Attached files
| file | filename |
|---|---|
| 8-K - 8-K - IRIDEX CORP | irix-8k_20171108.htm |

Investor Presentation November 2017 Exhibit 99.1

Forward Looking Statements Participation in this presentation requires that you be aware of the Federal legislation regarding forward-looking statements. Accordingly, during the course of this presentation we may make forward-looking statements regarding future events or the future performance of the Company. We caution you that such statements are just predictions that involve risks and uncertainties, and that actual events or results could differ materially. We discuss a number of the risks in our business in detail in the Company’s SEC reports, including our latest Form 10-K and our latest Form 10-Q.

Investment Highlights 80M people with Glaucoma; 25M diagnosed Estimated 100M people with diabetes-related eye disease MicroPulse® Patented and Trademarked Significant data supports clinical and economic benefit Entering rapidly growing Glaucoma market Expanding focus on captive disposables business model Leverage and stability for future expansion Strong global brand built over 30 years Underserved and Growing Patient Population Differentiated Laser Technology Recent Transformational Shift in Business Model Established Revenue Base and Worldwide Customers Source: Market Scope estimate as of 2015
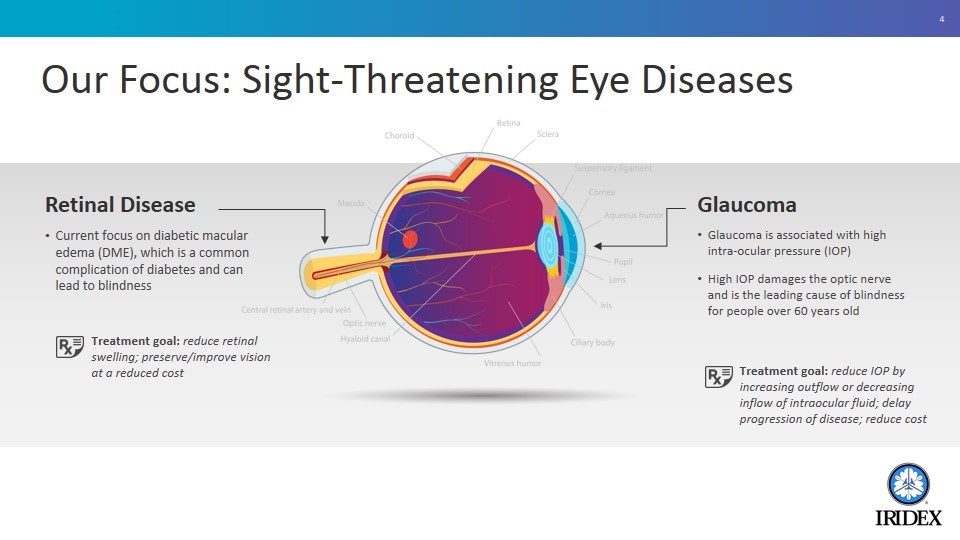
