Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Nabriva Therapeutics plc | a17-26050_18k.htm |
This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements about our future expectations, plans and prospects, including but not limited to statements about, our redomicile from Austria to Ireland, the development of our product candidates, such as plans for the design, conduct and timelines of Phase 3 clinical trials of lefamulin for CABP, the clinical utility of lefamulin for CABP and our plans for filing of regulatory approvals and efforts to bring lefamulin to market, the development of lefamulin for additional indications, the development of additional formulations of lefamulin, plans to pursue research and development of other product candidates, and other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties inherent in the initiation and conduct of clinical trials, availability and timing of data from clinical trials, whether results of early clinical trials or trials in different disease indications will be indicative of the results of ongoing or future trials, uncertainties associated with regulatory review of clinical trials and applications for marketing approvals, the availability or commercial potential of product candidates, the sufficiency of cash resources, whether research programs will result in product candidates that are advanced into future clinical trials, and need for additional financing and such other important factors as are set forth under the caption "Risk Factors" in the annual and quarterly reports we file with the United States Securities and Exchange Commission. In addition, the forward-looking statements included in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. Safe Harbor and Disclaimer

Expert team advancing pleuromutilins, a new class of antibiotics, for systemic (IV and oral) use in humans Lefamulin in two global, pivotal Phase 3 trials for moderate-to-severe CABP with topline data received in September 2017 and expected for LEAP 2 in the spring of 2018 Investing in pre-commercial activities to maximize US commercial potential and evaluating ex-US partner(s) Current cash resources expected to fund operations into Q4 2018. 2017- A Transformational Year for Nabriva Building a Fully Integrated Anti-Infectives Company
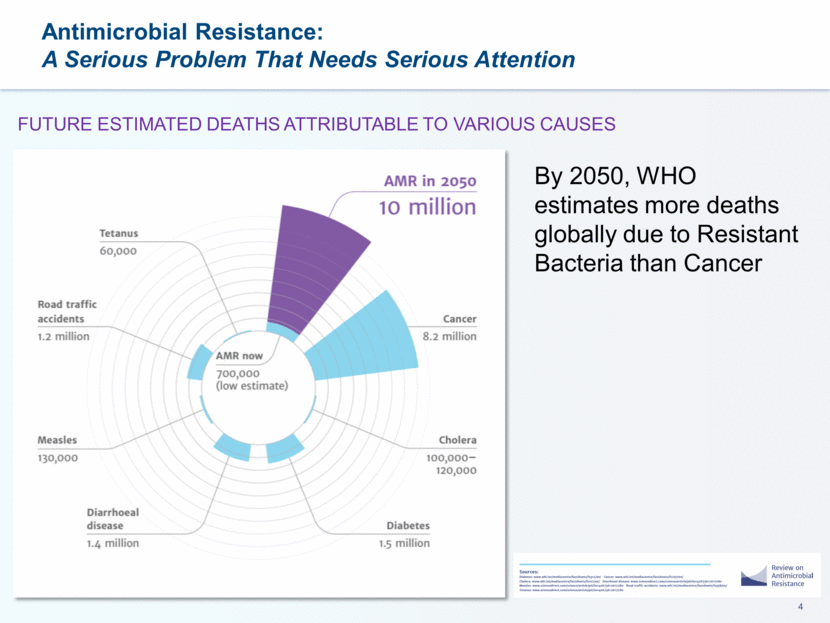
By 2050, WHO estimates more deaths globally due to Resistant Bacteria than Cancer FUTURE ESTIMATED DEATHS ATTRIBUTABLE TO VARIOUS CAUSES Antimicrobial Resistance: A Serious Problem That Needs Serious Attention
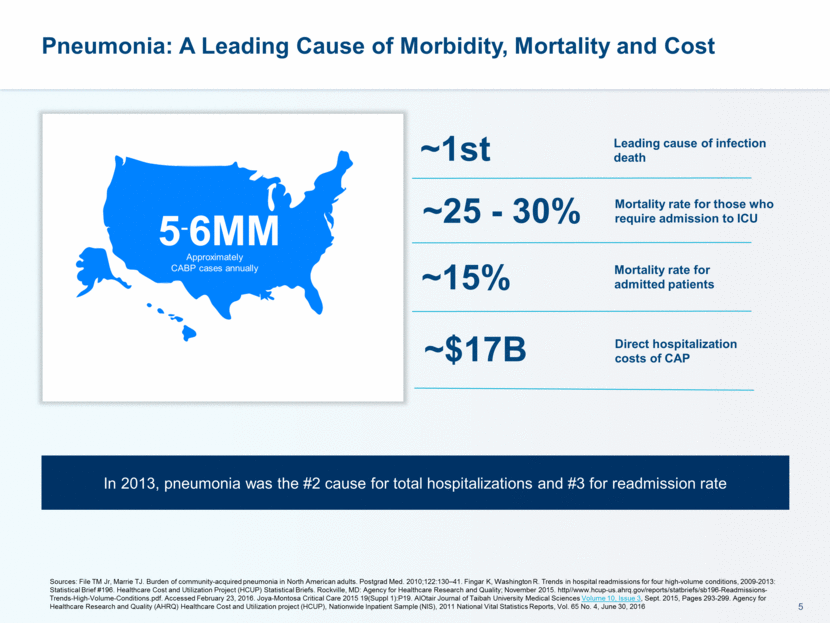
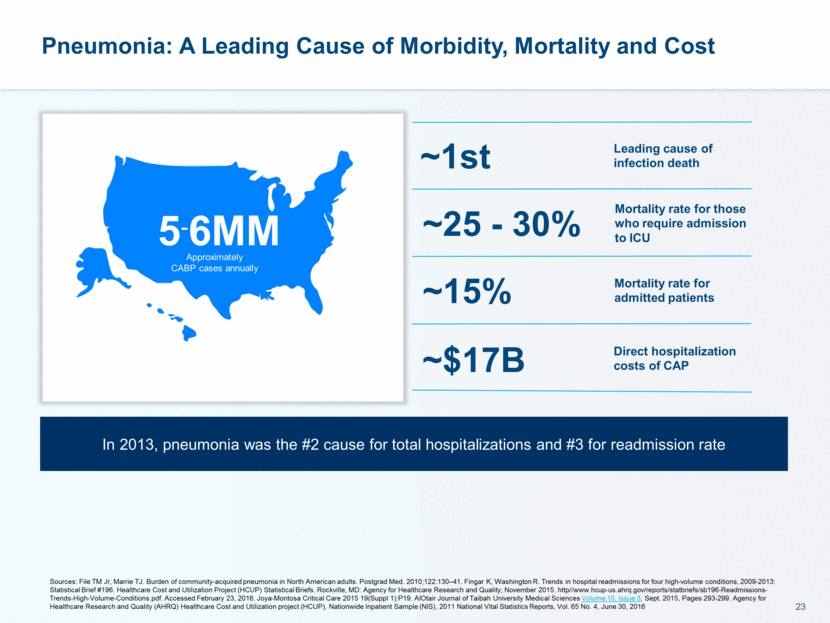
Pneumonia: A Leading Cause of Morbidity, Mortality and Cost 5-6MM Approximately CABP cases annually Mortality rate for admitted patients Mortality rate for those who require admission to ICU Leading cause of infection death ~15% ~25 - 30% ~1st Sources: File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130–41. Fingar K, Washington R. Trends in hospital readmissions for four high-volume conditions, 2009-2013: Statistical Brief #196. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; November 2015. http//www.hcup-us.ahrq.gov/reports/statbriefs/sb196-Readmissions-Trends-High-Volume-Conditions.pdf. Accessed February 23, 2016. Joya-Montosa Critical Care 2015 19(Suppl 1):P19. AlOtair Journal of Taibah University Medical Sciences Volume 10, Issue 3, Sept. 2015, Pages 293-299. Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost and Utilization project (HCUP), Nationwide Inpatient Sample (NIS), 2011 National Vital Statistics Reports, Vol. 65 No. 4, June 30, 2016 ~$17B Direct hospitalization costs of CAP In 2013, pneumonia was the #2 cause for total hospitalizations and #3 for readmission rate
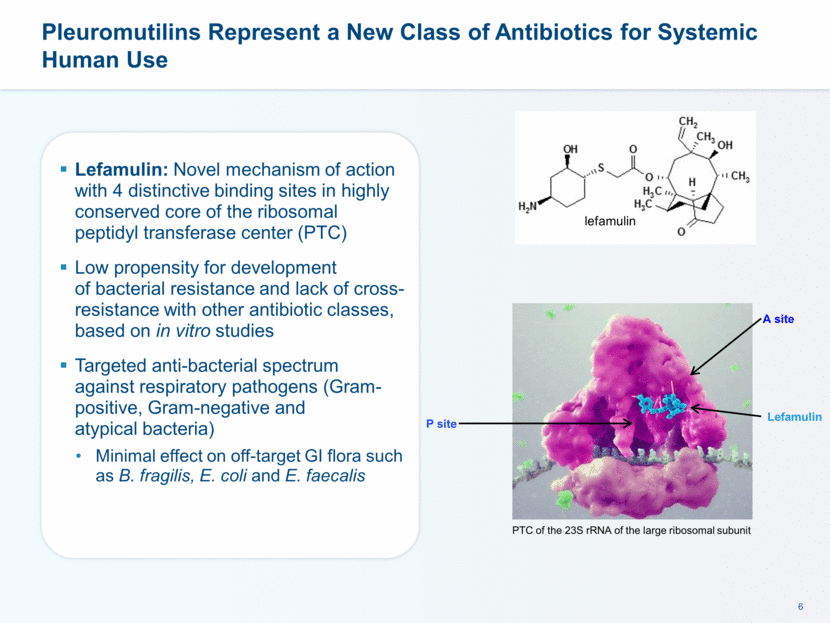
Lefamulin A site P site PTC of the 23S rRNA of the large ribosomal subunit Pleuromutilins Represent a New Class of Antibiotics for Systemic Human Use Lefamulin: Novel mechanism of action with 4 distinctive binding sites in highly conserved core of the ribosomal peptidyl transferase center (PTC) Low propensity for development of bacterial resistance and lack of cross-resistance with other antibiotic classes, based on in vitro studies Targeted anti-bacterial spectrum against respiratory pathogens (Gram-positive, Gram-negative and atypical bacteria) Minimal effect on off-target GI flora such as B. fragilis, E. coli and E. faecalis lefamulin
LEAP 1 Trial Design and Demographics
