Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - HEAT BIOLOGICS, INC. | htbx_8k.htm |
EXHIBIT 99.1

NASDAQ:HTBX May 31, 2017

This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements’’ within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2016 and our quarterly report on Form 10-Q for the subsequent quarters (collectively, our “SEC Filings”). In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law.You should read carefully the factors described in the “Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business. 2 Forward Looking Statements
Investment Opportunity 3 Favorable Safety Profile TECHNOLOGIES Multiple Platform Signals in lung cancer with CHECKPOINT Inhibitors POTENTIAL BEST-IN-CLASS Activation of Cancer Killing CD8+ T Cells ROBUST Pipeline of COMBINATION Therapies Turning COLD Tumors HOT
4 Mission Our Mission is to Activate CD8+ ”Killer” T cells to Turn “COLD” Tumors “HOT” Cold Hot Our goal is to combine with checkpoint inhibitors and other immunotherapies to dramatically increase their effectiveness
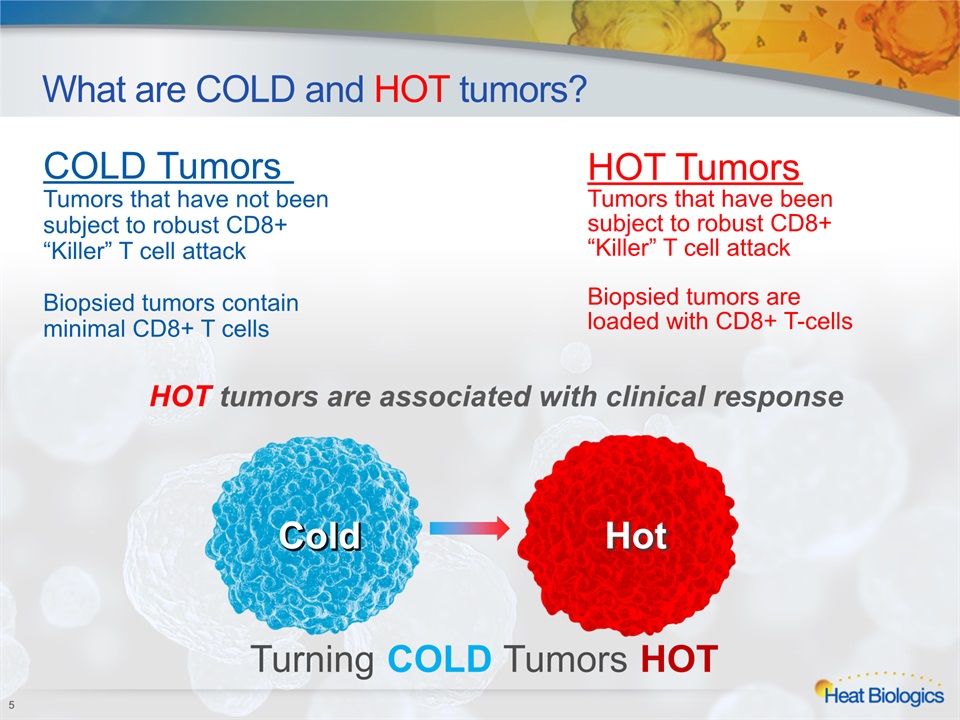
What are COLD and HOT tumors? 5 Turning COLD Tumors HOT Cold Hot COLD Tumors Tumors that have not been subject to robust CD8+ “Killer” T cell attackBiopsied tumors contain minimal CD8+ T cells HOT TumorsTumors that have been subject to robust CD8+ “Killer” T cell attack Biopsied tumors are loaded with CD8+ T-cells HOT tumors are associated with clinical response
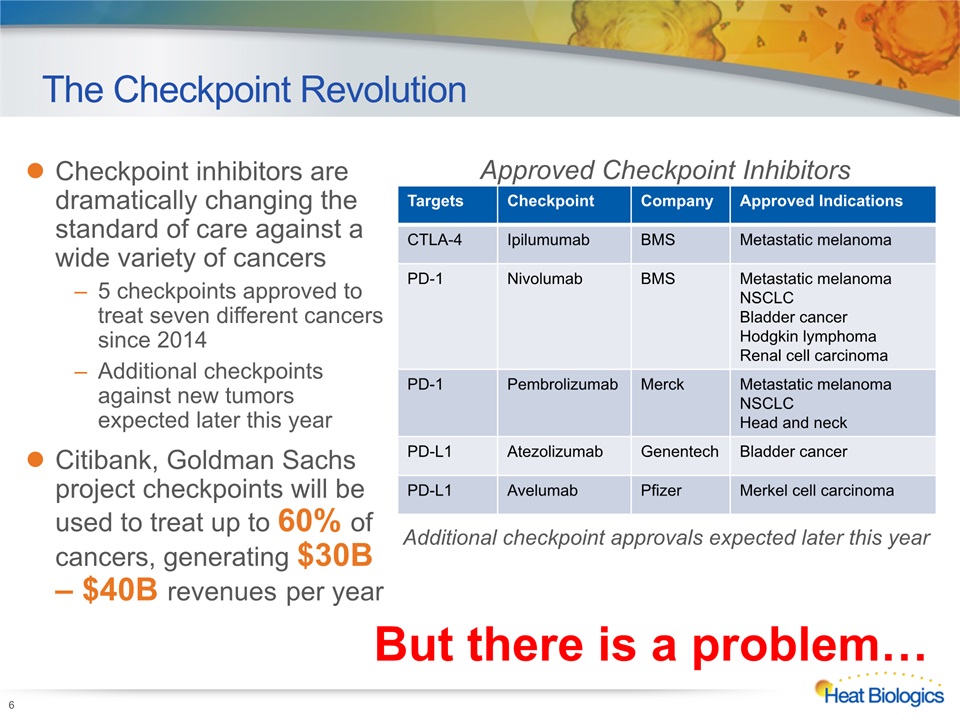
Checkpoint inhibitors are dramatically changing the standard of care against a wide variety of cancers5 checkpoints approved to treat seven different cancers since 2014Additional checkpoints against new tumors expected later this yearCitibank, Goldman Sachs project checkpoints will be used to treat up to 60% of cancers, generating $30B – $40B revenues per year 6 The Checkpoint Revolution But there is a problem… Targets Checkpoint Company Approved Indications CTLA-4 Ipilumumab BMS Metastatic melanoma PD-1 Nivolumab BMS Metastatic melanomaNSCLCBladder cancerHodgkin lymphomaRenal cell carcinoma PD-1 Pembrolizumab Merck Metastatic melanomaNSCLCHead and neck PD-L1 Atezolizumab Genentech Bladder cancer PD-L1 Avelumab Pfizer Merkel cell carcinoma Approved Checkpoint Inhibitors Additional checkpoint approvals expected later this year
