Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - Karyopharm Therapeutics Inc. | d529132dex321.htm |
| EX-31.2 - EX-31.2 - Karyopharm Therapeutics Inc. | d529132dex312.htm |
| EX-31.1 - EX-31.1 - Karyopharm Therapeutics Inc. | d529132dex311.htm |
| EX-23.1 - EX-23.1 - Karyopharm Therapeutics Inc. | d529132dex231.htm |
| EX-21.1 - EX-21.1 - Karyopharm Therapeutics Inc. | d529132dex211.htm |
| EX-12.1 - EX-12.1 - Karyopharm Therapeutics Inc. | d529132dex121.htm |
| 10-K - 10-K - Karyopharm Therapeutics Inc. | d529132d10k.htm |
Exhibit 10.30
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. Double asterisks denote omissions.
LICENSE AGREEMENT
by and between
KARYOPHARM THERAPEUTICS INC.
and
ONO PHARMACEUTICAL CO., LTD.
TABLE OF CONTENTS
| 1. | DEFINITIONS | 1 | ||||
| 2. | DEVELOPMENT | 14 | ||||
| 3. | REGULATORY MATTERS. | 21 | ||||
| 4. | COMMERCIALIZATION OF THE LICENSED PRODUCTS | 23 | ||||
| 5. | GOVERNANCE. | 26 | ||||
| 6. | MANUFACTURE AND SUPPLY | 31 | ||||
| 7. | LICENSES | 34 | ||||
| 8. | CERTAIN FINANCIAL TERMS. | 38 | ||||
| 9. | CONFIDENTIALITY AND PUBLICATION | 45 | ||||
| 10. | REPRESENTATIONS, WARRANTIES AND COVENANTS; DISCLAIMER | 49 | ||||
| 11. | INDEMNIFICATION; LIMITATION OF LIABILITY; INSURANCE | 53 | ||||
| 12. | INTELLECTUAL PROPERTY OWNERSHIP, PROTECTION AND RELATED MATTERS; BRAND NAME | 55 | ||||
| 13. | TERM AND TERMINATION | 63 | ||||
| 14. | MISCELLANEOUS | 67 | ||||
SCHEDULES
| Schedule 1.43 | Karyopharm Third Party Agreements | |
| Schedule 1.46 | KPT-8602 | |
| Schedule 1.74 | Selinexor | |
| Schedule 2.1 | Relevant Clinical Studies | |
| Schedule 2.2 | Overview Plan | |
| Schedule 10.2.2 | Karyopharm Patents as of Effective Date | |
| Schedule 11.1 | Agreed Indemnification Language to be incorporated in the Clinical Supply Agreement (and the Commercial Supply Agreement, if any) | |
-i-
Confidential
LICENSE AGREEMENT
THIS LICENSE AGREEMENT (this “Agreement”), effective as of October 11, 2017 (the “Effective Date”), is made and entered into by and between Karyopharm Therapeutics Inc., a corporation organized and existing under the laws of the State of Delaware, having an address at 85 Wells Avenue, Suite 210, Newton, MA 02459 USA (“Karyopharm”), and Ono Pharmaceutical Co., Ltd., a corporation organized and existing under the laws of Japan, having an address at 8-2, Kyutaro-machi 1-chome, Chuo-ku, Osaka, Osaka 541-8564, Japan (“Ono”). Karyopharm and Ono are sometimes referred to herein individually as a “Party” and collectively as the “Parties.”
RECITALS:
WHEREAS, Karyopharm owns or Controls certain intellectual property relating to the Licensed Compounds and the Licensed Products (each as defined below);
WHEREAS, Ono desires to Develop and Commercialize Licensed Compounds and Licensed Products in the Field in the Ono Territory (each as defined below);
WHEREAS, Karyopharm and Ono believe that a license for such purpose on the terms and conditions of this Agreement would be desirable.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants herein contained, the Parties hereby agree as follows:
| 1. | DEFINITIONS |
Unless specifically set forth to the contrary herein, the following terms, whether used in the singular or plural, shall have the respective meanings set forth below:
1.1 “Affiliate” means, as to a specified Person, another Person that, directly or indirectly, controls, is controlled by, or is under common control with the Person specified, for so long as such control continues. An entity will be regarded as in control of another entity if: (a) it owns, directly or indirectly, more than fifty percent (50%) of the voting securities or capital stock of such entity, or has other comparable ownership interest with respect to any entity other than a corporation; or (b) it possesses, directly or indirectly, the power to direct or cause the direction of the management and policies of the corporation or non-corporate business entity, as applicable, whether through the ownership or control of voting securities, by contract or otherwise.
1.2 “Annual Net Sales” means the Net Sales generated over any given Ono Fiscal Year.
1.3 “Back-Up Compound” means, with respect to a Lead Compound, any compound that (a) is Developed by Karyopharm for use in the Field, (b) inhibits the nuclear export protein Exportin 1, or XPO1, and (c) is designated by JOC as a back-up compound to such Lead Compound in accordance with Section 2.7.2.
1.4 “Business Day” means any day other than a day which is a Saturday, a Sunday, any day banks are authorized or required to be closed in the United States or Japan or any day within Karyopharm’s corporate holidays (for Karyopharm’s obligations) or Ono’s corporate holidays (for Ono’s obligations).
1.5 “Calendar Quarter” means the respective periods of three (3) consecutive calendar months ending on March 31, June 30, September 30, and December 31 of each Calendar Year; provided that the first Calendar Quarter of the Term shall begin on the Effective Date and end on the first to occur of March 31, June 30, September 30 or December 31 thereafter and the last Calendar Quarter of the Term shall end on the last day of the Term.
1.6 “Calendar Year” means each successive period of twelve (12) calendar months commencing on January 1 and ending on December 31; provided that the first Calendar Year of the Term shall begin on the Effective Date and end on the first December 31 thereafter and the last Calendar Year of the Term shall end on the last day of the Term.
1.7 “CDE” means the Center of Drug Evaluation, Taiwan and any successor Governmental Authority having substantially the same function.
1.8 “CDISC” means Clinical Data Interchange Standards Consortium which is an interdisciplinary nonprofit organization that establishes international standards for data collection, interchange, application, and storage for the purpose of promoting interoperation of clinical research data.
1.9 “Clinical Data” means all information relating to Licensed Compounds and/or Licensed Products made, collected or otherwise generated in the performance of or in connection with any Clinical Study, including any data, reports and results relating thereto (including clinical data and other related information generated in compliance with CDISC standards).
1.10 “Clinical Study” means a clinical trial in humans, including a Phase I Study, Phase II Study, Phase III Study, an Ono Post-Registration Study, a Karyopharm post-registration study or a Global Clinical Study.
1.11 “Combination Product” means any pharmaceutical product containing both a Licensed Product component and one or more other active pharmaceutical ingredients.
1.12 “Commence” or “Commencement” means, with respect to a Clinical Study of a Licensed Product, the first dosing of the first human subject with such Licensed Product in such Clinical Study.
1.13 “Commercialization” or “Commercialize” means any and all activities directed to marketing, promoting, distributing, importing, exporting, offering to sell and/or selling a Licensed Product and activities directed to obtaining pricing and reimbursement approvals, as applicable.
1.14 “Commercially Reasonable Efforts” means the carrying out of obligations in a diligent and sustained manner using such effort and employing such resources as would normally be exerted or employed by a Party or its Affiliates for a product that is of similar market potential
- 2 -
Confidential
at a similar stage in its Development or product life, taking into account all relevant factors, including the potential profitability of the product, the costs and risks of Developing, Manufacturing, having Manufactured, use and Commercializing the product, scientific, safety and regulatory concerns, product profile, the competitiveness of the marketplace and the proprietary position of the product.
Without limiting the foregoing,
(a) in relation to Development activities, including for purposes of obtaining Regulatory Approval of a product, “Commercially Reasonable Efforts” require that such Party: (i) assign responsibility for the relevant activities to specific employees who are responsible for progress and monitor such progress on a regular basis; (ii) set and consistently seek to achieve specific and meaningful objectives and timelines for carrying out such activities; and (iii) consistently make and implement decisions and allocate resources consistent with the efforts described above; and
(b) in relation to requiring Related Party to conduct certain activities under this Agreement, “Commercially Reasonable Efforts” require that (i) to the extent that such Related Party is its Affiliate, each Party oblige such Related Party to accept terms and conditions equivalent to those set forth in this Agreement, (ii) to the extent that such Related Party is a Third Party Licensee or a Sublicensee, each Party negotiate with such Related Party and use good faith efforts to persuade it to accept terms and conditions, which, to the maximum extent, will be consistent with those set forth in this Agreement and (iii) each Party exercise all of its rights and performs the obligations under any agreement between such Party and such Related Party in a commercially appropriate and timely manner so that the purpose of this Agreement contemplated in each Section will be achieved.
1.15 “Confidential Information” means any and all information and data, including all scientific, non-clinical, pre-clinical, clinical, regulatory, Manufacturing, marketing, financial, trade secret and commercial information or Data, whether communicated in writing or orally or by any other method, which is provided by or on behalf of one Party or any of its Related Parties (the “Disclosing Party”) to the other Party or any of its Related Parties (the “Receiving Party”) in connection with this Agreement. Notwithstanding anything to the contrary set forth herein, (a) Karyopharm Technology (other than Joint IP) is the Confidential Information of Karyopharm; (b) Ono Technology (other than Joint IP) is the Confidential Information of Ono; and (c) Joint IP which has not yet been publicly disclosed shall be deemed to be the Confidential Information of both Parties; and (d) the terms of this Agreement shall be deemed to be the Confidential Information of both Parties. All information and data disclosed prior to the Effective Date by or on behalf of either Party under, and subject to, the Confidentiality Agreement, dated as of April 24, 2017, between the Parties (the “Prior CDA”) shall be deemed the Confidential Information hereunder and such Party shall be deemed the Disclosing Party of such information and data hereunder and the other Party shall be deemed the Receiving Party hereunder. “Confidential Information” shall not include information or data, to the extent that such information or data:
1.15.1 is lawfully in the Receiving Party’s possession prior to disclosure by the Disclosing Party and was not acquired directly or indirectly from Disclosing Party, as documented by the Receiving Party’s business records;
- 3 -
Confidential
1.15.2 is generally known to the public prior to its receipt by the Receiving Party, or thereafter becomes generally known to the public through no fault of the Receiving Party or any of its Related Parties with whom the Receiving Party shared the Confidential Information;
1.15.3 is subsequently disclosed to the Receiving Party by a Third Party that lawfully has possession of and the right to disclose such Confidential Information without the breach of any contractual, legal or fiduciary obligation to the Disclosing Party or any Third Party and provided that such Third Party is not disclosing on behalf of the Disclosing Party; or
1.15.4 is independently developed by the Receiving Party without use of or reference to Disclosing Party’s Confidential Information, as documented by the Receiving Party’s business records.
1.16 “Control” means, subject to the provisions of Section 14.1, with respect to a Party and/or its Related Party, as the case may be, and any Know-How, Patent Right or other intellectual property right, the possession (whether by ownership or license, other than a license granted to such Party pursuant to this Agreement) of the ability of such Party or any such Related Party to transfer, grant access to, or grant a license or sublicense of, such Know-How, Patent Right or other intellectual property right as provided for herein without violating the terms of any agreement or other arrangement with any Third Party.
1.17 “Cost of Manufacturing” means, to the extent that Manufacturing of a Licensed Compound, Licensed Product or any component thereof is performed by a Party itself or by its Affiliate, the actual consolidated, fully burdened cost incurred by such a Party and its Affiliates to Manufacture such a Licensed Compound, Licensed Product or any component thereof, including: (a) direct labor costs (salaries, wages, incentive compensation, share-based compensation and employee benefits); (b) direct materials and packaging costs; (c) operating costs of facilities and equipment, excluding any surplus or idle capacity costs; (d) a charge for depreciation, repairs and maintenance costs of facilities and equipment; (e) quality and in-process control costs; (f) a charge for overhead costs for raw material and manufacturing administration and management, materials management, storage and handling, and manufacturing and employee training; (g) charges for spoilage, scrap, rework costs and expired goods; and (h) inbound and outbound freight, shipping insurance, excise taxes and customs duties; in each of the above cases to the extent reasonably allocable to Manufacture of such Licensed Compound, Licensed Product or component as determined in accordance with GAAP or IFRS, as applicable, consistently applied.
1.18 “Cover,” “Covering” or “Covers” means that in the absence of ownership of or a license granted under a Valid Claim, the Development, Manufacture, having Manufactured, use or Commercialization of a Licensed Compound or a Licensed Product would infringe such Valid Claim (or, in the case of a Valid Claim that has not yet issued, would infringe such Valid Claim if it were to issue).
1.19 “Data” means any and all scientific, technical, test and patient exposure data pertaining to any Licensed Compound or Licensed Product that are necessary or useful for the Development, Manufacture, having Manufactured, use and/or Commercialization of each
- 4 -
Confidential
Licensed Compound and Licensed Product in the Field and that are Controlled by Ono and/or its Related Parties or Controlled by Karyopharm and/or its Related Parties, including research data, clinical pharmacology data, non-clinical data, pre-clinical data and Clinical Data.
1.20 “Development” or “Develop” means non-clinical, pre-clinical and clinical research and development activities, including the design or identification of a compound, drug metabolism and pharmacokinetics, translational research, toxicology, pharmacology toxicology studies, statistical analysis and report writing, pre-clinical testing, formulation development, Clinical Studies, regulatory affairs (including preparation for NDA submission and other submission-related activities), product approval and registration activities, and all other activities necessary to seek, obtain and maintain Regulatory Approval; provided, however, that Development shall not include Commercialization and Manufacturing.
1.21 “Executive Officer” means the Chief Executive Officer or his or her designee in the case of Karyopharm, and Executive Director or his or her designee in the case of Ono.
1.22 “Field” means the diagnosis, treatment and/or prevention of cancer in humans.
1.23 “First Commercial Sale” means, with respect to a country in the Ono Territory, the first sale for end use or consumption of the Licensed Product in such country after all Regulatory Approvals legally required for such sale have been granted by the Regulatory Authority of such country or, if Regulatory Approval is not required, after the date on which sales are permitted by applicable Law.
1.24 “GAAP” means generally accepted accounting principles of the United States.
1.25 “GCP” means the current standards for clinical studies for pharmaceuticals, as set forth in the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (“ICH”) guidelines and applicable regulations promulgated thereunder, as amended from time to time.
1.26 “Global Clinical Study” means a combined Phase I Study and Phase II Study, a combined Phase II Study and Phase III Study, a Phase II Study or a Phase III Study, of a Licensed Product in the Field which includes sufficient [**] clinical sites and/or [**] patients to achieve Regulatory Approval in both the Ono Territory and the Karyopharm Territory for the Indication associated with such Clinical Study.
1.27 “Global Common Costs” means the direct development costs that are incurred by a Party in connection with the Global Common Activity.
1.28 “GLP” means the current standards for laboratory activities for pharmaceuticals, as set forth in the FDA’s Good Laboratory Practice regulations or the Good Laboratory Practice principles of the Organization for Economic Co-Operation and Development, as amended from time to time, and such standards of good laboratory practice as are required by the MHLW and other organizations and Governmental Authorities in countries in which a Licensed Product is intended to be sold, to the extent such standards are not less stringent than United States Good Laboratory Practice.
- 5 -
Confidential
1.29 “GMP” means all Laws and guidelines applicable to Manufacture of the Licensed Compound or Licensed Product, including (a) the FD&C Act (21 U.S.C. 321 et seq.); (b) relevant United States regulations in Title 21 of the United States Code of Federal Regulations (including Parts 11, 210, and 211); (c) European Community Directives 2001/83/EC and 2003/94/EC; (d) the EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use, as set out in Volume 4 of the European Commission’s Rules governing medicinal products in the EU; (e) those standards required by the MHLW; (f) ICH, Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients; (g) similar standards and Laws to those in (a) through (f), as are in effect at the time of Manufacture of the Licensed Compound and/or Licensed Product; and (h) all additional Regulatory Authority documents or regulations that replace, amend, modify, supplant or complement any of the foregoing.
1.30 “Governmental Authority” means any multinational, federal, national, state, provincial, local or other entity, office, commission, bureau, agency, political subdivision, instrumentality, branch, department, authority, board, court, arbitral or other tribunal, official or officer, exercising executive, judicial, legislative, police, regulatory, administrative or taxing authority or functions of any nature pertaining to government.
1.31 “IFRS” means International Financial Reporting Standards.
1.32 “IND” means an Investigational New Drug application, Clinical Trial Application or similar application or submission for approval to conduct human clinical investigations filed with or submitted to a Regulatory Authority in conformance with the requirements of such Regulatory Authority.
1.33 “Indication” means an indication by tumor type but not by line therapy of each tumor or cancer. By way of explanation, (a) an “Indication” shall be considered the same if the subject cancer has the same organ of origin even if they are, for example, of a different histologic or genetic subtype, a different cell type or a different line of therapy, and (b) “Indication” shall be considered different if the subject cancers have different organs of origin.
1.34 “Investigator Sponsored Clinical Study” means a clinical study or research of a Licensed Product in the Field that is sponsored and conducted by a physician, physician group or other Third Party not acting on behalf of a Party or its Related Party and who does not have a license from a Party or its Related Party to Commercialize such Licensed Product, pursuant to an IND owned by such Third Party in the case of a clinical study, and with respect to which a Party or its Related Party provides clinical supplies of the Licensed Product, funding or other support for such clinical study or research.
1.35 “Joint IP” means, collectively, Joint Patent Rights and Joint Know-How.
1.36 “Joint Know-How” means (a) any Know-How generated in course of the Global Clinical Study which is related to the combination of a Licensed Product and a commercial product or product in clinical development in either case owned by or licensed to Ono and (b) any Know-How that is (i) not solely related to Licensed Product or a commercial product or product in clinical development in either case owned by or licensed to Ono and (ii) first made or identified, discovered or developed jointly by director(s), officer(s), employee(s), agent(s) or consultant(s)
- 6 -
Confidential
acting on behalf of Karyopharm or its Affiliates, on the one hand, and director(s), officer(s), employee(s), agent(s) or consultant(s) acting on behalf of Ono or its Affiliates, on the other hand. All other Know-How generated in relation to the Global Clinical Study shall not be Joint Know-How and shall be Karyopharm Know-How and subject to the license grant to Ono described in Section 7.1.1.
1.37 “Joint Operating Committee” or “JOC” means the joint operating committee as more fully described in Section 5.1.
1.38 “Joint Patent Rights” means any Patent Rights that (a) Cover Joint Know-How and (b) are Controlled by Karyopharm and Ono.
1.39 “Karyopharm Know-How” means all Know-How, which, as of the Effective Date and during the Term, is Controlled by Karyopharm and/or any of its Affiliates, which (a) are not generally known, (b) are not Covered by a Karyopharm Patent Rights, (c) relates to any Licensed Compound and/or Licensed Product and (d) are necessary or useful for the research, Development, Manufacture, having Manufactured, use and/or Commercialization of each Licensed Compound and Licensed Product in the Field; provided, however, that Karyopharm Know-How excludes the Joint Know-How.
1.40 “Karyopharm Patent Rights” means all Patent Rights which, as of the Effective Date of and during the Term, are Controlled by Karyopharm and/or any of its Affiliates, and which claim or Cover, are necessary or useful for or would be practiced by the research, Development, Manufacture, having Manufactured, use, and/or Commercialization of each Licensed Compound and Licensed Product in the Field; provided, however, that Karyopharm Patent Rights excludes Joint Patent Rights.
1.41 “Karyopharm Technology” means, collectively, Karyopharm Know-How, Karyopharm Patent Rights and Karyopharm’s interest in Joint IP.
1.42 “Karyopharm Territory” means all countries and territories of the world other than the Ono Territory.
1.43 “Karyopharm Third Party Agreements” means (a) those agreements listed on Schedule 1.43 and (b) any agreements entered into as of the Effective Date by Karyopharm or any of its Affiliates pursuant to which Karyopharm Controls any Karyopharm Technology or receives funding to develop any Karyopharm Technology or any Licensed Compound or Licensed Product (unless solely for use outside the Field).
1.44 “Knowledge” means actual knowledge of department head and working team member(s) on function-by-function basis for or on behalf of each Party. For clarity, such actual knowledge shall be obtained and finalized after making due and appropriate inquiry with respect to the particular matter in question.
1.45 “Know-How” means all technical information, know-how and data, including trade secrets, inventions (whether patentable or not), discoveries, methods, specifications, processes, expertise, technology, Data, other non-clinical, pre-clinical and Clinical Data and results
- 7 -
Confidential
(including pharmacological, toxicological, biological, chemical, physical, safety and manufacturing data and results), analytical and quality control data and results, regulatory filings and documents, and other information. “Know-How” excludes in any event any Patent Rights.
1.46 “KPT-8602” means the compound known as KPT-8602, which is described on Schedule 1.46.
1.47 “Law” means any law, statute, rule, regulation, court order, ordinance or other pronouncement having the effect of law, of any Governmental Authority, including (a) good clinical practices and adverse event reporting requirements, guidance from the ICH or other generally accepted conventions, and all rules, regulations, requirements and guidances of applicable Regulatory Authorities, (b) all export control and sanctions laws, and (c) the rules of any stock exchange or listing entity.
1.48 “Lead Compound” means Selinexor or KPT-8602, as applicable.
1.49 “Licensed Compound” means a Lead Compound, which Lead Compound may be replaced by a Back-Up Compound in accordance with Section 2.7.2.
1.50 “Licensed Product” means any pharmaceutical product comprising or containing a Licensed Compound as an active ingredient, in any dosage form or formulation. As used in this Agreement, except where not appropriate in context, “Licensed Product” also means the Licensed Compound contained in the relevant Licensed Product. In calculation of the Royalty Term pursuant to Section 8.4.1, to the extent that a Licensed Compound is contained as a sole active ingredient, any formulation, including but not limited to a tablet, a capsule, a powder, a granule, a liquid, an intravenous, a subcutaneous injection or a patch formulation, any such formulated Licensed Product shall be deemed as same Licensed Product. Further, it is understood by the Parties that a Combination Product containing a Licensed Compound as one of active ingredients shall be deemed as a Licensed Product, which is different from a Licensed Product containing a Licensed Compound as a sole active ingredient.
1.51 “Losses” means any losses, liabilities, damages, costs, fees and expenses (including reasonable attorneys’ fees and litigation expenses) arising out of or relating to suits or claims brought by Third Party (including product liability claims).
1.52 “Manufacturing” or “Manufacture” means, as applicable, all activities associated with the production, manufacture, processing, filling, finishing, quality assurance testing and release, stability studies, process validation, analytical development, packaging, labeling, shipping and storage of a pharmaceutical product, (including production of drug substance and drug product, in bulk form, for preclinical studies, Clinical Studies or Commercialization); provided, however, that Manufacturing shall not include Development and Commercialization. When used as a verb, “to Manufacture” and “Manufacturing” mean to engage in Manufacture, and “Manufactured” has a corresponding meaning.
1.53 “Mechanism of Action” means the binding to the nuclear export protein, Exportin 1, or XPO1, causing inhibition of the activity of XPO1 or otherwise reducing the nuclear export of XPO1’s cargo proteins.
- 8 -
Confidential
1.54 “MFDS” means the Ministry of Food and Drug Safety in the Republic of Korea and any successor Governmental Authority having substantially the same function.
1.55 “MHLW” means the Japanese Ministry of Health, Labour and Welfare and any successor Governmental Authority having substantially the same function.
1.56 “NDA” means a New Drug Application, Biologics License Application, Marketing Authorization Application or similar application or submission filed with a Regulatory Authority in a country or group of countries to obtain marketing approval for a pharmaceutical product in such country or such group of countries.
1.57 “Net Sales” means the gross amount invoiced on sales of Licensed Products in the Field within the Ono Territory by Ono or any of its Related Parties to any Third Party, less the following sum incurred by Ono or any of its Related Parties, with respect to the sale of such Licensed Products, calculated in accordance with IFRS as consistently applied:
1.57.1 normal trade, cash, quantity and other customary discounts actually given to Third Parties in the ordinary course of business;
1.57.2 rebates, credits and allowances given by reason of rejections returns, damaged or defective product or recalls;
1.57.3 government-mandated rebates and any other compulsory payments, credits, adjustments and rebates actually paid or deducted;
1.57.4 any sales-based contributions actually made for “Contributions for Drug Induced Suffering”, “Contribution for Measure for Drug Safety” or any other contributions for aiding drug suffering in the amount determined by and payable to PMDA or any other Governmental Authority or industry organization in the Ono Territory;
1.57.5 price adjustments, allowances, credits, chargeback payments, discounts, rebates, fees and reimbursements or similar payments granted or made to managed care organizations, group purchasing organizations or other buying groups, pharmacy benefit management companies, health maintenance organizations and any other providers of health insurance coverage, health care organizations or other health care institutions (including hospitals), health care administrators, patient assistance or other similar programs, or to federal state/provincial, local and other governments, including their agencies, or to wholesalers, distributors or other trade customers;
1.57.6 a fixed amount of [**] percent ([**]%) of gross sales to cover reasonable and customary freight, shipping, insurance and other transportation expenses;
1.57.7 sales, consumption or excise taxes, tariffs and duties, and other taxes and government charges directly related to the sale, delivery or use of Licensed Product (but not including taxes assessed directly against the income derived from such sale); and
- 9 -
Confidential
1.57.8 a reasonable deduction to reflect amounts previously included in Net Sales of Licensed Product that are written off as uncollectible after reasonable collection efforts, in accordance with standard practices of Ono.
