Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - SELECTA BIOSCIENCES INC | exhibit991_earningsrelease.htm |
| 8-K - 8-K - SELECTA BIOSCIENCES INC | selectabiosciences8-k33117.htm |

May 11, 2017
First Quarter 2017 Conference Call

Safe Harbor / Disclaimer
2
Any statements in this presentation about the future expectations, plans and prospects of Selecta Biosciences, Inc. (“the company”),
including without limitation, statements regarding the development of its pipeline, the company's expectations about receiving
additional payments from Spark Therapeutics, Inc. under the license agreement and/or the stock purchase agreement, the progress of
the Phase 1/2 clinical program of SEL-212, the potential of SEL-212 to treat severe gout patients and resolve their debilitating
symptoms, the announcement of data, conference presentations, the ability of the company’s SVP platform, including SVP-
Rapamycin, to mitigate immune response and create better therapeutic outcomes, the potential treatment applications for products
utilizing the SVP platform in areas such as enzyme therapy, gene therapy, oncology therapy, vaccines and treatments for allergies and
autoimmune diseases, any future development of the company’s discovery programs in peanut allergy and/or celiac disease, the
potential of the company’s two gene therapy product candidates to enable repeat administration, the progress of the company’s Phase
I clinical trial in SELA-070, statements regarding the ability of SELA-070 to achieve smoking cessation and relapse prevention,
whether SELA-070 will induce a strong and durable immune response in smokers, whether SELA-070 triggers the production of a high
level of anti-nicotine antibodies and ultimately prevents nicotine from crossing the blood-brain barrier, the sufficiency of the company’s
cash, cash equivalents, investments, and restricted cash and other statements containing the words “anticipate,” “believe,” “continue,”
“could,” “estimate,” “expect,” “hypothesize,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” and
similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995.
Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors,
including, but not limited to, the following: the uncertainties inherent in the initiation, completion and cost of clinical trials including their
uncertain outcomes, the availability and timing of data from ongoing and future clinical trials and the results of such trials, whether
preliminary results from a particular clinical trial will be predictive of the final results of that trial or whether results of early clinical trials
will be indicative of the results of later clinical trials, the unproven approach of the company’s SVP technology, potential delays in
enrollment of patients, undesirable side effects of the company’s product candidates, its reliance on third parties to manufacture its
product candidates and to conduct its clinical trials, the company’s inability to maintain its existing or future collaborations or licenses,
its inability to protect its proprietary technology and intellectual property, potential delays in regulatory approvals, the availability of
funding sufficient for its foreseeable and unforeseeable operating expenses and capital expenditure requirements, substantial
fluctuation in the price of its common stock, a significant portion of the company’s total outstanding shares have recently become
eligible to be sold into the market, and other important factors discussed in the “Risk Factors” section of the company’s Annual Report
on Form 10-K filed with the Securities and Exchange Commission, or SEC, on March 28, 2017, and in other filings that the company
makes with the SEC. In addition, any forward-looking statements included in this presentation represent the company’s views only as
of the date of its publication and should not be relied upon as representing its views as of any subsequent date. The company
specifically disclaims any obligation to update any forward-looking statements included in this presentation.