Our Focus: Sight-Threatening Eye Diseases Retinal Disease Current focus on diabetic macular edema (DME), which is a common complication of diabetes and can lead to blindness Glaucoma Glaucoma is associated with high intra-ocular pressure (IOP) High IOP damages the optic nerve and is the leading cause of blindness for people over 60 years old Treatment goal: reduce IOP by increasing outflow or decreasing inflow of intraocular fluid; delay progression of disease; reduce cost Treatment goal: reduce retinal swelling; preserve/improve vision at a reduced cost

Shortcomings in the Treatment of Glaucoma and Retinal Disease IRIDEX’s MicroPulse® Laser Therapy delivers a differentiated laser treatment that provides safe, effective, and proven treatment for targeted sight-threatening eye disease PHARMACEUTICALS Requires continuous lifetime treatment High cost Significant compliance issue Side effects LEGACY LASERS Lack durable outcomes Not repeatable Side effects INVASIVE SURGERY High complication rate High failure rate Limited in scope Irreversible
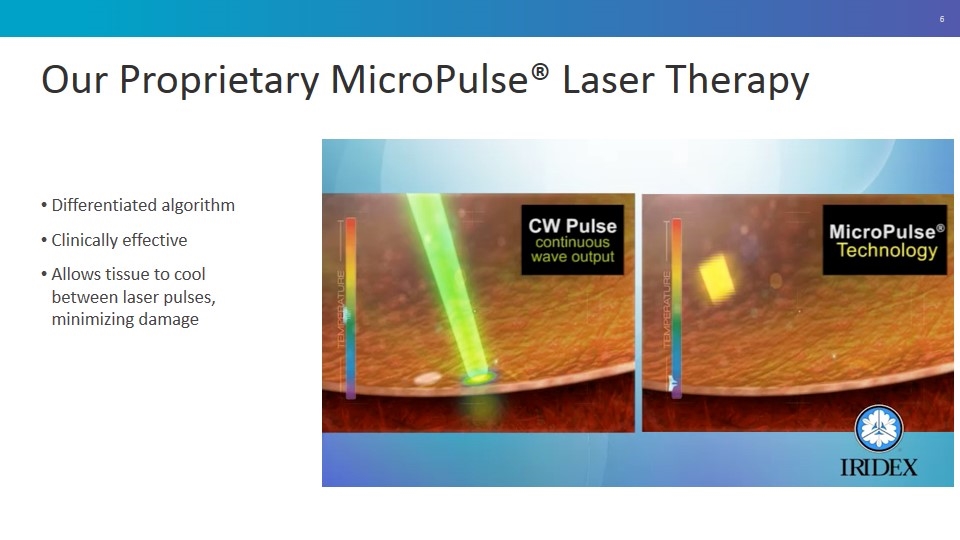
Our Proprietary MicroPulse® Laser Therapy Differentiated algorithm Clinically effective Allows tissue to cool between laser pulses, minimizing damage

Glaucoma Business Overview
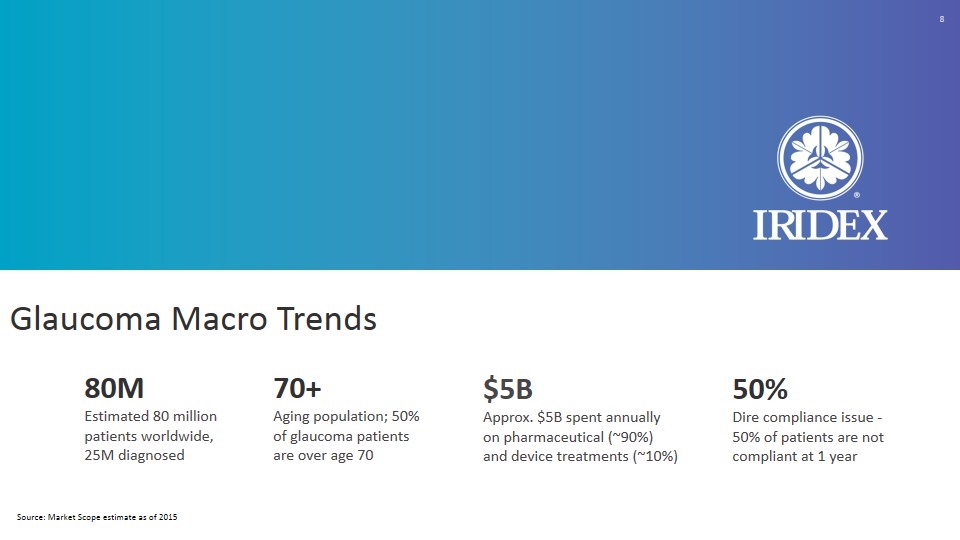
Glaucoma Macro Trends 80M Estimated 80 million patients worldwide, 25M diagnosed 70+ Aging population; 50% of glaucoma patients are over age 70 $5B Approx. $5B spent annually on pharmaceutical (~90%) and device treatments (~10%) 50% Dire compliance issue - 50% of patients are not compliant at 1 year Source: Market Scope estimate as of 2015

