, but if they are, we will likely need to modify our operations to comply. While we cannot predict changes to the regulatory environment under the DQSA, we believe we comply in all material respects with all applicable requirements of a non-outsourcing-facility pharmacy.
Infusion services
The FDA regulates certain medical devices (e.g., infusion pumps) essential to the Company’s infusion services. An infusion pump, like any medical device, is subject to failure. Since 2010, due to the relatively large number of adverse events associated with the use of infusion pumps, FDA has increased its oversight of infusion pumps. Changes have included higher levels of scrutiny, intensifying manufacturer engagement and bolstering user education and adverse event reporting. The shifting regulatory climate around infusion pumps; the requirement to maintain high levels of proficiency in using and training patients in the safe use of infusion pumps; cybersecurity issues, including modification and misuse of infusion pumps, and unauthorized use of information that is stored on or accessed from infusion pumps; and, finally, the need to stay current in infusion pump design and “best practices,” present elements of risk. Nevertheless, we believe we comply in all material respects with all applicable requirements and that our employees are adequately trained and equipped to use these devices.
Fraud and Abuse Laws
Anti-Kickback Laws
The federal Anti-Kickback Statute makes it a felony for a person or entity to knowingly and willfully offer, pay, solicit, or receive any remuneration with the intent of inducing the referral of an individual or the purchase, lease or order (or the arranging for or recommending of the purchase, lease or order) of healthcare items or services paid for in whole or in part by a federal healthcare program such as Medicare or Medicaid. Courts have held that there is a violation of the statute even if only one purpose of a payment arrangement is to induce referrals, even if there are other lawful purposes. Violations of the federal Anti-Kickback Statute could subject us to criminal and/or civil penalties, including suspension or exclusion from Medicare and Medicaid programs and other government-funded healthcare programs. In addition, submission of a claim for items or services generated in violation of the Anti-Kickback Statute may also be the basis of liability under the federal False Claims Act (“False Claims Act”).
The federal Anti-Kickback Statute has been interpreted broadly by courts, the OIG and other administrative bodies. For example, although the term “remuneration” is not defined in the federal Anti-Kickback Statute, it has been broadly interpreted to include anything of value, including for example, gifts, donations, the furnishing of supplies or equipment, credit arrangements, payments of cash, waivers of payment, ownership interests and providing any item, service, or compensation for something other
12
than fair market value. Because of the broad scope of the statute, there are several statutory exceptions to the law, and federal regulations establish certain safe harbors from liability. For example, there are safe harbors relating to certain discounts received from vendors, investment interests, group purchasing organizations, managed care and waivers of copayment obligations. A practice that does not fall within a safe harbor is not necessarily unlawful, but may be subject to increased scrutiny and challenge by government enforcement authorities.
Governmental entities have investigated pharmacies and their dealings with pharmaceutical manufacturers concerning, among other things, retail distribution, sales and marketing practices and product conversion or product switching programs. Governmental entities have also investigated pharmacies with respect to their relationships with physicians and other referral sources, including marketing practices. There can be no assurance that we will not receive subpoenas or be requested to produce documents in pending investigations or litigation from time to time. In addition, we may be the target or subject of one or more such investigations or named parties in corresponding actions.
In 2003, the OIG released Compliance Program Guidance for Pharmaceutical Manufacturers (the “Guidance”), which provides voluntary, nonbinding guidance in devising effective compliance programs to assist companies that develop, manufacture, market and sell pharmaceutical products or biological products. The Guidance sets forth the fundamental elements of a pharmaceutical manufacturer’s compliance program and principles that should be considered when creating, implementing, and maintaining an effective compliance program. While we are not a manufacturer, we believe that many aspects of it are useful to our business and therefore we currently maintain a compliance program that includes the key compliance program elements described in the Guidance. We believe the fundamental elements of our compliance programs are consistent with the principles, policies and intent of the Guidance.
A number of states have enacted anti-kickback laws that may apply not only to state-sponsored healthcare programs, but also to items or services that are paid for by private insurance and self-pay patients. State anti-kickback laws can vary considerably in their applicability and scope and sometimes have fewer statutory and regulatory exceptions than does the federal law. Our management carefully considers the importance of such anti-kickback laws when structuring each company’s operations and believes that each of our respective companies is in compliance therewith.
The Stark Laws
The federal physician self-referral law, commonly known as the “Stark Law,” prohibits physicians from referring Medicare and Medicaid patients for “designated health services” to an entity with which the physician, or an immediate family member of the physician, has a direct or indirect financial relationship, unless the financial relationship is structured to meet an applicable exception. Designated health services include outpatient prescription drugs, DME and supplies, parenteral and enteral nutrient, equipment and supplies, and home health services. An entity that bills Medicare or Medicaid for designated health services that result from a prohibited referral is required to refund amounts collected pursuant to the prohibited referral on a timely basis. Penalties for violation of the Stark Law include denial of payment, civil monetary penalties and exclusion from federal healthcare programs. A knowing violation of the Stark Law can also constitute a violation of the federal False Claims Act. Our management carefully considers the Stark Law and its accompanying regulations in structuring our financial relationships with physicians and believes we are in compliance therewith.
We are also subject to state statutes and regulations that prohibit self-referral arrangements. The laws and exceptions or safe harbors may vary from the federal Stark Law and vary significantly from state to state. Some of these state statutes mirror the federal Stark Law while others are broader. For example, some state statutes and regulations apply to services reimbursed by governmental as well as private payors, and some extend to providers other than physicians. Violation of these laws may result in prohibition of payment for services rendered, loss of pharmacy or health provider licenses, fines and criminal penalties. The state laws are often vague and, in many cases, have not been widely interpreted by courts or regulatory agencies. We believe we are in compliance with such laws.
Statutes Prohibiting False Claims and Fraudulent Billing Activities
A range of federal civil and criminal laws target the submission of false claims and fraudulent billing activities. One of the most significant of these laws is the federal False Claims Act, which provides for liability of treble damages and civil penalties for knowingly making or causing to be made false claims in order to secure reimbursement from government-sponsored programs, such as Medicare and Medicaid. Investigations or actions commenced under the False Claims Act may be brought either by the government or by private individuals on behalf of the government, through a “whistleblower” or “qui tam” action. The False Claims Act authorizes the payment of a portion of any recovery to the individual bringing suit. Such actions are initially required to be filed under seal pending their review by the Department of Justice. If the government intervenes in the lawsuit and prevails, the whistleblower (or plaintiff filing the initial complaint) may share with the federal government in any settlement or judgment.
13
If the government does not intervene in the lawsuit, the whistleblower plaintiff may pursue the action independently. The False Claims Act may result in substantial financial penalties for small billing errors that are replicated in a large number of claims, as each individual claim could be deemed to be a separate violation of the False Claims Act. Significantly, the Affordable Care Act amended the False Claims Act to impose liability for knowing failures to return overpayments which, under the Affordable Care Act’s 60-Day Rule, include failures to report and return an overpayment to the government within 60 days after it is identified.
The False Claims Act has been used by the federal government and private whistleblowers to bring enforcement actions under fraud and abuse laws like the federal Anti-Kickback Statute and the Stark Law, increasing potential financial exposure for alleged violations. Such actions are based on the theory that when an entity submits a claim, it either expressly or impliedly certifies that it has provided the underlying services in compliance with material laws, including the Anti-Kickback Statute or Stark Law. Liability may result even if the claims are otherwise billed accurately for appropriate and medically necessary services. These actions are costly and time-consuming to defend.
Some states also have enacted statutes similar to the False Claims Act which may include criminal penalties, substantial fines, and treble damages, and some allow individuals to bring qui tam actions. Federal law provides an incentive to states to enact false claims laws comparable to the federal False Claims Act.
In recent years, federal and state governments have launched several initiatives aimed at uncovering practices that violate false claims or fraudulent billing laws. We have experienced increasing audit activity by enforcement entities, and we may be the subject of future audits. We believe we have procedures in place to ensure the accuracy of our claims. While we believe we are in compliance with Medicaid and Medicare billing rules and requirements, there can be no assurance that regulators would agree with the methodology employed by us in billing for our products and services. A material disagreement with governmental agencies on the manner in which we provide or bill for products or services could have a material adverse effect on our business and Consolidated Financial Statements.
Civil Monetary Penalties Act
The Civil Monetary Penalties Law authorizes the U.S. Secretary of HHS to impose civil money penalties, assessments and program supervision or exclusion for various forms of fraud and abuse involving the Medicare and Medicaid programs. Penalties of up to $100,000 for each violation may be imposed, depending on the specific misconduct involved. These penalties are updated annually based on changes to the consumer price index. In some cases, violations of the Civil Monetary Penalty Law may result in penalties of up to three times the remuneration offered, paid, solicited or received, and may also result in exclusion from government healthcare programs. The availability of the Civil Money Penalties Law to enforce alleged fraud and abuse violations has increased the potential for enforcement actions, as it requires a lower burden of proof than some criminal statutes, and it has increased the potential financial exposure for such actions. These actions are costly and time-consuming to defend.
Other Fraud & Abuse Laws
We are also subject to additional fraud and abuse laws, including federal criminal statutes that prohibit, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Federal enforcement authorities may exclude from Medicare and Medicaid any business entities and any investors, officers and managing employees associated with business entities that have committed health care fraud. Officers and managing employees may be excluded even if they had no knowledge of the fraud.
We may also be subject to laws promoting transparency of financial relationships with providers and other potential referral sources. For example, the Physician Payment Sunshine Act requires pharmaceutical, biological, device, and medical supply manufacturers to report payments or other transfers of value to physicians and teaching hospitals, as well as physician ownership and investment interests. These initiatives may result in increased scrutiny by government enforcement authorities or impact our public reputation.
Confidentiality, Privacy and HIPAA
Many of our activities involve the receipt, use and/or disclosure of confidential medical, pharmacy or other health-related information concerning individual patients, including the disclosure of such confidential information to an individual's health plan.
The Health Insurance Portability and Accountability Act of 1996 (“HIPPA”) and its implementing regulations, as amended, give people greater control over the privacy of their medical information. The federal privacy regulations (the “Privacy
14
Regulations”) are designed to protect health-related information that could be used to identify an individual, also known as protected health information (“PHI”). Among numerous other requirements, the Privacy Regulations: (i) limit permissible uses and disclosures of PHI; (ii) limit most uses and disclosures of PHI to the minimum necessary to accomplish the intended purpose; (iii) require patient authorization for uses and disclosures of PHI unless an exception applies; and (iv) guarantee patients the right to access their medical records and to receive an accounting of certain disclosures. The federal security regulations (the “Security Regulations”) set certain standards regarding the storage, utilization of, access to and transmission of electronic PHI. The federal breach notification regulations (the “Breach Notification Regulations”) require notification to individuals, the federal government and, in some cases, the media in the event of a breach of unsecured PHI.
These regulations apply to “covered entities,” which include most healthcare providers and health plans, and some of these regulations apply to “business associates,” which are persons or entities that perform or assist in performing services or activities for or on behalf of a covered entity, if the performance of those services or activities involves the creation, receipt, maintenance or transmission of PHI. HIPAA also requires that a covered entity and its business associates enter into written contracts whereby the business associate agrees to restrict its use and disclosure of PHI. We provide a varied line of services to patients and other entities. When we are acting as a pharmacy or health care provider, we function as a covered entity. There may also be situations when we act on behalf of another covered entity as a business associate.
The requirements imposed by HIPAA are extensive, and it has required substantial cost and effort to assess and implement measures to comply with those requirements. We have taken and intend to continue to take steps that we believe are reasonably necessary to ensure our policies and procedures are in compliance with the Privacy Regulations, the Security Regulations and the Breach Notification Regulations. The requirements imposed by HIPAA have increased our burden and costs of regulatory compliance (including our health improvement programs and other information-based products), altered our reporting and reduced the amount of information we can use or disclose if patients do not authorize such uses or disclosures.
Some federal and state privacy-related laws are more restrictive than HIPAA and could result in additional penalties. For example, the Federal Trade Commission uses its consumer protection authority to initiate enforcement actions in response to data breaches. In addition, most states have enacted privacy and security laws, including laws that protect particularly sensitive medical information (such as HIV status or mental health records) and breach notification laws that may impose an obligation to notify persons if their personal information has or may have been accessed by an unauthorized person. Some of these laws apply to our business and have increased and will continue to increase our burden and costs of privacy and security-related regulatory compliance.
Employees
As of December 31, 2017, we had 1,727 full-time, 49 part-time and 378 per diem employees. Per diem employees are defined as those available on an as-needed basis. None of our employees are represented by any union and, in our opinion, relations with our employees are satisfactory.
Available Information
We maintain a website at www.bioscrip.com. The information contained on our website is not incorporated by reference into this Annual Report and should not be considered part of this report. We file annual, quarterly and current reports, proxy statements and other information with the SEC. We make available, free of charge through our website, our reports on Forms 10-K, 10-Q, and 8-K, and amendments to those reports, as soon as reasonably practicable after they are filed with or furnished to the SEC.
We have adopted a Code of Business Conduct and Ethics policy for our Company, including our directors, officers and employees. Our Code of Business Conduct and Ethics policy and the charters of the Audit Committee, Management Development and Compensation Committee, and Governance, Compliance and Nominating Committee of our board of directors are available on our website at www.bioscrip.com.
Item 1A. | Risk Factors |
Risks Related to Our Business
Pressures relating to downturns in the economy could adversely affect our business and consolidated financial statements.
Medicare and other federal and state payors account for a significant portion of our revenues. During economic downturns and periods of stagnant or slow economic growth, federal and state budgets are typically negatively affected, resulting in reduced
15
reimbursements or delayed payments by the federal and state government health care coverage programs in which we participate, including Medicare, Medicaid and other federal or state assistance plans. Government programs could also slow or temporarily suspend payments, negatively impacting our cash flow and increasing our working capital needs and interest payments. We have seen, and believe we will continue to see, Medicare and state Medicaid programs institute measures aimed at controlling spending growth, including reductions in reimbursement rates.
Higher unemployment rates and significant employment layoffs and downsizings may lead to lower numbers of patients enrolled in employer-provided plans. Adverse economic conditions could also cause employers to stop offering, or limit, healthcare coverage, or modify program designs, shifting more costs to the individual and exposing us to greater credit risk from patients or the discontinuance of therapy.
Existing and new government legislative and regulatory action could adversely affect our business and financial results.
Our business is subject to numerous federal, state and local laws and regulations. See “Business - Government Regulation.” Changes in these regulations may require extensive changes to our systems and operations that may be difficult to implement. Untimely compliance or noncompliance with applicable laws and regulations could adversely affect the continued operation of our business as a result of civil or criminal penalties, including, but not limited to: imposition of monetary penalties; suspension of payments from government programs; loss of required government certifications or approvals; suspension or exclusion from participation in government reimbursement programs; or loss of licensure. Reductions in reimbursement by Medicare, Medicaid and other governmental payors could adversely affect our business as well. The law and regulations to which we are subject include, but are not limited to, the federal Anti-Kickback Statute and Stark Law, and state counter parts; HIPAA; False Claims Act; Civil Monetary Penalties Act; regulations promulgated by the FDA, U.S. Federal Trade Commission, DEA, HHS and CMS, and regulations of individual state regulatory authorities. In that regard, our business and consolidated financial statements could be affected by one or more of the following:
• | federal and state laws and regulations governing the purchase, distribution, management, compounding, dispensing and reimbursement of prescription drugs and related services, including state and federal controlled substances laws and regulations; |
• | rules and regulations issued pursuant to HIPAA and HITECH; and other federal and state laws affecting the use, disclosure and transmission of health information, such as state security breach notification laws and state laws limiting the use and disclosure of prescriber information; |
• | administration of Medicare and state Medicaid programs, including legislative changes and/or rulemaking and interpretation; |
• | federal and state laws and regulations that require reporting and public dissemination of payments to and between various health care providers and other industry participants; |
• | government regulation of the development, administration, review and updating of formularies and drug lists; |
• | managed care reform and plan design legislation, including state laws regarding out-of-network charges and participation; and |
• | federal or state laws governing our relationships with physicians or others in a position to refer to us. |
The Affordable Care Act and other healthcare reform efforts could have a material adverse effect on our business.
In recent years, healthcare reform efforts at federal and state levels of government have resulted in sweeping changes to the delivery and financing of health care. The Affordable Care Act is the most prominent of these efforts. However, there is substantial uncertainty regarding its net effect and its future. The presidential administration and certain members of Congress continue to attempt to repeal or make significant changes to the Affordable Care Act, its implementation and its interpretation. It is impossible to predict the full impact of the Affordable Care Act and related regulations or the impact of its modification on our operations in light of the uncertainty regarding whether, when or how the law will be changed and what alternative reforms, if any, may be enacted. Health reform efforts may adversely affect our customers, which may cause them to reduce or delay use of our products and services. As such, we cannot predict the impact of the Affordable Care Act on our business, operations or financial performance.
Federal actions and legislation may reduce reimbursement rates from governmental payors and adversely affect our results of operations.
The Budget Control Act of 2011 requires automatic spending reductions to reduce the federal deficit, including Medicare spending reductions of up to 2% per fiscal year. CMS began imposing a 2% reduction on Medicare claims on April 1, 2013. These reductions have been extended through 2027.
16
In addition, the Affordable Care Act provides for material reductions in the growth of Medicare program spending. From time to time, CMS revises the reimbursement systems used to reimburse health care providers, which may result in reduced Medicare payments. Because most states must operate with balanced budgets and because the Medicaid program is often a state’s largest program, some states have enacted or may consider enacting legislation designed to reduce their Medicaid expenditures. Further, many states have taken steps to reduce coverage and/or enroll Medicaid recipients in managed care programs. The current economic environment has increased the budgetary pressures on many states, and these budgetary pressures have resulted, and likely will continue to result, in decreased spending, or decreased spending growth, for Medicaid programs and the Children’s Health Insurance Program in many states. In addition, the Cures Act significantly reduced the amount paid by Medicare for the drug costs, while delaying the implementation of a clinical services payment, though Congress passed a temporary transitional service payment that takes effect January 1, 2019.
In some cases, Third Party Payors rely on all or portions of Medicare payment systems to determine payment rates. Changes to government health care programs that reduce payments under these programs may negatively impact payments from Third Party Payors. Current or future health care reform and deficit reduction efforts, changes in other laws or regulations affecting government health care programs, changes in the administration of government health care programs and changes by Third Party Payors could have a material, adverse effect on our financial position and results of operations.
We face periodic reviews and billing audits by governmental and private payors, and these audits could have adverse findings that may negatively impact our business.
As a result of our participation in the Medicare and Medicaid programs, we are subject to various governmental reviews and audits to verify our compliance with these programs and applicable laws and regulations. We also are subject to audits under various government programs in which third party firms engaged by CMS conduct extensive reviews of claims data and medical and other records to identify potential improper payments under the Medicare program. Private pay sources may also conduct audits. Disputes with payors can arise from these reviews. Payors can claim that payments based on certain billing practices or billing errors were made incorrectly. If billing errors are identified in the sample of reviewed claims, the billing error can be extrapolated to all claims filed which could result in a larger overpayment than originally identified in the sample of reviewed claims. Our costs to respond to and defend claims, reviews and audits may be significant and could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. Moreover, an adverse claim, review or audit could result in:
• | required refunding or retroactive adjustment of amounts we have been paid by governmental or private payors; |
• | state or Federal agencies imposing fines, penalties and other sanctions on us; |
• | suspension or exclusion from the Medicare program, state programs, or one or more private payor networks; or |
• | damage to our business and reputation in various markets. |
These results could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
If any of our pharmacies fail to comply with the conditions of participation in the Medicare program, that pharmacy could be terminated from Medicare, which could adversely affect our consolidated financial statements.
Our pharmacies must comply with the extensive conditions of participation in the Medicare program. These conditions vary depending on the type of facility, but, in general, require our facilities to meet specified standards relating to licensure, personnel, patient rights, patient care, patient records, physical site, administrative reporting and legal compliance. If a pharmacy fails to meet any of the Medicare supplier standards, that pharmacy could be terminated from the Medicare program. We respond in the ordinary course to deficiency notices issued by surveyors, and none of our pharmacies has ever been terminated from the Medicare program for failure to comply with the supplier standards. Any termination of one or more of our pharmacies from the Medicare program for failure to satisfy the Medicare supplier standards could adversely affect our consolidated financial statements.
We cannot predict the impact of changing requirements on compounding pharmacies.
Compounding pharmacies are closely monitored by federal and state governmental agencies. We believe that our compounding is done in safe environments and we have clinically appropriate policies and procedures in place. We only compound pursuant to a patient specific prescription and do so in compliance with USP 797 standards. In 2013, Congress passed the DQSA, which creates a new category of compounders called outsourcing facilities, which are regulated by the FDA. We do not believe that our current compounding practices qualify us as an outsourcing facility and therefore we continue to operate consistently with USP 797 standards. Should state regulators or the FDA disagree, or should our business practices change to qualify us as an outsourcing facility, there is a risk of regulatory action and/or increased resources required to comply with federal requirements imposed
17
pursuant to the DQSA on outsourcing facilities that could significantly increase our costs or otherwise affect our results of operations. Furthermore, we cannot predict the overall impact of increased scrutiny on compounding pharmacies.
Competition in the healthcare industry may adversely affect our business.
The healthcare industry is very competitive. Our competitors include large and well-established companies that may have greater financial, marketing and technological resources than we do. Some of our competitors are under common control with, or owned by, pharmaceutical wholesalers and distributors, managed care organizations, pharmacy benefit managers or retail pharmacy chains and may be better positioned with respect to the cost-effective distribution of pharmaceuticals. In addition, some of our competitors may have secured long-term supply or distribution arrangements for prescription pharmaceuticals necessary to treat certain chronic disease states on price terms substantially more favorable than the terms currently available to us. As a result of such advantageous pricing, we may be less price competitive than some of these competitors with respect to certain pharmaceutical products. ACOs and other clinical integration models may result in lower reimbursement rates. Some of our competitors may negotiate exclusivity provisions with managed care plans or otherwise interfere with the ability of managed care companies to contract with us. Increasing consolidation in the payer and supplier industries, including vertical integration efforts among insurers and providers and suppliers, and cost-reduction strategies by large employer groups and their affiliates may limit our ability to negotiate favorable terms and conditions in our contracts and otherwise intensify competitive pressure. In addition, our competitive position could be adversely affected by any inability to obtain access to new biotech pharmaceutical products.
Changes in the case mix of patients, as well as payment methodologies, payor mix or pricing could adversely affect our consolidated financial statements.
The sources and amounts of our patient revenue are determined by a number of factors, including the mix of patients and the rates of reimbursement among payors. Changes in the case mix of the patients, payment methodologies, payor mix or pricing among private pay, Medicare and Medicaid may significantly affect our consolidated financial statements.
Changes in industry pricing benchmarks could adversely affect our financial performance.
Contracts within our business generally use certain published benchmarks to establish pricing for the reimbursement of prescription medications dispensed by us. These benchmarks include AWP, wholesale acquisition cost and average manufacturer price. Many of our contracts utilize the AWP benchmark. Publication of the AWP benchmark was expected to cease in 2011 as a result of the settlement of class-action lawsuits brought against First DataBank and Medi-Span, third-party publishers of various pricing benchmarks. However, Medi-Span continues to publish the AWP benchmark and has indicated that it will continue to do so until a new benchmark is widely accepted. Several industry participants have explored establishing a new benchmark but there is not currently a viable generally accepted alternative to the AWP benchmark. Without a suitable pricing benchmark in place many of our contracts will have to be modified and could potentially change the economic structure of our agreements.
Contract renewals, or lack thereof, with key revenue sources and key business relationships could result in less favorable pricing, loss of exclusivity and/or reduced distribution and access to customers, which could have an adverse effect on our business, financial condition and results of operations.
We have contractual and business relationships with key revenue sources, including Third Party Payors. Our future growth and success depends on our ability to maintain these relationships and renew such contracts on acceptable terms. However, we may not be able to continue to maintain these relationships. We may have disputes with Third Party Payors regarding these contractual relationships; these disputes may also disrupt our ongoing contractual relationships with these payors. Any break in these key business relationships could result in lost contracts and reduce our access to certain customers and distribution channels. Further, when these contracts near expiration, we may not be able to successfully renegotiate acceptable terms. Any increase in pricing or loss of exclusivity could result in reduced margins. Accordingly, it is possible that our ongoing efforts to renew contracts and business relationships with such key revenue sources as Third Party Payors could result in less favorable pricing or even reduced access to customers and distribution channels, any of which could have an adverse effect on our business, financial condition and results of operations. In addition, even when such contracts are renewed, they may be renewed for only a short term or may be terminable on relatively short notice.
We and certain of our former directors and executive officers were named as defendants in a derivative complaint and we may be subject to similar lawsuits in the future.
Certain of our current and former directors and executive officers were named as defendants in a derivative complaint (the “Derivative Complaint”) that generally alleged that certain defendants breached their fiduciary duties with respect to the Company’s
18
public disclosures, oversight of Company operations, secondary stock offerings and stock sales. The Company was also named as a nominal defendant in the Derivative Complaint. The Derivative Complaint also contended that certain defendants aided and abetted those alleged breaches. On April 18, 2017, the Court granted the defendants’ motion to dismiss, and on November 27, 2017 the Delaware Supreme Court affirmed the dismissal. Additional demands and lawsuits related to the same facts and circumstances, however, could be pursued in the future.
In that event, there is no assurance that any defenses will be successful or that insurance will be available or adequate to fund any settlement, judgment or litigation costs associated with this action. Certain of the defendants may also seek indemnification from the Company pursuant to certain indemnification agreements, for which there may be no insurance coverage.
Any conclusion in this matter or in any related manner adverse to us would have an adverse effect on our financial condition and business and the Company. We could incur substantial costs not covered by our directors’ and officers’ liability insurance, suffer a significant adverse impact on our reputation and divert management’s attention and resources from other priorities, including the execution of business plans and strategies that are important to our ability to grow our business, any of which could have an adverse effect on our business.
Pending and future litigation could subject us to significant monetary damages and/or require us to change our business practices.
We are subject to risks relating to asserted claims, litigation and other proceedings in connection with our operations. We are or may face claims or become a party to a variety of legal actions that affect our business, including breach of contract actions, employment and employment discrimination-related suits, employee benefit claims, stockholder suits and other securities laws claims, and tort claims. We may incur substantial expenses in defending such claims or litigation, regardless of merit, and such claims or litigation could result in a significant diversion of the efforts of our management personnel. Successful claims against us may result in monetary liability or a material disruption in the conduct of our business. See Item 3-Legal Proceedings for a description of material proceedings pending against us. We believe that these suits are without merit and, to the extent not already concluded, intend to contest them vigorously. However, an adverse outcome in one or more of these suits may have a material adverse effect on our consolidated results of operations, consolidated financial position and/or consolidated cash flow from operations, or may require us to make material changes to our business practices.
We periodically respond to subpoenas and requests for information from governmental agencies. To our knowledge, we are not a target or a potential subject of a criminal investigation. But we cannot predict with certainty whether we may in the future become a target or potential target of an investigation or the subject of further inquiries or ultimately settlements with respect to the subject matter of any subpoenas. In addition to potential monetary liability arising from suits and proceedings, from time to time we incur costs in providing documents to government agencies. Current pending claims and associated costs may be covered by our insurance, but certain other costs are not insured. Such costs may increase and/or continue to be material to our performance in the future.
In addition, as we continue our strategic assessment and cost reduction efforts, there is an increased risk of employment and workers compensation-related litigation and/or administrative claims brought against us. We would defend against any and all such litigation and claims, as appropriate. Such claims could have a material adverse effect on our consolidated financial statements in any particular reporting period.
Our acquisition strategy exposes us to a variety of operational and financial risks.
A principal element of our historic business strategy has been to grow by acquiring other companies and assets in the home infusion and complementary businesses. Growth, especially rapid growth, through acquisitions exposes us to a variety of operational and financial risks. We summarize the most significant of these risks below.
Integration risks. We must integrate our acquisitions with our existing operations. This process includes the integration of the various components of our business (including the following) and of the businesses we have acquired or may acquire in the future:
• | health care professionals and employees who are not familiar with our policies and procedures; |
• | clients who may terminate their relationships with us; |
• | key employees who may seek employment elsewhere; |
• | patients who may elect to switch to another health care provider; |
• | regulatory compliance programs; and |
• | disparate operating, information and record keeping systems and technology platforms. |
19
Integrating an acquisition could be expensive and time consuming and could disrupt our ongoing business, negatively affect cash flow and distract management and other key personnel from day-to-day operations.
We may not be able to combine successfully the operations of acquired companies with our operations, and, even if such integration is accomplished, we may never realize the potential benefits of the acquisition. The integration of acquisitions requires significant attention from management, may impose substantial demands on our operations or other projects and may impose challenges on the combined business including, but not limited to, consistencies in business standards, procedures, policies and business cultures. If we fail to complete ongoing integration efforts, we may never fully realize the potential benefits of the related acquisitions.
Benefits may not materialize. When evaluating potential acquisition targets, we identify potential synergies and cost savings that we expect to realize upon the successful completion of the acquisition and the integration of the related operations. We may, however, be unable to achieve or may otherwise never realize the expected benefits. Our ability to realize the expected benefits from improvements to companies we acquire are subject to significant business, economic and competitive uncertainties and contingencies, many of which are beyond our control, such as changes to government regulation governing or otherwise impacting our industry, reductions in reimbursement rates from Third Party Payors, reductions in service levels under our contracts, operating difficulties, client preferences, changes in competition and general economic or industry conditions. If we are unsuccessful in implementing these improvements or if we do not achieve our expected results, it may adversely impact our results of operations.
Assumptions of unknown liabilities. Companies that we acquire may have unknown or contingent liabilities, including, but not limited to, liabilities for failure to comply with healthcare laws and regulations. We may incur material liabilities for the past activities of acquired operations. Such liabilities and related legal or other costs and/or resulting damage to our reputation could negatively impact our business through lower-than-expected operating results, charges for impairment of acquired intangible assets or otherwise.
Competing for acquisitions. We face competition for acquisition candidates primarily from other home infusion and other healthcare companies. Some of our competitors have greater resources than we do. As a result, we may pay more to acquire a target business or may agree to less favorable deal terms than we would have otherwise. Accurately assessing the value of acquisition candidates is often very challenging. Also, suitable acquisitions may not be available due to unfavorable terms.
Further, the cost of an acquisition could result in a dilutive effect on our results of operations, depending on various factors, including employee severance costs, certain legal and professional fees, training costs, redundant wage costs, impacts recorded from the change in contingent consideration obligations, and other costs related to contract terminations and closed branches/offices.
Improving financial results. Some of the operations we have acquired or may acquire in the future may have had significantly lower operating margins than our current operations. If we fail to improve the operating margins of the companies we acquire, operate such companies profitably or effectively integrate the operations of the acquired companies, our results of operations could be negatively impacted.
Acquisitions, strategic investments and strategic relationships involve certain risks.
We may pursue opportunistic acquisitions, strategic investments in, or strategic relationships with businesses and technologies. Acquisitions may entail numerous risks, including difficulties in assessing values for acquired businesses, intangible assets and technologies, difficulties in the assimilation of acquired operations and products, diversion of management’s attention from other business concerns, assumption of unknown material liabilities of acquired companies, amortization of acquired intangible assets which could reduce future reported earnings, and potential loss of clients or key employees of acquired companies. We may not be able to successfully fully integrate the operations, personnel, services or products that we have acquired or may acquire in the future. Strategic investments may also entail some of the risks described above. If these investments are unsuccessful, we may need to incur charges against earnings. We may also pursue a number of strategic relationships. These relationships and others we may enter into in the future may be important to our business and growth prospects. We may not be able to maintain these relationships or develop new strategic alliances.
We may incur significant costs in connection with our evaluation of new business opportunities and suitable acquisition candidates.
Our management intends to identify, analyze and evaluate potential new business opportunities, including possible acquisition and merger candidates. We may incur significant costs, such as due diligence and legal and other professional fees and expenses,
20
as part of these efforts. Notwithstanding these efforts and expenditures, we may not be able to identify an appropriate new business opportunity, or any acquisition opportunity, in the near term, or at all.
If our remedial measures are insufficient to address material weaknesses and we are unable to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results, timely file our periodic reports, maintain our reporting status or prevent fraud.
During fiscal year 2017, management identified a material weakness in our internal control over financial reporting with respect to the continuous risk assessment process and monitoring activities meant to identify possible risks of material misstatement in our financial reporting processes. In connection with management’s assessment of the effectiveness of our internal control over financial reporting as of December 31, 2017, we concluded there was a material weakness related to internal controls as described in Item 9A - Controls and Procedures. While we have implemented certain measures that we believe will remediate this material weakness, we can provide no assurance that our remediation efforts will be effective. The Company’s remediation plan is also described in Item 9A - Controls and Procedures.
Under standards established by the Public Company Accounting Oversight Board, a material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented, detected or corrected on a timely basis.
If additional material weaknesses or significant deficiencies in our internal control over financial reporting are discovered or occur in the future, there exists a risk that our consolidated financial statements may contain material misstatements that are unknown to us at that time, and such misstatements could require us to restate our financial results. Our management or our independent registered public accounting firm may identify other material weaknesses in our internal control over financial reporting in the future. The existence of a material weakness in our internal control over financial reporting may result in current and potential stockholders losing confidence in our financial reporting, which could negatively impact the market price of our common stock (“Common Stock”).
