Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Foamix Pharmaceuticals Ltd. | zk1820967.htm |
Exhibit 99.1

Nasdaq: FOMXJanuary 2018

Disclaimer To the extent that statements contained in this presentation are not descriptions of historical facts regarding Foamix, they are forward-looking statements reflecting management’s current beliefs and expectations. Forward-looking statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “intends,” or “continue,” or the negative of these terms or other comparable terminology. Forward-looking statements contained in this presentation include, but are not limited to, (i) statements regarding the timing of anticipated clinical trials for our product candidates; (ii) the timing of receipt of clinical data for our product candidates; (iii) our expectations regarding the potential safety, efficacy, or clinical utility of our product candidates; (iv) the size of patient populations targeted by our product candidates and market adoption of our product candidates by physicians and patients; (v) the timing or likelihood of regulatory filings and approvals; and (vi) our revenues under our agreements with our licensees, including Bayer HealthCare and other companies. Although we believe that the expectations reflected in the forward-looking statements are reasonable, various factors may cause differences between our expectations and actual results, including, but not limited to, unexpected safety or efficacy data, unexpected side effects observed during preclinical studies or in clinical trials, lower than expected enrollment rates in clinical trials, changes in expected or existing competition, changes in the regulatory environment for our product candidates and our need for future capital, the inability to protect our intellectual property, and the risk that we become a party to unexpected litigation or other disputes. You should read the documents filed by Foamix with the SEC, including our prospectuses, the Risk Factors set forth therein and the documents filed as exhibits to our registration statements, of which the prospectuses are a part, completely and with the understanding that our actual future results may be materially different from what we expect. You may obtain those documents by visiting EDGAR on the SEC website at www.sec.gov. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future.The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the foam technology or product candidates of Foamix. 2

Foamix Value Proposition 3 Late-stage Products with Potential to Differentiate vs. Market LeadersGlobal, Unencumbered Rights to Lead Programs Innovative Platform Allows for Consistent, Organic Innovation Extensive IP portfolio Strong Cash Position + Recurring Revenue = Runway into 2019Experienced Commercial / R&D Teams in Dermatology

Differentiated Foam Technology with Multiple Platforms Patented1 - United States: 60 US patents granted - Worldwide: 168 Patents grantedCapability to formulate multiple drugs Suitable for a variety of target sitesDermal alternative to oral delivery 4 1. As of July 26, 2017 Cream Foams (Emulsion or Emollient) Ointment Foams (Petrolatum-based) Waterless Hydrophilic Foams (Enhanced penetration) Oil Foams Hydroethanolic Foams Saccharide Foams (For wounds and burns) Potent Solvent Foams(High solubility and delivery) Suspension Foams (Concentrated suspensions) Nano-Emulsion Foams (Enhanced penetration)

5 Name Track record Location David DomzalskiCEO President, Foamix USHead of Commercial Operations at Warner Chilcott & LEO US Ilan HadarCFO & Country Manager Held finance roles at Israeli subsidiaries of Pfizer, HP and BAE Systems Israel Iain Stuart, PhDSVP, Research & Development VP Clinical Development, FoamixVP Medical Affairs, LEO US Mutya HarschGeneral Counsel & SVP Legal Affairs Special Counsel, Mergers & AcquisitionsAssistant General Counsel US Mitchell Shirvan, PhDVP, Innovation & Discovery Head of R&D, CNS division at Teva CEO of MacroCure Israel Yohan Hazot VP, Pharmaceutical Development Led multiple product developments in dermatology Israel Russell Elliott, DPhilVP, Drug Development VP, Product Development at Stiefel, a GSK companyLed product development at Procter & Gamble US Alvin HowardVP, Regulatory Affairs SVP Regulatory Affairs at Warner ChilcottLed approvals of 14 NDA and sNDAs US Experienced Management Team

Late Stage Pipeline 6 Product Candidate Preclinical Phase 2 Phase 3 Milestones Minocycline Foam FMX101 (4%) for Moderate-Severe Acne Study 04 / 05 TLR announcedLong-term safety study completed3rd Phase 3 initiated August 2017TLR - mid 2018NDA filing - H2 2018 FMX103 (1.5%)for Moderate-Severe Rosacea Phase 2 completedPhase 3 initiated June 2017TLR - mid 2018NDA filing - 2019