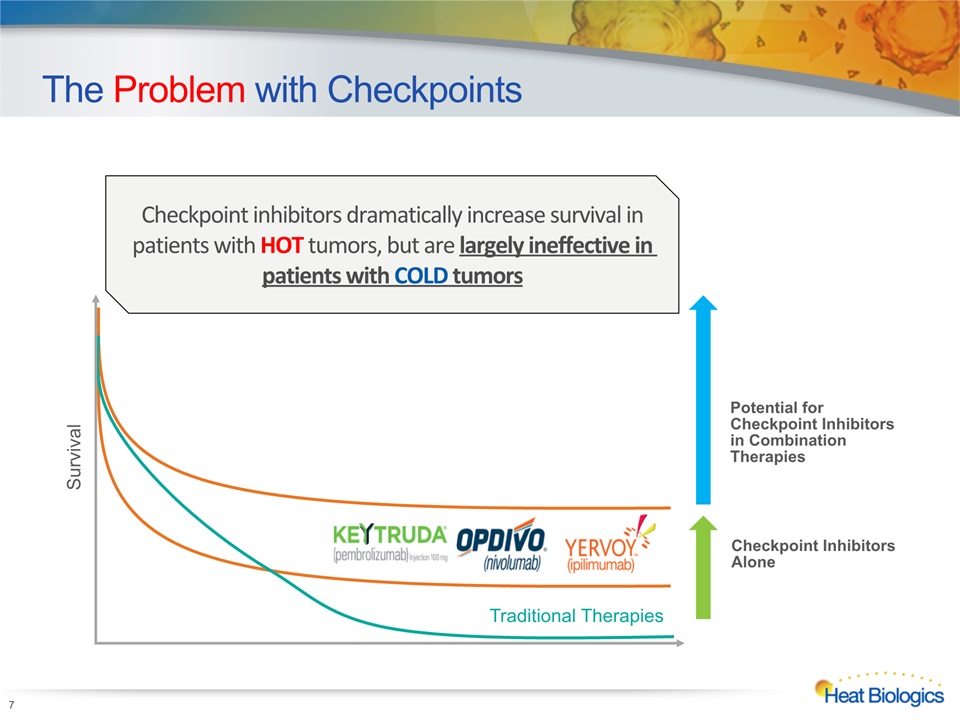
7 The Problem with Checkpoints Survival Traditional Therapies Checkpoint Inhibitors Alone Checkpoint inhibitors dramatically increase survival in patients with HOT tumors, but are largely ineffective in patients with COLD tumors Potential for Checkpoint Inhibitors in Combination Therapies
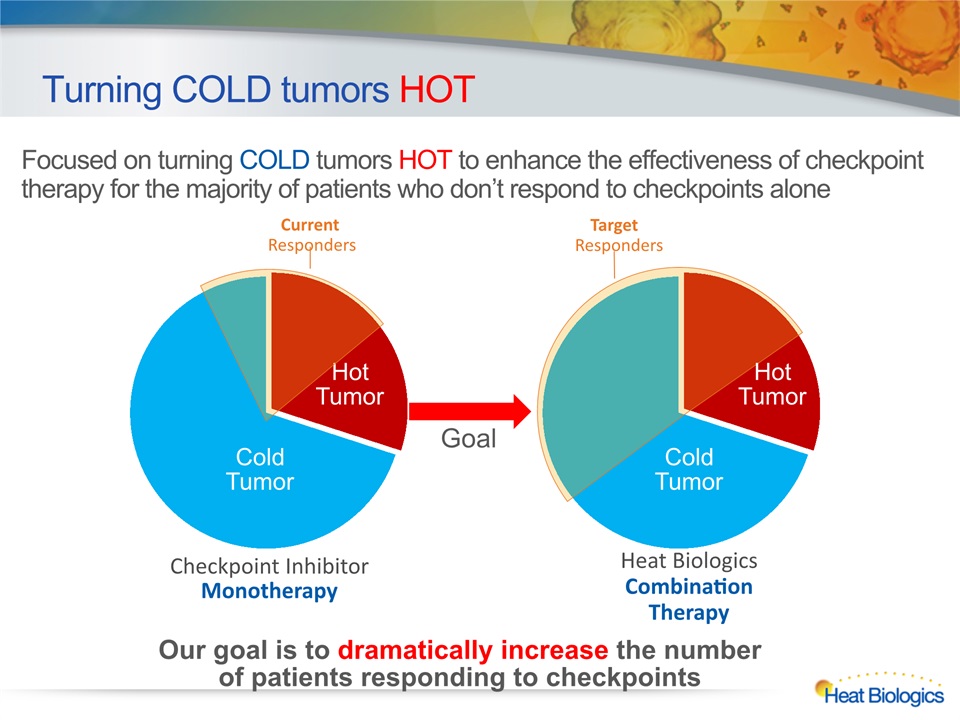
Turning COLD tumors HOT Focused on turning COLD tumors HOT to enhance the effectiveness of checkpoint therapy for the majority of patients who don’t respond to checkpoints alone Checkpoint Inhibitor Monotherapy Heat BiologicsCombination Therapy Cold Tumor Cold Tumor Hot Tumor Hot Tumor Current Responders Target Responders Goal Our goal is to dramatically increase the number of patients responding to checkpoints
Activates CD8+ T cells, a critical component of effective anti-cancer immunityActivated T cells invade tumor siteSimple once-a-week intradermal injectionWell-tolerated with combinationsClinical and preclinical data supports activity with ImPACT and checkpoint inhibitors 9 Checkpoint Combination Advantages PD1/PD-L1CTLA-4Lag-3TIM-3Plus Others Heat’s ImPACT Technology
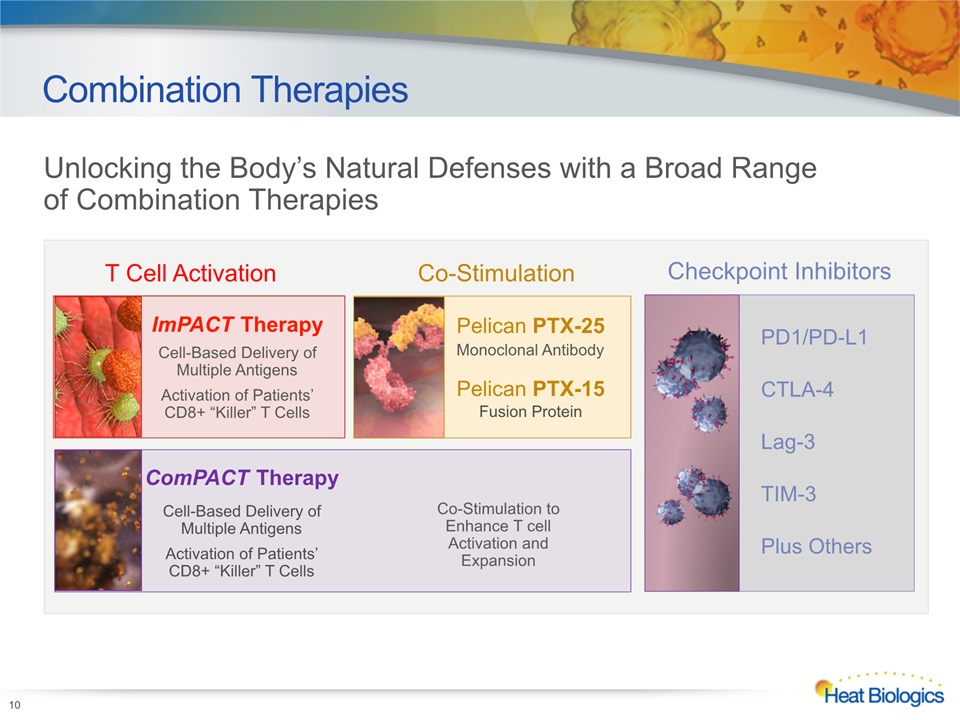
Combination Therapies 10 T Cell Activation ImPACT TherapyCell-Based Delivery of Multiple Antigens Activation of Patients’CD8+ “Killer” T Cells ComPACT TherapyCell-Based Delivery ofMultiple Antigens Activation of Patients’CD8+ “Killer” T Cells Co-Stimulation Pelican PTX-25Monoclonal AntibodyPelican PTX-15Fusion Protein Co-Stimulation to Enhance T cell Activation and Expansion Checkpoint Inhibitors PD1/PD-L1CTLA-4Lag-3TIM-3Plus Others Unlocking the Body’s Natural Defenses with a Broad Range of Combination Therapies
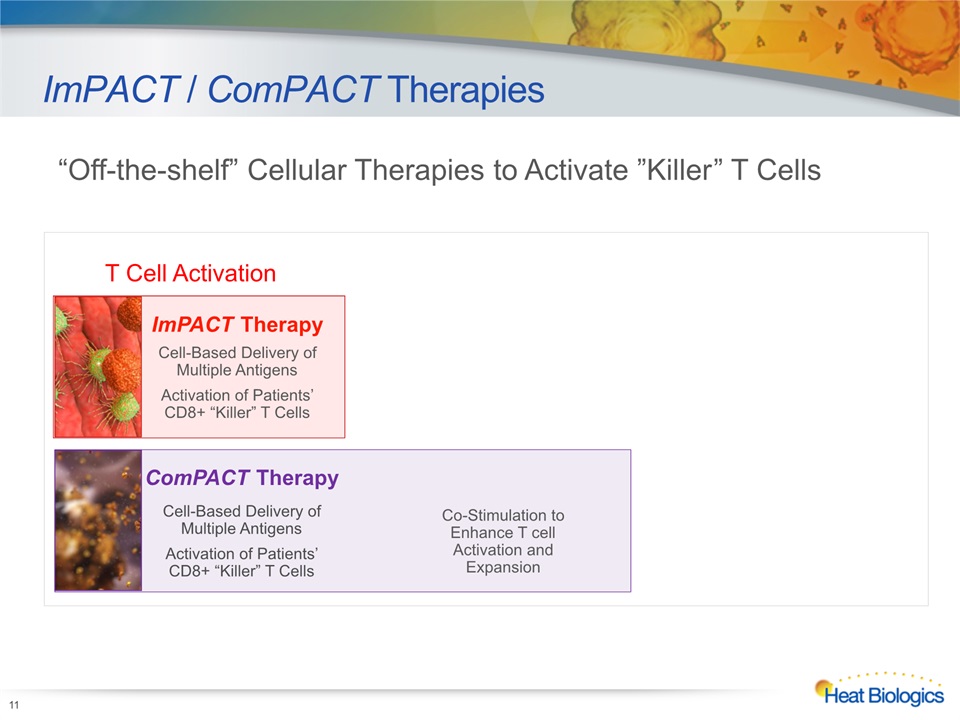
