Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Zynerba Pharmaceuticals, Inc. | a18-3969_18k.htm |
January 2018 Dedicated to the development and commercialization of innovative transdermal pharmaceutically-produced cannabinoid treatments for rare and near-rare neuropsychiatric conditions in patients with high unmet medical needs

Disclaimer The statements in this presentation may include forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements, among other things relate to the future operations, opportunities or financial performance of Zynerba Pharmaceuticals, Inc. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. These and other risks are described in our filings with the Securities and Exchange Commission, available at www.sec.gov. Any forward-looking statements that the Company makes in this presentation speak only as of the date of this PRESENTATION. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this presentation. © 2018 Zynerba Pharmaceuticals, Inc. All rights reserved. Zynerba is a trademark of Zynerba Pharmaceuticals, Inc. All other trademarks and registered trademarks are property of their respective owners. 2

Two patent protected compounds: ZYN002 (CBD gel) and ZYN001 (THC pro-drug patch) Focused on high unmet medical needs Fragile X syndrome (FXS): ~71K US patients; no approved products Developmental and epileptic encephalopathies (DEE): ~45K US patients Adult refractory focal epilepsy: ~500K US patients remain uncontrolled on existing AEDs Tourette Syndrome (TS): ~200K US patients have the most severe form of TS Opportunities for efficient development and commercialization strategy Orphan drug designation provides opportunity for rapid development/approval Other regulatory designations available; if granted, can accelerate approval of drugs meeting criteria Targeted physician audience = modest commercial investment Potential for consistent Orphan drug pricing across indications (>$25K per patient per year for ZYN002) Experienced team with proven development and commercialization track record in transdermal delivery, orphan diseases, neurology, and psychiatry Well capitalized with cash runway well into 2019 Multiple expected near term milestones Zynerba Pharmaceuticals A Rare/Near-Rare Neuropsychiatric Company 3
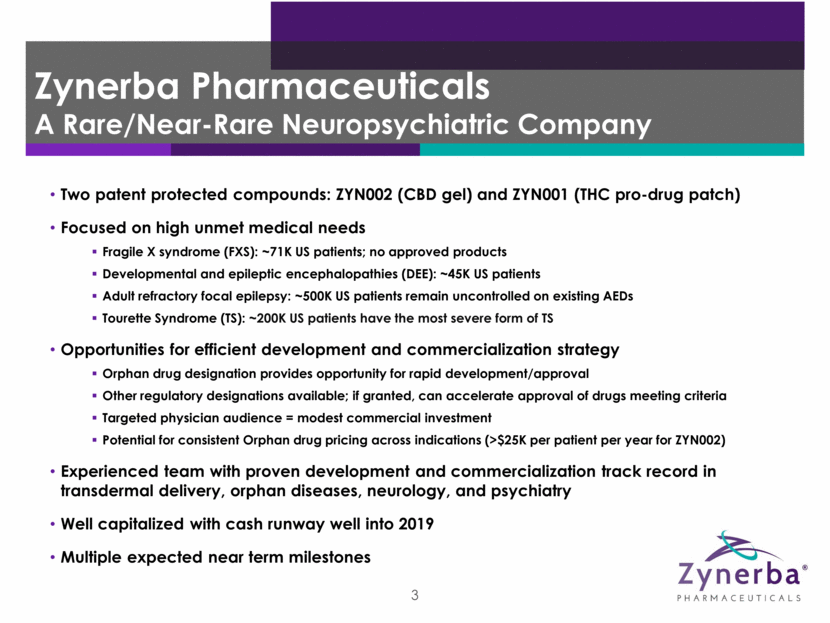
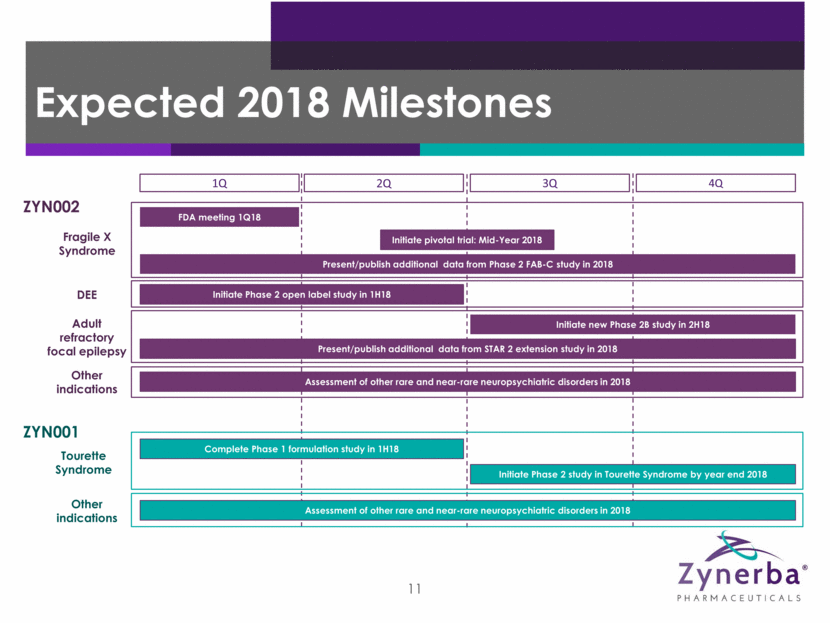
4 Expected 2018 Milestones 1Q 2Q 3Q 4Q ZYN002 Fragile X Syndrome FDA meeting 1Q18 DEE Initiate Phase 2 open label study in 1H18 Adult refractory focal epilepsy Initiate new Phase 2B study in 2H18 Present/publish additional data from STAR 2 extension study in 2018 ZYN001 Tourette Syndrome Complete Phase 1 formulation study in 1H18 Initiate Phase 2 study in Tourette Syndrome by year end 2018 Other indications Assessment of other rare and near-rare neuropsychiatric disorders in 2018 Other indications Assessment of other rare and near-rare neuropsychiatric disorders in 2018 Initiate pivotal trial: Mid-Year 2018 Present/publish additional data from Phase 2 FAB-C study in 2018
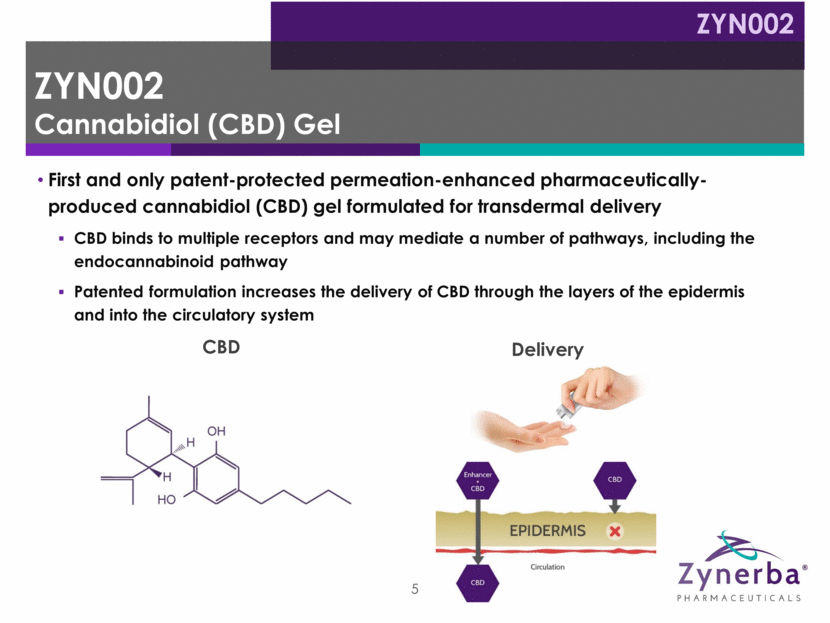
