Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Fortress Biotech, Inc. | v472763_ex99-1.htm |
| 8-K - FORM 8-K - Fortress Biotech, Inc. | v472763_8k.htm |
Exhibit 99.2

Non - Confidential Materials Corporate Presentation August 2017

Non - Confidential Materials Forward Looking Statements This presentation may contain “forward - looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements include, but are not limited to, any statements relating to our growth strategy and product development programs and any other statements that are not historical facts. Forward - looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price. Factors that could cause actual results to differ materially from those currently anticipated include: risks related to our growth strategy; risks relating to the results of research and development activities; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; uncertainties relating to preclinical and clinical testing; our dependence on third party suppliers; our ability to attract, integrate, and retain key personnel; the early stage of products under development; our need for and continued access to additional funds; government regulation; patent and intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as may be required by law. 2

Non - Confidential Materials Fortress Biotech: Our Unique Approach What we do: Acquire, develop and commercialize novel biopharmaceutical products in all stages of development and across multiple therapeutic areas directly within Fortress Biotech and through our subsidiaries. Our business strategy: Build subsidiaries around marketed products and product candidates that create a pipeline providing our shareholders with a diversified long - term revenue stream. Program candidates 4 17 Marketed Clinical Development 15 Pre - clinical 3
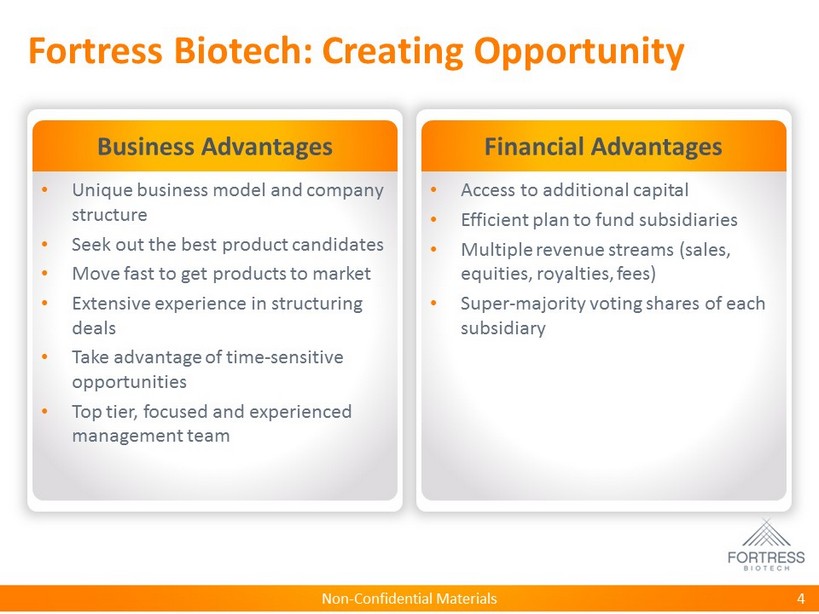
Non - Confidential Materials Fortress Biotech: Creating Opportunity Business Advantages • Unique business model and company structure • Seek out the best product candidates • Move fast to get products to market • Extensive experience in structuring deals • Take advantage of time - sensitive opportunities • Top tier, focused and experienced management team Financial Advantages • Access to additional capital • Efficient plan to fund subsidiaries • Multiple revenue streams (sales, equities, royalties, fees) • Super - majority voting shares of each subsidiary 4
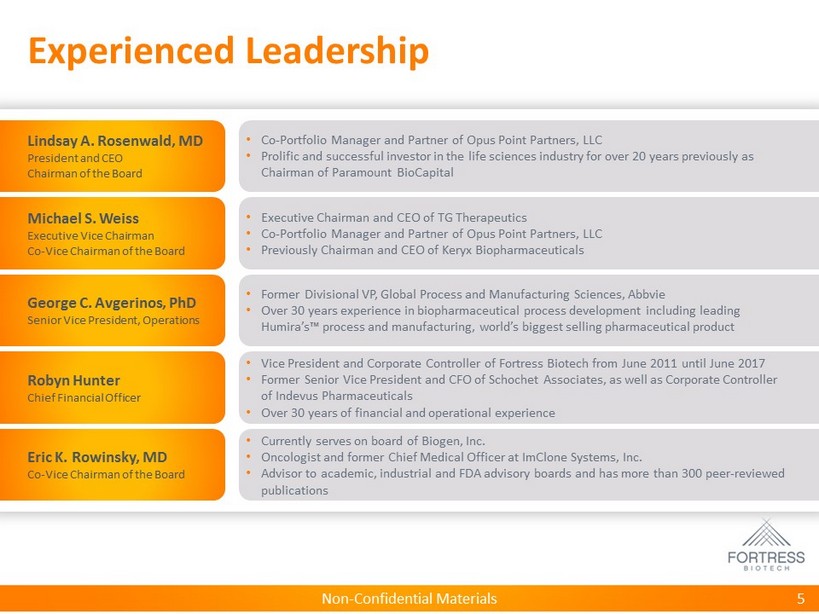