In addition, the existence of material weaknesses in our internal control over financial reporting may affect our ability to timely file periodic reports under the Exchange Act and may consequently result in the SEC revoking the registration of our Common Stock, the NASDAQ Global Market delisting our Common Stock or a default or an event of default under our Notes Facilities and our 2021 Notes (each, as defined below). Any of these events could have a material adverse effect on the market price of our Common Stock or on our business, financial condition and results of operations.
We may be subject to liability claims for damages and other expenses that are not covered by insurance.
A successful product or professional liability claim in excess of our insurance coverage could harm our consolidated financial statements. Various aspects of our business may subject us to litigation and liability for damages. For example, a prescription drug dispensing error could result in a patient receiving the wrong or incorrect amount of medication, leading to personal injury or death. Our business and consolidated financial statements could suffer if we pay damages or defense costs in connection with a claim that is outside the scope of any applicable contractual indemnity or insurance coverage.
Changes in our relationships with pharmaceutical suppliers, including changes in drug availability or pricing, could adversely affect our business and financial results.
We have contractual relationships with pharmaceutical manufacturers to purchase the drugs that we dispense. Any changes to these relationships, including, but not limited, to loss of a manufacturer relationship, drug shortages or changes in pricing, could have an adverse effect on our business and financial results.
We purchase a majority of our pharmaceutical products from one vendor and a disruption in our purchasing arrangements could adversely impact our business.
We purchase a majority of our prescription products, subject to certain minimum periodic purchase levels and excluding purchases of therapeutic plasma products, from a single wholesaler, AmerisourceBergen Drug Corporation (“ABDC”), pursuant to a prime vendor agreement. The term of this agreement extends until December 2019, subject to extension for up to two additional years. Any significant disruption in our relationship with ABDC, or in ABDC’s supply and timely delivery of products to us, would make it difficult and possibly more costly for us to continue to operate our business until we are able to execute a replacement wholesaler agreement. If that were to occur, we may not be able to find a replacement wholesaler on a timely basis. Further, such wholesaler may not be able to fulfill our demands on similar financial terms and service levels. If we are unable to identify a
21
replacement on substantially similar financial terms and/or service levels, our consolidated financial statements may be materially and adversely affected.
A disruption in supply could adversely impact our business.
We also source pharmaceuticals, medical supplies and equipment from other manufacturers, distributors and wholesalers. Most of the pharmaceuticals that we purchase are available from multiple sources, and we believe they are available in sufficient quantities to meet our needs and the needs of our patients. We keep safety stock to ensure continuity of service for reasonable, but limited, periods of time. Should a supply disruption result in the inability to obtain especially high margin drugs and compound components necessary for patient care, our consolidated financial statements could be negatively impacted.
Prescription volumes may decline, and our net revenues and profitability may be negatively impacted, when products are withdrawn from the market or when increased safety risk profiles of specific drugs result in utilization decreases.
We dispense significant volumes of prescription medications from our pharmacies. Our dispensing volume is the principal driver of revenue and profitability. When products are withdrawn by manufacturers, or when increased safety risk profiles of specific drugs or classes of drugs result in utilization decreases, physicians may cease writing or reduce the numbers of prescriptions written for these higher-risk drugs. Additionally, negative media reports regarding drugs with higher safety risk profiles may result in reduced consumer demand for such drugs. In cases where there are no acceptable prescription drug equivalents or alternatives for these prescription drugs, our prescription volumes, net revenues, profitability and cash flows may decline.
Home infusion joint ventures formed with hospitals could adversely affect our financial results.
The home infusion industry is currently seeing renewed activity in the formation of equity-based infusion joint ventures formed with hospitals. This activity stems, in part, from hospitals seeking to position themselves for new paradigms in the delivery of coordinated healthcare and new methods of payment, including an emerging interdisciplinary care model forming that is being labeled as an ACO. These organizations are encouraged by the Affordable Care Act. These entities are designed to save money and improve quality of care by better integrating care, with the healthcare provider possibly sharing in the financial benefits of the new efficiencies.
Participation in equity-based joint ventures offer hospitals and other providers an opportunity to more efficiently transfer patients to less expensive care settings, while keeping the patient within its network. Additionally, it provides many hospitals with a mechanism to invest accumulated profits in a growing sector with attractive margins.
If these home infusion joint ventures continue to expand, then we could lose referrals and our consolidated financial statements could be adversely affected. Also, there are risks and costs associated with joint venture participation. We consider joint ventures with hospitals from time to time.
A shortage of qualified registered nursing staff, pharmacists and other professionals could adversely affect our ability to attract, train and retrain qualified personnel and could increase operating costs.
Our business relies significantly on its ability to attract and retain nursing staff, pharmacists and other professionals who possess the skills, experience and licenses necessary to meet the requirements of their job responsibilities. From time to time and particularly in recent years, there have been shortages of nursing staff, pharmacists and other professionals in certain local and regional markets. As a result, we are often required to compete for personnel with other healthcare systems and our competitors. Our ability to attract and retain personnel depends on several factors, including our ability to provide them with engaging assignments and competitive salaries and benefits. We may not be successful in any of these areas.
In addition, where labor shortages arise in markets in which we operate, we may face higher costs to attract personnel, and we may have to provide them with more attractive benefit packages than originally anticipated or are being paid in other markets where such shortages don’t exist at the time. In either case, such circumstances could cause our profitability to decline. Finally, if we expand our operations into geographic areas where healthcare providers historically have unionized or unionization occurs in our existing geographic areas, negotiating collective bargaining agreements may have a negative effect on our ability to timely and successfully recruit qualified personnel and on our financial results. If we are unable to attract and retain nursing staff, pharmacists and other professionals, the quality of our services may decline and we could lose patients and referral sources.
22
Introduction of new drugs or accelerated adoption of existing lower margin drugs could cause us to experience lower revenues and profitability when prescribers prescribe these drugs for their patients or they are mandated by third party payors.
The pharmaceutical industry pipeline of new drugs includes many drugs that over the long term may replace older, more expensive therapies. As a result of such older drugs going off patent and being replaced by generic substitutes, new and less expensive delivery methods (such as when an infusion or injectable drug is replaced with an oral drug) or additional products are added to a therapeutic class, thereby increasing price competition among competing manufacturer’s products in that therapeutic category. In such cases, manufacturers have the ability to increase drug acquisition costs or lower the selling price of replaced products. This could have the effect of lowering our revenues and/or margins.
Acts of God such as major weather disturbances could disrupt our business.
We operate in a network of prescribers, providers, patients and facilities that can be negatively impacted by local weather disturbances and other force majeure events. For example, in anticipation of major weather events, patients with impaired health may be moved to alternate sites. After a major weather event, availability of electricity, clean water and transportation can impact our ability to provide service in the home. In addition, acts of God and other force majeure events may cause a reduction in our business or increased costs, such as increased costs in our operations as we incur overtime charges or redirect services to other locations, delays in our ability to work with payors, hospitals, physicians and other strategic partners on new business initiatives, and disruption to referral patterns as patients are moved out of facilities affected by such events or are unable to return to sites of service in the home.
Failure to develop new services or adapt to changes and trends within the industry may adversely affect our business.
We operate in a highly competitive environment. We develop new services from time to time to assist our clients. If we are unsuccessful in developing innovative services, our ability to attract new clients and retain existing clients may suffer.
Technology, including the ability to capture and report outcomes, is also an important component of our business as we continue to utilize new and better channels to communicate and interact with our clients, members and business partners. If our competitors are more successful than us in employing this technology, our ability to attract new clients, retain existing clients and operate efficiently may suffer. Any significant shifts in the structure of the healthcare products and services industry in general could alter the industry dynamics and adversely affect our ability to attract or retain clients. Our failure to anticipate or appropriately adapt to changes in the industry could negatively impact our competitive position and adversely affect our business and results of operations.
Cybersecurity risks could compromise our information and expose us to liability, which may harm our ability to operate effectively and may cause our business and reputation to suffer.
Cybersecurity refers to the combination of technologies, processes and procedures established to protect information technology systems and data from unauthorized access, attack, or damage. We rely on our information systems to provide security for processing, transmission and storage of confidential information about our patients, customers and personnel, such as names, addresses and other individually identifiable information protected by HIPAA and other privacy laws. Cyber-attacks are increasingly more common, including in the health care industry. The regulatory environment surrounding information security and privacy is increasingly demanding, with the frequent imposition of new and changing requirements. Compliance with changes in privacy and information security laws and with rapidly evolving industry standards may result in our incurring significant expense due to increased investment in technology and the development of new operational processes.
We have not experienced any known attacks on our information technology systems that compromised any confidential information. We maintain our information technology systems with safeguard protection against cyber-attacks including passive intrusion protection, firewalls and virus detection software. However, these safeguards do not ensure that a significant cyber-attack could not occur. Although we have taken steps to protect the security of our information systems and the data maintained in those systems, it is possible that our safety and security measures will not prevent the systems’ improper functioning or damage or the improper access or disclosure of personally identifiable information such as in the event of cyber-attacks.
Security breaches, including physical or electronic break-ins, computer viruses, attacks by hackers and similar breaches can create system disruptions or shutdowns or the unauthorized disclosure of confidential information. If personal information or protected health information is improperly accessed, tampered with or disclosed as a result of a security breach, we may incur significant costs to notify and mitigate potential harm to the affected individuals, and we may be subject to sanctions and civil or criminal penalties if we are found to be in violation of the privacy or security rules under HIPAA or other similar federal or state laws protecting confidential personal information. In addition, a security breach of our information systems could damage our
23
reputation, subject us to liability claims or regulatory penalties for compromised personal information and could have a material adverse effect on our business, financial condition and results of operations.
The success of our business depends on maintaining a well-secured business and technology infrastructure.
We are dependent on our infrastructure, including our information systems, for many aspects of our business operations. A fundamental requirement for our business is the secure storage and transmission of protected health information and other confidential data. Our business and operations may be harmed if we do not maintain our business processes and information systems in a secure manner, and maintain and continually improve the integrity of our confidential information. Although we have developed systems and processes that are designed to protect information against security breaches, failure to protect our confidential information or mitigate harm caused by such breaches may adversely affect our operating results. Malfunctions in our business processes, breaches of our information systems or the failure to maintain effective and up-to-date information systems could disrupt our business operations, result in customer and member disputes, damage our reputation, expose us to risk of loss or litigation, result in regulatory violations and related costs and penalties, increase administrative expenses or lead to other adverse consequences.
Our business is dependent on the services provided by third party information technology vendors.
Our information technology infrastructure includes hosting services provided by third parties. While we believe these third parties are high-performing organizations with secure platforms and customary certifications, they could suffer a security breach or business interruption which in turn could impact our operations negatively. In addition, changes in pricing terms charged by our technology vendors may adversely affect our financial performance.
Our failure to maintain controls and processes over billing and collecting could have a significant negative impact on our consolidated financial statements.
The collection of accounts receivable is a significant challenge, and requires constant focus and involvement by management and ongoing enhancements to information systems and billing center operating procedures. If we are unable to properly bill and collect our accounts receivable, our results could be materially and adversely affected.
Delays in payment may adversely affect our working capital.
Our business is characterized by delays from the time we provide services to the time we receive payment for these services. If we have difficulty in obtaining documentation, experience information system problems or experience other issues that arise with Medicare or other payors, we may encounter additional delays in our payment cycle.
In addition, timing delays may cause working capital shortages. Working capital management, including prompt and diligent billing and collection, is an important factor in achieving our financial results and maintaining liquidity. It is possible that documentation support, system problems, Medicare or other provider issues or industry trends may extend our collection period, which may materially adversely affect our working capital, and our working capital management procedures may not successfully mitigate this risk.
Our ability to use net operating loss carryforwards to offset future taxable income for U.S. federal tax purposes is subject to limitation and risk that could further limit our ability to utilize our net operating losses.
Under U.S. federal income tax law, a corporation’s ability to utilize its net operating losses (“NOLs”) to offset future taxable income may be significantly limited if it experiences an “ownership change” as defined in Section 382 of the Internal Revenue Code, as amended. In general, an ownership change will occur if there is a cumulative change in a corporation’s ownership by “5-percent shareholders” that exceeds 50 percentage points over a rolling three-year period. A corporation that experiences an ownership change will generally be subject to an annual limitation on the use of its pre-ownership change NOLs equal to the value of the corporation immediately before the ownership change, multiplied by the long-term tax-exempt rate (subject to certain adjustments). The annual limitation for a taxable year generally is increased by the amount of any “recognized built-in gains” for such year and the amount of any unused annual limitation in a prior year. On December 22, 2017, a law commonly known as the Tax Cuts and Jobs Act (“TCJA”) was enacted in the United States. Certain provisions of the TCJA impact the ability to utilize NOLs generated in 2018 and forward; any limitation to our annual use of NOLs could require us to pay a greater amount of U.S. federal (and in some cases, state) income taxes, which could reduce our after-tax income from operations for future taxable years and adversely impact our financial condition.
24
Changes to federal and state income tax laws and regulations could adversely affect our position or income taxes and estimated income liabilities.
We are subject to both state and federal income taxes in the U.S. and our operations, plans and results are affected by tax and other initiatives. The TCJA will impact our financial results beginning in 2018. Among other things, the TCJA reduces the U.S. corporate income tax rate to 21%, this reduction resulted in changes in the valuation of our deferred tax asset and liabilities.
We are also subject to regular reviews, examinations, and audits by the Internal Revenue Service and other taxing authorities with respect to our taxes. There are uncertainties and ambiguities in the application of the TCJA and it is possible that the IRS could issue subsequent guidance or take positions on audit that differ from our interpretations and assumptions. Although we believe our tax estimates are reasonable, if a taxing authority disagrees with the positions we have taken, we could face additional tax liability, including interest and penalties. Our effective tax rate could be adversely affected by changes in the mix of earnings in states with different statutory tax rates, changes in the valuation of deferred tax assets and liabilities, changes in tax laws and regulations, changes in our interpretations of tax laws, including the TCJA. Unanticipated changes in our tax rates or exposure to additional income tax liabilities could affect our profitability. There can be no assurance that payment of such additional amounts upon final adjudication of any disputes will not have a material impact on our results of operations and financial position.
The issuance of shares of our Preferred Stock reduced the percentage interests of our other stockholders, and any future exercise of the Class A and Class B Warrants or the 2017 Warrants will further reduce the percentage interests of our other stockholders.
On March 9, 2015, we entered into a securities purchase agreement (the “Purchase Agreement”) with Coliseum Capital Partners, L.P., Coliseum Capital Partners II, L.P., and Blackwell Partners, LLC, Series A (collectively, the “PIPE Investors”). Pursuant to the terms of the Purchase Agreement, we issued and sold to the PIPE Investors in a private placement an aggregate of (a) 625,000 shares of Series A Convertible Preferred Stock, par value $0.0001 per share (the “Series A Preferred Stock”), at a purchase price per share of $100.00, (b) 1,800,000 Class A warrants (the “Class A Warrants”), and (c) 1,800,000 Class B warrants (the “Class B Warrants” and, together with the Class A Warrants, the “PIPE Warrants”), for gross proceeds of $62.5 million. We also conducted a Rights Offering (as described below) pursuant to which we sold an additional 10,822 shares of Series A Preferred Stock along with the PIPE Warrants. On June 10, 2016, in order to facilitate the 2016 Equity Offering, the Company and the PIPE Investors agreed to exchange 614,177 shares of the existing Series A Preferred Stock for an identical number of shares of Series B Preferred Stock. On June 14, 2016, in order to facilitate the 2016 Equity Offering, the Company and the PIPE Investors agreed to exchange 614,177 shares of the Series B Preferred Stock for an identical number of shares of Series C Preferred Stock (the Series C Preferred Stock, together with the Series A Preferred Stock, the “Preferred Stock”). As a result of these exchanges, there are currently (a) 21,645 shares of Series A Preferred Stock outstanding, of which 10,823 shares are owned by the PIPE Investors, (b) no shares of Series B Preferred Stock outstanding, and (c) 614,177 shares of Series C Preferred Stock outstanding, all of which are owned by the PIPE Investors.
In addition, in connection with the Second Lien Note Facility, the Company also issued the 2017 Warrants to the purchasers of the Second Lien Notes pursuant to the Warrant Purchase Agreement. The 2017 Warrants entitle the purchasers of the 2017 Warrants to purchase shares of Common Stock, representing at the time of any exercise of the 2017 Warrants an equivalent number of shares equal to 4.99% of the Common Stock of the Company on a fully diluted basis, subject to the terms of the Warrant Agreement.
As of the date of this Annual Report, if all holders of the Preferred Stock converted their shares in full, and exercised the PIPE Warrants and the 2017 Warrants in full, their aggregate beneficial ownership would be approximately 22.9% of our outstanding Common Stock. The issuance of the Preferred Stock to the PIPE Investors reduced the relative voting power and percentage ownership interests of our other current stockholders. The future exercise of the PIPE Warrants by the holders of those securities will cause a further reduction in the relative voting power and percentage ownership interests of our other stockholders.
The PIPE Investors may exercise influence over us, including through their ability to influence matters requiring the approval of holders of our Common Stock or Preferred Stock.
Holders of the Preferred Stock are entitled to vote on an as-converted basis upon all matters upon which holders of our Common Stock have the right to vote. The shares of Preferred Stock owned by the PIPE Investors currently represent approximately 13% of the voting rights in respect of our share capital on an as-converted basis, and accordingly the PIPE Investors may have the ability to significantly influence the outcome of most matters submitted for the vote of our stockholders. The PIPE Investors are
25
currently the beneficial owners of 625,000 of the 635,822 shares of our Series A and Series C Preferred Stock.
Further, so long as shares of the Series C Preferred Stock represent at least 5% of our outstanding voting stock (on an as converted into Common Stock basis), the holders of our Series C Preferred Stock are entitled to designate one member of the Board by a majority of the voting power of the outstanding shares of Series C Preferred Stock. The PIPE Investors are currently the beneficial owners of all 614,177 issued and outstanding shares of our Series C Preferred Stock.
The PIPE Investors’ majority ownership of our Series A and Series C Preferred Stock will limit the ability of any current or future holders of such series of Preferred Stock to influence corporate matters requiring the approval of the holders of such series of Preferred Stock, including the right, voting as a separate class, to elect one director to our Board, and to approve certain amendments to our certificate of incorporation, or certain other changes, that would adversely affect the holders of the series of Preferred Stock. The PIPE Investors’ voting power of the Preferred Stock may also delay, defer or even prevent an acquisition by a third party or other change of control of our company to the extent that the consideration that would be received by the PIPE Investors and other holders of Preferred Stock in such acquisition or change of control is less than their liquidation preference, and may make some transactions more difficult or impossible without the support of the PIPE Investors, even if such events are in the best interests of our other stockholders. Accordingly, the ownership position and the governance rights of the PIPE Investors could discourage a third party from proposing a change of control or other strategic transaction with us. In any of these matters, the interests of the PIPE Investors may differ from or conflict with the interests of our other stockholders.
In addition, the PIPE Investors are in the business of making investments in companies and may, from time to time, acquire interests in businesses that directly or indirectly compete with our business, as well as businesses that are significant existing or potential customers.
Changes in future business conditions could cause business investments and/or recorded goodwill to become further impaired, and our financial condition and results of operations could suffer if there is an additional impairment of goodwill or other intangible assets with indefinite lives.
We are required to test intangible assets with indefinite lives, including goodwill, annually and on an interim basis if an event occurs or there is a change in circumstance to indicate that the carrying value of goodwill or indefinite-lived intangible assets may no longer be recoverable. When the carrying value of a reporting unit’s goodwill exceeds its implied fair value of goodwill, a charge to operations is recorded. If the carrying amount of an intangible asset with an indefinite life exceeds its fair value, a charge to operations is recognized. Either event would result in incremental expenses for that quarter, which would reduce any earnings or increase any loss for the period in which the impairment was determined to have occurred.
As previously disclosed, in 2015, we determined it was necessary to record a $251.9 million non-cash impairment charge related to goodwill associated with our Infusion Services business. The estimated impairment took into consideration our updated business outlook, pursuant to which we updated our future cash flow assumptions and calculated updated estimates of fair value. The estimated impairment loss was equal to the excess of the assets' carrying amount over its fair value as determined by an analysis of discounted future cash flows. In connection with our annual assessment of possible goodwill impairment during the fourth quarter of 2017, we concluded no further impairment charge was needed (see Note 7 - Goodwill and Intangible Assets).
Our goodwill impairment analysis is sensitive to changes in key assumptions used in our analysis, such as the degree of volatility in equity and debt markets and our stock price. If the assumptions used in our analysis are not realized, it is possible that an additional impairment charge may need to be recorded in the future. We cannot accurately predict the amount and timing of any impairment of goodwill or other intangible assets. Further, as we continue to work towards a turnaround of our business, we will need to continue to evaluate the carrying value of our goodwill. Any additional impairment charges that we may take in the future could be material to our results of operations and financial condition.
Failure to maintain effective internal control over our financial reporting could have an adverse effect on our ability to report our financial results on a timely and accurate basis.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934 (the “Exchange Act”), and is required to evaluate the effectiveness of these controls and procedures on a periodic basis and publicly disclose the results of these evaluations and related matters in accordance with the requirements of Section 404 of the Sarbanes-Oxley Act of 2002. Effective internal control over financial reporting is necessary for us to provide reliable financial reports, to help mitigate the risk of fraud and to operate successfully. However, testing and maintaining our internal control over financial reporting can be expensive and divert our management's attention from other business matters. Any failure to implement and maintain effective internal controls could result in material weaknesses or material misstatements in our consolidated financial statements.
26
If we fail to maintain effective internal control over financial reporting, or our independent registered public accounting firm is unable to provide us with an unqualified attestation report on our internal control, we may be required to take corrective measures or restate the affected historical financial statements. In addition, we may be subjected to investigations and/or sanctions by federal and state securities regulators, and/or civil lawsuits by security holders. Any of the foregoing could also cause investors to lose confidence in our reported financial information and in our company and would likely result in a decline in the market price of our stock and in our ability to raise additional financing if needed in the future.
New accounting pronouncements or new interpretations of existing standards could require us to make adjustments in our accounting policies that could affect our financial statements.
We prepare our consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (“GAAP”). The Financial Accounting Standards Board, the SEC, or other accounting organizations or governmental entities frequently issue new pronouncements or new interpretations of existing accounting standards. Changes in accounting standards, how the accounting standards are interpreted, or the adoption of new accounting standards can have a significant effect on our reported results, and could even retroactively affect previously reported transactions, and may require that we make significant changes to our systems, processes and controls.
Changes resulting from these new standards may result in materially different financial results and may require that we change how we process, analyze and report financial information and that we change financial reporting controls. Such changes in accounting standards may have an adverse effect on our business, financial position, and income, which may negatively impact our financial results.
In February 2016, the FASB issued ASU 2016-02—Leases (Topic 842), requiring lessees to recognize a right-of-use asset and a lease liability on the balance sheet for all leases with the exception of short-term leases. For lessees, leases will continue to be classified as either operating or finance leases in the income statement. The Company is evaluating the effect that the updated standard will have on its consolidated financial statements.
Risks Related to Our Indebtedness
We have incurred substantial indebtedness, which imposes operating and financial restrictions on us that, together with the resulting debt service obligations, may significantly limit our ability to execute our business strategy and may increase the risk of default under our debt obligations.
On June 29, 2017, the Company entered into (i) a first lien note purchase agreement, among the Company, which is the issuer under the agreement, the financial institutions and note purchasers from time to time party to the agreement, pursuant to which the Company issued first lien senior secured notes in an aggregate principal amount of $200.0 million; and (ii) a second lien note purchase agreement among the Company, which is the issuer under the agreement, the financial institutions and note purchasers from time to time party to the agreement, pursuant to which the Company (a) issued second lien senior secured notes in an aggregate initial principal amount of $100.0 million and (b) has the ability to draw upon the Second Lien Note Facility and issue second lien delayed draw senior secured notes in an aggregate initial principal amount of $10.0 million for a period of 18 months after the closing date, subject to certain terms and conditions. The Company used the proceeds of the sale of the First Lien Notes and the Initial Second Lien Notes to repay in full all amounts outstanding under the Prior Credit Agreements and extinguished the liability. Each of the Prior Credit Agreements was terminated following such repayment. The Notes accrue interest, payable monthly in arrears, at a floating rate. The First Lien Notes will amortize in equal quarterly installments equal to 0.625% of the aggregate principal amount of the First Lien Note Facility, commencing on September 30, 2019, and on the last day of each third month thereafter, with the balance payable at maturity. The First Lien Notes mature on August 15, 2020, provided that if the Company’s 2021 Notes (defined below) are refinanced prior to August 15, 2020, then the scheduled maturity date of the First Lien Notes shall be June 30, 2022. Our indebtedness includes many covenants and restrictions that may significantly limit the types of strategic relationships and our ability to execute our business strategy.
In addition, we have issued $200.0 million in aggregate principal amount of 8.875% senior notes due 2021 (the “2021 Notes”). See “Item 7 - Management’s Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources.” The 2021 Notes are our senior unsecured obligations and are fully and unconditionally guaranteed by all existing and future subsidiaries of the Company. Interest is payable semi-annually on February 15 and August 15.
The operating and financial restrictions and covenants of our debt instruments, including the Notes Facilities and the indenture governing the 2021 Notes, may adversely affect our ability to finance our future operations or capital needs or engage in other business activities that may be in our interest. The terms of the Notes Facilities require us to comply with certain financial covenants.
27
In addition, subject to a number of important exceptions, the Notes Facilities contain certain covenants and restrictions impacting our ability to, among other things:
• | incur or guarantee additional indebtedness or issue certain preferred stock; |
• | transfer or sell assets; |
• | make certain investments and loans; |
• | pay dividends or distributions, redeem subordinated indebtedness, or make other restricted payments; |
• | create or incur liens; |
• | incur dividend or other payment restrictions affecting certain subsidiaries; |
• | issue capital stock of our subsidiaries; |
• | enter into hedging transactions or sale and leaseback transactions; |
• | consummate a merger, consolidation or sale of all or substantially all of our assets or the assets of any of our subsidiaries; and |
• | enter into transactions with affiliates. |
The indenture governing the 2021 Notes contains similar restrictions. Our ability to comply with these covenants, including the financial covenants, may be affected by events beyond our control. Therefore, in order to engage in some corporate actions, we may need to seek permission from our lenders or the note holders, whose interests may be different from ours. We cannot guarantee that we will be able to obtain consent from these parties when needed. If we do not comply with the restrictions and covenants in our Notes Facilities, we may not be able to finance our future operations, make acquisitions or pursue business opportunities. The restrictions contained in our Notes Facilities may prevent us from taking actions that we believe would be in the best interest of our business and may make it difficult for us to successfully execute our business strategy or effectively compete with companies that are not similarly restricted.
A breach of any of these covenants or the inability to comply with the required financial ratio could result in a default under the Notes Facilities. If any such default occurs, the lenders under the respective Notes Facilities may elect to declare all of their respective outstanding debt, together with accrued interest and other amounts payable thereunder, to be immediately due and payable. Under such circumstances, we may not have sufficient funds or other resources to satisfy all of our obligations. In addition, the limitations imposed on our ability to incur additional debt and to take other corporate actions might significantly impair our ability to obtain other financing.
There can be no assurance that we will be granted future waivers or amendments to the restrictions in the Notes Facilities if for any reason we are unable to comply with such restrictions or that we will be able to refinance our debt on terms acceptable to us, or at all.
The lenders under the Notes Facilities also have the right in these circumstances to terminate any commitments they have to provide further borrowings. If we were unable to pay such amounts, the lenders under the Notes Facilities could recover amounts owed to them by foreclosing against the collateral pledged to them. We have pledged a substantial portion of our assets to the lenders under the Notes Facilities, including the equity of all of the Company’s subsidiaries.
In addition, the degree to which we are leveraged could:
• | make us more vulnerable to general adverse economic, regulatory and industry conditions; |
• | limit our flexibility in planning for, or reacting to, changes and opportunities in the markets in which we compete; |
• | place us at a competitive disadvantage compared to our competitors that have less debt; |
• | require us to dedicate a substantial portion of our cash flow to service our debt, reducing the availability of our cash flow and such proceeds to fund working capital, capital expenditures and other general corporate purposes; or |
• | restrict us from making strategic acquisitions or exploiting other business opportunities. |
To service our indebtedness and other obligations, we will require a significant amount of cash. Our ability to generate cash depends on many factors beyond our control, and any failure to meet our debt obligations could harm our business, financial condition and results of operations.
Our ability to make payments on and to refinance our indebtedness, including the First Lien Note Facility, for which principal payments are required beginning in 2019, the Second Lien Note Facility, and the 2021 Notes, and to fund working capital needs and planned capital expenditures will depend on our ability to generate cash in the future. A significant reduction in our operating cash flows resulting from changes in economic conditions, changes in government reimbursement rates or methods, increased competition or other events beyond our control could increase the need for additional or alternative sources of liquidity and could
28
have a material adverse effect on our business, consolidated financial statements, prospects and our ability to service our debt and other obligations.
We cannot assure you that our business will generate sufficient cash flows from operations or that future borrowings will be available to us under the Note Facilities or otherwise in an amount sufficient to enable us to pay our indebtedness, including our indebtedness under the First Lien Note Facility, the Second Lien Note Facility, and the 2021 Notes, or to fund our other liquidity needs. Our inability to pay our debts would require us to pursue one or more alternative strategies, such as selling assets, refinancing all or a portion of our indebtedness or selling equity capital. However, our alternative strategies may not be feasible at the time or may not provide adequate funds to allow us to pay our debts as they come due and fund our other liquidity needs. In addition, some alternative strategies are likely to require the prior consent of our Notes Facilities lenders, which we may not be able to obtain.
Despite our substantial indebtedness, we may still need to incur significantly more debt. This could exacerbate the risks associated with our substantial leverage.
We may need to incur substantial additional indebtedness, including additional secured indebtedness, in the future, in connection with future acquisitions, strategic investments and strategic relationships. Although the First Lien Note Facility, the Second Lien Note Facility and the indenture governing the 2021 Notes contain covenants and restrictions on the incurrence of additional debt, these restrictions are subject to a number of qualifications and exceptions and, under certain circumstances, debt incurred in compliance with these restrictions, including secured debt, could be substantial. Adding additional debt to current debt levels could exacerbate the leverage-related risks described above.
Item 1B. | Unresolved Staff Comments |
None.
Item 2. | Properties |
We currently lease all of our properties from third parties under various lease terms expiring over periods extending through 2027, in addition to a number of non-material month-to-month leases. Our properties mainly consist of infusion pharmacies equipped with clean room and compounding capabilities. Some infusion pharmacies are co-located with an ambulatory infusion center where patients receive infusion treatments. As of December 31, 2017 our property locations, all in support of our Infusion Services business, were as follows:
Birmingham, AL | Alexandria, LA | Omaha, NE | Knoxville, TN | |||
Burbank, CA | Baton Rouge, LA | Bedford, NH | Memphis, TN | |||
Irvine, CA | Covington, LA | Morris Plains, NJ | Austin, TX | |||
Ontario, CA | Hammond, LA | Somers Point, NJ | Houston, TX | |||
Cromwell, CT (two locations) | Houma, LA | Elmsford, NY | Richardson, TX | |||
Vernon, CT | Lafayette, LA | Forest Hills, NY | Annandale, VA | |||
Coral Springs, FL | Lake Charles, LA | Lake Success, NY | Ashland, VA | |||
Jacksonville, FL | Metairie, LA | Canfield, OH | Chantilly, VA | |||
Melbourne, FL | Monroe, LA | Cincinnati, OH | Newport News, VA | |||
Tampa, FL | Shreveport, LA | Columbus, OH | Norfold, VA | |||
Albany, GA | Southborough, MA | Sylvania, OH | Roanoke, VA | |||
Augusta, GA | Auburn, ME | Audubon, PA | Rutland, VT | |||
Norcross, GA | Eagan, MN | Dunmore, PA | Charleston, WV | |||
Savannah, GA | Chesterfield, MO | York, PA | Fairmount, WV | |||
Elmhurst, IL | Pearl, MS | Smithfield, RI | ||||
Silvis, IL | Charlotte, NC | Duncan, SC | ||||
Lexington, KY | Fayetteville, NC | Mount Pleasant, SC | ||||
Item 3. | Legal Proceedings |
29
The information set forth under Note 11, “Commitments and Contingencies,” in the Notes to the Consolidated Financial Statements under the caption “Legal Proceedings” included in Part II, Item 8 of this Annual Report is incorporated herein by reference.
Item 4. | Mine Safety Disclosures |
Item not applicable.