Notwithstanding anything in this Agreement to the contrary, the transfer of a Licensed Product between or among Ono and any of its Affiliates and Sublicensees will not be considered a sale.
Disposition of a Licensed Product for, or use of a Licensed Product in, Clinical Studies or other scientific testing, as free samples, or under compassionate use, patient assistance, or test marketing programs or other similar programs or studies shall not result in Net Sales.
Net Sales will be determined from books and records maintained in accordance with IFRS, consistently applied throughout Ono.
In the event a Licensed Product is sold in the form of a Combination Product, then the Net Sales for any such Combination Product shall be determined by multiplying the Net Sales of the Combination Product during the applicable royalty reporting period, by the fraction, A/(A+B+…+N), where A is the weighted (by sales volume) average sale price of the Licensed Product component when sold separately in finished form in the country in which the Combination Product is sold, and B+ … +N are the weighted (by sales volume) average sale prices of the other active pharmaceutical ingredients included in the Combination Product when sold separately in finished form in the country in which the Combination Product is sold, in each case during the applicable royalty reporting period or, if sales of both the Licensed Product component and the other active pharmaceutical ingredients did not occur in such period, then in the most recent royalty reporting period during the preceding twelve (12) months in which sales of both occurred, if any. In the event that such average sale price cannot be determined for the Licensed Product and/or all other active pharmaceutical ingredients included in the Combination Product, then the Parties will in good faith discuss and agree on a pro-rata allocation of the Net Sales that reflects the Licensed Product’s contribution to the Combination Product on an equitable basis.
1.58 “Ono Fiscal Year” means each successive period of twelve (12) calendar months commencing on April 1 of a particular Calendar Year and ending on March 31 of the immediately following Calendar Year.
1.59 “Ono Know-How” means (a) all Know-How, which, as of the Effective Date and during the Term, is Controlled by Ono and its Affiliates, which (i) are not generally known, (ii) are not Covered by a Ono Patent Rights, (iii) relates to any Licensed Compound and/or Licensed Product and (iv) are necessary or useful for the research, Development, Manufacture, having Manufactured, use and/or Commercialization of each Licensed Compound and Licensed Product in the Field and (b) any Know-How generated in course of the Global Clinical Study which is only related to a commercial product or product in clinical development in either case owned by or licensed to Ono; provided, however, that Ono Know-How excludes Joint Know-How.
- 10 -
Confidential
1.60 “Ono Patent Rights” means all Patent Rights Controlled by Ono and its Affiliates, as of the Effective Date and during the Term, which claim or Cover, are necessary or useful for or would be practiced by the research, Development, Manufacture, having Manufactured, use and/or Commercialization of Licensed Products in the Field; provided, however, that Ono Patent Rights excludes Joint Patent Rights.
1.61 “Ono Post-Registration Studies” means any clinical studies of a Licensed Product conducted by Ono or any of its Related Parties following receipt of Regulatory Approval for a Licensed Product necessary to maintain its Regulatory Approval.
1.62 “Ono Technology” means, collectively, Ono Know-How, Ono Patent Rights and Ono’s interest in Joint IP.
1.63 “Ono Territory” means the following countries, as may be amended in accordance with this Agreement: Japan, Republic of Korea, Republic of China (known as Taiwan), Hong Kong, Brunei, Cambodia, Indonesia, Laos, Malaysia, Mayanmar, Philippines, Singapore, Thailand, and Vietnam.
1.64 “Patent Rights” means (a) all issued patents (including extensions, restorations by existing or future extension or registration mechanisms, including patent term adjustments, patent term extensions, supplemental protection certificates or the equivalent thereof, substitutions, confirmations, re-registrations, re-examinations, and patents of addition), (b) patent applications (including all provisional applications, substitutions, requests for continuation, continuations, continuations-in-part, divisionals and renewals), (c) inventor’s certificates, and (d) all equivalents of the foregoing in any country of the world.
1.65 “Person” means any natural person, corporation, unincorporated organization, partnership, association, sole proprietorship joint stock company, joint venture, limited liability company, trust or government, or any agency or political subdivision of any government, or any other similar entity.
1.66 “Phase I Study” means a study in humans which provides for the introduction into humans of a pharmaceutical product, conducted in healthy volunteers or patients, to obtain initial information on product safety, tolerability, pharmacological activity or pharmacokinetics, as more fully defined in 21 C.F.R. § 312.21(a) (or the equivalent thereof outside the United States).
1.67 “Phase II Study” means a study in humans of the safety, dose ranging or efficacy of a pharmaceutical product, as further defined in 21 C.F.R. § 312.21(b) (or the equivalent thereof outside the United States).
1.68 “Phase III Study” means a study in humans of the efficacy and safety of a pharmaceutical product, which is prospectively designed to demonstrate whether such product is effective and safe for use in a particular indication in a manner sufficient (alone or together with one or more other such studies) to file an application for Regulatory Approval for the product.
1.69 “PMDA” means the Pharmaceuticals and Medical Devices Agency in Japan and any successor Governmental Authority having substantially the same function.
- 11 -
Confidential
1.70 “Regulatory Approval” means any and all approvals, licenses, registrations or authorizations of any Regulatory Authority that are necessary for the marketing and sale of a pharmaceutical product in a country or group of countries, including NDAs and orphan drug designations.
1.71 “Regulatory Authority” means any applicable government regulatory authority involved in granting approvals for the Development, Manufacturing or Commercialization of a pharmaceutical product, including, as applicable, the MHLW, PMDA, CDE and MFDS.
1.72 “Regulatory Exclusivity” means any exclusive marketing rights or data exclusivity rights conferred by any Regulatory Authority with respect to any Licensed Product that precludes the use of any Clinical Data collected and filed for such Licensed Product for the benefit of any Regulatory Approval for a generic or biosimilar product (for any use), including any orphan or pediatric exclusivity where applicable.
1.73 “Related Party” means (a) with respect to Karyopharm, Karyopharm’s Affiliates or any of its Third Party Licensee, and (b) with respect to Ono, Ono’s Affiliates and permitted Sublicensees.
1.74 “Selinexor” means the compound known as KPT-330, which is described on Schedule 1.74.
1.75 “Sublicensee” means a Third Party to whom Ono grants a sublicense under any Karyopharm Technology to (a) Develop, use or Commercialize a Licensed Compound or Licensed Product in the Field in the Ono Territory or (b) Manufacture or have Manufactured Licensed Compound or Licensed Product in the Field for the Ono Territory, pursuant to Section 7.2.1.
1.76 “Territory” means (a) with respect to Karyopharm, the Karyopharm Territory, and (b) with respect to Ono, the Ono Territory.
1.77 “Third Party” means a Person other than a Party and its Affiliates.
1.78 “Third Party Licensee” means Karyopharm’s licensees of the Karyopharm Technology in the Karyopharm Territory.
1.79 “United States” or “U.S.” means the United States of America and its territories, possessions and commonwealths.
1.80 “Valid Claim” means any claim in any (a) unexpired and issued patent that has not been disclaimed, revoked or held invalid by a final nonappealable decision of a court or other governmental agency of competent jurisdiction, or (b) to the extent that Karyopharm prosecutes in timely manner pursuant to Section 12.4.2(a), patent application that has not lapsed, in the case of a provisional patent application, or been cancelled, withdrawn or abandoned without the possibility of revival, nor has been pending for more than ten (10) years from the earliest priority date claimed for such application; provided, however, that, if, thereafter, a patent containing such claim matures into registered patent, such claim shall thereafter be considered a Valid Claim in accordance with subclause (a) above.
- 12 -
Confidential
1.81 Additional Definitions. Each of the following definitions is set forth in the Section of this Agreement indicated below:
| Definition: |
Section: | |
| 1974 Convention | 14.2 | |
| Agreement | Preamble | |
| Auditing Party | 8.6.1 | |
| Auditor | 8.6.1 | |
| Change in Control | 14.1 | |
| Clinical Quality Agreement | 6.1.2 | |
| Clinical Supply Agreement Collaboration Provisions |
6.1.2 7.1.3 | |
| Commercial Supply Agreement | 6.2.2 | |
| Common Brand Name | 12.9.1 | |
| Competitive Infringement | 12.5.1 | |
| CRO(s) | 2.1 | |
| Development Milestone Event | 8.2.1 | |
| Development Milestone Payment(s) | 8.2.1 | |
| Disclosing Party | 1.15 | |
| Disputes | 14.3.1 | |
| Effective Date | Preamble | |
| Generic Version | 8.4.2 | |
| Global Clinical Development Plan | 2.5.1 | |
| Global Clinical Study Proposal Global Common Activity |
2.5.2(a) 2.6.2 | |
| Global Development Working Group | 5.5 | |
| Indemnitee | 11.3 | |
| Infringement Action | 12.6.1 | |
| Initiating Party | 12.5.3 | |
| IP Working Group | 5.5 | |
| JOC Chairpersons | 5.1.3 | |
| Joint Patent Costs | 12.4.3 (c) | |
| Joint IP Prosecuting Party | 12.4.3 (a) | |
| Karyopharm | Preamble | |
| Karyopharm Development Plan | 2.4 | |
| Karyopharm Indemnitees | 11.1 | |
| [**] | [**] | |
| Liaison | 5.6 | |
| Manufacturing Technology Transfer Plan | 6.2.3 | |
| Ono | Preamble | |
| Ono Development Plan | 2.3 | |
| Ono Indemnitees | 11.2 | |
| Overview Plan | 2.2 | |
| Party/Parties | Preamble | |
| Patent Challenge | 13.4 | |
| Pre-Existing Affiliates | 14.1 |
- 13 -
Confidential
| Definition: |
Section: | |
| Prior CDA | 1.15 | |
| Receiving Party | 1.15 | |
| Relevant Clinical Studies | 2.1 | |
| Royalty Report | 8.5 | |
| Royalty Term | 8.4.1 | |
| Sales Milestone Event | 8.3.1 | |
| Sales Milestone Payment(s) | 8.3.1 | |
| SDEA | 3.3 | |
| SPC | 12.8.1 | |
| Subject Party | 12.6.1 | |
| Sublicense | 7.2.3 | |
| Term | 13.1 | |
| Third-Country Currency | 8.8.3 | |
| Working Group | 5.5 |
| 2. | DEVELOPMENT |
2.1 Overview. The Parties acknowledge and agree that the Development of the Licensed Products for each Licensed Compound on a global basis is desirable for maximizing such Licensed Products’ value, and therefore that exchange of each Party’s and/or its Related Party’s Development strategy in a transparent manner is imperative for such maximization. Prior to the Effective Date, Karyopharm has been engaged in the Development of the Licensed Compounds and the Licensed Products, including, with respect to each of the Licensed Products, sponsoring the conduct of the Clinical Studies set forth on Schedule 2.1 attached hereto (the “Relevant Clinical Studies”). Karyopharm shall use Commercially Reasonable Efforts to complete, at its own cost and expense, the Relevant Clinical Studies (including all pharmacovigilance aspects), including contracting and managing any contract research organization(s) (“CRO(s)”) that may be involved in such Relevant Clinical Studies, and Karyopharm shall keep the JOC informed of the status thereof conducted by Karyopharm or its Related Party. Subject to the terms of this Agreement and in accordance with this Article 2, with respect to each Licensed Product, Ono shall assume responsibility for the Development of Licensed Products in the Field in the Ono Territory and Karyopharm shall assume responsibility for the Development of Licensed Products in the Field in the Karyopharm Territory.
2.2 Overview Plan. The Parties have agreed to an overview of the key steps and timelines for Development of the Licensed Compounds and the Licensed Products in the Field in both the Ono Territory and the Karyopharm Territory as outlined in Schedule 2.2 attached hereto (the “Overview Plan”).
2.3 Development Plans for the Ono Territory. Within [**] after the Effective Date, Ono shall prepare and submit to the JOC for review and discussion a written plan for the Development of the Licensed Product in the Ono Territory (each, a “Ono Development Plan”)
- 14 -
Confidential
containing Selinexor setting forth the objectives of the Development to be conducted by Ono, a plan for the conduct of Clinical Studies by or on behalf of Ono or any of its Related Parties in the Ono Territory relating to the applicable Licensed Products containing Selinexor, the Development activities to be undertaken with respect to such Licensed Products containing Selinexor by or on behalf of Ono in the Field in the Ono Territory and a time table for the conduct of such activities, which Ono Development Plan shall be consistent with the Overview Plan and include a planning horizon of [**]. By the [**] of the Effective Date, Ono shall prepare and submit to the JOC for review and discussion an Ono Development Plan for the Licensed Product containing KPT-8602 setting forth the objectives of the Development to be conducted by Ono, the Development activities to be undertaken with respect to such Licensed Products containing KPT-8602 by or on behalf of Ono in the Field in the Ono Territory and a time table for the conduct of such activities. Ono will present any proposed amendments to each Ono Development Plan to the JOC for the JOC’s review and discussion reasonably in advance of Ono’s intention to implement such plans or amendments, including any amendments required under Section 2.7.2 below. For each Ono Development Plan, Ono shall also prepare and submit an updated Ono Development Plan to the JOC for the JOC’s review and discussion [**].
2.4 Development Plans for the Karyopharm Territory. Karyopharm shall and/or shall use Commercially Reasonable Efforts to cause its Related Party to prepare written plans for each Licensed Compound (each a “Karyopharm Development Plan”) setting forth the objectives of the Development to be conducted by Karyopharm and/or its Related Party with regard to such Licensed Compound and the Licensed Products containing such Licensed Compound in the Karyopharm Territory and written clinical development plans setting forth the Development activities and time tables regarding any Clinical Studies including Relevant Clinical Studies that Karyopharm and/or its Related Party conducts as of the Effective Date in the Karyopharm Territory or Karyopharm and/or its Related Party believes should be conducted within the Karyopharm Territory with regard to any Licensed Product. Within [**] after the Effective Date, Karyopharm shall prepare and submit to the JOC for review and discussion a Karyopharm Development Plan, which Karyopharm Development Plan shall be consistent with the Overview Plan and include a planning horizon of [**]. Karyopharm will present any proposed amendments to each Karyopharm Development Plan to the JOC for the JOC’s review and discussion reasonably in advance of Karyopharm’s intention to implement such plans or amendments. For each Karyopharm Development Plan, Karyopharm shall also prepare and submit an updated Karyopharm Development Plan to the JOC for the JOC’s review and discussion [**].
| 2.5 | Global Clinical Development Plan. |
2.5.1 Global Clinical Development Plan. Karyopharm shall and/or shall use Commercially Reasonable Efforts to cause its Related Party to prepare written global clinical development plans setting forth the Development activities and time tables regarding any Global Clinical Study that Karyopharm and/or its Related Party believes should be conducted with regard to any Licensed Product (each a “Global Clinical Development Plan”). Within [**] after the Effective Date, Karyopharm shall prepare and submit to the JOC for review and approval, an initial Global Clinical Development Plan setting forth a plan for the conduct of Global Clinical Studies by or on behalf of Karyopharm or any of its
- 15 -
Confidential
Related Parties relating to the applicable Licensed Products, the Development activities to be undertaken with respect to such Licensed Products by or on behalf of Karyopharm or any of its Related Parties in the Field and a time table for the conduct of such activities, which initial Global Development Plan shall be consistent with the Overview Plan and include a planning horizon of [**]. For each Global Clinical Development Plan, Karyopharm shall also prepare and submit an updated Global Clinical Development Plan to the JOC for the JOC’s review and approval [**].
2.5.2 Global Clinical Studies.
(a) From time to time during the Term, either Karyopharm (and/or its Related Party) or Ono (and/or its Related Party) may submit to the JOC a proposal for a Global Clinical Study that would support the filing of an NDA for the Licensed Product with Regulatory Authorities in both the Karyopharm Territory and the Ono Territory (a “Global Clinical Study Proposal”). Each such Global Clinical Study Proposal shall include a draft synopsis, proposed timelines for the conduct of such Global Clinical Studies. The JOC shall review and approve each such Global Clinical Study Proposal. If the JOC approves a Global Clinical Study Proposal within [**] after the date of submission to the JOC of such Global Clinical Study Proposal, Karyopharm shall prepare a Global Clinical Development Plan based on such Global Clinical Study Proposal. The Parties shall discuss in good faith the applicable Global Clinical Development Plan through the Working Group designated by the JOC, if necessary and Karyopharm shall consider and reflect Ono’s comments to such Global Clinical Development Plan to the extent that those comments are reasonable based on scientific, business, and/or other relevant considerations. Karyopharm shall provide such Global Clinical Development Plan for the JOC for review and approval. Ono shall be responsible for bearing all costs and expenses incurred for patients enrolled in such Global Clinical Study in the Ono Territory. In the event that the JOC does not approve the Global Clinical Study Proposal within [**] after such Global Clinical Study Proposal has been submitted to the JOC or the JOC does not approve the Global Clinical Development Plan within [**] after such Global Clinical Development Plan has been submitted to the JOC, such proposing Party shall be free to carry out at its own cost and expense the relevant Clinical Study(ies) described in such Global Clinical Study Proposal independently, within such proposing Party’s Territory.
(b) If Karyopharm or its Related Party proposes to expand a then-existing clinical-stage Development effort including any Relevant Clinical Study and any Clinical Study in accordance with Karyopharm Development Plan so that it would become a Global Clinical Study, then Karyopharm shall include such proposal in a Global Clinical Development Plan and Karyopharm shall provide Ono with such Global Clinical Development Plan through the JOC and Working Group designated by the JOC for Ono’s review. If Ono desires to participate in such Global Clinical Study, it shall provide written notice thereof to the JOC and Karyopharm within [**] after the date of the JOC’s receipt of the applicable Global Clinical Development Plan. The Parties shall discuss the applicable Global Clinical Development Plan through the Working Group designated by the JOC during such [**] period if necessary, and Karyopharm shall consider and reflect Ono’s comments to such Global Clinical Development Plan to the extent that those comments are reasonable based on scientific, business, and/or other relevant considerations. Karyopharm shall provide such Global Clinical Development Plan to the JOC for review and approval. Ono shall be responsible for bearing all costs and expenses incurred for patients enrolled in such Global Clinical Study in the Ono Territory after the date of JOC’s approval.
- 16 -
Confidential
(c) If Ono notifies Karyopharm in writing that Ono reasonably believes that a Clinical Study of a Licensed Product in the Field being conducted or determined to be conducted by Karyopharm and/or its Related Party in accordance with Karyopharm Development Plan or proposed by Karyopharm and/or its Related Party should be expanded to be a Global Clinical Study, Karyopharm shall expand such Clinical Study into a Global Clinical Study, and Karyopharm will include such proposal in a Global Clinical Development Plan that it provides to Ono through the JOC and Working Group designated by the JOC for the JOC’s review within [**] after the date of Karyopharm’s receipt of Ono’s written notification. The Parties shall discuss in good faith the applicable Global Clinical Development Plan through the Working Group designated by the JOC during such [**] period if necessary, and Karyopharm shall consider and reflect Ono’s comments to such Global Clinical Development Plan to the extent that those comments are reasonable based on scientific, business, and/or other relevant considerations. Karyopharm shall provide such Global Clinical Development Plan to the JOC for review and approval. Ono shall be responsible for bearing all costs and expenses incurred for patients enrolled in such Global Clinical Study in the Ono Territory after the date of JOC’s approval.
(d) In connection with any Global Clinical Development Plan or amendment thereto that includes any Global Clinical Study, Karyopharm may present comments on such Global Clinical Development Plan or amendment thereto from any Karyopharm’s Related Party outside of the Ono Territory with regard to the conduct of any such Global Clinical Study and the JOC shall consider in good faith any such comments.
(e) In case that amendment to the Global Clinical Development Plan is finally decided as the result of Karyopharm’s exercise of its deciding vote pursuant to Section 5.4.3(b), Ono may change the status of its part therein from “Global Clinical Study” to “Development in the Ono Territory”, which will be subject to Ono’s final deciding vote set forth in Section 5.4.3(a).
2.6 Responsibilities for Development Activities and Costs; No Conduct in Other Party’s Territory.
2.6.1 Ono Development Activities. Ono shall be responsible for the Development of the Licensed Products for each Licensed Compound in the Field in the Ono Territory, including the conduct of any Clinical Studies in the Ono Territory, in accordance with the terms of this Agreement. Ono shall be responsible for one hundred percent (100%) of all costs and expenses relating to Development activities that are conducted by or on behalf of Ono, including Global Clinical Studies in the Ono Territory. Ono will conduct all Development of the Licensed Products in the Field for the Ono Territory solely in accordance with the terms of this Agreement and the applicable Ono Development Plan or Global Clinical Development Plan, as applicable, as such Ono Development Plan or Global Clinical Development Plan may be amended or updated from time to time in accordance with this Agreement, and in accordance with all applicable Law.
- 17 -
Confidential
2.6.2 Karyopharm Development Activities. Karyopharm shall be responsible for, at its own cost and expense, all of its activities relating to the Development of the Licensed Compounds and the Licensed Products in the Karyopharm Territory and Global Common Activity, including the costs and expenses relating to its participation in Global Clinical Studies in the Karyopharm Territory or to conduct additional Development work in the Karyopharm Territory to Develop a Back-Up Compound. In addition, Karyopharm shall be responsible for all of its own costs and expenses relating to the preparation of any Global Clinical Study Plan and all Global Common Costs. “Global Common Activity” means any Development activity with regard to Global Clinical Study that is not specific to Development activities in Ono Territory or Karyopharm Territory: which includes, but not limited to, the project management, data management, pharmacovigilance support, statistical support and statistical analysis on global basis (i.e. both of Karyopharm Territory and Ono Territory).
2.6.3 No Conduct of Clinical Trials in Other Party’s Territory. During the Term, neither Party may conduct Clinical Studies or other Development activities with respect to a Licensed Product in the other Party’s Territory without such other Party’s prior written consent, which consent may be granted or withheld in the sole discretion of the other Party.
| 2.7 | Development in the Ono Territory. |
2.7.1 Diligence. With respect to each Licensed Compound, Ono will use Commercially Reasonable Efforts to Develop and to obtain Regulatory Approval for the Licensed Products in the Field in each country in the Ono Territory.
2.7.2 Amendment to Ono Development Plan Subsequent to Discontinuation of the Development of a Licensed Product. In the event that Ono determines not to continue the Development of a Licensed Product in the Field in the Ono Territory, Ono shall notify the JOC of its discontinuation of such Development activities in a written statement, which describes in reasonable detail the reasons that Ono determined to discontinue such Development activities. Karyopharm shall provide the JOC with all Know-How on all candidates for Back-Up Compounds Controlled by Karyopharm or its Affiliates as soon as practicable after its receipt through JOC of such notification by Ono. The Parties, through JOC, shall use Commercially Reasonable Efforts to determine a Back-Up Compound among such candidates (or not to select a Back-Up Compound), which replaces a Licensed Compound. Ono shall prepare an amendment to the Ono Development Plan to reflect any changes to the objectives, Development activities or time table set forth in the Ono Development Plan arising from the replacement of such discontinued Licensed Product with a substitute Licensed Product, if any, within [**] after the determination of Back-Up Compound by the JOC.
| 2.8 | Records; Reports; Information Sharing. |
- 18 -
Confidential
2.8.1 Development Activities. Each Party will provide the JOC with a [**] update regarding Development activities conducted by or on behalf of each Party and/or its Related Party under this Agreement. Each Party shall regularly inform the other Party, and shall formally provide written progress reports for the JOC on a [**] basis, summarizing the Development activities conducted by each Party and its Related Party, including any issues relating to meeting the obligations, objectives or timetables set forth each in the Ono Development Plan, the Karyopharm Development Plan or the Global Development Plan.
2.8.2 Scientific Records. Subject to Section 7.1.3, each Party shall and shall use Commercially Reasonable Efforts to cause its Related Party to maintain complete, current and accurate records of all Development work conducted by or on behalf of each Party and/or its Related Party, and all Clinical Data, Data and other Know-How resulting from such work. Such records shall fully and properly reflect all work done and results achieved in the performance of the Development activities in a good scientific manner appropriate for regulatory and patent purposes. Each Party shall, and shall use Commercially Reasonable Efforts to cause its Related Party to, document all Clinical Studies and other studies and research in formal written study reports in accordance with applicable guidelines (e.g., GCP, GLP, and GMP) and all other applicable Law. Subject to 7.1.3, each Party shall, and shall use Commercially Reasonable Efforts to cause its Related Party, to make all such Clinical Data, records and reports continuously available, within a reasonable period following their creation, to the other Party for inspection and review (including, to the extent reasonably requested, copying) through appropriate electronic data room facilities. Subject to applicable Law (including, but not limited to, the data privacy act in each country), each Party shall also have the right to review original versions of such records maintained by other Party and its Affiliates (and, to the extent permissible, its Related Parties) no more often than [**], at reasonable times, upon written request to other Party.