Shortcomings of Alternate Glaucoma Treatment Options Prescription Eye Drops High non-compliance rates Complex dosing regimens Adverse side effects High, recurring costs Legacy Lasers – SLT, ALT, MLT Effects dissipate over time Repeat procedures less effective MIGS devices Limited to use in cataract surgery Incisional procedure with implant left behind Long term efficacy unclear Traditional Surgery High complication rate High failure rate Limited long-term efficacy Significant post-operation patient management
Cyclo G6™ Addresses Significant Unmet Need Safe Non-incisional Easy to perform Titratable Repeatable Durable Treats full range of disease Cost effective
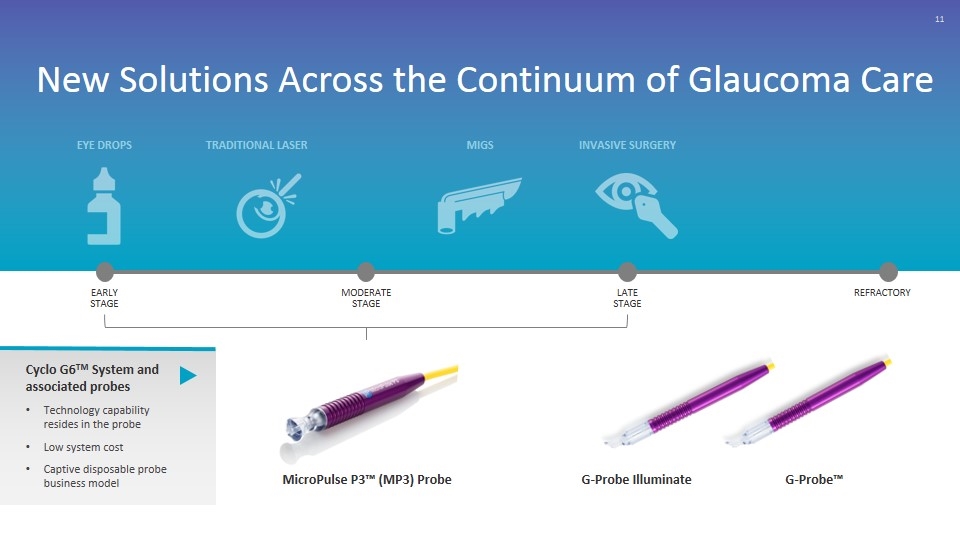
New Solutions Across the Continuum of Glaucoma Care EARLY STAGE MODERATE STAGE LATE STAGE REFRACTORY EYE DROPS TRADITIONAL LASER MIGS INVASIVE SURGERY Cyclo G6TM System and associated probes Technology capability resides in the probe Low system cost Captive disposable probe business model MicroPulse P3™ (MP3) Probe G-Probe Illuminate G-Probe™
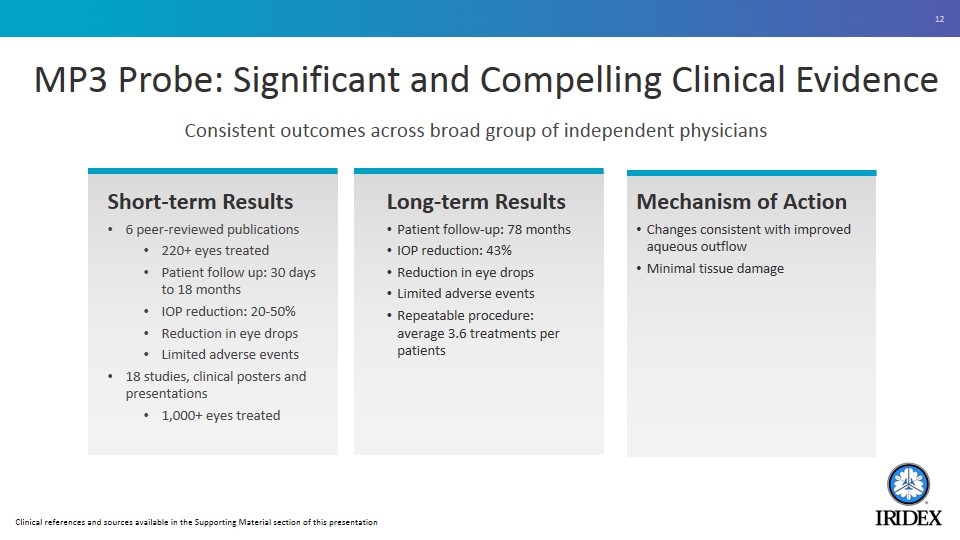
MP3 Probe: Significant and Compelling Clinical Evidence Consistent outcomes across broad group of independent physicians Short-term Results 6 peer-reviewed publications 220+ eyes treated Patient follow up: 30 days to 18 months IOP reduction: 20-50% Reduction in eye drops Limited adverse events 18 studies, clinical posters and presentations 1,000+ eyes treated Long-term Results Patient follow-up: 78 months IOP reduction: 43% Reduction in eye drops Limited adverse events Repeatable procedure: average 3.6 treatments per patients Mechanism of Action Changes consistent with improved aqueous outflow Minimal tissue damage Clinical references and sources available in the Supporting Material section of this presentation

