Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Blueprint Medicines Corp | bpmc-20170910ex992f48686.htm |
| EX-99.1 - EX-99.1 - Blueprint Medicines Corp | bpmc-20170910ex991574749.htm |
| 8-K - 8-K - Blueprint Medicines Corp | bpmc-20170910x8k.htm |
Exhibit 99.3
|
|
BLU-554 in FGFR4-driven Hepatocellular Carcinoma Monday, September 11, 2017 Clinical Development Program Update |
|
|
Conference call participants 2 Jeff Albers Chief Executive Officer, Blueprint Medicines Andy Boral, M.D. Chief Medical Officer, Blueprint Medicines Richard Kim, M.D. Associate Professor, Moffitt Cancer Center |
|
|
Forward-looking statements 3 This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. In this presentation, forward-looking statements include, without limitation, statements about plans and timelines for the clinical development of BLU-554 and the ability of Blueprint Medicines Corporation (the “Company”) to implement those clinical development plans; the potential benefits of BLU-554 in treating patients with hepatocellular carcinoma; the potential for fibroblast growth factor receptor 4 as a therapeutic target; plans and timelines for regulatory submissions, filings or discussions; expectations regarding potential milestones; and the Company’s strategy, business plans and focus. The Company has based these forward-looking statements on management’s current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks, uncertainties and other important factors, many of which are beyond the Company’s control and may cause actual results, performance or achievements to differ materially from those expressed or implied by any forward-looking statements. These risks and uncertainties include, without limitation, risks and uncertainties related to the delay of any current or future clinical trials or the development of the Company’s drug candidates, including BLU-285, BLU-554 and BLU-667; the Company's advancement of multiple early-stage efforts; the Company’s ability to successfully demonstrate the efficacy and safety of its drug candidates; the preclinical and clinical results for the Company’s drug candidates, which may not support further development of such drug candidates; actions or decisions of regulatory agencies or authorities, which may affect the initiation, timing and progress of current or future clinical trials; the Company’s ability to obtain, maintain and enforce patent and other intellectual property protection for any drug candidates it is developing; the Company’s ability to develop and commercialize companion diagnostics for its current and future drug candidates, including a companion diagnostic for BLU-554 with Ventana Medical Systems, Inc. and