Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AERIE PHARMACEUTICALS INC | d429549dex991.htm |
| 8-K - FORM 8-K - AERIE PHARMACEUTICALS INC | d429549d8k.htm |
 Roclatan TM Mercury 1 Phase 3 12-month Topline Results 1 For Investor Use Exhibit 99.2 |
 Important
Information Any discussion of the potential use or expected success of our
product candidates is subject to our product candidates being approved by
regulatory authorities. The information in this presentation is current
only as of its date and may have changed or may change in the future. We
undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in
this presentation is accurate or complete.
Certain statements in this presentation are “forward-looking statements”
within the meaning of the federal securities laws. Words such as
“may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,”
“potential” or similar expressions are intended to identify
these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could
cause actual results to differ materially from those contemplated by the statements. In
evaluating these statements, you should specifically consider various
factors that may cause our actual results to differ materially from any
forward-looking statements. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk
Factors” and “Management’s Discussion and Analysis of Financial
Condition and Results of Operations.” In particular, the topline
Mercury 1 data presented herein is preliminary and based solely on
information available to us as of the date of this press release and additional information about the results may be disclosed at any time. Such forward-looking statements only speak as of the date they
are made. We undertake no obligation to publicly update or revise any
forward-looking statements, whether because of new information,
future events or otherwise, except as otherwise required by law.
2 For Investor Use |
 Roclatan TM Achieves Positive 12-Month Safety and Efficacy Results • Safety data over the 12 months were consistent with previous Roclatan™
3-month results • There were no drug-related serious or systemic adverse events • The main adverse event for Roclatan™ was conjunctival hyperemia, which was reported in ~60% of patients, scored as mild for ~70% of these patients and sporadic • IOP-lowering effect of Roclatan™ through Month 12 remained stable and consistent with the primary efficacy analysis at Month 3, maintaining superiority over both latanoprost and Rhopressa™ – 1-3 mmHg greater than monotherapy with either latanoprost or Rhopressa™
throughout the duration of the study (i.e., Week 2, Week 6, Month 3, Month 6,
Month 9 and Month 12)
– At Month 12, Roclatan TM reduced mean diurnal IOPs to 16 mmHg or lower in 60% of patients, a significantly higher percentage than observed in the comparator arms
3 ++ Data on File Based on Mercury 1 Topline 12-month Product candidates have not approved by the FDA For Investor Use |
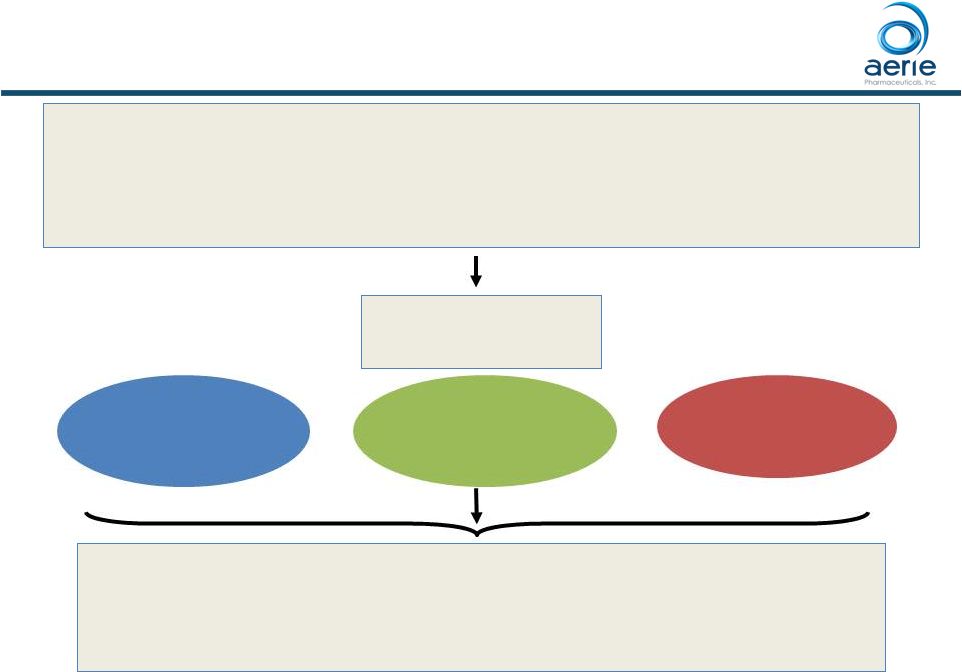 Mercury 1
Trial Design Patients randomized
1:1:1 Primary endpoints: • Efficacy: Mean IOP at nine time points (08:00, 10:00, and 16:00 at
Week 2, Week 6, and Month 3)
