Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Bionik Laboratories Corp. | v469012_8k.htm |
Exhibit 99.1

1 BIONIK LABORATORIES Investor Presentation June 2017 www.bioniklabs.com OTCQX: BNKL @ BionikLab # MobilityForLife

2 SAFE HARBOR STATEMENT www.bioniklabs.com WE DO NOT MAKE ANY REPRESENTATIONS OR WARRANTIES, EXPRESS OR IMPLIED, AS TO THE ACCURACY OR COMPLETENESS OF THE INFORMATION PROVIDED IN THIS DOCUMENT. WE RESERVE THE RIGHT TO AMEND, REPLACE AND/OR SUPPLEMENT THIS DOCUMENT AT ANY TIME AND UNDERTAKE NO OBLIGATION TO PROVIDE THE RECIPIENT WITH ACCESS TO ADDITIONAL INFORMATION. NOTHING IN THIS DOCUMENT IS, OR SHOULD BE RELIED UPON AS, A PROMISE OR REPRESENTATION AS TO THE FUTURE. THE INFORMATION CONTAINED IN THIS DOCUMENT DOES NOT PURPORT TO BE ALL - INCLUSIVE OR TO CONTAIN ALL THE INFORMATION AVAILABLE. THIS MEMORANDUM DOES NOT CONSTITUTE AN OFFER TO SELL OR A SOLICITATION OF AN OFFER TO BUY SECURITIES. THIS DOCUMENT MAY CONTAIN FORWARD - LOOKING STATEMENTS. THOSE STATEMENTS ARE SOMETIMES INDICATED BY WORDS SUCH AS “EXPECTS,” BELIEVES”, “WILL”, AND SIMILAR EXPRESSIONS. IN ADDITION, ANY STATEMENTS THAT REFER TO EXPECTATIONS, PROJECTIONS, OR CHARACTERIZATIONS OF FUTURE EVENTS OR CIRCUMSTANCES, INCLUDING ANY UNDERLYING ASSUMPTIONS, ARE FORWARD - LOOKING STATEMENTS. SUCH STATEMENTS ARE NOT GUARANTIES OF FUTURE PERFORMANCE AND ARE SUBJECT TO CERTAIN RISKS, UNCERTAINTIES, AND ASSUMPTIONS THAT ARE DIFFICULT TO PREDICT. THEREFORE, ACTUAL RETURNS COULD DIFFER MATERIALLY AND ADVERSELY FROM THOSE EXPRESSED OR IMPLIED IN ANY FORWARD - LOOKING STATEMENTS AS A RESULT OF VARIOUS

3 BIONIK: OVERVIEW – SIGNIFICANT GROWTH POTENTIAL www.bioniklabs.com • Founded in 2011 in Toronto, Canada by Michal Prywata • Two technology platforms – Rehabilitative and Assistive Robotics • Acquired MIT spinoff to expand product portfolio • Industry leading FDA cleared rehabilitative products in market • Additional products under development • Most comprehensive clinical evidence – in > 200 peer reviewed journals testing > 1,000 patients • Leveraging clinical relationships to access home market to significantly expand commercial opportunity • International strategy to expand business and development partnerships • Chinese JV agreement signed in May 2017 to launch in world’s largest market • Focus on revenues of commercial products and bringing new products to market

www.bioniklabs.com 4 Over 25 years of executive management experience with proven track record of building public and private technology companies. Former CFO and joint Interim CEO of Sanofi Canada, CFO of Intellivax, Gennum TSX:GNM (n/k/a Semtech NASDAQ:SMTC), Just Energy (NYSE/TSX:JE); and Founder of Tribute Pharmaceuticals Experienced commercial leader in medical and healthcare technology. Top - producing and results - oriented leader with 20+ years of accomplishments in developing commercial strategies for medical technology companies, building world class global organizations and growing multimillion - dollar revenues Over 25 years of finance and accounting leadership experience. Former CFO of SunOpta (NASDAQ: STKL) MANAGEMENT TEAM Peter Bloch, CPA, CA Chief Executive Officer Tim McCarthy Chief Commercial Officer Michal Prywata Founder and Chief Operating Officer Founder of Bionik. Experienced technology inventor, biomedical engineering experience, and superior knowledge of robotic technology. Track record of winning technology showcases, innovating, and developing technologies that address significant healthcare needs. Leslie N. Markow, CPA, CA, CPA (Illinois) C.Dir Chief Financial Officer

