Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ARADIGM CORP | d392227d8k.htm |

Inhaled Liposomal Ciprofloxacin in Patients With Non-Cystic Fibrosis Bronchiectasis and Chronic Pseudomonas aeruginosa: Results From Two Parallel Phase III Trials (ORBIT-3 and -4) C.S. Haworth1, A. Wanner2, J. Froehlich3, T. O'Neal3, A. Davis4, I. Gonda3, A. O'Donnell5 1Cambridge Centre For Lung Infection, Papworth Hospital, Cambridge, UK; 2University of Miami School of Medicine, Miami, FL, USA; 3Aradigm Corporation, Hayward, CA, USA; 4Grifols, Research Triangle Park, NC, USA; 5Georgetown University, Washington, DC, USA Exhibit 99.1
Background Patients with non-cystic fibrosis bronchiectasis (NCFBE) and Pseudomonas aeruginosa (PA) infection have a greater risk of: Frequent pulmonary exacerbations (PEs) Hospital admissions Decreased quality of life Higher mortality ARD-3150 is a once-daily inhaled antibiotic containing liposome encapsulated ciprofloxacin 150 mg/3 mL and free ciprofloxacin 60 mg/3 mL Administered through the PARI LCⓇ Sprint nebulizer Chalmers et al, AJRCCM 2014; Finch et al, Ann Am Thorac Soc 2015; Serisier et al, Thorax 2013
Background ORBIT-3 and ORBIT-4 were identical, 48-week, multinational, randomized, double-blind, placebo-controlled phase III trials in patients with NCFBE and chronic PA lung infections, followed by a 28-day open-label extension These trials were designed to evaluate the efficacy of once-daily ARD-3150 In delaying time to first exacerbation In decreasing the frequency of PEs