• Safety: Ocular and systemic safety during a 12-month treatment period Patients with open angle glaucoma (OAG) or ocular hypertension (OHT) with IOP >20 mmHg and < 36 mmHg N=718 subjects randomized at 58 US sites Rhopressa TM Netarsudil (AR-13324) 0.02% QD (PM) Latanoprost 0.005% QD (PM) 4 Product candidates have not approved by the FDA For Investor Use ClinicalTrials.gov Identifier: NCT02558400 Roclatan TM PG324 (netarsudil/latanoprost) QD (PM) |
 Disposition 5 ++ Data on File Based on Mercury 1 Topline 12-month Product candidates have not approved by the FDA For Investor Use Roclatan TM N = 238 Rhopressa TM N = 244 Latanoprost N = 236 Completed Month 12 159 (66.8%) 148 (60.7%) 203 (86.0%) Discontinued Prior to Month 12 79 (33.2%) 96 (39.3%) 33 (14.0%) Reasons for Discontinuation Adverse Event Withdrawal of Consent Non-Compliant Lost to Follow-up Lack of Efficacy Disallowed Concurrent Medication Investigator Decision Protocol Violation Other 47 (19.7%) 13 (5.5%) 0 5 (2.1%) 0 6 (2.5%) 2 (0.8%) 6 (2.5%) 0 53 (21.7%) 9 (3.7%) 1 (0.4%) 5 (2.0%) 13 (5.3%) 7 (2.9%) 2 (0.8%) 3 (1.2%) 3 (1.2%) 4 (1.7%) 8 (3.4%) 3 (1.3%) 4 (1.7%) 1 (0.4%) 5 (2.1%) 0 8 (3.4%) 0 |
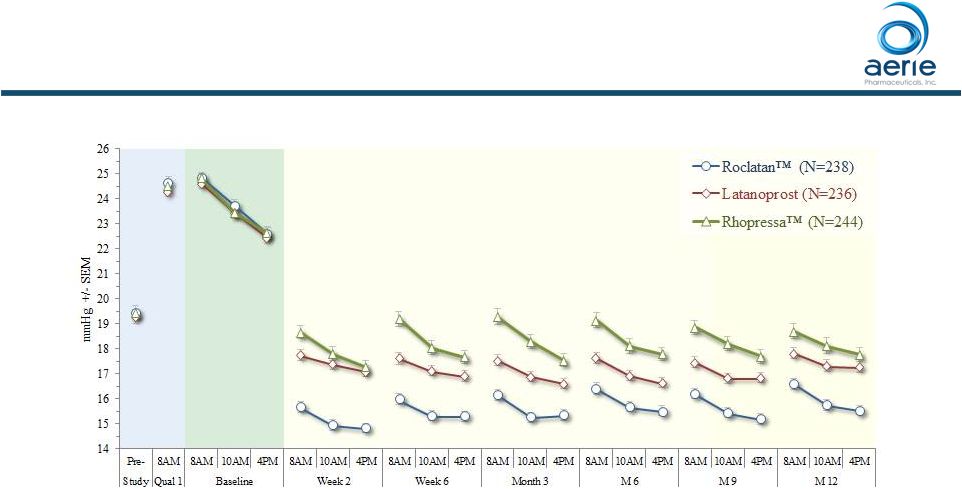 Roclatan TM Maintained Superior Efficacy Over Individual Components for 12 Months Mean IOP at Each Time Point (ITT) • Roclatan™ statistically superior to latanoprost and Rhopressa™ at all
time points • Roclatan™ IOP-lowering 1-3 mmHg greater than monotherapy through
Month 12 6 ++ Data on File Based on Mercury 1 Topline 12-month Product candidates have not approved by the FDA For Investor Use |
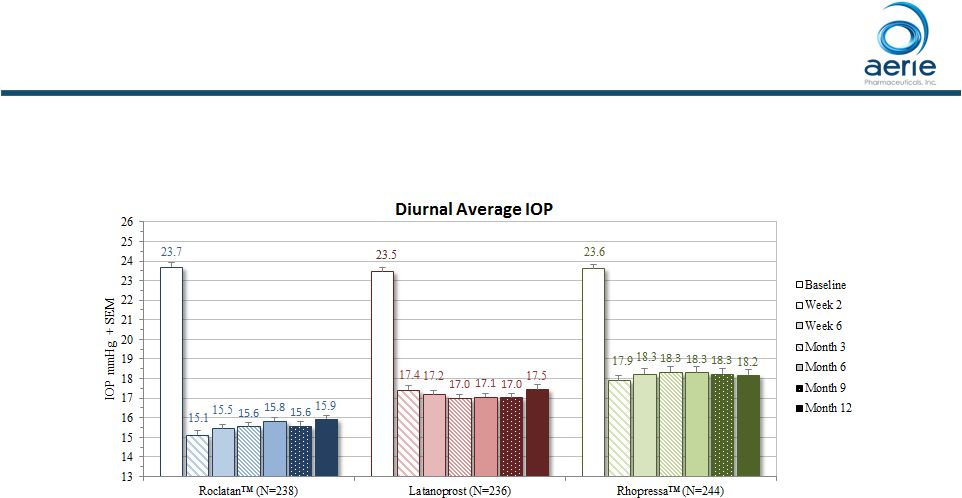 Roclatan TM Maintained Superior Efficacy Over Individual Components for 12 Months Mean Diurnal IOP at Each Visit (ITT) p<0.0001 at All Visits vs. Latanoprost and Rhopressa TM 7 ++ Data on File Based on Mercury 1 Topline 12-month Product candidates have not approved by the FDA For Investor Use |