www.bioniklabs.com 5 Key Advisors BOARD AND ADVISORS Board of Directors • Dr. Robert Hariri • Independent Director • Chairman, Founder, Chief Scientific Officer, and former Chief Executive Officer of Celgene Cellular Therapeutics; Board member of Myos Corporation and Provista Diagnostics • Marc Mathieu • Independent Director • Chief Marketing Officer of Samsung North America; Former Senior Vice President of Global Marketing at Unilever; Chairman and Co - Founder of We&Co • Peter Bloch, CPA, CA • Chairman and Chief Executive Officer • Michal Prywata • Founder and Chief Operating Officer • Hermano Krebs, Ph.D., M.S. • Principal Research Scientist and Lecturer at MIT • Neville Hogan, Ph.D., M.S, M.E. • Professor of Mechanical Engineering, Professor of Brain and Cognitive Sciences, and Director of the Newman Laboratory for Biomechanics and Human Rehabilitation at MIT • Andre Jacques Auberton - Herve • Co - Founder, President and CEO or Soitec for 23 years. • Built an international company in 10 countries, 5 manufacturing facilities (Europe, Asia, US) • Dr. Edward Lemaire • Clinical Trial Advisor & Investigator; Clinical Investigator, Centre for Rehabilitation Research, Clinical Epidemiology, Ottawa Hospital Research Institute

6 ADDRESSING MAJOR HEALTHCARE NEEDS WITH ROBOTS There is a rapidly growing need for quality of life solutions for the millions of mobility challenged people, while reducing the financial burden to the society. Our goal is to be the market leader in evidence based rehabilitative and assistive robotic technologies from hospital to home, allowing people to lead fuller and more independent lives. www.bioniklabs.com

7 UNADDRESSED GROWING IMPAIRED MOBILITY MARKET Roughly 50.5 MILLION people in the US have a mobility issue Extrapolated, that is over 1 BILLION globally These numbers are growing at pandemic rates as the population ages www.bioniklabs.com * https://www.census.gov/newsroom/releases/archives/miscellaneous/cb12 - 134.html

www.bioniklabs.com 8 STROKE EPIDEMIC: OUR MAJOR ENTRY POINT Source: Global and regional burden of stroke during 1990 – 2010: findings from the Global Burden of Disease Study 2010; 18 January 2014, Volume 383, No. 9913 Global stroke incidence per 100,000 people* Key Challenges • Stroke is the # 1 c ause of serious, long - term disability worldwide • Globally 16 Million new patients annually - will double by 2030 and double again by 2050 Key Healthcare Trends • Reimbursement shifting towards better patient outcomes vs patient volumes • Robots being adopted to improve patient outcomes

www.bioniklabs.com 9 IN - MARKET ROBOTS TACKLING STROKE EPIDEMIC • Designed to rehabilitate stroke patients with upper body neurological limitations • Interactive technology that continuously adapts and challenges the patient's ability • Allows the clinician to efficiently deliver personalized intensive sensorimotor therapy • Can overcome hypertonicity in even severely impaired neurologic patients • Can be used by clinicians as a stand - alone treatment option or in addition to the InMotion ARM • Add - on option to the InMotion ARM capable of continuously adapting to the needs of each patient • Provides assist - as - needed grasp and release training with flexible positioning InMotion ARM™ (FDA Listed ) InMotion WRIST™ (FDA Listed ) InMotion HAND™ (FDA Listed )

www.bioniklabs.com 10 BIONIK ROBOTS IMPROVING HEALTH OUTCOMES Bionik Robots Conventional • Focuses on habitual movement - repetition of a few functional motor skills. • Treatment protocols are non - reproducible • Difficult to quantify true recovery • Low intensity - 25 to 45 movements per session • Focuses on reducing impairment - neural plasticity and remapping of pathways • Reproducible to advanced clinical research standards • Bionik EVAL™ software quantifies true recovery • High intensity - over 1000 movements / session