Growing Leadership and Validation in the Field Reimbursement with permanent U.S. CPT code Growing list of relationships with prestigious institutions Increasing customers purchasing multiple systems Expanding probe utilization Increasing KOL support around the world
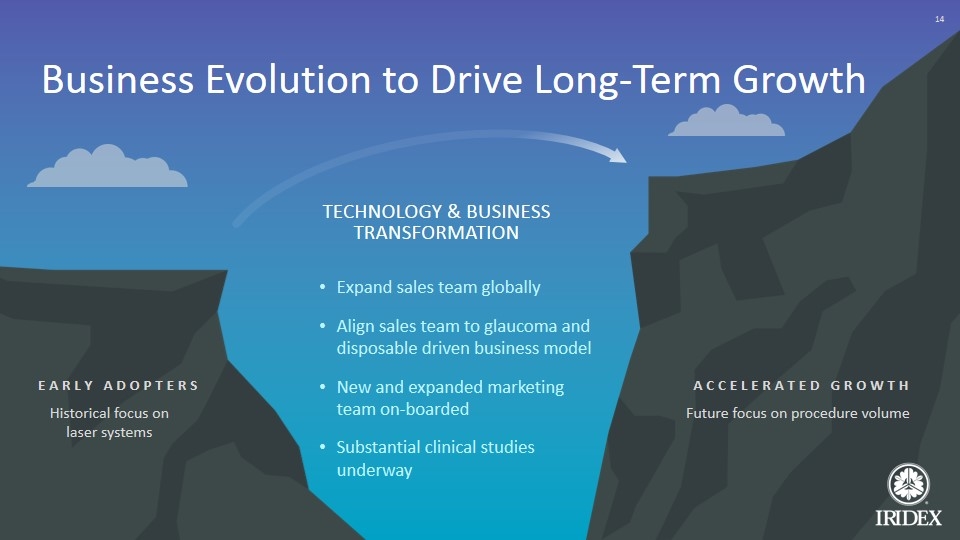
Expand sales team globally Align sales team to glaucoma and disposable driven business model New and expanded marketing team on-boarded Substantial clinical studies underway TECHNOLOGY & BUSINESS TRANSFORMATION EARLY ADOPTERS ACCELERATED GROWTH Business Evolution to Drive Long-Term Growth Historical focus on laser systems Future focus on procedure volume
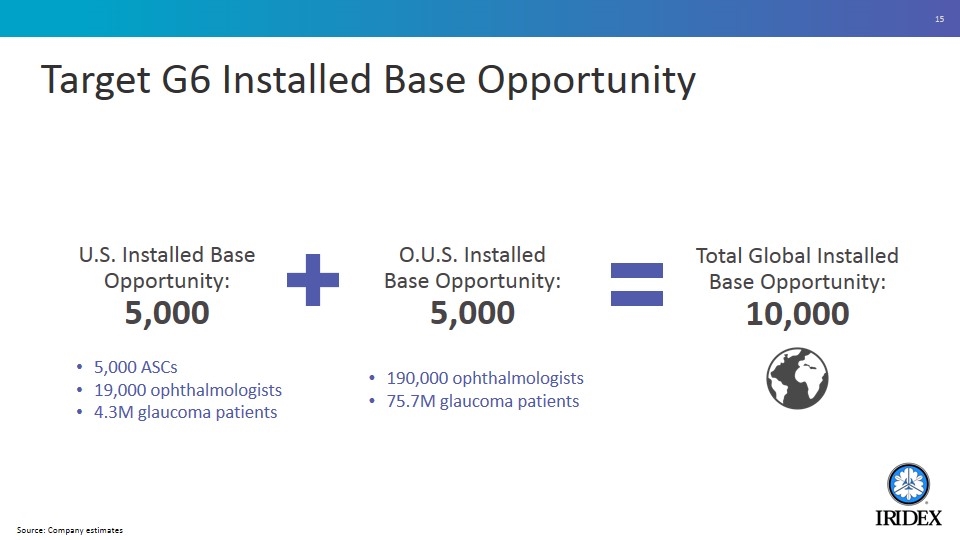
Target G6 Installed Base Opportunity 5,000 ASCs 19,000 ophthalmologists 4.3M glaucoma patients Total Global Installed Base Opportunity: 10,000 190,000 ophthalmologists 75.7M glaucoma patients O.U.S. Installed Base Opportunity: 5,000 U.S. Installed Base Opportunity: 5,000 Source: Company estimates
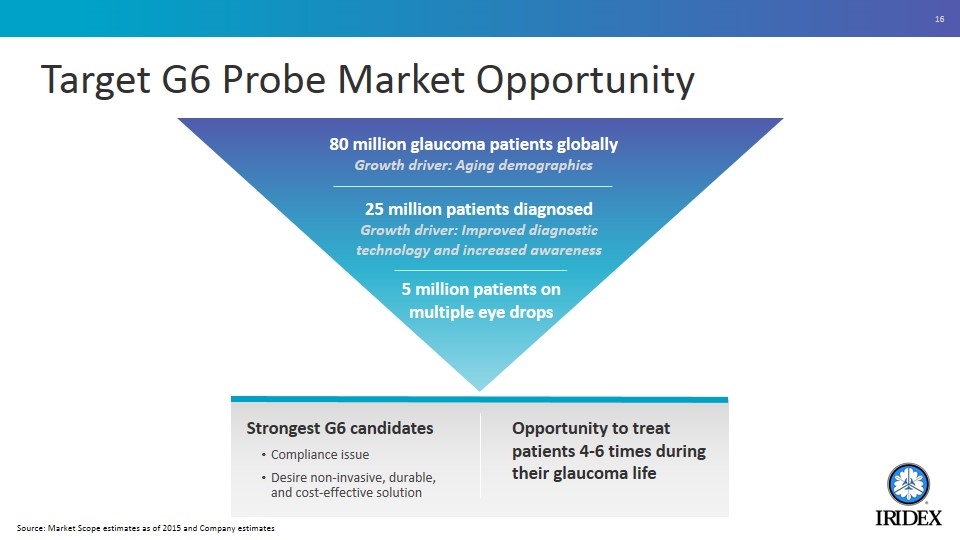
Target G6 Probe Market Opportunity Strongest G6 candidates Compliance issue Desire non-invasive, durable, and cost-effective solution 80 million glaucoma patients globally Growth driver: Aging demographics 25 million patients diagnosed Growth driver: Improved diagnostic technology and increased awareness 5 million patients on multiple eye drops Opportunity to treat patients 4-6 times during their glaucoma life Source: Market Scope estimates as of 2015 and Company estimates
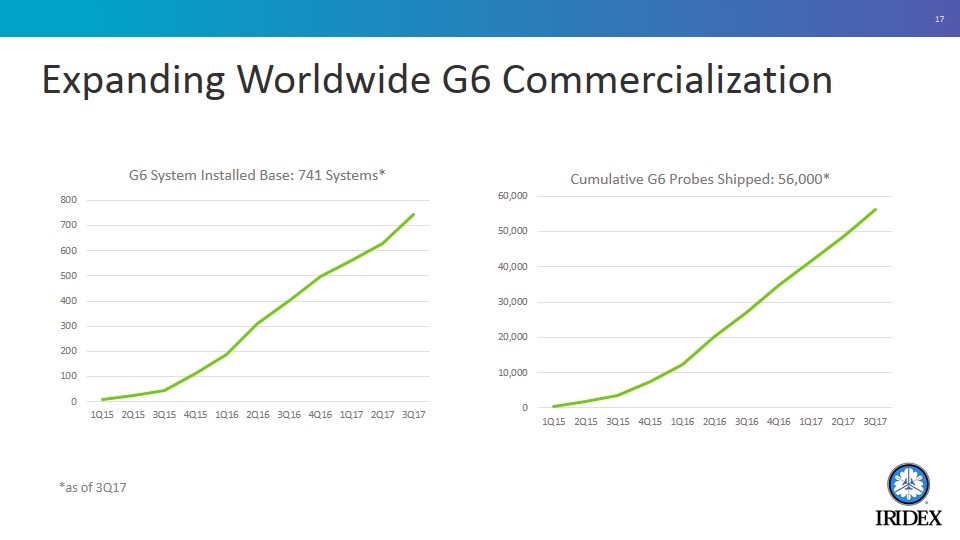
Expanding Worldwide G6 Commercialization *as of 3Q17

