Attached files
| file | filename |
|---|---|
| EX-23.2 - EX-23.2 - Athenex, Inc. | d358380dex232.htm |
| EX-23.1 - EX-23.1 - Athenex, Inc. | d358380dex231.htm |
Table of Contents
As filed with the Securities and Exchange Commission on May 18, 2017
Registration Statement No. 333-217928
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
ATHENEX, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | 2834 | 43-1985966 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
1001 Main Street, Suite 600
Buffalo, NY 14203
(716) 427-2950
(Address, including zip code and telephone number, including area code, of registrant’s principal executive offices)
Johnson Y.N. Lau
Chief Executive Officer
Athenex, Inc.
1001 Main Street, Suite 600
Buffalo, NY 14203
(716) 427-2950
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Karen A. Dewis, Esq. Michael J. Rosenthall, Esq. Sidley Austin LLP 1501 K Street NW Washington, DC 20005 (202) 736-8000 |
Chris K.H. Lin, Esq. Daniel Fertig, Esq. Simpson Thacher & Bartlett LLP 35th Floor ICBC Tower 3 Garden Road Central, Hong Kong +852 2514-7600 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ (Do not check if a smaller reporting company) | Smaller reporting company | ☐ | |||
| Emerging growth company | ☒ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☒
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We cannot sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting an offer to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
Subject to completion, dated May 18, 2017
Prospectus
Shares

COMMON STOCK
We are offering shares of our common stock. This is our initial public offering and no public market currently exists for our common stock. We currently expect the initial public offering price to be between $ and $ per share.
We have applied to have our stock listed on The NASDAQ Global Market under the symbol “ATNX.” We are an “emerging growth company” as defined by the Jumpstart Our Business Startups Act of 2012 and, as such, we have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings.
Investing in our common stock involves a high degree of risk. Please read “Risk Factors” beginning on page 14 of this prospectus before making a decision to invest in our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share |
Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||||
| Proceeds to Athenex, before expenses |
$ | $ | ||||||
| (1) | See “Underwriting” for a description of the compensation payable to the underwriters. |
Certain of our existing stockholders and their affiliated entities, including stockholders affiliated with certain of our directors, have indicated an interest in purchasing up to an aggregate of approximately $ million worth of shares of our common stock in this offering at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters could determine to sell more, less or no shares to any of these stockholders and any of these stockholders could determine to purchase more, less or no shares in this offering. The underwriters will receive the same underwriting discounts and commissions on any shares purchased by these stockholders as they will on any other shares sold to the public in this offering.
Delivery of the shares of our common stock is expected to be made on or about , 2017. We have granted the underwriters an option for a period of 30 days to purchase an additional shares of our common stock.
(in alphabetical order)
| Credit Suisse | J.P. Morgan | |||
| Deutsche Bank Securities | ||||
The date of this prospectus is , 2017
Table of Contents
| ii | ||||
| 1 | ||||
| 10 | ||||
| 14 | ||||
| 79 | ||||
| 81 | ||||
| 82 | ||||
| 83 | ||||
| 85 | ||||
| 87 | ||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
89 | |||
| 110 | ||||
| |
117 |
| ||
| 187 | ||||
| 196 | ||||
| 206 | ||||
| 212 | ||||
| 215 | ||||
| 220 | ||||
| 222 | ||||
| 227 | ||||
| 233 | ||||
| 234 | ||||
| CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
235 | |||
| 236 | ||||
| 237 | ||||
| F-1 |
Table of Contents
We have not, and the underwriters have not, authorized anyone to provide you with information other than that contained in this prospectus or in any free writing prospectus prepared by or on our behalf or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give to you. We are offering to sell shares of our common stock, and seeking offers to buy shares of our common stock, only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our common stock.
Through and including , 2017 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
Investors Outside the United States
Neither we nor any of the underwriters have taken any action to permit a public offering of the shares of our common stock or the possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
Market and Industry Data and Forecasts
Certain market and industry data and forecasts included in this prospectus were obtained from independent market research, industry publications and surveys, governmental agencies and publicly available information. We believe that these external sources are reliable, but we have not independently verified any of the data from third-party sources, nor have we ascertained the underlying assumptions relied upon therein. Similarly, independent market research and industry forecasts, which we believe to be reliable based upon our management’s knowledge of the industry, have not been independently verified. Notwithstanding the foregoing, the Company acknowledges that it remains liable for the use of the third-party data referenced herein.
Trademarks
We have proprietary rights to trademarks, trade names and service marks appearing in this prospectus that are important to our business. Solely for convenience, the trademarks, trade names and service marks may appear in this prospectus without the ® and TM symbols, but any such references are not intended to indicate, in any way, that we forgo or will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, trade names and service marks. All trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners.
Glossary
A glossary of scientific and technical terms used throughout this prospectus is included beginning on page 237.
ii
Table of Contents
This summary highlights information contained elsewhere in this prospectus. This summary is not complete and may not contain all the information you should consider before investing in our common stock. You should read this entire prospectus carefully, especially the risks of investing in our common stock discussed under the heading “Risk Factors,” and our consolidated financial statements and related notes included elsewhere in this prospectus before making an investment decision.
Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus to “Athenex” “the company,” “we,” “us” and “our” refer to Athenex, Inc. and its consolidated subsidiaries.
Overview
We are a global biopharmaceutical company dedicated to the discovery, development and commercialization of novel therapies for the treatment of cancer. Our mission is to improve the lives of cancer patients by creating more effective, safer and tolerable treatments. We have generated our clinical product candidates through our Orascovery and Src Kinase Inhibition research platforms, which are based on our understanding of human absorption biology and novel approaches to inhibiting kinase activity, respectively. We believe that our ability to overcome the challenges of oral delivery of chemotherapy and limitations associated with IV delivery, via our P-gp inhibitor, offers significant potential benefits to patient outcomes by allowing patients to stay on therapy longer and extending the potential opportunities to combine with other agents, including targeted and immunotherapies that would otherwise be too toxic in combination with IV chemotherapy. We have assembled a leadership team and have established global operations in the U.S. and China across the pharmaceutical value chain to execute our mission to become a global leader in bringing innovative cancer treatments to the market and improve health outcomes.
Orascovery platform
Our Orascovery platform is based on the novel oral P-glycoprotein, or P-gp, pump inhibitor molecule HM30181A. The P-gp pump is a plasma membrane protein on the cells of the gut which forms a localized drug transport system and limits effective oral absorption of known and widely used P-gp substrate cancer chemotherapeutic drugs such as paclitaxel, irinotecan and docetaxel, thus restricting current usage to intravenous, or IV, administration. IV chemotherapies’ adverse events are due in part to sharp increases in the blood concentration levels of the chemotherapeutic drugs, and infusion-related reactions caused in part by dilution agents used to facilitate IV administration. Although clinical trial results with IV chemotherapy have shown that the dose-limiting side effects are associated with treatment efficacy, these adverse events have limited the duration of treatment with this route of administration. Through sequential co-administration of HM30181A and oral paclitaxel (together, Oraxol), we are able to facilitate oral absorption of paclitaxel at therapeutic blood levels by blocking the P-gp pump. We believe oral administration of paclitaxel reduces blood concentration level fluctuations and eliminates infusion-related reactions related to IV administration improving patient tolerability and allowing for longer dosing durations to improve efficacy. In addition to Oraxol, we are advancing three other clinical candidates in this platform, Oratecan (HM30181A and oral irinotecan), Oradoxel (HM30181A and oral docetaxel) and Oratopo (HM30181A and oral topotecan) to target solid tumors.
Src Kinase Inhibition platform
Src Kinase, a tyrosine kinase protein involved in regulating cell growth, is strongly implicated with blocking metastasis. Defects in Src Kinase are implicated in a number of cancers, and inhibiting this protein may limit the growth or proliferation of cancerous cell types. The Src Kinase Inhibition platform refers to novel small molecule compounds that have multiple mechanisms of action, including the inhibition of the activity of Src Kinase and
1
Table of Contents
the inhibition of tubulin polymerization during cell division. We believe the combination of these mechanisms of action provide a broader range of anti-cancer activity as compared to either mechanism of action alone. Our key clinical product candidates in this platform are KX-01 ointment for pre-cancerous lesions and KX-02 for glioblastoma multiforme, or GBM.
In addition to our existing portfolio of clinical candidates, our research and development teams are evaluating additional applications of our novel technology platforms. For example, our novel Cytochrome P450, or CYP, and P-gp dual inhibitor technology could lead to the discovery of new drug candidates.
In advance of the launch of our proprietary product candidates in the U.S., our commercial team intends to market oncology and oncology symptom-related products, to fund our infrastructure build-out. We believe it is important to minimize supply chain disruptions for high potency oncology active pharmaceutical ingredients. We have thus internalized key components of the supply chain that we believe are integral to minimizing the associated risks. We have organized our business model into three segments: Oncology Innovation Platform, Commercial Platform and Global Supply Chain Platform—with operations in both the U.S. and China. Our global operations across the three segments are shown below:
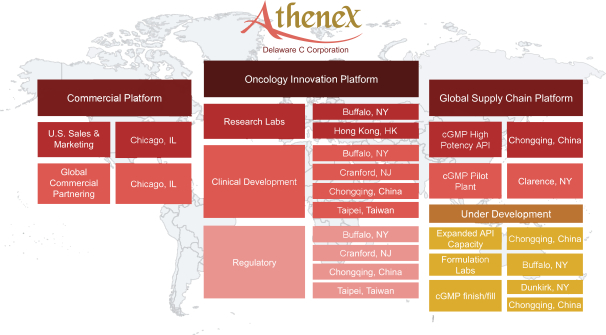
Our Global Supply Chain Platform manufactures active pharmaceutical ingredients, or API, for use internally in our research and development, clinical studies, and for sale to pharmaceutical customers globally. Our Commercial Platform currently markets the API produced by our Global Supply Chain Platform, including 14 products in the specialty and generic market segment in the U.S. and products under Section 503B of the Food, Drug and Cosmetics Act, or FDCA, through our compound pharmacy facility.
Our leadership team was carefully assembled to capture the global commercial market opportunities in novel drug development. Our executive officers are seasoned leaders with complementary skill sets across global pharmaceutical research and development, operations, supply chain and manufacturing, capital markets and mergers and acquisitions. We believe this characteristic is unique for a U.S.-based company and we believe we will be able to utilize this strength to create long term value for cancer patients, our employees and our
2
Table of Contents
shareholders. Our team is excited about the prospects of creating new paradigms in the treatment of cancer in developed markets and also driving our product candidates to emerging markets where patient access to treatments has historically been limited.
Based in Buffalo, New York, we were formed in 2003 and have been funded from inception by over $250 million in private financings and public-private partnerships with an estimated aggregate value of $375 million.
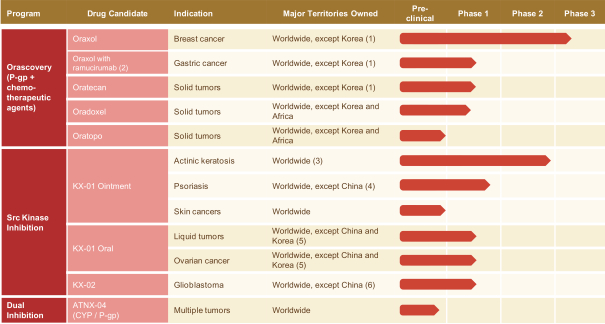
| (1) | Also excluding Taiwan, Singapore, Vietnam, Australia, New Zealand and Africa |
| (2) | Collaboration with Eli Lilly and Company, manufacturer of ramucirumab |
| (3) | Excluding Taiwan |
| (4) | Also excluding Taiwan, Macau, Hong Kong, Singapore and Malaysia |
| (5) | Also excluding Taiwan, Hong Kong, Singapore, Malaysia, Thailand, the Philippines, Indonesia and Vietnam |
| (6) | Also excluding Taiwan, Hong Kong and Singapore |
Our Orascovery Product Candidates
Oraxol (HM30181A and oral paclitaxel)
Oraxol is an oral dosage form of the widely used IV administered tubulin-stabilizing chemotherapeutic agent paclitaxel administered orally with the HM30181A molecule. We have been able to achieve similar paclitaxel exposures compared to the widely used 80 mg/m2 IV weekly dosing. We believe that Oraxol offers patients with paclitaxel-responsive tumors the possibility of oral therapy without the requirement for premedication to prevent infusion-related hypersensitivity-type reactions. Current clinical data suggests the potential for a better clinical response and tolerability profile, which is likely attributed to the pharmacokinetic profile achieved with oral dosing. Oraxol is presently in a Phase 3 trial in metastatic breast cancer and poised to enter into a combination study for treatment of advanced gastric cancer with ramucirumab through a clinical trial collaboration with Eli Lilly and Company, or Lilly.
3
Table of Contents
Oratecan (HM30181A and oral irinotecan)
Irinotecan given by IV is an anticancer agent that is used widely in the treatment of colorectal, lung, ovarian, cervical, pancreatic, upper gastrointestinal and brain cancer. In the product label for Camptosar (irinotecan), a variety of dosing regimens are approved for therapeutic use in metastatic colorectal cancer, including 350 mg/m2 given once every 3 weeks, weekly regimens of 125 mg/m2, and 180 or 240 mg/m2 every 2 weeks. The main objective supporting these dosing schedules has been to establish the highest dose possible that would lead to disruption of tumor growth, while allowing the bone marrow to recover from the chemotherapy-induced toxicity. Oratecan has been studied in combination with capecitabine in solid tumors in Korea and a Phase 1 study for Oratecan is ongoing in solid tumors in the U.S.
Oradoxel (HM30181A and oral docetaxel)
Docetaxel is an anticancer agent that is used widely in the treatment of breast, prostate, gastric, head and neck, and lung cancer. In the product label for Taxotere (docetaxel), a variety of doses ranging from 60-100 mg/m2 are approved for therapeutic use for these various indications, administered as a 1-hour infusion once every 3 weeks. The U.S. Food and Drug Administration, or FDA, allowed our Investigational New Drug Application, or IND, in the first quarter of 2016 and, most recently, we gained regulatory allowance for a Phase 1 clinical study in New Zealand.
Oratopo (HM30181A and oral topotecan)
Topotecan is an anticancer agent that can be used alone or in combination with other anticancer drugs to treat cervical cancer, ovarian cancer and lung cancer. In March 2017, the FDA allowed our IND for for Oratopo.
Our Src Kinase Inhibitor Product Candidates
KX-01
KX-01 is a compound developed under our Src Kinase Inhibition platform that, as a free base, has advantageous physical properties for topical ointment formulations. A topical ointment with KX-01 has shown promising results in a proof of concept clinical trial for actinic keratosis, or AK, a pre-cancerous skin lesion. We completed enrollment of an approximately 160-patient Phase 2a study of KX-01 for treatment of AK in 2016 and we have received allowance from FDA to conduct a Phase 3 study, which we expect to commence in the second half of 2017. An additional indication for psoriasis is being evaluated in a Phase 1 clinical trial led by our out-licensing partner. Since AK can lead to skin cancers, we are now investigating the initiation of a study in that indication, along with a study in T-cell lymphomas. These applications provide additional potential therapeutic utilities for KX-01 ointment and could represent significant potential market expansions beyond AK. KX-01 oral has shown activity against both solid and liquid tumors in patients, and we are planning further studies to focus our upcoming evaluation efforts in targeted indications.
KX-02
KX-02 is the second compound we developed using our Src Kinase Inhibition platform. Although KX-02 is an analog of KX-01, it has significantly different physical properties. These properties allow KX-02 to freely cross the blood-brain-barrier such that the concentration in the brain is equal to, or somewhat greater than, that in the plasma. This trait is uncommon for oncology drugs and highlights the potential for KX-02 as a novel therapy for unmet medical needs such as brain cancers, including GBM and brain metastases. The FDA has granted Orphan Drug Status to KX-02 for the treatment of gliomas. KX-02 is a non-ATP competitive Src Kinase inhibitor and tubulin polymerization inhibitor. Studies of KX-02 in preclinical syngeneic mouse GBM models resulted in the complete eradication, without recurrence, of the tumors in an average of approximately 30% of
4
Table of Contents
treated mice. KX-02’s multiple mechanisms of action, or MOAs, along with its ability to cross the blood-brain-barrier, make it a novel molecule for the treatment of brain tumors. KX-02 is currently in the early stages of a U.S. Phase 1 clinical trial in solid tumor patients. A Chinese IND has been filed by our development partner in China to start a study in primary brain tumors with KX-02 which we believe will commence in 2017.
Our Strengths
Transformative, oncology-focused and highly synergistic pipeline with late stage product candidates.
We believe we have a robust clinical pipeline. We have seven major clinical stage drug candidates, four of which are based on a proprietary Orascovery oral absorption technology, using our novel, highly-selective P-gp inhibitor in combination with widely-used cytotoxic agents enables oral administration of currently injectable only drugs. The remaining three are novel proprietary Src Kinase inhibitors that have multiple mechanisms of action.
We believe our extensive pipeline can create three impactful synergies:
| • | Drug synergy: We believe the potentially better clinical response and tolerability of Oraxol versus injectable paclitaxel opens the possibility for greater use of chemo and immuno-oncology therapies. There are also potential synergies between our pipeline products and existing anti-cancer products for potential best-in-combination or first-in-combination with immuno-oncology therapies; |
| • | Platform development and regulatory synergy: Approval of the first drug in each of our research and development platforms may serve as validation and facilitate approvals of other pipeline drug candidates using similar technology; and |
| • | Commercial synergy: We believe clinical and commercial success of our initial products will shift the oncology market from injectable to oral formulations. Adopting future products using our oral technologies should require less market education across physicians and providers. |
Proprietary research and development platforms capable of producing future drug candidates.
Since 2013 alone, our research and development platform has enabled us to file six U.S. investigational new drug applications, or INDs. We believe that our Orascovery platform can be applied to other existing drug therapies to achieve better pharmacokinetics, or PK, clinical response and tolerability profiles. Additionally, we have applied our research knowledge and experience of human oral absorption functions and have identified late stage novel molecule candidates which could represent additional future platform technologies.
Unique business model structured to capture value through commercialization and minimize supply chain risk.
We have a business model positioned to capture value by creating opportunities through multiple levers for growth. We have built a commercial sales and marketing infrastructure in the U.S. and intend to continue to further build out this segment. We expect this segment to start selling existing in-licensed therapeutically related oncology products in 2017 as a means of funding commercial infrastructure. Through acquisitions, we own elements of a high potency oncology supply chain. By utilizing capital efficient public-private partnerships in the U.S. and China, we expect to have built current good manufacturing practice, or cGMP, manufacturing facilities in both geographies.
A biopharmaceutical company with a focus on the U.S. and China oncology markets.
We have crafted our business model in order to capture the market opportunities in two of the largest pharmaceutical markets in the world. We have research and product development teams in both countries
5
Table of Contents
allowing us to leverage our innovation platform resources, including development and regulatory expertise, to maximize opportunities in both countries. We intend to serve as a technology bridge between these unique markets. We also expect to identify new technology opportunities through acquisitions, licensing or partnering in either market and position ourselves as a gateway to the other market.
Management team with industry-leading expertise and proven track record in leading global drug discovery, development and commercialization.
Our experienced senior management team members have held senior executives roles at multinational pharmaceutical companies and many led the development and regulatory approvals of pharmaceutical products in markets around the globe. Our research and development team members played a key role in the clinical development of numerous significant drugs including: Herceptin, Rituxan, Xeloda, Pegasys, PEG-Intron, Rebetol, IV Temodol, Requip, Suboxone, Subutex and Northera. The leadership of our commercial team has launched more than 50 novel and generic drugs and drug products into multiple markets around the world.
Our Mission and Strategy
Our mission is to improve the lives of cancer patients by creating more effective, safer and tolerable treatments. To achieve our mission, we intend to pursue the following strategies:
Rapidly and concurrently advance our clinical product candidates.
We intend to pursue the fastest feasible pathways to approval of our existing novel oral absorption technology. We are currently enrolling patients in a Phase 3 clinical trial of Oraxol. We plan to submit an NDA to the regulatory authorities in both New Zealand and Taiwan in 2017. We believe once we demonstrate the safety and effectiveness of this technology with Oraxol, the other drug candidates paired with this technology will face a more efficient development process. In addition, we expect complete primary endpoint data from the Phase 2a study of KX-01 ointment in the first half of 2017. If the data from this study confirms our previously completed proof of concept study data, we intend to launch a Phase 3 of KX-01 ointment registration study in 2017. We anticipate the development timeframe for our KX-02 drug candidate for GBM to be accelerated once we commence our partnered clinical program in China. Our licensing partner in China submitted a Clinical Trial Application, or CTA, for KX-02 to the China Food and Drug Administration, or CFDA, in 2016. We anticipate fast enrollment in China based on its large patient population which would accelerate the overall global development timeframe.
Leverage our global research and development operations to continue development of an oncology-focused product pipeline.
We have research and development operations in both the U.S. and China that are focused on both advancing our existing product pipeline and on developing additional novel clinical product candidates. We have developed a core competency in oral absorption technology and apply that skill to develop new methods of drug discovery. We believe that we can create substantial long term value by pursuing a robust, ongoing research and development program.
Build a proprietary commercial platform and selectively leverage collaborative relationships to achieve global drug sales, marketing and distribution.
We have begun building our U.S. commercial operation in preparation for future FDA approvals of our proprietary product candidates. We believe that our experienced product commercialization team can build an infrastructure that leverages both our global facilities and collaborative relationships to achieve global distribution of any FDA approved products in a timely and cost-effective manner.
6
Table of Contents
Continue to build-out our supply chain and cGMP manufacturing capabilities.
We believe internalization of our supply chain is uniquely suited to execute in both the U.S. and China, two of the world’s largest pharmaceutical markets. We intend to utilize cGMP manufacturing facilities from our public/private partnerships in both the China and U.S. markets as a mechanism to access both important markets and minimize supply disruptions. We intend to manufacture certain of our proprietary drugs and our partnered drugs commercialized around the world. Additionally, we expect that the expansion of our existing cGMP high potency API facilities will provide us with more flexibility, and control over high potency API as our drugs start to become commercialized. Our goal is to continue expanding this infrastructure and to leverage it to maintain future financial flexibility by optimizing our financial commitments and capital expenditures, which we believe will create value for shareholders.
Selectively pursue strategic M&A or licensing opportunities to complement our existing operations.
We have historically pursued acquisitions and in-licensing opportunities, and will continue to opportunistically target opportunities that will complement our existing portfolio and operations to create value for shareholders and support our business strategy and mission.
Risks Related to our Business
Despite our strengths and strategy described above, our ability to successfully operate our business is subject to numerous risks, including those that are generally associated with operating in the biopharmaceutical industry. Any of the factors set forth under the heading “Risk Factors” may limit our ability to successfully execute our business strategy. You should carefully consider all of the information set forth in this prospectus and, in particular, you should evaluate the specific factors set forth under the heading “Risk Factors” in deciding whether to invest in our common stock. Some of the principal risks relating to our business and our ability to execute our business strategy include:
| • | Our primary clinical candidates are still in the development stage and have not yet received regulatory approval, which may make it difficult to evaluate our current business and predict our future performance. |
| • | We incurred net losses in 2015 and 2016 and anticipate that we will continue to incur net losses for the foreseeable future. The report of our independent registered public accounting firm on our 2016 consolidated financial statements contains an explanatory paragraph regarding going concern, and we will need additional financing to fund our current operating plans and to continue as a going concern. |
| • | We currently do not generate substantial revenue from product sales and may never become profitable. |
| • | We will need to obtain additional financing to fund our operations, and if we are unable to obtain such financing, we may be unable to complete the development and commercialization of our drug candidates. |
| • | We depend substantially on the success of our proprietary drug candidates, which are in pre-clinical and clinical development. |
| • | We may not be successful in our efforts to identify or discover additional drug candidates. Due to our limited resources and access to capital, we must and have in the past decided to prioritize development of certain product candidates; these decisions may prove to have been wrong and may adversely affect our business. |
| • | Some of our drug candidates represent a novel approach to cancer treatment, which could result in delays in clinical development, heightened regulatory scrutiny, delays in our ability to achieve regulatory approval or commercialization, or market acceptance by physicians and patients of our drug candidates. |
7
Table of Contents
| • | Our product candidates may cause undesirable side effects that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any. |
| • | If clinical trials of our drug candidates fail to demonstrate safety and efficacy to the satisfaction of the FDA, CFDA, or other regulatory authorities or do not otherwise produce positive results, we may incur costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our drug candidates. |
| • | We rely on licensed intellectual property relating to certain of our lead product candidates. Any termination or loss of rights under those agreements would adversely affect our development or commercialization of our lead product candidates. |
| • | We are substantially dependent on our public-private partnerships and if we or our counterparties fail to meet the obligations of those agreements and we lose the benefits of those partnerships, it would materially impact our development, operations and prospectus. |
Implications of Being an Emerging Growth Company
As a company with less than $1.0 billion in revenues during our last fiscal year, we qualify and intend to characterize ourselves as an “emerging growth company” under the JOBS Act. An emerging growth company may take advantage of reduced reporting requirements and is relieved of certain other significant requirements that are otherwise generally applicable to public companies. As an emerging growth company:
| • | we may present only two years of audited financial statements and only two years of related management discussion and analysis of financial condition and results of operations; |
| • | we are exempt from the requirement to obtain an attestation and report from our auditors on management’s assessment of our internal control over financial reporting under the Sarbanes-Oxley Act of 2002, or Sarbanes-Oxley Act; |
| • | we are permitted to provide less extensive disclosure about our executive compensation arrangements; and |
| • | we are not required to give our stockholders non-binding advisory votes on executive compensation or golden parachute arrangements. |
We have elected to take advantage of the scaled disclosure requirements and other relief described above in this prospectus and may take advantage of these exemptions for so long as we remain an emerging growth company. In general, we will be an emerging growth company until the earliest of (i) the end of the fiscal year during which we have total annual gross revenues of $1.0 billion or more, (ii) the end of the fiscal year following the fifth anniversary of the completion of this offering, (iii) the date on which we have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt and (iv) the date on which we are deemed to be a “large accelerated filer,” which will occur at such time that we (a) have an aggregate worldwide market value of common equity securities held by non-affiliates of $700 million or more as of the last business day of our most recently completed second fiscal quarter, (b) have been required to file annual and quarterly reports under the Exchange Act for a period of at least 12 months and (c) have filed at least one annual report pursuant to the Exchange Act.
In addition to scaled disclosure and the other relief described above, the JOBS Act permits us an extended transition period for complying with new or revised accounting standards affecting public companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other
8
Table of Contents
public companies. We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements under the JOBS Act. Subject to certain conditions, as an emerging growth company, we may rely on certain of these exemptions, including without limitation, (i) providing an auditor’s attestation report on our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (ii) complying with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis.
Corporate Information
We were originally formed under the laws of the state of Delaware in November 2003 under the name Kinex Pharmaceuticals, LLC. In December 2012, we converted from a limited liability company to a Delaware corporation, Kinex Pharmaceuticals, Inc. In August 2015, we amended and restated our certificate of incorporation to change our name to Athenex, Inc. Our principal executive offices are located at 1001 Main Street, Suite 600, Buffalo, NY 14203, and our telephone number is (716) 427-2950. Our website address is www.athenex.com. The information contained on, or that can be accessed through, our website is not a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
9
Table of Contents
| Issuer |
Athenex, Inc. |
| Offering price per share |
$ |
| Common stock offered by us |
shares. |
| Common stock to be outstanding immediately after this offering |
shares ( shares if the underwriters exercise in full their option to purchase additional shares of common stock). |
| Underwriters’ option to purchase additional shares |
We have granted the underwriters a 30-day option to purchase up to additional shares at the public offering price less underwriting discounts and commissions. |
| Dividend policy |
We have never paid cash dividends on our common stock and we do not anticipate paying any cash dividends in the foreseeable future. See “Dividend Policy” for additional information. |
| Use of proceeds |
We intend to use net proceeds from this offering for development and regulatory activities for our Orascovery product candidates and platform and our Src Kinase Inhibition product candidates and platform, for research and development of our pre-clinical candidates. We anticipate using the remaining amounts for research and development of our pre-clinical candidates, and for working capital and other general corporate purposes. See “Use of Proceeds” for additional information. |
| Directed share program |
At our request, the underwriters have reserved for sale, at the initial public offering price, up to % of the shares to be sold in this offering to our directors, officers, employees, business associates and related persons. The number of shares of common stock available for sale to the general public will be reduced to the extent these individuals purchase such reserved shares. Any reserved shares that are not so purchased will be offered by the underwriters to the general public on the same basis as the other shares offered by this prospectus. See “Underwriting.” |
| Proposed NASDAQ symbol |
“ATNX” |
| Risk factors |
You should carefully read and consider the information set forth under “Risk Factors” beginning on page 14 and all other information included in this prospectus for a discussion of factors that you should consider before deciding to invest in shares of our common stock. |
The company and holders of approximately % of our common stock, including our officers, directors, and certain holders of the company’s common stock and options have agreed with the underwriters, subject to certain exceptions, not to dispose of or hedge any of their common stock, or securities convertible into or exchangeable for common stock during the period from the date of this prospectus continuing through the date 180 days after the date of this prospectus, except with the prior written consent of Credit Suisse Securities (USA) LLC and J.P. Morgan Securities LLC. This agreement does not apply to any existing employee benefit plans. See “Shares Eligible for Future Sale” and “Underwriting”.
10
Table of Contents
The number of shares of our common stock to be outstanding after this offering is based on (i) 41,348,568 shares of our common stock outstanding as of March 31, 2017, which includes 661,982 issued but unvested restricted shares, (ii) shares of our common stock to be issued upon the completion of this offering pursuant to the conversion of outstanding convertible notes, and (iii) the issuance of shares of our common stock to Hanmi and certain of our executive officers upon the completion of this offering pursuant to contractual obligations discussed elsewhere in this prospectus.
The number of shares of common stock to be outstanding after this offering excludes:
| • | 9,214,989 shares of common stock issuable upon the exercise of options outstanding as of March 31, 2017 at a weighted average exercise price of $6.25 per share; |
| • | 2,025,543 shares of common stock reserved for future grant or issuance under our stock option plans as of March 31, 2017; |
| • | 344,000 shares of common stock issuable upon the exercise of warrants to purchase our common stock outstanding as of March 31, 2017 at a weighted average exercise price of $0.44 per share; |
| • | shares of common stock (or approximately % of the total number of shares of our common stock outstanding immediately following the consummation of this offering, assuming no exercise of the underwriters’ option to purchase additional shares of our common stock) reserved for issuance pursuant to future awards under our 2017 Omnibus Incentive Plan, which will become effective on the day preceding the effectiveness of the registration statement to which this prospectus relates. |
Unless otherwise expressly stated or the context otherwise requires, the information in this prospectus assumes or reflects:
| • | no exercise of the underwriters’ option to purchase additional shares of our common stock; and |
| • | no exercise of outstanding options or warrants after March 31, 2017. |
Certain of our existing stockholders and their affiliated entities, including stockholders affiliated with certain of our directors, have indicated an interest in purchasing up to an aggregate of approximately $ million worth of shares of our common stock in this offering at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters could determine to sell more, less or no shares to any of these stockholders and any of these stockholders could determine to purchase more, less or no shares in this offering. The underwriters will receive the same underwriting discounts and commissions on any shares purchased by these stockholders as they will on any other shares sold to the public in this offering.
11
Table of Contents
SUMMARY CONSOLIDATED FINANCIAL AND OTHER DATA
The following selected statements of operations and comprehensive loss data and the cash flow data for the years ended December 31, 2015 and 2016 and the balance sheet data as of December 31, 2015 and 2016 are derived from our audited consolidated financial statements included elsewhere in this prospectus. The selected statements of operations and comprehensive loss data and the cash flow data for the three months ended March 31, 2016 and 2017 and the balance sheet data as of March 31, 2017 have been derived from our unaudited interim consolidated financial statements included elsewhere in this prospectus. In our opinion, these unaudited interim consolidated financial statements have been prepared on a basis consistent with our audited consolidated financial statements and contain all adjustments, consisting only of normal and recurring adjustments, necessary for a fair presentation of such financial data. You should read this data together with our audited consolidated financial statements, unaudited consolidated interim financial statements, and related notes appearing elsewhere in this prospectus and the information under the captions “Capitalization” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our historical results are not necessarily indicative of our future results, and our operating results for the three month period ended March 31, 2017 are not necessarily indicative of the results that may be expected for the fiscal year ending December 31, 2017 or any other interim periods or any future year or period. Our consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States, or U.S. GAAP.
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (In thousands, except share and per share data) |
||||||||||||||||
| Statements of Operations and Comprehensive Loss Data: |
||||||||||||||||
| Revenue: |
||||||||||||||||
| Product sales |
$ | 12,816 | $ | 19,394 | $ | 4,488 | $ | 3,900 | ||||||||
| License fees and consulting revenue |
314 | 392 | 95 | 598 | ||||||||||||
| Grant revenue |
814 | 765 | 46 | 83 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
13,944 | 20,551 | 4,629 | 4,581 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Costs and operating expenses: |
||||||||||||||||
| Cost of sales |
13,153 | 19,718 | 4,142 | 2,839 | ||||||||||||
| Research and development expenses |
24,463 | 60,624 | 6,746 | 26,408 | ||||||||||||
| Selling, general, and administrative expenses |
27,036 | 25,956 | 4,337 | 9,799 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and operating expenses |
64,652 | 106,298 | 15,225 | 39,046 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(50,708 | ) | (85,747 | ) | (10,596 | ) | (34,465 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Interest expense (income) |
1 | 1,891 | (46 | ) | 2,376 | |||||||||||
| Unrealized loss on derivative liability |
— | 533 | — | 4,276 | ||||||||||||
| Income tax (benefit) expense |
(54 | ) | (265 | ) | 100 | (92 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(50,655 | ) | (87,906 | ) | (10,650 | ) | (41,025 | ) | ||||||||
| Less: net loss attributable to non-controlling interests |
(55 | ) | (191 | ) | (32 | ) | (37 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to Athenex, Inc. |
$ | (50,600 | ) | $ | (87,715 | ) | $ | (10,618 | ) | $ | (40,988 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share attributable to Athenex, Inc. common stockholders, basic and diluted(1) |
$ | (1.50 | ) | $ | (2.19 | ) | $ | (0.27 | ) | $ | (1.01 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted-average shares used in computing net loss per share attributable to Athenex, Inc. common stockholders, basic and diluted(1) |
33,765,751 | 40,120,908 | 38,878,366 | 40,693,039 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pro forma net loss per share attributable to Athenex, Inc. common stockholders, basic and diluted(1) |
$ | $ | $ | $ | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pro forma weighted-average shares used in computing net loss per share attributable to Athenex, Inc. common stockholders, basic and diluted(1) |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Comprehensive loss |
$ | (50,906 | ) | $ | (88,796 | ) | $ | (10,656 | ) | $ | (40,486 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | See Note 17 to our audited consolidated financial statements appearing elsewhere in this prospectus for a description of the method used to calculate basic and diluted net loss per share attributable to Athenex, Inc. common stockholders and pro forma basic and diluted net loss per share attributable to Athenex, Inc. common stockholders. |
12
Table of Contents
| December 31, | March 31, | Pro Forma March 31, |
||||||||||||||
| 2015 | 2016 | 2017 | 2017 | |||||||||||||
| (in thousands) | ||||||||||||||||
| Selected Balance sheet data: |
||||||||||||||||
| Cash and cash equivalents |
$ | 43,495 | $ | 33,125 | $ | 26,034 | ||||||||||
| Marketable securities—current |
12,271 | 8,628 | 3,051 | |||||||||||||
| Goodwill |
37,996 | 37,552 | 37,574 | |||||||||||||
| Working capital* |
47,578 | 23,904 | 3,948 | |||||||||||||
| Total assets |
120,431 | 105,890 | 100,503 | |||||||||||||
| Long-term debt |
3,650 | 41,807 | 64,006 | |||||||||||||
| Total liabilities |
22,387 | 71,221 | 104,120 | |||||||||||||
| Non-controlling interests |
484 | 862 | 874 | |||||||||||||
| Total stockholders’ equity (deficit) |
$ | 98,044 | $ | 34,669 | $ | (3,617 | ) | |||||||||
| * | Working capital: total current assets—total current liabilities |
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (in thousands) | ||||||||||||||||
| Selected Cash flow data: |
||||||||||||||||
| Net cash used in operating activities |
$ | (33,756 | ) | $ | (47,870 | ) | $ | (7,556 | ) | $ | (20,753 | ) | ||||
| Net cash (used in) provided by investing activities |
(16,909 | ) | 2,659 | (755 | ) | 4,023 | ||||||||||
| Net cash provided by financing activities |
76,302 | 35,272 | 3,084 | 9,201 | ||||||||||||
| Net effect of foreign exchange rate changes |
337 | (431 | ) | 39 | 438 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net increase (decrease) in cash and cash equivalents |
25,974 | (10,370 | ) | (5,188 | ) | (7,091 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash and cash equivalents at beginning of period |
17,521 | 43,495 | 43,495 | 33,125 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash and cash equivalents at end of period |
$ | 43,495 | $ | 33,125 | $ | 38,307 | $ | 26,034 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
13
Table of Contents
Investing in our common stock involves a high degree of risk. You should consider carefully the following risk factors, as well as the other information in this prospectus, including the financial statements and related notes, before you decide to purchase our common stock. If any of the following risks actually occur, our business, financial condition and results of operations could be materially adversely affected, the value of our common stock could decline and you may lose all or part of your investment.
Risks Related to Our Financial Position and Need for Additional Capital
Our primary clinical candidates are still in the development stage and have not yet received regulatory approval, which may make it difficult to evaluate our current business and predict our future performance.
We are a globally-focused biopharmaceutical company formed in November 2003. Our operations to date have focused on organizing and staffing our company, business planning, raising capital, establishing our intellectual property portfolio and conducting preclinical studies and clinical trials of our drug candidates. We have not yet successfully completed large-scale, pivotal clinical trials, or obtained regulatory approvals for our drug candidates and have not yet established sales and marketing activities necessary for successful commercialization. Consequently, any predictions you make about our future success or viability may not be accurate. In addition, as a developing business, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown challenges.
We are focused on the discovery and development of innovative drugs for the treatment of cancers. The fact that we have not yet, among other things, demonstrated our ability to initiate or complete large-scale clinical trials or manufacture drugs at commercial scale, particularly in light of the rapidly evolving cancer treatment field, may make it difficult to evaluate our current business and predict our future performance. These constraints make any assessment of our future success or viability subject to significant uncertainty. We will encounter risks and difficulties frequently experienced by early-stage companies in rapidly evolving fields as we seek to transition to a company capable of supporting commercial activities. If we do not address these risks and difficulties successfully, our business will suffer.
We incurred net losses in 2015, 2016 and the three months ended March 31, 2017 and anticipate that we will continue to incur net losses for the foreseeable future.
Investment in pharmaceutical product development is highly speculative because it entails substantial upfront costs and expenses and significant risk that a drug candidate will fail to gain regulatory approval or become commercially viable. Since our formation, the company has relied on a combination of private securities offerings, public-private partnerships, the issuance of convertible notes and public grants to fund our operations. We have devoted most of our financial resources to research and development, including our non-clinical development activities and clinical trials. We have not generated substantial revenue from product sales to date, and we continue to incur significant development and other expenses related to our ongoing operations. As a result, we incurred losses in 2015, 2016 and the three months ended March 31, 2017. For the years ended December 31, 2015 and December 31, 2016 and the three months ended March 31, 2017, we reported net losses of $50.7 million, $87.9 million and $41.0 million, respectively, and had an accumulated deficit of $236.1 million as of March 31, 2017. Substantially all of our operating losses have resulted from costs incurred in connection with our research and development programs and from selling, general and administrative expenses associated with our operations.
We expect to continue to incur losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for, our drug candidates, and begin to commercialize approved drugs, if any. Typically, it takes many years to develop a new drug before it is available for treating patients. We may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. The size of our future net losses will depend, in part, on the rate of future growth of our expenses, our ability to generate revenue and the timing and amount of milestones and other
14
Table of Contents
required payments to third parties in connection with our potential future arrangements with third parties. If any of our drug candidates fail in clinical trials or do not gain regulatory approval, or if approved, fail to achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Our prior losses and expected future losses have had, and will continue to have, an adverse effect on our shareholders’ equity and working capital.
We expect our research and development expenses to continue to be significant in connection with our continued investment in our drug candidates and our ongoing and planned clinical trials for our drug candidates. Furthermore, if we obtain regulatory approval for our drug candidates, we expect to incur increased selling, general and administrative expenses. In addition, once we are a public company, we will incur additional costs associated with operating as a public company. As a result, we expect to continue to incur significant and increasing operating losses and negative cash flows from operations for the foreseeable future. These losses have had and will continue to have a material adverse effect on our stockholders’ equity, financial position, cash flows and working capital.
Our ability to continue as a going concern will require us to obtain additional financing to fund our current operations, which may be unavailable on acceptable terms, or at all.
Our recurring losses from operations and our current operating plans raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our consolidated financial statements as of and for the year ended December 31, 2016 with respect to this uncertainty. Our ability to continue as a going concern will require us to obtain additional financing to fund our current operating plans. We believe that the net proceeds from this offering and our existing cash and cash equivalents and short-term investments will be sufficient to fund our current operating plans through at least the next 12 months. We have based these estimates, however, on assumptions that may prove to be wrong, and we could spend our available financial resources much faster than we currently expect and need to raise additional funds sooner than we anticipate. If we are unable to raise capital when needed or on acceptable terms, we would be forced to delay, reduce or eliminate our research and drug development programs or commercialization efforts.
We currently do not generate substantial revenue from product sales and may never become profitable.
Our ability to generate revenue and become profitable depends upon our ability to successfully complete the development of, and obtain the necessary regulatory approvals for, our proprietary drug candidates, as we currently only have commercialized our API products. Our product sales, primarily from sales of API, totaled $12.8 million, $19.4 million and $3.9 million in the years ended December 31, 2015 and 2016 and the three months ended March 31, 2017, respectively. We expect to continue to incur substantial and increasing losses through the projected development and commercialization of our drug candidates. None of our proprietary drug candidates have been approved for marketing in the U.S., China or any other jurisdiction, and they may never receive such approval. Our ability to achieve revenue and profitability is dependent on our ability to complete the development of our proprietary drug candidates, obtain necessary regulatory approvals, and have our proprietary drugs manufactured and successfully marketed.
Even if we receive regulatory approval of our proprietary drug candidates for commercial sale, we do not know when they will generate revenue, if at all. Our ability to generate revenue from product sales of our drug candidates depends on a number of factors, including our ability to:
| • | complete research regarding, and non-clinical and clinical development of, our proprietary drug candidates; |
| • | formulate appropriate dosing protocols, drug preparations and capsule encapsulation methods; |
| • | obtain regulatory approvals and marketing authorizations for drug candidates for which we complete clinical trials; |
15
Table of Contents
| • | develop a sustainable and scalable manufacturing processes, including establishing and maintaining commercially viable supply relationships with third parties and establishing our own manufacturing capabilities and infrastructure; |
| • | compliantly launch and commercialize proprietary drug candidates for which we obtain regulatory approvals and marketing authorizations, either directly or with a collaborator or distributor; |
| • | obtain market acceptance of our proprietary drug candidates and their routes of administration as viable treatment options; |
| • | obtain adequate coverage and reimbursement for our proprietary drug candidates from government (including U.S. federal healthcare programs) and private payors; |
| • | identify, assess, acquire and/or develop new proprietary drug candidates; |
| • | address any competing technological and market developments; |
| • | negotiate and maintain favorable terms in any collaboration, licensing or other arrangements into which we may enter; |
| • | maintain, protect and expand our portfolio of intellectual property rights, including patents, trade secrets and know-how; |
| • | ability to successfully commercialize our 503B compound pharmacy products and U.S. specialty pharmaceutical products; |
| • | ability to further develop our API business; and |
| • | attract, hire and retain qualified personnel. |
In addition, because of the numerous risks and uncertainties associated with drug development, we are unable to predict the timing or amount of increased expenses, or when, or if, we will be able to achieve or maintain profitability. In addition, our expenses could increase beyond expectations if we are required by the FDA, CFDA, or regulatory authorities in other jurisdictions to perform studies in addition to those that we currently anticipate. Even if our proprietary drug candidates are approved for commercial sale, we anticipate incurring significant costs associated with the commercial launch of these drugs.
Our ability to become and remain profitable depends on our ability to generate revenue. Even if we are able to generate revenue from the sale of our drug candidates and API we manufacture for others, we may not become profitable and may need to obtain additional funding to continue operations. If we fail to become profitable or are unable to sustain profitability on a continuing basis, we may be unable to continue our operations at planned levels and be forced to reduce our operations. Even if we do achieve profitability, we may not be able to sustain or increase profitability. Our failure to become and remain profitable would decrease the value of our company and could impair our ability to raise capital, expand our business or continue our operations. Failure to become and remain profitable may adversely affect the market price of our common stock and our ability to raise capital and continue operations. A decline in the value of our company could also cause you to lose all or part of your investment.
We will need to obtain additional financing to fund our operations, and if we are unable to obtain such financing, we may be unable to complete the development and commercialization of our drug candidates.
We have financed our operations with a combination of private securities offerings, public-private partnerships, issuance of convertible notes and public grants. Through March 31, 2017, we have raised over $250 million in private financings. In addition, we have entered into public-private partnerships with an estimated aggregate value of $375 million. Our drug candidates will require the completion of regulatory review, significant sales and marketing efforts and substantial investment before they can provide us with any product sales revenue.
Our operations have consumed substantial amounts of cash since inception. The net cash used for our operating activities was $33.8 million, $47.9 million and $20.8 million for the years ended December 31, 2015
16
Table of Contents
and December 31, 2016 and the three months ended March 31, 2017, respectively. We expect to continue to spend substantial amounts on advancing the clinical development of our proprietary drug candidates, and launching and commercializing any proprietary drug candidates for which we receive regulatory approval, including building our own commercial organizations to address certain markets.
We will need to obtain additional financing to fund our future operations, including completing the development and commercialization of our proprietary drug candidates. We also need to obtain additional financing to conduct additional clinical trials for the approval of our proprietary drug candidates if requested by regulatory bodies, and completing the development of any additional proprietary drug candidates we might discover. Moreover, our research and development expenses and other contractual commitments are substantial and are expected to increase in the future.
Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed elsewhere in this “Risk Factors” section. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect. Our future funding requirements will depend on many factors, including, but not limited to:
| • | the progress, timing, scope and costs of our clinical trials, including the ability to timely enroll patients in our planned and potential future clinical trials; |
| • | the outcome, timing and cost of regulatory approvals by the FDA, CFDA and regulatory authorities in jurisdictions where we seek such approvals, including the possibility that the FDA, CFDA or regulatory authorities may require that we perform more studies than those that we currently expect; |
| • | our ability to secure adequate coverage and reimbursement for our proprietary drug candidates from government (including U.S. federal health care programs) and private payors; |
| • | the number and characteristics of drug candidates that we may in-license and develop; |
| • | our ability to successfully and compliantly launch and commercialize our drug candidates; |
| • | the amount of sales and other revenues from drug candidates that we may commercialize, if any, including the selling prices for such potential products and the availability of adequate reimbursement by third-party payors; |
| • | the amount of rebates or other price concessions we may owe under U.S. federal health care programs that cover and reimburse our proprietary drug candidates; |
| • | the amount and timing of the milestone and royalty payments we receive from our collaborators under our licensing arrangements; |
| • | the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
| • | selling and marketing costs associated with our potential products, including the cost and timing of expanding our marketing and sales capabilities; |
| • | the terms and timing of any potential future collaborations, licensing or other arrangements that we may establish; |
| • | cash requirements of any future acquisitions and/or the development of other drug candidates; |
| • | the costs of operating as a public company; |
| • | the cost and timing of completion of commercial-scale outsourced manufacturing activities; |
| • | the time and cost necessary to respond to technological and market developments; and |
| • | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights. |
17
Table of Contents
Until we can generate a sufficient amount of revenue, we may finance future cash needs through public or private equity offerings, debt financings, collaborations and strategic alliances. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. General market conditions or the market price of our common stock may not support capital raising transactions such as an additional public or private offering of our common stock or other securities. In addition, our ability to raise additional capital may be dependent upon our common stock being quoted on the NASDAQ stock market or upon obtaining shareholder approval to issue a sufficient number of shares of our common stock. There can be no assurance that we will be able to satisfy the criteria for continued listing on the NASDAQ stock market or that we will be able to obtain shareholder approval of such stock issuances if it is necessary. If adequate funds are not available to us on acceptable terms, or at all, we may be required to delay or reduce the scope of, or eliminate, one or more of our research or development programs or our commercialization efforts. We may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time. In addition, if we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or drug candidates or to grant licenses on terms that may not be favorable to us.
We believe that the net proceeds from this offering, together with existing cash and cash equivalents, will not be sufficient to enable us to complete all necessary development or commercially launch our proprietary drug candidates. If we are unable to raise capital when needed or on attractive terms, we will be forced to delay, reduce or eliminate our research and development programs or future commercialization efforts. Our inability to obtain additional funding when needed could seriously harm our business.
Raising additional capital may cause dilution to our shareholders, restrict our operations or require us to relinquish rights to our technologies or drug candidates.
We may seek additional funding through a combination of equity offerings, debt financings, collaborations and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a holder of our common stock. The incurrence of additional indebtedness or the issuance of certain equity securities could result in increased fixed payment obligations and could also result in certain additional restrictive covenants, such as limitations on our ability to incur additional debt or issue additional equity, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. In addition, issuance of additional equity securities, or the possibility of such issuance, may cause the market price of our common stock to decline. In the event that we enter into collaborations or licensing arrangements in order to raise capital, we may be required to accept unfavorable terms, including relinquishing or licensing to a third party on unfavorable terms our rights to technologies or proprietary drug candidates that we otherwise would seek to develop or commercialize ourselves or potentially reserve for future potential arrangements when we might be able to achieve more favorable terms.
Certain of our executive officers and employees have received grants of stock options and shares of restricted stock, which vest over time. Under certain circumstances, such vesting may be accelerated. The accelerated vesting of stock options and shares of restricted stock could result in dilution to our existing stockholders and lower the market price of our common stock.
An impairment of goodwill could have a material adverse effect on our results of operations.
Acquisitions frequently result in the recording of goodwill and other intangible assets. As of March 31, 2017, goodwill represented $37.6 million, or 37.4% of our total assets, primarily as a result of our acquisitions of QuaDPharma, LLC, or QuaDPharma, Comprehensive Drug Enterprises Limited, or CDE, and Polymed Therapeutics, Inc. and Chongqing Taihao Pharmaceutical Co Ltd, collectively Polymed. Goodwill is not
18
Table of Contents
amortized and is subject to impairment testing at least annually using a fair value based approach. The identification and measurement of goodwill impairment involves the estimation of the fair value of our reporting units. The estimates of fair value of reporting units are based on the best information available as of the date of the assessment and incorporate management assumptions about expected future cash flows and other valuation techniques. Future cash flows can be affected by changes in industry or market conditions, among other factors. The recoverability of goodwill is evaluated at least annually or more frequently when events or changes in circumstances indicate that the fair value of a reporting unit has more likely than not declined below its carrying value.
We cannot accurately predict the amount and timing of any future impairment of assets, and, going forward, we may be required to take goodwill or other asset impairment charges relating to certain of our reporting units. Any such charges would have an adverse effect on our financial results.
Our ability to utilize our net operating loss carryforwards and certain other tax attributes may be limited.
We have incurred operating losses that are treated as taxable losses for U.S. federal income tax purposes. To the extent that we continue to generate taxable losses, unused losses will carry forward to offset future taxable income, if any, until such unused losses expire. Under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, if a corporation undergoes an ownership change (generally defined as a greater than 50 percentage points change (by value) in its equity ownership over a rolling three-year period), the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be limited. We believe that we have experienced an ownership change in the past, which may affect our ability to utilize our net operating loss carryforwards. In addition, we may experience ownership changes in the future as a result of this offering or subsequent shifts in our stock ownership, some of which are outside our control. As of March 31, 2017, we had federal net operating loss carryforwards of approximately $146.0 million that could be limited by our past and any future ownership change, which could have an adverse effect on our future results of operations. Similar limitations will apply to our ability to carry forward any unused tax credits to offset future taxable income.
Risks Related to Clinical Development of Our Proprietary Drug Candidates
We depend substantially on the success of our proprietary drug candidates, which are in pre-clinical and clinical development.
As of the date of this prospectus, we had a total of more than 40 planned, ongoing and completed clinical trials for our drug candidates, including a Phase 2 and a Phase 3 clinical trial for KX-01 ointment and Oraxol, respectively. Our business and the ability to generate revenue related to product sales from our proprietary drug candidates will depend on the successful development, regulatory approval and commercialization for the treatment of patients with our drug candidates, which are still in development, and other drugs we may develop. Clinical development is a lengthy and expensive process with an uncertain outcome. The results of pre-clinical studies and early clinical trials of our drug candidates may not be predictive of the results of later-stage clinical trials. In the case of any trials we conduct, results have in the past, and may in the future, fail to meet the desired safety and efficacy endpoints, or differ from earlier trials due to the larger number of clinical trial sites and additional countries and populations involved in such trials. We have invested a significant portion of our efforts and financial resources in the development of our existing drug candidates. The success of our proprietary drug candidates will depend on several factors, including:
| • | successful enrollment in, and completion of, clinical studies; |
| • | receipt of regulatory approvals from the FDA, CFDA and other regulatory authorities for our drug candidates; |
| • | establishing commercial manufacturing capabilities, either by using our own facilities or making arrangements with third-party manufacturers; |
19
Table of Contents
| • | conducting our clinical trials safely and efficiently, and in many cases, relying on third parties to do so; |
| • | obtaining, maintaining and protecting our rights in our intellectual property, including patent, trade secrets, know-how and regulatory exclusivity; |
| • | ensuring we do not infringe, misappropriate or otherwise violate the patent, trade secret or other intellectual property rights of third parties; |
| • | competition with other drug candidates and drugs, including existing IV chemotherapy treatments, potential oncology biologics and other oral dosing technologies developed or being developed by competitors; and |
| • | continued acceptable safety profile for our drug candidates following regulatory approval, if and when received. |
If we do not achieve one or more of these requirements in accordance with our business plans or at all, we could experience significant delays in our ability to obtain approval for and/or to successfully commercialize our drug candidates, which would materially harm our business and we may not be able to generate sufficient revenues and cash flows to continue our operations.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. For example, our current lead product candidate, Oraxol, currently in Phase 3 clinical trials, has been in development by us since 2011. Failure can occur at any time during the clinical trial process. The results of preclinical studies and early clinical trials of our drug candidates may not be predictive of the results of later-stage clinical trials. Drug candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and initial clinical trials. In some instances, there can be significant variability in safety and/or efficacy results between different trials of the same product candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, including genetic differences, patient adherence to the dosing regimen and other trial protocols and the rate of dropout among clinical trial participants. In the case of any trials we conduct, results may differ from early trials due to the larger number of patients, clinical trial sites and additional countries and populations involved in such trials. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials. Our future clinical trial results may not be favorable.
We may not be successful in our efforts to identify or discover additional drug candidates. Due to our limited resources and access to capital, we must and have in the past decided to prioritize development of certain product candidates; these decisions may prove to have been wrong and may adversely affect our business.
To date, we have focused our drug discovery efforts on developing our cancer platform, particularly our Orascovery and Src Kinase Inhibition product candidates. If our cancer platform fails to identify potential drug candidates, our business could be materially harmed. Additionally our management, at the direction of our board of directors, has discretion in prioritizing which product candidates to develop.
Research programs to pursue the development of our drug candidates for additional indications and to identify new drug candidates and disease targets require substantial technical, financial and human resources whether or not we ultimately are successful. Our research programs may initially show promise in identifying potential indications and/or drug candidates, yet fail to yield results for clinical development for a number of reasons, including:
| • | the research methodology used may not be successful in identifying potential indications and/or drug candidates; |
20
Table of Contents
| • | potential drug candidates may, after further study, be shown to lack efficacy, have harmful adverse effects or other characteristics that indicate they are unlikely to be effective drugs; or |
| • | it may take greater human and financial resources to identify additional therapeutic opportunities for our drug candidates or to develop suitable potential drug candidates through internal research programs than we possess, thereby limiting our ability to diversify and expand our drug portfolio. |
Because we have limited financial and managerial resources, we focus on research programs and drug candidates for specific indications. As a result, we may forego or delay pursuit of opportunities with other drug candidates or for other indications that later prove to have greater commercial potential or a greater likelihood of success. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities.
Accordingly, there can be no assurance that we will be able to identify additional therapeutic opportunities for our drug candidates or to develop suitable potential drug candidates through internal research programs, which could materially adversely affect our future growth and prospects. We may focus our efforts and resources on potential drug candidates or other potential programs that ultimately prove to be unsuccessful.
If we encounter difficulties enrolling patients in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the trial until its conclusion. We and our research partners have from time to time and may in the future experience difficulties in patient enrollment in our clinical trials for a variety of reasons, including:
| • | the availability of a sizeable population of eligible patients; |
| • | the design of the trial; |
| • | our ability to recruit clinical trial investigators with the appropriate competencies and experience; |
| • | competing clinical trials for similar therapies or other new therapeutics; |
| • | clinicians’ and patients’ perceptions as to the potential advantages and side effects of the drug candidate being studied in relation to other available therapies, |
| • | our ability to obtain and maintain patient consents; |
| • | the failure of patients to complete a clinical trial; and |
| • | the availability of approved therapies that are similar in mechanism to our drug candidates. |
In addition, our clinical trials will compete with other clinical trials for drug candidates that are in the same therapeutic areas as our drug candidates, and this competition will reduce the number and types of patients available to us, because some patients who might have opted to enroll in our trials may instead opt to enroll in a trial being conducted by one of our competitors. Because the number of qualified clinical investigators is limited, we have conducted and expect to conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which will reduce the number of patients who are available for our clinical trials at such clinical trial sites.
Even if we are able to enroll a sufficient number of patients in our clinical trials, delays in patient enrollment may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of our drug candidates.
21
Table of Contents
Some of our drug candidates represent a novel approach to cancer treatment, which could result in delays in clinical development, heightened regulatory scrutiny, delays in our ability to achieve regulatory approval or commercialization, or market acceptance by physicians and patients of our drug candidates.
Some of our drug candidates, particularly those developed through our Orascovery platform, represent a departure from more commonly used methods for cancer treatment, and therefore represent a novel approach that carries inherent development risks. For instance, our Orascovery platform intends to facilitate the delivery of chemotherapy agents orally, as opposed to IV, while our Src Kinase inhibitor candidates operate by a new mechanism of action. To develop our Orascovery platform, we must successfully develop oral formulations of the active ingredients and ensure they can be delivered safely and consistently in capsule form. The need to further develop or modify in any way the protocols related to our drug candidates to demonstrate safety or efficacy may delay the clinical program, regulatory approval or commercialization, if approved. Our Src Kinase inhibitor platform is based on a novel molecule with an additional mechanism of action that is not found in other Src Kinase inhibitors. Because of this, unexpected safety and tolerability concerns may arise during the development process.
In addition, potential patients and their doctors may be inclined to use conventional standard-of-care treatments rather than enroll patients in any future clinical trial or to use our product candidates commercially once approved. This may have a material impact on our ability to generate revenues from our drug candidates. Further, given the novelty of the administration of our drug candidates, hospitals and physicians may prefer traditional treatment methods, may be reluctant to adopt the use of our products or may require a substantial amount of education and training, any of which could delay or prevent acceptance of our products by physicians and patients and materially hinder successful commercialization of our drug candidates.
Our products and product candidates may cause undesirable, or an increase in the frequency of, side effects that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any.
Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA, CFDA or other regulatory authorities. Further, if a product candidate receives marketing approval and we or others identify undesirable side effects caused by the product after the approval, or if drug abuse is determined to be a significant problem with an approved product, a number of potentially significant negative consequences could result, including:
| • | regulatory authorities may withdraw or limit their approval of the product; |
| • | regulatory authorities may require the addition of labeling statements, such as a “Black Box warning” or a contraindication; |
| • | we may be required to change the way the product is distributed or administered, conduct additional clinical trials or change the labeling of the product; |
| • | we may decide to remove the product from the marketplace; |
| • | we could be sued and held liable for injury caused to individuals exposed to or taking the product; and |
| • | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product candidate and could substantially increase the costs of commercializing an affected product or product candidate and significantly impact our ability to successfully commercialize or maintain sales of our product or product candidates and generate revenues.
22
Table of Contents
If clinical trials of our drug candidates fail to demonstrate safety and efficacy to the satisfaction of the FDA, CFDA or other regulatory authorities or do not otherwise produce positive results, we may incur costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our drug candidates.
We may experience various unexpected events during, or as a result of, clinical trials that could delay or prevent our ability to receive regulatory approval or commercialize our drug candidates, including:
| • | regulators, institutional review boards, or IRBs, or ethics committees may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| • | clinical trials of our drug candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon drug development programs; |
| • | the number of patients required for clinical trials of our drug candidates may be larger than we anticipate, enrollment may be insufficient or slower than we anticipate or patients may drop out at a higher rate than we anticipate; |
| • | our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; |
| • | we might have to suspend or terminate clinical trials of our drug candidates for various reasons, including a finding of a lack of clinical response or a finding that participants are being exposed to unacceptable health risks; |
| • | regulators, IRBs or ethics committees may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements; |
| • | the cost of clinical trials of our drug candidates may be greater than we anticipate; |
| • | the supply or quality of our drug candidates or other materials necessary to conduct clinical trials of our drug candidates may be insufficient or inadequate; and |
| • | our drug candidates may cause adverse events, have undesirable side effects or other unexpected characteristics, causing us or our investigators to suspend or terminate the trials. |
If we are required to conduct additional clinical trials or other testing of our drug candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical trials of our drug candidates or other testing, if the results of these trials or tests are not positive or are only modestly positive or if they raise safety concerns, we may:
| • | be delayed in obtaining regulatory approval for our drug candidates; |
| • | not obtain regulatory approval at all; |
| • | obtain approval for indications that are not as broad as intended; |
| • | have the drug removed from the market after obtaining regulatory approval; |
| • | be subject to additional post-marketing testing requirements; |
| • | be subject to restrictions on how the drug is distributed or used; or |
| • | be unable to obtain reimbursement for use of the drug. |
Delays in testing or approvals may result in increases in our drug development costs. We do not know whether any clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all.
Significant clinical trial delays also could shorten any periods during which we have the exclusive right to commercialize our drug candidates or allow our competitors to bring drugs to market before we do and impair our ability to commercialize our drug candidates and may harm our business and results of operations.
23
Table of Contents
Manufacturing risks, including our inability to manufacture API and clinical products used in the clinical trials of our proprietary product candidates could adversely affect our ability to commercialize our product candidates.
Our business strategy depends on our ability to manufacture API in sufficient quantities and on a timely basis so as to meet our needs to manufacture our product candidates for our clinical trials and to meet consumer demand for our future products, while adhering to product quality standards, complying with regulatory requirements and managing manufacturing costs. We are subject to numerous risks relating to our manufacturing capabilities, including:
| • | Our inability to manufacture API and clinical products in sufficient quantities to meet the needs of our clinical trials or to commercialize our products; |
| • | our inability to secure product components in a timely manner, in sufficient quantities or on commercially reasonable terms; |
| • | our failure to increase production of products to meet demand; |
| • | our inability to modify production lines to enable us to efficiently produce future products or implement changes in current products in response to regulatory requirements; |
| • | difficulty identifying and qualifying alternative suppliers for components in a timely manner; and |
| • | potential damage to or destruction of our manufacturing equipment or manufacturing facility. |
In addition, we conduct manufacturing operations at our facility in Chongqing, China to manufacture our proprietary product candidates. As a result, our business is subject to risks associated with doing business in China, including:
| • | adverse political and economic conditions, particularly those negatively affecting the trade relationship between the U.S. and China; |
| • | trade protection measures, such as tariff increases, and import and export licensing and control requirements; |
| • | potentially negative consequences from changes in tax laws; |
| • | difficulties associated with the Chinese legal system, including increased costs and uncertainties associated with enforcing contractual obligations in China; |
| • | historically lower protection of intellectual property rights; |
| • | unexpected or unfavorable changes in regulatory requirements; |
| • | possible patient or physician preferences for more established pharmaceutical products and medical devices manufactured in the U.S.; and |
| • | difficulties in managing foreign relationships and operations generally. |
These risks are likely to be exacerbated by our limited experience with our current products and manufacturing processes. If, as we expect, our need for API increases, or demand for our products increase, we will have to invest additional resources to purchase components, hire and train employees, and enhance our manufacturing processes. If we fail to increase our production capacity efficiently, our sales may not increase in line with our forecasts and our operating margins could fluctuate or decline. Any of these factors may affect our ability to manufacture our product and could reduce our revenues and profitability.
24
Table of Contents
Risks Related to Obtaining Regulatory Approval for Our Drug Candidates
The regulatory approval processes of the FDA, CFDA and other regulatory authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our drug candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA, CFDA and other regulatory authorities in jurisdictions where we seek such approval is unpredictable but typically takes many years following the commencement of preclinical studies and clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations or the type and amount of clinical data necessary to gain approval may change during the course of a drug candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any drug candidate, and it is possible that none of our existing drug candidates or any drug candidates we may discover, in-license or acquire and seek to develop in the future will ever obtain regulatory approval.
Our drug candidates could fail to receive regulatory approval from the FDA, CFDA or a regulatory authority for many reasons, including:
| • | disagreement with the design or implementation of our clinical trials; |
| • | failure to demonstrate that a drug candidate is safe and effective or safe, pure, and potent for its proposed indication; |
| • | failure of clinical trial results to meet the level of statistical significance required for approval; |
| • | failure to demonstrate that a drug candidate’s clinical and other benefits outweigh its safety risks; |
| • | disagreement with our interpretation of data from preclinical studies or clinical trials; |
| • | the insufficiency of data collected from clinical trials of our drug candidates to support the submission and filing of a new drug application, or NDA, or other submission or to obtain regulatory approval; |
| • | the FDA, CFDA or regulatory authority’s finding of deficiencies related to the product, manufacturing processes or facilities of ours or of third-party manufacturers with whom we contract for clinical and commercial supplies; and |
| • | changes in approval policies or regulations that render our preclinical and clinical data insufficient for approval. |
The FDA, CFDA or a regulatory authority may require more information, including additional preclinical or clinical data, to support approval, which may delay or prevent approval and our commercialization plans, or we may decide to abandon the development program. If we were to obtain approval, regulatory authorities may approve any of our drug candidates for fewer or more limited indications than we request, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve a drug candidate with a label that is not desirable for the successful commercialization of that drug candidate. In addition, if our drug candidate produces undesirable side effects or safety issues, the FDA may require the establishment of Risk Evaluation Mitigation Strategies, or REMS, or the CFDA or a regulatory authority may require the establishment of a similar strategy, that may, for instance, restrict distribution of our drug candidates and impose burdensome implementation requirements on us. Any of the foregoing scenarios could materially harm the commercial prospects of our drug candidates.
The approval process for pharmaceutical products outside the U.S. varies among countries and may limit our ability to develop, manufacture and sell our products internationally. Failure to obtain marketing approval in international jurisdictions would prevent our product candidates from being marketed abroad.
In order to market and sell our products internationally, we must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and
25
Table of Contents
may involve additional testing. We may conduct clinical trials for, and seek regulatory approval to market, our product candidates in countries other than the U.S. and the PRC. Depending on the results of clinical trials and the process for obtaining regulatory approvals in other countries, we may decide to first seek regulatory approvals of a product candidate in countries other than the U.S., or we may simultaneously seek regulatory approvals in the U.S. and other countries. If we seek marketing approval for a product candidate outside the U.S., we will be subject to the regulatory requirements of health authorities in each country in which we seek approval. With respect to marketing authorizations in China, we will be required to seek regulatory approval from the CFDA. The approval procedure varies among regions and countries and may involve additional testing, and the time required to obtain approval may differ from that required to obtain FDA approval.
Obtaining regulatory approvals from health authorities in countries outside the U.S. is likely to subject us to all of the risks associated with obtaining FDA approval described above. In addition, marketing approval by the FDA does not ensure approval by the health authorities of any other country, and marketing approvals by foreign health authorities do not ensure a similar approval by the FDA.
We are conducting, and may in the future conduct, clinical trials for our product candidates in sites outside the U.S. and the FDA may not accept data from trials conducted in such locations.
We have conducted, and may in the future conduct, certain of our clinical trials outside of the U.S. Although the FDA may accept data from clinical trials conducted outside the U.S., acceptance of this data is subject to certain conditions imposed by the FDA. There can be no assurance the FDA will accept data from any clinical trials we conduct outside of the U.S. If the FDA does not accept the data from any of our clinical trials conducted outside the U.S., it would likely result in the need for additional clinical trials in the U.S., which would be costly and time-consuming and could delay or prevent the commercialization of any of our product candidates.
Regulatory approval may be substantially delayed or may not be obtained for one or all of our drug candidates for a variety of reasons.
We may be unable to complete development of our drug candidates on schedule, if at all. The completion of the studies for our drug candidates will require funding beyond the proceeds of this offering. In addition, if regulatory authorities require additional time or studies to assess the safety or efficacy of our drug candidates, we may not have or be able to obtain adequate funding to complete the necessary steps for approval for any or all of our drug candidates. Preclinical studies and clinical trials required to demonstrate the safety and efficacy of our drug candidates are time consuming and expensive and together take several years or more to complete. For example, our current lead product candidate, Oraxol, currently in Phase 3 clinical trials, has been in development since 2011. Delays in clinical trials, regulatory approvals or rejections of applications for regulatory approval in the U.S., Taiwan, New Zealand, China or other markets may result from many factors, including:
| • | our inability to obtain sufficient funds required for a clinical trial; |
| • | regulatory requests for additional analyses, reports, data, non-clinical and preclinical studies and clinical trials; |
| • | regulatory questions regarding interpretations of data and results and the emergence of new information regarding our drug candidates or other products; |
| • | clinical holds, other regulatory objections to commencing or continuing a clinical trial or the inability to obtain regulatory approval to commence a clinical trial in countries that require such approvals; |
| • | failure to reach agreement with the FDA, CFDA or other regulators regarding the scope or design of our clinical trials; |
| • | delay or failure in obtaining authorization to commence a trial or inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a clinical trial; |
26
Table of Contents
| • | our inability to enroll a sufficient number of patients who meet the inclusion and exclusion criteria in a clinical trial; |
| • | our inability to conduct a clinical trial in accordance with regulatory requirements or our clinical protocols; |
| • | clinical sites and investigators deviating from trial protocol, failing to conduct the trial in accordance with regulatory requirements, or dropping out of a trial; |
| • | withdrawal of clinical trial sites from our clinical trials as a result of changing standards of care or the ineligibility of a site to participate in our clinical trials; |
| • | inability to identify and maintain a sufficient number of trial sites, many of which may already be engaged in other clinical trial programs, including some that may be for the same indication; |
| • | failure of our third-party clinical trial managers to satisfy their contractual duties or meet expected deadlines; |
| • | delay or failure in adding new clinical trial sites; |
| • | ambiguous or negative interim results, or results that are inconsistent with earlier results; |
| • | unfavorable or inconclusive results of clinical trials and supportive non-clinical studies, including unfavorable results regarding effectiveness of drug candidates during clinical trials; |
| • | feedback from the FDA, CFDA, an IRB, data safety monitoring boards, or comparable entities, or results from earlier stage or concurrent preclinical studies and clinical trials, that might require modification to the protocol; |
| • | unacceptable risk-benefit profile or unforeseen safety issues or adverse side effects; |
| • | decision by the FDA, CFDA, an IRB, comparable entities, or the company, or recommendation by a data safety monitoring board or comparable regulatory entity, to suspend or terminate clinical trials at any time for safety issues or for any other reason; |
| • | failure to demonstrate a benefit from using a drug or biologic; |
| • | lack of adequate funding to continue the clinical trial due to unforeseen costs or other business decisions; |
| • | our inability to reach agreements on acceptable terms with prospective contract research organizations, or CROs, and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| • | our inability to obtain approval from IRBs or ethics committees to conduct clinical trials at their respective sites; |
| • | manufacturing issues, including problems with manufacturing or timely obtaining from third parties sufficient quantities of a drug candidate for use in a clinical trial; and |
| • | difficulty in maintaining contact with patients after treatment, resulting in incomplete data. |
Changes in regulatory requirements and guidance may also occur, and we may need to amend clinical trial protocols submitted to applicable regulatory authorities to reflect these changes. Amendments may require us to resubmit clinical trial protocols to IRBs or ethics committees for re-examination, which may impact the costs, timing or successful completion of a clinical trial.
According to the Provisions for Drug Registration and the Reform Plan Regarding the Category of the Registration of Chemical Medicines promulgated by the CFDA, the registrations of chemical medicines in China are divided into five categories, among which, Category 1 means the registration of innovative drugs that are not
27
Table of Contents
marketed either domestically or abroad, and Category 5 for the registration of drugs that have been marketed abroad and are being registered for marketing in the PRC for the first time. Our drug candidates are all new therapeutic agents and we have built both research and development, clinical trial capacities, and commercial manufacturing facilities in China. As a result, we expect all of our current drug candidates to fall within the Category 1 application process, but cannot be sure we will be granted or be able to maintain Category 1 designation. We believe the local drug registration pathway, Category 1, is a faster and more efficient path to obtain approval in the Chinese market than the drug registration pathway for imported drugs under Category 5. Category 5 drug candidates may not qualify to benefit from fast track review with priority at the Clinical Trial Application stage. Category 1 drugs receive special examination and approval treatment. The advantages of such treatment include a separate pathway for Category 1 application to queue up for examination by the Center for Drug Evaluation of the CFDA, or the CDE, and a working mechanism for communication with the applicants for discussion of relevant technical issues. The applications for Category 1 drugs are handled with higher priority and enhanced communications with the CDE. Compared with Category 5 drugs, Category 1 drugs are qualified to apply for special examination and approval at both the Clinical Trial Application stage and the production registration application stage. If the special examination and approval are granted at the Clinical Trial Application stage, such treatment will apply to the production registration application stage without further approval. During the Clinical Trial Application stage, reduction or exemption of clinical trial may be available if Category 1 drugs are for orphan diseases or other special diseases. The advantages also include, by providing priority resources, shortening time limits to review and exam applications of Category 1 drugs’ clinical trials and of production registration, and to handle document submission and approval process. We cannot be sure that the CFDA will grant such priority treatment to any of our drugs candidates. Please see “Business—Government Regulation and Product Approval—PRC Government Regulation.”
If we experience delays in the completion of, or the termination of, a clinical trial, of any of our drug candidates, the commercial prospects of our drug candidates will be harmed, and our ability to generate revenues from the sale of any of those drug candidates will be delayed. In addition, any delays in completing our clinical trials will increase our costs, slow down our drug candidate development and approval process, and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our drug candidates.
Our drug candidates have caused and may cause undesirable adverse events or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following any regulatory approval.
Undesirable adverse events caused by our drug candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA, CFDA or other regulatory authority. Results of our trials could reveal a high and unacceptable severity or prevalence of adverse events. In such an event, our trials could be suspended or terminated and the FDA, CFDA or other regulatory authorities could order us to cease further development of, or deny approval of, our drug candidates for any or all targeted indications. Drug-related adverse events could affect patient recruitment or the ability of enrolled subjects to complete the trial, and could result in potential product liability claims. Any of these occurrences may significantly harm our reputation, business, financial condition and prospects.
In our clinical studies to date, we have observed the following serious adverse effects with respect to each of our product candidates:
| • | Oraxol - severe neutropenia, febrile neutropenia, sepsis, septic shock, altered state of consciousness, hypokalemia and cardiac arrest, dehydration, pneumonia, tracheal obstruction, death, nausea, vomiting, diarrhea, fatigue, abdominal and breast pain, anorexia, acute gastroenteritis, atrioventricular block, |
28
Table of Contents
| bacteremia, cerebral hemorrhage, constipation, disease progression, hematuria, liver dysfunction, neoplasm, pain, pancytopenia, pyrexia, syncope, urinary tract infection, urinary tract obstruction and death; |
| • | Oratecan - diarrhea, rash, gastrointestinal hemorrhage, vomiting, nausea, increased bilirubin, leukopenia, pulmonary embolism, asthenia, neutropenia, anorexia, increased alanine aminotransferase, increased aspartate aminotransferase, enteritis and acute kidney injury; |
| • | KX-01 oral - allergic reaction, bacteremia, fatigue, rash, syncope, tremor, dermatitis, neutropenic fever, hyponatremia, failure to thrive, lower extremity edema, mucositis, neutropenia, pancytopenia, thrombocytopenia, seizure and motor vehicle accident, embolic stroke, pneumonitis, fever, acute kidney injury, lung infection and increased blood platelet, albumin and bilirubin levels, abdominal pain, arm pain, pyrexia, rigors, tachypenia, oxygen desaturation pneumonia, anemia, elevated ALT and AST, dehydration and leukopenia; and |
| • | KX-02 - thromboembolic event. |
Additionally, if one or more of our drug candidates receives regulatory approval, and we or others later identify undesirable side effects caused by such drugs, a number of potentially significant negative consequences could result, including:
| • | we may suspend marketing of the drug; |
| • | regulatory authorities may withdraw approvals of the drug; |
| • | regulatory authorities may require additional warnings on the label; |
| • | we may be required to develop a REMS for the drug or, if a REMS is already in place, to incorporate additional requirements under the REMS, or to develop a similar strategy as required by a regulatory authority; |
| • | we may be required to conduct post-marketing studies; |
| • | we could be sued and held liable for harm caused to subjects or patients; and |
| • | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular drug candidate, if approved, and could significantly harm our business, results of operations and prospects.
We may seek Orphan Drug Exclusivity for some of our drug candidates, and we may be unsuccessful.
We have received Orphan Drug Designation from the FDA for our KX-02 proprietary product candidate. As part of our business strategy, we may seek Orphan Drug Designation for our product candidates and we may be unsuccessful. Regulatory authorities in some jurisdictions, including the U.S. and Europe, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a drug as an orphan drug if it is a drug intended to treat a rare disease or condition, which is generally defined as a disease with a patient population of fewer than 200,000 individuals in the U.S., or a patient population greater than 200,000 in the U.S. where there is no reasonable expectation that the cost of developing the drug will be recovered from sales in the U.S.
Generally, if a drug with an Orphan Drug Designation subsequently receives the first regulatory approval for the indication for which it has such designation, the drug is entitled to a period of marketing exclusivity, which precludes the FDA from approving another marketing application for the same drug for the same indication during the period of exclusivity, with certain limited exceptions. The applicable period is seven years in the U.S. and 10 years in Europe. The European exclusivity period can be reduced to six years if a drug no longer meets the criteria for Orphan Drug Designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan Drug Exclusivity may be lost if the FDA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
29
Table of Contents
Even if we obtain Orphan Drug Exclusivity for a drug candidate, exclusivity may not effectively protect the drug candidate from competition because different drugs can be approved for the same condition and the same drugs can be approved for a different condition but used off-label for any orphan indication we may obtain. Even after an orphan drug is approved, the FDA can subsequently approve a different drug for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care.
Risks Related to Commercialization of Our Drug Candidates
If we are not able to obtain, or experience delays in obtaining, required regulatory approvals, we will not be able to commercialize our drug candidates, and our ability to generate revenue will be materially impaired.
We currently do not have any proprietary drug candidates that have gained regulatory approval for sale in the U.S., China or any other country, and we cannot guarantee that we will ever obtain regulatory approval for marketable proprietary drugs. Our business is substantially dependent on our ability to complete the development of, obtain regulatory approval for and successfully commercialize drug candidates in a timely manner. We cannot commercialize drug candidates without first obtaining regulatory approval to market each drug from the FDA, CFDA or regulatory authorities in the relevant jurisdictions. Our proprietary drug candidates are currently undergoing various phases of FDA clinical trials. We cannot predict whether these trials and future trials will be successful or whether regulators will agree with our conclusions regarding the preclinical studies and clinical trials we have conducted to date.
Before obtaining regulatory approvals for the commercial sale of any drug candidate for a target indication, we must demonstrate in preclinical studies and well-controlled clinical trials, and, with respect to approval in the U.S., to the satisfaction of the FDA, that the drug candidate is safe and effective for use for that target indication and that the manufacturing facilities, processes and controls are adequate. An NDA must include extensive preclinical and clinical data and supporting information to establish the drug candidate’s safety and effectiveness. The NDA must also include significant information regarding the chemistry, manufacturing and controls for the drug. Obtaining approval of an NDA is a lengthy, expensive and uncertain process, and approval may not be obtained. If we submit an NDA to the FDA, the FDA decides whether to accept or reject the submission for filing. We cannot be certain that any submissions will be accepted for filing and review by the FDA.
Regulatory authorities outside of the U.S., such as the regulatory authorities in emerging markets, also have requirements for approval of drugs for commercial sale with which we must comply prior to marketing in those areas. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of our drug candidates. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and obtaining regulatory approval in one country does not mean that regulatory approval will be obtained in any other country. Approval processes vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking non-U.S. regulatory approval could require additional non-clinical studies or clinical trials, which could be costly and time-consuming. The non-U.S. regulatory approval process may include all of the risks associated with obtaining FDA approval and other risks specific to the relevant jurisdiction. For all of these reasons, we may not obtain non-U.S. regulatory approvals on a timely basis, if at all.
If we are unable to obtain regulatory approval for our drug candidates in one or more jurisdictions, or any approval contains significant limitations, our target market will be reduced and our ability to realize the full market potential of our drug candidates will be harmed. Furthermore, if we are not able to obtain, or experience delays in obtaining, required regulatory approvals, we will not be able to commercialize our drug candidates, and our ability to generate revenue will be materially impaired.
30
Table of Contents
Even if any of our drug candidates receives regulatory approval, they may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success.
If any of our drug candidates receives regulatory approval, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. For example, current cancer treatments like chemotherapy and radiation therapy are well established in the medical community, and doctors may continue to rely on these treatments to the exclusion of our drug candidates. In addition, physicians, patients and third-party payors may prefer other novel products to ours, and we may experience difficulties gaining acceptance for our orally administered drug candidates. We are also subject to regulatory restrictions on how we market our drug candidates. If our drug candidates do not achieve an adequate level of acceptance, we may not generate significant product sales revenues and we may not become profitable. The degree of market acceptance of our drug candidates, if approved for commercial sale, will depend on a number of factors, including:
| • | the clinical indications for which our drug candidates are approved; |
| • | physicians, hospitals, cancer treatment centers and patients considering our drug candidates as a safe and effective treatment; |
| • | the potential and perceived advantages of our drug candidates over alternative treatments; |
| • | the prevalence and severity of any side effects; |
| • | product labeling or product insert requirements of the FDA, CFDA or other regulatory authorities; |
| • | limitations or warnings contained in the labeling approved by the FDA, CFDA or other regulatory authorities; |
| • | the timing of market introduction of our drug candidates as well as competitive drugs; |
| • | the cost of treatment in relation to alternative treatments; |
| • | the amount of upfront costs or training required for physicians to administer our drug candidates; |
| • | the availability of adequate coverage, reimbursement and pricing by third-party payors and government authorities (including U.S. federal healthcare programs); |
| • | the willingness of patients to pay out-of-pocket in the absence of coverage and reimbursement by third-party payors and government authorities; |
| • | relative convenience and ease of administration, including as compared to alternative treatments and competitive therapies; and |
| • | the effectiveness of our sales and marketing efforts. |
If our drug candidates are approved but fail to achieve market acceptance among physicians, patients, hospitals, cancer treatment centers or others in the medical community, we will not be able to generate significant revenue. Even if our drugs achieve market acceptance, we may not be able to maintain that market acceptance over time if new products or technologies are introduced that are more favorably received than our drugs, are more cost effective or render our drugs obsolete.
Even if we receive regulatory approval for our drug candidates, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our drug candidates.
If our drug candidates are approved, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing studies, and submission of safety, efficacy, and other post-marketing information, including both federal and state requirements in the U.S. and requirements of regulatory authorities.
31
Table of Contents
Manufacturers and manufacturers’ facilities are required to comply with extensive requirements of the FDA, CFDA and regulatory authorities, including, in the U.S., ensuring that quality control and manufacturing procedures conform to current cGMP regulations. As such, we and our contract manufacturers will be subject to continual review and inspections to assess compliance with cGMP and adherence to commitments made in any NDA or other marketing application, and previous responses to inspection observations. Accordingly, we and others with whom we work must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production and quality control.
Any regulatory approvals that we receive for our drug candidates may be subject to limitations on the approved indicated uses for which the drug may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials and surveillance to monitor the safety and efficacy of the drug candidate. The FDA may also require a REMS program as a condition of approval of one or more of our drug candidates, which could entail requirements for long-term patient follow-up, a medication guide, physician communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. In addition, if the FDA, CFDA or a regulatory authority approves our drug candidates, we will have to comply with requirements including, for example, submissions of safety and other post-marketing information and reports, registration, and continued compliance with cGMPs and Good Clinical Practices, or GCPs, for any clinical trials that we conduct post-approval.
The FDA may impose consent decrees or withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the drug reaches the market. Later discovery of previously unknown problems with our drug candidates, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-marketing studies or clinical studies to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, among other things:
| • | restrictions on the marketing or manufacturing of our drugs, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| • | fines, untitled or warning letters, or holds on clinical trials; |
| • | refusal by the FDA to approve pending applications or supplements to approved applications filed by us or suspension or revocation of license approvals; |
| • | product seizure or detention, or refusal to permit the import or export of our drug candidates; and |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA, CFDA and other regulatory authorities actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability. The policies of the FDA, CFDA and of other regulatory authorities may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our drug candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the U.S. or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any regulatory approval that we may have obtained and we may not achieve or sustain profitability.
In addition, if we were able to obtain accelerated approval of any of our drug candidates, the FDA would require us to conduct a confirmatory study to verify the predicted clinical benefit and additional safety studies.
32
Table of Contents
Other regulatory authorities outside the U.S., such as the CFDA, may have similar requirements. The results from the confirmatory study may not support the clinical benefit, which would result in the approval being withdrawn. While operating under accelerated approval, we will be subject to certain restrictions that we would not be subject to upon receiving regular approval.
We market certain medical devices that, if modified, may be subject to FDA clearance and failure to obtain such clearance could adversely affect our financial condition or results of operations.
Through our subsidiary, Polymed, we currently market in-vitro diagnostic rapid test kits used in the performance of clinical laboratory tests (limited to drugs of abuse and pregnancy testing in the U.S.) under 510(k) clearance by the FDA pursuant to Section 510(k) of the Federal Food, Drug and Cosmetic Act. These products and our operations are subject to extensive regulation by the FDA and other federal and state authorities in the United States, as well as comparable authorities in foreign jurisdictions. After a device receives 510(k) marketing clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, will require a new 510(k) marketing clearance or, depending on the modification, Premarket Approval, or PMA. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k) or a PMA in the first instance, but the FDA can review that decision and disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or request the recall of the modified device until 510(k) marketing clearance or PMA approval is obtained. Also, in these circumstances, we may be subject to significant regulatory fines or penalties. In the event we make additional product enhancements to our 510(k)-cleared products, we cannot be assured that the FDA would agree with any of our decisions to not submit 510(k) premarket notifications for these modified devices.
Our manufacturing experience is limited and any failure by us to manufacture our products for commercial sale after receiving FDA approval would materially impact our revenue and financial condition.
The manufacture of drugs for commercial sale is subject to regulation by the FDA under cGMP regulations and by other regulators under other laws and regulations. We cannot assure you that we will continue to manufacture our products under cGMP regulations or other laws and regulations in sufficient quantities for commercial sale, or in a timely or economical manner.
Our manufacturing facilities require specialized personnel and are expensive to operate and maintain. Any delay in the regulatory approval or market launch of product candidates to be manufactured in these facilities will require us to continue to operate these expensive facilities and retain specialized personnel, which may increase our expected losses.
Through our public-private partnerships, additional cGMP manufacturing facilities for our use are currently being built in Dunkirk, New York and Chongqing, China. Our facility in Dunkirk, New York is being built pursuant to an agreement with Fort Schuyler Management Corporation, or FSMC, a not-for-profit corporation organized by the State of New York. Under the current arrangement, we will select and hire contractors for the project and oversee the development of the Dunkirk facility. Empire State Development, or ESD, the parent entity of FSMC, is responsible for the costs of construction and all equipment for the facility, up to an aggregate of $200 million, and ESD, not us, will own the facility and equipment. We have limited experience in overseeing the development of such a facility and we may not be able to complete the development within the timeframe expected, within the expected budget, or at all. If development of the Dunkirk facility is delayed or not completed it could materially adversely affect our operations and financial results.
Additionally, upon completion, both the Dunkirk and Chongqing facilities will need to be cGMP validated prior to operating. Validation is a lengthy process that must be completed before we can manufacture under cGMP guidelines. We cannot guarantee that the FDA or foreign regulatory agencies will approve any of the other facilities or, once they are approved, that such facilities will remain in compliance with cGMP regulations.
33
Table of Contents
The manufacture of pharmaceutical products is a highly complex process in which a variety of difficulties may arise from time to time. We may not be able to resolve any such difficulties in a timely fashion, if at all. If anything were to interfere with the continuing manufacturing operations in our facilities, it could materially adversely affect our business and financial condition.
Currently, many of our product candidates are manufactured in small quantities for use in clinical trials. We cannot assure you that we will be able to successfully scale up the manufacture of each of our product candidates in a timely or economical manner, or at all. If any of these product candidates are approved by the FDA or other drug regulatory authorities for commercial sale, we will need to manufacture them in larger quantities. If we are unable to successfully scale up our manufacturing capacity, the regulatory approval or commercial launch of such product candidate may be delayed or there may be a shortage in supply of such product candidate.
If we fail to develop manufacturing capacity and experience, fail to continue to contract for manufacturing on acceptable terms, or fail to manufacture our product candidates economically on a commercial scale or in accordance with cGMP regulations, our development programs will be materially adversely affected. This may result in delays in receiving FDA or foreign regulatory approval for one or more of our product candidates or delays in the commercial production of a product that has already been approved. Any such delays could materially adversely affect our business and financial condition.
The manufacture of API is highly regulated by FDA, CFDA and other regulatory bodies and is subject to current good manufacturing practice requirements and to inspection by such regulators, which may result in adverse findings and actions against certain API manufacturing facilities.
API manufacturing facilities are subject to regulation by the applicable regulatory bodies in the place of manufacture as well as the regulatory agency in the country to which the product is exported. For instance, FDA’s cGMP regulations apply to these facilities and violation of these, or other, regulations may result in adverse action against the facility, including cessation of manufacturing activities. Our API manufacturing facilities in Chongqing are also subject to regulation by the CFDA. If the FDA, CFDA or other regulators discover a problem at one facility, we may be subject to increased scrutiny and/or adverse actions across our operations, including fines or orders to cease manufacturing, which could have a material impact on our operations, clinical development, business strategy or results of operations.
We have limited experience in marketing proprietary drug products. If we are unable to establish such marketing and sales capabilities or enter into agreements with third parties to market and sell our proprietary drug candidates, we may not be able to generate sales revenue from such products.
We have limited sales, marketing and commercial product experience. We intend to continue to develop our in-house commercial organization and sales force for such products, which will require significant capital expenditures, management resources and time. We will have to compete with other pharmaceutical and biotechnology companies to recruit, hire, train and retain marketing and sales personnel.
If we are unable to establish internal sales, marketing and commercial distribution capabilities for our proprietary drug candidates, we will need to pursue collaborative arrangements for the sales and marketing of our proprietary drugs. However, there can be no assurance that we will be able to establish or maintain such collaborative arrangements, or if we are able to do so, that they will have effective sales forces. Any revenue we receive will depend upon the efforts of such third parties, which may not be successful. We may have less control over the marketing and sales efforts of such third parties which may present fraud and abuse and other regulatory considerations, and our revenue from product sales may be lower than if we had commercialized our proprietary drug candidates ourselves. We also face competition in our search for third parties to assist us with the sales and marketing efforts of our proprietary drug candidates.
There can be no assurance that we will be able to develop our in-house sales and commercial distribution capabilities or establish or maintain relationships with third-party collaborators to successfully commercialize any proprietary product, and as a result, we may not be able to generate sales revenue from such products.
34
Table of Contents
We face substantial competition, and our competitors may discover, develop or commercialize competing drugs before or more successfully than we do.
The development and commercialization of new drugs is highly competitive. We face competition with respect to our current drug candidates, and will face competition with respect to any drug candidates that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell drugs or are pursuing the development of drugs for the treatment of the types of cancer for which we are developing our drug candidates. Some of these competitive drugs and therapies are based on scientific approaches that are the same as or similar to our approach, and others are based on entirely different approaches. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize drugs that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any drugs that we may develop. Our competitors also may obtain approval from the FDA, CFDA or other regulatory authorities for their drugs more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market and/or slow our regulatory approval.
Many of the companies against which we are competing or against which we may compete in the future have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved drugs than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Even if we are able to commercialize any drug candidates, the drugs may become subject to unfavorable pricing regulations, third party reimbursement practices or healthcare reform initiatives, which could harm our business.
Successful sales of our drug candidates, if approved, depend on the availability of adequate coverage and reimbursement from third-party payors. Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Adequate coverage and reimbursement from governmental healthcare programs, such as Medicare and Medicaid in the U.S., and commercial payors are critical to new drug acceptance.
The regulations that govern regulatory approvals, pricing and reimbursement for new therapeutic products vary widely from country to country. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or licensing approval is granted. In some non-U.S. markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain regulatory approval for a drug in a particular country, but be subject to price regulations that delay our commercial launch of the drug and negatively impact the revenues we are able to generate from the sale of the drug in that country. For example, according to the guidance issued in March 2015 by the central government of the PRC, each province will decide which drugs to include in its provincial major illness reimbursement lists and the percentage of reimbursement, based on local funding. Adverse pricing limitations may hinder our ability to recover our investment in one or more drug candidates, even if our drug candidates obtain regulatory approval. For example, in China, according to a
35
Table of Contents
statement, Opinions on reforming the review and approval process for pharmaceutical products and medical devices, issued by the State Council in August 2015, the enterprises applying for new drug approval will be required to undertake that the selling price of new drug on PRC mainland market shall not be higher than the comparable market prices of the product in its country of origin or PRC’s neighboring markets, as applicable.
Our ability to commercialize any drugs successfully also will depend in part on the extent to which coverage and reimbursement for these drugs and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. Coverage and reimbursement by a third-party payor may depend upon a number of factors, including the third-party payor’s determination that use of a drug is:
| • | a covered benefit under its health plan; |
| • | safe, effective and medically necessary; |
| • | appropriate for the specific patient; |
| • | cost-effective; and |
| • | neither experimental nor investigational. |
We cannot be sure that reimbursement will be available for any drug that we commercialize and, if coverage and reimbursement are available, what the level of reimbursement will be. Reimbursement may impact the demand for, or the price of, any drug for which we obtain regulatory approval. Obtaining reimbursement for our drugs may be particularly difficult because of the higher prices often associated with branded drugs and drugs administered under the supervision of a physician. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize any drug candidate that we successfully develop.
In the U.S., no uniform policy of coverage and reimbursement for drugs exists among third-party payors. As a result, obtaining coverage and reimbursement approval of a drug from a government or other third-party payor is a time-consuming and costly process that could require us to provide to each payor supporting scientific, clinical and cost-effectiveness data for the use of our drugs on a payor-by-payor basis, with no assurance that coverage and adequate reimbursement will be obtained. Even if we obtain coverage for a given drug, the resulting reimbursement payment rates might not be adequate for us to achieve or sustain profitability or may require co-payments that patients find unacceptably high. Additionally, third-party payors may not cover, or provide adequate reimbursement for, long-term follow-up evaluations required following the use of our drugs. However, under Medicare Part D – Medicare’s outpatient prescription drug benefit – there are protections in place to ensure coverage and reimbursement for oncology products and all Part D prescription drug plans are required to cover substantially all anti-cancer agents.
The State Council required central and provincial authorities across the PRC to promote a medical insurance program for major illnesses, which targets covering at least 50% of the medical cost as incurred by treating major illnesses, but falls out of the coverage of the basic insurance programs. The State Council requires provincial authorities to increase reimbursement rates over the next three years.
We intend to seek approval to market our drug candidates in the U.S., China, and in other selected jurisdictions. If we obtain approval in one or more non-U.S. jurisdictions for our drug candidates, we will be subject to rules and regulations in those jurisdictions. In some non-U.S. countries, the pricing of drugs and biologics is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after obtaining regulatory approval of a drug candidate. In addition, market acceptance and sales of our drug candidates will depend significantly on the availability of adequate coverage and reimbursement from third-party payors for our drug candidates and may be affected by existing and future health care reform measures.
36
Table of Contents
Recently enacted and future legislation may increase the difficulty and cost for us to obtain regulatory approval of and commercialize our drug candidates and affect the prices we may obtain.
In the U.S., China and certain other jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay regulatory approval of our drug candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any drug candidates for which we obtain regulatory approval.
In the U.S., the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for physician-administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class. Cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for any approved products. While the MMA only applies to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
More recently, in March 2010, President Obama signed into law the Affordable Care Act, or ACA, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms.
Among the provisions of the Affordable Care Act of importance to our potential drug candidates are the following:
| • | an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologics; |
| • | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; |
| • | expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance; |
| • | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices; |
| • | extension of manufacturers’ Medicaid rebate liability; |
| • | expansion of eligibility criteria for Medicaid programs; |
| • | expansion of the entities eligible for discounts under the Public Health Service Act pharmaceutical pricing program; |
| • | new requirements to report payments and other transfers of value made to physicians or teaching hospitals; |
| • | a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; and |
| • | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. |
In addition, other legislative changes have been proposed and adopted in the U.S. since the Affordable Care Act was enacted. These changes included aggregate reductions to Medicare payments to providers of up to 2%
37
Table of Contents
per fiscal year, starting in 2013. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding.
We expect that the Affordable Care Act, as well as other healthcare reform legislative measures that have been since adopted or may be adopted in the future, may result in more rigorous coverage criteria and an additional downward pressure on the price that we receive for any approved drug. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our drugs. In particular, we expect that the new presidential administration and U.S. Congress will seek to modify, repeal, or otherwise invalidate all, or certain provisions of, the ACA. Since taking office, President Trump has continued to support the repeal of all or portions of the ACA. President Trump has also issued an executive order in which he stated that it is his administration’s policy to seek the prompt repeal of the ACA and directed executive departments and federal agencies to waive, defer, grant exemptions from, or delay the implementation of the provisions of the ACA to the maximum extent permitted by law. There is still uncertainty with respect to the impact President Trump’s administration and the U.S. Congress may have, if any, and any changes will likely take time to unfold. Such reforms could have an adverse effect on anticipated revenues from therapeutic candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop therapeutic candidates. However, we cannot predict the ultimate content, timing or effect of any healthcare reform legislation or the impact of potential legislation on us.
Other legislative and regulatory proposals have been made to expand post-approval requirements and restrict coverage and reimbursement and sales and promotional activities, for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether agencies such as the FDA or Centers for Medicare and Medicaid Services will issue new regulations, guidance or interpretations that may impact our drug candidates. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent regulatory approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
We may be subject, directly or indirectly, to applicable U.S. federal and state anti-kickback, false claims laws, physician payment transparency laws, fraud and abuse laws or similar healthcare and privacy and security laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and others play a primary role in the recommendation and prescription of any products for which we obtain regulatory approval. If we obtain FDA approval for any of our drug candidates and begin commercializing those drugs in the U.S., our operations may be subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act and physician payment transparency laws and regulations. These laws may impact, among other things, our proposed sales and marketing programs as well as any patient support programs we may consider offering. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, either the referral of an individual, or the purchase, lease, order or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs; |
| • | federal civil and criminal false claims laws and civil monetary penalty laws, such as the federal False Claims Act which imposes criminal and civil penalties, including civil whistleblower or qui tam |
38
Table of Contents
| actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment or approval from Medicare, Medicaid or other third-party payors that are false or fraudulent, including failure to timely return an overpayment received from the federal government or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | provisions of the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes referred to as the “HIPAA All-Payor Fraud Prohibition,” prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters; |
| • | provisions of HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 and their respective implementing regulations, which impose requirements on certain covered healthcare providers, health plans, and healthcare clearinghouses as well as their respective business associates that perform services for them that involve the use, or disclosure of, individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization; |
| • | the federal transparency requirements under the Affordable Care Act, including the provision commonly referred to as the Physician Payments Sunshine Act, which requires manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program to report annually to the U.S. Department of Health and Human Services information related to all payments or other transfers of value made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members unless a specific exclusion applies; and |
| • | federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers. |
Additionally, we are subject to state and non-U.S. equivalents of each of the healthcare laws described above, among others, some of which may be broader in scope and may apply regardless of the payor. Many U.S. states have adopted laws similar to the Federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare services reimbursed by any source, not just governmental payors, including private insurers. In addition, some states have passed laws that require pharmaceutical companies to comply with the April 2003 Office of Inspector General Compliance Program Guidance for Pharmaceutical Manufacturers and/or the Pharmaceutical Research and Manufacturers of America’s Code on Interactions with Healthcare Professionals. Several states also impose other marketing restrictions or require pharmaceutical companies to make marketing or price disclosures to the state. There are ambiguities as to what is required to comply with these state requirements and if we fail to comply with an applicable state law requirement we could be subject to penalties.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent health care reform legislation has strengthened these laws. For example, the Affordable Care Act, among other things, amends the intent requirement of the federal Anti-Kickback and criminal healthcare fraud statutes. As a result of such amendment, a person or entity no longer needs to have actual knowledge of these statutes or specific intent to violate them in order to have committed a violation. Moreover, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
39
Table of Contents
Violations of fraud and abuse laws may be punishable by criminal and/or civil sanctions, including penalties, fines and/or exclusion or suspension from federal and state healthcare programs such as Medicare and Medicaid and debarment from contracting with the U.S. government. In addition, private individuals have the ability to bring actions on behalf of the U.S. government under the Federal False Claims Act as well as under the false claims laws of several states.
Law enforcement authorities are increasingly focused on enforcing these laws, and it is possible that some of our practices may be challenged under these laws. Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, disgorgement, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations. In addition, the approval and commercialization of any of our drug candidates outside the U.S. will also likely subject us to non-U.S. equivalents of the healthcare laws mentioned above, among other non-U.S. laws.
If any of the physicians or other providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
We expect that the new presidential administration and U.S. Congress will seek to modify, repeal, or otherwise invalidate all, or certain provisions of, the ACA, which could impact provisions of the existing fraud and abuse, privacy and security or other health care laws and regulations. There is still uncertainty with respect to the impact President Trump’s administration and the U.S. Congress may have, if any, and any changes will likely take time to unfold. Such reforms could have an adverse effect on anticipated revenues from therapeutic candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop therapeutic candidates. However, we cannot predict the ultimate content, timing or effect of any healthcare reform legislation or the impact of potential legislation on us.
