Attached files
| file | filename |
|---|---|
| EX-32 - EXHIBIT 32.1 - IONIS PHARMACEUTICALS INC | exhibit32_1.htm |
| EX-31.2 - EXHIBIT 31.2 - IONIS PHARMACEUTICALS INC | exhibit31_2.htm |
| EX-31.1 - EXHIBIT 31.1 - IONIS PHARMACEUTICALS INC | exhibit31_1.htm |
| EX-23.1 - EXHIBIT 23.1 - IONIS PHARMACEUTICALS INC | exhibit23_1.htm |
| EX-21.1 - EXHIBIT 21.1 - IONIS PHARMACEUTICALS INC | exhibit21_1.htm |
| EX-10.17 - EXHIBIT 10.17 - IONIS PHARMACEUTICALS INC | exhibit10_17.htm |
| EX-10.10 - EXHIBIT 10.10 - IONIS PHARMACEUTICALS INC | exhibit10_10.htm |
| EX-10.4 - EXHIBIT 10.4 - IONIS PHARMACEUTICALS INC | exhibit10_4.htm |
| EX-4.2 - EXHIBIT 4.2 - IONIS PHARMACEUTICALS INC | exhibit4_2.htm |
| EX-4.1 - EXHIBIT 4.1 - IONIS PHARMACEUTICALS INC | exhibit14_1.htm |
| EX-3.1 - EXHIBIT 3.1 - IONIS PHARMACEUTICALS INC | exhibit3_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2017
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ___________ to ___________
Commission file number 0-19125
Ionis Pharmaceuticals, Inc.
(Exact name of Registrant as specified in its charter)
|
Delaware
|
33-0336973
|
|
|
(State or other jurisdiction of
incorporation or organization) |
(IRS Employer Identification No.)
|
|
2855 Gazelle Court, Carlsbad, CA
|
92010
|
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
760-931-9200
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Common Stock, $.001 Par Value
|
The Nasdaq Stock Market, LLC
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No
Indicate by check if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes No
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer
|
Accelerated filer
|
|
|
Non-accelerated filer
(Do not check if a smaller reporting company)
|
rr
|
Smaller reporting company
|
|
Emerging growth company
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act.
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes No
The approximate aggregate market value of the voting common stock held by non-affiliates of the Registrant, based upon the last sale price of the common stock reported on The Nasdaq Global Select Market was $5,158,628,572 as of June 30, 2017.*
The number of shares of voting common stock outstanding as of February 20, 2018 was 125,403,219.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive Proxy Statement to be filed on or about April 9, 2018 with the Securities and Exchange Commission in connection with the Registrant’s annual meeting of stockholders to be held on May 23, 2018 are incorporated by reference into Part III of this Report.
| * |
Excludes 22,738,285 shares of common stock held by directors and officers and by stockholders whose beneficial ownership is known by the Registrant to exceed 10 percent of the common stock outstanding at June 30, 2017. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the Registrant, or that such person is controlled by or under common control with the Registrant.
|
FORWARD-LOOKING STATEMENTS
This report on Form 10-K and the information incorporated herein by reference includes forward-looking statements regarding our business and the therapeutic and commercial potential of SPINRAZA, inotersen, volanesorsen and our technologies and products in development, including the business of Akcea Therapeutics, Inc., our majority owned affiliate. Any statement describing our goals, expectations, financial or other projections, intentions or beliefs, is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties, particularly those inherent in the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs. Our forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause our results to differ materially from those expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in this report on Form 10-K, including those identified in Item 1A entitled “Risk Factors”. Although our forward-looking statements reflect the good faith judgment of our management, these statements are based only on facts and factors currently known by us. As a result, you are cautioned not to rely on these forward-looking statements.
In this report, unless the context requires otherwise, “Ionis,” “Company,” “we,” “our,” and “us” refers to Ionis Pharmaceuticals, Inc. and its subsidiaries.
TRADEMARKS
"Ionis," the Ionis logo, and other trademarks or service marks of Ionis Pharmaceuticals, Inc. appearing in this report are the property of Ionis Pharmaceuticals, Inc. "Akcea," the Akcea logo, and other trademarks or service marks of Akcea Therapeutics, Inc. appearing in this report are the property of Akcea Therapeutics, Inc. This report contains additional trade names, trademarks and service marks of others, which are the property of their respective owners. Solely for convenience, trademarks and trade names referred to in this report may appear without the ® or TM symbols.
CORPORATE INFORMATION
We incorporated in California in 1989 and in January 1991 we changed our state of incorporation to Delaware. In December 2015, we changed our name to Ionis Pharmaceuticals, Inc. from Isis Pharmaceuticals, Inc. Our principal offices are in Carlsbad, California. We make available, free of charge, on our website, www.ionispharma.com, our reports on Forms 10-K, 10-Q, 8-K and amendments thereto, as soon as reasonably practical after we file such materials with the Securities and Exchange Commission. Any information that we include on or link to our website is not a part of this report or any registration statement that incorporates this report by reference. You may also read and copy our filings at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-732-0330. The SEC also maintains a website that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is www.sec.gov.
In December 2014, we formed Akcea Therapeutics, Inc., as a Delaware corporation, with its principal office in Cambridge, Massachusetts. Prior to Akcea’s IPO in July 2017, we owned 100 percent of Akcea’s stock. After Akcea’s IPO, we owned approximately 68 percent of Akcea.
2
IONIS PHARMACEUTICALS, INC.
FORM 10-K
For the Fiscal Year Ended December 31, 2017
Table of Contents
|
PART I
|
||
|
Page
|
||
|
Item 1.
|
Business
|
4
|
|
Item 1A.
|
Risk Factors
|
37
|
|
Item 1B.
|
Unresolved Staff Comments
|
46
|
|
Item 2.
|
Properties
|
46
|
|
Item 3.
|
Legal Proceedings
|
46
|
|
Item 4.
|
Mine Safety Disclosures
|
46
|
|
PART II
|
||
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
46
|
|
Item 6.
|
Selected Financial Data
|
47
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
48
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
69
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
69
|
|
Item 9.
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
|
69
|
|
Item 9A.
|
Controls and Procedures
|
69
|
|
Item 9B.
|
Other Information
|
71
|
|
PART III
|
||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
71
|
|
Item 11.
|
Executive Compensation
|
71
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
71
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
71
|
|
Item 14.
|
Principal Accounting Fees and Services
|
71
|
|
PART IV
|
||
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
72
|
|
Signatures
|
3
PART I
Item 1. Business
Overview
We are leaders in discovering and developing RNA-targeted therapeutics. We have created an efficient and broadly applicable drug discovery platform leveraging our expertise in antisense oligonucleotide therapeutics. Using this platform, we have developed a large, diverse and advanced pipeline of potentially first-in-class and/or best-in-class drugs that we believe can provide high value for patients with significant unmet medical needs. In this way, we believe we are fundamentally changing medicine with the goal to transform the lives of those suffering from severe, often life-threatening, diseases.
We made significant progress toward this goal with the commercial launch of SPINRAZA (nusinersen) for the treatment of spinal muscular atrophy, or SMA, in pediatric and adult patients. SMA is a leading genetic cause of death in infants marked by progressive, debilitating muscle weakness. SPINRAZA became the first and only approved drug to treat people with SMA and is now the standard of care for this debilitating disease. Our partner, Biogen, is responsible for global commercial activities. In January 2018, Biogen reported that SPINRAZA was available in over 30 global markets. Additionally, Biogen is continuing to purse regulatory approvals for SPINRAZA in countries around the world. In 2017, we earned $113 million in commercial revenue from SPINRAZA royalties. We also earned a $50 million milestone payment for the EU approval of SPINRAZA and a $40 million milestone payment for SPINRAZA pricing approval in Japan.
Our pipeline also contains two near-term, potentially transformative medicines for two different severe and rare diseases, each with significant commercial potential, inotersen and volanesorsen. We believe inotersen has the potential to become the preferred treatment option for many people with hereditary TTR amyloidosis, or hATTR. Our goal is to free these people from the burden of their disease. hATTR is a debilitating, progressive, fatal disease in which patients experience a progressive buildup of amyloid plaque deposits in tissues throughout the body. In May 2017, we reported positive top-line data from our Phase 3 study of inotersen, NEURO-TTR, in patients with hATTR with polyneuropathy. More than half of these patients also have cardiomyopathy. We are advancing inotersen to the market based on the positive data from our NEURO-TTR study. In November 2017, we filed for marketing authorization for inotersen to treat people with hATTR in the U.S. and EU. The Food and Drug Administration, or FDA, accepted the inotersen New Drug Application, or NDA, for Priority Review and set a Prescription Drug User Fee Act, or PDUFA, date of July 6, 2018. The European Medicines Agency, or EMA, also granted accelerated assessment to inotersen, which may reduce standard review time. We are on track in our pre-commercial preparations for a potential launch in mid-2018, if inotersen is approved. Our goals for inotersen are to maximize the commercial potential of the drug, maximize our commercial participation and continue to build our TTR franchise by moving IONIS-TTR-LRx forward rapidly. We plan to commercialize inotersen in North America ourselves and to seek a commercial partner in other geographic regions.
Akcea Therapeutics, Inc., or Akcea, our affiliate focused on developing and commercializing drugs for serious cardiometabolic diseases caused by lipid disorders, is working closely with us to develop volanesorsen to treat two severe and rare, genetically defined diseases, familial chylomicronemia syndrome, or FCS, and familial partial lipodystrophy, or FPL. FCS and FPL are orphan diseases characterized by severely high triglyceride levels that result in severe, daily symptoms and a high risk of life-threatening pancreatitis. We estimate that FCS and FPL each affect 3,000 to 5,000 people globally. The clinical development program for volanesorsen consists of three Phase 3 studies called APPROACH, BROADEN and COMPASS. In the first quarter of 2017, we and Akcea reported positive Phase 3 data from the APPROACH study in patients with FCS. In December 2016, we and Akcea reported positive results from the Phase 3 COMPASS study in patients with triglycerides above 500 mg/dL. Based on the positive data from our Phase 3 studies, Akcea filed for marketing authorization for volanesorsen in the U.S., EU and Canada in the third quarter of 2017. The FDA set a PDUFA date of August 30, 2018 for volanesorsen and an advisory committee meeting is scheduled for May 10, 2018. Volanesorsen was granted Priority Review in Canada. Akcea is on track in its pre-commercial preparations for a potential launch in mid-2018, if volanesorsen is approved.
In addition to preparing to commercialize volanesorsen, Akcea is focused on developing and commercializing three other clinical-stage drugs for serious cardiometabolic diseases caused by lipid disorders: AKCEA-APO(a)-LRx, AKCEA-ANGPTL3-LRx and AKCEA-APOCIII-LRx, each of which could potentially treat multiple patient populations. Moving these drugs into Akcea allows us to retain substantial value from them and ensures our core focus remains on innovation. Akcea completed its initial public offering, or IPO, and a concurrent private placement with Novartis in July 2017, raising over $180 million in net proceeds. As a result of Akcea’s IPO and as of February 2018, we owned approximately 68 percent of Akcea.
We are addressing a broad spectrum of diseases that affect millions of people, such as cardiovascular disease, clotting disorders, Alzheimer’s and Parkinson’s disease. We also are addressing rare diseases, such as acromegaly, amyotrophic lateral sclerosis, beta-thalassemia and Huntington’s disease. We are continuing to advance our mid-stage drugs in development, which have the potential to enter late-stage clinical development and progress toward the market over the next several years, like IONIS-HTTRx. IONIS-HTTRx is the first drug in clinical development to target the cause of Huntington’s disease, or HD, by reducing the production of toxic mutant huntingtin, or mHTT, protein. In December 2017, following successful completion of the Phase 1/2 study in which IONIS-HTTRx demonstrated dose-dependent reductions of the mHTT protein in patients with HD, Roche licensed IONIS-HTTRx for $45 million. We plan to report data from this Phase 1/2 study in early 2018. We have also initiated an open-label extension, or OLE, study for people who participated in the Phase 1/2 study. Roche is now responsible for all IONIS-HTTRx development, regulatory and commercialization activities and costs.
4
The depth of our knowledge and expertise with antisense technology, together with our strong financial position, provides us with the flexibility to determine the optimal development and commercialization strategy to maximize the near and longer-term value of our drugs. We have distinct partnering strategies that we employ based on the specific drug, therapeutic area and expertise and resources our potential partners may bring to the collaboration. For some drugs, we may choose to develop and commercialize them through wholly owned subsidiaries or majority owned affiliates like Akcea. In general, these are drugs, like volanesorsen, that we have the internal expertise to advance, that have a clear development path with manageable costs and that have the potential for initial rare disease indications. For other drugs, we may form partnerships that enable us to leverage our partner’s global expertise and resources needed to support large commercial opportunities, as we did with Bayer and Novartis.
We have established alliances with a cadre of leading global pharmaceutical companies that are working alongside us in developing our drugs, advancing our technology and preparing to commercialize our products. Our partners bring substantial resources and expertise that augment and build upon our internal capabilities. We have strategic partnerships with Biogen and AstraZeneca through which we can broadly expand our drug discovery efforts to new disease targets in specific therapeutic areas. We also have partnerships with Bayer, GSK, Janssen, Novartis and Roche. Each of these companies brings significant expertise and global resources to develop and potentially commercialize the drugs under these partnerships. In January 2017, we and Akcea initiated a collaboration with Novartis to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. As a leader in the cardiovascular disease space, Novartis brings significant resources and expertise that should support the development and commercialization of these two drugs for significant high-risk patient populations. The collaboration with Novartis should enable us to accelerate the development of these drugs for broader patient populations as Novartis plans to conduct a cardiovascular outcomes study for each of these drugs following successful completion of Phase 2 dose-ranging studies. In addition, Akcea has the right to co-commercialize these drugs using its specialized sales force focused on lipid specialists in select markets. We also form early stage research and development partnerships that allow us to expand the application of our technology to new therapeutic areas. For example, under our collaboration with Janssen, we have licensed IONIS-JBI1-2.5Rx and IONIS-JBI2-2.5Rx, two antisense drugs we discovered to treat autoimmune disorders in the gastrointestinal, or GI, tract. Our collaboration with Janssen combines our RNA-targeted technology platform and Janssen’s expertise in autoimmune disorders and therapeutic formulation. Lastly, we also work with a group of companies that can develop our drugs and utilize our technologies outside our primary areas of focus. We refer to these companies as satellite companies.
Through our partnerships, we have created a broad and sustaining base of research and development, or R&D, revenue in the form of license fees, upfront payments and milestone payments while spending prudently to advance our pipeline and technology. Our R&D revenue has consistently grown year over year since 2011. In 2017, we earned $386 million in R&D revenue. Moreover, we have the potential to earn over $13 billion in future milestone payments and licensing fees from our current partnerships. We also have the potential to share in the future commercial success of our inventions and drugs resulting from our partnerships through royalty arrangements. In late 2016, we began adding commercial revenue from SPINRAZA royalties to our existing R&D revenue base. Looking forward, we have the potential to increase our commercial revenue from SPINRAZA royalties from the continued growth we expect in the U.S., EU and in other markets globally. We also have the potential to further increase our commercial revenue with volanesorsen and inotersen. We believe we have the key elements in place to achieve sustained, long-term financial growth, with multiple drivers of revenue; a mature, broad and rapidly-advancing clinical pipeline; a partnership strategy that leverages our partner resources; and an innovative drug discovery technology platform that we continue to deploy across a range of therapeutic areas to address both rare and large patient populations.
Recent Pipeline and Technology Highlights
| ● |
SPINRAZA for SMA – one of the most successful orphan drug launches in history
|
| o |
SPINRAZA, commercialized by Biogen, generated 2017 global sales of $884 million
|
| o |
Results from the ENDEAR study and CHERISH study, in which people with infantile-onset and later-onset SMA, respectively, were treated with SPINRAZA, were published in The New England Journal of Medicine
|
| o |
Prestigious 2017 Prix Galien USA Award for Best Biotechnology Product awarded to us and Biogen for SPINRAZA
|
| o |
New collaboration with Biogen initiated to discover new antisense drugs with enhanced properties to treat SMA
|
| ● |
Inotersen for hATTR – potential to transform the lives of people with hATTR
|
| o |
Marketing applications accepted, no FDA Advisory Committee recommended, Priority Review in the U.S. and Accelerated Assessment in the EU
|
| o |
Preparations for global launch, planned for mid-2018, progressing
|
| o |
Phase 3 NEURO-TTR study met both primary endpoints demonstrating benefit compared to placebo in multiple measures of quality of life and disease severity; 50 percent of inotersen-treated patients experienced improvement from baseline in quality of life
|
| ● |
Volanesorsen for FCS and FPL – potential first treatment for people with FCS
|
| o |
Marketing applications accepted in the U.S., EU and Canada with Promising Innovative Medicine designation in the UK and Priority Review in Canada
|
| o |
Preparations for global launch for FCS, planned for mid-2018, progressing
|
| o |
Phase 3 APPROACH study met primary endpoint of reducing triglyceride levels in people with FCS
|
5
| ● |
Pipeline Programs (early and mid-stage) – advancing wholly owned and partnered programs
|
| o |
Positive results from seven Phase 2 studies reported, including:
|
o Positive data from Phase 1/2 study of IONIS-STAT3-2.5Rx in combination with AstraZeneca’s Imfinzi reported for people with head and neck cancer
| o |
Robust, dose-dependent reductions of mHTT observed in people with Huntington’s disease treated with IONIS-HTTRx
|
| o |
Positive clinical data on five LICA drugs reported, demonstrating consistent, positive performance and sustained target reduction with potential for monthly or less frequent dosing
|
| o |
Positive results from six Phase 1 studies reported
|
| o |
Nine Phase 2 studies and four Phase 1 studies initiated across multiple therapeutic areas to treat people with both broad and rare diseases
|
Drug Discovery and Development
Introduction to Drug Discovery
Proteins are essential working molecules in a cell. Almost all human diseases result from inappropriate protein production, improper protein activity or loss of a protein. Scientists use traditional drug discovery methods to design drugs to interact with the proteins in the body that are supporting or causing a disease. Antisense drugs are different from traditional small molecule or antibody drugs because antisense drugs can modify the production of proteins by targeting RNAs. In this way, antisense drugs can reduce the production of a disease-causing protein, modify the protein produced or increase the production of a protein that, when absent, causes disease. Antisense drugs also can treat disease by targeting and reducing RNAs that may be causing disease (so called “toxic RNAs”). RNAs are naturally occurring molecules in the body that primarily act as messengers that carry the information the cell needs to produce proteins from the DNA/genes to the protein making complex in the cell. When our antisense drugs bind to the specific RNAs of a particular gene, they will ultimately alter the production of the protein encoded in the target gene or, in the case of disease-causing RNAs, degrade the toxic RNA.
Our Development Projects
We are the leader in the discovery and development of an exciting class of RNA-targeted drugs called antisense oligonucleotide, or ASO, drugs, or just antisense drugs. With our proprietary drug discovery platform, we can rapidly identify drugs from a wealth of potential targets to treat a broad range of diseases. We focus our efforts in therapeutic areas in which our drugs will work best, efficiently screening many targets in parallel and carefully selecting the best drug candidates. By combining this efficiency with our rational approach to selecting disease targets, we have built a large and diverse portfolio of drugs we designed to treat a variety of health conditions, with an emphasis on severe and rare diseases, including neurodegenerative diseases, cardiometabolic diseases, and cancer. We are developing antisense drugs for systemic and local delivery (e.g., intrathecal for CNS diseases, intraocular for ophthalmic diseases, oral local for gastrointestinal diseases and aerosol for diseases of the lung). We expect to continue to add new drugs to our pipeline, building a broad proprietary portfolio of drugs to treat many diseases and creating opportunities to generate substantial revenue. We also continue to improve our scientific understanding of our drugs, including how our drugs impact the biological processes of the diseases we target.
With our expertise in discovering and characterizing novel antisense drugs, our scientists can optimize the properties of our antisense drugs for use with particular targets. Our scientists have made significant advances in chemical modifications we use in our antisense drugs, such as with our Generation 2+ antisense drugs, which have increased potency and an improved side effect profile over our earlier generation drugs. Our scientists have further improved upon our second-generation chemistry with our Generation 2.5 chemistry, an advancement that further increases the potency of our drugs, which broadens the tissues in which our drugs can work. We currently have nine Generation 2.5 drugs in development, and we expect that more of our future drugs will incorporate our Generation 2.5 chemistry. In addition to improving the chemical foundation of our drugs, we have also created LIgand-Conjugated Antisense, or LICA, technology, which we design to enhance the effective uptake of our drugs in particular tissues.
With our LICA technology we attached specific chemical structures or molecules to our antisense drugs. With our first LICA conjugate, a complex sugar-like molecule called N-acetylgalactosamine, or GalNac, we have shown an increase in drug potency from 20 to over 30-fold for liver targets, compared to non-conjugated antisense drugs. We currently have 12 LICA drugs in development, including IONIS-AZ4-2.5-LRx, a drug that combines our Generation 2.5 and LICA technology.
We have utilized our chemistry advancements, such as Generation 2.5 and LICA, to expand the therapeutic and commercial opportunities of our pipeline. These advancements, along with the manufacturing and analytical processes that are the same for all of our drugs, shorten our timeline from initial concept to the first human dose, when compared to early development timelines for other drug modalities like small molecule and antibody drugs.
6
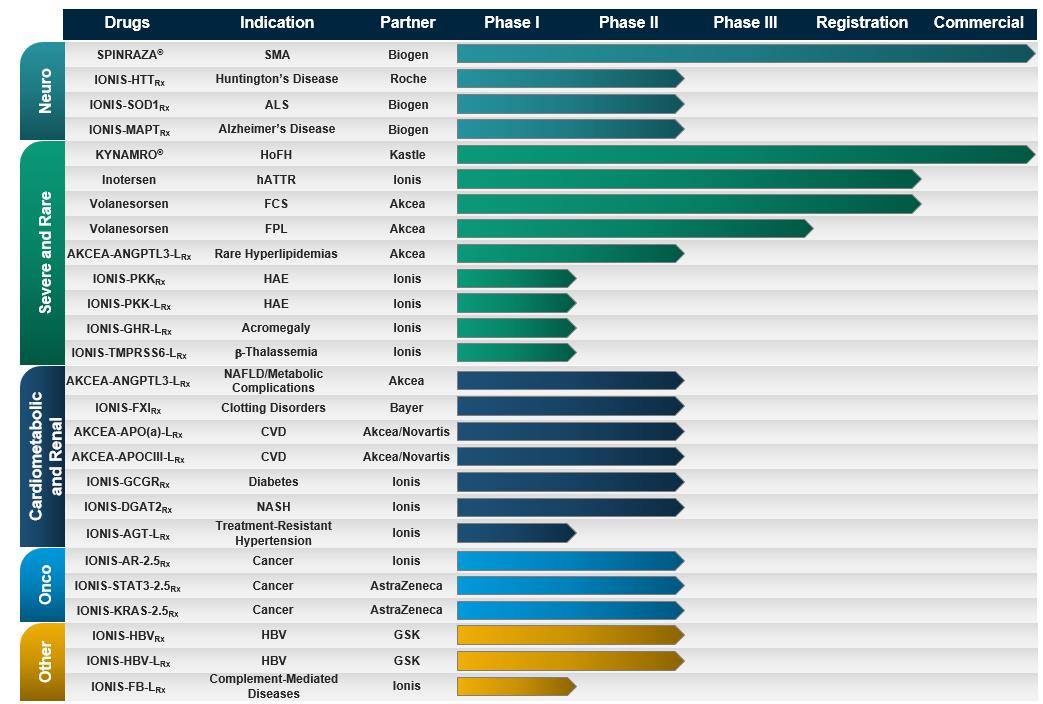

The above table lists the drugs in our pipeline that are in clinical trials, in registration for marketing authorization, or commercialized. The table includes the disease indication, a partner (if the drug is partnered), and the development status of each drug. Typically, the names of our drugs incorporate the target of the drug. For example, with IONIS-HTTRx, the RNA produced from the huntingtin gene, represented by the acronym HTT, is the target of the drug. Unless indicated otherwise, the majority of the drugs in our pipeline are Generation 2+ antisense drugs. We differentiate drugs that Akcea is developing by using “AKCEA”, instead of “IONIS” at the beginning of the drug name, such as AKCEA-ANGPTL3-LRx. We differentiate our Generation 2.5 drugs by adding a “2.5” notation at the end of the drug name, such as IONIS-STAT3-2.5Rx. We differentiate our LICA drugs by adding an “L” at the end of the drug name, such as IONIS-PKK-LRx. In 2016, we added IONIS-AZ4-2.5-LRx, a drug that combines our Generation 2.5 chemistry and LICA technology, to our preclinical pipeline. As the drugs in our pipeline advance in clinical development, we will adopt nonproprietary names given to each drug from the United States Adopted Names Council. For example, volanesorsen is a nonproprietary name that we obtained for IONIS-APOCIIIRx. Once we or our partners establish a brand name, we will adopt the brand name. For example, SPINRAZA is the brand name for nusinersen.
With a pipeline as large and advanced as ours, we have a number of clinical events each year as we initiate new clinical studies, complete and report data from clinical studies, and add numerous new drugs to our pipeline. In 2018, we plan to initiate five Phase 2 studies, report data on six Phase 2 studies and multiple proof-of-concept initial clinical trials and add three to five new drugs into development.
Our Marketed Drug
SPINRAZA – SPINRAZA (nusinersen) injection, for intrathecal use is a survival motor neuron-2 (SMN2)-directed antisense oligonucleotide indicated for the treatment of SMA in pediatric and adult patients. In July 2016, Biogen licensed SPINRAZA from us. We have transitioned all SPINRAZA development activities to Biogen as they are now responsible for all global development, regulatory and commercialization activities and costs. In January 2018, Biogen reported that SPINRAZA was available in over 30 global markets.
SMA is characterized by loss of motor neurons in the spinal cord and lower brain stem, resulting in severe and progressive muscular atrophy and weakness. Ultimately, individuals with the most severe type of SMA, infantile-onset SMA, can become paralyzed and have difficulty performing the basic functions of life, like breathing and swallowing. Due to a loss of, or defect in, the SMN1 gene, people with SMA do not produce enough survival motor neuron, or SMN, protein, which is critical for the maintenance of motor neurons. The severity of SMA correlates with the amount of SMN protein a patient can produce on his/her own. Patients with infantile-onset, or Type 1, SMA, the most severe life-threatening form of the disease, produce very little SMN protein and do not achieve the ability to sit without support or live beyond two years without respiratory support. Patients with later-onset, or Type 2 or Type 3 SMA, produce greater amounts of SMN protein and have less severe, but still life-altering, forms of SMA.
7
SPINRAZA is administered via intrathecal injection, which delivers therapies directly to the cerebrospinal fluid, or CSF, around the spinal cord, where motor neurons degenerate in people with SMA due to insufficient levels of SMN protein.
The safety and efficacy of SPINRAZA has been evaluated from multiple clinical studies in more than 270 patients, including two Phase 3 studies: ENDEAR, a randomized controlled study evaluating SPINRAZA in patients with infantile-onset SMA, and CHERISH, a randomized controlled study evaluating SPINRAZA in patients with later-onset SMA; as well as open-label studies in pre-symptomatic and symptomatic patients with, or likely to develop, Types 1, 2 and 3 SMA.
In the ENDEAR end of study analysis, or EOS, a statistically significant greater percentage of children with infant-onset SMA achieved improvement in motor milestones compared to untreated patients, with some infants in the SPINRAZA group achieving full head control, the ability to roll, sitting, and standing. Additionally, infants treated with SPINRAZA demonstrated a statistically significant improvement in event-free survival compared to untreated patients. In November 2017, results from EOS analysis, from the ENDEAR study, including the pre-specified primary endpoint, time to death or permanent ventilation, were published in The New England Journal of Medicine. SPINRAZA met the pre-specified primary endpoint at the ENDEAR EOS, demonstrating a statistically significant 47 percent reduction in the risk of death or permanent ventilation (p<0.01). In October 2017, Biogen presented a new analysis from the Phase 3 ENDEAR study that showed infants with SMA who initiated treatment earlier in the disease demonstrated greater benefit and improvement in motor function outcomes.
In the CHERISH pre-specified interim analysis, there was a statistically significant and clinically meaningful improvement in motor function in children with later-onset SMA treated with SPINRAZA compared to untreated children. In an EOS analysis, children receiving SPINRAZA experienced a highly statistically significant improvement in motor function compared to those who did not receive treatment. The New England Journal of Medicine published these results in February 2018.
Additionally, Biogen conducted NURTURE, a Phase 2 open-label study in pre-symptomatic infants. In an interim analysis, SPINRAZA-treated pre-symptomatic infants with SMA achieved motor milestones in timelines more consistent with normal development than what is observed in the natural history of patients with Type 1 SMA. At the time of the interim analysis, all patients were alive and did not require respiratory intervention. Three infants experienced AEs considered possibly related to SPINRAZA, all of which were resolved.
Further, in open-label studies, some patients achieved milestones that they would not be expected to achieve, such as the ability to sit unassisted, stand or walk, and maintained milestones at ages that they would expect to lose. The overall findings in the combined clinical studies to date support the effectiveness of SPINRAZA across the range of patients with SMA, and appear to support the early initiation of treatment.
In all clinical studies, SPINRAZA demonstrated a favorable safety profile. The most common side effects of SPINRAZA included lower and upper respiratory infections, constipation, headache, back pain, and post-lumbar puncture syndrome. For additional safety information, please see www.spinraza.com.
Our Drugs Under Regulatory Review for Marketing Authorization
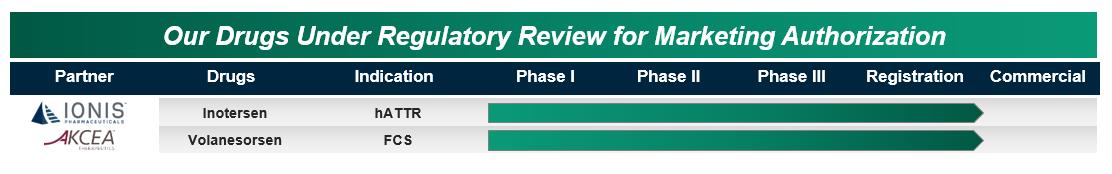
We have two drugs for which we successfully completed pivotal Phase 3 studies and are now under regulatory review for marketing authorization: inotersen and volanesorsen. These drugs are potentially transformative medicines for two different severe and rare diseases, each with significant commercial potential.
Inotersen – Inotersen is a Generation 2+ antisense drug we designed to treat people with hereditary TTR amyloidosis, or hATTR, a rare, progressive, fatal disease.
In people with hATTR, both the mutant and wild type, or wt, TTR protein builds up as fibrils in the tissues, such as peripheral nerves, heart, gastrointestinal system, eyes, kidneys, central nervous system, thyroid and bone marrow. The presence of TTR fibrils interferes with the normal function of these tissues. As the TTR protein fibrils enlarge, more tissue damage occurs and the disease worsens, resulting in poor quality of life and eventually death. We designed inotersen to reduce the production of the TTR protein, the underlying cause of ATTR. Inotersen is administered as a once weekly, self-administered, at-home, subcutaneous injection.
TTR amyloidosis that is the result of inherited mutations in the TTR gene is referred to as hATTR. There are an estimated 50,000 people worldwide with hATTR. There are two primary manifestations of hATTR: polyneuropathy and cardiomyopathy. Many people with hATTR often experience both manifestations, but often one symptom or the other is diagnosed first and is more pronounced. Polyneuropathy due to hATTR is caused by the accumulation of misfolded mutated TTR protein in the peripheral nerves. People with polyneuropathy due to hATTR experience ongoing debilitating nerve damage throughout their body resulting in the progressive loss of sensation in the extremities that progresses centrally, and progressive loss of motor functions, such as walking. These people also accumulate TTR in other major organs, which progressively compromise their function and eventually leads to death within five to fifteen years of disease onset. ATTR cardiomyopathy is caused by the accumulation of misfolded TTR protein in the cardiac muscle. ATTR can also result from normal, non-mutant, TTR protein forming fibrils, primarily in the heart. This form of the disease is referred to as wt-ATTR. It is estimated that more than 200,000 people worldwide have wt-ATTR. People with hATTR cardiomyopathy and wt-ATTR experience ongoing debilitating heart damage resulting in progressive heart failure, which results in death within 3 to 5 years from disease onset.
8
In May 2017, we completed the NEURO-TTR study, a randomized, double-blinded, placebo-controlled, international Phase 3 study in patients with polyneuropathy due to hATTR. Results from the study demonstrated benefit compared to placebo across both primary endpoints of the study: the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy, or Norfolk QoL-DN, and the modified Neuropathy Impairment Score +7, or mNIS+7, at both eight and 15 months of treatment. In addition, consistent and significant benefit was observed in both the Norfolk-QoL-DN and mNIS+7, independent of disease stage, types of mutation, previous treatment with TTR protein stabilizers or presence of cardiomyopathy. Inotersen-treated patients benefited significantly in the quality of life primary endpoint with 50 percent demonstrating improvement from baseline. Inotersen-treated patients achieved a mean 11.68 point benefit in the Norfolk QoL-DN score at 15 months of treatment compared to placebo-treated patients (mean change from baseline of 0.99 vs. 12.67, p=0.0006). In addition, clinically meaningful benefit compared to placebo was observed in the SF-36 physical component score, a measure of general health and quality of life. Inotersen-treated patients also benefited significantly in the co-primary endpoint of disease control, mNIS+7, with a mean 19.73-point benefit observed after 15 months of treatment, compared to placebo-treated patients (p = 0.00000004).
Two key safety issues were identified during the study: thrombocytopenia and safety signals related to renal function. Enhanced monitoring was implemented during the study to support early detection and management of these issues. Serious platelet and renal events were infrequent and manageable with routine monitoring, which has proven effective since implementation. Other serious adverse events were observed in 24.1 percent of inotersen-treated patients and 21.7 percent of placebo-treated patients. No cumulative toxicities have been identified with long-term exposure.
Adverse events occurring in ≥ 10 percent of patients and twice as frequently in inotersen-treated patients compared with placebo-treated patients, included thrombocytopenia/platelet count decreases, nausea, pyrexia, chills, vomiting, and anemia. Injection site reactions accounted for less than 1 percent of all injections and were mild or moderate in severity. There were no discontinuations due to injection site reactions.
The overall mortality rate in the NEURO-TTR study was 2.9 percent and was lower than mortality rates reported in other studies in patients with hATTR. There was a total of five deaths in the study, five (4.7 percent) in the inotersen arm and zero in the placebo arm. Four deaths in the inotersen arm were associated with disease progression and considered unrelated to treatment. As previously reported, there was one fatal intracranial hemorrhage in conjunction with serious thrombocytopenia. No serious thrombocytopenia was observed following implementation of more frequent monitoring.
Inotersen is currently under regulatory review for marketing authorization in the U.S. and EU for the treatment of hATTR. Inotersen has been granted Priority Review by the FDA and has a PDUFA date of July 6, 2018. The EMA also granted accelerated assessment to inotersen, which may reduce standard review time. In addition, an open-label extension study, or OLE, is ongoing for patients who have completed the NEURO-TTR study, in which all patients are treated with inotersen. We have also opened an expanded access program, or EAP, for eligible patients, beginning with sites in the U.S.
We plan to commercialize inotersen in North America ourselves and to seek a commercial partner in other geographic regions.
Volanesorsen – Volanesorsen is a Generation 2+ antisense drug Akcea and we are developing to treat people with FCS and FPL, which are severe, rare, genetically defined diseases characterized by extremely elevated triglyceride levels and a high risk of life-threatening pancreatitis.
Due to the high levels of triglycerides in their blood, people with FCS may suffer from many chronic health issues including severe, recurrent abdominal pain, fatigue, high risk of life-threatening pancreatitis and abnormal enlargement of the liver or spleen. In addition, people with FCS have to adhere to a very strict, low-fat diet. As a result of these factors, people with FCS and FPL are often unable to work, adding to the burden of the disease. While all the complications of FCS or FPL cause patients to have a lower quality of life, pancreatitis is the most serious consequence of the disease. In severe cases, patients can have bleeding into the pancreas, serious tissue damage, infection and cyst formation, as well as damage to other vital organs such as the heart, lungs and kidneys. We estimate there are 3,000 to 5,000 people with FCS in treatable markets and an additional 3,000 to 5,000 people with FPL globally.
Volanesorsen acts to reduce triglyceride levels by inhibiting the production of apolipoprotein C-III, or apoC-III, a protein that is a key regulator of triglyceride clearance. People who have low levels of apoC-III or reduced apoC-III function have lower levels of triglycerides and a lower incidence of cardiovascular disease, or CVD. By inhibiting the production of apoC-III, volanesorsen is able to increase triglyceride clearance in people with FCS, reducing their triglyceride levels.
The marketing application for volanesorsen for the treatment of FCS is based on data from the Phase 3 APPROACH and COMPASS studies. The pivotal APPROACH study, a one-year, randomized, placebo-controlled study in 66 patients with FCS (average baseline triglycerides of 2,209 mg/dL, or 25.0 mmol/L), achieved its primary endpoint of reduction in triglycerides at three months, with a 77 percent mean reduction in triglycerides, which translated into a 1,712 mg/dL (19.3 mmol/L) mean absolute triglyceride reduction in volanesorsen-treated patients. Akcea observed 50 percent of treated patients achieved triglyceride levels below 500 mg/dL, a commonly accepted threshold for pancreatitis risk. In addition, in the APPROACH study, treatment with volanesorsen was associated with a statistically significant reduced rate of pancreatitis attacks in the group of patients who had the highest incidence of pre-study pancreatitis, and reduced abdominal pain in patients reporting pain before treatment in the study. The COMPASS study, a six-month randomized placebo-controlled study in 113 patients with very high triglycerides (>500 mg/dL), also achieved its primary endpoint of reduction in triglycerides at three months, with a 71 percent mean reduction in triglycerides. In the COMPASS study, treatment with volanesorsen was associated with a statistically significant reduction in on-study pancreatitis attacks.
The most common adverse event in the studies was injection site reactions, which were mostly mild. In addition, declines in platelet counts were observed in many patients and some patients discontinued the study because of platelet declines. These platelet declines were not clinically significant in most patients and were generally well managed with monitoring and dose adjustment. Some patients discontinued participation in the APPROACH study due to other non-serious adverse events, including sweating and chills, severe fatigue, rash and injection site reaction. In the APPROACH study and the open label extension study, the potentially treatment-related serious adverse events, or SAEs, observed were serious platelet events (grade 4 thrombocytopenia), which resolved without complication after cessation of dosing. Enhanced monitoring was implemented during the study to support early detection and management of these issues. Since implementation of the enhanced monitoring, serious platelet events have been infrequent. In the COMPASS study, the most common adverse event in the volanesorsen-treated group of patients was injection site reactions, which were mostly mild, and a potentially treatment-related SAE of serum sickness reaction, from which the patient fully recovered. There have been no deaths and no treatment-related bleeding or cardiovascular events in any volanesorsen clinical study.
9
Akcea and we continue to conduct the BROADEN study, a Phase 3 clinical trial in patients with FPL, which continues to enroll, with data expected in 2019.
An open-label extension study is ongoing for patients with FCS who have completed or meet the study criteria for the APPROACH and COMPASS studies. Patients in the BROADEN study are also eligible to roll over into an open-label extension study upon completing dosing in the pivotal study.
Volanesorsen for the treatment of FCS is currently under regulatory review for marketing authorization in the U.S., EU and Canada. Volanesorsen has a PDUFA date of August 30, 2018 and an advisory committee meeting is scheduled for May 10, 2018. Volanesorsen has been granted priority review in Canada and a Promising Innovative Medicine, or PIM, designation by the United Kingdom’s Medicines and Healthcare Products Regulatory Agency, or MHRA. The U.S. and European regulatory agencies have granted Orphan Drug Designation to volanesorsen for the treatment of people with FCS. The European regulatory agency has also granted Orphan Drug Designation to volanesorsen for the treatment of FPL. In addition, Akcea and we have an ongoing OLE study of volanesorsen in people with FCS, in which all patients are treated with volanesorsen. Akcea and we also opened an EAP for eligible patients. Our EAP program is being initiated on a country-by-country basis globally and is currently available in select countries in Europe.
Akcea plans to globally commercialize volanesorsen for both FCS and FPL, if approved.
See our separate section below where we further discuss Akcea, our commercial affiliate.
Neurological Disease Franchise
We are discovering and developing antisense drugs to treat people with inadequate treatment options for both rare and common neurological diseases. According to the National Institute of Neurological Disorders and Stroke, or NINDS, at the National Institutes of Health, or NIH, a third of the 7,000 known rare diseases are neurological disorders or thought to include a neurological component.
Ionis Neurological Disease Pipeline
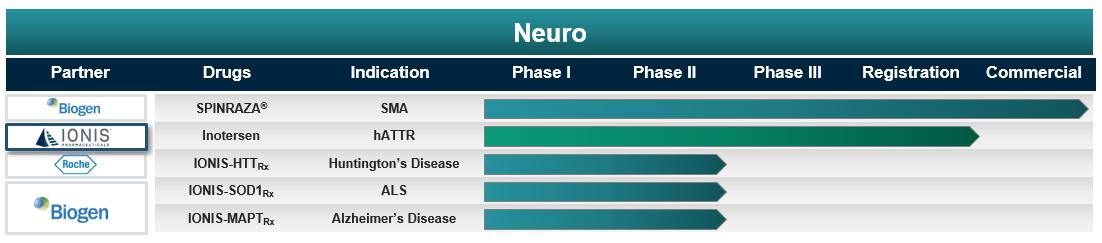
SPINRAZA – See the drug description under “Our Marketed Drug” section above.
Inotersen – See the drug description under “Our Drugs Under Regulatory Review for Marketing Authorization” section above.
IONIS-HTTRx – IONIS-HTTRx is a Generation 2+ antisense drug we designed to target the underlying cause of HD by reducing the production of the toxic mHTT protein. We and Roche entered into a collaboration to develop and commercialize antisense drugs to treat HD in April 2013. Roche licensed IONIS-HTTRx from us in December 2017. Roche is now responsible for all IONIS-HTTRx development, regulatory and commercialization activities and costs, including managing the ongoing OLE and all future studies.
HD is a rare, inherited, genetic brain disorder that results in the progressive deterioration of mental abilities and physical control. In the U.S., there are approximately 30,000 individuals with symptomatic HD and more than 200,000 people at risk of inheriting HD. HD is a triplet repeat disorder and is one of a large family of genetic diseases in which the body mistakenly repeats certain gene sequences. The resulting mHTT protein is toxic and gradually damages neurons in the brain. Symptoms of HD usually appear between the ages of 30 to 50 years and continually worsen over a 10 to 25-year period. Ultimately, the weakened individual succumbs to pneumonia, heart failure or other complications. Presently, there is no effective disease-modifying treatment, and current approaches only focus on managing the severity of some disease symptoms.
In December 2017, we announced that we had completed a randomized, placebo-controlled, dose escalation, Phase 1/2a clinical study of IONIS-HTTRx in patients with early stage HD. Dose-dependent reductions of mHTT were observed among patients treated with IONIS-HTTRx, with a safety and tolerability profile supporting continued development.
The FDA and EMA have granted Orphan Drug Designation for IONIS-HTTRx to treat people with HD.
10
IONIS-SOD1Rx – IONIS-SOD1Rx is a Generation 2+ antisense drug we designed to reduce the production of superoxide dismutase 1, or SOD1, which is the best understood genetic cause of familial amyotrophic lateral sclerosis, or ALS. We are collaborating with Biogen to develop IONIS-SOD1Rx to treat people with an inherited form of ALS, SOD1-ALS.
ALS is a rare, fatal, neurodegenerative disorder. People with ALS suffer progressive degeneration of the motor neurons, which results in a declining quality of life and ultimately death. The second most common familial form of ALS is SOD1-ALS, in which people have a mutation in the SOD1 gene that causes a progressive loss of motor neurons. As a result, people with SOD1-ALS experience muscle weakness, loss of movement, difficulty in breathing and swallowing and eventually succumb to their disease. Currently, treatment options for people with ALS are extremely limited with no drugs that significantly slow disease progression.
Biogen is evaluating IONIS-SOD1Rx in a randomized, placebo-controlled, dose escalation, Phase 1/2 clinical study in patients with ALS, including patients with SOD1-ALS.
IONIS-MAPTRx – IONIS-MAPTRx is a Generation 2+ antisense drug designed to selectively reduce production of the tau protein in the brain. We are collaborating with Biogen to develop IONIS-MAPTRx to treat people with Alzheimer's disease, or AD, and frontotemporal dementia, or FTD.
Microtubule-associated protein tau, or MAPT, or tau, is thought to be a contributor or cause of certain neurodegenerative diseases, known as tauopathies, that are characterized by the deposition of abnormal tau protein in neurons and glia in the brain. These disorders include AD, Progressive Supranuclear Palsy, or PSP, and some forms of FTD.
We and Biogen are evaluating IONIS-MAPTRx in a Phase 1/2a, randomized, placebo-controlled, dose-escalation study to evaluate the safety and activity of once-monthly intrathecal injections in patients with mild AD.
Severe and Rare Disease Franchise
Our severe and rare disease franchise is the largest franchise in our pipeline. We are discovering and developing antisense drugs to treat people with severe and rare diseases who need new treatment options. We believe our antisense technology could offer effective therapies for these people. According to the NIH there are approximately 5,000 to 8,000 rare diseases, many life-threatening or fatal. Unfortunately, people with many of these severe and rare diseases have few effective therapies available. Since most of these diseases are genetic or have a genetic component, parents often pass the disease to their children, creating a legacy of the disease resulting in profound effects on the family.
Due to the severe nature of these diseases and the lack of available treatments, there is an opportunity for more flexible and efficient development paths to the market. This means that, in some cases, the studies necessary for us to demonstrate proof-of-concept with a particular drug may also be the studies that complete our marketing registration package, thereby providing us with a relatively rapid path to market for potential new treatments for these devastating and often fatal diseases. For example, SPINRAZA was approved five years after we began the Phase 1 study for it.
IONIS’ Severe and Rare Disease Pipeline
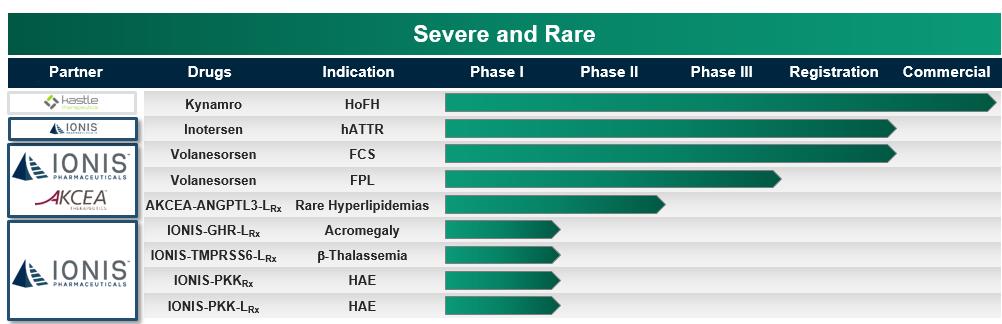
Kynamro – Kynamro (mipomersen sodium) injection is an oligonucleotide inhibitor of apolipoprotein B-100 synthesis indicated as an adjunct to lipid-lowering medications and diet, to reduce low density lipoprotein-cholesterol, or LDL-C, apolipoprotein B, total cholesterol, and non-high density lipoprotein-cholesterol in patients with homozygous familial hypercholesterolemia, or HoFH. Kynamro is approved for use in people with HoFH in the U.S. and several other countries. In 2016 Kastle acquired the global rights to develop and commercialize Kynamro and also began marketing and selling Kynamro.
Inotersen – See the drug description under “Our Drugs Under Regulatory Review for Marketing Authorization” section above.
Volanesorsen – See the drug description under “Our Drugs Under Regulatory Review for Marketing Authorization” section above.
AKCEA-ANGPTL3-LRx – See the drug description under the “Akcea Therapeutics” section below.
11
IONIS-GHR-LRx – IONIS-GHR-LRx is a LICA drug we designed to reduce the production of the growth hormone receptor, or GHr, to decrease the circulating level of insulin-like growth factor-1, or IGF-1. IGF-1 is a hormone primarily produced in the liver that plays an important role in childhood growth and has anabolic effects in adults. Several different diseases result from abnormally low or high levels of IGF-1, or an inappropriate response to this hormone. When produced in excess, IGF-1 results in acromegaly, a chronic, slowly progressing and life-threatening disease.
We have completed a Phase 1, double-blind, placebo-controlled, dose-escalation study of IONIS-GHR-LRx in healthy volunteers. Results from the Phase 1 study demonstrated an acceptable safety profile supportive of continued development.
IONIS-TMPRSS6-LRx – IONIS-TMPRSS6-LRx is a LICA drug we designed to reduce the production of transmembrane protease, serine 6, or TMPRSS6, to treat anemia and iron toxicity in people with β-thalassemia; a disease caused by mutations in the beta globin gene. TMPRSS6 is a protein produced in the liver that plays an important role in the regulation of the body's iron homeostasis through the control of the iron regulatory protein hepcidin. Inhibition of TMPRSS6 leads to increased production of hepcidin, which results in more effective red blood cell production in the bone marrow and reduced iron toxicity in the liver as a result of improved control of iron availability. Results from preclinical and clinical studies suggest that reducing levels of TMPRSS6 may be an effective strategy to control iron availability, improve liver iron toxicity and increase red blood cell production under conditions of β-thalassemia.
We are currently evaluating IONIS-TMPRSS6-LRx in a randomized, double-blind, placebo-controlled, dose-escalation Phase 1 study in healthy volunteers.
IONIS-PKKRx and IONIS-PKK-LRx – IONIS-PKKRx and IONIS-PKK-LRx are antisense drugs we designed to reduce the production of prekallikrein, or PKK, to treat people with hereditary angioedema, or HAE. HAE is a rare genetic disease that is characterized by rapid and painful attacks of inflammation in the hands, feet, limbs, face, abdomen, larynx and trachea and can be fatal if swelling occurs in the larynx. PKK plays an important role in the activation of inflammatory mediators associated with acute attacks of HAE. By inhibiting the production of PKK, IONIS-PKKRx and IONIS-PKK-LRx could be effective prophylactic approaches to preventing HAE attacks. In patients with frequent or severe attacks, doctors may use prophylactic treatment approaches to prevent or reduce the severity of HAE attacks. However, current prophylactic treatment approaches are very limited and have major tolerability issues due to challenging administration requirements leaving patients with few therapeutic options.
We have completed a Phase 1 study evaluating IONIS-PKKRx in healthy volunteers and we are exploring potential development options. We are currently evaluating IONIS-PKK-LRx in a Phase 1, randomized, double-blind, placebo-controlled, dose-escalation study in healthy volunteers. The Phase 1 study is evaluating single and multiple doses of IONIS-PKK-LRx administered subcutaneously.
Cardiometabolic and Renal Disease Franchise
Cardiovascular disease, or CVD, is an important area of focus for us. According to the World Health Organization, or WHO, cardiovascular disease was the number 1 cause of death globally. An estimated 17.7 million people died from CVDs in 2015, representing 31 percent of all global deaths. The drugs in our cardiovascular franchise target the key components of cardiovascular disease, including various atherogenic lipids, inflammation and thrombosis. Metabolic disorders are chronic diseases that affect millions of people. There is a significant need for new therapies for these people. According to the Centers for Disease Control and Prevention, diabetes affects more than 29 million people in the U.S., or nine percent of the population, with type 2 diabetes constituting 90 to 95 percent of those cases.
IONIS’ Cardiometabolic and Renal Disease Pipeline
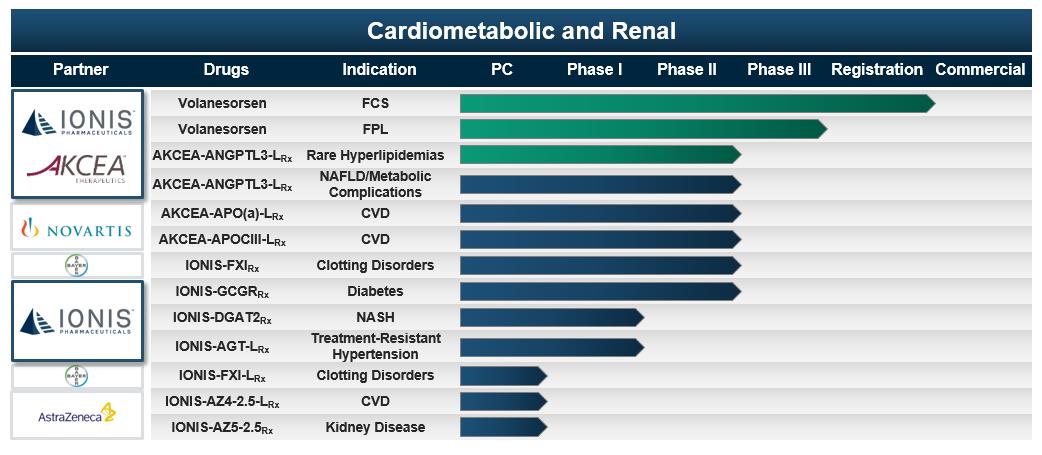
12
Volanesorsen – See the drug description under “Our Drugs Under Regulatory Review for Marketing Authorization” section below.
AKCEA-ANGPTL3-LRx – See the drug description under the “Akcea Therapeutics” section below.
AKCEA-APO(a)-LRx, – See the drug description under the “Akcea Therapeutics” section below.
AKCEA-APOCIII-LRx – See the drug description under the “Akcea Therapeutics” section below.
IONIS-FXIRx – IONIS-FXIRx is a Generation 2+ antisense drug we designed to reduce the production of Factor XI. Factor XI is a clotting factor produced in the liver that is important in the growth of blood clots. High levels of Factor XI increase the risk of thrombosis, which is the formation of a blood clot inside blood vessels. Thrombosis can cause heart attacks and strokes. People who are deficient in Factor XI have a lower incidence of thromboembolic events with minimal increase in bleeding risk. Although currently available anticoagulants reduce the risk of thrombosis, physicians associate these anticoagulants with increased bleeding, which can be fatal. Given the mechanism of Factor XI inhibition, we believe that our drug has the potential to be used broadly as an anti-thrombotic in many different therapeutic settings for which additional safe and well tolerated anti-thrombotic drugs are needed.
We completed a Phase 2 open-label, comparator-controlled global study evaluating IONIS-FXIRx in people undergoing total knee replacement surgery. The study compared the safety and activity of IONIS-FXIRx to enoxaparin. In this study patients treated with 300 mg of IONIS-FXIRx experienced a seven-fold lower rate of venous thromboembolic events, such as blood clots in a deep vein or in a lung, compared to those patients treated with enoxaparin. The data from this study were published in the New England Journal of Medicine in December 2014. In May 2015, we exclusively licensed IONIS-FXIRx to Bayer.
In November 2016, we completed a Phase 2 double-blinded, randomized, placebo-controlled study of IONIS-FXIRx in people with end-stage renal disease on hemodialysis. In this Phase 2 study, patients treated with IONIS-FXIRx achieved statistically significant, dose-dependent reductions in Factor XI activity. There were no clinically meaningful reductions in platelets and no treatment-related major or clinically relevant non-major bleeding events.
We are currently evaluating IONIS-FXIRx in a Phase 2b study in approximately 200 people with end-stage renal disease on hemodialysis to finalize dose selection.
IONIS-GCGRRx – IONIS-GCGRRx is a Generation 2+ antisense drug we designed to reduce the production of glucagon receptors, or GCGR, to treat people with type 2 diabetes. GCGR is a receptor for the hormone glucagon. Glucagon is a hormone that opposes the action of insulin and stimulates the liver to produce glucose, particularly in type 2 diabetes. We are developing IONIS-GCGRRx to provide better glucose control for people with type 2 diabetes. In people with advanced diabetes, uncontrolled glucagon action can lead to significant increases in blood glucose level. In addition, reducing GCGR produces more active glucagon-like peptide-1, or GLP-1, a hormone that preserves pancreatic function and enhances insulin secretion. We are developing IONIS-GCGRRx with Suzhou Ribo Life Sciences Co., for the treatment of diabetes in China.
In January 2017, we reported results from a Phase 2 dose optimization study in which patients treated with IONIS-GCGRRx achieved robust and sustained, statistically significant improvements in hemoglobin A1c, or HbA1c, and other measures of glucose control after 26 weeks of treatment. Additionally, IONIS-GCGRRx-treated patients experienced a mean increase in total GLP-1 from baseline compared to a decline in placebo-treated patients. The safety and tolerability profile of IONIS-GCGRRx in the Phase 2 program supports continued development.
In March 2017, we licensed IONIS-GCGRRx to Ribo to develop and commercialize the drug in China.
IONIS-DGAT2Rx – IONIS-DGAT2Rx is a Generation 2+ antisense drug we designed to reduce the production of DGAT2, or diacylglycerol acyltransferase 2, to treat people with nonalcoholic steatohepatitis, or NASH. NASH is a common liver disease characterized by excessive triglycerides in the liver with concurrent inflammation and cellular damage. As NASH progresses, scarring, or fibrosis, begins to accumulate in the liver. Ultimately, cirrhosis of the liver develops and the liver can no longer function normally. Currently, it is estimated that two to three percent of the general population have NASH. However, with the growing obesity epidemic, the number of people with NASH should also continue to rise. About 20 percent of people with NASH are reported to have a liver that does not function properly due to long-term damage, known as cirrhosis. Of those with NASH-related cirrhosis, 30 to 40 percent experience liver-related death. Currently, liver transplantation is the only treatment for advanced cirrhosis and liver failure. Because of the high prevalence of NASH, it has recently become the third most common indication for liver transplantation in the U.S.
DGAT2 is an enzyme that catalyzes the final step in triglyceride synthesis in the liver. Reducing the production of DGAT2 should therefore decrease triglyceride synthesis in the liver. In animal models of obesity and fatty liver disease, antisense inhibition of DGAT2 significantly improved non-alcoholic fatty liver disease, or NAFLD, lowered blood lipid levels and reversed diet-induced insulin resistance. NASH is a more severe form of NAFLD.
We are evaluating IONIS-DGAT2Rx in a Phase 2 randomized, placebo-controlled, dose-escalation study in patients with type 2 diabetes and NAFLD.
IONIS-AGT-LRx – IONIS-AGT-LRx is a LICA drug we designed to reduce the production of angiotensinogen to decrease blood pressure in people with treatment resistant hypertension, or TRH. Despite availability of generic antihypertensive agents, TRH is a major contributor to cardiovascular and renal disease.
We are evaluating IONIS-AGT-LRx in a blinded, randomized, placebo-controlled, dose-escalation Phase 1/2a study in healthy volunteers.
13
Cancer Franchise and Other Drugs in Development
Cancer is an area of significant unmet medical need. Cancer is an extremely complex disease that involves a large number of targets. Using our antisense technology, we can validate multiple potential cancer targets from a variety of different cancers, and rapidly identify anti-cancer drugs, which in many cases are the same or similar sequences to those used to validate the target. We preferentially select anti-cancer targets that provide a multi-faceted approach to treating cancer.
Our cancer franchise consists of anti-cancer antisense drugs that act upon biological targets associated with cancer progression and/or treatment resistance. We have a strategic alliance with AstraZeneca, which includes an anti-cancer collaboration that expands our anti-cancer efforts and supports a robust clinical development plan for IONIS-STAT3-2.5Rx and IONIS-KRAS-2.5Rx. AstraZeneca brings significant experience that enables the identification of novel genetic and epigenetic targets for cancer. Combining AstraZeneca’s expertise with our drug discovery technology, we plan to expand our cancer franchise with a number of promising new anti-cancer targets. We also have a collaboration agreement with University of Texas MD Anderson Cancer Center to identify cancer targets and create novel antisense drugs to treat cancer together.
Our Generation 2.5 chemistry enhances the potency and effectiveness of our antisense drugs, and potentially allows us to extend the applicability of our technology to cancers that are difficult to treat. For instance, STAT3 is a protein known to be important in carcinogenesis, however, it has been difficult to approach with traditional drug modalities. Data from a Phase 1b/2 clinical study of IONIS-STAT3-2.5Rx in combination with Imfinzi (durvalumab), AstraZeneca’s programmed death ligand (PD-L1) blocking antibody showed evidence of antitumor activity in people with advanced solid tumors and recurrent metastatic head and neck cancer.
In addition to cancer programs, we continue to advance other drugs in development that are outside of our core therapeutic areas, such as IONIS-FB-LRx for compliment-mediated diseases, and the antiviral drugs we and GSK are developing.