Retinal Disease Business Overview
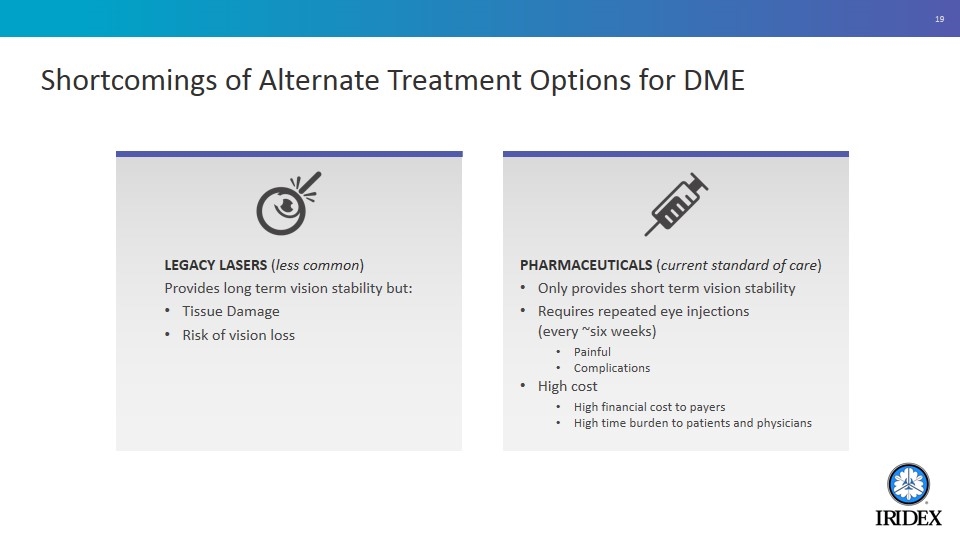
Shortcomings of Alternate Treatment Options for DME PHARMACEUTICALS (current standard of care) Only provides short term vision stability Requires repeated eye injections (every ~six weeks) Painful Complications High cost High financial cost to payers High time burden to patients and physicians LEGACY LASERS (less common) Provides long term vision stability but: Tissue Damage Risk of vision loss
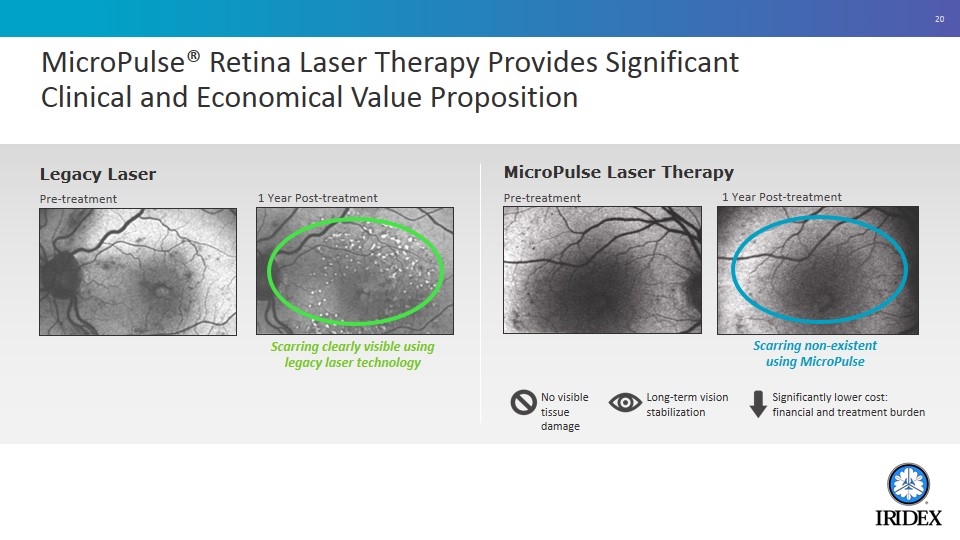
MicroPulse® Retina Laser Therapy Provides Significant Clinical and Economical Value Proposition Scarring clearly visible using legacy laser technology Scarring non-existent using MicroPulse Legacy Laser Pre-treatment 1 Year Post-treatment MicroPulse Laser Therapy Pre-treatment 1 Year Post-treatment No visible tissue damage Long-term vision stabilization Significantly lower cost: financial and treatment burden
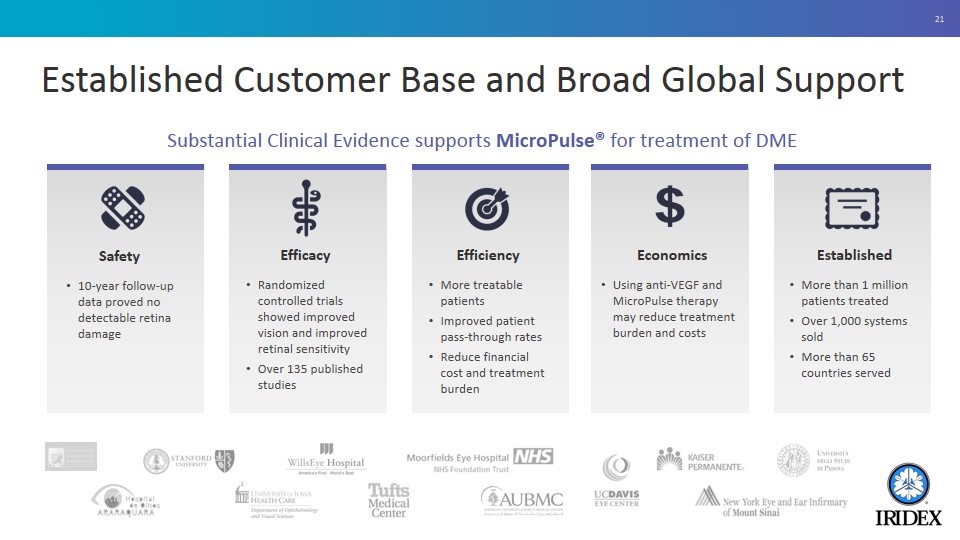
Established Customer Base and Broad Global Support Substantial Clinical Evidence supports MicroPulse® for treatment of DME Safety 10-year follow-up data proved no detectable retina damage Efficacy Randomized controlled trials showed improved vision and improved retinal sensitivity Over 135 published studies Efficiency More treatable patients Improved patient pass-through rates Reduce financial cost and treatment burden Economics Using anti-VEGF and MicroPulse therapy may reduce treatment burden and costs Established More than 1 million patients treated Over 1,000 systems sold More than 65 countries served
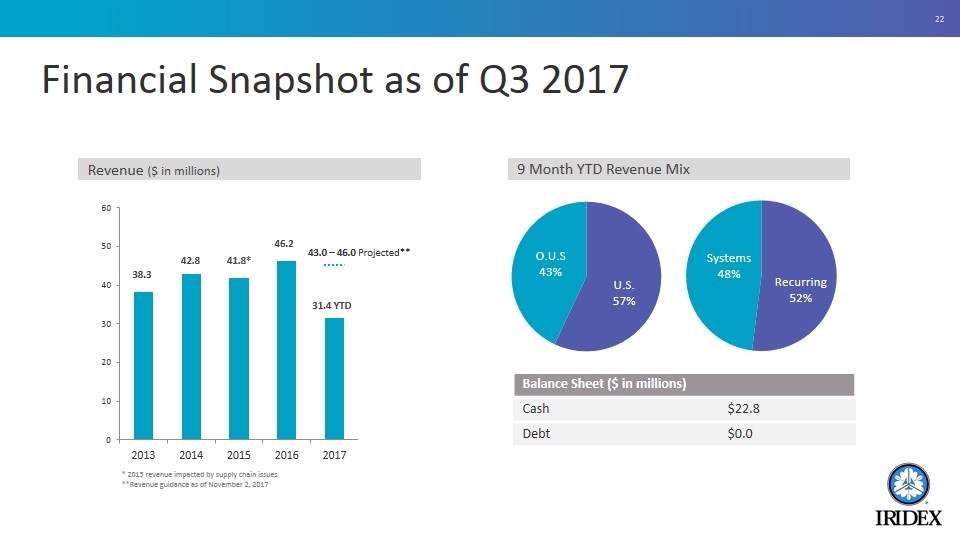