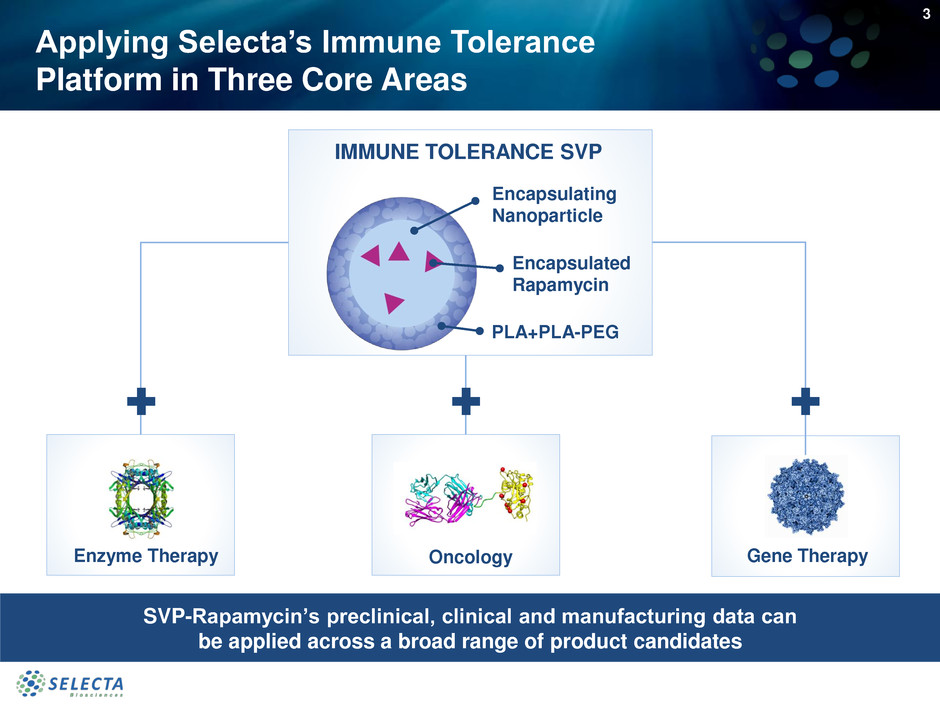
Applying Selecta’s Immune Tolerance
Platform in Three Core Areas
3
IMMUNE TOLERANCE SVP
Encapsulated
Rapamycin
Encapsulating
Nanoparticle
PLA+PLA-PEG
Gene TherapyEnzyme Therapy
SVP-Rapamycin’s preclinical, clinical and manufacturing data can
be applied across a broad range of product candidates
Oncology

SEL-212 for the
Treatment of Severe Gout
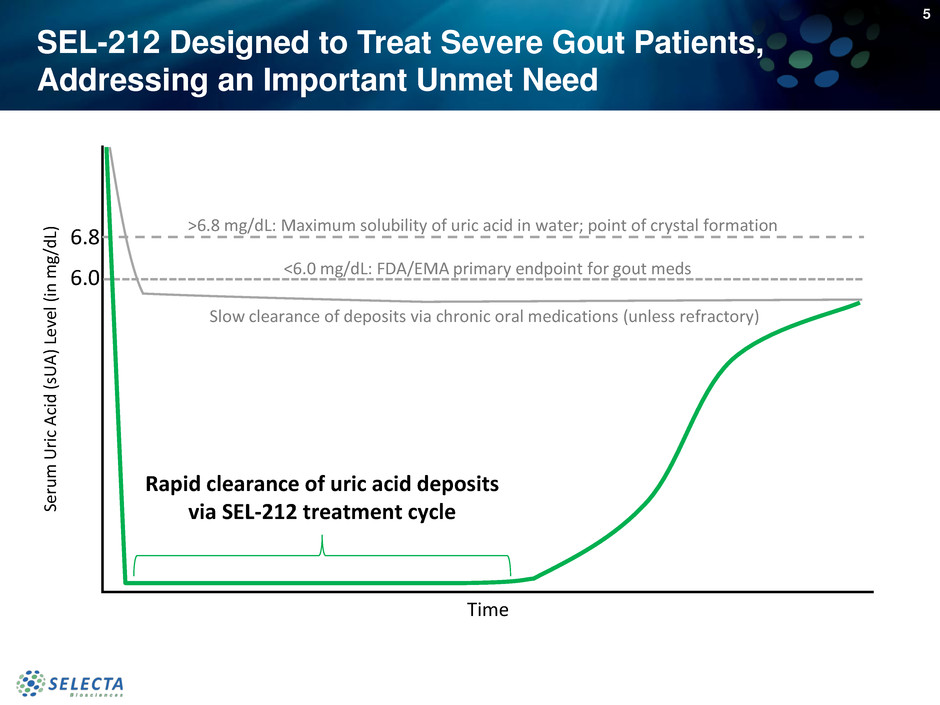
SEL-212 Designed to Treat Severe Gout Patients,
Addressing an Important Unmet Need
5
6.8
6.0
Seru
m
Uric
Aci
d
(
sU
A
)
Le
vel
(
in m
g/
d
L) >6.8 mg/dL: Maximum solubility of uric acid in water; point of crystal formation
<6.0 mg/dL: FDA/EMA primary endpoint for gout meds
Time
Slow clearance of deposits via chronic oral medications (unless refractory)
Rapid clearance of uric acid deposits
via SEL-212 treatment cycle

Trial Completion
Phase 2 Trial Overview
6
• Patients with symptomatic gout and serum uric acid levels >6 mg/dL