PD1/PD-L1CTLA-4Lag-3TIM-3Plus Others Pelican PTX-25Monoclonal AntibodyPelican PTX-15Fusion Protein ImPACT / ComPACT Therapies 11 T Cell Activation ImPACT TherapyCell-Based Delivery of Multiple Antigens Activation of Patients’CD8+ “Killer” T Cells ComPACT TherapyCell-Based Delivery ofMultiple Antigens Activation of Patients’CD8+ “Killer” T Cells Co-Stimulation to Enhance T cell Activation and Expansion “Off-the-shelf” Cellular Therapies to Activate ”Killer” T Cells
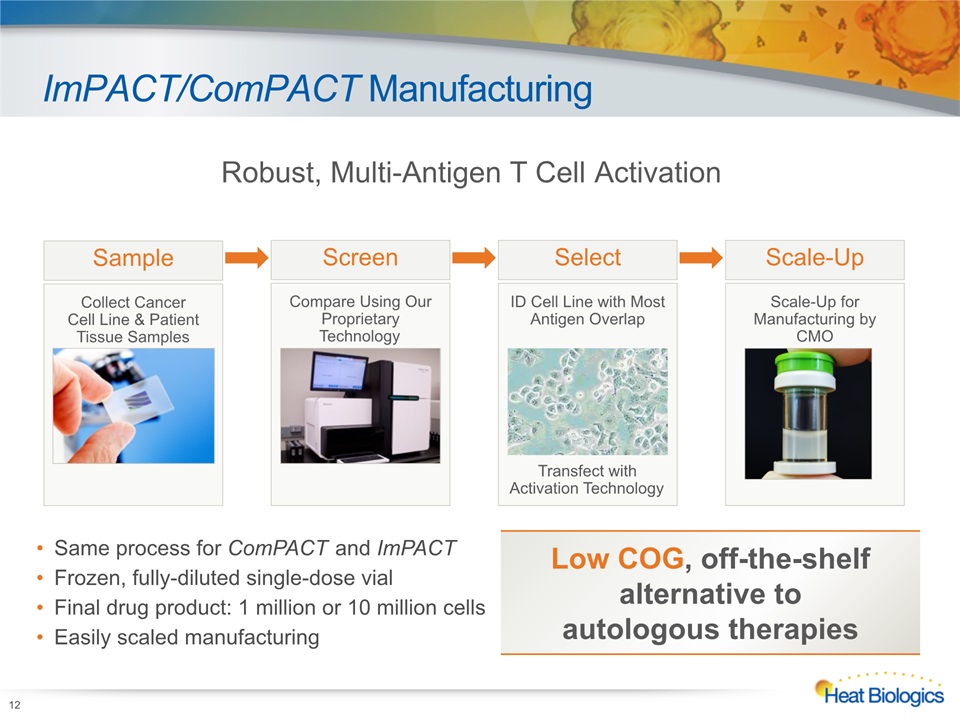
ImPACT/ComPACT Manufacturing 12 Robust, Multi-Antigen T Cell Activation Compare Using Our Proprietary Technology Screen ID Cell Line with Most Antigen OverlapTransfect with Activation Technology Select Scale-Up for Manufacturing by CMO Scale-Up Collect CancerCell Line & Patient Tissue Samples Sample Same process for ComPACT and ImPACTFrozen, fully-diluted single-dose vialFinal drug product: 1 million or 10 million cellsEasily scaled manufacturing Low COG, off-the-shelf alternative to autologous therapies
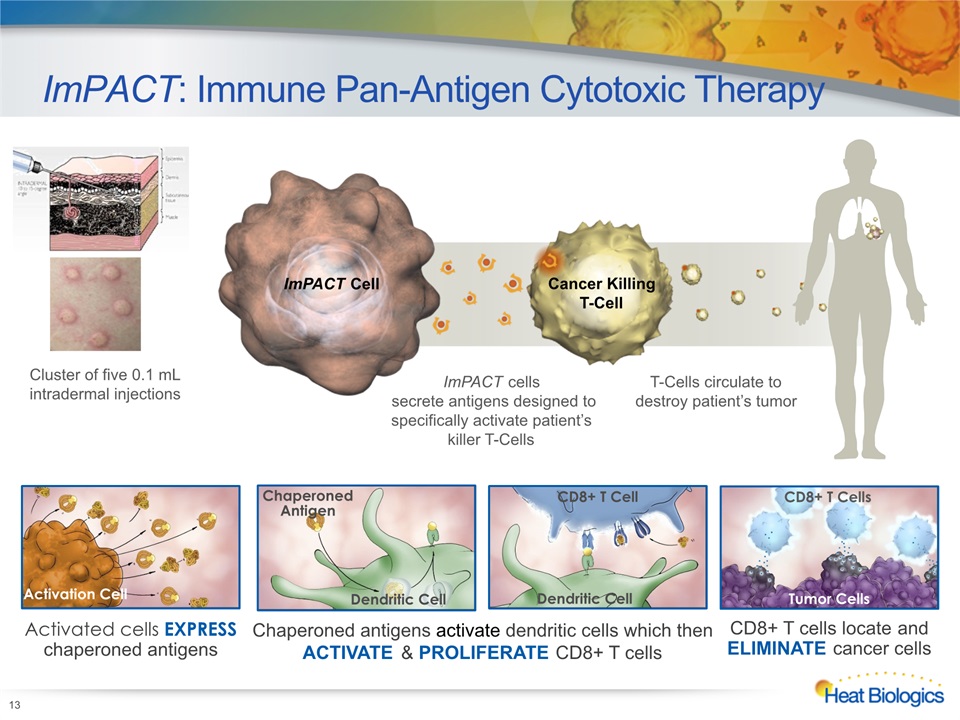
ImPACT: Immune Pan-Antigen Cytotoxic Therapy 13 ImPACT Cell Cancer Killing T-Cell ImPACT cells secrete antigens designed to specifically activate patient’s killer T-Cells T-Cells circulate to destroy patient’s tumor Cluster of five 0.1 mL intradermal injections Activated cells EXPRESS chaperoned antigens Activation Cell CD8+ T Cell Dendritic Cell Chaperoned antigens activate dendritic cells which thenACTIVATE & PROLIFERATE CD8+ T cells Dendritic Cell ChaperonedAntigen CD8+ T cells locate and ELIMINATE cancer cells Tumor Cells CD8+ T Cells
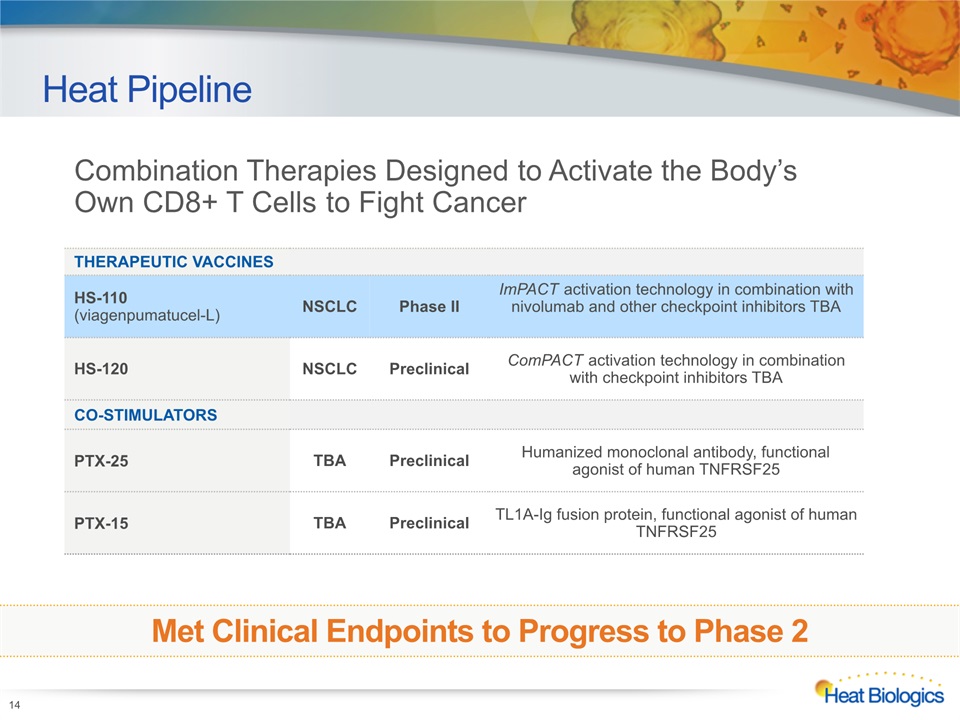
Heat Pipeline 14 Heat Biologics Corporate Model Deck THERAPEUTIC VACCINES HS-110(viagenpumatucel-L) NSCLC Phase II ImPACT activation technology in combination with nivolumab and other checkpoint inhibitors TBA HS-120 NSCLC Preclinical ComPACT activation technology in combination with checkpoint inhibitors TBA CO-STIMULATORS PTX-25 TBA Preclinical Humanized monoclonal antibody, functional agonist of human TNFRSF25 PTX-15 TBA Preclinical TL1A-Ig fusion protein, functional agonist of human TNFRSF25 Combination Therapies Designed to Activate the Body’s Own CD8+ T Cells to Fight Cancer Met Clinical Endpoints to Progress to Phase 2