FMX101 Topical Minocycline Foam 4%For Moderate-to-Severe Acne 7

Acne – the US Market ~50 million people of all ages and ethnicities have acne in the US1Moderate-to-severe acne affects ~10 million people in the US >14 million physician visits per year for treatment of acne2Classification by severity / current therapies (1) AAD. Acne Stats and Facts. www.aad.org/media-resources/stats-and-facts/conditions. Accessed March 30, 2016. (2) GlobalData, EpiCast. Acne Vulgaris Epidemiology Forecast to 2022;33-34.Mancini AJ. Adv Stud Med. 2008;8:100-105.Symphony Health Services PHAST: 2016 Branded Only (accessed 1.18.17), Select Market >75% rosacea weighting removed 8 Current Branded Market (United States)3 US Dollars TRxs Oral antibiotics $1.1 billion 1.2 mm Topical drugs $2 billion 4.3 mm Total $3.1 billion 5.5 mm Mild AcneLess than 30 lesions<15 Inflammatory lesions Moderate Acne <50 Inflammatory lesions Severe Acne>50 Inflammatory lesions Isotretinoin Topicalcombinations Topical Oral antibiotics

Acne – the US Market (2016)Large Market Potential Despite Lack of Innovation Source: Symphony Health Services PHAST (accessed 1.18.17). (1) market shares of the oral branded prescription acne drug market and the topical branded prescription acne drug market according to the total number of prescriptions. Top Oral Brands US Dollars TRxs 1 SOLODYNMinocycline, Valeant $596,079,817 565,040 2 ACTICLATEDoxycycline, Almirall $321,951,635 388,084 3 DORYXDoxycycline, Mayne $160,887,307 180,863 Top Topical Brands US Dollars TRxs 1 EPIDUO FRANCHISEAdapalene+BPO, Galderma $494,186,838 1,179,329 2 ACZONE FRANCHISEDapsone, Allergan $455,752,396 946,637 3 ONEXTON/ACANYAClindamycin+BPO, Valeant $225,357,468 503,547 4 RETIN-A FRANCHISETretinoin, Valeant $210,905,896 273,051 5 ZIANAClindamycin+tretinoin, Valeant $101,534,314 150,964 9 Top brands oral and topical formulations (LTM December, 2016)

Phase 3: Design of Each Pivotal Study (x2)Studies FX2014-04 & FX2014-05 Self-apply, once daily, in the evening, for 12 weeksInclusion criteriaAt least 20 inflammatory and 25 non-inflammatory lesionsIGA 6 point scale – Moderate or Severe (Grade 3 or 4) Co-Primary Efficacy EndpointsMean change from baseline in inflammatory lesion countProportion of subjects with IGA scores of “Clear” or “Almost Clear”, with improvement of at least 2 grades from baseline 10 12-week, randomized, double-blind, vehicle controlled, in subjects with moderate-to-severe acne; followed by 9 month open label safety extension Week 12(End of treatment) 12 Months Week 3 Week 6 Week 9 Double-blinded PhaseRandomized (2:1), double-blindN = 450 Minocycline foam 4% – 9 months of treatment Open Label Safety Extension Subjects who complete one of the randomized, Phase 3 studies may enter the open-label phase Minocycline Foam 4%Foam vehicle Baseline

Acne Phase 3 11 Baseline Data Total number of subjects: 466 (Study 04) 495 (Study 05)Mean age: 20.3 20.6Male/Female: M=42.9% F=57.1% M=41.4% F=58.6%Ethnicity: W=62.7% B=27.0% O=10.3% W=74.1% B=20.8% O=5.0% Study 04 Study 05 FMX-101, 4%(N = 307) Vehicle(N = 159) FMX-101, 4%(N = 333) Vehicle(N = 162) Baseline Inflammatory Lesion Counts Mean (SD) 32.2(8.4) 31.6(8.6) 31.6(8.6) 32.3(8.0) Median 31 30 30 31 Range (min-max) 20-50 20-76 20-69 20-50 Baseline Non-inflammatory Lesion Counts Mean (SD) 49.5(18.0) 46.5(16.6) 50.5(19.5) 50.9(19.9) Baseline Total Lesion Counts Mean (SD) 81.7(21.3) 78.1(19.7) 81.5(21.9) 83.1(23.2) Baseline IGA Score, n (%) 3 – Moderate 255(83.1) 137(86.2) 296(88.9) 148(91.4) 4 – Severe 52(16.9) 22(13.8) 37(11.1) 14(8.6)