• Safety, tolerability and pharmacokinetics of multiple doses of
SEL-212 and pegsiticase alone
• Reduction of ADA levels
• Reduction of serum uric acid levels
• Multiple ascending dose cohorts
• Control cohorts: pegsiticase alone every 28 days for up to five doses
• All other cohorts: SEL-212 every 28 days for three doses followed by
two doses of pegsiticase alone
• Dosing stopped upon failure to control serum uric acid
• Expected by the end of 2017
• 58 patients dosed at 10 active U.S. clinical sites
Enrollment Criteria
Primary/Secondary
Endpoints
Design
Dosing
Stopping Rules
As of May 10, 2017
Clinicaltrials.gov NCT02959918
No/not
diagnosed tophi
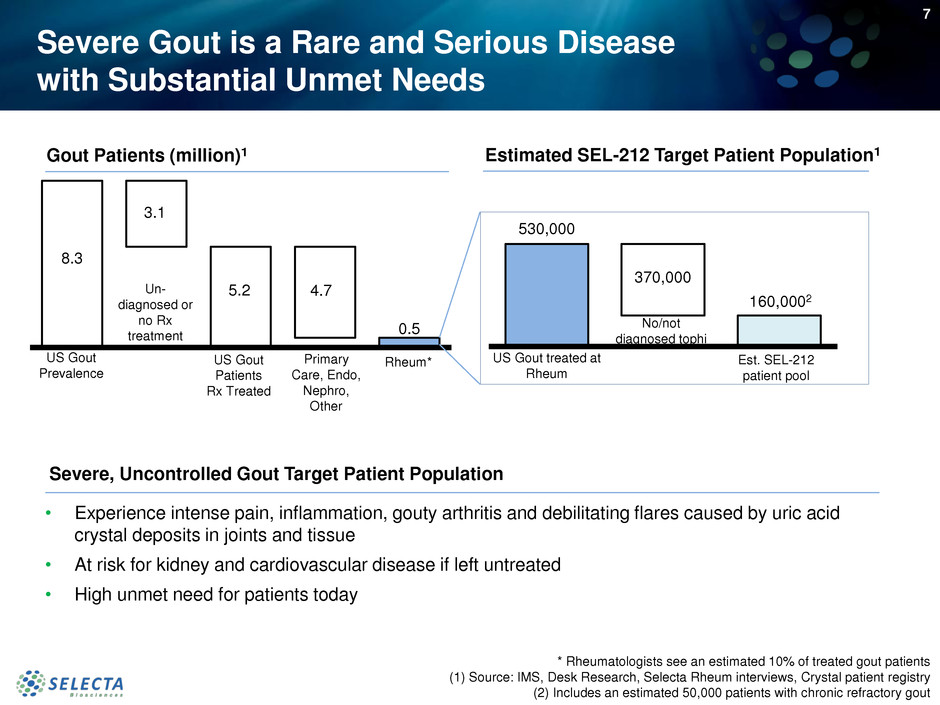
Severe Gout is a Rare and Serious Disease
with Substantial Unmet Needs
8.3
3.1
5.2 4.7
0.5
US Gout
Patients
Rx Treated
Primary
Care, Endo,
Nephro,
Other
Rheum*
Gout Patients (million)1
530,000
370,000
