Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ZOGENIX, INC. | d462623d8k.htm |

September 2017 STUDY 1 PHASE 3 TOP-LINE RESULTS Exhibit 99.1

Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests," "assuming," "designed" and similar expressions are intended to identify forward-looking statements. These statements are based on the company's current beliefs and expectations. These forward-looking statements include statements regarding ZX008’s potential as a treatment for seizures associated with Dravet syndrome; the timing of topline results from Study 1504; and regulatory submission timelines for ZX008. The inclusion of forward-looking statements should not be regarded as a representation by Zogenix that any of its plans will be achieved. Actual results may differ from those set forth in this release due to the risks and uncertainties inherent in Zogenix's business, including, without limitation: the top-line data Zogenix has reported is based on preliminary analysis of key efficacy and safety data, and such data may change following a more comprehensive review of the data related to the clinical trial and such top-line data may not accurately reflect the complete results of the trial, and the FDA may not agree with Zogenix’s interpretation of such results; the uncertainties associated with the clinical development and regulatory approval of product candidates such as ZX008, including potential delays in the enrollment and completion of clinical trials; the potential that earlier clinical trials and studies may not be predictive of future results; Zogenix's reliance on third parties to conduct its clinical trials, enroll patients, manufacture its preclinical and clinical drug supplies; unexpected adverse side effects or inadequate therapeutic efficacy of ZX008 that could limit approval and/or commercialization, or that could result in recalls or product liability claims; Zogenix's ability to fully comply with numerous federal, state and local laws and regulatory requirements, as well as rules and regulations outside the United States, that apply to its product development activities; and other risks described in Zogenix's prior press releases as well as in public periodic filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Zogenix undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement.
ZX008 Phase 3 Study 1 Top-Line Results Trial met primary endpoint of the change in the mean monthly convulsive seizure frequency Focused on Developing and Commercializing Therapies for Rare CNS Disorders All key secondary endpoints positive and demonstrated statistical significance
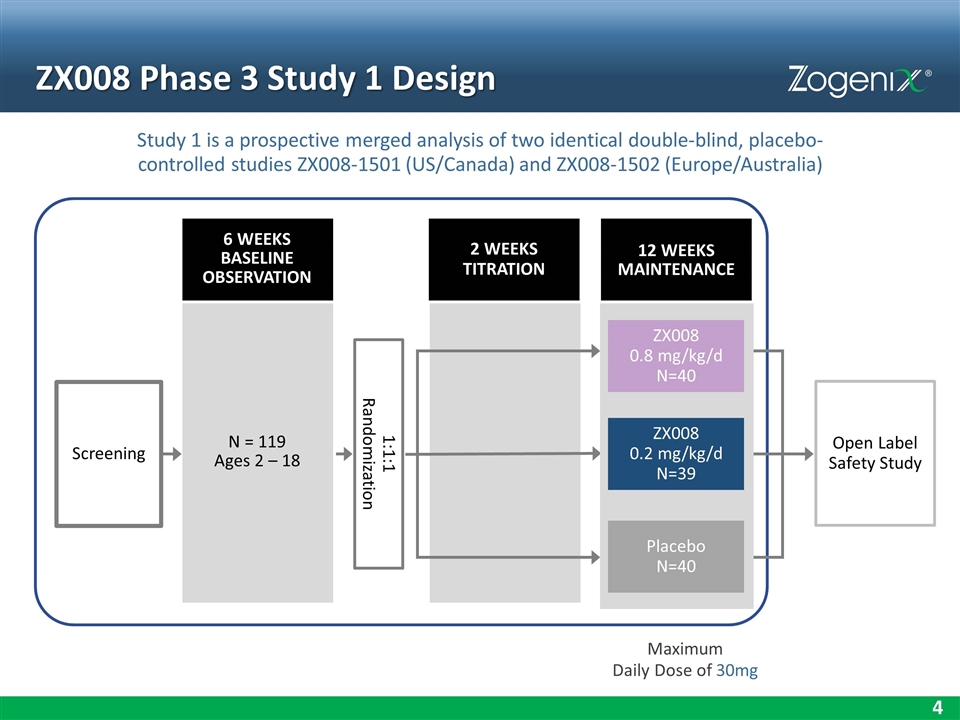