IONIS’ Oncology/Other Pipeline
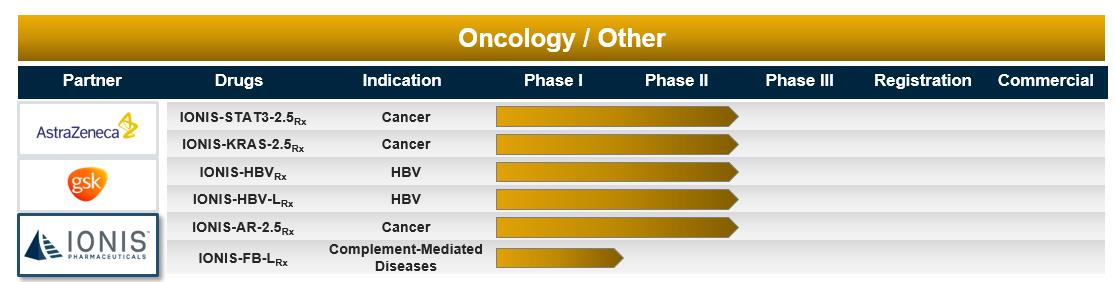
IONIS-STAT3-2.5Rx – IONIS-STAT3-2.5Rx, also referred to as AZD9150, is a Generation 2.5 antisense drug we designed to reduce the production of signal transducer and activator of transcription 3, or STAT3, to treat people with cancer. STAT3 is a protein involved in the translation of key factors critical for tumor cell growth and survival. STAT3 is over-active in a variety of cancers, including brain, lung, breast, bone, liver and multiple myeloma. Physicians believe that overactivity in STAT3 prevents cancer cell death and promotes tumor cell growth. IONIS-STAT3-2.5Rx is a part of our collaboration with AstraZeneca to discover and develop anti-cancer drugs. We believe the significant potency we observed in our preclinical studies with IONIS-STAT3-2.5Rx broadens the therapeutic opportunities for IONIS-STAT3-2.5Rx into many different types of cancer in which STAT3 is implicated.
In September 2017, we and AstraZeneca announced data from a Phase 1b/2 study of IONIS-STAT3-2.5Rx in combination with Imfinzi (durvalumab), AstraZeneca’s PD-L1 blocking antibody, in people with advanced solid tumors and recurrent metastatic head and neck cancer. The treatment combination demonstrated a 29 percent (8/28) objective response rate with four partial responses, or PR, and four complete responses, or CR, of which one was a CR in target lesions only. An additional eight people on the treatment combination had stable disease, or SD, at 12 weeks, resulting in an overall disease control rate of 57 percent (16/28). A complete response was seen in a person with recurrent/metastatic squamous cell carcinoma of the head and neck that was refractory to previous PD-L1 treatment. IONIS-STAT3-2.5Rx in combination with Imfinzi demonstrated a safety and tolerability profile supporting continued development.
IONIS-KRAS-2.5Rx - IONIS-KRAS-2.5Rx, also referred to as AZD4785, is a Generation 2.5 antisense drug designed to selectively inhibit KRAS, one of the most frequently mutated genes in cancer. KRAS mutations are thought to underlie the pathogenesis of up to 30 percent of human tumors. The KRAS protein is involved in regulating cell division and tumor cell survival. IONIS-KRAS-2.5Rx is a part of our collaboration with AstraZeneca to discover and develop anti-cancer drugs.
AstraZeneca is evaluating IONIS-KRAS-2.5Rx in a Phase 1/2, open-label, multi-center, dose-escalation study in people with advanced solid tumors for whom KRAS may be an important driver of tumor survival.
IONIS-HBVRx and IONIS-HBV-LRx – IONIS-HBVRx and IONIS-HBV-LRx are antisense drugs we designed to reduce the production of viral proteins associated with hepatitis B virus, or HBV. These include proteins associated with infection and replication, including the hepatitis B surface antigen, which is present in both acute and chronic infections and is associated with a poor prognosis in people with chronic HBV infection. IONIS-HBV-LRx is our first anti-infective drug in development that incorporates our LICA technology. Together with GSK, we are evaluating IONIS-HBVRx and IONIS-HBV-LRx to treat HBV infection.
14
HBV infection is a serious health problem that can lead to significant and potentially fatal health conditions, including cirrhosis, liver failure and liver cancer. Chronic HBV infection is one of the most common persistent viral infections in the world. Currently available therapies, although effective in reducing circulating HBV in the blood, do not effectively inhibit HBV antigen production and secretion, which are associated with poor prognosis and increased risk of liver cancer.
We and GSK are evaluating both IONIS-HBVRx and IONIS-HBV-LRx in Phase 2 studies in people with HBV infection.
IONIS-AR-2.5Rx – IONIS-AR-2.5Rx is a Generation 2.5 antisense drug we designed to treat people with prostate cancer by reducing the production of all known forms of androgen receptor, or AR, including variants of the AR gene. Prostate cancer is the second leading cause of cancer deaths in American men. Prostate cancer growth, proliferation and progression are all androgen-dependent and AR function is involved in disease progression at all stages of prostate cancer. For people diagnosed with metastatic prostate cancer, current treatments largely involve opposing the action of androgens by blocking the AR or removing circulating androgens. Although androgen deprivation therapy approaches are initially effective in delaying disease progression, people with metastatic prostate cancer will progress in their disease. Resistance to current therapies is frequent and can occur through a variety of mechanisms, including the activation of AR signaling in tumor cells through the amplification, over expression and mutation of the AR gene. Because IONIS-AR-2.5Rx can inhibit the production of all known forms of AR, we believe that this drug has the potential to be useful in treating people with all stages of prostate cancer, including those who are resistant to current therapies.
AstraZeneca completed an open-label, dose-escalation, Phase 1/2 clinical study of IONIS-AR-2.5Rx in people with advanced tumors for which the androgen receptor pathway is potentially a contributing factor. The drug exhibited a good safety and tolerability profile supportive of continued development. In March 2017, we licensed IONIS-AR-2.5Rx to Ribo to develop and commercialize the drug in China.
IONIS-FB-LRx - IONIS-FB-LRx is a LICA drug we designed to reduce the production of complement factor B, or FB. FB is produced predominantly in the liver and circulates at high levels throughout the vascular system where it plays a pivotal role in an innate immunogenic cascade. Genetic association studies have shown that overaction of this cascade has been associated with the development of several complement-mediated diseases, including dry age-related macular degeneration, or AMD. FB, which plays a pivotal role in this cascade, is produced predominately in the liver and circulates at high levels throughout the vascular system, including in capillaries in the eye.
In May 2017, we reported data from a randomized, placebo-controlled, dose-escalation Phase 1 study evaluating IONIS-FB-LRx in 54 healthy volunteers. Subjects treated with a single dose of IONIS-FB-LRx achieved dose-dependent reductions in plasma FB of up to 50 percent. Treatment with multiple doses of IONIS-FB-LRx during a six-week period resulted in greater reductions in circulating FB levels. The safety and tolerability profile of IONIS-FB-LRx supports further clinical development.
We are currently evaluating IONIS-FB-LRx in a Phase 2 study in people with dry AMD.
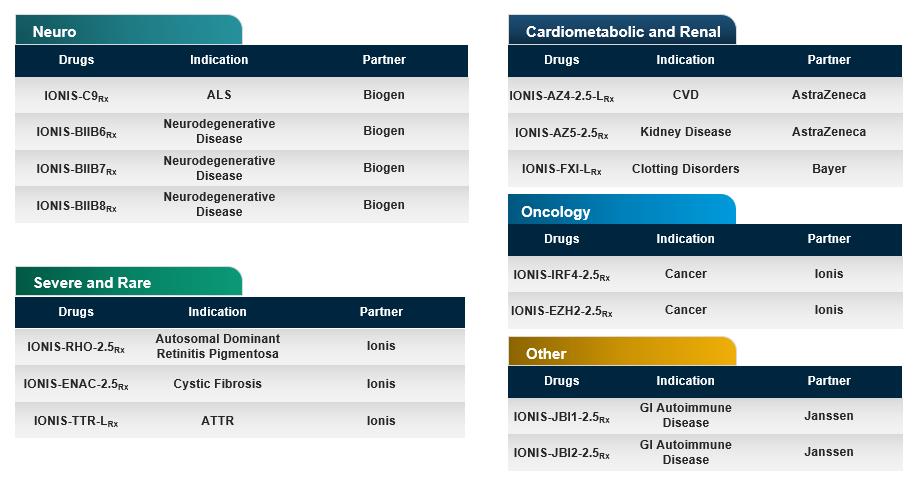
Preclinical Drugs in Development
The efficiency and broad applicability of our technology provides us with nearly unlimited targets against which to develop drugs. On average, it takes 12 to 18 months to complete the preclinical studies necessary to support clinical development. Over the last year we added eight new drugs to our preclinical pipeline.
IONIS’ Preclinical Pipeline
15
Akcea Therapeutics: Our Affiliate Focused on Developing and Commercializing Drugs to Treat People with Serious Cardiometabolic Diseases Caused by Lipid Disorders
Akcea Therapeutics is our development and commercialization affiliate that we formed in late 2014 to focus on developing and commercializing drugs to treat people with serious cardiometabolic diseases caused by lipid disorders. As part of its formation, we granted Akcea exclusive rights to develop and commercialize volanesorsen, AKCEA-APO(a)-LRx, AKCEA-APOCIII-LRx and AKCEA-ANGPTL3-LRx. These four novel drugs are based on our antisense technology and have the potential to treat multiple indications. We describe each of these drugs in more detail below.
Akcea is assembling the global infrastructure to develop the drugs in its pipeline, to commercialize them with a focus on lipid specialists as the primary call point and to create the specialized support required to address rare disease patient populations. Akcea and we entered into a collaboration, option and license agreement with Novartis, in which Akcea granted Novartis an exclusive option to license AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Both these drugs have the potential to treat people who are at high cardiovascular risk due to inadequately treated lipid disorders. After Akcea completes the Phase 2 development of each of these drugs, Novartis has the option to license each drug. If Novartis licenses one or both drugs, it plans to, for each licensed drug, use commercially reasonable efforts to conduct, at its expense, a Phase 3 cardiovascular outcome study in a high-risk patient population and will be responsible for the worldwide development and commercialization activities. Novartis brings significant resources and expertise that should support the development and commercialization of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx for significant high-risk patient populations. Akcea also plans to co-commercialize any such drug through its specialized sales force focused on lipid specialists in selected markets.
This report includes financial information for this separate business segment in Note 7, Segment Information and Concentration of Business Risk, in the Notes to the Consolidated Financial Statements.
Volanesorsen – Volanesorsen is a Generation 2+ antisense drug under regulatory review for marketing authorization in the U.S., EU and Canada for the treatment of people with FCS. Akcea and we are also developing volanesorsen to treat people with FPL. For more information on the regulatory and development plan for volanesorsen, see the drug description under “Our Drugs under Regulatory Review for Marketing Authorization” section above.
AKCEA-APO(a)-LRx – AKCEA-APO(a)-LRx is a LICA drug we designed to reduce the production of apolipoprotein(a), or Apo(a), protein in the liver to offer a direct approach for reducing lipoprotein(a), or Lp(a). Lp(a) is an independent risk factor for CVD that is composed of an apolipoprotein(a) protein bound to an LDL-cholesterol particle. Akcea initiated a collaboration with Novartis in January 2017 to advance AKCEA-APO(a)-LRx.
Akcea is developing AKCEA-APO(a)-LRx for people who are at significant risk of CVD because of their elevated levels of Lp(a). AKCEA-APO(a)-LRx inhibits the production of the Apo(a) protein, thereby reducing Lp(a). Lp(a) is a very atherogenic and thrombogenic form of LDL. Elevated Lp(a) is recognized as an independent, genetic cause of coronary artery disease, heart attack, stroke and peripheral arterial disease. Inhibiting the production of Apo(a) in the liver reduces the level of Lp(a) in blood, potentially slowing down or reversing cardiovascular disease in people with hyperlipoproteinemia(a), a condition in which individuals have levels of Lp(a) greater than 60 mg/dL.
Lp(a) is difficult to inhibit using other technologies, such as small molecules and antibodies; there are multiple genetically-determined forms of the Apo(a) molecule and creating a small molecule or antibody that can interact with multiple targets is difficult. We believe antisense technology is particularly well suited to address hyperlipoproteinemia(a) because it specifically targets the RNA that codes for all forms of the Apo(a) molecule. As a result, it can stop the production of all the forms of the protein. Furthermore, we believe addressing elevated Lp(a) is the next important horizon in lipid-focused treatment and, through Akcea’s collaboration with Novartis, it plans to develop AKCEA-APO(a)-LRx to treat people with established cardiovascular disease in whom hyperlipoproteinemia(a) likely plays a causal role.
Akcea and we completed a Phase 1/2a study with AKCEA-APO(a)-LRx in patients with hyperlipoproteinemia(a) and reported results at the American Heart Association, or AHA, annual meeting in November 2015. In this clinical study, we observed significant and sustained reductions in Lp(a) of up to 97 percent with a mean reduction of 79 percent after only a single, small volume dose of AKCEA-APO(a)-LRx. With multiple doses of AKCEA-APO(a)-LRx, we observed even greater reductions of Lp(a) of up to 99 percent with a mean reduction of 92 percent. Based on these results, Akcea started a Phase 2b dose-ranging study of AKCEA-APO(a)-LRx in patients with hyperlipoproteinemia(a) and established CVD. Akcea completed enrollment in this study in January 2018 and expects to report data from this study in the second half of 2018.
AKCEA-ANGPTL3-LRx – AKCEA-ANGPTL3-LRx is a LICA drug we designed to reduce the production of the angiopoietin-like 3, or ANGPTL3, protein. Akcea and we are developing AKCEA-ANGPTL3-LRx to treat multiple lipid disorders.
People with elevated levels of the angiopoietin-like 3, or ANGPTL3, protein have high LDL-C and triglyceride levels. Studies show this elevation is associated with an increased risk of premature heart attacks, increased arterial wall thickness, increased liver fat and multiple metabolic disorders, such as insulin resistance. In contrast, people with lower levels of ANGPTL3 have lower LDL-C and triglyceride levels, and thus lower risk of heart attacks, lower prevalence of fatty liver and lower incidence of metabolic disorders. In preclinical studies, an analog of AKCEA-ANGPTL3-LRx inhibited the production of the ANGPTL3 protein in the liver, resulting in lower liver fat accumulation and lower blood levels of LDL-C, triglycerides and very low density lipoprotein cholesterol, or VLDL-C. In addition, our preclinical data and initial Phase 1 data suggest that inhibiting the production of ANGPTL3 could improve other lipid parameters, including triglyceride levels and total cholesterol, as well as metabolic parameters, such as insulin sensitivity.
16
We and Akcea have completed a Phase 1/2 program for AKCEA-ANGPTL3-LRx in patients with elevated triglycerides. We and Akcea reported results for the initial cohort from this study at the AHA meeting in November 2016 and published the data in the New England Journal of Medicine. In the fourth quarter of 2017, we and Akcea initiated a study of AKCEA-ANGPTL3-LRx in patients with nonalcoholic fatty liver disease, or NAFLD, with metabolic complications, which include hypertriglyceridemia, type 2 diabetes or nonalcoholic steatohepatitis, or NASH. We expect data from this study in 2019. Further, in the fourth quarter of 2017, we and Akcea initiated a study of AKCEA-ANGPTL3-LRx in patients with rare hyperlipidemias, including patients with FCS. If we and Akcea find that AKCEA-ANGPTL3-LRx can effectively lower triglyceride levels in patients with rare hyperlipidemias, including patients with FCS, through a different mechanism of action from volanesorsen, it may represent an opportunity to expand our FCS franchise. As part of our exploratory rare hyperlipidemia clinical program, we and Akcea are also studying AKCEA-ANGPTL3-LRx in patients with FPL and in patients with HoFH. Additional potential indications for which we may consider developing AKCEA-ANGPTL3-LRx include other rare hyperlipidemias and lipodystrophies.
AKCEA-APOCIII-LRx – is a LICA drug we designed to inhibit the production of apoC-III, the same protein inhibited by volanesorsen, for the broad population of people who are at risk for cardiometabolic disease due to their elevated triglyceride levels. Akcea and we initiated a collaboration with Novartis in January 2017 to advance AKCEA-APOCIII-LRx.
ApoC-III impacts triglyceride levels and may also increase inflammatory processes. This combination of effects makes apoC-III a promising target for people with LDL-C already controlled on statin therapy, but for whom triglycerides remain poorly controlled. We believe that the enhancements offered by our LICA technology can provide greater patient convenience by allowing for significantly lower doses and less frequent administration, compared to volanesorsen. We and Akcea conducted a Phase 1/2 study of AKCEA-APOCIII-LRx in people with elevated triglycerides and reported results from this study in the fourth quarter of 2017.
Under our and Akcea’s collaboration with Novartis, we intend to complete the Phase 2 program required to define the appropriate dose and regimen to support a planned cardiovascular outcome study. We and Akcea initiated a Phase 2b dose-ranging study of AKCEA-APOCIII-LRx in patients with hypertriglyceridemia and established CVD in the first quarter of 2018 and plan to report data from this study in 2019.
Satellite Company Drugs in Development
We have successfully developed novel drugs we designed to treat many different diseases. In therapeutic areas that are outside of our core areas of development, we have licensed our drugs to highly focused satellite companies that have the specific expertise and resources to continue developing the drugs. For our satellite company drugs, we refer to the drug by the partner’s name or compound number, such as plazomicin or ATL1103. We have listed these drugs below in our Satellite Company pipeline.
IONIS’ Satellite Company Pipeline
* Named Patient Supply (see below).
Plazomicin – Plazomicin is an aminoglycoside drug that Achaogen is developing for the treatment of multi-drug resistant gram-negative bacterial infections. Aminoglycosides are a group of antibiotics that inhibit bacterial protein synthesis used to treat serious bacterial infections. In 2006, we licensed our proprietary aminoglycoside program to Achaogen. Achaogen discovered plazomicin based on technology licensed from us. Achaogen conducted two Phase 3 studies for plazomicin, CARE and EPIC. In December 2016, Achaogen announced that it completed two Phase 3 studies of plazomicin. The EPIC trial met its primary endpoint in patients with complicated urinary tract infections. The CARE trial demonstrated reduction in mortality in patients with serious multi-drug resistant infection due to carbapenem-resistant Enterobacteriaceae, or CRE compared with colistin, one of the few remaining antibiotics for treatment of infections due to CRE. Plazomicin was well tolerated in both Phase 3 studies.
17
Achaogen submitted an NDA to the FDA for plazomicin for the treatment of complicated urinary tract infections, including kidney infections and bloodstream infections due to certain Enterobacteriaceae in people who have limited to no alternative treatment options. FDA granted the NDA Priority Review and set a target action date under the PDUFA date of June 25, 2018. Achaogen plans to submit a MAA to the EMA in 2018.
The FDA has granted Fast Track Status for the development and regulatory review of plazomicin to treat serious and life-threatening CRE infections. In addition, plazomicin has received Qualified Infectious Disease Product, or QIDP, designation from the FDA. The QIDP designation provides certain incentives for the development of new antibiotics, including priority review and an additional five years of market exclusivity.
Alicaforsen – Alicaforsen is an antisense drug we designed to reduce the production of intercellular adhesion molecule 1, or ICAM-1. Ulcerative colitis, or UC, is an inflammatory bowel disease of the colon, a part of the large intestine, and pouchitis is an inflammation of the surgically constructed internal pouch created in people with UC who have had their diseased colons removed. In 2007, we licensed alicaforsen to Atlantic Pharmaceuticals for pouchitis, UC and other inflammatory diseases. Atlantic Pharmaceuticals is developing alicaforsen for the treatment of UC and currently supplies alicaforsen in response to physicians' requests under international Named Patient Supply regulations for people with inflammatory bowel disease. In 2017, under a rolling submission agreement with the FDA, Atlantic Pharmaceuticals filed the nonclinical data package of its NDA for alicaforsen to treat pouchitis. Alicaforsen has also been granted FDA Fast-Track designation, plus U.S. and European Orphan Drug designations for this indication.
ATL1103 – ATL1103 is an antisense drug we designed to reduce the production of the growth hormone receptor, or GHr, to treat people with acromegaly. Acromegaly is a serious, chronic, life-threatening disease triggered by excess secretion of GHr by benign pituitary tumors. In 2001, we licensed ATL1103 to Antisense Therapeutics Limited, or ATL. ATL conducted a successful Phase II trial of ATL1103 with the trial having met its primary efficacy endpoint by showing a statistically significant average reduction in sIGF-1 levels. ATL has also completed a high dose study of ATL1103 in adults with acromegaly in Australia.
RG-012 – RG-012 is an anti-miR, or an antisense oligonucleotide inhibitor of microRNA, targeting microRNA-21, or miR-21, to treat people with Alport syndrome, a life-threatening genetic kidney disease with no approved therapy. While there is little known information on the progression of this disease, researchers believe that miR-21 plays a critical role due to the observed increased miR-21 levels in animal models of Alport syndrome and in people with chronic kidney disease. Regulus is developing RG-012 in a strategic alliance with Genzyme, a Sanofi company, to treat Alport syndrome. In September 2017, Regulus initiated HERA, the Phase 2 randomized, double-blinded, placebo-controlled study evaluating the safety and efficacy of RG-012 in people with Alport syndrome.
RGLS4326 – RGLS4326 is an anti-miR, or an antisense oligonucleotide inhibitor of microRNA, designed to inhibit miR-17 to treat people with autosomal dominant polycystic kidney disease, or ADPKD, using a unique chemistry design to preferentially target the kidney. ADPKD, caused by the mutations in the PKD1 or PKD2 genes, is among the most common human monogenetic disorders and a leading genetic cause of end-stage renal disease. Approximately 1 in 1,000 people bear a mutation in either PKD1 or PKD2 genes worldwide. Preclinical studies with RGLS4326 have demonstrated a reduction in kidney cyst formation, improved kidney weight/body weight ratio, decreased cyst cell proliferation, and preserved kidney function in mouse models of ADPKD.
RGLS4326 is being studied in a Phase I randomized, double-blind, placebo-controlled, single ascending dose study designed to investigate the safety, tolerability, pharmacokinetics and pharmacodynamics of RGLS4326 administered subcutaneously in healthy volunteers.
ATL1102 – ATL1102 is an antisense drug we designed to reduce the production of CD49d, a sub-unit of Very Late Antigen-4, or VLA-4, for the treatment of people with multiple sclerosis, or MS. Results from preclinical studies demonstrated that inhibition of VLA-4 could positively affect a number of inflammatory diseases, including MS. In 2001, we licensed ATL1102 to ATL. ATL completed a chronic toxicology study in primates and a Phase 2a efficacy and safety trial. ATL1102 was shown by ATL to reduce MS lesions in the Phase 2a clinical trial and has also completed toxicology studies to support a potential future Phase 2b study in people with MS.
IONIS-DNM2-2.5Rx – IONIS-DNM2-2.5Rx is a Generation 2.5 antisense drug targeting dynamin 2 for the treatment of centronuclear myopathy, or CNM. CNM is a term for a group of rare, genetic, muscle disorders affecting children and young adults. These disorders are characterized by muscle weakness that can range from mild to profound. CNM, caused by mutations in the DNM2 gene, is highly variable in presentation and severity, presenting at birth, during childhood or in adulthood. When DNM2-related CNM occurs during infancy or early childhood, common symptoms include reduced muscle strength, generalized weakness, facial and eye muscle weakness and paralysis of muscles surrounding the eye. Affected children may exhibit delays in attaining motor milestones, such as holding their head up. Facial weakness can cause infants to have a weak sucking ability and/or experience difficulties swallowing, potentially resulting in feeding difficulties. Eventually, affected individuals can develop breathing complications, and sometimes infection of the lungs causes death in early infancy.
IONIS-DNM2-2.5Rx is currently in IND-enabling preclinical studies.
Antisense Technology
Our antisense technology is an innovative platform for discovering first-in-class or best-in-class drugs for treating disease. We believe this technology represents an important advance in the way we treat disease. Unlike most other drug technologies that work by affecting existing proteins in the body, antisense drugs target RNA, the intermediary that conveys genetic information from a gene to the protein synthesis machinery in the cell. By targeting RNA instead of proteins, we can use antisense technology to increase, decrease or alter the production of specific proteins. The unique properties of antisense technology provide several advantages over traditional drug discovery technologies.
18
These advantages include:
| ● |
Direct intervention in the disease process at the genetic level by targeting RNA: antisense technology represents a direct route from gene to drug. The explosion in genomic information and RNA biology has led to the discovery of many new disease-causing proteins and RNAs, and has created new opportunities that are only accessible to antisense technology.
|
| ● |
Precise specificity: we design antisense drugs to target a single RNA, which minimizes or eliminates the possibility our drugs will bind to unintended targets which can cause unwanted side effects.
|
| ● |
Good drug properties: antisense drugs distribute well throughout the body without the need for special formulations or vehicles. They also have a relatively long half-life of approximately two to four weeks in most tissues outside of the brain and spinal cord and three to four months in brain and spinal cord, which means patients and/or healthcare providers can dose our drugs weekly, monthly or even less frequently depending on the drug and target tissue.
|
| ● |
Ability to combine with other drugs: because antisense drugs do not interact with the enzymes that metabolize or break down other drugs, physicians can use our drugs in combination with other drugs.
|
| ● |
Broad applications to multiple disease targets, multiple tissues and multiple mechanisms: there are virtually no “undruggable” targets with antisense technology.
|
| ● |
Efficient discovery and early development: because of the efficiency of our antisense technology, our drug discovery and early development costs and success rates compare favorably to small molecule or antibody drug discovery and development.
|
We develop antisense drugs to potentially treat a wide range of diseases in a number of different therapeutic areas from severe and rare diseases to diseases that affect large patient populations. We focus our efforts on diseases in which there is a large unmet medical need with limited or no current treatments or in diseases for which we believe our drugs have a competitive advantage over existing therapies.
Technology Overview
We use our core technology platform to discover and develop drugs that affect targets in the body at the genetic level. Genes contain the information necessary to produce proteins. A gene is made up of nucleotides containing the nucleoside bases: adenine, thymine, guanine, and cytosine, commonly known as A, T, G and C, which are linked together to form a two-stranded structure that resembles a twisted ladder, known as deoxyribonucleic acid, or DNA. The nucleotides on one side of the ladder bind weakly to complementary nucleotides on the other strand according to specific rules; for example, A pairs with T and G pairs with C, creating the ladder’s rungs (Figure 1). Scientists call this highly specific nucleotide pairing hybridization. The sequence or order of these nucleotides establishes the cell’s recipes for making proteins. Each protein’s instructions reside in a corresponding segment of DNA known as a gene.
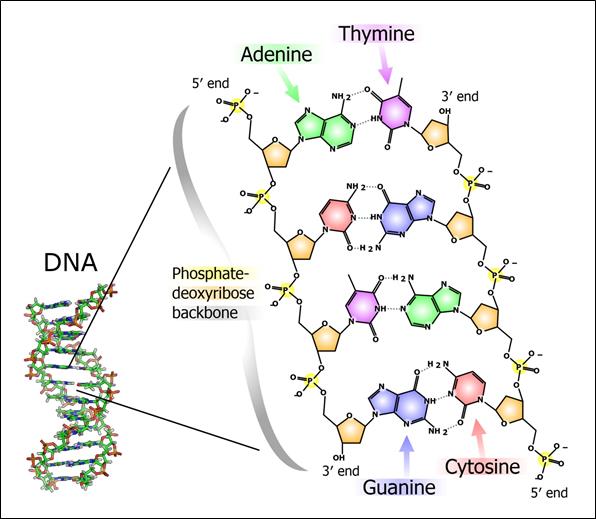
Figure 1: Illustration of DNA.
19
The instructions for making a protein are transcribed from a gene, or DNA into a different genetic molecule called messenger RNA. This process starts with the partial uncoiling of the two complementary strands of the DNA. One strand acts as a template and information stored in the DNA template strand is copied into a complementary RNA (Figure 2) by an enzyme called RNA polymerase, or RNAP. Messenger RNA, or mRNA, are mature, fully processed RNA that code for proteins.
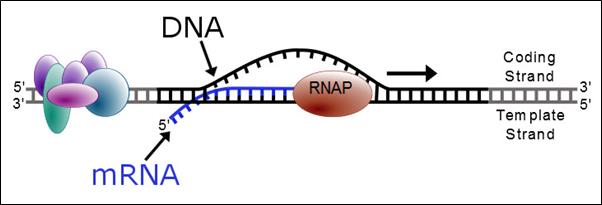
Figure 2: Transcription of information contained in a gene, or DNA, to mRNA.
Ribosomes, the cell’s factories for manufacturing proteins, translate mRNA into proteins. The ribosome reads the encoded information, the mRNA’s nucleotide sequence, and in doing so, strings together amino acids to form a specific protein (Figure 3).
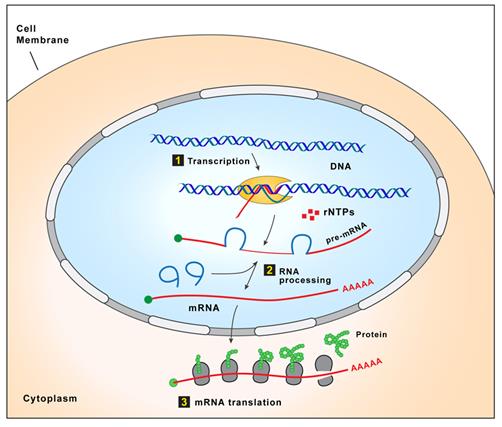
Figure 3: Translation of the protein-coding information contained in mRNA to protein.
We primarily use our antisense technology to interrupt the cell’s protein production process by preventing the mRNA instructions from reaching the ribosome, thus inhibiting the production of the protein. We can also design antisense drugs to increase protein production for diseases caused by the lack of a particular protein or modify the processing (or splicing) of the mRNA, which can alter the composition of the protein. The mRNA sequence of nucleotides that carries the information for protein production is called the ‘sense’ strand. Scientists call the complementary nucleotide chain that binds specifically to the sense strand the “antisense” strand. We use the information contained in mRNA to design chemical structures, that we call antisense oligonucleotides, or ASOs, or antisense drugs, which resemble DNA and RNA and are the complement of RNA. Our antisense drugs bind with high selectivity to the mRNA they were designed to target. Since each mRNA codes for a specific protein, this selectivity provides a level of specificity that is better than traditional drugs. As a result, we can design antisense drugs that selectively inhibit the disease-causing member of a protein family without interfering with other members of the protein family that might be necessary for normal cellular or bodily functions. This unique specificity means that antisense drugs may be less toxic than traditional drugs because we can design them to minimize the impact on unintended targets.
20
We have developed the majority of the drugs in our pipeline using our advanced screens to produce drugs with what we believe have the best possible safety and tolerability profiles. We refer to our drugs that have passed these advanced screens as Generation 2+ drugs. We continue to advance our antisense technology to create even more potent drugs that we can use in more tissues and against more targets. These advances allow us to expand the mechanisms through which we can use our drugs. These advancements provide us with greater opportunities to use our antisense drugs to treat a greater number of diseases and reach more patient populations. Today several of our early stage drugs and those entering our pipeline use our most advanced antisense technology, including our next generation chemistries, Generation 2.5, and our LICA technology.
Generation 2.5 chemistry is an Ionis advancement that we have demonstrated increases the potency of our drugs by up to ten-fold over our Generation 2+ drugs. This increase in potency enables our drugs to engage targets in a broader array of tissues. We have published data demonstrating that our Generation 2.5 drugs generally have enhanced potency over our Generation 2+ drugs and are broadly distributed throughout the body to multiple tissues including liver, kidney, lung, muscle, adipose, adrenal gland, peripheral nerves and tumor tissues. Our Generation 2.5 drugs constitute some of our recently added new drugs to our pipeline.
LICA (LIgand-Conjugated Antisense) is a chemical technology we developed at Ionis that involves the attachment of a molecule called a ligand that binds with receptors on the surfaces of cells in a highly specific manner. Because these receptors are often found only on certain cell types, LICA allows us to increase effective delivery of our antisense drugs with higher specificity to certain cell types that express these receptors relative to non-conjugated antisense drugs. We have demonstrated with multiple Generation 2+ LICA drugs that our LICA technology for liver targets can increase potency by up to more than thirty-fold over our non-LICA Generation 2+ drugs. We have also combined our LICA technology with our Generation 2.5 chemistry drugs to further increase potency. Although we designed our first LICA drugs to inhibit targets in the liver, we are also developing LICA conjugation technology that we can use to target other tissues and initial results are promising.
Antisense Targets and Mechanisms
There are more than a dozen different antisense mechanisms that we can exploit with our antisense technology. The majority of the drugs in our pipeline bind to mRNAs and inhibit the production of disease-causing proteins. However, our antisense technology is broadly applicable to many different antisense mechanisms, including modulation of RNA splicing, RNA interference, or RNAi, and enhancing protein translation to increase protein production. We have also recently published research showing that we can use our proprietary oligonucleotide technology with CRISPR/Cas9, a gene editing system that uses RNA to activate, target and edit specific sites on DNA. Our work in this area provides an important step toward development of potential therapeutic applications for CRISPR technology.
When using antisense technology to inhibit the production of disease-causing proteins or reduce levels of harmful RNAs, our antisense drugs bind to the target RNA via highly specific nucleotide pairing, or hybridization, and recruit a cellular enzyme called ribonuclease H1, or RNase H1, to degrade the target RNA. The antisense drug itself remains intact during this process, so it can remain active against additional target RNA molecules and repeatedly trigger their degradation (Figure 4). Examples of our clinical development stage antisense drugs that use the RNase H1 mechanism to reduce disease protein production include, volanesorsen, inotersen, IONIS-FXIRx, IONIS-FXI-LRx, AKCEA-APO(a)-LRx, IONIS-HTTRx, and others.
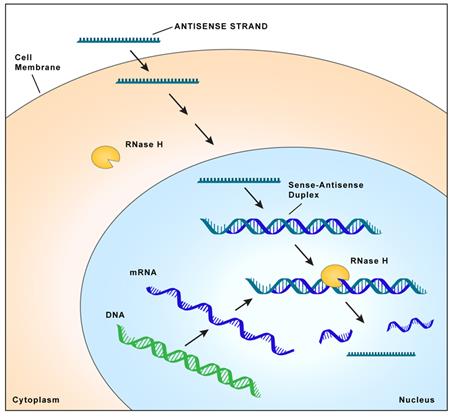
Figure 4: Antisense drug using the RNase H mechanism of action.
21
SPINRAZA is an example of an antisense drug that modulates RNA splicing to increase protein production of the SMN protein (Figure 5), which is critical to the health and survival of nerve cells in the spinal cord that are responsible for neuro-muscular function. The SMN protein is deficient in people with SMA. There are a number of other diseases, including cystic fibrosis and Duchenne muscular dystrophy, which may be treated by modulating splicing using antisense technology.
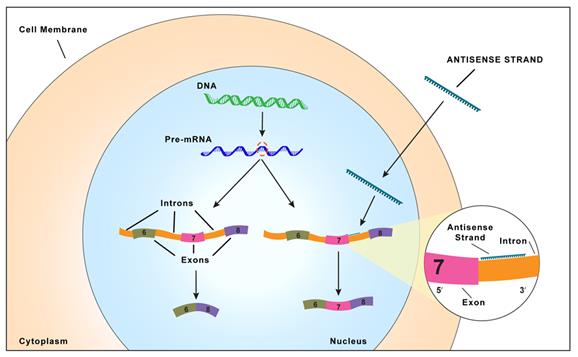
Figure 5: Antisense drug altering splicing of the SMN2 mRNA.
Another class of RNA targets for our antisense technology are microRNAs. To date, scientists have identified more than 700 microRNAs in the human genome, and have shown that the absence or presence of specific microRNAs in various cells is associated with specific human diseases, including cancer, viral infection, metabolic disorders and inflammatory disease. To fully exploit the therapeutic opportunities of targeting microRNAs, we co-founded Regulus Therapeutics as a company focused on the discovery, development and commercialization of microRNA-based therapeutics.
We are also making progress in designing antisense drugs to target long, non-coding RNAs, or lncRNAs and RNAs that possess a toxic function in human diseases. Many of these RNAs, such as lncRNAs, do not make proteins but often cause disease by regulating the function of other genes or proteins. In 2014, we published a paper in Nature in which we were the first to show that targeted reduction of an lncRNA with an antisense compound can ameliorate certain cognitive deficits in a mouse model of Angelman syndrome, or AS. Moreover, these studies demonstrate the potential therapeutic benefits of an antisense drugs for the treatment of AS.
Because the efficiency of our core technology platform can support multiple target-based antisense research programs without significantly increasing costs, we can develop antisense drugs to target a broad range of diseases, efficiently producing a large and broad proprietary portfolio of drugs. We are currently pursuing antisense drug discovery programs focused on various severe and rare diseases, cardiometabolic diseases, neurologic diseases, cancer and other diseases.
Collaborative Arrangements and Licensing Agreements
Partnering Strategy
We have established alliances with a cadre of leading global pharmaceutical companies that are working alongside us in developing our drugs, advancing our technology, preparing to commercialize our drugs and selling our drugs. Our partners include the following companies, among others: AstraZeneca, Biogen, Bayer, GSK, Janssen, Novartis and Roche. Our partners bring resources and expertise that augment and build upon our internal capabilities. The depth of our knowledge and expertise with antisense technology together with our strong financial position provides us the flexibility to partner our drugs at what we believe is the optimal time to maximize the near- and long-term value of our drugs. We have distinct partnering strategies that we employ based on the specific program, therapeutic area and the expertise and resources our potential partners may bring to the collaboration.
| ● |
We have strategic partnerships through which we can broadly expand our drug discovery efforts to new disease targets in specific therapeutic areas. Our partners provide expertise, tools and resources to complement our drug discovery efforts. For instance, we established a broad strategic alliance with Biogen that pairs Biogen’s extensive resources and expertise in neurodegenerative diseases with our antisense technology. Together we are creating a franchise of novel drugs for neurodegenerative diseases that has the potential to expand both our pipeline and Biogen’s pipeline with promising new drugs. Most recently, we entered into a new collaboration agreement with Biogen to identify new antisense drugs for the treatment of SMA.
|
| ● |
We have partnerships with companies that bring significant expertise and global resources to develop and potentially commercialize drugs for a particular therapeutic area. For example, in January 2017, we and Akcea initiated a collaboration with Novartis to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. As a leader in the cardiovascular disease space, Novartis brings significant resources and expertise that should support the development and commercialization of these two drugs for significant high-risk patient populations. The collaboration with Novartis should enable us to accelerate the development of these drugs for broader patient populations as Novartis plans to conduct a cardiovascular outcome study for each of these drugs.
|
22
| ● |
We also form early stage research and development partnerships that allow us to expand the application of our technology to new therapeutic areas. For example, we established a collaboration with Janssen in December 2014, which brings together our RNA-targeted technology platform and Janssen’s expertise in autoimmune disorders and therapeutic formulation to discover and develop antisense drugs to treat autoimmune disorders in the GI tract. Thus far, Janssen has licensed two drugs under our collaboration.
|
| ● |
We also work with a consortium of companies that can exploit our drugs and technologies outside our primary areas of focus. We refer to these companies as satellite companies. Through our satellite company collaborations, we expand the reach and potential of RNA-targeting therapeutics into disease areas that are outside of our core focus. For example, in October 2017, Achaogen submitted an NDA to the FDA for plazomicin. Plazomicin is an aminoglycoside Achaogen discovered based on the technology we licensed to Achaogen and we are eligible to earn milestone payments and royalties under our licensing agreement.
|
Financial Impact of Our Partnerships
Through our partnerships, we have created a broad and sustaining base of R&D revenue in the form of license fees, upfront payments and milestone payments while spending prudently to advance our pipeline and technology. Since 2007, we have received more than $2.4 billion in cash from upfront and licensing fees, equity purchase payments, milestone payments and research and development funding from our partnerships. We have the potential to earn over $13 billion in future milestone payments, licensing fees and other payments from our current partnerships. We also have the potential to share in the future commercial success of our inventions and drugs resulting from our partnerships through royalty arrangements. For example, during 2017 we earned $112.5 million in commercial revenue from SPINRAZA sales, adding a significant revenue stream to our broad base of R&D revenue.
Strategic Partnerships
AstraZeneca
Cardiometabolic and Renal Diseases Collaboration
In July 2015, we and AstraZeneca formed a strategic collaboration to discover and develop antisense therapies for treating cardiovascular and metabolic diseases primarily focused on targets in the kidney, and renal diseases. As part of the agreement, we granted AstraZeneca an exclusive license to IONIS-AZ4-2.5-LRx, a drug we designed to treat cardiovascular disease and our first drug that combines our Generation 2.5 and LICA technology. We also granted AstraZeneca the option to license a drug for each additional target advanced under this research collaboration. In February 2018, AstraZeneca licensed a second drug under our collaboration, IONIS-AZ5-2.5Rx, a drug we designed to treat a genetically associated form of kidney disease. AstraZeneca is responsible for all further global development, regulatory and commercialization activities and costs for IONIS-AZ4-2.5-LRx and IONIS-AZ5-2.5Rx and any other future drug development candidates AstraZeneca accepts.
Under the terms of the agreement, we received a $65 million upfront payment. We are eligible to receive license fees and milestone payments of up to more than $4 billion as drugs under this collaboration advance. In addition, we are eligible to receive tiered royalties up to the low teens on sales from any product that AstraZeneca successfully commercializes under this collaboration agreement. From inception through February 2018, we have generated over $120 million in payments under this collaboration, including $30 million when AstraZeneca licensed IONIS-AZ5-2.5Rx in February 2018.
Oncology Collaboration
In December 2012, we entered into a collaboration agreement with AstraZeneca to discover and develop antisense drugs to treat cancer. As part of the agreement, we granted AstraZeneca an exclusive license to develop and commercialize IONIS-STAT3-2.5Rx for the treatment of cancer and an option to license up to three anti-cancer drugs under separate research programs. AstraZeneca is responsible for all global development, regulatory and commercialization activities for IONIS-STAT3-2.5Rx. We and AstraZeneca have evaluated IONIS-STAT3-2.5Rx in people with advanced metastatic hepatocellular carcinoma and advanced lymphoma. AstraZeneca is evaluating IONIS-STAT3-2.5Rx in combination with Imfinzi (durvalumab), AstraZeneca’s anti-PD-L1 drug, in people with head and neck cancer and in people with diffuse large B cell lymphoma. Under the research program, we are responsible for identifying a development candidate for each of the three anti-cancer research programs. AstraZeneca has the option to license drugs resulting from each of the three anti-cancer research programs, and if AstraZeneca exercises its option for a drug, it will be responsible for all further global development, regulatory and commercialization activities and costs for such drug. The first development candidate identified under the anti-cancer research program was IONIS-KRAS-2.5Rx, which AstraZeneca licensed from us in December 2016. IONIS-KRAS-2.5Rx is a Generation 2.5 antisense drug we designed to directly target KRAS, one of the most frequently mutated genes in cancer.
Under the terms of the agreement, we received $31 million in upfront payments. We are eligible to receive milestone payments and license fees from AstraZeneca as programs advance in development. In addition, we are eligible to receive tiered royalties up to the low to mid-teens on sales from any drugs resulting from these programs. If AstraZeneca successfully develops IONIS-STAT3-2.5Rx, IONIS-KRAS-2.5Rx and two other drugs under the research program, we could receive license fees and milestone payments of up to more than $750 million. From inception through February 2018, we have generated more than $95 million in payments under this collaboration.
For additional details about our collaboration agreements with AstraZeneca, including other financial information and termination provisions, see Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
23
Biogen
We have several strategic collaborations with Biogen focused on using antisense technology to advance the treatment of neurological disorders. These collaborations combine our expertise in creating antisense drugs with Biogen's expertise in developing therapies for neurological disorders. We developed and licensed to Biogen SPINRAZA, our approved drug to treat people with SMA. Additionally, we and Biogen are currently developing six other drugs to treat neurodegenerative diseases under these collaborations, including IONIS-SOD1Rx for ALS, IONIS-MAPTRx (formerly IONIS-BIIB4Rx) for AD and IONIS-C9Rx (formerly IONIS-BIIB5Rx) for ALS, IONIS-BIIB6Rx, IONIS-BIIB7Rx and IONIS-BIIB8Rx to treat undisclosed neurodegenerative diseases. In addition to these drugs, we and Biogen are evaluating numerous additional targets to develop drugs to treat neurological diseases. Most recently, in December 2017 we entered into a collaboration with Biogen to identify new antisense drugs for the treatment of SMA. From inception through February 2018, we have generated over $810 million from our Biogen collaborations.
SPINRAZA
In January 2012, we entered into a collaboration agreement with Biogen to develop and commercialize SPINRAZA, an RNA-targeted therapy for the treatment of SMA. In December 2016, the FDA approved SPINRAZA for the treatment of SMA in pediatric and adult patients. In January 2018, Biogen reported that SPINRAZA was available in over 30 markets. Through December 2017, we have earned $113.4 million in commercial revenue from SPINRAZA royalties. In addition to SPINRAZA royalties, from inception through February 2018, we have generated over $435 million in payments for SPINRAZA, including $90 million of milestone payments for the approval of SPINRAZA in the EU and Japan during 2017. We are receiving tiered royalties up to the mid-teens on any sales of SPINRAZA. We have exclusively in-licensed patents related to SPINRAZA from Cold Spring Harbor Laboratory and the University of Massachusetts. We paid Cold Spring Harbor Laboratory and the University of Massachusetts nominal amounts for license fees and milestone payments we received in 2017. We also pay a low single digit royalty on sales of SPINRAZA. Additionally, we owe a low single digit royalty on future sales of SPINRAZA. Biogen is responsible for all further global development, regulatory and commercialization activities and costs for SPINRAZA.
New antisense drugs for the treatment of SMA
In December 2017, we entered into a collaboration agreement with Biogen to identify new antisense drugs for the treatment of SMA. Biogen will have the option to license therapies arising out of this collaboration following the completion of preclinical studies. Upon licensing, Biogen will be responsible for all further global development, regulatory and commercialization activities and costs for such therapies. Under the collaboration agreement, we received a $25 million upfront payment in December 2017. In total over the term of our collaboration, we are eligible to receive up to $1.2 billion in license fees, milestone payments and other payments. In addition, we are eligible to receive tiered royalties from the mid-teens to mid-20 percent range on net sales.
Neurology
In December 2012, we and Biogen entered into a collaboration agreement to develop and commercialize novel antisense drugs to up to three targets to treat neurodegenerative diseases. We are responsible for the development of each of the drugs through the completion of the initial Phase 2 clinical study for such drug. Biogen has the option to license a drug from each of the three programs through the completion of the first Phase 2 study for each program. We are currently advancing IONIS-MAPTRx (formerly IONIS-BIIB4Rx) for AD under this collaboration. If Biogen exercises its option for a drug, it will assume all further global development, regulatory and commercialization activities and costs for that drug. Under the terms of the agreement, we received an upfront payment of $30 million. Over the term of the collaboration, we are eligible to receive up to an additional $210 million in a license fee and milestone payments per program, plus a mark-up of the cost estimate of the Phase 1 and 2 studies. In addition, we are eligible to receive tiered royalties up to the mid-teens on sales from any drugs resulting from each of the three programs. From inception through February 2018, we have generated over $55 million in payments under this collaboration, including $10 million we received in 2017 for initiating a Phase 1/2a study of IONIS-MAPTRx.
Strategic Neurology
In September 2013, we and Biogen entered into a long-term strategic relationship focused on applying antisense technology to advance the treatment of neurodegenerative diseases. As part of the collaboration, Biogen gained exclusive rights to the use of our antisense technology to develop therapies for neurological diseases and has the option to license drugs resulting from this collaboration. The exclusivity for neurological diseases will last through September 2019, and may be extended for any drug development programs Biogen is pursuing under the collaboration. We will usually be responsible for drug discovery and early development of antisense drugs and Biogen will have the option to license antisense drugs after Phase 2 proof of concept. In October 2016, we expanded our collaboration to include additional research activities we will perform. If Biogen exercises its option for a drug, it will assume all further global development, regulatory and commercialization responsibilities and costs for that drug. We are currently advancing five drugs, IONIS-SOD1Rx for ALS, IONIS-C9Rx (formerly IONIS-BIIB5Rx), IONIS-BIIB6Rx, IONIS-BIIB7Rx and IONIS-BIIB8Rx under this collaboration. Biogen will be responsible for all of the drug discovery and development activities for drugs using other modalities.
Under the terms of the agreement, we received an upfront payment of $100 million and are eligible to receive milestone payments, license fees and royalty payments for all drugs developed through this collaboration, with the specific amounts dependent upon the modality of the molecule advanced by Biogen. For each antisense molecule that is chosen for drug discovery and development under this collaboration, we are eligible to receive up to approximately $260 million in a license fee and milestone payments. In addition, we are eligible to receive tiered royalties up to the mid-teens on sales from any antisense drugs developed under this collaboration. If other modalities are chosen, such as small molecules or monoclonal antibodies, we are eligible to receive up to $90 million in milestone payments. In addition, we are eligible to receive tiered single-digit royalties on sales from any drugs using non-antisense modalities developed under this collaboration. From inception through February 2018, we have generated nearly $170 million in payments under this collaboration, including $15 million in milestone payments we received in 2017 for validating two undisclosed neurological disease targets.
For additional details about our collaboration agreements with Biogen, including other financial information and termination provisions, see Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
24
Research, Development and Commercialization Partners
Bayer
In May 2015, we entered into an exclusive license agreement with Bayer to develop and commercialize IONIS-FXIRx for the prevention of thrombosis. We were responsible for completing a Phase 2 study of IONIS-FXIRx in people with end-stage renal disease on hemodialysis. Under the terms of the agreement, we received a $100 million upfront payment in the second quarter of 2015. In February 2017, we amended our agreement with Bayer to advance IONIS-FXIRx and to initiate development of IONIS-FXI-LRx, which Bayer licensed. In conjunction with the decision to advance these programs, we received a $75 million payment from Bayer. We are conducting a Phase 2b study evaluating IONIS-FXIRx in people with end-stage renal disease on hemodialysis to finalize dose selection. Additionally, we plan to develop IONIS-FXI-LRx through Phase 1. Following these studies and Bayer's decision to further advance these programs, Bayer will be responsible for all global development, regulatory and commercialization activities and costs for both drugs. We are eligible to receive additional milestone payments as each drug advances toward the market. In total over the term of our collaboration, we are eligible to receive up to $385 million in license fees, milestone and other payments. In addition, we are eligible to receive tiered royalties in the low to high 20 percent range on gross margins of both drugs combined. From inception through February 2018, we have generated over $175 million under this collaboration.
For additional details about our collaboration agreement with Bayer, including other financial information and termination provisions, see Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
GSK
In March 2010, we entered into an alliance with GSK using our antisense drug discovery platform to discover and develop new drugs against targets for rare and serious diseases, including infectious diseases and some conditions causing blindness. In August 2017, as part of a reprioritization of its pipeline and strategic review of its Rare Diseases business, GSK declined its options for inotersen, our Phase 3 drug to treat people with ATTR, and IONIS-FB-LRx (formerly IONIS-GSK4-LRx), an antisense drug to treat complement-mediated diseases. We are continuing to advance each of these drugs independently.
GSK, consistent with its focus on treatments for infectious diseases, continues to advance two drugs targeting hepatitis B virus, or HBV, under our collaboration: IONIS-HBVRx and IONIS-HBV-LRx. GSK is currently conducting Phase 2 studies for both of these drugs, which we designed to reduce the production of viral proteins associated with HBV infection. In March 2016, we and GSK amended the development plan for IONIS-HBVRx to allow GSK to conduct all further development activities for this program. GSK has the exclusive option to license the drugs resulting from this alliance at Phase 2 proof-of-concept for a license fee.
Under our agreement, if GSK successfully develops these drugs and achieves pre-agreed sales targets, we could receive license fees and milestone payments of more than $262 million. In addition, we are eligible to receive tiered royalties up to the mid-teens on sales from any product that GSK successfully commercializes under this alliance. From inception through February 2018, we have generated more than $162 million in payments under this alliance with GSK.
For additional details about our collaboration agreement with GSK, including other financial information and termination provisions, see Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
Janssen Biotech, Inc.
In December 2014, we entered into a collaboration agreement with Janssen Biotech, Inc. to discover and develop antisense drugs that can be locally administered, including oral delivery, to treat autoimmune disorders of the GI tract. Janssen has the option to license drugs from us through the designation of a development candidate for up to three programs. Prior to option exercise we are responsible for the discovery activities to identify a development candidate. If Janssen exercises an option for one of the programs, it will be responsible for the global development, regulatory and commercial activities under that program. Under our collaboration, Janssen licensed IONIS-JBI1-2.5Rx in July 2016 and IONIS-JBI2-2.5Rx in November 2017. Under the terms of the agreement, we received $35 million in upfront payments. We are eligible to receive up to more than $800 million in milestone payments and license fees for these programs. In addition, we are eligible to receive tiered royalties up to the near teens on sales from any drugs resulting from this collaboration. From inception through February 2018, we generated more than $70 million in payments under this collaboration, including $10 million in 2017 for the license of IONIS-JBI2-2.5Rx and the initiation of a Phase 1 study of IONIS-JBI1-2.5Rx.
For additional details about our collaboration agreement with Janssen, including other financial information and termination provisions, see Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
Novartis
In January 2017, we and Akcea initiated a collaboration with Novartis to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Under the collaboration agreement, Novartis has an exclusive option to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Akcea is responsible for completing a Phase 2 program, conducting an end-of-Phase 2 meeting with the FDA and providing active pharmaceutical ingredient, or API, for each drug. If Novartis exercises an option for one of these drugs, Novartis will be responsible for all further global development, regulatory and commercialization activities and costs for such drug.
25
Akcea received a $75 million upfront payment in the first quarter of 2017, of which it retained $60 million and paid us $15 million as a sublicense fee. If Novartis exercises its option for a drug, Novartis will pay Akcea a license fee equal to $150 million for each drug it licenses. In addition, Akcea is eligible to receive up to $600 million and $530 million in milestone payments related to AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx, respectively. Akcea plans to co-commercialize any licensed drug commercialized by Novartis in selected markets, under terms and conditions that it plans to negotiate with Novartis in the future, through the specialized sales force Akcea is building to commercialize volanesorsen. Akcea is also eligible to receive tiered royalties in the mid-teens to low twenty percent range on net sales of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Akcea will pay 50 percent of these license fees, milestone payments and royalties to us as a sublicense fee.
In conjunction with this collaboration, we and Akcea entered into a Stock Purchase Agreement, or SPA, with Novartis. As part of the SPA, Novartis purchased 1.6 million shares of our common stock for $100 million in the first quarter of 2017 and purchased $50 million of Akcea’s common stock at the IPO price concurrent with the IPO in July 2017.
For additional details about our and Akcea’s collaboration agreement with Novartis, including other financial information and termination provisions, see Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
Roche
In April 2013, we formed an alliance with Hoffman-La Roche Inc. and F. Hoffmann-La Roche Ltd., collectively Roche, to develop treatments for HD based on our antisense technology. Under the agreement, we discovered and developed IONIS-HTTRx, an antisense drug targeting HTT protein, through completion of our Phase 1/2a clinical study in people with early stage HD. In December 2017, upon completion of the Phase 1/2a study, Roche exercised its option to license IONIS-HTTRx and is now responsible for the global development, regulatory and commercialization activities for IONIS-HTTRx. Under the terms of the agreement, we received an upfront payment of $30 million in April 2013. We are eligible to receive up to $365 million in a license fee and milestone payments. In addition, we are eligible to receive up to $137 million in milestone payments for each additional drug successfully developed. We are also eligible to receive tiered royalties up to the mid-teens on sales from any product resulting from this alliance. From inception through February 2018, we have generated over $105 million in payments under this alliance with Roche, including $48 million in 2017 primarily for the license of IONIS-HTTRx.
For additional details about our collaboration agreement with Roche, including other financial information and termination provisions, see Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
Satellite Company Partnerships
Achaogen, Inc.
In 2006, we exclusively licensed to Achaogen, Inc. specific know-how, patents and patent applications relating to aminoglycosides. In connection with the license, Achaogen issued to us $1.5 million of Achaogen stock. Achaogen is developing plazomicin, an aminoglycoside Achaogen discovered based on the technology we licensed to Achaogen. If Achaogen successfully develops and commercializes two drugs under our agreement, we will receive payments totaling up to $49.3 million for the achievement of key clinical, regulatory and sales events. The FDA set a PDUFA date of June 25, 2018 for plazomicin. Achaogen also plans to submit an MAA to the EMA in 2018. Through February 2018, we have generated $7 million in milestone payments from Achaogen. We are also eligible to receive low single digit royalties on sales of drugs resulting from the program. Achaogen is solely responsible for the continued development, regulatory and commercialization activities of plazomicin.
Alnylam Pharmaceuticals, Inc.
In March 2004, we entered into an alliance with Alnylam to develop and commercialize RNAi therapeutics. Under the terms of the agreement, we exclusively licensed to Alnylam our patent estate relating to antisense motifs and mechanisms and oligonucleotide chemistry for double-stranded RNAi therapeutics in exchange for a $5 million technology access fee, participation in fees from Alnylam’s partnering programs, as well as future milestone and royalty payments from Alnylam. For each drug Alnylam develops under this alliance, we may receive up to $3.4 million in milestone payments. We also have the potential to earn a portion of payments that Alnylam receives from licenses of our technology it grants to its partners, plus royalties. We retained rights to a limited number of double-stranded RNAi therapeutic targets and all rights to single-stranded RNAi, or ssRNAi, therapeutics.
In turn, Alnylam nonexclusively licensed to us its patent estate relating to antisense motifs and mechanisms and oligonucleotide chemistry to research, develop and commercialize single-stranded antisense therapeutics, ssRNAi therapeutics, and to research double-stranded RNAi compounds. We also received a license to develop and commercialize double-stranded RNAi drugs targeting a limited number of therapeutic targets on a nonexclusive basis. If we develop or commercialize an RNAi-based drug using Alnylam’s technology, we will pay Alnylam up to $3.4 million in milestone payments for specified development and regulatory events, plus royalties. To date, we do not have an RNAi-based drug in development.
In 2015, we and Alnylam entered into an alliance in which we formed an intellectual property cross-license under which we and Alnylam each obtained exclusive license rights to four therapeutic programs. Alnylam granted us an exclusive, royalty-bearing license to its chemistry, RNA targeting mechanism and target-specific intellectual property for oligonucleotides against four targets, including FXI and Apo(a) and two other targets. In exchange, we granted Alnylam an exclusive, royalty-bearing license to our chemistry, RNA targeting mechanism and target-specific intellectual property for oligonucleotides against four other targets. Alnylam also granted us a royalty-bearing, non-exclusive license to new platform technology arising from May 2014 through April 2019 for single-stranded antisense therapeutics. In turn, we granted Alnylam a royalty-bearing, non-exclusive license to new platform technology arising from May 2014 through April 2019 for double-stranded RNAi therapeutics. Through February 2018, we have generated over $73 million from Alnylam.
26
Antisense Therapeutics Limited
In 2001, we licensed ATL1102 and ATL1103 to ATL, an Australian company publicly traded on the Australian Stock Exchange. ATL completed a Phase 2a efficacy and safety trial and has also completed a chronic toxicology study in primates to support a potential Phase 2b trial of ATL1102 in people with MS. In addition, ATL is currently developing ATL1103 for growth and sight disorders. We are eligible to receive royalties on sales of ATL1102 and ATL1103. We may also receive a portion of the fees ATL receives if it licenses ATL1102 or ATL1103.
Atlantic Pharmaceuticals Limited
In March 2007, we licensed alicaforsen to Atlantic Pharmaceuticals, a UK-based specialty pharmaceutical company founded in 2006. Atlantic Pharmaceuticals is developing alicaforsen for the treatment of UC and other inflammatory diseases. Atlantic Pharmaceuticals is initially developing alicaforsen for pouchitis, a UC indication, followed by UC and other inflammatory diseases. In 2017, under a rolling submission agreement with the FDA, Atlantic Pharmaceuticals filed the nonclinical data package of its NDA for alicaforsen to treat pouchitis. Alicaforsen has also been granted FDA Fast-Track designation, plus U.S. and European Orphan Drug designations for this indication. In exchange for the exclusive, worldwide license to alicaforsen, we received a $2 million upfront payment from Atlantic Pharmaceuticals in the form of equity. In 2010, Atlantic Pharmaceuticals began supplying alicaforsen under international named patient supply regulations for people with IBD for which we receive royalties. Under the agreement, we could receive milestone payments totaling up to $1.4 million for the achievement of regulatory milestones for multiple indications.
Dynacure, SAS
In October 2016, we entered into a collaboration with Dynacure to discover, develop and commercialize an antisense drug for the treatment of neuromuscular diseases. We and Dynacure shared research responsibilities to identify a drug candidate. In November 2017, Dynacure licensed IONIS-DNM2-2.5Rx, a drug targeting dynamin 2 for the treatment of centronuclear myopathy, from us. Upon licensing, Dynacure assumed all responsibility for development and commercialization for IONIS-DMN2-2.5Rx. Under the terms of the agreement, we obtained a 15 percent equity ownership in Dynacure upon the initiation of the collaboration. We received additional equity and convertible notes in Dynacure for the license of IONIS-DMN2-2.5Rx in 2017. We recorded a full valuation allowance for all of the equity and convertible debt we received from Dynacure because realization of value from the equity is uncertain. If Dynacure advances a target under this collaboration, we could receive cash or equity up to more than $210 million in a license fee and milestone payments for specified development, regulatory and sales events. In addition, we are eligible to receive royalties on future product sales of the drug under this collaboration.
