Attached files
| file | filename |
|---|---|
| EX-10.25 - EXHIBIT 10.25 - GNC HOLDINGS, INC. | ex1025conformedtermloancre.htm |
| EX-32.1 - EXHIBIT 32.1 - GNC HOLDINGS, INC. | gnc-ex321_20171231x10k.htm |
| EX-31.2 - EXHIBIT 31.2 - GNC HOLDINGS, INC. | gnc-ex312_20171231.htm |
| EX-31.1 - EXHIBIT 31.1 - GNC HOLDINGS, INC. | gnc-ex311_20171231x10k.htm |
| EX-23.1 - EXHIBIT 23.1 - GNC HOLDINGS, INC. | exhibit231to201710-k.htm |
| EX-21.1 - EXHIBIT 21.1 - GNC HOLDINGS, INC. | gnc-ex211_20171231x10k.htm |
| EX-10.33 - EXHIBIT 10.33 - GNC HOLDINGS, INC. | ex1033_executiveseverancep.htm |
| EX-10.27 - EXHIBIT 10.27 - GNC HOLDINGS, INC. | ex1027conformedtermloansec.htm |
| EX-10.26 - EXHIBIT 10.26 - GNC HOLDINGS, INC. | ex1026conformedablsecurity.htm |
| EX-10.24 - EXHIBIT 10.24 - GNC HOLDINGS, INC. | ex1024conformedablcreditagr.htm |
| EX-10.23 - EXHIBIT 10.23 - GNC HOLDINGS, INC. | ex1023conformedamendmentag.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One) | |
ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2017 | |
OR | |
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to | |
Commission file number: 001-35113
GNC Holdings, Inc.
(Exact name of registrant as specified in its charter)
DELAWARE (state or other jurisdiction of Incorporation or organization) | 20-8536244 (I.R.S. Employer Identification No.) | |
300 Sixth Avenue Pittsburgh, Pennsylvania (Address of principal executive offices) | 15222 (Zip Code) | |
Registrant's telephone number, including area code: (412) 288-4600
Securities registered pursuant to section 12(b) of the Act:
Title of each class | Name of each exchange on which registered | |
Class A common stock, par value $0.001 per share | New York Stock Exchange | |
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ý No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of "accelerated filer and large accelerated filer" in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ý | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) | Smaller reporting company o | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No ý
The aggregate market value of all common stock (based upon the closing price of the New York Stock Exchange) of the registrant held by non-affiliates of the registrant as of June 30, 2017 was approximately $0.6 billion.
As of February 22, 2018, the number of outstanding shares of Class A common stock, par value $0.001 per share (the "common stock"), of GNC Holdings, Inc. was 83,662,233 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Certain information in the Company's definitive Proxy Statement for the 2018 Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission pursuant to Regulation 14A, not later than 120 days after the end of the fiscal year, is incorporated by reference in Part III of this Form 10-K.
1
TABLE OF CONTENTS
Page | |||
2
FORWARD LOOKING STATEMENTS
This Annual Report on Form 10-K (this "Annual Report") contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 with respect to our financial condition, results of operations and business. Forward-looking statements include statements that may relate to our plans, objectives, goals, strategies, future events, future revenues or performance, capital expenditures, financing needs and other information that is not historical information. Forward-looking statements can often be identified by the use of terminology such as "subject to," "believe," "anticipate," "plan," "expect," "intend," "estimate," "project," "may," "will," "should," "would," "could," "can," the negatives thereof, variations thereon and similar expressions, or by discussions of strategy.
All forward-looking statements, including, without limitation, our examination of historical operating trends, are based upon our current expectations and various assumptions. We believe there is a reasonable basis for our expectations and beliefs, but they are inherently uncertain. We may not realize our expectations and our beliefs may not prove correct. A detailed discussion of risk and uncertainties that could cause actual results and events to differ materially from such forward-looking statements is included in the section titled "Risk Factors" (Item 1A of this Form 10-K).
Consequently, forward-looking statements should be regarded solely as our current plans, estimates and beliefs. You should not place undue reliance on forward-looking statements. We cannot guarantee future results, events, levels of activity, performance or achievements. The forward-looking statements included in this Annual Report on Form 10-K are made as of the date of this filing. We do not undertake and specifically decline any obligation to update, republish or revise forward-looking statements to reflect future events or circumstances or to reflect the occurrence of unanticipated events.
3
PART I
Item 1. BUSINESS.
GNC Holdings, Inc. (together with its subsidiaries, referred to as "Holdings", "GNC", "the Company", "we", "us" and "our" unless specified otherwise) is headquartered in Pittsburgh, Pennsylvania and our common stock trades on the New York Stock Exchange (the "NYSE") under the symbol "GNC." Our business was founded in 1935 by David Shakarian who opened our first health food store in Pittsburgh, Pennsylvania.
Our principal executive office is located at 300 Sixth Avenue, Pittsburgh, Pennsylvania 15222, and our telephone number is (412) 288-4600. We maintain and make available on GNC.com, free of charge, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports as soon as reasonably practical after we electronically file or furnish them to the United States Securities and Exchange Commission (the "SEC").
Business Strategy
Key elements of our business strategy are detailed below:
Leading retailer of nutritional supplements. GNC has been in business for more than 80 years and the Company is built on a core foundation as a manufacturer and retailer of high-quality nutritional supplements. Based on our worldwide network of approximately 9,000 locations and our online channels, we believe we are the leading global specialty retailer of health, wellness and performance products.
Our objective is to offer a broad and deep mix of products for consumers interested in living well, whether they are looking to treat a health-related issue, maintain their overall wellness, or improve their performance. Our premium, value-added offering includes proprietary GNC-branded products and other nationally recognized third-party brands.
We believe our vertically integrated model, depth of exclusive products and range of merchandise, combined with the customer support and service we offer, differentiates us and allows us to effectively compete against food, drug and mass channel players, specialty stores, independent vitamin, supplement and natural food shops and online retailers.
Product development and innovation. Through our facility in Greenville, South Carolina we manufacture high-quality, innovative nutritional supplement products that can ultimately be purchased only through our store locations, GNC.com, our Amazon.com marketplace and our wholesale partners. High quality ingredients are rigorously tested before going into GNC products with many quality checks including raw materials, purity, and potency.
We believe our sector-leading innovation capability is a significant competitive advantage. GNC has demonstrated strength in developing unique, branded, and scientifically verified products and has a long history of delivering new ingredients and reformulations. We directly employ scientists, nutritionists, formulators, chemists, engineers and quality control experts and have access to a wide range of world-class medical research facilities and consultants.
A differentiated customer experience. Our strategy is to deliver a compelling experience at every customer touch point. We operate in a highly personalized, aspirational sector and believe that the nutritional supplement consumer often desires and seeks out product expertise and knowledgeable customer service.
We differentiate ourselves from competitors through our well-trained sales associates, who are aided in becoming trusted advisors with regular training that focuses on solution-based selling, and through in-store technology including tablets that allow them to view customers’ purchase history and preferences. With that knowledge, and help from sales tools built into the tablet platform, associates can engage customers in conversation, share product information, testimonials and before and after pictures, recommend solutions and help customers add complimentary products and build wellness regimens.
Our loyalty programs allow us to develop and maintain a large and loyal customer base, provide targeted offers and information, and connect with our customers on a regular basis. We harness data generated by these programs to understand customers’ buying behaviors and needs, so we can deliver a stronger experience, bring like-minded consumers into the channel and make well-informed decisions about the business.
Omni-channel development. We believe our diversified, multi-channel model, which includes company-owned stores, domestic and international franchise locations, wholesale locations and e-commerce, can differentiate us from online-only competitors. Our strategy is to give consumers a seamless, integrated experience across digital, mobile and store channels and in every interaction they have with GNC.
Through GNC.com and our Amazon.com storefront, which carries our full product assortment, customers can research and purchase our products online. We believe our store base is a competitive advantage, allowing customers to experience our
4
products and get expert advice from an associate.
Our omni-channel model can enhance the customer experience and increase the lifetime value of a GNC customer, and we are implementing strategies to blend our digital, online and in-store platforms. These initiatives include increased cross-channel marketing, online and in-store subscription services, giving customers the option of picking up online purchases in GNC stores, shipping products purchased via e-commerce directly from stores, and additional educational content, information and advice on GNC.com.
International growth. We see opportunity to expand internationally within the large global supplement market which is expected to continue to grow. In particular, our recently announced partnership with Harbin Pharmaceutical Group Holdings., Ltd. allows us to further expand our business in China. Harbin’s expertise in distribution and regulation is the ideal match for our highly valued brand and assortment of products in the China market. Refer to Item 8, “Financial Statements and Supplementary Data,” Note 18, “Subsequent Events” for more information.
Driving constructive industry dialogue. We remain focused, together with other industry leaders and industry trade associations, on raising the bar for transparency and quality throughout the dietary supplement industry. We believe that over time the implementation of higher standards and more stringent industry self-regulation regarding manufacturing practices, ingredient traceability and product transparency will prove beneficial for the industry and lead to improved dialogue with regulators, stronger consumer trust and greater confidence in our industry.
Segments
We generate revenues from our three segments, which are U.S. and Canada, International and Manufacturing / Wholesale. The following table outlines our segments. For a description of operating (loss) income by segment, our total assets by segment, our total revenues by geographic area, see Note 16, "Segments," to our audited Consolidated Financial Statements included in this Annual Report.
Year ended December 31, | ||||||||||||||||||||
2017 | 2016 | 2015 | ||||||||||||||||||
($ in millions) | ||||||||||||||||||||
U.S. and Canada | $ | 1,993.4 | 81.3 | % | $ | 2,058.0 | 81.0 | % | $ | 2,168.0 | 80.8 | % | ||||||||
International | 177.4 | 7.2 | % | 160.7 | 6.3 | % | 183.0 | 6.8 | % | |||||||||||
Manufacturing / Wholesale (1) | 216.0 | 8.8 | % | 235.7 | 9.3 | % | 235.7 | 8.8 | % | |||||||||||
Other (2) | 66.2 | 2.7 | % | 85.6 | 3.4 | % | 96.6 | 3.6 | % | |||||||||||
Total revenue | $ | 2,453.0 | 100.0 | % | $ | 2,540.0 | 100.0 | % | $ | 2,683.3 | 100.0 | % | ||||||||
(1) Excludes intersegment sales.
(2) Includes Lucky Vitamin in all periods and Discount Supplements in 2015 both of which have been sold.
Although we believe that none of our segment operations experience significant seasonal fluctuations, historically we have experienced, and expect to continue to experience, the lowest amount of revenue in our fourth quarter compared with the first three quarters of the year.
U.S. and Canada
Our U.S. and Canada segment generates revenues primarily from sales of products to customers at our company-owned stores in the United States, Canada and Puerto Rico, through product sales to franchisees, royalties on franchise retail sales and franchise fees and through GNC.com and our market place on Amazon.
Company-Owned Retail Stores in the U.S. and Canada
As of December 31, 2017, we operated 3,423 company-owned stores across all 50 states and the District of Columbia in the United States, in Canada and Puerto Rico. Most of our company-owned stores in the United States are between 1,000 and 2,000 square feet and are located primarily in strip shopping centers and shopping malls.
Domestic Franchise Stores
As of December 31, 2017, there were 1,099 domestic franchise stores. Our franchise stores in the United States are typically between 1,000 and 2,000 square feet, and approximately 90% are located in strip shopping centers. We believe we have good relationships with our franchisees, as evidenced by our domestic franchisee renewal rate of approximately 92% between
5
2012 and 2017. Currently, we have 485 franchisees operating stores in the United States. We do not rely heavily on any single franchise operator in the United States, where our largest franchisee owns and/or operates 85 store locations.
All of our franchise stores in the United States offer both our proprietary products and third-party products, with a product selection similar to that of our company-owned stores.
Revenues from our franchisees in the United States accounted for approximately 16% of our total U.S. and Canada segment revenues for the year ended December 31, 2017. New franchisees in the United States are required to pay an initial fee of $40,000 for a franchise license. Existing GNC franchise operators may purchase an additional franchise license for a $30,000 fee. Once a store begins operations, franchisees are required to pay us a continuing royalty of 6% of sales and contribute 3% of sales to a national advertising fund. Our standard franchise agreements for the United States are effective for an initial ten-year period with unlimited five-year renewal options. At the end of the initial term and each of the renewal periods, the renewal fee is generally 33% of the franchise fee that is then in effect. The franchisee renewal option is generally at our election. Franchisees must meet certain conditions to exercise the franchisee renewal option. Our franchisees in the United States receive limited geographical exclusivity and are required to utilize the standard GNC store format.
Generally, we negotiate lease terms to secure locations at cost-effective rates, which we typically sublease to our franchisees at cost. Franchisees must meet certain minimum standards and duties prescribed by our franchise operations manual, and we conduct periodic field visit reports to ensure our minimum standards are maintained. If a franchisee does not meet specified performance and appearance criteria, we are permitted to terminate the franchise agreement. In these situations, we may take possession of the location, inventory and equipment, and operate the store as a company-owned store or refranchise the location.
Websites
GNC.com continues to represent a significant part of our business. The ability to purchase our products through the internet also offers a convenient method for repeat customers to evaluate and purchase new and existing products. This additional sales channel has enabled us to market and sell our products in regions where we have limited or no retail operations. We may offer products on our website that are not available at our retail locations, enabling us to broaden the assortment of products available to our customers. We also offer our full product assortment on our market place on Amazon, the revenue of which are included in our GNC.com business unit.
International
Our International segment generates revenue primarily from our international franchisees through product sales, royalties and franchise fees and also includes our China operations and The Health Store.
International Franchise Stores
As of December 31, 2017, there were 1,999 international franchise locations operating in 50 international countries (including distribution centers where retail sales are made). The international franchise locations are typically smaller and, depending upon the country and cultural preferences, are located in mall, strip shopping center, street or store-within-a-store locations. In addition, some international franchisees sell on the internet and distribute to other retail outlets in their respective countries. Typically, our international stores have a store format and signage similar to our United States franchise stores. We believe that our franchise program enhances our brand awareness and market presence and will enable us to continue to expand our store base internationally with limited capital expenditures.
Our international franchise stores generally offer a more limited product selection than our franchise stores in the United States, primarily due to regulatory constraints.
Revenues from our international franchisees accounted for approximately 74% of our total international segment revenues for the year ended December 31, 2017. In 2017, new international franchisees were required to pay an initial fee of approximately $25,000 for a franchise license for each full size store, $12,500 for a franchise license for a store-within-a-store and continuing royalty fees. Our international franchise program has enabled us to expand into international markets with limited investment.
We enter into development agreements with international franchisees for either full-size stores or store-within-a-store locations. We enter into distribution agreements for wholesale distribution centers and, in some cases, limited internet distribution. The development agreement grants the franchisee the right to develop a specific number of stores in a territory, often the entire country. The franchisee then enters into a franchise agreement for each location. The full-size store franchise agreement has an initial ten-year term with two five-year renewal options. The franchisee typically has the option to renew the agreement at 33% of the current initial franchise fee that is then being charged to new franchisees. Franchise agreements for international store-within-a-store locations have an initial term of five years, with two five-year renewal options. At the end of the initial term and each of the renewal periods, the franchisee has the option to renew the store-within-a-store agreement for up to a maximum of 50% of the franchise fee that is then in effect. Our international franchisees often receive exclusive franchising rights to the entire
6
country, generally excluding United States military bases. Our international franchisees must meet minimum standards and duties similar to our United States franchisees.
Manufacturing / Wholesale
Our Manufacturing / Wholesale segment is comprised of our manufacturing operations in South Carolina and our wholesale partner relationships. Our manufacturing facility supplies our U.S. and Canada and International segments with proprietary product and also manufactures products for other third parties. Our wholesale partner business includes the sale of products to wholesale customers, the largest of which include Rite Aid, Sam's Club and PetSmart.
Our manufacturing operations are designed to ensure low-cost production of a variety of products of different quantities, sizes and packaging configurations while maintaining strict levels of quality control. Our manufacturing procedures are designed to promote consistency and quality in our finished goods. We conduct sample testing on raw materials and finished products, including weight, purity and micro bacterial testing. The principal raw materials used in the manufacturing process are natural and synthetic vitamins, herbs, minerals and gelatin. We maintain multiple sources for the majority of our raw materials, although certain materials are single-sourced due to the unique nature of the material. In 2017, our largest vendor supplied approximately10% of our raw materials.
To increase brand awareness and promote access to customers who may not frequent specialty nutrition stores, we entered into a strategic alliance with Rite Aid in December 1998 to open GNC franchise "store-within-a-store" locations. As of December 31, 2017, we had 2,418 Rite Aid store-within-a-store locations. Through this strategic alliance, we generate revenues from sales to Rite Aid of our products at wholesale prices, the manufacture of Rite Aid private label products, retail sales of certain consigned inventory and license fees. We are Rite Aid's sole supplier for a number of Rite Aid private label supplements, pursuant to a supply agreement with Rite Aid that extends through 2018. The operating license and consignment agreement that comprise our store-within-a store alliance with Rite Aid each extend through 2019.
Other
Revenue also included the results of additional websites, DiscountSupplements.co.uk, beginning in October 2013 and through December 31, 2015, and LuckyVitamin.com, beginning in August 2011 and through September 30, 2017. We sold substantially all of the assets of our Discount Supplements subsidiary effective December 31, 2015 and Lucky Vitamin subsidiary effective September 30, 2017.
Products
We are a global specialty retailer of health, wellness and performance products, including protein, performance supplements, weight management supplements, vitamins, herbs and greens, wellness supplements, health and beauty, food and drink and other general merchandise. Refer to Item 8, "Financial Statements and Supplementary Data," Note 16, "Segments" for revenue by product category. Our domestic stores offer an extensive mix of brands across multiple categories and products. Through our GNC.com and our Amazon market place, we offer additional products to customers. This variety is designed to provide our customers with a wide selection of products to fit their specific needs and to generate a high number of transactions with purchases from multiple product categories.
We offer a wide range of high-quality nutritional supplements sold under our GNC proprietary brand names, approximately half of which we manufacture. Sales of our proprietary brands at our U.S. company-owned and franchise stores, GNC.com and wholesale partners including Rite Aid, PetSmart and Sam's Club represented 44% and 46% of total system-wide retail product sales in 2017 and 2016, or $960 million and $1,013 million, respectively. We also offer products through nationally recognized third-party brand names. Sales of our third-party products at our U.S. company-owned and franchise stores, GNC.com and wholesale partners represented approximately 56% and 54% of total system-wide retail product sales in 2017 and 2016, or $1,204 million and $1,189 million, respectively, and together with proprietary sales yielded total U.S. system-wide sales of $2,164 and $2,202 million. In 2017 and 2016, we did not have a material concentration of sales from any single product or product line. Our largest vendor supplies approximately 15% of our third-party products.
Effective with the launch of the "One New GNC" on December 29, 2016, the Gold Card Member Pricing program was discontinued in all domestic company-owned and franchise stores and we introduced a free points-based loyalty program, which enables customers to earn points based on their purchases. Points earned by members are valid for one year and may be redeemed for cash discounts on any product we sell at both company-owned or franchise locations. In addition, we offer a paid membership program, "PRO Access," for $39.99 per year, which provides members with the delivery of sample boxes throughout the membership year, as well as the offering of certain other benefits including the opportunity to earn triple points on a periodic basis. The boxes include sample merchandise and other materials.
7
Product Distribution
Products are delivered to retail stores and customers who make purchases through one of our websites, via a third party transportation network, primarily through our distribution centers located in: Leetsdale, Pennsylvania; Whitestown, Indiana; Anderson, South Carolina, and Phoenix, Arizona. Our distribution centers support our company-owned stores as well as franchise stores and Rite Aid locations. Each of our distribution centers has a quality control department that monitors products received from our vendors to ensure they meet our quality standards. Internet purchases are fulfilled and shipped directly from our distribution centers to our consumers using a third-party transportation service, or directly by Amazon for certain market place orders.
Employees
As of December 31, 2017, we had approximately 16,600 employees, including approximately 6,400 full-time and 10,200 part-time employees. None of our employees belong to a union or are party to any collective bargaining or similar agreement. We consider our relationship with our employees to be good.
Competition
The United States nutritional supplements retail industry is a large, highly fragmented and growing industry, with no single industry participant accounting for a majority of total industry retail sales. Competition is based on price, quality and assortment of products, customer service, convenience of store locations and websites, marketing support and availability of new products. In addition, the market is highly sensitive to the introduction of new products.
We compete with both publicly and privately owned companies, which are highly fragmented in terms of geographical market coverage and product categories. We also compete with other specialty retailers, supermarkets, drugstores, mass merchants, multi-level marketing organizations, mail-order companies, other internet sites and a variety of other smaller participants. In the United States, many of our competitors have national brands that are heavily advertised and are manufactured by large pharmaceutical and food companies and other retailers. Most supermarkets, drugstores and mass merchants have narrow product offerings limited primarily to simple vitamins, herbs and popular third-party sports and diet products. Our international competitors also include large international pharmacy chains and major international supermarket chains, as well as other large U.S.-based companies with international operations. Our wholesale and manufacturing operations compete with other wholesalers and manufacturers of third-party nutritional supplements.
Trademarks and Other Intellectual Property
We believe trademark protection is particularly important to the maintenance of the recognized brand names under which we market our products. We own or have rights to material trademarks or trade names that we use in conjunction with the sale of our products, including the GNC brand name. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position. We protect our intellectual property rights through a variety of methods, including trademark, patent and trade secret laws, as well as confidentiality agreements and proprietary information agreements with vendors, employees, consultants and others who have access to our proprietary information. Protection of our intellectual property often affords us the opportunity to enhance our position in the marketplace by precluding our competitors from using or otherwise exploiting our technology and brands. We are also a party to several intellectual property license agreements relating to certain of our products. The duration of our trademark registrations is generally 10, 15 or 20 years, depending on the country in which the marks are registered, and we can renew the registrations. The scope and duration of our intellectual property protection varies throughout the world by jurisdiction and by individual product. Our global trademark portfolio, with the aforementioned registration durations, consists of our core marks for our business and our proprietary product brands which drive significant brand awareness for all of our reportable segments. Our proprietary product formulas and recipes, maintained as trade secrets, are significant to our reportable segments as they are the foundation for effective products with high quality.
Insurance and Risk Management
We are self-insured for certain losses related to workers' compensation and general liability insurance and maintain stop-loss coverage with third-party insurers to limit our liability exposure. We were self-insured for health insurance through December 31, 2017 and effective January 1, 2018 are now fully insured. We face an inherent risk of exposure to product liability claims in the event that, among other things, the use of products sold by us results in injury. We carry product liability insurance with a deductible/retention of $4.0 million per claim with an aggregate cap on retained losses of $10.0 million per policy year. We have the ability to refer claims to most of our vendors and their insurers to pay the costs associated with any claims arising from such vendors' products. In most cases, our insurance covers such claims that are not adequately covered by a vendor's insurance and provides for excess secondary coverage above the limits provided by our product vendors.
8
We also purchase insurance to cover auto liability, network and cyber security, privacy liability and other casualty and property risks. We self-insure certain property and casualty risks such as property damage due to our analysis of the risk, the frequency and severity of a loss and the cost of insurance for the risk.
Government Regulation
Product Regulation
Domestic
The processing, formulation, safety, manufacturing, packaging, labeling, advertising and distribution of our products are subject to regulation by one or more federal agencies, including the U.S. Food and Drug Administration (the "FDA"), the Federal Trade Commission (the "FTC"), the Consumer Product Safety Commission (the "CPSC"), the United States Department of Agriculture (the "USDA") and the Environmental Protection Agency (the "EPA"), and by various agencies of the states and localities in which our products are sold.
The Dietary Supplement Health and Education Act of 1994 ("DSHEA") amended the Federal Food, Drug, and Cosmetic Act (the "FDC Act") to establish a new framework governing the composition, safety, labeling, manufacturing and marketing of dietary supplements. Generally, under the FDC Act, dietary ingredients that were marketed in the United States prior to October 15, 1994 may be used in dietary supplements without notifying the FDA. "New" dietary ingredients (i.e., dietary ingredients that were "not marketed in the United States before October 15, 1994") must be the subject of a new dietary ingredient notification submitted to the FDA unless the ingredient has been "present in the food supply as an article used for food" without being "chemically altered." A new dietary ingredient notification must provide the FDA evidence of a "history of use or other evidence of safety" establishing that use of the dietary ingredient "will reasonably be expected to be safe." A new dietary ingredient notification must be submitted to the FDA at least 75 days before the initial marketing of the new dietary ingredient. The FDA may determine that a new dietary ingredient notification does not provide an adequate basis to conclude that a dietary ingredient is reasonably expected to be safe. Such a determination could prevent the marketing of such dietary ingredient. In 2011 and 2016, the FDA issued draft guidance governing the notification of new dietary ingredients. Although FDA guidance is not mandatory, and companies are free to use an alternative approach if the approach satisfies the requirements of applicable laws and regulations, FDA guidance is a strong indication of the FDA's "current thinking" on the topic discussed in the guidance, including its position on enforcement. At this time, it is difficult to determine whether the draft guidance, if finalized, would have a material impact on our operations. However, if the FDA were to enforce the applicable statutes and regulations in accordance with the draft guidance as written, such enforcement could require us to incur additional expenses, which could be significant, and negatively impact our business in several ways, including, but not limited to, enjoining the manufacturing of our products until the FDA determines that we are in compliance and can resume manufacturing, increasing our liability and reducing our growth prospects.
The FDA or other agencies could take actions against products or product ingredients that in its determination present an unreasonable health risk to consumers that would make it illegal for us to sell such products. In addition, the FDA could issue consumer warnings with respect to the products or ingredients in such products that are sold in our stores. Such actions or warnings could be based on information received through FDC Act-mandated reporting of serious adverse events.
We take a number of actions to ensure the products we sell comply with the FDC Act. Some of these actions include maintaining and continuously updating a list of restricted ingredients that will be prohibited from inclusion in any products that are sold in our stores or on our websites. Vendors selling product to us for the sale of such products by us will be required to warrant to us that the products sold to us do not contain any of these restricted ingredients. In addition, we have developed and maintain a list of ingredients that we believe comply with the applicable provisions of the FDC Act. As is common in our industry, we rely on our third-party vendors to ensure that the products they manufacture and sell to us comply with all applicable regulatory and legislative requirements. In general, we seek representations and warranties, indemnification and/or insurance from our vendors. However, even with adequate insurance and indemnification, any claims of non-compliance could significantly damage our reputation and consumer confidence in our products. In addition, the failure of such products to comply with applicable regulatory and legislative requirements could prevent us from marketing the products or require us to recall or remove such products from the market, which in certain cases could materially and adversely affect our business, financial condition and results of operations. In the past, we have attempted to offset any losses related to recalls and removals with reformulated or alternative products; however, there can be no assurance that we would be able to offset all or any portion of losses related to any future removal or recall.
The FDC Act permits "statements of nutritional support" to be included in labeling for dietary supplements without FDA pre-market approval. Such statements must be submitted to the FDA within 30 days of marketing. Such statements may describe how a particular dietary ingredient affects the structure, function or general well-being of the body, or the mechanism of action by which a dietary ingredient may affect body structure, function or well-being, but may not expressly or implicitly represent that a dietary supplement will diagnose, cure, mitigate, treat or prevent a disease. A company that uses a statement of nutritional support
9
in labeling must possess scientific evidence substantiating that the statement is truthful and not misleading. If the FDA determines that a particular statement of nutritional support is an unacceptable drug claim, conventional food claim or an unauthorized version of a "health claim," or, if the FDA determines that a particular claim is not adequately supported by existing scientific data or is false or misleading, we would be prevented from using the claim.
In addition, DSHEA provides that so-called "third-party literature," e.g., a reprint of a peer-reviewed scientific publication linking a particular dietary ingredient with health benefits, may be used "in connection with the sale of a dietary supplement to consumers" without the literature being subject to regulation as labeling. The literature: (1) must not be false or misleading; (2) may not "promote" a particular manufacturer or brand of dietary supplement; (3) must present a balanced view of the available scientific information on the subject matter; (4) if displayed in an establishment, must be physically separate from the dietary supplements; and (5) should not have appended to it any information by sticker or any other method. If the literature fails to satisfy each of these requirements, we may be prevented from disseminating such literature with our products, and any dissemination could subject our product to regulatory action as an illegal drug.
In June 2007, pursuant to the authority granted by the FDC Act as amended by DSHEA, the FDA published detailed current Good Manufacturing Practice ("cGMP") regulations that govern the manufacturing, packaging, labeling and holding operations of dietary supplement manufacturers. The cGMP regulations, among other things, impose significant recordkeeping requirements on manufacturers. The cGMP requirements are in effect for all dietary supplement manufacturers, and the FDA is conducting inspections of dietary supplement manufacturers pursuant to these requirements. There remains considerable uncertainty with respect to the FDA's interpretation of the regulations and their actual implementation in manufacturing facilities.
In addition, the FDA's interpretation of the regulations will likely change over time as the agency becomes more familiar with the industry and the regulations. The failure of a manufacturing facility to comply with the cGMP regulations renders products manufactured in such facility "adulterated," and subjects such products and the manufacturer to a variety of potential FDA enforcement actions. In addition, under the Food Safety Modernization Act ("FSMA"), which was enacted in January 2011, the manufacturing of dietary ingredients contained in dietary supplements will be subject to similar or even more burdensome manufacturing requirements, which will likely increase the costs of dietary ingredients and will subject suppliers of such ingredients to more rigorous inspections and enforcement. The FSMA will also require importers of food, including dietary supplements and dietary ingredients, to conduct verification activities to ensure that the food they might import meets applicable domestic requirements.
The FDA has broad authority to enforce the provisions of federal law applicable to dietary supplements, including powers to issue a public warning or notice of violation letter to a company, publicize information about illegal products, detain products intended for import, require the reporting of serious adverse events, require a recall of illegal or unsafe products from the market, and request the Department of Justice to initiate a seizure action, an injunction action or a criminal prosecution in the United States courts.
The FSMA expands the reach and regulatory powers of the FDA with respect to the production and importation of food, including dietary supplements. The expanded reach and regulatory powers include the FDA's ability to order mandatory recalls, administratively detain domestic products, and require certification of compliance with domestic requirements for imported foods associated with safety issues. FMSA also gave FDA the authority to administratively revoke manufacturing facility registrations, effectively enjoining manufacturing of dietary ingredients and dietary supplements without judicial process. The regulation of dietary supplements may increase or become more restrictive in the future.
The FTC exercises jurisdiction over the advertising of dietary supplements and over-the-counter drugs and has instituted numerous enforcement actions against dietary supplement companies for failure to have adequate substantiation for claims made in advertising or for the use of false or misleading advertising claims. We continue to be subject to a consent decree issued by the FTC in 1994 covering hair care products.
The FTC continues to monitor our advertising and, from time to time, requests substantiation with respect to such advertising to assess compliance with the outstanding consent decree and with the Federal Trade Commission Act. Our policy is to use advertising that complies with the consent decree and applicable regulations. Nevertheless, there can be no assurance that inadvertent failures to comply with the consent decree and applicable regulations will not occur.
Some of the products sold by franchise stores are purchased by franchisees directly from other vendors and these products do not flow through our distribution centers. Although franchise contracts contain strict requirements for store operations, including compliance with federal, state and local laws and regulations, we cannot exercise the same degree of control over franchisees as we do over our company-owned stores.
As a result of our efforts to comply with applicable statutes and regulations, we have from time to time reformulated, eliminated or relabeled certain of our products and revised certain provisions of our sales and marketing program.
10
Foreign
Our products sold in foreign countries are also subject to regulation under various national, local and international laws that include provisions governing, among other things, the formulation, manufacturing, packaging, labeling, advertising and distribution of dietary supplements and over-the-counter drugs. Government regulations in foreign countries may prevent or delay the introduction, or require the reformulation, of certain of our products.
New Legislation or Regulation
Legislation may be introduced which, if passed, would impose substantial new regulatory requirements on dietary supplements. We cannot determine what effect additional domestic or international governmental legislation, regulations, or administrative orders, when and if promulgated, would have on our business in the future. New legislation or regulations may require the reformulation of certain products to meet new standards, require the recall or discontinuance of certain products not capable of reformulation, impose additional record keeping or require expanded documentation of the properties of certain products, expanded or different labeling or scientific substantiation.
Franchise Regulation
We must comply with regulations adopted by the FTC and with the laws of several states that regulate the offer and sale of franchises. The FTC's Trade Regulation Rule on Franchising and certain state laws require that we furnish prospective franchisees with a franchise offering circular containing information prescribed by the Trade Regulation Rule on Franchising and applicable state laws and regulations.
We also must comply with a number of state laws that regulate some substantive aspects of the franchisor-franchisee relationship. These laws may limit a franchisor's business practices in a number of ways, including limiting the ability to:
•terminate or not renew a franchise without good cause;
•interfere with the right of free association among franchisees;
•disapprove the transfer of a franchise;
•discriminate among franchisees with regard to franchise terms and charges, royalties and other fees; and
•place new stores near existing franchises.
To date, these laws have not precluded us from seeking franchisees in any given area and have not had a material adverse effect on our operations. Bills concerning the regulation of certain aspects of franchise relationships have been introduced into Congress on several occasions during the last decade, but none have been enacted. Revisions to the FTC rule have also been proposed by the FTC and currently are in the comment stage of the rulemaking process.
Our international franchise agreements and franchise operations are regulated by various foreign laws, rules and regulations. These laws may limit a franchisor's business practices in a number of ways. To date, these laws have not precluded us from seeking franchisees in any given area and have not had a material adverse effect on our operations.
Environmental Compliance
In March 2008, the South Carolina Department of Health and Environmental Control (the "DHEC") requested that we investigate contamination associated with historical activities at our South Carolina facility. These investigations have identified chlorinated solvent impacts in soils and groundwater that extend offsite from our facility. We entered into a Voluntary Cleanup Contract with the DHEC regarding the matter on September 24, 2012. Pursuant to such contract, we have completed additional investigations with the DHEC's approval. The Company installed and began operating a pilot vapor extraction system under a portion of the facility in the second half of 2016, which was an immaterial cost to the Company, with DHEC's approval to assess the effectiveness of such a remedial system. After an initial period of monitoring, in October of 2017 the DHEC approved a work plan for extended monitoring of such system and the contamination into 2021. We will continue to consult with the DHEC on the next steps in the work after their review of the results of the extended monitoring is complete. At this stage of the investigation, however, it is not possible to estimate the timing and extent of any additional remedial action that may be required, the ultimate cost of remediation, or the amount of our potential liability. Therefore, no liability has been recorded in the Company's Consolidated Financial Statements.
In addition to the foregoing, we are subject to numerous federal, state, local and foreign environmental and health and safety laws and regulations governing our operations, including the handling, transportation and disposal of our non-hazardous and hazardous substances and wastes, as well as emissions and discharges from its operations into the environment, including discharges to air, surface water and groundwater. Failure to comply with such laws and regulations could result in costs for remedial
11
actions, penalties or the imposition of other liabilities. New laws, changes in existing laws or the interpretation thereof, or the development of new facts or changes in their processes could also cause us to incur additional capital and operating expenditures to maintain compliance with environmental laws and regulations and environmental permits. We are also subject to laws and regulations that impose liability and cleanup responsibility for releases of hazardous substances into the environment without regard to fault or knowledge about the condition or action causing the liability. Under certain of these laws and regulations, such liabilities can be imposed for cleanup of previously owned or operated properties, or for properties to which substances or wastes that were sent in connection with current or former operations at its facilities. The presence of contamination from such substances or wastes could also adversely affect our ability to sell or lease our properties, or to use them as collateral for financing. From time to time, we have incurred costs and obligations for correcting environmental and health and safety noncompliance matters and for remediation at or relating to certain of our properties or properties at which our waste has been disposed. However, compliance with the provisions of national, state and local environmental laws and regulations has not had a material effect upon our capital expenditures, earnings, financial position, liquidity or competitive position. We believe we have complied with, and are currently complying with, our environmental obligations pursuant to environmental and health and safety laws and regulations and that any liabilities for noncompliance will not have a material adverse effect on our business, financial performance or cash flows. However, it is difficult to predict future liabilities and obligations, which could be material.
12
Item 1A. RISK FACTORS.
The following risk factors could cause our financial performance to differ significantly from the goals, plans, objectives, intentions and expectations expressed in this Annual Report. If any of the following risks and uncertainties actually occur, our business, financial condition, results of operations or cash flows could be materially and adversely affected.
Risks Relating to Our Business and Industry
While we believe we will have sufficient cash on hand and amounts available under our new asset-based Revolving Credit Facility to fund the $151.9 million under our Term Loan Facility and an excess cash flow payment, which could be material, due in March 2019, if actual results are below our projections by an amount greater than what is required to satisfy these obligations, it could have a material adverse impact on our ability to sustain operations.
As of December 31, 2017, the Company had principal of $1,131.2 million outstanding under its Term Loan Facility with an original maturity date of March 2019. After the effectiveness of the Amendment to our Senior Credit Facility described in Item 8, "Financial Statements and Supplementary Data," Note 18, "Subsequent Events," the amount due in March 2019 under the Term Loan facility is now $151.9 million. We are also required under the Amendment to make an excess cash flow payment in March 2019, which could be material, based on the projected Consolidated Net First Lien Leverage Ratio for the year ending December 31, 2018.
We expect to close the Securities Purchase Agreement described below under the risk factor titled "There can be no assurance that the conditions to the Securities Purchase Agreement with Harbin Pharmaceutical Group Holdings Co., Ltd. will be satisfied or that a satisfactory definitive agreement to enter into a China joint venture will be reached" in the second half of 2018, which will result in approximately $300 million of aggregate proceeds. Under the Amendment, $100 million of the net proceeds from the Securities Purchase Agreement are required to be utilized to pay the Term Loan Facility due March 2021. The remaining net proceeds, after deducting legal and advisory fees, would be available to satisfy the $151.9 million due under the Term Loan Facility in March 2019. However, there is no assurance that the Securities Purchase Agreement will close prior to March 2019 when the obligation is due.
In the event that Securities Purchase Agreement doesn’t close, management has concluded that the Company will have the ability to satisfy the $151.9 million Term Loan Facility and excess cash flow payments due in March 2019 with projected cash on hand and amounts available under the new $100 million asset-based Revolving Credit Facility. If actual results are below the Company’s projections by an amount greater than what is required to satisfy these obligations, management has the ability to reduce certain discretionary payments and will consider certain asset sales, as necessary, to maximize cash available.
As described in Item 8, "Financial Statements and Supplementary Data," Note 7, "Long-Term Debt / Interest Expense," we have concluded that it is probable the Company will have sufficient cash on hand and available liquidity to satisfy the obligations that are due in March 2019. However, we cannot provide full assurance that the Company will be able to satisfy the above obligations. If the Company is unable to satisfy these obligations through normal operations or strategic measures described above, we would be in default, which could have a material adverse impact on our ability to sustain operations.
Our current and historical effective tax rate may not be indicative of future rates.
On December 22, 2017, United States tax reform legislation known as The Tax Cuts and Jobs Act of 2017 (“2017 Tax Act”) was enacted. The 2017 Tax Act made significant changes to the Internal Revenue Code, including a reduction in the corporate tax rate from 35% to 21%. This rate reduction is effective for tax years beginning after December 31, 2017. The 2017 Tax Act also imposed a potential one-time repatriation and taxation of a company's foreign earnings. See Item 8, “Financial Statements and Supplementary Data,” Note 4, “Income Taxes” for more information. As a result, our future effective tax rate will likely differ significantly from current and historical rates due to the 2017 Tax Act. Furthermore, in light of our global earnings mix, future changes in domestic and international tax laws in the various jurisdictions in which we operate as well as changes to our tax positions could also impact the effective tax rate on a prospective basis.
Resources devoted to product innovation may not yield new products that achieve commercial success.
Our ability to develop new and innovative GNC-branded products depends on, among other factors, our ability to understand evolving customer and market trends and our ability to translate these insights into commercially viable new products. If we are unable to do so, our customer relationships and product sales could be harmed significantly. Furthermore, the nutritional supplements industry is characterized by rapid and frequent changes in demand for products and new product introductions. Our failure to accurately predict these trends could negatively impact consumer opinion of our stores as a source for the latest products. This could harm our customer relationships and cause losses to our market share. The development of new and innovative products also requires significant investment in research and development and testing of new ingredients, formulas and possibly new production processes. The R&D process can be expensive and prolonged and entails considerable uncertainty. Products may appear
13
promising in development but fail to reach market within the expected time frame, or at all. We may face significant challenges with regard to a key product launch. Further, products also may fail to achieve commercial viability. Finally, there is no guarantee that our development teams will be able to successfully respond to competitive products that could render some of our offerings obsolete. Development of a new product, from discovery through testing to the store shelf, typically takes between four to seven months, but may require an even longer timeline if clinical trials are involved. Each of these time periods can vary considerably from product to product.
We continue to explore new strategic initiatives, including our One New GNC model, but we may not be able to successfully execute on, or realize the expected benefit from the implementation of, our strategic initiatives, and our pursuit of new strategic initiatives may pose significant costs and risks.
We conducted a vast array of consumer tests, pilot programs and other market research throughout 2015 and 2016 as part of our comprehensive review of our customers’ experience. Based on this work, we launched our One New GNC single-tier pricing model and new customer loyalty programs, myGNC Rewards and PRO Access, at the end of 2016. Our future operating results are dependent, in part, on our management’s success in implementing these and other strategic initiatives. Also, our short-term operating results could be unfavorably impacted by the opportunity and financial costs associated with the implementation of these strategic plans, and we may not realize the expected benefits from such strategies. In addition, we may not be successful in achieving the intended objectives of these strategic initiatives in a timely manner or at all.