Estimated SEL-212 Target Patient Population1
US Gout treated at
Rheum
Est. SEL-212
patient pool
Un-
diagnosed or
no Rx
treatment
US Gout
Prevalence
* Rheumatologists see an estimated 10% of treated gout patients
(1) Source: IMS, Desk Research, Selecta Rheum interviews, Crystal patient registry
(2) Includes an estimated 50,000 patients with chronic refractory gout
Severe, Uncontrolled Gout Target Patient Population
160,0002
7
• Experience intense pain, inflammation, gouty arthritis and debilitating flares caused by uric acid
crystal deposits in joints and tissue
• At risk for kidney and cardiovascular disease if left untreated
• High unmet need for patients today

Oncology
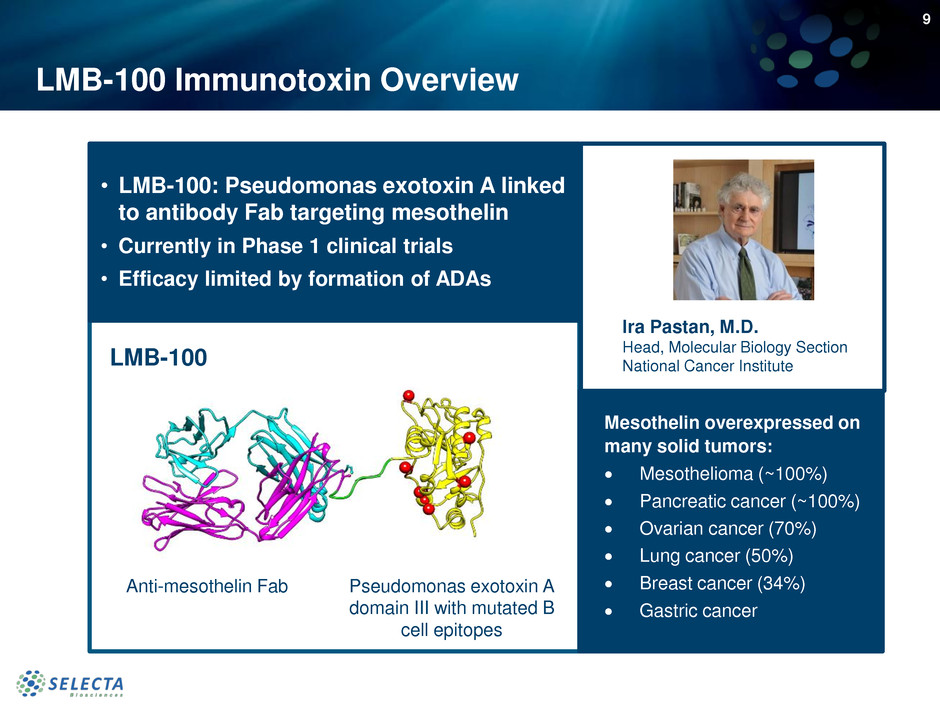
LMB-100 Immunotoxin Overview
• LMB-100: Pseudomonas exotoxin A linked
to antibody Fab targeting mesothelin
• Currently in Phase 1 clinical trials
• Efficacy limited by formation of ADAs
Ira Pastan, M.D.