Non - Confidential Materials Experienced Leadership 5 • Co - Portfolio Manager and Partner of Opus Point Partners, LLC • Prolific and successful investor in the life sciences industry for over 20 years previously as Chairman of Paramount BioCapital Lindsay A. Rosenwald, MD President and CEO Chairman of the Board • Executive Chairman and CEO of TG Therapeutics • Co - Portfolio Manager and Partner of Opus Point Partners, LLC • Previously Chairman and CEO of Keryx Biopharmaceuticals Michael S. Weiss Executive Vice Chairman Co - Vice Chairman of the Board • Currently serves on board of Biogen, Inc. • Oncologist and former Chief Medical Officer at ImClone Systems, Inc. • Advisor to academic, industrial and FDA advisory boards and has more than 300 peer - reviewed publications Eric K. Rowinsky, MD Co - Vice Chairman of the Board • Vice President and Corporate Controller of Fortress Biotech from June 2011 until June 2017 • Former Senior Vice President and CFO of Schochet Associates, as well as Corporate Controller of Indevus Pharmaceuticals • Over 30 years of financial and operational experience Robyn Hunter Chief Financial Officer • Former Divisional VP, Global Process and Manufacturing Sciences, Abbvie • Over 30 years experience in biopharmaceutical process development including leading Humira’s™ process and manufacturing, world’s biggest selling pharmaceutical product George C. Avgerinos, PhD Senior Vice President, Operations
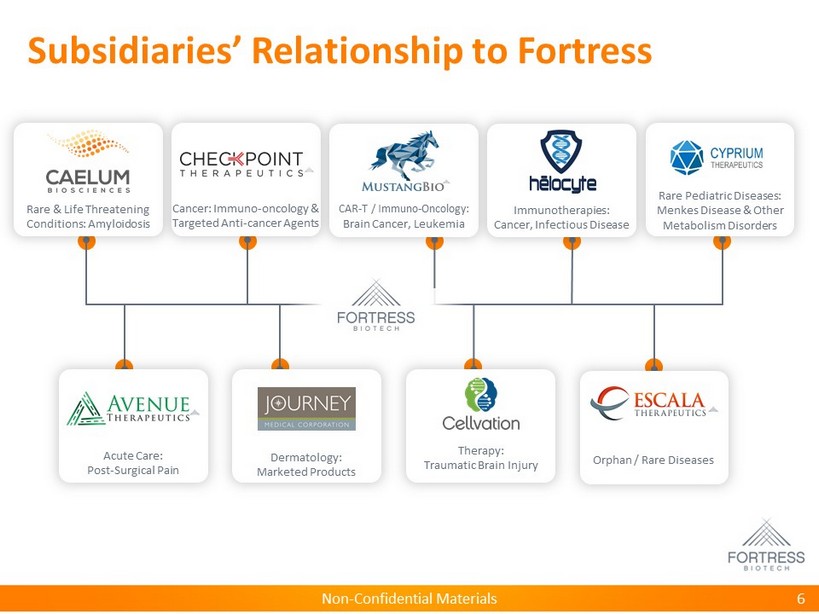
Non - Confidential Materials Subsidiaries’ Relationship to Fortress 6 Rare & Life Threatening Conditions: Amyloidosis Rare Pediatric Diseases: Menkes Disease & Other Metabolism Disorders Cancer: Immuno - oncology & Targeted Anti - cancer Agents CAR - T / Immuno - Oncology: Brain Cancer, Leukemia Acute Care: Post - Surgical Pain Immunotherapies: Cancer, Infectious Disease Orphan / Rare Diseases Dermatology: Marketed Products Therapy: Traumatic Brain Injury
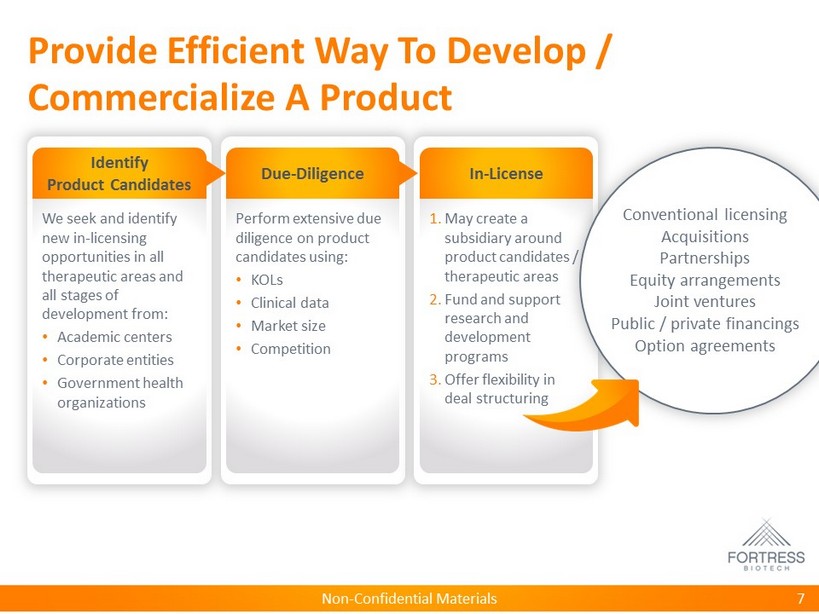
Non - Confidential Materials Provide Efficient Way To Develop / Commercialize A Product 7 Identify Product Candidates We seek and identify new in - licensing opportunities in all therapeutic areas and all stages of development from: • Academic centers • Corporate entities • Government health organizations Due - Diligence Perform extensive due diligence on product candidates using: • KOLs • Clinical data • Market size • Competition In - License 1. May create a subsidiary around product candidates / therapeutic areas 2. Fund and support research and development programs 3. Offer flexibility in deal structuring Conventional licensing Acquisitions Partnerships Equity arrangements Joint ventures Public / private financings Option agreements