ORBIT-3 & ORBIT-4 Clinical Site Countries
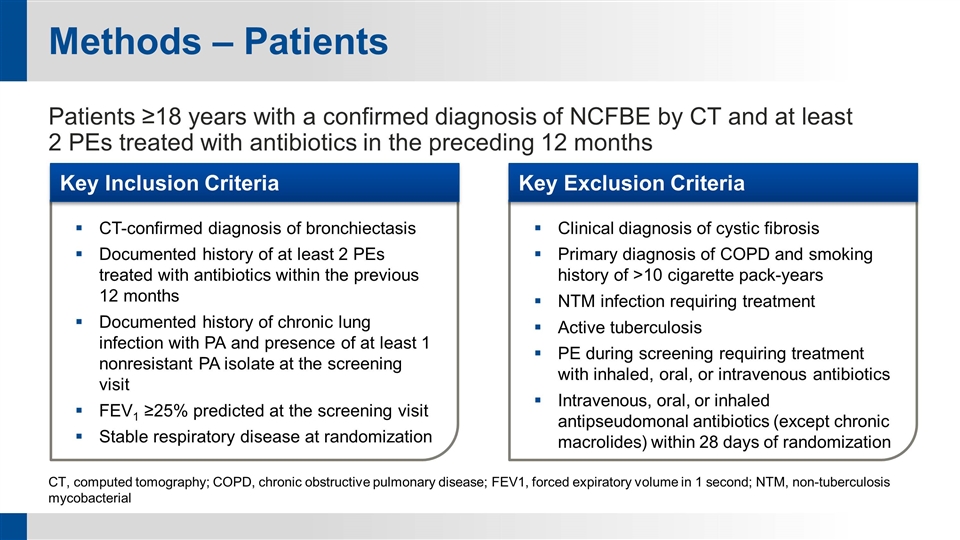
Methods – Patients Patients ≥18 years with a confirmed diagnosis of NCFBE by CT and at least 2 PEs treated with antibiotics in the preceding 12 months CT, computed tomography; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; NTM, non-tuberculosis mycobacterial CT-confirmed diagnosis of bronchiectasis Documented history of at least 2 PEs treated with antibiotics within the previous 12 months Documented history of chronic lung infection with PA and presence of at least 1 nonresistant PA isolate at the screening visit FEV1 ≥25% predicted at the screening visit Stable respiratory disease at randomization Clinical diagnosis of cystic fibrosis Primary diagnosis of COPD and smoking history of >10 cigarette pack-years NTM infection requiring treatment Active tuberculosis PE during screening requiring treatment with inhaled, oral, or intravenous antibiotics Intravenous, oral, or inhaled antipseudomonal antibiotics (except chronic macrolides) within 28 days of randomization Key Exclusion Criteria Key Inclusion Criteria
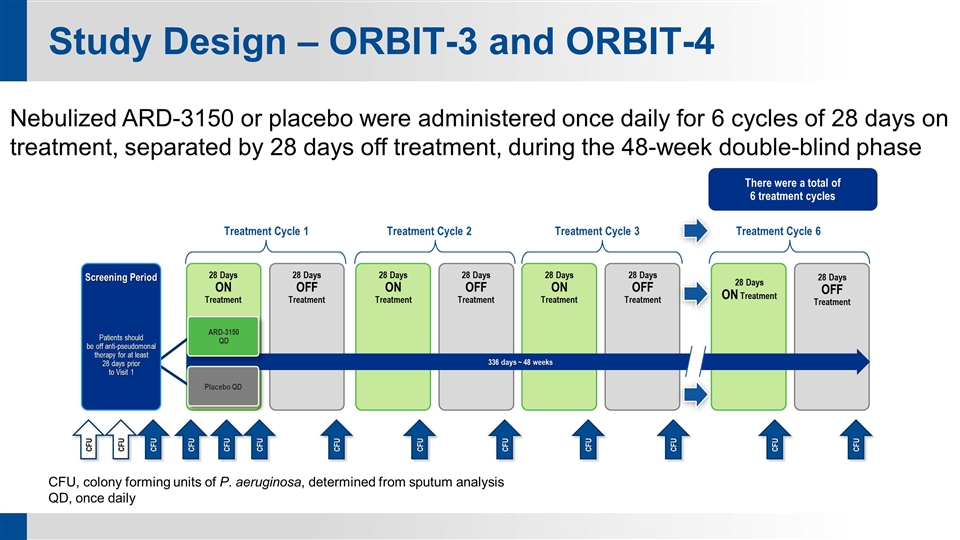