We recognized impairment charges during 2017 and may recognize additional such charges in the future, which could adversely affect our results of operations and financial condition.
We evaluate goodwill and our indefinite-lived brand intangible asset for impairment on at least an annual basis. We evaluate property and equipment and definite-lived intangible assets for recoverability when indicators of impairment exist.We will recognize an impairment charge if: our $324.4 million indefinite-lived brand intangible asset has a carrying value that exceeds its estimated fair value; our $141.0 million of goodwill has a carrying value for an applicable reporting unit that exceeds its fair value; or our property and equipment and definite-lived intangible assets totaling $286.3 million at December 31, 2017 have estimated future undiscounted cash flows that are less than the applicable carrying values. In assessing fair value, we rely primarily on a discounted cash flow analysis, as well as other generally accepted valuation methodologies. These analyses rely on the judgments and estimates of management, which involve inherent uncertainties. Impairment losses are significantly affected by estimates of future operating cash flows and estimates of fair value as well as the Company's total market capitalization. Estimates of future operating cash flows are identified from strategic long-term plans, which are based upon experience, knowledge, and expectations; however, these estimates can be affected by such factors as future operating results, future store profitability, future volumes, revenue and expense growth rates and asset disposal values and future economic conditions, all of which can be difficult to predict accurately. Any significant deterioration in macroeconomic conditions could affect the fair value of our long-lived assets and could result in future impairment charges, which would adversely affect our results of operations. We recorded long-lived asset impairment charges of $457.8 million in fiscal 2017. While we currently believe that the fair values of our long-lived assets exceed their respective carrying values, changes in our estimates and assumptions regarding the future performance of our business could result in further impairment charges, which may have a material adverse effect on our results of operations.
Our inability to attract, train and retain highly qualified associates could adversely impact our business, financial condition and results of operations.
Our success depends on the continued contributions of our store and field associates, and the loss of these contributions could have a material adverse effect on our business. We must attract, train and retain a large and growing number of qualified associates, while controlling related labor costs and maintaining our core values. Our ability to control labor and benefit costs is subject to numerous external factors, including regulatory changes, prevailing wage rates, and healthcare and other insurance costs. We compete with other retail and non-retail businesses for these store and field associates and invest significant resources in training and motivating them. There is no assurance that we will be able to attract or retain qualified store and field associates in the future, which could have a material adverse effect on our business, financial condition and results of operations.
We operate in a highly competitive industry. Our failure to compete effectively could adversely affect our market share, revenues and growth prospects.
The United States nutritional supplements retail industry is large and highly fragmented. Participants include specialty retailers, supermarkets, drugstores, mass merchants, multi-level marketing organizations, on-line merchants, mail-order companies and a variety of other smaller participants. We believe that the market is also highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market. In the United States, we compete for sales with heavily advertised national brands manufactured by large pharmaceutical and food companies, as well as other retailers. In addition, as some products become more mainstream, we experience increased price competition for those products as more participants enter the market. Our international competitors include large international pharmacy chains, major international supermarket chains and other large
14
U.S.-based companies with international operations. Our wholesale and manufacturing operations compete with other wholesalers and manufacturers of third-party nutritional supplements. We may not be able to compete effectively and our attempts to do so may require us to reduce our prices, which may result in lower margins. Failure to effectively compete could adversely affect our market share, revenues and growth prospects.
Unfavorable publicity or consumer perception of our products, the ingredients they contain and any similar products distributed by other companies could cause fluctuations in our operating results and could have a material adverse effect on our reputation, the demand for our products and our ability to generate revenues and the market price of our common stock.
We are highly dependent upon consumer perception of the safety and quality of our products and the ingredients they contain, as well as that of similar products distributed by other companies. Consumer perception of products and the ingredients they contain can be significantly influenced by scientific research or findings, national media attention and other publicity about product use. A product may be received favorably, resulting in high sales associated with that product that may not be sustainable as consumer preferences change. Future scientific research or publicity could be unfavorable to our industry or any of our particular products or the ingredients they contain and may not be consistent with earlier favorable research or publicity. A future research report or publicity that is perceived by our consumers as less favorable or that questions earlier research or publicity could have a material adverse effect on our ability to generate revenues. As such, period-to-period comparisons of our results should not be relied upon as a measure of our future performance. Adverse publicity in the form of published scientific research or otherwise, whether or not accurate, that associates consumption of our products or the ingredients they contain or any other similar products distributed by other companies with illness or other adverse effects, that questions the benefits of our or similar products, or that claims that such products are ineffective could have a material adverse effect on our reputation, the demand for our products, our ability to generate revenues and the market price of our common stock.
Our substantial debt could adversely affect our results of operations and financial condition and otherwise adversely impact our operating income and growth prospects.
On February 28, 2018, we amended our Senior Credit Facility, which consisted of an extension of the maturity date for $704.3 million of the $1,131.2 million Term Loan Facility from March 2019 to March 2021. However, if more than $50.0 million of our Notes have not been repaid, discharged, prepaid, refinanced or converted prior to May 2020 (“Existing Indenture Discharge”), the maturity date becomes May 2020. The amendment (the “Amendment”) also includes:
• | the termination of the Revolving Credit Facility, which was replaced with a new $100 million asset-based Revolving Credit Facility with a maturity date of August 2022 (which maturity date will become May 2020 if the Existing Indenture Discharge has not occurred); and |
• | a $275 million asset-based Term Loan Facility advanced on a “first-in, last-out” basis with a maturity date of December 2022 (which maturity date will become May 2020 if the Existing Indenture Discharge has not occurred). |
After the effectiveness of the Amendment, we will owe $151.9 million on our Term Loan Facility due in March 2019. The Amendment requires annual aggregate principal payments of at least $43 million related to the $704.3 million of the Term Loan Facility with a maturity of March 2021. There are no scheduled amortization payments associated with the $275.0 million asset-based Term Loan Facility and payments associated with the $151.9 million Term Loan Facility are consistent with past terms.
We also have $188.6 million principal amount of 1.5% convertible senior notes due 2020 outstanding as of December 31, 2017 that the Company issued in a private offering in August 2015 (the "Notes") (net of $20.6 million related to the conversion feature and discount). On December 20, 2017, the Company executed exchange agreements with certain holders of the Notes to exchange, in privately negotiated transactions, $98.9 million aggregate principal amount of the Notes for an aggregate of 14.6 million newly issued shares of the Company's Class A common stock, $0.001 par value per share, together with $0.5 million of cash, representing accrued and unpaid interest on the Notes being exchanged. The remaining debt of the Notes currently bear interest at a rate of 1.50% per year, payable semiannually in arrears on February 15 and August 15 each year prior to their maturity in August 2020.
Our substantial debt could materially affect our financial condition. For example, it could:
• | increase our vulnerability to general adverse economic and industry conditions; |
• | require us to use all or a large portion of our cash flow from operations to pay principal and interest on our debt, thereby reducing the availability of our cash flow to fund working capital, capital expenditures and other business activities; |
• | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
• | restrict us from making strategic acquisitions or capitalizing on business opportunities; |
• | place us at a competitive disadvantage compared with our competitors that have less debt; and |
15
• | limit our ability to borrow additional funds or pay cash dividends. |
We may be able to incur additional debt in the future, including collateralized debt. Although the Senior Credit Facility contains restrictions on the incurrence of additional debt, these restrictions are subject to a number of qualifications and exceptions. If we add to our current level of debt, the risks described above would be greater.
We may not have the ability to raise the funds necessary to settle conversions of the Notes or to repurchase the Notes upon a fundamental change, and our future debt may contain limitations on our or the subsidiary guarantors’ ability to pay cash upon conversion or repurchase of the Notes.
Holders of the Notes will have the right to require us to repurchase their notes upon the occurrence of certain “fundamental changes,” as defined in the Indenture governing the Notes, at a repurchase price equal to 100% of the principal amount of the Notes to be repurchased, plus accrued and unpaid interest, if any. In addition, upon conversion of the Notes, unless we elect to deliver solely shares of our common stock to settle such conversion (other than paying cash in lieu of delivering any fractional share), we will be required to make cash payments in respect of the Notes being converted. However, we may not have enough available cash or be able to obtain financing at the time we are required to make repurchases of notes surrendered therefor or pay cash upon conversions of notes being converted. In addition, our ability to repurchase the Notes or to pay cash upon conversions of the Notes may be limited by law, by regulatory authority or by agreements governing our existing or future indebtedness. Our failure to repurchase the Notes at a time when the repurchase is required by the Indenture or to pay any cash payable on future conversions of the Notes as required by the Indenture would constitute a default under the Indenture. A default under the indenture or the fundamental change itself could also lead to a default under agreements governing our future indebtedness. If the repayment of the related indebtedness were to be accelerated after any applicable notice or grace periods, we may not have sufficient funds to repay the indebtedness and repurchase the Notes or make cash payments upon conversions thereof.
The conditional conversion feature of the Notes, if triggered, may adversely affect our financial condition and operating results.
In the event the conditional conversion feature of the Notes is triggered, holders of Notes will be entitled to convert the Notes at any time during specified periods at their option. If one or more holders elect to convert their Notes, unless we elect to satisfy our conversion obligation by delivering solely shares of our common stock (other than paying cash in lieu of delivering any fractional share), we would be required to settle a portion or all of our conversion obligation through the payment of cash, which could adversely affect our liquidity. In addition, even if holders do not elect to convert their Notes, we could be required under applicable accounting rules to reclassify all or a portion of the outstanding principal of the Notes as a current rather than long-term liability, which could result in a material reduction of our net working capital.
The accounting method for convertible debt securities that may be settled in cash, such as the Notes, could have a material effect on our reported financial results.
In May 2008, the Financial Accounting Standards Board ("FASB"), issued FASB Staff Position No. APB 14-1, Accounting for Convertible Debt Instruments That May Be Settled in Cash Upon Conversion (Including Partial Cash Settlement), which has subsequently been codified as Accounting Standards Codification ("ASC") 470-20, Debt with Conversion and Other Options. Under ASC 470-20, an entity must separately account for the liability and equity components of the convertible debt instruments (such as the notes) that may be settled entirely or partially in cash upon conversion in a manner that reflects the issuer’s economic interest cost. The effect of ASC 470-20 on the accounting for the Notes is that the equity component is required to be included in the additional paid-in capital section of stockholders’ equity on our Consolidated Balance Sheet, and the value of the equity component would be treated as original issue discount for purposes of accounting for the debt component of the Notes. As a result, we will be required to record a greater amount of non-cash interest expense in current periods presented as a result of the amortization of the discounted carrying value of the Notes to their face amount over the term of the Notes. We will report lower net income in our financial results because ASC 470-20 will require interest to include both the current period’s amortization of the debt discount and the instrument’s coupon interest, which could adversely affect our reported or future financial results, the trading price of our common stock and the trading price of the Notes.
In addition, under certain circumstances, convertible debt instruments (such as the Notes) may use the treasury stock method to calculation dilution for earnings per share. Under the treasury stock method, the underlying convertible shares are anti-dilutive if the Company's average stock price is less than the conversion price, which historically has been the case. We no longer intend to settle the principal portion of Notes in cash, and as such, will apply the if-converted method to calculate dilution in future periods, which may adversely impact our diluted earnings per share.
16
Future sales of our common stock in the public market could lower the market price for our common stock and adversely impact the trading price of the Notes.
In the future, we may sell additional shares of our common stock to raise capital. In addition, a substantial number of shares of our common stock is reserved for issuance upon the exercise of stock options, vesting of restricted stock units, and upon conversion of the Notes. We cannot predict the size of future issuances or the effect, if any, that they may have on the market price for our common stock. The issuance and sale of substantial amounts of common stock, or the perception that such issuances and sales may occur, could adversely affect the trading price of the Notes and the market price of our common stock and impair our ability to raise capital through the sale of additional equity securities.
Our ability to continue to access credit on the terms previously obtained for the funding of our operations and capital projects may be limited due to changes in credit markets.
In the past, the credit markets and the financial services industry have experienced disruption characterized by the bankruptcy, failure, collapse or sale of various financial institutions, increased volatility in securities prices, diminished liquidity and credit availability and intervention from the United States and other governments. Continued concerns about the systemic impact of potential long-term or widespread downturn, energy costs, geopolitical issues, the availability and cost of credit, the global commercial and residential real estate markets and related mortgage markets and reduced consumer confidence have contributed to increased market volatility. The cost and availability of credit has been and may continue to be adversely affected by these conditions. We cannot be certain that funding for our capital needs will be available from our existing financial institutions and the credit markets if needed, and if available, to the extent required and on acceptable terms. In connection with the Amendment described above, the $704.3 million Term Loan Facility matures on March 2021, the new asset-based Revolving Credit Facility matures in August 2022 and the $275.0 million asset-based Term Loan Facility matures on December 2020 (all of which the maturity dates will become May 2020 if the Existing Indenture Discharge has not occurred). If we cannot renew or refinance these facilities upon their maturity or, more generally, obtain funding when needed, in each case on acceptable terms, we may be unable to adequately fund our operating expenses and fund required capital expenditures, which may have an adverse effect on our revenues and results of operations.
We require a significant amount of cash to service our debt. Our ability to generate cash depends on many factors, some of which are beyond our control, and, as a result, we may not be able to make payments on our debt obligations.
We may be unable to generate sufficient cash flow from operations or to obtain future borrowings under our credit facilities or otherwise in an amount sufficient to enable us to pay our debt or to fund our other liquidity needs. In addition, because we conduct our operations through our operating subsidiaries, we depend on those entities for dividends and other payments to generate the funds necessary to meet our financial obligations, including payments on our debt. Under certain circumstances, legal and contractual restrictions, as well as the financial condition and operating requirements of our subsidiaries, may limit our ability to obtain cash from our subsidiaries. If we do not have sufficient liquidity, we may need to refinance or restructure all or a portion of our debt on or before maturity, sell assets or borrow more money, which we may not be able to do on terms satisfactory to us or at all. In addition, any refinancing could be at higher interest rates and may require us to comply with more onerous covenants which could further restrict our business operations.
A default on any of our debt obligations could trigger certain acceleration clauses and cause those and our other obligations to become due and payable subject to defined rights to cure. Upon an acceleration of any of our debt, we may not be able to make payments under our other outstanding debt.
Restrictions in the agreements governing our existing and future indebtedness may prevent us from taking actions that we believe would be in the best interest of our business.
The agreements governing our existing indebtedness contain, and the agreements governing our future indebtedness will likely contain, customary restrictions on us or our subsidiaries, including covenants that restrict us or our subsidiaries, as the case may be, from:
• | incurring additional indebtedness and issuing preferred stock; |
• | granting liens on our assets; |
• | making investments; |
• | consolidating or merging with, or acquiring, another business; |
• | selling or otherwise disposing of our assets; |
• | paying dividends and making other distributions to our stockholders; |
17
• | entering into transactions with our affiliates; and |
• | incurring capital expenditures in excess of limitations set within the agreement. |
Our new $100 million asset-based Revolving Credit Facility requires that, for so long as availability under the Revolving Credit Facility is below a certain level, we meet a Fixed Charge Coverage Ratio of (a) consolidated earnings before interest, taxes, depreciation and amortization, or EBITDA, less certain capital expenditures and taxes, to (b) the sum of cash interest expense, scheduled amortization and certain dividends and distributions. If we fail to satisfy such ratio, then we will be restricted from drawing available borrowings under the asset-based Revolving Credit Facility and any amount outstanding may become due and payable subject to defined rights to cure, which may impair our liquidity.
Our ability to comply with these covenants and other provisions of the Senior Credit Facility may be affected by changes in our operating and financial performance, changes in general business and economic conditions, adverse regulatory developments or other events beyond our control. The breach of any of these covenants could result in a default under our debt obligations, which could cause those and other obligations to become due and payable subject to defined rights to cure. In addition, these restrictions may prevent us from taking actions that we believe would be in the best interest of our business and may make it difficult for us to successfully execute our business strategy or effectively compete with companies that are not similarly restricted.
Our use of derivative instruments for hedging purposes may result in financial losses.
We may from time to time utilize derivative instruments to manage our exposure to fluctuations in fuel and certain other commodity prices, interest rates and foreign currency exchange rates. We could recognize losses on these contracts as a result of volatility in the market values of the underlying commodities or to the extent that a counterparty fails to perform. In the absence of actively-quoted market prices and pricing information from external sources, the valuation of these instruments involves judgment or use of estimates. Furthermore, changes in the value of derivatives designated under hedge accounting to the extent not fully offset by changes in the value of the hedged transaction can result in ineffectiveness losses that may have an adverse effect on our results of operations.
The price of our common stock historically has been volatile.
The market price for our common stock has varied during the twelve-month period ended December 31, 2017 between a high of $11.45 on January 4, 2017 and a low of $3.35 on December 28, 2017. Our stock price is likely to continue to be volatile and subject to significant price and volume fluctuations in response to market and other factors, including those factors discussed under the heading “Risk Factors” in this Annual Report, as well as: variations in our quarterly operating results from our expectations or those of securities analysts or other investors; revisions in analyst estimates or announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; or the sale of substantial amounts of our common stock.
We depend on the services of key executives and other skilled professionals and any failure to attract or retain such individuals could affect our business strategy and adversely impact our performance and results of operations.
Our senior executives are instrumental in setting our strategic direction, operating our business, identifying, recruiting and training key personnel, identifying opportunities and arranging necessary financing. In addition, other key employees below the executive level, who are skilled professionals with deep knowledge of our business, are critical to the execution and success of our strategy. Losing the services of any of these individuals could adversely affect our business. Furthermore, to the extent that we must replace one or more of these individuals or hire additional senior executives or other professionals to support our growing business, we may be unable to identify candidates of sufficient experience and capabilities in a timely fashion, which could negatively impact our business and operations.
If our risk management methods are not effective, our business, reputation and financial results may be adversely affected.
We have methods to identify, monitor and manage our risks; however, these methods may not be fully effective. Some of our risk management methods may depend upon evaluation of information regarding markets, customers or other matters that are publicly available or otherwise accessible by us. That information may not in all cases be accurate, complete, up-to-date or properly evaluated. If our methods are not fully effective or we are not successful in monitoring or evaluating the risks to which we are or may be exposed, our business, reputation, financial condition and operating results could be materially and adversely affected. In addition, our insurance policies may not provide adequate coverage.
Compliance with new and existing governmental regulations could increase our costs significantly and adversely affect our results of operations.
The processing, formulation, safety, manufacturing, packaging, labeling, advertising and distribution of our products are subject to federal laws and regulation by one or more federal agencies, including the FDA, the FTC, the CPSC, the USDA, and
18
the EPA. These activities are also regulated by various state, local and international laws and agencies of the states and localities in which our products are sold. Government regulations may prevent or delay the introduction, or require the reformulation, of our products, which could result in lost revenues and increased costs to us. For instance, the FDA regulates, among other things, the composition, safety, manufacture, labeling and marketing of dietary supplements (including vitamins, minerals, herbs, and other dietary ingredients for human use). The FDA may not accept the evidence of safety for any new dietary ingredient that we may wish to market, may determine that a particular dietary supplement or ingredient presents an unacceptable health risk based on the required submission of serious adverse events or other information, and may determine that a particular claim or statement of nutritional value that we use to support the marketing of a dietary supplement is an impermissible drug claim, is not substantiated, or is an unauthorized version of a "health claim." See Item 1, "Business—Government Regulation—Product Regulation" for additional information. Any of these actions could prevent us from marketing particular dietary supplement products or making certain claims or statements with respect to those products. The FDA could also require us to remove a particular product from the market. Any future recall or removal would result in additional costs to us, including lost revenues from any products that we are required to remove from the market, any of which could be material. Any product recalls or removals could also lead to an increased risk of litigation and liability, substantial costs, and reduced growth prospects.
Additional or more stringent laws and regulations of dietary supplements and other products have been considered from time to time. These developments could require reformulation of some products to meet new standards, recalls or discontinuance of some products not able to be reformulated, additional record-keeping requirements, increased documentation of the properties of some products, additional or different labeling, additional scientific substantiation, or other new requirements. Any of these developments could increase our costs significantly. In addition, regulators' evolving interpretation of existing laws could have similar effects.
Our failure to comply with FTC regulations and the consent decree imposed on us by the FTC could result in substantial monetary penalties and could adversely affect our operating results.
The FTC exercises jurisdiction over the advertising of dietary supplements and has instituted numerous enforcement actions against dietary supplement companies, including us, for failure to have adequate substantiation for claims made in advertising or for the use of false or misleading advertising claims. As a result of these enforcement actions, we are currently subject to a consent decree that limits our ability to make certain claims with respect to our hair care products. See Item 1, "Business—Government Regulation—Product Regulation" for more information. Failure by us or our franchisees to comply with the consent decree and applicable regulations could result in substantial monetary penalties, which could have a material adverse effect on our financial condition or results of operations.
We may incur material product liability claims, which could increase our costs and adversely affect our reputation, revenues and operating income.
As a retailer, distributor and manufacturer of products designed for human consumption, we are subject to product liability claims if the use of our products is alleged to have resulted in injury. Our products consist of vitamins, minerals, herbs and other ingredients that are classified as foods or dietary supplements and are not subject to pre-market regulatory approval in the United States. Our products could contain contaminated substances, and some of our products contain ingredients that do not have long histories of human consumption. Previously unknown adverse reactions resulting from human consumption of these ingredients could occur.
In addition, third-party manufacturers produce many of the products we sell. We rely on these manufacturers to ensure the integrity of their ingredients and formulations. As a distributor of products manufactured by third parties, we may also be liable for various product liability claims for products we do not manufacture. Although our purchase agreements with our third-party vendors typically require the vendor to indemnify us to the extent of any such claims, any such indemnification is limited by its terms. Moreover, as a practical matter, any such indemnification is dependent on the creditworthiness of the indemnifying party and its insurer, and the absence of significant defenses by the insurers. We may be unable to obtain full recovery from the insurer or any indemnifying third-party in respect of any claims against us in connection with products manufactured by such third-party.
We have been and may be subject to various product liability claims, including, among others, that our products include inadequate instructions for use or inadequate warnings concerning possible side effects and interactions with other substances. See Item 3, "Legal Proceedings."
Even with adequate insurance and indemnification, product liability claims could significantly damage our reputation and consumer confidence in our products. Our litigation expenses could increase as well, which also could have a material adverse effect on our results of operations even if a product liability claim is unsuccessful or is not fully pursued.
19
We may experience product recalls, which could reduce our sales and margin and adversely affect our results of operations.
We may be subject to product recalls, withdrawals or seizures if any of the products we formulate, manufacture or sell are believed to cause injury or illness or if we are alleged to have violated governmental regulations in the manufacturing, labeling, promotion, sale or distribution of such products.
As is common in our industry, we rely on our third-party vendors to ensure that the products they manufacture and sell to us comply with all applicable regulatory and legislative requirements as well as the integrity of ingredients and proper formulation. In general, we seek representations and warranties, indemnification and/or insurance from our vendors. However, even with adequate insurance and indemnification, any claims of non-compliance could significantly damage our reputation and consumer confidence in our products, and could materially and adversely affect the market price of our common stock. In addition, the failure of such products to comply with the representations and warranties regarding such products that we receive from our third-party vendors, including compliance with applicable regulatory and legislative requirements, could prevent us from marketing the products or require us to recall or remove such products from the market, which in certain cases could materially and adversely affect our business, financial condition and results of operation. In the past, due to frequently changing consumer preferences in the dietary supplement space, we have offset losses related to recalls and removals with reformulated or alternative products; however, there can be no assurance that we would be able to offset all or any portion of losses related to any future removal or recall. As a result of the indeterminable level of product substitution and reformulated product sales, we cannot reliably determine the potential impact of any such recall or removal on our business, financial condition or results of operation.
Our operations are subject to environmental and health and safety laws and regulations that may increase our cost of operations or expose us to environmental liabilities.
Our operations are subject to environmental and health and safety laws and regulations, and some of our operations require environmental permits and controls to prevent and limit pollution of the environment. We could incur significant costs as a result of violations of, or liabilities under, environmental laws and regulations, or to maintain compliance with such environmental laws, regulations or permit requirements. For example, in March 2008, the South Carolina Department of Health and Environmental Control (the "DHEC") requested that we investigate contamination associated with historical activities at our South Carolina facility. These investigations have identified chlorinated solvent impacts in soils and groundwater that extend offsite from our facility. We entered into a Voluntary Cleanup Contract with the DHEC regarding the matter on September 24, 2012. Pursuant to such contract, we have completed additional investigations with the DHEC's approval and the DHEC is currently reviewing the results. We will consult with the DHEC on the next steps in the work after their review of the results of the investigation is complete. At this stage of the investigation, however, it is not possible to estimate the timing and extent of any remedial action that may be required, the ultimate cost of remediation, or the amount of our potential liability.
In addition to the foregoing, we are subject to numerous federal, state, local and foreign environmental and health and safety laws and regulations governing our operations, including the handling, transportation and disposal of our non-hazardous and hazardous substances and wastes, as well as emissions and discharges from its operations into the environment, including discharges to air, surface water and groundwater. Failure to comply with such laws and regulations could result in costs for remedial actions, penalties or the imposition of other liabilities. New laws, changes in existing laws or the interpretation thereof, or the development of new facts or changes in their processes could also cause us to incur additional capital and operating expenditures to maintain compliance with environmental laws and regulations and environmental permits. We also are subject to laws and regulations that impose liability and cleanup responsibility for releases of hazardous substances into the environment without regard to fault or knowledge about the condition or action causing the liability. Under certain of these laws and regulations, such liabilities can be imposed for cleanup of previously owned or operated properties, or for properties to which substances or wastes that were sent in connection with current or former operations at its facilities. The presence of contamination from such substances or wastes could also adversely affect our ability to sell or lease our properties, or to use them as collateral for financing.
We are not insured for a significant portion of our claims exposure, which could materially and adversely affect our operating income and profitability.
We have procured insurance independently for the following areas: (1) general liability; (2) product liability; (3) directors and officers liability; (4) network security and privacy liability; (5) property losses; (6) workers' compensation; and (7) various other areas. In addition, although we believe that we will continue to be able to obtain insurance in these areas in the future, because of increased selectivity by insurance providers, we may only be able to obtain such insurance at increased rates and/or with reduced coverage levels. Furthermore, we are self-insured for other areas, including: (1) physical damage to our vehicles for field personnel use; and (2) physical damages that may occur at company-owned stores. We are not insured for some property and casualty risks due to the frequency and severity of a loss, the cost of insurance and the overall risk analysis. In addition, we carry product liability insurance coverage that requires us to pay deductibles/retentions with primary and excess liability coverage above the retention amount. Because of our deductibles and self-insured retention amounts, we have significant exposure to fluctuations in the number and severity of claims. We currently maintain product liability insurance with a retention of $4.0 million per claim with an aggregate
20
cap on retained loss of $10.0 million. We could raise our deductibles/retentions, which would increase our already significant exposure to expense from claims. If any claim exceeds our coverage, we would bear the excess expense, in addition to our other self-insured amounts. If the frequency or severity of claims or our expenses increase, our operating income and profitability could be materially and adversely affected.
Because we rely on our manufacturing operations to produce a significant amount of the products we sell, disruptions in our manufacturing system or losses of manufacturing certifications could adversely affect our sales and customer relationships.
Our manufacturing operations produced approximately 25% of the products we sold in each of the years ended December 31, 2017 and 2016. Other than powders, chewables and liquids, nearly all of our proprietary products are produced in our manufacturing facility located in Greenville, South Carolina. In 2017, our largest vendor supplied approximately 10% of our raw materials. However, in the event any of our third-party suppliers or vendors becomes unable or unwilling to continue to provide raw materials in the required volumes and quality levels or in a timely manner, we would be required to identify and obtain acceptable replacement supply sources. If we are unable to identify and obtain alternative supply sources in a timely manner or at all, our business could be adversely affected. Any significant disruption in our operations at our Greenville, South Carolina facility for any reason, including regulatory requirements, an FDA determination that the facility is not in compliance with the cGMP regulations, the loss of certifications, power interruptions, fires, hurricanes, war or other force of nature, could disrupt our supply of products, adversely affecting our sales and customer relationships.
An increase in the price and shortage of supply of key raw materials could adversely affect our business.
Our products are composed of certain key raw materials. If the prices of these raw materials were to increase significantly, the prices our contract manufacturers and third-party manufacturers charge us for our GNC-branded products and third-party products could increase significantly and we may not be able to pass on such increases to our customers. A significant increase in the price of raw materials that cannot be passed on to customers could have a material adverse effect on our results of operations and financial condition. In addition, if we no longer are able to obtain products from one or more of our suppliers on terms reasonable to us or at all, our revenues could suffer. Events such as the threat of political or social unrest, or the perceived threat thereof, may also have a significant impact on raw material prices and transportation costs for our products. In addition, the interruption in supply of certain key raw materials essential to the manufacturing of our products may have an adverse impact on our suppliers' ability to provide us with the necessary products needed to maintain our customer relationships and an adequate level of sales.
Difficulties with our vendors may adversely impact our business.
Our performance depends on our ability to purchase products at sufficient levels and at competitive prices from vendors who can deliver products in a timely and efficient manner and in compliance with our vendor standards and all applicable laws and regulations. We currently have a large number of vendor relationships. Generally, we do not have any long-term purchase agreements or other contractual assurances of continued supply, pricing or access to new products, and any vendor could discontinue selling to us at any time. Historically, we have not relied on any single vendor for our products and have not had difficulties replacing vendors for various products we sell. However, in the future there is no assurance that we will continue to be able to acquire desired products in sufficient quantities or on terms acceptable to us, or be able to develop relationships with new vendors to replace any discontinued vendors. Our inability to acquire suitable products in the future or our failure to replace any one or more vendors may have a material adverse effect on our business, results of operations and financial condition. In addition, any significant change in the payment terms that we have with our suppliers could adversely affect our liquidity.
Many of our suppliers are small firms that produce a limited number of items. These smaller vendors generally have limited resources, production capacities and operating histories, and some of our vendors have limited the distribution of their products in the past. Accordingly, these vendors may be susceptible to cash flow issues, downturns in economic conditions, production difficulties, quality control issues and difficulty delivering agreed-upon quantities on schedule and in compliance with regulatory requirements. If a vendor fails to deliver on its commitments, whether due to financial difficulties or other reasons, we could experience products out-of-stocks that could lead to lost sales. In addition, although we generally have charge-back privileges in our vendor agreements, there is no assurance that we would be able, if necessary, to return product to these vendors, obtain refunds of our purchase price or obtain reimbursement or indemnification from any of our vendors should we so desire, and from time to time, we may be in litigation with one or more vendors. Many of these suppliers require extensive advance notice of our requirements in order to supply products in the quantities we need. This long lead time requires us to place orders far in advance of the time when certain products will be offered for sale, exposing us to shifts in consumer demand and discretionary spending.
Other supplier problems that we could face include product shortages, excess supply, risks related to the terms of our contracts with suppliers, risks associated with contingent workers, supplier financial weaknesses, inability of suppliers to borrow funds in the credit markets, disputes with suppliers and risks related to our relationships with single source suppliers, as described
21
above. Given the importance of third-party suppliers to our business, if any of these risks materializes, our ability to obtain raw materials and products and our results of operations may suffer.
A significant disruption to our distribution network, our network or communication systems or to the timely receipt of inventory could adversely impact sales and operations or increase our transportation costs, which would decrease our profits.
We rely on our ability to replenish depleted inventory in our stores through deliveries to our distribution centers from vendors and then from the distribution centers or direct ship vendors to our stores by various means of transportation, including shipments by sea and truck. Unexpected delays in those deliveries or increases in transportation costs (including through increased fuel costs) could significantly decrease our ability to make sales and earn profits. In addition, labor shortages in the transportation industry or long-term disruptions to the national and international transportation infrastructure that lead to delays or interruptions of deliveries could negatively affect our business.
In addition, our network and communications systems are dependent on third-party providers and are vulnerable to system interruption and damage, which could limit our ability to operate our business and could have a material adverse effect on our business, financial condition or results of operations. Our systems and operations and those of our third-party internet service providers are vulnerable to damage or interruption from fire, flood, earthquakes, power loss, server failure, telecommunications and internet service failure, acts of war or terrorism, computer viruses and denial-of-service attacks, physical or electronic breaches, sabotage, human error and similar events. Any of these events could lead to system interruptions, including the nonavailability or nonfunctionality of our website, processing and order fulfillment delays and loss of critical data for us, our suppliers or our internet service providers, and could prevent us from processing customer purchases. Because we are dependent on third-party service providers for the implementation and maintenance of certain aspects of our systems and operations, which may be outside of our control, we may not be able to remedy such interruptions in a timely manner, if at all. Accordingly, any computer, internet, network or system disruptions could have a a material adverse effect on our business, financial condition or results of operations.
If we fail to protect our brand name, competitors may adopt trade names that dilute the value of our brand name, and prosecuting or defending infringement claims could cause us to incur significant expenses or prevent us from manufacturing, selling or using some aspect of our products, which could adversely affect our revenues and market share.
We have invested significant resources to promote our GNC brand name in order to obtain the public recognition that we have today. Because of the differences in foreign trademark laws concerning proprietary rights, our trademarks may not receive the same degree of protection in foreign countries as they do in the United States. Also, we may not always be able to successfully enforce our trademarks against competitors or against challenges by others. For example, we are currently engaged in trademark disputes in foreign jurisdictions over "GNC", "LIVE WELL" and other similar trademarks and trademark applications. Our failure to successfully protect our trademarks could diminish the value and effectiveness of our past and future marketing efforts and could cause customer confusion. This could in turn adversely affect our revenues, profitability and the market price of our common stock.
We are currently and may in the future be subject to intellectual property litigation and infringement claims, which could cause us to incur significant expenses or prevent us from manufacturing, selling or using some aspect of our products. Claims of intellectual property infringement also may require us to enter into costly royalty or license agreements. However, we may be unable to obtain royalty or license agreements on terms acceptable to us or at all. Claims that our technology or products infringe on intellectual property rights could be costly and would divert the attention of management and key personnel, which in turn could adversely affect our revenues and profitability.
A substantial amount of our revenue is generated from our franchisees, and our revenues could decrease significantly if our franchisees do not conduct their operations profitably or if we fail to attract new franchisees.
Our franchise operations generated approximately 18% of our revenues in each of the years ended December 31, 2017 and 2016. Our revenues from franchise stores depend on the franchisees' ability to operate their stores profitably and adhere to our franchise standards. The closing of franchise stores or the failure of franchisees to comply with our policies could adversely affect our reputation and could reduce the amount of our franchise revenues. These factors could have a material adverse effect on our revenues and operating income.
If we are unable to attract new franchisees or to convince existing franchisees to open additional stores, any growth in royalties from franchise stores will depend solely upon increases in revenues at existing franchise stores. In addition, our ability to open additional franchise locations is limited by the territorial restrictions in our existing franchise agreements as well as our ability to identify additional markets in the United States and other countries. If we are unable to open additional franchise locations, we will have to sustain additional growth internally by attracting new and repeat customers to our existing locations.
22
Franchisee support of our marketing and advertising programs is critical to our success.
The support of our franchisees is critical for the success of our marketing programs and other strategic initiatives we seek to undertake, and the successful execution of these initiatives will depend on our ability to maintain alignment with our franchisees. While we can mandate certain strategic initiatives through enforcement of our franchise agreements, we need the active support of our franchisees if the implementation of these initiatives is to be successful. In addition, our efforts to build alignment with franchisees may result in a delay in the implementation of our marketing and advertising programs and other key initiatives. Although we believe that our current relationships with our franchisees are generally good, there can be no assurance that our franchisees will continue to support our marketing programs and strategic initiatives. The failure of our franchisees to support our marketing programs and strategic initiatives could adversely affect our ability to implement our business strategy and could materially harm our business, results of operations and financial condition.
Our franchisees are independent operators and we have limited influence over their operations.
Our revenues substantially depend upon our franchisees' sales volumes, profitability and financial viability. However, our franchisees are independent operators and we cannot control many factors that impact the profitability of their stores. Pursuant to the franchise agreements, we can, among other things, mandate signage, equipment and hours of operation, establish operating procedures and approve suppliers, distributors and products. However, the quality of franchise store operations may be diminished by any number of factors beyond our control. Consequently, franchisees may not successfully operate stores in a manner consistent with our standards and requirements or standards set by federal, state and local governmental laws and regulations. In addition, franchisees may not hire and train qualified managers and other personnel. While we ultimately can take action to terminate franchisees that do not comply with the standards contained in our franchise agreements, any delay in identifying and addressing problems could harm our image and reputation, and our franchise revenues and results of operations could decline.
Franchise regulations could limit our ability to terminate or replace underperforming franchises, which could adversely impact franchise revenues.
Our franchise activities are subject to federal, state and international laws regulating the offer and sale of franchises and the governance of our franchise relationships. These laws impose registration, extensive disclosure requirements and bonding requirements on the offer and sale of franchises. In some jurisdictions, the laws relating to the governance of our franchise relationship impose fair dealing standards during the term of the franchise relationship and limitations on our ability to terminate or refuse to renew a franchise. We may, therefore, be required to retain an underperforming franchise and may be unable to replace the franchisee, which could adversely impact franchise revenues. In addition, we cannot predict the nature and effect of any future legislation or regulation on our franchise operations.
We have limited influence over the decision of franchisees to invest in other businesses or incur excessive indebtedness.
Our franchisees are independent operators and, therefore, we have limited influence over their ability to invest in other businesses or incur excessive indebtedness. In some cases, these franchisees have used the cash generated by their stores to expand their other businesses or to subsidize losses incurred by such businesses. Additionally, as independent operators, franchisees do not require our consent to incur indebtedness. Consequently, our franchisees have in the past, and may in the future, experience financial distress as a result of over leveraging. To the extent that our franchisees use the cash from their stores to subsidize their other businesses or experience financial distress, due to over-leverage or otherwise, it could negatively affect (1) our operating results as a result of delayed or reduced payments of royalties, advertising fund contributions and rents for properties we lease to them, (2) our future revenue, earnings and cash flow growth and (3) our financial condition. In addition, lenders that are adversely affected by franchisees who default on their indebtedness may be less likely to provide current or prospective franchisees necessary financing on favorable terms or at all.
Economic, political and other risks associated with our international operations could adversely affect our revenues and international growth prospects.
As of December 31, 2017, we had 228 company-owned Canadian stores, 11 company-owned The Health Store stores located in Ireland, 5 company-owned stores located in China, and 1,999 international franchise locations in approximately 50 international countries (including distribution centers where retail sales are made). As part of our business strategy, we intend to expand our international franchise presence. Our international operations are subject to a number of risks inherent to operating in foreign countries, and any expansion of our international operations will increase the effects of these risks. These risks include, among others:
•political and economic instability of foreign markets;
•foreign governments' restrictive trade policies;
•inconsistent product regulation or sudden policy changes by foreign agencies or governments;
23
•the imposition of, or increase in, duties, taxes, government royalties or non-tariff trade barriers;
•difficulty in collecting international accounts receivable and potentially longer payment cycles;
•difficulty of enforcing contractual obligations of foreign franchisees;
•increased costs in maintaining international franchise and marketing efforts;
•problems entering international markets with different cultural bases and consumer preferences;
•compliance with laws and regulations applicable to international operations, such as the Foreign Corrupt Practices Act and regulations promulgated by the Office of Foreign Asset Control;
•fluctuations in foreign currency exchange rates; and
•operating in new, developing or other markets in which there are significant uncertainties regarding the interpretation, application and enforceability of laws and regulations relating to contract and intellectual property rights.
Any of these risks could have a material adverse effect on our international operations and our growth strategy.
We may be unable to successfully expand our operations into new international markets.
If the opportunity arises, we may expand our operations into new and high-growth international markets. However, there is no assurance that we will expand our operations in such markets in our desired time frame. To expand our operations into new international markets, we may enter into business combination transactions, make acquisitions or enter into strategic partnerships, joint ventures or alliances, any of which may be material. We may enter into these transactions to acquire other businesses or products to expand our products or take advantage of new developments and potential changes in the industry. Our lack of experience operating in new international markets and our lack of familiarity with local economic, political and regulatory systems could prevent us from achieving the results that we expect on our anticipated time frame or at all. If we are unsuccessful in expanding into new or high-growth international markets, it could adversely affect our operating results and financial condition.
We expect to close the Securities Purchase Agreement and the associated China joint venture described below under the risk factor titled "There can be no assurance that the conditions to the Securities Purchase Agreement with Harbin Pharmaceutical Group Holdings Co., Ltd. will be satisfied or that a satisfactory definitive agreement to enter into a China joint venture will be reached" in the second half of 2018. However, there is no assurance that the Securities Purchase Agreement will close or that the China JV will be successful.
We must successfully maintain and/or upgrade our information technology systems, and our failure to do so could have a material adverse effect on our business, financial condition or results of operations.
We rely on various information technology systems to manage our operations. Over the last several years, we have implemented, and we continue to implement, modifications and upgrades to such systems, including changes to legacy systems, replacing legacy systems with successor systems with new functionality, and acquiring new systems with new functionality. These types of activities subject us to inherent costs and risks associated with replacing and changing these systems, including impairment of our ability to fulfill customer orders, potential disruption of our internal control structure, substantial capital expenditures, additional administration and operating expenses, retention of sufficiently skilled personnel to implement and operate the new systems, demands on management time and other risks and costs of delays or difficulties in transitioning to or integrating new systems into our current systems. These implementations, modifications and upgrades may not result in productivity improvements at a level that outweighs the costs of implementation, or at all. In addition, the difficulties with implementing new technology systems may cause disruptions in our business operations and have a material adverse effect on our business, financial condition or results of operations.
