Attached files
| file | filename |
|---|---|
| EX-10.1 - EXHIBIT 10.1 - OptiNose, Inc. | formindemnificationagreeme.htm |
| EX-32.2 - EXHIBIT 32.2 - OptiNose, Inc. | optn12-31x201710xkex322.htm |
| EX-32.1 - EXHIBIT 32.1 - OptiNose, Inc. | optn12-31x201710xkex321.htm |
| EX-31.2 - EXHIBIT 31.2 - OptiNose, Inc. | optn12-31x201710xkex312.htm |
| EX-31.1 - EXHIBIT 31.1 - OptiNose, Inc. | optn12-31x201710xkex311.htm |
| EX-23.1 - EXHIBIT 23.1 - OptiNose, Inc. | auditorconsent10-kex231.htm |
| EX-21.1 - EXHIBIT 21.1 - OptiNose, Inc. | listofsubsidiariesex211.htm |
| EX-10.19 - EXHIBIT 10.19 - OptiNose, Inc. | optinose_athyriumxnotepurc.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý | Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 | |
For the fiscal year ended December 31, 2017
OR
o | Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 | |
For the transition period from ___________ to ______________.
Commission file number: 001-38241

OPTINOSE, INC.
(Exact name of registrant as specified in its charter)
Delaware | 42-1771610 | |
(State of other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) | |
1020 Stony Hill Road, Suite 300
Yardley, Pennsylvania 19067
(Address of principal executive offices, including zip code)
Yardley, Pennsylvania 19067
(Address of principal executive offices, including zip code)
(267) 364-3500
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class | Name of each exchange on which registered |
Common Stock, $0.001 par value | The Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer o | Accelerated filer o |
Non-accelerated filer ý (Do not check if a smaller reporting company) | Smaller reporting company o |
Emerging growth company ý | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ý
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).Yes o No ý
As of June 30, 2017 (the last business day of the registrant's most recently completed second fiscal quarter), the registrant's common stock was not listed on any exchange or over-the-counter market. The registrant's common stock began trading on the Nasdaq Global Select Market on October 13, 2017. As of March 12, 2017, the aggregate market value of the registrant's common stock held by non-affiliates was approximately $70.7 million based on the number of shares held by non-affiliates as of March 12, 2017 and the last reported sale price of the registrant's common stock on March 12, 2017.
The number of shares of common stock outstanding at March 1, 2018 was 37,865,740 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its 2018 annual meeting of stockholders are incorporated by reference into Part III of this Form 10-K where indicated. Such definitive proxy statement will be filed with the U.S. Securities and Exchange Commission within 120 days after the year ended December 31, 2017.
_________________________
Unless the context otherwise requires, all references in this Form 10-K to "Optinose," "Company," "we," "us," and "our" refer to OptiNose, Inc. and its subsidiaries.
_________________________
Trademark Notice
OPTINOSE® and XHANCE™ are trademarks or registered trademarks of ours in the United States. This 10-K contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights to these trademarks and trade names. All other trademarks, trade names and service marks appearing in this 10-K are the property of their respective owners. We do not intend our use or display of other companies' trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Form 10-K contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These statements include, among others, statements relating to:
▪ | the potential advantages of XHANCETM and our product candidates; |
▪ | the planned launch of XHANCE in retail pharmacies in the United States in early April 2018; |
▪ | our expectation to achieve 65% coverage of commercial lives during launch; |
▪ | our ability to secure broad market access in the commercial segment for XHANCE by targeting Tier 3 payor coverage, single step edit with no prior authorization; |
▪ | planned product development activities, studies and clinical trials, including our plans to initiate additional clinical trials of XHANCE in the fourth quarter of 2018 in pursuit of a follow-on indication for chronic sinusitis; |
▪ | our expectation that our existing cash and cash equivalents will be sufficient to fund our operations and debt service obligations through the end of 2019; |
▪ | our plans to commercial plans and objectives for XHANCE and our product candidates; |
▪ | the size and growth potential of the markets for XHANCE and our product candidates, and our ability to service those markets; |
▪ | our ability to develop sales and marketing capabilities, whether alone or with potential future collaborators; |
▪ | the rate and degree of market acceptance of XHANCE and our product candidates; |
▪ | our ability to maintain regulatory approval of XHANCE and our product candidates; |
▪ | our ability to attract collaborators with development, regulatory and commercialization expertise; |
▪ | regulatory developments in the United States and foreign countries; |
▪ | our ability to operate our business without infringing the intellectual property rights of others; |
▪ | the scope and duration of patent protection and other barriers to entry that we expect to benefit XHANCE and our product candidates; |
▪ | the performance of our third-party suppliers, manufacturers and contract sales organizations; |
▪ | the success of competing products that are or become available; |
▪ | our expectations regarding our ability to obtain and adequately maintain sufficient intellectual property protection for XHANCE and our other product candidates and to avoid claims of infringement; |
▪ | our expectations regarding the period during which we qualify as an emerging growth company under the JOBS Act; and |
▪ | the accuracy of our estimates regarding expenses, future revenue, capital requirements and need for additional financing; |
as well as other statements relating to our future operations, financial performance and financial condition, prospects, strategies, objectives or other future events. Forward-looking statements appear primarily in the sections of this Form 10-K entitled "Item 1 - Business," "Item 1A - Risk Factors," "Item 7 - Management's Discussion and Analysis of Financial Condition and Results of Operations," and "Item 7 - Quantitative and Qualitative Disclosures About Market Risk." In some cases, you can identify forward-looking statements by words such as "may,” "will,” "could," "would," "should," "expect," "intend," "plan," "anticipate," "target," "believe," "estimate," "predict," "project," "potential," "continue," "ongoing," "scheduled" and similar expressions, although not all forward-looking statements contain these identifying words.
1
Forward-looking statements are based upon our current expectations and assumptions and are subject to a number of known and unknown risks, uncertainties and other factors that could cause actual results to differ materially and adversely from those expressed or implied by such statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed under section "Item 1A - Risk Factors" of this Form 10-K. As a result, you should not place undue reliance on forward-looking statements.
Additionally, the forward-looking statements contained in this Form 10-K represent our views only as of the date of this Form 10-K (or any earlier date indicated in such statement). While we may update certain forward-looking statements from time to time, we specifically disclaim any obligation to do so, even if new information becomes available in the future. However, you are advised to consult any further disclosures we make on related subjects in the reports that we file with the SEC.
The foregoing cautionary statements are intended to qualify all forward-looking statements wherever they may appear in this Form 10-K. For all forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
This Form 10-K also contains estimates, projections and other information concerning our industry, our business, and the markets for certain diseases, including data regarding the estimated size of those markets, and the incidence and prevalence of certain medical conditions. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources.
2
PART I
ITEM 1. BUSINESS
Overview
Our Company
We are a specialty pharmaceutical company focused on the development and commercialization of products for patients treated by ear, nose and throat, or ENT, and allergy specialists. Our lead product, XHANCETM (fluticasone propionate) nasal spray, 93 mcg, is a therapeutic utilizing our proprietary Optinose Exhalation Delivery System, or EDS, that delivers a topically-acting and potent anti-inflammatory corticosteroid for the treatment of chronic rhinosinusitis with nasal polyps and, if approved, chronic rhinosinusitis without nasal polyps. Chronic rhinosinusitis is a serious nasal inflammatory disease that is currently treated using therapies, such as intranasal steroids, or INS, that have significant limitations. We believe XHANCE has a differentiated clinical profile with the potential to become part of the standard of care for this disease because it is able to deliver medication to the primary site of inflammation high and deep in the nasal passages in regions not adequately reached by current INS. We also believe that payors will respond favorably to XHANCE's clinical, cost, and quality-of-care profile, as compared to current and potential future costly drug therapy and surgical treatment options.
On September 18, 2017, the U.S. Food and Drug Administration, or FDA, approved our new drug application, or NDA, for XHANCE for the treatment of nasal polyps in patients 18 years of age or older. Based upon our research of over 300 launches between 2010 and 2016, we believe the evidence suggests that the success of a launch is highly dependent upon four critical factors: level of unmet need that exists within the market, level of clinical differentiation of a brand, market access and brand awareness. Therefore, rather than rushing our product to the market immediately following approval, our Company employed a different, purposeful launch model that would enable our commercial team to build market access for XHANCE and achieve critical levels of customer awareness to facilitate adoption upon making XHANCE available in the market.
Since the FDA approved of our NDA for XHANCE, we have been focused on executing our integrated launch plan with the objective of making XHANCE widely available through retail pharmacies in the second quarter of 2018; we now believe XHANCE will be available in retail pharmacies in early April 2018. The key strategies in our integrated launch plan include: (i) build a robust supply chain network and quality management system, (ii) drive awareness and appreciation of the clinical differentiation of XHANCE, (iii) design and deploy our customer facing model, (iv) engage commercial payors with the objective of securing tier 3 coverage, and (v) develop our internal capabilities (Finance, HR, IT, Data Analytics and Compliance) to support a commercial stage company. We have made progress in each of these key strategic areas:
• | Commercial Supply Chain. We have entered into commercial supply agreements with our key suppliers, spent significant time with our suppliers to oversee product production and quality management, and manufactured our initial commercial supply of XHANCE. We have contracted with a third-party logistics partner and are in the process of finalizing agreements with our distribution partners. |
• | Brand Awareness. We have executed a broad, multi-channel awareness campaign leveraging digital, non- personal promotion and journal advertising and have already reached over 10,000 ENT physicians and allergists with disease state and branded messages. In November 2017, we launched a nurse educator team of approximately 85 nurse professionals who have since called on approximately 5,000 ENT physicians and allergists and delivered over 10,000 presentations. The focus of their interactions with healthcare professionals include: (i) introducing Optinose and highlighting the unmet medical need and limitations of current treatments, (ii) increasing awareness about XHANCE along with differentiating the Optinose EDS, and (iii) familiarizing healthcare professionals with the proper administration of XHANCE. Initial reaction to core brand messages among target physicians has been positive. |
• | Customer Model. We have defined a sales force footprint of approximately 120 territories targeting approximately 14,000 ENT physicians, allergists and “specialty like” primary care physicians and are deploying a hybrid sales model that combines an internal sales leadership team with a fully dedicated contract sales force to call on our target customer universe. We have prioritized approximately 80 territories within our sales force footprint to deploy at launch based upon an expectation that we will achieve an estimated 65% commercial market access within each of those territories during launch. Most of the initial |
3
80 territory managers have completed training and are already engaging approximately 8,000 ENTs, allergists and primary care physician targets to promote XHANCE for the treatment of nasal polyps. Additionally, in March 2018, we introduced the XHANCE Xperience program to offer select physicians and their patients an opportunity to gain initial experience with XHANCE. Physicians can enroll a limited number of eligible patients in this program. Patients will receive up to two XHANCE prescriptions at no cost to them ($0 co-pay), and physicians will receive an opportunity for feedback on patient experience. We believe the positive experience that physicians and patients have with XHANCE in this program will help drive demand for XHANCE during the retail launch, starting in early April 2018.
• | Market Access. We have been engaging payors with the goal of securing tier 3 commercial coverage, primarily with a single step edit and no prior authorization. We have engaged with approximately 40 plans representing approximately 85% of commercial lives. In meeting with potential payors, we have shared what we believe is our compelling economic value proposition. Our analyses show that XHANCE will have a comparatively low pharmacy budget impact and our clinical trial data suggest that XHANCE may produce an offsetting benefit by helping reduce the rate of surgery with its related costs. For an insurance plan, this could represent a potential overall cost reduction for the population of patients with nasal polyps, as the overall cost of XHANCE could be less than the offsetting costs related to the reduction in surgeries. During clinical studies, XHANCE was also associated with an improvement in reported work productivity in treated patients, which should be valued by employers and patients. Further, we believe the cost of XHANCE to insurance plans will likely be significantly less than the projected costs of monoclonal antibodies that are currently in development for the treatment of nasal polyps. We expect to achieve approximately 65% coverage of commercial lives during the retail launch of XHANCE, and will seek to increase the number of covered lives during the first year. We have contracted with the Centers for Medicare and Medicaid Services regarding certain government covered lives. Further, we plan to introduce a co-pay assistance program and other patient affordability programs to appropriately support patient access to XHANCE. |
• | Infrastructure. We continue to develop our internal capabilities and grew from 21 employees as of January 1, 2017 to 82 employees as of March 1, 2018 to support a commercial stage company. We have implemented an enterprise resource planning system to expand our operational and commercial finance capabilities. We have implemented a robust healthcare compliance program to guide our staff’s and our partners' compliance with rules and regulations regarding pharmaceutical sales. In managing our growth, we have remained focused on fostering our One Mission culture. |
In additional to XHANCE’s existing indication for nasal polyps, we plan to initiate additional clinical trials in the fourth quarter of 2018 to seek a follow-on indication for the treatment of chronic sinusitis in order to broaden our market opportunity. XHANCE is the second commercial product that we have developed utilizing an Optinose EDS. Our first commercial product, indicated for the acute treatment of migraines in adults, was licensed in 2013 to Avanir Pharmaceuticals, Inc., or Avanir, and was approved by the FDA in January 2016.
The Unmet Need
Chronic rhinosinusitis is a serious nasal inflammatory disease characterized by chronic inflammation affecting tissues high and deep in the nasal passages, including the area where the openings from the sinuses normally ventilate and drain. This disease significantly impacts the quality of life and daily functioning of an estimated 30 million adults in the United States. The U.S. healthcare system spends approximately $60 billion annually in direct costs treating patients with chronic rhinosinusitis and its associated symptoms, including an estimated $5 billion on sinus surgeries. In the United States, physicians perform over 500,000 sinus surgeries each year, and we estimate that over seven million adults have undergone sinus surgery to treat chronic rhinosinusitis with and without nasal polyps.
In medical literature and medical practice, chronic rhinosinusitis is commonly divided into two subgroups: chronic rhinosinusitis with nasal polyps and chronic rhinosinusitis without nasal polyps. Chronic rhinosinusitis patients with and without nasal polyps suffer from chronic inflammation of the lining of the deep nasal passages and sinuses. Patients with chronic rhinosinusitis with nasal polyps also develop non-cancerous polyps on these chronically inflamed surfaces, typically originating in the deep crevices or sinus cavities on both sides of the nose. We estimate that up to 10 million adults in the United States have chronic rhinosinusitis with nasal polyps.
Both subgroups of chronic rhinosinusitis share the same four defining diagnostic symptoms: nasal congestion/obstruction; facial pain and pressure; purulent runny nose and postnasal drip; and loss of sense of smell and taste. Additional symptoms may include headaches, chronic sleep problems, fatigue, frequent episodes of acute
4
rhinosinusitis and mood disorders. There is evidence suggesting that the harm to a sufferer's quality of life from chronic rhinosinusitis, as measured in multiple domains, such as bodily pain, social functioning and mental health, is comparable to or worse than other serious diseases, including chronic obstructive pulmonary disease, congestive heart failure and angina. As a result, many patients eventually seek surgery for symptom relief.
Although the term chronic rhinosinusitis is often used in medical literature and medical practice, the FDA does not recognize chronic rhinosinusitis as a single indication for drug development purposes. Instead, the FDA recognizes chronic sinusitis, defined as inflammation of the sinuses with a duration longer than eight weeks, and nasal polyps, defined as non-cancerous polyps on the inflamed tissue of the nasal passages and sinuses, as separate indications for drug development purposes. For purposes of this 10-K, we use the terms chronic sinusitis and nasal polyps when referring to FDA treatment indications and our clinical trials, and use the term chronic rhinosinusitis with and without nasal polyps when referring to disease and economic data reported in the medical literature, medical practice and our estimates of XHANCE's market opportunity.
Current Treatment Limitations
Multiple current clinical practice guidelines specify the use of INS early in the treatment algorithm for chronic rhinosinusitis with and without nasal polyps. Steroids are generally pharmacologically effective at treating inflammation. However, conventional INS, including nasal sprays and nasal aerosols, are topically-acting and unable to effectively and consistently place the steroids onto the primary site of inflammation and nasal polyp origin, high and deep in the nasal passages. These products deposit a majority of the drug in the front of the nose or on the floor of the nasal passages, reducing their effectiveness and leaving many patients without sufficient symptomatic relief. These recognized limitations cause some physicians to seek out alternative treatment regimens such as high-volume steroid nasal rinses. This approach, however, has not been well studied, is difficult to administer, can be costly and may risk systemic side effects. Physicians may also prescribe oral steroids on an episodic basis to patients who have not received sufficient symptomatic relief from INS. Oral steroids, which are often effective in reducing inflammation and nasal polyps, offer only temporary benefit and are limited by the risk of significant systemic side effects associated with both short- and long-term use.
In cases where patients remain symptomatic despite medical management, physicians often recommend various forms of sinus surgery to help restore normal sinus ventilation or drainage. The effectiveness of sinus surgery can vary significantly and many patients experience persistent or recurrent symptoms and surgery may not address the underlying cause of inflammation. In patients with nasal polyps, regrowth of the nasal polyps has been reported in as high as 60% of cases within four years. In addition, it has been reported that 80% of patients who had surgery within the past two years continued to have symptoms. Because sinus surgery is often not curative and may not address the underlying cause of the inflammation, many patients continue to receive short- and long-term courses of INS after surgery.
Our Solution
XHANCE combines an Optinose EDS with a liquid formulation of fluticasone propionate, a well-characterized, second-generation corticosteroid. XHANCE is designed to deliver medication into the high and deep regions of the nasal passages where both nasal polyps and inflamed and swollen membranes can obstruct normal sinus ventilation and drainage. In multiple studies utilizing advanced imaging, an Optinose EDS produced a differentiated pattern of drug delivery in healthy subjects with significant drug deposited in the high and deep regions of the nasal passages, areas not well accessed by conventional INS delivery mechanisms. We believe XHANCE has the potential to become part of the standard of care for the treatment of patients with chronic rhinosinusitis before they progress to more costly treatment alternatives. We also believe that the current treatment practice of postoperative INS use could support XHANCE's adoption as a maintenance therapy to improve outcomes following sinus surgery.
We conducted five clinical trials evaluating over 1,500 adult patients, including two randomized, double-blinded, placebo-controlled Phase 3 pivotal clinical trials in adults with nasal polyps and two supportive open-label Phase 3 clinical trials in adults with symptoms of chronic sinusitis with or without nasal polyps. In both Phase 3 pivotal clinical trials, patients treated with XHANCE experienced statistically significant reductions of both nasal congestion/obstruction symptoms and total polyp grade, which were the co-primary endpoints. Treatment benefits were also observed in all four defining symptoms of chronic rhinosinusitis, as well as in polyp elimination, quality of life measures, need for sinus surgery and patient global impression of change. In addition, the magnitude of improvement for patients treated by XHANCE in our Phase 3 pivotal clinical trials, as measured by the Sinonasal Outcome Test-22, a validated clinical outcome assessment, was comparable to the reported benefits in third-party studies of endoscopic sinus surgery, or ESS, and balloon sinus dilation. XHANCE had an adverse event profile generally comparable to the profile reported in similarly designed studies with conventional INS. In our supportive
5
open-label Phase 3 clinical trials, which evaluated approximately 900 patients with symptoms of chronic sinusitis with and without nasal polyps for a period of up to one year, XHANCE was generally well tolerated and produced results on efficacy endpoints similar to those observed in our Phase 3 pivotal clinical trials. In these supportive trials, we observed comparable symptom improvements in patients with and without nasal polyps and continuing incremental polyp reduction and symptom improvement through 12 months.
We believe XHANCE will offer a cost-effective treatment solution to payors who are increasingly being asked to pay for multiple high-cost therapies for a variety of diseases priced at tens of thousands of dollars per year. We priced XHANCE comparably to the only other branded INS that is currently approved to treat nasal polyps, but at a price higher than generic INS products. We expect XHANCE to be adopted by physicians at a natural point in the care pathway for use in patients with chronic rhinosinusitis with or without nasal polyps before they progress to costly surgical interventions or biologic monoclonal antibodies in development for nasal polyps. Sinus surgery costs between $8,500 and $16,000 per procedure, and we expect that biologic monoclonal antibodies for the treatment of nasal polyps will cost approximately $35,000 per year based on the doses being studied in nasal polyps and the current costs per dose in other indications. We believe XHANCE will offer a cost-effective clinical benefit to payors that will reduce the perceived need for multiple step-edits and prior authorizations, which we believe will increase the likelihood of successful commercial adoption of XHANCE.
U.S. Market Opportunity
Our initial target market for XHANCE will consist of ENT physicians, allergists and primary care physicians in the United States that most frequently prescribe INS. This group of approximately 5,000 primary care physicians, which we refer to as high-decile INS-prescribing primary care physicians, account for approximately 25% of all INS prescriptions written by primary care physicians. We refer to these ENT physicians, allergists and high-decile INS-prescribing primary care physicians collectively as the specialty segment of our target market. We believe the approximately 15,000 physicians in this specialty segment together treat an estimated 3.5 million U.S. patients with chronic rhinosinusitis, an estimated 1.2 million of whom have chronic rhinosinusitis with nasal polyps. We believe the total annual U.S. market opportunity for XHANCE in this specialty segment is over $3.4 billion, of which approximately one-third consists of patients with chronic rhinosinusitis with nasal polyps. If we are able to obtain approval for the follow-on indication of chronic sinusitis, we intend to broaden our commercialization efforts to target additional primary care physicians that we believe treat an additional estimated 6.25 million U.S. patients with chronic rhinosinusitis, an estimated one-third of whom have chronic rhinosinusitis with nasal polyps. We refer to these additional primary care physicians as the primary care segment of our target market. We believe the total additional annual U.S. market opportunity for XHANCE in this primary care segment is over $6.0 billion, of which approximately one-third consists of patients with chronic rhinosinusitis with nasal polyps. Therefore, we estimate the total annual U.S. market opportunity for the combined specialty and primary care segments is over $9.5 billion, of which approximately one-third consists of patients with chronic rhinosinusitis with nasal polyps.
Intellectual Property and Barriers to Entry
XHANCE benefits from substantial intellectual property and other technical barriers to entry, including regulatory and drug delivery complexities. Our XHANCE U.S. patent portfolio consists of 9 issued device and method of use patents expiring through 2030, three issued design patents expiring through 2030 and 12 patent applications that, if granted, would expire through 2034. The 9 issued device and method of use patents are published in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book.
We believe the unique features of an Optinose EDS, as well as its delivery of a topically-acting drug, will present generic and 505(b)(2) NDA competitors of XHANCE with human factors engineering challenges specific to drug-device combination products and chemistry, manufacturing and controls challenges unique to suspension and respiratory products. We also believe that any future substitutable generic competitors would be required to conduct, among other things, non-inferiority clinical trials demonstrating equivalent efficacy and safety outcomes to establish clinical bioequivalence to XHANCE. We believe these clinical trials would require a significant amount of time and capital investment and present clinical development uncertainties.
Our Management Team
We are led by a management team with an average of over 20 years of experience developing and commercializing products at large, multinational pharmaceutical and medical device companies, such as Johnson & Johnson, Sanofi-Aventis, Bristol Myers-Squibb, Takeda and Novartis. Our management team's experience is complemented by its expertise at growing emerging healthcare companies, such as Cephalon, NuPathe and Take Care Health System. Our team previously developed our first product using an exhalation delivery system, Onzetra Xsail. We
6
believe the experience of our management team and our broad network of relationships with leaders within the industry and the medical community provide us with insight into product development and identification of product opportunities that benefit patients and physicians in the ENT and allergy specialty segments.
Our Growth Strategy
Our goal is to become a leading specialty pharmaceutical company dedicated to developing proprietary products that become a part of the standard of care for diseases in the ENT and allergy segments. We also plan to expand the use of the Optinose EDS into additional indications with significant unmet needs, including potential nose-to-brain drug delivery for central nervous system disorders. The key elements of our strategy are to:
▪ | Commercialize XHANCE in the ENT and allergy specialty segments in the United States. We plan to deploy an efficient, go-to-market commercialization model and have completed the build out of our commercial team and organization. Our fully dedicated specialty sales force of approximately 80 territory managers completed training and initiated promotion of XHANCE in early March 2018 to a defined prescriber base of approximately 6,000 ENT and allergy specialists, as well as approximately 2,000 high-decile INS-prescribing primary care physicians. Because we expect to secure broader market access over the next 12 to 18 months, we intend to increase the size of our sales force to approximately 120 territory managers based upon an expanded target audience of approximately 14,000 specialists or specialty-like primary care physicians. Additionally, we expect to target an additional approximately 1,000 physicians through digital and non-personal promotion in areas where we do not have territory managers. We believe these approximately 15,000 physicians treat an estimated 3.5 million chronic rhinosinusitis patients, an estimated 1.2 million of whom have chronic rhinosinusitis with nasal polyps. |
▪ | Pursue pipeline development of XHANCE for chronic sinusitis to broaden our market opportunity. We plan to seek a follow-on indication for XHANCE for the treatment of chronic sinusitis. We believe XHANCE would be the first drug therapy product approved for the treatment of chronic sinusitis. In the future, as appropriate, we plan to broaden our marketing to additional primary care physicians that we believe treat an additional estimated 6.25 million patients in the U.S. with chronic rhinosinusitis, an estimated one-third of whom have chronic rhinosinusitis with nasal polyps. In addition, at some point in the future, we intend to consider directing promotional resources to an additional estimated 20 million adult chronic rhinosinusitis sufferers who are not regularly under the care of physicians for this disease using programs such as direct-to-consumer and direct-to-patient promotion. |
▪ | Develop a pipeline of additional products focused on the ENT and allergy specialty segments. We are evaluating the use of the Optinose EDS to deliver other drugs or drug combinations, including antibiotics, anticholinergics, antihistamines, mucolytics, leukotriene inhibitors and other medication classes, to treat diseases primarily managed by ENT and allergy specialists. We also intend to explore complementary drug, diagnostic or device technologies or products to make effective use of our commercial infrastructure. We also plan to evaluate strategic licensing, acquisition, development and commercial partnerships that could increase our commercial efficiencies. |
▪ | Explore business development activities for Optinose EDS outside of the ENT and allergy segments. We are exploring the possibility of using an Optinose EDS to support development of central nervous system treatments, particularly those enabled by nose-to-brain drug delivery. We are in the early stages of clinical development of OPN-300, which combines an Optinose EDS with oxytocin for the treatment of Prader-Willi syndrome and autism spectrum disorder. We are in preclinical development of OPN-021, which combines an Optinose EDS with orexin-A, for the treatment of narcolepsy or symptoms of other diseases potentially amenable to the same pharmacologic activity, such as Parkinson's disease. We intend to evaluate business development activities to capture value through the continued development of these assets. |
▪ | Expand XHANCE into international markets. We have begun an initial assessment of the development and commercialization of XHANCE for markets outside the United States and plan to conduct further strategic evaluation of such markets now that XHANCE has been approved in the United States. We also intend to explore strategic collaboration opportunities in Europe and the rest of the world in order to maximize the commercial potential and the availability of XHANCE to patients. |
7
Chronic Rhinosinusitis and Market Opportunity
Chronic Rhinosinusitis
Chronic rhinosinusitis is a serious nasal inflammatory disease significantly impacting patients' quality of life and daily functioning. Chronic rhinosinusitis, unlike allergic rhinitis, is characterized by chronic inflammation affecting tissues high and deep in the nasal passages, including the area where the openings from the sinuses normally ventilate and drain, causing symptoms that persist for a period of 8 to 12 weeks or longer. Chronic rhinosinusitis patients typically suffer from these symptoms four to six months a year, with symptoms often persisting for many years.
In medical literature and medical practice, chronic rhinosinusitis is commonly divided into two subgroups: chronic rhinosinusitis with nasal polyps and chronic rhinosinusitis without nasal polyps. Chronic rhinosinusitis patients with and without nasal polyps suffer from chronic inflammation of the lining of the deep nasal passages and sinuses. Patients with chronic rhinosinusitis with nasal polyps also develop non-cancerous polyps on these chronically inflamed surfaces, typically originating in the deep crevices or sinus cavities on both sides of the nose. We estimate that up to 10 million adults in the United States have chronic rhinosinusitis with nasal polyps. Both subgroups of chronic rhinosinusitis share the same four defining diagnostic symptoms: nasal congestion/obstruction; facial pain and pressure; purulent runny nose, and postnasal drip; and loss of sense of smell and taste. Additional symptoms may include headaches, chronic sleep problems, fatigue, frequent episodes of acute rhinosinusitis and mood disorders. There is evidence suggesting that the harm to a sufferer's quality of life from chronic rhinosinusitis, as measured in multiple domains, such as bodily pain, social functioning and mental health, is comparable to or worse than other serious diseases, including chronic obstructive pulmonary disease, congestive heart failure and angina. As a result, many patients eventually seek surgery for symptom relief.
Although the term chronic rhinosinusitis is often used in medical literature and medical practice, the FDA does not recognize chronic rhinosinusitis as a single indication for drug development purposes. Instead, the FDA recognizes chronic sinusitis, defined as inflammation of the sinuses with a duration longer than eight weeks, and nasal polyps defined as non-cancerous polyps on the inflamed tissue of the nasal passages and sinuses, as separate indications for drug development purposes.
The American Academy of Otolaryngology-Head and Neck Surgery estimates that approximately 30 million adults in the United States have chronic rhinosinusitis, and it is estimated that up to 10 million adults have chronic rhinosinusitis with nasal polyps. Chronic rhinosinusitis imposes a significant healthcare burden on insurers and employers. The U.S. healthcare system spends approximately $60 billion annually in direct costs treating patients with chronic rhinosinusitis and its associated symptoms, including an estimated $5 billion on sinus surgeries. In the United States, physicians perform over 500,000 sinus surgeries each year, and we estimate that over seven million adults have undergone sinus surgery to treat chronic rhinosinusitis with and without nasal polyps. Chronic rhinosinusitis has been reported to account for an aggregate of 73 million restricted activity days per year. Additionally, people with chronic rhinosinusitis have been reported to be absent from work because of this disease 6.5% of the time and to suffer a 38% loss of productivity.
U.S. Market Opportunity
We estimate that approximately 9.75 million chronic rhinosinusitis patients are currently being treated in physician offices in the United States. We derived this estimate from a large patient claims database that reflects actual treatment patterns of chronic rhinosinusitis over a two-year period from 2010 to 2012. We also estimate that approximately 10,000 ENT and allergy specialists, as well as approximately 5,000 high-decile INS-prescribing primary care physicians, treat approximately 36% of all chronic rhinosinusitis patients in the United States, or approximately 3.5 million patients, an estimated 1.2 million of whom have chronic rhinosinusitis with nasal polyps. In accordance with multiple published clinical practice guidelines, physicians typically medically manage chronic rhinosinusitis patients by prescribing INS despite the fact that there are no FDA-approved products for the treatment of chronic sinusitis without nasal polyps. We initially intend to target approximately 15,000 physicians in the specialty segment. If we obtain the follow-on indication for chronic sinusitis, we intend to broaden our marketing outreach to additional primary care physicians that treat an additional estimated 6.25 million U.S. patients with chronic rhinosinusitis, an estimated one-third of whom have chronic rhinosinusitis with nasal polyps. We may also direct promotional resources to an additional estimated 20 million chronic rhinosinusitis sufferers who are not regularly under the care of physicians for this disease using programs such as direct-to-consumer and direct-to-patient promotion.
8
Based on internal estimates, we believe the total annual U.S. market opportunity for XHANCE in the specialty segment is over $3.4 billion, of which approximately one-third consists of patients with chronic rhinosinusitis with nasal polyps. Based on these same estimates, we believe the total additional annual U.S. market opportunity for XHANCE in the primary care segment is over $6.0 billion, of which approximately one-third consists of patients with chronic rhinosinusitis with nasal polyps. Therefore, we estimate the total annual U.S. market opportunity for the combined specialty and primary care segments is over $9.5 billion, of which approximately one-third consists of patients with chronic rhinosinusitis with nasal polyps.
Treatment Landscape
The treatment of chronic rhinosinusitis with and without nasal polyps typically begins with medical management. In cases where patients remain symptomatic despite medical management, physicians often recommend various forms of sinus surgery to help restore normal sinus ventilation and drainage. The following is a brief description of the current and potential future treatment landscape for chronic rhinosinusitis with and without nasal polyps:
Current Therapies
▪ | Intranasal Steroids. Multiple published clinical practice guidelines generally recommend topically-acting INS as the first line of prescription therapy for the treatment of chronic rhinosinusitis with and without polyps. As a result, physicians typically prescribe INS nasal sprays or nasal aerosols despite the fact that there are no FDA-approved products for the treatment of chronic sinusitis without nasal polyps. Therefore, the majority of chronic rhinosinusitis sufferers being treated have tried INS. We estimate that physicians in the United States prescribe approximately 17 million INS prescriptions each year for the treatment of chronic rhinosinusitis, which includes, among other INS products, a generic fluticasone propionate nasal spray. Nasonex, or mometasone furoate nasal spray, is currently the only other branded INS approved by the FDA for the treatment of nasal polyps. A generic version of Nasonex, mometasone furoate monohydrate, was approved by the FDA for, among other indications, the treatment of nasal polyps and launched in 2016. Physicians not only prescribe INS as a standalone therapy, but also typically prescribe INS following sinus surgery as some third-party clinical trials suggest that INS treatment can improve symptoms and delay symptom recurrence. |
▪ | Oral steroids. Physicians may prescribe oral steroids on an episodic basis to patients who have not received sufficient symptomatic relief from INS. Oral steroids are often effective at treating the underlying inflammation associated with the disease and reducing postoperative scarring, but the benefit is temporary. As inflammation returns, many patients resume INS therapy. |
▪ | Other medical management. Physicians commonly employ a variety of other non-surgical treatments in the medical management of chronic rhinosinusitis, including nasal saline rinses, multi-week courses of antibiotics, leukotriene antagonists, decongestants, aspirin desensitization and antifungals. The recognized limitations of drug deposition with current INS cause some physicians to seek out alternative treatment regimens, such as high doses of locally compounded liquid budesonide in high-volume nasal rinses. Chronic rhinosinusitis is one of the most common reasons for adult outpatient antibiotic use in the United States, comprised of approximately 37 million prescriptions per year. |
▪ | Sinus surgery and other procedures. Physicians generally recommend surgical treatment of chronic rhinosinusitis with and without nasal polyps only after patients fail medical management. The primary surgical alternative is ESS, which attempts to open the sinus drainage pathways while preserving as much bone and sinus tissue lining as possible. The physician typically uses rigid steel instruments and powered cutting tools to remove inflamed tissue, including any nasal polyps, and underlying bone to create a larger passage through the nasal anatomy to the sinuses. At the conclusion of the procedure, patients often have their nasal passages packed with a material that acts as a spacer to prevent surgical adhesions and control bleeding. Patients typically require one or more follow-up debridement treatments in which the physician may remove more tissue, crusting, scabs or scar tissue at the area of surgery in order to keep the sinus drainage pathway open and promote proper healing. |
Several companies have developed less invasive technologies for the treatment of chronic rhinosinusitis since the introduction of ESS, such as balloon sinus dilation devices and steroid-releasing sinus implants. Balloon sinus dilation employs a high pressure inflated balloon to open blocked sinus pathways to increase ventilation and mucus drainage. Steroid-releasing sinus implants are used to hold open the surgically enlarged sinus, while releasing a steroid over a period of time in order to reduce postoperative sinus inflammation and scarring.
9
Potential Future Therapies
Several biologic monoclonal antibodies, some of which are already approved for other indications, are being developed for the treatment of nasal polyps, and are believed to inhibit specific pathways of inflammation present in nasal polyps. These biologic monoclonal antibodies include omalizumab, reslizumab, mepolizumab and dupilumab.
Limitations of Therapies
The current and potential future therapies to treat patients suffering from chronic rhinosinusitis with and without nasal polyps have a number of limitations, including:
▪ | Limited efficacy of INS treatments using traditional nasal sprays and nasal aerosols. Although steroids are generally pharmacologically effective, conventional INS, including nasal sprays and nasal aerosols, are unable to effectively and consistently place the steroids onto the primary site of inflammation and nasal polyp origin, high and deep in the nasal passages. These products deposit a majority of the drug in the front of the nose or on the floor of the nasal passages, reducing their effectiveness and leaving many patients without sufficient symptomatic relief. |
▪ | Short-term benefits of oral steroids outweighed by significant side effects. Oral steroids offer only temporary benefit and are limited by the risk of significant systemic side effects associated with both short- and long-term use. These side effects include, among others, weight gain; increased risk of infections; loss of bone mineral density; death of bone tissue; cataract formation; glaucoma; adrenal suppression; and psychiatric complications, including mania, depression, and psychosis. |
▪ | Varying degrees of efficacy with other medical management. Other non-surgical treatments have varying degrees of supporting data and efficacy. In addition, high-volume steroid nasal rinses are difficult to administer, can be costly, may risk systemic side effects due to the absorption of the steroid into the body, can be associated with fluid draining from the nose after the procedure and are difficult for patients to comply with over prolonged courses of outpatient therapy. |
▪ | Sinus surgery and other procedures are costly and may not be a complete solution. The effectiveness of sinus surgery varies significantly and many patients experience persistent or recurrent symptoms. Reports have shown that nasal polyp regrowth following surgery occurs in as high as 60% of cases within four years. In addition, it has been reported that 80% of patients who had surgery within the past two years continued to have symptoms. Because sinus surgery is often not curative and may not address the underlying cause of the inflammation, many patients receive short- and long-term courses of INS after surgery and approximately 20% of patients elect surgical revisions. Postoperative scarring and persistent inflammation are common and can compromise symptom outcomes and also negatively impact the ability of the sinuses to heal. Sinus surgery is also a costly procedure, with estimated costs ranging from $8,500 to $16,000 per procedure. While balloon sinus dilation has the ability to open sinuses in a less invasive manner, it also may not address the underlying cause of the inflammation associated with chronic rhinosinusitis and is costly. Similarly, steroid-releasing sinus implants have limited duration of anti-inflammatory effect, are costly and face reimbursement challenges. |
▪ | Potential future biologic monoclonal antibodies treatment may be costly, difficult to administer or have negative side effects. The risks and benefits associated with the use of biologic monoclonal antibodies for the treatment of nasal polyps are not yet fully established. We expect the use of biologic monoclonal antibodies for the treatment of nasal polyps to be costly, with estimated costs of approximately $35,000 per year based on the doses being studied in nasal polyps and the current costs per dose in other indications. These drugs also require subcutaneous injections or intravenous administration that require frequent physician office visits. We believe the systemic nature of these treatments, which target components of the immune response, may result in more adverse side effects than treatments with topically-acting steroids. |
Our Solution
XHANCE
XHANCE combines an Optinose EDS with a liquid formulation of fluticasone propionate, a potent, well-characterized, second-generation anti-inflammatory corticosteroid for the treatment of serious nasal diseases characterized by chronic inflammation, such as chronic rhinosinusitis. XHANCE is designed to deliver fluticasone propionate into the high and deep regions of the nasal passages where nasal polyps or inflamed and swollen
10
membranes can obstruct normal sinus ventilation and drainage. On September 18, 2017, the FDA approved our NDA for XHANCE for the treatment of nasal polyps in patients 18 years of age or older. We also plan to initiate additional clinical trials of XHANCE in the fourth quarter of 2018 to seek a follow-on indication for the treatment of chronic sinusitis. Similar to our NDA for XHANCE for the treatment of nasal polyps, we believe we will be able to use the FDA's Section 505(b)(2) regulatory pathway for potential U.S. approval for XHANCE for the treatment of chronic sinusitis.
We believe XHANCE could become a part of the standard of care for the treatment of patients with chronic rhinosinusitis with and without nasal polyps before they progress to more costly treatment alternatives and could also be adopted as a maintenance therapy to improve outcomes following sinus surgery. We believe the following factors could contribute to the potential success of XHANCE:
▪ | High patient dissatisfaction with current INS treatments. In a market research study that we commissioned, we surveyed 438 patients with chronic sinusitis with and without nasal polyps. In this study, approximately 80% of the patients reported being frustrated with the symptom relief offered from their current INS medication and approximately 90% of the patients reported they would be interested in using a new product if it would improve symptom relief. |
▪ | Strong physician interest in XHANCE product profile. We surveyed approximately 700 physicians, consisting of 400 ENT and allergy specialists and 300 primary care physicians that currently treat patients with chronic sinusitis with and without nasal polyps. Approximately 75% of these physicians, including both specialists and primary care physicians, agreed, in part, that INS medications do not work well in patients with chronic sinusitis due to their belief that conventional INS do not sufficiently reach the high and deep regions of the nasal passages where inflammation occurs. In addition, 70% to 80% of these physicians reported that they would "definitely" or "probably" prescribe their patients a product with a clinical profile similar to XHANCE. |
▪ | Fluticasone propionate is the most widely-prescribed INS in the United States. XHANCE contains fluticasone propionate, a potent, well-characterized, second-generation, anti-inflammatory corticosteroid with a low bioavailability, meaning that only a small percentage of the drug is absorbed into the body. Corticosteroids provide multiple anti-inflammatory mechanisms of action and are used in forms such as pills, creams, inhalers and nasal sprays, to treat many sites of inflammation. |
▪ | XHANCE was designed to overcome the limitations of current INS therapies by delivering medication high and deep in the nasal passages. In multiple studies utilizing advanced imaging, an Optinose EDS produced a differentiated pattern of drug delivery with significant drug deposited at the primary site of inflammation high and deep in the nasal passages where nasal polyps or inflamed and swollen membranes produce nasal symptoms and can obstruct normal sinus ventilation and drainage. |
▪ | Strong clinical data demonstrating safety and efficacy. In two randomized, double-blinded, placebo-controlled Phase 3 pivotal clinical trials evaluating adult patients with nasal polyps, we met our co-primary endpoints of statistically significant reductions of nasal congestion/obstruction symptoms and total polyp grade. XHANCE also produced treatment benefits in all four defining symptoms of chronic rhinosinusitis, as well as in polyp elimination, quality of life measures, need for sinus surgery and patient global impression of change. In two supportive open-label Phase 3 clinical trials evaluating approximately 900 patients with symptoms of chronic sinusitis with and without nasal polyps for a period of up to one year, XHANCE was generally well tolerated. In these supportive trials, we observed comparable symptom improvements in patients with and without nasal polyps and continuing incremental polyp reduction and symptom improvement through 12 months. |
▪ | XHANCE is easy to use. In a market study that we commissioned, 98% of patients reported that XHANCE was easy to use after four weeks of use and 93% stated the ease of use was comparable to other INS. |
▪ | Potential for broad payor access. In a market research study that we commissioned, we surveyed 26 health insurance plans representing over 150 million covered lives. Most payors reacted positively to a profile of XHANCE with respect to its product design, mechanism of action and efficacy results based upon our clinical data. This research further suggested that market access for XHANCE will be dependent on XHANCE's pricing. A majority of payors surveyed in our study indicated that they do not intend to actively manage INS products priced below a certain dollar threshold and many surveyed payors indicated that they would provide access without prior authorization to INS products priced within a certain dollar range. The surveyed payors reported the following potential coverage based on the XHANCE profile: (i) no step edits |
11
on plans covering approximately 27% of commercial lives, meaning that payors would not require patients to use generic INS before seeking reimbursement for XHANCE, (ii) a single step edit on plans covering approximately 48% of commercial lives, (iii) a prior authorization requirement on plans covering approximately 10% of commercial lives and (iv) no coverage by plans covering approximately 15% of commercial lives. In addition to this market research study, we obtained formulary data for INS from various sources representing approximately 159 million covered lives. These data indicate that health insurance plans covering 84% of commercial lives do not require prior authorization in the INS category for contracted products. We are actively engaging payors to secure broad market access for XHANCE in the commercial segment by targeting Tier 3 payor coverage, single step edit with no prior authorization. This level of coverage indicates that payors would require patients to use a generic INS as a first step in treating their disease prior to the payor covering XHANCE. However, such coverage would not require the prior authorization of the payor. Tier 3 payor coverage requires a patient co-pay that is higher than that required for generics or drugs within a payor's formulary. We intend to contract with Commercial and Medicare Part D plans and Medicaid to accelerate physician adoption of XHANCE.
▪ | Cost-Effective Solution. We have priced XHANCE comparably to the only other branded INS that is approved to treat nasal polyps, but at a price higher than generic INS products. We believe XHANCE will offer a cost-effective, clinical benefit to payors when compared to surgery and expensive monoclonal antibodies and that this benefit will reduce the perceived need for multiple step-edits and prior authorizations, which we believe will increase the likelihood of successful commercial adoption of XHANCE. |
Optinose EDS
Our exhalation delivery systems enable the development of drug-device combination products intended for self-administration. We have developed both a liquid delivery system and a powder delivery system utilizing natural functional behaviors of the upper nasal airways to offer better drug deposition. These systems are designed to overcome many limitations inherent in conventional nasal spray and nasal aerosol delivery systems, most notably, enabling higher and deeper intranasal drug delivery.
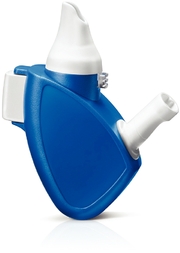
Liquid EDS
The liquid EDS depicted below, which is the EDS used in XHANCE, consists of the primary drug container for the liquid drug formulation, an amber glass vial sealed by a crimp-fitted metering spray pump, enclosed within a proprietary liquid delivery subassembly. The nasal spray applicator, which is a component of the subassembly, is attached to the pump and extends to the top of the nosepiece of the liquid delivery subassembly. The EDS includes a flexible mouthpiece and an asymmetrically-shaped nosepiece as part of a mechanism that uses the patient's exhaled breath to naturally seal closed the soft palate and to facilitate delivery of drug to the nasal passages through the sealing nosepiece. The nosepiece is designed to create a seal with the nostril and also to expand and stent the upper part of the nasal valve, which is an important anatomical structure that is the narrowest part of the entire respiratory tract and a barrier that causes most medication delivered by conventional INS to deposit in the front part of the nose.
12

Powder EDS
The powder EDS depicted below, which is the EDS used in Onzetra Xsail, consists of a reusable device body incorporating a flexible mouthpiece to adjust to individual anatomic variations, and a white button piercing assembly to pierce the medication capsule. Disposable nosepieces are provided in a foil pouch to be inserted into the drug delivery device body. Each pre-filled nosepiece section contains a medication capsule containing a dry powder formulation and a clear release tab. The capsule is pierced by pressing and releasing the white button piercing assembly. The flexible mouthpiece and an asymmetrically-shaped nosepiece are part of the mechanism that uses the patient's exhaled breath to naturally seal closed the soft palate and to facilitate delivery of drug to the nasal passages through the sealing nosepiece. The medication capsule is intended for single dose administration and is not refillable or removable from the nosepiece.
Following drug administration, the disposable nosepiece, including the dose-expended medication capsule, is then removed and discarded.
13
How an Optinose EDS works
When exhaling into an EDS, the soft palate automatically elevates and creates an air-tight seal separating the nasal cavity from the throat and lungs. This natural action is the same as that which prevents air from escaping from the nose when trying to blow up a balloon or blow a trumpet. The exhaled air is then routed through the EDS which introduces medication into the air flow and then directs the air and medication through the sealing nosepiece. The positive air pressure, which is the opposite of the negative pressure produced by sniffing with ordinary nasal sprays, acts to dynamically expand the nasal valve and the narrowed nasal passages, helping to "float" the drug around obstructing anatomic barriers and fill one side of the nasal cavity. This enables high and deep deposition of medication in the nasal passages. The positive air pressure, proportional to the pressure on the other side of the soft palate, helps to open a passage between the two sides of the nasal cavity, behind the back edge of the nasal septum. The picture below illustrates this action, which allows the exhaled air pressure to escape from the opposite nostril.