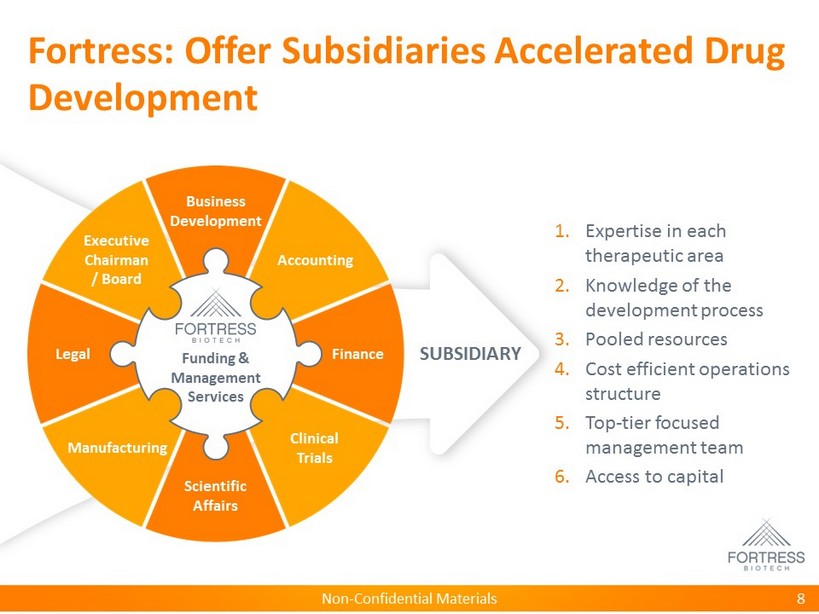
Non - Confidential Materials Fortress: Offer Subsidiaries Accelerated Drug Development 8 Scientific Affairs SUBSIDIARY 1. Expertise in each therapeutic area 2. Knowledge of the development process 3. Pooled resources 4. Cost efficient operations structure 5. Top - tier focused management team 6. Access to capital Funding & Management Services Business Development Finance Scientific Affairs Legal Accounting Clinical Trials Manufacturing Executive Chairman / Board
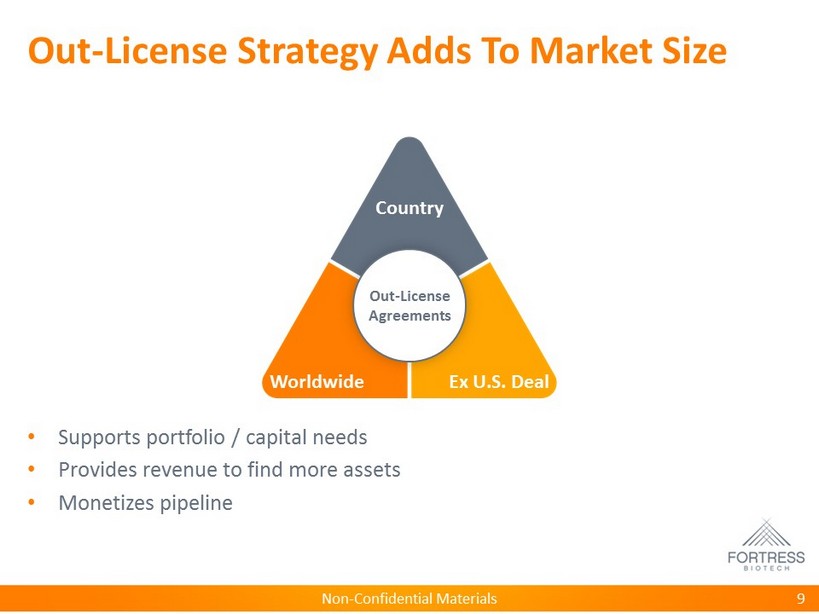
Non - Confidential Materials Out - License Strategy Adds To Market Size • Supports portfolio / capital needs • Provides revenue to find more assets • Monetizes pipeline 9 Country Worldwide Ex U.S. Deal Out - License Agreements
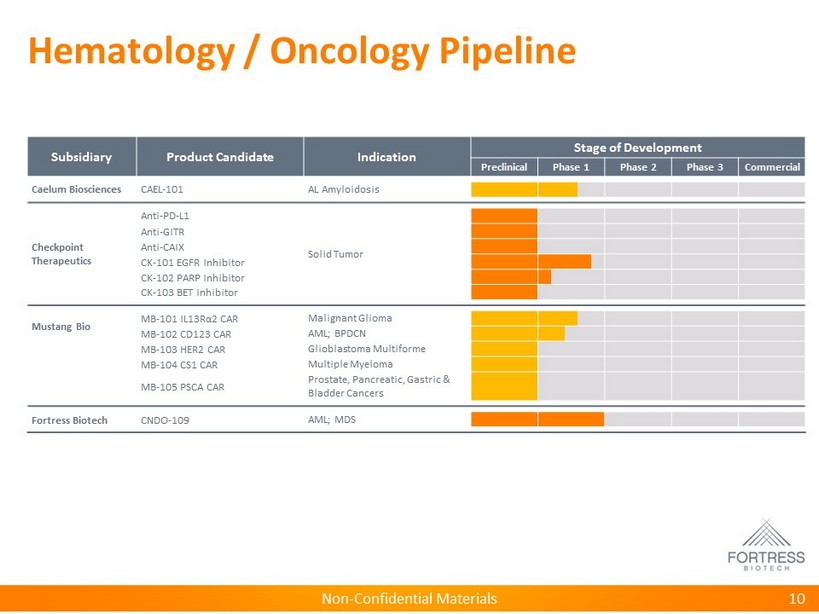
Non - Confidential Materials Hematology / Oncology Pipeline Subsidiary Product Candidate Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Commercial Caelum Biosciences CAEL - 101 AL Amyloidosis Checkpoint Therapeutics Anti - PD - L1 Solid Tumor Anti - GITR Anti - CAIX CK - 101 EGFR Inhibitor CK - 102 PARP Inhibitor CK - 103 BET Inhibitor Mustang Bio MB - 101 IL13R α 2 CAR Malignant Glioma MB - 102 CD123 CAR AML; BPDCN MB - 103 HER2 CAR Glioblastoma Multiforme MB - 104 CS1 CAR Multiple Myeloma MB - 105 PSCA CAR Prostate, Pancreatic, Gastric & Bladder Cancers Fortress Biotech CNDO - 109 AML; MDS 10
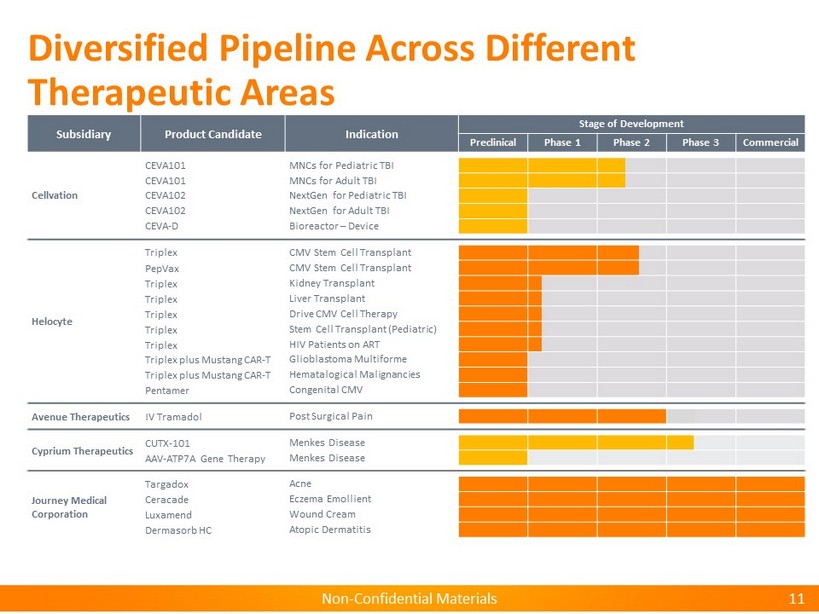
Non - Confidential Materials Non - Confidential Materials Diversified Pipeline Across Different Therapeutic Areas 11 Subsidiary Product Candidate Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Commercial Cellvation CEVA101 MNCs for Pediatric TBI CEVA101 MNCs for Adult TBI CEVA102 NextGen for Pediatric TBI CEVA102 NextGen for Adult TBI CEVA - D Bioreactor – Device Helocyte Triplex CMV Stem Cell Transplant PepVax CMV Stem Cell Transplant Triplex Kidney Transplant Triplex Liver Transplant Triplex Drive CMV Cell Therapy Triplex Stem Cell Transplant (Pediatric) Triplex HIV Patients on ART Triplex plus Mustang CAR - T Glioblastoma Multiforme Triplex plus Mustang CAR - T Hematalogical Malignancies Pentamer Congenital CMV Avenue Therapeutics IV Tramadol Post Surgical Pain Cyprium Therapeutics CUTX - 101 Menkes Disease AAV - ATP7A Gene Therapy Menkes Disease Journey Medical Corporation Targadox Acne Ceracade Eczema Emollient Luxamend Wound Cream Dermasorb HC Atopic Dermatitis