P<.01* P<.01* 12 *ANCOVA, Intent to Treat (ITT) Population, multiple imputation N=307 N=159 N=333 N=162 FMX101 Vehicle FMX101 Vehicle Acne Phase 3 Efficacy ResultsReduction of Inflammatory Lesion Count at Week 12 In Study 04, absolute change in inflammatory lesion count for the FMX101, 4% treatment group was -14.16 versus -11.17 in vehicle (p=0.0071) In Study 05, absolute change in inflammatory lesion count for the FMX101, 4% treatment group was -13.46 versus -10.72 in vehicle (p=0.0058) In the Pooled Analysis, absolute change in inflammatory lesion count for the FMX101, 4% treatment group was -13.79 versus -10.94 in vehicle (p=0.0001) FMX101 Vehicle P<.01* N=640 N=321

P<.05* P<.05* P>0.21* 13 N=307 N=159 N=333 N=162 FMX101 Vehicle FMX101 Vehicle Acne Phase 3 Efficacy ResultsIGA Treatment Success at Week 12 [Score Clear (0) or Almost Clear (1)] In Study 04, IGA Treatment Success for FMX101, 4% treatment group was 8.09% versus 4.77% in vehicle (p=0.2178) In Study 05, IGA Treatment Success for FMX101, 4% treatment group was 14.67% versus 7.89% in vehicle (p=0.0423) In the Pooled Analysis, IGA Treatment Success for FMX101, 4% treatment group was 11.51% versus 6.34% in vehicle (p=0.0188) N=640 N=321 FMX101 Vehicle *Cochran–Mantel–Haenszel test stratified by investigational site, Intent to Treat (ITT) population, multiple imputation

P<.05* 14 N=307 N=159 N=333 N=162 FMX101 Vehicle FMX101 Vehicle Acne Phase 3 Secondary Efficacy EndpointReduction of Non-Inflammatory Lesion Count at Week 12 In Study 04, absolute change in non-inflammatory lesion count for the FMX101, 4% treatment group was -16.45 versus -10.30 in vehicle (p=0.0042) In Study 05, absolute change in non-inflammatory lesion count for the FMX101, 4% treatment group was -13.20 versus -7.00 in vehicle (p=0.0320) In the Pooled Analysis, absolute change in inflammatory lesion count for the FMX101, 4% treatment group was -14.76 versus -8.64 in vehicle (p=0.0011) FMX101 Vehicle P<.01* N=640 N=321 *ANCOVA, Intent to Treat (ITT) Population, multiple imputation P<.01*

Acne Phase 3 Secondary Efficacy Endpoint% Change in Inflammatory Lesion Count at Weeks 3, 6, 9 and 12^ 15 Weeks % Reduction of IL Weeks % Reduction of IL ^ANCOVA, Intent to Treat (ITT) Population, multiple imputation In Study 04, percent change in inflammatory lesion count for the FMX101, 4% treatment group at week 12 was -44% versus -34% in vehicle (p=0.0033) In Study 05, percent change in inflammatory lesion count for the FMX101, 4% treatment group at week 12 was -43% versus -34% in vehicle (p=0.0097) Statistical significance demonstrated at all timepoints (beginning at Week 3) for both Study 04 & 05 −44% −34% ‡P≤.01; †P ≤.001; *P ≤.0001 * † ‡ † −43% −34% ‡ * † Study 04 - IL Count % Change Study 05 - IL Count % Change *