ZX008 Phase 3 Study 1 Design Maximum Daily Dose of 30mg Screening Open Label Safety Study N = 119 Ages 2 – 18 1:1:1 Randomization Placebo N=40 ZX008 0.8 mg/kg/d N=40 ZX008 0.2 mg/kg/d N=39 6 WEEKS BASELINE OBSERVATION 2 WEEKS TITRATION 12 WEEKS MAINTENANCE Study 1 is a prospective merged analysis of two identical double-blind, placebo-controlled studies ZX008-1501 (US/Canada) and ZX008-1502 (Europe/Australia)
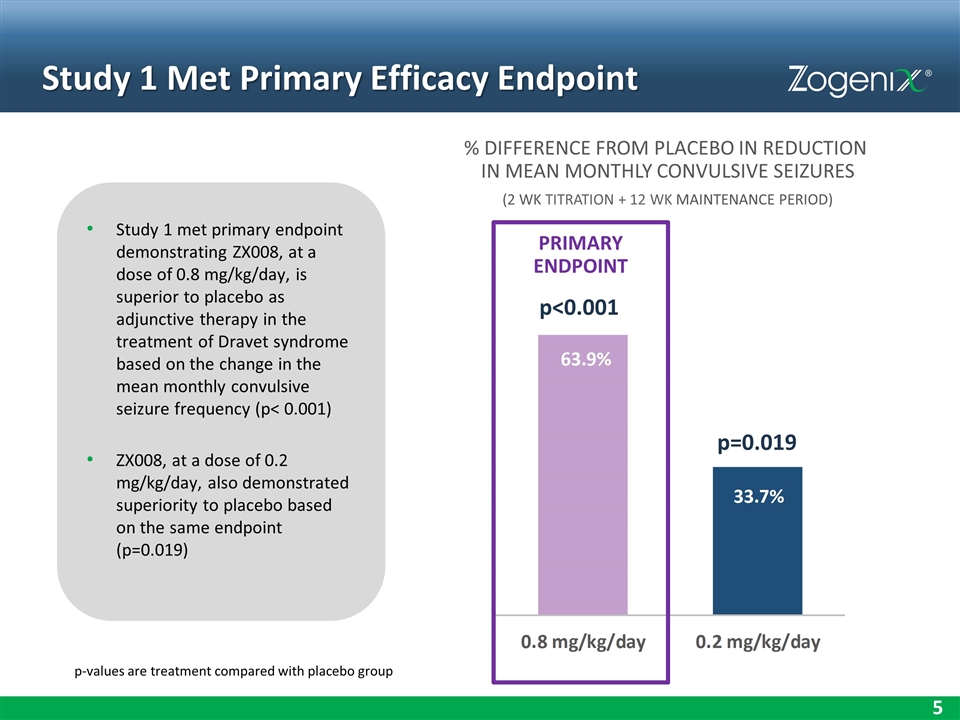
Study 1 Met Primary Efficacy Endpoint Study 1 met primary endpoint demonstrating ZX008, at a dose of 0.8 mg/kg/day, is superior to placebo as adjunctive therapy in the treatment of Dravet syndrome based on the change in the mean monthly convulsive seizure frequency (p< 0.001) ZX008, at a dose of 0.2 mg/kg/day, also demonstrated superiority to placebo based on the same endpoint (p=0.019) % DIFFERENCE FROM PLACEBO IN REDUCTION IN MEAN MONTHLY CONVULSIVE SEIZURES (2 WK TITRATION + 12 WK MAINTENANCE PERIOD) p=0.019 PRIMARY ENDPOINT p-values are treatment compared with placebo group p<0.001
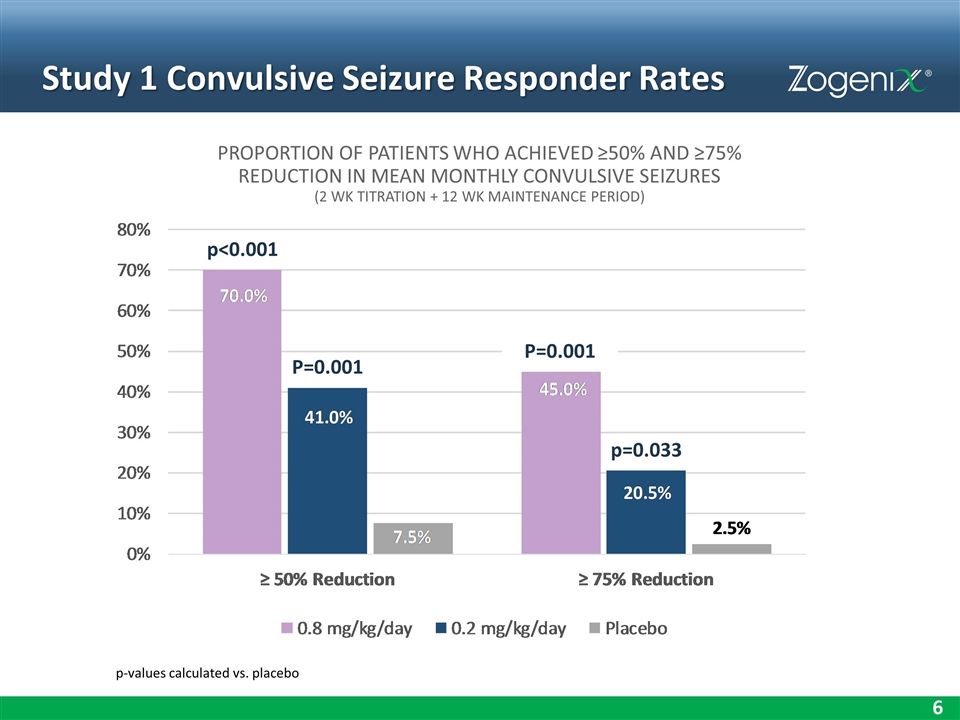
Study 1 Convulsive Seizure Responder Rates p-values calculated vs. placebo p=0.033 p<0.001 P=0.001 P=0.001 PROPORTION OF PATIENTS WHO ACHIEVED ≥50% AND ≥75% REDUCTION IN MEAN MONTHLY CONVULSIVE SEIZURES (2 WK TITRATION + 12 WK MAINTENANCE PERIOD)
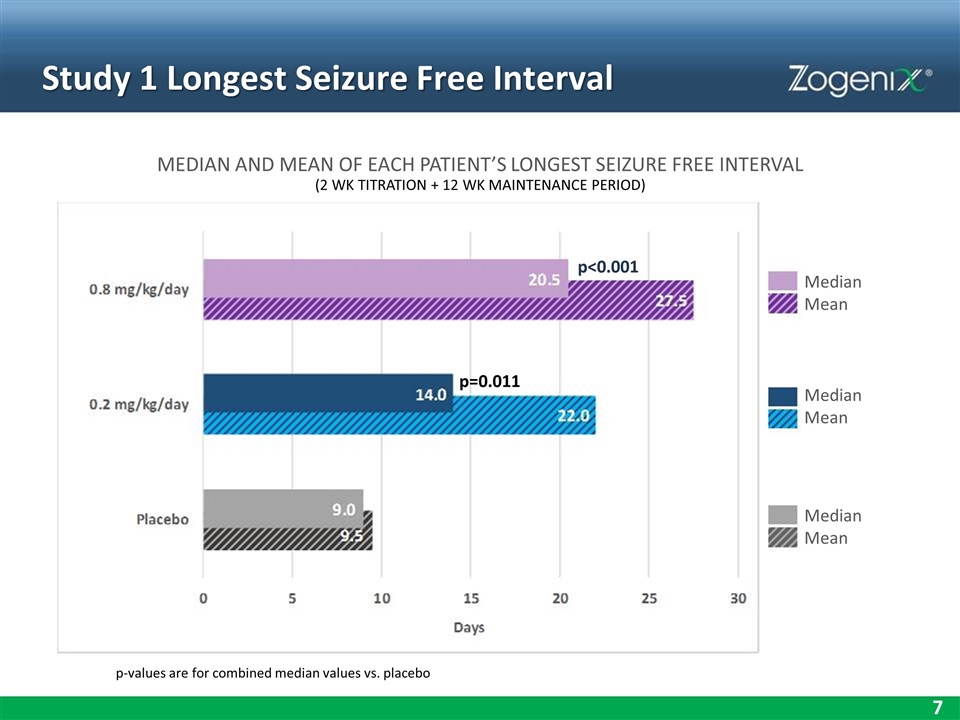