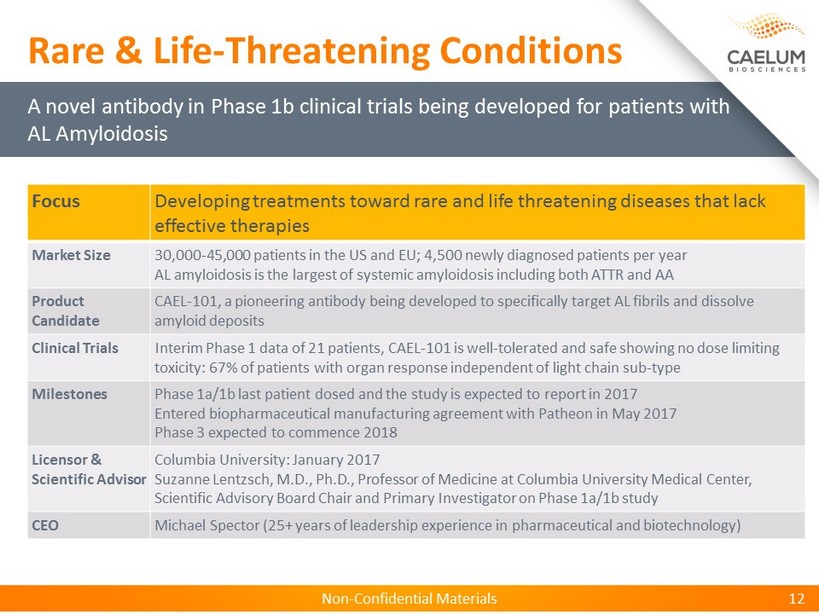
Non - Confidential Materials Non - Confidential Materials 12 Focus Developing treatments toward rare and life threatening diseases that lack effective therapies Market Size 30,000 - 45,000 patients in the US and EU; 4,500 newly diagnosed patients per year AL amyloidosis is the largest of systemic amyloidosis including both ATTR and AA Product Candidate CAEL - 101, a pioneering antibody being developed to specifically target AL fibrils and dissolve amyloid deposits Clinical Trials Interim Phase 1 data of 21 patients, CAEL - 101 is well - tolerated and safe showing no dose limiting toxicity: 67 % of patients with organ response independent of light chain sub - type Milestones Phase 1a/1b last patient dosed and the study is expected to report in 2017 Entered biopharmaceutical manufacturing agreement with Patheon in May 2017 Phase 3 expected to commence 2018 Licensor & Scientific Advisor Columbia University: January 2017 Suzanne Lentzsch, M.D., Ph.D., Professor of Medicine at Columbia University Medical Center, Scientific Advisory Board Chair and Primary Investigator on Phase 1a/1b study CEO Michael Spector (25+ years of leadership experience in pharmaceutical and biotechnology) A novel antibody in Phase 1b clinical trials being developed for patients with AL Amyloidosis Rare & Life - Threatening Conditions
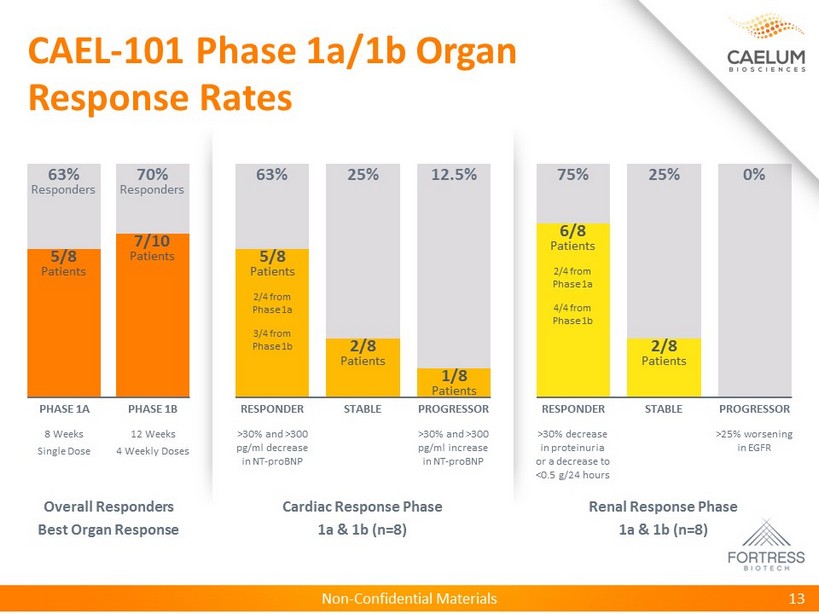
Non - Confidential Materials CAEL - 101 Phase 1a/1b Organ Response Rates 13 Renal Response Phase 1a & 1b (n=8) Cardiac Response Phase 1a & 1b (n=8) Overall Responders Best Organ Response 8 Weeks Single Dose PHASE 1A 63% Responders 12 Weeks 4 Weekly Doses PHASE 1B 70% Responders >30% and >300 pg/ml decrease in NT - proBNP RESPONDER 63% STABLE 25% >30% and >300 pg/ml increase in NT - proBNP PROGRESSOR 12.5% STABLE 25% RESPONDER 75% >30% decrease in proteinuria or a decrease to <0.5 g/24 hours PROGRESSOR 0% >25% worsening in EGFR 5/8 Patients 7/10 Patients 5/8 Patients 2/4 from Phase 1a 3/4 from Phase 1b 2/8 Patients 1/8 Patients 2/8 Patients 6/8 Patients 2/4 from Phase 1a 4/4 from Phase 1b