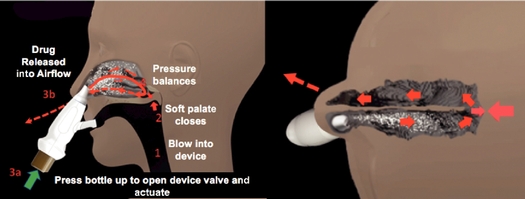
The drug delivery mechanism of an Optinose EDS is designed to overcome the drug deposition shortcomings of conventional nasal sprays and nasal aerosols. In conventional nasal sprays and nasal aerosols, the medication is inhaled or sniffed into the nose creating negative pressure within the nasal passages, which does not facilitate the expansion of the nasal valve or the nasal passages and may obstruct the drug from reaching deep into the nose where most nasal polyps and inflamed and swollen sinus membranes exist.
The pattern of drug deposition produced by conventional nasal sprays and an Optinose EDS has been evaluated in multiple studies using a combination of advanced imaging modalities to depict the regions of the nasal passages where drug is deposited after administration in human volunteers. In an open label, crossover study conducted by a third party in nine patients with allergic rhinitis, investigators examined the nasal deposition of radio-labeled materials that allow for traceability following use of Qnasl (HFA-beclomethasone, nasal aerosol), Flonase (fluticasone propionate, nasal spray) and Nasonex (mometasone furoate monohydrate, nasal spray). In this study, gamma cameras were used to capture emitted radiation from these tracers to create two-dimensional images in a similar process to the capture of x-ray images. These gamma images were merged with magnetic resonance images, or MRI, to quantify regional deposition within the nasal passages. The images below illustrate how the pattern of drug deposition in the nasal passages produced by Qnasl, Flonase and Nasonex was concentrated in the front and lower regions of the nasal passages, as opposed to the high and deep regions of the nasal passages targeted in the treatment of chronic rhinosinusitis.
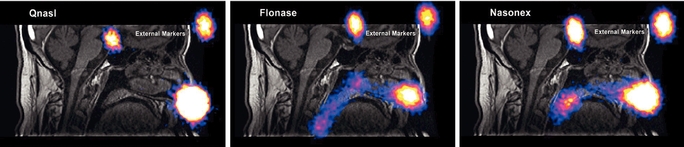
14
Reprinted with permission from JOURNAL OF AEROSOL MEDICINE & PULMONARY DRUG DELIVERY 28/8, 2015, by Leach et al, published by Mary Ann Liebert, Inc., New Rochelle, NY.
We conducted six deposition studies evaluating 53 healthy subjects that produced approximately 250 images. As depicted in the representative figures below, an Optinose EDS produced a differentiated pattern of drug delivery with significantly more drug deposited in the high and deep regions of the nasal passages.
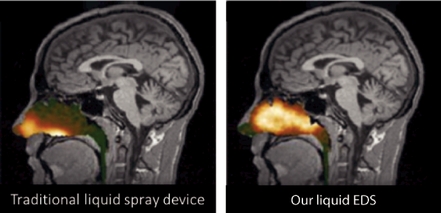
The pictures above use gamma camera image information, which was then superimposed on the corresponding MRI section. These images represent deposition in healthy subjects two minutes after delivery using a traditional liquid nasal spray and a version of our liquid EDS device. Deposition with traditional liquid nasal spray was greatest in the front parts of the nose, whereas deposition with an Optinose EDS was greatest in the high and deep regions of the nose.
The pictures below illustrate how an Optinose EDS (with exhalation) places medication higher and deeper in the nasal passages than a conventional nasal spray (without sniffing) in nasal cast models. As depicted below, although conventional nasal spray systems can reach, and therefore treat, large nasal polyps, they are not suitable for reaching nasal polyps or inflammation in the higher and deeper regions where obstruction of the sinus openings occurs.
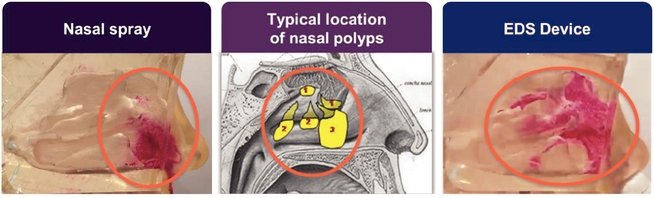
An Optinose EDS is also designed to address user dissatisfaction with standard nasal delivery by reducing drug drip-out from the front and back of the nose and the bad taste that often accompanies drug entering the throat. By reducing the loss of drug to non-targeted sites, such as the gastrointestinal tract by swallowing, or lungs, an Optinose EDS has the potential to improve the efficiency of drug activity and to improve tolerability by reducing off-target effects.
15
Our Pipeline
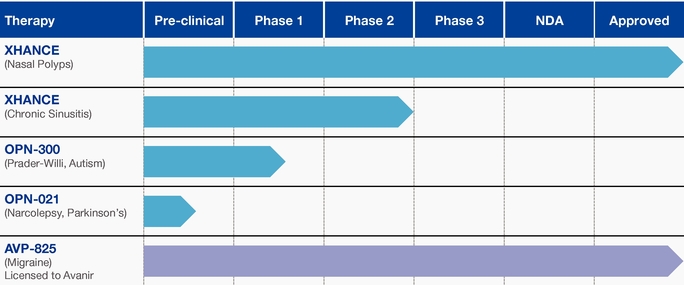
XHANCE for Chronic Sinusitis
We plan to initiate additional clinical trials of XHANCE in the fourth quarter of 2018 to seek a follow-on indication for the treatment of chronic sinusitis. We believe XHANCE would be the first drug therapy product approved for the treatment of chronic sinusitis. In the future, as appropriate, we intend to broaden our commercialization efforts to additional primary care physicians that we believe treat an additional estimated 6.25 million U.S. patients with chronic rhinosinusitis, an estimated one-third of whom have chronic rhinosinusitis with nasal polyps. In addition, at some point in the future, we intend to consider directing promotional resources to an additional estimated 20 million adults who are not regularly under the care of physicians for this disease using programs such as direct-to-consumer and direct-to-patient promotion.
Other Product Candidates
Although our initial focus is to prioritize the development of XHANCE in the ENT and allergy specialty segments, we have applied an Optinose EDS to other product candidates in our pipeline across a broad range of disease areas. By placing drug high and deep in the nose, in regions where cranial nerves connect directly with the brain, we believe it may be possible to deliver medications directly into the brain and avoid the difficulties of getting drug past the blood-brain barrier. This may enable treatment of brain diseases using small or large molecules that otherwise do not readily enter the nervous system.
OPN-300
We have engaged in early clinical development activities for OPN-300, which combines an Optinose EDS with oxytocin. Oxytocin is a small, naturally occurring peptide currently used to stimulate lactation in breastfeeding women. Oxytocin acts as a neurotransmitter in the brain and has recently been considered a potential novel treatment alternative in several brain disorders due to a growing body of evidence of its critical role in social cognition and behavior. Because oxytocin is a peptide with poor oral bioavailability, nasal administration with an Optinose EDS may allow for improved delivery. With standard liquid nasal spray delivery, only a small amount of the drug reaches systemic circulation. It is estimated that less than 0.01% of oxytocin in the blood enters the brain across the blood-brain barrier.
OPN-300 is being developed to target two orphan indications: Prader-Willi syndrome, a rare genetic disorder that is the leading genetic cause of obesity; and autism spectrum disorder. We conducted a Phase 1 clinical trial in late 2013 using OPN-300 in healthy volunteers. In that trial, a low dose of oxytocin delivered using an Optinose EDS produced a statistically significantly greater social-cognitive effect as measured with functional magnetic resonance imaging, performance on cognitive tests, and physiological markers, than intravenous administration of the same active ingredient that produced blood levels that were not statistically different. We believe this clinical trial supports the possibility of direct nose-to-brain activity of medication delivered using an Optinose EDS. We recently completed a second pilot clinical trial of OPN-300 in adult male patients with autism spectrum disorder. In that trial, adult men with autism spectrum disorder receiving nasal oxytocin showed statistically significant differences in interpretation of
16
facial expressions. We are preparing for additional clinical development activities in pursuit of an indication for Prader-Willi syndrome.
OPN-021
We are in preclinical development of OPN-021, which combines an Optinose EDS with orexin-A, also known as hypocretin-A, a peptide that acts as a neurotransmitter in the brain. OPN-021 is being developed for the treatment of narcolepsy or symptoms of other diseases potentially amenable to the same pharmacologic activity, such as Parkinson's disease. Narcolepsy is a chronic neurodegenerative disease caused by a deficiency of orexin-producing neurons in the lateral hypothalamus region of the brain. It is clinically characterized by excessive daytime sleepiness, sudden and uncontrollable muscle weakness or paralysis and by intrusions into wakefulness of physiological aspects of rapid eye movement sleep. We are in the process of developing the formulation for OPN-021 and are planning to initiate a Phase 1 clinical trial when a suitable formulation is prepared.
Other
We are evaluating the use of an Optinose EDS to deliver other drugs or drug combinations, including antibiotics, anticholinergics, antihistamines, mucolytics, leukotriene inhibitors and other medication classes used to treat diseases primarily managed by ENT and allergy specialists. We have also identified several other product candidates with the potential to leverage an Optinose EDS to create clinically differentiated drug treatments for indications such as central nervous system disorders and pain. We will continue to evaluate opportunities to develop product candidates indicated for markets outside of our ENT and allergy focus through business development activities.
Our Commercial Strategy
We are implementing our commercial strategy for XHANCE to focus on the following three phases of penetrating the chronic rhinosinusitis markets and becoming part of the standard of care treatment:
▪ | Efficient entry in the ENT and Allergy specialty segments: We plan to deploy an efficient, go-to-market commercialization model and have completed the build out of our commercial team and organization. Our fully dedicated specialty sales force of approximately 80 territory managers completed training and initiated promotion of XHANCE in early March 2018 to a defined prescriber base of approximately 6,000 ENT and allergy specialists, as well as approximately 2,000 high-decile INS-prescribing primary care physicians. Because we expect to secure broader market access over the next 12 to 18 months, we intend to increase the size of our sales force to approximately 120 territory managers based upon an expanded target audience of approximately 14,000 specialists or specialty like primary care physicians. Additionally, we expect to target an additional approximately 1,000 physicians through digital and non-personal promotion in areas where we do not have territory managers. We believe these approximately 15,000 physicians treat an estimated 3.5 million chronic rhinosinusitis patients, an estimated 1.2 million of whom have chronic rhinosinusitis with nasal polyps. |
▪ | Facilitate broader adoption: We intend to pursue a follow-on indication of XHANCE for the treatment of chronic sinusitis. Upon approval for the follow-on indication, we intend to broaden our commercialization efforts to target primary care physicians that we believe treat an additional estimated 6.25 million U.S. patients with chronic rhinosinusitis, an estimated one-third of whom have chronic rhinosinusitis with nasal polyps. We may target these physicians through a commercial partnership. |
▪ | Activate patient demand: At some point in the future, we intend to consider directing promotional resources to an additional estimated 20 million chronic rhinosinusitis sufferers who are not regularly under the care of physicians for this disease using programs such as direct-to-consumer and direct-to-patient promotion. |
We intend to efficiently launch XHANCE into the ENT and allergy specialty segments by utilizing the following strategies:
▪ | Define a clear patient type for XHANCE. We intend to focus on moderate-to-severely symptomatic patients who have not achieved satisfactory results with currently available INS. |
▪ | Establish a compelling brand position in the medical continuum of care. In an effort to establish our brand position within the continuum of care, we intend to, among other things, educate physicians, payors and patients on XHANCE's unique mechanism of action and differentiated efficacy profile. |
17
▪ | Develop a meaningful payor-friendly value proposition. We intend to establish a meaningful value proposition for physicians, payors and patients by highlighting the potential for XHANCE to reduce or delay the need for surgical intervention, reduce antibiotic prescribing and increase patient satisfaction with treatment outcomes. We believe the health economic data related to XHANCE are compelling. Our analyses show that XHANCE will have a comparatively low pharmacy budget impact and our clinical trial data suggest that XHANCE may produce an offsetting benefit by helping reduce the rate of surgery with its related costs. For an insurance plan, this could represent a potential overall cost reduction for the population of patients with chronic rhinosinusitis with nasal polyps, as the overall cost of XHANCE would be less than the offsetting costs related to the reduction in surgeries. During clinical studies, XHANCE was also associated with an improvement in reported work productivity in treated patients, which should be valued by employers and patients. Further, we believe the cost of XHANCE to insurance plans will likely be significantly less than the projected costs of monoclonal antibodies that are currently in development for the treatment of nasal polyps. |
▪ | Drive awareness, adoption and access. We are engaging with physicians and payors to educate both constituencies about XHANCE and its benefits, with the goal of securing broad market access for the expected availability of XHANCE through retail pharmacies in early April 2018. |
◦ | Physicians: We are utilizing a clinical nurse educator team to target ENT and allergy specialists to (i) introduce us and highlight the unmet medical need and limitations of current treatments, (ii) increase awareness about XHANCE along with differentiating the EDS and (iii) familiarize healthcare professionals with the proper administration of XHANCE. In addition, beginning in early March 2018 our dedicated sales force started to introduce the XHANCE Xperience program to select physicians. |
◦ | Payors: We are engaging with payors prior to launch with the objective of securing broad market access in the commercial segment by targeting Tier 3 payor coverage, single step edit with no prior authorization. Specifically, we are targeting pharmaceutical benefit managers, national plans and regional plans representing, in the aggregate, up to approximately 160 million of the estimated 180 million U.S. covered commercial lives. |
◦ | Patients: We are building a patient and physician support infrastructure in an effort to accelerate physician adoption and reduce the risk of patient abandonment during the fulfillment process. We expect that this infrastructure may include (i) patient samples, (ii) a co-pay assistance program for patients who have commercial coverage, (iii) savings cards for cash payors, (iv) reimbursement support programs for the retail channel, (v) a "specialized" distribution channel to assist patients with the complexities of the payor landscape, and (vii) a patient assistance program to provide access to XHANCE to people who have no or inadequate insurance. |
Since FDA approval on September 18, 2017, the commercial team has been executing a differentiated, purposeful launch model with the objective of enabling our commercial team to achieve levels of customer awareness, design and implement our customer model, and build market access for XHANCE to facilitate adoption upon making XHANCE broadly available through retail pharmacies in the second quarter of 2018; we now believe XHANCE will be available in retail pharmacies in early April 2018. Our progress across each of these key strategic areas is described below:
▪ | Drive product awareness and appreciation of the clinical differentiation of XHANCE |
◦ | Executed broad multi-channel awareness campaign leveraging digital, non-personal promotion and journal advertising and have already reached over 10,000 ENT and allergists |
◦ | Launched approximately 85 person nurse educator team calling on a universe of about 7,000 ENT and allergists. This team has reached approximately 5,000 ENT and allergy prescribers and delivered approximately 10,000 presentations to their target audience. Initial reaction to core brand messages among target physicians has been positive. |
◦ | Finalized XHANCE core marketing strategies and launch tactic including a compliant, value optimizing and cost-effective promotion mix to appropriately engage our target audience |
◦ | Introduced the XHANCE Xperience program to select physicians in early March 2018. This program will offer these physicians and their patients an opportunity to gain initial experience with XHANCE |
18
▪ | Design and deploy our customer facing model |
◦ | Designed hybrid sales model that leverages a fully dedicated contract sales organization reporting into an Optinose Sales Leadership Team |
◦ | Defined footprint of approximately 120 territories that will ultimately target approximately about 14,000 ENTs, allergists and “specialty like” primary care physicians |
◦ | Recruited, hired and trained 11 Optinose Regional Business Leaders with an average of approximately 11 years of sales leadership experience |
◦ | In partnership with our contract sales organization, approximately 80 Territory Managers, or TMs, were recruited, hired and trained. The TMs have an average of 13 years of pharmaceutical sales experience and over 70% have experience in the respiratory therapeutic category. The TMs were deployed in early March 2018 in geographies where we expect to have greater than 65% coverage of commercial lives during launch. |
▪ | Engage commercial payors with the objective of securing tier 3 commercial coverage |
◦ | Developed compelling economic value proposition |
◦ | Created value pack, budget impact model, supporting health economics and outcomes research, or HEOR, payor messages and payor partnership models |
◦ | Completed pricing study to inform pricing decision and contracting strategy and will launch with a Wholesaler Acquisition Cost, or WAC, of $425 per unit of XHANCE |
◦ | Created HEOR-related communication tools for use with payors |
◦ | Completed development of our clinical and economic evidence of pharmaceuticals in support of formulary consideration using the Academy of Managed Care Pharmacy, or AMCP, Dossier format |
◦ | Engaged with approximately 40 plans representing approximately 85% of commercial lives |
◦ | Intend to introduce co-pay assistance program and other patient affordability programs to appropriately support patient access to XHANCE |
XHANCE Clinical Development
Overview
We have evaluated XHANCE in the following five clinical trials comprised of over 1,500 patients:
▪ | two randomized, double-blinded, placebo-controlled Phase 3 pivotal clinical trials designed to compare the safety and efficacy of XHANCE to a placebo EDS in adults with bilateral nasal polyps, which we refer to as NAVIGATE I and NAVIGATE II or collectively, our pivotal clinical trials; |
▪ | two open-label Phase 3 clinical trials to evaluate the safety of XHANCE in adults with symptoms of chronic sinusitis with or without nasal polyps, which we refer to as EXHANCE-3 and EXHANCE-12 or collectively, our supportive clinical trials; and |
▪ | one Phase 1, open-label, randomized, single-dose, bioavailability study to compare the bioavailability of fluticasone propionate from XHANCE to Flonase and Flovent HFA in healthy patients and patients with mild-to-moderate asthma. |
Clinical Trial Highlights
Our Phase 3 clinical development program included a population of patients generally reflective of our intended patient population, with approximately 90% having previously tried currently available INS and almost one-third having previously undergone sinus surgery. Key results from our Phase 3 clinical trial program include:
▪ | In our pivotal clinical trials, XHANCE produced statistically significant benefits on both of the co-primary endpoints: a reduction of nasal congestion/obstruction symptoms at week 4 and a reduction in total polyp grade at week 16. |
19
▪ | Patients with nasal polyps generally experienced greater improvements in symptoms and reductions in polyp grade with longer duration of use. |
▪ | In our pivotal clinical trials, approximately 16% of patients treated with XHANCE had nasal polyps eliminated in at least one nostril after 16 weeks of treatment, and approximately 27% had nasal polyps eliminated in at least one nostril after an additional eight weeks of treatment. In our supportive clinical trials, we observed complete response rates in at least one nostril of 48% of patients in EXHANCE-3 and 47.1% of patients in EXHANCE-12. |
▪ | In our pivotal clinical trials, XHANCE produced improvement across all four defining symptoms of chronic rhinosinusitis. |
▪ | Over 85% of patients receiving XHANCE across our pivotal clinical trials reported improvement, and approximately two-thirds reported being "much" or "very much" improved, compared to approximately one-third of patients in the placebo EDS group. In our supportive clinical trials, approximately 70% of patients with symptoms of chronic sinusitis, both with and without nasal polyps, reported that they were "much" or "very much" improved after treatment with XHANCE. |
▪ | On a Sinonasal Outcome Test-22, the improvement with the 186- and 372-microgram, or mcg, doses of XHANCE was superior to the placebo EDS in both NAVIGATE I and NAVIGATE II. The magnitude of improvement associated with treatment with XHANCE was approximately 20 points. Although cross-trial comparisons have significant limitations and must be interpreted with caution, in a previous third-party study evaluating a large cohort (n=1468) of patients who were underwent sinus surgery, the degree of change on this outcome measure was approximately 18 points. |
▪ | After 12 months of treatment with XHANCE in our supportive clinical trials, at least 50% of patients had a Sinonasal Outcome Test-22 score that was at or below 9.3, which is the average score that has been reported for healthy individuals. |
▪ | XHANCE was well tolerated and had an adverse event profile generally similar to that observed in several comparably designed third party studies, including those of mometasone furoate in nasal polyps patients and of fluticasone propionate formulations in polyposis and allergic rhinitis patients. |
Phase 3 Pivotal Clinical Trials (NAVIGATE I and NAVIGATE II)
We have conducted two independent but comparable randomized double-blinded, placebo controlled Phase 3 clinical trials to examine the safety and efficacy of XHANCE versus a placebo EDS in adults with bilateral nasal polyps and moderate nasal congestion/obstruction. These clinical trials, which we refer to as NAVIGATE I and NAVIGATE II, also provided dose-ranging information to support the selection of clinically appropriate dose(s) for commercialization of XHANCE and served as pivotal clinical trials in our NDA for the treatment of adults with nasal polyps. These pivotal clinical trials were conducted in the United States, Canada, South Africa and several European countries.
Study Design
Each pivotal clinical trial included a single-blind EDS-placebo lead-in and a placebo EDS control group, a multi-center, multi-national study population to increase generalizability, an assessment of the efficacy of multiple doses (93, 186 or 372 mcg twice daily) over a 16-week period and experts in nasal endoscopy to assess objective efficacy outcomes and adverse events, or AEs, in all patients. Patients who completed the double-blinded phase of the pivotal clinical trials were allowed to continue in an open-label extension phase in which all patients received 372 mcg of XHANCE twice daily for up to eight additional weeks. All patients and investigators remained blinded to the original treatment during the open-label phase, allowing for a comparison of as-randomized initial treatments through the end of the open-label extension phase at week 24. We treated a total of 646 adults across both pivotal clinical trials with 568 adults completing the open-label extension phase.
Each of NAVIGATE I and NAVIGATE II had co-primary endpoints of (i) change in subjective nasal congestion/obstruction symptoms from baseline to week 4 and (ii) change in objectively-measured total (bilateral) nasal polyp grade from baseline to week 16. The severity of nasal symptoms was recorded by patients in an electronic diary immediately before dosing in the morning (AM) and evening (PM), and was measured using 7-day average instantaneous AM diary scores. Total (bilateral) nasal polyp grading was assessed with nasoendoscopy and is based on polyp protrusion past certain anatomical landmarks. These grading assessments were performed at screening (baseline) and at weeks 4, 8, 12, 16 (which was the end of the double-blinded phase) and 24 (which was
20
the end of the open-label phase) using a 0 to 3 point scale for each nostril, with 0 representing no polyps and 3 representing severe polyposis. The scores for each nostril were summed to yield a range of 0 to 6 for both nostrils.
These trials also evaluated several secondary endpoints, including the impact of XHANCE treatment on surgical eligibility and changes in the Sinonasal Outcome Test-22 score, which considers the core defining signs and symptoms of nasal polyps and the impact on functioning, quality of life and sleep. We also conducted a complete response analysis to evaluate the percentage of patients with a recorded nasal polyp grade of zero on at least one side of the nasal cavity.
Efficacy Results
The 186- and 372-mcg treatment groups achieved statistically significant reductions in the primary assessments of congestion severity at week 4 and reductions in polyp grade at week 16 relative to a placebo EDS. In NAVIGATE I, the differences from the placebo EDS generally increased with each increasing dose of XHANCE for both co-primary endpoints, meaning that administering higher doses to a patient led to a greater decrease in nasal congestion/obstruction symptoms and bilateral nasal polyp grade. In NAVIGATE II, the 186-mcg group achieved the largest numerical reduction in the primary assessment of congestion symptom severity, and the 372-mcg group achieved the largest numerical reduction in the primary assessment of polyp grade. On average, patients in both pivotal clinical trials had moderate nasal polyps (with an average bilateral score of approximately 3.9) at baseline. Patients treated with 372 mcg had the largest mean change in polyp grade in each pivotal clinical trial, with decreases in grade after 16 weeks of 1.1 and 1.4 in NAVIGATE I and NAVIGATE II, respectively. There was also a consistent decrease in average polyp grade over time through 24 weeks.
The following table summarizes the mean change in congestion scores in each of the pivotal clinical trials:
Mean Changes from Baseline in AM Congestion Score After 4 Weeks of Treatment in
Adult Patients with Nasal Polyps
Difference from Placebo EDS | ||||||||||||||
Treatment | N | Baseline Score (Standard Deviation) | Mean (Standard Error) Change from Baseline | Mean | 95% confidence interval | p-value(1) | ||||||||
NAVIGATE I | ||||||||||||||
XHANCE 372 mcg | 79 | 2.29 (0.44) | –0.62 (0.08) | –0.38 | –0.57, –0.19 | <0.001 | ||||||||
XHANCE 186 mcg | 80 | 2.24 (0.42) | –0.54 (0.08) | –0.30 | –0.48, –0.11 | 0.002 | ||||||||
Placebo EDS | 82 | 2.31 (0.41) | –0.24 (0.07) | |||||||||||
NAVIGATE II | ||||||||||||||
XHANCE 372 mcg | 82 | 2.25 (0.42) | –0.62 (0.07) | –0.38 | –0.58, –0.18 | <0.001 | ||||||||
XHANCE 186 mcg | 80 | 2.20 (0.37) | –0.68 (0.07) | –0.45 | –0.65, –0.25 | <0.001 | ||||||||
Placebo EDS | 79 | 2.29 (0.43) | –0.24 (0.07) | |||||||||||
________________________________________________________________________________________________________________________
(1) | The p-value, or probability value, is a measure of statistical significance reflecting the likelihood that an observed result occurred by chance. |
21
The following charts summarize the mean change in bilateral nasal polyps score in each of the pivotal clinical trials:
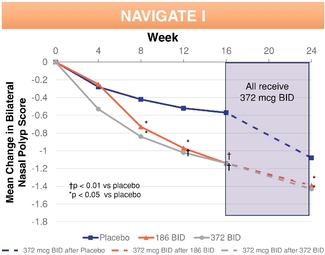
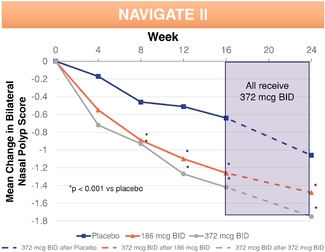
In addition to the co-primary efficacy endpoints described above, we also assessed a number of secondary endpoints in the pivotal clinical trials, including the following:
▪ | Sinonasal Outcome Test-22. In a Sinonasal Outcome Test-22, which broadly assesses the impact of nasal polyps on certain outcomes, including the symptoms of nasal polyps, functioning and quality of life, the change observed with the 186- and 372-mcg doses of XHANCE was superior to the placebo EDS in both of the pivotal clinical trials. The magnitude of improvement associated with treatment with XHANCE was approximately 20 points. |
▪ | Quality of Sleep. A positive impact of XHANCE on sleep was shown for the 372-mcg dose in both pivotal clinical trials through the "Sleep" sub-scale of the Sinonasal Outcome Test-22 and, in NAVIGATE II, a positive effect was further shown across a number of the sub-scales of the MOS-Sleep-R, a validated set of measures commonly used in clinical studies to assess changes in sleep quality. |
▪ | Defining Symptoms. The 186- and 372-mcg treatment groups, in pooled data for NAVIGATE I and NAVIGATE II, achieved statistically significant improvement in all four of the core defining symptoms of nasal polyps at the end of the double-blinded phase. |
▪ | Patient Global Impression of Change. Patient global impression of change is a summary measure of treatment benefit from the perspective of the patient measuring their perception of improvement or worsening of their condition. At the end of the double-blinded phase, the percentage of patients who were improved was substantially higher with XHANCE compared with the placebo EDS. Of the patients receiving 186 or 372 mcg of XHANCE, 86% reported improvement combined across both pivotal clinical trials, and 65.9% reported being "much" or "very much" improved. A post-hoc analysis of a subgroup of patients in the NAVIGATE I and II trials who were using a marketed INS at the time of study entry showed similar results, with 65% of patients treated with 186 or 372 mcg of XHANCE reporting being "much" or "very much improved" after 16 weeks of treatment compared with 28% of patients treated with the placebo EDS. |
▪ | Need for Surgery. Surgical eligibility was assessed using standardized criteria defined prior to trial initiation. Surgery was not necessarily planned or pending for these patients. The proportion of patients considered eligible for surgery among the 186-mcg and 372-mcg dose groups combined across both pivotal trials was reduced by 54% after 16 weeks of treatment with XHANCE versus 36% with the EDS-placebo group and was reduced by approximately 64% after the additional eight weeks of active treatment with the 372-mcg dose. |
▪ | Complete Response Analysis. The polyp grading scale is neither linear nor a direct measure of polyp mass, making it difficult to interpret mean change scores. Therefore, we also performed a complete response analysis to evaluate the percentage of patients who had nasal polyps eliminated on at least one side of the nasal passages. The percentage of patients who had nasal polyps eliminated on at least one side of the nasal passages at the end of the double-blinded phase was 14.1% in the 186- and 372-mcg dose groups combined across both pivotal clinical trials, compared to 7.8% of placebo EDS recipients. By the end of 24 weeks, after all patients received up to an additional eight weeks of active treatment with the 372-mcg dose, the complete response rate was 17.3% in patients previously treated with the placebo EDS compared |
22
to 26.2% in patients who previously received XHANCE across the 186- and 372-mcg dose groups in both pivotal clinical trials.
Safety Results
XHANCE was generally well tolerated across the 186- and 372-mcg dose groups in NAVIGATE I and NAVIGATE II. The most commonly reported AEs in the active treatment groups in the pivotal clinical trials, which are shown in the table below, were associated with local effects at the site of administration in the nasal passages or associated with the underlying disease. Most local AEs were not spontaneously reported but were identified as a result of active monitoring of all patients at scheduled intervals by endoscopic nasal examination at each visit. The majority of these AEs were reported to be mild and were observed to resolve with continued use of XHANCE. A total of six patients in the pivotal clinical trials experienced a total of seven serious adverse events, or SAEs, only one of which, in a patient in the placebo group, was determined to be treatment-related. 5.0% of subjects treated with XHANCE 186 mcg twice daily and 1.2% of subjects treated with 372 mcg twice daily discontinued from the clinical trials prior to the open-label extension phase based of adverse reactions compared to 4.3% of subjects treated with placebo.
Summary of Adverse Events with XHANCE Reported in ≥ 3% of Patients with Nasal Polyps and More Common Than Placebo EDS in Phase 3 Pivotal Clinical Trials
XHANCE | |||
Adverse Event | Placebo EDS (N = 161) n (%) | 186 mcg bid (N = 160) n (%) | 372 mcg bid (N = 161) n (%) |
Epistaxis1 | 4 (2.5) | 19 (11.9) | 16 (9.9) |
Nasopharyngitis | 8 (5.0) | 3 (1.9) | 12 (7.5) |
Nasal septal ulceration2 | 3 (1.9) | 11 (6.9) | 12 (7.5) |
Nasal congestion | 6 (3.7) | 7 (4.4) | 9 (5.6) |
Acute sinusitis | 6 (3.7) | 7 (4.4) | 8 (5.0) |
Headache | 5 (3.1) | 8 (5.0) | 6 (3.7) |
Pharyngitis | 2 (1.2) | 2 (1.3) | 5 (3.1) |
Nasal mucosal ulceration2 | 2 (1.2) | 6 (3.8) | 4 (2.5) |
Nasal mucosal erythema | 6 (3.7) | 9 (5.6) | 8 (5.0) |
Nasal septal erythema | 3 (1.9) | 6 (3.8) | 7 (4.3) |
________________________________________________________________________________________________________________________
bid = twice daily.
N = number of patients; n = number of patients in subset.
1 Includes spontaneous adverse reaction reports.
2 Includes ulcerations and erosions
Phase 3 Open-Label Clinical Trials (EXHANCE-3 and EXHANCE-12)
We also conducted two supportive, open-label Phase 3 clinical trials in adults with symptoms of chronic sinusitis with or without nasal polyps. The supportive clinical trials, which we refer to as EXHANCE-3 and EXHANCE-12, were conducted in the United States with a primary objective to assess the safety of twice-daily intranasal administration of the 372 mcg dose of XHANCE in an expanded number of patients and over an extended period of time. We also assessed a variety of objective and subjective efficacy parameters, including an assessment of each patient's symptoms and functioning and qualification for surgical intervention.
Study Design
Eligibility for enrollment, endpoint and study design were similar in EXHANCE-3 and EXHANCE-12 with the exception of duration (3 months in the case of EXHANCE-3 and 12 months in the case of EXHANCE-12). Across both supportive clinical trials, a total of 898 adults were treated, including 762 adults with chronic sinusitis without nasal polyps and 136 adults with symptoms of chronic sinusitis with nasal polyps.
Safety Results
XHANCE was generally well tolerated. As shown in the table below, 59.2% of patients in the supportive clinical trials experienced at least one treatment-emergent AE, with the most common being similar to those in the XHANCE
23
treatment groups of the pivotal clinical trials. The most common AEs were local (in the nose) and not systemic. Most AEs were mild and resolved with continued use of XHANCE. A total of 12 patients experienced a total of 14 SAEs in the supportive clinical trials, none of which were deemed treatment-related. Approximately 80% of patients completed the supportive clinical trials, with approximately 5% discontinuing due to an AE and 1% discontinuing for lack of efficacy.
Summary of Adverse Events Reported in ≥3% of Patients in EXHANCE 3 AND EXHANCE 12
________________________________________________________________________________________________________________________
Adverse Event | XHANCE 372 mcg (N = 898) n (%) |
Patients with at least 1 Adverse Event | 532 (59.2) |
Epistaxis1 | 73 (8.1) |
Nasal mucosal disorder (erythema or ulceration not at the nasal septum) | 109 (12.1) |
Nasal septum disorder (erythema) | 71 (7.9) |
Nasal septum ulceration | 53 (5.9) |
Acute sinusitis | 48 (5.3) |
Upper respiratory tract infection | 46 (5.1) |
Headache | 44 (4.9) |
Nasal congestion | 34 (3.8) |
Cough | 27 (3.0) |
________________________________________________________________________________________________________________________
1 Includes spontaneous adverse reaction reports.
Efficacy Results
Efficacy was also measured in EXHANCE-3 and EXHANCE-12. Key efficacy results from EXHANCE-3 and EXHANCE-12 included:
▪ | On the Lund-Mackay scale, which is an endoscopic objective assessment of disease in the nasal passages, scores for edema, nasal discharge and nasal polyps decreased through up to 12 months of treatment, with similar benefits observed in patients who did or did not have nasal polyps at baseline. Among those patients entering the clinical trials with endoscopic evidence of edema within the nasal cavity, approximately 35% with polyps and 53% without polyps in EXHANCE-3 and 50% with polyps and 56% without polyps in EXHANCE-12 no longer had observable edema by the end of the study. |
▪ | Patients with nasal polyps experienced improvement in nasal polyp grades. As observed in the pivotal clinical trials, mean nasal polyp grading scale scores improved more with longer durations of treatment. In addition, the percentage of nasal polyp patients with a polyp grade of 0 on at least one side of the nose was 47.1% in EXHANCE-12 and 48.0% in EXHANCE-3 by the end of their participation in the study. |
▪ | Mean total Sinonasal Outcome Test-22 scores improved throughout both supportive clinical trials. After 12 months of treatment with XHANCE in our supportive clinical trials, at least 50% of patients had a score that was at or below 9.3, which is the average score that has been reported for healthy individuals. |
Phase 1 Bioavailability Clinical Trial
We performed a Phase 1, open-label, randomized, single-dose, bioavailability clinical trial of XHANCE and Flonase in healthy patients and XHANCE and Flovent HFA in patients with mild-to-moderate asthma. We conducted the Phase 1 clinical trial to establish a bridge between XHANCE, which consists of our fluticasone propionate formulation combined with an Optinose EDS, and Flonase and Flovent HFA, the reference listed drugs for our NDA. We chose fluticasone propionate in part because it has limited absorption into the body. In our NDA, we relied in part on the FDA's previous findings of safety for Flonase and Flovent HFA, including non-clinical toxicology findings and findings of systemic safety risks related to hypothalamic-pituitary-adrenal, or HPA, axis suppression, which is a known side effect of corticosteroids. To do so, we were required to establish that the systemic exposure, or the
24
amount of drug absorbed into the body, to fluticasone propionate following use of XHANCE did not exceed the exposure produced by Flovent HFA.
Study Design
Part one of the clinical trial was a three-way, three-treatment, three-sequence crossover study in healthy patients in which patients were randomized to a sequence containing the following treatments: 186 mcg (1 × 93 mcg to each nostril) of XHANCE; 372 mcg (2 × 93 mcg to each nostril) of XHANCE; and 400 mcg (4 × 50 mcg to each nostril) of Flonase. The primary objective of part one was to assess and compare the systemic exposure of a single dose of 186 mcg and 372 mcg of XHANCE with 400 mcg of Flonase in healthy patients. If one or both of the test doses resulted in a systemic exposure that was at least 125% of that of Flonase, then part two was to be conducted. Part two of the clinical trial was a two-way, two-treatment, two-sequence crossover study in mild-to-moderate asthmatic patients in which patients were randomized to a sequence containing the following: 372 mcg (4 × 93 mcg) of XHANCE and 440 mcg (2 × 220 mcg) of Flovent HFA. The primary objective of part two was to assess and compare the systemic exposure produced by a single dose of 372 mcg of XHANCE with 440 mcg of Flovent HFA in mild-to-moderate asthmatic patients. A total of 112 adults were examined across both parts of the clinical trial.
Results
In part one of the clinical trial, peak and total exposure to fluticasone propionate was higher following 372 mcg of XHANCE compared to 400 mcg of Flonase. Peak exposure to fluticasone propionate was also higher for 186 mcg of XHANCE than 400 mcg of Flonase, but total exposure was higher for 400 mcg of Flonase than 186 mcg of XHANCE. In part two of the clinical trial, doses of 372 mcg of XHANCE produced systemic exposure substantially lower than that of 440 mcg of Flovent HFA. In particular, peak plasma of the drug, or Cmax, and the total amount of absorption, known as the area under the curve from time 0 to infinity, or AUC0-∞, were approximately 37% and 50% lower following administration of 372 mcg of XHANCE relative to 440 mcg of Flovent HFA, respectively. We believe these results support our use of Flonase and Flovent HFA as referenced listed drugs in our NDA for XHANCE.
Regulatory Exclusivity and Barriers to Entry
XHANCE benefits from substantial intellectual property and regulatory barriers to entry, including the following:
▪ | Strong patent protection. Our XHANCE U.S. patent portfolio consists of nine issued device and method of use patents expiring through 2030, three issued design patents expiring through 2030 and U.S. patent applications that, if granted, would expire through 2034. We rely primarily on the protections afforded by device and method of use patents. Our issued U.S. patents and patent applications for XHANCE are based on an Optinose EDS, including the combination of this technology with fluticasone propionate. |
▪ | Complex drug-delivery system. We believe the unique features of an Optinose EDS, as well as its delivery of a topical-acting corticosteroid, affords us significant protection against generic competition, as well as against a potential 505(b)(2) NDA, that seeks to reference XHANCE in order to obtain approval for a therapeutically equivalent, substitutable competitor product. XHANCE, utilizing our proprietary EDS, presents human factors engineering complexities for drug-device combination products and chemistry, manufacturing and controls challenges unique to suspension and respiratory products. Any future substitutable generic entrant will need to have considerable combination product know-how to develop and validate a substitutable drug delivery device or technology to compete with an Optinose EDS. |
▪ | Clinical and regulatory complexity. We have conducted a clinical development program comprised of over 1,500 patients to support our NDA for XHANCE to treat nasal polyps, including human factors studies and Phase 3 clinical trial assessments evaluating and validating the use of an Optinose EDS. As with other drugs that primarily have local activity, we believe the regulatory pathway for products seeking approval as substitutable generic equivalents to XHANCE will be more complex and costly than the pharmacokinetic studies generally required for systemically-acting medications. We believe current FDA guidance for substitutable INS generally requires the demonstration of "clinical bioequivalence," which has caused developers to conduct non-inferiority clinical trials. Clinical trials in nasal polyps are different from those that have been performed to support approval of generic INS for allergic rhinitis. We believe potential generic competitors to XHANCE must not only demonstrate efficacy versus placebo, but must also show equivalent efficacy and safety outcomes to establish clinical bioequivalence to XHANCE, requiring a significant amount of time and capital investment and presenting clinical development uncertainties. |
25
▪ | Three-year regulatory exclusivity. The FDA has granted XHANCE a three-year period of regulatory exclusivity that will end in September 2020. This exclusivity means that we are afforded at least three years in which to market our product free of generic or 505(b)(2) competition post-NDA approval. |
Intellectual Property
We strive to protect our proprietary technology that we believe is important to our business, including seeking and maintaining patents intended to cover our product candidates and technologies that are important to the development of our business. We also rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection, as well as know-how, trademarks, continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position. We internally developed our intellectual property related to the Optinose EDS, AVP-825, XHANCE and our product candidates. We have sought and intend to continue to seek appropriate patent protection for our product candidates, as well as other proprietary technologies and their uses by filing patent applications in the United States and selected other countries.
Patents
As of December 31, 2017, we owned a total of 43 U.S. patents and 43 pending U.S. patent applications. These U.S. patents will expire between 2020 and 2030. These U.S. patent applications, subject to issuance, would be projected to expire between 2020 and 2035, with potential patent term adjustments that would extend the patent term. In addition to our U.S. intellectual property, we also own 213 foreign issued patents, which will expire between 2020 and 2033 and 117 foreign patent applications, which will expire between 2020 and 2035, subject to issuance.
Our XHANCE U.S. patent portfolio consists of 9 issued device and method of use patents, three issued design patents and 12 pending patent applications. The 9 device and method of use patents expire between 2020 and 2030, the three design patents expire between 2029 and 2030 and the 12 pending patent applications would be projected to expire, subject to issuance, between 2022 and 2034, with potential patent term adjustments that would extend the patent term. The 9 device and method of use patents are published in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an abbreviated NDA, or ANDA, or a 505(b)(2) NDA. If any of these potential generic competitors claim that their product will not infringe XHANCE's listed patents, or that such patents are invalid, then they must send notice to us once the ANDA or 505(b)(2) NDA has been accepted for filing by the FDA. We may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification, which would automatically prevent the FDA from approving the ANDA or 505(b)(2) NDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit, or a decision in the infringement case that is favorable to the ANDA or 505(b)(2) NDA applicant.
The rest of our patent portfolio largely relates to patents and applications owned by us and directed to AVP-825 and other product candidates, including OPN-300 and OPN-021.
Trade Secrets and Other Proprietary Information
We seek to protect our proprietary information, including our trade secrets and proprietary know-how, by requiring our employees, consultants and other advisors to execute confidentiality agreements upon the commencement of their employment or engagement. These agreements generally provide that all confidential information developed or made known during the course of the relationship with us be kept confidential and not be disclosed to third parties except in specific circumstances. In the case of our employees, the agreements also typically provide that all inventions resulting from work performed for us, utilizing our property or relating to our business and conceived or completed during employment shall be our exclusive property to the extent permitted by law. Where appropriate, agreements we obtain with our consultants also typically contain similar assignment of invention provisions. Further, we generally require confidentiality agreements from business partners and other third parties that receive our confidential information.
Trademarks
We also rely on trademarks and trade designs to develop and maintain our competitive position. OPTINOSE®, XHANCETM and Breath Powered® are trademarks or registered trademarks of ours in the United States.
AVP-825 License Agreement
In July 2013, we, through our wholly-owned subsidiary, OptiNose AS, entered into a license agreement, or the AVP-825 License Agreement, with Avanir pursuant to which we granted an exclusive license to Avanir to further
26
develop and commercialize AVP-825, a combination of an Optinose EDS with a lose-dose sumatriptan powder, for the acute treatment of migraines in adults, in the United States, Canada and Mexico, which we refer to collectively as the Licensed Territory. AVP-825 was approved by the FDA in January 2016 for the acute treatment of migraines in adults and became commercially available in May 2016 under the brand name Onzetra Xsail.
We have received $70.0 million in aggregate licensing revenues to date, consisting of an up-front fee of $20.0 million received in 2013, a $2.5 million payment received in June 2014 upon the achievement of a development milestone and a $47.5 million payment received in February 2016 upon FDA approval of AVP-825. We are eligible to receive up to an additional $50.0 million upon the achievement of annual sales milestones and tiered low double-digit royalty payments once and if net sales of the product exceed a specified cumulative threshold.
Unless earlier terminated in accordance its terms, the AVP-825 License Agreement, including the royalty payments, will remain in effect on a country-by-country basis in the Licensed Territory until the commercial launch of a generic product in such country, at which time the AVP-825 License Agreement, including the royalty payments, will expire as to that particular country. In the United States, which to date is the only jurisdiction in the Licensed Territory in which AVP-825 has been approved for marketing, the commercial launch of a generic version of AVP-825 can occur as soon as the FDA grants marketing approval to a product as a generic to AVP-825. Several patents with respect to AVP-825 are published in the Orange Book, with the last patent expiring in December 2030. A sponsor of a generic version of AVP-825 must use AVP-825 as a reference listed drug in its ANDA, thereby requiring the sponsor to make one of several certifications regarding each AVP-825 patent listed in the Orange Book. A "Paragraph III" certification is the sponsor's statement that it will wait for the applicable patent to expire before obtaining approval for its product. A "Paragraph IV" certification is an assertion that the applicable patent does not block approval of the generic product, either because the patent is invalid or unenforceable or because the patent, even if valid, is not infringed by the generic product. If a sponsor of a generic version of AVP-825 files a "Paragraph III" certification with respect to each of the AVP-825 patents listed in the Orange Book, then the earliest the FDA will grant marketing approval to the generic product is December 2030. If, however, a sponsor of a generic version of AVP-825 files a "Paragraph IV" certification challenging AVP-825's Orange Book-listed patents, then, within 20 days of the FDA accepting the filing, the sponsor must provide notice to us and Avanir that an ANDA has been filed with the FDA, and provide the factual and legal basis for the sponsor's assertion that the patent is invalid or not infringed. If we or Avanir file suit against the applicant for patent infringement within 45 days of receiving the Paragraph IV notice, the FDA is prohibited from approving the ANDA application for a period of 30 months or the resolution of the underlying suit, whichever is earlier. The FDA may approve the proposed product before the expiration of the 30-month stay if a court finds the patent invalid or not infringed or if the court shortens the period because the parties have failed to cooperate in expediting the litigation. If the sponsor is unsuccessful in its defense of non-infringement or unable to prove invalidity of the listed patents, the court could issue an injunction prohibiting the launch of the generic version of AVP-825 until the last patent expires in December 2030.
Avanir may terminate the AVP-825 License Agreement in its sole discretion upon prior written notice to us as described in the agreement. We may terminate the AVP-825 License Agreement if Avanir commences any legal or administrative proceeding to revoke or challenge the validity of certain of the intellectual property we licensed to Avanir pursuant to the AVP-825 License Agreement. In addition, the AVP-825 License Agreement provides for customary termination rights in the event of a breach of the AVP-825 License Agreement by the other party.
Manufacturing and Distribution
Manufacturing
As of February 2018, our third-party manufacturers have met our manufacturing requirements for the initial commercial supply of XHANCE and for the XHANCE Xperience program.
We currently contract with third parties for the manufacture, testing and storage of our product candidates. In our experience, contract manufacturers, or CMOs, are generally cost-efficient and reliable and therefore we currently have no plans to build our own clinical or commercial manufacturing capabilities. Because we rely on CMOs, we employ personnel with extensive technical, manufacturing, analytical and quality experience to oversee contract manufacturing and testing activities, and to compile manufacturing and quality information for our regulatory submissions. Manufacturing is subject to extensive regulations that impose various procedural and documentation requirements, and which govern record-keeping, manufacturing processes and controls, personnel, quality control and quality assurance, among other activities. Our systems and our contractors are required to comply with these regulations, and we assess this compliance regularly through monitoring of performance and a formal audit program.