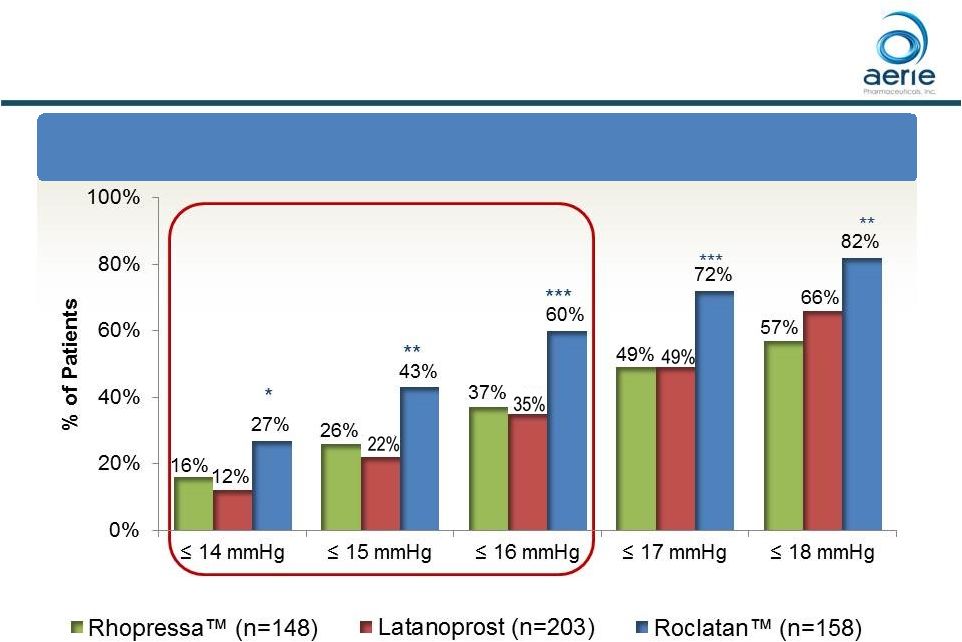 Roclatan TM Phase 3 Month 12 Responder Analysis: Goal is to Achieve Lowest IOP Possible At Month 12: % of Patients with IOP Reduced to 18 mmHg or Lower 8 *p<0.05, **p<0.01, ***p<0.0001 ++ Data on File Based on Mercury 1 Topline 12-month Product candidates have not approved by the FDA For Investor Use |
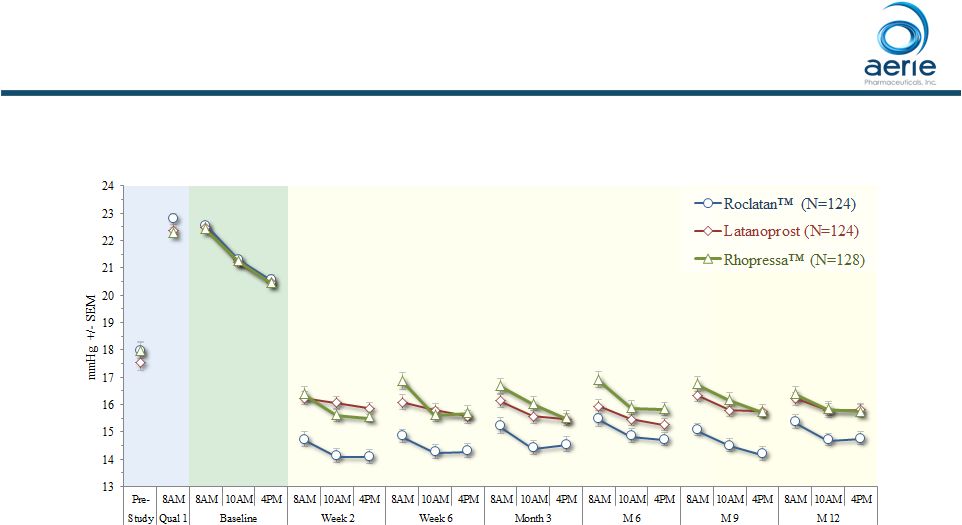 Efficacy
in Subjects with Baseline IOP <25 mmHg Mean IOP at Each Time Point
(ITT) •
Rhopressa TM efficacy similar to latanoprost and stable for 12 months 9 ++ Data on File Based on Mercury 1 Topline 12-month Product candidates have not approved by the FDA For Investor Use |
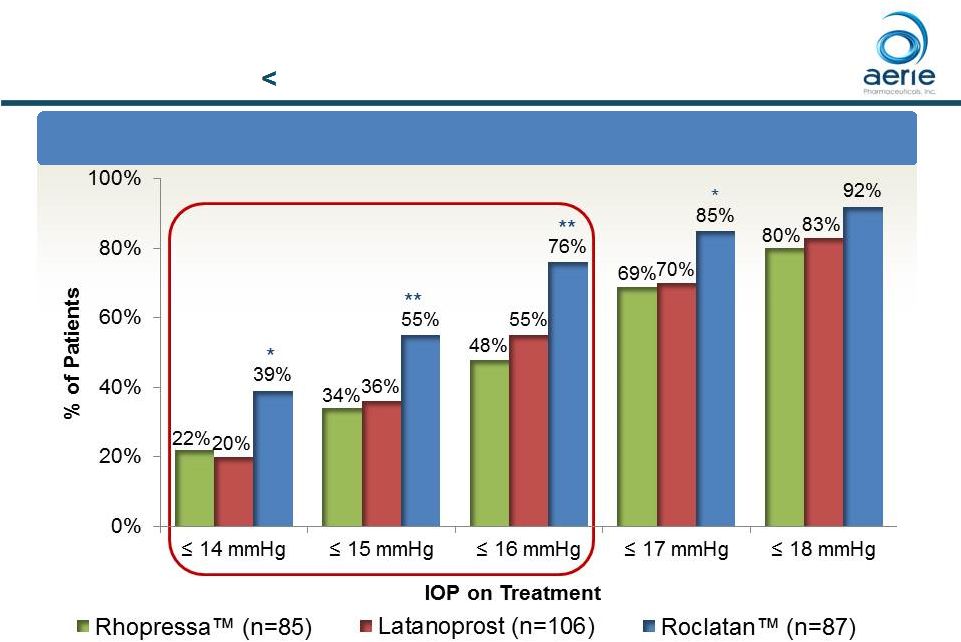 At Month
12: % of Patients with IOP Reduced to 18 mmHg or Lower
10 Roclatan TM Responder Analysis Baseline IOP 25 mmHg *p<0.05, **p<0.01 ++ Data on File Based on Mercury 1 Topline 12-month Product candidates have not approved by the FDA For Investor Use |
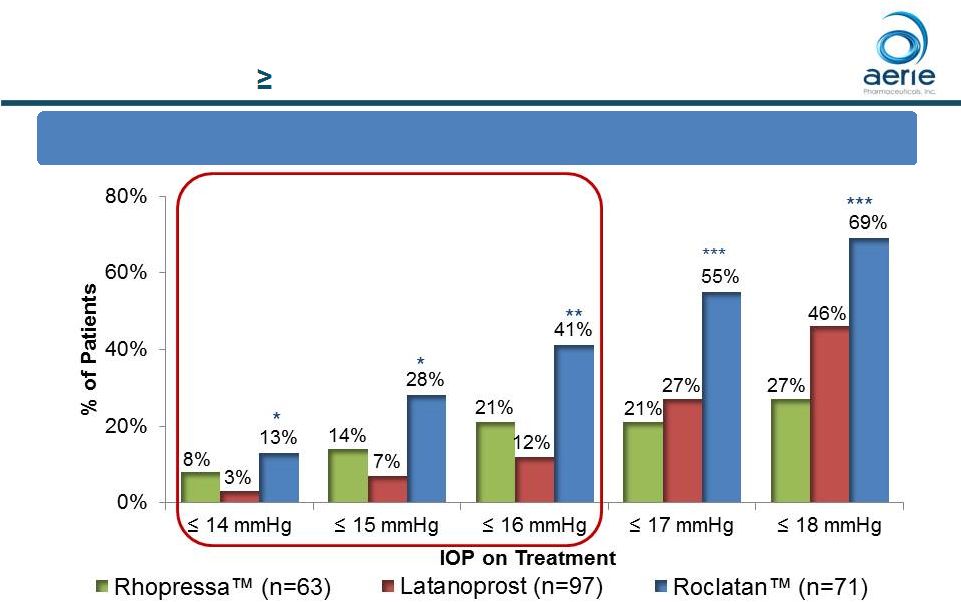 At Month
12: % of Patients with IOP Reduced to 18 mmHg or Lower
Roclatan TM Responder Analysis Baseline IOP 25 mmHg 11 *p<0.05 vs Latanoprost **p<0.05 vs Rhopressa TM , p<0.0001 Latanoprost ***p<0.0001 vs Rhopressa TM , p<0.01 Latanoprost ++ Data on File Based on Mercury 1 Topline 12-month Product candidates have not approved by the FDA For Investor Use |
 Safety/Tolerability Overview of Roclatan