Non - Confidential Materials Non - Confidential Materials 14 Focus Acquire and develop novel immuno - oncology and targeted cancer agents alone and in combination to treat patients with solid tumors Market Size Anti - PD - (L)1 >$30B, Anti - GITR > $1B, CK - 101 EGFR > $3B, CK - 103 BET > $1B Product Candidates Two immuno - oncology “I/O” antibodies, licensed from Dana Farber Four targeted anti - cancer agents Clinical Trials CK - 101 (EGFR Inhibitor) Phase 1/2 study ongoing Milestones 3Q 2017: Anti - PD - L1 Phase 1 initiation expected YE 2017: CK - 101 (EGFR Inhibitor) Phase 2 expected initiation YE 2017: CK - 103 (BET Inhibitor) target IND filing 2018: Anti - GITR target IND expected TGTX Collaboration Joint development of anti - PD - L1 and anti - GITR mAbs, and BET inhibitor program with Checkpoint developing solid tumor indications and TG in liquid tumors Funding ~$32M (3/31/17) to support development programs through 2018 CEO James Oliviero (15+ years of leadership experience in pharmaceutical and biotechnology, previously senior management of Keryx, achieving a new drug approval) Building a platform to combine targeted agents with immuno - oncology agents to maximize anti - cancer effect Immuno - Oncology
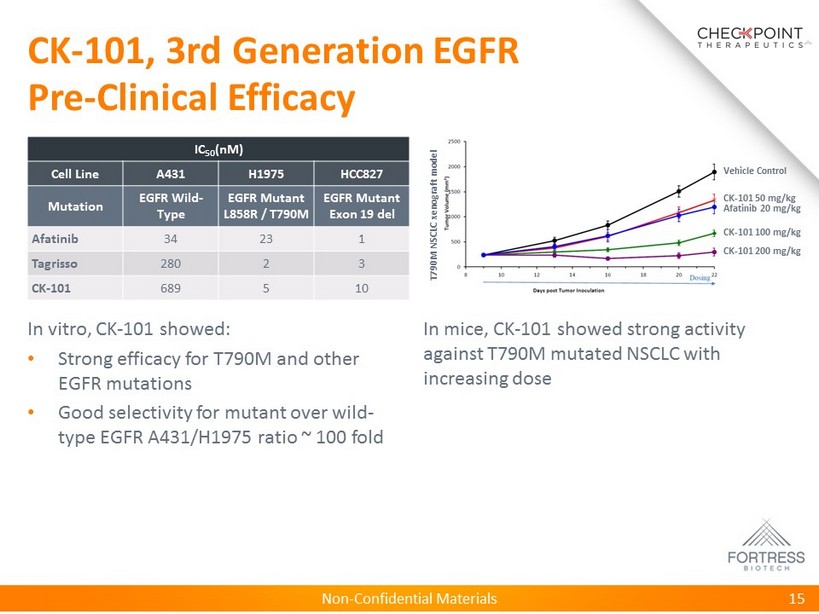
Non - Confidential Materials CK - 101, 3rd Generation EGFR Pre - Clinical Efficacy 15 Vehicle Control CK - 101 50 mg/kg CK - 101 100 mg/kg CK - 101 200 mg/kg Afatinib 20 mg/kg IC 50 ( nM ) Cell Line A431 H1975 HCC827 Mutation EGFR Wild - Type EGFR Mutant L858R / T790M EGFR Mutant Exon 19 del Afatinib 34 23 1 Tagrisso 280 2 3 CK - 101 689 5 10 In vitro, CK - 101 showed: • Strong efficacy for T790M and other EGFR mutations • Good selectivity for mutant over wild - type EGFR A431/H1975 ratio ~ 100 fold In mice, CK - 101 showed strong activity against T790M mutated NSCLC with increasing dose T790M NSCLC xenograft model
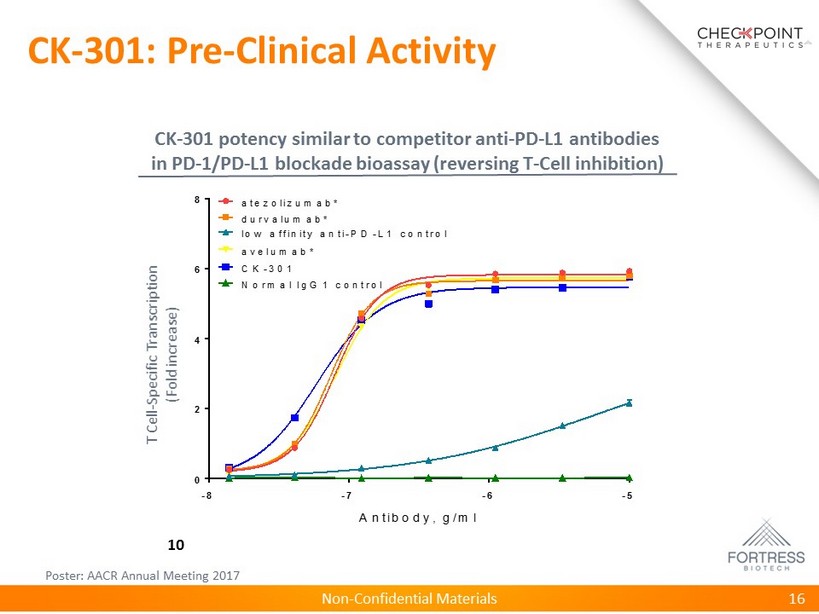
Non - Confidential Materials CK - 301: Pre - Clinical Activity 16 T Cell - Specific Transcription (Fold increase) 10 Poster: AACR Annual Meeting 2017 CK - 301 potency similar to competitor anti - PD - L1 antibodies in PD - 1/PD - L1 blockade bioassay (reversing T - Cell inhibition) -8 -7 -6 -5 0 2 4 6 8 Antibody, g/m l F o l d i n c r e a s e o v e r b c k g r d atezolizum ab* durvalum ab* low affinity anti-P D -L1 control avelum ab* C K -301 N orm al IgG 1 control
Non - Confidential Materials Non - Confidential Materials Focus Two lead CAR - T programs targeting IL13R α 2 and CD123, for the treatment of Glioblastoma Multiforme and AML/BPDCN, respectively Market Size In U.S., Japan and five major EU markets per year… ~30,000 newly diagnosed GBMs (malignant brain tumor) ~30,000 newly diagnosed cases of AML (acute myeloid leukemia) Product Candidates MB - 101 IL13R α 2 - specific CAR - T cells which have no current competition MB - 102 CD123 - specific CAR - T cells which have been validated in ultra orphan indication Clinical Trials Two Phase 1 trials ongoing with preliminary safety data from at least 6 patients in both CAR - T programs Milestones Phase 1 data readouts early 2018 NEJM case study demonstrates MB - 101 achieved complete remission in patient with recurrent GBM Licensor City of Hope Scientific Advisors Dr. Stephen Forman, City of Hope Dr. Christine Brown, City of Hope Focus Two clinical stage CAR - T programs & three preclinical stage CAR - T programs Market Size In the U.S., Japan and five major EU markets there are 30,000 newly diagnosed GBMs (malignant brain tumor) and 30,000 newly diagnosed cases of AML (acute myeloid leukemia) Product Candidates MB - 101 IL13R α 2 - specific CAR - T cells for GBM MB - 102 CD123 - specific CAR - T cells for AML & blastic plasmacytoid dendritic cell neoplasm, an ultra - orphan indication MB - 103 HER2 - specific CAR - T for GBM MB - 104 CS1 - specific CAR - T for multiple myeloma MB - 105 PSCA - specific CAR - T for prostate, pancreatic, gastric, & bladder cancers Clinical Trials One Phase 1 trial ongoing for each of the 2 lead CAR - T programs, with preliminary safety data from at least 6 patients in each Milestones Phase 1 data readouts early 2018 Licensor City of Hope Scientific Advisors Dr. Stephen Forman, City of Hope Dr. Christine Brown, City of Hope Funding ~$95M (3/31/17 ) CEO Manuel Litchman , M.D. (20+ years of experience in pharma & biotech , including senior leadership positions in licensing, development and general management at Novartis and Arvinas LLC) 17 Robust CAR - T platform technology in partnership with pioneers in CAR - T technologies from City of Hope, recently raising a $95M private placement financing Aggressive Forms of Cancer
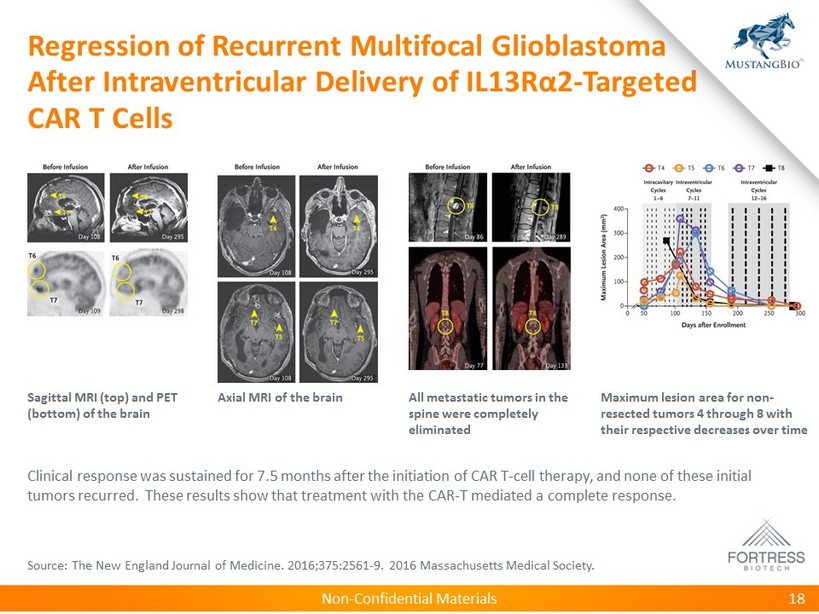
Non - Confidential Materials Regression of Recurrent Multifocal Glioblastoma After Intraventricular Delivery of IL13R α 2 - Targeted CAR T Cells 18 Sagittal MRI (top) and PET (bottom) of the brain Axial MRI of the brain All metastatic tumors in the spine were completely eliminated Maximum lesion area for non - resected tumors 4 through 8 with their respective decreases over time Clinical response was sustained for 7.5 months after the initiation of CAR T - cell therapy, and none of these initial tumors recurred. These results show that treatment with the CAR - T mediated a complete response. Source: The New England Journal of Medicine. 2016;375:2561 - 9. 2016 Massachusetts Medical Society.