2.8.3 Data Transfer.
(a) Within [**] after the Effective Date, Karyopharm shall transfer in electronic format to Ono all technical, and regulatory documents and Data Controlled by Karyopharm that are necessary or useful for Ono to conduct the Development activities and to perform Ono’s obligation or exercise Ono’s rights hereunder, existing as of the Effective Date. The Parties acknowledge that Karyopharm may be requested to arrange notarization or other certification of certain elements of the Data of Karyopharm and its Affiliates for official purposes. With Ono’s guidance or to the extent required for Development as requested by Regulatory Authorities for the Licensed Products in the Ono Territory, Karyopharm shall provide Ono with copies of documents covering (i) Data authenticity documents relevant to the Licensed Compounds and Licensed Products, (ii) authorization to use Data provided by Karyopharm, (iii) documentation perfecting the patent license provisions of this Agreement, and (iv) GLP documents; provided, however, that the foregoing shall reflect Karyopharm’s work conducted prior to the Effective Date and none of the foregoing shall require Karyopharm to perform or conduct further research, laboratory, Manufacturing or other work solely for the Ono Territory,
- 19 -
Confidential
including any work to establish GLP or GMP compliance, except pursuant to Section 2.8.3(b) below. Karyopharm’s provision of those documents shall be made in accordance with the time-schedule agreed upon between the Parties (to the extent feasible, within [**] after the Effective Date).
(b) If Ono believes it would be desirable to have additional work performed by Karyopharm to assist in the transition of Development activities from Karyopharm to Ono or an on-site transfer, the Parties may jointly develop a written scope of work to be performed by Karyopharm and Ono, including timelines, terms, costs, and resource requirements, to be mutually agreed by both Parties. The JOC will review and provide comments on any such scope of work before such scope of work is executed by both Parties. Out-of-pocket costs incurred by the Parties for such additional work shall be [**].
2.8.4 Information Sharing. During the Term, subject to Section 7.1.3, each Party shall provide the other Party with all Know-How Controlled by such Party and/or use Commercially Reasonable Efforts to provide the other Party with all Know-How Controlled by its Related Party, that is generated during the Term of this Agreement, that has not previously been provided hereunder and that is necessary or useful for the Development or Commercialization of the Licensed Compounds or Licensed Products in the Field in the other Party’s Territory, in each case promptly upon request by the other Party. The Party providing such Party’s and/or its Related Party’s Know-How shall provide the same in electronic form to the extent the same exists in electronic form, and shall provide copies for all other materials comprising such Know-How (including, for example, original patient report forms and other original source data). Any Data provided by one Party to the other Party under this Section 2.8.4 shall be provided in the original language in which such Data was generated, provided that, with respect to Data relating to any Global Clinical Study, if such original language is not English, then the Party supplying such Data shall also provide English translations thereof and the expense for such English translations shall be [**]. The Parties will cooperate and reasonably agree upon formats and procedures to facilitate the orderly and efficient exchange of such Know How.
2.8.5 Rights of Reference and Access to Data. Subject to Section 7.1.3, each Party shall have the right to cross-reference the regulatory filings and Regulatory Approvals (and each Party’s Related Party’s regulatory filings and Regulatory Approvals) related to the Licensed Products, and to access such regulatory filings and such Regulatory Approvals and any Data therein and use such Data in connection with the performance of its obligations and exercise of its rights under this Agreement, including inclusion of such Data in its own regulatory filings for a Licensed Product free of charge. Each Party hereby will grant, and will cause its Related Party to grant, to the other Party and its Related Party a “Right of Reference,” as that term is defined in 21 C.F.R. § 314.3(b) in the United States, or an equivalent right of access/reference in any other country or region, to any Data, including such Party’s or its Related Party’s clinical dossiers, Controlled by such Party or such Related Party that relates to the Licensed Product for use by the other Party to Develop and Commercialize the Licensed Product in
- 20 -
Confidential
the Field pursuant to this Agreement. Each Party shall provide a signed statement to this effect, if requested by the other Party, in accordance with 21 C.F.R. § 314.50(g)(3) or the equivalent as required in any country or region or otherwise provide appropriate notification of such right of the other Party to the applicable Regulatory Authority and shall cause its Related Party to provide such signed statement. Each Party will provide, and will cause its Related Party to provide, cooperation to the other Party to effect the foregoing.
2.8.6 Investigator Sponsored Clinical Studies. Ono shall have the right to authorize the protocol for each Investigator Sponsored Clinical Study in the Ono Territory and support such Investigator Sponsored Clinical Study at Ono’s own discretion, provided, however, Ono agrees to inform Karyopharm of all such Investigator Sponsored Clinical Study(ies) in a timely manner and each proposal shall be subject to review and comment by a Working Group designated by the JOC. Karyopharm shall have the right to authorize the protocol for each Investigator Sponsored Clinical Study in the Karyopharm Territory and support such Clinical Study at Karyopharm’s own discretion, provided, however, Karyopharm agrees to inform Ono of all such Investigator Sponsored Clinical Study(ies) in a timely manner and each proposal shall be subject to review and comment by a Working Group designated by the JOC. Neither Party shall authorize or support an Investigator Sponsored Clinical Study in the other Party’s Territory without such other Party’s prior written consent, which consent may be granted or withheld in the sole discretion of the other Party.
| 3. | REGULATORY MATTERS |
| 3.1 | Regulatory Filings and Interactions. |
3.1.1 Responsibilities. Each Party will own the INDs, the NDAs and related regulatory documents submitted to the applicable Regulatory Authorities for its Development activities with respect to each Licensed Product in the Field, and for Commercialization in its Territory with respect to each Licensed Product in the Field. Each Party will (a) oversee, monitor and coordinate all regulatory actions, communications and filings with, and submissions to, each Regulatory Authority, (b) be responsible for interfacing, corresponding and meeting with each Regulatory Authority and (c) be responsible for maintaining all regulatory filings, in each case of (a)-(c) with respect to its Development activities with respect to each Licensed Product in the Field, and with respect to Commercialization of each Licensed Product in the Field in its Territory.
3.1.2 Communications and Cooperation. Karyopharm shall cooperate in good faith with Ono pertaining to Ono’s Development activities and regulatory affairs with respect to each Licensed Product in the Field in the Ono Territory at Ono’s sole cost and expense. Ono will, as to each Licensed Product in the Field in the Ono Territory, (a) notify Karyopharm in writing of all material communications from a Regulatory Authority within [**] after receipt thereof, including a brief description in English of the principal issues raised, (b) provide
- 21 -
Confidential
Karyopharm with a summary translation of such material communications in English as soon as reasonably possible, and (c) provide the complete copies of the original correspondence in its original language to Karyopharm upon request. Ono shall provide Karyopharm with reasonable advance notice of all substantive meetings with the Regulatory Authorities in the Ono Territory pertaining to each Licensed Product in the Field, or with as much advance notice as practicable under the circumstances. Karyopharm may, at its own cost, attend such meetings with Regulatory Authorities as an observer upon reasonable advance notice to Ono, subject to Ono’s prior written consent and receipt of any required permissions of such Regulatory Authorities. Karyopharm will, as to each Licensed Product in the Field in the Karyopharm Territory, (a) notify Ono in writing of all material communications from a Regulatory Authority within [**] after receipt thereof, including a brief description in English of the principal issues raised, (b) provide Ono with a summary translation of such material communications in English as soon as reasonably possible, and (c) provide the complete copies of the original correspondence in its original language to Ono upon request. Karyopharm shall provide Ono with reasonable advance notice of all substantive meetings with the Regulatory Authorities in the Karyopharm Territory pertaining to each Licensed Product in the Field, or with as much advance notice as practicable under the circumstances. Ono may, at its own cost, attend such meetings with Regulatory Authorities as an observer upon reasonable advance notice to Karyopharm subject to Karyopharm’s prior written consent and receipt of any required permissions of such Regulatory Authorities.
3.1.3 Without limiting the obligations under Section 3.1.2, Ono shall provide to Karyopharm copies of the proposed labeling for the Licensed Product in the local language to be filed in the Ono Territory. Additionally, Ono shall provide Karyopharm with (a) a copy of the NDA in electronic format, provided that in cases where the NDA was not filed electronically, Ono will provide the electronic files used to generate such submission, and (b) copies of the final labeling for the Licensed Product in the local language in all countries in the Ono Territory in which Ono obtains Regulatory Approvals. Karyopharm shall and/or shall cause its Related Party to provide Ono with copies of the proposed labeling for the Licensed Product in the local language to be filed by Karyopharm in United States, France, Germany, Italy, Spain and the United Kingdom. Additionally, (a) Karyopharm shall, and shall cause its Related Party, to provide Ono with a copy of the NDA filed by Karyopharm and/or its Related Party with the FDA and the EMA, in each case in electronic format, provided that in cases where the NDA was not filed electronically, Karyopharm will and/or will cause its Related Party to provide the electronic files used to generate such submission, and (b) Karyopharm shall and/or shall cause its Related Party to provide Ono with copies of the final labeling for the Licensed Product in the local language in all countries in the Karyopharm Territory in which Karyopharm and/or its Related Party obtain(s) Regulatory Approvals.
- 22 -
Confidential
3.1.4 Submissions. In addition, Ono shall provide the JOC with written notice of the fact of (a) the filing and submitting for Regulatory Approval (including orphan drug applications and designations) regarding each Licensed Product in the Field in the Ono Territory in a timely manner; (b) whether Regulatory Approval is obtained or denied regarding each Licensed Product in the Field in the Ono Territory in a timely manner; and (c) the filing of any IND for each Licensed Product in the Field in the Ono Territory as soon as practicable after such event; provided, however, that in all circumstances, Ono shall inform the JOC of such event prior to public disclosure of such event by Ono except to the extent such public disclosure is required by Law.
3.2 Costs of Regulatory Affairs. Each Party shall be responsible for all costs incurred by or on behalf of it in connection with applying for Regulatory Approval with respect to each Licensed Product in the Field in each country in its own Territory and related regulatory affairs activities.
3.3 Pharmacovigilance. Prior to Ono’s filing IND for its first Clinical Study with respect to the Licensed Products, the Parties will negotiate and finalize a Safety Data Exchange Agreement (the “SDEA”) to be agreed upon in writing, that will define the pharmacovigilance responsibilities of the Parties and include safety data exchange procedures governing the coordination of collection, investigation, reporting, and exchange of information concerning any adverse experiences, and any product quality and product complaints associated with adverse experiences, related to the Licensed Products, sufficient to enable each Party (and their respective Related Parties, if any) to comply with its legal and regulatory obligations. The SDEA shall be modified in writing before obtaining the Regulatory Approval for such Licensed Products in either Territory, to enable each Party (and their respective Related Parties, if any) to comply with its legal and regulatory obligations. The Parties shall use Commercially Reasonable Efforts to amend the SDEA to add as parties any Related Parties.
| 4. | COMMERCIALIZATION OF THE LICENSED PRODUCTS |
| 4.1 | Responsibility, Cost and Diligence. |
4.1.1 Ono’s Commercialization Activities. Subject to the terms of this Agreement, Ono shall be solely responsible for all Commercialization activities relating to the Licensed Products in the Field in the Ono Territory. Ono shall be responsible for one hundred percent (100%) of all costs relating to Commercialization activities that are conducted by or on behalf of Ono. With respect to each Licensed Compound, Ono shall use Commercially Reasonable Efforts to Commercialize the Licensed Products in the Field in each country within the Ono Territory (including obtaining all required pricing and reimbursement approvals) as promptly as possible following receipt by Ono or its Related Parties of Regulatory Approval for such Licensed Product in such country. Such Commercialization efforts may include conducting Ono post-registration studies in the Ono Territory as may be necessary to expand the potential market for Licensed Products in the Field, planning and implementation, distribution, booking of sales, pricing and reimbursement, establishing and developing appropriate opinion leaders, promoting Licensed Products with managed care organizations and establishing Licensed Products with formularies.
- 23 -
Confidential
4.1.2 Karyopharm’s Commercialization Activities. Karyopharm shall be responsible for, at its own cost and expense, all of its activities relating to Commercialization of the Licensed Products in the Karyopharm Territory, including planning and implementation, distribution, booking of sales, pricing and reimbursement.
4.2 Reporting Obligations. Ono shall provide Karyopharm with written notice of each First Commercial Sale of a Licensed Product in a country in the Ono Territory within [**] after such event; provided, however, that in all circumstances, Ono shall inform Karyopharm of such event prior to public disclosure of such event by Ono. Ono shall also provide such other information, including its sales department structure, sales marketing structure and medical affairs structure to the JOC as Ono deems necessary or useful for Karyopharm and shall keep Karyopharm and the JOC reasonably informed of Ono’s Commercialization activities with respect to Licensed Products.
4.3 Commercialization by Karyopharm. Karyopharm shall provide information regarding its Commercialization activities, including its sales department structure, sales marketing structure and medical affairs structure to the JOC as Karyopharm deems necessary or useful for Ono and shall keep Ono and the JOC reasonably informed of Karyopharm’s Commercialization activities with respect to Licensed Products.
4.4 Sales and Distribution. Each Party and its Related Parties shall be responsible for booking sales and shall warehouse and distribute Licensed Products in the Field in its Territory. Moreover, each Party and its Related Parties shall be solely responsible for handling all returns of Licensed Product in the Field sold in its Territory, as well as all aspects of Licensed Product order processing, invoicing and collection, distribution, inventory and receivables of Licensed Products sold in its Territory.
4.5 Recalls, Market Withdrawals or Corrective Actions. In the event that any Regulatory Authority issues or requests a recall or market withdrawal or takes a similar action in connection with the Licensed Product in the Field in any part of a Party’s Territory, or in the event either Party determines that an event, incident or circumstance has occurred that may result in the need for such a recall, market withdrawal or similar action in its own Territory, the Party notified of such a recall, market withdrawal or similar action, or the Party that desires such a recall, market withdrawal or similar action, shall within [**] advise the other Party thereof by telephone, facsimile or e-mail, followed immediately by a notice in accordance with Section 14.10. Each Party, in consultation with the other Party but in its own discretion, shall decide whether to conduct such a recall, market withdrawal or similar action in its own Territory and the manner in which any such a recall, market withdrawal or similar action shall be conducted (except in the case of a government mandated recall, market withdrawal or similar action when such Party may act without such advance notice but shall notify the other Party as soon as possible). Subject to the terms and conditions of the Supply Agreement, each Party shall bear the expense of any such a recall, market withdrawal or similar action in its own Territory. Each Party will make available all of its pertinent records that may be reasonably requested by the other Party in order to effect such a recall, market withdrawal or similar action in the other Party’s Territory.
- 24 -
Confidential
| 4.6 | Ex-Territory Sales; Export Monitoring. |
4.6.1 Ex-Territory Sales. Subject to applicable Law, neither Party shall engage in any advertising or promotional activities relating to any Licensed Product in the Field directed primarily to customers or other buyers or users of such Licensed Product in the Field located outside its Territory or accept orders for such Licensed Product in the Field from, or sell such Licensed Product in the Field into, such other Party’s Territory for its own account, and if a Party receives any order for such Licensed Products in the Field in the other Party’s Territory, it shall refer such orders to the other Party.
4.6.2 Export Monitoring. Each Party and its Related Parties will use Commercially Reasonable Efforts at its own cost to monitor and prevent exports of each Licensed Product from its own Territory for Commercialization in the other Party’s Territory, or commercial use in the Ono Territory outside the Field of a Licensed Product sold by Ono or its Related Parties, using methods commonly used in the industry for such purpose, and shall promptly inform the other Party of any such exports of any Licensed Product from its Territory of which it becomes aware, and the actions taken to prevent such exports, to the extent permitted by applicable Law. Each Party shall, at the other Party’s cost, take reasonable actions requested in writing by the other Party that are consistent with applicable Law to prevent exports of the Licensed Products from its Territory for Commercialization in the other Party’s Territory or the use of Licensed Product, to the extent permitted by applicable Law.
| 4.7 | Promotional Materials. |
4.7.1 Karyopharm Promotional Materials. Subject to Section 7.1.3, Karyopharm shall, and shall use Commercially Reasonable Efforts to cause its Related Party to, provide Ono with copies of core promotional materials (written, printed, video or graphic advertising, promotional, educational and communication materials) developed and used in the Karyopharm Territory by Karyopharm or its Related Parties in Commercializing the Licensed Product to support Ono’s Commercialization activities for the Licensed Product in the Ono Territory, including materials relating to marketing strategies for the Licensed Product in the Karyopharm Territory pursued by Karyopharm or its Related Parties, where reasonably requested by Ono. Ono may use information contained in such promotional materials, free of charge, for preparation of promotional materials relating to the Licensed Product for use by Ono or its Related Parties in connection with Commercialization of the Licensed Product in the Field in the Ono Territory and for no other purpose, unless the Parties agree otherwise in writing.
- 25 -
Confidential
4.7.2 Ono Promotional Materials. Subject to Section 7.1.3, Ono shall, and shall use Commercially Reasonable Efforts to cause its Related Parties to, provide Karyopharm with copies of core promotional materials (written, printed, video or graphic advertising, promotional, educational and communication materials) developed and used in the Ono Territory by Ono or its Related Parties in Commercializing the Licensed Product to support Karyopharm’s Commercialization activities for the Licensed Product in the Karyopharm Territory, including materials relating to marketing strategies for the Licensed Product in the Ono Territory pursued by Ono or its Related Parties, where reasonably requested by Karyopharm. Karyopharm may use information contained in such promotional materials, free of charge, for preparation of promotional materials relating to the Licensed Product for use by Karyopharm or its Related Parties in connection with Commercialization of the Licensed Product in the Karyopharm Territory and for no other purpose, unless the Parties agree otherwise in writing.
| 5. | GOVERNANCE. |
5.1 Joint Operating Committee. Within [**] after the Effective Date, Karyopharm and Ono shall designate their representatives to the joint operating committee (the “Joint Operating Committee” or “JOC”) to oversee and monitor Development, Manufacturing, use and Commercialization activities, and to serve as the decision-making body for certain activities, under this Agreement with respect to the Licensed Compounds and the Licensed Products in the Field. The Parties anticipate that the JOC will not be involved in day-to-day implementation of such activities under this Agreement.
5.1.1 Composition of the Joint Operating Committee. The JOC shall be comprised of an equal number of representatives of each Party, which number shall be three (3) representatives of each Party. The JOC representatives shall be senior-level employees of the appointing Party having appropriate experience, expertise and decision-making authority. All JOC representatives shall have appropriate expertise and ongoing familiarity with the Licensed Products in the Field and this Agreement. Either Party may replace its respective JOC representatives at any time with prior written notice to the other Party; provided that the criteria for composition of the JOC set forth in the preceding sentence shall continue to be satisfied following any such replacement of a Party’s representative on JOC. An alternate member designated by a Party may serve temporarily in the absence of a member of the JOC for such Party. Each Party may invite its employees involved in Development, Manufacturing, use or Commercialization of the Licensed Product for JOC meetings with the prior notice to the other Party. All representatives on the JOC, and all other attendees at a JOC meeting, shall be subject to confidentiality obligations, whether in a written agreement or by operation of law, no less stringent than the requirements of Section 9.1.
5.1.2 JOC Responsibilities. The JOC shall have the following responsibilities:
- 26 -
Confidential
(a) review and discuss each Ono Development Plan, and all amendments and updates to such Ono Development Plan;
(b) as part of its review and discussion of an Ono Development Plan, take into account the comments or views of Karyopharm or any of Karyopharm’s Related Parties outside of the Ono Territory as Karyopharm may present to the JOC;
(c) review progress reports provided by Ono with respect to its Development activities;
(d) monitor and provide Ono with feedback regarding the conduct of Development activities by or on behalf of Ono;
(e) review and discuss each Karyopharm Development Plan, and all amendments and updates to such Karyopharm Development Plan;
(f) review progress reports provided by Karyopharm with respect to Development activities by Karyopharm and its Related Party;
(g) coordinate Development activities conducted by Ono and its Related Parties with the activities conducted by Karyopharm and its Related Parties in their respective Territories, including regulatory and pharmacovigilance requirements and matters;
(h) subject to the escalation provision in Section 5.4, review and approve each Global Clinical Study Proposal (it being confirmed that such review shall be subject to and supported by information sharing exchange in the Global Development Working Group pursuant to Section 5.5);
(i) subject to the escalation provision in Section 5.4, review and approve each Global Clinical Development Plan and any amendments to such Global Clinical Development Plan (it being confirmed that such review shall be subject to and supported by information sharing exchange in the Global Development Working Group pursuant to Section 5.5);
(j) review and discuss each Party’s and/or its Related Party’s long term Development strategy in the respective Territory in a timely manner (it being confirmed that such review and discussion shall be subject to and supported by information sharing exchange in the Global Development Working Group pursuant to Section 5.5);
(k) designate a Back-Up Compound among its candidates provided by Karyopharm in accordance with Section 2.7.2;
(l) review and provide comments on scope of work for additional work relating to Data transfer as specified in Section 2.8.3(b);
(m) oversee the manufacturing and supply relationship between the Parties with respect to Manufacture of Licensed Compounds and Licensed Products in case Licensed Compounds and Licensed Products are supplied by the Karyopharm to Ono for the Ono Territory;
- 27 -
Confidential
(n) review and provide comments with respect to the commercialization plan and marketing strategy of Karyopharm and/or any of Karyopharm’s Related Party in the Karyopharm Territory and Ono and/or any of Ono’s Related Party in the Ono Territory and any material updates or amendments thereto;
(o) providing a forum for the Parties to discuss Commercialization of Licensed Products in the Field worldwide, including coordination regarding Licensed Product positioning and messaging, key opinion leader relationship management, medical affairs, and marketing and selling materials; and
(p) performing such other activities as the Parties shall determine to be the responsibility of the JOC.
5.1.3 JOC Chairperson. Each Party shall designate one (1) of their JOC representative to be a co-chairperson. The JOC shall be co-chaired by one (1) representative selected by Karyopharm and one (1) representative selected by Ono (the “JOC Chairpersons”). Either Party shall have the right to change their JOC Chairperson by written notice to the other Party. The JOC Chairpersons’ responsibilities shall include (a) scheduling meetings in accordance with Section 5.2.1, but more frequently if a Party request in accordance with Section 5.2.1 or the JOC determines it necessary; (b) setting agendas for meetings with solicited input from other members; (c) coordinating the delivery of draft minutes to the JOC for review and final approval; and (d) conducting meetings, including ensuring that objectives for each meeting are set and achieved.
| 5.2 | Meetings. |
5.2.1 The JOC shall meet no less frequently than [**] until the [**] and [**] during the Term. In addition to the regular meetings, either Party may request an ad-hoc meeting of the JOC to solve any specific issues from time to time. In the event that an urgent issue or matter that requires prompt action by the JOC arises, each Party may call an emergency meeting of the JOC to attempt to resolve such issue or matter. Such meeting (or other means of communication) shall take place by teleconference or videoconference (unless otherwise mutually agreed by the Parties) as promptly as possible.
5.2.2 The location for the in-person meetings of the JOC shall, respectively, alternate between Karyopharm’s headquarters and Ono’s headquarters (or such other locations as are mutually agreed by the Parties). Alternatively, the JOC may meet by means of teleconference, videoconference or other similar communications equipment, but at least [**] of the JOC shall be conducted in person.
- 28 -
Confidential
5.2.3 All proceedings for the JOC shall take place in English. Each Party shall bear its own expenses relating to participation at such meetings by its representatives.
5.3 Minutes. The Liaison of a Party who is hosting the meeting (in person or via teleconference, videoconference or other similar communications equipment) shall arrange each meeting of the JOC and shall prepare minutes of the meeting, which shall provide a description in reasonable detail of the discussions held at the meeting and a list of any actions, decisions or determinations approved by the JOC.
| 5.4 | Decision-Making. |
5.4.1 Any decisions of the JOC shall be made by unanimous vote. With respect to decisions of the JOC, each Party shall have one (1) vote, exercised through its representatives on the JOC on behalf of such Party. For each meeting of the JOC, a quorum exists so long as there is at least one (1) representative of each Party present at the meeting. Action on any matter may be taken at a meeting, by teleconference, videoconference or by written consent. The JOC shall attempt to resolve any and all disputes before it for decision by consensus.
5.4.2 If the JOC is unable to reach unanimous agreement after working in good faith to reach a consensus and taking into account all reasonable medical, scientific, and clinical considerations and considering each Party’s comments or requests on such matters that would adversely impact the safety, commercial value or reputation of the Licensed Products, with respect to a dispute relating to a JOC responsibility described in Section 5.1.2 other than those set forth Sections 5.1.2(h) and 5.1.2(i) for a period in excess of [**], then the dispute shall be submitted to the Executive Officers for resolution. If the JOC is unable to reach unanimous agreement after working in good faith to reach a consensus and taking into account all reasonable medical, scientific, and clinical considerations and considering each Party’s comments or requests on such matters that would adversely impact the safety, commercial value or reputation of the Licensed Products, with respect to a dispute relating to a JOC responsibility set forth Sections 5.1.2(h) or 5.1.2(i) for a period in excess of [**], then the dispute shall be reviewed by a Working Group (as defined below) designated by the JOC, which shall within [**] submit to the JOC a written analysis of the dispute and recommendations for resolving the dispute. If the Working Group fails to timely submit such analysis and recommendations or if the JOC is unable to reach unanimous agreement with respect to such dispute for a period in excess of [**] following the receipt of such analysis and recommendation, then the dispute shall be submitted to the Executive Officers for resolution.