Lastly, political, economic, and regulatory influences are subjecting the health care industry in the United States to fundamental change. Initiatives to reduce the federal budget and debt and to reform health care coverage are increasing cost-containment efforts. We anticipate that federal agencies, Congress, state legislatures, and the private sector will continue to review and assess alternative health care benefits, controls on health care spending, and other fundamental changes to the healthcare delivery system. Any proposed or actual changes could limit coverage for or the amounts that federal and state governments will pay for health care products and services, which could also result in reduced demand for our products or additional pricing pressures, and limit or eliminate our spending on development projects and affect our ultimate profitability.
We intend to market our drugs, if approved, in a variety of international markets and we are exploring the licensing of commercialization rights or other forms of collaboration worldwide, which exposes us to additional risks of conducting business in additional international markets.
We conduct business operations in regions including the U.S., China, Taiwan and New Zealand, and non-U.S. markets are an important component of our growth strategy. If we fail to obtain licenses or enter into collaboration arrangements with third parties in these markets, or if these parties are not successful, our revenue-generating growth potential will be adversely affected.
40
Table of Contents
Moreover, international business relationships subject us to additional risks that may materially adversely affect our ability to attain or sustain profitable operations, including:
| • | initiatives to develop an international sales, marketing and distribution organization may increase our expenses, divert our management’s attention from the acquisition or development of drug candidates or cause us to forgo profitable licensing opportunities in these geographies; |
| • | efforts to enter into collaboration or licensing arrangements with third parties in connection with our international sales, marketing and distribution efforts may increase our expenses or divert our management’s attention from the acquisition or development of drug candidates; |
| • | changes in a specific country’s or region’s laws, regulations or political and cultural climate or economic condition; |
| • | differing regulatory requirements for drug approvals and marketing internationally; |
| • | difficulty of effective enforcement of contractual provisions and intellectual property rights in local jurisdictions; |
| • | potentially reduced protection for intellectual property rights; |
| • | potential third-party patent rights; |
| • | unexpected changes in tariffs, trade barriers and regulatory requirements, such as Export Administration Regulations promulgated by the U.S. Department of Commerce and fines, penalties or suspension or revocation of export privileges; |
| • | economic weakness, including inflation or political instability, particularly in non-U.S. economies and markets; |
| • | compliance with tax, employment, immigration and labor laws for employees traveling abroad; |
| • | the effects of applicable non-U.S. tax structures and potentially adverse tax consequences; |
| • | currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incidental to doing business in another country; |
| • | workforce uncertainty and labor unrest, particularly in non-U.S. countries where labor unrest is more common than in the U.S.; |
| • | the potential for so-called parallel importing, which is what happens when a local seller, faced with high or higher local prices, opts to import goods from a non-U.S. market with low or lower prices rather than buying them locally; |
| • | failure of our employees and contracted third parties to comply with Office of Foreign Asset Control rules and regulations and the Foreign Corrupt Practices Act; |
| • | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| • | business interruptions resulting from geo-political actions, including war and terrorism, or natural disasters, including earthquakes, volcanoes, typhoons, floods, hurricanes and fires. |
These and other risks may materially adversely affect our ability to obtain or sustain revenue from international markets.
41
Table of Contents
The use of legal, regulatory, and legislative strategies by both brand and generic competitors, including but not limited to “authorized generics” and regulatory petitions, as well as the potential impact of proposed and newly enacted legislation, may increase costs associated with the introduction or marketing of our generic products, could delay or prevent such introduction, and could adversely affect our results of operations.
Our competitors, both branded and generic, often pursue strategies to prevent, delay, or eliminate competition from generic alternatives to branded products. These strategies include, but are not limited to:
· entering into agreements whereby other generic companies will begin to market an authorized generic, a generic equivalent of a branded product, at the same time or after generic competition initially enters the market;
| • | launching a generic version of their own branded product prior to or at the same time or after generic competition initially enters the market; |
| • | filing petitions with the FDA or other regulatory bodies seeking to prevent or delay approvals, including timing the filings so as to thwart generic competition by causing delays of our product approvals; |
| • | seeking to establish regulatory and legal obstacles that would make it more difficult to demonstrate bioequivalence or to meet other requirements for approval, and/or to prevent regulatory agency review of applications, such as through the establishment of patent linkage (laws and regulations barring the issuance of regulatory approvals prior to patent expiration); |
| • | initiating legislative or other efforts to limit the substitution of generic versions of brand pharmaceuticals; |
| • | filing suits for patent infringement and other claims that may delay or prevent regulatory approval, manufacture, and/or scale of generic products; |
| • | introducing “next-generation” products prior to the expiration of market exclusivity for the reference product, which often materially reduces the demand for the generic or the reference product for which we seek regulatory approval; |
| • | persuading regulatory bodies to withdraw the approval of brand name drugs for which the patents are about to expire and converting the market to another product of the brand company on which longer patent protection exists; |
| • | obtaining extensions of market exclusivity by conducting clinical trials of brand drugs in pediatric populations or by other methods; and |
| • | seeking to obtain new patents on drugs for which patent protection is about to expire. |
If any other actions by our competitors and other third parties to prevent or delay activities necessary to the approval, manufacture, or distribution of our products are successful, our entry into the market and our ability to generate revenues associated with new products may be delayed, reduced, or eliminated, which could have a material adverse effect on our business, financial condition, results of operations, cash flows, and/or share price.
Our compounded preparations and the pharmacy compounding industry are subject to regulatory and customer scrutiny, which may impair our growth and sales.
Formulations prepared and dispensed by compounding pharmacies contain FDA-approved ingredients, but are not themselves approved by the FDA. As a 503B outsourcing facility, our compounded formulations are not subject to the FDA approval process. Certain compounding pharmacies have been the subject of widespread negative media coverage in recent years, and the actions of these pharmacies have resulted in increased scrutiny of compounding pharmacy activities from the FDA and state governmental agencies. For example, the FDA has in the past requested that a number of compounding pharmacies conduct a recall of all non-expired, purportedly sterile drug products and cease sterile compounding operations due to lack of sterility assurance, and additional compounding pharmacies have suspended sterile production or voluntarily recalled certain sterile compounding products after an FDA inspection of the relevant facilities. As a result of this exercise of caution, some physicians may be hesitant to prescribe, and some patients may be hesitant to purchase and use, these compounded formulations.
42
Table of Contents
If a compounded drug formulation provided through our compounding services leads to patient injury or death or results in a product recall, we may be exposed to significant liabilities and reputational harm.
The production, labeling and packaging of compounded drugs is inherently risky. The success of our compounded formulations and pharmacy operations depends to a significant extent upon perceptions of the safety and quality of our products. We could be adversely affected if our formulations are subject to negative publicity. We could also be adversely affected if any of our formulations or other products, any similar products sold by other companies, or any products sold by other compounding outsourcing facilities, prove to be, or are asserted to be, harmful to patients. There are a number of factors that could result in the injury or death of a patient who receives one of our compounded formulations, including quality issues, manufacturing or labeling flaws, improper packaging or unanticipated or improper uses of the products, any of which could result from human or other error. Any of these situations could lead to a recall of, or safety alert relating to, one or more of our products. Similarly, to the extent any of the components of approved drugs or other ingredients used by us to produce compounded formulations have quality or other problems that adversely affect the finished compounded preparations, our sales could be adversely affected. In addition, in the ordinary course of business, we may voluntarily retrieve products in response to a customer complaint. Because of our dependence upon medical and patient perceptions, any adverse publicity associated with illness or other adverse effects resulting from the use or misuse of our products, any similar products sold by other companies or any other compounded formulations, could have a material adverse impact on our business, results of operations and financial condition.
Risks Related to Our Intellectual Property
A significant portion of our intellectual property portfolio currently comprises pending patent applications that have not yet been issued as granted patents, and if our pending patent applications fail to issue our business will be adversely affected. If we are unable to obtain and maintain patent protection for our technology and drugs, our competitors could develop and commercialize technology and drugs similar or identical to ours, and our ability to successfully commercialize our technology and drugs may be adversely affected.
Our success depends in large part on our ability to obtain and maintain patent protection in the U.S., the PRC and other countries with respect to our proprietary technology and drug candidates. We have sought to protect our proprietary position by filing patent applications in the U.S., the PRC and other countries related to novel technologies and drug candidates that we consider are important to our business. As of March 31, 2017, we owned more than 100 granted patents and more than 40 pending patent applications worldwide, including one pending international patent application under the Patent Cooperation Treaty, or PCT, which we plan to file nationally in the U.S. and other jurisdictions. The process of obtaining patent protection is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. There can be no assurance that our pending patent applications will result in issued patents in the U.S. or non-U.S. jurisdictions in which such applications are pending. Moreover, even our issued patents do not guarantee us the right to practice our technology in relation to the commercialization of our platforms’ product candidates. The area of patent and other intellectual property rights in biotechnology is an evolving one with many risks and uncertainties, and third parties may have blocking patents that could be used to prevent us from commercializing our patented technologies, platforms and product candidates and practicing our proprietary technology. There can also be no assurance that a third party will not challenge the validity of our patents or that we will obtain sufficient claim scope in those patents to prevent a third party from competing successfully with our drug candidates.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued that protect our technology or drug candidates, or that effectively prevent others from commercializing competitive technologies and drug
43
Table of Contents
candidates. Changes in either the patent laws or interpretation of the patent laws in the U.S. and other countries may diminish the value of our patents or narrow the scope of our patent protection. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions. Under the America Invents Act enacted in 2011, the U.S. moved to a first-to-file system in early 2013 from the previous system under which the first to make the claimed invention was entitled to the patent. Assuming the other requirements for patentability are met, the first to file a patent application is entitled to the patent.
Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative technologies or drug candidates in a non-infringing manner. The issuance of a patent is not conclusive as to its scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the U.S. and abroad, which proceedings are time-consuming, costly and of uncertain outcome. We may become involved in interference, inter partes review, post grant review, ex parte reexamination, derivation, opposition or similar other proceedings challenging our patent rights or the patent rights of others. Such challenges may result in patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to stop or prevent us from stopping others from using or commercializing similar or identical technology and drug candidates, or limit the duration of the patent protection of our technology and drug candidates. Given the amount of time required for the development, testing and regulatory review of new drug candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing drug candidates similar or identical to ours.
We may not be able to protect our intellectual property rights throughout the world.
The patent positions of companies like ours are generally uncertain and involve complex legal and factual questions. No consistent policy regarding the scope of claims allowable in patents has emerged in the U.S. The patent situation outside of the U.S. is even more uncertain. Changes in the patent laws and rules, either by legislation, judicial decisions, or regulatory interpretation in the U.S. and other countries may diminish our ability to protect our inventions and enforce our intellectual property rights, and more generally could affect the value of our intellectual property.
In addition, the laws of certain non-U.S. countries do not protect intellectual property rights to the same extent as U.S. federal and state laws do. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the U.S., or from selling or importing drugs made using our inventions in and into the U.S. or non-U.S. jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own drugs and further, may export otherwise infringing drugs to non-U.S. jurisdictions where we have patent protection, but where enforcement rights are not as strong as those in the U.S. These drugs may compete with our drug candidates and our patent rights or other intellectual property rights may not be effective or adequate to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in certain jurisdictions, including China. The legal systems of some countries do not favor the enforcement of patents, trade secrets and other intellectual property, particularly those relating to biopharmaceutical products, which could make it difficult in those jurisdictions for us to stop the infringement or misappropriation of our patents or other intellectual property rights, or the marketing of competing drugs in violation of our proprietary rights. Proceedings to enforce our patent and other intellectual property rights in non-U.S. jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business.
44
Table of Contents
Furthermore, such proceedings could put our patents at risk of being invalidated, held unenforceable, or interpreted narrowly, could put our patent applications at risk of not issuing, and could provoke third parties to assert claims of infringement or misappropriation against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop.
We may become involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time-consuming and unsuccessful and our patent rights relating to our drug candidates could be found invalid or unenforceable if challenged in court or before the U.S. Patent and Trademark Office or comparable non-U.S. authority.
Competitors may infringe our patent rights or misappropriate or otherwise violate our intellectual property rights. To counter infringement or unauthorized use, litigation may be necessary in the future to enforce or defend our intellectual property rights, to protect our trade secrets or to determine the validity and scope of our own intellectual property rights or the proprietary rights of others. Such litigation can be expensive and time-consuming. Any claims that we assert against perceived infringers could also provoke these parties to assert counterclaims against us alleging that we infringe their intellectual property rights. Many of our current and potential competitors have the ability to dedicate substantially greater resources to enforce and/or defend their intellectual property rights than we can. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating our intellectual property. Litigation could result in substantial costs and diversion of management resources, which could harm our business and financial results. In addition, in an infringement proceeding, a court may decide that patent rights or other intellectual property rights owned by us are invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patent rights or other intellectual property rights do not cover the technology in question. An adverse result in any litigation proceeding could put our patent, as well as any patents that may issue in the future from our pending patent applications, at risk of being invalidated, held unenforceable or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
If we initiate legal proceedings against a third party to enforce any patent, or any patents that may issue in the future from our patent applications, that relates to one of our drug candidates, the defendant could counterclaim that such patent rights are invalid or unenforceable. In patent litigation in the U.S., defendant counterclaims alleging invalidity or unenforceability are commonplace, and there are numerous grounds upon which a third party can assert invalidity or unenforceability of a patent. Third parties may also raise similar claims before administrative bodies in the U.S. or abroad, even outside the context of litigation. Such mechanisms include ex parte re-examination, inter partes review, post-grant review, derivation and equivalent proceedings in non-U.S. jurisdictions, such as opposition proceedings. Such proceedings could result in revocation or amendment to our patents in such a way that they no longer cover and protect our drug candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity of our patents, for example, we cannot be certain that there is no invalidating prior art of which we, our patent counsel, and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our drug candidates. Such a loss of patent protection could have a material adverse impact on our business.
We may not be able to prevent misappropriation of our trade secrets or confidential information, particularly in countries where the laws may not protect those rights as fully as in the U.S. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
45
Table of Contents
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
Although we are not currently experiencing any claims challenging the inventorship of our patents or ownership of our intellectual property, we may in the future be subject to claims that former employees, collaborators or other third parties have an interest in our patents or other intellectual property as inventors or co-inventors. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our drug candidates. Litigation may be necessary to defend against these and other claims challenging inventorship. If we fail in defending any such claims, in addition to paying monetary damages, we may lose rights such as exclusive ownership of, or right to use, our patent rights or other intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
If we are sued for infringing intellectual property rights of third parties, such litigation could be costly and time-consuming and could prevent or delay us from developing or commercializing our drug candidates.
Our commercial success depends in part on our avoiding infringement of the patents and other intellectual property rights of third parties. There is a substantial amount of litigation involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including litigation in the U.S. courts, inter partes review, post grant review, interference and ex parte reexamination proceedings before the U.S. Patent and Trademark Office, or USPTO, or oppositions and other comparable proceedings in non-U.S. jurisdictions. Numerous issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing drug candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our drug candidates or manufacturing processes may give rise to claims of infringement of the patent rights of others.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents of which we are currently unaware with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our drug candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our drug candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of any of our drug candidates, any molecules formed during the manufacturing process or any final product itself, the holders of any such patents may be able to prevent us from commercializing such drug candidate unless we obtain a license under the applicable patents, or until such patents expire or they are finally determined to be held invalid or unenforceable. Similarly, if any third-party patent were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture or methods of use, including combination therapy or patient selection methods, the holders of any such patent may be able to block our ability to develop and commercialize the applicable drug candidate unless we obtain a license, limit our uses, or until such patent expires or is finally determined to be held invalid or unenforceable. In either case, such a license may not be available on commercially reasonable terms or at all.
Third parties who bring successful claims against us for infringement of their intellectual property rights may obtain injunctive or other equitable relief, which could prevent us from developing and commercializing one or more of our drug candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and employee resources from our business. In the event of a successful claim of infringement or misappropriation against us, we may have to pay substantial damages, including treble damages and attorneys’ fees in the case of willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing drug candidates, which may be impossible or require substantial time and monetary expenditure and undertaking additional preclinical studies, clinical trials or regulatory review. In the event of an adverse result in any such litigation, or even in the absence
46
Table of Contents
of litigation, we may need to obtain licenses from third parties to advance our research or allow commercialization of our drug candidates. We cannot predict whether any required license would be available at all or whether it would be available on commercially reasonable terms, and we may fail to obtain any of these licenses on commercially reasonable terms, if at all. In the event that we are unable to obtain such a license, we would be unable to further develop and commercialize one or more of our drug candidates, which could harm our business significantly. We may also elect to enter into license agreements in order to settle patent infringement claims or to resolve disputes prior to litigation and any such license agreements may require us to pay royalties and other fees that could significantly harm our business.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical personnel, management personnel, or both from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the market price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
If our products conflict with the intellectual property rights of third parties, we may incur substantial liabilities and we may be unable to commercialize products in a profitable manner or at all.
We seek to launch generic pharmaceutical products either where patent protection or other regulatory exclusivity of equivalent branded products has expired, where patents have been declared invalid or where products do not infringe on the patents of others. However, at times, we may seek approval to market generic products before the expiration of patents relating to the branded versions of those products, based upon our belief that such patents are invalid or otherwise unenforceable or would not be infringed by our products. Our success depends in part on our ability to operate without infringing the patents and proprietary rights of third parties. The manufacture, use and sale of generic versions of products has been subject to substantial litigation in the pharmaceutical industry. These lawsuits relate to the validity and infringement of patents or proprietary rights of third parties. If our products were found to be infringing on the intellectual property rights of a third-party, we could be required to cease selling the infringing products, causing us to lose future sales revenue from such products and face substantial liabilities for patent infringement, in the form of either payment for the innovator’s lost profits or a royalty on our sales of the infringing product. These damages may be significant and could materially adversely affect our business. Any litigation, regardless of the merits or eventual outcome, would be costly and time consuming and we could incur significant costs and/or a significant reduction in revenue in defending the action and from the resulting delays in manufacturing, marketing or selling any of our products subject to such claims.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the USPTO and other patent agencies in several stages over the lifetime of the patent. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment, and other similar provisions during the patent application process. Although an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent
47
Table of Contents
application include failure to respond to official actions within prescribed time limits, non-payment of fees, and failure to properly legalize and submit formal documents. In any such event, our competitors might be able to enter the market, which would have a material adverse effect on our business.
The terms of our patents may not be sufficient to effectively protect our drug candidates and business.
In most countries in which we file patent applications, including the U.S., the term of an issued patent is generally 20 years from the earliest claimed filing date of a non-provisional patent application in the applicable country. With respect to any issued patents in the U.S., we may be entitled to obtain a patent term extension or extend the patent expiration date provided we meet the applicable requirements for obtaining such patent term extensions. Although various such extensions may be available, the life of a patent and the protection it affords is by definition limited. Even if patents covering our drug candidates are obtained, we may be open to competition from other companies as well as generic medications once the patent life has expired for a drug. If patents are issued on our currently pending patent applications, the resulting patents will be expected to expire on dates ranging from 2025 to 2038, excluding any potential patent term extension or adjustment. Upon the expiration of our issued patent or patents that may issue from our pending patent applications, we will not be able to assert such patent rights against potential competitors and our business and results of operations may be adversely affected.
In addition, the rights granted under any issued patents may not provide us with protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may independently develop similar technologies. For these reasons, we may have competition for our technologies, platforms and product candidates. Moreover, because of the extensive time required for development, testing and regulatory review of a potential product, it is possible that, before any particular product candidate can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of the patent.
If we do not obtain additional protection under the Hatch-Waxman Amendments and similar legislation in other countries extending the terms of our patents, if issued, relating to our drug candidates, our business may be materially harmed.
Depending upon the timing, duration and specifics of FDA regulatory approval for our drug candidates, one or more of our U.S. patents, if issued, may be eligible for limited patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent term extension of up to five years as compensation for patent term lost during drug development and the FDA regulatory review process. Patent term extensions, however, cannot extend the remaining term of a patent beyond a total of 14 years from the date of drug approval by the FDA, and only one patent can be extended for a particular drug.
The application for patent term extension is subject to approval by the USPTO, in conjunction with the FDA. We may not be granted an extension because of, for example, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain a patent term extension for a given patent or the term of any such extension is less than we request, the period during which we will have the right to exclusively market our drug will be shortened and our competitors may obtain earlier approval of competing drugs, and our ability to generate revenues could be materially adversely affected.
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect our drug candidates.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patent rights. Obtaining and enforcing patents in the biopharmaceutical industry involves
48
Table of Contents
both technological and legal complexity, and is therefore costly, time-consuming, and inherently uncertain. In addition, the U.S. has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents once obtained, if any. Depending on decisions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. For example, in a recent case, Assoc. for Molecular Pathology v. Myriad Genetics, Inc., the U.S. Supreme Court held that certain claims to naturally-occurring substances are not patentable. Although we do not believe that our currently-issued patents and any patents that may issue from our pending patent applications directed to our drug candidates if issued in their currently pending forms, as well as patent rights licensed by us, will be found invalid based on this decision, we cannot predict how future decisions by the courts, the U.S. Congress or the USPTO may impact the value of our patent rights. There could be similar changes in the laws of foreign jurisdictions that may impact the value of our patent rights or our other intellectual property rights.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed. We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
In addition to our issued patents and pending patent applications, we rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position and to protect our drug candidates. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties that have access to them, such as our employees, corporate collaborators, outside scientific collaborators, sponsored researchers, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. These agreements provide that all confidential information concerning our business or financial affairs developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual, and which are related to our current or planned business or research and development or made during normal working hours, on our premises or using our equipment or proprietary information, are our exclusive property. In many cases our confidentiality and other agreements with consultants, outside scientific collaborators, sponsored researchers and other advisors require them to assign to us or grant us licenses to inventions they invent as a result of the work or services they render under such agreements or grant us an option to negotiate a license to use such inventions. However, any of these parties may breach such agreements and disclose our proprietary information, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive and time-consuming, and the outcome is unpredictable. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us and our competitive position would be harmed.
Furthermore, many of our employees, including our senior management, were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Some of these employees, including each member of our senior management, executed proprietary rights, non-disclosure and non-competition agreements in connection with such previous employment. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. We are not aware of any threatened or pending claims related to these matters or concerning the agreements with our senior management, but in the future litigation may be necessary to defend against such claims. If we fail in defending any such claims, in
49
Table of Contents
addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, while we typically require our employees, consultants and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own, which may result in claims by or against us related to the ownership of such intellectual property. If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our management and scientific personnel.
We also seek to preserve the integrity and confidentiality of our proprietary technology and processes by maintaining physical security of our premises and physical and electronic security of our information technology systems. Although we have confidence in these individuals, organizations, and systems, agreements or security measures may be breached and we may not have adequate remedies for any breach. To the extent that our employees, contractors, consultants, collaborators, and advisors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
We may not be successful in obtaining or maintaining necessary rights for our development pipeline through acquisitions and in-licenses.
Because our programs may involve additional drug candidates that may require the use of proprietary rights held by third parties, the growth of our business may depend in part on our ability to acquire and maintain licenses or other rights to use these proprietary rights. We may be unable to acquire or in-license any compositions, methods of use, or other third-party intellectual property rights from third parties that we identify. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to required third-party intellectual property rights, our business, financial condition and prospects for growth could suffer.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could be required to pay monetary damages or could lose license rights that are important to our business.
We have entered into license agreements with third parties providing us with rights under various third-party patents and patent applications, including the rights to prosecute patent applications and to enforce patents. Certain of these license agreements impose and, for a variety of purposes, we may enter into additional licensing and funding arrangements with third parties that also may impose diligence, development or commercialization timelines and milestone payment, royalty, insurance and other obligations on us. Certain of these license agreements provide us with the exclusive right to practice technologies in major markets including North America, South America, European Union, Australia, New Zealand, Eastern Europe, China, Taiwan, Hong Kong, Macau and parts of Southeast Asia, although the right to practice the technologies and any inventions arising out of such technologies outside of these territories may be reserved to the licensing company. In addition, under certain of our existing licensing agreements, we are obligated to pay royalties on net product sales of our drug candidates once commercialized, pay a percentage of sublicensing revenues, make other specified payments relating to our drug candidates or pay license maintenance and other fees. We also have diligence and clinical
50
Table of Contents
development obligations under certain of these agreements that we are required to satisfy. If we fail to comply with our obligations under our current or future license agreements, our counterparties may have the right to terminate these agreements, in which event we might not be able to develop, manufacture or market any drug or drug candidate that is covered by the licenses provided for under these agreements or we may face claims for monetary damages or other penalties under these agreements. Such an occurrence could diminish the value of these products and our company. Termination of the licenses provided for under these agreements or reduction or elimination of our rights under these agreements may result in our having to negotiate new or reinstated agreements with less favorable terms, or cause us to lose our rights under these agreements, including our rights to important intellectual property or technology.
In particular, our ability to stop third parties from making, using, selling, offering to sell, or importing any of our patented inventions, either directly or indirectly, will depend in part on our success in obtaining, defending, and enforcing patent claims that cover our technology, inventions, and improvements. With respect to both licensed and company-owned intellectual property, we cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents that may be granted to us in the future will be commercially useful in protecting our platforms and product candidates and the methods used to manufacture those platforms and product candidates. Our issued patents and those that may issue in the future may be challenged, invalidated, or circumvented, which could limit our ability to stop competitors from marketing related platforms or product candidates or limit the length of the term of patent protection that we may have for our technologies, platforms, and product candidates.
We also rely on trade secret protection for our confidential and proprietary information. Although we take steps to protect our confidential and proprietary information as trade secrets, including through contractual means with our employees and consultants, third parties may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or technology. Thus, we may not be able to meaningfully protect our trade secrets. It is our policy to require our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us.
If our licensing and sublicensing activities result in non-compliance with our licensing agreements, our business relationships with our licensing partners may suffer and we may be required to pay monetary damages or rescind or amend existing agreements which are important to our business.
We have entered into agreements with third parties under which we have granted licenses to use certain of our patents and patent applications, including the rights to develop, seek regulatory approval for and sell products using our KX-01 and KX-02 products. We have also entered into similar agreements sublicensing the intellectual property for the Orascovery platform, which we have licensed from Hanmi. We have granted exclusive patent rights to certain of these partners and have granted them certain additional rights with respect to the intellectual property we have licensed to them. From time to time we may engage in other licensing transactions in which we acquire licenses to certain intellectual property or sublicense intellectual property rights. If we fail to comply with or are found to have violated the terms of any of our licenses, we may be required to rescind or amend our license agreements or pay damages to license counterparties or other rightsholders. This may also negatively impact our relationships with our licensing and sublicencing partners for our candidate platforms. For further information regarding the terms of our licenses, please see “Business—License and Collaboration Agreements”.
51
Table of Contents
Risks Related to Our Reliance on Third Parties
We depend on our agreements with Hanmi Pharmaceutical Co. Ltd, or Hanmi, to provide rights to the intellectual property relating to certain of our lead product candidates. Any termination or loss of significant rights under those agreements would adversely affect our development or commercialization of our lead product candidates.
We have licensed the intellectual property rights related to HM30181A, an integral part of our current product candidates, from Hanmi pursuant to two license agreements. If, for any reason, our license agreements are terminated or we otherwise lose those rights, it would adversely affect our business. Our license agreements with Hanmi impose on us obligations relating to exclusivity, territorial rights, development, commercialization, funding, payment, diligence, sublicensing, insurance, intellectual property protection and other matters. If we breach any material obligations, or use the intellectual property licensed to us in an unauthorized manner, we may be required to pay damages to Hanmi and Hanmi may have the right to terminate our license, which could result in us being unable to develop, manufacture and sell our product candidates that incorporate HM30181A.
In addition, under our 2013 license agreement with Hanmi, we have granted Hanmi a one-time right of first negotiation that, at Hanmi’s discretion, requires us to negotiate in good faith the sale of our rights in Oraxol and Oratecan under such agreement to Hanmi at a purchase price determined by an internationally-recognized investment banking firm with an office in Hong Kong at any time prior to the earlier of (i) our first commercial sale of products using such technology or (ii) receipt by Hanmi of written notice from our company of the sublicense of the rights in an applicable product to a third party. If Hanmi exercises this right of first negotiation and we reach an agreement to sell our rights under that licensing agreement, our ability to continue to develop certain of our product candidates would be significantly impaired and would adversely affect our business and results of operations.
Each of our license agreements with Hanmi expires on the earlier of (i) expiration of the last of Hanmi’s patent rights licensed under the agreement or (ii) invalidation of Hanmi’s patent rights which are the subject of the agreement, provided that the term will automatically be extended for consecutive one year periods unless either party gives notice to the other at least 90 days prior to expiration of the patent rights licensed under the agreement or before the then current annual expiration date of the agreement. The patent rights licensed to us under the agreements with Hanmi have expiry dates ranging from 2023 to 2033, unless the terms of such licensed patents are able to be extended in accordance with applicable laws and regulations. Subject to certain conditions, Hanmi may also terminate the license agreements if we fail to comply with certain development milestones set out in each of the agreements. The agreements also contain customary termination rights for either party, such as in the event of a breach of the agreement or the initiation of bankruptcy proceedings by the other party or by mutual agreement. For further information regarding the license terms, right of first negotiation and termination provisions of the Hanmi in-license agreements, please see “Business—License and Collaboration Agreements—Hanmi Licensing Agreements—In-Licenses.”
We may rely on third parties to conduct our preclinical studies and clinical trials. If these third parties do not successfully carry out their contractual duties, meet expected deadlines, perform satisfactorily or operate in compliance with laws and regulations, we may not be able to obtain regulatory approval for or commercialize our drug candidates and our business could be substantially harmed.
We have relied upon and may, in the future, rely upon third-party CROs to monitor and manage data for our ongoing preclinical and clinical programs. We rely on these parties for execution of our preclinical studies and clinical trials, and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, and regulatory requirements and scientific standards, and our reliance on the CROs does not relieve us of our regulatory responsibilities. We and our CROs are required to comply with GCPs, which are regulations and guidelines enforced by the FDA, CFDA and other regulatory authorities for all of our drugs in clinical development. Regulatory authorities enforce these GCPs through periodic inspections of trial sponsors, principal investigators
52
Table of Contents
and trial sites. If we or any of our CROs fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, CFDA or regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP regulations. In addition, our clinical trials must be conducted with product produced under cGMP regulations. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process.
If any of our relationships with these third-party CROs terminate, we may not be able to enter into arrangements with alternative CROs or to do so on commercially reasonable terms. In addition, our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing clinical, non-clinical and preclinical programs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements, environmental, health and safety laws and regulations, or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our drug candidates. As a result, our results of operations and the commercial prospects for our drug candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
Our total revenue is highly dependent on a limited number of API customers, and the loss of, or any significant decrease in business from, any one or more of our major API customers could adversely affect our financial condition and results of operations.
We have in the past derived, and, until we further diversify our sources of revenue from the sales of new products and drugs, we believe that we will continue to derive, a significant portion of our revenue from a limited number of customers. We generated 46%, 62% and 50% in the years ended December 31, 2015 and 2016 and the three months ended March 31, 2017, respectively, of our total revenue from our two largest customers, Intas Pharmaceuticals and Ebewe Pharmaceuticals, during those periods. No other customer accounted for 10% or more of our total revenue during those periods. While our business model relies on API sales to generate the substantial majority of our revenue, we expect our total revenue will continue to be highly dependent on a limited number of API customers.
There are a number of factors that could cause us to lose major customers. We do not enter into long-term sales contracts with customers, but sell API to them based on short-term purchase orders. Accordingly, these customers may choose to use other suppliers with little or no notice, based upon considerations of price, quality, shipping time, competitive or other reasons. In addition, our API customers use the API to manufacture drugs, and they are subject to regulation and oversight by the FDA and other relevant regulatory agencies. If for any reason, any such customer violates an FDA regulation that results in their being prohibited from manufacturing drugs, they would no longer purchase API from us. Such sanctions or regulatory action against drug manufacturers could happen without notice, and our revenue stream could be adversely affected without notice.
The loss of any of our major API customers could materially and adversely affect our financial condition and results of operations.
Additionally, Polymed, our wholly owned subsidiary, sells API to third parties for use in those third parties’ products, which may be manufactured in cGMP facilities. In the event Polymed’s customers fail to remain in compliance with cGMP regulations, their operations may be adversely impacted, causing them to cancel or cease API orders from Polymed. Any decrease in orders by Polymed’s customers may impact Polymed’s revenue and, as a result, our overall financial condition.
53
Table of Contents
If our Global Supply Chain Platform is insufficient, we may rely on third parties to manufacture at least a portion of our drug candidate supplies, and for at least a portion of the manufacturing process of our drug candidates, if approved. Our business could be harmed if those third parties fail to provide us with sufficient quantities of product or fail to do so at acceptable quality levels or prices.
Although we currently have a facility that may be used as our clinical-scale manufacturing and processing facility, we partially rely on outside vendors to manufacture supplies and process our drug candidates. We have not yet begun to manufacture or process our drug candidates on a commercial scale and may not be able to do so for any of our drug candidates.
We have limited experience in managing the manufacturing process, and our process may be more difficult or expensive than the approaches currently in use.
Although we do intend to further develop our manufacturing facilities, and those leased to us under our public-private partnerships, we may also use third parties as part of our manufacturing process. Our reliance on third-party manufacturers may expose us to the following risks:
| • | we may be unable to identify manufacturers on acceptable terms or at all because the number of potential manufacturers is limited and the FDA, CFDA or other regulatory authorities must approve any manufacturers. This approval would require new testing and cGMP-compliance inspections by FDA, CFDA or other regulatory authorities. In addition, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of our drugs. |
| • | our manufacturers may have little or no experience with manufacturing our drug candidates, and therefore may experience quality issues or require a significant amount of support from us in order to implement and maintain the infrastructure and processes required to manufacture our drug candidates. |
| • | our third-party manufacturers might be unable to timely manufacture our drug or produce the quantity and quality required to meet our clinical and commercial needs, if any. |
| • | contract manufacturers may not be able to execute our manufacturing procedures and other logistical support requirements appropriately. |
| • | our future contract manufacturers may not perform as agreed, may not devote sufficient resources to our drugs, or may not remain in the contract manufacturing business for the time required to supply our clinical trials or to successfully produce, store and distribute our drugs. |
| • | we may not own, or may have to share, the intellectual property rights to any improvements made by our third-party manufacturers in the manufacturing process for our drugs. |
| • | our third-party manufacturers could breach or terminate their agreement with us. |
| • | raw materials and components used in the manufacturing process, particularly those for which we have no other source or supplier, may not be available or may not be suitable or acceptable for use due to material or component defects. |
| • | our contract manufacturers and critical reagent suppliers may be subject to inclement weather, as well as natural or man-made disasters. |
| • | our contract manufacturers may have unacceptable or inconsistent product quality success rates and yields. |
Each of these risks could delay or prevent the completion of our clinical trials or the approval of any of our drug candidates by the FDA, CFDA or other regulatory authorities, result in higher costs or adversely impact commercialization of our drug candidates. In addition, we will rely on third parties to perform certain specification tests on our drug candidates prior to delivery to patients. If these tests are not conducted appropriately and test data are not reliable, patients could be put at risk of serious harm and the FDA, CFDA or other regulatory authorities could place significant restrictions on our company until deficiencies are remedied.
54
Table of Contents
The manufacture of drug and biological products is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls.
Currently, raw materials used in our manufacturing activities, including the pacific yew used in many of the API products we manufacture, are supplied by multiple suppliers. We have agreements for the supply of such raw materials with manufacturers or suppliers that we believe have sufficient capacity to meet our demands. In addition, we believe that adequate alternative sources for such supplies exist. However, there is a risk that, if supplies are interrupted, it would materially harm our business.
Manufacturers of drug and biological products often encounter difficulties in production, particularly in scaling up or out, validating the production process, and assuring high reliability of the manufacturing process (including the absence of contamination). These problems include logistics and shipping, difficulties with production costs and yields, quality control, including stability of the product, product testing, operator error, availability of qualified personnel, as well as compliance with strictly enforced federal, state and non-U.S. regulations. Furthermore, if contaminants are discovered in our supply of our drug candidates or in the manufacturing facilities, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. We cannot assure you that any stability failures or other issues relating to the manufacture of our drug candidates will not occur in the future. Additionally, our manufacturers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or unstable political environments. If our manufacturers were to encounter any of these difficulties, or otherwise fail to comply with their contractual obligations, our ability to provide our drug candidate to patients in clinical trials would be jeopardized. Any delay or interruption in the supply of clinical trial supplies could delay the completion of clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, require us to begin new clinical trials at additional expense or terminate clinical trials completely.
If third-party manufacturers fail to comply with pharmaceutical manufacturing regulations, our financial results and financial condition will be adversely affected.
Before a third party can begin commercial manufacture of our drug candidates and potential drugs, contract manufacturers are subject to regulatory inspections of their manufacturing facilities, processes and quality systems. Due to the complexity of the processes used to manufacture drug and biological products and our drug candidates, any potential third-party manufacturer may be unable to initially pass federal, state or international regulatory inspections in a cost effective manner in order for us to obtain regulatory approval of our drug candidates. If our contract manufacturers do not pass their inspections by the FDA, CFDA or other regulatory authorities, our commercial supply of drug product or substance will be significantly delayed and may result in significant additional costs, including the delay or denial of any marketing application for our drug candidates. In addition, drug and biological manufacturing facilities are continuously subject to inspection by the FDA, CFDA and other regulatory authorities, before and after drug approval, and must comply with cGMPs. Our contract manufacturers may encounter difficulties in achieving quality control and quality assurance and may experience shortages in qualified personnel. In addition, contract manufacturers’ failure to achieve and maintain high manufacturing standards in accordance with applicable regulatory requirements, or the incidence of manufacturing errors, could result in patient injury, product liability claims, product shortages, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could seriously harm our business, reputation or corporate image. If a third-party manufacturer with whom we contract is unable to comply with manufacturing regulations, we may also be subject to fines, unanticipated compliance expenses, recall or seizure of our drugs, product liability claims, total or partial suspension of production and/or enforcement actions, including injunctions, and criminal or civil prosecution. These possible sanctions could materially adversely affect our financial results and financial condition.
Furthermore, changes in the manufacturing process or procedure, including a change in the location where the product is manufactured or a change of a third-party manufacturer, could require prior review by the FDA,
55
Table of Contents
CFDA or other regulatory authorities and/or approval of the manufacturing process and procedures in accordance with the FDA or CFDA’s regulations, or comparable requirements. This review may be costly and time consuming and could delay or prevent the launch of a product. The new facility will also be subject to pre-approval inspection. In addition, we have to demonstrate that the product made at the new facility is equivalent to the product made at the former facility by physical and chemical methods, which are costly and time consuming. It is also possible that the FDA, CFDA or other regulatory authorities may require clinical testing as a way to prove equivalency, which would result in additional costs and delay.
We have entered into collaborations and may form or seek collaborations or strategic alliances or enter into additional licensing arrangements in the future, and we may not realize the benefits of such alliances or licensing arrangements.
We have partnered with companies such as Hanmi, Gland Pharma Limited and SunGen Pharma LLC and may form or seek strategic alliances, create joint ventures or collaborations, or enter into additional licensing arrangements with third parties that we believe will complement or augment our development and commercialization efforts with respect to our drug candidates and any future drug candidates that we may develop. Any of these relationships may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute our existing shareholders, or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for our drug candidates because they may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view our drug candidates as having the requisite potential to demonstrate safety and efficacy. If and when we collaborate with a third party for development and commercialization of a drug candidate, we can expect to relinquish some or all of the control over the future success of that drug candidate to the third party.
Further, collaborations involving our drug candidates are subject to numerous risks, which may include the following:
| • | collaborators have significant discretion in determining the efforts and resources that they will apply to a collaboration; |
| • | collaborators may not pursue development and commercialization of our drug candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in their strategic focus due to the acquisition of competitive drugs, availability of funding, or other external factors, such as a business combination that diverts resources or creates competing priorities; |
| • | collaborators may delay clinical trials, provide insufficient funding for a clinical trial, stop a clinical trial, abandon a drug candidate, repeat or conduct new clinical trials, or require a new formulation of a drug candidate for clinical testing; |
| • | collaborators could independently develop, or develop with third parties, drugs that compete directly or indirectly with our drugs or drug candidates; |
| • | a collaborator with marketing and distribution rights to one or more drugs may not commit sufficient resources to their marketing and distribution; |
| • | collaborators may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability; |
| • | disputes may arise between us and a collaborator that cause the delay or termination of the research, development or commercialization of our drug candidates, or that result in costly litigation or arbitration that diverts management attention and resources; |
56
Table of Contents
| • | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable drug candidates; and |
| • | collaborators may own or co-own intellectual property covering our drugs that results from our collaborating with them, and in such cases, we would not have the exclusive right to commercialize such intellectual property. |
As a result, if we enter into collaboration agreements and strategic partnerships or license our drugs, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations and company culture, which could delay our timelines or otherwise adversely affect our business. We also cannot be certain that, following a strategic transaction or license, we will achieve the revenue or specific net income that justifies such transaction. If we are unable to reach agreements with suitable collaborators on a timely basis, on acceptable terms, or at all, we may have to curtail the development of a drug candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to fund and undertake development or commercialization activities on our own, we may need to obtain additional expertise and additional capital, which may not be available to us on acceptable terms or at all. If we fail to enter into collaborations and do not have sufficient funds or expertise to undertake the necessary development and commercialization activities, we may not be able to further develop our drug candidates or bring them to market and generate product sales revenue, which would harm our business prospects, financial condition and results of operations.
We have engaged and will rely on a single vendor to manage our order to cash cycle and our distribution activities in the U.S., and the loss or disruption of service from this vendor could adversely affect our operations and financial condition.
Our U.S. customer management, order processing, invoicing, cash application, chargeback and rebate processing and distribution and logistics activities are managed by Dohmen Life Science Services, or DLSS, a managed services provider with a focus on life sciences companies. If we were to lose the availability of DLSS’s services due to a dispute, termination of or inability to renew the contract, or other factors such as fire, natural disaster or other disruption, such loss could have a material adverse effect on our operations. Although multiple providers of such services exist, there can be no assurance that we could secure another source to handle these transactions on acceptable terms or otherwise to our specifications in the event of a disruption of services at operational centers.
Risks Related to Our Industry, Business and Operation
Our future success depends on our ability to retain our Chief Executive Officer and other key executives and to attract, retain and motivate qualified personnel. Additionally, certain members of our leadership may engage in other business ventures that may have interests in conflict with ours.
We are highly dependent on Dr. Lau, our Chief Executive Officer, and the other principal members of our management and scientific teams. We do not maintain “key person” insurance for any of our executives or other employees. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives.
To induce valuable employees to remain at our company, in addition to salary and cash incentives, we have provided stock option grants that vest over time. The value to employees of these equity grants that vest over time may be significantly affected by changes in the price of our common stock that are beyond our control, and may at any time be insufficient to counteract more lucrative offers from other companies. Although we have employment agreements with our key employees, any of our employees could leave our employment at any time, with or without notice.
57
Table of Contents
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel or consultants will also be critical to our success. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our discovery and preclinical development and commercialization strategy. The loss of the services of our executive officers or other key employees and consultants could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy.
Furthermore, replacing executive officers and key employees or consultants may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain regulatory approval of and commercialize products. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these key personnel or consultants on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel.
We may choose to hire part-time employees or use consultants. As a result, certain of our employees, officers, directors and consultants may not devote all of their time to our business, and may from time to time serve as officers, directors and consultants of other companies. These other companies may have interests in conflict with ours. For instance, Dr. Johnson Lau, who serves as our Chief Executive Officer and Chairman, Dr. Manson Fok and Mr. Song-Yi Zhang, who serve on our board of directors, are also directors of Avalon Global Holdings Limited, or Avalon, a shareholder of ours. In addition, Dr. David Hangauer, our former Chief Scientific Officer and one of our founders, recently retired in December 2016, and now maintains an advisory relationship with our company.
We also face competition for the hiring of scientific and clinical personnel from universities and research institutions. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high quality personnel, our ability to pursue our growth strategy will be limited.
We are substantially dependent on our public-private partnerships and if we or our counterparties fail to meet the obligations of those agreements and we lose the benefits of those partnerships, it would materially impact our development, operations and prospects.
Our long-term public-private partnerships with governments and government agencies, including in certain emerging markets, include agreements to build and/or maintain manufacturing facilities for us. For example, we entered into an agreement with FSMC, whereby FSMC agreed to fund the costs of construction of a new manufacturing facility in Dunkirk, New York. FSMC is responsible for the costs of construction and of all equipment for the facility, up to an amount not to exceed $225 million, and shall retain ownership of the facility and the equipment. We are entitled to lease the facility and all equipment at a rate of $1.00 per year for an initial 10-year term, and for the same rate if we elect to extend the lease for an additional 10-year term. We are responsible for all operating costs and expenses for the facility. In exchange, we have committed to spending $1.5 billion on operational expenses in the Dunkirk facility in our first 10-year term in the facility, and an additional $1.5 billion on operational expenses if we elect to extend the lease for a second 10-year term. We have also committed to hiring 450 permanent employees within the first 5 years at the Dunkirk facility. We have also entered into similar arrangements with FSMC relating to our headquarters, and Chongqing Maliu Riverside Development & Investment Co., Ltd. relating to a plant in Chongqing, PRC, under which we have committed to achieving certain operating, revenue and tax generation milestones. If we are unable to comply with our obligations under these arrangements, including the milestones we have committed to achieve, we may lose access to the properties covered by such arrangements which could disrupt our operations and manufacturing activities, cause us to divert resources to finding alternative facilities, which would not have any subsidies, and would have a significant impact on our operations and financial performance. Furthermore, there is no guarantee that the counterparties to our public-private partnerships will comply with the terms of the agreements, including
58
Table of Contents
that their ability to fund their capital commitments under the agreements may be subject to their ability to raise additional capital and that construction timetables may not be met, nor is there guarantee that the successors to such counterparties will continue to comply with terms of the agreements, regardless of existence of such government stipulations as a guideline released on November 4, 2016 by the State Council of China, which provides that, among others governments and relevant departments at all levels shall strictly keep policy commitments lawfully made to society and administrative counterparties, shall carefully perform all the contracts lawfully entered into with investment subjects in activities like attraction of investment and public-private partnership, shall not breach contracts with such excuses as government transition and replacement of leaders, and shall bear legal and economic liability in event of their infringements and contract breaches. If our public-private partnership counterparties or their successors fail to comply with their obligations under these arrangements, our development programs and prospects will be materially adversely affected. Public-private partnerships are also subject to risks associated with government and government agency counterparties, including risks related to government relations compliance, sovereign immunity, shifts in the political environment, changing economic and legal conditions and social dynamics.
We will need to increase the size and capabilities of our organization, and we may experience difficulties in managing our growth.
As of March 31, 2017, we had 437 employees and consultants and most of our employees are full-time. As our development and commercialization plans and strategies develop, and as we transition into operating as a public company, we must add a significant number of additional managerial, operational, sales, marketing, financial and other personnel. Future growth will impose significant added responsibilities on members of management, including:
| • | identifying, recruiting, integrating, maintaining, and motivating additional employees; |
| • | managing our internal development efforts effectively, including the clinical and FDA or other comparable authority review process for our drug candidates, while complying with our contractual obligations to contractors and other third parties; and |
| • | improving our operational, financial and management controls, reporting systems and procedures. |
Our future financial performance and our ability to commercialize our drug candidates will depend, in part, on our ability to effectively manage any future growth, and our management may also have to divert a disproportionate amount of its attention away from day-to-day activities in order to devote a substantial amount of time to managing these growth activities. In addition, we expect to incur additional costs in hiring, training and retaining such additional personnel.
If we are not able to effectively expand our organization by hiring new employees and expanding our groups of consultants and contractors, we may not be able to successfully implement the tasks necessary to further develop and commercialize our drug candidates and, accordingly, may not achieve our research, development and commercialization goals.
We previously identified material weaknesses in our internal control over financial reporting. If we fail to maintain effective internal control over financial reporting, we may not be able to accurately report our consolidated financial results.
In connection with the preparation of our consolidated financial statements as of and for the years ended December 31, 2014 and 2015, we and our independent registered public accounting firm identified material weaknesses related to our internal controls regarding: (1) the thoroughness of review and determination of appropriate accounting treatment for complex, non-routine transactions, including consideration of fair value concepts, notably for purchase price allocation and accounting for stock options; (2) the precision of review and
59
Table of Contents
application of available information through retrospective review to record accounting estimates accurately based on known, available information; and (3) the precision of review and evaluation of capitalization policies to ensure amounts capitalized as assets related to items for which an identifiable benefit has been received and will be realizable. We have concluded that such material weaknesses have been remediated as of December 31, 2016.
Management believes that at such times we did not have the appropriate resources or personnel to provide a full review of complex transactions to allow us, in the normal course of business, to prevent or identify a material misstatement on a timely basis and that constituted material weaknesses in our internal control over financial reporting under the standards established by the U.S. Public Company Accounting Oversight Board. Under standards established by the Public Company Accounting Oversight Board, a deficiency in internal control over financial reporting exists when the design or operation of a control does not allow management or personnel, in the normal course of performing their assigned functions, to prevent or detect misstatements on a timely basis. A material weakness is a deficiency or combination of deficiencies in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be prevented or detected and corrected on a timely basis. Management implemented a remediation plan to address these material weaknesses identified. In addition, management has hired additional accounting personnel with appropriate experience to assist us in applying U.S. GAAP technical accounting guidance and financial reporting, with a particular emphasis on events outside the ordinary course of business and complex transactions including estimates. As a newly public company, we will also need to establish and maintain effective disclosure and financial controls and make changes in our corporate governance practices including our board and committee practices. We cannot assure that there will not be additional material weaknesses and significant deficiencies that our independent registered public accounting firm or we will identify in the future. If we identify such issues or if we are unable to produce accurate and timely financial statements, our stock price may be adversely affected and we may be unable to maintain compliance with applicable listing requirements. Moreover, there is no assurance that we will be able to recruit additional accounting, financial and legal personnel or maintain such personnel. Even if we are able to hire appropriate personnel, our existing operating expenses and operations will be impacted by the direct costs of their employment and the indirect consequences relating to the diversion of management resources from product development efforts.
Our employees, independent contractors, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of fraud, misconduct or other illegal activity by our employees, independent contractors, consultants, commercial partners and vendors. Misconduct by these parties could include intentional, reckless and negligent conduct that fails to: comply with the laws of the FDA and other similar non-U.S. regulatory authorities; provide true, complete and accurate information to the FDA and other similar non-U.S. regulatory authorities; comply with manufacturing standards we have established; comply with healthcare fraud and abuse and privacy laws in the U.S. and similar non-U.S. fraudulent misconduct laws; or report financial information or data accurately or to disclose unauthorized activities to us. If we obtain FDA approval of any of our drug candidates and begin commercializing those drugs in the U.S., our potential exposure under U.S. laws will increase significantly and our costs associated with compliance with such laws are also likely to increase. These laws may impact, among other things, our current activities with principal investigators and research patients, as well as proposed and future sales, marketing and education programs. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, structuring and commission(s), certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials, which could result in regulatory sanctions and cause serious harm to our reputation. It is not always possible to identify and deter misconduct by employees and other parties, and the
60
Table of Contents
precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
If we engage in future acquisitions or strategic partnerships, this may increase our capital requirements, dilute our shareholders, cause us to incur debt or assume contingent liabilities, and subject us to other risks.
We may evaluate various acquisitions and strategic partnerships, including licensing or acquiring complementary products, intellectual property rights, technologies or businesses. Any potential acquisition or strategic partnership may entail numerous risks, including:
| • | increased operating expenses and cash requirements; |
| • | assimilation of operations, intellectual property and products of an acquired company, including difficulties associated with integrating new personnel; |
| • | the diversion of our management’s attention from our existing product programs and initiatives in pursuing such a strategic merger or acquisition; |
| • | retention of key employees, the loss of key personnel, and uncertainties in our ability to maintain key business relationships; |
| • | risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing drugs or drug candidates and regulatory approvals; and |
| • | our inability to generate revenue from acquired technology and/or products sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs. |
In addition, if we undertake acquisitions, we may issue dilutive securities, assume or incur debt obligations, incur large one-time expenses and acquire intangible assets that could result in significant future amortization expense. Moreover, we may not be able to locate suitable acquisition opportunities and this inability could impair our ability to grow or obtain access to technology or products that may be important to the development of our business.
Our business is subject to applicable laws and regulations relating to sanctions, anti-money laundering and anti-bribery practices, the violation of which could adversely affect our operations.
We must comply with all applicable economic sanctions, anti-money laundering and anti-bribery laws and regulations of the U.S. and other foreign jurisdictions where we operate, including the PRC. U.S. laws and regulations applicable to us include the economic trade sanctions laws and regulations administered by the U.S. Department of the Treasury’s Office of Foreign Assets Control, or OFAC, as well as certain laws administered by the U.S. Department of State. Our business is also subject to anti-money laundering laws and regulations, including the Proceeds of Crime Act 2002, the Terrorism Act 2000 and the Money Laundering Regulations 2007 in the U.K., the Bank Secrecy Act of 1970, the Money Laundering Control Act of 1986 and the USA PATRIOT Act of 2001 in the U.S. and equivalent or similar legislation in the other countries where we do business. In addition, we are subject to the Foreign Corrupt Practices Act of 1977, or FCPA, and other anti-bribery laws such as the U.K. Bribery Act 2010 that generally prohibit the corrupt provision of anything of value to foreign governments and their officials and political parties for the purpose of influencing official conduct or obtaining or retaining an undue business advantage. Applicable anti-bribery laws also may prohibit commercial bribery.
We have operations, conduct clinical trials, deal with government entities, including hospitals and public health regulators, and have contracts in countries known to experience corruption and commercial bribery. Our
61
Table of Contents
activities in these countries create the risk of unauthorized payments or offers of payments by our employees, brokers or agents that could be in violation of various laws, including the FCPA, even though these parties are not always subject to our control and supervision. There is no assurance that our existing safeguards and procedures will be completely effective in ensuring compliance with such laws, and our employees, brokers or agents may engage in conduct for which we may be held responsible. Violations of the FCPA or other anti-bribery laws may result in severe criminal or civil sanctions, and we may be subject to other liabilities, which could negatively affect our reputation, business, operating results, and financial condition.
Regulations administered by OFAC govern transactions with countries and persons subject to U.S. trade sanctions. We are also subject to U.S. Government restrictions on transactions with specific entities and individuals, including, without limitation, those set forth on the Entity List, the Specially Designated Nationals List, the Denied Persons List, the Unverified List, and the U.S. State Department’s lists of debarred parties and sanctioned entities, and we may also be subject to restrictions on transactions with specific entities and individuals subject to the sanctions administered by the United Nations Security Council, the European Union, Her Majesty’s Treasury, or other relevant sanctions authority. These regulations prohibit us from entering into or facilitating unlicensed transactions with, for the benefit of, or in some cases involving the property and property interests of such persons, governments, or countries designated by the relevant sanctions authority under one or more sanctions regimes. Failure to comply with these sanctions and embargoes may result in material fines, sanctions or other penalties being imposed on us or other governmental investigations. In addition, various state and municipal governments, universities and other investors maintain prohibitions or restrictions on investments in companies that do business involving sanctioned countries or entities.
International economic and trade sanctions are complex and subject to frequent change, including jurisdictional reach and the lists of countries, entities, and individuals subject to the sanctions. Current or future economic and trade sanctions regulations or developments might have a negative impact on our business or reputation, and we may incur significant costs related to current, new, or changing sanctions programs, as well as investigations, fines, fees or settlements, which may be difficult to predict. In addition, companies subject to SEC reporting obligations are required under Section 13 of the Exchange Act to disclose in their periodic reports specified dealings or transactions involving Iran or other individuals and entities targeted by certain sanctions promulgated by OFAC that the reporting company or any of its affiliates engaged in during the period covered by the relevant periodic report. In some cases Section 13 requires companies to disclose transactions even if they are permissible under U.S. law. The SEC is required to post this notice of disclosure pursuant to Section 13 on its website and report to the President and certain congressional committees regarding such filings.
On January 16, 2016, OFAC issued General License H, which authorized certain transactions relating to Iran. Pursuant to General License H, certain of our non-U.S. subsidiaries may conduct business relating to Iran. SEC guidance to date indicates that activities authorized by General License H generally are not subject to disclosure under Section 13, but should applicable SEC guidance or disclosure requirements change, or should our non-U.S. subsidiaries engage in activities subject to disclosure under Section 13, we may be required to disclose certain Iran-related transactions in future periodic reports with the SEC. Even if such activity is permitted under applicable law, disclosure could harm our reputation and have a negative impact on our business. Our non-U.S. subsidiaries also remain subject to OFAC secondary sanctions governing trade with Iran, and any violations of OFAC secondary sanctions regulations could negatively affect our reputation, business, operating results, and financial condition.
Although we have policies and controls in place that are designed to ensure compliance with these laws and regulations, it is possible that an employee or intermediary could fail to comply with applicable laws and regulations. In such event, we could be exposed to civil penalties, criminal penalties and other sanctions, including fines or other punitive actions, and the government may seek to impose modifications to business practices, including cessation of business activities in sanctioned countries, and modifications to compliance programs, which may increase compliance costs. In addition, such violations could damage our business and/or our reputation. Such criminal or civil sanctions, penalties, other sanctions, and damage to our business and/or reputation could have a material adverse effect on our financial condition and results of operations.
62
Table of Contents
Any failure to comply with applicable regulations and industry standards or obtain various licenses and permits could harm our reputation and our business, results of operations and prospects.
A number of governmental agencies or industry regulatory bodies in the U.S., and in non-U.S. jurisdictions including the PRC, impose strict rules, regulations and industry standards governing pharmaceutical and biotechnology research and development and manufacturing and marketing activities, which apply to us. Our failure to comply with such regulations could result in the termination of ongoing research or manufacturing and marketing, administrative penalties imposed by regulatory bodies or the disqualification of data for submission to regulatory authorities. This could harm our reputation, prospects for future work and operating results. For example, if we were to treat research animals inhumanely or in violation of international standards set out by the Association for Assessment and Accreditation of Laboratory Animal Care, it could revoke any such accreditation and the accuracy of our animal research data could be questioned.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of or exposure to hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage, use or disposal of biological or hazardous materials.
In addition, we may be required to incur substantial costs to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
If we face allegations of noncompliance with the law and encounter sanctions, our reputation, revenues and liquidity may suffer, and our drugs could be subject to restrictions or withdrawal from the market.
Any government investigation of alleged violations of law could require us to expend significant time and resources in response, and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenues from our drugs. If regulatory sanctions are applied or if regulatory approval is withdrawn, the value of our company and our operating results will be adversely affected. Additionally, if we are unable to generate revenues from our product sales, our potential for achieving profitability will be diminished and the capital necessary to fund our operations will be increased.
Our internal computer systems, or those used by our CROs or other contractors or consultants, may fail or suffer security breaches.
Despite the implementation of security measures, our internal computer systems and those of our current and future CROs and other contractors and consultants are vulnerable to damage from computer viruses and
63
Table of Contents
unauthorized access. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we partially rely on our third-party research institution collaborators for research and development of our drug candidates and other third parties for the manufacture of our drug candidates and to conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business. Certain data breaches must be reported to affected individuals and the government, and in some cases to the media, under provisions of HIPAA, other U.S. federal and state law, and requirements of non-U.S. jurisdictions, and financial penalties may also apply. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and reputational damage and the further development and commercialization of our drug candidates could be delayed.
We are aware of a security breach that occurred in March 2017. That incident occurred when the credentials of an approved consultant were compromised, and the consultant’s credentials were used to access the remote desktop server and active directory server of our wholly-owned subsidiary, Athenex Pharma Solutions, or APS. Upon discovery of the breach, we immediately took steps to void the compromised credentials and reset all credentials having access to APS’ systems. These particular APS information systems are independent of ours, and did not contain any drug candidate, clinical trial or patient-specific data. However, information stored on APS’ systems may have been vulnerable during the intrusion. We have retained a third party security firm to determine the extent of the breach and what, if any, data may have been compromised, and this analysis is ongoing. To help mitigate future incidents we have enhanced security measures required for access by consultants. Notwithstanding such measures, we cannot be certain that no future security breaches will occur or that future breaches will not result in a material disruption of our development programs and our business operations.
Business disruptions could seriously harm our future revenue and financial condition and increase our costs and expenses.
Our operations, and those of our third-party research institution collaborators, CROs, suppliers and other contractors and consultants, could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or man-made disasters or business interruptions, for which we are predominantly self-insured. In addition, we partially rely on our third-party research collaborators for conducting research and development of our drug candidates, and they may be affected by government shutdowns or withdrawn funding. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. Our ability to obtain clinical supplies of our drug candidates could be disrupted if the operations of these suppliers are affected by a man-made or natural disaster or other business interruption. Damage or extended periods of interruption to our corporate, development or research facilities due to fire, natural disaster, power loss, communications failure, unauthorized entry or other events could cause us to cease or delay development of some or all of our drug candidates. Although we maintain property damage and business interruption insurance coverage on these facilities, our insurance might not cover all losses under such circumstances and our business may be seriously harmed by such delays and interruption.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our drug candidates or our 503B products.
We face an inherent risk of product liability as a result of the clinical testing of our drug candidates and will face an even greater risk if we commercialize any of our clinical candidates. For example, we may be sued if our drug candidates or 503B products we plan to manufacture cause or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the drug, negligence, strict liability or a breach of warranties. Claims could also be asserted under
64
Table of Contents
state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our drug candidates. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| • | decreased demand for our drugs; |
| • | injury to our reputation; |
| • | withdrawal of clinical trial participants and inability to continue clinical trials; |
| • | initiation of investigations by regulators; |
| • | costs to defend the related litigation; |
| • | a diversion of management’s time and our resources; |
| • | substantial monetary awards to trial participants or patients; |
| • | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| • | loss of revenue; |
| • | exhaustion of any available insurance and our capital resources; |
| • | the inability to commercialize any drug candidate; and |
| • | a decline in the price of our common stock. |
Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of drugs we develop, alone or with collaborators. Although we currently carry clinical trial insurance, which we believe to be adequate for our current operations, the amount of such insurance coverage may not be adequate now, or in the future, and we may be unable to maintain such insurance, or we may not be able to obtain additional or replacement insurance at a reasonable cost, if at all. Our insurance policies may also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We may have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. Even if our agreements with any future corporate collaborators entitle us to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
Additionally, we may be sued if the products that we commercialize, market or distribute for our partners cause or are perceived to cause injury or are found to be otherwise unsuitable, and may result in:
| • | decreased demand for those products; |
| • | damage to our reputation; |
| • | costs incurred related to product recalls; |
| • | limiting our opportunities to enter into future commercial partnership; and |
| • | a decline in the price of our common stock. |
We have limited insurance coverage, and any claims beyond our insurance coverage may result in our incurring substantial costs and a diversion of resources.
We maintain property insurance policies covering physical damage to, or loss of, our buildings and their improvements, equipment, office furniture and inventory. We hold employer’s liability insurance generally covering death or work-related injury of employees. We hold public liability insurance covering certain incidents involving third parties that occur on or in the premises of the company. We hold directors and officers liability
65
Table of Contents
insurance. We do not maintain key-man life insurance on any of our senior management or key personnel, or business interruption insurance. Our insurance coverage may be insufficient to cover any claim for product liability, damage to our fixed assets or employee injuries. Any liability or damage to, or caused by, our facilities or our personnel beyond our insurance coverage may result in our incurring substantial costs and a diversion of resources.
We may increasingly become a target for public scrutiny, including complaints to regulatory agencies, negative media coverage, including social media and malicious reports, all of which could severely damage our reputation and materially and adversely affect our business and prospects.
We focus on the development of drugs used in the treatment of cancers, and such drugs may be the subject of regulatory, watchdog and media scrutiny and coverage, which also the possibility of heightened attention from the public, the media and our participants. In addition, members of our management and board include high-profile public figures who may be the subject of media and public scrutiny and attention. From time to time, these objections or allegations, regardless of their veracity, may result in public protests or negative publicity, which could result in government inquiry or harm our reputation. Corporate transactions we or related parties undertake may also subject us to increased media exposure and public scrutiny. There is no assurance that we would not become a target for public scrutiny in the future or such scrutiny and public exposure would not severely damage our reputation as well as our business and prospects.
In addition, our directors and management have been in the past, and may continue to be, subject to scrutiny by the media and the public regarding their activities in and outside our company, which may result in unverified, inaccurate or misleading information about them being reported by the press. Negative publicity about our directors or management, even if untrue or inaccurate, may harm our reputation.
Our business, financial condition and results of operations may be adversely affected by global economic conditions.
Our business and operating results could be affected by global economic conditions. When global economic conditions deteriorate or economic uncertainty continues, customers and potential customers may delay or cancellation of plans to purchase our products, governments may reduce healthcare expenditures, and other payors may reduce their reimbursement coverage or reimbursement rates. Our sensitivity to economic cycles and any related fluctuations in the businesses of our customers or potential customers could have a material adverse impact on our business and financial results. Although we are uncertain about the extent to which global financial market disruptions or a slowdown of the U.S. or Chinese economy would impact our business in the long term, there is a risk that our business, results of operations and prospects would be materially and adversely affected by any global economic downturn or the slowdown of the U.S. or Chinese economy.
If our manufacturing facilities are damaged or destroyed or production at such facilities is otherwise interrupted, our business and prospects would be negatively affected.
If our manufacturing facilities or the equipment in them is damaged or destroyed, we may not be able to quickly or inexpensively replace our manufacturing capacity or replace it at all. In the event of a temporary or protracted loss of the facilities or equipment, we might not be able to transfer manufacturing to a third party. Even if we could transfer manufacturing to a third party, the shift would likely be expensive and time-consuming, particularly since the new facility would need to comply with the necessary regulatory requirements and we would need FDA, CFDA or and other comparable regulatory agency approval before selling any drugs manufactured at that facility. Such an event could delay our clinical trials or reduce our product sales if and when we are able to successfully commercialize one or more of our drug candidates.
66
Table of Contents
Any interruption in manufacturing operations at our manufacturing facilities could result in our inability to satisfy the demands of our clinical trials or commercialization. A number of factors could cause interruptions, including:
| • | equipment malfunctions or failures; |
| • | malfunctions or compromise by third party actors of our technology systems; |
| • | work stoppages; |
| • | damage to or destruction of either facility due to natural disasters; |
| • | regional power shortages; |
| • | product tampering; or |
| • | terrorist activities. |
Any disruption that impedes our ability to manufacture our drug candidates in a timely manner could materially harm our business, financial condition and operating results.
Currently, we maintain insurance coverage against damage to our property and equipment. However, our insurance coverage may not reimburse us, or may not be sufficient to reimburse us, for any expenses or losses we may suffer. We may be unable to meet our requirements for our drug candidates if there were a catastrophic event or failure of our manufacturing facilities or processes.
Risks Related to Our Doing Business in the PRC
The pharmaceutical industry in China is highly regulated and such regulations are subject to change which may affect approval and commercialization of our drugs.
Certain of our research operations and manufacturing facilities are in China. The pharmaceutical industry in China is subject to comprehensive government regulation and supervision, encompassing the approval, registration, manufacturing, packaging, licensing and marketing of new drugs. In recent years, the regulatory framework in China regarding the pharmaceutical industry has undergone significant changes, and we expect that it will continue to undergo significant changes. Any such changes or amendments may result in increased compliance costs on our business or cause delays in or prevent the successful development or commercialization of our drug candidates in China and reduce the current benefits we believe are available to us from developing and manufacturing drugs in China. Chinese authorities have become increasingly vigilant in enforcing laws in the pharmaceutical industry and any failure by us or our partners to maintain compliance with applicable laws and regulations or obtain and maintain required licenses and permits may result in the suspension or termination of our business activities in China. We believe our strategy and approach is aligned with the Chinese government’s policies, but we cannot ensure that our strategy and approach will continue to be aligned.
Fluctuations in exchange rates could result in foreign currency exchange losses, which may adversely affect our financial condition, results of operations and cash flows.
We incur portions of our expenses, and may in the future derive revenues, in currencies other than U.S. dollars, in particular, the Renminbi. As a result, we are exposed to foreign currency exchange risk as our results of operations and cash flows are subject to fluctuations in foreign currency exchange rates. For example, a portion of our clinical trial activities are conducted outside of the U.S., and associated costs may be incurred in the local currency of the country in which the trial is being conducted, which costs could be subject to fluctuations in currency exchange rates. We currently do not engage in hedging transactions to protect against uncertainty in future exchange rates between particular foreign currencies and the U.S. dollar. A decline in the value of the U.S. dollar against currencies in countries in which we conduct clinical trials could have a negative impact on our research and development costs.
67
Table of Contents
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions and the foreign exchange policy adopted by the PRC and other non-U.S. governments. Specifically in the PRC, on July 21, 2005, the PRC government changed its policy of pegging the value of the Renminbi to the U.S. dollar. Following the removal of the U.S. dollar peg, the Renminbi appreciated more than 20% against the U.S. dollar over the following three years. Between July 2008 and June 2010, this appreciation halted and the exchange rate between the Renminbi and the U.S. dollar remained within a narrow band. Since June 2010, the PRC government has allowed the Renminbi to appreciate slowly against the U.S. dollar again, and it has appreciated more than 10% since June 2010. In April 2012, the PRC government announced that it would allow more Renminbi exchange rate fluctuation and in August 2015, China’s central bank executed a 2% devaluation in the Renminbi. From December 31, 2015 to December 31, 2016, the Renminbi depreciated approximately 6.7% against the U.S. dollar. It remains unclear what further fluctuations may occur or what impact this will have on the currency.
It is difficult to predict how market forces or PRC, U.S. or other government policies may impact the exchange rate between the Renminbi, U.S. dollar and other currencies in the future. There remains significant international pressure on the PRC government to adopt a more flexible currency policy, which could result in greater fluctuation of the Renminbi against the U.S. dollar. Substantially all of our revenues are denominated in U.S. dollars and our costs are denominated in U.S. dollars and Renminbi, and a large portion of our financial assets is denominated in U.S. dollars. To the extent that we need to convert U.S. dollars we receive from this offering into Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar would have an adverse effect on the Renminbi amount we would receive. Conversely, if we decide to convert our Renminbi into U.S. dollars for other business purposes, appreciation of the U.S. dollar against the Renminbi would have a negative effect on the U.S. dollar amount we would receive. We cannot predict the impact of foreign currency fluctuations, and foreign currency fluctuations in the future may adversely affect our financial condition, results of operations and cash flows.
Changes in the political and economic policies of the PRC government may materially and adversely affect our business, financial condition and results of operations and may result in our inability to sustain our growth and expansion strategies.
A significant portion of our operations are in the PRC. Accordingly, our financial condition and results of operations are affected to a large extent by economic, political and legal developments in the PRC.
The PRC economy differs from the economies of most developed countries in many respects, including the extent of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. Although the PRC government has implemented measures emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets, and the establishment of improved corporate governance in business enterprises, a substantial portion of productive assets in China is still owned by the government. In addition, the PRC government continues to play a significant role in regulating industry development by imposing industrial policies. The PRC government also exercises significant control over China’s economic growth by allocating resources, controlling payment of foreign currency-denominated obligations, setting monetary policy, regulating financial services and institutions and providing preferential treatment to particular industries or companies.
While the PRC economy has experienced significant growth in the past three decades, growth has been uneven, both geographically and among various sectors of the economy. The PRC government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures may benefit the overall PRC economy, but may also have a negative effect on us. Our financial condition and results of operation could be materially and adversely affected by government control over capital investments or changes in tax regulations that are applicable to us and consequently have a material adverse effect on our businesses, financial condition and results of operations.
68
Table of Contents
There are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations.
A portion of our operations are conducted in the PRC through our PRC subsidiaries, and are governed by PRC laws, rules and regulations. Our PRC subsidiaries are subject to laws, rules and regulations applicable to foreign investment in China. The PRC legal system is a civil law system based on written statutes. Unlike the common law system, prior court decisions may be cited for reference but have limited precedential value.
In 1979, the PRC government began to promulgate a comprehensive system of laws, rules and regulations governing economic matters in general. The overall effect of legislation over the past three decades has significantly enhanced the protections afforded to various forms of foreign investment in China. However, China has not developed a fully integrated legal system, and recently enacted laws, rules and regulations may not sufficiently cover all aspects of economic activities in China or may be subject to significant degrees of interpretation by PRC regulatory agencies. In particular, because these laws, rules and regulations are relatively new, and because of the limited number of published decisions and the nonbinding nature of such decisions, and because the laws, rules and regulations often give the relevant regulator significant discretion in how to enforce them, the interpretation and enforcement of these laws, rules and regulations involve uncertainties and can be inconsistent and unpredictable. In addition, the PRC legal system is based in part on government policies and internal rules, some of which are not published on a timely basis or at all, and which may have a retroactive effect. As a result, we may not be aware of our violation of these policies and rules until after the occurrence of the violation.
Any administrative and court proceedings in China may be protracted, resulting in substantial costs and diversion of resources and management attention. Since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy than in more developed legal systems. These uncertainties may impede our ability to enforce the contracts we have entered into and could materially and adversely affect our business, financial condition and results of operations.
Substantial uncertainties exist with respect to the enactment timetable, the final version, interpretation and implementation of draft PRC Foreign Investment Law and how it may impact the viability of our current corporate governance.
The Ministry of Commerce published a discussion draft of the proposed Foreign Investment Law in January 2015 aiming to, upon its enactment, replace the trio of existing laws regulating foreign investment in China, namely, the Sino-foreign Equity Joint Venture Enterprise Law, the Sino-foreign Cooperative Joint Venture Enterprise Law and the Wholly Foreign-invested Enterprise Law, together with their implementation rules and ancillary regulations. The draft Foreign Investment Law embodies an expected PRC regulatory trend to rationalize its foreign investment regulatory regime in line with prevailing international practice and the legislative efforts to unify the corporate legal requirements for both foreign and domestic investments. The Ministry of Commerce has solicited comments on this draft and substantial uncertainties exist with respect to its enactment timetable, the final version, interpretation and implementation. The draft Foreign Investment Law, if enacted as proposed, may materially impact the viability of our current corporate governance if we, in the future, have PRC shareholders.
Among other things, the draft Foreign Investment Law expands the definition of foreign investment and introduces the principle of “actual control” in determining whether a company is considered a foreign-invested enterprise, or an FIE. The draft Foreign Investment Law specifically provides that entities established in China but “controlled” by foreign investors will be treated as FIEs, whereas an entity set up in a foreign jurisdiction would nonetheless be, upon market entry clearance by the Ministry of Commerce or its local counterparts, treated as a PRC domestic investor provided that the entity is “controlled” by PRC entities and/or citizens. In this connection, “control” is broadly defined in the draft law to cover the following summarized categories: (1) holding 50% of more of the shares, equity or voting rights of the subject entity; (2) holding less than 50% of
69
Table of Contents
the voting rights of the subject entity but having the power to secure at least 50% of the seats on the board or other equivalent decision making bodies, or having the voting power to exert material influence on the board, the shareholders’ meeting or other equivalent decision making bodies; or (3) having the power to exert decisive influence, via contractual or trust arrangements, over the subject entity’s operations, financial matters or other key aspects of business operations. Once an entity is determined to be an FIE, it will be subject to the foreign investment restrictions or prohibitions, if the FIE is engaged in the industry listed in the “negative list” which will be separately issued by the State Council later. Unless the underlying business of the FIE falls within the negative list, which calls for market entry clearance by the Ministry of Commerce or its local counterparts, prior approval from the government authorities as mandated by the existing foreign investment legal regime would no longer be required for establishment of the FIE.
The draft Foreign Investment Law, if enacted as proposed, may also materially impact our corporate governance practice and increase our compliance costs. For instance, the draft Foreign Investment Law imposes stringent ad hoc and periodic information reporting requirements on foreign investors and the applicable FIEs. Aside from investment implementation report and investment amendment report that are required at each investment and alteration of investment specifics, an annual report is mandatory, and large foreign investors meeting certain criteria are required to report on a quarterly basis. Any company found to be non-compliant with these information reporting obligations may potentially be subject to fines and/or administrative or criminal liabilities, and the persons directly responsible may be subject to criminal liabilities.
PRC regulations relating to investments in offshore companies by PRC residents may subject our future PRC-resident beneficial owners or our PRC subsidiaries to liability or penalties, limit our ability to inject capital into our PRC subsidiaries or limit our PRC subsidiaries’ ability to increase their registered capital or distribute profits.
The State Administration of Foreign Exchange, or SAFE promulgated the Circular on Relevant Issues Concerning Foreign Exchange Control on Domestic Residents’ Offshore Investment and Financing and Roundtrip Investment through Special Purpose Vehicles, or SAFE Circular 37, on July 4, 2014, which replaced the former circular, commonly known as SAFE Circular 75, promulgated by SAFE on October 21, 2005. SAFE Circular 37 and other SAFE rules require PRC residents to register with banks or local branches of SAFE (only in the case of supplement registration) in connection with their direct establishment or indirect control of an offshore entity, for the purpose of overseas investment and financing, with such PRC residents’ legally owned assets or equity interests in domestic enterprises or offshore assets or interests, referred to in SAFE Circular 37 as a “special purpose vehicle”. SAFE Circular 37 further requires amendment to the registration in the event of any significant changes with respect to the special purpose vehicle, such as increase or decrease of capital contributed by PRC individuals, share transfer or exchange, merger, division or other material events. In the event that a PRC shareholder holding interests in a special purpose vehicle fails to fulfill the required registration, the PRC subsidiaries of that special purpose vehicle may be prohibited from making profit distributions to the offshore parent and from carrying out subsequent cross-border foreign exchange activities, and the special purpose vehicle may be restricted in its ability to contribute additional capital into its PRC subsidiary. Moreover, failure to comply with the various registration requirements described above could result in liability under PRC law for evasion of foreign exchange controls.
We believe that certain of our shareholders are PRC residents under SAFE Circular 37. These certain shareholders have undertaken to (i) apply to register with local SAFE branch or its delegated commercial bank as soon as possible after exercising their options, and (ii) indemnify and hold harmless us and our subsidiaries against any loss suffered arising from their failure to complete the registration. We do not have control over the shareholders and our other beneficial owners and cannot assure you that all of our PRC-resident beneficial owners have complied with, and will in the future comply with, SAFE Circular 37 and subsequent implementation rules. The failure of PRC-resident beneficial owners to register or amend their SAFE registrations in a timely manner pursuant to SAFE Circular 37 and subsequent implementation rules, or the failure of future PRC-resident beneficial owners of our company to comply with the registration procedures set
70
Table of Contents
forth in SAFE Circular 37 and subsequent implementation rules, may subject such beneficial owners or our PRC subsidiaries to fines and legal sanctions. Furthermore, SAFE Circular 37 is unclear how this regulation, and any future regulation concerning offshore or cross-border transactions, will be interpreted, amended and implemented by the relevant PRC government authorities, and we cannot predict how these regulations will affect our business operations or future strategy. Failure to register or comply with relevant requirements may also limit our ability to contribute additional capital to our PRC subsidiaries and limit our PRC subsidiaries’ ability to distribute dividends to us. These risks could in the future have a material adverse effect on our business, financial condition and results of operations.
We may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise Income Tax Law, and we may therefore be subject to PRC income tax on our global income.
Under the PRC Enterprise Income Tax Law and its implementing rules, both of which came into effect on January 1, 2008, enterprises established under the laws of jurisdictions outside of China with “de facto management bodies” located in China may be considered PRC tax resident enterprises for tax purposes and may be subject to the PRC enterprise income tax at the rate of 25% on their global income. “De facto management body” refers to a managing body that exercises substantive and overall management and control over the production and business, personnel, accounting books and assets of an enterprise. The State Administration of Taxation has issued guidance, known as Circular 82 that provides certain specific criteria for determining whether the “de facto management body” of a Chinese-controlled offshore-incorporated enterprise is located in China. Although Circular 82 only applies to offshore enterprises controlled by PRC enterprises, not those, such as us, controlled by foreign enterprises or individuals, the determining criteria set forth in Circular 82 may reflect the State Administration of Taxation’s general position on how the “de facto management body” test should be applied in determining the tax resident status of offshore enterprises, regardless of whether they are controlled by PRC enterprises. Currently, our management is located in the U.S., and we generate a portion of our revenues within the PRC and a portion outside the PRC. We believe that neither we nor any of our subsidiaries outside of China is a PRC resident enterprise for PRC tax purposes. However, the tax resident status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body”. If we were to be considered a PRC resident enterprise, we would be subject to PRC enterprise income tax at the rate of 25% on our global income. In such case, our profitability and cash flow may be materially reduced as a result of our global income being taxed under the PRC Enterprise Income Tax Law.
Dividends payable to our foreign investors and gains on the sale of our common stock by our foreign investors may become subject to PRC tax law.
Under the PRC Enterprise Income Tax Law and its implementing rules issued by the State Council, in general, a 10% PRC withholding tax is applicable to dividends payable to investors that are non-resident enterprises that do not have an establishment or place of business in the PRC or which have such establishment or place of business but the dividends are not effectively connected with such establishment or place of business, to the extent such dividends are derived from sources within the PRC. Similarly, any gain realized on the transfer of shares of our common stock by such investors is also subject to PRC tax at a current rate of 10%, subject to any reduction or exemption set forth in relevant tax treaties, if such gain is regarded as income derived from sources within the PRC. If we are deemed a PRC resident enterprise, dividends paid on our common stock, and any gain realized from the transfer of our common stock, would be treated as income derived from sources within the PRC and would as a result be subject to PRC taxation. Furthermore, if we are deemed a PRC resident enterprise, dividends payable to individual investors who are non-PRC residents and any gain realized on the transfer of common stock by such investors may be subject to PRC tax at a current rate of 20%, subject to any reduction or exemption set forth in applicable tax treaties. It is unclear whether we or any of our subsidiaries established outside China are considered a PRC resident enterprise, holders of our common stock would be able to claim the benefit of income tax treaties or agreements entered into between China and other countries or areas. If dividends payable to our non-PRC investors or gains from the transfer of our common stock by such investors are subject to PRC tax, the value of your investment in our common stock may decline significantly.
71
Table of Contents
We and our shareholders face uncertainties with respect to indirect transfers of equity interests in PRC resident enterprises by their non-PRC holding companies.
Pursuant to a notice, or Circular 698, issued by the State Administration of Taxation, where a non-resident enterprise conducts an “indirect transfer” by transferring the equity interests of a PRC resident enterprise indirectly via disposing of the equity interests of an overseas holding company, and such overseas holding company is located in a tax jurisdiction that: (1) has an effective tax rate less than 12.5%; or (2) does not tax foreign income of its residents, the non-resident enterprise, being the transferor, shall report to the relevant tax authority of the PRC resident enterprise such indirect transfer. Using a “substance over form” principle, the PRC tax authority may disregard the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of reducing, avoiding or deferring PRC tax. As a result, gains derived from such indirect transfer may be subject to PRC enterprise income tax, currently at a rate of 10%. In 2015, the State Administration of Taxation issued a circular, known as Circular 7, which replaced or supplemented certain previous rules under Circular 698. Circular 7 sets out a wider scope of indirect transfer of PRC assets that might be subject to PRC enterprise income tax, and more detailed guidelines on the circumstances when such indirect transfer is considered to lack a bona fide commercial purpose and thus regarded as avoiding PRC tax. The conditional reporting obligation of the non-PRC investor under Circular 698 is replaced by a voluntary reporting by the transferor, the transferee or the underlying PRC resident enterprise being transferred. Furthermore, if the indirect transfer is subject to PRC enterprise income tax, the transferee has an obligation to withhold tax from the sale proceeds, unless the transferor reports the transaction to the PRC tax authority under Circular 7. Late payment of applicable tax will subject the transferor to default interest. Gains derived from the sale of shares by investors through a public stock exchange are not subject to the PRC enterprise income tax pursuant to Circular 7 where such shares were acquired in a transaction through a public stock exchange.
As newly implemented, there is uncertainty as to the application of Circular 7. Circular 7 may be determined by the tax authorities to be applicable to our offshore restructuring transactions or sale of the shares of our offshore subsidiaries where non-resident enterprises, being the transferors, were involved. The PRC tax authorities may pursue such non-resident enterprises with respect to a filing regarding the transactions and request our PRC subsidiaries to assist in the filing. As a result, we and our non-resident enterprises in such transactions may become at risk of being subject to filing obligations or being taxed under Circular 7, and may be required to expend valuable resources to comply with Circular 7 or to establish that we and our non-resident enterprises should not be taxed under Circular 7, for our previous and future restructuring or disposal of shares of our offshore subsidiaries, which may have a material adverse effect on our financial condition and results of operations.
Restrictions on currency exchange may limit our ability to utilize our revenue effectively.
The PRC government imposes controls on the convertibility of Renminbi into foreign currencies and, in certain cases, the remittance of currency out of China. A portion of our revenue may in the future be denominated in Renminbi. Shortages in availability of foreign currency may then restrict the ability of our PRC subsidiaries to remit sufficient foreign currency to our offshore entities for our offshore entities to pay dividends or make other payments or otherwise to satisfy our foreign currency denominated obligations. The Renminbi is currently convertible under the “current account,” which includes dividends, trade and service-related foreign exchange transactions, but not under the “capital account”, which includes foreign direct investment and loans, including loans we may secure from our onshore subsidiaries. Currently, our PRC subsidiaries, which are wholly-foreign owned enterprises, may purchase foreign currency for settlement of “current account transactions,” including payment of dividends to us, without the approval of SAFE by complying with certain procedural requirements. However, the relevant PRC governmental authorities may limit or eliminate our ability to purchase foreign currencies in the future for current account transactions. Since a portion of our future revenue may be denominated in Renminbi, any existing and future restrictions on currency exchange may limit our ability to utilize revenue generated in Renminbi to fund our business activities outside of the PRC or pay dividends in
72
Table of Contents
foreign currencies to our shareholders, including holders of our common stock. Foreign exchange transactions under the capital account remain subject to limitations and require approvals from, or registration with, SAFE and other relevant PRC governmental authorities. This could affect our ability to obtain foreign currency through debt or equity financing for our subsidiaries.
Recent litigation and negative publicity surrounding China-based companies listed in the U.S. may result in increased regulatory scrutiny of us and negatively impact the trading price of our common stock and could have a material adverse effect upon our business, including its results of operations, financial condition, cash flows and prospects.
We believe that litigation and negative publicity surrounding companies with operations in China, including concerning the directors and officers of such companies, that are listed in the U.S. have negatively impacted stock prices for such companies. Various equity-based research organizations have published reports on China-based companies after examining, among other things, their corporate governance practices, related party transactions, sales practices and financial statements that have led to special investigations and stock suspensions on national exchanges, including as a result of purported whistle-blowing or leaking by employees or former employees. Any similar scrutiny of us, regardless of its lack of merit, could result in a diversion of management resources and energy, potential costs to defend ourselves against rumors, decreases and volatility in the trading price of our common stock, and increased directors and officers insurance premiums and could have a material adverse effect upon our business, including its results of operations, financial condition, cash flows and prospects.
Risks Related to Our Common Stock and This Offering
An active public market for our common stock may not develop and our common stock may trade below the public offering price.
Prior to this offering, there has been no public market for our common stock. We intend to apply to have our stock listed on the NASDAQ. However, a liquid public market for our common stock may not develop. If an active trading market for our common stock does not develop after this offering, the market price and liquidity of our common stock may be materially and adversely affected. The public offering price for our common stock has been determined by negotiation among us and the underwriters based upon several factors, and the price at which our common stock trade after this offering may decline below the public offering price. Investors in our common stock may experience a significant decrease in the value of their investment regardless of our operating performance or prospects.
The trading price of our common stock is likely to be volatile, which could result in substantial losses to you.
The trading price of our common stock is likely to be volatile and could fluctuate widely in response to a variety of factors, many of which are beyond our control. In addition, the performance and fluctuation of the market prices of other companies with a portion of their business operations located in China that have listed their securities in the U.S. may affect the volatility in the price of and trading volumes for our common stock. Some of these companies have experienced significant volatility, including significant price declines after their initial public offerings. The trading performances of these companies’ securities at the time of or after their offerings may affect the overall investor sentiment towards other companies with significant China operations listed in the U.S. and consequently may impact the trading performance of our common stock.
In addition to market and industry factors, the price and trading volume for our common stock may be highly volatile for specific business reasons, including:
| • | announcements of regulatory approval or a complete response letter, or specific label indications or patient populations for its use, or changes or delays in the regulatory review process; |
| • | announcements of therapeutic innovations or new products by us or our competitors; |
73
Table of Contents
| • | adverse actions taken by regulatory agencies with respect to our clinical trials, manufacturing supply chain or sales and marketing activities; |
| • | any adverse changes to our relationship with manufacturers or suppliers; |
| • | the results of our testing and clinical trials; |
| • | the results of our efforts to acquire or license additional drug candidates; |
| • | variations in the level of expenses related to our existing drug candidates or preclinical and clinical development programs; |
| • | any intellectual property infringement actions in which we may become involved; |
| • | announcements concerning our competitors or the pharmaceutical industry in general; |
| • | achievement of expected product sales and profitability; |
| • | manufacture, supply or distribution shortages; |
| • | variations in our results of operations; |
| • | announcements about our earnings that are not in line with analyst expectations, the risk of which is enhanced because it is our policy not to give guidance on earnings; |
| • | publication of operating or industry metrics by third parties, including government statistical agencies, that differ from expectations of industry or financial analysts; |
| • | changes in financial estimates by securities research analysts; |
| • | announcements made by us or our competitors of new product and service offerings, acquisitions, strategic relationships, joint ventures or capital commitments; |
| • | press reports or other negative publicity, whether or not true, about our business; |
| • | additions to or departures of our management; |
| • | fluctuations of exchange rates between the Renminbi and the U.S. dollar; |
| • | release or expiry of lock-up or other transfer restrictions on our outstanding common stock; |
| • | sales or perceived potential sales of additional common stock; |
| • | sales of our common stock by us, our executive officers and directors or our shareholders in the future; |
| • | general economic and market conditions and overall fluctuations in the U.S. equity markets; |
| • | changes in accounting principles; and |
| • | changes or developments in the PRC or global regulatory environment. |
Any of these factors may result in large and sudden changes in the volume and trading price of our common stock. In the past, following periods of volatility in the market price of a company’s securities, shareholders have often instituted securities class action litigation against that company. If we were involved in a class action suit, it could divert the attention of management, and, if adversely determined, have a material adverse effect on our financial condition and results of operations.
In addition, the stock market, in general, and small pharmaceutical and biotechnology companies have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. Further, the current decline in the financial markets and related factors beyond our control may cause our common stock price to decline rapidly and unexpectedly.
74
Table of Contents
We may be subject to securities litigation, which is expensive and could divert management attention.
The price of our common stock may be volatile, and in the past companies that have experienced volatility in the market price of their common stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
Upon completion of this offering, we will become subject to the periodic reporting requirements of the U.S. Securities Exchange Act of 1934, as amended, or the Exchange Act. Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management, and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the U.S. Securities and Exchange Commission, or SEC.
We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected.
Substantial future sales or perceived potential sales of our common stock or other equity securities in the public market could cause the price of our common stock to decline significantly.
Sales of our common stock or other equity securities in the public market after this offering, or the perception that these sales could occur, could cause the market price of our common stock to decline significantly. Upon completion of this offering, we will have shares of common stock outstanding, assuming the underwriters do not exercise their option to purchase additional shares. The shares of our common stock outstanding after this offering will be available for sale, upon the expiration of the lock-up periods described elsewhere in this prospectus beginning from the date of this prospectus (if applicable to such holder), subject to volume and other restrictions as applicable under Rules 144 and 701 under the Securities Act. Any or all of these shares may be released prior to the expiration of the applicable lock-up period at the discretion of one of the designated representatives. To the extent shares are released before the expiration of the applicable lock-up period and sold into the market, the market price of our common stock could decline significantly.
We are currently an “emerging growth company.” As a result of the reduced disclosure requirements applicable to emerging growth companies, our common stock may be less attractive to investors.
We are currently an “emerging growth company,” as defined in the JOBS Act. For so long as we remain an emerging growth company, we are permitted and intend to rely on some of the exemptions from certain reporting requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include but are not limited to not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
75
Table of Contents
We cannot predict whether investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and the price of our common stock may be more volatile.
We have broad discretion as to the use of proceeds from this offering and may not use the proceeds effectively.
Our management will retain broad discretion as to the allocation of the proceeds and may spend these proceeds in ways in which you may not agree. The failure of our management to apply these funds effectively could result in unfavorable returns and uncertainty about our prospects, each of which could cause the price of our common stock to decline.
Because we do not expect to pay dividends in the foreseeable future, you must rely on price appreciation of our common stock for return on your investment.
We intend to retain most, if not all, of our available funds and earnings to fund the development and growth of our business. As a result, we do not expect to pay any cash dividends in the foreseeable future. Therefore, you should not rely on an investment in our common stock as a source for any future dividend income.
Our board of directors has significant discretion as to whether to distribute dividends. Even if our board of directors decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on, among other things, our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors deemed relevant by our board of directors. Accordingly, the return on your investment in our common stock will likely depend entirely upon any future price appreciation of our common stock. There is no guarantee that our common stock will appreciate in value or even maintain the price at which you purchased our common stock. You may not realize a return on your investment in our common stock and you may even lose your entire investment in our common stock. See “Dividend Policy.”
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, the market price for our common stock and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. If research analysts do not establish and maintain adequate research coverage or if one or more of the analysts who covers us downgrades our common stock or publishes inaccurate or unfavorable research about our business, the market price for our common stock would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, we could lose visibility in the financial markets, which, in turn, could cause the market price or trading volume for our common stock to decline significantly.
As the public offering price is substantially higher than our net tangible book value per share of our common stock, you will incur immediate and substantial dilution.
If you purchase common stock in this offering, you will pay more for your common stock than the amount paid by existing shareholders for their common stock on a per share basis. As a result, you will experience immediate and substantial dilution of $ per share of common stock (assuming no exercise of outstanding options to acquire shares of common stock and no exercise of the underwriters’ option to purchase additional shares of common stock), representing the difference between our pro forma net tangible book value per share of common stock as of March 31, 2017, after giving effect to this offering and the initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus. In addition, you will experience further dilution upon the issuance of shares to holders of our convertible loan agreements and to Hanmi and certain of our executive officers upon the completion of this offering pursuant to contractual obligations. All of the shares issuable upon the exercise of currently outstanding options or convertible loan agreements will be issued at a purchase price on a per share basis that is less than the public offering price per share of common stock in this offering.
76
Table of Contents
Certain of our existing stockholders and/or their affiliates have agreed to purchase up to % of the shares in this offering. The public float of our common stock after this offering will be approximately % of our outstanding shares of common stock, which could adversely affect the trading market for our common stock.
Certain of our principal stockholders and/or their affiliates have agreed to purchase, individually, up to an aggregate of $ million of shares of common stock in this offering (or % of the shares we are offering), at the initial public offering price. If such stockholders purchase all shares they have agreed to purchase, the number of shares beneficially owned after this offering by all such stockholders and our directors and executive officers as a group will increase to , and the percentage of common stock beneficially owned by them after this offering will increase to %. The public float of our common stock after this offering will be approximately % of our outstanding shares of common stock, which could adversely affect the liquidity of the trading market for our common stock from what it would have been had these shares been purchased by unaffiliated investors. Reducing the public float and liquidity of our common stock can affect the market price of our common stock and make it more difficult to sell the shares purchased in this offering at current market prices, or at all. See “Principal Stockholders” for more information regarding beneficial ownership of our common stock.
Our directors, executive officers and principal stockholders will continue to have substantial control over us after this offering, which could limit your ability to influence the outcome of key transactions, including a change of control.
Our directors, executive officers and our stockholders who own greater than 5% of our outstanding common stock, together with their affiliates, will beneficially own, in the aggregate, approximately % of our outstanding common stock after this offering, based on the number shares outstanding as of March 31, 2017. As a result, these stockholders, if acting together, will be able to influence or control matters requiring approval by our stockholders, including the election of directors and the approval of mergers, acquisitions or other extraordinary transactions. In addition, these stockholders, acting together, would have the ability to control the management and affairs of our company. They may also have interests that differ from yours and may vote in a way with which you disagree and which may be adverse to your interests. This concentration of ownership may have the effect of delaying, preventing or deterring a change of control of our company, could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of our company and might ultimately affect the market price of our common stock.
In addition, our directors and executive officers, will beneficially own in the aggregate approximately % of our outstanding common stock after this offering, based on the number shares outstanding as of March 31, 2017. As such, our directors and executive officers could have considerable influence over matters such as approving a potential acquisition of us. Our directors and executive officers’ investment in and position in our company could also discourage others from pursuing any potential acquisition of us, which could have the effect of depriving the holders of our common stock of the opportunity to sell their shares at a premium over the prevailing market price.
Anti-takeover provisions in our charter documents may discourage our acquisition by a third party, which could limit our shareholders’ opportunity to sell their shares at a premium.
Our amended and restated certificate of incorporation and bylaws include provisions that could limit the ability of others to acquire control of our company, could modify our structure or could cause us to engage in change-of-control transactions. These provisions could have the effect of depriving our shareholders of an opportunity to sell their shares at a premium over prevailing market prices by discouraging third parties from seeking to obtain control in a tender offer or similar transaction.
77
Table of Contents
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of the NASDAQ Stock Market and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices including our board and committee practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
We are evaluating these rules and regulations, and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, we will first be required to furnish a report by our management on our internal control over financial reporting for the year ending December 31, 2017. However, while we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404. If we identify one or more material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
78
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains certain forward-looking statements that involve risks and uncertainties. These forward-looking statements reflect our current views with respect to, among other things, future events and our financial performance. These statements are often, but not always, made through the use of words or phrases such as “may,” “might,” “should,” “could,” “predict,” “potential,” “believe,” “expect,” “continue,” “will,” “anticipate,” “seek,” “estimate,” “intend,” “plan,” “projection,” “would,” “annualized” and “outlook,” or the negative version of those words or other comparable words or phrases of a future or forward-looking nature. These forward-looking statements are not historical facts, and are based on current expectations, estimates and projections about our industry, management’s beliefs and certain assumptions made by management, many of which, by their nature, are inherently uncertain and beyond our control. Accordingly, we caution you that any such forward-looking statements are not guarantees of future performance and are subject to risks, assumptions, estimates and uncertainties that are difficult to predict. Although we believe that the expectations reflected in these forward-looking statements are reasonable as of the date made, actual results may prove to be materially different from the results expressed or implied by the forward-looking statements.
A number of important factors could cause our actual results to differ materially from those indicated in these forward-looking statements, including those factors identified in “Risk Factors” or “Management’s Discussion and Analysis of Financial Condition and Results of Operations” or the following:
| • | our plans to develop and commercialize our product candidates; |
| • | our ongoing and planned clinical trials; |
| • | economic, political, regulatory and other risks associated with our international operations; |
| • | the timing of and our ability to obtain and maintain regulatory approvals for our product candidates; |
| • | our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
| • | our ability to identify additional products or product candidates with significant commercial potential that are consistent with our commercial objectives; |
| • | the rate and degree of market acceptance and clinical utility of our products; |
| • | our commercialization, marketing and manufacturing capabilities and strategy; |
| • | significant competition in our industry; |
| • | costs of litigation and the failure to successfully defend lawsuits and other claims against us; |
| • | our ability to receive research funding and achieve anticipated milestones under our collaborations; |
| • | our intellectual property position; |
| • | our ability to maintain licenses of key technology and intellectual property on commercially reasonable terms; |
| • | costs of compliance and our failure to comply with new and existing governmental regulations including, but not limited to, tax regulations; |
| • | loss or retirement of key members of management; |
| • | costs of compliance and our failure to comply with new and existing governmental regulations including, but not limited to, tax regulations; |
| • | our ability to maintain our partnerships with the local governments in New York State and Chongqing Municipality; |
79
Table of Contents
| • | failure to successfully execute our growth strategy, including any delays in our planned future growth; and |
| • | our failure to maintain effective internal controls. |
The foregoing factors should not be considered exhaustive and should be read together with the other cautionary statements included in this prospectus. If one or more events related to these or other risks or uncertainties materialize, or if our underlying assumptions prove to be incorrect, actual results may differ materially from what we anticipate. Accordingly, you should not place undue reliance on any such forward-looking statements. Any forward-looking statement speaks only as of the date on which it is made, and we do not undertake any obligation to update or review any forward-looking statement, whether as a result of new information, future developments or otherwise.
This prospectus also contains estimates, projections and other information concerning our industry, our business and the markets for certain drugs, including data regarding the estimated size of those markets, their projected growth rates and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained these industry, business, market and other data from reports, research surveys, studies and similar data prepared by third parties, industry, medical and general publications, government data and similar sources. In some cases, we do not expressly refer to the sources from which these data are derived.
80
Table of Contents
We estimate that our net proceeds from the sale of shares of our common stock in this offering will be approximately $ million, or $ million if the underwriters fully exercise their option to purchase additional shares, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
A $1.00 increase (decrease) in the assumed public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) our expected net proceeds from this offering by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. An increase (decrease) of 1.0 million shares in the number of shares offered by us would increase the net proceeds to us from this offering by approximately $ million, assuming the assumed initial public offering price of $ per share remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The principal purposes of this offering are to increase our financial flexibility, create a public market for our common stock, and to facilitate our access to the public equity markets. We currently expect to use the net proceeds from this offering as follows:
| • | Approximately $28 million for Phase 3 clinical trials of Oraxol for treatment of metastatic breast cancer; |
| • | Approximately $12 million for additional clinical development and regulatory activities for our Orascovery platform; |
| • | Approximately $20 million for Phase 3 clinical trials of KX-01 ointment for treatment of actinic keratosis; |
| • | Approximately $25 million for clinical and pre-clinical research and development; and |
| • | The remainder for working capital, capital expenditures and general corporate purposes. |
We may use a portion of the net proceeds of this offering for the acquisition or licensing, as the case may be, of additional technologies, other assets or businesses, or for other strategic investments or opportunities, although we have no definitive understandings, agreements or commitments to do so as of the date hereof.
Our expected use of the net proceeds from this offering is based upon our present plans and business condition. Based on those current plans and assumptions, we do not expect that the net proceeds from this offering, combined with our current operating capital, will be sufficient to enable us to complete all necessary development or commercially launch our current drug candidates. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual use of proceeds will vary depending on numerous factors, including the factors described under the heading “Risk Factors”. As a result, management will retain broad discretion over the allocation of the net proceeds from this offering, and investors will be relying on the judgment of our management regarding the application of the net proceeds.
Pending the use of the net proceeds of this offering, we intend to invest the net proceeds in high-quality, short-term interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
81
Table of Contents
We have never declared or paid cash dividends on our common stock and we do not intend to pay any cash dividends on our common stock for the foreseeable future. We currently intend to retain future earnings, if any, for use in the operation of our business and to fund future growth. Any future determination related to our dividend policy will be made at the discretion of our board of directors in light of conditions then-existing, including factors such as our results of operations, financial condition and requirements, business conditions and covenants under any applicable contractual arrangements.