27
We have entered into the following key supply agreements for the commercial manufacture and supply of XHANCE:
▪ | In July 2017, we entered into a supply agreement with Hovione Inter Ltd, or Hovione, for the supply of fluticasone propionate, the active pharmaceutical ingredient included in the liquid suspension formulation. This agreement has a term of five years from commercial launch of XHANCE, subject to earlier termination or extension in accordance with the terms of the agreement. Either we or Hovione may terminate the agreement prior to that date for uncured material breach or insolvency of the other party. We may also terminate the agreement in the event Hovione, among other things, (i) loses any required FDA approval rendering it unable to fulfill its contractual obligations, (ii) is engaged in felonious or fraudulent activities or (iii) does not submit a Corrective and Preventive Action plan to the FDA within a specified period of time of being notified of deficiencies in Hovione's facility. |
▪ | In August 2017, we entered into a manufacture and supply agreement with Contract Pharmaceuticals Limited Canada, or CPL, for the formulation and assembly of the finished drug product during the fill/pack operation. This agreement has a term of five years from the date on which we provide a purchase order for validation batches to CPL, subject to earlier termination or extension in accordance with the terms of the agreement. Either we or CPL may terminate the agreement prior to that date by mutual consent or for uncured material breach by or insolvency of the other party. We may also terminate the agreement if, among other things, any intellectual property of any third party is reasonably alleged by a third party to be infringed, misappropriated or otherwise violated by the manufacture, import, use, sale or distribution of XHANCE or if any regulatory authority requires us to cease production of the sale of XHANCE. |
▪ | In August 2017, we entered into a manufacturing services agreement with Ximedica, LLC for the manufacture of the liquid delivery sub-assembly, which consists of injection molded parts and other purchased components. This agreement has a term of two years from September 18, 2017, subject to earlier termination in accordance with the terms of the agreement. Either we or Ximedica may terminate the agreement prior to that date for uncured material breach or insolvency of the other party. We may also terminate the agreement for any reason upon prior written notice or if Ximedica (i) fails an inspection or suffers a disciplinary action by a governmental authority and fails to cure such issue within a specified period of time or (ii) fails to gain recommendation for approval by the FDA to manufacture the liquid delivery subassembly component to be manufactured pursuant to the agreement. |
We believe our third-party manufacturers have adequate capacity to manufacture sufficient quantities of XHANCE to meet anticipated commercial demands.
Distribution
We have established a distribution channel in the United States for the commercialization of XHANCE. We will be selling XHANCE to wholesale pharmaceutical distributors, who, in turn, will sell XHANCE to pharmacies, hospitals and other customers. We have also established a full-service pharmacy channel to whom we will sell XHANCE, who, in turn, will sell XHANCE directly to patients. We have contracted with a third-party logistics provider for key services related to logistics, warehousing and inventory management, and distribution. Further, our third-party logistics provider will provide customer order fulfillment services and accounts receivable management.
The initial supply of XHANCE necessary for our XHANCE Xperience program has been manufactured and delivered to a full-service pharmacy which coordinates fulfillment, shipping product directly to patients. XHANCE has already been provided to initial patients in this program. Our initial commercial supply of XHANCE has been delivered to our third-party logistics provider and we expect XHANCE to be in retail distribution channels by late March 2018 so that it is available in retail pharmacies in early April 2018.
Competition
Our industry is highly competitive and subject to rapid and significant technological change as research provides a deeper understanding of the pathology of diseases and new technologies and treatments are developed. We believe our scientific knowledge, technology, and development capabilities provide us with substantial competitive advantages, but we face potential competition from multiple sources, including large pharmaceutical, biotechnology, specialty pharmaceutical and, to a lesser degree, medical device companies.
XHANCE will compete primarily with INS, oral steroids and other medical management products, including locally compounded liquid budesonide in high-volume nasal rinses. XHANCE will also compete with surgical procedures, balloon sinus dilation products and steroid-releasing sinus implants. Key competitive factors affecting the
28
commercial success of XHANCE and any other product candidates we may develop are likely to be efficacy, safety and tolerability profile, reliability, convenience of administration, price and reimbursement.
The only other branded INS on the market indicated for the treatment of nasal polyps is Nasonex, which is marketed by Merck & Co., Inc. A generic version of Nasonex, mometasone furoate monohydrate, was approved by the FDA for, among other indications, the treatment of nasal polyps and launched in 2016. In addition, Beconase AQ, which is an INS marketed by GlaxoSmithKline, is indicated for the prophylaxis of nasal polyps after surgical resection. There are no products approved for the treatment of chronic sinusitis without nasal polyps. There are two categories of INS: first-generation INS products, which include Rhinocort, Nasacort AQ and Qnasl; and second-generation INS products, which include Flonase, Veramyst, Omnaris and Zetonna. The primary difference between first- and second-generation INS products is that first-generation INS are absorbed into the blood to a greater extent than second-generation INS, with systemic bioavailability ranging from 10% to 50% compared to a systemic bioavailability with fluticasone propionate, a second-generation INS, of less than 2%. Many of the most widely-prescribed INS products are available in generic form and some, such as Flonase (which contains the same active pharmaceutical ingredient as XHANCE), are available over-the-counter.
Several companies are also currently developing biologic monoclonal antibodies for the treatment of nasal polyps. These biologic monoclonal antibodies, which inhibit specific pathways of inflammation present in nasal polyps, include omalizumab, reslizumab, mepolizumab and dupilumab. Omalizumab has been studied in investigator-initiated Phase 2 clinical trials. GlaxoSmithKline has studied mepolizumab in a sponsor-initiated Phase 2 clinical trial and has initiated patient enrollment in a Phase 3 clinical trial with study completion anticipated in 2019. Dupilumab has been studied in a sponsor-initiated Phase 2 clinical trial and Sanofi is currently investigating it in two Phase 3 clinical trials that is expected to be completed in the second half of 2018. If these biologic monoclonal antibodies are successfully developed and approved for marketing, they could represent significant competition for XHANCE.
Research and Development
We expense research and development costs as they are incurred. For the years ended December 31, 2017, 2016 and 2015, we incurred total research and development expenses of approximately $16.8 million, $15.3 million, and $22.2 million respectively.
Government Regulation
As a pharmaceutical company that operates in the United States, we are subject to extensive regulation by the FDA and other federal, state, and local regulatory agencies. The Federal Food, Drug and Cosmetic Act, or the FD&C Act, and FDA's implementing regulations set forth, among other things, requirements for the testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record-keeping, reporting, distribution, import, export, advertising and promotion of our product candidates. Although the discussion below focuses on regulation in the United States, because that is currently our primary focus, we anticipate seeking approval for, and marketing, our products in other countries in the future. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the United States, although there can be important differences.
Development and Approval
Under the FD&C Act, FDA approval of an NDA is required before any new drug can be marketed in the United States. NDAs require extensive studies and submission of a large amount of data by the applicant.
Preclinical Testing. Before testing any compound in human patients in the United States, a company must generate extensive preclinical data. Preclinical testing generally includes laboratory evaluation of product chemistry and formulation, as well as toxicological and pharmacological studies in several animal species to assess the quality and safety of the product. Certain animal studies must be performed in compliance with the FDA's Good Laboratory Practice, or GLP, regulations and the U.S. Department of Agriculture's Animal Welfare Act.
IND Application. Human clinical trials in the United States cannot commence until an investigational new drug, or IND, application is submitted and becomes effective. A company must submit preclinical testing results to the FDA as part of the IND, and the FDA must evaluate whether there is an adequate basis for testing the drug in initial clinical studies in human volunteers. Unless the FDA raises concerns, the IND becomes effective 30 days following its receipt by the FDA. Once human clinical trials have commenced, the FDA may stop a clinical trial by placing it on "clinical hold" because of concerns about the safety of the product being tested, or for other reasons.
29
Clinical Trials. Clinical trials involve the administration of a drug to healthy human volunteers or to patients, under the supervision of a qualified investigator. The conduct of clinical trials is subject to extensive regulation, including compliance with the FDA's bioresearch monitoring regulations and Good Clinical Practice, or GCP, requirements, which establish standards for conducting, recording data from, and reporting the results of, clinical trials, and are intended to assure that the data and reported results are credible and accurate, and that the rights, safety, and well-being of study participants are protected. Clinical trials must be conducted under protocols that detail the study objectives, parameters for monitoring safety, and the efficacy criteria, if any, to be evaluated. Each protocol is reviewed by the FDA as part of the IND. In addition, each clinical trial must be reviewed and approved by, and conducted under the auspices of, an Institutional Review Board, or IRB. Companies sponsoring the clinical trials, investigators, and IRBs also must comply with, as applicable, regulations and guidelines for obtaining informed consent from the study patients, following the protocol and investigational plan, adequately monitoring the clinical trial, and timely reporting of AEs. Foreign studies conducted under an IND must meet the same requirements that apply to studies being conducted in the United States. Data from a foreign study not conducted under an IND may be submitted in support of an NDA if the study was conducted in accordance with GCP and the FDA is able to validate the data.
A study sponsor is required to publicly post specified details about certain clinical trials and clinical trial results on government or independent websites (e.g., http://clinicaltrials.gov). Human clinical trials typically are conducted in three sequential phases, although the phases may overlap with one another:
▪ | Phase 1 clinical trials involve the initial administration of the investigational drug to humans, typically to a small group of healthy human patients, but occasionally to a group of patients with the targeted disease or disorder. Phase 1 clinical trials generally are intended to determine the metabolism and pharmacologic actions of the drug, the side effects associated with increasing doses, and, if possible, to gain early evidence of effectiveness. |
▪ | Phase 2 clinical trials generally are controlled studies that involve a relatively small sample of the intended patient population, and are designed to develop initial data regarding the product's effectiveness, to determine dose response and the optimal dose range, and to gather additional information relating to safety and potential AEs. |
▪ | Phase 3 clinical trials are conducted after preliminary evidence of effectiveness has been obtained, and are intended to gather the additional information about safety and effectiveness necessary to evaluate the drug's overall risk-benefit profile, and to provide a basis for physician labeling. Generally, Phase 3 clinical development programs consist of expanded, large-scale studies of patients with the target disease or disorder to obtain statistical evidence of the efficacy and safety of the drug at the proposed dosing regimen. |
The sponsoring company, the FDA, or the IRB may suspend or terminate a clinical trial at any time on various grounds, including a finding that the patients are being exposed to an unacceptable health risk. Further, success in early-stage clinical trials does not assure success in later-stage clinical trials. Data obtained from clinical activities are not always conclusive and may be subject to alternative interpretations that could delay, limit or prevent regulatory approval.
NDA Submission and Review. The FD&C Act provides two pathways for the approval of new drugs through an NDA. An NDA under Section 505(b)(1) of the FD&C Act is a comprehensive application to support approval of a product candidate that includes, among other things, data and information to demonstrate that the proposed drug is safe and effective for its proposed uses, that production methods are adequate to ensure its identity, strength, quality, and purity of the drug, and that proposed labeling is appropriate and contains all necessary information. A 505(b)(1) NDA contains results of the full set of preclinical studies and clinical trials conducted by or on behalf of the applicant to characterize and evaluate the product candidate.
Section 505(b)(2) of the FD&C Act provides an alternate regulatory pathway to obtain FDA approval for new formulations or new uses of previously approved drug products. Specifically, Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The applicant may rely to some extent upon the FDA's findings of safety and effectiveness for an approved product that acts as the reference listed drug, or RLD, and submit its own product-specific data — which may include data from preclinical studies or clinical trials conducted by or on behalf of the applicant — to address differences between the product candidate and the RLD. We obtained FDA approval of XHANCE through the Section 505(b)(2) regulatory approval pathway, with Flonase and Flovent HFA as the RLDs. Flonase and Flovent HFA contain fluticasone propionate, which is also used in XHANCE.
30
The submission of an NDA under either Section 505(b)(1) or Section 505(b)(2) generally requires payment of a substantial user fee to the FDA. The FDA reviews applications to determine, among other things, whether a product is safe and effective for its intended use and whether the manufacturing controls are adequate to assure and preserve the product's identity, strength, quality, and purity. For some NDAs, the FDA may convene an advisory committee to seek insights and recommendations on issues relevant to approval of the application. Although the FDA is not bound by the recommendation of an advisory committee, the agency usually has followed such recommendations.
Our product and product candidates include products that combine drug and device components in a manner that the FDA considers to meet the definition of a "combination product" under FDA regulations. The FDA exercises significant discretion over the regulation of combination products, including the discretion to require separate marketing applications for the drug and device components in a combination product. For XHANCE, FDA's Center for Drug Evaluation and Research, or CDER, had primary jurisdiction for review of the NDA, and both the drug and device were reviewed under one marketing application. However, for a drug-device combination product CDER typically consults with the Center for Devices and Radiological Health in the NDA review process.
The FDA may determine that a Risk Evaluation and Mitigation Strategy, or REMS, is necessary to ensure that the benefits of a new product outweigh its risks, and the product can therefore be approved. A REMS may include various elements, ranging from a medication guide or patient package insert to limitations on who may prescribe or dispense the drug, depending on what the FDA considers necessary for the safe use of the drug. Under the Pediatric Research Equity Act, certain applications for approval must also include an assessment, generally based on clinical study data, of the safety and effectiveness of the subject drug in relevant pediatric populations. Before approving an NDA, the FDA will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with current Good Manufacturing Practice, or cGMP, requirements and adequate to assure consistent production of the product within required specifications.
Once an NDA submission has been accepted for filing — which occurs, if at all, within 60 days after submission of the NDA — the FDA's goal for a non-priority review of an NDA is ten months. The review process can be and often is significantly extended, however, by FDA requests for additional information, studies, or clarification. After review of an NDA, the FDA may decide to not approve the application or may issue a complete response letter outlining the deficiencies in the submission. The complete response letter also may request additional information, including additional preclinical or clinical data. Even if such additional information and data are submitted, the FDA may decide that the NDA still does not meet the standards for approval. Data from clinical trials are not always conclusive and the FDA may interpret data differently than the sponsor.
Obtaining regulatory approval often takes a number of years, involves the expenditure of substantial resources, and depends on a number of factors, including the severity of the disease in question, the availability of alternative treatments, and the risks and benefits demonstrated in clinical trials. Additionally, as a condition of approval, the FDA may impose restrictions that could affect the commercial success of a drug or require post-approval commitments, including the completion within a specified time period of additional clinical studies, which often are referred to as "Phase 4" or "post-marketing" studies. For example, the FDA originally required us to conduct a randomized, double-blind, placebo controlled, parallel group clinical study in children and adolescents 6 to 17 years of age with bilateral nasal polyps associated with nasal congestion to assess the safety, efficacy, pharmacokinetics, and pharmacodynamics of XHANCE in improving nasal polyp grade and symptoms (nasal congestion/obstruction, sense of smell, rhinorrhea and facial pain or pressure). On October 30, 2017, the FDA notified us that in response to our request it had modified the required age range to 12 to 17 years of age. We submitted our final protocol to the FDA with respect to the pediatric study by January 2018 as required, and we are required to complete the study by January 2022 and submit a final report with respect to the study by July 2022.
Post-approval modifications to the drug, such as changes in indications, labeling, or manufacturing processes or facilities, may require a sponsor to develop additional data or conduct additional preclinical studies or clinical trials, to be submitted in a new or supplemental NDA, which would require FDA approval.
Post-Approval Regulation
Once approved, products are subject to continuing regulation by the FDA. If ongoing regulatory requirements are not met or if safety problems occur after the product reaches the market, the FDA may at any time withdraw product approval or take actions that would limit or suspend marketing. Additionally, the FDA may require post-marketing studies or clinical trials if new safety information develops.
31
Good Manufacturing Practices. Companies engaged in manufacturing drug products or their components must comply with applicable cGMP requirements and product-specific regulations enforced by the FDA and other regulatory agencies. Compliance with cGMP includes adhering to requirements relating to organization and training of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, quality control and quality assurance, packaging and labeling controls, holding and distribution, laboratory controls, and records and reports. The FDA regulates and inspects equipment, facilities, and processes used in manufacturing pharmaceutical products prior to approval. If, after receiving approval, a company makes a material change in manufacturing equipment, location, or process (all of which are, to some degree, incorporated in the NDA), additional regulatory review and approval may be required. The FDA also conducts regular, periodic visits to re-inspect equipment, facilities, and processes following the initial approval of a product. Failure to comply with applicable cGMP requirements and conditions of product approval may lead the FDA to seek sanctions, including fines, civil penalties, injunctions, suspension of manufacturing operations, operating restrictions, withdrawal of FDA approval, seizure or recall of products, and criminal prosecution. Although we periodically monitor the FDA compliance of our third-party manufacturers, we cannot be certain that our present or future third-party manufacturers will consistently comply with cGMP and other applicable FDA regulatory requirements.
It is also likely that we will need to comply with some of FDA's manufacturing regulations for devices. FDA has discretion in determining post-approval compliance requirements for products that combine a drug substance with a delivery system device. In addition to cGMP, FDA may require that our drug-device combination product, if approved, comply with the Quality System Regulation, or QSR, which sets forth the FDA's manufacturing quality standards for medical devices.
Advertising and Promotion. The FDA and other federal regulatory agencies closely regulate the marketing and promotion of drugs through, among other things, standards and regulations for direct-to-consumer advertising, advertising and promotion to healthcare professionals, communications regarding unapproved uses, industry-sponsored scientific and educational activities, and promotional activities involving the Internet. A product cannot be commercially promoted before it is approved. After approval, product promotion can include only those claims relating to safety and effectiveness that are consistent with the labeling approved by the FDA. Healthcare providers are permitted to prescribe drugs for "off-label" uses — that is, uses not approved by the FDA and not described in the product's labeling — because the FDA does not regulate the practice of medicine. However, FDA regulations impose restrictions on manufacturers' communications regarding off-label uses. Broadly speaking, a manufacturer may not promote a drug for off-label use, but under certain conditions may engage in non-promotional, balanced, scientific communication regarding off-label use. Failure to comply with applicable FDA requirements and restrictions in this area may subject a company to adverse publicity and enforcement action by the FDA, the Department of Justice, or the Office of the Inspector General of the Department of Health and Human Services, as well as state authorities. This could subject a company to a range of penalties that could have a significant commercial impact, including civil and criminal fines and agreements that materially restrict the manner in which a company promotes or distributes a drug.
Other Requirements. NDA holders must comply with other regulatory requirements, including submitting annual reports, reporting information about adverse drug experiences, and maintaining certain records.
Hatch-Waxman Act
The Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, establishes two abbreviated approval pathways for pharmaceutical products that are in some way follow-on versions of already approved products.
Generic Drugs. A generic version of an approved drug is approved by means of an ANDA, by which the sponsor demonstrates that the proposed product is the same as the approved, brand-name drug, which is referred to as the RLD. Generally, an ANDA must contain data and information showing that the proposed generic product and RLD (i) have the same active ingredient, in the same strength and dosage form, to be delivered via the same route of administration, (ii) are intended for the same uses, and (iii) are bioequivalent. This is instead of independently demonstrating the proposed product's safety and effectiveness, which are inferred from the fact that the product is the same as the RLD, which the FDA previously found to be safe and effective.
505(b)(2) NDAs. As discussed above, if a product is similar, but not identical, to an already approved product, it may be submitted for approval via an NDA under section 505(b)(2) of the FD&C Act. Unlike an ANDA, this does not excuse the sponsor from demonstrating the proposed product's safety and effectiveness. Rather, the sponsor is permitted to rely to some degree on the FDA's finding that the RLD is safe and effective, and must submit its own
32
product-specific data of safety and effectiveness to an extent necessary because of the differences between the products. An NDA approved under 505(b)(2) may in turn serve as an RLD for subsequent applications from other sponsors.
RLD Patents. In an NDA, a sponsor must identify patents that claim the drug substance or drug product or a method of using the drug. When the drug is approved, those patents are among the information about the product that is listed in the FDA publication, Approved Drug Products with Therapeutic Equivalence Evaluations, which is referred to as the Orange Book. The sponsor of an ANDA or 505(b)(2) application seeking to rely on an approved product as the RLD must make one of several certifications regarding each listed patent. A "Paragraph III" certification is the sponsor's statement that it will wait for the patent to expire before obtaining approval for its product. A "Paragraph IV" certification is an assertion that the patent does not block approval of the later product, either because the patent is invalid or unenforceable or because the patent, even if valid, is not infringed by the new product.
Regulatory Exclusivities. The Hatch-Waxman Act provides periods of regulatory exclusivity for products that would serve as RLDs for an ANDA or 505(b)(2) application. If a product is a "new chemical entity," or NCE — generally meaning that the active moiety has never before been approved in any drug — there is a period of five years from the product's approval during which the FDA may not accept for filing any ANDA or 505(b)(2) application for a drug with the same active moiety. An ANDA or 505(b)(2) application may be submitted after four years, however, if the sponsor of the application makes a Paragraph IV certification.
A product that is not an NCE may qualify for a three-year period of exclusivity if the NDA contains new clinical data, derived from studies conducted by or for the sponsor, that were necessary for approval. In that instance, the exclusivity period does not preclude filing or review of an ANDA or 505(b)(2) application; rather, the FDA is precluded from granting final approval to the ANDA or 505(b)(2) application until three years after approval of the RLD. Additionally, the exclusivity applies only to the conditions of approval that required submission of the clinical data.
Once the FDA accepts for filing an ANDA or 505(b)(2) application containing a Paragraph IV certification, the applicant must within 20 days provide notice to the RLD NDA holder and patent owner that the application has been submitted, and provide the factual and legal basis for the applicant's assertion that the patent is invalid or not infringed. If the NDA holder or patent owner files suit against the ANDA or 505(b)(2) applicant for patent infringement within 45 days of receiving the Paragraph IV notice, the FDA is prohibited from approving the ANDA or 505(b)(2) application for a period of 30 months or the resolution of the underlying suit, whichever is earlier. If the RLD has NCE exclusivity and the notice is given and suit filed during the fifth year of exclusivity, the 30-month stay does not begin until five years after the RLD approval. The FDA may approve the proposed product before the expiration of the 30-month stay if a court finds the patent invalid or not infringed or if the court shortens the period because the parties have failed to cooperate in expediting the litigation.
Patent Term Restoration. A portion of the patent term lost during product development and FDA review of an NDA is restored if approval of the application is the first permitted commercial marketing of a drug containing the active ingredient. The patent term restoration period is generally one-half the time between the effective date of the IND or the date of patent grant (whichever is later) and the date of submission of the NDA, plus the time between the date of submission of the NDA and the date of FDA approval of the product. The maximum period of restoration is five years, and the patent cannot be extended to more than 14 years from the date of FDA approval of the product. Only one patent claiming each approved product is eligible for restoration and the patent holder must apply for restoration within 60 days of approval. The U.S. Patent and Trademark Office, or PTO, in consultation with the FDA, reviews and approves the application for patent term restoration. When any of our products is approved, we intend to seek patent term restoration for an applicable patent when it is appropriate.
Other Exclusivities
Pediatric Exclusivity. Section 505A of the FD&C Act provides for six months of additional exclusivity or patent protection if an NDA sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The data does not need to show that the product is effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA's request, the additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by FDA within the statutory time limits, whatever statutory or regulatory periods of exclusivity or Orange Book listed patent protection that cover the drug are extended by six months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot approve an ANDA or 505(b)(2) application owing to regulatory exclusivity or listed patents. When any product is approved, we will evaluate seeking pediatric exclusivity as appropriate.
33
Orphan Drug Exclusivity. The Orphan Drug Act provides incentives for the development of drugs intended to treat rare diseases or conditions, which generally are diseases or conditions affecting less than 200,000 individuals in the United States. If a sponsor demonstrates that a drug is intended to treat a rare disease or condition, the FDA grants orphan drug designation to the product for that use. The benefits of orphan drug designation include research and development tax credits and exemption from user fees. A drug that is approved for the orphan drug designated indication generally is granted seven years of orphan drug exclusivity. During that period, the FDA generally may not approve any other application for the same product for the same indication, although there are exceptions, most notably when the later product is shown to be clinically superior to the product with exclusivity. We may seek orphan drug designation and exclusivity for OPN-300, which we are developing for the treatment of Prader-Willi syndrome and autism spectrum disorder.
U.S. Healthcare Reform
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, which we refer to together as the Affordable Care Act, is a sweeping measure intended to expand healthcare coverage within the United States, primarily through the imposition of health insurance mandates on employers and individuals and expansion of the Medicaid program. This law substantially changes the way healthcare is financed by both governmental and private insurers and significantly impacts the pharmaceutical industry. Changes that may affect our business include those governing enrollment in federal healthcare programs, reimbursement changes, benefits for patients within a coverage gap in the Medicare Part D prescription drug program (commonly known as the "donut hole"), rules regarding prescription drug benefits under the health insurance exchanges, changes to the Medicaid Drug Rebate program, expansion of the Public Health Service's 340B drug pricing discount program, or 340B program, fraud and abuse, and enforcement. These changes impact existing government healthcare programs and are resulting in the development of new programs, including Medicare payment for performance initiatives and improvements to the physician quality reporting system and feedback program. Details of the changes to the Medicaid Drug Rebate program and the 340B program are discussed under the risk factor "If we are able to successfully commercialize XHANCE and if we participate in but fail to comply with our reporting and payment obligations under the Medicaid drug rebate program, or other governmental pricing programs, we could be subject to additional reimbursement requirements, penalties, sanctions and fines which could have a material adverse effect on our business, financial condition, results of operations and growth prospects" in the "Risk Factors" section of this 10-K.
Some states have elected not to expand their Medicaid programs to individuals with an income of up to 133% of the federal poverty level, as is permitted under the Affordable Care Act. For each state that does not choose to expand its Medicaid program, there may be fewer insured patients overall, which could impact our sales of products for which we receive regulatory approval, business and financial condition. Where new patients receive insurance coverage under any of the new Medicaid options made available through the Affordable Care Act, the possibility exists that manufacturers may be required to pay Medicaid rebates on drugs used under these circumstances, a decision that could impact manufacturer revenues. In addition, the federal government has announced delays in the implementation of key provisions of the Affordable Care Act.
Certain legislative changes to and regulatory changes under the Affordable Care Act have occurred in the 115th U.S. Congress and under the Trump Administration. For example, the Tax Cuts and Jobs Act enacted on December 22, 2017, eliminated the shared responsibility payment for individuals who fail to maintain minimum essential coverage under section 5000A of the Internal Revenue Code of 1986, commonly referred to as the individual mandate, beginning in 2019. In addition, the Bipartisan Budget Act of 2018 increased the Affordable Care Act required manufacturer point-of-sale discount from 50% to 70% off the negotiated price for Medicare Part D beneficiaries during their coverage gap period beginning in 2019. Additional legislative changes to and regulatory changed under the Affordable Care Act remain possible.
We expect that the Affordable Care Act, as currently enacted or as it may be amended or replaced in the future, and other healthcare reform measures that may be adopted in the future could have a material adverse effect on our industry generally and on our ability to maintain or increase sales of products for which we receive regulatory approval or to successfully commercialize our product candidates, if approved.
U.S. Tax Reform - 2017 U.S. Tax Reform
On December 22, 2017, the United States enacted major tax reform legislation, Public Law No. 115-97, commonly referred to as the Tax Cuts and Jobs Act (2017 Tax Act). The 2017 Tax Act imposes a repatriation tax on accumulated earnings of foreign subsidiaries, implements a territorial tax system together with a current tax on certain foreign earnings and lowers the general corporate income tax rate to 21%.
34
Coverage and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any products for which we may obtain regulatory approval. Sales of any of our products will depend, in part, on the extent to which the costs of the products will be covered by third-party payors, including government healthcare programs such as Medicare and Medicaid, and private payors, such as commercial health insurers and managed care organizations. Third-party payors determine which drugs they will cover and the amount of reimbursement they will provide for a covered drug. In the U.S., there is no uniform system among payors for making coverage and reimbursement decisions. In addition, the process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the FDA-approved products for a particular indication.
In order to secure coverage and reimbursement for our products we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costly studies required to obtain FDA or other comparable regulatory approvals. Even if we conduct pharmacoeconomic studies, our products and product candidates may not be considered medically necessary or cost-effective by payors. Further, a payor's decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved.
In the past, payors have implemented reimbursement metrics and periodically revised those metrics as well as the methodologies used as the basis for reimbursement rates, such as average sales price, or ASP, average manufacturer price, or AMP, and actual acquisition cost. The existing data for reimbursement based on these metrics is relatively limited, although certain states have begun to survey acquisition cost data for the purpose of setting Medicaid reimbursement rates. The Centers for Medicare and Medicaid Services, or CMS, surveys and publishes retail pharmacy acquisition cost information in the form of National Average Drug Acquisition Cost, or NADAC, files to provide state Medicaid agencies with a basis of comparison for their own reimbursement and pricing methodologies and rates.
Participation in the Medicaid Drug Rebate program would require us to pay a rebate for each unit of drug reimbursed by Medicaid. The amount of the "basic" portion of the rebate for each product is set by law as the larger of: (i) 23.1% of quarterly AMP, or (ii) the difference between quarterly AMP and the quarterly best price available from us to any commercial or non-governmental customer, or Best Price. AMP must be reported on a monthly and quarterly basis and Best Price is reported on a quarterly basis only. In addition, the rebate also includes the "additional" portion, which adjusts the overall rebate amount upward as an "inflation penalty" when the drug's latest quarter's AMP exceeds the drug's AMP from the first full quarter of sales after launch, adjusted for increases in the Consumer Price Index-Urban. The upward adjustment in the rebate amount per unit is equal to the excess amount of the current AMP over the inflation-adjusted AMP from the first full quarter of sales. The rebate amount is recomputed each quarter based on our report to CMS of current quarterly AMP and Best Price for our drug. The terms of our participation in the program would impose a requirement for us to report revisions to AMP or Best Price within a period not to exceed 12 quarters from the quarter in which the data was originally due. Any such revisions could have the impact of increasing or decreasing our rebate liability for prior quarters, depending on the direction of the revision.
Federal law requires that any manufacturer that participates in the Medicaid Drug Rebate program also participate in the 340B program in order for federal funds to be available for the manufacturer's drugs under Medicaid and Medicare Part B. The 340B program requires participating manufacturers to agree to charge statutorily defined covered entities no more than the 340B "ceiling price" for the manufacturer's covered outpatient drugs. These 340B covered entities include a variety of community health clinics and other entities that receive health services grants from the Public Health Service, as well as hospitals that serve a disproportionate share of low-income patients. The 340B ceiling price is calculated using a statutory formula, which is based on the AMP and rebate amount for the covered outpatient drug as calculated under the Medicaid Drug Rebate program. Any changes to the definition of AMP and the Medicaid rebate amount under the Affordable Care Act or other legislation could affect our 340B ceiling price calculations and negatively impact our results of operations. Health care reform also made changes to the 340B program by expanding the list of covered entities eligible for 340B participation to include certain free-standing cancer hospitals, critical access hospitals, rural referral centers and sole community hospitals. Health care reform obligates the Secretary of the U.S. Department of Health and Human Services, (HHS), to create regulations
35
and processes to improve the integrity of the 340B program. On January 5, 2017, the U.S. Health Resources and Services Administration (HRSA), an agency of the HHS that administers the 340B drug pricing program, issued a final regulation regarding the calculation of 340B ceiling price and the imposition of civil monetary penalties on manufacturers that knowingly and intentionally overcharge covered entities. The effective date of the regulation has been delayed until July 1, 2018. Implementation of this final rule and the issuance of any other final regulations and guidance could affect our obligations under the 340B program in ways that we cannot presently anticipate. In addition, legislation may be introduced that, if passed, would further expand the 340B program to additional covered entities or would require participating manufacturers to agree to provide 340B discounted pricing on drugs used in the inpatient setting.
In the U.S. Medicare program, outpatient prescription drugs may be covered under Medicare Part D. Medicare Part D is a voluntary prescription drug benefit, through which Medicare beneficiaries may enroll in prescription drug plans offered by private entities for coverage of outpatient prescription drugs. Part D plans include both stand-alone prescription drug benefit plans and prescription drug coverage as a supplement to Medicare Advantage plans provided for under Medicare Part C.
Coverage and reimbursement for covered outpatient drugs under Part D are not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. Any formulary used by a Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee. Although Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, they have some flexibility to establish those categories and classes and are not required to cover all of the drugs in each category or class. Medicare Part D prescription drug plans may use formularies to limit the number of drugs that will be covered in any therapeutic class and/or impose differential cost sharing or other utilization management techniques.
The availability of coverage under Medicare Part D may increase demand for products for which we receive marketing approval. However, in order for the products that we market to be included on the formularies of Part D prescription drug plans, we likely will have to offer pricing that is lower than the prices we might otherwise obtain. Changes to Medicare Part D that give plans more freedom to limit coverage or manage utilization, and other cost reduction initiatives in the program could decrease the coverage and price that we receive for any approved products and could seriously harm our business.
In order to be eligible to have our products paid for with federal funds under the Medicaid and Medicare Part B programs and purchased by certain federal agencies and grantees, we expect to participate in the U.S. Department of Veterans Affairs, or VA, Federal Supply Schedule, or FSS, pricing program. Under this program, we would be obligated to make our "innovator" drugs available for procurement on an FSS contract and charge a price to four federal agencies — the VA, U.S. Department of Defense, or DoD, Public Health Service and U.S. Coast Guard — that is no higher than the statutory Federal Ceiling Price, or FCP. The FCP is based on the non-federal average manufacturer price, or Non-FAMP, which we calculate and report to the VA on a quarterly and annual basis. We also expect to participate in the Tricare Retail Pharmacy program, under which we would pay quarterly rebates on utilization of innovator products that are dispensed through the Tricare Retail Pharmacy network to Tricare beneficiaries. The rebates are calculated as the difference between the annual Non-FAMP and FCP.
Pricing and rebate calculations vary across products and programs, are complex, and are often subject to interpretation by manufacturers, governmental or regulatory agencies, and the courts. Civil monetary penalties can be applied if a manufacturer is found to have knowingly submitted any false price information to the government or fails to submit the required price data on a timely basis. Such conduct also could be grounds for CMS to terminate the manufacturer's Medicaid drug rebate agreement, in which case federal payments may not be available under Medicaid or Medicare Part B for the manufacturer's covered outpatient drugs. In addition, claims submitted to federally-funded healthcare programs, such as Medicare and Medicaid, for drugs priced based on incorrect pricing data provided by a manufacturer can implicate the federal Civil False Claims Act.
The containment of healthcare costs has become a priority of federal, state and foreign governments, and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures, and foreign governments have shown significant interest in implementing cost-containment programs to limit the growth of government-paid healthcare costs, including price controls, restrictions on reimbursement, and requirements for substitution of generic products for branded prescription drugs. For example, there have been several recent U.S. Congressional inquiries and proposed federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the cost of drugs, and reform government program reimbursement methodologies for drug products.
36
Beginning April 1, 2013, Medicare payments for all items and services, including drugs, were reduced by 2% under the sequestration (i.e., automatic spending reductions) required by the Budget Control Act of 2011, as amended by the American Taxpayer Relief Act of 2012. Subsequent legislation extended the 2% reduction, on average, to 2025. If Congress does not take action in the future to modify these sequestrations, Medicare Part D plans could seek to reduce their negotiated prices for drugs. Other legislative or regulatory cost containment legislation could have a similar effect.
Further, the Affordable Care Act may reduce the profitability of drug products. It expanded manufacturers' rebate liability under the Medicaid program from fee-for-service Medicaid utilization to include the utilization of Medicaid managed care organizations as well, increased the minimum Medicaid rebate due for most innovator drugs, and capped the total rebate amount for innovator drugs at 100% of AMP. The Affordable Care Act and subsequent legislation also changed the definition of AMP. On February 1, 2016, CMS issued final regulations to implement the changes to the Medicaid drug rebate program under the Affordable Care Act. These regulations became effective on April 1, 2016.
The Affordable Care Act requires pharmaceutical manufacturers of branded prescription drugs to pay a branded prescription drug fee to the federal government. Each such manufacturer pays a prorated share of the branded prescription drug fee of $4.1 billion in 2018, based on the dollar value of its branded prescription drug sales to certain federal programs identified in the law. The Affordable Care Act also expanded the Public Health Service's 340B program to include additional types of covered entities. Substantial new provisions affecting compliance have also been enacted, which may affect our business practices with healthcare practitioners, and a significant number of provisions are not yet, or have only recently become, effective. It appears likely that the Affordable Care Act will continue the pressure on pharmaceutical pricing, especially under the Medicare and Medicaid programs, and may also increase our regulatory burdens and operating costs.
Legislative changes to and regulatory changes under the Affordable Care Act remain possible in the 115th U.S. Congress and under the Trump Administration, as discussed above under the heading "U.S. Healthcare Reform." In addition, there likely will continue to be proposals by legislators at both the federal and state levels, regulators, and third-party payors to contain healthcare costs. Thus, even if we obtain favorable coverage and reimbursement status for any products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Additional information regarding these programs is discussed under the risk factor "If we are able to successfully commercialize XHANCE and if we participate in but fail to comply with our reporting and payment obligations under the Medicaid drug rebate program, or other governmental pricing programs, we could be subject to additional reimbursement requirements, penalties, sanctions and fines which could have a material adverse effect on our business, financial condition, results of operations and growth prospects" in the "Risk Factors" section of this 10-K.
Healthcare Fraud and Abuse Laws
In addition to FDA restrictions on marketing of pharmaceutical products, our business will be subject to healthcare fraud and abuse regulation and enforcement by both the federal government and the states in which we conduct our business, particularly once third-party reimbursement becomes available for one or more of our products. These laws include, but are not limited to, anti-kickback and false claims statutes.
The federal Anti-kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or in kind, to induce or in return for purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any healthcare item or service reimbursable, in whole or in part, under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. A violation of the Anti-Kickback Statute may be established without proving actual knowledge of the statute or specific intent to violate it. The Affordable Care Act amended federal law to provide that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. Although there are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution, the exceptions and safe harbors are drawn narrowly and practices that involve remuneration to those who prescribe, purchase, or recommend pharmaceuticals, including certain discounts, or engaging such individuals as consultants, speakers or advisors, may be subject to scrutiny if they do not fit squarely within the exception or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability. Moreover, there are no safe harbors for many common practices, such as educational and research grants, charitable donations, product support and patient assistance
37
programs. Arrangements that implicate the Anti-Kickback Statute and do not fit within an exception or safe harbor are reviewed on a case-by-case basis to determine whether, based on the facts and circumstances, they violate the statute.
The federal civil False Claims Act prohibits, among other things, any person from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds, or knowingly making, using, or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. Actions under the federal civil False Claims Act may be brought by private individuals known as qui tam relators in the name of the government. In recent years, several pharmaceutical and other healthcare companies have faced enforcement actions under the federal civil False Claims Act for, among other things, providing free product to customers with the expectation that the customers would bill federal programs for the product, inflating prices reported to private price publication services which are used to set drug payment rates under government healthcare programs, and other interactions with prescribers and other customers including interactions that may have affected customers' billing or coding practices on claims submitted to the federal government. Other companies have faced enforcement actions for causing false claims to be submitted because of the company's marketing the product for unapproved, and thus non-reimbursable, uses. Federal enforcement agencies also have shown increased interest in pharmaceutical companies' product and patient assistance programs, including reimbursement and co-pay support services, and a number of investigations into these programs have resulted in significant civil and criminal settlements.
The Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, which we refer to collectively as HIPAA, also created several new federal crimes, including healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits, among other things, knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payors. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items or services.
The majority of states also have statutes or regulations similar to the federal anti-kickback and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Several states now require pharmaceutical companies to report expenses relating to the marketing and promotion of pharmaceutical products in those states and to report gifts and payments to individual health care providers in those states. Some of these states also prohibit certain marketing-related activities including the provision of gifts, meals, or other items to certain health care providers. In addition, several states require pharmaceutical companies to implement compliance programs or marketing codes.
The Physician Payments Sunshine Act, implemented as the Open Payments program, and its implementing regulations, requires certain manufacturers of drugs, devices, biologics and medical supplies that are reimbursable under Medicare, Medicaid, or the Children's Health Insurance Program to report annually to CMS information related to certain payments made in the previous calendar year and other transfers of value to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members.
Compliance with such laws and regulations will require substantial resources. Because of the breadth of these various fraud and abuse laws, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Such a challenge could have material adverse effects on our business, financial condition and results of operations. In the event governmental authorities conclude that our business practices do not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations, they may impose sanctions under these laws, which are potentially significant and may include civil monetary penalties, damages, exclusion of an entity or individual from participation in government health care programs, criminal fines and imprisonment, additional reporting requirements if we become subject to a corporate integrity agreement or other settlement to resolve allegations of violations of these laws, as well as the potential curtailment or restructuring of our operations. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity.
38
Healthcare Privacy Laws
We may be subject to laws and regulations covering data privacy and the protection of health-related and other personal information. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing focus on privacy and data protection issues which may affect our business. Numerous federal and state laws and regulations, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use, disclosure, and protection of personal information. Failure to comply with such laws and regulations could result in government enforcement actions and create liability for us (including the imposition of significant penalties), private litigation and/or adverse publicity that could negatively affect our business. In addition, healthcare providers who prescribe our products and research institutions we collaborate with are subject to privacy and security requirements under HIPAA.
Foreign Corrupt Practices Act
In addition, the U.S. Foreign Corrupt Practices Act prohibits corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any official of another country, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in that capacity.
Employees
As of March 1, 2017, we had a total of 78 full-time employees (including 3 in the United Kingdom and 1 in Norway) and 4 part-time employees. We have no collective bargaining agreements with our employees and none are represented by labor unions. We consider our current relations with our employees to be good.
Properties
Our principal office is located in Yardley, Pennsylvania, where we lease approximately 30,000 square feet of office space pursuant to a lease that expires in May 2021. We also lease facilities in Ewing, New Jersey, Oslo, Norway and Swindon, England. We believe our facilities are adequate to meet our current needs, although we may seek to negotiate new leases or evaluate additional or alternate space for our operations. We believe appropriate alternative space will be readily available on commercially reasonable terms.
Legal Proceedings
We are not currently a party to any legal proceedings.
Corporate Information
We were incorporated under the laws of the State of Delaware in May 2010. Our predecessor entity OptiNose AS was formed under the laws of Norway in September 2000. In 2010, OptiNose AS became our subsidiary as part of an internal reorganization. Our corporate office is located at 1020 Stony Hill Road, Suite 300, Yardley, PA 19067. Our telephone number is (267) 364-3500. We maintain an Internet website at www.optinose.com. The information contained on our website is not incorporated by reference into this Form 10-K.
We file reports, proxy and information statements and other information with the SEC. We make available free of charge under the “Investors—SEC Filings” section of our website all of our filings with the SEC, including our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statements and amendments to such documents, each of which is provided on our website as soon as reasonably practicable after we electronically file the information with the SEC.
The public may read and copy any materials we file with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Additionally, the SEC maintains a website (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, including us.
ITEM 1A. RISK FACTORS
Investing in our common stock involves a high degree of risk. Before deciding to invest in our common stock, you should consider carefully the risks and uncertainties described below, together with general economic and business risks and all of the other information contained in this 10-K, including our consolidated financial statements and the
39
related notes and the section titled "Management's Discussion and Analysis of Financial Condition and Results of Operations." If any of the following risks actually occur, our business, financial condition, results of operations and prospects could be harmed. In that event, the price of our common stock could decline and you could lose all or part of your investment. This 10-K also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of specific factors, including the risks described below. See "Note Regarding Forward-Looking Statements."
Risks Related to Our Financial Position and Capital Resources
We have incurred significant losses since our inception and anticipate that we will incur continued losses in the future.
We are a specialty pharmaceutical company with a limited operating history. To date, we have focused primarily on developing XHANCE as well as other product candidates using our proprietary Breath Powered exhalation delivery system, or EDS, technology. Since inception, we have incurred significant net losses and expect to continue to incur net losses for the foreseeable future. To date, we have generated revenue primarily from our license agreement, or the AVP-825 License Agreement, with Avanir Pharmaceuticals, Inc., or Avanir, pursuant to which we granted them the exclusive right to further develop and commercialize AVP-825 for the acute treatment of migraines in adults. We had net income of $22.6 million for the year ended December 31, 2016 due primarily to the achievement of a milestone under the AVP-825 License Agreement. However, we incurred net losses of $48.9 million and $28.3 million for the years ended December 31, 2017 and 2015, respectively. We incurred net losses in all other prior periods. As of December 31, 2017, we had an accumulated deficit of $211.3 million.
We expect to incur losses for the foreseeable future, and we expect these losses to increase as we:
▪ | begin to commercialize XHANCE and further scale up external manufacturing and distribution capabilities to commercialize XHANCE or any other product candidate for which we may obtain regulatory approval; |
▪ | adapt our regulatory compliance efforts to incorporate requirements applicable to marketed drugs; |
▪ | continue clinical development activities for XHANCE, including the U.S. Food and Drug Administration, or FDA, mandated pediatric studies, and seek regulatory approval for XHANCE for a follow-on indication for the treatment of chronic sinusitis; |
▪ | seek to discover and develop, in-license or acquire additional products, product candidates and technology; |
▪ | maintain, expand and protect our intellectual property portfolio; |
▪ | hire additional commercial, clinical, manufacturing and scientific personnel; |
▪ | add operational, financial and management information systems and personnel, including personnel to support commercialization efforts; and |
▪ | incur additional legal, accounting and other expenses in operating as a public company. |
Because of the numerous risks and uncertainties associated with drug development and commercialization, we are unable to accurately predict the timing or amount of expenses or when, or if, we will be able to achieve profitability. If we are required by regulatory authorities to perform studies in addition to those expected, or if there are any delays in the initiation and completion of our clinical trials or the development of any of our product candidates, our expenses could increase.
We may never achieve or maintain profitability.
Our ability to become and remain profitable will depend on our ability to generate revenue. Although we may be entitled to future milestone payments and royalties under the AVP-825 License Agreement, to date we have not commercialized any of our other product candidates and will therefore depend upon our ability to successfully commercialize XHANCE and any of our other product candidates or any other product candidates that we may in-license or acquire in the future. We do not know when XHANCE or any of our other product candidates, if approved, will generate revenue for us, if at all. Our ability to generate revenue from our current or future products and product candidates will depend on a number of factors, including:
▪ | our ability to successfully commercialize XHANCE for the treatment of nasal polyps; |
40
▪ | our ability to successfully complete required clinical trials and submit a supplemental new drug application to the FDA and obtain regulatory approval for XHANCE for the treatment of chronic sinusitis; |
▪ | our ability to complete and submit applications to, and obtain regulatory approval from, foreign regulatory authorities, if we choose to commercialize XHANCE outside the United States; |
▪ | the size of the markets in the territories for which we gain regulatory approval; |
▪ | the performance of our contract sales organization in marketing and promoting XHANCE; |
▪ | our ability to develop a commercial organization capable of sales, marketing and distribution for XHANCE and any of our other product candidates for which we may obtain marketing approval; |
▪ | our ability to maintain commercially reasonable agreements with wholesalers, distributors and other third parties in our supply chain; |
▪ | our success in establishing a commercially viable price for our products; |
▪ | our success in defending against potential generic competition and other developments in our market generally; |
▪ | our ability to manufacture commercial quantities of our products at acceptable cost levels; |
▪ | our ability to obtain coverage and adequate reimbursement from third parties, including government payors; and |
▪ | our ability to successfully complete development activities, including the necessary clinical trials, with respect to our other product candidates. |
XHANCE, as well as any of our other product candidates, if approved for commercial sale, may not gain market acceptance or achieve commercial success. If our addressable market is not as significant as we estimate or the treatment population is narrowed by competition, physician choice or clinical practice guidelines, we may not generate significant revenue from sales of XHANCE. In addition, we would anticipate incurring significant costs associated with commercializing any approved product. We may not achieve profitability soon after generating product sales, if ever. If we are unable to generate product revenues, we will not become profitable and may be unable to continue operations without continued funding.