Head, Molecular Biology Section
National Cancer InstituteLMB-100
Anti-mesothelin Fab Pseudomonas exotoxin A
domain III with mutated B
cell epitopes
Mesothelin overexpressed on
many solid tumors:
Mesothelioma (~100%)
Pancreatic cancer (~100%)
Ovarian cancer (70%)
Lung cancer (50%)
Breast cancer (34%)
Gastric cancer
9
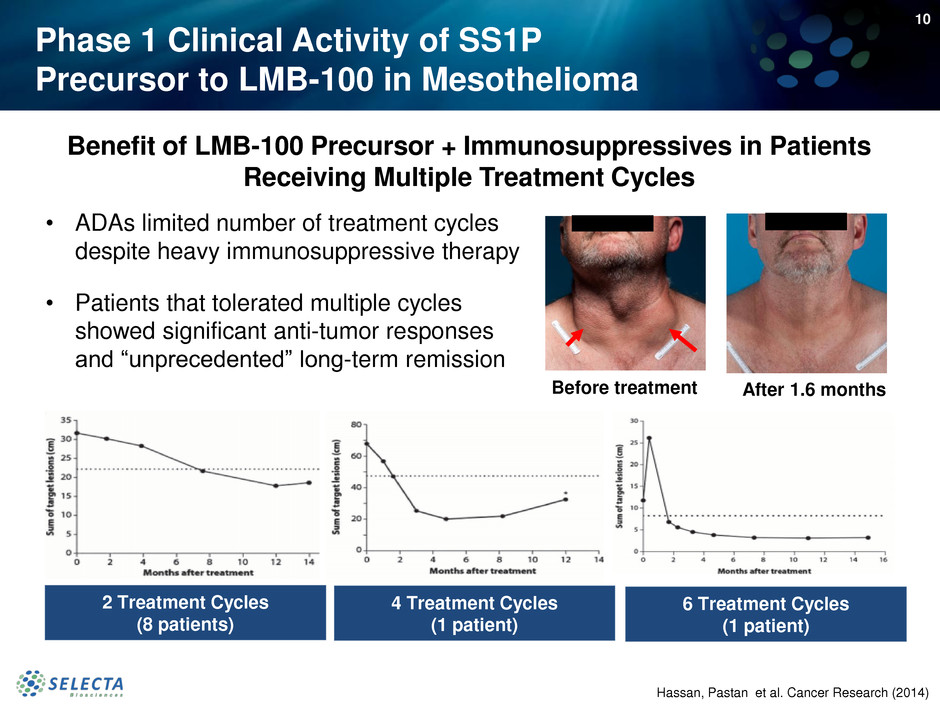
Phase 1 Clinical Activity of SS1P
Precursor to LMB-100 in Mesothelioma
• ADAs limited number of treatment cycles
despite heavy immunosuppressive therapy
• Patients that tolerated multiple cycles
showed significant anti-tumor responses
and “unprecedented” long-term remission
Benefit of LMB-100 Precursor + Immunosuppressives in Patients
Receiving Multiple Treatment Cycles
Before treatment After 1.6 months
Hassan, Pastan et al. Cancer Research (2014)
2 Treatment Cycles
(8 patients)
4 Treatment Cycles
(1 patient)
6 Treatment Cycles
(1 patient)
10
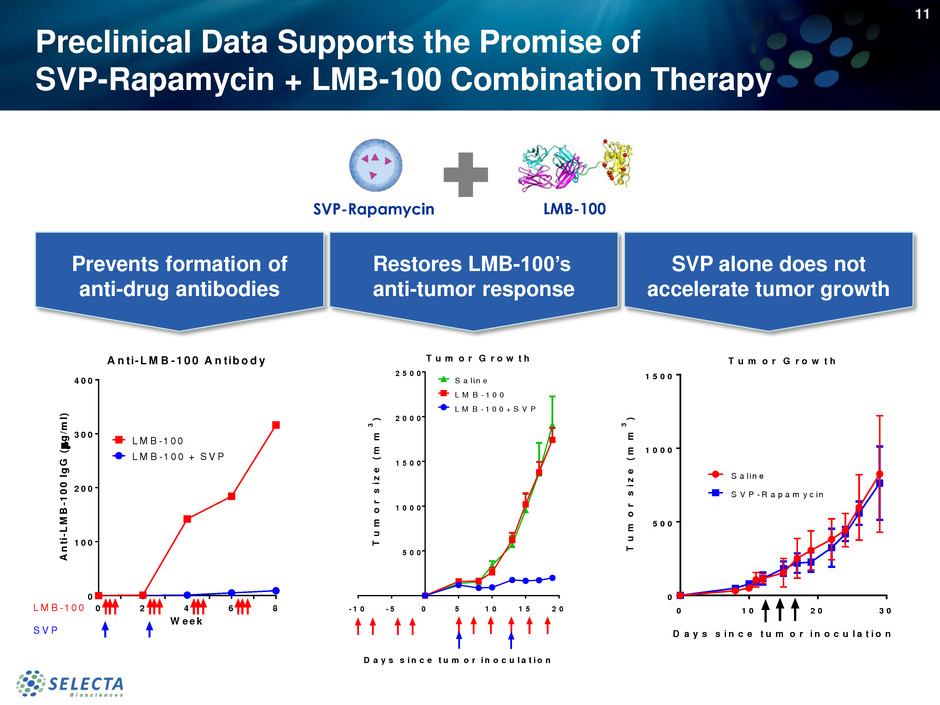
Preclinical Data Supports the Promise of
SVP-Rapamycin + LMB-100 Combination Therapy