5.4.3 If such dispute cannot be resolved for a period in excess of [**] following the escalation to the Executive Officers, then, such dispute shall be subject to this Section 5.4.3:
- 29 -
Confidential
(a) Subject to Section 5.4.4 and 5.4.5, Ono shall have the deciding vote with respect to any aspects of such matter relating to the Ono Territory, including the Ono Development Plan; provided that, any and all deciding votes shall be in good faith, and after good faith consideration of Karyopharm’s comments or requests on such matters that would adversely impact the safety, commercial value or reputation of the Licensed Products, and with due regard for the impact of such deciding vote on Development and Commercialization of the Licensed Products in the Karyopharm Territory and consistency in all material respects with the terms of this Agreement.
(b) Subject to Section 5.4.4 and 5.4.5, Karyopharm shall have the deciding vote with respect to any aspects of such matter relating to the Karyopharm Territory, as well as any amendment to a Global Clinical Development Plan; provided that, any and all deciding votes shall be in good faith, and after good faith consideration of Ono’s comments or requests on such matters that would adversely impact the safety, commercial value or reputation of the Licensed Products, and with due regard for the impact of such deciding vote on Development and Commercialization of the Licensed Products in the Ono Territory and consistency in all material respects with the terms of this Agreement.
5.4.4 Neither Party shall have the deciding vote on, and the JOC shall not have decision-making authority regarding, any of the following matters, which shall be mutually agreed to by the Parties:
(a) any matter that would materially adversely impact the safety, commercial value or reputation of a Licensed Product in the Ono Territory;
(b) the imposition of any requirements on the other Party to undertake obligations beyond those for which it is responsible, or to forgo any of its rights, under this Agreement;
(c) the imposition of any requirements that the other Party take or decline to take any action that would result in a violation of any Law or any agreement with any Third Party or the infringement of intellectual property rights of any Third Party;
(d) any matters that would excuse such Party from any of its obligations under this Agreement; or
(e) modifying the terms of this Agreement or taking any action to expand or narrow the responsibilities of the JOC.
5.4.5 Notwithstanding anything to the contrary set forth herein,
(a) the decision-making Party shall make its decision in good faith, subject to the terms and conditions of this Agreement;
(b) in no event may the decision-making Party unilaterally determine that it has fulfilled any obligations hereunder or that the non-deciding Party has breached any obligations hereunder; and
- 30 -
Confidential
(c) Ono may not make a decision that would cause Karyopharm to be in breach of a provision of a Karyopharm Third Party Agreement.
5.5 Working Groups. Upon mutual agreement, the Parties may establish other committees or working groups (each, a “Working Group”) as they deem appropriate. These Working Groups shall report to the JOC depending on the subject matter of such Working Group’s oversight. Each Working Group shall have equal number of representatives from each Party. Working Group may be established on an ad hoc basis for purposes of a specific project. In no event shall the authority of a Working Group exceed that of the JOC. The Parties agree to the establishment of a joint intellectual property working group (the “IP Working Group”) and a global development working group (the “Global Development Working Group”) after the Effective Date. The IP Working Group shall (a) review and discuss intellectual property matters relating to a Licensed Compound or Licensed Product in the Field, and (b) in the case of Joint IP, determining which Party shall be the Joint IP Prosecuting Party for such Joint IP. The Global Development Working Group shall use Commercially Reasonable Effort to share and exchange enough information on such matters in advance of the JOC so that the JOC members may make enough preparation and have discussion in efficient and effective manner.
5.6 Liaisons. Within [**] following the Effective Date, each Party shall appoint a representative (“Liaison”) to facilitate communications between the Parties (including, coordinating the exchange of Data and Know-How of each Party as required under this Agreement) and to act as a liaison between the Parties with respect to such other matters as the Parties may mutually agree in order to maximize the efficiency of the collaboration. Each Party may replace its Liaison with an alternative representative at any time with prior written notice to the other Party. Each Party’s Liaisons shall be entitled to attend all JOC meetings. Each Liaison may bring any matter to the attention of the JOC where such Liaison reasonably believes that such matter requires attention of the JOC. Each Liaison shall be responsible with creating and maintaining a collaborative work environment within the JOC.
5.7 Authority. The JOC will have only the powers assigned expressly to it in this Article 5, and will not have any power to amend or modify the terms of this Agreement or waive compliance with this Agreement. In furtherance thereof, each Party will retain the rights, powers and discretion granted to it under this Agreement and no such rights, powers or discretion will be delegated to or vested in the JOC unless such delegation or vesting of rights is expressly provided for in this Agreement or the Parties expressly so agree in writing.
5.8 Committee involving Karyopharm’s Related Party. In the case a Related Party of Karyopharm is responsible for Development and/or Commercialization of License Compound and Licensed Product in the United States, France, Germany, Italy, Spain and/or the United Kingdom, upon Ono’s request, Karyopharm shall use Commercially Reasonable Efforts to arrange and establish a committee constituted by representatives of Karyopharm, such Related Party and Ono to oversee and monitor Development and/or Commercialization of the Licensed Compound and Licensed Product on global basis.
| 6. | MANUFACTURE AND SUPPLY |
| 6.1 | Clinical Supply. |
- 31 -
Confidential
6.1.1 Clinical Supply. Subject to Section 6.2.3, Karyopharm shall use Commercially Reasonable Efforts to Manufacture and supply all quantities of the Licensed Compounds and/or Licensed Products for use by Ono in the Development of Licensed Products in the Ono Territory, including to obtain Regulatory Approval in the Ono Territory. It is confirmed that, as Karyopharm’s Commercially Reasonable Efforts set forth in this Section, Karyopharm shall supply to Ono [**] tablets of selinexor as initial requirement for Development in the Ono Territory within [**] from the Effective Date and subsequent requirements of clinical supplies for Development in the Ono Territory pursuant to the terms and conditions of the Clinical Supply Agreement. Ono’s purchase price for the Licensed Compounds and Licensed Products shall be [**].
6.1.2 Clinical Supply Agreement. The Manufacturing and supply by Karyopharm of the Licensed Compounds and Licensed Products for Development purposes shall be governed by a mutually acceptable clinical supply agreement (the “Clinical Supply Agreement”) and a mutually acceptable clinical quality agreement (the “Clinical Quality Agreement”) which shall include the terms and conditions as are reasonable and customary for an agreement governing the Manufacturing and supply of compounds and products similar to the Licensed Compounds and the Licensed Products for clinical supply purposes. The Clinical Supply Agreement and Clinical Quality Agreement shall be finalized within [**] period after the Effective Date. In no event shall Karyopharm be obligated to supply quantities of Licensed Compounds and/or Licensed Products in excess of amounts reasonably necessary to satisfy Ono’s and its Related Party’s Development requirements in the Field in the Ono Territory.
| 6.2 | Commercial Supply. |
6.2.1 Commercial Supply. With respect to supply of each of the Licensed Products for Commercialization, in the case Ono does not elect to Manufacture the Licensed Compounds and/or Licensed Products by itself, Ono may purchase all of its requirements for the Licensed Compounds and/or Licensed Products for the Ono Territory from Karyopharm. In the case Ono elects to Manufacture the Licensed Compounds and/or Licensed Products by itself, Ono may Manufacture the Licensed Compounds and/or Licensed Products for Development and Commercialization purposes.
6.2.2 Commercial Supply Agreement. If Ono desires to have Karyopharm to supply and Manufacture the Licensed Compounds and/or Licensed Products for the Ono Territory for Commercialization purposes, such Manufacture and supply by Karyopharm shall be covered by a mutually acceptable commercial supply agreement (the “Commercial Supply Agreement”) which shall include the terms and conditions as are reasonable and customary for an agreement governing the Manufacturing and supply of compounds and/or products similar to the Licensed Compounds and/or the Licensed Products for Commercialization purposes. The purchase price for the Licensed Compounds and/or the Licensed Products shall be [**]. Until the Commercial Supply Agreement is executed,
- 32 -
Confidential
Karyopharm shall continue to Manufacture and supply the Licensed Compounds and/or the Licensed Products to Ono pursuant to the terms and conditions of the Clinical Supply Agreement, except that the purchase price for the Licensed Compounds and/or the Licensed Products shall be as set forth in this Section 6.2.2.
6.2.3 Ono’s Right to Manufacture. If Ono elects to Manufacture the Licensed Compounds and/or the Licensed Products by itself in the Field for the Ono Territory for Development and Commercialization purposes, then within [**] after receipt of such request, Karyopharm shall, in accordance with this Section 6.2.3, transfer to Ono, an Affiliate of Ono, or a Third Party manufacturer approved by Karyopharm (such approval not to be unreasonably withheld, delayed or conditioned), the Karyopharm Know-How reasonably necessary or useful to enable Manufacture of the applicable Licensed Compounds and/or Licensed Products for Development and Commercialization for the Ono Territory in the Field and not previously transferred to Ono, such Affiliate of Ono or any such Third Party manufacturer. Such Know-How transfer by Karyopharm shall be conducted using Commercially Reasonable Efforts and shall include copies or samples of relevant documentation, materials, analytical assays for the Licensed Compounds and/or the Licensed Products and other embodiments of such Karyopharm Know-How. Upon such Know-How transfer, Karyopharm shall also make available its qualified technical personnel on a reasonable basis to consult with Ono, such Affiliate of Ono or such Third Party manufacturer with respect to such Karyopharm Know-How. The details of how such Know-How transfer, including a specific list of Karyopharm Know-How to be transferred by Karyopharm, shall be set forth in a written technology transfer plan (the “Manufacturing Technology Transfer Plan”) executed by the Parties for the purpose of ensuring the complete and timely transfer of such Karyopharm Know-How and the protection of Karyopharm’s rights in such Karyopharm Know-How. Manufacturing Technology Transfer Plan shall take into consideration, among other things, the Development and Commercialization activities in the Ono Territory, and the other responsibilities for key employees of Karyopharm. Ono shall pay the amounts set forth in the Manufacturing Technology Transfer Plan for the work and transfer performed by Karyopharm and shall reimburse Karyopharm for its out-of-pocket costs incurred in the course of such transfer pursuant to this Section 6.2.3. Karyopharm shall have no obligation to transfer any Karyopharm Know-How to Ono until the full execution of the Manufacturing Technology Transfer Plan by both Parties. For clarity, during the transfer of such Karyopharm Know-How, Karyopharm shall continue to Manufacture and supply the Licensed Compounds and the Licensed Product to Ono in accordance with Clinical Supply Agreement or Commercial Supply Agreement.
6.3 Related Substance Supply. Subject to the terms of the applicable Supply Agreement, Ono shall have the right to purchase from Karyopharm, and Karyopharm shall supply to Ono, related substance of the Licensed Compounds (e.g., reference standard, internal standard and impurities) necessary for Ono to conduct non-clinical studies, preclinical studies or Clinical Studies, including, but not limited to analytical test method development and/or validation, for regulatory submissions or Commercialization in the Ono Territory, at [**].
- 33 -
Confidential
| 7. | LICENSES |
| 7.1 | License Grants. |
7.1.1 License Grants to Ono. Subject to the terms and conditions of this Agreement, Karyopharm hereby grants Ono (a) an exclusive (even as to Karyopharm and its Affiliates), royalty-bearing, transferable, sublicenseable (including through multiple tiers) (in accordance with Section 7.2) license under the Karyopharm Technology to Develop, use and Commercialize the Licensed Compounds and the Licensed Products in the Field in the Ono Territory; and (b) subject to election by Ono and the full execution by the Parties of the Manufacturing Technology Transfer Plan, a non-exclusive, royalty-free, non-transferable (except as provided in Section 14.1), sublicenseable (including through multiple tiers) (in accordance with Section 7.2) license under the Karyopharm Technology to Manufacture or have Manufactured Licensed Compounds and Licensed Products in or outside of the Ono Territory solely for Development and Commercialization in the Field in the Ono Territory.
7.1.2 License Grants to Karyopharm. Ono hereby grants Karyopharm a non-transferable (except as provided in Section 14.1), sublicenseable (including through multiple tiers) (subject to Section 7.2), non-exclusive, royalty-free license under Ono Technology to Develop, Manufacture, have Manufactured, use and Commercialize Licensed Compounds and Licensed Products in the Field in the Karyopharm Territory.
7.1.3 License Grants to Karyopharm’s Related Party. Karyopharm shall not grant to its Affiliate or any Third Party a license under Karyopharm Technology, which license would materially conflict with Section 7.1.1 (License Grant to Ono). Parties agree that the breach of this provision by Karyopharm shall constitute a “material breach” of this Agreement by Karyopharm.
Karyopharm hereby acknowledges that Ono’s rights and status as the exclusive licensee under Karyopharm Technology for the Licensed Products in the Field in the Ono Territory set forth in each of Sections 2.8.2 (Scientific Records), 2.8.4 (Information Sharing), 2.8.5 (Rights of Reference and Access to Data), 3.1.3 and 4.7.1 (Karyopharm Promotional Materials) constitutes essential benefit to enter into this Agreement, provided, however, that, such acknowledgement shall not be made without prejudice to any other benefit conferred to Ono under this Agreement.
Karyopharm shall be responsible for the compliance of its Third Party Licensees with the requirements of Karyopharm’s Related Parties in Sections 2.8.2 (Scientific Records), 2.8.4 (Information Sharing), 2.8.5 (Rights of Reference and
- 34 -
Confidential
Access to Data), 3.1.3 and 4.7.1 (Karyopharm Promotional Materials) (collectively, the “Collaboration Provisions”). Karyopharm shall notify Ono in writing whether a Third Party Licensee has agreed to the terms of the Collaboration Provisions promptly after execution of the applicable agreement. If a Third Party Licensee does not agree to any or all of the Collaboration Provisions then Karyopharm shall not grant to such Third Party Licensee the applicable reciprocal rights which may include access to Ono’s Data or Ono Technology or which may include (a) the grant of any sublicense under Ono Patent Rights and Ono Know-How to such Third Party Licensee or (b) the grant to such Third Party Licensee of access to Data generated by Ono and Ono Technology or (c) a right of reference with respect to Ono’s regulatory filings and Ono’s Regulatory Approvals and Ono’s promotional materials. For example, if a Third Party Licensee does not agree to grant to Ono the right to cross reference its regulatory filings and Regulatory Approvals related to the Licensed Products, then Karyopharm will not grant to the Third Party Licensee the right to cross reference Ono’s regulatory filings and Regulatory Approvals related to the Licensed Products.
If a Third Party Licensee agrees to any or all of the Collaboration Provisions, the applicable reciprocal rights concerning the Licensed Products to be made available by such Third Party Licensee to Ono through Karyopharm shall be incorporated into Karyopharm Technology and shall be granted to Ono under the terms and conditions of this Agreement. Notwithstanding anything herein to the contrary, to the extent that (a) the Party is required by the Regulatory Authority to make available certain Know-How Controlled by Related Party of the other Party (e.g. included, but not limited to, safety information or investigator brochure for Clinical Study conducted by such Related Party) in order to obtain and maintain IND, NDA or Regulatory Approval of Licensed Product or (b) the Party wishes to utilize the Data Controlled by Related Party of the other Party, which is generated from Global Clinical Study, the other Party shall procure and make available to the requesting Party such Know-How and Data, respectively, free of charge.
| 7.2 | Sublicensing Terms. |
7.2.1 Ono’s Right to Sublicense. Ono may sublicense the rights granted under Section 7.1 to its Related Party with the prior written approval by Karyopharm (such approval not to be unreasonably withheld, delayed or conditioned), provided that Ono is not required for such written approval by Karyopharm and any prior notice to Karyopharm in the cases that:
(a) Ono sublicenses its rights under the Karyopharm Technology to any of its Affiliates;
(b) Ono uses competent and GCP compliant CROs, clinical trial sites or any other Third Party to perform portions of the Development of a Licensed Product to the extent consistent with its normal business practices and Ono Development Plan;
- 35 -
Confidential
(c) Ono uses Third Party manufacturer to Manufacture Licensed Compound and/or Licensed Product in the Field for the Development and Commercialization for the Ono Territory subject to Section 6.2.3 to the extent that (i) Licensed Compound and/or Licensed Product is Manufactured under direction by Ono and in the manufacturing method approved by Ono and (ii) the specification therefor is approved by Ono; or
(d) Ono engages reasonably qualified Third Parties to assist with Commercialization of the Licensed Products through co-promotion, and distributor arrangements and may sublicense its rights granted under Section 7.1 to such Third Parties to the extent that such arrangements are commercially reasonable and co-promotion and distribution by such Third Parties is made under direction by Ono or strategy given by Ono.
Ono shall promptly provide to Karyopharm written notice of any such grant by Ono in accordance with this Section 7.2.1 except for above (a) through (d) setting forth in reasonable detail the nature of such grant and the identity of the Sublicensee. Any sublicense agreement shall contain confidentiality, reporting, audit and access to data and information obligations comparable to those set forth herein and require the express compliance of such Sublicensee to the requirements set forth in Section 7.1.1(b).
7.2.2 Karyopharm Right to Sublicense. Subject to Section 7.1.3 Karyopharm may sublicense the rights granted under Section 7.1 to its Related Party with the prior written notice to Ono, provided that Karyopharm is not required to make such notice to Ono in the cases that:
(a) Karyopharm sublicenses its rights under the Ono Technology to any of its Affiliates;
(b) Karyopharm uses competent and GCP compliant CROs, clinical trial sites or any other Third Party to perform portions of the Development of a Licensed Product to the extent consistent with its normal business practices;
(c) Karyopharm uses Third Party manufacturer to Manufacture Licensed Compound and/or Licensed Product; or
(d) Karyopharm engages reasonably qualified Third Parties to assist with Commercialization of the Licensed Products through co-promotion, co-marketing and distributor arrangements and may sublicense its rights granted under Section 7.1 to such Third Parties to the extent such arrangements are commercially reasonable.
Karyopharm shall promptly provide to Ono written notice of any such grant by Karyopharm in accordance with this Section 7.2.2 except for above (a) through (d) setting forth in reasonable detail the nature of such grant and the identity of the sublicensee. Any sublicense agreement shall contain confidentiality, reporting, audit and access to data and information obligations comparable to those set forth herein.
7.2.3 Sublicense Requirements. Each sublicense granted by a Party pursuant to Section 7.2 (a “Sublicense”) to a Third Party shall be in writing
- 36 -
Confidential
and shall be consistent with the relevant restrictions and limitations set forth in this Agreement. No Sublicense shall diminish, reduce or eliminate any obligation of either Party under this Agreement. Each Party shall remain responsible for its obligations under this Agreement and shall be responsible for the performance of any of its Sublicensees or sublicensees in accordance with applicable terms and conditions of this Agreement.
| 7.3 | Exclusivity. |
7.3.1 Karyopharm Exclusivity. During [**], neither Karyopharm nor any of its Affiliates may engage, either alone or in combination with or through any Third Party, in the Development, use and/or Commercialization of (a) any Licensed Compounds and/or Licensed Products in or outside the Field in the Ono Territory; or (b) any compounds and/or products with the Mechanism of Action as a primary mechanism of action in the Field in the Ono Territory.
7.3.2 Ono Exclusivity. During [**], neither Ono nor any of its Affiliates may engage, either alone or in combination with or through any Third Party, in the Development, use and/or Commercializing of any compound or product with the Mechanism of Action as a primary mechanism of action in the Field in the Ono Territory, except that Ono may exercise its rights with respect to the Licensed Compounds and Licensed Products in the Field in the Ono Territory pursuant to this Agreement. If Ono or any of its Affiliates wishes to conduct any such activity, it may provide written notice of such interest to Karyopharm, and Karyopharm may, in its sole discretion, provide its written consent to Ono so that Ono or such Affiliate may conduct such activity.
7.4 No Other Rights. Except as otherwise expressly provided in this Agreement, under no circumstances shall a Party, as a result of this Agreement, obtain any ownership interest, license right or other right in any Know-How, Patent Rights or other intellectual property rights of the other Party, including items owned, controlled or developed by the other Party, or provided by the other Party to the receiving Party at any time pursuant to this Agreement. For clarity, except for Ono’s license and rights under Section 7.1,1 relating to Manufacturing of the Licensed Compound and the Licensed Product and other related provisions, (a) in no event will the licenses or rights granted to Ono under this Agreement with respect to the Licensed Compounds and the Licensed Products extend outside the Field and the Ono Territory; (b) Ono’s rights with respect to the Licensed Compounds and Licensed Products, and Ono’s rights with respect to any regulatory documents and any interactions with Regulatory Authorities with respect to the Licensed Compounds and Licensed Products, shall not extend outside the Field and the Ono Territory; and (c) Ono shall not, directly or indirectly, use, exploit or exercise any rights under any Karyopharm Technology, or otherwise conduct any Development, use, or Commercialization activities with respect to any Licensed Compound or Licensed Product, outside the Field or outside the Ono Territory. In addition, for clarity, (a) in no event will the licenses or rights granted to Karyopharm under this Agreement with respect to the Licensed Compounds and the Licensed Products extend outside the Field and the Karyopharm Territory; (b) Karyopharm’s rights under the Ono Technology with respect to the Licensed Compounds and Licensed Products, and
- 37 -
Confidential
Karyopharm’s rights with respect to any regulatory documents and any interactions with Regulatory Authorities with respect to the Licensed Compounds and Licensed Products, shall not extend outside the Karyopharm Territory; and (c) Karyopharm shall not, directly or indirectly use, exploit or exercise any rights under any Ono Technology, or otherwise conduct any Development, use, Manufacturing, having Manufactured or Commercialization activities with respect to any Licensed Compound or Licensed Product outside the Field and outside the Karyopharm Territory.
| 8. | CERTAIN FINANCIAL TERMS |
8.1 Upfront Fee. In consideration for the rights, licenses and options granted by Karyopharm to Ono under this Agreement, Ono shall pay Karyopharm a non-refundable, non-creditable upfront payment of Two and a Half Billion Japanese Yen (JPY2,500,000,000), within [**] of receipt by Ono of invoice for such upfront fee and the taxation documents expressly described in Section 8.11.
| 8.2 | Development Milestone Payments. |
8.2.1 Ono shall make the non-refundable, non-creditable milestone payments to Karyopharm set forth below in this Section 8.2.1 (the “Development Milestone Payment(s)”), each payable once, within [**] of receipt by Ono of an invoice for corresponding Development Milestone Payment and taxation documents expressly described in Section 8.11.
| Development Milestone Event – Multiple Myeloma |
Development Milestone Payment (in JPY) | |||
| [**] | [**] | |||
| [**] |
[**] | [**] | ||
| [**] |
[**] | [**] | ||
- 38 -
Confidential
| Development Milestone Event – Additional Indications* |
Development Milestone Payment (in JPY) | |||
| [**] |
[**] | |||
| [**] |
[**] | |||
| [**] |
[**] | |||
| Development Milestone Event – ASEAN and Hong Kong countries | ||||
| [**] |
[**] | |||
The total amount of the Development Milestone Payments to be paid by Ono to Karyopharm shall not exceed Ten Billion and One Hundred Fifty Million Japanese Yen (¥10,150,000,000).
* Each Development Milestone Payment for the Indications as set forth in this section 8.2.1 shall be made only once with respect to the achievement of each milestone event (the “Development Milestone Event”) above, regardless of subsequent or repeated achievement of such Development Milestone Event by any Licensed Compound or Licensed Product. [**].
8.2.2 Each Development Milestone Payment by Ono to Karyopharm hereunder shall be payable only once, regardless of the number of times achieved with respect to a Licensed Product.
8.2.3 Ono shall provide Karyopharm with written notice of the achievement by Ono or any of its Related Parties of any Development Milestone Event with respect to a Licensed Product set forth in Section 8.2.1 within [**] after the occurrence of such Development Milestone Event; provided, however, that Ono shall inform Karyopharm of such Development Milestone Event prior to any public disclosure of such Development Milestone Event by Ono.
| 8.3 | Sales Milestone Payments. |
8.3.1 Ono shall provide Karyopharm with written notice of the achievement by Ono or any of its Related Parties of any milestone event with respect to a Licensed Product set forth in this Section 8.3.1 (the “Sales Milestone Event”) within [**] after the occurrence of each Sales Milestone Event. Ono shall make the non-refundable, non-creditable payments to Karyopharm set forth below in this Section 8.3.1 (the “Sales Milestone Payment(s)”) within [**] of receipt by Ono of an invoice for corresponding Sales Milestone Payment and taxation documents expressly described in Section 8.11:
- 39 -
Confidential
| Sales Milestone Event |
Sales Milestone Payment (in JPY) | |
| (i) First achievement of Annual Net Sales of the Licensed Products in the Ono Fiscal Year greater than [**] | [**] | |
| (ii) First achievement of Annual Net Sales of the Licensed Products in the Ono Fiscal Year greater than [**] | [**] | |
| (iii) First achievement of Annual Net Sales of the Licensed Products in the Ono Fiscal Year greater than [**] | [**] | |
The total amount of the Sales Milestone Payments to be paid by Ono to Karyopharm shall not exceed Nine Billion Japanese Yen (¥9,000,000,000).