Even if we achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would depress the value of our company and could impair our ability to raise capital, expand our business, maintain our development efforts, obtain drug approvals, diversify our offerings or continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
We will likely require additional capital to fund our operations and, if we fail to obtain necessary financing, we may be unable to complete the commercialization of XHANCE and the development of our other product candidates.
Our operations have consumed substantial amounts of cash. To date, we have financed our operations primarily through the sale and issuance of common and preferred stock, debt, licensing revenues under the AVP-825 License Agreement and research grants. We expect to continue to spend substantial amounts to commercialize XHANCE and to advance the clinical development of XHANCE for the treatment of chronic sinusitis and our other product candidates. As of December 31, 2017, we had cash and cash equivalents of $234.9 million. We believe our existing cash and cash equivalents will be sufficient to fund our operations and debt service obligations through the end of 2019. During this period, we expect to launch XHANCE in the United States, continue our clinical development plans to seek approval for XHANCE for the treatment of chronic sinusitis and continue our early-stage development efforts with respect to our other product candidates. Our estimate of the period of time through which our financial resources will be adequate to support our operations is based on assumptions that may prove to be wrong, and we could deplete our available capital resources sooner than we currently expect.
Our future funding requirements, both near and long-term, will depend on many factors, including, but not limited to:
• | the success of our commercialization of XHANCE for the treatment of nasal polyps including, among other things, patient and physician acceptance of XHANCE and our ability to obtain adequate coverage and reimbursement for XHANCE; |
41
▪ | the cost and timing of commercialization activities for XHANCE, including product manufacturing, marketing, sales and distribution; |
▪ | revenue received from commercial sales of XHANCE; |
▪ | our clinical development plans for XHANCE, including FDA-mandated pediatric studies and clinical trials for the follow-on indication for the treatment of chronic sinusitis; |
▪ | the outcome, timing and cost of the regulatory approval process of XHANCE for chronic sinusitis by the FDA, including the potential for the FDA to require that we perform more studies and clinical trials than those that we currently expect; |
▪ | the costs involved in preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights, and; |
▪ | the cost of defending intellectual property disputes, including patent infringement actions brought by third parties against us; |
▪ | potential future licensing revenue from the AVP-825 License Agreement; |
▪ | fluctuations in the three-month LIBOR-based floating interest rate of our senior secured notes; |
▪ | the initiation, progress, timing, costs and results of clinical trials for our other product candidates; and |
▪ | the extent to which we in-license or acquire other products, product candidates or technologies. |
We cannot be certain that additional funding will be available when needed on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts, when required or on acceptable terms, we also could be required to:
▪ | seek strategic collaborations to assist in the commercialization of XHANCE in the United States and other markets; |
▪ | significantly delay, scale back or discontinue the development of XHANCE for the treatment of chronic sinusitis; |
▪ | relinquish or license on unfavorable terms our rights to an Optinose EDS technology or other product candidates that we otherwise would seek to develop or commercialize ourselves; |
▪ | delay, limit, reduce or terminate the drug development of our current or future product candidates, or seek collaborators for one or more of our current or future product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available; or |
▪ | significantly curtail our operations. |
Although we have the ability to obtain an additional $25 million through the issuance of additional senior secured notes pursuant to the Note Purchase Agreement, the availability of this $25 million is subject to certain conditions that we may not be able to meet.
Our failure to comply with the covenants or other terms of the Note Purchase Agreement, including as a result of events beyond our control, could result in a default under the Note Purchase Agreement that could materially and adversely affect the ongoing viability of our business.
On December 29, 2017, we entered into a Note Purchase Agreement with Athyrium Opportunities III Acquisition LP, as collateral agent, or the Collateral Agent, and the purchasers party thereto, or the Purchasers, that provides for the issuance of up to $100 million of senior secured notes, or the Notes. $75 million of the Notes were issued on December 29, 2017, of which $50 million were issued by OptiNose AS and $25 million were issued by OptiNose US, Inc., or the Issuers. The remaining $25 million of Notes, or the Delayed Draw Notes, may be issued by OptiNose US, Inc. and sold to the Purchasers between April 1, 2019 and August 14, 2019, subject to us and our consolidated subsidiaries achieving trailing four quarter net revenues (as calculated pursuant to the terms of the Note Purchase Agreement) of $15 million, a pro forma ratio of total debt to trailing four quarter net revenues not exceeding 6.50 to 1.00 and other specified conditions.
The unpaid principal amount under the Notes is due and payable on June 29, 2023, or the Maturity Date. The proceeds of the Notes will be used to provide ongoing working capital to support the launch and commercialization
42
of XHANCE, as well as for general corporate purposes. The Notes bear interest at a per annum rate of three-month LIBOR rate (subject to a 1.0% floor) plus 9.0%. The interest rate was 10.75% at December 31, 2017. The Issuers are required to make quarterly interest-only payments until the Maturity Date. In addition, the Issuers paid an upfront fee of 1% of the aggregate commitment amount of the Notes on December 29, 2017. We are also required to pay an exit fee of 2% of any principal payments (whether mandatory, voluntary or at maturity) made throughout the term of the Note Purchase Agreement.
In addition, each Note holder may elect to accelerate the repayment of all unpaid principal and accrued interest under such holder’s Note upon consummation of a specified change of control transaction or occurrence of certain events of default (as specified in the Note Purchase Agreement), including, among other things:
• | our default in a payment obligation under the Notes; |
• | our breach of the restrictive covenants or other terms of the Notes; |
• | our breach of reporting obligations; |
• | our failure to properly maintain the collateral; and |
• | certain specified insolvency and bankruptcy-related events. |
Subject to any applicable cure period set forth in the Notes, all amounts outstanding with respect to the Notes (principal and accrued interest) would become due and payable immediately upon an event of default. Our assets or cash flow may not be sufficient to fully repay our obligations under the Notes if the obligations thereunder are accelerated upon any events of default. Further, if we are unable to repay, refinance or restructure our obligations under the Notes, the holders of such Notes could proceed to protect and enforce their rights under the Notes by exercising such remedies as are available to the holders thereunder and in respect thereof under applicable law, either by suit in equity or by action at law, or both, whether for specific performance of any covenant or other agreement contained in the Notes or in aid of the exercise of any power granted in the Notes. The foregoing would materially and adversely affect the ongoing viability of our business.
Our Note Purchase Agreement contains restrictions that limit our flexibility in operating our business.
The Note Purchase Agreement contains various covenants that limit our ability to engage in specified types of transactions without our lenders’ prior consent. These covenants limit our ability to, among other things:
• | sell, transfer, lease or dispose of our assets; |
• | create, incur or assume additional indebtedness; |
• | encumber or permit liens on certain of our assets; |
• | make restricted payments, including paying dividends on, repurchasing or making distributions with respect to our common stock; |
• | make specified investments (including loans and advances); |
• | consolidate, merge, sell or otherwise dispose of all or substantially all of our assets; |
• | enter into certain transactions with our affiliates; |
• | grant certain license rights related to our products, technology and other intellectual property rights; and |
• | permit our cash and cash equivalents held in certain deposit accounts to be less than $10,000,000 at any time. |
The covenants in our Note Purchase Agreement and related security agreements may limit our ability to take certain actions that may be in our long-term best interests. In the event that we breach one or more covenants, our lenders may choose to declare an event of default and require that we immediately repay all amounts outstanding, plus penalties and interest, terminate their commitments to extend further credit and foreclose on the collateral granted to them to secure such indebtedness. Such repayment could have a material adverse effect on our business, operating results and financial condition.
43
Provisions of the Notes for certain potential payments to the holders of such Notes that could impede a sale of the Company.
Subject to certain exceptions, the Issuers are required to make mandatory prepayments of the Notes, with the proceeds of assets sales, extraordinary receipts and prohibited debt issuances, and upon the occurrence of a change of control. In addition, the Issuers may make voluntary prepayments of the Notes, in whole or in part. All mandatory and voluntary prepayments of the Notes are subject to the payment of prepayment premiums as follows: (i) if prepayment occurs prior to the second anniversary of the applicable date of issuance, an amount equal to the amount by which (a) the present value of 102% of the principal prepaid plus all interest that would have accrued on such principal through such second anniversary exceeds (b) the amount of principal prepaid, (ii) if prepayment occurs on or after the second anniversary of the applicable date of issuance but prior to the third anniversary of such issuance, an amount equal to 2% of the principal prepaid, and (iii) if prepayment occurs on or after the third anniversary of the applicable date of issuance but prior to the fourth anniversary of such issuance, an amount equal to 1% of the principal prepaid. No prepayment premium is due on any principal prepaid after the fourth anniversary of the applicable date of issuance of any Notes. These provisions may make it more costly for a potential acquirer to engage in a business combination transaction with us. Provisions that have the effect of discouraging, delaying or preventing a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock and could also affect the price that some investors are willing to pay for our common stock.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
We do not have any committed external source of funds other than potential milestone payments and royalties under the AVP-825 License Agreement and the additional $25 million that may become available under the Note Purchase Agreement between April 1, 2019 and August 14, 2019, subject to us and our consolidated subsidiaries achieving trailing four quarter net revenues (as calculated pursuant to the terms of the Note Purchase Agreement) of $15 million, a pro forma ratio of total debt to trailing four quarter net revenues not exceeding 6.50 to 1.00 and other specified conditions. Until such time, if ever, as we can generate substantial revenue, we may seek additional capital through a combination of private and public equity offerings, debt financings, collaborations, strategic alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financings may be coupled with an equity component, such as warrants to purchase shares, which could also result in dilution of our existing stockholders' ownership. The incurrence of additional indebtedness would result in increased fixed payment obligations and could also result in certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business and may result in liens being placed on our assets and intellectual property. If we were to default on such indebtedness, we could lose such assets and intellectual property.
If we raise additional funds through collaborations, or strategic alliance, marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, product candidates or future revenue streams or grant licenses on terms that are not favorable to us.
Our ability to use our net operating loss carryforwards and other tax attributes may be limited.
As of December 31, 2017, we had U.S. net operating loss, or NOL, carryforwards of approximately $30.3 million available to offset future U.S. taxable income and U.S. research and development (R&D) tax credits of $2.4 million. These amounts are potentially subject to annual utilization limits under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended. Our U.S. NOL carryforwards will begin to expire in 2030 if not utilized. In addition, we have total foreign NOL carryforwards of $96.6 million as of December 31, 2017 in our Norwegian and United Kingdom subsidiaries. These foreign NOL carryforwards do not expire but can only be used to offset profits generated in Norway or the United Kingdom, respectively.
Our U.S. NOL and tax credit carryforwards could expire unused and be unavailable to offset future income tax liabilities because of their limited duration or because of restrictions under U.S. tax law. Under Sections 382 and 383, if a corporation undergoes an “ownership change”, generally defined as a greater than 50% change, by value, in equity ownership during a three-year period, the corporation’s ability to offset pre-change tax attributes, such as NOLs and R&D tax credits, against post-change income or tax may be limited. We have not performed an analysis under Section 382 and cannot predict or otherwise determine whether our federal tax attribute carryforwards may be limited. As a result, if we have taxable income in the future, our ability to use existing U.S. NOL and R&D tax
44
credit carryforwards to reduce U.S. taxable income or tax liability may be subject to limitation. This could result in increased future tax liabilities. Similar rules at the state level may also limit our ability to use state NOLs. Also, there may be periods when the use of NOLs is suspended or otherwise limited at the state level, which could accelerate or permanently increase state taxes owed.
We may have ownership changes in the future due to further changes in our stock ownership. Some of these ownership changes could be outside of our control. If an ownership change occurs and our ability to use our historical net operating loss and tax credit carryforwards is limited, it could adversely impact our future operating results by increasing our tax obligations.
Foreign exchange risks and controls may affect our financial position and results of operations.
Through the operation of our subsidiaries based in the United Kingdom and Norway, we are exposed to foreign currency fluctuations and exchange rate risks. In addition to the operations of our foreign subsidiaries, we also contract with vendors that are located outside the United States, and in some cases make payment of invoices denominated in foreign currencies. We are subject to fluctuations in foreign currency rates in connection with these arrangements and we do not currently hedge our foreign currency exchange rate risk. In addition, because we maintain our consolidated financial statements in U.S. dollars, our financial results are vulnerable to fluctuations in the exchange rate between the U.S. dollar and foreign currencies, such as the British pound sterling, the euro, and the Norwegian krone. In preparing our consolidated financial statements, we must convert all non-U.S. dollar results to U.S. dollars, which impacts our results of operations, is reflected as a component of our stockholder's equity (deficit), and may be credited or charged to operations and reflected in other income (expense), net. The impact of changes in exchange rates has not been significant historically. However, changes in exchange rates could cause significant changes in our financial position and results of operations in the future.
Risks Related to Commercialization of XHANCE
We have no history of commercializing drugs, which may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
Although our predecessor and subsidiary OptiNose AS commenced operations in 2000, our operations to date have been largely focused on raising capital and developing AVP-825 and XHANCE, including undertaking preclinical studies and conducting clinical trials. While we conducted the pre-approval stages of clinical development for AVP-825, Avanir was responsible for completing the clinical development of, obtaining regulatory approval for, and initiating the commercial launch of that product under our license agreement with them. To date, we have manufactured the initial commercial supply for the launch of XHANCE but we have not yet demonstrated our ability to successfully manufacture for on-going commercial scale or, with the exception of AVP-825, arrange for a third party to do so on our behalf, or conduct sales, marketing and distribution activities necessary for successful product commercialization. Consequently, any predictions about our future success or viability may not be as accurate as they could be if we had a longer history of successfully developing and commercializing drugs.
If we are unable to successfully commercialize XHANCE, our business, financial condition and results of operations may be materially adversely affected and the price of our common stock may decline.
Our ability to successfully commercialize XHANCE depends on many factors, including:
▪ | our ability to manufacture commercial quantities of XHANCE at a reasonable cost and with sufficient speed to meet commercial demand; |
▪ | our ability to manage our third-party sales organization to market, promote and sell XHANCE; |
▪ | our success in educating physicians, patients and caregivers about the benefits, administration and use of XHANCE; |
▪ | the availability, perceived advantages, relative cost, relative safety and relative efficacy of competing products; |
▪ | the availability of coverage and adequate reimbursement for XHANCE; |
▪ | our ability to contract with wholesalers and/or specialty pharmaceutical distributors on acceptable terms; |
▪ | the effectiveness of our marketing campaigns; |
▪ | our effective use of promotional resources; |
45
▪ | a continued acceptable safety profile for XHANCE; |
▪ | our ability to obtain and maintain appropriate state licenses in the states in which we intend to sell XHANCE; and |
▪ | our ability to successfully defend any challenges to our intellectual property relating to XHANCE. |
Many of these matters are beyond our control and are subject to other risks described elsewhere in this "Risk Factors" section. Accordingly, we cannot assure you that we will be able to successfully commercialize or generate revenue from XHANCE. If we cannot do so, or are significantly delayed in doing so, our business, financial condition and results of operations may be materially adversely affected and the price of our common stock may decline.
The commercial success of XHANCE will depend upon its acceptance by multiple stakeholders, including physicians, patients and healthcare payors.
Physicians may not prescribe XHANCE, in which case we would not generate the revenues we anticipate. The degree of market acceptance of XHANCE will depend on a number of factors, including:
▪ | demonstration of clinical safety and efficacy; |
▪ | relative convenience and ease of administration; |
▪ | pricing and cost-effectiveness; |
▪ | availability of alternative treatments and perceived advantages over such alternative treatments; |
▪ | the clinical indications for which XHANCE is approved; |
▪ | the prevalence and severity of any AEs; |
▪ | restrictions placed on XHANCE in connection with its approval; |
▪ | limitations or warnings contained in the FDA-approved label for XHANCE; |
▪ | the effectiveness of our or any future collaborators' sales and marketing strategies; |
▪ | consolidation among healthcare providers, which increases the impact of the loss of any relationship; |
▪ | our ability to obtain and maintain sufficient third-party coverage and adequate reimbursement; and |
▪ | the willingness of patients to pay out-of-pocket in the absence of third-party coverage. |
If XHANCE does not achieve an adequate level of acceptance by physicians, patients and healthcare payors, we may not generate sufficient revenue in order to become or remain profitable.
If third-party payors do not reimburse patients for XHANCE or if reimbursement levels are set too low for us to sell XHANCE at a profit, our ability to successfully commercialize XHANCE and our results of operations will be harmed.
Our ability to commercialize XHANCE successfully will depend in part on the extent to which coverage and adequate reimbursement for XHANCE will be available in a timely manner from third-party payors, including governmental healthcare programs such as Medicare and Medicaid, commercial health insurers and managed care organizations. Government authorities and other third-party payors, such as private health insurers and health maintenance organizations, determine which medications they will cover and establish reimbursement levels. Reimbursement decisions by particular third-party payors depend upon a number of factors, including each third-party payor's determination that use of a product is:
▪ | a covered benefit under its health plan; |
▪ | appropriate and medically necessary for the specific condition or disease; |
▪ | cost effective; and |
▪ | neither experimental nor investigational. |
46
Obtaining coverage and reimbursement approval for XHANCE from government authorities or other third-party payors may be a time consuming and costly process that could require us to provide supporting scientific, clinical and cost-effectiveness data, including expensive pharmacoeconomic studies beyond the data required to obtain marketing approval, for the use of XHANCE to each government authority or other third-party payor. We may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement from government authorities or other third-party payors.
Third-party payors may deny reimbursement for covered products if they determine that a medical product was not used in accordance with cost-effective diagnosis methods, as determined by the third-party payor, or was used for an unapproved indication. Third-party payors also may refuse to reimburse for procedures and devices deemed to be experimental. Third-party payors may also limit coverage to specific products on an approved list, or formulary, which might not include all of the FDA-approved products for a particular indication.
Third-party payors are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for medical products and services. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Levels of reimbursement may also decrease in the future, and future legislation, regulation or reimbursement policies of third-party payors may adversely affect the demand for and reimbursement available for XHANCE, which in turn, could negatively impact pricing. Further, payors, including healthcare insurers, pharmacy benefit managers and group purchasing organizations, increasingly seek ways to reduce their costs. Many payors continue to adopt benefit plan changes that shift a greater portion of prescription costs to patients. Such measures include more limited benefit plan designs, higher patient co-pay or co-insurance obligations and limitations on patients’ use of commercial manufacturer co-pay payment assistance programs (including through co-pay accumulator adjustment or maximization programs). Payors also increasingly seek price discounts or rebates in connection with the placement of our products on their formularies or those they manage. Payors may also control costs by imposing restrictions on access to or usage of our products, such as by requiring prior authorizations or "step-edits," and may choose to exclude certain indications for which our products are approved or even choose to exclude coverage entirely. For example, insurers may establish a step-edit system that requires a patient to first use a lower price alternative product prior to becoming eligible for reimbursement of a higher price product. Some providers may not complete the burdensome administrative process required to demonstrate or document that the patients for whom XHANCE has been prescribed meet the payors’ utilization management criteria (i.e., prior authorizations or step-edits) and, as a result, patients will not gain access to XHANCE treatment. Further, other patients may obtain coverage for XHANCE but abandon their prescriptions rather than pay their co-pay payment which would result in a significant shortfall in achieving revenue expectations and negatively impact our business, prospects, results of operations and financial condition.
Significant consolidation in the health insurance industry has resulted in a few large insurers and pharmacy benefit managers exerting greater pressure in pricing and usage negotiations with drug manufacturers, significantly increasing discounts and rebates required of manufacturers and limiting patient access and usage. Further consolidation among insurers, pharmacy benefit managers and other payors, including through integrated delivery systems, would increase the negotiating leverage such entities have over us and other drug manufacturers. Ultimately, further discounts, rebates, coverage or plan changes, restrictions or exclusions as described above could have a material adverse effect on sales of our affected products.
If we are unable to differentiate XHANCE from current and future products or existing methods of treatments, our ability to successfully commercialize XHANCE would be adversely affected.
We initially intend to commercialize XHANCE for the treatment of nasal polyps and seek FDA approval for a follow-on indication of XHANCE for the treatment of chronic sinusitis. Currently, Nasonex, marketed by Merck, is the only other branded drug therapy approved by the FDA for the treatment of nasal polyps. A generic version of Nasonex, mometasone furoate monohydrate, was approved by the FDA for, among other indications, the treatment of nasal polyps and launched in 2016. In addition, Beconase AQ, which is an INS marketed by GlaxoSmithKline, is indicated for the prophylaxis of nasal polyps after surgical resection. We are not aware of any product approved for the treatment of chronic sinusitis. In addition to competition from Nasonex and Beconase AQ, we will also need to differentiate XHANCE from other products and treatments identified in current clinical practice guidelines for the treatment of chronic rhinosinusitis with and without nasal polyps. Such products and treatments include the use of nasal rinses, decongestants, over-the-counter and INS products, oral steroids, antibiotics, and sinus surgery and other procedures, including functional endoscopic sinus surgery, balloon sinus dilation and steroid-releasing sinus implants. In addition, several biologic monoclonal antibodies are in clinical development for the treatment of nasal polyps, including omalizumab, reslizumab, mepolizumab and dupilumab. If we are unable to achieve significant
47
differentiation for XHANCE against these other products and treatments, including on the basis of efficacy, safety and tolerability profile, reliability, convenience of administration, price and reimbursement, the opportunity for XHANCE to be commercialized successfully would be adversely affected.
If the market opportunities for XHANCE are smaller than we believe, our revenue may be adversely affected, and our business may suffer.
Our initial target market for XHANCE will consist of ENT physicians, allergists and high-decile INS-prescribing primary care physicians that we believe treat an estimated 3.5 million U.S. patients with chronic rhinosinusitis, an estimated 1.2 million of whom have chronic rhinosinusitis with nasal polyps. If we are able to obtain a follow-on indication of XHANCE for the treatment of chronic sinusitis, we intend to broaden our reach and target primary care physicians that we believe treat an additional estimated 6.25 million patients with chronic rhinosinusitis, an estimated one-third of whom have chronic rhinosinusitis with nasal polyps.
Our projections of both the number of people who suffer from chronic rhinosinusitis with and without nasal polyps, as well as the subset of people with these diseases who have the potential to benefit from the use of XHANCE, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys we commissioned, prescription data or other market research and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of chronic rhinosinusitis or nasal polyps. The number of patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for XHANCE may be limited or may not be amenable to treatment with XHANCE, and new patients may become increasingly difficult to identify or gain access to, which would adversely affect our results of operations and our business.
Clinical practice guidelines and recommendations published by various organizations could have significant influence on the use of XHANCE.
Government agencies may promulgate clinical practice guidelines directly applicable to XHANCE. In addition, professional societies, practice management groups, private health and science foundations and organizations involved in various diseases from time to time may also publish guidelines or recommendations to the healthcare and patient communities. Recommendations of government agencies or these other groups or organizations may relate to such matters as usage, dosage, route of administration and use of concomitant therapies. Recommendations or guidelines suggesting the reduced use of XHANCE or the use of competitive or alternative products as the standard of care to be followed by patients and healthcare providers could result in decreased use of XHANCE.
If we are unable to maintain agreements with third parties to market and sell XHANCE we may be unable to generate any revenue for XHANCE.
We currently have limited sales, marketing or distribution capabilities. We have contracted to use an outsourced contract sales organization, or CSO, to promote XHANCE to our defined specialty audience of ENT and allergy specialists and high-decile INS-prescribing primary care physicians. Our CSO may not dedicate sufficient resources to the commercialization of XHANCE or may otherwise fail in its commercialization due to factors beyond our control. Additionally, our CSO may fail to comply with applicable legal or regulatory requirements, or may enter into agreements with other parties that have products and services that could compete with XHANCE.
In the event that we fail to successfully launch and commercialize XHANCE through our CSO, we may also enter into a strategic collaboration with a third party. We face significant competition in seeking appropriate strategic collaborators, and these strategic collaborations can be intricate and time-consuming to negotiate and document. We may not be able to negotiate strategic collaborations on acceptable terms, or at all. We are unable to predict when, if ever, we will enter into any strategic collaborations because of the numerous risks and uncertainties associated with establishing strategic partnerships.
XHANCE may become associated with undesirable adverse reactions or have other properties that could result in significant negative consequences following regulatory approval.
If we or others identify adverse events, or AEs, associated with XHANCE, a number of potentially significant negative consequences could result, including:
▪ | we may be forced to suspend marketing of XHANCE; |
▪ | the FDA may withdraw its approval of XHANCE or impose restrictions on its distribution; |
48
▪ | the FDA may require additional warnings or contradictions in the label that could diminish the usage or otherwise limit the commercial success of XHANCE; |
▪ | we may be required to conduct additional post-marketing studies; |
▪ | we could be sued and held liable for harm caused to patients; and |
▪ | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of XHANCE.
If the FDA or other applicable regulatory authorities approve generic or similar products that compete with XHANCE, or if the FDA or other applicable regulatory authorities change or create new pathways that may expedite approval of such products, it could decrease our expected sales of XHANCE.
Once an NDA, including a Section 505(b)(2) application, is approved, the product covered thereby becomes a "listed drug" which can, in turn, be cited by potential competitors in support of approval of an abbreviated NDA, or ANDA. The FD&C Act, FDA regulations and other applicable regulations and policies provide incentives to manufacturers to create modified, non-infringing versions of a drug to facilitate the approval of an ANDA for generic substitutes. Manufacturers may be able to bring a generic product to market in a much more cost-efficient pathway than we currently anticipate. If the costs involved in bringing such a product to market are significantly less than our costs with respect to the development of XHANCE, companies that produce generic equivalents to XHANCE may be able to offer their products at lower prices. Further, if the timeline for bringing such a product to market is expedited, companies that produce generic equivalents to XHANCE may compete with XHANCE faster than we currently anticipate. For example, the FDA has communicated a priority to build on initiatives to accelerate generic entry of complex generics, which include locally acting nasal drug products. If the FDA provides for alternatives to comparative clinical endpoint bioequivalence studies for generic versions of locally-acting orally inhaled and nasal drug products, companies that product generic equivalents to XHANCE may compete with XHANCE faster than we currently anticipate and a significant percentage of any future sales of XHANCE could be lost to such generic products. Moreover, in addition to generic competition, we could face competition from other companies seeking approval of products that are similar to ours using the Section 505(b)(2) pathway. Such applicants may be able to rely on XHANCE or other approved drug products or published literature to develop drug products that are similar to ours. The introduction of a drug product similar to our products or product candidates could expose us to increased competition, leading to a decrease in sales of XHANCE. Competition that we may face from generic or similar versions of XHANCE could materially and adversely impact our future revenue, profitability, and cash flows.
Even though we have obtained regulatory approval for XHANCE, we will still face extensive FDA regulatory requirements and may face future regulatory difficulties.
Even though we have obtained regulatory approval in the United States for XHANCE, the FDA and state regulatory authorities may still impose significant restrictions on the indicated uses or marketing of XHANCE, or impose ongoing requirements for potentially costly post-approval studies or post-marketing surveillance. For example, as part of its approval of XHANCE for the treatment of nasal polyps in adults, the FDA required that we conduct a randomized, double-blind, placebo controlled clinical study in children and adolescents with nasal polyposis to assess the safety, efficacy, and pharmacokinetics of XHANCE in this population. The FDA originally indicated the study was to be conducted in children and adolescents 6 to 17 years of age. On October 30, 2017, the FDA notified us that in response to our request it had modified the required age range to 12 to 17 years of age. We submitted our final protocol to the FDA with respect to the pediatric study by January 2018 as required, and we are required to complete the study by January 2022 and to submit a final report with respect to the study by July 2022. Because the Optinose EDS for XHANCE was designed for use in adult patients, we may discover that the dimensions of this EDS make it unsuitable for use in pediatric patient populations. As such, this pediatric study may also require us to undergo a costly and time-consuming development process to design and manufacture as appropriate a modified EDS to conduct these studies.
We are also subject to ongoing FDA requirements governing the labeling, packaging, storage, distribution, safety surveillance, advertising, promotion, record-keeping and reporting of safety and other post-marketing information. The holder of an approved NDA is obligated to monitor and report AEs and any failure of a product to meet the specifications in the NDA. The holder of an approved NDA must also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. Advertising and promotional materials must comply with FDA regulations and may be subject to other potentially applicable federal and state laws. The applicable regulations in countries outside the United States grant similar powers to the competent authorities and impose similar obligations on companies.
49
In addition, manufacturers of drug products and their facilities are subject to payment of substantial user fees and continual review and periodic inspections by the FDA and other regulatory authorities for compliance with current Good Manufacturing Practice, or cGMP, regulations and adherence to commitments made in the NDA. Since XHANCE is a combination product, we will also need to comply with some of the FDA's manufacturing regulations for devices. In addition to cGMP, the FDA requires that our drug-device combination product comply with the Quality System Regulation, or QSR, which sets forth the FDA's manufacturing quality standards for medical devices, and other applicable government regulations and corresponding foreign standards. If we, or a regulatory authority, discover previously unknown problems with XHANCE, such as AEs, of unanticipated severity or frequency, or problems with a facility where the product is manufactured, a regulatory authority may impose restrictions relative to XHANCE or the manufacturing facility, including requiring recall or withdrawal of the product from the market, suspension of manufacturing, or other FDA action or other action by foreign regulatory authorities.
If we fail to comply with applicable regulatory requirements following approval of XHANCE, a regulatory authority may:
▪ | issue a warning letter asserting that we are in violation of the law; |
▪ | seek an injunction or impose civil or criminal penalties or monetary fines; |
▪ | suspend, modify or withdraw regulatory approval; |
▪ | suspend any ongoing clinical trials; |
▪ | refuse to approve a pending NDA or a pending application for marketing authorization or supplements to an NDA or to an application for marketing authorization submitted by us; |
▪ | seize our product candidate; and/or |
▪ | refuse to allow us to enter into supply contracts, including government contracts. |
Our relationships with physicians, patients and payors in the U.S. are subject to applicable anti-kickback, fraud and abuse laws and regulations. Our failure to comply with these laws could expose us to criminal, civil and administrative sanctions, reputational harm, and could harm our results of operations and financial conditions.
Our current and future operations with respect to the commercialization of XHANCE are subject to various U.S. federal and state healthcare laws and regulations. These laws impact, among other things, our proposed sales, marketing, support and education programs and constrain our business and financial arrangements and relationships with third-party payors, healthcare professionals and others who may prescribe, recommend, purchase or provide XHANCE, and other parties through which we market, sell and distribute XHANCE. Finally, our current and future operations are subject to additional healthcare-related statutory and regulatory requirements and enforcement by foreign regulatory authorities in jurisdictions in which we conduct our business. The laws are described in greater detail in the previous section under "Business — Government Regulation — Healthcare Fraud and Abuse Laws," and include, but are not limited to:
▪ | the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving or paying any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, lease, order, or arranging for or recommending the purchase, lease or order of, any good or service, for which payment may be made, in whole or in part, under federal healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
▪ | the U.S. civil False Claims Act (which can be enforced through "qui tam," or whistleblower actions, by private citizens on behalf of the federal government), prohibits any person from, among other things, knowingly presenting, or causing to be presented false or fraudulent claims for payment of government funds or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the U.S. federal government; |
▪ | the U.S. federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or |
50
covering up a material fact or making any materially false statement, in connection with the delivery of, or payment for healthcare benefits, items or services by a healthcare benefit program, which includes both government and privately funded benefits programs; similar to the U.S. federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
▪ | state laws and regulations, including state anti-kickback and false claims laws, that may apply to our business practices, including but not limited to, research, distribution, sales and marketing arrangements and claims involving healthcare items or services reimbursed by any third-party payor, including private insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the U.S. federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; and state laws and regulations that require drug manufacturers to file reports relating to pricing and marketing information, which requires tracking gifts and other remuneration and items of value provided to healthcare professionals and entities; and |
▪ | the Physician Payments Sunshine Act, implemented as the Open Payments program, and its implementing regulations, requires certain manufacturers of drugs, devices, biologics and medical supplies that are reimbursable under Medicare, Medicaid, or the Children's Health Insurance Program to report annually to CMS information related to certain payments made in the preceding calendar year and other transfers of value to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. |
The shifting commercial compliance environment and the need to build and maintain robust and expandable systems to comply with different compliance or reporting requirements in multiple jurisdictions increase the possibility that a healthcare or pharmaceutical company may fail to comply fully with one or more of these requirements. Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices do not comply with applicable fraud and abuse or other healthcare laws and regulations or guidance. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, additional oversight and reporting requirements if we become subject to a corporate integrity agreement to resolve allegations of non-compliance with these laws and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, they may be subject to the same criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could harm our financial condition and divert resources and the attention of our management from operating our business.
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity in addition to the aforementioned potential regulatory actions. The occurrence of any event or penalty described above may inhibit our ability to commercialize XHANCE and generate revenues which would have a material adverse effect on our business, financial condition and results of operations.
If we are able to successfully commercialize XHANCE and if we participate in but fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate Program, or other governmental pricing programs, we could be subject to additional reimbursement requirements, penalties, sanctions and fines which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
We participate in the Medicaid Drug Rebate Program, and other governmental pricing programs, and therefore we will be obligated to pay certain specified rebates and report pricing information with respect to XHANCE. Pricing and rebate calculations are complex and are often subject to interpretation by us, governmental or regulatory agencies and the courts. We cannot assure you that our submissions will not be found by the Centers for Medicare & Medicaid Services, or CMS, to be incomplete or incorrect. Governmental agencies may also make changes in program interpretations, requirements or conditions of participation, some of which may have implications for amounts previously estimated or paid. The Medicaid rebate amount is computed each quarter based on our submission to CMS of our current average manufacturer price, or AMP, and best price for the quarter.
51
If we become aware that our reporting for a prior quarter was incorrect, or has changed as a result of recalculation of the pricing data, we are obligated to resubmit the corrected data for a period not to exceed twelve quarters from the quarter in which the data originally were due, and CMS may request or require restatements for earlier periods as well. Such restatements and recalculations increase our costs for complying with the laws and regulations governing the Medicaid Drug Rebate Program. Any corrections to our rebate calculations could result in an overage or underage in our rebate liability for past quarters, depending on the nature of the correction. Price recalculations also may affect the ceiling price at which we are required to offer our products to certain covered entities, such as safety-net providers, under the Public Health Service's 340B drug pricing program, or the 340B program, and under other similar government pricing programs. These programs are described in greater detail in the previous section under "Business — Government Regulation — Coverage and Reimbursement."
We will also be liable for errors associated with our submission of pricing data. In addition to retroactive rebates and the potential for 340B program refunds, if we are found to have knowingly submitted false AMP, or best price information to the government, we may be liable for civil monetary penalties in the amount of $181,071 per item of false information. If we are found to have made a misrepresentation in the reporting of our average sales price, we may be liable for civil monetary penalties of up to $13,066 for each misrepresentation for each day in which the misrepresentation was applied. Our failure to submit monthly/quarterly AMP and best price data on a timely basis could result in a civil monetary penalty of $18,107 per day for each day the information is late beyond the due date. Such failure also could be grounds for CMS to terminate our Medicaid drug rebate agreement, pursuant to which we participate in the Medicaid program. In the event that CMS terminates our rebate agreement, federal payments may not be available under Medicaid for XHANCE. On January 5, 2017, the U.S. Health Resources and Services Administration (HRSA), an agency of the HHS that administers the 340B drug pricing program issued a final regulation regarding the calculation of 340B ceiling price and the imposition of civil monetary penalties on manufacturers that knowingly and intentionally overcharge covered entities. The effective date of the regulation has been delayed until July 1, 2018. Implementation of this final rule and the issuance of any other final regulations and guidance could affect our obligations under the 340B program in ways that we cannot presently anticipate. In addition, legislation may be introduced that, if passed, would further expand the 340B program to additional covered entities or would require participating manufacturers to agree to provide 340B discounted pricing on drugs used in the inpatient setting.
Federal law requires that a company must participate in the U.S. Department of Veterans Affairs, or VA, Federal Supply Schedule, or FSS, pricing program to be eligible to have its products paid for with federal funds. As part of this program, we would be obligated to make XHANCE available for procurement on an FSS contract under which we must comply with standard government terms and conditions and charge a price that is no higher than the statutory Federal Ceiling Price, or FCP, to four federal agencies (VA, U.S. Department of Defense, or DOD, Public Health Service, and U.S. Coast Guard). The FCP is based on the Non-Federal Average Manufacturer Price, or Non-FAMP, which we calculate and report to the VA on a quarterly and annual basis. If we overcharge the government in connection with our FSS contract or Section 703 Agreement, whether due to a misstated FCP or otherwise, we are required to refund the difference to the government. Failure to make necessary disclosures and/or to identify contract overcharges can result in allegations against us under the U.S. civil False Claims Act and other laws and regulations. Unexpected refunds to the government, and responding to a government investigation or enforcement action, would be expensive and time-consuming, and could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Regulatory approval for any approved product is limited by the FDA to those specific indications and conditions for which clinical safety and efficacy have been demonstrated.
Our promotional materials, statements and training methods must comply with applicable laws and regulations, including FDA's prohibition of the promotion of unapproved, or off-label, use. Physicians may use our products off-label, as the FDA does not restrict or regulate a physician's independent choice of treatment within the practice of medicine. As healthcare professionals frequently prescribe corticosteroids for the treatment of chronic nasal inflammatory diseases, such as chronic rhinosinusitis, doctors could prescribe XHANCE for the treatment of chronic sinusitis and other chronic nasal inflammatory diseases, even though the FDA has granted approval of XHANCE only for the treatment of nasal polyps. If the FDA determines that our promotional materials, statements or activities constitute promotion of an off-label use, we could be required to modify our promotional materials, statements or training methods or subject us to regulatory or enforcement actions, such as the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine, disgorgement of money, operating restrictions or criminal penalties. We may also be subject to actions by other governmental entities or private parties, such as the U.S. civil False Claims Act, civil whistleblower or "qui tam" actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional, materials or activities to constitute promotion of an off-
52
label use, which could result in significant fines or penalties under other statutory authorities. In that event, our reputation could be damaged and market adoption of XHANCE could be impaired.
Even though we have obtained FDA approval for XHANCE in the United States, we may never obtain approval for or successfully commercialize it outside of the United States, which would limit our ability to realize its full market potential.
In order to market XHANCE outside of the United States, we must obtain marketing authorizations and comply with numerous and varying regulatory requirements of other countries regarding quality, safety and efficacy. Clinical trials conducted in one country may not be accepted by foreign regulatory authorities, and regulatory approval in one country does not mean that regulatory approval will be obtained in any other country. Approval processes vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking foreign regulatory approval could result in difficulties and costs for us and require additional non-clinical studies or clinical trials, which could be costly and time consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of XHANCE in those countries. While our management team has experience in obtaining foreign regulatory approvals at other companies, we do not have any product candidates approved for sale in any foreign jurisdiction, and we, as a company, do not have experience in obtaining regulatory approval in international markets. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approval in international markets is delayed, our target market for XHANCE will be reduced and we would not be able to realize the full market potential of XHANCE.
The Affordable Care Act and any changes in healthcare law may increase the difficulty and cost for us to commercialize XHANCE and affect the prices we may obtain.
The United States and many foreign jurisdictions have enacted or proposed legislative and regulatory changes affecting the healthcare system that could restrict or regulate post-approval activities and affect our ability to profitably sell XHANCE. The United States government, state legislatures and foreign governments also have shown significant interest in implementing cost-containment programs to limit the growth of government-paid healthcare costs, including price controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively the Affordable Care Act was intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. These intended reforms are described in greater detail in the previous section under "Business — Government Regulation — U.S. Healthcare Reform."
Among the provisions of the Affordable Care Act that have been implemented since enactment and are of importance to the commercialization of XHANCE are the following:
▪ | an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs or biologic agents; |
▪ | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; |
▪ | expansion of healthcare fraud and abuse laws, including the U.S. civil False Claims Act and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance; |
▪ | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for a manufacturer's outpatient drugs to be covered under Medicare Part D; |
▪ | extension of manufacturers' Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; |
▪ | a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted, or injected; |
▪ | expansion of eligibility criteria for Medicaid programs; |
53
▪ | expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; |
▪ | requirements to report certain financial arrangements with physicians and teaching hospitals; |
▪ | a requirement to annually report certain information regarding drug samples that manufacturers and distributors provide to physicians; and |
▪ | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. |
Legislative changes to or regulatory changes under the Affordable Care Act remain possible in the 115th U.S. Congress and under the Trump Administration. In January 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the Affordable Care Act to waive, defer, grant exemptions from, or delay the implementation of any provision of the Affordable Care Act that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. The Tax Cuts and Jobs Act enacted on December 22, 2017, eliminated the shared responsibility payment for individuals who fail to maintain minimum essential coverage under section 5000A of the Internal Revenue Code of 1986, commonly referred to as the individual mandate, beginning in 2019. In addition, the Bipartisan Budget Act of 2018 increased the Affordable Care Act required manufacturer point-of-sale discount from 50% to 70% off the negotiated price for Medicare Part D beneficiaries during their coverage gap period beginning in 2019. We expect that the Affordable Care Act, as currently enacted or as it may be amended in the future, and other healthcare reform measures that may be adopted in the future could have a material adverse effect on our industry generally and on our ability to maintain or increase sales of XHANCE or to successfully commercialize XHANCE
We expect that the Affordable Care Act, as well as other healthcare reform measures that have and may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for XHANCE and could seriously harm our future revenues. Any reduction in reimbursement from Medicare, Medicaid, or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize XHANCE.
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of XHANCE and any other product candidates that we may develop.
We currently face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials, and this risk will increase significantly as we commercialize XHANCE and other product candidates that we may develop. We may face product liability claims, regardless of FDA approval for commercial manufacturing and sale as product liability claims may be brought against us by patients who have used XHANCE in any of our clinical trials, future patients, healthcare providers or others using, administering or selling our products, if and when approved. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
▪ | decreased demand for XHANCE; |
▪ | injury to our reputation and significant negative media attention; |
▪ | termination of clinical trial sites or entire trial programs that we conduct in the future relating to XHANCE or our other product candidates; |
▪ | withdrawal of clinical trial participants from any future clinical trial relating to XHANCE or our other product candidates; |
▪ | significant costs to defend the related litigation; |
▪ | substantial monetary awards to patients; |
▪ | loss of revenue; |
▪ | diversion of management and scientific resources from our business operations; and |
54
▪ | an increase in product liability insurance premiums or an inability to maintain product liability insurance coverage. |
We currently carry product liability insurance with coverage up to $10.0 million in the aggregate, with a per incident limit of $10.0 million, which may not be adequate to cover all liabilities that we may incur. Further, we may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise. Our inability to maintain sufficient product liability insurance at an acceptable cost could prevent or inhibit the commercialization of XHANCE or the development of our other product candidates.
Additionally, any agreements we may enter into in the future with collaborators in connection with the development or commercialization of XHANCE or any of our other product candidates may entitle us to indemnification against product liability losses, but such indemnification may not be available or adequate should any claim arise. In addition, several of our agreements require us to indemnify third parties and these indemnifications obligations may exceed the coverage under our product liability insurance policy. For example, the AVP-825 License Agreement provides for reciprocal indemnification obligations for each of the parties in the event that a product liability claim arises from, among other things, one party's development, manufacture, sale or commercialization activities for AVP-825.
Risks Related to Clinical Development and Regulatory Approval of XHANCE for the Treatment of Chronic Sinusitis and Our Other Product Candidates
The design and execution of clinical trials to support FDA-approval of XHANCE for the treatment of chronic sinusitis is subject to substantial risk and uncertainty.
We intend to initiate a clinical program to support a follow-on indication of XHANCE for the treatment of chronic sinusitis. Similar to our NDA for XHANCE for the treatment of nasal polyps, we believe we may also be able to use the Section 505(b)(2) pathway for potential U.S. approval for XHANCE for the treatment of chronic sinusitis. Because there is no FDA-approved product for the treatment of chronic sinusitis, we believe there is substantial risk and uncertainty in planning and conducting adequate clinical trials to meet FDA requirements to support approval for this indication. If the clinical program required by the FDA is more costly or time-consuming than anticipated, we may decide to not pursue this follow-on indication. Additionally, if we do conduct clinical trials for this indication, XHANCE may not demonstrate sufficient efficacy or safety to support FDA approval. If we do not obtain a follow-on indication for the treatment of chronic sinusitis, our promotion of XHANCE will be limited to nasal polyps, which would limit our potential sales of XHANCE.
The regulatory approval processes of the FDA are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory agency. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate's clinical development. It is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain regulatory approval.
Our product candidates could fail to receive regulatory approval for many reasons, including the following:
▪ | the FDA may not accept our NDA filing; |
▪ | the FDA may disagree with the design, scope or implementation of our clinical trials; |
▪ | we may be unable to demonstrate to the satisfaction of the FDA that a product candidate is safe and effective for its proposed indication; |
▪ | we may be unable to demonstrate that a product candidate's clinical and other benefits outweigh its safety risks; |
▪ | the FDA may disagree with our interpretation of data from preclinical studies or clinical trials; |
▪ | the data collected from clinical trials of our product candidates may not be sufficient to support the submission of an NDA; |
55
▪ | the FDA may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
▪ | the approval policies or regulations of the FDA may change in a manner rendering our clinical data insufficient for approval. |
With the exception of our NDA submission for XHANCE, we have not previously submitted an NDA or any similar drug approval filing to the FDA for any product candidate, and we cannot be certain that any of our current product candidates will receive regulatory approval. If we do not receive regulatory approval for our product candidates, we may not be able to continue our operations. Even if we successfully obtain regulatory approval to market one or more of our product candidates, our revenue will be dependent, to a significant extent, upon the size of the markets in the territories for which we gain regulatory approval. If the markets for patients or indications that we are targeting are not as significant as we estimate, we may not generate significant revenue from sales of such products, if approved.
Clinical development is a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results. Clinical failure can occur at any stage of clinical development.
Clinical trials are expensive, can take many years to complete and have highly uncertain outcomes. Failure can occur at any time during the clinical trial process as a result of inadequate performance of a drug, inadequate adherence by patients or investigators to clinical trial protocols, or other factors. Drug candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through earlier clinical trials. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials as a result of a lack of efficacy or adverse safety profiles, despite promising results in earlier trials. Our clinical trials for the follow-on indication of XHANCE for the treatment of chronic sinusitis or our other product candidates may not be successful or may be more expensive or time-consuming than we currently expect. If clinical trials for these product candidates fail to demonstrate safety or efficacy to the satisfaction of the FDA, the FDA may not approve that product candidate and we would not be able to commercialize it, which could impair our ability to gain or maintain profitability.
Delays in clinical trials are common and have many causes, and any delay could result in increased costs to us and jeopardize or delay our ability to obtain regulatory approval and commence product sales.