Regulus Therapeutics Inc.
In September 2007, we and Alnylam established Regulus as a company focused on the discovery, development and commercialization of microRNA-targeting therapeutics. We and Alnylam retain rights to develop and commercialize, on pre-negotiated terms, microRNA therapeutic products that Regulus decides not to develop either by itself or with a partner. Regulus is addressing therapeutic opportunities that arise from alterations in microRNA expression. Since microRNAs may act as master regulators of the genome, affecting the expression of multiple genes in a disease pathway, microRNA therapeutics define a new platform for drug discovery and development. MicroRNAs may also prove to be an attractive new tool for characterizing diseases. Regulus’ focus is on drug discovery and development efforts for diseases with significant unmet medical need in organs to which we have been able to preferentially deliver oligonucleotide therapeutics effectively, such as the liver and kidney. Regulus currently has two drugs in clinical development. Regulus is studying RGLS4326 in a Phase 1 single ascending dose study designed to investigate the safety, tolerability, pharmacokinetics and pharmacodynamics of RGLS4326 administered subcutaneously in healthy volunteers. We are eligible to receive royalties on any future product sales of these drugs.
Suzhou Ribo Life Science Co., Ltd.
In April 2017, we entered into a collaboration with Ribo to develop and commercialize RNA-targeted therapeutics in China. We licensed IONIS-AR-2.5Rx, IONIS-GCGRRx and IONIS-EZH2-2.5Rx to Ribo under our collaboration to develop and commercialize these drugs in China. In addition, Ribo will be responsible for conducting a multi-year research and drug discovery program to identify drugs that utilize our ssRNAi technology. Following the identification of a development candidate, Ribo may exercise its option to license each drug by paying us a license fee. For each drug that Ribo licenses, Ribo will be responsible for all development and commercialization activities and costs in China. We retained the rights to develop and commercialize ssRNAi technology and all drugs under the collaboration outside of China. Ribo will provide us a royalty-free license to the data and intellectual property created under the collaboration.
Under the agreement, we received an up-front payment of $2 million. We also obtained a nine percent equity ownership in Ribo. We are eligible to receive up to $153 million in license fees and milestone payments. In addition, we are eligible to receive tiered royalties up to the mid-twenty percent range on sales from any drugs resulting from this collaboration. From inception through February 2018, we have generated $2 million in payments under this collaboration with Ribo.
The University of Texas MD Anderson Cancer Center
In May 2016, we entered into a collaboration agreement with the University of Texas MD Anderson Cancer Center to identify cancer targets and create novel antisense drugs to treat cancer together. In the collaboration, we and MD Anderson are working together to validate novel “undruggable” cancer targets selected based on human genomic data. We are leading the drug discovery efforts against mutually agreed upon novel targets and MD Anderson is leading development activities through clinical proof of concept. Following clinical proof of concept, we and MD Anderson plan to identify a partner to complete development and to commercialize each drug with us leading business development efforts. Under the five year collaboration, we and MD Anderson will evenly share costs specific to our collaboration.
For additional details about our satellite company arrangements, see Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
27
External Project Funding
We are pursuing discovery and development projects that provide us with new therapeutic applications for antisense drugs. These programs represent opportunities for us and our technology. In some cases, we have funded these studies through support from our partners or disease advocacy groups and foundations.
CHDI Foundation, Inc.
Starting in November 2007, CHDI provided financial and scientific support to our Huntington’s disease drug discovery program through our development collaboration. In April 2013, we formed an alliance with Roche to develop treatments for Huntington’s disease. Under the terms of our agreement with CHDI, we will reimburse CHDI for a portion of its support of our Huntington’s disease program out of the payments we receive from Roche.
Cystic Fibrosis Foundation
In August 2016, we entered into a collaboration agreement with the Cystic Fibrosis Foundation to discover and advance a drug for the treatment of Cystic Fibrosis. Under this agreement, we received upfront payments of $1 million and we are eligible to receive additional milestone payments of up to $2 million. Under the agreement, we and the Cystic Fibrosis Foundation will evenly share the first $3 million of costs specific to our collaboration. We are obligated to pay the Cystic Fibrosis Foundation up to $18 million upon achieving specific regulatory and sales events if we advance a drug under our collaboration. From inception through February 2018, we generated nearly $3 million under this collaboration, including $1 million we earned in 2017 for advancing IONIS-ENAC-2.5Rx.
The Ludwig Institute; Center for Neurological Studies
In October 2005, we entered into a collaboration agreement with the Ludwig Institute, the Center for Neurological Studies and researchers from these institutions to discover and develop antisense drugs for ALS and other neurodegenerative diseases. Under this agreement, we agreed to pay the Ludwig Institute and Center for Neurological Studies modest milestone payments and royalties on any antisense drugs resulting from the collaboration.
For additional details about our external project funding collaborations, see Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
Intellectual Property Sale and Licensing Agreements
We have a broad patent portfolio covering our products and technologies. We believe our patent estate represents the largest and most valuable nucleic acid therapeutics-oriented patent estate in the pharmaceutical industry. While the principal purpose of our intellectual property portfolio is to protect our products and those of our partners described above, our intellectual property is a strategic asset that we are exploiting to generate near-term revenues and that we expect will also provide us with revenue in the future. We have an active intellectual property sales and licensing program in which we sell or license aspects of our intellectual property to companies. Through this program, we also license our non-antisense patents. To date, we have generated more than $510 million from our intellectual property sale and licensing program that helps support our internal drug discovery and development programs.
In-Licensing Arrangements
University of Massachusetts
We have a license agreement with the University of Massachusetts under which we acquired an exclusive license to the University of Massachusetts’ patent rights related to SPINRAZA. We paid the University of Massachusetts nominal amounts for license fees and milestone payments we received. We also pay a low single digit royalty on sales of SPINRAZA.
Cold Spring Harbor Laboratory
We have a collaboration and license agreement with the Cold Spring Harbor Laboratory under which we acquired an exclusive license to the Cold Spring Harbor Laboratory’s patent rights related to SPINRAZA. We paid Cold Spring Harbor Laboratory nominal amounts for license fees and milestone payments we received in 2017 and a low single digit royalty on sales of SPINRAZA. Additionally, we owe a low single digit royalty on future sales of SPINRAZA.
For additional details about our Intellectual Property Sale and Licensing arrangements, see Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
Manufacturing
In the past, except for small quantities, it was generally expensive and difficult to produce chemically modified oligonucleotides like the antisense drugs we use in our research and development programs. As a result, we have dedicated significant resources to develop ways to improve manufacturing efficiency and capacity. Since we can use variants of the same nucleotide building blocks and the same type of equipment to produce our oligonucleotide drugs, we found that the same techniques we used to efficiently manufacture one oligonucleotide drug could help improve the manufacturing processes for many other oligonucleotide drugs. By developing several proprietary chemical processes to scale up our manufacturing capabilities, we have greatly reduced the cost of producing oligonucleotide drugs. For example, we have significantly reduced the cost of raw materials through improved yield efficiency, while at the same time increasing our capacity to make the drugs. Through both our internal research and development programs and collaborations with outside vendors we may achieve even greater efficiency and further cost reductions.
28
Our drug substance manufacturing facility is located in a 28,700 square foot building in Carlsbad, California. We purchased this building in 2017. In addition, we have a 25,800 square foot building that houses support functions for our manufacturing activities. We lease this facility under a lease that has an initial term ending in June 2021 with an option to extend the lease for up to two additional five-year periods. Our manufacturing facility is subject to periodic inspections by the FDA to ensure that it is operating in compliance with current Good Manufacturing Practices, or cGMP, requirements.
As part of our collaborations we may agree to manufacture clinical trial materials and/or commercial supply for our partners. For example, in the past we have manufactured clinical supply materials for AstraZeneca, Bayer, Biogen, GSK and Novartis. We believe that with reasonably anticipated benefits from increases in scale and improvements in chemistry, we can manufacture antisense drugs at commercially competitive prices.
We believe we have sufficient manufacturing capacity to meet our current internal research, development and potential commercial needs, including the ongoing Phase 3 clinical trial we have for volanesorsen, as well as our current and future obligations under existing agreements with our partners for research, development and commercial needs. Specifically, we have the following in place for our approved drug, SPINRAZA and our drugs currently under regulatory review, volanesorsen and inotersen:
SPINRAZA
Pursuant to our collaboration with Biogen, Biogen is responsible for SPINRAZA drug supply. Biogen has contracted with us to manufacture API for SPINRAZA through September 2018.
Volanesorsen
We have supplied Akcea either through our manufacturing processes or through our outside vendors, including API and finished drug product to complete its ongoing clinical study for volanesorsen. We have also supplied the API and the finished drug product for volanesorsen’s launch. We believe the API and drug product is adequate for at least the first two years of volanesorsen’s launch. Akcea plans to leverage our relationships with contract manufacturing organizations, or CMO's, to procure its own long-term raw material and drug supplies at competitive prices in the future.
Inotersen
For inotersen’s commercial launch, we are using CMOs to produce custom raw materials, API and finished goods. Our CMO partners have extensive technical expertise and cGMP experience. We believe our current network of CMO partners are capable of providing sufficient quantities to meet anticipated commercial demands. Additionally, we continue to evaluate further relationships with additional suppliers to increase overall capacity as well as reduce further risks. While we believe that there are alternate sources of supply that can satisfy our commercial requirements, we cannot be certain that identifying and establishing relationships with such sources, if necessary, would not result in significant delay or material additional costs. We also cannot provide assurance that we will not experience a disruption in supply from our current CMO partners.
CMOs are subject to the FDA’s cGMP requirements and other rules and regulations prescribed by foreign regulatory authorities. We depend on our CMO partners for continued compliance with cGMP requirements and applicable foreign standards.
We have manufactured limited supplies of our LICA drugs for our preclinical and clinical studies. We have purchased additional supplies of our LICA drugs through a CMO. LICA enables lower doses than unconjugated oligonucleotides. With our expertise in optimizing manufacturing of oligonucleotides, we believe we can develop new processes to scale up manufacturing of our LICA drugs at commercially competitive prices.
Patents and Proprietary Rights
Our success depends, in part, on our ability to obtain patent protection for our products in the United States and other countries. We own or have exclusively licensed a substantial patent estate with numerous issued patents worldwide protecting our products and, more generally, our platform for development and commercialization of oligonucleotide therapeutics. We focus our resources on patents and new patent applications that drive value for our company.
We own or control patents that provide exclusivity for products in our pipeline and patents that provide exclusivity for our core technology in the field of antisense more generally. Our core technology patents include claims to chemically modified nucleosides and oligonucleotides as well as antisense drug designs utilizing these chemically modified nucleosides. These core claims are each independent of specific therapeutic target, nucleic acid sequence, or clinical indication. We also own a large number of patents claiming antisense compounds having nucleic acid sequences complementary to therapeutic target nucleic acids, independent of the particular chemical modifications incorporated into the antisense compound. Most importantly, we seek and obtain issued patent claims to specifically protect each of our drugs. For example, we file and seek to obtain claims covering each drug’s nucleic acid sequence and precise drug design. In sum, we maintain our competitive advantage in the field of antisense technology by protecting our core platform technology, which applies to most of our drugs, and by creating multiple layers of patent protection for each of our specific drugs in development.
|
Type of Patent Claim
|
Description
|
|||
|
1. Chemically Modified Nucleosides and Oligonucleotides
2. Antisense Drug Design Motifs
3. Therapeutic Methods
4. Antisense Sequence
5. Drug Composition
|
 |
1. Target and sequence independent
2. Sequence independent
3. Chemistry independent
4. Specific claim to drug candidates
|
29
Chemically Modified Nucleosides and Oligonucleotides
The most broadly applicable of our patents are those that claim modified nucleosides and oligonucleotides comprising the modified nucleosides that we incorporate into our antisense drugs to increase their therapeutic efficacy. Nucleosides and chemically modified nucleosides are the basic building blocks of our antisense drugs, therefore claims that cover any oligonucleotide incorporating one of our proprietary modified nucleosides can apply to a wide array of antisense mechanisms of action as well as several therapeutic targets. Of particular note are our patents covering our proprietary 2’-O-(2-methoxy) ethyl, or “MOE,” modified nucleosides, incorporated into many of our second generation development compounds, as well as our constrained-ethyl nucleosides, or “cEt” nucleosides incorporated into our Generation 2.5 compounds.
The following are some of our patents in this category in key jurisdictions (U.S., Europe and Japan):
|
Jurisdiction
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
|
United States
|
7,101,993
|
|
OLIGONUCLEOTIDES CONTAINING 2’O-MODIFIED PURINES
|
|
2023
|
|
Covers certain MOE nucleosides and oligonucleotides containing these nucleotides.
|
|
|
United States
|
7,399,845
|
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
|
2027
|
|
Covers our cEt nucleosides and oligonucleotides containing these nucleoside analogs.
|
|
|
United States
|
7,741,457
|
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
|
2027
|
|
Covers our cEt nucleosides and oligonucleotides containing these nucleoside analogs.
|
|
|
United States
|
8,022,193
|
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
|
2027
|
|
Covers oligonucleotides containing cEt nucleoside analogs.
|
|
|
United States
|
7,569,686
|
COMPOUNDS AND METHODS FOR SYNTHESIS OF BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Covers methods of synthesizing our cEt nucleosides.
|
||||
|
Europe
|
EP1984381
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Covers our cEt nucleosides and oligonucleotides containing these nucleoside analogs.
|
||||
|
Europe
|
EP2314594
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Covers our cEt oligonucleotides and methods of use.
|
||||
|
Japan
|
JP5342881
|
6-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
2027
|
Covers our cEt nucleosides and oligonucleotides containing these nucleoside analogs.
|
Antisense Drug Design Motifs
Other of our patents claim oligonucleotides comprising antisense drug design motifs, or patterns of nucleoside modifications at specified positions in the oligonucleotide. Patent claims covering our antisense drug design motifs are independent of nucleic acid sequence, so they cover oligonucleotides having the recited motif, regardless of cellular target or clinical indication. The claimed motifs generally confer properties that optimize oligonucleotides for a particular antisense mechanism of action, such as ribonuclease H, or RNase H, RNAi, or splicing. We have designed oligonucleotides incorporating motifs, which we refer to as chimeric compounds or gapmers to exploit the RNase H mechanism to achieve target RNA reduction. Almost all of our drugs, including volanesorsen and inotersen, but excluding SPINRAZA, contain this gapmer antisense drug design motif. We own a U.S. patent that covers all of our second generation MOE gapmer antisense drugs until March of 2023.
In addition, we have pursued patent claims to antisense drug design motifs incorporating bicyclic nucleoside analogs, which include both locked nucleic acids, or “LNA” and cEt. In Europe, we have been granted claims drawn to certain short gapmer oligonucleotides with bicyclic nucleosides, which include locked nucleic acids, in the wings for the treatment of cardiovascular or metabolic disorders. We have also successfully obtained issued patent claims covering our Generation 2.5 gapmer antisense drug design motifs that incorporate our cEt modified nucleosides. Santaris opposed granted European patents EP2092065 and EP2410053. In April 2015, the claims of EP2092065 were successfully upheld in amended form and in January 2017, EP2410053 was upheld with only minor amendment. The following patents are some examples of our issued patents in this category in key jurisdictions:
|
Jurisdiction
|
|
Patent/
Application No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
7,015,315
|
|
GAPPED OLIGONUCLEOTIDES
|
|
2023
|
|
2’-O-alkyl-O-alkyl gapmer oligonucleotides.
|
|
Europe
|
|
EP2021472
|
|
COMPOUNDS AND METHODS FOR MODULATING GENE EXPRESSION
|
|
2027
|
|
Short gapmer oligonucleotides, having wings of 2 bicyclic nucleosides, and a gap of 10 deoxynucleotides for the treatment of cardiovascular or metabolic disorders
|
|
United States
|
|
7,750,131
|
|
5’-MODIFIED BICYCLIC NUCLEIC ACID ANALOGS
|
|
2027
|
|
5’-Methy BNA containing gapmer compounds
|
|
Europe
|
|
EP2092065
|
|
ANTISENSE COMPOUNDS
|
|
2027
|
|
Gapmer compounds having wings comprised of 2’-modifed and LNA nucleosides
|
|
Europe
|
EP2410053
|
ANTISENSE COMPOUNDS
|
2027
|
Gapmer compounds having wings comprised of 2’-MOE and bicyclic nucleosides
|
||||
|
Japan
|
JP 5665317
|
ANTISENSE COMPOUNDS
|
2027
|
Gapmer compounds having wings comprised of 2’-MOE and bicyclic nucleosides
|
||||
|
Europe
|
EP2673361
|
OLIGOMERIC COMPOUNDS COMPRISING BICYCLIC NUCLEOTIDES AND USES THEREOF
|
2032
|
Gapmer having at least one bicyclic nucleoside, 2’-modified nucleoside, and 2’-deoxynucleoside in either the 5’- or 3’-wing.
|
30
LIgand-Conjugated Antisense (LICA) Technology
We have also pursued patent claims to new chemistries created to enhance targeting of antisense drugs to specific tissues and cells in order to improve a drug’s properties. Our N-acetyl-galactosamine, or GalNAc, LICA drugs are designed to provide an increase in potency for targets in the liver. We have successfully obtained issued patent claims covering our LICA technology conjugated to any modified oligonucleotide, including gapmers, double-stranded siRNA compounds, and fully modified oligonucleotides. The following patents are some examples of our issued patents in this category:
|
Jurisdiction
|
|
Patent/
Application No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
9,127,276
|
|
CONJUGATED ANTISENSE COMPOUNDS AND THEIR USE
|
|
2034
|
|
Covers our primary THA LICA conjugated to any group of nucleosides, including gapmers, double-stranded siRNA compounds, and fully modified oligonucleotides
|
|
United States
|
|
9,181,549
|
|
CONJUGATED ANTISENSE COMPOUNDS AND THEIR USE
|
|
2034
|
|
Covers our primary THA conjugate having our preferred linker and cleavable moiety conjugated to any oligomeric compound or any nucleoside having a 2’-MOE modification or a cEt modification
|
Therapeutic Methods of Treatment and Antisense Drug Sequences
In addition to our broad core patents, we also own hundreds of patents, worldwide, with claims to antisense compounds having particular sequences and compounds directed to particular therapeutically important targets or methods of achieving clinical endpoints using these antisense compounds. These “Target” patents also include claims reciting the specific nucleic acid sequences utilized by our products, independent of chemical modifications and motifs. In addition, our product specific patents typically include claims combining specific nucleic acid sequences with nucleoside modifications and motifs. In this way, we seek patent claims narrowly tailored to protect our products specifically, in addition to the broader core antisense patents described above.
Survival Motor Neuron and SPINRAZA
SPINRAZA is protected from generic competition in the United States until at least 2030 and in Europe until 2026 by a suite of patents. These issued patents include: (i) the Bennett patent related to methods of altering mRNA processing (e.g., splicing, the mechanism of action of SPINRAZA) with a fully modified 2’MOE oligonucleotide, (ii) a patent licensed from the University of Massachusetts drawn to antisense compounds having the sequence of SPINRAZA, independent of chemical modification and uses of such compounds for treating SMA, and (iii) joint patents with Cold Spring Harbor Laboratory claiming fully modified 2’MOE compositions targeting SMN2, including the precise composition of matter of SPINRAZA and methods of using such compositions. Those patents protect SPINRAZA from generic and antisense innovator competition in the United States until at least 2030. We have filed for patent term extension, to potentially extend the term beyond 2030. With Biogen’s license of SPINRAZA, we assigned our interest in these patents to Biogen. The table below lists the key U.S. and European issued patents protecting SPINRAZA:
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
6,210,892
|
|
ALTERATION OF CELLULAR BEHAVIOR BY MODULATION OF MRNA PROCESSING
|
|
2018
|
|
Altering mRNA processing with a fully modified 2’MOE oligonucleotide.
|
|
United States
|
|
8,361,977
|
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING
|
|
2030
|
|
Sequence and chemistry (full 2’-MOE) of SPINRAZA
|
|
Europe
|
|
1910395
|
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING
|
|
2026
|
|
Sequence and chemistry (full 2’-MOE) of SPINRAZA
|
|
United States
|
|
7,838,657
|
|
SPINAL MUSCULAR ATROPHY (SMA) TREATMENT VIA TARGETING OF SMN2 SPLICE SITE INHIBITORY SEQUENCES
|
|
2027
|
|
Oligonucleotides having sequence of SPINRAZA (chemistry independent)
|
|
United States
|
|
8,110,560
|
|
SPINAL MUSCULAR ATROPHY (SMA) TREATMENT VIA TARGETING OF SMN2 SPLICE SITE INHIBITORY SEQUENCES
|
|
2025
|
|
Methods of using antisense oligonucleotides having sequence of SPINRAZA to alter splicing of SMN2 and/or to treat SMA
|
|
United States
|
8,980,853
|
COMPOSITIONS AND METHODS FOR MODULATION OF SMN2 SPLICING IN A SUBJECT
|
2030
|
Methods of administering SPINRAZA
|
31
Apolipoprotein C-III and volanesorsen
We have obtained patent claims in the United States drawn to the use of antisense compounds complementary to a broad active region of human Apo C-III, including the site targeted by volanesorsen. We have secured similar claims to compounds complementary to any site on human Apo C-III in Australia. We have also obtained issued patent claims to the specific antisense sequence and chemical composition of volanesorsen in the United States, Australia, Canada, Hong Kong and Europe. The issued U.S. claims protect volanesorsen from generic competition in the United States until at least 2023. In addition, upon approval of volanesorsen by the FDA, we will seek patent term extension to recapture a portion of the term lost during FDA regulatory review, extending the term of this patent beyond 2023. We are pursuing additional patent applications designed to protect volanesorsen worldwide. The table below lists the issued patents in key jurisdictions:
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
9,624,496
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
2023
|
Antisense compound specifically hybridizable within the nucleotide region of apoCIII targeted by volanesorsen
|
||||
|
United States
|
|
7,598,227
|
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
|
2023
|
|
Methods of treating hyperlipidemia, lowering cholesterol levels or lowering triglyceride levels with volanesorsen
|
|
United States
|
|
7,750,141
|
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
|
2023
|
|
Antisense sequence and chemistry of volanesorsen
|
|
Europe
|
|
EP1622597
|
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
|
2024
|
|
Antisense sequence and chemistry of volanesorsen
|
|
Europe
|
EP2441449
|
MODULATION OF APOLIPOPROTEIN C-III EXPRESSION
|
2024
|
Antisense compound specifically hybridizable within the nucleotide region of apoCIII targeted by volanesorsen
|
||||
|
United States
|
9,157,082
|
MODULATION OF APOLIPOPROTEIN CIII (APOCIII) EXPRESSION
|
2032
|
Methods of using APOCIII antisense oligonucleotides for reducing pancreatitis and chylomicronemia and increasing HDL
|
||||
|
Japan
|
JP 6203707
|
MODULATION OF APOLIPOPROTEIN CIII (APOCIII) EXPRESSION
|
2032
|
Methods of using APOCIII antisense oligonucleotides having the sequence of volanesorsen for treating pancreatitis
|
||||
|
United States
|
9,593,333
|
MODULATION OF APOLIPOPROTEIN C-III (APOCIII) EXPRESSION IN LIPOPROTEIN LIPASE DEFICIENT (LPLD) POPULATIONS
|
2034
|
Methods of using APOCIII specific inhibitors for treating lipoprotein lipase deficiency
|
Transthyretin and inotersen
We obtained issued claims covering inotersen in the United States. The issued U.S. claims protect inotersen from generic competition in the United States until at least 2031. We are also pursuing additional patent applications designed to protect inotersen in foreign jurisdictions. The table below lists the current issued patents protecting inotersen in key jurisdictions:
|
Jurisdiction
|
|
Patent No.
|
|
Title
|
|
Expiration
|
|
Description of Claims
|
|
United States
|
|
8,101,743
|
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
|
2025
|
|
Antisense sequence and chemistry of inotersen
|
|
United States
|
|
8,697,860
|
|
DIAGNOSIS AND TREATMENT OF DISEASE
|
|
2031
|
|
Composition of inotersen
|
|
United States
|
9,061,044
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
2031
|
Sodium salt composition of inotersen
|
||||
|
United States
|
9,399,774
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
2031
|
Methods of treating transthyretin amyloidosis by administering inotersen
|
||||
|
Japan
|
JP5896175
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
2031
|
Composition of inotersen
|
||||
|
Europe
|
EP2563920
|
MODULATION OF TRANSTHYRETIN EXPRESSION
|
2031
|
Composition of inotersen
|
We seek patent protection in significant markets and/or countries for each drug in development. We also seek to maximize patent term. In some cases, the patent term can be extended to recapture a portion of the term lost during FDA regulatory review. The patent exclusivity period for a drug will prevent generic drugs from entering the market. Patent exclusivity depends on a number of factors including initial patent term and available patent term extensions based upon delays caused by the regulatory approval process.
Manufacturing Patents
We also own patents claiming methods of manufacturing and purifying oligonucleotides. These patents claim methods for improving oligonucleotide drug manufacturing, including processes for large-scale oligonucleotide synthesis and purification. These methods allow us to manufacture oligonucleotides at lower cost by, for example, eliminating expensive manufacturing steps.
We also rely on trade secrets, proprietary know-how and continuing technological innovation to develop and maintain a competitive position in antisense therapeutics.
32
Government Regulation
Regulation by government authorities in the United States and other countries is a significant component in the development, manufacture and commercialization of pharmaceutical products and services. In addition to regulations enforced by the FDA and relevant foreign regulatory authorities, we are also subject to regulation under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act and other present and potential future federal, state and local regulations.
Extensive regulation by United States and foreign governmental authorities governs the development, manufacture and sale of our drugs. In particular, our drugs are subject to a number of approval requirements by the FDA in the United States under the Federal Food, Drug and Cosmetic Act, or FDCA, and other laws and by comparable agencies in those foreign countries in which we conduct business. The FDCA and other various federal, state and foreign statutes govern or influence the research, testing, manufacture, safety, labeling, storage, recordkeeping, approval, promotion, marketing, distribution, post-approval monitoring and reporting, sampling, quality, and import and export of our drugs. State, local, and other authorities also regulate pharmaceutical manufacturing facilities and procedures.
Our manufacturing facility and our CMOs are subject to periodic inspection by the FDA and other foreign equivalents to ensure that they are operating in compliance with cGMP requirements. In addition, marketing authorization for each new drug may require a rigorous manufacturing pre-approval inspection by regulatory authorities. Post approval, there are strict regulations regarding changes to the manufacturing process, and, depending on the significance of the change, changes may require prior FDA approval. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use.
The FDA must approve any new unapproved drug before a manufacturer can market it in the United States. In order to obtain approval, we and our partners must complete clinical studies and prepare and submit an NDA to the FDA. If the FDA approves a drug, it will issue an approval letter authorizing commercial marketing of the drug and may require a risk evaluation and mitigation strategy, or REMS, to help ensure the benefits of the drug outweigh the potential risks. The requirements for REMS can materially affect the potential market and profitability of our drugs. In foreign jurisdictions, the drug approval process is similarly demanding.
Numerous regulatory authorities in addition to the FDA, including, in the United States, the Centers for Medicare & Medicaid Services, other divisions of the U.S. Department of Health and Human Services, the U.S. Department of Justice, and similar foreign, state and local government authorities, regulate sales, promotion and other activities following drug approval. Only those claims relating to safety and efficacy that the FDA has approved may be used in labeling. Promotional communications regarding a drug must be consistent with the information in the drug’s approved labeling. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses.
For any approved drug, domestic and foreign sales of the drug depend, in part, on the availability and amount of reimbursement by third party payors, including governments and private health plans. Private health plans may seek to manage cost and use of our drugs by implementing coverage and reimbursement limitations. Governments may also regulate or influence coverage, reimbursement and/or pricing of our drugs to control cost or affect use. Within the EU a variety of payors pay for drugs, with governments being the primary source of payment. Negotiating pricing with governmental authorities can delay commercialization. Such pricing and reimbursement factors could impact our ability and that of our commercial partners, including Akcea, to successfully commercialize approved drugs.
In the United States and foreign jurisdictions, the legislative landscape continues to evolve. There have been a number of legislative and regulatory changes to the healthcare system that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the United States federal and state levels and by foreign governments that seek to reduce healthcare costs. There has also been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in efforts to bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs.
Other healthcare laws that may affect our ability to operate include the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, which governs the conduct of certain electronic healthcare transactions and protects the security and privacy of protected health information; analogous state laws governing the privacy and security of health information, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect; and the Physician Payments Sunshine Act, which requires manufacturers of drugs, devices, biologics, and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians and teaching hospitals, and ownership and investment interests held by physicians and their immediate family members.
Our operations may be directly, or indirectly through our customers, distributors, or other business partners, subject to various federal and state fraud and abuse laws, including, without limitation, anti-kickback statutes and false claims statutes. These laws may impact, among other things, our commercialization partners’ proposed sales, marketing and education programs.
The U.S. Foreign Corrupt Practices Act, or FCPA, prohibits certain individuals and entities, including us, from promising, paying, offering to pay, or authorizing the payment of anything of value to any foreign government official, directly or indirectly, to obtain or retain business or an improper advantage. If we violate the FCPA, it could result in large civil and criminal penalties as well as an adverse effect on our reputation, operations, and financial condition. We could also face collateral consequences such as debarment and the loss of export privileges.
33
Competition
Our Business in General
Some of our drugs may compete with existing therapies for market share. In addition, there are a number of companies pursuing the development of oligonucleotide-based technologies and the development of pharmaceuticals utilizing these technologies. These companies include specialized pharmaceutical firms and large pharmaceutical companies acting either independently or together with biopharmaceutical companies. Our drugs are differentiated from traditional small molecule drugs by their chemistry, how they move in the body, how they act in the body, delivery technology, and formulations.
Our approved products and our products under development address numerous markets. The diseases our drugs target for which we have or may receive marketing authorization will determine our competition. For some of our products, an important factor in competition may be the timing of market introduction of competitive products. Accordingly, the relative speed with which we can develop products, complete the clinical trials and marketing authorization processes and supply commercial quantities of the products to the market are important competitive factors. We expect to compete among products approved for sale based on a variety of factors, including, among other things, product efficacy, safety, mechanism of action, dosing convenience, marketing and sales strategy and tactics, availability, price, and reimbursement.
The current key competition for SPINRAZA, our marketed drug for the treatment of people with SMA, and volanesorsen and inotersen, our drugs currently under regulatory review for the treatment of people with FCS and hATTR, respectively, is set forth below.
SPINRAZA
We believe that the following drugs could compete with SPINRAZA:
|
Drug
|
Company
|
Drug Description
|
Phase
|
Admin/Dosing
|
Efficacy(1)
|
Safety(1)
|
|
AVXS-101
|
AveXis
|
Gene therapy that corrects the SMN1 gene using the
AAV9 Vector
|
Pivotal
|
Infusion
|
As of January 20, 2017, in the Phase 1 OLE, the 12 patients taking the proposed therapeutic dose of AVXS-101 were event free and were a median age of 20.2 months at their last follow up appointment. Additionally, 10 out of the 12 patients achieved the ability to sit unassisted for at least 5 seconds, including one patient whose achievement of this milestone was confirmed after January 20, 2017.
|
Generally well tolerated to date, no new treatment-related SAEs or AEs observed
|
|
RG7916
|
PTC Therapeutics/ Roche/ SMA Foundation
|
A small molecule drug that modulates splicing of the SMN2 gene
|
2
|
Oral
|
None reported
|
Safe and well tolerated at all doses and had no drug-related or safety-related study withdrawals.
|
|
LMI070
|
Novartis
|
A small molecule drug that modulates splicing of the
SMN2 gene
|
1/2
|
Oral
|
None reported
|
Study was placed on clinical hold in May 2016 due to safety findings reported in animal studies. The clinical hold was removed in September 2017 and dosing resumed along with additional monitoring.
|
| (1) |
Taken from public documents including respective company press releases, company presentations, and scientific presentations.
|
We believe that SPINRAZA’s closest competitor is AVXS-101. AVXS-101 is currently in a pivotal study for infants with Type 1 SMA using natural history as a comparator. Avexis initiated this study in September 2017 and plans to enroll 15 patients. AveXis has incorporated EU specific Scientific Advice from the EMA into its European pivotal study. While the data released thus far on the AVXS-101 study is encouraging, it is still early in development, having just initiated its first of two pivotal studies. In addition, other gene therapies have had difficulty providing lasting therapeutic benefit. Also AveXis has stated it needs to scale its manufacturing capabilities to be able to manufacture larger quantities of AVXS-101 GMP drug for their pivotal studies in Type 1, ongoing Phase 1 studies in Type 2 patients, and future studies in patients with Type 3 SMA. Further, no company has yet to successfully commercialize a gene replacement therapy, which may create significant barriers for AVXS-101.
34
Volanesorsen
We believe that the following drugs could compete with volanesorsen:
|
Drug
|
Company
|
Drug Description
|
Phase
|
Admin/Dosing
|
Efficacy(1)
|
Safety(1)
|
|
Metreleptin
|
Novelion Therapeutics
|
A synthetic form of the hormone leptin
|
3
|
Reconstituted subcutaneous injection
|
44.4% mean reduction in triglycerides at four months in patients with abnormal triglyceride levels
|
Anti-metreleptin antibodies, hypoglycemia, hypersensitivity, risk of T-cell lymphoma
|
|
Gemcabene
|
Gemphire Therapeutics
|
Monocalcium salt of a dialkyl ether dicarboxylic acid
|
2
|
Oral, once-daily
|
In a post hoc analysis (n=9) of patients with triglycerides >500 mg/dl, reductions of 59% and 60% from 150mg and 300mg doses, respectively, were observed
|
In a recent study, in the gemcabene-treatment group, the
most frequently occurring adverse events were headache and infection
|
| (1) |
Taken from public documents including respective company press releases, company presentations, and scientific presentations.
|
Metreleptin is being tested in people with FPL who also have NASH. In December 2016, Novelion submitted a marketing authorization application to the EMA seeking approval for Metreleptin as replacement therapy to treat complications of leptin deficiency in a small subset of people with FPL and in people with generalized lipodystrophy, or GL. An investigator-sponsored study is currently ongoing with the support of Novelion to evaluate Metreleptin in people with FPL who also have NASH. Metreleptin does not affect apoC-III levels. ApoC-III levels have been shown to be elevated in people with FPL, and directly correlate to triglyceride levels.
Gemcabene is being studied in people with severe hypertriglyceridemia, defined as triglycerides above 500 mg/dL and Gemphire expects to report top-line results from its Phase 2 study in the second quarter of 2018.
Volanesorsen for the treatment of FCS is currently under regulatory review for marketing authorization in the U.S., EU and Canada. To date, volanesorsen has shown the highest percent of triglyceride reductions compared to existing treatments, such as fibrates, regardless of starting triglyceride levels prior to dosing with volanesorsen. Based on our broad Phase 2 data for the treatment of different patients including people with FCS, we believe that volanesorsen will work equally well as a single agent or in combination with other triglyceride-lowering drugs on the market. If regulatory authorities require us to implement platelet monitoring procedures in the commercial setting, which have yet to be determined, it could impact the future competitive profile of volanesorsen.
Inotersen
We believe that the following drugs could compete with inotersen:
|
Drug
|
Company
|
Drug Description
|
Phase
|
Admin/Dosing
|
Efficacy(1)
|
Safety(1)
|
|
Patisiran
|
Alnylam
|
An RNAi drug formulated with lipid nanoparticles to inhibit TTR mRNA
|
Registration
|
Infusion every 3 weeks with pre-treatment with steroids
|
84.3% mean reduction in TTR at 18 months
|
Most common adverse events more frequently observed in patisiran arm vs. placebo were peripheral edema (29.7% vs.
22.1%) and infusion-related reactions (18.9% vs. 9.1%)
|
|
Tafamidis
|
Pfizer
|
A small molecule drug to stabilize TTR Protein
|
3 to support refiling in the U.S., Approved in the EU
|
Daily oral capsule
|
In 45% of people taking Tafamidis, nerve function either improved or stabilized, compared with 30% of patients taking placebo
|
Urinary tract infection, vaginal infection, upper abdominal pain and diarrhea
|
|
Diflunisal
|
N/A Generic
|
A non-steroid anti-inflammatory agent
|
Approved (but not for ATTR)
|
Daily oral capsule/doses
|
Improved nerve function as shown by lower Neuropathy Impairment Score plus 7 nerve tests, or NIS+7. The NIS+7 score increased by 25.0 points in the placebo group versus 8.7 points in the diflunisal group
|
In two studies repurposing diflunisal for use in TTR amyloidosis, drug-related adverse events that led to discontinuation were: gastrointestinal bleeding, low platelets, deterioration of renal function, congestive heart failure, glaucoma and nausea.
|
|
Tolcapone
|
SOM Biotech
|
Small molecule repurposed generic drug
|
2
|
Daily oral dose
|
Shows binding and stabilization of TTR in humans
|
No drug related adverse events reported
|
|
ALN-TTRsc02
|
Alnylam
|
An RNAi drug conjugated with GalNAC to inhibit TTR mRNA in liver cells
|
1
|
Monthly or quarterly
|
In healthy volunteers, a single dose showed mean max TTR knockdown of 97%
|
Injection site reactions were reported
|
| (1) |
Taken from public documents including respective company press releases, company presentations, and scientific presentations. Diflunisal efficacy and safety came from the published papers of two investigator sponsored studies, Berk JL, Suhr OB, Obici L, et al. Repurposing Diflunisal for Familial Amyloid Polyneuropathy: A Randomized Clinical Trial. JAMA. 2013;310(24):2658-2667 and Sekijima YS, Toja K, Morita H, et al. Safety and efficacy of long-term diflunisal administration in hereditary transthyretin (ATTR) amyloidosis. Amyloid. 2015;22(2):79-83.
|
35
We believe that of the drugs that are in development or on the market, inotersen’s closest competitor is patisiran. Alnylam is developing patisiran for hATTR. Patisiran is an intravenously administered RNAi molecule that is formulated with lipid nanoparticles to enable delivery of the drug to the liver. It is administered via an infusion by a healthcare provider in a clinical setting every three weeks. People receiving patisiran are pretreated with steroids to prevent infusion related reactions. In October 2016, Alnylam discontinued development of revusiran, its drug for the cardiomyopathy form of TTR amyloidosis, due to a safety finding in its Phase 3 study. Revusiran was a subcutaneously administered RNAi molecule that was Alnylam’s first generation GalNAc drug and was dosed at 500 mg per week as two subcutaneous injections. Alnylam completed Phase 1 studies of its second generation GalNAC, ALN-TTRsc02. Because we have a PDUFA date of July 6, 2018 for inotersen and Alnylam’s PDUFA date for patisiran is August 11, 2018, we believe that inotersen could be the first RNA-targeted drug on the market for the treatment of people with hATTR. We also believe that the overall product profile of inotersen. as a once weekly, subcutaneous injection with no pretreatment has advantages to the drugs detailed above, however potential platelet and renal monitoring requirements in the commercial setting, which have yet to be determined, could impact the future competitive profile of inotersen.
Employees
As of February 20, 2018, we employed 547 people, including 100 Akcea employees. A significant number of our management and professional employees have had prior experience with pharmaceutical, biotechnology or medical product companies. Collective bargaining agreements do not cover any of our employees, and management considers relations with our employees to be good.
Executive Officers of Ionis
The following sets forth certain information regarding our executive officers as of February 20, 2018:
|
Name
|
|
Age
|
|
Position
|
|
Stanley T. Crooke, M.D., Ph.D.
|
|
72
|
|
Chairman, Chief Executive Officer and President
|
|
Brett P. Monia, Ph.D.
|
|
56
|
Chief Operating Officer and Senior Vice President, Drug Discovery and Corporate Development
|
|
|
C. Frank Bennett, Ph.D.
|
|
61
|
|
Senior Vice President, Antisense Research
|
|
Sarah Boyce
|
46
|
Chief Business Officer
|
||
|
Richard S. Geary, Ph.D.
|
|
60
|
|
Senior Vice President, Development
|
|
Elizabeth L. Hougen
|
|
56
|
|
Senior Vice President, Finance and Chief Financial Officer
|
|
Patrick R. O’Neil, Esq.
|
|
44
|
|
Senior Vice President, Legal, General Counsel, Chief Compliance Officer and Corporate Secretary
|
Management Transitions
In January 2018 Brett Monia, a founder of Ionis, and head of Drug Discovery, and the inotersen program, was promoted to Chief Operating Officer. In his new role, in addition to continuing to play a key role in drug discovery and development including taking responsibility for the research to development transition, Dr. Monia assumed responsibilities for the company’s regulatory, patient advocacy, human resources and business functions including corporate communications, investor relations, business development, alliance management and competitive intelligence. B. Lynne Parshall, who has been with Ionis for 27 years, became Senior Strategic Advisor to Ionis and remains a member of the Board of Directors of Ionis and Akcea. In addition to supporting Dr. Monia in his transition, Ms. Parshall is continuing to be involved in strategic planning, business development, and with Ionis’ important relationships with Biogen and Akcea.
STANLEY T. CROOKE, M.D., Ph.D.
Chairman, Chief Executive Officer and President
Dr. Crooke is a founder of Ionis and has been Chief Executive Officer and a Director since January 1989. He was elected Chairman of the Board in February 1991. Prior to founding Ionis, from 1980 until January 1989, Dr. Crooke was employed by SmithKline Beckman Corporation, a pharmaceutical company, where his titles included President of Research and Development of SmithKline and French Laboratories.
BRETT P. MONIA, Ph.D.
Chief Operating Officer and Senior Vice President, Drug Discovery and Corporate Development
Dr. Monia was promoted to Chief Operating Officer in January 2018 and to Senior Vice President, Drug Discovery and Corporate Development in January 2012. From February 2009 to January 2012, Dr. Monia served as our Vice President, Drug Discovery and Corporate Development and from October 2000 to February 2009, he served as our Vice President, Preclinical Drug Discovery. From October 1989 to October 2000 he held various positions within our Molecular Pharmacology department.
C. FRANK BENNETT, Ph.D.
Senior Vice President, Antisense Research
Dr. Bennett was promoted to Senior Vice President, Antisense Research in January 2006. From June 1995 to January 2006, Dr. Bennett served as our Vice President, Research. From March 1993 to June 1995, he was Director, Molecular Pharmacology, and from May 1992 to March 1993, he was an Associate Director in our Molecular and Cellular Biology department. Prior to joining Ionis in 1989, Dr. Bennett was employed by SmithKline and French Laboratories in various research positions. He is an external member of the Scientific Advisory Board of Experimental Therapeutics Center in Singapore.
36
SARAH BOYCE
Chief Business Officer
Ms. Boyce joined Ionis in January 2015 as our Chief Business Officer. Prior to joining Ionis, Ms. Boyce was Vice President, Head of International Business Strategy and Operations at Forest Laboratories, Inc. from 2012 to 2014. She was Vice President, Global Head Nephrology Therapeutics Area of Alexion Pharmaceuticals from 2010 to 2011. She held various positions at Novartis Group AG, including Vice President, Global Program Head, Pediatric and Specialty from 2000 to 2010. Prior to that, Ms. Boyce held positions at Bayer Pharmaceuticals and Roche.
RICHARD S. GEARY, Ph.D.
Senior Vice President, Development
Dr. Geary was promoted to Senior Vice President, Development in August 2008. From August 2003 to August 2008, Dr. Geary served as our Vice President, Preclinical Development. From November 1995 to August 2003, he held various positions within the Preclinical Development department. Prior to joining Ionis in 1995, Dr. Geary was Senior Research Scientist and Group Leader for the bioanalytical and preclinical pharmacokinetics group in the Applied Chemistry Department at Southwest Research Institute.
ELIZABETH L. HOUGEN
Senior Vice President, Finance and Chief Financial Officer
Ms. Hougen was promoted to Senior Vice President, Finance and Chief Financial Officer in January 2013. From January 2007 to December 2012, Ms. Hougen served as our Vice President, Finance and Chief Accounting Officer and from May 2000 to January 2007, she served as our Vice President, Finance. Prior to joining Ionis in 2000, Ms. Hougen was Executive Director, Finance and Chief Financial Officer for Molecular Biosystems, Inc., a public biotechnology company.
PATRICK R. O’NEIL, Esq.
Senior Vice President, Legal, General Counsel, Chief Compliance Officer and Corporate Secretary
Mr. O’Neil was promoted to Senior Vice President, Legal and General Counsel in January 2013. Mr. O'Neil also serves as our Chief Compliance Officer and Corporate Secretary. From September 2010 to January 2013, Mr. O’Neil served as our Vice President, Legal and General Counsel and from January 2009 to September 2010, he served as our Vice President, Legal and Senior Transactions Counsel. From October 2001 to January 2009 he held various positions within our Legal department. Prior to joining Ionis, Mr. O’Neil was an associate at Cooley LLP.
Item 1A. RISK FACTORS
Investing in our securities involves a high degree of risk. You should consider carefully the following information about the risks described below, together with the other information contained in this report and in our other public filings in evaluating our business. If any of the following risks actually occur, our business could be materially harmed, and our financial condition and results of operations could be materially and adversely affected. As a result, the trading price of our securities could decline, and you might lose all or part of your investment.
Risks Associated with our Ionis Core and Akcea Therapeutics Businesses
If the market does not accept our drugs, including SPINRAZA, volanesorsen and inotersen, we are not likely to generate revenues or become consistently profitable.
Even if our drugs are authorized for marketing, including SPINRAZA, volanesorsen and inotersen, our success will depend upon the medical community, patients and third party payors accepting our drugs as medically useful, cost-effective and safe. Even when the FDA or foreign regulatory authorities authorize our or our partners' drugs for commercialization, doctors may not prescribe our drugs to treat patients. We and our partners may not successfully commercialize additional drugs.
Additionally, in many of the markets where we may sell our drugs in the future, if we cannot agree with the government regarding the price we can charge for our drugs, then we may not be able to sell our drugs in that market. Similarly, cost control initiatives by governments or third party payors could decrease the price received for our drugs or increase patient coinsurance to a level that makes our drugs, including SPINRAZA, volanesorsen and inotersen, unaffordable.
The degree of market acceptance for our drugs, including SPINRAZA, volanesorsen and inotersen, depends upon a number of factors, including the:
| ● |
receipt and scope of marketing authorizations;
|
| ● |
establishment and demonstration in the medical and patient community of the efficacy and safety of our drugs and their potential advantages over competing products;
|
| ● |
cost and effectiveness of our drugs compared to other available therapies;
|
| ● |
patient convenience of the dosing regimen for our drugs; and
|
| ● |
reimbursement policies of government and third-party payors.
|
37
Based on the profile of our drugs, physicians, patients, patient advocates, payors or the medical community in general may not accept and/or use any drugs that we may develop. For example, in the clinical studies with volanesorsen and inotersen, declines in platelet counts were observed in many patients and some patients discontinued the studies because of platelet declines. In addition, in the inotersen NEURO-TTR study, safety signals related to renal function were observed. Therefore, we expect the product label for volanesorsen and inotersen will require periodic platelet monitoring and the product label for inotersen will require periodic renal monitoring, which could negatively affect our ability to attract and retain patients for these drugs. We believe that the enhanced monitoring we have implemented to support early detection and management of these issues can help manage these safety issues so that patients can continue treatment. Since implementation of the enhanced monitoring, serious platelet events have been infrequent. While we believe we and Akcea can better maintain patients on inotersen and volanesorsen through patient-centric commercial approachs where we and Akcea plan to have greater involvement with physicians and patients, if we and Akcea cannot effectively maintain patients on inotersen or volanesorsen, we may not be able to generate substantial revenue from inotersen or volanesorsen sales.
If we or our partners fail to compete effectively, our drugs, including SPINRAZA, volanesorsen and inotersen, will not contribute significant revenues.
Our competitors engage in drug discovery throughout the world, are numerous, and include, among others, major pharmaceutical companies and specialized biopharmaceutical firms. Other companies engage in developing antisense technology. Our competitors may succeed in developing drugs that are:
| ● |
priced lower than our drugs;
|
| ● |
reimbursed more favorably by government and other third-party payors than our drugs;
|
| ● |
safer than our drugs;
|
| ● |
more effective than our drugs; or
|
| ● |
more convenient to use than our drugs.
|
These competitive developments could make our drugs, including SPINRAZA, volanesorsen and inotersen, obsolete or non-competitive.
Certain of our partners are pursuing other technologies or developing other drugs either on their own or in collaboration with others, including our competitors, to treat the same diseases our own collaborative programs target. Competition may negatively impact a partner's focus on and commitment to our drugs and, as a result, could delay or otherwise negatively affect the commercialization of our drugs, including SPINRAZA, volanesorsen and inotersen.
Many of our competitors have substantially greater financial, technical and human resources than we do. In addition, many of these competitors have significantly greater experience than we do in conducting preclinical testing and human clinical studies of new pharmaceutical products in obtaining FDA and other regulatory authorizations of such products and in commercializing such products. Accordingly, our competitors may succeed in obtaining regulatory authorization for products earlier than we do. Marketing and sales capability is another factor relevant to the competitive position of our drugs, and we will primarily rely on our partners, and Akcea to provide this capability.
There are several pharmaceutical and biotechnology companies engaged in the development or commercialization of products against targets that are also targets of products in our development pipeline. For example, AVXS-101, RG7916, and LMI070 could compete with SPINRAZA and metreleptin and Gemcabene could compete with volanesorsen; patisiran, tafamadis, diflunisal, tolcapone, PRX004 and ALN-TTRsc02 could compete with inotersen.
Following approval, our drugs, including SPINRAZA, volanesorsen and inotersen could be subject to regulatory limitations.
Following approval of a drug, we and our partners must comply with comprehensive government regulations regarding the manufacture, marketing and distribution of drug products. We or our partners may not obtain the labeling claims necessary or desirable to successfully commercialize our drug products, including SPINRAZA, volanesorsen and inotersen.
The FDA and foreign regulatory authorities have the authority to impose significant restrictions on an approved drug product through the product label and on advertising, promotional and distribution activities.
In addition, when approved, the FDA or a foreign regulatory authority may condition approval on the performance of post-approval clinical studies or patient monitoring, which could be time consuming and expensive. If the results of such post-marketing studies are not satisfactory, the FDA or a foreign regulatory authority may withdraw marketing authorization or may condition continued marketing on commitments from us or our partners that may be expensive and/or time consuming to fulfill.
If we or others identify side effects after any of our drug products are on the market, or if manufacturing problems occur subsequent to regulatory approval, we or our partners may lose regulatory approval, or we or our partners may need to conduct additional clinical studies and/or change the labeling of our drug products including SPINRAZA, volanesorsen and inotersen.
We depend on our collaboration with Biogen for the development and commercialization of SPINRAZA.
We have entered into a collaborative arrangement with Biogen to develop and commercialize SPINRAZA. We entered into this collaboration primarily to:
| ● |
fund our development activities for SPINRAZA;
|
| ● |
seek and obtain regulatory approvals for SPINRAZA; and
|
| ● |
successfully commercialize SPINRAZA.
|
38
We are relying on Biogen to obtain additional regulatory approvals for SPINRAZA, and successfully commercialize SPINRAZA. In general, we cannot control the amount and timing of resources that Biogen devotes to our collaboration. If Biogen fails to further develop SPINRAZA, obtain additional regulatory approvals for SPINRAZA, or commercialize SPINRAZA, or if Biogen’s efforts are not effective, our business may be negatively affected.
Our collaboration with Biogen may not continue for various reasons. Biogen can terminate our collaboration at any time. If Biogen stops developing or commercializing SPINRAZA, we would have to seek or spend additional funding and SPINRAZA's development and commercialization may be harmed or delayed.
Our collaboration with Biogen may not result in the continued successful commercialization of SPINRAZA. If Biogen does not continue to successfully commercialize SPINRAZA, we will receive limited revenues for SPINRAZA.
If we cannot effectively build and manage a distribution, medical affairs, market access, marketing and sales infrastructure for inotersen, or have a commercial partner perform these functions, it could delay, harm or preclude the commercial launch of inotersen and the related product revenue.
We plan to commercialize inotersen in North America ourselves and to seek a commercial partner in other geographic regions. We currently have a limited commercial infrastructure to distribute, market and sell inotersen. If approved, to commercialize inotersen, we must build these capabilities, have a commercial partner perform these functions, or make arrangements with third parties to perform these services. There are significant risks involved in building and managing a commercial infrastructure. We may not be successful in doing so.
We may contract with, and rely on, third party specialty pharmacies to distribute inotersen. A specialty pharmacy is a pharmacy that specializes in dispensing medications for complex or chronic conditions, a process that requires a high level of patient education and ongoing management. Absent a commercial partner, our management team will need to devote a significant amount of attention to building and managing this distribution network. The use of specialty pharmacies involves certain risks, including but not limited to risks that these organizations will not provide us with accurate or timely information, not effectively sell or support our drug products, not satisfy financial obligations to us, or cease operations.
We may also build a specialty sales force in each global region we expect to market inotersen, leverage the sales infrastructure of a commercial partner, or utilize a third-party marketing and sales organization. It will be expensive and time consuming for us to build and establish our own sales force and related compliance protocols to market inotersen. We will have to compete with other companies to recruit, hire, train, manage and retain sales personnel.
We will also incur expenses prior to product launch to develop our distribution, medical affairs, market access, marketing and sales infrastructure. If there is a delay in the commercial launch of inotersen, we will incur additional expenses for having developed these capabilities earlier than required and prior to realizing any revenue from sales of inotersen.
If we cannot effectively build and manage our distribution, medical affairs, market access, marketing and sales infrastructure, or find a suitable commercial partner to perform such functions, the commercial launch and sales of inotersen may be delayed, less successful or precluded. Such events may result in decreased sales and lower revenue, which could have a material adverse effect on our business, prospects, financial condition and results of operations.
If government or other third-party payors fail to provide adequate coverage and payment rates for our drugs, including SPINRAZA, inotersen and volanesorsen, our revenue will be limited.
In both domestic and foreign markets, sales of our current and future products will depend in part upon the availability of coverage and reimbursement from third-party payors. The majority of people in the United States who would fit within our target patient populations for our drugs have their healthcare supported by a combination of Medicare coverage, other government health programs such as Medicaid, managed care providers, private health insurers and other organizations. Coverage decisions may depend upon clinical and economic standards that disfavor new drug products when more established or lower cost therapeutic alternatives are already available or subsequently become available. Assuming coverage is approved, the resulting reimbursement payment rates might not be enough to make our drugs affordable.
Third-party payors, whether foreign or domestic, or governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs. In addition, in the United States, no uniform policy of coverage and reimbursement for drug products exists among third-party payors. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. Further, we believe that future coverage and reimbursement will likely be subject to increased restrictions both in the United States and in international markets. For example, in the United States, recent health reform measures have resulted in reductions in Medicare and other healthcare funding, and there have been several U.S. Congressional inquiries and proposed federal legislation designed to, among other things, reform government program reimbursement methodologies for drug products and bring more transparency to drug pricing. Third-party coverage and reimbursement for our products or drugs may not be available or adequate in either the United States or international markets, which would negatively affect the potential commercial success of our products, our revenue and our profits.
39
If Biogen cannot manufacture finished drug product for SPINRAZA or the post-launch supply of the active drug substance for SPINRAZA, SPINRAZA may not achieve or maintain commercial success.
Biogen is responsible for the long term supply of both SPINRAZA drug substance and finished drug product. Biogen may not be able to reliably manufacture SPINRAZA drug substance and drug product to support the long term commercialization of SPINRAZA. If Biogen cannot reliably manufacture SPINRAZA drug substance and drug product, SPINRAZA may not achieve or maintain commercial success, which will harm our ability to generate revenue.
If we or our partners fail to obtain regulatory approval for our drugs, including volanesorsen, inotersen, and additional approvals for SPINRAZA, we or our partners cannot sell them in the applicable markets.
We cannot guarantee that any of our drugs, including volanesorsen and inotersen, will be considered safe and effective, or will be approved for commercialization. In addition, we cannot guarantee that SPINRAZA will be approved in additional markets or for additional indications. We and our partners must conduct time-consuming, extensive and costly clinical studies to show the safety and efficacy of each of our drugs before they can be approved for sale. We must conduct these studies in compliance with FDA regulations and with comparable regulations in other countries.
We and our partners may not obtain necessary regulatory approvals on a timely basis, if at all, for our drugs. It is possible that regulatory agencies will not approve our drugs including, volanesorsen and inotersen for marketing or additional marketing authorizations for SPINRAZA. If the FDA or another regulatory agency believes that we or our partners have not sufficiently demonstrated the safety or efficacy of any of our drugs, including SPINRAZA, volanesorsen and inotersen, the agency will not approve the specific drug or will require additional studies, which can be time consuming and expensive and which will delay or harm commercialization of the drug. For example, the FDA or foreign regulatory authorities could claim that we have not tested volanesorsen in a sufficient number of patients to demonstrate volanesorsen is safe and effective in patients with FCS or FPL to support an application for marketing authorization, especially since a small number of patients in the APPROACH FCS study experienced severe thrombocytopenia, a condition where the patient has severely low platelet levels. In such a case, we may need to conduct additional clinical studies before obtaining marketing authorization, which would be expensive and cause delays.
The FDA’s Division of Metabolism and Endocrinology Products advisory committee is scheduled to discuss and advise the FDA on the risk-benefit profile of volanesorsen for the treatment of FCS on May 10, 2018. In advance of this advisory committee meeting, we, Akcea and the FDA will submit briefing documents for the committee’s review, and these briefing documents will be made available to the public and may include information from the volanesorsen development program that have not previously been disclosed. Historically, for some companies, disclosure of information in this manner has led to increased volatility in their stock price. The advisory committee and FDA may interpret nonclinical and clinical data differently than we and our experts have. Press coverage and public scrutiny of the materials that will be discussed at the advisory committee meeting may negatively affect the potential for the NDA for volanesorsen to receive approval or the trading price of our securities. Even if we and Akcea ultimately obtain approval for volanesorsen, the matters discussed at the advisory committee meeting could limit Akcea’s ability to successfully commercialize volanesorsen.
Failure to receive marketing authorization for our drugs, volanesorsen and inotersen, or additional authorizations for SPINRAZA, or delays in these authorizations could prevent or delay commercial introduction of the drug, and, as a result, could negatively impact our ability to generate revenue from product sales.
If the results of clinical testing indicate that any of our drugs are not suitable for commercial use we may need to abandon one or more of our drug development programs.
Drug discovery and development has inherent risks and the historical failure rate for drugs is high. Antisense drugs are a relatively new approach to therapeutics. If we cannot demonstrate that our drugs are safe and effective for human use, we may need to abandon one or more of our drug development programs.
In the past, we have invested in clinical studies of drugs that have not met the primary clinical end points in their Phase 3 studies. Similar results could occur in clinical studies for our drugs, including the study of volanesorsen in patients with FPL. If any of our drugs in clinical studies, including volanesorsen, do not show sufficient efficacy in patients with the targeted indication, it could negatively impact our development and commercialization goals for the drug and our stock price could decline.
Even if our drugs are successful in preclinical and human clinical studies, the drugs may not be successful in late-stage clinical studies.
Successful results in preclinical or initial human clinical studies, including the Phase 2 results for some of our drugs in development, may not predict the results of subsequent clinical studies, including the Phase 3 study of volanesorsen in patients with FPL. There are a number of factors that could cause a clinical study to fail or be delayed, including:
| ● |
the clinical study may produce negative or inconclusive results;
|
| ● |
regulators may require that we hold, suspend or terminate clinical research for noncompliance with regulatory requirements;
|
| ● |
we, our partners, the FDA or foreign regulatory authorities could suspend or terminate a clinical study due to adverse side effects of a drug on subjects in the trial;
|
| ● |
we may decide, or regulators may require us, to conduct additional preclinical testing or clinical studies;
|
| ● |
enrollment in our clinical studies may be slower than we anticipate;
|
| ● |
people who enroll in the clinical study may later drop out due to adverse events, a perception they are not benefiting from participating in the study, fatigue with the clinical study process or personal issues;
|
| ● |
the cost of our clinical studies may be greater than we anticipate; and
|
| ● |
the supply or quality of our drugs or other materials necessary to conduct our clinical studies may be insufficient, inadequate or delayed.
|
40
In addition, our current drugs, including SPINRAZA, volanesorsen and inotersen, are chemically similar to each other. As a result, a safety observation we encounter with one of our drugs could have, or be perceived by a regulatory authority to have, an impact on a different drug we are developing. This could cause the FDA and other regulators to ask questions or take actions that could harm or delay our ability to develop and commercialize our drugs or increase our costs. For example, the FDA or other regulatory agencies could request, among other things, any of the following regarding one of our drugs: additional information or commitments before we can start or continue a clinical study, protocol amendments, increased safety monitoring, additional product labeling information, and post-approval commitments. Similarly, we have an ongoing Phase 3 study of volanesorsen in patients with FPL, an ongoing open label extension study of volanesorsen in patients with FCS, an ongoing open label extension study of inotersen and expanded access programs for each drug. Adverse events or results from these studies could negatively impact our current or planned marketing approval applications for volanesorsen in patients with FCS, for inotersen or the commercial opportunity for each product.