8.3.2 Sections 8.2.2 shall apply for the Sales Milestone Payment. If a Sales Milestone Event described in a clause in Section 8.3.1 occurs before or concurrently with another Sales Milestone Event described in a preceding clause in Section 8.3.1, Ono shall also pay the Sales Milestone Payment described in such earlier clause when the Sales Milestone Payment described in such later clause is paid. By way of example, if, during an Ono Fiscal Year, Annual Net Sales of Licensed Products first exceed the thresholds set forth in Sections 8.3.1(i) and (ii), Ono shall pay Karyopharm the Sales Milestone Payments set forth in both Sections 8.3.1(i) and (ii).
| 8.4 | Royalties. |
8.4.1 Royalties Payable on Licensed Products. Subject to Sections 1.50, 1.80, 8.4.1, 8.4.2 and 8.4.3, during the Royalty Term, Ono shall pay to Karyopharm a royalty of (a) [**] percent ([**]%) on Net Sales of a Licensed Product by Ono and its Related Parties of all Licensed Products in the Ono Territory. For purpose of this Agreement, the “Royalty Term” means the period which commences upon the date of First Commercial Sale and continues until the later of (a) the date of expiration of the first Regulatory Exclusivity period applicable to the Licensed Product in such country in the Ono Territory (which shall not be extended by grant of the second Regulatory Exclusivity Period); (b) the date of expiration of all Karyopharm Patent Rights, whose Valid Claim Covers such Licensed Product, or (c) the tenth (10th) anniversary of the First Commercial Sale of such Licensed Product in the Field (which shall not be extended by launch of any other formulation or dosage form of the same API as in such Licensed Product). Such Royalty Term shall be established on Licensed Product-by-Licensed Product and country-by-country basis.
- 40 -
Confidential
8.4.2 Royalty Reduction by Entry of Generic Version If at any time during the period from the date of First Commercial Sale of a Licensed Product until the tenth (10th) anniversary of such First Commercial Sale of such Licensed Product, any Third Party (other than a Sublicensee) makes a Generic Version (defined below) of such Licensed Product commercially available in such country in the Ono Territory, then the royalty rate applicable to Net Sales of such Licensed Product in such country shall be reduced to [**] percent ([**]%). If (a) at any time following the tenth (10th) anniversary of the First Commercial Sale of a Licensed Product, any Third Party (other than a Sublicensee) makes a Generic Version of such Licensed Product commercially available in such country in the Ono Territory or (b) prior to the tenth (10th) anniversary of the First Commercial Sale of a Licensed Product, any Third Party (other than a Sublicensee) makes a Generic Version of such Licensed Product commercially available in such country in the Ono Territory and such Generic Version is marketed as of such tenth (10th) anniversary, then [**] shall be payable on the Net Sales of such Licensed Product in such country during the remainder of the Royalty Term. “Generic Version” means a product that: (a) contains as an active pharmaceutical ingredient a chemical composition that is assigned the same INN (international nonproprietary name) or JAN (Japanese Accepted Names for Pharmaceuticals) as is assigned to active pharmaceutical ingredient contained in the corresponding Licensed Product being marketed in the Ono Territory; (b) obtained marketing approval in a country in the Ono Territory by means of an abridged procedure that relies (i) in whole or in part on the safety and efficacy data contained in the NDA for such Licensed Product submitted by Ono in such country, and (ii) on establishing bioequivalence to the Licensed Product; and (c) is legally marketed in the Ono Territory by an entity other than Ono, its Affiliates or its Sublicensees without infringing any Valid Claim of Karyopharm Patent Rights.
8.4.3 Stacking. The applicable royalty due pursuant to Section 8.4.1 shall be adjusted as follows: If Ono or any of its Affiliates determines in good faith that, in order to avoid infringement of any Third Party’s Patent Right, it is reasonably necessary to obtain a license after the Effective Date from a Third Party under one or more Valid Claims owned or licensable by such Third Party Covering the composition of matter, method of making (including method of formulation if no other comparable method of formulation is available without requiring Ono or its Related Party to obtain a license) or method of use of a Licensed Product in order for Ono and its Related Parties to Manufacture or have Manufactured in the Field for a country in the Ono Territory such Licensed Product, or Develop, use or Commercialize in the Field in a country in the Ono Territory such Licensed Product and to make payments under such license, and Ono or any Related Party actually enters into any such license, then the amount of Ono’s royalty payments under Section 8.4.1 for such Licensed Product in such country in a Calendar Quarter may be reduced by [**] percent ([**]%) of the
- 41 -
Confidential
royalties actually paid by Ono or any of its Affiliates to such Third Party to the extent applicable to such Licensed Product in such country during such Calendar Quarter; provided, however, that in no event shall the amounts paid to Karyopharm be reduced below [**] percent ([**]%) of the amounts that would otherwise have been payable, as determined pursuant to Section 8.4.1, and Ono may carry such royalty amounts which should be reduced in accordance with Section 8.4.2 and 8.4.3 forward for reduction against milestone payments and royalties payable with respect to Net Sales of such Licensed Product in such country in future Calendar Quarters until fully applied.
8.4.4 Limits on Deductions. In no event shall the cumulative effect of the adjustments in Section 8.4.3 reduce the royalties payable to Karyopharm pursuant to Section 8.4.1 to less than [**] percent ([**]%) of the amounts that would otherwise have been payable, as determined pursuant to Section 8.4.
8.5 Reports; Payment of Royalty. During the Term, following the First Commercial Sale of the Licensed Product in any country in the Ono Territory, Ono shall furnish to Karyopharm (a) an estimate within [**] after the end of each Calendar Quarter of the Net Sales of each Licensed Product in each country of the Ono Territory and the royalties payable under this Agreement; and (b) a written report (each, a “Royalty Report”) within [**] after the end of each Calendar Quarter showing, on a Licensed Product-by-Licensed Product and country-by-country basis, the Net Sales of each Licensed Product in each country of the Ono Territory and the royalties payable under this Agreement, along with (i) gross sales of the Licensed Product in the Ono Territory in the relevant Calendar Quarter on a country-by-country basis, (ii) Net Sales in the relevant Calendar Quarter on a country-by-country basis, (iii) all relevant exchange rate conversions in accordance with Section 8.8.3, (iv) all deductions in accordance with Sections 1.57 and 8.4 and (v) the amount of any payment due from Ono to Karyopharm, calculated in accordance with this Article 8. Simultaneously with the delivery of each such Royalty Report, Ono shall pay to Karyopharm the total amounts due under Section 8.4 for the period covered by such Royalty Report subject to Ono’s receipt of taxation documents expressly described in Section 8.11. Ono and its Related Parties involved in Commercializing Licensed Products shall keep complete and accurate records in sufficient detail to enable the royalties and other payments payable hereunder to be determined. In addition, Ono and its Related Parties shall comply with any applicable reporting requirements under the Karyopharm Third Party Agreements.
| 8.6 | Audits. |
8.6.1 Ono shall keep, and shall require its Related Parties to keep, full, true and accurate books of account containing all particulars that may be necessary for the purpose of calculating the amounts payable by Ono under this Agreement, including, as applicable, records of Development activities and Net Sales, and the amount of Joint Patent Costs payable by Karyopharm with respect to Joint Patent Rights pursuant to Section 12.4.3. Karyopharm shall keep, and shall require its Related Parties to keep, full, true and accurate books of account containing all particulars that may be necessary for the purpose of calculating the amounts payable by Ono under this Agreement, including, as applicable, records
- 42 -
Confidential
of Manufacturing activities and Karyopharm’s Cost of Manufacturing for the Licensed Compound and Licensed Product, and Joint Patent Costs payable by Ono with respect to Joint Patent Rights pursuant to Section 12.4.3. Such books of accounts shall be kept at each Party’s principal place of business for a period of at least [**] Calendar Years after the date on which the relevant Development or Manufacturing activity or Net Sales occurred or the relevant Joint Patent Costs were incurred. Either Party (the “Auditing Party”) has the right, at its expense (except as set forth below), to engage an independent, certified public accountant selected by such Auditing Party and reasonably acceptable to the other Party (the “Auditor”) to perform, on behalf of such Auditing Party, an audit of such books and records that are deemed necessary by such Auditor to report on the correctness of any report or payments made or to have been made under this Agreement.
8.6.2 The Auditing Party shall provide reasonable, but at least [**], prior written notice to the other Party of any requested audit and shall conduct such audit during regular business hours in such a manner as to not unnecessarily interfere with the other Party’s normal business activities. Any audit shall be limited to records for the [**] Calendar Years prior to audit notification. The Auditing Party shall not perform an audit more frequently than [**] nor more frequently than [**] with respect to records covering any specific period of time, except if Karyopharm is required to do so pursuant to a Karyopharm Third Party Agreement.
8.6.3 The Auditing Party shall use all such records of the other Party only for the purpose of verifying payments due hereunder, and shall treat such records as the other Party’s Confidential Information. The Auditor shall be obligated to execute a reasonable confidentiality agreement with the other Party prior to commencing any such audit. The Auditor shall only share the results of the audit, not the underlying records, with the Auditing Party. Any final audit report shall be shared by the Auditing Party with the other Party.
8.6.4 Notwithstanding Section 8.6.1, if the audit uncovers an underpayment in any Calendar Quarter by a Party, or an overpayment in any Calendar Quarter by a Party, that exceeds [**] percent ([**]%) of the total payment owed, then the reasonable out-of-pocket fees incurred by the Auditing Party with respect to such audit shall be paid by the other Party. In the event such audit leads to the discovery of a discrepancy to the Auditing Party’s detriment, the other Party will pay any undisputed underpaid amount of the discrepancy, plus interest on the underpayment at a rate per annum equal set forth in Section 8.9 within [**] after receipt by the other Party of an invoice for corresponding payment and taxation documents delivered from the Auditing Party, subject to the other Party’s receipt of such final audit report from the Auditor.
8.7 Karyopharm Third Party Agreement Payments. Karyopharm shall be solely responsible for any royalties or other payments due under the Karyopharm Third Party Agreements.
- 43 -
Confidential
8.8 Exchange Rate.
8.8.1 Payment Method. All payments to be made by Ono under this Agreement shall be made by bank wire transfer in immediately available funds to bank account as may be designated by Karyopharm from time to time. The first designated bank account of Karyopharm shall be as follows:
| Account name: |
[**] | |||
| Account number: |
[**] | |||
| Bank name: |
[**] | |||
| Beneficiary Address: |
[**] | |||
| Swift code: |
[**] | |||
| Routing/Transit for Wires: |
[**] | |||
| Routing/Transit for ACH |
[**] | |||
8.8.2 Currency Conversion. All amounts specified in this Agreement are in Japanese Yen. All payments hereunder shall be made in US Dollars. In the case Ono makes payment under this Agreement, all such payment shall be converted into US Dollars at the exchange rate (TTS rate) for the conversion of Japanese Yen into US Dollars posted by the Bank of Tokyo-Mitsubishi UFJ, Ltd., on the date on which Ono will make the applicable payment hereunder, provided that no deduction from any amount shall be made in respect of bank fees or charges.
8.8.3 Conversion of Net Sales. In the case of Net Sales made in one or more currencies other than Japanese Yen during a Calendar Quarter (each a “Third-Country Currency”), Net Sales will be converted from each relevant Third-Country Currency to such Third-Country Currency’s equivalent in Japanese Yen. The amount of Net Sales made during any Calendar Quarter shall be determined by converting the portion of such Net Sales made in each Third-Country Currency into Japanese Yen, using the exchange rate for the conversion of foreign currency into Japanese Yen posted by the Bank of Tokyo-Mitsubishi UFJ, Ltd., between the relevant Third-Country Currency, on the one hand, and Japanese Yen, on the other hand. All currency conversions described in this Section 8.8.3 shall be made in accordance with IFRS, to the extent reasonable and consistently applied.
8.9 Late Payments. Any amount owed by a Party to the other Party under this Agreement that is not paid on or before the date such payment is due shall bear interest at a rate per annum equal to [**] percent ([**]%) per month, calculated based on the number of days such payments are paid after the date such payments are due, compounded monthly and computed on the basis of a year of three hundred sixty-five (365) days for the actual number of days’ payment is delinquent.
8.10 Blocked Payments. If, by reason of Law in any jurisdiction in the Ono Territory, it becomes impossible or illegal for Ono to transfer milestone payments, royalties or other payments under this Agreement to Karyopharm, (a) Ono shall promptly notify Karyopharm; and
- 44 -
Confidential
(b) Ono shall pay Karyopharm the amounts due from an account in another jurisdiction in the Ono Territory; provided, however, that if there is no jurisdiction in the Ono Territory from which it is legal for Ono to transfer payments to Karyopharm (i) Ono shall deposit such payments in local currency in the relevant jurisdiction to the credit of Karyopharm in a recognized banking institution designated by Karyopharm or, if none is designated by Karyopharm within a period of [**], in a recognized banking institution selected by Ono and identified in a written notice given to Karyopharm, and (ii) Karyopharm may terminate this Agreement if Ono is not permitted by Law to transfer payments to Karyopharm for a period of [**].
8.11 Taxes. In the event that Ono is required to withhold and pay over any tax to the Governmental Authorities in any country in the Ono Territory in respect of any payment to Karyopharm, the amount thereof shall be deducted from the payment to be made by Ono and timely and properly paid over to such Governmental Authorities, provided that Ono shall furnish Karyopharm with copies of receipts and other documentation evidencing such withholding. Karyopharm shall provide to Ono any taxation documents (Form 3 and Form 17), and the Residency Certificate (Form 6166) of Karyopharm issued by the US Internal Revenue Service (which Residency Certificate is effective for three (3) years after its issuance to a public company) and other documents that may be reasonably necessary in order for Ono not to withhold tax or to withhold tax at a reduced rate under an applicable bilateral income tax treaty. Without limiting the foregoing, the Parties shall exercise their reasonable efforts to ensure that any withholding taxes imposed are reduced as far as possible under the provisions of any applicable tax treaty, and shall cooperate in filing any forms required for such reduction. Each Party shall cooperate with the other and furnish the other Party with appropriate documents to secure application of the most favorable rate of withholding tax under applicable Law (or exemption from such withholding tax payments, as applicable).
| 9. | CONFIDENTIALITY AND PUBLICATION |
| 9.1 | Nondisclosure Obligation. |
9.1.1 Except with the prior written consent of the Disclosing Party or as otherwise set forth herein, during the Term and for a period of [**] after any termination or expiration of this Agreement, all Confidential Information of the Disclosing Party (a) shall be maintained in confidence by the Receiving Party, (b) shall not be disclosed by the Receiving Party to an Affiliate or Third Party, and (c) shall not be used by the Receiving Party for any purpose except to perform its obligations and to exploit its rights under this Agreement.
9.1.2 Notwithstanding the obligations of confidentiality and non-use set forth above, subject to Section 7.1.3, the Receiving Party may provide the Disclosing Party’s Confidential Information, and disclose the existence and terms of this Agreement, as may be reasonably required in order to perform its obligations and to exploit its rights under this Agreement, and specifically (a) to its Related Parties, and the Receiving Party’s and its Related Parties’ employees, directors, officers, agents, consultants, advisors or other Third Parties who need to
- 45 -
Confidential
know the Confidential Information of the Disclosing Party for the performance of its obligations or to exercises its rights hereunder (or for such Persons to determine their interest in performing such activities) in accordance with this Agreement, in each case who are under an obligation of confidentiality with respect to such Confidential Information that is no less stringent than the terms of this Section 9.1, provided that, it being understood that, notwithstanding any other provision of this Agreement, in the case of disclosures made to clinical trial sites, investigators, CROs or other Third Parties involved in the Development of the Licensed Compound or Licensed Product, the duration for the obligation of confidentiality and non-use provided in a Party’s agreement with such clinical trial sites, investigators, CROs or other Third Parties may be less than the duration for the obligation of confidentiality and non-use in this Agreement so long as such agreement specifies a duration for the obligation of confidentiality and non-use at least [**] from the expiration or termination date of such agreement with clinical trial sites, investigators, CROs or other Third Parties; (b) to Governmental Authorities or Regulatory Authorities in order to seek or obtain Patent Rights or Regulatory Approvals in a manner not inconsistent with this Agreement or to perform its obligations or exploit its rights under this Agreement; provided that such Confidential Information shall be disclosed only to the extent reasonably necessary to do so; (c) to the extent required by Governmental Authority or Law; and (d) to its actual or bona fide prospective acquirers, underwriters, investors, lenders or other financing sources, its actual or bona fide prospective collaborators, licensors, licensees or strategic partners, and to its consultants and advisors with respect to any actual or bona fide prospective acquisition, sale, financing or collaboration of the Receiving Party, in each case who are under an obligation of confidentiality with respect to such information that is no less stringent than the terms of this Section 9.1, provided that such Confidential Information is disclosed only to the extent reasonably necessary to do so. In addition, Karyopharm may disclose Ono’s Confidential Information to the counterparty to any Karyopharm Third Party Agreement, which is under an obligation of confidentiality with respect to such Confidential Information that is no less stringent than the terms of this Section 9.1, to comply with such Third Party Agreement, provided that such Confidential Information is disclosed only to the extent reasonably necessary to do so and Karyopharm shall be fully responsible for compliance with such an obligation of confidentiality and non-use by such counterparty to any Karyopharm Third Party Agreement. If the Receiving Party is required by Governmental Authority or Law to disclose the Disclosing Party’s Confidential Information, the Receiving Party shall, to the extent consistent with Law, promptly inform the Disclosing Party of the required disclosure in order to provide the Disclosing Party an opportunity to challenge or limit the disclosure obligations. Confidential Information that is required to be disclosed by Governmental Authority or Law shall remain otherwise subject to the confidentiality and non-use provisions of this Section 9.1. If a Party concludes that a copy of this Agreement shall be filed with the U.S. Securities and Exchange Commission or similar regulatory agency in a country other than the United States, then, to the extent consistent with Law, such Party will provide the other
- 46 -
Confidential
Party with a copy of this Agreement showing any provisions hereof as to which the Party proposes to request confidential treatment, will provide the other Party with an opportunity to comment on any such proposed redactions and to suggest additional redactions, and will take such Party’s reasonable and timely comments into consideration before filing this Agreement.
| 9.2 | Publication and Publicity. |
9.2.1 Publication. Ono and Karyopharm each acknowledge the other Party’s interest in publishing certain key results of the activities conducted under this Agreement. Ono may publish or publicly disclose such results with respect to a Clinical Study or Development activity that is part of Ono’s Development Plan in which Karyopharm is not participating, subject to Karyopharm’s right to review as required in this Section 9.2.1. Karyopharm may publish or publicly disclose results from any of its activities, subject to Ono’s right to review as required in this Section 9.2.1. Each Party also recognizes the mutual interest in obtaining valid patent protection and in protecting trade secret information. Consequently, except for disclosures permitted pursuant to Section 9.1 and 9.2.2(a) through (c), in case Ono wishes to make a publication or public presentation that pertains to results concerning Licensed Compound(s) or Licensed Product(s) which has not been previously disclosed, Ono shall deliver to the other Party a copy of the proposed written publication or presentation at least [**] prior to submission for publication or presentation. Except for disclosures permitted pursuant to Section 9.1 and 9.2.2(a) through (c), in case that Karyopharm wishes to make (i) a publication or public presentation that pertains to results concerning Licensed Compound(s) or Licensed Product(s) in an internationally recognized publication or conference or (ii) a publication or public presentation that, Karyopharm reasonably judges, contains Confidential Information of Ono, Karyopharm shall deliver to Ono a copy of the proposed written publication or presentation at least [**] prior to submission for publication or presentation. The reviewing Party shall have the right (a) to propose modifications to the publication or presentation for patent reasons, trade secret reasons or business reasons, and the publishing Party will remove all Confidential Information of the reviewing Party if requested by the reviewing Party, and (b) to request a reasonable delay in publication or presentation in order to protect patentable information. If the reviewing Party requests a delay, the publishing Party shall delay submission or presentation for a period of [**] (or such shorter period as may be mutually agreed by the Parties considering the time constraints for submission to a particular conference or journal) to enable the non-publishing Party to file patent applications protecting such Party’s rights in such information in accordance with Article 12 or may agree to remove the patentable information from the publication or presentation in question. With respect to any proposed publications or disclosures by investigators or academic or non-profit collaborators working with a Party, such materials shall be subject to review and delay under this Section 9.2 to the extent that such Party has the right and ability (after using Commercially Reasonable Efforts to obtain such right and ability) to do so.
9.2.2 Publicity. Except as set forth in Section 9.1 above and Section 9.2.2(a) through (c) below, the terms of this Agreement may not be disclosed by either Party, and no Party shall use the name, trademark, trade name or logo of the other Party
- 47 -
Confidential
or its employees in any publicity, news release or disclosure relating to this Agreement, its subject matter or the activities of the Parties hereunder without the prior express written permission of the other Party, except as may be required by Law or expressly permitted by the terms hereof.
(a) Following the execution of this Agreement, the Parties shall issue a joint press release of which content shall be mutually agreed between the Parties. After such initial press release, except as provided in Section 9.2.2(b), neither Party shall issue a press release or public statement relating to this Agreement without the prior written approval of the other Party, which approval shall not be unreasonably withheld, conditioned or delayed, except that a Party may (i) once a press release or other public statement has been made by a Party in accordance with this Agreement, make subsequent public disclosure of any of the information contained in such press release or other written statement, without the approval of the other Party, and (ii) upon prior written notice, to the extent permitted under applicable Law, to the other Party, issue a press release or public announcement as required, in the reasonable judgment of such Party, by Law.
(b) In addition, either Party may, upon prior written notice, to the extent permitted under applicable Law, to the other Party, issue a press release or make a public disclosure relating to such Party’s Development, use, Manufacturing, having Manufactured or Commercialization activities with respect to Licensed Products in the Field in accordance with this Agreement and Karyopharm may, upon a prior written notice to Ono, issue a press release or make a public disclosure relating to the Development, use, Manufacturing, having Manufactured or Commercialization activities with respect to Licensed Products outside the Field, provided that such press release or public disclosure does not disclose Confidential Information of the other Party. The Party wishing to issue such press release or make such a public disclosure shall provide the other Party with such draft at least [**] prior to such press release or public disclosure for other Party’s review. The reviewing Party shall have the right to propose modifications to such press release or public disclosure for patent reasons or, trade secret reasons or business reasons, and the publishing Party will remove all Confidential Information of the reviewing Party if requested by the reviewing Party. Notwithstanding the foregoing, a Party may issue such press release or make such a public disclosure without [**] prior written notice to the other Party if in the reasonable judgment of such Party, such press release or public disclosure within the period shorter than [**] is required by Law, provided that the Party shall provide the other Party with a copy of such press release or other public disclosure no later than when it is issued or released. Either Party may publicly announce or disclose without regard to the preceding requirements of this Section 9.2.2(b) any information that was previously publicly disclosed pursuant to this Section 9.2.2(b). Furthermore, either Party may issue a full translation of a press release or public disclosure to be issued by the other Party.
(c) Each Party shall be entitled to include the name and picture of the other Party within a list of collaborators with consent of the other Party. Once a Party obtains such consent from the other Party, such Party may use the name and picture of such other Party in a Party’s annual report, company brochure or website and so on, and such Party may continue to use them in the same.
- 48 -
Confidential
| 10. | REPRESENTATIONS, WARRANTIES AND COVENANTS; DISCLAIMER |
10.1 Mutual Representations and Warranties. Each Party represents and warrants to the other Party that as of the Effective Date:
10.1.1 It is duly organized and validly existing under the Law of its jurisdiction of incorporation or formation, and has full corporate or other power and authority to enter into this Agreement, and to carry out the provisions hereof;
10.1.2 It is duly authorized to execute and deliver this Agreement, and to perform its obligations hereunder, and the individual(s) executing this Agreement on its behalf has been duly authorized to do so by all requisite corporate action;
10.1.3 This Agreement is legally binding upon it and enforceable in accordance with its terms, subject to the general principles of equity and to bankruptcy, insolvency, moratorium and other similar Law affecting the enforcement of creditors’ rights generally;
10.1.4 The execution, delivery and performance of this Agreement by it does not conflict with any agreement, instrument or understanding, oral or written, to which it is a party and by which it may be bound, or with its charter or by-laws;
10.1.5 Neither the execution and delivery of this Agreement nor the performance hereof by such Party requires such Party to obtain any permit, authorization or consent from any Governmental Authority (except for any intellectual property rights, INDs, Regulatory Approvals, pricing or reimbursement approvals, Manufacturing-related approvals or similar approvals necessary for Manufacture or having Manufactured in the Field for the Ono Territory, or Development, use or Commercialization in the Field in the Ono Territory, of the Licensed Product as set forth herein), or, to its and its Affiliates’ Knowledge, from any other Person;
10.1.6 It has not granted any right to any Third Party that would conflict with the rights granted to the other Party hereunder; and
10.1.7 Neither it nor any of its Affiliates has been debarred or is subject to debarment and neither Party nor any of its Affiliates has, to its Knowledge, used in any capacity, in connection with its Development of the Licensed Compound or the Licensed Product, any Person that has been debarred pursuant to Section 306 of the U.S. Federal Food, Drug, and Cosmetic Act, as amended, or any comparable Law in any country, or that is the subject of a conviction described in such section or any comparable Law in any country.