Non - Confidential Materials Non - Confidential Materials 19 Focus Develop novel immunotherapies for the prevention and treatment of CMV that can cause life - threatening disease in those with weak immune systems Market Size CDC estimates 50 - 80% infected with Cytomegalovirus (CMV) by age of 40 CMV in Allogeneic Stem Cell Transplant: U.S. Incidence ~8,000 / EU Incidence ~15,000 CMV in Allogeneic Solid Organ Transplant: U.S. Incidence ~8,000 / EU Incidence ~15,000 Product Candidates PepVax : HLA - restricted, single antigen CMV vaccine Triplex: First universal, multi - antigen CMV vaccine Clinical Trials PepVax : Phase 2 ongoing, multi - center, double - blind trial for stem cell transplant (n=96) Phase 1b showed safe, effective and Published in Lancet Dec 2015 Triplex: Phase 2 ongoing, multi - center, double - blind trial for stem cell transplant (n=115) Phase 1 showed safe, immunogenic. Presented ASH 2015. Published in Blood Nov 2016 Upcoming Milestones Triplex: Phase 2 primary endpoint by 1H2018 PepVax : Phase 2 primary endpoint by 2H2018 Licensor City of Hope Funding $8M+ in current grant funding, other grants in progress CEO Frank Taffy (15+ years of experience at Forest Labs and Life Tech in corporate development and operations) Three novel biologic immunotherapies (two in Phase 2) targeting billion dollar orphan market Cytomegalovirus (CMV): Common Virus

Non - Confidential Materials Phase 1 Studies: Journal Publications Phase 1b (Completed, Published in The Lancet ) Design Single - Center (City of Hope) Study in 36 Allogeneic HSCT CMV(+)Recipients Randomized (1:1) between Vaccine Arm (VA) and Observation Arm (OA) Dosing Schedule Two subcutaneous vaccinations after transplant • Day 28 • Day 56 1˚ Endpoint Overall safe and well - tolerated Published in The Lancet Haematology (12/28/2015) 2˚ Endpoint • Increase in CD8+ T - cells • Reduced CMV Reactivation, 6% vs.33%,p=0.044 • Reduced Relapse, 6% vs. 28%, p=0.015 • Reduced Death, 0vs. 39% Phase 1 (Completed, Published in Blood ) 20 Vaccine (n=18 ) Observation (n=18) Patients with serious adverse events 4 (22%) 9 (50%) Disease relapse 1 (6%) 5 (28%) Death 0 (0%) 7 (39%) CMV viraemia (≥500 gc/mL ) 1 (6%) 6 (33%) Design Single - Center (City of Hope) Dose Escalation (three levels) in 24 Healthy Volunteers (CMV +/ - ) Dosing Schedule Two IM injections four weeks apart Last patient dosed 4/2015 1˚ Endpoint Safe and well - tolerated in all dose cohorts Presented at ASH (December 2015) Published in Blood (November2016) 2˚ Endpoint ↑pp65 - , IE1 - , IE2 - specific CD8 and CD4 T - cells Particularly pronounced increase in T - cells in those with low baseline levels
Proprietary Materials Non - Confidential Materials 21 Focus Developing novel therapies for the treatment of rare, fatal pediatric diseases, with initial focus on Menkes disease and related copper metabolism disorders Market Size Menkes disease is a rare X - linked pediatric disease caused by gene mutations of copper transporter ATP7A, which affects approximately one in 100,000 newborns per year. Product Candidate CUTX - 101 (Copper Histidinate injection) is being developed to replenish copper levels in patients with Menkes disease. A preclinical AAV - based ATP7A gene therapy is being developed to deliver working copies of ATP7A to Menkes patients. Both programs have FDA Orphan Drug Designations. Clinical Trials In Phase 1/2 clinical studies conducted at NICHD, early treatment of Menkes patients with CUTX - 101 led to an improvement in neurodevelopmental outcomes and survival. Milestones Natural History Study of untreated Menkes patients in 1H2017 FDA meetings to confirm regulatory pathway in 2017 Licensor & Scientific Advisor Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), part of the National Institutes of Health (NIH): March 2017 (CRADA & Exclusive License Agreement) Stephen G. Kaler, M.D., Senior Investigator and Head, Section on Translational Neuroscience, Molecular Medicine Branch, NICHD Principal Investigator for Menkes disease clinical studies CEO Lung S. Yam, M.D., Ph.D. (Senior Advisor, Opus Point Partners; BD Consultant involved in identifying and in - licensing of multiple assets to Fortress and affiliated companies) A novel therapy in Phase 3 clinical trial being developed for patients with AL Menkes Disease Rare & Fatal Pediatric Diseases