Any failure or delay in the clinical studies, including the Phase 3 study for volanesorsen in patients with FPL, could reduce the commercial potential or viability of our drugs.
If we cannot manufacture our drugs or contract with a third party to manufacture our drugs at costs that allow us to charge competitive prices to buyers, we cannot market our products profitably.
To successfully commercialize any of our drugs, we or our partner would need to establish large-scale commercial manufacturing capabilities either on our own or through a third party manufacturer. We and Akcea will rely on third party manufacturers to supply the drug substance and drug product for inotersen and volanesorsen. In addition, as our drug development pipeline increases and matures, we will have a greater need for clinical trial and commercial manufacturing capacity. We have limited experience manufacturing pharmaceutical products of the chemical class represented by our drugs, called oligonucleotides, on a commercial scale for the systemic administration of a drug. There are a small number of suppliers for certain capital equipment and raw materials that we use to manufacture our drugs, and some of these suppliers will need to increase their scale of production to meet our projected needs for commercial manufacturing. Further, we must continue to improve our manufacturing processes to allow us to reduce our drug costs. We may not be able to manufacture our drugs at a cost or in quantities necessary to make commercially successful products.
Also, manufacturers, including us, must adhere to the FDA's current Good Manufacturing Practices regulations and similar regulations in foreign countries, which the applicable regulatory authorities enforce through facilities inspection programs. We and our contract manufacturers may not comply or maintain compliance with Good Manufacturing Practices, or similar foreign regulations. Non-compliance could significantly delay or prevent receipt of marketing authorization for our drugs, including authorizations for SPINRAZA, volanesorsen and inotersen, or result in enforcement action after authorization that could limit the commercial success of our drugs, including SPINRAZA, volanesorsen and inotersen.
We depend on third parties to conduct our clinical studies for our drugs and any failure of those parties to fulfill their obligations could adversely affect our development and commercialization plans.
We depend on independent clinical investigators, contract research organizations and other third-party service providers to conduct our clinical studies for our drugs and expect to continue to do so in the future. For example, we use clinical research organizations, such as Icon Clinical Research Limited, INC Research Toronto, Inc. and Medpace for the clinical studies for our drugs, including volanesorsen and inotersen. We rely heavily on these parties for successful execution of our clinical studies, but do not control many aspects of their activities. For example, the investigators are not our employees. However, we are responsible for ensuring that these third parties conduct each of our clinical studies in accordance with the general investigational plan and approved protocols for the study. Third parties may not complete activities on schedule, or may not conduct our clinical studies in accordance with regulatory requirements or our stated protocols. The failure of these third parties to carry out their obligations or a termination of our relationship with these third parties could delay or prevent the development, marketing authorization and commercialization of our drugs, including authorizations for volanesorsen and inotersen or additional authorizations for SPINRAZA.
Risks Associated with our Businesses as a Whole
We have incurred losses, and our business will suffer if we fail to consistently achieve profitability in the future.
Because drug discovery and development requires substantial lead-time and money prior to commercialization, our expenses have generally exceeded our revenue since we were founded in January 1989. As of December 31, 2017, we had an accumulated deficit of approximately $1.2 billion and stockholders’ equity of approximately $418.7 million. Most of the losses resulted from costs incurred in connection with our research and development programs and from selling, general and administrative costs associated with our operations. Most of our income has come from collaborative arrangements, including commercial revenue from royalties and R&D revenue, with additional income from research grants and the sale or licensing of our patents, as well as interest income. We may incur additional operating losses over the next several years, and these losses may increase if we cannot increase or sustain revenue. We may not successfully develop any additional products or achieve or sustain future profitability.
Our ability to use our net operating loss carryovers and certain other tax attributes may be limited.
As described above, we have incurred net losses. Under the Internal Revenue Code of 1986, as amended, or the Code, a corporation is generally allowed a deduction for net operating losses, or NOLs, carried over from a prior taxable year. Under that provision, we can carryforward our NOLs to offset our future taxable income, if any, until such NOLs are used or expire. The same is true of other unused tax attributes, such as tax credits.
41
As of December 31, 2017, we had federal and California net operating loss carryforwards of approximately $561.1 million and $887.1 million, respectively. The federal net operating loss carryforwards will begin to expire, if not utilized, beginning in 2024. These net operating loss carryforwards could expire unused and be unavailable to offset future income tax liabilities. Under the Tax Cut and Jobs Act of 2017, or the Tax Act, federal net operating losses incurred in 2018 and in future years may be carried forward indefinitely, but the deductibility of such federal net operating losses is limited. It is uncertain if and to what extent various states will conform to the newly enacted federal tax law. In addition, under Section 382 of the Internal Revenue Code of 1986, as amended, and corresponding provisions of state law, if a corporation undergoes an “ownership change,” which is generally defined as a greater than 50 percent change, by value, in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income or taxes may be limited. It is possible that we have experienced an ownership change limitation. We may experience ownership changes in the future as a result of subsequent shifts in our stock ownership, some of which may be outside of our control. If an ownership change occurs and our ability to use our net operating loss carryforwards is materially limited, it would harm our future operating results by effectively increasing our future tax obligations.
Since corporate partnering is a significant part of our strategy to fund the development and commercialization of our development programs, if any of our collaborative partners fail to fund our collaborative programs, or if we cannot obtain additional partners, we may have to delay or stop progress on our drug development programs.
To date, corporate partnering has played a significant role in our strategy to fund our development programs and to add key development resources. We plan to continue to rely on additional collaborative arrangements to develop and commercialize our unpartnered drugs. However, we may not be able to negotiate favorable collaborative arrangements for these drug programs. If we cannot continue to secure additional collaborative partners, our revenues could decrease and the development of our drugs could suffer.
Our corporate partners are developing and/or funding many of the drugs in our development pipeline. If any of these pharmaceutical companies stops developing and/or funding these drugs, our business could suffer and we may not have, or be willing to dedicate, the resources available to develop these drugs on our own.
Our collaborators can terminate their relationships with us under certain circumstances, many of which are outside of our control. For example, as part of a reprioritization of its pipeline and strategic review of its rare disease business, GSK declined its option on inotersen and IONIS-FB-LRx.
Even with funding from corporate partners, if our partners do not effectively perform their obligations under our agreements with them, it would delay or stop the progress of our drug development and commercial programs.
In addition to receiving funding, we enter into collaborative arrangements with third parties to:
| ● |
conduct clinical studies;
|
| ● |
seek and obtain marketing authorization; and
|
| ● |
manufacture, market and sell our drugs.
|
Once we have secured a collaborative arrangement to further develop and commercialize one of our drug development programs, such as our collaborations with AstraZeneca, Bayer, Biogen, GSK, Novartis and Roche these collaborations may not continue or result in commercialized drugs, or may not progress as quickly as we first anticipated.
For example, a collaborator such as AstraZeneca, Bayer, Biogen, GSK, Novartis or Roche, could determine that it is in its financial interest to:
| ● |
pursue alternative technologies or develop alternative products that may be competitive with the drug that is part of the collaboration with us;
|
| ● |
pursue higher-priority programs or change the focus of its own development programs; or
|
| ● |
choose to devote fewer resources to our drugs than it does for its own drugs.
|
If any of these occur, it could affect our partner's commitment to the collaboration with us and could delay or otherwise negatively affect the commercialization of our drugs, including SPINRAZA.
If we do not progress in our programs as anticipated, the price of our securities could decrease.
For planning purposes, we estimate and may disclose the timing of a variety of clinical, regulatory and other milestones, such as when we anticipate a certain drug will enter the clinic, when we anticipate completing a clinical study, or when we anticipate filing an application for, or obtaining, marketing authorization. We base our estimates on present facts and a variety of assumptions. Many underlying assumptions are outside of our control. If we do not achieve milestones in accordance with our or our investors' expectations, including milestones related to SPINRAZA, volanesorsen and inotersen the price of our securities could decrease.
42
If we cannot protect our patents or our other proprietary rights, others may compete more effectively against us.
Our success depends to a significant degree upon whether we can continue to develop and secure intellectual property rights to proprietary products and services. However, we may not receive issued patents on any of our pending patent applications in the United States or in other countries. In addition, the scope of any of our issued patents may not be sufficiently broad to provide us with a competitive advantage. Furthermore, other partners may successfully challenge, invalidate or circumvent our issued patents or patents licensed to us so that our patent rights do not create an effective competitive barrier or revenue source.
Intellectual property litigation could be expensive and prevent us from pursuing our programs.
From time to time we have to defend our intellectual property rights. If we are involved in an intellectual property dispute, we sometimes need to litigate to defend our rights or assert them against others. Disputes can involve arbitration, litigation or proceedings declared by the United States Patent and Trademark Office or the International Trade Commission or foreign patent authorities. Intellectual property litigation can be extremely expensive, and this expense, as well as the consequences should we not prevail, could seriously harm our business. For example, in November 2013 we filed a patent infringement lawsuit against Gilead Sciences Inc. in the United States District Court of the Northern District of California. Intellectual property lawsuits may be costly and may not be resolved in our favor.
If a third party claims that our drugs or technology infringe its patents or other intellectual property rights, we may have to discontinue an important product or product line, alter our products and processes, pay license fees or cease certain activities. We may not be able to obtain a license to needed intellectual property on favorable terms, if at all. There are many patents issued or applied for in the biotechnology industry, and we may not be aware of patents or patent applications held by others that relate to our business. This is especially true since patent applications in the United States are filed confidentially for the first 18 months. Moreover, the validity and breadth of biotechnology patents involve complex legal and factual questions for which important legal issues remain.
If we fail to obtain timely funding, we may need to curtail or abandon some of our programs.
Many of our drugs are undergoing clinical studies or are in the early stages of research and development. All of our drug programs will require significant additional research, development, preclinical and/or clinical testing, marketing authorization and/or commitment of significant additional resources prior to their successful commercialization. As of December 31, 2017, we had cash, cash equivalents and short-term investments equal to $1.0 billion. If we do not meet our goals to successfully commercialize our drugs, including SPINRAZA, volanesorsen and inotersen, or to license our drugs and proprietary technologies, we will need additional funding in the future. Our future capital requirements will depend on many factors, such as the following:
| ● |
successful commercialization for SPINRAZA;
|
| ● |
marketing approvals for volanesorsen and inotersen;
|
| ● |
the profile and launch timing of our drugs, including volanesorsen and inotersen;
|
| ● |
changes in existing collaborative relationships and our ability to establish and maintain additional collaborative arrangements;
|
| ● |
continued scientific progress in our research, drug discovery and development programs;
|
| ● |
the size of our programs and progress with preclinical and clinical studies;
|
| ● |
the time and costs involved in obtaining marketing authorizations; and
|
| ● |
competing technological and market developments, including the introduction by others of new therapies that address our markets.
|
If we need additional funds, we may need to raise them through public or private financing. Additional financing may not be available at all or on acceptable terms. If we raise additional funds by issuing equity securities, the shares of existing stockholders will be diluted and the price, as well as the price of our other securities, may decline. If adequate funds are not available or not available on acceptable terms, we may have to cut back on one or more of our research, drug discovery or development programs. Alternatively, we may obtain funds through arrangements with collaborative partners or others, which could require us to give up rights to certain of our technologies or drugs.
The loss of key personnel, or the inability to attract and retain highly skilled personnel, could make it more difficult to run our business and reduce our likelihood of success.
We are dependent on the principal members of our management and scientific staff. We do not have employment agreements with any of our executive officers that would prevent them from leaving us. The loss of our management and key scientific employees might slow the achievement of important research and development goals. It is also critical to our success that we recruit and retain qualified scientific personnel to perform research and development work. We may not be able to attract and retain skilled and experienced scientific personnel on acceptable terms because of intense competition for experienced scientists among many pharmaceutical and health care companies, universities and non-profit research institutions. In addition, failure to succeed in clinical studies may make it more challenging to recruit and retain qualified scientific personnel.
If the price of our securities continues to be highly volatile, this could make it harder for you to liquidate your investment and could increase your risk of suffering a loss.
The market price of our common stock, like that of the securities of many other biopharmaceutical companies, has been and is likely to continue to be highly volatile. These fluctuations in our common stock price may significantly affect the trading price of our securities. During the 12 months preceding December 31, 2017, the market price of our common stock ranged from $65.51 to $37.26 per share. Many factors can affect the market price of our securities, including, for example, fluctuations in our operating results, announcements of collaborations, clinical study results, technological innovations or new products being developed by us or our competitors, governmental regulation, marketing authorization, changes in payors' reimbursement policies, developments in patent or other proprietary rights, public concern regarding the safety of our drugs and general market conditions.
43
We are exposed to potential product liability claims, and insurance against these claims may not be available to us at a reasonable rate in the future or at all.
Our business exposes us to potential product liability risks that are inherent in the testing, manufacturing, marketing and sale of therapeutic products, including potential product liability claims related to SPINRAZA, volanesorsen and inotersen. We have clinical study insurance coverage and commercial product liability insurance coverage. However, this insurance coverage may not be adequate to cover claims against us, or be available to us at an acceptable cost, if at all. Regardless of their merit or eventual outcome, products liability claims may result in decreased demand for our drug products, injury to our reputation, withdrawal of clinical study volunteers and loss of revenues. Thus, whether or not we are insured, a product liability claim or product recall may result in losses that could be material.
Because we use biological materials, hazardous materials, chemicals and radioactive compounds, if we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
Our research, development and manufacturing activities involve the use of potentially harmful biological materials as well as materials, chemicals and various radioactive compounds that could be hazardous to human health and safety or the environment. We store most of these materials and various wastes resulting from their use at our facilities in Carlsbad, California pending ultimate use and disposal. We cannot completely eliminate the risk of contamination, which could cause:
| ● |
interruption of our research, development and manufacturing efforts;
|
| ● |
injury to our employees and others;
|
| ● |
environmental damage resulting in costly clean up; and
|
| ● |
liabilities under federal, state and local laws and regulations governing health and human safety, as well as the use, storage, handling and disposal of these materials and resultant waste products.
|
In such an event, we may be held liable for any resulting damages, and any liability could exceed our resources. Although we carry insurance in amounts and types that we consider commercially reasonable, we do not have insurance coverage for losses relating to an interruption of our research, development or manufacturing efforts caused by contamination, and the coverage or coverage limits of our insurance policies may not be adequate. If our losses exceed our insurance coverage, our financial condition would be affected. We manufacture the finished drug product for volanesorsen and inotersen at third party contract manufacturers.
If a natural or man-made disaster strikes our research, development or manufacturing facilities or otherwise affects our business, it could delay our progress developing and commercializing our drugs.
We manufacture our research and clinical supplies in a manufacturing facility located in Carlsbad, California. The facilities and the equipment we and our contract manufacturers use to research, develop and manufacture our drugs would be costly to replace and could require substantial lead time to repair or replace. Our facilities or our contract manufacturers may be harmed by natural or man-made disasters, including, without limitation, earthquakes, floods, fires and acts of terrorism; and if our facilities are affected by a disaster, our development and commercialization efforts would be delayed. Although we possess insurance for damage to our property and the disruption of our business from casualties, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all. In addition, our development and commercialization activities could be harmed or delayed by a shutdown of the U.S. government, including the FDA.
Our business and operations would suffer in the event of computer system failures.
Despite the implementation of security measures, our internal computer systems, and those of our clinical research organizations, manufacturers, commercial partners and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. If issues were to arise and cause interruptions in our operations, it could result in a material disruption of our drug programs. For example, the loss of clinical study data from completed or ongoing clinical studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development or commercialization of our drugs, including SPINRAZA, volanesorsen and inotersen could be harmed or delayed.
Provisions in our certificate of incorporation, other agreements and Delaware law may prevent stockholders from receiving a premium for their shares.
Our certificate of incorporation provides for classified terms for the members of our board of directors. Our certificate also includes a provision that requires at least 66 2/3 percent of our voting stockholders to approve a merger or certain other business transactions with, or proposed by, any holder of 15 percent or more of our voting stock, except in cases where certain directors approve the transaction or certain minimum price criteria and other procedural requirements are met.
Our certificate of incorporation also requires that any action required or permitted to be taken by our stockholders must be taken at a duly called annual or special meeting of stockholders and may not be taken by written consent. In addition, only our board of directors, chairman of the board or chief executive officer can call special meetings of our stockholders. We have in the past, and may in the future, implement a stockholders' rights plan, also called a poison pill, which could make it uneconomical for a third party to acquire our company on a hostile basis. In addition, our board of directors has the authority to fix the rights and preferences of, and issue shares of preferred stock, which may have the effect of delaying or preventing a change in control of our company without action by our stockholders.
44
The provisions of our convertible senior notes could make it more difficult or more expensive for a third party to acquire us. Upon the occurrence of certain transactions constituting a fundamental change, holders of the notes will have the right, at their option, to require us to repurchase all of their notes or a portion of their notes, which may discourage certain types of transactions in which our stockholders might otherwise receive a premium for their shares over the then current market prices.
These provisions, as well as Delaware law, including Section 203 of the Delaware General Corporation Law, and other of our agreements, may discourage certain types of transactions in which our stockholders might otherwise receive a premium for their shares over then current market prices, and may limit the ability of our stockholders to approve transactions that they think may be in their best interests.
Future sales of our common stock in the public market could adversely affect the trading price of our securities.
Future sales of substantial amounts of our common stock in the public market, or the perception that such sales could occur, could adversely affect trading prices of our securities. For example, we may issue approximately 10.3 million shares of our common stock upon conversion of our convertible senior notes. The addition of any of these shares into the public market may have an adverse effect on the price of our securities.
Our business is subject to changing regulations for corporate governance and public disclosure that has increased both our costs and the risk of noncompliance.
Each year we are required to evaluate our internal controls systems in order to allow management to report on and our Independent Registered Public Accounting Firm to attest to, our internal controls as required by Section 404 of the Sarbanes-Oxley Act. As a result, we continue to incur additional expenses and divert our management's time to comply with these regulations. In addition, if we cannot continue to comply with the requirements of Section 404 in a timely manner, we might be subject to sanctions or investigation by regulatory authorities, such as the SEC, the Public Company Accounting Oversight Board, or PCAOB, or The Nasdaq Global Select Market. Any such action could adversely affect our financial results and the market price of our common stock.
The SEC and other regulators have continued to adopt new rules and regulations and make additional changes to existing regulations that require our compliance. On July 21, 2010, the Dodd-Frank Wall Street Reform and Protection Act, or the Dodd-Frank Act, was enacted. There are significant corporate governance and executive compensation-related provisions in the Dodd-Frank Act that require the SEC to adopt, or where the SEC has adopted, additional rules and regulations in these areas such as "say on pay" and proxy access. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business.
The recently passed comprehensive tax reform bill could adversely affect our business and financial condition.
The Tax Act significantly revises the Internal Revenue Code of 1986, as amended. The Tax Act, among other things, contains significant changes to corporate taxation, including reduction of the corporate tax rate from a top marginal rate of 35 percent to a flat rate of 21 percent, limitation of the tax deduction for interest expense to 30 percent of adjusted earnings (except for certain small businesses), limitation of the deduction for net operating losses to 80 percent of current year taxable income and elimination of net operating loss carrybacks, one time taxation of offshore earnings at reduced rates regardless of whether they are repatriated, elimination of U.S. tax on foreign earnings (subject to certain important exceptions), immediate deductions for certain new investments instead of deductions for depreciation expense over time, and modifying or repealing many business deductions and credits. Notwithstanding the reduction in the corporate income tax rate, the overall impact of the new federal tax law is uncertain and our business and financial condition could be adversely affected. In addition, it is uncertain if and to what extent various states will conform to the newly enacted federal tax law. The impact of this tax reform on holders of our common stock is also uncertain and could be adverse. We urge our stockholders to consult with their legal and tax advisors with respect to this legislation and the potential tax consequences of investing in or holding our common stock.
We could be subject to additional tax liabilities.
We are subject to U.S. federal, state, local and sales taxes in the U.S. and foreign income taxes, withholding taxes and transaction taxes in foreign jurisdictions. Significant judgment is required in evaluating our tax positions and our worldwide provision for taxes. During the ordinary course of business, there are many activities and transactions for which the ultimate tax determination is uncertain. In addition, our tax obligations and effective tax rates could be adversely affected by changes in the relevant tax, accounting and other laws, regulations, principles and interpretations, including those relating to income tax nexus, by recognizing tax losses or lower than anticipated earnings in jurisdictions where we have lower statutory rates and higher than anticipated earnings in jurisdictions where we have higher statutory rates, by changes in foreign currency exchange rates, or by changes in the valuation of our deferred tax assets and liabilities. We may be audited in various jurisdictions, and such jurisdictions may assess additional taxes, sales taxes and value-added taxes against us. Although we believe our tax estimates are reasonable, the final determination of any tax audits or litigation could be materially different from our historical tax provisions and accruals, which could have a material adverse effect on our operating results or cash flows in the period for which a determination is made.
Negative conditions in the global credit markets and financial services and other industries may adversely affect our business.
The global credit markets, the financial services industry, the U.S. capital markets, and the U.S. economy as a whole have in the past experienced periods of substantial turmoil and uncertainty characterized by unprecedented intervention by the U.S. federal government and the failure, bankruptcy, or sale of various financial and other institutions. It is possible that a crisis in the global credit markets, the U.S. capital markets, the financial services industry or the U.S. economy may adversely affect our business, vendors and prospects, as well as our liquidity and financial condition. More specifically, our insurance carriers and insurance policies covering all aspects of our business may become financially unstable or may not be sufficient to cover any or all of our losses and may not continue to be available to us on acceptable terms, or at all.
45
Item 1B. Unresolved Staff Comments
Not applicable.
Item 2. Properties
As of February 20, 2018, we occupied the following properties:
|
Property Description
|
Location
|
Square Footage
|
Owned or Leased
|
Initial Lease Term End Date
|
Lease Extension Options
|
|||||
|
Ionis laboratory and office space facility
|
Carlsbad, CA
|
176,000
|
Owned
|
|||||||
|
Ionis manufacturing facility
|
Carlsbad, CA
|
28,700
|
Owned
|
|||||||
|
Ionis manufacturing support facility
|
Carlsbad, CA
|
25,800
|
Leased
|
2021
|
Two, five-year options to extend
|
|||||
|
Akcea office space facility
|
Cambridge, MA
|
6,100
|
Leased
|
2018
|
None
|
|||||
|
Akcea office space facility
|
Cambridge, MA
|
3,100
|
Leased
|
2020
|
None
|
|||||
|
239,700
|
We believe our existing facilities are adequate for our requirements in the foreseeable future and that we have sufficient manufacturing capacity to meet our current and future obligations under existing agreements with our partners for commercial, research and development needs and to produce launch quantities for volanesorsen and inotersen. Akcea will need additional space in the future as it continues to build its development, commercial and support teams. Akcea is currently searching for a new office facility and believes it can find suitable space on commercially reasonable terms.
Item 3. Legal Proceedings
Gilead Litigation
In August 2013, Gilead Sciences Inc. filed a suit in the United States District Court of Northern District of California related to United States Patent Nos. 7,105,499 and 8,481,712, which are jointly owned by Merck Sharp & Dohme Corp. and Ionis Pharmaceuticals, Inc. In the suit Gilead asked the court to determine that Gilead's activities do not infringe any valid claim of the named patents and that the patents are not valid. We and Merck Sharp & Dohme Corp. filed our answer denying Gilead's noninfringement and invalidity contentions, contending that Gilead's commercial sale and offer for sale of sofosbuvir prior to the expiration of the '499 and '712 patents infringes those patents, and requesting monetary damages to compensate for such infringement. In the trial for this case held in March 2016, the jury upheld all ten of the asserted claims of the patents-in-suit. The jury then decided that we and Merck are entitled to four percent of $5 billion in past sales of sofosbuvir. Gilead has stated it would appeal the jury’s finding of validity. In the meantime, Gilead asserted two additional non-jury defenses: waiver and unclean hands. Although the judge rejected the waiver defense, she granted Gilead’s motion claiming that the patents are unenforceable against it under the doctrine of unclean hands. We believe this ruling is contrary to the relevant law and the facts of the case. Accordingly, in July 2016, together with Merck we appealed the decision to the Court of Appeals for the Federal Circuit. Gilead cross-appealed on the issue of validity. Briefing on the appeals is now complete and oral arguments were held in February 2018. Under our agreement with Merck, Merck is responsible for the costs of this suit.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
| Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
Our common stock is traded publicly through The Nasdaq Global Select Market under the symbol “IONS.” The following table presents quarterly information on the price range of our common stock. This information indicates the high and low sale prices reported by The Nasdaq Global Select Market. These prices do not include retail markups, markdowns or commissions.
|
|
HIGH
|
LOW
|
||||||
|
2017
|
||||||||
|
First Quarter
|
$
|
56.91
|
$
|
37.29
|
||||
|
Second Quarter
|
$
|
55.73
|
$
|
37.26
|
||||
|
Third Quarter
|
$
|
60.01
|
$
|
43.75
|
||||
|
Fourth Quarter
|
$
|
65.51
|
$
|
50.02
|
||||
|
2016
|
||||||||
|
First Quarter
|
$
|
62.68
|
$
|
30.93
|
||||
|
Second Quarter
|
$
|
46.75
|
$
|
19.59
|
||||
|
Third Quarter
|
$
|
40.82
|
$
|
23.26
|
||||
|
Fourth Quarter
|
$
|
57.00
|
$
|
24.58
|
||||
As of February 20, 2018, there were approximately 560 stockholders of record of our common stock. Because many of our shares are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these record holders.
46
We have never paid dividends and do not anticipate paying any dividends in the foreseeable future.
Set forth below is a table and chart comparing the total return on an indexed basis of $100 invested on December 31, 2012 in our common stock, the Nasdaq Composite Index (total return) and the Nasdaq Biotechnology Index. The total return assumes reinvestment of dividends.
Performance Graph (1)
*$100 invested on December 31, 2012 in stock or index, including reinvestment of dividends. Fiscal year ending December 31.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN
Among Ionis Pharmaceuticals, Inc., the Nasdaq Composite Index,
and the Nasdaq Biotechnology Index
|
|
Dec-12
|
Dec-13
|
Dec-14
|
Dec-15
|
Dec-16
|
Dec-17
|
||||||||||||||||||
|
Ionis Pharmaceuticals, Inc.
|
$
|
100.00
|
$
|
381.61
|
$
|
591.38
|
$
|
593.20
|
$
|
458.14
|
$
|
481.80
|
||||||||||||
|
Nasdaq Composite Index
|
$
|
100.00
|
$
|
141.63
|
$
|
162.09
|
$
|
173.33
|
$
|
187.19
|
$
|
242.29
|
||||||||||||
|
Nasdaq Biotechnology Index
|
$
|
100.00
|
$
|
174.05
|
$
|
230.33
|
$
|
244.29
|
$
|
194.95
|
$
|
228.29
|
||||||||||||
| (1) |
This section is not “soliciting material,” is not deemed “filed” with the SEC, is not subject to the liabilities of Section 18 of the Exchange Act and is not to be incorporated by reference in any of our filings under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
|
Item 6. Selected Financial Data
This selected financial data should be read in conjunction with our audited consolidated financial statements and accompanying notes and Management’s Discussion and Analysis of Financial Condition and Results of Operations included elsewhere in this Annual Report on Form 10-K. Our historical consolidated financial information may not be indicative of our future
47
performance. Set forth below are our selected consolidated financial data (in thousands, except per share amounts):
|
|
Years Ended December 31,
|
|||||||||||||||||||
|
|
2017
|
2016
|
2015
|
2014
|
2013
|
|||||||||||||||
|
Consolidated Statement of Operations Data:
|
||||||||||||||||||||
|
Revenue
|
$
|
507,666
|
$
|
346,620
|
$
|
283,703
|
$
|
214,161
|
$
|
147,285
|
||||||||||
|
Research, development and patent expenses
|
$
|
374,644
|
$
|
344,320
|
$
|
322,292
|
$
|
241,751
|
$
|
184,033
|
||||||||||
|
Net loss attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
(5,970
|
)
|
$
|
(86,556
|
)
|
$
|
(88,278
|
)
|
$
|
(38,984
|
)
|
$
|
(60,644
|
)
|
|||||
|
Basic net income (loss) per share attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
0.08
|
$
|
(0.72
|
)
|
$
|
(0.74
|
)
|
$
|
(0.33
|
)
|
$
|
(0.55
|
)
|
||||||
|
Diluted net income (loss) per share attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
0.08
|
$
|
(0.72
|
)
|
$
|
(0.74
|
)
|
$
|
(0.33
|
)
|
$
|
(0.55
|
)
|
||||||
|
Shares used in computing basic net income (loss) per share
|
124,016
|
120,933
|
119,719
|
117,691
|
110,502
|
|||||||||||||||
|
Shares used in computing diluted net income (loss) per share
|
126,098
|
120,933
|
119,719
|
117,691
|
110,502
|
|||||||||||||||
|
|
As of December 31,
|
|||||||||||||||||||
|
|
2017
|
2016
|
2015
|
2014
|
2013
|
|||||||||||||||
|
Consolidated Balance Sheet:
|
||||||||||||||||||||
|
Cash, cash equivalents and short-term investments
|
$
|
1,022,715
|
$
|
665,223
|
$
|
779,183
|
$
|
728,832
|
$
|
656,761
|
||||||||||
|
Working capital
|
$
|
943,243
|
$
|
664,148
|
$
|
688,127
|
$
|
721,265
|
$
|
637,698
|
||||||||||
|
Total assets
|
$
|
1,322,024
|
$
|
912,467
|
$
|
947,900
|
$
|
946,471
|
$
|
843,267
|
||||||||||
|
Long-term debt and other obligations, less current portion
|
$
|
678,564
|
$
|
679,118
|
$
|
598,234
|
$
|
588,896
|
$
|
367,065
|
||||||||||
|
Accumulated deficit
|
$
|
(1,187,398
|
)
|
$
|
(1,181,428
|
)
|
$
|
(1,094,872
|
)
|
$
|
(1,006,594
|
)
|
$
|
(967,610
|
)
|
|||||
|
Stockholders’ equity
|
$
|
418,719
|
$
|
99,565
|
$
|
200,790
|
$
|
257,780
|
$
|
378,390
|
||||||||||
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This financial review presents our operating results for each of the three years in the period ended December 31, 2017, and our financial condition at December 31, 2017. Except for the historical information contained herein, the following discussion contains forward-looking statements which are subject to known and unknown risks, uncertainties and other factors that may cause our actual results to differ materially from those expressed or implied by such forward-looking statements. We discuss such risks, uncertainties and other factors throughout this report and specifically under Item 1A of Part I of this report, “Risk Factors.” In addition, the following review should be read in conjunction with the information presented in our consolidated financial statements and the related notes to our consolidated financial statements as indexed on page F-1.
Overview
We are leaders in discovering and developing RNA-targeted therapeutics. We have created an efficient and broadly applicable drug discovery platform leveraging our expertise in antisense oligonucleotide therapeutics. Using this platform, we have developed a large, diverse and advanced pipeline of potentially first-in-class and/or best-in-class drugs that we believe can provide high value for patients with significant unmet medical needs. In this way, we believe we are fundamentally changing medicine with the goal to transform the lives of those suffering from severe, often life-threatening, diseases.
We made significant progress toward this goal with the commercial launch of SPINRAZA (nusinersen) for the treatment of SMA in pediatric and adult patients. SMA is a leading genetic cause of death in infants marked by progressive, debilitating muscle weakness. SPINRAZA became the first and only approved drug to treat people with SMA and is now the standard of care for this debilitating disease. Our partner, Biogen, is responsible for global commercial activities. In January 2018, Biogen reported that SPINRAZA was available in over 30 global markets. Additionally, Biogen is continuing to purse regulatory approvals for SPINRAZA in countries around the world. In 2017, we earned $113 million in commercial revenue from SPINRAZA royalties. We also earned a $50 million milestone payment for the EU approval of SPINRAZA and a $40 million milestone payment for SPINRAZA pricing approval in Japan.
Our pipeline also contains two near-term, potentially transformative medicines for two different severe and rare diseases, each with significant commercial potential, inotersen and volanesorsen. We believe inotersen has the potential to become the preferred treatment option for many people with hereditary TTR amyloidosis, or hATTR. Our goal is to free these people from the burden of their disease. hATTR is a debilitating, progressive, fatal disease in which patients experience a progressive buildup of amyloid plaque deposits in tissues throughout the body. In May 2017, we reported positive top-line data from our Phase 3 study of inotersen, NEURO-TTR, in patients with hATTR with polyneuropathy. More than half of these patients also have cardiomyopathy. We are advancing inotersen to the market based on the positive data from our NEURO-TTR study. In November 2017, we filed for marketing authorization for inotersen to treat people with hATTR in the U.S. and EU. The Food and Drug Administration, or FDA, accepted the inotersen New Drug Application, or NDA, for Priority Review and set a Prescription Drug User Fee Act, or PDUFA, date of July 6, 2018. The European Medicines Agency, or EMA, also granted accelerated assessment to inotersen, which may reduce standard review time. We are on track in our pre-commercial preparations for a potential launch in mid-2018, if inotersen is approved. Our goals for inotersen are to maximize the commercial potential of the drug, maximize our commercial participation and continue to build our TTR franchise by moving IONIS-TTR-LRx forward rapidly. We plan to commercialize inotersen in North America ourselves and to seek a commercial partner in other geographic regions.
48
Akcea Therapeutics, Inc., or Akcea, our affiliate focused on developing and commercializing drugs for serious cardiometabolic diseases caused by lipid disorders, is working closely with us to develop volanesorsen to treat two severe and rare, genetically defined diseases, familial chylomicronemia syndrome, or FCS, and familial partial lipodystrophy, or FPL. FCS and FPL are orphan diseases characterized by severely high triglyceride levels that result in severe, daily symptoms and a high risk of life-threatening pancreatitis. We estimate that FCS and FPL each affect 3,000 to 5,000 people globally. The clinical development program for volanesorsen consists of three Phase 3 studies called APPROACH, BROADEN and COMPASS. In the first quarter of 2017, we and Akcea reported positive Phase 3 data from the APPROACH study in patients with FCS. In December 2016, we and Akcea reported positive results from the Phase 3 COMPASS study in patients with triglycerides above 500 mg/dL. Based on the positive data from our Phase 3 studies, Akcea filed for marketing authorization for volanesorsen in the U.S., EU and Canada in the third quarter of 2017. The FDA set a PDUFA date of August 30, 2018 for volanesorsen and an advisory committee meeting is scheduled for May 10, 2018. Volanesorsen was granted Priority Review in Canada. Akcea is on track in its pre-commercial preparations for a potential launch in mid-2018, if volanesorsen is approved.
In addition to preparing to commercialize volanesorsen, Akcea is focused on developing and commercializing three other clinical-stage drugs for serious cardiometabolic diseases caused by lipid disorders: AKCEA-APO(a)-LRx, AKCEA-ANGPTL3-LRx and AKCEA-APOCIII-LRx, each of which could potentially treat multiple patient populations. Moving these drugs into Akcea allows us to retain substantial value from them and ensures our core focus remains on innovation. Akcea completed its initial public offering, or IPO, and a concurrent private placement with Novartis in July 2017, raising over $180 million in net proceeds. As a result of Akcea’s IPO and as of February 2018, we owned approximately 68 percent of Akcea.
We are addressing a broad spectrum of diseases that affect millions of people, such as cardiovascular disease, clotting disorders, Alzheimer’s and Parkinson’s disease. We also are addressing rare diseases, such as acromegaly, amyotrophic lateral sclerosis, beta-thalassemia and Huntington’s disease. We are continuing to advance our mid-stage drugs in development, which have the potential to enter late-stage clinical development and progress toward the market over the next several years, like IONIS-HTTRx. IONIS-HTTRx is the first drug in clinical development to target the cause of Huntington’s disease, or HD, by reducing the production of toxic mutant huntingtin, or mHTT, protein. In December 2017, following successful completion of the Phase 1/2 study in which IONIS-HTTRx demonstrated dose-dependent reductions of the mHTT protein in patients with HD, Roche licensed IONIS-HTTRx for $45 million. We plan to report data from this Phase 1/2 study in early 2018. We have also initiated an open-label extension, or OLE, study for people who participated in the Phase 1/2 study. Roche is now responsible for all IONIS-HTTRx development, regulatory and commercialization activities and costs.
We have established alliances with a cadre of leading global pharmaceutical companies that are working alongside us in developing our drugs, advancing our technology and preparing to commercialize our products. Our partners bring substantial resources and expertise that augment and build upon our internal capabilities. We have strategic partnerships with Biogen and AstraZeneca through which we can broadly expand our drug discovery efforts to new disease targets in specific therapeutic areas. We also have partnerships with Bayer, GSK, Janssen, Novartis and Roche. Each of these companies brings significant expertise and global resources to develop and potentially commercialize the drugs under these partnerships. Lastly, we also work with a group of companies that can develop our drugs and utilize our technologies outside our primary areas of focus. We refer to these companies as satellite companies.
Through our partnerships, we have created a broad and sustaining base of research and development, or R&D, revenue in the form of license fees, upfront payments and milestone payments while spending prudently to advance our pipeline and technology. Our R&D revenue has consistently grown year over year since 2011. In 2017, we earned $386 million in R&D revenue. Moreover, we have the potential to earn over $13 billion in future milestone payments and licensing fees from our current partnerships. We also have the potential to share in the future commercial success of our inventions and drugs resulting from our partnerships through royalty arrangements. In late 2016, we began adding commercial revenue from SPINRAZA royalties to our existing R&D revenue base. Looking forward, we have the potential to increase our commercial revenue from SPINRAZA royalties from the continued growth we expect in the U.S., EU and in other markets globally. We also have the potential to further increase our commercial revenue with volanesorsen and inotersen. We believe we have the key elements in place to achieve sustained, long-term financial growth, with multiple drivers of revenue; a mature, broad and rapidly-advancing clinical pipeline; a partnership strategy that leverages our partner resources; and an innovative drug discovery technology platform that we continue to deploy across a range of therapeutic areas to address both rare and large patient populations.
Financial Highlights
The following is a summary of our financial results (in thousands):
|
2017
|
2016
|
2015
|
||||||||||
|
Total revenue
|
$
|
507,666
|
$
|
346,620
|
$
|
283,703
|
||||||
|
Total operating expenses
|
$
|
483,132
|
$
|
392,936
|
$
|
359,465
|
||||||
|
Income (loss) from operations
|
$
|
24,534
|
$
|
(46,316
|
)
|
$
|
(75,762
|
)
|
||||
|
Net loss
|
$
|
(17,296
|
)
|
$
|
(86,556
|
)
|
$
|
(88,278
|
)
|
|||
|
Net loss attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
(5,970
|
)
|
$
|
(86,556
|
)
|
$
|
(88,278
|
)
|
|||
|
Cash, cash equivalents and short-term investments
|
$
|
1,022,715
|
$
|
665,223
|
$
|
779,183
|
||||||
We had a net loss attributable to our common stockholders of $6 million for 2017, compared to $87 million for 2016. Our net loss improved significantly due to the substantial revenue we earned in 2017. During 2017, we added $113 million of commercial revenue from SPRINRAZA royalties. Additionally, we earned R&D revenue of $386 million, including $90 million in milestone payments related to SPINRAZA, a nearly 20 percent increase over 2016.
49
Our operating expenses for 2017 were $483 million, and increased compared to $393 million for 2016. The increase in operating expenses was primarily due to higher SG&A expenses as we prepare to commercialize volanesorsen and inotersen in 2018. Our SG&A expenses also increased in 2017 compared to 2016 because of fees we owed under our in-licensing agreements related to SPINRAZA. Additionally, stock-based compensation expense increased year over year primarily due to Akcea stock option grants made to new employees as Akcea continues to build out its organization and additional stock option and RSU grants under the Ionis plan. During each of the years above, we were conducting several Phase 3 studies for SPINRAZA, volanesorsen and inotersen along with advancing numerous earlier-stage drugs. We are projecting an increase in our operating expenses for 2018, compared to 2017 primarily due to the cost of preparing for the launch of inotersen and volanesorsen.
During 2017, we received more than $580 million in payments from our partners, primarily from Novartis, Bayer and Biogen reflecting the successes of our partnered programs and drugs. In addition to cash and revenue, our partners provide expertise and additional resources, which we believe will maximize the commercial value of our partnered drugs. Additionally, our 2017 cash balance increased from the proceeds Akcea received from its IPO and Novartis’ concurrent strategic investment. We believe our strong financial position should enable us to continue to execute on our corporate goals throughout 2018 and beyond.
Business Segments
In 2015, we began reporting our financial results in two reportable segments, Ionis Core, and Akcea Therapeutics. Segment income (loss) from operations includes revenue less operating expenses attributable to each segment.
In our Ionis Core segment we are exploiting a novel drug discovery platform we created to generate a broad pipeline of first-in-class or best-in-class drugs for us and our partners. Our Ionis Core segment generates revenue from a multifaceted partnering strategy and includes multiple streams of revenue including license fees, milestone payments and royalties, among others.
Akcea Therapeutics is a late-stage biopharmaceutical company focused on developing and commercializing drugs to treat people with serious cardiometabolic diseases caused by lipid disorders. Prior to Akcea’s IPO in July 2017, we owned 100 percent of Akcea’s stock. After Akcea’s IPO, we owned approximately 68 percent of Akcea. We did not change our reportable segments as a result of Akcea’s IPO.
Critical Accounting Policies
We prepare our consolidated financial statements in conformity with accounting principles generally accepted in the United States. As such, we make certain estimates, judgments and assumptions that we believe are reasonable, based upon the information available to us. These judgments involve making estimates about the effect of matters that are inherently uncertain and may significantly impact our quarterly or annual results of operations and financial condition. Each quarter, our senior management reviews the development, selection and disclosure of such estimates with the audit committee of our board of directors. In the following paragraphs, we describe the specific risks associated with these critical accounting policies and we caution that future events rarely develop exactly as one may expect, and that best estimates may require adjustment.
The following are our significant accounting policies, which we believe are the most critical to aid in fully understanding and evaluating our reported financial results:
| |
Assessing the propriety of revenue recognition and associated deferred revenue;
|
| |
Determining the proper valuation of investments in marketable securities;
|
| |
Determining the appropriate cost estimates for unbilled preclinical studies and clinical development activities;
|
| |
Estimating the impact of the Tax Act and our net deferred income tax asset valuation allowance;
|
| |
Determining the fair value of convertible debt without the conversion feature; and
|
| |
Valuing premiums under our and Akcea’s Novartis collaboration.
|
Descriptions of these critical accounting policies follow.
Additionally, in January 2018, we adopted the new revenue recognition accounting guidance. As a result, our critical accounting policy for revenue recognition and associated deferred revenue will be updated in our 2018 consolidated financial statements. We are adopting the new standard on a retrospective basis, which means that starting with our first quarter financial statements for 2018 we will begin showing all periods presented using the new standard. The primary impact to our revenue relates to when we recognize milestone payments. Through 2017 under the existing accounting guidance, we recognized milestone payments we earned for performing R&D activities in full when achieved. Under the new guidance starting in 2018, we will now amortize those milestone payments over the period of time we are obligated to perform R&D activities for our partners. For example, in 2017 we initiated a Phase 1/2a clinical study of IONIS-MAPTRx in patients with mild AD. We earned a $10 million milestone payment from Biogen related to the initiation of this study. In 2017, we recognized the entire $10 million as revenue. Under the new standard, we will recognize this milestone payment over the period we are providing R&D services for Biogen. We will continue to recognize milestone payments we earn based on our partner’s activities in full when the milestone is achieved. For example, in 2017 we earned a $50 million milestone payment from Biogen for the EU approval of SPINRAZA. Under both the new and old standard, we account for this milestone payment the same by recognizing the entire amount upon achievement of the event.
Revenue Recognition
We generally recognize revenue when we have satisfied all contractual obligations and are reasonably assured of collecting the resulting receivable. We are often entitled to bill our customers and receive payment from our customers in advance of recognizing the revenue. In the instances in which we have received payment from our customers in advance of recognizing revenue, we include the amounts in deferred revenue on our consolidated balance sheet.
50
Commercial Revenue: SPINRAZA royalties and Licensing and other royalty revenue
We often enter into agreements to license and sell our technology on an exclusive or non-exclusive basis in exchange for upfront fees, license fees, milestone payments and/or royalties. We generally recognize as revenue immediately license payments with stand-alone value when the license is delivered and we are reasonably assured of collecting the resulting receivable. We recognize royalty revenue in the period in which the counterparty sells the related product, unless we are unable to obtain information to estimate the royalty. For example, in 2017 we recorded SPINRAZA royalty revenue of $112.5 million.
Research and development revenue under collaborative agreements
Arrangements with multiple deliverables
Our collaboration agreements typically contain multiple elements, or deliverables, including technology licenses or options to obtain technology licenses, research and development services, and in certain cases manufacturing services, and we allocate the consideration to each unit of accounting based on the relative selling price of each deliverable.
Amendments to agreements
From time to time we amend our collaboration agreements. For these agreements, before we identify our deliverables and allocate consideration to each unit of accounting, we must determine if the amendment should be accounted for as a separate agreement, or if the amendment and any undelivered elements for the original agreement should be accounted for as a single new arrangement.
For example, in May 2015, we entered into an exclusive license agreement with Bayer to develop and commercialize IONIS-FXIRx for the prevention of thrombosis. As part of the agreement, Bayer paid us a $100 million upfront payment. At the onset of the agreement, we were responsible for completing a Phase 2 study of IONIS-FXIRx in patients with end-stage renal disease on hemodialysis and for providing an initial supply of API. In February 2017, we amended our agreement with Bayer to advance IONIS-FXIRx and to initiate development of IONIS-FXI-LRx, which Bayer licensed. As part of the 2017 amendment, Bayer paid us $75 million. We are also eligible to receive milestone payments and tiered royalties on gross margins of IONIS-FXIRx and IONIS-FXI-LRx.
Under the 2017 amendment, there was a substantial increase in the consideration we are eligible to receive and a significant change in the deliverables we will provide to Bayer. As a result, we concluded that the amendment should be evaluated with the undelivered elements of the original agreement as a single new arrangement. Therefore, we evaluated our original and 2017 amended agreements with Bayer together to determine our deliverables. We concluded that the 2017 amendment did not impact the items we already delivered to Bayer.
Identifying deliverables and units of accounting
We evaluate the deliverables in our collaboration agreements to determine whether they meet the criteria to be accounted for as separate units of accounting or whether they should be combined with other deliverables and accounted for as a single unit of accounting. When the delivered items in an arrangement have "stand-alone value" to our customer, we account for the deliverables as separate units of accounting. Delivered items have stand-alone value if they are sold separately by any vendor or the customer could resell the delivered items on a stand-alone basis. For example, our 2017 amended agreement with Bayer has multiple elements. We evaluated the deliverables in this arrangement when we entered into the 2017 amended agreement and determined that certain of the deliverables have stand-alone value. Below is a list of the three units of accounting under our 2017 amended agreement:
| ● |
The exclusive license we granted to Bayer to develop and commercialize IONIS-FXI-LRx for the treatment of thrombosis;
|
| ● |
The development services we agreed to perform for IONIS-FXI-LRx and IONIS-FXIRx; and
|
| ● |
The remaining undelivered IONIS-FXIRx API that was part of the original agreement.
|
We determined that each of these three units of accounting have stand-alone value. The license we granted to Bayer has stand-alone value because it gives Bayer the exclusive right to develop IONIS-FXI-LRx or to sublicense its rights. The development services and the remaining undelivered supply of API each have stand-alone value because Bayer or another third party could provide these items without our assistance.
Measurement and allocation of arrangement consideration
Our collaborations may provide for various types of payments to us including upfront payments, funding of research and development, milestone payments, licensing fees and royalties on product sales. We initially allocate the amount of consideration that is fixed and determinable at the time the agreement is entered into and exclude contingent consideration. We allocate the consideration to each unit of accounting based on the relative selling price of each deliverable. We use the following hierarchy of values to estimate the selling price of each deliverable: (i) vendor-specific objective evidence of fair value; (ii) third-party evidence of selling price; and (iii) best estimate of selling price, or BESP. BESP reflects our best estimate of what the selling price would be if we regularly sold the deliverable on a stand-alone basis. We recognize the revenue allocated to each unit of accounting as we deliver the related goods or services. If we determine that we should treat certain deliverables as a single unit of accounting, then we recognize the revenue ratably over our estimated period of performance.
51
We determined that the allocable arrangement consideration for the Bayer 2017 amended agreement was $76.3 million, comprised of the $75 million we received as part of the amendment and the remaining amount of the $100 million upfront payment we had not yet recognized into revenue, related to the undelivered API. We allocated the consideration based on the relative BESP of each unit of accounting. We engaged a third party, independent valuation specialist to assist us with determining BESP. We estimated the selling price of the license granted for IONIS-FXI-LRx by using the relief from royalty method. Under this method, we estimated the amount of income, net of taxes, for IONIS-FXI-LRx. We then discounted the projected income to present value. The significant inputs we used to determine the projected income of the license included:
| ● |
Estimated future product sales;
|
| ● |
Estimated royalties on future product sales;
|
| ● |
Contractual milestone payments;
|
| ● |
Expenses we expect to incur;
|
| ● |
Income taxes; and
|
| ● |
An appropriate discount rate.
|
We estimated the selling price of the development services by using our internal estimates of the cost to perform the specific services and estimates of expected cash outflows to third parties for services and supplies over the expected period that we will perform the development services. The significant inputs we used to determine the selling price of the development services included:
| ● |
The number of internal hours we will spend performing these services;
|
| ● |
The estimated cost of work we will perform;
|
| ● |
The estimated cost of work that we will contract with third parties to perform; and
|
| ● |
The estimated cost of API we will use.
|
For purposes of determining BESP of the services we will perform and the API we will deliver in our 2017 amended Bayer transaction, accounting guidance required us to include a markup for a reasonable profit margin.
Based on the units of accounting under the 2017 amended agreement, we allocated the $76.3 million of allocable consideration as follows:
| ● |
$64.9 million to the IONIS-FXI-LRx exclusive license;
|
| ● |
$11.0 million for development services for IONIS-FXI-LRx and IONIS-FXIRx; and
|
| ● |
$0.4 million for the remaining delivery of IONIS-FXIRx API.
|
Assuming a constant selling price for the other elements in the arrangement, if there was an assumed 10 percent increase or decrease in the estimated selling price of the IONIS-FXI-LRx license, we determined that the revenue we would have allocated to the IONIS-FXI-LRx license would change by approximately one percent, or $0.7 million, from the amount we recorded.
Timing of revenue recognition
We recognize revenue as we deliver each item under the arrangement and the related revenue is realizable and earned. For example, we recognized revenue for the exclusive license we granted Bayer for IONIS-FXI-LRx in the first quarter of 2017 because that was when we delivered the license. We also recognize revenue over time as we provide services. Our collaborative agreements typically include a research and/or development project plan outlining the activities the agreement requires each party to perform during the collaboration. We estimate our period of performance at the inception of the agreement when the agreements we enter into do not clearly define such information. We then recognize revenue from development services ratably over such period. In certain instances, the period of performance may change as the development plans for our drugs progress. If our estimates and judgments change over the course of our collaboration agreements, it may affect the timing and amount of revenue that we recognize in future periods. We recognize any changes in estimates on a prospective basis.
The following are the periods over which we are recognizing revenue for each of our units of accounting under our 2017 amended Bayer agreement:
| ● |
We recognized the portion of the consideration attributed to the IONIS-FXI-LRx license in the first quarter of 2017 because we delivered the license and earned the revenue;
|
| ● |
We are recognizing the amount attributed to the development services for IONIS-FXI-LRx and IONIS-FXIRx over the period of time we are performing the services; and
|
| ● |
We are recognizing the amount attributed to the remaining API supply as we deliver it to Bayer.
|
Multiple agreements
From time to time, we may enter into separate agreements at or near the same time with the same partner. We evaluate such agreements to determine whether they should be accounted for individually as distinct arrangements or whether the separate agreements are, in substance, a single multiple element arrangement. We evaluate whether the negotiations are conducted jointly as part of a single negotiation, whether the deliverables are interrelated or interdependent, whether fees in one arrangement are tied to performance in another arrangement, and whether elements in one arrangement are essential to another arrangement. Our evaluation involves significant judgment to determine whether a group of agreements might be so closely related that they are, in effect, part of a single arrangement. For example, in the first quarter of 2017, we and Akcea entered into two separate agreements with Novartis at the same time: a collaboration agreement and a SPA.
52
Akcea entered into a collaboration agreement with Novartis to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Under the collaboration agreement, Akcea received a $75 million upfront payment. For each drug, Akcea is responsible for completing a Phase 2 program, conducting an end-of-Phase 2 meeting with the FDA and delivering API. Under the collaboration agreement, Novartis has an exclusive option to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. If Novartis exercises an option for one of these drugs, Novartis will pay Akcea a $150 million license fee and will assume all further global development, regulatory and commercialization activities and costs for the licensed drug. Akcea is also eligible to receive a development milestone payment, milestone payments if Novartis achieves pre-specified regulatory milestones, commercial milestones and tiered royalties on net sales from each drug under the collaboration.
Under the SPA, Novartis purchased 1.6 million shares of Ionis’ common stock for $100 million in the first quarter of 2017 and paid a premium over the weighted average trading price at the time of purchase. Additionally, the SPA required Novartis to purchase $50 million of Akcea’s common stock in a concurrent private placement with Akcea’s IPO in July 2017.
We evaluated the Novartis agreements to determine whether we should treat the agreements separately or as a single arrangement. We considered that the agreements were negotiated concurrently and in contemplation of one another. Additionally, the same individuals were involved in the negotiations of both agreements. Based on these facts and circumstances, we concluded that we should treat both agreements as a single arrangement and evaluate the provisions of the agreements on a combined basis. Refer to Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements for further discussion of the accounting treatment for the Novartis collaboration.
Milestone payments
Our collaborations often include contractual milestones, which typically relate to the achievement of pre-specified development, regulatory and/ or commercialization events. These three categories of milestone events reflect the three stages of the life-cycle of our drugs, which we describe in more detail in the following paragraphs.
Prior to the first stage in the life-cycle of our drugs, we perform a significant amount of work using our proprietary antisense technology to design chemical compounds that interact with specific genes that are good targets for drug discovery. From these research efforts, we hope to identify a development candidate. The designation of a development candidate is the start of the development stage, which is the first stage in the life-cycle of our drugs. A development candidate is a chemical compound that has demonstrated the necessary safety and efficacy in preclinical animal studies to warrant further study in humans.
During the first step of the development stage, we or our partners study our drugs in Investigational New Drug, or IND,-enabling studies, which are animal studies intended to support an IND application and/or the foreign equivalent. An approved IND allows us or our partners to study our development candidate in humans. If the regulatory agency approves the IND, we or our partners initiate a Phase 1 clinical trial in which we typically enroll a small number of healthy volunteers to ensure the development candidate is safe for use in patients. If we or our partners determine that a development candidate is safe based on the Phase 1 data, we or our partners initiate Phase 2 studies that are generally larger studies in patients with the primary intent of determining the preliminary efficacy and safety of the development candidate.
The final step in the development stage is Phase 3 studies to gather the necessary safety and efficacy data to request marketing authorization from the FDA and/or foreign equivalents. Phase 3 studies typically involve larger numbers of patients and can take up to several years to complete.
If the data gathered during the Phase 3 trials demonstrates acceptable safety and efficacy results, we or our partner will submit an application to the FDA and/or its foreign equivalents for marketing authorization. This stage of the drug’s life-cycle is the regulatory stage.
If the FDA or a foreign equivalent grants marketing authorization for a drug, it moves into the commercialization stage. During this stage we or our partner will market and sell the drug to patients. Although our partner may ultimately be responsible for marketing and selling a partnered drug, our efforts to discover and develop a drug that is safe, effective and reliable contributes significantly to our partner’s ability to successfully sell the drug. The FDA and its foreign equivalents have the authority to impose significant restrictions on an approved drug through the product label and on advertising, promotional and distribution activities. Therefore, our efforts designing and executing the necessary animal and human studies are critical to obtaining claims in the product label from the regulatory agencies that would allow us or our partner to successfully commercialize our drug. Further, the patent protection afforded our drugs as a result of our initial patent applications and related prosecution activities in the United States and foreign jurisdictions are critical to our partner’s ability to sell our drugs without competition from generic drugs. The potential sales volume of an approved drug is dependent on several factors including the size of the patient population, market penetration of the drug, and the price charged for the drug.
Generally, the milestone events contained in our partnership agreements coincide with the progression of our drugs from development, to marketing authorization and then to commercialization. The process of successfully discovering a new development candidate, having it approved and ultimately selling it for a profit is highly uncertain. As such, the milestone payments we may earn from our partners involve a significant degree of risk to achieve. Therefore, as a drug progresses through the stages of its life-cycle, the value of the drug generally increases.
Development milestones in our partnerships may include the following types of events:
| ● |
Designation of a development candidate. Following the designation of a development candidate, IND-enabling animal studies for a new development candidate generally take 12 to 18 months to complete.
|
| ● |
Initiation of a Phase 1 clinical trial. Generally, Phase 1 clinical trials take one to two years to complete.
|
| ● |
Initiation or completion of a Phase 2 clinical trial. Generally, Phase 2 clinical trials take one to three years to complete.
|
| ● |
Initiation or completion of a Phase 3 clinical trial. Generally, Phase 3 clinical trials take two to four years to complete.
|
53
Regulatory milestones in our partnerships may include the following types of events:
| ● |
Filing of regulatory applications for marketing authorization such as a New Drug Application, or NDA, in the United States or a Marketing Authorization Application, or MAA, in Europe. Generally, it takes six to twelve months to prepare and submit regulatory filings.
|
| ● |
Obtaining marketing authorization in a major market, such as the United States, Europe or Japan. Generally it takes one to two years after an application is submitted to obtain authorization from the applicable regulatory agency.
|
Commercialization milestones in our partnerships may include the following types of events:
| ● |
First commercial sale in a particular market, such as in the United States or Europe.
|
| ● |
Product sales in excess of a pre-specified threshold, such as annual sales exceeding $1 billion. The amount of time to achieve this type of milestone depends on several factors including but not limited to the dollar amount of the threshold, the pricing of the product and the pace at which customers begin using the product.
|
We assess whether a substantive milestone exists at the inception of our agreements. When a substantive milestone is achieved, we recognize revenue related to the milestone payment immediately. For our licensing and collaboration agreements in which we are involved in the discovery and/or development of the related drug or provide the partner with access to new technologies we discover, we have determined that the majority of future development, regulatory and commercialization milestones are substantive. For example, we consider most of the milestones associated with our strategic alliance with Biogen substantive because we are using our antisense drug discovery platform to discover and develop new drugs against targets for neurological diseases. In evaluating if a milestone is substantive we consider whether:
| ● |
Substantive uncertainty exists as to the achievement of the milestone event at the inception of the arrangement;
|
| ● |
The achievement of the milestone involves substantive effort and can only be achieved based in whole or in part on our performance or the occurrence of a specific outcome resulting from our performance;
|
| ● |
The amount of the milestone payment appears reasonable either in relation to the effort expended or to the enhancement of the value of the delivered items;
|
| ● |
There is no future performance required to earn the milestone; and
|
| ● |
The consideration is reasonable relative to all deliverables and payment terms in the arrangement.
|
If any of these conditions are not met, we do not consider the milestone to be substantive and we defer recognition of the milestone payment and recognize it as revenue over our estimated period of performance, if any. Further information about our collaborative arrangements can be found in Note 6, Collaborative Arrangements and Licensing Agreements, in the Notes to the Consolidated Financial Statements.
Option to license
In several of our collaboration agreements, we provide our partner with an option to obtain a license to one or more of our drugs. When we have a multiple element arrangement that includes an option to obtain a license, we evaluate if the option is a deliverable at the inception of the arrangement. We do not consider the option to be a deliverable if we conclude that it is substantive and not priced at a significant and incremental discount. We consider an option substantive if, at the inception of the arrangement, we are at risk as to whether our collaboration partner will choose to exercise its option to obtain the license. In those circumstances, we do not include the associated license fee in the allocable consideration at the inception of the agreement. Rather, we account for the license fee when our partner exercises its option. For example, during 2017, we earned license fee revenue when three of our partners, Bayer, Janssen and Roche, exercised their options to license three of our drugs, which under the respective agreements we concluded to be substantive options at inception. As a result, in 2017 we recognized the related revenue immediately in research and development revenue under collaborative agreements on our statement of operations as these amounts relate to drugs in development under research and development collaboration arrangements.