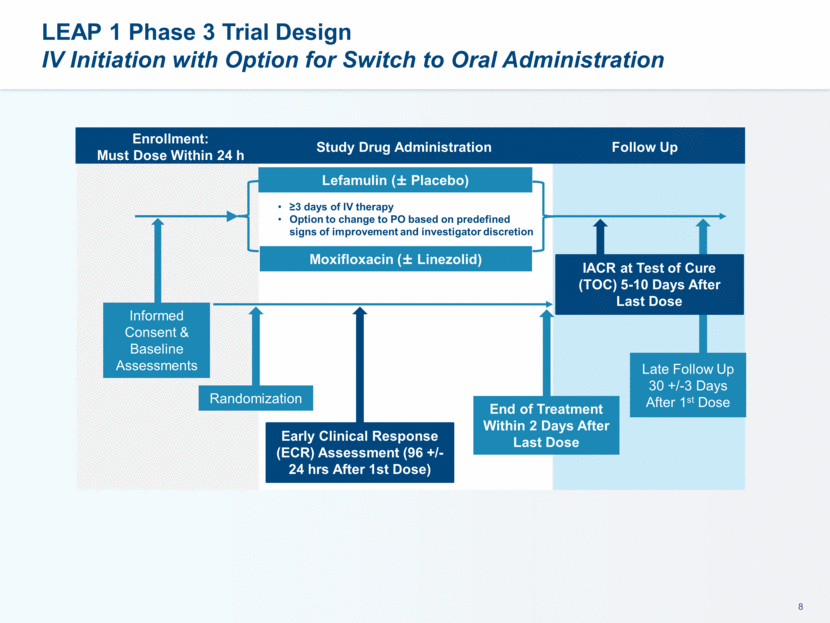
LEAP 1 Phase 3 Trial Design IV Initiation with Option for Switch to Oral Administration Lefamulin (± Placebo) Moxifloxacin (± Linezolid) Informed Consent & Baseline Assessments Randomization Early Clinical Response (ECR) Assessment (96 +/- 24 hrs After 1st Dose) End of Treatment Within 2 Days After Last Dose Late Follow Up 30 +/-3 Days After 1st Dose ≥3 days of IV therapy Option to change to PO based on predefined signs of improvement and investigator discretion Follow Up Study Drug Administration Enrollment: Must Dose Within 24 h IACR at Test of Cure (TOC) 5-10 Days After Last Dose
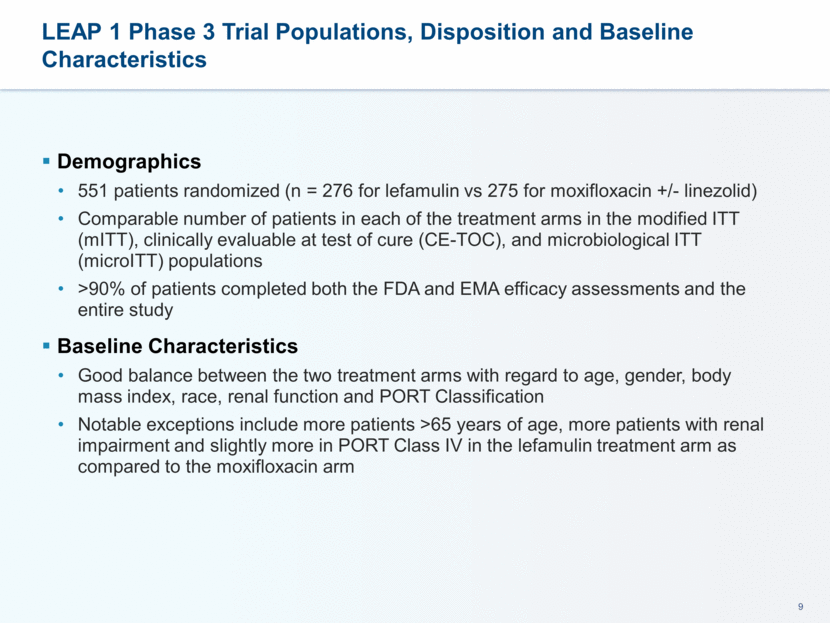
LEAP 1 Phase 3 Trial Populations, Disposition and Baseline Characteristics Demographics 551 patients randomized (n = 276 for lefamulin vs 275 for moxifloxacin +/- linezolid) Comparable number of patients in each of the treatment arms in the modified ITT (mITT), clinically evaluable at test of cure (CE-TOC), and microbiological ITT (microITT) populations >90% of patients completed both the FDA and EMA efficacy assessments and the entire study Baseline Characteristics Good balance between the two treatment arms with regard to age, gender, body mass index, race, renal function and PORT Classification Notable exceptions include more patients >65 years of age, more patients with renal impairment and slightly more in PORT Class IV in the lefamulin treatment arm as compared to the moxifloxacin arm 9
LEAP 1 Trial Efficacy Results

LEAP 1 Trial Efficacy Results Lefamulin Meets FDA and EMA Primary Endpoints
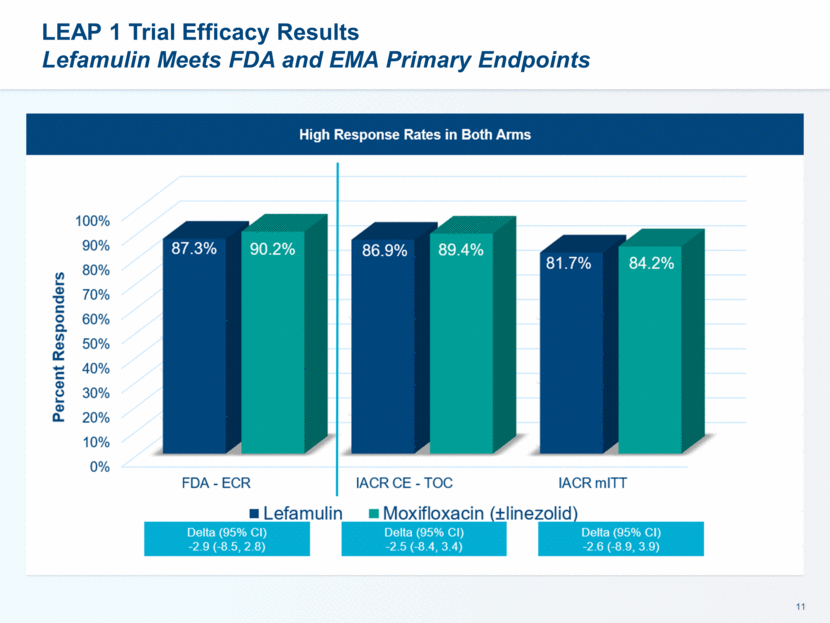
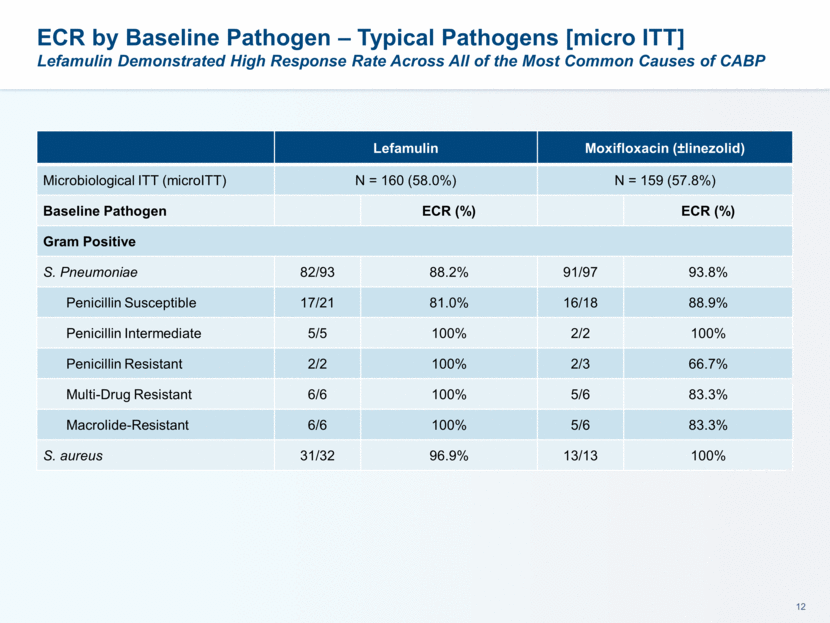
ECR by Baseline Pathogen – Typical Pathogens [micro ITT] Lefamulin Demonstrated High Response Rate Across All of the Most Common Causes of CABP Lefamulin Moxifloxacin (±linezolid) Microbiological ITT (microITT) N = 160 (58.0%) N = 159 (57.8%) Baseline Pathogen ECR (%) ECR (%) Gram Positive S. Pneumoniae 82/93 88.2% 91/97 93.8% Penicillin Susceptible 17/21 81.0% 16/18 88.9% Penicillin Intermediate 5/5 100% 2/2 100% Penicillin Resistant 2/2 100% 2/3 66.7% Multi-Drug Resistant 6/6 100% 5/6 83.3% Macrolide-Resistant 6/6 100% 5/6 83.3% S. aureus 31/32 96.9% 13/13 100%
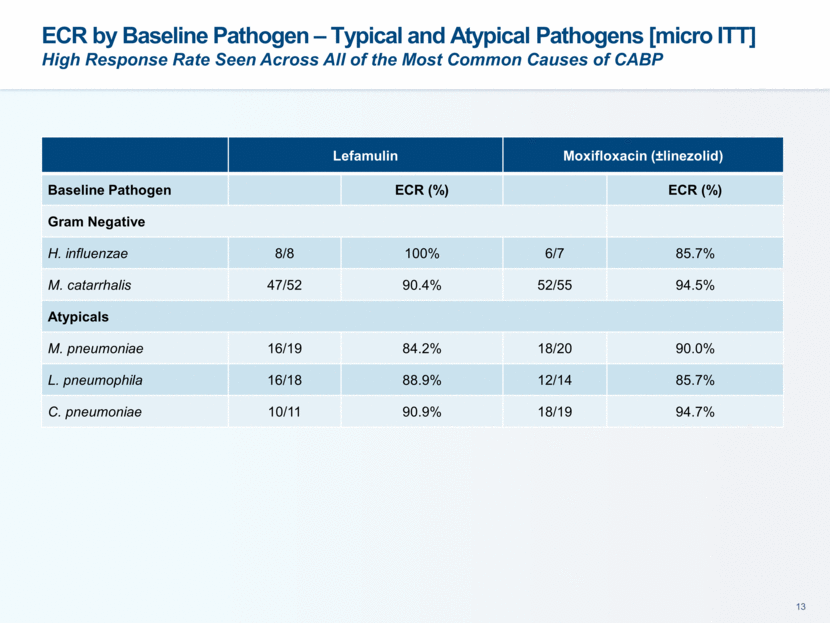
ECR by Baseline Pathogen – Typical and Atypical Pathogens [micro ITT] High Response Rate Seen Across All of the Most Common Causes of CABP Lefamulin Moxifloxacin (±linezolid) Baseline Pathogen ECR (%) ECR (%) Gram Negative H. influenzae 8/8 100% 6/7 85.7% M. catarrhalis 47/52 90.4% 52/55 94.5% Atypicals M. pneumoniae 16/19 84.2% 18/20 90.0% L. pneumophila 16/18 88.9% 12/14 85.7% C. pneumoniae 10/11 90.9% 18/19 94.7%
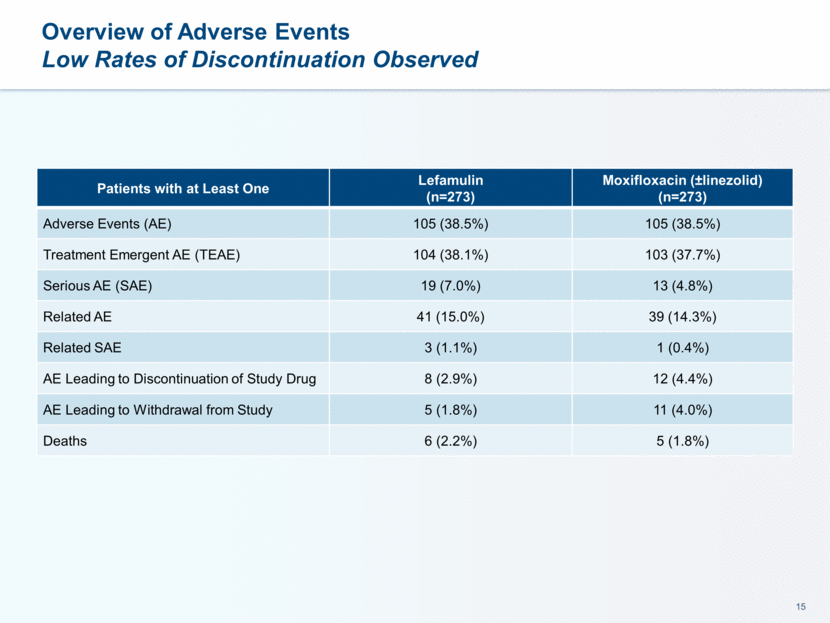
LEAP 1 Trial Safety and Tolerability Data

Overview of Adverse Events Low Rates of Discontinuation Observed Patients with at Least One Lefamulin (n=273) Moxifloxacin (±linezolid) (n=273) Adverse Events (AE) 105 (38.5%) 105 (38.5%) Treatment Emergent AE (TEAE) 104 (38.1%) 103 (37.7%) Serious AE (SAE) 19 (7.0%) 13 (4.8%) Related AE 41 (15.0%) 39 (14.3%) Related SAE 3 (1.1%) 1 (0.4%) AE Leading to Discontinuation of Study Drug 8 (2.9%) 12 (4.4%) AE Leading to Withdrawal from Study 5 (1.8%) 11 (4.0%) Deaths 6 (2.2%) 5 (1.8%)
Treatment Emergent Adverse Events of Interest TEAEs in 6.6% and 13.0% of subjects receiving lefamulin and moxifloxacin (±linezolid), respectively No cases of Clostridium difficile infection were reported in either treatment group Diarrhea was observed in 0.7% and 7.7% of subjects receiving lefamulin and moxifloxacin (±linezolid), respectively TEAEs in 0.7% and 1.5% of subjects receiving lefamulin and moxifloxacin (±linezolid), respectively Low incidence of liver enzyme elevation in both treatment groups consistent with CABP patient population No evidence of hepatotoxicity Gastrointestinal System Organ Class (SOC) Hepatobiliary SOC Cardiac Disorders SOC TEAEs in 2.9% and 4.0% of subjects receiving lefamulin and moxifloxacin (±linezolid), respectively Changes in QT interval of potential clinical concern were uncommon and of similar frequency between treatment groups
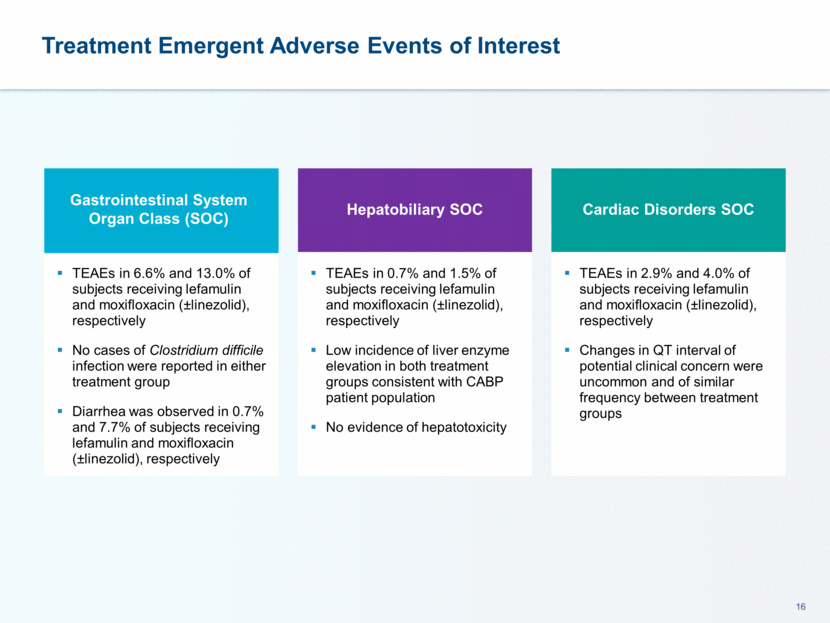
TEAEs > 2% for Study Medication: Safety Population Lefamulin was Well Tolerated with a Lower Incidence of Diarrhea Preferred Term Lefamulin (n=273) Moxifloxacin (±linezolid) (n=273) Hypokalemia 8 (2.9%) 6 (2.2%) Nausea 8 (2.9%) 6 (2.2%) Insomnia 8 (2.9%) 5 (1.8%) Infusion Site Pain 8 (2.9%) 0 (0.0%) Infusion Site Phlebitis 6 (2.2%) 3 (1.1%) ALT Increase 5 (1.8%) 6 (2.2%) Hypertension 2 (0.7%) 6 (2.2%) Diarrhea 2 (0.7%) 21 (7.7%)