82
Table of Contents
The following table sets forth our cash and cash equivalents and our capitalization as of March 31, 2017:
| • | on an actual basis; |
| • | on a pro forma basis to give effect to the issuance of shares of our common stock, pursuant to (i) the conversion of outstanding convertible notes into shares of our common stock upon the completion of this offering, and (ii) issuance of shares to Hanmi and certain of our executive officers upon the completion of this offering pursuant to contractual obligations; and |
| • | on a pro forma as adjusted basis, giving effect to (i) the pro forma adjustments set forth above; and (ii) the sale and issuance of shares of our common stock by us in this offering, based upon the initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
You should read this table in conjunction with the sections entitled “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Description of Capital Stock” and our consolidated financial statements and related notes included elsewhere in this prospectus.
| As of March 31, 2017 | ||||||||||||
| (in thousands, except share and per share data) |
||||||||||||
| Actual | Pro Forma | Pro Forma as Adjusted(1) |
||||||||||
| Cash and cash equivalents |
$ | 26,034 | $ | $ | ||||||||
|
|
|
|
|
|
|
|||||||
| Stockholders’ (deficit) equity: |
||||||||||||
| Common stock, $0.001 par value; 250,000,000 shares authorized, actual; shares authorized, pro forma and pro forma as adjusted; 40,686,586 shares outstanding, actual; shares outstanding, pro forma; shares outstanding, pro forma as adjusted |
$ | 42 | ||||||||||
| Undesignated preferred stock, $ par value per share; no shares authorized, issued and outstanding, actual; shares authorized, no shares issued and outstanding, actual, pro forma and pro forma as adjusted |
||||||||||||
| Additional paid-in capital |
239,769 | |||||||||||
| Accumulated other comprehensive loss |
(802 | ) | ||||||||||
| Accumulated deficit |
(236,094 | ) | ||||||||||
| Less: treasury stock, at cost; 1,672,920 shares at March 31, 2017 |
(7,406 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ (deficit) equity |
(3,617 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | (3,617 | ) | $ | $ | |||||||
|
|
|
|
|
|
|
|||||||
| (1) | A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus, would increase (decrease) each of cash and cash equivalents, additional paid-in capital, total stockholders’ equity and total capitalization by $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) each of cash and cash equivalents, additional paid-in capital, total stockholders’ equity and total capitalization by $ million, assuming the assumed initial public offering price of $ per share remains the same and after deducting the estimated underwriting discounts and commissions and |
83
Table of Contents
| estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and terms of this offering determined at pricing. |
If the underwriters’ option to purchase additional shares is exercised in full, pro forma as adjusted cash and cash equivalents, additional paid-in capital, total stockholders’ equity and total capitalization as of March 31, 2017 would be $ million, $ million, $ million and $ million, respectively.
The table above does not include:
| • | 9,214,989 shares of common stock issuable upon the exercise of options to purchase our common stock outstanding as of March 31, 2017 at a weighted average exercise price of $6.25 per share; |
| • | 344,000 shares of common stock issuable upon the exercise of warrants outstanding as of March 31, 2017; and |
| • | 2,025,543 shares of common stock reserved for future grant or issuance under our stock option plans as of March 31, 2017. |
84
Table of Contents
If you invest in our common stock in this offering, your interest will be diluted to the extent of the difference between the initial public offering price per share of our common stock in this offering and the pro forma as adjusted net tangible book value per share of our common stock immediately after this offering.
As of March 31, 2017, our historical net tangible book value was approximately $ million, or $ per share of common stock. Historical net tangible book value per share represents our total assets less goodwill, intangible assets and deferred offering costs, less total liabilities, divided by the number of our outstanding shares of common stock.
As of March 31, 2017, our pro forma net tangible book value was approximately $ million, or $ . per share of common stock. Our pro forma net tangible book value per share represents the amount of our total tangible assets reduced by the amount of our total liabilities and divided by the total number of shares of our common stock outstanding as of March 31, 2017, assuming upon the closing of this offering.
After giving further effect to (i) the sale of shares of our common stock in this offering, at the initial public offering price of $ per share, after deducting underwriting discounts and commissions and estimated offering expenses payable by us and (ii) the issuance of shares to holders of our convertible loan agreements and to Hanmi and certain of our executive officers upon the completion of this offering pursuant to contractual obligations, our pro forma as adjusted net tangible book value as of March 31, 2017 would have been approximately $ , or $ per share. This represents an immediate increase in pro forma as adjusted net tangible book value of $ per share to our existing stockholders and an immediate dilution of $ per share to investors purchasing shares in this offering.
The following table illustrates this dilution:
| Assumed initial public offering price per share |
$ | |||||||
| Historical net tangible book value per share as of March 31, 2017 |
$ | |||||||
| Pro forma decrease attributable to issuance of common stock pursuant to contractual obligations |
||||||||
| Pro forma net tangible book value per share as of March 31, 2017 |
||||||||
| Increase in pro forma net tangible book value per share attributable to new investors purchasing shares in this offering |
||||||||
|
|
|
|||||||
| Pro forma, as adjusted net tangible book value per share after this offering |
||||||||
|
|
|
|||||||
| Dilution per share to investors in this offering |
$ | |||||||
|
|
|
A $1.00 increase or decrease in the assumed initial public offering price of $ per share would increase or decrease our pro forma net tangible book value by approximately $ , our pro forma net tangible book value per share after this offering by approximately $ and dilution per share to new investors by approximately $ , assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same.
If the underwriters exercise their option to purchase additional shares of our common stock in full, the pro forma as adjusted net tangible book value per share after this offering would be $ per share, and the dilution in pro forma net tangible book value per share to new investors in this offering would be $ per share.
85
Table of Contents
The following table summarizes on an as adjusted basis as of March 31, 2017, the difference between the number of shares of common stock purchased from us, the total consideration paid and the average price per share paid by existing stockholders and by new investors, assuming an initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us:
| Shares Purchased | Total Consideration(1) | Average Price per Share |
||||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||||
| Existing stockholders |
% | $ | % | $ | ||||||||||||||||
| New investors |
$ | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
100 | % | $ | 100 | % | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| (1) | A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus, would increase (decrease) the total consideration paid to us by new investors and total consideration paid to us by all stockholders by $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) the total consideration paid to us by new investors and total consideration paid to us by all stockholders by $ million, assuming the assumed initial public offering price of $ per share remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
If the underwriters exercise their option to purchase additional shares in full, our existing stockholders would own % and our new investors would own % of the total number of shares of our common stock outstanding after our initial public offering.
The foregoing discussion and tables assume no exercise of any stock options or warrants outstanding as of March 31, 2017. To the extent that these options and warrants are exercised, new investors will experience further dilution. As of March 31, 2017, options to purchase 9,214,989 shares of common stock were outstanding at a weighted average exercise price of $6.25 per share and warrants to purchase 344,000 shares of common stock were outstanding.
86
Table of Contents
SELECTED HISTORICAL FINANCIAL INFORMATION AND OTHER DATA
The following selected statements of operations and comprehensive loss data and the cash flow data for the years ended December 31, 2015 and 2016 and the balance sheet data as of December 31, 2015 and 2016 are derived from our audited consolidated financial statements included elsewhere in this prospectus. The selected statements of operations and comprehensive loss data and the cash flow data for the three months ended March 31, 2016 and 2017 and the balance sheet data as of March 31, 2017 have been derived from our unaudited interim consolidated financial statements included elsewhere in this prospectus. In our opinion, these unaudited interim consolidated financial statements have been prepared on a basis consistent with our audited consolidated financial statements and contain all adjustments, consisting only of normal and recurring adjustments, necessary for a fair presentation of such financial data. You should read this data together with our audited consolidated financial statements, unaudited consolidated interim financial statements, and related notes appearing elsewhere in this prospectus and the information under the captions “Capitalization” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our historical results are not necessarily indicative of our future results, and our operating results for the three month period ended March 31, 2017 are not necessarily indicative of the results that may be expected for the fiscal year ending December 31, 2017 or any other interim periods or any future year or period. Our consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States, or U.S. GAAP.
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (In thousands, except share and per share data) |
||||||||||||||||
| Statements of Operations and Comprehensive Loss Data: |
||||||||||||||||
| Revenue: |
||||||||||||||||
| Product sales |
$ | 12,816 | $ | 19,394 | $ | 4,488 | $ | 3,900 | ||||||||
| License fees and consulting revenue |
314 | 392 | 95 | 598 | ||||||||||||
| Grant revenue |
814 | 765 | 46 | 83 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
13,944 | 20,551 | 4,629 | 4,581 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Costs and operating expenses: |
||||||||||||||||
| Cost of sales |
13,153 | 19,718 | 4,142 | 2,839 | ||||||||||||
| Research and development expenses |
24,463 | 60,624 | 6,746 | 26,408 | ||||||||||||
| Selling, general, and administrative expenses |
27,036 | 25,956 | 4,337 | 9,799 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and operating expenses |
64,652 | 106,298 | 15,225 | 39,046 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(50,708 | ) | (85,747 | ) | (10,596 | ) | (34,465 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Interest expense (income) |
1 | 1,891 | (46 | ) | 2,376 | |||||||||||
| Unrealized loss on derivative liability |
— | 533 | — | 4,276 | ||||||||||||
| Income tax (benefit) expense |
(54 | ) | (265 | ) | 100 | (92 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(50,655 | ) | (87,906 | ) | (10,650 | ) | (41,025 | ) | ||||||||
| Less: net loss attributable to non-controlling interests |
(55 | ) | (191 | ) | (32 | ) | (37 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to Athenex, Inc. |
$ | (50,600 | ) | $ | (87,715 | ) | $ | (10,618 | ) | $ | (40,988 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share attributable to Athenex, Inc. common stockholders, basic and diluted(1) |
$ | (1.50 | ) | $ | (2.19 | ) | $ | (0.27 | ) | $ | (1.01 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted-average shares used in computing net loss per share attributable to Athenex, Inc. common stockholders, basic and diluted(1) |
33,765,751 | 40,120,908 | 38,878,366 | 40,693,039 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pro forma net loss per share attributable to Athenex, Inc. common stockholders, basic and diluted(1) |
$ | $ | $ | $ | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pro forma weighted-average shares used in computing net loss per share attributable to Athenex, Inc. common stockholders, basic and diluted(1) |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Comprehensive loss |
$ | (50,906 | ) | $ | (88,796 | ) | $ | (10,656 | ) | $ | (40,486 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | See Note 17 to our audited consolidated financial statements appearing elsewhere in this prospectus for a description of the method used to calculate basic and diluted net loss per share attributable to Athenex, Inc. common stockholders and pro forma basic and diluted net loss per share attributable to Athenex, Inc. common stockholders. |
87
Table of Contents
| December 31, | March 31, | Pro Forma March 31, |
||||||||||||||
| 2015 | 2016 | 2017 | 2017 | |||||||||||||
| (in thousands) | ||||||||||||||||
| Selected Balance sheet data: |
||||||||||||||||
| Cash and cash equivalents |
$ | 43,495 | $ | 33,125 | $ | 26,034 | ||||||||||
| Marketable securities—current |
12,271 | 8,628 | 3,051 | |||||||||||||
| Goodwill |
37,996 | 37,552 | 37,574 | |||||||||||||
| Working capital* |
47,578 | 23,904 | 3,948 | |||||||||||||
| Total assets |
120,431 | 105,890 | 100,503 | |||||||||||||
| Long-term debt |
3,650 | 41,807 | 64,006 | |||||||||||||
| Total liabilities |
22,387 | 71,221 | 104,120 | |||||||||||||
| Non-controlling interests |
484 | 862 | 874 | |||||||||||||
| Total stockholders’ equity (deficit) |
$ | 98,044 | $ | 34,669 | $ | (3,617 | ) | |||||||||
| * | Working capital: total current assets—total current liabilities |
| Year Ended December 31, |
Three Months Ended March 31, |
|||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (in thousands) | ||||||||||||||||
| Selected Cash flow data: |
||||||||||||||||
| Net cash used in operating activities |
$ | (33,756 | ) | $ | (47,870 | ) | $ | (7,556 | ) | $ | (20,753 | ) | ||||
| Net cash (used in) provided by investing activities |
(16,909 | ) | 2,659 | (755 | ) | 4,023 | ||||||||||
| Net cash provided by financing activities |
76,302 | 35,272 | |
3,084 |
|
9,201 | ||||||||||
| Net effect of foreign exchange rate changes |
337 | (431 | ) | 39 | 438 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net increase (decrease) in cash and cash equivalents |
25,974 | (10,370 | ) | (5,188 | ) | (7,091 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash and cash equivalents at beginning of period |
17,521 | 43,495 | 43,495 | 33,125 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash and cash equivalents at end of period |
$ | 43,495 | $ | 33,125 | $ | 38,307 | $ | 26,034 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
88
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with “Our Selected Consolidated Financial Data,” our consolidated financial statements and the related notes and the related notes appearing elsewhere in this prospectus. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should read the “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” sections of this prospectus for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. The terms “Company,” “Athenex,” “we,” “our” or “us” as used herein refer to Athenex, Inc. and its consolidated subsidiaries unless otherwise stated or indicated by context.
Overview
We are a global biopharmaceutical company dedicated to the discovery, development and commercialization of novel therapies for the treatment of cancer. Our mission is to improve the lives of cancer patients by creating more effective, safer and tolerable treatments. We have generated our clinical product candidates through our Orascovery and Src Kinase Inhibition research platforms, which are based on our understanding of human absorption biology and novel approaches to inhibiting kinase activity, respectively. We believe that our ability to overcome the challenges of oral delivery of chemotherapy and limitations associated with IV delivery, via our P-gp inhibitor, offers significant potential benefits to patient outcomes by allowing patients to stay on therapy longer and extending the potential opportunities to combine with other agents, including targeted and immunotherapies that would otherwise be too toxic in combination with IV chemotherapy. We have assembled a leadership team and have established global operations in the U.S. and China across the pharmaceutical value chain to execute our mission to become a global leader in bringing innovative cancer treatments to the market and improve health outcomes.
Basis of Presentation
Our financial data presented herein as of and for the years ended December 31, 2015 and 2016 have been derived from our audited consolidated financial statements, which were prepared in accordance with U.S. GAAP, and should be read in conjunction with those statements which are included elsewhere in this prospectus. Our financial data presented herein as of March 31, 2017 and for the three months ended March 31, 2016 and 2017 have been derived from our unaudited interim consolidated financial statements, which were prepared in accordance with U.S. GAAP, and should be read in conjunction with those statements which are included elsewhere in this prospectus.
We acquired three businesses during the periods included in our consolidated financial statements within this prospectus. These acquisitions were: (1) in September 2014, we acquired QuaDPharma, LLC, or QuaDPharma, which provides cGMP manufacturing, packaging and laboratory services to support our research and development and also to third parties; (2) in June 2015, we acquired Polymed Therapeutics, Inc. and Chongqing Taihao Pharmaceutical Co Ltd, collectively Polymed. Polymed markets and sells API and medical devices throughout the world. Through its cGMP facility in Chongqing, China, Polymed manufactures API and conducts research and development of novel drugs and therapies; and (3) in July 2015, we acquired Comprehensive Drug Enterprises Limited, or CDE, which primarily performs research and development activities related to the development of transmucosal drug delivery and also in support of our Oncology Innovation Platform.
We have three segments operating in North America and Asia: our Oncology Innovation Platform, our Commercial Platform and our Global Supply Chain Platform. Our Oncology Innovation Platform include two core research and development centers, one in Hong Kong, China and one in the U.S.
89
Table of Contents
Factors Affecting our Results of Operations
Since inception, we have devoted substantially all of our resources to research and development of our lead product candidates under our Orascovery and Src Kinase Inhibition platforms. We have incurred significant net losses since inception. As of March 31, 2017, we had an accumulated deficit of approximately $236.1 million. Our recurring losses from operations and negative cash flows from operations have raised substantial doubt regarding our ability to continue as a going concern, and as a result, our independent registered public accounting firm has noted this in the opinion they issued on our consolidated financial statements for the year ended December 31, 2016. As a result of the acquisitions of QuaDPharma in 2014 and Polymed in 2015, we started to generate revenue from those businesses. Product sales totaled $12.8 million and $19.4 million for the years ended December 31, 2015 and 2016, respectively, and $4.5 million and $3.9 million for the three months ended March 31, 2016 and 2017, respectively.
We believe that revenue generated from our Global Supply Chain Platform will continue to grow at a steady pace in the years ahead. We expect that our Commercial Platform will start generating revenue in 2017. However, due to both unforeseeable factors such as global political and economic changes and foreseeable factors such as market competition, revenue generated from these segments might not be sufficient to meet their operating costs. Therefore, we will continue to require substantial additional capital beyond the expected proceeds from this offering to continue our clinical development and potential commercialization activities. The amount and timing of our future funding requirements will depend on many factors, including the pace and results of our clinical development efforts. We cannot assure you that we will be profitable or generate positive cash flow from operating activities in the next three years, or at all.
Research and Development Expenses
We believe our ability to successfully develop innovative oncology drug candidates through our Oncology Innovation Platforms will be the primary factor affecting our long-term competitiveness, as well as our future growth and development. All of the drug candidates of our Oncology Innovation Platform are still in the development stage. Creating innovative oncology drugs requires a significant investment of resources over a prolonged period of time. As a result of this commitment, our pipeline of drug candidates has been steadily advancing and expanding, with six clinical stage drug candidates in different regulatory approval stages in various countries as of March 31, 2017.
Our research and development expenses since inception have primarily included:
| • | Employee compensation related expenses for research and development personnel, including salaries, benefits and equity compensation expense; |
| • | Expenses incurred for payments to contract research organizations, investigators and clinical trial sites that conduct our clinical studies; |
| • | Costs of acquiring, developing, and manufacturing clinical study materials; |
| • | Facilities, depreciation, and other expenses, which include office leases and other overhead expenses; and |
| • | Costs associated with pre-clinical activities and regulatory operations. |
Research and development expenses incurred totaled $24.5 million and $60.6 million for the years ended December 31, 2015 and 2016, respectively, and $6.7 million and $26.4 million for the three months ended March 31, 2016 and 2017, respectively. These figures represent 37.8%, 57.0%, 44.3% and 67.6% of our total costs and operating expenses for the respective periods.
We have not historically tracked or recorded research and development expenses on a project-by-project basis primarily because we use our employee and infrastructure resources across multiple research and
90
Table of Contents
development projects and it is not practical for us to allocate such costs on a project-by-project basis. Due to the numerous risks and uncertainties associated with our research and development activities, we cannot determine with certainty the duration and completion costs of the current and future preclinical studies and clinical trials. We expect our research and development expenses to increase for the foreseeable future as we continue the development of our drug candidates.
We have historically been able to fund the research and development expenses for our Oncology Innovation Platform via a range of sources, including through private equity and debt financings and to a lesser extent through the profits of our Global Supply Chain Platform, and through grant funding in the U.S., Hong Kong and China. We intend to continue making significant investments in research and development to bring our existing oncology drug products to the market.
We expect that the net proceeds to us from this offering will also be an important source of funds for our research and development.
Ability to Commercialize Our Drug Candidates
Our ability to generate revenue from our drug candidates depends on our ability to successfully complete clinical trials and obtain regulatory approvals for them in the U.S., Europe, China and other major markets.
Our major drug candidates supported by our Orascovery platform are being rapidly developed and clinically tested simultaneously in multiple countries, including the U.S. Nonetheless, we cannot be certain if any of our drug candidates will successfully complete clinical trials or receive necessary regulatory approvals. Even if such approvals are granted, we will need to thereafter establish manufacturing infrastructure and engage in extensive marketing prior to generating any revenue from such drugs, and the ultimate commercial success of our drugs will depend on their acceptance by patients, the medical community and third-party payors and their ability to compete effectively with other therapies on the market.
As a first step towards commercialization, we have started to establish our sales, marketing, and distribution infrastructure through our Commercial Platform. We have entered into a number of agreements to sell, market and distribute compound pharmacy 503B and specialty products. Those specialty products being launched in the near future are expected to generate cash that will help subsidize the build-out of the commercialization team in the U.S., the ongoing clinical trials and research and development projects for the Athenex proprietary pipeline. We intend to have our internal marketing and sales force for the launching of our proprietary oncology products when they obtain regulatory approval.
Key Components of Results of Operations
Revenue
We derive our consolidated revenue primarily from (i) the sales of API and medical devices by our Global Supply Chain Platform; (ii) licensing and collaboration projects conducted by our Oncology Innovation Platform, which generates revenue in the form of upfront payments, milestone payments and payments received for providing research and development services for our collaboration projects and for other third parties; and (iii) grant awards from government agencies and universities for our continuing research and development efforts.
91
Table of Contents
The following table sets forth the components of our consolidated revenue and the amount as a percentage of total revenue for the periods indicated.
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||||||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||||||||||||||||||
| (in thousands) | % | (in thousands) | % | (in thousands) | % | (in thousands) | % | |||||||||||||||||||||||||
| Product sales |
$ | 12,816 | 92 | % | $ | 19,394 | 94 | % | $ | 4,488 | 97 | % | $ | 3,900 | 85 | % | ||||||||||||||||
| Licensing fees and consulting revenue |
314 | 2 | % | 392 | 2 | % | 95 | 2 | % | 598 | 13 | % | ||||||||||||||||||||
| Grant revenue |
814 | 6 | % | 765 | 4 | % | 46 | 1 | % | 83 | 2 | % | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total revenue |
$ | 13,944 | $ | 20,551 | $ | 4,629 | $ | 4,581 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
We do not anticipate revenue being generated from sales of our product candidates conducted by our Oncology Innovation Platform until we have obtained regulatory approval. We cannot assure you that we will succeed in achieving regulatory approval for our drug candidates as planned, or at all.
Cost of Sales
We manufacture our clinical products in our cGMP facility in New York and APIs at our cGMP facility in China. Cost of sales primarily includes the cost of raw materials, labor costs, manufacturing overhead expenses, reserves for expected scrap, as well as transportation costs. Cost of sales also includes depreciation expense for production equipment, changes to our excess and obsolete inventory reserves, and certain direct costs such as shipping costs, net of costs charged to customers.
Research and Development Expenses
Research and development expenses consist of the costs associated with our research and development activities, conducting preclinical studies and clinical trials and activities related to regulatory filings. The following table sets forth the components of our research and development expenses and the amount as a percentage of total research and development expenses for the periods indicated.
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||||||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||||||||||||||||||
| (in thousands) | % | (in thousands) | % | (in thousands) | % | (in thousands) | % | |||||||||||||||||||||||||
| Wages, benefits, and related costs |
$ | 6,660 | 27 | % | $ | 19,531 | 32 | % | $ | 3,246 | 48 | % | $ | 2,771 | 11 | % | ||||||||||||||||
| Clinical trial costs |
8,617 | 35 | % | 14,438 | 24 | % | 2,256 | 33 | % | 6,164 | 23 | % | ||||||||||||||||||||
| Preclinical research costs |
7,946 | 33 | % | 5,449 | 9 | % | 798 | 12 | % | 588 | 2 | % | ||||||||||||||||||||
| Drug licensing costs |
— | 0 | % | 17,690 | 29 | % | — | 0 | % | 15,178 | 58 | % | ||||||||||||||||||||
| Other research and development costs |
1,240 | 5 | % | 3,516 | 6 | % | 446 | 7 | % | 1,707 | 6 | % | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total research and development expenses |
$ | 24,463 | $ | 60,624 | $ | 6,746 | $ | 26,408 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
Our current research and development activities mainly relate to the clinical development of the following programs:
Orascovery platform—Comprised of our in-licensed and novel P-gp inhibitor, HM30181A, that is combined with various chemotherapeutic agents and enables them to be absorbed into the blood when given orally:
| • | Oraxol, combining HM30181A with an oral dosage form of paclitaxel; |
| • | Oratecan, combining HM30181A with an oral dosage form of irinotecan; |
92
Table of Contents
| • | Oradoxel, combining HM30181A with an oral dosage form of docetaxel; and |
| • | Oratopo, combining HM30181A with an oral dosage form of topotecan. |
Src Kinase Inhibition platform—Targets the tyrosine kinase protein in regulating cell growth that leads to blockade of metastasis:
| • | KX-01 ointment, Src kinase inhibitor that is being topically administered to treat skin cancers and pre-cancers; |
| • | KX-01 oral, Src kinase inhibitor that is being orally administered to treat certain solid and liquid tumors; and |
| • | KX-02, Src kinase inhibitor that is orally administered to treat brain cancer, such as glioblastoma multiforme (GBM). |
We expense research and development costs as incurred. We record costs for certain development activities, such as clinical trials, based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment or clinical site activations. We do not allocate employee related costs, depreciation, rental and other indirect costs to specific research and development programs because these costs are deployed across multiple product programs under research and development.
We cannot determine with certainty the duration, costs and timing of the current or future preclinical or clinical studies of our drug candidates. The duration, costs, and timing of clinical studies and development of our drug candidates will depend on a variety of factors, including:
| • | The scope, rate of progress, and costs of our ongoing, as well as, any additional clinical studies and other research and development activities; |
| • | Future clinical study results; |
| • | Uncertainties in clinical study enrollment rates; |
| • | Significant and changing government regulation; and |
| • | The timing and receipt of any regulatory approvals. |
A change in the outcome of any of these variables with respect to the development of a drug candidate could mean a significant change in the costs and timing associated with the development of that drug candidate.
Research and development activities are central to our business model. We expect our research and development expenses to continue to increase for the foreseeable future as we continue to support the clinical trials of Oraxol, Oratecan, Oradoxel, Oratopo, KX-01 ointment, KX-01 oral and KX-02, as well as initiate and prepare for additional clinical and preclinical studies. We also expect spending to increase in the research and development for API and specialty products. There are numerous factors associated with the successful commercialization of any of our drug candidates, including future trial design and various regulatory requirements, many of which cannot be determined with accuracy at this time based on our stage of development. Additionally, future commercial and regulatory factors beyond our control will impact our clinical development programs and plans.
Selling, General and Administrative Expenses
Selling, general and administrative, or SG&A, expenses primarily consist of compensation, including salary, employee benefits and stock-based compensation expenses for sales and marketing personnel, and for administrative personnel that support our general operations such as, executive management, legal counsel, financial accounting, information technology, and human resources personnel. SG&A expenses also includes professional fees for legal, patents, consulting, auditing and tax services, as well as other direct and allocated
93
Table of Contents
expenses for rent and maintenance of facilities, insurance and other supplies used in the selling, general and administrative activities. We expect to incur additional SG&A expenses in connection with our becoming a public company, which may increase further when we are no longer able to rely on the “emerging growth company” exemption pursuant to the JOBS Act.
Despite the decrease in SG&A expenses in recent periods, we anticipate that our SG&A expenses will increase in future periods to support increases in our research and development and commercialization activities. We expect these increases will likely result in increased headcount, increased share compensation charges, expanded infrastructure and increased costs for insurance. We also anticipate increases to legal, compliance, accounting and investor and public relations expenses associated with being a public company.
Critical Accounting Policies and Significant Judgments and Estimates
Our discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities and the disclosure of contingent assets and liabilities at the date of our financial statements and the reported amounts of revenue and expenses during the periods. We evaluate our estimates and judgments on an ongoing basis, including but not limited to, estimating the useful lives of long-lived assets, assessing the impairment of long-lived assets, stock-based compensation expenses, and the realizability of deferred income tax assets. We base our estimates on historical experience, known trends and events, contractual milestones and other various factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Changes in the accounting estimates are likely to occur from period to period. Actual results could be significantly different from these estimates. We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management’s judgment and estimates.
Revenue Recognition
We recognize product revenue when there is persuasive evidence of an arrangement, the price is fixed or determinable, collectability is reasonably assured, and upon shipment to customers or acceptance by customers.
We recognize revenue related to the license of certain intellectual properties and related consulting services when earned and when realized or realizable. Amounts received in advance are recorded as deferred revenue until earned. Out-license revenue is earned upon the achievement of milestone events by the licensees. Milestone events include execution of license agreements, completion of clinical studies of varying stages in various territories, regulatory approval of drugs in specific jurisdictions, among other events. After the licensees obtain regulatory approval and begin sales, we are entitled to royalties on net sales on most out-license agreements. Currently, all out-license agreements are in the milestone stage; no licensees have yet obtained regulatory approval of the licensed drugs.
Certain of our out-license agreements contain multiple elements and are accounted for in accordance with ASC 605-25 – Revenue Recognition – Multiple-Element Arrangements. We identify the deliverables included within the arrangement and evaluate which deliverables represent separate units of accounting. The consideration received is then allocated among the separate units of accounting based on each unit’s relative selling price. We generally consider non-refundable milestone payments to be achieved as a result of our efforts to be substantive and recognize them as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met. Revenue related to agreements with multiple elements or milestone payments are not significant in the periods presented.
We receive certain grant award funding to support our continuing research and developing efforts. We consider these grants to be operating revenue as they support our primary operating activities. Revenue is recognized when earned and when realized or realizable. Revenue from grant awards is deemed to be earned when all eligibility criteria are met.
94
Table of Contents
Research and Development Expenses
Research and development expenses represent costs associated with developing our proprietary drug candidates, our collaboration agreements for such drugs, and our ongoing clinical studies.
Clinical trial costs are a significant component of our research and development expenses. We have a history of contracting with third parties that perform various clinical trial activities on our behalf in the ongoing development of our drug candidates. Expenses related to clinical trials are accrued based on our estimates of the actual services performed by the third parties for the respective period. If the contracted amounts are revised or the scope of a contract is revised, we will modify the accruals accordingly on a prospective basis and will do so in the period in which the facts that give rise to the revision become reasonably certain.
Stock-Based Compensation and Fair Value of our Common Stock
Awards Granted to Employees
We apply FASB Accounting Standard Codification, or ASC, 718, Compensation- Stock Compensation to account for our employee stock-based compensation. In accordance with ASC 718, we determine whether an award should be classified and accounted for as a liability award or equity award. All of our grants of stock-based awards to employees were classified as equity awards and are recognized in the financial statements based on their grant-date fair values. We recognize compensation expense using the straight-line method for all employee equity awards over the requisite service period, which is generally the vesting period of the equity grant. We estimate the grant date fair value of stock options using the Black-Scholes option pricing model.
The valuation model for stock-based compensation expense requires us to make assumptions and judgments about the variables used in the calculation including the expected term (period of time that the options granted are expected to be outstanding), the expected volatility of our common stock, an assumed risk-free interest rate, the dividend rate and the estimated forfeitures of unvested stock options. The following table summarizes the weighted-average variables used to determine the fair value of stock options:
| Year Ended December 31, |
Three Months Ended March 31, |
|||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| Expected dividend yield |
— | % | — | % | — | % | — | % | ||||||||
| Expected stock price volatility |
62 | % | 65 | % | 65 | % | 65 | % | ||||||||
| Risk-free interest rate |
1.56 | % | 1.29 | % | 1.23 | % | 1.94 | % | ||||||||
| Expected life of options (in years) |
5.9 | 6.0 | 6.2 | 6.3 | ||||||||||||
| • | Fair Value of Common Stock. As discussed below, the fair value of the shares of our common stock underlying the stock options has historically been determined based on recent third-party sales of our common stock in private equity financing events. Because there has been no public market for our common stock, our board of directors has determined the fair value of our common stock at the time of grant generally by looking to recent transactions including our common stock with third parties, as well as valuations when the grants were contemporaneous. The price of a share of our common stock in the transactions referred to were determined by a number of objective and subjective factors. See below for further discussion. |
| • | Expected Dividend Yield. The expected dividend was assumed to be zero as we have never paid dividends and have no current plans to do so in the foreseeable future. |
| • | Expected Stock Price Volatility. Since there has been no public market for our common stock and lack of company-specific historical volatility, we have determined the stock price volatility for options granted based on an analysis of the historical stock price volatility of a peer group of publicly traded biotechnology and pharmaceutical companies. In evaluating similarity, we considered factors such as industry, business model, stage of life cycle, and size. |
95
Table of Contents
| • | Risk-free Interest Rate. The risk-free interest rate is based on the U.S. Treasury yield in effect at the time of the grant for zero-coupon U.S. Treasury notes with remaining terms similar to the expected term of the options. |
| • | Expected Term. Since there is not sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term, we utilize the simplified method to estimate the life of our stock options. All stock options granted meet the criteria of plain vanilla awards, and therefore, the estimated term is a function of the vesting period and the contractual life of the award. |
| • | Expected Forfeiture Rate. Forfeiture rates are estimated based on historical and future expectations of employee turnover rates and are adjusted to reflect future changes in circumstances and facts, if any. Stock-based compensation expense is recorded net of estimated forfeitures such that expense is recorded only for those stock-based awards that are expected to vest. To the extent we revise these estimates in the future, the stock-based payments could be materially impacted in the period of revision, as well as in following periods. |
Valuation Approaches
Our board of directors has historically estimated the fair value of our common stock relying on the consummation of transactions with third parties. In addition to relying on the consummation of transactions with third parties, our Board may have considered many objective and subjective factors to determine the common stock fair market value at the valuation date. The following factors, among others, were considered:
| • | Our business strategy and stage of development; |
| • | Our financial condition and operating results, including our projected results; |
| • | The financial condition and operating results of publicly owned companies with similar lines of business and their historical volatility; |
| • | External market conditions that could affect companies in the life sciences sector; |
| • | The likelihood of a liquidity event such as an initial public offering, a merger, or the sale of our company; and |
| • | Passage of time relative to any stock transactions with third parties. |
The dates of our valuations have historically coincided with significant events, such as equity financings, and would therefore not always fall on the same dates as when options have been granted. However, we have historically granted the majority of our equity awards following one or more of such significant events. Therefore, our board of directors has historically used the valuation closest to the grant date of options granted in determining the exercise prices.
We considered multiple types of approaches in the preparation of our valuations as follows:
| • | Option-Pricing Method Backsolve, or OPM Backsolve. The OPM backsolve method derives the implied equity value for a company from a recent transaction involving the company’s own securities issued on an arm’s length basis. |
| • | Probability-Weighted Expected Return Method. Using the probability-weighted expected return, or PWERM, method, the value of a company’s common stock is estimated based upon the analysis of future values for the company assuming various possible future liquidity events including an initial public offering, or IPO, a sale, or a merger. Share value is based upon the probability-weighted present value of expected future net cash flows, considering each of the possible future events, as well as the rights and preferences of each share class. The PWERM is complex as it requires numerous assumptions relating to the potential future outcomes of equity. |
96
Table of Contents
| • | Hybrid Method. The hybrid method is a hybrid between the PWERM and OPM, estimating the probability-weighted value across multiple scenarios but using OPM to estimate the allocation of value within one or more of those scenarios. |
The key subjective factors and assumptions used in our valuations primarily consisted of (1) the implied value of a share of our common stock resulting from transactions consummated with unrelated, third parties, (2) the selection of the appropriate valuation model, (3) the financial forecasts utilized to determine future cash flow and necessary capital requirements, (4) the probability and timing of the various possible liquidity events, and (5) the discount for lack of marketability of our common stock.
Discussion of Specific Valuation Inputs
Over time, a combination of factors caused changes in the fair value of our common stock. The following summarizes the changes in value since January 1, 2015 and the major factors that caused each change:
July 2014 to March 2015. On June 20, 2014, we held a private placement offering of common stock. We issued 720,000 shares of common stock in exchange for $4.0 million ($5.50 per share) to a third party. Since the last valuation in February 2014, we received an allowance of our IND submission for Oratecan (oral formulation of irinotecan) from the FDA. Based on our valuation and the sale of common stock at arms-length to a third party, our board of directors determined the fair value of our common stock to be $5.50 per share.
April 2015 to June 2015. On April 17, 2015, we held a private placement offering of common stock. We issued 7,160,000 shares of common stock in exchange for $53.7 million ($7.50 per share) to various third parties. In negotiating the arm’s length sale of stock to a third party, we considered the development stage of our drug products, the overall economic condition, recent business acquisitions, and company forecasts. Since the last valuation in June 2014, we acquired the QuaDPharma business for $5.5 million. This acquisition improved our clinical production process, helped control our supply chain, provided a cGMP facility, and contributed to future cash flows through sales to their existing customers. During this period, we also executed our public-private partnership with the State of New York. Our clinical research team successfully completed a Phase I clinical study of KX-01 in the U.S. and we launched Phase I clinical studies of KX-02 and KX-01 Ointment. We also licensed the Orascovery Program (oral absorption platform) in India. In June 2015, we also acquired Polymed for $30.8 million. This acquisition has grown, and we expect it to continue to grow, our business globally, and it has contributed significantly to our forecasted cash flows with the introduction of API sales, and helped control our supply chain. All of these factors were considered in our board’s discounted cash flow, or DCF, valuation and drove the increase in the value of our stock. Based on our valuation and the sale of common stock at arms-length to unrelated third parties, our board of directors determined the fair value of our common stock to be $7.50 per share.
July 2015 to June 2016. In July 2015, we executed a stock-for-stock transaction to purchase a research and development company, CDE, that valued our common stock at $9.00 per share. We negotiated the consideration based on an independent third party’s risk-adjusted projection which was built on CDE’s projected revenue on a standalone basis. We also considered the development stage of the drug products, the overall economic condition and recent business acquisitions. All of these factors were considered and drove the increase in the value of common our stock. This reassessed stock price was used in the acquisition of CDE. Based on the implied valuation of our company in this transaction consummated with a third party, our board of directors determined the fair value of our common stock to be $9.00 per share. After that, there were no significant events impacting the business that would have warranted a significant change to fair value over this period.
July 2016 to March 2017. In September 2016, our board of directors determined the fair value of our common stock to be $11.00 per share. This determination was made by considering a combination of the
97
Table of Contents
backsolve valuation technique, which had yielded a fair value of $9.00 per share, and changes in our business. The changes in our business included, among other things: the receipt of an FDA allowance letter in January 2016 that allows us to proceed with our Oradoxel IND clinical study; evaluating data results from ongoing studies, including Oraxol and KX-01 ointment, in the third quarter of 2016; and the execution of a non-exclusive definitive license agreement in September 2016 to market 21 products (8 of which have been approved by the FDA). These changes resulted in the hiring of additional experienced pharmaceutical experts and the establishment of our Commercial Platform in the third quarter of 2016.
Stock Options Granted
The following table illustrates our stock option grant information since January 1, 2015:
| Grant Date |
Number of Shares Subject to Options Granted |
Option Exercise Price |
Estimated Fair Value of Common Stock per Share at Grant Date |
Intrinsic Value per Underlying Share at Grant Date |
||||||||||||
| February 5, 2015 |
162,000 | $ | 5.50 | $ | 5.50 | $ | — | |||||||||
| March 20, 2015 |
366,000 | $ | 5.50 | $ | 5.50 | $ | — | |||||||||
| May 22, 2015 |
3,488,000 | $ | 7.50 | $ | 7.50 | $ | — | |||||||||
| June 11, 2015 |
496,392 | $ | 7.50 | $ | 7.50 | $ | — | |||||||||
| July 26, 2015 |
304,536 | $ | 9.00 | $ | 9.00 | $ | — | |||||||||
| October 17, 2015 |
211,400 | $ | 9.00 | $ | 9.00 | $ | — | |||||||||
| February 28, 2016 |
247,500 | $ | 9.00 | $ | 9.00 | $ | — | |||||||||
| April 18, 2016 |
125,000 | $ | 9.00 | $ | 9.00 | $ | — | |||||||||
| June 19, 2016 |
465,800 | $ | 9.00 | $ | 9.00 | $ | — | |||||||||
| July 1, 2016 |
80,000 | $ | 9.00 | $ | 9.00 | $ | — | |||||||||
| September 18, 2016 |
22,000 | $ | 11.00 | $ | 11.00 | $ | — | |||||||||
| October 17, 2016 |
10,000 | $ | 11.00 | $ | 11.00 | $ | — | |||||||||
| December 31, 2016 |
159,100 | $ | 11.00 | $ | 11.00 | $ | — | |||||||||
| January 3, 2017 |
15,500 | $ | 11.00 | $ | 11.00 | $ | — | |||||||||
| January 30, 2017 |
500 | $ | 11.00 | $ | 11.00 | $ | — | |||||||||
The intrinsic value of all outstanding vested and unvested options as of March 31, 2017 was $43.7 million based on a stock price fair value of $11.00 per share and based on 9,214,989 shares of common stock issuable upon the exercise of options outstanding as of March 31, 2017 with a weighted-average exercise price of $6.25 per share.
Business Acquisitions, Intangible Assets, Goodwill, and Contingent Consideration
We account for acquired businesses using the purchase method of accounting, which requires that the assets acquired and liabilities assumed be recorded at the date of acquisition at their respective estimated fair values. The cost to acquire businesses has been allocated to the underlying net assets of the acquired businesses based on estimates of their respective fair values. Total consideration for the three acquisitions was $5.5 million for QuaDPharma, $30.8 million for Polymed and $14.9 million for CDE. Any excess of the purchase price over the estimated fair values of the net assets acquired is recorded as goodwill. The amount of goodwill recorded was $4.6 million for QuaDPharma, $22.2 million for Polymed and $11.4 million for CDE.
The judgments made in determining the estimated fair value assigned to each class of assets acquired and liabilities assumed, as well as asset lives, can materially impact our results of operations. The fair values of intangible assets are determined using one of three valuation approaches: market, income, or cost. The selection of a particular method depends on the reliability of the available data and the nature of the asset. The market approach values the asset based on available market pricing for comparable assets. The income approach values the asset based on the present value of risk adjusted cash flows projected to be generated by the asset. The cost approach values the asset by determining the current cost of replacing that asset with another asset of equivalent
98
Table of Contents
economic utility. Because this process involves management making estimates with respect to future sales volumes, pricing, new product launches, government reform actions, anticipated cost environment and overall market conditions, and because these estimates form the basis for the determination of whether or not an impairment charge should be recorded, these estimates are considered to be critical accounting estimates. Definite-lived intangible assets are amortized over the expected life of the asset. If the carrying value of the indefinite-lived intangible asset exceeds management’s estimate of fair value or the projects have been abandoned, the asset is impaired, and we would record an impairment charge accordingly.
Goodwill is recorded when the purchase price of an acquisition exceeds the fair value of the net tangible and identified intangible assets acquired. Goodwill is allocated to our reporting units based on the relative expected fair value provided by the acquisition. Reporting units may be operating segments as a whole or an operation one level below an operating segment, referred to as a component, or a combination thereof.
We perform an annual impairment assessment on October 1, or more frequently if indicators of potential impairment exist, to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying value. Impairment test approaches with a weighting of the Discounted Cash Flow (income approach) and Guideline Public Company method (market approach) are utilized. Weighting will most likely be greater for the market approach given the lack of historical results to be able to rely significantly on financial projections. The performance of the goodwill impairment test involves a two-step process. The first step is to estimate the fair value of each reporting unit and compare the fair value to the carrying value. For reporting units in which the step-one impairment assessment concludes that it is more likely than not that the fair value of the reporting unit exceeds the carrying value of the net assets assigned to that reporting unit, goodwill is not considered to be impaired and we do not perform additional analysis. For reporting units in which the step-one impairment assessment concludes that it is more likely than not that the carrying value of the net assets assigned to that reporting unit exceeds the fair value of the reporting unit, we must perform the second step, which is to measure the amount of impairment. We then record the impairment loss equal to the difference between the fair value and the carrying value. None of our reporting units are at risk of failing step one of the impairment test, as the fair value is substantially in excess of the carrying value for each reporting unit.
We record contingent consideration resulting from a business acquisition at its estimated fair value on the acquisition date. Each reporting period thereafter, we revalue these obligations and record increases or decreases in their fair value as an adjustment to contingent consideration recorded within selling, general and administrative expenses within the consolidated statements of operations and comprehensive loss. Changes in the fair value of the contingent consideration obligations can result from adjustments to the discount rates, payment periods, and adjustments in the probability of achieving future development steps, regulatory approvals, market launches, sales targets, and profitability.
Significant judgment is employed in determining the assumptions utilized as of the acquisition date and for each subsequent measurement period. Accordingly, changes in assumptions described above could have a material impact on our consolidated results of operations.
99
Table of Contents
Results of Operations
The following table sets forth a summary of our unaudited condensed consolidated results of operations for the three months ended March 31, 2016 and 2017, together with the changes in those items in dollars and percentages. This information should be read together with our consolidated financial statements and related notes included elsewhere in this prospectus. Our operating results in any period are not necessarily indicative of the results that may be expected for any future period.
| Three Months Ended March 31, | ||||||||||||||||
| 2016 | 2017 | Change | ||||||||||||||
| (in thousands) | (in thousands) | (in thousands) | % | |||||||||||||
| Revenue |
$ | 4,629 | $ | 4,581 | $ | (48 | ) | (1 | )% | |||||||
| Cost of sales |
(4,142 | ) | (2,839 | ) | 1,303 | (31 | ) | |||||||||
| Research and development expenses |
(6,746 | ) | (26,408 | ) | (19,662 | ) | 291 | |||||||||
| Selling, general and administrative expense |
(4,337 | ) | (9,799 | ) | (5,462 | ) | 126 | |||||||||
| Interest income (expense) |
46 | (2,376 | ) | (2,422 | ) | NM | ||||||||||
| Unrealized loss on derivative liability |
— | (4,276 | ) | (4,276 | ) | NM | ||||||||||
| Income tax (expense) benefit |
(100 | ) | 92 | 192 | (192 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
(10,650 | ) | (41,025 | ) | (30,375 | ) | 278 | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Less: net loss attributable to non-controlling interests |
(32 | ) | (37 | ) | (5 | ) | NM | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss attributable to Athenex, Inc. |
$ | (10,618 | ) | $ | (40,988 | ) | $ | (30,370 | ) | |||||||
|
|
|
|
|
|
|
|||||||||||
Three Months Ended March 31, 2016 Compared to Three Months Ended March 31, 2017
Revenue
Revenue remained stable at $4.6 million in the three months ended March 31, 2017, compared to $4.6 million in the three months ended March 31, 2016. These results in the three months ended March 31, 2017 included a decrease in API sales of $1.0 million compared to the same period in the prior year, offset by an increase in contract manufacturing revenue and license and consulting fees of $0.3 million and $0.5 million, respectively.
Cost of Sales
Our cost of sales decreased by $1.3 million in the three months ended March 31, 2017 compared to the three months ended March 31, 2016. This decrease was primarily caused by the corresponding decrease in API sales, which experienced low gross margins in the first quarter of 2017.
Research and Development Expenses
Our research and development expenses increased by $19.7 million to $26.4 million in the three months ended March 31, 2017, from $6.7 million in the three months ended March 31, 2016, primarily due to increased licensing fees and increased payments for our proprietary drug programs, and included the following:
| • | $15.0 million increase in licensing fees related to three licensing agreements; |
| • | $3.9 million increase in costs of clinical studies; and |
| • | $1.0 million increase in R&D expenses. |
The above increases in research and development expenses were offset by $0.5 million of decreases in stock-based compensation expense related to research and development personnel.
100
Table of Contents
Selling, General and Administrative Expenses
Our selling, general and administrative expenses increased by $5.5 million to $9.8 million in the three months ended March 31, 2017, from $4.3 million in the three months ended March 31, 2016, primarily due to the establishment of our Commercial Platform in the third quarter of 2016, and included the following:
| • | $3.6 million increase in employee compensation expenses, primarily in our Commercial Platform; and |
| • | $1.4 million increase in bad debt expense, professional fees and foreign currency transaction loss. |
Year Ended December 31, 2016 Compared to Year Ended December 31, 2015
The following table sets forth a summary of our consolidated results of operations for the years ended December 31, 2016 and 2015, together with the changes in those items in dollars and percentage. This information should be read together with our consolidated financial statements and related notes included elsewhere in this prospectus. Our operating results in any period are not necessarily indicative of the results that may be expected for any future period.
| Year Ended December 31, | ||||||||||||||||
| 2015 | 2016 | Change | ||||||||||||||
| (in thousands) | (in thousands) | (in thousands) | % | |||||||||||||
| Revenue |
$ | 13,944 | $ | 20,551 | $ | 6,607 | 47 | |||||||||
| Cost of sales |
(13,153 | ) | (19,718 | ) | (6,565 | ) | 50 | |||||||||
| Research and development expenses |
(24,463 | ) | (60,624 | ) | (36,161 | ) | 148 | |||||||||
| Selling, general, and administrative expenses |
(27,036 | ) | (25,956 | ) | 1,080 | (4 | ) | |||||||||
| Interest expense |
(1 | ) | (1,891 | ) | (1,890 | ) | NM | |||||||||
| Unrealized loss on derivative liability |
— | (533 | ) | (533 | ) | — | ||||||||||
| Income tax benefit |
54 | 265 | 211 | 391 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
(50,655 | ) | (87,906 | ) | (37,251 | ) | 74 | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Less: net loss attributable to non-controlling interests |
(55 | ) | (191 | ) | (136 | ) | 247 | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss attributable to Athenex, Inc. |
$ | (50,600 | ) | $ | (87,715 | ) | $ | (37,115 | ) | |||||||
|
|
|
|
|
|
|
|||||||||||
Revenue
Our revenue increased by $6.6 million, or 47%, from the year ended December 31, 2015 to the year ended December 31, 2016, primarily due to an increase from sales primarily of API, of $6.5 million, a portion of which increase was due to the inclusion of Polymed in our consolidated financial statements for the full twelve months of activity in 2016 compared to only seven months of activity in 2015.
Cost of Sales
Our cost of sales similarly increased as a result of the June 1, 2015 acquisition of Polymed. Polymed’s cost of sales in 2016 was $14.2 million compared to the seven-month cost of sales of $9.2 million included in the consolidated operating results for the year ended December 31, 2015, a $5.0 million, or 54%, increase. Also, cost of sales at QuaDPharma increased by $1.8 million as a result of increased costs to support the internal production of clinical supplies.
Research and Development Expenses
Our research and development expenses increased by $36.2 million, or 148%, to $60.6 million in the year ended December 31, 2016 from $24.5 million in the year ended December 31, 2015, primarily due to the advancement of our clinical and preclinical pipeline, and included the following:
| • | $17.7 million increase as a result of the increased costs of drug licensing; |
| • | $12.9 million increase in employee compensation expenses, including wages and benefits, as well as stock-based compensation, primarily attributable to increased headcount during 2016; |
101
Table of Contents
| • | $7.4 million increase in costs of clinical studies, primarily for Oraxol and KX-01 ointment; and |
| • | $0.5 million increase in the office related costs. |
The increases in research and development expenses were offset by $2.3 million of decreases in preclinical studies costs related to drugs that entered into clinical study phases.
Selling, General and Administrative Expenses
Our selling, general and administrative expenses decreased by $1.1 million, or 4%, from $27.0 million in the year ended December 31, 2015 to $26.0 million in the year ended December 31, 2016 primarily due to a decrease of expenses on professional services, and included the following:
| • | $3.8 million decrease in professional fees, which included a decrease of $2.0 million of accounting, legal, and consulting fees associated with our public-private partnerships, a decrease of $1.1 million of legal fees for certain litigation settlement, and a decrease of $0.7 million of business acquisition related costs; and |
| • | This was partially offset by a $1.2 million increase of employee compensation, including wages and benefits, as well as stock-based compensation, primarily attributable to increased headcount, a $0.8 million increase in office related costs, and a $0.7 million increase in loss on disposal of long-lived assets. |
Internal Control over Financial Reporting
In connection with the audit of our consolidated financial statements as of and for the year ended December 31, 2015, we and our independent registered public accounting firm identified three material weaknesses in our internal control over financial reporting. As defined in the standards established by the PCAOB, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis.
The material weaknesses that had been identified related to (1) thoroughness of review and determination of appropriate accounting treatment for complex, non-routine transactions, including consideration of fair value concepts, notably for purchase price allocation and accounting for stock options; (2) the precision of review and application of available information through retrospective review to record accounting estimates accurately based on known, available information; and (3) the precision of review and evaluation of capitalization policies to ensure amounts capitalized as assets relate to items for which an identifiable benefit has been received and will be realizable. The weaknesses described contributed to certain material adjustments that were required in 2015.
We have implemented a number of measures to address these material weaknesses that had been identified in 2015 and remediated such weaknesses as of December 31, 2016. We have hired additional financial and accounting staff with appropriate U.S. GAAP and SEC reporting experience, and we have allocated additional resources to improve financial control functions, to introduce formal business performance review processes, and to prepare and review the consolidated financial statements and related disclosures in accordance with U.S. GAAP and SEC reporting requirements.
We, and our independent registered public accounting firm, were not required to perform an evaluation of our internal control over financial reporting as of December 31, 2016 in accordance with the provisions of the Sarbanes-Oxley Act. Accordingly, we cannot assure you that we have identified all, or that we will not in the future have additional, material weaknesses. Material weaknesses may still exist when we report on the effectiveness of our internal control over financial reporting as required by reporting requirements under Section 404 of the Sarbanes-Oxley Act after the completion of this offering.
Liquidity and Capital Resources
Since our inception, we have incurred net losses and negative cash flows from our operations. Substantially all of our losses have resulted from funding our research and development programs and selling, general and
102
Table of Contents
administrative costs associated with our operations. We incurred net losses of $50.7 million and $87.9 million for the years ended December 31, 2015 and 2016, respectively and $10.7 million and $41.0 million for the three months ended March 31, 2016 and 2017, respectively. As of March 31, 2017, we had an accumulated deficit of $236.1 million. Our primary use of cash is to fund research and development costs. Our operating activities used $33.8 million and $47.9 million of cash during the years ended December 2015 and 2016, respectively and $7.6 million and $20.8 million for the three months ended March 31, 2016 and 2017, respectively. As of March 31, 2017, we had cash and cash equivalents of $26.0 million and marketable securities of $3.1 million.
Historically, we have financed our operations principally through proceeds from private placements of equity and debt securities; to a much lesser extent, cash generated from operations, primarily from the collection of accounts receivable resulting from sales by our Global Supply Chain Platform. However, the revenue generated might not be sufficient to meet the demand of their operating costs. Therefore, we will continue to require substantial additional capital beyond the expected proceeds from this offering to continue our research and development and commercialization activities.
Cash kept in our subsidiaries in China is subject to PRC regulations restricting transfer of funds overseas. Thus, our PRC subsidiaries are restricted in their ability to transfer their net assets to us as cash dividends, loans or advances. As of March 31, 2017, we had cash and cash equivalents of approximately $4.5 million at our Chinese subsidiaries. Although we do not currently require any such dividends, loans or advances from our PRC subsidiaries to fund our operations, should we require additional sources of liquidity in the future, such restrictions may have a material adverse effect on our liquidity and capital resources. The following table shows a summary of our cash flows:
| Year Ended December 31, |
Three Months Ended March 31, |
|||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (in thousands) | ||||||||||||||||
| Net cash (used in) operating activities |
$ | (33,756 | ) | $ | (47,870 | ) | $ | (7,556 | ) | $ | (20,753 | ) | ||||
| Net cash (used in) provided by investing activities |
(16,909 | ) | 2,659 | (755 | ) | 4,023 | ||||||||||
| Net cash provided by financing activities |
76,302 | 35,272 | 3,084 | 9,201 | ||||||||||||
| Net effect of foreign exchange rate changes |
337 | (431 | ) | 39 | 438 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net increase (decrease) in cash and cash equivalents |
$ | 25,974 | $ | (10,370 | ) | $ | (5,188 | ) | $ | (7,091 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
Net Cash (Used in) Operating Activities
The use of cash in all periods presented resulted primarily from our net losses adjusted for non-cash charges and changes in components of working capital. The primary use of our cash in all periods presented was to fund our research and development, regulatory and other clinical trial costs, and related supporting administration. Our prepaid expenses and other current assets, accounts payable and accrued expense balances in all periods presented were affected by the timing of vendor invoicing and payments.
During the three months ended March 31, 2017, operating activities used $20.8 million of cash, which resulted principally from our net loss of $41.0 million, adjusted for non-cash charges of $15.7 million, and by cash provided from our operating assets and liabilities of $4.7 million partially offset by $0.1 million charge in deferred income taxes. Our net non-cash charges during the three months ended March 31, 2017 primarily consisted of $0.8 million depreciation and amortization expense, $2.1 million of stock-based compensation expense, $4.3 million unrealized loss on derivative liability, $1.2 million amortization of a debt discount, and $7.0 million of license fees settled through the issuance of a convertible bond.
During the three months ended March 31, 2016, operating activities used $7.6 million of cash, which resulted principally from our net loss of $10.7 million, adjusted for non-cash charges of $3.5 million, offset by cash used in our operating assets and liabilities of $0.5 million. Our non-cash charges during the three months ended March 31, 2016 primarily consisted of $0.4 million depreciation and amortization expense and $2.9 million of stock-based compensation expense.
103
Table of Contents
During the year ended December 31, 2016, operating activities used $47.9 million of cash, which resulted principally from our net loss of $87.9 million, adjusted for non-cash charges of $24.7 million. This was partially offset by $0.5 million change in deferred income taxes, and by cash provided from our operating assets and liabilities of $15.8 million. Our net non-cash charges during the year ended December 31, 2016 primarily consisted of $2.0 million depreciation and amortization expense, $19.5 million of stock-based compensation expense, and $1.0 million of loss on disposal of assets and impairment charges.
During the year ended December 31, 2015, our operating activities used $33.8 million of cash, which resulted principally from our net loss of $50.7 million, adjusted for non-cash charges of $16.9 million and offset by $0.3 million change in deferred income taxes, and by cash provided from our operating assets and liabilities of $0.2 million. Our net non-cash charges during the year ended December 31, 2015 primarily consisted of $0.9 million of depreciation and amortization expense, $15.5 million of stock-based compensation expense, and a $0.5 million increase from changes in fair value of contingent consideration.
Net Cash (Used in) Provided by Investing Activities
Net cash provided by investing activities was $4.0 million for the three months ended March 31, 2017 compared to $0.8 million used in investing activities for the three months ended March 31, 2016. The increase in cash from investing activities was primarily due to cash provided by the sale of marketable securities.
Net cash from investing activities was $2.7 million for the year ended December 31, 2016, compared to $16.9 million cash used in investing activities for the year ended December 31, 2015. The difference in cash from investing activities was primarily due to a net increase of $8.7 million in cash received from sales of marketable securities and a decrease in acquisition-related net cash payments of $9.4 million.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was $9.2 million for the three months ended March 31, 2017 compared to $3.1 million cash provided by financing activities for the three months ended March 31, 2016. The change was primarily attributable to the proceeds from the issuance of convertible bonds for $10.0 million in the three months ended March 31, 2017.
Net cash provided by financing activities was $35.3 million for the year ended December 31, 2016 compared to $76.3 million cash provided by financing activities for the year ended December 31, 2015. The decrease was primarily due to fewer sales of securities in the year ended December 31, 2016 compared to the year ended December 31, 2015.
Indebtedness
We had $41.8 million and $64.0 million of debt as of December 31, 2016 and March 31, 2017, respectively. This consisted of three seller promissory notes that were negotiated as part of the Polymed acquisition, a mortgage under CDE, and convertible loan agreements entered into in 2016 and the first quarter of 2017.
The Polymed promissory notes have a 36 month maturity beginning on July 1, 2015 and ending on June 1, 2018 with a 6% stated interest rate. The outstanding principal on the Polymed promissory notes was $1.6 million and $1.3 million as of December 31, 2016 and March 31, 2017, respectively.
In connection with the acquisition of CDE, we assumed a mortgage liability associated with the manufacturing plant asset in the PRC. The mortgage payments extend through July 30, 2017. The remaining mortgage principal payment of $0.8 million is due in 2017.
In September and October 2016 and January and April 2017, we executed convertible loan financing agreements with existing and new investors, approximately $38.0 million of which were entered into prior to
104
Table of Contents
December 31, 2016 and an additional $30.0 million of which were issued thereafter. The loans will automatically and mandatorily be converted into a number of shares of common stock equal to the outstanding amount of the convertible loans divided by the discount prices (which is variable based on the actual date of the event). The loans issued have a maturity date of October 1, 2018, except for the April 2017 loans, which have a maturity date of April 20, 2019. The stated interest rate on the loans is 10% per annum. If the convertible loans have not been converted into common stock prior to the maturity date, we must pay full interest from the date of the loan advancement to the date of repayment. The outstanding convertible loans will be converted into shares of our common stock upon the completion of this offering, assuming an initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus.
Additionally in March 2017, in connection with an amendment to a licensing agreement, we issued a $7.0 million convertible bond to Hanmi in lieu of an upfront payment. At the election of Hanmi, the convertible bond will be converted into a number of shares of common stock equal to the outstanding amount of the convertible bond divided by the discount price (which is variable based on the date of conversion) at certain specified dates. If Hanmi has not elected to convert the convertible bond to common stock prior to October 1, 2018, Hanmi may elect to convert the convertible bond to: (i) common stock equal to the outstanding amount of the convertible bond divided by the discount price, or (ii) cash equal to $7.0 million plus interest at 10% per annum accruing from March 7, 2017 to October 1, 2018.
Servicing our convertible notes may require a significant amount of cash, and we may not have sufficient cash flow or the ability to raise the funds necessary to satisfy our obligations under such notes, and our future debt may contain limitations on our ability to pay cash upon conversion or repurchase of such notes.
Capital Expenditures
Our liquidity position and capital requirements are subject to a number of factors. For example, our cash inflow and outflow may be impacted by the following:
| • | Our ability to generate revenue; and |
| • | Fluctuations in working capital. |
Our primary short term capital needs, which are subject to change, include expenditures related to:
| • | Continuous support of the development and research of our proprietary drug products; |
| • | Build out of our new API plant in China and improvements in our existing manufacturing capacity and efficiency; |
| • | New research and product development efforts; and |
| • | Support of our commercialization efforts related to our current and future products. |
Although we believe the foregoing items reflect our most likely uses of cash in the short term, we cannot predict with certainty all of our short-term cash uses or the timing or amounts of cash used. If cash generated from operations is insufficient to satisfy our working capital and capital expenditure requirements, we may be required to sell additional equity or debt securities or obtain credit financing. This capital may not be available on satisfactory terms, if at all. Furthermore, any additional equity financing may be dilutive to our stockholders, and debt financing, if available, may include restrictive covenants.
In 2015, we entered into two public-private partnerships. New York State is investing in a 315,000 square foot, ISO Class 5 high potency oral and sterile injectable pharmaceutical manufacturing facility, which will be built in Dunkirk, New York. We have agreed to utilize this facility to manufacture our proprietary products upon approval of the drugs and completion of the facility. The estimated cost of the facility will be approximately $200 million, and we will be able to occupy the space on concessionary terms. In Chongqing, China, funded by
105
Table of Contents
the Banan District government, a GMP API and a GMP pharmaceutical manufacturing plant will be built, which we will occupy on concessionary terms. We plan to utilize these plants to manufacture API and the finished drugs in which these API will be used. We do not have significant construction period risks. New York State and the Banan District government will fund a majority of the construction costs and hold ownership of the manufacturing and office facilities.
Future Capital Requirements
Our independent registered public accounting firm included an explanatory paragraph in its report on our consolidated financial statements as of and for the year ended December 31, 2016, noting the existence of substantial doubt about our ability to continue as a going concern. This uncertainty arose from management’s review of our results of operations and financial condition and its conclusion that, based on our operating plans, we did not have sufficient existing working capital to sustain operations for a period of one year from the issuance of our consolidated financial statements as of and for the year ended December 31, 2016. We believe that we will be able to obtain additional working capital through equity financings or other arrangements, including this offering, to fund our current operating plans through at least the next 12 months. To the extent that we raise additional capital through future equity financings, the ownership interest of our stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our existing common stockholders. If we raise additional funds through the issuance of debt securities, these securities could contain covenants that would restrict our operations. There can be no assurance that such additional financing, if available, can be obtained on terms acceptable to us. If we are unable to obtain such additional financing, we would need to reevaluate our future operating plans.
Contractual Obligations
A summary of our contractual obligations as of December 31, 2016 is as follows:
| Payments Due by Period | ||||||||||||||||||||
| Less than 1 year |
1 to 3 years | 3 to 5 years | More than 5 years |
Total Amounts Committed |
||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Operating leases |
$ | 801 | $ | 2,674 | $ | 3,043 | $ | 5,959 | $ | 12,477 | ||||||||||
| Long-term debt |
766 | — | — | — | 766 | |||||||||||||||
| Long-term debt—related parties |
1,123 | 496 | — | — | 1,619 | |||||||||||||||
| Convertible bonds |
— | 38,000 | — | — | 38,000 | |||||||||||||||
| Licensing fees |
12,988 | — | — | — | 12,988 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| $ | 15,678 | $ | 41,170 | $ | 3,043 | $ | 5,959 | $ | 65,850 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The operating leases include (1) the rental of our global headquarters in the Conventus Center for Collaborative Medicine in Buffalo, NY and (2) the rental of our research and development facility in the IC Development Centre in Hong Kong and (3) the rental of the Commercial Platform headquarters in Chicago, IL. Of the total amounts committed, $9.7 million are committed for the rental of our Buffalo, NY global headquarters, $0.5 million are committed for our Hong Kong research and development facility, and $2.3 million are committed for our Commercial Platform headquarters.
Off Balance Sheet Arrangements
We do not maintain any off balance sheet partnerships, arrangements, or other relationships with unconsolidated entities or others, often referred to as structured finance or special purpose entities, which are established for the purpose of facilitating off balance sheet arrangements or other contractually narrow or limited purposes.
106
Table of Contents
Quantitative and Qualitative Disclosures about Market Risk
Foreign Currency Exchange Risk
A significant portion of our business is located outside the United States and, as a result, we generate revenue and incur expenses denominated in currencies other that the U.S. dollar, a majority of which is denominated in Renminbi. In 2015 and 2016, approximately 13% and 7%, respectively, of our sales, excluding intercompany sales, were denominated in foreign currencies. As a result, our revenue can be significantly impacted by fluctuations in foreign currency exchange rates. We expect that foreign currencies will represent a lower percentage of our sales in the future due to the anticipated growth of our U.S. business. Our international selling, marketing, and administrative costs related to these sales are largely denominated in the same foreign currencies, which somewhat mitigates our foreign currency exchange risk rate exposure.
Interest Rate Risk
We are exposed to market risks in the ordinary course of our business. Our cash and cash equivalents include cash in readily available checking and money market accounts. These securities are not dependent on interest rate fluctuations that may cause the principal amount of these assets to fluctuate.
Our primary exposure to market risk relates to fluctuations in the interest rates which are affected by changes in the general level of PRC and U.S. interest rates. Given the short-term nature of our cash equivalents, we believe that a sudden change in market interest rates would not be expected to have a material impact on our financial condition and/or results of operation. While we believe our cash and cash equivalents do not contain excessive risk, we cannot provide assurance that in the future investments will not subject to adverse changes in market value.
Credit Risk
We had cash and cash equivalents of $43.5 million and $33.1 million and marketable securities of $14.1 million and $8.6 million at December 31, 2015 and 2016, respectively, and cash and cash equivalents of $26.0 million and marketable securities of $3.1 million at March 31, 2017. Substantially all of our bank deposits are in major financial institutions, which we believe are of high credit quality. The primary objectives of our investment activities are to preserve principle, provide liquidity and maximize income without significant increasing risk.
We make periodic assessments of the recoverability of trade and other receivables and amounts due from related parties. Our historical experience in collection of receivables falls within the recorded allowances, and we believe that we have made adequate provision for uncollectible receivables.
Inflation
Inflationary factors, such as increases in our cost of sales and SG&A expenses, may adversely affect our operating results. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, a high rate of inflation in the future may have an adverse effect on our ability to maintain and increase our net income and SG&A expenses as a percentage of our revenue if the selling prices of our products do not increase as much or more than these increased costs.
Jumpstart Our Business Startups Act of 2012 (JOBS Act)
In April 2012, the JOBS Act was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. Thus, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
107
Table of Contents
We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements under the JOBS Act. Subject to certain conditions, as an emerging growth company, we may rely on certain of these exemptions, including without limitation, (i) providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (ii) complying with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We will remain an emerging growth company until the earlier of (a) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (b) the last day of the fiscal year following the fifth anniversary of the date of the completion of this offering; (c) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (d) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission.
Recent Accounting Pronouncements
Recent Accounting Pronouncements Not Yet Adopted
In May 2014, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update, or ASU, No. 2014-09, “Revenue from Contracts with Customers (Topic 606)”, which requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. The ASU will replace most existing revenue recognition guidance in U.S. GAAP when it becomes effective. The new standard is effective for the Company on January 1, 2018 with early adoption permitted only for annual reporting periods beginning after December 15, 2016. The standard permits the use of either the retrospective or cumulative effect transition method. The Company’s evaluation of the effects of ASU 2014-09 and the selection of a transition method is ongoing and not yet complete. The Company anticipates that the standard may impact the accounting related to its out-license agreements, however, such agreements are not currently significant to the consolidated financial statements.
In February 2016, the FASB issued ASU No. 2016-02, “Leases (Topic 842)” which requires that lessees distinguish between finance and operating leases and recognize the assets and liabilities that arise from the leases on the company’s balance sheet. This ASU is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years, and is required to be applied on a modified retrospective basis. The Company is evaluating the effect of this standard on its consolidated financial statements.
In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments,” which modifies the measurement of expected credit losses of certain financial instruments. ASU 2016-13 is effective for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years. The Company is evaluating the effect of this standard on its consolidated financial statements.
In November 2016, the FASB issued ASU 2016-18, “Statement of Cash Flows (Topic 230): Restricted Cash. The primary purpose of this ASU is to reduce the diversity in practice that exists in the classification and presentation of changes in restricted cash on the statement of cash flows. This ASU will require that a statement of cash flows explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash or cash equivalents. Therefore, amounts generally described as restricted cash and restricted cash equivalents should be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. This ASU is effective for fiscal years beginning after December 15, 2017. This ASU is required to be applied retrospectively. Early adoption is permitted, including adoption in an interim period. The Company is evaluating the effect of this standard on its consolidated financial statements.
108
Table of Contents
Recently Adopted Accounting Pronouncements
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern” which requires management to assess if there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures in certain circumstances. In connection with each annual and interim period, management must assess if there is substantial doubt about an entity’s ability to continue as a going concern within one year after the financial statement issuance date. Disclosures are required if conditions give rise to substantial doubt. The amendment is effective for the first annual period ending after December 15, 2016 with early adoption permitted for annual or interim reporting periods for which the financial statements have not previously been issued. The Company adopted the guidance for the year ended December 31, 2016. The adoption resulted in expanded disclosure of the principal conditions that raise substantial doubt, management’s evaluation of those conditions, and management’s plans to mitigate these conditions.
In July 2015, the FASB issued ASU No. 2015-11, “Inventory (Topic 330): Simplifying the Measurement of Inventory.” This ASU requires inventory to be measured at the lower of cost or net realizable value. The provisions of this ASU are effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. The amendment is required to be applied prospectively, and early adoption is permitted. The Company adopted ASU 2015-11 effective January 1, 2017. Adoption of ASU 2015-11 did not have a significant impact on the consolidated financial statements.
In March 2016, the FASB issued ASU No. 2016-09, “Compensation—Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting” which changes how companies account for certain aspects of stock-based awards to employees, including the accounting for income taxes, forfeitures, and statutory tax withholding requirements, as well as the classification in the statement of cash flows. Effective January 1, 2017, the Company adopted ASU 2016-09. The standard eliminated the requirement to defer recognition of excess tax benefits related to employee share-based awards until they are realized through a reduction to income taxes payable. The Company applied the modified retrospective method and there was no net cumulative-effect adjustment to retained earnings on January 1, 2017 as the increase in deferred income tax assets for previously unrecognized excess tax benefits was fully offset by a valuation allowance. As permitted by the ASU, the Company will continue to use an estimated forfeiture rate in determining stock-based compensation expense.
In August 2016, the FASB issued ASU No. 2016-15, “Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments,” which addresses the classification of certain cash transactions on the statement of cash flows. ASU 2016-15 is effective for fiscal years beginning after December 15, 2018, and interim periods within fiscal years beginning after December 15, 2019. The Company early adopted ASU 2016-15 as of January 1, 2016 and applied its provisions retrospectively through the earliest period presented, which did not have a significant impact on its consolidated financial statements.
In January 2017, the FASB issued ASU 2017-04, “Intangibles—Goodwill and Other (Topic 350) Simplifying the Test for Goodwill Impairment.” The primary purpose of the ASU is to simplify the subsequent measurement of goodwill by eliminating Step 2 from the goodwill impairment test. The ASU also applies the same test of goodwill to all reporting units, now including those with a zero or negative carrying amount of net assets. This ASU is required to be adopted on a prospective basis and is effective for any goodwill impairment tests in fiscal years beginning after December 15, 2019, although early adoption is permitted for any impairment tests performed after January 1, 2017. The Company has adopted the new guidance on a prospective basis during the first quarter of 2017. The adoption of this ASU has not impacted the Company’s consolidated financial statements.
109
Table of Contents
Cancer is a broad group of diseases in which cells divide and grow in an uncontrolled fashion, forming malignancies that can invade other parts of the body. In normal tissues, the rates of new cell growth and cell death are tightly regulated and kept in balance. In cancerous tissues, however, this balance is disrupted as a result of mutations, causing unregulated cell growth that leads to tumor formation and growth. While tumors can grow at different paces, the dividing cells will nevertheless accumulate and the normal organization of the tissue will become disrupted. Cancers can subsequently spread throughout the body by processes known as invasion and metastasis. Once cancer spreads to sites beyond the primary tumor, it becomes less curable.
Cancer is the second leading cause of death in high-income countries (following cardiovascular diseases) and the third leading cause of death in low- and middle-income countries (following cardiovascular diseases and infectious and parasitic diseases). The most common risk factors for cancer include aging, family history, alcohol and tobacco use, sun and radiation exposure, poor diet and lack of physical activity. In 2012, there were an estimated 14.1 million new cases and 8.2 million cancer-related deaths worldwide. With a growing and aging global population, the annual incidence is expected to grow to 21.7 million by 2030.
Cancer statistics in the United States
An estimated 1.7 million new cases of cancer are expected to be diagnosed and 0.6 million people will die from cancer in the U.S. in 2016, according to the National Cancer Institute. Prostate and breast cancer are the leading cancer types in the U.S. representing 21% and 29% of total new cancer cases in men and women, respectively. The economic burden of cancer remains high for both cancer patients and society as a whole. The Agency for Healthcare Research and Quality estimates that the direct medical costs for cancer totaled $88.7 billion in 2011, of which 50% were for hospital outpatient or doctor office visits, 35% for inpatient hospital stays and 11% for prescription drugs. The cost of cancer care has grown quickly due in part to high prices on novel therapeutics creating an increased focus on development of cost effective treatment regimens. According to the IMS Institute for Healthcare Informatics, 60% of the increase in U.S. oncology costs from 2010–2015 can be attributed to new therapies launched since 2010. Though the annualized mean cost of care varies greatly between cancer types, the cost of care is highest in the last year of life.
Cancer statistics in China
China represents a significant opportunity, forecasted to be the second largest pharmaceutical market in the world in 2017, including traditional and modern medications. According to the China Pharmaceuticals & Healthcare Report published in July 2016, the Chinese pharmaceutical market was forecasted to grow strongly from $109 billion in 2015, and is expected to grow at a compound annual growth rate, or CAGR, of 7.7% over the next five years reaching $158 billion by 2020.
China provides an opportunity to access largely untapped clinical trial pools and develop drugs for a population for whom global standard therapies are not available. Because of China’s large population size, representing approximately one-fifth of the world population and, according to data from the World Health Organization and Globocan, in 2012 China accounted for approximately 22% of global new cancer cases. The most common cancers in China include lung, stomach, liver and esophageal cancer and account for over half of all types of cancer diagnosed.
110
Table of Contents
The following table illustrates the estimated number of incident cases for the top cancer sites:
2012 incidences by cancer types in China and worldwide (millions)
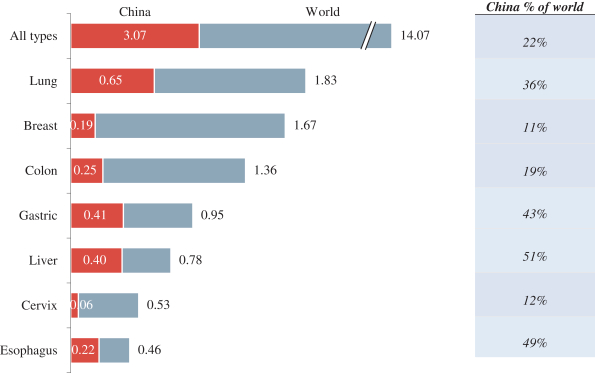
Source: World Health Organization International Agency for Research on Cancer, Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012
Currently available cancer treatments include chemotherapy, surgery and radiation
The most common treatments for cancer include surgery and radiation therapy which remove, kill or shrink cancer cells in a specific area and chemotherapy which works systemically to remove cancer at a specific anatomic location in the body. Newer methods include targeted therapy, which targets specific biological molecules in the human body that play a role in the spread of cancer, and immunotherapy, which uses the patient’s own immune system to help fight cancer. Cancer is often treated with some combination of these therapies.
Chemotherapy was first used as an effective treatment for cancer in the 1940s and is still a key first-line treatment option of cancer patients. Chemotherapy can be administered as monotherapy or in combination with either another chemotherapy drug or targeted therapies. Key uses of chemotherapy include use as a first line agent to reduce tumor size before surgery or radiation therapy, and to destroy cancer cells that remain after a surgical procedure or radiation therapy. The Company is currently running trials of its chemotherapeutic products both as monotherapy and in combination with targeted treatments.
Innovative branded chemotherapy, such as Abraxane and Onivyde, has demonstrated an ability to contribute to significant growth rates within their respective chemotherapeutic categories. According to QuintilesIMS reported sales data, the worldwide product sales of branded and generic paclitaxel products including, Abraxane and Taxol was $1.6 billion in 2012 and $2.0 billion in 2015, representing a CAGR of 8.5%. The worldwide product sales of Irinotecan, docetaxel and topotecan (branded and generic) are estimated to be $427 million, $1.1 billion and $57 million in 2015, respectively. Together, the worldwide product sales of paclitaxel, irinotecan, docetaxel and topotecan (branded and generic) was estimated to be $3.9 billion in 2012 and $3.6 billion in 2015.
111
China World China% of world All types 3. 3.0707 14.07 22% Lung 0.650.65 0.40 1.83 36% Breast 0.190.650.65 0.19 0.06 1.67 11% Colon 0.25 0.25 0.22 1.36 19% Gastric 0.41 0.41 0.95 43% Liver 0.400.4000.40. 0.78 51% Cervix 0.060.061 0.53 12% Esophagus 0.22 0.46 49%
Table of Contents
A key limitation to traditional intravenous chemotherapy drugs is that they have a fairly narrow range for dose safety and effectiveness. Insufficient dosing of a drug will not treat the cancer well and excessive dosing may cause life-threatening side effects. Chemotherapy is commonly given in regular intervals or cycles where doses are separated over time allowing the patient to recover from side effects. A meta-analysis of clinical studies found that breast cancer patients taking more frequent doses of paclitaxel may have better outcomes than patients taking less frequent doses. Consistent frequent intravenous dosing has limitations in patient tolerability over a sustained period of time. We believe that making paclitaxel or other cytotoxic drugs more tolerable will increase the dosage patients can take over time and, thereby, increase the efficacy of the drug.
U.S. market share of spending by formulation and oncology segment
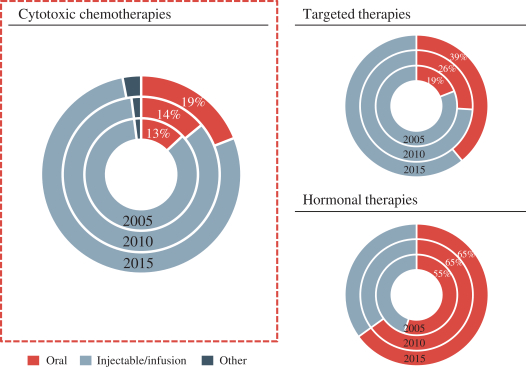
Common cancer treatments include surgery, radiation, chemotherapy, immunotherapy, targeted therapy and hormone therapy. The diagrams above illustrate, for each of cytotoxic chemotherapies, targeted therapies and hormonal therapies, the percentage of such therapies administered orally, by injection or intravenously and by other methods. Traditional cytotoxic chemotherapies use cell-killing toxic drugs to stop or slow the growth of cancer cells. Targeted therapies, such as small-molecule drugs and monoclonal antibodies, are manufactured drugs and antibodies that attempt to stop the growth of cancers by preventing cancer cells from growing, dividing and spreading. Hormonal therapies generally fall into two groups, those that block the body’s ability to produce hormones and those that interfere with how hormones behave in the body, in each case to stop or slow the growth of cancers that rely on hormones to grow.
Our Orascovery product candidates will target the oral segment of the cytotoxic chemotherapies market. We believe changes in the U.S. reimbursement landscape to a more outcomes and performance-based payment model will incentivize physicians to choose more cost effective oral chemotherapy and will reduce incentives for prescribing infusible / injectable treatments. Our Src Kinase Inhibitor product candidates will target the targeted therapies market.
112
Table of Contents
Key Chemotherapy Indications
We are developing many of our drug candidates against multiple indications, which in some cases are common to one or more of our drug candidates. The current treatment landscape and market potential of the primary cancer types on which our Orascovery Platform is focused are discussed below.
Gastric Cancer
Gastric cancer occurs when malignant cells form in the lining of the stomach. There were an estimated 951,000 new cases of gastric cancer and 723,000 gastric cancer related deaths worldwide in 2012, according to the American Cancer Society. CA Cancer Journal estimates that, in 2015, there were approximately 25,000 and 680,000 new gastric cancer cases in the U.S. and China, respectively. According to EvaluatePharma reported sales and consensus forecast data, the worldwide product sales of gastric cancer treatments was approximately $1.0 billion in 2015 and is expected remain nearly constant through 2022, in the absence of the introduction of new novel therapies.
Breast Cancer
Breast cancer is an uncontrolled growth of breast cells that starts in the tissues of the breast and can spread to nearby lymph nodes, the liver, lungs, bone and brain. Breast cancer is the most commonly diagnosed cancer among women in majority of countries worldwide. Based on tumor size, extent of spread and patient preference, treatment usually involves surgery to remove the tumor and surrounding tissue or a complete mastectomy, which involves the removal of the entire breast. In addition, chemotherapy, radiation therapy, hormone therapy and targeted therapy may be used after surgery.
In 2012, there were an estimated 1.7 million new cases of breast cancer and 521,900 breast cancer deaths in women worldwide, according to the American Cancer Society. CA Cancer Journal estimates that, in 2015, there were approximately 234,000 and 272,000 new breast cancer cases in the U.S. and China, respectively. According to EvaluatePharma reported sales and consensus forecast data, the worldwide product sales of breast cancer treatments was approximately $13.8 billion in 2015, and is expected to grow to $25.5 billion in 2022, representing a CAGR of 9.1%.
Lung Cancer
Lung cancer is cancer that starts in the lungs and can spread to other parts of the body. Tobacco use is the most important risk factor for lung cancer. Lung cancer rates have been decreasing in several Western countries where the tobacco epidemic began and peaked earlier, such as the U.S., the United Kingdom and Denmark. On the other hand, in countries where the epidemic was established more recently and smoking has just began to increase, such as China and India, lung cancer rates are likely to increase for the next few decades.
Treatment of lung cancer is based on the type and stage of cancer. Lung cancer can be classified as small cell or non-small cell. For early stage non-small cell lung cancers, the treatment of choice is usually surgery but chemotherapy, sometimes in combination with radiation therapy, may be used as well. For more advanced-stage non-small cell lung cancers, the main treatment is usually chemotherapy, targeted drugs, or some combination of the two. For small cell lung cancers, chemotherapy as monotherapy or in combination with radiation is the primary treatment.
In 2012, there were an estimated 1.8 million new cases of lung cancer and 1.6 million lung cancer related deaths worldwide, according to the American Cancer Society. CA Cancer Journal estimates that, in 2015, there were approximately 221,000 and 733,000 new lung cancer cases in the U.S. and China, respectively. According to EvaluatePharma reported sales and consensus forecast data, the worldwide product sales of lung cancer treatments was approximately $7.7 billion in 2015, and is expected to grow to $25.3 billion in 2022, representing a CAGR of 18.5%.
113
Table of Contents
Colorectal Cancer
Colorectal cancer or bowel cancer starts in the inner wall of the colon or rectum. Early stages of colon cancer are generally treated though surgery and adjuvant therapies. Toward the later stages, a combination of chemotherapy, radiation, and targeted therapies are used. Targeted therapies include drugs which generally act on EGFR and VEGFR to inhibit the growth of new blood vessels. However, tumors with certain genetic mutations, such as mutation in codons 12, 13, or 61 of the KRAS gene, do not benefit from these targeted treatments.
Treatment of rectal cancer involves surgery combined with other therapies such as adjuvant and/or neoadjuvant chemotherapy, radiation therapies and targeted therapies. Advanced stages of rectal cancer are rarely completely treated by removing all the tumors through surgery, given that these treatments merely help to relieve, delay, or prevent symptoms in order to prolong life. Targeted therapies for the treatment of colorectal cancer have become popular over the years with biological therapies, such as Erbitux and Avastin, more commonly used than small molecules treatments, such as Stivarga.
In 2012, there were an estimated 1.4 million new cases of colorectal cancer and 694,000 colorectal cancer related deaths worldwide, according to the American Cancer Society. CA Cancer Journal estimates that, in 2015, there were approximately 132,000 and 376,000 new colorectal cancer cases in the U.S. and China, respectively. According to EvaluatePharma reported sales and consensus forecast data, the worldwide product sales of colorectal cancer treatments was approximately $7.2 billion in 2015 and is expected remain nearly constant through 2022, in the absence of the introduction of new novel therapies.
Actinic Keratosis
AK is characterized by scaly crusty lesions resulting from the intraepidermal proliferation of atypical keratinocyotes caused by prolonged exposure to ultraviolet radiation. Generally, appearance on the skin is most likely to be located on sun exposed areas such as the face, ears, bald scalp, neck, back of hands, forearms, and lips.
AK is a very common condition with an estimated prevalence of over 58 million patients, according to the Skin Cancer Foundation, and an earlier study published by the National Ambulatory Medical Care Survey showed that, between 1990 and 1999, AK was found in approximately 14% of patients visiting dermatologists in the U.S. The condition is more prevalent in individuals with fair skin and in older populations as the cumulative effect of solar radiation becomes greater with age. AK lesions are a concern because, if left untreated, 10-15% will develop into skin cancers. If left untreated they may also bleed, ulcerate, become infected, or grow large and invade the surrounding tissues and metastasize 3% of the time.
Treatment options for AK include destructive therapies, such as surgery cryotherapy and dermabrasion, topical medications, such as topical fluorouracil, imiquimod, ingenol mebutate and diclofenac, chemical peels, and photodynamic therapy. Generally, lesion-directed treatments such as cryotherapy and surgical procedures are the primary approach for isolated lesions. Field-directed therapies, such as topical fluorouracil, imiquimod and ingenol mebutate are especially useful for treating areas with multiple lesions.
One major disadvantage of current dermal agents is a severe reaction of the skin around the treatment area, which is especially undesirable as AK is most likely to be located in highly visible areas such as the face, ears, bald scalp, neck, back of the hands, forearms and lips. Many of these dermal agents, such as topical 5-fluorouracil, are known cytotoxins and damage both precancerous and normal cells. We believe that a favorable side effect profile of KX-01 when applied topically, coupled with similar efficacy will be unique in the dermal AK market. The company believes that KX-01 topical ointment will achieve significant market share of the current AK dermal market as well as expand the market use of dermal agents post-surgery. We completed enrollment of an approximately 160-patient Phase 2a study of KX-01 in 2016 and early data suggested similar efficacy to current agents with a much improved side effect profile during treatment.
114
Table of Contents
According to QuintilesIMS reported sales data, the worldwide product sales of pharmaceutical products indicated for actinic keratosis including Carac and Diclofenac was $3.5 billion in 2012 and $4.5 in 2015, representing a CAGR of 8.4%. We believe the problematic side effect profiles of existing therapies present a market opportunity.
Glioblastoma
Glioblastoma multiforme, or GBM, is the most common and the most malignant of the glial tumors. GBM is a fast-growing glioma that develops from glial cells that support the health of the nerve cells within the brain. The histological features that distinguish GBM from all other grades are the presence of necrosis, or dead cells, and increase of blood vessels around the tumor. These tumors are always rapidly growing and highly malignant. GBM can arise in the brain “de novo” or evolve from lower-grade astrocytomas or oligodendriogliomas. Hallmark symptoms manifest as the rapidly growing tumors interfere with the normal functions of the brain. Headaches, seizures, memory loss and changes in behavior are common while some patients also experience loss in movement or sensation on one side of the body, language dysfunction or cognitive impairment. Currently, the cause of glioblastoma is unknown and the disease cannot be prevented.
The National Cancer Institute estimates that 23,770 adults will be diagnosed with brain and other nervous system cancer in 2016 and that 16,050 of these diagnoses will result in death. GBM has an incidence of 2 to 3 per 100,000 adults per year and accounts for 52% of all primary brain tumors. GBM most commonly occurs in adults aged 45-70 and disproportionately affects men relative to women. Though the tumors are highly malignant within the brain, they rarely spread elsewhere in the body.
Effective treatment of GBM is a substantial unmet need as many patients die soon after diagnosis even if treated with surgery, which is the current standard of care, followed by radiation and chemotherapy. Patients treated with optimal therapy have a median survival of approximately 12 months, with fewer than 25% of patients surviving up to 2 years and fewer than 10% of patients surviving up to 5 years.
Generally, the first step in treatment of GBM is surgery. The primary objective of surgery is to remove as much of the tumor as possible without injuring the surrounding brain tissue needed for normal neurological function. However, due to the position of GBM tumors in the brain and the tentacle like shape of the cancerous cells that grow into the surrounding tissue, it is generally impossible to remove the entire tumor. After the surgery is completed, radiation therapy to selectively kill the remaining tumor cells that have infiltrated the surrounding normal brain tissue can begin. Concomitant and adjuvant chemotherapy with temozolomide, or TMZ, is the current standard of care. TMZ is generally administered daily during radiation therapy then prescribed for 6 to 12 cycles of 28 days after radiation. The goal of chemotherapy is long-term tumor control; however, this is the case in only about 2% of patients. Survival among patients with GBM remains poor.
According to QuintilesIMS reported sales data, the worldwide product sales of glioblastoma treatments was approximately $702 million in 2015.
Changes in the U.S. oncology reimbursement landscape may benefit our products
Since 2005, physician administered injectable and infused drugs have been reimbursed by Medicare Part B at the Average Sales Price, or ASP, plus 6 percent. In this ‘buy and bill’ environment, physicians purchase drugs at ASP and receive 106% of that price as reimbursement. Many oncology drugs, including all approved versions of paclitaxel, are injectable and physician administered and are therefore subject to this reimbursement paradigm. In an effort to develop new payment and delivery models designed to improve the effectiveness and efficiency of specialty care and to eliminate perverse incentives that may occur under the buy and bill paradigm, CMS has undertaken an initiative to overhaul this existing system as part of its Oncology Care Model, or OCM, demonstration project.
115
Table of Contents
The OCM model differs from the current paradigm because payment to physicians is tied to an episode of care rather than on a fee for service basis. Under OCM, physicians receive two payments during “episodes” of care. An episode of care starts when a patient begins chemotherapy and lasts for a period of six months, but additional episodes may be initiated if patients require more than six months of chemotherapy. The first is a monthly capitation payment, called Monthly Enhanced Oncology Services payment, of $160 per Medicare beneficiary per month during an episode, intended to cover the costs of managing care. The second payment is a performance-based incentive tied to OCM episodes of care. These performance payments are awarded to physician practices based on key performance indicators in relation to benchmarks for the episode of care provided by CMS. If the physicians’ expenditures are below the target price for the episodes of care, they have an opportunity to receive a bonus. The size of the bonus is tied in part to how well the provider performs on key quality measures. Since launching in July 1, 2016, OCM has selected nearly 200 physician group practices and 17 health insurance companies to participate in the OCM pilot program.
The OCM is designed to change reimbursement of participating healthcare providers from being tied to dispensing or using oncology products within the physician’s office and replace it with reimbursement for episodes of care. This shift in physician reimbursement is expected to remove incentives to use more expensive oncology products delivered in the office and instead promote delivering the most efficacious and cost-effective therapy. We believe that our orally administered product candidates, if they receive regulatory approval, offer an opportunity to reduce costs and provide superior treatment to patients and therefore believe that OCM may facilitate adoption of our products by physicians whose reimbursement will be less influenced by the price of the drugs they prescribe.
116
Table of Contents
Overview
We are a global biopharmaceutical company dedicated to the discovery, development and commercialization of novel therapies for the treatment of cancer. Our mission is to improve the lives of cancer patients by creating more effective, safer and tolerable treatments. We have generated our clinical product candidates through our Orascovery and Src Kinase Inhibition research platforms, which are based on our understanding of human absorption biology and novel approaches to inhibiting kinase activity, respectively. We believe that our ability to overcome the challenges of oral delivery of chemotherapy and limitations associated with IV delivery, via our P-gp inhibitor, offers significant potential benefits to patient outcomes by allowing patients to stay on therapy longer and extending the potential opportunities to combine with other agents, including targeted and immunotherapies that would otherwise be too toxic in combination with IV chemotherapy. We have assembled a leadership team and have established global operations in the U.S. and China across the pharmaceutical value chain to execute our mission to become a global leader in bringing innovative cancer treatments to the market and improve health outcomes.
Cancer is a major health problem worldwide. The global market for cancer treatments reached $107 billion in 2015 and is expected to reach $150 billion in 2020. Common treatments for cancer include surgery, radiation therapy, chemotherapy, and such newer methods as targeted therapy; however, chemotherapy remains one of the key treatment options for cancer patients and is traditionally administrated intravenously. A major part of cancer treatment consists of IV chemotherapy. The limitations of IV chemotherapy involve repeated painful IV line insertions, potential anaphylactic reactions, expensive hospital visits, toxic side effects and poor quality of life for cancer patients. To address this unmet medical need, development of oral chemotherapy that is more effective and more tolerable, can be taken easily orally at home, avoiding weekly IV infusions and hospital visits (e.g. paclitaxel) is urgently needed. Oral administration of many IV chemotherapy drugs has been unsuccessful because human intestinal cells have a P-gp pump that pumps out chemotherapy drugs (e.g. paclitaxel, doxetaxel, irinotecan etc.) before they can be absorbed. Many attempts at new drug development of P-gp inhibitors failed clinically because of lack of clinical efficacy or significant toxicities.
We believe that oral administration can overcome key limitations and challenges around IV administration of certain cytotoxic chemotherapies, and our Orascovery platform will establish a new paradigm in the use of oral anti-cancer drugs for cancer treatments. Our Orascovery platform is based on the novel P-glycoprotein, or P-gp, pump inhibitor molecule HM30181A, which we in-licensed in 2011 from Hanmi, a major Korean pharmaceutical company focusing on research and development. The P-gp pump is a plasma membrane protein on the cells of the gut which forms a localized drug transport system and prevents oral absorption at therapeutic levels of many well-known, widely used P-gp substrate cancer chemotherapeutic drugs such as paclitaxel, irinotecan and docetaxel, limiting their current delivery to IV. These chemotherapy agents are widely used to treat multiple types of cancer. A cancer patient’s inability to tolerate IV chemotherapies has limited the effectiveness of IV anti-cancer therapies. Co-administration of HM30181A with oral paclitaxel facilitates the oral absorption of paclitaxel by blocking P-gp in intestinal cells and enables oral dosing at therapeutic blood levels which have not been successfully and safely achieved to date without the use of HM30181A. We have learned through clinical studies that this technology allows for certain active chemotherapeutic agents to be absorbed into the blood orally as compared to IV, and may enable some patients to tolerate many cycles of treatment. Oraxol, our leading Orascovery drug candidate is composed of HM30181A, co-administered with an oral dosage form of paclitaxel. We have three other major clinical product candidates in this platform, Oratecan, Oradoxel and Oratopo, which include HM30181A co-administered with an oral formulation of the widely used IV-administered chemotherapeutic agents, irinotecan, docetaxel and topotecan.
Oral administration can overcome key limitations and challenges around IV administration of certain cytotoxic chemotherapies, such as dosing, tolerability and efficacy, and we believe the Orascovery approach will establish a new paradigm in the use of oral anti-cancer drugs for cancer treatments in at least three ways. First, with the use of
117
Table of Contents
HM30181A, clinicians may be able to consistently deliver oral doses of certain chemotherapeutic drugs over a greater number of cycles and duration of time. Second, we believe active drug exposure of chemotherapeutic agents in the patient over time is a critical element in determining efficacy and we can achieve greater tolerability with administration of HM30181A in combination with chemotherapeutic drugs as compared to the current IV standards of care. Third, in light of better tolerability of standard chemotherapies delivered orally, combination with immuno-oncology and targeted anti-cancer treatments can be potentially optimized compared to current treatment paradigms.
We are rapidly advancing our lead Orascovery drug candidate, Oraxol. In 2015, we started enrolling patients in a Phase 3 Oraxol study which combines the dosing of our 15 milligram tablet of HM30181A paired with the dosing of our oral formulation of paclitaxel in a head to head comparison to IV formulation of paclitaxel. In October 2016, we entered into a clinical study collaboration with Eli Lilly and Company to evaluate Oraxol in combination with Lilly’s approved monoclonal antibody Cyramza (ramucirumab) to treat gastric, gastric-esophageal and esophageal cancer. One of the milestones we expect to reach in 2017 for Oraxol is our first 90 patient interim analysis from our Phase 3 study in metastatic breast cancer.
We have also developed novel small molecule compounds through our Src Kinase Inhibition platform. The Src Kinase inhibition platform refers to novel small molecule compounds that have differentiated multiple-mechanisms of actions including: (1) the inhibition of the activity of Src Kinase and (2) the inhibition of tubulin polymerization. We believe the combination of the two mechanisms of action provides a broader range of anti-cancer activity compared to either mechanism of action alone. Our three key clinical product candidates in this platform are KX-01 ointments for actinic keratosis, or AK, pre-cancerous lesions and psoriasis; KX-01 oral for solid and liquid tumors and potential skin cancer indications and pre-cancerous lesions and KX-02 for glioblastoma multiforme, or GBM.
We completed enrollment of an approximately 160-patient Phase 2a study of KX-01 ointment for treatment of AK across 16 sites in 2016 and we have received allowance from the FDA to conduct a Phase 3 study, which we expect to commence in the second half of 2017. AK is a common disease, with a prevalence of approximately 58 million patients in the United States. If left untreated, 10-15% of AK lesions will develop into skin cancers. Our Phase 1 clinical study and preliminary data from our Phase 2 clinical study demonstrated a complete response rate of up to 43%, with no severe local skin reactions reported with the dosing regimen studied. Currently available treatments are limited by severe local skin reactions such as vesicultation, postulation, erosion and ulceration, with low patient compliance. We believe physicians and patients have avoided topical treatments because of the pronounced side effects of the current treatments such as ingenol mebutate, imiquimod, fluorouracil, and that an ointment product with good clinical activity and a favorable side effect profile could capture substantial new market share for treatment of this condition.
In addition to our existing portfolio of clinical candidates, our research and development teams are evaluating additional applications of Orascovery, and developing new platforms based on our knowledge of absorption biology. For example, we are exploring a CYP and P-gp dual inhibitor technology to generate new product candidates.
118
Table of Contents
The following pipeline chart sets forth certain information concerning our key innovative drug product candidates:

| (1) | Also excluding Taiwan, Singapore, Vietnam, Australia, New Zealand and Africa |
| (2) | Collaboration with Eli Lilly and Company, manufacturer of ramucirumab |
| (3) | Excluding Taiwan |
| (4) | Also excluding Taiwan, Macau, Hong Kong, Singapore and Malaysia |
| (5) | Also excluding Taiwan, Hong Kong, Singapore, Malaysia, Thailand, the Philippines, Indonesia and Vietnam |
| (6) | Also excluding Taiwan, Hong Kong and Singapore |
To date, we and our partners have conducted, or are conducting, clinical trials in the U.S., South Korea, New Zealand, Taiwan, Argentina, Chile, Colombia, Ecuador, Guatemala, Honduras, the Dominican Republic, Peru, and China.
119
| Program | Drug Candidate |
Indication | Major Territories Owned |
Pre- clinical |
Phase 1 |
Phase 2 |
Phase 3 |
|||||||||||||||||||||||||
| Orascovery (P-gp + cytotoxic) | Oraxol | Breast cancer | |
U.S., EU, China, India |
|
|||||||||||||||||||||||||||
| |
Oraxol with ramucirumab |
|
|
Gastric cancer |
|
|
U.S., EU, China, India |
|
||||||||||||||||||||||||
| Oratecan | |
Gastric cancer |
|
|
U.S., EU, China, India |
|
||||||||||||||||||||||||||
| Oradoxel | Solid tumors | |
U.S., EU, China, India |
|
||||||||||||||||||||||||||||
| Src Kinase Inhibition | Oratopo | Solid tumors | |
U.S., EU, China, India |
|
|||||||||||||||||||||||||||
| |
KX-01 Ointment |
|
Solid tumors | |
U.S., EU, China, India |
|
||||||||||||||||||||||||||
| Solid tumors | |
U.S., EU, China, India |
|
|||||||||||||||||||||||||||||
| KX-01 Oral | |
Actinic Keratosis |
|
World, except China | ||||||||||||||||||||||||||||
| Skin cancers | World | |||||||||||||||||||||||||||||||
| KX-02 Oral | AML | World, except China | ||||||||||||||||||||||||||||||
| |
Liquid tumors |
|
World, except China | |||||||||||||||||||||||||||||
| |
ATNX-04 |
(CYP / P-gp) |
|
Ovarian cancer |
|
World, except China | ||||||||||||||||||||||||||
| Glioblastoma | World, except China | |||||||||||||||||||||||||||||||
| Dual Inhibition | |
Multiple tumors |
|
World | ||||||||||||||||||||||||||||
Table of Contents
In advance of the launch of our proprietary product candidates in the U.S., our commercial team intends to market oncology and oncology symptom-related products, to fund our infrastructure build-out. We believe it is important to minimize supply chain disruptions for high potency oncology active pharmaceutical ingredients. We have thus internalized key components of the supply chain that we believe are integral to minimizing the associated risks. We have organized our business model into three segments: Oncology Innovation Platform, Commercial Platform and Global Supply Chain Platform—with operations in both the U.S. and China. Our global operations across the three segments are shown below:

Our Global Supply Chain Platform manufactures API for use internally in our research and development and clinical studies, and for sale to pharmaceutical customers globally. Our Commercial Platform currently markets the APIs produced by our Global Supply Chain Platform, including 14 products in the specialty and generic market segment in the U.S. and three products under Section 503B of the FDCA through our compound pharmacy facility.
Based in Buffalo, New York, we were formed in 2003 and have been funded from inception by over $250 million in private financings and public-private partnerships with an estimated aggregate value of $375 million.
Our leadership team was carefully assembled to capture the global commercial market opportunities in novel drug development. Our executive officers are seasoned leaders with complementary skill sets across global pharmaceutical research and development, operations, supply chain and manufacturing, capital markets and mergers and acquisitions. We believe this characteristic is unique for a U.S.-based company and we believe we will be able to utilize this strength to create long term value for cancer patients, our employees and our shareholders. Our team is excited about the prospects of creating new paradigms in the treatment of cancer in developed markets and also driving our product candidates to emerging markets where patient access to treatments has historically been limited.