Privacy protection is increasingly demanding, and we may be exposed to risks and costs associated with security breaches, data loss, credit card fraud and identity theft that could cause us to incur unexpected expenses and loss of revenue as well as other risks.
The protection of customer, employee, vendor, franchisee and other business data is critical to us. Federal, state, provincial and international laws and regulations govern the collection, retention, sharing and security of data that we receive from and about our employees, customers, vendors and franchisees. The regulatory environment surrounding information security and privacy has been increasingly demanding in recent years, and may see the imposition of new and additional requirements by states and the federal government as well as foreign jurisdictions in which we do business. Compliance with these requirements may result in cost increases due to necessary systems changes and the development of new processes to meet these requirements by us and our franchisees. In addition, customers and franchisees have a high expectation that we will adequately protect their personal information. If we or our service provider fail to comply with these laws and regulations or experience a significant breach of
24
customer, employee, vendor, franchisee or other company data, our reputation could be damaged and result in an increase in service charges, suspension of service, lost sales, fines or lawsuits.
The use of credit payment systems makes us more susceptible to a risk of loss in connection with these issues, particularly with respect to an external security breach of customer information that we or third parties (including those with whom we have strategic alliances) under arrangements with us control. A significant portion of our sales require the collection of certain customer data, such as credit card information. In order for our sales channel to function, we and other parties involved in processing customer transactions must be able to transmit confidential information, including credit card information, securely over public networks. In the event of a security breach, theft, leakage, accidental release or other illegal activity with respect to employee, customer, vendor, franchisee third-party, with whom we have strategic alliances or other company data, we could become subject to various claims, including those arising out of thefts and fraudulent transactions, and may also result in the suspension of credit card services. This could cause consumers to lose confidence in our security measures, harm our reputation as well as divert management attention and expose us to potentially unreserved claims and litigation. Any loss in connection with these types of claims could be substantial. In addition, if our electronic payment systems are damaged or cease to function properly, we may have to make significant investments to fix or replace them, and we may suffer interruptions in our operations in the interim. In addition, we are reliant on these systems, not only to protect the security of the information stored, but also to appropriately track and record data. Any failures or inadequacies in these systems could expose us to significant unreserved losses, which could materially and adversely affect our earnings and the market price of our common stock. Our brand reputation would likely be damaged as well.
General economic conditions, including a prolonged weakness in the economy, may affect consumer purchases, which could adversely affect our sales and the sales of our business partners.
Our results, and those of our business partners to whom we sell, are dependent on a number of factors impacting consumer spending, including general economic and business conditions; consumer confidence; wages and employment levels; the housing market; consumer debt levels; availability of consumer credit; credit and interest rates; fuel and energy costs; energy shortages; taxes; general political conditions, both domestic and abroad; and the level of customer traffic within department stores, malls and other shopping and selling environments. Consumer product purchases, including purchases of our products, may decline during recessionary periods. A prolonged downturn or an uncertain outlook in the economy may materially adversely affect our business, revenues and profits and the market price of our common stock.
Natural disasters (whether or not caused by climate change), unusually adverse weather conditions, pandemic outbreaks, terrorist acts and global political events could cause permanent or temporary distribution center or store closures, impair our ability to purchase, receive or replenish inventory or cause customer traffic to decline, all of which could result in lost sales and otherwise adversely affect our financial performance.
The occurrence of one or more natural disasters, such as hurricanes, fires, floods and earthquakes (whether or not caused by climate change), unusually adverse weather conditions, pandemic outbreaks, terrorist acts or disruptive global political events, such as civil unrest in countries in which our suppliers are located, or similar disruptions could adversely affect our operations and financial performance. To the extent these events result in the closure of one or more of our distribution centers, a significant number of stores, a manufacturing facility or our corporate headquarters, or impact one or more of our key suppliers, our operations and financial performance could be materially adversely affected through an inability to make deliveries to our stores and through lost sales. In addition, these events could result in increases in fuel (or other energy) prices or a fuel shortage, delays in opening new stores, the temporary lack of an adequate work force in a market, the temporary or long-term disruption in the supply of products from some local and overseas suppliers, the temporary disruption in the transport of goods from overseas, delay in the delivery of goods to our distribution centers or stores, the temporary reduction in the availability of products in our stores and disruption to our information systems. These events also could have indirect consequences, such as increases in the cost of insurance, if they were to result in significant loss of property or other insurable damage. We estimate that Hurricanes Harvey, Irma and Maria impacted our 2017 same store sales adversely by 0.2%.
Our holding company structure makes us dependent on our subsidiaries for our cash flow and subordinates the rights of our stockholders to the rights of creditors of our subsidiaries in the event of an insolvency or liquidation of any of our subsidiaries.
Holdings is a holding company and, accordingly, substantially all of our operations are conducted through its subsidiaries. Holdings' subsidiaries are separate and distinct legal entities. As a result, Holdings' cash flow depends upon the earnings of its subsidiaries. In addition, Holdings depends on the distribution of earnings, loans or other payments by its subsidiaries. Holdings' subsidiaries have no obligation to provide it with funds for its payment obligations. If there is an insolvency, liquidation or other reorganization of any of Holdings' subsidiaries, Holdings' stockholders will have no right to proceed against their assets. Creditors of those subsidiaries will be entitled to payment in full from the sale or other disposal of the assets of those subsidiaries before Holdings, as a stockholder, would be entitled to receive any distribution from that sale or disposal.
25
There can be no assurance that the conditions to the Securities Purchase Agreement with Harbin Pharmaceutical Group Holdings Co., Ltd. will be satisfied or that a satisfactory definitive agreement to enter into a China joint venture will be reached.
On February 13, 2018, we entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) with Harbin Pharmaceutical Group Holdings Co., Ltd. (the “Investor”), pursuant to which we agreed to issue and sell to the Investor, and the Investor agreed to purchase, 299,950 shares of a newly created series of convertible perpetual preferred stock of the Company designated as “Series A Convertible Preferred Stock” (the “Convertible Preferred Stock”), for a purchase price of $1,000 per share, or an aggregate of approximately $300 million. In addition, the Securities Purchase Agreement provides for the parties to use their respective reasonable best efforts to negotiate in good faith definitive documentation with respect to a commercial joint venture in China (the “China JV”) pursuant to which, among other things, the joint venture would be granted an exclusive right to use our trademarks and manufacture and distribute our products in China (excluding Hong Kong, Taiwan and Macau). The joint venture would be controlled 65% by the Investor and 35% by the Company.
Consummation of the sale of the Convertible Preferred Stock is subject to several conditions set forth in the Securities Purchase Agreement, including without limitation (a) approval by our stockholders, (b) no governmental entity having prohibited or made illegal the transactions contemplated by the Securities Purchase Agreement, (c) customary anti-trust approvals, (d) entry into the China JV, (e) accuracy or representations and warranties and compliance with covenants set forth in the Stock Purchase Agreement, (f) consummation of a refinancing of our Existing Credit Agreement on terms reasonably satisfactory to the Investor, (g) appointment of certain individuals selected by the Investor to the Company’s Board of Directors and (h) certain customary filings with the Secretary of State of Delaware and the NYSE. In addition, entry into the China JV will require the satisfactory negotiation and execution of definitive agreement between the Company and the Investor.
There can be no assurance that the conditions to the Securities Purchase Agreement will be satisfied or that a satisfactory definitive agreement to enter into the China JV will be reached. If these transactions are not consummated, we will not achieve the expected benefits thereof and will be subject to the risks described above, any of which could affect our share price and future business and financial results. In addition, delays in consummating these transactions or the failure to consummate these transactions at all may result in our incurring significant additional costs in connection with such delay or termination of the Securities Purchase Agreement, significant diversion of management’s time and attention from other business matters, and/or failing to achieve the anticipated benefits associated with these transactions.
Item 1B. UNRESOLVED STAFF COMMENTS.
None.
26
Item 2. PROPERTIES.
As of December 31, 2017, there were 8,955 GNC store locations globally (including distribution centers where retail sales are made). In our U.S. and Canada segment substantially all of our stores are located on leased premises that typically range in size from 1,000 to 2,000 square feet. Most all of our domestic franchisees are located on premises we lease and then sublease to our respective franchisees. All of our franchise stores in the international markets are owned or leased directly by our franchisees. No single store is material to our operations. The table below presents our consolidated stores by location in the U.S. and international countries as of December 31, 2017.
Location | Company-Owned | Domestic Franchise | International Franchise* | ||||||||
Alabama | 36 | 15 | Argentina | 2 | |||||||
Alaska | 17 | — | Aruba | 1 | |||||||
Arizona | 74 | 3 | Australia | 1 | |||||||
Arkansas | 24 | 4 | Bahrain | 6 | |||||||
California | 283 | 131 | Bangladesh | 1 | |||||||
Colorado | 74 | 5 | Bolivia | 30 | |||||||
Connecticut | 45 | 2 | Brunei | 3 | |||||||
Delaware | 17 | 3 | Bulgaria | 9 | |||||||
District of Columbia | 7 | 1 | Cayman Islands | 2 | |||||||
Florida | 279 | 110 | Chile | 182 | |||||||
Georgia | 111 | 37 | Costa Rica | 25 | |||||||
Hawaii | 27 | — | Dominican Republic | 3 | |||||||
Idaho | 12 | 3 | Ecuador | 1 | |||||||
Illinois | 120 | 53 | El Salvador | 15 | |||||||
Indiana | 64 | 18 | Guam | 3 | |||||||
Iowa | 29 | 5 | Guatemala | 61 | |||||||
Kansas | 31 | 5 | Honduras | 6 | |||||||
Kentucky | 42 | 7 | Hong Kong | 89 | |||||||
Louisiana | 49 | 14 | India | 49 | |||||||
Maine | 9 | — | Indonesia | 58 | |||||||
Maryland | 67 | 22 | Latvia | 1 | |||||||
Massachusetts | 81 | 4 | Lebanon | 11 | |||||||
Michigan | 85 | 36 | Lithuania | 1 | |||||||
Minnesota | 61 | 21 | Malaysia | 83 | |||||||
Mississippi | 24 | 16 | Mexico | 631 | |||||||
Missouri | 57 | 11 | Mongolia | 7 | |||||||
Montana | 7 | 3 | Montenegro | 2 | |||||||
Nebraska | 9 | 13 | Myanmar | 1 | |||||||
Nevada | 32 | 11 | Nigeria | 10 | |||||||
New Hampshire | 18 | 6 | Oman | 6 | |||||||
New Jersey | 99 | 45 | Pakistan | 7 | |||||||
New Mexico | 23 | 2 | Panama | 16 | |||||||
New York | 208 | 48 | Paraguay | 3 | |||||||
North Carolina | 125 | 22 | Peru | 38 | |||||||
North Dakota | 9 | — | Philippines | 43 | |||||||
Ohio | 118 | 39 | Qatar | 8 | |||||||
Oklahoma | 27 | 17 | Romania | 7 | |||||||
Oregon | 36 | 3 | Russia | 17 | |||||||
Pennsylvania | 151 | 34 | Saudi Arabia | 61 | |||||||
Rhode Island | 15 | — | Singapore | 61 | |||||||
South Carolina | 47 | 21 | South Africa | 154 | |||||||
South Dakota | 7 | 4 | South Korea | 157 | |||||||
Tennessee | 55 | 24 | Sri Lanka | 1 | |||||||
Texas | 145 | 223 | Taiwan | 50 | |||||||
Utah | 38 | 6 | Thailand | 34 | |||||||
Vermont | 4 | — | Trinidad | 9 | |||||||
Virginia | 95 | 29 | Turks & Caicos | 2 | |||||||
Washington | 65 | 15 | UAE | 19 | |||||||
West Virginia | 22 | 6 | Ukraine | 1 | |||||||
Wisconsin | 65 | 2 | Vietnam | 11 | |||||||
Wyoming | 9 | — | |||||||||
Puerto Rico | 36 | — | |||||||||
Military bases in other U.S. territories | 5 | — | |||||||||
U.S. Subtotal | 3,195 | 1,099 | |||||||||
Canada | 228 | — | |||||||||
Ireland | 11 | — | |||||||||
China | 5 | — | |||||||||
Total | 3,439 | 1,099 | Total | 1,999 | |||||||
* Includes distribution centers where retail sales are made. | |||||||||||
27
In our Manufacturing / Wholesale segment, there are 2,418 GNC franchise "store-within-a-store" locations under our strategic alliance with Rite Aid.
In addition to the above, we own and lease the following locations to support our store operations:
Location | Approximate Square Footage (in 000s) | Own or Lease | |||
Corporate Headquarters: | |||||
Pittsburgh, PA | 253 | Own | |||
Nutra Manufacturing: | |||||
Greenville, SC (1) | 280 | Own | |||
Distribution Centers: | |||||
Anderson, SC (1) | 813 | Own | |||
Indianapolis, IN | 343 | Lease | |||
Leetsdale, PA | 217 | Lease | |||
Phoenix, AZ | 112 | Lease | |||
Other Locations / Offices: | |||||
Boston, MA | 2 | Own | |||
Tustin, CA | 2 | Lease | |||
Mississauga, Ontario | 5 | Lease | |||
Dublin, Ireland | <7 | Lease | |||
Shanghai, China | 1 | Lease | |||
(1) We manufacture approximately half of our proprietary products at our manufacturing facility in Greenville, South Carolina. The Anderson, South Carolina location is used for packaging, materials receipt, lab testing, warehousing and distribution. Both the Greenville and Anderson facilities are leased on a long-term basis pursuant to "fee-in-lieu-of-taxes" arrangements with the counties in which the facilities are located, but we retain the right to purchase each of the facilities at any time during the lease for $1.00, subject to a loss of property tax benefits. The land and building of these facilities are recorded within property and equipment on our Consolidated Balance Sheet.
Our manufacturing facility is used by the Manufacturing / Wholesale segment. Distribution centers are used by all of our segments. Retail stores are used by the U.S. and Canada and International segments depending upon location.
Item 3. LEGAL PROCEEDINGS.
DMAA/Aegeline Claims. Prior to December 2013, we sold products manufactured by third parties that contained derivatives from geranium known as 1.3-dimethylpentylamine/ dimethylamylamine/ 13-dimethylamylamine, or "DMAA," which were recalled from our stores in November 2013, and/or Aegeline, a compound extracted from bael trees. As of December 31, 2017 we were named in the following 32 personal injury lawsuits involving products containing DMAA and/or Aegeline:
• | Susan Straub individually and as Administratrix of the Estate of Shane Staub v. USPlabs, LLC and General Nutrition Holdings, Inc, Common Pleas Court of Philadelphia County, Pennsylvania (Case No. 140502403), filed May 20, 2014 |
• | Justin Carolyne, et al. v. USPlabs, LLC, GNC Corporation, et al. Superior Court of California, County of Los Angeles (Case No. BC508212), filed May 22, 2013 |
• | Jeremy Reed, Timothy Anderson, Dan Anderson, Nadia Black, et al. v. USPlabs, LLC, et al., GNC, Superior Court for California, County of San Diego (Case No. 37-2013-00074052-CU-PL-CTL), filed November 1, 2013 |
• | Kenneth Waikiki v. USPlabs, LLC, Doyle, Geissler, USPlabs OxyElite, LLC, et al. and GNC Corporation, et al., United States District Court for the District of Hawaii (Case No. 3-00639 DMK), filed November 21, 2013 |
• | Nicholas Akau v. USPlabs, LLC, GNC Corporation, et al., United States District Court for the District of Hawaii (Case No. CV 14-00029), filed January 23, 2014 |
• | Melissa Igafo v. USPlabs, LLC, GNC Corporation, et al., United States District Court for the District of Hawaii (Case No. CV 14-00030), filed January 23, 2013 |
• | Calvin Ishihara v. USPlabs, LLC, GNC Corporation, et al., United States District Court for the District of Hawaii (Case No. CV 14-00031), filed January 23, 2014 |
28
• | Gaye Anne Mattson v. USPlabs, LLC, GNC Corporation, et al., United States District for the District of Hawaii (Case No. CV 14-00032), filed January 23, 2014 |
• | Thomas Park v. GNC Holdings, Inc., USPlabs, LLC, Superior Court of California, County of San Diego (Case No. 37-2014-110924), filed September 8, 2014 |
• | Nicholas Olson, Adrian Chavez, Rebecca Fullerton, Robert Gunter, Davina Maes and Edwin Palm v. GNC Corporation, USPlabs, LLC, Superior Court of California, County of Orange (Case No. 2014-00740258) filed August 18, 2014 |
• | Mereane Carlisle, Charles Paio, Chanelle Valdez, Janice Favella and Christine Mariano v. USPlabs, LLC et al., United states District Court for the District of Hawaii (Case No. CV14-00029), filed January 23, 2014 |
• | Nichole Davidson, William Dunlao, Gina Martin, Lee Ann Miranda, Yuka Colescott, Sherine Cortinas, and Shawna Nishimoto v. GNC Corporation and USPlabs, LLC, United States District Court for the District of Hawaii (Case No. 14-cv-00364) filed October 24, 2014 |
• | Rodney Ofisa, Christine Mosca, Margaret Kawamoto as guardian for Jane Kawamoto (a minor), Ginny Pia, Kimberlynne Tom, Faituitasi Tuioti, Ireneo Rabang, and Tihane Laupola v. GNC Corporation and USPlabs, LLC, United States District Court for the District of Hawaii (Case No. CV14-00365) filed October 24, 2014 |
• | Palani Pantohan, Deborah Cordiero, J. Royal Kanamu, Brent Pascula, Christie Shiroma, Justan Chun, Kasey Grace and Adam Miyasato v. USPlabs, LLC. et al., United States District Court for the District of Hawaii (Case No. CV14-00366) filed August 15, 2014 |
• | Keahi Pavao, Derek Kamiya, as personal representative of the Estate of Sonnette Marras, Gary Powell, on behalf of and as conservator for M.P.C.F.S.M., a minor child, R.P.O.C.S.S.M., a minor child, M.P.C.I.H.S.M., a minor child, M.K.C.S.M., a minor child, Michael Soriano, and Lance Taniguchi v. USPlabs, LLC, et al. United States District Court for the District of Hawaii (Case No. 14-cv-00367) filed October 24, 2014 |
• | Kai Wing Tsui and John McCutchen v. GNC Corporation, USPlabs, LLC, Superior Court of California, County of Los Angeles (Case No. BC559542), filed October 6, 2014 |
• | Dennis Balila, Melinda Jean Collins, Janice Samson, Mia Fagley, Clayton Goo, Joliana Kurtz and Mae Kwan v. USPlabs, LLC et al., California Superior Court, San Diego County (Case No. 37-2015-00008455), filed March 13, 2015 |
• | Cuong Bahn, Ismael Flores, Chue Xiong, Leilani Groden, Trudy Jenkins, and Mary Hess v. USPlabs, LLC et al., California Superior Court, Orange County (Case No. 30-2015-00776749), filed March 12, 2015 |
• | Alexis Billones, Austin Ashworth, Karen Litre, Nancy Murray, Wendy Ortiz, Edward Pullen, and Corazon Vu v. USPlabs, LLC et al., California Superior Court, Los Angeles County (Case No. BC575264), filed March 13, 2015 |
• | Asofiafia Morales, Richard Ownes, Lynn Campbell, Joseph Silzgy, Delphone Smith-Dean, Nicole Stroud, Barrett Mincey and Amanda Otten v. USPlabs, LLC et al., California Superior Court, Los Angeles County (Case No. BC575262), filed March 13, 2015 |
• | Laurie Nadura, Angela Abril-Guthmiller, Sarah Rogers, Jennifer Apes, Ellen Beedie, Edmundo Cruz, and Christopher Almanza v. USPlabs, LLC et al., California Superior Court, Monterey County (Case No. M131321), filed March 13, 2015 |
• | Cynthia Novida, Demetrio Moreno, Mee Yang, Tiffone Parker, Christopher Tortal, David Patton and Raymond Riley v. USPlabs, LLC et al., California Superior Court, San Diego County (Case No. 37-2015-00008404), filed March 13, 2015 |
• | Johanna Stussy, Lai Uyeno, Gwenda Tuika-Reyes, Zeng Vang, Kevin Williams, and Kristy Williams v. USPlabs, LLC, et al., California Superior Court, Santa Clara County (Case No. 115CV78045), filed March 13, 2015 |
• | Natasiri Tali, Tram Dobbs, Mauela Reyna-Perez, Kimberly Turvey, Meagan Van Dyke, Hang Nga Tran, Shea Steard, and Jimmy Tran v. USPlabs, LLC et al., California Superior Court, Los Angeles County (Case No. BC575263), filed March 13, 2015 |
29
• | Issam Tnaimou, Benita Rodriguez, Marcia Rouse, Marcel Macy, Joseph Worley, Joanne Zgrezepski, Crystal Franklin, Deanne Fry, and Caron Jones, in her own right, o/b/h Joshua Jones and o/b/o The Estate of James Jones v. USPlabs, LLC et al., California Superior Court, Monterey County (Case No. M131322), filed March 13, 2015 |
• | Kuulei Hirota v. USPlabs, LLC et al., First Circuit Court, State of Hawaii (Case No. 15-1-0847-05), filed May 1, 2015 |
• | Roel Vista v. USPlabs, LLC, GNC Corporation et al., California Superior Court, County of Santa Clara (Case No. CV-14-0037), filed January 24, 2014 |
• | Larry Tufts v. USPlabs, LLC, GNC Corporation et al., Court of Common Pleas for the County of Jasper, South Carolina (Case No. 2016-CP-27-0257), filed June 16, 2016 |
• | Dominic Little, David Blake Allen, Jeff Ashworth, Naomi Book and Stanley Book as Conservators of the Estate of Justin Book, Martin Sanchez, John Bainter, Rich Wolnik, Brian Norris, Joseph Childs, Jimi Hernandez and Novallie Hill v. USPlabs, LLC, et al., California Superior Court, Los Angeles County (Case No. BC534065), filed January 23, 2014 |
• | David Ramirez, Michelle Sturgill, Joseph losefa, Yanira Bernal, Jacob Michels, Cynthia Gaona and Tamara Gandara v. USPlabs, LLC, et al., California Superior Court Orange County (Case No. 30-2015-00783256-CU-PL-CXC), filed April 16, 2015 |
• | Thad Estrada v. USPlabs, LLC, et al., United States District Court for the District of Hawaii (Case No. CV-15-00228), filed June 17, 2016 |
• | Calwin Williams v. USPlabs, LLC, et al., Circuit Court of Jackson County, State of Missouri at Independence (Case No. 1716-CV-23399), filed September 28, 2017 |
The proceedings associated with the majority of these personal injury cases, which generally seek indeterminate money damages, are in the early stages, and any liabilities that may arise from these matters are not probable or reasonably estimable at this time.
We are contractually entitled to indemnification by our third-party vendor with regard to these matters, although our ability to obtain full recovery in respect of any such claims against us is dependent upon the creditworthiness of our vendor and/or its insurance coverage and the absence of any significant defenses available to its insurer.
Other Legal Proceedings. For additional information regarding certain legal proceedings to which we are a party, see Item 8, "Financial Statements and Supplementary Data," Note 11, "Commitments and Contingencies."
Item 4. MINE SAFETY DISCLOSURES
This Item 4 is not applicable.
30
PART II
Item 5. | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF SECURITIES. |
Market Information
Since March 31, 2011, our common stock has been traded on the NYSE under the symbol "GNC." As of February 22, 2018, there were 83,662,233 shares of common stock outstanding, the closing price of our common stock was $4.05 per share, and we had 26 stockholders of record (including 21 holders of restricted stock).
The following table presents the high and low sales prices and dividend declared by quarter for the common stock, as reported by the NYSE:
2017 quarter ended | High | Low | Dividend per Share | ||||||||
March 31 | $ | 11.45 | $ | 6.95 | $ | — | |||||
June 30 | $ | 9.20 | $ | 6.65 | $ | — | |||||
September 30 | $ | 10.95 | $ | 7.74 | $ | — | |||||
December 31 | $ | 8.91 | $ | 3.35 | $ | — | |||||
2016 quarter ended | High | Low | Dividend per Share | ||||||||
March 31 | $ | 32.74 | $ | 23.13 | $ | 0.20 | |||||
June 30 | $ | 35.90 | $ | 23.23 | $ | 0.20 | |||||
September 30 | $ | 28.11 | $ | 18.92 | $ | 0.20 | |||||
December 31 | $ | 22.32 | $ | 10.29 | $ | 0.20 | |||||
2015 quarter ended | High | Low | Dividend per Share | ||||||||
March 31 | $ | 49.66 | $ | 41.43 | $ | 0.18 | |||||
June 30 | $ | 49.06 | $ | 40.93 | $ | 0.18 | |||||
September 30 | $ | 51.69 | $ | 39.65 | $ | 0.18 | |||||
December 31 | $ | 43.09 | $ | 22.64 | $ | 0.18 | |||||
Dividends
In February 2017, the Board of Directors approved our recommendation to suspend the quarterly dividend. The dividend suspension is part of a broader plan to utilize a greater portion of our free cash to reduce debt.
31
Issuer Purchases of Equity Securities
Period (1) | Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs (2) | Dollar Value of Shares That May Yet be Purchased Under the Plan or Program | |||||||||
October 1 to October 31, 2017 | — | $ | — | — | $ | 197,795,011 | |||||||
November 1 to November 30, 2017 | — | $ | — | — | $ | 197,795,011 | |||||||
December 1 to December 31, 2017 | — | $ | — | — | $ | 197,795,011 | |||||||
Total | — | $ | — | — | |||||||||
(1) Other than as set forth in the table above, we made no purchases of shares of Class A common stock for the quarter ended December 31, 2017.
(2) In August 2015, the Board approved a $500.0 million multi-year repurchase program in addition to the $500.0 million multi-year program approved in August 2014, bringing the aggregate share repurchase program to $1.0 billion of Holdings' common stock. As of December 31, 2017, $197.8 million remains available for purchase under the program.
32
Stock Performance Graph
The graph below matches GNC Holdings, Inc.'s cumulative 5-Year total shareholder return on common stock with the cumulative total returns of the S&P 500 index and the S&P 500 Retail index. The graph tracks the performance of a $100 investment in our common stock and in each index (with the reinvestment of all dividends) from December 31, 2012 to December 31, 2017.
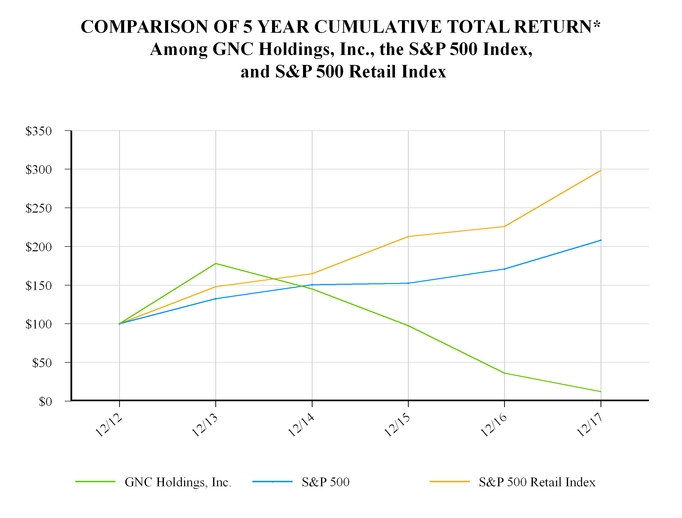
*$100 invested on December 31, 2012 in stock or index, including reinvestment of dividends.
Fiscal year ending December 31.
Copyright© 2017 Standard & Poor's, a division of S&P Global. All rights reserved
Fiscal year ending December 31.
Copyright© 2017 Standard & Poor's, a division of S&P Global. All rights reserved
33
Item 6. SELECTED FINANCIAL DATA.
The selected consolidated financial data presented below as of December 31, 2017 and 2016 and for the years ended December 31, 2017, 2016 and 2015 are derived from our audited Consolidated Financial Statements and Footnotes included in this Annual Report. The selected consolidated financial data presented below as of December 31, 2015, 2014 and 2013 and for the years ended December 31, 2014 and 2013 are derived from our audited Consolidated Financial Statements and Footnotes not included in this Annual Report. The Consolidated Financial Statements for the year ended December 31, 2017 were impacted significantly by long-lived asset impairment charges, the convertible debt exchange and enacted tax reform legislation. The Consolidated Financial Statements for the year ended December 31, 2016 were significantly impacted by long-lived asset impairment charges and gains on refranchising.
34
You should read the following financial information together with the information under Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our audited Consolidated Financial Statements and related notes in Item 8, "Financial Statements and Supplementary Data."
As of and for the Year ended December 31, | |||||||||||||||||||
(in millions, except per share data) | 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||
Statement of Operations Data: (1) | |||||||||||||||||||
Revenue | $ | 2,453.0 | $ | 2,540.0 | $ | 2,683.3 | $ | 2,655.0 | $ | 2,664.3 | |||||||||
Cost of sales, including warehousing, distribution and occupancy | 1,653.0 | 1,679.9 | 1,698.7 | 1,674.8 | 1,673.8 | ||||||||||||||
Gross profit | 800.0 | 860.1 | 984.6 | 980.2 | 990.5 | ||||||||||||||
Selling, general and administrative | 603.6 | 575.2 | 567.3 | 554.8 | 533.7 | ||||||||||||||
Gains on refranchising | (0.4 | ) | (19.1 | ) | (7.6 | ) | (9.9 | ) | (2.7 | ) | |||||||||
Long-lived asset impairments | 457.8 | 476.6 | 28.3 | — | — | ||||||||||||||
Other (income) loss net (2) | (0.5 | ) | 0.4 | 3.5 | (4.2 | ) | (1.0 | ) | |||||||||||
Operating (loss) income | (260.5 | ) | (173.0 | ) | 393.1 | 439.5 | 460.5 | ||||||||||||
Interest expense, net | 64.2 | 60.4 | 50.9 | 46.7 | 53.0 | ||||||||||||||
Gain on convertible debt and debt refinancing costs | (11.0 | ) | — | — | — | — | |||||||||||||
(Loss) Income before income taxes | (313.7 | ) | (233.4 | ) | 342.2 | 392.8 | 407.5 | ||||||||||||
Income tax (benefits) expense | (164.8 | ) | 52.9 | 122.9 | 136.9 | 142.5 | |||||||||||||
Net (loss) income | $ | (148.9 | ) | $ | (286.3 | ) | $ | 219.3 | $ | 255.9 | $ | 265.0 | |||||||
Weighted average shares outstanding: | |||||||||||||||||||
Basic | 68.8 | 69.4 | 83.9 | 90.5 | 96.5 | ||||||||||||||
Diluted | 68.8 | 69.4 | 84.2 | 90.9 | 97.4 | ||||||||||||||
(Loss) Earnings per share: | |||||||||||||||||||
Basic | $ | (2.16 | ) | $ | (4.12 | ) | $ | 2.61 | $ | 2.83 | $ | 2.75 | |||||||
Diluted | $ | (2.16 | ) | $ | (4.12 | ) | $ | 2.60 | $ | 2.81 | $ | 2.72 | |||||||
Dividends declared per share | $ | — | $ | 0.80 | $ | 0.72 | $ | 0.64 | $ | 0.60 | |||||||||
Balance Sheet Data: | |||||||||||||||||||
Cash and cash equivalents | $ | 64.0 | $ | 34.5 | $ | 56.5 | $ | 133.8 | $ | 226.2 | |||||||||
Working capital (3)(4) | 478.1 | 478.6 | 504.3 | 628.4 | 715.2 | ||||||||||||||
Total assets (4) | 1,516.6 | 2,055.8 | 2,543.5 | 2,670.6 | 2,734.8 | ||||||||||||||
Total current and non-current long-term debt | 1,297.0 | 1,540.5 | 1,449.2 | 1,337.9 | 1,341.8 | ||||||||||||||
Stockholders' (deficit) equity | (162.0 | ) | (95.0 | ) | 468.6 | 756.0 | 815.6 | ||||||||||||
Statement of Cash Flows: | |||||||||||||||||||
Net cash provided by operating activities | $ | 220.5 | $ | 208.2 | $ | 354.5 | $ | 303.8 | $ | 239.4 | |||||||||
Net cash used in investing activities | (23.8 | ) | (22.4 | ) | (45.6 | ) | (75.5 | ) | (78.3 | ) | |||||||||
Net cash used in financing activities | (168.1 | ) | (207.5 | ) | (384.5 | ) | (321.0 | ) | (94.3 | ) | |||||||||
Capital expenditures | 32.1 | 59.6 | 45.8 | 70.5 | 50.2 | ||||||||||||||
(1) | Figures may not sum due to rounding |
(2) | In 2017, other income principally related to $1.2 million of foreign currency gains and $1.0 million related to insurance and lease settlements, partially offset by a $1.7 million loss attributed to the sale of substantially all of the assets of the Lucky Vitamin e-commerce business. |
In 2016, other loss principally related to $0.4 million of foreign currency losses.
In 2015, other loss principally related to a $2.7 million loss on sale of Discount Supplements and $0.8 million of foreign currency losses.
35
In 2014, other income principally related to a $4.4 million reversal of a contingent purchase price liability partially offset by $0.2 million of foreign currency losses.
In 2013, other income principally related to a $1.0 million reversal of a contingent purchase price liability.
(3) | Defined as current assets less current liabilities. |
(4) | Includes the adoption of ASU 2015-17 relating to the presentation of deferred tax assets and liabilities as non-current on the balance sheet. The company reclassified current deferred income tax assets formerly presented within total current assets as a reduction to deferred income taxes presented within total long-term liabilities on the Consolidated Balance Sheet for 2016, 2015, 2014, and 2013 of $12.8 million, $10.9 million, $7.6 million, and $3.8 million, respectively. The reclassification in 2016 also included a $0.1 million increase to other long-term assets. |
36
The following table summarizes our stores for the periods indicated:
Year Ended December 31, | ||||||||||||||
2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||
U.S. & Canada | ||||||||||||||
Company-owned (a): | ||||||||||||||
Beginning of period balance | 3,513 | 3,584 | 3,487 | 3,332 | 3,178 | |||||||||
Store openings | 59 | 69 | 115 | 183 | 170 | |||||||||
Acquired franchise stores (b) | 60 | 21 | 44 | 25 | 16 | |||||||||
Franchise conversions (c) | (2 | ) | (102 | ) | (33 | ) | (25 | ) | (9 | ) | ||||
Store closings | (207 | ) | (59 | ) | (29 | ) | (28 | ) | (23 | ) | ||||
End of period balance | 3,423 | 3,513 | 3,584 | 3,487 | 3,332 | |||||||||
Domestic Franchise: | ||||||||||||||
Beginning of period balance | 1,178 | 1,084 | 1,070 | 1,012 | 949 | |||||||||
Store openings | 29 | 33 | 32 | 70 | 74 | |||||||||
Acquired franchise stores (b) | (60 | ) | (21 | ) | (44 | ) | (25 | ) | (16 | ) | ||||
Franchise conversions (c) | 2 | 102 | 33 | 25 | 9 | |||||||||
Store closings | (50 | ) | (20 | ) | (7 | ) | (12 | ) | (4 | ) | ||||
End of period balance | 1,099 | 1,178 | 1,084 | 1,070 | 1,012 | |||||||||
International (d) | ||||||||||||||
Beginning of period balance | 1,973 | 2,095 | 2,150 | 2,034 | 1,840 | |||||||||
Store openings (e) | 243 | 108 | 144 | 208 | 325 | |||||||||
Store closings (f) | (201 | ) | (230 | ) | (199 | ) | (92 | ) | (131 | ) | ||||
End of period balance | 2,015 | 1,973 | 2,095 | 2,150 | 2,034 | |||||||||
Store-within-a-store (Rite Aid): | ||||||||||||||
Beginning of period balance | 2,358 | 2,327 | 2,269 | 2,215 | 2,181 | |||||||||
Store openings | 70 | 41 | 59 | 60 | 41 | |||||||||
Store closings | (10 | ) | (10 | ) | (1 | ) | (6 | ) | (7 | ) | ||||
End of period balance | 2,418 | 2,358 | 2,327 | 2,269 | 2,215 | |||||||||
Total Stores | 8,955 | 9,022 | 9,090 | 8,976 | 8,593 | |||||||||
_______________________________________________________________________________
(a) | Includes Canada. |
(b) | Stores that were acquired from franchisees and subsequently converted into company-owned stores. |
(c) | Company-owned store locations sold to franchisees. |
(d) | Includes franchise locations in approximately 50 countries (including distribution centers where sales are made) and company-owned stores located in Ireland (The Health Store) and China. |
(e) | In 2017, store openings include 145 stores-within-a-store in South Africa not formerly included in the store count. Effective at the end of the third quarter of 2017, these stores were subject to royalties on retail sales and as a result, have been included in the store count. |
(f) | In 2017, store closings include 68 store-within-a-store locations in Peru which did not contribute significantly to revenue. |
37
Item 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
You should read the following discussion in conjunction with Item 6, "Selected Financial Data" and our audited Consolidated Financial Statements and the related notes included in Item 8, "Financial Statements and Supplementary Data." The discussion in this section contains forward-looking statements that involve risks and uncertainties. See Part I, Item 1A, "Risk Factors" in this Annual Report for a discussion of important factors that could cause actual results to differ materially from those described or implied by the forward-looking statements contained herein.
Overview
We are a global specialty retailer of health, wellness and performance products, including protein, performance supplements, weight management supplements, vitamins, herbs and greens, wellness supplements, health and beauty, food and drink and other general merchandise. We derive our revenues principally from: product sales through our company-owned stores; the internet primarily through our websites, GNC.com and prior to the sale of its assets on September 30, 2017, LuckyVitamin.com, as well as third-party websites; domestic and international franchise activities; and sales of products manufactured in our facility to third parties. We sell products through a worldwide network of approximately 9,000 locations operating under the GNC brand name.
We believe the competitive strengths that position us as a leader in the specialty nutritional supplement space include our: well-recognized brand; stable base of long-term customers; geographically diverse store base; vertically integrated operations and differentiated service model designed to enhance the customer experience.
Our Current Strategy
Prior to 2017, we had been experiencing declining traffic trends leading to decreasing same store sales in our retail stores. After extensive consumer research and market analysis we determined that our business model needed to be reimagined. Listening to the customer and addressing their key issues led to the development and launch of the One New GNC in December 2016. Customers responded to the simplified pricing model, enhanced loyalty programs and product innovation during 2017 driving positive comparable store sales in the fourth quarter of 2017 of 5.7%, including GNC.com.
Management has been focused on the following key areas to move the business forward:
• | Loyalty programs. As of December 31, 2017, we had 11.4 million members in our myGNC Rewards program, of which 0.8 million members were enrolled in our PRO Access program. We see opportunity to leverage this extensive customer relationship management database in the more future to more efficiently serve our customers. |
• | Proprietary products and innovation capabilities. We believe that product innovation is critical to our growth, brand image superiority and competitive advantage. Through market research, interactions with customers and partnerships with leading industry vendors, we work to identify shifting consumer trends that can inform our product development process. We believe that our brand portfolio of proprietary products, which are available in our stores, on GNC.com, on our market place on Amazon.com and other third-party websites, advances GNC's brand presence and our general reputation as a leading retailer of health and wellness products. |
We recently launched Slimvance, a natural weight loss product that delivers clinically proven results, in stores in December 2017 with the official launch in January 2018. Slimvance, along with a suite of complimentary products that are part of our BodyDynamics product line. Slimvance is the first of several new, exclusive products planned for this year and we are pleased with the results to date.
• | Customer experience. We are continuing to work on improvements in product availability and the in-store shopping experience. We are investing in our online and omnichannel capabilities to better meet consumer demand without regard to the place and time a customer is interested in GNC. We believe that developing the capability to leverage all of our sales channels to deliver a consistent and high-quality customer experience will differentiate us from other competitors, particularly online-only options. Our store base is a competitive advantage over online-only competitors especially as we continue to develop our in-store associates to deliver thoughtful assistance throughout the shopping experience. Specifically, we have been focused on the following: |
◦ | We made significant changes in our e-commerce pricing and promotion strategy in 2016, which eliminated channel conflict and bulk sales. These changes allowed GNC to successfully launch on Amazon in January 2017. In addition, the recently completed re-platforming of the website from a third-party to a cloud-based solution provides more flexibility and control for new features and enhancements including advanced personalization capability, improved merchandising and opportunity for omnichannel expansion, which is creating the ability to better optimize the e-commerce business; |
38
◦ | Technology, training and incentives for store associates have been refocused on loyalty, building basket, and growing our proprietary GNC brand. For example, tablets can now be used by store associates to enroll customers into the loyalty programs and facilitate product recommendations supporting customer regimens. In addition, in-store subscription services were piloted in December 2017 with a full rollout to all domestic corporate stores expected in the first half of 2018. In addition, other initiatives including single sign-on functionality, accessibility to membership rewards on GNC.com, improved online content and buy online/ship to store, are being developed and are expected to be available at varying points during 2018; and |
◦ | Supply chain focus has been reducing overall enterprise inventory including lower weeks of supply while maintaining in-stocks. Our focus has been to reduce lead time, strengthen our forecasting practices and optimize our product assortment at the store level. Additional focus has also been placed on consistency of in-stocks across all channels and testing store service improvements. Our inventory decreased from $583.2 million at December 31, 2016 to $506.9 million at December 31, 2017. |
Key Performance Indicators
The primary key performance indicators that senior management focuses on include revenue and operating income for each segment, which are discussed in detail within "Results of Operations", as well as same store sales growth.