FMX-101 Phase 3 Long-Term Safety Results 16 Subject Demographics & Disposition - Open Label Phase Total number of subjects (04 | 05): 284 | 373 Mean age (04 | 05): 20.3 | 19.8 Male/Female (04 | 05): M=44.4% F=55.6% | M=44.8% F=55.2% Ethnicity (04 | 05): W=69.0% B=19.4% O=11.6% | W=76.4% B=19.3% O=4.3% Study 04 Study 05 Subject Disposition FMX-1011 Vehicle1 Overall FMX-1011 Vehicle1 Overall Subjects entering Open-Label 193 91 284 256 117 373 Subjects in Safety Population, n (%) 193 (100.0) 91 (100.0) 284 (100.0) 256 (100.0) 117 (100.0) 373 (100.0) Subjects completing 52 weeks of therapy, n (%)2 122 (63.2) 50 (54.9) 172 (60.6) 169 (66.0) 73 (62.4) 242 (64.9) Number of Subjects discontinuing Open-Label (%) 71 (36.8) 41 (45.1) 112 (39.4) 87 (34.0) 44 (37.6) 131 (35.1) Reason for Discontinuation, n (%) Adverse event (including clinically significant lab results) 1 (0.5) 1 (1.1) 2 (0.7) 2 (0.8) 3 (2.6) 5 (1.3) Lost to follow-up 23 (11.9) 9 (9.9) 32 (11.3) 18 (7.0) 9 (7.7) 27 (7.2) Subject request 24 (12.4) 13 (14.3) 37 (13.0) 33 (12.9) 18 (15.4) 51 (13.7) Poor protocol adherence 0 (0) 1 (1.1) 1 (0.4) 3 (1.2) 0 (0) 3 (0.8) Administrative3 21 (10.9) 12 (13.2) 33 (11.6) 26 (10.2) 12 (10.3) 38 (10.2) Other 2 (1.0) 5 (5.5) 7 (2.5) 5 (2.0) 2 (1.7) 7 (1.9) 1. Treatment received from preceding double-blinded phase.2. Subjects are identified as completing open-label if they enrolled in the open-label and did not discontinue.3. Study closed early due to high roll-over into open-label study and lower than anticipated discontinuation rate. 291 subjects completed 52 weeks of FMX-101 therapy
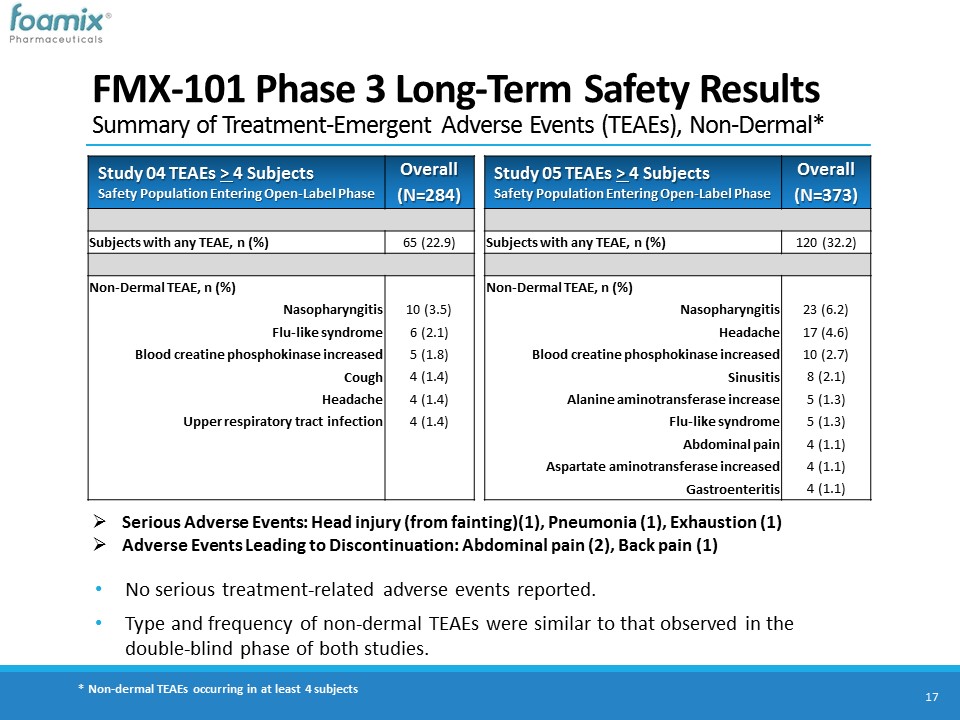
FMX-101 Phase 3 Long-Term Safety ResultsSummary of Treatment-Emergent Adverse Events (TEAEs), Non-Dermal* 17 No serious treatment-related adverse events reported. Type and frequency of non-dermal TEAEs were similar to that observed in the double-blind phase of both studies. Study 04 TEAEs > 4 SubjectsSafety Population Entering Open-Label Phase Overall (N=284) Subjects with any TEAE, n (%) 65 (22.9) Non-Dermal TEAE, n (%) Nasopharyngitis 10 (3.5) Flu-like syndrome 6 (2.1) Blood creatine phosphokinase increased 5 (1.8) Cough 4 (1.4) Headache 4 (1.4) Upper respiratory tract infection 4 (1.4) Serious Adverse Events: Head injury (from fainting)(1), Pneumonia (1), Exhaustion (1)Adverse Events Leading to Discontinuation: Abdominal pain (2), Back pain (1) * Non-dermal TEAEs occurring in at least 4 subjects Study 05 TEAEs > 4 SubjectsSafety Population Entering Open-Label Phase Overall (N=373) Subjects with any TEAE, n (%) 120 (32.2) Non-Dermal TEAE, n (%) Nasopharyngitis 23 (6.2) Headache 17 (4.6) Blood creatine phosphokinase increased 10 (2.7) Sinusitis 8 (2.1) Alanine aminotransferase increase 5 (1.3) Flu-like syndrome 5 (1.3) Abdominal pain 4 (1.1) Aspartate aminotransferase increased 4 (1.1) Gastroenteritis 4 (1.1)