Valuation of Investments in Marketable Securities
We consider all liquid investments with maturities of three months or less when we purchase them to be cash equivalents. Our short-term investments have initial maturities of greater than three months from date of purchase. We classify our short-term investments as “available-for-sale” and carry them at fair market value based upon prices for identical or similar items on the last day of the fiscal period. We record unrealized gains and losses as a separate component of comprehensive income (loss) and include net realized gains and losses in gain (loss) on investments. We use the specific identification method to determine the cost of securities sold.
We use a three-tier fair value hierarchy to prioritize the inputs used in our fair value measurements. These tiers include: Level 1, defined as observable inputs such as quoted prices in active markets for identical assets, which includes our money market funds and treasury securities classified as available-for-sale securities and our investment in equity securities in publicly held biotechnology companies; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable, which includes our fixed income securities and commercial paper classified as available-for-sale securities; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring us to develop our own assumptions. We classify the majority of our securities as Level 2. We obtain the fair value of our Level 2 investments from our custodian bank or from a professional pricing service. We validate the fair value of our Level 2 investments by understanding the pricing model used by the custodian banks or professional pricing service provider and comparing that fair value to the fair value based on observable market prices.
54
Estimated Liability for Clinical Development Costs
We record accrued liabilities related to expenses for which service providers have not yet billed us. These liabilities are for products or services that we have received, specifically related to ongoing preclinical studies and clinical trials. These costs primarily relate to third-party clinical management costs, laboratory and analysis costs, toxicology studies and investigator grants. We have numerous drugs in concurrent preclinical studies and clinical trials at several clinical sites throughout the world. In order to ensure that we have adequately provided for ongoing preclinical and clinical development costs during the period in which we incur such costs, we maintain an accrual to cover these costs. We update our estimate for this accrual on at least a quarterly basis. The assessment of these costs is a subjective process that requires judgment. Upon settlement, these costs may differ materially from the amounts accrued in our consolidated financial statements. Our historical accrual estimates have not been materially different from our actual amounts.
Estimating the Impact of the Tax Cuts and Jobs Act of 2017 and Our Net Deferred Income Tax Asset Valuation Allowance
On December 22, 2017, the Tax Act was signed into law. The Tax Act makes broad and complex changes to the U.S. tax code. The changes include, but are not limited to, reducing the U.S. federal corporate tax rate from 35 percent to 21 percent, imposing a mandatory one-time transition tax on certain unrepatriated earnings of foreign subsidiaries, introducing bonus depreciation that will allow for full expensing of qualified property, eliminating the corporate alternative minimum tax, or AMT, and changing how existing AMT credits can be realized, and modifying or repealing many business tax deductions and credits.
The SEC staff issued guidance to address the application of U.S. GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the Tax Act.
In accordance with the SEC guidance, the amounts we presented are preliminary and our best estimate of the impact of the Tax Act in the period ending December 31, 2017 based on our understanding of the Tax Act and guidance available as of the date of this filing. We remeasured our existing net U.S. deferred tax assets using the enacted tax rate and other known significant changes to the tax code. This resulted in a total decrease in these assets by $107.3 million which was fully offset by a decrease in our valuation allowance. In addition, we recorded a $7.7 million tax benefit related to our cumulative prior year AMT tax credit carryovers, which are now included as part of a long-term income tax receivable because under the Tax Act, AMT credits are refundable from 2018 through 2021.
We account for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in our financial statements or tax returns. In addition, deferred tax assets are recorded for the future benefit of utilizing net operating losses and research and development credit carryforwards. Valuation allowances are provided when necessary to reduce deferred tax assets to the amount expected to be realized.
We apply the authoritative accounting guidance prescribing a threshold and measurement attribute for the financial recognition and measurement of a tax position taken or expected to be taken in a tax return. We recognize liabilities for uncertain tax positions based on a two-step process. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step requires us to estimate and measure the tax benefit as the largest amount that is more than 50 percent likely to be realized upon ultimate settlement.
We are required to use significant judgment in evaluating our uncertain tax positions and determining our provision for income taxes. Although we believe our reserves are reasonable, no assurance can be given that the final tax outcome of these matters will not be different from that which is reflected in our historical income tax provisions and accruals. We adjust these reserves for changing facts and circumstances, such as the closing of a tax audit or the refinement of an estimate. To the extent that the final tax outcome of these matters is different than the amounts recorded, such differences may impact the provision for income taxes in the period in which such determination is made.
We are also required to use significant judgment in determining any valuation allowance recorded against our deferred tax assets. In assessing the need for a valuation allowance, we consider all available evidence, including scheduled reversal of deferred tax liabilities, past operating results, the feasibility of tax planning strategies and estimates of future taxable income. Estimates of future taxable income are based on assumptions that are consistent with our plans. The assumptions we use represent our best estimates and involve inherent uncertainties and the application of our judgment. Should actual amounts differ from our estimates, the amount of our tax expense and liabilities we recognize could be materially impacted.
We record a valuation allowance to reduce the balance of our net deferred tax assets to the amount we believe is more-likely-than-not to be realized. We have incurred historical financial statement losses and as a result we have a full valuation allowance recorded against our net deferred tax assets. We regularly assess the future realization of our net deferred tax assets and will reduce the valuation allowance in any such period in which we determine that all, or a portion, of our deferred tax assets are more-likely-than-not to be realized.
We do not provide for a U.S. income tax liability and foreign withholding taxes on undistributed foreign earnings of our foreign subsidiaries.
55
Convertible Debt
We account for our convertible debt instruments that may be settled in cash upon conversion (including partial cash settlement) by separating the liability and equity components of the instruments in a manner that reflects our nonconvertible debt borrowing rate. We determine the carrying amount of the liability component by measuring the fair value of similar debt instruments that do not have the conversion feature. If a similar debt instrument does not exist, we estimate the fair value by using assumptions that market participants would use in pricing a debt instrument, including market interest rates, credit standing, yield curves and volatilities. Determining the fair value of the debt component requires the use of accounting estimates and assumptions. These estimates and assumptions are judgmental in nature and could have a significant impact on the determination of the debt component and the associated non-cash interest expense.
We assigned a value to the debt component of our convertible notes equal to the estimated fair value of similar debt instruments without the conversion feature, which resulted in us recording the debt at a discount. We are amortizing our debt issuance costs and our debt discount over the life of the convertible notes as additional non-cash interest expense utilizing the effective interest method. For additional information, see Note 3, Long-Term Obligations and Commitments, in the Notes to the Consolidated Financial Statements.
Valuation of Premiums under our and Akcea’s Novartis Collaboration
During the first quarter of 2017, we valued the premiums under the SPA agreement with Novartis. These premiums included the premium Novartis paid us related to its $100 million purchase of our stock in the first quarter of 2017 and the premium we could have received related to Novartis’ potential purchase of our stock. These valuations required us to use level 3 inputs, which we consider to be a critical accounting policy for our results for 2017.
We determined the fair value of the premium we received and the future premium we could have received by using the stated premium in the SPA and applying a lack of marketability discount. We included a lack of marketability discount in our valuation of the premiums because Novartis received unregistered shares as part of Novartis’ $100 million equity purchase and we would have issued unregistered shares to Novartis if it had purchased our common stock. Additionally, for the future potential stock purchase, we estimated the probability of an Akcea IPO. At the inception of the agreements, we calculated the following fair values:
| ● |
$28.4 million for the premium paid by Novartis for its purchase of our common stock in the first quarter of 2017; and
|
| ● |
$5.0 million for the potential premium Novartis would have paid if it had purchased our common stock in the future at a premium.
|
Because Akcea completed its IPO before April 2018, Novartis will not purchase additional shares of Ionis stock. Therefore, this asset no longer had any value and we wrote-off the remaining potential premium Novartis would have paid to us if an Akcea IPO did not occur. We wrote off the amount to other expenses on our consolidated statement of operations during the third quarter of 2017. See further discussion about our valuation of the potential premium in our Fair Value Measurements policy in Note 1, Organization and Significant Accounting Policies, in the Notes to the Consolidated Financial Statements.
Results of Operations
Years Ended December 31, 2017 and December 31, 2016
Revenue
Total revenue for 2017 was $507.7 million, compared to $346.6 million for 2016. See below for our discussion of the changes in our revenue.
Commercial Revenue
SPINRAZA Royalties
2017 was the first full year in which we earned commercial revenue from SPINRAZA royalties. Commercial revenue from SPINRAZA royalties for 2017 was $112.5 million, compared to $0.9 million in 2016.
Licensing and Other Royalty Revenue
Our revenue from licensing activities and other royalties for 2017 was $9.5 million, compared to $19.8 million for 2016. During 2016 we earned $15 million from Kastle when it acquired the global rights to develop and commercialize Kynamro.
Research and Development Revenue Under Collaborative Agreements
Research and development revenue under collaborative agreements for 2017 was $385.6 million, compared to $325.9 million for 2016. The change in our R&D revenue was primarily due to increased amortization from the upfront payment Akcea received in 2017 from the collaboration with Novartis. Our R&D revenue for 2017 primarily consisted of the following:
| ● |
$118 million in milestone payments from Biogen, including $90 million in approval milestone payments for SPINRAZA, $15 million in milestone payments for validating two undisclosed neurological disease targets and $10 million for initiating a Phase 1/2a study of IONIS-MAPTRx;
|
| ● |
$65 million from Bayer for the license of IONIS-FXI-LRx;
|
| ● |
$48 million from Roche primarily for the license of IONIS-HTTRx;
|
| ● |
$10 million from Janssen for the license of IONIS-JBI2-2.5Rx and initiation of a Phase 1 study of IONIS-JBI1-2.5Rx:
|
| ● |
$115 million from the amortization of upfront fees; and
|
| ● |
$29.6 million primarily from services we performed for our partners.
|
56
Our R&D revenue may fluctuate quarterly based on the nature and timing of payments under agreements with our partners and consists primarily of revenue from the amortization of upfront fees, milestone payments and license fees.
Operating Expenses
Operating expenses for 2017 were $483.1 million, and increased compared to $392.9 million for 2016. Our operating expenses increased year over year principally due to higher SG&A expenses as we prepare to commercialize volanesorsen and inotersen. Our SG&A expenses also increased in 2017 compared to 2016 because of fees we owed under our in-licensing agreements related to SPINRAZA.
Our operating expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
305,352
|
$
|
260,233
|
||||
|
Akcea Therapeutics
|
146,332
|
73,363
|
||||||
|
Elimination of intercompany activity
|
(54,527
|
)
|
(12,768
|
)
|
||||
|
Subtotal
|
397,157
|
320,828
|
||||||
|
Non-cash compensation expense related to equity awards
|
85,975
|
72,108
|
||||||
|
Total operating expenses
|
$
|
483,132
|
$
|
392,936
|
||||
In order to analyze and compare our results of operations to other similar companies, we believe it is important to exclude non-cash compensation expense related to equity awards from our operating expenses. We believe non-cash compensation expense is not indicative of our operating results or cash flows from our operations. Further, we internally evaluate the performance of our operations excluding it.
Research, Development and Patent Expenses
Our research, development and patent expenses consist of expenses for antisense drug discovery, antisense drug development, manufacturing and operations and R&D support expenses.
The following table sets forth information on research, development and patent expenses (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Research, development and patent expenses, excluding non-cash compensation expense related to equity awards
|
$
|
310,123
|
$
|
289,221
|
||||
|
Non-cash compensation expense related to equity awards
|
64,521
|
55,099
|
||||||
|
Total research, development and patent expenses
|
$
|
374,644
|
$
|
344,320
|
||||
For 2017, our research, development and patent expenses were $310.1 million, compared to $289.2 million for 2016. Our research, development and patent expenses increased slightly primarily related to expenses such as regulatory filings, manufacturing initial launch supplies and medical affairs activities in support of inotersen and volanesorsen. If you exclude these expenses, our research, development and patent expenses decreased year-over-year; demonstrating we can prudently manage our research, development and patent expenses, even while advancing and expanding our pipeline, because of the efficiency of antisense technology and the contributions of our partners. All amounts exclude non-cash compensation expense related to equity awards.
Our research, development and patent expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
246,390
|
$
|
238,106
|
||||
|
Akcea Therapeutics
|
118,260
|
63,883
|
||||||
|
Elimination of intercompany activity
|
(54,527
|
)
|
(12,768
|
)
|
||||
|
Subtotal
|
310,123
|
289,221
|
||||||
|
Non-cash compensation expense related to equity awards
|
64,521
|
55,099
|
||||||
|
Total research, development and patent expenses
|
$
|
374,644
|
$
|
344,320
|
||||
Antisense Drug Discovery
We use our proprietary antisense technology to generate information about the function of genes and to determine the value of genes as drug discovery targets. We use this information to direct our own antisense drug discovery research, and that of our partners. Antisense drug discovery is also the function that is responsible for advancing our antisense core technology.
As we continue to advance our antisense technology, we are investing in our drug discovery programs to expand our and our partners’ drug pipelines. We anticipate that our existing relationships and collaborations, as well as prospective new partners, will continue to help fund our research programs and contribute to the advancement of the science by funding core antisense technology research.
57
Our antisense drug discovery expenses were as follows (in thousands) and are part of our Ionis Core business segment:
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Antisense drug discovery expenses, excluding non-cash compensation expense related to equity awards
|
$
|
56,160
|
$
|
51,028
|
||||
|
Non-cash compensation expense related to equity awards
|
15,203
|
13,589
|
||||||
|
Total antisense drug discovery expenses
|
$
|
71,363
|
$
|
64,617
|
||||
Antisense drug discovery expenses for 2017 were $56.2 million and were slightly higher compared to $51.0 million for 2016, due to expenses we incurred related to advancing our early stage research programs. All amounts exclude non-cash compensation expense related to equity awards.
Antisense Drug Development
The following table sets forth research and development expenses for our major antisense drug development projects (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
SPINRAZA
|
$
|
10,996
|
$
|
43,868
|
||||
|
Volanesorsen
|
22,524
|
26,285
|
||||||
|
Inotersen
|
24,880
|
22,939
|
||||||
|
Other antisense development projects
|
70,009
|
42,999
|
||||||
|
Development overhead expenses
|
43,784
|
39,398
|
||||||
|
Total antisense drug development, excluding non-cash compensation expense related to equity awards
|
172,193
|
175,489
|
||||||
|
Non-cash compensation expense related to equity awards
|
25,737
|
20,116
|
||||||
|
Total antisense drug development expenses
|
$
|
197,930
|
$
|
195,605
|
||||
Antisense drug development expenses were $172.2 million for 2017 and were essentially flat compared to $175.5 million for 2016. As we projected, the expenses for SPINRAZA and volanesorsen declined in 2017. Specifically, we have transitioned all further development of SPINRAZA to Biogen and we are finishing our Phase 3 volanesorsen trial in people with FCS. Additionally, we completed our Phase 3 inotersen trial in people with hATTR with polyneuropathy. Our 2017 expenses included $4.8 million of expenses related to regulatory filing activities for volanesorsen and inotersen. Additionally, during 2017, we made investments in our other antisense development projects, including AKCEA-APO(a)-LRx and IONIS-FXIRx. All amounts exclude non-cash compensation expense related to equity awards.
Our antisense drug development expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
122,163
|
$
|
132,418
|
||||
|
Akcea Therapeutics
|
98,425
|
43,071
|
||||||
|
Elimination of intercompany activity
|
(48,395
|
)
|
—
|
|||||
|
Subtotal
|
172,193
|
175,489
|
||||||
|
Non-cash compensation expense related to equity awards
|
25,737
|
20,116
|
||||||
|
Total antisense drug development expenses
|
$
|
197,930
|
$
|
195,605
|
||||
We may conduct multiple clinical trials on a drug candidate, including multiple clinical trials for the various indications we may be studying. Furthermore, as we obtain results from trials we may elect to discontinue clinical trials for certain drug candidates in certain indications in order to focus our resources on more promising drug candidates or indications. Our Phase 1 and Phase 2 programs are clinical research programs that fuel our Phase 3 pipeline. When our products are in Phase 1 or Phase 2 clinical trials, they are in a dynamic state in which we may adjust the development strategy for each product. Although we may characterize a product as "in Phase 1" or "in Phase 2," it does not mean that we are conducting a single, well-defined study with dedicated resources. Instead, we allocate our internal resources on a shared basis across numerous products based on each product's particular needs at that time. This means we are constantly shifting resources among products. Therefore, what we spend on each product during a particular period is usually a function of what is required to keep the products progressing in clinical development, not what products we think are most important. For example, the number of people required to start a new study is large, the number of people required to keep a study going is modest and the number of people required to finish a study is large. However, such fluctuations are not indicative of a shift in our emphasis from one product to another and cannot be used to accurately predict future costs for each product. And, because we always have numerous drugs in preclinical and early stage clinical research, the fluctuations in expenses from drug to drug, in large part, offset one another. If we partner a drug, it may affect the size of a trial, its timing, its total cost and the timing of the related costs.
58
Medical Affairs
Our medical affairs function is responsible for performing further research regarding our drugs to ensure appropriate medical use. In addition, members of our medical affairs team educate the medical community about the diseases our drugs are designed to treat.
Expenditures in our medical affairs function include personnel costs and outside services.
Our medical affairs expenses were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Medical affairs expenses, excluding non-cash compensation expense related to equity awards
|
$
|
9,097
|
$
|
3,568
|
||||
|
Non-cash compensation expense related to equity awards
|
2,588
|
1,264
|
||||||
|
Total medical affairs expenses
|
$
|
11,685
|
$
|
4,832
|
||||
Medical affairs expenses were $9.1 million for 2017 and were higher compared to $3.6 million for 2016. The increase was primarily due to the build-out of our medical affairs teams and associated activities to educate the medical community on FCS and hATTR. All amounts exclude non-cash compensation expense related to equity awards.
Our medical affairs expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
1,771
|
$
|
—
|
||||
|
Akcea Therapeutics
|
7,326
|
3,568
|
||||||
|
Subtotal
|
9,097
|
3,568
|
||||||
|
Non-cash compensation expense related to equity awards
|
2,588
|
1,264
|
||||||
|
Total medical affairs expenses
|
$
|
11,685
|
$
|
4,832
|
||||
Manufacturing and Operations
Expenditures in our manufacturing and operations function consist primarily of personnel costs, specialized chemicals for oligonucleotide manufacturing, laboratory supplies and outside services. Our manufacturing and operations function is responsible for providing drug supplies to antisense drug development, Akcea and our collaboration partners. Our manufacturing procedures include testing to satisfy good laboratory and good manufacturing practice requirements.
Our manufacturing and operations expenses were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Manufacturing and operations expenses, excluding non-cash compensation expense related to equity awards
|
$
|
43,526
|
$
|
30,148
|
||||
|
Non-cash compensation expense related to equity awards
|
6,904
|
6,113
|
||||||
|
Total manufacturing and operations expenses
|
$
|
50,430
|
$
|
36,261
|
||||
Manufacturing and operations expenses were $43.5 million for 2017 and were higher compared to $30.1 million for 2016. $11 million of the increase in manufacturing expenses was related to volanesorsen and inotersen to prepare for the planned launches in mid-2018, if approved. All amounts exclude non-cash compensation expense related to equity awards.
Our manufacturing and operations expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
39,098
|
$
|
27,341
|
||||
|
Akcea Therapeutics
|
10,440
|
15,455
|
||||||
|
Elimination of intercompany activity
|
(6,012
|
)
|
(12,648
|
)
|
||||
|
Subtotal
|
43,526
|
30,148
|
||||||
|
Non-cash compensation expense related to equity awards
|
6,904
|
6,113
|
||||||
|
Total manufacturing and operations expenses
|
$
|
50,430
|
$
|
36,261
|
||||
59
R&D Support
In our research, development and patent expenses, we include support costs such as rent, repair and maintenance for buildings and equipment, utilities, depreciation of laboratory equipment and facilities, amortization of our intellectual property, informatics costs, procurement costs and waste disposal costs. We call these costs R&D support expenses.
The following table sets forth information on R&D support expenses (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Personnel costs
|
$
|
11,432
|
$
|
11,560
|
||||
|
Occupancy
|
8,236
|
7,891
|
||||||
|
Patent expenses
|
2,095
|
3,945
|
||||||
|
Depreciation and amortization
|
249
|
245
|
||||||
|
Insurance
|
1,735
|
1,344
|
||||||
|
Other
|
5,400
|
4,003
|
||||||
|
Total R&D support expenses, excluding non-cash compensation expense related to equity awards
|
29,147
|
28,988
|
||||||
|
Non-cash compensation expense related to equity awards
|
14,089
|
14,017
|
||||||
|
Total R&D support expenses
|
$
|
43,236
|
$
|
43,005
|
||||
R&D support expenses for 2017 were $29.1 million and were flat compared to $29.0 million for 2016. All amounts exclude non-cash compensation expense related to equity awards.
Our R&D support expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
27,198
|
$
|
27,319
|
||||
|
Akcea Therapeutics
|
2,069
|
1,789
|
||||||
|
Elimination of intercompany activity
|
(120
|
)
|
(120
|
)
|
||||
|
Subtotal
|
29,147
|
28,988
|
||||||
|
Non-cash compensation expense related to equity awards
|
14,089
|
14,017
|
||||||
|
Total R&D support expenses
|
$
|
43,236
|
$
|
43,005
|
||||
Selling, General and Administrative Expenses
Selling, general and administrative expenses include costs associated with the pre-commercialization activities for our drugs and costs to support our company, our employees and our stockholders. These costs include personnel and outside costs in the areas of pre-commercialization, legal, human resources, investor relations, and finance. Additionally, we include in selling, general and administrative expenses such costs as rent, repair and maintenance of buildings and equipment, depreciation and utilities costs that we need to support the corporate functions listed above. We also include fees we owed under our in-licensing agreements related to SPINRAZA in our SG&A expenses.
The following table sets forth information on selling, general and administrative expenses (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Selling, general and administrative expenses, excluding non-cash compensation expense related to equity awards
|
$
|
87,034
|
$
|
31,607
|
||||
|
Non-cash compensation expense related to equity awards
|
21,454
|
17,009
|
||||||
|
Total selling, general and administrative expenses
|
$
|
108,488
|
$
|
48,616
|
||||
Selling, general and administrative expenses were $87.0 million for 2017 and significantly increased compared to $31.6 million for 2016. The increase in SG&A expenses was principally due to the cost of preparing to commercialize volanesorsen and inotersen in mid-2018 and from fees we owed under our in-licensing agreements related to SPINRAZA. We project our expenses will increase if SPINRAZA sales continue to grow and as we continue to prepare to launch inotersen and Akcea continues to prepare to launch volanesorsen. All amounts exclude non-cash compensation expense related to equity awards.
Our selling, general and administrative expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Ionis Core
|
$
|
58,962
|
$
|
22,127
|
||||
|
Akcea Therapeutics
|
28,072
|
9,480
|
||||||
|
Non-cash compensation expense related to equity awards
|
21,454
|
17,009
|
||||||
|
Total selling general and administrative expenses
|
$
|
108,488
|
$
|
48,616
|
||||
60
Akcea Therapeutics, Inc.
The following table sets forth information on operating expenses (in thousands) for our Akcea Therapeutics business segment:
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Development and patent expenses
|
$
|
118,260
|
$
|
63,883
|
||||
|
General and administrative expenses
|
28,072
|
9,480
|
||||||
|
Total operating expenses, excluding non-cash compensation expense related to equity awards
|
146,332
|
73,363
|
||||||
|
Non-cash compensation expense related to equity awards
|
17,539
|
10,149
|
||||||
|
Total Akcea Therapeutics operating expenses
|
$
|
163,871
|
$
|
83,512
|
||||
Operating expenses for Akcea were $146.3 million for 2017 and increased compared to $73.4 million for 2016.
$48.4 million of the increase in Akcea’s development and patent expenses was for one-time sublicensing expenses related to the Novartis collaboration recorded in the first quarter of 2017. $33.4 million of these expenses were non-cash and the remaining $15 million was paid to us. For each period presented, we allocated a portion of Ionis’ R&D support expenses, which are included in development and patent expenses in the table above, to Akcea for work we performed on behalf of Akcea.
Akcea’s G&A expenses increased in 2017, compared to 2016, primarily due to Akcea continuing to build its commercial infrastructure and advance the pre-commercialization activities necessary to successfully launch volanesorsen in mid-2018, if approved. During the first quarter of 2017, we and Akcea reported positive results from the APPROACH Phase 3 study of volanesorsen in people with FCS. During the third quarter of 2017, Akcea, working closely with us, filed for marketing approval in the U.S., EU and Canada. For each period presented, we allocated a portion of Ionis' G&A expenses, which were included in Akcea’s G&A expenses in the table above, to Akcea for work we performed on Akcea’s behalf.
All amounts exclude non-cash compensation expense related to equity awards.
Investment Income
Investment income for 2017 was $7.8 million compared to $5.5 million for 2016. Investment income increased primarily due a higher average cash balance and an improvement in the market conditions during 2017 compared to 2016.
Interest Expense
Interest expense for 2017 was $44.8 million, compared to $38.8 million for 2016. The increase was primarily non-cash expense related to amortization of debt issuance costs for our 1 percent notes.
Interest expense includes non-cash amortization of the debt discount and debt issuance costs plus interest expense payable in cash for our 1 percent and 2¾ percent notes, non-cash interest expense related to the long-term financing liability, which was replaced by mortgage debt for our primarily R&D and manufacturing facilities beginning in July 2017 and other miscellaneous debt.
In July 2017, we purchased the building that houses our primary R&D facility and the building that houses our manufacturing facility for $79.4 million and $14.0 million, respectively. As a result of the purchase of our primary R&D facility, we extinguished the financing liability we had previously recorded on our balance sheet. We financed the purchase of the buildings with mortgage debt of $51.3 million with an interest rate of 3.88 percent for our primary R&D facility and mortgage debt of $9.1 million with an interest rate of 4.2 percent for our manufacturing facility. Both mortgages mature in August 2027. The non-cash interest expense for our long-term financing liability was replaced with lower mortgage interest expense.
The following table sets forth information on interest expense (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Convertible notes:
|
||||||||
|
Non-cash amortization of the debt discount and debt issuance costs
|
$
|
32,536
|
$
|
25,115
|
||||
|
Interest expense payable in cash
|
7,090
|
6,684
|
||||||
|
Non-cash interest expense for long-term financing liability
|
3,352
|
6,693
|
||||||
|
Interest on mortgage for primary R&D and manufacturing facilities
|
1,103
|
—
|
||||||
|
Other
|
671
|
303
|
||||||
|
Total interest expense
|
$
|
44,752
|
$
|
38,795
|
||||
61
Loss on Extinguishment of Financing Liability for Leased Facility
We recognized a loss on extinguishment of the financing liability for leased facility of $7.7 million in 2017. The loss represents the difference between the amount we previously recorded as a financing liability for the leased facility and the purchase price we paid for our primary R&D facility in July 2017. This loss was non-cash and nonrecurring.
Early Retirement of Debt
As a result of the debt exchange we completed in December 2016, we recorded a $4.0 million non-cash loss on early retirement of debt, reflecting the early retirement of the majority of our remaining 2¾ percent convertible notes in December 2016. We did not recognize any loss on early retirement of debt in 2017.
Other Expenses
Other expenses were $3.5 million for 2017 and primarily consisted of the previously capitalized fair value of the potential premium we would have received from Novartis if Akcea had not completed its IPO. This expense was non-cash and nonrecurring.
Income Tax Benefit (Expense)
We are subject to U.S. federal, state and foreign taxes. In 2017, we recorded a net income tax benefit of $6.0 million, compared to income tax expense of $2.9 million in 2016. Our tax expense flipped from an expense position in 2016 to a benefit position in 2017 primarily due to a $7.7 million reduction in our valuation allowance. As a result of the Tax Act, we reduced our valuation allowance because we are entitled to receive a tax refund for our cumulative prior year alternative minimum tax credit carryforwards. At December 31, 2017 we retained a full valuation allowance against the net balance of our remaining deferred tax assets.
Net Loss
We had a net loss of $17.3 million for 2017, compared to $86.6 million for 2016. Our net loss improved for 2017 compared to 2016 primarily due to the addition of commercial revenue from SPINRAZA royalties and increased R&D revenue.
Net Operating Loss and Tax Credit Carryforwards
At December 31, 2017, we had federal and California tax net operating loss carryforwards of approximately $561.1 million and $887.1 million, respectively. Our federal tax loss carryforwards begin to expire in 2024. A portion of our California tax loss carryforwards continued to expire in 2017. At December 31, 2017, we also had federal and California research and development tax credit carryforwards of approximately $233.3 million and $56.2 million, respectively. Our Federal research and development tax credit carryforwards will begin to expire in 2018. Our California research and development tax credit carryforwards are available indefinitely.
Net Loss Attributable to Noncontrolling Interest in Akcea Therapeutics, Inc.
As a result of Akcea’s IPO, beginning in July 2017, we no longer own 100 percent of Akcea. From the closing of Akcea’s IPO on July 19, 2017 through the end of 2017, we owned approximately 68 percent of Akcea. As a result, we adjusted our financial statements to reflect the portion of Akcea we no longer own, which was 32 percent at December 31, 2017. Accordingly, our consolidated statement of operations now includes a new line called “Net loss attributable to noncontrolling interests in Akcea”, our noncontrolling interest in Akcea for 2017 was $11.3 million. We also added a corresponding account to our consolidated balance sheet called “Noncontrolling interest in Akcea Therapeutics, Inc.”
Net Loss Attributable to Ionis Pharmaceuticals, Inc. Common Stockholders and Net Income (Loss) per Share
We had a net loss attributable to our common stockholders’ of $6.0 million for 2017, compared to $86.6 million in 2016. Basic and diluted net income per share for 2017 was $0.08 compared to a net loss per share of $0.72 for 2016.
Years Ended December 31, 2016 and December 31, 2015
Revenue
Total revenue for 2016 was $346.6 million compared to $283.7 million for 2015.
Commercial Revenue
SPINRAZA Royalties
SPINRAZA was approved by the FDA in December 2017. Commercial revenue from SPINRAZA royalties for 2016 was $0.9 million.
Licensing and Other Royalty Revenue
Our revenue from licensing activities and royalties for 2016 was $19.8 million, compared to $2.3 million for 2015. Our revenue from licensing and royalties for 2016 primarily consisted of the $15 million we earned from Kastle when it acquired the global rights to develop and commercialize Kynamro.
62
Research and Development Revenue Under Collaborative Agreements
Research and development revenue under collaborative agreements for 2016 was $325.9 million compared to $281.4 million for 2015. We earned $115.7 million in milestone payments and $91.2 million when Bayer licensed IONIS-FXIRx during 2016 compared to milestone payments of $135.0 million in 2015. Our revenue in 2016 was primarily comprised of:
| |
$170 million from Biogen for FDA approval, licensing and advancing the Phase 3 program for SPINRAZA;
|
| |
$53 million from AstraZeneca for advancing and licensing IONIS-KRAS-2.5Rx and selecting IONIS-AZ4-2.5-LRx to move into development;
|
| |
$15 million from Janssen for licensing IONIS-JBI1-2.5Rx and selecting an additional development candidate;
|
| |
$7.5 million from Biogen for advancing IONIS-SOD1Rx, IONIS-BIIB4Rx and IONIS-BIIB6Rx;
|
| |
$61 million from the amortization of upfront fees; and
|
| |
$19.4 million primarily from the manufacturing services we performed for our partners.
|
Operating Expenses
Operating expenses for 2016 were $392.9 million, and increased compared to $359.5 million for 2015. The increase in operating expenses was primarily due to:
| |
During 2016, we were conducting five Phase 3 studies and three open-label extension studies for SPINRAZA, inotersen and volanesorsen. We completed target enrollment in four of these Phase 3 studies at the end of 2015, and as a result, these studies were in their most expensive stage during 2016.
|
| |
Akcea’s operating expenses increased as it continued to build its commercial infrastructure and advance the pre-commercialization activities necessary to successfully launch volanesorsen, if approved for marketing.
|
| |
Our non-cash compensation expense related to equity awards increased due to an increase in the exercise price of the stock options we have granted over the past several years.
|
Our operating expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Ionis Core
|
$
|
260,233
|
$
|
256,674
|
||||
|
Akcea Therapeutics
|
73,363
|
46,252
|
||||||
|
Elimination of intercompany activity
|
(12,768
|
)
|
(2,775
|
)
|
||||
|
Subtotal
|
320,828
|
300,151
|
||||||
|
Non-cash compensation expense related to equity awards
|
72,108
|
59,314
|
||||||
|
Total operating expenses
|
$
|
392,936
|
$
|
359,465
|
||||
Research, Development and Patent Expenses
The following table sets forth information on research, development and patent expenses (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Research, development and patent expenses, excluding non-cash compensation expense related to equity awards
|
$
|
289,221
|
$
|
278,654
|
||||
|
Non-cash compensation expense related to equity awards
|
55,099
|
43,638
|
||||||
|
Total research, development and patent expenses
|
$
|
344,320
|
$
|
322,292
|
||||
Our research, development and patent expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Ionis Core
|
$
|
238,106
|
$
|
240,061
|
||||
|
Akcea Therapeutics
|
63,883
|
41,368
|
||||||
|
Elimination of intercompany activity
|
(12,768
|
)
|
(2,775
|
)
|
||||
|
Subtotal
|
289,221
|
278,654
|
||||||
|
Non-cash compensation expense related to equity awards
|
55,099
|
43,638
|
||||||
|
Total research, development and patent expenses
|
$
|
344,320
|
$
|
322,292
|
||||
For 2016, total research, development and patent expenses were $289.2 million, compared to $278.7 million for 2015, and were slightly higher primarily due to the progression of our drugs in Phase 3 development. All amounts exclude non-cash compensation expense related to equity awards.
63
Antisense Drug Discovery
Our antisense drug discovery expenses were as follows (in thousands) and are part of our Ionis Core business segment:
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Antisense drug discovery expenses, excluding non-cash compensation expense related to equity awards
|
$
|
51,028
|
$
|
49,331
|
||||
|
Non-cash compensation expense related to equity awards
|
13,589
|
11,914
|
||||||
|
Total antisense drug discovery expenses
|
$
|
64,617
|
$
|
61,245
|
||||
Antisense drug discovery expenses for 2016 were $51.0 million and were slightly higher compared to $49.3 million for 2015, All amounts exclude non-cash compensation expense related to equity awards.
Antisense Drug Development
The following table sets forth research and development expenses for our major antisense drug development projects (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
SPINRAZA
|
$
|
43,868
|
$
|
35,164
|
||||
|
Volanesorsen
|
26,285
|
21,348
|
||||||
|
Inotersen
|
22,939
|
19,560
|
||||||
|
Other antisense development products
|
42,999
|
59,599
|
||||||
|
Development overhead expenses
|
39,398
|
36,117
|
||||||
|
Total antisense drug development, excluding non-cash compensation expense related to equity awards
|
175,489
|
171,788
|
||||||
|
Non-cash compensation expense related to equity awards
|
20,116
|
16,108
|
||||||
|
Total antisense drug development expenses
|
$
|
195,605
|
$
|
187,896
|
||||
Antisense drug development expenditures were $175.5 million for 2016 compared to $171.8 million for 2015. Expenses in 2016 were slightly higher compared to 2015 primarily due to the progression of our drugs in Phase 3 development. As drugs move forward to more advanced stages of development, including into larger, longer clinical studies, the costs of development increase. Our other antisense development project expenses declined in 2016, compared to 2015, primarily due to completing the FOCUS FH Phase 3 study of Kynamro in 2015 and our shift to LICA drugs, which were in less expensive stages of development. All amounts exclude non-cash compensation expense related to equity awards.
Our antisense drug development expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Ionis Core
|
$
|
132,418
|
$
|
137,092
|
||||
|
Akcea Therapeutics
|
43,071
|
34,696
|
||||||
|
Non-cash compensation expense related to equity awards
|
20,116
|
16,108
|
||||||
|
Total antisense drug development expenses
|
$
|
195,605
|
$
|
187,896
|
||||
Medical Affairs
Our medical affairs expenses were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Medical affairs expenses, excluding non-cash compensation expense related to equity awards
|
$
|
3,568
|
$
|
429
|
||||
|
Non-cash compensation expense related to equity awards
|
1,264
|
100
|
||||||
|
Total medical affairs expenses
|
$
|
4,832
|
$
|
529
|
||||
Medical affairs expenses were $3.6 million for 2016 and were higher compared to $0.4 million for 2015. The increase was primarily due to the build-out of our medical affairs team and associated activities to educate the medical community on FCS. All amounts exclude non-cash compensation expense related to equity awards. All of our medical affairs expenses for 2016 and 2015 related to our Akcea segment.
64
Manufacturing and Operations
Our manufacturing and operations expenses were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Manufacturing and operations expenses, excluding non-cash compensation expense related to equity awards
|
$
|
30,148
|
$
|
28,588
|
||||
|
Non-cash compensation expense related to equity awards
|
6,113
|
4,563
|
||||||
|
Total manufacturing and operations expenses
|
$
|
36,261
|
$
|
33,151
|
||||
Manufacturing and operations expenses for 2016 were $30.1 million and were slightly higher compared to $28.6 million for 2015. All amounts exclude non-cash compensation expense related to equity awards.
Our manufacturing and operations expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Ionis Core
|
$
|
27,341
|
$
|
25,632
|
||||
|
Akcea Therapeutics
|
15,455
|
5,611
|
||||||
|
Elimination of intercompany activity
|
(12,648
|
)
|
(2,655
|
)
|
||||
|
Subtotal
|
30,148
|
28,588
|
||||||
|
Non-cash compensation expense related to equity awards
|
6,113
|
4,563
|
||||||
|
Total manufacturing and operations expenses
|
$
|
36,261
|
$
|
33,151
|
||||
R&D Support
The following table sets forth information on R&D support expenses (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Personnel costs
|
$
|
11,560
|
$
|
10,210
|
||||
|
Occupancy
|
7,891
|
7,854
|
||||||
|
Patent expenses
|
3,945
|
2,785
|
||||||
|
Depreciation and amortization
|
245
|
2,911
|
||||||
|
Insurance
|
1,344
|
1,320
|
||||||
|
Other
|
4,003
|
3,438
|
||||||
|
Total R&D support expenses, excluding non-cash compensation expense related to equity awards
|
28,988
|
28,518
|
||||||
|
Non-cash compensation expense related to equity awards
|
14,017
|
10,953
|
||||||
|
Total R&D support expenses
|
$
|
43,005
|
$
|
39,471
|
||||
R&D support expenses for 2016 were $29.0 million, and were essentially flat compared to $28.5 million for 2015. All amounts exclude non-cash compensation expense related to equity awards.
Our R&D support expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Ionis Core
|
$
|
27,319
|
$
|
28,005
|
||||
|
Akcea Therapeutics
|
1,789
|
633
|
||||||
|
Elimination of intercompany activity
|
(120
|
)
|
(120
|
)
|
||||
|
Subtotal
|
28,988
|
28,518
|
||||||
|
Non-cash compensation expense related to equity awards
|
14,017
|
10,953
|
||||||
|
Total R&D support expenses
|
$
|
43,005
|
$
|
39,471
|
||||
General and Administrative Expenses
The following table sets forth information on general and administrative expenses (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
General and administrative expenses, excluding non-cash compensation expense related to equity awards
|
$
|
31,607
|
$
|
21,497
|
||||
|
Non-cash compensation expense related to equity awards
|
17,009
|
15,676
|
||||||
|
Total general and administrative expenses
|
$
|
48,616
|
$
|
37,173
|
||||
65
General and administrative expenses for 2016 were $31.6 million and increased compared to $21.5 million for 2015 primarily due to expenses associated with Akcea building its organization. All amounts exclude non-cash compensation expense related to equity awards.
Our general and administrative expenses by segment were as follows (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Ionis Core
|
$
|
22,127
|
$
|
16,613
|
||||
|
Akcea Therapeutics
|
9,480
|
4,884
|
||||||
|
Non-cash compensation expense related to equity awards
|
17,009
|
15,676
|
||||||
|
Total general and administrative expenses
|
$
|
48,616
|
$
|
37,173
|
||||
Akcea Therapeutics, Inc.
The following table sets forth information on operating expenses (in thousands) for our Akcea Therapeutics business segment:
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Development and patent expenses
|
$
|
63,883
|
$
|
41,368
|
||||
|
General and administrative expenses
|
9,480
|
4,884
|
||||||
|
Total operating expenses, excluding non-cash compensation expense related to equity awards
|
73,363
|
46,252
|
||||||
|
Non-cash compensation expense related to equity awards
|
10,149
|
6,496
|
||||||
|
Total Akcea Therapeutics operating expenses
|
$
|
83,512
|
$
|
52,748
|
||||
Akcea’s operating expenses were $73.4 million for 2016 and increased compared to $46.3 million for 2015. The increase in expenses was primarily because Akcea was conducting more and later-stage clinical studies in 2016 than it conducted in 2015, including the continuation of the Phase 3 studies for volanesorsen in people with FCS and FPL. In 2016, we began charging Akcea for Ionis’ internal development costs associated with the ongoing work we are performing for Akcea's drugs. For each period presented, we allocated a portion of Ionis' R&D support expenses, which are included in research and development expenses in the table above, to Akcea for work we performed on behalf of Akcea.
Akcea also incurred additional general and administrative costs as it continued to build its organization and advance the pre-commercialization activities necessary to launch volanesorsen, if approved for marketing. For each year presented, we allocated a portion of Ionis' general and administrative expenses, which are included in general and administrative expenses in the table above, to Akcea for work we performed on Akcea’s behalf. All amounts exclude non-cash compensation expense related to equity awards.
Investment Income
Investment income for 2016 totaled $5.5 million compared to $4.4 million for 2015. The increase in investment income was primarily due an improvement in the market conditions during 2016 compared to 2015.
Interest Expense
The following table sets forth information on interest expense (in thousands):
|
Year Ended
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Convertible notes:
|
||||||||
|
Non-cash amortization of the debt discount and debt issuance costs
|
$
|
25,115
|
$
|
23,208
|
||||
|
Interest expense payable in cash
|
6,684
|
6,683
|
||||||
|
Non-cash interest expense for long-term financing liability
|
6,693
|
6,665
|
||||||
|
Other
|
303
|
176
|
||||||
|
Total interest expense
|
$
|
38,795
|
$
|
36,732
|
||||
Interest expense for 2016 was $38.8 million, and was relatively flat compared to $36.7 million in 2015.
Gain on Investment in Regulus Therapeutics Inc.
In 2015, we recorded a gain on our investment in Regulus of $20.2 million related to our sale of a portion of our Regulus common stock.
66
Early Retirement of Debt
As a result of the debt exchange we completed in December 2016, we recorded a $4.0 million non-cash loss on early retirement of debt, reflecting the early retirement of the majority of our remaining 2¾ percent convertible notes in December 2016. We did not recognize any loss on early retirement of debt in 2015.
Income Tax Expense (Benefit)
In 2016, we recorded a net tax expense of $2.9 million, compared to $0.4 million in 2015. Our tax expense increased in 2016 compared to 2015 primarily due to the taxable income resulting from our strong financial performance in 2016 and excess tax benefits related to stock-based compensation. Included in our tax expense for 2015 is $4.3 million of tax benefit we recorded in 2015 related to a tax refund we received in 2015 from the State of California Franchise Tax Board related to the California franchise taxes we paid for the tax year ended December 31, 2009.
Net Loss and Net Loss Per Share
Net loss for 2016 was $86.6 million compared $88.3 million for 2015. Basic and diluted net loss per share for the year ended December 31, 2016 was $0.72 compared to $0.74 for 2015. We had a lower net loss in 2016 primarily due to the increase in revenue we earned in 2016 compared to 2015.
Liquidity and Capital Resources
We have financed our operations with revenue primarily from research and development collaborative agreements Beginning in December 2016 we added commercial revenue from SPINRAZA royalties. From our inception through December 31, 2017, we had earned approximately $2.6 billion in revenue. We also financed our operations through the sale of our equity securities and the issuance of long-term debt. From the time we were founded through December 31, 2017, we had raised net proceeds of approximately $1.2 billion from the sale of our equity securities, not including the $182.4 million Akcea received in net proceeds from its IPO in July 2017. Additionally, we had borrowed approximately $1.4 billion under long-term debt arrangements to finance a portion of our operations over the same time period.
At December 31, 2017, we had cash, cash equivalents and short-term investments of $1.0 billion and stockholders’ equity of $418.7 million. In comparison, we had cash, cash equivalents and short-term investments of $665.2 million and stockholders’ equity of $99.6 million at December 31, 2016. During 2017, we received more than $580 million in payments from our partners, primarily from Novartis, Bayer and Biogen. Additionally, our cash balance at December 31, 2017 included the proceeds from Akcea’s IPO and Novartis’ strategic investment received in the third quarter of 2017.
In July 2017, we purchased two buildings that house our primary R&D facility and our manufacturing facility for $79.4 million and $14.0 million, respectively. In conjunction with the purchase of the buildings we obtained a $51.4 million mortgage for our primary R&D facility and a $9.1 million mortgage for our manufacturing facility. Both mortgages mature in August 2027. We expect these transactions will result in cash and expense savings for us.
At December 31, 2017, we had consolidated working capital of $943.2 million compared to $664.1 million at December 31, 2016. Working capital increased in 2017 primarily due to the increase in our cash, cash equivalents and short-term investments as a result of the substantial payments we received from partners and Akcea’s IPO during 2017.
As of December 31, 2017, our debt and other obligations totaled $759.8 million compared to $774.2 million at December 31, 2016.
The following table summarizes our contractual obligations as of December 31, 2017. The table provides a breakdown of when obligations become due. We provide a more detailed description of the major components of our debt in the paragraphs following the table:
|
|
Payments Due by Period (in millions)
|
|||||||||||||||||||
|
Contractual Obligations
(selected balances described below)
|
Total
|
Less than
1 year
|
1-3 years
|
3-5 years
|
After
5 years
|
|||||||||||||||
|
Convertible senior notes (principal and interest payable)
|
$
|
712.9
|
$
|
6.9
|
$
|
13.7
|
$
|
692.3
|
$
|
—
|
||||||||||
|
Building mortgage payments
|
$
|
83.2
|
$
|
2.4
|
$
|
4.8
|
$
|
5.1
|
$
|
70.9
|
||||||||||
|
Financing arrangements (principal and interest payable)
|
$
|
13.0
|
$
|
0.3
|
$
|
12.7
|
$
|
—
|
$
|
—
|
||||||||||
|
Other obligations (principal and interest payable)
|
$
|
1.1
|
$
|
0.1
|
$
|
0.1
|
$
|
0.1
|
$
|
0.8
|
||||||||||
|
Operating leases
|
$
|
2.1
|
$
|
0.9
|
$
|
1.1
|
$
|
0.1
|
$
|
—
|
||||||||||
|
Total
|
$
|
812.3
|
$
|
10.6
|
$
|
32.4
|
$
|
697.6
|
$
|
71.7
|
||||||||||
Our contractual obligations consist primarily of our convertible debt. In addition, we also have facility mortgages, facility leases, equipment financing arrangements and other obligations. Due to the uncertainty with respect to the timing of future cash flows associated with our unrecognized tax benefits, we are unable to make reasonably reliable estimates of the period of cash settlement with the respective taxing authorities. Therefore, we have excluded $78 million of gross unrecognized tax benefits from our contractual obligations table above.
67
In November 2014, we completed a $500 million offering of convertible senior notes, which mature in 2021 and bear interest at 1 percent. We used a substantial portion of the net proceeds from the issuance of the 1 percent convertible senior notes to repurchase $140 million in principal of our 2¾ percent convertible senior notes. As a result, the principal balance of the 2¾ percent notes following the repurchase in November 2014 was $61.2 million.
In December 2016, we issued an additional $185.5 million of 1 percent convertible senior notes in exchange for the redemption of $61.1 million of our 2¾ percent convertible senior notes. At December 31, 2017, we had a nominal amount of our 2¾ percent convertible senior notes outstanding. At December 31, 2017, we had the following 1 percent convertible senior notes outstanding (amounts in millions except price per share data):
|
1 Percent Convertible
Senior Notes
|
||||
|
Outstanding principal balance
|
$
|
685.5
|
||
|
Original issue date ($500 million of principal)
|
November 2014
|
|||
|
Additional issue date ($185.5 million of principal)
|
December 2016
|
|||
|
Maturity date
|
November 2021
|
|||
|
Interest rate
|
1 percent
|
|||
|
Conversion price per share
|
$
|
66.81
|
||
|
Total shares of common stock subject to conversion
|
10.3
|
|||
Interest is payable semi-annually for the 1 percent notes. The notes are convertible under certain conditions, at the option of the note holders. We settle conversions of the notes, at our election, in cash, shares of our common stock or a combination of both. We may not redeem the 1 percent notes prior to maturity, and no sinking fund is provided for them. Holders of the 1 percent notes may require us to purchase some or all of their notes upon the occurrence of certain fundamental changes, as set forth in the indenture governing the 1 percent notes, at a purchase price equal to 100 percent of the principal amount of the notes to be purchased, plus accrued and unpaid interest.
Financing Arrangements
In June 2015, we entered into a five-year revolving line of credit agreement with Morgan Stanley Private Bank, National Association, or Morgan Stanley. We amended the credit agreement in February 2016 to increase the amount available for us to borrow. Under the amended credit agreement, Morgan Stanley will provide a maximum of $30 million of revolving credit for general working capital purposes. Any loans under the credit agreement have interest payable monthly in arrears at a borrowing rate based on our option of:
|
(i)
|
a floating rate equal to the one-month London Interbank Offered Rate, or LIBOR, in effect plus 1.25 percent per annum;
|
|
(ii)
|
a fixed rate equal to LIBOR plus 1.25 percent for a period of one, two, three, four, six, or twelve months as elected by us; or
|
|
(iii)
|
a fixed rate equal to the LIBOR swap rate during the period of the loan.
|
Additionally, we pay 0.25 percent per annum, payable quarterly in arrears, for any amount unused under the credit facility. As of December 31, 2017, we had $12.5 million in outstanding borrowings under the credit facility with a 2.31 percent fixed interest rate and a maturity date of September 2019, which we used to fund our capital equipment needs consistent with our historical practice to finance these costs.
The credit agreement includes customary affirmative and negative covenants and restrictions. We are in compliance with all covenants of the credit agreement.
Research and Development and Manufacturing Facilities
In July 2017, we purchased the building that houses our primary R&D facility for $79.4 million. As a result of the purchase, we extinguished the financing liability we had previously recorded on our balance sheet. The difference between the purchase price of the facility and the carrying value of our financing liability at the time of the purchase was $7.7 million. We recognized this amount as a loss on extinguishment of financing liability for leased facility in our consolidated results of operations in the third quarter of 2017.
We purchased our manufacturing facility in July 2017 for $14.0 million. We previously accounted for the lease on this facility as an operating lease. We capitalized the purchase price of the building as a fixed asset in the third quarter of 2017.
We financed the purchase of our primary R&D facility and manufacturing facility, with mortgage debt of $51.3 million and $9.1 million, respectively. Our primary R&D facility mortgage has an interest rate of 3.88 percent. Our manufacturing facility mortgage has an interest rate of 4.20 percent. During the first five years of both mortgages we are only required to make interest payments. Both mortgages mature in August 2027.
Other Obligations
In addition to contractual obligations, we had outstanding purchase orders as of December 31, 2017 for the purchase of services, capital equipment and materials as part of our normal course of business.
68
We plan to continue to enter into collaborations with partners to provide for additional revenue to us and we may incur additional cash expenditures related to our obligations under any of the new agreements we may enter into. We currently intend to use our cash, cash equivalents and short-term investments to finance our activities. However, we may also pursue other financing alternatives, like issuing additional shares of our common stock, issuing debt instruments, refinancing our existing debt, or securing lines of credit. Whether we use our existing capital resources or choose to obtain financing will depend on various factors, including the future success of our business, the prevailing interest rate environment and the condition of financial markets generally.
Off-Balance Sheet Arrangements
We have not entered into, nor do we currently have, any off-balance sheet arrangements (as defined under SEC rules).
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We are exposed to changes in interest rates primarily from our long-term debt arrangements and, secondarily, investments in certain short-term investments. We primarily invest our excess cash in highly liquid short-term investments of the U.S. Treasury and reputable financial institutions, corporations, and U.S. government agencies with strong credit ratings. We typically hold our investments for the duration of the term of the respective instrument. We do not utilize derivative financial instruments, derivative commodity instruments or other market risk sensitive instruments, positions or transactions to manage exposure to interest rate changes. Accordingly, we believe that, while the securities we hold are subject to changes in the financial standing of the issuer of such securities, we are not subject to any material risks arising from changes in interest rates, foreign currency exchange rates, commodity prices, equity prices or other market changes that affect market risk sensitive instruments.
Item 8. Financial Statements and Supplementary Data
We filed our consolidated financial statements and supplementary data required by this item as exhibits hereto, and listed them under Item 15(a)(1) and (2), and incorporate them herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or Exchange Act) that are designed to ensure that information we are required to disclose in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. We designed and evaluate our disclosure controls and procedures recognizing that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance and not absolute assurance of achieving the desired control objectives.
As of the end of the period covered by this report on Form 10-K, we carried out an evaluation of our disclosure controls and procedures under the supervision of, and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer. Based on our evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2017.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rules 13a-15(f). Our internal control over financial reporting is a process designed under the supervision of our Chief Executive Officer and Chief Financial Officer to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our financial statements for external purposes in accordance with United States generally accepted accounting principles.
As of December 31, 2017, we assessed the effectiveness of our internal control over financial reporting based on the criteria for effective internal control over financial reporting under the 2013 “Internal Control—Integrated Framework,” issued by the Committee of Sponsoring Organizations, or COSO, of the Treadway Commission, under the supervision of, and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer. Based on that assessment, our management concluded that we maintained effective internal control over financial reporting as of December 31, 2017.
Attestation Report of the Registered Public Accounting Firm
Ernst & Young LLP, an independent registered public accounting firm, audited the effectiveness of our internal control over financial reporting as of December 31, 2017, as stated in their attestation report, which is included elsewhere herein.
Changes in Internal Control over Financial Reporting
The above assessment did not identify any change in our internal control over financial reporting that occurred during our latest fiscal quarter and that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
69
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Ionis Pharmaceuticals, Inc.
Opinion on Internal Control over Financial Reporting
We have audited Ionis Pharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework) (the COSO criteria). In our opinion, Ionis Pharmaceuticals, Inc. (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2017, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of Ionis Pharmaceuticals, Inc. as of December 31, 2017 and 2016, and the related consolidated statements of operations, comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2017, and the related notes and our report dated February 28, 2018 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| /s/ ERNST & YOUNG LLP
|
|
|
|
|
|
San Diego, California
|
|
|
February 28, 2018
|
|
70
Item 9B. Other Information
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
We incorporate by reference the information required by this Item with respect to directors and the Audit Committee from the information under the caption “ELECTION OF DIRECTORS,” including in particular the information under “Nominating, Governance and Review Committee” and “Audit Committee,” contained in our definitive Proxy Statement, which we will file with the Securities and Exchange Commission within 120 days after the end of the fiscal year ended December 31, 2017 (the “Proxy Statement”).
We incorporate by reference the required information concerning our Code of Ethics from the information under the caption “Code of Ethics and Business Conduct” contained in the Proxy Statement. Our Code of Ethics and Business Conduct is posted on our website at www.ionispharma.com(1). We intend to disclose future amendments to, or waivers from, our Code of Ethics and Business Conduct on our website.
Item 1, Part I of this Report contains information concerning our executive officers. We incorporate by reference the information required by this Item concerning compliance with Section 16(a) of the Exchange Act from the information under the caption “Section 16(a) Beneficial Ownership Reporting Compliance” contained in the Proxy Statement.
________________
|
(1)
|
Any information that is included on or linked to our website is not part of this Form 10-K.
|
Item 11. Executive Compensation
We incorporate by reference the information required by this item to the information under the caption “EXECUTIVE COMPENSATION,” “Compensation Committee Interlocks and Insider Participation” and “COMPENSATION COMMITTEE REPORT” contained in the Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
We incorporate by reference the information required by this item to the information under the captions “SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT” contained in the Proxy Statement.
Securities Authorized for Issuance under Equity Compensation Plans
The following table sets forth information regarding outstanding options and shares reserved for future issuance under our equity compensation plans as of December 31, 2017.
|
Plan Category
|
Number of Shares
to be Issued Upon Exercise of Outstanding Options |
Weighted Average
Exercise Price of Outstanding Options |
Number of Shares
Remaining Available for Future Issuance |
|
|||||||||
|
Equity compensation plans approved by stockholders(a)
|
9,396,796
|
$
|
44.52
|
8,158,366
|
(b)
|
||||||||
|
Total
|
9,396,796
|
$
|
44.52
|
8,158,366
|
|
||||||||
________________
| (a) |
Consists of four Ionis plans: 1989 Stock Option Plan, Amended and Restated 2002 Non-Employee Directors’ Stock Option Plan, 2011 Equity Incentive Plan and Employee Stock Purchase Plan, or ESPP.
|
| (b) |
Of these shares, 668,232 remained available for purchase under the ESPP as of December 31, 2017. The ESPP incorporates an evergreen formula pursuant to which on January 1 of each year, we automatically increase the aggregate number of shares reserved for issuance under the plan by 150,000 shares.
|
Item 13. Certain Relationships and Related Transactions, and Director Independence
We incorporate by reference the information required by this item to the information under the captions “Independence of the Board of Directors” and “Certain Relationships and Related Transactions” contained in the Proxy Statement.
Item 14. Principal Accounting Fees and Services
We incorporate by reference the information required by this item to the information under the caption “Ratification of Selection of Independent Auditors” contained in the Proxy Statement.
71
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a)(1) Index to Financial Statements
We submitted the consolidated financial statements required by this item in a separate section beginning on page F-1 of this Report.
(a)(2) Index to Financial Statement Schedules
We omitted these schedules because they are not required, or are not applicable, or the required information is shown in the consolidated financial statements or notes thereto.