a companion diagnostic for BLU-285 with QIAGEN Manchester Limited; and the success of the Company’s cancer immunotherapy collaboration with F. Hoffmann-La Roche Ltd and Hoffmann-La Roche Inc. These and other risks and uncertainties are described in greater detail under “Risk Factors” in the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2017, as filed with the Securities and Exchange Commission (“SEC”) on August 2, 2017, and any other filings the Company may make with the SEC in the future. The Company cannot guarantee future results, outcomes, levels of activity, performance, developments, or achievements, and there can be no assurance that the Company’s expectations, intentions, anticipations, beliefs, or projections will result or be achieved or accomplished. The forward-looking statements in this presentation are made only as of the date hereof, and except as required by law, the Company undertakes no obligation to update any forward-looking statements contained in this presentation as a result of new information, future events or otherwise. This presentation also contains estimates, projections and other statistical data made by independent parties and by the Company relating to market size and growth and other data about the Company’s industry. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of the Company’s future performance and the future performance of the markets in which the Company operates are necessarily subject to a high degree of uncertainty and risk. |
|
|
Jeff Albers Chief Executive Officer Blueprint Medicines |
|
|
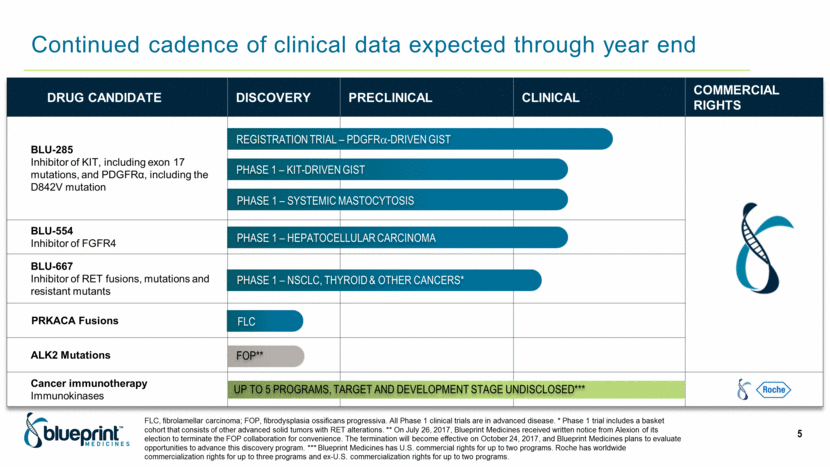
Drug candidate Discovery Preclinical Clinical Commercial rights BLU-285 Inhibitor of KIT, including exon 17 mutations, and PDGFRα, including the D842V mutation BLU-554 Inhibitor of FGFR4 BLU-667 Inhibitor of RET fusions, mutations and resistant mutants PRKACA Fusions ALK2 Mutations Cancer immunotherapy Immunokinases PHASE 1 – KIT-DRIVEN GIST REGISTRATION TRIAL – PDGFR-DRIVEN GIST PHASE 1 – SYSTEMIC MASTOCYTOSIS PHASE 1 – HEPATOCELLULAR CARCINOMA PHASE 1 – NSCLC, THYROID & OTHER CANCERS* Up to 5 programs, TARGET AND DEVELOPMENT STAGE UNDISCLOSED*** FLC FLC, fibrolamellar carcinoma; FOP, fibrodysplasia ossificans progressiva. All Phase 1 clinical trials are in advanced disease. * Phase 1 trial includes a basket cohort that consists of other advanced solid tumors with RET alterations. ** On July 26, 2017, Blueprint Medicines received written notice from Alexion of its election to terminate the FOP collaboration for convenience. The termination will become effective on October 24, 2017, and Blueprint Medicines plans to evaluate opportunities to advance this discovery program. *** Blueprint Medicines has U.S. commercial rights for up to two programs. Roche has worldwide commercialization rights for up to three programs and ex-U.S. commercialization rights for up to two programs. FOP** Continued cadence of clinical data expected through year end 5 |