TM • There were no drug-related serious adverse events (SAEs) and no evidence of treatment-related systemic effects • The most common adverse event was conjunctival hyperemia with ~60% incidence, scored as mild on biomicroscopy for ~70% of these patients and sporadic • Other ocular AEs – AEs occurring in ~5-18% of subjects receiving Roclatan TM included: cornea verticillata, conjunctival hemorrhage, eye pruritus, lacrimation increased, visual acuity reduced, blepharitis and punctate keratitis. 12 ++ Data on File Based on Mercury 1 Topline 12-month Product candidates have not approved by the FDA For Investor Use |
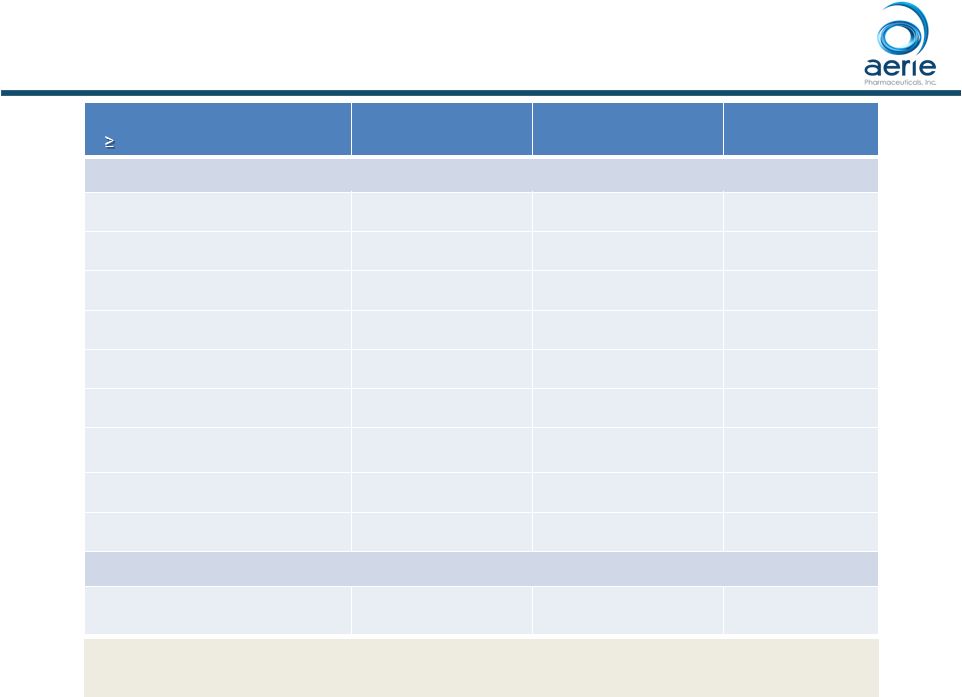 Roclatan TM Phase 3 Safety Profile Adverse Events
( 5.0% in any group)
Roclatan n=238 Rhopressa n=243 Latanoprost n=237 Eye Disorders Conjunctival Hyperemia 150 (63.0%) 125 (51.4%) 52 (21.9%) Conjunctival Hemorrhage 31 (13.0%) 44 (18.1%) 3 (1.3%) Cornea Verticillata 42 (17.6%) 33 (13.6%) 0 Eye Pruritus 27 (11.3%) 22 (9.1%) 3 (1.3%) Punctate Keratitis 12 (5.0%) 18 (7.4%) 10 (4.2%) Lacrimation Increased 17 (7.1%) 20 (8.2%) 1 (0.4%) Visual Acuity Reduced 13 (5.5%) 13 (5.3%) 6 (2.5%) Vision Blurred 11 (4.6%) 15 (6.2%) 3 (1.3%) Blepharitis 14 (5.9%) 8 (3.3%) 5 (2.1%) Administration Site Conditions Instillation site pain 55 (23.1%) 60 (24.7%) 18 (7.6%) Patients with known contraindications or hypersensitivity to latanoprost were excluded 13 ++ Data on File Based on Mercury 1 Topline 12-month Product candidates have not approved by the FDA For Investor Use TM TM |
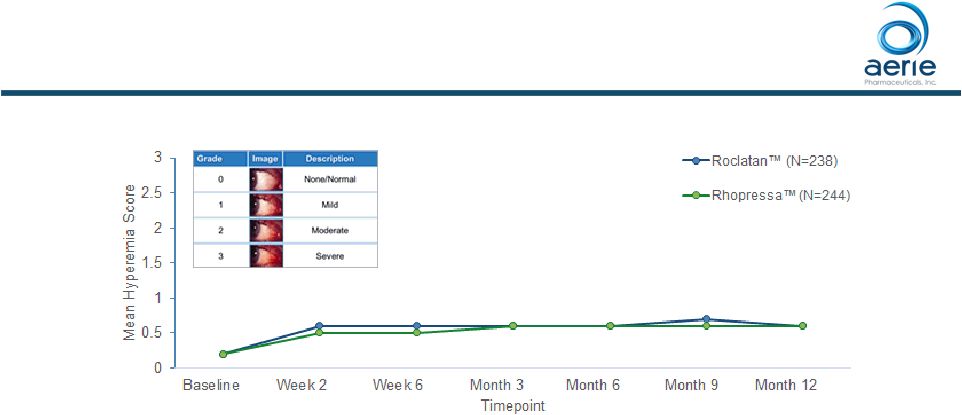 14 ++ Data on File Based on Mercury 1 Topline 12-month Product candidates have not approved by the FDA For Investor Use – Hyperemia severity did not increase with continued dosing – Hyperemia was sporadic • Only ~10% of patients had hyperemia on each study visit day from week 2 to month 12 (~7% Rhopressa™, ~3% latanoprost) – Only ~8% of all patients discontinued due to hyperemia (~7% of all patients at Month 3) Roclatan™ Conjunctival Hyperemia Was Sporadic And Severity Did Not Increase With Continued Dosing |