30
PART II
Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Our Common Stock, par value $0.0001 per share, is traded on the Nasdaq Global Market under the symbol “BIOS”. The following table represents the range of high and low per share sale prices for our Common Stock for the indicated periods:
High | Low | ||||||||
2017 | First Quarter | $ | 2.27 | $ | 1.26 | ||||
Second Quarter | $ | 2.99 | $ | 1.40 | |||||
Third Quarter | $ | 3.25 | $ | 2.35 | |||||
Fourth Quarter | $ | 2.93 | $ | 1.91 | |||||
2016 | First Quarter | $ | 2.52 | $ | 1.28 | ||||
Second Quarter | $ | 3.00 | $ | 2.07 | |||||
Third Quarter | $ | 2.92 | $ | 2.51 | |||||
Fourth Quarter | $ | 3.33 | $ | 1.02 | |||||
As of March 23, 2018, there were 181 stockholders of record of our Common Stock. On March 23, 2018, the closing sale price of our Common Stock on the Nasdaq Global Market was $2.46 per share.
We have never paid cash dividends on our Common Stock and do not anticipate doing so in the foreseeable future. Our Notes Facilities contain covenants and restrictions impacting our ability to pay dividends.
Information regarding securities authorized for issuance under our equity compensation plans required by this Item 5 is included in our definitive proxy statement to be filed with the SEC on or before April 30, 2018 in connection with our 2018 Annual Meeting of Stockholders and is hereby incorporated by reference.
The information disclosed in Part II Item 7 under the headings “First Quarter 2017 Private Placement,” “2017 Warrants” and “Second Quarter 2017 Private Placement” is hereby incorporated by reference. The Company relied on Section 4(a)(2) of the Securities Act for the issuance of the 2017 Warrants and the Common Shares issued in both the First Quarter 2017 Private Placement and the Second Quarter 2017 Private Placement.
31
The following graph compares our total cumulative return to holders of our Common Stock with the total cumulative returns of the Nasdaq Composite Index and the Nasdaq Health Services Index for the five-year period from December 31, 2012 through December 31, 2017. The graph shows the performance of a $100 investment in our Common Stock and in each index as of December 31, 2012.
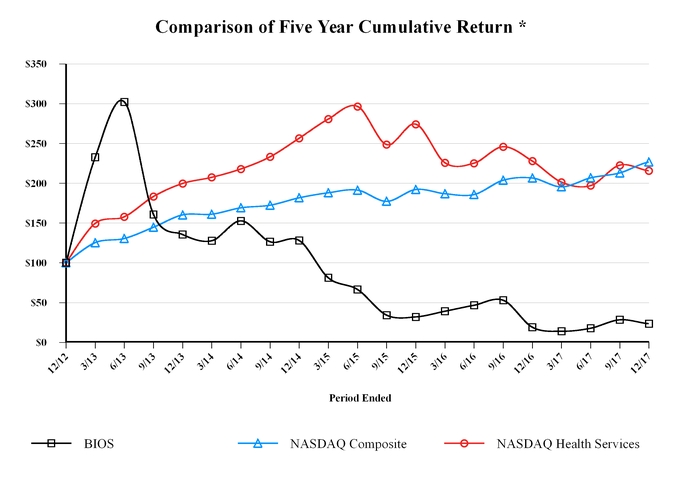
Year Ended December 31, | |||||||||||||||||||||||
2012 | 2013 | 2014 | 2015 | 2016 | 2017 | ||||||||||||||||||
BioScrip, Inc. | $ | 100.00 | $ | 135.53 | $ | 128.02 | $ | 32.05 | $ | 19.05 | $ | 23.58 | |||||||||||
Nasdaq Composite Index | $ | 100.00 | $ | 160.32 | $ | 181.80 | $ | 192.21 | $ | 206.63 | $ | 226.78 | |||||||||||
Nasdaq Health Services Index | $ | 100.00 | $ | 199.82 | $ | 256.70 | $ | 274.30 | $ | 227.91 | $ | 215.79 | |||||||||||
* $100 invested on December 31, 2012 in stock or index including reinvestment of dividends.
Item 6. Selected Consolidated Financial Data
The selected consolidated financial data presented below should be read in conjunction with, and is qualified in its entirety by reference to, Management’s Discussion and Analysis of Financial Condition and Results of Operations and our Consolidated Financial Statements and the Notes thereto appearing elsewhere in this Annual Report. Acquisitions during the periods below include HomeChoice beginning February 2013, CarePoint Business beginning August 2013, and Home Solutions beginning September 2016. Divestitures during this period include the sale of the Home Health Business in March 2014, and the sale of the PBM Business in August 2015. All historical amounts have been restated to reclassify amounts directly associated with these divested operations as discontinued operations. The amounts below are not necessarily indicative of what the actual results would have been if the Home Health Business and the PBM Business were divested at the beginning of the period.
32
December 31, | |||||||||||||||||||
Balance Sheet Data | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||
(in thousands) | |||||||||||||||||||
Working capital (1) | $ | 82,561 | $ | 43,180 | $ | 29,574 | $ | 25,347 | $ | 44,417 | |||||||||
Total assets (2) | 603,092 | 604,985 | 528,416 | 801,204 | 846,660 | ||||||||||||||
Total debt | 480,588 | 451,934 | 418,121 | 423,803 | 435,579 | ||||||||||||||
Stockholders’ equity (deficit) | (84,752 | ) | (33,621 | ) | (81,515 | ) | 216,589 | 354,583 | |||||||||||
Total assets of discontinued operations | — | — | — | 22,294 | 90,198 | ||||||||||||||
Year Ended December 31, | |||||||||||||||||||
Statement of Operations Data | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||
(in thousands, except per share amounts) | |||||||||||||||||||
Net revenue | $ | 817,190 | $ | 935,589 | $ | 982,223 | $ | 922,654 | $ | 696,473 | |||||||||
Gross profit, excluding depreciation expense | 269,242 | 262,082 | 259,952 | 250,753 | 206,650 | ||||||||||||||
Other operating expenses | 163,273 | 169,781 | 165,328 | 165,728 | 127,200 | ||||||||||||||
Bad debt expense | 23,697 | 26,608 | 42,444 | 80,587 | 19,516 | ||||||||||||||
General and administrative expenses | 39,625 | 38,798 | 42,474 | 49,314 | 47,897 | ||||||||||||||
Change in fair value of equity linked liabilities | 3,587 | (10,450 | ) | — | — | — | |||||||||||||
Impairment of goodwill | — | — | 251,850 | — | — | ||||||||||||||
Restructuring, acquisition, integration, and other expenses, net (3) | 12,662 | 15,859 | 24,405 | 30,206 | 18,062 | ||||||||||||||
Depreciation and amortization expense | 27,725 | 22,025 | 22,864 | 22,943 | 20,226 | ||||||||||||||
Interest expense (4) | 52,072 | 37,572 | 36,938 | 40,918 | 44,130 | ||||||||||||||
Loss on extinguishment of debt | 13,453 | — | — | — | — | ||||||||||||||
Loss (gain) on dispositions | 581 | (3,954 | ) | — | — | — | |||||||||||||
Loss from continuing operations, before income taxes | (67,433 | ) | (34,157 | ) | (326,351 | ) | (138,943 | ) | (70,381 | ) | |||||||||
Income tax benefit (expense) | 4,130 | (2,015 | ) | 21,532 | (11,193 | ) | (1,260 | ) | |||||||||||
Loss from continuing operations, net of income taxes | (63,303 | ) | (36,172 | ) | (304,819 | ) | (150,136 | ) | (71,641 | ) | |||||||||
(Loss) income from discontinued operations, net of income taxes | (893 | ) | (6,593 | ) | 4,691 | 2,452 | 1,987 | ||||||||||||
Net loss | $ | (64,196 | ) | $ | (42,765 | ) | $ | (300,128 | ) | $ | (147,684 | ) | $ | (69,654 | ) | ||||
Accrued dividends on preferred stock | (9,376 | ) | (8,392 | ) | (6,120 | ) | — | — | |||||||||||
Deemed dividends on preferred stock | (701 | ) | (692 | ) | (3,690 | ) | — | — | |||||||||||
Net loss attributable to common stockholders | $ | (74,273 | ) | $ | (51,849 | ) | $ | (309,938 | ) | $ | (147,684 | ) | $ | (69,654 | ) | ||||
Loss per common share: | |||||||||||||||||||
Loss from continuing operations, basic and diluted | $ | (0.59 | ) | $ | (0.48 | ) | $ | (4.58 | ) | $ | (2.19 | ) | $ | (1.11 | ) | ||||
(Loss) income from discontinued operations, basic and diluted | (0.01 | ) | (0.07 | ) | 0.07 | 0.04 | 0.03 | ||||||||||||
Net loss, basic and diluted (5) | $ | (0.60 | ) | $ | (0.55 | ) | $ | (4.51 | ) | $ | (2.15 | ) | $ | (1.08 | ) | ||||
Weighted average common shares outstanding, basic and diluted | 123,791 | 93,740 | 68,710 | 68,476 | 64,560 | ||||||||||||||
(1) | Working capital calculation excludes current assets of discontinued operations and current liabilities of discontinued operations. |
(2) | Total assets exclude total assets of discontinued operations as of December 31, 2014, and 2013. |
(3) | Restructuring, acquisition, integration and other expenses include non-operating costs associated with restructuring, acquisition, and integration initiatives such as employee severance costs, certain legal and professional fees, training costs, redundant wage costs, impacts recorded from the change in contingent consideration obligations, and other costs related to contract terminations and closed branches/offices. |
33
(4) | Interest expense includes interest income, interest expense, and amortization of deferred financing cost. |
(5) | Net income (loss) per diluted share excludes the effect of all common stock equivalents for all years as their inclusion would be anti-dilutive to loss per share from continuing operations. |
(6) | Certain amounts have been revised to reflect immaterial corrections. See Note 1 - Nature of Business. |
Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations (MD&A) is designed to assist the reader in understanding our Consolidated Financial Statements, the changes in certain key items in those financial statements from year-to-year and the primary factors that accounted for those changes, as well as how certain accounting principles affect our Consolidated Financial Statements.
Except for the historical information contained herein, the following discussion contains forward-looking statements that are subject to known and unknown risks, uncertainties and other factors that may cause our actual results to differ materially from those expressed or implied by such forward-looking statements. We discuss such risks, uncertainties and other factors throughout this Annual Report and specifically under the caption “Cautionary Note Regarding Forward-Looking Statements” and under “Item 1A. Risk Factors” in this Annual Report. In addition, the following discussion of financial condition and results of operations should be read in conjunction with the Consolidated Financial Statements and Notes thereto appearing elsewhere in this Annual Report.
Business Overview
We are a national provider of infusion solutions. We work with physicians, hospital systems, skilled nursing facilities, and healthcare payors to provide patients access to post-acute care services. We operate with a commitment to bring customer-focused healthcare infusion therapy services into the home or alternate site setting. By collaborating with the full spectrum of healthcare professionals and the patient, we aim to provide cost-effective care that is driven by clinical excellence, customer service and values that promote positive outcomes and an enhanced quality of life for those whom we serve. As of December 31, 2017, we had a total of 66 service locations in 27 states.
Our platform provides nationwide service capabilities and the ability to deliver clinical management services that offer patients a high-touch, community-based and home-based care environment. Our core services are provided in coordination with, and under the direction of, the patient’s physician. Our multidisciplinary team of clinicians, including pharmacists, nurses, dietitians and respiratory therapists, work with the physician to develop a plan of care suited to our patient’s specific needs. Whether in the home, physician office, ambulatory infusion center, skilled nursing facility or other alternate sites of care, we provide products, services and condition-specific clinical management programs tailored to improve the care of individuals with complex health conditions such as gastrointestinal abnormalities, infectious diseases, cancer, multiple sclerosis, organ and blood cell transplants, bleeding disorders, immune deficiencies and heart failure.
Segments
Following the sale of our PBM Business on August 27, 2015 (as further discussed below), Infusion Services is the only remaining operating segment. On an ongoing basis we will no longer report operating segments unless a change in the business necessitates the need to do so.
Strategic Assessment and Transactions
We continually perform strategic assessments of our business and operations. The assessments examine our market strengths and opportunities and compare our position to that of our competitors. As a result of these ongoing assessments, we have focused our growth on investments in the Infusion Services business, which remains the primary driver of our growth strategy. Recent transactions which represent execution of the strategic assessments include:
• | On August 27, 2015, we completed the sale of substantially all of our pharmacy benefit management services segment (the “PBM Business”) pursuant to an Asset Purchase Agreement dated as of August 9, 2015 (the “PBM Asset Purchase Agreement”), by and among the Company, BioScrip PBM Services, LLC and ProCare Pharmacy Benefit Manager Inc. (the “PBM Buyer”). Under the PBM Asset Purchase Agreement, the PBM Buyer agreed to acquire substantially all of the assets used solely in connection with the PBM Business and to assume certain PBM Business liabilities (the “PBM Sale”). On the closing date, pursuant to the terms of the PBM Asset Purchase Agreement, we received total cash consideration of approximately $24.6 million, including an adjustment for estimated closing date net working capital. |
34
On October 20, 2015, we finalized working capital adjustment negotiations in relation to the PBM Sale whereby we agreed to repay approximately $1.0 million to the PBM Buyer. We used the net proceeds from the PBM Sale to pay down a portion of our outstanding debt.
• | On September 9, 2016, we acquired substantially all of the assets and assumed certain liabilities of Home Solutions and its subsidiaries (the “Home Solutions Transaction”) pursuant to an Asset Purchase Agreement dated June 11, 2016 (as amended, the “Home Solutions Agreement”), by and among Home Solutions, a Delaware corporation, certain subsidiaries of Home Solutions, the Company and HomeChoice Partners, Inc., a Delaware corporation. Home Solutions, a privately held company, provides home infusion and home nursing products and services to patients suffering from chronic and acute medical conditions. The aggregate consideration paid by the Company in the Transaction was equal to (i) $67.5 million in cash (the “Cash Consideration); plus (ii) (a) 3,750,000 shares of Company common stock (the “Transaction Closing Equity Consideration”) and (b) the right to receive contingent equity securities of the Company, in the form of restricted shares of Company common stock (the “RSUs”), issuable in two tranches, Tranche A and Tranche B, with different vesting conditions (collectively, the “Contingent Shares”). |
Regulatory Matters Update
Approximately 16% of revenue for the year ended December 31, 2017 was derived directly from Medicare, state Medicaid programs and other government payors. We also provide services to beneficiaries of Medicare, Medicaid and other government-sponsored healthcare programs through managed care entities. Medicare Part D, for example, is administered through managed care entities. In the normal course of business, we and our customers are subject to legislative and regulatory changes impacting the level of reimbursement received from the Medicare and state Medicaid programs.
State Medicaid Programs
Over the last several years, increased Medicaid spending, combined with slow state revenue growth, led many states to institute measures aimed at controlling spending growth. Spending cuts have taken many forms including reducing eligibility and benefits, eliminating certain types of services, and provider reimbursement reductions. In addition, some states have been moving beneficiaries to managed care programs in an effort to reduce costs.
Each individual state Medicaid program represents less than 5% of our consolidated revenue for the year ended December 31, 2017 and no individual state Medicaid reimbursement reduction is expected to have a material effect on our Consolidated Financial Statements. We are continually assessing the impact of the state Medicaid reimbursement cuts as states propose, finalize and implement various cost-saving measures. These measures may include strategies to reduce coverage, restrict enrollment, or enroll more beneficiaries in managed care programs.
Given the reimbursement pressures, we continue to improve operational efficiencies and reduce costs to mitigate the impact on results of operations where possible. In some cases, reimbursement rate reductions may result in negative operating results, and we would likely exit some or all services where rate reductions result in unacceptable returns to our stockholders.
Medicare
Medicare currently covers home infusion therapy for selected therapies primarily through the durable medical equipment benefit. The Cures Act changed the new payment system for certain home infusion therapy services paid under Medicare Part B. The Cures Act significantly reduced the amount paid by Medicare for the drug costs, and also provides for the implementation of a clinical services payment. Under the Cures Act, the services payment does not take effect until 2021. However, the Bipartisan Budget Act of 2018 provides for a temporary transitional payment, starting January 1, 2019, for Medicare Part B home infusion services. This temporary benefit will continue until January 1, 2021, when the services payment in the Cures Act takes effect. We have taken steps to mitigate the impact of the Cures Act on our business, but the Act has had material negative impact on our revenues and profitability.
Approximately 7% and 8% of revenue for the years ended December 31, 2017 and 2016, respectively, was derived from Medicare.
Critical Accounting Estimates
Our Consolidated Financial Statements have been prepared in accordance with United States GAAP. In preparing our financial statements, we are required to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and
35
expenses during the reporting period. We evaluate our estimates and judgments on an ongoing basis. We base our estimates and judgments on historical experience and on various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses for the period presented. Our actual results may differ from these estimates, and different assumptions or conditions may yield different estimates. The following discussion highlights what we believe to be the critical accounting estimates and judgments made in the preparation of our Consolidated Financial Statements.
The following discussion is not intended to be a comprehensive list of all the accounting estimates or judgments made in the preparation of our financial statements and in many cases the accounting treatment of a particular transaction is specifically dictated by GAAP, with no need for management’s judgment on its application. See our audited Consolidated Financial Statements and notes thereto appearing elsewhere in this Annual Report, which contain a description of our accounting policies and other disclosures required by GAAP.
Revenue Recognition
We generate revenue principally through the provision of home infusion services to provide clinical management services and the delivery of cost effective prescription medications.
Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Subtopic 605-25, Revenue Recognition: Multiple-Element Arrangements (“ASC 605-25”), addresses situations in which there are multiple deliverables under one revenue arrangement with a customer and provides guidance in determining whether multiple deliverables should be recognized separately or in combination.
For infusion-related therapies, we frequently provide multiple deliverables of drugs and related nursing services. After applying the criteria of ASC 605-25, we concluded that separate units of accounting exist in revenue arrangements with multiple deliverables. If the drug is shipped, the drug revenue is recognized at the time of shipment, and nursing revenue is recognized on the date of service. We allocate revenue consideration based on the relative fair value as determined by our best estimate of selling price to separate the revenue where there are multiple deliverables under one revenue arrangement. We recognize infusion nursing revenue as the estimated net realizable amounts from patients and payors for services rendered and products provided. This revenue is recognized as the treatment plan is administered to the patient and is recorded at amounts estimated to be received under reimbursement or payment arrangements with payors.
Allowance for Doubtful Accounts
The allowance for doubtful accounts is based on estimates of losses related to receivable balances. The risk of collection varies based upon the service/product, the payor (commercial health insurance and government) and the patient’s ability to pay the amounts not reimbursed by the payor. We estimate the allowance for doubtful accounts based upon several factors including the age of the outstanding receivables, the historical experience of collections, adjusting for current economic conditions and, in some cases, evaluating specific customer accounts for the ability to pay. Collection agencies are employed and legal action is taken when we determine that taking collection actions is reasonable relative to the probability of receiving payment on amounts owed. Management judgment is used to assess the collectability of accounts and the ability of our customers to pay. Judgment is also used to assess trends in collections and the effects of systems and business process changes on our expected collection rates. We review the estimation process quarterly and make changes to the estimates as necessary. When it is determined that a customer account is uncollectible, that balance is written off against the existing allowance.
36
The following table shows the aging of our net accounts receivable (net of allowance for contractual adjustments and prior to allowance for doubtful accounts), aged based on date of service and categorized based on the three primary overall types of accounts receivable characteristics (in thousands):
December 31, 2017 | December 31, 2016 | |||||||||||||||||||||||||||
0 - 180 days | Over 180 days | Total | % of Total | 0 - 180 days | Over 180 days | Total | % of Total | |||||||||||||||||||||
Government | $ | 20,602 | $ | 10,082 | $ | 30,684 | $ | 19,891 | $ | 8,278 | $ | 28,169 | ||||||||||||||||
Commercial | 63,767 | 18,779 | 82,546 | 95,018 | 19,849 | 114,867 | ||||||||||||||||||||||
Patient | 2,577 | 7,627 | 10,204 | 3,955 | 6,825 | 10,780 | ||||||||||||||||||||||
Gross accounts receivable | $ | 86,946 | $ | 36,488 | 123,434 | $ | 118,864 | $ | 34,952 | 153,816 | ||||||||||||||||||
Allowance for doubtful accounts | (37,912 | ) | 30.7 | % | (44,730 | ) | 29.1 | % | ||||||||||||||||||||
Net accounts receivable | $ | 85,522 | $ | 109,086 | ||||||||||||||||||||||||
At December 31, 2017, our allowance for doubtful accounts was $37.9 million, or 30.7% of gross accounts receivable, as compared to $44.7 million, or 29.1% of gross accounts receivable, at December 31, 2016. The allowance for doubtful accounts decreased by approximately $3.0 million during 2017 due to a change in estimate resulting from stabilized collections including more predictable cash receipts from our payors.
Allowance for Contractual Discounts
We are reimbursed by payors for products and services we provide. Payments for medications and services covered by payors average less than billed charges. We monitor revenue and receivables from payors for each of our branches and record an estimated contractual allowance for certain revenue and receivable balances as of the revenue recognition date to properly account for anticipated differences between amounts estimated in our billing system and amounts ultimately reimbursed by payors. Accordingly, the total revenue and receivables reported in our financial statements are recorded at the amounts expected to be received from these payors. For the significant portion of our Infusion Services revenue, the contractual allowance is estimated based on several criteria, including unbilled claims, historical trends based on actual claims paid, current contract and reimbursement terms and changes in customer base and payor/product mix. Contractual allowance estimates are adjusted to actual amounts as cash is received and claims are settled. We do not believe these changes in estimates are material. The billing functions for the remaining portion of our revenue are largely computerized, which enables on-line adjudication (i.e., submitting charges to third-party payors electronically, with simultaneous feedback of the amount the primary insurance plan expects to pay) at the time of sale to record net revenue, exposure to estimating contractual allowance adjustments is limited to this portion of the business.
Goodwill and Intangible Assets
Goodwill and indefinite-lived intangible assets are not subject to amortization and, in accordance with ASC Topic 350, Intangibles – Goodwill and Other, we evaluate goodwill and indefinite lived intangible assets for impairment on an annual basis and whenever events or circumstances exist that indicate that the carrying value of goodwill or indefinite-lived intangible assets may no longer be recoverable.
Management may choose to undertake a qualitative assessment in order to assess whether a quantitative analysis is required. In determining whether management will utilize the qualitative assessment in any one year, management will consider overall economic factors as well as the passage of time since last quantitative assessment.
In January 2017, the FASB issued authoritative guidance that simplifies the measurement of goodwill impairment to a single-step test. The guidance eliminates step two of the goodwill impairment test; the measurement of goodwill impairment will now be the amount by which a reporting unit’s carrying value exceeds its fair value, not to exceed the carrying amount of goodwill. Under the revised guidance, failing step one will result in goodwill impairment. The Company adopted the new guidance on January 1, 2017 on a prospective basis.
2017 Warrants
The 2017 Warrants are reflected as a liability in other non-current liabilities on the balance sheet and are adjusted to fair value at the end of each reporting period through earnings. The 2017 Warrants entitle the purchasers of the Warrants to purchase shares of Common Stock, representing at the time of any exercise an equivalent number of shares equal to 4.99% of the Common Stock of the Company on a fully diluted basis. The exercise price and the number of shares that may be acquired upon exercise of the
37
2017 Warrants is subject to adjustment in certain situations, including price based anti-dilution protection and standard anti-dilution protections if the Company effects a stock split, subdivision, reclassification or combination of its Common Stock or fixes a record date for the making of a dividend or distribution to stockholders of cash or certain assets.
Off-Balance Sheet Arrangements
As of December 31, 2017, we did not have any material off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of Regulation S-K.
Results of Operations
The following consolidated statements have been derived from our audited consolidated financial statements included in this Annual Report on Form 10-K. The discussion set forth below compares our annual results of operations with the results of prior years. Certain amounts below have been revised to reflect immaterial corrections, see Note 1 - Nature of Business.
Year Ended December 31, (in thousands) | As a Percentage of Revenue | |||||||||||||||||||
2017 | 2016 | 2015 | 2017 | 2016 | 2015 | |||||||||||||||
Net revenue | $ | 817,190 | $ | 935,589 | $ | 982,223 | 100.0 | % | 100.0 | % | 100.0 | % | ||||||||
Gross profit, excluding depreciation expense | 269,242 | 262,082 | 259,952 | 32.9 | % | 28.0 | % | 26.5 | % | |||||||||||
Other operating expenses | 163,273 | 169,781 | 165,328 | 20.0 | % | 18.1 | % | 16.8 | % | |||||||||||
Bad debt expense | 23,697 | 26,608 | 42,444 | 2.9 | % | 2.8 | % | 4.3 | % | |||||||||||
General and administrative expenses | 39,625 | 38,798 | 42,474 | 4.8 | % | 4.1 | % | 4.3 | % | |||||||||||
Change in fair value of equity linked liabilities | 3,587 | (10,450 | ) | — | 0.4 | % | (1.1 | )% | — | % | ||||||||||
Impairment of goodwill | — | — | 251,850 | — | % | — | % | 25.6 | % | |||||||||||
Restructuring, acquisition, integration, and other expenses, net | 12,662 | 15,859 | 24,405 | 1.5 | % | 1.7 | % | 2.5 | % | |||||||||||
Depreciation and amortization expense | 27,725 | 22,025 | 22,864 | 3.4 | % | 2.4 | % | 2.3 | % | |||||||||||
Interest expense | 52,072 | 37,572 | 36,938 | 6.4 | % | 4.0 | % | 3.8 | % | |||||||||||
Loss on extinguishment of debt | 13,453 | — | — | 1.6 | % | — | % | — | % | |||||||||||
Loss (gain) on dispositions | 581 | (3,954 | ) | — | 0.1 | % | (0.4 | )% | — | % | ||||||||||
Loss from continuing operations, before income taxes | (67,433 | ) | (34,157 | ) | (326,351 | ) | (8.3 | )% | (3.7 | )% | (33.2 | )% | ||||||||
Income tax benefit (expense) | 4,130 | (2,015 | ) | 21,532 | 0.5 | % | (0.2 | )% | 2.2 | % | ||||||||||
Loss from continuing operations, net of income taxes | (63,303 | ) | (36,172 | ) | (304,819 | ) | (7.7 | )% | (3.9 | )% | (31.0 | )% | ||||||||
Income (loss) from discontinued operations, net of income taxes | (893 | ) | (6,593 | ) | 4,691 | (0.1 | )% | (0.7 | )% | 0.5 | % | |||||||||
Net loss | $ | (64,196 | ) | $ | (42,765 | ) | $ | (300,128 | ) | (7.9 | )% | (4.6 | )% | (30.6 | )% | |||||
Revenue. Revenue for the year ended December 31, 2017 decreased approximately $118.4 million, or 13%, to $817.2 million, compared to revenue of $935.6 million for the year ended December 31, 2016. The decrease in net revenue primarily reflects the Company’s shift in strategy to focus on growing its core revenue mix, including the impact of UnitedHealthcare contract transition effective September 30, 2017, the impact of the Cures Act, and the impact of the Company’s exit from the Hepatitis C market in 2016, partially offset by additional revenues resulting from the acquisition of Home Solutions. Revenue for the year ended December 31, 2016 decreased approximately $46.6 million, or 5%, to approximately $935.6 million, compared to revenue of $982.2 million for the year ended December 31, 2015. The decrease in revenue in 2016 as compared to 2015 is the result of decreases in patient service volumes, specifically in our lower margin chronic business, the divestiture of our Hepatitis C business, partially offset by additional revenues resulting from the Home Solutions acquisition, and an increase in patient service volume primarily in our core nutrition therapies and chronic infused therapies.
Gross Profit. Gross profit consists of revenue less cost of revenue (excluding depreciation expense). The cost of revenue primarily includes the costs of prescription medications, supplies, nursing services, shipping and other direct and indirect costs. The increase in gross profit during 2017 as compared to 2016 of $7.2 million, or 3%, to $269.2 million, compared to gross profit
38
of $262.1 million for the year ended December 31, 2016, was primarily driven by the Home Solutions acquisition, an improved mix of higher margin core therapy revenues versus lower margin non-core therapy revenues, and a decreased cost of prescription medicines and supplies as a result of improved supply chain management, partially offset by the Company’s shift in strategy to focus on growing its core revenue mix, including the UnitedHealthcare contract transition effective September 30, 2017. The increase in gross profit in 2016 of $2.1 million, or 1%, as compared to $260.0 million for the year ended December 31, 2015 was the result of the acquisition of Home Solutions, improved supply chain management, and an increase in patient service volume primarily in our core nutrition therapies and chronic infused therapies, offset partially by the impact of decreases in patient service volumes, specifically in our lower margin chronic business, and the divestiture of our Hepatitis C business
Other Operating Expenses. Other operating expenses consist primarily of wages and benefits, travel expenses, and professional service and field office expenses for our healthcare professionals engaged in providing infusion services to our patients. Other operating expenses for the year ended December 31, 2017 decreased by approximately $6.5 million, or 4%, to $163.3 million, compared to expenses of $169.8 million for the year ended December 31, 2016. The decrease was primarily the result of restructuring and other workforce optimization efforts. Other operating expenses for the year ended December 31, 2016 increased by approximately $4.5 million, or 3%, to $169.8 million, compared to expenses of $165.3 million for the year ended December 31, 2015, reflecting the impact of the Home Solutions acquisition and increased wage, benefit, and other field office costs.
Bad Debt Expenses. Bad debt expense for the year ended December 31, 2017 decreased by approximately $2.9 million, or 11%, to $23.7 million, compared to $26.6 million for the year ended December 31, 2016. Bad debt expense decreased primarily due to a change in estimate resulting from stabilized collections, including more predictable cash receipts from our payors. The decrease in bad debt expense of $15.8 million, or 37%, in 2016 as compared to expense of $42.4 million for the year ended December 31, 2015 was the result of continued focus on improvement of billing and collection efforts to ensure timely cash receipts, as well as a change in estimate associated with the allowance for doubtful accounts. The change in estimate had the effect of lowering the doubtful accounts allowance, overall, due to improved collection experience evidenced by more predictable cash receipts from our payors.
General and Administrative Expenses. General and administrative expenses for the year ended December 31, 2017 increased by approximately $0.8 million, or 2.1%, to $39.6 million, compared to $38.8 million for the year ended December 31, 2016. General and administrative expenses consist of wages and benefits for corporate overhead personnel and certain corporate level professional service fees, including legal, accounting, and IT fees. The increase is primarily due to increased wages and benefits expense. The decrease in general and administrative expenses of $3.7 million, or 9%, in 2016 as compared to expense of $42.5 million during the year ended December 31, 2015 resulted from the reduction in the use and cost of various professional services combined with reductions in the number of corporate personnel and their associated wage and benefits costs.
Change in Fair Value of Equity Linked Liabilities. The increase in fair value of equity linked liabilities of $14.0 million is attributable to a $3.6 million charge during the year ended December 31, 2017 representative of the change in the estimated fair value of the 2017 Warrants issued in connection with the Second Lien Note Facility. The year ended December 31, 2016 saw a $10.5 million gain on the reversal of a liability recorded in connection with contingent equity securities, in the form of restricted shares of Company common stock (the “RSUs”), issuable in connection with the Home Solutions Transaction. We did not incur such charges or benefit from contingent equity linked liabilities during 2015.
Goodwill Impairment. The Company did not record any impairment charges as a result of its goodwill impairment assessments performed during the years ended December 31, 2017 and 2016. During the year ended December 31, 2015 we performed a goodwill impairment assessment due to a significant decline in market capitalization which resulted in a market value significantly lower than the fair value of the business. We recorded a goodwill impairment charge of $251.9 million for the year ended December 31, 2015 related to our Infusion Services business.
Restructuring, Acquisition, Integration, and Other Expenses, net. Restructuring, acquisition, integration, and other expenses include costs associated with restructuring, acquisition and integration initiatives such as employee severance costs, certain legal and professional fees, training costs, redundant wage costs, impacts recorded from the change in contingent consideration obligations, and other costs related to contract terminations and closed branches/offices. Restructuring, acquisition, integration, and other expenses, net decreased by $3.2 million, or 20%, during the year ended December 31, 2017 to $12.7 million from $15.9 million primarily due to lower expenses related to the Home Solutions acquisition and integration, partially offset by restructuring and other workforce optimization efforts during 2017. Restructuring, acquisition, integration, and other expenses decreased by $8.5 million during the year ended December 31, 2016 as compared to the year ended December 31, 2015, as a result of the completion of cost cutting measures associated with the financial improvement plan, partially offset by increases associated with the Home Solutions acquisition.
39
Depreciation and Amortization Expenses. Depreciation and amortization expenses include the depreciation of property and equipment and the amortization of intangible assets such as customer relationships, managed care contracts, licenses, trademarks, trade names, and non-compete agreements with estimable lives. During the years ended December 31, 2017 and 2016, we recorded depreciation expenses of $15.9 million and $15.8 million, respectively. The decrease in depreciation expense is attributable to a decline in capital expenditures. Amortization expense increased during the year ended December 31, 2017 to $11.8 million, or 91%, from $6.2 million. The increase in amortization expense is attributable to the corresponding increase in intangible assets associated with the acquisition of Home Solutions in the third quarter of 2016. During the years ended December 31, 2016 and 2015, we recorded depreciation expense of $15.8 million and $17.7 million, respectively, and amortization expense of intangibles of $6.2 million and $5.1 million, respectively. The decrease in depreciation expense was driven by a decline in capital expenditures.