|
|
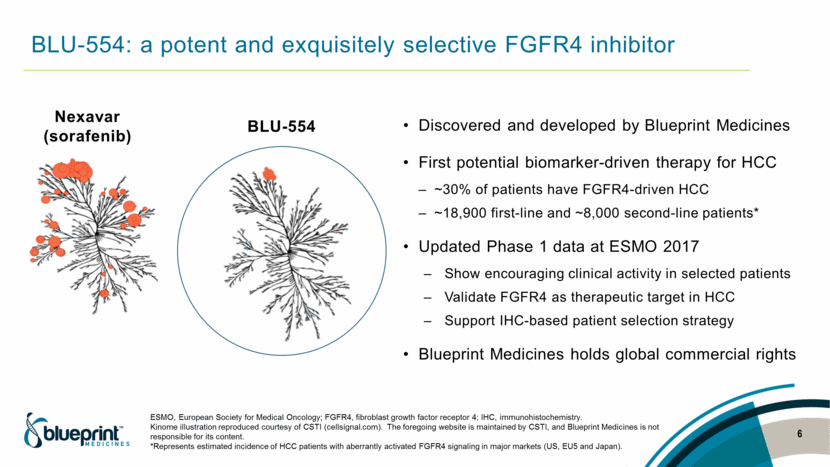
BLU-554: a potent and exquisitely selective FGFR4 inhibitor Discovered and developed by Blueprint Medicines First potential biomarker-driven therapy for HCC ~30% of patients have FGFR4-driven HCC ~18,900 first-line and ~8,000 second-line patients* Updated Phase 1 data at ESMO 2017 Show encouraging clinical activity in selected patients Validate FGFR4 as therapeutic target in HCC Support IHC-based patient selection strategy Blueprint Medicines holds global commercial rights BLU-554 Nexavar (sorafenib) ESMO, European Society for Medical Oncology; FGFR4, fibroblast growth factor receptor 4; IHC, immunohistochemistry. Kinome illustration reproduced courtesy of CSTI (cellsignal.com). The foregoing website is maintained by CSTI, and Blueprint Medicines is not responsible for its content. *Represents estimated incidence of HCC patients with aberrantly activated FGFR4 signaling in major markets (US, EU5 and Japan). 6 |
|
|
Phase 1 safety and clinical activity of BLU-554 in advanced hepatocellular carcinoma Richard Kim1, Debashis Sarker2, Teresa Macarulla3, Thomas Yau4, Su Pin Choo5, Tim Meyer6, Antoine Hollebecque7, Jonathan Whisenant8, Max Sung9, Jung-Hwan Yoon10, Ho Yeong Lim11, Andrew Zhu12, Joong-Won Park13, Sandrine Faivre14, Vincenzo Mazzaferro15, Hongliang Shi16, Terri Alvarez-Diaz16, Oleg Schmidt-Kittler16, Corinne Clifford16, Beni Wolf16, Yoon-Koo Kang17 1Gastrointestinal Oncology, Moffitt Cancer Center, Tampa, United States, 2Early Phase Trials Unit, Guy’s Hospital, London, United Kingdom,3Medical Oncology, Vall d’Hebron University Hospital, Barcelona, Spain, 4Department of Medicine, Queen Mary Hospital, Hong Kong, Hong Kong, 5Medical Oncology, National Cancer Centre, Singapore, Singapore, 6Oncology, UCL Cancer Institute, London, United Kingdom, 7Oncology, Institut Gustave Roussy, Villejuif, France, 8Internal Medicine, Huntsman Cancer Institute, Salt Lake City, United States, 9Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, United States, 10Oncology, Seoul National University Hospital, Seoul, Republic of Korea, 11Department of Medicine, Divisions of Hematology-Oncology, Samsung Medical Center, Seoul, Republic of Korea, 12Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, United States, 13Center for Liver Cancer, National Cancer Center, Goyang, Republic of Korea, 14Oncology, Beaujon University Hospital, Clichy, France, 15Department of Surgery, Liver Transplantation and Gastroenterology, Fondazione Istituto Nazionale Tumori (National Cancer Institute) IRCCS, Milan, Italy, 16Clinical Development, Blueprint Medicines Corporation, Cambridge, United States, 17Oncology, Asan Medical Center, Seoul, Republic of Korea 7 |