Prevents formation of
anti-drug antibodies
Restores LMB-100’s
anti-tumor response
SVP alone does not
accelerate tumor growth
SVP-Rapamycin LMB-100
T u m o r G r o w t h
D a y s s i n c e t u m o r i n o c u l a t i o n
T
u
m
o
r
s
iz
e
(
m
m
3
)
0 1 0 2 0 3 0
0
5 0 0
1 0 0 0
1 5 0 0
S a l in e
S V P - R a p a m y c in
- 1 0 - 5 0 5 1 0 1 5 2 0
5 0 0
1 0 0 0
1 5 0 0
2 0 0 0
2 5 0 0
D a y s s i n c e t u m o r i n o c u l a t i o n
T
u
m
o
r
s
i
z
e
(
m
m
3
)
L M B - 1 0 0
S a l i n e
L M B - 1 0 0 + S V P
T u m o r G r o w t h
11
W e e k
A
n
ti
-L
M
B
-1
0
0
I
g
G
(
g
/m
l)
0 2 4 6 8
0
1 0 0
2 0 0
3 0 0
4 0 0
L M B -1 0 0
S V P
L M B -1 0 0
L M B -1 0 0 + S V P
A n ti-L M B -1 0 0 A n tib o d y

Gene Therapy
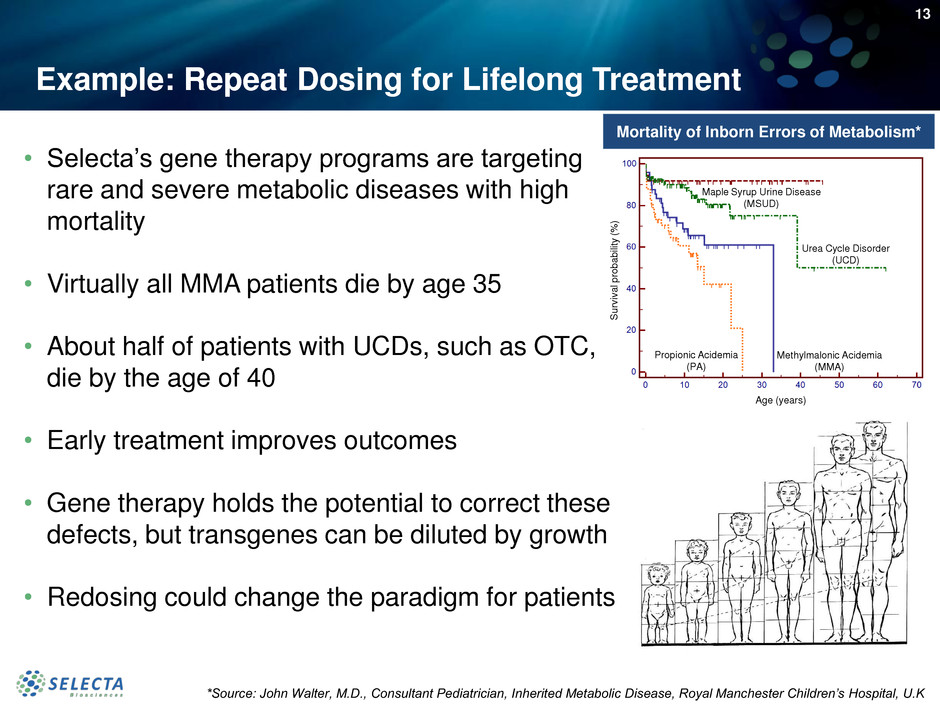
Example: Repeat Dosing for Lifelong Treatment
• Selecta’s gene therapy programs are targeting
rare and severe metabolic diseases with high
mortality
• Virtually all MMA patients die by age 35
• About half of patients with UCDs, such as OTC,
die by the age of 40