First and only patent-protected permeation-enhanced pharmaceutically-produced cannabidiol (CBD) gel formulated for transdermal delivery CBD binds to multiple receptors and may mediate a number of pathways, including the endocannabinoid pathway Patented formulation increases the delivery of CBD through the layers of the epidermis and into the circulatory system 5 CBD Delivery ZYN002 Cannabidiol (CBD) Gel ZYN002
Positive open label Phase 2 data (Sept. 2017): Achieved primary and numerous secondary endpoints with statistical significance vs. baseline Extremely well tolerated U.S. Orphan Drug Designation for use of CBD as a treatment of Fragile X (Feb. 2016) Meeting scheduled with FDA in 1Q2018 to discuss development strategy including clinical pivotal program Expect to initiate pivotal Phase 2/3 program in pediatric and adolescent FXS patients mid-year 2018 Results expected in 2019 6 Fragile X Syndrome (FXS) Pivotal-Ready Program ZYN002 FXS Inherited autism spectrum disorder found in ~71,000 U.S. patients Most common inherited intellectual disability Symptoms including significant social, behavioral, and cognitive deficits Symptoms linked to deficiencies in the endocannabinoid system caused by FMR1 mutation
Third party clinical data show impact of CBD on seizures and behavioral issues in children Expect to initiate Phase 2 study in 1H2018 24 week multi-dose study in 48 DEE patients (3-18 years) Two Australian sites Results expected in 2019 7 Developmental & Epileptic Encephalopathies (DEE) New Phase 2 Program in 2018 ZYN002 Category including a number of rare and ultra-rare severe brain disorders that manifest with seizures in children ~45,000 U.S. children with DEE Includes Doose, Dravet, Lennox-Gastaut, and West Syndromes, etc. All highly resistant to treatment DEE
Learnings from Phase 2 STAR 1 study and open label STAR 2 extension provide input into Phase 2B trial design STAR 2 data suggest clinically meaningful response with longer term use of ZYN002 Consistent improvements in median seizure rate at three, six and nine months of treatment with ZYN002 Modifications include increased baseline seizure frequency, patient count, and trial duration Expect to initiate ~300 patient double blind placebo controlled study in 2H2018 8 Adult Refractory Focal Epilepsy Phase 2B Anticipated in 2018 ZYN002 Focal seizures are the most common epilepsy in adults Substantial US market ~500,000 refractory adults New treatment options with improved quality of life (safety and efficacy) needed Adult Refractory Focal Seizures
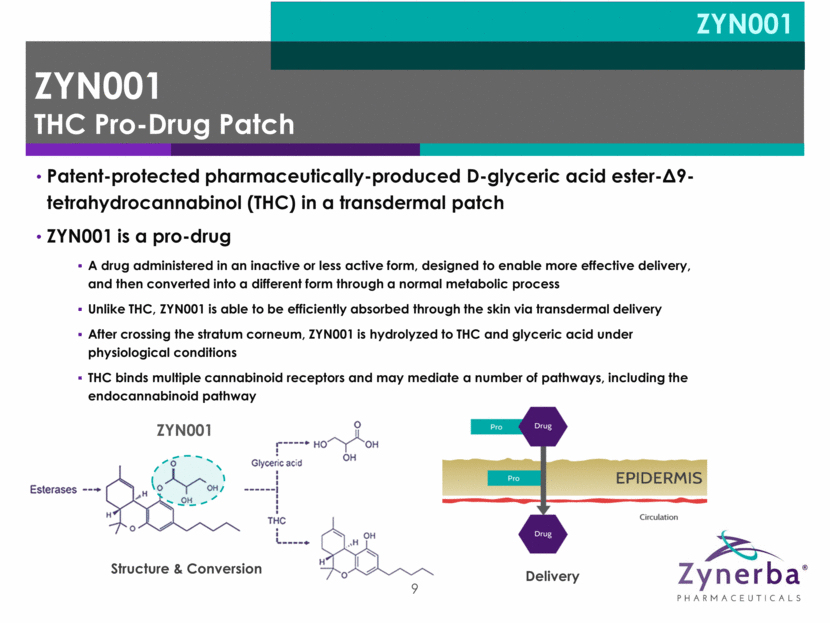
9 Patent-protected pharmaceutically-produced D-glyceric acid ester-Δ9-tetrahydrocannabinol (THC) in a transdermal patch ZYN001 is a pro-drug A drug administered in an inactive or less active form, designed to enable more effective delivery, and then converted into a different form through a normal metabolic process Unlike THC, ZYN001 is able to be efficiently absorbed through the skin via transdermal delivery After crossing the stratum corneum, ZYN001 is hydrolyzed to THC and glyceric acid under physiological conditions THC binds multiple cannabinoid receptors and may mediate a number of pathways, including the endocannabinoid pathway Structure & Conversion Delivery ZYN001 ZYN001 THC Pro-Drug Patch ZYN001