|
|
BLU-554 is an investigational agent currently in development by Blueprint Medicines Corporation (Blueprint Medicines) Dr Richard Kim is an investigator for Blueprint Medicines’ ongoing Phase 1 studies in advanced HCC Dr Richard Kim has the following disclosures: Research: Blueprint Medicines, Bayer, BMS and Eisai Consultant: Lilly, BMS, Eisai, Bayer Speaker: Lilly Disclosures 8 HCC, hepatocellular carcinoma |
|
|
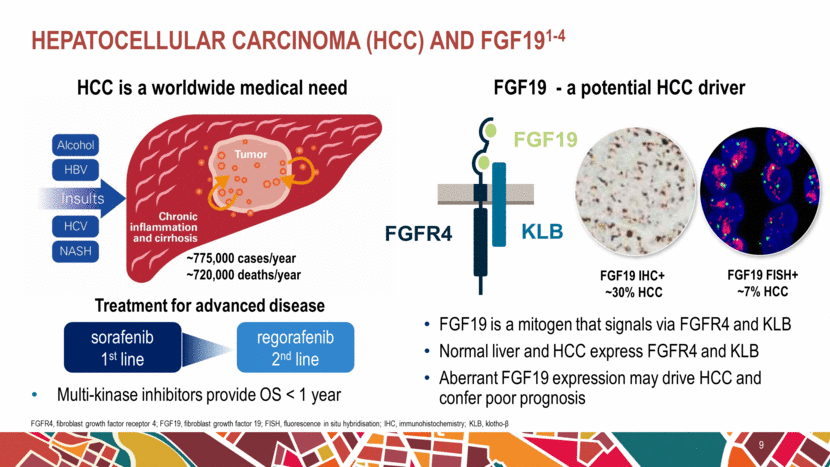
Hepatocellular Carcinoma (HCC) and FGF191-4 9 FGFR4, fibroblast growth factor receptor 4; FGF19, fibroblast growth factor 19; FISH, fluorescence in situ hybridisation; IHC, immunohistochemistry; KLB, klotho-β Multi-kinase inhibitors provide OS < 1 year FGF19 - a potential HCC driver FGF19 is a mitogen that signals via FGFR4 and KLB Normal liver and HCC express FGFR4 and KLB Aberrant FGF19 expression may drive HCC and confer poor prognosis Treatment for advanced disease sorafenib 1st line regorafenib 2nd line ~775,000 cases/year ~720,000 deaths/year HCC is a worldwide medical need FGF19 IHC+ ~30% HCC FGF19 FISH+ ~7% HCC FGFR4 KLB FGF19 |
|
|
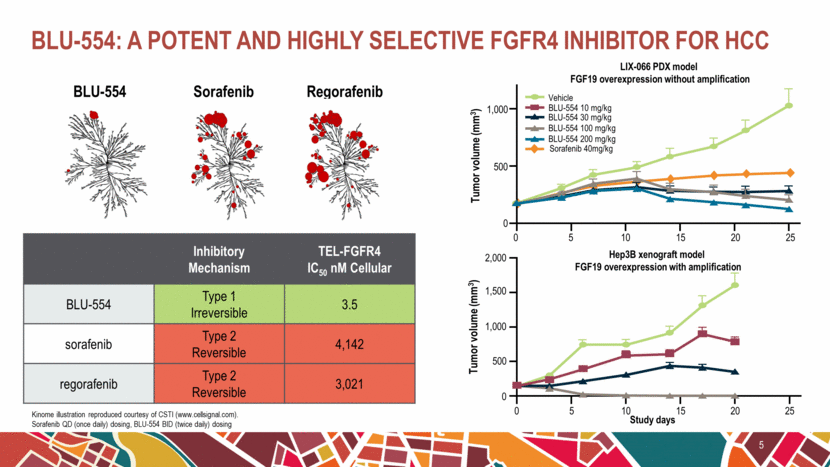
BLU-554: a potent and highly selective FGFR4 inhibitor for HCC 5 BLU-554 Sorafenib Regorafenib Inhibitory Mechanism TEL-FGFR4 IC50 nM Cellular BLU-554 Type 1 Irreversible 3.5 sorafenib Type 2 Reversible 4,142 regorafenib Type 2 Reversible 3,021 LIX-066 PDX model FGF19 overexpression without amplification 0 Tumor volume (mm3) Hep3B xenograft model FGF19 overexpression with amplification Study days 2,000 1,500 1,000 500 0 5 Tumor volume (mm3) 0 10 15 20 25 5 0 10 15 20 25 1,000 500 Vehicle BLU-554 30 mg/kg BLU-554 100 mg/kg BLU-554 200 mg/kg BLU-554 10 mg/kg Sorafenib 40mg/kg Kinome illustration reproduced courtesy of CSTI (www.cellsignal.com). Sorafenib QD (once daily) dosing, BLU-554 BID (twice daily) dosing |
|
|
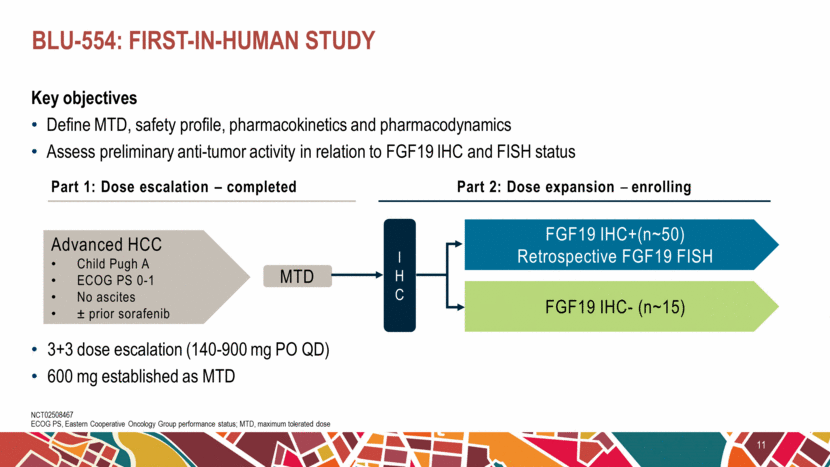
Key objectives Define MTD, safety profile, pharmacokinetics and pharmacodynamics Assess preliminary anti-tumor activity in relation to FGF19 IHC and FISH status BLU-554: first-in-human study 11 NCT02508467 ECOG PS, Eastern Cooperative Oncology Group performance status; MTD, maximum tolerated dose Part 1: Dose escalation – completed Part 2: Dose expansion – enrolling Advanced HCC Child Pugh A ECOG PS 0-1 No ascites ± prior sorafenib MTD 3+3 dose escalation (140-900 mg PO QD) 600 mg established as MTD IHC FGF19 IHC- (n~15) FGF19 IHC+(n~50) Retrospective FGF19 FISH |