Interest Expense. Interest expense consists of interest expense and amortization of deferred financing costs reduced by an immaterial amount of interest income. During the years ended December 31, 2017 and 2016, we recorded interest expense of $52.1 million and $37.6 million, respectively, including $1.3 million and $3.6 million of amortization of deferred financing costs, respectively. The increase in interest expense of $14.5 million, or 39%, in 2017 as compared to 2016 is the result of the changes in debt structure (see Note 10 - Debt), which also resulted in a higher effective interest rate specific to the amortization of the discount associated with the 2017 Warrants. During the years ended December 31, 2016 and 2015, we recorded interest expense of $37.6 million and $36.9 million, respectively, including $3.6 million and $2.9 million of amortization of deferred financing costs, respectively. The increase in interest expense in 2016 as compared to 2015 was primarily attributable to the increase of $0.7 million in amortization of deferred financing costs in 2016 as compared to 2015 associated with changes in debt structure during the year ended December 31, 2015.
Loss on Extinguishment of Debt. The loss on extinguishment of debt of $13.5 million during the year ended December 31, 2017 is attributable to the Company’s entry into the Notes Facilities and the associated extinguishment of the Senior Credit Facilities and the Prior Credit Agreements (see Note 10 - Debt).
Income Tax Benefit (Expense). Our income tax provision for the year ended December 31, 2017 reflects a $4.1 million benefit, compared to a provision of $2.0 million during the year ended December 31, 2016. The primary driver of the change was the reversal of the valuation allowance, which created an income tax benefit in 2017. The reversal of the valuation allowance was the result of new federal NOL carryforward rules enacted under TJCA, which prescribe an indefinite federal NOL carryforward period for NOLs generated in 2018 and beyond (subject to a 20% reduction). The 2017 income tax benefit of $4.1 million includes a federal tax benefit of $23.7 million and a state tax benefit of $4.6 million, a $41.6 million adjustment related to deferred tax asset valuation allowances and other adjustments of $2.0 million, offset by a $67.7 million adjustment associated with the impact of the change in the corporate tax rate brought about by the enactment of the TCJA. The 2016 income tax expense of $2.0 million includes a federal tax benefit of $11.9 million and a state tax benefit of $1.4 million at statutory tax rates, offset by a $14.7 million adjustment related to deferred tax asset valuation allowances and other adjustments of $0.7 million. The 2015 income tax benefit of $21.5 million includes a federal tax benefit of $114.2 million and state tax benefit of $8.4 million at statutory rates, offset by a $57.6 million adjustment to deferred tax asset valuation allowances, a goodwill impairment adjustment of $43.4 million, and other adjustments of $0.2 million.
Non-GAAP Measures
The following table reconciles GAAP loss from continuing operations, net of income taxes to consolidated Adjusted EBITDA. Adjusted EBITDA is net income (loss) adjusted for interest expense, income tax expense (benefit), depreciation and amortization, loss (gain) on dispositions, change in fair value of equity linked liabilities, impairments, loss on extinguishment of debt, and stock-based compensation expense. Adjusted EBITDA also excludes restructuring, acquisition, integration and other expenses including costs associated with restructuring, acquisition, and integration initiatives such as employee severance costs, certain legal and professional fees, training costs, redundant wage costs, impacts recorded from the change in contingent consideration obligations, and other costs related to contract terminations and closed branches/offices.
Consolidated Adjusted EBITDA is a measure of earnings that management monitors as an important indicator of financial performance, particularly future earnings potential and recurring cash flow. Consolidated Adjusted EBITDA is also a primary objective of the management bonus plan. Inclusion of Consolidated Adjusted EBITDA is intended to provide investors insight into the manner in which management views the performance of the Company.
Non-GAAP financial measures have limitations as analytical tools and should not be considered in isolation or as a substitute for our financial results prepared in accordance with GAAP. Our calculation of Non-GAAP Adjusted EBITDA, as presented, may differ from similarly titled measures reported by other companies. We encourage investors to review these reconciliations and we qualify our use of non-GAAP financial measures with cautionary statements as to their limitations.
40
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(in thousands) | |||||||||||
Loss from continuing operations, net of income taxes | $ | (63,303 | ) | $ | (36,172 | ) | $ | (304,819 | ) | ||
Interest expense | (52,072 | ) | (37,572 | ) | (36,938 | ) | |||||
Loss on extinguishment of debt | (13,453 | ) | — | — | |||||||
(Loss) gain on dispositions | (581 | ) | 3,954 | — | |||||||
Income tax benefit (expense) | 4,130 | (2,015 | ) | 21,532 | |||||||
Depreciation and amortization expense | (27,725 | ) | (22,025 | ) | (22,864 | ) | |||||
Impairment of goodwill | — | — | (251,850 | ) | |||||||
Stock-based compensation expense | (2,360 | ) | (1,801 | ) | (4,513 | ) | |||||
Change in fair value of equity linked liabilities | (3,587 | ) | 10,450 | — | |||||||
Restructuring, acquisition, integration, and other expenses, net | (12,662 | ) | (15,859 | ) | (24,405 | ) | |||||
Consolidated Adjusted EBITDA | $ | 45,007 | $ | 28,696 | $ | 14,219 | |||||
Adjusted EBITDA increased during the year ended December 31, 2017 compared to the prior year primarily due to increased gross profit resulting from improved gross profit margins driven by increased core revenue mix and supply chain management, as well as restructuring and integration efforts which optimized operations.
Liquidity and Capital Resources
Sources and Uses of Funds
We utilize funds generated from operations for general working capital needs, capital expenditures and acquisitions.
Net cash provided by operating activities from continuing operations was $5.6 million for the year ended December 31, 2017, a $41.1 million improvement, compared to cash used in operating activities from continuing operations of $35.5 million for the year ended December 31, 2016. Cash interest payments increased $10.7 million to $45.4 million in 2017, compared to $34.7 million during 2016. These higher cash interest payments during 2017 were more than offset by the favorable impacts of increased Adjusted EBITDA, lower restructuring, acquisition, integration, and other expenses, net, and working capital management. Net cash used in operating activities from continuing operations was $35.5 million for the year ended December 31, 2016, a $27.2 million improvement, compared to $62.7 million for the year ended December 31, 2015, resulting from the favorable impacts of increased Adjusted EBITDA, lower restructuring, acquisition, integration, and other expenses, net, and working capital management.
Net cash used in investing activities from continuing operations during the year ended December 31, 2017 was $13.6 million compared to $73.2 million of cash used during the same period in 2016. Fluctuations in investing cash flows during the year ended December 31, 2017, as compared to the same period in 2016, were primarily attributable to a year over year decrease in cash consideration paid for acquisitions of $67.5 million associated with the prior year acquisition of Home Solutions, Inc and a year over year decrease in purchases of property and equipment of $1.2 million, offset by year over year decreases of $4.2 million and $5.0 million associated with proceeds received in divestitures and investment in restricted cash balances required to be maintained as collateral in accordance with the Notes Facilities, respectively. During the year ended December 31, 2016 we received proceeds of $4.2 million from dispositions, primarily attributable to the strategic divestiture of the Hepatitis C business. Capital expenditures were $8.7 million and $9.9 million for the years ended December 31, 2017 and 2016, respectively, resulting in $1.2 million decreased use of cash. Net proceeds from the sale of the PBM Business of $24.6 million are included in net cash provided by investing activities from discontinued operations in the year ended December 31, 2015.
Net cash provided by financing activities was $44.3 million and $109.7 million during the years ended December 31, 2017 and 2016, respectively. The cash provided in 2017 includes the net proceeds of approximately $20.8 million from the First Quarter 2017 Private Placement and Second Quarter 2017 Private Placement (each defined below), $23.1 million from the Priming Credit Agreement, and $294.4 million from the Notes Facilities offset by repayments of $55.9 million on our Revolving Credit Facility and by $236.8 million of principal payments made on the Term Loan Facility and the Priming Credit Agreement. Cash provided in 2016 results from $83.3 million from the 2016 Equity Offering (defined below) and by advances of $104.3 million offset by repayments of $64.0 million on our Revolving Credit Facility (defined below) and $12.6 million of principal payments made on the Term Loan Facility.
41
At December 31, 2017, we had net working capital (excluding current assets and current liability of discontinued operations) of $82.6 million, including $39.5 million of cash on hand, compared to $43.2 million of net working capital at December 31, 2016. The $39.4 million increase in working capital results primarily from the increase in our cash and cash equivalents and restricted cash of $34.8 million. Additional liquidity of $10.0 million is provided by the delayed draw capacity in our Second Lien Note Facility described below. At December 31, 2017, we had outstanding letters of credit totaling $4.8 million, collateralized by restricted cash of $5.0 million.
Debt Facilities
The Company was previously obligated under (i) a senior secured first-lien revolving credit facility in an aggregate principal amount of $75.0 million (the “Revolving Credit Facility”), (ii) a senior secured first-lien term loan B in an aggregate principal amount of $250.0 million (the “Term Loan B Facility”) and (iii) a senior secured first-lien delayed draw term loan B in an aggregate principal amount of $150.0 million (the “Delayed Draw Term Loan Facility” and, together with the Revolving Credit Facility and the Term Loan B Facility, the “Senior Credit Facilities”) with SunTrust Bank, Jefferies Finance LLC and Morgan Stanley Senior Funding, Inc., originally entered on July 31, 2013 and amended from time to time.
On January 6, 2017, the Company entered into a credit agreement (the “Priming Credit Agreement” and, together with the Senior Credit Facilities, the “Prior Credit Agreements”) with certain existing lenders under the Senior Credit Facilities and SunTrust, as administrative agent for itself and the lenders. The Priming Credit Agreement provided an aggregate borrowing commitment of $25.0 million, which was fully drawn at closing.
On June 29, 2017, the Company entered into (i) a first lien note purchase agreement (the “First Lien Note Facility”), among the Company, which is the issuer under the agreement, the financial institutions and note purchasers from time to time party to the agreement (the “First Lien Note Purchasers”), and Wells Fargo Bank, National Association, in its capacity as collateral agent for itself and the First Lien Note Purchasers (the “First Lien Collateral Agent”), pursuant to which the Company issued first lien senior secured notes in an aggregate principal amount of $200.0 million (the “First Lien Notes”); and (ii) a second lien note purchase agreement (the “Second Lien Note Facility” and, together with the First Lien Note Facility, the “Notes Facilities”) among the Company, which is the issuer under the agreement, the financial institutions and note purchasers from time to time party to the agreement (the “Second Lien Note Purchasers”), and Wells Fargo Bank, National Association, in its capacity as collateral agent for itself and the Second Lien Note Purchasers (the “Second Lien Collateral Agent” and, together with the First Lien Collateral Agent, the “Collateral Agent”), pursuant to which the Company (a) issued second lien senior secured notes in an aggregate initial principal amount of $100.0 million (the “Initial Second Lien Notes”) and (b) has the ability to draw upon the Second Lien Note Facility and issue second lien delayed draw senior secured notes in an aggregate initial principal amount of $10.0 million for a period of 18 months after the Closing Date, subject to certain terms and conditions (the “Second Lien Delayed Draw Notes” and, together with the Initial Second Lien Notes, the “Second Lien Notes”; the Second Lien Notes, together with the First Lien Notes, the “Notes”). Funds managed by Ares Management L.P. (“Ares”) acted as lead purchasers for the Notes Facilities.
The Company used the proceeds of the sale of the First Lien Notes and the Initial Second Lien Notes pursuant to the Notes Facilities to repay in full all amounts outstanding under the Prior Credit Agreements and extinguished the liability. Each of the Prior Credit Agreements was terminated following such repayment. The Company used the remaining proceeds of $15.9 million of the Notes Facilities, net of $0.2 million in associated costs, and the Second Quarter 2017 Private Placement for working capital and general corporate purposes.
The First Lien Notes accrue interest, payable monthly in arrears, at a floating rate or rates equal to, at the option of the Company, (i) the base rate (defined as the highest of the Federal Funds Rate plus 0.5% per annum, the Prime Rate as published by The Wall Street Journal and the one-month London Interbank Offered Rate (“LIBOR”) (subject to a 1.0% floor) plus 1.0%), or (ii) the one-month LIBOR rate (subject to a 1.0% floor), plus a margin of 6.0% if the base rate is selected or 7.0% if the LIBOR Option is selected. The First Lien Notes mature on August 15, 2020, provided that if the Company’s existing 8.875% Senior Notes due 2021 (the “2021 Notes”) are refinanced prior to August 15, 2020, then the scheduled maturity date of the First Lien Notes shall be June 30, 2022.
The First Lien Notes will amortize in equal quarterly installments equal to 0.625% of the aggregate principal amount of the First Lien Note Facility, commencing on September 30, 2019, and on the last day of each third month thereafter, with the balance payable at maturity. The First Lien Notes are pre-payable at the Company’s option at specified premiums to the principal amount that will decline over the term of the First Lien Note Facility. If the First Lien Notes are prepaid prior to the second anniversary of the Closing Date, the Company will be required to pay a make-whole premium based on the present value (using a discount rate based on the specified treasury rate plus 50 basis points) of all remaining interest payments on the First Lien Notes being prepaid prior to the second anniversary of the Closing Date, plus 4.0% of the principal amount of First Lien Notes being prepaid. On or after the second anniversary of the Closing Date, the prepayment premium is 4.0%, which declines to 2.0% on or after the
42
third anniversary of the Closing Date, and declines to 0.0% on or after the fourth anniversary of the Closing Date. At any time, the Company may pre-pay up to $50.0 million in aggregate principal amount of the First Lien Notes from internally generated cash without incurring any make-whole or prepayment premium. The occurrence of certain events of default may increase the applicable rate of interest by 2.0% and could result in the acceleration of the Company’s obligations under the First Lien Note Facility prior to stated maturity and an obligation of the Company to pay the full amount of its obligations under the First Lien Note Facility.
The First Lien Note Facility contains customary events of default that include, among others, non-payment of principal, interest or fees, violation of covenants, inaccuracy of representations and warranties, bankruptcy and insolvency events, material judgments, cross-defaults to material indebtedness and events constituting a change of control. In addition, the obligations under the First Lien Note Facility will be guaranteed by joint and several guarantees from the Company’s subsidiaries.
In connection with the First Lien Note Facility, the Company, its subsidiaries and the First Lien Collateral Agent entered into a First Lien Guaranty and Security Agreement, dated as of June 29, 2017 (the “First Lien Guaranty and Security Agreement”). Pursuant to the First Lien Guaranty and Security Agreement, the obligations under the First Lien Notes will be secured by first priority liens on, and security interests in, substantially all of the assets of the Company and its subsidiaries.
The Second Lien Notes accrue interest, payable monthly in arrears, at a floating rate or rates equal to, at the option of the Company, (i) one-month LIBOR (subject to a 1.25% floor) plus 9.25% per annum in cash, (ii) one-month LIBOR (subject to a 1.25% floor) plus 11.25% per annum, which amount will be capitalized on each interest payment date, or (iii) one-month LIBOR (subject to a 1.25% floor) plus 10.25% per annum, of which one-half LIBOR plus 4.625% per annum will be payable in cash and one-half LIBOR plus 5.625% per annum will be capitalized on each interest payment date, provided that, in each case, if any permitted refinancing indebtedness with which the 2021 Notes are refinanced requires or permits the payment of cash interest, all of the interest on the Second Lien Notes shall be paid in cash. The Second Lien Notes mature on August 15, 2020, provided that if the 2021 Notes are refinanced prior to August 15, 2020, then the scheduled maturity date of the Second Lien Notes shall be June 30, 2022.
The Second Lien Notes are not subject to scheduled amortization installments. The Second Lien Notes are pre-payable at the Company’s option at specified premiums to the principal amount that will decline over the term of the Second Lien Note Facility. If the Second Lien Notes are prepaid prior to the third anniversary of the Closing Date, the Company will need to pay a make-whole premium based on the present value (using a discount rate based on the specified treasury rate plus 50 basis points) of all remaining interest payments on the Second Lien Notes being prepaid prior to the third anniversary of the Closing Date, plus 4.0% of the principal amount of Second Lien Notes being prepaid. On or after the third anniversary of the Closing Date, the prepayment premium is 4.0%, which declines to 2.0% on or after the fourth anniversary of the Closing Date, and declines to 0.0% on or after the fifth anniversary of the Closing Date. The occurrence of certain events of default may increase the applicable rate of interest by 2.0% and could result in the acceleration of the Company’s obligations under the Second Lien Note Facility prior to stated maturity and an obligation of the Company to pay the full amount of its obligations under the Second Lien Note Facility.
The Second Lien Note Facility contains customary events of default that include, among others, non-payment of principal, interest or fees, violation of covenants, inaccuracy of representations and warranties, bankruptcy and insolvency events, material judgments, cross-defaults to material indebtedness and events constituting a change of control. In addition, the obligations under the Second Lien Note Facility will be guaranteed by joint and several guarantees from the Company’s subsidiaries.
In connection with the Second Lien Note Facility, the Company, its subsidiaries and the Second Lien Collateral Agent entered into a Second Lien Guaranty and Security Agreement, dated as of June 29, 2017 (the “Second Lien Guaranty and Security Agreement”). Pursuant to the Second Lien Guaranty and Security Agreement, the obligations under the Second Lien Notes will be secured by second priority liens on, and security interests in, substantially all of the assets of the Company and its subsidies.
In connection with the First Lien Note Facility and the Second Lien Note Facility, the Company, the First Lien Collateral Agent and the Second Lien Collateral Agent, entered into an intercreditor agreement containing customary provisions to, among other things, subordinate the lien priority of the liens granted under the Second Lien Note Facility to the liens granted under the First Lien Note Facility.
43
Issuance of 2021 Notes
On February 11, 2014, we issued $200.0 million aggregate principal amount of 8.875% senior notes due in 2021 (the “2021 Notes”) with net proceeds to us of approximately $194.5 million. The 2021 Notes are senior unsecured obligations of the Company and are fully and unconditionally guaranteed by all existing and future subsidiaries of the Company. As of December 31, 2017, we do not have any independent assets or operations and, as a result, our direct and indirect subsidiaries (other than minor subsidiaries), each being 100% owned by us, are fully and unconditionally, jointly and severally, providing guarantees on a senior unsecured basis to the 2021 Notes. The 2021 Notes were offered in the United States to qualified institutional buyers in reliance on Rule 144A under the Securities Act and outside the United States to non-U.S. persons in reliance on Regulation S under the Securities Act pursuant to an Indenture dated February 11, 2014, by and among the Company, the guarantors named therein and U.S. Bank National Association, as trustee.
Interest on the 2021 Notes accrues at the rate of 8.875% per annum and is payable semi-annually in cash in arrears on February 15 and August 15 of each year, commencing on August 15, 2014. The debt discount of $5.0 million at issuance is being amortized as interest expense through maturity which will result in the accretion over time of the outstanding debt balance to the principal amount. The 2021 Notes are the Company’s senior unsecured obligations and rank equally in right of payment with all of its other existing and future senior unsecured indebtedness and senior in right of payment to all of its existing and future subordinated indebtedness.
PIPE Transaction
On March 9, 2015, we entered into a securities purchase agreement (the “Purchase Agreement”) with Coliseum Capital Partners, L.P., Coliseum Capital Partners II, L.P., and Blackwell Partners, LLC, Series A, (collectively, the “PIPE Investors”). Pursuant to the terms of the Purchase Agreement, we issued and sold to the PIPE Investors in a private placement (the “PIPE Transaction”) an aggregate of (a) 625,000 shares of Series A Preferred Stock at a purchase price per share of $100.00, (b) 1,800,000 Class A Warrants, and (c) 1,800,000 Class B Warrants (and together with Class A Warrants, the “PIPE Warrants”), for gross proceeds of $62.5 million. The initial conversion price for the Series A Preferred Stock is $5.17. The PIPE Warrants may be exercised to acquire shares of Common Stock. Pursuant to an addendum (the “Warrant Addendum”), dated as of March 23, 2015, to the Warrant Agreement, dated as of March 9, 2015, with the PIPE Investors, the PIPE Investors paid the Company $0.5 million in the aggregate, and the per share exercise price of the Class A Warrants and Class B Warrants was set at $5.17 and $6.45, respectively, reduced from $5.295 to $5.17 and from $6.595 to $6.45, respectively.
We repaid approximately $45.3 million of the Revolving Credit Facility indebtedness and accrued interest, representing 77% of the PIPE Transaction’s net proceeds.
Series A, Series B, and Series C Convertible Preferred Stock
In connection with the PIPE Transaction, the Company authorized 825,000 shares and issued to the PIPE Investors 625,000 shares of Series A Preferred Stock at $100.00 per share. We are required, pursuant to the terms of the Certificate of Designations governing the Series A Preferred Stock and the Warrant Agreement governing the PIPE Warrants, to at all times reserve sufficient shares of common stock to allow for the conversion of the Series A Preferred Stock and exercise of the PIPE Warrants.
The Series A Preferred Stock may, at the option of the holder, be converted into Common Stock and receive a Liquidation Preference upon voluntary or involuntary liquidation, dissolution, or winding up of the Company as described in the Company’s Annual Report. The Company may pay a noncumulative cash dividend on each share of the Series A Preferred Stock. In the event the Company does not declare and pay a cash dividend, the Liquidation Preference of the Series A Preferred Stock will be increased to an amount equal to the Liquidation Preference in effect at the start of the applicable quarterly dividend period, plus an amount equal to such then applicable Liquidation Preference multiplied by 11.5% per annum.
On June 10, 2016, in order to allow the shares of common stock reserved for issuance for the conversion of the Series A Preferred Stock and exercise of the PIPE Warrants to be released from reservation and sold pursuant to the 2016 Equity Offering (see below), we entered into an Exchange Agreement with the PIPE Investors (the “Series B Exchange Agreement”) pursuant to which the PIPE Investors agreed:
i) to exchange 614,177 shares of the existing Series A Preferred Stock for an identical number of shares of Series B Convertible Preferred Stock (the “Series B Preferred Stock”), which have the same terms as the Series A Preferred Stock, except that the terms of the Series B Preferred Stock include the authority of the holders of the Series B Preferred Stock to waive the requirement that the Company reserve a sufficient number of shares of common stock reserved at all times to allow for the conversion of the Series B Preferred Stock; and
44
ii) to waive the requirement under the Warrant Agreement governing the PIPE Warrants to reserve 3,600,000 shares of our common stock for the exercise of the PIPE Warrants.
On June 14, 2016, the Company entered into another Exchange Agreement (the “Series C Exchange Agreement”) with the PIPE Investors, pursuant to which the PIPE Investors agreed to exchange their shares of Series B Preferred Stock issued pursuant to the Series B Exchange Agreement on a one for one basis for shares of a new series of preferred stock of the Company (the “Series C Preferred Stock” and, together with the Series A Preferred Stock and the Series B Preferred Stock, the “Preferred Stock”), designated “Series C Convertible Preferred Stock.”
Under the terms of the Series C Exchange Agreement, the PIPE Investors agreed to exchange 614,177 shares of the Series B Preferred Stock for an identical number of shares of Series C Preferred Stock, which have the same terms as the Series B Preferred Stock, except that the terms of the Series C Preferred Stock provide that the 11.5% per annum rate of non-cash dividends payable on the shares of the Series C Preferred Stock will be reduced based on the achievement by the Company of specified “Consolidated EBITDA” as defined in the Senior Credit Facilities. In addition, pursuant to the Series C Exchange Agreement, the PIPE Investors agreed to waive the requirement under the Warrant Agreement governing the PIPE Warrants held by the PIPE Investors to reserve 3,600,000 shares of our common stock for the exercise of the PIPE Warrants.
The transactions effected pursuant to the Series C Exchange Agreement ensured there were a sufficient number of authorized shares of common stock to undertake the 2016 Equity Offering. In the Series C Exchange Agreement, the Company agreed that within four months of the date of the Series C Exchange Agreement, a special meeting of our stockholders would be called to seek approval to the Charter Amendment so as to allow the Company to reserve sufficient shares for the conversion of the Series C Preferred Stock and the exercise of the PIPE Warrants. This approval was obtained at a special meeting held on November 30, 2016.
As a result of the exchanges discussed above, there are currently (a) 21,645 shares of Series A Preferred Stock outstanding, of which 10,823 shares are owned by the PIPE Investors, (b) no shares of Series B Preferred Stock outstanding, and (c) 614,177 shares of Series C Preferred Stock outstanding, all of which are owned by the PIPE Investors.
Rights Offering
On June 30, 2015, we commenced a rights offering (the “Rights Offering”) pursuant to which we distributed subscription rights to purchase units consisting of (1) Series A Preferred Stock, each share convertible into shares of Common Stock at a conversion price of $5.17 per share, (2) Class A warrants to purchase one share of Common Stock at a price of $5.17 per share (the “Public Class A Warrants”), and (3) Class B warrants to purchase one share of Common Stock at a price of $6.45 per share (the “Public Class B Warrants” and, together with the Public Class A Warrants, the “Public Warrants”). The Rights Offering was completed on July 31, 2015. Our stockholders exercised subscription rights to purchase 10,822 units, consisting of an aggregate of 10,822 shares of the Series A Preferred Stock, 31,025 Public Class A Warrants, and 31,025 Public Class B Warrants, at a subscription price of $100.00 per unit. Pursuant to the Rights Offering, we raised gross proceeds of approximately $1.1 million.
With the exception of the expiration date, the PIPE Class A Warrants issued pursuant to the PIPE Transaction, as amended by the Warrant Addendum, have the same terms as the Public Class A Warrants issued pursuant to the Rights Offering.
Shelf Registration Statement
The Company filed a shelf registration statement on Form S-3 under the Securities Act on April 1, 2016, which was declared effective May 2, 2016 (the “2016 Shelf”). Under the 2016 Shelf at the time of effectiveness, the Company had the ability to raise up to $200.0 million, in one or more transactions, by selling Common Stock, preferred stock, debt securities, warrants, units and rights. Subsequent to the 2016 Equity Offering (defined below) the Company has the ability to raise $109.6 million by selling Common Stock, preferred stock, debt securities, warrants, units and rights.
2016 Equity Offering
On June 22, 2016 the Company completed an underwritten public offering of 45,200,000 shares of Common Stock, including 5,200,000 shares of Common Stock issued upon the underwriters’ full exercise of the over-allotment option, at a public offering price of $2.00 per share, less underwriting discounts and commissions and offering expenses payable by us (the “2016 Equity Offering”). The Company received net proceeds of approximately $83.3 million from the 2016 Equity Offering, after deducting underwriting discounts and commissions and offering expenses.
45
A portion of the net proceeds from the 2016 Equity Offering was used to fund the Cash Consideration (as defined below) and pay fees and expenses in connection with the closing of the Home Solutions Transaction.
Home Solutions Transaction
On September 9, 2016, the Company acquired substantially all of the assets and assumed certain liabilities of Home Solutions and its subsidiaries pursuant to the Home Solutions Agreement dated June 11, 2016, by and among Home Solutions, a Delaware corporation, certain subsidiaries of Home Solutions, the Company and HomeChoice Partners, Inc., a Delaware corporation. Home Solutions, a privately held company, provided home infusion and home nursing products and services to patients suffering from chronic and acute medical conditions. On June 16, 2016, the Company, HomeChoice Partners, Inc. and Home Solutions entered into an amendment to the Home Solutions Agreement (the “First Amendment”), which modified the terms of the consideration payable by the Company to Home Solutions thereunder. On September 2, 2016, the same parties entered into a second amendment to the Home Solutions Agreement (the “HS Second Amendment”), which amended the Home Solutions Agreement to eliminate the condition to closing that the Company receive stockholder approval to increase its authorized share capital (the “Charter Amendment”) and facilitated the timely consummation of the Transaction. The HS Second Amendment instead provided that the Company will hold a stockholder meeting after the closing of the Transaction to seek stockholder approval of the Charter Amendment, and if the approval is not obtained at the first special meeting, the Company will submit the proposal on a twice per year basis beginning in 2017, at either the annual meeting or a special meeting of stockholders. This approval was obtained at a special meeting held on November 30, 2016. On September 9, 2016, in connection with the consummation of the Transaction, the parties entered into a third amendment (the “Third Amendment”) to the Home Solutions Agreement, which provided for non-material amendments to the closing mechanics, defined terms, acquired and excluded assets, and covenants of the Home Solutions Agreement.
Under the Home Solutions Agreement, the Company did not purchase, among other things, (a) any accounts receivable associated with governmental payors, (b) cash assets, (c) certain non-transferable assets (e.g., state licenses and Medicare and Medicaid certifications and personnel and employment records), (d) the equity of Home Solutions and its subsidiaries; (e) certain tax assets, (f) causes of actions related to any of the items specified as excluded assets or excluded liabilities in the Home Solutions Agreement, (g) any privileged materials, documents or records of Home Solutions related to such excluded assets or excluded liabilities, or (h) intercompany receivables.
The aggregate consideration paid by the Company in the Transaction was equal to (i) $67.5 million in cash (the “Cash Consideration”); plus (ii) (a) 3,750,000 shares of Company common stock (the “Transaction Closing Equity Consideration”) and (b) the right to receive contingent equity securities of the Company, in the form of restricted shares of Company common stock, issuable in two tranches, Tranche A and Tranche B, with different vesting conditions (collectively, the “Contingent Shares”). The number of shares of Company common stock in Tranche A will be approximately 3.1 million. The number of shares of Company common stock in Tranche B will be approximately 4.0 million. Upon close of the Transaction the RSUs had no intrinsic value, but are reported in our consolidated financial statements at their estimated fair value at the date of issuance. The Home Solutions Agreement provides Home Solutions with certain customary registration rights that required us, within 30 days following the closing of the Transaction, to file a registration statement for the selling stockholder’s resale of the Transaction Closing Equity Consideration and the Contingent Shares pursuant to the Securities Act. The Company filed the registration statement on October 7, 2016 and it was declared effective on October 27, 2016.
The Company will issue the shares of our Common Stock issuable to Home Solutions pursuant to the RSUs in Tranche A promptly, and in any event within five business days, following the earlier of (a) the closing price of our Common Stock, as reported by Nasdaq, averaging $4.00 per share or above over 20 consecutive trading days during the period beginning on September 9, 2016 and ending December 31, 2019, or (b) a change of control that occurs on or prior to December 31, 2017 or a change of control thereafter but on or prior to December 31, 2019, pursuant to which the consideration payable per share equals or exceeds $4.00 per share. The Company will issue the shares of our Common Stock issuable to Home Solutions pursuant to the RSUs in Tranche B promptly, and in any event within five business days, following the earlier of (a) the closing price of our Common Stock, as reported by Nasdaq, averaging $5.00 per share or above over 20 consecutive trading days during the period beginning on September 9, 2016 and ending December 31, 2019, or (b) a change of control that occurs on or prior to December 31, 2017, or a change of control thereafter but on or prior to December 31, 2019, pursuant to which the consideration payable per share equals or exceeds $5.00 per share. The Home Solutions Agreement provides for a cash settlement option related to the RSUs, effective June 15, 2021, if, and only if, authorized shares are unavailable when the vesting conditions of Tranche A and Tranche B are met.
The Cash Consideration and the Transaction Closing Equity Consideration were paid at closing and were funded by cash on-hand and borrowings from our Revolving Credit Facility.
46
First Quarter 2017 Private Placement
On March 1, 2017, the Company entered into a Stock Purchase Agreement (the “First Quarter Stock Purchase Agreement”) with Venor Capital Master Fund Ltd., Map 139 Segregated Portfolio of LMA SPC, Venor Special Situations Fund II LP and Trevithick LP (the “First Quarter Stockholders”). Pursuant to the First Quarter Stock Purchase Agreement, the Company sold an aggregate of 3.3 million shares of its common stock (the “First Quarter Shares”) for aggregate gross proceeds of approximately $5.1 million in a private placement transaction (the “First Quarter 2017 Private Placement”). The purchase price for each Share was $1.5366, which was negotiated between the Company and the Stockholders based on the volume-weighted average price of the Company’s common stock on the Nasdaq Global Market on March 1, 2017. Proceeds from the First Quarter 2017 Private Placement were used for working capital and general corporate purposes.
2017 Warrants
In connection with the Second Lien Note Facility, the Company also issued warrants (the “2017 Warrants”) to the purchasers of the Second Lien Notes pursuant to a Warrant Purchase Agreement dated as of June 29, 2017 (the “2017 Warrant Purchase Agreement”). The 2017 Warrants entitle the purchasers of the 2017 Warrants to purchase shares of Common Stock, representing at the time of any exercise of the 2017 Warrants an equivalent number of shares equal to 4.99% of the Common Stock of the Company on a fully diluted basis, subject to the terms of the Warrant Agreement governing the 2017 Warrants, dated as of June 29, 2017 (the “2017 Warrant Agreement”); provided, however, the 2017 Warrants may not be converted to the extent that, after giving effect to such conversion, the holders of the 2017 Warrants would beneficially own, in the aggregate, in excess of (i) 19.99% of the shares of Common Stock outstanding as of June 29, 2017 (the “Closing Date”) minus (ii) the shares of Common Stock that were sold pursuant to the Second Quarter 2017 Private Placement (as defined below) (the “Conversion Cap”). The Conversion Cap will not apply to the 2017 Warrants if the Company obtains the approval of its stockholders for the removal of the Conversion Cap, which the Company is required to take certain steps to attempt to obtain, subject to the terms of the Warrant Agreement.