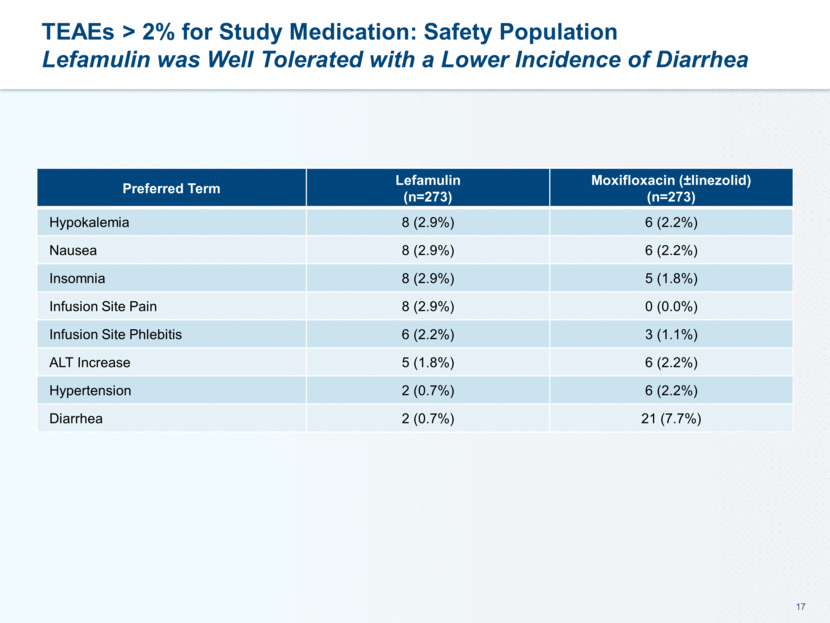
Maximum Post Baseline Liver Enzymes: Safety Population Low Incidence of Elevated Liver Function Tests Lab Parameter Lefamulin Moxifloxacin (±linezolid) Any Post Baseline ALT >3xULN 19/268 (7.1%) 17/267 (6.4%) >5xULN 6/268 (2.2%) 5/267 (1.9%) >10xULN 1/268 (0.4%) 0/267 (0.0%) Any Post Baseline AST >3xULN 11/268 (4.1%) 7/267 (2.6%) >5xULN 2/268 (0.7%) 2/267 (0.7%) >10xULN 1/268 (0.4%) 0/267 (0.0%) Any Post Baseline Total Bilirubin >1.5xULN 3/268 (1.1%) 3/267 (1.1%) >2xULN 0/268 (0.0%) 2/267 (0.7%) Any Post Baseline Alkaline Phosphatase >2xULN 5/268 (1.9%) 5/267 (1.9%) No subject met laboratory criteria for Hy’s Law
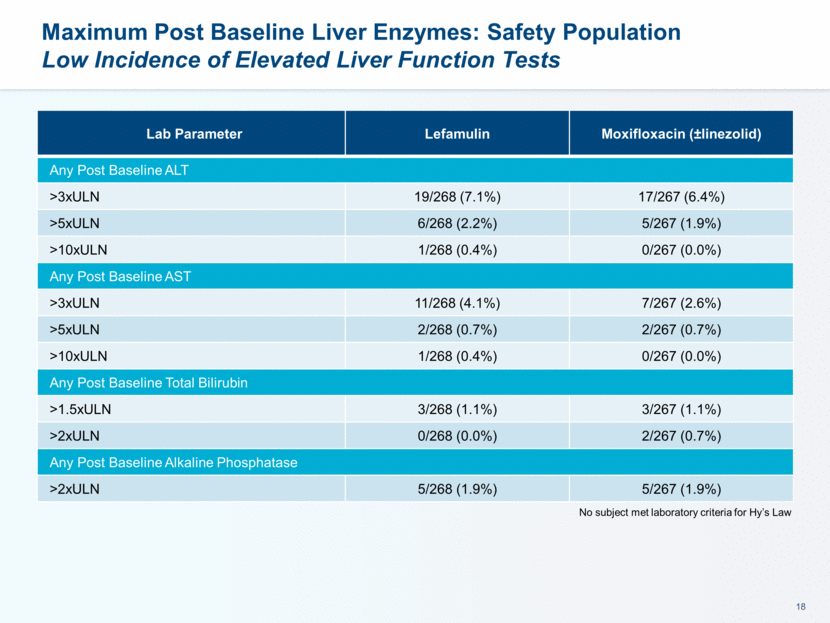
Maximum Post Dose QTcF Changes (Day 3) Parameter Lefamulin Moxifloxacin (±linezolid) Post Dose Increase 30 - 60 msec 12 (4.6%) 14 (5.4%) Post Dose Increase > 60 msec 0 (0.0%) 1 (0.4%) Post Dose Value > 500 msec 1 (0.4%) 1 (0.4%)
LEAP 1 Phase 3 Clinical Trial Highlights Lefamulin is the first of a new class of antibiotics with favorable Phase 3 data in CABP in more than 15 years Lefamulin’s efficacy data - in our first trial treating adults with CABP - was comparable to a gold standard therapy Lefamulin showed a favorable tolerability profile compared to moxifloxacin (±linezolid) with no unexpected safety signals or evidence of off target activity Lefamulin IV, with an option to switch to oral administration, met all primary FDA and EMA endpoints of non-inferiority compared to moxifloxacin (±linezolid)
LEAP 2: Next Significant Clinical Milestone LEAP 1 (IV to Oral) Trial 551 adult patients with PORT Risk Class ≥III (moderate to severe) 1:1 randomization: lefamulin vs. moxifloxacin ± linezolid Rx for 7 days (10 days for MRSA) PORT Risk Class III vs. IV and V ≥25% of patients will have a PORT risk class of IV or V Special Protocol Assessment with FDA LEAP 2 (Oral) Trial ~740 adult patients with PORT Risk Class II-IV (moderate) 1:1 randomization: lefamulin vs. moxifloxacin 5 days lefamulin / 7 days moxifloxacin PORT Risk Class II vs. III and IV ≥50% of patients will have a PORT risk class of III or IV Double-blind, randomized, active comparator >100 multinational centers
5-6MM Pts/year in the US, #1 cause of Infectious Deaths, #2 cause of Hospitalization and #3 cause of Hospital Readmissions, and it’s very costlyA Current available treatments for CABP have limitations due to increased resistance and Collateral Damage Lefamulin has profile well suited for CABP with efficacy comparable to a Gold Standard, a favorable tolerability profile, no unexpected safety signals or evidence of off target activity in a Phase 3 trial For patients with CABP, lefamulin has the potential to become a therapy of choice for 1) In-Hospital, 2) Transition of Care and 3) Community Lefamulin: A Significant Commercial Opportunity in CABP A: Sources on Slide 23
Pneumonia: A Leading Cause of Morbidity, Mortality and Cost 5-6MM Approximately CABP cases annually Sources: File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130–41. Fingar K, Washington R. Trends in hospital readmissions for four high-volume conditions, 2009-2013: Statistical Brief #196. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; November 2015. http//www.hcup-us.ahrq.gov/reports/statbriefs/sb196-Readmissions-Trends-High-Volume-Conditions.pdf. Accessed February 23, 2016. Joya-Montosa Critical Care 2015 19(Suppl 1):P19. AlOtair Journal of Taibah University Medical Sciences Volume 10, Issue 3, Sept. 2015, Pages 293-299. Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost and Utilization project (HCUP), Nationwide Inpatient Sample (NIS), 2011 National Vital Statistics Reports, Vol. 65 No. 4, June 30, 2016 Mortality rate for admitted patients Mortality rate for those who require admission to ICU Leading cause of infection death ~15% ~25 - 30% ~1st ~$17B Direct hospitalization costs of CAP In 2013, pneumonia was the #2 cause for total hospitalizations and #3 for readmission rate
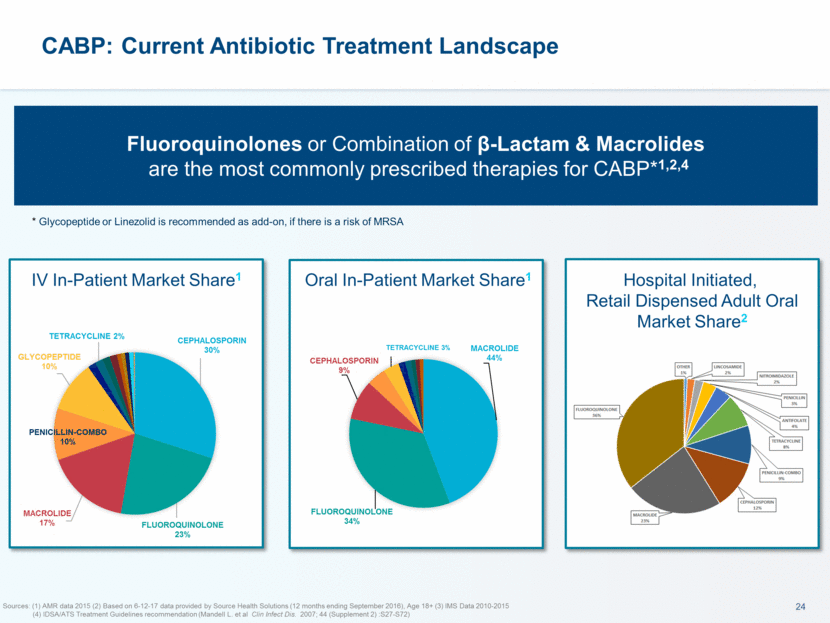