Study Design – ORBIT-3 and ORBIT-4 CFU, colony forming units of P. aeruginosa, determined from sputum analysis QD, once daily Nebulized ARD-3150 or placebo were administered once daily for 6 cycles of 28 days on treatment, separated by 28 days off treatment, during the 48-week double-blind phase Patients should be off anti-pseudomonal therapy for at least 28 days prior to Visit 1 Treatment Cycle 1 Treatment Cycle 2 Treatment Cycle 3 There were a total of 6 treatment cycles Treatment Cycle 6 Screening Period 28 Days ON Treatment 28 Days OFF Treatment 28 Days ON Treatment 28 Days OFF Treatment 28 Days ON Treatment 28 Days OFF Treatment 28 Days ON Treatment 28 Days OFF Treatment CFU CFU CFU CFU CFU CFU CFU CFU CFU CFU CFU CFU CFU 336 days ~ 48 weeks ARD-3150 QD Placebo QD
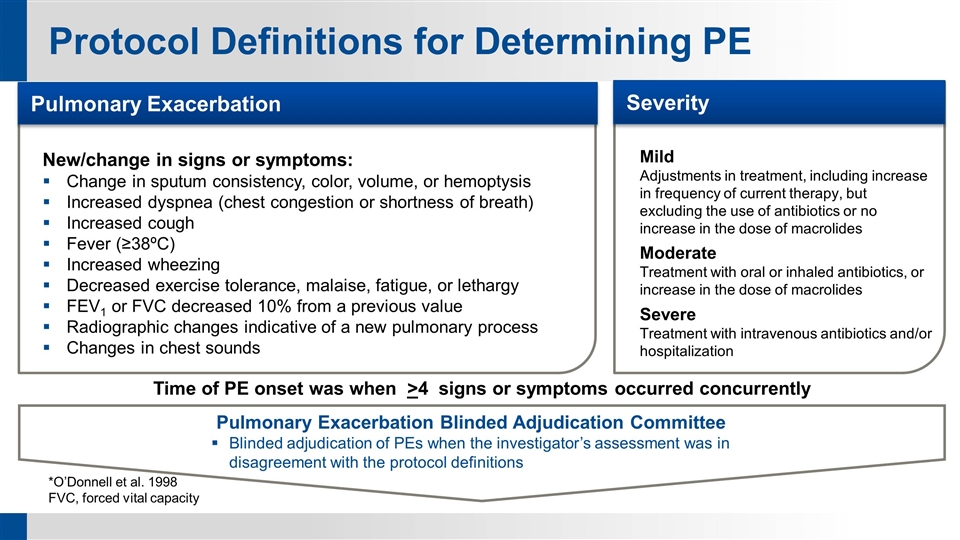
Protocol Definitions for Determining PE *O’Donnell et al. 1998 FVC, forced vital capacity Pulmonary Exacerbation Blinded Adjudication Committee Blinded adjudication of PEs when the investigator’s assessment was in disagreement with the protocol definitions New/change in signs or symptoms: Change in sputum consistency, color, volume, or hemoptysis Increased dyspnea (chest congestion or shortness of breath) Increased cough Fever (≥38ºC) Increased wheezing Decreased exercise tolerance, malaise, fatigue, or lethargy FEV1 or FVC decreased 10% from a previous value Radiographic changes indicative of a new