As fully defined below, we have clarified the definition of same store sales in the first quarter of 2017, which now excludes sales from our membership programs, including the Gold Card program discontinued in the U.S. in December 2016 and the new loyalty PRO Access program launched in connection with the One New GNC. Same store sales now include only product sales. The table below presents the key components of same store sales.
2017 | 2016 | ||||||||||||||||||||||||||||
U.S. Company-Owned Same Store Sales, including GNC.com | Q1 3/31 | Q2 6/30 | Q3 9/30 | Q4 12/31 | YTD 12/31 | Q1 3/31 | Q2 6/30 | Q3 9/30 | Q4 12/31 | YTD 12/31 | |||||||||||||||||||
Total same store sales | (3.9 | )% | (0.9 | )% | 1.3 | % | 5.7 | % | 0.2 | % | (2.3 | )% | (3.9 | )% | (8.6 | )% | (11.3 | )% | (6.3 | )% | |||||||||
Drivers of same store sales: | |||||||||||||||||||||||||||||
Number of transactions | 9.3 | % | 12.3 | % | 12.4 | % | 11.7 | % | 11.6 | % | (4.1 | )% | (5.5 | )% | (6.6 | )% | (6.5 | )% | (5.6 | )% | |||||||||
Average transaction amount | (12.1 | )% | (11.8 | )% | (9.9 | )% | (5.4 | )% | (10.2 | )% | 1.8 | % | 1.7 | % | (2.2 | )% | (5.2 | )% | (0.8 | )% | |||||||||
Contribution to same store sales: | |||||||||||||||||||||||||||||
Domestic retail same store sales | (3.6 | )% | (0.5 | )% | (1.2 | )% | 0.2 | % | (1.4 | )% | (1.9 | )% | (3.4 | )% | (6.5 | )% | (6.6 | )% | (4.5 | )% | |||||||||
GNC.com contribution to same store sales | (0.3 | )% | (0.4 | )% | 2.5 | % | 5.5 | % | 1.6 | % | (0.4 | )% | (0.5 | )% | (2.1 | )% | (4.7 | )% | (1.8 | )% | |||||||||
Total same store sales | (3.9 | )% | (0.9 | )% | 1.3 | % | 5.7 | % | 0.2 | % | (2.3 | )% | (3.9 | )% | (8.6 | )% | (11.3 | )% | (6.3 | )% | |||||||||
Same store sales for company-owned stores include point-of-sale retail sales from all domestic stores which have been operating for twelve full months following the opening period. We are an omnichannel retailer with capabilities that allow a customer to use more than one channel when making a purchase, including in-store and through e-commerce channels, which include our wholly-owned website GNC.com and third-party websites, including Amazon (the sales from which are included in the GNC.com business unit) where product assortment and price are controlled by us, in which purchases are fulfilled by direct shipment to the customer from one of our distribution facilities as well as third-party e-commerce vendors. In-store sales are reduced by sales originally consummated online or through mobile devices and subsequently returned in-store. Sales of membership programs, including the new PRO Access loyalty program and former Gold Card program, which is no longer offered in the U.S., as well as the net change in the deferred points liability associated with the myGNC Rewards program, are excluded from same store sales.
Same store sales are calculated on a daily basis for each store and exclude the net sales of a store for any period if the store was not open during the same period of the prior year. When a store’s square footage has been changed as a result of reconfiguration or relocation in the same mall or shopping center, the store continues to be treated as a same store. If, during the period presented, a store was closed, relocated to a different mall or shopping center, or converted to a franchise store or a company-owned store, sales from that store up to and including the closing day or the day immediately preceding the relocation or conversion are included as same store sales as long as the store was open during the same period of the prior year. Corporate stores are included in same store sales after the thirteenth month following a relocation or conversion to a company-owned store.
39
We also provide retail comparable same stores sales of our franchisees as well as our Canada business if meaningful to current results. While retail sales of franchisees are not included in the Consolidated Financial Statements, the metric serves as a key performance indicator of our franchisees, which ultimately impacts wholesale sales and royalties and fees received from franchisees. We compute same store sales for our franchisees and Canada business consistent with the description of corporate same store sales above. Same store sales for international franchisees and Canada exclude the impact of foreign exchange rate changes relative to the U.S. dollar.
Non-GAAP Measures
We have included operating (loss) income for our reportable segments below under "Results of Operations" adjusted to exclude the impact of the long-lived asset impairments and gains on refranchising because we believe it represents an effective supplemental means by which to measure our segment’s operating performance. We believe that this metric is useful to investors as it enables our management and our investors to evaluate and compare our segment’s results from operations in a more meaningful and consistent manner by excluding specific items that are not reflective of ongoing operating results. However, this metric is not a measurement of our segment’s performance under GAAP and should not be considered as
an alternative to net income, operating income or any other performance measures derived in accordance with GAAP, or as an alternative to GAAP cash flow from operating activities, or as a measure of our profitability or liquidity.
40
Results of Operations
The following information presented was derived from our audited Consolidated Financial Statements and accompanying notes included in Item 8, "Financial Statements and Supplementary Data."
(Expressed as a percentage of total consolidated revenue)
Year ended December 31, | ||||||||
2017 (*) | 2016 (*) | 2015 (*) | ||||||
Revenues: | ||||||||
U.S. and Canada | 81.3 | % | 81.0 | % | 80.8 | % | ||
International | 7.2 | % | 6.3 | % | 6.8 | % | ||
Manufacturing / Wholesale: | ||||||||
Intersegment revenues | 9.4 | % | 8.6 | % | 10.0 | % | ||
Third Party | 8.8 | % | 9.3 | % | 8.8 | % | ||
Subtotal Manufacturing / Wholesale | 18.2 | % | 17.9 | % | 18.8 | % | ||
Other | 2.7 | % | 3.4 | % | 3.6 | % | ||
Elimination of intersegment revenue | (9.4 | )% | (8.6 | )% | (10.0 | )% | ||
Total net revenues | 100.0 | % | 100.0 | % | 100.0 | % | ||
Operating expenses: | ||||||||
Cost of sales, including warehousing, distribution and occupancy | 67.4 | % | 66.1 | % | 63.3 | % | ||
Gross Profit | 32.6 | % | 33.9 | % | 36.7 | % | ||
Selling, general and administrative expenses | 24.6 | % | 22.6 | % | 21.1 | % | ||
Gains on refranchising | 0.0 | % | (0.8 | )% | (0.3 | )% | ||
Long-lived asset impairments | 18.7 | % | 18.8 | % | 1.1 | % | ||
Other (income) loss, net | — | % | — | % | 0.1 | % | ||
Total operating expenses | 110.6 | % | 106.8 | % | 85.3 | % | ||
Operating (loss) income: | ||||||||
U.S. and Canada | (10.0 | )% | (4.1 | )% | 14.1 | % | ||
International | 2.5 | % | 2.2 | % | 2.4 | % | ||
Manufacturing / Wholesale | 2.0 | % | (0.8 | )% | 3.2 | % | ||
Unallocated corporate costs and other: | ||||||||
Corporate costs | (4.2 | )% | (4.1 | )% | (3.7 | )% | ||
Other | (0.8 | )% | — | % | (1.4 | )% | ||
Subtotal unallocated corporate and other costs | (5.0 | )% | (4.1 | )% | (5.1 | )% | ||
Total operating (loss) income | (10.6 | )% | (6.8 | )% | 14.7 | % | ||
Interest expense, net | 2.6 | % | 2.4 | % | 1.9 | % | ||
Gain on convertible debt and debt refinancing costs | (0.4 | )% | — | % | — | % | ||
(Loss) income before income taxes | (12.8 | )% | (9.2 | )% | 12.8 | % | ||
Income tax (benefit) expense | (6.7 | )% | 2.1 | % | 4.6 | % | ||
Net (loss) income | (6.1 | )% | (11.3 | )% | 8.2 | % | ||
(*) Figures may not sum due to rounding
41
Comparison of the Years Ended December 31, 2017 (current year) and 2016 (prior year)
Revenues
Our consolidated net revenues decreased $87.0 million, or 3.4%, to $2,453.0 million in the current year compared with $2,540.0 million in 2016. The decrease was the result of lower sales in our U.S. and Canada and Manufacturing / Wholesale segments, partially offset by higher sales in our International segment. In addition, the sale of Lucky Vitamin on September 30, 2017 contributed to the decrease in revenue, which was formerly presented in the U.S. and Canada segment and is now reflected within Other as explained in Item 8, "Financial Statements and Supplementary Data," Note 16, "Segments."
U.S. and Canada. Revenues in our U.S. and Canada segment decreased $64.6 million, or 3.1%, to $1,993.4 million in the current year compared with $2,058.0 million in 2016. E-commerce sales (which now exclude Lucky Vitamin) were 6.4% of U.S. and Canada revenue for the year ended December 31, 2017, compared with 5.2% in the year ended December 31, 2016. The $64.6 million decrease in revenue in the current year as compared with the prior year was primarily due to the following:
•The change in our loyalty programs resulted in a decrease to revenue of $35.9 million. Gold Card sales in the U.S. decreased $42.4 million, which includes the impact from the recognition of $24.4 million in deferred revenue in the first quarter of 2017, net of $1.4 million of applicable coupon redemptions. The reduction was offset by a $6.5 million net increase to revenue related to the new loyalty programs as PRO Access membership fees were partially offset by the change in the free myGNC Rewards deferred points liability;
•The decrease in the number of corporate stores from 3,513 at December 31, 2016 to 3,423 at December 31, 2017 contributed an approximate $21 million decrease to revenue;
•A decrease in domestic franchise revenue of $3.7 million to $319.8 million in the current year compared with $323.5 million in 2016 (which excludes $3.2 million of Gold Card sales associated with the change in our loyalty programs as explained above) due to the impact of a decrease in retail same store sales of 2.4% and a decrease in the number of franchise stores from 1,178 at December 31, 2016 to 1,099 at December 31, 2017;
•A year-over-year decrease in our Canada business (not currently under the One New GNC) of $6.8 million was primarily due to a reduction in same store sales of 10.9%; and
•Partially offsetting the above decreases was an increase in U.S. company-owned same store sales of 0.2%, which includes GNC.com sales, which resulted in a $5.1 million increase to revenue. GNC.com contributed 1.6% to the increase in same store sales due to increased sales through Amazon as well as the change to better align our web promotions to our stores in August 2016. Same store sales for company-owned stores decreased 1.4% in the current year due to lower sales in Protein, Vitamins, Weight Management Supplements, Food/Drink, and Wellness Supplement categories, partially offset by higher sales in the Performance Supplements, Health and Beauty, and Herbs/Greens categories. The decrease in company-owned same store sales includes an estimated 0.2% from the impact of Hurricanes Harvey, Irma and Maria.
International. Revenues in our International segment increased $16.7 million, or 10.4%, to $177.4 million in 2017 compared with $160.7 million in 2016. Revenues from our China business increased by $14.5 million in the current year compared with the prior year largely due to higher cross-border e-commerce sales. Revenue from our international franchisees increased $1.3 million in the current year compared to the prior year despite reporting a decrease in retail same store sales of 0.6%.
Manufacturing / Wholesale. Revenues in our Manufacturing / Wholesale segment, excluding intersegment revenues, decreased $19.6 million in the current year compared to the prior year. Third-party contract manufacturing sales decreased by $6.6 million, or 4.9%, to $127.9 million in the current year compared with $134.5 million in 2016 primarily due to lower demand associated with decreased sales with certain customers. Sales to our wholesale partners decreased $12.9 million, or 12.8% to $88.2 million for the year ended December 31, 2017 compared with $101.1 million in 2016 primarily due to lower demand and the termination of Drugstore.com that occurred in September 2016. Intersegment sales increased $12.7 million, or 5.8%, from $218.8 million in the prior year to $231.5 million in the current year reflecting our increasing focus on proprietary products.
Other. The sale of Lucky Vitamin in September 2017 resulted in a decrease to revenue of $19.5 million in the current year compared with the prior year, which had a very insignificant impact on operating income as explained further below.
Cost of Sales and Gross Profit
Cost of sales, which includes product costs, warehousing, distribution and occupancy costs, decreased $26.9 million, or 1.6%, to $1,653.0 million in the current year compared with $1,679.9 million in 2016. Gross profit decreased $60.1 million from $860.1 million in the prior year to $800.0 million in the current year, and as a percentage of revenue, decreased from 33.9% in the prior year to 32.6% in the current year primarily due to lower domestic retail product margin rate and occupancy expense deleverage associated with lower sales. The decrease in domestic retail product margin rate was primarily due to the impact of
42
pricing and loyalty program changes associated with the One New GNC including the impact of discontinuing the Gold Card program in the U.S., partially offset by the favorable comparative effect of prior year reserves on certain third-party and proprietary inventory and deep discounts on excess vitamins inventory nearing expiration in the first quarter of 2016.
Selling, General and Administrative ("SG&A") Expense
SG&A expense, including compensation and related benefits, advertising and other expenses, increased $28.4 million, or 4.9%, to $603.6 million in the current year compared with $575.2 million in 2016. As a percentage of revenue, SG&A expense was 24.6% in the current year compared with 22.6% in 2016. Compensation and related benefits increased $18.0 million, primarily due to higher wages and benefits for store and field associates and $3.3 million of stock-based compensation and other executive placement costs associated with the hiring of our new Chief Executive Officer, partially offset by severance expense of $4.5 million associated with the departure of our former Chief Executive Officer. Marketing expense increased by $15.1 million to support incremental online advertising and higher China sales. Partially offsetting the above increases was a decrease in other SG&A expenses of $4.7 million due to lower legal-related expenses, partially offset by higher commissions to support GNC.com sales.
Gains on Refranchising
Gains on refranchising were $0.4 million for the year ended December 31, 2017 resulting from the sale of two company-owned stores. We sold 102 company-owned stores to franchisees in the prior year, of which 84 related to one franchisee, resulting in total refranchising gains of $19.1 million.
Long-Lived Asset Impairments
We recorded $457.8 million in non-cash long-lived asset impairments, consisting of $395.6 million related to the brand name (of which $394.0 million was allocated to the U.S. and Canada segment and $1.6 million was allocated to the International segment), $24.3 million related to goodwill in our Wholesale reporting unit, $19.4 million related to Lucky Vitamin and the remaining related to property and equipment at certain of our underperforming stores.
We recorded $476.6 million in non-cash long-lived asset impairments in the prior year consisting of $471.1 million related to goodwill (Domestic Stores, Manufacturing and Canada reporting units for $366.4 million, $90.5 million and $14.2 million, respectively) and the remaining related to property and equipment.
Refer to Item 8, “Financial Statements and Supplementary Data,” Note 5, “Goodwill and Intangible Assets” and Note 6, “Property, Plant and Equipment, Net” for more information.
Other (income) loss, Net
Other income, net, in the current year of $0.5 million consisted of $1.2 million in foreign currency gains and gains of $1.0 million related to insurance and lease settlements, partially offset by a $1.7 million loss attributed to the sale of substantially all of the assets of the Lucky Vitamin e-commerce business. Other loss, net in the prior year of $0.4 million relates to foreign currency losses.
Operating Loss
As a result of the foregoing, consolidated operating loss was $260.4 million in the current year compared with operating loss of $172.9 million in 2016. Operating loss in the current and prior year was impacted significantly by non-cash long-lived asset impairment charges of $457.8 million and $476.6 million, respectively, as described above. Operating loss in the prior year was also impacted significantly by refranchising gains of $19.1 million.
U.S and Canada. Operating loss was $246.1 million in the current year compared with a loss of $104.9 million in 2016. As explained above, long-lived asset impairments were recorded in the current year and prior year, which impacted the U.S. and Canada segment by $412.5 million and $386.0 million, respectively. Excluding these items and gains on refranchising of $0.4 million and $19.1 million in the current year and prior year, respectively, operating income was $166.0 million in the current year or 8.3% of segment revenue compared with $262.0 million in the prior year or 12.7% of segment revenue.
The decrease in operating income compared with the prior year was primarily due to lower domestic retail product margin rate as explained above under "Cost of Sales and Gross Profit," higher salaries and benefits of $15.9 million associated with higher store and field wages and an increase in marketing expense of $13.7 million primarily related to incremental online advertising. Also contributing to the decrease in operating income rate was expense deleverage associated with lower revenues.
International. Operating income was $60.6 million in the current year compared with $55.4 million in 2016. As explained above, a long-lived asset impairment of $1.6 million was recorded in the current year. Excluding this non-cash impairment charge, operating income was $62.2 million, or 35.1% of segment revenue, in the current year compared with 34.5% of segment revenue in the prior year. The increase in operating income was primarily associated with our China business largely from cross-
43
border e-commerce sales, partially offset by higher marketing to support the increase in China sales and the comparative effect of a bad debt allowance associated with a franchisee that was recorded in the prior year.
Manufacturing / Wholesale. Operating income was $48.0 million in the current year compared with operating loss of $20.0 million in 2016. Operating income in the current year and operating loss in the prior year was significantly impacted by goodwill impairment charges of $24.3 million and $90.5 million, respectively. Excluding these non-cash impairment charges, operating income was $72.3 million, or 16.1% of segment revenue in the current year compared with $70.5 million, or 15.5% of segment revenue in the prior year. The increase in operating income was primarily due to higher intersegment sales, which resulted in favorable manufacturing variances.
Other. Operating loss was $20.8 million in the current year primarily due to $19.4 million of non-cash long-lived asset impairments recorded in the second quarter and $1.7 million of a loss on sale relating to the Lucky Vitamin e-commerce business.
Corporate costs. Corporate costs decreased $1.3 million to $102.1 million in the current year compared with $103.4 million in 2016 primarily due to severance expense in the prior year of $4.5 million associated with the departure of our former Chief Executive Officer and lower legal-related charges, partially offset by $3.3 million of stock-based compensation and other executive placement costs associated with the hiring of our new Chief Executive Officer in 2017.
Interest Expense
Interest expense was $64.2 million for the year ended December 31, 2017 compared with $60.4 million in 2016 primarily due to a higher interest rate on the Term Loan Facility.
Gains on convertible debt and debt refinancing costs
The convertible debt exchange resulted in a gain of $15.0 million and together with other legal, investing banking and rating agency fees resulted in a net gain of $11.0 million. Refer to Item 8, "Financial Statements and Supplementary Data," Note 7, "Long-Term Debt /Interest Expense" for more information.
Income Tax (Benefit) Expense
We recognized an income tax benefit of $164.8 million in the current year. The current year effective tax rate was significantly impacted by a $90.5 million reduction to net deferred tax liabilities, which were revalued using a lower corporate tax rate associated with Tax Cuts and Jobs Act of 2017 and, a $24.3 million goodwill impairment charge, the majority of which is not deductible for tax purposes. The tax rate was also impacted by a reduction to a valuation allowance of $3.8 million.
In the prior year, we recognized tax expense of $52.9 million. The effective tax rate in 2016 was significantly impacted by $471.1 million of goodwill impairments, the majority of which were not deductible for tax purposes. The prior year tax rate was also impacted by an increase to the valuation allowance of $4.4 million.
The valuation allowance in each period was adjusted based on a change in circumstances, including anticipated future earnings, which caused a change in judgment about the realizability of certain deferred tax assets related to net operating losses.
Net Loss
As a result of the foregoing, consolidated net loss was $148.9 million in the current year compared with net loss of $286.3 million.
Diluted Loss Per Share
Diluted loss per share was $2.16 in the current year compared with diluted loss per share of $4.12 in the prior year.
Comparison of the Years Ended December 31, 2016 and 2015
Revenues
Our consolidated net revenues decreased $143.3 million, or 5.3%, to $2,540.0 million for the year ended December 31, 2016 compared with $2,683.3 million in 2015. The decrease was the result of lower sales in our U.S. and Canada and International segments.
U.S. and Canada. Revenues in our U.S. and Canada segment decreased $110.0 million, or 5.1%, to $2,058.0 million for the year ended December 31, 2016 compared with $2,168.0 million in 2015. E-commerce sales were 5.2% of U.S. and Canada revenue for the year ended December 31, 2016, compared with 6.6% in the year ended December 31, 2015.
44
Negative domestic retail same store sales which includes corporate stores and GNC.com, resulted in a $109.1 million decrease in revenue year-over-year. Negative same store sales were primarily due to lower sales in the Vitamin, Food/Drink, Protein and Weight Management categories, partially offset by improvement in the Health and Beauty and Performance Supplements categories and include the impact of a significant decrease in our GNC.com sales due in part to a meaningful reduction in our web promotions as well as lower point-of-sale gold card sales. In addition, our total corporate stores decreased from 3,584 at December 31, 2015 to 3,513 at December 31, 2016.
Revenues from our domestic franchisees decreased by $5.2 million to $326.7 million for the year ended December 31, 2016 compared with $331.9 million in 2015. This decrease was due to the impact of our franchisees having negative retail same stores sales of 6.8% in 2016, partially offset by an increase in the number of franchise stores from 1,084 at December 31, 2015 to 1,178 at December 31, 2016. Revenue from our company-owned stores in Canada decreased $6.2 million year-over-year primarily due to negative same store sales of 7.1% and the unfavorable impact of foreign exchange rate changes.
Gold Card revenue (including the impact of deferred revenue) recognized in our company-owned U.S. stores in 2016 was $62.2 million compared with $59.2 million in 2015. The sale of Gold Cards ended on December 18, 2016 and the Gold Card Member Pricing program was discontinued in all domestic company-owned and franchise stores on December 28, 2016 in connection with the introduction of One New GNC. As a part of the launch, we provided former Gold Card customers within the one year membership period with a coupon equivalent to a reimbursement of the unexpired portion of their Gold Card membership fee. During the fourth quarter of 2016, we accelerated recognition of $4.0 million of Gold Card deferred revenue, as a portion of the coupons were redeemed relating to the pilot markets and system-wide after the launch of the One New GNC.
International. Revenues in our International segment decreased $22.3 million, or 12.2%, to $160.7 million for the year ended December 31, 2016 compared with $183.0 million in 2015. Despite our international franchisees reporting an increase in retail same store sales of 0.8% in 2016, revenue from franchisees decreased $28.1 million primarily relating to challenges in several markets as well as a net decrease in the number of franchise stores from 2,095 at December 31, 2015 to 1,973 at December 31, 2016. Partially offsetting the above decrease was an increase in revenue of $5.6 million associated with our China business.
Manufacturing / Wholesale. Revenues in our Manufacturing / Wholesale segment, excluding intersegment revenues, were flat at $235.7 million in each of the years ended December 31, 2016 and 2015. Third-party contract manufacturing sales increased by $15.6 million, or 13.2%, to $134.5 million for the year ended December 31, 2016 compared with $118.9 million in 2015. This increase was offset by a decrease in sales to our wholesale partners of $15.7 million, or 13.4% to $101.1 million for the year ended December 31, 2016 compared with $116.8 million in 2015. Intersegment sales decreased $48.6 million from $267.4 million for the year ended December 31, 2015 to $218.8 million in 2016 due to lower proprietary sales in our U.S. and Canada and International segments.
Other. Revenue decreased by $11.0 million due to the sale of Discount Supplements in the fourth quarter of 2015, partially offset by an increase of $13.1 million from Lucky Vitamin, the assets of which were sold on September 30, 2017.
Cost of Sales and Gross Profit
Cost of sales decreased $18.8 million, or 1.1%, to $1,679.9 million for the year ended December 31, 2016 compared with $1,698.7 million in 2015. Gross profit decreased $124.5 million from $984.6 million for the year ended December 31, 2016 to $860.1 million in 2015, and as a percentage of revenue, decreased from 36.7% in 2015 to 33.9% for the year ended December 31, 2016. The decrease in gross profit rate was primarily due to occupancy expense deleverage associated with negative same store sales and lower domestic retail product margin rate due to the impact of promotional pricing and reserves on certain third-party product that could not be returned to vendors as well as higher estimated reserves on certain proprietary products as a result of sales trends.
SG&A Expense
SG&A expense increased $7.9 million, or 1.4%, to $575.2 million, for the year ended December 31, 2016 compared with $567.3 million in 2015. As a percentage of revenue, SG&A expense was 22.6% for the year ended December 31, 2016 compared with 21.1% in 2015. The increase in SG&A expense was due to increases in compensation and related benefits of $11.3 million and marketing of $4.6 million, partially offset by a decrease in other SG&A expenses of $8.0 million.
The increase in compensation and related benefits of $11.3 million or 3.3% for the year ended December 31, 2016 as compared with the year ended December 31, 2015 was primarily due to severance expense of $4.5 million recorded in 2016 associated with the departure of our former Chief Executive Officer as well as higher salaries expense, the majority of which relates to store and field associates. Partially offsetting the above increases is a decrease of $2.8 million as a result of the comparative effect of the correction of an immaterial error in 2015 as further explained in Item 8, "Financial Statements," Note 2, "Basis of Presentation" as well as lower sales incentives and certain other benefits.
45
The increase in marketing expense of $4.6 million was primarily due to the launch of One New GNC in December 2016.
The decreases in other SG&A expenses of $8.0 million or 5.2% was primarily due to lower commissions associated with the lower GNC.com sales and lower legal-related charges, partially offset by the comparative effect of a decrease in bad debt expense associated with a reduction in the previously established allowance for certain of our international franchisees based on cash collected, and higher IT expenses.
Gains on Refranchising
Gains on refranchising were $19.1 million for the year ended December 31, 2016 compared with $7.6 million in 2015. We sold 102 company-owned stores to franchisees in 2016, of which 84 related to one franchisee, compared with 33 company-owned stores in 2015.
Long-Lived Asset Impairments
We recorded $471.1 million in goodwill impairments that together with charges on property and equipment resulted in $476.6 million of non-cash long-lived asset impairments in 2016. We recorded a $28.3 million impairment charge in the third quarter of 2015 relating to our Discount Supplements business which was sold in the fourth quarter of 2015. Refer to Item 8, "Financial Statements and Supplementary Data," Note 5, "Goodwill and Intangible Assets, Net" and Note 6, "Property, Plant and Equipment, Net" for more information.
Other Loss, Net
Other loss, net, in the year ended December 31, 2016 of $0.4 million relates to foreign currency losses. Other loss, net in the year ended December 31, 2015 includes a loss of $2.7 million relating to the sale of the assets of Discount Supplements and losses on foreign currency of $0.8 million.
Operating (Loss) Income
As a result of the foregoing, consolidated operating loss was $172.9 million for the year ended December 31, 2016 compared with operating income of $393.1 million in 2015. Operating (loss) income was impacted significantly by long-lived asset impairment charges and gains on refranchising for each year ended December 31, 2016 and 2015.
U.S. and Canada. Operating loss was $104.9 million for the year ended December 31, 2016 compared with income of $379.3 million in 2015. As explained above, long-lived asset impairments were recorded in 2016 in the U.S. and Canada segment totaling $386.0 million and in 2015 of $28.3 million relating to Discount Supplements. Excluding these non-cash impairment charges and gains on refranchising of $19.1 million and $7.6 million in 2016 and 2015, respectively, operating income was $262.0 million or 12.7% of segment revenue for the year ended December 31, 2016 as compared with $371.7 million or 17.1% of segment revenue in 2015. The decrease was primarily due to expense deleverage in occupancy and salaries expenses associated with a decrease in company-owned same store sales, the launch of the One New GNC (which lowered operating income by approximately $10 million) and lower domestic retail product margin rate as described above under "Cost of Sales and Gross Profit."
International. Operating income decreased $9.1 million, or 14.1%, to $55.4 million for the year ended December 31, 2016 compared with $64.5 million in 2015, and as a percentage of segment revenue was 34.5% and 35.2%, respectively. The decrease in operating income percentage was primarily due to the comparative effect of a 2015 reduction in the previously established bad debt allowance associated with certain of our franchisees as well as higher marketing and deleverage in salaries and benefits. Partially offsetting the decrease in operating income as a percentage of segment revenue was higher product margin rates due in part to a higher mix of proprietary sales.
Manufacturing / Wholesale. Operating loss was $20.0 million for the year ended December 31, 2016 compared with income of $86.2 million in 2015. As explained above, operating loss for the year ended December 31, 2016 was significantly impacted by a $90.5 million goodwill impairment. Excluding this non-cash charge, operating income was $70.5 million, or 15.5% of segment revenue, for the year ended December 31, 2016 compared with 17.1% of segment revenue in 2015. The decrease in operating income as a percentage of segment revenue was primarily due to lower intersegment sales, which resulted in unfavorable manufacturing variances, and a higher mix of third-party contract manufacturing sales, which generally contribute lower margins.
Other. Operating income increased by $38.4 million primarily due to $28.3 million of long-lived asset impairments and a $2.7 million loss on sale related to Discount Supplements in 2015.
Corporate costs. Corporate costs increased by $5.1 million, or 5.1%, to $103.4 million in the year ended December 31, 2016 compared to $98.3 million in 2015 primarily due to higher salaries expense, severance of $4.5 million related to the departure of the former Chief Executive Officer and higher IT expense, partially offset by lower legal-related charges.
46
Interest Expense
Interest expense increased $9.5 million, or 18.7%, to $60.4 million for the year ended December 31, 2016 compared with $50.9 million in 2015. The increase in interest expense was due to the convertible debt issuance in August 2015, the majority of which relates to non-cash interest expense associated with the amortization of the conversion feature, and amounts drawn under the Revolving Credit Facility, partially offset by a lower balance on the Term Loan Facility.
Income Tax Expense
We recognized $52.9 million of income tax expense for the year ended December 31, 2016 compared with $122.9 million in 2015. The tax rate for 2016 was significantly impacted by $471.1 million of goodwill impairments, the majority of which were not deductible for tax purposes. The tax rate for 2016 was also impacted by an increase to the valuation allowance of $4.4 million.
The tax rate for the year ended December 31, 2015 was impacted by a discrete tax benefit of $11.8 million due to the effect of a worthless stock deduction resulting from excess tax basis in the common shares of Discount Supplements and a decrease in the liability for uncertain tax positions of $3.3 million due in part to the expiration of certain statutes of limitation with respect to the 2011 fiscal year. The tax rate for 2015 was also impacted by an increase to the valuation allowance of $2.3 million.
The valuation allowance in each period was adjusted based on a change in circumstances, including anticipated future earnings, which caused a change in judgment about the realizability of certain deferred tax assets related to net operating losses.
Net (Loss) Income
As a result of the foregoing, we recorded a net loss of $286.3 million for the year ended December 31, 2016, compared with net income of $219.3 million in 2015.
Diluted (Loss) Earnings Per Share
Diluted loss per share was $4.12 for the year ended December 31, 2016 due to the decrease in net income partially offset by a 17.5% decrease in the weighted average diluted shares outstanding in 2016 as a result of 7.9 million shares repurchased in 2016, the significant majority of which were in the first quarter.
47
Liquidity and Capital Resources
On February 28, 2018, we amended our Senior Credit Facility, which consisted of an extension of the maturity date for $704.3 million of the $1,131.2 million Term Loan Facility from March 2019 to March 2021. However, if more than $50.0 million of our Notes have not been repaid, discharged, prepaid, refinanced or converted prior to such date (“Existing Indenture Discharge”), the maturity date becomes May 2020. The amendment (the “Amendment”) also includes:
• | the termination of the Revolving Credit Facility, which was replaced with a new $100 million asset-based Revolving Credit Facility with a maturity date of August 2022 (which maturity date will become May 2020 if the Existing Indenture Discharge has not occurred); and |
• | a $275 million asset-based Term Loan Facility advanced on a “first-in, last-out” basis with a maturity date of December 2022 (which maturity date will become May 2020 if the Existing Indenture Discharge has not occurred). |
After the effectiveness of the Amendment, the amount due in March 2019 under the Term Loan is now $151.9 million. We are also required under the Amendment to make an excess cash flow payment in March 2019, which could be material, based on the projected Consolidated Net First Lien Leverage Ratio for the year ending December 31, 2018. We expect to close the Securities Purchase Agreement described in Item 8, "Financial Statements and Supplementary Data," Note 18, "Subsequent Events" in the second half of 2018, which will result in approximately $300 million of aggregate proceeds. Under the Amendment, $100 million of the net proceeds from the Securities Purchase Agreement are required to be utilized to pay the Term Loan Facility due March 2021. The remaining net proceeds, after deducting legal and advisory fees, would be available to satisfy the $151.9 million due under the Term Loan Facility in March 2019. There is no assurance that the Securities Purchase agreement will close prior to March 2019 when the obligation is due.
In the event that Securities Purchase Agreement doesn’t close, management has concluded that the Company will have the ability to satisfy the $151.9 million Term Loan Facility and the excess cash flow payments due in March 2019 with projected cash on hand and amounts available under the new $100 million asset-backed Revolving Credit Facility. If actual results are below the Company’s projections by an amount greater than what is required to satisfy these obligations, management has the ability to reduce certain discretionary payments and will consider certain asset sales, as necessary, to maximize cash available.
The Amendment requires annual aggregate principal payments of at least $43 million related to the $704.3 million of the Term Loan Facility with a maturity of March 2021. There are no scheduled amortization payments associated with the $275.0 million asset-based Term Loan Facility and payments associated with the $151.9 million Term Loan Facility are consistent with past terms. We expect our primary uses of the cash in the near future will be for debt repayment, capital expenditures and working capital requirements.
Cash Provided by Operating Activities
Cash provided by operating activities was $220.5 million, $208.2 million and $354.5 million during the years ended December 31, 2017, 2016 and 2015 respectively. The increase in cash flow from operations in the current year compared with the prior year was primarily due to favorable working capital changes primarily within inventory resulting from supply chain optimization as explained above under "Our Current Strategy," partially offset by reduced operating performance. The decrease in cash flow from operations in 2016 as compared with 2015 was primarily due to reduced operating performance and higher inventory purchases.
Cash Used in Investing Activities
We used cash from investing activities of $23.8 million, $22.4 million and $45.6 million for the years ended December 31, 2017, 2016 and 2015 respectively, of which capital expenditures were $32.1 million, $59.6 million and $45.8 million. The decrease in capital expenditures in 2017 compared with 2016 primarily relates to the prior year investments for our strategic initiatives and IT infrastructure including new registers and tablets in our stores coupled with a focus on debt repayment in the current year. The increase in capital expenditures in 2016 compared with 2015 primarily relate to the aforementioned 2016 investments.
We completed an asset sale of Lucky Vitamin on September 30, 2017 for a purchase price of $6.4 million, net of closing fees, the proceeds of which were received in October 2017. The prior year period includes the refranchising of 102 stores for $39.2 million, which includes the sale of 84 stores to one franchisee for $28.6 million of net proceeds.
In 2018, we expect our capital expenditures to be approximately $28 million, which includes investments for store development, IT infrastructure and maintenance. We anticipate funding our 2018 capital requirements with cash flows from operations.
48
Cash Used in Financing Activities
For the year ended December 31, 2017, cash used in financing activities was $168.1 million, primarily consisting of net payments of $127.0 million under our Revolving Credit Facility. In addition, based on the results of the year ended December 31, 2016, our Consolidated Net Senior Secured Leverage Ratio required us to make an excess cash flow payment on our outstanding Term Loan Facility. On April 10, 2017, we made a payment of $39.7 million, of which $28.2 million was paid with borrowings from the Revolving Credit Facility and $11.5 million was paid with cash on hand. The excess cash flow payment described above satisfies the $1.1 million quarterly principal amount owed through the maturity of the Term Loan Facility.
For the year ended December 31, 2016, cash used in financing activities was $207.5 million, primarily consisting of the repurchase of an aggregate of $229.2 million in shares of common stock under share repurchase programs and dividends paid to our stockholders of $55.3 million, partially offset with net borrowings of $84.0 million under our Revolving Credit Facility.
For the year ended December 31, 2015, cash used in financing activities was $384.5 million, which includes the repurchase of an aggregate of $479.8 million in shares of common stock under share repurchase programs and dividends paid to our stockholders of $59.6 million. In August 2015, we issued $287.5 million principal amount of 1.5% convertible senior notes due 2020 (the "Notes") and in connection with this transaction, we paid down $164.3 million of our Term Loan Facility and paid $8.2 million of debt issuance costs. In 2015, we borrowed $43.0 million under our Revolving Credit Facility.
Indebtedness
Senior Credit Facility. Refer to "Liquidity and Capital Resources" above for information regarding the Amendment to our Senior Credit Facility in February 2018. All information regarding the Company's Senior Credit Facility within this section is as of December 31, 2017 prior to the Amendment.
General Nutrition Centers, Inc. is party to a Senior Credit Facility, consisting of the Term Loan Facility and the Revolving Credit Facility. The Senior Credit Facility permits us to prepay a portion or all of the outstanding balance without incurring penalties (except London Interbank Offering Rate ("LIBOR") breakage costs). GNC Corporation, our indirect wholly owned subsidiary ("GNC Corporation"), and Centers' existing and future domestic subsidiaries have guaranteed Centers' obligations under the Senior Credit Facility. In addition, the Senior Credit Facility is collateralized by first priority pledges (subject to permitted liens) of substantially all of the assets of Centers, including its equity interests and the equity interests of its domestic subsidiaries.
As of December 31, 2017 and 2016, the interest rate on the Senior Credit Facility was 4.1% and 3.3%, respectively. At December 31, 2017, we did not have any borrowings outstanding on its Revolving Credit Facility. We are also required to pay an annual fee of 2.75% on outstanding letters of credit and an annual commitment fee of 0.5% on the undrawn portion of the Revolving Credit Facility.
The interest rate under the Senior Credit Facility is at a rate, at our option, per annum equal to (A) or (B) as defined below.
(A) The sum of (i) the applicable margin of 1.25% with respect to borrowings under the Revolving Credit Facility and 1.5% with respect to amounts outstanding under the Term Loan Facility plus (ii) the greater of:
• | the prime rate (as publicly announced by JPMorgan Chase Bank, N.A. as its prime rate in effect); |
• | the overnight federal funds effective rate plus 0.50%; |
• | one month LIBOR plus 1.0%; or |
• | 1.75% applicable to the Term Loan Facility only. |
(B) The sum of (i) the applicable margin of 2.25% with respect to borrowings under the Revolving Credit Facility and 2.5% with respect to amounts outstanding under the Term Loan Facility plus (ii) the greater of:
• | LIBOR; or |
• | 0.75% applicable to the Term Loan Facility only. |
The Senior Credit Facility, including amendments, contains customary covenants, including incurrence covenants and certain other limitations on the ability of GNC Corporation, Centers, and Centers' subsidiaries to, among other things, make optional payments in respect of other debt instruments, pay dividends or other payments on capital stock, and enter into arrangements that restrict their ability to pay dividends or grant liens.
On March 4, 2016, we amended the Revolving Credit Facility to extend its maturity from March 2017 to September 2018 and increase total availability from $130.0 million to $300.0 million. In December 2017, we reduced the amount available under the Revolving Credit Facility from $300.0 million to $225.0 million. As of December 31, 2017, we had $219.1 million available under the Revolving Credit Facility subject to a defined leverage ratio after consideration of $5.9 million utilized to secure letters of credit and no borrowings outstanding, which was subsequently terminated and replaced with an asset-based Revolving Credit facility as described above.
49
Convertible Senior Notes. On August 10, 2015, we issued $287.5 million principal amount of Notes. The Notes will mature on August 15, 2020, unless earlier repaid, discharged, refinanced or converted by the holders subject to restrictions through May 15, 2020. The Notes bear interest at a rate of 1.5% per annum. In connection with this transaction, we paid down $164.3 million of our outstanding Term Loan Facility.
On December 20, 2017, we exchanged in privately negotiated transactions $98.9 million in aggregate principal amount of the Notes for an aggregate of 14.6 million newly issued shares of the Company’s Class A common stock, which had a value of $71.7 million at the time of the exchange. At December 31, 2017, the Company had $188.6 million of principal outstanding on the Notes.
Contractual Obligations
Refer to "Liquidity and Capital Resources" above for a description of the Amendment to the Senior Credit Facility made in February 2018. The following table summarizes our future minimum non-cancelable contractual obligations at December 31, 2017 prior the Amendment:
Payments due by period | |||||||||||||||||||
(in millions) | Total | 2018 | 2019-2020 | 2021-2022 | After 2022 | ||||||||||||||
Long-term debt obligations (1) | $ | 1,319.8 | $ | — | $ | 1,319.8 | $ | — | $ | — | |||||||||
Scheduled interest payments (2) | 74.1 | 54.4 | 19.7 | — | — | ||||||||||||||
Operating lease obligations (3) | 572.7 | 149.8 | 210.6 | 119.3 | 93.0 | ||||||||||||||
Purchase commitments (4) | 22.8 | 14.6 | 7.8 | 0.4 | — | ||||||||||||||
Total | $ | 1,989.4 | $ | 218.8 | $ | 1,557.9 | $ | 119.7 | $ | 93.0 | |||||||||
(1) | These balances consist of the following debt obligations: (a) $1,131.2 million of outstanding borrowings under the Term Loan Facility including $0.9 million of unamortized original issuance discount due in March 2019 and (b) $188.6 million of outstanding borrowings under the Notes including the unamortized conversion feature of $18.1 million and original issuance discount of $2.5 million due in August 2020. |
(2) | The interest that will accrue on the long-term obligations includes variable rate payments, which are estimated using the associated LIBOR index as of December 31, 2017. Interest under the Senior Credit Facility currently accrues based on a one-month LIBOR. |
(3) | Consists of the following contractual payments excluding optional renewals: (a) $651.5 million for company-owned retail and franchise stores; (b) $104.7 million of sublease income from franchisees; and (c) $25.9 million relating to various leases for warehouses, vehicles, and various equipment at our facility. Operating lease obligations exclude insurance, taxes, maintenance, percentage rent and other costs. These amounts are subject to fluctuation from year to year. For each of the years ended December 31, 2017, 2016 and 2015 these amounts collectively represented approximately 35% of the aggregate costs associated with our company-owned retail store operating leases. |
(4) | These balances represent amounts owed under advertising and technology-related agreements. Excludes unrecognized tax benefits of $5.8 million at December 31, 2017 because we are unable to reliably estimate the timing of any required payments. |
Impact of Inflation
Refer to Item 7A. "Quantitative and Qualitative Disclosures About Market Risk" for more information.