We may experience delays in clinical trials of our product candidates or the time required to complete clinical trials for our product candidates may be longer than anticipated. Our future clinical trials may not begin on time, have an effective design, enroll a sufficient number of patients, or be completed on schedule, if at all. Our clinical trials can be delayed for a variety of reasons, including, but not limited to:
▪ | inability to raise funding necessary to initiate or continue a clinical trial; |
▪ | delays in obtaining regulatory approval to commence a clinical trial; |
▪ | delays in reaching agreement with the FDA or foreign regulatory authorities on final trial design or the scope of the development program; |
▪ | imposition of a clinical hold following an inspection of our clinical trial operations or trial sites by the FDA or foreign regulatory authorities; |
▪ | delays in reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites; |
▪ | delays in obtaining required institutional review board, or IRB, approval; |
▪ | delays in recruiting suitable patients to participate in a clinical trial; |
▪ | patients' delays or failure to complete participation in a clinical trial or return for post-treatment follow-up; |
▪ | clinical sites dropping out of a clinical trial; |
▪ | time required to add new clinical sites; or |
▪ | delays by our contract manufacturing organizations, or CMOs, to produce and deliver a sufficient supply of clinical trial materials. |
56
If clinical trials for our product candidates are delayed for any of the above reasons or other reasons, our development costs may increase, our approval process could be delayed and our ability to commercialize our product candidates could be materially harmed.
We will need to identify proprietary names for our product candidates that are acceptable to FDA, and any delay associated with doing so may adversely impact our business.
Any proprietary name we propose to use with our product candidates in the United States must be reviewed and accepted by the FDA, regardless of whether we have registered it, or applied to register it, as a trademark. The FDA reviews any proposed product name, including an evaluation of potential for confusion with other product names. The FDA may also object to a product name if it believes the name inappropriately implies medical claims or contributes to an overstatement of efficacy. If the FDA objects to any proposed proprietary product name, we may be required to expend significant additional resources in an effort to identify a suitable proprietary product name that would qualify under applicable laws, not infringe the existing rights of third parties and be acceptable to the FDA.
Our product candidates, if approved, may require REMS, which may significantly increase our costs.
Our product candidates, if approved, may require REMS. The REMS may include requirements for special labeling or medication guides for patients, special communication plans to healthcare professionals and restrictions on distribution and use. We cannot predict the specific scope or magnitude of REMS that may be required as part of the FDA's approval of our other product candidates. Depending on the extent of the REMS requirements, our costs to commercialize our product candidates may increase significantly and distribution restrictions could limit sales. Similar requirements may arise in countries outside of the United States.
Changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols submitted to applicable regulatory authorities to reflect these changes. Amendments may require us to resubmit clinical trial protocols to IRBs or ethics committees for re-examination, which may impact the costs, timing or successful completion of a clinical trial.
The FDA's and other regulatory authorities' policies may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our other product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained.
If we are required to conduct additional clinical trials or other studies with respect to our product candidates beyond those that we currently contemplate, or if we are unable to successfully complete our clinical trials or other studies, we may be delayed in obtaining regulatory approval of any of our product candidates, we may not be able to obtain regulatory approval at all or we may obtain approval of indications that are not as broad as intended. Our product development costs will also increase if we experience delays in testing or approvals, and we may not have sufficient funding to complete the testing and approval process for our product candidates. Significant clinical trial delays could allow our competitors to bring products to market before we do and impair our ability to commercialize our products if and when approved. If any of this occurs, our business would be harmed.
Risks Related to Our Reliance on Third Parties
If we encounter difficulties in maintaining commercial manufacturing and supply agreements with our third-party manufacturers and suppliers of XHANCE, our ability to commercialize XHANCE would be impaired.
We do not own any manufacturing facilities. We currently have no plans to build our own clinical or commercial scale manufacturing facility. We lack the resources to manufacture and test, on a commercial scale, the technical performance of XHANCE and our other product candidates. We currently rely, and expect to continue to rely, on a limited number of experienced personnel and CMOs and suppliers who assist in the production, assembly, test, supply, storage and distribution of XHANCE and its components in our clinical trials and FDA registration, and we control only some of the aspects of their activities. We may not be able to maintain terms that are favorable to us. We may not be able to enter into commercial manufacturing and supply agreements with any necessary third parties, should such additional agreements become necessary. If we are unable to enter into such agreements or maintain existing agreements, each on commercially reasonable terms, our ability to commercialize XHANCE would be impaired, and our business, financial condition and results of operations would be materially adversely affected.
57
If we encounter issues with our contract manufacturers or suppliers, we may need to qualify alternative manufacturers or suppliers, which could impair our ability to sufficiently and timely manufacture and supply XHANCE.
We currently depend on contract manufacturers and suppliers for XHANCE and its components. Although we could obtain each of these components from other third-party suppliers, we would need to qualify and obtain FDA approval for another contract manufacturer or supplier as an alternative source for each such component, which could be costly and cause significant delays. Each of our current commercial manufacturing and supply agreements include limitations on our ability to utilize alternative manufacturers or suppliers for these components above certain specified thresholds during the terms of the agreements, which impairs our ability to fully implement any future manufacturing strategies to prevent supply shortages or quality issues.
In addition, some of our suppliers, including our active pharmaceutical ingredient, or API, supplier and our contract manufacturers, conduct their manufacturing operations for us at a single facility. Unless and until we qualify additional facilities, we may face limitations in our ability to respond to manufacturing and supply issues. For example, if regulatory, manufacturing or other problems require one of these manufacturers or suppliers to discontinue production at their respective facility, or if the equipment used for the production of XHANCE in these facilities is significantly damaged or destroyed by fire, flood, earthquake, power loss or similar events, the ability of such manufacturer or supplier to provide components or API needed for XHANCE, or to manufacture XHANCE may be significantly impaired. In the event that these parties suffer a temporary or protracted loss of its facility or equipment, we would still be required to obtain FDA approval to qualify a new manufacturer or supplier, as applicable, as an alternate manufacturer or source for the respective component before any components manufactured by such manufacturer or by such supplier could be sold or used.
Any production shortfall that impairs the supply of XHANCE or any of these components could have a material adverse effect on our business, financial condition and results of operations and adversely affect our ability to satisfy demand for XHANCE, which could adversely affect our product sales and operating results materially.
If third-party manufacturers, wholesalers and distributors fail to devote sufficient time and resources to XHANCE or their performance is substandard, our product supply may be impacted.
Our reliance on a limited number of manufacturers, wholesalers and distributors exposes us to the following risks, any of which could limit commercial supply of our products, result in higher costs, or deprive us of potential product revenues:
▪ | our CMOs, or other third parties we rely on, may encounter difficulties in achieving the volume of production needed to satisfy commercial demand, may experience technical issues that impact quality or compliance with applicable and strictly enforced regulations governing the manufacture of pharmaceutical products, and may experience shortages of qualified personnel to adequately staff production operations; |
▪ | our wholesalers and distributors could become unable to sell and deliver XHANCE for regulatory, compliance and other reasons; |
▪ | our CMOs, wholesalers and distributors could default on their agreements with us to meet our requirements for commercial supply of XHANCE; |
▪ | our CMOs, wholesalers and distributors may not perform as agreed or may not remain in business for the time required to successfully produce, store, sell and distribute our products and we may incur additional cost; and |
▪ | if our CMOs, wholesalers and distributors were to terminate our arrangements or fail to meet their contractual obligations, we may be forced to delay our commercial programs. |
Our reliance on third parties reduces our control over our product candidate development and commercialization activities but does not relieve us of our responsibility to ensure compliance with all required legal, regulatory and scientific standards. For example, the FDA and other regulatory authorities require that our product candidates and any products that we may eventually commercialize be manufactured according to cGMP and similar foreign standards. Any failure by our third-party manufacturers to comply with cGMP or failure to scale up manufacturing processes, including any failure to deliver sufficient quantities of product candidates in a timely manner, could lead to a delay in, or failure to obtain, regulatory approval of any of our product candidates or supply our commercial volume of XHANCE. In addition, such failure could be the basis for the FDA to issue a warning or untitled letter, withdraw approvals for product candidates previously granted to us, or take other regulatory or legal action,
58
including recall or seizure, total or partial suspension of production, suspension of ongoing clinical trials, refusal to approve pending applications or supplemental applications, detention or product, refusal to permit the import or export of products, injunction, imposing civil penalties or pursuing criminal prosecution.
Manufacturing issues may arise that could increase product and regulatory approval costs or delay commercialization.
As we further scale up manufacturing of XHANCE and conduct required stability testing, issues may arise involving product-packaging and third-party equipment malfunctions. These issues may require refinement or resolution in order to continue with commercial marketing of XHANCE. In addition, quality issues may arise during scale-up and of commercial manufacturing processes. Any issues in our product or delivery devices could result in increased scrutiny by regulatory authorities, delays in our regulatory approval process, increases in our operating expenses, or failure to obtain or maintain approval for our products.
We rely on third parties to conduct our clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, or if they terminate their agreement with us, we may not be able to obtain regulatory approval for or commercialize our product candidates.
We have relied upon and plan to continue to rely upon CROs to monitor and manage data for our prospective preclinical and clinical programs. We rely on these parties for execution of our clinical trials, and we control only some of the aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies and clinical trials are conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on the CROs does not relieve us of our regulatory responsibilities. We and our CROs are required to comply with federal regulations and current Good Clinical Practices, or GCP, which are international standards meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, advisors and monitors. GCPs are enforced by the FDA and foreign regulatory authorities in the form of International Conference on Harmonization, or ICH, guidelines for all of our product candidates in clinical development. Regulatory authorities enforce these GCP through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of our CROs fail to comply with applicable GCP and other regulations, including as a result of any recent changes in such regulations, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP requirements. In addition, our clinical trials must be conducted with product produced under cGMP requirements. While we have agreements governing activities of our CROs, we have limited influence over their actual performance. Failure to comply with applicable regulations in the conduct of the clinical trials for our product candidates may require us to repeat preclinical studies and clinical trials, which would increase our operating expenses and delay the regulatory approval process.
Our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing clinical and preclinical programs. If our CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons or if we receive additional FDA notices that do require corrective action, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, the commercial prospects for our product candidates would be harmed, our costs could increase substantially and our ability to generate revenue could be delayed.
Switching or adding additional CROs involves additional cost and requires management time and focus. Identifying, qualifying and managing performance of third-party service providers can be difficult, time-consuming and cause delays in our development programs. In addition, there is a natural transition period when a new CRO commences work and the new CRO may not provide the same type or level of services as the original provider. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects. If any of our relationships with our CROs terminate, we may not be able to enter into arrangements with alternative CROs or to do so on commercially reasonable terms. As a result, delays may occur, which can materially impact our ability to meet our desired clinical development timelines.
Because we have relied on third parties, our internal capacity to perform these functions is limited. Outsourcing these functions involves risks that third parties may not perform to our standards, may not produce results in a timely manner or may fail to perform at all. In addition, the use of third-party service providers requires us to
59
disclose our proprietary information to these parties, which could increase the risk that this information will be misappropriated. We currently have a small number of employees, which limits the internal resources we have available to identify and monitor our third-party providers. To the extent we are unable to identify and successfully manage the performance of third-party service providers in the future, our ability to advance our product candidates through clinical trials will be compromised. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
Risks Related to Our Business Operations and Industry
Our future success depends on our ability to retain and have the full attention of our key executives and to attract, retain and motivate other qualified personnel.
We are highly dependent on the management, development, clinical, financial and business development expertise of our executive team and, in particular, the services of Peter K. Miller, our Chief Executive Officer, and Ramy A. Mahmoud, our President and Chief Operating Officer. Each of Mr. Miller and Dr. Mahmoud is employed by us at will and is permitted to terminate his employment with us at any time. We entered into employment agreements with Mr. Miller and Dr. Mahmoud in October 2017, but Mr. Miller and Dr. Mahmoud continue to be employed at will. We do not maintain "key person" insurance for any of our executives or other employees. The loss of the services of Mr. Miller or Dr. Mahmoud could impede the achievement of our development and commercialization objectives.
Recruiting and retaining qualified employees for our business, including scientific, technical and sales and marketing personnel, will also be critical to our success. Competition for skilled personnel in our industry is intense and the turnover rate can be high. We may not be able to attract and retain personnel on acceptable terms given the competition among numerous pharmaceutical companies for individuals with similar skill sets. In addition, failure to succeed in our commercialization efforts or in the performance of any future clinical studies may make it more challenging to recruit and retain qualified personnel. The inability to recruit or loss of the services of any executive or key employee could impede the progress of our research, development and commercialization objectives.
We will need to grow the size of our organization, and we may experience difficulties in managing this growth.
Implementation of our development and commercialization strategies will require additional managerial, operational, sales, marketing, financial and other resources. Our current management, personnel and systems may not be adequate to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, employee turnover and reduced productivity. Future growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of our existing or future product candidates. Future growth would impose significant added responsibilities on members of management, including:
▪ | managing the commercialization XHANCE and any other products for which we obtain marketing approval; |
▪ | overseeing our preclinical studies and clinical trials effectively; |
▪ | identifying, recruiting, maintaining, motivating and integrating additional employees, including any sales and marketing personnel engaged in connection with the commercialization of any approved product; |
▪ | managing our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors and other third parties; and |
▪ | improving our managerial, development, operational and financial systems and procedures. |
As our operations expand, we will need to manage additional relationships with various strategic collaborators, suppliers and other third parties. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to manage our development efforts and clinical trials effectively and hire, train and integrate additional management, administrative and sales and marketing personnel. Failure to accomplish any of these activities could prevent us from successfully growing our company.
60
We are subject to intense competition and, if we are unable to compete effectively, our product candidates, if approved, may not reach their commercial potential.
The development and commercialization of new drugs is highly competitive and subject to rapid and significant technological change as research provides a deeper understanding of the pathology of diseases and new technologies and treatments are developed. We face competition with respect to XHANCE from INS, oral steroids and other medical management products, and will face competition with respect to any other product candidates that we may seek to develop or commercialize in the future, from many different sources, including large pharmaceutical, biotechnology, specialty pharmaceutical and, to a lesser degree, medical device companies.
The key competitive factors that we expect to impact the commercial success of XHANCE and any other product candidates we may develop are likely to be their efficacy, safety and tolerability profile, reliability, convenience of administration, price and reimbursement. Nasonex, marketed by Merck, is currently the only other branded drug therapy approved by the FDA for the treatment of nasal polyps, which is our initial indication for XHANCE. A generic version of Nasonex, mometasone furoate monohydrate, was approved by the FDA for, among other indications, the treatment of nasal polyps and launched in 2016. In addition, Beconase AQ, which is an INS marketed by GlaxoSmithKline, is indicated for the prophylaxis of nasal polyps after surgical resection. We are not aware of any drug therapy approved by the FDA or foreign regulatory agencies for the treatment of chronic sinusitis.
Even though they have not been approved for the treatment of such indications, published clinical practice guidelines do recommend the use of INS products for the treatment of chronic rhinosinusitis and nasal polyps in an effort to maximize medical therapy prior to surgical intervention. Currently approved branded INS products include Rhinocort, marketed by AstraZeneca, Nasacort AQ, marketed by sanofi-aventis, Beconase AQ, Flonase, and Veramyst, each marketed by GlaxoSmithKline, Qnasl, marketed by Teva Pharmaceuticals, and Omnaris and Zetonna, each marketed by Sunovion Pharmaceuticals. Due to the limitations of current treatments, several companies are investigating the treatment of nasal polyps with biologic monoclonal antibodies. To date, four biologic monoclonal antibodies have been studied in nasal polyps: omalizumab, reslizumab, mepolizumab and dupilumab. Most of these INS and biologics companies, as well as other potential competitors, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products.
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a small number of our competitors. Accordingly, our competitors may be more successful than we may be in obtaining FDA approval of drugs and achieving widespread market acceptance. Our competitors' drugs, or drugs they may develop in the future, may be more effective, or more effectively marketed and sold, than any drug we may commercialize and may render XHANCE or any of our other product candidates we may develop obsolete or non-competitive before we can recover the expenses of developing and commercializing XHANCE or any of our other product candidates. Our competitors may also obtain FDA or other regulatory approval of products more rapidly than expected or may obtain better or preferred market access by offering large rebates to payors or by other means. We may not have accurately or completely predicted the development of new and improved or low-cost surgical interventions, alternative medical therapies or other market-disrupting events. If we are unable to manufacture, distribute, stimulate demand reaching the predicted market share, overcome barriers to access or otherwise effectively commercialize the product, all of which factors may be influenced by current or future competition, then our opportunity to generate revenue from the sale of XHANCE or any of our other product candidates, if approved, will be compromised.
Our long-term growth depends on our ability to develop and commercialize additional ENT products.
It is important to our business that we continue to build a more complete product offering within the ENT and allergy markets. We are evaluating the use of our proprietary EDS technology to develop new product candidates for the ENT and allergy markets. Developing additional product candidates is expensive and time-consuming and could divert management's attention away from the commercialization of XHANCE. Even if we are successful in developing additional product candidates, the success of any new product candidates or enhancement to any existing product candidates will depend on several factors, including our ability to:
▪ | properly identify and anticipate ENT and allergy physician and patient needs; |
▪ | develop, obtain necessary regulatory clearances or approvals, and introduce new product candidates or product enhancements in a timely manner; |
61
▪ | demonstrate, if required, the safety and efficacy of new product candidates with data from preclinical studies and clinical trials; |
▪ | avoid infringing upon the intellectual property rights of third parties; |
▪ | comply with all regulations relating to the marketing of new product candidates, including any new or modified EDS technologies; and |
▪ | provide adequate training to potential users of our product candidates. |
If we are unsuccessful in developing and commercializing additional product candidates in other areas of the ENT and allergy markets, our ability to gain and maintain profitability may be impaired.
We may acquire other assets or businesses, or form collaborations or make investments in other companies or technologies, which could negatively impact our operating results, dilute our stockholders' ownership, increase our debt or cause us to incur significant expense.
As part of our business strategy, we may pursue acquisitions of assets, including preclinical, clinical or commercial-stage products or product candidates, businesses or strategic alliances and collaborations, to expand our existing technologies and operations. We may not identify or complete these transactions in a timely manner, on a cost-effective basis, or at all, and we may not realize the anticipated benefits of any such transaction. We may not be able to find suitable acquisition candidates, and if we make any acquisitions, we may not be able to complete technology transfers and integrate these acquisitions successfully into our existing business and we may incur additional debt or assume unknown or contingent liabilities as part of the transaction. Integration of an acquired company or assets may also disrupt ongoing operations, require the hiring of additional personnel and the implementation of additional internal systems and infrastructure, especially the acquisition of commercial assets, and require management resources that would otherwise focus on developing our existing business. We may not be able to find suitable strategic collaborators or identify other investment opportunities, and we may experience losses related to any such investments.
To finance any acquisitions or collaborations, we may choose to issue debt or shares of our common or preferred stock as consideration. Any such issuance of shares would dilute the ownership of our stockholders. If the price of our common stock is low or volatile, we may not be able to acquire other assets or companies or fund a transaction using our stock as consideration. Alternatively, it may be necessary for us to raise additional funds for acquisitions through public or private financings. Additional funds may not be available on terms that are favorable to us, or at all.
Our employees, collaborators, independent contractors, principal investigators, consultants, vendors and CROs may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk that our employees, collaborators, independent contractors, principal investigators, consultants, vendors and CROs may engage in fraudulent or other illegal activity with respect to our business. Misconduct by these employees could include intentional, reckless and/or negligent conduct or unauthorized activity that violates:
▪ | FDA regulations, including those laws requiring the reporting of true, complete and accurate information to the FDA; |
▪ | manufacturing standards; |
▪ | federal and state healthcare fraud and abuse laws and regulations; or |
▪ | laws that require the true, complete and accurate reporting of financial information or data. |
In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Misconduct by these parties could also involve individually identifiable information, including, without limitation, the improper use of information obtained in the course of clinical trials, or illegal misappropriation of drug product, which could result in regulatory sanctions and serious harm to our reputation. Any incidents or any other conduct that leads to an employee receiving an FDA debarment could result in a loss of business from third parties and severe reputational harm.
62
In October 2017, we adopted a Code of Business Conduct and Ethics to govern and deter such behaviors, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and curtailment of our operations.
The security of our information technology systems may be compromised, and confidential information, including non-public personal information that we maintain, could be improperly disclosed.
Our information technology systems may be vulnerable to physical or electronic intrusions, computer viruses or other attacks. As part of our business, we and our vendors maintain large amounts of confidential information, including non-public personal information on patients, our employees and business partners. Breaches in security could result in the loss or misuse of this information, which could, in turn, result in potential regulatory actions or litigation, including material claims for damages, interruption to our operations, damage to our reputation or otherwise have a material adverse effect on our business, financial condition and operating results. We expect to have appropriate information security policies and systems in place in order to prevent unauthorized use or disclosure of confidential information, including non-public personal information, however, there can be no assurance that such use or disclosure will not occur.
If we fail to comply with data protection laws and regulations, we could be subject to government enforcement actions, which could include civil or criminal penalties, as well as private litigation and/or adverse publicity, any of which could negatively affect our operating results and business.
We may be subject to laws and regulations that address privacy and data security of patients who use our product candidates in the United States and in states in which we conduct our business. In the United States, numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act) govern the collection, use, disclosure, and protection of health-related and other personal information. For instance, HIPAA imposes certain obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information and imposes notification obligations in the event of a breach of the privacy or security of individually identifiable health information on entities subject to HIPAA and their business associates that perform certain activities that involve the use or disclosure of protected health information on their behalf. Certain of these laws and regulations are described in greater detail in the previous section under "Business — Government Regulation — Healthcare Privacy Laws." Failure to comply with applicable data protection laws and regulations could result in government enforcement actions and create liability for us, which could include civil and/or criminal penalties, as well as private litigation and/or adverse publicity that could negatively affect our operating results and business.
Our business and operations would suffer in the event of computer system failures, cyberattacks or a deficiency in our cybersecurity.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures, cyberattacks or cyber-intrusions over the Internet, attachments to emails, persons inside our organization, or persons with access to systems inside our organization. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. Such an event could cause interruption of our operations. For example, the loss of data from completed or ongoing clinical trials for our product candidates could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of or damage to our data, or inappropriate disclosure of confidential, proprietary or personal information, we could incur material legal claims and liability and damage to our reputation and the development and commercialization of our product candidates could be delayed.
63
If we fail to successfully manage the implementation of our new global enterprise resource planning system, it could adversely affect our operations and operating results.
We are in the process of implementing a new global enterprise resource planning (ERP) system. This system will replace many of our existing operating and financial systems. Such an implementation is a major undertaking, both financially and from a management and personnel perspective. Any disruptions, delays or deficiencies in the design and implementation of our new ERP system could adversely affect our ability to process financial transactions, fulfill contractual obligations or otherwise operate our business.
We are subject to risks inherent in foreign operations.
We currently operate portions of our business through our foreign subsidiaries, including through our Norwegian subsidiary, OptiNose AS, which currently owns a substantial portion of our intellectual property and conducts development activities, and our United Kingdom subsidiary OptiNose UK Ltd., which performs research and development for the Optinose EDS technology as well as other services. We have committed, and intend to continue to commit, resources to our international operations. We are subject to a number of risks associated with our international business operations and activities that may increase liability, costs, and require significant management attention. These risks include:
▪ | compliance with the laws of the United States, the United Kingdom, Norway, and other countries that apply to our international operations, including import and export legislation; |
▪ | compliance with foreign data protection laws and regulations in the United Kingdom, Norway and other countries that apply to our international operations; |
▪ | the complexities and expenses of administering a business abroad; |
▪ | complications in compliance with, and unexpected changes in, tariffs, trade barriers, price and exchange controls and other foreign regulatory requirements, including potential trade conflicts, changes to trade agreements/treaties, and the implementation of trade restrictions; |
▪ | instability in economic or political conditions, including inflation, recession and actual or anticipated military conflicts, social upheaval or political uncertainty; |
▪ | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; |
▪ | uncertainties of laws and enforcement relating to the protection of intellectual property or secured technology; |
▪ | litigation in foreign court systems; |
▪ | language barriers; |
▪ | changes in tax laws and regulations in the jurisdictions in which we operate; |
▪ | compliance with tax, employment, immigration and labor laws, regulations and restrictions for employees living or traveling abroad; |
▪ | difficulties staffing and managing foreign operations; and |
▪ | workforce uncertainty in countries where labor unrest is more common than in the United States; |
There can be no assurance that the policies and procedures we implement to address or mitigate these risks will be successful, that our personnel will comply with them or that we will not experience these factors in the future or that they will not have a material adverse effect on our business, results of operations and financial condition.
Our corporate structure and foreign operations may have adverse tax consequences and expose us to additional tax liabilities.
A substantial portion of our intellectual property, including rights to XHANCE and rights under the AVP-825 License Agreement, are owned by OptiNose AS, our Norwegian subsidiary. In addition, as we plan for the commercial launch of XHANCE, commercial functions may be conducted by a current or potential future foreign subsidiary.
64
We operate pursuant to written intercompany service and related agreements, or transfer pricing agreements. These transfer pricing agreements establish transfer prices for intellectual property licenses, production, marketing, management, technology development and other services performed by our group companies for other group companies. Transfer prices are prices that one company in a group of related companies charge to another member of the group for goods, services or the use of property. If two or more affiliated companies are located in different countries, the tax laws or regulations of each country generally will require that transfer prices be consistent with those between unrelated companies dealing at arm's length. Our transfer pricing arrangements are not binding on applicable tax authorities, and, if tax authorities in any country were successful in challenging our transfer prices as not representing arm's length transactions, they could require us to adjust our transfer prices and thereby reallocate our income. A reallocation of taxable income from one tax jurisdiction to another tax jurisdiction could result in a higher tax liability to us. In addition, if the country from which the income is reallocated does not agree with the reallocation, both countries could tax the same income, resulting in double taxation.
If we generate sales of XHANCE in the United States or otherwise generate any other sales or revenues, a portion of the income we generate may be allocated to one or more of our current or future foreign subsidiaries and repatriation of any cash from our foreign subsidiaries to the United States may trigger significant adverse tax consequences. If we generate cash through our foreign operations or if the cash generated by our U.S. operations is not sufficient to fund our U.S. operations, we may face challenges applying any such cash held by our foreign subsidiaries to support the growth of our U.S. operations and any strategic opportunities in the United States. If we are forced to repatriate any foreign-held cash, we could incur a significant tax charge, and our business, operating results or financial condition could be adversely impacted.
Income earned by our foreign subsidiaries may give rise to United States corporate income tax, even if there are no distributions to the United States, to the extent that our foreign subsidiaries generate income that is subject to Subpart F or similar provisions of the U.S. Internal Revenue Code, such as the new global intangible low-taxed income provisions, generally referred to in this paragraph as Subpart F. Subpart F income includes, for example, certain "passive" income, certain income from intercompany transactions involving our foreign subsidiaries, foreign subsidiary income over a legislative threshold, and certain income of any foreign subsidiary which makes an "investment in U.S. property", such as holding the stock in, or making a loan to, a U.S. corporation. Any income taxable under Subpart F is currently taxable in the United States at federal corporate income tax rates of up to 21.0%, even if it is not distributed to us. We have not treated any of our foreign subsidiaries' income as being Subpart F income pursuant to available exemptions for which we believe we qualify. We may, however, be required to do so and pay taxes on the prior and future income of our foreign subsidiaries if we do not qualify for an available exemption.
Comprehensive tax reform legislation could adversely affect our business and financial condition.
On December 22, 2017, the US government signed into law comprehensive tax legislation, referred to as the Tax Cuts and Jobs Act, or the Tax Act. The Tax Act introduced significant changes to the US tax laws. The Tax Act, among other things, contains significant changes to corporate taxation, including but not limited to the reduction of the corporate tax rate from a top rate of 35% to a flat rate of 21%, limitation of the tax deduction for interest expense to 30% of adjusted earnings (except for certain small businesses), limitation of the deduction for net operating losses to 80% of current year taxable income in respect of losses arising in taxable years beginning after 2017 and elimination of net operating loss carrybacks, one time taxation of offshore earnings at reduced rates regardless of whether they are repatriated, immediate deductions for certain new investments instead of deductions for depreciation expense over time, and modifying or repealing many business deductions and credits (including reducing the business tax credit for certain clinical testing expenses incurred in the testing of certain drugs for rare diseases or conditions generally referred to as “orphan drugs”). Any federal net operating loss carryovers for taxable years beginning after 2017 will be carried forward indefinitely pursuant to the Tax Act. The Tax Act also limits deductions for compensation in excess of $1 million, which could impact our ability to deduct such corporate expenses. We continue to examine the impact the Tax Act may have on our business. Notwithstanding the reduction in the federal corporate income tax rate, the overall impact of the Tax Act is uncertain and our business and financial condition could be adversely affected.
We may be exposed to liabilities under the U.S. Foreign Corrupt Practices Act and other U.S. and foreign anti-corruption anti-money laundering, export control, sanctions, and other trade laws and regulations, and any determination that we violated these laws could have a material adverse effect on our business.
We are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, and various economic and trade sanctions regulations administered by the U.S. Treasury Department's Office of Foreign Assets Control. We are also subject to the U.S. Foreign Corrupt
65
Practices Act of 1977, as amended, or FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, the United Kingdom Bribery Act 2010, the Proceeds of Crime Act 2002, and possibly other anti-bribery and anti-money laundering laws in countries outside of the United States in which we conduct our activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees and third-party intermediaries from authorizing, promising, offering, providing, soliciting, or accepting, directly or indirectly, improper payments or benefits to or from any person whether in the public or private sector. As we commercialize XHANCE and any other product candidates that we may develop, we may engage with third-party manufacturers and collaborators who operate abroad and are required to obtain certain necessary permits, licenses and other regulatory approvals with respect to our business. Our activities abroad create the risk of unauthorized payments or offers of payments by employees, consultants, sales agents or distributors, even though they may not always be subject to our control. It is our policy to implement safeguards to discourage these practices by our employees, consultants, sales agents and distributors. However, our existing safeguards and any future improvements may prove to be less than effective, and the employees, consultants, sales agents, or distributors of our company may engage in conduct for which we might be held responsible, even if we do not explicitly authorize such activities.
Noncompliance with anti-corruption, anti-money laundering, export control, sanctions, and other trade laws could subject us to whistleblower complaints, investigations, sanctions, settlements, prosecution, other enforcement actions, disgorgement of profits, significant fines, damages, other civil and criminal penalties or injunctions, suspension and/or debarment from contracting with certain persons, the loss of export privileges, reputational harm, adverse media coverage and other collateral consequences. If any subpoenas or investigations are launched, or governmental or other sanctions are imposed, or if we do not prevail in any possible civil or criminal litigation, our business, results of operations and financial condition could be materially harmed. Responding to any action will likely result in a materially significant diversion of management's attention and resources and significant defense and compliance costs and other professional fees. In addition, the U.S. government may seek to hold us liable for successor liability FCPA violations committed by companies in which we invest or that we acquire. As a general matter, enforcement actions and sanctions could harm our business, results of operations, and financial condition.
Risks Related to Our Intellectual Property
If we are unable to protect our intellectual property rights or if our intellectual property rights are inadequate to protect our technology, XHANCE or our other product candidates, our competitors could develop and commercialize technology similar to ours, and our competitive position could be harmed.
Our commercial success will depend in large part on our ability to obtain and maintain patent and other intellectual property protection in the United States and other countries with respect to our proprietary technology and products. We rely on trade secret, patent, copyright and trademark laws, and confidentiality and other agreements with employees and third parties, all of which offer only limited protection. Our strategy is to seek patent protection for XHANCE, our other product candidates and their compositions, their methods of use and processes for their manufacture, and any other aspects of inventions that are commercially important to the development of our business.
The patent prosecution process is expensive and time-consuming, and we and any future licensors and licensees may not be able to apply for or prosecute patents on certain aspects of our product candidates or delivery technologies at a reasonable cost, in a timely fashion, or at all. We may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the rights to patents licensed to third parties. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. It is also possible that we or any future licensors or licensees, will fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Therefore, our patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. It is possible that defects of form in the preparation or filing of our patents or patent applications may exist, or may arise in the future, such as with respect to proper priority claims, inventorship, claim scope or patent term adjustments. If any future licensors or licensees, are not fully cooperative or disagree with us as to the prosecution, maintenance, or enforcement of any patent rights, such patent rights could be compromised and we might not be able to prevent third parties from making, using, and selling competing products. If there are material defects in the form or preparation of our patents or patent applications, such patents or applications may be invalid or unenforceable. Moreover, our competitors may independently develop equivalent knowledge, methods, and know-how. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business, financial condition, and operating results.
66
The patent positions of pharmaceutical companies generally are highly uncertain, involve complex legal and factual questions and have in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of any patents that issue, are highly uncertain. The steps we have taken to protect our proprietary rights may not be adequate to preclude misappropriation of our proprietary information or infringement of our intellectual property rights, both inside and outside the United States. Further, the examination process may require us to narrow the claims of pending patent applications, which may limit the scope of patent protection that may be obtained if these applications issue. The rights that may be granted under future issued patents may not provide us with the proprietary protection or competitive advantages we are seeking. If we are unable to obtain and maintain patent protection for our technology and products, or if the scope of the patent protection obtained is not sufficient, our competitors could develop and commercialize technology and products similar or superior to ours, and our ability to successfully commercialize our technology and products may be impaired.
As of December 31, 2017, we owned a total of 43 U.S. patents and 43 pending U.S. patent applications. These U.S. patents will expire between 2020 and 2030. With respect to these patent rights, we do not know whether any of our patent applications will result in issued patents or, if any of our patent applications do issue, whether such patents will protect our technology and drugs, in whole or in part, or whether such patents will effectively prevent others from commercializing competitive technologies and products. There is no guarantee that any of our issued or granted patents will not later be found invalid or unenforceable.
The laws of foreign countries may not protect our rights to the same extent as the laws of the United States or vice versa. For example, European patent law restricts the patentability of methods of treatment of the human body more than United States law does. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing or in some cases not at all, until they are issued as a patent. Therefore, we cannot be certain that we were the first to make the inventions claimed in our pending patent applications, that we were the first to file for patent protection of such inventions, or that we have found all of the potentially relevant prior art relating to our patents and patent applications that could invalidate one or more of our patents or prevent one or more of our patent applications from issuing. Even if patents do successfully issue and even if such patents cover our product candidates, third parties may initiate oppositions, interferences, re-examinations, post-grant reviews, inter partes reviews, nullification or derivation actions in court or before patent offices or similar proceedings challenging the validity, enforceability, or scope of such patents, which may result in the patent claims being narrowed or invalidated. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our product candidates, or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties.
Furthermore, the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and drugs, or limit the duration of the patent protection of our technology and drugs. Given the amount of time required for the development, testing and regulatory review of new drug candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing drugs similar or identical to ours.
We may become involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents or the patents of any party from whom we may license patents from in the future. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In a patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the U.S. Patent and Trademark Office, or USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. A court may decide that a patent of ours or of any of our future licensors is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. In addition, to the extent that we have to file patent litigation in a
67
federal court against a U.S. patent holder, we would be required to initiate the proceeding in the state of incorporation or residency of such entity. With respect to the validity question, for example, we cannot be certain that no invalidating prior art exists. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated, found unenforceable, or interpreted narrowly, and it could put our patent applications at risk of not issuing. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on one or more of our products or certain aspects of the Optinose EDS technology. Such a loss of patent protection could compromise our ability to pursue our business strategy.
Interference proceedings brought by the USPTO may be necessary to determine the priority of inventions with respect to our patents and patent applications or those of our collaborators or licensors. An unfavorable outcome could require us to cease using the technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if a prevailing party does not offer us a license on terms that are acceptable to us. Litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distraction of our management and other employees. We may not be able to prevent, alone or with any of our future licensors, misappropriation of our proprietary rights, particularly in countries where the laws may not protect those rights as fully as in the United States. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
Moreover, we may be subject to a third-party pre-issuance submission of prior art to the USPTO or other foreign patent offices, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or drugs and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize drugs without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop, or commercialize current or future product candidates.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on XHANCE, our other product candidates and the Optinose EDS technology throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States may be less extensive than those in the United States. In addition, the laws and practices of some foreign countries do not protect intellectual property rights, especially those relating to life sciences, to the same extent as federal and state laws in the United States. For example, novel formulations of existing drugs and manufacturing processes may not be patentable in certain jurisdictions, and the requirements for patentability may differ in certain countries, particularly developing countries. Also, some foreign countries, including European Union countries, India, Japan and China, have compulsory licensing laws under which a patent owner may be compelled under certain circumstances to grant licenses to third parties. Consequently, we may have limited remedies if patents are infringed or if we are compelled to grant a license to a third party, and we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions into or within the United States or other jurisdictions. This could limit our potential revenue opportunities. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products, and may export otherwise infringing products to territories where we have patent protection, but where enforcement is not as strong as that in the United States. These products may compete with our products in jurisdictions where we do not have any issued patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from competing with us in these jurisdictions. Furthermore, the prevalence of counterfeit medicines, which is one that has been deliberately and fraudulently mislabeled as to its identity and source, is a significant and growing industry-wide issue that could impact our revenue and our reputation for which we may have limited or no recourse. Accordingly, our efforts to enforce intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from our intellectual property. We may not prevail in any lawsuits that we initiate in these foreign countries and the damages or other remedies awarded, if any, may not be commercially meaningful.
68
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and applications are required to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and applications. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process and after a patent has issued. There are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which could be uncertain and could harm our business.
Our commercial success depends upon our ability to develop, manufacture, market and sell XHANCE and our other product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. While our product candidates are in preclinical studies and clinical trials, we believe that the use of our product candidates in these preclinical studies and clinical trials falls within the scope of the exemptions provided by 35 U.S.C. Section 271(e) in the United States, which exempts from patent infringement liability activities reasonably related to the development and submission of information to the FDA. As XHANCE and our other product candidates progress toward commercialization, the possibility of a patent infringement claim against us increases. For instance, our use of the Section 505(b)(2) regulatory pathway for the follow-on indication of chronic sinusitis or any of our other product candidates will require us to provide a Paragraph IV certification to the NDA and patent holders of the RLD pursuant to the Hatch-Waxman Act if the RLD is covered by Orange Book-listed patents. If the NDA or patent holder files a patent infringement lawsuit against us within 45 days of its receipt of notice of our certification, the FDA is prevented from approving our Section 505(b)(2) NDA until the earliest of 30 months, expiration of the patents, settlement of the lawsuit or a court decision in the infringement case that is favorable to us. Accordingly, we may invest significant time and expense in the development of our product candidates only to be subject to significant delay and expensive and time-consuming patent litigation before our product candidates may be commercialized. There can be no assurance that our product candidates do not infringe other parties' patents or other proprietary rights and competitors or other parties may assert that we infringe their proprietary rights in any event.
There is considerable intellectual property litigation in the biotechnology and pharmaceutical industries. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our product candidates, including interference or derivation proceedings before the USPTO. Numerous U.S. and foreign issued patents and pending patent applications owned by third parties exist in the fields in which we are developing our drug candidates. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future.
If we are found to infringe a third party's intellectual property rights, we could be required to obtain a license from such third party to continue commercializing our product candidates. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if a license can be obtained on acceptable terms, the rights may be non-exclusive, which could give our competitors access to the same technology or intellectual property rights licensed to us. If we fail to obtain a required license, we may be unable to effectively market product candidates based on our technology, which could limit our ability to generate revenue or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations. Alternatively, we may need to redesign our infringing products, which may be impossible or require substantial time and monetary expenditure. Under certain circumstances, we could be forced, including by court order, to cease commercializing our product candidates. In addition, in any such proceeding or litigation, we could be found liable for substantial monetary damages, potentially including treble damages and attorneys' fees, if we are found to have willfully infringed. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could harm our business. Any claims by third parties that we have misappropriated their confidential information or trade secrets could have a similar negative impact on our business.
The cost to us in defending or initiating any litigation or other proceeding relating to patent or other proprietary rights, even if resolved in our favor, could be substantial, and litigation would divert our management's attention. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could compromise our commercialization efforts, delay our research and
69
development efforts and limit our ability to continue our operations. There could also be public announcements of the results of the hearing, motions, or other interim proceedings or developments. If securities analysts or investors perceive those results to be negative, it could cause the price of shares of our common stock to decline.
Our competitors may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner.
Our competitors may seek to market generic versions of any of our approved products by submitting ANDAs to the FDA or new products that use our approved products as the RLD, in each case where our competitors claim that our patents are invalid, unenforceable or not infringed. Alternatively, our competitors may seek approval to market their own products that are the same as, similar to or otherwise competitive with XHANCE and any future product candidates we may develop. In these circumstances, we may need to defend or assert our patents, by means including filing lawsuits alleging patent infringement requiring us to engage in complex, lengthy and costly litigation or other proceedings. In any of these types of proceedings, a court or government agency with jurisdiction may find our patents invalid, unenforceable or not infringed. We may also fail to identify patentable aspects of our research and development before it is too late to obtain patent protection. Even if we have valid and enforceable patents, these patents still may not provide protection against competing products or processes sufficient to achieve our business objectives.
Changes in either U.S. or foreign patent law or interpretation of such laws could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other pharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the pharmaceutical industry involve both technological and legal complexity, and it therefore is costly, time-consuming and inherently uncertain. In addition, on September 16, 2011, the Leahy-Smith America Invents Act, or the AIA, was signed into law. The AIA includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation.
An important change introduced by the AIA is that, as of March 16, 2013, the United States transitioned to a "first-to-file" system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that files a patent application in the USPTO after that date, but before us, could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by the third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application.
Among some of the other changes introduced by the AIA are changes that limit where a patentee may file a patent infringement suit and providing opportunities for third parties to challenge any issued patent in the USPTO. This applies to all of our U.S. patents, even those issued before March 16, 2013. Because of a lower evidentiary standard necessary to invalidate a patent claim in USPTO proceedings compared to the evidentiary standard in United States federal court, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action.
Depending on decisions by the U.S. Congress, the federal courts, the USPTO, or similar authorities in foreign jurisdictions, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
We may be subject to claims asserting that our employees, consultants, independent contractors and advisors have wrongfully used or disclosed confidential information and/or alleged trade secrets of their current or former employers or claims asserting ownership of what we regard as our own intellectual property.
Although we try to ensure that our employees, consultants, independent contractors and advisors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these individuals or we have inadvertently or otherwise used or disclosed confidential information and/or intellectual property, including trade secrets or other proprietary information, of the companies that any such individual currently or formerly worked for or provided services to. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property
70
rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to our business.
In addition, while we require our employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that we regard as our own. The assignment of intellectual property rights may not be self-executing or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property.
Intellectual property rights do not prevent all potential threats to competitive advantages we may have.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and intellectual property rights may not adequately protect our business or permit us to maintain our competitive advantage.
The following examples are illustrative:
▪ | Others may be able to make drug and device components that are the same as or similar to XHANCE and our other product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed; |
▪ | We or any of our licensors or collaborators might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed; |
▪ | We or any of our licensors or collaborators might not have been the first to file patent applications covering certain of our inventions; |
▪ | Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
▪ | The prosecution of our pending patent applications may not result in granted patents; |
▪ | Granted patents that we own or have licensed may not cover our products or may be held not infringed, invalid or unenforceable, as a result of legal challenges by our competitors; |
▪ | With respect to granted patents that we own or have licensed, especially patents that we either acquire or in-license, if certain information was withheld from or misrepresented to the patent examiner, such patents might be held to be unenforceable; |
▪ | Patent protection on our product candidates may expire before we are able to develop and commercialize the product, or before we are able to recover our investment in the product; |
▪ | Our competitors might conduct research and development activities in the United States and other countries that provide a safe harbor from patent infringement claims for such activities, as well as in countries in which we do not have patent rights, and may then use the information learned from such activities to develop competitive products for sale in markets where we intend to market our product candidates; |
▪ | We may not develop additional proprietary technologies that are patentable; |
▪ | The patents of others may have an adverse effect on our business; and |
▪ | We may choose not to file a patent application for certain technologies, trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property. |
Should any of these events occur, they could significantly harm our business, financial condition, results of operations and prospects.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patent protection for certain aspects of our product candidates and delivery technologies, we also consider trade secrets, including confidential and unpatented know-how important to the maintenance of our competitive position. We protect trade secrets and confidential and unpatented know-how, in part, by customarily
71
entering into non-disclosure and confidentiality agreements with parties who have access to such knowledge, such as our employees, outside scientific and commercial collaborators, CROs, CMOs, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants that obligate them to maintain confidentiality and assign their inventions to us. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. In addition, our trade secrets may otherwise become known, including through a potential cybersecurity breach, or may be independently developed by competitors.
Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts in the United States and certain foreign jurisdictions are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
If our trademarks are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
We expect to rely on trademarks as one means to distinguish any of our product candidates that are approved for marketing from the products of our competitors. OPTINOSE®, XHANCETM and Breath Powered® are trademarks or registered trademarks of ours in the United States. Our trademarks may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks or may be forced to stop using these names, which we need for name recognition by potential partners or customers in our markets of interest. If we are unable to establish name recognition based on our trademarks, we may not be able to compete effectively.
Risks Related to Ownership of Our Common Stock and Our Status as a Public Company
An active market for our common stock may not develop.
We completed our initial public offering in October 2017, but prior to that offering there was no market for our shares of common stock. Although our common stock is listed on Nasdaq, an active market for our common stock may not develop. The lack of an active market may impair your ability to sell your shares at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the fair market value of your shares. Further, an inactive market may also impair our ability to raise capital by selling shares of our common stock and may impair our ability to enter into strategic collaborations or acquire companies or products by using our shares of common stock as consideration.
The price of our common stock may be volatile and you may lose all or part of your investment.
The market price of our common stock is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. In addition to the factors discussed in this "Risk Factors" section and elsewhere in this 10-K, these factors include:
▪ | our ability to successfully commercialize XHANCE; |
▪ | any delay in our regulatory approval or filings for XHANCE for a follow-on indication for the treatment of chronic sinusitis or any other product candidate we may develop, and any adverse development or perceived adverse development with respect to the applicable regulatory authority's review of such filings, including without limitation the FDA's issuance of a "refusal to file" letter, a request for additional information, or a CRL; |
▪ | the success of competitive products or technologies; |
▪ | adverse regulatory actions with respect to our product candidates, including the failure to receive regulatory approval, or our competitors' products or product candidates; |
▪ | actual or anticipated changes in our growth rate relative to our competitors; |
▪ | announcements by us or our competitors of significant acquisitions or divestitures, strategic collaborations, joint ventures, collaborations or capital commitments; |
72
▪ | the commencement, enrollment or results of planned clinical trials of our product candidates or any future clinical trials we may conduct, or any changes generally in the development status of our product candidates or those of our competitors; |
▪ | regulatory or legal developments in the United States and other countries; |
▪ | the outcome of any investigations or regulatory scrutiny of our operations or litigation that may be brought against us; |
▪ | developments or disputes concerning patent applications, issued patents or other proprietary rights; |
▪ | the recruitment or departure of key personnel; |
▪ | the level of expenses related to any of our product candidates or clinical development programs; |
▪ | actual or anticipated variations in our quarterly operating results; |
▪ | the number and characteristics of our efforts to in-license or acquire additional product candidates or products; |
▪ | introduction of new products or services by us or our competitors; |
▪ | failure to meet the estimates and projections of the investment community or financial guidance that we may otherwise provide to the public; |
▪ | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
▪ | actual or anticipated changes in estimates as to development timelines that we may provide to the public; |
▪ | variations in our financial results or those of companies that are perceived to be similar to us; |
▪ | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
▪ | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
▪ | announcement or expectation of additional financing efforts; |
▪ | sales of our common stock by us, our insiders or our other stockholders; |
▪ | significant lawsuits, including patent or stockholder litigation; |
▪ | changes in the structure of healthcare payment systems; |
▪ | market conditions in the pharmaceutical and biotechnology sectors; |
▪ | general political, economic, industry and market conditions; |
▪ | investors' general perception of our company and our business; |
▪ | publication of research reports about us, our competitors or our industry, or positive or negative recommendations or withdrawal of research coverage by securities or industry analysts; and |
▪ | other events or factors, many of which are beyond our control. |
In addition, the stock market in general, and pharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. The realization of any of the above risks or any of a broad range of other risks stated above could have a material adverse effect on the market price of our common stock.