10 Potential for THC in Tourette Syndrome (TS) Neurodevelopmental disorder characterized by motor / vocal tics Evident in early childhood ~200K US pts have most severe form (National Institute of Neurological Disorders and Stroke) Up to 1:100 exhibit milder and less complex symptoms Central cannabinoid receptor system believed to play role in Tourette Syndrome pathology Tourette Syndrome Rationale Third party double blind, placebo controlled studies show activity of THC in TS Phase 1 formulation work expected to be completed in 1H2018 Phase 2 study in Tourette Syndrome expected to initiate in late 2H2018 ZYN001
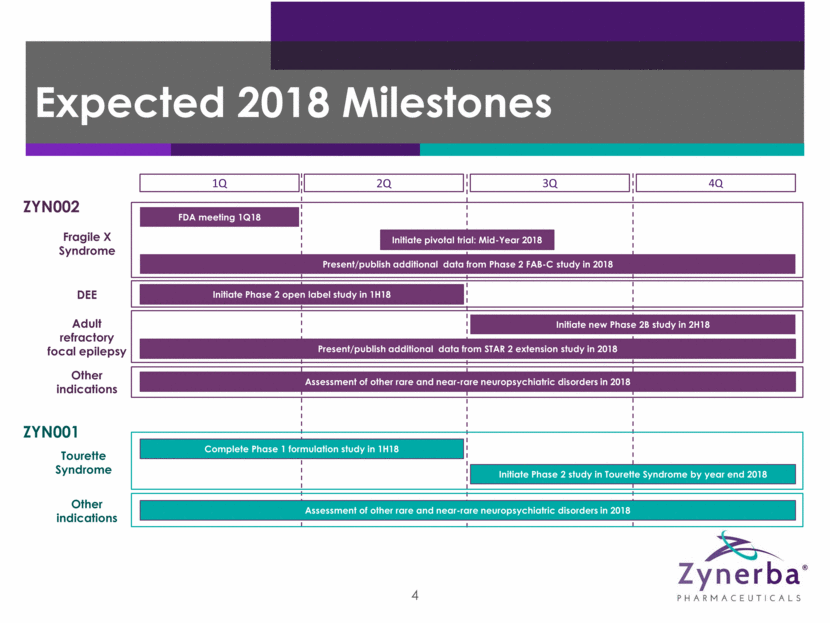
11 Expected 2018 Milestones 1Q 2Q 3Q 4Q ZYN002 Fragile X Syndrome FDA meeting 1Q18 DEE Initiate Phase 2 open label study in 1H18 Adult refractory focal epilepsy Initiate new Phase 2B study in 2H18 Present/publish additional data from STAR 2 extension study in 2018 ZYN001 Tourette Syndrome Complete Phase 1 formulation study in 1H18 Initiate Phase 2 study in Tourette Syndrome by year end 2018 Other indications Assessment of other rare and near-rare neuropsychiatric disorders in 2018 Other indications Assessment of other rare and near-rare neuropsychiatric disorders in 2018 Initiate pivotal trial: Mid-Year 2018 Present/publish additional data from Phase 2 FAB-C study in 2018
January 2018 Dedicated to the development and commercialization of innovative transdermal pharmaceutically-produced cannabinoid treatments for rare and near-rare neuropsychiatric conditions in patients with high unmet medical needs

ZYN002 CBD Gel Clinical Program Fragile X Syndrome

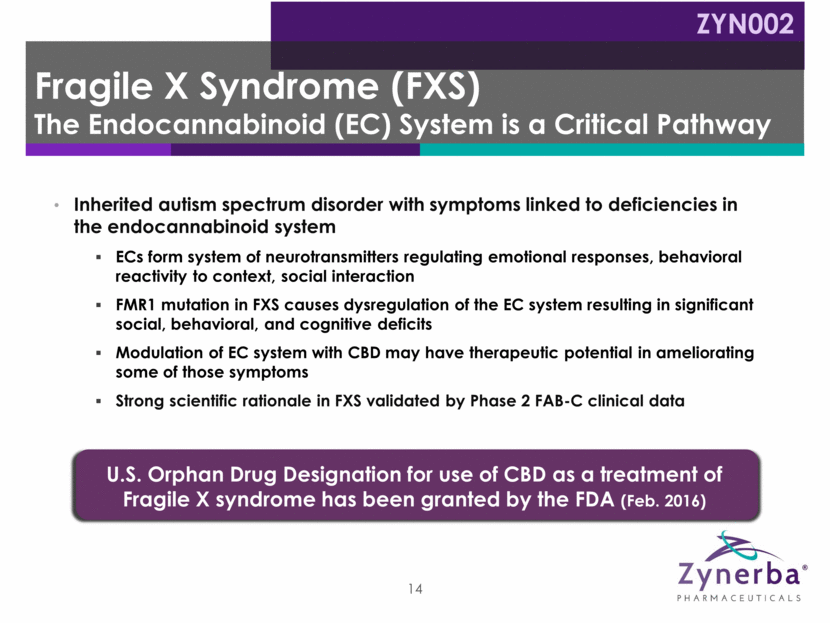
Inherited autism spectrum disorder with symptoms linked to deficiencies in the endocannabinoid system ECs form system of neurotransmitters regulating emotional responses, behavioral reactivity to context, social interaction FMR1 mutation in FXS causes dysregulation of the EC system resulting in significant social, behavioral, and cognitive deficits Modulation of EC system with CBD may have therapeutic potential in ameliorating some of those symptoms Strong scientific rationale in FXS validated by Phase 2 FAB-C clinical data 14 U.S. Orphan Drug Designation for use of CBD as a treatment of Fragile X syndrome has been granted by the FDA (Feb. 2016) Fragile X Syndrome (FXS) The Endocannabinoid (EC) System is a Critical Pathway ZYN002
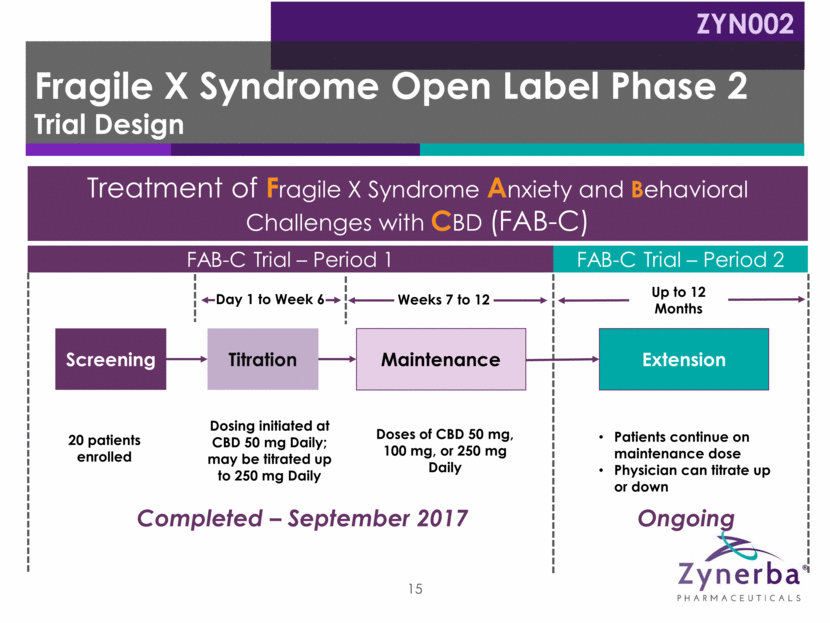
Maintenance Dosing initiated at CBD 50 mg Daily; may be titrated up to 250 mg Daily Screening Titration Weeks 7 to 12 Day 1 to Week 6 Doses of CBD 50 mg, 100 mg, or 250 mg Daily 20 patients enrolled Treatment of Fragile X Syndrome Anxiety and Behavioral Challenges with CBD (FAB-C) FAB-C Trial – Period 1 FAB-C Trial – Period 2 Up to 12 Months Extension Patients continue on maintenance dose Physician can titrate up or down Fragile X Syndrome Open Label Phase 2 Trial Design Completed – September 2017 Ongoing ZYN002 15