pulmonary process Changes in chest sounds Pulmonary Exacerbation Mild Adjustments in treatment, including increase in frequency of current therapy, but excluding the use of antibiotics or no increase in the dose of macrolides Moderate Treatment with oral or inhaled antibiotics, or increase in the dose of macrolides Severe Treatment with intravenous antibiotics and/or hospitalization Severity Time of PE onset was when >4 signs or symptoms occurred concurrently
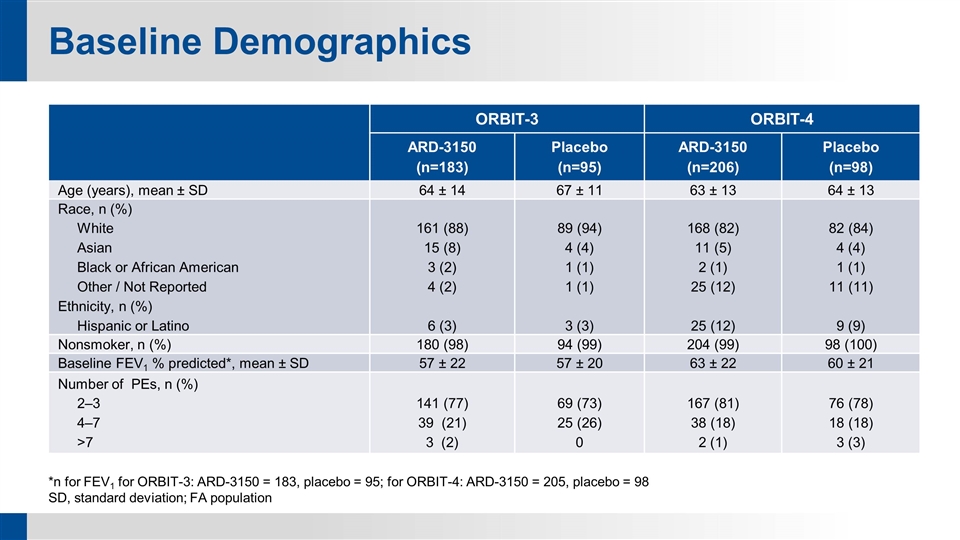
Baseline Demographics ORBIT-3 ORBIT-4 ARD-3150 (n=183) Placebo (n=95) ARD-3150 (n=206) Placebo (n=98) Age (years), mean ± SD 64 ± 14 67 ± 11 63 ± 13 64 ± 13 Race, n (%) White Asian Black or African American Other / Not Reported Ethnicity, n (%) Hispanic or Latino 161 (88) 15 (8) 3 (2) 4 (2) 6 (3) 89 (94) 4 (4) 1 (1) 1 (1) 3 (3) 168 (82) 11 (5) 2 (1) 25 (12) 25 (12) 82 (84) 4 (4) 1 (1) 11 (11) 9 (9) Nonsmoker, n (%) 180 (98) 94 (99) 204 (99) 98 (100) Baseline FEV1 % predicted*, mean ± SD 57 ± 22 57 ± 20 63 ± 22 60 ± 21 Number of PEs, n (%) 2‒3 4‒7 >7 141 (77) 39 (21) 3 (2) 69 (73) 25 (26) 0 167 (81) 38 (18) 2 (1) 76 (78) 18 (18) 3 (3) *n for FEV1 for ORBIT-3: ARD-3150 = 183, placebo = 95; for ORBIT-4: ARD-3150 = 205, placebo = 98 SD, standard deviation; FA population
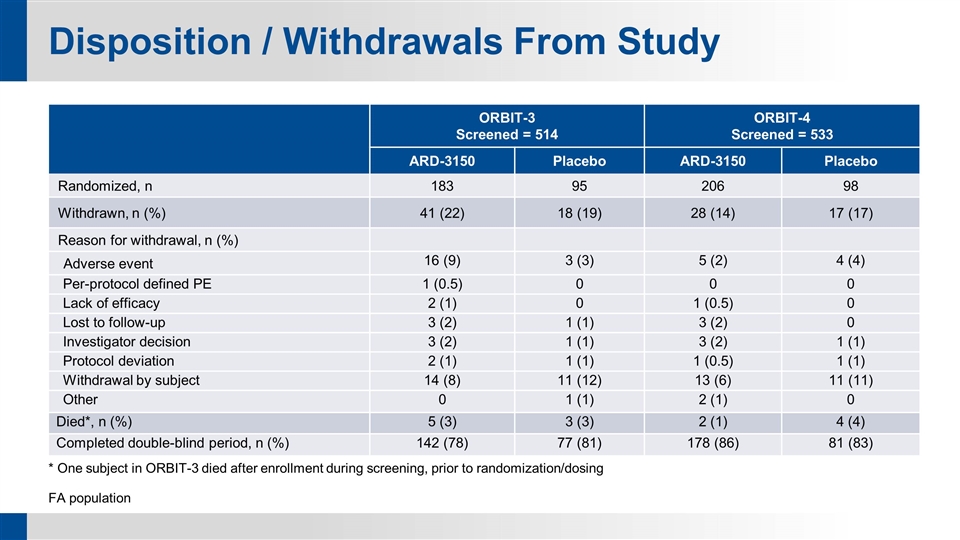