FMX-101 Phase 3 Long-Term Safety ResultsSummary of Treatment-Emergent Adverse Events (TEAEs), Application Site 18 Type and frequency of application site TEAEs were similar to that observed in double-blinded phase Study 04 TEAEs >1 % FrequencySafety Population Entering Open-Label Phase Overall (N=284) Application Site TEAEs, n (%)† Application site acne 3 (1.1) Study 05 TEAEs >1 % FrequencySafety Population Entering Open-Label Phase Overall (N=373) Application Site TEAEs, n (%)† None 0 (0.0) Adverse Events Leading to Discontinuation: Worsening of acne (2), Contact dermatitis (1), Localized facial edema (1) †Application Site TEAEs (ALL): Cyst (1), Rash (1), Dermatitis (1), Discoloration (1), Hypersensitivity (1), Injury (1), Edema (1)

FMX-101 Phase 3 Long-Term Safety Results 19 Facial Local Tolerability Assessments at Week 52, Scale 0 (none) to 3 (severe) Facial Local Tolerability Assessments, FMX-101 Population at Week 52 Study 04FMX-101, n (%)n=114 Study 05FMX-101, n (%)n=148 Assessed Severity 0=None 1=Mild 2=Moderate 0=None 1=Mild 2=Moderate Erythema 109 (95.6) 5 (4.4) 0 130 (87.8) 17 (11.5) 1 (0.7) Dryness 104 (91.2) 10 (8.8) 0 144 (97.3) 4 (2.7) 0 Hyperpigmentation† 109 (95.6) 4 (3.5) 1 (0.9) 133 (89.9) 13 (8.8) 2 (1.3) Skin Peeling 110 (96.5) 4 (3.5) 0 147 (99.3) 1 (0.7) 0 Itching 113 (99.1) 1 (0.9) 0 147 (99.3) 1 (0.7) 0 * Based on observed cases† Hyperpigmentation most commonly used to describe localized post-inflammatory darkening of the affected skin >95%* of signs and symptoms were classified as “none” or “mild” Degree and frequency of facial local tolerability scores were similar to that observed in double-blinded phase where >95% of signs and symptoms were classified as “none” or “mild”No severe (3) local tolerability scores were recorded