120
Table of Contents
Our Strengths
Transformative, oncology-focused and highly synergistic pipeline with late stage product candidates.
We believe we have a robust clinical pipeline. We have seven major clinical stage drug candidates, four of which are based on a proprietary Orascovery oral absorption technology, using our novel, highly-selective P-gp inhibitor in combination with widely-used cytotoxic agents enabling oral administration of currently injectable only drugs. The remaining three are novel proprietary Src Kinase inhibitors that have multiple mechanisms of action.
We believe our extensive pipeline can create three impactful synergies:
| • | Drug synergy: We believe that the potentially better clinical response and tolerability of Oraxol versus injectable paclitaxel opens the possibility for greater use of chemo and immuno-oncology therapies. There are also potential synergies between our pipeline products and existing anti-cancer products for potential best-in-combination or first-in-combination with immuno-oncology therapies. |
| • | Platform development and regulatory synergy: Approval of the first drug in each of our research and development platforms may serve as validation and facilitate approvals of other pipeline drug candidates using similar technology. |
| • | Commercial synergy: We believe clinical and commercial success of our initial products may facilitate a shift in the oncology market from injectable formulations to oral. Once physicians gain experience and confidence in our oral formulations, adopting future products using our oral technologies should require less market conditioning. |
Proprietary research and development platforms capable of producing future drug candidates.
Since 2013 alone, our research and development platform has enabled us to file six U.S. investigational new drug applications, or INDs. In addition to the current clinical pipeline, our team is working both on new applications of our existing technologies and on innovating new platform technologies. We believe that our Orascovery platform can be applied to other existing drug therapies to achieve better PK, clinical response and tolerability profiles. Additionally, we have applied our research knowledge and experience of human oral absorption functions and have identified late stage novel molecule candidates which could represent additional future platform technologies. Our research scientists have also developed proprietary systems to identify and screen new molecules, including target binding sites, leading to a more efficient and effective innovation process. We believe these systems and platforms will likely lead to additional clinical candidates.
Unique business model structured to capture value through commercialization and minimize supply chain risk.
We have a business model positioned to capture value by creating opportunities through multiple levers for growth. We are internalizing our commercial infrastructure and supply chain through a variety of organic methods, acquisitions, and partnerships which we believe will enable us to capture greater shareholder value as well as minimize supply chain risk for our proprietary oncology product pipeline. We have built a commercial sales and marketing infrastructure in the U.S. and intend to continue to further build out this segment. We expect this segment to start selling existing in-licensed therapeutically related oncology products in 2017 as a means of funding our commercial infrastructure. Through acquisitions, we own elements of a high potency oncology supply chain, which we believe are important to control in order to minimize risk of disruption. By utilizing capital efficient public-private partnerships in the U.S. and China, we expect to have built cGMP manufacturing facilities in both geographies. We expect to continue to further build out this model using a variety of different methods, including organic growth, strategic acquisitions and collaborations.
121
Table of Contents
A biopharmaceutical company with a near-term focus on the U.S. and China oncology markets.
We have crafted our business model in order to capture the market opportunities in two of the largest pharmaceutical markets in the world. The U.S. represents the largest pharmaceutical market in the world. China has identified biotechnology as one of its strategic industries of focus. In addition, there are several unique aspects to the two markets whereby most biopharmaceutical companies are not well positioned to capture both U.S. and China markets. We have research and product development teams in both countries allowing us to leverage our innovation platform resources, including development and regulatory expertise, to maximize opportunities in both countries. We intend to serve as a technology bridge between these unique markets. We also expect to identify new technology opportunities through acquisitions, licensing or partnering in either market and position ourselves as a gateway to the other market.
Management team with industry-leading expertise and proven track record in leading global drug discovery, development and commercialization.
Our experienced senior management team members have held senior executive roles at multinational pharmaceutical companies and many led the development and regulatory approvals of pharmaceutical products in markets around the globe. Our research and development team members played a key role in the clinical development of numerous significant drugs, including Herceptin, Rituxan, Xeloda, Pegasys, PEG-Intron, Rebetol, IV Temodol, Requip, Suboxone, Subutex and Northera. The leadership of our commercial team has launched more than 50 novel and generic drugs and drug products in multiple markets around the world. A number of our executive officers have significant experience in corporate governance matters, public capital markets transactions, licensing and partnerships, and mergers and acquisitions. We believe this unique combination of executive skill sets has the potential to create substantial long term value for investors.
Our Mission and Strategy
We have a comprehensive and experienced leadership team who have come together under one organization to achieve our mission. Our mission is to improve the lives of cancer patients by creating more effective, safer and tolerable treatments. To achieve our mission, we intend to execute the following strategies:
Rapidly advance our clinical product candidates.
We intend to pursue the fastest feasible pathways to approval of our existing novel oral absorption technology. We are currently enrolling patients in a Phase 3 clinical trial of Oraxol, the program pairing the novel absorption enhancing tablet with the oral capsule formulation of paclitaxel. We plan to submit an NDA to the regulatory authorities in both New Zealand and Taiwan in the next 12-18 months. We believe that if we demonstrate the safety and effectiveness of this technology with Oraxol, the other drug candidates paired with this technology will face a more efficient development process. In addition, we expect complete primary endpoint data from this Phase 2a study of KX-01 ointment in the first half of 2017. If the data from this study confirms our previously completed proof of concept study data, we intend to launch a Phase 3 of KX-01 ointment registration study in 2017. We anticipate the development timeframe for our KX-02 drug candidate for GBM to be accelerated once we commence our partnered clinical program in China. Our licensing partner in China submitted a CTA for KX-02 to the CFDA in 2016. We anticipate fast enrollment in China based on its large patient population, which would accelerate the overall global development timeframe.
Leverage our global research and development operations to continue development of an oncology-focused product pipeline.
We have research and development operations in both the U.S. and China that are focused on both advancing our existing product pipeline and on developing additional novel clinical product candidates. We have developed a core competency in oral absorption technology and apply that skill to develop new methods of drug discovery. We believe that we can create substantial long term value by pursuing a robust, ongoing research and development program.
122
Table of Contents
Build a proprietary commercial platform and selectively leverage collaborative relationships to achieve global drug sales, marketing and distribution.
We have begun building our U.S. commercial operation in preparation for future FDA approvals of our proprietary product candidates. We believe that our experienced product commercialization team can build an infrastructure that leverages both our global facilities and collaborative relationships to achieve global distribution of any FDA approved products in a timely and cost-effective manner.
Continue to build-out our supply chain and cGMP manufacturing capabilities.
We believe internalization of our supply chain is uniquely suited to execute in both the U.S. and China, two of the world’s largest pharmaceutical markets. We intend to utilize cGMP manufacturing facilities from our public/private partnerships in both the China and U.S. markets as a mechanism to access both important markets and minimize supply disruptions. We intend to manufacture certain of our proprietary drugs and our partnered drugs commercialized around the world. Additionally, we expect that the expansion of our existing cGMP high potency API facilities will provide us with more flexibility, and control over high potency APIs as our drugs start to become commercialized. Our goal is to continue expanding this infrastructure and to leverage it to maintain future financial flexibility by optimizing our financial commitments and capital expenditures, which we believe will create value for shareholders.
Selectively pursue strategic M&A or licensing opportunities to complement our existing operations.
We have historically pursued acquisitions and in-licensing opportunities, and will continue to opportunistically target opportunities that will complement our existing portfolio and operations to create value for shareholders and support our business strategy and mission.
Our Orascovery Platform
We are developing a series of orally administered chemotherapeutic agents using our proprietary P-gp pump inhibitor delivery system. The technology enables the oral administration of many cancer agents, which currently are only given by IV due to poor oral absorption. Oral administration of certain cytotoxic chemotherapies can potentially overcome several key challenges in IV administration of those molecules. We believe that our Orascovery platform overcomes these challenges by allowing more frequent dosing over longer periods of time, which we believe will lead to better tolerability and allow for higher total dosage and longer time exposure to the chemotherapeutic agent. Further, we believe additional agents like immuno-oncology and targeted therapy can be better optimized with longer administration of oral chemotherapy agents.
Chemotherapeutic agents such as paclitaxel, irinotecan, docetaxel and topotecan are clinically proven and widely used but have historically been limited to IV administration. The combined worldwide revenue of marketed formulations of these agents is estimated to be $1.9 billion in 2015 and is expected to grow at a CAGR of 9.6% to reach $3.6 billion by 2022. We believe our pipeline products, which leverage our proprietary delivery system that enables oral administration of these chemotherapeutic agents, will substantially expand the use of these chemotherapeutic agents. Additionally, we believe that there is a substantial opportunity for our products to be used in combination with targeted therapies. Furthermore, as shown by Abraxane, novel technology applied to a traditional chemotherapy agent may achieve pricing premiums if data demonstrates superior efficacy and tolerability as compared to current standards of care. We believe our pipeline products will be able to capture a large untapped market and achieve significantly larger market potential than the revenue generated by existing formulations, due to (1) increasing adoption of oral therapy due to patient preference, (2) the potential for improved response rates through greater exposure (based on our predictive model), (3) the potential for improved tolerability (based on our predictive model), and (4) the possibility to expand the market through combination therapies with immune-oncology therapy and oral targeted treatments, of which 39% are already oral.
123
Table of Contents
The table below shows our planned and ongoing clinical trials for our major Orascovery drug candidates.
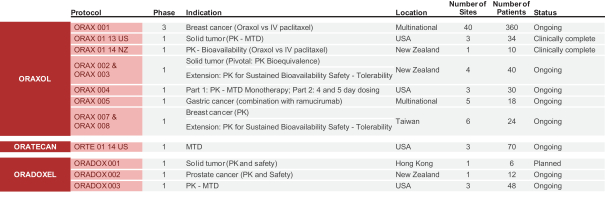
PK and MTD denote pharmacokinetics and maximum tolerated dose, respectively.
HM30181A—Our Novel P-gp Pump Inhibitor
Overview
The novel P-gp inhibition by HM30181A forms the cornerstone of our Orascovery platform, and enables the administration of oral dosing formats of paclitaxel (Oraxol), irinotecan (Oratecan), docetaxel (Oradoxel), and topotecan (Oratopo) each of which is currently under clinical development. The feature that distinguishes HM30181A from other small molecule P-gp inhibitors is this novel compound is specific to P-gp, does not interfere significantly with the activity of other related transporters, and does not significantly inhibit cytochrome 3A4, an enzyme that is important in the metabolism of commonly used drugs. HM30181A is minimally absorbed following oral administration. This localizes P-gp inhibitory activity in the gastrointestinal tract, limiting the potential for interaction at additional systemic sites where P-gp is expressed. Inhibition of gastrointestinal P-gp significantly improves the absorption of chemotherapy agents to achieve systemic exposure profiles which enhance the efficacy and may reduce toxicity of these established chemotherapeutic agents. Based on its pharmacological profile and low systemic absorption, HM30181A is not expected to cause drug-to-drug interactions other than enhancement of oral absorption of medications which are P-gp substrates.
Background—Chemotherapy Treatments
IV paclitaxel is used widely for the treatment of breast, ovarian, and lung cancer. Due to its poor solubility, paclitaxel is usually dissolved in ethanol and polyethoxylated castor oil, which is a major cause of IV hypersensitivity reactions. As a result, premedication with steroids and antihistamines is required to minimize these adverse reactions. Additional common toxicities associated with IV administration of paclitaxel include neuropathy, neutropenia, and alopecia. These side effects limit dose intensification and often require reduction in dosing.
124
Table of Contents
As a single agent or in combination, IV paclitaxel is administered at a variety of doses and regimens that are approved for therapeutic use for various indications, including 135 and 175 mg/m2 administered as both 3- and 24- hour infusions once every 3 weeks. Over the past fifteen years, there has been great interest in dose dense therapy with paclitaxel, switching from the conventional every three-week regimen to administering the drug once weekly. Dose dense treatment with paclitaxel has various advantages that can lead to an increase in the overall exposure, as measured by AUC, over a treatment cycle, while balancing the adverse event profile normally observed, such as neutropenia. This concept is consistent with the hypothesis of maintaining sufficient drug concentrations above a threshold target value for an extended duration.
Based on various clinical trials conducted across multiple tumor types, the weekly regimen of paclitaxel can lead to an increase in response rate, progression free survival, and overall survival. For example, in a clinical trial investigating different dosing schedules of paclitaxel for the treatment of breast cancer in the adjuvant setting, dose-dense paclitaxel given as 80 mg/m2 weekly led to an improvement in disease-free and overall survival, with a 5-year survival rate of 81.5% versus 76.9%. In addition, weekly paclitaxel (80 mg/m2) has a benefit in response rate (42% vs. 29%), time to tumor progression (TTP) (9 vs. 5 months), and median overall survival (24 vs. 12 months) over conventional 175 mg/m2 every three weeks.
A recent analysis compiling data from 29 clinical trials of paclitaxel given as monotherapy investigated the relationship between paclitaxel dose and dosing regimen versus safety and efficacy. This average weekly dose from the every 3-week regimen (175—210 mg/m2) of paclitaxel produced a response rate of 30%, while the weekly regimen of 80 mg/m2 showed a response rate of 37%. In another analysis, a trend towards reduced grade 3 neuropathy with weekly paclitaxel was observed. Together, several clinical trials along with analyses, which evaluated efficacy and safety for dose-dense paclitaxel, suggested a trend of larger therapeutic window and a better safety-efficacy profile for weekly paclitaxel.
Irinotecan is a potent anticancer drug that is marketed under the trade name, Camptosar. Irinotecan is mainly administered to patients with metastatic colorectal cancer (mCRC), but also in glioblastoma, lung, ovarian, cervical, upper gastrointestinal cancer, and pancreatic cancer. The active metabolite of Irinotecan, SN-38, is a type 1 DNA topoisomerase inhibitor with potent antitumor activity and wide antitumor spectrum. We believe that oral administration of irinotecan will more efficiently generate, SN-38, resulting in the potential for better clinical response with reduced toxicity. Oratecan is intended for oral administration for the treatment of irinotecan-responsive cancers.
Docetaxel is a potent anticancer drug within the class of antimicrotubule agents that is marketed under the trade name Taxotere. Docetaxel is mainly administered to patients with breast, lung, prostate, gastric, and head and neck cancers. Docetaxel has potent activity with a wide antitumor spectrum. As a single-agent therapy, docetaxel is administered by IV infusion over 1 hour at a dose of 60-100 mg/m2 for breast cancer and 75 mg/m2 for non-small cell lung cancer given once every 3 weeks. Docetaxel is also used in combination with doxorubicin and cyclophosphamide (adjuvant treatment of breast cancer), cisplatin (lung), topical fluorouracil (head and neck and gastric), and prednisone (prostate). Docetaxel causes dose-limiting toxicities that are more common at higher doses. One significant dose-limiting toxicity is fluid retention that we believe is associated (at least in part) with the IV formulation that contains polysorbate 80, a nonionic and emulsifier frequently used in food and cosmetics. Hypersensitivity reactions may also be attributable to IV administration of polysorbate 80. We believe that oral administration of docetaxel with HM30181A will provide therapeutic exposures of the drug, and result in the potential for better clinical response with reduced toxicity.
Topotecan is a potent anticancer drug under the class of camptothecins that is marketed under the trade name, Hycamtin. Topotecan is mainly administered to patients with lung, ovarian, and cervical cancer. Clinical activity has been shown in combination with the taxanes, docetaxel and paclitaxel, for the treatment of a variety of tumors, including lung cancer. Topotecan causes dose-limiting toxicities. These side effects mainly include neutropenia, late onset diarrhea and nausea and vomiting.
125
Table of Contents
Mechanism of Action of HM30181A
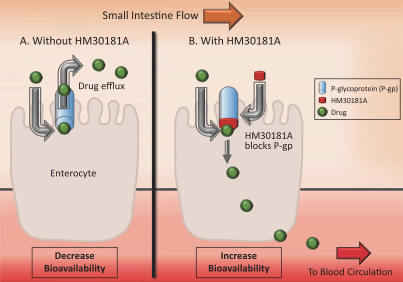
P-glycoprotein plays an important physiologic role as a transporter protein at multiple barrier sites, including the gastrointestinal tract and the blood brain barrier. The demonstrated role of P-gp in limiting intestinal absorption of multiple cancer chemotherapies highlighted the potential utility of a small molecule P-gp inhibitor for enabling oral administration of P-gp substrate drugs otherwise restricted to IV dosing. HM30181A was originally identified by Hanmi as a highly selective and potent P-gp inhibitor, capable of elevating the oral bioavailability of paclitaxel from less than 5% (in the absence of HM30181A) to 41% in rats. Unlike previously developed small molecule P-gp inhibitors, HM30181A is designed to not be systemically absorbed in the gastrointestinal tract following oral administration, with only small amounts detectable in the plasma even after relatively high doses. This unique property made HM30181A a good candidate for co-administration with P-gp substrate drugs, such as paclitaxel, which normally exhibit poor oral bioavailability and are therefore limited to IV routes of dosing.
126
Table of Contents
Pre-clinical and Clinical Development of HM30181A
In vitro Activity
HM30181A was first discovered as a novel P-gp inhibitor in 2006. A subsequent published study demonstrated selective activity against P-gp, with low nanomolar inhibitory activity reported (IC50=0.6 nM in an in vitro assay of P-gp function, where the lower the number the higher the potency) and more potent than cyclosporin A, tariquidar and elacridar, which are previously tested P-gp inhibitors, as shown in the first figure below. In similar assays, HM30181A did not inhibit the transporter proteins MRP1, MRP2, or MRP3 at the concentrations evaluated and only marginally inhibited BCRP transporter activity (IC50 = 3,717 nM, as shown in the second figure below).
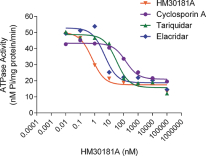
|
|
In vivo Activity
In preclinical studies, HM30181A demonstrated poor absorption from the gastrointestinal tract following oral administration in rats and dogs. The low systemic exposure to HM30181A may at least partially account for the good tolerability observed thus far in pre-clinical toxicology studies. In a single dose rat study, no mortality was noted and there were no test article-related clinical signs or body weight changes, and no gross necropsy findings 15 days after treatment with single oral doses of HM30181A as high as 2000 mg/kg. Likewise, the highest dose evaluated (200 mg/kg) was well tolerated in repeat dose studies in both rats and dogs (once daily up to 13 weeks), with no dose-related mortality.
Multiple pre-clinical studies have evaluated the in vivo pharmacologic effect of HM30181A, generally in the context of a co-administered P-gp substrate, such a paclitaxel. In each case, co-administration of HM30181A significantly enhanced systemic exposure of the co-administered substrate. In murine models of human cancer, oral co-administration of HM30181A with oral paclitaxel or docetaxel confers anti-tumor activity comparable to the IV dosing route.
Clinical Development
HM30181A belongs to a new class of P-gp inhibitor that has high potency, specificity and local action at the intestine cells. Oraxol consists of two drug products, a paclitaxel capsule and a HM30181A tablet. The 15 mg HM30181A tablet has an established room temperature shelf life of 36 months. The 30 mg paclitaxel liquid-
127
Table of Contents
filled, hard gelatin capsule has an established room temperature shelf life of 18 months. Oratecan consists of two drug products, a irinotecan tablet and a HM30181A tablet. The irinotecan 20 mg tablet has an established room temperature shelf life of at least 18 months.
Phase 1 clinical trials showed HM30181A has a good clinical safety profile and was not significantly absorbed systemically in humans. It has been given in amounts of up to 900 mg as a single dose, and up to 360 mg/day for 5 days, without major toxicities. The clinical dose we use currently in our Phase 3 clinical trial is 15 mg/day.
In three separate PK studies of HM30181A conducted in healthy subjects, a total of 81 individuals received single oral doses of HM30181A tablets in single doses of up to 900 mg and 30 individuals received multiple doses of HM30181A tablets ranging from 60 to 360 mg per day for 5 days. HM30181A was well-tolerated, with mostly mild GI side effects at high doses. At the current clinical dose of 15 mg given once daily for up to 5 days, the Cmax in systemic circulation is low.
Our Orascovery Product Candidates
Oraxol (HM30181A tablet + Oral Paclitaxel)
Overview of Clinical Findings
Oraxol has been administered to more than 150 patients through March 31, 2017 across multiple clinical studies in advanced malignances and gastric cancer. There were four completed Phase 1 and 2 clinical studies. No MTD was reached. Overall, Oraxol has been well-tolerated by cancer patients. Studies have indicated that anti-cancer activity of paclitaxel may be related to blood exposure in the patient. Oraxol administration results in similar blood concentration of paclitaxel over time as achieved with IV paclitaxel. We believe oral dosing of paclitaxel can provide a longer drug exposure over a target drug concentration than intravenous paclitaxel, which may translate to better clinical response. We have observed anti-cancer activity in a Phase 1/2 study of patients in gastric cancer with Oraxol monotherapy, where the overall survival in the study for 43 subjects was 10.7 months, which compared favorably to historical data for ramucirumab, the only FDA approved drug for second line treatment of gastric cancer. A randomized, placebo controlled Phase 3 clinical study of ramucirumab reported 5.2 months of overall survival.
Completed Clinical Studies
HM-OXL-101 Phase 1 MTD Study
The Phase 1 MTD study was conducted by Hanmi in South Korea in 24 subjects with advanced solid cancer, with a “3+3” design in which cycles were 28 days and dosing with HM30181A tablets and an oral liquid formulation of paclitaxel was given on Days 1, 8, and 15 of each cycle for three cycles. Premedication was not required prior to treatment with Oraxol. Paclitaxel doses evaluated ranged from 60 to 420 mg/m2. HM30181A doses were half of paclitaxel doses (30 to 210 mg/m2). The MTD was not reached in this study and dose escalation was stopped after 420 mg/m2 because the drug exposure at doses above 300 mg/m2 reached a plateau.
HM-OXL-201 Phase 2 Gastric Cancer Study
The Phase 1/2 gastric cancer study was conducted by Hanmi in South Korea. HM-OXL-201 was an open-label Phase 2, single-arm clinical trial of Oraxol for second line treatment of advanced gastric cancer patients. This trial included dosing Oraxol at 150 mg/m2 per day, for 2 consecutive days per week, for 3 weeks out of a 4-week cycle. A total of 46 subjects enrolled in this study. Oraxol was well tolerated by gastric cancer patients. The results of the Phase 2 portion of this clinical trial showed treatment with Oraxol resulted in a median overall survival of 10.7 months.
128
Table of Contents
ORAX-01-13-US Phase 1 MTD Study
This Phase 1 MTD study was conducted by us in the U.S. and is clinically complete. The objective of this study was to demonstrate the MTD of Oraxol. Study ORAX-01-13-US was a standard “3+3” Phase 1b study to determine the MTD of Oraxol in subjects with advanced malignancies. Oraxol dosing was 270 mg (approximately 150 mg/m2) per day starting at the 2 days of treatment per week for 3 weeks out of a 4 week cycle. Subjects in subsequent cohorts received 3, 4 or 5 days of treatment per week for 3 weeks out of a 4 week cycle. Premedication was not required prior to Oraxol treatment. A total of 33 subjects were enrolled in this study, including 9 subjects in an expansion cohort at the highest weekly dose tested (5 days per week of dosing). The MTD was not reached in this study, showing daily oral dosing of Oraxol was well tolerated.
ORAX-01-14-NZ Phase 1 Bioavailability Study
This Phase 1 bioavailability study was conducted by us in conjunction with ZenRX Limited, or ZenRx, in New Zealand and is clinically complete. The objective of this study was to determine the absolute bioavailability of Oraxol, and to compare the extent of absorption of Oraxol to that of IV paclitaxel. This study showed that Oraxol can achieve blood paclitaxel concentrations over time that are comparable in total exposure to IV paclitaxel.
The following figure shows the mean plasma concentrations of paclitaxel following intravenous administration of 80 mg/m2, as compared to oral administrations of 274 mg/m2 orally dosed daily for two days:
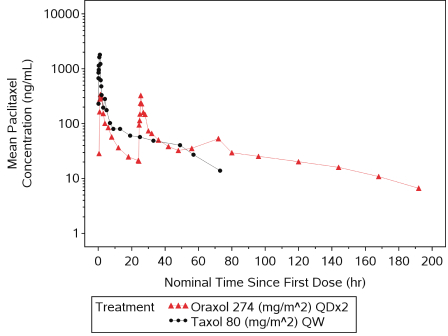
In this study the aggregate Oraxol AUC was 7,052 ng*hr/mL for the 274 mg/m2 dosing regimen (N=2) versus IV Taxol AUC of 6,628 ng*hr/mL (N=2). Oraxol dose escalation above 274 mg/m2 did not further increase exposure (N=2). Based on this, we chose a dose regimen of 15 mg HM30181A + 205 mg/m2 of Oraxol daily over three consecutive days each week for future study, that we believe will produce similar exposure to paclitaxel as 80 mg/m2 IV Taxol given weekly. In addition, this 3 day dosing regimen provides a longer time above the target plasma concentration and could lead to better anti-cancer efficacy.
129
Table of Contents
Overview of Safety Observations in the four completed Oraxol Studies
The MTD for Oraxol was not reached in these studies. Oraxol was well tolerated by cancer patients even when given without premedication for hypersensitivity type reactions, in contrast to the premedication requirement for IV paclitaxel. No hypersensitivity type reactions were observed. No new toxicity, apart from those typically observed with paclitaxel, was observed. Infusion related reactions, including hypersensitivity type reactions, have not been observed. Additionally, severe toxicities associated with IV administration of paclitaxel, including neuropathy, neutropenia and alopecia, are expected to be at a lower incidence and grade for Oraxol.
In our Oraxol clinical studies to date, the serious adverse effects observed include severe neutropenia, febrile neutropenia, sepsis, septic shock, altered state of consciousness, hyopkalemia and cardiac arrest, dehydration, pneumonia, tracheal obstruction, death, nausea, vomiting, diarrhea, fatigue, abdominal and breast pain, anorexia, acute gastroenteritis atrioventricular block, bacteremia, cerebral hemorrhage, constipation, disease progression, hematuria, liver dysfunction, neoplasm, pain, pancytopenia, pyrexia, syncope, urinary tract infection and urinary tract obstruction and death.
Current and Planned Clinical Studies
Phase 1 Bioequivalence Study
The Phase 1 AUC bioequivalence study is being conducted by us in conjunction with ZenRx in New Zealand and is currently ongoing. The study of approximately 40 patients was designed to compare the area under the curve of Oraxol at the estimated clinical dose to that of IV paclitaxel. We are evaluating the bioavailability, safety and tolerability of the bioequivalence of Oraxol Phase 3 dosing regimen of 15 mg HM30181A + 205 mg/m2 of Oraxol daily over three consecutive days each week. The following chart shows interim PK results from the first 6 completed patients, indicating that this dosing regimen could achieve similar exposure to weekly AUC to 80 mg/m2 of IV paclitaxel:
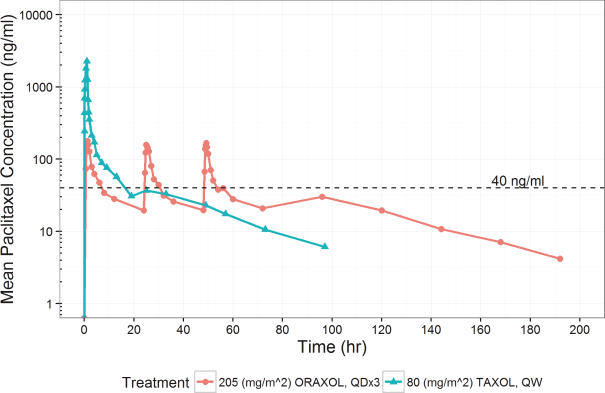
130
Table of Contents
Phase 1 MTD Study of Oraxol in Combination with Ramucirumab
We are conducting a Phase 1 MTD study of Oraxol in combination with ramucirumab in patients with advanced gastric cancer in the U.S. and Asia, through a clinical trial collaboration with Lilly. We are commencing a study of up to 18 patients in a dose escalation study of Oraxol in combination with a fixed dose of ramucirumab to determine the MTD. In a published Lilly-sponsored Phase 3 study comparing ramucirumab in combination with IV paclitaxel to IV paclitaxel alone, the single agent IV paclitaxel arm of the study showed a median overall survival of 7.4 months as compared to 9.6 months with IV paclitaxel in combination with ramucirumab. In a Phase 1/2 study for end stage gastric cancer, single agent Oraxol dosing at 150 mg/m2 for two consecutive days each week, three weeks on, one week off, resulted in a median overall survival of 10.7 months. The objective of this study is to define the MTD of daily Oraxol dosing, starting at 200 mg/m2 for 3 days in a week, three weeks on, one week off, in combination with ramucirumab, which will be dosed every other week.
The following list shows overall survival rates from the randomized, Phase 3 studies for Lilly’s FDA approved drug, ramucirumab, approved in combination with IV paclitaxel for second line treatment of advanced gastric cancer:
| Phase 3 clinical study of ramucirumab vs placebo (n=355) | 5.2 months ramucirumab 3.8 months placebo | |
| Phase 3 trial of ramucirumab plus IV paclitaxel vs IV paclitaxel (n=665) | 9.6 months ramucirumab + IV paclitaxel 7.4 months IV paclitaxel | |
Phase 3 Study for Treatment of Metastatic Breast Cancer
Our Phase 3 study of Oraxol for the treatment of metastatic breast cancer is an open-label, randomized, multicenter study in approximately 360 adult female subjects. The study contains a screening period, a treatment period of 18-21 weeks, and a treatment extension period up to a total of 48 weeks. The study is currently being conducted in 8 countries in Central and South America and will have up to 50 sites participating. Subjects will be randomized to either Oraxol or IV paclitaxel in a 2:1 ratio. The study is designed with two interim analyses which will be conducted after 90 and 180 evaluable subjects have been treated. Tumor assessments will be performed utilizing RECIST v1.1 guidelines by a blinded central radiologist group in the U.S. The blinded U.S. radiology group will measure tumor response rates with scans at week 10, 16 and week 19.
Our Oraxol dosing regimen consists of 3 days consecutive dosing, each week, of: a 15 mg tablet HM30181A one hour before dosing an oral formulation of paclitaxel of 205 mg/m2. There are no weeks off from the Oraxol arm. The comparator IV paclitaxel arm is the labeled dosing regimen of 175 mg/m2 paclitaxel IV one week out of three.
The chart below shows the simulated comparison of one cycle of the labeled dose of IV Taxol of 175 mg/m2 and Oraxol dosed daily at 205 mg/m2 for three consecutive days per week over a similar three week period. While the expected aggregate Oraxol AUC over the cycle is similar to IV Taxol at 15,240 as compared to 15,000, the expected time exposure of Oraxol in the patient’s blood at a therapeutic level is projected to be longer. For Oraxol, the time above 40 ng/mL is forecasted to equal 108 hours per three week cycle as compared to 54 hours for IV Taxol. We believe time exposure of the active pharmaceutical ingredient in the patient’s blood is an important consideration in determining efficacy. In addition, the lower Cmax with Oraxol is believed to be associated with better long term tolerability of Oraxol.
131
Table of Contents
Simulated PK comparison of Oraxol 205 mg/m2 QDx3 per week for 3 weeks vs. IV Taxol 175 mg/m2 one week out of three weeks cycle:
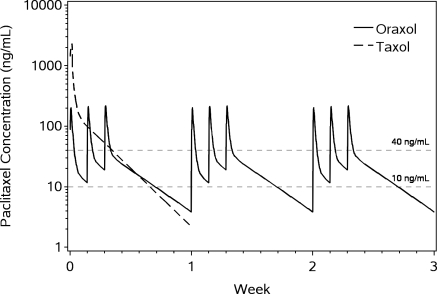
In summary, we believe Oraxol’s longer paclitaxel exposure over time and lower Cmax (based on our predictive model) as compared to the labeled dose of IV paclitaxel could translate into superior clinical response and improved tolerability.
Oratecan (HM30181A Tablet + Oral Irinotecan)
Completed Clinical Studies
Hanmi conducted three Oratecan Phase 1 studies, two as monotherapy (HM-OTE-101, HM-OTE-102), and one in combination with capecitabine (HM-OTE-103), in a total of 54 Korean patients with advanced solid tumors. The tumor types in these clinical trials were mostly gastric and colorectal cancers. MTD for Oraetecan as monotherapy was defined as 100 mg/m2 per 3 week cycle, either given as once daily for 5 consecutive days for 1 week (20 mg/day), or 2 weeks (10 mg/day), of a 3 week cycle. Anti-cancer activity was observed in these studies.
HM-OTE-101 Phase 1 MTD Oratecan Study
Oratecan was administered to 20 patients with advanced solid tumors on Days 1 to 5 during a 21-day cycle. Irinotecan daily doses ranged from 5 to 30 mg/m2, and HM30181A doses were 60 mg. MTD was identified at 20 mg/m2 per day for 5 days of a 3 week cycle. Adverse events were typical of events seen with IV irinotecan. Common adverse events included nausea (90%), diarrhea (65%), and vomiting (55%). Four subjects had dose-limiting toxicity (DLT) events (diarrhea, neutropenia, nausea/vomiting, and AST elevation). At the MTD, the SN-38 Cmax on Days 1 and 5 were 9 and 12 ng/mL. Estimated SN-38 cycle exposure (AUC) was 373 ng*hr/mL. In this study Oratecan montherapyin patients with advanced solid tumors resulted in a disease control rate of 44%.
HM-OTE-102 Phase 1 MTD Oratecan Study
Oratecan was given once daily for 5 consecutive days each week for 2 weeks during a 21-day cycle to 13 subjects with advanced solid tumors. Irinotecan doses ranged from 5 to 20 mg/m2. MTD was identified at 10 mg/m2 per day. Adverse events were similar to those observed following IV irinotecan and included diarrhea, nausea, and anorexia. Five subjects had a dose limiting toxicity, or DLT, in Cycle 1. At the MTD, the resulting SN-38 Cmax on Days 1 and 12 were 5 and 4 ng/mL. Estimated cycle exposure (AUC) for SN-38 was 423 ng*hr/mL.
132
Table of Contents
The following table shows the rate of control of the disease following administration of Oratecan once daily for five days for two out of every three weeks.
| Disease control rate (DCR)* | ||||||||
| Dose Level |
N | # of DCR (%) | ||||||
| HM30181A tablet 60 mg + irinotecan HCl tablet 5 mg/m2 |
3 | 3 (100%) | ||||||
| HM30181A tablet 60 mg + irinotecan HCl tablet 10 mg/m2 |
5 | 3 (60.0%) | ||||||
| HM30181A tablet 60 mg + irinotecan HCl tablet 15 mg/m2 |
1 | 1 (100%) | ||||||
| HM30181A tablet 60 mg + irinotecan HCl tablet 20 mg/m2 |
2 | 1 (50.0%) | ||||||
| Total |
11 | 8 (72.7%) | ||||||
| * | DCR determined by the total number of subjects with complete response, partial response or stable disease, divided by the number of subjects at each dose level. |
HM-OTE-103 Phase 1 MTD Study of Oratecan in Combination with Capecitabine
This study was to determine the MTD of Oratecan in combination with capecitabine. Oratecan was administered to 21 subjects on Days 1 to 5 during a 21-day cycle. Irinotecan doses ranged from 10 to 20 mg/m2 per day, with HM30181A (15 mg) in combination with capecitabine at 800-1000 mg/m2 for 14 days. The MTD of Oratecan, in combination with capecitabine at the 1000 mg/m2 dose was identified at 15 mg/m2 per day. Adverse drug reactions in the study included diarrhea, nausea, anorexia, and vomiting. At the MTD of 15 mg/m2, the SN-38 Cmax on Days 1 and 5 were 5 and 8 ng/mL. Estimated SN-38 cycle exposure (AUC) was 390 ng-hr/mL. In this study of combination of Oratecan with capecitabine in patients with a variety of solid tumors (mostly GI cancers), 10 out of 18 (56%) patients had either stable disease or a partial response.
Overview of Safety Observations in completed Oratecan Studies
In our Oratecan clinical studies to date, the serious adverse effects observed include diarrhea, rash, gastrointestinal hemorrhage, anorexia, vomiting, nausea, enteritis, increased bilirubin, leukopenia, pulmonary embolism, asthenia, neutropenia, increased alanine aminotransferase, increased aspartate aminotransferase and acute kidney injury.
Current and Planned Clinical Development
ORTE-01-14-US Phase 1 MTD Study
The Phase 1 MTD study is being conducted by us and is currently ongoing. This study is to determine the MTD of Oratecan, when given once every 3 weeks, in subjects with advanced malignancies. In previous studies, Oratecan was given once daily for 5 days every 3 weeks and achieved total cycle SN-38 exposure, as measured by AUC, similar to IV administration of irinotecan. This once every 3-week dosing strategy is being evaluated in order to assess if we can further increase SN-38 exposure while avoiding toxicity.
Phase 1 Bioavailability Study
We are currently planning a Phase 1 bioavailability study of Oratecan to be conducted in conjunction with ZenRx in New Zealand. The objective is to determine the absolute bioavailability of Oratecan and to compare the extent of absorption of Oratecan to that of IV irinotecan based on levels of SN-38 This study will allow us to determine the exposure of SN-38 following Oratecan administration that will be equivalent to the SN-38 levels observed with the IV route of administration.
Oradoxel (HM30181A Tablet + Oral Docetaxel)
Preclinical Activity and Evaluation
The effectiveness of HM30181A to inhibit the P-gp pump’s ability to transport docetaxel out of cells was first demonstrated in vitro by an increase in the potency of docetaxel by 1,788-fold in a uterine sarcoma cell line.
133
Table of Contents
In rat oral PK studies, the plasma concentrations of docetaxel versus time, shown below, demonstrates a significant increase upon co-administration of HM30181A with docetaxel. In this experiment, docetaxel is formulated in the proposed clinical formulation. The chart and table below show the plasma levels when docetaxel was dosed in oral formulations in the absence of HM30181A and in the presence of HM30181A. This data shows a large increase in the plasma levels of docetaxel when HM30181A was also dosed orally in all three oral dosing formulations tested. Oradoxel was tested in preclinical human prostate cancer murine model as shown in the table below. Overall, Oradoxel was more active than docetaxel given orally without a P-gp inhibitor and was similar to the efficacy of IV docetaxel administration. At a dose of 25 mg/kg docetaxel with HM30181A a percent of tumor control of 4.8% was achieved which is comparable to the standard 10 mg/kg IV dosing regimen of docetaxel (2.9%). Without P-gp pump inhibition by HM30181A, oral administration of docetaxel demonstrated less inhibition of tumor growth, with a percent of control of 50.5%, consistent with reduced absorption of oral docetaxel when dosed without HM30181A.
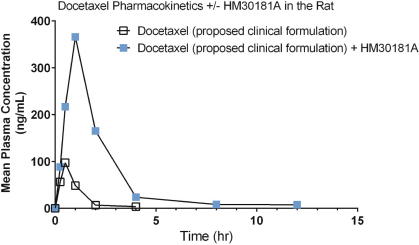
Docetaxel +/- HM30181A in Prostate Cancer Murine Model
| Treatment |
Mean (±SD) Tumor Weight on Day 21 Post Treatment |
T/C (%)a | ||
| Control |
0.348 ± 0.047 | — | ||
| Docetaxel (10 mg/kg, IV) |
0.01 ± 0 | 2.87 | ||
| Docetaxel (25 mg/kg. Oral) plus HM30181A |
0.017 ± 0.003 | 4.78 | ||
| Docetaxel (25 mg/kg Oral) |
0.176 ±0.035 | 50.53 |
Current and Planned Clinical Studies
Based on the preclinical mouse efficacy data and rat and dog toxicology data, we expect that oral administration of docetaxel together with HM30181A will be efficacious and well tolerated in the clinic. We believe it may minimize the dose-limiting toxicities associated with docetaxel therapy, such as fluid retention, and allow increasing dosing to obtain superior efficacy.
The U.S. FDA allowed our IND in the first quarter of 2016 and, most recently, we gained regulatory allowance for a clinical trial in New Zealand. The U.S. study will be a Phase 1 dose escalation trial for Oradoxel in patients with various solid tumors with a starting dose of 35 mg/m2 given once every 3 weeks. In New Zealand, a Phase 1 study will be conducted to identify the absolute bioavailability of Oradoxel in solid tumor patients.
134
Table of Contents
Oratopo (HM30181A Tablet + Oral Topotecan)
Preclinical Activity and Evaluation
In rat oral PK studies, the plasma concentrations of topotecan versus time, shown below, demonstrates a significant increase upon co-administration with HM30181A. This effect is evident when topotecan is formulated in saline or the marketed product, Hycamtin. In preclinical murine models with human tumor transplants, including ovarian cancer, oral topotecan in combination with HM30181A was more active than oral topotecan alone following administration at a dose of topotecan 1 mg/kg once daily for five days per week, as illustrated in the lower chart below.
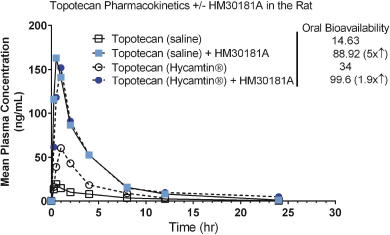
Topotecan +/- HM30181A in Ovarian Cancer Murine Model
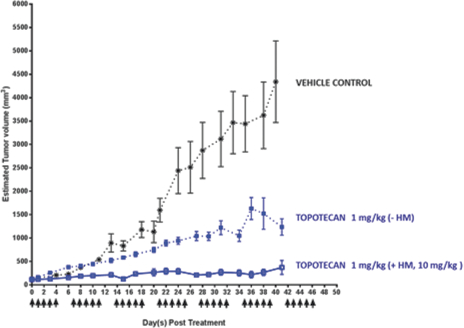
135
Table of Contents
Current and Planned Clinical Studies
IND enabling studies have been conducted. To date, dose-range finding studies along with GLP toxicology and toxicokinetic studies following single and multiple daily doses are being conducted with oral Topotecan in combination with HM30181A. These studies have been recently completed to establish the maximum tolerated dose for the 5-day regimen to support our proposed clinical dosing regimen. We recently completed cGMP manufacturing of oral irinotecan formulation. We filed an IND application for oral topotecan in combination with HM30181A in February 2017 and it was allowed in March 2017.
136
Table of Contents
Our Src Kinase Inhibition Platform
The table below shows our planned and ongoing clinical trials for our major Kinase inhibition drug candidates.
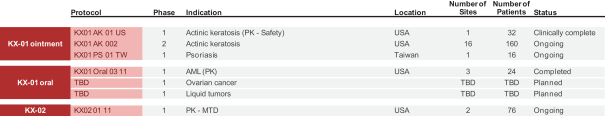
PK and MTD denote pharmacokinetics and maximum tolerated dose, respectively.
KX-01
Mechanism of Action
KX-01 is a novel small molecule, which we discovered and developed, which demonstrates at least two MOAs relevant to the control of cancer and hyper-proliferative disorders: Src tyrosine kinase inhibition (non-ATP competitive) and tubulin polymerization inhibition. Src plays a demonstrated role in regulating multiple aspects of tumor development, growth, and metastases, and its inhibition limits such tumor activity. Interfering with tubulin polymerization activity is a clinically validated mechanism for treating cancer. For both targets KX-01 binds at a novel binding site. Taken together, these two MOAs provide for a potent means of treating cancer and other hyper-proliferative disorders.
The first MOA defined for KX-01 is Src tyrosine kinase inhibition. We have demonstrated the correlation of KX-01 inhibition of Src auto-phosphorylation (a measure of Src activity) and cell growth during the proliferation phase of tumor cells in both c-Src527F/NIH-3T3, a cell line derived from fibroblasts with enhanced Src activity, and HT29, a cell line derived from colon cancer cells. Through in vitro tests, KX-01 is shown to induce caspase 3 cleavage and PARP cleavage, which are both markers for cell death/apoptosis, as well as p53 induction, which is a protein involved in tumor suppression. Unlike most known Src inhibitors, KX-01 is unique in that it is not an ATP competitive inhibitor of Src but rather it is believed to be a substrate competitive inhibitor, which means high specificity for the intended binding target. A computational model for how KX-01 is predicted to bind in the peptide substrate site of Src is depicted in the figure below, as noted in a NMR/paramagnetic probe study conducted and published by Wyeth LLC.
137
Table of Contents
The second MOA defined for KX-01 is inhibition of tubulin polymerization, a step essential for cell growth. We have demonstrated the ability of KX-01 to inhibit tubulin polymerization in vivo within tumors in a mouse xenograft, and showed synergistic activity with paclitaxel to cause tubulin fragmentation, a process to interrupt cell growth.
The two MOAs of KX-01 are intended to control cell growth and proliferation of cancer cell types as well as cell types involved in hyper-proliferative diseases. Specifically, KX-01 has been shown to have potent activity in controlling cell growth in keratinocytes, or skin cells (with IC50 = 32 nM). This activity demonstrates the potential of these compounds to control hyper-proliferative diseases of the skin, examples being actinic keratosis and psoriasis.
The following table shows the broad spectrum activity against many cancer types. GI50 represents the concentration of KX-01 to be used to inhibit 50% of tumor cell growth. The lower the numerical value of GI50 the higher the potency of KX-01.
| Human Tumor Cell Line |
KX-01 GI50 (nM) |
Human Leukemia Cell Line |
KX-01 GI50 (nM) | |||||
| HT29 (Colon) |
25 | K562 (CML) | 13 | |||||
| SKOV-3 (Ovarian) |
10 | K562R (Gleevec resistant CML) | 0.64 | |||||
| PC3-MM2 (Prostate) |
9 | MOLT-4 (ALL) | 13 | |||||
| L3.6pl (Pancreas) |
25 | CCRF-HSB-2 (ALL) | 12 | |||||
| MDA-MB-231 (Breast) |
20 | Jurkat (Adult T Cell Leukemia) | 10 | |||||
| A549 (Lung) |
9 | Ba/F3 + WT BCR-Abl | 85 | |||||
| HuH7 (Liver) |
9 | Ba/F3 + E225K (Gleevec Resistant) | 80 | |||||
| 769-P (Kidney) |
45 | Ba/F3 + T315l (Gleevec & Dasatinib Resistant) | 35 | |||||
| SNU-1 (Gastric) |
6 | KG-1 (AML) | 16 | |||||
| RPMI8226 (Multiple Myeloma) | 40 | |||||||
| RL (non-Hodgkin’s lymphoma) | 19 | |||||||
Research Background
Topical formulation development studies carried out by our licensing partner, PharmaEssentia, in Taiwan resulted in a suitable clinical formulation. KX-01 topical formulations are initially being tested by PharmaEssentia in psoriasis clinical trials in Taiwan.
In parallel, we are evaluating a topical KX-01 ointment in the clinic for AK in the U.S. The most common cause of AK is exposure to ultraviolet radiation from the sun or tanning beds. This exposure can lead to oncogenic changes, such as inactivation of p53, and consequential hyper-proliferation of mutated keratinocytes. If left untreated 10-15% of AKs can progress to skin cancer. KX-01 inhibits the proliferation of keratinocytes and up-regulates p53 so its utility in clinically treating AK was of interest. Clinical trials with a KX-01 topical ointment for treating AK have produced encouraging early results. KX-01 ointment 1% has a room temperature shelf life of at least 6 months.
KX-01 Ointment for Topical Indications
Completed Clinical Study
Phase 1 Study
The Phase 1 study of KX-01 ointment for treatment of AK was conducted by us in the U.S. and is clinically complete. This is a four cohort, PK and safety study of 3 days or 5 days, with treatment of 25 cm2 or 100 cm2 applied to the forearm. The results for the Phase 1 studies showed that with 1% KX-01 ointment being applied for 5 consecutive days in on a 25 cm2 area of the forearm; 50% (4 of 8 subjects) had complete response (100% clearance). This was achieved with very good local and systemic tolerability. We believe that the high clearance rate with low skin toxicity compares well to existing treatments on the market.
138
Table of Contents
Clinical data thus far indicate that KX-01 ointment produces a complete response without severe adverse skin reactions in some patients, as shown in the images below:
Skin reaction from KX-01 ointment in a subject who had a 100% response
|
|
|
Current and Planned Clinical Development
Phase 2a Study
We completed enrollment in 2016 of an approximately 160-patient Phase 2a clinical study in the U.S. of KX-01 ointment for treatment of AK on the face and scalp. This is an open label, two sequential cohort study of approximately 80 patients each with treatment of KX-01 ointment 1% for either 5 days or 3 days. The primary objective is to evaluate the complete response rate, which is defined as 100% clearance of such patient’s AK at Day 57 after treatment. Additionally, we seek to further investigate the findings from the Phase 1 proof of concept study indicating that KX-01 ointment has a favorable side effect profile. We completed a face-to-face meeting with the FDA and the FDA has allowed a Phase 3 study of KX-01 for treatment of AK, which we expect to commence in the second half of 2017.
Initial data from patients in the study shows that the KX-01 dosing regimen used in this study is devoid of severe LSRs. There were no Grade 4 LSRs reported thus far.
Maximal Local Skin Reactions for Study KX-01-AK-002 Phase 2a Trial: Percentage of subjects with most severe LSRs as assessed by study investigators (Grade 4)
| Local Skin Responses |
Grade | KX-01-AK-002a N=67 (%) |
||||||
| Erythema |
4 | 0 | ||||||
| Flaking/Scaling |
4 | 0 | ||||||
| Crusting |
4 | 0 | ||||||
| Swelling |
4 | 0 | ||||||
| Vesiculation/Pustulation |
4 | 0 | ||||||
| Erosion/Ulceration |
4 | 0 | ||||||
| (a) | Ongoing preliminary data. |
Cross-table tabulation of Complete Clearance Rate:
| KX-01% Days(a) | ||||
| Face |
15/35 | (43%) | ||
| Scalp |
9/32 | (28%) | ||
| (a) | Ongoing preliminary data. |
139
Table of Contents
Skin reaction example from KX-01 ointment in a subject who had a 100% response
|
|
|
| ||
| Day 1 |
Day 8 | Day 57 |
As demonstrated above in the pictures, the patient in our Phase 2 study who experienced a 100% response experienced no severe skin reaction. Since skin toxicity is likely to be a significant consideration of clinicians and patients, especially on the face and scalp, we believe that the market is likely to expand for topical treatments options for this pre-cancerous skin condition. To the extent that skin toxicity has been limiting the market, we believe that KX-01 ointment may significantly expand the market as a result of a low skin toxicity.
KX-01 Oral
Completed Clinical Studies
KX-01 oral capsules are available in strengths of 20 mg and 80 mg and have a room temperature shelf life of 48 months. KX-01 oral has been evaluated in several early dose finding studies against both solid and liquid tumors. Initial clinical results indicate activity against both solid and liquid tumors in patients. We are planning further probing studies to focus our evaluation in certain of those indications where activity was observed.
Phase 1 and Phase 2a US Study—Complete
A Phase 1 clinical trial in solid tumor patients identified the MTD for continuous twice daily oral dosing at 40 mg/dose, with a favorable PK profile, and indications of activity. In this trial, 44 patients were enrolled in 9 dose cohorts. The drug was well tolerated and the dose limiting toxicities were mainly elevated levels of aspartate transaminase, or AST, and alanine transaminase, or ALT, which were readily reversible. 11 patients had stable disease for at least 4 months, including patients with ovarian, carcinoid, papillary thyroid, prostate, pancreas and head and neck cancer. An ovarian cancer patient had stable disease for 16 months and a KX-01 oral induced a large decrease in the ovarian cancer CA-125 biomarker, which correlates well with clinical response. As shown in the table below, this patient had failed nine prior drug, and drug combination therapies, thereby showing a clear benefit from KX-01 oral treatment.
| Therapy |
Duration (months) | |||
| Carboplatin, Paclitaxel |
5 | |||
| Gemcitabine, Carboplatin |
2 | |||
| Gemcitabine, Taxotere |
2 | |||
| Doxil |
1 | |||
| Cisplatin, Cyclophosphamide, Epirubicin |
4 | |||
| Tamoxifen |
2 | |||
| Topotecan |
3 | |||
| Abraxane, Avastin |
4 | |||
| Folfox, Avastin |
1 | |||
| KX-01 oral |
16 | |||
A subsequent Phase 2a clinical study in men with bone-metastatic castration-resistant prostate cancer using the twice daily 40 mg/dose was conducted. 31 patients were dosed with KX-01 oral at 40 mg/dose twice daily until disease progression or unacceptable toxicity. The primary endpoint was 24-week progression-free survival (PFS). The designated clinical endpoints were not met with KX-01 oral at this dose.
140
Table of Contents
A Phase 1b clinical study in elderly AML patients was conducted using once daily dosing. The doses tested were 40, 80, 120, 140 and 160 mg of KX-01. 24 patients were recruited with a median age of 76 years (range 63 to 86 years). Most had been previously treated for their disease, generally with decitabine or azacitidine. The MTD is estimated to be 105 mg of KX-01 oral daily.
Overview of Safety Observations in completed KX-01 Oral Studies
In our KX-01 Oral clinical studies to date, the serious adverse effects observed include allergic reaction, bacteremia, fatigue, rash, syncope, tremor, dermatitis, neutropenic fever, hyponatremia, failure to thrive, lower extremity edema, mucositis, neutropenia, pancytopenia, thrombocytopenia, seizure and motor vehicle accident, embolic stroke, pneumonitis, fever, acute kidney injury, lung infection and increased blood platelet, albumin and bilirubin levels, abdominal pain, arm pain, pyrexia, rigors, tachypenia, oxygen desaturation, pneumonia, anemia, elevated ALT and AST, dehydration and leukopenia.
Current and Planned Clinical Development
Our licensing collaborator, Hanmi Pharmaceuticals is completing a Phase 1b clinical trial in South Korea, combining escalating continuous once daily doses of KX-01 with a standard Taxol IV treatment of 80 mg/m2 once weekly for 3 out of 4 weeks. This study is clinically completed and awaiting a study report.
Phase 1 Probing Studies in Hong Kong and Taiwan—Planned
We are planning probing studies in Asia in 2017 to evaluate KX-01 oral treatment in ovarian and liquid tumors for cancer indications where previous activities were observed.
KX-02
Research Background
KX-02 is a closely related structural analog of KX-01 and demonstrates a similar dual MOA of inhibition of Src activity and microtubule polymerization. KX-02 was designed to readily cross the blood-brain-barrier, or BBB. The KX-02 levels in the mouse brain meet or exceed that in the plasma at the same time points after oral dosing, demonstrating that KX-02 readily crosses the BBB. We believe that this ability to cross the BBB provides a rationale for investigating brain cancers and metastases in the brain as potential therapeutic applications for what is traditionally considered an unmet medical need.
141
Table of Contents
Preclinical evaluation and activity
In Vivo Activity
Based on pre-clinical data demonstrating activity against a number of brain cancer cell lines and demonstrated BBB penetration, KX-02 was tested orally in a mouse GBM tumor model, wherein the mice with a fully competent immune system are implanted with mouse GBM tumor cells into the brain in order to simulate a human patient. When compared with TMZ, the current standard of treatment, in one particular study KX-02 produced long term survival mice, as compared to TMZ, which extended survival but did not result in any long term survivors. Across multiple experiments, an average of approximately 30% long term survival mice were produced when dosed with KX-02 at 30 mg/kg once daily, as shown in the figure below.
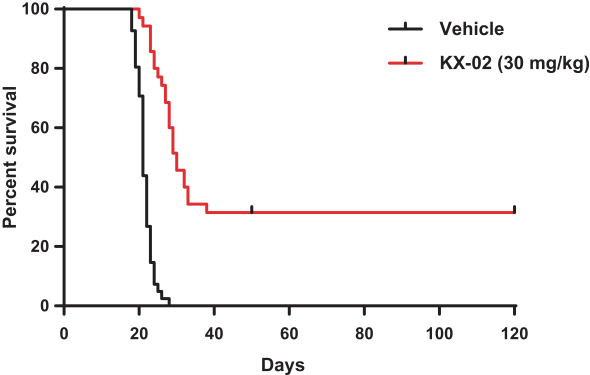
To visualize tumor growth in situ, animals treated with KX-02 were compared to those that had been treated with placebo alone using MRI. The KX-02 treated mice that eventually survived long term did not have tumors whereas the placebo mice have large tumors at the time points evaluated.
The potential role of an adaptive immune response in the responses of the mice treated with KX-02 was demonstrated in three ways. First, when the same mouse study was repeated with mice lacking an adaptive immune system no cures were obtained. Second, when mice that had been cured by KX-02 were re-challenged with mouse GBM cells they failed to develop tumors, whereas untreated mice readily show tumor growth. Third, a larger infiltration of lymphocytes into brain tumor tissues was seen in mice treated with KX-02 as compared to those treated with a placebo, and these lymphocytes stained as CD3 and CD8 immune cells. Accordingly, KX-02 also appears to facilitate the immune system’s recognition of brain tumor tissues as foreign cells, in addition to its Src and tubulin-targeted activities. In our KX-02 clinical studies to date, the only serious adverse effects observed were thromboembolic events.
142
Table of Contents
Current and Planned Clinical Studies
Phase 1 Study
We are currently enrolling a Phase 1 study in the U.S. of KX-02 for treatment of solid tumors in order to determine the MTD, PK, and safety profile. This trial is currently in the dose escalating phase.
In 2012, we out-licensed KX-02 to Xiangxue Pharmaceuticals for development and marketing in greater China and clinical trials are currently being planned. As part of that collaboration, Xiangxue recently submitted an IND to the CFDA for subsequent clinical trials in China. We anticipate the development timeframe for our KX-02 drug candidate for glioblastoma to be accelerated once we commence our partnered clinical program in China.
Intranasal Granisetron (GNS)
The development of supportive therapy is complementary to our oncology drug platform. Chemotherapy-induced nausea and vomiting, commonly referred to as chemotherapy-induced nausea and vomiting, or CINV, which is a common side effect of cytotoxic chemotherapy treatments. Granisetron is a 5-hydroxytryptamine 3, or 5-HT3, receptor antagonist, a class of anti-emetic drugs that are commonly used in the prevention of CINV. Our subsidiary, Comprehensive Drug Enterprises, has developed intranasal formulations of granisetron for further evaluation in the clinic.
Current Therapies and Limitations
Currently, granisetron is dosed in IV, oral and patch forms to treat CINV. We believe these dosing regimens have disadvantages including time to effectiveness, and lack of ability to control the symptoms effectively at home.
Current and Planned Clinical Studies
We believe intranasal delivery will possess a number of advantages for the patient. These advantages include a rapid delivery of therapeutic drug levels for quick relief of CINV as well as the ability to self-dose outside of the hospital and IV settings while experiencing CINV whereupon oral administration would be difficult. The intranasal route of administration of GNS leads to more rapid achievement of systemic concentrations of the drug compared to the oral route.
A Phase 1 parallel-group study of GNS was conducted in Taiwan to assess the PK, safety, and tolerability of GNS. A total of 50 healthy subjects, 25 male and 25 female, were divided into the following treatment groups: 1 mg Kytril administered IV over 30 seconds to 10 patients, 1 mg Kytril tablets administered orally to 10 patients, and either 0.5, 1, or 2 mg of intranasal GNS was administered to 10 patients. The results showed that the drug concentrations were dose proportional following intranasal delivery of GNS and the side effects were acceptable.
We are presently evaluating the market opportunity in various geographies for the development of an intranasal route of delivery in order to determine the next path clinically for this drug candidate.
Our Research and Development
We are an innovative oncology company with drug discovery, drug formulation, clinical development, and API/drug product manufacturing facilities in both the U.S. and China. The U.S. drug discovery, clinical development, and formulation research facilities are largely concentrated in Buffalo, New York and Cranford, New Jersey. The range of capabilities at these facilities includes medicinal chemistry, biochemistry, cell biology, formulation, chemical manufacturing and control, quality control, pharmacokinetics/pharmacodynamics (PK/
143
Table of Contents
PD), data management, as well as pharmacovigilance, clinical development and regulatory expertise functions. Animal efficacy, PK/PD, and toxicology studies are carried out at various contract research organizations, or CROs, around the world in order to eliminate the need for an in-house animal facility.
In China, our research and development center in Hong Kong is closely integrated with our research and development center in Buffalo. This center concentrates on drug formulation development and evaluation. When we acquired Comprehensive Drug Enterprises, or CDE, in 2015, we added scale to our formulation and research personnel in Hong Kong. The clinical oral formulation of docetaxel is an example of a discovery emanating from our Hong Kong research and development center. Higher strength paclitaxel powder tablet formulations, to be introduced into our future clinical evaluations of the Oraxol drug product, are a second example of the formulation development work being successfully carried out at the Hong Kong research and development center.
Our proprietary Dual (CYP/P-gp) Inhibitor Program
We are developing a proprietary class of “dual” absorption enhancers that inhibit both the P-gp transporter and the CYP enzymes within the gastrointestinal tract. There are many barriers that limit the oral absorption of drugs in humans. The P-gp transporter is a major barrier to absorption of active chemotherapy drugs. However, certain other drugs with P-gp liabilities may also have liabilities for other barriers such as metabolizing enzymes, primarily the cytochrome P450, or CYP, class of enzymes. These CYPs limit oral absorption of certain drugs by metabolizing drugs within the gastrointestinal tract before they can be absorbed. This class of dual absorption enhancers has shown potential to significantly improve the oral bioavailability of certain other drugs in laboratory tests, and may expand the application of our oral absorption platform to drugs where the CYP barrier to oral absorption is also important. These dual absorption enhancers may lead to better performing next-generation oral medicines in our pipeline of clinical products.
The development of these dual absorption enhancers is at the preclinical stage. Proof of concept, providing increased oral bioavailability in preclinical species, has been obtained with several absorption enhancers and candidate drugs. Currently additional filters such as patentability/freedom to operate, physical-chemical characterization, pre-formulation studies, manufacturing analysis and preliminary toxicity testing are being applied to our first group of lead candidates for clinical development. In 2017, we expect to identify our lead molecule for a future IND enabling study.
Commercial Platform
We believe the value creation potential is higher for biopharmaceutical companies able to commercialize their proprietary products as compared to companies who have a partner to commercialize. The infrastructure investment and build-out of a commercial team prior to regulatory approval is typically costly and requires years of investment. In 2016, we launched a commercial platform in the U.S. to begin building out this infrastructure in advance of our launch of proprietary products. As part of our capital efficient strategy, we anticipate that our commercial team will market and sell a variety of in-licensed pharmaceutical products, which are therapeutically related to our proprietary portfolio, to cover the cost and expense of the infrastructure investment.
Using our resources to commercialize products in oncology may create more value for investors than marketing product rights pre-commercialization. We believe commercialization risks can be offset by establishing oncology manufacturing operations (API, Manufacturing, etc.) and commercial operations (Multi-source Oncology, Pharmacy, Hospitals, etc.).
Our Commercial Operations
Target Audiences: U.S. Oncology Market
The U.S. Oncology market is highly complex with Gatekeepers, Influencers and Prescribers influencing sales of oncology products. Launching a commercial operation in preparation for a proprietary drug approval is
144
Table of Contents
risky, difficult and expensive. Any commercial oncology organization must be able to market to Gatekeepers, Influencers and Prescribers in the oncology market at launch. Gatekeepers include hospitals (including pharmacies and therapeutics committees), buying groups, oncology managed care organizations, specialty distributors and pharmacists. Influencers in the oncology market include Key Opinion Leader physicians, regional cancer centers (as defined by the National Cancer Institute) and the U.S. government. Prescribers include oncologists and dermatologists.
Key hurdles in establishing Commercial Operations in the oncology market include the unpredictability of timing for U.S. FDA approval and the limited time to establish market relationships post approval, competition with companies with broader oncology offerings, identifying key influencers in the local oncology market and the unpredictability of timing with U.S. FDA approval. Another hurdle is recruiting key senior business leaders since they are responsible for recruiting a successful Oncology Sales and Marketing team. For all these reasons, establishing commercial operations in the oncology market is risky and expensive.
In order to manage the risks and capture post commercial oncology economics, we plan to launch two oncology product lines in 2017—Multisource Oncology products and 503B Compounded Oncology Products. We intend to support these two product lines with a sales and marketing organization to target Gatekeepers. Our National Accounts organization will target Gatekeepers and the U.S. Government for these two oncology product lines. Regional Cancer Centers will also be targeted for Multisource Oncology and Compound Pharmacy products.
503B Compound Pharmacy Products
We plan to directly manufacture our own products in our 503B Compound Pharmacy. We expect to use our internal cGMP operations, and selected contract manufacturers to make both sterile to sterile products and products from sterile bulk API. We plan to source certain of our API from our own internal supply chain to make products from sterile API bulk. We also plan to buy API from other sources. For sterile to sterile products, we expect to source the sterile vials and bags from national suppliers. This second oncology business is expected to further expand our offering to the U.S. oncology market.
U.S. Specialty Pharmaceuticals
Our U.S. Specialty Pharmaceuticals business sources products through licensing agreements with various partners, who we collectively refer to as our Global Partner network. The company has unique commercial expertise in multisource oncology products and has developed a number of Global Partners that develop and manufacture multisource products for the U.S. market. This Global Partner network supplies the products the company markets in the U.S. Specialty Pharmaceutical business. The company is launching a commercial oncology business in the U.S. by launching multisource oncology and therapeutically related products supplied by our Global Partner network. We anticipate structuring collaborations whereby we split the profits with the Global Partners and market the products to Gatekeepers and Influencers in the U.S. oncology market. This will help the company prepare to launch proprietary oncology products into the U.S. market.
As of March 31, 2017, we have the non-exclusive commercial rights to market and sell 14 FDA approved products and 20 products pending FDA approval.
Gland Agreement
In August 2016, we entered into two binding term sheets with Gland Pharma Limited, or Gland, pursuant to which we expect to enter into a definitive agreement for a non-exclusive license to market 21 of Gland’s products. Gland has obtained FDA approval for 8 of such products and has filed an abbreviated new drug application, or ANDA, in the U.S. for the remaining 13 products. For each of the licensed products we will pay a license fee to Gland. The maximum amount of such licenses is $7.3 million, of which $1.15 million is contingent
145
Table of Contents
on regulatory approval for 13 products for which Gland has filed ANDAs. We will pay a portion of such $1.15 million upon regulatory approval of each of these 13 licensed drugs. Additionally, during the terms of the agreements we have a profit sharing arrangement pursuant to which we will pay to Gland between 20% and 60% of the net profits from sales of each of the licensed products, depending on the product. The initial term of each of the of the Gland license agreements is five years from the launch of each product licensed pursuant to the agreement, subject to automatic renewal for additional two year terms, unless terminated by either party upon provision to the other party at least 90 days’ notice in advance of a renewal date.
In February 2017, we entered into an additional binding term sheet with Gland, pursuant to which we expect to enter into a definitive agreement for a non-exclusive license to market an additional 6 of Gland’s products. Gland has obtained FDA approval for 4 of such products and has filed an ANDA in North America for the remaining 2 products. For each of the licensed products we will pay a license fee to Gland. The maximum amount of such licenses is $3.15 million, of which $312,500 is contingent on regulatory approval for 2 products for which Gland has filed ANDAs. We will pay a portion of such $312,500 upon regulatory approval of each of these 2 licensed drugs. Additionally, during the term of the agreement we have a profit sharing arrangement pursuant to which we will pay to Gland between 25% and 40% of the net profits from sales of each of the licensed products, depending on the product. The initial term the Gland license agreement is five years from the launch of each product licensed pursuant to the agreement, subject to automatic renewal for additional two year terms, unless terminated by either party upon provision to the other party at least 90 days’ notice in advance of a renewal date.
SunGen Agreement
In September 2016, we entered into a joint venture agreement with SunGen Pharma LLC, or SunGen, which we refer to as the SunGen Agreement, to create a joint venture to develop and commercialize certain specialty pharmaceuticals. We and SunGen have agreed to negotiate in good faith a limited liability company operating agreement to govern the joint venture, which we agreed will be owned 51% by SunGen and 49% by us.
Initially, SunGen has committed the sales and marketing rights for two specialty pharmaceuticals—terbutaline drug products and injectable lincomycin, as well as other related lincomycin products which are in the development stage to the joint venture. We have agreed to pay any manufacturing costs of terbutaline, our portion of the development and manufacturing costs of lincomycin, $375,000, and marketing and sales expenses for both. We have agreed to equal profit sharing of all profits of the joint venture, except for any profits arising from sales of terbutaline injectable products, which shall be allocated 75% to SunGen and 25% to us.
In November 2016, we signed an addendum to the agreement to add Desmopressin Acetate Injection to the existing SunGen joint venture agreement. Through the joint venture, we and SunGen have agreed to pay to a third party an aggregate of $200,000 for the rights to the product. We and SunGen will each pay $40,000 upfront, with an additional $60,000 payable by each of us upon FDA approval of the product. In addition we and SunGen have each agreed to pay 25% of the one-time ANDA filing fee of $70,480, and 4% each of the annual FDA facility fees. The term of the addendum is ten years from the date of ANDA approval by the FDA, and renews automatically for one year periods, unless terminated in writing six months prior to the end of the term or automatic extension. 50% of the profits from the sale of Desmopressin Acetate under this addendum will be payable to the third party, with the remaining 50% paid to the joint venture between us and SunGen.
The term of the SunGen Agreement shall continue for 99 years, unless the parties mutually agree to terminate it earlier. The agreement also contains customary termination rights for either party in the event of a breach of the agreement by the other party.
Pemetrexed, Supply Agreement Term Sheet
In December 2016, we entered into a binding term sheet with Nang-Kuang Pharmaceutical Co., LTD and CANDA NK-2, LLC, two affiliated pharmaceutical suppliers, pursuant to which we will enter into a definitive
146
Table of Contents
agreement for exclusive distribution of a generic injectable oncology product for the U.S. market. The generic product is not currently marketed in the U.S. pending the outcome of an ongoing challenge to the validity of patent protection of the branded product currently on the market. The ANDA for this product has been filed by the suppliers with the FDA and is awaiting approval. Upon signing of the term sheet, we were obligated to make a prepayment for a total of $12.0 million, of which $3.0 million was paid in January and February 2017, with the remaining $9.0 million due on May 1, 2017.
Under the agreement, we will make two additional product transfer payments, one equal to a premium between 10% and 20% over the cost incurred by the suppliers to produce and ship the product after confirmation of each purchase order of the product and, after receiving such product, we will make the other payment quarterly to the suppliers of between 40% and 60% of the earnings from sales, less certain expenses, of the product.
The initial term of the definitive agreement will begin on the signing date and continue through ten years after the date of our first commercial sale of the product, subject to automatic renewals of successive two-year terms, unless terminated by either party with six months’ notice prior to the expiration of the initial term or any renewal term. The agreement will also contain customary termination rights for either party in the event of a material breach of the agreement by the other party or bankruptcy or insolvency. In addition, we will be able to terminate the agreement with 30 days’ notice to the suppliers if the net profits from sales of the product, less certain expenses, equals zero or less and the parties cannot agree on reductions to the actual cost of the products.
Amphastar Agreement
In February 2017, we entered into a definitive agreement with Amphastar Pharmaceuticals, Inc., or Amphastar, to acquire 14 ANDAs and inventory for certain APIs. The agreement requires payments of up to $6.4 million, of which $1.0 million was paid upon execution of the agreement, $1.0 million is due within thirty days following May 1, 2017, $3.0 million is due within thirty days of receiving FDA approval of site transfer to sell Prochlorperazine Edisylate Injection USP, and $1.4 million is due within thirty days of receiving FDA approval of site transfer of a second product. If the conditions relating to the third and fourth payments described above are not met by December 31, 2017, we shall make such payments within 30 days of December 31, 2017 regardless of whether such conditions are ever met. In addition to the payments described above, we have agreed to pay Amphastar a royalty fee equal to 2% of our net sales relating to the 14 ANDAs and API inventory transferred to us by Amphstar for a period of 10 years from the execution of the agreement.
Summary of Commercial Strategy & Source of Supply Chain
| Product line |
Proprietary Oral / |
Multisource / Specialty |
503B Products |
API | ||||
| Launch date |
To be determined | 2017 | 2017 | Ongoing | ||||
| Commercialization regions |
U.S., EU, China | U.S. | U.S. | U.S., EU, China | ||||
| Manufacturing sites |
Clarence, NY, Dunkirk, NY, Chongqing, China |
Partner network, Dunkirk, NY, Chongqing, China | Clarence, NY, Dunkirk, NY
|
Chongqing, China
|
Global Supply Chain Platform
We believe it is important to minimize potential disruptions associated with a high potency oncology pharmaceutical supply chain. Therefore, we have begun the process of internalizing key components of the supply chain we believe are integral to minimizing these risks and retaining value for shareholders. For example, the World Health Organization lists paclitaxel as an essential medicine. Paclitaxel is derived from the bark of the Pacific yew tree and harvestable trees for the starting biomass are globally limited in supply. While current
147
Table of Contents
supply of the starting biomass for paclitaxel may be sufficient to meet global paclitaxel API demand, we believe future shortages are possible if we are successful in the commercialization of one of our lead drug candidates, Oraxol. We believe this increased demand could lead to shortages of paclitaxel API potentially leading to market and supply disruptions.
Our research group evaluated the purity and potency of some of the largest global suppliers of paclitaxel API. In 2015, we acquired one of these suppliers, Polymed Therapeutics Inc., or Polymed, and Chongqing Taihao Pharmaceutical Co. Ltd., or Taihao. Taihao is a cGMP manufacturer of high potency oncology API based in Chongqing, China and Polymed is the U.S. marketing entity for Taihao’s API in North America and Europe. Historical production and sales of API by this subsidiary were to third parties. We anticipate a greater share of Taihao’s manufacturing capacity will be used for our internal needs in the future and, therefore, sales to third parties may decrease. Historically, Polymed sold certain of these API products internationally to mostly large multi-national pharmaceutical companies. For the years ended December 31, 2015 and 2016, 29% and 38%, respectively, of our total revenue came from Intas Pharmaceuticals and 17% and 24%, respectively, came from Ebewe Pharmaceuticals. We have not entered into any agreements with any of our customers, and no other customer accounted for sales representing more than 10% of our consolidated total revenue for the years ended December 31, 2015 and 2016.
In 2014 we sought to obtain better control over our manufacturing of high potency oncology drugs used in global clinical studies, and in the third quarter of 2014 acquired QuaDPharma, one of our suppliers based in Clarence, New York. The number of our clinical studies has grown since the close of the acquisition. We are currently standardizing and leveraging the acquired cGMP systems and operating procedures in anticipation of developing multi-cGMP large scale manufacturing plants in both the U.S. and China. Our Commercial Platform has also initiated an assessment for a transition and potential expansion of these facilities to produce FDA shortage products.
Facilities, Manufacturing and Quality Assurance
Our facilities are located in the U.S., China and Hong Kong, and we own and lease a variety of manufacturing, research and development, distribution, packaging, laboratory, office and warehouse space. Our GMP operating facilities are inspected by the FDA, and we believe that all of our facilities are being operated in material compliance with the FDA’s cGMP regulations where applicable.
Our material facilities in Buffalo, New York, Dunkirk, New York and Chongqing, China have been, and are being, paid for through public-private partnerships, and will be available for our exclusive use at below-market rates, for the foreseeable future. These material facilities are described further in the descriptions of our public-private partnerships below.
New York State Partnership
In May 2015, we entered into an agreement with Fort Schuyler Management Corporation, or FSMC, a not-for-profit corporation owned by the State of New York, for a medical technology research, development, innovation, and commercialization alliance. Under the agreement, FSMC will pay up to $25 million for the construction of our North American headquarters and formulation lab and equipment in Buffalo, New York. We moved into the North American Headquarters in October 2015 and will lease from FSMC for a 10-year term, with an option to extend the term for an additional 10 years. For the first three years of the lease, we will pay rent to FSMC equal to 35% of FSMC’s operating costs for the space and thereafter will pay 100% of FSMC’s operating costs for the space for the remainder of the term. Under the agreement, we are obligated to spend $100 million in the Buffalo area over the initial 10-year term of the lease, and an additional $100 million during the second 10-year term, if we elect to extend the lease. We also committed to hiring 250 permanent employees within the first 5 years in the Buffalo area.
148
Table of Contents
Under the same May 2015 agreement, FSMC also agreed to fund the costs of construction of a new manufacturing facility in Dunkirk, New York. Under the current arrangement, we will select and hire contractors for the project and oversee the development of the facility. Empire State Development, the parent entity of FSMC, is responsible for the costs of construction and all equipment for the facility, up to an aggregate of $200 million, and ESD, not us, will own the facility and equipment. We are entitled to lease the facility and all equipment at a rate of $1.00 per year for an initial 10-year term, and for the same rate if we elect to extend the lease for an additional 10-year term. We are responsible for all operating costs and expenses for the facility. In exchange, we have committed to spending $1.5 billion on operational expenses in our first 10-year term in the facility, and an additional $1.5 billion on operational expenses if we elect to extend the lease for a second 10-year term. We also committed to hiring 450 employees within the first 5 years at our Dunkirk facility.
China Partnership
In October 2015, we entered into an agreement with Chongqing Maliu Riverside Development & Investment Co., Ltd., or CQ, which is wholly owned by the Finance Bureau of Banan district of Chongqing, and is authorized to be responsible for investments, financing, infrastructure construction, operations and management in the Chongqing Maliu Riverside Development Zone. Our agreement with CQ provides for the construction of both a formulation plant and an API plant in the PRC. After entering into the agreement, and pursuant to its terms, we established a PRC-based subsidiary that is responsible for the operations of both facilities in July 2016. CQ is now responsible for construction of both facilities according to U.S. GMP standards. Although the agreement contemplates completion by the end of 2016, we estimate the construction of the new API plant will be completed by the end of 2017 and that we will begin utilizing the facility in 2018. The land and buildings will be owned by CQ, and we will lease the facilities, rent-free, for the first 10-year term, with an option to extend the lease for an additional 10-year term, during which, if we are profitable, we will pay a monthly rent of 5 RMB per square meter of space occupied. We are responsible for the costs of all equipment for the facilities, and we have committed to occupying and beginning to use the facilities within six months of the completion of construction. We have also committed to achieving certain operational, revenue and tax generation milestones within certain time periods once we commence operations.
Our goal is to use our public-private partnerships as a capital efficient method for large scale cGMP manufacturing within our supply chain and to facilitate market access in China. We believe our current facilities will be adequate and suitable for our operations for the foreseeable future.
To date, we have utilized a combination of acquisitions and public-private partnerships to internalize certain key components of our manufacturing and supply chain. We expect to continue to use a combination of collaborations and acquisitions to continue to build out elements of our supply chain where needed as a mechanism to minimize execution risk and retain value for our shareholders.
Segment and Geographic Information
For financial reporting purposes, we present three distinct financial reporting segments based on criteria established by U.S. GAAP: (1) Oncology Innovation Platform, for the discovery and development of cancer supportive therapies, (2) Commercial Platform, the manufacturing of clinical and commercial pharmaceutical products, and (3) Global Supply Chain Platform, the manufacturing and marketing of API and medical devices. For a description of our revenues and long-lived assets by geographic location and more information on our reporting segments, please see Note 2 of the Notes to our consolidated financial statements included elsewhere in this prospectus.
Intellectual Property
We strive to protect and enhance the proprietary technologies, inventions, products and product candidates, methods of manufacture, methods of using our products and product candidates, and improvements thereof that
149
Table of Contents
are commercially important to our business. We protect our proprietary intellectual property position by, among other things, filing patent applications in the U.S. and in jurisdictions outside of the U.S. covering our proprietary technologies, inventions, products and product candidates, methods, and improvements that are important to the development and implementation of our business. We also rely on trade secrets, know-how, continuing innovation, and licensing opportunities to develop, strengthen and maintain our proprietary intellectual property position. As of March 31, 2017, we own more than 100 granted patents and more than 40 pending patent applications worldwide. In addition, we have in-licensed patents and patent applications relating to our Orascovery platform technology from Hanmi. In our Orascovery platform, the lead compound is covered as composition-of-matter in granted patents in the U.S. and other territories, such as China and Europe. These patents will expire in October 2023 or 2024, excluding any potential patent term adjustments and/or patent term extensions that may be available. The lead compounds in our Src Kinase Inhibition platform are covered as composition-of-matter in granted patents in the US and other territories, including China and Europe. These patents will begin to expire in December 2025, excluding any potential patent term adjustments and/or patent term extensions that may be available.
The term of individual patents depends upon the laws of the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing of a non-provisional patent application. In the U.S., the term of a patent may be lengthened by patent term adjustment to compensate the patentee for administrative delays by the USPTO in examining and granting the patent, or may be shortened if the patent is terminally disclaimed over an earlier-filed patent. In addition, a patent term may be extended to restore a portion of the term effectively lost as a result of FDA regulatory review. However, the restoration period cannot be longer than five years and cannot extend the remaining term of a patent beyond a total of 14 years from the date of FDA approval, and only one patent applicable to an approved drug may be extended. Similar extensions as compensation for regulatory delays are available in Europe and other jurisdictions. We intend to seek patent term extensions where these are available. However there is no guarantee that the applicable authorities, including the FDA in the U.S., will agree with our assessment of whether such extensions should be granted, and we cannot predict the length of the extensions even if they are granted. The actual protection afforded by a patent varies on a claim-by-claim basis, from country-to-country, and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country, and the validity and enforceability of the patent. For a granted patent to remain in force most countries require the payment of annuities or maintenance fees, either yearly or at certain intervals during the term of a patent. If an annuity or maintenance fee is not paid, the patent may lapse irrevocably.
We own 16 granted patents in the U.S. and 99 granted patents in other countries relating to our Src Kinase Inhibition technology. In addition, we own 37 pending applications relating to the Src Kinase Inhibition technology in the U.S. and other countries. These patents and pending patent applications contain composition-of-matter claims to our lead product candidates and their analogs, claims to pharmaceutical compositions comprising such candidates, and claims to methods of making and method of treatment using such candidates. Not accounting for any patent term adjustment, patent term extension or terminal disclaimer, and assuming that all annuity and/or maintenance fees are paid, the patents and, if granted, patent applications, will expire from 2025 to 2038.
In addition, we have one pending patent application in each of the Patent Cooperation Treaty, or PCT, the Gulf Cooperation Council, Jordan and Taiwan. The PCT application can be filed worldwide by entering national stage in various member states by January 21, 2018. These pending patent applications relate to therapeutic combinations of orally administered paclitaxel and a P-gp inhibitor. Not accounting for any patent term adjustment, patent term extension or terminal disclaimer, and assuming that all annuity and/or maintenance fees are paid, the patent applications, if granted, will expire in 2036.
150
Table of Contents
License and Collaboration Agreements
Hanmi Licensing Agreements
Out-License
In April 2011, we entered into a license agreement with Hanmi, which we refer to as the Hanmi Out-License, pursuant to which we granted to Hanmi an exclusive, sublicensable license to use certain of our intellectual property for development and commercialization of products containing KX-01 in certain territory including South Korea, China, Taiwan, Hong Kong, Singapore, Malaysia, Thailand, the Philippines, Indonesia and Vietnam. We also granted to Hanmi a right of first refusal for any KX-01 related formulation or pharmaceutical that we develop and intend to grant an exclusive license for in the territory covered by the Hanmi Out-License. Hanmi is responsible for all development, manufacturing and commercialization, and the related costs and expenses, of any product candidates resulting from the agreement.
We received an upfront payment of $1.5 million from Hanmi upon effectiveness of the agreement, and we may be entitled to receive an aggregate of $4.0 million in additional development and regulatory milestone payments. We are also eligible to receive tiered royalty payments in the teens on net sales of each product commercialized by Hanmi utilizing the intellectual property subject to the Hanmi Out-License. Such royalties will be reduced as competing generic products gain market share in the applicable country.
The term of the Hanmi Out-License expires on the earlier of (i) expiration of the last of our patent rights licensed under the agreement or (ii) invalidation of our patent rights which are the subject of the agreement, provided that in each case the term will automatically be extended for consecutive one-year periods unless either party gives notice to the other at least 90 days prior to expiration of the patent rights licensed under the agreement or before the then-current annual expiration date of the agreement. Prior to the expiration of the term of the agreement, Hanmi may terminate the agreement in its sole discretion by providing six months’ notice to us. Subject to certain conditions, we may also terminate the agreement if Hanmi fails to comply with certain development timelines set forth in the Hanmi Out-License. The agreement also contains customary termination rights for either party, such as in the event of a breach of the agreement or the initiation of bankruptcy proceedings by the other party.
In September 2012, we entered into a memorandum of understanding with Hanmi, related to the Hanmi Out-License, pursuant to which we agreed to jointly enter into an agreement with a contract manufacturing organization to manufacture KX-01 for our and Hanmi’s needs, and to determine cost-sharing between us and Hanmi for such services. We have subsequently sourced certain clinical supplies from Formex L.L.C., pursuant to this agreement.
In-Licenses
In December 2011 and June 2013 we entered into two separate in-licensing agreements with Hanmi pursuant to which Hanmi granted us licenses to certain patents and know-how with respect to Hanmi’s Orascovery Program to research, discover and develop compounds that enhance or increase the oral absorption of active pharmaceutical ingredients.
The December 2011 agreement, which we refer to as the 2011 Hanmi Agreement, granted us an exclusive, sublicensable license for development and commercialization activities utilizing Hanmi’s patents and know-how related to the Orascovery Program in a certain territory including North America, South America, the European Union, Australia, New Zealand, Russia, Eastern Europe, Taiwan and Hong Kong, and a non-exclusive license to utilize the same intellectual property in manufacturing worldwide for sales inside those territories. The June 2013 agreement, which we refer to as the 2013 Hanmi Agreement, granted us an exclusive, sublicensable license comparable to the 2011 Hanmi Agreement solely for China. The 2011 Hanmi Agreement was amended in November 2012 to add Macau and Singapore to the territory governed by the agreement; in October 2013 to add Malaysia, Thailand, Vietnam, the Philippines and Indonesia; in March 2015 to add India; and again in March 2017 to add Japan.
151
Table of Contents
Upon effectiveness of the 2011 Hanmi Agreement we made an upfront payment of $250,000 to Hanmi, and we will pay Hanmi tiered royalty payments in the teens based on aggregate net sales of any products using the licensed intellectual property in the territory. Such royalties will be reduced if competing generic products gain market share in the applicable country. Depending on when we receive regulatory approval of a product using the intellectual property licensed from Hanmi in the U.S. or Europe, we may be obligated to pay Hanmi a regulatory bonus worth $24,000,000, to be paid (i) upon the occurrence of a liquidity event, if the regulatory approval has already been received, or (ii) upon receipt of the regulatory approval, if such approval is received after a liquidity event. We will also be required to pay Hanmi an exit bonus, in shares of our common stock, worth $5,000,000 upon the completion of this offering. In connection with the March 2015 amendment to the 2011 Hanmi Agreement, we made an upfront payment of $50,000 to Hanmi. Additionally, in connection with the March 2017 amendment to the 2011 Hanmi Agreement, we issued a $7.0 million convertible bond to Hanmi in lieu of an upfront payment. At the election of Hanmi, the convertible bond will be converted into a number of shares of common stock equal to the outstanding amount of the convertible bond divided by the discount price (which is variable based on the date of conversion) at certain specified dates. If Hanmi has not elected to convert the convertible bond to common stock prior to October 1, 2018, Hanmi may elect to convert the convertible bond to: (i) common stock equal to the outstanding amount of the convertible bond divided by the discount price, or (ii) cash equal to $7.0 million plus interest at 10% per annum accruing from March 7, 2017 to October 1, 2018. Pursuant to the March 2017 amendment to the 2011 Hanmi Agreement, we will be required to make payments to Hanmi worth up to $6.0 million in our common stock or in cash upon the occurrence of certain regulatory and sales milestones.
Upon effectiveness of the 2013 Hanmi Agreement we made an upfront payment of $100,000 to Hanmi, and we will pay Hanmi tiered royalty payments in the teens based on net sales of any products using the licensed intellectual property in China. The royalties shall be reduced if competing generic products gain market share in China. We also granted to Hanmi a one-time right of first negotiation to purchase all of our rights in Oraxol or Oratecan under the agreement during development and prior that, at Hanmi’s discretion, requires us to negotiate in good faith the sale of our rights under such agreement to Hanmi at a purchase price determined by an internationally-recognized investment banking firm with an office in Hong Kong at any time prior to the earlier of (i) our first commercial sale of products using such technology or (ii) receipt by Hanmi of written notice from our company of the sublicense of the rights in an applicable product to a third party.
Under each agreement, we are responsible for all clinical studies and development and commercialization activities, and the related expenses, resulting from the agreements. Each of the 2011 Hanmi Agreement and the 2013 Hanmi Agreement expires on the earlier of (i) expiration of the last of Hanmi’s patent rights licensed under the agreement or (ii) invalidation of Hanmi’s patent rights which are the subject of the agreement, provided that the term will automatically be extended for consecutive one year periods unless either party gives notice to the in each case other at least 90 days prior to expiration of the patent rights licensed under the agreement or before the then-current annual expiration date of the agreement. The patent rights licensed to us under the 2011 and 2013 Hanmi Agreements have expiry dates ranging from in 2023 to 2033, unless the terms of such licensed patents are able to be extended in accordance with applicable laws and regulations.
Hanmi may also terminate the 2011 Hanmi Agreement if (i) we fail to file an IND with the FDA for Oraxol within 6 months of the latest of (x) our receipt from Hanmi of all English translations necessary for the filing of an IND with the FDA, (y) the date we and Hanmi agree that all studies necessary for the filing of an IND with the FDA have been completed, or (z) the date of the final study report for the last of any additional studies that are necessary for the filing of an IND with the FDA, or (ii) we fail to commence clinical studies for Oraxol within twelve months after the date of approval of an IND by the FDA.
The 2013 Hanmi Agreement may be terminated by Hanmi if (i) we fail to file an IND for Oraxol with the CFDA within six months after the latest of (w) completion of all Chinese translations necessary for the filing of an IND with the CFDA, (x) completion of all manufacturing and toxicology studies necessary for the filing of an IND with the CFDA, (y) the date we and Hanmi agree that all studies necessary for the filing of an IND with the
152
Table of Contents
CFDA have been completed, or (z) the date of the final study report for the last of any additional studies that are necessary for the filing of an IND with the CFDA, or (ii) we fail to commence clinical studies for Oraxol within twelve months after the date of approval of an IND by the CFDA.
Such clinical development milestones in respect of the termination right in both the 2011 Hanmi Agreement and the 2013 Hanmi Agreement may be extended for 12 months if we reasonably request.
Prior to the expiration of the term of each agreement, we may terminate the agreement in our sole discretion, by providing six months notice to Hanmi. Subject to certain conditions. The agreements also contain customary termination rights for either party, such as in the event of a breach of the agreement or the initiation of bankruptcy proceedings by the other party or by mutual agreement.
ZenRx License Agreement
In April 2013, we entered into a license agreement with ZenRx, which we refer to as the ZenRx License, pursuant to which we granted to ZenRx an exclusive, sublicensable license to use certain of our intellectual property to develop and commercialize Oratecan and Oraxol in Australia and New Zealand, and a non-exclusive license to manufacture a certain compound, but only for use in Oratecan and Oraxol. ZenRx is responsible for all development, manufacturing and commercialization, and the related costs and expenses, of any product candidates resulting from the agreement.
We received a $50,000 payment from ZenRx upon effectiveness of the agreement, and we may be entitled to receive up to an aggregate of $1.4 million in additional development, regulatory and sales milestone payments. We will also be eligible to receive tiered royalties in the mid-teens on net sales of each product commercialized by ZenRx utilizing the intellectual property that is the subject of the ZenRx License. Such royalties will be reduced by 40% when competing generic products have 30% of the market share in the applicable country, and will be eliminated entirely when competing generic products have 60% of the market share in the applicable country.
As an incentive to ZenRx to further development and commercialization of Oratecan and Oraxol in the territory, if ZenRx obtains certain regulatory approvals in the territory prior to regulatory approval of those products in either the U.S. or South Korea, we may be required to make payments to ZenRx, at ZenRx’s option, either up to $600,000 in cash or $350,000 in cash plus $250,000 worth of our common stock.
The term of the ZenRx License expires on the earlier of (i) expiration of the last of our patent rights licensed under the agreement or (ii) invalidation of our patent rights which are the subject of the agreement, provided that the term will automatically be extended for consecutive one year periods unless either party gives notice to the other at least 90 days prior to expiration of the patent rights licensed under the agreement or before the then current annual expiration date of the agreement. Prior to the expiration of the term of the agreement, ZenRx may terminate the agreement in its sole discretion, by providing three months notice to us. Subject to certain conditions, we may also terminate the agreement if ZenRx fails to comply with certain development timelines set forth in the ZenRx License. The agreement also contains customary termination rights for either party, such as in the event of a breach of the agreement or the initiation of bankruptcy proceedings by the other party.
PharmaEssentia License Agreements
In December 2011 and December 2013, we entered into two separate out-licensing agreements with PharmaEssentia Corp., or PharmaEssentia, pursuant to which we granted to PharmaEssentia certain licenses to our intellectual property for use in development and commercialization of certain products in specific territories.
The December 2011 agreement, which we refer to as the 2011 PharmaEssentia Agreement, granted an exclusive, sublicensable license to use any pharmaceutical preparation containing KX-01 or KX-02 for use in
153
Table of Contents
treating psoriasis or other non-malignant skin conditions in a territory that includes China, Taiwan, Macau, Hong Kong, Singapore and Malaysia. In December 2016, we agreed to amend the 2011 PharmaEssentia Agreement such that the field under the license agreement does not include AK for any country in the territory except Taiwan.
We received a $40,000 payment from PharmaEssentia upon effectiveness of the 2011 PharmaEssentia Agreement, and we may be entitled to an aggregate of up to $1.6 million in additional development and regulatory milestone payments, $250,000 of which may be paid in the form of PharmaEssentia stock. PharmaEssentia has discretion to offer to make such payment in the form of its stock, and we have discretion as to whether to accept such payment in the form of its stock. We will also be eligible to receive tiered royalties ranging from the high single-digits to teens on net sales of each product commercialized by PharmaEssentia utilizing the intellectual property that is the subject of the 2011 PharmaEssentia Agreement. Such royalties will be reduced by 40% when competing generic products have 30% of the market share in the applicable country, and will be eliminated entirely when competing generic products have 60% of the market share in the applicable country.
The December 2013 agreement, which we refer to as the 2013 PharmaEssentia Agreement, granted an exclusive, sublicensable license for development and commercialization of Oraxol and Oratecan in Taiwan and Singapore. Under the agreement, PharmaEssentia may also have the right to expand its license to include China, if Hanmi does not exercise its right of first refusal to such a product candidate under the Hanmi Out-License. In December 2016, we agreed to amend the 2013 PharmaEssentia Agreement to also include Vietnam in the territories covered by the license, provided that, if PharmaEssentia has not completed a submission for regulatory approval in Vietnam by 2021, the rights under the license in Vietnam will be returned to us.
We received a $50,000 payment from PharmaEssentia upon effectiveness of the 2013 PharmaEssentia Agreement, and we may be entitled to an aggregate of up to $2.0 million in additional development, regulatory and sales milestone payments, and we may also be obligated to pay PharmaEssentia an aggregate of $1.0 million in incentives if PharmaEssentia achieves certain milestones within designated timeframes. We will also be eligible to receive tiered royalties in the mid-teens on net sales of each product commercialized by PharmaEssentia utilizing the intellectual property that is the subject of the 2013 PharmaEssentia Agreement. Such royalties will be reduced by 40% when competing generic products have 30% of the market share in the applicable country, and will be eliminated entirely when competing generic products have 60% of the market share in the applicable country.
Under each agreement, PharmaEssentia is responsible for all clinical studies and development and commercialization activities, and the related expenses, resulting from the agreements. Each of the 2011 PharmaEssentia Agreement and the 2013 PharmaEssentia Agreement expire on the earlier of (i) expiration of the last of our patent rights licensed under the agreement or (ii) invalidation of our patent rights which are the subject of the agreement, provided that the term will automatically be extended for consecutive one year periods unless either party gives notice to the other at least 90 days prior to expiration of the patent rights licensed under the agreement or before the then current annual expiration date of the agreement.
Prior to the expiration of the term of each agreement, PharmaEssentia may terminate the agreement in its sole discretion, by providing six months notice to us. Subject to certain conditions, we may also terminate the agreement if PharmaEssentia fails to comply with certain development timelines set out in each of the agreements. The agreements also contain customary termination rights for either party, such as in the event of a breach of the agreement or the initiation of bankruptcy proceedings by the other party.
Guangzhou Xiangxue License Agreement
In May 2012, we entered into a license agreement with Guangzhou Xiangxue New Drug Discovery and Development Company Limited, or Xiangxue, which we refer to as the Xiangxue License, pursuant to which we
154
Table of Contents
granted to Xiangxue an exclusive, sublicensable license to use certain of our intellectual property to develop and commercialize products containing KX-02 in all indications for brain tumors in China, Taiwan, Hong Kong and Singapore. Xiangxue is responsible for all development, manufacturing and commercialization, and the related costs and expenses, of any product candidates resulting from the agreement.
We received a $750,000 payment from Xiangxue upon effectiveness of the agreement, and we may be entitled to receive an aggregate of up to $5.3 million in additional development and regulatory milestone payments. We will also be eligible to receive royalties in the teens on net sales of each product commercialized by Xiangxue utilizing the intellectual property that is the subject of the Xiangxue License. Such royalties will be reduced by 40% when competing generic products have 30% of the market share in the applicable country, and will be eliminated entirely when competing generic products have 60% of the market share in the applicable country.
The term of the Xiangxue License expires on the earlier of (i) expiration of the last of our patent rights licensed under the agreement or (ii) invalidation of our patent rights which are the subject of the agreement, provided that the term will automatically be extended for consecutive one year periods unless either party gives notice to the other at least 90 days prior to expiration of the patent rights licensed under the agreement or before the then current annual expiration date of the agreement. Prior to the expiration of the term of the agreement, Xiangxue may terminate the agreement in its sole discretion, by providing six months notice to us. Subject to certain conditions, we may also terminate the agreement if Xiangxue fails to comply with certain development timelines set forth in the Xiangxue License. The agreement also contains customary termination rights for either party, such as in the event of a breach of the agreement or the initiation of bankruptcy proceedings by the other party.
Eli Lilly and Company Agreement
In October 2016, we entered into a Clinical Trial Collaboration and Supply Agreement with Eli Lilly and Company, which we refer to as the Lilly Agreement, under which we and Lilly will conduct a Phase 1b trial of Oraxol in combination with Lilly’s ramucirumab in patients with gastric, gastro-esophageal and esophageal cancers. Under the terms of the Lilly Agreement we will act as the sponsor of the study and will hold the IND/CTA relating to the study, while all clinical data generated under the study will be jointly owned by us and Lilly. Other than Lilly’s obligation to supply ramucirumab to us, we will be responsible for all other costs associated with the conduct of the study.
The Lilly Agreement will remain in effect until the study contemplated by the agreement has been completed. The agreement also contains customary termination rights for either party, such as in the event of a breach of the agreement by the other party, or in the event a regulatory authority takes any action against or raises any objection to the study.
DLSS Agreement
In August 2016, we entered into an agreement with Dohmen Life Science Services, or DLSS, pursuant to which DLSS will provide a variety of logistics and related services to us, including warehousing and shipment services, order-to-cash services, contract administration services and chargeback processing.
The initial term of the DLSS Agreement is three years following the initial delivery date, and will automatically renew for successive 12-month periods, unless either we or DLSS give notice of intent to terminate at least 90 days in advance of such automatic renewal. We may also have the opportunity to terminate the DLSS Agreement within 30 days of receiving notice of certain price increases by DLSS. The agreement also contains customary termination rights for either party, such as in the event of a breach of the agreement.
155
Table of Contents
Competition
The biopharmaceutical industry and the oncology subsector are characterized by rapid evolution of technologies, fierce competition and strong defense of intellectual property. Any product candidates that we successfully develop and commercialize will have to compete with existing therapies and new therapies that may become available in the future. While we believe that our product candidates, platforms and scientific expertise in the field of biotechnology and oncology provide us with competitive advantages, a wide variety of institutions, including large biopharmaceutical companies, specialty biotechnology companies, academic research departments and public and private research institutions, are actively developing potentially competitive products and technologies. We face substantial competition from biotechnology and biopharmaceutical companies developing oncology products. These competitors generally fall within the following categories:
Oral administration: Taxol, Abraxane, Cynviloq, Camptosar, Onivyde, Taxotere and Hycamtin;
Src Kinase inhibitors: Picato and Temodar.
Many of our competitors, either alone or with strategic partners, have substantially greater financial, technical and human resources than we do. Accordingly, our competitors may be more successful than us in obtaining approval for treatments and achieving widespread market acceptance, rendering our treatments obsolete or non-competitive. Accelerated merger and acquisition activity in the biotechnology and biopharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors. These companies also compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical study sites and patient registration for clinical studies, acquiring technologies complementary to, or necessary for, our programs and for sales in the API business. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Our commercial opportunity could be substantially limited in the event that our competitors develop and commercialize products that are more effective, safer, less toxic, more convenient or less expensive than our comparable products. In geographies that are critical to our commercial success, competitors may also obtain regulatory approvals before us, resulting in our competitors building a strong market position in advance of our products’ entry. We believe the factors determining the success of our programs will be the efficacy, safety and convenience of our product candidates and our access to supply of API.
Government Regulation and Product Approval
Governmental authorities in the U.S., at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, approval, quality control, labeling, packaging, promotion, storage, advertising, distribution, post-approval monitoring, marketing and export and import of products such as those we are developing. Our therapeutic candidates must be approved by the FDA through the NDA process before they may be legally marketed in the U.S. and will be subject to similar requirements in other countries prior to marketing in those countries. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources.
U.S. Government Regulation
In the U.S., the FDA regulates drugs under the FDCA and its implementing regulations. Failure to comply with the applicable U.S. requirements at any time during the product development or approval process, or after approval, may subject an applicant to administrative or judicial sanctions, any of which could have a material adverse effect on us. These sanctions could include:
| • | refusal to approve pending applications; |
| • | withdrawal of an approval; |
156
Table of Contents
| • | imposition of a clinical hold; |
| • | warning or untitled letters; |
| • | seizures or administrative detention of product; |
| • | total or partial suspension of production or distribution; or |
| • | injunctions, fines, restitution, disgorgement, refusal of government contracts, or civil or criminal penalties. |
NDA approval processes
The process required by the FDA before a therapeutic product may be marketed in the U.S. generally involves the following:
| • | completion of extensive nonclinical laboratory tests, animal studies and formulation studies conducted according to GLPs, and other applicable regulations; |
| • | submission to the FDA of an IND, which must be allowed before human clinical trials may begin; |
| • | performance of adequate and well-controlled human clinical trials according to Good Clinical Practices, or GCPs, to establish the safety and efficacy of the product candidate for its intended use; |
| • | submission to the FDA of an NDA or; |
| • | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the product candidate is produced to assess readiness for commercial manufacturing and conformance to the manufacturing-related elements of the application, to conduct a data integrity audit, and to assess compliance with cGMPs to assure that the facilities, methods and controls are adequate to preserve the product candidate’s identity, strength, quality and purity; |
| • | potential FDA audit of the clinical trial sites that generated the data in support of the NDA; and |
| • | FDA review and approval of the NDA. |
Once a pharmaceutical candidate is identified for development, it enters the preclinical or nonclinical testing stage. Nonclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. Such studies must generally be conducted in accordance with the FDA’s GLPs. An IND sponsor must submit the results of the nonclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. Some nonclinical testing may continue even after the IND is submitted. In addition to including the results of the nonclinical studies, the IND will also include a protocol detailing, among other things, the objectives of the clinical trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated if the first phase lends itself to an efficacy determination. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, places the IND on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can begin. A clinical hold may occur at any time during the life of an IND and may affect one or more specific studies or all studies conducted under the IND.