The 2017 Warrants have a 10-year term and an initial exercise price of $2.00 per share, and may be exercised by payment of the exercise price in cash or surrender of shares of Common Stock into which the 2017 Warrants are being converted in an aggregate amount sufficient to pay the exercise price. The exercise price and the number of shares that may be acquired upon exercise of the 2017 Warrants is subject to adjustments in certain situations, including price based anti-dilution protection whereby, subject to certain exceptions, if the Company later issues Common Stock or certain Common Stock Equivalents (as defined in the Warrant Agreement) at a price less than either the then-current market price per share or exercise price of the 2017 Warrant, then the exercise price will be decreased and the percentage of shares of Common Stock issuable upon exercise of the 2017 Warrants will remain the same, giving effect to such issuance. Additionally, the 2017 Warrants have standard anti-dilution protections if the Company effects a stock split, subdivision, reclassification or combination of its Common Stock or fixes a record date for the making of a dividend or distribution to stockholders of cash or certain assets. Upon the occurrence of certain business combinations the 2017 Warrants will be converted into the right to acquire shares of stock or other securities or property (including cash) of the successor entity. The 2017 Warrants are reflected as a liability in other non-current liabilities on the consolidated balance sheet and are adjusted to fair value at the end of each reporting period through an adjustment to earnings. The fair value of the 2017 Warrants, subsequent to a remeasurement adjustment of $3.6 million, is $20.5 million at December 31, 2017.
Second Quarter 2017 Private Placement
On June 29, 2017, the Company entered into a Stock Purchase Agreement (the “Second Quarter Stock Purchase Agreement”) with a fund managed by Ares (the “Second Quarter Stock Purchaser”). Pursuant to the terms of the Second Quarter Stock Purchase Agreement, the Company issued and sold to the Second Quarter Stock Purchaser in a private placement (the “Second Quarter 2017 Private Placement”) 6,359,350 shares of Common Stock (the “Second Quarter Shares”) at a price of $2.50 per share, for proceeds of approximately $15.9 million, net of $0.2 million in associated costs.
Second Quarter Registration Rights Agreement
In connection with the 2017 Warrants and the Second Quarter 2017 Private Placement, the Company entered into a Registration Rights Agreement (the “Second Quarter 2017 Registration Rights Agreement”) with the holders of the 2017 Warrants and the Second Quarter Stock Purchaser. Pursuant to the Second Quarter 2017 Registration Rights Agreement, subject to certain exceptions, the Company is required, upon the request of the Second Quarter Stock Purchaser and holders of the 2017 Warrants, to register the resale of the Second Quarter Shares and the shares of Common Stock issuable upon exercise of the 2017 Warrants. Pursuant to the terms of the Second Quarter 2017 Registration Rights Agreement, these registration rights will not become effective until twelve months after the Closing Date, and the costs incurred in connection with such registrations will be borne by the Company.
47
Income Taxes
At December 31, 2017, the Company had federal net operating loss (“NOL”) carryforwards of approximately $410.3 million, of which $12.9 million is subject to an annual limitation, which will begin expiring in 2026 and later. The Company has post-apportioned state NOL carryforwards of approximately $450.4 million, the majority of which will begin expiring in 2018 and later.
Future Cash Requirements
Net cash provided by operating activities from continuing operations totaled $5.6 million during the year ended December 31, 2017. Our working capital position as of December 31, 2017 reflects a $39.4 million improvement versus December 31, 2016. As of December 31, 2017, we had $39.5 million of unrestricted cash on hand and, until December 2018, $10.0 million of delayed draw capacity under the Second Lien Notes Facility, maturing on August 15, 2020, to supplement our working capital needs.
If we cannot successfully execute our strategic plans we will likely require additional or alternative sources of liquidity, including additional borrowings.
On June 29, 2017, we entered into the Notes Facilities pursuant to which we issued new senior secured notes and refinanced our existing senior secured credit facilities. Please refer to “Debt Facilities” in this section.
We regularly evaluate market conditions and financing options to improve our current liquidity profile and enhance our financial flexibility. These options may include opportunities to raise additional funds through the issuance of various forms of equity and/or debt securities or other instruments, the sale of assets or refinancing all or a portion of our indebtedness. However, there is no assurance that, if necessary, we would be able to raise capital to provide required liquidity.
Additionally, we may pursue our operational and strategic plan and will also review a range of strategic alternatives, which could include, among other things, transitioning chronic therapies to alliance partners, a potential sale or merger of our company, or continuing to pursue our operational and strategic plan. Additionally, we may pursue joint venture arrangements, additional business acquisitions and other transactions designed to expand our business.
As of the filing of this Annual Report, we expect that our cash on hand, cash from operations, and available borrowings under the Second Lien Delayed Draw Senior Secured Notes will be sufficient to fund our anticipated working capital, scheduled interest repayments and other cash needs for at least the next 12 months. Principal payments on the Notes Facilities are not required until September 30, 2019.
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates realization of assets and satisfaction of liabilities in the ordinary course of business. As such, they do not include any adjustments to the recoverability and reclassification of recorded amounts that might be necessary should we be unable to continue as a going concern.
The following table sets forth our contractual obligations affecting future cash flows as of December 31, 2017 (in thousands):
Payments Due in Year Ending December 31, | |||||||||||||||||||||||||||
Contractual Obligations | Total | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 and Beyond | ||||||||||||||||||||
Long-term debt (1) | $ | 633,558 | $ | 45,046 | $ | 47,519 | $ | 332,118 | $ | 208,875 | $ | — | $ | — | |||||||||||||
Operating lease obligations | 25,716 | 7,739 | 5,010 | 3,688 | 2,559 | 1,829 | 4,891 | ||||||||||||||||||||
Capital lease obligations (1) | 2,863 | 1,722 | 754 | 387 | — | — | — | ||||||||||||||||||||
Total | $ | 662,137 | $ | 54,507 | $ | 53,283 | $ | 336,193 | $ | 211,434 | $ | 1,829 | $ | 4,891 | |||||||||||||
(1) | Includes principal and estimated interest. |
48
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
We are exposed to market risk from changes in interest rates related to our outstanding debt. At December 31, 2017, we had total debt of $480.6 million of which $284.0 million is related to the Notes and is subject to floating interest rates. The First Lien Note bears interest at a floating rate or rates equal to, at the option of the Company, (i) the base rate (defined as the highest of the Federal Funds Rate plus 0.5% per annum, the Prime Rate as published by The Wall Street Journal and the one-month London Interbank Offered Rate (“LIBOR”) (subject to a 1.0% floor) plus 1.0%), or (ii) the one-month LIBOR rate (subject to a 1.0% floor), plus a margin of 6.0% if the base rate is selected or 7.0% if the LIBOR Option is selected. The Second Lien Note bears interest at a floating rate or rates equal to, at the option of the Company, (i) one-month LIBOR (subject to a 1.25% floor) plus 9.25% per annum in cash, (ii) one-month LIBOR (subject to a 1.25% floor) plus 11.25% per annum, which amount will be capitalized on each interest payment date, or (iii) one-month LIBOR (subject to a 1.25% floor) plus 10.25% per annum, of which one-half LIBOR plus 4.625% per annum will be payable in cash and one-half LIBOR plus 5.625% per annum will be capitalized on each interest payment date, provided that, in each case, if any permitted refinancing indebtedness with which the 2021 Notes are refinanced requires or permits the payment of cash interest, all of the interest on the Second Lien Notes shall be paid in cash. As of December 31, 2017, the Eurodollar rate is approximately 1.6%; an increase in the current market rate of 1.00% would result in an increase in annual interest expense of approximately $3.0 million.
On February 11, 2014, we issued $200.0 million in aggregate principal amount of the 2021 Notes. The interest rate on the 2021 Notes, 8.875%, is fixed and not subject to market risk.
We regularly assess the significance of interest rate market risk as part of our treasury operations and as circumstances change and enter into instruments to hedge variable rate interest expense as appropriate in accordance with the terms of the Debt Facilities. We do not use financial instruments for trading or other speculative purposes and are not a party to any derivative financial instruments at this time.
At December 31, 2017, financial assets with carrying values approximating fair value include cash and cash equivalents and accounts receivable. Financial liabilities with carrying values approximating fair value include accounts payable and capital leases. The carrying value of these financial assets and liabilities approximates fair value due to their short maturities. The fair value of our long-term debt under our Note Facilities subject to variable interest rates and the 2021 Notes is disclosed in Note 10 of the Notes to the Consolidated Financial Statements.
49
Item 8. | Financial Statements and Supplementary Data |
Report of Independent Registered Public Accounting Firm
To the stockholders and board of directors
BioScrip, Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of BioScrip, Inc. and subsidiaries (the “Company”) as of December 31, 2017 and 2016, the related consolidated statements of operations, stockholders’ (deficit) equity, and cash flows for each of the years in the three‑year period ended December 31, 2017, and the related notes and financial statement schedule (collectively, the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the three‑year period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company’s internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission”, and our report dated March 26, 2018 expressed an adverse opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ KPMG LLP
We have served as the Company’s auditor since 2014.
Denver, Colorado
March 26, 2018
50
BIOSCRIP, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(in thousands, except for share amounts)
December 31, | |||||||
2017 | 2016 | ||||||
ASSETS | |||||||
Current assets | |||||||
Cash and cash equivalents | $ | 39,457 | $ | 9,569 | |||
Restricted cash | 4,950 | — | |||||
Receivables, less allowance for doubtful accounts of $37,912 and $44,730 at December 31, 2017 and 2016, respectively | 85,522 | 109,086 | |||||
Inventory | 38,044 | 36,165 | |||||
Deferred taxes | 1,098 | — | |||||
Prepaid expenses and other current assets | 18,620 | 18,507 | |||||
Total current assets | 187,691 | 173,327 | |||||
Property and equipment, net | 26,973 | 32,678 | |||||
Goodwill | 367,198 | 365,947 | |||||
Intangible assets, net | 19,114 | 31,043 | |||||
Other non-current assets | 2,116 | 1,990 | |||||
Total assets | $ | 603,092 | $ | 604,985 | |||
LIABILITIES AND STOCKHOLDERS’ (DEFICIT) EQUITY | |||||||
Current liabilities | |||||||
Current portion of long-term debt | $ | 1,722 | $ | 18,521 | |||
Accounts payable | 65,963 | 64,420 | |||||
Amounts due to plan sponsors | 4,621 | 3,679 | |||||
Accrued interest | 6,706 | 6,705 | |||||
Accrued expenses and other current liabilities | 26,118 | 36,822 | |||||
Total current liabilities | 105,130 | 130,147 | |||||
Long-term debt, net of current portion | 478,866 | 433,413 | |||||
Deferred taxes | — | 2,281 | |||||
Other non-current liabilities | 21,769 | 763 | |||||
Total liabilities | 605,765 | 566,604 | |||||
Series A convertible preferred stock, $.0001 par value; 825,000 shares authorized; 21,645 shares issued and outstanding as of December 31, 2017 and 2016; and $2,916 and $2,603 liquidation preference as of December 31, 2017 and 2016, respectively | 2,827 | 2,462 | |||||
Series C convertible preferred stock, $.0001 par value; 625,000 shares authorized; 614,177 shares issued and outstanding; and, $84,555 and $75,491 liquidation preference as of December 31, 2017 and 2016, respectively. | 79,252 | 69,540 | |||||
Stockholders’ (deficit) equity | |||||||
Preferred stock, $.0001 par value; 5,000,000 shares authorized; no shares issued and outstanding as of December 31, 2017 and 2016, respectively | — | — | |||||
Common stock, $.0001 par value; 250,000,000 and 125,000,000 shares authorized; 127,634,012 and 117,682,543 shares issued and outstanding as of December 31, 2017 and 2016, respectively | 13 | 12 | |||||
Treasury stock, 5,106 shares outstanding, at cost, as of December 31, 2017 and no shares outstanding as of December 31, 2016. | (16 | ) | — | ||||
Additional paid-in capital | 624,762 | 611,682 | |||||
Accumulated deficit | (709,511 | ) | (645,315 | ) | |||
Total stockholders’ (deficit) equity | (84,752 | ) | (33,621 | ) | |||
Total liabilities and stockholders’ (deficit) equity | $ | 603,092 | $ | 604,985 | |||
See accompanying Notes to the Consolidated Financial Statements.
51
BIOSCRIP, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share amounts)
Years Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Net revenue | $ | 817,190 | $ | 935,589 | $ | 982,223 | |||||
Cost of revenue (excluding depreciation expense) | 547,948 | 673,507 | 722,271 | ||||||||
Gross profit | 269,242 | 262,082 | 259,952 | ||||||||
Other operating expenses | 163,273 | 169,781 | 165,328 | ||||||||
Bad debt expense | 23,697 | 26,608 | 42,444 | ||||||||
General and administrative expenses | 39,625 | 38,798 | 42,474 | ||||||||
Change in fair value of equity linked liabilities | 3,587 | (10,450 | ) | — | |||||||
Impairment of goodwill | — | — | 251,850 | ||||||||
Restructuring, acquisition, integration, and other expenses, net | 12,662 | 15,859 | 24,405 | ||||||||
Depreciation and amortization expense | 27,725 | 22,025 | 22,864 | ||||||||
Interest expense | 52,072 | 37,572 | 36,938 | ||||||||
Loss on extinguishment of debt | 13,453 | — | — | ||||||||
Loss (gain) on dispositions | 581 | (3,954 | ) | — | |||||||
Loss from continuing operations, before income taxes | (67,433 | ) | (34,157 | ) | (326,351 | ) | |||||
Income tax benefit (expense) | 4,130 | (2,015 | ) | 21,532 | |||||||
Loss from continuing operations, net of income taxes | (63,303 | ) | (36,172 | ) | (304,819 | ) | |||||
(Loss) income from discontinued operations, net of income taxes | (893 | ) | (6,593 | ) | 4,691 | ||||||
Net loss | (64,196 | ) | (42,765 | ) | (300,128 | ) | |||||
Accrued dividends on preferred stock | (9,376 | ) | (8,392 | ) | (6,120 | ) | |||||
Deemed dividends on preferred stock | (701 | ) | (692 | ) | (3,690 | ) | |||||
Loss attributable to common stockholders | $ | (74,273 | ) | $ | (51,849 | ) | $ | (309,938 | ) | ||
Loss per common share: | |||||||||||
Loss from continuing operations, basic and diluted | $ | (0.59 | ) | $ | (0.48 | ) | $ | (4.58 | ) | ||
(Loss) Income from discontinued operations, basic and diluted | (0.01 | ) | (0.07 | ) | 0.07 | ||||||
Net loss, basic and diluted | $ | (0.60 | ) | $ | (0.55 | ) | $ | (4.51 | ) | ||
Weighted average common shares outstanding, basic and diluted | 123,791 | 93,740 | 68,710 | ||||||||
See accompanying Notes to the Consolidated Financial Statements.
52
BIOSCRIP, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ (DEFICIT) EQUITY
(in thousands)
Preferred Stock | Common Stock | Treasury Stock | Additional Paid-in Capital | Accumulated Deficit | Total Stockholders' (Deficit) Equity | |||||||||||||||||
Balance at December 31, 2014 | $ | — | $ | 8 | $ | (10,679 | ) | $ | 529,682 | $ | (302,422 | ) | $ | 216,589 | ||||||||
Exercise of stock options | — | — | — | 2 | — | 2 | ||||||||||||||||
Surrender of stock to satisfy minimum tax withholding | — | — | (58 | ) | — | — | (58 | ) | ||||||||||||||
Issuance of Series A convertible preferred stock and warrants | — | — | — | 6,581 | — | 6,581 | ||||||||||||||||
Accrued dividends on preferred stock | — | — | — | (6,120 | ) | — | (6,120 | ) | ||||||||||||||
Deemed dividends on preferred stock | — | — | — | (3,690 | ) | — | (3,690 | ) | ||||||||||||||
Compensation under employee stock compensation plan | — | — | — | 5,309 | — | 5,309 | ||||||||||||||||
Net loss | — | — | — | — | (300,128 | ) | (300,128 | ) | ||||||||||||||
Balance at December 31, 2015 | — | 8 | (10,737 | ) | 531,764 | (602,550 | ) | (81,515 | ) | |||||||||||||
Net proceeds of public stock offering | — | 4 | — | 83,263 | — | 83,267 | ||||||||||||||||
Surrender of stock to satisfy minimum tax withholding | — | — | (33 | ) | — | — | (33 | ) | ||||||||||||||
Surrender of stock - settlement | — | — | (255 | ) | 255 | — | — | |||||||||||||||
Shares issued in connection with the acquisition of Home Solutions, Inc. | — | — | 11,025 | (1,088 | ) | — | 9,937 | |||||||||||||||
Equity linked liabilities reclassified to equity upon approval of Charter Amendment | — | — | — | 2,847 | — | 2,847 | ||||||||||||||||
Accrued dividends on preferred stock | — | — | — | (8,392 | ) | — | (8,392 | ) | ||||||||||||||
Deemed dividends on preferred stock | — | — | — | (692 | ) | — | (692 | ) | ||||||||||||||
Compensation under employee stock compensation plan | — | — | — | 3,725 | — | 3,725 | ||||||||||||||||
Net loss | — | — | — | — | (42,765 | ) | (42,765 | ) | ||||||||||||||
Balance at December 31, 2016 | — | 12 | — | 611,682 | (645,315 | ) | (33,621 | ) | ||||||||||||||
Net proceeds from private placements | — | 1 | — | 20,776 | — | 20,777 | ||||||||||||||||
Exercise of stock options | — | — | — | 21 | — | 21 | ||||||||||||||||
Surrender of stock to satisfy minimum tax withholding | — | — | (16 | ) | — | — | (16 | ) | ||||||||||||||
Accrued dividends on preferred stock | — | — | — | (9,376 | ) | — | (9,376 | ) | ||||||||||||||
Deemed dividends on preferred stock | — | — | — | (701 | ) | — | (701 | ) | ||||||||||||||
Compensation under employee stock compensation plans | — | — | — | 2,360 | — | 2,360 | ||||||||||||||||
Net loss | — | — | — | — | (64,196 | ) | (64,196 | ) | ||||||||||||||
Balance at December 31, 2017 | $ | — | $ | 13 | $ | (16 | ) | $ | 624,762 | $ | (709,511 | ) | $ | (84,752 | ) | |||||||
See accompanying Notes to the Consolidated Financial Statements.
53
BIOSCRIP, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
Years Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Cash flows from operating activities: | |||||||||||
Net loss | $ | (64,196 | ) | $ | (42,765 | ) | $ | (300,128 | ) | ||
Less: Income (loss) from discontinued operations, net of income taxes | (893 | ) | (6,593 | ) | 4,691 | ||||||
Loss from continuing operations, net of income taxes | (63,303 | ) | (36,172 | ) | (304,819 | ) | |||||
Adjustments to reconcile net loss from continuing operations to net cash provided by (used in) operating activities: | |||||||||||
Depreciation and amortization | 27,725 | 22,025 | 22,864 | ||||||||
Impairment of goodwill | — | — | 251,850 | ||||||||
Amortization of deferred financing costs and debt discount | 6,998 | 4,042 | 3,440 | ||||||||
Change in fair value of contingent consideration | — | (4,597 | ) | (30 | ) | ||||||
Change in fair value of equity linked liabilities | 3,587 | (10,450 | ) | — | |||||||
Change in deferred income tax | (3,379 | ) | 2,045 | (20,089 | ) | ||||||
Compensation under stock-based compensation plans | 2,360 | 1,801 | 4,513 | ||||||||
Loss (gain) on dispositions | 581 | (3,954 | ) | — | |||||||
Loss on extinguishment of debt | 13,453 | — | — | ||||||||
Changes in assets and liabilities, net of acquired businesses: | |||||||||||
Receivables, net of bad debt expense | 23,564 | (2,219 | ) | 18,760 | |||||||
Inventory | (2,544 | ) | 10,016 | (5,769 | ) | ||||||
Prepaid expenses and other assets | (239 | ) | (893 | ) | (734 | ) | |||||
Accounts payable | 689 | (15,977 | ) | (23,381 | ) | ||||||
Amounts due to plan sponsors | 942 | 308 | (1,377 | ) | |||||||
Accrued interest | 1 | (192 | ) | 45 | |||||||
Accrued expenses and other liabilities | (4,805 | ) | (1,305 | ) | (8,020 | ) | |||||
Net cash provided by (used in) operating activities from continuing operations | 5,630 | (35,522 | ) | (62,747 | ) | ||||||
Net cash used in operating activities from discontinued operations | (6,393 | ) | (7,019 | ) | (1,483 | ) | |||||
Net cash used in operating activities | (763 | ) | (42,541 | ) | (64,230 | ) | |||||
Cash flows from investing activities: | |||||||||||
Cash consideration paid for acquisitions, net of cash acquired | — | (67,516 | ) | — | |||||||
Purchases of property and equipment, net | (8,680 | ) | (9,870 | ) | (12,056 | ) | |||||
Proceeds from dispositions | — | 4,177 | — | ||||||||
Investment in restricted cash | (4,950 | ) | — | — | |||||||
Net cash used in investing activities from continuing operations | (13,630 | ) | (73,209 | ) | (12,056 | ) | |||||
Net cash provided by investing activities from discontinued operations | — | — | 24,565 | ||||||||
Net cash (used in) provided by investing activities | (13,630 | ) | (73,209 | ) | 12,509 | ||||||
Cash flows from financing activities: | |||||||||||
Proceeds from private issuances, net | 20,777 | 83,267 | — | ||||||||
Proceeds from issuance of convertible preferred stock and warrants, net of issuance costs | — | — | 59,691 | ||||||||
Proceeds from priming credit agreement, net | 23,060 | — | |||||||||
Deferred and other financing costs | (980 | ) | — | (2,630 | ) | ||||||
Borrowings on revolving credit facility | 563 | 104,300 | 203,663 | ||||||||
Repayments on revolving credit facility | (55,863 | ) | (64,000 | ) | (193,663 | ) | |||||
Borrowing of long-term debt | 294,446 | — | — | ||||||||
Principal payments of long-term debt | (236,770 | ) | (12,550 | ) | — | ||||||
Repayments of capital leases | (1,072 | ) | (1,073 | ) | (395 | ) | |||||
Net proceeds from exercise of employee stock compensation plans | 120 | (202 | ) | (108 | ) | ||||||
Net cash provided by financing activities | 44,281 | 109,742 | 66,558 | ||||||||
Net change in cash and cash equivalents | 29,888 | (6,008 | ) | 14,837 | |||||||
Cash and cash equivalents - beginning of period | 9,569 | 15,577 | 740 | ||||||||
Cash and cash equivalents - end of period | $ | 39,457 | $ | 9,569 | $ | 15,577 | |||||
DISCLOSURE OF CASH FLOW INFORMATION: | |||||||||||
Cash paid during the period for interest | $ | 45,376 | $ | 34,696 | $ | 34,302 | |||||
Cash paid during the period for income taxes, net of refunds | $ | 649 | $ | (372 | ) | $ | 114 | ||||
DISCLOSURE OF NON-CASH TRANSACTIONS: | |||||||||||
Issuance of 3,750,000 shares in connection with the Home Solutions acquisition | $ | — | $ | 9,938 | $ | — | |||||
Capital lease obligations incurred to acquire property and equipment | $ | 1,825 | $ | 2,314 | $ | — | |||||
See accompanying Notes to the Consolidated Financial Statements.
54
BIOSCRIP, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – NATURE OF BUSINESS
Corporate Organization and Business
BioScrip, Inc. and subsidiaries (the “Company” or “BioScrip”) is a national provider of infusion service that partners with physicians, hospital systems, skilled nursing facilities and healthcare payors to provide patients access to post-acute care services. The Company operates with a commitment to bring customer-focused infusion therapy services into the home or alternate-site setting. By collaborating with the full spectrum of healthcare professionals and the patient, the Company aims to provide cost-effective care that is driven by clinical excellence, customer service and values that promote positive outcomes and an enhanced quality of life for those whom it serves.
The Company’s platform provides nationwide service capabilities and the ability to deliver clinical management services that offer patients a high-touch, community-based and home-based care environment. The Company’s core services are provided in coordination with, and under the direction of, the patient’s physician. The Company's multidisciplinary team of clinicians, including pharmacists, nurses, dietitians and respiratory therapists, work with the physician to develop a plan of care suited to the patient’s specific needs. Whether in the home, physician office, ambulatory infusion center, skilled nursing facility or other alternate sites of care, the Company provides products, services and condition-specific clinical management programs tailored to improve the care of individuals with complex health conditions such as gastrointestinal abnormalities, infectious diseases, cancer, multiple sclerosis, organ and blood cell transplants, bleeding disorders, immune deficiencies and heart failure.
On August 27, 2015, the Company completed the sale of substantially all of the Company’s Pharmacy Benefit Management Services segment (the “PBM Business”) to ProCare Pharmacy Benefit Manager Inc. (see Note 6 - Discontinued Operations). As a result of the sale of the PBM Business, the Company no longer has multiple operating segments. The change reflects how the Company's chief operating decision maker reviews the Company’s results in terms of allocating resources and assessing performance.
Basis of Presentation
The Company’s Consolidated Financial Statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”).
Reclassifications
Certain prior period financial statement amounts have been reclassified to conform to current period presentation.
Immaterial Error Correction
During the fourth quarter of 2017, the Company determined that certain prior period balances contained errors, predominantly due to a failure to appropriately account for and resolve transactions specific to suspense and clearing accounts. Management evaluated the materiality of the errors quantitatively and qualitatively, and concluded that they were not material to the financial statements of any period presented, but has elected to correct them in the accompanying prior period consolidated financial statements.
55
The following tables set forth the effect these corrections had on the Company’s December 31, 2016 and 2015 statements of operations:
Year Ended December 31, 2016 | Year Ended December 31, 2015 | ||||||||||||||||||||||
Previously Reported | Corrections | As Revised | Previously Reported | Corrections | As Revised | ||||||||||||||||||
Net revenue | $ | 935,589 | $ | — | $ | 935,589 | $ | 982,223 | $ | — | $ | 982,223 | |||||||||||
Gross profit | 265,631 | (3,549 | ) | 262,082 | 260,915 | (963 | ) | 259,952 | |||||||||||||||
Total Operating Expenses | 263,702 | (1,081 | ) | 262,621 | 548,562 | 803 | 549,365 | ||||||||||||||||
Interest expense | 38,235 | (663 | ) | 37,572 | 37,313 | (375 | ) | 36,938 | |||||||||||||||
Loss from continuing operations, net of income taxes | (34,367 | ) | (1,805 | ) | (36,172 | ) | (303,428 | ) | (1,391 | ) | (304,819 | ) | |||||||||||
Loss from discontinued operations, net of income taxes | (7,139 | ) | 546 | (6,593 | ) | 3,721 | 970 | 4,691 | |||||||||||||||
Net loss | $ | (41,506 | ) | $ | (1,259 | ) | $ | (42,765 | ) | $ | (299,707 | ) | $ | (421 | ) | $ | (300,128 | ) | |||||
The following tables set forth the effect these corrections had on the Company’s December 31, 2016 balance sheet.
Year Ended December 31, 2016 | |||||||||
Previously Reported | Corrections | As Revised | |||||||
Total assets | $ | 607,740 | $ | (2,755 | ) | $ | 604,985 | ||
Total liabilities | 567,301 | (697 | ) | 566,604 | |||||
Additional paid-in capital | 611,844 | (162 | ) | 611,682 | |||||
Accumulated deficit | (643,419 | ) | (1,896 | ) | (645,315 | ) | |||
Total stockholders' equity | (31,563 | ) | (2,058 | ) | (33,621 | ) | |||
Total liabilities and stockholders' equity | $ | 607,740 | $ | (2,755 | ) | $ | 604,985 | ||
The accumulated deficit correction above includes a $0.4 million adjustment as of January 1, 2015 related to prior periods.
Certain amounts disclosed in the accompanying notes to the financial statements have been revised to reflect the corrections.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Consolidation
The Consolidated Financial Statements include the accounts of the Company and its wholly-owned subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make certain estimates and assumptions. These estimates and assumptions affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Fair Value Measurements
The fair value measurement accounting standard, ASC Topic 820, Fair Value Measurement (“ASC 820”), provides a framework for measuring fair value and defines fair value as the price that would be received to sell an asset or paid to transfer a liability. Fair value is a market-based measurement that should be determined using assumptions that market participants would use in pricing an asset or liability. The standard establishes a valuation hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability developed
56
based on independent market data sources. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability developed based upon the best information available.
The valuation hierarchy is composed of three categories. The categorization within the valuation hierarchy is based on the lowest level of input that is significant to the fair value measurement. The categories within the valuation hierarchy are described as follows:
• | Level 1 - Inputs to the fair value measurement are quoted prices in active markets for identical assets or liabilities. |
• | Level 2 - Inputs to the fair value measurement include quoted prices in active markets for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly. |
• | Level 3 - Inputs to the fair value measurement are unobservable inputs or valuation techniques. |
Cash and Cash Equivalents
Highly liquid investments with a maturity of three months or less when purchased are classified as cash equivalents. Restricted cash consists of cash balances held by financial institutions as collateral for letters of credit. These balances are reclassified to cash and cash equivalents when the underlying obligation is satisfied, or in accordance with the governing agreement. Restricted cash balances expected to become unrestricted during the next twelve months are recorded as current assets. As of December 31, 2017, the Company had a restricted cash balance, in a money market account, of approximately $5.0 million to cash collateralize outstanding letters of credit.
Receivables
Receivables include amounts due from government sources, such as Medicare and Medicaid programs, Managed Care Organizations and other commercial insurance, amounts due from patient co-payments, and service fees resulting from the distribution of certain drugs through retail pharmacies.
Allowance for Doubtful Accounts
The allowance for doubtful accounts is based on estimates of losses related to receivable balances. The risk of collection varies based upon the product, the payor (commercial health insurance and government) and the patient’s ability to pay the amounts not reimbursed by the payor. We estimate the allowance for doubtful accounts based on several factors including the age of the outstanding receivables, the historical experience of collections, adjusting for current economic conditions, and, in some cases, evaluating specific customer accounts for the ability to pay. We also consider qualitative factors. Collection agencies are employed and legal action is taken when we determine that taking collection actions is reasonable relative to the probability of receiving payment on amounts owed. Management judgment is used to assess the collectability of accounts and the ability of our customers to pay. Judgment is also used to assess trends in collections and the effects of systems and business process changes on our expected collection rates. The Company reviews the estimation process quarterly and makes changes to the estimates as necessary. When it is determined that a customer account is uncollectible, that balance is written off against the existing allowance.
Collectability of Accounts Receivable
57
The following table sets forth the aging of our net accounts receivable (net of allowance for contractual adjustments and prior to allowance for doubtful accounts), aged based on date of service and categorized based on the three primary overall types of accounts receivable characteristics (in thousands):
December 31, 2017 | December 31, 2016 | ||||||||||||||||||||||||||||
0 - 180 days | Over 180 days | Total | % of Total | 0 - 180 days | Over 180 days | Total | % of Total | ||||||||||||||||||||||
Government | $ | 20,602 | $ | 10,082 | $ | 30,684 | $ | 19,891 | $ | 8,278 | $ | 28,169 | |||||||||||||||||
Commercial | 63,767 | 18,779 | 82,546 | 95,018 | 19,849 | 114,867 | |||||||||||||||||||||||
Patient | 2,577 | 7,627 | 10,204 | 3,955 | 6,825 | 10,780 | |||||||||||||||||||||||
Gross accounts receivable | $ | 86,946 | $ | 36,488 | 123,434 | $ | 118,864 | $ | 34,952 | 153,816 | |||||||||||||||||||
Allowance for doubtful accounts | (37,912 | ) | 30.7 | % | (44,730 | ) | 29.1 | % | |||||||||||||||||||||
Net accounts receivable | $ | 85,522 | $ | 109,086 | |||||||||||||||||||||||||
At December 31, 2017, our allowance for doubtful accounts was $37.9 million, or 30.7% of gross accounts receivable, as compared to $44.7 million, or 29.1% of gross accounts receivable, at December 31, 2016. The allowance for doubtful accounts decreased by approximately $3.0 million during 2017 due to a change in estimate resulting from stabilized collections including more predictable cash receipts from our payors.
Allowance for Contractual Discounts
The Company is reimbursed by payors for products and services the Company provides. Payments for medications and services covered by payors average less than billed charges. The Company monitors revenue and receivables from payors for each of our branches and records an estimated contractual allowance for certain revenue and receivable balances as of the revenue recognition date to properly account for anticipated differences between amounts estimated in our billing system and amounts reimbursed. Accordingly, the total revenue and receivables reported in our financial statements are recorded at the amounts expected to be received from the payor. For the significant portion of the Company’s revenue, the contractual allowance is estimated based on several criteria, including unbilled claims, historical trends based on actual claims paid, current contract and reimbursement terms and changes in customer base and payor/product mix. Contractual allowance estimates are adjusted to actual amounts as cash is received and claims are settled. We do not believe these changes in estimates are material. The billing functions for the remaining portion of the Company’s revenue are largely computerized, which enables on-line adjudication (i.e., submitting charges to third-party payors electronically with simultaneous feedback of the amount the primary insurance plan expects to pay) at the time of sale to record net revenue, exposure to estimating contractual allowance adjustments is limited on this portion of the business.