FMX-101 Phase 3 Patient Satisfaction Questionnaire at Week 52, FMX-101 Population Entering Open-Label (n=251)* 20 Scale 1- Very Satisfied 2- Satisfied 3- Somewhat Satisfied 4- Dissatisfied 5- Very Dissatisfied 85% of patients satisfied or very satisfied with ease of use of FMX-101 68% of patients satisfied or very satisfied with the product feel of FMX-101 85% of subjects satisfied or very satisfied with FMX-101 Q3. How satisfied are you with how this product feels on your skin after treatment? Q2. How satisfied are you with how easy this product is to use? * Based on observed cases of subjects who completed 52 weeks of FMX-101 therapy Q1. Overall, how satisfied are you with this product?
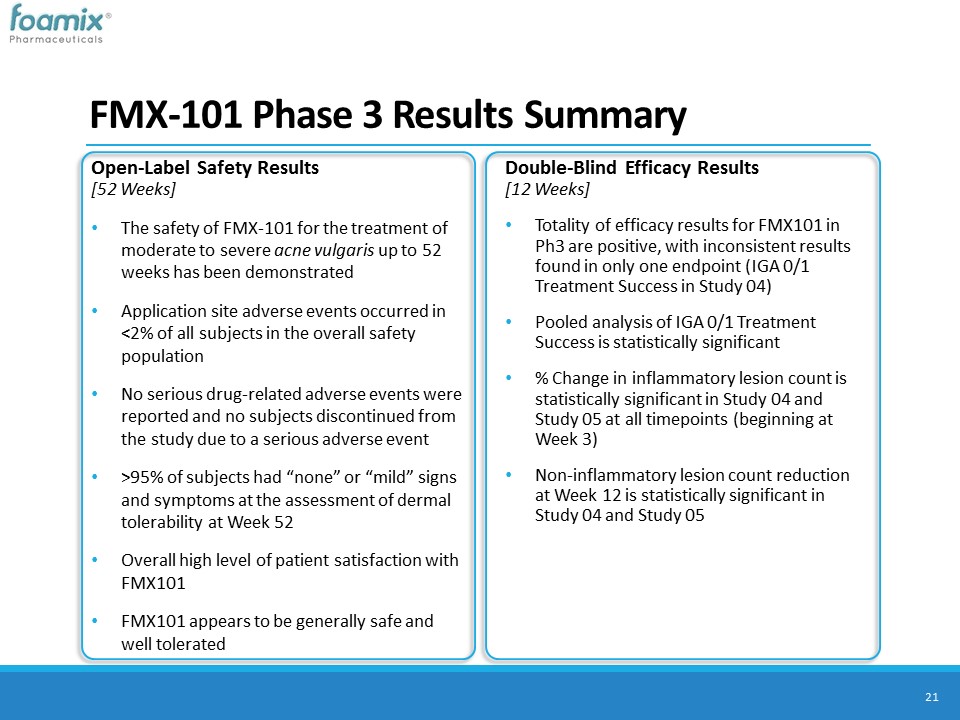
FMX-101 Phase 3 Results Summary 21 Double-Blind Efficacy Results[12 Weeks]Totality of efficacy results for FMX101 in Ph3 are positive, with inconsistent results found in only one endpoint (IGA 0/1 Treatment Success in Study 04) Pooled analysis of IGA 0/1 Treatment Success is statistically significant % Change in inflammatory lesion count is statistically significant in Study 04 and Study 05 at all timepoints (beginning at Week 3)Non-inflammatory lesion count reduction at Week 12 is statistically significant in Study 04 and Study 05 Open-Label Safety Results [52 Weeks]The safety of FMX-101 for the treatment of moderate to severe acne vulgaris up to 52 weeks has been demonstrated Application site adverse events occurred in <2% of all subjects in the overall safety population No serious drug-related adverse events were reported and no subjects discontinued from the study due to a serious adverse event>95% of subjects had “none” or “mild” signs and symptoms at the assessment of dermal tolerability at Week 52Overall high level of patient satisfaction with FMX101 FMX101 appears to be generally safe and well tolerated