A significant portion of our total outstanding shares are restricted from resale but may be sold into the market in the near future. Sales of a substantial number of shares of our common stock in the public market could cause our stock price to fall, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. If our stockholders sell, or the market perceives that the holders of a large number of shares intend to sell, substantial
73
amounts of our common stock in the public market, the market price of our common stock could decline significantly.
As of March 1, 2018, there were 37,865,740 outstanding shares of our common stock. Various shareholders who held shares of our common stock before our October 2017 initial public offering are currently restricted from selling their shares for a 180-day period, which is until the close of the market on April 9, 2018, pursuant to a contractual lock-up agreements with the underwriters and/or pursuant to the terms of our Second Amended and Restated Shareholders' Agreement. The representatives of the underwriters may, in their sole discretion and at any time or from time to time before the termination of the 180-day period, release all or any portion of the securities subject to lock-up agreements. Holders of an aggregate of 30,482,155 shares of our common stock have rights, subject to specified conditions, that require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. If we were to register the resale of these shares, they could be freely sold in the public market. Additionally, these shares are also eligible for sale without registration under Rule 144, subject to volume limitations applicable to affiliates. If these additional shares are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common stock could decline.
Future issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that we may require additional capital in the future to execute our business plan. To raise capital, we may sell substantial amounts of common stock or securities convertible into or exchangeable for common stock. These future issuances of common stock or common stock-related securities, together with the exercise of outstanding options, warrants and any additional shares issued in connection with acquisitions, if any, may result in material dilution to our investors. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences and privileges senior to those of holders of our common stock.
Our principal stockholders and management own a majority of our stock and are able to exert significant control over matters subject to stockholder approval, which could prevent new investors from influencing significant corporate decisions.
Our executive officers, directors, beneficial owners of 5% or more of our capital stock and their respective affiliates, in the aggregate, beneficially own approximately 82.2% of our outstanding common stock as of March 1, 2018. Entities associated with Avista Capital Partners II, L.P., or Avista, our largest stockholder, collectively hold as a group approximately 48.6% of our outstanding common stock as of March 1, 2018. As a result, Avista can significantly influence the outcome of matters requiring stockholder approval, including the election of directors, amendments of our organizational documents, or approval of any merger, sale of assets or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may feel are in your best interest. The interests of Avista may not always coincide with your interests or the interests of other stockholders and they may act in a manner that advances their best interests and not necessarily those of other stockholders, including seeking a premium value for their common stock. For instance, under the terms of our fourth amended and restated certificate of incorporation, neither Avista nor any of its respective representatives on our board of directors are required to offer us any transaction opportunity of which they become aware, and they could take any such opportunity for themselves or offer it to other companies in which they have an investment, unless that opportunity is expressly offered to a person serving on our board of directors solely in his or her capacity as one of our directors. These actions might affect the prevailing market price for our common stock. In addition, because Avista and certain of our other principal stockholders have held their shares for several years, they may be more interested in selling our company to an acquiror than other investors, or they may want us to pursue strategies that deviate from the interests of other stockholders. Such concentration of ownership control may also:
▪ | delay, defer or prevent a change in control; |
▪ | entrench our management and/or the board of directors; or |
▪ | impede a merger, consolidation, takeover or other business combination involving us that other stockholders may desire. |
We may also take actions that our other stockholders do not view as beneficial, which may adversely affect our results of operations and financial condition and cause the value of your investment to decline.
74
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders, and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our fourth amended and restated certificate of incorporation and our amended and restated bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders, or remove our current management. These include provisions that :
▪ | permit our board of directors to issue up to five million shares of preferred stock, with any rights, preferences and privileges as it may designate, which issuance could result in the loss of voting control by other stockholders; |
▪ | provide that our board of directors will be classified into three classes with staggered, three-year terms and that, subject to the rights of Avista to remove its respective director nominees with or without cause, directors may only be removed for cause by the affirmative vote of the holders of at least a majority of the voting power of outstanding shares of our capital stock; |
▪ | subject to any director nomination rights afforded Avista, provide that all vacancies on our board of directors, including as a result of newly created directorships, may, except as otherwise required by law, be filled only by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
▪ | following the date that Avista ceases to hold a majority of the outstanding shares of our common stock, require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent; |
▪ | provide that, with the exception of director nominees submitted by Avista pursuant to our Stockholders Agreement, dated October 2, 2017 with Avista, stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide advance notice in writing, and also specify requirements as to the form and content of a stockholder's notice; |
▪ | require that the amendment of certain provisions of our certificate of incorporation relating to anti-takeover measures may only be approved by a vote of 662/3% of our outstanding common stock; |
▪ | require that the amendment of our bylaws be approved by the affirmative vote of a majority of directors then in office or 662/3% of our outstanding common stock entitled to vote thereon; |
▪ | not provide for cumulative voting rights, thereby allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election; and |
▪ | provide that special meetings of our stockholders may be called only by the chairman or vice chairman of our board of directors, our chief executive officer, a majority of our board of directors or, for so long as Avista holds a controlling ownership interest in our common stock, by the holders of a majority of our outstanding common stock. |
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, who are responsible for appointing the members of our management. Under our fourth amended and restated certificate of incorporation, we have elected not to be governed by Section 203 of the Delaware General Corporation Law until such time that Avista ceases to own 15% or more of our capital stock. Our fourth amended and restated certificate of incorporation does, however, contain a provision that generally mirrors Section 203 of the Delaware General Corporation Law, except that it excludes Avista and its affiliates from the definition of "interested stockholder." At such time that Avista ceases to own 15% or more of our capital stock, we will be governed by the provisions of Section 203 of the Delaware General Corporation Law. These provisions may discourage, delay or prevent someone from acquiring us or merging with us whether or not it is desired by or beneficial to our stockholders. Under Section 203, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other things, prior to the time the stockholder has become an interested stockholder, the board of directors has approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder.
These provisions of our fourth amended and restated certificate of incorporation, our amended and restated bylaws and Delaware law could have the effect of discouraging potential acquisition proposals and delaying or preventing a
75
change in control. They could also have the effect of discouraging others from making tender offers for our common stock, including transactions that may be in your best interests or provide an opportunity for our stockholders to receive a premium for their shares of our common stock. These provisions could also affect the price that some investors are willing to pay for our common stock.
Our certificate of incorporation also provides that the Court of Chancery of the State of Delaware will be the exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders' ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our fourth amended and restated certificate of incorporation provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders; any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, our certificate of incorporation or our bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine. Our fourth amended and restated certificate of incorporation also provides that the United States District Court for the District of Delaware and any appellate courts thereof will be the exclusive forum for resolving any such complaint for which subject matter jurisdiction of such claim is vested exclusively in the federal courts of the United States of America. These choice of forum provisions may limit a stockholder's ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. Alternatively, if a court were to find the choice of forum provisions contained in our certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions.
We may fail to qualify for continued listing on Nasdaq which could make it more difficult for investors to sell their shares.
We list our common stock on The Nasdaq Global Market. We will need to satisfy the continued listing requirements of The Nasdaq Stock Market, LLC, or Nasdaq, for inclusion on The Nasdaq Global Market to maintain such listing, including, among other things, the maintenance of a minimum bid price of $1.00 per share and stockholders' equity of at least $10.0 million. There can be no assurance that we will be able to maintain compliance with the continued listing requirements or that our common stock will not be delisted from Nasdaq in the future. If our common stock is delisted by Nasdaq, we could face significant material adverse consequences, including:
▪ | a limited availability of market quotations for our securities; |
▪ | reduced liquidity with respect to our securities; |
▪ | a determination that our shares are a "penny stock," which will require brokers trading in our shares to adhere to more stringent rules, possibly resulting in a reduced level of trading activity in the secondary trading market for our shares; |
▪ | a limited amount of news and analyst coverage for our company; and |
▪ | a decreased ability to issue additional securities or obtain additional financing in the future. |
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. If one or more of the analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain and you may never receive a return on your investment.
We have never declared or paid cash dividends on our capital stock, and you should not rely on an investment in our common stock to provide dividend income. We currently intend to retain all of our future earnings, if any, to
76
finance the growth and development of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. In addition, the terms of the Note Purchase Agreement precludes us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
We may be subject to securities litigation, which is expensive and could divert our management's attention.
As we operate in the pharmaceutical industry, we may be especially vulnerable to volatility in the market price of our common stock, especially to the extent that various factors affect the common stock of companies in our industry. In the past companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management's attention from other business concerns, which could seriously harm our business, and could also require us to make substantial payments to satisfy judgments or to settle litigation.
We are an "emerging growth company" and intend to take advantage of reduced disclosure and governance requirements applicable to emerging growth companies, which could result in our common stock being less attractive to investors.
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and we are eligible to and intend to take advantage of some of the exemptions from reporting requirements applicable to other public companies, but not to emerging growth companies, including, but not limited to, an exemption from the auditor attestation requirement of Section 404 of the Sarbanes-Oxley Act, reduced disclosure about executive compensation arrangements pursuant to the rules applicable to smaller reporting companies and no requirement to seek non-binding advisory votes on executive compensation or golden parachute arrangements. We will remain an emerging growth company until the earliest of (1) the beginning of the first fiscal year following the fifth anniversary of our initial public offering, or January 1, 2023, (2) the beginning of the first fiscal year after our annual gross revenue is $1.07 billion or more, (3) the date on which we have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt securities and (4) the end of any fiscal year in which the market value of our common stock held by non-affiliates exceeded $700.0 million as of the end of the second quarter of that fiscal year.
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, for complying with new or revised accounting standards. An emerging growth company can therefore delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we have chosen to "opt out" of such extended transition period and, as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
We cannot predict if investors will find our common stock less attractive as a result of our taking advantage of these exemptions. If some investors find our common stock less attractive as a result of our choices, there may be a less active trading market for our common stock and our stock price may be more volatile. We may also be unable to raise additional capital as and when we need it.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to timely and accurately report our financial condition, results of operations or cash flows, which may adversely affect investor confidence in us and, as a result, the value of our common stock.
We are subject to the reporting requirements of the Securities Exchange Act of 1934, or the Exchange Act, as well as the Sarbanes-Oxley Act and the rules and regulations of the stock market on which our common stock is listed. The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal control over financial reporting. Commencing with our fiscal year ending December 31, 2018, we will be required, under Section 404 of the Sarbanes-Oxley Act, to include in our Form 10-K filing for that year a report by management on, among other things, the effectiveness of our internal control over financial reporting. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a control deficiency, or combination of control deficiencies, in internal control over financial reporting that results in more than a reasonable possibility that a material misstatement of annual or interim consolidated financial statements will not be prevented or detected on a timely basis. Section 404 of the Sarbanes-Oxley Act also generally requires an attestation from our independent registered public accounting firm on the
77
effectiveness of our internal control over financial reporting. However, for as long as we remain an emerging growth company as defined in the JOBS Act, we intend to take advantage of an exemption from the independent registered public accounting firm attestation requirement.
Our compliance with Section 404's requirement to furnish a report by management requires that we incur substantial accounting expense and expend significant management efforts. Prior to our initial public offering in October 2017, we were never required to test our internal control within a specified period, and, as a result, we may experience difficulty in meeting these reporting requirements in a timely manner. We currently do not have an internal audit function. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion, which could potentially subject us to sanctions or investigations by the Securities and Exchange Commission, or the SEC, or other regulatory authorities.
We may identify weaknesses in our system of internal financial and accounting controls and procedures that could result in a material misstatement of our consolidated financial statements. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Our internal control over financial reporting will not prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system's objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud will be detected.
Any failure to maintain internal control over financial reporting could severely inhibit our ability to timely and accurately report our financial condition, results of operations or cash flows. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness in our internal control over financial reporting once that firm begins the testing procedures over internal controls, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of our common stock could decline, and we could be subject to sanctions or investigations by Nasdaq, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
We are subject to the periodic reporting requirements of the Exchange Act. Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management, recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations reflect the reality that judgments can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected. If that were to happen, the market price of our stock could decline and we could be subject to sanctions or investigations by Nasdaq, the SEC or other regulatory authorities.
We have incurred and will continue to incur increased costs as a result of operating as a public company, and our management is required to devote substantial time to new compliance initiatives.
Prior to the consummation of our initial public offering in October 2017, we were not subject to public company reporting obligations. We now incur significant additional legal, accounting, administrative and other costs and expenses as a public company. Compliance with the Sarbanes-Oxley Act, the Dodd-Frank Act of 2010, the Exchange Act, as well as rules of the SEC and Nasdaq, for example, resulted in, and will result in further, significant initial costs to us as well as ongoing increases in our legal, audit and financial compliance costs, particularly after we are no longer an "emerging growth company." In addition, changing laws, regulations and standards relating to corporate governance and public disclosure, including regulations implemented by Nasdaq and the SEC, may increase legal and financial compliance costs and make some activities more time-consuming. These laws, regulations and standards are subject to varying interpretations and, as a result, their application in practice may
78
evolve over time as new guidance is provided by regulatory and governing bodies. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management's time and attention from revenue-generating activities to compliance activities. Any changes that we make to comply with these obligations may not be sufficient to allow us to satisfy our obligations as a public company on a timely basis, or at all.
The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and financial condition. Our board of directors, management and other personnel need to devote a substantial amount of time to these compliance initiatives. Moreover, failure to comply with these rules and regulations might make it more difficult and more expensive for us to obtain director and officer liability insurance, or we might be forced to accept reduced policy limits and coverage or incur substantially higher costs to maintain the same or similar coverage.
79
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our principal office is located in Yardley, Pennsylvania, where we lease approximately 30,000 square feet of office space pursuant to a lease that expires in May 2021. We also lease facilities in Ewing, New Jersey, Oslo, Norway and Swindon, England. We believe our facilities are adequate to meet our current needs, although we may seek to negotiate new leases or evaluate additional or alternate space for our operations. We believe appropriate alternative space will be readily available on commercially reasonable terms.
ITEM 3. LEGAL PROCEEDINGS
We are not currently a party to any legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR RESISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock has been publicly traded on The Nasdaq Global Select Market under the symbol “OPTN” since October 13, 2017. Prior to that time, there was no public market for our common stock. Shares sold in our initial public offering on October 12, 2017 were priced at $16.00 per share. The following table sets forth the high and low sales price of our common stock, as reported by the Nasdaq Global Select Market for the periods indicated:
High | Low | |||||||
Year Ended December 31, 2017 | ||||||||
Fourth Quarter (beginning October 13, 2017) | $ | 21.46 | $ | 15.01 | ||||
The last reported sale price of shares of our common stock on March 12, 2018 was $17.36 per share.
Stock Performance Graph
The graph set forth below compares the cumulative total stockholder return on our common stock between October 13, 2017 (the first date that shares of our common stock were publicly traded) and December 31, 2017, with the cumulative total return of (a) the Nasdaq Biotechnology Index and (b) the Nasdaq Composite Index, over the same period. This graph assumes the investment of $100 after the market closed on October 13, 2017 in each of our common stock, the Nasdaq Biotechnology Index and the Nasdaq Composite Index. The graph assumes our closing sales price on October 13, 2017 of $19.00 per share as the initial value of our common stock and not the initial offering price to the public in our initial public offering of $16.00 per share.
80
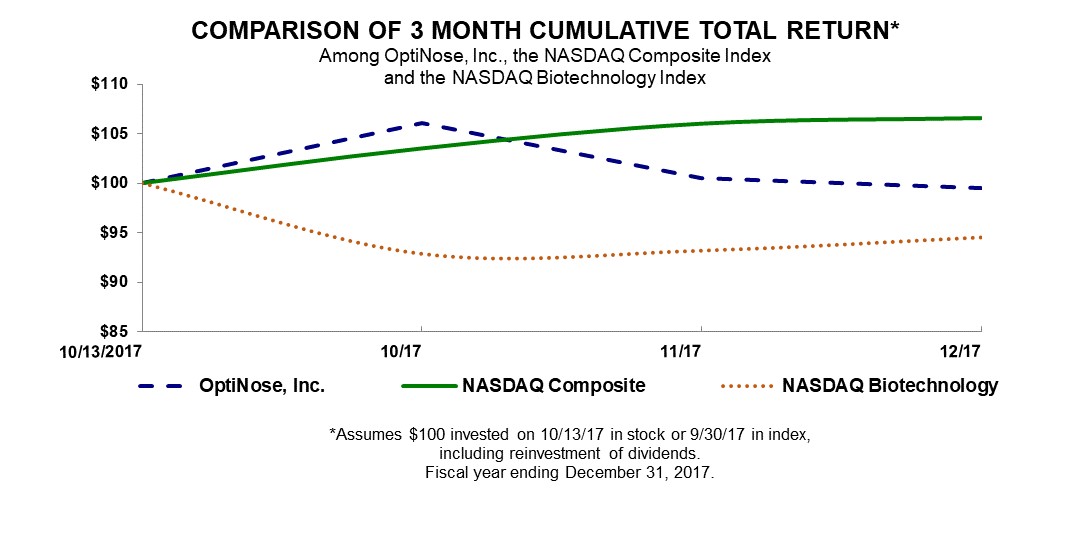
Holders of Record
As of March 1, 2018, there were 37,865,740 shares of our common stock outstanding. There were approximately 40 stockholders of record at March 1, 2018. Because many of our shares are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these record holders.
Dividends
We have never declared or paid any cash dividend on our common stock and our board of directors does not intend to do so in the foreseeable future. In addition, the Note Purchase Agreement precludes us from paying dividends. The declaration and payment of dividends in the future, of which there can be no assurance, will be determined by the board of directors in light of conditions then existing, including earnings, financial condition, capital requirements and other factors.
Securities Authorized for Issuance under Equity Compensation Plans
Information required by Item 5 of Form 10-K regarding our equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report on Form 10-K.
Recent Sales of Unregistered Securities
Set forth below is information regarding securities issued by us during the year ended December 31, 2017 that were
not registered under the Securities Act. Also included is the consideration, if any, received by us for such securities,
and information relating to the section of the Securities Act, or the rule of the SEC, under which exemption from
registration was claimed:
(a) Issuances of Capital Stock.
1. | In March 2017, we issued and sold an aggregate of 1,065,451 shares of our Series D Preferred Stock to certain new and existing investors at a purchase price of $32.85 per share, for aggregate consideration of $35.0 million. |
2. | In April 2017 and May 2017, we issued and sold to certain of our existing stockholders an additional 52,127 shares of Series D Preferred Stock at a purchase price of $32.85 per share, for aggregate consideration of $1.7 million. |
(b) Convertible Notes
1. | In September 2015, we issued and sold convertible notes in the aggregate principal amount of $15.0 million. In March 2017, concurrently with our Series D Preferred Stock financing, the notes were converted |
81
into an aggregate of 687,474 shares of our Series C-2 Preferred Stock at a conversion price of approximately $28.40 per share.
(c) Stock Option Grants
1. | 1. On January 23, 2017, we granted a stock option to purchase a total of 158,834 shares of common stock at an exercise price of $5.14 per share to one executive officer pursuant to our 2010 Stock Incentive Plan. |
2. | On January 30, 2017, we granted stock options to purchase a total of 144,395 shares of common stock at an exercise price of $5.14 per share to one executive officer pursuant to our 2010 Stock Incentive Plan. |
3. | On February 13, 2017, we granted stock options to purchase a total of 20,214 shares of common stock at an exercise price of $5.14 per share to two employees pursuant to our 2010 Stock Incentive Plan. |
4. | On February 20, 2017, we granted a stock option to purchase 5,775 shares of common stock at an exercise price of $5.14 per share to one employee pursuant to our 2010 Stock Incentive Plan. |
5. | On February 27, 2017, we granted a stock option to purchase 5,775 shares of common stock at an exercise price of $5.14 per share to one employee pursuant to our 2010 Stock Incentive Plan. |
The offers, sales and issuances of the securities described in paragraphs (a) and (b) above were exempt from registration under the Securities Act in reliance on Regulation D of the Securities Act.
With respect to the shares of Series C-2 Preferred Stock issued upon conversion of the convertible notes in March 2017 described in paragraph (b), the issuance of such shares was exempt from registration under Section 3(a)(9) of the Securities Act.
The grants of stock options described in paragraph (c) above to our executive officers and directors were exempt from registration under Section 4(a)(2) of the Securities Act as transactions not involving any public offering. The remaining grants of stock options described in paragraph (c) above were exempt from registration under the Securities Act in reliance on Rule 701 as offers and sales of securities under written compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships, to information about us.
All purchasers of securities in transactions exempt from registration pursuant to Regulation D described above represented to us in connection with their purchase that they were accredited investors, as defined in Rule 501 under the Securities Act, and were acquiring the securities for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from the registration requirements of the Securities Act.
All of the foregoing securities are deemed restricted securities for purposes of the Securities Act. The certificates representing the issued securities described in this Item 5 included appropriate legends setting forth that the applicable securities have not been registered and reciting the applicable restrictions on transfer. There were no underwriters employed in connection with any of the transactions set forth in this Item 5. Except as set forth above, we did not sell any shares of our common stock or our preferred stock, or grant any stock options or restricted stock awards, during the year ended December 31, 2017 that were not registered under the Securities Act of 1933, as amended, or the Securities Act, and that have not otherwise been described in a Quarterly Report on Form 10-Q.
Issuer Purchases of Equity Securities
We did not purchase any of our registered equity securities during the period covered by this Annual Report on Form 10-K.
Use of Proceeds from Registered Securities
Our IPO was effected through a Registration Statement on Form S-1 (File No. 333-220515) that was declared effective by the SEC on October 12, 2017. On October 17, 2017, 8,625,000 shares of our common stock were sold at a price to the public of $16.00 per share, for aggregate gross proceeds of $138.0 million. As of the date of filing this report, the offering has terminated, and all of the securities registered pursuant to the offering were sold prior to termination. Jefferies and Piper Jaffray acted as lead joint book-running managers in the IPO, and BMO Capital Markets and RBC Capital Markets acted as joint book-running managers in the IPO.
82
On October 17, 2017 we received proceeds from the IPO of $128.3 million, which was net of underwriting discounts and commissions of approximately $9.7 million. Of this amount, we paid offering expenses of approximately $2.8 million. The balance of the funds totaling approximately $125.5 million shall be used in a manner consistent with the use of proceeds described in the final prospectus for the IPO filed with the SEC on October 12, 2017 (the "Final Prospectus").
The foregoing expenses are a reasonable estimate of the expenses incurred by us in the offering and do not represent the exact amount of expenses incurred. All of the foregoing expenses were direct or indirect payments to persons other than (i) our directors, officers or any of their associates; (ii) persons owning 10% or more of our common stock; or (iii) our affiliates.
There has been no material change in the use of proceeds from the IPO as described in the Final Prospectus. During the period from the closing of our IPO to December 31, 2017, we used $13.8 million of the proceeds as follows:
• | approximately $7.7 million to support the planned launch of XHANCE, including investments in marketing and sales, inventory and our commercial infrastructure; |
• | approximately $1.7 million to fund further development efforts for XHANCE; and |
• | approximately $4.4 million to fund other working capital and general corporate purposes, including costs of operating as a public company. |
The foregoing amounts represent the Company’s reasonable estimate of the amount of net offering proceeds applied to such activities instead of the actual amount of net offering proceeds used.
ITEM 6. SELECTED CONSOLIDATED FINANCIAL DATA
The selected financial data set forth below is derived from our audited consolidated financial statements and may not be indicative of future operating results. This section should be read together with our consolidated financial statements and accompanying notes and Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations" appearing elsewhere in this Form 10-K. The selected consolidated financial data in this section are not intended to replace our consolidated financial statements and the related notes. Our historical results are not necessarily indicative of the results that may be expected in the future.
Years Ended December 31, | ||||||||||||
(in thousands, except share and per share data) | 2017 | 2016 | 2015 | |||||||||
Consolidated Statement of Operations Data: | ||||||||||||
Licensing revenues | $ | — | $ | 47,500 | $ | 85 | ||||||
Operating expenses: | ||||||||||||
Research and development | 16,832 | 15,311 | 22,156 | |||||||||
Selling, general and administrative | 31,698 | 6,869 | 6,006 | |||||||||
Total operating expenses | 48,530 | 22,180 | 28,162 | |||||||||
(Loss) income from operations | (48,530 | ) | 25,320 | (28,077 | ) | |||||||
Other (income) expense, net: | ||||||||||||
Interest (income) expense | 607 | 3,374 | 791 | |||||||||
Other (income) expense | (235 | ) | (667 | ) | (554 | ) | ||||||
Total other (income) expense, net | 372 | 2,707 | 237 | |||||||||
Net (loss) income | (48,902 | ) | 22,613 | (28,314 | ) | |||||||
Accretion of redeemable convertible preferred stock | (13,065 | ) | (13,114 | ) | (12,061 | ) | ||||||
Net (loss) income attributable to common stockholders | $ | (61,967 | ) | $ | 9,499 | $ | (40,375 | ) | ||||
Net (loss) income per share of common stock, | ||||||||||||
basic | $ | (5.63 | ) | $ | 0.40 | $ | (9.97 | ) | ||||
diluted | $ | (5.63 | ) | $ | 0.32 | $ | (9.97 | ) | ||||
Weighted average common shares outstanding, | ||||||||||||
basic | 10,999,121 | 4,054,316 | 4,049,668 | |||||||||
diluted | 10,999,121 | 4,980,181 | 4,049,668 | |||||||||
83
As of December 31, | ||||||||||||
(in thousands) | 2017 | 2016 | 2015 | |||||||||
Consolidated Balance Sheet Data: | ||||||||||||
Cash and cash equivalents | $ | 234,854 | $ | 36,797 | $ | 15,198 | ||||||
Working capital(1) | 223,390 | 34,765 | 8,624 | |||||||||
Total assets | 241,136 | 41,551 | 16,009 | |||||||||
Convertible notes | — | 15,256 | 14,480 | |||||||||
Long term debt, net | 71,863 | — | — | |||||||||
Redeemable convertible preferred stock | — | 168,173 | 155,059 | |||||||||
Accumulated deficit | (211,269 | ) | (151,102 | ) | (161,255 | ) | ||||||
Total stockholders' deficit | 154,496 | (151,197 | ) | (161,392 | ) | |||||||
________________________________________________________________________________________________________________________
(1) | Working capital is calculated as current assets minus current liabilities. |
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our historical consolidated financial statements and the related notes thereto appearing in this Annual Report. In addition to historical information, some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report, includes forward‑looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this Annual Report, our actual results could differ materially from the results described in or implied by the forward‑looking statements contained in the following discussion and analysis.
Company Overview
We are a specialty pharmaceutical company focused on the development and commercialization of products for patients treated by ear, nose and throat, or ENT, and allergy specialists. Our lead product, XHANCETM (fluticasone propionate) nasal spray, 93 mcg, is a therapeutic utilizing our proprietary Optinose Exhalation Delivery System, or EDS, that delivers a topically-acting and potent anti-inflammatory corticosteroid for the treatment of chronic rhinosinusitis with nasal polyps and, if approved, chronic rhinosinusitis without nasal polyps. Chronic rhinosinusitis is a serious nasal inflammatory disease that is currently treated using therapies, such as intranasal steroids, or INS, that have significant limitations. We believe XHANCE has a differentiated clinical profile with the potential to become part of the standard of care for this disease because it is able to deliver medication to the primary site of inflammation high and deep in the nasal passages in regions not adequately reached by current INS. We also believe that payors will respond favorably to XHANCE's clinical, cost, and quality-of-care profile, as compared to current and potential future costly drug therapy and surgical treatment options.
On September 18, 2017, the U.S. Food and Drug Administration, or FDA, approved our new drug application, or NDA, for XHANCE for the treatment of nasal polyps in patients 18 years of age or older. Based upon our research of over 300 launches between 2010 and 2016, we believe the evidence suggests that the success of a launch is highly dependent upon four critical factors: level of unmet need that exists within the market, level of clinical differentiation of a brand, market access and brand awareness. Therefore, rather than rushing our product to the market immediately following approval, our Company employed a different, purposeful launch model that would enable our commercial team to build market access for XHANCE and achieve critical levels of customer awareness to facilitate adoption upon making XHANCE available in the market.
Since the FDA approved of our NDA for XHANCE, we have been focused on executing our integrated launch plan with the objective of making XHANCE widely available through retail pharmacies in the second quarter of 2018; we now believe XHANCE will be available in retail pharmacies in early April 2018. The key strategies in our integrated launch plan include: (i) build a robust supply chain network and quality management system, (ii) drive awareness and appreciation of the clinical differentiation of XHANCE, (iii) design and deploy our customer facing model, (iv) engage commercial payors with the objective of securing tier 3 coverage, and (v) develop our internal capabilities (Finance, HR, IT, Data Analytics and Compliance) to support a commercial stage company. We have made progress in each of these key strategic areas:
• | Commercial Supply Chain. We have entered into commercial supply agreements with our key suppliers, spent significant time with our suppliers to oversee product production and quality management, and |
84
manufactured our initial commercial supply of XHANCE. We have contracted with a third-party logistics partner and are in the process of finalizing agreements with our distribution partners.
• | Brand Awareness. We have executed a broad, multi-channel awareness campaign leveraging digital, non- personal promotion and journal advertising and have already reached over 10,000 ENT physicians and allergists with disease state and branded messages. In November 2017, we launched a nurse educator team of approximately 85 nurse professionals who have since called on approximately 5,000 ENT physicians and allergists and delivered over 10,000 presentations. The focus of their interactions with healthcare professionals include: (i) introducing Optinose and highlighting the unmet medical need and limitations of current treatments, (ii) increasing awareness about XHANCE along with differentiating the Optinose EDS, and (iii) familiarizing healthcare professionals with the proper administration of XHANCE. Initial reaction to core brand messages among target physicians has been positive. |
• | Customer Model. We have defined a sales force footprint of approximately 120 territories targeting approximately 14,000 ENT physicians, allergists and “specialty like” primary care physicians and are deploying a hybrid sales model that combines an internal sales leadership team with a fully dedicated contract sales force to call on our target customer universe. We have prioritized approximately 80 territories within our sales force footprint to deploy at launch based upon an expectation that we will achieve an estimated 65% commercial market access within each of those territories during launch. Most of the initial 80 territory managers have completed training and are already engaging approximately 8,000 ENTs, allergists and primary care physician targets to promote XHANCE for the treatment of nasal polyps. Additionally, in March 2018, we introduced the XHANCE Xperience program to offer select physicians and their patients an opportunity to gain initial experience with XHANCE. Physicians can enroll a limited number of eligible patients in this program. Patients will receive up to two XHANCE prescriptions at no cost to them ($0 co-pay), and physicians will receive an opportunity for feedback on patient experience. We believe the positive experience that physicians and patients have with XHANCE in this program will help drive demand for XHANCE during the retail launch, starting in early April 2018. |
• | Market Access. We have been engaging payors with the goal of securing tier 3 commercial coverage, primarily with a single step edit and no prior authorization. We have engaged with approximately 40 plans representing approximately 85% of commercial lives. In meeting with potential payors, we have shared what we believe is our compelling economic value proposition. Our analyses show that XHANCE will have a comparatively low pharmacy budget impact and our clinical trial data suggest that XHANCE may produce an offsetting benefit by helping reduce the rate of surgery with its related costs. For an insurance plan, this could represent a potential overall cost reduction for the population of patients with nasal polyps, as the overall cost of XHANCE could be less than the offsetting costs related to the reduction in surgeries. During clinical studies, XHANCE was also associated with an improvement in reported work productivity in treated patients, which should be valued by employers and patients. Further, we believe the cost of XHANCE to insurance plans will likely be significantly less than the projected costs of monoclonal antibodies that are currently in development for the treatment of nasal polyps. We expect to achieve approximately 65% coverage of commercial lives during the retail launch of XHANCE, and will seek to increase the number of covered lives during the first year. We have contracted with the Centers for Medicare and Medicaid Services regarding certain government covered lives. Further, we plan to introduce a co-pay assistance program and other patient affordability programs to appropriately support patient access to XHANCE. |
• | Infrastructure. We continue to develop our internal capabilities and grew from 21 employees as of January 1, 2017 to 82 employees as of March 1, 2018 to support a commercial stage company. We have implemented an enterprise resource planning system to expand our operational and commercial finance capabilities. We have implemented a robust healthcare compliance program to guide our staff’s and our partners' compliance with rules and regulations regarding pharmaceutical sales. In managing our growth, we have remained focused on fostering our One Mission culture. |
In additional to XHANCE’s existing indication for nasal polyps, we plan to initiate additional clinical trials in the fourth quarter of 2018 to seek a follow-on indication for the treatment of chronic sinusitis in order to broaden our market opportunity. XHANCE is the second commercial product that we have developed utilizing an Optinose EDS. Our first commercial product, indicated for the acute treatment of migraines in adults, was licensed in 2013 to Avanir Pharmaceuticals, Inc., or Avanir, and was approved by the FDA in January 2016.
85
Financial Operations Overview
The following discussion sets forth certain components of our consolidated statements of operations as well as factors that impact those items.
Licensing revenues
To date, we have not generated any revenues from product sales. Substantially all of our revenue to date has been derived from the AVP-825 License Agreement. We do not expect to generate significant product revenue until we commercialize XHANCE and our other product candidates.
In July 2013, we, through our wholly owned subsidiary, OptiNose AS, entered into the AVP-825 License Agreement under which we granted an exclusive license to Avanir to further develop and commercialize AVP-825 (now marketed as Onzetra Xsail). Under the terms of the AVP-825 License Agreement, we have received $70.0 million in aggregate licensing revenues to date in connection with the initial signing and the achievement of development milestones, including a $47.5 million payment upon FDA approval of AVP-825 in the first quarter of 2016. We are eligible to receive up to an additional $50.0 million upon the achievement of annual sales milestones and tiered low double-digit royalty payments once and if net sales of the product exceed a specified cumulative threshold. We do not expect to generate any additional revenue from the AVP-825 License Agreement in the near term.
Research and development expense
Research and development expense consists substantially of costs incurred in connection with the development and pursuit of regulatory approval for XHANCE for the treatment of nasal polyps. We expense research and development costs as incurred. These expenses include:
▪ | personnel expenses, including salaries, benefits and stock-based compensation expense; |
▪ | costs of funding research performed by third parties, including pursuant to agreements with contract research organizations, or CROs, as well as investigative sites and consultants that conduct our preclinical studies and clinical trials; |
▪ | expenses incurred under agreements with contract manufacturing organizations, or CMOs, including manufacturing scale-up expenses and the cost of acquiring and manufacturing preclinical study and clinical trial materials; |
▪ | consultant fees and expenses associated with outsourced professional scientific development services; |
▪ | expenses for regulatory activities, including filing fees paid to regulatory agencies and costs incurred to compile and respond to filings with the FDA; |
▪ | costs incurred to maintain, expand and protect our patent portfolio as it relates to product candidates in development; and |
▪ | allocated expenses for facility costs, including rent, utilities, depreciation and maintenance. |
Certain regulatory, patent and pre-commercialization expenses classified as research and development expenses prior to the FDA approval of XHANCE in September 2017 have been classified as selling, general and administrative expenses if incurred post approval of XHANCE to the extent that these expenses support the commercialization of XHANCE.
We typically use our employee, consultant and infrastructure resources across our research and development programs. Although we track certain outsourced development costs by product candidate, we do not allocate personnel costs or other internal costs to specific product candidates.
We plan to incur research and development expenses for the foreseeable future as we expect to continue the development of XHANCE for a follow-on indication for the treatment of chronic sinusitis and our other product candidates. At this time, due to the inherently unpredictable nature of preclinical and clinical development, and given the preliminary nature of our clinical trial design for XHANCE for a follow-on indication for the treatment of chronic sinusitis and the FDA-mandated pediatric studies for XHANCE, and the early stage of our other product candidates, we are unable to estimate with any certainty the costs we will incur and the timelines we will require in our continued development efforts.
86
Selling, general and administrative expense
Selling, general and administrative expense consists primarily of personnel expenses, including salaries, benefits and stock-based compensation expense, for employees in executive, finance, accounting, business development, legal and human resource functions. General and administrative expense also includes corporate facility costs, including rent, utilities, depreciation and maintenance, not otherwise included in research and development expense, as well as regulatory fees and professional fees for legal, patent, accounting and other consulting services.
We anticipate that our general and administrative expenses will increase in 2018 as compared to 2017 as a result of an expanded infrastructure and an increased headcount to support the commercialization of XHANCE. We also anticipate higher corporate infrastructure costs including, but not limited to accounting, legal, human resources, consulting and investor relations expenses, as well as increased director and officer insurance premiums, associated with operating as a public company.
Sales and marketing related expenses consist of market research and other market preparation and pre-commercial activities ahead of making XHANCE available through retail pharmacies, expected to occur in early April 2018, as well as salaries and related benefits for employees focused on such efforts. We anticipate that our sales and marketing and related expenses will increase in 2018 as compared to 2017 as a result of an increase in headcount and the commercial launch of XHANCE in the United States, including the engagement of a dedicated contract sales organization.
Interest (income) expense
Interest (income) expense consists of interest earned on our cash and cash equivalents held with institutional banks and interest expense related to our long-term debt and amounts amortized and accrued under our convertible notes that were converted into preferred stock in March 2017.
Other (income) expense
Other (income) expense consists primarily of grant and other income as a result of government cost reimbursements for research and development activities over a contractually defined period, as well as foreign currency (income) losses due to exchange rate fluctuations on transactions denominated in a currency other than our functional currency.
Critical accounting policies
Our consolidated financial statements are prepared in accordance with U.S. generally accepted accounting principles, or GAAP. The preparation of our consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of expenses during the reported period. We base our estimates on historical experience, known trends and events and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions and conditions.
While our significant accounting policies are described in more detail in the notes to our consolidated financial statements appearing elsewhere in this report, we believe that the following accounting policies are those most critical to the preparation of our consolidated financial statements.
Revenue recognition
We have generated revenue primarily through licensing arrangements, which generally contain multiple elements, or deliverables, including licenses and research and development activities we perform on behalf of the licensee. Revenues are recognized when (1) persuasive evidence of an arrangement exists, (2) delivery has occurred or services have been rendered, (3) the price is fixed or determinable and (4) collectability is reasonably assured.
Currently, our only source of revenue is the AVP-825 License Agreement. The AVP-825 License Agreement includes licensed rights to patented technology, non-refundable up-front license fees, research services, and regulatory and sales milestones as well as royalty payments.
87
For arrangements with multiple elements, we recognize revenue in accordance with the Financial Accounting Standards Board, or FASB, Accounting Standards Update, or ASU, No. 2009-13, Multiple-Deliverable Revenue Arrangements, which provides guidance for separating and allocating consideration in a multiple element arrangement. The selling prices of deliverables under an arrangement may be derived using third-party evidence, or TPE, or a best estimate of selling price, or BESP, if vendor-specific objective evidence of selling price, or VSOE, is not available. The objective of BESP is to determine the price at which we would transact a sale if each element within the AVP-825 License Agreement was sold on a standalone basis. Deliverables under the arrangement are separate units of accounting if the delivered item has value to the customer on a standalone basis and if the arrangement includes a general right of return relative to the delivered item, and delivery or performance of the undelivered item is considered probable and substantially within our control. The arrangement consideration that is fixed or determinable at the inception of the arrangement is allocated to the separate units of accounting based on their relative selling prices. The appropriate revenue recognition model is applied to each element and revenue is accordingly recognized as each element is delivered. Management exercises significant judgment in determining whether a deliverable is a separate unit of accounting.
In determining the separate units of accounting for our licensing arrangements, we evaluate whether the license has standalone value to the licensee based on consideration of the relevant facts and circumstances for each arrangement. Factors considered in this determination include the research and development capabilities of the licensee and the availability of relevant research expertise in the marketplace. In addition, we consider whether or not the licensee could use the license for its intended purpose without the receipt of the remaining deliverables, the value of the license was dependent on the undelivered items and the licensee or other vendors could provide the undelivered items.
Whenever we determine that an element is delivered over a period of time, revenue is recognized using either a proportional performance model, if a pattern of performance can be determined, or a straight-line model over the period of performance, which is typically the research and development term.
Development milestones may be triggered either by the results of our research efforts or by events external to it, such as regulatory approval to market a product. Consideration that is contingent upon achievement of a development milestone is recognized in its entirety as revenue in the period in which the milestone is achieved, but only if the consideration earned from the achievement of a milestone meets all the criteria for the milestone to be considered substantive at the inception of the arrangement. For a milestone to be considered substantive, the consideration earned by achieving the milestone must be commensurate with either our performance to achieve the milestone or the enhancement of the value of the item delivered as a result of a specific outcome resulting from our performance to achieve the milestone, relate solely to past performance and be reasonable relative to all deliverables and payment terms in the collaboration agreement. As of December 31, 2016, all development milestones have been achieved under the AVP-825 License Agreement.
Royalties and sales milestones are recorded as earned in accordance with the contract terms when third party sales can be reliably measured and collectability is reasonably assured.
Research and development expenses
Research and development expense consists primarily of costs incurred in connection with development and regulatory approval of XHANCE, as well as costs associated with developing commercial manufacturing capabilities for XHANCE. We expense research and development costs as incurred.
At the end of each reporting period, we compare payments made to third-party service providers to the estimated progress toward completion of the applicable research or development objectives. Such estimates are subject to change as additional information becomes available. Depending on the timing of payments to the service providers and the progress that we estimate has been made as a result of the service provided, we may record net prepaid or accrued expenses relating to these costs. To date, we have not made any material adjustments to our prior estimates of accrued research and development expenses.
Stock-based compensation
We account for stock-based compensation awards in accordance with the FASB Accounting Standards Codification, or ASC, Topic 718, Compensation — Stock Compensation, or ASC 718. ASC 718 requires all stock-based compensation awards to employees to be recognized as expense based on their grant date fair values. We
88
use the Black-Scholes option pricing model to value our stock option awards and we account for forfeitures of stock option awards as they occur. For awards issued to employees, we recognize compensation expense on a straight-line basis over the requisite service period, which is generally the vesting period of the award. Stock-based awards issued to nonemployees are revalued at each reporting period until the award vests in accordance with ASC Topic 505, Equity. The resulting increase or decrease in value, if any, is recognized as expense or income, respectively, during the period the related services are rendered. Expense for awards with performance conditions is estimated and adjusted on a quarterly basis based upon our assessment of the probability that the performance condition will be met. We have not issued awards where vesting is subject to market conditions; however, if we were to grant such awards in the future, recognition would be based on the derived service period.
Estimating the fair value of options requires the input of subjective assumptions, including the estimated fair value of our common stock, the expected life of the option, stock price volatility, the risk-free interest rate and expected dividends. The assumptions used in our Black-Scholes option-pricing model represent management's best estimates and involve a number of variables, uncertainties and assumptions and the application of management's judgment, as they are inherently subjective. If any assumptions change, our stock-based compensation expense could be materially different in the future.
These assumptions used in our Black-Scholes option-pricing model are estimated as follows:
• | Expected Term. Due to the lack of a public market for the trading of our common stock and the lack of sufficient company-specific historical data, the expected term of employee options is determined using the "simplified" method, as prescribed in SEC's Staff Accounting Bulletin, or SAB, No. 107, whereby the expected life equals the arithmetic average of the vesting term and the original contractual term of the option. The expected term of nonemployee options is equal to the contractual term. |
• | Expected Volatility. The expected volatility is based on historical volatilities of similar entities within our industry which were commensurate with the expected term assumption as described in SAB No. 107. |
• | Risk-Free Interest Rate. The risk-free interest rate is based on the interest rate payable on U.S. Treasury securities in effect at the time of grant for a period that is commensurate with the assumed expected term. |
• | Expected Dividends. The expected dividend yield is 0% because we have not historically paid, and do not expect for the foreseeable future to pay, a dividend on our common stock. |
The following table reflects the weighted average assumptions used to estimate the fair value of options granted during the periods presented.
December 31, | |||||||
2017 | 2016 | ||||||
Expected term (in years) | 6.06 | 6.08 | |||||
Expected volatility | 78.67 | % | 74.29 | % | |||
Risk free interest rate | 2.06 | % | 2.22 | % | |||
Expected dividend yield | 0.00 | % | 0.00 | % | |||
Fair value of common stock | $ | 9.68 | $ | 5.14 | |||
No awards were granted during the year ended December 31, 2015.
For information about our employee stock purchase plan, see Note 13 to the Consolidated Financial Statements.
Inventory
Prior to receiving FDA approval for XHANCE in September 2017, inventory purchases were expensed as incurred and recorded as a component of research and development expense. Subsequent to receiving FDA approval, inventories are stated at the lower of cost or net realizable value, net of reserves for excess and obsolete inventory. Cost is computed using standard cost (which approximates actual cost) on a first-in, first-out basis. At December 31, 2017, inventory consisted of raw materials and sub-assemblies.
Recent Accounting Pronouncements
See Note 3 of our audited consolidated financial statement beginning on page F-1 of this Annual Report on Form
89
10-K for a description of recent accounting pronouncements applicable to our consolidated financial statements.
JOBS Act
The JOBS Act, permits an “emerging growth company” such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies until those standards would otherwise apply to private companies. We have irrevocably elected to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards when they are required to be adopted by public companies that are not emerging growth companies.
Consolidated Results of Operations
Comparison of the years ended December 31, 2017 and 2016
The following table sets forth our selected consolidated statements of operations data for the periods indicated (in thousands):
Year Ended December 31, | |||||||
2017 | 2016 | ||||||
Licensing revenues | $ | — | $ | 47,500 | |||
Operating expenses: | |||||||
Research and development | 16,832 | 15,311 | |||||
Selling, general and administrative | 31,698 | 6,869 | |||||
Total operating expenses | 48,530 | 22,180 | |||||
(Loss) income from operations | (48,530 | ) | 25,320 | ||||
Other (income) expense: | |||||||
Interest (income) expense | 607 | 3,374 | |||||
Other (income) expense | (235 | ) | (667 | ) | |||
Total other (income) expense | 372 | 2,707 | |||||
Net (loss) income | $ | (48,902 | ) | $ | 22,613 | ||
Licensing Revenues
Licensing revenues were $47.5 million for the year ended December 31, 2016 and were attributable to the achievement of a development milestone under the terms of the AVP-825 License Agreement as a result of FDA approval of Onzetra Xsail in January 2016. There were no licensing revenues during the year ended December 31, 2017.