|
|
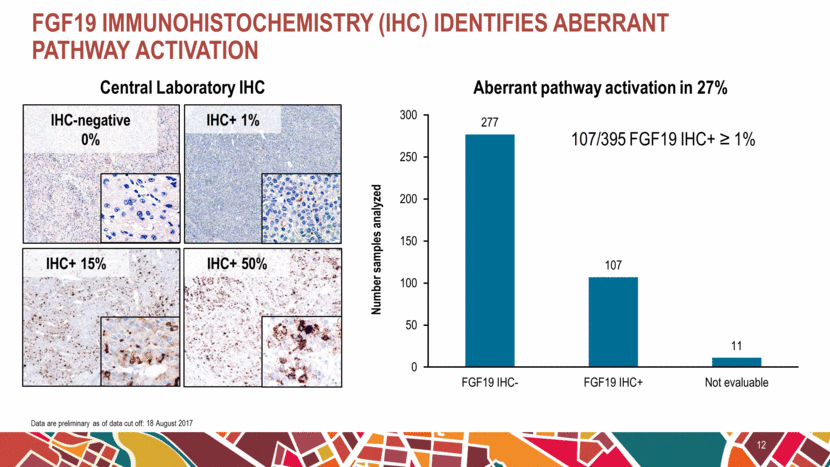
FGF19 immunohistochemistry (IHC) identifies aberrant pathway activation 12 107/395 FGF19 IHC+ ≥ 1% Aberrant pathway activation in 27% Central Laboratory IHC IHC-negative 0% IHC+ 1% IHC+ 15% IHC+ 50% Data are preliminary as of data cut off: 18 August 2017 277 107 11 0 50 100 150 200 250 300 FGF19 IHC- FGF19 IHC+ Not evaluable Number samples analyzed |
|
|
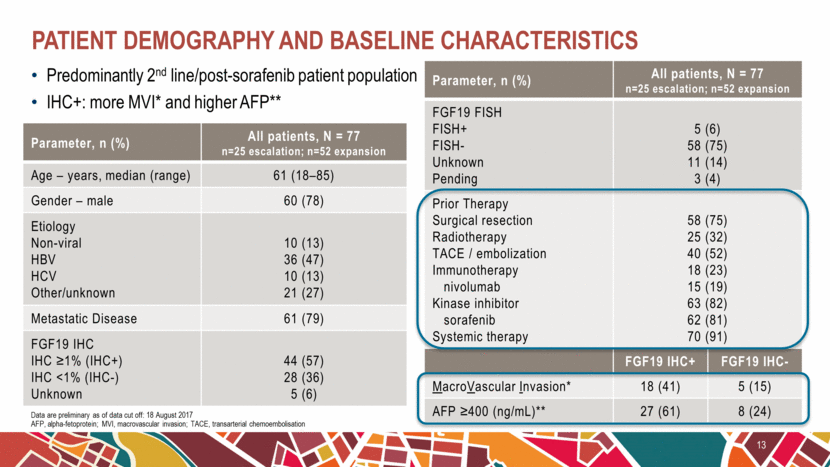
Predominantly 2nd line/post-sorafenib patient population IHC+: more MVI* and higher AFP** Patient demography and baseline characteristics 13 Data are preliminary as of data cut off: 18 August 2017 AFP, alpha-fetoprotein; MVI, macrovascular invasion; TACE, transarterial chemoembolisation Parameter, n (%) All patients, N = 77 n=25 escalation; n=52 expansion Age – years, median (range) 61 (18–85) Gender – male 60 (78) Etiology Non-viral HBV HCV Other/unknown 10 (13) 36 (47) 10 (13) 21 (27) Metastatic Disease 61 (79) FGF19 IHC IHC ≥1% (IHC+) IHC <1% (IHC-) Unknown 44 (57) 28 (36) 5 (6) Parameter, n (%) All patients, N = 77 n=25 escalation; n=52 expansion FGF19 FISH FISH+ FISH- Unknown Pending 5 (6) 58 (75) 11 (14) 3 (4) Prior Therapy Surgical resection Radiotherapy TACE / embolization Immunotherapy nivolumab Kinase inhibitor sorafenib Systemic therapy 58 (75) 25 (32) 40 (52) 18 (23) 15 (19) 63 (82) 62 (81) 70 (91) FGF19 IHC+ FGF19 IHC- MacroVascular Invasion* 18 (41) 5 (15) AFP ≥400 (ng/mL)** 27 (61) 8 (24) |
|
|
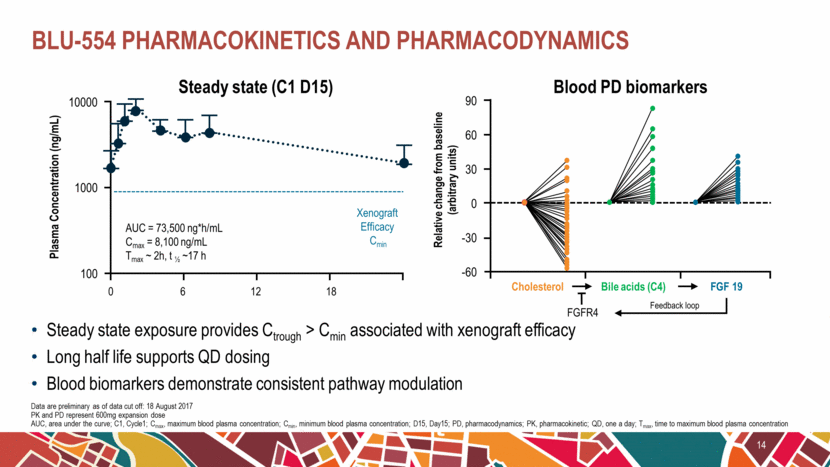
Steady state exposure provides Ctrough > Cmin associated with xenograft efficacy Long half life supports QD dosing Blood biomarkers demonstrate consistent pathway modulation BLU-554 pharmacokinetics and pharmacodynamics 14 Data are preliminary as of data cut off: 18 August 2017 PK and PD represent 600mg expansion dose AUC, area under the curve; C1, Cycle1; Cmax, maximum blood plasma concentration; Cmin, minimum blood plasma concentration; D15, Day15; PD, pharmacodynamics; PK, pharmacokinetic; QD, one a day; Tmax, time to maximum blood plasma concentration Steady state (C1 D15) Blood PD biomarkers Plasma Concentration (ng/mL) AUC = 73,500 ng*h/mL Cmax = 8,100 ng/mL Tmax ~ 2h, t ½ ~17 h 10000 1000 100 18 12 6 0 FGFR4 Relative change from baseline (arbitrary units) Cholesterol 90 60 30 0 -30 -60 Bile acids (C4) FGF 19 Feedback loop Xenograft Efficacy Cmin |
|
|
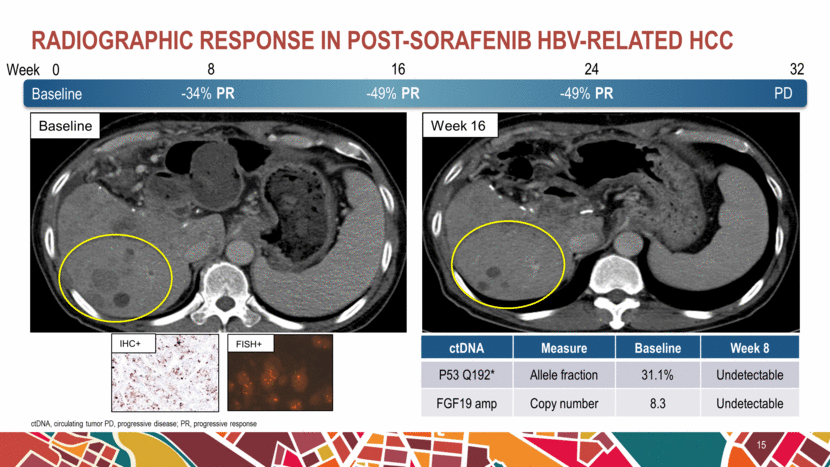
Radiographic response in post-sorafenib HBV-related HCC 15 ctDNA, circulating tumor PD, progressive disease; PR, progressive response 0 8 16 24 32 -34% PR -49% PR -49% PR PD Week Baseline Week 16 Baseline ctDNA Measure Baseline Week 8 P53 Q192* Allele fraction 31.1% Undetectable FGF19 amp Copy number 8.3 Undetectable IHC+ FISH+ |
