Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Aldeyra Therapeutics, Inc. | d352977dex992.htm |
| 8-K - FORM 8-K - Aldeyra Therapeutics, Inc. | d352977d8k.htm |

Allergic Conjunctivitis Phase 2b Results June 14, 2017 Exhibit 99.1

Disclaimers and Forward-Looking Statements This presentation and various remarks which may be made during this presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations , expenses and financing needs, business strategies and plans, research, development and regulatory plans or expectations, trends, the structure , timing and success of Aldeyra’s planned or pending clinical trials, market sizing, pricing and reimbursement, competitive position, regulatory matters, industry environment and potential growth opportunities, among other things. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan” or similar expressions and the negatives of those terms. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These statements reflect Aldeyra’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the development and clinical plans for Aldeyra’s product candidates and Aldeyra’s continuing review and quality control analysis of clinical data. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Aldeyra’s Annual Report on Form 10-K for the year ended December 31, 2016 and Quarterly Report on Form 10-Q for the quarter ended March 31, 2017, which are on file with the Securities and Exchange Commission (SEC) and available on the SEC's website at www.sec.gov. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information in this presentation is provided only as of June 14, 2017, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law.

Allergic Conjunctivitis Phase 2b Key Conclusions The results confirm the activity of ADX-102 in allergic conjunctivitis. Aldeyra plans to move forward aggressively to Phase 3 testing. Aldeyra believes that the differentiated clinical response to ADX-102 supports a compelling product opportunity in a significant, unaddressed segment of the allergic conjunctivitis population, potentially representing one of the largest ophthalmic markets worldwide. The data provide further validation of the anti-inflammatory potential of Aldeyra’s novel aldehyde trap platform.
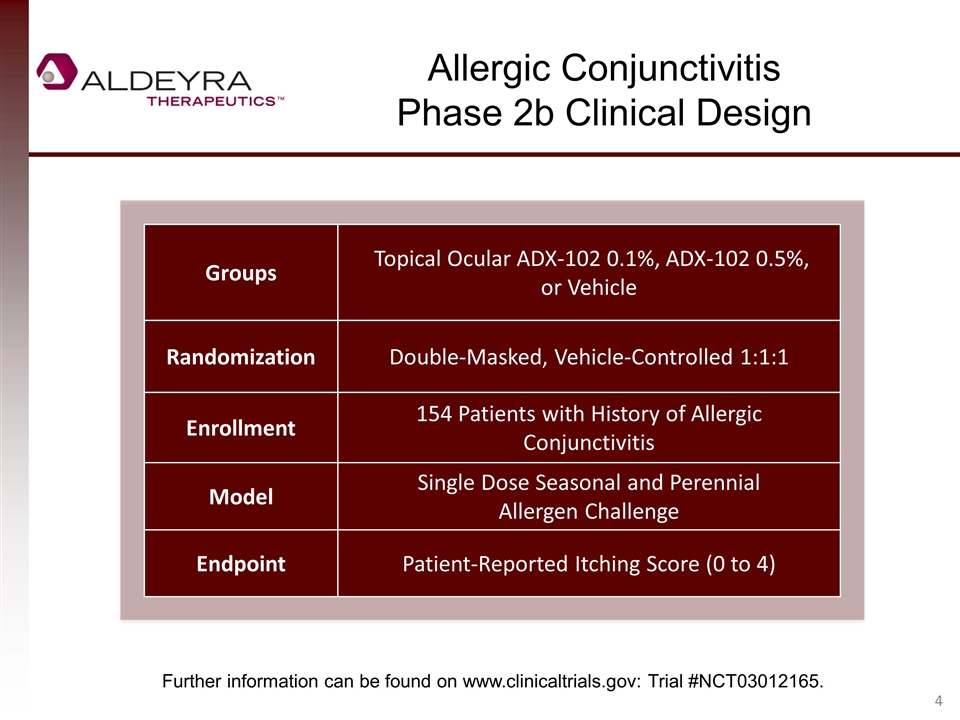
Allergic Conjunctivitis Phase 2b Clinical Design Groups Topical Ocular ADX-102 0.1%, ADX-102 0.5%, or Vehicle Randomization Double-Masked, Vehicle-Controlled 1:1:1 Enrollment 154 Patients with History of Allergic Conjunctivitis Model Single Dose Seasonal and Perennial Allergen Challenge Endpoint Patient-Reported Itching Score (0 to 4) Further information can be found on www.clinicaltrials.gov: Trial #NCT03012165.