(a)(3) Index to Exhibits
72
INDEX TO EXHIBITS
|
Exhibit Number
|
Description of Document
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation filed June 19, 1991.
|
|
|
3.2
|
Certificate of Amendment to Restated Certificate of Incorporation filed June 17, 2014. - Filed as an exhibit to the Registrant’s Notice of Annual Meeting and Proxy Statement, for the 2014 Annual Meeting of Stockholders, filed with the SEC on April 25, 2014, and incorporated herein by reference.
|
|
|
3.3
|
Certificate of Amendment to Restated Certificate of Incorporation filed December 18, 2015. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 18, 2015 and incorporated herein by reference.
|
|
|
3.4
|
Amended and Restated Bylaws. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 18, 2015 and incorporated herein by reference.
|
|
|
4.1
|
Certificate of Designation of the Series C Junior Participating Preferred Stock. - Filed as an exhibit to Registrant’s Report on Form 8-K dated filed December 13, 2000 and incorporated herein by reference.
|
|
|
4.2
|
Specimen Common Stock Certificate.
|
|
|
4.3
|
Indenture, dated as of August 13, 2012, between the Registrant and Wells Fargo Bank, National Association, as trustee, including Form of 2¾ percent Convertible Senior Note due 2019. - Filed as an exhibit to the Registrant’s Report on Form 8-K filed August 13, 2012 and incorporated herein by reference.
|
|
|
4.4
|
Indenture, dated as of November 17, 2014, between the Registrant and Wells Fargo Bank, National Association, as trustee, including Form of 1.00 percent Convertible Senior Note due 2021. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed November 21, 2014 and incorporated herein by reference.
|
|
|
10.1
|
Form of Indemnity Agreement entered into between the Registrant and its Directors and Officers with related schedule. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012 and incorporated herein by reference.
|
|
|
10.2*
|
Registrant’s 1989 Stock Option Plan, as amended. - Filed as an exhibit to Registrant’s Notice of Annual Meeting and Proxy Statement for the 2012 Annual Meeting of Stockholders, filed with the SEC on April 16, 2012, and incorporated herein by reference.
|
|
|
10.3*
|
Registrant’s Amended and Restated 2000 Employee Stock Purchase Plan. - Filed as an exhibit to Registrant’s Notice of Annual Meeting and Proxy Statement for the 2009 Annual Meeting of Stockholders, filed with the SEC on April 20, 2009, and incorporated herein by reference.
|
|
|
10.4
|
Form of Employee Confidential Information and Inventions Agreement.
|
|
|
10.5
|
Patent Rights Purchase Agreement between the Registrant and Gilead Sciences, Inc., dated December 18, 1998. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1998 and incorporated herein by reference.
|
|
|
10.6
|
Collaboration and License Agreement between the Registrant and Hybridon, Inc., dated May 24, 2001. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s report on Form 10-Q as amended for the quarter ended June 30, 2001 and incorporated herein by reference.
|
|
|
10.7
|
Amendment #1 to the Research, Development and License Agreement dated May 11, 2011 by and between the Registrant and Glaxo Group Limited. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2011 and incorporated herein by reference.
|
|
|
10.8
|
Amended and Restated Collaboration and License Agreement between the Registrant and Antisense Therapeutics Ltd dated February 8, 2008. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2008 and incorporated herein by reference.
|
|
|
10.9
|
Amended and Restated License Agreement between the Registrant and Atlantic Pharmaceuticals Limited dated November 30, 2009. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report as Form 10-K for the year ended December 31, 2009 and incorporated herein by reference.
|
73
|
10.10
|
Amended and Restated Strategic Neurology Drug Discovery and Development Collaboration, Option and License Agreement between the Registrant and Biogen MA Inc. dated October 20, 2017. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
|
|
10.11
|
Stock Purchase Agreement among the Registrant, Akcea Therapeutics, Inc. and Novartis Pharma AG dated January 5, 2017. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2017 and incorporated herein by reference.
|
|
|
10.12
|
Amendment #1 between the Registrant and Bayer AG dated February 10, 2017. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2017 and incorporated herein by reference.
|
|
|
10.13
|
Registrant’s Amended and Restated 10b5-1 Trading Plan dated September 12, 2013. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and incorporated herein by reference.
|
|
|
10.14*
|
Registrant’s Amended and Restated 2002 Non-Employee Directors’ Stock Option Plan, as amended. - Filed as an exhibit to the Registrant’s Notice of Annual Meeting and Proxy Statement, for the 2014 Annual Meeting of Stockholders, filed with the SEC on April 25, 2014, and incorporated herein by reference.
|
|
10.15*
|
Form of Restricted Stock Unit Agreement for Restricted Stock Units granted under the Ionis Pharmaceuticals, Inc. Amended and Restated 2002 Non-Employee Directors’ Stock Option Plan. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2012 and incorporated herein by reference.
|
|
|
10.16*
|
Amended and Restated Severance Agreement dated December 3, 2008 between the Registrant and Stanley T. Crooke. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed December 5, 2008 and incorporated herein by reference.
|
|
|
Research Collaboration, Option and License Agreement between the Registrant and Biogen MA Inc. dated December 19, 2017. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment.
|
||
|
10.18*
|
Ionis Pharmaceuticals, Inc. 2011 Equity Incentive Plan - Filed as an exhibit to the Registrant’s Notice of 2011 Annual Meeting of Stockholders and Proxy Statement filed with the SEC on April 28, 2011, and incorporated herein by reference.
|
|
|
10.19*
|
Form of Option Agreement under the 2011 Equity Incentive Plan. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference.
|
|
|
10.20*
|
Form of Restricted Stock Unit Agreement for Restricted Stock Units granted under the 2011 Equity Incentive Plan. - Filed as an exhibit to the Registrant’s Registration Statement on Form S-8 filed with the SEC on August 8, 2011, and incorporated herein by reference.
|
|
|
10.21
|
Loan Agreement between Ionis Gazelle, LLC and UBS AG dated July 18, 2017. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.22*
|
Form of Option Agreement under the 1989 Stock Option Plan. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference.
|
|
|
10.23*
|
Form of Option Agreement for Options Granted after March 8, 2005 under the 2002 Non-Employee Director’s Stock Option Plan. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2004 and incorporated herein by reference.
|
|
|
10.24
|
Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated March 30, 2010. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010 and incorporated herein by reference.
|
|
|
10.25
|
Loan Agreement between Ionis Faraday, LLC and UBS AG dated July 18, 2017. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.26
|
Research Agreement dated August 10, 2011 between the Registrant and CHDI Foundation, Inc. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2011 and incorporated herein by reference.
|
|
|
10.27
|
Guaranty between the Registrant and UBS AG dated July 18, 2017. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
74
|
10.28
|
Development, Option and License Agreement between the Registrant and Biogen Idec International Holding Ltd. dated January 3, 2012. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2012 and incorporated herein by reference.
|
|
|
10.29
|
DMPK Research, Development, Option and License Agreement between the Registrant and Biogen Idec MA Inc. dated June 27, 2012. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2012 and incorporated herein by reference.
|
|
|
10.30
|
Amendment #2 to Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated October 30, 2012. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012 and incorporated herein by reference.
|
|
|
10.31
|
Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated December 7, 2012. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012 and incorporated herein by reference.
|
|
10.32
|
Neurology Drug Discovery and Development Collaboration, Option and License Agreement between the Registrant and Biogen Idec MA Inc. dated December 10, 2012. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2012 and incorporated herein by reference.
|
|
|
10.33
|
HTT Research, Development, Option and License Agreement among the Registrant, F. Hoffmann-La Roche Ltd and Hoffman-La Roche Inc. dated April 8, 2013. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2013 and incorporated herein by reference.
|
|
|
10.34
|
Letter Agreement between the Registrant and CHDI Foundation, Inc. dated April 8, 2013. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2013 and incorporated herein by reference.
|
|
|
10.35
|
Strategic Neurology Drug Discovery and Development Collaboration, Option and License Agreement between the Registrant and Biogen Idec MA Inc. dated September 5, 2013. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and incorporated herein by reference.
|
|
|
10.36
|
Amendment #1 to Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated August 13, 2013. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and incorporated herein by reference.
|
|
10.37
|
Letter Agreement Amendment between the Registrant and Biogen Idec International Holding Ltd dated January 27, 2014. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 and incorporated herein by reference.
|
|
|
10.38
|
Amendment No. 3 to the Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated July 10, 2013. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2014 and incorporated herein by reference.
|
|
|
10.39
|
Amendment #4 to the Research, Development and License Agreement between the Registrant and Glaxo Group Limited dated April 10, 2014. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2014 and incorporated herein by reference.
|
|
|
10.40
|
Amendment #5 to the Research, Development and License Agreement among the Registrant, Glaxo Group Limited and GlaxoSmithKline Intellectual Property Development Limited dated June 27, 2014. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2014 and incorporated herein by reference.
|
|
|
10.41
|
Exclusive License Agreement between the Registrant and the University of Massachusetts dated January 14, 2010. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and incorporated herein by reference.
|
75
|
10.42
|
Amended and Restated Collaboration and License Agreement between the Registrant and Cold Spring Harbor Laboratory dated October 26, 2011. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and incorporated herein by reference.
|
|
|
10.43
|
Amendment to Amended and Restated Collaboration and License Agreement between the Registrant and Cold Spring Harbor Laboratory dated March 14, 2014. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2014 and incorporated herein by reference.
|
|
|
10.44
|
Amendment #1 to the Development, Option and License Agreement between the Registrant and Biogen Idec International Holding Ltd. dated December 15, 2014. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2014 and incorporated herein by reference.
|
|
|
10.45
|
Research Collaboration, Option and License Agreement between the Registrant and Janssen Biotech Inc. dated December 22, 2014. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2014 and incorporated herein by reference.
|
|
|
10.46
|
Amendment No.2 to the Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated October 15, 2014. Portions of this exhibit have been omitted and separately filed with the SEC with a request for confidential treatment. - Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2014 and incorporated herein by reference.
|
|
|
10.47
|
Strategic Collaboration Agreement between the Registrant and AstraZeneca AB dated July 31, 2015. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference.
|
|
|
10.48
|
Amendment #6 to Research, Development and License Agreement between the Registrant, Glaxo Group Limited and GlaxoSmithKline Intellectual Property Development Limited dated September 2, 2015. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference.
|
|
|
10.49
|
Amendment Number One to the Second Amended and Restated Strategic Collaboration and License Agreement between the Registrant and Alnylam Pharmaceuticals, Inc. dated July 13, 2015. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2015 and incorporated herein by reference.
|
|
|
10.50
|
License Agreement between the Registrant and Bayer Pharma AG dated May 1, 2015. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2015 and incorporated herein by reference.
|
|
10.51
|
Line of Credit Agreement between the Registrant and Morgan Stanley Private Bank, National Association dated June 16, 2015. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2015 and incorporated herein by reference.
|
|
|
10.52
|
Second Amended and Restated Strategic Collaboration and License Agreement between the Registrant and Alnylam Pharmaceuticals, Inc. dated January 8, 2015. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2015 and incorporated herein by reference.
|
|
|
10.53
|
Amendment #1 to HTT Research, Development, Option and License Agreement between the Registrant, F. Hoffmann-La Roche Ltd and Hoffmann-La Roche Inc. dated January 9, 2015. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2015 and incorporated herein by reference.
|
|
|
10.54
|
Amendment No.1 to Loan Documents between the Registrant and Morgan Stanley Private Bank, National Association dated December 30, 2015. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed January 5, 2016 and incorporated herein by reference.
|
|
|
10.55
|
Amendment No.2 to Line of Credit Agreement between the Registrant and Morgan Stanley Private Bank, National Association dated February 24, 2016. Filed as an exhibit to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2015 and incorporated herein by reference.
|
|
|
10.56
|
Amendment No.3 to the Collaboration, License and Development Agreement between the Registrant and AstraZeneca AB dated January 18, 2016. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2016 and incorporated herein by reference.
|
76
|
10.57
|
Amendment #7 to the Research, Development and License Agreement among the Registrant, Glaxo Group Limited and GlaxoSmithKline Intellectual Property Development Limited dated March 4, 2016. Portions of this exhibit have been omitted and separately filed with the SEC. - Filed as an exhibit to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2016 and incorporated herein by reference.
|
|
|
10.58
|
First Amendment to Research Collaboration, Option and License Agreement between the Registrant and Janssen Biotech Inc. dated December 21, 2016. Portions of this exhibit have been omitted and separately filed with the SEC.
|
|
|
10.59
|
Letter Agreement between the Registrant and Biogen MA Inc. dated October 28, 2016. Portions of this exhibit have been omitted and separately filed with the SEC. Portions of this exhibit have been omitted and separately filed with the SEC.
|
|
|
10.60
|
Guaranty between the Registrant and UBS AG dated July 18, 2017. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.61
|
Environmental Indemnity Agreement among the Registrant, Ionis Gazelle, LLC and UBS AG dated July 18, 2017. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.62
|
Environmental Indemnity Agreement among the Registrant, Ionis Faraday, LLC and UBS AG dated July 18, 2017. - Filed as an exhibit to the Registrant’s Current Report on Form 8-K filed July 21, 2017 and incorporated herein by reference.
|
|
|
10.63*
|
Amendment to Ionis Pharmaceuticals, Inc. 2011 Equity Incentive Plan. - Filed as an exhibit to the Registrant’s Notice of Annual Meeting and Proxy Statement, for the 2017 Annual Meeting of Stockholders, filed with the SEC on April 10, 2017, and incorporated herein by reference.
|
|
|
14.1
|
Registrant’s Code of Ethics and Business Conduct.
|
|
|
21.1
|
List of Subsidiaries for the Registrant.
|
|
|
23.1
|
Consent of Independent Registered Public Accounting Firm.
|
|
|
24.1
|
Power of Attorney – Included on the signature page of this Annual Report on Form 10-K.
|
|
|
31.1
|
Certification by Chief Executive Officer Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
31.2
|
Certification by Chief Financial Officer Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
32.1+
|
Certification Pursuant to 18 U.S.C. Section 1350 as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101
|
The following financial statements from the Ionis Pharmaceuticals, Inc. Annual Report on Form 10-K for the year ended December 31, 2016, formatted in Extensive Business Reporting Language (XBRL): (i) consolidated balance sheets, (ii) consolidated statements of operations, (iii) consolidated statements of stockholders’ equity, (iv) consolidated statements of cash flows, and (v) notes to consolidated financial statements (detail tagged).
|
| * |
Indicates management compensatory plans and arrangements as required to be filed as exhibits to this Report pursuant to Item 14(c).
|
| + |
This certification is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing under the Securities Act of 133, as amended, or the Securities Exchange Act of 1934, as amended.
|
77
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized on the 28th day of February, 2018.
|
|
IONIS PHARMACEUTICALS, INC.
|
|
|
|
|
|
|
|
By:
|
/s/ STANLEY T. CROOKE
|
|
|
|
Stanley T. Crooke, M.D., Ph.D.
|
|
|
|
Chairman of the Board, President and Chief Executive Officer (Principal executive officer)
|
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Stanley T. Crooke and Elizabeth L. Hougen, or any of them, his or her attorney-in-fact, each with the power of substitution, for him or her in any and all capacities, to sign any amendments to this Report, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his or her substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
Signatures
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ STANLEY T. CROOKE
|
|
Chairman of the Board, President, and Chief Executive Officer
|
|
February 28, 2018
|
|
Stanley T. Crooke, M.D., Ph.D.
|
|
(Principal executive officer)
|
|
|
|
|
|
|
|
|
|
/s/ ELIZABETH L. HOUGEN
|
|
Senior Vice President, Finance and Chief Financial Officer
|
|
February 28, 2018
|
|
Elizabeth L. Hougen
|
|
(Principal financial and accounting officer)
|
|
|
|
/s/ B. LYNNE PARSHALL
|
|
Director and Senior Strategic Advisor
|
|
February 28, 2018
|
|
B. Lynne Parshall, J.D.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ SPENCER R. BERTHELSEN
|
|
Director
|
|
February 28, 2018
|
|
Spencer R. Berthelsen, M.D.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ BREAUX CASTLEMAN
|
|
Director
|
|
February 28, 2018
|
|
Breaux Castleman
|
|
|
|
|
|
|
|
|
|
|
|
/s/ JOSEPH KLEIN
|
|
Director
|
|
February 28, 2018
|
|
Joseph Klein, III
|
|
|
|
|
|
|
|
|
|
|
|
/s/ JOSEPH LOSCALZO
|
|
Director
|
|
February 28, 2018
|
|
Joseph Loscalzo, M.D., Ph.D.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ FREDERICK T. MUTO
|
|
Director
|
|
February 28, 2018
|
|
Frederick T. Muto, Esq.
|
|
|
|
|
|
|
|
|
|
|
|
/s/ JOSEPH H. WENDER
|
|
Director
|
|
February 28, 2018
|
|
Joseph H. Wender
|
|
|
|
|
78
IONIS PHARMACEUTICALS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
|
Page
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
Consolidated Balance Sheets at December 31, 2017 and 2016
|
F-3
|
|
Consolidated Statements of Operations for the years ended December 31, 2017, 2016 and 2015
|
F-4
|
|
Consolidated Statements of Comprehensive Loss for the years ended December 31, 2017, 2016 and 2015
|
F-5
|
|
Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2017, 2016 and 2015
|
F-6
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2017, 2016 and 2015
|
F-7
|
|
Notes to Consolidated Financial Statements
|
F-9
|
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Ionis Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Ionis Pharmaceuticals, Inc. (the Company) as of December 31, 2017 and 2016, and the related consolidated statements of operations, comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2017, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework) and our report dated February 28, 2018 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
|
/s/ ERNST & YOUNG LLP
|
|
We have served as the Company’s auditor since 1989
San Diego, California
February 28, 2018
F-2
IONIS PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share data)
|
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
129,630
|
$
|
84,685
|
||||
|
Short-term investments
|
893,085
|
580,538
|
||||||
|
Contracts receivable
|
62,955
|
108,043
|
||||||
|
Inventories
|
9,982
|
7,489
|
||||||
|
Other current assets
|
72,332
|
17,177
|
||||||
|
Total current assets
|
1,167,984
|
797,932
|
||||||
|
Property, plant and equipment, net
|
121,907
|
92,845
|
||||||
|
Patents, net
|
22,004
|
20,365
|
||||||
|
Deposits and other assets
|
10,129
|
1,325
|
||||||
|
Total assets
|
$
|
1,322,024
|
$
|
912,467
|
||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$
|
24,886
|
$
|
21,120
|
||||
|
Accrued compensation
|
25,151
|
24,186
|
||||||
|
Accrued liabilities
|
66,618
|
36,013
|
||||||
|
Current portion of long-term obligations
|
1,621
|
1,185
|
||||||
|
Current portion of deferred contract revenue
|
106,465
|
51,280
|
||||||
|
Total current liabilities
|
224,741
|
133,784
|
||||||
|
Long-term deferred contract revenue
|
72,708
|
91,198
|
||||||
|
1 percent convertible senior notes
|
533,111
|
500,511
|
||||||
|
Long-term obligations, less current portion
|
12,974
|
15,050
|
||||||
|
Long-term financing liability for leased facility
|
—
|
72,359
|
||||||
|
Long-term mortgage debt
|
59,771
|
—
|
||||||
|
Total liabilities
|
903,305
|
812,902
|
||||||
|
Stockholders’ equity:
|
||||||||
|
Common stock, $0.001 par value; 300,000,000 shares authorized, 124,976,373 and 121,636,273 shares issued and outstanding at December 31, 2017 and December 31, 2016, respectively
|
125
|
122
|
||||||
|
Additional paid-in capital
|
1,549,904
|
1,311,229
|
||||||
|
Accumulated other comprehensive income (loss)
|
(31,759
|
)
|
(30,358
|
)
|
||||
|
Accumulated deficit
|
(1,187,398
|
)
|
(1,181,428
|
)
|
||||
|
Total Ionis stockholders' equity
|
330,872
|
99,565
|
||||||
|
Noncontrolling interest in Akcea Therapeutics, Inc.
|
87,847
|
—
|
||||||
|
Total stockholders’ equity
|
418,719
|
99,565
|
||||||
|
Total liabilities and stockholders’ equity
|
$
|
1,322,024
|
$
|
912,467
|
||||
See accompanying notes.
F-3
IONIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except for per share amounts)
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Revenue:
|
||||||||||||
|
Commercial revenue:
|
||||||||||||
|
SPINRAZA royalties
|
$
|
112,540
|
$
|
883
|
$
|
—
|
||||||
|
Licensing and other royalty revenue
|
9,519
|
19,839
|
2,343
|
|||||||||
|
Total commercial revenue
|
122,059
|
20,722
|
2,343
|
|||||||||
|
Research and development revenue under collaborative agreements
|
385,607
|
325,898
|
281,360
|
|||||||||
|
Total revenue
|
507,666
|
346,620
|
283,703
|
|||||||||
|
Expenses:
|
||||||||||||
|
Research, development and patent
|
374,644
|
344,320
|
322,292
|
|||||||||
|
Selling, general and administrative
|
108,488
|
48,616
|
37,173
|
|||||||||
|
Total operating expenses
|
483,132
|
392,936
|
359,465
|
|||||||||
|
Income (loss) from operations
|
24,534
|
(46,316
|
)
|
(75,762
|
)
|
|||||||
|
Other income (expense):
|
||||||||||||
|
Investment income
|
7,805
|
5,472
|
4,377
|
|||||||||
|
Interest expense
|
(44,752
|
)
|
(38,795
|
)
|
(36,732
|
)
|
||||||
|
Gain on investment in Regulus Therapeutics Inc.
|
374
|
—
|
20,211
|
|||||||||
|
Loss on extinguishment of financing liability for leased facility
|
(7,689
|
)
|
—
|
—
|
||||||||
|
Loss on early retirement of debt
|
—
|
(3,983
|
)
|
—
|
||||||||
|
Other expenses
|
(3,548
|
)
|
—
|
—
|
||||||||
|
Loss before income tax benefit (expense)
|
(23,276
|
)
|
(83,622
|
)
|
(87,906
|
)
|
||||||
|
Income tax benefit (expense)
|
5,980
|
(2,934
|
)
|
(372
|
)
|
|||||||
|
Net loss
|
(17,296
|
)
|
(86,556
|
)
|
(88,278
|
)
|
||||||
|
Net loss attributable to noncontrolling interest in Akcea Therapeutics, Inc.
|
11,326
|
—
|
—
|
|||||||||
|
Net loss attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
(5,970
|
)
|
$
|
(86,556
|
)
|
$
|
(88,278
|
)
|
|||
|
Basic net income (loss) per share
|
$
|
0.08
|
$
|
(0.72
|
)
|
$
|
(0.74
|
)
|
||||
|
Shares used in computing basic net income (loss) per share
|
124,016
|
120,933
|
119,719
|
|||||||||
|
Diluted net income (loss) per share
|
$
|
0.08
|
$
|
(0.72
|
)
|
$
|
(0.74
|
)
|
||||
|
Shares used in computing diluted net income (loss) per share
|
126,098
|
120,933
|
119,719
|
|||||||||
See accompanying notes.
F-4
IONIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Net loss
|
$
|
(17,296
|
)
|
$
|
(86,556
|
)
|
$
|
(88,278
|
)
|
|||
|
Unrealized losses on investments, net of tax
|
(960
|
)
|
(17,219
|
)
|
(33,101
|
)
|
||||||
|
Reclassification adjustment for realized (gains) losses included in net loss
|
(374
|
)
|
447
|
(20,211
|
)
|
|||||||
|
Currency translation adjustment
|
(67
|
)
|
(21
|
)
|
—
|
|||||||
|
Comprehensive loss
|
(18,697
|
)
|
(103,349
|
)
|
(141,590
|
)
|
||||||
|
Comprehensive loss attributable to noncontrolling interest in Akcea Therapeutics, Inc.
|
11,421
|
—
|
—
|
|||||||||
|
Comprehensive loss attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
(7,276
|
)
|
$
|
(103,349
|
)
|
$
|
(141,590
|
)
|
|||
See accompanying notes.
F-5
IONIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
Years Ended December 31, 2017, 2016 and 2015
(In thousands)
|
Common Stock
|
Additional Paid in
|
Accumulated
Other
Comprehensive
|
Accumulated
|
Total Ionis
Stockholders'
|
Noncontrolling
Interest in Akcea
|
Total
Stockholders’
|
||||||||||||||||||||||||||
|
Description
|
Shares
|
Amount
|
Capital
|
Income (Loss)
|
Deficit
|
Equity
|
Therapeutics, Inc.
|
Equity
|
||||||||||||||||||||||||
|
Balance at December 31, 2014
|
118,443
|
$
|
118
|
$
|
1,224,509
|
$
|
39,747
|
$
|
(1,006,594
|
)
|
$
|
257,780
|
$
|
—
|
$
|
257,780
|
||||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
(88,278
|
)
|
(88,278
|
)
|
—
|
(88,278
|
)
|
|||||||||||||||||||||
|
Change in unrealized gains (losses), net of tax
|
—
|
—
|
—
|
(53,312
|
)
|
—
|
(53,312
|
)
|
—
|
(53,312
|
)
|
|||||||||||||||||||||
|
Issuance of common stock in connection with employee stock plans
|
1,908
|
2
|
24,888
|
—
|
—
|
24,890
|
—
|
24,890
|
||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
59,314
|
—
|
—
|
59,314
|
—
|
59,314
|
||||||||||||||||||||||||
|
Excess tax benefits from stock-based compensation awards
|
—
|
—
|
396
|
—
|
—
|
396
|
—
|
396
|
||||||||||||||||||||||||
|
Balance at December 31, 2015
|
120,351
|
$
|
120
|
$
|
1,309,107
|
$
|
(13,565
|
)
|
$
|
(1,094,872
|
)
|
$
|
200,790
|
$
|
—
|
$
|
200,790
|
|||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
(86,556
|
)
|
(86,556
|
)
|
—
|
(86,556
|
)
|
|||||||||||||||||||||
|
Change in unrealized gains (losses), net of tax
|
—
|
—
|
—
|
(16,772
|
)
|
—
|
(16,772
|
)
|
—
|
(16,772
|
)
|
|||||||||||||||||||||
|
Foreign currency translation
|
—
|
—
|
—
|
(21
|
)
|
—
|
(21
|
)
|
—
|
(21
|
)
|
|||||||||||||||||||||
|
Issuance of common stock in connection with employee stock plans
|
1,285
|
2
|
13,706
|
—
|
—
|
13,708
|
—
|
13,708
|
||||||||||||||||||||||||
|
2¾ percent convertible senior notes redemption, equity portion
|
—
|
—
|
(128,888
|
)
|
—
|
—
|
(128,888
|
)
|
—
|
(128,888
|
)
|
|||||||||||||||||||||
|
1 percent convertible senior notes, equity portion, net of issuance costs
|
—
|
—
|
43,335
|
—
|
—
|
43,335
|
—
|
43,335
|
||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
72,108
|
—
|
—
|
72,108
|
—
|
72,108
|
||||||||||||||||||||||||
|
Excess tax benefits from stock-based compensation awards
|
—
|
—
|
1,861
|
—
|
—
|
1,861
|
—
|
1,861
|
||||||||||||||||||||||||
|
Balance at December 31, 2016
|
121,636
|
$
|
122
|
$
|
1,311,229
|
$
|
(30,358
|
)
|
$
|
(1,181,428
|
)
|
$
|
99,565
|
$
|
—
|
$
|
99,565
|
|||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
(5,970
|
)
|
(5,970
|
)
|
—
|
(5,970
|
)
|
|||||||||||||||||||||
|
Change in unrealized gains (losses), net of tax
|
—
|
—
|
—
|
(1,334
|
)
|
—
|
(1,334
|
)
|
—
|
(1,334
|
)
|
|||||||||||||||||||||
|
Foreign currency translation
|
—
|
—
|
—
|
(67
|
)
|
—
|
(67
|
)
|
—
|
(67
|
)
|
|||||||||||||||||||||
|
Novartis stock purchase
|
1,631
|
2
|
71,737
|
—
|
—
|
71,739
|
—
|
71,739
|
||||||||||||||||||||||||
|
Issuance of common stock in connection with employee stock plans
|
1,709
|
1
|
22,931
|
—
|
—
|
22,932
|
—
|
22,932
|
||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
85,975
|
—
|
—
|
85,975
|
—
|
85,975
|
||||||||||||||||||||||||
|
Issuance of Akcea Therapeutics, Inc. common stock in conjunction with initial public offering
|
—
|
—
|
157,270
|
—
|
—
|
157,270
|
—
|
157,270
|
||||||||||||||||||||||||
|
Noncontrolling interest in Akcea Therapeutics, Inc. in conjunction with initial public offering
|
—
|
—
|
(90,351
|
)
|
—
|
—
|
(90,351
|
)
|
90,381
|
30
|
||||||||||||||||||||||
|
Noncontrolling interest in Akcea Therapeutics, Inc.
|
—
|
—
|
(8,887
|
)
|
—
|
—
|
(8,887
|
)
|
(2,534
|
)
|
(11,421
|
)
|
||||||||||||||||||||
|
Balance at December 31, 2017
|
124,976
|
$
|
125
|
$
|
1,549,904
|
$
|
(31,759
|
)
|
$
|
(1,187,398
|
)
|
$
|
330,872
|
$
|
87,847
|
$
|
418,719
|
|||||||||||||||
See accompanying notes.
F-6
IONIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Operating activities:
|
||||||||||||
|
Net loss
|
$
|
(17,296
|
)
|
$
|
(86,556
|
)
|
$
|
(88,278
|
)
|
|||
|
Adjustments to reconcile net loss to net cash provided by (used in) operating activities:
|
||||||||||||
|
Depreciation
|
6,708
|
7,481
|
6,984
|
|||||||||
|
Amortization of patents
|
1,641
|
1,552
|
1,381
|
|||||||||
|
Amortization of licenses
|
—
|
—
|
1,873
|
|||||||||
|
Amortization of premium on investments, net
|
6,752
|
6,813
|
7,812
|
|||||||||
|
Amortization of debt issuance costs
|
1,616
|
1,225
|
1,133
|
|||||||||
|
Amortization of convertible senior notes discount
|
30,920
|
23,890
|
22,075
|
|||||||||
|
Amortization of long-term financing liability for leased facility
|
3,659
|
6,693
|
6,665
|
|||||||||
|
Stock-based compensation expense
|
85,975
|
72,108
|
59,314
|
|||||||||
|
Gain on investment in Regulus Therapeutics Inc.
|
(374
|
)
|
—
|
(20,211
|
)
|
|||||||
|
Loss on extinguishment of financing liability for leased facility
|
7,689
|
—
|
—
|
|||||||||
|
Loss on early retirement of debt
|
—
|
3,983
|
—
|
|||||||||
|
Non-cash losses related to patents, licensing, property, plant and equipment and strategic investments
|
3,302
|
2,297
|
1,881
|
|||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Contracts receivable
|
45,088
|
(96,687
|
)
|
(7,453
|
)
|
|||||||
|
Inventories
|
(2,493
|
)
|
(590
|
)
|
(609
|
)
|
||||||
|
Other current and long-term assets
|
(58,367
|
)
|
1,603
|
(4,394
|
)
|
|||||||
|
Long-term income tax receivable
|
(9,114
|
)
|
—
|
—
|
||||||||
|
Accounts payable
|
1,784
|
(10,677
|
)
|
9,211
|
||||||||
|
Income taxes
|
435
|
1,069
|
—
|
|||||||||
|
Accrued compensation
|
965
|
8,121
|
3,763
|
|||||||||
|
Accrued liabilities and deferred rent
|
28,564
|
4,720
|
(2,140
|
)
|
||||||||
|
Deferred contract revenue
|
36,695
|
(59,150
|
)
|
22,118
|
||||||||
|
Net cash provided by (used in) operating activities
|
174,149
|
(112,105
|
)
|
21,125
|
||||||||
|
Investing activities:
|
||||||||||||
|
Purchases of short-term investments
|
(877,810
|
)
|
(300,912
|
)
|
(493,467
|
)
|
||||||
|
Proceeds from the sale of short-term investments
|
557,369
|
364,572
|
419,584
|
|||||||||
|
Purchases of property, plant and equipment
|
(34,764
|
)
|
(7,107
|
)
|
(7,692
|
)
|
||||||
|
Acquisition of licenses and other assets, net
|
(3,093
|
)
|
(4,421
|
)
|
(4,056
|
)
|
||||||
|
Purchase of strategic investments
|
(2,500
|
)
|
—
|
—
|
||||||||
|
Proceeds from the sale of Regulus Therapeutics, Inc.
|
2,507
|
4,467
|
25,527
|
|||||||||
|
Proceeds from the sale of strategic investments
|
—
|
—
|
52
|
|||||||||
|
Net cash (used in) provided by investing activities
|
(358,291
|
)
|
56,599
|
(60,052
|
)
|
|||||||
|
Financing activities:
|
||||||||||||
|
Proceeds from equity, net
|
22,931
|
13,417
|
24,888
|
|||||||||
|
Proceeds from issuance of common stock in Akcea Therapeutics, Inc. from its initial public offering, net of underwriters' discount
|
110,438
|
—
|
—
|
|||||||||
|
Proceeds from building mortgage debt, net of issuance costs
|
59,750
|
—
|
—
|
|||||||||
|
Proceeds from the issuance of common stock to Novartis
|
71,737
|
—
|
—
|
|||||||||
|
Proceeds from borrowing on line of credit facility
|
—
|
4,000
|
8,500
|
|||||||||
|
Proceeds from the sale of Akcea Therapeutics, Inc. common stock to Novartis in a private placement
|
50,000
|
—
|
—
|
|||||||||
|
Offering costs paid
|
(2,037
|
)
|
(818
|
)
|
—
|
|||||||
|
Payment to settle financing liability for leased facility
|
(80,133
|
)
|
—
|
—
|
||||||||
|
Excess tax benefits from stock-based compensation awards
|
—
|
1,861
|
396
|
|||||||||
|
Principal payments on debt and capital lease obligations
|
(3,599
|
)
|
(7,066
|
)
|
(9,058
|
)
|
||||||
|
Net cash provided by financing activities
|
229,087
|
11,394
|
24,726
|
|||||||||
|
Net increase (decrease) in cash and cash equivalents
|
44,945
|
(44,112
|
)
|
(14,201
|
)
|
|||||||
|
Cash and cash equivalents at beginning of year
|
84,685
|
128,797
|
142,998
|
|||||||||
|
Cash and cash equivalents at end of year
|
$
|
129,630
|
$
|
84,685
|
$
|
128,797
|
||||||
F-7
IONIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
Supplemental disclosures of cash flow information:
|
2017 | 2016 | 2015 | |||||||||
|
Interest paid
|
$
|
8,035
|
$
|
7,313
|
$
|
6,800
|
||||||
|
Supplemental disclosures of non-cash investing and financing activities:
|
||||||||||||
|
Amounts accrued for capital and patent expenditures
|
$
|
1,983
|
$
|
3,439
|
$
|
1,162
|
||||||
|
1 percent convertible senior notes principal issued related to our December 2016 debt exchange
|
$
|
—
|
$
|
185,450
|
$
|
—
|
||||||
|
2¾ percent convertible senior notes principal extinguished related to our December 2016 debt exchange
|
$
|
—
|
$
|
61,099
|
$
|
—
|
||||||
|
Unpaid deferred offering costs
|
$
|
—
|
$
|
291
|
$
|
—
|
||||||
See accompanying notes.
F-8
IONIS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Significant Accounting Policies
Basis of Presentation
In our consolidated financial statements we included the accounts of Ionis Pharmaceuticals, Inc. ("we", "us" or "our") and the consolidated results of our majority-owned affiliate, Akcea Therapeutics, Inc., which we formed in December 2014. In July 2017, Akcea completed an initial public offering, or IPO, and therefore beginning in July 2017, we no longer own 100 percent of Akcea. As of July 19, 2017, the closing of the IPO, we owned approximately 68 percent of Akcea. Refer to the noncontrolling interest in Akcea section in this note for further information related to our accounting for our investment in Akcea.
Organization and Business Activity
We incorporated in California on January 10, 1989. In conjunction with our IPO, we reorganized as a Delaware corporation in April 1991. We were organized principally to develop human therapeutic drugs using antisense technology. In December 2015, we changed our name from Isis Pharmaceuticals, Inc. to Ionis Pharmaceuticals, Inc.
Basic and Diluted Net Income (Loss) per Share
We compute basic net income (loss) per share by dividing the net income (loss) attributable to our common stockholders by our weighted average number of common shares outstanding during the period.
The calculation of total net income (loss) attributable to our common stockholders for 2017 considered our net income for Ionis on a stand-alone basis plus our share of Akcea’s net loss. To calculate the portion of Akcea’s net loss attributable to our ownership, we multiplied Akcea’s loss per share by the weighted average shares we owned in Akcea during the year. Prior to Akcea’s IPO, we owned Akcea Series A convertible preferred stock, which included a six percent cumulative dividend. Upon completion of Akcea’s IPO in July 2017, our preferred stock was converted into common stock on a 1:1 basis. The preferred stock dividend was not paid at the IPO because it was not a liquidation event or a change in control. For 2017, Akcea used a two-class method to compute its net income (loss) per share because it had both common and preferred shares outstanding during the year. The two-class method required Akcea to calculate its net income (loss) per share for each class of stock by dividing total distributable losses applicable to preferred and common stock, including the six percent cumulative dividend contractually due to Series A convertible preferred shareholders, by the weighted average of preferred and common shares outstanding during the requisite period. Since Akcea used the two-class method, accounting rules required us to include our portion of Akcea's net income (loss) per share for both Akcea's common and preferred shares which we owned in our calculation of basic and diluted net income per share for 2017. As a result of this calculation, our total net income available to Ionis common stockholders for the calculation of net income per share is different than net income (loss) attributable to Ionis Pharmaceuticals, Inc. common stockholders in the consolidated statements of operations.
We calculated our basic net income per share for 2017 as follows (in thousands, except per share amounts):
|
Year Ended December 31, 2017
|
Weighted
Average Shares
Owned in Akcea
|
Akcea’s
Net Loss
Per Share
|
Ionis’
Portion of
Akcea’s Net Loss
|
|||||||||
|
Common shares
|
20,669
|
$
|
(2.82
|
)
|
$
|
(58,332
|
)
|
|||||
|
Preferred shares
|
15,748
|
(1.55
|
)
|
(24,344
|
)
|
|||||||
|
Akcea’s net loss attributable to our ownership
|
$
|
(82,676
|
)
|
|||||||||
|
Ionis’ stand-alone net income
|
92,336
|
|||||||||||
|
Net income available to Ionis common stockholders
|
$
|
9,661
|
||||||||||
|
Weighted average shares outstanding
|
124,016
|
|||||||||||
|
Basic net income per share
|
$
|
0.08
|
||||||||||
For 2017, we had net income available to Ionis common stockholders. As a result, we computed diluted net income per share using the weighted-average number of common shares and dilutive common equivalent shares outstanding during those periods. Diluted common equivalent shares for 2017 consisted of the following (in thousands except per share amounts):
|
Year Ended December 31, 2017
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per-Share
Amount
|
|||||||||
|
Net income available to Ionis common stockholders
|
$
|
9,661
|
124,016
|
$
|
0.08
|
|||||||
|
Effect of dilutive securities:
|
||||||||||||
|
Shares issuable upon exercise of stock options
|
—
|
1,619
|
||||||||||
|
Shares issuable upon restricted stock award issuance
|
—
|
459
|
||||||||||
|
Shares issuable related to our ESPP
|
—
|
4
|
||||||||||
|
Income available to Ionis common stockholders, plus assumed conversions
|
$
|
9,661
|
126,098
|
$
|
0.08
|
|||||||
For 2017, the calculation excluded the 1 percent and 2¾ percent notes because the effect on diluted earnings per share was anti-dilutive.
F-9
As we incurred a net loss for 2016 and 2015, we did not include dilutive common equivalent shares in the computation of diluted net loss per share because the effect would have been anti-dilutive. Common stock from the following would have had an anti-dilutive effect on net loss per share:
| |
percent convertible senior notes;
|
| |
2¾ percent convertible senior notes;
|
| |
Dilutive stock options;
|
| |
Unvested restricted stock units; and
|
| |
Employee Stock Purchase Plan, or ESPP.
|
Revenue Recognition
We generally recognize revenue when we have satisfied all contractual obligations and are reasonably assured of collecting the resulting receivable. We are often entitled to bill our customers and receive payment from our customers in advance of recognizing the revenue. In the instances in which we have received payment from our customers in advance of recognizing revenue, we include the amounts in deferred revenue on our consolidated balance sheet.
Commercial Revenue: SPINRAZA royalties and Licensing and other royalty revenue
We often enter into agreements to license and sell our technology on an exclusive or non-exclusive basis in exchange for upfront fees, license fees, milestone payments and/or royalties. We generally recognize as revenue immediately license payments with stand-alone value when the license is delivered and we are reasonably assured of collecting the resulting receivable. We recognize royalty revenue in the period in which the counterparty sells the related product, unless we are unable to obtain information to estimate the royalty. For example, in 2017 we recorded SPINRAZA royalty revenue of $112.5 million.
Research and development revenue under collaborative agreements
Arrangements with multiple deliverables
Our collaboration agreements typically contain multiple elements, or deliverables, including technology licenses or options to obtain technology licenses, research and development services, and in certain cases manufacturing services, and we allocate the consideration to each unit of accounting based on the relative selling price of each deliverable.
Amendments to agreements
From time to time we amend our collaboration agreements. For these agreements, before we identify our deliverables and allocate consideration to each unit of accounting, we must determine if the amendment should be accounted for as a separate agreement, or if the amendment and any undelivered elements for the original agreement should be accounted for as a single new arrangement.
For example, in May 2015, we entered into an exclusive license agreement with Bayer to develop and commercialize IONIS-FXIRx for the prevention of thrombosis. As part of the agreement, Bayer paid us a $100 million upfront payment. At the onset of the agreement, we were responsible for completing a Phase 2 study of IONIS-FXIRx in people with end-stage renal disease on hemodialysis and for providing an initial supply of active pharmaceutical ingredient, or API. In February 2017, we amended our agreement with Bayer to advance IONIS-FXIRx and to initiate development of IONIS-FXI-LRx, which Bayer licensed. As part of the 2017 amendment, Bayer paid us $75 million. We are also eligible to receive milestone payments and tiered royalties on gross margins of IONIS-FXIRx and IONIS-FXI-LRx.
Under the 2017 amendment, there was a substantial increase in the consideration we are eligible to receive and a significant change in the deliverables we will provide to Bayer. As a result, we concluded that the amendment should be evaluated with the undelivered elements of the original agreement as a single new arrangement. Therefore, we evaluated our original and 2017 amended agreements with Bayer together to determine our deliverables. We concluded that the 2017 amendment did not impact the items we already delivered to Bayer.
Identifying deliverables and units of accounting
We evaluate the deliverables in our collaboration agreements to determine whether they meet the criteria to be accounted for as separate units of accounting or whether they should be combined with other deliverables and accounted for as a single unit of accounting. When the delivered items in an arrangement have "stand-alone value" to our customer, we account for the deliverables as separate units of accounting. Delivered items have stand-alone value if they are sold separately by any vendor or the customer could resell the delivered items on a stand-alone basis. For example, our 2017 amended agreement with Bayer has multiple elements. We evaluated the deliverables in this arrangement when we entered into the 2017 amended agreement and determined that certain of the deliverables have stand-alone value. Below is a list of the three units of accounting under our 2017 amended agreement:
| ● |
The exclusive license we granted to Bayer to develop and commercialize IONIS-FXI-LRx for the treatment of thrombosis;
|
| ● |
The development services we agreed to perform for IONIS-FXI-LRx and IONIS-FXIRx; and
|
| ● |
The remaining undelivered IONIS-FXIRx API that was part of the original agreement.
|
F-10
We determined that each of these three units of accounting have stand-alone value. The license we granted to Bayer has stand-alone value because it gives Bayer the exclusive right to develop IONIS-FXI-LRx or to sublicense its rights. The development services and the remaining undelivered supply of API each have stand-alone value because Bayer or another third party could provide these items without our assistance.
Measurement and allocation of arrangement consideration
Our collaborations may provide for various types of payments to us including upfront payments, funding of research and development, milestone payments, licensing fees and royalties on product sales. We initially allocate the amount of consideration that is fixed and determinable at the time the agreement is entered into and exclude contingent consideration. We allocate the consideration to each unit of accounting based on the relative selling price of each deliverable. We use the following hierarchy of values to estimate the selling price of each deliverable: (i) vendor-specific objective evidence of fair value; (ii) third-party evidence of selling price; and (iii) best estimate of selling price, or BESP. BESP reflects our best estimate of what the selling price would be if we regularly sold the deliverable on a stand-alone basis. We recognize the revenue allocated to each unit of accounting as we deliver the related goods or services. If we determine that we should treat certain deliverables as a single unit of accounting, then we recognize the revenue ratably over our estimated period of performance.
We determined that the allocable arrangement consideration for the Bayer 2017 amended agreement was $76.3 million, comprised of the $75 million we received as part of the amendment and the remaining amount of the $100 million upfront payment we had not yet recognized into revenue, related to the undelivered API. We allocated the consideration based on the relative BESP of each unit of accounting. We engaged a third party, independent valuation specialist to assist us with determining BESP. We estimated the selling price of the license granted for IONIS-FXI-LRx by using the relief from royalty method. Under this method, we estimated the amount of income, net of taxes, for IONIS-FXI-LRx. We then discounted the projected income to present value. The significant inputs we used to determine the projected income of the license included:
| ● |
Estimated future product sales;
|
| ● |
Estimated royalties on future product sales;
|
| ● |
Contractual milestone payments;
|
| ● |
Expenses we expect to incur;
|
| ● |
Income taxes; and
|
| ● |
An appropriate discount rate.
|
We estimated the selling price of the development services by using our internal estimates of the cost to perform the specific services and estimates of expected cash outflows to third parties for services and supplies over the expected period that we will perform the development services. The significant inputs we used to determine the selling price of the development services included:
| ● |
The number of internal hours we will spend performing these services;
|
| ● |
The estimated cost of work we will perform;
|
| ● |
The estimated cost of work that we will contract with third parties to perform; and
|
| ● |
The estimated cost of API we will use.
|
For purposes of determining BESP of the services we will perform and the API we will deliver in our 2017 amended Bayer transaction, accounting guidance required us to include a markup for a reasonable profit margin.
Based on the units of accounting under the 2017 amended agreement, we allocated the $76.3 million of allocable consideration as follows:
| ● |
$64.9 million to the IONIS-FXI-LRx exclusive license;
|
| ● |
$11.0 million for development services for IONIS-FXI-LRx and IONIS-FXIRx; and
|
| ● |
$0.4 million for the remaining delivery of IONIS-FXIRx API.
|
Assuming a constant selling price for the other elements in the arrangement, if there was an assumed 10 percent increase or decrease in the estimated selling price of the IONIS-FXI-LRx license, we determined that the revenue we would have allocated to the IONIS-FXI-LRx license would change by approximately one percent, or $0.7 million, from the amount we recorded.
Timing of revenue recognition
We recognize revenue as we deliver each item under the arrangement and the related revenue is realizable and earned. For example, we recognized revenue for the exclusive license we granted Bayer for IONIS-FXI-LRx in the first quarter of 2017 because that was when we delivered the license. We also recognize revenue over time as we provide services. Our collaborative agreements typically include a research and/or development project plan outlining the activities the agreement requires each party to perform during the collaboration. We estimate our period of performance at the inception of the agreement when the agreements we enter into do not clearly define such information. We then recognize revenue from development services ratably over such period. In certain instances, the period of performance may change as the development plans for our drugs progress. If our estimates and judgments change over the course of our collaboration agreements, it may affect the timing and amount of revenue that we recognize in future periods. We recognize any changes in estimates on a prospective basis.
F-11
The following are the periods over which we are recognizing revenue for each of our units of accounting under our 2017 amended Bayer agreement:
| ● |
We recognized the portion of the consideration attributed to the IONIS-FXI-LRx license in the first quarter of 2017 because we delivered the license and earned the revenue;
|
| ● |
We are recognizing the amount attributed to the development services for IONIS-FXI-LRx and IONIS-FXIRx over the period of time we are performing the services; and
|
| ● |
We are recognizing the amount attributed to the remaining API supply as we deliver it to Bayer.
|
Multiple agreements
From time to time, we may enter into separate agreements at or near the same time with the same partner. We evaluate such agreements to determine whether they should be accounted for individually as distinct arrangements or whether the separate agreements are, in substance, a single multiple element arrangement. We evaluate whether the negotiations are conducted jointly as part of a single negotiation, whether the deliverables are interrelated or interdependent, whether fees in one arrangement are tied to performance in another arrangement, and whether elements in one arrangement are essential to another arrangement. Our evaluation involves significant judgment to determine whether a group of agreements might be so closely related that they are, in effect, part of a single arrangement. For example, in the first quarter of 2017, we and Akcea entered into two separate agreements with Novartis at the same time: a collaboration agreement and a stock purchase agreement, or SPA.
Akcea entered into a collaboration agreement with Novartis to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Under the collaboration agreement, Akcea received a $75 million upfront payment. For each drug, Akcea is responsible for completing a Phase 2 program, conducting an end-of-Phase 2 meeting with the Food and Drug Administration, or FDA, and delivering API. Under the collaboration agreement, Novartis has an exclusive option to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. If Novartis exercises an option for one of these drugs, Novartis will pay Akcea a $150 million license fee and will assume all further global development, regulatory and commercialization activities and costs for the licensed drug. Akcea is also eligible to receive a development milestone payment, milestone payments if Novartis achieves pre-specified regulatory milestones, commercial milestones and tiered royalties on net sales from each drug under the collaboration.
Under the SPA, Novartis purchased 1.6 million shares of Ionis’ common stock for $100 million in the first quarter of 2017 and paid a premium over the weighted average trading price at the time of purchase. Additionally, the SPA required Novartis to purchase $50 million of Akcea’s common stock in a concurrent private placement with Akcea’s IPO in July 2017.
We evaluated the Novartis agreements to determine whether we should treat the agreements separately or as a single arrangement. We considered that the agreements were negotiated concurrently and in contemplation of one another. Additionally, the same individuals were involved in the negotiations of both agreements. Based on these facts and circumstances, we concluded that we should treat both agreements as a single arrangement and evaluate the provisions of the agreements on a combined basis. Refer to Note 6, Collaborative Arrangements and Licensing Agreements for further discussion of the accounting treatment for the Novartis collaboration.
Milestone payments
Our collaborations often include contractual milestones, which typically relate to the achievement of pre-specified development, regulatory and/ or commercialization events. These three categories of milestone events reflect the three stages of the life-cycle of our drugs, which we describe in more detail in the following paragraphs.
Prior to the first stage in the life-cycle of our drugs, we perform a significant amount of work using our proprietary antisense technology to design chemical compounds that interact with specific genes that are good targets for drug discovery. From these research efforts, we hope to identify a development candidate. The designation of a development candidate is the start of the development stage, which is the first stage in the life-cycle of our drugs. A development candidate is a chemical compound that has demonstrated the necessary safety and efficacy in preclinical animal studies to warrant further study in humans.
During the first step of the development stage, we or our partners study our drugs in Investigational New Drug, or IND,-enabling studies, which are animal studies intended to support an IND application and/or the foreign equivalent. An approved IND allows us or our partners to study our development candidate in humans. If the regulatory agency approves the IND, we or our partners initiate a Phase 1 clinical trial in which we typically enroll a small number of healthy volunteers to ensure the development candidate is safe for use in patients. If we or our partners determine that a development candidate is safe based on the Phase 1 data, we or our partners initiate Phase 2 studies that are generally larger studies in patients with the primary intent of determining the preliminary efficacy and safety of the development candidate.
The final step in the development stage is Phase 3 studies to gather the necessary safety and efficacy data to request marketing authorization from the FDA and/or foreign equivalents. Phase 3 studies typically involve larger numbers of patients and can take up to several years to complete.
If the data gathered during the Phase 3 trials demonstrates acceptable safety and efficacy results, we or our partner will submit an application to the FDA and/or its foreign equivalents for marketing authorization. This stage of the drug’s life-cycle is the regulatory stage.
F-12
If the FDA or a foreign equivalent grants marketing authorization for a drug, it moves into the commercialization stage. During this stage we or our partner will market and sell the drug to patients. Although our partner may ultimately be responsible for marketing and selling a partnered drug, our efforts to discover and develop a drug that is safe, effective and reliable contributes significantly to our partner’s ability to successfully sell the drug. The FDA and its foreign equivalents have the authority to impose significant restrictions on an approved drug through the product label and on advertising, promotional and distribution activities. Therefore, our efforts designing and executing the necessary animal and human studies are critical to obtaining claims in the product label from the regulatory agencies that would allow us or our partner to successfully commercialize our drug. Further, the patent protection afforded our drugs as a result of our initial patent applications and related prosecution activities in the United States and foreign jurisdictions are critical to our partner’s ability to sell our drugs without competition from generic drugs. The potential sales volume of an approved drug is dependent on several factors including the size of the patient population, market penetration of the drug, and the price charged for the drug.
Generally, the milestone events contained in our partnership agreements coincide with the progression of our drugs from development, to marketing authorization and then to commercialization. The process of successfully discovering a new development candidate, having it approved and ultimately selling it for a profit is highly uncertain. As such, the milestone payments we may earn from our partners involve a significant degree of risk to achieve. Therefore, as a drug progresses through the stages of its life-cycle, the value of the drug generally increases.
Development milestones in our partnerships may include the following types of events:
| ● |
Designation of a development candidate. Following the designation of a development candidate, IND-enabling animal studies for a new development candidate generally take 12 to 18 months to complete.
|
| ● |
Initiation of a Phase 1 clinical trial. Generally, Phase 1 clinical trials take one to two years to complete.
|
| ● |
Initiation or completion of a Phase 2 clinical trial. Generally, Phase 2 clinical trials take one to three years to complete.
|
| ● |
Initiation or completion of a Phase 3 clinical trial. Generally, Phase 3 clinical trials take two to four years to complete.
|
Regulatory milestones in our partnerships may include the following types of events:
| ● |
Filing of regulatory applications for marketing authorization such as a New Drug Application, or NDA, in the United States or a Marketing Authorization Application, or MAA, in Europe. Generally, it takes six to twelve months to prepare and submit regulatory filings.
|
| ● |
Obtaining marketing authorization in a major market, such as the United States, Europe or Japan. Generally it takes one to two years after an application is submitted to obtain authorization from the applicable regulatory agency.
|
Commercialization milestones in our partnerships may include the following types of events:
| ● |
First commercial sale in a particular market, such as in the United States or Europe.
|
| ● |
Product sales in excess of a pre-specified threshold, such as annual sales exceeding $1 billion. The amount of time to achieve this type of milestone depends on several factors including but not limited to the dollar amount of the threshold, the pricing of the product and the pace at which customers begin using the product.
|
We assess whether a substantive milestone exists at the inception of our agreements. When a substantive milestone is achieved, we recognize revenue related to the milestone payment immediately. For our licensing and collaboration agreements in which we are involved in the discovery and/or development of the related drug or provide the partner with access to new technologies we discover, we have determined that the majority of future development, regulatory and commercialization milestones are substantive. For example, we consider most of the milestones associated with our strategic alliance with Biogen substantive because we are using our antisense drug discovery platform to discover and develop new drugs against targets for neurological diseases. In evaluating if a milestone is substantive we consider whether:
| ● |
Substantive uncertainty exists as to the achievement of the milestone event at the inception of the arrangement;
|
| ● |
The achievement of the milestone involves substantive effort and can only be achieved based in whole or in part on our performance or the occurrence of a specific outcome resulting from our performance;
|
| ● |
The amount of the milestone payment appears reasonable either in relation to the effort expended or to the enhancement of the value of the delivered items;
|
| ● |
There is no future performance required to earn the milestone; and
|
| ● |
The consideration is reasonable relative to all deliverables and payment terms in the arrangement.
|
If any of these conditions are not met, we do not consider the milestone to be substantive and we defer recognition of the milestone payment and recognize it as revenue over our estimated period of performance, if any. Further information about our collaborative arrangements can be found in Note 6, Collaborative Arrangements and Licensing Agreements.
Option to license
In several of our collaboration agreements, we provide our partner with an option to obtain a license to one or more of our drugs. When we have a multiple element arrangement that includes an option to obtain a license, we evaluate if the option is a deliverable at the inception of the arrangement. We do not consider the option to be a deliverable if we conclude that it is substantive and not priced at a significant and incremental discount. We consider an option substantive if, at the inception of the arrangement, we are at risk as to whether our collaboration partner will choose to exercise its option to obtain the license. In those circumstances, we do not include the associated license fee in the allocable consideration at the inception of the agreement. Rather, we account for the license fee when our partner exercises its option. For example, during 2017, we earned license fee revenue when three of our partners, Bayer, Janssen and Roche, exercised their options to license three of our drugs, which under the respective agreements we concluded to be substantive options at inception. As a result, in 2017 we recognized the related revenue immediately in research and development revenue under collaborative agreements on our statement of operations as these amounts relate to drugs in development under research and development collaboration arrangements.
F-13
Research, Development and Patent Expenses
Our research and development expenses include wages, benefits, facilities, supplies, external services, clinical trial and manufacturing costs and other expenses that are directly related to our research and development operations. We expense research and development costs as we incur them. When we make payments for research and development services prior to the services being rendered, we record those amounts as prepaid assets on our consolidated balance sheet and we expense them as the services are provided. For the years ended December 31, 2017, 2016 and 2015, research and development expenses were $372.5 million, $340.4 million and $319.5 million, respectively. A portion of the costs included in research and development expenses are costs associated with our partner agreements. For the years ended December 31, 2017, 2016 and 2015, research and development costs of approximately $59.5 million, $187.1 million and $161.7 million, respectively, were related to our partner agreements.
We capitalize costs consisting principally of outside legal costs and filing fees related to obtaining patents. We amortize patent costs over the useful life of the patent, beginning with the date the United States Patent and Trademark Office, or foreign equivalent, issues the patent. The weighted average remaining amortizable life of our issued patents was 10.1 years at December 31, 2017.
The cost of our patents capitalized on our consolidated balance sheet at December 31, 2017 and 2016 was $30.8 million and $28.8 million, respectively. Accumulated amortization related to patents was $8.8 million and $8.4 million at December 31, 2017 and 2016, respectively.
Based on our existing patents, we estimate amortization expense related to patents in each of the next five years to be the following:
|
Years Ending December 31,
|
Amortization
(in millions)
|
|||
|
2018
|
$
|
1.6
|
||
|
2019
|
$
|
1.4
|
||
|
2020
|
$
|
1.3
|
||
|
2021
|
$
|
1.3
|
||
|
2022
|
$
|
1.2
|
||
We review our capitalized patent costs regularly to ensure that they include costs for patents and patent applications that have future value. When we identify patents and patent applications that we are not actively pursuing, we write off any associated costs. In 2017, 2016 and 2015, patent expenses were $2.1 million, $3.9 million and $2.8 million, respectively, and included non-cash charges related to the write-down of our patent costs to their estimated net realizable values of $0.4 million, $2.3 million and $1.1 million, respectively.
Accrued Liabilities
Our accrued liabilities consisted of the following (in thousands):
|
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Clinical expenses
|
$
|
16,347
|
$
|
23,428
|
||||
|
In-licensing expenses
|
33,790
|
6,430
|
||||||
|
Other miscellaneous expenses
|
16,481
|
6,155
|
||||||
|
Total accrued liabilities
|
$
|
66,618
|
$
|
36,013
|
||||
Noncontrolling Interest in Akcea Therapeutics, Inc.
In July 2017, Akcea completed an IPO. Akcea raised $193.8 million of aggregate gross proceeds from the IPO, including $50.0 million from a private placement by Novartis. Akcea’s net proceeds were $182.4 million. As part of Akcea’s IPO, we invested $25.0 million. In conjunction with the IPO, the shares of Akcea’s series A convertible preferred stock we owned converted into shares of Akcea’s common stock. Additionally, the amount outstanding under Akcea’s line of credit with us converted into shares of Akcea’s common stock.
Prior to Akcea’s IPO in July 2017, we owned 100 percent of Akcea’s stock and consolidated 100 percent of Akcea’s results in our financial statements. In connection with Akcea’s IPO, shares of Akcea’s common stock were sold to third parties. We owned approximately 68 percent of Akcea after the IPO and at December 31, 2017. The shares third parties own represent an interest in Akcea’s equity that is not controlled by us. However, as we continue to maintain overall control of Akcea through our voting interest, we reflect the assets, liabilities and results of operations of Akcea in our consolidated financial statements. The noncontrolling interest attributable to other owners of Akcea’s common stock is reflected in a separate line on the statement of operations and a separate line within stockholders’ equity in our consolidated financial statements. In addition, we recorded a noncontrolling interest adjustment to account for the stock options that Akcea grants for its common stock, which if exercised, will dilute our ownership in Akcea. This adjustment was reflected as a reclassification within stockholders’ equity from additional paid-in capital to noncontrolling interest in Akcea equal to the amount of stock-based compensation expense Akcea had recognized from inception through the IPO. Going forward, each period we will reclassify Akcea’s stock-based compensation expense in a similar fashion.
F-14
Concentration of Credit Risk
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash equivalents, short-term investments and receivables. We place our cash equivalents and short-term investments with reputable financial institutions. We primarily invest our excess cash in commercial paper and debt instruments of the U.S. Treasury, financial institutions, corporations, and U.S. government agencies with strong credit ratings and an investment grade rating at or above A-1, P-1 or F-1 by Moody’s, Standard & Poor’s, or S&P, or Fitch, respectively. We have established guidelines relative to diversification and maturities that maintain safety and liquidity. We periodically review and modify these guidelines to maximize trends in yields and interest rates without compromising safety and liquidity.
Cash, Cash Equivalents and Short-Term Investments
We consider all liquid investments with maturities of three months or less when we purchase them to be cash equivalents. Our short-term investments have initial maturities of greater than three months from date of purchase. We classify our short-term investments as “available-for-sale” and carry them at fair market value based upon prices for identical or similar items on the last day of the fiscal period. We record unrealized gains and losses as a separate component of comprehensive income (loss) and include net realized gains and losses in gain (loss) on investments. We use the specific identification method to determine the cost of securities sold.
We have equity investments of less than 20 percent in privately and publicly held biotechnology companies that we received as part of a technology license or partner agreement. At December 31, 2017, we held equity investments in one publicly held company, Antisense Therapeutics Limited, or ATL. Furthermore, we held cost method investments in five companies, Atlantic Pharmaceuticals Limited, Dynacure SAS, Kastle Therapeutics, Seventh Sense Biosystems and Suzhou Ribo Life Science Co., Ltd.
We account for our equity investments in publicly held companies at fair value and record unrealized gains and losses related to temporary increases and decreases in the stock as a separate component of comprehensive income (loss). We account for our equity investments in privately held companies under the cost method of accounting because we own less than 20 percent and do not have significant influence over their operations. Realization of our equity position in these private companies is usually uncertain. When realization of our investment is uncertain, we record a full valuation allowance. In determining if and when a decrease in market value below our cost in our equity positions is temporary or other-than-temporary, we examine historical trends in the stock price, the financial condition of the company, near term prospects of the company and our current need for cash. If we determine that a decline in value in either a public or private investment is other-than-temporary, we recognize an impairment loss in the period in which the other-than-temporary decline occurs.
Inventory Valuation
We capitalize the costs of raw materials that we purchase for use in producing our drugs because until we use these raw materials they have alternative future uses. We include in inventory raw material costs for drugs that we manufacture for our partners under contractual terms and that we use primarily in our clinical development activities and drug products. We can use each of our raw materials in multiple products and, as a result, each raw material has future economic value independent of the development status of any single drug. For example, if one of our drugs failed, we could use the raw materials for that drug to manufacture our other drugs. We expense these costs when we begin to manufacture API for a particular drug. We reflect our inventory on the balance sheet at the lower of cost or market value under the first-in, first-out method, or FIFO. We review inventory periodically and reduce the carrying value of items we consider to be slow moving or obsolete to their estimated net realizable value. We consider several factors in estimating the net realizable value, including shelf life of raw materials, alternative uses for our drugs and clinical trial materials, and historical write-offs. We did not record any inventory write-offs for the years ended December 31, 2017, 2016 or 2015. Total inventory was $10.0 million and $7.5 million as of December 31, 2017 and 2016, respectively.