The manufacture of investigational drugs for the conduct of human clinical trials is subject to cGMP requirements. Investigational drugs and API imported into the U.S. are also subject to regulation by the FDA relating to their labeling and distribution. Further, the export of investigational drug products outside of the U.S. may be subject to regulatory requirements of the receiving country as well as U.S. export requirements under the FDCA.
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with GCP requirements, which include, among other things, the requirements that all research
157
Table of Contents
subjects provide their informed consent in writing for their participation in any clinical trial. Investigators must also provide certain information to the clinical trial sponsors to allow the sponsors to make certain financial disclosures to the FDA. Clinical trials must be conducted under protocols detailing the objectives of the trial, dosing procedures, research subject selection and exclusion criteria and the safety and effectiveness criteria to be evaluated. Each protocol, and any subsequent material amendment to the protocol, must be submitted to the FDA as part of the IND, and progress reports detailing the status of the clinical trials must be submitted to the FDA annually. Sponsors also must report to the FDA serious and unexpected adverse reactions in a timely manner. Reporting requirements also apply to, among other things, any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator’s brochure and any findings from other studies or animal or in vitro testing that suggest a significant risk in humans exposed to the product candidate. An institutional review board , or IRB, at each institution participating in the clinical trial must review and approve the protocol before a clinical trial commences at that institution and must also approve the information regarding the trial and the consent form that must be provided to each research subject or the subject’s legal representative, monitor the study until completed and otherwise comply with IRB regulations. There are also requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries within a certain timeframe for public dissemination on the National Institutes of Health clinicaltrials.gov website.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined.
| • | Phase 1—The product candidate is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and elimination. In the case of some therapeutic candidates for severe or life-threatening diseases, such as cancer, especially when the product candidate may be inherently too toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients. |
| • | Phase 2—Clinical trials are performed on a limited patient population intended to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| • | Phase 3—Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to establish the overall risk-benefit ratio of the product and provide an adequate basis for product labeling. |
A pivotal study is a clinical study that adequately meets regulatory agency requirements for the evaluation of a product candidate’s efficacy and safety such that it can be used to justify the approval of the product. Generally, pivotal studies are also Phase 3 studies but may be Phase 2 studies, with the agreement of FDA, if the trial design provides a reliable assessment of clinical benefit, particularly in situations where there is an unmet medical need.
In the case of a 505(b)(2) NDA, which is a marketing application in which the sponsor may rely on investigations that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use from the person by or for whom the investigations were conducted, some of the above-described studies and nonclinical studies may not be required or may be abbreviated. The applicant may rely upon the FDA’s prior findings of safety and efficacy for a previously approved product or on published scientific literature in support of its application. Bridging studies, including clinical studies, may be needed, however, to demonstrate the applicability of the studies that were previously conducted by other sponsors to the drug that is the subject of the marketing application.
Human clinical trials are inherently uncertain and Phase 1, Phase 2 and Phase 3 testing may not be successfully completed or may not be completed at all. The FDA or the sponsor may suspend a clinical trial at any time for a variety of reasons, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the product candidate has been associated with unexpected serious harm to patients.
158
Table of Contents
During the development of a new product candidate, sponsors are given opportunities to meet with the FDA at certain points; specifically, prior to the submission of an IND, at the end of Phase 2 and before an NDA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date and for the FDA to provide advice on the next phase of development. Sponsors typically use the meeting at the end of Phase 2 to discuss their Phase 2 clinical results and present their plans for the pivotal Phase 3 clinical trial that they believe will support the approval of the new therapeutic. If a Phase 3 clinical trial is the subject of discussion at the end of Phase 2 meeting with the FDA, a sponsor may be able to request a Special Protocol Assessment, or SPA, the purpose of which is to reach agreement with the FDA on the Phase 3 clinical trial protocol design and analysis that will form the primary basis of an efficacy claim. The agreement will be binding on the FDA and may not be changed by the sponsor or the FDA after the trial begins except with the written agreement of the sponsor and the FDA or if the FDA determines that a substantial scientific issue essential to determining the safety or efficacy of the product candidate was identified after the testing began.
Post-approval trials, sometimes referred to as “Phase 4” clinical trials, may be required after initial marketing approval. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication.
Concurrent with clinical trials, sponsors usually complete additional animal safety studies, develop additional information about the chemistry and physical characteristics of the product candidate and finalize a process for manufacturing commercial quantities of the product candidate in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and the manufacturer must develop methods for testing the quality, purity and potency of the product candidate. Additionally, for NDA products, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its proposed shelf-life.
The results of product development, nonclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests and other control mechanisms, proposed labeling and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. Under the Prescription Drug User Fee Act, or PDUFA, as amended, each NDA must be accompanied by a significant user fee. The FDA adjusts the PDUFA user fees on an annual basis. PDUFA also imposes an annual product fee for products and an annual establishment fee on facilities used to manufacture prescription drug products. Fee waivers or reductions are available in certain circumstances, such as where a waiver is necessary to protect the public health, where the fee would present a significant barrier to innovation, or where the applicant is a small business submitting its first human therapeutic application for review. Product candidates that are designated as orphan drugs are also not subject to user fees unless the application contains an indication other than an orphan indication.
Within 60 days following submission of the application, the FDA reviews a NDA submitted to determine if it is substantially complete before the agency accepts it for filing. The FDA may refuse to accept any NDA that it deems incomplete or not properly reviewable at the time of submission, and may request additional information. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review of the NDA. The FDA reviews an NDA, whether the product is safe and effective for its intended use, and in each case, whether the product is being manufactured in accordance with cGMP. The FDA may refer applications for novel products or products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations when making decisions.
159
Table of Contents
During the product approval process, the FDA also will determine whether a Risk Evaluation and Mitigation Strategy, or REMS, plan is necessary to assure the safe use of the product. If the FDA concludes that an REMS plan is needed, the sponsor of the NDA must submit a proposed REMS plan prior to approval. The FDA has authority to require an REMS plan under the Food and Drug Administration Amendments Act of 2007 (the “FDAAA”) when necessary to ensure that the benefits of a drug outweigh the risks. In determining whether an REMS plan is necessary, the FDA must consider the size of the population likely to use the drug, the seriousness of the disease or condition to be treated, the expected benefit of the drug, the duration of treatment, the seriousness of known or potential adverse events, and whether the drug is a new molecular entity. A REMS plan may be required to include various elements, such as a medication guide or patient package insert, a communication plan to educate health care providers of the risks, limitations on who may prescribe or dispense the drug, or other measures that the FDA deems necessary to assure the safe use of the drug. In addition, the REMS plan must include a timetable to assess the strategy at 18 months, three years, and seven years after the strategy’s approval.
The FDA may also require an REMS plan for a drug that is already on the market if it determines, based on new safety information, that an REMS plan is necessary to ensure that the product’s benefits outweigh its risks.
Before approving a NDA, the FDA will inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving a NDA, the FDA will typically inspect one or more clinical sites to assure that the clinical trials were conducted in compliance with IND trial requirements and GCP requirements. To assure cGMP and GCP compliance, an applicant must incur significant expenditure of time, money and effort in the areas of training, record keeping, production and quality control.
Notwithstanding the submission of relevant data and information, the FDA may ultimately decide that the NDA does not satisfy its regulatory criteria for approval and deny approval. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than the applicant. If the agency decides not to approve the NDA in its then present form, the FDA will issue a complete response letter that describes all of the specific deficiencies in the NDA identified by the FDA. The deficiencies identified may be minor, for example, requiring labeling changes, or major, for example, requiring additional clinical trials. Additionally, the complete response letter may include recommended actions that the applicant might take to place the application in a condition for approval. If a complete response letter is issued, the applicant must either resubmit the NDA, addressing all of the deficiencies identified in the letter, withdraw the application, or request an opportunity for a hearing.
Even if a product receives regulatory approval, the approval may be significantly limited to specific indications and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling. The FDA may impose restrictions and conditions on product distribution, prescribing, or dispensing in the form of a risk management plan, or otherwise limit the scope of any approval. In addition, the FDA may require post marketing clinical trials, sometimes referred to as “Phase 4” clinical trials, designed to further assess a drug’s safety and effectiveness, and testing and surveillance programs to monitor the safety of approved products that have been commercialized.
Expedited Review and Approval
The FDA has various programs, including Fast Track, priority review, accelerated approval and breakthrough therapy designation, which are intended to expedite or simplify the process for reviewing therapeutic candidates, or provide for the approval of a product candidate on the basis of a surrogate endpoint. Even if a product candidate qualifies for one or more of these programs, the FDA may later decide that the product candidate no longer meets the conditions for qualification or that the time period for FDA review or
160
Table of Contents
approval will be lengthened. Generally, therapeutic candidates that are eligible for these programs are those for serious or life-threatening conditions, those with the potential to address unmet medical needs and those that offer meaningful benefits over existing treatments. For example, Fast Track is a process designed to facilitate the development and expedite the review of therapeutic candidates to treat serious or life-threatening diseases or conditions and fill unmet medical needs. Priority review is designed to give therapeutic candidates that offer major advances in treatment or provide a treatment where no adequate therapy exists an initial review within six months as compared to a standard review time of ten months.
Although Fast Track and priority review do not affect the standards for approval, the FDA will attempt to facilitate early and frequent meetings with a sponsor of a Fast Track designated product candidate and expedite review of the application for a product candidate designated for priority review. Accelerated approval, which is described in Subpart H of 21 CFR Part 314, provides for an earlier approval for a new product candidate that is (1) intended to treat a serious or life-threatening disease or condition; (2) generally provides a meaningful advantage over available therapies; and (3) demonstrates an effect on either a surrogate endpoint that is reasonably likely to predict clinical benefit or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, or IMM, and is reasonably likely to predict an effect on IMM or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. A surrogate endpoint is a laboratory measurement or physical sign used as an indirect or substitute measurement representing a clinically meaningful outcome. As a condition of approval, the FDA may require that a sponsor of a product candidate receiving accelerated approval perform post-marketing studies to verify and describe the predicted effect on irreversible morbidity or mortality or other clinical endpoint, and the product may be subject to accelerated withdrawal procedures.
In the Food and Drug Administration Safety and Innovation Act, or FDASIA, which was signed into law in July 2012, the U.S. Congress encouraged the FDA to utilize innovative and flexible approaches to the assessment of therapeutic candidates under accelerated approval. The law required the FDA to issue related guidance and also promulgate confirming regulatory changes. In May 2014, the FDA published a final Guidance for Industry titled “Expedited Programs for Serious Conditions—Drugs and Biologics,” which provides guidance on FDA programs that are intended to facilitate and expedite development and review of new therapeutic candidates as well as threshold criteria generally applicable to concluding that a product candidate is a candidate for these expedited development and review programs.
In addition to the Fast Track, accelerated approval and priority review programs discussed above, the FDA’s “Expedited Programs” guidance also describes the breakthrough therapy designation. A breakthrough therapy is defined as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or conditions, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. Drugs designated as breakthrough therapies are eligible for, among other things, the Fast Track designation, intensive guidance on an efficient drug development program, and a commitment from FDA to involve senior managers and experienced review staff in a proactive collaborative, cross-disciplinary review.
In December 2016, the 21st Century Cures Act, or Cures Act, was signed into law. The Cures Act included numerous provisions that may be relevant to our product candidates, including provisions designed to speed development of innovative and breakthrough therapies. The Cures Act amends the FDCA and the Public Health Service Act, or PHSA, to reauthorize and expand funding for the National Institutes of Health, or NIH, and to authorize FDA to increase spending on innovation projects. Central to the Cures Act are provisions that enhance and accelerate FDA’s processes for reviewing and approving new drugs and supplements to approved NDAs. These include, but are not limited to, provisions that (i) require FDA to establish a program to evaluate the potential use of real world evidence to help to support the approval of a new indication for an approved drug and to help to support or satisfy post-approval study requirements, (ii) provide that FDA may rely upon qualified data summaries to support the approval of a supplemental application with respect to a qualified indication for an already approved drug, (iii) require FDA to issue guidance for purposes of assisting sponsors in incorporating
161
Table of Contents
complex adaptive and other novel trial designs into proposed clinical protocols and applications for new drugs, (iv) affirm that FDA should continue to expedite the approval of breakthrough therapies, and (v) require FDA to establish a process for the qualification of drug development tools for use in supporting or obtaining FDA approval for investigational use of a drug. The Cures Act also includes a provision which requires certain manufacturers or distributors of an investigational drug to make their policies on the availability of certain expanded access programs publicly available. Because the Cures Act was enacted recently and the FDA may take several years to develop these policies, it is difficult to know whether or how the Cures Act will directly affect our business.
Abbreviated New Drug Applications for Generic Drugs
NDA applicants are required to list with the FDA each patent with claims covering the applicant’s product or method of using the product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic or 505(b)(2) applicants in support of approval of an abbreviated new drug application, or ANDA, or a 505(b)(2) application. An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown to be bioequivalent to the listed drug. Other than the requirement for bioequivalence testing, ANDA applicants are not required to conduct, or submit results of, pre-clinical or clinical tests to prove the safety or effectiveness of their drug product. Drugs approved in this way can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA or 505(b)(2) applicant is required to make a certification to the FDA concerning any patents listed for the approved NDA product in the FDA’s Orange Book. Specifically, the ANDA or 505(b)(2) applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The ANDA applicant may also elect to submit a section viii statement certifying that its proposed ANDA labeling does not contain (or carves out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent. If the applicant does not challenge the listed patents, the ANDA or 505(b)(2) application will not be approved until all the listed patents claiming the referenced product have expired.
A certification that the ANDA or 505(b)(2) product will not infringe the already approved product’s listed patents, or that such patents are invalid, is called a Paragraph IV certification. If the ANDA or 505(b)(2) applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA or 505(b)(2) application has been received by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA or 505(b)(2) application until the earlier of 30 months, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA applicant.
The ANDA or 505(b)(2) application will not be approved until any applicable non-patent exclusivity listed in the Orange Book for the referenced product has expired.
Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of FDA approval of the use of our drug candidates, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Act. The Hatch-Waxman Act permits a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the
162
Table of Contents
remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA plus the time between the submission date of an NDA and the approval of that application, except that this review period is reduced by any time during which the applicant failed to exercise due diligence. Only one patent applicable to an approved product is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, if available, we intend to apply for restorations of patent term for some of our currently owned patents beyond their current expiration dates, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant NDA; however, there can be no assurance that any such extension will be granted to us.
Market exclusivity provisions under the FDCA can also delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the U.S. to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant Orphan Drug Designation to therapeutic candidates intended to treat a rare disease or condition, which is generally a disease or condition that affects either (1) fewer than 200,000 individuals in the U.S., or (2) or more than 200,000 individuals in the U.S. and for which there is no reasonable expectation that the cost of developing and making available in the U.S. a product candidate for this type of disease or condition will be recovered from sales in the U.S. for that product candidate. Orphan Drug Designation must be requested before submitting an NDA. We have received Orphan Drug Designation for KX-02. After the FDA grants Orphan Drug Designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan Drug Designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product candidate that has Orphan Drug Designation subsequently receives the first FDA approval for the disease for which it has such designation, the product candidate is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same product candidate for the same indication, except under limited circumstances, for seven years. Orphan drug exclusivity, however, could also block the approval of one of our therapeutic candidates for seven years if a competitor obtains approval of the same product candidate as defined by the FDA or if our product candidate is determined to be contained within the competitor’s product candidate for the same indication or disease.
Pediatric Exclusivity and Pediatric Use
Under the Best Pharmaceuticals for Children Act (the “BPCA”), certain therapeutic candidates may obtain an additional six months of exclusivity if the sponsor submits information requested in writing by the FDA,
163
Table of Contents
referred to as a Written Request, relating to the use of the active moiety of the product candidate in children. Although the FDA may issue a Written Request for studies on either approved or unapproved indications, it may only do so where it determines that information relating to that use of a product candidate in a pediatric population, or part of the pediatric population, may produce health benefits in that population.
In addition, the Pediatric Research Equity Act, or PREA, requires a sponsor to conduct pediatric studies for most therapeutic candidates, for a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration. Under PREA, original NDAs and supplements thereto must contain a pediatric assessment unless the sponsor has received a deferral or waiver. The required assessment must assess the safety and effectiveness of the product candidate for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric subpopulation for which the product candidate is safe and effective. The sponsor or FDA may request a deferral of pediatric studies for some or all of the pediatric subpopulations. A deferral may be granted for several reasons, including a finding that the product candidate is ready for approval for use in adults before pediatric studies are complete or that additional safety or effectiveness data needs to be collected before the pediatric studies begin. The law requires the FDA to send a PREA Non-Compliance letter to sponsors who have failed to submit their pediatric assessments required under PREA, have failed to seek or obtain a deferral or deferral extension or have failed to request approval for a required pediatric formulation. It further requires the FDA to post the PREA Non-Compliance letter and sponsor’s response.
As part of the FDASIA, the U.S. Congress made a few revisions to the BPCA and PREA, which were slated to expire on September 30, 2012, and made both laws permanent.
Post-Approval Requirements
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements is not maintained or if problems occur after the product candidate reaches the market. Later discovery of previously unknown problems with a product candidate may result in restrictions on the product candidate or even complete withdrawal of the product candidate from the market. After approval, some types of changes to the approved product candidate, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval. In addition, the FDA may under some circumstances require testing and surveillance programs to monitor the effect of approved therapeutic candidates that have been commercialized, and the FDA under some circumstances has the power to prevent or limit further marketing of a product candidate based on the results of these post-marketing programs.
Any therapeutic candidates manufactured or distributed pursuant to FDA approvals for prescription drugs are subject to continuing regulation by the FDA, including, among other things:
| • | reporting and record-keeping requirements; |
| • | reporting of adverse experiences with the product candidate; |
| • | providing the FDA with updated safety and efficacy information; |
| • | product sampling and distribution requirements; |
| • | notifying the FDA and gaining its approval of specified manufacturing or labeling changes; and |
| • | complying with FDA promotion and advertising requirements, which include, among other things, standards for direct-to-consumer advertising, restrictions on promoting products for uses or in patient populations that are not described in the product’s approved labeling, limitations on industry-sponsored scientific and educational activities and requirements for promotional activities involving the internet. |
Therapeutic manufacturers and other entities involved in the manufacture and distribution of approved therapeutic products are required to register their establishments with the FDA and obtain licenses in certain
164
Table of Contents
states and are subject to periodic unannounced inspections by the FDA and some state agencies for compliance with cGMPs and other laws. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes extensive procedural, substantive and record-keeping requirements. In addition, changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require FDA approval before being implemented. FDA regulations would also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers used. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of FDA-regulated products, including drugs, are required to register and disclose certain clinical trial information, which is publicly available at www.clinicaltrials.gov. Information related to the product, patient population, phase of investigation, study sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to disclose the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed under certain limited circumstances. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs. The government recently released a regulation and policy to expand and enhance the requirements related to registering and reporting the results of which may result in greater enforcement of these requirements in the future.
Regulation of Compounding Pharmacies
Compounding is a practice in which a licensed pharmacist, a licensed physician, or in the case of an outsourcing facility, a person under the supervision of a licensed pharmacists, combines, mixes, or alters ingredients of a drug to create a medication tailored to the needs of an individual patient. We are engaged in the compounding of sterile drugs as an outsourcing facility registered with FDA. The Compounding Quality Act, or CQA, allows an entity that compounds sterile drugs to register as an outsourcing facility. Once registered (including a fee), an outsourcing facility must meet certain conditions in order to be exempt from the FDCA’s approval requirements and the requirement to label products with adequate directions for use. Under the CQA, the drugs must be compounded in compliance with CGMP by or under the direct supervision of a licensed pharmacist in a registered facility pursuant to Section 503B(a). The outsourcing facility must also report specific information about the products that it compounds, including a list of all of the products it compounded during the previous six months, and information about the compounded products, such as the source of the ingredients used to compound pursuant to Section 503B(b)(2). In addition, the outsourcing facility must meet other conditions described in the new law, including reporting adverse events and labeling its compounded products with certain information pursuant to Section 503B(b)(5) and Section 503B(a)(10). Registered outsourcing facilities are prohibited from selling compounded drugs through a wholesale distributor. Registered outsourcing facilities are subject to FDA inspection, and FDA conducts inspections on a risk-based frequency under Section 503B(b)(4).
Pharmaceutical Coverage, Reimbursement and Pricing
Significant uncertainty exists as to the coverage and reimbursement status of any products for which we may obtain regulatory approval. In the U.S., sales of any products for which we may receive regulatory approval for commercial sale will depend in part on the availability of coverage and reimbursement from third-party payors. Third-party payors include government authorities such as Medicare, Medicaid, TRICARE and the Veterans Administration, managed care providers, private health insurers and other organizations.
The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the Department of Health and Human Services as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. Effective in 2010, the ACA made several changes to the Medicaid Drug Rebate Program, including
165
Table of Contents
increasing pharmaceutical manufacturers’ rebate liability by raising the minimum basic Medicaid rebate on most branded prescription drugs from 15.1% of average manufacturer price, or AMP, to 23.1% of AMP, and adding a new rebate calculation for “line extensions” (i.e., new formulations, such as extended release formulations) of solid oral dosage forms of branded products, as well as potentially impacting their rebate liability by modifying the statutory definition of AMP. The ACA also expanded the universe of Medicaid utilization subject to drug rebates by requiring pharmaceutical manufacturers to pay rebates on Medicaid managed care utilization as of 2010 and by expanding the population potentially eligible for Medicaid drug benefits. CMS will expand Medicaid rebate liability to the territories of the United States as well, beginning in 2017, if the territories elect to enroll in the Medicaid Drug Rebate Program. In addition, the ACA provides for the public availability of retail survey prices and certain weighted average AMPs under the Medicaid program. The implementation of this requirement by CMS may also provide for the public availability of pharmacy acquisition cost data, which could influence our decisions related to setting product prices and offering related discounts.
In order for a pharmaceutical product to receive federal reimbursement under the Medicare Part B and Medicaid programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B drug pricing program. The required 340B discount on a given product is calculated based on the AMP and Medicaid rebate amounts reported by the manufacturer. Effective in 2010, the Affordable Care Act expanded the types of entities eligible to receive discounted 340B pricing; although, under the current state of the law, with the exception of children’s hospitals, these newly eligible entities will not be eligible to receive discounted 340B pricing on orphan drugs when used for the orphan indication. In addition, as 340B drug pricing is determined based on AMP and Medicaid rebate data, the revisions to the Medicaid rebate formula and AMP definition described above could cause the required 340B discount to increase.
The process for determining whether a payor will provide coverage for a product is typically separate from the process for setting the reimbursement rate that the payor will pay for the product. Third-party payors may limit coverage to specific products on an approved list or formulary which might not include all of the FDA-approved products for a particular indication. Also, third-party payors may refuse to include a particular branded drug on their formularies or otherwise restrict patient access to a branded drug when a less costly generic equivalent or other alternative is available. However, under Medicare Part D—Medicare’s outpatient prescription drug benefit—there are protections in place to ensure coverage and reimbursement for oncology products and all Part D prescription drug plans are required to cover substantially all anti-cancer agents. However, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be available. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
Third-party payors are increasingly challenging the price and examining the medical necessity and cost- effectiveness of medical products and services, in addition to their safety and efficacy. In order to obtain coverage and reimbursement for any product that might be approved for sale, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of any products, in addition to the costs required to obtain regulatory approvals. Our drug candidates may not be considered medically necessary or cost-effective. If third-party payors do not consider a product to be cost-effective compared to other available therapies, they may not cover an approved product as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow a company to sell its products at a profit.
The U.S. government and state legislatures have shown significant interest in implementing cost containment programs to limit the growth of government-paid health care costs, including price controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. For example, in March 2016, the Centers for Medicare and Medicaid Services, or CMS, issued a proposed rule seeking to reduce reimbursement for drugs paid under Medicare Part B to 102.5% of Average Sales Price and to implement a variety of value-based purchasing tools. Further, the Affordable Care Act, contains provisions that may reduce the profitability of drug products, including, for example, increased rebates for drugs
166
Table of Contents
reimbursed by Medicaid programs and the extension of Medicaid rebates to Medicaid managed care plans. Several other provisions of the Affordable Care Act focused on cost containment include:
| • | The Patient-Centered Outcomes Research Institute, which was established to identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. The research conducted by the Patient-Centered Outcomes Research Institute may affect the market for certain pharmaceutical products. |
| • | The Independent Payment Advisory Board which, since 2014, has had authority to recommend certain changes to the Medicare program to reduce expenditures by the program when spending exceeds a certain growth rate and such changes could result in reduced payments for prescription drugs. Under certain circumstances, these recommendations will become law unless Congress enacts legislation that will achieve the same or greater Medicare cost savings. However, as of late 2016, the President has yet to nominate anyone to serve on the board. |
| • | The Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. Funding has been allocated to support the mission of the Center for Medicare and Medicaid Innovation from 2011 to 2019. |
| • | Effective in 2011, the Affordable Care Act imposed an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs, apportioned among these entities according to their market share in certain government healthcare programs, although this fee would not apply to sales of certain products approved exclusively for orphan indications. |
| • | Effective in 2011, the Affordable Care Act imposed a requirement on manufacturers of branded drugs to provide a 50% discount off the negotiated price of branded drugs dispensed to Medicare Part D patients in the coverage gap (i.e., the “donut hole” or the period of consumer payment for prescription medicine costs which lies between the initial coverage limit and the catastrophic—coverage threshold). |
The adoption of government controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could also limit payments for pharmaceuticals.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Generic Drugs
Given that we manufacture and market generic drug products, our business may be impacted by laws and policies governing the coverage, pricing, and reimbursement of generic drugs. Generic drugs are the of innovator medicines and are typically more affordable in comparison to the innovator’s products. Sales of generic medicines have benefitted from policies encouraging generic substitution and a general increasing acceptance of generic drugs on the part of healthcare insurers, consumers, physicians and pharmacists. However, while the U.S. generics market is one of the largest in the world, with generic prescription sales of approximately $70 billion in 2016, the recent trend of rising generic drug prices has drawn scrutiny from the U.S. government. Specifically, beginning in 2014 generic drug pricing became the subject of Congressional inquiries and media attention, and many generic drug manufacturers became the targets of government investigations.
In addition, under amendments to the Medicaid Drug Rebate Statute in 2015, generic drug manufacturers are now required to pay an inflation penalty if price increases on generic drugs exceed the rate of inflation.
167
Table of Contents
Specifically, the Bipartisan Budget Act of 2015 (“BBA ‘15”) amended section 1927(c)(3) of the Social Security Act to require manufacturers of non-innovator multiple source (N) drugs to pay additional Medicaid rebates if a drug’s AMP increases at a rate that exceeds the rate of inflation. Manufacturers of generic drugs must calculate the additional Medicaid rebates for noninnovator drugs beginning with the rebates that are calculated for the first quarter of 2017.
Also, the ACA revised the methodology for setting Medicaid generic drug reimbursement in order to further limit the reimbursement of generic drugs under the Medicaid program. Specifically, effective April 1, 2016, the Federal Upper Limit (“FUL”), which establishes the government’s maximum payment amount for certain generic drugs, is no less than 175% of the weighted average of the most recently reported monthly AMPs for pharmaceutically and therapeutically equivalent multiple source drug products that are available for purchase by retail community pharmacies on a nationwide basis. Similarly, reimbursement for generic drugs is also limited in Medicare Part B, as the Average Sales Price (the metric upon which reimbursement is based) for multiple-source drugs included within the same multiple-source drug billing and payment code is the volume-weighted average of the various manufacturers’ ASPs for those drug products.
Laboratory Testing Services Coverage and Reimbursement
Given that we market medical devices in the form of in vitro diagnostic devices, or IVDs, used in the performance of clinical laboratory tests, currently limited to drugs of abuse, pregnancy, and alcohol testing in the U.S., and cardiac marker and infectious disease testing in Asia, our business may be impacted by laws and policies governing the coding, coverage, reimbursement, and demand for clinical laboratory services. With regard to the clinical laboratory services performed on Medicare beneficiaries, health care providers utilizing such tests generally either are paid under prospective payment systems for most tests performed on hospital inpatients and outpatients, or must bill the Medicare Part B program directly in compliance with applicable coding, coverage and reimbursement rules, and accept the amount paid by the Medicare contractor under the Medicare Clinical Laboratory Fee Schedule, or CLFS, as payment in full. Currently, Medicare does not require the beneficiary to pay a co-payment for clinical laboratory services paid under the CLFS. Pursuant to Section 216 of-the federal Protecting Access to Medicare Act of 2014, or PAMA, CMS is modernizing the CLFS by creating a market-based reimbursement system which will require clinical laboratories subject to the law to report certain private payor prices and test volumes, and CMS to set new payment rates for CLFS tests based on the weighted median of reported prices. Under PAMA, price reporting will begin in 2017 and new prices will take effect in 2018, with updates occurring every one to three years thereafter. It is unclear how this new law will affect testing services that use our products at this time, but as a general matter CMS has indicated that prices of many clinical laboratory tests will decrease under PAMA. In addition, state Medicaid programs are prohibited from paying more (and in many instances, pay significantly less) than Medicare, and payment is subject to state-specific coverage, reimbursement, and laboratory law requirements. Certain state Medicaid programs also require Medicaid recipients to pay co-payment amounts for clinical laboratory services. Likewise, payment by private payors is subject to payor-determined coverage and reimbursement policies that vary considerably and are subject to change without notice. Finally, there is increasing legislative attention to opioid abuse in the United States, including passage of the Comprehensive Addiction and Recovery Act of 2016 which, among other things, strengthens state prescription drug monitoring programs and expands educational efforts for certain populations, which may increase the need for drugs of abuse testing. Changes like these related to clinical laboratory services, and any other changes related to coverage or reimbursement may impact the demand for and pricing of some of our products which could adversely affect our ability to operate our business and our financial results.
Reimbursement for Compounded Drugs
Given that we intend to compound and sell compounded products, some of which may include active pharmaceutical ingredients (“APIs”) that we manufacture, our business may be impacted by the downstream coverage and reimbursement of compounded products. Generally, federal reimbursement is available for compounded drugs, but is typically dependent upon whether the individual ingredients or bulk drug substances
168
Table of Contents
that make up the compounded product are FDA-approved. While the majority of the APIs we sell are FDA-approved, some of our API products have not yet received FDA approval.
There is a national payment policy for compounded drugs under Medicare Part B, but the policy is unclear because it does not stipulate whether payment is available for ingredients that are bulk drug substances, which are generally not FDA-approved. Under Medicare Part B, claims for compounded drugs are typically submitted using a billing code for “not otherwise classified drugs”, and CMS contractors who process Part B claims may conduct further reviews of outpatient claims to determine whether the drug billed under a nonspecific billing code is a compounded drug and to identify its ingredients in order to make payment decisions. However, CMS contractors who process Part B claims do not always collect information on the FDA-approval status of drug ingredients and, therefore, payment may be made for ingredients that are not FDA-approved products. Therefore, there is uncertainty as to whether Medicare payments for compounded drugs are consistent with the Medicare Part B policy.
Under Medicare Part D, federal payments are not available for non-FDA-approved products—including bulk drug substances—and inactive ingredients used to make a compounded drug. Insurers that offer Medicare Part D benefits and Part D-only sponsors, generally, pay pharmacies for each ingredient in the compounded drug that is an FDA-approved product and is otherwise eligible for reimbursement under Part D. However, with respect to non-FDA approved bulk drug substances, insurers that offer Medicare Part D benefits and Part D-only sponsors may choose to pay for such bulk substances but may not submit these payments as part of the Part D transaction data CMS uses to determine federal payments to Part D plans.
Healthcare Fraud and Abuse Laws and Compliance Requirements
If we obtain regulatory approval of our products, we may be subject to various federal and state laws targeting fraud and abuse in the healthcare industry. These laws may impact, among other things, our proposed sales and marketing programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering, or paying remuneration (a term interpreted broadly to include anything of value, including, for example, gifts, discounts, and credits), directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order, or recommendation of, an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; |
| • | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment to Medicare, Medicaid, or other third-party payors that are false or fraudulent, or making a false statement or record material to payment of a false claim or avoiding, decreasing, or concealing an obligation to pay money owed to the federal government; |
| • | provisions of the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes, referred to as the “HIPAA All-Payor Fraud Prohibition,” that prohibit knowingly and willfully executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
| • | the federal transparency laws, including the federal Physician Payment Sunshine Act, which was part of the Affordable Care Act, that require manufacturers of certain drugs and biologics to track and disclose payments and other transfers of value they make to U.S. physicians and teaching hospitals as well as physician ownership and investment interests in the manufacturer, and that such information is subsequently made publicly available in a searchable format on a CMS website; |
169
Table of Contents
| • | provisions of HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, state transparency reporting and compliance laws; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and which may not have the same effect, thus complicating compliance efforts. |
The Affordable Care Act broadened the reach of the fraud and abuse laws by, among other things, amending the intent requirement of the federal Anti-Kickback Statute and the applicable criminal healthcare fraud statutes contained within 42 U.S.C. § 1320a-7b. Pursuant to the statutory amendment, a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. In addition, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act or the civil monetary penalties statute. Many states have adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
The federal False Claims Act prohibits anyone from, among other things, knowingly presenting, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services that are false or fraudulent. Although we would not submit claims directly to payors, manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. In addition, our future activities relating to the reporting of wholesaler or estimated retail prices for our products, the reporting of prices used to calculate Medicaid rebate information and other information affecting federal, state, and third-party reimbursement for our products, and the sale and marketing of our products, are subject to scrutiny under this law. For example, pharmaceutical companies have been prosecuted under the federal False Claims Act in connection with their alleged off-label promotion of drugs, purportedly concealing price concessions in the pricing information submitted to the government for government price reporting purposes, and allegedly providing free product to customers with the expectation that the customers would bill federal health care programs for the product. Penalties for a False Claims Act violation include three times the actual damages sustained by the government, plus mandatory civil penalties of between $10,781 and $21,563 for each separate false claim, the potential for exclusion from participation in federal healthcare programs, and, although the federal False Claims Act is a civil statute, conduct that results in a False Claims Act violation may also implicate various federal criminal statutes. In addition, private individuals have the ability to bring actions under the federal False Claims Act and certain states have enacted laws modeled after the federal False Claims Act.
Medical Devices
Through our subsidiary, Polymed, we currently market in-vitro diagnostic rapid test kits used in the performance of clinical laboratory tests (limited to drugs of abuse and pregnancy testing in the U.S.) pursuant to clearance under Section 510(k) of the FDAA by the FDA. These products and our operations are subject to extensive regulation by the FDA and other federal and state authorities in the United States, as well as comparable authorities in foreign jurisdictions. Our test kits are subject to regulation as medical devices in the United States under the FDCA, and related regulations enforced by the FDA. The FDA regulates, among other things, the development, design, non-clinical and clinical research, manufacturing, safety, efficacy, labeling, packaging, storage, installation, servicing, recordkeeping, premarket clearance or approval, import, export, adverse event reporting, advertising, promotion, marketing and distribution of medical devices to ensure that medical devices distributed domestically are safe and effective for their intended uses and otherwise meet the requirements of the FDCA.
170
Table of Contents
In addition, the Clinical Laboratory Improvement Amendments of 1988, or CLIA, established quality standards for laboratory testing to ensure the accuracy, reliability and timeliness of patient test results regardless of where the test was performed. Pursuant to CLIA, the FDA categorizes diagnostic tests into three categories based on their complexity in the testing process and risk of harm in reporting erroneous results: (1) waived tests, (2) moderate complexity tests, and (3) high complexity tests. Laboratories that perform only waived tests and hold a Certificate of Waiver under CLIA (including most physician office laboratories) are subject to minimal regulation as compared with laboratories that perform moderate or high complexity tests. To obtain a CLIA waived categorization for diagnostic tests that are intended for home use or for use by laboratories holding a Certificate of Waiver, we must demonstrate to FDA that the tests are simple to use with a low risk of error. Foreign countries may require similar or more onerous approvals to manufacture or market our products or to allow the use of our products in certain settings. Most of our test strips are categorized as CLIA waived, but some of our test strips are categorized as moderate in complexity.
FDA Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device commercially distributed in the United States requires either FDA clearance of a 510(k) premarket notification submission, granting of a de novo classification request, or approval of a premarket approval application, or PMA. Under the FDCA, medical devices are classified into one of three classes—Class I, Class II or Class III—depending on the degree of risk associated with each medical device. Class I includes devices with the lowest risk to the patient and are subject to the FDA’s general controls for medical devices, which include compliance with the applicable portions of the Quality System Regulation, or QSR, facility registration and product listing, reporting of adverse medical events, and truthful and non-misleading labeling, advertising, and promotional materials. Class II devices are subject to the FDA’s general controls, and special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. These special controls can include performance standards, post-market surveillance, patient registries and FDA guidance documents.
Most Class I devices are exempt from the 510(k) requirements. Manufacturers of most Class II devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA requesting permission to commercially distribute the device. The FDA’s permission to commercially distribute a device subject to a 510(k) premarket notification is generally known as 510(k) clearance. Devices deemed by the FDA to pose the greatest risks, such as life sustaining, life supporting or some implantable devices, or devices that have a new intended use, or use advanced technology that is not substantially equivalent to that of a legally marketed device, are placed in Class III, requiring approval of a PMA. Our currently marketed products are Class II devices subject to 510(k) clearance.
510(k) Marketing Clearance and De Novo Pathways
To obtain 510(k) clearance, a premarket notification submission must be submitted to the FDA demonstrating that the proposed device is “substantially equivalent” to a predicate device. A predicate device is a legally marketed device that is not subject to premarket approval, i.e., a device that was legally marketed prior to May 28, 1976 (pre-amendments device) and for which a PMA is not required, a device that has been reclassified from Class III to Class II or I, or a device that was found substantially equivalent another device cleared through the 510(k) process. The FDA’s 510(k) review process usually takes from three to six months, but may take longer. The FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence.
If the FDA agrees that the device is substantially equivalent to a predicate device, it will grant 510(k) clearance to market the device. If the FDA determines that the device is “not substantially equivalent” to a previously cleared device, the device is automatically designated as a Class III device. The device sponsor must then fulfill more rigorous PMA requirements, or can request a risk-based classification determination for the device in accordance with the “de novo” process, which may determine that the new device is of low to moderate
171
Table of Contents
risk and that it can be appropriately be regulated as a Class I or II device. If a de novo request is granted, the device may be legally marketed and a new classification is established. If the device is classified as Class II, the device may serve as a predicate for future 510(k) submissions.
PMA Approval Pathway
Class III devices require PMA approval before they can be marketed. The PMA process is more demanding than the 510(k) process. In a PMA the manufacturer must demonstrate that the device is safe and effective, and the PMA must be supported by extensive data, including data from preclinical studies and human clinical trials. The FDA will approve the new device for commercial distribution if it determines that the data and information in the PMA constitute valid scientific evidence and that there is reasonable assurance that the device is safe and effective for its intended use(s). The FDA may approve a PMA with post-approval conditions intended to ensure the safety and effectiveness of the device, including, among other things, restrictions on labeling, promotion, sale and distribution, and collection of long-term follow-up data from patients in the clinical trial that supported PMA approval or requirements to conduct additional clinical trials post-approval. Failure to comply with the conditions of approval can result in material adverse enforcement action, including withdrawal of the approval.
Our products are not currently subject to PMA requirements. However, we may in the future develop devices that will require the submission of a PMA, or FDA may find that some of our proposed uses are not substantially equivalent to previously cleared and marketed devices, and thus a PMA is required.
Clinical Trials
Clinical trials are almost always required to support a PMA and are sometimes required to support a 510(k) submission. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with the FDA’s investigational device exemption, or IDE, regulations which govern investigational device labeling, prohibit promotion of the investigational device, and specify an array of recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. If the device presents a “significant risk,” to human health, as defined by the FDA, the FDA requires the device sponsor to submit an IDE application to the FDA, which must be approved prior to commencing human clinical trials. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a subject. An IDE application must be supported by appropriate data, such as animal and laboratory test results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE will automatically become effective 30 days after receipt by the FDA unless the FDA notifies us that the investigation may not begin. If the FDA determines that there are deficiencies or other concerns with an IDE for which it requires modification, the FDA may permit a clinical trial to proceed under a conditional approval.
During a clinical trial, the sponsor is required to comply with applicable FDA requirements, and the clinical investigators are also subject to FDA’s regulations. Both must comply with good clinical practice requirements, or GCPs, which among other things requires that informed consent be obtained from each research subject, that the investigational plan and study protocol be followed, that the disposition of the investigational device be controlled, and that reporting and recordkeeping requirements are followed. Additionally, after a trial begins, we, the FDA or the IRB could suspend or terminate a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a clinical trial is completed, there can be no assurance that the data generated during a clinical trial will meet the safety and effectiveness endpoints or otherwise produce results that will lead the FDA to grant marketing clearance or approval.
172
Table of Contents
Post-Market Regulation
After a device is cleared or approved for marketing, numerous and pervasive regulatory requirements continue to apply. These include:
| • | establishment registration and device listing with the FDA; |
| • | Quality System Regulation, or QSR, requirements, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process; |
| • | labeling regulations and requirements related to promotional activities, including FDA prohibitions against the promotion of investigational products, or “off-label” uses of cleared or approved products; |
| • | clearance or approval of product modifications to 510(k)-cleared devices that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared devices; |
| • | medical device reporting requirements, which require that a manufacturer report to the FDA if a device it markets may have caused or contributed to a death or serious injury, or has malfunctioned and the device or a similar device that it markets would be likely to cause or contribute to a death or serious injury, if the malfunction were to recur; |
| • | correction, removal and recall reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; |
| • | the FDA’s mandatory recall authority, whereby the agency can order device manufacturers to recall from the market a product that is in violation of governing laws and regulations; and |
| • | post-market surveillance activities and regulations, which apply when deemed by the FDA to be necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
Our manufacturing processes are required to comply with the applicable portions of the QSR, which cover the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation and servicing of finished devices intended for human use. The QSR also requires, among other things, maintenance of a device master file, device history file, and complaint files. As a manufacturer, we and our third-party manufacturers are subject to periodic scheduled or unscheduled inspections by the FDA. Our failure to maintain compliance with the QSR requirements could result in the shut-down of, or restrictions on, our manufacturing operations and the recall or seizure of our product. The discovery of previously unknown problems with our product, including unanticipated adverse events or adverse events of increasing severity or frequency, whether resulting from the use of the device within the scope of its clearance or off-label by a physician in the practice of medicine, could result in restrictions on the device, including the removal of the product from the market or voluntary or mandatory device recalls.
New Legislation and Regulations
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the testing, approval, manufacturing, marketing, coverage and reimbursement of products regulated by the FDA or other government agencies. In addition to new legislation, FDA and healthcare fraud and abuse and coverage and reimbursement regulations and policies are often revised or interpreted by the agency in ways that may significantly affect our business and our products. In particular, we expect that the new presidential administration and U.S. Congress will seek to modify, repeal, or otherwise invalidate all, or certain provisions of, the U.S. healthcare reform legislation. Since taking office, President Trump has continued to support the repeal of all or portions of the Affordable Care Act. President Trump has also issued an executive order in which he stated that it is his administration’s policy to seek the prompt repeal of
173
Table of Contents
the Affordable Care Act and directed executive departments and federal agencies to waive, defer, grant exemptions from, or delay the implementation of the provisions of the Affordable Care Act to the maximum extent permitted by law. There is still uncertainty with respect to the impact President Trump’s administration and the U.S. Congress may have, if any, and any changes will likely take time to unfold. Such reforms could have an adverse effect on anticipated revenues from therapeutic candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop therapeutic candidates. However, we cannot predict the ultimate content, timing or effect of any healthcare reform legislation or the impact of potential legislation on us.
Furthermore, in the U.S., the health care industry is subject to political, economic, and regulatory influences. Initiatives to reduce the federal budget and debt and to reform health care coverage are increasing cost-containment efforts. We anticipate that federal agencies, Congress, state legislatures, and the private sector will continue to review and assess alternative health care benefits, controls on health care spending, and other fundamental changes to the healthcare delivery system. Any proposed or actual changes could limit coverage for or the amounts that federal and state governments will pay for health care products and services, which could also result in reduced demand for our products or additional pricing pressures, and limit or eliminate our spending on development projects and affect our ultimate profitability. We are not able to predict whether further legislative changes will be enacted or whether FDA or healthcare fraud and abuse or coverage and reimbursement regulations, guidance, policies or interpretations will be changed or what the effect of such changes, if any, may be.
Foreign Corrupt Practices Act
The FCPA prohibits any U.S. individual or business from corruptly offering, paying, promising, or authorizing the provision of anything of value, directly or indirectly, to any foreign official, foreign political party or official thereof, or candidate for foreign political office to obtain or retain business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring the issuer to maintain books and records that accurately and fairly reflect all transactions of the issuer and its controlled subsidiaries, and to devise and maintain an adequate system of internal accounting controls.
Environment
We are subject to inspections by the FDA for compliance with cGMP and other U.S. regulatory requirements, including U.S. federal, state and local regulations regarding environmental protection and hazardous and controlled substance controls, among others. Environmental laws and regulations are complex, change frequently and have tended to become more stringent over time. We have incurred, and may continue to incur, significant expenditures to ensure we are in compliance with these laws and regulations. We would be subject to significant penalties for failure to comply with these laws and regulations.
PRC Government Regulation
In the PRC, we operate in an increasingly complex legal and regulatory environment. We are subject to a variety of PRC laws, rules and regulations affecting many aspects of our business. This section summarizes the principal PRC laws, rules and regulations relevant to our business and operations.
Foreign Investment in Pharmaceutical Industry
The Foreign Investment Industrial Guidance Catalogue (2015 Version), or the Foreign Investment Catalogue jointly promulgated by the National Development and Reform Commission, or NDRC, and the Ministry of Commerce, or MOFCOM, in March 2015 and effective in April 2015 and replaced the previous versions. The Foreign Investment Catalogue divides foreign investments in the pharmaceutical industry into four categories: encouraged, permitted, restricted or prohibited. In September 2016, the National People’s Congress
174
Table of Contents
Standing Committee adopted a decision on amending the law of foreign invested companies which became effective from October 1, 2016. Upon the effectiveness of the decision, the establishment of the foreign invested enterprise and its subsequent changes will be required to be filed with the relevant authorities instead of obtaining approvals from relevant commerce authorities, except for the foreign invested enterprises which are subject to the special administrative measures regarding foreign investment entry. In October 2016, NDRC and MOFCOM jointly issued a notice according to which the industries falling within the categories in which foreign investment is prohibited or restricted and those falling within the encouraged category subject to relevant requirements of equity or senior management under the Foreign Investment Catalogue, will be subject to the special administrative measures for foreign investment entry.
General Regulations on China Food and Drug Administration
In the PRC, the CFDA monitors and supervises the administration of pharmaceutical products, as well as medical devices and equipment. The CFDA’s primary responsibility includes evaluating, registering and approving new drugs, generic drugs, imported drugs and traditional Chinese medicines; approving and issuing permits for the manufacture, export and import of pharmaceutical products and medical appliances; approving the establishment of enterprises for pharmaceutical manufacture and distribution; formulating administrative rules and policies concerning the supervision and administration of food, cosmetics and pharmaceuticals; and handling significant accidents involving these products. The local provincial drug administrative authorities are responsible for supervision and administration of drugs within their respective administrative regions.
The PRC Drug Administration Law promulgated by the Standing Committee of the National People’s Congress in 1984 and the Implementing Measures of the PRC Drug Administration Law promulgated by the Ministry of Health, or the MOH, in 1989 set forth the legal framework for the administration of pharmaceutical products, including the research, development and manufacturing of drugs.
The PRC Drug Administration Law was revised in February 2001, December 2013, and again in April 2015 respectively. The purpose of the revisions was to strengthen the supervision and administration of pharmaceutical products and to ensure the quality and safety of those products for human use. The revised PRC Drug Administration Law applies to entities and individuals engaged in the development, production, trade, application, supervision and administration of pharmaceutical products. It regulates and prescribes a framework for the administration of pharmaceutical preparations of medical institutions and for the development, research, manufacturing, distribution, packaging, pricing and advertisement of pharmaceutical products. Revised Implementing Measures of the PRC Drug Administration Law promulgated by the State Council took effect in September 2002 and was revised in February 2016, providing detailed implementing regulations for the revised PRC Drug Administration Law.
Under these regulations, we need to follow related regulations for preclinical research, clinical trials and production of new drugs.
Good Laboratories Practice Certification for Preclinical Research
To improve the quality of preclinical research, the CFDA promulgated the Administrative Measures for Good Laboratories Practice of Preclinical Laboratory in 2003 and began to conduct the certification program of Good Laboratories Practice, or the GLP. Under CFDA Circular 214, the CFDA decides whether an institution is qualified for undertaking pharmaceutical preclinical research upon the evaluation of the institution’s organizational administration, its research personnel, its equipment and facilities and its operation and management of preclinical pharmaceutical projects. If all requirements are met, a GLP Certification will be issued by the CFDA and the result will be published on the CFDA’s website.
Approval for Clinical Trials and Production of New Drugs
According to the Provisions for Drug Registration promulgated by the CFDA in 2007, the PRC Drug Administration Law, the Provisions on the Administration of Special Examination and Approval of Registration
175
Table of Contents
of New Drugs, or the Special Examination and Approval Provisions issued by the CFDA in 2009, and the Circular on Information Publish Platform for Pharmaceutical Clinical Trials issued by the CFDA in 2013, we must comply with the following procedures and obtain several approvals for clinical trials and production of new drugs.
Clinical Trial Application
Upon completion of its preclinical research, a research institution must apply for approval of a Clinical Trial Application before conducting clinical trials.
Domestic Category 1 New Drugs Are Eligible for Special Examination and Approval
According to the Provisions for Drug Registration, drug registration applications are divided into three different types, namely Domestic New Drug Application, Domestic Generic Drug Application, and Imported Drug Application. Drugs fall into one of three categories, namely chemical medicine, biological product, or traditional Chinese or natural medicine. The registrations of chemical medicines are divided into six categories, among which, a Category 1 drug is a new drug that has never been marketed in any country. All of our clinical-stage drug candidates qualify as domestic Category 1 new drugs.
In March 2016, the CFDA promulgated the Work Plan for Reforming the Chemical Medicines Registration Classification System, under which, the registrations of chemical medicines are divided into five categories as follows:
Category 1: Innovative drugs that are not marketed both domestically and abroad. These drugs contain new compounds with clear structures and pharmacological effects and they have clinical value.
Category 2: Modified new drugs that are not marketed both domestically and abroad. With known active components, the drug’s structure, phase, prescription manufacturing process, administration route and indication are optimized and it has obvious clinical advantage.
Category 3: The drugs that are imitated by domestic applicants to original drugs that have been marketed abroad but not domestically. These kinds of drugs are supposed to have the same quality and effects with original drugs. Original drugs are the foremost drugs that are approved to be marketed domestically and /or abroad with complete and full safety and validity data as marketing evidence.
Category 4: The drugs that are imitated by domestic applicants to original drugs that have been marketed domestically. These kinds of drugs are supposed to have the same quality and effects with original drugs.
Category 5: The drugs that have been marketed abroad are applied to be marketed domestically.
The registration of Category 1 or Category 2 drugs above will be subject to the requirements of Domestic New Drug Application under the Provisions for Drug Registration, Domestic Generic Drug Application will be applicable to Category 3 or Category 4 drugs registration, and Imported Drug Application will be applicable to Category 5 drugs registration. The applicants whose registration applications for chemical medicines have been accepted by the CFDA before the date of promulgation of the Work Plan for Reforming the Chemical Medicines Registration Classification System can choose to continue the applications process according to the Provisions for Drug Registration or to comply with the new categories under the Work Plan for Reforming the Chemical Medicines Registration Classification System
According to the Special Examination and Approval Provisions, the CFDA conducts special examination and approval for new drugs registration application when:
(1) active ingredients and their preparations extracted from plants, animals and minerals, and newly discovered medical materials and their preparations have not been marketed in China;
176
Table of Contents
(2) the chemical raw material medicines as well as the preparations and biological products thereof haven’t been approved for marketing home and abroad;
(3) the new drugs are for treating AIDS, malignant tumors and rare diseases, etc., and have obvious advantages in clinic treatment; or
(4) the new drugs are for treating diseases with no effective methods of treatment.
The Special Examination and Approval Provisions provide that the applicant may file for special examination and approval at the stage of Clinical Trial Application if the drug candidate falls within items (1) or (2), and for drug candidates that fall within items (3) or (4), the application for special examination and approval must be made when filing for production.
The registration of Category 1 or Category 2 drugs above will be subject to the requirements of Domestic New Drug Application under the Provisions for Drug Registration, Domestic Generic Drug Application will be applicable to Category 3 or Category 4 drugs registration, and Imported Drug Application will be applicable to Category 5 drugs registration. The applicants whose registration applications for chemical medicines have been accepted by the CFDA before the date of promulgation of the Reform Plan Regarding the Category of the Registration of Chemical Medicines can choose to continue the applications process according to the Provisions for Drug Registration or to comply with the new categories under the Reform Plan Regarding the Category of the Registration of Chemical Medicines.
We believe that certain of our products fall within items (2) and (3) above. Therefore, we may file an application for special examination and approval at the Clinical Trial Application stage, which may enable us to pursue a more expedited path to approval in China and bring therapies to patients more quickly.
The Advantages of Category 1 New Drugs over Category 5 Drugs
Under the Provisions for Drug Registration and the Work Plan for Reforming the Chemical Medicines Registration Classification System, Category 5 drugs are drugs which have already been marketed abroad by multinational companies, but are not yet approved in China and Category 5 drug registration will be subject to the requirements of the Imported Drug Application. Compared with the application for Category 5 drugs, the application for Category 1 domestic new drugs has a more straight-forward registration pathway. According to the Special Examination and Approval Provisions, where a special examination and approval treatment is granted, the application for clinical trial and manufacturing will be handled with priority and with enhanced communication with the Center for Drug Evaluation of the CFDA, or the CDE, which will establish a working mechanism for communicating with the applicants. If it becomes necessary to revise the clinical trial scheme or make other major alterations during the clinical trial, the applicant may file an application for communication. When an application for communication is approved, the CDE will arrange the communication with the applicant within one month.
In comparison, according to the Provisions for Drug Registration, the registration pathway for Category 5 drugs is complicated and evolving. Category 5 drug applications may be submitted after a company obtains an NDA approval and receive the CPP granted by a major regulatory authority, such as the FDA or the EMA. Multinational companies may need to apply for conducting MRCTs, which means that companies do not have the flexibility to design the clinical trials to fit the Chinese patients and standard-of-care. Category 5 drug candidates may not qualify to benefit from fast track review with priority at the Clinical Trial Application stage. Moreover, a requirement to further conduct local clinical trials can potentially delay market access by several years from its international NDA approval.
177
Table of Contents
Changes to the Review and Approval Process
In August 2015, the State Council issued a statement, Opinions on Reforming the Review and Approval Process for Pharmaceutical Products and Medical Devices, which contained several potential policy changes that could benefit the pharmaceutical industry:
| • | A plan to accelerate innovative drug approval with a special review and approval process, with a focus on areas of high unmet medical needs, including drugs for HIV, cancer, serious infectious diseases, orphan diseases and drugs on national priority lists. |
| • | A plan to adopt a policy which would allow companies to act as the marketing authorization holder and to hire contract manufacturing organizations to produce drug products. |
| • | A plan to improve the review and approval of clinical trials, and to allow companies to conduct clinical trials at the same time as they are in other countries and encourage local clinical trial organizations to participate in international multi-center clinical trials. |
In November 2015, the CFDA released the Circular Concerning Several Policies on Drug Registration Review and Approval, which further clarified the following policies potentially simplifying and accelerating the approval process of clinical trials:
| • | A one-time umbrella approval procedure allowing approval of all phases of a new drug’s clinical trials at once, rather than the current phase-by-phase approval procedure, will be adopted for new drugs’ clinical trial applications. |
| • | A fast track drug registration or clinical trial approval pathway will be available for the following applications: (1) registration of innovative new drugs treating HIV, cancer, serious infectious diseases and orphan diseases; (2) registration of pediatric drugs; (3) registration of geriatric drugs and drugs treating China-prevalent diseases; (4) registration of drugs sponsored by national science and technology grants; (5) registration of innovative drugs using advanced technology, using innovative treatment methods, or having distinctive clinical benefits; (6) registration of foreign innovative drugs to be manufactured locally in China; (7) concurrent applications for new drug clinical trials which are already approved in the U.S. or European Union, or concurrent drug registration applications for drugs which have applied for marketing authorization and passed onsite inspections in the U.S. or European Union and are manufactured using the same production line in China; and (8) clinical trial applications for drugs with urgent clinical need and patent expiry within three years, and marketing authorization applications for drugs with urgent clinical need and patent expiry within one year. |
In March 2016, the CFDA issued the Interim Provisions on the Procedures for Drug Clinical Trial Data Verification that provides procedural rules for CFDA’s on-site verification of clinical data before drug approvals.
Also in February 2016, the CFDA published the Opinions on Implementing a Prioritized Review System to Avoid Drug Review Backlogs, which introduces a prioritized review and approval pathway to clinical trial applications and registration applications of certain drugs as part of CFDA’s ongoing reform of its current drug review and approval system.
The CFDA issued the Procedures for Priority Examination and Approval of Medical Devices, or the Procedures on October 25, 2016, which shall come into effect on January 1, 2017. The Procedures, composed of 17 articles, specify that the priority in examination and approval shall be given, in relation to the applications of registering Class-III domestic, or Class-II and Class-III imported medical devices, when those applications fall within such categories as diagnosis or treatment of rare disease or malignant tumor with significant clinical advantage. According to the Procedures, the medical device technical evaluation center of the CFDA will tentatively decide on the applicants applying for their project given priority examination and approval, names of their products and the reception numbers and disclose such information on its website for a period of no less than five working days. The Procedures provide that for projects given priority in examination and approval, the
178
Table of Contents
medical device technical evaluation center shall communicate with applicants in an active way as required by applicable provisions in the course of evaluating relevant technologies, and may arrange for special talks when necessary; food and drug administrative departments at provincial level shall take the review of the registered quality management system of medical devices as priority; and the CFDA will prioritize their administrative examination and approval.
PRC Enterprise Income Tax Law and Its Implementation
The PRC Enterprise Income Tax Law, or EIT Law, and its implementation rules provide that from January 1, 2008, a uniform income tax rate of 25% is applied equally to domestic enterprises as well as foreign investment enterprises, and permit certain High and New Technologies Enterprises, or HNTEs, to enjoy preferential enterprise income tax rates subject to these HNTEs meeting certain qualification criteria.
The EIT Law and its implementation rules provide that a withholding tax at the rate of 10% is applicable to dividends and other distributions payable by a PRC resident enterprise to investors who are “non-resident enterprises” (that do not have an establishment or place of business in the PRC, or that have such establishment or place of business but the relevant dividend or other distribution is not effectively connected with the establishment or place of business). However, pursuant to the Arrangement between the Mainland and Hong Kong Special Administrative Region for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with respect to Taxes on Income effective on December 8, 2006, the withholding tax rate for dividends paid by a PRC resident enterprise is 5% if the Hong Kong enterprise owns at least 25% of the capital of the PRC enterprise; otherwise, the dividend withholding tax rate is 10%. According to the Notice of the PRC State Administration of Taxation on Issues relating to the Administration of the Dividend Provision in Tax Treaties promulgated on February 20, 2009 and effective on the same day, the corporate recipient of dividends distributed by PRC enterprises must satisfy the direct ownership thresholds at all times during the 12 consecutive months preceding the receipt of the dividends. The PRC State Administration of Taxation issued the Notice on How to Understand and Identify the Owner of Benefits in the PRC-HK Tax Agreement on October 27, 2009. Pursuant to these regulations, non-resident enterprises are required to obtain approval from the competent tax authorities in order to enjoy the favorable treatments under the treaties. However, if a company is deemed to be a pass-through entity rather than a qualified owner of benefits, it cannot enjoy the favorable tax treatments provided in the tax arrangement. In addition, if transactions or arrangements are deemed by the relevant tax authorities to be entered into mainly for the purpose of enjoying favorable tax treatments under the tax arrangement, such favorable tax treatments may be subject to adjustment by the relevant tax authorities in the future.
On July 27, 2011, the Ministry of Finance, the General Administration of Customs, and the State Administration of Taxation issued the Notice on the Relevant Tax Policies for the Implementation of the Strategy of Extensive Development of the Western Regions, under which from January 1, 2011 to December 31, 2020, a reduced enterprise income tax rate of 15% is applicable to the enterprises set up in the western regions as designated by the relevant PRC regulations with their main business in the encouraged industries. The encouraged industries are those listed in the Catalog of Encouraged Industries in the Western Regions as promulgated by NDRC. To qualify for the reduced tax rate, an enterprise must derive 70 percent or more of its revenue from the business listed in the Catalog of Encouraged Industries in the Western Regions.
Regulations Relating to Business Tax and Value-added Tax
Pursuant to the Temporary Regulations on Business Tax, which were promulgated by the State Council on December 13, 1993 and effective on January 1, 1994, as amended on November 10, 2008 and effective January 1, 2009, any entity or individual conducting business in a service industry is generally required to pay business tax at the rate of 5% on the revenues generated from providing such services.
In November 2011, the Ministry of Finance and the State Administration of Taxation, or SAT, promulgated the Pilot Plan for Imposition of Value-Added Tax to Replace Business Tax, or the Pilot Plan. Since January
179
Table of Contents
2012, the SAT has been implementing the Pilot Plan, which imposes value-added tax, or VAT, in lieu of business tax for certain industries in Shanghai. The Pilot Plan was expanded to other regions, including Beijing, in September 2012, and was further expanded nationwide beginning August 1, 2013. VAT is applicable at a rate of 6% in lieu of business taxes for certain services and 17% for the sale of goods and provision of tangible property lease services. VAT payable on goods sold or taxable services provided by a general VAT taxpayer for a taxable period is the net balance of the output VAT for the period after crediting the input VAT for the period. In March 2016, the Ministry of Finance and SAT jointly issued the Notice on Adjustment of Transfer Business Tax to Value Added Tax effective from May 2016, according to which PRC tax authorities have started imposing VAT on revenues from various service sectors, including real estate, construction, financial services and insurance, as well as other lifestyle service sectors, replacing the business tax.
Four Phases of Clinical Trials
A clinical development program consists of Phases 1, 2, 3 and 4. Phase 1 refers to the initial clinical pharmacology and safety evaluation studies in humans. Phase 2 refers to the preliminary evaluation of a drug candidate’s therapeutic effectiveness and safety for particular indication(s) in patients, provide evidence and support for the design of Phase 3 clinical trial, and settle the administrative dose regimen. Phase 3 refers to clinical trials undertaken to confirm the therapeutic effectiveness of a drug. Phase 3 is used to further verify the drug’s therapeutic effectiveness and safety on patients with target indication(s), to evaluate overall benefit-risk relationships of the drug, and ultimately to provide sufficient evidence for the review of drug registration application. Phase 4 refers to a new drug’s post-marketing study to assess therapeutic effectiveness and adverse reactions when the drug is widely used, to evaluate overall benefit-risk relationships of the drug when used among general population or specific groups, and to adjust the administration dose, etc.
New Drug Application
When Phase 1, 2 and 3 of the clinical trials have been completed, the applicant must apply to the CFDA for approval of a new drug application. The CFDA then determines whether to approve the application according to the comprehensive evaluation opinion provided by the CDE of the CFDA. We must obtain approval of a new drug application before our drugs can be manufactured and sold in the PRC market.
Good Manufacturing Practice
All facilities and techniques used in the manufacture of products for clinical use or for sale in the PRC must be operated in conformity with cGMP guidelines as established by the CFDA. Failure to comply with applicable requirements could result in the termination of manufacturing and significant fines.
Animal Test Permits
According to Regulations for the Administration of Affairs Concerning Experimental Animals promulgated by the State Science and Technology Commission in November 1988, as revised in January 2011 and July 2013, and Administrative Measures on the Certificate for Animal Experimentation promulgated by the State Science and Technology Commission and other regulatory authorities in January 2001, performing experimentation on animals requires a Certificate for Use of Laboratory Animals. Applicants must satisfy the following conditions:
| • | Laboratory animals must be qualified and sourced from institutions that have Certificates for Production of Laboratory Animals; |
| • | The environment and facilities for the animals’ living and propagating must meet state requirements; |
| • | The animals’ feed and water must meet state requirements; |
| • | The animals’ feeding and experimentation must be conducted by professionals, specialized and skilled workers, or other trained personnel; |
180
Table of Contents
| • | The management systems must be effective and efficient; and |
| • | The applicable entity must follow other requirements as stipulated by the PRC laws and regulations. |
We obtained a Certificate for Use of Laboratory Animals in 2012 regarding the scope of rats and mice.
Regulations Relating to Intellectual Property Rights
Patent
Pursuant to the Patent Law of the PRC and its implementation rules, patents in the PRC fall into three categories, namely invention patent, utility model and design patent. Invention patent refers to a new technical solution proposed in respect of a product, method or its improvement; utility model refers to a new technical solution that is practicable for application and proposed in respect of the shape, structure or a combination of both of a product; and design patent refers to the new design of a certain product in shape, pattern or a combination of both and in color, shape and pattern combinations aesthetically suitable for industrial application. Under the Patent Law of the PRC, the term of patent protection starts from the date the patent was filed. Patents relating to inventions are effective for twenty years from the initial date the patent application was filed, and the term for utility model and designed patents is ten years from the initial date the patent application was filed. The Patent Law of the PRC adopts the principle of “first to file,” which means where more than one person files a patent application for the same invention, a patent will be granted to the person who first filed the application.
Existing patents can become invalid or unenforceable due to a number of factors, including known or unknown prior art, deficiencies in patent application and lack of novelty in technology. In the PRC, a patent must have novelty, innovation and practical application. Under the Patent Law of PRC, novelty means that before a patent application is filed, no identical invention or utility model has been publicly disclosed in any publication in the PRC or abroad or has been publicly used or made known to the public by any other means, whether in or outside of China, nor has any other person filed with the patent authority an application that describes an identical invention or utility model and is published after the filing date. Patents in the PRC are filed with the State Intellectual Property Office, or SIPO. Normally, the SIPO publishes an application for a pharmaceutical invention 18 months after the application is filed, which may be shortened upon request by the applicant. The applicant must apply to the SIPO for a substantive examination within three years from the date the application is filed.
Article 20 of the Patent Law of the PRC provides that, for an invention or utility model completed in China, any applicant (not just Chinese companies and individuals), before filing a patent application outside of China, must first submit it to the SIPO for a confidential examination. Failure to comply with this requirement will result in the denial of any Chinese patent for the subject invention. This added requirement of confidential examination by the SIPO has raised concerns by foreign companies who conduct research and development activities in the PRC or outsource research and development activities to service providers in the PRC.
Patent Enforcement
Unauthorized use of patents without consent from owners of patents, forgery of the patents belonging to other persons, or engagement in other infringement acts against patent rights, will subject the infringers to tortious liabilities. Serious offences may be subject to criminal penalties.
When a dispute arises as a result of infringement of the patent owner’s patent right, PRC law requires that the parties first attempt to settle the dispute through consultation between them. However, if the dispute cannot be settled through consultation, the patent owner, or an interested party who believes the patent is being infringed, may either file a civil legal suit or file an administrative complaint with the relevant patent administration authority under the SIPO. A PRC court may issue a preliminary injunction upon the patent owner’s or an interested party’s request before instituting any legal proceedings or during the proceedings. Damages for infringement are calculated as either the loss suffered by the patent holder arising from the
181
Table of Contents
infringement or the benefit gained by the infringer from the infringement. If it is difficult to ascertain damages in this manner, damages may be determined by using a reasonable multiple of the license fee under a contractual license. As in other jurisdictions, with one notable exception, the patent owner in the PRC has the burden of proving that the patent is being infringed. However, if the owner of a manufacturing process patent alleges infringement of its patent, the alleged infringer has the burden of proving that it has not infringed. To our knowledge, there are no disputes as to our infringement of any third party’s patent.
Medical Patent Compulsory License
According to the Patent Law of the PRC, the SIPO may grant a compulsory license for manufacturing patented drugs and exporting them to countries or regions covered under relevant international treaties to which the People’s Republic of China has acceded.
Exemptions for Unlicensed Manufacture, Use and Import of Patented Drugs
According to the Patent Law of the PRC, any person may manufacture, use or import patented drugs for the purpose of providing information required for administrative examination and approval without authorization granted by the patent owner.
Trade Secrets
According to the Anti-Unfair Competition Law of the PRC, the term “trade secrets” refers to technical information and business information that is unknown to the public, that has utility and may create business interest or profit for its legal owners or holders, and that is maintained as a secret by its legal owners or holders.
Under this law, business persons are prohibited from employing the following methods to infringe trade secrets: (1) obtaining the trade secrets from the legal owners or holders by any unfair methods such as stealing, solicitation or coercion; (2) disclosing, using or permitting others to use the trade secrets obtained illegally under item (1) above; or (3) disclosing, using or permitting others to use the trade secrets, in violation of any contractual agreements or any requirements of the legal owners or holders to keep such trade secrets in confidence. If a third party knows or should have known of the above-mentioned illegal conduct but nevertheless obtains, uses or discloses trade secrets of others, the third party may be deemed to have committed a misappropriation of the others’ trade secrets. The parties whose trade secrets are being misappropriated may petition for administrative corrections, and regulatory authorities may stop any illegal activities and fine infringing parties in the amount of RMB 10,000—200,000. Alternatively, persons whose trade secrets are being misappropriated may file lawsuits in a PRC court for loss and damages caused by the misappropriation.
The measures to protect trade secrets include oral or written agreements or other reasonable measures to require the employees of, or persons in business contact with, legal owners or holders to keep trade secrets confidential. Once the legal owners or holders have asked others to keep trade secrets confidential and have adopted reasonable protection measures, the requested persons bear the responsibility for keeping the trade secrets confidential.
Recently Issued Policies on the Protection of Intellectual Property Rights
On November 4, 2016, the Central Committee of the Communist Party of China and the State Council jointly issued a Guideline on Improving the Property Rights Protection System and Providing Law-based Protection to Property Rights (the “Guideline”), effective on the date of its release. The Guideline proposes that the country will provide equal, comprehensive and law-based protection to all kinds of property rights and requires that the punishment of intellectual property rights violations should be strengthened and the limits on compensation for violating intellectual property rights laws should be increased. In addition, the Guideline proposes to explore the establishment of infringement punitive compensation system for such intellectual
182
Table of Contents
property rights as patent and copyright, including allowing for punitive damages for serious malicious tort. The Guideline also stipulates to perfect the foreign-related intellectual property rights enforcement mechanism, and strengthen the international cooperation in criminal case enforcement and investigation in foreign-related intellectual property crimes. On November 28, 2016, the Supreme People’s Court released the Implementation Opinions on Appropriately and Lawfully Handling Long-standing Historical Property Rights Cases and the Opinions on Giving Full Play to Judicial Functions to Enhance Judicial Protection of Property Rights (the “Opinions”), effective on the date of their releases. The Opinions stipulate that, among others, efforts shall be made to crack down on intellectual property rights infringement and crimes in accordance with relevant laws and regulations, provide stronger judicial protection to intellectual property rights, introduce judicial interpretations and guiding cases in due time, promote the lawful application of the punitive compensation system, and impose severer punishments on chain-type and industrialized crimes against intellectual property rights.
Regulations Relating to Environmental Protection
China has adopted extensive environmental laws and regulations with national and local standards for emissions control, discharge of waste water and storage and transportation, treatment and disposal of waste materials. At the national level, the relevant environmental protection laws and regulations include the PRC Environmental Protection Law, the PRC Law on the Prevention and Control of Air Pollution, the PRC Law on the Prevention and Control of Water Pollution, the PRC Law on the Promotion of Clean Production, the PRC Law on the Prevention and Control of Noise Pollution, the PRC Law on the Prevention and Control of Solid Waste Pollution, the PRC Recycling Economy Promotion Law, the PRC Law on Environmental Impact Assessment, the Administrative Regulations on the Levy and Use of Discharge Fees and the Measures for the Administration of the Charging Rates for Pollutant Discharge Fees. In recent years, the PRC Government has introduced a series of new policies designed to generally promote the protection of the environment. For instance, on November 10, 2016, the General Office of the State Council has released the Implementing Plan for the Permit System for Controlling the Discharge of Pollutants (the “Plan”). The Plan proposes the need of instituting a system for enterprises and public institutions to control their respective total amount of pollutants discharged, which shall be connected with the environmental impact assessment system organically. The Plan also stipulates that it is necessary to regulate the orderly issuance of pollutant discharge permits, to make a name list to manage the permission of pollutant discharge, to promote the administration of such permission system per industry, and to impose severer administration and control over enterprises and public institutions located at such places where environment quality fails to reach relevant standards. Furthermore, the Plan requires that a national pollutant discharge permit management information platform shall be established by 2017 to strengthen the information disclosure and social supervision.
Regulations Relating to Foreign Exchange and Dividend Distribution
Foreign Exchange Regulation
The Foreign Exchange Administration Regulations, most recently amended in August 2008, are the principal regulations governing foreign currency exchange in China. Under the PRC foreign exchange regulations, payments of current account items, such as profit distributions and trade and service-related foreign exchange transactions, may be made in foreign currencies without prior approval from the State Administration of Foreign Exchange, or SAFE, by complying with certain procedural requirements. In contrast, approval from or registration with appropriate government authorities is required when Renminbi is to be converted into a foreign currency and remitted out of China to pay capital expenses such as the repayment of foreign currency-denominated loans.
In August 2008, SAFE issued the Circular on the Relevant Operating Issues Concerning the Improvement of the Administration of the Payment and Settlement of Foreign Currency Capital of Foreign-Invested Enterprises, or SAFE Circular 142, regulating the conversion by a foreign-invested enterprise of foreign currency-registered capital into Renminbi by restricting how the converted Renminbi may be used. In addition, SAFE promulgated
183
Table of Contents
Notice on Issues concerning Further Clarifying and Regulating the Foreign Exchange Administration under Some Capital Accounts, or Circular 45, on November 9, 2011 to clarify the application of SAFE Circular 142. Under SAFE Circular 142 and Circular 45, Renminbi capital converted from foreign currency registered capital of a foreign-invested enterprise may only be used for purposes within the business scope approved by the applicable government authority and may not be used for equity investments within the PRC. In addition, SAFE strengthened its oversight of the flow and use of the Renminbi capital converted from foreign currency registered capital of foreign-invested enterprises. The use of such Renminbi capital may not be changed without SAFE’s approval, and such Renminbi capital may not, in any case, be used to repay Renminbi loans whose proceeds were not used. Furthermore, SAFE promulgated Notice on Issues Concerning Strengthening Administration of Foreign Exchange Services in November 2010, which tightens the regulation over settlement of net proceeds from overseas offerings, such as our initial public offering, and requires, among other things, the authenticity of settlement of net proceeds from offshore offerings to be closely examined and the net proceeds to be settled in the manner described in our prospectus or otherwise approved by our board of directors. Violations of these SAFE regulations may result in severe monetary or other penalties, including confiscation of earnings derived from such violation activities, a fine of up to 30% of the RMB funds converted from the foreign invested funds or in the case of a severe violation, a fine ranging from 30% to 100% of the Renminbi funds converted from the foreign-invested funds.
In November 2012, SAFE promulgated the Circular of Further Improving and Adjusting Foreign Exchange Administration Policies on Foreign Direct Investment, which substantially amends and simplifies the current foreign exchange procedure. Pursuant to this circular, the opening of various special purpose foreign exchange accounts, such as pre-establishment expenses accounts, foreign exchange capital accounts and guarantee accounts, the reinvestment of Renminbi proceeds by foreign investors in the PRC, and remittance of foreign exchange profits and dividends by a foreign-invested enterprise to its foreign shareholders no longer require the approval or verification of SAFE, and multiple capital accounts for the same entity may be opened in different provinces, which was not previously possible. In addition, SAFE promulgated the Circular on Printing and Distributing the Provisions on Foreign Exchange Administration over Domestic Direct Investment by Foreign Investors and the Supporting Documents in May 2013, which specifies that the administration by the SAFE or its local branches over direct investment by foreign investors in the PRC will be conducted by way of registration, and banks must process foreign exchange business relating to the direct investment in the PRC based on the registration information provided by SAFE and its branches.
Under the Circular of the SAFE on Further Improving and Adjusting the Policies for Foreign Exchange Administration under Capital Accounts promulgated by the SAFE on January 10, 2014 and effective from February 10, 2014, administration over the outflow of the profits by domestic institutions has been further simplified. In principle, a bank is no longer required to examine transaction documents when handling the outflow of profits of no more than the equivalent of $50,000 by a domestic institution. When handling the outflow of profits exceeding the equivalent of $50,000, the bank, in principle, is no longer required to examine the financial audit report and capital verification report of the domestic institution, provided that it must examine, according to the principle of transaction authenticity, the profit distribution resolution of the board of directors (or the profit distribution resolution of the partners) relating to this profit outflow and the original copy of its tax record-filing form. After each profit outflow, the bank must affix its seal to and endorsements on the original copy of the relevant tax record-filing form to indicate the actual amount of the profit outflow and the date of the outflow.
On March 30, 2015, SAFE promulgated the Circular on Reforming the Management Approach regarding the Settlement of Foreign Exchange Capital of Foreign-invested Enterprises, or SAFE Circular 19, which became effective on June 1, 2015. According to SAFE Circular 19, the foreign exchange capital of foreign-invested enterprises may be settled on a discretionary basis, meaning that the foreign exchange capital in the capital account of a foreign-invested enterprise for which the rights and interests of monetary contribution has been confirmed by the local foreign exchange bureau (or the book-entry registration of monetary contribution by the banks) can be settled at the banks based on the actual operational needs of the foreign-invested enterprise. The
184
Table of Contents
proportion of such discretionary settlement is temporarily determined as 100%. The Renminbi converted from the foreign exchange capital will be kept in a designated account, and if a foreign-invested enterprise needs to make further payment from such account, it still must provide supporting documents and go through the review process with the banks.
Furthermore, SAFE Circular 19 stipulates that the use of capital by foreign-invested enterprises must adhere to the principles of authenticity and self-use within the business scope of enterprises. The capital of a foreign-invested enterprise and capital in RMB obtained by the foreign-invested enterprise from foreign exchange settlement must not be used for the following purposes:
1. directly or indirectly used for the payment beyond the business scope of the enterprises or the payment prohibited by relevant laws and regulations;
2. directly or indirectly used for investment in securities, unless otherwise provided by relevant laws and regulations;
3. directly or indirectly used for granting the entrusted loans in RMB, unless permitted by the scope of business, repaying the inter-enterprise borrowing (including advances by the third party), or repaying the bank loans in RMB that have been sub-lent to the third party; and/or
4. paying the expenses related to the purchase of real estate that is not for self-use, except for the foreign-invested real estate enterprises.
On June 9, 2016, SAFE issued the Notice to Reform and Regulate the Administration Policies of Foreign Exchange Capital Settlement to further reform foreign exchange capital settlement nationwide.
Our PRC subsidiaries’ distributions to the offshore parent and carrying out cross-border foreign exchange activities shall comply with the various SAFE registration requirements described above.
Share Option Rules
Under the Administration Measures on Individual Foreign Exchange Control issued by the People’s Bank of China on December 25, 2006, all foreign exchange matters involved in employee share ownership plans and share option plans in which PRC citizens participate require approval from SAFE or its authorized branch. In addition, under the Notices on Issues concerning the Foreign Exchange Administration for Domestic Individuals Participating in Share Incentive Plans of Overseas Publicly-Listed Companies, or Share Option Rules, issued by the SAFE on February 15, 2012, PRC residents who are granted shares or share options by companies listed on overseas stock exchanges under share incentive plans are required to (1) register with the SAFE or its local branches; (2) retain a qualified PRC agent, which may be a PRC subsidiary of the overseas listed company or another qualified institution selected by the PRC subsidiary, to conduct the SAFE registration and other procedures with respect to the share incentive plans on behalf of the participants; and (3) retain an overseas institution to handle matters in connection with their exercise of share options, purchase and sale of shares or interests and funds transfers. We will make efforts to comply with these requirements upon completion of our initial public offering.
Regulation of Dividend Distribution
The principal laws, rules and regulations governing dividend distribution by foreign-invested enterprises in the PRC are the Company Law of the PRC, as amended, the Wholly Foreign-owned Enterprise Law and its implementation regulations, the Cooperative Joint Venture Law and its implementation regulations and the Equity Joint Venture Law and its implementation regulations. Under these laws, rules and regulations, foreign-invested enterprises may pay dividends only out of their accumulated profit, if any, as determined in accordance with PRC accounting standards and regulations. Both PRC domestic companies and wholly-foreign owned PRC enterprises are required to allocate at least 10% of their respective accumulated after-tax profits each year, if any,
185
Table of Contents
to fund certain capital reserve funds until the aggregate amount of these reserve funds have reached 50% of the registered capital of the enterprises. A PRC company is not permitted to distribute any profits until any losses from prior fiscal years have been offset. Profits retained from prior fiscal years may be distributed together with distributable profits from the current fiscal year.
Labor Laws and Social Insurance
Pursuant to the PRC Labor Law and the PRC Labor Contract Law, employers must execute written labor contracts with full-time employees. All employers must comply with local minimum wage standards. Violations of the PRC Labor Contract Law and the PRC Labor Law may result in the imposition of fines and other administrative and criminal liability in the case of serious violations.
In addition, according to the PRC Social Insurance Law, employers like our PRC subsidiaries in China must provide employees with welfare schemes covering pension insurance, unemployment insurance, maternity insurance, work-related injury insurance, medical insurance, and housing funds.
Employees
As of March 31, 2017, we had 429 full-time employees and 8 part-time employees. Of these, 186 are engaged in full-time research and development and laboratory operations and 251 are engaged in full-time selling, general and administrative functions. As of March 31, 2017, 39% of our personnel were located in the U.S. and 61% were located in Asia. We have also engaged and may continue to engage independent consultants and contractors to assist us with our operations. None of our employees are represented by a labor union or covered by a collective bargaining agreement. We have never experienced any employment related work stoppages, and we consider our relations with our employees to be good.
Legal Proceedings
From time to time we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not presently a party to any legal proceedings that, if determined adversely to us, would individually or taken together have a material adverse effect on our business, results of operations, financial condition or cash flows. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
186
Table of Contents
Executive Officers and Directors
The following table provides information with respect to our directors and executive officers as of March 31, 2017.
| Name |
Age | Position(s) | ||||
| Executive Officers |
||||||
| Johnson Lau, M.D. |
56 | Chief Executive Officer and Chairman of the Board | ||||
| Jeffrey Yordon |
68 | Chief Operating Officer and President, Athenex Pharmaceutical Division | ||||
| Nick Riehle |
64 | Chief Financial Officer | ||||
| Rudolf Kwan, M.B., B.S. |
64 | EVP, Chief Medical Officer | ||||
| Simon Pedder, Ph.D. |
56 | Chief Business and Strategy Officer, Proprietary Products | ||||
| William Zuo, Ph.D. |
55 | President, China | ||||
| Non-Employee Directors |
||||||
| Kim Campbell(3) |
70 | Director | ||||
| Manson Fok, M.B., B.S. |
60 | Director | ||||
| Antony Leung(1) |
65 | Director | ||||
| Jinn Wu, Ph.D.(2) |
68 | Director | ||||
| Song-Yi Zhang(2) |
61 | Director | ||||
| Michael Cannon(2)(3) |
71 | Director Nominee | ||||
| Sheldon Trainor-Degirolamo(1) |
53 | Director Nominee | ||||
| James Zukin(1)(3) |
68 | Director Nominee | ||||
| (1) | Member of the audit committee upon completion of this offering. |
| (2) | Member of the compensation committee upon completion of this offering. |
| (3) | Member of the nominating and corporate governance committee upon completion of this offering. |
The following is a biographical summary of the experience of our executive officers and directors:
Executive Officers
Johnson Lau
Dr. Lau has served as our Chief Executive Officer since 2011, and as Chairman of the Board since our inception in 2003. Dr. Lau has had extensive leadership experience in both scientific and business management. He previously served as Chairman and CEO of Ribapharm Inc., and oversaw the company’s initial public offering in 2002. Prior to Ribapharm, he served as SVP and Head of Research and Development for ICN Pharmaceuticals Inc. Prior to joining ICN, Dr. Lau served as the Senior Director of Antiviral Therapy Research at Schering-Plough. Dr. Lau has contributed more than 200 scientific publications, editorials/reviews and chapters in peer reviewed scientific journals and has edited two books. He was a former Managing Director at Roth Capital Partners and a Director of the Board of Chelsea Therapeutics, serving as the Chair of the Audit and Risk Management Committee as well as the Corporate Governance Committee. He is currently serving on the Board of Porton Fine Chemicals, and private companies including Avalon, Avagenex Limited, Aiviva Corporation, and Hong Kong X-tech Startup platform as a general partner and mentor. He is also an Executive Board Member of the charity Project Vision and is an honorary professor/adjunct professor of the University of Hong Kong, Hong Kong Polytechnic University and Chongqing Southwestern Hospital, and a member of the Advisory Board of the School of Biomedical Sciences of the Chinese University of Hong Kong. Dr. Lau received his medical degree, or MBBS, and medical doctorate degree, or M.D., from the University of Hong Kong. He is also a Fellow of the Royal College of Physicians.
We believe that Dr. Lau serves as a valuable member of our board of directors due to the perspective and experience he brings as our Chairman and CEO.
187
Table of Contents
Jeffrey Yordon
Mr. Yordon joined our company as President, Athenex Pharmaceutical Division in April 2016 and in February 2017 he was appointed as our Chief Operating Officer. Mr. Yordon has held multiple senior management positions in the pharmaceutical industry over the last 46 years. Mr. Yordon was the Founder, Chairman and CEO of Sagent Pharmaceuticals from 2007 until joining us in 2016. Prior to that Mr. Yordon was the COO of American Pharmaceutical Partners where he was a co-founder until the company was eventually sold to Fresenius. Mr. Yordon was the CEO of Faulding Pharmaceuticals, CEO and founder of YorPharm, COO of Gensia Pharmaceuticals and he was involved in the sale of each of these companies to Apotex, Teva and Hospira, respectively. Mr. Yordon was an Ernst & Young Entrepreneur of the Year in 2011, was inducted into the Chicago Entrepreneur Hall of Fame in 2014, won a prestigious Innovation Award from the City of Chicago, was appointed to the Chicago Innovation Council in 2014, was appointed by Governor Rauner to the Illinois Sports Facilities Authority in 2015, has been appointed to be the Chairman of the Board of the Northern Illinois University Foundation, is the Chair of the NIU Political Science Advisory Panel and is actively involved in the NIU Athletic program. Mr. Yordon received a B.A. in Political Science from Northern Illinois University.
Nick Riehle
Mr. Riehle joined our company in January 2017 and in February 2017 was appointed as our Chief Financial Officer. Previously, Mr. Riehle served as Vice President, Administration and Chief Financial Officer of Chelsea Therapeutics, Inc., a publicly-held biopharmaceutical company, from 2004 until 2014. Prior to that, Mr. Riehle served as Chief Financial Officer at HAHT Commerce, Inc., a software company, from 1996 until 2003 and in various roles at Nortel Networks and IBM. He also currently serves on the board of directors of a privately held media company. Mr. Riehle received his Bachelor of Commerce from McGill University, his MBA from York University and earned a Certified Management Accountant (CMA) designation in Ontario, Canada.
Rudolf Kwan
Dr. Kwan has served as our Chief Medical Officer since 2014 and has advised our company since 2008. Until February 2017, Dr. Kwan was engaged on a consultant basis. Dr. Kwan has over twenty years of experience in the pharmaceutical industry in global clinical development and operations. Before joining us, he served dual roles at Schering-Plough as Vice President and Regional Head of Asia Pacific Global Clinical Operations and Vice President of Global Clinical Development, CNS. In the clinical operations position, Dr. Kwan successfully recruited Heads of Clinical Operations for China, South East Asia, Australia, Taiwan and South Korea and set up the infrastructure to conduct global clinical trials in Asia Pacific for Schering-Plough. As Vice President of Global Clinical Development, CNS, he was responsible for the clinical development of all Schering-Plough’s central nervous system drugs, globally, where his achievements included overseeing development and execution of a bioequivalence registration strategy for a new formulation of Temodol for glioblastoma, which led to a simultaneous global registration. He also designed and executed multiple global development programs. He held similar positions at Chiron Corporation and was at Smith-Kline Beecham. Dr. Kwan obtained his medical degree (MBBS) from the University of Hong Kong, and received subsequent training at the University of Wales and is a member of the Royal College of Physicians in the United Kingdom. He was a member and Chair of the Data Monitoring and Safety Board and Protocol Review Board for the Clinical Trial Network of the National Institute on Drug Abuse of the U.S. National Institutes of Health, or NIH. He was also a member of several advisory panels and grant review panels for the NIH.
Simon Pedder
Dr. Pedder joined our company as Chief Business Development Officer in February 2016 and he now serves as our Chief Business and Strategy Officer for Proprietary Products. Dr. Pedder has had a long career in both drug development and commercialization. This includes recent leadership roles with publicly traded Biotechnology companies. He was President and CEO of Cellectar Biosciences from April 2014 to June 2015.
188
Table of Contents
He was President and CEO of Chelsea Therapeutics from May 2004 to July 2012. Previously he was Vice President of Oncology Pharma Business, and a company officer at Hoffmann-La Roche, as well he has served as the Life Cycle Leader and Global Project Leader of Pegasys/IFN and Head of Hepatitis Franchise at Roche. Formerly, he was a member of the faculty in the Department of Pharmacology in College of Medicine in the University of Saskatchewan, where he obtained his Ph.D in Pharmacology. During his longstanding career in pharmaceutical development, Dr. Pedder has led the late stage development and commercial launch of multiple proprietary pharmaceutical products. In addition to his Ph.D in Pharmacology, Dr. Pedder obtained a Master of Science in Toxicology from Concordia University, a Bachelor of Science in Environmental Studies from the University of Waterloo, and completed the Roche-sponsored Pharmaceutical Executive Management Program at Columbia Business School.
William Zuo
Dr. Zuo joined our company in 2015 as President of our China operations in conjunction with our acquisition of Polymed. Dr. Zuo served as President of Polymed Therapeutics since 1995 and Chairman of Chongqing Taihao Pharmaceutical since 2012. Dr. Zuo’s career has focused on the development, manufacture, and sale and marketing of various complex API on a global basis, especially injectable oncology API. Dr. Zuo was the CEO of the Fibrocell Science Group Companies in Asia from 2010 to 2013. Dr. Zuo oversaw the introduction of the U.S. FDA approved cell therapeutics product, LaViv, to the Asia market. He has overseen the construction of multiple cGMP facilities in China and has extensive experience with the Food and Drug Administrations in both China and the United States. Dr. Zuo received his Ph.D in Nanotechnology from Rice University where he worked extensively with Dr. Richard Smalley, the late Nobel Prize Scholar in Chemistry. Dr. Zuo also has Master degrees in Chemical Engineering and Applied Mathematics from Rice University.
Non-Employee Directors
Kim Campbell
The Right Honourable Kim Campbell has served as a member of our board of directors since 2015. Ms. Campbell served as Canada’s nineteenth and first female Prime Minister in 1993. More recently, Ms. Campbell has served as the Founding Principal of Peter Lougheed Leadership College at the University of Alberta since 2014, and as a professional speaker since 2001. She previously held cabinet portfolios as Minister of Justice and Attorney General, Minister of State for Indian Affairs and Northern Development and Minister of National Defence and Minister of Veterans’ Affairs. She was the first woman to hold the Justice and Defence portfolios, and the first woman to be Defence Minister of a NATO country. Ms. Campbell participated in major international meetings including the Commonwealth, NATO, the G-7 Summit and the United Nations General Assembly. After her tenure as Prime Minister, Ms. Campbell was a fellow at the Institute of Politics (Spring 1994) and the Joan Shorenstein Center for the Study of Press and Politics (1994-1995) at the Harvard Kennedy School of Government. She served as the Canadian Consul General in Los Angeles (1996-2000), then returned to Harvard to teach at the Center for Public Leadership at the Kennedy School (2001-2004).
Ms. Campbell is a founding member of the Club of Madrid, an organization of former heads of government and state who work to promote democratic values. She served as Secretary General (2004-2006). She has also served as its Acting President in 2002, its Vice President in 2003-2004 and served on its Board of Directors from 2007-2011. Ms. Campbell is a member and chair emerita of the Council of Women World Leaders (1993-2003). The Council’s membership consists of women who hold or have held the office of President or Prime Minister. Ms. Campbell is a member of the International Women’s Forum, a global organization of women of significant and diverse achievement. She served as its president (2003-2005) and was inducted into the IWF Hall of Fame in 2008.
Today, Ms. Campbell devotes the majority of her time to serving as the founding principal of the new Peter Lougheed Leadership College at the University of Alberta. She is also a trustee of the International Center for the
189
Table of Contents
Study of Radicalisation and Political Violence at King’s College London. She is a member of the Pacific Council on International Policy, the West Coast affiliate of the Council on Foreign Relations, and the Global Council of the Asia Society of New York. She is on the advisory board of Equal Voice and an honorary patron of Informed Opinions. She is also a senior advisor to the Crisis Group and an honorary board member of the Climate Action Reserve and previously served as a trustee of the Salk Institute for Biological Studies (2007-2010).
We believe that Ms. Campbell serves as a valuable member of our board of directors due to her extensive experience serving on the boards of directors of a variety of other entities over the course of her career.
Manson Fok
Dr. Fok has served as a member of our board of directors since 2015. Since October 2011, Dr. Fok has been the Chairman of Pedder Clinic, a private medical practice in Hong Kong. He is also the Dean, Faculty of Health Sciences at Macau University of Science and Technology, or MUST; Hospital Director of University Hospital at MUST; President of the Macau Healthcare Management and Promotion Association; Censor-in-Chief, World Chinese Doctors’ Association; Honorary Fellow, Chinese College of Surgeons; Committee member, The Council of Medical Affairs in Macau SAR, among many other leadership positions. Dr. Fok is also a director of Avalon Global. Dr. Fok was awarded the 2014 Gusi Peace Prize in Humanitarianism for his remarkable contributions to medical education, healthcare delivery and cross-border biotechnology developments that act as a bridge within Asia and across continents. In 2015, Dr. Fok was appointed as the CEO of the same Peace Prize Foundation to continue promoting peace, cooperation and healthcare development in the Asia-Pacific region. After receiving his medical degree (MBBS) from the University of Hong Kong in 1982, Dr. Fok was appointed faculty in the Surgical Unit of the University of Hong Kong. Dr. Fok has published many original research papers in high-ranking international medical journals and chapters in various academic books focusing on minimally invasive treatment for esophageal surgery.
We believe that Dr. Fok serves as a valuable member of our board of directors due to his extensive knowledge of cross-border biotechnology developments that act as a bridge between the U.S. and Asia.
Antony Leung
Mr. Leung has served as a member of our board of directors since March 2016. Since February 2014, Mr. Leung has been Group Chief Executive Officer of Nan Fung Group, a Hong Kong-based company focusing on property & investment businesses, and since April 2016 he has also served as its Chairman. Mr. Leung previously served as a Senior Managing Director and Chairman of Greater China of The Blackstone Group (HK) Limited from January 2007 through January 2014, and also as the Financial Secretary of Hong Kong Special Administrative Region. He is also Independent Non-Executive Director of China Merchants Bank, Chairman of charity organizations Heifer International Hong Kong and Food Angel, and Chairman of Harvard Business School Association of Hong Kong.
Mr. Leung has had extensive experience in financial services, including Chairman of Asia of JP Morgan Chase, Asia Head of Citi Private Bank, and Regional Head of Citi Investment Bank, Treasury and Greater China. In addition, he was independent director of Industrial and Commercial Bank of China, China Mobile (Hong Kong) Limited, American International Assurance (Hong Kong) Limited, and international advisory board member of China Development Bank. His past public service includes Non-Official Member of the Executive Council, member of the Exchange Fund Advisory Committee, Hong Kong Airport Authority, and Chairman of the Education Commission and University Grants Committee in Hong Kong.
Mr. Leung graduated from the University of Hong Kong in 1973, and attended Harvard Business School’s Program for Management Development and Advanced Management Program.
We believe that Mr. Leung serves as a valuable member of our board of directors due to his extensive knowledge of global capital markets and of the business environment in Hong Kong and the PRC.
190
Table of Contents
Jinn Wu
Dr. Wu has served as a member of our board of directors since 2007. In 1987, Dr. Wu founded Xenobiotic Laboratory, or XBL, in Plainsboro, New Jersey, a contract research organization that provides an extensive array of clinical and preclinical research services to the biotechnology and pharmaceutical industries, and he served as its President until 2014. Since then, Dr. Wu has served as Chief Scientific Officer and Senior Vice President of WuXi AppTec from 2015 to 2016 and, beginning in 2017, now serves as Scientific Strategic Advisor to WuXi AppTec. Dr. Wu earned a Ph.D in Natural Products and Medicinal Chemistry from Ohio State University and spent several years as a research scientist at FMC Corporation before founding XBL. He is an adjunct professor at the University of Medicine and Dentistry of New Jersey and is a member of the American Association of Pharmaceutical Scientists, the International Society for the Study of Xenobiotics, the American Society of Pharmacognosy and the American Chemical Society.
We believe that Dr. Wu serves as a valuable member of our board of directors due to his extensive medical experience and experience with clinical and preclinical research services.
Song-Yi Zhang
Mr. Zhang is the founder of Mandra Capital, an investment holding company focused on early stage opportunities in internet, life science, materials and technologies. Mr. Zhang has more than twenty years of investment banking and direct investment experience. In addition to his responsibilities as Chairman of Mandra Capital since its inception in 2002, Mr. Zhang serves as a director of SINA Corporation since April 2004, a company listed on the Nasdaq Stock Market, and an independent non-executive director of China Long Yuan Electric Power Group Corp. since July 2009 and China Renewal Energy Investment Limited from April 2008 to April 2013 and since January 2016, each a company listed on Hong Kong Stock Exchange. Prior to founding Mandra Capital, Mr. Zhang served as a Managing Director of Asia Merger, Acquisition and Divestiture Group, and the co-Head of Asia Resources and Infrastructure Group of Morgan Stanley, and a Senior Associate of Milbank, Tweed, Hadley & McCloy LLP. Mr. Zhang holds a Juris Doctor degree from Yale University.
We believe that Mr. Zhang serves as a valuable member of our board of directors due to his extensive experience investing in biotechnology companies.
Director Nominees
Michael Cannon
Mr. Cannon will join our board of directors effective upon the completion of this offering. Mr. Cannon has over forty years experience in the pharmaceutical industry, and has served as an Executive Director of BioSentinel, Inc. since July 2010. Previously, he worked at SICOR Inc., a Swiss-Italian API manufacturing company, in a variety of positions in manufacturing, quality and regulatory affairs, before transitioning to business development. Mr. Cannon was a member of the team that orchestrated the merger of SICOR with Gensia of San Diego in 1997 and after the merger he served as Chief Scientific Officer. He then served as a member of SICOR’s board and president of the biotech division until the company was sold to Teva in 2004. Mr. Cannon also serves on the boards of directors of Moleculin Biotech Inc. and three privately held companies. Mr. Cannon received a B.S. in Chemistry from Fordham College.
We believe that Mr. Cannon’s experience in both scientific and business development roles in the pharmaceutical industry, as well as his knowledge of our company, qualifies him to serve on our board of directors.
Sheldon Trainor-Degirolamo
Mr. Trainor-Degirolamo will join our board of directors effective upon the completion of this offering. Mr. Trainor-Degirolamo is the Founder and Managing Director of PacBridge Capital Partners (HK) Limited, a
191
Table of Contents
principal investment and advisory firm based in Hong Kong, which he founded in 2009. Prior to establishing PacBridge, Mr. Trainor-Degirolamo spent more than 20 years in roles of increasing responsibility in the financial services industry, including with Credit Suisse Australia, Morgan Stanley Asia and as Head of Investment Banking for Asia and as Vice Chairman of Merrill Lynch Asia. Since 2012, he has also served as an Executive Director of Macau Legend Development Company Ltd., a company listed on the Hong Kong Stock Exchange. Mr. Trainor-Degirolamo received his Bachelor of Commerce from the University of British Columbia.
We believe that Mr. Trainor-Degirolamo’s experience in investing and financial services, as well as his knowledge of our company, qualifies him to serve on our board of directors.
James Zukin
Mr. Zukin will join our board of directors effective upon the completion of this offering. Mr. Zukin is a co-founder of Houlihan Lokey, Inc., and served in various capacities from 1976 until his retirement in 2013, most recently as Senior Managing Director of Houlihan Lokey, Inc. and Chairman of its subsidiary, Houlihan Lokey Howard & Zukin Investment Consulting (Beijing) Co., Ltd. Mr. Zukin has served on the faculty of various World Bank, IFC, and IMF conferences. He serves as a delegate to the Paris Club meetings involving the private sector. Mr. Zukin frequently speaks at business conferences in the United States and Asia on M&A, financial restructuring and shareholder liquidity, among other topics. Mr. Zukin earned a B.A. in Economics from the University of California at Berkeley and an M.B.A. from Harvard Business School.
We believe that Mr. Zukin experience in investment advisory services in both the U.S. and Asia, as well as his knowledge of our company, qualifies him to serve on our board of directors.
Board of Directors and Director Independence
Upon completion of this offering, our board of directors will consist of eight non-employee directors and our Chief Executive Officer, Johnson Lau.
We intend to amend and restate our certificate of incorporation and bylaws in connection with this offering to provide that the number of directors may be determined from time to time by resolution of our board of directors. Upon the closing of this offering, we will have directors. Our board of directors will be divided into three classes, as follows:
| • | Class I, which initially will consist of , and , whose terms will expire at our annual meeting of stockholders to be held in 2017; |
| • | Class II, which initially will consist of , and , whose terms will expire at our annual meeting of stockholders to be held in 2018; and |
| • | Class III, which initially will consist of , and , whose terms will expire at our annual meeting of stockholders to be held in 2019. |
Upon the expiration of the initial term of office for each class of directors, each director in such class shall be elected for a term of three years and serve until a successor is duly elected and qualified or until his or her earlier death, resignation or removal. Any additional directorships resulting from an increase in the number of directors or a vacancy will be filled by the directors then in office.
Directors will only be removed with cause by the affirmative vote of at least a majority of the stock then entitled to vote at an election of directors. Because only one-third of our directors will be elected at each annual meeting, two consecutive annual meetings of stockholders could be required for the stockholders to change a majority of the board.