Financial Snapshot as of Q3 2017 * 2015 revenue impacted by supply chain issues **Revenue guidance as of November 2, 2017 Revenue ($ in millions) 9 Month YTD Revenue Mix Balance Sheet ($ in millions) Cash $22.8 Debt $0.0 43.0 – 46.0 Projected**

Long-Term Financial Goals $100+M in revenues 65% recurring, 35% systems 55+% gross margin 15+% operating margin

NEAR AND MID-TERM GOALS Expand US and International presence Improve design and processes to increase outsource production capacity Product enhancements to introduce new features and benefits Focused Execution to Achieve Goals Lower cost and improved reliability laser technologies New delivery modalities MicroPulse® technology for other eye diseases FUTURE PROGRAMS IN DEVELOPMENT

Thank You

Supporting Material
Video Resources Video 1: Summary of MicroPulse: Dr. Myers at AAO 2017 Video 2: Cyclo G6 Laser System Technology Video 3: Transscleral Cyclophotocoagulation with Cyclo G6 Video 4: Interview with Dr. Jacky Lee at ESCRS 2015 Video 5: Media Release on New Treatments for Glaucoma (Chinese with English subtitles) Video 6: News Report Dr. Harris on IQ577 for MLT and MP3
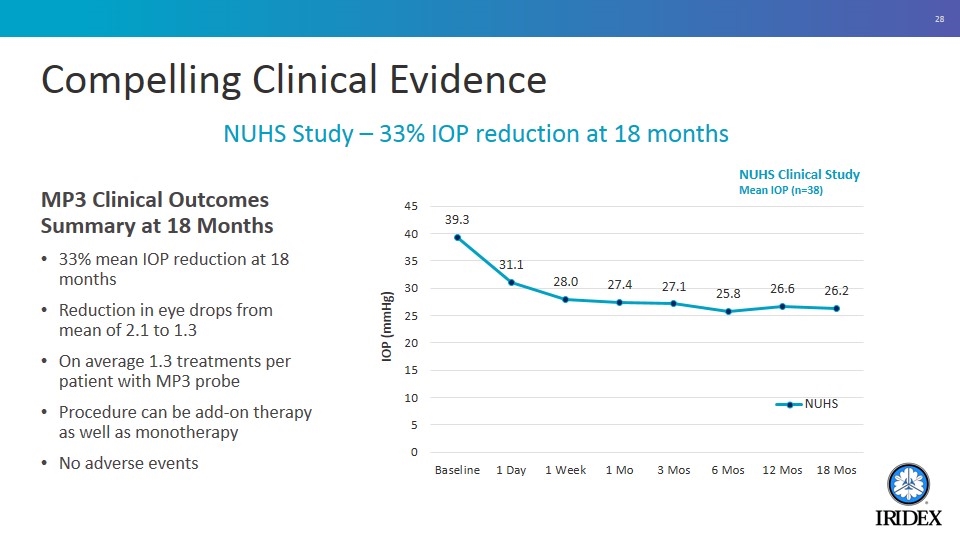
Compelling Clinical Evidence MP3 Clinical Outcomes Summary at 18 Months 33% mean IOP reduction at 18 months Reduction in eye drops from mean of 2.1 to 1.3 On average 1.3 treatments per patient with MP3 probe Procedure can be add-on therapy as well as monotherapy No adverse events NUHS Study – 33% IOP reduction at 18 months NUHS Clinical Study Mean IOP (n=38)
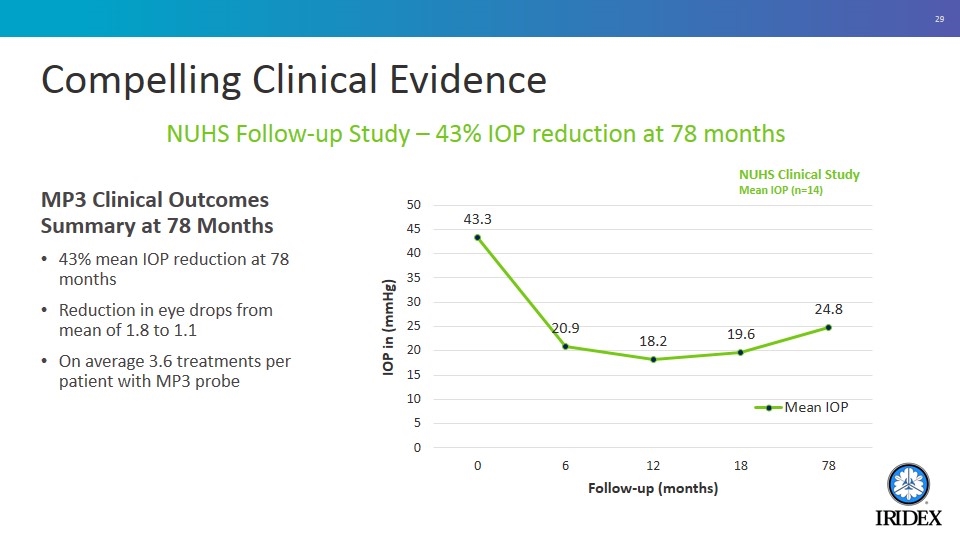