FMX101 3rd Phase 3 Study DesignStudy FX2017-22 22 12-week, randomized, double-blind, vehicle controlled, in subjects with moderate-to-severe acne Week 12(End of treatment) Week 3 Week 6 Week 9 Double-blinded Study (-22)Randomized (1:1), double-blindN=1,500 Topline results expected Mid-2018 Minocycline Foam 4%Foam vehicle Week 3 1 US Study, 1,500 subjects, ~80 sites, >9 years of ageSelf-apply, once daily, for 12 weeksInclusion CriteriaAt least 20 inflammatory and 25 non-inflammatory lesionsIGA 6 point scale – Moderate to Severe (Grade 3 or 4)Co-primary Efficacy Endpoints:Mean change from baseline in inflammatory lesion countProportion of subjects with IGA scores of “Clear” or “Almost Clear”, with improvement of at least 2 grades from baselineSafety Evaluations: AEs, physical exams, vitals, dermal tolerability, erythema assessments, labs

FMX103 Topical Minocycline Foam For Moderate-to-Severe Rosacea 23

Rosacea Chronic acneiform disorder affecting both the skin and the eyeAffects ~ 16 million adults in the US1Typical age of onset for rosacea – 30-60More common in Caucasian populationImpact on Quality of Life2“Devastating impact on emotional well being”Low self esteem Affects professional interactions2 primary subtypes1Erythemato-telangiectatic – facial flushing and rednessPapulopustular – acne-like papules and pustules (inflammatory lesions) 24 (1) National Rosacea Society. Rosacea Review; Winter 2010. http://www.rosacea.org/rr/2010/winter/article_1.php. Accessed May 16, 2016; (2) Wilkin J, et al. J Am Acad Dermatol. 2002;46:584-587. Pathogenesis and Treatment of Acne and Rosacea, Zouboulis et al., Eds, 2014, p 743-747 Data on file – Foamix Ltd. Study FX2015-10.

25 Market Leaders Rx Volume: ~3mm TRx(~2/3rds of Total Market - $$ & TRx) Top Brands $ USD TRxs 1 METROGEL/METRONIDAZOLE All forms, Galderma & Generics $255,414,358 1,350,300 2 ORACEA2Doxycyline, Galderma $219,689,492 338,160 3 FINACEA2Azelaic Acid, Bayer $148,738,638 520,061 4 SOOLANTRAIvermectin, Galderma $102,465,519 334,932 5 MINOCYCLINE (oral)Valeant & Generics $43,585,959 439,525 6 MIRVASO2Brimonidine, Galderma $40,517,131 99,378 Total Market $ Potential: ~$1.1 billion(Approximately 16 million people) Rosacea – the US Market (2016)Undifferentiated Market with Limited Competition Current Market (United States)1 US Dollars TRxs Oral antibiotics $270 million 1.1 mm Topical drugs $830 million 3.6 mm Total $1.1 billion 4.7 mm Symphony Health Services PHAST: 2016 Market Data (accessed 1.24.17), weighted values, rosacea usageSelect brands, unweighted values (>75% rosacea usage)

Phase 2 Clinical Study Design 26 12-week, randomized, double-blind, dose range-finding study in subjects with moderate-to-severe papulopustular rosacea Week 12(End of treatment) Week 16 Week: 6 10 Randomized (1:1:1), double-blindN=233 Inclusion criteriaAt least 12 papules and/or pustulesInvestigator’s Global Assessment (IGA): Moderate-to-SevereEfficacy endpointsAbsolute change in inflammatory lesion count at Week 12 compared to Baseline Investigator’s Global Assessment – IGA Proportion of subjects with IGA improvement of ≥2 gradesProportion of subjects with IGA scores of “Clear” / “Almost Clear”Safety & tolerability Minocycline foam 1.5%Minocycline foam 3%Foam Vehicle 1 2 4 8 0 Follow Up Once daily, in the evening, for 12 weeks