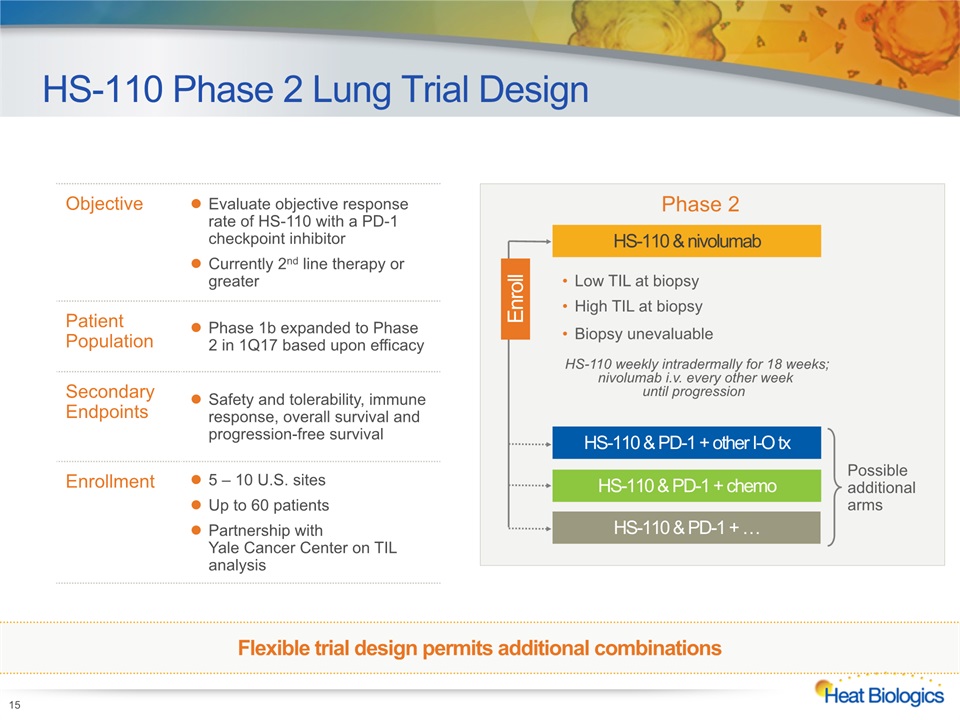
Objective Evaluate objective response rate of HS-110 with a PD-1 checkpoint inhibitorCurrently 2nd line therapy or greater Patient Population Phase 1b expanded to Phase 2 in 1Q17 based upon efficacy Secondary Endpoints Safety and tolerability, immune response, overall survival and progression-free survival Enrollment 5 – 10 U.S. sitesUp to 60 patientsPartnership with Yale Cancer Center on TIL analysis HS-110 Phase 2 Lung Trial Design 15 HS-110 weekly intradermally for 18 weeks; nivolumab i.v. every other week until progression HS-110 & PD-1 + other I-O tx HS-110 & PD-1 + chemo HS-110 & PD-1 + … Phase 2 Possible additional arms Low TIL at biopsy High TIL at biopsyBiopsy unevaluable HS-110 & nivolumab Enroll Flexible trial design permits additional combinations
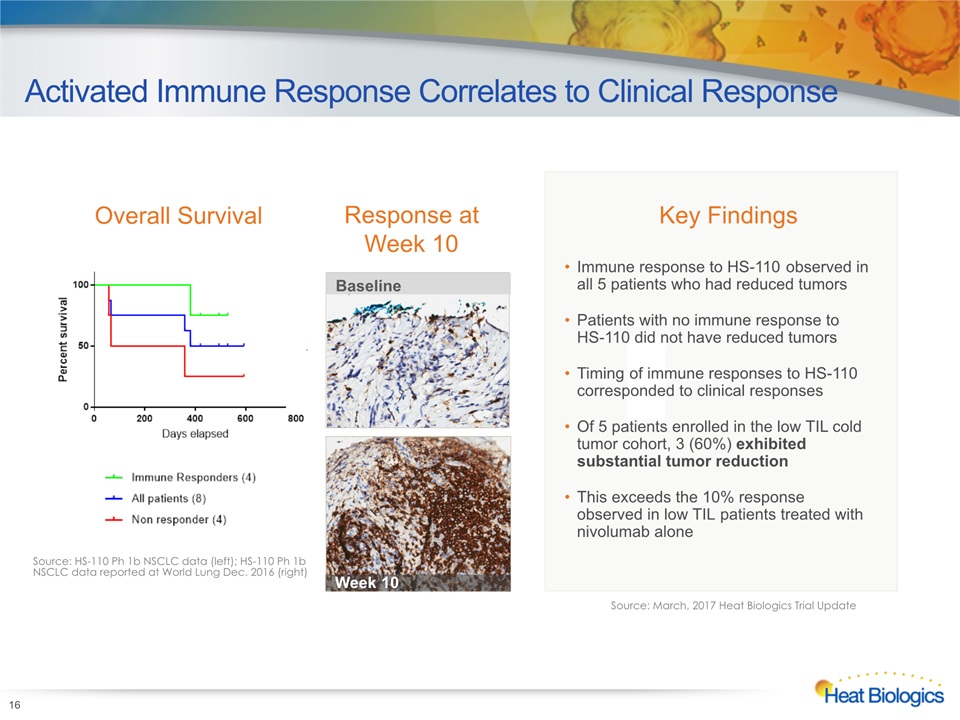
16 Activated Immune Response Correlates to Clinical Response Key Findings Source: HS-110 Ph 1b NSCLC data (left); HS-110 Ph 1b NSCLC data reported at World Lung Dec. 2016 (right) Immune response to HS-110 observed in all 5 patients who had reduced tumorsPatients with no immune response to HS-110 did not have reduced tumorsTiming of immune responses to HS-110 corresponded to clinical responsesOf 5 patients enrolled in the low TIL cold tumor cohort, 3 (60%) exhibited substantial tumor reduction This exceeds the 10% response observed in low TIL patients treated with nivolumab alone Overall Survival Response at Week 10 High CD8+ TIL (≥10%)* Baseline Week 10 Source: March, 2017 Heat Biologics Trial Update
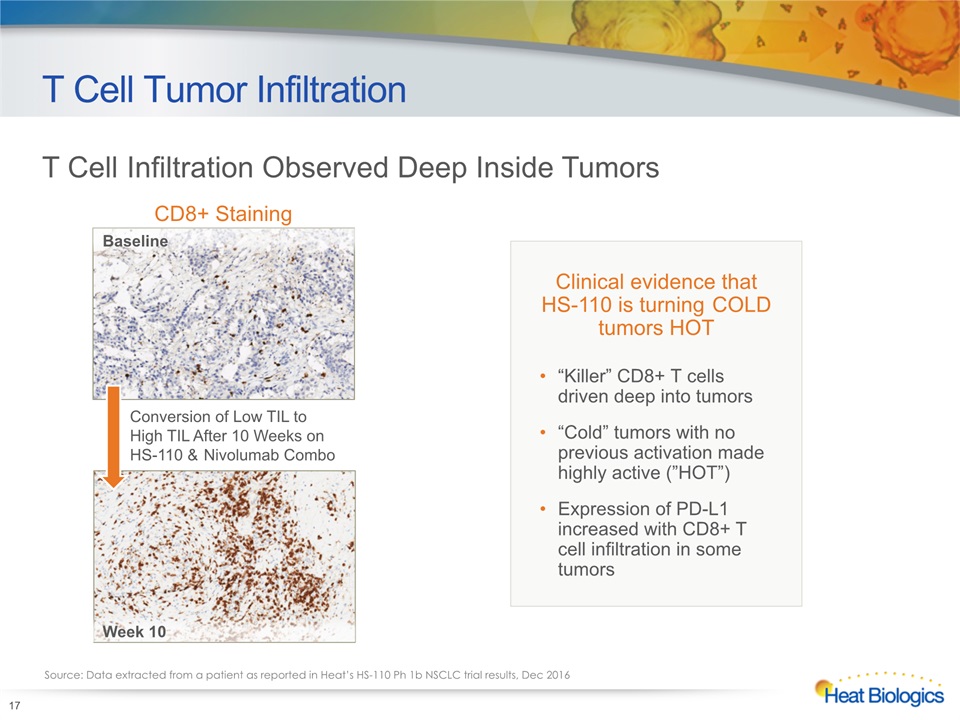
T Cell Tumor Infiltration 17 Clinical evidence that HS-110 is turning COLD tumors HOT“Killer” CD8+ T cells driven deep into tumors“Cold” tumors with no previous activation made highly active (”HOT”)Expression of PD-L1 increased with CD8+ T cell infiltration in some tumors T Cell Infiltration Observed Deep Inside Tumors Source: Data extracted from a patient as reported in Heat’s HS-110 Ph 1b NSCLC trial results, Dec 2016 CD8+ Staining Baseline Week 10 Conversion of Low TIL to High TIL After 10 Weeks onHS-110 & Nivolumab Combo