Disposition / Withdrawals From Study ORBIT-3 Screened = 514 ORBIT-4 Screened = 533 ARD-3150 Placebo ARD-3150 Placebo Randomized, n 183 95 206 98 Withdrawn, n (%) 41 (22) 18 (19) 28 (14) 17 (17) Reason for withdrawal, n (%) Adverse event 16 (9) 3 (3) 5 (2) 4 (4) Per-protocol defined PE 1 (0.5) 0 0 0 Lack of efficacy 2 (1) 0 1 (0.5) 0 Lost to follow-up 3 (2) 1 (1) 3 (2) 0 Investigator decision 3 (2) 1 (1) 3 (2) 1 (1) Protocol deviation 2 (1) 1 (1) 1 (0.5) 1 (1) Withdrawal by subject 14 (8) 11 (12) 13 (6) 11 (11) Other 0 1 (1) 2 (1) 0 Died*, n (%) 5 (3) 3 (3) 2 (1) 4 (4) Completed double-blind period, n (%) 142 (78) 77 (81) 178 (86) 81 (83) * One subject in ORBIT-3 died after enrollment during screening, prior to randomization/dosing FA population
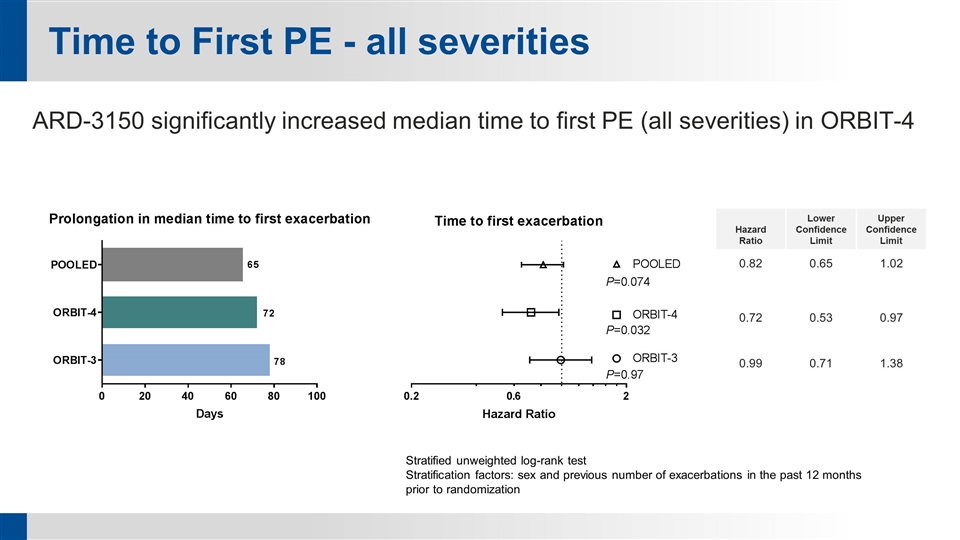
Time to First PE - all severities Hazard Ratio Lower Confidence Limit Upper Confidence Limit 0.82 0.65 1.02 0.72 0.53 0.97 0.99 0.71 1.38 Stratified unweighted log-rank test Stratification factors: sex and previous number of exacerbations in the past 12 months prior to randomization ARD-3150 significantly increased median time to first PE (all severities) in ORBIT-4
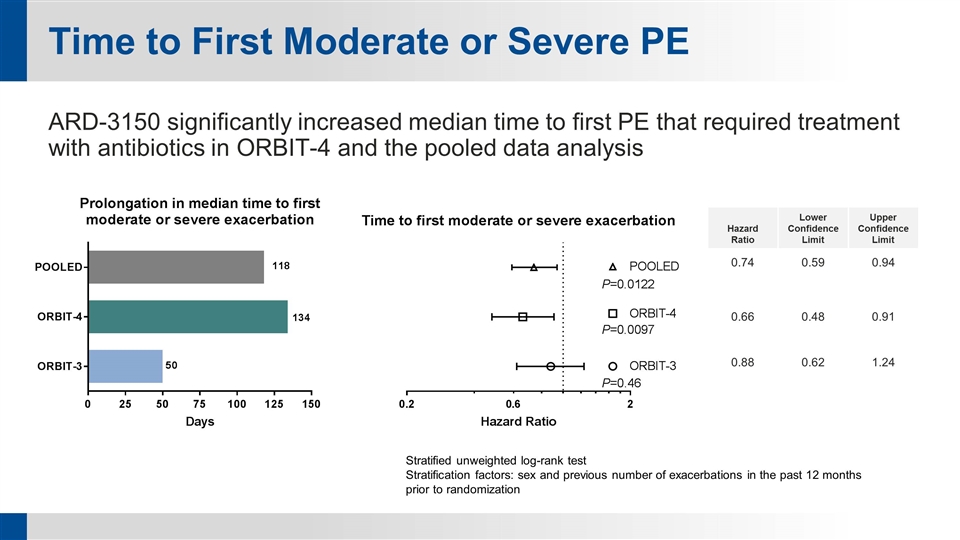
Time to First Moderate or Severe PE ARD-3150 significantly increased median time to first PE that required treatment with antibiotics in ORBIT-4 and the pooled data analysis Hazard Ratio Lower Confidence Limit