11 THE BIONIK ADVANTAGE: A PROVEN EFFECT ON BRAIN PLASTICITY InMotion technology assists the healthcare provider to: • Treat based on neuro science principles, not only functional movements • Deliver progressive “assist - as - needed” individualized treatment plans • Precisely reproduc e and measure treatment protocols • Provide high intensity treatment • Document patient progress • Treat multiple patients at same time Powerful clinical evaluation tool, also suitable for advanced clinical research applications – Biomarker for stroke* Circle Evaluation Test Measures range of motor coordination, joint independence and coordinated movement planning Point to Point Evaluation Measures control of changes in acceleration, speed, accuracy, and movement coordination S o u r c e:” R o b o t ic M e a s u r eme n t of A r m Mo v eme n t s Af t er St r o k e E s t a bli s h e s B i o m a r k e r s of M o t o r R ec o v er y. ” St r o k e jo u r n al of A H A January 2014, Volume 45, Issue 1 t0 t0 + 2 weeks t0 + 10 weeks
12 CLINICALLY & ECONOMICALLY PROVEN - 15 YEARS OF RESEARCH Clinical Validation Economic Validation • Proven to improve health outcomes • Over 200 published papers reviewing Bionik’s products • Over 1,000 patients studied in clinical trials at prestigious academic and clinical sites • Demonstrated clinical and economically significant results compared to conventional therapy • Studies meet the highest standards of scientific rigor – independent, randomized, controlled trials • NHS - World’s la rgest robotic st ud y being c ondu cte d exclusively w i t h Bionik r obo t s with over 720 patients Veteran Affairs Healthcare System clinical trial using Bionik’s Products* • 25% reduction in healthcare expenses at 36 weeks - Robot treated group consumed fewer healthcare resources - Reduced outpatient visits - Reduced readmissions • VA study a strong indicator for widespread clinical adoption within bundled reimbursement environment “ An Ec o n o m i c A n a l y s i s of R o b ot - Ass i s t e d T h er a p y f or Lo ng - T erm U pp er - L i m b I m p a i rme nt A f t e r S t r ok e ” T o dd Wa gn er , J o u r n a l of t h e Amer i c a n H e a r t Ass o c i a t i on , Vo l . 42 N o. 9 Se pt em b e r 2011 http:// newmanlab.mit.edu/people /
13 ROBOTIC THERAPY RECOMMENDATION BASED ON BIONIK’S DATA • Referencing Bionik’s cli n ica l trial s , AHA's Str o ke Division assigned highest recommendation level for r obo t a ssiste d t h er a py f o r upp er extremity m o t o r re habili tat ion of moderate to severe str o ke p at i ent s • VA stroke rehabilitation guidelines recommend robot assisted therapy for upper extremity motor rehabilitation: - VA Objective: “Develop and implement robot - assisted interventions as standard clinical practice for patients who have suffered neurological injury due to conditions such as stroke, spinal cord injury or multiple sclerosis” Sour c e: A m eri c an H e a rt A ss o c i a t io n Scien t i f ic St a t e m ent Publi s he d i n Stroke Miller et a l . ( 2010 ) ; 41 : 240 2 - 2448 D ep t . o f Ve t er a n s A ffa ir s a n d D ep t . o f D e f en s e, M a n ag e m ent o f S t roke R eh a bili t a t io n W orking G roup . “VA /D o D C lini c al Pr a ct i c e G uideline f or t he M a n ag e m ent o f S t roke R eh a bili t a t ion, G uideline Su mm a ry. ” W as hin g t on, D . C .: G overn m ent Printing Off i c e, O ct ober ( 2010 ) V er s. 2 . 0 , p . 3 7 U R L: ht tp : // www . h eal t hquali t y.va.go v. V A R e s e a r c h a n d D eve l opmen t S t r a t e gi c P la n : 200 9 - 2014