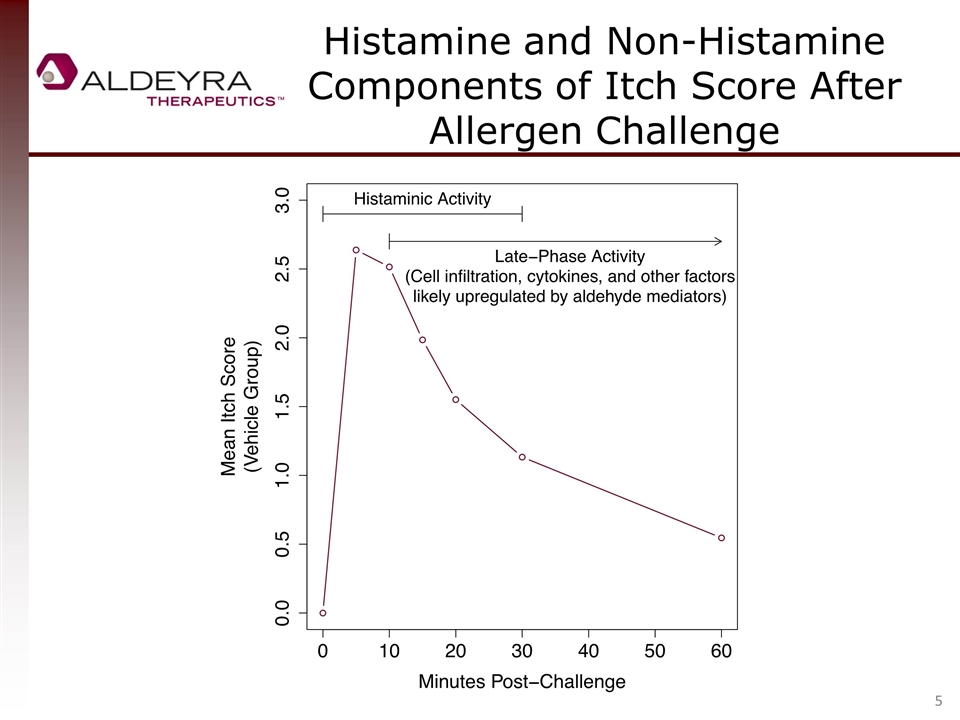
Histamine and Non-Histamine Components of Itch Score After Allergen Challenge
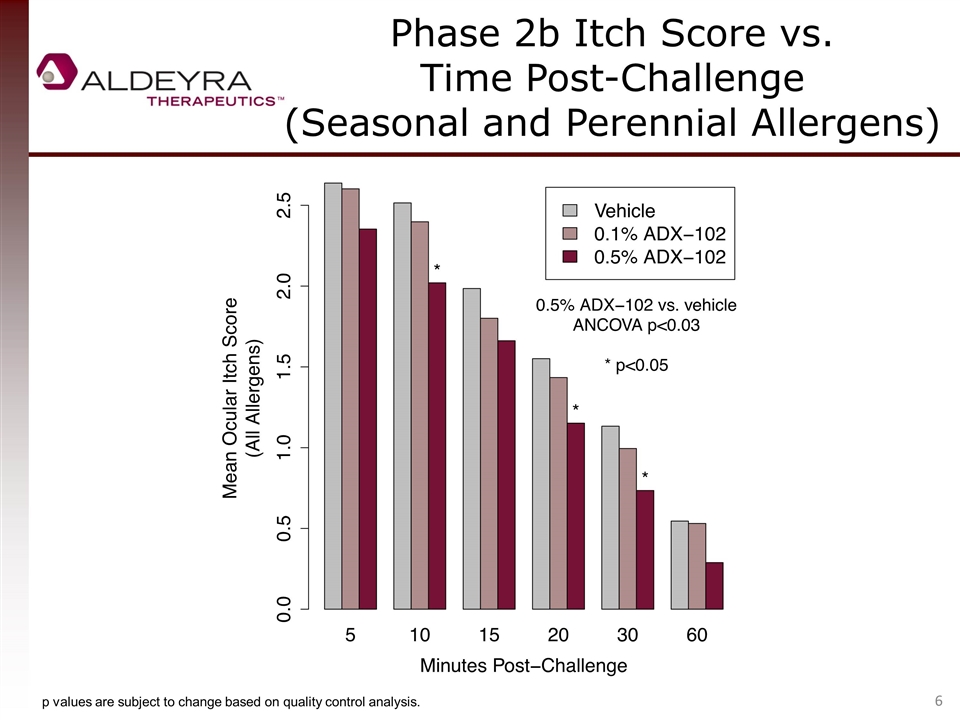
Phase 2b Itch Score vs. Time Post-Challenge (Seasonal and Perennial Allergens) p values are subject to change based on quality control analysis.