Inventory
Inventory is recorded at the lower of cost or market. Cost is determined using specific item or the first-in, first-out method. Inventory consists principally of purchased prescription drugs and related supplies. Included in inventory is a reserve for inventory waste and obsolescence.
Property and Equipment
Property and equipment is stated at cost less accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of assets as follows:
Asset | Useful Life | |||||
Computer hardware and software | 3 | years | - | 5 | years | |
Office equipment | 5 | years | ||||
Vehicles | 4 | years | - | 5 | years | |
Medical equipment | 13 | months | - | 5 | years | |
Furniture and fixtures | 5 | years | ||||
Leasehold improvements and assets leased under capital leases are depreciated using a straight-line basis over the related lease term or estimated useful life of the assets, whichever is less. The cost and related accumulated depreciation of assets sold
58
or retired are removed from the accounts with the gain or loss, if applicable, recorded in the statement of operations. Maintenance and repair costs are expensed as incurred.
Costs relating to the development of software for internal purposes are charged to expense until technological feasibility is established. Thereafter, the remaining software production costs up to the date placed into production are capitalized and included in Property and Equipment. Costs of customization and implementation of computer software purchased for internal use are likewise capitalized. Depreciation of the capitalized amounts commences on the date the asset is ready for its intended use and is calculated using the straight-line method over the estimated useful life of the software.
Goodwill
Goodwill is not subject to amortization but is instead tested for impairment annually and whenever events or circumstances exist that indicate that the carrying value of goodwill may no longer be recoverable. Management considers the Company’s business as a whole to be its reporting unit for the purpose of testing for impairment as, subsequent to the sale of the PBM Business, the Company no longer has multiple operating segments. Management may choose to undertake a qualitative assessment in order to assess whether a quantitative analysis is required. In determining whether management will utilize the qualitative assessment in any one year, management will consider overall economic factors as well as the passage of time between the last quantitative assessment.
In January 2017, the FASB issued authoritative guidance that simplifies the measurement of goodwill impairment to a single-step test. The guidance eliminates step two of the goodwill impairment test; the measurement of goodwill impairment will now be the amount by which a reporting unit’s carrying value exceeds its fair value, not to exceed the carrying amount of goodwill. Under the revised guidance, failing step one will result in goodwill impairment. The Company adopted the new guidance on January 1, 2017 on a prospective basis.
Intangible Assets
The Company evaluates the useful lives of its intangible assets to determine if they are finite or indefinite-lived. Finite-lived intangible assets, primarily acquired customer relationships, trademarks and non-compete agreements, are amortized on a straight-line basis over their estimated useful lives.
Impairment of Long Lived Assets
The Company evaluates whether events and circumstances have occurred that indicate the remaining estimated useful life of long lived assets, including intangible assets, may warrant revision or that the remaining balance of an asset may not be recoverable. The measurement of possible impairment is based on the ability to recover the balance of assets from expected future operating cash flows on an undiscounted basis. Impairment losses, if any, are determined based on the fair value of the asset, which are generally calculated as the present value of related cash flows using discount rates that reflect the inherent risk of the underlying business.
Amounts due to Plan Sponsors
Amounts due to Plan Sponsors primarily represent payments received from Plan Sponsors in excess of the contractually required reimbursement. These amounts are further evaluated in order to determine amounts to be refunded to Plan Sponsors.
Revenue Recognition
The Company generates revenue principally through the provision of infusion services to provide clinical management services and the delivery of cost effective prescription medications. Prescription drugs are dispensed either through a pharmacy participating in the Company’s pharmacy network or a pharmacy owned by the Company. Fee-for-service agreements includes pharmacy agreements, where we dispense prescription medications through the Company’s pharmacy facilities.
FASB ASC Subtopic 605-25, Revenue Recognition: Multiple-Element Arrangements (“ASC 605-25”), addresses situations in which there are multiple deliverables under one revenue arrangement with a customer and provides guidance in determining whether multiple deliverables should be recognized separately or in combination. The Company provides a variety of therapies to patients. For infusion-related therapies, the Company frequently provides multiple deliverables of drugs and related nursing services. After applying the criteria from ASC 605-25, the Company concluded that separate units of accounting exist in revenue arrangements with multiple deliverables. Drug revenue is recognized at the time the drug is shipped, and nursing revenue is recognized on the date of service. The Company allocates revenue consideration based on the relative fair value as determined
59
by the Company’s best estimate of selling price to separate the revenue where there are multiple deliverables under one revenue arrangement.
The Company also recognizes nursing revenue as the estimated net realizable amounts from patients and third party payors for the infusion services rendered and products provided. This revenue is recognized as the treatment plan is administered to the patient and is recorded at amounts estimated to be received under reimbursement or payment arrangements with payors.
Cost of Revenue
Cost of revenue includes the costs of prescription medications, shipping and other direct and indirect costs, and nursing services, offset by volume and prompt pay discounts received from pharmaceutical manufacturers and distributors and total manufacturer rebates.
Rebates
Manufacturers’ rebates are generally volume-based incentives that are earned and recorded upon purchase of the inventory. Rebates are recorded to cost of goods sold.
Lease Accounting
The Company accounts for operating leasing transactions by recording rent expense on a straight-line basis over the expected term of the lease starting on the date it gains possession of leased property. The Company includes tenant improvement allowances and rent holidays received from landlords and the effect of any rent escalation clauses, as adjustments to straight-line rent expense over the expected term of the lease.
Capital lease transactions are reflected as a liability at the inception of the lease based on the present value of the minimum lease payments or, if lower, the fair value of the property. Assets recorded under capital leases are depreciated in the same manner as owned property.
Income Taxes
As part of the process of preparing the Company’s Consolidated Financial Statements, management is required to estimate income taxes in each of the jurisdictions in which it operates. The Company accounts for income taxes under ASC Topic 740, Income Taxes (“ASC 740”). ASC 740 requires the use of the asset and liability method of accounting for income taxes. Under this method, deferred taxes are determined by calculating the future tax consequences attributable to differences between the financial accounting and tax bases of existing assets and liabilities. A valuation allowance is recorded against deferred tax assets when, in the opinion of management, it is more likely than not that the Company will not be able to realize the benefit from its deferred tax assets.
The Company files income tax returns, including returns for its subsidiaries, as prescribed by Federal tax laws and the tax laws of the state and local jurisdictions in which it operates. The Company’s uncertain tax positions are related to tax years that remain subject to examination and are recognized in the Consolidated Financial Statements when the recognition threshold and measurement attributes of ASC 740 are met. Interest and penalties related to unrecognized tax benefits are recorded as income tax expense.
Financial Instruments
The Company’s financial instruments consist mainly of cash and cash equivalents, receivables, accounts payable, and accrued interest. The carrying amounts of cash and cash equivalents, receivables, accounts payable, and accrued interest approximate fair value due to their fully liquid or short-term nature.
Accounting for Stock-Based Compensation
The Company accounts for stock-based compensation expense under the provisions of ASC Topic 718, Compensation – Stock Compensation (“ASC 718”). At December 31, 2017, the Company has one stock-based compensation plan pursuant to which incentive stock options (“ISOs”), non-qualified stock options (“NQSOs”), stock appreciation rights (“SARs”), restricted stock, performance shares and performance units may be granted to employees and non-employee directors. Option and stock awards are typically settled by issuing authorized but unissued shares of the Company.
60
The Company accounts for its stock-based awards to employees and non-employee directors using the fair value method. The fair value of each option award is based on several criteria including, but not limited to, the valuation model used and associated input factors including principally stock price volatility and, to a lesser extent, expected term, dividend rate, and risk-free interest rate. The input factors used in the valuation model are based on subjective future expectations combined with management judgment. The fair value of each stock award is determined based on the closing price of the underlying common stock on the date of grant. The fair value of the award is amortized to expense on a straight-line basis over the requisite service period. The Company expenses restricted stock awards based on vesting requirements, including time elapsed, market conditions and/or performance conditions. Because of these requirements, the weighted average period for which the expense is recognized varies. The Company expenses SAR awards based on vesting requirements. In addition, because they are settled with cash, the fair value of the SAR awards are revalued on a quarterly basis.
Recent Accounting Pronouncements
In July 2017, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2017-11—Earnings Per Share (Topic 260), Distinguishing Liabilities From Equity (Topic 480), and Derivatives and Hedging (Topic 815): I. Accounting for Certain Financial Instruments with Down Round Features and II. Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Nonpublic Entities and Certain Mandatorily Redeemable Noncontrolling Interests with a Scope Exception. ASU 2017-11 eliminates the requirement that a down round feature precludes equity classification when assessing whether an instrument is indexed to an entity’s own stock. A freestanding equity-linked financial instrument no longer would be accounted for as a derivative liability at fair value as a result of the existence of a down round feature. The effective date for ASU 2017-11 is for annual or any interim periods beginning after December 15, 2018. Early adoption is permitted. The adoption of this standard is not expected to have a material impact on the Company’s consolidated financial statements.
In May 2017, the FASB issued ASU 2017-09—Compensation–Stock Compensation (Topic 718): Scope of Modification Accounting. ASU 2017-09 modifies when a change to the terms or conditions of a share-based payment award must be accounted for as a modification. The new guidance requires modification accounting if the fair value, vesting condition or the classification of the award is not the same immediately before and after a change to the terms and conditions of the award. The effective date for ASU 2017-09 is for annual or any interim periods beginning after December 15, 2017. The Company will adopt this ASU effective January 1, 2018. The adoption of this standard will not materially impact the Company’s consolidated financial statements.
In January 2017, the FASB issued ASU 2017-04—Intangibles–Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment. ASU 2017-04 modifies the accounting for goodwill impairment. The guidance removes Step 2 of the goodwill impairment test, which requires a hypothetical purchase price allocation. A goodwill impairment will now be the amount by which a reporting unit’s carrying value exceeds its fair value, not to exceed the carrying amount of goodwill. The effective date for ASU 2017-04 is for annual or any interim periods beginning after December 15, 2019. The adoption of this standard did not materially impact the Company’s consolidated financial statements.
In November 2016, the FASB issued ASU 2016-18—Statement of Cash Flows (Topic 230): Restricted Cash. ASU 2016-18 requires that a statement of cash flows explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash or restricted cash equivalents. Therefore, amounts generally described as restricted cash and restricted cash equivalents should be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. The effective date for ASU 2016-18 is for annual or any interim periods beginning after December 15, 2017. The Company will adopt this ASU effective January 1, 2018. The adoption of this standard will not materially impact the Company’s consolidated financial statements.
In August 2016, the FASB issued ASU 2016-15—Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments. ASU 2016-15 provides guidance for eight specific cash flow issues with respect to how cash receipts and cash payments are classified in the statements of cash flows, with the objective of reducing diversity in practice. The effective date for ASU 2016-15 is for annual periods beginning after December 15, 2017, and interim periods within those fiscal years. The Company adopted this ASU effective January 1, 2018. The adoption of this standard did not materially impact the Company’s consolidated financial statements.
In February 2016, the FASB issued ASU 2016-02—Leases (Topic 842), requiring lessees to recognize a right-of-use asset and a lease liability on the balance sheet for all leases with the exception of short-term leases. For lessees, leases will continue to be classified as either operating or finance leases in the income statement. The effective date of the new standard for public companies is for fiscal years beginning after December 15, 2018 and interim periods within those fiscal years. Early adoption is permitted. The new standard may be adopted using a modified retrospective transition and requires application of the new guidance at the beginning of the earliest comparative period presented. The Company is evaluating the effect that the updated standard will have on its consolidated financial statements.
61
In May 2014, the FASB issued ASU 2014-09—Revenue from Contracts with Customers (Topic 606). The guidance requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. The FASB delayed the effective date to annual reporting periods beginning after December 15, 2017, including interim reporting periods within that reporting period. The Company did not elect early adoption and applied the modified retrospective approach upon adoption which results in application of the new guidance only to contracts that are not completed at the adoption date and does not require adjustment of prior reporting periods. Assessment of the new guidance is not anticipated to result in an opening balance sheet adjustment. The Company anticipates the new standard will result in the reclassification of a substantial portion of amounts previously reported as bad debt expense to contra revenue upon implementation during fiscal year 2018.
NOTE 3 – LOSS PER SHARE
Loss Per Share
The Company presents basic and diluted loss per share (“LPS”) for its common stock, par value $.0001 per share (“Common Stock”). Basic LPS is calculated by dividing the net loss attributable to common stockholders of the Company by the weighted average number of shares of Common Stock outstanding during the period. Diluted LPS is determined by adjusting the profit or loss attributable to stockholders and the weighted average number of shares of Common Stock outstanding adjusted for the effects of all dilutive potential common shares comprised of options granted, unvested restricted stocks, stock appreciation rights, warrants and Series A and Series C Convertible Preferred Stock. Potential Common Stock equivalents that have been issued by the Company related to outstanding stock options, unvested restricted stock and warrants are determined using the treasury stock method, while potential common shares related to Series A and Series C Convertible Preferred Stock are determined using the “if converted” method.
The Company's Series A and Series C Convertible Preferred Stock, par value $.0001 per share (together, the “Preferred Stock”), is considered a participating security, which means the security may participate in undistributed earnings with Common Stock. The holders of the Preferred Stock would be entitled to share in dividends, on an as-converted basis, if the holders of Common Stock were to receive dividends. The Company is required to use the two-class method when computing LPS when it has a security that qualifies as a participating security. The two-class method is an earnings allocation formula that determines LPS for each class of common stock and participating security according to dividends declared (or accumulated) and participation rights in undistributed earnings. In determining the amount of net earnings to allocate to common stockholders, earnings are allocated to both common and participating securities based on their respective weighted-average shares outstanding during the period. Diluted LPS for the Company’s Common Stock is computed using the more dilutive of the two-class method or the if-converted method.
The following table sets forth the computation of basic and diluted loss per common share (in thousands, except for per share amounts):
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Numerator: | |||||||||||
Loss from continuing operations, net of income taxes | $ | (63,303 | ) | $ | (36,172 | ) | $ | (304,819 | ) | ||
(Loss) income from discontinued operations, net of income taxes | (893 | ) | (6,593 | ) | 4,691 | ||||||
Net loss | (64,196 | ) | (42,765 | ) | (300,128 | ) | |||||
Accrued dividends on Preferred Stock | (9,376 | ) | (8,392 | ) | (6,120 | ) | |||||
Deemed dividends on Preferred Stock | (701 | ) | (692 | ) | (3,690 | ) | |||||
Loss attributable to common stockholders | $ | (74,273 | ) | $ | (51,849 | ) | $ | (309,938 | ) | ||
Denominator - Basic and Diluted: | |||||||||||
Weighted average number of common shares outstanding | 123,791 | 93,740 | 68,710 | ||||||||
Loss Per Common Share: | |||||||||||
Loss from continuing operations, basic and diluted | $ | (0.59 | ) | $ | (0.48 | ) | $ | (4.58 | ) | ||
(Loss) income from discontinued operations, basic and diluted | (0.01 | ) | (0.07 | ) | 0.07 | ||||||
Loss per common share, basic and diluted | $ | (0.60 | ) | $ | (0.55 | ) | $ | (4.51 | ) | ||
62
The loss attributable to common stockholders is used as the basis of determining whether the inclusion of common stock equivalents would be anti-dilutive. Accordingly, the computation of diluted shares for the years ended December 31, 2017, 2016 and 2015 excludes the effect of securities issued in connection with the PIPE Transaction and the Rights Offering (see Note 4 - Stockholders’ Deficit), as well as stock options and restricted stock awards, as their inclusion would be anti-dilutive to loss attributable to common stockholders.
NOTE 4 – STOCKHOLDERS’ DEFICIT
Carrying Value of Series A Preferred Stock
As of December 31, 2017, the following values were accreted and recorded as a reduction of additional paid in capital in Stockholders’ Deficit and a deemed dividend on the consolidated Statement of Operations. In addition, dividends were accrued at 11.5% from the date of issuance to December 31, 2017. The following table sets forth the activity recorded during the year ended December 31, 2017 related to the Series A Preferred Stock (in thousands) issued for both the PIPE Transaction and the Rights Offering:
Series A Preferred Stock carrying value at December 31, 2015 | $ | 62,918 | |
Exchange of Series A for Series C | (60,776 | ) | |
Accretion of discount related to issuance costs | 40 | ||
Dividends recorded through December 31, 2016 1 | 280 | ||
Series A Preferred Stock carrying value at December 31, 2016 | $ | 2,462 | |
Accretion of discount related to issuance costs | 53 | ||
Dividends recorded through December 31, 2017 1 | 312 | ||
Series A Preferred Stock carrying value at December 31, 2017 | $ | 2,827 | |
1 Dividends recorded reflect the increase in the Liquidation Preference associated with unpaid dividends.
Carrying Value of Series C Preferred Stock
As of December 31, 2017, the following values were accreted and recorded as a reduction of additional paid in capital in Stockholders’ Deficit and a deemed dividend on the consolidated Statement of Operations. In addition, dividends were accrued at 11.5% from the date of issuance to December 31, 2017. The following table sets forth the activity recorded during the year ended December 31, 2017 related to the Series C Preferred Stock (in thousands):
Series C Preferred Stock carrying value at December 31, 2015 | $ | — | |
Exchange of Series A for Series C | 60,776 | ||
Accretion of discount related to issuance costs | 652 | ||
Dividends recorded through December 31, 2016 1 | 8,112 | ||
Series C Preferred Stock Carrying Value at December 31, 2016 | $ | 69,540 | |
Accretion of discount related to issuance costs | 648 | ||
Dividends recorded through December 31, 2017 1 | 9,064 | ||
Series C Preferred Stock carrying value at December 31, 2017 | $ | 79,252 | |
1 Dividends recorded reflect the increase in the Liquidation Preference associated with unpaid dividends.
First Quarter 2017 Private Placement
On March 1, 2017, the Company entered into a Stock Purchase Agreement (the “First Quarter Stock Purchase Agreement”) with Venor Capital Master Fund Ltd., Map 139 Segregated Portfolio of LMA SPC, Venor Special Situations Fund II LP and Trevithick LP (the “First Quarter Stockholders”). Pursuant to the First Quarter Stock Purchase Agreement, the Company sold an aggregate of 3.3 million shares of its common stock (the “First Quarter Shares”) for aggregate gross proceeds of approximately $5.1 million in a private placement transaction (the “First Quarter 2017 Private Placement”). The purchase price for each Share was $1.5366, which was negotiated between the Company and the First Quarter Stockholders based on the volume-weighted average price of the Company's common stock on the Nasdaq Global Market on March 1, 2017.
63
Proceeds from the First Quarter 2017 Private Placement were used for working capital and general corporate purposes.
2017 Warrants
In connection with the Second Lien Note Facility (as defined below), the Company issued warrants (the “2017 Warrants”) to the purchasers of the Second Lien Notes (as defined below) pursuant to a Warrant Purchase Agreement dated as of June 29, 2017 (the “Warrant Purchase Agreement”). The 2017 Warrants entitle the purchasers of the Warrants to purchase shares of Common Stock, representing at the time of any exercise of the 2017 Warrants an equivalent number of shares equal to 4.99% of the Common Stock of the Company on a fully diluted basis, subject to the terms of the Warrant Agreement governing the 2017 Warrants, dated as of June 29, 2017 (the “Warrant Agreement”); provided, however, the 2017 Warrants may not be converted to the extent that, after giving effect to such conversion, the holders of the 2017 Warrants would beneficially own, in the aggregate, in excess of (i) 19.99% of the shares of Common Stock outstanding as of June 29, 2017 (the “Closing Date”) minus (ii) the shares of Common Stock that were sold pursuant to the Second Quarter 2017 Private Placement (as defined below) (the “Conversion Cap”). The Conversion Cap will not apply to the 2017 Warrants if the Company obtains the approval of its stockholders for the removal of the Conversion Cap, which the Company is required to take certain steps to attempt to obtain, subject to the terms of the Warrant Agreement.
The 2017 Warrants have a 10 year term and an initial exercise price of $2.00 per share, and may be exercised by payment of the exercise price in cash or surrender of shares of Common Stock into which the 2017 Warrants are being converted in an aggregate amount sufficient to pay the exercise price. The exercise price and the number of shares that may be acquired upon exercise of the 2017 Warrants are subject to adjustment in certain situations, including price based anti-dilution protection whereby, subject to certain exceptions, if the Company later issues Common Stock or certain Common Stock Equivalents (as defined in the Warrant Agreement) at a price less than either the then-current market price per share or exercise price of the 2017 Warrants, then the exercise price will be decreased and the percentage of shares of Common Stock issuable upon exercise of the 2017 Warrants will remain the same, giving effect to such issuance. Additionally, the 2017 Warrants have standard anti-dilution protections if the Company effects a stock split, subdivision, reclassification or combination of its Common Stock or fixes a record date for the making of a dividend or distribution to stockholders of cash or certain assets. Upon the occurrence of certain business combinations the 2017 Warrants will be converted into the right to acquire shares of stock or other securities or property (including cash) of the successor entity. The 2017 Warrants are reflected as a liability in other non-current liabilities on the balance sheet and are adjusted to fair value at the end of each reporting period through an adjustment to earnings. The fair value of the 2017 Warrants, subsequent to a remeasurement adjustment of $3.6 million, is $20.5 million at December 31, 2017.
Second Quarter 2017 Private Placement
On June 29, 2017, the Company entered into a Stock Purchase Agreement (the “Second Quarter Stock Purchase Agreement”) with a fund managed by Ares Management L.P. (“Ares” or the “Second Quarter Stock Purchaser”). Pursuant to the terms of the Second Quarter Stock Purchase Agreement, the Company issued and sold to the Second Quarter Stock Purchaser in a private placement (the “Second Quarter 2017 Private Placement”) 6,359,350 shares of Common Stock (the “Second Quarter Shares”) at a price of $2.50 per share, for proceeds of approximately $15.9 million, net of $0.2 million in associated costs.
Second Quarter Registration Rights Agreement
In connection with the 2017 Warrants and the Second Quarter 2017 Private Placement, the Company entered into a Registration Rights Agreement (the “Second Quarter 2017 Registration Rights Agreement”) with the holders of the 2017 Warrants and the Second Quarter Stock Purchaser. Pursuant to the Second Quarter 2017 Registration Rights Agreement, subject to certain exceptions, the Company is required, upon the request of the Second Quarter Stock Purchaser and holders of the 2017 Warrants, to register the resale of the Second Quarter Shares and the shares of Common Stock issuable upon exercise of the 2017 Warrants. Pursuant to the terms of the Second Quarter 2017 Registration Rights Agreement, these registration rights will not become effective until twelve months after the Closing Date, and the costs incurred in connection with such registrations will be borne by the Company.
Shelf Registration Statement
The Company filed a shelf registration statement on Form S-3 under the Securities Act on April 1, 2016, which was declared effective May 2, 2016 (the “2016 Shelf”). Under the 2016 Shelf at the time of effectiveness, the Company had the ability to raise up to $200.0 million, in one or more transactions, by selling Common Stock, preferred stock, debt securities, warrants, units and rights.
Authorized Shares
64
On November 30, 2016, the stockholders of the Company approved an amendment to the Company’s Second Amended and Restated Certificate of Incorporation to increase the number of shares of Common Stock that the Company is authorized to issue from 125 million shares to 250 million shares (the “Charter Amendment”).
Treasury Stock
During the year ended December 31, 2017, 5,106 shares were surrendered to satisfy tax withholding obligations on the vesting of restricted stock awards. The Company did not hold any shares of treasury stock at December 31, 2016 as the balance was utilized to issue shares, reflected as consideration, in the Home Solutions acquisition.
NOTE 5 – ACQUISITIONS
Home Solutions
On September 9, 2016, the Company acquired substantially all of the assets and assumed certain liabilities of Home Solutions, Inc. (HS Infusion Holdings, Inc. and its subsidiaries pursuant to the Asset Purchase Agreement dated June 11, 2016, by and among Home Solutions, a Delaware corporation, certain subsidiaries of Home Solutions, the Company and HomeChoice Partners, Inc., a Delaware corporation) pursuant to the Home Solutions Agreement. Home Solutions, a privately held company, provides home infusion and home nursing products and services to patients suffering from chronic and acute medical conditions. The aggregate consideration paid by the Company in the Transaction was equal to (i) $67.5 million in cash (the “Cash Consideration); plus (ii) (a) 3,750,000 shares of Company common stock (the “Transaction Closing Equity Consideration”) and (b) the right to receive contingent equity securities of the Company, in the form of restricted shares of Company common stock (the “RSUs”), issuable in two tranches, Tranche A and Tranche B, with different vesting conditions (collectively, the “Contingent Shares”). The number of shares of Company common stock in Tranche A will be approximately 3.1 million. The number of shares of Company common stock in Tranche B will be approximately 4.0 million. Upon close of the Transaction the RSUs had no intrinsic value, but were reported as a liability in our consolidated financial statements at their estimated fair value at the date of issuance. Upon approval of the Charter Amendment, as defined below, on November 30, 2016, the date at which sufficient shares were available should the RSUs vest and become issuable, the liability was remeasured to its then-current fair value and reclassified to equity.
The following table sets forth the consideration transferred in connection with the acquisition of Home Solutions as of September 9, 2016 (in thousands):
Cash | $ | 67,516 | |
Equity issued at closing | 9,938 | ||
Capital lease obligation assumed | 301 | ||
Fair value of contingent consideration | 15,400 | ||
Total consideration | $ | 93,155 | |
65
The following table sets forth the estimate of fair value of the assets acquired and liabilities assumed upon acquisition of Home Solutions as of September 9, 2016 (in thousands):
Accounts receivable | $ | 11,956 | |
Inventories | 3,199 | ||
Prepaids and other assets | 852 | ||
Total current assets | $ | 16,007 | |
Property and equipment | 4,350 | ||
Goodwill | 58,468 | ||
Managed care contracts | 24,600 | ||
Licenses | 5,400 | ||
Trade name | 1,800 | ||
Non-compete agreements | 200 | ||
Other long-term assets | 891 | ||
Total assets | $ | 111,716 | |
Accounts payable | 14,575 | ||
Accrued liabilities | 3,986 | ||
Total liabilities | $ | 18,561 | |
Net assets acquired | $ | 93,155 | |
The excess of the purchase price over the fair value of the tangible and identifiable intangible assets acquired and liabilities assumed in the acquisition was allocated to goodwill. The value of the goodwill represents the value the Company expects to be created by combining the operations of the companies, including the ability to cross-sell its services on a national basis with an expanded footprint in home infusion and the opportunity to focus on higher margin therapies.
In accordance with ASC Topic 805 Business Combinations (“ASC 805”), the allocation of the purchase price is subject to adjustment during the measurement period after the closing date (September 9, 2016) when additional information on assets and liability valuations becomes available. During the measurement period, the Company recorded adjustments to the fair value of assumed liabilities and goodwill based on revised estimates of the shortfall amount described below. The Company finalized its valuation of certain assets and liabilities recorded pursuant to the acquisition including intangible assets and contingent consideration.
Under the Home Solutions Agreement, the Company did not purchase, among other things, any accounts receivable associated with governmental payors. However, the Home Solutions Agreement stipulates that collections of government receivables, as of the first anniversary of the closing date, in an amount less than the amount estimated as government receivables in the Closing Certificate (such difference, the “Shortfall Amount”), must be paid by the Company to the seller. On October 4, 2017, the Company and Home Solutions agreed to defer the measurement of the Shortfall Amount from the first anniversary of the closing date to December 31, 2017 in exchange for a payment by the Company of $0.5 million, which would be credited toward any amount ultimately owed to Home Solutions. The Company also recognized, as of September 30, 2017, a liability of $0.3 million, reflected in current liabilities and allocated in the purchase price, in anticipation of a shortfall in actual collections. As of December 31, 2017, the Shortfall Amount was $0.4 million, and an additional $0.1 million is reflected in current liabilities and earnings in anticipation of payment of the shortfall in collections of accounts receivable associated with governmental payors.
Acquisition and Integration Expense
66
Acquisition and integration expenses in restructuring, acquisition, integration, and other expenses, net in the accompanying Consolidated Statements of Operations for the years ended December 31, 2017, 2016 and 2015 include the following costs related to the Home Solutions, CarePoint Business, and the HomeChoice acquisitions (in thousands):
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Legal and professional fees | $ | 528 | $ | 3,059 | $ | 1,033 | |||||
Financial advisory fees | — | 5,087 | — | ||||||||
Facilities consolidation and discontinuation | — | 1,323 | 488 | ||||||||
Other | — | 653 | 219 | ||||||||
Total | $ | 528 | $ | 10,122 | $ | 1,740 | |||||
NOTE 6 – DISCONTINUED OPERATIONS
Sale of PBM Services
On August 27, 2015, the Company completed the sale of substantially all of the Company’s PBM Services segment (as defined above, the “PBM Business”) pursuant to an Asset Purchase Agreement dated as of August 9, 2015 (the “Asset Purchase Agreement”), by and among the Company, BioScrip PBM Services, LLC and ProCare Pharmacy Benefit Manager Inc. (the “PBM Buyer”).
The operating results included in discontinued operations of the PBM Business for the years ended December 31, 2017, 2016 and 2015 are summarized as follows (in thousands):
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Revenue | $ | — | $ | — | $ | 44,375 | |||||
Gross profit | $ | — | $ | — | $ | 9,763 | |||||
Other operating expenses | — | 1,015 | 5,444 | ||||||||
Bad debt expense | — | — | (45 | ) | |||||||
(Loss) income from operations | — | (1,015 | ) | 4,364 | |||||||
Gain on sale before income taxes | — | — | (11,424 | ) | |||||||
Financial advisory fee and legal expenses | — | 614 | 1,731 | ||||||||
Other income and (expenses), net | (893 | ) | 4,922 | 928 | |||||||
(Loss) income before income taxes | (893 | ) | (6,551 | ) | 13,129 | ||||||
Income tax expense | — | — | 206 | ||||||||
(Loss) income from discontinued operations, net of income taxes | $ | (893 | ) | $ | (6,551 | ) | $ | 12,923 | |||
Sale of Home Health Business
On March 31, 2014, the Company completed the sale of substantially all of the Company’s Home Health Services segment (the “Home Health Business”) pursuant to the Stock Purchase Agreement dated as of February 1, 2014 (the “Stock Purchase Agreement”).
The operating results included in discontinued operations of the Home Health Business for the years ended December 31, 2017, 2016 and 2015 are summarized as follows (in thousands):
67
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Revenue | $ | — | $ | — | $ | — | |||||
Gross profit | $ | — | $ | — | $ | — | |||||
Other operating expenses | — | — | 417 | ||||||||
Loss from operations | — | — | (417 | ) | |||||||
Financial advisor fee and legal expenses | — | (44 | ) | — | |||||||
Other costs and expenses | — | (118 | ) | 861 | |||||||
Income (loss) before income taxes | — | 162 | (1,278 | ) | |||||||
Income tax expense (benefit) | — | — | — | ||||||||
Income (loss) from discontinued operations, net of income taxes | $ | — | $ | 162 | $ | (1,278 | ) | ||||
Pharmacy Services Asset Sale
On February 1, 2012, the Company entered into a Community Pharmacy and Mail Business Purchase Agreement by and among Walgreen Co. and certain subsidiaries and the Company and certain subsidiaries (collectively, the “Sellers”) with respect to the sale of certain assets, rights and properties relating to the Sellers’ traditional and specialty pharmacy mail operations and community retail pharmacy stores.
The operating results included in discontinued operations of the divested traditional and specialty pharmacy mail operations and community pharmacies for the years ended December 31, 2017, 2016 and 2015 are summarized as follows (in thousands):
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Revenue | $ | — | $ | — | $ | — | |||||
Gross profit | $ | — | $ | — | $ | — | |||||
Other operating expenses | — | 185 | 4,485 | ||||||||
Legal fees and settlement expense | — | 2 | 1,312 | ||||||||
Other expense, including gain on sale | — | 17 | 1,157 | ||||||||
Loss from discontinued operations, net of income taxes | $ | — | $ | (204 | ) | $ | (6,954 | ) | |||
On December 28, 2016, in response to a lawsuit filed by the Sellers alleging that the Company and certain of its subsidiaries breached certain non-compete provisions contained in the Community Pharmacy and Mail Business Purchase Agreement, an arbitrator awarded Walgreens $5.8 million in damages constituting approximately 3.0% of the total sales Walgreens claimed were made in violation of the agreement. The Company filed a motion to vacate the arbitration award but on July 19, 2017, the Court confirmed the arbitration award. Following that decision, the parties entered into a global settlement of all disputes related to the non-compete provisions and the lawsuit was dismissed. The Company paid the settlement amount in August 2017.