14 BIONIK EXCLUSIVELY SELECTED FOR WORLD’S LARGEST ROBOTIC STUDY • Land m a rk UK st ud y: World’s la rgest st ud y c ondu cte d w i t h r obo t i c s f o r re habili tat ion • Funded by the research arm of the National Health Service (NHS), the largest and oldest single - payer healthcare system in the world • Exclusively uses Bionik’s upper body products RATULS - Three arm randomized controlled trial designed to determine whether robot assisted training improves upper limb function post stroke compared to enhanced upper limb therapy or conventional therapy 720 s ub jec t s a c r o s s f ou r s t ud y ce nt er s i n U n i t e d K i n g do m Pr i m a ry ou t c o me: • U p p er li m b fun c t i on me as u r e d b y t h e A c t i on R e s e a r c h A rm T e s t ( A R A T ) S e c o nd a ry o u t c o m e s • U p p er li m b i m p ai rmen t ( F u g l - M e y er T e s t ) • A c t i v i t ie s of da il y li v i n g ( B a rt h e l A D L I nd e x) • Qua li t y of li f e ( S t r o k e I m pa ct S c a l e, E Q - 5 D - 5 L ) • A d v e r s e e v e nt s i n c l ud i n g upp e r li m b pa i n (nu m e r i c al r a t i n g s c a le ) R obot A ssisted T raining for the U pper L imb after S troke (RATULS) “This is currently the best available technology for robot assisted training for patients with moderate to severe upper limb impairment post stroke. It has CE medical approval and is supported by an infrastructure for production, distribution and maintenance.”* *Robot Assisted Training for the Upper Limb after Stroke: Study Protocol Version 3; August 2, 2016

www.bioniklabs.com 15 ROBOTS IN DEVELOPMENT TACKLING STROKE EPIDEMIC • Exoskeletal robotic system using the same design principles • Designed in close collaboration with the Newman Laboratory for Biomechanics and Human Rehabilitation • Currently available in multiple clinics for research in the U.S. • Upper body stroke technology redesigned and adapted for use in the home • Extends the continuum of care from the hospital to the home InMotion ANKLEBOT™ InMotion HOME™
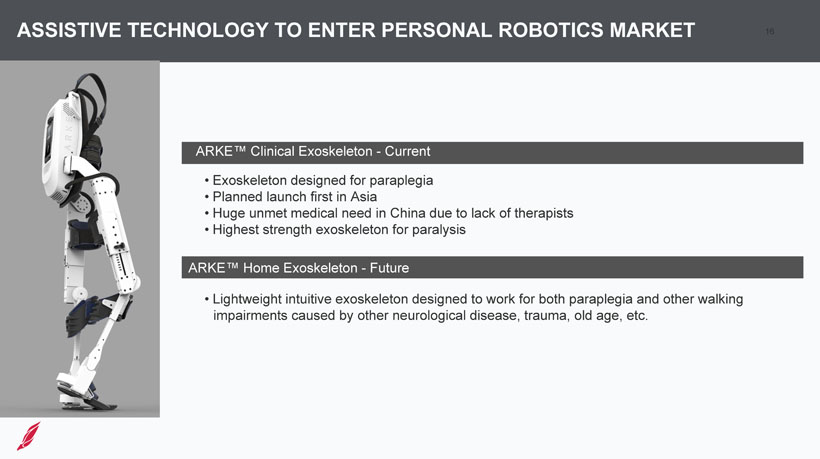
16 ASSISTIVE TECHNOLOGY TO ENTER PERSONAL ROBOTICS MARKET • Exoskeleton designed for paraplegia • Planned launch first in Asia • Huge unmet medical need in China due to lack of therapists • Highest strength exoskeleton for paralysis • Lightweight intuitive exoskeleton designed to work for both paraplegia and other walking impairments caused by other neurological disease, trauma, old age, etc. ARKE™ Clinical Exoskeleton - Current ARKE™ Home Exoskeleton - Future