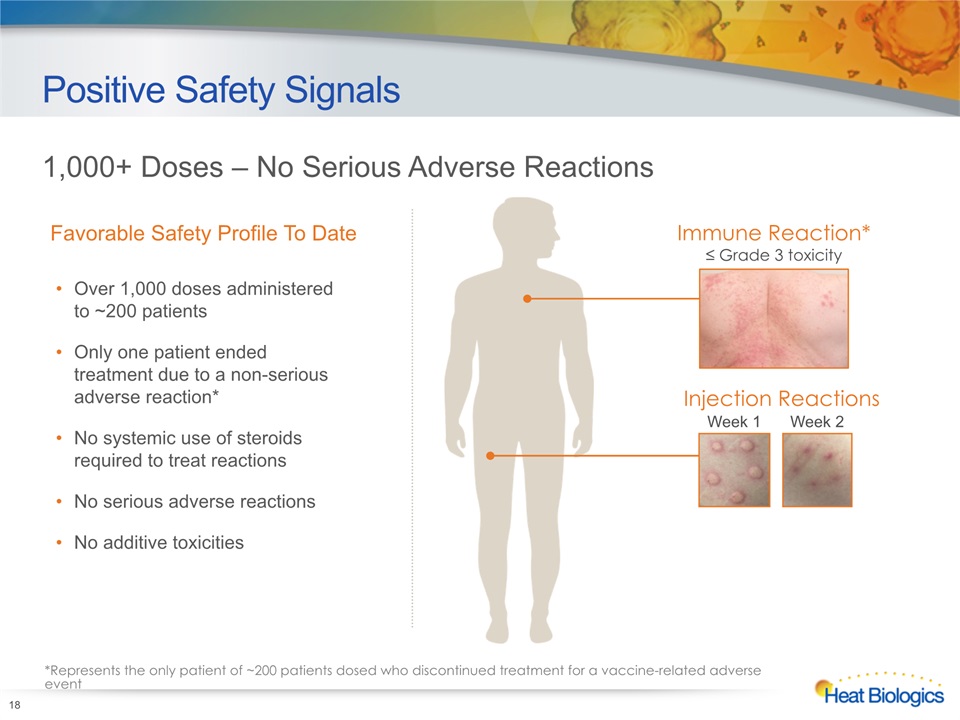
Positive Safety Signals Over 1,000 doses administered to ~200 patientsOnly one patient ended treatment due to a non-serious adverse reaction*No systemic use of steroids required to treat reactionsNo serious adverse reactions No additive toxicities 18 1,000+ Doses – No Serious Adverse Reactions Favorable Safety Profile To Date *Represents the only patient of ~200 patients dosed who discontinued treatment for a vaccine-related adverse event Immune Reaction*≤ Grade 3 toxicity Injection Reactions Week 1 Week 2

ComPACT Platform Technology 19 gp96-Ig OX40L-Fc The first potential dual-acting immunotherapy designed to deliver T cell activation and costimulation in a single product – combination therapy without additive costs
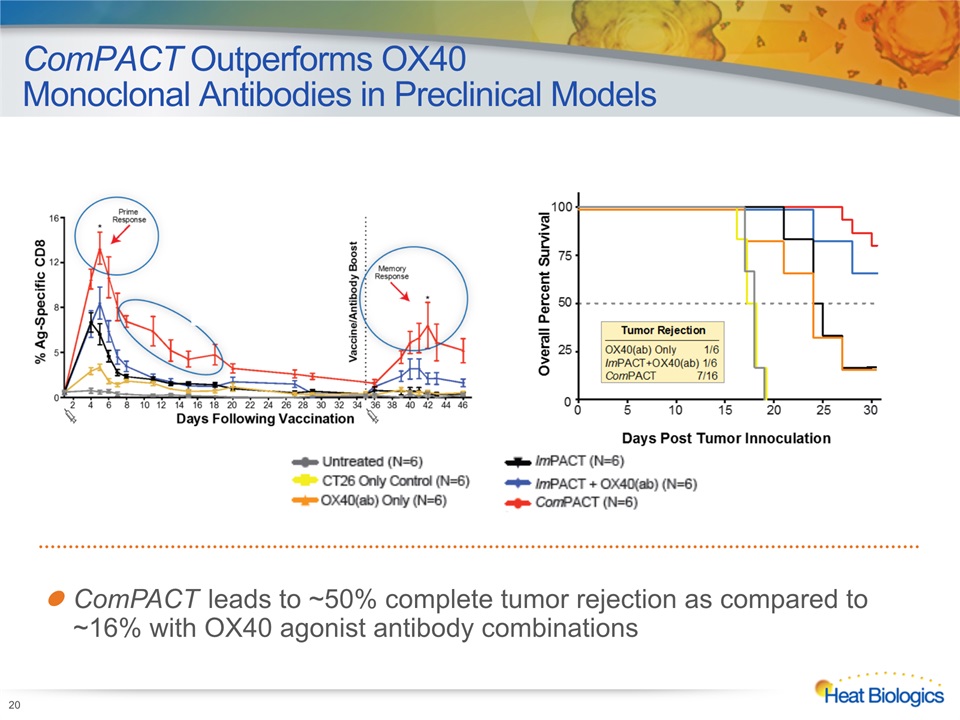
ComPACT Outperforms OX40 Monoclonal Antibodies in Preclinical Models 20 ComPACT leads to ~50% complete tumor rejection as compared to ~16% with OX40 agonist antibody combinations
T Cell Co-Stimulation 21 Heat Biologics Corporate Model Deck CO-STIMULATORS PTX-25 TBA Preclinical Humanized monoclonal antibody, functional agonist of human TNFRSF25 PTX-15 TBA Preclinical TL1A-Ig fusion protein, functional agonist of human TNFRSF25 Co-Stimulation Pelican PTX-25Monoclonal AntibodyPelican PTX-15Fusion Protein T Cell Co-stimulation to Enhance Immune Response Against Cancer

22 Heat has acquired 80% controlling interest in PelicanPelican will operate as a subsidiary in TexasPTX-25 - potential best-in-class co-stimulator Pre-clinical synergy with Heat’s ImPACT and checkpoint therapy$15.2M CPRIT Grant to fund clinical development Heat Biologics Acquires Pelican Therapeutics
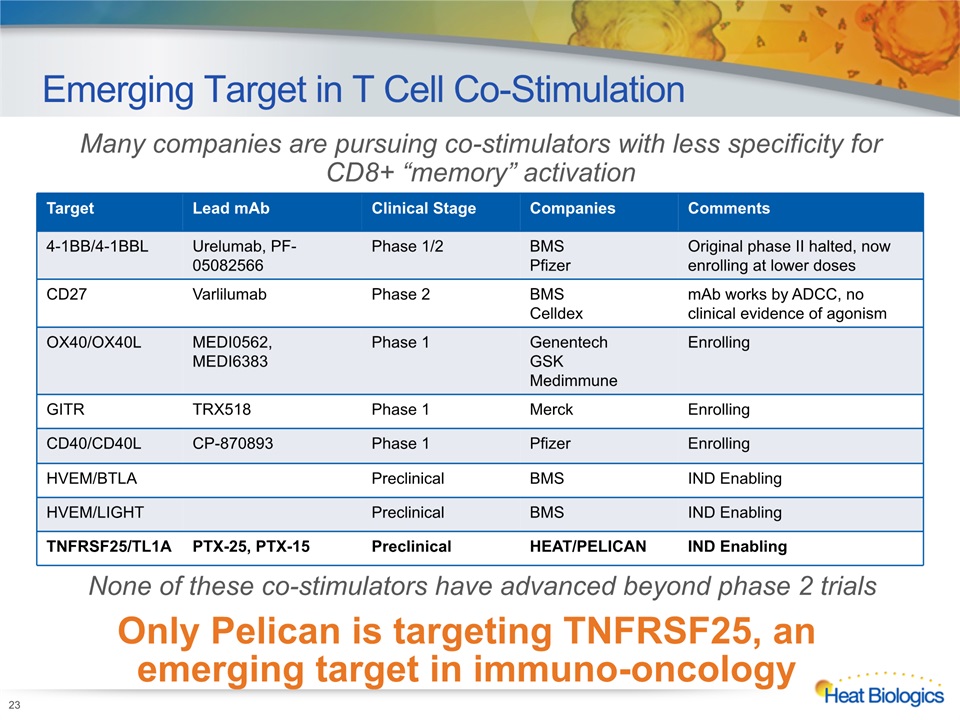