 Consistent statistically superior efficacy over both latanoprost and
Rhopressa™ at all time points demonstrated in 2 Phase 3 trials
(Mercury 1 and Mercury 2)
IOP-lowering effect was greater (1-3 mmHg) than monotherapy
with either latanoprost
or Rhopressa™ throughout the duration of
the study Stable efficacy through 12 months Well tolerated with no evidence of treatment-related serious or systemic effects 15 Roclatan TM Once-Daily Performance Summary Product candidates have not approved by the FDA For Investor Use ++ Data on File Based on Mercury 1 and Mercury 2 |
 Rhopressa TM efficacy similar to latanoprost with baseline IOP < 25 mmHg Rhopressa TM maintained consistent IOP lowering across all baseline IOPs including 25 mmHg Stable efficacy through 12 months Adverse event profile consistent with previous studies 16 Rhopressa TM Once-Daily Performance Summary Product candidates have not approved by the FDA For Investor Use ++ Data on File Based on Mercury 1 Topline 12-month |
 Rhopressa TM • PDUFA February 28, 2018 • - Expected FDA Advisory Committee • Initiating clinical program for Japan market (Phase 1 and 2 to be conducted in the U.S. in Japanese patients) • - To commence in Q3/Q4 2017 Roclatan TM • NDA filing expected 1H 2018 • Mercury 3 (Europe): 6-month study, comparing to Ganfort® • - To commence in Q3 2017 17 Key Upcoming Milestones Product candidates have not approved by the FDA For Investor Use |