Off Balance Sheet Arrangements
The majority of our contractual obligations not presented on our Consolidated Balance Sheet relate to operating leases for our stores. Future scheduled lease payments under non-cancelable operating leases as of December 31, 2017 are described under the heading "Operating lease obligations" in the table above. We have not created, and are not a party to, any off-balance sheet entities for the purpose of raising capital, incurring debt or operating our business. We do not have any off-balance sheet arrangements or relationships with entities that are not consolidated into our financial statements that have or are reasonably likely to have a material current or future effect on our financial condition, changes in financial condition, revenues, expenses, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Estimates
Use of Estimates
Certain amounts in our audited Consolidated Financial Statements require the use of estimates, judgments, and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts
50
of revenues and expenses during the periods presented. Our accounting policies are described in Note 2, "Basis of Presentation and Summary of Significant Accounting Policies" to our audited Consolidated Financial Statements included in Item 8, "Financial Statements and Supplementary Data." Our critical accounting policies and estimates are described in this section. An accounting estimate is considered critical if:
•the estimate requires us to make assumptions about matters that were uncertain at the time the estimate was made;
•different estimates reasonably could have been used; or
•changes in the estimate that would have a material impact on our financial condition or our results of operations are likely to occur from period to period.
We believe that the accounting estimates used are appropriate and the resulting balances are reasonable. However, actual results could differ from the original estimates, requiring adjustments to these balances in future periods.
Allowance for Doubtful Accounts
The majority of our domestic store and e-commerce revenues are received as cash at the point of sale. The majority of our franchise and wholesale revenues are billed with varying terms for payment. An allowance for doubtful accounts is established based on the financial condition of our franchisees and other third-party customers and historical write-off experience. Our allowance for doubtful accounts was $3.9 million and $4.6 million at December 31, 2017 and 2016, respectively.
Inventory
Inventory components consist of raw materials, work-in-process, finished product and packaging supplies. Inventory includes costs associated with distribution and transportation costs, as well as manufacturing overhead, which are capitalized and expensed as merchandise is sold. Inventory is recorded on a first in/first out basis ("FIFO") at the lower of cost or net realizable value, net of obsolescence, shrinkage and vendor allowances. We regularly review our inventory levels in order to identify slow moving and short dated products, using factors such as amount of inventory on hand, the remaining shelf life, current and expected market conditions, historical trends and the likelihood of recovering the inventory costs based on anticipated demand.
Impairment of Definite-Long-Lived Assets
Fixed assets and definite-lived intangible assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset group may not be recoverable. If the sum of the undiscounted future cash flows is less than the carrying value of the asset group, we recognize an impairment loss, measured as the amount by which the carrying value exceeds the fair value of the long-lived asset. Estimates of cash flows require significant judgment and certain assumptions about future volume, revenue and expense growth rates and asset disposal values. While we make estimates based on historical experience, current expectations and assumptions that we believe are reasonable, actual results, including future cash flows, could differ from our estimates resulting in required impairment charges. We recorded $26.5 million of definite-long-lived asset impairment charges in 2017 of which $7.9 million relates the trade name and property and equipment of the Lucky Vitamin e-commerce business, the assets of which were sold on September 30 2017. The remaining amount relates to certain of our underperforming corporate stores and to a lesser extent the impact of Hurricane Maria on our stores located in Puerto Rico. We had $5.4 million impairment charge related to certain of our stores in 2016. Refer to Item 8 "Financial Statements and Supplementary Data," Note 7 "Property, Plant and Equipment, Net" for more information.
Goodwill and Indefinite-Lived Intangible Assets
As described in Item 8 "Financial Statements and Supplementary Data," Note 6 "Goodwill and Intangible Assets," based on a significant decline in our share price in the fourth quarter of 2017 and previous challenges associated with our refinancing efforts of the long term debt, we concluded a triggering event occurred in the fourth quarter requiring an impairment test of our indefinite-lived brand name intangible asset and all reporting units. The fourth quarter of 2017 impairment testing resulted in non-cash impairment charges for the brand name and goodwill of $395.6 and $24.3 million, respectively.
The fair value of the indefinite-lived brand intangible asset was estimated using a relief from royalty method (an income approach). Key assumptions included in the determination of the estimated fair value include, but are not limited to, projected future revenues, the royalty rate and the discount rate.
The following table represents a sensitivity analysis on the indefinite-lived brand intangible asset depicting the percent increase in the $395.6 million charge recorded had the fair value been estimated with a 1.0% increase in the discount rate used and a 0.5% decrease in the royalty rate used at December 31, 2017 (after current year impairment charge).
51
Assumption Change | % Increase in Impairment Charge | |
1.0% increase in discount rate | 4.5% | |
0.5% decrease in royalty rate | 17.6% | |
For the goodwill impairment test, we determined the fair values of our reporting units at December 31, 2016 using a discounted cash flow method (income approach) weighted 50% and a guideline company method (market approach) weighted 50%. The key assumptions under the income approach include, but are not limited to, future cash flow assumptions and the discount rate. The guideline company method involves analyzing transaction and financial data of publicly-traded companies to develop multiples, which are adjusted to account for differences in growth prospects and risk profiles of the reporting unit and the comparable entities.
The following table illustrates the amount of goodwill allocated to each reporting unit as well as the deficit, if any, created between the fair value and the carrying value of each reporting unit that would occur given hypothetical reductions in their respective fair values under step one at December 31, 2017 (after current year impairment charges). In connection with the adoption of ASU 2017-04, the fair value of the Wholesale reporting unit approximates its carrying value after giving consideration to the current period impairment charges recorded. Any future reductions to the fair value of this reporting unit would result in an additional impairment charge.
Goodwill | 10% | 20% | 30% | ||||||||||||
(in thousands) | |||||||||||||||
GNC.com | $ | 9,251 | $ | — | $ | — | $ | — | |||||||
International Franchise | 37,772 | — | — | — | |||||||||||
The Health Store | 5,936 | — | (541 | ) | (1,364 | ) | |||||||||
Manufacturing | 61,542 | — | (17,550 | ) | (39,786 | ) | |||||||||
Wholesale | 26,528 | (6,063 | ) | (12,388 | ) | (18,712 | ) | ||||||||
Total | $ | 141,029 | $ | (6,063 | ) | $ | (30,479 | ) | $ | (59,862 | ) | ||||
The following table represents a sensitivity analysis on goodwill for the Wholesale and Manufacturing reporting units (under the income approach without consideration to the market approach) depicting the percentage excess (deficit) of fair value compared with carrying value:
Assumption Change | Wholesale | Manufacturing | |||
1% increase in discount rate | (5.8)% | 5.4% | |||
1% decrease in long-term growth rate | (2.1)% | 9.9% | |||
Although we believe we have used reasonable estimates and assumptions to calculate the fair values of the indefinite-lived brand intangible asset and the reporting units with goodwill balances, these estimates and assumptions could be materially different from actual results. If actual market conditions are less favorable than those projected, or if events occur or circumstances change that would reduce the fair values below the respective carrying values, we may be required to conduct an interim test and possibly recognize impairment charges, which may be material, in future periods.
Leases
Many of our lease agreements contain escalation clauses under which, if fixed and determinable, rent expense is recognized on a straight-line basis over the lives of the leases, including renewal periods that are reasonably assured. Certain of our leases also contain clauses for rent to be paid as a percentage of sales, which are based on a percentage of retail sales or a percentage of retail sales in excess of stipulated amounts (contingent rent). Contingent rent is recorded as rent expense when attainment of the target is considered probable and is recognized in proportion to the retail sales contributing to the achievement of the target. We regularly review projected sales for applicable stores to determine if the target has been achieved and the extent that projected sales will exceed the target to determine the appropriate amount of contingent rent expense to record.
52
Self-Insurance
We are self-insured for certain losses related to health insurance, workers' compensation and general liability insurance and we maintain stop-loss coverage with third-party insurers to limit our liability exposure. Liabilities associated with these losses are estimated by considering historical claims experience, estimated lag time to report and pay claims, average cost per claim and other actuarial factors. Effective January 1, 2018, we are now fully insured for health coverage.
Income Taxes
We compute our annual tax rate based on the statutory tax rates and tax planning opportunities available to us in the various jurisdictions in which we earn income. Significant judgment is required in determining our annual tax rate and in evaluating uncertainty in our tax positions. We recognize a benefit for tax positions that we believe will more likely than not be sustained upon examination. The amount of benefit recognized is the largest amount of benefit that we believe has more than a 50% probability of being realized upon settlement. We regularly monitor our tax positions and adjust the amount of recognized tax benefit based on our evaluation of information that has become available since the end of our last financial reporting period.
We record valuation allowances to reduce deferred tax assets to the amount that is more likely than not to be realized. When assessing the need for valuation allowances, we consider future taxable income and ongoing prudent and feasible tax planning strategies. Should a change in circumstances lead to a change in judgment about the realizability of deferred tax assets in future years, we would adjust related valuation allowances in the period that the change in circumstances occurs. As of December 31, 2017 and 2016, we had a valuation allowance of $17.5 million and $21.3 million, respectively, principally related to certain state and foreign net operating loss carryforwards.
Recently Issued Accounting Pronouncements
Refer to Item 8, "Financial Statements and Supplementary Data," Note 2, "Basis of Presentation and Summary of Significant Accounting Policies."
53
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Market risk represents the risk of changes in the value of market risk sensitive instruments caused by fluctuations in interest rates, foreign exchange rates and commodity prices. Changes in these factors could cause fluctuations in the results of our operations and cash flows. In the ordinary course of business, we are primarily exposed to foreign currency, interest rate and fuel and certain other commodity risks. We have not entered into any derivative instruments during 2017 but we may in the future utilize derivative instruments to manage our exposure to fluctuations in fuel and certain other commodity prices, interest rates and foreign currency exchange rates.
Interest Rate Market Risk
The Senior Credit Facility is subject to changing interest rates. Although changes in interest rates do not impact our operating income, the changes could affect the fair value of such debt and related interest payments. An increase of 1% on our variable rate debt balance at December 31, 2017 would result in an increase to interest expense, net of $12.6 million for the year ended December 31, 2017, after giving effect to the 0.75% floor on the Term Loan Facility. Refer to Item 7 "Management's Discussion and Analysis of Financial Condition and Results of Operations - Liquidity" for more information. A decrease in the current interest rates would have no impact on interest expense due to an interest rate floor that exists under the Senior Credit Facility.
Foreign Currency Exchange Rate Market Risk
We are subject to the risk of foreign currency exchange rate changes in the conversion from local currencies to the U.S. dollar of the reported financial position and operating results of our non-U.S. based subsidiaries. The primary currencies to which we are exposed to fluctuations are the Canadian Dollar, the Chinese Renminbi, Hong Kong dollar, the British Pound, and the Euro. The impact of foreign exchange rate changes primarily related to the Canadian dollar resulted in an increase of approximately $2 million to U.S. and Canada segment revenue in 2017 compared with 2016. In addition, since our international franchisees pay for product sales and royalties to us in the U.S. dollar, any strengthening of the U.S. dollar relative to our franchisees' local currency could adversely impact our revenue.
We have intercompany balances with foreign entities that are routinely settled primarily relating to product sales and management fees. Gains or losses resulting from these foreign currency transactions were not material for the fiscal years ended December 31, 2017, 2016 and 2015.
Fuel Price Market Risk
We rely on our ability to replenish depleted inventory through deliveries to our distribution centers and stores by various means of transportation, including shipments by sea and truck. We are exposed to fluctuations in fuel prices in these arrangements which may have a favorable or unfavorable impact to our Consolidated Financial Statements.
Impact of Inflation
Inflationary factors such as increases in the cost of our products and overhead costs may adversely affect our operating results. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, a high rate of inflation in the future may have an adverse effect on our ability to maintain current levels of margins and SG&A expenses as a percentage of revenue if the selling prices of our products do not increase with inflation.
54
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
TABLE OF CONTENTS
Page | |
55
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of GNC Holdings, Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of GNC Holdings, Inc. and its subsidiaries as of December 31, 2017 and 2016, and the related consolidated statements of operations, comprehensive (loss) income, stockholders’ (deficit) equity and cash flows for each of the three years in the period ended December 31, 2017, including the related notes and financial statement schedules of (i) condensed financial information of GNC Holdings, Inc. as of December 31, 2017 and 2016 and for each of the three years in the period ended December 31, 2017, and (ii) valuation and qualifying accounts for each of the three years in the period ended December 31, 2017 appearing under Item 15(a)(2) (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2017 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Annual Report on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the
56
company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Pittsburgh, Pennsylvania
March 1, 2018
We have served as the Company’s auditor since 2003.
57
GNC HOLDINGS, INC. AND SUBSIDIARIES
Consolidated Balance Sheets
(in thousands, except per share amounts)
December 31, | |||||||
2017 | 2016 | ||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 64,001 | $ | 34,464 | |||
Receivables, net | 126,650 | 129,178 | |||||
Inventory (Note 3) | 506,858 | 583,212 | |||||
Prepaid and other current assets | 42,320 | 39,400 | |||||
Total current assets | 739,829 | 786,254 | |||||
Long-term assets: | |||||||
Goodwill (Note 5) | 141,029 | 176,062 | |||||
Brand name (Note 5) | 324,400 | 720,000 | |||||
Other intangible assets, net (Note 5) | 99,715 | 111,229 | |||||
Property, plant and equipment, net (Note 6) | 186,562 | 232,292 | |||||
Deferred income taxes (Note 4) | 1,737 | 78 | |||||
Other long-term assets | 23,289 | 29,927 | |||||
Total long-term assets | 776,732 | 1,269,588 | |||||
Total assets | $ | 1,516,561 | $ | 2,055,842 | |||
Current liabilities: | |||||||
Accounts payable | $ | 153,018 | $ | 179,933 | |||
Current portion of long-term debt (Note 7) | — | 12,562 | |||||
Deferred revenue and other current liabilities (Note 8) | 108,672 | 115,171 | |||||
Total current liabilities | 261,690 | 307,666 | |||||
Long-term liabilities: | |||||||
Long-term debt (Note 7) | 1,297,023 | 1,527,891 | |||||
Deferred income taxes (Note 4) | 64,121 | 259,203 | |||||
Other long-term liabilities | 55,721 | 56,129 | |||||
Total long-term liabilities | 1,416,865 | 1,843,223 | |||||
Total liabilities | 1,678,555 | 2,150,889 | |||||
Commitments and contingencies (Note 11) | |||||||
Stockholders' deficit: | |||||||
Common stock, $0.001 par value, 300,000 shares authorized: | |||||||
Class A, 129,558 shares issued, 83,567 shares outstanding and 45,991 shares held in treasury at December 31, 2017 and 114,390 shares issued, 68,399 shares outstanding and 45,991 shares held in treasury at December 31, 2016 | 130 | 114 | |||||
Additional paid-in capital | 1,001,315 | 922,687 | |||||
Retained earnings | 567,741 | 716,198 | |||||
Treasury stock, at cost (Note 12) | (1,725,349 | ) | (1,725,349 | ) | |||
Accumulated other comprehensive loss | (5,831 | ) | (8,697 | ) | |||
Total stockholders' deficit | (161,994 | ) | (95,047 | ) | |||
Total liabilities and stockholders' deficit | $ | 1,516,561 | $ | 2,055,842 | |||
The accompanying notes are an integral part of the Consolidated Financial Statements.
58
GNC HOLDINGS, INC. AND SUBSIDIARIES
Consolidated Statements of Operations
(in thousands, except per share amounts)
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Revenue | $ | 2,453,038 | $ | 2,540,016 | $ | 2,683,298 | |||||
Cost of sales, including warehousing, distribution and occupancy | 1,652,991 | 1,679,897 | 1,698,655 | ||||||||
Gross profit | 800,047 | 860,119 | 984,643 | ||||||||
Selling, general, and administrative | 603,561 | 575,218 | 567,296 | ||||||||
Gains on refranchising | (384 | ) | (19,112 | ) | (7,580 | ) | |||||
Long-lived asset impairments (Note 5) | 457,794 | 476,553 | 28,333 | ||||||||
Other (income) loss, net | (511 | ) | 407 | 3,487 | |||||||
Operating (loss) income | (260,413 | ) | (172,947 | ) | 393,107 | ||||||
Interest expense, net (Note 7) | 64,221 | 60,443 | 50,936 | ||||||||
Gain on convertible debt and debt refinancing costs (Note 7) | (10,996 | ) | — | — | |||||||
(Loss) income before income taxes | (313,638 | ) | (233,390 | ) | 342,171 | ||||||
Income tax (benefit) expense (Note 4) | (164,787 | ) | 52,860 | 122,872 | |||||||
Net (loss) income | $ | (148,851 | ) | $ | (286,250 | ) | $ | 219,299 | |||
(Loss) earnings per share (Note 13): | |||||||||||
Basic | $ | (2.16 | ) | $ | (4.12 | ) | $ | 2.61 | |||
Diluted | $ | (2.16 | ) | $ | (4.12 | ) | $ | 2.60 | |||
Weighted average common shares outstanding (Note 13): | |||||||||||
Basic | 68,789 | 69,409 | 83,927 | ||||||||
Diluted | 68,789 | 69,409 | 84,186 | ||||||||
Dividends declared per share | $ | — | $ | 0.80 | $ | 0.72 | |||||
The accompanying notes are an integral part of the Consolidated Financial Statements.
59
GNC HOLDINGS, INC. AND SUBSIDIARIES
Consolidated Statements of Comprehensive (Loss) Income
(in thousands)
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Net (loss) income | $ | (148,851 | ) | $ | (286,250 | ) | $ | 219,299 | |||
Other comprehensive income (loss): | |||||||||||
Foreign currency translation gain (loss) | 2,866 | 952 | (7,439 | ) | |||||||
Release of cumulative translation loss to earnings related to substantial liquidation of Discount Supplements | — | — | 1,619 | ||||||||
Other comprehensive income (loss) | 2,866 | 952 | (5,820 | ) | |||||||
Comprehensive (loss) income | $ | (145,985 | ) | $ | (285,298 | ) | $ | 213,479 | |||
The accompanying notes are an integral part of the Consolidated Financial Statements.
60
GNC HOLDINGS, INC. AND SUBSIDIARIES
Consolidated Statements of Stockholders' (Deficit) Equity
(in thousands, except per share amounts)
Common Stock | ||||||||||||||||||||||||||||||
Class A | Additional Paid-In Capital | Retained Earnings | Accumulated Other Comprehensive (Loss) Income | Total Stockholders' (Deficit) Equity | ||||||||||||||||||||||||||
Shares | Dollars | Treasury Stock | ||||||||||||||||||||||||||||
Balance at December 31, 2014 | 88,335 | $ | 113 | $ | (1,016,381 | ) | $ | 877,566 | $ | 898,574 | $ | (3,829 | ) | $ | 756,043 | |||||||||||||||
Comprehensive income | — | — | — | — | 219,299 | (5,820 | ) | 213,479 | ||||||||||||||||||||||
Purchase of treasury stock | (12,414 | ) | — | (479,799 | ) | — | — | — | (479,799 | ) | ||||||||||||||||||||
Dividends declared | — | — | — | — | (59,725 | ) | — | (59,725 | ) | |||||||||||||||||||||
Exercise of stock options | 80 | 1 | — | 1,743 | — | — | 1,744 | |||||||||||||||||||||||
Restricted stock awards | 290 | — | — | — | — | — | — | |||||||||||||||||||||||
Minimum tax withholding requirements | (15 | ) | — | — | (574 | ) | — | — | (574 | ) | ||||||||||||||||||||
Excess tax benefit from stock-based compensation | — | — | — | 604 | — | — | 604 | |||||||||||||||||||||||
Stock-based compensation | — | — | — | 6,280 | — | — | 6,280 | |||||||||||||||||||||||
Issuance of convertible senior notes, net | — | — | — | 30,509 | — | — | 30,509 | |||||||||||||||||||||||
Balance at December 31, 2015 | 76,276 | $ | 114 | $ | (1,496,180 | ) | $ | 916,128 | $ | 1,058,148 | $ | (9,649 | ) | $ | 468,561 | |||||||||||||||
Comprehensive loss | — | — | — | — | (286,250 | ) | 952 | (285,298 | ) | |||||||||||||||||||||
Purchase of treasury stock | (7,926 | ) | — | (229,169 | ) | — | — | — | (229,169 | ) | ||||||||||||||||||||
Dividends declared | — | — | — | — | (55,700 | ) | — | (55,700 | ) | |||||||||||||||||||||
Exercise of stock options | 24 | — | — | 353 | — | — | 353 | |||||||||||||||||||||||
Restricted stock awards | 74 | — | — | — | — | — | — | |||||||||||||||||||||||
Minimum tax withholding requirements | (49 | ) | — | — | (1,169 | ) | — | — | (1,169 | ) | ||||||||||||||||||||
Excess tax benefit from stock-based compensation | — | — | — | (1,458 | ) | — | — | (1,458 | ) | |||||||||||||||||||||
Stock-based compensation | — | — | — | 8,833 | — | — | 8,833 | |||||||||||||||||||||||
Balance at December 31, 2016 | 68,399 | $ | 114 | $ | (1,725,349 | ) | $ | 922,687 | $ | 716,198 | $ | (8,697 | ) | $ | (95,047 | ) | ||||||||||||||
Comprehensive loss | — | — | — | — | (148,851 | ) | 2,866 | (145,985 | ) | |||||||||||||||||||||
Dividend forfeitures on restricted stock | — | — | — | — | 394 | — | 394 | |||||||||||||||||||||||
Restricted stock awards | 574 | 1 | — | — | — | — | 1 | |||||||||||||||||||||||
Minimum tax withholding requirements | (32 | ) | — | — | (253 | ) | — | — | (253 | ) | ||||||||||||||||||||
Stock-based compensation | — | — | — | 8,359 | — | — | 8,359 | |||||||||||||||||||||||
Exchange of convertible senior notes (Note 7) | 14,626 | 15 | — | 70,522 | — | — | 70,537 | |||||||||||||||||||||||
Balance at December 31, 2017 | 83,567 | — | $ | 130 | $ | (1,725,349 | ) | $ | 1,001,315 | — | $ | 567,741 | — | $ | (5,831 | ) | — | $ | (161,994 | ) | ||||||||||
The accompanying notes are an integral part of the Consolidated Financial Statements.
61
GNC HOLDINGS, INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
(in thousands)
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Cash flows from operating activities: | |||||||||||
Net (loss) income | $ | (148,851 | ) | $ | (286,250 | ) | $ | 219,299 | |||
Adjustments to reconcile net (loss) income to net cash provided by operating activities: | |||||||||||
Depreciation and amortization expense | 56,809 | 60,038 | 57,237 | ||||||||
Amortization of debt costs | 13,160 | 12,698 | 6,421 | ||||||||
Stock-based compensation | 8,359 | 8,833 | 6,280 | ||||||||
Long-lived asset impairments | 457,794 | 476,553 | 28,333 | ||||||||
Gains on refranchising | (384 | ) | (19,112 | ) | (7,580 | ) | |||||
Gain on convertible debt exchange and debt financing costs | (10,996 | ) | — | — | |||||||
Deferred income tax (benefit) expense | (196,586 | ) | (31,026 | ) | 450 | ||||||
Changes in assets and liabilities: | |||||||||||
(Increase) decrease in receivables | (448 | ) | 11,053 | 422 | |||||||
Decrease (increase) in inventory | 72,070 | (33,496 | ) | 5,381 | |||||||
(Increase) decrease in prepaid and other current assets | (4,498 | ) | (11,955 | ) | 776 | ||||||
(Decrease) increase in accounts payable | (23,960 | ) | 26,980 | 22,375 | |||||||
(Decrease) increase in deferred revenue and accrued liabilities | (6,712 | ) | (8,282 | ) | 9,841 | ||||||
Other operating activities | 4,751 | 2,164 | 5,298 | ||||||||
Net cash provided by operating activities | 220,508 | 208,198 | 354,533 | ||||||||
Cash flows from investing activities: | |||||||||||
Capital expenditures | (32,123 | ) | (59,579 | ) | (45,827 | ) | |||||
Refranchising proceeds | 3,983 | 39,177 | 3,374 | ||||||||
Store acquisition costs | (1,989 | ) | (2,018 | ) | (3,196 | ) | |||||
Proceeds from the sale of Lucky Vitamin | 6,367 | — | — | ||||||||
Net cash used in investing activities | (23,762 | ) | (22,420 | ) | (45,649 | ) | |||||
Cash flows from financing activities: | |||||||||||
Borrowings under Revolving Credit Facility | 317,500 | 234,500 | 43,000 | ||||||||
Payments on Revolving Credit Facility | (444,500 | ) | (150,500 | ) | — | ||||||
Payments on Term Loan Facility | (40,853 | ) | (4,550 | ) | (169,060 | ) | |||||
Proceeds from issuance of convertible senior notes | — | — | 287,500 | ||||||||
Debt issuance costs | — | (1,827 | ) | (8,225 | ) | ||||||
Proceeds from exercise of stock options | — | 353 | 1,743 | ||||||||
Gross excess tax benefits from stock-based compensation | — | 162 | 604 | ||||||||
Minimum tax withholding requirements | (253 | ) | (1,169 | ) | (574 | ) | |||||
Cash paid for treasury stock | — | (229,169 | ) | (479,799 | ) | ||||||
Dividends paid to shareholders | — | (55,336 | ) | (59,647 | ) | ||||||
Net cash used in financing activities | (168,106 | ) | (207,536 | ) | (384,458 | ) | |||||
Effect of exchange rate changes on cash and cash equivalents | 897 | (240 | ) | (1,798 | ) | ||||||
Net increase (decrease) in cash and cash equivalents | 29,537 | (21,998 | ) | (77,372 | ) | ||||||
Beginning balance, cash and cash equivalents | 34,464 | 56,462 | 133,834 | ||||||||
Ending balance, cash and cash equivalents | $ | 64,001 | $ | 34,464 | $ | 56,462 | |||||
The accompanying notes are an integral part of the Consolidated Financial Statements.
62
GNC HOLDINGS, INC. AND SUBSIDIARIES
Supplemental Cash Flow Information
(in thousands)
Year Ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Cash paid during the period for: | |||||||||||
Income taxes | $ | 35,476 | $ | 93,216 | $ | 121,006 | |||||
Interest | 51,205 | 47,597 | 42,911 | ||||||||
As of December 31, | |||||||||||
Non-cash investing activities: | 2017 | 2016 | 2015 | ||||||||
Capital expenditures in current liabilities | $ | 1,683 | $ | 7,556 | $ | 6,018 | |||||
Non-cash financing activities: | |||||||||||
Issuance of shares associated with exchange of convertible senior notes | 71,670 | — | — | ||||||||
The accompanying notes are an integral part of the Consolidated Financial Statements.
63
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1. NATURE OF BUSINESS
GNC Holdings, Inc., a Delaware corporation ("Holdings," and collectively with its subsidiaries and, unless the context requires otherwise, its and their respective predecessors, the "Company"), is a global specialty retailer of health, wellness and performance products, including protein, performance supplements, weight management supplements, vitamins, herbs and greens, wellness supplements, health and beauty, food and drink and other general merchandise.
The Company is vertically integrated as its operations consist of purchasing raw materials, formulating and manufacturing products and selling the finished products through its three reportable segments, which effective in the second quarter of 2016 include U.S. and Canada, International, and Manufacturing / Wholesale (refer to Note 16, "Segments" for more information). Corporate retail store operations are located in the United States, Canada, Puerto Rico, China and Ireland. In addition, the Company offers products on the internet through GNC.com, third-party websites, and prior to the sale of its assets on September 30, 2017, LuckyVitamin.com (see Note 5, "Goodwill and Intangible Assets" for more information). The Company also offered product on the internet through A1 Sports Limited d/b/a Discount Supplements (“Discount Supplements”) up to and including December 31, 2015 when the assets of Discount Supplements were sold and operations were ceased. Franchise locations exist in the United States and approximately 50 other countries. The Company operates its primary manufacturing facility in South Carolina and distribution centers in Arizona, Indiana, Pennsylvania and South Carolina. The Company manufactures approximately half of its branded products and merchandises various third-party products. Additionally, the Company licenses the use of its trademarks and trade names.
The processing, formulation, packaging, labeling and advertising of the Company's products are subject to regulation by various federal agencies, including the Food and Drug Administration, the Federal Trade Commission, the Consumer Product Safety Commission, the United States Department of Agriculture and the Environmental Protection Agency. These activities are also regulated by various agencies of the states and localities in which the Company's products are sold.
NOTE 2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying Consolidated Financial Statements and Footnotes have been prepared by the Company in accordance with accounting principles generally accepted in the United States of America ("GAAP") and with the instructions to Form 10-K and Regulation S-X. The Company's annual reporting period is based on a calendar year.
Summary of Significant Accounting Policies
Principles of Consolidation. The Consolidated Financial Statements include the accounts of Holdings and all of its subsidiaries. All intercompany transactions have been eliminated in consolidation.
Use of Estimates. The preparation of financial statements in conformity with GAAP requires management to make estimates that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management bases these estimates on assumptions that it believes to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions.
Cash and Cash Equivalents. The Company considers cash and cash equivalents to include all cash and liquid deposits and investments with an original maturity of three months or less. Payments due from banks for third-party credit and debit cards generally process within 24 to 72 hours, and are classified as cash equivalents.
Receivables, net. The Company extends credit terms for sales of product to its franchisees, wholesale partners and contract manufacturing customers. Receivables consist principally of unpaid invoices for product sales, franchisee royalties and sublease payments. The Company also has notes receivables with certain of its franchisees that were $6.8 million and $12.1 million at December 31, 2017 and 2016, respectively, and are primarily recorded within other long-term assets on the Consolidated Balance Sheets. As of the first quarter of 2016, the Company discontinued offering franchisees loans. Franchisees secure financing from lending institutions, which include but are not limited to the small business administration and national banks with franchise programs. These loans generally require the Company to subordinate its first lien position on inventory and furniture and fixtures at predetermined amounts.
64
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The Company monitors the financial condition of its customers and establishes an allowance for doubtful accounts for balances estimated to be uncollectible. In addition to considering the aging of receivable balances and assessing the financial condition, the Company considers collateral including inventory and fixed assets for domestic franchisees and letters of credit for international franchisees. The allowance for doubtful accounts was $3.9 million and $4.6 million at December 31, 2017 and 2016, respectively.
Inventory. Inventory components consist of raw materials, work-in-process, finished product and packaging supplies. Inventories are stated at the lower of cost or net realizable value on a first in/first out basis ("FIFO"). Inventory includes costs associated with distribution and transportation, as well as manufacturing overhead, which are capitalized and expensed as merchandise is sold. Inventory is recorded net of obsolescence, shrinkage and vendor allowances for product costs. The Company regularly reviews its inventory levels in order to identify slow moving and short dated products, using factors such as amount of inventory on hand, remaining shelf life, current and expected market conditions, historical trends and the likelihood of recovering the inventory costs based on anticipated demand.
Property, Plant and Equipment. Property, plant and equipment expenditures are recorded at cost. Depreciation and amortization are recognized using the straight-line method over the estimated useful life of the assets. The estimated useful lives are as follows:
Building | 30 yrs |
Machinery and equipment | 3-10 yrs |
Building and leasehold improvements | 3-15 yrs |
Furniture and fixtures | 5-8 yrs |
Software | 3-5 yrs |
Building improvements are depreciated over their estimated useful life or the remaining useful life of the related building, whichever period is shorter. Improvements to leased premises are depreciated over the estimated useful life of the improvements or the related leases including renewals that are reasonably assured, whichever period is shorter. Expenditures that materially increase the value or clearly extend the useful life of property, plant and equipment are capitalized while repair and maintenance costs incurred in the normal course of operations are expensed as incurred.
Goodwill and Indefinite-Lived Intangible Asset. The Company was acquired by Ares Corporate Opportunities Fund II L.P. and Ontario Teachers’ Pension Plan Board in March 2007 and subsequently completed an initial public offering in 2011 of its common stock. In connection with this acquisition, the Company recorded approximately $600 million of goodwill and a $720 million indefinite-lived intangible asset related to its brand name.
Goodwill is allocated to the Company's reporting units, which are at or below the level of an operating segment as defined by Accounting Standards Codification ("ASC") 280 "Segment Reporting." The Company formally evaluates the carrying amount of goodwill for each of its reporting units in the fourth quarter. In addition, the Company performs an evaluation on an interim basis if it determines that recent events or prevailing conditions indicate a potential impairment of goodwill. A significant amount of judgment is involved in determining whether an indicator of impairment has occurred between annual impairment tests. These indicators include, but are not limited to, overall financial performance such as adverse changes in recent forecasts of operating results, industry and market considerations, a sustained decrease in the share price of the Company's common stock, updated business plans and regulatory and legal developments.
In connection with the adoption of Accounting Standards Update ("ASU") 2017-04 as explained below, if the carrying value of a reporting unit exceeds its fair value, an impairment charge is recorded for the difference as an operating expense in the period incurred.
The Company's indefinite-lived intangible brand asset is also evaluated annually in the fourth quarter for impairment and on an interim basis if events or changes in circumstances between annual tests indicate that the asset might be impaired. If the carrying value of the intangible asset exceeds its fair value, an impairment loss is recognized in an amount equal to the difference. Refer to Note 5, "Goodwill and Intangible Assets" for a description of impairment charges recorded.
Impairment of Definite-Long-lived Assets. The Company evaluates whether the carrying values of property, plant and equipment and definite-lived intangible assets have been impaired whenever events or changes in circumstances indicate that the carrying amounts may not be recoverable based on estimated undiscounted future cash flows. Factors that may trigger an impairment
65
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
review include significant changes in the intended use of assets, significant negative industry or economic trends, underperforming stores and anticipated store closings. If it is determined that the carrying value of the applicable asset group is not recoverable, an impairment loss is recognized for the amount the carrying value of the long-lived asset exceeds its estimated fair value. Refer to Note 6, "Property, Plant and Equipment, Net" for a description of impairment charges recorded.
Revenue Recognition. Within the U.S. and Canada segment, retail sales in company-owned stores are recognized at the point of sale, net of sales tax. Revenue related to e-commerce sales is recognized upon delivery to customers and includes shipping charges. A provision for anticipated returns is recorded through a reduction of sales and cost of sales (for product that can be resold or returned to vendors) in the period that the related sales are recorded.
Revenue is deferred on sales of the Company's Gold Cards and subsequently recognized over the one year membership period. The Gold Card Member Pricing program which provided members product discounts was discontinued in all domestic company-owned and franchise stores on December 28, 2016 in connection with the introduction of the One New GNC. As a part of this launch, the Company provided former Gold Card customers that were within the membership period of generally one year with a coupon which was equivalent to a reimbursement of the unexpired portion of their Gold Card membership fee. As of December 31, 2016, the Company had $24.4 million of deferred Gold Card revenue which was recognized in the first quarter of 2017 over the coupon redemption period which expired in March 2017, net of $1.4 million of applicable redemptions.
Effective with the launch of the One New GNC on December 29, 2016, the Company introduced myGNC Rewards, a free points-based loyalty program system-wide in the U.S. The program enables customers to earn points based on their purchases. Points earned by members are valid for one year and may be redeemed for cash discounts on any product the Company sells at both company-owned or franchise locations. The Company defers the estimated standalone selling price of points related to this program as a reduction to revenue as points are earned by allocating a portion of the transaction price the customer pays to a loyalty program liability within deferred revenue and other current liabilities on the Consolidated Balance Sheet. The estimated selling price of each point is based on the estimated value of product for which the point is expected to be redeemed, net of points not expected to be redeemed, based on historical redemption. When a customer redeems earned points, revenue is recognized with a corresponding reduction to the program liability.
Also effective with the launch of the One New GNC, the Company began offering a paid membership program, PRO Access, for $39.99 per year, which provides members with the delivery of sample boxes throughout the membership year, as well as the offering of certain other benefits including the opportunity to earn triple points on a periodic basis. The boxes include sample merchandise and other materials. The Company defers the membership price paid within deferred revenue and other current liabilities on the Consolidated Balance Sheet and recognizes revenue as the underlying performance obligations are satisfied.
Revenue from gift cards is recognized when the gift card is redeemed. Gift cards do not have expiration dates and are not required to be escheated to government authorities. Utilizing historical redemption rates, the Company recognizes revenue for amounts not expected to be redeemed proportionately as other gift card balances are redeemed.
Revenues from domestic and international franchisees include product sales, royalties and franchise fees and are recorded within the U.S. and Canada segment for domestic franchisees and the International segment for international franchisees. The Company's franchisees purchase a significant amount of the products they sell in their retail stores from the Company at wholesale prices. Revenue on product sales to franchisees is recognized when risk of loss, title and insurable risks have transferred to the franchisee, net of estimated returns and allowances. Franchise fees are paid in advance, deferred and recognized by the Company at the time of a franchise store opening. Franchise royalties are recognized as a percentage of the franchisees' retail sales in the period the franchisees' sales occur.
The Manufacturing / Wholesale segment sells product to the Company's other segments, which is eliminated in consolidation, and third-party customers. Revenue is recognized when risk of loss, title and insurable risks have transferred to the customer, net of estimated returns and allowances.
Refer to "Revenue Recognition Update" below for the impact of ASU 2014-09 on the Company effective in the first quarter of 2018.
Cost of Sales. The Company purchases products directly from third-party vendors and manufactures its own products. Cost of sales includes product costs, vendor allowances, inventory obsolescence, shrinkage, manufacturing overhead, warehousing, distribution, shipping and store occupancy costs. Store occupancy costs include rent, common area maintenance charges, real estate and other asset-based taxes, general maintenance, utilities, depreciation, lease incentives and certain insurance expenses.
66
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Vendor Allowances. The Company receives allowances/credits from various vendors based on either sales or purchase volumes, right of return for expired product and non-saleable customer returns, and cooperative advertising. As the right of offset exists under these arrangements, credit earned under these arrangements are recorded as a reduction in the vendors' accounts payable balances on the Consolidated Balance Sheet and represent the estimated amounts due to the Company under the provisions of such contracts. Amounts expected to be received from vendors relating to the purchase of merchandise inventories are recognized as a reduction to cost of sales as the merchandise is sold. Amounts that represent a reimbursement of costs incurred, such as advertising, are recorded as a reduction to the related expense in the period that the expense is incurred. The Company recorded a reduction to cost of sales of $86.7 million, $94.9 million and $98.7 million for the years ended December 31, 2017, 2016 and 2015, respectively, for vendor allowances associated with the purchase and sale of merchandise.
Research and Development. Research and development costs arising from internally generated projects are expensed as incurred. The Company recognized approximately $6 million to $8 million in each of the years ended December 31, 2017, 2016 and 2015 relating to research and development.
Advertising Expenditures. The Company recognizes the costs of advertising, promotion and marketing programs the first time the communication takes place. The Company administers national advertising funds on behalf of its franchisees. In accordance with the franchisee contracts, the Company collects advertising fees from the franchisees and utilizes the proceeds to coordinate various advertising and marketing campaigns. The Company recognized advertising expense of $89.2 million, $74.1 million and $69.5 million for the years ended December 31, 2017, 2016 and 2015, respectively, net of $15.3 million, $15.7 million and $16.0 million received from the Company's franchisees.
Leases. The Company has various operating leases for company-owned and franchise store locations, distribution centers, and equipment generally with an initial term of five years, which may include renewal options for varying terms thereafter. Leases for franchise store locations are subleased to franchisees. The Company is the primary lessee for the majority of the franchise store locations and makes rental payments to the landlord directly, and then bills the franchisee for reimbursement. The Company records rental income received from franchisees as revenue. If a franchisee defaults on its sublease, the Company has in the past converted, any such franchise store into a company-owned store and fulfilled the remaining lease obligation.
Leases generally include amounts relating to base rent, percent rent and other charges such as common area maintenance and real estate taxes. Periodically, the Company receives varying amounts of reimbursements from landlords to compensate the Company for costs incurred in the construction of stores. These reimbursements are recorded as deferred rent within other long-term liabilities on the Consolidated Balance Sheet and are amortized as a reduction to rent expense over the life of the related lease. The expenditures made by the Company are recorded as an increase to leasehold improvements within property, plant and equipment, net. Many of the Company’s lease agreements contain escalation clauses under which, if fixed and determinable, rent expense and rent income is recognized on a straight-line basis over the lives of the leases, including renewal periods that are reasonably assured. Certain of the Company's leases also contain clauses for rent to be paid as a percentage of sales, which are based on a percentage of retail sales or a percentage of retail sales in excess of stipulated amounts (contingent rent). Contingent rent is recorded as rent expense when attainment of the target is considered probable and is recognized in proportion to the retail sales contributing to the achievement of the target.