17 SIGNIFICANT OPPORTUNITY FROM B2B TO B2C Rehab Robotics Assistive Robotics CLINICAL: Addressable Market estimated at $1B+ per year • With average selling price of $100k per robot • In Market • Clinical robots are point of entry for the home market HOME: Addressable Market estimated at $4B+ per year • With average home rental at $ 4,500 per person • In Development CLINICAL: Addressable Market estimated at $200M+ per year • With average selling price of $75k per robot • Planned launch into Chinese market HOME (Planned future development) : Potential Market estimated at $22B+ per year • Targeted average selling price of $5,000 - $7,000 • Market size based on number of wheelchair users and individuals requiring canes, crutches, and walkers. • Starting to build relationships and licensing technology • Market includes elderly population • Amortized over 30 years, not including growth

www.bioniklabs.com 18 PRODUCT ROADMAP PRODUCT DEVELOPMENT PRE - CLINICAL CLINICAL IN MARKET MILESTONE InMotion ARM In Market InMotion HAND In Market InMotion WRIST In Market; Updated product Q4 ’17 InMotion AHW V2 Commercial Launch Q3 ’17 ARKE Lower Body Exoskeleton Launch ARKE 2.5 in Asia InMotion ANKLEBOT Advance as funding allows InMotion HOME Commercial Launch 2018 ARKE Home Bionik experience + Licensing + Partnerships

19 18 MONTH GROWTH STRATEGY 1. Offer clinically proven products from hospital to home ▪ Deploy our new commercial version upper body robots ▪ Expand clinical robot offering into lower extremity ▪ Extend clinical robot offering into home 2. Increase clinical install - base ▪ Target high volume inpatient centres to expose technology and build pipeline ▪ Leverage rental model with our financial partner to reduce capital purchasing cycles 3. Access rapidly growing Asian markets ▪ Leverage our Chinese joint venture distribution agreement

20 HIGH GROWTH REVENUE POSITIONING: SNOWBALL EFFECT B2B to B2C 5% cash pay patients 20 patients per month @ $4,500 per patient = $1M annually per clinical installation • 99% of stroke patients are discharged from Acute Care hospitals to Post Acute Care (PAC) settings • Just 100 “high volume” clinics could generate $100 million in annual revenue from home robot rental - Customer can discharge 20 cash pay patients (5%) per month with our home robot rental products

21 FINANCIAL SNAPSHOT STOCK TICKER SYMBOL OTCQX: BNKL Market Capitalization as of June 9 , 2017 $ 2 8 . 1 M Shares Outstanding* 96. 8 M Warrants at exercise price of $1.40 16.4 M Warrants at exercise price of $0.80 1.2 M Amount received in grants to date $4 M *Includes 47,909,336 Exchangeable Shares which have the same economic and voting rights as common stock.

22 SUMMARY • Proprietary robotics product line in market focused on stroke rehabilitation and assistive technology solutions from hospital to home • Proven clinical evidence differentiates product portfolio and puts Bionik in a leading position for reimbursement • High growth revenue potential: snowball effect going from B2B to B2C • Highly experienced team executing on robust commercial strategy to fuel revenue generation • Advancing growth strategy with late - stage distribution and development negotiations to expand reach into key markets • Planned NASDAQ up list prior to year end

www.bioniklabs.com 23 Headquarters 483 Bay Street, Office N105 Toronto, ON M5G 2C9 Canada Investor Relations Counsel Amato and Partners, LLC admin@amatoandpartners.com PR Contact matt@fischtankpr.com BIONIK LABORATORIES