Non - Confidential Materials Non - Confidential Materials 22 IV Tramadol For Post - Surgical Pain IV Tramadol, if approved, would be the only Schedule IV intravenous opioid in the U.S. Focus IV tramadol for the treatment of post - surgical pain Market Size IV analgesics sells ~$1bn per year in the U.S. IV acetaminophen sells >$250MM with ~3 to 4% of the unit volume Product Candidate Intravenous (IV) Tramadol, an opioid without the typical side effects of narcotics , for the treatment of moderate to moderately severe pain Regulatory Path 505b(2) Status Phase 3 ready Funding IPO with $38 million in gross proceeds in June 2017 CEO Lucy Lu, M.D. (15+ years of experience in biotech and equity research)
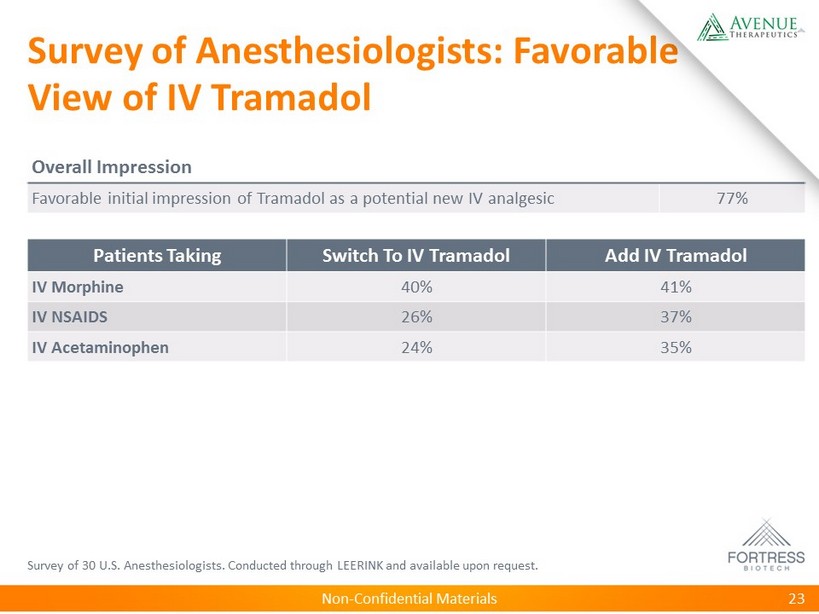
Non - Confidential Materials Survey of Anesthesiologists: Favorable View of IV Tramadol Survey of 30 U.S. Anesthesiologists. Conducted through LEERINK and available upon request. 23 Patients Taking Switch To IV Tramadol Add IV Tramadol IV Morphine 40% 41% IV NSAIDS 26% 37% IV Acetaminophen 24% 35% Overall Impression Favorable initial impression of Tramadol as a potential new IV analgesic 77%

Non - Confidential Materials 24 Focus Identify, develop and commercialize innovative, differentiated prescription dermatology products through a highly efficient and potent sales and marketing model Product Portfolio Targadox ( doxycyline tablets ) : Sever e acne Ceracade (skin emulsion) : Atopic and various types of dermatitis Luxamend (wound cream) : Wounds from superficial to full thickness and 1 st and 2 nd degree burns Dermasorb HC (hydrocortisone lotion) Kit: Seborrheic dermatitis Market Highlights Journey targets the top 5,000 prescribing dermatologists reaching more than 70% of our market Increased sales force from 15 to 30 representatives in 2017 Targadox is the fastest growing branded doxycycline in 2017 Luxamend is the #1 prescribed brand in the prescription wound market in 2017 CEO Claude Maraoui (25+ years commercializing dermatology products; previously Vice President of Sales at Medicis , responsible for 1.2 billion in revenue and 240 sales representatives. Prior roles include head of North America sales and head of Marketing for Medicis Aesthetics makers of Restylane and Dysport ) Team of industry experts successfully launched four dermatology products in 12 months Innovative Dermatology Products
Non - Confidential Materials 25 Focus Develop novel biologic therapies for TBI treatment Market Size 200,000 adults / 50,000 children with TBI Product Candidate CEVA101: Autologous bone - marrow derived mononuclear cells Clinical Trials Two ongoing Phase 2 studies, one adult and one pediatric Phase 1 in Adult TBI: Published in Stem Cells , November 2016 Milestone Phase 2 data in Children by 1H2018, in Adults by 1H2019 Potential for accelerated approval in Japan Potential for pediatric voucher Licensor Two technology platforms from University of Texas Health Science Center Funding NIH/DOD Grant Funding: $10M+ CEO Frank Taffy ( 15+ years of experience at Forest Labs and Life Tech in corporate development and operations) No approved reparative therapy for treatment of severe TBI. Now have CEVA101, a biologic, that minimizes the secondary injury associated with TBI. CEVA101: Severe Traumatic Brain Injury
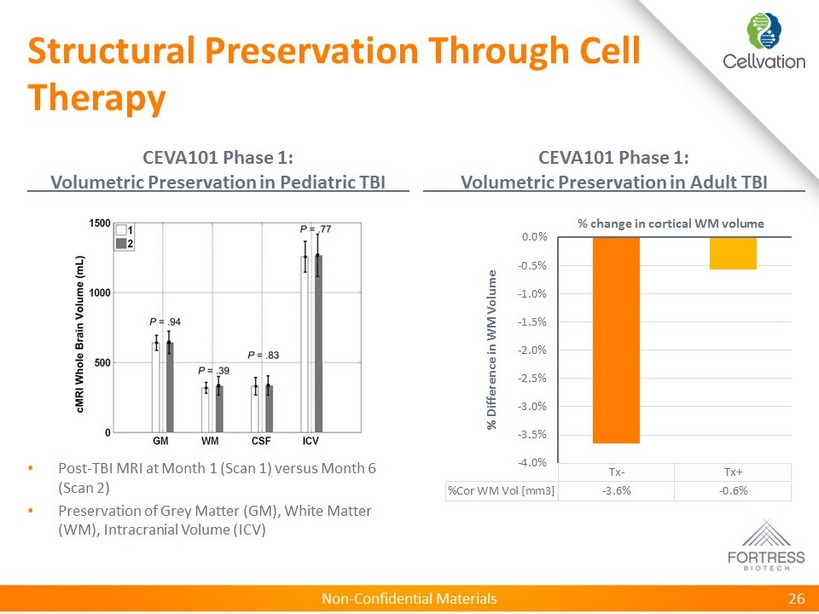
Non - Confidential Materials Structural Preservation Through Cell Therapy CEVA101 Phase 1: Volumetric Preservation in Pediatric TBI CEVA101 Phase 1: Volumetric Preservation in Adult TBI 26 • Post - TBI MRI at Month 1 (Scan 1) versus Month 6 (Scan 2) • Preservation of Grey Matter (GM), White Matter (WM), Intracranial Volume (ICV) Tx- Tx+ %Cor WM Vol [mm3] -3.6% -0.6% -4.0% -3.5% -3.0% -2.5% -2.0% -1.5% -1.0% -0.5% 0.0% % Difference in WM Volume % change in cortical WM volume
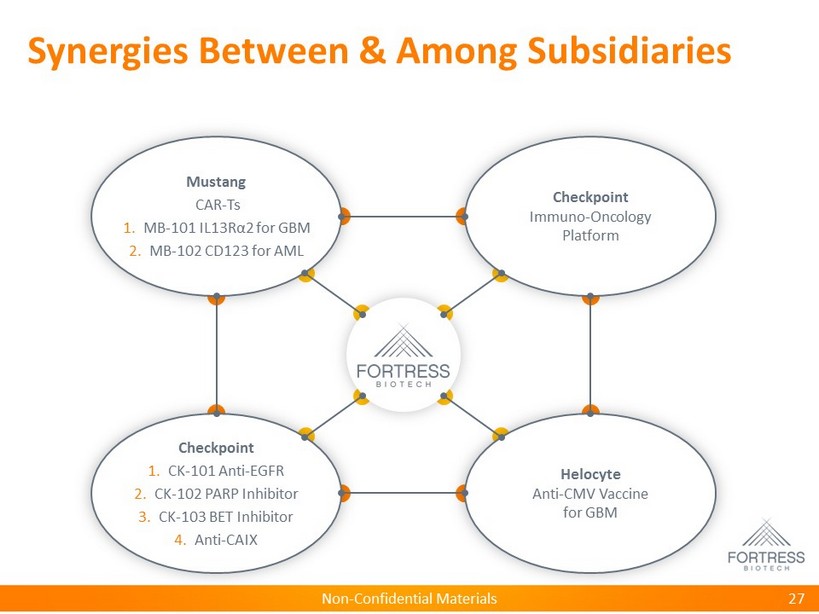
Non - Confidential Materials Checkpoint Immuno - Oncology Platform Helocyte Anti - CMV Vaccine for GBM Checkpoint 1. CK - 101 Anti - EGFR 2. CK - 102 PARP Inhibitor 3. CK - 103 BET Inhibitor 4. Anti - CAIX Mustang CAR - Ts 1. MB - 101 IL13R α 2 for GBM 2. MB - 102 CD123 for AML Synergies Between & Among Subsidiaries 27
Non - Confidential Materials Accelerated Drug Development Model Fortress Subsidiaries Are Creating A Pipeline of Therapies For Life - Threatening Diseases 28 Diversified Pipeline Experienced, Proven Leadership

Non - Confidential Materials Corporate Presentation August 2017