Contingencies. The Company records accruals for outstanding legal matters when it believes it is probable that a loss will be incurred and the amount of such loss can be reasonably estimated. The Company evaluates, on a quarterly basis, developments in legal matters that could affect the amount of any accrual and developments that would make a loss contingency both probable and reasonably estimable. If both of the conditions above are not met, disclosure is made when there is at least a reasonable possibility that a loss contingency has been incurred. As facts concerning contingencies evolve and become known, management reassesses the likelihood of a probable loss and makes appropriate adjustments to its financial statements.
Pre-Opening Expenditures. The Company recognizes the cost associated with the opening of new stores, which consist primarily of rent, marketing, payroll and recruiting costs, as incurred.
Income Taxes. The Company accounts for income taxes using the asset and liability method. Under this method, deferred tax assets and liabilities result from (i) the future tax impact of temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and (ii) differences between the recorded value of assets acquired in business combinations accounted for as purchases for financial reporting purposes and their corresponding tax bases. Deferred income tax assets are reduced by a valuation allowance if it is more likely than not that some portion of the deferred income tax asset will not be realized.
67
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The Company recognizes the tax benefit from an uncertain tax position only if it is at least more likely than not that the tax position will be sustained upon examination by the taxing authorities based on the technical merits of the position. The amount of the tax benefit that is recognized is measured as the largest amount of benefit that is more likely than not to be realized upon effective settlement. The Company classifies interest and penalties accrued in connection with unrecognized tax benefits as income tax expense in its Consolidated Statements of Operations.
Refer to Note 4, "Income Taxes," for more information.
Self-Insurance. The Company is self-insured for certain losses related to health insurance, workers' compensation and general liability insurance and maintains stop-loss coverage with third-party insurers to limit its liability exposure. Liabilities associated with these losses are estimated by considering historical claims experience, estimated lag time to report and pay claims, average cost per claim and other actuarial factors. Effective January 1, 2018, the Company is now fully insured.
Stock-Based Compensation. The Company utilizes the Black-Scholes model to calculate the fair value of time-based stock option awards. The Company utilizes a Monte Carlo simulation for its performance awards with a market condition, which requires various inputs and assumptions, including the Company's own stock price. The grant-date fair value of all other stock-based compensation, including time-based and performance-based restricted stock awards, is based on the closing price for a share of the Company's common stock on the New York Stock Exchange (the "NYSE") on the grant date.
Compensation expense for time-based stock options and restricted stock awards is recognized over the applicable vesting period, net of expected forfeitures. Compensation expense for performance-based shares with a market condition is recognized over the applicable vesting period, net of expected forfeitures, regardless of whether the market condition is achieved. Compensation expense related to the performance-based awards is recognized over the applicable vesting period, net of expected forfeitures, and adjusted as necessary to reflect changes in the probability that the vesting criteria will be achieved. The Company regularly reviews the probability of achieving the performance condition on these awards.
Refer to Note 14, "Stock-Based Compensation" for more information.
Earnings Per Share. Basic earnings per share ("EPS") is computed by dividing net income by the weighted average number of shares of common stock outstanding for the period. The Company uses the treasury stock method to compute diluted EPS for its stock-based compensation to the extent that awards with performance and market conditions are probable of being achieved and stock options are in-the-money, which assumes that outstanding stock awards were converted into common stock, and the resulting proceeds (which includes unrecognized compensation expense for all awards and the exercise price associated with stock options) were used to acquire shares of common stock at the average market price during the reporting period. Refer to Note 13, "Earnings Per Share" for information on the Company's underlying shares of its convertible debt in the computation of EPS.
Foreign Currency. For all active foreign operations, the functional currency is generally the local currency. Assets and liabilities of foreign operations are translated into the Company's reporting currency, the U.S. dollar, using period-end exchange rates, while income and expenses are translated using the average exchange rates for the reporting period. Translation gains and losses are recorded as part of accumulated other comprehensive loss on the Consolidated Balance Sheet. The Company has intercompany balances with its foreign entities that are routinely settled primarily relating to product sales and management fees. Gains or losses resulting from these foreign currency transactions are included in the Consolidated Statements of Operations and were not material in the fiscal years ended December 31, 2017, 2016 and 2015.
Correction of Immaterial Error
During the quarter ended March 31, 2015, the Company identified a $2.8 million error relating to prior periods in the calculation of the portion of the accrued payroll liability relating to certain amounts paid to store employees. The impact of this error was not material to any prior period. In addition, the cumulative impact of the correction was not material to the Company's Consolidated Financial Statements for the quarter ended March 31, 2015 or the year ended December 31, 2015. Consequently, the Company corrected the error in the first quarter of 2015 by increasing SG&A expense on the Consolidated Statement of Operations and deferred revenue and other current liabilities on the Consolidated Balance Sheet by $2.8 million. The impact to net income was a decrease of $1.8 million for the year ended December 31, 2015. This correction had no impact on cash flows in 2015.
68
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Other (Income) Loss, Net
Other (income) loss, net, in the year ended December 31, 2017 includes $1.2 million of foreign currency gains and $1.0 million related to insurance and lease settlements, partially offset by a $1.7 million loss attributed to the sale of substantially all of the assets of the Lucky Vitamin e-commerce business. The year ended December 31, 2016 includes $0.4 million of foreign currency losses. The year ended December 31, 2015 includes a loss of $2.7 million attributable to the closure and related asset sale of Discount Supplements and $0.8 million of foreign currency losses.
Recently Adopted Accounting Pronouncements
In January 2017, the Financial Accounting Standards Board (the "FASB") issued ASU 2017-04, which simplifies the subsequent measurement of goodwill by eliminating Step 2 from the goodwill impairment test. Under the new guidance, an entity will recognize an impairment charge for the amount by which the carrying value exceeds the fair value. This standard is effective for annual or any interim goodwill impairment tests in fiscal years beginning after December 15, 2019. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. The Company has adopted this ASU in the second quarter of 2017. Refer to Note 5, "Goodwill and Intangible Assets" for a description of the goodwill impairment charges recorded in 2017.
In March 2016, the FASB issued ASU 2016-09, which includes multiple provisions intended to simplify various aspects of accounting and reporting for share-based payments. The difference between the deduction for tax purposes and the compensation cost of a share-based payment award results in either an excess tax benefit or deficiency. Formerly, these excess tax benefits were recognized in additional paid-in capital and tax deficiencies (to the extent there were previous tax benefits) were recognized as an offset to accumulated excess tax benefits. If no previous tax benefit existed, the deficiencies were recognized in the income statement as an increase to income tax expense. The changes require all excess tax benefits and tax deficiencies related to share-based payments be recognized as income tax expense or benefit in the income statement. Gross excess tax benefits in the cash flow statement have also changed from the prior presentation as a financing activity to being classified as an operating activity. Lastly, excess tax benefits are no longer included in the assumed proceeds of the diluted EPS calculation, which results in stock-based awards being more dilutive. This standard is effective prospectively for annual reporting periods, and interim periods therein, beginning after December 15, 2016. The Company has adopted this ASU in the first quarter of 2017, which did not have a material impact to the Consolidated Financial Statements.
In November 2015, the FASB issued ASU 2015-17, which requires an entity to classify deferred tax assets and liabilities as noncurrent on the balance sheet. This standard is effective for annual reporting periods, and interim periods therein, beginning after December 15, 2016. The Company has adopted this ASU during the first quarter of fiscal 2017, with retrospective application. The Company reclassified $12.9 million of current deferred income tax assets formerly presented within total current assets as a $12.8 million reduction to deferred income taxes presented within total long-term liabilities and a $0.1 million increase to other long-term assets at December 31, 2016 on the Consolidated Balance Sheet to conform to the current year presentation.
In July 2015, the FASB issued ASU 2015-11, which requires an entity that determines the cost of inventory by methods other than last-in, first-out and the retail inventory method to measure inventory at the lower of cost and net realizable value. This standard is effective for annual reporting periods, and interim periods therein, beginning after December 15, 2016. Accordingly, the Company has adopted this ASU in the first quarter of 2017, which did not have a material effect on the Company’s Consolidated Financial Statements.
Recently Issued Accounting Pronouncements
In May 2017, the FASB issued ASU 2017-09, which amends the scope of modification accounting for share-based payment arrangements. This standard states that an entity should account for the effects of a modification unless all of the following are met: 1) The fair value of the modified award is the same as the fair value of the original award immediately before the original award is modified (if the modification does not affect any of the inputs to the valuation technique that the entity uses to value the award, the entity is not required to estimate the value immediately before and after the modification); 2) The vesting conditions of the modified award are the same as the vesting conditions of the original award immediately before the original award is modified; and 3) The classification of the modified award as an equity instrument or a liability instrument is the same as the classification of the original award immediately before the original award is modified. The standard is effective for all entities for annual periods, and interim periods within those annual periods, beginning after December 15, 2017. Early adoption is permitted. The Company does not expect the impact of the new standard to have a material impact on the Consolidated Financial Statements.
69
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
In August 2016, the FASB issued ASU 2016-15, which addresses changes to the classification of certain cash receipts and cash payments within the statement of cash flows in order to address diversity in practice. In November 2016, the FASB issued ASU 2016-18, which requires that a statement of cash flows explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash or restricted cash equivalents. Therefore, amounts generally described as restricted cash and restricted cash equivalents should be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. Both standards are effective for annual reporting periods, and interim periods therein, beginning after December 15, 2017. The Company does not expect the impact of the new standard to have a material impact on the Consolidated Financial Statements.
In February 2016, the FASB issued ASU 2016-02, which requires lessees to recognize a right-of-use asset and a lease liability, initially measured at the present value of the lease payments for all leases with a term greater than 12 months. This standard is effective for annual reporting periods, and interim periods therein, beginning after December 15, 2018 and is required to be applied using a modified retrospective approach for all leases existing at, or entered into after, the beginning of the earliest comparative period presented. The Company has a significant number of leases, and as a result, expects this guidance to have a material impact on its Consolidated Balance Sheet, which is currently being evaluated.
Revenue Recognition Update
In May 2014, the FASB issued ASU 2014-09, which updates revenue recognition guidance relating to contracts with customers. This standard states that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This standard is effective for annual reporting periods, and interim periods therein, beginning after December 15, 2017. The standard may be applied retrospectively to each prior period presented (full retrospective method) or retrospectively with the cumulative effect recognized as of the date of adoption (modified retrospective method). The Company intends on applying the full retrospective method upon adoption in the first quarter of 2018.
The new standard will not impact recognition of point-of-sale revenue in company-owned stores, most wholesale sales, royalties and sublease revenue, together which account for approximately 90% of the Company’s revenue. The new standard will have no impact on the timing or classification of the Company’s cash flows as reported in the Consolidated Statement of Cash Flows and is not expected to have a significant impact on the Company’s Consolidated Statement of Operations. The Company will record a reduction to opening retained earnings, net of tax, at December 31, 2015 of approximately $20 million to $25 million primarily relating to deferred franchise fees. Below is a description of expected changes resulting from the new standard.
Franchise fees. The Company's current accounting policy for franchise fees and license fees received for new store openings and renewals is to recognize these fees when earned per the contract terms, which is when a new store opens or at the start of a new term. In accordance with the new guidance, these fees will be deferred and recognized over the applicable license term as the Company satisfies the performance obligation of granting the customer access to the rights of the Company’s intellectual property. This change will impact all of the Company’s reportable segments. In addition, franchise fees received as part of a sale of a company-owned store to a franchisee will be recorded as described above as part of revenue and will no longer be presented as part of gains on refranchising. The Company does not anticipate the impact of this change to be material to the Company’s Consolidated Statement of Operations. The opening balance sheet adjustment to retained earnings at December 31, 2015 will include an increase to deferred revenue of $35 million to $40 million, net of a deferred taxes. Deferred revenue for franchise fees will be recorded within current liabilities and long-term liabilities on the Consolidated Balance Sheet in future periods.
Cooperative advertising and other franchise support fees. The Company currently classifies advertising and other franchise support fees received from domestic franchisees of approximately $23 million to $25 million each year as a reduction to selling, general and administrative expense and cost of sales on the Consolidated Statement of Operations. In accordance with the new guidance, these fees will be required to be classified as revenue within the U.S. and Canada segment. The new standard will not impact the timing of recognition of this income or on the Consolidated Balance Sheet.
Specialty manufacturing. The Company currently recognizes revenue for products manufactured and sold to customers at a point in time when risk of loss, title and insurable risks have transferred to the customer, net of estimated returns and allowances. Under the new standard, revenue is required to be recognized over time as manufacturing occurs if the customized goods have no alternative use to the manufacturer, and the manufacturer has an enforceable right to payment for performance completed to date. This change will impact contract manufacturing sales to third-parties recorded in the Manufacturing / Wholesale segment. The
70
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Company does not anticipate that the impact of this change will be material to the Consolidated Statement of Operations. The Company will record a reduction to inventory as applicable custom manufacturing services are completed with a corresponding contract asset including the applicable markup recorded within other current assets on the Consolidated Balance Sheet. The opening balance sheet adjustment to retained earnings at December 31, 2015 is not expected to be significant, which will include a decrease to inventory with a corresponding increase to a contract asset (for a slightly higher amount representing the markup) of approximately $20 million, net of a deferred taxes.
E-Commerce revenues. The Company currently records revenue to its e-commerce customers upon delivery. Under the new guidance, the Company will recognize revenue upon shipment based on meeting the transfer of control criteria. The Company has made a policy election to treat shipping and handling as costs to fulfill the contract, and as a result, any fees received from customers will be included in the transaction price allocated to the performance obligation of providing goods with a corresponding amount accrued within cost of sales for amounts paid to applicable carriers. The new standard is not expected to have a material impact on the timing of recognition of this income or on the Consolidated Balance Sheet. The Company is not revising prior period balances for e-commerce revenues because the changes are not material.
Loyalty. Effective with the launch of the One New GNC on December 29, 2016, the Company introduced a free points-based myGNC Rewards loyalty program system-wide in the U.S. The Company is utilizing the new revenue recognition standard to account for this program, the difference of which is immaterial relative to the current standard. Refer to "Revenue recognition" above for information on the Company's accounting policy for this loyalty program.
Other Revisions
Certain amounts in the Consolidated Financial Statements for prior year periods have been revised to confirm to the current period's presentation. The impact to prior periods for these revisions was not significant with no impact on previously reported operating income, net income, cash flows from operations or stockholders' equity.
NOTE 3. INVENTORY
The net realizable value of inventory consisted of the following:
December 31, | |||||||
2017 | 2016 | ||||||
(in thousands) | |||||||
Finished product ready for sale (*) | $ | 435,216 | $ | 509,209 | |||
Work-in-process, bulk product and raw materials (*) | 66,592 | 67,275 | |||||
Packaging supplies | 5,050 | 6,728 | |||||
Inventory | $ | 506,858 | $ | 583,212 | |||
(*) The December 31, 2016 balances have been revised for an $18.0 million correction in the classification of certain amounts between finished product ready for sale and work-in-process, bulk product and raw materials. The correction had no impact on total inventory.
NOTE 4. INCOME TAXES
(Loss) income before income taxes consisted of the following components:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(in thousands) | |||||||||||
Domestic | $ | (301,948 | ) | $ | (212,095 | ) | $ | 365,362 | |||
Foreign | (11,690 | ) | (21,295 | ) | (23,191 | ) | |||||
(Loss) income before income taxes | $ | (313,638 | ) | $ | (233,390 | ) | $ | 342,171 | |||
71
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Income tax (benefit) expense consisted of the following components:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(in thousands) | |||||||||||
Current: | |||||||||||
Federal | $ | 23,965 | $ | 67,326 | $ | 104,711 | |||||
State | 4,458 | 9,928 | 13,414 | ||||||||
Foreign | 3,376 | 6,632 | 4,297 | ||||||||
Total current income tax expense | 31,799 | 83,886 | 122,422 | ||||||||
Deferred: | |||||||||||
Federal | (182,217 | ) | (32,397 | ) | 3,193 | ||||||
State | (13,773 | ) | (1,110 | ) | (1,412 | ) | |||||
Foreign | (596 | ) | 2,481 | (1,331 | ) | ||||||
Total deferred income tax (benefit) expense | (196,586 | ) | (31,026 | ) | 450 | ||||||
Total income tax (benefit) expense | $ | (164,787 | ) | $ | 52,860 | $ | 122,872 | ||||
Income tax (benefit) expense reflected in the accompanying Consolidated Statements of Operations varies from the amounts that would have been provided by applying the United States federal statutory income tax rate of 35% to (loss) income before income taxes as shown below:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(in thousands) | |||||||||||
U.S. federal statutory income tax | $ | (109,773 | ) | $ | (81,686 | ) | $ | 119,760 | |||
Increase (reduction) resulting from: | |||||||||||
State income tax, net of federal tax benefit | (6,678 | ) | 6,316 | 11,976 | |||||||
Nondeductible goodwill | 6,219 | 132,800 | — | ||||||||
Brand name impairment | 50,957 | — | — | ||||||||
Exchange of convertible senior notes | (9,526 | ) | — | — | |||||||
Other permanent differences | 2,513 | 633 | 1,369 | ||||||||
International operations, net of foreign tax credits | (1,087 | ) | 3,454 | 13,035 | |||||||
Worthless stock tax benefit | — | — | (11,634 | ) | |||||||
Federal tax credits and income deductions | (2,698 | ) | (6,030 | ) | (8,554 | ) | |||||
Tax impact of uncertain tax positions and other | (4,224 | ) | (2,627 | ) | (3,080 | ) | |||||
Impact of 2017 Tax Act | (90,490 | ) | — | — | |||||||
Income tax (benefit) expense | $ | (164,787 | ) | $ | 52,860 | $ | 122,872 | ||||
On December 22, 2017, tax reform legislation known as The Tax Cuts and Jobs Act of 2017 (“2017 Tax Act”) was enacted. The 2017 Tax Act made significant changes to the Internal Revenue Code, including a reduction in the corporate tax rate from 35% to 21%, which is effective for tax years beginning after December 31, 2017. The Company has calculated the impact of the 2017 Tax Act as part of the year end income tax provision. The Company has recorded a non-cash income tax benefit of $90.5 million in 2017, related to the remeasurement of its deferred tax assets and liabilities to reflect the effects of these temporary differences at enacted tax rates expected to be in effect when taxes are actually paid or recovered.
On December 22, 2017, Staff Accounting Bulletin No. 118 ("SAB 118") was issued to address the application of GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the 2017 Tax Act. The accompanying Consolidated Financial Statements include an immaterial provisional tax impact related to deemed repatriated earnings. The ultimate impact may differ from these provisional amounts, due to, among other things, additional analysis,
72
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
changes in interpretations and assumptions the Company has made and additional regulatory guidance that may be issued. Any subsequent adjustment will be recorded to current tax expense in the quarter of 2018 when the analysis is complete.
The adjustment explained above related to the 2017 Tax Act was recorded prior to long-lived asset impairment charges primarily related to the Company's indefinite-lived brand asset explained in Note 5, "Goodwill and Intangible Assets." The reduction to the applicable deferred tax liability associated with the indefinite-lived intangible asset impaired utilized the updated statutory rate associated with the 2017 Tax Act resulting in a reconciling item in the table above, which begins with the 35% statutory rate enacted in the year ended December 31, 2017. In addition, the effective tax rates in 2017 and 2016 were significantly impacted by goodwill impairment charges totaling $24.3 million and $471.1 million, respectively, the majority of which is not deductible for income tax purposes.
Deferred tax assets and liabilities consisted of the following at December 31:
2017 | 2016 | ||||||||||||||||||||||
Assets | Liabilities | Net | Assets | Liabilities | Net | ||||||||||||||||||
(in thousands) | |||||||||||||||||||||||
Deferred tax assets (liabilities): | |||||||||||||||||||||||
Operating reserves | $ | 6,213 | $ | — | $ | 6,213 | $ | 9,774 | $ | — | $ | 9,774 | |||||||||||
Deferred revenue | 1,897 | — | 1,897 | 4,439 | — | 4,439 | |||||||||||||||||
Prepaid expenses | — | (3,873 | ) | (3,873 | ) | — | (4,556 | ) | (4,556 | ) | |||||||||||||
Intangible assets | — | (102,602 | ) | (102,602 | ) | — | (300,253 | ) | (300,253 | ) | |||||||||||||
Fixed assets | 15,420 | — | 15,420 | 16,006 | — | 16,006 | |||||||||||||||||
Stock-based compensation | 4,586 | — | 4,586 | 4,597 | — | 4,597 | |||||||||||||||||
Net operating loss and credit carryforwards | 28,244 | — | 28,244 | 26,628 | — | 26,628 | |||||||||||||||||
Long-term rent liabilities | 6,946 | — | 6,946 | 8,604 | — | 8,604 | |||||||||||||||||
Convertible senior notes | — | (5,755 | ) | (5,755 | ) | — | (12,581 | ) | (12,581 | ) | |||||||||||||
Other | 4,018 | — | 4,018 | 9,541 | — | 9,541 | |||||||||||||||||
Valuation allowance | (17,478 | ) | — | (17,478 | ) | (21,324 | ) | — | (21,324 | ) | |||||||||||||
Total net deferred taxes | $ | 49,846 | $ | (112,230 | ) | $ | (62,384 | ) | $ | 58,265 | $ | (317,390 | ) | $ | (259,125 | ) | |||||||
At December 31, 2017 and 2016, the Company had deferred tax assets relating to foreign and state NOLs with lives ranging from 5 to 20 years. As of December 31, 2017 and 2016, a valuation allowance was provided for certain NOLs, as the Company currently believes that these NOLs may not be realizable prior to their expiration. In 2017, the Company reduced its valuation allowance by $3.8 million. During 2016 and 2015, the Company increased its valuation allowance by $4.4 million and $2.3 million, respectively. The valuation allowance was adjusted in 2017 based on a change in circumstances, including anticipated future earnings, which caused a change in judgment about the realizability of certain deferred tax assets related to NOLs.
The Company does not have any material undistributed earnings of international subsidiaries at December 31, 2017 as these subsidiaries are considered to be branches for United States tax purposes, to have incurred cumulative NOLs, or to have only minimal undistributed earnings.
GNC Holdings, Inc. files a consolidated federal tax return and various consolidated and separate tax returns as prescribed by the tax laws of the state, local and international jurisdictions in which it and its subsidiaries operate. The statutes of limitation for the Company’s U.S. federal income tax returns are closed for years through 2013. The Company has various state and local jurisdiction tax years open to possible examination (the earliest open period is generally 2011), and the Company also has certain state and local tax filings currently under audit.
73
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
A reconciliation of the beginning and ending amount of unrecognized tax benefits, excluding penalties and interest, is as follows:
December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(in thousands) | |||||||||||
Balance of unrecognized tax benefits at beginning of period | $ | 6,456 | $ | 7,282 | $ | 11,652 | |||||
Additions for tax positions taken during current period | 748 | 289 | 1,345 | ||||||||
Additions for tax positions taken during prior periods | 192 | 1,031 | 543 | ||||||||
Reductions for tax positions taken during prior periods | (675 | ) | (1,378 | ) | (6,258 | ) | |||||
Settlements | (947 | ) | (768 | ) | — | ||||||
Balance of unrecognized tax benefits at end of period | $ | 5,774 | $ | 6,456 | $ | 7,282 | |||||
The Company's liability for uncertain tax positions, excluding penalties and interest, decreased by $0.7 million during the current year due in part to the expiration of certain statutes of limitation with respect to the 2013 fiscal year and the payment of settlements to satisfy open audits.
As of December 31, 2017, the Company is not aware of any positions for which it is reasonably possible that the total amount of unrecognized tax benefits will significantly increase or decrease within the next 12 months. Accrued interest and penalties were $2.0 million and $1.9 million at December 31, 2017 and 2016, respectively. At December 31, 2017, the amount of unrecognized tax benefits that, if recognized, would affect the effective tax rate was $7.8 million, including the impact of accrued interest and penalties. While it is often difficult to predict the final outcome or the timing of resolution of any particular uncertain tax position, the Company believes that its unrecognized tax benefits reflect the most likely outcome. The Company adjusts these unrecognized tax benefits, as well as the related interest, in light of changing facts and circumstances. Settlement of any particular position could require the use of cash. Favorable resolution would be recognized as a reduction to the effective income tax rate in the period of resolution.
NOTE 5. GOODWILL AND INTANGIBLE ASSETS
Impairment Charges
Based on the decline in the Company's share price of over 50% in the fourth quarter of 2017 and the previous challenges associated with the Company’s efforts to refinance its long-term debt, management concluded a triggering event occurred in the fourth quarter requiring an impairment test of its indefinite-lived intangible brand name asset and the goodwill of all reporting units.
The Company recorded the following impairment charges. Refer to Note 6, "Property, Plant and Equipment, Net" for more information on the property and equipment charges.
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(in thousands) | |||||||||||
Brand name | $ | 395,600 | $ | — | $ | — | |||||
Goodwill | 24,283 | 471,132 | — | ||||||||
Property and equipment | 18,555 | 5,421 | — | ||||||||
Lucky Vitamin (*) | 19,356 | — | — | ||||||||
Discount Supplements (*) | — | — | 28,333 | ||||||||
Total long-lived asset impairment charges | $ | 457,794 | $ | 476,553 | $ | 28,333 | |||||
74
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(*) Includes goodwill, intangible assets and property and equipment as explained below.
Brand Name
Management performed an impairment test for its $720.0 million indefinite-lived brand intangible asset, and concluded that the estimated fair value under the relief from royalty method (income approach) was less than its carrying value, which resulted in an impairment charge of $395.6 million. The brand name impairment test was performed in totality as it represents a single unit of account and the charge was allocated to the U.S. and Canada and International segments for $394.0 million and $1.6 million, respectively. Key assumptions included in the estimation of the fair value include, but are not limited to, the following:
• | Future cash flow assumptions - Future cash flow assumptions include retail sales from the Company’s corporate stores and GNC.com, as well as retail sales from domestic and international franchisees. Retail sales were based on organic growth and were derived from historical experience and assumptions regarding future growth. The Company's analysis incorporated an assumed period of cash flows of 10 years with a terminal value. |
• | Royalty rate - The royalty rate used considers external market evidence and internal financial metrics. Based on a review of market based royalty rates coupled with a review of available returns after the consideration of property, plant and equipment, working capital and other intangible assets, the royalty rate utilized in the impairment test decreased relative to the impairment test performed at December 31, 2016. |
• | Discount rate - The discount rate was based on the measure used in the goodwill impairment test described below adjusted for the risk associated with the specific brand name asset. The discount rate used in the analysis was 18.5%. |
Goodwill
The Domestic Stores and Canada reporting units have no remaining goodwill balance after prior year impairment charges. The results of the goodwill impairment test performed in the fourth quarter of 2017 indicated no impairment for the GNC.com, International Franchise, Manufacturing and The Health Store reporting units. However, the Wholesale reporting unit had a fair value below its carrying value, which resulted in a $24.3 million goodwill impairment charge for the difference, which was recorded within the Manufacturing / Wholesale segment. The goodwill impairment test performed in the fourth quarter of 2017 satisfies the Company's annual testing requirement.
The results of the impairment testing indicated that the Manufacturing and The Health Store reporting units, which have goodwill balances of $61.5 million and $5.9 million, respectively, had fair values that exceeded their carrying values by less than 15%. In addition, in connection with the adoption of ASU 2017-04, the fair value of the Wholesale reporting unit approximates its carrying value after giving consideration to the current period impairment charges recorded. Any future reductions to the fair value of this reporting unit would result in an additional impairment charge. If actual market conditions are less favorable than those projected, or if events occur or circumstances change that would reduce the fair values of the Wholesale, Manufacturing and The Health Store reporting units below their respective carrying values, management may be required to conduct an interim test and possibly recognize additional impairment charges in future periods.
The Company estimated the fair values of its reporting units in the fourth quarter of 2017 using a discounted cash flow method (income approach) weighted 50% and a guideline company method (market approach) weighted 50%. The key assumptions used under the income approach were, but not limited to, the following:
• | Future cash flow assumptions - The Company's projections for its reporting units were based on organic growth and were derived from historical experience and assumptions regarding future growth and profitability trends. The Company's analysis incorporated an assumed period of cash flows of 8 years with a terminal value. |
• | Discount rate - The discount rate was based on an estimated weighted average cost of capital ("WACC") for each reporting unit. The components of WACC are the cost of equity and the cost of debt, each of which requires judgment by management to estimate. The Company developed its cost of equity estimate based on perceived risks and predictability of future cash flows. The WACC used to estimate the fair values of the Company's reporting units was |
75
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
generally within a range of 17.0% to 18.0%. Any difference between the WACC among reporting units is primarily due to the precision with which management expects to be able to predict the future cash flows of each reporting unit.
The guideline company method involves analyzing transaction and financial data of publicly-traded companies to develop multiples, which are adjusted to account for differences in growth prospects and risk profiles of the reporting unit and the comparable.
Lucky Vitamin
During the second quarter of 2017, in order for the Company to focus on strategic changes around the One New GNC, the Company considered strategic alternatives for the Lucky Vitamin e-commerce business, which was considered a triggering event requiring an interim goodwill impairment review of the Lucky Vitamin reporting unit as of June 30, 2017. As previously disclosed in the Company's Annual Report on Form 10-K for the year ended December 31, 2016, the estimated fair value for the Lucky Vitamin reporting unit exceeded its carrying value by less than 20% as of December 31, 2016.
The Company estimated the fair value of the Lucky Vitamin reporting unit using a discounted cash flow method (income approach) and a guideline company method (market approach), each of which took into account the expectations regarding the potential strategic alternatives for the Lucky Vitamin business being explored in the second quarter of 2017. The key assumptions used under the income approach included, but were not limited to, the following:
• | Future cash flow assumptions - The Company's projections for Lucky Vitamin were based on organic growth and were derived from historical experience and assumptions regarding future growth and profitability trends. The Company's analysis incorporated an assumed period of cash flows of 8 years with a terminal value. |
• | Discount rate - The discount rate was based on Lucky Vitamin's estimated WACC, which was derived consistently as explained above under "Goodwill." At June 30, 2017, the WACC used to estimate the fair value of the Lucky Vitamin reporting unit was 18.0%. |
As a result of the review, the Company concluded that the carrying value of the Lucky Vitamin reporting unit exceeded its fair value, which resulted in a non-cash goodwill impairment charge of $11.5 million being recorded in the second quarter of 2017. There was no remaining goodwill balance on the Lucky Vitamin reporting unit after the impact of this charge. As a result of the impairment indicator described above, the Company also performed an impairment analysis with respect to its definite-long-lived assets on the Lucky Vitamin reporting unit, consisting of a trade name and property and equipment. The fair value of the trade name was determined using a relief from royalty method (income approach) and the fair value of the property and equipment was determined using an income approach. Based on the results of the analyses, the Company concluded that the carrying value of the Lucky Vitamin trade name and property and equipment exceed their fair values resulting in an impairment charge of $4.2 million and $3.7 million, respectively. All of the aforementioned non-cash charges totaling $19.4 million are recorded in long-lived asset impairments in the Consolidated Statement of Operations within the U.S. and Canada segment.
The Company completed an asset sale of Lucky Vitamin on September 30, 2017, resulting in a loss of $1.7 million recorded within other (income) loss, net on the Consolidated Statement of Operations consisting of the net assets sold subtracted from the purchase price of $6.4 million, which includes fees paid to a third-party. The proceeds were received in October 2017.
Discount Supplements 2015 Charge
During the third quarter of 2015, due to the declining financial performance and the Company’s decision to review strategic options for the business, a triggering event occurred requiring an interim goodwill impairment review of the Discount Supplements reporting unit as of September 30, 2015. The Company estimated the fair value of the Discount Supplements reporting unit at September 30, 2015 using a discounted cash flow method (income approach).
As a result of the review, the Company concluded that the carrying value of the Discount Supplements reporting unit exceeded its fair value and proceeded to step two of the impairment analysis. Based on the results of step two, the Company concluded that this reporting unit's goodwill was fully impaired; as a result, a goodwill impairment charge of $23.3 million was recorded in the third quarter of 2015.
76
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
As a result of the impairment indicators, the Company also performed an impairment analysis with respect to the definite-long-lived assets of Discount Supplements, consisting of trade name and website intangibles and property and equipment. The fair value of these assets were determined using various income approaches. Based on the results of the analyses, the Company recorded impairment charges of $4.4 million on the trade name and website intangible assets and $0.6 million on property and equipment. All of the aforementioned charges totaling $28.3 million were recorded in long-lived asset impairments in the Consolidated Statement of Operations for the year ended December 31, 2015.
The Company sold substantially all of the assets of Discount Supplements in the fourth quarter of 2015 and recorded a $2.7 million loss within other (income) loss net on the Consolidated Statement of Operations.
Change in Reporting Units
In connection with the Company's change in reportable segments described in Note 16, "Segments," the Company's Domestic Retail and Domestic Franchise reporting units were combined into one Domestic Stores reporting unit, consistent with how the segment manager reviews this business effective in the second quarter of 2016.
Goodwill Roll-Forward
The following table summarizes the Company's goodwill activity by reportable segment:
U.S. and Canada | International | Manufacturing / Wholesale | Other (*) | Total | |||||||||||||||
(in thousands) | |||||||||||||||||||
Goodwill at December 31, 2015 | $ | 392,410 | $ | 43,177 | $ | 202,841 | $ | 11,464 | $ | 649,892 | |||||||||
2016 Activity: | |||||||||||||||||||
Impairments | (380,644 | ) | — | (90,488 | ) | — | (471,132 | ) | |||||||||||
Acquired franchise stores | 1,372 | — | — | — | 1,372 | ||||||||||||||
Translation effect of exchange rates | 12 | (183 | ) | — | — | (171 | ) | ||||||||||||
Derecognition associated with refranchising | (3,899 | ) | — | — | — | (3,899 | ) | ||||||||||||
Total 2016 activity | (383,159 | ) | (183 | ) | (90,488 | ) | — | (473,830 | ) | ||||||||||
Balance at December 31, 2016: | |||||||||||||||||||
Gross | 389,895 | 42,994 | 202,841 | 11,464 | 647,194 | ||||||||||||||
Accumulated impairments | (380,644 | ) | — | (90,488 | ) | — | (471,132 | ) | |||||||||||
Goodwill | $ | 9,251 | $ | 42,994 | $ | 112,353 | $ | 11,464 | $ | 176,062 | |||||||||
2017 Activity: | |||||||||||||||||||
Impairments | $ | — | $ | — | $ | (24,283 | ) | $ | (11,464 | ) | $ | (35,747 | ) | ||||||
Translation effect of exchange rates | — | 714 | — | — | 714 | ||||||||||||||
Total 2017 activity | — | 714 | (24,283 | ) | (11,464 | ) | (35,033 | ) | |||||||||||
Balance at December 31, 2017: | |||||||||||||||||||
Gross | 389,895 | 43,708 | 202,841 | — | 636,444 | ||||||||||||||
Accumulated impairments | (380,644 | ) | — | (114,771 | ) | — | (495,415 | ) | |||||||||||
Goodwill | $ | 9,251 | $ | 43,708 | $ | 88,070 | $ | — | $ | 141,029 | |||||||||
(*) In connection with the sale of the assets of Lucky Vitamin in the third quarter of 2017, as described above, the gross goodwill and accumulated impairment was derecognized.
77
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Intangible Assets
The following table reflects the gross carrying amount and accumulated amortization for each major intangible asset:
December 31, 2017 | December 31, 2016 | ||||||||||||||||||||||||
Weighted- Average Life | Gross | Accumulated Amortization/ Impairment | Carrying Amount | Gross | Accumulated Amortization | Carrying Amount | |||||||||||||||||||
(in thousands) | |||||||||||||||||||||||||
Brand name | Indefinite | $ | 720,000 | $ | (395,600 | ) | $ | 324,400 | $ | 720,000 | $ | — | $ | 720,000 | |||||||||||
Retail agreements | 30.3 | 31,000 | (11,513 | ) | 19,487 | 31,000 | (10,460 | ) | $ | 20,540 | |||||||||||||||
Franchise agreements | 25.0 | 70,000 | (30,217 | ) | 39,783 | 70,000 | (27,417 | ) | 42,583 | ||||||||||||||||
Manufacturing agreements | 25.0 | 70,000 | (30,217 | ) | 39,783 | 70,000 | (27,417 | ) | 42,583 | ||||||||||||||||
Other intangibles (*) | 6.8 | 683 | (377 | ) | 306 | 10,201 | (5,467 | ) | 4,734 | ||||||||||||||||
Franchise rights | 3.0 | 7,486 | (7,130 | ) | 356 | 7,486 | (6,697 | ) | 789 | ||||||||||||||||
Total | $ | 899,169 | $ | (475,054 | ) | $ | 424,115 | $ | 908,687 | $ | (77,458 | ) | $ | 831,229 | |||||||||||
(*) In connection with the sale of the assets of Lucky Vitamin in the third quarter of 2017, as described above, the gross trade name and accumulated amortization/impairment was derecognized.
Amortization expense during the years ended December 31, 2017, 2016 and 2015 was $7.4 million, $8.2 million and $10.3 million, respectively.
The following table represents future amortization expense of definite-lived intangible assets at December 31, 2017:
Years ending December 31, | Amortization expense | ||
(in thousands) | |||
2018 | $ | 6,974 | |
2019 | 6,835 | ||
2020 | 6,771 | ||
2021 | 6,693 | ||
2022 | 6,653 | ||
Thereafter | 65,789 | ||
Total future amortization expense | $ | 99,715 | |
Store Acquisitions
For the years ended December 31, 2017, 2016 and 2015, the Company acquired 60, 21 and 44 franchise stores, respectively. These acquisitions are accounted for utilizing the acquisition method of accounting, and the Company allocated the purchase price by recognizing acquired inventory, fixed assets, franchise rights and other net assets at fair value with any excess being recorded as goodwill. For the years ended December 31, 2017, 2016 and 2015, the impact of these store acquisitions was not material.
78
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 6. PROPERTY, PLANT AND EQUIPMENT, NET
Property, plant and equipment, net, consisted of the following:
December 31, | |||||||
2017 | 2016 | ||||||
(in thousands) | |||||||
Land, buildings and improvements | $ | 73,287 | $ | 72,119 | |||
Machinery and equipment | 170,107 | 172,261 | |||||
Leasehold improvements | 146,830 | 144,667 | |||||
Furniture and fixtures | 108,085 | 108,998 | |||||
Software | 50,098 | 64,264 | |||||
Construction in progress | 1,710 | 6,346 | |||||
Total property, plant and equipment | 550,117 | 568,655 | |||||
Less: accumulated depreciation | (341,267 | ) | (330,942 | ) | |||
Less: impairment | (22,288 | ) | (5,421 | ) | |||
Net property, plant and equipment | $ | 186,562 | $ | 232,292 | |||
The Company recognized depreciation expense on property, plant and equipment of $49.4 million, $51.8 million, and $47.0 million for the years ended December 31, 2017, 2016 and 2015, respectively, which is included in occupancy expense as part of cost of sales and SG&A expense on the Consolidated Statements of Operations.
Fixed Assets Impairments
Management evaluated its property, plant, and equipment and recorded $18.6 million and $5.4 million of impairment charges within the U.S. and Canada segment for the years ended December 31, 2017 and 2016, respectively, presented as long-lived asset impairments in the accompanying Consolidated Statement of Operations. These impairments primarily relate to certain of the Company's underperforming stores and to a lesser extent the 2017 impact of Hurricane Maria on the Company's stores located in Puerto Rico. For individual underperforming stores, the impairment test was performed at the individual store level as this is the lowest level which identifiable cash flows are largely independent of other groups of assets and liabilities.
Underperforming stores were generally defined as those with historical and expected future losses or stores that management intends on closing in the near term. If the undiscounted estimated future cash flows were less than the carrying value of the asset group, an impairment charge was calculated by subtracting the estimated fair value of property and equipment from its carrying value. Fair value was estimated using a discounted cash flow method (income approach) utilizing the undiscounted cash flows estimated in the first step of the test. Refer to Note 5, "Goodwill and Intangible Assets" for fixed asset impairments related to Lucky Vitamin in 2017 and Discount Supplements in 2015 prior to their respective sales.
NOTE 7. LONG-TERM DEBT / INTEREST EXPENSE
As of December 31, 2017, the Company had principal of $1,131.2 million outstanding under its Term Loan Facility with an original maturity date of March 2019. After the effectiveness of the Amendment to our Senior Credit Facility described in Note 18, "Subsequent Events," the amount due in March 2019 under the Term Loan Facility is now $151.9 million. The Company is also required under the Amendment to make an excess cash flow payment in March 2019, which could be material, based on the projected Consolidated Net First Lien Leverage Ratio for the year ending December 31, 2018.
The Company expects to close the Securities Purchase Agreement described in Note 18, "Subsequent Events" in the second half of 2018, which will result in approximately $300 million of aggregate proceeds. Under the Amendment, $100.0 million of the net proceeds from the Securities Purchase Agreement are required to be utilized to pay the Term Loan Facility due March 2021. The remaining net proceeds, after deducting legal and advisory fees, would be available to satisfy the $151.9 million due under the Term Loan Facility in March 2019. However, there is no assurance that the Securities Purchase Agreement will close prior to March 2019 when the obligation is due.
In the event that Securities Purchase Agreement doesn’t close, management has concluded that the Company will have the ability to satisfy the $151.9 million Term Loan Facility and the excess cash flow payments due in March 2019 with projected
79
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
cash on hand and amounts available under the new $100 million asset-backed Revolving Credit Facility. If actual results are below the Company’s projections by an amount greater than what is required to satisfy these obligations, management has the ability to reduce certain discretionary payments and will consider certain sales, as necessary, to maximize cash available.