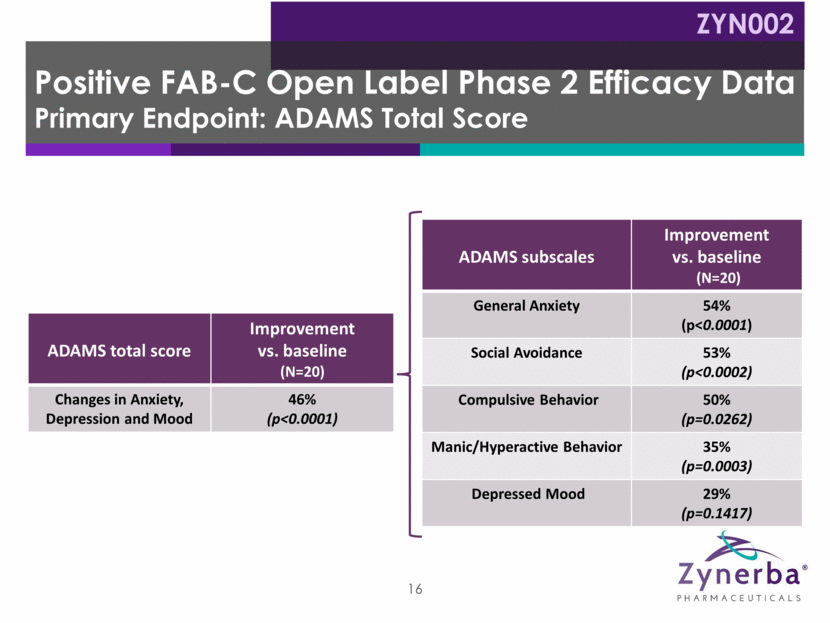
ADAMS total score Improvement vs. baseline (N=20) Changes in Anxiety, Depression and Mood 46% (p<0.0001) ADAMS subscales Improvement vs. baseline (N=20) General Anxiety 54% (p<0.0001) Social Avoidance 53% (p<0.0002) Compulsive Behavior 50% (p=0.0262) Manic/Hyperactive Behavior 35% (p=0.0003) Depressed Mood 29% (p=0.1417) 16 Positive FAB-C Open Label Phase 2 Efficacy Data Primary Endpoint: ADAMS Total Score ZYN002
17 ABC-FXS subscale Improvement vs. baseline (N=20) Stereotypy: "Repetitive Movements" 59% (p=0.0006) Social Avoidance: "Seeks Isolation" 55% (p=0.0005) Socially Unresponsive/Lethargic: "Does Not Pay Attention" 53% (p=0.0034) Inappropriate Speech: "Repeats Words or Phrases" 43% (p=0.0018) Irritability: "Has Temper Tantrums" 42% (p=0.0096) Hyperactivity: "Disrupts Group Activities" 33% (p=0.0194) Positive FAB-C Open Label Phase 2 Efficacy Data Key Secondary Endpoint: ABC-FXS ZYN002
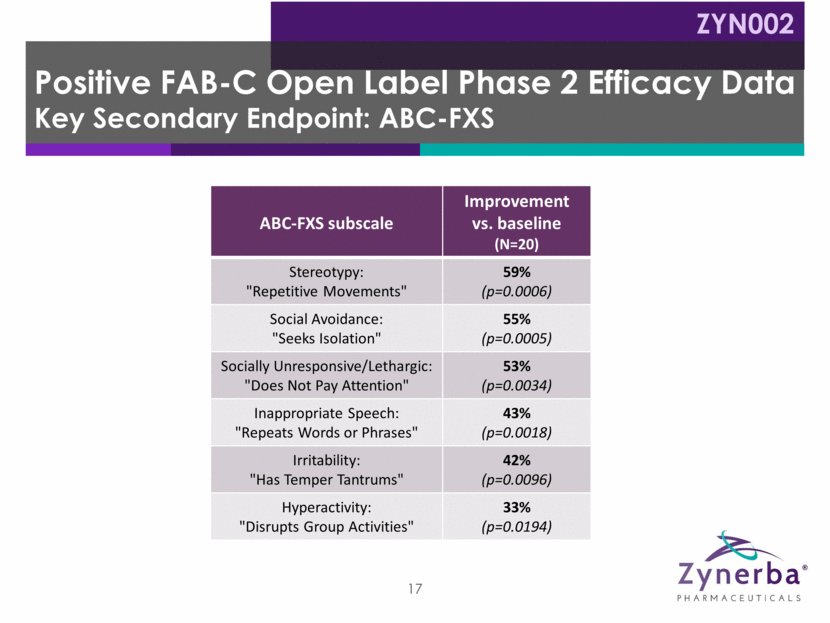
18 Manic/hyperactive Behavior Depressed Mood Social Avoidance General Anxiety Compulsive Behavior Baseline: 9.4 Baseline: 8.1 8.7 Baseline: 2.8 Baseline: 3.0 3.1 Baseline: 10.2 Baseline: 6.9 7.5 Baseline: 10.0 Baseline: 7.9 8.3 Baseline: 2.8 Baseline: 3.0 3.4 * Ligsay, A., Van Dijck, A., Nguyen, D. V., Lozano, R., Chen, Y., Bickel, E. S., et al. (2017). A randomized double-blind, placebo-controlled trial of ganaxolone in children and adolescents with fragile x syndrome. Journal of Neurodevelopmental Disorders, 9:26. 54.0% 15.7% 21.5% 52.9% 16.0% 15.9% 50.0% 17.7% 10.0% 35.1% 10.3% 8.6% 28.6% 16.1% -16.7% ADAMS Subscales Week 12: Percent Improvement vs. 3rd party data* ZYN002 0 10 20 30 40 ZYN002 -20 -10 0 10 20 30 Ganaxolone Placebo -20 -10 0 10 20 30 ZYN002 0 10 20 30 40 50 60 Ganaxolone Placebo 0 10 20 30 40 50 60 ZYN002 0 10 20 30 40 50 60 Ganaxolone Placebo 0 10 20 30 40 50 60 ZYN002 0 10 20 30 40 50 60 Ganaxolone Placebo 0 10 20 30 40 50 60 ZYN002
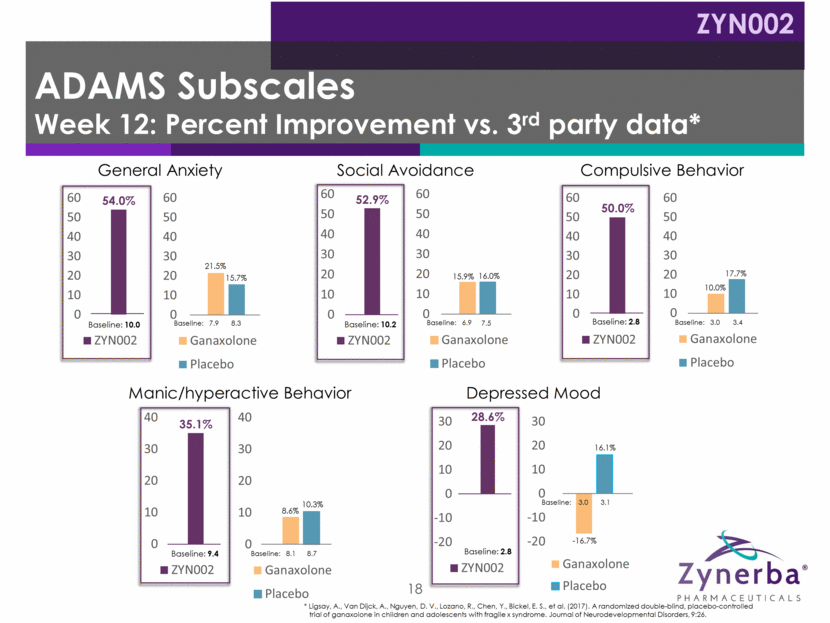
19 Irritability Temper Tantrums Hyperactivity Disrupts Group Activities Socially Unresponsive / Lethargic Does Not Pay attention Social Avoidance Seeks Isolation Stereotypy Repetitive Movements Baseline: 18.2 Baseline: 18.9 19.6 Baseline: 14.5 Baseline: 13.9 13.9 Baseline: 8.7 Baseline: 6.6 6.9 Baseline: 5.1 Baseline: 3.5 3.1 Baseline: 7.9 Baseline: 7.4 7.1 Inappropriate Speech Repeats Words / Phrases Baseline: 6.1 Baseline: 6.0 6.3 * Ligsay, A., Van Dijck, A., Nguyen, D. V., Lozano, R., Chen, Y., Bickel, E. S., et al. (2017). A randomized double-blind, placebo-controlled trial of ganaxolone in children and adolescents with fragile x syndrome. Journal of Neurodevelopmental Disorders, 9:26. 41.8% 17.9% 16.9% 33.1% 11.5% 18.7% 52.9% 17.4% 15.2% 54.9% 9.7% 31.4% 59.5% 9.9% 23.0% 42.6% 15.9% 15.0% ABC-FXS Subscales Week 12: Percent Improvement vs. 3rd Party Data* ZYN002 0 10 20 30 40 50 ZYN002 0 10 20 30 40 Ganaxolone Placebo 0 10 20 30 40 ZYN002 0 10 20 30 40 50 60 Ganaxolone Placebo 0 10 20 30 40 50 60 ZYN002 0 10 20 30 40 50 60 Ganaxolone Placebo 0 10 20 30 40 50 60 ZYN002 0 10 20 30 40 50 60 Ganaxolone Placebo 0 10 20 30 40 50 60 ZYN002 0 10 20 30 40 50 Ganaxolone Placebo 0 10 20 30 40 50 ZYN002