Research and development expense
Research and development expenses were $16.8 million and $15.3 million for the years ended December 31, 2017 and 2016, respectively. The $1.5 million increase was attributable primarily to:
▪ | a $1.2 million increase related to the preparation of contract manufacturing capabilities prior to the receipt of FDA approval of XHANCE in September of 2017 in anticipation of the expected commercial launch of XHANCE in the U.S. for the treatment of nasal polyps; |
▪ | a $1.3 million increase in personnel and bonus expenses, including non-cash stock-based compensation, due to increases in headcount, as well as increases in bonus expense as a result of the achievement of Company performance targets; and |
▪ | a $1.1 million increase in medical affairs spending in connection with the conduct, reporting and planning for current and future research programs and to prepare for our planned clinical trials for a follow-on indication for XHANCE for the treatment of chronic sinusitis. |
These increases were offset primarily by:
▪ | a $1.3 million decrease in regulatory expenses as a result of the substantial completion in 2016 of our NDA submission activities for XHANCE for the treatment of nasal polyps; and |
▪ | a $0.7 million decrease in expenses as a result of the completion of an anthropometric study in preparation for the FDA mandated pediatric studies as well as the completion of other early research projects. |
90
Selling general and administrative expense
Selling, general and administrative expenses were $31.7 million and $6.9 million for the years ended December 31, 2017 and 2016, respectively. The $24.8 million increase was due primarily to:
▪ | a $9.7 million increase in sales and marketing expenses related to our preparation for the expected commercial launch of XHANCE in the U.S. for the treatment of nasal polyps; |
▪ | a $9.1 million increase in personnel and bonus expenses, including non-cash stock-based compensation, due to increases in headcount, as well as increases in bonus expense as a result of the achievement of Company performance targets; |
▪ | a $4.4 million increase in professional fees and consultant expenses related to the completion of our IPO, the support of our expanding infrastructure to prepare for the expected commercial launch of XHANCE and as a result of operating as a public company; and |
▪ | $1.7 million increase in facilities and administrative expenses to support expanding business operations. |
Interest (income) expense, net
Interest expense, net, was $0.6 million and $3.4 million for the years ended December 31, 2017 and 2016, respectively. The $2.8 million decrease was due primarily to the conversion of convertible notes to shares of preferred stock in March 2017.
Other (income) expense, net
Other income, net, was $0.2 million and $0.7 million for the years ended December 31, 2017 and 2016, respectively. The income in both periods was attributable primarily to grant eligible research and development expenses incurred by OptiNose AS, our Norwegian subsidiary.
Comparison of the years ended December 31, 2016 and 2015
The following table sets forth our selected consolidated statements of operations data for the periods indicated (in thousands):
Year Ended December 31, | |||||||
2016 | 2015 | ||||||
Licensing revenues | $ | 47,500 | $ | 85 | |||
Operating expenses: | |||||||
Research and development | 15,311 | 22,156 | |||||
Selling, general and administrative | 6,869 | 6,006 | |||||
Total operating expenses | 22,180 | 28,162 | |||||
Income (loss) from operations | 25,320 | (28,077 | ) | ||||
Other (income) expense: | |||||||
Interest (income) expense | 3,374 | 791 | |||||
Other (income) expense | (667 | ) | (554 | ) | |||
Total other (income) expense | 2,707 | 237 | |||||
Net income (loss) | $ | 22,613 | $ | (28,314 | ) | ||
Licensing Revenues
Licensing revenues were $47.5 million and $0.1 million for the years ended December 31, 2016 and 2015, respectively. Licensing revenues earned during the year ended December 31, 2016 were attributable to the achievement of a development milestone under the terms of the AVP-825 License Agreement as a result of FDA approval of Onzetra Xsail in January 2016. Licensing revenues earned during the year ended December 31, 2015 were attributable to research and development services provided in connection with the AVP-825 License Agreement.
Research and development expense
Research and development expenses were $15.3 million and $22.2 million for the years ended December 31, 2016 and 2015, respectively. The $6.9 million decrease was attributable primarily to the $10.0 million decrease in clinical
91
development and chemistry, manufacturing and controls expenses in connection with the substantial completion of our Phase 3 clinical trial development program of XHANCE for the treatment of nasal polyps in 2015.
This decrease was offset primarily by:
• | a $2.2 million increase in personnel and bonus expense; |
• | a $0.8 million increase in regulatory and intellectual property maintenance costs in connection with the submission of our NDA for XHANCE for the treatment of nasal polyps; and |
• | a $0.1 million increase in rent and other operating expenses in connection our new corporate office lease. |
Selling, general and administrative expense
Selling, general and administrative expenses were $6.9 million and $6.0 million for the years ended December 31, 2016 and 2015, respectively. The $0.9 million increase was due primarily to:
• | a $1.5 million increase in personnel and bonus expense; |
• | a $0.6 million increase in professional service expenses as a result of our preparations to become a public company and for the expected commercial launch of XHANCE for the treatment of nasal polyps; and |
• | a $0.1 million increase in rent and other operating expenses in connection with our new corporate office lease. |
• |
These increases were offset by a $1.3 million decrease in marketing-related expenses incurred in connection with market research and commercial feasibility studies that we commissioned for XHANCE in 2015.
Interest (income) expense, net
Interest expense, net, was $3.4 million and $0.8 million for the years ended December 31, 2016 and 2015, respectively. The $2.6 million increase was attributable primarily to the September 2015 issuance of $15.0 million in convertible notes.
Other (income) expense, net
Other income, net, was $0.7 million and $0.6 million for the years ended December 31, 2016 and 2015, respectively. The $0.1 million increase was due primarily to an increase in grant eligible research and development expenses incurred by OptiNose AS, our Norwegian subsidiary.
Liquidity and Capital Resources
Since inception, we have incurred significant net losses and expect to continue to incur net losses for the foreseeable future. We had net income of $22.6 million for the year ended December 31, 2016 due primarily to the achievement of a milestone under the AVP-825 License Agreement. However, we incurred net losses of $48.9 million for the year ended December 31, 2017. As of December 31, 2017, we had an accumulated deficit of $211.3 million.
We have funded our operations primarily through the sale of stock and the issuance of debt, as well as through licensing revenues received under the terms of the AVP-825 License Agreement. As of December 31, 2017, we had $234.9 million in cash and cash equivalents.
In October 2017, we completed our initial public offering, or IPO, selling 8,625,000 shares of our common stock at a price of $16.00 per share. As a result of the IPO, we received approximately $125.5 million in net proceeds, after deducting underwriting discounts and commissions of approximately $9.7 million and offering expenses of approximately $2.9 million payable by us.
In December 2017, we entered into a Note Purchase Agreement with Athyrium Opportunities III Acquisition LP, as collateral agent, or the Collateral Agent, and the purchasers party thereto, or the Purchasers, that provides for the issuance of up to $100.0 million of senior secured notes, or the Notes, of which $75.0 million of the Notes were issued on December 29, 2017, of which $50.0 million were issued by OptiNose AS and $25 million were issued by
92
OptiNose US, Inc., or the Issuers. The remaining $25.0 million of Notes, or the Delayed Draw Notes, may be issued by OptiNose US, Inc. and sold to the Purchasers between April 1, 2019 and August 14, 2019, subject to achieving trailing four quarter net revenues (as calculated pursuant to the terms of the Note Purchase Agreement) of $15.0 million and a pro forma ratio of total debt to trailing four quarter net revenues not exceeding 6.50 to 1.00, and certain other conditions.
The unpaid principal amount under the Notes is due and payable on June 29, 2023, or the Maturity Date. The Notes bear interest at a per annum rate of three-month LIBOR rate (subject to a 1.0% floor) plus 9.0%. The Issuers are required to make quarterly interest-only payments until the Maturity Date. In addition, the Issuers paid an upfront fee of 1% of the aggregate principal amount of the Notes on the Closing Date. We are also required to pay an exit fee of 2% of any principal payments (whether mandatory, voluntary or at maturity) made throughout the term of the Note Purchase Agreement. Subject to certain exceptions, the Issuers are required to make mandatory prepayments of the Notes, with the proceeds of assets sales extraordinary receipts and prohibited debt issuances and upon the occurrence of a change of control. In addition, the Issuers may make voluntary prepayments of the Notes, in whole or in part. All mandatory and voluntary prepayments of the Notes are subject to the payment of prepayment premiums as follows: (i) if prepayment occurs prior to the second anniversary of the applicable date of issuance, an amount equal to the amount by which (a) the present value of 102% of the principal prepaid plus all interest that would have accrued on such principal through such second anniversary exceeds (b) the amount of principal prepaid, (ii) if prepayment occurs on or after the second anniversary of the applicable date of issuance but prior to the third anniversary of such issuance, an amount equal to 2% of the principal prepaid, and (iii) if prepayment occurs on or after the third anniversary of the applicable date of issuance but prior to the fourth anniversary of such issuance, an amount equal to 1% of the principal prepaid. No prepayment premium is due on any principal prepaid after the fourth anniversary of the applicable date of issuance of any Notes.
The Note Purchase Agreement contains affirmative and negative covenants customary for financings of this type, including limitations on our and our subsidiaries’ ability, among other things, to incur additional debt, grant or permit additional liens, make investments and acquisitions, merge or consolidate with others, dispose of assets, grant certain license rights to our products, technology and other intellectual property rights, pay dividends and distributions, repay junior indebtedness and enter into affiliate transactions, in each case, subject to certain exceptions. In addition, the Note Purchase Agreement contains financial covenants requiring us to maintain at all times (i) at least $10 million of cash and cash equivalents and (ii) following the issuance of the Delayed Draw Notes or upon entering into certain exclusive licenses of XHANCE, a total debt to trailing four quarter XHANCE net revenue ratio of not more than 6.50 to 1.00 initially, and thereafter declining quarterly by equal half-steps to a ratio of not more than 3.00 to 1.00.
The following table shows a summary of our cash flows for the periods indicated (in thousands):
Year Ended December 31, | ||||||||||||
2017 | 2016 | 2015 | ||||||||||
Net cash (used in) provided by operating activities | $ | (35,651 | ) | $ | 21,720 | $ | (28,714 | ) | ||||
Net cash used in investing activities | (2,406 | ) | (215 | ) | (80 | ) | ||||||
Net cash provided by financing activities | 236,125 | 55 | 19,123 | |||||||||
Effects of exchange rates on cash and cash equivalents | (11 | ) | 39 | (14 | ) | |||||||
Net increase (decrease) in cash and cash equivalents | $ | 198,057 | $ | 21,599 | $ | (9,685 | ) | |||||
Operating activities
Cash provided by (used in) operating activities decreased by $57.4 million, from $21.7 million for the year ended December 31, 2016 to $(35.7) million for the year ended December 31, 2017. The decrease in cash provided by operating activities was attributable primarily to the net income generated from the $47.5 million of licensing revenue earned in connection with the achievement of a development milestone under the terms of the AVP-825 License Agreement resulting from FDA approval of Onzetra Xsail in January 2016. No revenue was generated from the AVP-825 License Agreement during the year ended December 31, 2017.
Cash provided by (used in) operating activities increased by $50.4 million, from $(28.7) million for the year ended December 31, 2015 to $21.7 million for the year ended December 31, 2016. The $50.4 million increase in cash provided by operating activities was attributable primarily to the net income generated from the $47.5 million of
93
licensing revenue earned in connection with the achievement of a development milestone under the terms of the AVP-825 License Agreement resulting from FDA approval of Onzetra Xsail in January 2016.
Investing activities
Cash used in investing activities increased $2.2 million from $0.2 million for the year ended December 31, 2016 to $2.4 million for the year ended December 31, 2017. The increase was related to purchases of equipment in connection with our preparation for the commercial launch of XHANCE.
Cash used in investing activities increased $0.1 million from $0.1 million for the year ended December 31, 2015 to $0.2 million for the year ended December 31, 2016. The $0.1 million increase was related to increased purchases of equipment.
Financing activities
Cash provided by financing activities increased to $236.1 million for the year ended December 31, 2017 from $0.1 million for the year ended December 31, 2016. During 2017, we received $36.4 million in net proceeds from the sale of our Series D Preferred Stock, $125.5 million in net proceeds from our IPO and $71.9 million in net proceeds from the issuance of the Notes.
Cash provided by financing activities decreased $19.0 million from $19.1 million for the year ended December 31, 2015 to $0.1 million for the year ended December 31, 2016. During 2015, we received $4.8 million in net proceeds from the sale of our Series C-1 Preferred Stock and $14.3 million in net proceeds from the sale and issuance of our convertible promissory notes that were subsequently converted to Series C-2 Preferred Stock in March 2017. During 2016, we received $0.1 million in cash from stock option exercises.
Future funding requirements
We expect to continue to incur significant expenses in connection with our ongoing activities, particularly as we:
▪ | deploy a contract specialty sales force, which initially consists of approximately 80 territory managers, to market XHANCE for the treatment of nasal polyps and build commercial infrastructure to support sales and marketing for XHANCE; |
▪ | initiate advertising and other promotional activities to support the commercialization of XHANCE; |
▪ | continue clinical development activities for XHANCE, including FDA-mandated pediatric studies and clinical trials for a follow-on indication for the treatment of chronic sinusitis; |
▪ | hire additional staff and add operational, financial and information systems to execute our business plan; |
▪ | maintain, expand and protect our patent portfolio; |
▪ | contract to manufacture XHANCE and our other product candidates; |
▪ | service our debt obligations under the Notes issued in December 2017; |
▪ | continue research and development activities for our other product candidates; and |
▪ | operate as a public company. |
Our future funding requirements, both near and long-term, will depend on many factors, including, but not limited to:
▪ | the success of our commercialization of XHANCE for the treatment of nasal polyps including, among other things, patient and physician acceptance of XHANCE and our ability to obtain adequate coverage and reimbursement for XHANCE; |
▪ | the cost and timing of commercialization activities for XHANCE, including product manufacturing, marketing, sales and distribution; |
▪ | revenue received from commercial sales of XHANCE; |
▪ | our clinical development plans for XHANCE, including FDA-mandated pediatric studies and clinical trials for the follow-on indication for the treatment of chronic sinusitis; |
94
▪ | the outcome, timing and cost of the regulatory approval process of XHANCE for chronic sinusitis by the FDA, including the potential for the FDA to require that we perform more studies and clinical trials than those that we currently expect; |
▪ | the costs involved in preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights, and; |
▪ | the cost of defending intellectual property disputes, including patent infringement actions brought by third parties against us; |
▪ | potential future licensing revenue from the AVP-825 License Agreement; |
▪ | fluctuations in the three-month LIBOR-based floating interest rate of our Notes; |
▪ | the initiation, progress, timing, costs and results of clinical trials for our other product candidates; and |
▪ | the extent to which we in-license or acquire other products, product candidates or technologies. |
Although it is difficult to predict future liquidity requirements, we believe that our existing cash and cash equivalents will enable us to fund our operations as well as our debt service obligations through the end of 2019. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we expect. We expect that we will need to raise additional capital in the future to further the commercialization of XHANCE for the treatment of nasal polyps, to complete the clinical development of XHANCE for a follow-on indication for the treatment of chronic sinusitis, and to support the development of our other product candidates. Additional funds may not be available on a timely basis, on favorable terms, or at all, and such funds, if raised, may not be sufficient to enable us to continue to implement our long-term business strategy. If we cannot expand our operations or otherwise capitalize on our business opportunities because we lack sufficient capital, our business, financial condition and results of operations could be materially adversely affected and we may need to delay or curtail our operations until such funding is received. Additionally, we may never become profitable, or if we do, we may not be able to sustain profitability on a recurring basis.
Contractual obligations and commitments
The following table summarizes our contractual obligations at December 31, 2017:
Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | ||||||||||||||||
(in thousands) | ||||||||||||||||||||
Operating leases(1) | $ | 187 | $ | 187 | $ | — | $ | — | $ | — | ||||||||||
Long-term debt(2) | 121,494 | 7,928 | 16,327 | 16,349 | 80,890 | |||||||||||||||
Purchase obligations (3) | 16,354 | 14,019 | 2,335 | — | — | |||||||||||||||
Total | $ | 138,035 | $ | 22,134 | $ | 18,662 | $ | 16,349 | $ | 80,890 | ||||||||||
(1) Reflects obligations pursuant to our office leases in Yardley, Pennsylvania, Ewing, New Jersey, Oslo, Norway and Swindon, England. In January 2018, we amended our existing office lease agreement for our headquarters in Yardley, PA, or the Lease Amendment. Under the terms of the Lease Amendment, our leased office space was increased from approximately 20,050 square feet to approximately 30,000 square feet and the term of the lease was extended from March 31, 2018 to May 31, 2021, or the Extended Term.The rent payments during the Extended Term will be approximately $2.8 million in the aggregate and we will also be required to pay our proportionate share of certain operating costs and property taxes applicable to the leased premises. Because the Lease Amendment was executed after December 31, 2017, obligations under the Lease Amendment are not included in the table above.
(2) Reflects principal, interest obligations and exit fees pursuant to the Note Purchase Agreement entered into on December 29, 2017. The Notes bear interest at 9.0% plus the three-month LIBOR rate, subject to a 1.0% floor. The Company is required to make quarterly, interest only payments until the maturity date. Interest amounts included above are calculated at the quarterly rate as of December 31, 2017.
(3) Reflects non-cancellable services under an agreement we entered into in November 2017 with a contract sales organization for the recruitment, deployment and management of a contract sales force to market XHANCE in the United States. Subject to certain limited exceptions, we may not terminate this agreement until after the first anniversary of the deployment of the sales force (which occurred in March 2018). We estimate the expenses related to the non-cancellable services during this period to be approximately $16.4 million. Thereafter, we may terminate the agreement subject to potential early termination fees ranging from $0.1 million to $0.7 million.
95
Off-balance sheet arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
ITEM 7A. QUALITATIVE AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to various market risks, which may result in potential losses arising from adverse changes in market rates, such as interest rates and foreign exchange rates. We do not enter into derivatives or other financial instruments for trading or speculative purposes and do not believe we are exposed to material market risk with respect to our cash and cash equivalents.
Through the operation of our subsidiaries based in the United Kingdom and Norway, we are exposed to foreign exchange rate risks. In addition to the operations of our foreign subsidiaries, we also contract with vendors that are located outside the United States, and in some cases make payment of invoices denominated in foreign currencies. We are subject to fluctuations in foreign currency rates in connection with these arrangements. We do not currently hedge our foreign currency exchange rate risk. As of December 31, 2017, we had minimal liabilities denominated in foreign currencies.
As of December 31, 2017, we had cash and cash equivalents of $234.9 million. We do not engage in any hedging activities against changes in interest rates. Because of the short-term maturities of our cash and cash equivalents, we do not believe that an immediate 10% increase in interest rates would have a significant impact on the realized value of our investments.
The interest rate on our senior secured notes under the Note Purchase Agreement is variable based on the three-month London Interbank Offered Rate (LIBOR) and, therefore, is affected by changes in market interest rates. As of December 31, 2017, we had $75.0 million of aggregate principal amount of senior secured notes outstanding under the Note Purchase Agreement. As of December 31, 2017, the three-month LIBOR was 1.75%. A hypothetical 1% increase the the three-month LIBOR would result in $0.8 million in incremental annual interest expense under the outstanding senior secured notes.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information required by Item 8 including the financial statements and notes thereto, and report of the independent registered public accounting firm thereon, are included in this Form 10-K as set forth in the “Index to Consolidated Financial Statements” on page F-1.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
The term "disclosure controls and procedures," as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange
Act, refers to controls and procedures that are designed to ensure that information required to be disclosed by a
company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC's rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that such information is accumulated and communicated to a company's management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure.
In designing and evaluating our disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the disclosure controls and procedures are met. Additionally, in designing disclosure controls and procedures, our management was required to apply its judgment in evaluating the cost-benefit relationship of possible disclosure controls and procedures. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will
96
succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a control system, misstatements due to error or fraud may occur and not be detected.
Our Chief Executive Officer and our Chief Financial Officer evaluated the effectiveness of our disclosure controls and procedures, as defined in Rules 13a 15(e) and 15d 15(e) under the Exchange Act, as of the end of the period covered by this report. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2017.
Management's Report on Internal Control over Financial Reporting
This Annual Report on Form 10-K does not include a report of management’s assessment regarding internal control over financial reporting due to a transition period established by the rules of the SEC for newly public companies.
Attestation Report of the Registered Public Accounting Firm
This Annual Report on Form 10-K does not include an attestation report of our registered public accounting firm
due to an exemption established by the JOBS Act for “emerging growth companies.”
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during our the quarter ended December 31, 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORTE GOVERNANCE
The information required by this item is incorporated by reference to our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days of the fiscal year ended December 31, 2017.
Our Board has adopted a written Code Conduct applicable to all officers, directors and employees, which is available on our website (www.optinose.com) under "Corporate Governance" within the “Investors” section. We intend to satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding amendment to, or waiver from, a provision of this Code and by posting such information on the website address and location specified above.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item is incorporated by reference to our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days of the fiscal year ended December 31, 2017.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item is incorporated by reference to our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days of the fiscal year ended December 31, 2017.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item is incorporated by reference to our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days of the fiscal year ended December 31, 2017.
97
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this item is incorporated by reference to our Proxy Statement for the 2018 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days of the fiscal year ended December 31, 2017.
PART IV
ITEM 15. EXIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) (1) Consolidated Financial Statements.
The Consolidated Financial Statements are filed as part of this report. See the Index to the Consolidated Financial Statements on page F-1.
(2) Consolidated Financial Statement Schedule.
Schedules are omitted because they are not applicable, or are not required, or because the information is included in the Consolidated Financial Statements and Notes thereto.
(3) The exhibits listed under Item 15(b), which are incorporated herein by reference, are filed or furnished as part of this report or are incorporated into this report by reference.
(b) Exhibits.
Incorporated by Reference | ||||||||||
Exhibit Number | Exhibit Description | Form | Date | Number | Filed Herewith | |||||
2.1 | S-1 | 9/18/17 | 2.1 | |||||||
3.1 | 8-K | 10/18/17 | 3.1 | |||||||
3.2 | 8-K | 10/18/17 | 3.2 | |||||||
4.1 | S-1/A | 10/3/17 | 4.1 | |||||||
4.2 | S-1 | 9/18/17 | 4.2 | |||||||
4.3 | S-1 | 9/18/17 | 4.3 | |||||||
4.4 | S-1 | 9/18/17 | 4.4 | |||||||
10.1 | x | |||||||||
10.2 | 8-K | 10/18/17 | 10.1 | |||||||
10.3 | 8-K | 10/18/17 | 10.2 | |||||||
10.4 | 8-K | 10/18/17 | 10.3 | |||||||
10.5 | 8-K | 10/18/17 | 10.4 | |||||||
10.6 | 8-K | 10/18/17 | 10.5 | |||||||
10.7 | S-1/A | 10/3/17 | 10.7 | |||||||
10.8 | S-1/A | 10/3/17 | 10.8 | |||||||
10.9 | S-1/A | 10/3/17 | 10.9 | |||||||
98
10.10 | S-1/A | 10/3/17 | 10.10 | |||||||
10.11 | S-1 | 9/18/17 | 10.11 | |||||||
10.12 | S-1 | 9/18/17 | 10.12 | |||||||
10.13 | S-1 | 9/18/17 | 10.13 | |||||||
10.14 | S-1 | 9/18/17 | 10.14 | |||||||
10.15 | S-1 | 9/18/17 | 10.15 | |||||||
10.16 | S-1 | 9/18/17 | 10.16 | |||||||
10.17 | S-1/A | 10/3/17 | 10.17 | |||||||
10.18 | S-1/A | 10/3/17 | 10.18 | |||||||
10.19 | x | |||||||||
21.1 | x | |||||||||
23.1 | x | |||||||||
31.1 | x | |||||||||
31.2 | x | |||||||||
32.1 | x | |||||||||
32.2 | x | |||||||||
101.INS | XBRL Instance Document. | x | ||||||||
101.SCH | XBRL Taxonomy Extension Schema Document. | x | ||||||||
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document. | x | ||||||||
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document. | x | ||||||||
101.LAB | XBRL Taxonomy Extension Label Linkbase Document. | x | ||||||||
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document. | x | ||||||||
† | Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for confidential treatment granted pursuant to Rule 406 under the Securities Act of 1933. | |||||||||
+ | Indicates management contract or compensatory plan. | |||||||||
ITEM 16. FORM 10-K SUMMARY
Not applicable.
99
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized in the capacities and on the date indicated.
OPTINOSE, INC. | ||||||
Date: March 13, 2018 | By: | /s/ PETER K. MILLER | ||||
Name: | Peter K. Miller | |||||
Title: | Chief Executive Officer | |||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ PETER K. MILLER | Chief Executive Officer and Director (Principal Executive Officer) | March 13, 2018 | ||
Peter K. Miller | ||||
/s/ KEITH A. GOLDAN | Chief Financial Officer (Principal Finance Officer and Principal Accounting Officer) | March 13, 2018 | ||
Keith A. Goldan | ||||
/s/ JOSEPH C. SCODARI | Chairman of the Board of Directors | March 13, 2018 | ||
Joseph C. Scodari | ||||
/s/ LARRY G. PICKERING | Vice Chairman of the Board of Directors | March 13, 2018 | ||
Larry G. Pickering | ||||
/s/ SRIRAM VENKATARAMAN | Director | March 13, 2018 | ||
Sriram Venkataraman | ||||
/s/ WILLIAM F. DOYLE | Director | March 13, 2018 | ||
William F. Doyle | ||||
/s/ JOSHUA A. TAMAROFF | Director | March 13, 2018 | ||
Joshua A. Tamaroff | ||||
/s/ WILHELMUS GROENHUYSEN | Director | March 13, 2018 | ||
Wilhelmus Groenhuysen | ||||
/s/ SANDRA L. HELTON | Director | March 13, 2018 | ||
Sandra L. Helton | ||||
100
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Page | ||
Audited Consolidated Financial Statements | ||
F-1
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors and of OptiNose, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of OptiNose, Inc. (the Company) as of December 31, 2017 and 2016, the related consolidated statements of operations, comprehensive income (loss), redeemable convertible preferred stock and stockholders’ equity (deficit) and cash flows for each of the three years in the period ended December 31, 2017, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2016.
Philadelphia, Pennsylvania
March 13, 2018
F-2
OptiNose, Inc.
Consolidated Balance Sheets
(in thousands, except share and per share data)
December 31, | |||||||
2017 | 2016 | ||||||
Assets | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 234,854 | $ | 36,797 | |||
Grants and other receivables | 46 | 384 | |||||
Inventory | 2,013 | — | |||||
Deposits and other current assets | 1,254 | 3,494 | |||||
Total current assets | 238,167 | 40,675 | |||||
Property and equipment, net | 2,564 | 323 | |||||
Deposits and other assets — long-term | 405 | 553 | |||||
Total assets | $ | 241,136 | $ | 41,551 | |||
Liabilities, redeemable convertible preferred stock and stockholders' equity (deficit) | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 5,893 | $ | 3,369 | |||
Accrued expenses | 8,698 | 2,541 | |||||
Deferred other income | 186 | — | |||||
Total current liabilities | 14,777 | 5,910 | |||||
Convertible notes payable, net | — | 15,256 | |||||
Long-term debt, net | 71,863 | — | |||||
Accrued interest | — | 3,409 | |||||
Total liabilities | 86,640 | 24,575 | |||||
Commitments and contingencies (Note 11) | |||||||
Redeemable convertible preferred stock, $0.001 par value: | |||||||
Series A, No shares authorized, issued and outstanding at December 31, 2017; 285,480 shares authorized, issued and outstanding at December 31, 2016 | — | 5,381 | |||||
Series B-1, No shares authorized, issued and outstanding at December 31, 2017; 35,680 shares authorized, issued and outstanding at December 31, 2016 | — | 673 | |||||
Series B-2, No shares authorized, issued and outstanding at December 31, 2017; 782,600 shares authorized, issued and outstanding at December 31, 2016 | — | 14,760 | |||||
Series C, No shares authorized, issued and outstanding at December 31, 2017; 4,115,344 shares authorized, issued and outstanding at December 31, 2016 | — | 105,738 | |||||
Series C-1, No shares authorized, issued and outstanding at December 31, 2017; 1,656,410 shares authorized, issued and outstanding at December 31, 2016 | — | 41,621 | |||||
Total redeemable convertible preferred stock | — | 168,173 | |||||
Stockholders' equity (deficit): | |||||||
Common stock, $0.001 par value; 200,000,000 and 10,624,486 shares authorized at December 31, 2017 and December 31, 2016 , respectively; 37,802,556 and 4,067,717 shares issued and outstanding at December 31, 2017 and December 31, 2016, respectively | 38 | 4 | |||||
Additional paid-in capital | 365,838 | — | |||||
Accumulated deficit | (211,269 | ) | (151,102 | ) | |||
Accumulated other comprehensive loss | (111 | ) | (99 | ) | |||
Total stockholders' equity (deficit) | 154,496 | (151,197 | ) | ||||
Total liabilities, redeemable convertible preferred stock and stockholders' equity (deficit) | $ | 241,136 | $ | 41,551 | |||
See accompanying notes to consolidated financial statements
F-3
OptiNose, Inc.
Consolidated Statements of Operations
(in thousands, except share and per share data)
Year Ended December 31, | ||||||||||||
2017 | 2016 | 2015 | ||||||||||
Licensing revenues | $ | — | $ | 47,500 | $ | 85 | ||||||
Operating expenses: | ||||||||||||
Research and development | 16,832 | 15,311 | 22,156 | |||||||||
Selling, general and administrative | 31,698 | 6,869 | 6,006 | |||||||||
Total operating expenses | 48,530 | 22,180 | 28,162 | |||||||||
(Loss) income from operations | (48,530 | ) | 25,320 | (28,077 | ) | |||||||
Other (income) expense: | ||||||||||||
Grant and other income | (162 | ) | (727 | ) | (643 | ) | ||||||
Interest income | (303 | ) | (143 | ) | (28 | ) | ||||||
Interest expense | 910 | 3,517 | 819 | |||||||||
Foreign currency (gains) losses | (73 | ) | 60 | 89 | ||||||||
Net (loss) income | $ | (48,902 | ) | $ | 22,613 | $ | (28,314 | ) | ||||
Deemed dividend | 11,969 | 11,005 | 9,992 | |||||||||
Accretion to redemption value | 1,096 | 2,109 | 2,069 | |||||||||
Net (loss) income attributable to common stockholders | $ | (61,967 | ) | $ | 9,499 | $ | (40,375 | ) | ||||
Net (loss) income per share of common stock, | ||||||||||||
basic | $ | (5.63 | ) | $ | 0.40 | $ | (9.97 | ) | ||||
diluted | $ | (5.63 | ) | $ | 0.32 | $ | (9.97 | ) | ||||
Weighted average common shares outstanding, | ||||||||||||
basic | 10,999,121 | 4,054,316 | 4,049,668 | |||||||||
diluted | 10,999,121 | 4,980,181 | 4,049,668 | |||||||||
See accompanying notes to consolidated financial statements
F-4
OptiNose, Inc.
Consolidated Statements of Comprehensive Income and Loss
(in thousands)
Year Ended December 31, | ||||||||||||
2017 | 2016 | 2015 | ||||||||||
Net (loss) income | $ | (48,902 | ) | $ | 22,613 | $ | (28,314 | ) | ||||
Other comprehensive (loss) income: | ||||||||||||
Foreign currency translation adjustment | (12 | ) | 42 | (13 | ) | |||||||
Comprehensive (loss) income | $ | (48,914 | ) | $ | 22,655 | $ | (28,327 | ) | ||||
See accompanying notes to consolidated financial statements
F-5
OptiNose, Inc.
Consolidated Statements of Changes in Redeemable Convertible Preferred Stock and
Stockholders' Equity (Deficit)
(in thousands, except share data)
Redeemable Convertible Preferred Stock | Stockholders' Deficit | |||||||||||||||||||||||||||||
Common Stock | Additional Paid -in Capital | Accumulated Deficit | Accumulated Other Comprehensive Loss | Total Stockholders' Deficit | ||||||||||||||||||||||||||
Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||
Balance at January 1, 2015 | 6,638,885 | $ | 138,160 | 4,049,668 | $ | 4 | $ | — | $ | (121,468 | ) | $ | (128 | ) | $ | (121,592 | ) | |||||||||||||
Stock compensation expense | — | — | — | — | 588 | — | — | 588 | ||||||||||||||||||||||
Balance at Sale of Series C-1 preferred stock | 236,629 | 4,838 | — | — | — | — | — | — | ||||||||||||||||||||||
Accretion of Series C & Series C-1 preferred stock to redemption value | — | 2,069 | — | — | (588 | ) | (1,481 | ) | — | (2,069 | ) | |||||||||||||||||||
Accretion of Series C & Series C-1 preferred stock in lieu of 8% dividend | — | 9,992 | — | — | — | (9,992 | ) | — | (9,992 | ) | ||||||||||||||||||||
Foreign currency translation adjustment | — | — | — | — | — | — | (13 | ) | (13 | ) | ||||||||||||||||||||
Net loss | — | — | — | — | — | (28,314 | ) | — | (28,314 | ) | ||||||||||||||||||||
Balance at December 31, 2015 | 6,875,514 | $ | 155,059 | 4,049,668 | $ | 4 | $ | — | $ | (161,255 | ) | $ | (141 | ) | $ | (161,392 | ) | |||||||||||||
Stock compensation expense | — | — | — | — | 599 | — | — | 599 | ||||||||||||||||||||||
Exercise of common stock options | — | — | 18,049 | — | 55 | — | — | 55 | ||||||||||||||||||||||
Accretion of Series C & Series C-1 preferred stock to redemption value | — | 2,109 | — | — | (654 | ) | (1,455 | ) | — | (2,109 | ) | |||||||||||||||||||
Accretion of Series C & Series C-1 preferred stock in lieu of 8% dividend | — | 11,005 | — | — | — | (11,005 | ) | — | (11,005 | ) | ||||||||||||||||||||
Foreign currency translation adjustment | — | — | — | — | — | — | 42 | 42 | ||||||||||||||||||||||
Net loss | — | — | — | — | — | 22,613 | — | 22,613 | ||||||||||||||||||||||
Balance at December 31, 2016 | 6,875,514 | $ | 168,173 | 4,067,717 | $ | 4 | $ | — | $ | (151,102 | ) | $ | (99 | ) | $ | (151,197 | ) | |||||||||||||
Conversion of convertible debt to Series C-2 preferred stock | 687,474 | 19,527 | — | — | — | — | — | — | ||||||||||||||||||||||
Sale of Series D preferred stock, net of issuance costs | 1,117,578 | 36,494 | — | — | — | — | — | — | ||||||||||||||||||||||
Stock compensation expense | — | — | — | — | 5,096 | — | — | 5,096 | ||||||||||||||||||||||
Accretion of Series C, Series C-1 & Series D preferred stock to redemption value | — | 1,096 | — | — | (1,096 | ) | — | — | (1,096 | ) | ||||||||||||||||||||
Accretion of Series C, Series C-1, Series C-2 & Series D preferred stock in lieu of 8% dividend | — | 11,969 | — | — | (704 | ) | (11,265 | ) | — | (11,969 | ) | |||||||||||||||||||
Sale of common stock upon consummation of initial public offering, net of issuance costs | — | — | 8,625,000 | 9 | 125,462 | — | — | 125,471 | ||||||||||||||||||||||
Reclassification of redeemable convertible preferred stock upon consummation of initial public offering | (8,680,566 | ) | (237,259 | ) | 25,068,556 | 25 | 237,234 | — | — | 237,259 | ||||||||||||||||||||
Exercise of stock options, net of shares withheld for income taxes | — | — | 41,283 | — | (154 | ) | — | — | (154 | ) | ||||||||||||||||||||
Foreign currency translation adjustment | — | — | — | — | — | — | (12 | ) | (12 | ) | ||||||||||||||||||||
Net loss | — | — | — | — | — | (48,902 | ) | — | (48,902 | ) | ||||||||||||||||||||
Balance at December 31, 2017 | — | $ | — | 37,802,556 | $ | 38 | $ | 365,838 | $ | (211,269 | ) | $ | (111 | ) | $ | 154,496 | ||||||||||||||
See accompanying notes to consolidated financial statements
F-6
OptiNose, Inc.
Consolidated Statements of Cash Flows
(in thousands)
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Operating activities: | |||||||||||
Net (loss) income | $ | (48,902 | ) | $ | 22,613 | $ | (28,314 | ) | |||
Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: | |||||||||||
Depreciation and amortization | 164 | 83 | 75 | ||||||||
Stock-based compensation | 5,096 | 599 | 588 | ||||||||
Amortization of debt discount and issuance costs | 198 | 776 | 195 | ||||||||
Loss on sale of equipment | 2 | — | — | ||||||||
Changes in operating assets and liabilities: | |||||||||||
Grants and other receivables | 337 | 65 | 144 | ||||||||
Deposits and other assets | 2,387 | (3,888 | ) | 1,504 | |||||||
Inventory | (2,013 | ) | — | — | |||||||
Accounts payable | 392 | (130 | ) | 450 | |||||||
Accrued expenses | 5,834 | (1,097 | ) | (3,814 | ) | ||||||
Accrued interest | 668 | 2,740 | 669 | ||||||||
Deferred other income | 186 | (41 | ) | (211 | ) | ||||||
Cash (used in) provided by operating activities | (35,651 | ) | 21,720 | (28,714 | ) | ||||||
Investing activities: | |||||||||||
Purchases of property and equipment | (2,406 | ) | (215 | ) | (80 | ) | |||||
Cash used in investing activities | (2,406 | ) | (215 | ) | (80 | ) | |||||
Financing activities: | |||||||||||
Proceeds from the sale of Series C-1 preferred stock | — | — | 5,000 | ||||||||
Proceeds from the sale of Series D preferred stock | 36,712 | — | — | ||||||||
Proceeds from the sale of common stock units in connection with initial public offering | 138,000 | — | — | ||||||||
Proceeds from issuance of convertible notes payable, net | — | — | 14,285 | ||||||||
Proceeds from long-term debt | 75,000 | — | — | ||||||||
Proceeds from the exercise of stock options | 134 | 55 | — | ||||||||
Payments of withholdings on shares withheld for income taxes | (288 | ) | — | — | |||||||
Cash paid for financing costs | (13,433 | ) | — | (162 | ) | ||||||
Cash provided by financing activities | 236,125 | 55 | 19,123 | ||||||||
Effects of exchange rate changes on cash and cash equivalents | (11 | ) | 39 | (14 | ) | ||||||
Net increase in cash and cash equivalents | 198,057 | 21,599 | (9,685 | ) | |||||||
Cash and cash equivalents at beginning of period | 36,797 | 15,198 | 24,883 | ||||||||
Cash and cash equivalents at end of period | $ | 234,854 | $ | 36,797 | $ | 15,198 | |||||
Supplemental disclosure of noncash financing activities: | |||||||||||
Deemed dividend | $ | 11,969 | $ | 11,005 | $ | 9,992 | |||||
Accretion to redemption value | $ | 1,096 | $ | 2,109 | $ | 2,069 | |||||
Financing costs within accounts payable and accrued expenses | $ | 2,454 | $ | — | $ | — | |||||
Conversion of convertible notes payable and accrued interest into Series C-2 preferred stock | $ | 19,527 | $ | — | $ | — | |||||
Conversion of redeemable convertible preferred stock into common stock | $ | 237,259 | $ | — | $ | — | |||||
See accompanying notes to consolidated financial statements
F-7
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
1. Organization and Description of Business
OptiNose, Inc. (the Company) was incorporated in Delaware in May 2010 (inception) and has facilities in Yardley, Pennsylvania, Ewing, New Jersey, Oslo, Norway and Swindon, England. The Company's predecessor entity, OptiNose AS, was formed under the laws of Norway in September 2000. In 2010, OptiNose AS became a wholly-owned subsidiary of the Company as part of an internal reorganization.
The Company is a specialty pharmaceutical company focused on the development and commercialization of products for patients treated by ear, nose and throat (ENT) and allergy specialists. The Company's first two products approved by the United States Food and Drug Administration (FDA) utilize its proprietary Exhalation Delivery Systems (EDS), which are capable of deep intranasal deposition of medication. OptiNose developed its first product, Onzetra® Xsail® (sumatriptan nasal power) through the completion of Phase III clinical trials and subsequently out-licensed the product to Avanir Pharmaceuticals, Inc (Avanir). Onzetra Xsail received FDA approval and was launched in the United States (US) in 2016. The Company's second product, XHANCETM (fluticasone propionate) nasal spray, 93 mcg, is a therapeutic that utilizes its EDS to deliver a topically-acting corticosteroid for the treatment of chronic rhinosinusitis with nasal polyps and, if approved, chronic rhinosinusitis without nasal polyps. On September 18, 2017, the FDA approved the Company's New Drug Application (NDA) for XHANCE for the treatment of nasal polyps in patients 18 years of age or older, and XHANCE is in development for the treatment of chronic sinusitis.
In October 2017, the Company completed an initial public offering (IPO) of its common stock, selling 8,625,000 shares at $16.00 per share. As a result of the IPO, the Company received $125,471 in net proceeds, after deducting discounts and commissions of $9,660 and offering expenses of approximately $2,869 payable by the Company. Upon consummation of the IPO, all of the outstanding shares of the Company’s redeemable convertible preferred stock were converted into an aggregate of 25,068,556 shares of common stock.
2. Liquidity
Since inception, the Company's operations have focused on organization and staffing, business planning, raising capital, establishing an intellectual property portfolio, conducting preclinical studies and clinical trials, pursuing regulatory approvals and most recently, preparing for the launch of XHANCE. The Company has not generated any revenue from product sales to date. As of December 31, 2017, the Company had cash and cash equivalents of $234,854.
During the year ended December 31, 2017, the Company sold 1,117,578 shares of Series D preferred stock, which resulted in gross proceeds to the Company of $36,712 (Note 12). Additionally, in October 2017, the Company completed an IPO of its common stock, selling 8,625,000 shares at $16.00 per share. As a result of the IPO, the Company received $125,471 in net proceeds. In December 2017, the Company entered into a note purchase agreement which provided for the issuance of up to $100,000 of senior secured notes, of which $75,000 (Note 9) of such notes were issued immediately.
The Company may need to secure additional capital in the future through equity or debt financings, partnerships, collaborations, or other sources in order to service the Company's existing obligations under outstanding notes, including repayment, and to carry out all of the Company's planned development and commercial activities. If additional capital is not secured when required, the Company may need to delay or curtail its operations until such funding is received. The Company is subject to a number of risks similar to other life sciences companies, including, but not limited to, successful discovery and development of its product candidates, raising additional capital, the development by its competitors of new technological innovations, protection of proprietary technology and market acceptance of the Company's products.
F-8
OptiNose, Inc.
Notes to Unaudited Interim Consolidated Financial Statements (Continued)
(in thousands, except share and per share data)
3. Summary of Significant Accounting Policies
Basis of presentation
The accompanying consolidated financial statements have been prepared in conformity with US generally accepted accounting principles (GAAP). Any reference in these notes to applicable guidance is meant to refer to GAAP as found in the Accounting Standards Codification (ASC) and Accounting Standards Updates (ASU) of the Financial Accounting Standards Board (FASB).
Principles of consolidation
The consolidated financial statements include the accounts of OptiNose, Inc. and its wholly-owned subsidiaries, OptiNose US, Inc., OptiNose AS and OptiNose UK Ltd. All inter-company balances and transactions have been eliminated in consolidation.
Use of estimates
The preparation of the consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and reported amounts of expenses during the reporting period. Due to the uncertainty of factors surrounding the estimates or judgments used in the preparation of the consolidated financial statements, actual results may materially vary from these estimates. Estimates and assumptions are periodically reviewed and the effects of revisions are reflected in the consolidated financial statements in the period they are determined to be necessary.
Stock Split
On October 10, 2017, the Company effected a 2.8879-for-1 reclassification, or stock split, of the Company's common stock in connection with its initial public offering, or the IPO. All common share and per share amounts in these consolidated financial statements and notes thereto have been retroactively adjusted for all periods presented to reflect the stock split.
Cash and cash equivalents
All highly liquid investments purchased with an original maturity date of three months or less at the date of purchase are considered to be cash equivalents. The Company generally invests its cash in deposits with high credit quality financial institutions. Additionally, the Company performs periodic evaluations of the relative credit standing of these financial institutions.
The Company maintains its cash and cash equivalent balances at foreign and domestic financial institutions. Bank deposits with Norwegian banks are insured up to approximately 2,000 Norwegian krone by the Norwegian Banks' Guarantee Fund. Bank deposits with US banks are insured up to $250 by the Federal Deposits Insurance Corporation. The Company had uninsured cash balances of $233,772 and $35,866 at December 31, 2017 and 2016, respectively.
Fair value of financial instruments
The Company measures certain assets and liabilities at fair value, which is defined as the price that would be received to sell an asset or paid to transfer a liability (the exit price) in an orderly transaction between market participants at the measurement date. The FASB accounting guidance outlines a valuation framework and creates a fair value hierarchy in order to increase the consistency and comparability of fair value measurements and the related disclosures. In determining fair value, the Company uses quoted prices and observable inputs. Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from independent sources. The fair value hierarchy is broken down into three levels based on the source of the inputs as follows:
• | Level 1 — Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities. |
F-9
OptiNose, Inc.
Notes to Unaudited Interim Consolidated Financial Statements (Continued)
(in thousands, except share and per share data)
• | Level 2 — Valuations based on observable inputs (other than Level 1 quoted prices), such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data. |
• | Level 3 — Valuations based on inputs that are unobservable and models that are significant to the overall fair value measurement. |
At December 31, 2017 and 2016, the Company's financial instruments included cash and cash equivalents, grants receivable, accounts payable, and accrued expenses. The carrying amounts reported in the Company's financial statements for these instruments approximates their respective fair values because of the short-term nature of these instruments. In addition, at December 31, 2017, the Company believes the carrying value of debt approximates fair value as the interest rates are reflective of the rate the Company could obtain on debt with similar terms and conditions. At December 31, 2017 and 2016, there were no financial assets or liabilities measured at fair value on a recurring basis.
The Company's financial instruments also included convertible debt at December 31, 2016 (Note 8).
Inventory
Prior to receiving FDA approval for XHANCE in September 2017, inventory purchases were expensed as incurred and recorded as a component of research and development expense. Subsequent to receiving FDA approval, inventories are stated at the lower of cost or net realizable value, net of reserves for excess and obsolete inventory. Cost is computed using standard cost (which approximates actual cost) on a first-in, first-out basis. Inventory consisted of the following:
December 31, | |||||||
2017 | 2016 | ||||||
Raw materials | $ | 1,385 | $ | — | |||
Work-in-process | 628 | — | |||||
Finished goods | — | — | |||||
Total | $ | 2,013 | $ | — | |||
Deposits and other assets
Deposits and other assets consist primarily of prepaid insurance, payments made in advance to outsourced contract manufacturers and equipment suppliers, as well as a receivable due from the FDA at December 31, 2016 related to a Prescription Drug User Fee Act (PDUFA) New Drug Application fee that the FDA refunded to the Company in March 2017.
Throughout 2017 and 2016, the Company made upfront payments to outsourced plastic mold development manufacturers and equipment suppliers for molds and equipment that are expected to be used for the commercial production of XHANCE. The Company received the majority of this equipment in 2017. For equipment received prior to FDA approval, the Company recorded the cost associated with the equipment purchase as a component of research and development expense if there was no alternative future use of the equipment without FDA approval. Conversely, deposits on equipment received after the September 18, 2017 FDA approval of XHANCE were capitalized as fixed assets when the equipment was received and therefore classified as long-term deposits at December 31, 2017.
Property and equipment
Property and equipment is recorded at cost less accumulated depreciation. Significant additions or improvements are capitalized, and expenditures for repairs and maintenance are charged to expense as incurred. Gains and
F-10
OptiNose, Inc.
Notes to Unaudited Interim Consolidated Financial Statements (Continued)
(in thousands, except share and per share data)
losses on disposal of assets are included in the consolidated statements of operations. Depreciation is calculated on a straight-line basis over the estimated useful lives of the respective assets.
The estimated useful lives of equipment are as follows:
Computer equipment | 3 years | ||||||
Software | 3 years | ||||||
Machinery & production equipment | 5-10 years | ||||||
Furniture & fixtures | 3-5 years | ||||||
Leasehold improvements | Shorter of lease term or useful life | ||||||
Long lived assets
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future net cash flows expected to be generated. Impairment charges are recognized at the amount by which the carrying amount of an asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or the fair value less costs to sell. The Company has not recognized any impairment or disposition of long-lived assets for the years ended December 31, 2017 , 2016 or 2015.