Management assessed the Company's ability to continue as a going concern in accordance with ASU 2014-15, "Disclosures of Uncertainties about an Entity's Ability to Continue as a Going Concern." Management has concluded that it is probable the Company will have sufficient cash on hand and available liquidity to satisfy the obligations that are due in March 2019.
Refer to Note 18, "Subsequent Events" for information regarding the Company's Amendment of its Senior Credit Facility in February 2018. All information regarding the Company's Senior Credit Facility within this footnote is as of December 31, 2017 prior to the Amendment.
Long-term debt consisted of the following:
December 31, | |||||||
2017 | 2016 | ||||||
(in thousands) | |||||||
Term Loan Facility (net of $0.9 million and $1.6 million discount) | $ | 1,130,320 | $ | 1,170,486 | |||
Revolving Credit Facility | — | 127,000 | |||||
Notes | 167,988 | 245,273 | |||||
Debt issuance costs | (1,285 | ) | (2,306 | ) | |||
Total debt | $ | 1,297,023 | $ | 1,540,453 | |||
Less: current maturities | — | (12,562 | ) | ||||
Long-term debt | $ | 1,297,023 | $ | 1,527,891 | |||
At December 31, 2017, the Company's future annual contractual obligations on long-term debt are detailed below:
Year Ending December 31, | Term Loan Facility (1) | Convertible Notes (2) | Total | ||||||||
(in thousands) | |||||||||||
2019 | $ | 1,131,197 | $ | — | $ | 1,131,197 | |||||
2020 | — | 188,565 | 188,565 | ||||||||
Total | $ | 1,131,197 | $ | 188,565 | $ | 1,319,762 | |||||
(1) Includes the unamortized original issuance discount of $0.9 million.
(2) Includes unamortized conversion feature of $18.1 million and original issuance discount of $2.5 million.
Senior Credit Facility
In March 2011, General Nutrition Centers, Inc. ("Centers"), a wholly owned subsidiary of Holdings, entered into the Senior Credit Facility, consisting of the Term Loan Facility and the Revolving Credit Facility. The Senior Credit Facility permits the Company to prepay a portion or all of the outstanding balance without incurring penalties (except London Interbank Offering Rate ("LIBOR") breakage costs). GNC Corporation, the Company's indirect wholly owned subsidiary, and Centers' existing and future domestic subsidiaries have guaranteed Centers' obligations under the Senior Credit Facility. In addition, the Senior Credit Facility is collateralized by first priority pledges (subject to permitted liens) of substantially all of Centers' assets, including its equity interests and the equity interests of its domestic subsidiaries.
The Company amended the Revolving Credit Facility on March 4, 2016, to extend its maturity from March 2017 to September 2018 and increase total availability from $130.0 million to $300.0 million. In December 2017, the Company reduced the amount available under the Revolving Credit Facility from $300.0 million to $225.0 million. As of December 31, 2017, the Company had $219.1 million available under the Revolving Credit Facility after consideration of $5.9 million utilized to secure letters of credit and no borrowings outstanding, which was subsequently terminated and replaced with the asset-based Revolving Credit Facility as described in Note 18, "Subsequent Events."
80
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
As of December 31, 2017 and 2016, the Company's interest rate on its Term Loan Facility was 4.1% and 3.3%, respectively. The Revolving Credit Facility had a weighted average interest rate of 2.7% at December 31, 2016. The Company is also required to pay an annual fee of 2.75% on outstanding letters of credit and an annual commitment fee of 0.5% on the undrawn portion of the Revolving Credit Facility.
The Senior Credit Facility contains customary covenants, including incurrence covenants and certain other limitations on the ability of GNC Corporation, Centers, and Centers' subsidiaries to, among other things, make optional payments in respect of other debt instruments, pay dividends or other payments on capital stock, and enter into arrangements that restrict their ability to pay dividends or grant liens. The Company is currently in compliance with the terms of its Senior Credit Facility.
Convertible Debt
Issuance and Terms
On August 10, 2015, the Company issued $287.5 million principal amount of 1.5% convertible senior notes due 2020 in a private offering (the "Notes"). The Notes are governed by the terms of an indenture between the Company and BNY Mellon Trust Company, N.A., as the Trustee (the "Indenture"). The Notes mature on August 15, 2020, unless earlier repaid, discharged, refinanced or converted by the holders subject to restrictions through May 15, 2020. The Notes bear interest at a rate of 1.5% per annum, and additionally are subject to special interest in connection with any failure of the Company to perform certain of its obligations under the Indenture.
The Notes are unsecured obligations and do not contain any financial covenants or restrictions on the payments of dividends, the incurrence of indebtedness or the issuance or repurchase of securities by the Company or any of its subsidiaries. Certain events are considered “events of default” under the Notes, which may result in the acceleration of the maturity of the Notes, as described in the indenture governing the Notes. The Notes are fully and unconditionally guaranteed by certain operating subsidiaries of the Company (“Subsidiary Guarantors”) and are subordinated to the Subsidiary Guarantors obligations from time to time with respect to the Senior Credit Facility and ranks equal in right of payment with respect to the Subsidiary Guarantor’s other obligations.
The initial conversion rate applicable to the Notes is 15.1156 shares of common stock per $1,000 principal amount of Notes, which is equivalent to an initial conversion price of $66.16 per share. The conversion rate is subject to adjustment upon the occurrence of certain specified events, but will not be adjusted for any accrued and unpaid special interest. In addition, upon the occurrence of a “make-whole fundamental change" as defined in the Indenture, the Company will, in certain circumstances, increase the conversion rate by a number of additional shares for a holder that elects to convert its Notes in connection with such make-whole fundamental change.
Prior to May 15, 2020, the Notes are convertible only under the following circumstances: (1) during any calendar quarter commencing after September 30, 2015, if, for at least 20 trading days (whether or not consecutive) during the 30 consecutive trading day period ending on the last trading day of the immediately preceding calendar quarter, the last reported sale price of the Company’s common stock on such trading day is greater than or equal to 130% of the applicable conversion price on such trading day; (2) during the 5 consecutive business day period after any ten consecutive trading day period in which, for each day of that period, the trading price per $1,000 principal amount of Notes for such trading day was less than 98% of the product of the last reported sale price of the Company’s common stock and the applicable conversion rate on such trading day; or (3) upon the occurrence of specified corporate transactions. On and after May 15, 2020, until the close of business on the second scheduled trading day immediately preceding the maturity date, holders may convert all or a portion of their Notes at any time, regardless of the foregoing circumstances. Upon conversion, the Notes will be settled, at the Company’s election, in cash, shares of the Company’s common stock, or a combination of cash and shares of the Company’s common stock. If the Company has not delivered a notice of its election of settlement method prior to the final conversion period, it will be deemed to have elected combination settlement with a dollar amount per note to be received upon conversion of $1,000.
Exchange
On December 20, 2017, the Company exchanged in privately negotiated transactions $98.9 million in aggregate principal amount of the Notes for an aggregate of 14.6 million newly issued shares of the Company’s Class A common stock, which had a value of $71.7 million at the time of the exchange. The Company accounted for the transaction as a troubled debt restructuring as a result of satisfying the below criteria.
81
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
• | Based on previous challenges associated with the Company’s refinancing efforts of its long term debt at the time of the convertible debt exchange. |
• | The holders of the convertible debt completed the exchange for a value lower than the face amount of the notes. As a result, management concluded a concession was granted to the Company. |
The convertible debt exchange resulted in a gain of $15.0 million, which includes the unamortized conversion feature of $9.6 million, unamortized discount of $1.4 million and other third party fees of $1.2 million and together with legal, investment banking and rating agency fees associated with the Company’s refinancing efforts, the Company recorded a net gain of $11.0 million in the fourth quarter of 2017.
Notes by Component
The Notes consist of the following components:
As of December 31, | |||||||
2017 | 2016 | ||||||
(in thousands) | |||||||
Liability component | |||||||
Principal | $ | 188,565 | $ | 287,500 | |||
Conversion feature | (18,065 | ) | (37,179 | ) | |||
Discount related to debt issuance costs | (2,512 | ) | (5,048 | ) | |||
Net carrying amount | $ | 167,988 | $ | 245,273 | |||
Equity component | |||||||
Conversion feature | $ | 49,680 | $ | 49,680 | |||
Debt issuance costs | (1,421 | ) | (1,421 | ) | |||
Deferred taxes (*) | (16,620 | ) | (17,750 | ) | |||
Net amount recorded in additional paid-in capital | $ | 31,639 | $ | 30,509 | |||
* The balance at December 31, 2017 includes $1.1 million related to the tax provision that was allocated to additional paid-in capital associated with the exchange explained above.
82
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Interest Expense
Interest expense consisted of the following:
For the year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(in thousands) | |||||||||||
Senior Credit Facility: | |||||||||||
Term Loan Facility coupon | $ | 41,477 | $ | 38,821 | $ | 42,147 | |||||
Revolving Credit Facility | 4,685 | 4,689 | 805 | ||||||||
Amortization of discount and debt issuance costs | 2,413 | 2,444 | 2,583 | ||||||||
Total Senior Credit Facility | 48,575 | 45,954 | 45,535 | ||||||||
Notes: | |||||||||||
Coupon | 4,272 | 4,313 | 1,702 | ||||||||
Amortization of conversion feature | 9,496 | 9,092 | 3,410 | ||||||||
Amortization of discount and debt issuance costs | 1,251 | 1,140 | 412 | ||||||||
Total Notes | 15,019 | 14,545 | 5,524 | ||||||||
Interest income and other | 627 | (56 | ) | (123 | ) | ||||||
Interest expense, net | $ | 64,221 | $ | 60,443 | $ | 50,936 | |||||
83
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 8. DEFERRED REVENUE AND OTHER CURRENT LIABILITIES
Deferred revenue and other current liabilities consisted of the following:
December 31, | |||||||
2017 | 2016 | ||||||
(in thousands) | |||||||
Deferred revenue | $ | 34,802 | $ | 38,044 | |||
Accrued compensation and related benefits | 32,177 | 34,700 | |||||
Accrued occupancy | 8,732 | 8,815 | |||||
Accrued sales tax | 3,022 | 2,626 | |||||
Accrued interest | 2,124 | 2,383 | |||||
Accrued income taxes | — | 1,454 | |||||
Other current liabilities | 27,815 | 27,149 | |||||
Total deferred revenue and other current liabilities | $ | 108,672 | $ | 115,171 | |||
Deferred revenue at December 31, 2016 includes $24.4 million associated with the domestic company-owned Gold Card Member Pricing program, which was discontinued on December 28, 2016 in connection with the introduction of the One New GNC and replaced with a points-based loyalty program, myGNC Rewards, and a paid membership program, PRO Access. Refer to Note 2, "Basis of Presentation and Summary of Significant Accounting Policies" for more information.
NOTE 9. FAIR VALUE MEASUREMENTS AND FINANCIAL INSTRUMENTS
ASC 820, "Fair Value Measurements and Disclosures" defines fair value as a market-based measurement that should be determined based on the assumptions that marketplace participants would use in pricing an asset or liability. As a basis for considering such assumptions, the standard establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value as follows:
Level 1 — observable inputs such as quoted prices in active markets for identical assets and liabilities; |
Level 2 — observable inputs such as quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, other inputs that are observable, or can be corroborated by observable market data; and |
Level 3 — unobservable inputs for which there are little or no market data, which require the reporting entity to develop its own assumptions. |
The carrying amounts of cash and cash equivalents, receivables, accounts payable, accrued liabilities and the Revolving Credit Facility approximate their respective fair values. Based on the interest rates currently available and their underlying risk, the carrying value of franchise notes receivable recorded primarily in Other long-term assets approximates its fair value.
The carrying value and estimated fair value of the Term Loan Facility, net of discount, and Notes (net of the equity component classified in stockholders' equity and discount) were as follows:
December 31, 2017 | December 31, 2016 | ||||||||||||||
Carrying Amount | Fair Value | Carrying Amount | Fair Value | ||||||||||||
(in thousands) | |||||||||||||||
Term Loan Facility | $ | 1,130,320 | $ | 930,592 | $ | 1,170,486 | $ | 1,100,257 | |||||||
Notes | 167,988 | 85,044 | 245,273 | 185,794 | |||||||||||
The fair values of the Term Loan Facility and the Notes were determined using trading values in markets that are not active, which are considered Level 2 inputs.
As described in Note 5, "Goodwill and Intangible Assets, Net," and Note 6, "Property, Plant and Equipment, Net," the Company recorded long-lived asset impairments in the years ended December 31, 2017, 2016 and 2015. This resulted in the following assets being measured at fair value on a non-recurring basis using Level 3 inputs:
•the indefinite-lived brand name intangible asset at December 31, 2017;
•goodwill at December 31, 2017 for the Wholesale reporting unit;
•goodwill at December 31, 2016 for the Domestic Stores, Canada and Manufacturing reporting units; and
•property and equipment at certain of the Company's stores at December 31, 2017 and 2016.
NOTE 10. LONG-TERM LEASE OBLIGATIONS
The Company's rent expense, which is recorded within cost of sales on the Consolidated Statements of Operations, was as follows:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(in thousands) | |||||||||||
Company-owned and franchise stores: | |||||||||||
Rent on operating leases | $ | 193,398 | $ | 193,830 | $ | 187,346 | |||||
Landlord related taxes | 27,872 | 27,747 | 25,765 | ||||||||
Common operating expenses | 45,866 | 45,375 | 44,184 | ||||||||
Percent and contingent rent | 17,870 | 19,435 | 21,109 | ||||||||
Total company-owned and franchise stores | 285,006 | 286,387 | 278,404 | ||||||||
Other | 22,446 | 19,905 | 16,568 | ||||||||
Total rent expense | $ | 307,452 | $ | 306,292 | $ | 294,972 | |||||
The Company recorded sublease revenue, within revenue on the Consolidated Statements of Operations, of $49.0 million, $47.6 million and $44.1 million in the years ended December 31, 2017, 2016 and 2015, respectively, relating to subleases with its franchisees, which includes rental income and other occupancy related items.
Minimum future rent obligations for non-cancelable operating leases, excluding optional renewal periods, were as follows for the years ending December 31 and exclude landlord related taxes, common operating expenses, and percent and contingent rent.
Company-Owned and Franchise Stores | Sublease Income from Franchisees | Other * | Rent on Operating Leases, net of Sublease Revenue | ||||||||||||
(in thousands) | |||||||||||||||
2018 | $ | 176,770 | $ | (31,936 | ) | $ | 4,984 | $ | 149,818 | ||||||
2019 | 139,772 | (25,382 | ) | 4,314 | 118,704 | ||||||||||
2020 | 107,685 | (19,611 | ) | 3,836 | 91,910 | ||||||||||
2021 | 81,289 | (13,115 | ) | 2,618 | 70,792 | ||||||||||
2022 | 53,743 | (6,947 | ) | 1,638 | 48,434 | ||||||||||
Thereafter | 92,234 | (7,744 | ) | 8,548 | 93,038 | ||||||||||
Total future obligations | $ | 651,493 | $ | (104,735 | ) | $ | 25,938 | $ | 572,696 | ||||||
* Includes various leases for warehouses, vehicles, and various equipment at our facility
84
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 11. COMMITMENTS AND CONTINGENCIES
The Company is engaged in various legal actions, claims and proceedings arising in the normal course of business, including claims related to breach of contracts, products liabilities, intellectual property matters and employment-related matters resulting from the Company's business activities.
The Company's contingencies are subject to substantial uncertainties, including for each such contingency the following, among other factors: (i) the procedural status of the case; (ii) whether the case has or may be certified as a class action suit; (iii) the outcome of preliminary motions; (iv) the impact of discovery; (v) whether there are significant factual issues to be determined or resolved; (vi) whether the proceedings involve a large number of parties and/or parties and claims in multiple jurisdictions or jurisdictions in which the relevant laws are complex or unclear; (vii) the extent of potential damages, which are often unspecified or indeterminate; and (viii) the status of settlement discussions, if any, and the settlement posture of the parties. Consequently, except as otherwise noted below with regard to a particular matter, the Company cannot predict with any reasonable certainty the timing or outcome of the legal matters described below, and the Company is unable to estimate a possible loss or range of loss. If the Company ultimately is required to make a payment in connection with an adverse outcome in any of the matters discussed below, it is possible that it could have a material adverse effect on the Company's business, financial condition, results of operations or cash flows.
As a manufacturer and retailer of nutritional supplements and other consumer products that are ingested by consumers or applied to their bodies, the Company has been and is currently subjected to various product liability claims. Although the effects of these claims to date have not been material to the Company, it is possible that current and future product liability claims could have a material adverse effect on its business or financial condition, results of operations or cash flows. The Company currently maintains product liability insurance with a deductible/retention of $4.0 million per claim with an aggregate cap on retained loss of $10.0 million per policy year. The Company typically seeks and has obtained contractual indemnification from most parties that supply raw materials for its products or that manufacture or market products it sells. The Company also typically seeks to be added, and has been added, as an additional insured under most of such parties' insurance policies. However, any such indemnification or insurance is limited by its terms and any such indemnification, as a practical matter, is limited to the creditworthiness of the indemnifying party and its insurer, and the absence of significant defenses by the insurers. Consequently, the Company may incur material product liability claims, which could increase its costs and adversely affect its reputation, revenue and operating income.
During the year ended December 31, 2017, the Company recorded $4.4 million in net legal-related charges associated with the Holland and Barrett license litigation, E-Commerce pricing matters and an update to the Pennsylvania fluctuating workweek wage issue. During the year ended December 31, 2016, the Company recorded $5.1 million in legal-related charges associated with a Pennsylvania fluctuating workweek wage issue, the Jason Olive case and a government regulation matter. These amounts were individually immaterial and are explained below in more detail.
The Company entered into a settlement agreement with a third-party in December 2017 and recognized a gain, net of legal costs, the proceeds of which were received in the fourth quarter of 2017. The settlement represents estimated losses incurred as certain key aspects of the Company's media campaign around the One New GNC launch were not executed as planned. This gain was recorded as a reduction to marketing and legal expense within selling, general and administrative expense on the accompanying Consolidated Statement of Operations for the year ended December 31, 2017.
Litigation
DMAA / Aegeline Claims. Prior to December 2013, the Company sold products manufactured by third parties that contained derivatives from geranium known as 1.3-dimethylpentylamine/ dimethylamylamine/13-dimethylamylamine, or "DMAA," which were recalled from the Company's stores in November 2013, and/or Aegeline, a compound extracted from bael trees. As of December 31, 2017, the Company was named in 32 personal injury lawsuits involving products containing DMAA and/or Aegeline.
As a general matter, the proceedings associated with these personal injury cases, which generally seek indeterminate money damages, are in the early stages, and any losses that may arise from these matters are not probable or reasonably estimable at this time.
The Company is contractually entitled to indemnification by its third-party vendors with regard to these matters, although the Company’s ability to obtain full recovery in respect of any such claims against it is dependent upon the creditworthiness of the vendors and/or their insurance coverage and the absence of any significant defenses available to its insurer.
85
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
California Wage and Break Claims. On February 29, 2012, former Senior Store Manager, Elizabeth Naranjo, individually and on behalf of all others similarly situated sued General Nutrition Corporation in the Superior Court of the State of California for the County of Alameda. The complaint contains eight causes of action, alleging, among other matters, meal, rest break, and overtime violations. As of December 31, 2017, an immaterial liability has been accrued in the accompanying financial statements. The Company intends to conduct further discovery and file a motion to decertify the class action prior to the trial, which is scheduled for July 2018.
Pennsylvania Fluctuating Workweek. On September 18, 2013, Tawny Chevalier and Andrew Hiller commenced a class action in the Court of Common Pleas of Allegheny County, Pennsylvania. Plaintiff asserted a claim against the Company for a purported violation of the Pennsylvania Minimum Wage Act (PMWA), challenging the Company's utilization of the "fluctuating workweek" method to calculate overtime compensation, on behalf of all employees who worked for the Company in Pennsylvania and who were paid according to the fluctuating workweek method. In October 2014, the Court entered an order holding that the use of the fluctuating workweek method violated the PMWA. In September 2016, the Court entered judgment in favor of Plaintiffs and the class in an immaterial amount, which has been recorded as a charge in the accompanying Consolidated Financial Statements. Plaintiffs subsequently filed a petition for an award of attorney's fees, costs and incentive payment. The court awarded an immaterial amount in legal fees. The Company appealed from the adverse judgment and the award of attorney's fees. On December 22, 2017, the Pennsylvania Superior Court held that the Company correctly determined the "regular rate" by dividing weekly compensation by all hours worked (rather than 40), but held that the regular rate must be multiplied by 1.5 (rather than 0.5) to determine the amount of overtime owed. Taking accumulated interest into account, the net result of the Superior Court's decision was to reduce the Company's liability by an immaterial amount, which has been reflected in the accompanying Consolidated Financial Statements. The Company filed a petition for appeal to the Pennsylvania Supreme Court on January 22, 2018.
Jason Olive v. General Nutrition Corp. In April 2012, Jason Olive filed a complaint in the Superior Court of California, County of Los Angeles, for misappropriation of likeness in which he alleges that the Company continued to use his image in stores after the expiration of the license to do so in violation of common law and California statutes. Mr. Olive is seeking compensatory, punitive and statutory damages and attorneys’ fees and costs. The trial in this matter began on July 20, 2016 and concluded on August 8, 2016. The jury awarded plaintiff immaterial amounts for actual damages and emotional distress damages, which are accrued in the accompanying Consolidated Financial Statements. The jury refused to award plaintiff any of the profits he sought to disgorge, or punitive damages. The court entered judgment in the case on October 14, 2016. In addition to the verdict, the Company and Mr. Olive sought attorneys' fees and other costs from the Court. The Court refused to award attorney's fees to either side but awarded plaintiff an immaterial amount for costs. Plaintiff has appealed the judgment, and separately, the order denying attorney's fees. The Company has cross-appealed the judgment and the Court's denial of attorney fees. The appeals are currently pending.
Oregon Attorney General. On October 22, 2015, the Attorney General for the State of Oregon sued GNC in Multnomah County Circuit Court for alleged violations of Oregon’s Unlawful Trade Practices Act, in connection with its sale in Oregon of certain third-party products. The Company is vigorously defending itself against these allegations. Along with its Amended Answer and Affirmative Defenses, the Company filed a counterclaim for declaratory relief, asking the court to make certain rulings in favor of the Company. USPlabs, LLC and SK Laboratories have been joined to the case as defendants to the Company's counterclaim but have yet to enter an appearance. In September 2017, SK Laboratories filed a motion to dismiss for lack of personal jurisdiction. USP Laboratories also filed a motion to dismiss for improper venue or in the alternative motion to stay the litigation pending the criminal trial of USPlabs. The Company is contesting both motions, which are pending. As any losses that may arise from this matter are not probable or reasonably estimable at this time, no liability has been accrued in the accompanying Consolidated Financial Statements. Moreover, the Company does not anticipate that any such losses are likely to have a material impact on the Company, its business or results of operations. The Company is contractually entitled to indemnification and defense by its third-party vendors. Ultimately, however, the Company's ability to obtain full recovery in respect of any such claims against it is dependent upon the creditworthiness of its vendors and/or their insurance coverage and the absence of any significant defenses available to their insurers.
Holland and Barrett License Litigation. On September 18, 2014, the Company's wholly-owned affiliate General Nutrition Investment Company ("GNIC") commenced proceedings in the UK High Court to determine if the license agreement from March 2003 between GNIC and Holland & Barrett International Ltd and Health and Diet Centers Ltd. (“Defendants”) was validly terminated. GNIC alleged that termination of the entire agreement was warranted due to several material breaches by Defendants, and that the agreement should be terminated related to five licensed GNC trademarks for lack of use for more than five years. On April 7, 2017, the Court issued its judgment that found that GNIC's notice of termination was invalid and while there were several breaches of the agreement, none were sufficiently material to justify termination. Under UK procedural rules, GNIC is required
86
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
to pay some portion of Defendant’s legal costs. As a result, the Company recorded a charge of $2.1 million in the first quarter of 2017 and subsequently reached an agreement with the Defendants in relation to costs. The Defendants appealed part of the Court's judgment concerning findings in relation to the licensed GNIC trademarks, and that appeal will be heard the UK's Court of Appeal in June 2018.
E-Commerce Pricing Matters. In April 2016, Jenna Kaskorkis, et al. filed a complaint against General Nutrition Centers, Inc. followed by similar cases brought forth by Ashley Gennock in May 2016 and Kenneth Harrison in December 2016. Plaintiffs allege that the Company's promotional pricing on its website was misleading and did not fairly represent promotions based on average retail prices over a trended period of time being consistent with prices advertised as promotional. The Company attended a mediation with counsel for all plaintiffs and has reached tentative agreement in the third quarter of 2017 on many of the key terms of a settlement. The matters have been effectively stayed while the parties remain in discussions. The Company currently expects any settlement to be in a form that does not require the recording of a contingent liability, except an immaterial amount the Company has accrued in the accompanying Consolidated Financial Statements.
Government Regulation
In November 2013, the Company received a subpoena from the U.S. Department of Justice ("DOJ") for information related to its investigation of a third party product vendor, USPlabs, LLC. The Company fully cooperated with the investigation of the vendor and the related products, all of which were discontinued in 2013. In December 2016, the Company reached agreement with the DOJ in connection with the Company's cooperation, which agreement acknowledges the Company relied on the representations and written guarantees of USPlabs and the Company's representation that it did not knowingly sell products not in compliance with the FDCA. Under the agreement, which includes an immaterial payment to the federal government, the Company will take a number of actions to broaden industry-wide knowledge of prohibited ingredients and improve compliance by vendors of third party products. These actions are in keeping with the leadership role the Company has taken in setting industry quality and compliance standards, and the Company's commitment over the course of the agreement (60 months) to support a combination of its and the industry's initiatives. Some of these actions include maintaining and continuously updating a list of restricted ingredients that will be prohibited from inclusion in any products that are sold by the Company. Vendors selling products to the Company for the sale of such products by the Company will be required to warrant that the products sold do not contain any of these restricted ingredients. In addition, the Company will develop and maintain a list of ingredients that the Company believes comply with the applicable provisions of the FDCA.
Environmental Compliance
In March 2008, the South Carolina Department of Health and Environmental Control (the "DHEC") requested that the Company investigate contamination associated with historical activities at its South Carolina facility. These investigations have identified chlorinated solvent impacts in soils and groundwater that extend offsite from the facility. The Company entered into a Voluntary Cleanup Contract with the DHEC regarding the matter on September 24, 2012. Pursuant to such contract, the Company has completed additional investigations with the DHEC's approval. The Company installed and began operating a pilot vapor extraction system under a portion of the facility in the second half of 2016, which was an immaterial cost to the Company, with DHEC's approval to assess the effectiveness of such a remedial system. After an initial period of monitoring, in October of 2017, the DHEC approved a work plan for extended monitoring of such system and the contamination into 2021. The Company will continue to consult with the DHEC on the next steps in the work after their review of the results of the extended monitoring is complete. At this stage of the investigation, however, it is not possible to estimate the timing and extent of any additional remedial action that may be required, the ultimate cost of remediation, or the amount of the Company's potential liability. Therefore, no liability has been recorded in the Company's Consolidated Financial Statements.
In addition to the foregoing, the Company is subject to numerous federal, state, local and foreign environmental and health and safety laws and regulations governing its operations, including the handling, transportation and disposal of the Company's non-hazardous and hazardous substances and wastes, as well as emissions and discharges from its operations into the environment, including discharges to air, surface water and groundwater. Failure to comply with such laws and regulations could result in costs for remedial actions, penalties or the imposition of other liabilities. New laws, changes in existing laws or the interpretation thereof, or the development of new facts or changes in their processes could also cause the Company to incur additional capital and operating expenditures to maintain compliance with environmental laws and regulations and environmental permits. The Company is also subject to laws and regulations that impose liability and cleanup responsibility for releases of hazardous substances into the environment without regard to fault or knowledge about the condition or action causing the liability. Under certain of these laws and regulations, such liabilities can be imposed for cleanup of previously owned or operated properties, or for properties to which substances or wastes that were sent in connection with current or former operations at its facilities. The presence of contamination
87
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
from such substances or wastes could also adversely affect the Company's ability to sell or lease its properties, or to use them as collateral for financing. From time to time, the Company has incurred costs and obligations for correcting environmental and health and safety noncompliance matters and for remediation at or relating to certain of the Company's properties or properties at which the Company's waste has been disposed. However, compliance with the provisions of national, state and local environmental laws and regulations has not had a material effect upon the Company's capital expenditures, earnings, financial position, liquidity or competitive position. The Company believes it has complied with, and is currently complying with, its environmental obligations pursuant to environmental and health and safety laws and regulations and that any liabilities for noncompliance will not have a material adverse effect on its business, financial performance or cash flows. However, it is difficult to predict future liabilities and obligations, which could be material.
Commitments
In addition to operating leases obtained in the normal course of business, the Company maintains certain purchase commitments with various vendors to ensure its operational needs are fulfilled. As of December 31, 2017, such future purchase commitments were $22.8 million. Other commitments related to the Company's business operations cover varying periods of time and are not significant. All of these commitments are expected to be fulfilled with no adverse consequences to the Company's operations or financial condition.
NOTE 12. STOCKHOLDERS' EQUITY
Treasury Stock
In August 2015, the Board approved a $500.0 million multi-year repurchase program in addition to the $500.0 million multi-year program approved in August 2014, bringing the aggregate share repurchase program to $1.0 billion of Holdings' common stock. Holdings repurchased $229.2 million and $479.8 million of common stock during 2016 and 2015, respectively. No shares were repurchased in 2017. As of December 31, 2017, $197.8 million remains available for purchase under the program, which is not expected to be utilized in the future.
Preferred Stock
Holdings is authorized to issue up to 60.0 million shares of preferred stock, par value $0.001 per share. No shares of preferred stock were issued to date.
NOTE 13. EARNINGS PER SHARE
The following table represents the Company's basic and dilutive weighted average shares:
Year ended December 31, | ||||||||
2017 | 2016 | 2015 | ||||||
(in thousands) | ||||||||
Basic weighted average shares | 68,789 | 69,409 | 83,927 | |||||
Effect of dilutive stock-based compensation awards | — | — | 259 | |||||
Diluted weighted averages shares | 68,789 | 69,409 | 84,186 | |||||
For the year ended December 31, 2017 and December 31, 2016, all 4.0 million and 1.5 million outstanding stock-based awards, respectively, were excluded from the computation of diluted EPS because the Company was in a net loss position and as a result, inclusion of the awards would have been anti-dilutive. For the year ended December 31, 2015, the following awards were not included in the computation of diluted EPS because the impact of applying the treasury stock method was antidilutive or because certain conditions have not been met with respect to the Company's performance-based awards.
88
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Antidilutive: | ||
Time-based | 161 | |
Contingently issuable: | ||
Performance-based | 139 | |
Total stock-based awards | 300 | |
In connection with the exchange of the Company's Notes as described in Note 7, "Long-Term Debt / Interest Expense," the Company issued 14.6 million shares, which are included in basic and diluted earnings per share for the weighted average days they were outstanding in 2017. The remaining underlying convertible shares were anti-dilutive in all periods presented. The Company no longer has the intent to settle the principal portion of Notes in cash, and as such, will apply the if-converted method to calculate dilution in future periods with net income.
NOTE 14. STOCK-BASED COMPENSATION
Stock and Incentive Plans
The Company has outstanding stock-based compensation awards that were granted by the compensation committee of Holdings' Board of Directors (the "Compensation Committee") under the following two stock-based employee compensation plans:
•the GNC Holdings, Inc. 2015 Stock and Incentive Plan (the "2015 Stock Plan") amended and adopted in May 2015, formerly the GNC Holdings, Inc. 2011 Stock and Incentive Plan adopted in March 2011; and
•the GNC Acquisition Holdings Inc. 2007 Stock Incentive Plan adopted in March 2007 (as amended, the "2007 Stock Plan").
Both plans have provisions that allow for the granting of stock options, restricted stock and other stock-based awards and are available to eligible employees, directors, consultants or advisors as determined by the Compensation Committee. The Company will not grant any additional awards under the 2007 Stock Plan. Up to 11.5 million shares of common stock may be issued under the 2015 Stock Plan (subject to adjustment to reflect certain transactions and events specified in the 2015 Stock Plan for any award grant), of which 4.6 million shares remain available for issuance as of December 31, 2017.
Non-Plan Inducement Awards
On September 11, 2017, in connection with the appointment of the Company's new Chief Executive Officer, the Company made the following non-plan inducement awards:
• | "make-whole" restricted stock awards consisting of the following: |
◦ | $600,000, which are 67,000 fully vested restricted shares with transfer restrictions that lapse on the earliest to occur of a Change in Control of the Company, the third anniversary of grant or death, disability or other separation from service for any reason; |
◦ | $950,000, which are 106,000 restricted shares that vested on December 29, 2017; and |
◦ | $1,200,000, which are 134,000 unvested restricted shares scheduled to vest in three equal installments on each of the first three anniversaries of grant subject to acceleration to cover any applicable income and payroll tax withholding resulting from the recognition of ordinary income pursuant to a Section 83(b) election ("Section 83(b) Tax Liability"); and |
• | time-vested awards consisting of 212,000 restricted shares and 519,000 stock options in the amount of $1,900,000 each, which are scheduled to vest in three equal installments on each of the first three anniversaries of grant. |
The Company recognized $2.6 million in stock-based compensation in 2017, primarily relating to the make-whole awards, which includes the impact of acceleration of vesting associated with the Section 83(b) Tax Liability that together with executive recruitment and other expenses resulted in $3.3 million of charges for the year ended December 31, 2017 recorded within SG&A expense on the accompanying Consolidated Statement of Operations.
Stock-Based Compensation Activity
The following table sets forth a summary of all stock-based compensation awards outstanding under all plans:
December 31, 2017 | December 31, 2016 | ||||
Time-based stock options | 2,605,167 | 913,960 | |||
Time-based restricted stock awards | 1,039,380 | 312,245 | |||
Performance-based restricted stock awards | — | 101,384 | |||
Performance-based restricted stock awards with a market condition | 367,150 | 165,635 | |||
Total share awards outstanding | 4,011,697 | 1,493,224 | |||
The Company recognized $8.4 million, $8.8 million and $6.3 million of total non-cash stock-based compensation expense for the years ended December 31, 2017, 2016 and 2015, respectively. At December 31, 2017, there was $16.7 million of total unrecognized compensation cost related to non-vested stock-based compensation, net of expected forfeitures, for all awards previously made that are expected to be recognized over a weighted-average period of 1.7 years.
In 2017, there were no stock options exercised. Cash received from the exercise of options was $0.4 million and $1.7 million in 2016 and 2015, respectively, which was recorded as additional paid-in capital on the accompanying Consolidated Balance Sheets and presented as a cash inflow from financing activities on the accompanying Consolidated Statements of Cash Flows.
On July 28, 2016, the Company announced the departure from the Company and resignation from the Board of Michael G. Archbold, its former Chief Executive Officer. During the year ended December 31, 2016 in connection with Mr. Archbold's departure, the company recognized $4.5 million in severance expense of which $2.3 million relates to the acceleration of non-cash stock-based compensation.
Stock Options
Time-based stock options were granted using the Black-Scholes model with exercise prices at the Company's stock price on the date of grant which typically vest at 25% per year over a four-year period except for the non-plan inducement awards as explained above. The following table sets forth a summary of stock options under all plans.
Total Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value (in thousands) | |||||||||
Outstanding at December 31, 2016 | 913,960 | $ | 26.53 | $ | 71 | |||||||
Granted | 2,298,093 | $ | 8.53 | |||||||||
Exercised | — | $ | — | $ | — | |||||||
Forfeited and expired | (606,886 | ) | $ | 21.60 | ||||||||
Outstanding at December 31, 2017 | 2,605,167 | $ | 11.84 | 8.3 | $ | — | ||||||
Exercisable at December 31, 2017 | 374,294 | $ | 22.17 | 2.8 | $ | — | ||||||
During the years ended December 31, 2016 and 2015, the total intrinsic value of options exercised was $0.3 million, and $2.0 million, respectively. The assumptions used in the Company's Black Scholes valuation were as follows:
Year ended December 31, | |||||
2017 | 2016 | 2015 | |||
Dividend yield | 0% | 2.3% - 3.8% | 1.5% - 2.4% | ||
Expected term | 6 - 6.3 years | 6.3 years | 6.3 years | ||
Volatility | 38.2% - 40.8% | 30.1% - 30.7% | 31.1% - 38.3% | ||
Risk free rate | 1.8% - 2.1% | 1.3% - 1.9% | 1.3% - 1.9% | ||
89
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The option term has been estimated by considering both the vesting period and the contractual term. Volatility was estimated giving consideration to a peer group and the Company's own volatility. The Black Scholes valuation resulted in a weighted average grant date fair value in 2017, 2016 and 2015 of $3.50, $6.23 and $15.64, respectively.
Restricted Stock Awards
Under the 2015 Stock Plan, the Company granted time-based and performance-based restricted stock and restricted stock units as well as, performance restricted shares with a market condition. Time-based awards vest over a period of three years. Performance-based awards vest after a period of three years and the achievement of performance targets; based on the extent to which the targets are achieved, vested shares may range from 0% to 200% of the original share amount. Performance restricted shares with a market condition vest after a period of three years and the achievement of total shareholder return compared with that of a selected group of peer companies. Total shareholder return is defined as share price appreciation plus the value of dividends paid during the three year vesting period. Vested shares may range from 0% to 200% of the original target.
Key assumptions used in the Monte Carlo simulation for the performance restricted shares with a market condition granted during the year ended December 31, 2017 includes a volatility of 34.6% for the applicable peer group and a risk-free rate of 1.46%. Key assumptions used in the Monte Carlo simulation for awards granted in 2016 include peer group volatility of 34.2% and a risk-free rate of 0.89%.
The following table sets forth a summary of restricted stock awards granted under all plans:
Time-Based | Performance-Based | Performance Restricted Shares with a Market Condition | ||||||||||||||||||
Shares | Wtd Avg Grant Date Fair Value | Shares | Wtd Avg Grant Date Fair Value | Shares | Wtd Avg Grant Date Fair Value | |||||||||||||||
Outstanding at December 31, 2016 | 312,245 | $ | 30.81 | 101,384 | $ | 48.99 | 165,635 | $ | 34.28 | |||||||||||
Granted | 1,294,315 | $ | 8.19 | — | $ | — | 365,292 | $ | 8.03 | |||||||||||
Vested | (388,418 | ) | $ | 18.05 | — | $ | — | — | $ | — | ||||||||||
Forfeited | (178,762 | ) | $ | 15.71 | (101,384 | ) | $ | 48.99 | (163,777 | ) | $ | 18.02 | ||||||||
Outstanding at December 31, 2017 | 1,039,380 | $ | 10.01 | — | $ | 48.99 | 367,150 | $ | 15.42 | |||||||||||
The total intrinsic value of time-based restricted stock awards vested was $3.0 million, $3.1 million and $2.4 million for the years ended December 31, 2017, 2016 and 2015, respectively. The total intrinsic value of time-based restricted stock awards outstanding at December 31, 2017 was $3.8 million. The total intrinsic value of performance restricted shares with a market condition outstanding at December 31, 2017 assuming vesting at 100% was $1.4 million. The weighted average grant date fair value of time-based and performance-based stock awards granted was $45.95 and $47.86 in 2015, respectively.
NOTE 15. RETIREMENT PLANS
The Company sponsors a 401(k) defined contribution savings plan covering substantially all employees who have attained age 21. Full time employees who have completed 30 days of service and part time employees who have completed 1,000 hours of service are eligible to participate in the plan. The plan provides for employee contributions of 1% to 80% of individual compensation into deferred savings, subject to IRS limitations. The plan provides for Company contributions upon the employee meeting the eligibility requirements. The Company match consists of both a fixed and a discretionary match. The fixed match is 50% on the first 3% of employee contributions and the discretionary match could be up to an additional 50% match on the 3% deferral. A discretionary match can be approved at any time by the Company.
90
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
An employee becomes vested in the Company match portion as follows:
Years of Service | Percent Vested | |
0-1 | 0 | % |
1-2 | 33 | % |
2-3 | 66 | % |
3+ | 100 | % |
The Company made cash contributions to the 401(k) plan of $2.1 million, $1.9 million and $1.5 million for the years ended December 31, 2017, 2016 and 2015, respectively.
The Company has a Non-qualified Deferred Compensation Plan that provides benefits payable to certain eligible employees upon scheduled in-service distribution, termination, or retirement. This plan allows participants the opportunity to defer pretax amounts ranging from 2% to 80% of their base compensation and up to 100% of bonuses. During 2017, 2016 and 2015, the Company elected to match a percentage of the contributions from employees. For each of the years ended December 31, 2017, 2016 and 2015 this contribution was $0.3 million.
NOTE 16. SEGMENTS
The Company's organizational structure and the financial reporting utilized by the Company's chief operating decision maker (its chief executive officer) to assess performance and allocate resources changed effective in the second quarter of 2016, which resulted in a change in the Company's reportable segments.