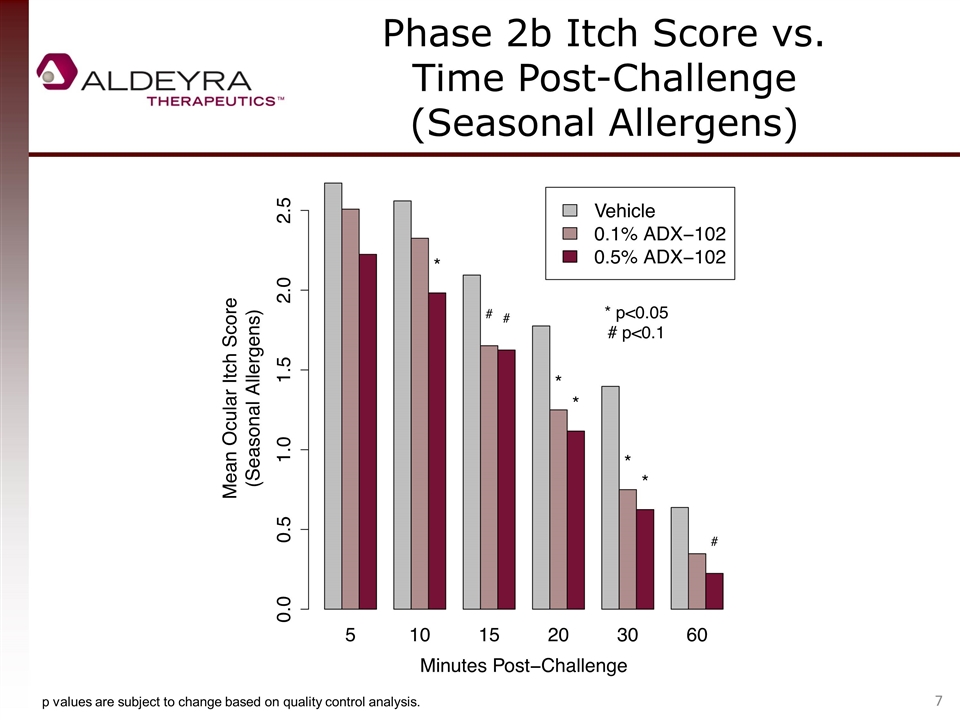
Phase 2b Itch Score vs. Time Post-Challenge (Seasonal Allergens) p values are subject to change based on quality control analysis.