192
Table of Contents
We have entered into a voting agreement with Mandra Health Limited that, subject to certain requirements, requires us to use our best efforts to include a director nominee of Mandra’s choosing in any slate of nominees to be recommended by our board of directors to our shareholders for election. For additional information about this agreement, please see “Certain Relationships and Related Party Transactions—Mandra Voting Agreement.”
There is no family relationship between any director, executive officer or person nominated to become a director or executive director.
Our board has determined that each of Ms. Campbell and Messrs. Cannon, Leung, Trainor-Degirolamo, Wu and Zukin are “independent” as defined in the currently applicable listing standards of the NASDAQ Global Market. Dr. Lau is not independent because he is one of our executive officers.
Board Committees
Our board directs the management of our business as provided by Delaware law and conducts its business through meetings of the board of directors, an audit committee, a compensation committee, and a nominating and corporate governance committee. Further, from time to time, other committees may be established under the direction of the board when necessary to address specific issues. The composition of the board committees will comply, when required, with the applicable rules of the NASDAQ Global Market and applicable law.
Compensation Committee
Upon completion of this offering, our compensation committee will consist of Dr. Wu as chair, Mr. Cannon and Mr. Zhang, each of Dr. Wu and Mr. Cannon is an independent director, as defined in the NASDAQ Global Market qualification standards. Mr. Zhang is not an independent director. The NASDAQ listing standards and SEC rules provide a grace period for meeting the compensation committee independence requirements for a company conducting an initial public offering. That grace period provides that one member of the committee must be independent at the time of listing, a majority of the members must be independent within 90 days of listing, and all committee members must be independent within one year of listing. The functions of this committee include:
| • | reviewing our company’s overall compensation strategy, including base salary, incentive compensation and equity-based grants, to assure that it promotes stockholder interests and supports our strategic and tactical objectives, and that it provides for appropriate rewards and incentives for our management and employees; |
| • | reviewing and recommending to the Board compensation for our executive officers and the board of directors; |
| • | administering and, if necessary, revising our company’s 401(k) plan, any deferred compensation plans, and any additional employee benefit plans; |
| • | reviewing with management our company’s major compensation-related risk exposures and the steps management has taken, or should consider taking, to monitor or mitigate such exposures; and |
| • | overseeing our company’s compliance with regulatory requirements associated with compensation of our directors, executive officers and other employees, including reviewing executive compensation disclosures, any conflict of interest disclosure with regard to any compensation consultant retained by the compensation committee, and any other compensation disclosure prepared in response to disclosure requirements to the extent applicable to us. |
Compensation Committee Interlocks and Insider Participation
During the last fiscal year, Messrs. Fok, Wu and Zhang served as the members of our compensation committee.
193
Table of Contents
Audit Committee
Upon completion of this offering, our audit committee will consist of Mr. Zukin as chair, Mr. Leung and Mr. Trainor-Degirolamo, each of whom is an independent director, as defined in the NASDAQ Global Market qualification standards.
Mr. Zukin qualifies as an “audit committee financial expert” as that term is defined in the rules and regulations established by the SEC. The functions of this committee include:
| • | overseeing our company’s corporate accounting and financial reporting practices, the audit of the our financial statements by the company’s independent registered public accounting firm, and our internal audit function; |
| • | monitoring the periodic reviews of the adequacy of the accounting and financial reporting processes and systems of internal control that are conducted by the independent registered public accounting firm and our company’s senior management, and internal audit function; |
| • | appointing the independent registered public accounting firm; determining and approving the fees paid to such firm and reviewing and evaluating the qualifications, independence and performance of such firm; |
| • | reviewing and evaluating the organization and performance of our company’s internal audit function; and |
| • | preparing a report to be included in our company’s annual proxy statement as required by the rules and regulations of the SEC under U.S. federal securities laws. |
Both our independent registered public accounting firm and internal financial personnel regularly meet privately with the audit committee and have unrestricted access to this committee.
Nominating and Governance Committee
Upon completion of this offering, our nominating and governance committee will consist of Ms. Campbell as chair, Mr. Cannon and Mr. Zukin. All members of the nominating and governance committee are independent directors, as defined in the NASDAQ Global Market qualification standards. The functions of this committee include:
| • | identifying, considering and recommending to the board candidates for nomination to the board of directors consistent with criteria approved by the board of directors; |
| • | developing and recommending corporate governance guidelines and policies for our company; |
| • | overseeing the evaluation of the board of directors and its committees, including an annual evaluation of the corporate governance committee; and |
| • | advising the board of directors on corporate governance matters and performance matters relating to the board of directors, including recommendations regarding the structure and composition of the board of directors and its committees. |
Indemnification Agreements
We have entered into indemnification agreements with certain of our current and former directors and executive officers and will enter into indemnification agreements with our other directors and officers in connection with this offering. These agreements will require us to indemnify these individuals to the fullest extent permitted under Delaware law against liabilities that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified. We also intend to enter into indemnification agreements with our future directors and executive officers.
194
Table of Contents
Certain Legal Proceedings
Mr. Zukin, who will join our board of directors effective upon the completion of this offering, pled no contest in the State of California in August 2015 to reckless driving causing bodily injury as a result of an accident that occurred in July 2014.
Letter Agreement with Former Officer
On December 8, 2016, we entered into a letter agreement with one of our former executive officers and directors, Flint Besecker. Please see “Certain Relationships and Related Party Transactions—Letter Agreement with Former Officer.”
Code of Conduct and Ethics
Our board of directors has adopted a code of conduct and ethics that establishes the standards of ethical conduct applicable to all directors, officers and employees of our company and addresses, among other things, conflicts of interest, corporate opportunities, regulatory reporting, medical device laws, communications and confidentiality requirements. We intend to amend the code in connection with this offering to also address, among other things, compliance with disclosure controls and procedures and internal controls over financial reporting. We intend to disclose any amendments to the code, or any waivers of its requirements, on our website to the extent required by the applicable rules and exchange requirements. The inclusion of our website address in this prospectus does not include or incorporate by reference into this prospectus the information on or accessible through our website. The audit committee is responsible for applying and interpreting our code of conduct and ethics in situations where questions are presented to it.
195
Table of Contents
EXECUTIVE AND DIRECTOR COMPENSATION
Director Compensation
The compensation and benefits for service as a member of the board of directors is determined by our board of directors. We reimburse each of our directors for any out-of-pocket expenses in connection with attending meetings of our board of directors and committees of the board of directors.
Historically, certain of our non-employee directors have been granted options to purchase shares of our common stock as compensation for their service on our board of directors. These grants were in lieu of cash or other compensation and were not awarded according to any schedule or on an annual basis.
Director Compensation Table
The following table shows information regarding the compensation earned during the year ended December 31, 2016 by our non-employee directors.
| Name |
Fees Earned or Paid in Cash ($) |
Stock Awards ($) |
Option Awards ($)(1) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||
| Kim Campbell |
20,000 | 20,000 | ||||||||||||||||||
| Manson Fok |
20,000 | 20,000 | ||||||||||||||||||
| Antony Leung |
20,000 | 249,931 | 269,931 | |||||||||||||||||
| Jinn Wu |
20,000 | 20,000 | ||||||||||||||||||
| Song-Yi Zhang |
20,000 | 20,000 | ||||||||||||||||||
| (1) | The amounts in this column reflect the aggregate grant date fair value of the stock options under FASB ASC Topic 718, which was determined using the Black-Scholes Method and the assumptions set forth in “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Estimates—Stock-Based Compensation and Fair Value of our Common Stock.” |
196
Table of Contents
The following table summarizes the outstanding equity awards held by our non-employee directors as of December 31, 2016.
| Name |
Number of Securities Underlying Unexercised Options Exercisable (#) |
Number of Securities Underlying Unexercised Options Unexercisable (#) |
Option Exercise Price ($) |
Option Expiration Date |
||||||||||||
| Kim Campbell |
24,000 | 24,000 | 9.00 | 10/17/2025 | ||||||||||||
| Manson Fok |
80,000 | — | 4.55 | 1/2/2023 | ||||||||||||
| 48,000 | — | 4.55 | 5/13/2023 | |||||||||||||
| 20,000 | — | 4.55 | 10/11/2023 | |||||||||||||
| 40,000 | — | 4.55 | 6/12/2024 | |||||||||||||
| 48,000 | — | 7.50 | 5/18/2025 | |||||||||||||
| 24,000 | 24,000 | 9.00 | 10/17/2025 | |||||||||||||
| Antony Leung |
— | 48,000 | 9.00 | 2/28/2026 | ||||||||||||
| Jinn Wu |
16,000 | — | 2.25 | 4/24/2017 | ||||||||||||
| 16,000 | — | 4.55 | 5/9/2021 | |||||||||||||
| 16,000 | — | 4.55 | 3/26/2022 | |||||||||||||
| 90,000 | — | 4.55 | 1/2/2023 | |||||||||||||
| 10,000 | — | 4.55 | 2/12/2024 | |||||||||||||
| 24,000 | — | 5.50 | 2/5/2025 | |||||||||||||
| 30,000 | — | 5.50 | 2/5/2025 | |||||||||||||
| 48,000 | 48,000 | 5.50 | 3/20/2025 | |||||||||||||
| Song-Yi Zhang |
80,000 | — | 7.50 | 5/18/2025 | ||||||||||||
| 24,000 | 24,000 | 9.00 | 10/17/2025 | |||||||||||||
Executive Compensation
As an emerging growth company, we have opted to comply with the executive compensation disclosure rules applicable to “smaller reporting companies,” as such term is defined in the rules promulgated under the Securities Act, which require compensation disclosure for our principal executive officer and the two most highly compensated executive officers other than our principal executive officer. Our named executive officers for the year ended December 31, 2016 are: Johnson Lau, our Chief Executive Officer and Chairman of the Board, Jeffrey Yordon, President, Athenex Pharmaceutical Division and Simon Pedder, EVP, Chief Business Development Officer. Since December 31, 2016, Mr. Yordon was also appointed as our Chief Operating Officer, and Dr. Pedder was appointed as our Chief Business and Strategy Officer for Proprietary Products.
The primary objective of our compensation policies and programs with respect to executive compensation is to serve our stockholders by attracting, retaining and motivating talented and qualified executives. We focus on providing a competitive compensation package that provides, at the discretion of our board of directors, long-term incentives for the achievement of corporate and individual performance objectives. Decisions regarding executive compensation are the primary responsibility of our compensation committee. Our board of directors regularly assesses our compensation policies for any practices that are reasonably likely to have a material adverse effect on our company; as of the end of December 31, 2016, our board of directors concluded that our compensation policies did not present any such risks to the company.
In 2016, we compensated our named executive officers through a mix of base salary and equity compensation at levels that we believed were comparable to those of executives at companies of similar size and stage of development, and that rewarded our named executive officers for their contributions.
We have not yet established a formal policy with respect to our allocations between long-term equity compensation and short-term incentive compensation. As a private company, our compensation plans and the
197
Table of Contents
amount of each compensation element to pay our named executive officers were generally developed by our management and approved by our board of directors on an individual, case-by-case basis utilizing a number of factors, including publicly available data and our general business conditions and objectives, as well as our subjective determination with respect to each executive’s individual contributions to such objectives.
Summary Compensation Table
The following table shows information regarding the compensation earned during the year ended December 31, 2016 by our named executive officers.
| Name and Principal Position |
Year | Salary ($) |
Bonus ($) |
Stocks Awards ($) |
Option Awards ($)(1) |
Nonqualified deferred compensation earnings ($) |
All Other Compensation ($)(2) |
Total ($) |
||||||||||||||||||||||||
| Johnson Lau |
2016 | 200,000 | — | — | — | 300,000 | 21,687 | 521,687 | ||||||||||||||||||||||||
| Chief Executive Officer and Chairman of the Board |
||||||||||||||||||||||||||||||||
| Jeffrey Yordon |
2016 | 254,538 | — | 1,800,000 | 785,184 | — | 2,237 | 2,841,959 | ||||||||||||||||||||||||
| Chief Operating Officer and President, Athenex Pharmaceutical Division |
||||||||||||||||||||||||||||||||
| Simon Pedder |
2016 | 172,308 | — | 536,126 | — | 16,645 | 725,079 | |||||||||||||||||||||||||
| Chief Business and Strategy Officer, Proprietary Products |
||||||||||||||||||||||||||||||||
| (1) | The amounts in this column reflect the aggregate grant date fair value of the stock options under FASB ASC Topic 718, which was determined using the Black-Scholes Method and the assumptions set forth in “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Estimates—Stock-Based Compensation and Fair Value of our Common Stock.” |
| (2) | The amounts reflect (i) $8,000, $2,237 and $5,538 in 401(k) matching contributions for each of Dr. Lau, Mr. Yordon and Dr. Pedder, respectively, and (ii) $13,687 and $11,107 in company-paid health insurance premiums for each of Dr. Lau and Dr. Pedder, respectively. |
Narrative to Summary Compensation Table
We are currently evaluating various compensation programs to implement upon the completion of this offering. The disclosures below describe our historical compensation practices.
Annual Salary
We review compensation annually for our named executive officers. In setting base salaries and bonuses and granting equity incentive awards, we consider compensation for comparable positions in the market, the historical compensation levels of our executives, individual performance as compared to our expectations and objectives, our desire to motivate our employees to achieve short- and long-term results that are in the best interests of our stockholders, and a long-term commitment to our company. We do not target a specific competitive position or a specific mix of compensation among base salary, bonus or long-term incentives.
Our board of directors has historically determined the compensation for our executive officers and more recently delegated this authority to the compensation committee, other than with respect to our chief executive officer. Our compensation committee typically reviews and discusses management’s proposed compensation
198
Table of Contents
with the chief executive officer for all executive officers other than the chief executive officer. Based on those discussions and its discretion, the compensation committee then approves the compensation for our executive officers. Our board of directors, without members of management present, discusses the compensation committee’s report on these matters and approves the compensation of our chief executive officer. To date, our compensation committee has not engaged a compensation consultant or adopted a peer group of companies for purposes of determining executive compensation.
Long-Term Incentives
Our 2013 Plan authorizes us to make grants to eligible recipients of non-qualified stock options. To date, we have granted only stock options to our named executive officers under the 2013 Plan.
We typically grant stock options at the start of employment to each named executive officer and our other employees. To date, we have not maintained a practice of granting additional equity on an annual basis, but we have granted additional equity following significant equity financings, and we have retained discretion to provide additional targeted grants in certain circumstances.
We award our equity grants on the date our board of directors approves the grant. We set the option exercise price and grant date fair value based on our per-share valuation on the date of grant. For grants in connection with initial employment, vesting begins on the initial date of employment. Time vested stock option grants to our executives and most employees typically vest 25% on each anniversary of the vesting commencement date over either a three- or four-year period.
Outstanding Equity Awards at 2016 Year-End
The following table summarizes the outstanding equity awards held by our named executive officers as of December 31, 2016.
| Name |
Number of Securities Underlying Unexercised Options Exercisable (#) |
Number of Securities Underlying Unexercised Options Unexercisable (#) |
Option Exercise Price ($) |
Option Expiration Date |
Number of shares or units of stock that have not vested (#) |
Market value of shares or units of stock that have not vested ($)(1) |
||||||||||||||||||
| Lau |
60,000 | — | 4.25 | 12/3/2018 | ||||||||||||||||||||
| 41,944 | — | 4.55 | 5/9/2021 | |||||||||||||||||||||
| 198,056 | — | 4.55 | 5/9/2021 | |||||||||||||||||||||
| 150,000 | — | 4.55 | 3/26/2022 | |||||||||||||||||||||
| 1,200,000 | — | 4.55 | 1/2/2023 | |||||||||||||||||||||
| 466,667 | 933,333 | 7.50 | 5/22/2025 | |||||||||||||||||||||
| Yordon |
— | 150,000 | 9.00 | 6/19/2026 | 150,000 | 1,650,000 | ||||||||||||||||||
| Pedder |
15,000 | 5,000 | 4.55 | 5/13/2023 | ||||||||||||||||||||
| — | 100,000 | 9.00 | 2/28/2026 | |||||||||||||||||||||
| (1) | At December 31, 2016, the fair market value of our common stock was $11.00. |
Pension Benefits
We do not have any qualified or non-qualified defined benefit pension plans.
Non-qualified Deferred Compensation
Our Restated Non-Qualified Deferred Compensation Plan, or Deferred Compensation Plan, allows certain of our officers to defer portions of their compensation to be paid at specific times after their separation from the company.
199
Table of Contents
Named Executive Officer Employment Agreements
We have entered into employment agreements with certain of our officers, including certain of our named executive officers. Our employment agreements with our named executive officers are summarized below.
Johnson Lau
We entered into an amended and restated employment agreement, dated June 1, 2015, with our current Chief Executive Officer and Board Chairman, Johnson Lau. Under the agreement, Dr. Lau is entitled to receive a base salary, subject to adjustment by our board of directors from time to time, deferred compensation, and certain equity awards.
Dr. Lau is entitled to receive a grant of 400,000 shares of our common stock, provided our valuation is greater than $200 million, immediately prior to the occurrence of certain liquidity events, including the completion of this offering. This stock incentive will only take effect if the liquidity event occurs while Mr. Lau is our chief executive officer.
Pursuant to the terms of his employment agreement, we issued to Dr. Lau 880,000 shares of our common stock in exchange for a promissory note, which has since been satisfied. See “Certain Relationships and Related Party Transactions—Contractual Arrangements—Share Purchases.”
Jeffrey Yordon
We entered into an employment agreement, effective February 21, 2017, with Jeffrey Yordon, our Chief Operating Officer and President of Athenex Pharmaceutical Division. Under the agreement, Mr. Yordon is entitled to receive a base salary, consideration for a cash bonus at the discretion of our Compensation Committee, and an award of an option to purchase 230,000 shares of our common stock subject to vesting in equal installments over three years to be granted upon the completion of this offering.
Mr. Yordon’s employment agreement has an initial term of three years, after which it will automatically renew for additional one-year terms until terminated pursuant to its terms. The agreement may be terminated by Mr. Yordon, either with or without good reason, as such term is defined in the agreement, and by us, either with or without cause, as such term is defined in the agreement. Pursuant to the agreement, depending on the circumstances of his termination, Mr. Yordon may be entitled to certain payments or benefits on or after termination. The agreement also contains customary non-solicitation, non-competition and confidentiality provisions.
Simon Pedder
We entered into an employment agreement, effective February 21, 2017, with Simon Pedder, our Chief Business and Strategy Officer, Proprietary Products. Under the agreement, Dr. Pedder is entitled to receive a base salary, consideration for a cash bonus at the discretion of our Compensation Committee, and an award of an option to purchase 130,000 shares of our common stock subject to vesting in equal installments over four years to be granted upon the completion of this offering.
Dr. Pedder’s employment agreement has an initial term of three years, after which it will automatically renew for additional one-year terms until terminated pursuant to its terms. The agreement may be terminated by Dr. Pedder, either with or without good reason, as such term is defined in the agreement, and by us, either with or without cause, as such term is defined in the agreement. Pursuant to the agreement, depending on the circumstances of his termination, Dr. Pedder may be entitled to certain payments or benefits on or after termination. The agreement also contains customary non-solicitation, non-competition and confidentiality provisions.
200
Table of Contents
Employee Benefit Plans
Our employees, including our named executive officers, are eligible to receive various employee benefits. These benefits include the following: medical, dental, and vision care plans; a 401(k) plan; group term life insurance; accidental death and dismemberment; short term and long term disability insurance, flexible spending accounts for unreimbursed medical expenses and dependent care accounts; Employee Assistance Program, holidays and paid time off.
401(k) Plan
Our employees are eligible to participate in our 401(k) plan. Our 401(k) plan is intended to qualify as a tax-qualified plan under Section 401(a) of the Code. Our 401(k) plan provides that each participant may contribute a portion of his or her pre-tax compensation, up to a statutory limit, which for most employees is $18,000 in 2016. Participants who are 50 years or older can also make “catch-up” contributions, which in 2016 may be up to an additional $6,000 (or a combined maximum of $24,000). Employee contributions are held and invested by the plan’s trustee. Our 401(k) plan also permits us to make discretionary contributions and matching contributions. We make matching contributions to our employees of an amount equal to 50% of their elective deferral which does not exceed 8% of their compensation.
Equity Incentive Plans
2004 Common Unit Option Plan
Our board of directors adopted our 2004 Common Unit Option Plan, or 2004 Plan, and our stockholders approved it, in 2004, when we were organized as a Delaware limited liability company. Our 2004 Plan provided for the grant of options to purchase units representing interests in the limited liability company to our employees, directors, and consultants and any parent’s or subsidiary’s employees, directors and consultants. In conjunction with our conversion from a Delaware limited liability company to a Delaware corporation in 2012, we amended and restated our 2004 Plan such that all outstanding unit options under the 2004 Plan became fully vested as of October 1, 2012. Upon our conversion into a corporation, these fully vested unit options were converted into fully vested options to purchase shares of our common stock. We discontinued issuing options under the 2004 Plan in 2011.
As of March 31, 2017, options to purchase 41,944 shares remained outstanding under our 2004 Plan. No other awards were made under the 2004 Plan.
If any change is made in, or other event occurs with respect to, our common stock without the receipt of consideration by us, through merger, consolidation, reorganization, recapitalization, reincorporation, stock and certain other dividends, stock split, combination of shares, exchange of shares, change in corporate structure or other transaction, the shares subject to the 2004 Plan or subject to any option or award granted under the 2004 Plan would be similarly adjusted.
2007 Common Unit Option Plan
Our board of directors adopted our 2007 Common Unit Option Plan, or 2007 Plan, and our stockholders approved it, in 2007, when we were organized as a Delaware limited liability company. Our 2004 Plan provided for the grant of options to purchase units representing interests in the limited liability company to our employees, directors, and consultants and any parent’s or subsidiary’s employees, directors and consultants. In conjunction with our conversion from a Delaware limited liability company to a Delaware corporation in 2012, we amended and restated our 2007 Plan such that all outstanding unit options under the 2007 Plan became fully vested as of October 1, 2012. Upon our conversion into a corporation, these fully vested unit options were converted into fully vested options to purchase shares of our common stock. We discontinued issuing options under the 2007 Plan in 2012.
201
Table of Contents
As of March 31, 2017, options to purchase 1,007,388 shares remained outstanding under our 2007 Plan. No other awards were made under the 2007 Plan.
If any change is made in, or other event occurs with respect to, our common stock without the receipt of consideration by us, through merger, consolidation, reorganization, recapitalization, reincorporation, stock and certain other dividends, stock split, combination of shares, exchange of shares, change in corporate structure or other transaction, the shares subject to the 2007 Plan or subject to any option or award granted under the 2007 Plan would be similarly adjusted.
2013 Common Stock Option Plan
Our board of directors adopted our 2013 Common Stock Option Plan, or 2013 Plan, in 2012. Our 2013 Plan provides for the grant of non-qualified stock options to our employees, directors, and consultants and any parent’s or subsidiary’s employees, directors and consultants. We will cease issuing awards under the 2013 Plan upon the implementation of the 2017 Plan, which is described below. Following our initial public offering, we will grant equity awards under our 2017 Plan.
As of March 31, 2017, we had reserved 10,200,000 shares of our common stock for issuance under our 2013 Plan. As of March 31, 2017, options to purchase 8,165,657 of these shares remained outstanding and 2,025,543 of these shares remained available for future grant. No other awards have been made under the 2013 Plan.
If any change is made in, or other event occurs with respect to, our common stock without the receipt of consideration by us, through merger, consolidation, reorganization, recapitalization, reincorporation, stock and certain other dividends, stock split, combination of shares, exchange of shares, change in corporate structure or other transaction, the shares subject to the 2013 Plan or subject to any option or award granted under the 2013 Plan would be similarly adjusted.
2017 Omnibus Incentive Plan
Our 2017 Omnibus Incentive Plan, or 2017 Plan, was adopted by our Board of Directors on and approved by our stockholders thereafter. The 2017 Plan will become effective prior to the completion of this offering. The 2017 Plan will provide for the grant of incentive stock options, within the meaning of Section 422 of the Code, to our employees and any parent and subsidiary employees, and for the grant of non-qualified stock options, stock appreciation rights, restricted stock, restricted stock units, dividend equivalent rights, cash-based awards (including annual cash incentives and long-term cash incentives), and any combination thereof to our employees, directors, and consultants and to employees, directors, and consultants of certain affiliated entities.
In connection with this offering, we have reserved for issuance under the 2017 Plan shares of our common stock equal to the sum of: (i) shares of common stock; and (ii) up to shares of our common stock underlying awards granted under our 2013 Plan and outstanding when the 2017 Plan becomes effective that are forfeited, canceled, or expire (whether voluntarily or involuntarily). In addition, the 2017 Plan provides for an annual increase to the number of shares of our common stock available for issuance thereunder on the first business day of each calendar year beginning with the calendar year following the calendar year in which the 2017 Plan becomes effective, equal to the lesser of (i) shares, (ii) percent of the number of shares of our common stock outstanding as of the last day of the immediately preceding calendar year, and (iii) a lesser number of shares determined by the administrator (as defined below).
Our Board of Directors or a committee of our Board of Directors, which we refer to as the “administrator” in this description, will administer the 2017 Plan. In the case of awards intended to qualify as “performance-based compensation” within the meaning of Section 162(m) of the Code, the administrator will consist of two or more “outside directors” within the meaning of Section 162(m) of the Code. The administrator will have the power to determine and interpret the terms and conditions of the awards, including, as applicable, the employees,
202
Table of Contents
directors, and consultants who will receive awards, the exercise price, the number of shares subject to each award, the vesting schedule and exercisability of the awards, the restrictions on transferability of awards, and the form of consideration payable upon exercise. The administrator also will have the authority to reduce the exercise price of any outstanding stock options and the base appreciation amount of any stock appreciation rights if the exercise price or base appreciation amount exceeds the fair market value of the underlying shares, and to cancel such options and stock appreciation rights in exchange for new awards, in each case without stockholder approval.
The 2017 Plan will allow for the grant of incentive stock options that qualify under Section 422 of the Code only to our employees and employees of any of our parents or subsidiaries. Non-qualified stock options may be granted to our employees and directors and those of certain of our affiliates. The per share exercise price of all options granted under the 2017 Plan must be equal to at least the per share fair market value of our common stock on the date of grant. The term of an incentive stock option may not exceed 10 years, except that with respect to any employee who owns more than 10% of the voting power of all classes of our outstanding stock or any parent or subsidiary corporation as of the grant date, the term must not exceed 5 years, and the exercise price must equal at least 110% of the fair market value on the grant date.
After the continuous service of an employee, director or consultant terminates, he or she may exercise his or her option, to the extent vested, for the period of time specified in the option agreement. However, an option may not be exercised later than the expiration of its term.
The 2017 Plan will allow for the grant of stock appreciation rights. Stock appreciation rights allow the recipient to receive the appreciation in the fair market value of our common stock between the date of grant and the exercise date. The administrator will determine the terms of stock appreciation rights, including when such rights become exercisable and whether to pay the increased appreciation in cash or with shares of our common stock, or a combination thereof, except that the base appreciation amount used to determine the cash or shares to be issued pursuant to the exercise of a stock appreciation right will be no less than 100% of the fair market value per share on the date of grant. After the continuous service of an employee, director or consultant terminates, he or she may exercise his or her stock appreciation right, to the extent vested, only to the extent provided in the stock appreciation right agreement.
The 2017 Plan will allow for the grant of restricted stock. Restricted stock awards are shares of our common stock that vest in accordance with terms and conditions, if any, established by the administrator. The administrator will determine the number of shares of restricted stock granted to any employee, director or consultant. The administrator may impose whatever conditions, if any, on vesting it determines to be appropriate. For example, the administrator may set restrictions based on the achievement of specific performance goals. Shares of restricted stock that do not vest are subject to our right of repurchase or forfeiture.
The 2017 Plan will allow for the grant of restricted stock units. Restricted stock units are awards that will result in payment to a recipient at the end of a specified period only if the vesting criteria established by the administrator, if any, are achieved or the award otherwise vests. The administrator may impose whatever conditions, if any, to vesting, or restrictions and conditions, if any, to payment that it determines to be appropriate. The administrator may set restrictions based on the achievement of specific performance goals or on the continuation of service or employment. Payments of earned restricted stock units may be made, in the administrator’s discretion, in cash, with shares of our common stock or other securities, or a combination thereof.
The 2017 Plan also will allow for the grant of awards denominated in cash that may be settled in cash or shares of common stock, which may be subject to restrictions, as established by the administrator. Prior to the first stockholder meeting at which directors are to be elected to our Board of Directors that occurs after the close of the third calendar year following the calendar year in which this offering occurs, the maximum aggregate amount of cash that may be issued pursuant to awards under the 2017 Plan to employees who would otherwise be covered by Section 162(m) of the Code will be $ . Section 162(m) of the Code generally applies to a public company’s chief executive officer and its three other most highly compensated executive officers, other than its chief financial officer.
203
Table of Contents
The administrator will determine the provisions, terms, and conditions of each award including vesting schedules, forfeiture provisions, form of payment (cash, shares, or other consideration) upon settlement of the award, payment contingencies, and satisfaction of any performance criteria. The performance criteria established by the administrator for any awards intended to qualify as “performance-based compensation” for purposes of Section 162(m) of the Code, will be one of, or combination of, the following: net earnings or net income (before or after taxes); earnings per share; revenues or sales (including net sales or revenue growth); net operating profit; return measures (including return on assets, net assets, capital, invested capital, equity, sales, or revenue); cash flow (including operating cash flow, free cash flow, cash flow return on equity, and cash flow return on investment); earnings before or after taxes, interest, depreciation, or amortization; gross or operating margins; productivity ratios; share price (including growth measures and total stockholder return); expense targets; margins; operating efficiency; market share; working capital targets and change in working capital; economic value added or EVA® (net operating profit after tax minus the sum of capital multiplied by the cost of capital); or net operating income. The performance criteria may be applicable to our company, our affiliates or any individual segments of our company or any affiliate and may be measured over any specified period, on an absolute basis or relative to a pre-established target, to previous years’ results or to a designated comparison group, in each case as specified by the administrator.
The 2017 Plan will allow for the transfer of awards under the 2017 Plan only (i) by will, (ii) by the laws of descent and distribution and (iii) for awards other than incentive stock options, to the extent authorized by the administrator to certain persons or entities. Only the recipient of an incentive stock option may exercise such award during his or her lifetime.
In the event of certain changes in our capitalization, to prevent enlargement of the benefits or potential benefits available under the 2017 Plan, the administrator will make adjustments to one or more of the number of shares that are covered by outstanding awards, the exercise or purchase price of outstanding awards, the numerical share limits contained in the 2017 Plan, and any other terms that the administrator determines require adjustment.
The 2017 Plan provides that in the event of certain corporate transactions, as such term is defined in the 2017 Plan, the portion of each outstanding award that is neither continued by us nor assumed or replaced by the successor entity or its parent will automatically terminate. In connection with a corporate transaction or change in control, as such term is defined in the 2017 Plan, the administrator has the authority to provide for the full or partial automatic vesting and exercisability of one or more outstanding unvested awards under the 2017 Plan and the release from restrictions on transfer or forfeiture rights of such awards on such terms and conditions as the administrator may specify. In addition, any incentive stock option accelerated in connection with a corporate transaction or change in control will remain exercisable as an incentive stock option to the extent the dollar limitation under the Code is not exceeded, with any excess becoming a nonqualified stock option.
The 2017 Plan will automatically terminate 10 years following the date it becomes effective, unless we terminate it sooner. In addition, our Board of Directors has the authority to amend, suspend or terminate the 2017 Plan provided such action does not impair the rights under any outstanding award.
2017 Employee Stock Purchase Plan
Our 2017 Employee Stock Purchase Plan, or ESPP, was adopted by our Board of Directors in and will become effective prior to the completion of this offering and will enable eligible employees of ours and designated affiliates to purchase shares of our common stock at a discount following its effectiveness. In this description, we sometimes refer to an eligible employee’s right to purchase shares of our common stock under the ESPP as an “option”. Purchases will be accomplished through participation in discrete offering periods. The ESPP is intended to qualify as an employee stock purchase plan under Section 423 of the Code. We initially reserved shares of our common stock for issuance under the ESPP. In addition, the ESPP provides for an annual increase to the number of shares of our common stock available for issuance thereunder on the first
204
Table of Contents
business day of each calendar year beginning with the calendar year following the calendar year in which the ESPP becomes effective, equal to the lesser of (i) shares, (ii) percent of the number of shares of our common stock outstanding as of the last day of the immediately preceding calendar year, and (iii) a lesser number of shares determined by the administrator (as defined below).
Our Board of Directors or a committee designated by the board, which we refer to as the “administrator” in this description, will administer the ESPP. Our employees generally are eligible to participate in the ESPP (except for employees (i) whose customary employment is 20 hours or less per week, (ii) whose customary employment is for not more than 5 months in any calendar year, (iii) who have not been employed for such continuous period as the administrator may require (up to a maximum of 2 years), or (iv) who are citizens or residents of a non-U.S. jurisdiction under certain circumstances, although the administrator may permit such categories of employees to participate in the ESPP in its discretion). Employees who are 5% stockholders, or would become 5% stockholders as a result of their participation in the ESPP, are ineligible to participate in the ESPP. We may impose additional restrictions on eligibility. Under the ESPP, eligible employees generally will be able to acquire shares of our common stock by accumulating funds through payroll deductions. Our eligible employees will be able to select a rate of payroll deduction between % and % of their eligible compensation.
When an offering period commences, our employees who meet the eligibility requirements and wish to participate in the ESPP will be required to enroll in a timely manner. Once an employee is enrolled, participation will be automatic in subsequent offering periods. An employee’s participation automatically ends upon termination of employment for any reason.
It is anticipated that the offering periods will be for six months. The duration of the first offering period may be shorter or longer.
No participant will have the right to purchase our shares in an amount, when aggregated with purchase rights under all our employee stock purchase plans that are also in effect in the same calendar year(s), that has a fair market value of more than $25,000, determined as of the first day of the applicable offering period, for each calendar year in which that right is outstanding. In addition, no participant will be permitted to purchase more than shares during any one offering period or such lesser amount determined by the administrator. The purchase price for shares of our common stock purchased under the ESPP may be as low as 85% of the lower of the fair market value of our common stock on the first trading day of the applicable offering period or the last trading day of the applicable offering period.
In the event of certain corporate transactions (as defined in the ESPP), each option will be assumed by the successor corporation or a parent or subsidiary of the successor corporation, unless the administrator determines to shorten the offering period then in progress, in which case the options will either be exercised automatically or we will pay the option holder an amount equal to the excess, if any, of (x) the fair market value of the shares subject to the options over (y) the purchase price due had the options been exercised automatically.
The ESPP will terminate on the anniversary of its adoption by our Board of Directors, unless it is terminated earlier by the administrator. The administrator may at any time and for any reason terminate or amend the ESPP. Except in connection with certain corporate transactions or changes in capitalization, no such termination can adversely affect options previously granted, provided that the ESPP may be terminated by the administrator under certain circumstances if the administrator determines that the termination of the ESPP or one or more offering periods is in our best interests or in the best interests of our stockholders. Except as described in the previous sentence, or in connection with certain corporate transactions or changes in capitalization, no amendment may make any change in any option theretofore granted which adversely affects the rights of any participant without the consent of affected participants. To the extent necessary to comply with Section 423 of the Code (or any successor rule or provision or any other applicable law), we will obtain stockholder approval of any amendment in such a manner and to such a degree as required.
205
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following is a summary of each transaction or series of similar transactions since January 1, 2014, or any currently proposed transaction, to which we were or are a party in which:
| • | the amount involved exceeded or exceeds $120,000; and |
| • | any of our directors or executive officers, any holder of 5% of any class of our voting capital stock or any member of their immediate family had or will have a direct or indirect material interest. |
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to such securities.
Convertible Note Financings
We completed convertible note financings between September 2016 and February 2017, in which we issued and sold to 10 investors an aggregate of approximately $48.0 million principal amount of notes convertible into shares of our common stock. We completed an additional convertible note financing in April 2017 in which we sold to one investor a convertible note with a principal amount of $20.0 million. Upon completion of this offering, the notes will automatically convert into a number of shares of our common stock equal to the outstanding principal amount of such notes divided by a % discount to the initial public offering price. The investors include one of the holders of more than 5% of our outstanding common stock, Advance Data Services Limited, controlled by Ma Huateng, an entity affiliated with Antony Leung, one of our directors, Dr. Manson Fok, one of our directors, and Mandra. The table below shows the principal amount of convertible notes purchased by each related party and the number of shares of common stock each note will automatically convert into upon completion of this offering.
| Related Party |
Principal Amount of Convertible Note |
Shares of Common Stock Upon Completion of Offering |
||||||
| Advance Data Services Limited(1) |
$ | 10,000,000 | ||||||
| Sunderland Global Limited |
$ | 10,000,000 | ||||||
| Manson Fok |
$ | 2,000,000 | ||||||
| Mandra Medical Limited |
$ | 2,000,000 | ||||||
| (1) | Pursuant to the terms of the convertible loan agreement, another entity controlled by Ma Huateng assigned the agreement and all of its rights thereunder to Advance Data Services Limited in April 2017. |
206
Table of Contents
Sales of Preferred Stock and Common Stock
From time to time since January 1, 2014, we have financed our operations through sales of shares of our common stock and sales of our Series A preferred stock, which were subsequently converted into common stock on a 1-to-1.1 basis, effective May 8, 2014. The table below sets forth the date, purchase price and number of shares purchased for each sale of shares of our common stock or Series A preferred stock, on an as-converted basis, to parties who were, at the time of such sale, any of our directors, executive officers or holders of 5% of any class of our voting capital stock or any member of their immediate family had or will have a direct or indirect material interest:
| Date of Sale |
Name of Related Party |
Aggregate Purchase Price ($) |
Shares of Common Stock Purchased |
|||||||
| March 26, 2014 |
Flint Besecker | $ | 2,000,000 | 440,000 | ||||||
| March 26, 2014 |
Johnson Lau | $ | 4,000,000 | 880,000 | ||||||
| March 29, 2014 |
Mandra Health Limited | $ | 5,000,006 | 1,000,000 | ||||||
| August 13, 2014 |
Mandra Health Limited | $ | 3,000,000 | 600,000 | ||||||
| September 8, 2014 |
Mandra Health Limited | $ | 5,000,000 | 1,000,000 | ||||||
| January 22, 2015 |
Flint Besecker | $ | 550,000 | 100,000 | ||||||
| January 22, 2015 |
Johnson Lau | $ | 550,000 | 100,000 | ||||||
| January 30, 2015 |
Mandra Health Limited | $ | 12,867,768 | 2,557,776 | ||||||
| February 5, 2015 |
Flint Besecker | $ | 3,300,000 | 600,000 | ||||||
| February 5, 2015 |
Johnson Lau | $ | 3,300,000 | 600,000 | ||||||
| February 5, 2015 |
Jinn Wu | $ | 176,000 | 32,000 | ||||||
| April 17, 2015 |
Charter Link International Ltd. | $ | 10,500,000 | 1,400,000 | ||||||
| April 17, 2015 |
Advance Data Services Limited(1) | $ | 30,000,000 | 4,000,000 | ||||||
| April 17, 2015 |
Manson Fok | $ | 1,000,020 | 133,336 | ||||||
| May 31, 2015 |
William Zuo | $ | 6,300,000 | 840,000 | ||||||
| June 5, 2015 |
Mandra Health Limited | $ | 6,600,000 | 1,200,000 | ||||||
| July 17, 2015 |
Flint Besecker | $ | 944,352 | 104,928 | ||||||
| July 17, 2015 |
Rudolf Kwan | $ | 187,092 | 20,788 | ||||||
| July 17, 2015 |
Mandra Health Limited | $ | 5,000,006 | 853,148 | ||||||
| March 1, 2016 |
Mandra Health Limited | $ | 5,000,003 | 666,667 | ||||||
| March 31, 2016 |
William Zuo | $ | 2,499,993 | 277,777 | ||||||
| May 26, 2016 |
Mandra Health Limited | $ | 3,499,988 | 466,665 | ||||||
| (1) | Pursuant to the terms of our shareholders’ agreement, another entity controlled by Ma Huateng, which purchased the shares of our common stock on April 17, 2015, transferred ownership of those shares to Advance Data Services Limited in April 2017. |
Shareholders Agreement
We entered into a shareholders agreement with certain of our shareholders, dated May 8, 2014, as amended from time to time, which prescribes the size of our board of directors and grants the shareholders party to the agreement with certain information rights. The agreement also provides that no shareholder shall, voluntarily or involuntarily, transfer all or any part of his or her shares of common stock without the prior written approval of the board of directors, subject to certain permitted transfers. This agreement will be terminated upon the closing of this offering.
Additional Shareholder Rights
Charter Link
We entered into a purchase agreement for our previously outstanding Series A convertible preferred stock, dated February 18, 2013 and amended on July 14, 2013, with Charter Link International Ltd., or Charter Link, for an aggregate price of $5,000,000. Among other things, the agreement gives Charter Link an option to
207
Table of Contents
purchase additional shares of our common stock in the event of certain issuances by us of our common stock. This option will be terminated upon the closing of this offering. The agreement also gives Charter Link the right to designate an individual to our board of directors who shall serve until our first annual shareholders meeting, and to require us to include the name of such nominee in the slate of nominees for director that is submitted to our shareholders at each subsequent annual shareholders meeting, as long as Charter Link continues to beneficially own more than 5% of our outstanding common stock. The agreement also provides that all of our directors, founders and another shareholder, must vote all their shares from time to time for the election of such nominee. This right will be terminated upon the closing of this offering.
Mandra Health Limited
We entered into a stock purchase and option agreement, dated March 4, 2014, with Mandra Health Limited, or Mandra, which provided for the purchase of our Series A convertible preferred stock by Mandra over two tranches, as well as an option to purchase additional shares, for an aggregate sales price of $25,000,000. Among other things, the agreement also gives Mandra the preemptive right to purchase all or part of its pro rata share, based on its percentage ownership of our then-outstanding common stock, of any new securities we issue to new investors. This preemptive right shall terminate upon the closing of this offering.
Voting Agreements
Mandra Health Limited
We entered into a voting agreement, dated March 4, 2014, with Mandra and certain other shareholders whereby Mandra shall be entitled to nominate one nominee for appointment or election to our board of directors for as long as Mandra beneficially owns 10% or more of our then-outstanding common stock on a fully-diluted basis. Upon the closing of this offering, we will be required to use our best efforts to include Mandra’s nominee on any slate of nominees to be recommended to our shareholders for election to the board of directors. The agreement shall terminate upon the earlier of: (i) seven years from the date of the agreement, (ii) a written agreement by and among the parties thereto, (iii) the fifth anniversary of the closing of this offering or (iv) the date Mandra no longer beneficially owns 10% or more of our then-outstanding common stock on a fully-diluted basis. Upon completion of this offering, Mandra will own approximately % of our outstanding common stock, assuming an initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus.
Manson Fok
We entered into a voting agreement, dated March 1, 2013, with Dr. Manson Fok and certain other shareholders whereby such shareholders agreed to vote all shares beneficially owned by them at any annual or special meeting of our shareholders or in any action by written consent for the election of one nominee of Dr. Fok to our board of directors. The shareholders party to this agreement also agreed not to vote to or consent to remove any director nominated by Dr. Fok unless Dr. Fok consents, approves and recommends such removal. The agreement shall terminate upon the earlier of: (i) seven years from the date of the agreement, (ii) a written agreement by and among the parties thereto, (iii) the fifth anniversary of the closing of this offering or (iv) the date Dr. Fok no longer beneficially owns at least 5% of the then-outstanding shares of our common stock. Upon completion of this offering, Dr. Fok will own approximately % of our outstanding common stock, assuming an initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus.
CDE Acquisition
In July 2015, we acquired all of the outstanding equity interests in CDE in a stock-for-stock transaction valued at approximately $14.9 million. Prior to the transaction, certain of our directors, officers and stockholders,
208
Table of Contents
including Dr. Lau, Mr. Fok, Mr. Zhang and Dr. Kwan, had a financial interest in CDE. In addition, Dr. Lau and Mr. Fok served as directors of CDE. Consequently, our board of directors established a special committee of disinterested directors with authority to review, evaluate, negotiate, and approve or reject the terms and conditions of the transaction and to retain its own financial and legal advisors to assist in connection therewith. Following negotiation of the transaction between the committee and its advisors and representatives of CDE, the special committee determined that the proposed terms of the transaction were fair to us from a financial point of view and approved our entry into the definitive agreements and consummation of the transactions contemplated thereby.
Contractual Arrangements
Avalon BioMedical (Management) Limited
Since 2015, CDE has entered into a series of agreements with Avalon BioMedical (Management) Limited, or Avalon BioMedical, under which Avalon BioMedical will occupy space and use certain services and facilities and pay to CDE 30% in 2015 and 50% in 2016 of the total services expenses and rent payment incurred for the Hong Kong research and development facility. Pursuant to such agreements, Avalon BioMedical paid to CDE approximately $0.2 million and $0.3 million in 2015 and 2016, respectively. Dr. Lau, our Chief Executive Officer and Chairman, and Dr. Fok and Mr. Zhang, two of our directors, collectively have a controlling interest in, and serve on the board of directors of, Avalon Global Holdings Limited, the indirect parent of Avalon BioMedical. As of December 31, 2016, Avalon BioMedical held 678,880 shares of our common stock. We do not hold any interest in Avalon BioMedical.
Chongqing Taisheng Biotechnology Co., Ltd.
We purchase certain pharmaceutical ingredients from Chongqing Taisheng Biotechnology Co., Ltd., or Taisheng, a company which is owned by Dr. William Zuo, one of our officers. Purchases from Taisheng amounted to $0.1 million and $0.2 million for the years ended December 31, 2015 and 2016, respectively. We believe that the prices we pay to Taisheng for these ingredients are comparable to prices available from third parties selling similar ingredients of similar quality.
Xenobiotic Laboratory
Xenobiotic Laboratory, or XBL, is a contract research company. Dr. Lau, our Chairman and CEO, and Dr. Wu, one of our directors, owned 30% and 70%, respectively, of XBL until it was sold to a third party in September 2014. Prior to that sale, Dr. Lau and Dr. Wu also served as directors of XBL. Up until the date XBL was sold to a third party in 2014, we paid approximately $188,000 to XBL for contract research services.
ZenRx Limited
ZenRx is a contract research company located in New Zealand. ZenRx conducts certain clinical development with our company and we have entered into the ZenRx License with ZenRx. See “Business—License and Collaboration Agreements—ZenRx License Agreement.” Dr. Rudolf Kwan, our Chief Medical Officer, serves on the board of directors of ZenRx, and in 2014, 2015 and 2016, we paid approximately $0.1 million, $0.1 million and less than $0.1 million, respectively, to ZenRx for clinical research.
Dr. William Zuo
Dr. Zuo, who serves as President of our China operation, was entitled to certain payments following our acquisition of Polymed in 2015. In 2015 and 2016, we paid Dr. Zuo $2.5 million and $2.5 million worth of our common stock in connection with such acquisition. See Management’s Discussion and Analysis of Financial Position—Liquidity And Capital Resources—Indebtedness.”
209
Table of Contents
Dr. Rudolf Kwan
Dr. Kwan, our Chief Medical Officer, provided services to us as a consultant until February 2017 through RSJ Consulting LLC, a limited liability company of which he is the principal. We have paid consulting fees of $144,000, $149,333 and $164,000 to RSJ Consulting LLC in each of 2014, 2015 and 2016, respectively. We have not paid any fees to RSJ Consulting LLC for any other services other than the consulting services of Dr. Kwan.
Dr. Jane Fang
We have entered into a consulting agreement with Dr. Jane Fang, who is the wife of Dr. Lau, our Chairman and CEO to provide consulting advice related to the development of our KX-01 ointment, reporting to Dr. Kwan, our Chief Medical Officer. We have paid consulting fees of $1,600, $0 and $127,000 to Dr. Fang in each of 2014, 2015 and 2016, respectively.
Equity Issuances
We have entered into employment agreements and arrangements with certain of our executive officers and have granted stock options to our executive officers and certain of our directors. For additional information about these agreements and transactions, see “Executive and Director Compensation—Director Compensation” and “Executive and Director Compensation—Executive Compensation.”
Letter Agreement with Former Officer
On December 8, 2016, we entered into a letter agreement with one of our former executive officers and directors, Flint Besecker, pursuant to which Mr. Besecker agreed to take on a lesser role within the Company and to return to the Company certain executive compensation benefits previously granted to him in order to, among other things, provide us with additional flexibility to grant incentive stock awards to other employees of the Company in the future. Under the December 8, 2016 agreement, among other things, Mr. Besecker (i) resigned from his roles as Chief Financial Officer and Chief Operating Officer and agreed to accept a new, part-time role in Strategic Operations, (ii) resigned from our Board of Directors, (iii) agreed to terminate both the vested and unvested portion of an option to purchase 1,280,000 shares of our common stock previously granted to him with an exercise price of $7.50 per share, (iv) agreed to resell to us, for nominal consideration, 546,667 shares of our common stock previously purchased by him with the proceeds of a loan from the Company that was forgivable over time, (v) agreed to resell to us, for nominal consideration, 100,000 shares of our common stock owned by him as a result of an incentive stock award previously made to him, and (v) agreed to certain continuing confidentiality, non-competition and transfer restrictions as a former executive officer of the Company. Mr. Besecker subsequently resigned from his position in Strategic Operations, and since January 13, 2017 has no longer been employed with our Company.
Share Purchases
In 2014, we sold to Dr. Lau and Flint Besecker, an executive officer at the time, 880,000 and 440,000 shares, respectively, of our common stock in exchange for promissory notes in the amounts of $4,000,000 and $2,000,000, respectively. In 2015, we sold to Dr. Lau and Mr. Besecker, an executive officer at the time, 600,000 shares each of our common stock in exchange for promissory notes in the amount of $3.3 million each. Pursuant to the terms of the promissory notes, one-third of the principal amount outstanding would be forgiven each year over a three-year period, contingent on their continued employment. In October 2016, our board of directors accelerated the forgiveness of the full remaining value of each of the promissory notes described above and extinguished the notes and, in connection therewith, repurchased 174,309 and 148,416 shares from Dr. Lau and Mr. Besecker, respectively, to satisfy certain tax obligations.
210
Table of Contents
Directed Share Program
At our request, the underwriters have reserved for sale, at the initial public offering price, up to % of the shares offered by this prospectus for sale to some of our directors, officers, employees and related persons as part of a directed share program. The directed share program will not limit the ability of such directors, officers and their family members, or holders of more than 5% of our capital stock, to purchase more than $120,000 in value of our common stock. We do not currently know the extent to which these related persons will participate in our directed share program, if at all, or the extent to which they will purchase more than $120,000 in value of our common stock.
Participation in this Offering
Certain of our existing stockholders and their affiliated entities, including stockholders affiliated with certain of our directors, have indicated an interest in purchasing up to an aggregate of approximately $ million worth of shares of our common stock in this offering at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters could determine to sell more, less or no shares to any of these stockholders and any of these stockholders could determine to purchase more, less or no shares in this offering. The underwriters will receive the same underwriting discounts and commissions on any shares purchased by these stockholders as they will on any other shares sold to the public in this offering.
Indemnification Agreements with Officers and Directors
See “Management—Indemnification Agreements.”
Policies and Procedures for Related Party Transactions
Following this offering, pursuant to the written charter of our audit committee adopted in 2017, our audit committee of the board of directors will be responsible for reviewing and approving, prior to our entry into any such transaction, all related party transactions and potential conflict of interest situations involving a principal stockholder, a member of the board of directors or senior management. In addition, our company policies require that our officers and employees avoid using their positions for purposes that are, or give the appearance of being, motivated by a desire for personal gain.
211
Table of Contents
The following table indicates information as of March 31, 2017, except for footnote 2, which is as of April 26, 2017, regarding the ownership of our common stock, both before and after giving effect to the sale of common stock offered in this offering, for:
| • | each person who is known by us to own more than 5% of our shares of common stock; |
| • | each named executive officer; |
| • | each of our directors; and |
| • | all of our directors and executive officers as a group. |
The following table does not reflect any shares of common stock that may be purchased in this offering pursuant to our directed share program described under “Underwriting.”
The number of shares beneficially owned and the percentage of shares beneficially owned are based on 41,348,568 shares of our common stock outstanding as of March 31, 2017, which includes 661,982 issued but unvested restricted shares. Unvested shares of restricted stock are subject to our right to repurchase such shares until such shares have vested. These shares are deemed to be outstanding and beneficially owned by the person holding those shares for the purpose of computing the percentage ownership of that person and for the purpose of computing the percentage ownership of any other person. Percentage ownership of our common stock after this offering (assuming no exercise of the underwriters’ over-allotment option to purchase additional shares) assumes our sale of shares in this offering. Unless otherwise indicated in the footnotes to the table, and subject to community property laws where applicable, the following persons have sole voting and investment control with respect to the shares beneficially owned by them.
Beneficial ownership for the purposes of the following table is determined in accordance with the rules and regulations of the SEC. These rules generally provide that a person is the beneficial owner of securities if such person has or shares the power to vote or direct the voting thereof, or to dispose or direct the disposition thereof or has the right to acquire such powers within 60 days. Also, in accordance with SEC rules, shares of common stock that may be acquired by an individual or group within 60 days of March 31, 2017, pursuant to the exercise of options, warrants or other rights, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. Except as otherwise indicated, the address of each of the persons in this table is c/o Athenex, Inc., 1001 Main Street, Suite 600, Buffalo, NY 14203.
| Shares Beneficially Owned Prior to the Offering |
Percentage of Shares Beneficially Owned |
|||||||||||
| Before Offering |
After Offering |
|||||||||||
| Name of Beneficial Owner |
||||||||||||
| 5% Stockholders: |
||||||||||||
| Mandra Entities(1) |
6,715,784 | 16.24 | % | |||||||||
| Ma Huateng(2) |
4,080,000 | 9.85 | % | |||||||||
| Pharminex, LLC(3) |
2,693,776 | 6.51 | % | |||||||||
| Charter Link International Ltd.(4) |
2,500,000 | 6.05 | % | |||||||||
| Directors and Named Executive Officers: |
||||||||||||
| Johnson Lau(5) |
5,746,583 | 13.08 | % | |||||||||
| Simon Pedder(6) |
45,000 | * | ||||||||||
| Jeffrey Yordon(7) |
183,709 | * | ||||||||||
| Kim Campbell(8) |
24,000 | * | ||||||||||
| Manson Fok(9) |
2,181,216 | 5.24 | % | |||||||||
| Antony Leung(10) |
690,664 | 1.67 | % | |||||||||
| Jinn Wu(11) |
521,208 | 1.25 | % | |||||||||
212
Table of Contents
| Shares Beneficially Owned Prior to the Offering |
Percentage of Shares Beneficially Owned |
|||||||||||
| Before Offering |
After Offering |
|||||||||||
| Song-Yi Zhang(12) |
7,502,664 | 18.10 | % | |||||||||
| Michael Cannon(13) |
36,528 | * | ||||||||||
| Sheldon Trainor-Degirolamo(14) |
943,334 | 2.28 | % | |||||||||
| James Zukin(15) |
531,100 | 1.28 | % | |||||||||
| All executive officers, directors and director nominees as a group(16) (14 persons) |
18,738,547 | 41.43 | % | |||||||||
| (1) | Consists of (i) 6,428,608 shares of common stock held of record by Mandra Medical Limited and (ii) 287,176 shares of common stock held of record by Mandra Health Limited. Mandra Medical Limited and Mandra Health Limited are collectively referred to as the Mandra Entities. Each of Mandra Health Limited and Mandra Medical Limited are wholly-owned subsidiaries of Beansprouts Limited. Song-Yi Zhang, a member of our board of directors, together with his spouse, owns all of the outstanding interests in Beansprouts Limited and shares voting and dispositive power over the shares held by it. The amount above does not include $2.0 million worth of convertible notes issued to Mandra Medical Limited in January 2017 that will automatically convert into shares of our common stock upon the completion of this offering. The address for each of Mandra Medical Limited, Mandra Health Limited and Beansprouts Limited is c/o Newhaven Trustees (BVI) Limited, 3rd Floor, J&C Building, P.O. Box 933, Road Town, Tortola, British Virgin Islands, VG1110. |
| (2) | Consists of (i) 4,000,000 shares of common stock held by Advance Data Services Limited, which acquired the shares by transfer from another entity controlled by Ma Huateng, pursuant to our shareholders’ agreement, on April 26, 2017, and (ii) 80,000 shares of common stock issuable upon the exercise of options exercisable within 60 days of March 31, 2017 held of record by Ma Huateng. Ma Huateng is a Director of Advance Data Services Limited and has sole voting and dispositive power over the shares held by Advance Data Services Limited. The amount above does not include $10.0 million worth of convertible notes issued to an entity controlled by Ma Huateng in September 2016, which were assigned to Advance Data Services Limited pursuant to the terms of the convertible note agreement in April 2017, which will automatically convert into shares of our common stock upon the completion of this offering. The address for Advance Data Services Limited is 29/F Three Pacific Place, 1 Queen’s Road East, Wanchai, Hong Kong. |
| (3) | Charles Lannon, John Bellomo and Jonathan Swiatkowski are the directors of Pharminex, LLC and share voting and dispositive power over the shares held by Pharminex, LLC. The address for Pharminex, LLC is 6467 Main Street, Williamsville, New York 14221. |
| (4) | Edward Cheng Seng Chong and Paul Cheng Link Man are the directors of Charter Link International Ltd. and share voting and dispositive power over the shares held by Charter Link International Ltd. The address for Charter Link International, Inc. is Room 1802 Harbour Centre, 25 Harbour Road, Wanchai, Hong Kong. |
| (5) | Consists of (i) 2,322,722 shares of common stock, (ii) 2,583,334 shares of common stock issuable upon the exercise of options exercisable within 60 days of March 31, 2017, (iii) 161,647 shares of restricted common stock, subject to certain repurchase rights, held by Dr. Lau’s spouse, and (iv) 678,880 shares of common stock held by Avalon Biomedical (Management) Limited (“Avalon Biomedical”), an indirect wholly-owned subsidiary of Avalon Global Holdings Limited (“Avalon Global”). Dr. Lau owns all of the outstanding interests in Creative Decade Global Limited, which owns 30% of the outstanding interests in Avalon Global, and Dr. Lau serves on the board of directors of Avalon Global and has shared voting and dispositive power with respect to the shares held by Avalon Biomedical. |
| (6) | Consists of 45,000 shares of common stock issuable upon the exercise of options exercisable within 60 days of March 31, 2017. |
| (7) | Consists of (i) 33,709 shares of common stock and (ii) 150,000 shares of restricted common stock subject to certain repurchase rights. |
| (8) | Consists of 24,000 shares of common stock issuable upon the exercise of options exercisable within 60 days of March 31, 2017. |
| (9) | Consists of (i) 1,242,336 shares of common stock, (ii) 260,000 shares of common stock issuable upon the exercise of options exercisable within 60 days of March 31, 2017 and (iii) 678,880 shares of common stock held by Avalon Biomedical, an indirect wholly-owned subsidiary of Avalon Global. Dr. Fok, together with his spouse, |
213
Table of Contents
| owns all of the outstanding interests in Sino Glory Developments Limited, which owns 30% of the outstanding interests in Avalon Global, and Dr. Fok serves on the board of directors of Avalon Global and shares voting and dispositive power with respect to the has shared held by Avalon Biomedical. The amount above does not include $2.0 million worth of convertible notes issued to Dr. Fok in January 2017 that will automatically convert into shares of our common stock upon the completion of this offering. |
| (10) | Consists of (i) 24,000 shares of common stock issuable upon the exercise of options within 60 days of March 31, 2017 and (ii) 666,664 shares of common stock owned by Sunderland Global Limited. Sunderland Global Limited is an indirect wholly-owned subsidiary of Nan Fung Group. Mr. Leung is the CEO of Nan Fung Group and has shared voting and dispositive power over the shares held by Sunderland Global Limited. The amount above does not include $10.0 million worth of convertible notes issued to Sunderland Global Limited in September 2016 that will automatically convert into shares of our common stock upon the completion of this offering. |
| (11) | Consists of (i) 223,208 shares of common stock, and (ii) 298,000 shares of common stock issuable upon the exercise of options exercisable within 60 days of March 31, 2017. |
| (12) | Consists of (i) the shares described in footnote (1) above, (ii) 4,000 shares of common stock, (iii) 104,000 shares of common stock issuable upon the exercise of options exercisable within 60 days of March 31, 2017, and (iv) 678,880 shares of common stock held by Avalon Biomedical, an indirect wholly-owned subsidiary of Avalon Global. Mr. Zhang, together with his spouse, indirectly owns all of the outstanding interests in Mandra Medical Limited, which owns 30% of the outstanding interests in Avalon Global, and Mr. Zhang serves on the board of directors of Avalon Global and has shared voting and dispositive power with respect to the shares held by Avalon Biomedical. |
| (13) | Consists of 36,528 shares of common stock held by Cannon Venture No. 2 LP. Michael Cannon, who has been nominated to join our Board upon the completion of this offering, is the general partner of Cannon Venture No. 2 LP and has sole voting and dispositive power over the shares held by it. |
| (14) | Consists of 943,334 shares of common stock held by PacBridge Partners V Investment Co Ltd. Sheldon Trainor-Degirolamo, who has been nominated to join our Board upon the completion of this offering, is the sole director and shareholder of PacBridge Partners V Investment Co Ltd. and has sole voting and dispositive power over the shares held by it. |
| (15) | Consists of (i) 465,100 shares of common stock held directly by James Zukin, who has been nominated to join our Board upon the completion of this offering and (ii) 66,000 shares of common stock held by the James Zukin Trust dated 11/4/05, of which Mr. Zukin is the trustee and beneficiary. Mr. Zukin has sole voting and dispositive power over the shares held by the Trust. Mr. Zukin is a member of Pharminex, LLC, but does not have voting or dispositive power over the shares of our common stock held by it. |
| (16) | Consists of (i) 14,858,213 shares of common stock and (ii) 3,880,334 shares of common stock issuable upon the exercise of options exercisable within 60 days of March 31, 2017. |
214
Table of Contents
The following description of certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws to be adopted prior to the closing of this offering does not purport to be complete. You should also refer to the copies of our amended and restated certificate of incorporation and amended and restated bylaws which have been filed with the SEC as exhibits to our registration statement, of which this prospectus forms a part. The description of common stock and preferred stock reflect changes to our capital structure that will occur upon the closing of this offering in accordance with the terms of the amended and restated certificate of incorporation that will be adopted by us immediately prior to the closing of this offering.
Upon the closing of this offering, our authorized capital stock will consist of shares of common stock, par value $0.001 per share, and shares of preferred stock, par value $0.001 per share.
Common Stock
As of March 31, 2017, there were 41,348,568 shares of our common stock outstanding, which includes 661,982 issued but unvested restricted shares, held of record by 333 stockholders. Under the amended and restated certificate of incorporation and amended and restated bylaws, holders of common stock are entitled to one vote per share on all matters submitted to a vote of our stockholders and do not have cumulative voting rights in the election of directors. This means that, subject to any rights of holders of outstanding shares of our preferred stock, if any, to vote in the election of directors, the holders of a majority of our outstanding shares of common stock can elect all of the directors standing for election by holders of our common stock and the holders of the remaining outstanding shares of our common stock will not be able to elect any such directors. The shares of common stock offered by this prospectus, when issued, will be fully paid and non-assessable and will not be subject to any redemption or sinking fund provisions. Holders of common stock do not have any preemptive, subscription or conversion rights.
Holders of common stock are entitled to receive dividends if and when declared by the board of directors out of legally available funds, subject to the rights of preferred stockholders, if any, and the terms of any existing or future agreements between us and our lenders. We presently intend to retain future earnings, if any, for use in the operation and expansion of our business. We do not anticipate paying cash dividends on our common stock in the foreseeable future. See “Dividend Policy.” In the event of our liquidation, dissolution or winding up, common stockholders are entitled to share ratably in all assets legally available for distribution after payment of or provision for all of our debts and other liabilities, and subject to the prior rights of any holders of outstanding shares of our preferred stock, if any.
Preferred Stock
Upon the closing of this offering, our board of directors will be authorized, without vote or action by the stockholders, to issue from time to time up to an aggregate of shares of preferred stock in one or more series and to fix or alter the designations, preferences, rights and any qualifications, limitations or restrictions of the shares of each of these series, including the dividend rights, dividend rates, conversion rights, voting rights, terms and rights of redemption, including without limitation sinking fund provisions, redemption price or prices, liquidation preferences and the number of shares constituting any series. The issuance of preferred stock may have the effect of delaying, deferring or preventing a change in control of us without further action by our stockholders and may adversely affect the dividend, liquidation and voting and other rights of the holders of common stock. The issuance of preferred stock with voting and conversion rights may adversely affect the voting power of the holders of common stock, including the loss of voting control to others. We currently have no plans to issue any shares of preferred stock.
We believe that the ability to issue preferred stock without the expense and delay of a special stockholders’ meeting will provide us with increased flexibility in structuring possible future financings and acquisitions, and
215
Table of Contents
in meeting other corporate needs that might arise. This also permits the board of directors to issue preferred stock containing terms which could impede the completion of a takeover attempt. This could discourage an acquisition attempt or other transaction which stockholders might believe to be in their best interests or in which they might receive a premium for their stock over the then market price of the stock.
Options and Warrants
As of March 31, 2017, options to purchase a total of 9,214,989 shares of our common stock were outstanding with a weighted average exercise price of $6.25 per share, and up to additional shares of our common stock were reserved for future issuance under our 2017 Plan. After this offering, we intend to cease granting awards under our 2013 Plan, and instead grant awards, including options, under our 2017 Plan, which is expected to be adopted in connection with this offering. For a more complete discussion of our equity incentive plans, please see “—Employee Benefit Plans.”
As of March 31, 2017, warrants to purchase up to an aggregate of 344,000 shares of our common stock were outstanding at a weighted average exercise price of $0.44 per share.
Anti-takeover Provisions of Delaware Law, Our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws
The provisions of the Delaware General Corporation Law, or DGCL, our amended and restated certificate of incorporation and our amended and restated bylaws may have the effect of delaying, deferring or discouraging another person from acquiring control of us by means of a tender offer, a proxy contest or otherwise, or removing incumbent officers and directors. These provisions, some of which are described below, may discourage certain types of coercive takeover practices and takeover bids that our board of directors may consider inadequate and to encourage any person seeking to acquire control of us to first negotiate with our board of directors. We believe the benefits of increased protection of our ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweighs the disadvantages of discouraging takeover or acquisition proposals because, among other things, negotiation of these proposals could result in an improvement of their terms.
Classified Board
Our amended and restated certificate of incorporation will provide that subject to the rights of holders of any series of preferred stock with respect to director elections, the board shall be divided into three (3) classes. The initial term of the directors who are members of Class I will expire at the first annual meeting of stockholders following the closing of this offering, the initial term of the directors who are members of Class II will expire at the second annual meeting of stockholders following the closing of this offering, and the initial term of the directors who are members of Class III will expire at the third annual meeting of stockholders following the closing of this offering. At each annual meeting of stockholders, commencing with the first annual meeting of stockholders following the closing of this offering, each of the successors elected to replace the directors of a class whose term shall have expired at such annual meeting shall be elected to hold office until the third annual meeting next succeeding his or her election and until his or her respective successor shall have been duly elected and qualified. Our stockholders will elect only one class of directors each year. We believe that classification of our board of directors will help to assure the continuity of our business strategies and policies. The classified board provision could have the effect of making the replacement of incumbent directors more time consuming and difficult. At least two annual meetings of our stockholders will generally be required to effect a change in a majority of our board of directors.
Removal of Directors; Filling Vacancies and Newly Created Directorships
Our amended and restated certificate of incorporation and amended and restated bylaws will provide that subject to any limitations imposed by law and the rights of the holders of any series of our preferred stock, the
216
Table of Contents
board of directors or any individual director may be removed from office by our stockholders only for cause. Subject to the rights of holders of any series of outstanding preferred stock, vacancies in the board and newly created directorships shall, unless otherwise provided by law, be filled solely by the affirmative vote of a majority of directors then in office, or by a sole remaining director, and shall not be filled by stockholders.
No Written Consent of Stockholders
Our amended and restated certificate of incorporation will provide that all stockholder actions are required to be taken by a vote of the stockholders at an annual or special meeting, and that stockholders may not take any action by written consent in lieu of a meeting.
Special Meetings of Stockholders
Our amended and restated certificate of incorporation will provide that special meetings of stockholders may only be called by the chairman of our board of directors, our chief executive officer, or our board of directors. In addition, our amended and restated bylaws prohibit the conduct of any business at a special meeting other than as specified in the notice of such meeting.
Advance Notice Requirements and Procedures
Our amended and restated bylaws will establish advance notice procedures with regard to stockholder proposals relating to the nomination of candidates for election as directors or new business to be brought before meetings of our stockholders. These procedures will provide that notice of stockholder nominations and proposals must be timely given in writing to our secretary prior to the meeting at which the action is to be taken. Generally, to be timely, notice must be received at our principal executive offices not later than the ninetieth (90th) day nor earlier than the one hundred twentieth (120th) day prior to the first anniversary of the annual meeting for the preceding year. The notice must contain certain information specified in the bylaws. These provisions may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed. These provisions may also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our company.
Amendment to Certificate of Incorporation and Bylaws
Our amended and restated certificate of incorporation will provide that the affirmative votes of the holders of at least seventy-five percent (75%) of the voting power of all of the then-outstanding shares of our voting stock will be required to amend certain provisions of our certificate of incorporation, including provisions relating to the size of our board of directors, term and classification of directors, rights of holders of preferred stock relating to director elections, removal of directors, filling board vacancies and newly-created directorships, special meetings of stockholders, shareholder action by written consent, limitation of liability and indemnification, and amendment of the certificate of incorporation and bylaws.
The affirmative votes of the holders of at least seventy-five percent (75%) of the voting power of all of the then-outstanding shares of our voting stock will be required to amend or repeal our bylaws. In addition, our amended and restated bylaws may be amended by our board of directors.
Blank Check Preferred Stock
Our amended and restated certificate of incorporation will provide for authorized shares of preferred stock. The existence of authorized but unissued shares of preferred stock may enable our board of directors to render more difficult or to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise. For example, if in the due exercise of its fiduciary obligations, our board of directors were
217
Table of Contents
to determine that a takeover proposal is not in the best interests of us or our stockholders, our board of directors could cause shares of preferred stock to be issued without stockholder approval in one or more private offerings or other transactions that might dilute the voting or other rights of the proposed acquirer or insurgent stockholder or stockholder group. In this regard, our certificate of incorporation grants our board of directors broad power to establish the rights and preferences of authorized and unissued shares of preferred stock. The issuance of shares of preferred stock could decrease the amount of earnings and assets available for distribution to holders of shares of common stock. The issuance may also adversely affect the rights and powers, including voting rights, of these holders and may have the effect of delaying, deterring or preventing a change in control of us.
Section 203 of the DGCL
Upon the closing of this offering, we will be subject to the provisions of Section 203 of the DGCL, as amended. In general, Section 203 prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes, among other things, a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns, or did own within three years prior to the determination of interested stockholder status, 15 percent or more of the corporation’s voting stock.
Under Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions:
| • | before the stockholder became interested, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| • | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85 percent of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder), shares owned by persons who are directors and also officers, and employee stock plans, in some instances; or |
| • | at or after the time the stockholder became interested, the business combination was approved by the board of directors of the corporation and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least 66 2/3 percent of the outstanding voting stock which is not owned by the interested stockholder. |
A Delaware corporation may “opt out” of these provisions with an express provision in its original certificate of incorporation or an express provision in its certificate of incorporation or bylaws resulting from a stockholders’ amendment approved by at least a majority of the outstanding voting shares. We have not opted out of these provisions. As a result, mergers or other takeover or change in control attempts of us may be discouraged or prevented.
Delaware as Sole and Exclusive Forum
Our amended and restated certificate of incorporation will provide that, unless a majority of our board of directors consents in writing to an alternative forum, the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have jurisdiction, another state court located within the State of Delaware, or, if no court located within the State of Delaware has jurisdiction, the federal district court for the District of Delaware) shall, to the fullest extent permitted by law, be the sole and exclusive forum for (i) any derivative action or proceeding brought on behalf of us, (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders, (iii) any action asserting a claim against us
218
Table of Contents
arising pursuant to any provision of the DGCL, as amended, or our certificate of incorporation or bylaws, or (iv) any action asserting a claim against us or any of our directors, officers or employees governed by the internal affairs doctrine of the State of Delaware.
NASDAQ Global Market Listing
We have applied to have our common stock listed on the NASDAQ Global Market under the symbol “ATNX”.
Convertible Loans and Registration Rights
Between September 2016 and April 2017 we entered into convertible loan agreements with an aggregate principal amount of $75.0 million. While the convertible loan agreements do not confer any specific registration rights to the loan holders, the agreements do provide, upon conversion of each loan into shares of our common stock, that we will grant to the loan holders registration rights for such issued common stock that are comparable to any registration rights held by other shareholders. Currently, no other shareholders hold any registration rights with respect to any of our outstanding shares of common stock.
Upon the closing of this offering, $68.0 million of the convertible loans will automatically convert into shares of our common stock, which is based on a 20.0% discount to the initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus. The remaining $7.0 million of the convertible loans, held by Hanmi, may be converted at Hanmi’s option at discounted rates from 20.0% to 22.5% at various dates up to the maturity date of October 1, 2018.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock will be Computershare Trust Company, Inc.
219
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock, and we cannot predict the effect, if any, that market sales of shares of our common stock or the availability of shares of our common stock for sale will have on the market price of our common stock prevailing from time to time. Nevertheless, sales of substantial amounts of our common stock, including shares issued upon exercise of outstanding options or warrants, or the perception that these sales could occur in the public market after this offering could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through the sale of our equity securities.
Immediately after the closing of this offering, based on the number of shares outstanding as of March 31, 2017, we will have shares of common stock outstanding. All of the shares sold in this offering (other than any shares sold pursuant to our directed share program, which are subject to lock-up agreements) will be freely tradable without restriction under the Securities Act unless purchased by our “affiliates”, as that term is defined in Rule 144 under the Securities Act.
The remaining shares of our common stock will be “restricted securities” under Rule 144.
Subject to the lock-up and market stand-off agreements described below and the provisions of Rule 144 and 701 under the Securities Act, these restricted securities will be available for sale in the public market as follows:
| Date Available for Sale |
Shares |
Description | ||
| Date of Prospectus |
Shares eligible for sale pursuant to Rule 144 that are not subject to lock-up or market stand-off | |||
| 90 Days after Date of Prospectus |
Shares eligible for sale pursuant to Rules 144 and 701 that are not subject to lock-up or market stand-off | |||
| 180 Days after Date of Prospectus |
Release of lock-up and market stand-off; shares eligible for sale pursuant to Rules 144 and 701 |
Rule 144
In general, under Rule 144 as currently in effect, any person who is or has been an affiliate of ours during the 90 days immediately preceding the sale and who has beneficially owned shares for at least six months is entitled to sell, within any three-month period commencing 90 days after the date of this prospectus, a number of shares that does not exceed the greater of:
| • | 1% of the then-outstanding shares of common stock, which will equal approximately shares immediately after this offering; and |
| • | the average weekly trading volume during the four calendar weeks preceding the sale, subject to the filing of a Form 144 with respect to the sale. |
Sales under Rule 144 by our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us.
A person who is not deemed to have been an affiliate of ours at any time during the 90 days immediately preceding the sale and who has beneficially owned his or her shares for at least six months is entitled to sell his or her shares under Rule 144 without regard to the limitations described above, subject only to the availability of current public information about us during the six months after the initial six-month holding period is met. After a nonaffiliate has beneficially owned his or her shares for one year or more, he or she may freely sell his or her shares under Rule 144 without complying with any Rule 144 requirements.
220
Table of Contents
We are unable to estimate the number of shares that will be sold under Rule 144, since this will depend on the market price for our common stock, the personal circumstances of the sellers and other factors. Prior to the offering, there has been no public market for the common stock, and there can be no assurance that a significant public market for the common stock will develop or be sustained after the offering. Any future sale of substantial amounts of the common stock in the open market may adversely affect the market price of the common stock offered by this prospectus.
Rule 701
In general, under Rule 701 under the Securities Act, any of our employees, directors, consultants or advisors who purchased shares from us in connection with a qualified compensatory stock or option plan or other written agreement and in compliance with Rule 701, is eligible to resell those shares 90 days after the effective date of this offering in reliance on Rule 144, but without compliance with the various restrictions, including the holding period, contained in Rule 144.
Lock-up and Market Stand-Off Agreements
We, holders of approximately % of our common stock, including our directors and executive officers, and certain of our other stockholders have agreed that, subject to certain exceptions, they will not sell any common stock without the prior written consent of Credit Suisse Securities (USA) LLC and J.P. Morgan Securities LLC for a period of 180 days from the date of this prospectus.
Employee Benefit Plans
Any employee or consultant, other than certain executive officers in some cases, who purchased his or her shares under a written compensatory plan or contract is entitled to rely on the resale provisions of Rule 701, which permits non-affiliates to sell their Rule 701 shares without having to comply with the public information, holding period, volume limitation or notice provisions of Rule 144 and permits affiliates to sell their Rule 701 shares without having to comply with the Rule 144 holding period restrictions, in each case commencing 90 days after the date of this prospectus. As of the holders of options to purchase approximately shares of common stock will be eligible to sell their shares upon the expiration of the 180-day lockup period, subject to the vesting of those options.
We intend to file a registration statement on Form S-8 under the Securities Act as soon as practicable after the completion of the offering to register shares of common stock subject to outstanding stock options or reserved for issuance under our stock plans. This registration will permit the resale of these shares by non-affiliates in the public market without restriction under the Securities Act, upon completion of the lock-up period described above. Shares registered under the Form S-8 registration statement held by affiliates will be subject to Rule 144 volume limitations. See “Management—Executive Compensation” and “—Employee Benefit Plans.”
221
Table of Contents
The following is a general summary of certain PRC and United States federal income tax consequences relevant to an investment in our common stock. The discussion is not intended to be, nor should it be construed as, legal or tax advice to any particular prospective purchaser. The discussion is based on laws and relevant interpretations thereof in effect as of the date of this prospectus, all of which are subject to change or different interpretations, possibly with retroactive effect. The discussion does not address U.S. state or local tax laws, or tax laws of jurisdictions other than the People’s Republic of China and the United States. You should consult your own tax advisors with respect to the consequences of acquisition, ownership and disposition of our common stock.
People’s Republic of China Taxation
We are a holding company incorporated in the U.S., and we gain a material portion of our income by way of dividends from our PRC subsidiaries. The EIT Law and its implementation rules, both of which became effective on January 1, 2008, provide that China-sourced income of foreign enterprises, such as dividends paid by a PRC subsidiary to its equity holders that are non-resident enterprises, will normally be subject to PRC withholding tax at a rate of 10%, unless any such foreign investor’s jurisdiction of incorporation has a tax treaty with China that provides for a different withholding arrangement.
Under the EIT Law, an enterprise established outside of China with a “de facto management body” within China is considered a “resident enterprise,” which means that it is treated in a manner similar to a Chinese enterprise for enterprise income tax purposes. Although the implementation rules of the EIT Law define “de facto management body” as a managing body that exercises substantive and overall management and control over the production and business, personnel, accounting books and assets of an enterprise, the only official guidance for this definition currently available is set forth in Circular 82, issued by the State Administration of Taxation, which provides guidance on the determination of the tax residence status of a Chinese-controlled offshore incorporated enterprise, defined as an enterprise that is incorporated under the laws of a foreign country or territory and that has a PRC enterprise or enterprise group as its primary controlling shareholder. Although Athenex does not have a PRC enterprise or enterprise group as our primary controlling shareholder and is therefore not a Chinese-controlled offshore incorporated enterprise within the meaning of Circular 82, in the absence of guidance specifically applicable to us, we have applied the guidance set forth in Circular 82 to evaluate the tax residence status of Athenex and its subsidiaries organized outside the PRC.
According to Circular 82, a Chinese-controlled offshore incorporated enterprise will be regarded as a PRC tax resident by virtue of having a “de facto management body” in China and will be subject to PRC enterprise income tax on its worldwide income only if all of the following criteria are met:
| • | the primary location of the day-to-day operational management is in the PRC; |
| • | decisions relating to the enterprise’s financial and human resource matters are made or are subject to approval by organizations or personnel in the PRC; |
| • | the enterprise’s primary assets, accounting books and records, company seals, and board and shareholders meeting minutes are located or maintained in the PRC; and |
| • | 50% or more of voting board members or senior executives habitually reside in the PRC. |
We do not believe that we meet any of the conditions outlined in the immediately preceding paragraph. Athenex and its offshore subsidiaries are incorporated outside the PRC. As a holding company, our key assets and records, including the resolutions and meeting minutes of our board of directors and the resolutions and meeting minutes of our shareholders, are located and maintained outside the PRC. In addition, we are not aware of any offshore holding companies with a corporate structure similar to ours that has been deemed a PRC “resident enterprise” by the PRC tax authorities. Accordingly, we believe that Athenex and its offshore
222
Table of Contents
subsidiaries should not be treated as a “resident enterprise” for PRC tax purposes if the criteria for “de facto management body” as set forth in Circular 82 were deemed applicable to us. However, as the tax residency status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body” as applicable to our offshore entities, we will continue to monitor our tax status.
The implementation rules of the EIT Law provide that, (i) if the enterprise that distributes dividends is domiciled in the PRC or (ii) if gains are realized from transferring equity interests of enterprises domiciled in the PRC, then such dividends or capital gains are treated as China-sourced income. It is not clear how “domicile” may be interpreted under the EIT Law, and it may be interpreted as the jurisdiction where the enterprise is a tax resident. Therefore, if we are considered to be a PRC tax resident enterprise for PRC tax purposes, any dividends we pay to our overseas shareholders which are non-resident enterprises as well as gains realized by such shareholders from the transfer of our shares may be regarded as China-sourced income and as a result become subject to PRC withholding tax at a rate of up to 10%.
Furthermore, if we are considered a PRC resident enterprise and the competent PRC tax authorities consider dividends we pay with respect to our shares and the gains realized from the transfer of our shares to be income derived from sources within the PRC, such dividends and gains we pay to our overseas shareholders who are non-resident individuals may be subject to PRC individual income tax at a rate of 20%, unless any such non-resident individuals’ jurisdiction has a tax treaty with China that provides for a preferential tax rate or a tax exemption. It is also unclear whether, if we are considered a PRC resident enterprise, holders of our shares would be able to claim the benefit of income tax treaties or agreements entered into between China and other countries or areas.
See “Risk Factors—Risks Related to Doing Business in the People’s Republic of China—We may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise Income Tax Law, and we may therefore be subject to PRC income tax on our global income.” and “Risk Factors—Risks Related to Doing Business in the People’s Republic of China—Dividends payable to our foreign investors and gains on the sale of our common stock by our foreign investors may become subject to PRC tax law.”
United States
The following is a general discussion of the material U.S. federal income and estate tax consequences of the ownership and disposition of our common stock by a beneficial owner that is a “non-U.S. holder.” For purposes of this discussion, a “non-U.S. holder” is a person or entity that does not own or has not owned, actually or constructively, more than 5% of our common stock, and is for U.S. federal income tax purposes:
| • | a non-resident alien individual, other than certain former citizens and residents of the U.S.; |
| • | a corporation, or other entity treated as a corporation for U.S. federal income tax purposes, created or organized under the laws of a jurisdiction other than the U.S., any state thereof or the District of Columbia; |
| • | an estate, other than an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust, other than if a court within the U.S. is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have the authority to control all substantial decisions of the trust or if the trust has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. |
A “non-U.S. holder” does not include an individual who is present in the U.S. for 183 days or more in the taxable year of disposition of our stock and is not otherwise a resident of the United States for U.S. federal income tax purposes. Such an individual is urged to consult his or her own tax advisor regarding the U.S. federal income tax consequences of the sale, exchange or other disposition of our common stock.
223
Table of Contents
This discussion is based on the Code, and administrative pronouncements, judicial decisions and final, temporary and proposed Treasury Regulations, changes to any of which subsequent to the date of this prospectus may affect the tax consequences described herein, possibly with a retroactive effect. This discussion does not address all aspects of U.S. federal income and estate taxation that may be relevant to non-U.S. holders in light of their particular circumstances and does not address any tax consequences arising under the laws of any state, local or foreign jurisdiction.
The discussion below is limited to non-U.S. holders that hold our shares of common stock as capital assets within the meaning of the Code and that are not subject to special rules under the Code (e.g., financial institutions, governments or agencies or instrumentalities thereof, controlled foreign corporations, passive foreign investment companies, insurance companies, tax-exempt organizations, broker-dealers and traders in securities or commodities, persons that hold our common stock as part of a “straddle,” “hedge,” “conversion transaction,” “synthetic security” or integrated investment or other risk reduction strategy, and persons subject to the alternative minimum tax or Medicare contribution tax).
If a partnership, or any entity treated as a partnership for U.S. federal income tax purposes, is a holder of our common stock, the tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of the partnership. A holder that is a partnership, and the partners in such partnership, should consult their own tax advisers regarding the tax consequences of the acquisition, holding and disposition of our common stock.
Prospective holders are urged to consult their tax advisors with respect to the particular tax consequences to them of acquiring, holding and disposing of our common stock, including the consequences under the laws of any state, local or foreign jurisdiction.
Distributions
As discussed in the section entitled “Dividend Policy,” we do not anticipate paying any dividends on our common stock in the foreseeable future. In the event that we do pay dividends, any dividends paid to a non-U.S. holder of our common stock to the extent made out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles) generally will be subject to withholding tax at a 30% rate, or a reduced rate specified by an applicable income tax treaty. In order to obtain a reduced rate of withholding under an applicable income tax treaty, a non-U.S. holder must provide an Internal Revenue Service Form W-8BEN (or other applicable form) certifying its entitlement to benefits under the treaty. Special certification and other requirements apply to certain non-U.S. holders that are pass-through entities rather than corporations or individuals.
A non-U.S. holder of our common stock eligible for a reduced rate of U.S. withholding tax pursuant to an income tax treaty may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the Internal Revenue Service.
The withholding tax does not apply to dividends paid to a non-U.S. holder that provides a Form W-8ECI, certifying that the dividends are effectively connected with the non-U.S. holder’s conduct of a trade or business within the U.S. and, if required by an applicable income tax treaty, are attributable to a U.S. permanent establishment (“effectively connected dividends”). Instead, effectively connected dividends will be subject to regular U.S. income tax as if the non-U.S. holder were a U.S. resident, subject to any applicable income tax treaty providing otherwise. A non-U.S. corporation receiving effectively connected dividends may also be subject to an additional “branch profits tax,” currently at the rate of 30% (or a lower rate prescribed under an applicable income tax treaty).
To the extent dividends on our common stock, if any, exceed our current and accumulated earnings and profits, they will first reduce the non-U.S. holder’s adjusted basis in our common stock, but not below zero, and then will be treated as gain to the extent of any excess, and taxed in the same manner as gain realized from a sale or other disposition of common stock as described in the next section.
224
Table of Contents
Gain on Disposition of Common Stock
A non-U.S. holder generally will not be subject to U.S. federal income tax on gain realized on a sale or other disposition of common stock unless:
| • | the gain is effectively connected with a trade or business of the non-U.S. holder in the U.S., subject to an applicable income tax treaty providing otherwise; or |
| • | we are or have been a “U.S. real property holding corporation,” as defined below, at any time within the five-year period preceding the disposition or during the non-U.S. holder’s holding period, whichever period is shorter. |
An individual non-U.S. holder described in the first bullet point immediately above will be subject to tax on the net gain derived from the sale under regular graduated U.S. federal income tax rates. If a non-U.S. holder that is a foreign corporation falls under the first bullet point immediately above, it will be subject to tax on its net gain in the same manner as if it were a United States person as defined under the Code and, in addition, may be subject to the branch profits tax equal to 30% of its effectively connected earnings and profits or at such lower rate as may be specified by an applicable income tax treaty.
We are not, and do not anticipate becoming, a U.S. real property holding corporation. Generally, a corporation is a “U.S. real property holding corporation” if the fair market value of its U.S. real property interests (as defined in the Code and the applicable Treasury regulations) equals or exceeds 50% of the aggregate fair market value of its worldwide real property interests and its other assets used or held for use in a trade or business. Even if we were to become a U.S. real property holding corporation, gain on the sale or other disposition of common stock by a non-U.S. holder generally would not be subject to U.S. federal income tax, provided that the common stock is regularly traded on an established securities market and the non-U.S. holder does not actually or constructively own more than 5% of the common stock during the shorter of (1) the five-year period ending on the date of the disposition or (2) the period of time during which the holder held such shares.
Information Reporting Requirements and Backup Withholding
Information returns will be filed with the Internal Revenue Service in connection with payments of dividends to a non-U.S. holder. Copies of the information returns reporting such dividends and withholding may also be made available to the tax authorities in the country in which the non-U.S. holder resides under the provisions of an applicable income tax treaty. Unless a non-U.S. holder complies with certification procedures to establish that it is not a U.S. person, information returns may be filed with the Internal Revenue Service in respect of the proceeds from a sale or other disposition of common stock and the non-U.S. holder may be subject to U.S. backup withholding on payments of dividends or on the proceeds from a sale or other disposition of common stock. The certification procedures required to claim a reduced rate of withholding under a treaty will satisfy the certification requirements necessary to avoid the backup withholding tax as well. The amount of any backup withholding from a payment to a non-U.S. holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is furnished to the Internal Revenue Service in a timely manner.
Federal Estate Tax
Individual non-U.S. holders and entities the property of which is potentially includible in such an individual’s gross estate for U.S. federal estate tax purposes (for example, a trust funded by a non-U.S. holder individual and with respect to which the individual has retained certain interests or powers), should note that, absent an applicable treaty benefit, the common stock will be treated as U.S. situs property subject to U.S. federal estate tax.
225
Table of Contents
Additional Withholding Requirements
U.S. source payments to certain foreign financial institutions and non-financial foreign entities may be subject to withholding under the U.S. Foreign Account Tax Compliance Act, or FATCA. Subject to certain exceptions, FATCA, as it has been supplemented by published IRS guidance, generally provides that a 30% withholding tax will be imposed on payments to certain foreign financial institutions and certain non-financial foreign entities of certain types of U.S. source income, including dividends, and that, beginning on January 1, 2019, a 30% withholding tax will be imposed on payments to a foreign financial institution or a non-financial foreign entity of proceeds from the sale of property that could give rise to U.S. source dividends. However, such withholding taxes may be avoided by the foreign financial institution if the foreign financial institution enters into an agreement (an “FFI Agreement”) with the IRS to disclose the name, address and taxpayer identification number of certain U.S. persons (as defined for U.S. Federal income tax purposes) that are account holders in the foreign financial institution (which includes certain equity holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) and may be avoided by the non-financial foreign entity if it either certifies that it does not have any substantial direct or indirect U.S. owners or provides information regarding substantial direct or indirect U.S. owners of the entity. Treasury Regulations gradually phase in the information that must be disclosed by the foreign financial institution pursuant to the terms of an FFI Agreement. Prospective investors are encouraged to consult with their own tax advisors regarding the possible implications of FATCA on their ownership of common stock.
226
Table of Contents
We are offering the shares of common stock described in this prospectus through a number of underwriters. Credit Suisse Securities (USA) LLC and J.P. Morgan Securities LLC are acting as joint book-running managers of the offering and as representatives of the underwriters. We have entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover of this prospectus, the number of shares of common stock listed next to its name in the following table:
| Name |
Number of shares |
|||
| Credit Suisse Securities (USA) LLC |
||||
| J.P. Morgan Securities LLC |
||||
| Deutsche Bank Securities Inc. |
||||
| Total |
||||
The underwriters are committed to purchase all the shares of common stock offered by us if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may also be increased or the offering may be terminated.
The underwriters propose to offer the shares of common stock directly to the public at the initial public offering price set forth on the cover of this prospectus and to certain dealers at that price less a concession not in excess of $ per share. After the initial public offering of the shares, the offering price and other selling terms may be changed by the underwriters. Sales of shares made outside of the U.S. may be made by affiliates of the underwriters.
The underwriters have an option to buy up to additional shares of common stock from us to cover sales of shares by the underwriters which exceed the number of shares specified in the table above. The underwriters have 30 days from the date of this prospectus to exercise this option to purchase additional shares of common stock. If any shares are purchased with this option to purchase additional shares, the underwriters will purchase shares in approximately the same proportion as shown in the table above. If any additional shares of common stock are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered.
The underwriting fee is equal to the public offering price per share of common stock less the amount paid by the underwriters to us per share of common stock. The underwriting fee is $ per share. The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Without exercise of option to purchase additional shares |
With full exercise of option to purchase additional shares |
|||||||
| Per share |
$ | $ | ||||||
| Total |
$ | $ | ||||||
We estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions, will be approximately $ million. We have agreed to reimburse the underwriters for expenses of an amount of up to $ relating to the clearance of this offering with the Financial Industry Regulatory Authority. Credit Suisse Securities (USA) LLC has been granted the right to participate in future financings by the Company; this right is deemed to constitute 1% in underwriting compensation for this offering pursuant to FINRA Rule 5110.
A prospectus in electronic format may be made available on the web sites maintained by one or more underwriters, or selling group members, if any, participating in the offering. The underwriters may agree to
227
Table of Contents
allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make Internet distributions on the same basis as other allocations.
Directed Share Program
At our request, the underwriters have reserved for sale, at the initial public offering price, up to % of the common stock offered by this prospectus for sale to our director nominees, officers and certain of our employees and other persons associated with us. Pursuant to the underwriting agreement, the sales will be made by J.P. Morgan Securities LLC, an underwriter of this offering, through a Directed Share Program. If these persons purchase reserved common stock, it will reduce the number of shares of common stock available for sale to the general public. Any reserved shares of common stock that are not so purchased will be offered by the underwriters to the general public on the same terms as the other shares of common stock offered by this prospectus.
The company and holders of approximately % of our common stock, including our officers, directors, and certain holders of the company’s common stock and options have agreed with the underwriters, subject to certain exceptions, not to dispose of or hedge any of their common stock, or securities convertible into or exchangeable for common stock during the period from the date of this prospectus continuing through the date 180 days after the date of this prospectus, except with the prior written consent of Credit Suisse Securities (USA) LLC and J.P. Morgan Securities LLC. This agreement does not apply to any existing employee benefit plans. See “Shares Eligible for Future Sale” for a discussion of certain transfer restrictions.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act.
Certain of the underwriters are expected to make offers and sales both inside and outside the United States through their respective selling agents. Any offers or sales in the United States will be conducted by broker-dealers registered with the SEC.
The address of Credit Suisse Securities (USA) LLC is Eleven Madison Avenue, New York, New York 10010, United States of America. The address of J.P. Morgan Securities LLC is 383 Madison Avenue, New York, New York 10179, United States of America.
We have applied to have our common stock approved for listing/quotation on The NASDAQ Global Market under the symbol “ATNX.”
In connection with this offering, the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling shares of common stock in the open market for the purpose of preventing or retarding a decline in the market price of the common stock while this offering is in progress. These stabilizing transactions may include making short sales of the common stock, which involves the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in this offering, and purchasing shares of common stock on the open market to cover positions created by short sales. Short sales may be “covered” shorts, which are short positions in an amount not greater than the underwriters’ option to purchase additional shares referred to above, or may be “naked” shorts, which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their option to purchase additional shares, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market compared to the price at which the underwriters may purchase shares through the option to purchase additional shares. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market that could adversely affect investors who purchase in this offering. To the extent that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
228
Table of Contents
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of the common stock, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase common stock in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
These activities may have the effect of raising or maintaining the market price of the common stock or preventing or retarding a decline in the market price of the common stock, and, as a result, the price of the common stock may be higher than the price that otherwise might exist in the open market. If the underwriters commence these activities, they may discontinue them at any time. The underwriters may carry out these transactions on The NASDAQ Global Market, in the over-the-counter market or otherwise.
Prior to this offering, there has been no public market for our common stock. The initial public offering price will be determined by negotiations between us and the representatives of the underwriters. In determining the initial public offering price, we and the representatives of the underwriters expect to consider a number of factors including:
| • | the information set forth in this prospectus and otherwise available to the representatives; |
| • | our prospects and the history and prospects for the industry in which we compete; |
| • | an assessment of our management; |
| • | our prospects for future earnings; |
| • | the general condition of the securities markets at the time of this offering; |
| • | the recent market prices of, and demand for, publicly traded common stock of generally comparable companies; and |
| • | other factors deemed relevant by the underwriters and us. |
Neither we nor the underwriters can assure investors that an active trading market will develop for our common stock, or that the shares will trade in the public market at or above the initial public offering price.
Selling restrictions
General
Other than in the U.S., no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Canada
The shares may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the shares must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
229
Table of Contents
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the Securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the Securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor. Pursuant to section 3A-3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriters conflicts of interest in connection with this offering.
United Kingdom
This document is only being distributed to and is only directed at persons who are “qualified investors” (as defined in the Prospectus Directive) who are (i) persons having professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”) (ii) high net worth entities, and (iii) other persons to whom it may lawfully be communicated, falling with Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). The securities are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such securities will be engaged in only with, relevant persons. This document and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by any recipients to any other person in the United Kingdom. Any person who is not a relevant person should not act or rely on this document or any of its contents.
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”), with effect from and including the date on which the Prospectus Directive was implemented in that Relevant Member State no offer of securities described in this prospectus may be made to the public in that Relevant Member State other than:
| • | to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| • | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive) per Relevant Member State, subject to obtaining the prior consent of the underwriters; or |
| • | in any other circumstances falling within Article 3(2) of the Prospectus Directive; |
provided that no such offer of securities described in this prospectus shall result in a requirement for the publication by us or any underwriter of a prospectus pursuant to Article 3 of the Prospectus Directive
For the purposes of this provision, the expression an “offer of securities to the public” in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Relevant Member State. The expression “Prospectus Directive” means Directive 2003/71/EC (as amended, including by Directive 2010/73/EU) and includes any relevant implementing measure in each Relevant Member State.
Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or the SIX, or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing
230
Table of Contents
Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this prospectus nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this prospectus nor any other offering or marketing material relating to the offering, the Company, or the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or the CISA. The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
China
This prospectus does not constitute a public offer of the shares offered by this prospectus, whether by sale or subscription, in China. The shares are not being offered or sold directly or indirectly in China to or for the benefit of, legal or natural persons of the PRC.
Further, no legal or natural persons of China may directly or indirectly purchase any of the shares without obtaining all prior Chinese governmental approvals that are required, whether statutorily or otherwise. Persons who come into possession of this prospectus are required by the issuer and its representatives to observe these restrictions.
Hong Kong
The shares may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap.32, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”), (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares of common stock are subscribed or purchased under Section 275 by a relevant person which is: (a) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments
231
Table of Contents
and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for 6 months after that corporation or that trust has acquired the shares under Section 275 except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is given for the transfer; or (3) by operation of law.
Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (the Financial Instruments and Exchange Law) and each underwriter has agreed that it will not offer or sell any securities, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Law and any other applicable laws, regulations and ministerial guidelines of Japan.
Certain of the underwriters and their affiliates have provided in the past to us and our affiliates and may provide from time to time in the future certain commercial banking, financial advisory, investment banking and other services for us and such affiliates in the ordinary course of their business, for which they have received and may continue to receive customary fees and commissions. In addition, from time to time, certain of the underwriters and their affiliates may effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future.
232
Table of Contents
The validity of the issuance of the shares of common stock offered hereby will be passed upon for us by our counsel, Sidley Austin LLP, Washington, District of Columbia. Certain legal matters as to PRC law will be passed upon for us by Commerce & Finance Law Offices and certain legal matters as to British Virgin Islands law will be passed upon for us by Ogier. The underwriters are being represented by Simpson Thacher & Bartlett LLP with respect to certain legal matters as to United States federal securities and New York state law. Certain legal matters as to PRC law will be passed upon for the underwriters by King & Wood Mallesons.
233
Table of Contents
The consolidated financial statements of Athenex Inc. and its subsidiaries (the “Company”) as of December 31, 2015 and 2016 and for the years then ended, included in this Prospectus and the related financial statement schedules included elsewhere in the Registration Statement have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report appearing herein and elsewhere in this Registration Statement (which report expresses an unqualified opinion on the consolidated financial statements and financial statement schedules and includes an explanatory paragraph regarding a going concern uncertainty). Such consolidated financial statements and financial statement schedules have been so included in reliance upon the report of such firm given their authority as experts in accounting and auditing.
The combined financial statements of Polymed Therapeutics, Inc. and Chongqing Taihao Pharmaceutical Co., Ltd. as of December 31, 2014 and 2013 and for the years then ended, included in this prospectus have been audited by Freed Maxick CPAs, P.C., an independent auditor, as stated in their report herein. Such combined financial statements have been so included in reliance upon the report of such firm given their authority as experts in accounting and auditing.
234
Table of Contents
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS
ON ACCOUNTING AND FINANCIAL DISCLOSURE
On July 26, 2015, our board of directors approved the dismissal of Freed Maxick CPAs, P.C., or Freed Maxick, as our independent accountants. We engaged Deloitte as our independent registered public accounting firm on December 10, 2015 to audit our consolidated financial statements as of December 31, 2015 and for the year then ended. Subsequent to their appointment, we engaged Deloitte to reaudit our consolidated financial statements as of December 31, 2014 and for the year then ended.
The reports of Freed Maxick on our consolidated financial statements did not contain any adverse opinion or disclaimer of opinion, nor were such reports qualified or modified as to uncertainty, audit scope, or accounting principles.
During the year ended December 31, 2014 and through July 26, 2015, Freed Maxick did not have any disagreement with us on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreement, if not resolved to the satisfaction of Freed Maxick, would have caused it to make reference to the subject matter of the disagreement in connection with its report on our consolidated financial statements.
We did not consult with Deloitte on any financial or accounting reporting matters in the period before its appointment.
We delivered a copy of this disclosure to Freed Maxick and requested that they furnish us a letter addressed to the SEC stating whether they agree with the above statements. In their letter to the SEC dated November 3, 2016, attached as Exhibit 16.1 to the Registration Statement of which this prospectus is a part, Freed Maxick states that they agree with the statements above concerning their firm.
235
Table of Contents
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 with the SEC under the Securities Act with respect to the common stock offered by this prospectus. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information with respect to us and our common stock, please see the registration statement and the exhibits and schedules filed with the registration statement. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. The registration statement, including its exhibits and schedules, may be inspected without charge at the public reference room maintained by the SEC, located at 100 F Street, N.E., Room 1580, Washington, D.C. 20549, and copies of all or any part of the registration statement may be obtained from such offices upon the payment of the fees prescribed by the SEC. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains an Internet website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the site is www.sec.gov.
Upon completion of this offering, we will become subject to the information and periodic reporting requirements of the Exchange Act, and, in accordance therewith, will file periodic reports, proxy statements and other information with the SEC. Such periodic reports, proxy statements and other information will be available for inspection and copying at the public reference room and on the SEC website referred to above.