• Early treatment improves outcomes
• Gene therapy holds the potential to correct these
defects, but transgenes can be diluted by growth
• Redosing could change the paradigm for patients
13
Propionic Acidemia
(PA)
Methylmalonic Acidemia
(MMA)
Urea Cycle Disorder
(UCD)
Maple Syrup Urine Disease
(MSUD)
Age (years)
Sur
v
iv
a
l proba
b
ili
ty
(%
)
Mortality of Inborn Errors of Metabolism*
*Source: John Walter, M.D., Consultant Pediatrician, Inherited Metabolic Disease, Royal Manchester Children’s Hospital, U.K

Smoking Cessation
and Relapse Prevention
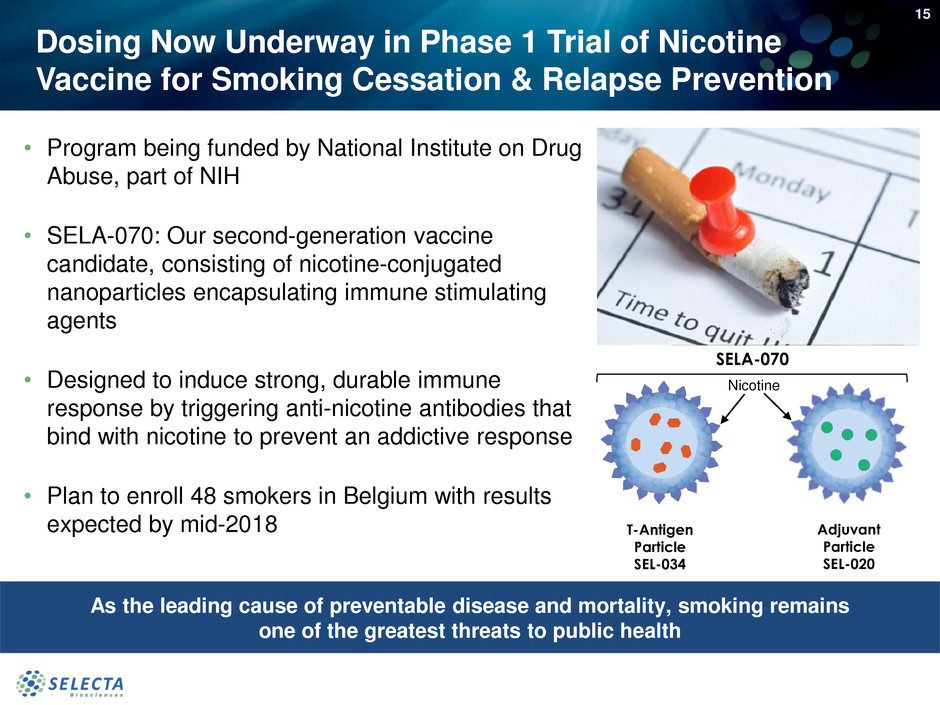
Dosing Now Underway in Phase 1 Trial of Nicotine
Vaccine for Smoking Cessation & Relapse Prevention
• Program being funded by National Institute on Drug
Abuse, part of NIH
• SELA-070: Our second-generation vaccine
candidate, consisting of nicotine-conjugated
nanoparticles encapsulating immune stimulating
agents
• Designed to induce strong, durable immune
response by triggering anti-nicotine antibodies that
bind with nicotine to prevent an addictive response