Emerging Target in T Cell Co-Stimulation 23 Many companies are pursuing co-stimulators with less specificity for CD8+ “memory” activation Only Pelican is targeting TNFRSF25, an emerging target in immuno-oncology Target Lead mAb Clinical Stage Companies Comments 4-1BB/4-1BBL Urelumab, PF-05082566 Phase 1/2 BMSPfizer Original phase II halted, now enrolling at lower doses CD27 Varlilumab Phase 2 BMSCelldex mAb works by ADCC, no clinical evidence of agonism OX40/OX40L MEDI0562, MEDI6383 Phase 1 GenentechGSKMedimmune Enrolling GITR TRX518 Phase 1 Merck Enrolling CD40/CD40L CP-870893 Phase 1 Pfizer Enrolling HVEM/BTLA Preclinical BMS IND Enabling HVEM/LIGHT Preclinical BMS IND Enabling TNFRSF25/TL1A PTX-25, PTX-15 Preclinical HEAT/PELICAN IND Enabling None of these co-stimulators have advanced beyond phase 2 trials

24 Potential Best-in-Class Co-Stimulator PTX-25 is a potential best-in-class T cell co-stimulator specific to “killer” CD8+ “memory” T cellsPre-clinical studies show advantages over competing T cell co-stimulator programs based on CD8+ T cell specificity$15.2 million New Company Product Development Award from the Cancer Prevention and Research Institute of Texas (CPRIT)Propels PTX25 through a ~70-patient first-in-man clinical program Advance multiple products through preclinical development, and at least one through Phase I trials Antigen-specific T cell proliferationIncreased effector cytokine production Increased effector immune functionIncreased survival in preclinical models Pre-clinical studies show advantages over competing T cell co-stimulator programs based on CD8+ T cell specificity
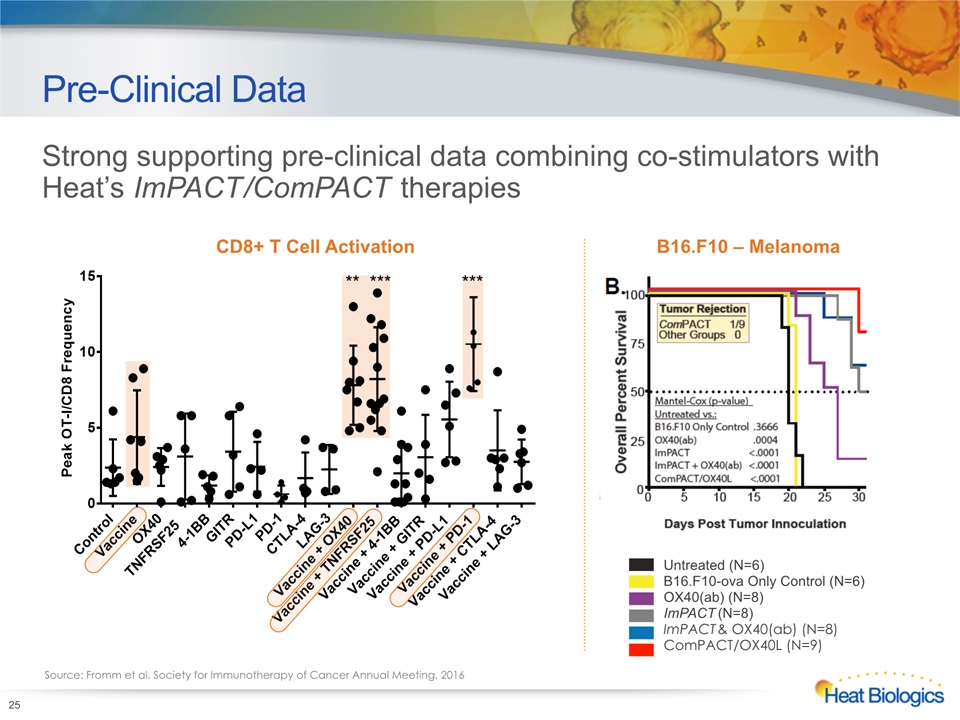
Pre-Clinical Data 25 Untreated (N=6)B16.F10-ova Only Control (N=6)OX40(ab) (N=8)ImPACT (N=8)ImPACT & OX40(ab) (N=8)ComPACT/OX40L (N=9) Strong supporting pre-clinical data combining co-stimulators with Heat’s ImPACT/ComPACT therapies Source: Fromm et al. Society for Immunotherapy of Cancer Annual Meeting, 2016 CD8+ T Cell Activation B16.F10 – Melanoma
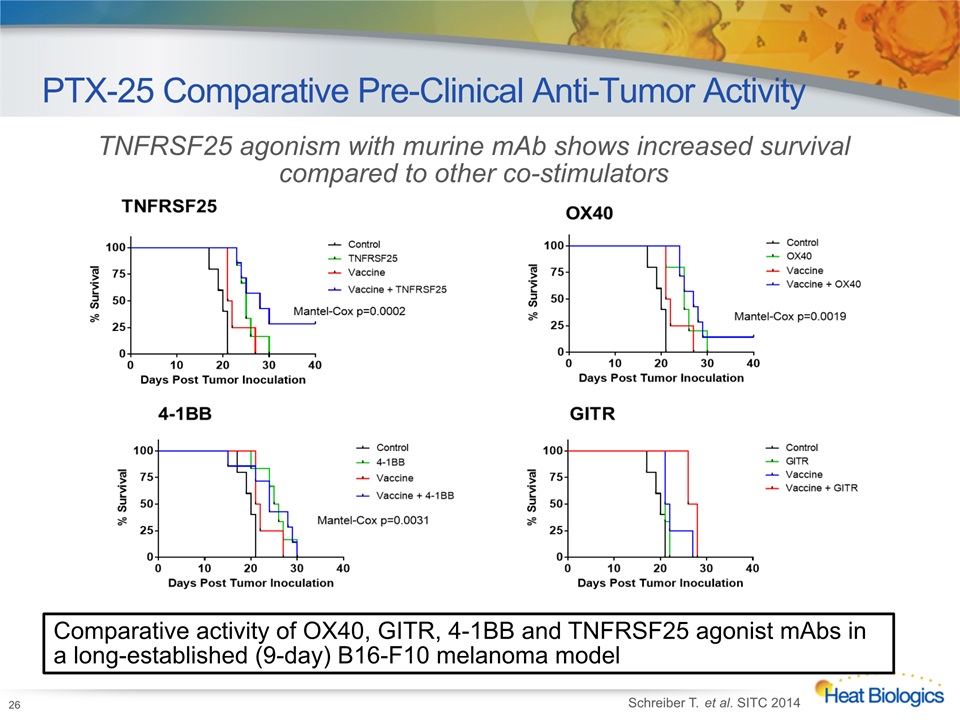
PTX-25 Comparative Pre-Clinical Anti-Tumor Activity 26 TNFRSF25 agonism with murine mAb shows increased survival compared to other co-stimulators Schreiber T. et al. SITC 2014 Comparative activity of OX40, GITR, 4-1BB and TNFRSF25 agonist mAbs in a long-established (9-day) B16-F10 melanoma model
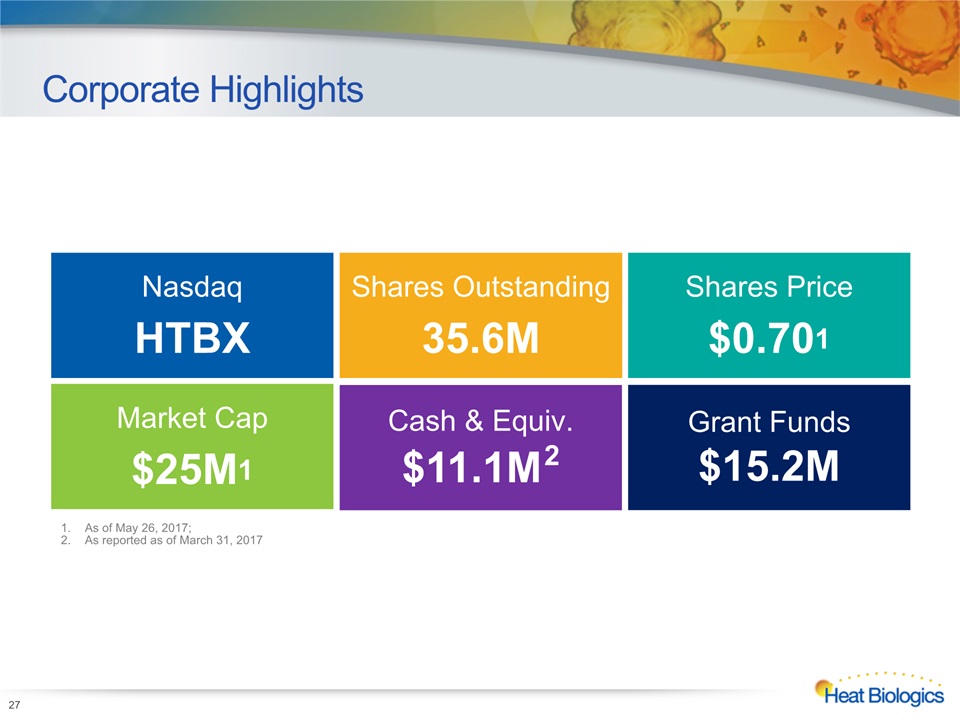