Compelling Clinical Evidence MP3 Clinical Outcomes Summary at 78 Months 43% mean IOP reduction at 78 months Reduction in eye drops from mean of 1.8 to 1.1 On average 3.6 treatments per patient with MP3 probe NUHS Follow-up Study – 43% IOP reduction at 78 months NUHS Clinical Study Mean IOP (n=14)
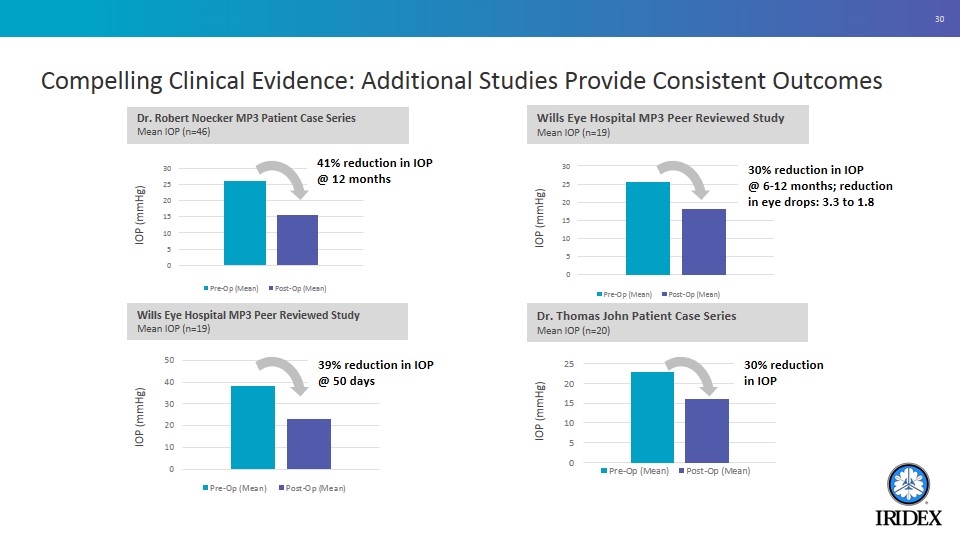
Compelling Clinical Evidence: Additional Studies Provide Consistent Outcomes Dr. Thomas John Patient Case Series Mean IOP (n=20) 30% reduction in IOP IOP (mmHg) Wills Eye Hospital MP3 Peer Reviewed Study Mean IOP (n=19) 30% reduction in IOP @ 6-12 months; reduction in eye drops: 3.3 to 1.8 IOP (mmHg) 41% reduction in IOP @ 12 months IOP (mmHg) Dr. Robert Noecker MP3 Patient Case Series Mean IOP (n=46) 39% reduction in IOP @ 50 days IOP (mmHg) Wills Eye Hospital MP3 Peer Reviewed Study Mean IOP (n=19)

Compelling Clinical Evidence: Additional Studies Provide Consistent Outcomes Drs. Thomas Samuelson and Mark Hansen, Minnesota Eye Consultants Reviewed 119 procedures and concluded that “MicroPulse is an effective, non-invasive treatment to lower IOP and is a viable option for all types of glaucoma at various stages.” Doheny Eye Center UCLA, Dr. Brian Francis 43% IOP reduction @ approximately 2 months follow-up (N=20) University of Missouri, Dr. Rohit Krishna 43% IOP reduction @ 6 months follow-up (N=30) Wills Eye Hospital, Dr. Marlene Moster 50% IOP reduction @ an average of 7.5 months follow-up (N=78) University of Washington, Dr. Murray Johnstone “Clinically used MicroPulse parameters induce outflow system configuration changes generally associated with improved aqueous flow.” UCSF, Dr. Shan Lin “No presence