Sources: (1) AMR data 2015 (2) Based on 6-12-17 data provided by Source Health Solutions (12 months ending September 2016), Age 18+ (3) IMS Data 2010-2015 (4) IDSA/ATS Treatment Guidelines recommendation (Mandell L. et al Clin Infect Dis. 2007; 44 (Supplement 2) :S27-S72) CABP: Current Antibiotic Treatment Landscape Fluoroquinolones or Combination of β-Lactam & Macrolides are the most commonly prescribed therapies for CABP*1,2,4 * Glycopeptide or Linezolid is recommended as add-on, if there is a risk of MRSA IV In-Patient Market Share1 Oral In-Patient Market Share1 Hospital Initiated, Retail Dispensed Adult Oral Market Share2
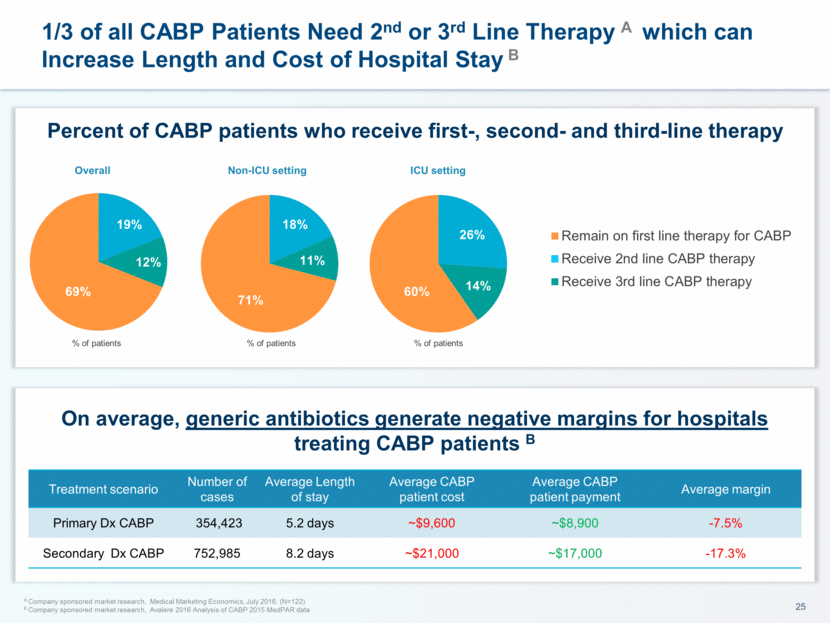
1/3 of all CABP Patients Need 2nd or 3rd Line Therapy A which can Increase Length and Cost of Hospital Stay B A Company sponsored market research, Medical Marketing Economics, July 2016, (N=122) B Company sponsored market research, Avalere 2016 Analysis of CABP 2015 MedPAR data Percent of CABP patients who receive first-, second- and third-line therapy Non-ICU setting ICU setting % of patients % of patients Overall % of patients Treatment scenario Number of cases Average Length of stay Average CABP patient cost Average CABP patient payment Average margin Primary Dx CABP 354,423 5.2 days ~$9,600 ~$8,900 -7.5% Secondary Dx CABP 752,985 8.2 days ~$21,000 ~$17,000 -17.3% On average, generic antibiotics generate negative margins for hospitals treating CABP patients B
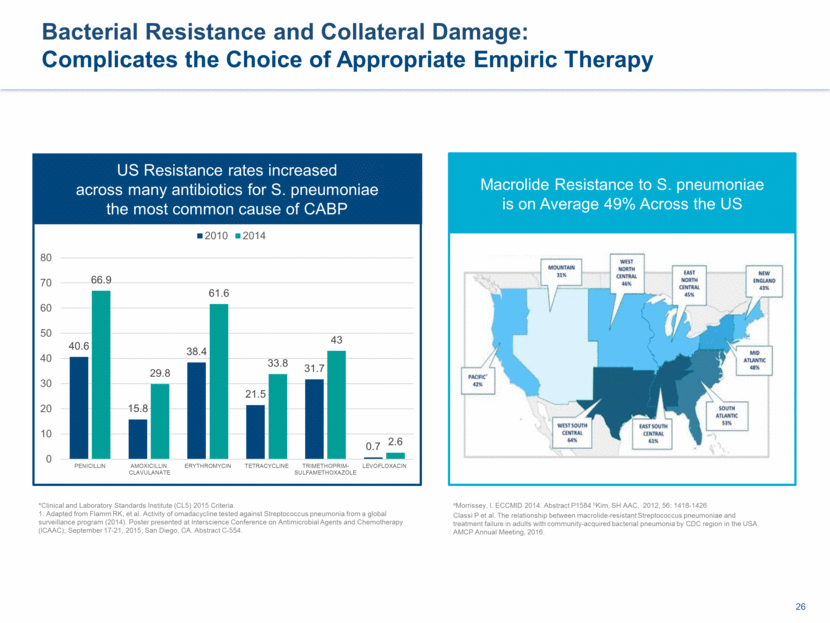
Bacterial Resistance and Collateral Damage: Complicates the Choice of Appropriate Empiric Therapy aMorrissey, I. ECCMID 2014. Abstract P1584 bKim, SH AAC, 2012, 56: 1418-1426 Classi P et al. The relationship between macrolide-resistant Streptococcus pneumoniae and treatment failure in adults with community-acquired bacterial pneumonia by CDC region in the USA. AMCP Annual Meeting, 2016. US Resistance rates increased across many antibiotics for S. pneumoniae the most common cause of CABP Macrolide Resistance to S. pneumoniae is on Average 49% Across the US *Clinical and Laboratory Standards Institute (CL5) 2015 Criteria. 1. Adapted from Flamm RK, et al. Activity of omadacycline tested against Streptococcus pneumonia from a global surveillance program (2014). Poster presented at Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); September 17-21, 2015; San Diego, CA. Abstract C-554. 40.6 15.8 38.4 21.5 31.7 0.7 66.9 29.8 61.6 33.8 43 2.6 0 10 20 30 40 50 60 70 80 PENICILLIN AMOXICILLIN CLAVULANATE ERYTHROMYCIN TETRACYCLINE TRIMETHOPRIM- SULFAMETHOXAZOLE LEVOFLOXACIN 2010 2014
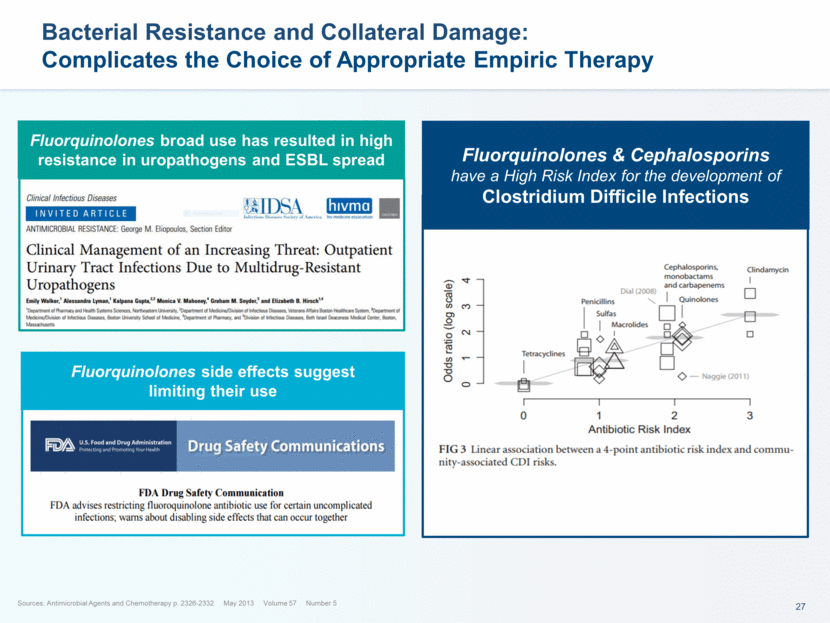
Bacterial Resistance and Collateral Damage: Complicates the Choice of Appropriate Empiric Therapy Fluorquinolones & Cephalosporins have a High Risk Index for the development of Clostridium Difficile Infections Sources: Antimicrobial Agents and Chemotherapy p. 2326-2332 May 2013 Volume 57 Number 5 Fluorquinolones broad use has resulted in high resistance in uropathogens and ESBL spread Fluorquinolones side effects suggest limiting their use
Antibiotic Stewardship: Appropriate Antibiotic Therapy is Essential 1. Dellit TH et.al., Clin Infect Dis (2007) 2. Amin AN et.al., Mayo Clin Proc (2014) 3. Slama TG, Alpesh Amin et.al., Am Jour Med (2005) Overuse of Broad Spectrum Antibiotics Antimicrobial Resistance & Collateral Damage (e.g. C. Difficile Infection) Higher Mortality Increased LOS Poor Treatment Outcomes Readmissions Higher Economic Burden to Hospitals Effective January 1, 2017, Joint Commission & CMS require that all hospitals have Antibiotic Stewardship Committees (ASC) Key Principle of the Antibiotic Stewardship Appropriate initial therapy should be directed against most-likely causative organisms
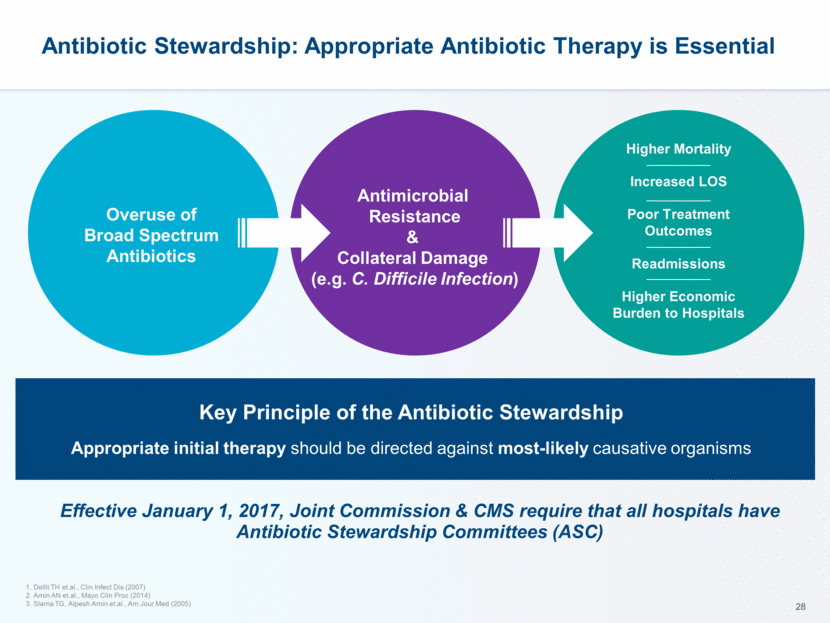
Lefamulin: Well Suited to Treat CABP - Consistent with all the Key Principles of Antimicrobial Stewardship - New Class New Mechanism of Action Effective against resistant pathogens and no cross –resistance to antibiotics commonly used for CABP Low propensity for resistance development Complete Spectrum of Coverage of main CABP pathogens Potent activity as monotherapy including against multi-drug resistant strains (DRSP, MRMP, MRSA) Minimal collateral damage to GI flora and low liability for C. difficile infection expected Excellent PK-PD Profile No loading dose with oral formulation due to rapid absorption with therapeutic concentrations on day 1 No dose adjustment for renal or hepatic insufficiency (expected) and limited food interaction potential Flexible IV & Oral Formulations Twice daily treatment for both IV and oral Ability to switch from any IV in-hospital antibiotic could allow earlier discharge or avoidance of hospitalization Short Course Of Monotherapy 7 days in the IV to Oral (LEAP 1) study 5 days in the Oral (LEAP 2) study Study still ongoing, expected results in Spring 2018 Favorable Safety & Tolerability Profile DRSP = Drug-resistant S. pneumoniae; MRMP = Macrolide-resistant M. pneumoniae; MRSA = Methicillin-resistant S. aureus Favorable tolerability profile with no unexpected safety signals or evidence of off target activity Should enhance patient compliance, especially in outpatient setting