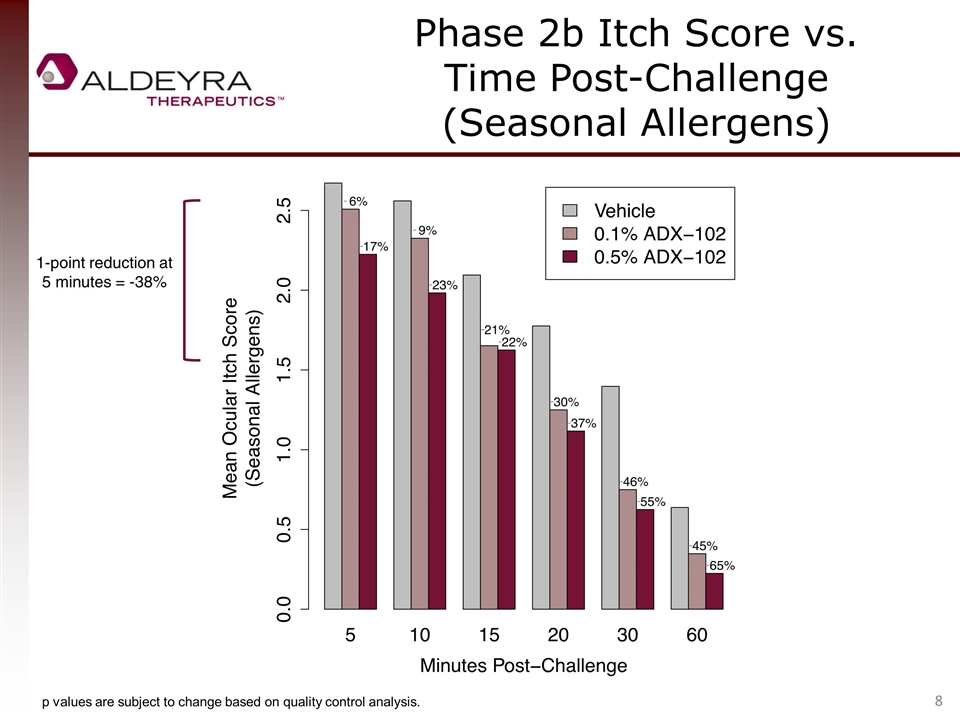
Phase 2b Itch Score vs. Time Post-Challenge (Seasonal Allergens) 1-point reduction at 5 minutes = -38% p values are subject to change based on quality control analysis.
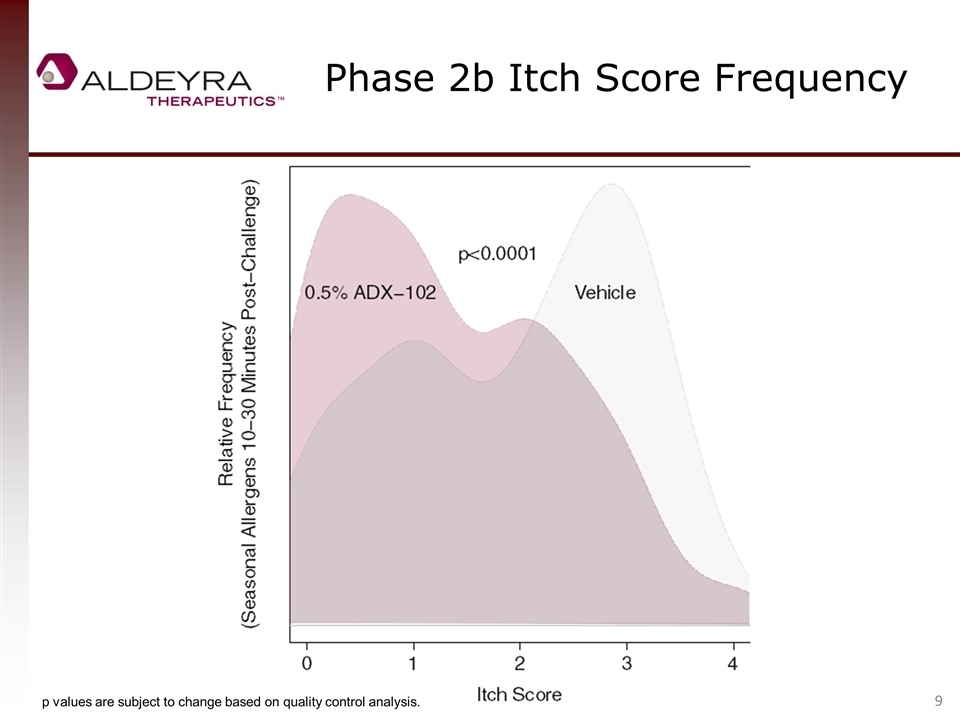
Phase 2b Itch Score Frequency p values are subject to change based on quality control analysis.