We also maintain a website at www.athenex.com. Upon completion of this offering, you may access these materials at our website free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on our website is not a part of this prospectus and the inclusion of our website address in this prospectus is an inactive textual reference only.
236
Table of Contents
As used herein, the terms set forth below shall have the meanings, or are explained, as follows:
| 503B |
A section of the FDCA that allows a drug compounding entity to qualify as an “outsourcing facility,” which may qualify for exemptions from certain FDA approval requirements. |
| 505(b)(2) |
A pathway for FDA approval, in reliance on previously produced clinical data, of a drug that is not new but makes changes to a previously approved drug, including to its recommended dose, its formulation, its route of administration, its strength, or the drugs with which it is combined. |
| absolute bioavailability |
A measure of the amount of drug available in the body achieved through a dosing route (such as oral) relative to the amount of drug available given through an intravenous route. Typically expressed as a percentage. |
| absorption biology |
Study of the body’s ability to modulate drugs distribution. |
| absorption enhancers |
Materials that affect the ability of a drug to distribute into the body. |
| actinic keratosis |
Actinic keratosis (AK), also called solar keratosis, are scaly, crusty growths (lesions) caused by damage from the sun’s ultraviolet (UV) rays. They typically appear on sun-exposed areas such as the face, bald scalp, lips, and the back of the hands, and are often elevated, rough in texture, and resemble warts |
| active chemotherapeutic agents |
The active component of drugs that lead to the destruction of cancerous cells. |
| active pharmaceutical ingredients |
Active component of pharmaceutical dosage form. |
| adaptive immune response |
The body’s attempt to protect itself by neutralizing a specific antigen that the body has previously responded to. |
| adjuvant therapy |
Additional cancer treatment given after the primary treatment to lower the risk that the cancer will come back. Adjuvant therapy may include chemotherapy, radiation therapy, hormone therapy, targeted therapy, or biological therapy. |
| adverse events |
An adverse event is any undesirable experience associated with the use of a medical product in a patient. |
| alanine transaminase |
A protein, found commonly in the liver, that can be measured and serve as a marker of liver health. |
| alopecia |
Hair loss. |
| anaphylactic |
Related to a life-threatening allergic response. |
| anorexia |
A lack or loss of appetite. |
237
Table of Contents
| anti-emetic |
A drug for treatment and/or prevention of nausea and vomiting. |
| antihistamines |
A class of drugs that target the histamine receptors in the body. Commonly used to treat allergic responses. |
| apoptosis |
The death of cells that occurs as a normal and controlled part of an organism’s growth or development. |
| area under the curve |
A measure of total drug exposure over time. |
| aspartate transaminase |
A protein, found commonly in the liver, that can be measured and serve as a marker of liver health. |
| assay |
A test to measure a metric of a material. Examples of metrics used in pharmaceuticals include potency, purity, or pharmacological responses. |
| azacitdine |
An injectable drug use to treat cancer. This drug targets DNA which results in the cell death. Trade name is Vidaza. |
| bioavailability |
The proportion or a drug relative to dose that is available to the body to elicit a pharmacological response. |
| biomarker |
A biological specimen that can be measured to monitor a physiological process or pharmacologic response to a drug. |
| camptothecins |
A class of chemotherapy drugs derived from the natural product camptothecin. |
| capecitabine |
An anti-cancer chemotherapeutic drug. Trade name is Xeloda |
| caspase 3 cleavage |
Caspase 3 is a protein associated with cell death. The cleavage of this protein can serve as a marker of cell death. |
| chemotherapy |
The use of chemical drugs to treat a disease, such as cancer, especially with cytotoxic agents. |
| cisplatin |
An injectable drug used to treat many different types of cancer. This drug targets DNA replication which results in death of proliferating cells. |
| concomitant chemotherapy |
The use of combinations of chemotherapeutic drugs to treat a disease, such as cancer. |
| codons |
A portion of the genetic code, composed of three nucleotides. Mutations in the codons of particular genes may be associated with response to targeted therapies. |
| cyclophosphamide |
An anti-cancer chemotherapeutic drug. This drug targets DNA replication which results in death of proliferating cells. |
238
Table of Contents
| cyclosporin A |
An immunosuppressive drug typically used in treating immune disorders. This drug is a substrate and inhibitor of P-glycoprotein (PGP) and is frequently used as a reference compound for PGP-mediated drug-drug interaction studies. |
| cytotoxic chemotherapies |
Cell-killing toxic drugs used to stop or slow the growth of cancer cells. |
| decitabine |
An anti-cancer chemotherapeutic drug. This drug targets DNA synthesis which results in death of proliferating cells. Trade name is Dacogen. |
| diclofenac |
A nonsteroidal anti-inflammatory drug used topically to treat diseases such as Actinic Keratosis. |
| doxorubicin |
An injectable drug used to treat many types of cancer. It interacts with DNA and interferes with the process of DNA replication resulting in death of replicating cells. |
| elacridar |
A P-glycoprotein inhibiting drug. |
| enterocyte |
A cell comprising the intestinal lining. |
| erythema |
A reddening of the skin, usually in patches, as a result of injury or irritation causing dilation of the blood capillaries. |
| fibroblasts |
A cell in connective tissue that produces collagen and other fibers. |
| fluorouracil (5-FU) |
A drug that interferes with DNA synthesis to block cell growth. Used topically to treat Actinic Keratosis. Also known as 5-FU. An example trade name is Efudex. |
| glial |
Non-neuronal cells that maintain homeostasis, form myelin, and provide support and protection for neurons in the central and peripheral nervous systems. |
| glioblastoma multiforme |
A highly malignant, rapidly growing type of brain tumor that arises from glial cells in the brain. |
| glioma |
See glioblastoma multiforme. |
| hormonal therapies |
Therapies designed to block the body’s ability to produce hormones or to interfere with how hormones behave in the body, in each case to stop or slow the growth of cancers that rely on hormones to grow. |
| HT29 |
A cancer cell line derived from human colon cancer. |
| imiquimod |
A drug that acts on the immune system used topically to treat Actinic Keratosis. An example trade name is Aldara. |
| immune-therapies |
Therapies that engage the immune system to treat disease. |
239
Table of Contents
| immuno-oncology |
The use of the therapies that engage the immune system to treat cancer. |
| in vitro |
Performed or taking place in a test tube, culture dish, or elsewhere outside a living organism. |
| in vivo |
Performed or taking place in a living organism. |
| ingenol mebutate |
A drug that induced cell death used topically to treat Actinic Keratosis. The current trade name is Picato. |
| irinotecan |
A drug used to treat many types of cancer. This drug interacts with the protein topoisomerase which results in cell damage and death. Trade name is Camptosar. |
| keratinocytes |
Skin cells. |
| kinase |
An enzyme that catalyzes the transfer of a phosphate group from one molecule to another. An ubiquitous family of proteins involved in many cellular processes including, most relevantly, cell growth and proliferation. |
| liquid tumor |
An informal name for cancers of the blood cells, bone marrow, or lymphatic system. Examples include leukemia and lymphoma. |
| lymphocytes |
A subset of white blood cells; cells that participate in immune responses. |
| malignant |
A malignant disease or tumor is one likely to progress and become worse; malignant tumors have the capability and tendency to spread. |
| microtubule polymerization |
The process by which microtubules form in cells; microtubules are key in ensuring cell structure and stability. |
| monoclonal antibody |
Antibodies that are produced by exact clones of a parent cell. These antibodies will bind specifically to one given substance; they can be used as markers of the presence of that substance. |
| MTD |
maximum tolerated dose |
| neoadjuvant |
An initial therapy given to a patient before the main treatment. e.g. chemotherapy before surgery is performed. |
| neuropathy |
A term referring to the damage of nerves. Often causes weakness, numbness or pain. |
| neutropenia |
A state characterized by a very low concentration of neutrophils (a type of white blood cell); these cells are the primary defense against infections. |
| non-small cell |
Used to describe the difference between the two major types of lung cancer: small cell and non-small cell. The different types of cancer are treated very differently. Non-small cell lung cancer comprises about 80% of all lung cancers. |
240
Table of Contents
| oligodendriogliomas |
A type of glioma found in the brain or central nervous system. |
| paclitaxel |
A prominent anticancer drug used as a treatment in many different types of cancers. The drug targets cellular microtubules and promotes arrest of cellular proliferation and eventual cell death. Traditionally administered intravenously with excipients know to have toxic side effects. An example trade name is Taxol*. |
| PARP cleavage |
The primary job of the enzyme PARP is to repair DNA that has been damaged (via metabolic, chemical, or radiation sources). If the DNA damage is severe, PARP can be inactivated by cleavage. When this occurs, the cell undergoes programmed cell death to release its energy to surrounding cells. |
| pharmacodynamics |
The branch of pharmacology concerned with the effects of drugs and the mechanism of their action. Referred to as the study of how a drug affects the body. |
| pharmacokinetics |
The branch of pharmacology concerned with the movement of drugs within the body. Referred to as the study of how the body affects the drug during its time within it. |
| photodynamic therapy |
A treatment that uses a combination of light and special drugs, called photosensitizing agents, to kill cancer cells. The drugs only work after they have been activated by light. |
| plasma membrane protein |
A class of proteins that are embedded in either the cell membrane or another lipid membrane. |
| prednisone |
A corticosteroid drug that is a potent immunosuppressant. It can be used with chemotherapy to prevent allergic reactions to other drugs, or to treat nausea and vomiting. |
| psoriasis |
An autoimmune disease which results in red, scaly patches on the skin. |
| ramucirumab |
Ramucirumab (trade name Cyramza) is a human monoclonal antibody developed for the treatment of solid tumors. It is approved by the FDA for single agent use or as a combination with paclitaxel, docetaxel or FOLFIRI. Ramucirumab prevents the formation of new blood vessels by blocking the action of the vascular endothelial growth factor receptor 2 (VEGFR2). |
| receptor antagonist |
A molecule that binds to a specific protein receptor, preventing the receptor from responding to a biological signal. |
| small cell |
Small cell carcinoma, or small-cell lung cancer, is a type of malignant cancer that most commonly arises within the lung. Compared to non-small cell carcinoma, small-cell carcinoma grows and spreads more quickly. |
| small molecule P-gp inhibitors |
Organic molecules with a low molecular weight that bind to the P-glycoprotein transporter and prevent its function. |
241
Table of Contents
| small molecule treatments |
A treatment comprised of a drug that is an organic molecule with low molecular weight. |
| Src Kinase / Src Kinase inhibition |
Src Kinase refers to a protein in humans that is involved in cell signaling, cell proliferation and cell growth. Increased expression of the Src gene is observed in many cancers, and is associated with increased tumor proliferation. Inhibition of Src Kinase proteins is a method to control tumor growth. |
| Src tyrosine kinase |
See Src Kinase / Src Kinase inhibition. |
| systemic |
Affecting or pervasive to the whole body. |
| target binding sites |
A region on a protein, or a piece of RNA or DNA, where a biologically active molecule or drug attaches reversibly. This binding has a biological effect on the protein, RNA, or DNA. |
| targeted therapy |
A type of cancer treatment using drugs that precisely identify and attack cancer cells, while doing little to no damage to normal cells. |
| tariquidar |
Tariquidar is a small molecule inhibitor of P-glycoprotein. It is currently undergoing research as an adjuvant treatment against multidrug resistance in cancer. |
| taxanes |
Taxanes are a class of diterpene small molecule drugs that are widely used as chemotherapy agents. Paclitaxel and docetaxel are examples of taxanes. |
| temozolomide |
Temozolomide is an oral chemotherapy drug used as a treatment for some brain cancers. Trade names include Temodar. |
| topical fluorouracil |
See fluorouracil. |
| topoisomerase inhibitor |
Topoisomerases are enzymes that facilitate the coiling or uncoiling of DNA, which alters whether or not enzymes or other molecules can interact with the DNA. Topoisomerase inhibitors prevent this function, and can be used for chemotherapy or as antibacterial agents. |
| topotecan |
Topotecan (trade name Hycamtin) is a chemotherapeutic agent that is a topoisomerase inhibitor. It is approved to treat a number of cancer types, including ovarian and lung cancer. |
| toxicokinetic |
A description of the rate at which a chemical will enter the body as well as a description of what will happen to the chemical once it is in the body. |
| toxicology |
A branch of pharmacology concerned with studying the adverse effects of chemicals or drugs on living organisms. |
| transporter proteins |
Proteins embedded within cell membranes which transport substances across the cell membrane. |
| transporters |
See transporter proteins. |
242
Table of Contents
| tubulin polymerization / inhibition of tubulin polymerization |
The cellular process of formation, remodeling, and destruction of microtubules which are comprised primarily of the protein tubulin. These microtubules serve as part of the cell’s structure, transport system, and are involved in cell division. |
| uterine sarcoma cell |
Uterine sarcoma is a malignant cancer originating in the uterus. |
| vesicultation |
The formation of vesicles: small structures within cells composed of fluid contained by a roughly spherical lipid bilayer. Vesicles are useful for the processes of secretion, uptake, and transport within cells. |
| vesicultation / pustulation |
For vesiculation, see vesiculation above. Pustulation is the formation of a pustule: a lesion or bump filled with pus. |
| xenograft |
A tissue graft or transplant from a donor of a different species. An example is taking a human cancer cell line and implanting in a mouse to test prospective drugs for therapeutic efficacy. |
243
Table of Contents
| INDEX TO FINANCIAL STATEMENTS | ||||
| Consolidated Financial Statements of Athenex, Inc. |
||||
| F-2 | ||||
| F-3 | ||||
| Consolidated Statements of Operations and Comprehensive Loss |
F-4 | |||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| Combined Financial Statements of Polymed Therapeutics, Inc. and Chongqing Taihao Pharmaceutical Co., Ltd. |
||||
| F-40 | ||||
| F-41 | ||||
| F-42 | ||||
| F-43 | ||||
| Combined Statements of Cash Flow for the years ended December 31, 2014 and 2013 |
F-44 | |||
| F-45 | ||||
| Combined Interim Financial Statements of Polymed Therapeutics, Inc. and Chongqing Taihao Pharmaceutical Co., Ltd. |
||||
| Combined Balance Sheets as of March 31, 2015 and December 31, 2014 |
F-54 | |||
| F-55 | ||||
| Combined Cash Flows for the three months ended March 31, 2015 and 2014 |
F-56 | |||
| F-57 | ||||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors of Athenex, Inc.
Buffalo, New York
We have audited the accompanying consolidated balance sheets of Athenex, Inc. and subsidiaries (the “Company”) as of December 31, 2015 and 2016, and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for the years then ended. Our audits also included the financial statement schedules listed in the Index at Item 16. These consolidated financial statements and financial statement schedules are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements and financial statement schedules based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of Athenex, Inc. and subsidiaries as of December 31, 2015 and 2016, and the results of their operations and their cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such financial statement schedules, when considered in relation to the basic consolidated financial statements taken as a whole, present fairly in all material respects the information set forth therein.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company’s recurring losses from operations and negative cash flows from operations raise substantial doubt about its ability to continue as a going concern. Management’s plans concerning these matters are also discussed in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Deloitte & Touche LLP
Williamsville, New York
May 1, 2017
F-2
Table of Contents
ATHENEX, INC. AND SUBSIDIARIES
(In thousands, except share and per share data)
| December 31, | March 31, 2017 |
Pro Forma March 31, 2017 |
||||||||||||||
| 2015 | 2016 | |||||||||||||||
| (unaudited) | ||||||||||||||||
| Assets |
||||||||||||||||
| Current assets: |
||||||||||||||||
| Cash and cash equivalents |
$ | 43,495 | $ | 33,125 | $ | 26,034 | ||||||||||
| Marketable securities—current |
12,271 | 8,628 | 3,051 | |||||||||||||
| Accounts receivable, net of allowance for doubtful accounts of $478, $155 and $655, respectively |
3,832 | 2,777 | 1,933 | |||||||||||||
| Inventories, net |
3,256 | 4,240 | 6,835 | |||||||||||||
| Prepaid expenses and other current assets |
2,340 | 3,153 | 4,583 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total current assets |
65,194 | 51,923 | 42,436 | |||||||||||||
| Property and equipment, net |
6,289 | 5,810 | 7,645 | |||||||||||||
| Investment |
487 | 340 | 351 | |||||||||||||
| Marketable securities—long-term |
1,868 | — | — | |||||||||||||
| Goodwill |
37,996 | 37,552 | 37,574 | |||||||||||||
| Intangible assets, net |
7,158 | 8,464 | 9,594 | |||||||||||||
| Other long-term assets |
1,439 | 1,801 | 2,903 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets |
$ | 120,431 | $ | 105,890 | $ | 100,503 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities and stockholders’ equity (deficit) |
||||||||||||||||
| Current liabilities: |
||||||||||||||||
| Accounts payable |
$ | 6,271 | $ | 7,174 | $ | 9,856 | ||||||||||
| Accrued expenses |
3,627 | 18,956 | 26,711 | |||||||||||||
| Accrued legal liability |
450 | — | — | |||||||||||||
| Current portion of contingent consideration |
5,974 | — | — | |||||||||||||
| Current portion of long-term debt—related parties |
1,064 | 1,123 | 1,140 | |||||||||||||
| Current portion of long-term debt |
230 | 766 | 781 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total current liabilities |
17,616 | 28,019 | 38,488 | |||||||||||||
| Long-term liabilities: |
||||||||||||||||
| Deferred compensation |
1,463 | 2,174 | 2,327 | |||||||||||||
| Deferred rent |
255 | 904 | 1,112 | |||||||||||||
| Deferred income tax liability |
697 | 206 | 108 | |||||||||||||
| Long-term debt—related parties |
1,619 | 496 | 203 | |||||||||||||
| Long-term debt |
737 | — | — | |||||||||||||
| Convertible bonds |
— | 14,498 | 24,107 | |||||||||||||
| Convertible bonds—related parties |
— | 16,129 | 19,585 | |||||||||||||
| Derivative liability |
— | 8,795 | 18,190 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
22,387 | 71,221 | 104,120 | |||||||||||||
| Commitments and contingencies (Note 20) |
||||||||||||||||
| Stockholders’ equity (deficit): |
||||||||||||||||
| Common stock, par value $0.001 per share, 250,000,000 shares authorized at December 31, 2015 and 2016 and March 31, 2017; 40,330,124, 42,342,706 and 42,359,506 issued at December 31, 2015 and 2016 and March 31, 2017, respectively; 39,907,796, 40,685,786 and 40,686,586 shares outstanding at December 31, 2015 and 2016 and March 31, 2017, respectively |
40 | 42 | 42 | |||||||||||||
| Additional paid-in capital |
206,679 | 237,581 | 239,769 | |||||||||||||
| Accumulated other comprehensive loss |
(223 | ) | (1,304 | ) | (802 | ) | ||||||||||
| Accumulated deficit |
(107,391 | ) | (195,106 | ) | (236,094 | ) | ||||||||||
| Less: treasury stock, at cost; 422,328, 1,656,920, and 1,672,920 shares at December 31, 2015 and 2016 and March 31, 2017, respectively |
(1,545 | ) | (7,406 | ) | (7,406 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Athenex, Inc. stockholders’ equity (deficit) |
97,560 | 33,807 | (4,491 | ) | ||||||||||||
| Non-controlling interests |
484 | 862 | 874 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total stockholders’ equity (deficit) |
98,044 | 34,669 | (3,617 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities and stockholders’ equity (deficit) |
$ | 120,431 | $ | 105,890 | $ | 100,503 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Table of Contents
ATHENEX, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except share and per share data)
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Revenue: |
||||||||||||||||
| Product sales |
$ | 12,816 | $ | 19,394 | $ | 4,488 | $ | 3,900 | ||||||||
| License fees and consulting revenue |
314 | 392 | 95 | 598 | ||||||||||||
| Grant revenue |
814 | 765 | 46 | 83 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
13,944 | 20,551 | 4,629 | 4,581 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Costs and operating expenses: |
||||||||||||||||
| Cost of sales |
13,153 | 19,718 | 4,142 | 2,839 | ||||||||||||
| Research and development expenses |
24,463 | 60,624 | 6,746 | 26,408 | ||||||||||||
| Selling, general, and administrative expenses |
27,036 | 25,956 | 4,337 | 9,799 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and operating expenses |
64,652 | 106,298 | 15,225 | 39,046 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(50,708 | ) | (85,747 | ) | (10,596 | ) | (34,465 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Interest expense (income) |
1 | 1,891 | (46 | ) | 2,376 | |||||||||||
| Unrealized loss on derivative liability |
— | 533 | — | 4,276 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before income tax benefit |
(50,709 | ) | (88,171 | ) | (10,550 | ) | (41,117 | ) | ||||||||
| Income tax (benefit) expense |
(54 | ) | (265 | ) | 100 | (92 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(50,655 | ) | (87,906 | ) | (10,650 | ) | (41,025 | ) | ||||||||
| Less: net loss attributable to non-controlling interests |
(55 | ) | (191 | ) | (32 | ) | (37 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to Athenex, Inc. |
$ | (50,600 | ) | $ | (87,715 | ) | $ | (10,618 | ) | $ | (40,988 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Unrealized gain (loss) on investment, net of income taxes |
91 | (33 | ) | (4 | ) | 3 | ||||||||||
| Foreign currency translation adjustment, net of income taxes |
(397 | ) | (1,048 | ) | (34 | ) | 499 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Comprehensive loss |
$ | (50,906 | ) | $ | (88,796 | ) | $ | (10,656 | ) | $ | (40,486 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share attributable to Athenex, Inc. common stockholders, basic and diluted (Note 17) |
$ | (1.50 | ) | $ | (2.19 | ) | $ | (0.27 | ) | $ | (1.01 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted-average shares used in computing net loss per share attributable to Athenex, Inc. common stockholders, basic and diluted (Note 17) |
33,765,751 | 40,120,908 | 38,878,366 | 40,693,039 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pro forma net loss per share attributable to Athenex, Inc. common stockholders, basic and diluted (unaudited) (Note 17) |
$ | $ | ||||||||||||||
|
|
|
|
|
|||||||||||||
| Pro forma weighted-average shares used in computing net loss per share attributable to Athenex, Inc. common stockholders, basic and diluted (unaudited) (Note 17) |
||||||||||||||||
|
|
|
|
|
|||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
ATHENEX, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(In thousands, except share data)
| Common Stock | Additional paid-in capital |
Accumulated deficit |
Accumulated other comprehensive income (loss) |
Treasury Stock | Total Athenex, Inc. stockholders’ equity (deficit) |
Non- controlling interests |
Total stockholders’ equity (deficit) |
|||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2014 |
23,649,044 | $ | 24 | $ | 86,064 | $ | (56,791 | ) | $ | 83 | (422,328 | ) | $ | (1,545 | ) | $ | 27,835 | $ | — | $ | 27,835 | |||||||||||||||||||
| Issuance of common stock |
13,050,924 | 13 | 85,355 | — | — | — | — | 85,368 | — | 85,368 | ||||||||||||||||||||||||||||||
| Issuance of common stock in connection with acquisition of Polymed |
1,538,464 | 1 | 11,537 | — | — | — | — | 11,538 | — | 11,538 | ||||||||||||||||||||||||||||||
| Issuance of common stock in connection with acquisition of CDE |
1,651,264 | 1 | 14,860 | — | — | — | — | 14,861 | — | 14,861 | ||||||||||||||||||||||||||||||
| Cost of equity raise |
— | (13 | ) | — | — | — | — | (13 | ) | — | (13 | ) | ||||||||||||||||||||||||||||
| Stock awarded to directors and officers |
410,668 | 1 | 2,559 | — | — | — | — | 2,560 | — | 2,560 | ||||||||||||||||||||||||||||||
| Stock-based compensation cost |
— | — | 8,932 | — | — | — | — | 8,932 | — | 8,932 | ||||||||||||||||||||||||||||||
| Notes receivable from officers |
— | — | (6,632 | ) | — | — | — | — | (6,632 | ) | — | (6,632 | ) | |||||||||||||||||||||||||||
| Vesting of restricted stock |
— | — | 4,035 | — | — | — | — | 4,035 | — | 4,035 | ||||||||||||||||||||||||||||||
| Stock options exercised |
29,760 | — | 61 | — | — | — | — | 61 | — | 61 | ||||||||||||||||||||||||||||||
| Repurchase of stock options and warrants |
— | — | (79 | ) | — | — | — | — | (79 | ) | (79 | ) | ||||||||||||||||||||||||||||
| Non-controlling interests |
— | — | — | — | — | — | — | — | 539 | 539 | ||||||||||||||||||||||||||||||
| Net loss |
— | — | — | (50,600 | ) | — | — | — | (50,600 | ) | (55 | ) | (50,655 | ) | ||||||||||||||||||||||||||
| Other comprehensive loss, net of tax |
— | — | — | — | (306 | ) | — | — | (306 | ) | — | (306 | ) | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at December 31, 2015 |
40,330,124 | 40 | 206,679 | (107,391 | ) | (223 | ) | (422,328 | ) | (1,545 | ) | 97,560 | 484 | 98,044 | ||||||||||||||||||||||||||
| Issuance of common stock |
1,133,332 | 1 | 8,499 | — | — | — | — | 8,500 | — | 8,500 | ||||||||||||||||||||||||||||||
| Issuance of common stock in connection with satisfaction of contingent consideration |
315,810 | 1 | 2,842 | — | — | — | — | 2,843 | — | 2,843 | ||||||||||||||||||||||||||||||
| Stock-based compensation cost |
— | — | 10,977 | — | — | — | — | 10,977 | — | 10,977 | ||||||||||||||||||||||||||||||
| Vesting of restricted stock |
50,000 | — | 8,534 | — | — | — | — | 8,534 | — | 8,534 | ||||||||||||||||||||||||||||||
| Repurchase of common stock |
— | — | — | — | — | (1,234,592 | ) | (5,861 | ) | (5,861 | ) | — | (5,861 | ) | ||||||||||||||||||||||||||
| Stock options and warrants exercised |
513,440 | — | 50 | — | — | — | — | 50 | — | 50 | ||||||||||||||||||||||||||||||
| Non-controlling interests |
— | — | — | — | — | — | — | — | 569 | 569 | ||||||||||||||||||||||||||||||
| Net loss |
— | — | — | (87,715 | ) | — | — | — | (87,715 | ) | (191 | ) | (87,906 | ) | ||||||||||||||||||||||||||
| Other comprehensive loss, net of tax |
— | — | — | — | (1,081 | ) | — | — | (1,081 | ) | — | (1,081 | ) | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at December 31, 2016 |
42,342,706 | 42 | 237,581 | (195,106 | ) | (1,304 | ) | (1,656,920 | ) | (7,406 | ) | 33,807 | 862 | 34,669 | ||||||||||||||||||||||||||
| Stock-based compensation cost |
— | — | 1,605 | — | — | — | — | 1,605 | — | 1,605 | ||||||||||||||||||||||||||||||
| Restricted stock expense |
— | — | 540 | — | — | — | — | 540 | — | 540 | ||||||||||||||||||||||||||||||
| Repurchase of common stock |
— | — | — | — | — | (16,000 | ) | — | — | — | — | |||||||||||||||||||||||||||||
| Stock options and warrants exercised |
16,800 | — | 43 | — | — | — | — | 43 | — | 43 | ||||||||||||||||||||||||||||||
| Non-controlling interests |
— | — | — | — | — | — | — | — | 49 | 49 | ||||||||||||||||||||||||||||||
| Net loss |
— | — | — | (40,988 | ) | — | — | — | (40,988 | ) | (37 | ) | (41,025 | ) | ||||||||||||||||||||||||||
| Other comprehensive income, net of tax |
— | — | — | — | 502 | — | — | 502 | — | 502 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at March 31, 2017 (unaudited) |
42,359,506 | $ | 42 | $ | 239,769 | $ | (236,094 | ) | $ | (802 | ) | (1,672,920 | ) | $ | (7,406 | ) | $ | (4,491 | ) | $ | 874 | $ | (3,617 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
ATHENEX, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Cash flows from operating activities: |
||||||||||||||||
| Net loss |
$ | (50,655 | ) | $ | (87,906 | ) | $ | (10,650 | ) | $ | (41,025 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||||||
| Depreciation and amortization |
888 | 2,026 | 417 | 816 | ||||||||||||
| Stock-based compensation expense |
15,527 | 19,511 | 2,910 | 2,145 | ||||||||||||
| Change in fair value of contingent consideration |
491 | 53 | 53 | — | ||||||||||||
| Change in fair value of derivative liability |
— | 533 | — | 4,276 | ||||||||||||
| Amortization of debt discount |
— | 889 | — | 1,184 | ||||||||||||
| Deferred rent expense |
— | 649 | 153 | 208 | ||||||||||||
| Loss on disposal of assets and impairment changes |
31 | 1,034 | — | 79 | ||||||||||||
| Research and development license fees settled with convertible bond |
— | — | — | 7,000 | ||||||||||||
| Deferred income taxes |
(270 | ) | (491 | ) | 63 | (92 | ) | |||||||||
| Changes in operating assets and liabilities, net of effects of acquisitions: |
||||||||||||||||
| Receivables, net |
1,081 | 1,055 | (146 | ) | 844 | |||||||||||
| Prepaid expenses and other assets |
(1,394 | ) | (359 | ) | (44 | ) | (1,430 | ) | ||||||||
| Inventories, net |
786 | (984 | ) | (786 | ) | (2,595 | ) | |||||||||
| Accounts payable and accrued expenses |
(241 | ) | 16,120 | 474 | 7,837 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash used in operating activities |
(33,756 | ) | (47,870 | ) | (7,556 | ) | (20,753 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash flows from investing activities: |
||||||||||||||||
| Proceeds from sale of property and equipment |
— | 335 | — | — | ||||||||||||
| Purchase of property and equipment |
(3,310 | ) | (1,487 | ) | (158 | ) | (1,554 | ) | ||||||||
| (Payment) receipt of refundable deposit |
(1,000 | ) | 1,000 | — | — | |||||||||||
| Payments for licenses |
(50 | ) | (2,700 | ) | — | — | ||||||||||
| Acquisition of Polymed, net of cash acquired |
(11,076 | ) | — | — | — | |||||||||||
| Acquisition of CDE, net of cash acquired |
1,699 | — | — | — | ||||||||||||
| Purchases of marketable securities |
(15,787 | ) | (9,750 | ) | (4,000 | ) | (3,051 | ) | ||||||||
| Sale of marketable securities |
12,615 | 15,261 | 3,403 | 8,628 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash (used in) provided by investing activities |
(16,909 | ) | 2,659 | (755 | ) | 4,023 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash flows from financing activities: |
||||||||||||||||
| Proceeds from sale of stock |
78,768 | 8,500 | 5,000 | — | ||||||||||||
| Proceeds from issuance of convertible bonds |
— | 38,000 | — | 10,000 | ||||||||||||
| Costs incurred related to the sale of stock |
(1,013 | ) | (1,537 | ) | — | (630 | ) | |||||||||
| Proceeds from exercise of stock options |
61 | 50 | 14 | 43 | ||||||||||||
| Investment from non-controlling interest |
539 | 569 | 519 | 49 | ||||||||||||
| Payment of contingent consideration |
— | (3,184 | ) | — | — | |||||||||||
| Repurchase of options and warrants |
(79 | ) | — | — | — | |||||||||||
| Repayment of long-term debt |
(724 | ) | (1,265 | ) | (233 | ) | (261 | ) | ||||||||
| Purchase of treasury stock |
(1,250 | ) | (5,861 | ) | (2,216 | ) | — | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash provided by financing activities |
76,302 | 35,272 | 3,084 | 9,201 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net increase (decrease) in cash and cash equivalents |
25,637 | (9,939 | ) | (5,227 | ) | (7,529 | ) | |||||||||
| Cash and cash equivalents, beginning of period |
17,521 | 43,495 | 43,495 | 33,125 | ||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
337 | (431 | ) | 39 | 438 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash and cash equivalents, end of period |
$ | 43,495 | $ | 33,125 | $ | 38,307 | $ | 26,034 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Supplemental cash flow disclosures: |
||||||||||||||||
| Interest paid |
$ | 92 | $ | 144 | $ | 40 | $ | 19 | ||||||||
| Income taxes paid |
$ | 146 | $ | 329 | $ | 98 | $ | 15 | ||||||||
| Non-cash investing and financing activities: |
||||||||||||||||
| Stock issued in connection with the acquisition of QuaDPharma |
$ | — | $ | 343 | $ | 343 | $ | — | ||||||||
| Stock issued in connection with the acquisition of Polymed |
$ | 11,538 | $ | 2,500 | $ | 2,500 | $ | — | ||||||||
| Stock issued in connection with the acquisition of CDE |
$ | 14,861 | $ | — | $ | — | $ | — | ||||||||
| Accrued purchases of property and equipment |
$ | 239 | $ | 348 | $ | 85 | $ | 1,080 | ||||||||
| Fair value of acquisition-related contingent consideration |
$ | 4,488 | $ | — | $ | — | $ | — | ||||||||
| Notes payable assumed in connection with the acquisition of Polymed |
$ | 3,275 | $ | — | $ | — | $ | — | ||||||||
| Mortgage assumed in connection with the acquisition of CDE |
$ | 1,099 | $ | — | $ | — | $ | — | ||||||||
| Note receivable for restricted stock granted to officers |
$ | 6,600 | $ | — | $ | — | $ | — | ||||||||
| Cost of equity raise in accounts payable and accrued expenses |
$ | — | $ | 264 | $ | — | $ | 735 | ||||||||
| Accrued purchases of pharmaceutical licenses |
$ | — | $ | — | $ | — | $ | 1,550 | ||||||||
| Convertible bond issued in lieu of licensing cash payment |
$ | — | $ | — | $ | — | $ | 7,000 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
ATHENEX, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(INFORMATION AS OF MARCH 31, 2017 AND FOR THE THREE MONTHS ENDED MARCH 31, 2016 AND 2017 IS UNAUDITED)
| 1. | COMPANY AND NATURE OF BUSINESS |
Description of Business
Athenex, Inc. (the “Company” or “Athenex”), originally under the name Kinex Pharmaceuticals LLC (“Kinex”), formed in November 2003, commenced operations on February 5, 2004, and operated as a limited liability company until it was incorporated in the State of Delaware under the name Kinex Pharmaceuticals, Inc. on December 31, 2012. The Company changed its name to Athenex, Inc. on August 26, 2015.
Athenex is a global biopharmaceutical company dedicated to the discovery, development and commercialization of novel therapies for the treatment of cancer. The Company’s mission is to improve the lives of cancer patients by creating more effective, safer and tolerable treatments. The Company has generated its clinical product candidates through its Orascovery and Src Kinase Inhibition research platforms, which are based on their understanding of human absorption biology and novel kinase binding selection, respectively. The Company has assembled a leadership team and have established global operations in the U.S. and China across the pharmaceutical value chain to execute its mission to become a global leader in bringing innovative cancer treatments to the market and improve health outcomes. The Company’s primary activities since commencement have been conducting research and development internally and through corporate collaborators, in-licensing and out-licensing pharmaceutical compounds and technology, and conducting clinical trials.
On June 1, 2015, the Company acquired Polymed Therapeutics, Inc. and Chongqing Taihao Pharmaceutical Co Ltd (collectively, “Polymed”). Located in Texas, Polymed markets and sells Active Pharmaceutical Ingredients (“API”) and medical devices in North America, Europe, and India. Polymed, through its cGMP facility in Chongqing, China, also manufactures API and conducts research and development of novel drugs and therapies.
On July 17, 2015, the Company acquired Comprehensive Drug Enterprises Ltd (“CDE”). CDE primarily performs research and development activities related to the development of transmucosal drug delivery. CDE also manufactures and markets several patented and trademarked pharmaceuticals in China, Hong Kong, and Taiwan, including PromptolTM and AvertiTM and has been approved to market an Antipruritic Hydrogel in China by The China Food and Drug Administration (“CFDA”).
Significant Risks and Uncertainties
The Company has incurred operating losses since its inception and, as a result, as of December 31, 2016 and March 31, 2017 had an accumulated deficit of $195.1 million and $236.1 million, respectively. Operations have been funded primarily through the sale of common stock and, to a lesser extent, from convertible bond financing grant funding. The Company will require significant additional funds in order to conduct clinical trials and to fund its operations. There can be no assurances, however, that additional funding will be available on favorable terms, or at all. If adequate funds are not available, the Company may be required to delay, modify, or terminate its research and development programs or reduce its planned commercialization efforts. The Company believes that it will be able to obtain additional working capital through equity financings or other arrangements to fund operations, including an initial public offering (IPO); however, there can be no assurance that such additional financing, if available, can be obtained on terms acceptable to the Company. If the Company is unable to obtain such additional financing, the Company will need to reevaluate future operating plans. Accordingly, there is substantial doubt regarding the Company’s ability to continue as a going concern.
These consolidated financial statements have been prepared on a going concern basis, which implies the Company will continue to realize its assets and discharge its liabilities in the normal course of the business. The Company’s recurring losses from operations and negative cash flows from operations have raised substantial
F-7
Table of Contents
doubt regarding its ability to continue as a going concern. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of this uncertainty.
Athenex is subject to a number of risks similar to other biopharmaceutical companies, including, but not limited to, the lack of available capital, possible failure of preclinical testing or clinical trials, inability to obtain marketing approval of product candidates, competitors developing new technological innovations, market acceptance of the Company’s products, and protection of proprietary technology. If the Company does not successfully commercialize or partner any of its product candidates, it will be unable to generate sufficient product revenue to achieve profitability.
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation and Principles of Consolidation
These consolidated financial statements reflect the accounts and operations of the Company and those of its subsidiaries in which the Company has a controlling financial interest. Intercompany transactions and balances have been eliminated.
Use of Estimates
These consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities as of the date of the consolidated financial statements and the reported amount of revenue and expenses during the reporting period. Such management estimates include those relating to assumptions used in contract research accruals, measurement of acquired assets and assumed liabilities in business combinations, allowance for doubtful accounts, inventory reserves, the valuation of the derivative liability, income taxes, the estimated useful life and recoverability of long-lived assets, and the valuation of stock-based awards. Actual results could differ from those estimates.
Unaudited Interim Financial Information
The accompanying interim consolidated balance sheet as of March 31, 2017, interim consolidated statements of operations and comprehensive loss and cash flows for the three months ended March 31, 2016 and 2017, and the interim consolidated statement of stockholders’ deficit for the three months ended March 31, 2017 are unaudited. The interim unaudited financial statements have been prepared on the same basis as the annual audited financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments necessary for the fair presentation of the Company’s financial position as of March 31, 2017 and the results of operations and its cash flows for the three months ended March 31, 2016 and 2017. The financial data and other information disclosed in these notes as of March 31, 2017 and for the three months ended March 31, 2016 and 2017 are also unaudited. The results as of and for the three months ended March 31, 2017 are not necessarily indicative of results to be expected for the year ending December 31, 2017, any other interim periods or any future annual or interim period.
Unaudited Pro Forma Balance Sheet and Stockholders’ Equity
The Company has presented an unaudited pro forma balance sheet including stockholders’ equity as of March 31, 2017 in order to show the assumed effect on the consolidated balance sheet of the obligation to issue common stock immediately prior to a qualified initial public offering (IPO) as discussed in Note 17. The unaudited pro forma stockholders’ equity does not give effect to any proceeds from the assumed IPO of the Company’s common stock.
F-8
Table of Contents
Functional Currency
Assets and liabilities of subsidiaries that prepare financial statements in currencies other than the U.S. dollar are translated using rates of exchange as of the balance sheet date and the statements of operations and comprehensive loss are translated at the average rates of exchange for each reporting period. The Company recorded a foreign currency translation loss of $0.4 million and $1.0 million for the years ended December 31, 2015 and 2016, respectively and a loss of less than $0.1 million and a gain of $0.5 million for the three months ended March 31, 2016 and 2017, respectively.
Cash, Cash Equivalents, and Marketable Securities
The Company considers all highly liquid investments with an original maturity of three months or less at the date of purchase to be cash equivalents. The Company deposits its cash primarily in checking, money market accounts, as well as certificates of deposit. The Company generally does not enter into investments for trading or speculative purposes, rather to preserve its capital for the purpose of funding operations.
Accounts Receivable, net
Accounts receivable are recorded at the invoiced amount. On a periodic basis, the Company evaluates its accounts receivable and establishes an allowance for doubtful accounts, based upon a history of past write-offs, the age of the receivables, and current credit conditions.
Inventories, net
Inventories for clinical trials are stated at the lower-of-cost-or-market, with approximate cost being determined on a first-in-first-out basis. Active pharmaceutical ingredient (“API”) inventory is stated at the lower-of-cost-or-market, with approximate cost being determined on a weighted average basis.
The Company provides inventory write-downs based on excess and obsolete inventories determined primarily by future demand forecasts. The write-down is measured as the difference between the cost of the inventory and market, based upon assumptions about future demand, and is charged to the provision for inventory, which is a component of cost of sales. At the point of the loss recognition, a new, lower cost basis for that inventory is established, and subsequent changes in facts and circumstances do not result in the restoration or increase in that newly established cost basis.
Property and Equipment, net
Property and equipment are recorded at cost or acquisition date fair value in a business acquisition. Depreciation is recorded over the estimated useful lives of the related assets using the straight-line method. Leasehold improvements are amortized on a straight-line basis over the shorter of the useful life or term of the lease. Upon retirement or disposal, the cost and related accumulated depreciation are removed from the consolidated balance sheets and the resulting gain or loss is recorded to general and administrative expense in the consolidated statements of operations and comprehensive loss. Routine expenditures for maintenance and repairs are expensed as incurred.
Estimated useful lives for property and equipment are as follows:
| Property and Equipment |
Estimated Useful Life | |
| Land |
Not depreciated | |
| Equipment |
5 - 8 years | |
| Furniture and fixtures |
5 years | |
| Computer hardware |
3 years | |
| Leasehold improvements |
Lesser of estimated useful life or remaining lease term | |
| Construction in process |
Not depreciated |
F-9
Table of Contents
Fair Value of Financial Instruments
Financial instruments consist of cash and cash equivalents, marketable securities, an investment, accounts receivable, accounts payable, accrued liabilities, and debt. Marketable securities, the investment, and the embedded derivative liability are stated at fair value. Cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities, and debt, are stated at their carrying value, which approximates fair value due to the short time to the expected receipt or payment date of such amounts.
Investment
The Company’s investment is classified as an available-for-sale security which is reported at fair value with unrealized gains and losses, net of related income taxes, recorded as a separate component of accumulated other comprehensive income (loss) in stockholders’ equity until realized. Unrealized losses that are considered to be other-than-temporary are expensed in the consolidated statements of operations and comprehensive loss as an impairment charge.
The Company considers available evidence in evaluating potential other-than-temporary impairments of its investment, including the duration and extent to which fair value is less than cost, and the Company’s ability and intent to hold the investment. Realized gains and losses on sales of the securities are included in the consolidated statement of operations and comprehensive loss as financial income or expenses. Unrealized gains and losses resulting from changes in the fair value of the securities are recognized in other comprehensive income.
Business Acquisitions
The Company accounts for acquired businesses using the acquisition method of accounting, which requires that assets acquired and liabilities assumed be recognized at their estimated fair values as of the acquisition date. Identifiable amortizing intangible assets are recorded on the consolidated balance sheet at fair value and amortized over their estimated useful lives. Acquisition-related costs are expensed as incurred. Any excess of the consideration transferred over the estimated fair values of the net assets acquired is recorded as goodwill.
Goodwill
The Company tests goodwill for impairment annually on October 1st, the Company’s annual goodwill impairment measurement date, or more frequently if a triggering event occurs. The Company has three operating segments: Oncology Innovation Platform, Commercial Platform, and Global Supply Chain Platform which has two components: Polymed and QuaDPharma. Accordingly, the Company has four reporting units: Oncology Innovation Platform, Commercial Platform, Polymed, and QuaDPharma, all of which have discrete financial information that are reviewed by segment managers. Goodwill is assigned to three reporting units: Oncology Innovation Platform, Polymed, and QuaDPharma. Impairment is determined for goodwill using the two-step approach. The first step is the estimation of fair value of each reporting unit, which is compared to the carrying value. If step one indicates that impairment potentially exists, the second step is performed to measure the amount of impairment, if any. Goodwill impairment exists when the implied fair value of goodwill is less than its carrying value. The Company concluded that there was no impairment of goodwill for the years ended December 31, 2015 and 2016 or for the three months ended March 31, 2017.
Intangible Assets, net
Intangible assets arising from a business acquisition are recognized at fair value as of the acquisition date. The Company amortizes intangible assets using the straight-line method. When the straight-line method of amortization is utilized, the estimated useful life of the intangible asset is shortened to assure the recognition of amortization expense corresponds with the expected cash flows. Other purchased intangibles, including certain licenses, are capitalized at cost and amortized on a straight-line basis over the license life, when a future economic benefit is probable and measurable. If a future economic benefit is not probable or measurable, the license costs are expensed as incurred within research and development expenses.
F-10
Table of Contents
Impairment of Long-Lived Assets
The Company reviews the recoverability of its long-lived assets, excluding goodwill, when events or changes in circumstances occur that indicate that the carrying value of the asset may not be recoverable. The assessment of possible impairment is based on the ability to recover the carrying value of the assets from the expected future cash flows (undiscounted and without interest expense) of the related operations. If these cash flows are less than the carrying value of such assets, an impairment loss for the difference between the estimated fair value and carrying value is recorded. The Company concluded that there was no impairment of long-lived assets for the year ended December 31, 2015 and the three months ended March 31, 2016. An impairment charge of $0.3 million was recorded for the year ended December 31, 2016 and $0.1 million for the three months ended March 31, 2017. See Note 5—Goodwill and Intangible Assets, net for additional details.
Contingent Consideration
Contingent consideration arising from a business acquisition is included as part of the purchase price and is recorded at fair value as of the acquisition date. Subsequent to the acquisition date, the Company remeasures contingent consideration arrangements at fair value at each reporting period until the contingency is resolved. The changes in fair value are recognized within selling, general, and administrative expenses in the Company’s consolidated statement of operations and comprehensive loss. Changes in fair values reflect new information about the likelihood of the payment of the contingent consideration and the passage of time.
Derivative Liability
The Company has an outstanding derivative instrument related to certain features embedded within the Company’s outstanding convertible bonds. The embedded derivative is accounted for as a derivative liability and it is re-measured to fair value as of each balance sheet date. The related remeasurement adjustments are recognized in the consolidated statements of operations and comprehensive loss. The Company records adjustments to the fair value of the derivative liability until the conversion or repayment of the convertible bonds occurs as discussed further in Note 10—Debt.
Treasury Stock
The Company records treasury stock activities under the cost method whereby the cost of the acquired stock is recorded as treasury stock. The Company’s accounting policy upon the formal retirement of treasury stock is to deduct the par value from common stock and to reflect any excess of cost over par value as a reduction to additional paid-in capital (to the extent created by previous issuances of the stock) and then accumulated deficit.
Revenue Recognition
The Company recognizes product revenue when there is persuasive evidence of an arrangement, the price is fixed or determinable, collectability is reasonably assured, and upon shipment to or acceptance by customers. Service revenue is recognized in the period such services have been rendered.
The Company receives certain grant award funding to support its continuing research and development efforts. The Company considers these grants to be operating revenue as they support the Company’s primary operating activities. Revenue is recognized when earned and when realized or realizable. Revenue from grant awards is deemed to be earned when all eligibility criteria are met.
The Company recognizes revenue related to the license of certain intellectual properties and related consulting services when earned and when realized or realizable. Amounts received in advance are recorded as deferred revenue until earned.
F-11
Table of Contents
Certain of the Company’s out-license agreements contain multiple elements and are accounted for in accordance with Accounting Standards Codification (“ASC”) 605-25 – Revenue Recognition – Multiple-Element Arrangements. The Company identifies the deliverables included within the arrangement and evaluates which deliverables represent separate units of accounting. The consideration received is then allocated among the separate units of accounting based on each unit’s relative selling price. The Company generally considers non-refundable milestone payments to be achieved as a result of the Company’s efforts to be substantive and recognizes them as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met. Revenue related to agreements with multiple elements or milestone payments were not significant in the periods presented.
Research and Development Expenses
Costs for research and development (“R&D”) of products, including payroll, contractor expenses, and supplies, are expensed as incurred. Clinical trial and other development costs incurred by third parties are expensed as the contracted work is performed. Where contingent milestone payments are due to third parties under research and development arrangements, the obligations are recorded when the milestone results are probable of being achieved.
Deferred Offering Costs
Deferred offering costs consist of qualified legal, accounting and other direct costs related to the efforts to raise capital through a public sale of the Company’s common stock. There were no such qualified offering costs deferred as of December 31, 2015, and $1.8 million and $2.9 million deferred as of December 31, 2016 and March 31, 2017, respectively.
Deferred Rent
Rent expense is recognized on a straight-line basis over the term of the lease. Lease incentives, including rent holidays provided by lessors and rent escalation provisions, are accounted for as deferred rent.
Comprehensive Income (Loss)
Comprehensive income (loss) is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. Changes in unrealized gains and losses on investments and foreign currency translation adjustments represent the differences between the Company’s net loss and comprehensive loss.
Stock-Based Compensation
Awards granted to employees
The Company recognizes stock-based compensation based on the grant date fair value of stock options granted to employees, officers, and directors. The Company used the Black-Scholes option pricing model to calculate the grant date fair value of stock options and warrants. The Black-Scholes option pricing model requires inputs for risk-free interest rate, dividend yield, volatility, fair value of common stock, and expected lives of the options. The risk-free rate for periods within the expected life of the option is based on the U.S. Treasury yield curve in effect at the time of the grant. No dividend yield is used, consistent with the Company’s history. Expected volatility is based on historical volatilities of the stock prices of peer biopharmaceutical companies. The fair value of common stock is based on the most recent sale price of the Company’s common stock. The Company uses the simplified method for determining the expected lives of options. The Company recognizes compensation expenses based on the grant date fair value of stock options on a straight-line basis over the requisite service period, which is generally the vesting period.
F-12
Table of Contents
Stock grants
The Company grants common stock to key officers and directors and records the fair value of these grants, based on the fair value of the common stock on the grant date, as compensation expense throughout the requisite service period.
Awards granted to non-employees
The Company has accounted for equity instruments issued to non-employees in accordance with the provisions of ASC 718, Compensation—Stock Compensation, and ASC 505, Equity. All transactions in which goods or services are received in exchange for equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. The expense is recognized in the same manner as if the Company had paid cash for the services provided by the non-employees.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes. Deferred income tax assets and liabilities are recognized for the estimated future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Deferred income tax expense or benefit is the result of changes in the deferred income tax assets and liabilities. Valuation allowances are established when necessary to reduce deferred income tax assets where, based upon the available evidence, management concludes that it is more-likely-than not that the deferred income tax assets will not be realized. In evaluating its ability to recover deferred income tax assets, the Company considers all available positive and negative evidence, including its operating results, ongoing tax planning and forecasts of future taxable income on a jurisdiction-by-jurisdiction basis. Because of the uncertainty of the realization of the deferred income tax assets, the Company has recorded a valuation allowance against its deferred income tax assets.
Reserves are provided for tax benefits for which realization is uncertain. Such benefits are only recognized when the underlying tax position is considered more likely than not to be sustained on examination by a taxing authority, assuming they possess full knowledge of the position and facts. Interest and penalties related to uncertain tax positions are recognized in income tax expense (benefit); however, the Company currently has no interest or penalties related to income taxes.
Segment and Geographic Information
The Company’s chief operating decision-maker, its Chief Executive Officer, reviews its operating results on an aggregate basis and at the operating segment level for purposes of allocating resources and evaluating financial performance. The Company has three business platforms which are the operating segments: (1) Oncology Innovation Platform, for the discovery and development of cancer supportive therapies, (2) Commercial Platform, the manufacturing and selling of commercial pharmaceutical products, and (3) Global Supply Chain Platform, the cGMP manufacturing and marketing of API, medical devices, and clinical products. Each operating segment has a segment manager who is held accountable for operations and operating results. Accordingly, the Company operates in three reportable segments.
Concentration of Credit Risk, Other Risks and Uncertainties
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents and investments. Cash equivalents are deposited in interest-bearing money market accounts and certificates of deposit. Although the Company deposits the cash with multiple financial institutions, cash balances may occasionally be in excess of the amounts insured by the Federal Deposit Insurance Corporation. The Company also has significant assets and liabilities held in its overseas biomanufacturing
F-13
Table of Contents
facility in China, Taihao, and therefore is subject to foreign currency fluctuation. Also, due to government restrictions on transferring funds out of China, the total restricted net assets of the Company’s consolidated subsidiaries was $13.5 million, $15.7 million and $17.0 million as of December 31, 2015 and 2016 and March 31, 2017, respectively.
Recent Accounting Pronouncements Not Yet Adopted
In May 2014, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (“ASU”) No. 2014-09, “Revenue from Contracts with Customers (Topic 606)”, which requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. The ASU will replace most existing revenue recognition guidance in U.S. GAAP when it becomes effective. The new standard is effective for the Company on January 1, 2018 with early adoption permitted only for annual reporting periods beginning after December 15, 2016. The standard permits the use of either the retrospective or cumulative effect transition method. The Company’s evaluation of the effects of ASU 2014-09 and the selection of a transition method is ongoing and not yet complete. The Company anticipates that the standard may impact the accounting related to its out-license agreements, however, such agreements are not currently significant to the consolidated financial statements.
In February 2016, the FASB issued ASU No. 2016-02, “Leases (Topic 842)” which requires that lessees distinguish between finance and operating leases and recognize the assets and liabilities that arise from the leases on the balance sheet. This ASU is required to be adopted retrospectively and is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years, and is required to be applied on a modified retrospective basis. The Company is evaluating the effect of this standard on its consolidated financial statements.
In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments,” which modifies the measurement of expected credit losses of certain financial instruments. ASU 2016-13 is required to be adopted retrospectively and is effective for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years. The Company is evaluating the effect of this standard on its consolidated financial statements.
In November 2016, the FASB issued ASU 2016-18, “Statement of Cash Flows (Topic 230): Restricted Cash. The primary purpose of this ASU is to reduce the diversity in practice that exists in the classification and presentation of changes in restricted cash on the statement of cash flows. This ASU will require that a statement of cash flows explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash or cash equivalents. Therefore, amounts generally described as restricted cash and restricted cash equivalents should be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. This ASU is effective for fiscal years beginning after December 15, 2017. This ASU is required to be applied retrospectively. Early adoption is permitted, including adoption in an interim period. The Company is evaluating the effect of this standard on its consolidated financial statements.
Recently Adopted Accounting Pronouncements
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern” which requires management to assess if there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures in certain circumstances. In connection with each annual and interim period, management must assess if there is substantial doubt about an entity’s ability to continue as a going concern within one year after the financial statement issuance date. Disclosures are required if conditions give rise to substantial doubt. The amendment is effective for the first annual period ending after December 15, 2016 with early adoption permitted for annual or interim reporting periods for which the financial statements have not previously been issued. The Company adopted the guidance for the year ended
F-14
Table of Contents
December 31, 2016. The adoption resulted in expanded disclosure of the principal conditions that raise substantial doubt, management’s evaluation of those conditions, and management’s plans to mitigate these conditions.
In July 2015, the FASB issued ASU No. 2015-11, “Inventory (Topic 330): Simplifying the Measurement of Inventory.” This ASU requires inventory to be measured at the lower of cost or net realizable value. The provisions of this ASU are effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. The amendment is required to be applied prospectively, and early adoption is permitted. The Company adopted ASU 2015-11 effective January 1, 2017. Adoption of ASU 2015-11 did not have a significant impact on the consolidated financial statements.
In March 2016, the FASB issued ASU No. 2016-09, “Compensation- Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting” which changes how companies account for certain aspects of stock-based awards to employees, including the accounting for income taxes, forfeitures, and statutory tax withholding requirements, as well as the classification in the statement of cash flows. Effective January 1, 2017, the Company adopted ASU 2016-09. The standard eliminated the requirement to defer recognition of excess tax benefits related to employee share-based awards until they are realized through a reduction to income taxes payable. The Company applied the modified retrospective method and there was no net cumulative-effect adjustment to retained earnings on January 1, 2017 as the increase in deferred income tax assets for previously unrecognized excess tax benefits was fully offset by a valuation allowance. As permitted by the ASU, the Company will continue to use an estimated forfeiture rate in determining stock-based compensation expense.
In August 2016, the FASB issued ASU No. 2016-15, “Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments,” which addresses the classification of certain cash transactions on the statement of cash flows. ASU 2016-15 is effective for fiscal years beginning after December 15, 2018, and interim periods within fiscal years beginning after December 15, 2019. The Company early adopted ASU 2016-15 as of January 1, 2016 and applied its provisions retrospectively through the earliest period presented, which did not have a significant impact on its consolidated financial statements.
In January 2017, the FASB issued ASU 2017-04, “Intangibles—Goodwill and Other (Topic 350) Simplifying the Test for Goodwill Impairment.” The primary purpose of the ASU is to simplify the subsequent measurement of goodwill by eliminating Step 2 from the goodwill impairment test. The ASU also applies the same test of goodwill to all reporting units, now including those with a zero or negative carrying amount of net assets. This ASU is required to be adopted on a prospective basis and is effective for any goodwill impairment tests in fiscal years beginning after December 15, 2019, although early adoption is permitted for any impairment tests performed after January 1, 2017. The Company has adopted the new guidance on a prospective basis during the first quarter of 2017. The adoption of this ASU has not impacted the Company’s consolidated financial statements.
| 3. | INVENTORIES, NET |
Inventories, net, consist of the following (in thousands):
| December 31, | March 31,
2017 |
|||||||||||
| 2015 | 2016 | |||||||||||
| (unaudited) | ||||||||||||
| Raw materials and purchased parts |
$ | 630 | $ | 977 | $ | 1,344 | ||||||
| Work in progress |
3,084 | 3,656 | 3,725 | |||||||||
| Finished goods |
381 | 536 | 2,705 | |||||||||
|
|
|
|
|
|
|
|||||||
| Inventories, gross |
4,095 | 5,169 | 7,774 | |||||||||
| Less: inventory reserve |
(839 | ) | (929 | ) | (939 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total inventories, net |
$ | 3,256 | $ | 4,240 | $ | 6,835 | ||||||
|
|
|
|
|
|
|
|||||||
F-15
Table of Contents
| 4. | PROPERTY AND EQUIPMENT, NET |
Property and equipment, net, consists of the following (in thousands):
| December 31, | March 31,
2017 |
|||||||||||
| 2015 | 2016 | |||||||||||
| (unaudited) | ||||||||||||
| Land |
$ | 1,214 | $ | 1,120 | $ | 1,129 | ||||||
| Equipment |
3,218 | 3,955 | 4,539 | |||||||||
| Furniture and fixtures |
429 | 836 | 846 | |||||||||
| Computer hardware |
228 | 338 | 733 | |||||||||
| Leasehold improvements |
581 | 948 | 1,266 | |||||||||
| Construction in process |
1,598 | 670 | 1,561 | |||||||||
|
|
|
|
|
|
|
|||||||
| Property and equipment, gross |
7,268 | 7,867 | 10,074 | |||||||||
| Less: accumulated depreciation |
(979 | ) | (2,057 | ) | (2,429 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Property and equipment, net |
$ | 6,289 | $ | 5,810 | $ | 7,645 | ||||||
|
|
|
|
|
|
|
|||||||
Depreciation expense amounted to $0.5 million and $1.2 million for the years ended December 31, 2015 and 2016 and $0.2 million and $0.4 million for the three months ended March 31, 2016 and 2017, respectively.
| 5. | GOODWILL AND INTANGIBLE ASSETS, NET |
Goodwill
The changes in the carrying amount of goodwill for each reporting unit with goodwill for the years ended December 31, 2015 and 2016 and for the three months ended March 31, 2017 are as follows (in thousands):
| QuaDPharma | Polymed | Oncology Innovation Platform |
Total | |||||||||||||
| Balance as of December 31, 2014 |
$ | 4,586 | $ | — | $ | — | $ | 4,586 | ||||||||
| Goodwill acquired in connection with acquisitions |
— | 22,204 | 11,379 | 33,583 | ||||||||||||
| Effect of currency translation adjustment |
— | (173 | ) | — | (173 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as of December 31, 2015 |
4,586 | 22,031 | 11,379 | 37,996 | ||||||||||||
| Effect of currency translation adjustment |
— | (439 | ) | (5 | ) | (444 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as of December 31, 2016 |
$ | 4,586 | $ | 21,592 | $ | 11,374 | $ | 37,552 | ||||||||
| Effect of currency translation adjustment |
— | 46 | (24 | ) | 22 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as of March 31, 2017 (unaudited) |
$ | 4,586 | $ | 21,638 | $ | 11,350 | $ | 37,574 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Intangible Assets, Net
The Company’s identifiable intangible assets, net, consist of the following (in thousands):
| December 31, 2015 | ||||||||||||||||
| Cost/Fair Value | Accumulated Amortization |
Impairments | Net | |||||||||||||
| Amortizable intangible assets: |
||||||||||||||||
| Licenses |
$ | 400 | $ | 100 | $ | — | $ | 300 | ||||||||
| QuaDPharma customer list |
204 | 39 | — | 165 | ||||||||||||
| Polymed customer list |
1,593 | 157 | — | 1,436 | ||||||||||||
| Polymed technology |
3,712 | 180 | — | 3,532 | ||||||||||||
| Indefinite-lived intangible assets: |
||||||||||||||||
| CDE in-process research and development (IPR&D) |
1,884 | — | — | 1,884 | ||||||||||||
| Effect of currency translation adjustment |
(159 | ) | — | — | (159 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total intangible assets, net |
$ | 7,634 | $ | 476 | $ | — | $ | 7,158 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-16
Table of Contents
| December 31, 2016 | ||||||||||||||||
| Cost/Fair Value | Accumulated Amortization |
Impairments | Net | |||||||||||||
| Amortizable intangible assets: |
||||||||||||||||
| Licenses |
$ | 3,100 | $ | 315 | $ | — | $ | 2,785 | ||||||||
| QuaDPharma customer list |
204 | 58 | 146 | — | ||||||||||||
| Polymed customer list |
1,593 | 414 | — | 1,179 | ||||||||||||
| Polymed technology |
3,712 | 437 | — | 3,275 | ||||||||||||
| Indefinite-lived intangible assets: |
||||||||||||||||
| CDE in-process research and development (IPR&D) |
1,884 | — | 248 | 1,636 | ||||||||||||
| Effect of currency translation adjustment |
(411 | ) | — | — | (411 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total intangible assets, net |
$ | 10,082 | $ | 1,224 | $ | 394 | $ | 8,464 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| March 31, 2017 (unaudited) | ||||||||||||||||
| Cost/Fair Value | Accumulated Amortization |
Impairments | Net | |||||||||||||
| Amortizable intangible assets: |
||||||||||||||||
| Licenses |
$ | 4,650 | $ | 510 | $ | — | $ | 4,140 | ||||||||
| Polymed customer list |
1,593 | 480 | — | 1,113 | ||||||||||||
| Polymed technology |
3,712 | 510 | — | 3,202 | ||||||||||||
| Product rights |
531 | 33 | — | 498 | ||||||||||||
| Indefinite-lived intangible assets: |
||||||||||||||||
| CDE in-process research and development (IPR&D) |
1,104 | — | 79 | 1,025 | ||||||||||||
| Effect of currency translation adjustment |
(384 | ) | — | — | (384 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total intangible assets, net |
$ | 11,206 | $ | 1,533 | $ | 79 | $ | 9,594 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
As of December 31, 2016, licenses at cost include an Orascovery license of $0.4 million and a license purchased from Gland Pharma Ltd (“Gland”) of $2.7 million. The Orascovery license with Hanmi Pharmaceuticals Co. Ltd. (“Hanmi”) was purchased directly from Hanmi and is being amortized on a straight-line basis over a period of 12.75 years, the remaining life of the license agreement at the time of purchase. During the three months ended March 31, 2017, the Company purchased additional licenses from Gland for $1.6 million. The licenses purchased from Gland are being amortized on a straight-line basis over a period of 5 years, the remaining life of the license agreement at the time of purchase.
The remaining intangible assets were acquired in connection with the acquisitions of QuaDPharma, Polymed, and CDE, as further described in Note 11—Business Acquisitions. Intangible assets are amortized using an economic consumption model over their useful lives. The QuaDPharma customer list is amortized on a straight-line basis over 7 years. The Polymed customer list and technology are amortized on a straight-line basis over 6 and 12 years, respectively. The CDE IPR&D will not be amortized until the in-process research and development projects are completed. IPR&D will be tested annually for impairment, unless conditions exist causing an earlier impairment test (i.e. abandonment of an in-process project). No impairment charges were recorded during 2015. During the year ended December 31, 2016, impairment charges of $0.2 million and $0.1 million were recorded within research and development costs and selling, general, and administrative costs, respectively, in the 2016 consolidated statement of operations and comprehensive loss. The charge of $0.2 million was due to the impairment of CDE’s IPR&D. One drug development project included within IPR&D was abandoned and therefore, the related balance was written off as impaired. The charge of $0.1 million was due to the impairment of the QuaDPharma customer list. This was due to the business model change of QuaDPharma from a contract manufacturer to a facility primarily producing FDA shortage products under 503B regulations, which changed the Company’s anticipated use of the customer list. Additionally, during the three months ended March 31, 2017, one drug development project was abandoned and therefore, the related balance of $0.1 million was written off as
F-17
Table of Contents
impaired and is included within research and development expenses in the consolidated statement of operations and comprehensive loss for the three months ended March 31, 2017. The weighted-average useful life for all intangible assets was 8.37 years.
The Company recorded $0.4 million and $0.8 million of amortization expense for the years ended December 31, 2015 and 2016 and $0.2 million and $0.4 million for the three months ended March 31, 2016 and 2017, respectively.
The Company expects amortization expense related to its finite-lived intangible assets for the next 5 years and thereafter to be as follows as of December 31, 2016 (in thousands):
| Year ending December 31: |
Estimated Amortization Expense |
|||
| 2017 |
$ | 1,146 | ||
| 2018 |
1,146 | |||
| 2019 |
1,146 | |||
| 2020 |
1,146 | |||
| 2021 |
818 | |||
| Thereafter |
1,837 | |||
|
|
|
|||
| $ | 7,239 | |||
|
|
|
|||
| 6. | FAIR VALUE MEASUREMENTS |
ASC 820, Fair Value Measurements, establishes a framework for measuring fair value. That framework provides a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1 measurements) and the lowest priority to unobservable inputs (level 3 measurements). The three levels of the fair value hierarchy under the ASC 820 are described as follows:
Level 1—Inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets that the plan has the ability to access.
Level 2—Inputs to the valuation methodology include:
| • | Quoted prices for similar assets or liabilities in active markets; |
| • | Quoted prices for identical or similar assets or liabilities in inactive markets; |
| • | Inputs other than quoted prices that are observable for the asset or liability; |
| • | Inputs that are derived principally from or corroborated by observable market data by correlation or other means; and |
| • | If the asset or liability has a specified (contractual) term, the level 2 input must be observable for substantially the full term of the asset or liability. |
Level 3—Inputs to the valuation methodology are unobservable, supported by little or no market activity, and that are significant to the fair value measurement.
Transfers between levels, if any, are recorded as of the beginning of the reporting period in which the transfer occurs; there were no transfers between Levels 1, 2 or 3 of any financial assets or liabilities during 2015 or 2016 and during the three months ended March 31, 2016 and 2017.
F-18
Table of Contents
The following tables represent the fair value hierarchy for those assets and liabilities that the Company measures at fair value on a recurring basis (in thousands):
| Fair Value Measurements at December 31, 2015 Using: | ||||||||||||||||
| Total | Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
|||||||||||||
| Assets: |
||||||||||||||||
| Money market funds |
$ | 35,195 | $ | 35,195 | $ | — | $ | — | ||||||||
| Marketable securities—certificates of deposit |
14,139 | 14,139 | — | — | ||||||||||||
| Investment |
487 | 487 | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets |
$ | 49,821 | $ | 49,821 | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities: |
||||||||||||||||
| Contingent consideration—QuaDPharma |
$ | 1,133 | $ | — | $ | — | $ | 1,133 | ||||||||
| Contingent consideration—Polymed |
4,841 | — | — | 4,841 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
$ | 5,974 | $ | — | $ | — | $ | 5,974 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Fair Value Measurements at December 31, 2016 Using: | ||||||||||||||||
| Total | Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
|||||||||||||
| Assets: |
||||||||||||||||
| Money market funds |
$ | 6,522 | $ | 6,522 | $ | — | $ | — | ||||||||
| Marketable securities—certificates of deposit |
8,628 | 8,628 | — | — | ||||||||||||
| Investment |
340 | 340 | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets |
$ | 15,490 | $ | 15,490 | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities: |
||||||||||||||||
| Derivative liability |
$ | 8,795 | $ | — | $ | — | $ | 8,795 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
$ | 8,795 | $ | — | $ | — | $ | 8,795 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Fair Value Measurements at March 31, 2017 (unaudited) Using: | ||||||||||||||||
| Total | Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
|||||||||||||
| Assets: |
||||||||||||||||
| Money market funds |
$ | 21 | $ | 21 | $ | — | $ | — | ||||||||
| Marketable securities—certificates of deposit |
3,051 | 3,051 | — | — | ||||||||||||
| Investment |
351 | 351 | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets |
$ | 3,423 | $ | 3,423 | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities: |
||||||||||||||||
| Derivative liability |
$ | 18,190 | $ | — | $ | — | $ | 18,190 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
$ | 18,190 | $ | — | $ | — | $ | 18,190 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The Company classifies its certificates of deposit and money market funds within Level 1 because it uses quoted market prices to determine their fair value.
F-19
Table of Contents
The Company owns 68,000 shares of PharmaEssentia, a company publicly traded on the Taiwan OTC Exchange (“TWO”). As of December 31, 2015 and 2016 and March 31, 2017, the Company’s investment in PharmaEssentia is valued at the closing price reported on the active market in which the security is traded. This investment is classified as a level 1 investment.
The Company accounted for the acquisitions of QuaDPharma, Polymed, and CDE as business combinations under the acquisition method of accounting. All assets and liabilities were measured at fair value as of the acquisition date. As a result of the purchases, the Company became liable for contingent consideration (“earn-out”) payable to certain previous owners of QuaDPharma and Polymed. These earn-outs are measured at fair value using level 3 inputs. All business combinations are described in Note 11—Business Acquisitions and the fair value of the earn-outs are discussed further in Note 12—Contingent Consideration.
The Company bifurcated the embedded derivative feature from its convertible bonds and recorded such as a long-term liability. The derivative liability was measured at fair value as of the issuance date and remeasured at fair value at the end of the reporting period. The liability is measured at fair value using level 3 inputs. The derivative liability is discussed further in Note 10—Debt.
The following table sets forth a summary of the changes in the fair value of the Company’s Level 3 financial instruments (in thousands):
| Derivative Liability | ||||
| Balance as of December 31, 2015 |
$ | — | ||
| Issuance of convertible bonds with embedded derivative |
8,262 | |||
| Change in fair value |
533 | |||
|
|
|
|||
| Balance as of December 31, 2016 |
8,795 | |||
| Issuance of convertible bonds with embedded derivative |
5,119 | |||
| Change in fair value |
4,276 | |||
|
|
|
|||
| Balance as of March 31, 2017 (unaudited) |
$ | 18,190 | ||
|
|
|
|||
| 7. | ACCRUED EXPENSES |
Accrued expenses consist of the following (in thousands):
| December 31, | March 31, 2017 |
|||||||||||
| 2015 | 2016 | |||||||||||
| (unaudited) | ||||||||||||
| Accrued wages and benefits |
$ | 444 | $ | 1,828 | $ | 1,244 | ||||||
| Accrued clinical expenses |
204 | 1,080 | 1,402 | |||||||||
| Accrued operating expenses |
891 | 1,057 | 3,539 | |||||||||
| Deferred revenue |
88 | 237 | 206 | |||||||||
| Accrued cost of equity raise |
— | 264 | 160 | |||||||||
| Accrued consulting costs |
2,000 | 515 | 500 | |||||||||
| Accrued R&D licensing fees |
— | 12,988 | 15,717 | |||||||||
| Accrued interest |
— | 987 | 2,163 | |||||||||
| Accrued withholding tax on foreign license |
— | — | 1,050 | |||||||||
| Accrued construction in process |
— | — | 730 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total accrued expenses |
$ | 3,627 | $ | 18,956 | $ | 26,711 | ||||||
|
|
|
|
|
|
|
|||||||
In December 2016, the Company entered into a binding term sheet with Nang Kuang Pharmaceutical Co., LTD (“NK”), which requires the Company to pay $12.0 million in license fees. Such amount is recorded as accrued R&D licensing fees above and included within research and development expenses in the 2016 consolidated statement of operations and comprehensive loss.
F-20
Table of Contents
| 8. | INCOME TAXES |
Income tax expense (benefit) was $0.1 million and ($0.1) million for the three months ended March 31, 2016 and 2017, respectively. Current year income tax benefit is attributable to changes in deferred income tax liabilities that were recognized in connection with the Company’s acquisitions. The Company and its other subsidiaries were in a cumulative loss position as of March 31, 2017.
The components of loss before income tax benefit consist of the following (in thousands):
| Year Ended December 31, | ||||||||
| 2015 | 2016 | |||||||
| Domestic |
$ | (47,428 | ) | $ | (83,714 | ) | ||
| Foreign |
(3,281 | ) | (4,457 | ) | ||||
|
|
|
|
|
|||||
| $ | (50,709 | ) | $ | (88,171 | ) | |||
|
|
|
|
|
|||||
The components of the income tax benefit consist of the following (in thousands):
| Year Ended December 31, | ||||||||
| 2015 | 2016 | |||||||
| Current: |
||||||||
| Federal |
$ | — | $ | — | ||||
| State |
80 | 61 | ||||||
| Foreign |
136 | 165 | ||||||
|
|
|
|
|
|||||
| 216 | 226 | |||||||
| Deferred: |
||||||||
| Federal |
(15,488 | ) | (26,386 | ) | ||||
| State |
(2,948 | ) | (4,165 | ) | ||||
| Foreign |
(529 | ) | (848 | ) | ||||
|
|
|
|
|
|||||
| (18,965 | ) | (31,399 | ) | |||||
| Change in valuation allowance |
18,695 | 30,908 | ||||||
|
|
|
|
|
|||||
| $ | (54 | ) | $ | (265 | ) | |||
|
|
|
|
|
|||||
The income tax benefit differs from the federal statutory rate due to the following:
| Year Ended December 31, | ||||||||
| 2015 | 2016 | |||||||
| Statutory rate |
34.0 | % | 34.0 | % | ||||
| State taxes, net of federal benefit |
5.7 | 5.4 | ||||||
| Foreign rate differential |
(0.9 | ) | (0.8 | ) | ||||
| Valuation allowance |
(36.9 | ) | (35.1 | ) | ||||
| Other |
(1.8 | ) | (3.2 | ) | ||||
|
|
|
|
|
|||||
| 0.1 | % | 0.3 | % | |||||
|
|
|
|
|
|||||
F-21
Table of Contents
Deferred tax assets (liabilities) consist of the following (in thousands):
| December 31, | ||||||||
| 2015 | 2016 | |||||||
| Intangible assets |
$ | 1,048 | $ | 8,534 | ||||
| Property and equipment |
19 | 28 | ||||||
| Stock-based compensation |
8,464 | 9,437 | ||||||
| Net operating loss carryforwards |
21,266 | 43,807 | ||||||
| Other |
1,341 | 1,840 | ||||||
|
|
|
|
|
|||||
| Gross deferred income tax assets |
32,138 | 63,646 | ||||||
| Less valuation allowance |
(31,400 | ) | (62,308 | ) | ||||
|
|
|
|
|
|||||
| Net deferred income tax assets |
738 | 1,338 | ||||||
|
|
|
|
|
|||||
| Intangible assets |
(1,281 | ) | (1,296 | ) | ||||
| Property and equipment |
(154 | ) | (248 | ) | ||||
|
|
|
|
|
|||||
| Gross deferred income tax liabilities |
(1,435 | ) | (1,544 | ) | ||||
|
|
|
|
|
|||||
| Net deferred income tax liabilities |
$ | (697 | ) | $ | (206 | ) | ||
|
|
|
|
|
|||||
For the years ended December 31, 2015 and 2016 and for the three months ended March 31, 2016 and 2017, the primary difference between the income tax calculated at the statutory rate and the effective rate is the valuation allowance established against the Company’s net deferred tax assets.
As of December 31, 2016, there exists $106.7 million federal net operating losses and $93.3 million of state net operating losses, respectively, which may be carried forward to offset future years’ tax liabilities and expire beginning in 2027. In addition, there exists $9.0 million of foreign net operating losses as of December 31, 2016 which may be carried forward indefinitely.
The Company considers whether any positions taken on the Company’s income tax returns would be considered uncertain tax positions that may require the recognition of a liability. The Company has concluded that there are no material uncertain tax positions as of December 31, 2015 and 2016 and as of March 31, 2017. The Company recognizes interest and penalties related to unrecognized tax benefits as a component of income benefit in the consolidated statement of operations and comprehensive loss. There were no amounts recognized for interest and penalties related to unrecognized tax benefits during the years ended December 31, 2015 and 2016 and during the three months ended March 31, 2016 and 2017. The income tax returns for the taxable years 2012 to 2015 in the U.S., China, and Hong Kong remain open and subject to income tax audits.
Provision has not been made for U.S. taxes on undistributed earnings of foreign subsidiaries. Those earnings have been and will continue to be indefinitely reinvested.
Under the provisions of Section 382 of the Internal Revenue Code (“IRC”), net operating loss and credit carryforwards and other tax attributes may be subject to limitation if there has been a significant change in ownership of the Company, as defined by the IRC. Future owner of equity shifts, including an initial public offering, could result in limitations on net operating loss carryforwards.
| 9. | DEFERRED COMPENSATION |
The Company has a non-qualified deferred compensation plan for certain key employees. In connection with the agreements between the Company and certain members of management, the employees have agreed to defer a portion of their salary to the future which is payable upon their retirement or separation of service with the Company. The deferred compensation accrues interest at 4% annually which is included with the total balance due. The Company incurred $0.5 million and $0.7 million of deferred compensation expense included within research and development expenses ($0.3 million and $0.4 million) and selling, general, and administrative expenses ($0.2 million and $0.3 million) during the years ended December 31, 2015 and 2016, respectively. The Company incurred $0.1 million and $0.2 million of deferred compensation expense included within research and development expenses ($0.1 million and $0.1 million) and selling, general, and administrative expenses (less
F-22
Table of Contents
than $0.1 million and $0.1 million) during the three months ended March 31, 2016 and 2017, respectively. The related liability as of December 31, 2015 and 2016 and March 31, 2017 totaled $1.5 million, $2.2 million, and $2.3 million, respectively.
| 10. | DEBT |
The Company’s debt as of December 31, 2015 and 2016 and as of March 31, 2017 amounted to $3.7 million, $41.8 million and $64.0 million, respectively. This consisted of three seller promissory notes that were negotiated as part of the Polymed acquisition (for details of the acquisition, refer to Note 11—Business Acquisitions), a mortgage, convertible bonds issued in 2016 and 2017 and a related derivative liability. As of December 31, 2015 and 2016 and March 31, 2017, the balances of this debt are as follows (in thousands):
| December 31, | March 31, 2017 |
|||||||||||
| 2015 | 2016 | |||||||||||
| (unaudited) | ||||||||||||
| Current portion of promissory notes to related parties |
$ | 1,064 | $ | 1,123 | $ | 1,140 | ||||||
| Current portion of mortgage |
230 | 766 | 781 | |||||||||
| Long-term portion of promissory notes to related parties |
1,619 | 496 | 203 | |||||||||
| Long-term portion of mortgage |
737 | — | — | |||||||||
| Convertible bonds, net of debt discount of $3,502 and $6,893 as of December 31, 2016 and March 31, 2017, respectively |
— | 14,498 | 24,107 | |||||||||
| Convertible bonds—related parties, net of debt discount of $3,871 and $4,415 as of December 31, 2016 and March 31, 2017, respectively |
— | 16,129 | 19,585 | |||||||||
| Derivative liability |
— | 8,795 | 18,190 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 3,650 | $ | 41,807 | $ | 64,006 | ||||||
|
|
|
|
|
|
|
|||||||
The promissory notes have a 36 month maturity beginning on July 1, 2015 and ending on June 1, 2018 with a 6% stated interest rate. The mortgage payments extend through July 30, 2017. Future minimum principal payments on these promissory notes and mortgage consist of $1.9 million and $0.2 million due in the remaining nine months of 2017 and the year ending December 31, 2018, respectively.
In September and October 2016 and January 2017, the Company issued convertible bonds with an aggregate principal value of $38.0 million and $10.0 million, respectively, and a maturity date of October 1, 2018. The bonds will be converted into shares of common stock upon the completion of an IPO. If such event does not occur prior to the maturity date, the bonds will become due, including interest of 10% per annum.
In March 2017, the Company signed an amendment to its license agreement with Hanmi, under which the Company received the rights to develop and sell drugs under the Orascovery program in additional territories, including Japan. This license amendment required an upfront fee of $7.0 million payable to Hanmi upon the execution of the agreement. In lieu of the payment, the Company issued a convertible bond to Hanmi with a par value of $7.0 million. This bond carries an interest rate of 10% per annum and a maturity date of October 1, 2018. Hanmi has the option to convert the bond to shares of common stock at discounted rates from 20% to 22% at various dates up to the maturity date. This amendment includes additional regulatory milestone payments and royalties based on sales. The occurrence of any milestone triggering events have not been deemed to be probable and no sales have yet occurred.
The conversion feature of these convertible bonds has been accounted for as an embedded derivative liability, which is measured at fair value and totaled $8.3 million and $5.1 million on the borrowing dates in 2016 and 2017, respectively. This resulted in a debt discount in the same amount, which is amortized to interest expense over the term of the debt using the effective interest method. As of March 31, 2017, the fair value of the derivative liability is $18.2 million. The fair value measurement of the derivative liability is determined using unobservable Level 3 inputs. These inputs include (a) the estimated amount and timing of projected cash flows and (b) the probability and timing of the achievement of the factors on which the derivative is based. Significant increases (decreases) in any of those input could result in a lower or higher fair value measurement. The
F-23
Table of Contents
unrealized loss due to changes in the derivative liability amounted to $0.5 million for the year ended December 31, 2016 and $4.3 million for the three months ended March 31, 2017.
| 11. | BUSINESS ACQUISITIONS |
Polymed
In June 2015, the Company finalized the acquisition of 100% of the outstanding shares of Polymed Therapeutics Inc. and Chongqing Taihao Pharmaceutical Co. Ltd. (collectively, “Polymed”). Polymed markets and sells API and medical devices in North America, Europe, and Asia from its locations in Texas and China. Polymed also develops new compounds, processing techniques, and manufactures API at Taihao, a cGMP facility in Chongqing, China. The Company believed that the acquisition was essential to control its supply chain, develop business globally, and to generate capital to fund operations.
The total cash purchase price paid by the Company during 2015 amounted to $11.0 million. This included $9.2 million of cash paid, $2.2 million of debt paid on Polymed’s behalf, less $0.4 million of cash acquired. In addition, the Company issued promissory notes in the amount of $3.3 million to the sellers (refer to Note 10—Debt). Further, the Company issued 1,538,464 shares of common stock valued at $7.50 per share as part of the consideration paid, which was the fair value of the common stock at the acquisition date.
In accordance with the acquisition agreement, there are provisions for contingent consideration up to a maximum of $5.0 million upon achievement of certain consolidated net revenue goals. On the acquisition date, the Company recorded the fair value of this contingent consideration as a liability based on the probabilities of Polymed achieving the performance thresholds and the present value of such payments. Refer to Note 12—Contingent Consideration for further details.
The net assets acquired have been recorded at fair value. To estimate the fair value of the identifiable intangible assets acquired, the Company utilized the income method which requires assumptions of projected revenue and expenses and an estimated discount rate, among other inputs. The following table summarizes the purchase price and the initial estimates of the fair values of assets and liabilities acquired at the date of acquisition (in thousands):
| Consideration: |
||||
| Cash |
$ | 9,285 | ||
| Debt repaid |
2,234 | |||
| Promissory notes |
3,275 | |||
| Stock issued (1,538,464 shares at $7.50) |
11,538 | |||
| Contingent consideration |
4,488 | |||
|
|
|
|||
| Purchase price |
$ | 30,820 | ||
|
|
|
|||
| Net assets acquired: |
||||
| Cash |
$ | 443 | ||
| Accounts receivable |
4,527 | |||
| Inventories |
3,876 | |||
| Other assets |
199 | |||
| Property and equipment |
1,011 | |||
| Customer list |
1,593 | |||
| Technology |
3,712 | |||
| Accounts payable and other accruals |
(5,718 | ) | ||
| Deferred income tax liability |
(987 | ) | ||
| Customer deposits |
(40 | ) | ||
|
|
|
|||
| Total identifiable net assets |
8,616 | |||
| Goodwill |
22,204 | |||
|
|
|
|||
| Total purchase price allocation |
$ | 30,820 | ||
|
|
|
F-24
Table of Contents
Goodwill in the amount of $22.2 million was recorded for the excess of the purchase price over the fair value of the assets acquired and liabilities assumed. The goodwill and intangible assets acquired in connection with this acquisition are not deductible for income tax purposes. This acquisition was made to benefit the Company’s Global Supply Chain Platform and therefore is included as a component of such segment.
The operating results of Polymed have been included within the Company’s Global Supply Chain Platform operating segment from the date of acquisition. Polymed added $11.5 million and $18.8 million of revenue for the years ended December 31, 2015 and 2016, respectively. Polymed contributed a net loss of $1.3 million and net income of $0.1 million for the years ended December 31, 2015 and 2016, respectively.
Comprehensive Drug Enterprises
On July 17, 2015, the Company executed a Sale and Purchase Agreement to purchase 100% of the shares of Comprehensive Drug Enterprises Ltd. (“CDE”). CDE is a Hong Kong-based biopharmaceutical company with a focus on the development of transmucosal drug delivery, with special emphasis on sublingual and nasal administration of pharmaceuticals. Additionally, CDE owns 100% of the shares of Maxinase Life Sciences Limited and owns 95% of the shares of MJ Medical Gel Systems (“HKMJ”). HKMJ has one wholly-owned subsidiary, Chongqing MJ Medical Sciences Co Ltd. and holds a majority interest (66.6%) in Chongqing MJ Medical Devices Co Ltd. For each of the entities in which the Company has a majority interest but is not wholly-owned, the Company consolidated the financial results of that company in its consolidated financial statements and records a non-controlling interest. The non-controlling interests are classified as equity in the consolidated balance sheets and totaled $0.5 million and $0.9 million as of December 31, 2015 and 2016, respectively. The Company believed that the acquisition of CDE was essential to expand its research efforts, add short-cycle symptom therapeutic drug candidates to the product portfolio, and add efficiencies to the manufacturing occurring in Chongqing, China.
This transaction was executed with a stock-for-stock exchange, with Athenex being the surviving parent company. For each share of CDE outstanding prior to the acquisition, the Company issued 0.023 shares of its common stock. Each Athenex share was valued as $9 and a total of 1,651,264 shares were issued as part of this transaction as the consideration transferred. The purchase price of CDE amounted to $14.9 million, however, as a cashless acquisition, the cash effect was the $1.7 million of cash acquired.
The net assets acquired have been recorded at fair value. To estimate the fair value of the identifiable intangible assets acquired, the Company utilized the cost method. The intangible asset, in-process research and development, is not amortized and is held as an indefinite-lived asset until the research and development projects are completed or abandoned. The following table summarizes the purchase price and the initial estimates of the fair values of assets and liabilities acquired at the date of acquisition (in thousands):
| Consideration: |
||||
| Stock issued (1,651,264 shares at $9) |
$ | 14,861 | ||
|
|
|
|||
| Purchase price |
$ | 14,861 | ||
|
|
|
|||
| Net assets acquired: |
||||
| Cash |
$ | 1,699 | ||
| Accounts receivable |
107 | |||
| Inventories |
166 | |||
| Other assets |
449 | |||
| Property and equipment |
1,803 | |||
| In-process research & development |
1,884 | |||
| Investment |
144 | |||
| Accounts payable and other accruals |
(1,671 | ) | ||
| Mortgage liability |
(1,099 | ) | ||
|
|
|
|||
| Total identifiable net assets |
3,482 | |||
| Goodwill |
11,379 | |||
|
|
|
|||
| Total purchase price allocation |
$ | 14,861 | ||
|
|
|
F-25
Table of Contents
Goodwill in the amount of $11.4 million was recorded for the excess of the purchase price over the fair value of the assets acquired and liabilities assumed. The goodwill and intangible assets acquired in connection with this acquisition are not deductible for income tax purposes. This acquisition was made to benefit the Company’s R&D efforts and therefore, is included in the Oncology Innovation Platform.
The operating results of CDE have been included within the Company’s Oncology Innovation Platform operating segment from the date of acquisition. CDE added $0.5 million and $0.7 million of revenue for the years ended December 31, 2015 and 2016, respectively. CDE contributed a net loss of $0.4 million and $1.8 million for the years ended December 31, 2015 and 2016.
Pro-forma Financial Information (Unaudited)
The pro forma results presented below include the effects of the Company’s 2015 acquisitions as if the acquisitions occurred on January 1, 2014. The pro forma net loss for the year ended December 31, 2015 includes the following adjustments: (1) additional amortization resulting from assets which arose during purchase accounting, (2) additional expenses for the change in the fair value of contingent consideration if the original measurement period was the beginning of the prior reporting period, (3) additional interest expense for loans that were used to fund the acquisitions, (4) removal of interest expense related to loans which were repaid in connection with the acquisitions, (5) removal of direct acquisition-related costs which would not have been incurred had the businesses been owned on the beginning of the prior reporting period, and (6) the deferred tax effect if the intangible assets and purchase accounting were recorded as of the beginning of the prior reporting period. The pro forma results do not include any anticipated synergies or other expected benefits of the acquisitions. The unaudited pro forma financial information is for informational purposes only and is not necessarily indicative of either future results of operations of the combined entity or results that might have been achieved had the acquisitions been consummated as of the beginning of the prior reporting period. The following table presents the unaudited pro forma consolidated financial information for 2015 (in thousands):
| Unaudited pro forma financial information (Athenex, Polymed, and CDE consolidated) |
Year Ended December 31, |
|||
| 2015 | ||||
| Consolidated revenue |
$ | 21,032 | ||
| Consolidated net loss |
$ | (51,682 | ) | |
Acquisition-Related Costs
Acquisition-related costs, including legal and regulatory and consulting costs, amounted to $0.2 million and $0.6 million for the acquisitions of Polymed and CDE, respectively, and are included within selling, general, and administrative expenses in the Company’s 2015 consolidated statement of operations and comprehensive loss.
| 12. | CONTINGENT CONSIDERATION |
The fair value measurements of contingent consideration liabilities are determined using unobservable Level 3 inputs. These inputs include (a) the estimated amount and timing of projected cash flows; (b) the probability of the achievement of the factors on which the contingency is based; and (c) the risk-adjusted discount rate used to present value the probability-weighted cash flows. Significant increases (decreases) in any of those inputs could result in a lower or higher fair value measurement.
F-26
Table of Contents
QuaDPharma
The following table represents a reconciliation of the contingent consideration liability related to the acquisition of QuaDPharma in 2014 measured on a recurring basis using level 3 inputs as of December 31, 2015 and 2016 (in thousands):
| Balance as of December 31, 2014 |
$ | 995 | ||
| Adjustment to fair value |
138 | |||
|
|
|
|||
| Balance as of December 31, 2015 |
1,133 | |||
| Adjustment to fair value |
(106 | ) | ||
| Satisfied through issuance of common stock |
(343 | ) | ||
| Paid in cash |
(684 | ) | ||
|
|
|
|||
| Balance as of December 31, 2016 |
$ | — | ||
|
|
|
The increase of the contingent consideration related to QuaDPharma was due to the time value of money from the initial measurement date (QuaDPharma acquisition date) to the final date of the payout. This adjustment to the contingent consideration liability is included within selling, general, and administrative expenses in the Company’s consolidated statements of operations and comprehensive loss. As of December 31, 2015, the QuaDPharma contingent consideration was classified as a current liability, as the Company was required to pay this obligation in 2016. On March 31, 2016, the Company exercised its option, to pay up to 50% of the earn-out liability in common stock, and issued 38,033 shares of common stock at $9.00 per share. In May 2016, cash payments totaling $0.7 million were made, satisfying the contingent consideration liability in full.
Polymed
The following table represents a reconciliation of the contingent consideration liability related to the acquisition of Polymed measured on a recurring basis using level 3 inputs as of December 31, 2015 and December 31, 2016:
| Acquisition date fair value of contingent consideration |
$ | 4,488 | ||
| Adjustment to fair value |
353 | |||
|
|
|
|||
| Balance as of December 31, 2015 |
4,841 | |||
| Adjustment to fair value |
159 | |||
| Satisfied through issuance of common stock |
(2,500 | ) | ||
| Paid in cash |
(2,500 | ) | ||
|
|
|
|||
| Balance as of December 31, 2016 |
$ | — | ||
|
|
|
The increase of the contingent consideration related to Polymed was due to the time value of money from the initial measurement date (Polymed acquisition date) to the final date of the payout. This adjustment to the contingent consideration liability is included within selling, general, and administrative expenses in the Company’s consolidated statements of operations and comprehensive loss. As of December 31, 2015, the Polymed contingent consideration was classified as a current liability as the Company was required to pay this obligation in 2016. On March 31, 2016, the Company exercised its option to pay up to 50% of the earn-out liability in common stock, and issued 277,777 shares of common stock at $9.00 per share. In April 2016, the remaining liability of $2.5 million was paid in cash.
| 13. | RELATED PARTY TRANSACTIONS |
During the years ended December 31, 2015 and 2016 and the three months ended March 31, 2016 and 2017, the Company entered into transactions with individuals and other companies that have financial interests in the Company. Related party transactions included the following:
| a. | The Company sold 2,520,000 shares of restricted stock to the executives of the Company: 1,200,000 in 2015 and 1,320,000 in 2014. To fund these stock purchases, the executives signed promissory notes in the amount |
F-27
Table of Contents
| of $6.6 million in 2015 and $6.0 million in 2014. The notes in 2015 purchased 1,200,000 shares at $5.50 share and the notes in 2014 purchased 1,320,000 shares at $4.55 per share. In an effort to retain the executives, it was negotiated that, based on the continued employment of those executives, the Company will forgive the notes over a three year period. Accordingly, the restricted shares vest and become non-restricted equally over the three year period. The Company has accounted for this related party transaction as a restricted stock offering, recognizing as an expense the value of the vested shares and the forgiveness of the notes over the 3-year period, contingent on the continued employment of the executive. The notes are reported as a reduction to additional paid-in capital. The Company accelerated the forgiveness of these promissory notes in 2016 and forgave the notes in full. The stock-based compensation expense recognized from these transactions was $4.0 million, $6.9 million $1.0 million and $0 and for the years ended December 31, 2015 and 2016 and the three months ended March 31, 2016 and 2017, respectively. Further, certain family members of executives hold unvested, restricted shares resulting from consulting services performed in years prior. |
| b. | Prior to the acquisition of CDE, certain directors, stockholders, and officers of the Company had a financial interest in CDE. Consequently, the Company’s board established a Special Committee of disinterested directors with authority to review, evaluate, negotiate, and approve or reject the terms and conditions of the transaction and to retain its own financial and legal advisors to assist in connection therewith. Following negotiation of the transaction between the committee and its advisors and representatives of CDE, the committee determined that the proposed terms of the transaction were fair from a financial point of view to the Company and approved the Company’s execution of the definitive agreements and consummation of the transactions contemplated thereby. |
| c. | In 2015, CDE signed an agreement with Avalon BioMedical (Management) (“Avalon”) under which Avalon will receive certain administrative services and will occupy space at CDE’s research location. Avalon reimburses CDE for these administrative services as incurred and pays CDE 30% of the total rent payment for the Hong Kong research and development facility (See Note 20—Commitments and Contingencies). Certain members of the Company’s board and management collectively have a controlling interest in Avalon. The Company does not hold any interest in Avalon and does not have any obligations to absorb losses or any rights to receive benefits from Avalon. As of December 31, 2015 and 2016 and March 31, 2017, Avalon held 678,880 shares of the Company’s common stock, which represents 1.7% of the Company’s total issued shares. Balances due from Avalon recorded on the consolidated balance sheets were not significant. |
| d. | The Company receives consulting and licensing revenue from PharmaEssentia, a company in which Athenex has an investment classified as available-for-sale (see Note 6—Fair Value Measurements). Revenue recorded from PharmaEssentia amounted to $0.1 million and $0 for the years ended December 31, 2015 and 2016, respectively and $0 and $0.5 million for the three months ended March 31, 2016 and 2017, respectively. |
| e. | The Company purchases certain pharmaceutical ingredients from Chongqing Taisheng Biotechnology Co., Ltd. (“Taisheng”), a company which is owned by a member of Athenex’s management. Purchases from Taisheng amounted to $0.1 million, $0.2 million, $0.2 million and $0 for the years ended December 31, 2015 and 2016 and the three months ended March 31, 2016 and 2017, respectively, and amounts owed to Taisheng were $0.2 million, $0.3 million, $0 and $0 as of the Polymed acquisition date, December 31, 2015, December 31, 2016, and March 31, 2017, respectively. |
| f. | The Company receives certain clinical development services from ZenRx Limited and subsidiaries (“ZenRx”), a company for which one of our executive officers serves on the board of directors. In connection with such services, the Company made payments to ZenRx of $0.2 million and less than $0.1 million for the years ended December 31, 2015 and 2016, respectively and $0 and $0 million for the three months ended March 31, 2016 and 2017, respectively. As of March 31, 2017, amounts owed to ZenRx were $0.2 million. In April 2013, the Company entered into a license agreement with ZenRx pursuant to which the Company granted an exclusive, sublicensable license to use certain of our intellectual property to develop and commercialize Oratecan and |
F-28
Table of Contents
| Oraxol in Australia and New Zealand, and a non-exclusive license to manufacture a certain compound, but only for use in Oratecan and Oraxol. ZenRx is responsible for all development, manufacturing and commercialization, and the related costs and expenses, of any product candidates resulting from the agreement. No revenue was earned from this license agreement in the periods presented in these consolidated financial statements. |
| g. | The Company receives certain consulting services from RSJ Consulting LLC (“RSJ”), a limited liability company for which one of our executive officers serves as the principal. Services incurred from RSJ amounted to $0.1 million and $0.2 million for the years ended December 31, 2015 and 2016, respectively, and less than $0.1 million and $0.1 million for the three months ended March 31, 2016 and 2017, respectively. |
| h. | The Company issued $20.0 million in convertible bonds in September 2016 to related parties. One of the holders of more than 5% of our outstanding common stock as of December 31, 2016, and an entity affiliated with one of our directors, were each issued $10.0 million in convertible bonds. Additionally, during the three months ended March 31, 2017 the Company issued $4.0 million in convertible bonds to two related parties. One of the holders of more than 5% of our outstanding common stock as of March 31, 2017 and a director of the Company were each issued $2.0 million in convertible bonds. |
| 14. | BUSINESS AND ECONOMIC COLLABORATIVE AGREEMENTS |
New York State
On May 1, 2015, the Company executed an agreement for a medical technology research, development, innovation, and commercialization alliance with Fort Schuyler Management Corporation (“FSMC”), a not-for-profit corporation existing under the laws of the State of New York (the “State”). The Company expects that $25 million will be invested by the State to build new corporate offices including a formulation lab with related equipment for the Company.
The Company, through its partnership with FSMC, Empire State Development (“ESD”), and The State University of New York (“SUNY”) Polytechnic, plans to execute a major expansion and establish a 315,000 square foot, ISO Class 5 high potency oral and sterile injectable pharmaceutical manufacturing facility in Dunkirk, New York. The Company expects that $200 million will be invested by the State to build the manufacturing plant. The Company does not have significant construction period risks and the State will fund a majority of the construction costs and hold ownership of the manufacturing and office facilities. As of December 31, 2016 and March 31, 2017, construction on these facilities had not yet been completed.
Chongqing Government Department of Economic Development
In October 2015, the Company completed and executed an agreement with the Banan District in Chongqing, China to construct one GMP API and one GMP pharmaceutical manufacturing plant on Banan sites identified and selected by the Company’s management. Under the terms of the agreement, Banan will provide the funding for the land and construction of the manufacturing plants according to Athenex specifications and the Company will equip the plant. This agreement allows the Company to expand its existing high potency oncology active pharmaceutical ingredient manufacturing capacity as well as its drug manufacturing capacity in China. The Company does not have significant construction period risks and the Banan District will fund a majority of the construction costs and hold ownership of the facilities. As of December 31, 2016 and March 31, 2017, construction on these facilities had not yet been completed.
In connection with these arrangements with FSMC and the Banan District we have committed to certain operational milestones. If we are unable to comply with such, we may lose access to these properties.
F-29
Table of Contents
| 15. | STOCKHOLDERS’ EQUITY |
Common Stock
As of December 31, 2015 and 2016 and March 31, 2017, 250 million common shares, par value $0.001, were authorized by the Company’s Board of Directors. The common shares are entitled to one vote per share and to receive dividends as declared.
During 2015, the Company issued 2,400,000 shares of common stock at $5.00 per share, 3,399,232 shares at $5.50 per share, 307,689 shares at $6.50 per share, 8,845,132 shares at $7.50 per share, 1,699,267 shares at $9.00 per share, and 29,760 shares from the exercise of options for a cumulative increase to equity of $114.3 million. During 2016, the Company issued 1,133,332 shares of common stock at $7.50 per share, 315,810 shares at $9.00 per share, 513,440 shares from the exercise of warrants and options, and 50,000 shares from the vesting of restricted stock units for a cumulative increase to equity of $19.9 million. During the three months ended March 31, 2017, the Company issued 16,800 shares of common stock from the exercise of options for a cumulative increase to equity of less than $0.1 million.
Treasury Stock
During 2016, the Company purchased 587,925 shares of common stock at a cost of $5.9 million. In 2016 and the three months ended March 31, 2017, the Company also purchased 646,667 and 16,000 shares of common stock, respectively, for de minimis amounts as the result of the cancellation of shares issued in connection with a restricted stock agreement.
Cost of Equity Raise
Costs incurred in raising equity, whether paid with cash or through the issuance of securities, are charged against the equity raised. These costs include legal fees and amounts paid to consultants and amounted to $0.1 million and $0 for the years ended December 31, 2015 and 2016, respectively. During 2016 and the three months ended March 31, 2017, the Company incurred $1.8 million and $1.1 million, respectively, of legal and consulting fees related to a potential initial public offering. These costs were deferred and included within other long-term assets on the consolidated balance sheet at $1.8 million and $2.9 million as of December 31, 2016 and March 31, 2017, respectively, and will be charged against the corresponding equity raised.
Common Stock Option Plans
The Company has three common stock option plans adopted in 2013, 2007 and 2004 (the “Plans”) which authorize the grant of up to 11,800,000 common stock options to employees, directors and consultants. Management has valued the options at their grant date using the Black-Scholes option pricing model. See Note 16—Stock-Based Compensation for more information.