68
NOTE 7 – GOODWILL AND INTANGIBLE ASSETS
Goodwill, and the changes in the carrying amount of goodwill for the years ended December 31, 2017 and 2016, are as follows (in thousands):
Infusion Services | |||
Balance at December 31, 2015 | $ | 308,729 | |
Acquisition of Home Solutions | 57,218 | ||
Balance at December 31, 2016 | 365,947 | ||
Adjustments associated with the acquisition of Home Solutions | 1,251 | ||
Balance at December 31, 2017 | $ | 367,198 | |
The Company evaluates goodwill for impairment on an annual basis and whenever events or circumstances exist that indicates that the carrying value of goodwill may no longer be recoverable. Management may choose to undertake a qualitative assessment (step zero approach) in order to assess whether a quantitative analysis is required. In determining whether management will utilize the qualitative assessment in any one year, management will consider overall economic factors as well as the passage of time between the last quantitative assessment. In January 2017, the FASB issued authoritative guidance that simplifies the measurement of goodwill impairment to a single-step test. The guidance eliminates step two of the goodwill impairment test; the measurement of goodwill impairment will now be the amount by which a reporting unit’s carrying value exceeds its fair value, not to exceed the carrying amount of goodwill. Under the revised guidance, failing step one will result in goodwill impairment. The Company adopted the new guidance on January 1, 2017 on a prospective basis.
During the third quarter of 2015, the Company recorded a total impairment charge of $251.9 million year to date, all of which related to our Infusion Services reporting unit. The Company evaluated goodwill for possible impairment during the years ending December 31, 2017 and 2016 and concluded no additional impairment charge was needed.
Intangible assets consisted of the following as of December 31, 2017 and 2016 (in thousands):
December 31, 2017 | December 31, 2016 | ||||||||||||||||||||||
Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | ||||||||||||||||||
Finite Lived Assets | |||||||||||||||||||||||
Infusion customer relationships | $ | 25,650 | $ | (25,650 | ) | $ | — | $ | 25,650 | $ | (23,768 | ) | $ | 1,882 | |||||||||
Managed care contracts | 25,000 | (8,403 | ) | 16,597 | 24,700 | (1,898 | ) | 22,802 | |||||||||||||||
Licenses | 5,400 | (3,681 | ) | 1,719 | 5,400 | (906 | ) | 4,494 | |||||||||||||||
Trade name | 1,800 | (1,181 | ) | 619 | 1,800 | (281 | ) | 1,519 | |||||||||||||||
Non-compete agreements | 1,700 | (1,521 | ) | 179 | 1,700 | (1,354 | ) | 346 | |||||||||||||||
$ | 59,550 | $ | (40,436 | ) | $ | 19,114 | $ | 59,250 | $ | (28,207 | ) | $ | 31,043 | ||||||||||
Finite lived intangible assets are amortized on a straight-line basis over their estimated useful lives as follows:
Estimated Useful Life | |||||||
Infusion customer relationships | 5 | months | - | 4 | years | ||
Managed care contracts | 4 | years | |||||
Licenses | 2 | years | |||||
Trade name | 2 | years | |||||
Non-compete agreements | 1 | year | - | 5 | years | ||
69
Total amortization expense of intangible assets was $11.8 million, $6.2 million, and $5.1 million for the years ended December 31, 2017, 2016, and 2015, respectively. Amortization expense is expected to be the following (in thousands):
Year ending December 31, | Estimated Amortization | ||
2018 | $ | 8,644 | |
2019 | 6,218 | ||
2020 | 4,252 | ||
2021 | — | ||
2022 | — | ||
Thereafter | — | ||
Total estimated amortization expense | $ | 19,114 | |
NOTE 8 – RESTRUCTURING, ACQUISITION, INTEGRATION, AND OTHER EXPENSE, NET
Restructuring, acquisition, integration and other expenses include costs associated with restructuring, acquisition and integration initiatives such as employee severance costs, certain legal and professional fees, training costs, redundant wage costs, impacts recorded from the change in contingent consideration obligations, and other costs related to contract terminations and closed branches/offices.
Restructuring, acquisition, integration, and other expenses, net in the Consolidated Statements of Operations for the years ended December 31, 2017, 2016, and 2015 consisted of the following (in thousands):
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Restructuring and other expense | $ | 12,134 | $ | 10,334 | $ | 22,635 | |||||
Acquisition and integration expenses | 528 | 10,122 | 1,740 | ||||||||
Change in fair value of contingent consideration | — | (4,597 | ) | 30 | |||||||
Total restructuring, acquisition, integration, and other expenses, net | 12,662 | 15,859 | 24,405 | ||||||||
NOTE 9 – PROPERTY AND EQUIPMENT
Property and equipment consists of the following (in thousands):
December 31, | |||||||
2017 | 2016 | ||||||
Computer and office equipment | $ | 31,371 | $ | 30,060 | |||
Software capitalized for internal use | 17,470 | 16,481 | |||||
Vehicles | 2,379 | 2,552 | |||||
Medical equipment | 36,230 | 32,086 | |||||
Work in progress | 2,478 | 4,370 | |||||
Furniture and fixtures | 5,534 | 5,319 | |||||
Leasehold improvements | 19,809 | 17,496 | |||||
Property and equipment, gross | 115,271 | 108,364 | |||||
Less: Accumulated depreciation | (88,298 | ) | (75,686 | ) | |||
Property and equipment, net | $ | 26,973 | $ | 32,678 | |||
Depreciation expense, including expense related to assets under capital lease, for the years ended December 31, 2017, 2016 and 2015 was $15.9 million, $15.8 million, and $17.7 million, respectively.
70
Impairment
The Company, which assesses the impairment of its assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable, has determined that no such events or changes have occurred and therefore, no impairment charge in relation to property, plant and equipment was incurred during the year ended December 31, 2017.
NOTE 10 – DEBT
As of December 31, 2017 and 2016, the Company’s debt consisted of the following (in thousands):
December 31, | |||||||
2017 | 2016 | ||||||
First Lien Note Facility, net of unamortized discount | $ | 198,324 | $ | — | |||
Second Lien Note Facility, net of unamortized discount | 85,694 | — | |||||
2021 Notes, net of unamortized discount | 197,363 | 196,670 | |||||
Revolving Credit Facility | — | 55,300 | |||||
Term Loan Facilities | — | 210,207 | |||||
Capital leases | 2,863 | 2,209 | |||||
Less: Deferred financing costs | (3,656 | ) | (12,452 | ) | |||
Total Debt | 480,588 | 451,934 | |||||
Less: Current portion | (1,722 | ) | (18,521 | ) | |||
Long-term debt, net of current portion | $ | 478,866 | $ | 433,413 | |||
Debt Facilities
The Company was previously obligated under (i) a senior secured first-lien revolving credit facility in an aggregate principal amount of $75.0 million (the “Revolving Credit Facility”), (ii) a senior secured first-lien term loan B in an aggregate principal amount of $250.0 million (the “Term Loan B Facility”) and (iii) a senior secured first-lien delayed draw term loan B in an aggregate principal amount of $150.0 million (the “Delayed Draw Term Loan Facility” and, together with the Revolving Credit Facility and the Term Loan B Facility, the “Senior Credit Facilities”) with SunTrust Bank (“SunTrust”), Jefferies Finance LLC and Morgan Stanley Senior Funding, Inc., originally entered on July 31, 2013 and amended from time to time.
On January 6, 2017, the Company entered into a credit agreement (the “Priming Credit Agreement” and, together with the Senior Credit Facilities, the “Prior Credit Agreements”) with certain existing lenders under the Senior Credit Facilities and SunTrust, as administrative agent for itself and the lenders. The Priming Credit Agreement provided an aggregate borrowing commitment of $25.0 million, which was fully drawn at closing.
On June 29, 2017 (the “Closing Date”), the Company entered into (i) a first lien note purchase agreement (the “First Lien Note Facility”), among the Company, which is the issuer under the agreement, the financial institutions and note purchasers from time to time party to the agreement (the “First Lien Note Purchasers”), and Wells Fargo Bank, National Association, in its capacity as collateral agent for itself and the First Lien Note Purchasers (the “First Lien Collateral Agent”), pursuant to which the Company issued first lien senior secured notes in an aggregate principal amount of $200.0 million (the “First Lien Notes”); and (ii) a second lien note purchase agreement (the “Second Lien Note Facility” and, together with the First Lien Note Facility, the “Notes Facilities”) among the Company, which is the issuer under the agreement, the financial institutions and note purchasers from time to time party to the agreement (the “Second Lien Note Purchasers”), and Wells Fargo Bank, National Association, in its capacity as collateral agent for itself and the Second Lien Note Purchasers (the “Second Lien Collateral Agent” and, together with the First Lien Collateral Agent, the “Collateral Agent”), pursuant to which the Company (a) issued second lien senior secured notes in an aggregate initial principal amount of $100.0 million (the “Initial Second Lien Notes”) and (b) has the ability to draw upon the Second Lien Note Facility and issue second lien delayed draw senior secured notes in an aggregate initial principal amount of $10.0 million for a period of 18 months after the Closing Date, subject to certain terms and conditions (the “Second Lien Delayed Draw Notes” and, together with the Initial Second Lien Notes, the “Second Lien Notes”; the Second Lien Notes, together with the First Lien Notes, the “Notes”). Funds managed by Ares are acting as lead purchasers for the Notes Facilities.
The Company used the proceeds of the sale of the First Lien Notes and the Initial Second Lien Notes pursuant to the Notes Facilities to repay in full all amounts outstanding under the Prior Credit Agreements and extinguished the liability. Each of the
71
Prior Credit Agreements was terminated following such repayment. The Company used the remaining proceeds of $15.9 million of the Notes Facilities, net of $0.2 million in issuance costs, from the Notes Facilities and the Second Quarter 2017 Private Placement for working capital and general corporate purposes.
The First Lien Notes accrue interest, payable monthly in arrears, at a floating rate or rates equal to, at the option of the Company, (i) the base rate (defined as the highest of the Federal Funds Rate plus 0.5% per annum, the Prime Rate as published by The Wall Street Journal and the one-month London Interbank Offered Rate (“LIBOR”) (subject to a 1.0% floor) plus 1.0%), or (ii) the one-month LIBOR rate (subject to a 1.0% floor), plus a margin of 6.0% if the base rate is selected or 7.0% if the LIBOR Option is selected. The First Lien Notes mature on August 15, 2020, provided that if the Company’s existing 8.875% Senior Notes due 2021 (the “2021 Notes”) are refinanced prior to August 15, 2020, then the scheduled maturity date of the First Lien Notes shall be June 30, 2022.
The First Lien Notes amortize in equal quarterly installments equal to 0.625% of the aggregate principal amount of the First Lien Note Facility, commencing on September 30, 2019, and on the last day of each third month thereafter, with the balance payable at maturity. The First Lien Notes are pre-payable at the Company’s option at specified premiums to the principal amount that will decline over the term of the First Lien Note Facility. If the First Lien Notes are prepaid prior to the second anniversary of the Closing Date, the Company will be required to pay a make-whole premium based on the present value (using a discount rate based on the specified treasury rate plus 50 basis points) of all remaining interest payments on the First Lien Notes being prepaid prior to the second anniversary of the Closing Date, plus 4.0% of the principal amount of First Lien Notes being prepaid. On or after the second anniversary of the Closing Date, the prepayment premium is 4.0%, which declines to 2.0% on or after the third anniversary of the Closing Date, and declines to 0.0% on or after the fourth anniversary of the Closing Date. At any time, the Company may pre-pay up to $50.0 million in aggregate principal amount of the First Lien Notes from internally generated cash without incurring any make-whole or prepayment premium. The occurrence of certain events of default may increase the applicable rate of interest by 2.0% and could result in the acceleration of the Company’s obligations under the First Lien Note Facility prior to stated maturity and an obligation of the Company to pay the full amount of its obligations under the First Lien Note Facility.
The First Lien Note Facility contains customary events of default that include, among others, non-payment of principal, interest or fees, violation of covenants, inaccuracy of representations and warranties, bankruptcy and insolvency events, material judgments, cross-defaults to material indebtedness and events constituting a change of control. In addition, the obligations under the First Lien Note Facility are guaranteed by joint and several guarantees from the Company’s subsidiaries.
In connection with the First Lien Note Facility, the Company, its subsidiaries and the First Lien Collateral Agent entered into a First Lien Guaranty and Security Agreement, dated as of June 29, 2017 (the “First Lien Guaranty and Security Agreement”). Pursuant to the First Lien Guaranty and Security Agreement, the obligations under the First Lien Notes are secured by first priority liens on, and security interests in, substantially all of the assets of the Company and its subsidiaries.
The Second Lien Notes accrue interest, payable monthly in arrears, at a floating rate or rates equal to, at the option of the Company, (i) one-month LIBOR (subject to a 1.25% floor) plus 9.25% per annum in cash, (ii) one-month LIBOR (subject to a 1.25% floor) plus 11.25% per annum, which amount will be capitalized on each interest payment date, or (iii) one-month LIBOR (subject to a 1.25% floor) plus 10.25% per annum, of which one-half LIBOR plus 4.625% per annum will be payable in cash and one-half LIBOR plus 5.625% per annum will be capitalized on each interest payment date, provided that, in each case, if any permitted refinancing indebtedness with which the 2021 Notes are refinanced requires or permits the payment of cash interest, all of the interest on the Second Lien Notes shall be paid in cash. The Second Lien Notes mature on August 15, 2020, provided that if the 2021 Notes are refinanced prior to August 15, 2020, then the scheduled maturity date of the Second Lien Notes shall be June 30, 2022.
In connection with the Second Lien Note Facility, the Company also issued the 2017 Warrants to the purchasers of the Second Lien Notes pursuant to the Warrant Purchase Agreement. The 2017 Warrants entitle the purchasers of the 2017 Warrants to purchase shares of Common Stock, representing at the time of any exercise of the 2017 Warrants an equivalent number of shares equal to 4.99% of the Common Stock of the Company on a fully diluted basis, subject to the terms of the Warrant Agreement. The 2017 Warrants, considered a derivative and subject to remeasurement at each reporting period, are reflected in other non-current liabilities in the consolidated balance sheet. The 2017 Warrants, subsequent to a remeasurement adjustment of $3.6 million, are carried at a fair value of $20.5 million at December 31, 2017.
The Second Lien Notes are not subject to scheduled amortization installments. The Second Lien Notes are pre-payable at the Company’s option at specified premiums to the principal amount that will decline over the term of the Second Lien Note Facility. If the Second Lien Notes are prepaid prior to the third anniversary of the Closing Date, the Company will need to pay a make-whole premium based on the present value (using a discount rate based on the specified treasury rate plus 50 basis points) of all remaining interest payments on the Second Lien Notes being prepaid prior to the third anniversary of the Closing Date, plus 4.0%
72
of the principal amount of Second Lien Notes being prepaid. On or after the third anniversary of the Closing Date, the prepayment premium is 4.0%, which declines to 2.0% on or after the fourth anniversary of the Closing Date, and declines to 0.0% on or after the fifth anniversary of the Closing Date. The occurrence of certain events of default may increase the applicable rate of interest by 2.0% and could result in the acceleration of the Company’s obligations under the Second Lien Note Facility prior to stated maturity and an obligation of the Company to pay the full amount of its obligations under the Second Lien Note Facility.
The Second Lien Note Facility contains customary events of default that include, among others, non-payment of principal, interest or fees, violation of covenants, inaccuracy of representations and warranties, bankruptcy and insolvency events, material judgments, cross-defaults to material indebtedness and events constituting a change of control. In addition, the obligations under the Second Lien Note Facility are guaranteed by joint and several guarantees from the Company’s subsidiaries.
In connection with the Second Lien Note Facility, the Company, its subsidiaries and the Second Lien Collateral Agent entered into a Second Lien Guaranty and Security Agreement, dated as of June 29, 2017 (the “Second Lien Guaranty and Security Agreement”). Pursuant to the Second Lien Guaranty and Security Agreement, the obligations under the Second Lien Notes are secured by second priority liens on, and security interests in, substantially all of the assets of the Company and its subsidies.
In connection with the First Lien Note Facility and the Second Lien Note Facility, the Company, the First Lien Collateral Agent and the Second Lien Collateral Agent, entered into an intercreditor agreement containing customary provisions to, among other things, subordinate the lien priority of the liens granted under the Second Lien Note Facility to the liens granted under the First Lien Note Facility.
2021 Notes
On February 11, 2014, the Company issued $200.0 million aggregate principal amount of the 2021 Notes. The 2021 Notes are senior unsecured obligations of the Company and are fully and unconditionally guaranteed by all existing and future subsidiaries of the Company. The 2021 Notes were offered in the United States to qualified institutional buyers in reliance on Rule 144A under the Securities Act of 1933, as amended (the “Securities Act”), and outside the United States to non-U.S. persons in reliance on Regulation S under the Securities Act pursuant to an Indenture (the “2021 Notes Indenture”), dated February 11, 2014, by and among the Company, the guarantors named therein and U.S. Bank National Association, as trustee.
Interest on the 2021 Notes accrues at a fixed rate of 8.875% per annum and is payable in cash semi-annually, in arrears, on February 15 and August 15 of each year, commencing on August 15, 2014. The debt discount of $5.0 million at issuance is being amortized as interest expense through maturity which will result in the accretion over time of the outstanding debt balance to the principal amount. The 2021 Notes are the Company’s senior unsecured obligations and rank equally in right of payment with all of its other existing and future senior unsecured indebtedness and senior in right of payment to all of its existing and future subordinated indebtedness.
The 2021 Notes are guaranteed on a full, joint and several basis by each of the Company’s existing and future domestic restricted subsidiaries that is a borrower under any of the Company’s credit facilities or that guarantees any of the Company’s debt or that of any of its restricted subsidiaries, in each case incurred under the Company’s credit facilities. As of December 31, 2017, the Company does not have any independent assets or operations, and as a result, its direct and indirect subsidiaries (other than minor subsidiaries), each being 100% owned by the Company, are fully and unconditionally, jointly and severally, providing guarantees on a senior unsecured basis to the 2021 Notes.
The 2021 Notes Indenture contains covenants that, among other things, limit the Company’s ability and the ability of certain of the Company’s subsidiaries to (i) grant liens on its assets, (ii) make dividend payments, other distributions or other restricted payments, (iii) incur restrictions on the ability of the Company’s restricted subsidiaries to pay dividends or make other payments, (iv) enter into sale and leaseback transactions, (v) merge, consolidate, transfer or dispose of substantially all of their assets, (vi) incur additional indebtedness, (vii) make investments, (viii) sell assets, including capital stock of subsidiaries, (ix) use the proceeds from sales of assets, including capital stock of restricted subsidiaries, and (x) enter into transactions with affiliates. In addition, the 2021 Notes Indenture requires, among other things, the Company to provide financial and current reports to holders of the 2021 Notes or file such reports electronically with the U.S. Securities and Exchange Commission (the “SEC”). These covenants are subject to a number of exceptions, limitations and qualifications set forth in the 2021 Notes Indenture.
Pursuant to the terms of the Second Amendment to the Senior Credit Facilities, the Company used the net proceeds of the 2021 Notes of approximately $194.5 million to repay $59.3 million of the Revolving Credit Facility and $135.2 million of the Term Loan Facilities.
Fair Value of Financial Instruments
73
The following details our financial instruments where the carrying value and the fair value differ:
Financial Instrument | Carrying Value as of December 31, 2017 | Markets for Identical Item (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | ||||||||
First Lien Note Facility | $ | 198,324 | $ | — | $ | — | $ | 200,578 | ||||
Second Lien Note Facility | 85,694 | — | — | 100,850 | ||||||||
2017 Warrants | 20,495 | — | 20,495 | — | ||||||||
2021 Notes | 197,363 | — | 183,561 | — | ||||||||
Total | $ | 501,876 | $ | — | $ | 204,056 | $ | 301,428 | ||||
The fair value hierarchy for disclosure of fair value measurements is as follows:
Level 1: Quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: Quoted prices, other than quoted prices included in Level 1, which are observable for the assets or liabilities, either directly or indirectly.
Level 3: Inputs that are unobservable for the assets or liabilities.
Financial assets with carrying values approximating fair value include cash and cash equivalents and accounts receivable. Financial liabilities with carrying values approximating fair value include accounts payable and capital leases. The carrying value of these financial assets and liabilities approximates fair value due to their short maturities.
Deferred Financing Costs
In connection with the Note Facilities and the 2021 Notes, the Company incurred underwriting fees, agent fees, legal fees and other expenses of approximately $4.1 million and $0.5 million, respectively. The deferred financing costs are reflected as additional issuance costs and amortized as a component of interest expense over the remaining term of the Note Facilities using the effective interest method.
Future Maturities
The estimated future maturities of the Company’s long-term debt, inclusive of $3.7 million in deferred financing costs and $2.6 million of unamortized discount on the 2021 notes, as of December 31, 2017, are as follows (in thousands):
Year Ending December 31, | Amount | |||
2018 | $ | 1,722 | ||
2019 | 3,254 | |||
2020 | 297,887 | |||
2021 | 200,000 | |||
2022 | — | |||
Thereafter | — | |||
Total future maturities | $ | 502,863 | ||
Interest Expense
The weighted average interest rate on the Company’s short-term borrowings under its Revolving Credit Facility during the years ended December 31, 2017 and 2016 was 10.3%.
Liquidity
As of the filing of this Annual Report, we expect that our cash on hand, cash from operations, and available borrowing under the Second Lien Delayed Draw Senior Secured Notes will be sufficient to fund our anticipated working capital, scheduled interest repayments and other cash needs for at least the next 12 months.
74
NOTE 11 – COMMITMENTS AND CONTINGENCIES
Legal Proceedings
The Company is a party to various legal, regulatory and governmental proceedings incidental to its business. Based on current knowledge, management does not believe that loss contingencies arising from pending legal, regulatory and governmental matters, including the matters described herein, will have a material adverse effect on the consolidated financial position or liquidity of the Company. However, in light of the inherent uncertainties involved in pending legal, regulatory and governmental matters, some of which are beyond the Company’s control, and the indeterminate damages sought in some of these matters, an adverse outcome in one or more of these matters could be material to the Company’s results of operations or cash flows for any particular reporting period.
With respect to all legal, regulatory and governmental proceedings, the Company considers the likelihood of a negative outcome. If the Company determines the likelihood of a negative outcome with respect to any such matter is probable and the amount of the loss can be reasonably estimated, the Company records an accrual for the estimated loss for the expected outcome of the matter. If the likelihood of a negative outcome with respect to material matters is reasonably possible and the Company is able to determine an estimate of the possible loss or a range of loss, whether in excess of a related accrued liability or where there is no accrued liability, the Company discloses the estimate of the possible loss or range of loss. However, the Company is unable to estimate a possible loss or range of loss in some instances based on the significant uncertainties involved in, and/or the preliminary nature of, certain legal, regulatory and governmental matters.
Breach of Contract Litigation in the Delaware Court of Chancery
On November 3, 2015, Walgreen Co. and various affiliates (“Walgreens”) filed a lawsuit in the Delaware Court of Chancery against the Company and certain of its subsidiaries (collectively, the “Defendants”). The complaint alleges that the Company breached certain non-compete provisions contained in the Community Pharmacy and Mail Business Purchase Agreement dated as of February 1, 2012, by and among Walgreens and certain subsidiaries and the Company and certain subsidiaries. The complaint seeks both money damages and injunctive relief. On December 7, 2015, the Defendants filed a motion to dismiss the case. Walgreens filed an answering brief on January 11, 2016, and the Defendants filed a reply on January 25, 2016. On March 11, 2016, the Court held oral argument on the Company’s motion to dismiss and granted the motion, holding that Walgreens’ breach of contract claims for money damages must be resolved in accordance with the 2012 Purchase Agreement’s alternative dispute resolution procedure. On March 15, 2016, Walgreens informed the Court that it would not be pursuing any claims for injunctive relief in the Court at that time, but instead would engage in the required alternative dispute resolution procedure. Walgreens requested that the Court keep the case open pending the results of that process. On March 16, 2016, the Court stayed the lawsuit and removed the trial from its calendar, but did not grant Walgreens any other relief or enjoin the Company from taking any action. On December 8, 2016, the parties submitted the dispute to an arbitrator. On December 28, 2016, the arbitrator rendered its decision, finding that the Company had not violated the non-compete, except for certain limited sales of oral oncology, HIV and transplant pharmaceuticals, constituting approximately 3 percent of the total sales that Walgreens claimed were made in violation of the agreement. The arbitrator also concluded that Walgreens was not entitled to recover its lost profits or lost revenues as a result of any such sales. Despite that ruling, the arbitrator awarded Walgreens $5.8 million in damages, or approximately 20 percent of the total amount requested. On January 13, 2017, the Company filed a motion to vacate the arbitration award. On February 10, 2017, Walgreens opposed the Company’s motion and filed a motion to confirm the arbitration award and for other relief. On July 19, 2017, the Court confirmed the arbitration award and denied Walgreens’ request for injunctive relief. Following that decision, the parties entered into a global settlement of all disputes related to the non-compete provisions and the lawsuit was dismissed. The Company paid the settlement amount in August 2017.
Derivative Lawsuit in the Delaware Court of Chancery
On May 7, 2015, a derivative complaint was filed in the Delaware Court of Chancery (the “Derivative Complaint”) by the Park Employees’ & Retirement Board Employees’ Annuity & Benefit Fund of Chicago (the “Derivative Plaintiff”). The Derivative Complaint names as defendants certain current and former directors of the Company, consisting of Richard M. Smith, Myron Holubiak, Charlotte Collins, Samuel Frieder, David Hubers, Richard Robbins, Stuart Samuels and Gordon Woodward (collectively, the “Director Defendants”), certain current and former officers of the Company, consisting of Kimberlee Seah, Hai Tran and Patricia Bogusz (collectively the “Officer Defendants”), Kohlberg & Co., L.L.C., Kohlberg Management V, L.L.C., Kohlberg Investors V, L.P., Kohlberg Partners V, L.P., Kohlberg TE Investors V, L.P., KOCO Investors V, L.P., and Jefferies LLC. The Company is also named as a nominal defendant in the Derivative Complaint. The Derivative Complaint was filed in the Delaware Court of Chancery as Park Employees and Retirement Board Employees’ Annuity and Benefit Fund of Chicago v. Richard M. Smith, Myron Z. Holubiak, Charlotte W. Collins, Samuel P. Frieder, David R. Huber, Richard L. Robbins, Stuart A. Samuels, Gordon H. Woodward, Kimberlee C. Seah, Hai V.Tran, Patricia Bogusz, Kohlberg & Co., L.L.C., Kohlberg Management V, L.L.C.,
75
Kohlberg Investors V, L.P., Kohlberg Partners V, L.P., Kohlberg TE Investors V, L.P., KOCO Investors V, L.P., Jefferies LLC and BioScrip, Inc., C.A. No. 11000-VCG (Del. Ch. Ct., May 7, 2015).
The Derivative Complaint alleged generally that certain defendants breached their fiduciary duties with respect to the Company’s public disclosures, oversight of Company operations, secondary stock offerings and stock sales. The Derivative Complaint also contended that certain defendants aided and abetted those alleged breaches. The damages sought were not quantified but included, among other things, claims for money damages, restitution, disgorgement, equitable relief, reasonable attorneys’ fees, costs and expenses, and interest. The Derivative Complaint incorporated the same factual allegations from In re BioScrip, Inc., Securities Litigation. On April 18, 2017, the Court granted the defendants’ motion to dismiss, and on November 27, 2017 the Delaware Supreme Court affirmed the dismissal. Additional demands and lawsuits related to the same facts and circumstances, however, could be pursued in the future. In that event, there is no assurance that any defenses will be successful or that insurance will be available or adequate to fund any settlement, judgment or litigation costs associated with this action. Certain of the defendants may also seek indemnification from the Company pursuant to certain indemnification agreements, for which there may be no insurance coverage.
While no assurance can be given as to the ultimate outcome of this matter, the Company believes that the final resolution of this action is not likely to have a material adverse effect on results of operations, financial position, liquidity or capital resources.
On December 18, 2017, a commercial payor of the Company sent a letter that claimed an alleged breach of the Company’s obligation under its provider contracts. No legal proceeding has been filed. The Company is not able to estimate the amount of any possible loss. The Company believes this claim is without merit and intends to vigorously defend against this claim if any such legal proceeding is commenced.
Government Regulation
Various federal and state laws and regulations affecting the healthcare industry do or may impact the Company’s current and planned operations, including, without limitation, federal and state laws prohibiting kickbacks in government health programs, federal and state antitrust and drug distribution laws, and a wide variety of consumer protection, insurance and other state laws and regulations. While management believes the Company is in substantial compliance with all existing laws and regulations material to the operation of its business, such laws and regulations are often uncertain in their application to our business practices as they evolve and are subject to rapid change. As controversies continue to arise in the healthcare industry, federal and state regulation and enforcement priorities in this area can be expected to increase, the impact of which cannot be predicted.
From time to time, the Company responds to investigatory subpoenas and requests for information from governmental agencies and private parties. The Company cannot predict with certainty what the outcome of any of the foregoing might be. While the Company believes it is in substantial compliance with all laws, rules and regulations that affects its business and operations, there can be no assurance that the Company will not be subject to scrutiny or challenge under one or more existing laws or that any such challenge would not be successful. Any such challenge, whether or not successful, could have a material effect upon the Company’s Consolidated Financial Statements. A violation of the federal Anti-Kickback Statute, for example, may result in substantial criminal penalties, as well as suspension or exclusion from the Medicare and Medicaid programs. Moreover, the costs and expenses associated with defending these actions, even where successful, can be significant. Further, there can be no assurance the Company will be able to obtain or maintain any of the regulatory approvals that may be required to operate its business, and the failure to do so could have a material effect on the Company’s Consolidated Financial Statements.
Leases
The Company leases its facilities and certain equipment under various operating leases with third parties. The majority of these leases contain escalation clauses that increase base rent payments based upon either the Consumer Price Index or an agreed upon schedule.
In addition, the Company utilizes capital leases agreements with third parties to obtain certain assets such as telecommunications equipment and vehicles. Interest rates on capital leases are both fixed and variable and range from 3% to 7%.
76
As of December 31, 2017, future minimum lease payments under operating and capital leases were as follows (in thousands):
Operating Leases | Capital Leases | Total | |||||||||
2018 | $ | 7,739 | $ | 1,722 | $ | 9,461 | |||||
2019 | 5,010 | 754 | 5,764 | ||||||||
2020 | 3,688 | 387 | 4,075 | ||||||||
2021 | 2,559 | — | 2,559 | ||||||||
2022 | 1,829 | — | 1,829 | ||||||||
2023 and Thereafter | 4,891 | — | 4,891 | ||||||||
Total Future Minimum Lease Payments | $ | 25,716 | $ | 2,863 | $ | 28,579 | |||||
Rent expense for leased facilities and equipment was approximately $7.7 million, $7.3 million and $7.2 million for the years ended December 31, 2017, 2016 and 2015, respectively
Purchase Commitments
As of December 31, 2017, the Company had no outstanding purchase commitments.
NOTE 12 – CONCENTRATION OF RISK
Customer and Credit Concentration Risk
The Company provides trade credit to its customers in the normal course of business. One commercial payor, United Healthcare, accounted for approximately 18%, 24% and 26% of revenue during the years ended December 31, 2017, 2016 and 2015, respectively. Medicare accounted for 7%, 8% and 7% of revenue during the years ended December 31, 2017, 2016 and 2015, respectively.
NOTE 13 – INCOME TAXES
The Company’s federal and state income tax benefit (expense) from continuing operations is summarized in the following table (in thousands):
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Current | |||||||||||
Federal | $ | 925 | $ | — | $ | — | |||||
State | (174 | ) | 30 | 76 | |||||||
Total current | 751 | 30 | 76 | ||||||||
Deferred | |||||||||||
Federal | 1,951 | (1,744 | ) | 18,293 | |||||||
State | 1,428 | (301 | ) | 3,163 | |||||||
Total deferred | 3,379 | (2,045 | ) | 21,456 | |||||||
Total tax benefit (expense) | $ | 4,130 | $ | (2,015 | ) | $ | 21,532 | ||||
77
The effect of temporary differences that give rise to a significant portion of deferred taxes is as follows (in thousands):
December 31, | |||||||
2017 | 2016 | ||||||
Deferred tax assets: | |||||||
Reserves not currently deductible | $ | 10,707 | $ | 19,249 | |||
Net operating loss carryforwards | 110,773 | 122,420 | |||||
Goodwill and intangibles (tax deductible) | 12,757 | 25,268 | |||||
Accrued expenses | 95 | 467 | |||||
Property basis differences | 2,813 | 2,578 | |||||
Stock based compensation | 2,371 | 6,887 | |||||
Other | — | 638 | |||||
Total deferred tax assets | 139,516 | 177,507 | |||||
Deferred tax liabilities: | |||||||
Other | (180 | ) | — | ||||
Less: valuation allowance | (138,238 | ) | (179,788 | ) | |||
Net deferred tax asset | 1,098 | (2,281 | ) | ||||
Deferred taxes | $ | 1,098 | $ | (2,281 | ) | ||
The Company continually assesses the necessity of a valuation allowance. Based on this assessment, the Company concluded that a valuation allowance, in the amount of $138.2 million and $179.8 million, was required as of December 31, 2017 and 2016, respectively. If the Company determines in a future period that it is more likely than not that part or all of the deferred tax assets will be realized, the Company will reverse part or all of the valuation allowance.