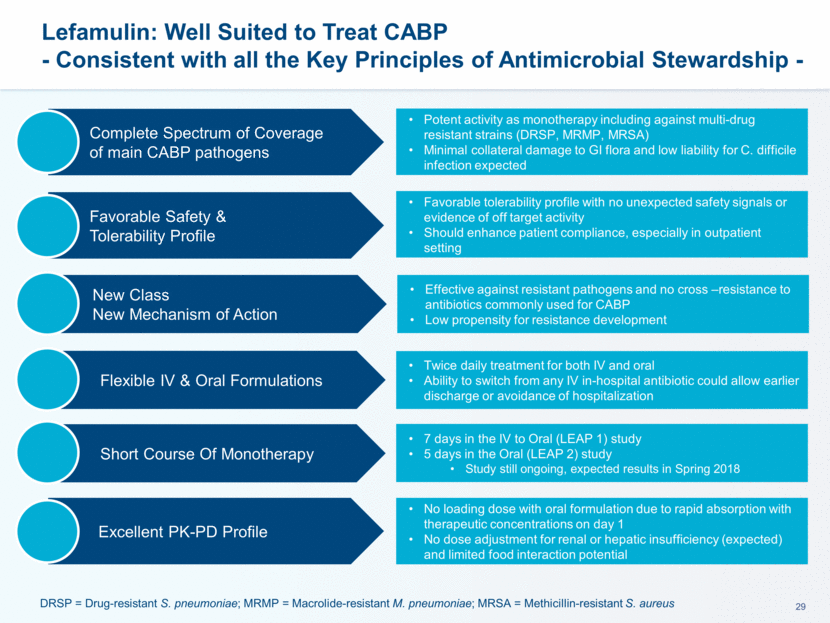
Lefamulin Innovative Profile is Well Suited for 3 Distinct and Significant Opportunities in the Treatment of CAPB Total Addressable Population Market Share Scenario*C 1st Empiric TherapyC: ~90% Hospital In Patient ~3.0MMA 2nd-3rd Line TherapyC: ~30% ~2.7 MM Patients ~0.9 MM Patients ~5% ~10% 135K Pts 90K Pts ~15% 150K Pts Transition of Care ~3.9MMA** ED Discharged Out-Patient IV to Oral (or Oral): ~100% ~0.9 MM Patients ~10% 230K Pts Community CABP ~2.3MMB Oral Treatment ~100% ~2.3MM Patients Potential Lefamulin Pts ~900K * Market share scenario approximately six years after launch, if approved. In the scenario, patients numbers & sales continue to grow until LOE, following the CABP epidemiology growth**~3.0MM Hospital Initiated Outpatients and ~0.9MM of ED Outpatients. See appendix for references. Discharged on OralC : ~80% ~2.4 MM Patients ~15% 300K Pts In-Hospital initiated Discharged Out-Patient
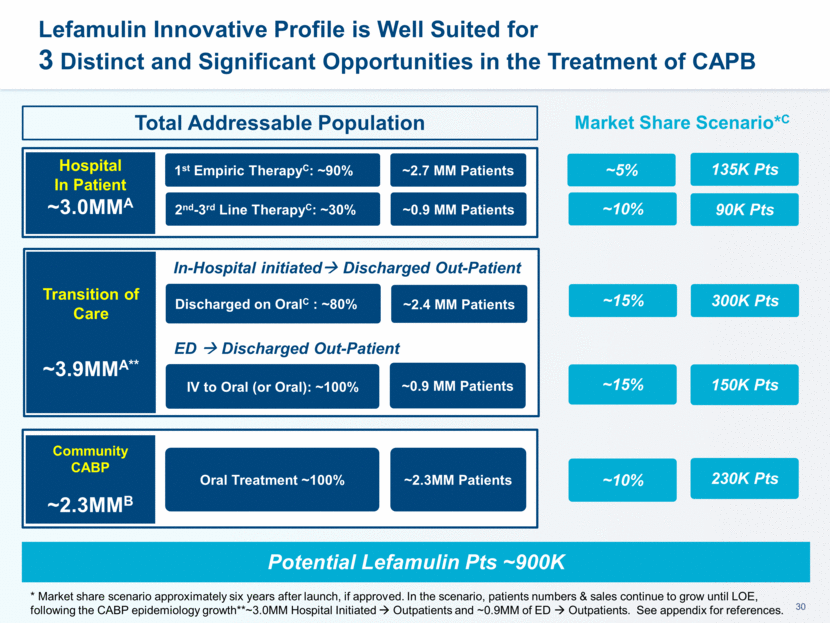
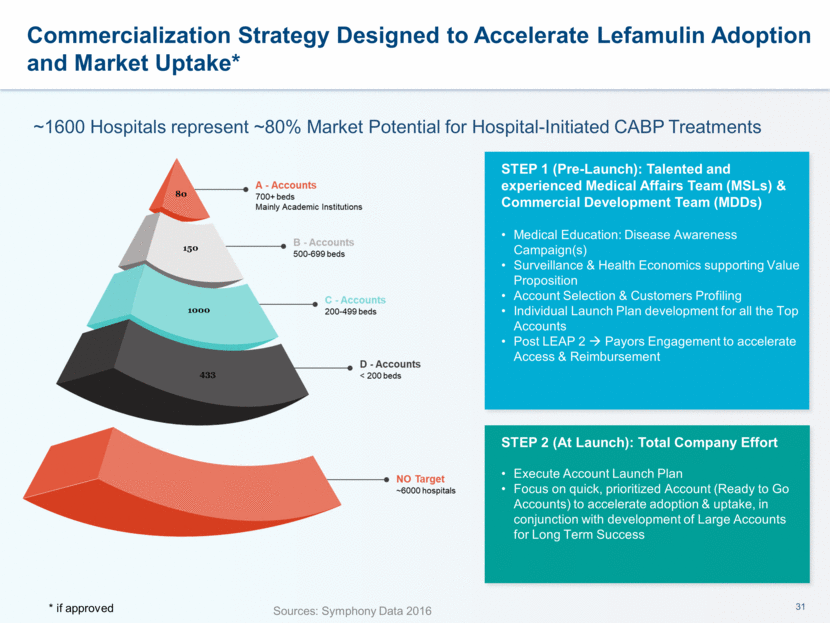
~1600 Hospitals represent ~80% Market Potential for Hospital-Initiated CABP Treatments Sources: Symphony Data 2016 STEP 1 (Pre-Launch): Talented and experienced Medical Affairs Team (MSLs) & Commercial Development Team (MDDs) Medical Education: Disease Awareness Campaign(s) Surveillance & Health Economics supporting Value Proposition Account Selection & Customers Profiling Individual Launch Plan development for all the Top Accounts Post LEAP 2 Payors Engagement to accelerate Access & Reimbursement STEP 2 (At Launch): Total Company Effort Execute Account Launch Plan Focus on quick, prioritized Account (Ready to Go Accounts) to accelerate adoption & uptake, in conjunction with development of Large Accounts for Long Term Success Commercialization Strategy Designed to Accelerate Lefamulin Adoption and Market Uptake* * if approved
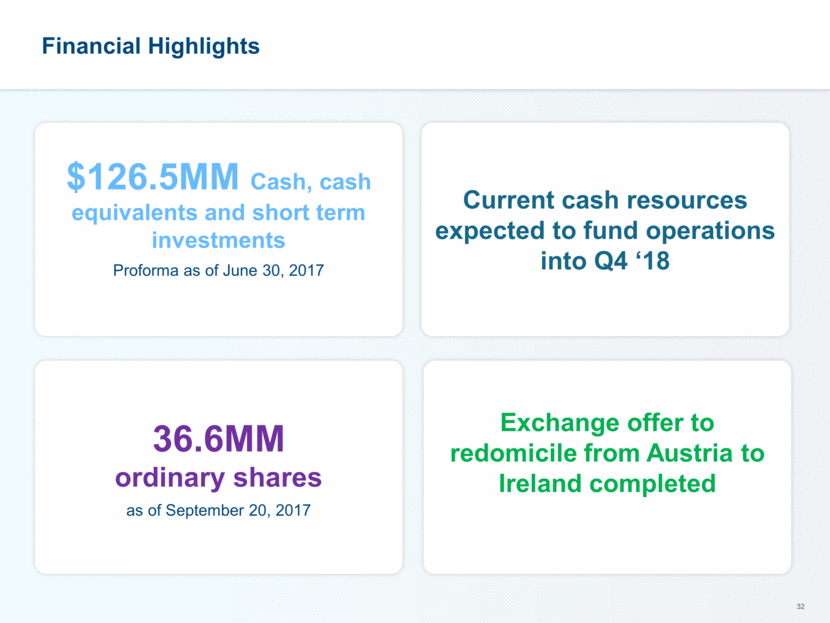
Financial Highlights $126.5MM Cash, cash equivalents and short term investments Proforma as of June 30, 2017 Current cash resources expected to fund operations into Q4 ‘18 36.6MM ordinary shares as of September 20, 2017 Exchange offer to redomicile from Austria to Ireland completed
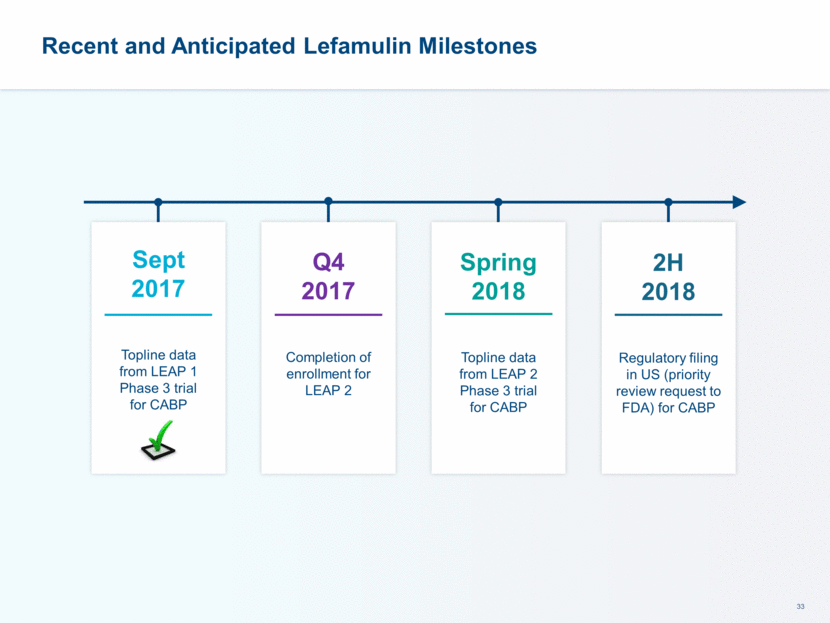
Sept 2017 Topline data from LEAP 1 Phase 3 trial for CABP Q4 2017 Completion of enrollment for LEAP 2 Recent and Anticipated Lefamulin Milestones Spring 2018 Topline data from LEAP 2 Phase 3 trial for CABP 2H 2018 Regulatory filing in US (priority review request to FDA) for CABP
Positioned for Long-Term Growth and Success CABP represents a significant medical need Rising rates of bacterial resistance to commonly used antibiotic therapies Utility of available treatment options becoming more limited Lefamulin is well-positioned to potentially address limitations of current antibiotics for the treatment of adult patients with CABP Well-designed Phase 3 development program consistent with regulatory guidance Potential first new class of antibiotics for CABP approved in more than 15 years Nabriva has an experienced team in place to successfully execute its strategy
Fall 2017 Developing Novel Anti-Infectives
Appendix
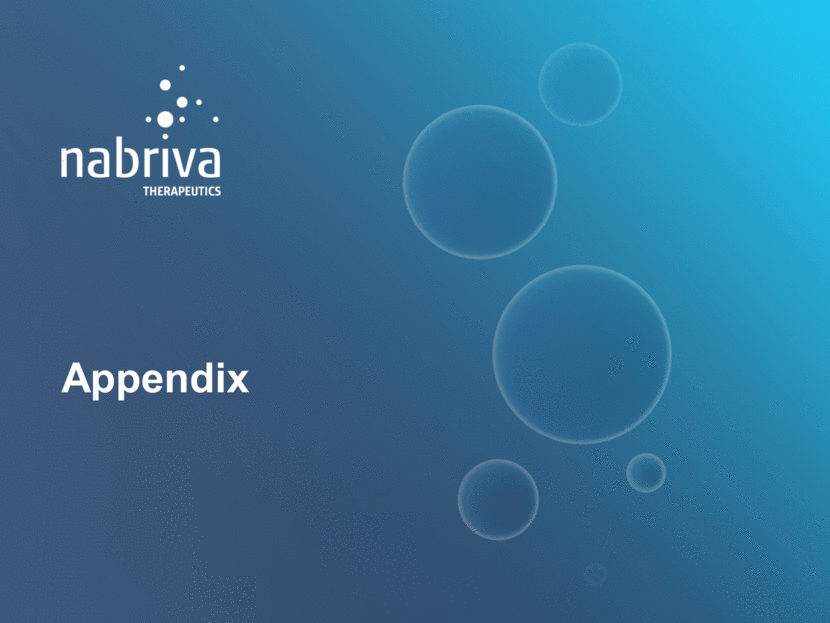
Lefamulin has a Differentiated Profile for CABP 37 Lefamulin Macrolides Cephalo-sporins Linezolid and Tedizolid Tetracyclines, including Omadacycline Fluoro-Quinolones Including Delafloxacin New Class, New MoA for CABP Ideal CABP Spectrum, Including Atypical & MRSA PK/PD Profile IV & Oral Short Course Low Propensity for Resistance Development Empiric monotherapy for CABP (data with both IV/Oral or Oral Only) Safety & Tolerability profile Risk of C. Difficile Infection Note: Nabriva management perspectives on product profiles (based on publicly available information), or in the case of lefamulin based on preclinical studies and clinical trials completed to date
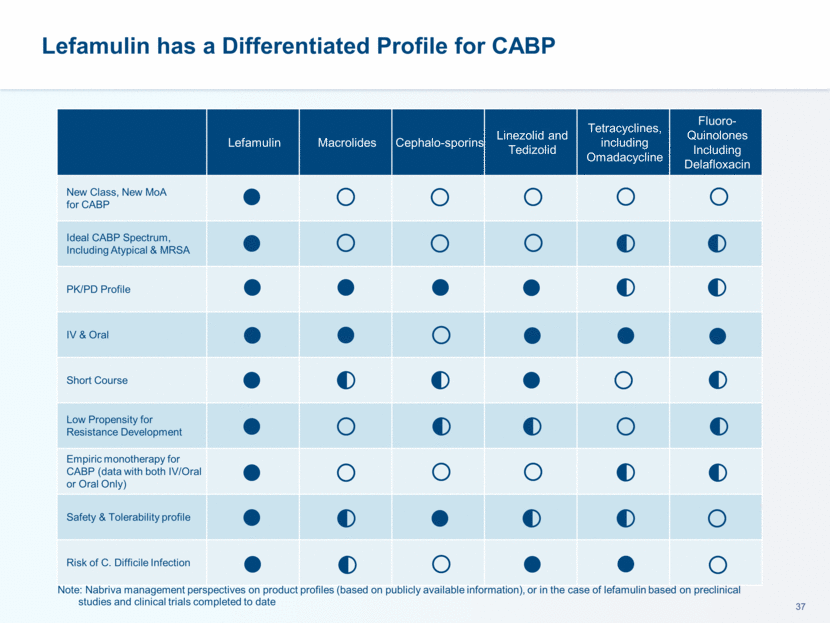