10.2 Representations and Warranties of Karyopharm. Karyopharm represents and warrants to Ono that as of the Effective Date:
- 49 -
Confidential
10.2.1 Karyopharm is the sole and exclusive owner of, or otherwise Controls, the Karyopharm Technology and has the right to grant the licenses to the Karyopharm Technology to Ono pursuant to this Agreement;
10.2.2 To the Knowledge of Karyopharm and its Affiliates, Schedule 10.2.2 is an accurate listing of all Karyopharm Patent Rights owned or Controlled by Karyopharm or its Affiliates as of the Effective Date that are necessary or useful for Manufacture or having Manufactured in the Field for the Ono Territory, or Development, use or Commercialization in the Field in the Ono Territory, of the Licensed Product;
10.2.3 Any Karyopharm Technology is free and clear of liens, charges or encumbrances;
10.2.4 To the Knowledge of Karyopharm and its Affiliates, any Karyopharm Patent Rights specified in Schedule 10.2.2 are not invalid or unenforceable in whole or part or, as to patent application, has not lapsed, or in the case of a provisional patent application has not been cancelled, withdrawn or abandoned without the possibility of revival;
10.2.5 To the Knowledge of Karyopharm and its Affiliates, except manufacture and supply of Licensed Compound as reagent by reagent supplier, there is no material infringement or misappropriation of any Karyopharm Technology by any Third Party;
10.2.6 To the Knowledge of Karyopharm and its Affiliates, any Person who was involved in the invention for the Karyopharm Patent Rights, has executed and delivered to Karyopharm or its Affiliates, as the case may be, an agreement assigning to Karyopharm (or its applicable Affiliate) all rights, titles and interests in and to all the inventions for the Karyopharm Patent Rights arising out of or relating to such Person’s activities with respect to the Licensed Compound and the Licensed Product;
10.2.7 Karyopharm and its Affiliates have generated, prepared, maintained and retained all Data and regulatory documentation that is required to be generated, maintained or retained pursuant to and in accordance with GLP and GCP and applicable Law in all material respects, and all such Data and regulatory documentation are true, complete and correct and what it purports to be. Karyopharm and its Affiliates have conducted, (and each of their respective contractors and consultants have conducted) its research and Development activities in accordance with applicable GLP and GCP and applicable Law in each case in all material respects, provided, however, with respect to any information provided by Karyopharm to Ono prior to the Effective Date relating to any Relevant Clinical Studies, Ono acknowledges and agrees that such information is preliminary, based on unaudited clinical site data and will not be finalized until the completion of data analysis, lock and transfer;
- 50 -
Confidential
10.2.8 Karyopharm has disclosed or made available to Ono all material information in its or its Affiliates’ possession and Control relating to the Licensed Compound and the Licensed Product, and the Development, Manufacture, use and Commercialization of the Licensed Compound and the Licensed Product as conducted prior to the Effective Date, including by providing or making available complete and correct copies of the following: (a) adverse event reports; (b) clinical study reports and material study data; and (c) FDA inspection reports, notices of adverse findings, warning letters, Regulatory Approval filings and other material regulatory documentation;
10.2.9 To the Knowledge of Karyopharm and its Affiliates, there are no, and there have been no, material safety issues relating to the Licensed Compound or the Licensed Product;
10.2.10 Except as has been disclosed to Ono by Karyopharm, Karyopharm and its Affiliates are not aware of any fact or circumstance that would reasonably be expected to materially adversely affect the acceptance or the subsequent approval, by Regulatory Authority of any filing, application or request for Regulatory Approval;
10.2.11 Karyopharm and its Affiliates are not aware of, and has not received, any written notice of (a) any actual or threatened claim that any issued patent or trade secret right owned or controlled by a Third Party would be infringed or misappropriated by the Development, Manufacture, use or Commercialization of the Licensed Products in the Field in the Ono Territory and Karyopharm Territory, or (b) any threatened claims or litigation seeking to invalidate or otherwise challenge the Karyopharm Patent Rights or Karyopharm’s or its Affiliates’ rights therein;
10.2.12 Karyopharm and its Affiliates have taken commercially reasonable steps to protect, preserve and maintain the confidentiality of all confidential or non-public information included in Karyopharm Know-How, including by disclosing such Karyopharm Know-How to Third Parties only under terms of confidentiality. To the Knowledge of Karyopharm and its Affiliates, no breach of such confidentiality obligations has been committed by any Third Party;
10.2.13 To the Knowledge of Karyopharm and its Affiliates, neither Karyopharm nor its Affiliates, nor any of its or their respective directors, officers, employees or agents has (a) committed an act, (b) made a statement or (c) failed to act or make statement, in any case ((a), (b) or (c)), that (x) would be or create an untrue statement of material fact or fraudulent statement to the FDA or any other Regulatory Authority with respect to the Development, Manufacture, having Manufactured, use or Commercialization of the Licensed Compound or the Licensed Product or (y) could reasonably be expected to provide a basis for the FDA or any other Regulatory Authority to invoke its policy respecting “Fraud, Untrue Statements of Material Facts, Bribery and Illegal Gratuities”, set forth in
- 51 -
Confidential
56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto or any analogous laws or policies, with respect to the Development, Manufacture, having Manufactured, use or Commercialization of the Licensed Compound or the Licensed Product; and
10.2.14 With respect to each Karyopharm Third Party Agreement set forth on Schedule 1.43, (a) Karyopharm is not in breach under such Third Party Agreement; (b) Karyopharm has not received any notice of breach under such Third Party Agreement; and (c) Karyopharm has previously provided Ono with access to true and complete copies of such Third Party Agreement; provided, however, that Confidential Information of Karyopharm or confidential information of its counterparty may have been redacted from such copies.
10.3 Mutual Covenants. During the Term, each Party covenants as follows;
10.3.1 It will not enter into any agreement, instrument or understanding, oral or written, with any Third Party which conflicts with this Agreement;
10.3.2 It will not grant any right to any Third Party that would conflict with the rights granted to the other Party hereunder; and
10.3.3 It will not, and it will use Commercially Reasonable Efforts to ensure its Related Parties will not conduct any activities which would be subject to debarment and neither Party will use in any capacity, and either Party will use Commercially Reasonable Efforts to ensure any Related Party will not use, in connection with the performance of its obligations under this Agreement, any Person that has been debarred pursuant to Section 306 of the U.S. Federal Food, Drug, and Cosmetic Act, as amended, or any comparable Law in any country, or that is the subject of a conviction described in such section or any comparable Law in any country. Each Party agrees to inform the other Party in writing immediately if it or any Person that is performing activities under this Agreement, is debarred or is subject to debarment or is the subject of a conviction described in such Section 306, or any comparable Law in any country, or if any action, suit, claim, investigation or legal or administrative proceeding is pending or, to the notifying Party’s Knowledge, is threatened, relating to the debarment or conviction of the notifying Party or any Person used in any capacity by such Party or any of its Related Party in connection with the performance of its obligations under this Agreement.
10.4 Karyopharm’s Covenants. During the Term, Karyopharm covenants as follows;
10.4.1 Any Karyopharm Technology will be free and clear of liens, charges or encumbrances except for any liens, charges or encumbrances that may be incurred in connection with a financing arrangement by Karyopharm, provided that such liens, charges or encumbrances will not affect the ability of Ono to Develop, use, obtain Regulatory Approval for and Commercialize the Licensed Products in the Field in each country in the Ono Territory and to Manufacture the Licensed Compound and Licensed Product in the Field for the Ono Territory; and
- 52 -
Confidential
10.4.2 With respect to each Karyopharm Third Party Agreement set forth on Schedule 1.43, Karyopharm will not conduct any activities that would be reasonably expected to result in a breach of any such Third Party Agreement which would affect the license grant to Ono under this Agreement; and
10.4.3 Karyopharm will obtain Katholieke Universiteit Leuven’s assurances that it will not act adversely to affect the license grant to Ono under this Agreement.
10.5 Warranty Disclaimer. EXCEPT AS OTHERWISE EXPRESSLY PROVIDED IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATION OR EXTENDS ANY WARRANTY OF ANY KIND, EITHER EXPRESS OR IMPLIED, TO THE OTHER PARTY WITH RESPECT TO ANY TECHNOLOGY, LICENSED COMPOUND, LICENSED PRODUCT, GOODS, SERVICES, RIGHTS OR SUBJECT MATTER OF THIS AGREEMENT AND EACH PARTY HEREBY DISCLAIMS ALL IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NON-INFRINGEMENT WITH RESPECT TO ANY AND ALL OF THE FOREGOING. EACH PARTY HEREBY DISCLAIMS ANY REPRESENTATION OR WARRANTY THAT THE DEVELOPMENT, USE, MANUFACTURE, HAVING MANUFACTURED OR COMMERCIALIZATION OF THE LICENSED COMPOUNDS OR THE LICENSED PRODUCTS PURSUANT TO THIS AGREEMENT WILL BE SUCCESSFUL OR THAT ANY PARTICULAR SALES LEVEL WITH RESPECT TO A LICENSED PRODUCT WILL BE ACHIEVED.
| 11. | INDEMNIFICATION; LIMITATION OF LIABILITY; INSURANCE |
11.1 Indemnification by Ono. Ono shall indemnify, hold harmless, and defend Karyopharm, its Related Parties, and their respective directors, officers, employees, agents and representatives (collectively, the “Karyopharm Indemnitees”) from and against any and all Losses to the extent arising out of or resulting from, directly or indirectly, (a) any breach of, or inaccuracy in, any representation or warranty made by Ono in this Agreement, or any breach or violation of any covenant or agreement of Ono in or in the performance of this Agreement, (b) the negligence or willful misconduct of Ono Indemnitees, in the performance of Ono’s obligations under this Agreement, or (c) the Development, Manufacture, having Manufactured, use or Commercialization of Licensed Compounds and Licensed Products by or on behalf of Ono Indemnitees. Additionally, it is agreed between Karyopharm and Ono that (i) the indemnification language described in Schedule 11.1 shall be incorporated in the Clinical Supply Agreement (and the Commercial Supply Agreement, if any) and (ii) Ono will owe and perform indemnification obligation under the Clinical Supply Agreement (and the Commercial Supply Agreement, if any). Ono shall have no obligation to indemnify, hold harmless and defend the Karyopharm Indemnitees to the extent that Karyopharm is obligated to indemnify, hold harmless and defend Ono Indemnitees under Section 11.2.
- 53 -
Confidential
11.2 Indemnification by Karyopharm. Karyopharm shall indemnify, hold harmless, and defend Ono, its Related Parties and their respective directors, officers, employees, agents and representatives (collectively, the “Ono Indemnitees”) from and against any and all Losses to the extent arising out of or resulting from, directly or indirectly, (a) any breach of, or inaccuracy in, any representation or warranty made by Karyopharm in this Agreement, or any breach or violation of any covenant or agreement of Karyopharm in or in the performance of this Agreement, (b) the negligence or willful misconduct of Karyopharm Indemnitees, in the performance of Karyopharm’s obligations under this Agreement, or (c) the Development, Manufacture, having Manufactured, use or Commercialization of Licensed Compounds and Licensed Products by or on behalf of Karyopharm Indemnitees. Additionally, it is agreed between Karyopharm and Ono that (i) the indemnification language described in Schedule 11.1 shall be incorporated in the Clinical Supply Agreement (and the Commercial Supply Agreement, if any) and (ii) Karyopharm will owe and perform indemnification obligation under the Clinical Supply Agreement (and the Commercial Supply Agreement, if any). Karyopharm shall have no obligation to indemnify, hold harmless and defend the Ono Indemnitees to the extent that Ono is obligated to indemnify, hold harmless and defend Karyopharm Indemnitees under Section 11.1.
11.3 Indemnification Procedure. In the event of any such claim against any Ono Indemnitee or Karyopharm Indemnitee (individually, an “Indemnitee”), the indemnified Party shall promptly notify the other Party in writing of the claim and the indemnifying Party shall manage and control, at its sole expense, the defense of the claim and its settlement. The indemnifying Party may settle the claim only with the consent of the applicable Indemnitees, which shall not be unreasonably withheld, conditioned or delayed; provided that an Indemnitee shall have no obligation to consent to any settlement of any such claim which imposes on such Indemnitee any liability or obligation which cannot be assumed and performed in full by the indemnifying Party. The applicable Indemnitee shall cooperate with the indemnifying Party and may, at its option and expense, be represented in any such action or proceeding. No Indemnitee may settle any such claim without the prior written consent of the indemnifying Party, which shall not be unreasonably withheld, delayed or conditioned. The indemnifying Party shall not be liable for any settlements, litigation costs or expenses incurred by any Indemnitee without the indemnifying Party’s written consent. Notwithstanding the foregoing, if the indemnifying Party believes that any of the exceptions to its obligation of indemnification of the Indemnitees set forth in Sections 11.1 or 11.2 may apply, the indemnifying Party shall promptly notify the Indemnitees, which shall then have the right to be represented in any such action or proceeding by separate counsel at their expense; provided that the indemnifying Party shall be responsible for payment of such expenses if the Indemnitees are ultimately determined to be entitled to indemnification from the indemnifying Party for the matters to which the Indemnitee notified the indemnifying Party of the application of Sections 11.1 or 11.2, as applicable.
11.4 Limitation of Liability. NEITHER PARTY WILL BE LIABLE FOR SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES ARISING OUT OF OR RELATING OT THIS AGREEMENT OR THE EXERCISE OF ITS RIGHTS HEREUNDER, OR LOST PROFITS ARISING FROM OR RELATING TO THIS AGREEMENT, REGARDLESS OF ANY NOTICE OF SUCH DAMAGES, EXCEPT AS A RESULT OF SUCH PARTY’S WILLFUL MISCONDUCT OR GROSS NEGLIGENCE OR A BREACH OF SECTION 7.3 OR ARTICLE 9. NOTHING IN THIS SECTION 11.4 IS INTENDED TO LIMIT OR RESTRICT THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF EITHER PARTY.
- 54 -
Confidential
11.5 Insurance. Either Party shall, at its own expense, procure and maintain product liability insurance or self-insurance in the amount sufficient to perform its obligation hereunder. Upon the other Party’s request, a Party shall promptly provide the other Party with copies of certificates of insurance evidencing such coverage.
| 12. | INTELLECTUAL PROPERTY OWNERSHIP, PROTECTION AND RELATED MATTERS; BRAND NAME |
12.1 Inventorship. For purposes of Section 12.2, inventorship for inventions and discoveries first made during the course of the performance of activities pursuant to this Agreement shall be determined in accordance with U.S. Patent Law for determining inventorship.
12.2 Ownership. Karyopharm shall own the entire right, title and interest in and to all inventions and discoveries (and Patent Rights claiming patentable inventions therein) first made or discovered solely by directors, officers or employees of Karyopharm or its Affiliates, or acquired solely by Karyopharm or its Affiliates. Ono shall own the entire right, title and interest in and to all inventions and discoveries (and Patent Rights claiming patentable inventions therein) first made or discovered solely by directors, officers or employees of Ono or its Affiliates, or acquired solely by Ono or its Affiliates. The Parties shall jointly own any Joint IP.
12.3 Disclosure. Each Party shall promptly disclose to the other Party Joint IP and all inventions made by a director, an officer or an employee of such Party or its Affiliates in the performance of its obligations under this Agreement.
12.4 Prosecution and Maintenance of Patent Rights.
12.4.1 Ono Technology.
(a) Subject to Section 12.4.1(b), Ono has the sole responsibility to, at Ono’s discretion and expense, file, prosecute, and maintain all Patent Rights comprising Ono Technology (other than Joint Patent Rights), in Ono’s name.
(b) In the event that Ono elects not to seek or continue to seek or maintain patent protection on any Ono Patent Rights in any country in the Karyopharm Territory, Karyopharm shall have the right (but not the obligation), at its expense, to seek, prosecute and maintain patent protection on such Ono Patent Rights in such country in the name of Ono. Ono shall use Commercially Reasonable Efforts to make available to Karyopharm and its authorized attorneys, agents or representatives, such of its employees as are reasonably necessary to assist Karyopharm in obtaining and maintaining the patent protection described under this Section 12.4.1(b). Ono shall sign or use Commercially Reasonable Efforts to have signed all legal documents necessary for Karyopharm to file and prosecute such patent applications or to obtain or maintain such patents.
- 55 -
Confidential
12.4.2 Karyopharm Technology.
(a) Subject to Section 12.4.2(b), Karyopharm has the sole responsibility to, at Karyopharm’s discretion and expense, file, conduct prosecution, and maintain, all Patent Rights comprising Karyopharm Technology (other than Joint Patent Rights), in Karyopharm’s name. Karyopharm shall prosecute and maintain the Karyopharm Patent Rights in the Ono Territory.
(b) Notwithstanding Section 12.4.2(a), Karyopharm shall consult with Ono on the preparation, filing, prosecution, and maintenance of all Karyopharm Patent Rights in the Ono Territory. Karyopharm shall furnish Ono with copies of proposed filings and documents received from or filed with the relevant patent offices with respect to Karyopharm Patent Rights and such other documents directly related to the prosecution and maintenance of Karyopharm Patent Rights in the Ono Territory reasonably necessary for Ono to exercise its rights under this Section 12.4.2(b), and, as applicable, in sufficient time prior to filing such document or making any payment due thereunder to allow for review and comment by Ono and shall consider in good faith timely comments from Ono thereon.
(c) In the event that Karyopharm elects not to seek or continue to seek or maintain patent protection on any Karyopharm Patent Right in any country in the Ono Territory, Ono shall have the right (but not the obligation), at its expense, to seek, prosecute and maintain patent protection on such Karyopharm Patent Right in such country in the Ono Territory in the name of Karyopharm. Karyopharm shall use Commercially Reasonable Efforts to make available to Ono and its authorized attorneys, agents or representatives, such of its employees as are reasonably necessary to assist Ono in obtaining and maintaining the patent protection described under this Section 12.4.2(c). Karyopharm shall sign or use Commercially Reasonable Efforts to have signed all legal documents necessary for Ono to file and prosecute such patent applications or to obtain or maintain such patents. Such Karyopharm Patent Right in such country shall not be taken into account in determining the Royalty Term.
12.4.3 Joint Patent Rights.
(a) Upon receiving notice of the creation of Joint Patent Rights, the IP Working Group shall determine which Party will be responsible of obtaining and maintaining Joint Patent Rights. Such Party (the “Joint IP Prosecuting Party”) shall file, prosecute, and maintain all Joint Patent Rights throughout the world, in the names of both Karyopharm and Ono. The Joint IP Prosecuting Party shall provide the other Party an opportunity to review and comment on material documents related to such filing, prosecution and maintenance in accordance with this Section 12.4.3, which comments the Joint IP Prosecuting Party shall consider in good faith. Each Party shall at its own cost, sign, or use Commercially Reasonable Efforts to have signed, all legal documents necessary to file and prosecute patent applications or to obtain or maintain patents in respect of such Joint Patent Rights.
(b) In the event that the Joint IP Prosecuting Party elects not to file or continue to prosecute or maintain patent protection on any Joint Patent Rights anywhere in the world, the other Party shall have the right (but not the obligation) to file, prosecute and maintain Joint Patent Rights in the names of both Karyopharm and Ono. If such other Party exercises such
- 56 -
Confidential
right, the Joint IP Prosecuting Party shall use Commercially Reasonable Efforts to make available to such other Party and its authorized attorneys, agents or representatives, such of its employees as are reasonably necessary to assist such other Party in obtaining and maintaining the patent protection described under this Section 12.4.3(b). Each Party shall at its own cost, sign or use Commercially Reasonable Efforts to have signed all legal documents necessary to file and prosecute such patent applications or to obtain or maintain such patents.
(c) The Parties shall [**] incurred for the common activities for patent filing, prosecution and maintenance of Joint Patent Rights and each Party shall be responsible for other costs for patent filing, prosecution and maintenance of Joint Patent Rights in its Territory (collectively, “Joint Patent Costs”). The Joint IP Prosecuting Party shall invoice the other Party such Joint Patent Costs which shall be incurred by the other Party in accordance with this Section 12.4.3(c) within [**] after the Calendar Quarter in which such Joint Patent Costs were incurred and the other Party shall pay such Joint Patent Costs within [**] after receipt of such invoice.
(d) Notwithstanding Section 12.4.3(c), if a Party does not wish to bear Joint Patent Costs which shall be incurred by such Party with respect to a Joint Patent Right in any country(ies), such Party may, by providing [**] prior written notice to the other Party, terminate its obligation to pay such Joint Patent Costs. Such Party shall promptly assign all of its right, title and interest in and to such Joint Patent Right in such country(ies) to the other Party upon such other Party’s written request.
12.4.4 Cooperation. Each Party shall: (a) use Commercially Reasonable Efforts to make its employees, agents and consultants reasonably available to the other Party (or to the other Party’s authorized attorneys, agents or representatives), to the extent reasonably necessary to enable such other Party to undertake patent prosecution in accordance with this Agreement; and (b) provide the other Party with copies of all material correspondence pertaining to prosecution with the patent offices wherever applicable to Patent Rights licensed to such other Party in such other Party’s Territory under this Agreement.
12.5 Enforcement.
12.5.1 Notices. Each Party shall promptly report in writing to the other Party any (a) known or suspected infringement (including any interference, opposition or invalidation proceedings) of any Karyopharm Technology, Ono Technology or Joint IP or (b) unauthorized use or misappropriation of any Confidential Information or Know-How of a Party, by a Third Party of which it becomes aware in its Territory, in each case to the extent such infringing, unauthorized or misappropriating activities involve, as to a Licensed Product or a competing product in the Field (“Competitive Infringement”), and shall provide the other Party with all available evidence of such Competitive Infringement.
12.5.2 Rights to Enforce.
(a) In the Karyopharm Territory; Ono Technology.
- 57 -
Confidential
(i) First Right. Karyopharm shall have the first right to initiate an infringement or other appropriate suit or action in the Karyopharm Territory against any Third Party with respect to any Competitive Infringement in the Karyopharm Territory of any Ono Technology with respect to a product competing with a Licensed Product in the Karyopharm Territory.
(ii) Step-In Right. If within [**] (or such shorter period of time as required by applicable Law to avoid loss of material enforcement rights) after Karyopharm’s receipt of a notice from Ono or notifying Ono of a Competitive Infringement with respect to any Ono Technology, with respect to a product competing with a Licensed Product in the Karyopharm Territory, Karyopharm does not take any action as described in Section 12.5.2(a)(i) and permitted hereunder against such Competitive Infringement in the Karyopharm Territory, Ono may in its sole discretion (but not the obligation), bring and control any legal or other appropriate action in connection therewith at its sole expense.
(b) In the Ono Territory; Ono Technology.
(i) First Right. Ono shall have the first right to initiate an infringement or other appropriate suit or action in the Ono Territory against any Third Party with respect to any Competitive Infringement in the Ono Territory of any Ono Technology with respect to a product competing with a Licensed Product in the Ono Territory.
(ii) Step-In Right. If within [**] (or such shorter period of time as required by applicable Law to avoid loss of material enforcement rights) after Ono’s receipt of a notice from Karyopharm or notifying Karyopharm of a Competitive Infringement with respect to any Ono Technology, with respect to a product competing with a Licensed Product in the Ono Territory, Ono does not take any action as described in Section 12.5.2(b)(i) and permitted hereunder against such Competitive Infringement in the Ono Territory, Karyopharm may in its sole discretion (but not the obligation), bring and control any legal or other appropriate action in connection therewith at its sole expense.
(c) In the Ono Territory; Karyopharm Technology.
(i) First Right. Karyopharm shall have the first right to initiate an infringement or other appropriate suit or action in the Ono Territory against any Third Party with respect to any Competitive Infringement in the Ono Territory of any Karyopharm Technology with respect to a product competing with a Licensed Product in the Ono Territory.
(ii) Step-In Right. If within [**] (or such shorter period of time as required by applicable Law to avoid loss of material enforcement rights) after Karyopharm’s receipt of a notice from Ono or notifying Ono of a Competitive Infringement with respect to any Karyopharm Technology, with respect to a product competing with a Licensed Product in the Ono Territory, Karyopharm does not take any action as described in Section 12.5.2(c)(i) and permitted hereunder against such Competitive Infringement in the Ono Territory, Ono may in its sole discretion (but not the obligation), bring and control any legal other appropriate action in connection therewith at its sole expense.
- 58 -
Confidential
(d) Joint IP. In the case of any Competitive Infringement of any Joint IP, the Parties shall promptly confer to consider such Competitive Infringement and the appropriate course of action in good faith.