27 ANCOVA; multiple imputation method‡P<.05; †P<.01; *P<.001 IGA Scale: 0=Clear; 1=Almost Clear; 2=Mild; 3=Moderate; 4=SevereITT Population; Cochran–Mantel–Haenszel test; multiple imputation method FMX103 – Phase 2 Efficacy Results Clinically and statistically significant lesion reduction and IGA score % Reduction of Papules & Pustules IGA = “Clear” or “Almost Clear” (Score of 0-1) and Improvement ≥2 Grades % Subjects with IGA = 0-1 XC P=.001 XC P<.05 25.3% 17.3% 7.7% −61.4% −55.5% −29.7% * * † Weeks * * % Reduction of Papules & Pustules * * No statistically significant difference between 1.5% and 3% dosesBased on these results, FMX103 1.5% has been selected for further development

28 Rosacea Phase 2 Efficacy Results Visible Effects on Moderate-to-Severe Rosacea Subjects Data on file – Foamix Ltd. Study FX2015-10. Baseline Visit Week-12 Visit

Rosacea Phase 2 Safety Results 29 Drug-related skin reactions (AEs):A total of 4 subjects discontinued the study due to an adverse event (3 in the 3% group and 1 in the vehicle group)No SAEs or drug-related systemic adverse events were reported FMX103 – Generally Safe and Well Tolerated No. Subjects FMX103 1.5% FMX103 3% Vehicle Eczema of face 0 1 1 Skin exfoliation/scaling 0 1 0 Erythema 0 0 1 Pruritus 0 0 1 Scab/Crust in treatment area 0 0 1 Skin burning 0 0 1 Worsening of Rosacea 0 2 0

FMX103 Phase 3 Study Design Studies FX2016-11, FX2016-12 & FX2016-13 30 12-week, randomized, double-blind, vehicle controlled, in subjects with moderate-to-severe papulopustular rosacea; followed by 9 month open label safety extension Week 12 (End of treatment) 12 Months Week 2 Week 4 Week 8 Double-blinded Study (-11,-12)Randomized (2:1), double-blindN=750 (X2) Minocycline Foam 1.5%Foam vehicle Minocycline foam 1.5% – 9 months of treatment Open Label Safety Extension Study (-13) Subjects who complete one of the randomized, Phase 3 studies may enter the open-label study 2 US Double-blinded Studies, ~80 sites, 750 subjects per study (N=1,500 subjects), >18 years of ageSelf-apply, once daily, for 12 weeksInclusion Criteria15 to 75 inflammatory lesions IGA 5 point scale – Moderate to Severe (Grade 3 or 4)Co-primary Efficacy Endpoints:Mean change from baseline in inflammatory lesion countProportion of subjects with IGA scores of “Clear” or “Almost Clear”, with improvement of at least 2 grades from baselineSafety Evaluations: AEs, physical exams, vitals, dermal tolerability, erythema assessments, labs Topline results expected Mid-2018

Planned Milestones 31 TLR (Studies 04 & 05) Mar 27 3rd Ph3 DB (Study 22) Ph3 LTS 3rd Ph3 TLR NDA PDUFA Ph3 DB (Studies 11 & 12) Ph3 TLR (DB) Ph3 LTS NDA PDUFA 2017 2020 H12017 H2 H12018 H2 H12019 H2 H12020 H2 FMX101, 4% (Acne) FMX103, 1.5% (Rosacea) TLR = Topline Results*Actual timelines may vary

Collaborations Development and licensing agreements with pharmaceutical companiesEach license agreement is product specific (Licensee’s drug) Licensed products are currently in preclinical, Phase 2, Phase 3 and commercial stagesFoamix owns the IP for the drug delivery platformFoamix retains the rights to develop products for the same indications using our foam technology in conjunction with other drugs 32 Upfront payments, contingent payments and royalties on sales of products that are commercializedTotal of $27.1 million cumulative revenues as of September 30, 2017Recurring royalties since Q4, 2015 Revenues

Strong Financial Position 33 Net proceeds: US $42.3 millionPrice per share: US $6.00 IPO – September 2014 Net proceeds: US $64.2 millionPrice per share: US $9.30 Follow-on offering, April 2015 Cash, cash equivalents and investments: US $89.4 millionNet cash used in operating activities YTD US $41.6 millionExisting cash provides sufficient financial runway to finance our clinical and business operations, into 2019 Cash position as of September 30, 2017 Net proceeds: US $54.1 millionPrice per share: US $9.50 Follow-on offering, September 2016

Nasdaq: FOMXJanuary 2018