LEAP 1 Phase 3 Trial Design FDA and EMA Primary Endpoints At 72 to 120 hours after first dose of study drug: Improvement in ≥ 2 of 4 CABP signs/symptoms No worsening in any CABP signs/symptoms Alive Did not receive non-study antibacterial therapy for the treatment of CABP Non-inferiority margin 12.5%, >90% power At 5-10 days after the last dose of study drug: Signs/symptoms of CABP have resolved/improved such that no additional antibacterial therapy is administered for the treatment of CABP Non-inferiority margin 10%, 80% power EMA endpoint: Investigator Assessment of Clinical Response (IACR) at Test of Cure (TOC) mITT and CE-TOC Population FDA Endpoint: Early Clinical Response (ECR) ITT Population
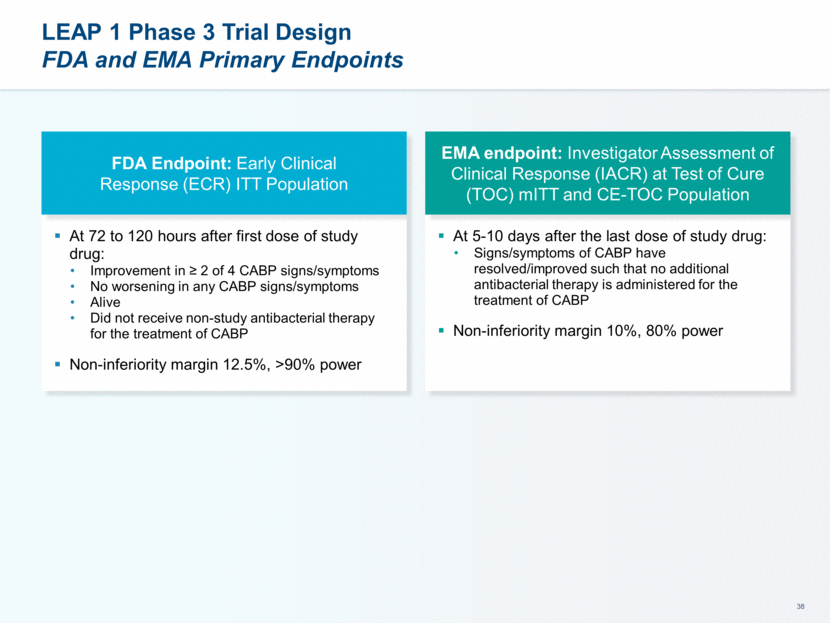
LEAP 1 and 2: Powering and Primary Objectives FDA Endpoint: Early Clinical Response (ECR) 72-120 hours after first dose of study drug Intent to Treat (ITT) Population LEAP 1 non-inferiority margin 12.5%, >90% power LEAP 2 non-inferiority margin 10%, 90% power EMA endpoint: Investigator Assessment of Clinical Response (IACR) at Test of Cure 5-10 days after the last dose of study drug Modified-ITT (mITT) and Clinically Evaluable Populations LEAP 1 non-inferiority margin 10%, 80% power LEAP 2 non-inferiority margin 10%, >90% power
LEAP 1 and 2: Treatment Scenarios LEAP 1 All patients: IV therapy for a minimum of three days before switching to oral therapy Patients will receive 7 days of lefamulin vs. 7 days moxifloxacin Patients receive 10 days if: MRSA pneumonia confirmed Both moxifloxacin and linezolid started at baseline (vs. lefamulin + placebo) If MRSA identified: moxifloxacin discontinued, linezolid continued LEAP 2 All patients will receive 5 days of lefamulin vs. 7 days moxifloxacin
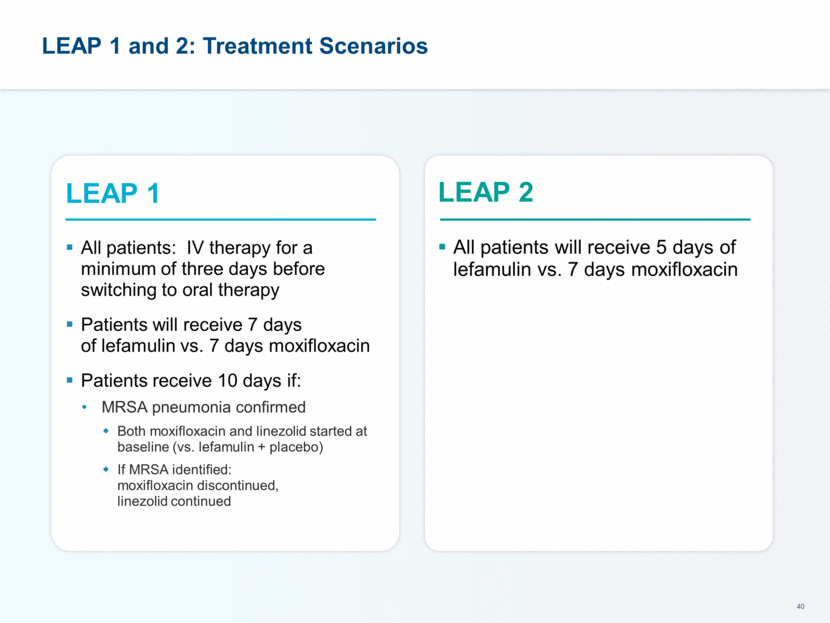
LEAP 1 Phase 3 Trial Design Symptom Assessment for ECR Symptom Absent (0) Mild (1) Moderate (2) Severe (3) Dyspnea Resolution (to pre-CABP baseline) or absence of dyspnea Dyspnea on exertion (e.g., climbing stairs) Dyspnea with normal/routine activities (e.g., walking) Dyspnea at rest or requiring oxygen therapy Cough Resolution (to pre-CABP baseline) or absence of cough Transient, does not interfere with normal activity Frequent, interferes with normal activity or sleep Constant, interferes with most or all activity or sleep Production of purulent sputum Resolution (to pre-CABP baseline) or absence of sputum production Sputum production rarely causes difficulty or distress Sputum production often causes difficulty or distress Constant difficulty with sputum production Chest pain Resolution or absence of chest pain related to CABP Transient, does not interfere with normal activity Frequent, interferes with normal activity or sleep Constant, interferes with most or all activity or sleep
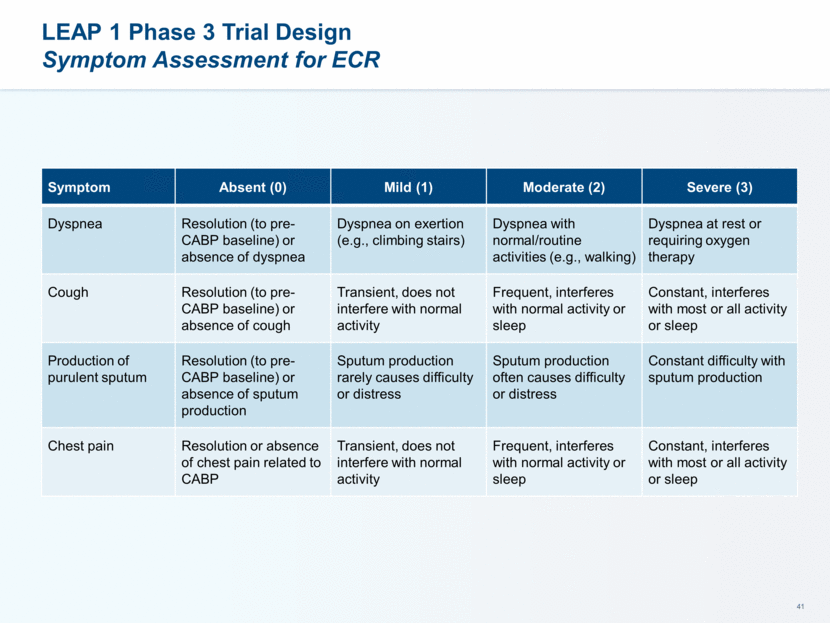
LEAP 1 Phase 3 Trial Populations and Disposition High Patient Completion and Microbiological Isolate Recovery Rates Category Lefamulin Moxifloxacin (±linezolid) TOTAL All Randomized 276 275 551 Modified ITT (mITT) 273 (98.9%) 273 (99.3%) 546 (99.1%) Clinically Evaluable-Test of Cure (CE-TOC) 236 (85.5%) 245 (89.1%) 481 (87.3%) Microbiological ITT (microITT) 160 (58.0%) 159 (57.8%) 319 (57.9%) Completed Study 249 (90.2%) 256 (93.1%) 505 (91.7%) Completed ECR Assessment 267 (96.7%) 261 (94.9%) 528 (95.8%) Completed TOC Assessment 260 (94.2%) 264 (96.0%) 524 (95.1%)
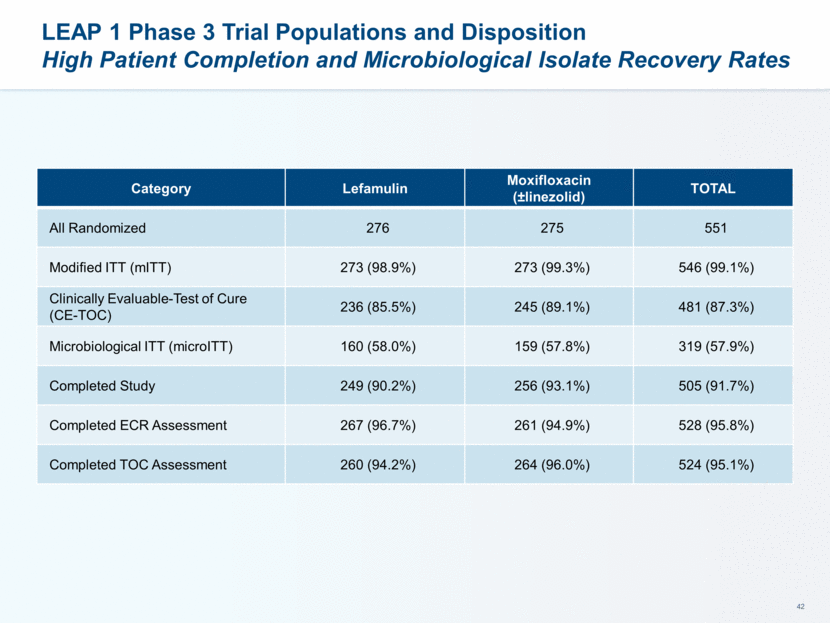
LEAP 1 Phase 3 Trial ITT Population Balanced Demographics Between Treatment Groups Category Lefamulin Moxifloxacin (±linezolid) TOTAL Age (Years; Mean) 61.0 59.6 60.3 <65 years 144 (52.2%) 167 (60.7%) 311 (56.4%) 65-74 74 (26.8%) 66 (24.0%) 140 (25.4%) ≥75 58 (21.0%) 42 (15.3%) 100 (18.1%) Gender Male 170 (61.6%) 160 (58.2%) 330 (59.9%) BMI (kg/m2) (Mean) 26.48 26.33 26.40 Race (White) 239 (86.6%) 239 (86.9%) 478 (86.8%) Renal Status Severe impairment (CrCl <30 ml/min) 3 (1.1%) 3 (1.1%) 6 (1.1%) Moderate impairment (CrCl 30-60ml/min) 61 (22.1%) 62 (22.5%) 123 (22.3%) Mild impairment (CrCl 60-<90 ml/min) 89 (32.2%) 74 (26.9%) 163 (29.6%) Normal function (CrCl >90 ml/min) 121 (43.8%) 135 (49.1%) 256 (46.5%)
LEAP 1 Phase 3 Trial PORT Risk Classification: ITT Population Moderate to Severe Pneumonia Patients PORT Class Lefamulin Moxifloxacin (±linezolid) TOTAL II 0 1 (0.4%) 1 (0.2%) III 196 (71.0%) 201 (73.1%) 397 (72.1%) IV 76 (27.5%) 70 (25.5%) 146 (26.5%) V 4 (1.4%) 3 (1.1%) 7 (1.3%)
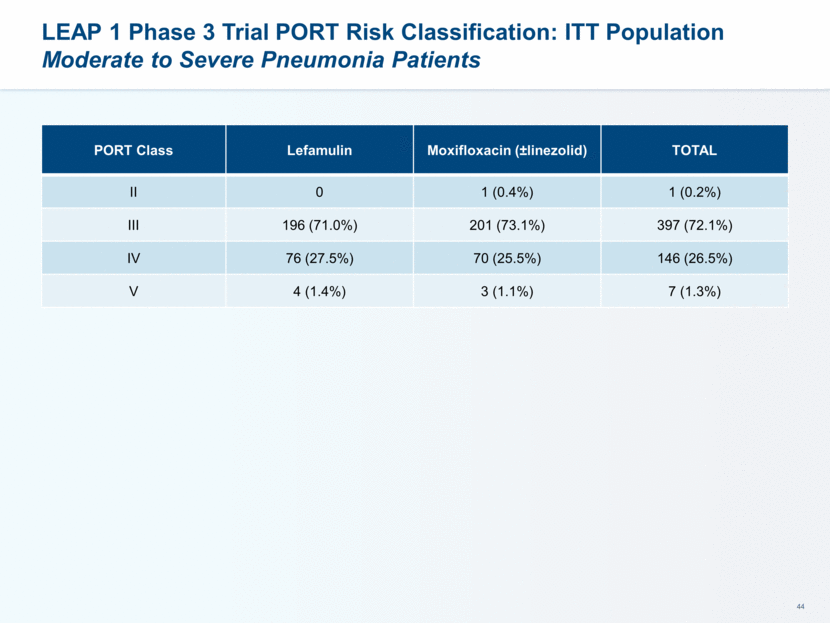