Upper Confidence Limit 0.74 0.59 0.94 0.66 0.48 0.91 0.88 0.62 1.24 Stratified unweighted log-rank test Stratification factors: sex and previous number of exacerbations in the past 12 months prior to randomization
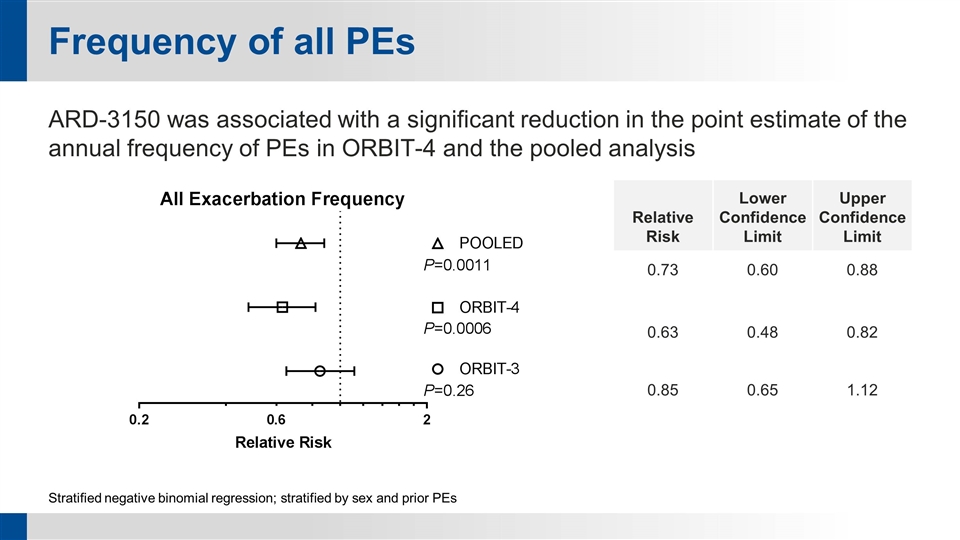
Frequency of all PEs ARD-3150 was associated with a significant reduction in the point estimate of the annual frequency of PEs in ORBIT-4 and the pooled analysis Stratified negative binomial regression; stratified by sex and prior PEs Relative Risk Lower Confidence Limit Upper Confidence Limit 0.73 0.60 0.88 0.63 0.48 0.82 0.85 0.65 1.12
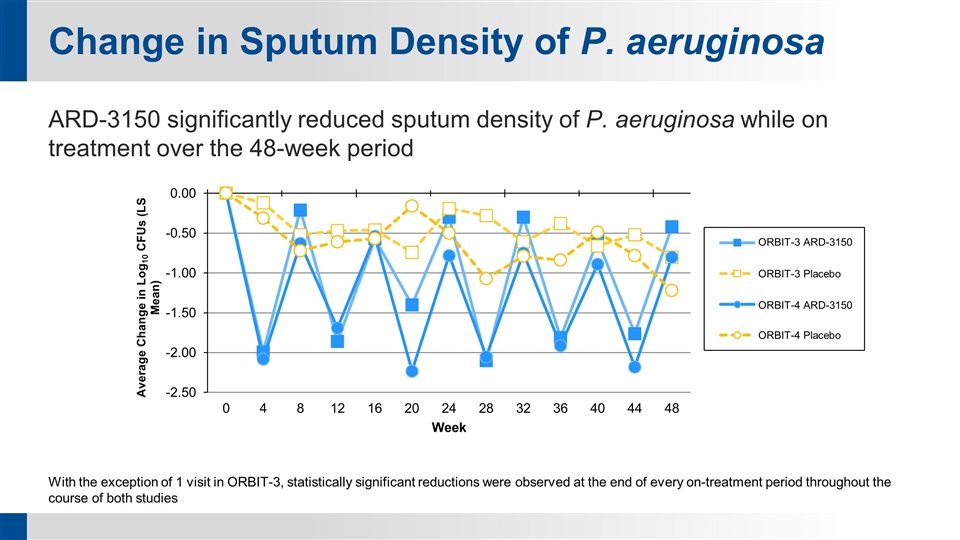
Change in Sputum Density of P. aeruginosa ARD-3150 significantly reduced sputum density of P. aeruginosa while on treatment over the 48-week period With the exception of 1 visit in ORBIT-3, statistically significant reductions were observed at the end of every on-treatment period throughout the course of both studies ORBIT-3 ARD-3150 ORBIT-3 Placebo ORBIT-4 ARD-3150 ORBIT-4 Placebo
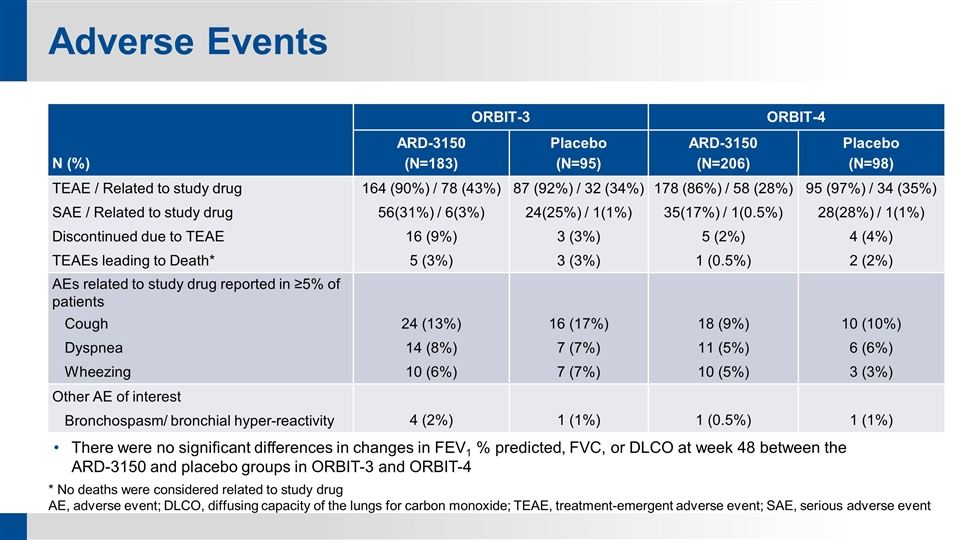
Adverse Events * No deaths were considered related to study drug AE, adverse event; DLCO, diffusing capacity of the lungs for carbon monoxide; TEAE, treatment-emergent adverse event; SAE, serious adverse event N (%) ORBIT-3 ORBIT-4 ARD-3150 (N=183) Placebo (N=95) ARD-3150 (N=206) Placebo (N=98) TEAE / Related