12.5.3 Procedures; Expenses and Recoveries. The Party having the right to initiate any infringement suit or action (including any interference, opposition or invalidation proceedings; the same hereinafter) under Section 12.5.2 above (the “Initiating Party”) shall have the sole and exclusive right to select counsel for any such suit or action and shall pay all expenses of the suit or action, including attorneys’ fees and court costs and reimbursement of the other Party’s reasonable out-of-pocket expense in rendering assistance requested by the Initiating Party. Should Initiating Party initiate an infringement suit or action under Section 12.5.2, Initiating Party agrees to discuss with the other Party ways to manage the potential risk to the other Party’s Patent Rights in connection with such suit or action, including limiting the number and scope of claims that are asserted in connection with such suit or action. Initiating Party shall use good faith efforts to employ any reasonable measures agreed to by the Parties to manage such potential risk. If required under applicable Law in order for the Initiating Party to initiate or maintain such suit, or if the Initiating Party is unable to initiate or prosecute such suit solely in its own name or it is otherwise advisable to obtain an effective legal remedy, the other Party shall join or participate in as a party to the suit and shall execute and cause its Affiliates to execute all documents necessary for the Initiating Party to initiate suit to prosecute and maintain such suit. In addition, at the Initiating Party’s request, the other Party shall provide reasonable assistance to the Initiating Party in connection with such an infringement suit at no charge to the Initiating Party except for reimbursement by the Initiating Party of reasonable out-of-pocket expenses incurred in rendering such assistance. The non-Initiating Party shall have the right to participate and be represented in any such suit by its own counsel at its own expense. Neither Party shall enter into any settlement of any Competitive Infringement described in Section 12.5.2 that admits to the invalidity or unenforceability of the Karyopharm Patent Rights, Ono Patent Rights and Joint Patent Rights, incurs any financial or other liability on the part of the other Party or requires an admission of liability, wrongdoing or fault on the part of the other Party, in each case without the other Party’s prior written consent, not to be unreasonably withheld, conditioned or delayed. If the Initiating Party obtains from a Third Party, in connection with such suit, any damages, license fees, royalties or other compensation (including any amount received in settlement of such suit), such amounts shall be allocated in all cases as follows:
(a) first, to reimburse each Party for all expenses of the suit incurred by the Parties, including attorneys’ fees and disbursements, court costs and other litigation expenses (on a pro rata basis, based on each Party’s respective expenses, to the extent the recovery is less than all such expenses); and
- 59 -
Confidential
(b) second, the remainder share be retained by the Initiating Party; provided that in the case of Section 12.5.2 (c), despite of which Party is the Initiating Party, such remainder shall be retained by [**].
12.6 Infringement of Third Party Patent Rights.
12.6.1 If the Development, Manufacture, having Manufactured, use or Commercialization of any Licensed Compound and/or Licensed Product in or outside the Ono Territory pursuant to this Agreement results in a claim, suit or proceeding alleging patent infringement against Karyopharm and/or Ono (or their respective Related Parties) (collectively, “Infringement Actions”), the Party subject to such Infringement Actions (the “Subject Party”) shall promptly notify the other Party in writing and shall discuss with the other Party the strategy for defending such Infringement Actions, but shall have the right to direct and control the defense thereof in its sole discretion and at its own expense, with counsel of its choice; provided that, the other Party may participate in the defense and/or settlement thereof, at its own expense with counsel of its choice. In any event, the Subject Party agrees to keep the other Party hereto reasonably informed of all material developments in connection with any such Infringement Action. Ono agrees not to settle such Infringement Action, or make any admissions or assert any position in such Infringement Action, in a manner that would adversely affect the allegedly infringing Licensed Compound and/or Licensed Product or the Development, Manufacture, having Manufactured, use or Commercialization of such Licensed Compound and/or Licensed Product in any country of the world, without the prior written consent of Karyopharm, which shall not be unreasonably withheld, delayed or conditioned; and Karyopharm agrees not to settle such Infringement Action, or make any admissions or assert any position in such Infringement Action, in a manner that would adversely affect the allegedly infringing Licensed Compound and/or Licensed Product, or the Development, Manufacture, having Manufactured, use or Commercialization of such Licensed Compound and/or Licensed Product, within the Ono Territory, without the prior written consent of Ono, which shall not be unreasonably withheld, delayed or conditioned.
12.6.2 Upon request by Ono, which shall be made within [**] following the Effective Date, Karyopharm shall use its Commercially Reasonable Efforts to clarify that Development, Manufacture, having Manufactured, use or Commercialization of the Licensed Compounds and the Licensed Products in the Field in the Ono Territory and in the Karyopharm Territory will not constitute infringement of the Patent Rights designated by Ono. Said Commercially Reasonable Efforts shall include, but are not limited to, Karyopharm’s filing of opposition to the grant of patent so that the Valid Claim of the matured Patent Rights will not include Development, Manufacture, having Manufactured, use or Commercialization of the Licensed Compounds and the Licensed Products in the Field in the Ono Territory and in the Karyopharm Territory. For clarity, such action is not required until the Valid Claim in question has granted. In the case that (i) the Patent Rights (x) in any country in the Ono Territory or (y) in any
- 60 -
Confidential
country in the Karyopharm Territory where Karyopharm Manufactures or has Manufactured the Licensed Compounds or the Licensed Products for the Ono Territory, become matured patent and (ii) Development, Manufacture, having Manufactured, use or Commercialization of the Licensed Compounds and the Licensed Products in the Field in the Ono Territory and in the Karyopharm Territory constitutes infringement of such Patent Rights, Karyopharm shall procure the patent license under the Patent Rights in the country in question at its sole cost and responsibility. If Karyopharm successfully procures patent license under the Patent Rights in the country in question, the Patent Rights shall be automatically deemed Karyopharm Technology and the license thereunder shall be granted to Ono in accordance with Section 7.1.1 (a). If Ono is required to make payment due to Karyopharm’s failure to procure the license under the Patent Rights, Section 11.2 shall be applicable to said Ono’s payment instead of Section 12.6.1.
12.7 Registration of License.
12.7.1 By Ono. Ono may register, in its discretion and at its expense, the licenses granted to Ono under this Agreement with the Japanese Patent Office (“Senyo-Jissiken Settei Toroku”) or any other appropriate Governmental Authority in any country of the Ono Territory; provided that the Parties agree that such registration is not intended to affect the allocation of prosecution and enforcement rights and obligations set forth in Article 12. If Ono determines to make any such registrations: (a) Karyopharm agrees, at Ono’s expense, to promptly take such actions and execute such documents as are reasonably requested by Ono in order to effect such registration(s) in the applicable country; and (b) in the event the licenses granted to Ono under this Agreement expire or are terminated or become non-exclusive, Ono shall promptly take such actions and execute such documents as are reasonably necessary and requested by Karyopharm to cancel such registration(s) in the applicable country with respect to the expired, terminated or revised license grant(s).
12.7.2 By Karyopharm. Karyopharm may register, in its discretion and at its expense, the licenses granted to Karyopharm under this Agreement with any appropriate Governmental Authority in any country of the Karyopharm Territory; provided that the Parties agree that such registration is not intended to affect the allocation of prosecution and enforcement rights and obligations set forth in Article 12. If Karyopharm determines to make any such registrations: (a) Ono agrees, at Karyopharm’s expense, to promptly take such actions and execute such documents as are reasonably requested by Karyopharm in order to effect such registration(s) in the applicable country; and (b) in the event the licenses granted to Karyopharm under this Agreement expire or are terminated, Karyopharm shall promptly take such actions and execute such documents as are reasonably necessary and requested by Ono to cancel such registration(s) in the applicable country with respect to the expired or terminated license grant(s).
- 61 -
Confidential
12.8 Patent Term Extensions.
12.8.1 Karyopharm shall reasonably cooperate with Ono to determine a mutually agreeable strategy to seek supplemental protection certificates (“SPC”), patent term extensions and restorations for Karyopharm Patent Rights, Ono Patent Rights or Joint Patent Rights in the Field in the Ono Territory, which may include seeking SPCs, extensions and restorations for Karyopharm Patent Rights, Ono Patent Rights or Joint Patent Rights in the Ono Territory, and the Parties, subject to the provisions of any Karyopharm Third Party Agreement, shall seek SPCs, extensions and restorations for the Karyopharm Patent Rights, Ono Patent Rights or Joint Patent Rights in the Ono Territory in accordance with that strategy. Where required under national Law, Karyopharm shall make the filings for such SPCs, extensions and restorations for Karyopharm Patent Rights, Ono Patent Rights or Joint Patent Rights in the Ono Territory in accordance with this Section 12.8.1. Each Party shall execute such authorizations and other documents and take such other actions as may be reasonably requested by the other Party to obtain any such SPCs, extensions and restorations for Karyopharm Patent Rights, Ono Patent Rights or Joint Patent Rights in the Ono Territory.
12.8.2 Ono shall reasonably cooperate with Karyopharm to determine a mutually agreeable strategy to seek SPCs, patent term extensions and restorations for Ono Patent Rights or Joint Patent Rights in the Field in the Karyopharm Territory, which may include seeking SPCs, extensions and restorations for Ono Patent Rights or Joint Patent Rights in the Karyopharm Territory, and the Parties shall seek SPCs, extensions and restorations for the Ono Patent Rights or Joint Patent Rights in the Karyopharm Territory in accordance with that strategy. Where required under national Law, Ono shall make the filings for such SPCs, extensions and restorations for Ono Patent Rights or Joint Patent Rights in the Karyopharm Territory in accordance with this Section 12.8.2. Each Party shall execute such authorizations and other documents and take such other actions as may be reasonably requested by the other Party to obtain any such SPCs, extensions and restorations for Ono Patent Rights or Joint Patent Rights in the Karyopharm Territory.
12.9 Brand Name
12.9.1 Brand Name of the Product. Both Parties acknowledge and agree that Commercialization of the Licensed Product under a common brand name or trademark throughout the world would be beneficial for both Parties in order to maximize the value of the Licensed Product. In furtherance of the foregoing, each Party shall have the right (but not the obligation) to propose to the other Party a limited number of brand names under consideration for use in Commercializing the Licensed Product and shall consider in good faith any
- 62 -
Confidential
comments the other Party has on such brand names. If the Parties select one brand name for, or a Party selects the same brand name that the other Party has decided to use, in Commercializing the Licensed Product (the “Common Brand Name”), then, subject to successful registration and approval of such Common Brand Name by the applicable Governmental Authorities, each Party shall use such Common Brand Name for Commercialization of the Licensed Product in its respective Territory. Karyopharm shall search the possibility of the registration worldwide, and if confirmed the possibility shall file the application for registration of the trademark rights for the Common Brand Name using counsel of its own choice at Karyopharm’s cost for the Karyopharm Territory and Ono’s cost for the Ono Territory. After registration, Karyopharm shall assign the rights to the Common Brand Name in the Ono Territory to Ono without requiring Ono any compensation for such assignment. The costs of procedure related to such assignment shall be borne by [**]. Karyopharm shall be responsible for the prosecution, registration and maintenance of such trademark rights in the Karyopharm Territory at Karyopharm’s sole costs. Karyopharm shall be responsible for the prosecution and registration of such trademark rights in the Ono Territory at Ono’s sole costs, and Ono shall be responsible for the maintenance of such trademark rights in the Ono Territory at Ono’s sole costs.
12.9.2 If the Parties do not reach an agreement on a Common Brand Name, each Party may use, for Commercializing the Licensed Product in countries in each Party’s respective Territory, its own trademark it considers appropriate and which is reasonably suitable for the Licensed Product in such countries. Both Parties shall own respectively all rights, title and interests in and to its own trademarks throughout the world and shall have the sole right to register, prosecute and maintain its trademarks using counsel of its own choice and at its own expense.
| 13. | TERM AND TERMINATION |
13.1 Term. This Agreement shall be effective as of the Effective Date and, unless terminated earlier, this Agreement shall continue in effect on a Licensed Product-by-Licensed Product and country-by-country basis until expiration of the last Royalty Term to expire under this Agreement (“Term”). Upon expiration of this Agreement on a Licensed Product-by-Licensed Product and country-by-country basis, Ono’s license pursuant to Section 7.1.1 shall become a fully paid-up, irrevocable, perpetual license, sublicensable without restriction on a Licensed Product-by-Licensed Product and country-by-country basis.
13.2 Termination by Ono
13.2.1 Termination without Cause. At any time, Ono shall have the right to terminate this Agreement, on a Licensed Product-by-Licensed Product and country-by-country basis, by upon one hundred and eighty (180) days advance written notice to Karyopharm.
- 63 -
Confidential
13.2.2 Termination for Safety or Efficacy Reason. Ono shall have the right to terminate this Agreement, on a Licensed Product-by-Licensed Product basis, for safety or efficacy reasons upon thirty (30) days written notice to Karyopharm or within a shorter period if required under applicable Law.
13.3 Termination by Either Party
13.3.1 Termination for Cause. This Agreement may be terminated at any time during the Term upon written notice by either Party if the other Party is in material breach of its obligations hereunder and has not cured such breach within [**] in the case of a payment breach, or within [**] in the case of all other breaches, after notice requesting cure of the breach.
13.3.2 Termination Upon Bankruptcy. Either Party may immediately terminate this Agreement if, at any time, the other Party becomes insolvent or an order is made or a resolution passed for the administration, winding-up or dissolution of such other Party (other than for the purposes of a solvent amalgamation or reconstruction) or an administrative or other receiver, manager, liquidator, administrator, trustee or similar officer is appointed over all or any substantial part of the assets of such other Party or such other Party enters into or proposes any composition or arrangement with its creditors generally or anything analogous to the foregoing occurs in any applicable jurisdiction.
13.4 Termination by Karyopharm for Challenges of Patent Rights. If during the Term Ono or any of its Related Party (a) commences or participates in any action or proceeding (including any patent opposition, re-examination or invalidation proceeding), or otherwise asserts any claim, challenging or denying the validity or enforceability of any Karyopharm Patent Right or any claim thereof or (b) assists any Person in bringing or prosecuting any action or proceeding (including any patent opposition, re-examination or invalidation proceeding) challenging or denying the validity or enforceability of any Karyopharm Patent Right or any claim thereof (each of (a) and (b), a “Patent Challenge”), then, to the extent permitted by Law, Karyopharm shall have the right, in its sole discretion, to give at least thirty (30) days prior written notice to Ono that Karyopharm may terminate this Agreement, and, unless Ono or such Related Party withdraws or causes to be withdrawn all such challenge(s) (or in the case of ex-parte proceedings, multi-party proceedings, or other Patent Challenges that Ono or Ono’s Related Party do not have the power to unilaterally withdraw or cause to be withdrawn, Ono and Ono’s Related Party ceases actively assisting any other party to such Patent Challenge and, to the extent Ono or a Ono Related Party is a party to such Patent Challenge, it withdraws from such Patent Challenge) within thirty (30) days after Ono’s receipt of notice regarding such Patent Challenge, Karyopharm will have the right to terminate this Agreement by providing written notice thereof to Ono.
13.5 Effect of Termination.
13.5.1 Termination by Ono Under Section 13.3. Without limiting any other legal or equitable remedies that either Party may have, if this Agreement is terminated by Ono pursuant to Section 13.3, then the provisions of Section 13.5.1(a)-(d) shall apply:
- 64 -
Confidential
(a) the license grants to Ono shall terminate;
(b) If such termination occurs prior to the date of the First Commercial Sale of a Licensed Product in Japan, Karyopharm shall pay Ono a royalty of [**] percent ([**]%) of net sales (with the same meaning as “Net Sales”, mutatis mutandis) of Licensed Product by Karyopharm or its Related Parties in the Ono Territory, and the provisions of Sections 8.4.2 through 8.11 and the defined terms therein shall apply, mutatis mutandis, with the references to “Karyopharm” and “Ono” switched;
(c) If such termination occurs on or after the date of the First Commercial Sale of a Licensed Product in Japan, Karyopharm shall pay Ono a royalty of [**] percent ([**]%) of net sales (with the same meaning as “Net Sales”, mutatis mutandis) of Licensed Product by Karyopharm or its Related Parties in the Ono Territory, and the provisions of Sections 8.4.2 through 8.11 and the defined terms therein shall apply, mutatis mutandis, with the references to “Karyopharm” and “Ono” switched; and
(d) the license grants to Karyopharm in Section 7.1.2 shall become a non-transferable (except as provided in Section 14.1), sublicenseable (including through multiple tiers) (subject to Section 7.2), non-exclusive, royalty-bearing license, and shall be expanded to include the Ono Territory.
13.5.2 Termination by Karyopharm Under Section 8.10, 13.3 or 13.4; Termination by Ono Under Section 13.2 or Termination Upon Mutual Written Agreement of the Parties. Without limiting any other legal or equitable remedies that either Party may have, if this Agreement is terminated by Karyopharm pursuant to Sections 8.10, 13.3 or 13.4, or by Ono pursuant to Section 13.2, or upon the mutual written agreement of the Parties, then the provisions of Section 13.5.2(a)-(k) shall apply:
(a) Upon termination notice from terminating Party to the other Party or mutual agreement for termination, as applicable, Ono shall responsibly wind-down any on-going Development, Manufacture, having Manufactured, use or Commercialization of the Licensed Compound or the Licensed Product. Ono shall be responsible for [**];
(b) the license grants to Ono shall terminate, and the license grants to Karyopharm in Section 7.1.2 (i) specific to the Licensed Compound or Licensed Product shall survive on a perpetual and irrevocable basis and (ii) not specific to the Licensed Compound or Licensed Product shall become a non-transferable (except as provided in Section 14.1), sublicenseable (including through multiple tiers) (subject to Section 7.2), non-exclusive, royalty-bearing license, and each license for (i) and (ii) shall be expanded to include the Ono Territory;
(c) Ono shall provide to Karyopharm a fair and accurate description of the status of the Development, Manufacture, use and Commercialization of the Licensed Product in the Field in the Ono Territory as of the effective date of termination;
(d) Ono shall as promptly as practicable transfer to Karyopharm or Karyopharm’s designee, to the extent practicable and necessary for Karyopharm or its Related
- 65 -
Confidential
Parties to Develop, Manufacture, have Manufactured, use or Commercialize the Licensed Compound and Licensed Product in the Ono Territory, (i) possession and ownership of all filings and approvals (including all INDs, NDAs, Regulatory Approvals and pricing and reimbursement approvals) relating to the Development, Manufacture, use or Commercialization of the Licensed Product, (ii) copies of data, reports, records and materials, and other sales and marketing related information Controlled by Ono, including non-clinical and Clinical Data relating to the Licensed Product and all adverse event data Controlled by Ono; provided that Ono shall use Commercially Reasonable Efforts to obtain for Karyopharm the right to access such data, reports, records, materials, and other sales and marketing related information, and (iii) records and materials in Ono’s possession containing Confidential Information of Karyopharm requested to be transferred by Karyopharm;
(e) if the effective date of termination is after the First Commercial Sale of the Licensed Product in any country in the Ono Territory, then, subject to Karyopharm’s election and subject to applicable Laws, Ono shall (i) appoint Karyopharm’s designee as its exclusive distributor of the Licensed Product in the Ono Territory and grant Karyopharm the right to appoint sub-distributors, or (ii) continue to distribute the Licensed Product in the Ono Territory, until such time as all Regulatory Approvals in the Ono Territory have been transferred to Karyopharm or its designee, and in the case of (ii), the license granted to Ono under Section 7.1.1 shall survive to the extent necessary to perform Ono’s obligation under this Section 13.5.2 (e);
(f) if Ono or its Related Parties are Manufacturing the Licensed Product, then, except for the termination pursuant to Section 13.2.2, at Karyopharm’s option, Ono shall supply the Licensed Product to Karyopharm in the Ono Territory at (A) [**]; or (B) [**], in each case (A) or (B) plus [**] percent ([**]%) thereof , until the earlier of (i) such time as all Regulatory Approvals in the Ono Territory have been transferred to Karyopharm or its designee, Karyopharm has obtained all necessary manufacturing approvals and Karyopharm has procured or developed its own source of the Licensed Product supply for the Ono Territory or (ii) [**] following the effective date of such termination;
(g) if Karyopharm so requests, and to the extent permitted under Ono’s or any of its Affiliates’ obligations to Third Parties at the time of termination, Ono shall use Commercially Reasonable Efforts to transfer to Karyopharm any Third Party agreements relating solely and exclusively to the Development, Manufacture or Commercialization of the Licensed Product to which Ono or any of its Affiliates is a party, subject to any required consents of such Third Party;
(h) Ono shall promptly transfer and assign to Karyopharm all of [**] used in Commercialization of the Licensed Product (but not any Ono house marks or any trademark containing the word “Ono” owned by Ono);
(i) Ono shall, upon Karyopharm’s written request, transfer to Karyopharm any inventory of Licensed Compounds and Licensed Products owned or controlled by Ono or its Affiliates as of the termination date at the (i) [**] for such Licensed Products or (ii) [**] for such supply, as applicable;
- 66 -
Confidential
(j) Ono shall provide any other assistance reasonably requested by Karyopharm for the purpose of allowing Karyopharm or its designee to proceed expeditiously with the Development, Manufacture, use and Commercialization of Licensed Compounds and Licensed Products in or for the Ono Territory for [**] from the effective date of termination of this Agreement; and
(k) Ono shall execute all documents and take all such further actions as may be reasonably requested by Karyopharm in order to give effect to the foregoing clauses.
13.5.3 Bankruptcy. All rights and licenses granted under or pursuant to this Agreement by a Party to the other Party, including those set forth in Section 7.1, are and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, licenses of right to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. The Parties agree that the Parties and their respective permitted Sublicensees or sublicensees, as Sublicensees or sublicensees of such rights under this Agreement, shall retain and may fully exercise all of their rights and elections under the U.S. Bankruptcy Code and any foreign counterpart thereto.
13.6 Effect of Expiration or Termination; Survival. Any expiration or termination of this Agreement shall be without prejudice to the rights of either Party against the other accrued or accruing under this Agreement prior to expiration or termination, including Articles 1, Article 8 (with respect to milestone payments and royalty payments accruing prior to, but not yet paid, as of the effective date of termination), 11, and 14 and Sections 4.5, 7.3.2, 7.4, 8.6, 8.7, 9.1, 9.2 (to the extent Confidential Information is included in a proposed disclosure), 10.5, 12.6, 12.7, 13.1, 13.5, 12.4.3, 12.4.4, and 13.6 which shall survive any expiration or termination of this Agreement. Except as otherwise set forth in this Article 13, upon termination or expiration of this Agreement all rights and obligations of the Parties under this Agreement will cease.
| 14. | MISCELLANEOUS |
14.1 Assignment / Change in Control. This Agreement may not be assigned or otherwise transferred, nor may any right or obligation hereunder be assigned or transferred, by either Party without the written consent of the other Party; provided, however, that either Party may, without the other Party’s written consent, assign this Agreement and its rights and obligations hereunder in whole or in part to (a) an Affiliate; or (b) the relevant Person in the context of a Change in Control. Each Party agrees that, notwithstanding any provisions of this Agreement to the contrary, (A) no Patent Right, Know-How or other intellectual property or other proprietary rights not Controlled by a Party prior to a Change in Control with respect to such Party or by any of its Affiliates who were its Affiliates prior to such Change in Control (such Party’s “Pre-Existing Affiliates”), or which first becomes Controlled by such Party’s Pre-Existing Affiliates following such Party’s Change in Control, will be deemed Controlled by such Party or its Affiliates for purposes of this Agreement after such Change in Control, other than any Patent Right that claims priority, directly or indirectly, to any other Patent Right first Controlled by such Party or its Pre-Existing Affiliates before such Change in Control and licensed to the other Party hereunder as of such Change in Control, which will be deemed Controlled by such Party or its Pre-Existing Affiliates thereafter no matter when such Patent Right is filed or issued, and (B) to
- 67 -
Confidential
the extent that certain Person, who starts to control Karyopharm as the result of Change in Control, is developing or commercializing any [**] in the Field, any Development and Commercialization of a Licensed Product in combination with [**] by the Parties shall be conducted subject to appropriate firewall procedures to segregate such activities (and the personnel conducting such activities) from the activities performed by or on behalf of such Person with respect to any [**] it is developing or commercializing, to ensure that [**] is disclosed to employees of such Person, who are developing or commercializing such Person’s [**]. Any purported assignment in violation of this Section 14.1 shall be void. For purposes of this Section 14.1, “Change in Control” means, with respect to a Party (a) the acquisition of beneficial ownership, directly or indirectly, by any Third Party of securities or other voting interest of such Party representing a majority or more of the combined voting power of such Party’s then outstanding securities or other voting interests, (b) any merger, reorganization, consolidation or business combination involving such Party with a Third Party that results in the holders of beneficial ownership (other than by virtue of obtaining irrevocable proxies) of the voting securities or other voting interests of such Party (or, if applicable, the ultimate parent of such Party) immediately prior to such merger, reorganization, consolidation or business combination ceasing to hold beneficial ownership of more than (50%) of the combined voting power of the surviving entity immediately after such merger, reorganization, consolidation or business combination, or (c) any sale, lease, exchange, contribution or other transfer (in one transaction or a series of related transactions) of all or substantially all of the assets of such Party to which this Agreement relates to a Third Party, other than a sale or disposition of such assets to an Affiliate of such Party.
14.2 Governing Law. This Agreement shall be construed and the respective rights or obligations of the Parties determined in accordance with the substantive Law of the State of New York, other than (a) its conflicts of laws principles; (b) the United Nations Convention on Contracts for the International Sale of Goods; (c) the 1974 Convention on the Limitation Period in the International Sale of Goods (the “1974 Convention”); and (d) the Protocol amending the 1974 Convention, done at Vienna April 11, 1980; provided, that with respect to matters involving enforcement of intellectual property rights, the Law of the applicable country shall apply.
14.3 Arbitration.
14.3.1 Subject to Section 14.3.4, any disputes, claims or controversies in connection with this Agreement, including any questions regarding its formation, existence, validity, enforceability, performance, interpretation, tort, breach or termination hereof (“Disputes”), shall be resolved amicably by negotiation between the Parties. Either Party may initiate such informal Dispute resolution by sending written notice of the Dispute to the other Party, and then appropriate representatives of the Parties shall meet for attempted resolution by good faith negotiations in person or via video-conference without delay from such notice. If such representatives are unable to resolve such Disputes within [**] of such notice, either Party may refer the matter by written notice to the Executive Officers for discussion and resolution. If such Executive Officers are unable to resolve such Dispute within [**] of such written notice, Section 14.3.2 shall apply.