Study 1 Longest Seizure Free Interval MEDIAN AND MEAN OF EACH PATIENT’S LONGEST SEIZURE FREE INTERVAL (2 WK TITRATION + 12 WK MAINTENANCE PERIOD) p-values are for combined median values vs. placebo Median Mean Median Mean Median Mean p<0.001 p=0.011
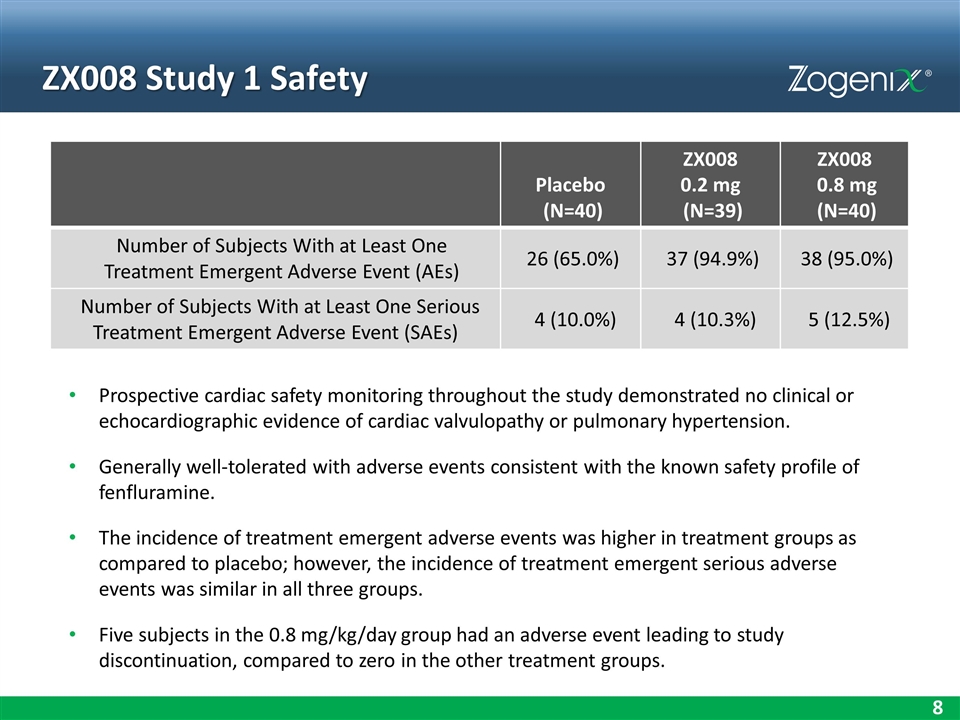
ZX008 Study 1 Safety Placebo (N=40) ZX008 0.2 mg (N=39) ZX008 0.8 mg (N=40) Number of Subjects With at Least One Treatment Emergent Adverse Event (AEs) 26 (65.0%) 37 (94.9%) 38 (95.0%) Number of Subjects With at Least One Serious Treatment Emergent Adverse Event (SAEs) 4 (10.0%) 4 (10.3%) 5 (12.5%) Prospective cardiac safety monitoring throughout the study demonstrated no clinical or echocardiographic evidence of cardiac valvulopathy or pulmonary hypertension. Generally well-tolerated with adverse events consistent with the known safety profile of fenfluramine. The incidence of treatment emergent adverse events was higher in treatment groups as compared to placebo; however, the incidence of treatment emergent serious adverse events was similar in all three groups. Five subjects in the 0.8 mg/kg/day group had an adverse event leading to study discontinuation, compared to zero in the other treatment groups.
ZX008 Phase 3 Study 1 Top-Line Results Trial met primary endpoint of the change in the mean monthly convulsive seizure frequency Focused on Developing and Commercializing Therapies for Rare CNS Disorders All key secondary endpoints positive and demonstrated statistical significance