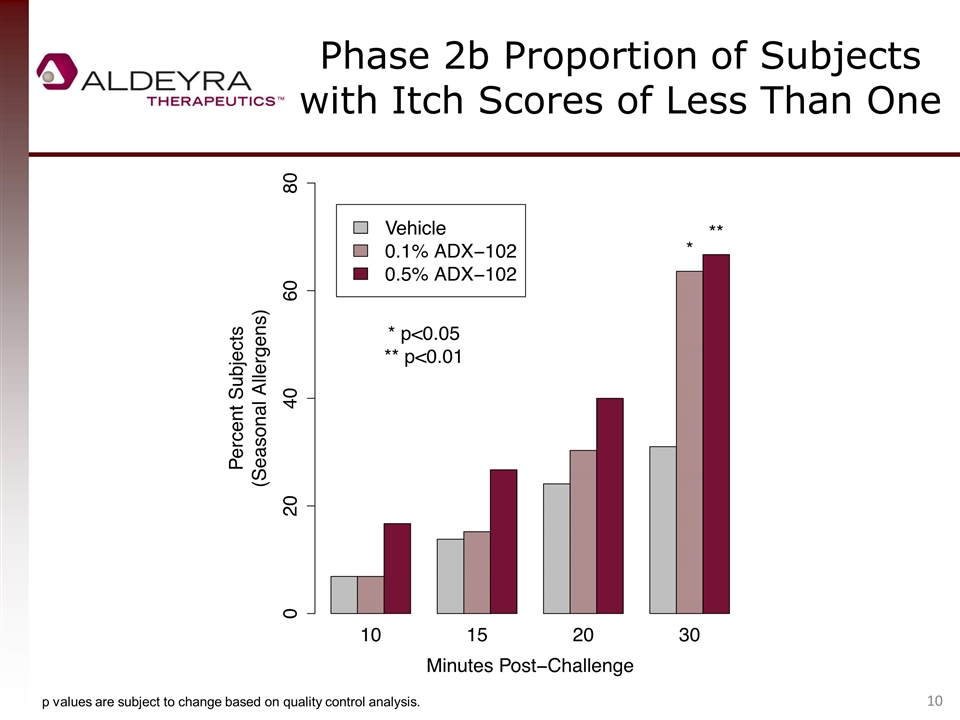
Phase 2b Proportion of Subjects with Itch Scores of Less Than One p values are subject to change based on quality control analysis.

Allergic Conjunctivitis Phase 2b Clinical Trial Summary One-point itch score reduction vs. control (a component of the primary endpoint of all allergen challenge allergic conjunctivitis trials to date) was not met, but approached in seasonal allergy patients (peak reduction of 0.8). 0.5% ADX-102 was statistically superior to control in reducing itching. One-point equivalent percent reductions of 38% were observed in seasonal allergy patients during the late inflammatory phase. The proportion of patients with minimal or zero itch was statistically superior to control at 30 minutes. A clear and highly consistent dose response was demonstrated, confirming the biological effect of drug. Both concentrations of drug were well tolerated; no serious adverse events were reported. The late inflammatory phase drug activity is differentiated from antihistamines, an effect that is, to our knowledge, unprecedented. Late phase inflammation is responsible for suboptimal response to antihistamines and persistent disease associated with serious and chronic forms of allergic conjunctivitis, an area of high medical need.

Allergic Conjunctivitis: A Common Unmet Medical Need Allergic conjunctivitis is estimated to affect 20% or more of worldwide population Symptoms include stinging, burning, tearing, pain, itching, redness Aldehydes may mediate IL-5 release and other pro-allergy cytokines An estimated 30 % - 40% of patients may not respond sufficiently to antihistamines

Allergic Conjunctivitis Phase 2b Key Conclusions The results confirm the activity of ADX-102 in allergic conjunctivitis. Aldeyra plans to move forward aggressively to Phase 3 testing. Aldeyra believes that the differentiated clinical response to ADX-102 supports a compelling product opportunity in a significant, unaddressed segment of the allergic conjunctivitis population, representing one of the largest ophthalmic markets worldwide. The data provide further validation of the anti-inflammatory potential of Aldeyra’s novel aldehyde trap platform.