|
|
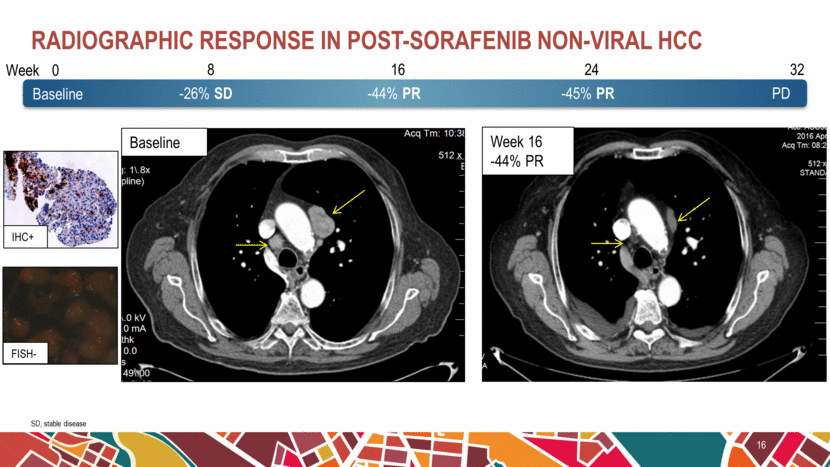
Radiographic response in post-sorafenib non-viral HCC 16 SD, stable disease 0 8 16 24 32 -26% SD -44% PR -45% PR PD Week Baseline IHC+ Week 16 -44% PR Baseline FISH- |
|
|
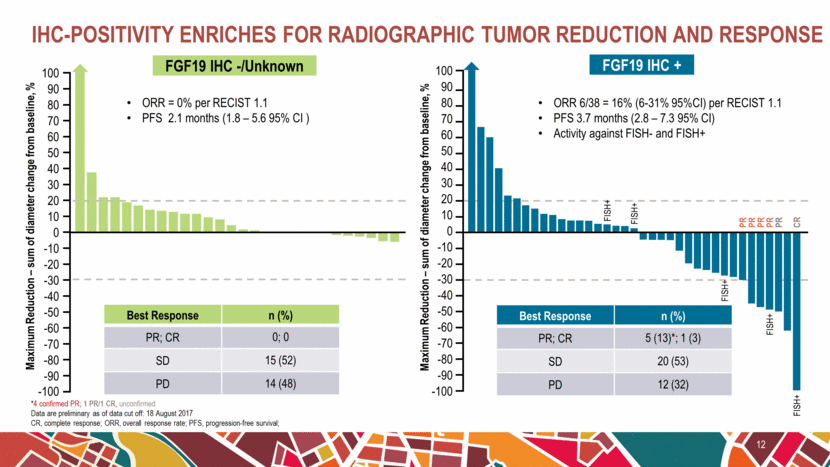
IHC-positivity enriches for radiographic tumor reduction and response 12 *4 confirmed PR; 1 PR/1 CR, unconfirmed Data are preliminary as of data cut off: 18 August 2017 CR, complete response; ORR, overall response rate; PFS, progression-free survival; Best Response n (%) PR; CR 5 (13)*; 1 (3) SD 20 (53) PD 12 (32) Best Response n (%) PR; CR 0; 0 SD 15 (52) PD 14 (48) ORR 6/38 = 16% (6-31% 95%CI) per RECIST 1.1 PFS 3.7 months (2.8 – 7.3 95% CI) Activity against FISH- and FISH+ 100 90 80 70 60 50 40 30 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 Maximum Reduction – sum of diameter change from baseline, % ORR = 0% per RECIST 1.1 PFS 2.1 months (1.8 – 5.6 95% CI ) Maximum Reduction – sum of diameter change from baseline, % FISH+ FISH+ FISH+ FISH+ PR PR PR PR CR FISH+ PR 100 90 80 70 60 50 40 30 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 FGF19 IHC -/Unknown FGF19 IHC + |
|
|
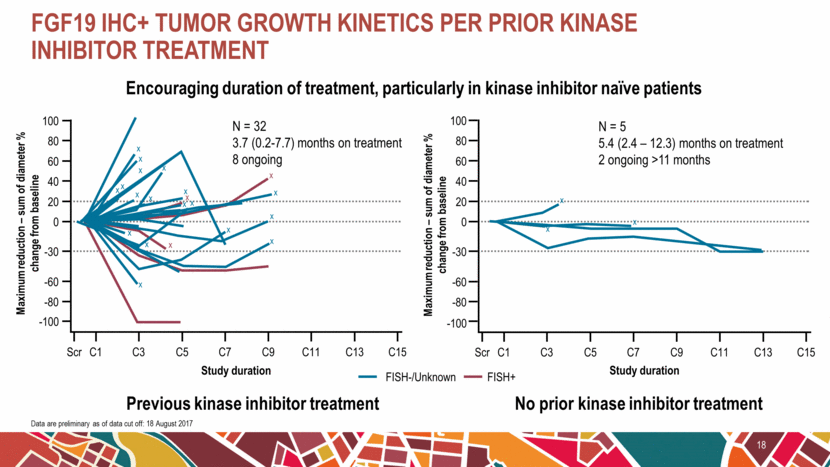
FGF19 IHC+ tumor growth kinetics per prior kinase inhibitor treatment 18 Data are preliminary as of data cut off: 18 August 2017 Encouraging duration of treatment, particularly in kinase inhibitor naïve patients Previous kinase inhibitor treatment Maximum reduction – sum of diameter % change from baseline No prior kinase inhibitor treatment Maximum reduction – sum of diameter % change from baseline Study duration FISH-/Unknown FISH+ Study duration Scr C1 -100 100 80 60 40 20 0 -80 -60 -30 C3 C5 C7 C9 C11 C13 C15 x x x x x x x x x x x x x x x x x x x x x -100 Scr C1 100 80 60 40 20 0 -80 -60 -30 C3 C5 C7 C9 C11 C13 C15 x x x N = 5 5.4 (2.4 – 12.3) months on treatment 2 ongoing >11 months N = 32 3.7 (0.2-7.7) months on treatment 8 ongoing |
|
|
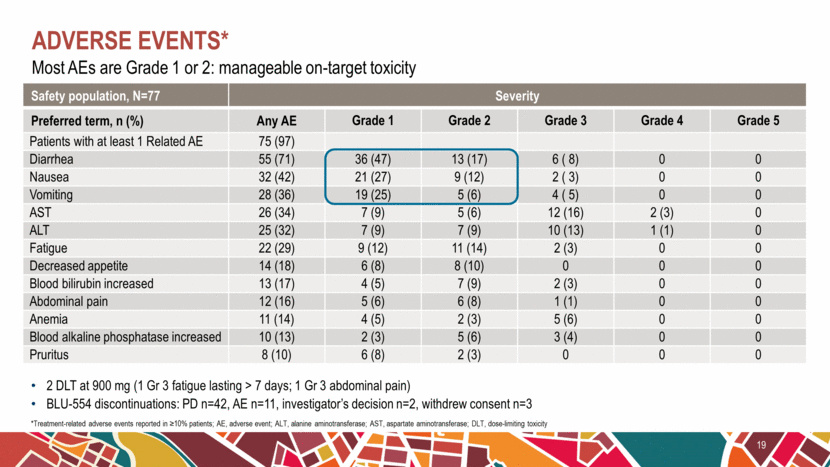
Most AEs are Grade 1 or 2: manageable on-target toxicity Adverse events* 19 *Treatment-related adverse events reported in ≥10% patients; AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DLT, dose-limiting toxicity 2 DLT at 900 mg (1 Gr 3 fatigue lasting > 7 days; 1 Gr 3 abdominal pain) BLU-554 discontinuations: PD n=42, AE n=11, investigator’s decision n=2, withdrew consent n=3 Safety population, N=77 Severity Preferred term, n (%) Any AE Grade 1 Grade 2 Grade 3 Grade 4 Grade 5 Patients with at least 1 Related AE 75 (97) Diarrhea 55 (71) 36 (47) 13 (17) 6 ( 