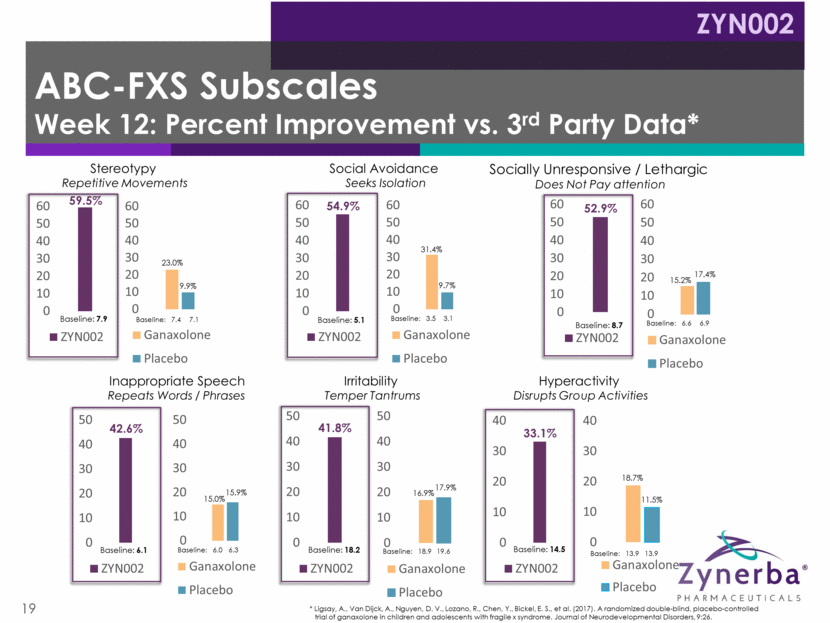
20 Very well tolerated, consistent with previously reported clinical data Two sibling patients discontinued due to worsening of pre-existing eczema Four other patients experienced an AE; no SAEs No drug-related GI events No THC was detected in the plasma 13 patients continued into open label extension; 12 remain as of 1/30/18 Eight patients at 3 months (6 months total on ZYN002) Four patients at 6 months (9 months total on ZYN002) Positive FAB-C Open Label Phase 2 Safety Data ZYN002
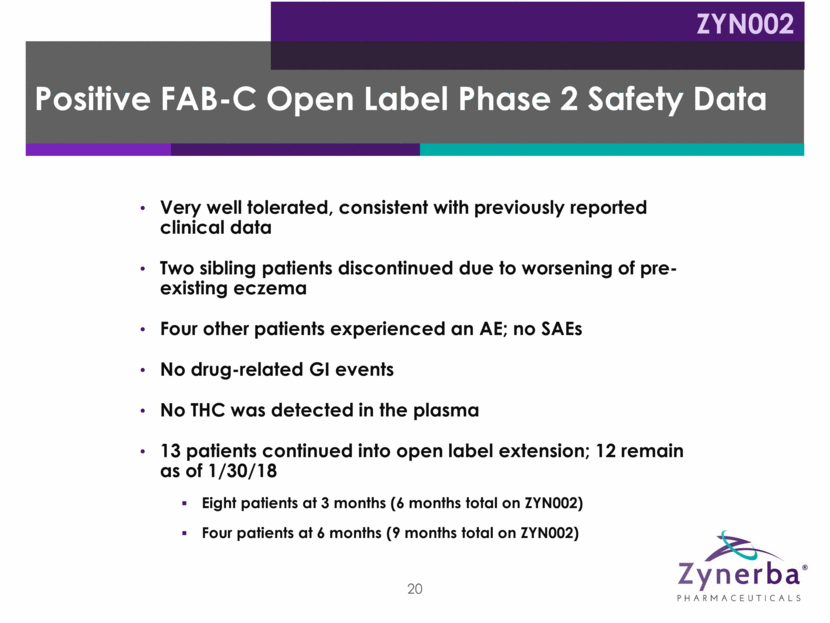
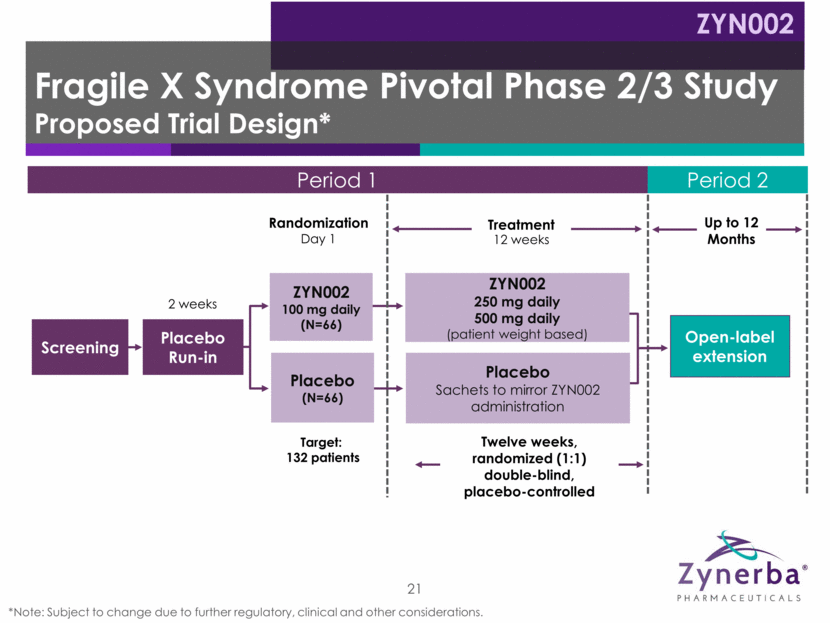
Period 1 Period 2 Up to 12 Months Fragile X Syndrome Pivotal Phase 2/3 Study Proposed Trial Design* ZYN002 Twelve weeks, randomized (1:1) double-blind, placebo-controlled Screening 21 Target: 132 patients Placebo Run-in 2 weeks Randomization Day 1 Treatment 12 weeks ZYN002 100 mg daily (N=66) Placebo (N=66) Open-label extension ZYN002 250 mg daily 500 mg daily (patient weight based) Placebo Sachets to mirror ZYN002 administration *Note: Subject to change due to further regulatory, clinical and other considerations.
22 Meeting scheduled with FDA in 1Q2018 to discuss development strategy including clinical pivotal program Goal to begin pivotal Phase 2/3 program in pediatric and adolescent patients with FXS mid-year 2018 Data expected in 2019 Assessing opportunity to present / publish full data set as soon as possible in 2018 Targeting three FXS meetings June-August 2019 Evaluating opportunities for FDA fast-track, breakthrough status, and/or priority review ZYN002 in Fragile X Syndrome Next Steps ZYN002
ZYN002 CBD Gel Clinical Program Developmental Epileptic Encephalopathies (DEE)

Category of rare and ultra-rare severe brain disorders that manifest with seizures or EEG abnormalities that can directly worsen cognition or behavior Often progressive; highly resistant to treatment Treatment of seizures or EEG abnormalities expected to improve the cognitive or behavioral deficits and reduce the seizures Third party clinical data show impact of CBD on seizures and behavioral issues Developmental and Epileptic Encephalopathies Doose Syndrome Dravet Syndrome Early Myoclonic Encephalopathy Epilepsy of Infancy With Migrating Focal Seizures Epilepsy with Generalized Tonic-Clonic Seizures alone (EGTCS) Juvenile Myoclonic Epilepsy (JME) Landau-Kleffner Syndrome Lennox-Gastaut Syndrome Ohtahara Syndrome (Early Infantile Epileptic Encephalopathy) West Syndrome / Infantile Spasms DEE category includes: 24 ZYN002
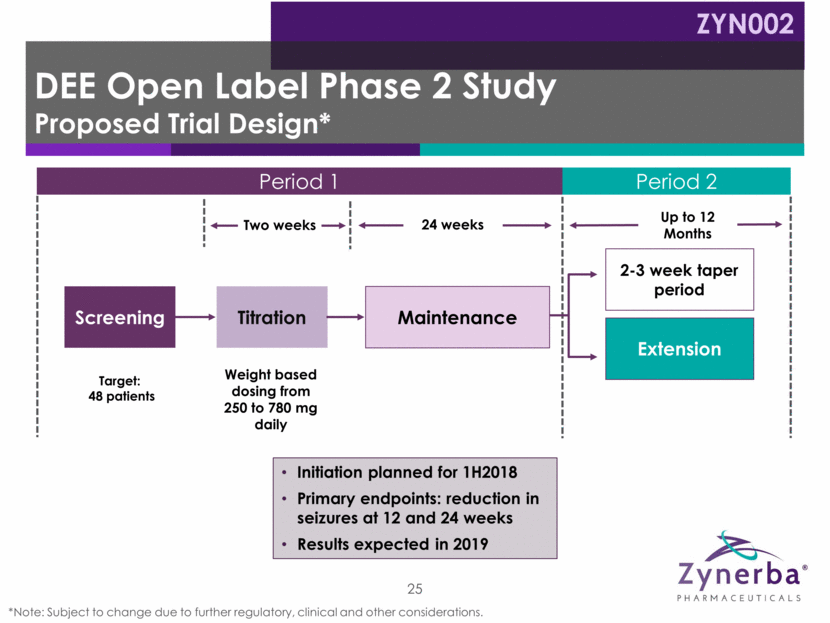