• Plan to enroll 48 smokers in Belgium with results
expected by mid-2018
15
As the leading cause of preventable disease and mortality, smoking remains
one of the greatest threats to public health
SELA-070
T-Antigen
Particle
SEL-034
Adjuvant
Particle
SEL-020
Nicotine
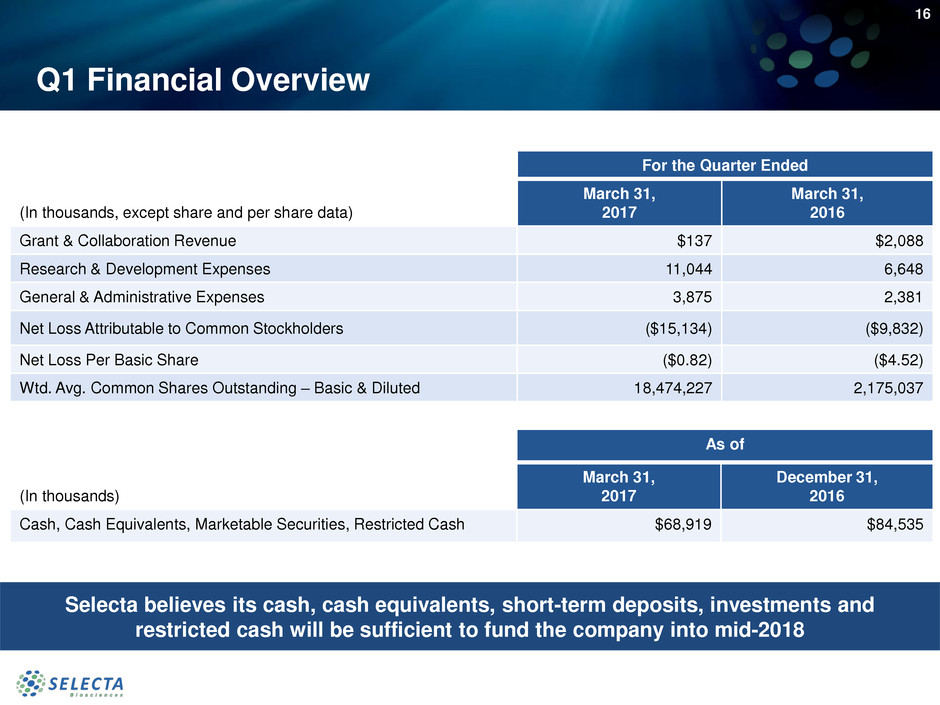
Q1 Financial Overview
For the Quarter Ended
(In thousands, except share and per share data)
March 31,
2017
March 31,
2016
Grant & Collaboration Revenue $137 $2,088
Research & Development Expenses 11,044 6,648
General & Administrative Expenses 3,875 2,381
Net Loss Attributable to Common Stockholders ($15,134) ($9,832)
Net Loss Per Basic Share ($0.82) ($4.52)
Wtd. Avg. Common Shares Outstanding – Basic & Diluted 18,474,227 2,175,037
As of
(In thousands)
March 31,
2017
December 31,
2016
Cash, Cash Equivalents, Marketable Securities, Restricted Cash $68,919 $84,535
Selecta believes its cash, cash equivalents, short-term deposits, investments and
restricted cash will be sufficient to fund the company into mid-2018
16