8) 0 0 Nausea 32 (42) 21 (27) 9 (12) 2 ( 3) 0 0 Vomiting 28 (36) 19 (25) 5 (6) 4 ( 5) 0 0 AST 26 (34) 7 (9) 5 (6) 12 (16) 2 (3) 0 ALT 25 (32) 7 (9) 7 (9) 10 (13) 1 (1) 0 Fatigue 22 (29) 9 (12) 11 (14) 2 (3) 0 0 Decreased appetite 14 (18) 6 (8) 8 (10) 0 0 0 Blood bilirubin increased 13 (17) 4 (5) 7 (9) 2 (3) 0 0 Abdominal pain 12 (16) 5 (6) 6 (8) 1 (1) 0 0 Anemia 11 (14) 4 (5) 2 (3) 5 (6) 0 0 Blood alkaline phosphatase increased 10 (13) 2 (3) 5 (6) 3 (4) 0 0 Pruritus 8 (10) 6 (8) 2 (3) 0 0 0 |
|
|
BLU-554 provides acceptable tolerability, pathway engagement and anti-tumor activity in heavily pre-treated FGF19 IHC+ patients Aberrant pathway activation (FGF19 IHC+) demonstrated in ~30% of HCC patients BLU-554 demonstrates clinical activity regardless of HCC etiology and prognostic factors These data validate FGFR4 as a therapeutic target and FGF19 IHC as selection marker for pathway activation in advanced HCC Planning is underway for further clinical development of BLU-554 in kinase inhibitor naïve, FGF19 IHC+ HCC alone and in combination with immunotherapy Conclusions 20 |
|
|
We thank the participating patients, their families, all study co-investigators, and research co-ordinators at the following institutions: Acknowledgements 21 We also thank Samantha Clark, BSc, of iMed Comms, an Ashfield company, who provided editorial writing support funded by Blueprint Medicines Moffitt Cancer Center, Tampa, United States Guy’s Hospital, London, United Kingdom, Vall d’Hebron University Hospital, Barcelona, Spain Queen Mary Hospital, HongKong, Hong Kong National Cancer Center, Singapore, Singapore UCL Cancer Institute, London, United Kingdom Institut Gustave Roussy, Villejuif, France Huntsman Cancer Institute, Salt Lake City, United States Asan Medical Center, Seoul, Republic of Korea Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, United States Seoul National University Hospital, Seoul, Republic of Korea Samsung Medical Center, Seoul, Republic of Korea Massachusetts General Hospital Cancer Center, Boston, United States Center for Liver Cancer, National Cancer Center, Goyang, Republic of Korea Beaujon University Hospital, Clichy, France Fondazione Istituto Nazionale Tumori (National Cancer Institute) IRCCS, Milan, Italy |
|
|
Llovet JM et al (2016) Nature Reviews Disease Primers 2: 1–23 Miura S et al (2012) BMC Cancer 12:56 Hyeon J et al (2013) Dig Dis Sci 58:1916-1922 Schultze et al. (2015)Nature Genetics 47:505–511 References 22 |
|
|
Andy Boral, M.D. Chief Medical Officer Blueprint Medicines |
|
|
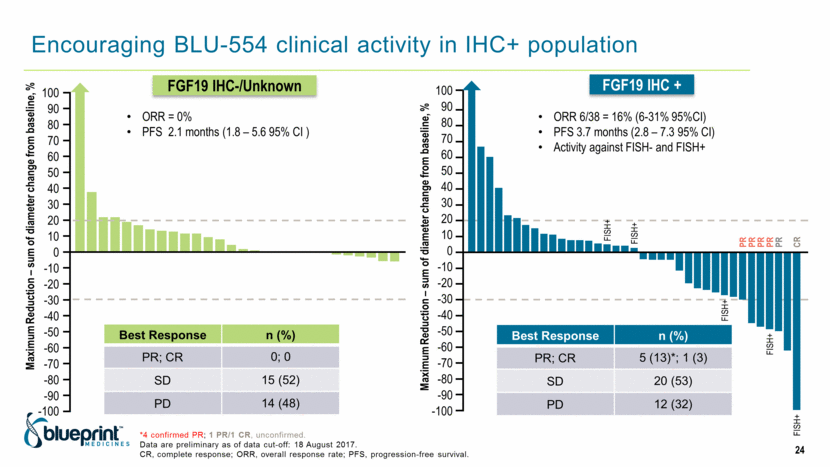