The Company aggregates its operating segments into three reportable segments, which include U.S. and Canada, International and Manufacturing / Wholesale. Warehousing and distribution costs have been allocated to each reportable segment based on estimated utilization and benefit. The Company's chief operating decision maker evaluates segment operating results based primarily on performance indicators, including revenue and operating income. Operating income of each reportable segment excludes certain items that are managed at the consolidated level, such as corporate costs. The Manufacturing / Wholesale segment manufactures and sells product to the U.S. and Canada and International segments at cost with a markup, which is eliminated at consolidation. In connection with the asset sales of Lucky Vitamin and Discount Supplements as described in Note 5, "Goodwill and Intangible Assets," their results are now included within Other for applicable prior periods to ensure comparability.
The following table presents key financial information for each of the Company's reportable segments. The company recorded $457.8 million and $476.6 million in long-lived asset impairments in the years ended December 31, 2017 and 2016, respectively, which significantly impacted the U.S. and Canada segment by $412.5 million and $386.0 million, and the Manufacturing / Wholesale segment by $24.3 million and $90.5 million. Refer to Note 5, "Goodwill and Intangible Assets" and Note 6, "Property, Plant and Equipment, Net" for more information.
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(in thousands) | |||||||||||
Revenue: | |||||||||||
U.S. and Canada | $ | 1,993,444 | $ | 2,058,011 | $ | 2,168,005 | |||||
International | 177,359 | 160,691 | 183,007 | ||||||||
Manufacturing / Wholesale | |||||||||||
Intersegment revenues | 231,495 | 218,761 | 267,377 | ||||||||
Third party | 216,053 | 235,678 | 235,680 | ||||||||
Subtotal Manufacturing / Wholesale | 447,548 | 454,439 | 503,057 | ||||||||
Total reportable segment revenues | 2,618,351 | 2,673,141 | 2,854,069 | ||||||||
Other | 66,182 | 85,636 | 96,606 | ||||||||
Elimination of intersegment revenues | (231,495 | ) | (218,761 | ) | (267,377 | ) | |||||
Total revenue | $ | 2,453,038 | $ | 2,540,016 | $ | 2,683,298 | |||||
Operating (loss) income: | |||||||||||
U.S. and Canada | $ | (246,097 | ) | $ | (104,943 | ) | $ | 379,320 | |||
International | 60,568 | 55,404 | 64,486 | ||||||||
Manufacturing / Wholesale | 47,990 | (19,961 | ) | 86,172 | |||||||
Total reportable segment operating (loss) income | (137,539 | ) | (69,500 | ) | 529,978 | ||||||
Unallocated corporate and other costs | |||||||||||
Corporate costs | (102,114 | ) | (103,362 | ) | (98,340 | ) | |||||
Other | (20,760 | ) | (85 | ) | (38,531 | ) | |||||
Unallocated corporate costs and other | (122,874 | ) | (103,447 | ) | (136,871 | ) | |||||
Total operating (loss) income | (260,413 | ) | (172,947 | ) | 393,107 | ||||||
Interest expense, net | 64,221 | 60,443 | 50,936 | ||||||||
Gain on convertible debt and debt refinancing costs | (10,996 | ) | — | — | |||||||
(Loss) income before income taxes | $ | (313,638 | ) | $ | (233,390 | ) | $ | 342,171 | |||
91
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Depreciation and amortization: | (in thousands) | ||||||||||
U.S. and Canada | $ | 35,571 | $ | 37,979 | $ | 33,936 | |||||
International | 2,455 | 2,475 | 2,524 | ||||||||
Manufacturing / Wholesale | 10,238 | 10,793 | 10,823 | ||||||||
Corporate and other | 8,545 | 8,791 | 9,954 | ||||||||
Total depreciation and amortization | $ | 56,809 | $ | 60,038 | $ | 57,237 | |||||
Capital expenditures: | |||||||||||
U.S. and Canada | $ | 20,614 | $ | 40,417 | $ | 27,545 | |||||
International | 277 | 518 | 716 | ||||||||
Manufacturing / Wholesale | 2,862 | 7,467 | 5,655 | ||||||||
Corporate and Other | 8,370 | 11,177 | 11,911 | ||||||||
Total capital expenditures | $ | 32,123 | $ | 59,579 | $ | 45,827 | |||||
Total revenues by geographic areas: | |||||||||||
United States | $ | 2,305,375 | $ | 2,402,649 | $ | 2,522,774 | |||||
Foreign | 147,663 | 137,367 | 160,524 | ||||||||
Total revenues | $ | 2,453,038 | $ | 2,540,016 | $ | 2,683,298 | |||||
As of December 31 | |||||||
2017 | 2016 | ||||||
Total assets: | (in thousands) | ||||||
U.S. and Canada | $ | 916,263 | $ | 1,452,482 | |||
International | 202,624 | 196,057 | |||||
Manufacturing / Wholesale | 302,772 | 338,108 | |||||
Corporate and other | 94,902 | 69,195 | |||||
Total assets | $ | 1,516,561 | $ | 2,055,842 | |||
Property, plant, and equipment, net: | |||||||
United States | $ | 181,118 | $ | 223,107 | |||
Foreign | 5,444 | 9,185 | |||||
Total property, plant and equipment, net | $ | 186,562 | $ | 232,292 | |||
92
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
U.S. and Canada Revenue
The following is a summary of revenue in the U.S. and Canada segment:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
U.S. company-owned product sales: | (in thousands) | ||||||||||
Protein | $ | 338,773 | $ | 369,150 | $ | 389,917 | |||||
Performance supplements | 281,532 | 254,753 | 246,662 | ||||||||
Weight management | 140,148 | 154,195 | 165,114 | ||||||||
Vitamins | 203,569 | 218,908 | 271,099 | ||||||||
Herbs / Greens | 66,324 | 63,356 | 70,924 | ||||||||
Wellness | 196,942 | 200,914 | 211,377 | ||||||||
Health / Beauty | 190,977 | 164,510 | 149,520 | ||||||||
Food / Drink | 94,390 | 105,134 | 124,865 | ||||||||
General merchandise | 28,931 | 28,786 | 27,384 | ||||||||
Total U.S. company-owned product sales | $ | 1,541,586 | $ | 1,559,706 | $ | 1,656,862 | |||||
Wholesale sales to franchisees | 242,521 | 250,779 | 257,497 | ||||||||
Royalties and franchise fees | 33,149 | 34,469 | 35,350 | ||||||||
Sublease income | 48,972 | 47,555 | 44,086 | ||||||||
Gold Card revenue recognized in U.S.(1) | 24,399 | 62,211 | 59,247 | ||||||||
Other (2) | 102,817 | 103,291 | 114,963 | ||||||||
Total U.S. and Canada revenue | $ | 1,993,444 | $ | 2,058,011 | $ | 2,168,005 | |||||
(1) | Gold Card was discontinued in December 2017 in connection with the launch of the One New GNC . Refer to Note 2, "Basis of Presentation and Summary of Significant Accounting Policies," for more information. |
(2) | Includes revenue primarily related to Canada operations. |
International Revenue
The following is a summary of the Company's revenue in the International reportable segment:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(in thousands) | |||||||||||
Wholesale sales to franchisees | $ | 104,384 | $ | 104,405 | $ | 130,719 | |||||
Royalties and franchise fees | 26,190 | 25,485 | 29,085 | ||||||||
Other (*) | 46,785 | 30,801 | 23,203 | ||||||||
Total International revenue | $ | 177,359 | $ | 160,691 | $ | 183,007 | |||||
(*) Includes revenue primarily related to China operations and The Health Store.
93
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Manufacturing / Wholesale Revenue
The following is a summary of the Company's revenue in the Manufacturing / Wholesale reportable segment:
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
(in thousands) | |||||||||||
Third-party contract manufacturing | $ | 127,883 | $ | 134,542 | $ | 118,852 | |||||
Intersegment sales | 231,495 | 218,761 | 267,378 | ||||||||
Wholesale partner sales | 88,170 | 101,136 | 116,827 | ||||||||
Total Manufacturing / Wholesale revenue | $ | 447,548 | $ | 454,439 | $ | 503,057 | |||||
94
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 17. QUARTERLY FINANCIAL INFORMATION
The results of operations for the three months ended December 31, 2017 were impacted significantly by long-lived asset impairment charges of $434.6 million, consisting of $395.6 million related to the brand name, $24.3 million related to goodwill and $14.7 million related to property, plant and equipment, a convertible debt exchange and other debt refinancing costs which resulted in a gain of $11.0, and the enacted tax reform legislation resulting in an income tax benefit of $90.5 million related to the remeasurement of the Company's net deferred tax assets and liabilities. The results of operations, during three months ended June 30, 2017, includes long-lived asset impairment charges of $19.4 million related to Lucky Vitamin, the assets of which were sold on September 30, 2017. The results of operations for the three months ended December 31, 2016 were impacted significant by long-lived asset impairment charges of $473.5 million, consisting of $471.1 million related to goodwill and $2.4 million related to property, plant and equipment. For more information on these items, refer to Note 4, "Income Taxes," Note 5, "Goodwill and Intangible Assets" and Note 7, "Long-Term Debt / Interest Expense."
The following table summarizes the Company's 2017 and 2016 quarterly results:
Three months ended (unaudited) | Year ended | ||||||||||||||||||
March 31, | June 30, | September 30, | December 31, | December 31, | |||||||||||||||
2017 | 2017 | 2017 | 2017 | 2017 | |||||||||||||||
(In thousands, except per share amounts) | |||||||||||||||||||
Total revenue | $ | 644,838 | $ | 640,994 | $ | 609,469 | $ | 557,737 | $ | 2,453,038 | |||||||||
Gross profit | 212,971 | 212,723 | 196,806 | 177,547 | 800,047 | ||||||||||||||
Operating income (loss) | 53,553 | 39,820 | 40,445 | (394,231 | ) | (260,413 | ) | ||||||||||||
Net income (loss) | 23,850 | 15,661 | 21,463 | (209,825 | ) | (148,851 | ) | ||||||||||||
Weighted average shares outstanding: | |||||||||||||||||||
Basic | 68,246 | 68,287 | 68,354 | 70,251 | 68,789 | ||||||||||||||
Diluted | 68,300 | 68,362 | 68,569 | 70,251 | 68,789 | ||||||||||||||
Earnings per share: | |||||||||||||||||||
Basic (1) | $ | 0.35 | $ | 0.23 | $ | 0.31 | $ | (2.99 | ) | $ | (2.16 | ) | |||||||
Diluted (1) | $ | 0.35 | $ | 0.23 | $ | 0.31 | $ | (2.99 | ) | $ | (2.16 | ) | |||||||
Three months ended (unaudited) | Year ended | ||||||||||||||||||
March 31, | June 30, | September 30, | December 31, | December 31, | |||||||||||||||
2016 | 2016 | 2016 | 2016 | 2016 | |||||||||||||||
(In thousands, except per share amounts) | |||||||||||||||||||
Total revenue | $ | 668,905 | $ | 673,218 | $ | 627,964 | $ | 569,929 | $ | 2,540,016 | |||||||||
Gross profit | 235,845 | 238,698 | 215,408 | 170,168 | 860,119 | ||||||||||||||
Operating income (loss) | 94,065 | 116,224 | 64,893 | (448,129 | ) | (172,947 | ) | ||||||||||||
Net income (loss) | 50,815 | 64,028 | 32,354 | (433,447 | ) | (286,250 | ) | ||||||||||||
Weighted average shares outstanding: | |||||||||||||||||||
Basic | 73,078 | 68,176 | 68,190 | 68,219 | 69,409 | ||||||||||||||
Diluted | 73,373 | 68,303 | 68,315 | 68,219 | 69,409 | ||||||||||||||
Earnings per share: | |||||||||||||||||||
Basic (1) | $ | 0.70 | $ | 0.94 | $ | 0.47 | $ | (6.35 | ) | $ | (4.12 | ) | |||||||
Diluted (1) | $ | 0.69 | $ | 0.94 | $ | 0.47 | $ | (6.35 | ) | $ | (4.12 | ) | |||||||
(1) Quarterly results for earnings per share may not add to full year results due to rounding.
95
GNC HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS (Continued)
NOTE 18. SUBSEQUENT EVENTS
On February 13, 2018, the Company entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) with Harbin Pharmaceutical Group Holdings Co., Ltd. (the “Investor”), pursuant to which the Company agreed to issue and sell to the Investor, 299,950 shares of a newly created series of convertible perpetual preferred stock of the Company, designated as “Series A Convertible Preferred Stock” (the “Convertible Preferred Stock”), for a purchase price of $1,000 per share, or an aggregate of approximately $300 million. The Convertible Preferred Stock is convertible into shares of the common stock of the Company (the “Common Stock”) at an initial conversion price of $5.35 per share, subject to customary antidilution adjustments. The closing of the issuance and sale of the shares of Convertible Preferred Stock pursuant to the Securities Purchase Agreement is subject to receipt of regulatory approvals necessary to complete the transaction in the United States and China, approval of the Company’s stockholders and other customary closing conditions.
In addition, the Securities Purchase Agreement provides for the parties to use their respective reasonable best efforts to negotiate in good faith definitive documentation with respect to a commercial joint venture in China pursuant to which, among other things, the joint venture would be granted an exclusive right to use our trademarks and manufacture and distribute our products in China (excluding Hong Kong, Taiwan and Macau). The joint venture would be controlled 65% by the Investor and 35% by the Company.
On February 28, 2018, the Company amended its Senior Credit Facility, which consisted of an extension of the maturity date for $704.3 million of the $1,131.2 million Term Loan Facility from March 2019 to March 2021. However, if more than $50.0 million of the Company's Notes have not been repaid, discharged, prepaid, refinanced or converted prior to such date (“Existing Indenture Discharge”), the maturity date becomes May 2020. The amendment (the “Amendment”) also includes:
• | the termination of the Revolving Credit Facility, which was replaced with a new $100 million asset-based Revolving Credit Facility with a maturity date of August 2022 (which maturity date will become May 2020 if the Existing Indenture Discharge has not occurred); and |
• | a $275.0 million asset-based Term Loan Facility advanced on a “first-in, last-out” basis with a maturity date of December 2022 (which maturity date will become May 2020 if the Existing Indenture Discharge has not occurred). |
After the effectiveness of the Amendment, the Company will owe $151.9 million on the Term Loan Facility with a maturity date of March 2019. The Amendment requires annual aggregate principal payments of at least $43 million related to the $704.3 million of the Term Loan Facility with a maturity of March 2021. There are no scheduled amortization payments associated with the $275.0 million asset-based Term Loan Facility, and payments associated with the $151.9 million Term Loan Facility are consistent with past terms.
96
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
Item 9A. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer ("CEO") and Chief Financial Officer ("CFO"), has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this Annual Report. Disclosure controls and procedures are designed to provide reasonable assurance that the information required to be disclosed in the reports that we file or submit under the Exchange Act has been appropriately recorded, processed, summarized and reported on a timely basis and are effective in ensuring that such information is accumulated and communicated to our management, including our CEO and CFO, as appropriate to allow timely decisions regarding required disclosure. Based on such evaluation, our CEO and CFO have concluded that, as of December 31, 2017, our disclosure controls and procedures are effective at the reasonable assurance level.
Management's Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements.
Our management, with the participation of our CEO and CFO, has assessed the effectiveness of our internal control over financial reporting based on the framework and criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013). Based on this assessment, our management has concluded that, as of December 31, 2017, our internal control over financial reporting was effective based on that framework.
Our independent registered public accounting firm, PricewaterhouseCoopers LLP, has audited the effectiveness of our internal control over financial reporting as of December 31, 2017, as stated in their report, which is included in Item 8, "Financial Statements and Supplementary Data" of this Annual Report.
Changes in Internal Control Over Financial Reporting
There have not been any changes in our internal controls over financial reporting that occurred during the last fiscal quarter, which have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. OTHER INFORMATION.
None.
97
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information with respect to this Item will be included in our definitive Proxy Statement to be filed with respect to our 2018 Annual Meeting to be held on May 22, 2018, which is incorporated herein by reference, under the captions "Election of Directors," "Executive Officers," "Other Board Information," and "Section 16(a) Beneficial Ownership Reporting Compliance."
Item 11. EXECUTIVE COMPENSATION
Information with respect to this Item will be included in our definitive Proxy Statement to be filed with respect to our 2018 Annual Meeting to be held on May 22, 2018, which is incorporated herein by reference, under the captions "Other Board Information", "Director Compensation," "Named Executive Officer Compensation" and "Compensation Discussion and Analysis;" provided, however, that the subsection entitled "Compensation Discussion and Analysis—Compensation Committee Report" shall be deemed to be furnished hereunder, but shall not be deemed to be incorporated by reference.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Equity Compensation Plans
The following table sets forth information regarding outstanding stock options and shares remaining available for future issuance under our equity compensation plans as of December 31, 2017:
Plan Category | Number of Securities to Be Issued upon Exercise of Outstanding Options, Warrants and Rights | Weighted Average Exercise Price of Outstanding Options, Warrants and Rights | Number of Securities Remaining Available for Future Issuance under Equity Compensation Plans | |||||||
Equity compensation plans approved by security holders (1) | ||||||||||
2007 Stock Plan | 107,220 | $ | 12.17 | — | ||||||
2015 Stock Plan | 1,978,821 | $ | 12.51 | 4,627,292 | (2)(3) | |||||
Subtotal | 2,086,041 | $ | 12.50 | 4,627,292 | ||||||
Equity compensation plans not approved by security holders (4) | 519,126 | $ | 8.95 | — | ||||||
Total | 2,605,167 | $ | 11.79 | 4,627,292 | ||||||
(1)Effective May 21, 2015, our GNC Holdings, Inc. 2011 Stock and Incentive Plan was amended and restated as the GNC Holdings, Inc. 2015 Stock and Incentive Plan (the “2015 Stock Plan”). The GNC Holdings, Inc. 2007 Stock Incentive Plan (the “2007 Stock Plan”) and the 2015 Stock Plan are the only equity compensation plans that we have adopted, and each of the 2007 Stock Plan and 2015 Stock Plan has been approved by our stockholders.
(2)Excludes 1,978,821 outstanding stock options as set forth in the first column, 96,969 shares of outstanding time vested restricted stock, zero shares of performance vesting restricted stock, 660,596 shares of outstanding time vesting restricted stock units and 367,150 of market vesting restricted stock units.
(3)Up to 11,500,000 shares of our common stock may be issued under the 2015 Stock Plan (subject to adjustment to reflect certain transactions and events specified in the 2015 Stock Plan for any award grant). If any award granted under the 2015 Stock Plan expires, terminates or is canceled without having been exercised in full, the number of shares underlying such unexercised award will again become available for issuance under the 2015 Stock Plan. The total number of shares of our common stock available for awards under the 2015 Stock Plan will be reduced by (i) the total number of stock options or stock appreciation rights exercised, regardless of whether any of the shares of our common stock underlying such awards are not actually issued to the participant as the result of a net settlement and (ii) any shares of our common stock used to pay any exercise price or tax withholding obligation. In addition, the number of shares of our common stock that are subject to restricted stock, performance shares or other stock-based awards that are not subject to the appreciation of the value of a share of our common stock ("Full Share Awards") is limited by counting shares granted pursuant to such Full Share Awards against the aggregate share reserve as 1.8 shares for every share granted. If any stock option, stock appreciation right or other stock-based award that is not a Full Share Award is canceled, expires or terminates unexercised for any reason, the shares covered by such awards will again be available for issuance under the 2015 Stock
98
Plan. If any shares of our common stock that are subject to Full Share Awards are forfeited for any reason, 1.8 shares of our common stock will again be available for issuance under the 2015 Stock Plan.
(4)The Company's non-shareholder approved plan is the inducement exception plan pursuant to NYSE rules for awards granted to Kenneth A. Martindale pursuant to his employment agreement ("Inducement Exception Awards"), under which no further grants may be made. The Inducement Exception Awards were made pursuant to the inducement award exception under the NYSE rules to induce an executive officer to join the Company. These awards were granted to Kenneth A. Martindale pursuant to his employment agreement and were made in order to attract and retain an executive of his unique caliber and experience. Refer to Item 8, "Financial Statements and Supplementary Data," Note 14, "Stock-Based Compensation" for details of the non-plan inducement awards.
Additional information with respect to this Item will be included in our definitive Proxy Statement to be filed with respect to our 2018 Annual Meeting to be held on May 22, 2018, which is incorporated herein by reference, under the caption "Security Ownership of Certain Beneficial Owners and Management."
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE.
Information with respect to this Item will be included in our definitive Proxy Statement to be filed with respect to our 2018 Annual Meeting to be held on May 22, 2018, which is incorporated herein by reference, under the captions "Certain Relationships and Related Transactions," and "Director Independence."
Item 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
Information with respect to this Item will be included in our definitive Proxy Statement to be filed with respect to our 2018 Annual Meeting to be held on May 22, 2018, which is incorporated herein by reference, under the caption "Ratification of Appointment of Auditors."
99
PART IV
Item 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES.
(a) | Documents filed as part of this Annual Report: |
(1) | Financial statements filed in Part II, Item 8 of this Annual Report: |
• | Report of Independent Registered Public Accounting Firm |
• | Consolidated Balance Sheets |
As of December 31, 2017 and December 31, 2016
• | Consolidated Statements of Operations |
For the years ended December 31, 2017, 2016 and 2015
• | Consolidated Statements of Comprehensive (Loss) Income |
For the years ended December 31, 2017, 2016 and 2015
• | Consolidated Statements of Stockholders' (Deficit) Equity |
For the years ended December 31, 2017, 2016 and 2015
• | Consolidated Statements of Cash Flows |
For the years ended December 31, 2017, 2016 and 2015
• | Notes to Consolidated Financial Statements |
100
(2) | Financial statement schedules: |
SCHEDULE I—CONDENSED FINANCIAL INFORMATION OF GNC HOLDINGS, INC.
GNC HOLDINGS, INC.
(Parent Company Only)
Balance Sheets
(in thousands)
December 31, | |||||||
2017 | 2016 | ||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 20 | $ | — | |||
Prepaids and other current assets | 9,743 | 191 | |||||
Total current assets | 9,763 | 191 | |||||
Long-term assets: | |||||||
Intercompany receivable | 164,300 | 164,300 | |||||
Investment in subsidiaries | (7,691 | ) | 149,933 | ||||
Total long-term assets | 156,609 | 314,233 | |||||
Total assets | $ | 166,372 | $ | 314,424 | |||
Current liabilities: | |||||||
Intercompany payable | 4,055 | 404 | |||||
Deferred revenue and other current liabilities | 1,143 | 2,274 | |||||
Total current liabilities | 5,198 | 2,678 | |||||
Long-term liabilities: | |||||||
Deferred tax liabilities | 5,742 | 12,550 | |||||
Convertible senior notes | 167,898 | 245,273 | |||||
Intercompany loan | 149,528 | 148,970 | |||||
Total long term liabilities | 323,168 | 406,793 | |||||
Total liabilities | 328,366 | 409,471 | |||||
Stockholders' deficit: | |||||||
Class A common stock | 130 | 114 | |||||
Additional paid-in capital | 1,001,315 | 922,687 | |||||
Retained earnings | 567,741 | 716,198 | |||||
Treasury stock, at cost | (1,725,349 | ) | (1,725,349 | ) | |||
Accumulated other comprehensive loss | (5,831 | ) | (8,697 | ) | |||
Total stockholders' deficit | (161,994 | ) | (95,047 | ) | |||
Total liabilities and stockholders' deficit | $ | 166,372 | $ | 314,424 | |||
See the accompanying note to the condensed parent-only financial statements.
101
SCHEDULE I—CONDENSED FINANCIAL INFORMATION OF GNC HOLDINGS, INC.
GNC HOLDINGS, INC.
(Parent Company Only)
Statements of Operations and Comprehensive (Loss) Income
(in thousands, except per share data)
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Selling, general and administrative | $ | 1,238 | $ | 1,543 | $ | 1,645 | |||||
Subsidiary loss (income) | 161,463 | 279,192 | (223,090 | ) | |||||||
Operating (loss) income | (162,701 | ) | (280,735 | ) | 221,445 | ||||||
Interest expense, net | 10,399 | 9,643 | 4,241 | ||||||||
Gains on convertible debt | (15,041 | ) | — | — | |||||||
(Loss) income before income taxes | (158,059 | ) | (290,378 | ) | 217,204 | ||||||
Income tax benefit | (9,208 | ) | (4,128 | ) | (2,095 | ) | |||||
Net (loss) income | $ | (148,851 | ) | $ | (286,250 | ) | $ | 219,299 | |||
Other comprehensive (loss) income: | |||||||||||
Foreign currency translation gain (loss) | $ | 2,866 | $ | 952 | $ | (7,439 | ) | ||||
Release of cumulative translation loss to earnings related to substantial liquidation of Discount Supplements | — | — | 1,619 | ||||||||
Other comprehensive income (loss) | 2,866 | 952 | (5,820 | ) | |||||||
Comprehensive (loss) income | $ | (145,985 | ) | $ | (285,298 | ) | $ | 213,479 | |||
(Loss) Earnings per share: | |||||||||||
Basic | $ | (2.16 | ) | $ | (4.12 | ) | $ | 2.61 | |||
Diluted | $ | (2.16 | ) | $ | (4.12 | ) | $ | 2.60 | |||
Weighted average common shares outstanding: | |||||||||||
Basic | 68,789 | 69,409 | 83,927 | ||||||||
Diluted | 68,789 | 69,409 | 84,186 | ||||||||
Dividends declared per share | $ | — | $ | 0.80 | $ | 0.72 | |||||
See the accompanying note to the condensed parent-only financial statements.
102
SCHEDULE I—CONDENSED FINANCIAL INFORMATION OF GNC HOLDINGS, INC.
GNC HOLDINGS, INC.
(Parent Company Only)
Statements of Cash Flows
(in thousands)
Year ended December 31, | |||||||||||
2017 | 2016 | 2015 | |||||||||
Cash flows from operating activities: | |||||||||||
Net (loss) income | $ | (148,851 | ) | $ | (286,250 | ) | $ | 219,299 | |||
Deficit (equity) in loss (income) of subsidiaries | 161,463 | 279,192 | (223,090 | ) | |||||||
Dividends received | — | 283,280 | 422,355 | ||||||||
Other operating activities | (12,339 | ) | 10,895 | 4,738 | |||||||
Net cash provided by operating activities | 273 | 287,117 | 423,302 | ||||||||
Cash flows from financing activities: | |||||||||||
Loan to a subsidiary | — | — | (164,300 | ) | |||||||
Proceeds from issuance of convertible notes | — | — | 287,500 | ||||||||
Debt issuance costs on convertible senior notes | — | (1,797 | ) | (8,225 | ) | ||||||
Proceeds from exercise of stock options | — | 353 | 1,744 | ||||||||
Minimum tax withholding requirements | (253 | ) | (1,169 | ) | (574 | ) | |||||
Repurchase of treasury stock | — | (229,169 | ) | (479,799 | ) | ||||||
Dividend payment | — | (55,336 | ) | (59,648 | ) | ||||||
Net cash used in financing activities | (253 | ) | (287,118 | ) | (423,302 | ) | |||||
Net increase (decrease) in cash and cash equivalents | 20 | (1 | ) | — | |||||||
Beginning balance, cash and cash equivalents | — | 1 | 1 | ||||||||
Ending balance, cash and cash equivalents | $ | 20 | $ | — | $ | 1 | |||||
See the accompanying note to the condensed parent-only financial statements.
103
GNC HOLDINGS, INC.
SCHEDULE I—NOTES TO THE CONDENSED FINANCIAL STATEMENTS (PARENT ONLY)
NOTE 1. BACKGROUND
These condensed parent company financial statements should be read in conjunction with the Consolidated Financial Statements of GNC Holdings, Inc. and subsidiaries. The Senior Credit Facility of General Nutrition Centers, Inc. ("Centers"), a wholly owned subsidiary of GNC Holdings, Inc., contains customary covenants, including incurrence covenants and certain other limitations on the ability of GNC Corporation, Centers, and Centers' subsidiaries to, among other things, make optional payments in respect of other debt instruments, pay dividends or other payments on capital stock, and enter into arrangements that restrict their ability to pay dividends or grant liens.
104
SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS
GNC Holdings, Inc. and Subsidiaries
Valuation and Qualifying Accounts
(in thousands)
Balance at Beginning of Period | Charged to Costs and Expenses | Deductions | Balance at End of Period | ||||||||||||
2015 | |||||||||||||||
Allowance for doubtful accounts | $ | 6,192 | $ | 2,679 | $ | (4,744 | ) | $ | 4,127 | ||||||
Reserve for sales returns | 4,949 | 67,283 | (67,385 | ) | 4,847 | ||||||||||
Tax valuation allowances | 14,653 | 2,266 | — | 16,919 | |||||||||||
2016 | |||||||||||||||
Allowance for doubtful accounts | $ | 4,127 | $ | 6,231 | $ | (5,747 | ) | $ | 4,611 | ||||||
Reserve for sales returns | 4,847 | 66,246 | (67,723 | ) | 3,370 | ||||||||||
Tax valuation allowances | 16,919 | 4,405 | — | 21,324 | |||||||||||
2017 | |||||||||||||||
Allowance for doubtful accounts | $ | 4,611 | $ | 3,109 | $ | (3,806 | ) | $ | 3,914 | ||||||
Reserve for sales returns | 3,370 | 57,356 | (57,627 | ) | 3,099 | ||||||||||
Tax valuation allowances | 21,324 | — | (3,845 | ) | 17,479 | ||||||||||
105
(1) | Exhibits: |
Listed below are all exhibits filed as part of this Annual Report. Certain exhibits are incorporated by reference from statements and reports previously filed by Holdings or Centers with the SEC pursuant to Rule 12b-32 under the Exchange Act:
3.1 | Amended and Restated Certificate of Incorporation of Holdings, as currently in effect. (Incorporated by reference to Exhibit 3.1 to Holdings' Quarterly Report on Form 10-Q (File No. 001-35113), filed August 1, 2013.) |
3.2 | Fifth Amended and Restated Bylaws of Holdings, as currently in effect. (Incorporated by reference to Exhibit 3.1 to Holdings' Current Report on Form 8-K (File No. 001-35113), filed October 23, 2012.) |
4.8 | Specimen of Class A Common Stock Certificate. (Incorporated by reference to Exhibit 4.8 to Holdings' Pre-Effective Amendment No. 3 to its Registration Statement on Form S-1 (File No. 333-169618), filed February 25, 2011.) |
4.9 | Indenture, dated as of August 10, 2015, by and among Holdings, the Subsidiary Guarantors party thereto and Bank of New York Mellon Trust Company, N.A., as Trustee (Incorporated by reference to Exhibit 10.1 to Holdings’ Quarterly Report on Form 10-Q (File No. 001-35113) filed July 28, 2016). |
10.1 | Lease Agreement, dated as of November 1, 1998, between Greenville County, South Carolina and General Nutrition Products, Inc. (Incorporated by reference to Exhibit 10.34 to Holdings' Pre-Effective Amendment No. 2 to its Registration Statement on Form S-1 (File No. 333-169618), filed February 10, 2011.) |
10.2 | GNC Live Well Later Non-Qualified Deferred Compensation Plan, effective February 1, 2002. (Incorporated by reference to Exhibit 10.14 to Centers' Registration Statement on Form S-4 (File No. 333-114502), filed April 15, 2004.) |
10.3 | Deferred Compensation Plan for Centers, effective January 1, 2009. (Incorporated by reference to Exhibit 10.32 to Centers' Annual Report on Form 10-K (File No. 333-114396), filed February 25, 2011.) |
10.4 | GNC Acquisition Holdings Inc. 2007 Stock Incentive Plan, adopted as of March 16, 2007. (Incorporated by reference to Exhibit 10.12 to Centers' Pre-Effective Amendment No. 1 to its Registration Statement on Form S-4 (File No. 333-144396), filed August 10, 2007.)** |
10.5 | Amendment No. 1 to the GNC Acquisition Holdings Inc. 2007 Stock Incentive Plan, dated as of February 12, 2008. (Incorporated by reference to Exhibit 10.11 to Centers' Annual Report on Form 10-K (File No. 333-144396), filed March 14, 2008.) ** |
10.6 | Form of Non-Qualified Stock Option Agreement Pursuant to the GNC Acquisition Holdings Inc. 2007 Stock Incentive Plan. (Incorporated by reference to Exhibit 10.13 to Centers' Pre-Effective Amendment No. 1 to its Registration Statement on Form S-4 (File No. 333-144396), filed August 10, 2007.) ** |
10.7 | GNC Holdings, Inc. 2011 Stock and Incentive Plan (Incorporated by reference to Exhibit 10.1 to Holdings' Registration Statement on Form S-8 (File No. 333-173578), filed April 18, 2011.) ** |
10.8 | Form of Non-Qualified Stock Option Agreement pursuant to the GNC Holdings, Inc. 2011 Stock and Incentive Plan. (Incorporated by reference to Exhibit 10.33 to Holdings Pre-Effective Amendment No. 5 to its Registration Statement on Form S-1 (File No. 333-169618), filed March 11, 2011.) ** |
10.9 | Form of Restricted Stock Agreement pursuant to the GNC Holdings, Inc. 2011 Stock and Incentive Plan. (Incorporated by reference to Exhibit 10.34 to Holdings' Registration Statement on Form S-1 (File No. 333-176721), filed September 7, 2011.) ** |
10.10 | Form of Restricted Stock Unit Agreement pursuant to the GNC Holdings, Inc. 2011 Stock and Incentive Plan (Incorporated by reference to Exhibit 10.1 to Holdings' Current Report on Form 8-K (File No. 001-35113), filed October 23, 2012.)** |
10.11 | Form of Performance-Vested Restricted Stock Unit Agreement pursuant to the GNC Holdings, Inc. 2011 Stock and Incentive Plan *,(Incorporated by reference to Exhibit 10.10.3 to Holdings' Annual Report on Form 10-K. (File No. 001-35113), filed February 26, 2013.)** |
106
10.12 | GNC Holdings, Inc. 2015 Stock and Incentive Plan (Incorporated by reference to Appendix A to Holdings' Schedule 14A Definitive Proxy Statement (File No. 001-35113), filed April 12, 2015** |
10.13 | Form of Non-Qualified Stock Option Agreement pursuant to the GNC Holdings, Inc. 2015 Stock and Incentive Plan. (Incorporated by reference to Exhbit 10.13 to Holdings' Annual Report on Form 10-K (File No. 001-35113), filed February 16, 2017.)** |
10.14 | Form of Restricted Stock Agreement pursuant to the GNC Holdings, Inc. 2015 Stock and Incentive Plan. (Incorporated by reference to Exhibit 10.14 to Holdings' Annual Report on Form 10-K (File No. 001-35113), filed February 16, 2017.)** |
10.15 | Form of Performance-Vested Restricted Stock Unit Agreement pursuant to the GNC Holdings, Inc. 2015 Stock and Incentive Plan. (Incorporated by reference to Exhibit 10.15 to Holdings' Annual Report on Form 10-K (File No. 001-35113), filed February 16, 2017.)** |
10.16 | Form of Time-Vested Restricted Stock Unit Agreement pursuant to the GNC Holdings, Inc. 2015 Stock and Incentive Plan. (Incorporated by reference to Exhibit 10.16 to Holdings' Annual Report on Form 10-K (File No. 001-35113), field February 16, 2017.)** |
10.17 | Form of Indemnification Agreement between Holdings and each of our directors and relevant schedule. (Incorporated by reference to Exhibit 10.3 to Holdings Quarterly Report on Form 10-Q (File No. 001-35113), filed October 29, 2015.) ** |
10.18 | Form of Indemnification Agreement between Holdings and certain officers and relevant schedule. (Incorporated by reference to Exhibit 10.2 to Holdings Quarterly Report on Form 10-Q (File No. 001-35113), filed October 29, 2015.) ** |
10.19 | GNC/Rite Aid Retail Agreement, dated December 8, 1998, between General Nutrition Sales Corporation and Rite Aid Corporation. (Incorporated by reference to Exhibit 10.24 to Centers' Pre-Effective Amendment No. 1 to its Registration Statement on Form S-4 (File No. 333-114502), filed August 9, 2004.)† |
10.20 | Amendment to the GNC/Rite Aid Retail Agreement, dated December 8, 1998, by and between General Nutrition Sales Corporation and Rite Aid Hdqtrs Corp. (Incorporated by reference to Exhibit 10.25 to Centers' Pre-Effective Amendment No. 1 to its Registration Statement on Form S-4 (File No. 333-114502), filed August 9, 2004.)† |
10.21 | Amendment to the GNC/Rite Aid Retail Agreement, effective as of May 1, 2004, between General Nutrition Sales Corporation and Rite Aid Hdqtrs Corp. (Incorporated by reference to Exhibit 10.26 to Centers' Pre-Effective Amendment No. 1 to its Registration Statement on Form S-4 (File No. 333-114502), filed August 9, 2004.)† |
10.22 | Amended and Restated GNC/Rite Aid Retail Agreement, dated July 31, 2007, between Nutra Sales Corporation (f/k/a General Nutrition Sales Corporation) and Rite Aid Hdqtrs. Corp. (Incorporated by reference to Exhibit 10.34 to Centers' Pre-Effective Amendment No. 1 to its Registration Statement on Form S-4 (File No. 333-144396), filed August 10, 2007.)† |
10.23 | |
10.24 | |
10.25 | |
107
10.26 | |
10.27 | |
10.28 | Directors' Non-Qualified Deferred Compensation Plan Effective as of January 1, 2013. (Incorporated by reference to Exhibit 10.1 to Holdings' Quarterly Report on Form 10-Q (File No. 001-35113), filed May 2, 2013.)** |
10.29 | Form of Holdings Director Restricted Stock Unit Award Agreement (Incorporated by reference to Exhibit 10.2 to Holdings' Quarterly Report on Form 10-Q (File No. 001-35113), filed May 2, 2013.)** |
10.30 | Mutual General Release and Waiver, dated as of August 12, 2014, among Joseph M. Fortunato, Centers and Holdings (Incorporated by reference to Exhibit 10.2 to Holdings Quarterly Report on Form 10-Q (File No. 001-35113), filed October 30, 2014.)** |
10.31 | Separation Agreement and Mutual General Release and Waiver, dated as of September 10, 2014, among Thomas R. Dowd, Holdings and Centers (Incorporated by reference to Exhibit 10.1 to Holdings Quarterly Report on Form 10-Q (File No. 001-35113), filed October 30, 2014** |
10.32 | Separation Agreement and Mutual General Release and Waiver, dated as of November 10, 2014, among Gerald J. Stubenhofer, Holdings and Centers. (Incorporated by reference to Exhibit 10.28 to Holdings' Annual Report on Form 10-K (File No. 001-35113), filed February 17, 2015.)** |
10.33 | |
10.34 | Employment Agreement by and among General Nutrition Centers, Inc., GNC Holdings, Inc. and Ken Martindale, (Incorporated by reference to Exhibit 10.1 of Form 8-K filed September 12, 2017 (File No.001-35113))** |
10.35 | Form of Inducement Restricted Stock Award Agreement between GNC Holdings, Inc. and Ken Martindale, (Incorporated by reference to Exhibit 10.2 of Form 8-K filed September 12, 2017 (File No.001-35113))** |
10.36 | Form of Inducement Non-Qualified Stock Option Award Agreement between GNC Holdings, Inc. and Ken Martindale (Incorporated herein by reference to Exhibit 10.3 of Form 8-K filed September 12, 2017 (File No.001-35113))** |
21.1 | |
23.1 | |
31.1 | |
31.2 | |
32.1 | |
101.INS | XBRL Instance Document |
101.SCH | XBRL Taxonomy Extension Schema |
101.CAL | XBRL Taxonomy Extension Calculation Linkbase |
101.LAB | XBRL Taxonomy Extension Label Linkbase |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase |
101.DEF | XBRL Taxonomy Extension Definition Linkbase |
_______________________________________________________________________________
* Filed herewith
108
** Management contract or compensatory plan or arrangement of the Company required to be filed as an exhibit.
*** Management contract or compensatory plan or arrangement of the Company required to be filed as an exhibit and filed herewith.
† Portions of this exhibit have been omitted pursuant to a request for confidential treatment. The omitted portions have been separately filed with the SEC.
109
Item 16. FORM 10-K SUMMARY.
We may voluntarily include a summary of information required by Form 10-K under this Item 16. We have elected not to include such summary information.
110
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
GNC HOLDINGS, INC. | ||
By: | /s/ KENNETH A. MARTINDALE | |
Kenneth A. Martindale Director, Chief Executive Officer Dated: Dated: March 1, 2018 | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
By: | /s/ KENNETH A. MARTINDALE | |
Kenneth A. Martindale Director, Chief Executive Officer (principal executive officer) Dated: March 1, 2018 | ||
By: | /s/ TRICIA K. TOLIVAR | |
Tricia K. Tolivar Chief Financial Officer (principal financial officer and principal accounting officer) Dated: March 1, 2018 | ||
By: | /s/ JEFFREY P. BERGER | |
Jeffrey P. Berger Director Dated: March 1, 2018 | ||
By: | /s/ ALAN D. FELDMAN | |
Alan D. Feldman Director Dated: March 1, 2018 | ||
By: | /s/ MICHAEL F. HINES | |
Michael F. Hines Director Dated: March 1, 2018 | ||
By: | /s/ AMY B. LANE | |
Amy B. Lane Director Dated: March 1, 2018 | ||
By: | /s/ PHILIP E. MALLOTT | |
Philip E. Mallott Director Dated: March 1, 2018 | ||
111
/s/ ROBERT F. MORAN | ||
Robert F. Moran Director Dated: March 1, 2018 | ||
By: | /s/ RICHARD J. WALLACE | |
Richard J. Wallace Director Dated: March 1, 2018 | ||
112