- 68 -
Confidential
14.3.2 All Disputes which remain unresolved under Section 14.3.1 shall be finally resolved under the Rules of Arbitration of the International Chamber of Commerce by three (3) arbitrators appointed in accordance with the said rules. Each Party shall nominate one (1) arbitrator, and the two (2) arbitrators so nominated shall nominate a third arbitrator, who shall act as the chairperson. The place of the arbitration shall be Boston, Massachusetts if the arbitration is demanded by Ono, and Osaka, Japan if the arbitration is demanded by Karyopharm. The language of the arbitration shall be English. If the tribunal orders production of documents, the tribunal shall take guidance from the IBA Rules on the Taking of Evidence in International Arbitration as current on the date of the commencement of the arbitration. The costs and expenses of translation of relevant documents and translators relating to the arbitration shall be deemed as the costs and expenses of the arbitration, and may be allocated to any Party in the award by the tribunal. The tribunal may include in its award an allocation to any Party of costs and expenses relating to the arbitration, excluding lawyers’ fee, as the tribunal deems reasonable. Each Party shall bear its own cost and expenses for its own lawyers. The award rendered by the tribunal shall be final and binding upon the Parties and may be entered in any court of appropriate jurisdiction. The Emergency Arbitrator Provisions and the Expedited Procedure Provisions shall not apply.
14.3.3 The existence and content of the arbitral proceedings, any information exchanged between Parties during the arbitral proceedings and any rulings or award shall be kept confidential by the Parties and members of the tribunal except (a) to the extent that disclosure may be required by a Party to fulfill a legal duty, protect or pursue a legal right, or enforce or challenge an award in bona fide legal proceedings before a court or other judicial authority, (b) with the consent of both Parties, (c) where needed for the preparation or presentation of a claim or defense in this arbitration, (d) where such information is already in the public domain other than as a result of a breach of this clause, or (e) by order of the tribunal upon application of a Party.
14.3.4 At any time, a Party may seek or obtain preliminary, interim or conservatory measures from the arbitrators or from a court.
14.3.5 Unless otherwise agreed by the Parties, a dispute between the Parties relating to the validity or enforceability of any Patent Right regarding this Agreement shall not be subject to arbitration and shall be submitted to a court or patent office of competent jurisdiction in the relevant country in which such patent was issued or, if not issued, in which the underlying patent application was filed. The Parties submit to the jurisdiction of such court or patent office and irrevocably waive any assertion that the case should be heard in a different venue or forum.
14.4 Entire Agreement; Amendments. This Agreement contains the entire understanding of the Parties with respect to the subject matter hereof, and supersedes all previous arrangements with respect to the subject matter hereof, whether written or oral, including the Prior CDA. This Agreement (including the Schedules hereto) may be amended, or any term hereof modified, only by a written instrument duly-executed by authorized representatives of both Parties.
- 69 -
Confidential
14.5 Severability. If any provision hereof should be held invalid, illegal or unenforceable in any respect in any jurisdiction, the Parties shall substitute, by mutual consent, valid, legal and enforceable provisions for such invalid, illegal or unenforceable provisions, which valid, legal and enforceable provisions in their economic effect are sufficiently similar to the invalid, illegal or unenforceable provisions that it can be reasonably assumed that the Parties would have entered into this Agreement with such valid, legal and enforceable provisions. In case such valid, legal and enforceable provisions cannot be agreed upon, the invalid, illegal or unenforceable of one or several provisions of this Agreement shall not affect the validity of this Agreement as a whole.
14.6 Headings. The captions to the Articles and Sections hereof are not a part of this Agreement and shall not affect in any way the meaning or interpretation of this Agreement, but are merely for convenience to assist in locating and reading the several Articles and Sections hereof.
14.7 Waiver of Rule of Construction. Each Party has had the opportunity to consult with counsel in connection with the review, drafting and negotiation of this Agreement. Accordingly, the rule of construction that any ambiguity in this Agreement shall be construed against the drafting Party shall not apply.
14.8 Interpretation. Except where the context expressly requires otherwise, (a) the use of any gender herein shall be deemed to encompass references to any other gender, and the use of the singular shall be deemed to include the plural (and vice versa); (b) the words “include”, “includes” and “including” shall be deemed to be followed by the phrase “without limitation” and shall not be interpreted to limit the provision to which it relates; (c) the word “will” shall be construed to have the same meaning and effect as the word “shall”; (d) any definition of or reference to any agreement, instrument or other document herein shall be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein); (e) any reference herein to any Person shall be construed to include the Person’s successors and assigns (subject to Section 14.1 with respect to a Party); (f) the words “herein”, “hereof” and “hereunder”, and words of similar import, shall be construed to refer to this Agreement in its entirety and not to any particular provision hereof; (g) all references herein to Articles, Sections, Exhibits or Schedules shall be construed to refer to Articles, Sections, Exhibits or Schedules of this Agreement, and references to this Agreement include all Exhibits and Schedules hereto; (h) the word “notice” means notice in writing (whether or not specifically stated) and shall include notices, consents, approvals and other written communications contemplated under this Agreement; (i) provisions that require that a Party, the Parties or any committee hereunder “agree,” “consent” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, approved minutes or otherwise (but excluding e-mail and instant messaging); (j) references to any specific Law, or article, section or other division thereof, shall be deemed to include the then-current amendments thereto or any replacement or successor Law; (k) the term “or” shall be interpreted in the inclusive sense commonly associated with the term “and/or”; (l) references to “$” or “dollars” shall mean U.S. Dollars; and (m) references to “day” shall mean a calendar day unless “Business Day” is specified.
- 70 -
Confidential
14.9 No Implied Waivers; Rights Cumulative. Neither Party shall be deemed to have waived any of its right, power, remedy or privilege under this Agreement, or provided by statute or at law or in equity or otherwise unless the waiver is made in writing, signed by a duly authorized representative of that Party. No failure on the part of Karyopharm or Ono to exercise, and no delay in exercising, any right, power, remedy or privilege under this Agreement, or provided by statute or at law or in equity or otherwise, shall impair, prejudice or constitute a waiver of any such right, power, remedy or privilege or be construed as a waiver of any breach of this Agreement or as an acquiescence therein, nor shall any single or partial exercise of any such right, power, remedy or privilege preclude any other or further exercise thereof or the exercise of any other right, power, remedy or privilege.
14.10 Notices. All notices which are required or permitted hereunder shall be in writing and sufficient if delivered personally, sent by facsimile (and promptly confirmed by personal delivery, registered or certified mail or overnight courier), sent by internationally-recognized overnight courier or sent by registered or certified mail, postage prepaid, return receipt requested, addressed as follows:
| If to Karyopharm, to: |
Karyopharm Therapeutics Inc. Attention: Chief Executive Officer 85 Wells Avenue, Suite 210 Newton, MA 02459 Facsimile No.: [**] | |
| With a copy to: |
Karyopharm Therapeutics Inc. Attention: General Counsel 85 Wells Avenue, Suite 210 Newton, MA 02459 Facsimile No.: [**] | |
| With a copy to: |
WilmerHale LLP 60 State Street Boston, MA 02109 USA Attention: Steven D. Singer, Esq. Facsimile No.: 1-(617) 526-5000 | |
| If to Ono, to: |
Ono Pharmaceutical Co., Ltd. 8-2, Kyutaromachi 1-chome, Chuo-ku, Osaka, 541-8564, Japan Attention: [**] Facsimile No.: [**] | |
| With a copy to: |
Ono Pharmaceutical Co., Ltd. Attention: [**] Facsimile No.: [**] | |
- 71 -
Confidential
or to such other address as the Party to whom notice is to be given may have furnished to the other Party in writing in accordance herewith. Any such notice shall be deemed to have been given: (a) when delivered if personally delivered or sent by facsimile on a business day (or if delivered or sent on a non-business day, then on the next business day); (b) on receipt if sent by overnight courier; or (c) on receipt if sent by mail.
Notwithstanding any provision of this Section 14.10, it is understood and agreed between the Parties that this Section 14.10 is not intended to govern the day-to-day communications necessary between the Parties in performing their duties, in due course, under the terms and conditions hereof.
14.11 Compliance with Law. Each Party and its Affiliates shall conduct, and shall use Commercially Reasonable Efforts to cause its Related Parties, contractors and consultants to conduct, all of its activities contemplated under this Agreement in accordance with all applicable Laws of the country in which such activities are conducted, as well as the US Foreign Corrupt Practices Act and the UK Bribery Act 2010, and all export control and sanctions Law of the United States. In addition, each Party shall not, shall ensure that its Affiliates do not, and shall use Commercially Reasonable Efforts to cause its Related Parties, contractors and consultants not to, take any action that would cause the other Party to violate any applicable anti-corruption or sanctions Laws.
14.12 Force Majeure. Neither Party shall be held liable to the other Party nor be deemed to have defaulted under or breached this Agreement for failure or delay in performing any obligation under this Agreement to the extent that such failure or delay is caused by or results from causes beyond the reasonable control of the affected Party, potentially including embargoes, war, acts of war (whether war be declared or not), insurrections, riots, civil commotions, strikes, lockouts or other labor disturbances, fire, floods, earthquake, tsunami or other acts of God. The affected Party shall notify the other Party of such force majeure circumstances as soon as reasonably practical, and shall promptly undertake all reasonable efforts necessary to cure such force majeure circumstances.
14.13 Independent Contractors. It is expressly agreed that Karyopharm and Ono shall be independent contractors and that the relationship between Karyopharm and Ono shall not constitute a partnership, joint venture or agency. Karyopharm shall not have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on Ono, without the prior written consent of Ono, and Ono shall not have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on Karyopharm without the prior written consent of Karyopharm.
14.14 Counterparts. This Agreement may be executed in two (2) or more counterparts, including by facsimile or PDF signature pages, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
- 72 -
Confidential
14.15 Binding Effect; No Third Party Beneficiaries. As of the Effective Date, this Agreement shall be binding upon and inure to the benefit of the Parties and their respective permitted successors and permitted assigns. Except as expressly set forth in this Agreement, no Person other than the Parties and their respective Affiliates and permitted successors and assignees hereunder shall be deemed an intended beneficiary hereunder or have any right to enforce any obligation of this Agreement.
[REMAINDER OF PAGE LEFT INTENTIONALLY BLANK]
- 73 -
Confidential
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the Effective Date.
| ONO PHARMACEUTICAL CO., LTD. | KARYOPHARM THERAPEUTICS INC. | |||||||
| BY: | /s/ Gyo Sagara | BY: | /s/ Michael G. Kauffman | |||||
| NAME: | Gyo Sagara | NAME: | Michael G. Kauffman | |||||
| TITLE: | President, Representative Director and CEO | TITLE: | Chief Executive Officer | |||||
Signature page to License Agreement
Confidential
SCHEDULE 1.43
KARYOPHARM THIRD PARTY AGREEMENTS
[**]
Research Agreement, made as of the 18th day of July, 2011, by and between The Multiple Myeloma Research Foundation, Inc. and Karyopharm1
| [**]. |
Confidential
SCHEDULE 1.46
KPT-8602
Confidential
| 1. | PHYSICAL, CHEMICAL, AND PHARMACEUTICAL PROPERTIES AND FORMULATIONS |
| 1.1. | Physical Properties |
White to off-white solid, Melting point: 225 °C
| 1.2. | Chemical Properties |
| 1.2.1. | Chemical Name |
(E)-3-(3-(3,5-bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)-2-(pyrimidin-5-yl)acrylamide
| 1.2.2. | Structural Formula |
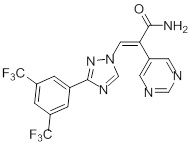
| 1.2.3. | Molecular Formula |
C17H10F6N6O
| 1.2.4. | Molecular Weight |
428.30 g/mol
| 1.3. | Solubility |
< [**] mg/mL in water; ³ [**] mg/mL in dimethylsulfoxide (DMSO); < [**] mg/mL in methanol
| 1.4. | Formulations |
The clinical dosage form for KPT-8602 is an [**] for oral administration. Initial supplies came in two strengths of active ingredient: 5 mg and 20 mg of the active pharmaceutical ingredient per tablet. Additional supplies for the clinic included the addition of a 10 mg tablet based on the 5 mg formulation and are size proportional (2x) to the 5 mg tablet. All inactive ingredients used in the tablets are either compendia or generally recognized as safe. Tablets are film coated for ease in handling.
| 1.5. | Storage Conditions |
KPT-8602 tablets are packaged in [**] with an [**] and can be stored at room temperature between 5 and 25oC. Instructions for the receipt, inspection, storage, preparation, administration, and disposal of KPT-8602 tablets are provided in the Pharmacy Manual at each clinical site.
Confidential
SCHEDULE 1.74
SELINEXOR
Confidential
| 2. | PHYSICAL, CHEMICAL, AND PHARMACEUTICAL PROPERTIES AND FORMULATIONS |
| 2.1. | Drug Substance Description |
| Product Name: | Selinexor (International Nonproprietary Name [INN]) | |
| Selinexor (United States Adopted Name [USAN]) | ||
| Company Code: | KPT-330 | |
| Chemical Name: | (Z)-3-(3-(3,5-bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)-N'-(pyrazin-2-yl)acrylohydrazide | |
| Molecular Formula: | C17H11F6N7O | |
| Molecular Weight: | 443.31 g/mol | |
| Pharmacologic Class: | Apoptosis inducing agent (Wu, 2006) | |
Figure 1: Chemical Structure of Selinexor
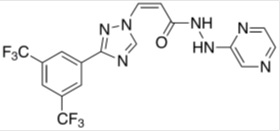
| 2.2. | Drug Product Formulation |
The selinexor dosage forms used in clinical studies are capsules and tablets for oral administration. Capsule strengths for selinexor were 1 mg, 5 mg and 20 mg; tablet strengths are 10 mg and 25 mg (supplied in bottles), and 20 mg tablets (supplied in blister packs).
Capsules of selinexor for oral administration are filled with a micronized mixture containing about [**]% by weight of the active pharmaceutical ingredient (API) (selinexor), and an approximately [**] mixture of [**] (the manufacturing procedure involves [**] of the active pharmaceutical ingredient and [**]). The capsules were used in early phase 1 studies but are no longer manufactured or used.
Selinexor 10 and 25 mg tablets (tablet formulation #1) were manufactured via [**] and contain approximately [**]% by weight of the API as well as, [**]. Additional inactive excipients include [**]. The tablets are coated with [**]. These tablet components/excipients are common to oral pharmaceuticals and/or compendial. Tablet formulation #1 (1st generation tablets) has been evaluated in a Phase 1b comparative bioavailability study KCP-330-003 and demonstrated similar exposure to the capsules. Tablet strengths are distinguished by tablet size and debossing (K10, K20 and K25 for 10 mg, 20 mg, and 25 mg tablets respectively).
Confidential
Selinexor 20 mg tablets (tablet formulation #2) were manufactured via [**] and contain [**]% by weight of the API as well as [**] (lubricant). Additional inactive excipients include [**] are blended with the [**], and [**]. The [**] to provide product formulation. Tablet formulation #2 (2nd generation tablets) has been evaluated in a Phase 1b comparative bioavailability study KCP-330-003 and demonstrated similar exposure to tablet formulation #1.
Twenty (20) milligram tablets (tablet formulation #2) are the preferred dosage form for current and future clinical trials. The use of the 20 mg tablets in CSTs began in December, 2014.
| 2.3. | Storage and Handling |
All selinexor formulations can be stored or shipped either at ambient temperature or refrigerated. Selinexor tablets (20 mg) are to be stored at room temperature (at or below 30°C) in clear blister strips that are composed of either [**] with a [**] or [**] with a [**]. The blistered packaged product will be placed in paperboard secondary packaging, to which labeling materials will be fixed.
Confidential
SCHEDULE 2.1
Relevant Clinical Studies
Selinexor
Multiple Myeloma
1. BOSTON (Phase III trial, ongoing): Randomized, controlled, open-label study of selinexor, bortezomib, and dexamethasone versus bortezomib and dexamethasone in patients with relapsed or refractory Multiple Myeloma (rrmm).
2. STORM (Phase IIb trial, ongoing): Open-label, single-arm study of selinexor plus low dose dexamethasone in patients with multiple myeloma previously treated with lenalidomide, pomalidomide, bortezomib, carfilzomib, and daratumumab, and refractory to prior treatment with glucocorticoids, an immunomodulatory agent, a proteasome inhibitor, and the anti-CD38 mAb daratumumab.
3. STOMP (Phase I/II trial, ongoing): Assessment of the efficacy and safety of four separate combination therapies for the treatment of patients with rrmm: selinexor + pomalidomide + dexamethasone, selinexor + bortezomib + dexamethasone, selinexor + lenalidomide + dexamethasone, and selinexor + daratumumab + dexamethasone.
Non-Hodgkin’s Lymphoma
4. SADAL (Phase IIb trial, ongoing): Randomized study of selinexor (60 mg versus 100 mg) in relapsed or refractory Diffuse Large B-Cell Lymphoma (DLBCL). (in consultation with FDA, protocol amended to remove the 100 mg arm and continue enrollment in only the 60 mg twice weekly arm).
Solid Tumors
5. SEAL (Phase II/III trial, ongoing): Randomized study of selinexor vs. placebo in patients with advanced, unresectable, dedifferentiated Liposarcoma.
KPT-8602
6. Phase I/II trial (ongoing): Dose escalation clinical trial for KPT-8602 in patients with to Pancreatic Cancer, Colorectal Cancer and Myelodysplastic Syndrome.
Confidential
SCHEDULE 2.2
Overview Plan
[**]
Confidential
SCHEDULE 10.2.22
KARYOPHARM PATENT RIGHTS
|
Docket |
Country |
Application |
Application |
Filing |
Publica- |
Public- |
Patent |
|||||||||||||||||||||||
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 3 pages were omitted. [**]
| 2 | Current as of Sept 8, 2017 |
Confidential
SCHEDULE 11.1
Agreed Indemnification Language to be incorporated
in the Clinical Supply Agreement (and the Commercial Supply Agreement, if any)
8.1 Supplier Indemnification. Subject to the provisions of Section 8.3 (of the Clinical Supply Agreement), Supplier (i.e. Karyopharm) shall defend, indemnify, and hold Purchaser, its Affiliates and Related Party and its and their successors, assigns, and their respective directors, officers, agents, and employees (collectively, the “Purchaser Indemnitees”) harmless with respect to all liability, damage, judgments, losses and expenses (including reasonable attorneys’ fees and court costs) whether for money or equitable relief, (collectively, “Losses”) arising out of, relating to, or resulting from suits, proceedings, claims, actions, demands, or threatened claims, actions or demands, in each case brought by a Third Party (each, a “Claim”) to the extent Claim arises out of or results from (a) bodily injury, risk of bodily injury, death, property damage or product liability arising from Supplier’s failure to supply Conforming Licensed Particle (i.e. the Licensed Product or any component or intermediate thereof or a Related Substance, which satisfies the conditions set forth in representation and warranty in Section 8.1 of the Clinical Supply Agreement) at the time of Delivery to Purchaser, (b) the breach or default by Supplier of any representation, warranty, covenant, or agreement of Supplier contained in the Agreement (i.e. the Clinical Supply Agreement) or (c) the negligence, fraud, fraudulent misrepresentation or willful misconduct of Supplier in the course of the performance of its obligations hereunder, except, in each case, to the extent any such Losses result from the negligence or willful misconduct of a Purchaser Indemnitee or from the breach or default by Purchaser of any representation, warranty, or covenant of Purchaser contained in this Agreement (i.e. the Clinical Supply Agreement). It is confirmed that Supplier will give Purchaser its representation and warranty as follows: at the time of Delivery, each Licensed Particle (i.e. the Licensed Product or any component or intermediate thereof or a Related Substance) supplied under this Agreement (i.e. the Clinical Supply Agreement) (a) will have been manufactured in accordance with the Specifications, the Law as applicable to Supplier with respect to such Licensed Particle, the Agreement (i.e. the Clinical Supply Agreement), any applicable Statement of Work and the Quality Agreement; (b) will have been manufactured in accordance with cGMP if the Statement of Work pursuant to which such Licensed Particle is supplied expressly specifies that such Licensed Particle is to be manufactured in accordance with cGMP; (c) will have a shelf life equal to or exceeding the Minimum Shelf Life (it being confirmed that, in case that Licensed Particle becomes out of the Specifications in course of the post release stability study therefor, such Licensed Particle shall be deemed to fail to have a Minimum Shelf Life); (d) will comply with all applicable Laws, and will not be adulterated or misbranded, (e) will not be an article that may not be introduced into interstate commerce under the provisions of §§404 or 505 of the United States Food, Drug, and Cosmetic Act (“FD&C Act”) (“Conforming Licensed Particle”) and (f) upon receipt by Supplier of full payment for a Delivery of a Licensed Particle, such Licensed Particle shall be free of all Third Party liens and security interests.
8.2 Purchaser Indemnification. Subject to the provisions of Section 8.3 (of the Clinical Supply Agreement), Purchaser shall defend, indemnify and hold Supplier, its Affiliates and Related Party, and its and their successors, assigns, and their respective directors, officers, agents,
Confidential
and employees (collectively, the “Supplier Indemnitees”), harmless with respect to all Losses arising out of, relating to, or resulting from Claims against a Supplier Indemnitee to the extent Claims arises out of or resulting from (a) the breach or default by Purchaser of any representation, warranty, covenant or agreement of Purchaser contained in this Agreement (i.e. the Clinical Supply Agreement); (b) the negligence or willful misconduct of Purchaser or its Representatives in the course of the performance of Purchaser’s obligations hereunder; (c) the negligence of Purchaser or its Representatives in connection with the storage, handling, or use of any Licensed Particle after delivery pursuant to Section 2.5 (of the Clinical Supply Agreement); or (d) manufacture, clinical development, use, sale, import, distribution, or other exploitation of any Licensed Particle, including Claims for bodily injury, risk of bodily injury, death or property damage and product liability resulting from, arising out of or relating to any clinical trials, or other products liability claims, except, in each case, to the extent any such Losses result from the negligence or willful misconduct of a Supplier Indemnitee or from the breach or default by Supplier of any representation, warranty, or covenant of Supplier contained in this Agreement (i.e. the Clinical Supply Agreement).
8.3 Limitations on Indemnification. The obligations to indemnify, defend, and hold harmless set forth in this ARTICLE VIII (of the Clinical Supply Agreement) shall be contingent upon the Party seeking indemnification (the “Indemnitee”): (a) notifying the indemnifying Party of a claim, demand or suit in timely manner; provided, however, that Indemnitee’s failure or delay in providing such notice shall not relieve the indemnifying Party of its indemnification obligation except to the extent the indemnifying Party is prejudiced thereby; (b) allowing the indemnifying Party or its insurers the right to assume direction and control of the defense of any claim, demand or suit; (c) using its best efforts to cooperate with the indemnifying Party or its insurers, at the indemnifying Party’s expense, in the defense of such claim, demand or suit; and (d) not settling or compromising any claim, demand or suit without prior written authorization of the indemnifying Party (not to be unreasonably withheld). The indemnifying Party will act reasonably and in good faith with respect to all matters relating to such claim, demand or suit and will not settle or otherwise resolve such claim, demand or suit without the Indemnitee’s prior written consent, which will not be unreasonably withheld, conditioned or delayed; provided that such consent will not be required with respect to any settlement involving only the payment of monetary awards for which the indemnifying Party will be fully-responsible. The Indemnitee shall have the right, at the Indemnitee’s expense, to employ one separate counsel and to participate in the defense of such claim, demand or suit; provided that the indemnifying Party shall bear the reasonable fees, costs and expenses of one such separate counsel and participation if the Indemnitee shall have reasonably determined, after consultation with counsel, that an actual or potential conflict of interest makes representation by the same counsel or the counsel selected by the indemnifying Party inappropriate.
8.4 LIMITATION OF LIABILITY; RIGHT TO OFFSET.
(a) Consequential Damages Limitation. In no event will any Party be liable to the other Party for any indirect, special, incidental, exemplary or consequential damages of any kind arising out of or in connection with this Agreement (i.e. the Clinical Supply Agreement), however caused and on any theory of liability (whether in contract, tort (including negligence), strict liability or otherwise), even if such Party was advised or otherwise aware of the likelihood of such damages. The foregoing limitations will not apply with respect to (a) the Party’s indemnification obligations under this Agreement (i.e. the Clinical Supply Agreement), (b) breach by a Party of ARTICLE X (Confidential Information) (of the Clinical Supply Agreement) or (c) intentional misconduct of a Party.
Confidential
(b) Offset. Following the determination of Losses subject to indemnification under Section 8.1 (of the Clinical Supply Agreement) by Supplier or Section 8.2 (of the Clinical Supply Agreement) by Purchaser by a court of competent jurisdiction, the Indemnitee shall have a right to offset such Losses against any current or future payment due to the indemnifying Party hereunder or under the License Agreement. In addition, either Party shall have a right to offset any damages owed to it of any kind arising out of or in connection with this Agreement (i.e. the Clinical Supply Agreement), however caused and on any theory of liability (whether in contract, tort (including negligence), strict liability or otherwise, against any current or future payment to the other Party hereunder or under the License Agreement.
Confidential