Encouraging BLU-554 clinical activity in IHC+ population *4 confirmed PR; 1 PR/1 CR, unconfirmed. Data are preliminary as of data cut-off: 18 August 2017. CR, complete response; ORR, overall response rate; PFS, progression-free survival. Best Response n (%) PR; CR 5 (13)*; 1 (3) SD 20 (53) PD 12 (32) Best Response n (%) PR; CR 0; 0 SD 15 (52) PD 14 (48) ORR 6/38 = 16% (6-31% 95%CI) PFS 3.7 months (2.8 – 7.3 95% CI) Activity against FISH- and FISH+ 100 90 80 70 60 50 40 30 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 Maximum Reduction – sum of diameter change from baseline, % ORR = 0% PFS 2.1 months (1.8 – 5.6 95% CI ) Maximum Reduction – sum of diameter change from baseline, % FISH+ FISH+ FISH+ FISH+ PR PR PR PR CR FISH+ PR 100 90 80 70 60 50 40 30 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 FGF19 IHC-/Unknown FGF19 IHC + 24 |
|
|
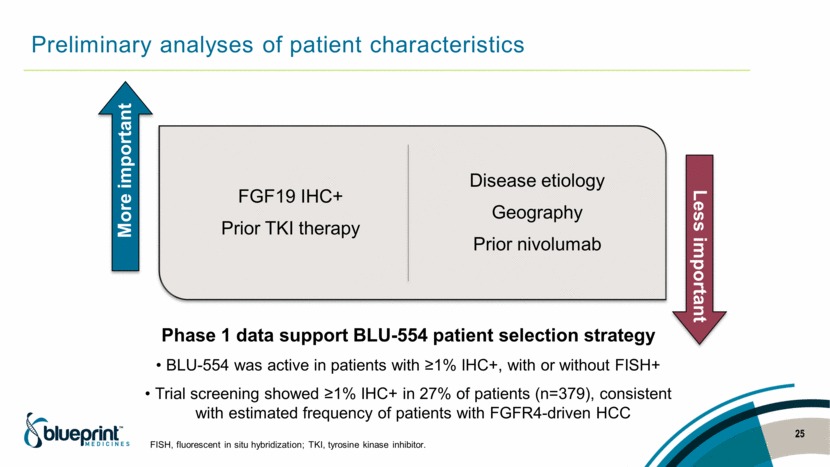
Preliminary analyses of patient characteristics 25 Phase 1 data support BLU-554 patient selection strategy BLU-554 was active in patients with ≥1% IHC+, with or without FISH+ Trial screening showed ≥1% IHC+ in 27% of patients (n=379), consistent with estimated frequency of patients with FGFR4-driven HCC FISH, fluorescent in situ hybridization; TKI, tyrosine kinase inhibitor. FGF19 IHC+ Prior TKI therapy Disease etiology Geography Prior nivolumab More important Less important |
|
|
Opportunities for BLU-554 in the evolving HCC landscape 26 Sorafenib Regorafenib 1L 2L+ Current paradigm |
|
|
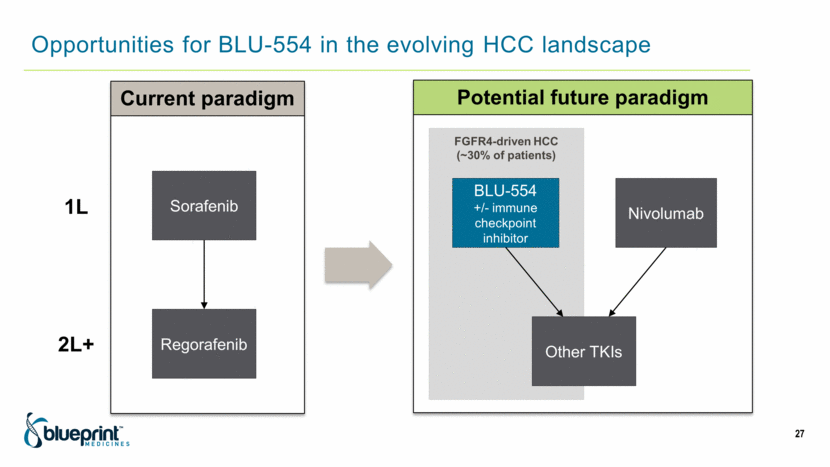
Opportunities for BLU-554 in the evolving HCC landscape 27 Sorafenib Regorafenib 1L 2L+ Current paradigm Potential future paradigm Nivolumab BLU-554 +/- immune checkpoint inhibitor Other TKIs FGFR4-driven HCC (~30% of patients) |
|
|
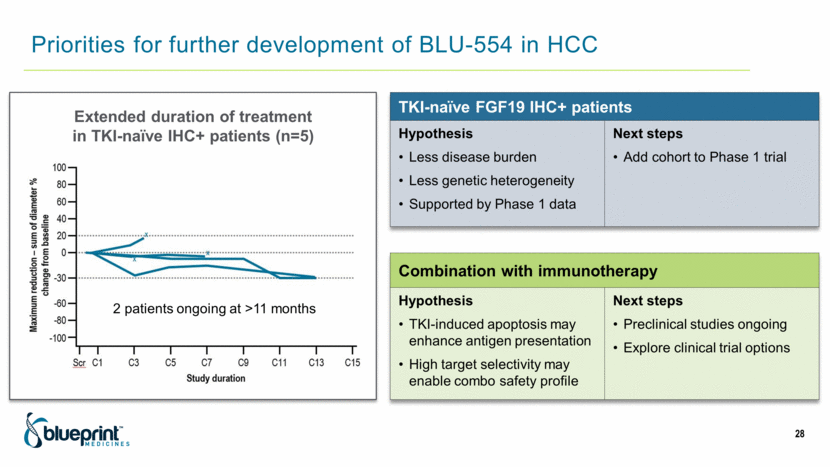
Priorities for further development of BLU-554 in HCC 28 TKI-naïve FGF19 IHC+ patients Hypothesis Less disease burden Less genetic heterogeneity Supported by Phase 1 data Next steps Add cohort to Phase 1 trial Combination with immunotherapy Hypothesis TKI-induced apoptosis may enhance antigen presentation High target selectivity may enable combo safety profile Next steps Preclinical studies ongoing Explore clinical trial options Extended duration of treatment in TKI-naïve IHC+ patients (n=5) 2 patients ongoing at >11 months |
|
|
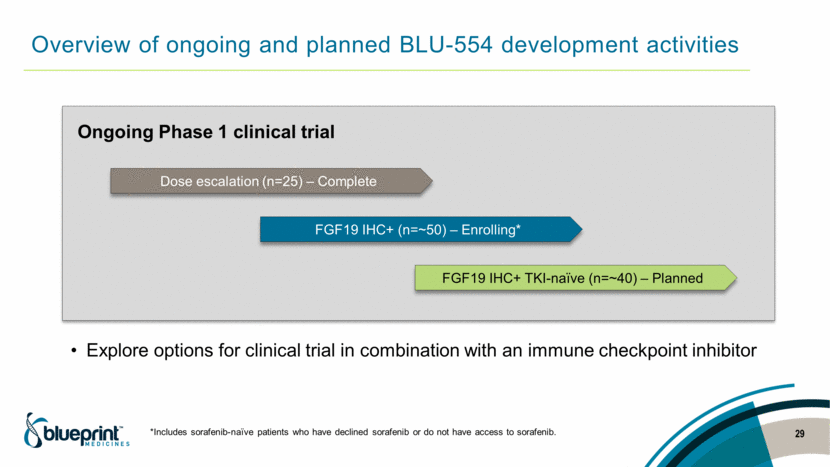
Overview of ongoing and planned BLU-554 development activities *Includes sorafenib-naïve patients who have declined sorafenib or do not have access to sorafenib. 29 Dose escalation (n=25) – Complete FGF19 IHC+ (n=~50) – Enrolling* FGF19 IHC+ TKI-naïve (n=~40) – Planned Ongoing Phase 1 clinical trial Explore options for clinical trial in combination with an immune checkpoint inhibitor |
|
|
Question & Answer |
|
|
Thank you |