Period 1 Period 2 DEE Open Label Phase 2 Study Proposed Trial Design* ZYN002 25 *Note: Subject to change due to further regulatory, clinical and other considerations. Weight based dosing from 250 to 780 mg daily Screening Titration 24 weeks Two weeks Up to 12 Months 2-3 week taper period Extension Target: 48 patients Initiation planned for 1H2018 Primary endpoints: reduction in seizures at 12 and 24 weeks Results expected in 2019 Maintenance
ZYN002 CBD Gel Clinical Program Adult Refractory Focal Epilepsy

Learnings from Phase 2 STAR 1 study and open label STAR 2 extension provide input into Phase 2B trial design STAR 2 data suggest clinically meaningful response with longer term use of ZYN002 Consistent improvements in median seizure rate at three, six and nine months of treatment with ZYN002 Modifications include increased baseline seizure frequency, patient count, and trial duration Expect to initiate ~300 patient double blind placebo controlled study in 2H2018 27 Adult Refractory Focal Epilepsy Phase 2B Anticipated in 2018 ZYN002 Focal seizures are the most common epilepsy in adults Substantial US market ~500,000 refractory adults New treatment options with improved quality of life (safety and efficacy) needed Adult Refractory Focal Seizures
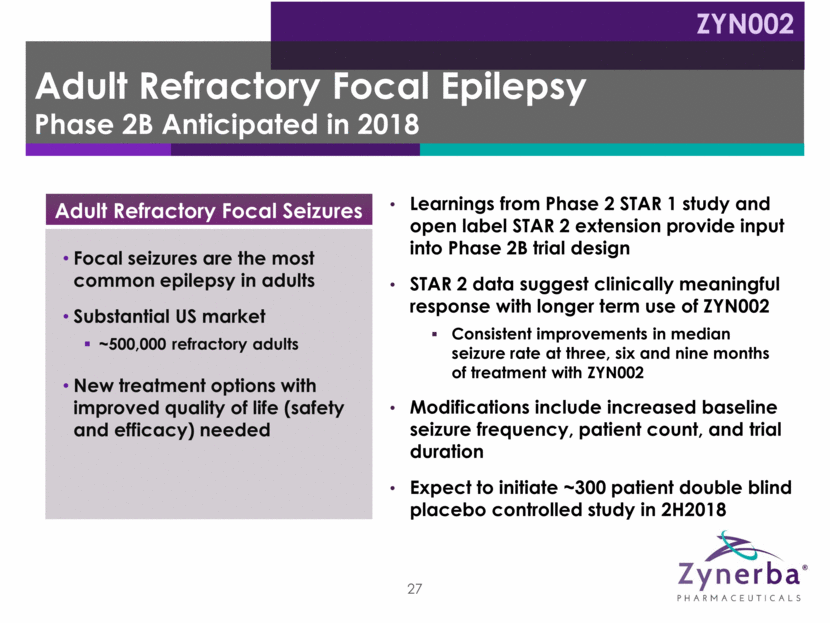
ZYN002 High-Dose CBD 195 mg BID ZYN002 Low-Dose CBD 97.5 mg BID Placebo Baseline Assess seizure frequency/type Open-Label Extension Synthetic Transdermal CAnnabidiol for the TReatment of Epilepsy ZYN002 CBD 195 mg BID STAR 2 Trial STAR 1 Trial Dose can be adjusted to: BID for 18 months BID for 12 weeks 8 weeks Randomized (1:1:1), double-blind, placebo-controlled - 188 patients randomized Epilepsy Phase 2 Clinical Study Trial Design Completed – August 2017 Ongoing ZYN002 Low-Dose CBD 97.5 mg BID ZYN002 High-Dose CBD 390 mg BID 28
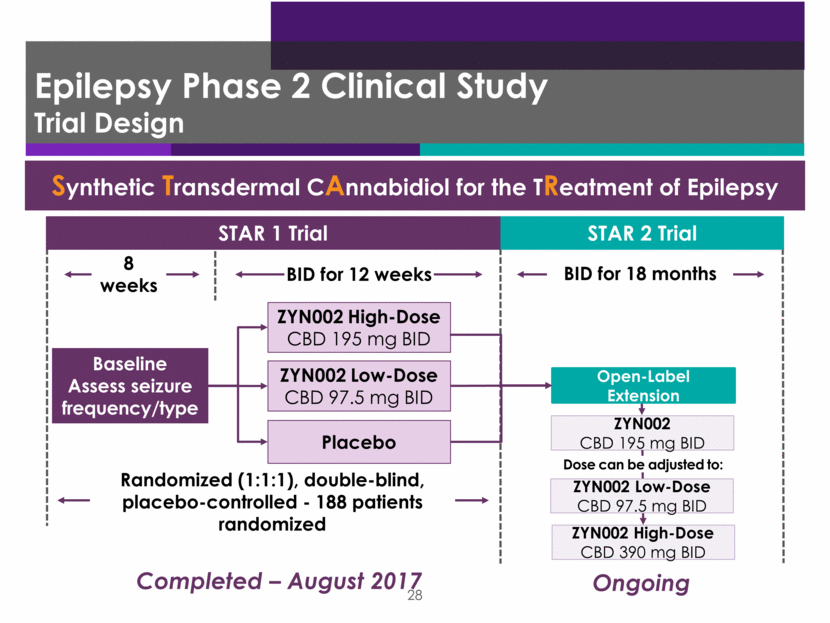
Epilepsy Phase 2 Clinical Study Demographics and Baseline Characteristics ZYN002 STAR 1 patients Placebo 195 mg ZYN002 390 mg ZYN002 Total Pts Randomized 63 63 62 188 Sex 43% male 57% female 51% male 49% female 42% male 58% female 45% male 55% female Pts Analyzed for efficacy 63 62 61 186 Pts completing study 62 57 55 174 Patients continuing into STAR 2 171 Baseline median seizure rate 10.5 14.0 10.1 10.6 (3-335) AEDs Median: 3.0 Mean: 2.5 Primary endpoint: Percent reduction in baseline seizures 8.7% 18.4% 14.0% 29
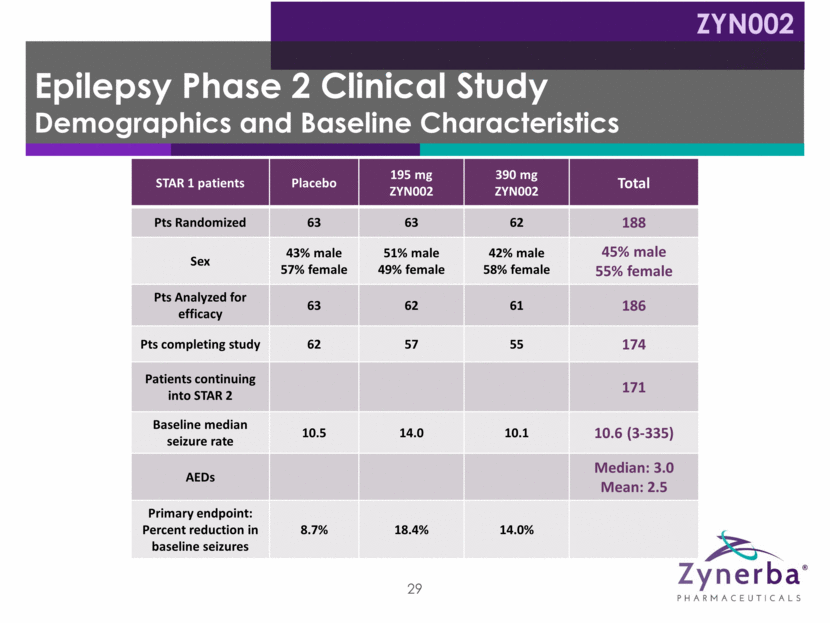
Epilepsy Phase 2 Clinical Study STAR 1 and STAR 2 Results Company believes study missed primary endpoint due to bimodal distribution of placebo patient responses : >50% reductions in focal seizures in ~¼ of placebo patients 13 of these 15 patients were female Strong separation from placebo seen at >15 baseline seizures Excellent tolerability *As of January 22, 2018 STAR 1 STAR 2 Low dropout rate: 92 patients remain in study vs 126 as of August 8, 2017* 75 patients have reached 6 mo. of drug exposure; 35 have reached 9 months* Excellent tolerability Data suggest clinically meaningful response with longer term use First cut of STAR 1 and STAR 2 data presented at the 2017 AES Meeting Additional data cuts to be presented in 2018 Learnings provide input to revised Phase 2B clinical trial design ZYN002 30