Property, Plant and Equipment
We carry our property, plant and equipment at cost and depreciate it on the straight-line method over its estimated useful life, which consists of the following (in thousands):
|
Estimated Useful Lives
|
December 31,
|
|||||||||||
|
(in years)
|
2017
|
2016
|
||||||||||
|
Computer software, laboratory, manufacturing and other equipment
|
3 to 10
|
$
|
66,558
|
$
|
63,287
|
|||||||
|
Building, building improvements and building systems
|
15 to 40
|
92,770
|
48,909
|
|||||||||
|
Land improvements
|
20 |
2,853
|
2,853
|
|||||||||
|
Leasehold improvements
|
5 to 15
|
26,748
|
41,736
|
|||||||||
|
Furniture and fixtures
|
5 to 10
|
6,161
|
5,937
|
|||||||||
|
195,090
|
162,722
|
|||||||||||
|
Less accumulated depreciation
|
(87,676
|
)
|
(80,075
|
)
|
||||||||
|
107,414
|
82,647
|
|||||||||||
|
Land
|
14,493
|
10,198
|
||||||||||
|
Total
|
$
|
121,907
|
$
|
92,845
|
||||||||
F-15
We depreciate our leasehold improvements using the shorter of the estimated useful life or remaining lease term. As a result of the purchase of our primary manufacturing facility in 2017, we reclassed previously capitalized leasehold improvements to building, building improvements and building systems. Additionally, during 2017 we made additional improvements and expansions of our buildings to accommodate the growth in our business.
Fair Value of Financial Instruments
We have estimated the fair value of our financial instruments. The amounts reported for cash, accounts receivable, accounts payable and accrued expenses approximate the fair value because of their short maturities. We report our investment securities at their estimated fair value based on quoted market prices for identical or similar instruments.
Long-Lived Assets
We evaluate long-lived assets, which include property, plant and equipment and patent costs, for impairment on at least a quarterly basis and whenever events or changes in circumstances indicate that we may not be able to recover the carrying amount of such assets. We recorded charges of $0.8 million, $2.3 million and $1.9 million for the years ended December 31, 2017, 2016 and 2015, respectively, related primarily to the write-down of intangible assets.
Use of Estimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ from those estimates.
Stock-Based Compensation Expense
We measure stock-based compensation expense for equity-classified awards, principally related to stock options, restricted stock units, or RSUs, and stock purchase rights under our ESPP based on the estimated fair value of the award on the date of grant. We recognize the value of the portion of the award that we ultimately expect to vest as stock-based compensation expense over the requisite service period in our Consolidated Statements of Operations. We reduce stock-based compensation expense for estimated forfeitures at the time of grant and revise in subsequent periods if actual forfeitures differ from those estimates.
We use the Black-Scholes model as our method of valuing option awards and stock purchase rights under our ESPP. On the grant date, we use our stock price and assumptions regarding a number of highly complex and subjective variables to determine the estimated fair value of stock-based payment awards. These variables include, but are not limited to, our expected stock price volatility over the term of the awards, and actual and projected employee stock option exercise behaviors. Option-pricing models were developed for use in estimating the value of traded options that have no vesting or hedging restrictions and are fully transferable. Because our employee stock options have certain characteristics that are significantly different from traded options, and because changes in the subjective assumptions can materially affect the estimated value, in management’s opinion, the existing valuation models may not provide an accurate measure of the fair value of our employee stock options. Although we determine the estimated fair value of employee stock options using an option-pricing model, that value may not be indicative of the fair value observed in a willing buyer/willing seller market transaction.
We recognize compensation expense for option awards using the accelerated multiple-option approach. Under the accelerated multiple-option approach (also known as the graded-vesting method), we recognize compensation expense over the requisite service period for each separately vesting tranche of the award as though the award were in substance multiple awards, which results in the expense being front-loaded over the vesting period.
The fair value of RSUs is based on the market price of our common stock on the date of grant. The RSUs we have granted vest annually over a four-year period.
See Note 4, Stockholders’ Equity, for additional information regarding our stock-based compensation plans.
Accumulated other comprehensive income (loss)
Accumulated other comprehensive income (loss) is primarily comprised of unrealized gains and losses on investments, net of taxes and adjustments we made to reclassify realized gains and losses on investments from other accumulated comprehensive income (loss) to our Consolidated Statement of Operations. The following table summarizes changes in accumulated other comprehensive income (loss) for the years ended December 31, 2017, 2016 and 2015 (in thousands):
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Beginning balance accumulated other comprehensive (loss) income
|
$
|
(30,358
|
)
|
$
|
(13,565
|
)
|
$
|
39,747
|
||||
|
Unrealized losses on securities, net of tax (1)
|
(960
|
)
|
(17,219
|
)
|
(33,101
|
)
|
||||||
|
Amounts reclassified from accumulated other comprehensive (loss) income (2)
|
(374
|
)
|
447
|
(20,211
|
)
|
|||||||
|
Currency translation adjustment
|
(67
|
)
|
(21
|
)
|
—
|
|||||||
|
Net other comprehensive loss for the period
|
(1,401
|
)
|
(16,793
|
)
|
(53,312
|
)
|
||||||
|
Ending balance accumulated other comprehensive loss
|
$
|
(31,759
|
)
|
$
|
(30,358
|
)
|
$
|
(13,565
|
)
|
|||
| (1) |
There was no tax expense for other comprehensive loss for the years ended December 31, 2017, 2016 or 2015.
|
| (2) |
Amounts for 2015 and 2017 are included in the separate line called “Gain on investment in Regulus Therapeutics Inc.” on our Consolidated Statement of Operations.
|
F-16
Convertible Debt
We account for convertible debt instruments, including our 1 percent and 2¾ percent notes that may be settled in cash upon conversion (including partial cash settlement) by separating the liability and equity components of the instruments in a manner that reflects our nonconvertible debt borrowing rate. We determine the carrying amount of the liability component by measuring the fair value of similar debt instruments that do not have the conversion feature. If no similar debt instrument exists, we estimate fair value by using assumptions that market participants would use in pricing a debt instrument, including market interest rates, credit standing, yield curves and volatilities. Determining the fair value of the debt component requires the use of accounting estimates and assumptions. These estimates and assumptions are judgmental in nature and could have a significant impact on the determination of the debt component, and the associated non-cash interest expense.
We assigned a value to the debt component of our convertible notes equal to the estimated fair value of similar debt instruments without the conversion feature, which resulted in us recording our debt at a discount. We are amortizing our debt issuance costs and debt discount over the life of the convertible notes as additional non-cash interest expense utilizing the effective interest method. For additional information, see Note 3, Long-Term Obligations and Commitments.
Segment Information
We have two operating segments, our Ionis Core segment and Akcea Therapeutics. Prior to Akcea’s IPO in July 2017, we owned 100 percent of Akcea’s stock. After Akcea’s IPO, we owned approximately 68 percent of Akcea. We did not change our reportable segments as a result of Akcea’s IPO. Akcea is a late-stage biopharmaceutical company focused on developing and commercializing drugs to treat people with serious cardiometabolic diseases caused by lipid disorders. We provide segment financial information and results for our Ionis Core segment and our Akcea Therapeutics segment based on the segregation of revenues and expenses that our chief decision maker reviews to assess operating performance and to make operating decisions. We allocate a portion of Ionis’ development, R&D support expenses and general and administrative expenses to Akcea for work we performed on behalf of Akcea.
Fair Value Measurements
We use a three-tier fair value hierarchy to prioritize the inputs used in our fair value measurements. These tiers include: Level 1, defined as observable inputs such as quoted prices in active markets for identical assets, which includes our money market funds and treasury securities classified as available-for-sale securities and our investment in equity securities in publicly held biotechnology companies; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable, which includes our fixed income securities and commercial paper classified as available-for-sale securities; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring us to develop our own assumptions. We classify the majority of our securities as Level 2. We obtain the fair value of our Level 2 investments from our custodian bank or from a professional pricing service. We validate the fair value of our Level 2 investments by understanding the pricing model used by the custodian banks or professional pricing service provider and comparing that fair value to the fair value based on observable market prices. During 2017 and 2016, there were no transfers between our Level 1 and Level 2 investments. When we recognize transfers between levels of the fair value hierarchy, we recognize the transfer on the date the event or change in circumstances that caused the transfer occurs. When we recognize transfers between levels of the fair value hierarchy, we recognize the transfer on the date the event or change in circumstances that caused the transfer occurs. During 2017 and 2016 we did not have any investments that were classified as Level 3 investments.
The following tables present the major security types we held at December 31, 2017 and 2016 that are regularly measured and carried at fair value. The tables segregate each security type by the level within the fair value hierarchy of the valuation techniques we utilized to determine the respective securities’ fair value (in thousands):
|
At
December 31, 2017
|
Quoted Prices in
Active Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
||||||||||
|
Cash equivalents (1)
|
$
|
86,262
|
$
|
86,262
|
$
|
—
|
||||||
|
Corporate debt securities (2)
|
647,461
|
—
|
647,461
|
|||||||||
|
Debt securities issued by U.S. government agencies (3)
|
136,325
|
—
|
136,325
|
|||||||||
|
Debt securities issued by the U.S. Treasury (3)
|
30,818
|
30,818
|
—
|
|||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states (4)
|
93,932
|
—
|
93,932
|
|||||||||
|
Total
|
$
|
994,798
|
$
|
117,080
|
$
|
877,718
|
||||||
|
At
December 31, 2016
|
Quoted Prices in
Active Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
||||||||||
|
Cash equivalents (1)
|
$
|
54,137
|
$
|
54,137
|
$
|
—
|
||||||
|
Corporate debt securities (3)
|
396,221
|
—
|
396,221
|
|||||||||
|
Debt securities issued by U.S. government agencies (3)
|
55,179
|
—
|
55,179
|
|||||||||
|
Debt securities issued by the U.S. Treasury (3)
|
29,286
|
29,286
|
—
|
|||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states (5)
|
109,111
|
—
|
109,111
|
|||||||||
|
Investment in Regulus Therapeutics Inc.
|
2,414
|
2,414
|
—
|
|||||||||
|
Total
|
$
|
646,348
|
$
|
85,837
|
$
|
560,511
|
||||||
| (1) |
Included in cash and cash equivalents on our consolidated balance sheet.
|
| (2) |
$11.9 million included in cash and cash equivalents on our consolidated balance sheet, with the difference included in short-term investments on our consolidated balance sheet.
|
F-17
| (3) |
Included in short-term investments on our consolidated balance sheet.
|
| (4) |
$3.5 million included in cash and cash equivalents on our consolidated balance sheet, with the difference included in short-term investments on our consolidated balance sheet.
|
| (5) |
$9.3 million included in cash and cash equivalents on our consolidated balance sheet, with the difference included in short-term investments on our consolidated balance sheet.
|
Novartis Future Stock Purchase
In January 2017, we and Akcea entered into a SPA with Novartis. As part of the SPA, Novartis was required to purchase $50 million of Akcea’s common stock at the IPO price or our common stock at a premium if an IPO did not occur by April 2018. Therefore, at the inception of the SPA, we recorded a $5.0 million asset representing the fair value of the potential future premium we could have received if Novartis purchased our common stock. We determined the fair value of the future premium by calculating the value based on the stated premium in the SPA and estimating the probability of an Akcea IPO. We also included a lack of marketability discount when we determined the fair value of the premium because we would have issued unregistered shares to Novartis if they had purchased our common stock. We measured this asset using Level 3 inputs and recorded it in other assets on our consolidated balance sheet. Because Akcea completed its IPO before April 2018, Novartis will not purchase additional shares of Ionis stock. Therefore, this asset no longer had any value and we wrote-off the remaining balance to other expenses on our third quarter 2017 consolidated statement of operations.
The following is a reconciliation of the potential premium we would have received if Akcea had not completed its IPO, measured at fair value on a recurring basis using significant unobservable inputs (Level 3) for 2017 (in thousands):
|
Year Ended
December 31, 2017
|
||||
|
Beginning balance of Level 3 instruments
|
$
|
—
|
||
|
Value of the potential premium we will receive from Novartis at inception of the SPA (January 2017)
|
5,035
|
|||
|
Write-off of premium to other expenses
|
(5,035
|
)
|
||
|
Ending balance of Level 3 instruments
|
$
|
—
|
||
Income Taxes
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act of 2017, or the Tax Act. The Tax Act makes broad and complex changes to the U.S. tax code. The changes include, but are not limited to, reducing the U.S. federal corporate tax rate from 35 percent to 21 percent, imposing a mandatory one-time transition tax on certain unrepatriated earnings of foreign subsidiaries, introducing bonus depreciation that will allow for full expensing of qualified property, eliminating the corporate alternative minimum tax, or AMT, and changing how existing AMT credits can be realized, and modifying or repealing many business tax deductions and credits.
The SEC staff issued guidance to address the application of U.S. GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the Tax Act.
We account for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in our financial statements or tax returns. In addition, deferred tax assets are recorded for the future benefit of utilizing net operating losses and research and development credit carryforwards. Valuation allowances are provided when necessary to reduce deferred tax assets to the amount expected to be realized.
We apply the authoritative accounting guidance prescribing a threshold and measurement attribute for the financial recognition and measurement of a tax position taken or expected to be taken in a tax return. We recognize liabilities for uncertain tax positions based on a two-step process. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation settlement. The second step is to estimate and measure the tax benefit as the largest amount that is more than 50 percent likely to be realized upon ultimate settlement.
We recognize interest and penalties related to unrecognized tax benefits within the income tax expense line in the accompanying consolidated statements of operations. Accrued interest and penalties are included within other long-term liabilities in the consolidated balance sheets.
We are required to use significant judgment in evaluating our uncertain tax positions and determining our provision for income taxes. Although we believe our reserves are reasonable, no assurance can be given that the final tax outcome of these matters will not be different from that which is reflected in our historical income tax provisions and accruals. We adjust these reserves for changing facts and circumstances, such as the closing of a tax audit or the refinement of an estimate. To the extent that the final tax outcome of these matters is different than the amounts recorded, such differences may impact the provision for income taxes in the period in which such determination is made.
We are also required to use significant judgment in determining any valuation allowance recorded against our deferred tax assets. In assessing the need for a valuation allowance, we consider all available evidence, including scheduled reversal of deferred tax liabilities, past operating results, the feasibility of tax planning strategies and estimates of future taxable income. Estimates of future taxable income are based on assumptions that are consistent with our plans. The assumptions we use represent our best estimates and involve inherent uncertainties and the application of our judgment. Should actual amounts differ from our estimates, the amount of our tax expense and liabilities we recognize could be materially impacted.
F-18
We record a valuation allowance to reduce the balance of our net deferred tax assets to the amount we believe is more-likely-than-not to be realized. We have incurred historical financial statement losses and as a result we had a full valuation allowance recorded against our net deferred tax assets for each of the years in these financial statements. We regularly assess the future realization of our net deferred tax assets and will reduce the valuation allowance in any such period in which we determine that all, or a portion, of our deferred tax assets are more-likely-than-not to be realized.
We do not provide for a U.S. income tax liability and foreign withholding taxes on undistributed foreign earnings of our foreign subsidiaries.
Impact of Recently Issued Accounting Standards
In May 2014, the FASB issued accounting guidance on the recognition of revenue from customers. Under this guidance, an entity will recognize revenue when it transfers control of promised goods or services to customers in an amount that reflects what the entity expects to receive in exchange for the goods or services. Further an entity will recognize revenue upon satisfying the performance obligation(s) under the related contract. Our performance obligation under our collaboration agreements is typically the research and development activities associated with the delivery of a drug candidate or drug to our partner. Under the current accounting guidance, we recognize revenue from milestone payments we earn under the milestone method from our collaboration agreements. Under the new guidance, the milestone method of revenue recognition is eliminated. Specifically, certain R&D milestone payments we previously recognized in full when we achieved a milestone will now be recognized over a period of time. If we achieve an R&D milestone payment related to activities we are performing under a collaboration agreement, we will recognize the associated revenue from the milestone payment over our estimated performance obligation period. For example, in 2017, we initiated a Phase 1/2a clinical study of IONIS-MAPTRx in patients with mild Alzheimer's disease. We earned a $10 million milestone payment from Biogen related to the initiation of this study. In 2017, we recognized the entire $10 million as revenue. Under the new standard, we will recognize this milestone payment over the period we are providing R&D services for Biogen. For milestones achieved for which we do not have a continuing performance obligation, we will continue to recognize the milestone payment in its entirety as revenue in the period in which our partner achieves the milestone. For example, in 2017, we earned a $50 million milestone payment from Biogen for the EU approval of SPINRAZA. Under both the new and old standard, we account for this milestone payment the same by recognizing the entire amount upon achievement of the event. This guidance does not change our recognition of commercial revenue from SPINRAZA royalties. We adopted this guidance on January 1, 2018 under the full retrospective approach, which requires us to recast our prior period amounts in the period of adoption.
Our adoption of the standard in 2018 will result in the recognition of additional revenue of approximately $17 million and approximately $27 million for 2017 and 2016, respectively. In addition, our adoption of the standard will result in an increase in our deferred revenue balance of approximately $39 million at December 31, 2017 and a corresponding adjustment to our accumulated deficit for the same amount. Since our collaboration revenue has no associated cost of sales, the impact to our net loss (income) is equal to our revenue adjustment for each period. Additionally, as a result of adopting this new guidance there is no impact to our income tax expense because we have a full valuation allowance. This new guidance also requires additional disclosures about the attributes of our revenue and balances associated with our contracts, which we will include in our first quarter of 2018 financial statements.
In January 2016, the FASB issued amended accounting guidance related to the recognition, measurement, presentation, and disclosure of certain financial instruments. The amended guidance requires us to measure and record equity investments, except those accounted for under the equity method of accounting that have a readily determinable fair value, at fair value and for us to recognize the changes in fair value in our net income (loss), instead of recognizing unrealized gains and losses through accumulated other comprehensive income, as we currently do under the existing guidance. The amended guidance also changes several disclosure requirements for financial instruments, including the methods and significant assumptions we use to estimate fair value. The guidance is effective for fiscal years, and interim periods within that year, beginning after December 15, 2017. We adopted this guidance on January 1, 2018. The adoption of this guidance did not have an impact on our financial results.
In February 2016, the FASB issued amended accounting guidance related to lease accounting, which will require us to record all leases with a term longer than one year on our balance sheet. When we record leases on our balance sheet under the new guidance, we will record a liability with a value equal to the present value of payments we will make over the life of the lease and an asset representing the underlying leased asset. The new accounting guidance requires us to determine if our leases are operating or financing leases. We will record expense for operating type leases on a straight-line basis as an operating expense. If we determine a lease is a financing lease, we will record both interest and amortization expense and generally the expense will be higher in the earlier periods of the lease. The new lease standard is effective for annual and interim periods beginning after December 15, 2018, with early adoption permitted. We must adopt the new standard on a modified retrospective basis, which requires us to reflect our leases on our consolidated balance sheet for the earliest comparative period presented. We plan to adopt this guidance on January 1, 2019. We are currently assessing the effects the new guidance will have on our consolidated financial statements and disclosures.
In June 2016, the FASB issued guidance that changes the measurement of credit losses for most financial assets and certain other instruments. If we have credit losses, this updated guidance requires us to record allowances for these instruments under a new expected credit loss model. This model requires us to estimate the expected credit loss of an instrument over its lifetime, which represents the portion of the amortized cost basis we do not expect to collect. This change will result in us remeasuring our allowance in each reporting period we have credit losses. The new standard is effective for annual and interim periods beginning after December 15, 2019. Early adoption is permitted for periods beginning after December 15, 2018. When we adopt the new standard, we will make any adjustments to beginning balances through a cumulative-effect adjustment to accumulated deficit on that date. We are currently assessing the timing of adoption as well as the effects it will have on our consolidated financial statements and disclosures.
In May 2017, the FASB issued clarifying guidance related to the accounting for modifications of stock-based payment awards. The new guidance is meant to clarify when modification accounting is required. We early adopted this guidance in our financial statements for the quarter ended June 30, 2017 and it did not have an effect on our consolidated financial statements and disclosures.
F-19
2. Investments
As of December 31, 2017, we had primarily invested our excess cash in debt instruments of the U.S. Treasury, financial institutions, corporations, and U.S. government agencies with strong credit ratings and an investment grade rating at or above A-1, P-1 or F-1 by Moody’s, Standard & Poor’s, or S&P, or Fitch, respectively. We have established guidelines relative to diversification and maturities that maintain safety and liquidity. We periodically review and modify these guidelines to maximize trends in yields and interest rates without compromising safety and liquidity.
The following table summarizes the contract maturity of the available-for-sale securities we held as of December 31, 2017:
|
One year or less
|
71
|
%
|
||
|
After one year but within two years
|
23
|
%
|
||
|
After two years but within three and one half years
|
6
|
%
|
||
|
Total
|
100
|
%
|
As illustrated above, at December 31, 2017, 94 percent of our available-for-sale securities had a maturity of less than two years.
All of our available-for-sale securities are available to us for use in our current operations. As a result, we categorize all of these securities as current assets even though the stated maturity of some individual securities may be one year or more beyond the balance sheet date.
At December 31, 2017, we had an ownership interest of less than 20 percentin five private companies and one public company with which we conduct business. The privately-held companies are Atlantic Pharmaceuticals Limited, Dynacure SAS, Kastle Therapeutics, Seventh Sense Biosystems and Suzhou Ribo Life Science CO. The publicly traded company is Antisense Therapeutics Limited, or ATL. We account for our equity investments in the privately-held companies under the cost method of accounting and we account for our equity investment in the publicly traded company at fair value. We record unrealized gains and losses as a separate component of comprehensive income (loss) and include net realized gains and losses in gain (loss) on investments.
During 2015 and 2017, we realized a net gain on our investment in Regulus of $20.2 million and $0.4 million, respectively, when we sold our stock in Regulus.
The following is a summary of our investments (in thousands):
|
Gross Unrealized
|
Estimated
|
|||||||||||||||
|
December 31, 2017
|
Cost (1)
|
Gains
|
Losses
|
Fair Value
|
||||||||||||
|
Available-for-sale securities:
|
||||||||||||||||
|
Corporate debt securities (2)
|
$
|
500,599
|
$
|
2
|
$
|
(752
|
)
|
$
|
499,849
|
|||||||
|
Debt securities issued by U.S. government agencies
|
83,926
|
—
|
(212
|
)
|
83,714
|
|||||||||||
|
Debt securities issued by the U.S. Treasury
|
29,428
|
—
|
(17
|
)
|
29,411
|
|||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states (2)
|
29,240
|
4
|
(122
|
)
|
29,122
|
|||||||||||
|
Total securities with a maturity of one year or less
|
643,193
|
6
|
(1,103
|
)
|
642,096
|
|||||||||||
|
Corporate debt securities
|
148,663
|
8
|
(1,059
|
)
|
147,612
|
|||||||||||
|
Debt securities issued by U.S. government agencies
|
52,779
|
—
|
(168
|
)
|
52,611
|
|||||||||||
|
Debt securities issued by the U.S. Treasury
|
1,409
|
—
|
(2
|
)
|
1,407
|
|||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states
|
65,550
|
—
|
(740
|
)
|
64,810
|
|||||||||||
|
Total securities with a maturity of more than one year
|
268,401
|
8
|
(1,969
|
)
|
266,440
|
|||||||||||
|
Total available-for-sale securities
|
$
|
911,594
|
$
|
14
|
$
|
(3,072
|
)
|
$
|
908,536
|
|||||||
|
Gross Unrealized
|
Estimated
|
|||||||||||||||
|
December 31, 2016
|
Cost (1)
|
Gains
|
Losses
|
Fair Value
|
||||||||||||
|
Available-for-sale securities:
|
||||||||||||||||
|
Corporate debt securities
|
$
|
195,087
|
$
|
25
|
$
|
(161
|
)
|
$
|
194,951
|
|||||||
|
Debt securities issued by U.S. government agencies
|
26,548
|
—
|
(10
|
)
|
26,538
|
|||||||||||
|
Debt securities issued by the U.S. Treasury
|
29,298
|
2
|
(14
|
)
|
29,286
|
|||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states (2)
|
72,775
|
2
|
(134
|
)
|
72,643
|
|||||||||||
|
Total securities with a maturity of one year or less
|
323,708
|
29
|
(319
|
)
|
323,418
|
|||||||||||
|
Corporate debt securities
|
202,408
|
36
|
(1,174
|
)
|
201,270
|
|||||||||||
|
Debt securities issued by U.S. government agencies
|
28,807
|
1
|
(167
|
)
|
28,641
|
|||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states
|
36,816
|
1
|
(349
|
)
|
36,468
|
|||||||||||
|
Total securities with a maturity of more than one year
|
268,031
|
38
|
(1,690
|
)
|
266,379
|
|||||||||||
|
Total available-for-sale securities
|
$
|
591,739
|
$
|
67
|
$
|
(2,009
|
)
|
$
|
589,797
|
|||||||
|
Equity securities:
|
||||||||||||||||
|
Regulus Therapeutics Inc.
|
$
|
2,133
|
$
|
281
|
$
|
—
|
$
|
2,414
|
||||||||
|
Total equity securities
|
$
|
2,133
|
$
|
281
|
$
|
—
|
$
|
2,414
|
||||||||
|
Total available-for-sale and equity securities
|
$
|
593,872
|
$
|
348
|
$
|
(2,009
|
)
|
$
|
592,211
|
|||||||
| (1) |
Our available-for-sale securities are held at amortized cost.
|
| (2) |
Includes investments classified as cash equivalents on our consolidated balance sheet.
|
F-20
Investments we consider to be temporarily impaired at December 31, 2017 are as follows (in thousands):
|
Less than 12 Months of
Temporary Impairment
|
More than 12 Months of
Temporary Impairment
|
Total Temporary
Impairment
|
||||||||||||||||||||||||||
|
Number of
Investments
|
Estimated
Fair Value
|
Unrealized
Losses
|
Estimated
Fair Value
|
Unrealized
Losses
|
Estimated
Fair Value
|
Unrealized
Losses
|
||||||||||||||||||||||
|
Corporate debt securities
|
476
|
$
|
551,446
|
$
|
(1,236
|
)
|
$
|
74,987
|
$
|
(575
|
)
|
$
|
626,433
|
$
|
(1,811
|
)
|
||||||||||||
|
Debt securities issued by U.S. government agencies
|
45
|
107,788
|
(262
|
)
|
27,538
|
(118
|
)
|
135,326
|
(380
|
)
|
||||||||||||||||||
|
Debt securities issued by the U.S. Treasury
|
7
|
30,818
|
(19
|
)
|
—
|
—
|
30,818
|
(19
|
)
|
|||||||||||||||||||
|
Debt securities issued by states of the U.S. and political subdivisions of the states
|
60
|
62,519
|
(545
|
)
|
24,572
|
(317
|
)
|
87,091
|
(862
|
)
|
||||||||||||||||||
|
Total temporarily impaired securities
|
588
|
$
|
752,571
|
$
|
(2,062
|
)
|
$
|
127,097
|
$
|
(1,010
|
)
|
$
|
879,668
|
$
|
(3,072
|
)
|
||||||||||||
We believe that the decline in value of our debt securities is temporary and primarily related to the change in market interest rates since purchase. We believe it is more likely than not that we will be able to hold these securities to maturity. Therefore, we anticipate full recovery of our debt securities’ amortized cost basis at maturity.
3. Long-Term Obligations and Commitments
The carrying value of our long-term obligations was as follows (in thousands):
|
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
1 percent convertible senior notes
|
$
|
533,111
|
$
|
500,511
|
||||
|
Long-term mortgage debt
|
59,771
|
—
|
||||||
|
Long-term financing liability for leased facility
|
—
|
72,359
|
||||||
|
Principal balance of fixed rate note with Morgan Stanley
|
12,500
|
12,500
|
||||||
|
Leases and other obligations
|
2,095
|
3,735
|
||||||
|
Total
|
$
|
607,477
|
$
|
589,105
|
||||
|
Less: current portion
|
(1,621
|
)
|
(1,185
|
)
|
||||
|
Total Long-Term Obligations
|
$
|
605,856
|
$
|
587,920
|
||||
Convertible Notes
In November 2014, we completed a $500 million offering of convertible senior notes, which mature in 2021 and bear interest at 1 percent. We raised $487 million of proceeds, net of issuance costs. We used a substantial portion of the net proceeds from the issuance of the 1 percent convertible senior notes to repurchase $140 million in principal of our 2¾ percent convertible senior notes at a price of $441.9 million, including accrued interest. As a result, the new principal balance of the 2¾ percent notes was $61.2 million.
In December 2016, we issued an additional $185.5 million of 1 percent convertible senior notes in exchange for the redemption of $61.1 million of our 2¾ percent convertible senior notes. As a result of the debt exchange we completed in December 2016, we recorded a $4.0 million non-cash loss on early retirement of debt, reflecting the early retirement of the majority of our remaining 2¾ percent convertible notes in December 2016.
At December 31, 2017, we had a nominal amount of our 2¾ percent convertible senior notes outstanding. At December 31, 2017 we had the following 1 percent convertible senior notes outstanding (amounts in millions except price per share data):
|
1 Percent
Convertible Senior Notes
|
||||
|
Outstanding balance
|
$
|
685.5
|
||
|
Original issue date ($500 million of principal)
|
November 2014
|
|||
|
Additional issue date ($185.5 million of principal)
|
December 2016
|
|||
|
Maturity date
|
November 2021
|
|||
|
Interest rate
|
1 percent
|
|||
|
Conversion price per share
|
$
|
66.81
|
||
|
Total shares of common stock subject to conversion
|
10.3
|
|||
Interest is payable semi-annually in arrears on May 15 and November 15 of each year for the 1 percent notes. The 1 percent notes are convertible at the option of the note holders prior to July 1, 2021 only under certain conditions. On or after July 1, 2021, the notes are initially convertible into approximately 10.3 million shares of common stock at a conversion price of approximately $66.81 per share. We will settle conversions of the notes, at our election, in cash, shares of our common stock or a combination of both. We may not redeem the 1 percent notes prior to maturity, and no sinking fund is provided for them. If we undergo a fundamental change, holders may require us to purchase for cash all or any portion of their 1 percent notes at a purchase price equal to 100 percent of the principal amount of the notes to be purchased, plus accrued and unpaid interest to, but excluding, the fundamental change purchase date.
F-21
We account for our convertible notes using an accounting standard that requires us to assign a value to our convertible debt equal to the estimated fair value of similar debt instruments without the conversion feature and to record the remaining portion in equity. As a result, we recorded our convertible notes at a discount, which we are amortizing as additional non-cash interest expense over the expected life of the respective debt. We determined our nonconvertible debt borrowing rate using a combination of the present value of the debt’s cash flows and a Black-Scholes valuation model. The following table summarizes the nonconvertible borrowing rate, effective interest rate and amortization period of our debt discount for our convertible notes:
|
1 Percent
Convertible Senior Notes
Issued in November 2014
|
1 Percent
Convertible Senior Notes
Issued in December 2016
|
||
|
Nonconvertible debt borrowing rate
|
7.4 percent
|
6.8 percent
|
|
|
Effective interest rate
|
7.8 percent
|
7.2 percent
|
|
|
Amortization period of debt discount
|
7 years
|
5 years
|
Interest expense for the year ended December 31, 2017, 2016 and 2015 included $32.5 million, $25.1 million and $23.2 million, respectively, of non-cash interest expense related to the amortization of the debt discount and debt issuance costs for our convertible notes.
The following table summarizes information about the equity and liability components of our outstanding 1 percent convertible notes (in thousands). We measured the fair values of the convertible notes outstanding based on quoted market prices, which is a Level 2 measurement:
|
December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Fair value of outstanding notes
|
$
|
727,420
|
$
|
700,969
|
||||
|
Principal amount of convertible notes outstanding
|
$
|
685,450
|
$
|
685,450
|
||||
|
Unamortized portion of debt discount
|
$
|
144,112
|
$
|
175,699
|
||||
|
Long-term debt
|
$
|
533,111
|
$
|
500,511
|
||||
|
Carrying value of equity component
|
$
|
219,011
|
$
|
219,011
|
||||
Financing Arrangements
Line of Credit Arrangement
In June 2015, we entered into a five-year revolving line of credit agreement with Morgan Stanley Private Bank, National Association, or Morgan Stanley. We amended the credit agreement in February 2016 to increase the amount available for us to borrow. Under the amended credit agreement, we can borrow up to a maximum of $30 million of revolving credit for general working capital purposes. Under the credit agreement interest is payable monthly in arrears on the outstanding principal at a borrowing rate based on our option of:
|
(i)
|
a floating rate equal to the one-month London Interbank Offered Rate, or LIBOR, in effect plus 1.25 percent per annum;
|
|
(ii)
|
a fixed rate equal to LIBOR plus 1.25 percent for a period of one, two, three, four, six, or twelve months as elected by us; or
|
|
(iii)
|
a fixed rate equal to the LIBOR swap rate during the period of the loan.
|
Additionally, after June 1, 2016, we pay 0.25 percent per annum, payable quarterly in arrears, for any amount unused under the credit facility. As of December 31, 2017, we had $12.5 million in outstanding borrowings under the credit facility with a 2.31 percent fixed interest rate and a maturity date of September 2019, which we used to fund our capital equipment needs and is consistent with our historical practice to finance these costs.
The credit agreement includes customary affirmative and negative covenants and restrictions. We are in compliance with all covenants of the credit agreement.
Research and Development and Manufacturing Facilities
In July 2017, we purchased the building that houses our primary R&D facility for $79.4 million. As a result of the purchase, we extinguished the financing liability we had previously recorded on our balance sheet. The difference between the purchase price of the facility and the carrying value of our financing liability at the time of the purchase was $7.7 million. We recognized this amount as a non-cash loss on extinguishment of financing liability for leased facility in our consolidated results of operations in the third quarter of 2017.
We also purchased our manufacturing facility in July 2017 for $14.0 million. We previously accounted for the lease on this facility as an operating lease. We capitalized the purchase price of the building as a fixed asset in the third quarter of 2017.
F-22
We financed the purchase of our primary R&D facility and our manufacturing facility, with mortgage debt of $51.3 million and $9.1 million, respectively. Our primary R&D facility mortgage has an interest rate of 3.88 percent. Our manufacturing facility mortgage has an interest rate of 4.20 percent. During the first five years of both mortgages we are only required to make interest payments. Both mortgages mature in August 2027.
Maturity Schedules
Annual debt and other obligation maturities, including fixed and determinable interest, at December 31, 2017 are as follows (in thousands):
|
2018
|
$
|
9,617
|
||
|
2019
|
22,082
|
|||
|
2020
|
9,330
|
|||
|
2021
|
694,774
|
|||
|
2022
|
2,809
|
|||
|
Thereafter
|
71,603
|
|||
|
Subtotal
|
$
|
810,215
|
||
|
Less: current portion
|
(53
|
)
|
||
|
Less: fixed and determinable interest
|
(51,465
|
)
|
||
|
Less: unamortized portion of debt discount
|
(144,791
|
)
|
||
|
Plus: Deferred rent
|
165
|
|||
|
Total
|
$
|
614,071
|
Operating Leases
We lease a facility adjacent to our manufacturing facility that has laboratory and office space that we use to support our manufacturing facility. We lease this space under a non-cancelable operating lease with an initial term ending in June 2021 and an option to extend the lease for up to two five-year periods. Additionally, Akcea leases office space in a building in Cambridge, Massachusetts. A portion of Akcea's operating lease expires in July 2018, with the other portion expiring in April 2020. We also lease office equipment under non-cancelable operating leases with terms through January 2021.
Annual future minimum payments under operating leases as of December 31, 2017 are as follows (in thousands):
|
Operating
Leases
|
||||
|
2018
|
$
|
864
|
||
|
2019
|
636
|
|||
|
2020
|
477
|
|||
|
2021
|
147
|
|||
|
Total minimum payments
|
$
|
2,124
|
||
Rent expense was $1.7 million for the year ended December 31, 2017. Rent expense was $2.0 million for each of the years ended December 31, 2016 and 2015. We recognized rent expense on a straight line basis over the lease term for the lease on our manufacturing facility, the lease on our building adjacent to our manufacturing facility and Akcea’s office space, which resulted in a deferred rent balance of $0.1 million and $2.1 million at December 31, 2017 and 2016, respectively.
4. Stockholders’ Equity
Preferred Stock
We are authorized to issue up to 15,000,000 shares of “blank check” Preferred Stock. As of December 31, 2017, there were no shares of Preferred Stock outstanding. We have designated Series C Junior Participating Preferred Stock but have no issued or outstanding shares as of December 31, 2017.
Common Stock
At December 31, 2017 and 2016, we had 300,000,000 shares of common stock authorized, of which 124,976,373 and 121,636,273 were issued and outstanding, respectively. As of December 31, 2017, total common shares reserved for future issuance were 18,419,727.
During the years ended December 31, 2017, 2016 and 2015, we issued 1,706,000, 1,285,000 and 1,908,000 shares of common stock, respectively, for stock option exercises, vesting of restricted stock units, and ESPP purchases. We received net proceeds from these transactions of $22.9 million, $13.7 million and $24.9 million in 2017, 2016 and 2015, respectively.
F-23
Stock Plans
1989 Stock Option Plan
In June 1989, our Board of Directors adopted, and the stockholders subsequently approved, a stock option plan that, as amended, provides for the issuance of non-qualified and incentive stock options for the purchase of up to 20,000,000 shares of common stock to our employees, directors, and consultants. The plan expires in January 2024. The 1989 Plan does not allow us to grant stock bonuses or restricted stock awards and prohibits us from repricing any options outstanding under the plan unless our stockholders approve the repricing. Options vest over a four-year period, with 25 percent exercisable at the end of one year from the date of the grant and the balance vesting ratably, on a monthly basis, thereafter and have a term of seven years. At December 31, 2017, a total of 1,603,403 options were outstanding, of which options to purchase 1,553,252 shares were exercisable, and 31,878 shares were available for future grant under the 1989 Plan.
2011 Equity Incentive Plan
In March 2011, our Board of Directors adopted, and the stockholders subsequently approved, a stock option plan that provides for the issuance of stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, and performance cash awards to our employees, directors, and consultants. In June 2015 and in May 2017, after receiving approval from our stockholders, we amended our 2011 Equity Incentive Plan to increase the total number of shares reserved for issuance. We increased the shares available under our 2011 Equity Incentive Plan from 5,500,000 to 11,000,000 in June 2015 and from 11,000,000 to 16,000,000 in May 2017. The plan expires in June 2021. The 2011 Plan does not allow us to reduce the exercise price of any outstanding stock options or stock appreciation rights or cancel any outstanding stock options or stock appreciation rights that have an exercise price or strike price greater than the current fair market value of the common stock in exchange for cash or other stock awards unless our stockholders approve such action. Currently we anticipate awarding only options and restricted stock unit awards to our employees, directors and consultants. Under the 2011 Plan, stock options cannot vest in a period of less than two years and restricted stock unit awards cannot vest in a period of less than three years. We have granted restricted stock unit awards to our employees under the 2011 Plan which vest annually over a four-year period. At December 31, 2017, a total of 7,120,643 options were outstanding, of which 3,201,717 were exercisable, 821,771 restricted stock unit awards were outstanding, and 6,822,389 shares were available for future grant under the 2011 Plan.
Under the 2011 Plan, we may issue a stock award with additional acceleration of vesting and exercisability upon or after a change in control. In the absence of such provisions, no such acceleration will occur. The stock options and restricted stock unit awards we issue to our chief executive officer and issued to B. Lynne Parshall in her former role as chief operating officer will accelerate upon a change of control, as defined in the 2011 Plan.
Corporate Transactions and Change in Control under 2011 Plan
In the event of certain significant corporate transactions, our Board of Directors has the discretion to take one or more of the following actions with respect to outstanding stock awards under the 2011 Plan:
| |
arrange for assumption, continuation, or substitution of a stock award by a surviving or acquiring entity (or its parent company);
|
| |
arrange for the assignment of any reacquisition or repurchase rights applicable to any shares of our common stock issued pursuant to a stock award to the surviving or acquiring corporation (or its parent company);
|
| |
accelerate the vesting and exercisability of a stock award followed by the termination of the stock award;
|
| |
arrange for the lapse of any reacquisition or repurchase rights applicable to any shares of our common stock issued pursuant to a stock award;
|
| |
cancel or arrange for the cancellation of a stock award, to the extent not vested or not exercised prior to the effective date of the corporate transaction, in exchange for cash consideration, if any, as the Board, in its sole discretion, may consider appropriate; and
|
| |
arrange for the surrender of a stock award in exchange for a payment equal to the excess of (a) the value of the property the holder of the stock award would have received upon the exercise of the stock award, over (b) any exercise price payable by such holder in connection with such exercise.
|
2002 Non-Employee Directors’ Stock Option Plan
In September 2001, our Board of Directors adopted, and the stockholders subsequently approved, an amendment and restatement of the 1992 Non-Employee Directors’ Stock Option Plan, which provides for the issuance of non-qualified stock options and restricted stock units to our non-employee directors. The name of the resulting plan is the 2002 Non-Employee Directors’ Stock Option Plan (the 2002 Plan). In June 2015, after receiving approval from our stockholders, we amended our 2002 Non-Employee Directors Stock Option Plan to increase the total number of shares reserved for issuance. We increased the shares available under our 2002 Non-Employee Directors Stock Option Plan from 1,200,000 to 2,000,000. Options under this plan expire ten years from the date of grant. Options granted become exercisable in four equal annual installments beginning one year after the date of grant. At December 31, 2017, a total of 672,750 options were outstanding, of which 427,125 were exercisable, 40,933 restricted stock unit awards were outstanding, and 635,867 shares were available for future grant under the 2002 Plan.
F-24
Employee Stock Purchase Plan
In June 2009, our Board of Directors adopted, and the stockholders subsequently approved, the amendment and restatement of the ESPP and we reserved an additional 150,000 shares of common stock for issuance thereunder. In each of the subsequent years, we reserved an additional 150,000 shares of common stock for the ESPP resulting in a total of 3,524,596 shares authorized under the plan as of December 31, 2017. The ESPP permits full-time employees to purchase common stock through payroll deductions (which cannot exceed 10 percent of each employee’s compensation) at the lower of 85 percent of fair market value at the beginning of the purchase period or the end of each six-month purchase period. Under the amended and restated ESPP, employees must hold the stock they purchase for a minimum of six months from the date of purchase. During 2017, employees purchased and we issued to employees 67,481 shares under the ESPP at a weighted average price of $27.51 per share. At December 31, 2017, there were 668,232 shares available for purchase under the ESPP.
Stock Option Activity
The following table summarizes the stock option activity for the year ended December 31, 2017 (in thousands, except per share and contractual life data):
|
Number of
Shares
|
Weighted
Average Exercise
Price Per Share
|
Average
Remaining
Contractual Term
(Years)
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
Outstanding at December 31, 2016
|
9,178
|
$
|
40.48
|
|||||||||||||
|
Granted
|
3,274
|
$
|
47.76
|
|||||||||||||
|
Exercised
|
(1,345
|
)
|
$
|
15.74
|
||||||||||||
|
Cancelled/forfeited/expired
|
(1,710
|
)
|
$
|
51.71
|
||||||||||||
|
Outstanding at December 31, 2017
|
9,397
|
$
|
44.52
|
4.42
|
$
|
92,288
|
||||||||||
|
Exercisable at December 31, 2017
|
5,182
|
$
|
39.10
|
3.33
|
$
|
80,167
|
||||||||||
The weighted-average estimated fair values of options granted were $25.42, $26.72 and $27.44 for the years ended December 31, 2017, 2016 and 2015, respectively. The total intrinsic value of options exercised during the years ended December 31, 2017, 2016 and 2015 were $49.5 million, $28.0 million and $84.7 million, respectively, which we determined as of the date of exercise. The amount of cash received from the exercise of stock options was $21.2 million, $12.6 million and $23.6 million for the years ended December 31, 2017, 2016 and 2015, respectively. For the year ended December 31, 2017, the weighted-average fair value of options exercised was $52.53. As of December 31, 2017, total unrecognized compensation cost related to non-vested stock-based compensation plans was $75.2 million. We will adjust the total unrecognized compensation cost for future changes in estimated forfeitures. We expect to recognize this cost over a weighted average period of 1.2 years.
Restricted Stock Unit Activity
The following table summarizes the RSU activity for the year ended December 31, 2017 (in thousands, except per share data):
|
Number of
Shares
|
Weighted
Average
Grant Date
Fair Value
Per Share
|
|||||||
|
Non-vested at December 31, 2016
|
778
|
$
|
47.68
|
|||||
|
Granted
|
420
|
$
|
48.88
|
|||||
|
Vested
|
(296
|
)
|
$
|
43.79
|
||||
|
Cancelled/forfeited
|
(39
|
)
|
$
|
48.90
|
||||
|
Non-vested at December 31, 2017
|
863
|
$
|
49.55
|
|||||
For the years ended December 31, 2017, 2016 and 2015, the weighted-average grant date fair value of RSUs granted was $48.88, $41.79 and $65.69 per RSU, respectively. As of December 31, 2017, total unrecognized compensation cost related to RSUs was $16.5 million. We will adjust the total unrecognized compensation cost for future changes in estimated forfeitures. We expect to recognize this cost over a weighted average period of 1.2 years.
Stock-based Compensation Expense and Valuation Information
The following table summarizes stock-based compensation expense for the years ended December 31, 2017, 2016 and 2015 (in thousands), which was allocated as follows and includes $17.5 million, $10.1 million and $6.5 million of stock-based compensation expense for Akcea employees in 2017, 2016 and 2015, respectively:
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Research, development and patent
|
$
|
64,521
|
$
|
55,099
|
$
|
43,638
|
||||||
|
Selling, general and administrative
|
21,454
|
17,009
|
15,676
|
|||||||||
|
Total
|
$
|
85,975
|
$
|
72,108
|
$
|
59,314
|
||||||
Determining Fair Value
Valuation. We measure stock-based compensation expense for equity-classified awards, principally related to stock options, RSUs, and stock purchase rights under the ESPP at the grant date, based on the estimated fair value of the award and we recognize the expense over the employee’s requisite service period. We value RSUs based on the market price of our common stock on the date of grant.
F-25
We use the Black-Scholes model to estimate the fair value of stock options granted and stock purchase rights under our ESPP. The expected term of stock options granted represents the period of time that we expect them to be outstanding. We estimate the expected term of options granted based on actual and projected exercise patterns. We recognize compensation expense for stock options granted, RSUs, and stock purchase rights under the ESPP using the accelerated multiple-option approach. Under the accelerated multiple-option approach (also known as the graded-vesting method), we recognize compensation expense over the requisite service period for each separately vesting tranche of the award as though the award were in substance multiple awards, which results in the expense being front-loaded over the vesting period.
For the years ended December 31, 2017, 2016 and 2015, we used the following weighted-average assumptions in our Black-Scholes calculations:
Employee Stock Options:
|
December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Risk-free interest rate
|
1.8
|
%
|
1.5
|
%
|
1.5
|
%
|
||||||
|
Dividend yield
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
||||||
|
Volatility
|
65.9
|
%
|
58.7
|
%
|
53.8
|
%
|
||||||
|
Expected life
|
4.5 years
|
4.5 years
|
4.5 years
|
|||||||||
Board of Director Stock Options:
|
December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Risk-free interest rate
|
2.2
|
%
|
1.3
|
%
|
2.1
|
%
|
||||||
|
Dividend yield
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
||||||
|
Volatility
|
61.2
|
%
|
53.1
|
%
|
52.2
|
%
|
||||||
|
Expected life
|
6.6 years
|
6.5 years
|
6.9 years
|
|||||||||
ESPP:
|
December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Risk-free interest rate
|
0.8
|
%
|
0.4
|
%
|
0.1
|
%
|
||||||
|
Dividend yield
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
||||||
|
Volatility
|
59.9
|
%
|
86.4
|
%
|
51.7
|
%
|
||||||
|
Expected life
|
6 months
|
6 months
|
6 months
|
|||||||||
Risk-Free Interest Rate. We base the risk-free interest rate assumption on observed interest rates appropriate for the term of our stock option plans or ESPP.
Dividend Yield. We base the dividend yield assumption on our history and expectation of dividend payouts. We have not paid dividends in the past and do not expect to in the future.
Volatility. We use an average of the historical stock price volatility of our stock for the Black-Scholes model. We computed the historical stock volatility based on the expected term of the awards.
Expected Life. The expected term of stock options we have granted represents the period of time that we expect them to be outstanding. We estimated the expected term of options we have granted based on actual and projected exercise patterns.
Forfeitures. We reduce stock-based compensation expense for estimated forfeitures. We estimate forfeitures at the time of grant and revise, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We estimate forfeitures based on historical experience. Our historical forfeiture estimates have not been materially different from our actual forfeitures.
5. Income Taxes
Loss before income tax (benefit) expense is comprised of (in thousands):
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
United States
|
$
|
(11,802
|
)
|
$
|
(83,622
|
)
|
$
|
(87,906
|
)
|
|||
|
Foreign
|
(11,474
|
)
|
—
|
—
|
||||||||
|
Loss before income tax (benefit) expense
|
$
|
(23,276
|
)
|
$
|
(83,622
|
)
|
$
|
(87,906
|
)
|
|||
F-26
Our income tax (benefit) expense was as follows (in thousands):
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Current:
|
||||||||||||
|
Federal
|
$
|
(7,460
|
)
|
$
|
1,067
|
$
|
379
|
|||||
|
State
|
1,246
|
1,867
|
(7
|
)
|
||||||||
|
Foreign
|
234
|
—
|
—
|
|||||||||
|
Total current income tax (benefit) expense
|
(5,980
|
)
|
2,934
|
372
|
||||||||
|
Deferred:
|
||||||||||||
|
Federal
|
—
|
—
|
—
|
|||||||||
|
State
|
—
|
—
|
—
|
|||||||||
|
Total deferred income tax (benefit) expense
|
—
|
—
|
—
|
|||||||||
|
Total income tax (benefit) expense
|
$
|
(5,980
|
)
|
$
|
2,934
|
$
|
372
|
|||||
The reconciliation between our effective tax rate on loss from continuing operations and the statutory U.S. tax rate is as follows (in thousands):
|
Years Ended December 31,
|
||||||||||||||||||||||||
|
2017
|
2016
|
2015
|
||||||||||||||||||||||
|
Pre-tax loss
|
$
|
(23,276
|
)
|
$
|
(83,622
|
)
|
$
|
(87,906
|
)
|
|||||||||||||||
|
Statutory rate
|
(8,147
|
)
|
35.0
|
%
|
(29,268
|
)
|
35.0
|
%
|
(30,767
|
)
|
35.0
|
%
|
||||||||||||
|
State income tax net of federal benefit
|
722
|
(3.1
|
)%
|
(276
|
)
|
0.3
|
%
|
1
|
0.0
|
%
|
||||||||||||||
|
Foreign
|
4,299
|
(18.3
|
)%
|
—
|
0.0
|
%
|
—
|
0.0
|
%
|
|||||||||||||||
|
Net change in valuation allowance
|
(76,409
|
)
|
328.3
|
%
|
55,927
|
(66.9
|
)%
|
69,499
|
(79.1
|
)%
|
||||||||||||||
|
Net operating loss expiration
|
3,987
|
(17.0
|
)%
|
—
|
0.0
|
%
|
—
|
0.0
|
%
|
|||||||||||||||
|
Tax credits
|
(32,769
|
)
|
140.8
|
%
|
(26,954
|
)
|
32.2
|
%
|
(41,284
|
)
|
47.0
|
%
|
||||||||||||
|
Deferred tax true-up
|
4,848
|
(20.6
|
)%
|
2,591
|
(3.1
|
)%
|
1,496
|
(1.7
|
)%
|
|||||||||||||||
|
Tax Cuts and Jobs Act
|
107,323
|
(461.1
|
)%
|
—
|
—
|
0.0
|
%
|
|||||||||||||||||
|
Nondeductible items
|
4,123
|
(17.9
|
)%
|
1,149
|
(1.4
|
)%
|
1,055
|
(1.2
|
)%
|
|||||||||||||||
|
Akcea deconsolidation adjustment at IPO
|
469
|
(2.0
|
)%
|
—
|
0.0
|
%
|
—
|
0.0
|
%
|
|||||||||||||||
|
Excess stock-based compensation
|
(14,337
|
)
|
61.0
|
%
|
—
|
0.0
|
%
|
—
|
0.0
|
%
|
||||||||||||||
|
Other
|
(89
|
)
|
0.6
|
%
|
(235
|
)
|
0.4
|
%
|
372
|
(0.4
|
)%
|
|||||||||||||
|
Effective rate
|
$
|
(5,980
|
)
|
25.7
|
%
|
$
|
2,934
|
(3.5
|
)%
|
$
|
372
|
(0.4
|
)%
|
|||||||||||
Significant components of our deferred tax assets and liabilities as of December 31, 2017 and 2016 are as follows (in thousands):
|
Years Ended December 31,
|
||||||||
|
2017
|
2016
|
|||||||
|
Deferred Tax Assets:
|
||||||||
|
Net operating loss carryovers
|
$
|
153,575
|
$
|
194,372
|
||||
|
R&D credits
|
240,290
|
193,845
|
||||||
|
Deferred revenue
|
42,055
|
54,203
|
||||||
|
Stock-based compensation
|
40,090
|
48,209
|
||||||
|
Intangible and capital assets
|
672
|
—
|
||||||
|
Other
|
12,164
|
26,228
|
||||||
|
Total deferred tax assets
|
$
|
488,846
|
$
|
516,857
|
||||
|
Deferred Tax Liabilities:
|
||||||||
|
Convertible debt
|
$
|
(32,391
|
)
|
$
|
(62,669
|
)
|
||
|
Intangible and capital assets
|
—
|
(2,030
|
)
|
|||||
|
Net deferred tax asset
|
$
|
456,455
|
$
|
452,158
|
||||
|
Valuation allowance
|
(456,455
|
)
|
(452,158
|
)
|
||||
|
Total net deferred tax assets and liabilities
|
$
|
—
|
$
|
—
|
||||
In accordance with the SEC guidance, we provided our best estimate of the impact of the Tax Act in the period ended December 31, 2017 based on our understanding of the Tax Act and guidance available as of the date of this filing. We remeasured our existing net U.S. deferred tax assets using the enacted rate and other known existing changes to the tax code. This resulted in a total decrease in these assets by $107.3 million which was fully offset by a decrease in the valuation allowance. In addition, we recorded a $7.7 million tax benefit related to our cumulative prior year AMT tax credit carryovers, which are now reflected as part of a long-term income tax receivable because under the Tax Act, AMT credits are refundable from 2018 through 2021.We also assessed the impact of the deemed repatriation of foreign earnings and the impact of the limitation on tax deductions for executive compensation under the applicable section of the tax code. We have recognized provisional amounts in our financial statements for these and other items. The ultimate impact may differ materially from these provisional amounts due to, among other things, additional analysis, changes in our interpretations and assumptions, additional regulatory guidance that may be issued, and other actions we may take as a result of the Tax Act.
F-27
At December 31, 2017, we had federal and California tax net operating loss carryforwards of approximately $561.1 million and $887.1 million, respectively. Our federal tax loss carryforwards will begin to expire in 2024, unless we use them before then. Our California loss carryforwards continued to expire in 2017. At December 31, 2017 we also had federal and California research and development tax credit carryforwards of approximately $233.3 million and $56.2 million, respectively. Our Federal research and development tax credit carryforwards begin to expire in 2018. Our California research and development tax credit carryforwards are available indefinitely.
Utilization of the net operating loss and tax credit carryforwards may be subject to an annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986, as amended, and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization.
We record a valuation allowance to reduce the balance of our net deferred tax assets to the amount we believe is more-likely-than-not to be realized. We have incurred historical financial statement losses since inception and as a result we have a full valuation allowance recorded against our net deferred tax assets. We regularly assess the future realization of our net deferred tax assets and will reduce the valuation allowance in any such period in which we determine that all, or a portion, of our deferred tax assets are more-likely-than-not to be realized.
Our valuation allowance increased by $4.3 million from December 31, 2016 to December 31, 2017. The net increase relates to increases from current year activity, offset by a decrease related to the remeasurement of our net deferred tax assets as required by the Tax Act.
Historically, we recognized excess tax benefits associated with stock-based compensation to stockholders' equity only when realized. We followed the with-and-without approach excluding any indirect effects of the excess tax deductions to determine when we should realize excess tax benefits relating to stock-based compensation. Under this approach, we did not realize our excess tax benefits related to stock-based compensation until after we utilize all our other tax benefits available to us. During the year ended December 31, 2016, we realized $1.9 million of such excess tax benefits, and accordingly, we recorded a corresponding credit to additional paid-in capital.
In March 2016, the FASB issued amended guidance to simplify certain aspects of accounting for stock-based payments. We adopted this amended guidance on January 1, 2017. Under the amended guidance, we recognize all excess tax benefits and tax deficiencies as income tax expense or benefit in the period in which they occur.
We analyze filing positions in all U.S. federal, state and foreign jurisdictions where we file income tax returns, and all open tax years in these jurisdictions to determine if we have any uncertain tax positions on any of our income tax returns. We recognize the impact of an uncertain tax position on an income tax return at the largest amount that the relevant taxing authority is more-likely-than not to sustain upon audit. We do not recognize uncertain income tax positions if they have less than 50 percent likelihood of the applicable tax authority sustaining our position.
The following table summarizes our gross unrecognized tax benefits (in thousands):
|
Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Beginning balance of unrecognized tax benefits
|
$
|
66,999
|
$
|
51,257
|
$
|
27,365
|
||||||
|
Settlement of prior period tax positions
|
—
|
(4,033
|
)
|
—
|
||||||||
|
Increase for prior period tax positions
|
1,520
|
7,928
|
215
|
|||||||||
|
Increase for current period tax positions
|
9,495
|
11,847
|
23,677
|
|||||||||
|
Ending balance of unrecognized tax benefits
|
$
|
78,014
|
$
|
66,999
|
$
|
51,257
|
||||||
Included in the balance of unrecognized tax benefits at December 31, 2017, is $63.6 million that could impact our effective tax rate, if recognized. None of the unrecognized tax benefits currently impact our effective tax rate due to the full valuation allowance we have recorded against our deferred tax assets.
We do not foresee any material changes to our gross unrecognized tax benefits within the next twelve months.
We recognize interest and/or penalties related to income tax matters in income tax expense. We did not recognize any accrued interest and penalties related to gross unrecognized tax benefits during the year ended December 31, 2017.
Due to the carryforward of unutilized net operating losses and research and development credits, we are subject to taxation in the United States and various state jurisdictions. Our tax years for 1998 through 2016 are subject to examination by the U.S. tax authorities and our tax years for 2003 through 2016 are subject to examination by the California tax authorities.
We do not provide for a U.S. income tax liability and foreign withholding taxes on undistributed foreign earnings of our foreign subsidiaries.
F-28
6. Collaborative Arrangements and Licensing Agreements
Strategic Partnerships
AstraZeneca
Cardiometabolic and Renal Diseases Collaboration
In July 2015, we and AstraZeneca formed a strategic collaboration to discover and develop antisense therapies for treating cardiovascular and metabolic diseases primarily focused on targets in the kidney and renal diseases. As part of the agreement, we granted AstraZeneca an exclusive license to IONIS-AZ4-2.5-LRx, a drug we designed to treat cardiovascular disease and our first drug that combines our Generation 2.5 and LIgand-Conjugated Antisense, or LICA, technology. We also granted AstraZeneca the option to license a drug for each additional target advanced under this research collaboration. In February 2018, AstraZeneca licensed a second drug under our collaboration, IONIS-AZ5-2.5Rx, a drug we designed to treat a genetically associated form of kidney disease. AstraZeneca is responsible for all further global development, regulatory and commercialization activities and costs for IONIS-AZ4-2.5-LRx and IONIS-AZ5-2.5Rx and any other future drug development candidates AstraZeneca accepts.
Under the terms of the agreement, we received a $65 million upfront payment. Since this agreement has multiple elements, we evaluated the deliverables in this arrangement and determined that none of the deliverables have stand-alone value because of the early stage of research for this collaboration. Therefore, we concluded there is one unit of accounting and we are amortizing the $65 million upfront payment through August 2021. We are eligible to receive license fees and substantive milestone payments of up to more than $4 billion as drugs under this collaboration advance, including up to $1.1 billion for the achievement of development milestones and up to $2.9 billion for regulatory milestones. From inception through December 2017, we have received $93 million in upfront fees, milestone payments, and other payments under this cardiometabolic and renal diseases collaboration, including a $25 million milestone payment we received when we moved the first development candidate into preclinical development, IONIS-AZ4-2.5-LRx in December 2016. Additionally, in February 2018, we earned $30 million when AstraZeneca licensed IONIS-AZ5-2.5Rx. We will earn the next milestone payment of $10 million under this collaboration if we advance a drug under our cardiometabolic research program with AstraZeneca. In addition, we are eligible to receive tiered royalties up to the low teens on sales from any product that AstraZeneca successfully commercializes under this collaboration agreement.