At December 31, 2017, the Company had federal net operating loss (“NOL”) carryforwards of approximately $410.3 million, of which $12.9 million is subject to an annual limitation, which will begin expiring in 2026 and later. The Company has post-apportioned state NOL carryforwards of approximately $450.4 million, the majority of which will begin expiring in 2018 and later.
The Company’s reconciliation of the statutory rate to the effective income tax rate from continuing operations is as follows (in thousands):
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Tax benefit at statutory rate | $ | 23,654 | $ | 11,907 | $ | 114,222 | |||||
State tax benefit, net of federal taxes | 4,587 | 1,398 | 8,414 | ||||||||
Change in valuation allowance | 41,550 | (14,725 | ) | (57,567 | ) | ||||||
Change in tax contingencies | 10 | 66 | 37 | ||||||||
Alternative minimum tax receivable | 925 | — | — | ||||||||
Corporate tax rate changes | (67,707 | ) | — | — | |||||||
Goodwill impairment | — | — | (43,362 | ) | |||||||
Other | 1,111 | (661 | ) | (212 | ) | ||||||
Tax benefit (expense) | $ | 4,130 | $ | (2,015 | ) | $ | 21,532 | ||||
As of December 31, 2017, the Company had $1.0 million of gross unrecognized tax benefits. A reconciliation of the beginning and ending amount of gross unrecognized tax benefits is as follows (in thousands):
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Unrecognized tax benefits balance at January 1, | $ | 1,021 | $ | 1,067 | $ | 1,096 | |||||
Lapse of statute of limitations | (7 | ) | (46 | ) | (29 | ) | |||||
Unrecognized tax benefits balance at December 31, | $ | 1,014 | $ | 1,021 | $ | 1,067 | |||||
78
The Company’s policy for recording interest and penalties associated with uncertain tax positions is to record such items as a component of income tax expense in the Consolidated Statements of Operations. As of December 31, 2017 and December 31, 2016, the Company had a nominal amount of accrued interest related to uncertain tax positions.
The Company files income tax returns, including returns for its subsidiaries, with federal, state and local jurisdictions. The Company’s uncertain tax positions are related to tax years that remain subject to examination. As of December 31, 2017, U.S. tax returns for the years 2014 through 2017 remain subject to examination by federal tax authorities. Tax returns for the years 2013 through 2017 remain subject to examination by state and local tax authorities for a majority of the Company's state and local filings.
On December 22, 2017, the President of the United States signed into law the Tax Cuts and Jobs Act of 2017 or US Federal Tax Reform (the “Reform”). The enactment included broad tax changes that are applicable to Bioscrip, Inc. Most notably, the Reform has established the U.S. corporate tax rate decrease from a high of 35% to a flat 21% income tax rate effective January 1, 2018.
These changes require Bioscrip, Inc. to re-measure deferred tax assets and liabilities. The Company uses the asset and liability approach for accounting for income taxes. Under that method, assets and liabilities are recorded for future tax consequences attributable to the difference between financial statement balances of assets and liabilities and their respective tax basis. Deferred tax assets and liabilities are measured using the enacted tax rates at which the temporary differences are expected to reverse. As a result of the decreased US corporate income tax rate from 35% to 21%, the Company has revalued its ending net deferred tax assets as of December 31, 2017. Due to the full valuation allowance against substantially all net deferred tax assets, the change in deferred tax rate to 21% does not have an impact on the Company’s financial statements.
NOTE 14 – STOCK-BASED COMPENSATION
BioScrip Equity Incentive Plans
Under the Company’s Amended and Restated 2008 Equity Incentive Plan (the “2008 Plan”), the Company may issue, among other things, incentive stock options, non-qualified stock options, stock appreciation rights (“SARs”), restricted stock grants, restricted stock units, performance shares and performance units to key employees and directors. While SARs are authorized under the 2008 Plan, they may also be issued outside of the plan. The 2008 Plan is administered by the Company’s Management Development and Compensation Committee (the “Compensation Committee”), a standing committee of the Board of Directors.
On November 30, 2016, at a special meeting, the stockholders approved (i) an amendment to the Company’s Second Amended and Restated Certificate of Incorporation to increase the number of shares of Common Stock that the Company is authorized to issue from 125 million shares to 250 million shares (the “Charter Amendment”); and (ii) an amendment to the 2008 Plan to (a) increase the number of shares of Common Stock in the aggregate that may be subject to awards by 5,250,000 shares, from 9,355,000 to 14,605,000 shares and (b) increase the annual grant caps under the Company’s 2008 Plan from 500,000 Options, 500,000 Stock Appreciation Rights and 350,000 Stock Grants and Restricted Stock Units that are intended to comply with the requirements of Section 162(m) of the Code to a cap of no more than a total of 3,000,000 Options, Stock Appreciation Rights, Stock Grants and Restricted Stock Units that are intended to comply with the requirements of Section 162(m) of the Code combined.
As of December 31, 2017, there were 5,245,719 shares that remained available for grant under the 2008 Plan.
Employee Stock Purchase Plan
On May 7, 2013, the Company’s stockholders approved the BioScrip, Inc. Employee Stock Purchase Plan (the “ESPP”). The ESPP Plan is administered by the Compensation Committee. The ESPP provides all eligible employees, as defined under the ESPP, the opportunity to purchase up to a maximum number of shares of Common Stock of the Company as determined by the Compensation Committee. Participants in the ESPP may acquire the Common Stock at a cost of 85% of the lower of the fair market value on the first or last day of the quarterly offering period. The Company filed a Registration Statement on Form S-8 to register 750,000 shares of Common Stock, par value $0.0001 per share, for issuance under the ESPP.
As of December 31, 2017, there were 53,462 shares that remained available for grant under the ESPP. During the year ended December 31, 2017, the ESPP’s third-party service provider purchased 265,608 shares on the open market and delivered these shares to the Company’s employees pursuant to the ESPP, and the Company recorded $0.1 million of expense related to the ESPP.
79
BioScrip/CHS Equity Plan
In connection with the May 8, 2014 amendment to the 2008 Plan noted above, the Company determined to cease issuance of awards under the BioScrip/CHS 2006 Equity Incentive Plan. As of December 31, 2017, no shares remained available under the BioScrip/CHS Plan.
Stock Options
Options granted under the Equity Compensation Plans: (a) typically vest over a three-year period and, in certain instances, fully vest upon a change in control of the Company, (b) have an exercise price that may not be less than 100% of its fair market value on the date of grant and (c) are exercisable for seven to ten years after the date of grant, subject to earlier termination in certain circumstances.
Option expense is amortized on a straight-line basis over the requisite service period. The Company recognized compensation expense related to stock options of $1.0 million, $3.4 million, and $4.8 million, in the years ended December 31, 2017, 2016 and 2015, respectively.
The weighted-average, grant-date fair value of options granted during the years ending December 31, 2017, 2016 and 2015 was $1.22, $0.72, and $2.25, respectively. The fair value of stock options granted was estimated on the date of grant using a binomial model for grants issued through June 30, 2015 and a Black-Scholes option-pricing model for grants issued beginning July 1, 2015. The assumptions used to compute the fair value of options for the years ending December 31, 2017, 2016 and 2015 were:
2017 | 2016 | 2015 | ||||||
Expected volatility | 73.2 | % | 68.1 | % | 62.3 | % | ||
Risk-free interest rate | 2.04 | % | 1.98 | % | 2.20 | % | ||
Expected life of options | 5.7 years | 4.8 years | 8.9 years | |||||
Dividend rate | — | — | — | |||||
A summary of stock option activity for the Equity Compensation Plans through December 31, 2017 was as follows:
Options | Weighted Average Exercise Price | Aggregate Intrinsic Value (thousands) | Weighted Average Remaining Contractual Life | |||||||||
Balance at December 31, 2016 | 5,265,370 | $ | 5.78 | $ | — | 4.4 years | ||||||
Granted | 1,618,092 | $ | 1.93 | $ | 1,499 | |||||||
Exercised | (146,667 | ) | $ | 2.37 | $ | 36 | ||||||
Forfeited and expired | (2,338,595 | ) | $ | 6.77 | $ | 325 | ||||||
Balance at December 31, 2017 | 4,398,200 | $ | 3.98 | $ | 2,639 | 5.5 years | ||||||
Outstanding options less expected forfeitures at December 31, 2017 | 4,210,163 | $ | 4.07 | $ | 2,465 | 5.4 years | ||||||
Exercisable at December 31, 2017 | 2,497,766 | $ | 5.59 | $ | 647 | 3.6 years | ||||||
Cash received from option exercises under share-based payment arrangements was $0.4 million for the year ended December 31, 2017 and nominal for the years ended 2016 and 2015.
80
The maximum term of stock options under these plans is ten years. Options outstanding as of December 31, 2017 expire on various dates ranging from January 2018 through March 2026. The following table outlines our outstanding and exercisable stock options as of December 31, 2017:
Options Outstanding | Options Exercisable | |||||||||||||||
Range of Option Exercise Price | Outstanding Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life | Options Exercisable | Weighted Average Exercise Price | |||||||||||
$0.00 - $2.06 | 1,253,866 | $ | 1.42 | 7.3 years | 257,604 | $ | 1.29 | |||||||||
$2.06 - $4.13 | 1,663,258 | $ | 2.49 | 6.0 years | 759,086 | $ | 2.71 | |||||||||
$4.13 - $6.19 | 210,500 | $ | 5.02 | 3.8 years | 210,500 | $ | 5.02 | |||||||||
$6.19 - $8.25 | 983,076 | $ | 7.20 | 3.5 years | 983,076 | $ | 7.20 | |||||||||
$10.31 - $12.38 | 175,000 | $ | 11.04 | 2.8 years | 175,000 | $ | 11.04 | |||||||||
$12.38 - $14.44 | 104,500 | $ | 13.09 | 4.9 years | 104,500 | $ | 13.09 | |||||||||
$16.50 - $18.57 | 8,000 | $ | 16.63 | 3.6 years | 8,000 | $ | 16.63 | |||||||||
All options | 4,398,200 | 2,497,766 | ||||||||||||||
As of December 31, 2017 there was $1.6 million of unrecognized compensation expense related to unvested option grants that is expected to be recognized over a weighted-average period of 2.2 years.
As compensation expense for options granted is recorded over the requisite service period of options, future stock-based compensation expense may be greater as additional options are granted.
Restricted Stock
Under the Equity Compensation Plans, stock grants subject solely to an employee’s or director’s continued service with the Company will not become fully vested less than (a) three years from the date of grant to employees and, in certain instances, may fully vest upon a change in control of the Company, and (b) one year from the date of grant for directors. Stock grants subject to the achievement of performance conditions will not vest less than one year from the date of grant. Such performance shares may vest after one year from grant. No such time restrictions applied to stock grants made under the Company’s prior equity compensation plans.
The Company recognized compensation expense related to restricted stock awards of $1.1 million, $0.5 million, and $0.4 million for the years ended December 31, 2017, 2016 and 2015, respectively.
Since the Company records compensation expense for restricted stock awards based on the vesting requirements, which generally includes time elapsed, market conditions and/or performance conditions, the weighted average period over which the expense is recognized varies. Also, future equity-based compensation expense may be greater if additional restricted stock awards are made.
A summary of restricted stock award activity through December 31, 2017 was as follows:
Restricted Stock | Weighted Average Grant Date Fair Value | Weighted Average Remaining Recognition Period | ||||||
Balance at December 31, 2016 | 547,356 | $ | 2.43 | 2.2 years | ||||
Granted | 1,563,922 | $ | 1.80 | |||||
Awards Vested | (145,402 | ) | $ | 3.71 | ||||
Canceled | (83,513 | ) | $ | 1.83 | ||||
Balance at December 31, 2017 | 1,882,363 | $ | 1.82 | 4.0 years | ||||
As of December 31, 2017, there was $2.0 million in unrecognized compensation expense related to unvested restricted stock awards. The total grant date fair value of awards vested during the years ended December 31, 2017, 2016 and 2015 was $2.8 million, $0.9 million, and $0.2 million, respectively. The total fair value of restricted stock awards vested during the years December 31, 2017, 2016 and 2015 was $0.4 million, $0.2 million, and $0.5 million, respectively.
81
Performance Units
Under the 2008 Plan, the Compensation Committee may grant performance units to key employees. The Compensation Committee will establish the terms and conditions of any performance units granted, including the performance goals, the performance period and the value for each performance unit. If the performance goals are satisfied, the Company would pay the key employee an amount in cash equal to the value of each performance unit at the time of payment. In no event may a key employee receive an amount in excess of $1.0 million with respect to performance units for any given year. As of December 31, 2017, 1,563,922 performance units have been granted under the 2008 Plan.
Stock Appreciation Rights
The Company has outstanding cash-based phantom stock appreciation rights (“SARs”), which are independent of the Company's 2008 Equity Incentive Plan, with respect to 100,000 shares of the Company's common stock. The SARs vest in three equal annual installments and will fully vest in connection with a change of control (as defined in the grantee’s employment agreement). The SARs may be exercised, in whole or in part, to the extent each SAR has been vested and will receive in cash the amount by which the closing stock price on the exercise date exceeds the Grant Price, if any. Upon the exercise of any SARs, as soon as practicable under the applicable federal and state securities laws, the grantee may be required to use the net after-tax proceeds of such exercise to purchase shares of the Common Stock from the Company at the closing stock price of the Common Stock on that date and hold such shares of Common Stock for a period of not less than one year from the date of purchase, except that the grantee will not be required to purchase any shares of Common Stock if the SAR is exercised on or after a change of control of the Company. The grantee’s right to exercise the SAR will expire on the earliest of (1) the tenth anniversary of the grant date, or (2) under certain conditions as a result of termination of the grantee’s employment.
A summary of SAR activity through December 31, 2017 was as follows:
Stock Appreciation Rights | Weighted Average Exercise Price | Weighted Average Remaining Recognition Period | ||||||
Balance at December 31, 2016 | 300,000 | $ | 6.48 | 0.0 years | ||||
Granted | — | |||||||
Exercised | — | |||||||
Canceled | (200,000 | ) | $ | 5.70 | ||||
Balance at December 31, 2017 | 100,000 | $ | 8.05 | 0.0 years | ||||
The SARs are recorded as a liability in other non-current liabilities in the Consolidated Balance Sheets. Compensation benefit related to the SARs for the years ended December 31, 2017, 2016 and 2015 was negligible. As of December 31, 2017 all outstanding SARs were fully vested. In addition, because they are settled with cash, the fair value of the SAR awards is revalued on a quarterly basis. During the years ended December 31, 2017, 2016 and 2015, the Company did not pay cash related to the exercise of SAR awards.
NOTE 15 – DEFINED CONTRIBUTION PLAN
The Company maintains a deferred compensation plan under Section 401(k) of the Internal Revenue Code. Under the Plan, employees may elect to defer up to 100% of their salary, subject to Internal Revenue Service limits, and the Company may make a discretionary matching contribution. The Company recorded matching contributions within general and administrative expenses in the Consolidated Statements of Operations of $1.3 million during the year ended December 31, 2015. The Company elected to forgo a matching contribution during the years ended December 31, 2017 and 2016.
NOTE 16 – SELECTED QUARTERLY FINANCIAL DATA (UNAUDITED)
During the fourth quarter of 2017, the Company determined that certain 2017 and 2016 prior period quarterly balances contained errors, predominately due to a failure to appropriately account for and resolve transactions specific to suspense and clearing accounts. There were also immaterial corrections in the third quarter of 2017 for interest expense and intangible asset amortization expense. Management evaluated the materiality of the errors, quantitatively and qualitatively, and concluded that they were not material, but elected to correct the accompanying table of selected quarterly financial data. See Note 1 - Nature of Business.
82
A summary of unaudited quarterly financial information for the years ended December 31, 2017 and 2016 is as follows (in thousands except per share data).
First Quarter | Second Quarter | Third Quarter | Fourth Quarter | ||||||||||||
Year ended December 31, 2017 | |||||||||||||||
Revenue | $ | 217,810 | $ | 218,106 | $ | 198,692 | $ | 182,582 | |||||||
Gross profit | 64,874 | 67,611 | 66,563 | 70,194 | |||||||||||
Loss from continuing operations, before income taxes | (18,801 | ) | (28,432 | ) | (12,998 | ) | (7,202 | ) | |||||||
Net (loss) income from discontinued operations, net of income taxes | (299 | ) | (373 | ) | 66 | (287 | ) | ||||||||
Net loss | $ | (19,719 | ) | $ | (29,523 | ) | $ | (12,992 | ) | $ | (1,962 | ) | |||
Loss per share from continuing operations, basic and diluted | $ | (0.18 | ) | $ | (0.26 | ) | $ | (0.12 | ) | $ | (0.03 | ) | |||
Loss per share from discontinued operations, basic and diluted | — | — | — | (0.01 | ) | ||||||||||
Loss per share, basic and diluted | $ | (0.18 | ) | $ | (0.26 | ) | $ | (0.12 | ) | $ | (0.04 | ) | |||
Year ended December 31, 2016 | |||||||||||||||
Revenue | $ | 238,462 | $ | 232,462 | $ | 224,542 | $ | 240,123 | |||||||
Gross profit | 63,302 | 63,266 | 61,721 | 73,793 | |||||||||||
Loss from continuing operations, before income taxes | (10,311 | ) | (8,770 | ) | (11,012 | ) | (4,064 | ) | |||||||
Net (loss) income from discontinued operations, net of income taxes | 504 | 169 | 107 | (7,373 | ) | ||||||||||
Net loss | $ | (9,830 | ) | $ | (8,750 | ) | $ | (11,327 | ) | $ | (12,858 | ) | |||
Loss per share from continuing operations, basic and diluted | $ | (0.17 | ) | $ | (0.14 | ) | $ | (0.11 | ) | $ | (0.06 | ) | |||
Income (loss) per share from discontinued operations, basic and diluted | — | — | — | (0.07 | ) | ||||||||||
Loss per share, basic and diluted | $ | (0.17 | ) | $ | (0.14 | ) | $ | (0.11 | ) | $ | (0.13 | ) | |||
Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
Item 9A. | Controls and Procedures |
(a) Evaluation of Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures that are designed to ensure that information required to be disclosed by the Company in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to the Company’s management, including its Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure. Under the supervision and with the participation of the Company’s management, including its Chief Executive Officer and Chief Financial Officer, management evaluated the effectiveness of the Company’s disclosure controls and procedures as of December 31, 2017. Based on that evaluation, the Company’s Chief Executive Officer and its Chief Financial Officer concluded that due to material weaknesses in our internal controls over financial reporting described below, the Company’s disclosure controls and procedures (as such term is defined under Rule 13a-15(e) promulgated under the Exchange Act) were not effective as of December 31, 2017.
83
(b) Management Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f). Our internal control over financial reporting is a process designed by management, under the supervision of our Chief Executive Officer and Chief Financial Officer and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. A deficiency exists when the design or operation of a control does not allow management or employees, in the normal course of performing their assigned functions, to prevent or detect misstatements on a timely basis.
Our management, led by our Chief Executive Officer and Chief Financial Officer, assessed the effectiveness of internal control over financial reporting as of December 31, 2017, using the criteria set forth in Internal Control- Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO 2013 Framework).
Management’s evaluation of the effectiveness of our internal control over financial reporting determined that the Company’s internal control over financial reporting was not effective as of December 31, 2017, because of the material weaknesses described below.
We did not conduct an effective continuous risk assessment process and monitoring activities to identify possible risks of material misstatement in our financial reporting processes and to establish effective internal controls to manage such risks. As a consequence we did not design, implement and operate effective process level controls:
• | to ensure spreadsheets used to calculate amortization of intangible assets, the valuation of equity-linked liabilities, amortization of discounts and deferred issuance costs of debt, and spreadsheets used to evaluate the going concern premise were reviewed in sufficient detail to identify formulaic and data input errors following a change in personnel responsible for operation of the control. |
• | to review the timely accurate resolution of transactions posted to accounts receivable, accounts payable, and accrued liability suspense accounts. |
• | to review the timely accurate recognition of physical inventory count differences at all branch locations in our inventory management system. |
• | to review the timely accurate recognition of transfers from CIP to in-use and the completeness and accuracy of fixed asset disposals. |
The control deficiencies described above resulted in immaterial misstatements in the preliminary consolidated financial statements as of and for the year ended December 31, 2017 that were corrected. However, these control deficiencies create a reasonable possibility that a material misstatement in our consolidated financial statements will not be prevented or detected on a timely basis and, therefore, we concluded that the deficiencies represented material weaknesses in our internal control over financial reporting as of December 31, 2017.
The independent registered public accounting firm, KPMG LLP, has expressed an adverse report on the operating effectiveness of our internal control over financial reporting as of December 31, 2017. KPMG LLP’s report appears on page 86.
Remediation Plans
Management is actively remediating the identified material weakness, and has identified the following remediation steps:
• | Enhance risk assessment processes and monitoring activities to ensure the Company designs, implements, and operates effective controls that are responsive to identified risks. |
84
• | Implementation of controls to validate key inputs and calculations used in spreadsheets used to determine financial statement amounts and disclosures. |
• | Implementation of controls to identify and clear unmatched transactions in suspense accounts. |
• | Implementation of monitoring controls to be operated by a centralized resource to ensure periodic counts of inventory and fixed assets are completed and differences are timely processed by our accounting systems. |
• | Enhance controls surrounding the timely and accurate recognition of fixed asset disposals and abandonments. |
Changes in Internal Control over Financial Reporting
Except for the identification of the material weaknesses described above, there have been no changes in internal control over financial reporting during the fourth quarter of 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
85
Report of Independent Registered Public Accounting Firm
To the stockholders and board of directors
BioScrip, Inc.:
Opinion on Internal Control Over Financial Reporting
We have audited BioScrip, Inc. and subsidiaries’ (the “Company”) internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, because of the effect of the material weaknesses, described below, on the achievement of of the objectives of the control criteria, the Company has not maintained effective internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated balance sheets of the Company as of December 31, 2017 and 2016, the related consolidated statements of operations, stockholders’ (deficit) equity, and cash flows for each of the years in the three-year period ended December 31, 2017, and the related notes and financial statement schedule (collectively, the consolidated financial statements), and our report dated March 26, 2018 expressed an unqualified opinion on those consolidated financial statements.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. Material weaknesses related to ineffective continuous risk assessment process and monitoring activities; and ineffective process level controls regarding the accuracy of certain spreadsheet formulas and data inputs, the accuracy of certain suspense accounts, the accuracy of physical inventory count differences, the accuracy of fixed asset CIP transfers, and the completeness and accuracy of fixed asset disposals. The material weaknesses were considered in determining the nature, timing, and extent of audit tests applied in our audit of the 2017 consolidated financial statements, and this report does not affect our report on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
86
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ KPMG LLP
Denver, Colorado
March 26, 2018
87
Item 9B. | Other Information |
None.
PART III
Item 10. | Directors, Executive Officers and Corporate Governance |
We have adopted a Code of Ethics that applies to all of our directors, officers and employees, including our principal executive, principal financial and principal accounting officers, or persons performing similar functions. Our Code of Ethics is posted on our website located at http://www.bioscrip.com/corporate-governance. We intend to disclose future amendments to certain provisions of the Code of Ethics, and waivers of the Code of Ethics granted to executive officers and directors.
The other information required by this item is incorporated by reference from the information contained in our definitive proxy statement to be filed with the SEC on or before April 30, 2018 in connection with our 2018 Annual Meeting of Stockholders.
Item 11. | Executive Compensation |
The information required by this item is incorporated by reference from the information contained in our definitive proxy statement to be filed with the SEC on or before April 30, 2018 in connection with our 2018 Annual Meeting of Stockholders.
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this item is incorporated by reference from the information contained in our definitive proxy statement to be filed with the SEC on or before April 30, 2018 in connection with our 2018 Annual Meeting of Stockholders.
Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this item is incorporated by reference from the information contained in our definitive proxy statement to be filed with the SEC on or before April 30, 2018 in connection with our 2018 Annual Meeting of Stockholders.
Item 14. | Principal Accountant Fees and Services |
The information required by this item is incorporated by reference from the information contained in our definitive proxy statement to be filed with the SEC on or before April 30, 2018 in connection with our 2018 Annual Meeting of Stockholders.
88
PART IV
Item 15. | Exhibits, Financial Statement Schedules |
(a). The following financial statements appear in Item 8 of this Form 10-K:
Page | |
1. Financial Statements: | |
Report of Independent Registered Public Accounting Firm | |
Consolidated Balance Sheets as of December 31, 2017 and 2016 | |
Consolidated Statements of Operations for the years ended December 31, 2017, 2016, and 2015 | |
Consolidated Statements of Stockholders’ (Deficit) Equity for the years ended December 31, 2017, 2016, and 2015 | |
Consolidated Statements of Cash Flows for the years ended December 31, 2017, 2016, and 2015 | |
Notes to Consolidated Financial Statements | |
2. Financial Statement Schedule: | |
Valuation and Qualifying Accounts for the years ended December 31, 2017, 2016, and 2015 | |
All other schedules not listed above have been omitted since they are not applicable or are not required.
89
Item 16. Summary
None
3. and (b) Exhibits | |
See Index of Exhibits. | |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on March 26, 2018.
BIOSCRIP, INC. |
/s/ Alex Schott |
Alex Schott |
Senior Vice President, Strategic Operations (Acting Principal Accounting Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
90
Signature | Title(s) | Date |
/s/ Daniel E. Greenleaf Daniel E. Greenleaf | Chief Executive Officer, President and Director (Principal Executive Officer) | March 26, 2018 |
/s/ Stephen Deitsch Stephen Deitsch | Chief Financial Officer and Treasurer (Principal Financial Officer) | March 26, 2018 |
/s/ Alex Schott Alex Schott | Senior Vice President, Strategic Operations (Acting Principal Accounting Officer) | March 26, 2018 |
/s/ R. Carter Pate R. Carter Pate | Non-Executive Chairman of the Board | March 26, 2018 |
/s/ David Golding David Golding | Director | March 26, 2018 |
/s/ Michael Goldstein Michael Goldstein | Director | March 26, 2018 |
/s/ Tricia Huong Thi Nguyen Tricia Huong Thi Nguyen | Director | March 26, 2018 |
/s/ Christopher Shackelton Christopher Shackelton | Director | March 26, 2018 |
/s/ Michael G. Bronfein Michael G. Bronfein | Director | March 26, 2018 |
/s/ Steven Neumann Steven Neumann | Director | March 26, 2018 |
91
Bioscrip, Inc. and Subsidiaries
Schedule II-- Valuation and Qualifying Accounts
(in thousands)
Balance at Beginning of Period | Write-Off of Receivables | Charged to Costs and Expenses | Balance at End of Period | ||||||||||||
Year ended December 31, 2015 | |||||||||||||||
Allowance for doubtful accounts | $ | 66,405 | $ | (49,160 | ) | $ | 42,444 | $ | 59,689 | ||||||
Year ended December 31, 2016 | |||||||||||||||
Allowance for doubtful accounts | $ | 59,689 | $ | (41,567 | ) | $ | 26,608 | $ | 44,730 | ||||||
Year ended December 31, 2017 | |||||||||||||||
Allowance for doubtful accounts | $ | 44,730 | $ | (30,515 | ) | $ | 23,697 | $ | 37,912 | ||||||
92
(Exhibits being filed with this Annual Report on Form 10-K)
Index to Exhibits
93
94
10.5† | Second Amendment to Bioscrip, Inc. 2008 Equity Incentive Plan dated November 28, 2016. (Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on December 2, 2016) |
10.6† | BIOSCRIP/CHS 2006 Equity Incentive Plan, as Amended and Restated. (Incorporated by reference to Exhibit 10.3 to the Company’s Form 8-K filed on May 2, 2011) |
10.7† | Amendment One to the Stock Grant Certificate under the BioScrip/CHS 2006 Equity Incentive Plan from the Company to Brian Stiver, dated September 8, 2016.(Incorporated by reference to Exhibit 10.3 to the Company’s Form 8-K filed on September 12, 2016) |
10.8† | Employee Stock Purchase Plan. (Incorporated by reference to the definitive proxy statement filed on April 2, 2013) |
10.9† | First Amendment to Employee Stock Purchase Plan. (Incorporated by reference to Exhibit 10.5 to the Company’s Form 10-Q filed on August 10, 2015) |
10.10† | Form of Restricted Stock Grant Certificate. (Incorporated by reference to Exhibit 99.3 to the Company's Registration Statement on Form S-8 filed on filed on May 16, 2008) |
10.11† | Form of Non-Qualified Stock Option Agreement 2008 Equity Incentive Plan. (Incorporated by reference to Exhibit 10.7 to the Company’s Form 10-K filed on March 2, 2015) |
10.12† | Form of Amendment One to Non-Qualified Stock Option Agreement 2008 Equity Incentive Plan (entered with Messrs. Kreger, Evans and Stiver). (Incorporated by reference to Exhibit 10.2 to the Company’s Form 8-K filed on September 12, 2016) |
10.13† | Form of Market-Based Cash Award Agreement. (Incorporated by reference to Exhibit 10.4 to the Company’s Form 10-Q filed on August 10, 2015) |
10.14† | Employment Offer Letter, dated January 30, 2009, by and between the Company and David Evans. (Incorporated by reference to Exhibit 10.23 to the Company’s Form 10-K/A filed on December 16, 2013) |
10.15† | Amended and Restated Employment Agreement, dated as of November 25, 2013, by and between the Company and Richard M. Smith. (Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on November 27, 2013) |
10.16† | First Amendment to Amended and Restated Employment Agreement, dated September 9, 2016, between Richard M. Smith and the Company. (Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on September 12, 2016) |
10.17† | Employment Offer Letter, dated March 10, 2009, by and between the Company and Brian Stiver. (Incorporated by reference to Exhibit 10.24 to the Company’s Form 10-K/A filed on June 6, 2014) |
10.18† | Employment Offer Letter, dated July 30, 2012, by and between the Company and Brian Stiver. (Incorporated by reference to Exhibit 10.25 to the Company’s Form 10-K/A filed on June 6, 2014) |
10.19† | Amendment, dated April 2, 2015, to the Employment Offer Letter by and between the Company and Brian Stiver. (Incorporated by reference to Exhibit 10.6 to the Company’s Form 10-Q filed on May 8, 2015) |
10.20† | Employment Offer Letter, dated December 1, 2013, by and between the Company and Karen Cain. (Incorporated by reference to Exhibit 10.17 to the Company’s Form 10-K filed on March 2, 2015) |
10.21† | Employment Offer Letter, dated as of April 26, 2015, by and between the Company and Jeffrey M. Kreger. (Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on April 28, 2015) |
10.22† | Offer Letter, dated as of April 10, 2017, by and between BioScrip, Inc. and Stephen M. Deitsch. (Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on April 20, 2017) |
10.23† | Offer Letter, dated as of November 21, 2017, by and between BioScrip, Inc. and Harriet Booker. (Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed November 28, 2017) |
10.24† | Offer Letter, dated as of November 29, 2017, by and between BioScrip, Inc. and Anthony “Tony” Lopez. (Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed December 1, 2017) |
10.25 | Form of Indemnification Agreement. (Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on March 14, 2013) |
95
96
97
10.59 | Second Lien Note Purchase Agreement, dated as of June 29, 2017, by and among the Company, the financial institutions and note purchasers from time to time party thereto, and Wells Fargo Bank, National Association as Collateral Agent (Incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on June 29, 2017) |
10.60 | Second Lien Guaranty and Security Agreement, dated as of June 29, 2017, by and the Company, the subsidiaries of the Company signatory thereto and Wells Fargo Bank, National Association as Collateral Agent (Incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on June 29, 2017) |
10.61 | Warrant Purchase Agreement, dated as of June 29, 2017, by and among the Company and the subscribers signatory thereto (Incorporated by reference to Exhibit 10.5 to the Company’s Current Report on Form 8-K filed on June 29, 2017) |
10.62 | Stock Purchase Agreement, dated as of June 29, 2017, by and among the Company and the purchaser signatory thereto (Incorporated by reference to Exhibit 10.6 to the Company’s Current Report on Form 8-K filed on June 29, 2017) |
21.1 * | |
23.1 * | |
31.1 * | |
31.2 * | |
32.1 * | |
32.2 * | |
101 | The following financial information from the Company’s Form 10-K for the fiscal year ended December 31, 2016, formatted in XBRL (Extensible Business Reporting Language): (i) Consolidated Statements of Operations for the fiscal years ended December 31, 2016, 2015 and 2014, (ii) Consolidated Balance Sheets as of December 31, 2016 and 2015, (iii) Consolidated Statements of Stockholders’ Equity for the fiscal years ended December 31, 2016, 2015 and 2014, (iv) Consolidated Statements of Cash Flows for the fiscal years ended December 31, 2016, 2015 and 2014, and (v) Notes to Consolidated Financial Statements. |
* | Filed herewith. |
** | Pursuant to Item 601(b)(2) of Regulation S-K, certain schedules and exhibits are omitted from some exhibits. The Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the U.S. Securities and Exchange Commission (the “SEC”) upon request. |
† | Designates the Company’s management contracts or compensatory plan or arrangement. |
# | The SEC has granted confidential treatment of certain provisions of these exhibits. Omitted material for which confidential treatment has been granted has been filed separately with the SEC. |
98