New Data Presented at AES STAR 1 and STAR 2 Efficacy Data 31 -8.70 N=63 -18.42 N=62 -14.03 N=61 -13.40 N=59 -32.68 N=55 -30.04 N=55 -26.53 N=24 -65.23 N=21 -48.45 N=18 STAR 1 STAR 2 Week 12 Month 6 Month 3 Median Percent Change in Seizure Rates Placebo ZYN002 195 mg ZYN002 390 mg Placebo (STAR 1) – ZYN002 390 mg (STAR 2) ZYN002 195 mg (STAR 1) – ZYN002 390 mg (STAR 2) ZYN002 390 mg (STAR 1) – ZYN002 390 mg (STAR 2) Median Percent Change in Seizure Rates at Week 12 (STAR 1) and Month 3 and 6 (STAR 2) STAR 2 results based on data collected through mid-August 2017 and in patients who reported seizure frequency data during the respective time period. Not all patients had reached 3 and 6 months in STAR 2 ZYN002
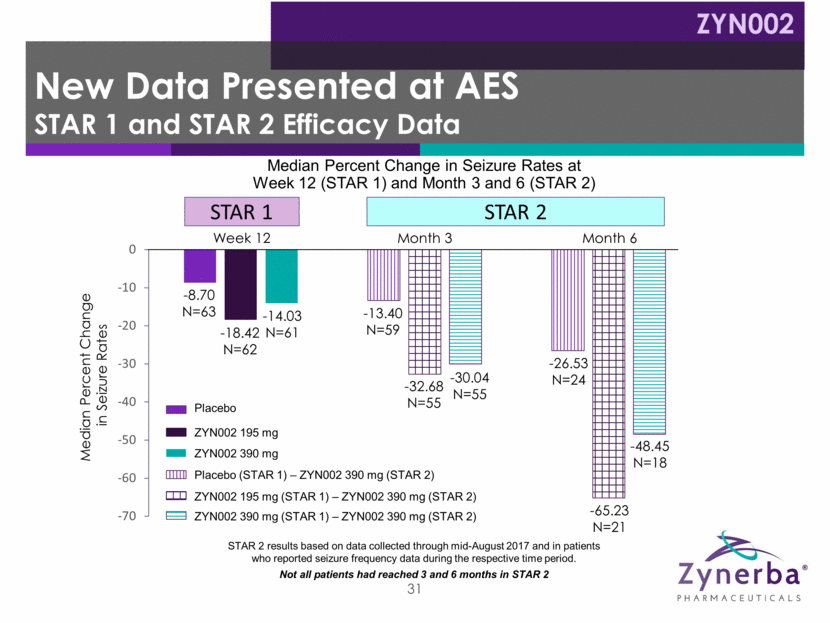
32 -18.4 N=62 -14.0 N=61 -13.4 N=59 -32.68 N=55 -30.04 N=55 -26.53 N=24 -65.23 N=21 -48.45 N=18 195 mg – 390 mg Placebo – 390 mg 390 mg – 390 mg Median Percent Change in Seizure Rates Dose Regimen in STAR 1 and STAR 2 STAR 2 results based on data collected through mid-August 2017 and in patients who reported seizure frequency data during the respective time period. Not all patients had reached 3 and 6 months in STAR 2 as of mid-August 2017 STAR 1 3 mos STAR 1 3 mos STAR 1 – 3 mos STAR 2 – 3 mos STAR 2 3 mos STAR 1 – 3 mos STAR 2 – 6 mos STAR 2 – 6 mos STAR 1 – 3 mos STAR 2 – 6 mos STAR 1 – 3 mos STAR 2 – 3 mos Consistent Results at Various Timeframes Three Months on ZYN002 ZYN002
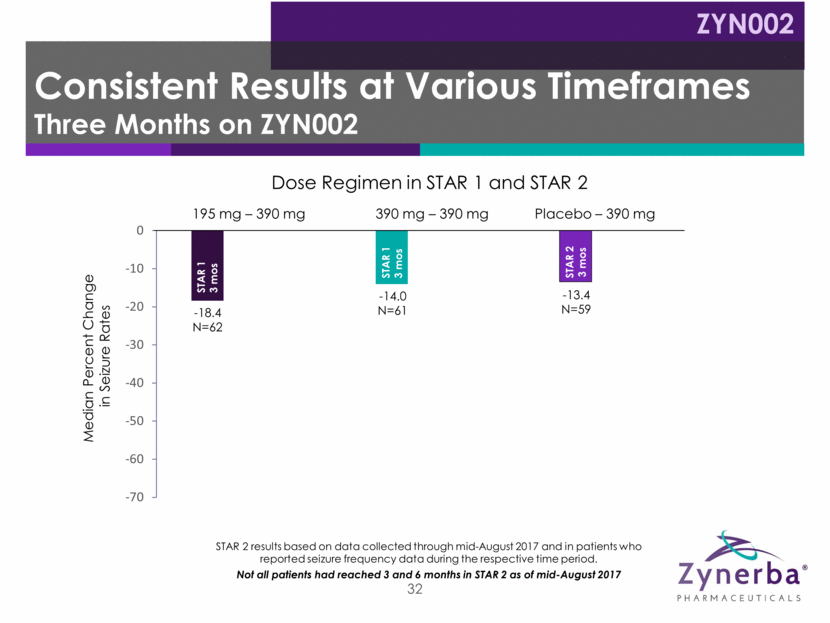
33 -18.4 N=62 -14.0 N=61 -13.4 N=59 -32.7 N=55 -30.0 N=55 -26.5 N=24 -65.23 N=21 -48.45 N=18 195 mg – 390 mg Placebo – 390 mg 390 mg – 390 mg Median Percent Change in Seizure Rates Dose Regimen in STAR 1 and STAR 2 STAR 2 results based on data collected through mid-August 2017 and in patients who reported seizure frequency data during the respective time period. Not all patients had reached 3 and 6 months in STAR 2 as of mid-August 2017 STAR 1 3 mos STAR 1 3 mos STAR 1 – 3 mos STAR 2 – 3 mos STAR 2 3 mos STAR 1 – 3 mos STAR 2 – 6 mos STAR 2 – 6 mos STAR 1 – 3 mos STAR 2 – 6 mos STAR 1 – 3 mos STAR 2 – 3 mos Consistent Results at Various Timeframes Six Months on ZYN002 ZYN002
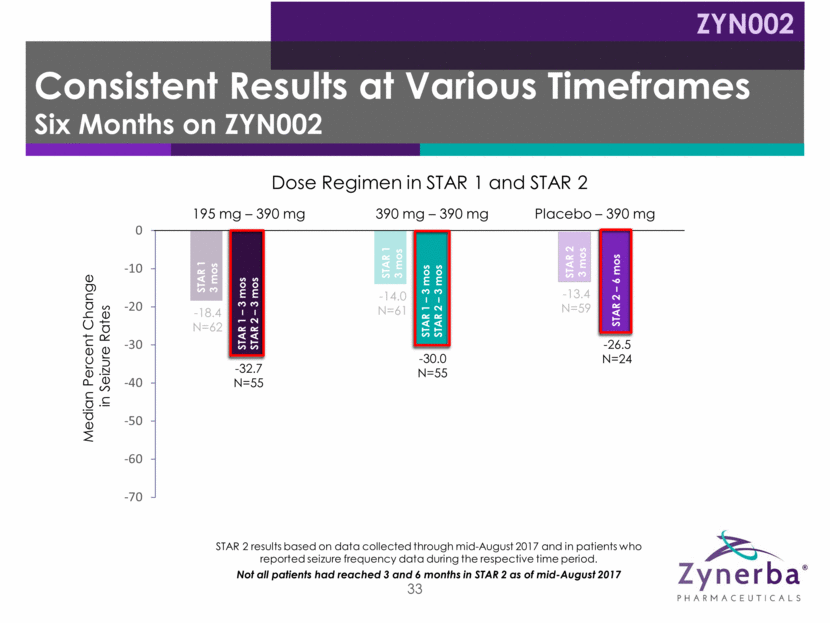
34 -18.4 N=62 -14.0 N=61 -13.4 N=59 -32.7 N=55 -30.0 N=55 -26.5 N=24 -65.2 N=21 -48.5 N=18 195 mg – 390 mg Placebo – 390 mg 390 mg – 390 mg Median Percent Change in Seizure Rates Dose Regimen in STAR 1 and STAR 2 STAR 2 results based on data collected through mid-August 2017 and in patients who reported seizure frequency data during the respective time period. Not all patients had reached 3 and 6 months in STAR 2 as of mid-August 2017 STAR 1 3 mos STAR 1 3 mos STAR 1 – 3 mos STAR 2 – 3 mos STAR 2 3 mos STAR 1 – 3 mos STAR 2 – 6 mos STAR 2 – 6 mos STAR 1 – 3 mos STAR 2 – 6 mos STAR 1 – 3 mos STAR 2 – 3 mos Consistent Results at Various Timeframes Nine Months on ZYN002 ZYN002
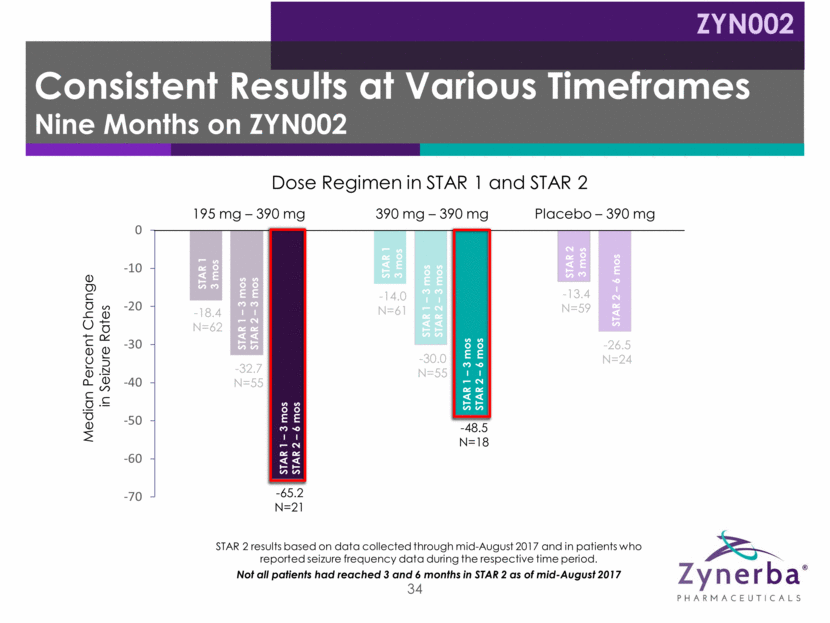
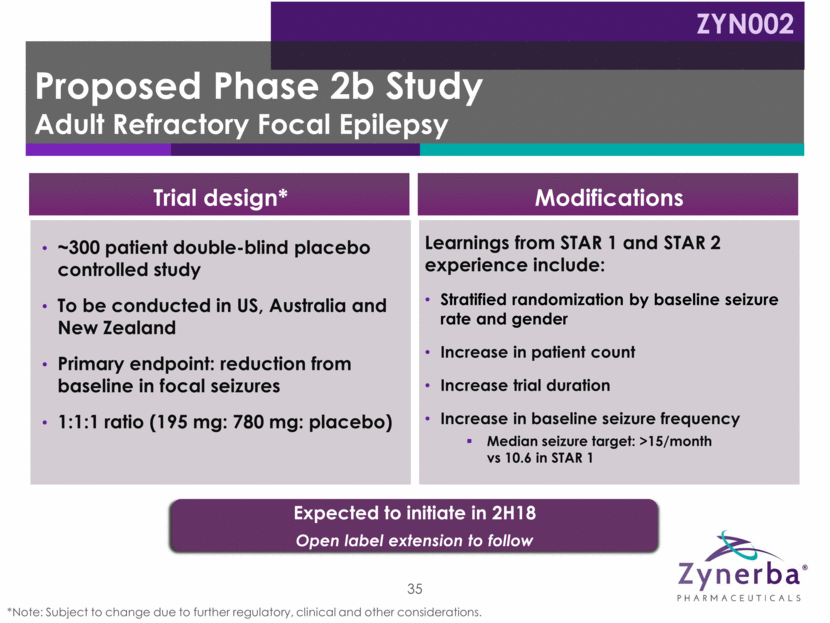