Corporate Highlights 27 As of May 26, 2017; As reported as of March 31, 2017 Market Cap$25M1 Cash & Equiv.$11.1M2 NasdaqHTBX Shares Outstanding35.6M Shares Price$0.701 Grant Funds$15.2M
Heat Biologics Highlights 28 Lower COG Compared to Personalized Therapies Phase II NSCLC Trial Innovative drugs designed to turn COLD tumors HOT Favorable Safety Profile TECHNOLOGIES Multiple Platform Signals in lung cancer with CHECKPOINT Inhibitors POTENTIAL BEST-IN-CLASS Activation of Cancer Killing CD8+ T Cells ROBUST Pipeline of COMBINATION Therapies
Cold Hot Turning COLD Tumors HOT
Appendix 30
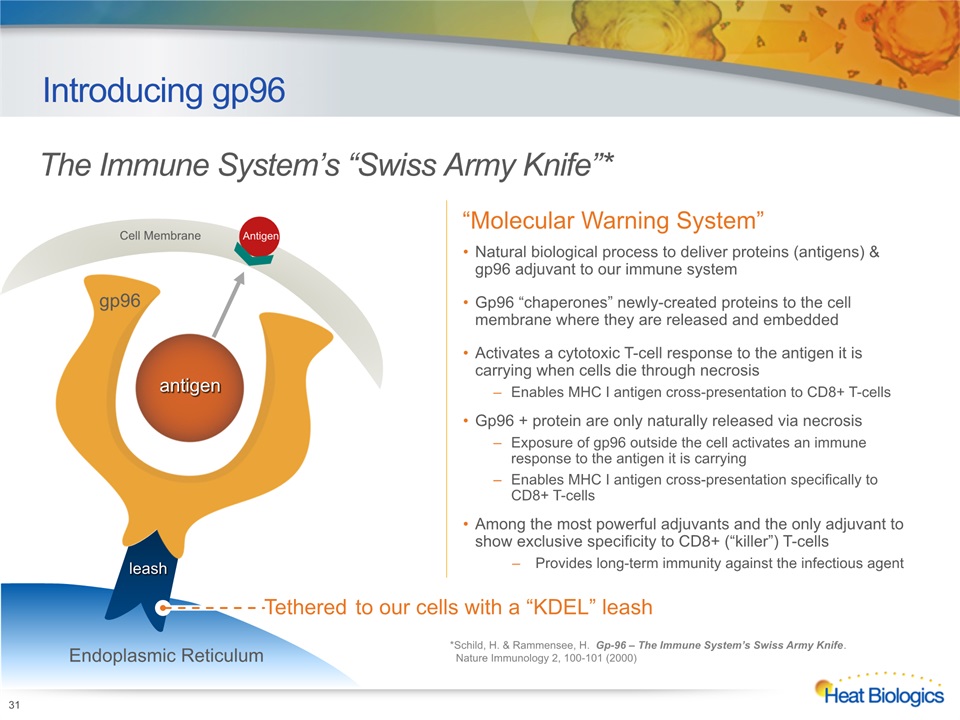
Introducing gp96 *Schild, H. & Rammensee, H. Gp-96 – The Immune System’s Swiss Army Knife. Nature Immunology 2, 100-101 (2000) “Molecular Warning System” Natural biological process to deliver proteins (antigens) & gp96 adjuvant to our immune systemGp96 “chaperones” newly-created proteins to the cell membrane where they are released and embeddedActivates a cytotoxic T-cell response to the antigen it is carrying when cells die through necrosisEnables MHC I antigen cross-presentation to CD8+ T-cellsGp96 + protein are only naturally released via necrosisExposure of gp96 outside the cell activates an immune response to the antigen it is carrying Enables MHC I antigen cross-presentation specifically to CD8+ T-cellsAmong the most powerful adjuvants and the only adjuvant to show exclusive specificity to CD8+ (“killer”) T-cellsProvides long-term immunity against the infectious agent Tethered to our cells with a “KDEL” leash gp96 antigen leash Endoplasmic Reticulum Cell Membrane Antigen 31 The Immune System’s “Swiss Army Knife”*

ImPACT Therapy Heat Biologics ImPACT technology reprograms cancer cells to continuously secrete their own antigens bound to heat shock protein gp96 to seek out and destroy a variety of tumours Genetically modify tumor cells by “severing the leash” that binds the gp96 to the endoplasmic reticulum of the cell and replacing it with a sequence that pumps gp96 out of the cellEnables living cancer cells to “pump-out” their own surface antigens along with their