Oncology Collaboration
In December 2012, we entered into a collaboration agreement with AstraZeneca to discover and develop antisense drugs to treat cancer. As part of the agreement, we granted AstraZeneca an exclusive license to develop and commercialize IONIS-STAT3-2.5Rx for the treatment of cancer and an option to license up to three anti-cancer drugs under separate research programs. AstraZeneca is responsible for all global development, regulatory and commercialization activities for IONIS-STAT3-2.5Rx. We and AstraZeneca have evaluated IONIS-STAT3-2.5Rx in people with advanced metastatic hepatocellular carcinoma and advanced lymphoma. AstraZeneca is evaluating IONIS-STAT3-2.5Rx in combination with Imfinzi (durvalumab), AstraZeneca’s programmed death ligand (PD-L1) blocking drug, in people with head and neck cancer. Under the research program, we are responsible for identifying a development candidate for each of the three anti-cancer research programs. AstraZeneca has the option to license drugs resulting from each of the three anti-cancer research programs, and if AstraZeneca exercises its option for a drug, it will be responsible for all further global development, regulatory and commercialization activities and costs for such drug. The first development candidate identified under the anti-cancer research program was IONIS-KRAS-2.5Rx, which AstraZeneca licensed from us in December 2016. IONIS-KRAS-2.5Rx is a Generation 2.5 antisense drug we designed to directly target KRAS, one of the most frequently mutated genes in cancer.
Under the terms of the agreement, we received $31 million in upfront payments. We recorded revenue of $11.5 million upon receipt of these payments and we have amortized $11.9 million into revenue as we have performed development activities under this collaboration. We recognized the remaining $7.6 million related to the option to license three drugs under the research program through February 2018. In January 2016, we and AstraZeneca amended the agreement for the research program. Under the amended terms of the agreement, we can earn an additional $5 million in milestone payments for advancing a drug under our research program.
We are eligible to receive milestone payments and license fees from AstraZeneca as programs advance in development. In addition, we are eligible to receive tiered royalties up to the low to mid-teens on sales from any drugs resulting from these programs. If AstraZeneca successfully develops IONIS-STAT3-2.5Rx, IONIS-KRAS-2.5Rx and two other drugs under the research program, we could receive license fees and substantive milestone payments of up to more than $750 million, including up to $226 million for the achievement of development milestones and up to $485 million for the achievement of regulatory milestones. From inception through December 2017, we have received $97.8 million in upfront fees, milestone payments, and other payments under this oncology collaboration. We will earn the next milestone payment of $17.5 million if we advance a drug under our cancer research program with AstraZeneca.
Each of our agreements with AstraZeneca will continue until the expiration of all payment obligations under the applicable agreement. In addition, the agreement, or any program under the applicable agreement, may terminate early under the following situations:
| ● |
AstraZeneca may terminate the agreement or any program at any time by providing written notice to us;
|
| ● |
AstraZeneca may terminate the agreement or any program by providing written notice if we undergo a change of control with a third party; and
|
| ● |
Either we or AstraZeneca may terminate the agreement or any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement, or the entire agreement if the other party becomes insolvent.
|
F-29
During 2017, 2016 and 2015 we earned revenue of $13.8 million, $64.9 million and $6.4 million, respectively, from our relationship with AstraZeneca, which represented three percent, 19 percent and two percent, respectively, of our total revenue for those periods. Our balance sheets at December 31, 2017 and 2016 included deferred revenue of $41.8 million and $51.5 million, respectively, related to our relationship with AstraZeneca.
Biogen
We have several strategic collaborations with Biogen focused on using antisense technology to advance the treatment of neurological disorders. These collaborations combine our expertise in creating antisense drugs with Biogen's expertise in developing therapies for neurological disorders. We developed and licensed to Biogen SPINRAZA, our approved drug to treat people with spinal muscular atrophy, or SMA. Additionally, we and Biogen are currently developing six other drugs to treat neurodegenerative diseases under these collaborations, including IONIS-SOD1Rx for ALS, IONIS-MAPTRx (formerly IONIS-BIIB4Rx) for Alzheimer’s disease and IONIS-C9Rx (formerly IONIS-BIIB5Rx), IONIS-BIIB6Rx, IONIS-BIIB7Rx and IONIS-BIIB8Rx to treat undisclosed neurodegenerative diseases. In addition to these drugs, we and Biogen are evaluating numerous additional targets to develop drugs to treat neurological diseases. Most recently, in December 2017 we entered into a collaboration with Biogen to identify new antisense drugs for the treatment of SMA. From inception through December 2017, we have received nearly $745 million from our Biogen collaborations.
SPINRAZA
In January 2012, we entered into a collaboration agreement with Biogen to develop and commercialize SPINRAZA, an RNA-targeted therapy for the treatment of SMA. In December 2016, the FDA approved SPINRAZA for the treatment of SMA in pediatric and adult patients. In January 2018, Biogen reported that SPINRAZA was available in over 30 global markets.
Our 2017 revenue included $112.5 million in commercial revenue from SPINRAZA royalties. In addition to SPINRAZA royalties, from inception through December 2017, we have received $436 million in payments for advancing SPINRAZA, including $90 million of milestone payments for the approval of SPINRAZA in the EU and Japan we earned during 2017. We are receiving tiered royalties up to the mid-teens on any sales of SPINRAZA. We have exclusively in-licensed patents related to SPINRAZA from Cold Spring Harbor Laboratory and the University of Massachusetts. We paid Cold Spring Harbor Laboratory and the University of Massachusetts nominal amounts for license fees and milestone payments we received in 2017. We also pay a low single digit royalty on sales of SPINRAZA. Additionally, we owe a low single digit royalty on future sales of SPINRAZA. Biogen is responsible for all further global development, regulatory and commercialization activities and costs for SPINRAZA.
New antisense drugs for the treatment of SMA
In December 2017, we entered into a collaboration agreement with Biogen to identify new antisense drugs for the treatment of SMA. Biogen will have the option to license therapies arising out of this collaboration following the completion of preclinical studies. Upon licensing, Biogen will be responsible for all further global development, regulatory and commercialization activities and costs for such therapies. Under the collaboration agreement, we received a $25 million upfront payment in December 2017, which we plan to amortize through December 2019. We will earn development and regulatory substantive milestone payments from Biogen if new drugs advance towards marketing approval. In total over the term of our collaboration, we are eligible to receive up to $1.2 billion in license fees, substantive milestone payments and other payments, including up to $80 million for the achievement of development milestones, up to $180 million for the achievement of commercialization milestones and $800 million for the achievement of sales milestones. In addition, we are eligible to receive tiered royalties from the mid-teens to mid-20 percent range on net sales. We will earn the next milestone payment of up to $45 millionfor the initiation of a Phase 3 study for a drug under this collaboration.
Neurology
In December 2012, we and Biogen entered into a collaboration agreement to develop and commercialize novel antisense drugs to up to three targets to treat neurodegenerative diseases. We are responsible for the development of each of the drugs through the completion of the initial Phase 2 clinical study for such drug. Biogen has the option to license a drug from each of the three programs through the completion of the first Phase 2 study for each program. We are currently advancing IONIS-MAPTRx (formerly IONIS-BIIB4Rx) for Alzheimer’s disease under this collaboration. If Biogen exercises its option for a drug, it will assume all further global development, regulatory and commercialization responsibilities and costs for that drug.
Under the terms of the agreement, we received an upfront payment of $30 million, which we are amortizing through December 2020. Over the term of the collaboration, we are eligible to receive up to $210 million in a license fee and substantive milestone payments per program, plus a mark-up of the cost estimate of the Phase 1 and 2 studies. We are eligible to receive up to $10 million in development milestone payments to support research and development of each program, plus a mark-up of the cost estimate of the Phase 1 and 2 studies. We are also eligible to receive up to $130 million in milestone payments per program if Biogen achieves pre-specified regulatory milestones. In addition, we are eligible to receive tiered royalties up to the mid-teens on sales from any drugs resulting from each of the three programs. From inception through December 2017, we have received $56 million in milestone payments and upfront fees under this collaboration, including $10 million milestone payment we received in 2017 for the initiation of a Phase 1/2a study of IONIS-MAPTRx. We will earn the next milestone payment of $7.5 million if we continue to advance IONIS-MAPTRx.
Strategic Neurology
In September 2013, we and Biogen entered into a long-term strategic relationship focused on applying antisense technology to advance the treatment of neurodegenerative diseases. As part of the collaboration, Biogen gained exclusive rights to the use of our antisense technology to develop therapies for neurological diseases and has the option to license drugs resulting from this collaboration. The exclusivity for neurological diseases will last through September 2019, and may be extended for any drug development programs Biogen is pursuing under the collaboration. We will usually be responsible for drug discovery and early development of antisense drugs and Biogen will have the option to license antisense drugs after Phase 2 proof of concept. In October 2016, we expanded our collaboration to include additional research activities we will perform. If Biogen exercises its option for a drug, it will assume all further global development, regulatory and commercialization responsibilities and costs for that drug. We are currently advancing five drugs, IONIS-SOD1Rx, IONIS-C9Rx (formerly IONIS-BIIB5Rx), IONIS-BIIB6Rx, IONIS-BIIB7Rx and IONIS-BIIB8Rx under this collaboration. Biogen will be responsible for all of the drug discovery and development activities for drugs using other modalities.
F-30
Under the terms of the agreement, we received an upfront payment of $100 million and are eligible to receive milestone payments, license fees and royalty payments for all drugs developed through this collaboration, with the specific amounts dependent upon the modality of the molecule advanced by Biogen. If we have a change of control during the first six years of the collaboration, we may be required to refund Biogen a portion of the $100 million upfront payment, with the amount of the potential refund decreasing ratably as we progress through the initial six-year term of the collaboration. We are amortizing the $100 million upfront payment through September 2019. Because the amortization period for the upfront payment will never be less than the initial six-year term of the collaboration, the amount of revenue we recognize from the upfront payment will never exceed the amount that Biogen could potentially require us to refund.
For each antisense molecule that is chosen for drug discovery and development under this collaboration, we are eligible to receive up to approximately $260 million in a license fee and substantive milestone payments. We are eligible to receive up to approximately $60 million for the achievement of research and development milestones, including amounts related to the cost of clinical trials, and up to $130 million for the achievement of regulatory milestones. In addition, we are eligible to receive tiered royalties up to the mid-teens on sales from any antisense drugs developed under this collaboration. If other modalities are chosen, such as small molecules or monoclonal antibodies, we are eligible to receive up to $90 million in substantive milestone payments, including up to $35 million for the achievement of research and development milestones and up to $55 million for the achievement of regulatory milestones. In addition, we are eligible to receive tiered single-digit royalties on sales from any drugs using non-antisense modalities developed under this collaboration. From inception through December 2017, we have received $165 million in upfront fees, milestone payments and other payments under this collaboration, including $15 million in milestone payments we received in 2017 for validating two undisclosed neurological disease targets. We will earn the next milestone payment of up to $10 million if we advance a program under this collaboration.
Each of our agreements with Biogen will continue until the earlier of the date all of Biogen's options to obtain the exclusive licenses under the applicable agreement expire unexercised or, if Biogen exercises its option, until the expiration of all payment obligations under the applicable agreement. In addition, each agreement, or any program under an agreement, may terminate early under the following situations:
| ● |
Biogen may terminate the agreement or any program at any time by providing written notice to us;
|
| ● |
Under specific circumstances, if we are acquired by a third party with a product that directly competes with a compound being developed under the agreement, Biogen may terminate the affected program by providing written notice to us;
|
| ● |
If, within a specified period of time, any required clearance of a transaction contemplated by an agreement under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, is not received, then either we or Biogen may terminate the affected program by providing written notice to the other party; and
|
| ● |
Either we or Biogen may terminate any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement with respect to the affected program, or the entire agreement if the other party becomes insolvent.
|
During 2017, 2016 and 2015, we earned revenue of $259.8 million, $207.9 million and $106.2 million, respectively, from our relationship with Biogen, which represented 51 percent, 60 percent and 37 percent, respectively, of our total revenue for those periods. Our balance sheets at December 31, 2017 and 2016 included deferred revenue of $69.3 million and $67.5 million, respectively, related to our relationship with Biogen.
Research, Development and Commercialization Partners
Bayer
In May 2015, we entered into an exclusive license agreement with Bayer to develop and commercialize IONIS-FXIRx for the prevention of thrombosis. We were responsible for completing a Phase 2 study of IONIS-FXIRx in people with end-stage renal disease on hemodialysis. Under the terms of the agreement, we received a $100 million upfront payment in the second quarter of 2015. We recorded revenue of $91.2 million related to the license for IONIS-FXIRx in June 2015 and we recognized the majority of the remaining amount related to development activities for IONIS-FXIRx through November 2016.
In February 2017, we amended our agreement with Bayer to advance IONIS-FXIRx and to initiate development of IONIS-FXI-LRx, which Bayer licensed. In conjunction with the decision to advance these programs, we received a $75 million payment from Bayer. We recorded revenue of $64.9 million related to the license for IONIS-FXI-LRx in February 2017, and we are recognizing the remaining amount over the period we are performing the ongoing development activities for IONIS-FXI-LRx and IONIS-FXIRx through May 2019. We are conducting a Phase 2b study evaluating IONIS-FXIRx in people with end-stage renal disease on hemodialysis to finalize dose selection. Additionally, we plan to develop IONIS-FXI-LRx through Phase 1. Following these studies and Bayer's decision to further advance these programs, Bayer will be responsible for all global development, regulatory and commercialization activities and costs for both drugs. We are eligible to receive additional milestone payments as each drug advances toward the market. In total over the term of our collaboration, we are eligible to receive up to $385 million in license fees, substantive milestone payments and other payments, including up to $125 million for the achievement of development milestones and up to $110 million for the achievement of commercialization milestones. In addition, we are eligible to receive tiered royalties in the low to high 20 percent range on gross margins of both drugs combined. From inception through December 2017, we have received over $175 million from our Bayer collaboration. We will earn the next milestone payment of $10 million if we advance a program under this collaboration.
F-31
Our agreement with Bayer will continue until the expiration of all payment obligations under the agreement. In addition, the agreement, or any program under the agreement, may terminate early under the following situations:
| ● |
Bayer may terminate the agreement or any program at any time by providing written notice to us;
|
| ● |
Either we or Bayer may terminate the agreement or any program by providing written notice to the other party upon the other party’s uncured failure to perform a material obligation under the agreement, or the entire agreement if the other party becomes insolvent.
|
During 2017, 2016 and 2015 we earned revenue of $69.2 million, $5.4 million and $93.4 million, respectively, from our relationship with Bayer, which represented 14 percent, two percent and 33 percent, respectively, of our total revenue for those periods. Our consolidated balance sheet at December 31, 2017 and 2016 included deferred revenue of $7.3 million and $1.4 million, respectively, related to our relationship with Bayer.
GSK
In March 2010, we entered into an alliance with GSK using our antisense drug discovery platform to discover and develop new drugs against targets for rare and serious diseases, including infectious diseases and some conditions causing blindness. Under the terms of the agreement, we received $38 million in upfront and expansion payments, which we amortized through September 2017.
In August 2017, as part of a reprioritization of its pipeline and strategic review of its Rare Diseases business, GSK declined its options for inotersen, our Phase 3 drug to treat people with TTR amyloidosis and IONIS-FB-LRx (formerly IONIS-GSK4-LRx), an antisense drug to treat complement-mediated diseases. We are continuing to advance each of these drugs independently.
GSK, consistent with its focus on treatments for infectious diseases, continues to advance two drugs targeting hepatitis B virus, or HBV, under our collaboration: IONIS-HBVRx and IONIS-HBV-LRx. GSK is currently conducting Phase 2 studies for both of these drugs, which we designed to reduce the production of viral proteins associated with HBV infection. In March 2016, we and GSK amended the development plan for IONIS-HBVRx to allow GSK to conduct all further development activities for this program. GSK has the exclusive option to license the drugs resulting from this alliance at Phase 2 proof-of-concept for a license fee.
Under our agreement, if GSK successfully develops these drugs and achieves pre-agreed sales targets, we could receive license fees and substantive milestone payments of $262 million, including up to $47.5 million for the achievement of development milestones, up to $120 million for the achievement of regulatory milestones and up to $70 million for the achievement of commercialization milestones. From inception through December 2017, we have received more than $162 million in payments under this alliance with GSK. We will earn the next milestone payment of up to $15 million for the initiation of a Phase 3 study for the HBV program. In addition, we are eligible to receive tiered royalties up to the mid-teens on sales from any product that GSK successfully commercializes under this alliance.
Our alliance with GSK will continue until the earlier of the date that all of GSK's options to obtain the exclusive licenses under the agreement expire unexercised or, if GSK exercises its option, until the expiration of all payment obligations under the agreement. In addition, the agreement, or any program under the agreement, may terminate early under the following situations:
| ● |
GSK may terminate any program, at any time by providing written notice to us; and
|
| ● |
Either we or GSK may terminate any program by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement with respect to the affected program, or the entire agreement if the other party becomes insolvent.
|
During 2017, 2016 and 2015, we earned revenue of $8.6 million, $12.3 million and $33.3 million respectively, from our relationship with GSK, which represented two percent, four percent and 12 percent, respectively, of our total revenue for those years. Our balance sheet at December 31, 2016 included deferred revenue of $2.1 million, related to our relationship with GSK.
Janssen Biotech, Inc.
In December 2014, we entered into a collaboration agreement with Janssen Biotech, Inc. to discover and develop antisense drugs that can be locally administered, including oral delivery, to treat autoimmune disorders of the gastrointestinal tract. Janssen has the option to license drugs from us through the designation of a development candidate for up to three programs. Prior to option exercise we are responsible for the discovery activities to identify a development candidate. If Janssen exercises an option for one of the programs, it will be responsible for the global development, regulatory and commercial activities under that program. Under the terms of the agreement, we received $35 million in upfront payments, which we amortized through November 2017. We are eligible to receive up to more than $800 million in license fees and substantive milestone payments for these programs, including up to $175 million for the achievement of development milestones, up to $440 million for the achievement of regulatory milestones and up to $180 million for the achievement of commercialization milestones. From inception through December 2017, we have received $61.8 million, including $15 million in license fees when Janssen licensed IONIS-JBI1-2.5Rx and IONIS-JBI2-2.5Rx from us in 2016 and 2017, respectively. We also received $5 million in January 2018 for the initiation of a Phase 1 study of IONIS-JBI1-2.5Rx in late 2017. In addition, we are eligible to receive tiered royalties up to the near teens on sales from any drugs resulting from this collaboration. We will earn the next milestone payment of $5 million if Janssen chooses another target to advance under this collaboration.
F-32
Our agreement with Janssen will continue until the earlier of the date that all of Janssen’s options to obtain the exclusive licenses under the agreement expire unexercised or, if Janssen exercises its option, until the expiration of all payment obligations under the agreement. In addition, the agreement, or any program under the agreement, may terminate early under the following situations:
| ● |
Janssen may terminate the agreement or any program at any time by providing written notice to us; and
|
| ● |
Either we or Janssen may terminate any program by providing written notice to the other party upon the other party’s uncured failure to perform a material obligation under the agreement, or the entire agreement if the other party becomes insolvent.
|
During 2017, 2016 and 2015 we earned revenue of $33.5 million, $27.3 million and $8.9 million, respectively, from our relationship with Janssen. Our balance sheet at December 31, 2016 included deferred revenue of $17.5 million related to our relationship with Janssen.
Novartis
In January 2017, we and Akcea initiated a collaboration with Novartis to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Under the collaboration agreement, Novartis has an exclusive option to develop and commercialize AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Akcea is responsible for completing a Phase 2 program, conducting an end-of-Phase 2 meeting with the FDA and providing API for each drug. If Novartis exercises an option for one of these drugs, Novartis will be responsible for all further global development, regulatory and commercialization activities and costs for such drug.
Akcea received a $75 million upfront payment in the first quarter of 2017, of which it retained $60 million and paid us $15 million as a sublicense fee. If Novartis exercises its option for a drug, Novartis will pay Akcea a license fee equal to $150 million for each drug it licenses. In addition, for AKCEA-APO(a)-LRx, Akcea is eligible to receive up to $600 million in substantive milestone payments, including $25 million for the achievement of a development milestone, up to $290 million for the achievement of regulatory milestones and up to $285 million for the achievement of commercialization milestones. In addition, for AKCEA-APOCIII-LRx, Akcea is eligible to receive up to $530 million in substantive milestone payments, including $25 million for the achievement of a development milestone, up to $240 million for the achievement of regulatory milestones and up to $265 million for the achievement of commercialization milestones. Akcea plans to co-commercialize any licensed drug commercialized by Novartis in selected markets, under terms and conditions that it plans to negotiate with Novartis in the future, through the specialized sales force Akcea is building to commercialize volanesorsen. Following Novartis’ exercise of its option for either drug, Akcea will earn the next milestone payment of $25 million if Novartis advances the Phase 3 study for either drug. Akcea is also eligible to receive tiered royalties in the mid-teens to low 20 percent range on net sales of AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx. Akcea will pay 50 percent of these license fees, milestone payments and royalties to us as a sublicense fee.
The agreement with Novartis will continue until the earlier of the date that all of Novartis’ options to obtain the exclusive licenses under the agreement expire unexercised or, if Novartis exercises its options, until the expiration of all payment obligations under the agreement. In addition, the agreement as a whole or with respect to any drug under the agreement, may terminate early under the following situations:
| ● |
Novartis may terminate the agreement as a whole or with respect to any drug at any time by providing written notice to us;
|
| ● |
Either we or Novartis may terminate the agreement with respect to any drug by providing written notice to the other party in good faith that we or Novartis has determined that the continued development or commercialization of the drug presents safety concerns that pose an unacceptable risk or threat of harm in humans or would violate any applicable law, ethical principles, or principles of scientific integrity;
|
| ● |
Either we or Novartis may terminate the agreement for a drug by providing written notice to the other party upon the other party’s uncured failure to perform a material obligation related to the drug under the agreement, or the entire agreement if the other party becomes insolvent; and
|
| ● |
We may terminate the agreement if Novartis disputes or assists a third party to dispute the validity of any of our patents.
|
In conjunction with this collaboration, we and Akcea entered into a SPA with Novartis. As part of the SPA, Novartis purchased 1.6 million shares of our common stock for $100 million in the first quarter of 2017. As part of the SPA, Novartis was required to purchase $50 million of Akcea’s common stock at the IPO price or our common stock at a premium if an IPO did not occur by April 2018.
To determine the amount of revenue to recognize under our agreements with Novartis, we first concluded that we would account for the collaboration and SPA agreements as a single multiple element arrangement. We next identified four separate units of accounting under the arrangement, each with stand-alone value:
| ● |
Development services for AKCEA-APO(a)-LRx;
|
| ● |
Development services for AKCEA-APOCIII-LRx;
|
| ● |
API for AKCEA-APO(a)-LRx; and
|
| ● |
API for AKCEA-APOCIII-LRx.
|
We then determined the total consideration under the arrangement was $180.0 million, which included the following:
| ● |
$75 million from the upfront payment;
|
| ● |
$100 million from our common stock Novartis purchased under the SPA, including $28.4 million for the premium paid by Novartis for its purchase of our common stock at a premium in the first quarter of 2017; and
|
| ● |
$5.0 million for the potential premium Novartis would have paid if they purchased our common stock in the future.
|
F-33
We first allocated $71.6 million of the consideration to equity based on the fair value of our common stock Novartis purchased. Next, we allocated the remaining consideration of $108.4 million based on the relative stand-alone selling price of each unit of accounting as follows:
| ● |
$64.0 million for the development services for AKCEA-APO(a)-LRx;
|
| ● |
$40.1 million for the development services for AKCEA-APOCIII-LRx;
|
| ● |
$1.5 million for the delivery of AKCEA-APO(a)-LRx API; and
|
| ● |
$2.8 million for the delivery of AKCEA-APOCIII-LRx API.
|
We are recognizing the amount attributed to the development services for AKCEA-APO(a)-LRx and AKCEA-APOCIII-LRx over the period of time we are performing the services, currently estimated to be through November 2018 and June 2019, respectively. We recognized the amount attributed to the API supply for AKCEA-APOCIII-LRx when we delivered it to Novartis in 2017. We will recognize the amount attributed to the API supply for AKCEA-APO(a)-LRx as we deliver it to Novartis. We determined at the inception that all milestones under its Novartis collaboration are substantive milestones and we will recognize any future exercise of an option to license a drug under the Novartis agreement in full in the period the option is exercised. Akcea is responsible for the development activities under this collaboration. As such, Akcea is recognizing the associated revenue in its statement of operations. Akcea pays us sublicense fees for payments that it receives under the collaboration and we recognize those fees as revenue and Akcea recognizes the fees as R&D expense. On a consolidated basis, we eliminate the sublicense fees.
During 2017, we earned revenue of $55.2 million from our relationship with Novartis, which represented 11 percent of our total revenue for 2017. Our balance sheet at December 31, 2017 included deferred revenue of $58.9 million related to our relationship with Novartis.
Roche
In April 2013, we formed an alliance with Hoffman-La Roche Inc. and F. Hoffmann-La Roche Ltd., collectively Roche, to develop treatments for Huntington's disease, or HD, based on our antisense technology. Roche has the option to license the drugs from us through the completion of the first Phase 1 trial. Under the agreement, we are responsible for the discovery and development of an antisense drug targeting huntingtin, or HTT, protein. We evaluated a drug targeting HTT, IONIS-HTTRx, in a Phase 1/2a clinical study in people with early stage HD.
In December 2017, upon completion of the Phase 1/2a study, Roche exercised its option to license IONIS-HTTRx and is now responsible for the global development, regulatory and commercialization activities for IONIS-HTTRx. Under the terms of the agreement, we received an upfront payment of $30 million in April 2013, which we amortized through September 2017. In December 2016, we updated development activities for IONIS-HTTRx and as a result we are eligible for an additional $3 million payment, which we earned in 2017. We are eligible to receive up to $365 million in a license fee and substantive milestone payments including up to $70 million for the achievement of development milestones, up to $170 million for the achievement of regulatory milestones and up to $80 million for the achievement of commercialization milestones. In addition, we are eligible to receive up to $136.5 million in milestone payments for each additional drug successfully developed. We are also eligible to receive tiered royalties up to the mid-teens on any sales of any product resulting from this alliance. From inception through December 2017, we have received $60 million in milestone payments and upfront fees under this alliance with Roche, not including the $45 million license fee we received in January 2018 for IONIS-HTTRx, which we recognized into revenue in 2017. We will earn the next milestone payment of $10 million if Roche initiates a Phase 2 trial for IONIS-HTTRx.
Our alliance with Roche will continue until the earlier of the date Roche's option to obtain the exclusive license under the agreement expires unexercised or, if Roche exercises its option, until the expiration of all payment obligations under the agreement. In addition, the agreement may terminate early under the following situations:
| ● |
Roche may terminate the agreement at any time by providing written notice to us; and
|
| ● |
Either we or Roche may terminate the agreement by providing written notice to the other party upon the other party's uncured failure to perform a material obligation under the agreement or if the other party becomes insolvent.
|
During 2017, 2016 and 2015, we earned revenue of $53.0 million, $7.1 million and $31.2 million, respectively from our relationship with Roche, which represented 10 percent, two percent and 11 percent, respectively, of our total revenue for those years. Our balance sheet at December 31, 2016 included deferred revenue of $1.7 million related to our relationship with Roche.
Satellite Company Partnerships
Achaogen, Inc.
In 2006, we exclusively licensed to Achaogen, Inc. specific know-how, patents and patent applications relating to aminoglycosides. In connection with the license, Achaogen issued to us $1.5 million of Achaogen stock. Achaogen is developing plazomicin, an aminoglycoside Achaogen discovered based on the technology we licensed to Achaogen. If Achaogen successfully develops and commercializes two drugs under our agreement, we will receive payments totaling up to $49.3 million for the achievement of key clinical, regulatory and sales events. The FDA set a Prescription Drug User Fee Act, or PDUFA, date of June 25, 2018 for plazomicin. Achaogen also plans to submit an MAA to the EMA in 2018. From inception through December 2017, we have earned $7 million in milestone payments from Achaogen. We will earn the next milestone payment of $7.5 million if Achaogen obtains regulatory approval for plazomicin in a major market. We are also eligible to receive low single digit royalties on sales of drugs resulting from the program. Achaogen is solely responsible for the continued development, regulatory and commercialization activities of plazomicin.
During 2017, 2016 and 2015, we did not earn any revenue from our relationship with Achaogen.
F-34
Alnylam Pharmaceuticals, Inc.
In March 2004, we entered into an alliance with Alnylam to develop and commercialize RNAi therapeutics. Under the terms of the agreement, we exclusively licensed to Alnylam our patent estate relating to antisense motifs and mechanisms and oligonucleotide chemistry for double-stranded RNAi therapeutics in exchange for a $5 million technology access fee, participation in fees from Alnylam’s partnering programs, as well as future milestone and royalty payments from Alnylam. For each drug Alnylam develops under this alliance, we may receive up to $3.4 million in milestone payments, including up to $1.1 million for the achievement of development milestones and $2.3 million for regulatory milestones. We will earn the next milestone payment of $0.8 million if Alnylam advances a drug in its pipeline. We also have the potential to earn royalties on drug sales and a portion of payments that Alnylam receives from licenses of our technology it grants to its partners, plus royalties. We retained rights to a limited number of double-stranded RNAi therapeutic targets and all rights to single-stranded RNAi, or ssRNAi, therapeutics.
In turn, Alnylam nonexclusively licensed to us its patent estate relating to antisense motifs and mechanisms and oligonucleotide chemistry to research, develop and commercialize single-stranded antisense therapeutics, ssRNAi therapeutics, and to research double-stranded RNAi compounds. We also received a license to develop and commercialize double-stranded RNAi drugs targeting a limited number of therapeutic targets on a nonexclusive basis. If we develop or commercialize an RNAi-based drug using Alnylam’s technology, we will pay Alnylam up to $3.4 million in milestone payments for specified development and regulatory events, plus royalties. To date, we do not have an RNAi-based drug in development.
In 2015, we and Alnylam entered into an alliance in which we formed an intellectual property cross-license under which we and Alnylam each obtained exclusive license rights to four therapeutic programs. Alnylam granted us an exclusive, royalty-bearing license to its chemistry, RNA targeting mechanism and target-specific intellectual property for oligonucleotides against four targets, including FXI and Apo(a) and two other targets. In exchange, we granted Alnylam an exclusive, royalty-bearing license to our chemistry, RNA targeting mechanism and target-specific intellectual property for oligonucleotides against four other targets. Alnylam also granted us a royalty-bearing, non-exclusive license to new platform technology arising from May 2014 through April 2019 for single-stranded antisense therapeutics. In turn, we granted Alnylam a royalty-bearing, non-exclusive license to new platform technology arising from May 2014 through April 2019 for double-stranded RNAi therapeutics. From inception through December 2017, we have received over $70 million from Alnylam.
During 2017, 2016 and 2015, we earned revenue from our relationship with Alnylam totaling $3.3 million, $1.1 million and $1.3 million, respectively.
Antisense Therapeutics Limited
In 2001, we licensed ATL1102 and ATL1103 to ATL, an Australian company publicly traded on the Australian Stock Exchange. ATL completed a Phase 2a efficacy and safety trial and has also completed a chronic toxicology study in primates to support a potential Phase 2b trial of ATL1102 in people with multiple sclerosis, or MS. In addition, ATL is currently developing ATL1103 for growth and sight disorders. We are eligible to receive royalties on sales of ATL1102 and ATL1103. We may also receive a portion of the fees ATL receives if it licenses ATL1102 or ATL1103. At December 31, 2017 and 2016, we owned less than 10 percent of ATL’s equity. During 2017, 2016 and 2015, we did not earn any revenue from our relationship with ATL.
Atlantic Pharmaceuticals Limited
In March 2007, we licensed alicaforsen to Atlantic Pharmaceuticals, a UK-based specialty pharmaceutical company founded in 2006. Atlantic Pharmaceuticals is developing alicaforsen for the treatment of ulcerative colitis, or UC, and other inflammatory diseases. Atlantic Pharmaceuticals is initially developing alicaforsen for pouchitis, a UC indication, followed by UC and other inflammatory diseases. In 2017, under a rolling submission agreement with the FDA, Atlantic Pharmaceuticals filed the nonclinical data package of its NDA for alicaforsen to treat pouchitis. Alicaforsen has also been granted FDA Fast-Track designation, plus U.S. and European Orphan Drug designations for this indication. In exchange for the exclusive, worldwide license to alicaforsen, we received a $2 million upfront payment from Atlantic Pharmaceuticals in the form of equity. Under the agreement, we could receive milestone payments totaling up to $1.4 million for the achievement of regulatory milestones for multiple indications. We will earn the next milestone payment of $0.6 million when Atlantic Pharmaceuticals completes its NDA submission for alicaforsen with the FDA. In 2010, Atlantic Pharmaceuticals began supplying alicaforsen under international named patient supply regulations for people with inflammatory bowel disease, or IBD, for which we receive royalties.
In 2010, 2013 and 2016, we agreed to sell Atlantic Pharmaceuticals alicaforsen drug substance in return for shares of Atlantic Pharmaceuticals’ common stock. Additionally, in 2013 we received an advance payment in the form of equity for the initial royalties that we will earn from Atlantic Pharmaceuticals. We recorded a full valuation allowance for all of the equity we received from Atlantic Pharmaceuticals, including the upfront payment, because realization of value from the equity is uncertain. At December 31, 2017 and 2016, we owned approximately 9 percent, respectively, of Atlantic Pharmaceuticals’ equity. Because the payments were made in equity, we did not record any revenue. During 2017 and 2016, we did not earn any revenue and during 2015, our revenue was negligible from our relationship with Atlantic Pharmaceuticals.
F-35
Dynacure, SAS
In October 2016, we entered into a collaboration with Dynacure to discover, develop and commercialize an antisense drug for the treatment of neuromuscular diseases. We and Dynacure shared research responsibilities and to identify a drug candidate. In November 2017, Dynacure licensed IONIS-DNM2-2.5Rx, a drug targeting dynamin 2 for the treatment of centronuclear myopathy, from us. Upon licensing, Dynacure assumed all responsibility for development and commercialization for IONIS-DMN2-2.5Rx. Under the terms of the agreement, we obtained a 15 percent equity ownership in Dynacure upon the initiation of the collaboration. We received additional equity and convertible notes in Dynacure for the license of IONIS-DMN2-2.5Rx in 2017. We recorded a full valuation allowance for all of the equity and convertible debt we received from Dynacure, because realization of value from the equity is uncertain. If Dynacure advances a target under this collaboration, we could receive cash or equity up to more than $210 million in a license fee and substantive milestone payments including up to $34.5 million for the achievement of development milestones, up to $111 million for the achievement of regulatory milestones and up to $60 million for the achievement of commercialization milestones. In addition, we are eligible to receive royalties on future product sales of the drug under this collaboration. We will receive additional equity or convertible notes in Dynacure if Dynacure initiates a Phase 1 study for a target under this collaboration. During 2017 and 2016, we did not earn any revenue from our relationship with Dynacure.
Regulus Therapeutics Inc.
In September 2007, we and Alnylam established Regulus as a company focused on the discovery, development and commercialization of microRNA-targeting therapeutics. We and Alnylam retain rights to develop and commercialize, on pre-negotiated terms, microRNA therapeutic products that Regulus decides not to develop either by itself or with a partner. Regulus is addressing therapeutic opportunities that arise from alterations in microRNA expression. Since microRNAs may act as master regulators of the genome, affecting the expression of multiple genes in a disease pathway, microRNA therapeutics define a new platform for drug discovery and development. MicroRNAs may also prove to be an attractive new tool for characterizing diseases. Regulus’ focus is on drug discovery and development efforts for diseases with significant unmet medical need in organs to which we have been able to preferentially deliver oligonucleotide therapeutics effectively, such as the liver and kidney. Regulus currently has two drugs in clinical development. In September 2017, Regulus initiated a Phase 2 study of RG-012, a drug to treat people with Alport syndrome. Regulus is studying RGLS4326 in a Phase 1 single ascending dose study designed to investigate the safety, tolerability, pharmacokinetics and pharmacodynamics of RGLS4326 administered subcutaneously in healthy volunteers. We are eligible to receive royalties on any future product sales of these drugs.
During 2017, 2016 and 2015, we did not earn any revenue from our relationship with Regulus. During 2015, we sold a portion of our Regulus stock, resulting in a gain of $20.2 million, and proceeds of $25.5 million. During 2016, we sold a portion of our Regulus stock for proceeds of $4.5 million. In January 2017, we sold our remaining investment in Regulus for proceeds of $2.5 million.
Suzhou Ribo Life Science Co., Ltd.
In April 2017, we entered into a collaboration with Ribo to develop and commercialize RNA-targeted therapeutics in China. We licensed IONIS-AR-2.5Rx, IONIS-GCGRRx and IONIS-EZH2-2.5Rx to Ribo under our collaboration to develop and commercialize these drugs in China. In addition, Ribo will be responsible for conducting a multi-year research and drug discovery program to identify drugs that utilize our ssRNAi technology. Following the identification of a development candidate, Ribo may exercise its option to license each drug by paying us a license fee. For each drug that Ribo licenses, Ribo will be responsible for all development and commercialization activities and costs in China. We retained the rights to develop and commercialize ssRNAi technology and all drugs under the collaboration outside of China. Ribo will provide us a royalty-free license to the data and intellectual property created under the collaboration.
Under the agreement, we received an up-front payment of $2 million, which we are amortizing through April 2020. We also obtained approximately nine percent equity ownership in Ribo. We are eligible to receive up to $152.9 million in substantive milestone and other payments, including $13.3 million for the achievement of development milestones and $138.4 million for the achievement of commercialization milestones. In addition, we are eligible to receive tiered royalties up to the mid-twenty percent range on sales from any drugs resulting from this collaboration. From inception through December 2017, we have received $2 million in milestone payments and upfront fees under this collaboration with Ribo. We will earn the next milestone payment of $3.3 million if Ribo advances a drug under this collaboration.
During 2017, we earned revenue of $0.7 million from our relationship with Ribo. Our balance sheet at December 31, 2017 included deferred revenue of $1.7 million related to our relationship with Ribo.
The University of Texas MD Anderson Cancer Center
In May 2016, we entered into a collaboration agreement with the University of Texas MD Anderson Cancer Center to identify cancer targets and create novel antisense drugs to treat cancer together. In the collaboration, we and MD Anderson are working together to validate novel “undruggable” cancer targets selected based on human genomic data. We are leading the drug discovery efforts against mutually agreed upon novel targets and MD Anderson is leading development activities through clinical proof of concept. Following clinical proof of concept, we and MD Anderson plan to identify a partner to complete development and to commercialize each drug with us leading business development efforts. Under the five-year collaboration, we and MD Anderson will evenly share costs specific to our collaboration.
F-36
External Project Funding
CHDI Foundation, Inc.
Starting in November 2007, CHDI provided financial and scientific support to our Huntington’s disease drug discovery program through our development collaboration. In April 2013, we formed an alliance with Roche to develop treatments for Huntington’s disease. Under the terms of our agreement with CHDI, we will reimburse CHDI for a portion of its support of our Huntington’s disease program out of the payments we receive from Roche. From inception through December 2017, we have made payments of $19.3 million to CHDI, associated with the progression of our Huntington’s disease program.
During 2017 and 2016, we did not earn any revenue from our relationship with CHDI. During 2015, our revenue earned from our relationship with CHDI was negligible.
Cystic Fibrosis Foundation
In August 2016, we entered into a collaboration agreement with the Cystic Fibrosis Foundation to discover and advance a drug for the treatment of Cystic Fibrosis. Under this agreement, we received upfront payments of $1 million, which we are amortizing through March 2018. We are eligible to receive additional milestone payments of up to $2 million. Under the agreement, we and the Cystic Fibrosis Foundation will evenly share the first $3 million of costs specific to our collaboration. We will pay the Cystic Fibrosis Foundation up to $18 million in payments upon achieving specific regulatory and sales events if we advance a drug under our collaboration. We will earn the next milestone payment of $0.8 million if we further advance IONIS-ENAC-2.5Rx. From inception through December 2017, we have received $2.7 million in milestone payments, upfront fees and other payments under this collaboration, including $1 million we received in 2017 for advancing IONIS-ENAC-2.5Rx.
During 2017 and 2016 we earned $1.9 million and $0.6 million, respectively, from our relationship with the Cystic Fibrosis Foundation.
The Ludwig Institute; Center for Neurological Studies
In October 2005, we entered into a collaboration agreement with the Ludwig Institute, the Center for Neurological Studies and researchers from these institutions to discover and develop antisense drugs for amyotrophic lateral sclerosis, or ALS, and other neurological diseases. Under this agreement, we agreed to pay the Ludwig Institute and Center for Neurological Studies modest milestone payments and royalties on any antisense drugs resulting from the collaboration.
In-Licensing Agreements
University of Massachusetts
We have a license agreement with the University of Massachusetts under which we acquired an exclusive license to the University of Massachusetts’ patent rights related to SPINRAZA. We paid the University of Massachusetts nominal amounts for license fees and milestone payments we received. We also pay a low single digit royalty on sales of SPINRAZA. During 2017 and 2016, we paid the University of Massachusetts $9.7 million and $0.4 million, respectively. The University of Massachusetts believes we owe them an additional amount pertaining to the license fees and milestones we received. At December 31, 2017, we had an accrued liability of $12.9 million, which reflects our estimate of the additional amount we could pay the University of Massachusetts for the license fees and milestones we received, assuming we reach agreement with the University of Massachusetts regarding the appropriate calculation of these sublicense fees.
Cold Spring Harbor Laboratory
We have a collaboration and license agreement with the Cold Spring Harbor Laboratory under which we acquired an exclusive license to the Cold Spring Harbor Laboratory’s patent rights related to SPINRAZA. We paid Cold Spring Harbor Laboratory nominal amounts for license fees and milestone payments we received in 2017 and a low single digit royalty on sales of SPINRAZA. Additionally, we owe a low single digit royalty on future sales of SPINRAZA. During 2017 and 2016, we paid Cold Spring Harbor Laboratory $13.1 million and $3.4 million, respectively.
7. Segment Information and Concentration of Business Risk
We have two reportable segments Ionis Core and Akcea Therapeutics. Prior to Akcea’s IPO in July 2017, we owned 100 percent of Akcea’s stock and consolidated 100 percent of Akcea’s results in our financial statements. After Akcea’s IPO, we owned approximately 68 percent of Akcea. As a result, beginning in the third quarter of 2017, we began adjusting our financial statements to reflect the noncontrolling interest that we no longer own in Akcea. Our reportable segments remain unchanged as a result of Akcea’s IPO. Segment income (loss) from operations includes revenue less operating expenses attributable to each segment.
In our Ionis Core segment we are exploiting a novel drug discovery platform we created to generate a broad pipeline of first-in-class and/or best-in-class drugs for us and our partners. Our Ionis Core segment generates revenue from a multifaceted partnering strategy and includes multiple streams of revenue including license fees, milestone payments and royalties, among others.
Akcea is a late-stage biopharmaceutical company focused on developing and commercializing drugs to treat people with serious cardiometabolic diseases caused by lipid disorders.
F-37
The following tables show our segment revenue and income (loss) from operations for 2017, 2016 and 2015 (in thousands), respectively.
|
2017
|
Ionis Core
|
Akcea Therapeutics
|
Elimination of
Intercompany Activity
|
Total
|
||||||||||||
|
Revenue:
|
||||||||||||||||
|
Commercial revenue:
|
||||||||||||||||
|
SPINRAZA royalties
|
$
|
112,540
|
$
|
—
|
$
|
—
|
$
|
112,540
|
||||||||
|
Licensing and other royalty revenue
|
9,519
|
—
|
—
|
9,519
|
||||||||||||
|
Total commercial revenue
|
122,059
|
—
|
—
|
122,059
|
||||||||||||
|
R&D revenue under collaborative agreements
|
384,805
|
55,209
|
(54,407
|
)
|
385,607
|
|||||||||||
|
Total segment revenue
|
$
|
506,864
|
$
|
55,209
|
$
|
(54,407
|
)
|
$
|
507,666
|
|||||||
|
Total operating expenses
|
$
|
373,788
|
$
|
163,871
|
$
|
(54,527
|
)
|
$
|
483,132
|
|||||||
|
Income (loss) from operations
|
$
|
133,076
|
$
|
(108,662
|
)
|
$
|
120
|
$
|
24,534
|
|||||||
|
2016
|
Ionis Core
|
Akcea Therapeutics
|
Elimination of
Intercompany Activity
|
Total
|
||||||||||||
|
Revenue:
|
||||||||||||||||
|
Commercial revenue:
|
||||||||||||||||
|
SPINRAZA royalties
|
$
|
883
|
$
|
—
|
$
|
—
|
$
|
883
|
||||||||
|
Licensing and other royalty revenue
|
19,839
|
—
|
—
|
19,839
|
||||||||||||
|
Total commercial revenue
|
20,722
|
—
|
—
|
20,722
|
||||||||||||
|
R&D revenue under collaborative agreements
|
338,546
|
—
|
(12,648
|
)
|
325,898
|
|||||||||||
|
Total segment revenue
|
$
|
359,268
|
$
|
—
|
$
|
(12,648
|
)
|
$
|
346,620
|
|||||||
|
Total operating expenses
|
$
|
322,192
|
$
|
83,512
|
$
|
(12,768
|
)
|
$
|
392,936
|
|||||||
|
Income (loss) from operations
|
$
|
37,076
|
$
|
(83,512
|
)
|
$
|
120
|
$
|
(46,316
|
)
|
||||||
|
2015
|
Ionis Core
|
Akcea Therapeutics
|
Elimination of
Intercompany Activity
|
Total
|
||||||||||||
|
Revenue:
|
||||||||||||||||
|
R&D revenue under collaborative agreements
|
$
|
284,015
|
$
|
—
|
$
|
(2,655
|
)
|
$
|
281,360
|
|||||||
|
Licensing and other royalty revenue
|
2,343
|
—
|
—
|
2,343
|
||||||||||||
|
Total segment revenue
|
$
|
286,358
|
$
|
—
|
$
|
(2,655
|
)
|
$
|
283,703
|
|||||||
|
Total operating expenses
|
$
|
309,492
|
$
|
52,748
|
$
|
(2,775
|
)
|
$
|
359,465
|
|||||||
|
Income (loss) from operations
|
$
|
(23,134
|
)
|
$
|
(52,748
|
)
|
$
|
120
|
$
|
(75,762
|
)
|
|||||
The following table shows our total assets by segment at December 31, 2017 and 2016 (in thousands), respectively.
|
Total Assets
|
Ionis Core
|
Akcea Therapeutics
|
Elimination of
Intercompany Activity
|
Total
|
||||||||||||
|
December 31, 2017
|
$
|
1,341,828
|
$
|
268,804
|
$
|
(288,608
|
)
|
$
|
1,322,024
|
|||||||
|
December 31, 2016
|
$
|
1,067,770
|
$
|
10,684
|
$
|
(165,987
|
)
|
$
|
912,467
|
|||||||
We have historically funded our operations from collaborations with corporate partners and a relatively small number of partners have accounted for a significant percentage of our revenue. Revenue from significant partners, which is defined as 10 percent or more of our total revenue, was as follows:
|
2017
|
2016
|
2015
|
||||||||||
|
Partner A
|
51
|
%
|
60
|
%
|
37
|
%
|
||||||
|
Partner B
|
14
|
%
|
2
|
%
|
33
|
%
|
||||||
|
Partner C
|
10
|
%
|
2
|
%
|
11
|
%
|
||||||
|
Partner D
|
11
|
%
|
0
|
%
|
0
|
%
|
||||||
|
Partner E
|
3
|
%
|
19
|
%
|
2
|
%
|
||||||
|
Partner F
|
2
|
%
|
4
|
%
|
12
|
%
|
||||||
Contracts receivables at December 31, 2017 and December 31, 2016 were comprised of approximately 84 percent and 92 percent for each year from two significant partners, respectively.
F-38
8. Employment Benefits
We have an employee 401(k) salary deferral plan, covering all employees. Employees could make contributions by withholding a percentage of their salary up to the IRS annual limit $18,000 and $24,000 in 2017 for employees under 50 years old and employees 50 years old or over, respectively. We made approximately $3.0 million, $1.7 million and $1.5 million in matching contributions for the years ended December 31, 2017, 2016 and 2015, respectively.
9. Legal Proceedings
From time to time, we are involved in legal proceedings arising in the ordinary course of our business. Periodically, we evaluate the status of each legal matter and assess our potential financial exposure. If the potential loss from any legal proceeding is considered probable and the amount can be reasonably estimated, we accrue a liability for the estimated loss. Significant judgment is required to determine the probability of a loss and whether the amount of the loss is reasonably estimable. The outcome of any proceeding is not determinable in advance. As a result, the assessment of a potential liability and the amount of accruals recorded are based only on the information available to us at the time. As additional information becomes available, we reassess the potential liability related to the legal proceeding, and may revise our estimates.
Gilead Litigation
In August 2013, Gilead Sciences Inc. filed a suit in the United States District Court of Northern District of California related to United States Patent Nos. 7,105,499 and 8,481,712, which are jointly owned by Merck Sharp & Dohme Corp. and Ionis Pharmaceuticals, Inc. In the suit Gilead asked the court to determine that Gilead's activities do not infringe any valid claim of the named patents and that the patents are not valid. We and Merck Sharp & Dohme Corp. filed our answer denying Gilead's noninfringement and invalidity contentions, contending that Gilead's commercial sale and offer for sale of sofosbuvir prior to the expiration of the '499 and '712 patents infringes those patents, and requesting monetary damages to compensate for such infringement. In the trial for this case held in March 2016, the jury upheld all ten of the asserted claims of the patents-in-suit. The jury then decided that we and Merck are entitled to four percent of $5 billion in past sales of sofosbuvir. Gilead has stated it would appeal the jury’s finding of validity. In the meantime, Gilead asserted two additional non-jury defenses: waiver and unclean hands. Although the judge rejected the waiver defense, she granted Gilead’s motion claiming that the patents are unenforceable against it under the doctrine of unclean hands. We believe this ruling is contrary to the relevant law and the facts of the case. Accordingly, in July 2016, together with Merck we appealed the decision to the Court of Appeals for the Federal Circuit. Gilead cross-appealed on the issue of validity. Briefing on the appeals is now complete and oral arguments were held in February 2018. Under our agreement with Merck, Merck is responsible for the costs of this suit.
10. Quarterly Financial Data (Unaudited)
The following financial information reflects all normal recurring adjustments, which are, in the opinion of management, necessary for a fair statement of the results of the interim periods. Summarized quarterly data for the years ended December 31, 2017 and 2016 are as follows (in thousands, except per share data).
|
2017 Quarters
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
||||||||||||
|
Revenue
|
$
|
110,304
|
$
|
104,152
|
$
|
120,911
|
$
|
172,299
|
||||||||
|
Operating expenses
|
$
|
96,315
|
$
|
105,823
|
$
|
107,002
|
$
|
173,992
|
||||||||
|
Income (loss) from operations
|
$
|
13,989
|
$
|
(1,671
|
)
|
$
|
13,909
|
$
|
(1,693
|
)
|
||||||
|
Net income (loss)
|
$
|
3,468
|
$
|
(11,206
|
)
|
$
|
(4,896
|
)
|
$
|
(4,662
|
)
|
|||||
|
Net income (loss) attributable to Ionis Pharmaceuticals, Inc. common stockholders
|
$
|
3,468
|
$
|
(11,206
|
)
|
$
|
(976
|
)
|
$
|
2,744
|
||||||
|
Basic net income (loss) per share (1) (2)
|
$
|
0.03
|
$
|
(0.09
|
)
|
$
|
0.00
|
$
|
0.02
|
|||||||
|
Diluted net income (loss) per share (1) (3)
|
$
|
0.03
|
$
|
(0.09
|
)
|
$
|
0.00
|
$
|
0.02
|
|||||||
|
2016 Quarters
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
||||||||||||
|
Revenue
|
$
|
36,874
|
$
|
38,470
|
$
|
110,927
|
$
|
160,349
|
||||||||
|
Operating expenses
|
$
|
91,526
|
$
|
87,397
|
$
|
94,819
|
$
|
119,194
|
||||||||
|
Income (loss) from operations
|
$
|
(54,652
|
)
|
$
|
(48,927
|
)
|
$
|
16,108
|
$
|
41,155
|
||||||
|
Net income (loss)
|
$
|
(62,917
|
)
|
$
|
(56,855
|
)
|
$
|
7,351
|
$
|
25,865
|
||||||
|
Basic net income (loss) per share (1)
|
$
|
(0.52
|
)
|
$
|
(0.47
|
)
|
$
|
0.06
|
$
|
0.21
|
||||||
|
Diluted net income (loss) per share (1) (4) (5)
|
$
|
(0.52
|
)
|
$
|
(0.47
|
)
|
$
|
0.06
|
$
|
0.21
|
||||||
| (1) |
We computed net income (loss) per share independently for each of the quarters presented. Therefore, the sum of the quarterly net income (loss) per share will not necessarily equal the total for the year.
|
| (2) |
As discussed in Note 1, Organization and Significant Accounting Policies, we compute basic net income (loss) per share by dividing the total net income (loss) attributable to our common stockholders by our weighted-average number of common shares outstanding during the period. The calculation of total net income (loss) attributable to our common stockholders for the three months ended December 31, 2017 and September 30, 2017 considered our net income for Ionis on a stand-alone basis plus our share of Akcea’s net loss for the periods. To calculate the portion of Akcea’s net loss attributable to our ownership, we multiplied Akcea’s income (loss) per share by the weighted average shares we owned in Akcea during the period.
|
F-39
Our basic net income (loss) per share for the three months ended December 31, 2017, was calculated as follows (in thousands, except per share amounts):
|
Three Months Ended December 31, 2017
|
Weighted
Average Shares
Owned in Akcea
|
Akcea’s
Net Loss
Per Share
|
Ionis’
Portion of
Akcea’s Net Loss
|
|||||||||
|
Common shares
|
45,448
|
$
|
(0.35
|
)
|
$
|
(15,955
|
)
|
|||||
|
Akcea’s net loss attributable to our ownership
|
$
|
(15,955
|
)
|
|||||||||
|
Ionis’ stand-alone net income
|
18,672
|
|||||||||||
|
Net income available to Ionis common stockholders
|
$
|
2,717
|
||||||||||
|
Weighted average shares outstanding
|
124,818
|
|||||||||||
|
Basic net income per share
|
$
|
0.02
|
||||||||||
For the three months ended December 31, 2017, we had net income available to Ionis common stockholders. As a result, we computed diluted net income per share using the weighted-average number of common shares and dilutive common equivalent shares outstanding during those periods. Diluted common equivalent shares for the three months ended December 31, 2017 consisted of the following (in thousands except per share amounts):
|
Three Months Ended December 31, 2017
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per-Share
Amount
|
|||||||||
|
Income available to common shareholders
|
$
|
2,717
|
124,818
|
$
|
0.02
|
|||||||
|
Effect of dilutive securities:
|
||||||||||||
|
Shares issuable upon exercise of stock options
|
—
|
1,532
|
||||||||||
|
Shares issuable upon restricted stock award issuance
|
—
|
507
|
||||||||||
|
Shares issuable related to our ESPP
|
—
|
5
|
||||||||||
|
Income available to common shareholders, plus assumed conversions
|
$
|
2,717
|
126,862
|
$
|
0.02
|
|||||||
For the three months ended December 31, 2017, the calculation excluded the 1 percent and 2¾ percent notes because the effect on diluted earnings per share was anti-dilutive.
Prior to Akcea’s IPO, we owned Akcea series A convertible preferred stock, which included a six percent cumulative dividend. Upon completion of Akcea’s IPO in July 2017, our preferred stock was converted into common stock on a 1:1 basis. The preferred stock dividend was not paid at the IPO because it was not a liquidation event or a change in control. During the three months ended September 30, 2017, Akcea used a two-class method to compute its net income (loss) per share because it had both common and preferred shares outstanding during the periods. The two-class method required Akcea to calculate its net income (loss) per share for each class of stock by dividing total distributable losses applicable to preferred and common stock, including the six percent cumulative dividend contractually due to series A convertible preferred shareholders, by the weighted-average of preferred and common shares outstanding during the requisite period. Since Akcea used the two-class method, accounting rules required us to include our portion of Akcea's net income (loss) per share for both Akcea's common and preferred shares which we owned in our calculation of basic and diluted net income (loss) per share for three months ended September 30, 2017. As a result of this calculation, our total net income (loss) available to Ionis common stockholders for the calculation of net income (loss) per share is different than net income (loss) attributable to Ionis Pharmaceuticals, Inc. common stockholders in the consolidated statements of operations.
Our basic net income (loss) per share for the three months ended September 30, 2017, was calculated as follows (in thousands, except per share amounts):
|
Three Months Ended September 30, 2017
|
Weighted
Average Shares
Owned in Akcea
|
Akcea’s
Net Income (Loss)
Per Share
|
Ionis’
Portion of
Akcea’s Net Loss
|
|||||||||
|
Common shares
|
36,556
|
$
|
(0.27
|
)
|
$
|
(9,870
|
)
|
|||||
|
Preferred shares
|
5,651
|
0.05
|
283
|
|||||||||
|
Akcea’s net loss attributable to our ownership
|
$
|
(9,587
|
)
|
|||||||||
|
Ionis’ stand-alone net income
|
9,168
|
|||||||||||
|
Net loss available to Ionis common stockholders
|
$
|
(419
|
)
|
|||||||||
|
Weighted average shares outstanding
|
124,370
|
|||||||||||
|
Basic net income per share
|
$
|
0.00
|
||||||||||
F-40
| (3) |
For the three months ended March 31, 2017 we had net income. As a result, we computed diluted net income per share using the weighted-average number of common shares and dilutive common equivalent shares outstanding during the period. Diluted common equivalent shares for the three months ended March 31, 2017 consisted of the following (in thousands):
|
|
Three Months Ended March 31, 2017
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per-Share
Amount
|
|||||||||
|
Income available to common shareholders
|
$
|
3,468
|
122,861
|
$
|
0.03
|
|||||||
|
Effect of dilutive securities:
|
||||||||||||
|
Shares issuable upon exercise of stock options
|
—
|
1,674
|
||||||||||
|
Shares issuable upon restricted stock award issuance
|
—
|
377
|
||||||||||
|
Shares issuable related to our ESPP
|
—
|
60
|
||||||||||
|
Income available to common shareholders, plus assumed conversions
|
$
|
3,468
|
124,972
|
$
|
0.03
|
|||||||
For the three months ended March 31, 2017, the calculation excludes the 1 percent and 2¾ percent notes because the effect on diluted earnings per share was anti-dilutive.
| (4) |
For the three months ended December 31, 2016, we had net income. As a result, we computed diluted net income per share using the weighted-average number of common shares and dilutive common equivalent shares outstanding during the period. Diluted common equivalent shares for the three months ended December 31, 2016 consisted of the following (in thousands):
|
|
Three Months Ended December 31, 2016
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per-Share
Amount
|
|||||||||
|
|
||||||||||||
|
Income available to common shareholders
|
$
|
25,865
|
121,340
|
$
|
0.21
|
|||||||
|
Effect of diluted securities:
|
||||||||||||
|
Shares issuable upon exercise of stock options
|
—
|
2,189
|
||||||||||
|
Shares issuable upon restricted stock award issuance
|
—
|
403
|
||||||||||
|
Shares issuable related to our ESPP
|
—
|
21
|
||||||||||
|
Income available to common shareholders, plus assumed conversions
|
$
|
25,865
|
123,953
|
$
|
0.21
|
|||||||
For the three months ended December 31, 2016, the calculation excludes the 1 percent and 2¾ percent notes because the effect on diluted earnings per share would be anti-dilutive.
| (5) |
For the three months ended September 30, 2016, we had net income. As a result, we computed diluted net income per share using the weighted-average number of common shares and dilutive common equivalent shares outstanding during the period. Diluted common equivalent shares for the three months ended September 30, 2016 consisted of the following (in thousands):
|
|
Three Months Ended September 30, 2016
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per-Share
Amount
|
|||||||||
|
|
||||||||||||
|
Income available to common shareholders
|
$
|
7,351
|
120,989
|
$
|
0.06
|
|||||||
|
Effect of diluted securities:
|
||||||||||||
|
Shares issuable upon exercise of stock options
|
—
|
2,129
|
||||||||||
|
Shares issuable upon restricted stock award issuance
|
—
|
202
|
||||||||||
|
Shares issuable related to our ESPP
|
—
|
58
|
||||||||||
|
Income available to common shareholders, plus assumed conversions
|
$
|
7,351
|
123,378
|
$
|
0.06
|
|||||||
For the three months ended September 30, 2016, the calculation excludes the 1 percent and 2¾ percent notes because the effect on diluted earnings per share would be anti-dilutive.
F-41