of suprachoroidal fluid or anatomical changes were found.” The study concluded, “MP-TCP is effective at lowering IOP in the majority of patients and appears safe without major complications.” Yale University study Study demonstrating minimal tissue disruption from MP3 Multi-Center Retrospective study 30% IOP reduction at three months Nisha Chadha, MD; Cataract & Refractive Surgery Today; September 2017 -New Laser Therapies For Glaucoma- “…MicroPulse technology offers a novel means of laser delivery that has been shown to be safe and effective…” Matthew E. Emanuel, MD Glaucoma Journal; Aug 2017 -MicroPulse Cyclophotocoagulation: Initial Results in Refractory Glaucoma- “…The out comes of our study are promising with good evidence of the IOP-lowering effects of MP-TSCPC and decreased need for ocular antihypertensive medications postlaser at 6 months”…” Shan Lin, MD UCSF -Micropulse transcleral diode laser cyclophotocoagulation: Mid to long term results- “MicroPulse TCP is effective in lowering IOP in the majority of patients in this study in a mid-long term follow up, and appears safe with no major complications”.
Clinical Data References Clin Experiment Ophthalmol 2010;38(3):266-72 EGS abstract, Prague, Czech Republic, June 19-22, 2016 ARVO Abstract, Baltimore, Maryland, May 7-11, 2016 Lasers Medical Science (2016)31:393-396, DOI 10.1007/s10103-015-1856-9 Journal of Clinical & Experimental Ophthalmology, December 2016 ASCRS Paper Presentation, Los Angeles, CA, May 2017 ARVO Abstract, Baltimore, MD, May 2017 AGS Abstract, Fort Lauderdale, FL, March 2016 AGS Abstract, San Diego, CA, February 2015