to study drug 164 (90%) / 78 (43%) 87 (92%) / 32 (34%) 178 (86%) / 58 (28%) 95 (97%) / 34 (35%) SAE / Related to study drug 56(31%) / 6(3%) 24(25%) / 1(1%) 35(17%) / 1(0.5%) 28(28%) / 1(1%) Discontinued due to TEAE 16 (9%) 3 (3%) 5 (2%) 4 (4%) TEAEs leading to Death* 5 (3%) 3 (3%) 1 (0.5%) 2 (2%) AEs related to study drug reported in ≥5% of patients Cough 24 (13%) 16 (17%) 18 (9%) 10 (10%) Dyspnea 14 (8%) 7 (7%) 11 (5%) 6 (6%) Wheezing 10 (6%) 7 (7%) 10 (5%) 3 (3%) Other AE of interest Bronchospasm/ bronchial hyper-reactivity 4 (2%) 1 (1%) 1 (0.5%) 1 (1%) There were no significant differences in changes in FEV1 % predicted, FVC, or DLCO at week 48 between the ARD-3150 and placebo groups in ORBIT-3 and ORBIT-4
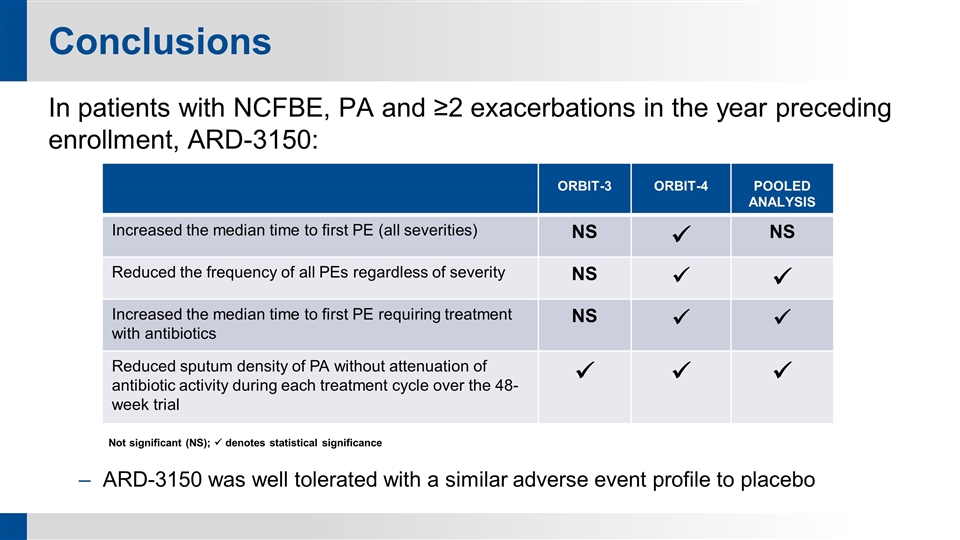
Conclusions In patients with NCFBE, PA and ≥2 exacerbations in the year preceding enrollment, ARD-3150: Not significant (NS); ü denotes statistical significance ARD-3150 was well tolerated with a similar adverse event profile to placebo ORBIT-3 ORBIT-4 POOLED ANALYSIS Increased the median time to first PE (all severities) NS ü NS Reduced the frequency of all PEs regardless of severity NS ü ü Increased the median time to first PE requiring treatment with antibiotics NS ü ü Reduced sputum density of PA without attenuation of antibiotic activity during each treatment cycle over the 48-week trial ü ü ü