ECR by PORT Risk Classification: ITT Population Lefamulin Demonstrated High Response Rates Across All Severities of CABP PORT Class Lefamulin Moxifloxacin (±linezolid) Treatment Difference (95% CI) II 0 1/1 (100%) -- III 175/196 (89.3%) 187/201 (93.0%) -3.7 (-9.8, 2.3) IV 63/76 (82.9%) 57/70 (81.4%) 1.5 (-12.3, 15.3) V 3/4 (75%) 3/3 (100%) -25.0 (-96.6, 46.6)
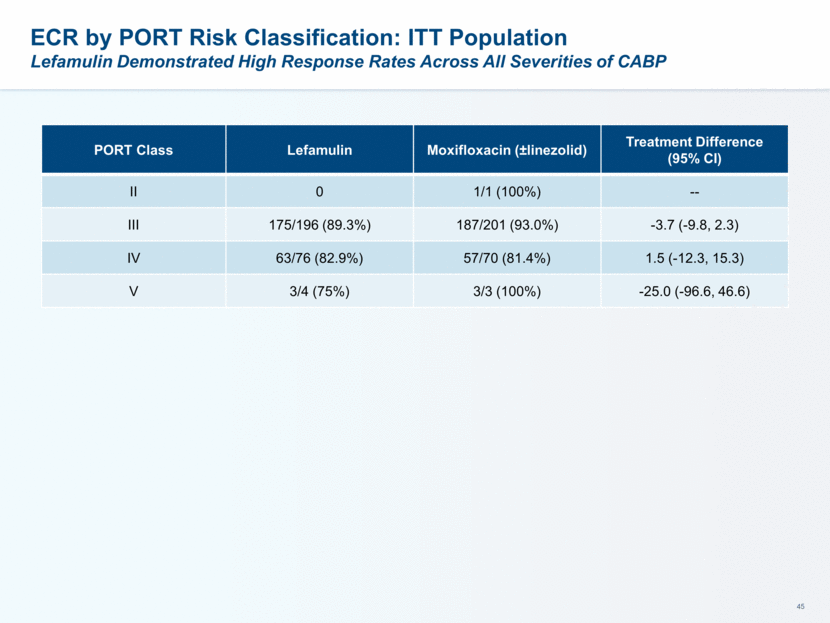
Lefamulin Spectrum of in vitro Activity Well Suited for CABP Extensively investigated against most prevalent pathogens Not affected by increasing antibiotic resistance observed in CABP pathogens: Cephalosporin/ fluoroquinolone/ macrolide/ penicillin resistance in S. pneumoniae Macrolide-resistant Mycoplasma spp. Note: S. pneumoniae resistant to macrolides or to levofloxacin showed 100% susceptibility to lefamulin (SENTRY 2015-16 surveillance with S. pneumoniae isolates collected world-wide) * Data from SENTRY 2015-16 world-wide surveillance program ** Waites KB et al. ASM-Microbe Meeting, June 19, 2016; Abstract#3972 Organism n MIC90 [mg/L] Organism n MIC90 [mg/L] S. pneumoniae* 2886 0.12 L. pneumophila 30 0.5 M. catarrhalis* 779 0.06 C. pneumoniae 50 0.04 H. influenzae* 1108 1 M. pneumoniae** 50 0.002 S. aureus (including HA-MRSA, CA-MRSA and MSSA) 3077 0.12
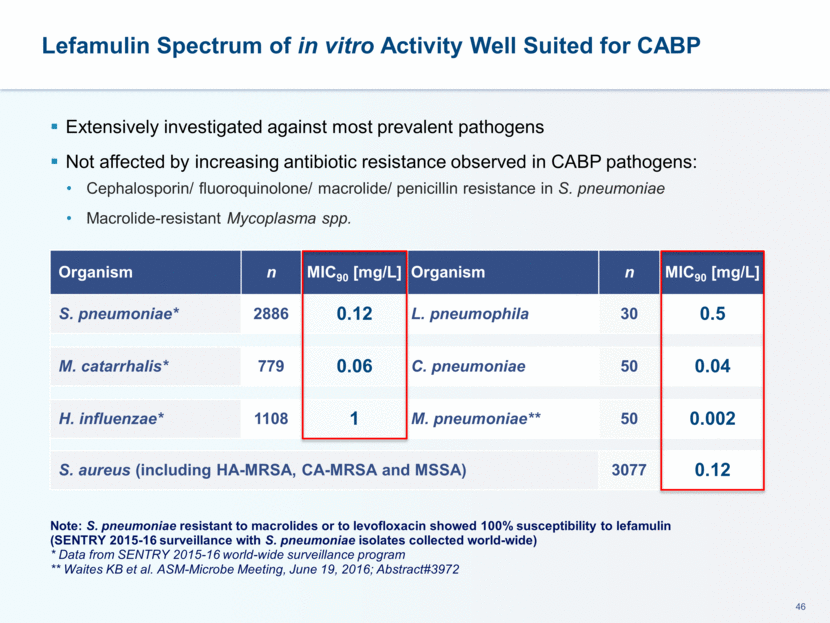
Therapeutic Effect Observed in Phase 2 Trial of Acute Bacterial Skin and Skin Structure Infections (ABSSSI) Lefamulin achieved clinical response rates comparable to vancomycin Lefamulin was well tolerated Most commonly reported adverse events included headache, nausea and infusion site pain or redness Lefamulin 100 mg Lefamulin 150 mg Vancomycin 1 g N = 70 N = 71 N = 66 ITT population at Test of Cure (TOC) 85.7 83.1 81.8 CE population at TOC 90.0 88.9 92.2 No increase in area of erythema plus absence of fever (%) 48-72h (ITT pop.) 85.7 83.1 80.3 20% reduction in area of erythema (%) at 48-72h (ITT pop.) 74.3 70.4 71.2 Treatment Group % response rate Lefamulin 100 mg or 150 mg IV q12h vs. vancomycin in 207 patients with ABSSSI
Lefamulin Related Tolerability Profile Observed in Phase 2 Trial of ABSSSI
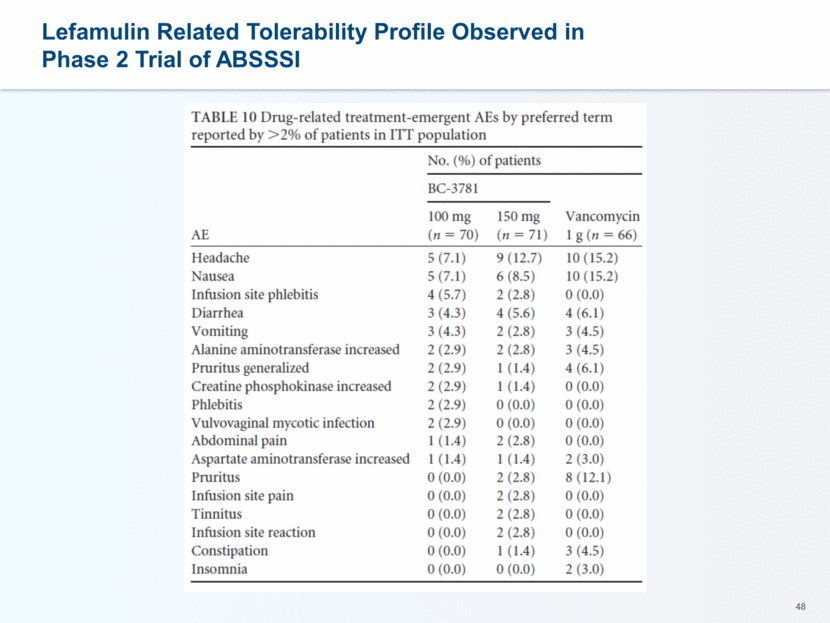
Lefamulin Clinical Development Plan Supports Differentiation in CABP Phase 3 CABP registration program initiated in the fall of 2015 Lefamulin Evaluation of Adults with Pneumonia (LEAP 1) trial (IV to oral): moderate-to-severe CABP (Pneumonia Outcomes Research Team (PORT) III, IV and V) February 2017- Confirmation of ~550 patients sample size April 2017 – Completed enrollment Data received in Sept 2017 Second trial (LEAP 2 – oral): moderate CABP (PORT II, III and IV) initiated April 2016 ~160 sites enrolling patients Anticipate completing enrollment in 4Q 2017 Data anticipated in spring 2018 Hepatic / Renal Impairment Additional Phase 1 studies LEAP 2: CABP Oral CP3, global, ~740 pts. LEAP 1: CABP IV to Oral CP3, global, 551 pts. 2016 2018 2017 Q1 Q4 Q4 Q2 Q3 Q4 Non-Clinical Activities Blue = Clinical Programs Q3 Q2 Q1 Q3 Q2 Q1 NDA submission target in 2H 2018
Lefamulin Manufacturing On Track for NDA Submission in 2018 Nabriva CMC/Manufacturing Team- extensive experience with new product approvals in the US and Europe Nabriva Quality Assurance Team- extensive experience with regulatory agency requirements in the US and Europe Drug Substance (API) Well known contract manufacturer in Europe with good regulatory inspection history API is very stable- expect 4-year retest date Plan to hold sufficient API inventory to reduce business interruption risk Plan to approve second API supplier to further reduce business interruption risk Drug Product (DP) Well known contract manufacturers in Europe with good regulatory inspection histories IV Product- expect 36-month shelf life with refrigerated storage Oral Product- expect 36-month shelf life with room temperature storage Plan to approve second IV and oral DP suppliers to reduce business interruption risk

Nabriva Has a Strong and Broad Patent Portfolio Nabriva currently has 19 worldwide pleuromutilin patent families active on file Most of the global pleuromutilin IP landscape is covered (including U.S., Europe, Japan) Lefamulin IP protected at least until 2028 by several layers Composition of matter granted in U.S., EP and Japan until 2028 Process patents ensure protection until 2031 Patent extension by up to 5 years in major jurisdictions BC-7013 IP protection until 2027 Composition of matter granted inter alia in U.S., EP and Japan