F-30
Table of Contents
| 16. | STOCK-BASED COMPENSATION |
Options granted have a contractual term of 10 years and generally vest over a 2-4 year period. A limited number of options vest immediately in certain circumstances. The following table summarizes the status of the Company’s stock option activity granted under the Plans to employees, directors, and consultants (in thousands, except stock option amounts):
| Stock Options |
Weighted- Average Exercise price |
Weighted- Average Remaining Contractual Term |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding at December 31,2014 |
5,355,316 | $ | 4.48 | 7.47 | $ | 5,485 | ||||||||||
| Granted |
5,028,328 | 7.44 | — | |||||||||||||
| Exercised |
(29,760 | ) | 2.05 | — | ||||||||||||
| Forfeited |
(594,200 | ) | 5.38 | — | ||||||||||||
| Expired |
(2,000 | ) | 1.88 | — | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2015 |
9,757,684 | 5.96 | 7.95 | 29,687 | ||||||||||||
| Granted |
1,109,400 | 9.34 | — | |||||||||||||
| Exercised |
(13,440 | ) | 3.71 | — | ||||||||||||
| Forfeited |
(257,084 | ) | 2.67 | — | ||||||||||||
| Cancelled |
(1,280,000 | ) | 7.50 | — | ||||||||||||
| Expired |
(35,871 | ) | 2.09 | — | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2016 |
9,280,689 | 6.26 | 7.26 | 43,994 | ||||||||||||
|
|
|
|||||||||||||||
| Vested and exercisable at December 31,2016 |
6,845,754 | 5.62 | 6.73 | 36,798 | ||||||||||||
|
|
|
|||||||||||||||
| Granted |
16,000 | 11.00 | — | |||||||||||||
| Exercised |
(16,800 | ) | 2.57 | — | ||||||||||||
| Forfeited |
(64,900 | ) | 9.31 | — | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at March 31,2017 (unaudited) |
9,214,989 | $ | 6.25 | 7.01 | 43,743 | |||||||||||
|
|
|
|||||||||||||||
| Vested and exercisable at March 31, 2017 (unaudited) |
7,212,481 | $ | 5.74 | 6.60 | 37,948 | |||||||||||
|
|
|
|||||||||||||||
The total fair-value of stock options vested and recorded as compensation expense during the years ended December 31, 2015 and 2016 and during the three months ended March 31, 2016 and 2017 was $8.9 million, $11.0 million, $1.9 million and $1.6 million, respectively. As of December 31, 2015 and 2016 and March 31, 2017, $16.2 million, $11.0 million and $ 9.2 million of unrecognized cost related to non-vested stock options is expected to be recognized over a weighted-average period of approximately 2.1 years, 1.6 years and 1.4 years, respectively. The total intrinsic value of options exercised was approximately $0.2 million and $0.1 million for the years ended December 31, 2015 and 2016, respectively and was less than $0.1 million and $0.1 million for the three months ended March 31, 2016 and 2017, respectively.
The Company determines the fair value of stock-based awards on the grant date using the Black-Scholes option pricing model, which is impacted by assumptions regarding a number of highly subjective variables. The following table summarizes the weighted-average assumptions used as inputs to the Black-Scholes model during the periods indicated:
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Weighted average grant date fair value |
$ | 4.25 | $ | 5.53 | $ | 5.33 | $ | 6.45 | ||||||||
| Expected dividend yield |
— | % | — | % | — | % | — | % | ||||||||
| Expected stock price volatility |
62 | % | 65 | % | 65 | % | 65 | % | ||||||||
| Risk-free interest rate |
1.56 | % | 1.29 | % | 1.23 | % | 1.94 | % | ||||||||
| Expected life of options (in years) |
5.9 | 6.0 | 6.2 | 6.3 | ||||||||||||
F-31
Table of Contents
Employee Stock Grants
The Company also grants common stock to key officers and directors as additional compensation in certain circumstances. The fair value of these grants is recorded as compensation expense throughout the requisite service period (See Note 13—Related Party Transactions for further detail on these grants). Compensation cost recorded for the restricted stock grants amounted to $4.0 million and $8.5 million for the years ended December 31, 2015 and 2016, respectively and $1.0 million and $0 for the three months ended March 31, 2016 and 2017, respectively. For all other grants to officers and directors, the Company recorded compensation cost of $2.6 million and $0 during the years ended December 31, 2015 and 2016, respectively and $0 for each of the three months ended March 31, 2016 and 2017.
Restricted Stock
The following table summarizes restricted stock activity:
| Shares of Restricted Stock |
Weighted Average Fair Value |
|||||||
| Nonvested at January 1, 2015 |
965,554 | $ | 4.55 | |||||
| Granted |
1,200,000 | 5.50 | ||||||
| Vested |
(806,667 | ) | 5.00 | |||||
|
|
|
|||||||
| Nonvested at December 31, 2015 |
1,358,887 | 5.13 | ||||||
| Granted |
711,982 | 9.00 | ||||||
| Vested |
(1,408,887 | ) | 5.27 | |||||
|
|
|
|||||||
| Nonvested at December 31, 2016 |
661,982 | $ | 9.00 | |||||
|
|
|
|||||||
No restricted stock was granted or vested in the three months ended March 31, 2017.
Warrants
The Company has granted warrants to purchase common stock. The Company determined the fair value of the warrants on the grant date using the Black-Scholes option pricing model, consistent with the valuations of stock options described above. During August 2015, the Company repurchased 106,668 warrants for $0.4 million. All warrants are fully vested and 844,000 were outstanding as of December 31, 2015 and 344,000 were outstanding as of December 31, 2016 and March 31, 2017.
Stock-Based Compensation Cost
The components of stock-based compensation and the amounts recorded within research and development expenses and selling, general, and administrative expenses in the Company’s consolidated statements of operations and comprehensive loss consisted of the following for the years ended December 31, 2015 and 2016 and for the three months ended March 31, 2017 (in thousands):
| Year Ended December 31, |
Three Months Ended March 31, |
|||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Stock options |
$ | 8,932 | $ | 10,977 | $ | 1,864 | $ | 1,605 | ||||||||
| Restricted stock expense |
4,035 | 8,534 | 1,046 | 540 | ||||||||||||
| Stock awarded to directors and officers |
2,560 | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total stock-based compensation expense |
$ | 15,527 | $ | 19,511 | $ | 2,910 | $ | 2,145 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cost of sales |
$ | — | $ | — | $ | — | $ | 22 | ||||||||
| Research and development expenses |
$ | 5,600 | $ | 8,573 | 1,554 | 462 | ||||||||||
| Selling, general, and administrative expenses |
9,927 | 10,938 | 1,356 | 1,661 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total stock-based compensation expense |
$ | 15,527 | $ | 19,511 | $ | 2,910 | $ | 2,145 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-32
Table of Contents
| 17. | NET LOSS AND PROFORMA NET LOSS PER SHARE ATTRIBUTABLE TO ATHENEX, INC. COMMON STOCKHOLDERS |
Net Loss Per Share Attributable to Common Stockholders
Basic net loss per share is calculated by dividing net loss attributable to common stockholders by the weighted-average number of common shares issued, outstanding, and vested during the period. Diluted net loss per share is computed by dividing net loss attributable to common stockholders by the weighted-average number of common share and common shares equivalents for the period using the treasury-stock method. For the purposes of this calculation, warrants for common stock and stock options are considered to be common stock equivalents and are only included in the calculation of diluted net loss per share when their effect is dilutive.
The following outstanding shares of common stock equivalents were excluded from the calculation of diluted net loss per share attributable to common stockholders for the periods presented because including them would have been antidilutive:
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Stock options and other common stock equivalents |
10,601,684 | 9,624,689 | 10,721,632 | 9,558,989 | ||||||||||||
| Unvested restricted shares |
1,162,221 | 948,484 | 1,253,887 | 661,982 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total potential dilutive shares |
11,763,905 | 10,573,173 | 11,975,519 | 10,253,821 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
As described in Note 10—Debt, the Company issued bonds that, upon the completion of an IPO, will be converted into shares of common stock. Upon this event, the number of outstanding shares will increase significantly and will consequently effect net loss per share.
Unaudited Pro Forma Basic and Diluted Net Loss Per Share
The denominator of the pro forma basic and diluted net loss per share attributable to common stockholders reflects the (i) issuance of additional shares of common stock that the Company would be required to issue at an IPO price of $ per share in accordance with a licensing agreement (see below); and (ii) the issuance of additional shares of common stock upon the achievement of a performance condition within employment contracts that will be achieved upon the completion of an IPO; and (iii) the issuance of additional shares of common stock upon an IPO as the result of the vesting of certain restricted stock.
The numerator of the pro forma basic and diluted net loss per share attributable to common stockholders includes the stock-based compensation expense associated with the vesting of the stock options upon the achievement of a performance condition and excludes the cost related to the issuance of the common stock associated with licensing agreements upon the completion of an IPO. The pro forma basic and diluted net loss per share attributable to common stockholders does not include the shares expected to be sold and related proceeds to be received from the IPO.
In 2011, the Company entered into a license agreement with Hanmi Pharmaceutical Ltd. (“Hanmi”), a publicly traded company existing under the laws of South Korea for the right to use certain intellectual property (the “Orascovery program”) in various jurisdictions. Under this agreement, the Company may be obligated to issue Hanmi shares of common stock upon the occurrence of (1) regulatory approval of a drug under the Orascovery program and (2) an IPO. If an IPO occurs prior to a regulatory approval, the Company will issue common stock
F-33
Table of Contents
to Hanmi in the amount of $5.0 million as an “exit bonus.” Subsequently, when regulatory approval is achieved, the Company will issue common stock to Hanmi in the amount of $24.0 million as a “regulatory bonus.” Conversely, if regulatory approval of a drug under the Orascovery program occurs prior to an IPO, the Company will issue common stock to Hanmi in the amount of $24.0 million as a regulatory bonus. Subsequently, when an IPO occurs, the Company will issue common stock to Hanmi in the amount of $4.0 million to $10.0 million as an exit bonus, with the amount being determined on a sliding scale derived from the pre-money valuation of the Company immediately prior to the IPO. The occurrence of these events has not yet been deemed to be probable and therefore, no liability has been recorded as of December 31, 2015 and 2016 and March 31, 2017.
The following table sets forth the computation of the Company’s unaudited pro forma basic and diluted net loss per share attributable to common stockholders for the following periods (in thousands, except share and per share data):
| Year Ended December 31, 2016 |
Three Months Ended March 31, 2017 |
|||||||
| (unaudited) | (unaudited) | |||||||
| Numerator: |
||||||||
| Net loss attributable to common stockholders |
$ | $ | ||||||
| Expense associated with the vesting of stock options and restricted stock upon the completion of an IPO per employment agreements and restricted stock agreements |
||||||||
|
|
|
|
|
|||||
| Net loss attributable to common stockholders used in computing pro forma net loss per share attributable to common stockholders, basic and diluted |
$ | $ | ||||||
|
|
|
|
|
|||||
| Denominator: |
||||||||
| Weighted-average shares used in computing net loss per share attributable to common stockholders, basic and diluted |
||||||||
| Weighted-average pro forma adjustment to reflect issuance of common shares in connection with employment and license agreements |
||||||||
| Weighted-average pro forma adjustment to reflect issuance of common shares resulting from vesting of restricted stock upon IPO |
||||||||
| Weighted-average pro forma adjustment to reflect issuance of common shares in connection with the convertible bonds |
||||||||
|
|
|
|
|
|||||
| Weighted-average shares used in computing pro forma net loss per share attributable to common stockholders, basic and diluted |
||||||||
|
|
|
|
|
|||||
| Pro forma net loss per share attributable to common stockholders, basic and diluted |
$ | $ | ||||||
|
|
|
|
|
|||||
F-34
Table of Contents
| 18. | ACCUMULATED OTHER COMPREHENSIVE INCOME (LOSS) |
The components and changes of accumulated other comprehensive income (loss), net of related income tax effects, are as follows (in thousands):
| Balance as of December 31, 2014 |
$ | 83 | ||
| Foreign currency translation adjustment |
(397 | ) | ||
| Unrealized gains on investment |
91 | |||
|
|
|
|||
| Balance as of December 31, 2015 |
(223 | ) | ||
| Foreign currency translation adjustment |
(1,048 | ) | ||
| Unrealized gains on investment |
(33 | ) | ||
|
|
|
|||
| Balance as of December 31, 2016 |
(1,304 | ) | ||
| Foreign currency translation adjustment |
499 | |||
| Unrealized gains on investment |
3 | |||
|
|
|
|||
| Balance as of March 31, 2017 (unaudited) |
$ | (802 | ) | |
|
|
|
| 19. | BUSINESS SEGMENT, GEOGRAPHIC, AND CONCENTRATION RISK INFORMATION |
The Company has three operating segments, which are organized based mainly on the nature of the business activities performed and regulatory environments in which they operate. The Company also considers the types of products from which the reportable segments derive their revenue (only applicable to two reportable segments). Each operating segment has a segment manager who is held accountable for operations and has discrete financial information that is regularly reviewed by the Company’s chief operating decision-maker. The Company’s operating segments are as follows:
Oncology Innovation Platform—This primary operating segment performs research and development on certain of the Company’s proprietary drugs, from the preclinical development of its chemical compounds, to the execution and analysis of its several clinical trials. This segment focuses specifically on the oral absorption cancer drug platform, the Src Kinase inhibitors, and the transmucosal drug delivery system. This segment performs research in the United States, Taiwan, Hong Kong, and mainland China.
Global Supply Chain Platform—This operating segment includes QuaDPharma and Polymed. QuaDPharma is a contract manufacturing company that provides small to mid-scale cGMP manufacturing of clinical and commercial products for pharmaceutical and biotech companies. QuaDPharma also performs microbiological and analytical testing for raw material and formulated products and is expanding to manufacture and sell pharmaceutical products under 503B regulations set forth by the U.S. Food and Drug Administration (“FDA”). Polymed markets and sells API and medical devices in North America, Europe, and Asia from its locations in Texas and China. Polymed also develops new compounds, processing techniques, and manufactures API at Taihao, a cGMP facility in Chongqing, China. A majority of the Company’s revenue is generated by this segment. The pharmaceutical manufacturing facilities being built in the Banan District in Chongqing, China (see Note 14—Business and Economic Collaborative Agreements) will be included within this segment and the Company anticipates that this segment will support the Oncology Innovation Platform segment when drugs in development are approved for market.
Commercial Platform—This operating segment includes Athenex Pharmaceutical Division, a newly-formed component that is focused on the manufacturing, distribution, and sales of generic pharmaceuticals. This segment provides services and products to external customers based mainly in the United States.
The segments operate in North America and Asia. The Company’s Oncology Innovation Platform segment operates and holds long-lived assets located in the United States, Taiwan, Hong Kong, and mainland China. The Commercial Platform segment operates and holds long-lived assets located in the United States. The Global
F-35
Table of Contents
Supply Chain Platform segment operates and holds long-lived assets located in the United States and China. For geographic segment reporting, product sales have been attributed to countries based on the location of the customer.
Segment information is as follows (in thousands):
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Net loss attributable to Athenex, Inc.: |
||||||||||||||||
| Oncology Innovation Platform |
$ | (50,257 | ) | $ | (64,837 | ) | $ | (10,415 | ) | $ | (28,259 | ) | ||||
| Global Supply Chain Platform |
(343 | ) | (11 | ) | (203 | ) | (1,148 | ) | ||||||||
| Commercial Platform |
— | (22,867 | ) | — | (11,581 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total consolidated net loss attributable to Athenex, Inc. |
$ | (50,600 | ) | $ | (87,715 | ) | $ | (10,618 | ) | $ | (40,988 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Total revenue: |
||||||||||||||||
| Oncology Innovation Platform |
$ | 973 | $ | 998 | $ | 141 | $ | 733 | ||||||||
| Global Supply Chain Platform |
17,998 | 26,581 | 5,356 | 6,296 | ||||||||||||
| Commercial Platform |
— | — | — | 77 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue for reportable segments |
18,971 | 27,579 | 5,497 | 7,106 | ||||||||||||
| Intersegment revenue |
(5,027 | ) | (7,028 | ) | (868 | ) | (2,525 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total consolidated revenue |
$ | 13,944 | $ | 20,551 | $ | 4,629 | $ | 4,581 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Total revenue by product group: |
||||||||||||||||
| API sales |
$ | 9,179 | $ | 15,331 | $ | 3,963 | $ | 3,000 | ||||||||
| Medical device sales |
1,966 | 2,338 | 357 | 335 | ||||||||||||
| Contract manufacturing revenue |
1,464 | 1,497 | 168 | 437 | ||||||||||||
| Commercial product sales |
224 | 228 | — | 128 | ||||||||||||
| License fees |
297 | 392 | 95 | 598 | ||||||||||||
| Grant revenue |
814 | 765 | 46 | 83 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total consolidated revenue |
$ | 13,944 | $ | 20,551 | $ | 4,629 | $ | 4,581 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Intersegment revenue is recorded by the selling segment when it is realized or realizable and all revenue recognition criteria are met. Upon consolidation, all intersegment revenue and related cost of sales are eliminated from the selling segment’s ledger.
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Total depreciation and amortization: |
||||||||||||||||
| Oncology Innovation Platform |
$ | 101 | $ | 195 | $ | 47 | $ | 92 | ||||||||
| Global Supply Chain Platform |
787 | 1,644 | 370 | 526 | ||||||||||||
| Commercial Platform |
— | 187 | — | 198 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total consolidated depreciation and amortization |
$ | 888 | $ | 2,026 | $ | 417 | $ | 816 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-36
Table of Contents
| December 31, | March 31, 2017 |
|||||||||||
| 2015 | 2016 | |||||||||||
| (unaudited) | ||||||||||||
| Total assets: |
||||||||||||
| Oncology Innovation Platform |
$ | 73,837 | $ | 53,022 | $ | 40,372 | ||||||
| Global Supply Chain Platform |
46,594 | 48,560 | 49,459 | |||||||||
| Commercial Platform |
— | 4,308 | 10,672 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total consolidated assets |
$ | 120,431 | $ | 105,890 | $ | 100,503 | ||||||
|
|
|
|
|
|
|
|||||||
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Total revenue: |
||||||||||||||||
| United States |
$ | 3,270 | $ | 3,573 | $ | 502 | $ | 713 | ||||||||
| India |
4,914 | 7,803 | 1,955 | 1,471 | ||||||||||||
| Austria |
2,323 | 5,197 | 1,392 | 1,002 | ||||||||||||
| China |
2,366 | 2,338 | 456 | 312 | ||||||||||||
| Taiwan |
84 | — | — | 500 | ||||||||||||
| Other foreign countries |
987 | 1,640 | 324 | 583 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total consolidated revenue |
$ | 13,944 | $ | 20,551 | $ | 4,629 | $ | 4,581 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| December 31, | March 31, 2017 |
|||||||||||
| 2015 | 2016 | |||||||||||
| (unaudited) | ||||||||||||
| Total property and equipment, net: |
||||||||||||
| United States |
$ | 2,621 | $ | 2,177 | $ | 3,930 | ||||||
| China |
3,668 | 3,633 | 3,715 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total consolidated property and equipment, net |
$ | 6,289 | $ | 5,810 | $ | 7,645 | ||||||
|
|
|
|
|
|
|
|||||||
Customer revenue and accounts receivable concentration amounted to the following for the identified periods. All customers relate to the Global Supply Chain Platform Segment.
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||
| 2015 | 2016 | 2016 | 2017 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Percentage of total revenue by customer: |
||||||||||||||||
| Customer A |
29 | % | 38 | % | 42 | % | 28 | % | ||||||||
| Customer B |
17 | % | 24 | % | 30 | % | 22 | % | ||||||||
| December 31, | March 31, 2017 |
|||||||||||
| 2015 | 2016 | |||||||||||
| (unaudited) | ||||||||||||
| Percentage of total accounts receivable by customer: |
||||||||||||
| Customer A |
46 | % | 50 | % | 28 | % | ||||||
| Customer B |
12 | % | 9 | % | 19 | % | ||||||
| Customer C |
11 | % | 0 | % | 0 | % | ||||||
F-37
Table of Contents
| 20. | COMMITMENTS AND CONTINGENCIES |
Rental and lease commitments
In August 2015, the Company entered into a lease agreement with FSMC to occupy a portion of the Conventus Center for Collaborative Medicine in Buffalo, NY. A deferred rent liability for this agreement of $0.3 million, $0.9 million and $1.0 million was recorded as of December 31, 2015, December 31, 2016 and March 31, 2017, respectively. Total rent expense related to this location, recognized on a straight-line basis, for the years ended December 31, 2015 and 2016 were $0.4 and $1.0 million, respectively, and $0.3 million for both of the three months ended March 31, 2016 and 2017.
In July 2015, CDE entered into an agreement to lease facilities in Hong Kong. Under the rental agreement, CDE will make monthly payments of less than $0.1 million for three years beginning on July 1, 2015. Total rent expense related to this location, recognized on a straight-line basis, amounted to $0.2 million and $0.3 million for the years ended December 31, 2015 and 2016, respectively, and $0.1 million for both of the three months ended March 31, 2016 and 2017.
In October 2016, the Company’s Commercial Platform entered into an agreement to lease office space in Chicago, IL. Under the lease agreement, the Company will make monthly payments based on an escalating scale over ten years. Total rent expense related to this location, recognized on a straight-line basis, amounted to less than $0.1 million for each of the year ended December 31, 2016 and the three months ended March 31, 2017. The Company has recorded a deferred rent liability of less than $0.1 million as of December 31, 2016 and $0.1 million as of March 31, 2017. In lieu of a security deposit, the Company issued an irrevocable letter of credit to the landlord in the amount of $0.3 million.
Future minimum payments under the non-cancelable leases consists of the following as of December 31, 2016 (in thousands):
| Year ending December 31: |
Minimum payments | |||
| 2017 |
$ | 801 | ||
| 2018 |
1,162 | |||
| 2019 |
1,512 | |||
| 2020 |
1,518 | |||
| 2021 |
1,525 | |||
| Thereafter |
5,959 | |||
|
|
|
|||
| $ | 12,477 | |||
|
|
|
|||
Legal Proceedings
The Company is not a party to any pending or known threatened legal proceedings that, in the opinion of the Company, would have a material impact on the Company’s consolidated financial statements.
| 21. | SUBSEQUENT EVENTS |
Subsequent Events (Annual)
The Company evaluated subsequent events through May 1, 2017, the date on which the December 31, 2016 consolidated financial statements were issued.
Subsequent Events (Interim)
For the three months ended March 31, 2017, the Company evaluated events through May 18, 2017, the date on which these consolidated interim financial statements were issued.
F-38
Table of Contents
In January and February 2017, the Company issued additional convertible bonds with an aggregate value of $10.0 million and a maturity date of October 1, 2018, of which $4.0 million were issued to related parties. The bonds will be converted into shares of common stock upon the completion of an IPO. If such event does not occur prior to the maturity date, interest will be payable based on a 10% annual stated interest rate.
In February 2017, the Company entered into a definitive agreement with Amphastar Pharmaceuticals, Inc., or Amphastar, to acquire 14 ANDAs and inventory for certain APIs. The agreement requires payments of up to $6.4 million, of which $1.0 million was paid upon execution of the agreement, $1.0 million is due within thirty days following May 1, 2017, $3.0 million is due within thirty days of receiving FDA approval of site transfer to sell Prochlorperazine Edisylate Injection USP, and $1.4 million is due within thirty days of receiving FDA approval of site transfer of a second product. If the conditions relating to the third and fourth payments described above are not met by December 31, 2017, the Company shall make such payments within 30 days of December 31, 2017 regardless of whether such conditions are ever met. In addition to the payments described above, the Company has agreed to pay Amphastar a royalty fee equal to 2% of its net sales relating to the 14 ANDAs and API inventory transferred to it by Amphastar for a period of 10 years from the execution of the agreement.
In February 2017, the Company entered into an additional binding term sheet with Gland Pharma Limited, pursuant to which we expect to enter into a definitive agreement for a non-exclusive license to market an additional 6 of Gland’s products. Gland has obtained FDA approval for 4 of such products and has filed an ANDA in North America for the Remaining 2 products. For each of the licensed products we will pay a license fee to Gland. The maximum amount of such licenses is $3.15 million, of which $312,500 is contingent on regulatory approval for 2 products for which Gland has filed ANDAs. We will pay a portion of such $312,500 upon regulatory approval of each of these 2 licensed drugs. Additionally, during the term of the agreement we have a profit sharing arrangement pursuant to which we will pay to Gland between 25% and 40% of the net profits from sales of each of the licensed products, depending on the product. The initial term the Gland license agreement is five years from the launch of each product licensed pursuant to the agreement, subject to automatic renewal for additional two year terms, unless terminated by either party upon provision to the other party at least 90 days’ notice in advance of a renewal date.
In March 2017, the Company signed an amendment to its license agreement with Hanmi, under which the Company received the rights to develop and sell drugs under the Orascovery program in additional territories, including Japan. This license amendment required an upfront fee of $7.0 million payable to Hanmi upon the execution of the agreement. In lieu of the payment, the Company issued a convertible bond to Hanmi with a par value of $7.0 million. This bond carries an interest rate of 10% per annum and a maturity date of October 1, 2018. Hanmi has the option to convert the bond to shares of common stock at discounted rates from 20.0% to 22.5% at various dates up to the maturity date. This amendment includes additional regulatory milestone payments and royalties based on sales. The occurrence of any milestone triggering events have not been deemed to be probable as no sales have occurred.
In April 2017, the Company issued additional convertible bonds with an aggregate value of $20.0 million and a maturity date of April 20, 2019. The bonds will be converted into shares of common stock upon the completion of an IPO. If such event does not occur prior to the maturity date, interest will be payable based on a 10% annual stated interest rate.
******
F-39
Table of Contents
To the Board of Directors
Athenex, Inc.
Buffalo, New York
Report on the Financial Statements
We have audited the accompanying combined financial statements of Polymed Therapeutics, Inc. and Chongqing Taihao Pharmaceutical Co., Ltd. (collectively “the Company”), which comprise the combined balance sheets as of December 31, 2014 and 2013, the related combined statements of operations and comprehensive loss, changes in stockholders’ equity and cash flows for the years then ended, and the related notes to the combined financial statements (collectively, the financial statements).
Management’s Responsibility for the Financial Statements
Management is responsible for the preparation and fair presentation of these combined financial statements in accordance with accounting principles generally accepted in the United States of America; this includes the design, implementation and maintenance of internal control relevant to the preparation and fair presentation of financial statements that are free from material misstatement, whether due to fraud or error.
Auditor’s Responsibility
Our responsibility is to express an opinion on these combined financial statements based on our audits. We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the financial statements. The procedures selected depend on the auditor’s judgment, including the assessment of the risks of material misstatement of the financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity’s preparation and fair presentation of the financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. Accordingly, we express no such opinion. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluating the overall presentation of the financial statements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the combined financial statements referred to above present fairly, in all material respects, the financial position of Polymed Therapeutics, Inc. and Chongqing Taihao Pharmaceutical Co., Ltd. as of December 31, 2014 and 2013, and the results of their operations and their cash flows for the years then ended in accordance with accounting principles generally accepted in the United States of America.
/s/ Freed Maxick CPAs, P.C.
Buffalo, New York
December 13, 2016
F-40
Table of Contents
POLYMED THERAPEUTICS, INC. AND CHONGQING TAIHAO PHARMACEUTICAL CO., LTD.
COMBINED BALANCE SHEETS
(In thousands, except share data)
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 465 | $ | 95 | ||||
| Accounts receivable, net of allowance for doubtful accounts of $50 and $50, respectively |
4,125 | 960 | ||||||
| Receivables from related parties |
232 | 223 | ||||||
| Inventories |
2,499 | 1,593 | ||||||
| Prepaid expenses |
179 | 250 | ||||||
| Other receivable |
241 | 215 | ||||||
|
|
|
|
|
|||||
| Total current assets |
7,741 | 3,336 | ||||||
| Property and equipment, net |
1,296 | 1,273 | ||||||
| Deferred tax asset |
646 | 575 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 9,683 | $ | 5,184 | ||||
|
|
|
|
|
|||||
| Liabilities and stockholders’ equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 4,586 | $ | 4,218 | ||||
| Accrued expenses |
439 | 1,166 | ||||||
| Line of credit |
750 | 360 | ||||||
| Notes payable |
1,303 | 745 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
7,078 | 6,489 | ||||||
| Long-term liabilities: |
||||||||
| Loans from related parties |
1,705 | 1,706 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
8,783 | 8,195 | ||||||
| Commitments and contingencies (Note 7) |
||||||||
| Stockholders’ equity |
||||||||
| Common stock—Polymed, no par value, 1,000,000 shares authorized, 100,000 issued and outstanding at December 31, 2014 and 2013. |
100 | 100 | ||||||
| Owners’ equity—Taihao |
1,876 | 1,390 | ||||||
| Additional paid-in capital—Taihao |
4,226 | 65 | ||||||
| Receivable from stockholder—Taihao |
(288 | ) | (123 | ) | ||||
| Accumulated deficit |
(5,036 | ) | (4,438 | ) | ||||
| Accumulated other comprehensive income (loss) |
22 | (5 | ) | |||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
900 | (3,011 | ) | |||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 9,683 | $ | 5,184 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these combined financial statements.
F-41
Table of Contents
POLYMED THERAPEUTICS, INC. AND CHONGQING TAIHAO PHARMACEUTICAL CO., LTD.
COMBINED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands)
| Year Ended December 31, | ||||||||
| 2014 | 2013 | |||||||
| Revenue: |
||||||||
| Product sales |
$ | 10,218 | $ | 9,772 | ||||
|
|
|
|
|
|||||
| Total revenue |
10,218 | 9,772 | ||||||
|
|
|
|
|
|||||
| Costs and operating expenses: |
||||||||
| Cost of product sales |
7,222 | 6,171 | ||||||
| General and administrative expenses |
2,229 | 3,533 | ||||||
| Selling and marketing expenses |
360 | 436 | ||||||
| Research and development expenses |
894 | — | ||||||
|
|
|
|
|
|||||
| Total costs and operating expenses |
10,705 | 10,140 | ||||||
|
|
|
|
|
|||||
| Operating loss |
(487 | ) | (368 | ) | ||||
|
|
|
|
|
|||||
| Interest expense |
183 | 110 | ||||||
|
|
|
|
|
|||||
| Loss before income taxes |
(670 | ) | (478 | ) | ||||
| Income tax expense (benefit) |
(72 | ) | 32 | |||||
|
|
|
|
|
|||||
| Net loss |
(598 | ) | (510 | ) | ||||
|
|
|
|
|
|||||
| Foreign currency translation adjustment, net of tax effect of zero |
27 | (39 | ) | |||||
|
|
|
|
|
|||||
| Comprehensive loss |
$ | (571 | ) | $ | (549 | ) | ||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these combined financial statements.
F-42
Table of Contents
POLYMED THERAPEUTICS, INC. AND CHONGQING TAIHAO PHARMACEUTICAL CO., LTD.
COMBINED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(In thousands)
| Common stock - Polymed |
Owners’ equity - Taihao |
Additional paid-in capital - Taihao |
Receivable from shareholder - Taihao |
Accumulated deficit |
Accumulated other comprehensive income (loss), net of tax effect |
Total stockholders’ equity |
||||||||||||||||||||||
| Balance at December 31, 2012 |
$ | 100 | $ | 1,390 | $ | 65 | $ | — | $ | (3,928 | ) | $ | 34 | $ | (2,339 | ) | ||||||||||||
| Net loss |
— | — | — | — | (510 | ) | — | (510 | ) | |||||||||||||||||||
| Receivable from stockholder |
— | — | — | (123 | ) | — | — | (123 | ) | |||||||||||||||||||
| Other comprehensive loss, net of tax |
— | — | — | — | — | (39 | ) | (39 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2013 |
100 | 1,390 | 65 | (123 | ) | (4,438 | ) | (5 | ) | (3,011 | ) | |||||||||||||||||
| Capital contribution |
— | 486 | 4,161 | — | — | — | 4,647 | |||||||||||||||||||||
| Net loss |
— | — | — | — | (598 | ) | — | (598 | ) | |||||||||||||||||||
| Receivable from stockholder |
— | — | — | (165 | ) | — | — | (165 | ) | |||||||||||||||||||
| Other comprehensive income, net of tax |
— | — | — | — | — | 27 | 27 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2014 |
$ | 100 | $ | 1,876 | $ | 4,226 | $ | (288 | ) | $ | (5,036 | ) | $ | 22 | $ | 900 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The accompanying notes are an integral part of these combined financial statements.
F-43
Table of Contents
POLYMED THERAPEUTICS, INC. AND CHONGQING TAIHAO PHARMACEUTICAL CO., LTD.
COMBINED STATEMENTS OF CASH FLOW
(In thousands)
| Year Ended December 31, |
||||||||
| 2014 | 2013 | |||||||
| Cash flows from operating activities |
||||||||
| Net loss |
(598 | ) | (510 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation and amortization |
300 | 312 | ||||||
| Deferred income taxes |
(72 | ) | 32 | |||||
| Changes in operating assets and liabilities: |
||||||||
| Receivables, net |
(3,165 | ) | (471 | ) | ||||
| Receivables from related parties |
(175 | ) | (346 | ) | ||||
| Prepaid expenses and other current assets |
45 | (128 | ) | |||||
| Inventories, net |
(906 | ) | 439 | |||||
| Accounts payable and accrued expenses |
(359 | ) | (248 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(4,930 | ) | (920 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from investing activities |
||||||||
| Purchase of property and equipment |
(327 | ) | (446 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
(327 | ) | (446 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from financing activities |
||||||||
| Proceeds from capital contributions |
4,647 | — | ||||||
| Proceeds from related party loans |
— | 611 | ||||||
| Proceeds from line of credit |
390 | 360 | ||||||
| Payment of notes payable |
(739 | ) | (569 | ) | ||||
| Proceeds from notes payable |
1,313 | 725 | ||||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
5,611 | 1,127 | ||||||
|
|
|
|
|
|||||
| Net increase (decrease) in cash and cash equivalents |
354 | (239 | ) | |||||
| Cash and cash equivalents, beginning of year |
95 | 394 | ||||||
| Effect of exchange rate changes on cash and cash equivalents |
16 | (60 | ) | |||||
|
|
|
|
|
|||||
| Cash and cash equivalents, end of year |
$ | 465 | $ | 95 | ||||
|
|
|
|
|
|||||
| Supplemental cash flow disclosures: |
||||||||
| Interest paid |
$ | 110 | $ | 183 | ||||
| Income taxes paid |
$ | 32 | $ | — | ||||
| Non-cash investing activities: |
||||||||
| Accrued purchases of property and equipment |
$ | 49 | $ | 34 | ||||
The accompanying notes are an integral part of these combined financial statements.
F-44
Table of Contents
POLYMED THERAPEUTICS, INC. AND CHONGQING TAIHAO PHARMACEUTICAL CO., LTD.
NOTES TO COMBINED FINANCIAL STATEMENTS
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
| 1. | COMPANY AND NATURE OF BUSINESS |
Description of Business
Polymed Therapeutics, Inc (“Polymed”) and Chongqing Taihao Pharmaceutical Co Ltd (“Taihao”) (collectively “the Company”) commenced operations in 2004. Though operated independently, they were related under common control during the years ended December 31, 2013 and 2014, as well as subsequently until the Company was acquired by Athenex, Inc. in June 2015 (See Note 11—Subsequent Events). Located in Texas, United States, Polymed is an S-corporation that markets and sells Active Pharmaceutical Ingredients (“API”) and medical devices in North America, Europe, and India. Taihao is an API manufacturing company located in Chongqing, China. Taihao owns and operates a current Good Manufacturing Practices (“cGMP”) facility where it manufactures API to be sold in China and abroad. Polymed facilitates the sale of Taihao’s product to their customers outside of China.
Significant Risks and Uncertainties
The Company incurred losses during the years ended December 31, 2014 and 2013 and had an accumulated deficit of $5.0 million as of December 31, 2014. Operations have been funded primarily through stockholders’ investments, borrowings, and the sale of the Company’s products.
The Company is subject to the risks inherent in conducting business globally and under the laws, regulations, and customs of various jurisdictions. These risks include, but are not limited to, changes in laws, regulations, and practices affecting the pharmaceutical and healthcare industries, adverse changes in the economies in which the Company or its partners and suppliers operate, fluctuation in exchange rates for transactions conducted in currencies other than the functional currency, differing local product preferences and product requirements, supply disruptions and increases in energy, insurance, and transportation costs, and compliance with a variety of national and local laws of countries in which the Company does business, including the restrictions on the import and export of certain intermediates, drugs, and technologies. If any or all of these events occur, the Company could struggle to generate sufficient product revenue and achieve profitability.
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation and Principles of Combination
These combined financial statements reflect the accounts and operations of Polymed and Taihao, which were related under common control as of and for the years ended December 31, 2014 and 2013. During the years ended December 31, 2014 and 2013, Polymed owned between 20 and 25% of the outstanding capital of Taihao and one of the shareholders of Polymed owned between 50 and 75% of the outstanding capital of Taihao. Intercompany transactions and balances have been eliminated.
Use of Estimates
These combined financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities as of the date of the combined financial statements and the reported amount of revenue and expenses during the reporting period. Such management estimates include those relating to assumptions used in allowances for doubtful accounts, income taxes, and the estimated useful life and recoverability of long-lived assets. Actual results could differ from those estimates.
F-45
Table of Contents
Functional Currency
Assets and liabilities of Taihao, which prepares financial statements in currencies other than the U.S. dollar (Chinese Yuan is considered the functional currency of Taihao), are translated using exchange rates as of the balance sheet date and the statements of operations are translated at the average exchange rates for each reporting period. The Company recorded a foreign currency translation gain/loss of less than $0.1 million in the years ended December 31, 2014 and 2013.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less at the date of purchase to be cash equivalents. The Company deposits its cash primarily in checking or money market accounts. The Company generally does not enter into investments for trading or speculative purposes, rather to preserve its capital for the purpose of funding operations.
Accounts Receivable, net
Accounts receivable are recorded at the invoiced amount net of an allowance for doubtful accounts, if necessary. On a periodic basis, the Company evaluates its accounts receivable and establishes an allowance for doubtful accounts, based upon a history of past write-off, the age of the receivables, and current credit conditions.
Inventories
Inventory is stated at the lower-of-cost-or-market, with cost being determined on a weighted average basis.
Property and Equipment, net
Property and equipment are initially recorded at cost. Depreciation is recorded over the estimated useful lives of the related assets using the straight-line method. Leasehold improvements are amortized on a straight-line basis over the shorter of the useful life or term of the lease. Upon retirement or disposal, the cost and related accumulated depreciation are removed from the combined balance sheets and the resulting gain or loss is recorded to general and administrative expense in the combined statements of operations. Routine expenditures for maintenance and repairs are expensed as incurred.
Estimated useful lives for property and equipment are as follows:
| Property and Equipment |
Estimated Useful Life | |||
| Plant and Machinery |
5 - 10 years | |||
| Office Equipment |
5 - 10 years | |||
| Leasehold improvements |
Lesser of 5 years or remaining lease term | |||
Impairment of Long-Lived Assets
The Company reviews the recoverability of its long-lived assets when events or changes in circumstances occur that indicate that the carrying value of the asset may not be recoverable. The assessment of possible impairment is based on the ability to recover the carrying value of the assets from the expected future cash flows (undiscounted and without interest expense) of the related operations. If these cash flows are less than the carrying value of such assets, an impairment loss for the difference between the estimated fair value and carrying value is recorded. There was no impairment loss recognized during the years ended December 31, 2014 and 2013.
F-46
Table of Contents
Fair Value of Financial Instruments
Financial instruments consist of cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities, and debt. Cash equivalents are stated at fair value. Accounts receivable, accounts payable, accrued liabilities, and debt are stated at their carrying value, which approximates fair value due to the short time to the expected receipt or payment date of such amounts.
Revenue Recognition
The Company recognizes product revenue when there is persuasive evidence of an arrangement, the price is fixed or determinable, collectability is reasonably assured, and upon delivery to and acceptance by customers.
Comprehensive Income (Loss)
Comprehensive income (loss) is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. Foreign currency translation adjustments represent the differences between the Company’s net loss and comprehensive loss.
Income Taxes
Taihao
Taihao uses the asset and liability method of accounting for income taxes. Deferred income tax assets and liabilities are recognized for the estimated future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply in the years in which those temporary differences are expected to be recovered or settled. Deferred income tax expense or benefit is the result of changes in the deferred income tax assets and liabilities. Valuation allowances are established when necessary to reduce deferred income tax assets where, based upon the available evidence, management concludes that it is more-likely-than not that the deferred income tax assets will not be realized. In evaluating its ability to recover deferred income tax assets, Taihao considers all available positive and negative evidence, including its operating results, ongoing tax planning and forecasts of future taxable income on a jurisdiction-by-jurisdiction basis.
Reserves are provided for tax benefits for which realization is uncertain. Such benefits are only recognized when the underlying tax position is considered more likely than not to be sustained on examination by a taxing authority, assuming they possess full knowledge of the position and facts. Interest and penalties related to uncertain tax positions are recognized in income tax (benefit) expense; however, Taihao currently has no interest or penalties related to income taxes. Taihao files income tax returns in the People’s Republic of China.
Polymed
Polymed has elected to have its income taxed as an S Corporation. The tax regulations provide that, in lieu of corporation income taxes, the S Corporation stockholders are taxed on their proportionate share of Polymed’s taxable income; consequently, no federal income taxes have been provided on S Corporation current income.
In accordance with US GAAP, the Company adopted the provisions of Accounting for Uncertainty in Income Taxes. As a result of implementation, the Company recognizes no increase in a liability for unrecognized tax expense and as a result recognized no interest or penalties. Polymed files income tax returns in the U.S. federal and state jurisdictions, whereas Taihao files income tax returns in China.
F-47
Table of Contents
Concentration of Credit Risk, Other Risks and Uncertainties
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents. Cash and cash equivalents are deposited in interest-bearing money market accounts. Although the Company deposits the cash with multiple financial institutions, cash balances may occasionally be in excess of the amounts insured by the Federal Deposit Insurance Corporation. The Company also has significant assets and liabilities held in its overseas biomanufacturing facility in China and therefore is subject to foreign currency fluctuation.
The Company had certain customers whose revenue individually represented 10% or more of the Company’s total revenue, or whose accounts receivable balances individually represented 10% or more of the Company’s total accounts receivable, as follows:
For the years ended December 31, 2014 and 2013, one customer accounted for 35% and three customers accounted for 63% of revenue, respectively. One customer accounted for 27% and two customers accounted for 33% of accounts receivable as of December 31, 2014 and 2013, respectively.
The Company has one major vendor that accounted for 32% and 12% of cost of product sales for the years ended December 31, 2014 and 2013, respectively. This vendor accounted for 15% and 27% of accounts payable as of December 31, 2014 and 2013, respectively.
Recent Accounting Pronouncements Not Yet Adopted
In May 2014, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (“ASU”) No. 2014-09, “Revenue from Contracts with Customers (Topic 606)”, which requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. The ASU will replace most existing revenue recognition guidance in U.S. GAAP when it becomes effective. The new standard is effective for the Company on January 1, 2018 with early adoption permitted only for annual reporting periods beginning after December 15, 2016. The standard permits the use of either the retrospective or cumulative effect transition method. The Company is evaluating the effect of this standard on its combined financial statements. The Company has not yet selected a transition method nor has it determined the effect of the standard on its ongoing financial reporting.
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern” which requires management to assess if there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures in certain circumstances. In connection with each annual and interim period, management must assess if there is substantial doubt about an entity’s ability to continue as a going concern within one year after the financial statement issuance date. Disclosures are required if conditions give rise to substantial doubt. The amendment is effective for the Company on January 1, 2017 with early adoption permitted for annual or interim reporting periods for which the financial statements have not previously been issued. The Company is evaluating the effect of this standard on its combined financial statements.
In July 2015, the FASB issued ASU No. 2015-11, “Inventory (Topic 330): Simplifying the Measurement of Inventory.” This ASU requires inventory to be measured at the lower of cost or net realizable value. The provisions of this ASU are effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. The amendment is required to be applied prospectively, and early adoption is permitted. The Company is evaluating the effect of this standard on its combined financial statements.
In February 2016, the FASB issued ASU No. 2016-02, “Leases (Topic 842)” which requires that lessees distinguish between finance and operating leases and recognize the assets and liabilities that arise from the leases on the company’s balance sheet. This ASU is effective for fiscal years beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2020. The Company is evaluating the effect of this standard on its combined financial statements.
F-48
Table of Contents
Recently Adopted Accounting Pronouncements
In November 2015, the FASB issued ASU No. 2015-17, “Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes” which requires that deferred tax liabilities and assets be classified as noncurrent in a classified balance sheet. This ASU is effective for financial statements issued for annual periods beginning after December 15, 2017, and interim periods within annual periods beginning after December 15, 2018. Earlier application is permitted as of the beginning of an interim or annual reporting period. The Company early adopted ASU 2015-17 as of December 31, 2014 and applied its provisions retrospectively, which did not have a significant impact on its combined financial statements.
| 3. | INVENTORIES |
Inventories as of December 31, 2014 and 2013 consisted of the following (in thousands):
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Raw materials and purchased parts |
$ | 226 | $ | 93 | ||||
| Work in progress |
1,959 | 1,486 | ||||||
| Finished goods |
314 | 14 | ||||||
|
|
|
|
|
|||||
| Inventories |
$ | 2,499 | $ | 1,593 | ||||
|
|
|
|
|
|||||
| 4. | PROPERTY AND EQUIPMENT, NET |
Property and equipment as of December 31, 2014 and 2013 consisted of the following (in thousands):
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Plant and machinery |
$ | 2,401 | $ | 2,292 | ||||
| Office equipment |
252 | 193 | ||||||
| Leasehold improvements |
424 | 275 | ||||||
|
|
|
|
|
|||||
| Property and equipment, gross |
3,077 | 2,760 | ||||||
| Less: accumulated depreciation |
(1,781 | ) | (1,487 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 1,296 | $ | 1,273 | ||||
|
|
|
|
|
|||||
Depreciation expense amounted to $0.3 million for each of the years ended December 31, 2014 and 2013.
| 5. | ACCRUED EXPENSES |
Accrued expenses as of December 31, 2014 and 2013 consisted of the following (in thousands):
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Accrued wages, benefits, and personnel expenses |
$ | 64 | $ | 56 | ||||
| Accrued operating taxes and government fees |
8 | 52 | ||||||
| Accrued marketing and selling expenses |
141 | 222 | ||||||
| Advances from customers |
89 | 291 | ||||||
| Accrued material purchases |
30 | 521 | ||||||
| Other accrued expenses |
107 | 24 | ||||||
|
|
|
|
|
|||||
| $ | 439 | $ | 1,166 | |||||
|
|
|
|
|
|||||
F-49
Table of Contents
| 6. | INCOME TAXES |
The components for the income tax expense (benefit) are as follows (in thousands):
| Year Ended December 31, |
||||||||
| 2014 | 2013 | |||||||
| Current: |
||||||||
| Federal |
$ | — | $ | — | ||||
| State |
— | — | ||||||
| Foreign |
— | — | ||||||
|
|
|
|
|
|||||
| — | — | |||||||
| Deferred: |
||||||||
| Federal |
— | — | ||||||
| State |
— | — | ||||||
| Foreign |
(72 | ) | 32 | |||||
|
|
|
|
|
|||||
| (72 | ) | 32 | ||||||
|
|
|
|
|
|||||
| Total income tax (benefit) expense |
$ | (72 | ) | $ | 32 | |||
|
|
|
|
|
|||||
The components for loss before income taxes are as follows (in thousands):
| Year Ended December 31, |
||||||||
| 2014 | 2013 | |||||||
| (Loss) income before income taxes: |
||||||||
| Domestic |
$ | (110 | ) | $ | (671 | ) | ||
| Foreign |
(560 | ) | 193 | |||||
|
|
|
|
|
|||||
| Total loss before income taxes: |
$ | (670 | ) | $ | (478 | ) | ||
|
|
|
|
|
|||||
The income tax benefit differs from the “expected” tax benefit computed by applying the U.S. federal corporate tax rate of 34% to the income or loss before income taxes primarily as a result of Polymed being taxed as a flow-through entity in the U.S. and statutory foreign tax rate differences.
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Gross deferred income tax assets |
$ | 646 | $ | 575 | ||||
| Less: valuation allowance |
— | — | ||||||
|
|
|
|
|
|||||
| Total net deferred income tax assets |
$ | 646 | $ | 575 | ||||
|
|
|
|
|
|||||
Deferred income tax assets result principally from net operating losses and an impairment of intangible assets. A valuation allowance is established for any deferred income tax assets for which realization is uncertain. There is no valuation allowance established for the years ended December 31, 2014 or 2013.
As of December 31, 2014 and 2013, the Company had a net operating loss carry forwards of approximately $0.2 million and $0.1 million, respectively, which will begin to expire in the year 2017. The Company files income tax returns in the U.S. federal and state jurisdictions and China.
The Company recognizes interest and penalties accrued related to unrecognized tax benefits in tax expense. No accrual was made for the payment of interest and penalties at December 31, 2014 or 2013.
F-50
Table of Contents
| 7. | DEBT, COMMITMENTS, AND CONTINGENCIES |
Debt
The Company’s debt amounted to $3.8 million and $2.8 million as of December 31, 2014 and 2013, respectively. This consisted of several borrowings held by Polymed and Taihao.
Polymed had a line of credit with a financial institution that had an outstanding balance of $0.8 million and $0.4 million as of December 31, 2014 and 2013, respectively. This line of credit was classified as short term, as it had a 12 month maturity beginning on June 10, 2013. On June 9, 2014, the maturity of the line of credit was extended by 12 months, ending on June 9, 2015. The line of credit had a variable interest rate and included several restrictive covenants. The interest rate was calculated as 1.50 percentage points over the selected interest rate index, which was 3.250% per annum at the time of the line of credit origination. As of December 31, 2014 and 2013, Polymed failed to meet certain of the financial covenants and these violations were not waived. However, no penalty was incurred and the line of credit was repaid subsequent to December 31, 2014. During 2015 the Company repaid $0.1 million. In connection with the acquisition by Athenex (Note 11—Subsequent Events), the remaining $0.7 million was repaid and the line of credit was closed.
Taihao had three promissory notes with financial institutions throughout the course of 2014 and 2013. These notes had total outstanding balances of $1.3 million and $0.7 million as of December 31, 2014 and 2013, respectively. The first note was drawn in 2008, had no stated interest rate and was repaid in full in 2014. This note had an outstanding balance of $0.1 million as of December 31, 2013. The second note had a 12 month maturity beginning on March 29, 2013 and ending on March 29, 2014 with an 18% stated interest rate. This note had an outstanding balance of $0.7 million as of December 31, 2013 and was repaid in full in 2014. The last note had a 12 month maturity beginning on January 20, 2014 and ending on January 20, 2015 with an 11% stated interest rate. This note had an outstanding balance of $1.3 million as of December 31, 2014 and was repaid in full in 2015.
Polymed had two loans, one from each of its two stockholders, which had a combined outstanding balance of $1.5 million as of December 31, 2014 and 2013. These loans had no specified maturity and a 0% stated interest rate. These loans were forgiven as part of the purchase agreement in connection with the acquisition by Athenex (Note 11—Subsequent Events). These amounts are included within Loans from related parties on the combined balance sheets and amounted to $1.5 million as of December 31, 2014 and 2013.
Taihao had a loan from its primary stockholder (whom also owned 50% of Polymed) with an outstanding balance of $0.2 million as of December 31, 2014 and 2013. This loan had no specified maturity and a 0% stated interest rate. This loan remained outstanding at the time of the acquisition by Athenex (Note 11—Subsequent Events).
Rental and lease commitments
In November 2012, Polymed entered into a lease agreement to occupy their office space. This is a non-cancelable lease with a term ending January 2019. The Company records rent expense on a straight-line basis. Rent payments were $0.2 million for the years ended December 31, 2014 and 2013. Future minimum lease payments amount to $0.2 million annually for the years ending December 31, 2015 through 2018 and less than $0.1 million for the year ending December 31, 2019.
Contingencies
From time to time, the Company may be subject to litigation in the normal course of business, which may result in contingent liabilities. During the years ended and as of December 31, 2014 and 2013, the Company was not a party to any pending or known threatened legal proceedings.
F-51
Table of Contents
| 8. | RELATED PARTY TRANSACTIONS |
During the years ended December 31, 2014 and 2013, the Company entered into transactions with individuals and other companies that have financial interests in the Company. Related party transactions included the following:
| a. | Polymed received loans from certain stockholders. These loans are more fully described in Note 7—Debt, Commitments, and Contingencies. |
| b. | Taihao held a liability due to its primary stockholder (whom also owned 50% of Polymed), which was outstanding as of December 31, 2014 and 2013. No interest was incurred in connection with this liability during the years ending December 31 2014 and 2013. See Note 7—Debt, Commitments, and Contingencies for further details. This amount is included within Loans from Related Parties on the combined balance sheets and amounted to $0.2 million as of December 31, 2014 and 2013. |
| c. | Taihao purchases certain pharmaceutical ingredients from Chongqing Taisheng Biotechnology Co., Ltd. (“Taisheng”), a company which is owned by the common stockholder of Polymed and Taihao. Purchases from Taisheng amounted to $0.5 million and $0.2 million for the years ended December 31, 2014 and 2013, respectively. Amounts owed to Taisheng were $0.4 million and $0.2 million as of December 31, 2014 and 2013, respectively. These amounts are included within Accounts Payable on the combined balance sheets. |
| d. | During 2014 and 2013, Taihao made payments to third parties on behalf of the common stockholder of Polymed and Taihao. Taihao recorded receivables in the amount of $0.5 million and $0.3 million that were outstanding as of December 31, 2014 and 2013, respectively. Subsequent to December 31, 2014 but prior to the acquisition by Athenex (see Note 11—Subsequent Events), $0.2 million was repaid by the stockholder and the remaining unpaid balance is shown as a reduction to equity. |
| e. | During 2013, the Company made prepayments totaling $1.475 million to a vendor owned by a previous owner of Taihao, whom did not have an ownership interest during the years ended December 31, 2013 or 2014, anticipating future delivery of product purchases. However, the vendor was not able to deliver and was only able to repay $0.2 million of these prepayments during 2014. The Company expensed the balance of this prepayment in the amount of $1.275 million during the year ended December 31, 2013 within general and administrative expenses on the combined statements of operations. |
| 9. | STOCKHOLDERS’ EQUITY |
Taihao Stockholders’ Equity
As a Chinese Limited Liability Company, Taihao’s Board of Directors authorized a specified amount in Chinese Yuan as owners’ equity. Each stockholders’ ownership percentage is proportionate to their share of the owners’ equity. Stockholders may be required to invest in excess of the authorized owners’ equity; this is recorded as additional paid-in capital.
As of December 31, 2014 and 2013, Taihao’s authorized owners’ equity amounted to $1.9 million and $1.4 million, respectively. During 2014, the Company authorized an increase of the owners’ equity by $0.5 million, which was issued to a new stockholder that invested $0.5 million of owners’ equity and $4.1 million of additional paid-in capital during 2014. Additional paid-in capital amounted to $4.2 million and $0.1 million as of December 31, 2014 and 2013, respectively. Total capital contributed to Taihao, excluding contributions from Polymed Therapeutics, Inc., which eliminate upon combination, amounted to $6.1 million and $1.5 million as of December 31, 2014 and 2013, respectively.
F-52
Table of Contents
| 10. | ACCUMULATED OTHER COMPREHENSIVE INCOME (LOSS) |
The components and changes of accumulated other comprehensive income (loss) as of and for the years ended December 31, 2014 and 2013, net of related income tax effects, are as follows (in thousands):
| Balance as of January 1, 2013 |
$ | 34 | ||
| Foreign currency translation adjustment, net of tax effect of zero |
(39 | ) | ||
|
|
|
|||
| Balance as of December 31, 2013 |
(5 | ) | ||
| Foreign currency translation adjustment, net of tax effect of zero |
27 | |||
|
|
|
|||
| Balance as of December 31, 2014 |
$ | 22 | ||
|
|
|
| 11. | SUBSEQUENT EVENTS |
In June 2015, all of the outstanding shares of Polymed and Taihao were purchased by Athenex, Inc. for total consideration of $30.8 million. Through December 13, 2016, both companies remain wholly owned subsidiaries of Athenex, Inc.
The Company has evaluated events through December 13, 2016, the date the combined financial statements were complete and approved for issuance.
******
F-53
Table of Contents
POLYMED THERAPEUTICS, INC. AND CHONGQING TAIHAO PHARMACEUTICAL CO., LTD.
COMBINED BALANCE SHEETS
(In thousands, except share data)
(Unaudited)
| March 31, | December 31, | |||||||
| 2015 | 2014 | |||||||
| (Note 2) | ||||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 544 | $ | 465 | ||||
| Accounts receivable, net of allowance for doubtful accounts of $50 and $50, respectively |
2,731 | 4,125 | ||||||
| Receivables from related parties |
233 | 232 | ||||||
| Inventories |
3,680 | 2,499 | ||||||
| Prepaid expenses |
86 | 179 | ||||||
| Other receivables |
537 | 241 | ||||||
|
|
|
|
|
|||||
| Total current assets |
7,811 | 7,741 | ||||||
| Property and equipment, net |
1,273 | 1,296 | ||||||
| Deferred tax asset |
743 | 646 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 9,827 | $ | 9,683 | ||||
|
|
|
|
|
|||||
| Liabilities and stockholders’ equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 4,202 | $ | 4,586 | ||||
| Accrued expenses |
1,660 | 439 | ||||||
| Line of credit |
750 | 750 | ||||||
| Notes payable |
— | 1,303 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
6,612 | 7,078 | ||||||
| Long-term liabilities: |
||||||||
| Loans from related parties |
2,705 | 1,705 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
9,317 | 8,783 | ||||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity: |
||||||||
| Common stock—Polymed, no par value, 1,000,000 shares authorized, 100,000 issued and outstanding at March 31, 2015 and December 31, 2014. |
100 | 100 | ||||||
| Owners’ equity—Taihao |
1,876 | 1,876 | ||||||
| Additional paid-in capital—Taihao |
4,226 | 4,226 | ||||||
| Receivable from stockholder—Taihao |
(288 | ) | (288 | ) | ||||
| Accumulated deficit |
(5,427 | ) | (5,036 | ) | ||||
| Accumulated other comprehensive income (loss) |
23 | 22 | ||||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
510 | 900 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 9,827 | $ | 9,683 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these combined financial statements.
F-54
Table of Contents
POLYMED THERAPEUTICS, INC. AND CHONGQING TAIHAO PHARMACEUTICAL CO., LTD.
COMBINED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands)
(Unaudited)
| Three Months Ended March 31, 2015 |
Three Months Ended March 31, 2014 |
|||||||
| Revenue: |
||||||||
| Product sales |
$ | 3,007 | $ | 2,101 | ||||
|
|
|
|
|
|||||
| Total revenue |
3,007 | 2,101 | ||||||
|
|
|
|
|
|||||
| Costs and operating expenses: |
||||||||
| Cost of product sales |
2,263 | 1,537 | ||||||
| General and administrative expenses |
610 | 691 | ||||||
| Selling and marketing expenses |
114 | 77 | ||||||
| Research and development expenses |
482 | — | ||||||
|
|
|
|
|
|||||
| Total costs and operating expenses |
3,469 | 2,305 | ||||||
|
|
|
|
|
|||||
| Operating loss |
(462 | ) | (204 | ) | ||||
|
|
|
|
|
|||||
| Interest expense |
23 | 37 | ||||||
|
|
|
|
|
|||||
| Loss before income taxes |
(485 | ) | (241 | ) | ||||
| Income tax benefit |
(94 | ) | (24 | ) | ||||
|
|
|
|
|
|||||
| Net loss |
(391 | ) | (217 | ) | ||||
|
|
|
|
|
|||||
| Foreign currency translation adjustment, net of tax effect of zero |
1 | 11 | ||||||
|
|
|
|
|
|||||
| Comprehensive loss |
$ | (390 | ) | $ | (206 | ) | ||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these combined financial statements.
F-55
Table of Contents
POLYMED THERAPEUTICS, INC. AND CHONGQING TAIHAO PHARMACEUTICAL CO., LTD.
COMBINED STATEMENTS OF CASH FLOW
(In thousands)
(Unaudited)
| Three Months Ended March 31, |
||||||||
| 2015 | 2014 | |||||||
| Cash flows from operating activities |
||||||||
| Net loss |
(391 | ) | (217 | ) | ||||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
||||||||
| Depreciation and amortization |
57 | 64 | ||||||
| Deferred income taxes |
(94 | ) | (24 | ) | ||||
| Changes in operating assets and liabilities: |
||||||||
| Receivables, net |
1,393 | (575 | ) | |||||
| Receivables from related parties |
(1 | ) | 617 | |||||
| Prepaid expenses and other current assets |
(203 | ) | 49 | |||||
| Inventories, net |
(1,181 | ) | (499 | ) | ||||
| Accounts payable and accrued expenses |
838 | (427 | ) | |||||
|
|
|
|
|
|||||
| Net cash provided by (used in) operating activities |
418 | (1,012 | ) | |||||
|
|
|
|
|
|||||
| Cash flows from investing activities |
||||||||
| Purchase of property and equipment |
(24 | ) | (13 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
(24 | ) | (13 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from financing activities |
||||||||
| Payment of related party loans |
— | (83 | ) | |||||
| Proceeds from related party loans |
1,000 | — | ||||||
| Proceeds from line of credit |
— | 390 | ||||||
| Payment of notes payable |
(1,303 | ) | — | |||||
| Proceeds from notes payable |
— | 968 | ||||||
|
|
|
|
|
|||||
| Net cash (used in) provided by financing activities |
(303 | ) | 1,275 | |||||
|
|
|
|
|
|||||
| Net increase in cash and cash equivalents |
91 | 250 | ||||||
| Cash and cash equivalents, beginning of period |
465 | 95 | ||||||
| Effect of exchange rate changes on cash and cash equivalents |
(12 | ) | 39 | |||||
|
|
|
|
|
|||||
| Cash and cash equivalents, end of period |
$ | 544 | $ | 384 | ||||
|
|
|
|
|
|||||
| Supplemental cash flow disclosures: |
||||||||
| Interest paid |
$ | 12 | $ | 30 | ||||
| Income taxes paid |
$ | — | $ | — | ||||
| Non-cash investing activities: |
||||||||
| Accrued purchases of property and equipment |
$ | 31 | $ | 26 | ||||
The accompanying notes are an integral part of these combined financial statements.
F-56
Table of Contents
POLYMED THERAPEUTICS, INC. AND CHONGQING TAIHAO PHARMACEUTICAL CO., LTD.
NOTES TO COMBINED FINANCIAL STATEMENTS
AS OF AND FOR THE THREE MONTHS ENDED MARCH 31, 2015 AND 2014
| 1. | COMPANY AND NATURE OF BUSINESS |
Description of Business
Polymed Therapeutics, Inc (“Polymed”) and Chongqing Taihao Pharmaceutical Co Ltd (“Taihao”) (collectively “the Company”) commenced operations in 2004. Though operated independently, they were related under common control during the years ended December 31, 2013 and 2014, as well as subsequently until the Company was acquired by Athenex, Inc. in June 2015 (See Note 8—Subsequent Events). Located in Texas, United States, Polymed is an S-corporation that markets and sells Active Pharmaceutical Ingredients (“API”) and medical devices in North America, Europe, and India. Taihao is an API manufacturing company located in Chongqing, China. Taihao owns and operates a current Good Manufacturing Practices (“cGMP”) facility where it manufactures API to be sold in China and abroad. Polymed facilitates the sale of Taihao’s product to their customers outside of China.
Significant Risks and Uncertainties
The Company incurred losses during the three months ended March 31, 2015 and 2014 and had an accumulated deficit of $5.4 million as of March 31, 2015. Operations have been funded primarily through stockholders’ investments, borrowings, and the sale of the Company’s products.
The Company is subject to the risks inherent in conducting business globally and under the laws, regulations, and customs of various jurisdictions. These risks include, but are not limited to, changes in laws, regulations, and practices affecting the pharmaceutical and healthcare industries, adverse changes in the economies in which the Company or its partners and suppliers operate, fluctuation in exchange rates for transactions conducted in currencies other than the functional currency, differing local product preferences and product requirements, supply disruptions and increases in energy, insurance, and transportation costs, and compliance with a variety of national and local laws of countries in which the Company does business, including the restrictions on the import and export of certain intermediates, drugs, and technologies. If any or all of these events occur, the Company could struggle to generate sufficient product revenue and achieve profitability.
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation and Principles of Combination
These combined financial statements reflect the accounts and operations of Polymed and Taihao, which were related under common control as of and for the three months ended March 31, 2015 and 2014. These statements are prepared only for the quarterly periods presented and therefore do not include all of the information and disclosures required by U.S. GAAP for a complete set of financial statements. These unaudited condensed combined financial statements and notes thereto should be read in conjunction with the audited combined financial statements and notes thereto as of and for the years ended December 31, 2014 and 2013. The condensed combined balance sheet as of December 31, 2014 has been derived from the audited financial statements at that date. The unaudited financial information for the interim periods presented herein reflects all adjustments, which, in the opinion of management, are necessary for a fair presentation of the financial condition and results of operations for the periods presented, with such adjustments consisting only of normal recurring adjustments. Results of operations for interim periods covered by these quarterly financial statements may not necessarily be indicative of results of operations for the full fiscal year or any other interim period.
The unaudited condensed combined financial statements include the accounts of Polymed and Taihao. All intercompany transactions and balances have been eliminated in the unaudited condensed combined financial statements.
F-57
Table of Contents
The complete disclosure of accounting principles is included within the audited financial statements as of and for the years ended December 31, 2014 and 2013. There were no changes of accounting principles during the three months ended March 31, 2015.
Use of Estimates
These combined financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities as of the date of the combined financial statements and the reported amount of revenue and expenses during the reporting period. Such management estimates include those relating to assumptions used in allowances for doubtful accounts, income taxes, and the estimated useful life and recoverability of long-lived assets. Actual results could differ from those estimates.
Fair Value of Financial Instruments
Financial instruments consist of cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities, and debt. Cash equivalents are stated at fair value. Accounts receivable, accounts payable, accrued liabilities, and debt are stated at their carrying value, which approximates fair value due to the short time to the expected receipt or payment date of such amounts.
Concentration of Credit Risk, Other Risks and Uncertainties
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents. Cash and cash equivalents are deposited in interest-bearing money market accounts. Although the Company deposits the cash with multiple financial institutions, cash balances may occasionally be in excess of the amounts insured by the Federal Deposit Insurance Corporation. The Company also has significant assets and liabilities held in its overseas biomanufacturing facility in China and therefore is subject to foreign currency fluctuation.
The Company had certain customers whose revenue individually represented 10% or more of the Company’s total revenue, or whose accounts receivable balances individually represented 10% or more of the Company’s total accounts receivable, as follows:
For the three months ended March 31, 2015 and 2014, two customers accounted for 53% and 65% of revenue, respectively. Two customers accounted for 44% accounts receivable as of March 31, 2015.
The Company has one major vendor that accounted for 19% and 26% of cost of product sales for the three months ended March 31, 2015 and 2014, respectively. This vendor accounted for 11% of accounts payable as of March 31, 2015.
| 3. | INVENTORIES |
Inventories as of March 31, 2015 and December 31, 2014 consisted of the following (in thousands):
| March 31, 2015 |
December 31, 2014 |
|||||||
| Raw materials and purchased parts |
$ | 622 | $ | 226 | ||||
| Work in progress |
2,644 | 1,959 | ||||||
| Finished goods |
414 | 314 | ||||||
|
|
|
|
|
|||||
| Inventories |
$ | 3,680 | $ | 2,499 | ||||
|
|
|
|
|
|||||
F-58
Table of Contents
| 4. | ACCRUED EXPENSES |
Accrued expenses as of March 31, 2015 and December 31, 2014 consisted of the following (in thousands):
| March 31, 2015 |
December 31, 2014 |
|||||||
| Accrued wages, benefits, and personnel expenses |
$ | 52 | $ | 64 | ||||
| Accrued operating taxes and government fees |
12 | 8 | ||||||
| Accrued marketing and selling expenses |
176 | 141 | ||||||
| Advances from customers |
80 | 89 | ||||||
| Accrued material purchases |
1,340 | 30 | ||||||
| Other accrued expenses |
— | 107 | ||||||
|
|
|
|
|
|||||
| $ | 1,660 | $ | 439 | |||||
|
|
|
|
|
|||||
| 5. | INCOME TAXES |
The components for the income tax expense (benefit) are as follows (in thousands):
| Three Months Ended March 31, |
||||||||
| 2015 | 2014 | |||||||
| Current: |
||||||||
| Federal |
$ | — | $ | — | ||||
| State |
— | — | ||||||
| Foreign |
— | — | ||||||
|
|
|
|
|
|||||
| — | — | |||||||
| Deferred: |
||||||||
| Federal |
— | — | ||||||
| State |
— | — | ||||||
| Foreign |
(94 | ) | (24 | ) | ||||
|
|
|
|
|
|||||
| (94 | ) | (24 | ) | |||||
|
|
|
|
|
|||||
| Total income tax (benefit) expense |
$ | (94 | ) | $ | (24 | ) | ||
|
|
|
|
|
|||||
The components for loss before income taxes are as follows (in thousands):
| Three Months Ended March 31, |
||||||||
| 2015 | 2014 | |||||||
| (Loss) income before income taxes: |
||||||||
| Domestic |
$ | 133 | $ | (81 | ) | |||
| Foreign |
(618 | ) | (160 | ) | ||||
|
|
|
|
|
|||||
| Total loss before income taxes: |
$ | (485 | ) | $ | (241 | ) | ||
|
|
|
|
|
|||||
| 6. | DEBT, COMMITMENTS, AND CONTINGENCIES |
Debt
Polymed had a line of credit with a financial institution that had an outstanding balance of $0.8 million as of March 31, 2015 and December 31, 2014. This line of credit was classified as short term, as it had a 12 month maturity beginning on June 10, 2013. On June 9, 2014, the maturity of the line of credit was extended by 12 months, ending on June 9, 2015. The line of credit had a variable interest rate and included several restrictive
F-59
Table of Contents
covenants. The interest rate was calculated as 1.50 percentage points over the selected interest rate index, which was 3.250% per annum at the time of the line of credit origination. As of December 31, 2014, Polymed failed to meet certain of the financial covenants and these violations were not waived. However, no penalty was incurred and the line of credit was repaid subsequent to March 31, 2015. Between April 1, 2015 and May 31, 2015, the Company repaid $0.1 million. In connection with the acquisition by Athenex (Note 8—Subsequent Events), the remaining $0.7 million was repaid and the line of credit was closed.
Taihao held a promissory note which had a 12 month maturity beginning on January 20, 2014 and ending on January 20, 2015 with an 11% stated interest rate. This note had an outstanding balance of $1.3 million as of December 31, 2014 and was repaid in full during January 2015.
Polymed had two loans, one from each of its two stockholders, which had a combined outstanding balance of $1.5 million as of December 31, 2014 and 2013. These loans had no specified maturity and a 0% stated interest rate. Polymed took another loan from a stockholder with an interest rate stated at 6% in the amount of $1.0 million during the three months ended March 31, 2015. These loans were forgiven as part of the purchase agreement in connection with the acquisition by Athenex (Note 8—Subsequent Events). These amounts are included within Loans from related parties on the combined balance sheets and amounted to $2.5 million and $1.5 million as of March 31, 2015 and December 31, 2014, respectively.
Taihao had a loan from its primary stockholder (whom also owned 50% of Polymed) with an outstanding balance of $0.2 million as of March 31, 2015 and December 31, 2014. This loan had no specified maturity and a 0% stated interest rate. This loan remained outstanding at the time of the acquisition by Athenex (Note 8—Subsequent Events).
Rental and lease commitments
In November 2012, Polymed entered into a lease agreement to occupy their office space. This is a non-cancelable lease with a term ending January 2019. The Company records rent expense on a straight-line basis. Rent payments were $0.2 million for the years ended December 31, 2014 and 2013 and $0.1 million for the three months ended March 31, 2015. Future minimum lease payments amount to $0.2 million annually for the years ending December 31, 2015 through 2018 and less than $0.1 million for the year ending December 31, 2019.
Contingencies
From time to time, the Company may be subject to litigation in the normal course of business, which may result in contingent liabilities. During the three months ended March 31, 2015 and the year ended December 31, 2014, the Company was not a party to any pending or known threatened legal proceedings.
| 7. | RELATED PARTY TRANSACTIONS |
During the three months ended March 31, 2015 and 2014, the Company entered into transactions with individuals and other companies that have financial interests in the Company. Related party transactions included the following:
| a. | Polymed received loans from certain stockholders. These loans are more fully described in Note 5—Debt, Commitments, and Contingencies. |
| b. | During 2014, Taihao made payments to third parties on behalf of the common stockholder of Polymed and Taihao. Taihao recorded a receivable in the amount of $0.5 million that was outstanding as of March 31, 2015 and December 31, 2014. Subsequent to March 31, 2015 but prior to the acquisition by Athenex (see Note 8—Subsequent Events), $0.2 million was repaid by the stockholder and the remaining unpaid balance is shown as a reduction to equity. |
F-60
Table of Contents
| 8. | STOCKHOLDERS’ EQUITY |
Taihao Stockholders’ Equity
As a Chinese Limited Liability Company, Taihao’s Board of Directors authorized a specified amount in Chinese Yuan as owners’ equity. Each stockholders’ ownership percentage is proportionate to their share of the owners’ equity. Stockholders may be required to invest in excess of the authorized owners’ equity; this is recorded as additional paid-in capital.
As of March 31, 2015 and December 31, 2014, Taihao’s authorized owners’ equity amounted to $1.9 million. During 2014, the Company authorized an increase of the owners’ equity by $0.5 million, which was issued to a new stockholder that invested during 2014. Additional paid-in capital amounted to $4.2 million as of March 31, 2015 and December 31, 2014. Total capital contributed to Taihao, excluding contributions from Polymed Therapeutics, Inc., which eliminate upon combination, amounted to $6.1 million as of March 31, 2015 and December 31, 2014. No additional capital investments were made during the three months ended March 31, 2015.
| 9. | SUBSEQUENT EVENTS |
In June 2015, all of the outstanding shares of Polymed and Taihao were purchased by Athenex, Inc. for total consideration of $30.8 million. Through December 13, 2016, both companies remain wholly owned subsidiaries of Athenex, Inc.
The Company has evaluated events through December 13, 2016, the date the combined financial statements were complete and approved for issuance.
******
F-61
Table of Contents
Shares
Common Stock
Prospectus
| Credit Suisse | J.P. Morgan | |||
| Deutsche Bank Securities | ||||
, 2017
Until (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses, other than underwriting discounts and commissions, payable in connection with the sale and distribution of the securities being registered. All amounts are estimated except the SEC and FINRA registration fees. All of the expenses below will be paid by us.
| Item |
||||
| SEC Registration fee |
$ | 11,590 | ||
| FINRA filing fee |
15,500 | |||
| NASDAQ Global Market listing fee |
* | |||
| Printing and mailing expenses |
* | |||
| Legal fees and expenses |
* | |||
| Accounting fees and expenses |
* | |||
| Transfer agent and registrar fees and expenses |
* | |||
| Miscellaneous |
* | |||
|
|
|
|||
| Total |
$ | * | ||
|
|
|
|||
| * | To be filed by amendment |
Item 14. Indemnification of Directors and Officers
Under Section 145 of the Delaware General Corporation Law, we can indemnify our directors and officers against liabilities they may incur in such capacities, including liabilities under the Securities Act of 1933, as amended, or the Securities Act. Our amended and restated certificate of incorporation to be in effect upon the closing of this offering (Exhibit 3.2 to this registration statement) provides that we will indemnify our directors and officers to the fullest extent permitted by law and require us to pay expenses incurred in defending or other participating in any proceeding in advance of its final disposition upon our receipt of an undertaking by the director or officer to repay such advances if it is ultimately determined that the director or officer is not entitled to indemnification. Our certificate of incorporation further provides that rights conferred under such certificate of incorporation do not exclude any other right such persons may have or acquire under the certificate of incorporation, the bylaws, any statute, agreement, vote of stockholders or disinterested directors or otherwise.
The amended and restated certificate of incorporation also provides that, pursuant to Delaware law, our directors shall not be liable for monetary damages for breach of the directors’ fiduciary duty of care to us and our stockholders. This provision in the amended and restated certificate of incorporation does not eliminate the duty of care, and in appropriate circumstances equitable remedies such as injunctive or other forms of non-monetary relief will remain available under Delaware law. In addition, each director will continue to be subject to liability for breach of the director’s duty of loyalty to us for acts or omissions not in good faith or involving intentional misconduct, or knowing violations of law, for actions leading to improper personal benefit to the director, and for payment of dividends or approval of stock repurchases or redemptions that are unlawful under Delaware law. The provision also does not affect a director’s responsibilities under any other law, such as the federal securities laws or state or federal environmental laws. We also maintain directors’ and officers’ liability insurance.
In addition, we have agreements to indemnify our directors and certain of our officers in addition to the indemnification provided for in the amended and restated certificate of incorporation. These agreements, among other things, indemnify our directors and some of our officers for certain expenses (including attorney’s fees), judgments, fines and settlement amounts incurred by such person in any action or proceeding, including any action by or in our right, on account of services by that person as a director or officer of our company or as a
II-1
Table of Contents
director or officer of our subsidiary, or as a director or officer of any other company or enterprise that the person provides services to at our request.
The underwriting agreement (Exhibit 1.1 to this registration statement) provides for indemnification by the underwriters of us and our officers and directors, and by us of the underwriters, for certain liabilities arising under the Securities Act or otherwise in connection with this offering.
Item 15. Recent Sales of Unregistered Securities
During the last three years we have issued unregistered securities as described below. The share and per share amounts below reflect shares of preferred stock on an as-converted to common stock basis:
Issuances of preferred stock
| 1. | From January 2014 through April 2014, we issued and sold an aggregate of 4,259,310 shares and 1,000,000 shares of our Series A preferred stock to accredited investors at a per share price of $4.55 per share and $5.00 per share, respectively, for aggregate cash consideration of $19,815,046. |
| 2. | On March 26, 2014, we issued and sold an aggregate of 1,320,000 shares of our Series A preferred stock to our executive officers at a purchase price of $4.55 per share for aggregate consideration of $6,000,000. |
Issuances of common stock
| 3. | On June 20, 2014, we issued and sold an aggregate of 720,000 shares of our common stock at a purchase price of $5.50 per share for an aggregate consideration of $3,960,000. |
| 4. | From August 2014 through April 2015, we issued and sold an aggregate of 4,000,000, 157,776 and 6,200,000 shares of our common stock at purchase prices of $5.00, $5.50 and $7.50, respectively, per share for aggregate consideration of $67,367,768. |
| 5. | On September 8, 2014, in connection with our acquisition of QuaDPharma, we issued an aggregate of 181,820 shares of our common stock at a purchase price of $5.50 per share for an aggregate consideration of $1,000,010. |
| 6. | In January through February 2015, we issued an aggregate of 1,496,000 shares of our common stock as stock compensation to certain of our officers and directors, with a value of $5.50 per share for an aggregate amount of $8,228,000. |
| 7. | In April and May of 2015, in connection with our acquisition of Polymed, we issued an aggregate of 1,538,464 shares of our common stock to the sellers, at a value of $7.50 per share, for an aggregate consideration of $11,538,480. |
| 8. | In May 2015, we issued and sold an aggregate of 960,000 shares of our common stock at a purchase price of $7.50 per share for an aggregate consideration of $7,200,000. |
| 9. | On June 5, 2015, we issued and sold an aggregate of 1,200,000 shares of our common stock at a purchase price of $5.50 per share for an aggregate consideration of $6,600,000. |
| 10. | On June 5, 2015, we issued and sold an aggregate of 80,000 shares of our common stock at a purchase price of $7.50 per share for an aggregate consideration of $600,000. |
| 11. | In July 2015, we issued to certain of our employees as stock compensation an aggregate of 66,668 shares and 48,000 shares of our common stock with values of $7.50 per share and $9.00 per share, respectively, for an aggregate amount of $932,010. |
| 12. | On July 17, 2015, we issued and sold 545,456 shares of our common stock at a purchase price of $5.50 per share and 307,692 shares of our common stock at a purchase price of $6.50, for an aggregate consideration |
II-2
Table of Contents
| of $5,000,006 pursuant to an earlier contractual agreement to issue such share upon achievement of certain milestones. |
| 13. | On July 17, 2015, we issued to certain seller affiliated parties in connection with our acquisition of CDE an aggregate of 1,651,264 shares of our common stock at a purchase price of $9.00 per share for an aggregate consideration of $14,861,376. |
| 14. | On March 1, 2016, we issued and sold an aggregate of 666,667 shares of our common stock at a purchase price of $7.50 per share for an aggregate consideration of $5,000,003. |
| 15. | On March 31, 2016, we issued to certain seller affiliated parties in connection with the earnouts from the QuaDPharma and Polymed acquisitions an aggregate of 315,810 shares of our common stock at a purchase price of $9.00 per share for an aggregate consideration of $2,842,290. |
| 16. | On May 26, 2016, we issued and sold an aggregate of 466,666 shares of our common stock at a purchase price of $7.50 per share for an aggregate consideration of $3,499,995. |
Issuances of warrants
| 17. | From March 2014 through January 2015, we issued a series of warrants to purchase an aggregate of 500,000 shares of our common stock, with an exercise price of less than $0.01 per share. |
Grants of stock options and issuances of common stock upon exercise of options
| 18. | Since January 1, 2014, we have granted stock options to purchase an aggregate of 7,358,804 shares of our common stock with exercise prices of $4.55, $5.50, $7.50, $9.00 and $11.00 per share to our employees, directors and consultants pursuant to our 2013 Plan. Since January 1, 2014, we have issued an aggregate of 60,000 shares of our common stock upon exercise of stock options granted pursuant to our 2004 Common Unit Option Plan, 2007 Common Unit Option Plan and 2013 Plan for aggregate consideration of $154,060 in cash. |
Issuances of Convertible Loan Agreements
| 19. | In September and October 2016 and January, February, March and April 2017, we issued and sold notes convertible into shares of our common stock with an aggregate principal amount of $75.0 million. |
We deemed the offers, sales and issuances of the securities described in paragraphs 1 through 18 and 20 above to be exempt from registration under the Securities Act, in reliance on Section 4(a)(2) of the Securities Act, including Regulation D and Rule 506 promulgated thereunder, relative to transactions by an issuer not involving a public offering. All purchasers of securities in transactions exempt from registration pursuant to Regulation D represented to us that they were accredited investors and were acquiring the shares for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
We deemed the grants of stock options and issuances of common stock upon exercise of stock options described in paragraph 19 above, except to the extent described above as exempt pursuant to Section 4(2) of the Securities Act, to be exempt from registration under the Securities Act in reliance on Rule 701 of the Securities Act as offers and sales of securities under compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships, to information about us.
All of the foregoing securities are deemed restricted securities for purposes of the Securities Act. The certificates representing the securities issued in the transactions described in this Item 15 included appropriate
II-3
Table of Contents
legends setting forth that the securities had not been offered or sold pursuant to a registration statement and describing the applicable restrictions on transfer of the securities. There were no underwriters employed in connection with any of the transactions set forth in this Item 15.
Item 16. Exhibits and Financial Statement Schedules
| (a) | Exhibits |
The exhibits to the registration statement are listed in the Exhibit Index attached hereto and incorporated by reference herein.
II-4
Table of Contents
| (b) | Financial Statement Schedules |
SCHEDULE I
CONDENSED FINANCIAL INFORMATION OF REGISTRANT (PARENT COMPANY ONLY)
CONDENSED BALANCE SHEETS
(In thousands, except share and per share data)
| December 31, | ||||||||
| 2015 | 2016 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 41,756 | $ | 28,677 | ||||
| Marketable securities—current |
12,271 | 8,628 | ||||||
| Accounts receivable, net |
3,327 | 2,620 | ||||||
| Inventories, net |
495 | 466 | ||||||
| Prepaid expenses and other current assets |
1,715 | 2,117 | ||||||
|
|
|
|
|
|||||
| Total current assets |
59,564 | 42,508 | ||||||
| Property and equipment, net |
4,836 | 4,039 | ||||||
| Investment |
487 | 340 | ||||||
| Marketable securities—long-term |
1,868 | — | ||||||
| Goodwill |
32,643 | 32,638 | ||||||
| Intangible assets, net |
3,725 | 5,542 | ||||||
| Investment in subsidiary |
10,589 | 14,289 | ||||||
| Due from subsidiary |
4,812 | 5,725 | ||||||
| Other long-term assets |
1,439 | 1,801 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 119,963 | $ | 106,882 | ||||
|
|
|
|
|
|||||
| Liabilities and stockholders’ equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable (intercompany payable of $906 and $1,027, respectively) |
$ | 5,021 | $ | 5,641 | ||||
| Accrued expenses |
3,628 | 18,848 | ||||||
| Accrued legal liability |
450 | — | ||||||
| Current portion of contingent consideration |
5,974 | — | ||||||
| Current portion of long-term debt—related parties |
1,064 | 1,123 | ||||||
| Current portion of long-term debt |
230 | 766 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
16,367 | 26,378 | ||||||
| Long-term liabilities: |
||||||||
| Deferred compensation |
1,463 | 2,174 | ||||||
| Deferred rent |
255 | 904 | ||||||
| Deferred income tax liability |
457 | 215 | ||||||
| Long-term debt—related parties |
1,619 | 496 | ||||||
| Long-term debt |
737 | — | ||||||
| Convertible bonds |
— | 14,498 | ||||||
| Convertible bonds—related parties |
— | 16,129 | ||||||
| Derivative liability |
— | 8,795 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
20,898 | 69,589 | ||||||
| Stockholders’ equity: |
||||||||
| Common stock, par value $0.001 per share, 250,000,000 shares authorized at December 31, 2015 and 2016; 40,330,124, and 42,342,706 issued at December 31, 2015 and 2016, respectively; 39,907,796, and 40,685,786 shares outstanding at December 31, 2015 and 2016, respectively |
40 | 42 | ||||||
| Additional paid-in capital |
206,679 | 237,581 | ||||||
| Accumulated other comprehensive loss |
167 | 41 | ||||||
| Accumulated deficit |
(106,760 | ) | (193,827 | ) | ||||
| Less: treasury stock, at cost; 422,328 shares at December 31, 2015 and 1,656,920 shares at December 31, 2016 |
(1,545 | ) | (7,406 | ) | ||||
|
|
|
|
|
|||||
| Total Athenex, Inc. stockholders’ equity |
98,581 | 36,431 | ||||||
| Non-controlling interests |
484 | 862 | ||||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
99,065 | 37,293 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 119,963 | $ | 106,882 | ||||
|
|
|
|
|
|||||
This statement should be read in conjunction with the notes to the consolidated financial statements included in the Company’s Form S-1
II-5
Table of Contents
SCHEDULE I
CONDENSED FINANCIAL INFORMATION OF REGISTRANT (PARENT COMPANY ONLY)
CONDENSED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except share and per share data)
| Year Ended December 31, | ||||||||
| 2015 | 2016 | |||||||
| Revenue: |
||||||||
| External revenue |
$ | 6,015 | $ | 8,799 | ||||
| Earnings from subsidiary |
6,187 | 10,348 | ||||||
|
|
|
|
|
|||||
| Total revenue |
12,202 | 19,147 | ||||||
|
|
|
|
|
|||||
| Costs and operating expenses: |
||||||||
| Cost of sales |
12,412 | 20,590 | ||||||
| Research and development expenses |
23,878 | 60,058 | ||||||
| Selling, general, and administrative expenses |
25,737 | 23,501 | ||||||
|
|
|
|
|
|||||
| Total costs and operating expenses |
62,027 | 104,149 | ||||||
|
|
|
|
|
|||||
| Operating loss |
(49,825 | ) | (85,002 | ) | ||||
|
|
|
|
|
|||||
| Interest expense |
193 | 1,904 | ||||||
| Unrealized loss on derivative liability |
— | 533 | ||||||
|
|
|
|
|
|||||
| Loss before income tax benefit |
(50,018 | ) | (87,439 | ) | ||||
| Income tax expense (benefit) |
6 | (182 | ) | |||||
|
|
|
|
|
|||||
| Net loss |
(50,024 | ) | (87,257 | ) | ||||
| Less: net loss attributable to non-controlling interests |
(55 | ) | (191 | ) | ||||
|
|
|
|
|
|||||
| Net loss attributable to Athenex, Inc. |
$ | (49,969 | ) | $ | (87,066 | ) | ||
|
|
|
|
|
|||||
| Unrealized gain (loss) on investment, net of income taxes |
91 | (33 | ) | |||||
| Foreign currency translation adjustment, net of income taxes |
— | (85 | ) | |||||
|
|
|
|
|
|||||
| Comprehensive loss |
$ | (49,878 | ) | $ | (87,184 | ) | ||
|
|
|
|
|
|||||
This statement should be read in conjunction with the notes to the consolidated financial statements included in the Company’s Form S-1
II-6
Table of Contents
SCHEDULE I
CONDENSED FINANCIAL INFORMATION OF REGISTRANT (PARENT COMPANY ONLY)
CONDENSED STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended December 31, | ||||||||
| 2015 | 2016 | |||||||
| Cash flows from operating activities: |
||||||||
| Net cash used in operating activities |
$ | (31,219 | ) | $ | (47,172 | ) | ||
|
|
|
|
|
|||||
| Cash flows from investing activities: |
||||||||
| Proceeds from sale of property and equipment |
— | 335 | ||||||
| Purchase of property and equipment |
(2,688 | ) | (623 | ) | ||||
| (Payment) Receipt of refundable deposit |
(1,000 | ) | 1,000 | |||||
| Payments for licenses |
(50 | ) | (2,700 | ) | ||||
| Acquisition of Polymed, net of cash acquired |
(11,076 | ) | — | |||||
| Acquisition of CDE, net of cash acquired |
1,699 | — | ||||||
| Investments in and advances to subsidiary |
(4,734 | ) | (4,613 | ) | ||||
| Purchases of marketable securities |
(15,787 | ) | (9,750 | ) | ||||
| Sale of marketable securities |
12,615 | 15,261 | ||||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
(21,021 | ) | (1,090 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from financing activities: |
||||||||
| Proceeds from sale of stock |
78,768 | 8,500 | ||||||
| Proceeds from issuance of convertible bonds |
— | 38,000 | ||||||
| Costs incurred related to the sale of stock |
(1,013 | ) | (1,538 | ) | ||||
| Proceeds from exercise of stock options |
61 | 50 | ||||||
| Investment from non-controlling interest |
539 | 569 | ||||||
| Payment of contingent consideration |
— | (3,184 | ) | |||||
| Repurchase of options and warrants |
(79 | ) | — | |||||
| Repayment of long-term debt |
(724 | ) | (1,264 | ) | ||||
| Purchase of treasury stock |
(1,250 | ) | (5,861 | ) | ||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
76,302 | 35,272 | ||||||
|
|
|
|
|
|||||
| Net increase (decrease) in cash and cash equivalents |
24,062 | (12,990 | ) | |||||
| Cash and cash equivalents, beginning of period |
17,295 | 41,756 | ||||||
| Effect of exchange rate changes on cash and cash equivalents |
399 | (89 | ) | |||||
|
|
|
|
|
|||||
| Cash and cash equivalents, end of period |
$ | 41,756 | $ | 28,677 | ||||
|
|
|
|
|
|||||
This statement should be read in conjunction with the notes to the consolidated financial statements included in the Company’s Form S-1
II-7
Table of Contents
Schedule II—Valuation and Qualifying Accounts
Activity in the following valuation and qualifying accounts consisted of the following (in thousands):
| Col. C - Additions | ||||||||||||||||||||
| Col. A |
Col. B Balance at Beginning of Period |
Charged to Costs & Expenses |
Charged to Other Accounts - Describe |
Col. D Deductions - Describe |
Col. E Balance at End of Period |
|||||||||||||||
| December 31, 2016 |
||||||||||||||||||||
| Allowance for doubtful accounts |
$ | 478 | $ | 267 | (1) | $ | — | $ | (590 | )(1) | $ | 155 | ||||||||
| Reserve for excess and obsolete inventory |
$ | 839 | $ | 175 | $ | — | $ | (85 | )(2) | $ | 929 | |||||||||
| Deferred tax asset valuation allowance |
$ | 31,400 | $ | — | $ | 30,908 | (3) | $ | — | $ | 62,308 | |||||||||
| December 31, 2015 |
||||||||||||||||||||
| Allowance for doubtful accounts |
$ | 199 | $ | 279 | (1) | $ | — | $ | — | $ | 478 | |||||||||
| Reserve for excess and obsolete inventory |
$ | — | $ | 839 | $ | — | $ | — | $ | 839 | ||||||||||
| Deferred tax asset valuation allowance |
$ | 12,512 | $ | — | $ | 18,888 | (3) | $ | — | $ | 31,400 | |||||||||
(1) Increases in the allowance for doubtful accounts consist of our provision for bad debts, which is included within selling, general, and administrative expenses on the consolidated statements of operations and comprehensive loss. Decreases in the allowances for doubtful accounts consist of the write-off of specific accounts and the recovery of previously reserved receivables.
(2) Increases in the reserve for excess and obsolete inventory are charged to expense within cost of sales on the consolidated statements of operations and comprehensive loss and are based on the difference, if any, between the cost of the inventory and market, based upon assumptions about future demand. Decreases in the reserve for excess and obsolete inventory consist of the write-off of specific items and the recovery of previously reserved inventory.
(3) Increases in the valuation allowance for deferred income tax assets offset the increase in our gross deferred tax assets, based on the expected realization of those future tax benefits.
Item 17. Undertakings
The registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit, or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus as filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) |
II-8
Table of Contents
| under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (2) | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and this offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-9
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Buffalo, State of New York, on May 18, 2017.
| ATHENEX, INC. | ||
| By: | /s/ Johnson Y.N. Lau | |
| Johnson Y.N. Lau | ||
| Chief Executive Officer and Board Chairman | ||
Pursuant to the requirements of the Securities Act, this Registration Statement on Form S-1 has been signed by the following persons in the capacities indicated on May 18, 2017:
| Signature |
Title | |
| /s/ Johnson Y.N. Lau Johnson Y.N. Lau |
Chief Executive Officer and Board Chairman (Principal Executive Officer) | |
| /s/ J. Nicholas Riehle J. Nicholas Riehle |
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | |
| * Kim Campbell |
Director | |
| * Manson Fok |
Director | |
| * Antony Leung |
Director | |
| * Jinn Wu |
Director | |
| * Song-Yi Zhang |
Director | |
| *By: | /s/ J. Nicholas Riehle | |
| J. Nicholas Riehle, Attorney-in-Fact | ||
II-10
Table of Contents
EXHIBIT INDEX
| Exhibit Number |
Description | |
| 1.1* | Form of Underwriting Agreement | |
| 3.1** | Amended and Restated Certificate of Incorporation of the Company | |
| 3.1.1** | Certificate of Amendment of Amended and Restated Certificate of the Company | |
| 3.1.2** | Certificate of Amendment of Amended and Restated Certificate of the Company | |
| 3.2* | Form of Amended and Restated Certificate of Incorporation of the Company, to be in effect upon closing of the Company’s initial public offering | |
| 3.3** | Amended and Restated Bylaws of the Company | |
| 3.4* | Form of Amended and Restated Bylaws of the Company, to be in effect upon closing of the Company’s initial public offering | |
| 4.1** | Specimen Common Stock Certificate | |
| 5.1* | Opinion of Sidley Austin LLP | |
| 10.1+** | Form of Director and Officer Indemnification Agreement | |
| 10.2+** | First Amended and Restated 2004 Unit Option Plan and Form of Unit Option Agreement | |
| 10.3+** | First Amended and Restated 2007 Unit Option Plan and Form of Unit Option Agreement | |
| 10.4+** | 2013 Common Stock Option Plan and Form of Stock Option Agreement | |
| 10.5+* | 2017 Omnibus Incentive Plan | |
| 10.6+* | 2017 Employee Stock Purchase Plan | |
| 10.7^** | License Agreement dated as of December 16, 2011, by and between Kinex Pharmaceuticals, LLC and Hanmi Pharmaceutical Ltd. | |
| 10.7.1** | Amendment No. 1 to License Agreement by and between Kinex Pharmaceuticals, LLC and Hanmi Pharmaceutical Ltd., effective as of November 9, 2012 | |
| 10.7.2** | Amendment No. 2 to License Agreement by and between Kinex Pharmaceuticals, LLC and Hanmi Pharmaceutical Ltd., effective as of October 21, 2013 | |
| 10.7.3** | Amendment No. 3 to License Agreement by and between Kinex Pharmaceuticals, LLC and Hanmi Pharmaceutical Ltd., effective as of March 3, 2015 | |
| 10.7.4^** | Amendment No. 4 to License Agreement and Convertible Loan Agreement by and between Athenex, Inc. and Hanmi Pharmaceutical Ltd., effective as of March 7, 2017 | |
| 10.8^** | License Agreement dated as of June 28, 2013, by and between Kinex Therapeutics (HK) Limited, Hanmi Pharmaceutical Co., Ltd. and Kinex Pharmaceuticals, Inc. | |
| 10.9^** | License Agreement dated as of April 2011, by and between Kinex Pharmaceuticals, LLC and Hanmi Pharmaceutical Ltd. | |
| 10.10^** | License Agreement dated as of December 8, 2011, by and between Kinex Pharmaceuticals, LLC and PharmaEssentia Corporation | |
| 10.10.1** | First Amendment to License Agreement by and between Athenex, Inc. and PharmaEssentia Corp., effective as of December 23, 2016 | |
| 10.11^** | License Agreement dated as of December 16, 2013, by and between Kinex Pharmaceuticals, Inc. and PharmaEssentia Corporation | |
II-11
Table of Contents
| Exhibit Number |
Description | |
| 10.11.1** | First Amendment to License Agreement by and between Athenex, Inc. and PharmaEssentia Corp., effective as of December 23, 2016 | |
| 10.12^** | License Agreement dated as of April 25, 2013, by and between Kinex Pharmaceuticals, Inc. and ZenRx Limited | |
| 10.13^** | License Agreement dated as of May 6, 2012, by and between Kinex Pharmaceuticals, LLC and Guangzhou Xiangxue New Drug Discovery and Development Company Limited | |
| 10.14^** | Binding Term Sheet for License effective as of August 1, 2016, by and between Athenex Pharmaceutical Division, LLC and Gland Pharma Limited | |
| 10.14.1^** | Binding Term Sheet for License effective as of August 26, 2016, by and between Athenex Pharmaceutical Division, LLC and Gland Pharma Limited | |
| 10.14.2^** | Binding Term Sheet for License effective as of February 22, 2017, by and between Athenex Pharmaceutical Division, LLC and Gland Pharma Limited | |
| 10.15^** | Joint Venture Agreement dated as of September 22, 2016, by and between SunGen Pharma LLC and Athenex Pharmaceutical Division, LLC | |
| 10.15.1^** | Addendum to Joint Venture Agreement by and between SunGen Pharma LLC and Athenex Pharmaceutical Division, LLC, effective November 29, 2016 | |
| 10.15.2** | Limited Liability Company Agreement of Peterson Athenex Pharmaceuticals, LLC | |
| 10.16^** | Service Agreement dated as of August 9, 2016 by and between Dohmen Life Science Services, LLC and Athenex Pharmaceutical Division, LLC | |
| 10.17^** | Clinical Trial Collaboration and Supply Agreement, dated as of October 24, 2016, by and among Athenex, Inc., Eli Lilly and Company and ImClone LLC | |
| 10.18** | Agreement for Medical Technology Research, Development, Innovation, and Commercialization Alliance, dated May 1, 2015, by and between Fort Schuyler Management Corporation and Kinex Pharmaceuticals, Inc. | |
| 10.18.1** | First Amendment to Agreement for Medical Technology Research, Development, Innovation, and Commercialization Alliance, by and between Fort Schuyler Management Corporation and Kinex Pharmaceuticals, Inc., effective as of July 21, 2015 | |
| 10.18.2** | Second Amendment to Agreement for Medical Technology Research, Development, Innovation, and Commercialization Alliance, by and between Fort Schuyler Management Corporation and Athenex, Inc., effective as of June 22, 2016 | |
| 10.19** | Sublease Agreement, dated July 21, 2015, by and between Fort Schuyler Management Corporation and Kinex Pharmaceuticals, Inc. | |
| 10.20** | Chongqing Public-Private Partnership Agreement (English translation of original foreign language agreement) | |
| 10.21^** | Binding Term Sheet for License dated December 29, 2016, by and between Athenex API Limited, Nang-Kuang Pharmaceutical Co., LTD and CANDA NK-2, LLC | |
| 10.22** | Asset Purchase Agreement, dated February 1, 2017, by and between Athenex, Inc. and Amphastar Pharmaceuticals, Inc. | |
| 10.23+** | Amended and Restated Employment Agreement by and between Johnson Lau and Kinex Pharmaceuticals, Inc., effective as of June 1, 2015 | |
| 10.24+** | Employment Agreement by and between Kinex Polymed Hong Kong Ltd. and William Zuo, PhD, effective as of June 1, 2015 | |
II-12
Table of Contents
| Exhibit Number |
Description | |
| 10.25+** | Employment Agreement by and between Athenex, Inc. and Rudolf Min-Fun Kwan, effective as of February 21, 2017 | |
| 10.26+** | Employment Agreement by and between Athenex, Inc. and Simon Pedder, effective as of February 20, 2017 | |
| 10.27+** | Employment Agreement by and between Athenex, Inc. and J. Nick Riehle, effective as of February 21, 2017 | |
| 10.28+** | Employment Agreement by and between Athenex, Inc. and Jeffrey Yordon, effective as of February 21, 2017 | |
| 10.29+** | Letter Agreement by and between Athenex, Inc. and Flint Besecker, dated December 8, 2016 | |
| 10.29.1+** | Amendment to Letter Agreement by and between Athenex, Inc. and Flint Besecker, dated April 17, 2017 | |
| 16.1** | Letter Regarding Change in Certifying Accountant | |
| 21.1** | Subsidiaries of Athenex, Inc. | |
| 23.1 | Consent of Deloitte & Touche LLP, Independent Registered Public Accounting Firm | |
| 23.2 | Consent of Freed Maxick CPAs, P.C. | |
| 23.3* | Consent of Sidley Austin LLP (included in Exhibit 5.1) | |
| 24.1** | Power of Attorney (included on signature pages hereto) | |
| 99.1** | Consent of Michael Cannon to be named as a director nominee | |
| 99.2** | Consent of Sheldon Trainor-Degirolamo to be named as a director nominee | |
| 99.3** | Consent of James Zukin to be named as a director nominee | |
| * | To be filed by amendment. |
| ** | Previously filed. |
| + | Indicates management contract or compensatory plan. |
| ^ | Confidential treatment is requested for certain confidential portions of this exhibit pursuant to Rule 406 under the Securities Act. In accordance with Rule 406, these confidential portions have been omitted from this exhibit and filed separately with the Commission. |
II-13