~300 patient double-blind placebo controlled study To be conducted in US, Australia and New Zealand Primary endpoint: reduction from baseline in focal seizures 1:1:1 ratio (195 mg: 780 mg: placebo) 35 Learnings from STAR 1 and STAR 2 experience include: Stratified randomization by baseline seizure rate and gender Increase in patient count Increase trial duration Increase in baseline seizure frequency Median seizure target: >15/month vs 10.6 in STAR 1 Trial design* Modifications Expected to initiate in 2H18 Open label extension to follow Proposed Phase 2b Study Adult Refractory Focal Epilepsy ZYN002 *Note: Subject to change due to further regulatory, clinical and other considerations.
Cash and cash equivalent position of $66.3 million (September 30, 2017) Additional $3.0 million in net proceeds from shares sold in September and October 2017 under our ATM program Recorded in the fourth quarter of 2017 Well capitalized, expect cash to fund operations well into 2019 36 Financial Strength

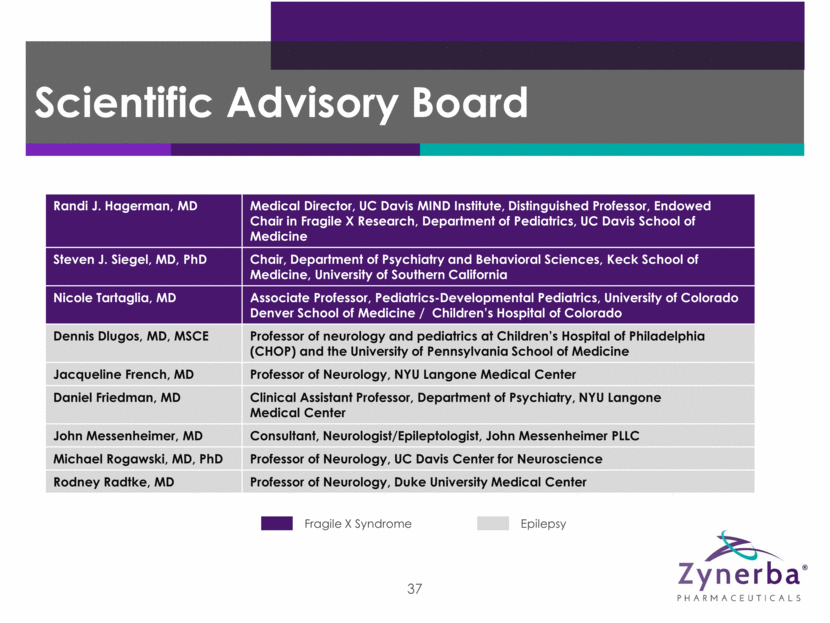
Randi J. Hagerman, MD Medical Director, UC Davis MIND Institute, Distinguished Professor, Endowed Chair in Fragile X Research, Department of Pediatrics, UC Davis School of Medicine Steven J. Siegel, MD, PhD Chair, Department of Psychiatry and Behavioral Sciences, Keck School of Medicine, University of Southern California Nicole Tartaglia, MD Associate Professor, Pediatrics-Developmental Pediatrics, University of Colorado Denver School of Medicine / Children’s Hospital of Colorado Dennis Dlugos, MD, MSCE Professor of neurology and pediatrics at Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania School of Medicine Jacqueline French, MD Professor of Neurology, NYU Langone Medical Center Daniel Friedman, MD Clinical Assistant Professor, Department of Psychiatry, NYU Langone Medical Center John Messenheimer, MD Consultant, Neurologist/Epileptologist, John Messenheimer PLLC Michael Rogawski, MD, PhD Professor of Neurology, UC Davis Center for Neuroscience Rodney Radtke, MD Professor of Neurology, Duke University Medical Center 37 Epilepsy Fragile X Syndrome Scientific Advisory Board
38 484.581.7505 investorrelations@zynerba.com www.zynerba.com @ZynerbaPharma Zynerba Zynerba Investor Contact Will Roberts, VP, Investor Relations and Corporate Communications 484.581.7489 NASDAQ: ZYNE Analyst Coverage* Piper Jaffray: Charles C. Duncan, PhD Cantor Fitzgerald: Elemer Piros, PhD Canaccord Genuity: Arlinda Lee, PhD Seaport Global: Corey Davis Oppenheimer: Derek Archila Jefferies: Biren Amin, PhD * Note: Any opinions, estimates or forecasts regarding Zynerba Pharmaceuticals, Inc.’s performance made by these analysts are theirs alone and do not represent opinions, forecasts or predictions of Zynerba Pharmaceuticals, Inc. or its management. Zynerba Pharmaceuticals, Inc. does not by its reference above imply its endorsement of or concurrence with such information, conclusions or recommendations. Investor Relations
January 2018 Dedicated to the development and commercialization of innovative transdermal pharmaceutically-produced cannabinoid treatments for rare and near-rare neuropsychiatric conditions in patients with high unmet medical needs