Antibiotic Stewardship Should Increasingly Drive Antibiotic Choices Effective January 1st 2017, Joint Commission & CMS require that all hospitals have Antibiotic Stewardship Committees (ASC) - Goalsa Achieve the best clinical outcome, while lessening or mitigating adverse events and limiting selective pressures Decrease excessive costs due to suboptimal antibiotic use Realize a fiduciary responsibility across the continuum of care Reduce the use of high-risk antibiotics associated with a high risk of C. Diff infections Increase use of oral antibiotics as a strategy to improve outcomes or decrease costs Reduce antibiotic therapy to the shortest effective duration Sources: a Policy Statement on Antimicrobial Stewardship by SHEA, IDSA, and Pediatric Infectious Diseases Society (PIDS)nfect Control Hosp Epidemiol 2012;33(4):322-327 IDSA/SHEA Antibiotic Stewardship guidelines for antibiotic use updated in 2016: Key Principle: Appropriate initial therapy should be directed against most-likely causative organism
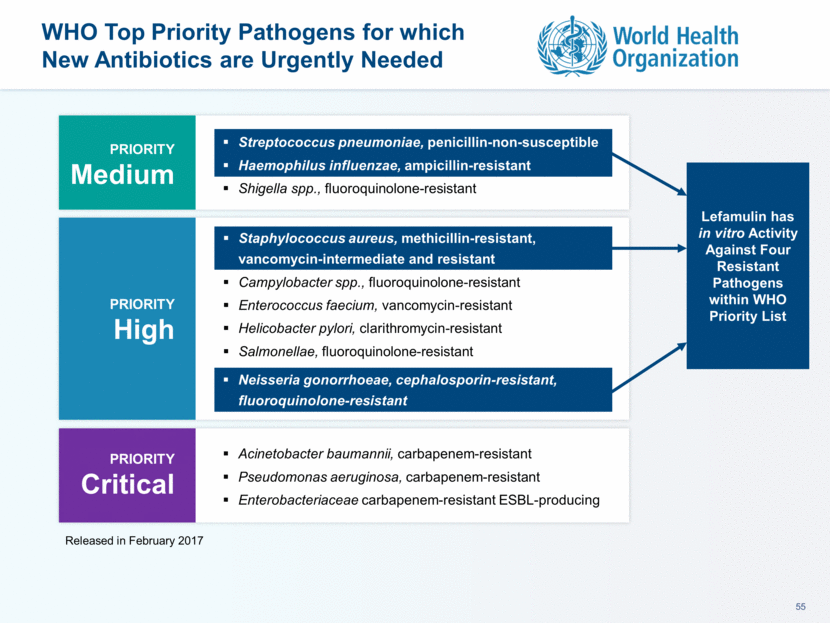
Non-ICU Inpatient B-lactam (IV or IM) + Macrolide (IV or PO) Respiratory Quinolone (IV or PO) B-lactam (IV or IM) + Doxycycline (IV or PO) ICU Inpatient B-lactam (IV) + Macrolide (IV) B-lactam (IV) + respiratory Quinolone (IV) B-lactam (IV) + Aminoglycoside (IV) + respiratory Quinolone (IV) Mandell L. et al Clin Infect Dis. 2007; 44 (Supplement 2) :S27-S72; modified to show Community Acquired MRSA occurring in hospital, not just in ICU Outpatient If healthy, no antimicrobial use in 3 months and no Risk Factors for Drug-Resistant S. pneumoniae (DRSP) Macrolide (PO) Doxycycline (PO) Co-morbidities or Risk Factors for DRSP B-lactam (PO) + Macrolide (PO) Respiratory Quinolone (PO) At Risk for CA-MRSA Add oxazolidinone (IV) or glycopeptide (IV) Lefamulin May Be Used in Multiple CABP Treatment Settings CABP Treatment Guidelines
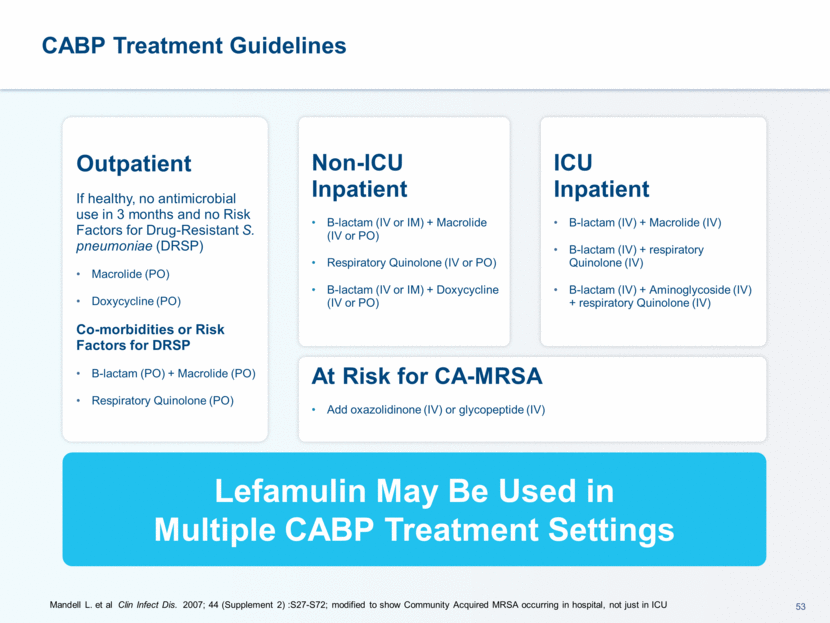
US legislation continues to drive discovery of novel antibiotics GAIN Act (Generating Antibiotic Incentives Now) ADAPT Act (Antibiotic Development to Advance Patient Treatment) DISARM Act (Developing an Innovative Strategy for Antimicrobial Resistant Microorganisms)- part of 21st Century Cures Act CDC Antibiotic Resistance Threats in US, 2013 FDA CABP clinical trial guidance issued 2014 IDSA/SHEA 2016 Stewardship guidelines IDSA/ATS 2016 HAP/VAP Treatment Guidelines Recent FDA Advisory Committees for Fluoroquinolones and Solithromycin Update to 2007 IDSA/ATS CABP Treatment Guidelines in progress We believe that a need exists for novel antibiotics for infectious diseases, including CABP Scientific, Regulatory and Government Agencies Focused on Infectious Diseases
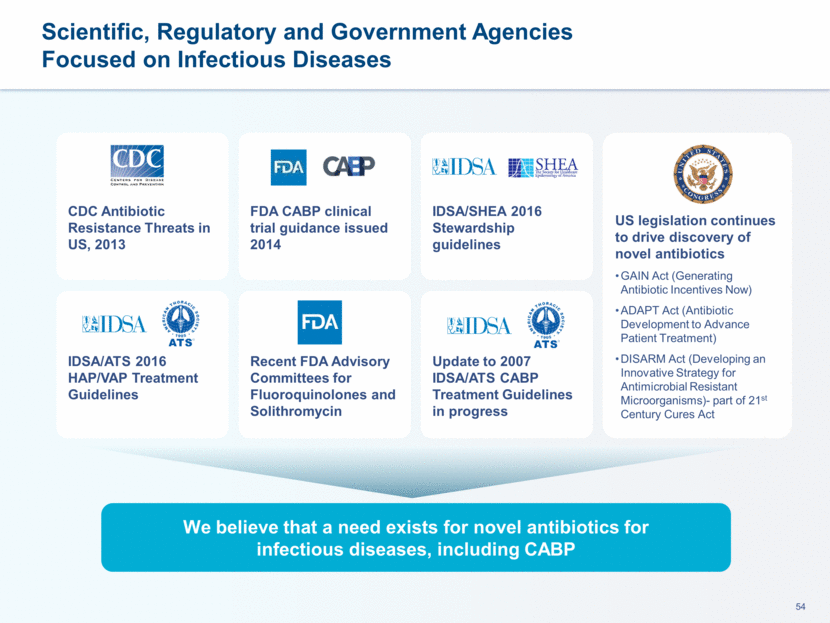
WHO Top Priority Pathogens for which New Antibiotics are Urgently Needed PRIORITY Critical Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa, carbapenem-resistant Enterobacteriaceae carbapenem-resistant ESBL-producing PRIORITY High Staphylococcus aureus, methicillin-resistant, vancomycin-intermediate and resistant Campylobacter spp., fluoroquinolone-resistant Enterococcus faecium, vancomycin-resistant Helicobacter pylori, clarithromycin-resistant Salmonellae, fluoroquinolone-resistant PRIORITY Medium Streptococcus pneumoniae, penicillin-non-susceptible Haemophilus influenzae, ampicillin-resistant Shigella spp., fluoroquinolone-resistant Lefamulin has in vitro Activity Against Four Resistant Pathogens within WHO Priority List Neisseria gonorrhoeae, cephalosporin-resistant, fluoroquinolone-resistant Released in February 2017
56 A: Healthcare Cost and Utilization Project (HCUP) 2013, Age 18+ (projected to 2029) B: Community Data (CDC 2009-2010), Age 18+ (projected to 2029) C: Company Sponsored Market Research, Medical Marketing Economics, July 2016, (N=122) References to Slide 30 (Commercial Assessment)

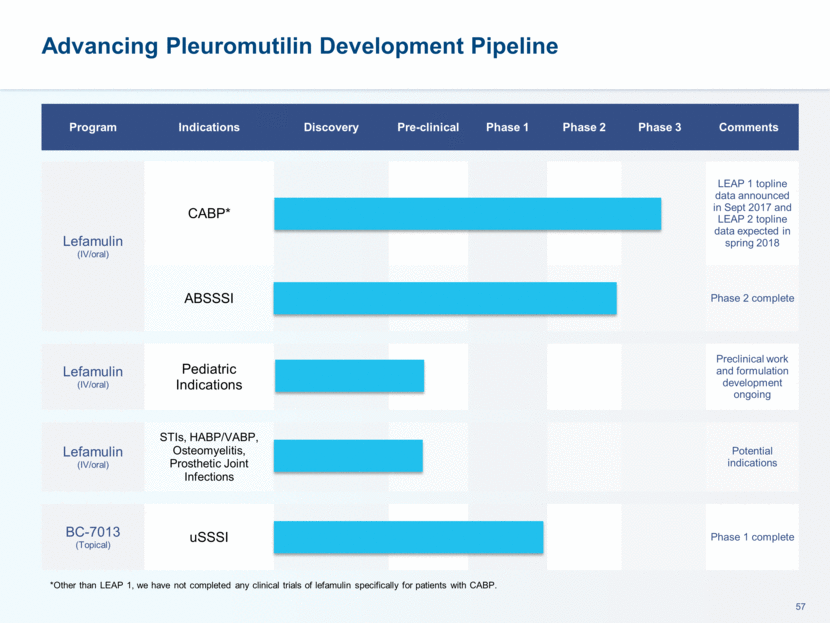
Advancing Pleuromutilin Development Pipeline Program Indications Discovery Pre-clinical Phase 1 Phase 2 Phase 3 Comments Lefamulin (IV/oral) CABP* LEAP 1 topline data announced in Sept 2017 and LEAP 2 topline data expected in spring 2018 ABSSSI Phase 2 complete Lefamulin (IV/oral) Pediatric Indications Preclinical work and formulation development ongoing Lefamulin (IV/oral) STIs, HABP/VABP, Osteomyelitis, Prosthetic Joint Infections Potential indications BC-7013 (Topical) uSSSI Phase 1 complete *Other than LEAP 1, we have not completed any clinical trials of lefamulin specifically for patients with CABP.