gp96 chaperoneMimics necrotic cell deathActivates a powerful pan-antigen cytotoxic T-cell immune response 32 “Severing the Leash” Heat Biologics ImPACT technology removes the leash that binds gp96 to the cell, replacing with a sequence that allows cells to continually secrete gp96 along with their “chaperoned” antigen Cell Membrane
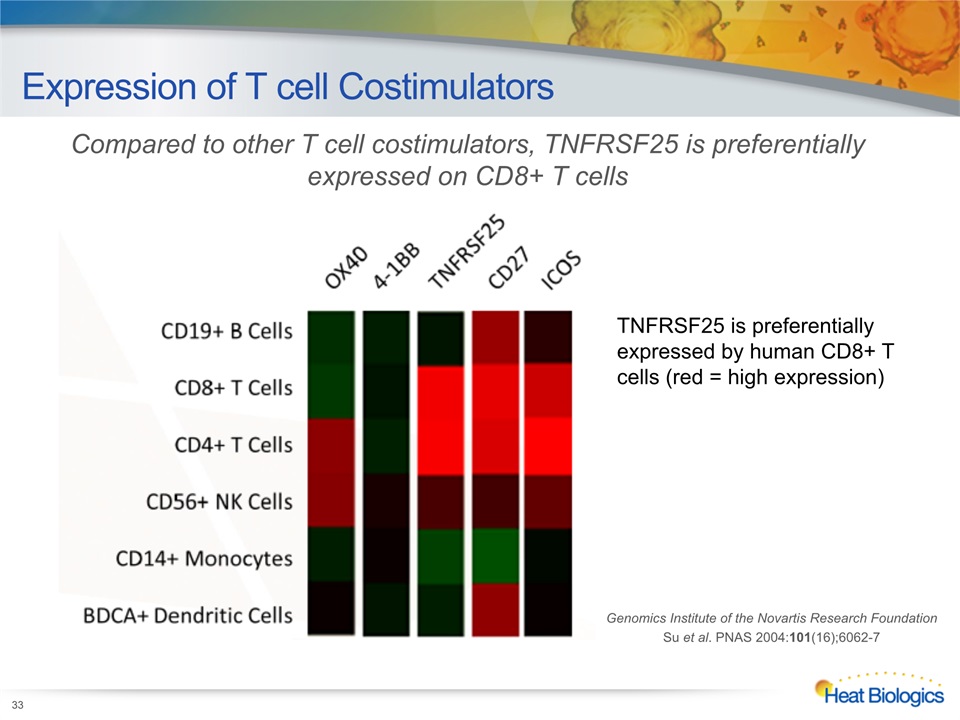
Expression of T cell Costimulators Genomics Institute of the Novartis Research FoundationSu et al. PNAS 2004:101(16);6062-7 TNFRSF25 is preferentially expressed by human CD8+ T cells (red = high expression) Compared to other T cell costimulators, TNFRSF25 is preferentially expressed on CD8+ T cells 33
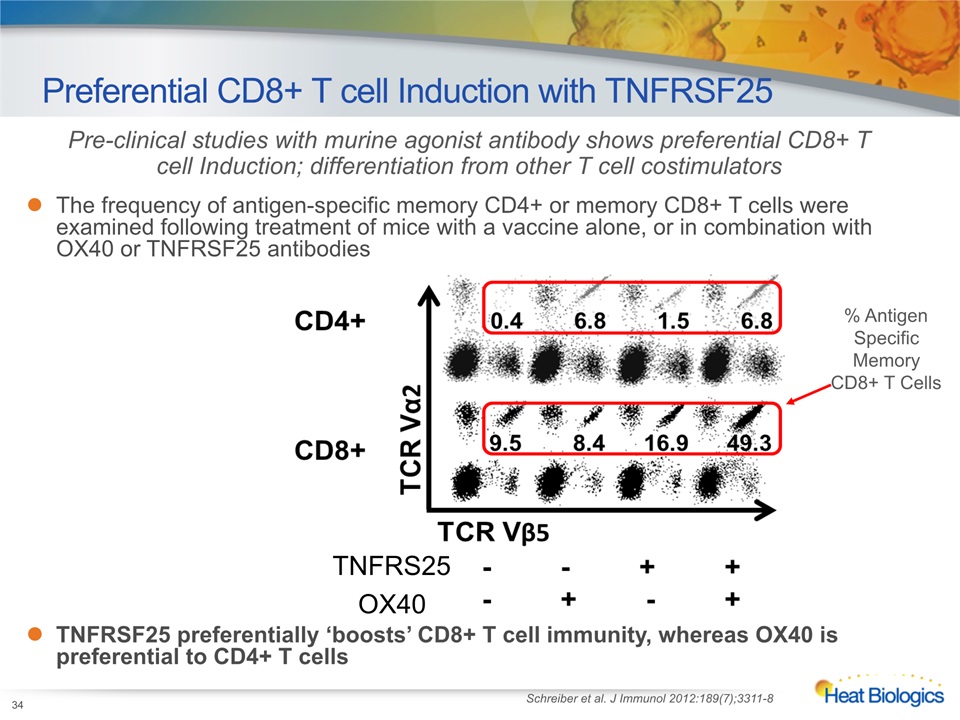
Preferential CD8+ T cell Induction with TNFRSF25 Pre-clinical studies with murine agonist antibody shows preferential CD8+ T cell Induction; differentiation from other T cell costimulators The frequency of antigen-specific memory CD4+ or memory CD8+ T cells were examined following treatment of mice with a vaccine alone, or in combination with OX40 or TNFRSF25 antibodiesTNFRSF25 preferentially ‘boosts’ CD8+ T cell immunity, whereas OX40 is preferential to CD4+ T cells Schreiber et al. J Immunol 2012:189(7);3311-8 % AntigenSpecific Memory CD8+ T Cells TNFRS25 OX40 34
Highlights from Pelican Pre-clinical Studies mAb to TNFRSF25, drives the development of antigen-specific CD8+ T cells (this effect mimics TLIA, the natural monogamous ligand of TNFRSF25)mAb to TNFRSF25 results in costimulation and expansion of antigen-experienced memory T cells, both CD4+ and CD8+notable is a significantly enhanced effect on memory CD8+ T cellsCostimulation occurs only in the context of TCR recognition of antigenTNFRSF25 appears to have superior activity in stimulating memory CD8+ cells relative to OX40, 4-1BB and GITRAgonism with TNFRSF25 mAb leads to increases in effector cytokine and effector immune function, and increases survival in mouse modelsIn mouse melanoma models, TNFRSF25 mAb results in increased survival compared to agonism of OX40, GITR, 4-1BB with respective agonist mAbs 35