Revenue recognition
Through December 31, 2017, the Company's revenues have been generated through licensing arrangements, which generally contain multiple elements, or deliverables, including licenses and research and development activities to be performed by the Company on behalf of the licensee. Revenues are recognized when (1) persuasive evidence of an arrangement exists, (2) delivery has occurred or services have been rendered, (3) the price is fixed or determinable and (4) collectibility is reasonably assured.
To date, the Company's revenues have been generated pursuant to the terms of a single license agreement (the AVP-825 License Agreement) with Avanir Pharmaceuticals, Inc. (Avanir) (Note 7). The AVP-825 License Agreement includes licensed rights to patented technology, non-refundable up-front license fees, research services, and regulatory and sales milestones as well as royalty payments. As of December 31, 2017, only possible sales milestones and royalty payments remain to be earned under the license AVP-825 License Agreements. Such amounts would only be earned after net sales in the US, Canada and Mexico exceed a certain threshold.
For arrangements with multiple elements, the Company recognizes revenue in accordance with the FASB ASU No. 2009-13, Multiple-Deliverable Revenue Arrangements, which provides guidance for separating and allocating consideration in a multiple element arrangement. The selling prices of deliverables under an arrangement may be derived using third-party evidence (TPE), or a best estimate of selling price (BESP), if vendor-specific objective evidence of selling price (VSOE) is not available. The objective of BESP is to determine the price at which the Company would transact a sale if the element within the License Agreement was sold on a standalone basis. Deliverables under the arrangement are separate units of accounting if (i) the delivered item has value to the customer on a standalone basis and (ii) if the arrangement includes a general right of return relative to the delivered item, and delivery or performance of the undelivered item is considered probable and substantially within the Company's control. The arrangement consideration that is fixed or determinable at the inception of the arrangement is allocated to the separate units of accounting based on their relative selling prices. The appropriate revenue recognition model is applied to each element and revenue is accordingly recognized as each element is delivered. Management exercises significant judgment in determining whether a deliverable is a separate unit of accounting.
In determining the separate units of accounting for the Company's collaborations, the Company evaluated whether the AVP-825 License Agreement has standalone value to the collaborator based on consideration of the relevant
F-11
OptiNose, Inc.
Notes to Unaudited Interim Consolidated Financial Statements (Continued)
(in thousands, except share and per share data)
facts and circumstances for each arrangement. Factors considered in this determination include the research and development capabilities of the collaborator and the availability of relevant research expertise in the marketplace. In addition, the Company considers whether or not (i) the collaborator could use the license for its intended purpose without the receipt of the remaining deliverables, (ii) the value of the license was dependent on the undelivered items and (iii) the collaborator or other vendors could provide the undelivered items.
Whenever the Company determines that an element is delivered over a period of time, revenue is recognized using either a proportional performance model, if a pattern of performance can be determined, or a straight-line model over the period of performance, which is typically the research and development term.
Development milestones may be triggered either by the results of the Company's research efforts or by events external to it, such as regulatory approval to market a product. Consideration that is contingent upon achievement of a development milestone is recognized in its entirety as revenue in the period in which the milestone is achieved, but only if the consideration earned from the achievement of a milestone meets all the criteria for the milestone to be considered substantive at the inception of the arrangement. For a milestone to be considered substantive, the consideration earned by achieving the milestone must (i) be commensurate with either the Company's performance to achieve the milestone or the enhancement of the value of the item delivered as a result of a specific outcome resulting from the Company's performance to achieve the milestone, (ii) relate solely to past performance and (iii) be reasonable relative to all deliverables and payment terms in the AVP-825 License Agreement. As of December 31, 2017, all development milestones have been achieved.
Advertising expenses
The Company expenses the costs of advertising, including promotional expenses, as incurred. Advertising expenses were $1,833, $26 and $44 for the years ended December 31, 2017, 2016 and 2015, respectively.
Research and development
Research and development costs are expensed as incurred. Research and development costs consist primarily of device development, clinical trial related costs, and regulatory related costs. The Company enters into agreements with contract research organizations (CROs) to facilitate, coordinate and perform agreed upon research and development activities for the Company's clinical trials. These CRO contracts typically call for the payment of fees for services at the initiation of the contract and/or upon the achievement of certain clinical trial milestones. The Company prepays certain CRO fees whereby the prepayments are recorded as a current or non-current prepaid asset and are amortized into research and development expense over the period of time the contracted research and development services were performed. The Company's CRO contracts generally also included other fees such as project management and pass through fees whereby the Company expenses these costs as incurred, using the Company's best estimate. Pass through fees include, but are not limited to, regulatory expenses, investigator fees, travel costs, and other miscellaneous costs. Pass through fees incurred are based on the amount of work completed for the clinical trials and are monitored through reporting provided by the Company's CROs.
Stock-based compensation
The Company measures and recognizes compensation expense for all stock options awarded to employees and nonemployees and shares issued under the employee stock purchase plan based on the estimated fair value of the awards on the respective grant dates. The Company uses the Black-Scholes option pricing model to value its stock option awards and shares issued under the employee stock purchase plan. The Company recognizes compensation expense for time-based awards on a straight-line basis over the requisite service period, which is generally the vesting period of the award. The Company recognizes compensation expense for performance based awards when the performance condition is probable of achievement. Stock-based awards issued to nonemployees are revalued at each reporting period until the award vests. The Company accounts for forfeitures of stock option awards as they occur.
Estimating the fair value of options and shares issued under the employee stock purchase plan requires the input of subjective assumptions, including the estimated fair value of the Company's common stock, the expected life of the
F-12
OptiNose, Inc.
Notes to Unaudited Interim Consolidated Financial Statements (Continued)
(in thousands, except share and per share data)
options, stock price volatility, the risk-free interest rate and expected dividends. The assumptions used in the Company's Black-Scholes option-pricing model represent management's best estimates and involve a number of variables, uncertainties and assumptions and the application of management's judgment, as they are inherently subjective.
Income taxes
Income taxes are accounted for under the asset and liability method. The Company recognizes deferred tax assets and liabilities for temporary differences between the financial reporting basis and the tax basis of the Company's assets and liabilities and the expected benefits of net operating loss carryforwards. The impact of changes in tax rates and laws on deferred taxes, if any, applied during the period in which temporary differences are expected to be settled, is reflected in the Company's financial statements in the period of enactment. The measurement of deferred tax assets is reduced, if necessary, if, based on weight of the evidence, it is more likely than not that some, or all, of the deferred tax assets will not be realized. As of December 31, 2017 and 2016, the Company has concluded that a full valuation allowance is necessary for all of its net deferred tax assets. The Company had no amounts recorded for uncertain tax positions, interest or penalties in the accompanying consolidated financial statements.
Grant income
Government grants are agreements that provide cost reimbursement for certain research and development activities over a contractually defined period. Income from government grants is recognized in the period in which related costs are incurred, provided that the conditions under which government grants were provided have been met and only perfunctory obligations are outstanding. Grant income received in excess of costs incurred is recognized as deferred other income.
Net income (loss) per common share
The Company used the two-class method to compute net income (loss) per common share for the year ended December 31, 2016 as the Company realized net income and had issued securities (redeemable convertible preferred stock) that entitled the holder to participate in dividends and earnings of the Company. Under this method, net income is reduced by the amount of any dividends earned and the accretion of redeemable convertible preferred stock to its redemption value during the period. The remaining earnings (undistributed earnings) are allocated to common stock and each series of redeemable convertible preferred stock to the extent that each preferred security may share in earnings as if all of the earnings for the period had been distributed. The total earnings allocated to common stock is then divided by the number of outstanding shares to which the earnings are allocated to determine the earnings per share. The two-class method is not applicable during periods with a net loss, as the holders of the redeemable convertible preferred stock have no obligation to fund losses. For the year ended December 31, 2016, the Company presented diluted net income per common share using the two-class method, which was more dilutive than the "if-converted" method.
Diluted net income (loss) per common share is computed under the two-class method by using the weighted-average number of shares of common stock outstanding, plus, for periods with net income attributable to common stockholders, the potential dilutive effects of stock options, warrants, and convertible debt. In addition, the Company analyzes the potential dilutive effect of the outstanding redeemable convertible preferred stock and convertible debt under the "if-converted" method when calculating diluted earnings per share, in which it is assumed that the outstanding redeemable convertible preferred stock or convertible debt converts into common stock at the beginning of the period or when issued if later. The Company reports the more dilutive of the approaches (two class or "if-converted") as their diluted net income per share during the period.
Basic net income (loss) per common share is determined by dividing net income (loss) applicable to common stockholders by the weighted average common shares outstanding during the period. For the years ended December 31, 2017 and 2015, the outstanding common stock option and common stock warrants have been excluded from the calculation of diluted net income (loss) per share because their effect would be anti-dilutive. Therefore, the weighted average shares used to calculate both basic and diluted net loss per share are the same.
F-13
OptiNose, Inc.
Notes to Unaudited Interim Consolidated Financial Statements (Continued)
(in thousands, except share and per share data)
The following table sets forth the computation of basic and diluted net income (loss) per share for the periods indicated:
Year Ended December 31, | ||||||||||||
2017 | 2016 | 2015 | ||||||||||
Basic net (loss) income per common share calculation: | ||||||||||||
Net (loss) income attributable to common stockholders | $ | (61,967 | ) | $ | 9,499 | $ | (40,375 | ) | ||||
Less: undistributed earnings to participating securities | — | (7,884 | ) | — | ||||||||
Net (loss) income attributable to common stockholders — basic | (61,967 | ) | 1,615 | (40,375 | ) | |||||||
Weighted average common shares outstanding — basic | 10,999,121 | 4,054,316 | 4,049,668 | |||||||||
Net (loss) income per share of common stock — basic | $ | (5.63 | ) | $ | 0.40 | $ | (9.97 | ) | ||||
Diluted net (loss) income per common share calculation: | ||||||||||||
Net (loss) income attributable to common stockholders | $ | (61,967 | ) | $ | 9,499 | $ | (40,375 | ) | ||||
Less: undistributed earnings to participating securities | — | (7,884 | ) | — | ||||||||
Net (loss) income attributable to common stockholders — diluted | (61,967 | ) | 1,615 | (40,375 | ) | |||||||
Weighted average common shares outstanding — basic | 10,999,121 | 4,054,316 | 4,049,668 | |||||||||
Stock options | — | 925,865 | — | |||||||||
Weighted average common shares outstanding — diluted | 10,999,121 | 4,980,181 | 4,049,668 | |||||||||
Net (loss) income per share of common stock — diluted | $ | (5.63 | ) | $ | 0.32 | $ | (9.97 | ) | ||||
Diluted net income (loss) per common share for the periods presented do not reflect the following potential common shares, as the effect would be antidilutive:
Year Ended December 31, | |||||||||
2017 | 2016 | 2015 | |||||||
Stock options | 2,141,367 | 2,346,073 | 3,413,178 | ||||||
Common stock warrants | 1,890,489 | 1,890,489 | 1,890,489 | ||||||
Convertible debt | — | 1,917,522 | 1,888,484 | ||||||
Convertible preferred stock | — | 19,855,772 | 19,855,772 | ||||||
Total | 4,031,856 | 26,009,856 | 27,047,923 | ||||||
Recent accounting pronouncements
In May 2017, the FASB issued ASU No. 2017-09, Stock Compensation - Scope of Modification Accounting. ASU 2017-09 provides guidance on which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting. The new standard is effective for fiscal years beginning after December 15, 2017. The Company is currently evaluating the potential impact of the adoption of this standard on its results of operations, financial position and cash flows and related disclosures.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842). The FASB issued the update to require the recognition of lease assets and liabilities on the balance sheet of lessees. The standard will be effective for fiscal years beginning after December 15, 2018, including interim periods within such fiscal years. The ASU requires a modified retrospective transition method with the option to elect a package of practical expedients. Early adoption is permitted. The Company is currently evaluating the potential impact of the adoption of this standard on its results of operations, financial position and cash flows and related disclosures.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606), which will replace numerous requirements in US GAAP, including industry-specific requirements. This guidance provides a five-step model to be applied to all contracts with customers, with an underlying principle that an entity will recognize revenue to depict the transfer of goods or services to customers at an amount that the entity expects to be entitled to in exchange for those goods or services. The new standard also defines accounting for certain costs related to origination and fulfillment of contracts with customers, including whether such costs should be capitalized.
F-14
OptiNose, Inc.
Notes to Unaudited Interim Consolidated Financial Statements (Continued)
(in thousands, except share and per share data)
This statement requires extensive quantitative and qualitative disclosures covering the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including disclosures on significant judgments made when applying the guidance and assets recognized from costs incurred to obtain or fulfill a contract. The guidance is effective for annual reporting periods beginning after December 15, 2017, and interim periods within that reporting period. An entity can elect to apply the guidance under one of the following two methods: (i) retrospectively to each prior reporting period presented — referred to as the full retrospective method or (ii) retrospectively with the cumulative effect of initially applying the standard recognized at the date of initial application in retained earnings — referred to as the modified retrospective method.
The Company has assessed the impact that ASU No. 2014-09 will have on its financial statements and related disclosures. To date, the Company has derived its revenues from a single licensing agreement with Avanir (the AVP-825 License Agreement). The consideration the Company has received to date includes an upfront payment, research and development funding and development milestone payments. Additionally, the Company is eligible to receive sales milestone payments and royalties in the future once net product sales exceed a certain threshold. The Company analyzed the performance obligations under the AVP-825 License Agreement, and the consideration received to date and that the Company may receive in the future, as part of its analysis of the impact of ASU 2014-09 on this arrangement. The Company has completed its initial assessment of the AVP-825 License Agreement, and currently does not expect the adoption of the ASU to have a material impact on its financial statements but is expected to result in expanded footnote disclosures. The Company will continue to monitor additional changes, modifications, clarifications or interpretations being undertaken by the FASB, which may impact our current conclusion.
The Company plans to adopt the new standard effective January 1, 2018 using the modified retrospective approach.
4. Deposits and Other Assets
Deposits and other assets consisted of the following:
December 31, | |||||||
2017 | 2016 | ||||||
Short-term | |||||||
Receivable due from the FDA | $ | — | $ | 2,038 | |||
Deposits on equipment | — | 1,201 | |||||
Prepaid expenses and other | 1,254 | 255 | |||||
Total short-term deposits and other assets | $ | 1,254 | $ | 3,494 | |||
Long-term | |||||||
Deposits on equipment | $ | 329 | $ | 499 | |||
Other | 76 | 54 | |||||
Total long-term deposits and other assets | 405 | 553 | |||||
$ | 1,659 | $ | 4,047 | ||||
F-15
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
5. Property and Equipment
Property and equipment, net, consisted of:
December 31, | |||||||
2017 | 2016 | ||||||
Computer equipment and software | $ | 307 | $ | 293 | |||
Furniture and fixtures | 89 | 121 | |||||
Machinery and equipment | 2,495 | 255 | |||||
Leasehold improvements | 28 | 28 | |||||
2,919 | 697 | ||||||
Less: accumulated depreciation | (355 | ) | (374 | ) | |||
$ | 2,564 | $ | 323 | ||||
Depreciation expense was $164, $83 and $75 for the years ended December 31, 2017, 2016 and 2015, respectively. During the year ended December 31, 2017, the Company disposed of $209 of fully depreciated assets.
6. Accrued Expenses
Accrued expenses consisted of:
December 31, | |||||||
2017 | 2016 | ||||||
Research and development expenses | $ | 80 | $ | 736 | |||
Selling, general and administrative expenses | 3,463 | 290 | |||||
Bonus expense | 4,163 | 1,390 | |||||
Payroll and benefit expenses | 633 | 125 | |||||
Interest expense | 45 | — | |||||
Other | 314 | — | |||||
$ | 8,698 | $ | 2,541 | ||||
7. AVP-825 License Agreement
In July 2013, the Company's wholly owned subsidiary, OptiNose AS, entered into the AVP-825 License Agreement with Avanir for the exclusive right to sell AVP-825 (now marketed as Onzetra® Xsail®), a product combining a low-dose powder form of sumatriptan with the Company's EDS technology platform, for the acute treatment of migraines in adults and any follow-on products under development that consist of a formulation that contains triptans as the sole active ingredient. Through December 31, 2017, under the terms of the AVP-825 License Agreement, the Company received aggregate cash payments of $70,000 in connection with the initial signing and the achievement of certain development milestones. Under the terms of the License Agreement, the Company is eligible to receive up to $50,000 upon the achievement of sales milestones as well as tiered low double-digit royalty payments on net sales in the US, Canada and Mexico after such cumulative sales exceed a certain threshold.
The Company determined that there were two deliverables under the AVP-825 License Agreement: (i) the license which was delivered in July 2013 and (ii) its obligation to provide certain research and development services in execution of the development plan and to share equally in certain qualified third party development costs through FDA approval. The Company concluded that the license had standalone value to Avanir and was separable from the research and development services, given Avanir has significant research capabilities in the field.
F-16
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
As a result, the license and research services qualify as separate units of accounting and the value of the license and the value of the research services were separately valued based upon the estimated selling price of each deliverable. The value attributable to the license was recognized up-front upon delivery of the license and the values attributable to the research services were deferred and recognized over the period in which the related services were to be delivered based upon a percentage of costs incurred in each respective reporting period. The estimated selling price of each deliverable was determined using the BESP. The BESP reflects the Company's best estimate of what the selling price would be if the deliverable was regularly sold on a stand-alone basis.
In conjunction with the AVP-825 License Agreement, the Company recognized $47,500 as licensing revenue during the year ended December 31, 2016. The revenue was related to the achievement of the FDA approval milestone in January 2016. The Company did not recognize any licensing revenue during the year ended December 31, 2017.
8. Convertible Notes
At December 31, 2017 and 2016, the Company's convertible notes payable, net, balance was as follows:
December 31, | |||||||
2017 | 2016 | ||||||
Face amount | $ | — | $ | 15,000 | |||
Front end fees | — | (75 | ) | ||||
Debt issuance costs | — | (44 | ) | ||||
Back end fees | — | 375 | |||||
Convertible notes payable, net | $ | — | $ | 15,256 | |||
On September 30, 2015, the Company entered into a Senior Secured Convertible Note Purchase Agreement (Notes) with various existing stockholders. The Notes provided the Company with up to $30,000 in capital available in two separate tranches. The first tranche of $15,000 closed on September 30, 2015. The second tranche of up to $15,000 was available to the Company until March 30, 2017 but was never drawn. The Notes bore an annual interest rate of 17% and were scheduled to mature on September 30, 2020 if not otherwise converted to Series C-2 shares. The Notes also bore front-end fees of $450, which were paid at issuance, and back end fees of $450 plus interest that was to be paid at maturity. The Notes could be repaid at any time in $100 increments, did not contain any prepayment penalties and were secured by assets of OptiNose Inc. and OptiNose US, Inc. At the option of the majority purchaser of the Notes after March 30, 2017, or prior to March 30, 2017 if an event of default occurred or was continuing under the Notes, all note principal along with any accrued interest and back end fees thereon, could be converted into Series C-2 shares of preferred stock at a conversion price based upon a Company valuation equal to the lower of fair market value or $300,000.
As of December 31, 2016, the fair value of the Notes was $21,814, which was estimated based on the as converted value of the Notes as of that date.
On March 24, 2017, in connection with the Series D Financing, the Notes and associated accrued interest and back end fees thereon totaling $19,527 converted into 687,474 shares of Series C-2 preferred stock at a per share conversion price of approximately $28.40.
The Company recorded interest expense of $862, $3,517 and $819 during the years ended December 31, 2017, 2016 and 2015, respectively, in conjunction with the Notes. Total coupon interest on the Notes and back end fees was $743 and $2,740 during the years ended December 31, 2017 and 2016, respectively. The front-end fees of $450 were recorded as debt discount at issuance and were amortized to interest expense over the 18 month loan conversion period. During the years ended December 31, 2017 and 2016, the Company recorded a total of $75 and $300 of interest expense, respectively, related to the front end fees. Additionally, back end fees of $450 were amortized to interest expense over the 18 month loan conversion period of which $90 and $300 have been recorded as interest expense and as an increase in the carrying amount of the Notes during the years ended December 31, 2017 and 2016, respectively. The Company also incurred $265 in debt issuance costs during the
F-17
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
year ended December 31, 2015 which were amortized to interest expense over the 18 month loan conversion period.
As of December 31, 2017, none of the Notes were outstanding.
9. Long-term Debt
On December 29, 2017, the Company entered into a Senior Secured Note Purchase Agreement (the Senior Secured Notes) with Athyrium Opportunities III Acquisition LP. The Senior Secured Notes provided the Company with up to $100,000 in capital, of which $75,000 was issued immediately. The remaining $25,000 (the Delayed Draw Notes) may be issued between April 1, 2019 and August 14, 2019, subject to the Company achieving trailing four quarter net revenues (as calculated pursuant to the terms of the Note Purchase Agreement) of $15,000 and a pro forma ratio of total debt to trailing four quarter net revenues not exceeding 6.50 to 1.00, and certain other conditions.
The Senior Secured Notes bear interest at 9.0% plus the three-month London Inter-bank Offered Rate (LIBOR) rate, subject to a 1.0% floor and are scheduled to mature on June 29, 2023. The interest rate was 10.75% at December 31, 2017. The Senior Secured Notes bore front-end fees of 1% of the aggregate principal amount, which were paid at issuance. The Company is also required to pay an exit fee of 2% of any principal payments (whether mandatory, voluntary, or at maturity) made throughout the term of the note purchase agreement.
The Company is required to make quarterly, interest only payments until the maturity date. The Company may make voluntary prepayments of the Senior Secured Notes, in whole or in part, and subject to certain exceptions, is required to make mandatory prepayments upon the occurrence of certain events as defined in the agreement, including, the occurrence of a change of control.
All mandatory and voluntary prepayments of the Senior Secured Notes are subject to the payment of prepayment premiums as follows: (i) if prepayment occurs prior to the second anniversary of the applicable date of issuance, an amount equal to the amount by which (a) the present value of 102% of the principal prepaid plus all interest that would have accrued on such principal through such second anniversary exceeds (b) the amount of principal prepaid, (ii) if prepayment occurs on or after the second anniversary of the applicable date of issuance but prior to the third anniversary of such issuance, an amount equal to 2% of the principal prepaid, and (iii) if prepayment occurs on or after the third anniversary of the applicable date of issuance but prior to the fourth anniversary of such issuance, an amount equal to 1% of the principal prepaid. No prepayment premium is due on any principal prepaid after the fourth anniversary of the applicable date of issuance of any Senior Secured Notes.
The Senior Secured Notes are secured by a pledge of substantially all of the Company’s assets and contains affirmative and negative covenants customary for financings of this type, including limitations on the Company’s and its subsidiaries’ ability to, among other things, incur additional debt, grant or permit additional liens, make investments and acquisitions, merge or consolidate with others, dispose of assets, grant certain license rights related to the Company's products, technology and other intellectual property rights, pay dividends and distributions, repay junior indebtedness and enter into affiliate transactions, in each case, subject to certain exceptions. In addition, the Senior Secured Notes contains financial covenants requiring the Company to maintain (i) at least $10,000 of cash and cash equivalents and (ii) following the issuance of the Delayed Draw Notes or upon entering into certain exclusive licenses of XHANCE, a total debt to trailing four quarter net revenue ratio of less than 6.50 to 1.00 initially, and thereafter declining quarterly by equal half-steps to a ratio of less than 3.00 to 1.00. As of December 31, 2017, the Company was in compliance with the covenants.
The Company recorded interest expense of $48 during the year ended December 31, 2017, in conjunction with the Senior Secured Notes. Interest expense included total coupon interest, back end fees, front end fees and the amortization of debt issuance costs. The front-end fees of $1,000 were recorded as debt discount at issuance and are being amortized to interest expense over the 5.5 year term of the loan. Additionally, back end fees of $2,000 are being amortized to interest expense and are recorded as, an increase in the carrying amount throughout the term of the Senior Secured Notes. The Company also incurred $2,140 in debt issuance costs during the year ended December 31, 2017 which are also being amortized to interest expense over the term of the Senior Secured Notes.
F-18
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
As of December 31, 2017, the long-term debt balance is comprised of the following:
December 31, | |||
2017 | |||
Face amount | $ | 75,000 | |
Front end fees | (999 | ) | |
Debt issuance costs | (2,139 | ) | |
Back end fees | 1 | ||
Long-term debt, net | $ | 71,863 | |
10. Employee Benefit Plans
For US employees, the Company maintains a defined contribution 401(k) retirement plan, which covers all employees. Employees are eligible on the first of the month following their date of hire. Under the 401(k) retirement plan, participating employees may defer up to 100% of their pre-tax salary but not more than statutory limits. In October 2017, the Company adopted a 401(k) matching program for its US employees. The Company matches 100% of the first 3% of participating employee contributions and 50% of the next 2% of participating employee contributions, subject to applicable IRC limits. For 2017, the 401(k) match was applied to eligible earnings starting January 1, 2017. As of December 31, 2017, approximately $230 related to the Company match applicable to 2017 employee contributions, which Company match will be made in the first quarter of 2018.There were no contributions to the plan by the Company in the years ended December 31, 2016 or 2015. The Company's contributions are made in cash. The Company's common stock is not currently an investment option available to participants in the 401(k) retirement plan.
For Norway and UK employees, the Company maintains defined contribution pension plans which meet statutory requirements of those jurisdictions. The Company incurred costs of $45, $24 and $24 related to the pension plans for the years ended December 31, 2017, 2016 and 2015, respectively.
11. Commitments and Contingencies
Leases
The Company leases office space under four operating leases. Rent expense is recognized as incurred.
The following is a schedule of future minimum annual payments as of December 31, 2017 under non-cancellable operating lease agreements:
For the years ending December 31: | |||
2018 | 187 | ||
Total future minimum lease payments as of December 31, 2017 | $ | 187 | |
Rent expense under these operating leases was $689, $407 and $312 for the years ended December 31, 2017, 2016 and 2015, respectively.
In January 2018, the Company amended its existing office lease agreement for the Company’s headquarters in Yardley, PA (the Lease Amendment). Under the terms of the Lease Amendment, the Company’s leased office space was increased from approximately 20,050 square feet to approximately 30,000 square feet, and the term of the lease was extended from March 31, 2018 to May 31, 2021 (the Extended Term), with an option to renew the lease for an additional three-year term. The Company’s rent payments during the Extended Term will be approximately
F-19
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
$2,750 in the aggregate, and the Company will also be required to pay its proportionate share of certain operating costs and property taxes applicable to the leased premises.
Purchase commitments
In November 2017, the Company entered into agreement with a contract sales organization for the recruitment, deployment and management of a contract sales force to market XHANCE in the US. Subject to certain limited exceptions, the Company may not terminate this agreement until after the first anniversary of the deployment of the sales force (which occurred in March 2018). The Company estimates the expenses related to the non-cancellable services during this period to be approximately $16,354. Thereafter, the Company may terminate the agreement subject to potential early termination fees ranging from $100 to $700.
As of December 31, 2017, the Company had no other outstanding non-cancellable purchase commitments related to inventory and other goods and services, including pre-commercial manufacturing scale-up and sales and marketing activities.
Employment agreements
The Company has entered into employment contracts with its officers and certain employees that provide for severance and continuation of benefits in the event of termination of employment by the Company without cause or by the employee for good reason. In addition, in the event of termination of employment following a change in control, the vesting of certain equity awards may be accelerated.
Litigation
Liabilities for loss contingencies arising from claims, assessments, litigation, fines, penalties, and other sources are recorded when it is probable that a liability has been incurred and the amount can be reasonably estimated. There are no matters currently outstanding.
12. Stockholders' equity (deficit)
Common stock
In October 2017, the Company increased the number of authorized common shares from 10,624,486 to 200,000,000 and completed an initial public offering (IPO) of its common stock, selling 8,625,000 shares at $16.00 per share. As a result of the IPO, the Company received $125,471 in net proceeds, after deducting discounts and commissions of $9,660 and offering expenses of approximately $2,869 payable by the Company.
Each share of common stock entitles the holder to one vote on all matters submitted to a vote of the Company’s stockholders. Subject to preferences that may apply to any outstanding preferred stock, holders of common stock are entitled to receive ratably any dividends that the Company’s board of directors may declare out of funds legally available for that purpose on a non-cumulative basis. No dividends had been declared through December 31, 2017.
Common stock warrants
As of December 31, 2017, the Company had 1,890,489 warrants outstanding to purchase shares of the Company's common stock with an exercise price of $8.16. The warrants expire on November 1, 2020.
Redeemable convertible preferred stock
During the year ended December 31, 2017, the Company sold 1,117,578 shares of Series D Preferred Stock at a per share purchase price of $32.85, resulting in gross proceeds to the Company of $36,712 (the Series D Financing). In connection with the Series D Financing, the Company's existing convertible notes and associated accrued interest and back end fees thereon totaling $19,527 converted into 687,474 shares of Series C-2 Preferred Stock at a per share conversion price of approximately $28.40 (Note 8).
In conjunction with the Series D financing, the number of authorized shares of common stock was increased from 10,624,486 to 13,067,149 and the number of authorized shares of preferred stock was increased from 6,875,514 to 8,932,851, of which 1,369,863 shares were designated as Series D shares and 687,474 shares were designated as Series C-2 shares. Also, the redemption date for all classes of the Company's preferred stock was extended to March 24, 2020 and the terms upon which all classes of Preferred Stock would mandatorily convert into common stock in connection with an underwritten public offering were revised to align with the terms of the Series C-2 preferred stock.
F-20
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
Upon consummation of the IPO in October 2017, all of the outstanding shares of the Company's redeemable convertible preferred stock were converted into an aggregate of 25,068,556 shares of common stock. In connection with the IPO, 5,000,000 shares of preferred stock, with a par value of $0.001 per share, were authorized for issuance. As of December 31, 2017, no preferred stock was issued or outstanding.
13. Stock-based compensation
The Company issues stock-based awards pursuant to its 2010 Stock Incentive Plan. Effective as of October 12, 2017, the Company's 2010 Stock Incentive Plan was amended and restated(A&R Plan). The A&R Plan provides for the grant of incentive stock options, non-statutory stock options, restricted stock awards, restricted stock units, deferred stock units, performance shares, stock appreciation rights and other equity-based awards. The Company's employees, officers, directors and other persons are eligible to receive awards under the A&R Plan. As of December 31, 2017, 6,894,445 shares of the Company's common stock were authorized to be issued under the A&R Plan, and 546,131 shares were reserved for future awards under the A&R Plan. The number of shares of the Company's common stock authorized under the A&R Plan will automatically increase on January 1st of each year, commencing on January 1, 2018 and continuing until the expiration of the A&R Plan, in an amount equal to four percent of the total number of shares of the Company's common stock outstanding on December 31st of the preceding calendar year, subject to the discretion of the Company's board of directors or compensation committee to determine a lesser number of shares shall be added for such year.
The amount, terms of grants, and exercisability provisions are determined and set by the Company's board of directors or compensation committee. The Company measures employee stock-based awards at grant-date fair value and records compensation expense on a straight-line basis over the vesting period of the award. Stock-based awards issued to non-employees are revalued until the award vests.
Service-based stock options
Options issued under the A&R Plan generally have a contractual life of up to 10 years and may be exercisable in cash or as otherwise determined by the board of directors. Vesting generally occurs over a period of not greater than four years. The following table summarizes the activity related to service-based stock option grants to employees and nonemployees for the years ended December 31, 2017:
Shares | Weighted average exercise price per share | Weighted average remaining contractual life | ||||||
Outstanding at December 31, 2016 | 1,894,083 | $ | 3.09 | 6.67 | ||||
Granted | 2,341,096 | 14.00 | ||||||
Exercised | (26,047 | ) | 2.34 | |||||
Expired | (2,887 | ) | 5.68 | |||||
Forfeited | (96,036 | ) | 9.29 | |||||
Outstanding at December 31, 2017 | 4,110,209 | $ | 9.16 | 7.89 | ||||
Exercisable at December 31, 2017 | 1,327,843 | $ | 2.36 | 4.53 | ||||
Vested and expected to vest at December 31, 2017 | 4,110,209 | $ | 9.16 | 7.89 | ||||
During the year ended December 31, 2017,time-based options to purchase 2,341,096 shares of common stock were granted to employees that generally vest over four years. The options had an estimated weighted average grant date fair value of $9.68. The grant date fair value of each option grant was estimated at the time of grant using the Black-Scholes option-pricing model.
The total aggregate intrinsic value of service-based options exercised during the years ended December 31, 2017 and 2016 was $435 and $20, respectively. There were no service-based options exercised during the year ended December 31, 2015. The aggregate intrinsic value of service-based options outstanding and service-based options exercisable as of December 31, 2017 was $40,102 and $21,961, respectively. At December 31, 2017, the unrecognized compensation cost related to unvested service-based stock options expected to vest was $22,198.
F-21
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
This unrecognized compensation will be recognized over an estimated weighted-average amortization period of 3.89 years.
Performance-based stock options
The Company has issued performance-based stock options under the A&R Plan which generally have a ten-year life from the date of grant and may vest upon the achievement of certain milestones in connection with the Company's development programs. Additionally, the Company has issued options in excess of the fair market value of common shares on the issuance date that are only exercisable upon a change in control or upon or after an initial public offering. Compensation expense for performance-based stock options is only recognized when management determines it is probable that the awards will vest.
The following table summarizes the activity related to performance-based stock option grants to employees and nonemployees for the years ended December 31, 2017:
Shares | Weighted average exercise price per share | Weighted average remaining contractual life | ||||||
Outstanding at December 31, 2016 | 2,171,760 | $ | 9.59 | 6.55 | ||||
Granted | — | — | ||||||
Exercised | (30,393 | ) | 2.44 | |||||
Forfeited | — | — | ||||||
Outstanding at December 31, 2017 | 2,141,367 | $ | 9.69 | 5.56 | ||||
Exercisable at December 31, 2017 | 1,708,181 | $ | 8.02 | 5.04 | ||||
Vested and expected to vest at December 31, 2017 | 2,141,367 | $ | 9.69 | 5.56 | ||||
The total aggregate intrinsic value of performance-based options exercised during the years ended December 31, 2017 and 2016 was $505 and $20 respectively. There were no performance-based options exercised during the year ended December 31, 2015. The aggregate intrinsic value of performance-based options outstanding and performance-based options exercisable as of December 31, 2017 was $19,713 and $18,592, respectively. As of December 31, 2017, there was $563 of unrecognized compensation cost related to unvested performance-based stock options that will vest and be expensed over an estimated weighted average amortization period of 2.68 years.
2017 Employee Stock Purchase Plan
The Company's 2017 Employee Stock Purchase Plan (the 2017 Plan) became effective on October 12, 2017. The 2017 Plan authorized the issuance of up to 144,395 shares of the Company's common stock pursuant to purchase rights granted to its employees or to employees of any of its participating affiliates. The number of shares of the Company's common stock that may be issued pursuant to rights granted under the 2017 Plan shall automatically increase on January 1st of each year, commencing on January 1, 2018 and continuing until the expiration of the 2017 Plan, in an amount equal to one percent of the total number of shares of the Company's common stock outstanding on December 31st of the preceding calendar year, subject to the discretion of the board of directors or compensation committee to determine a lesser number of shares shall be added for such year.
Under the 2017 Plan, eligible employees can purchase the Company’s common stock through accumulated payroll deductions at such times as are established by the administrator. The 2017 Plan is administered by the compensation committee. Under the 2017 Plan, eligible employees may purchase the Company’s common stock at discount of up to 85% of the lower of the fair market value of the Company's common stock on the first day of the offering period or on the last day of the offering period. Eligible employees may contribute up to 15% of their eligible compensation. Under the 2017 Plan, a participant may not accrue rights to purchase more than $25,000 worth of the Company’s common stock for each calendar year in which such right is outstanding.
Effective October 12, 2017, employees who elected to participate in the 2017 Plan commenced payroll withholdings that accumulate through June 30, 2018. Beginning on January 1, 2018, employees who elected to participate in the 2017 Plan will commence payroll withholdings that accumulate through June 30, 2018.
F-22
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
At the end of each of the current offering periods, shares of the Company’s common stock may be purchased at 85% of the lower of the fair market value of the Company’s common stock on the first or last day of the respective offering period. In accordance with the guidance in ASC 718-50 – Compensation – Stock Compensation, the ability to purchase shares of the Company’s common stock at the lower of the price on the first day of the offering period or the last day of the offering period (i.e. the purchase date) represents an option and, therefore, the 2017 Plan is a compensatory plan under this guidance. Accordingly, stock-based compensation expense is determined based on the option’s grant-date fair value as estimated by applying the Black Scholes option-pricing model and is recognized over the requisite service period of the option. The Company has recognized stock-based compensation expense of $106 during the year ended December 31, 2017 related to the 2017 Plan.
Stock-based compensation expense
The Company recorded stock-based compensation expense in the following expense categories of its accompanying consolidated statements of operations for the years ended December 31, 2017 and 2016:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Research and development | $ | 1,288 | $ | 362 | $ | 354 | |||||
General and administrative | 3,808 | 237 | 234 | ||||||||
Total stock-based compensation expense | $ | 5,096 | $ | 599 | $ | 588 | |||||
The Company utilized the Black-Scholes valuation model for estimating the fair value of stock options granted and the option component of the 2017 Plan. The Company calculated the fair value of each option grant and the option component of the 2017 Plan on the respective dates of grant using the following weighted average assumptions:
2010 A&R Stock Incentive Plan | 2017 Employee Stock Purchase Plan | ||||
December 31, | December 31, | ||||
2017 | 2017 | ||||
Risk free interest rate | 2.06 | % | 1.27 | % | |
Expected term (in years) | 6.06 | 0.72 | |||
Expected volatility | 78.67 | % | 91.18 | % | |
Annual dividend yield | 0.00 | % | 0.00 | % | |
Option valuation methods, including Black-Scholes, require the input of subjective assumptions, which are discussed below.
• | The expected term of employee options is determined using the "simplified" method, as prescribed in SEC's Staff Accounting Bulletin (SAB) No. 107, whereby the expected life equals the arithmetic average of the vesting term and the original contractual term of the option due to the Company's lack of sufficient historical data. The expected term of nonemployee options is equal to the contractual term. |
• | The expected volatility is based on historical volatilities of similar entities within the Company's industry which were commensurate with the expected term assumption as described in SAB No. 107. |
• | The risk-free interest rate is based on the interest rate payable on US Treasury securities in effect at the time of grant for a period that is commensurate with the assumed expected term. |
• | The expected dividend yield is 0% because the Company has not historically paid, and does not expect for the foreseeable future to pay, a dividend on its common stock. |
F-23
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
14. Income taxes
The Tax Cuts and Jobs Act, or the TCJA, was enacted on December 22, 2017 and became effective January 1, 2018. Among other changes, the TCJA significantly lowers the US corporate income tax rate, implements a territorial tax system and imposes a one-time transition tax on deemed repatriated earnings of foreign subsidiaries.
The TCJA reduces the US corporate income tax rate from 35% to 21%, effective January 1, 2018. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to reverse. As a result of the reduction in the US corporate income tax rate, the Company revalued its net deferred tax assets at December 31, 2017. Due to the full valuation allowance on the Company's deferred tax assets, no tax expense or benefit associated with the re-measurement was recognized in the Company's consolidated statement of operations for the year ended December 31, 2017. The change in the US corporate tax rate is also included in the Company’s deferred tax table.
The TCJA provided for a one-time transition tax on the deemed repatriation of post-1986 undistributed foreign subsidiary earnings and profits, or E&P. As the Company's foreign subsidiaries did not have consolidated accumulated E&P, the Company did not record any income tax expense related to the transition tax.
Due to the timing of and the substantial changes made by the TCJA , the Staff of the Securities and Exchange Commission, or the SEC, issued Staff Accounting Bulletin No. 118, or SAB 118, which provides registrants a measurement period to report the impact of the new US tax law. During the measurement period, provisional amounts for the effects of the law are recorded to the extent a reasonable estimate can be made. To the extent that all information necessary is not available, prepared or analyzed, companies may recognize provisional estimated amounts for a period of up to one year following enactment of the TCJA. Accordingly, the Company's preliminary estimate of the impact of the TCJA and the re-measurement of its deferred tax assets and liabilities is subject to finalization of its analysis of certain matters, such as developing interpretations of the TCJA provisions, changes to certain estimates and the filing of its tax returns. US Treasury regulations, administrative interpretations or court decisions interpreting the TCJA may require adjustments to the Company's initial estimates. The final determination of the TCJA provisions and re-measurement of the Company's deferred tax assets and liabilities will be completed as additional information becomes available, but no later than one year from the enactment of the TCJA.
Income taxes have been recorded on the following income (loss) before income tax expense:
Year ended December 31, | ||||||||
2017 | 2016 | |||||||
Domestic operations | $ | (30,463 | ) | $ | (4,967 | ) | ||
Foreign operations | (18,439 | ) | 27,580 | |||||
Income (loss) before provision for income taxes | $ | (48,902 | ) | $ | 22,613 | |||
F-24
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
A reconciliation of income tax expense (benefit) at the statutory federal income tax rate and income taxes as reflected in the financial statements is as follows:
Year ended December 31, | ||||||
2017 | 2016 | |||||
Income tax expense at statutory rate | 35.0 | % | 35.0 | % | ||
Permanent items | 0.5 | — | ||||
Foreign rate differential | (4.2 | ) | (12.2 | ) | ||
State taxes, net of federal benefit | 0.8 | (1.4 | ) | |||
Tax rate changes | (15.4 | ) | — | |||
Change in valuation allowance | (16.7 | ) | (21.4 | ) | ||
Effective income tax rate | 0.0 | % | 0.0 | % | ||
Tax rate changes includes both the impact of the reduction in the US tax rate under the TCJA and a reduction in the Norway tax rate.
The principal components of the Company’s deferred tax assets and liabilities were as follows:
Year ended December 31, | ||||||||
2017 | 2016 | |||||||
Deferred tax assets: | ||||||||
Accrued expenses and other | $ | 972 | $ | 539 | ||||
Fixed assets | 55 | — | ||||||
Interest expense | 783 | 677 | ||||||
Stock compensation | 1,701 | 1,092 | ||||||
Research and development | 2,485 | 2,183 | ||||||
Net operating losses | 29,636 | 22,695 | ||||||
Total deferred tax assets | 35,632 | 27,186 | ||||||
Deferred tax liabilities: | ||||||||
Fixed assets | — | (46 | ) | |||||
Total deferred tax liabilities: | — | (46 | ) | |||||
Less: Valuation allowance | (35,632 | ) | (27,140 | ) | ||||
Total net deferred tax assets (liabilities) | $ | — | $ | — | ||||
The TCJA, in addition to the changes indicated above, contained other provisions that may have a future impact on the Company. The provisions include limitations on the deductibility of interest based on the amount of adjusted taxable income, the deferral of research and development deductions, the acceleration of deductions related to fixed asset additions, changes to the utilization of net operating loss carry forwards and changes in the carry forward period, and global intangible low-taxed income provisions that subject foreign subsidiary income that exceeds an allowable return to current US taxation.
As of December 31, 2017, the Company had foreign net operating loss, or NOL, carry forwards of $96,616 primarily from its operations in Norway. As of December 31, 2017, the Company had federal and state NOL’s of $30,327 and $13,702, respectively. These domestic NOL carry forwards may be subject to an annual limitation in the event of cumulative changes in the ownership interest of significant stockholders over a three‑year period in excess of 50%. This could limit the amount of NOLs that the Company can utilize annually to offset future domestic taxable income or
F-25
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
tax liabilities, if any. The amount of the annual limitation, if any, will be determined based on the value of the Company immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. The federal and state NOL’s will expire beginning in 2030 and through 2037.
ASC 740 requires a valuation allowance to reduce the deferred tax assets reported if, based on the weight of available evidence, it is more likely than not that all or a portion of the deferred tax assets will not be realized. After consideration of all the evidence, both positive and negative, the Company has recorded a full valuation allowance against its deferred tax assets at December 31, 2017 and 2016, respectively, because the Company’s management has determined that is it more likely than not that these assets will not be fully realized. The Company experienced a net change in valuation allowance of $8,492 during the year ended December 31, 2017.
The Company files income tax returns in Norway, the UK, the US, and various states. The Company is subject to examination by federal, state and foreign jurisdictions. The Company’s tax years in the US are open under statute from inception to present. All open years may be examined to the extent that tax credit or net operating loss carryforwards are used in future periods.
The Company’s policy is to record interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2017, the Company had no accrued interest or penalties related to uncertain tax positions and no amounts have been recognized in the Company’s statement of operations.
15. Related-party transactions
Debt and equity transactions
All of the Company's convertible debt (see Note 8) was issued to holders of the Company's convertible preferred stock.
During the year-ended December 31, 2017, the Company reimbursed Avista Capital Holdings, LP and related parties $157 in expenses, primarily related to legal fees incurred in conjunction with our IPO in October 2017 and the issuance of Series D redeemable convertible preferred stock March 2017.
16. Quarterly Financial Information (unaudited)
The following table summarizes the unaudited consolidated financial results of operations for the quarters ended (amounts in thousands except per share data):
2017 Quarters Ended | |||||||||||||||
March 31, | June 30, | September 30, | December 31, | ||||||||||||
Revenues | $ | — | $ | — | $ | — | $ | — | |||||||
Research and development | 3,073 | 5,906 | 6,641 | 1,212 | |||||||||||
Selling, general and administrative | 4,230 | 2,431 | 6,553 | 18,484 | |||||||||||
Total operating expenses | 7,303 | 8,337 | 13,194 | 19,696 | |||||||||||
Income (loss) from operations | (7,303 | ) | (8,337 | ) | (13,194 | ) | (19,696 | ) | |||||||
Net income (loss) | (8,075 | ) | (8,208 | ) | (13,068 | ) | (19,551 | ) | |||||||
Net income (loss) attributable to common stockholders | (11,671 | ) | (12,836 | ) | (17,192 | ) | (20,268 | ) | |||||||
Basic net income (loss) per common share | $ | (2.87 | ) | $ | (3.16 | ) | $ | (4.23 | ) | $ | (0.64 | ) | |||
Diluted net income (loss) per common share | $ | (2.87 | ) | $ | (3.16 | ) | $ | (4.23 | ) | $ | (0.64 | ) | |||
F-26
OptiNose, Inc.
Notes to Consolidated Financial Statements
(in thousands, except share and per share data)
2016 Quarters Ended | |||||||||||||||
March 31, | June 30, | September 30, | December 31, | ||||||||||||
Revenues | $ | 47,500 | $ | — | $ | — | $ | — | |||||||
Research and development | 4,961 | 3,412 | 3,868 | 3,070 | |||||||||||
Selling, general and administrative | 2,250 | 1,046 | 1,761 | 1,812 | |||||||||||
Total operating expenses | 7,211 | 4,458 | 5,629 | 4,882 | |||||||||||
Income (loss) from operations | 40,289 | (4,458 | ) | (5,629 | ) | (4,882 | ) | ||||||||
Net income (loss) | 39,572 | (5,265 | ) | (6,106 | ) | (5,588 | ) | ||||||||
Net income (loss) attributable to common stockholders | 36,294 | (8,544 | ) | (9,385 | ) | (8,866 | ) | ||||||||
Basic net income (loss) per common share | $ | 1.52 | $ | (2.10 | ) | $ | (2.32 | ) | $ | (2.18 | ) | ||||
Diluted net income (loss) per common share | $ | 1.25 | $ | (2.10 | ) | $ | (2.32 | ) | $ | (2.18 | ) | ||||
F-27
