Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - AVRA Medical Robotics, Inc. | s106100_ex23-1.htm |
| EX-10.9 - EXHIBIT 10.9 - AVRA Medical Robotics, Inc. | s106100_ex10-9.htm |
| EX-10.8 - EXHIBIT 10.8 - AVRA Medical Robotics, Inc. | s106100_ex10-8.htm |
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM S-1 REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
AVRA MEDICAL ROBOTICS, INC.
(Exact name of registrant as specified in its charter)
| Florida | 3841 | 47-3478854 | ||
| (State or other jurisdiction of | (Primary Standard Industrial | (I.R.S. Employer | ||
| incorporation or organization) | Classification Code Number) | Identification No.) |
3259 Progress Drive
Suite 126
Orlando, FL 32826
(407) 956-2250
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive officer)
Barry F. Cohen
CEO
3259 Progress Drive
Suite 126
Orlando, FL 32826
(407) 956-2250
Name, address, including zip code, and telephone number,
including area code, of agent for service)
Copies to:
Dale S. Bergman, Esq.
Gutierrez Bergman Boulris, PLLC
100 Almeria Avenue, Suite 340
Coral Gables, Florida 33134
(305) 358-5100
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer ¨ |
| Non-accelerated filer ¨ | Smaller reporting company x |
| (Do not check if a smaller reporting company) |
CALCULATION OFADDITIONAL REGISTRATION FEE
| Title of each class of Securities to be Registered (1) |
Amount to be Registered |
Proposed Maximum Offering Price Per Share (2)(3) |
Proposed Maximum Aggregate Offering Price |
Amount of Registration Fee |
||||||||||||
| Common Stock, $0.0001 per share | 10,802,714 | $ | 2.00 | $ | 21,605,428 | $ | 2,504.07(4) |
(1) This registration statement covers the resale by our selling shareholders of up to 10,802,714 shares of common stock previously issued to such selling shareholders or underlying $480,000 in principal amount of our 7.5% Convertible Promissory Notes due June 30, 2017.
(2) The offering price has been estimated solely for the purpose of computing the amount of the registration fee in accordance with Rule 457. The proposed maximum offering price is based on the estimated high end of the range at which the common stock will initially be sold.
(3) The selling shareholders will offer their shares at $2.00 per share until the Company’s shares are quoted on the OTCQX or the OTCQB tiers of the over-the-counter-market operated by OTC Markets Group, Inc. and, assuming we secure this quotation, thereafter at prevailing market prices or privately negotiated prices.
(4) Of which $1,996.35 has heretofore been paid.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities nor may offers to buy these securities be accepted until the registration statement filed with the Securities and Exchange Commission becomes effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED MAY 11, 2017
PROSPECTUS
AVRA MEDICAL ROBOTICS, INC.
10,802,714 Shares of Common Stock
The selling shareholders named in this prospectus are offering up to 10,802,714 shares of common stock through this prospectus. We will not receive any proceeds from this offering and have not made any arrangements for the sale of these securities.
Our common stock is presently not traded on any market or securities exchange. Given the foregoing, the selling shareholders will offer their shares at an initial offering price of $2.00 per share until the shares are quoted on the OTCQX or the OTCQB tiers of the over-the counter operated by OTC Markets Group, Inc (“OTC Markets Group”). Although we intend to apply for quotation of our common stock on the OTCQX or the OTCQB tiers of the over-the counter market operated by OTC Markets Group, we may not secure this qualification and even if we do an active public market for our common stock may never materialize. If we secure this qualification, the sale price to the public of the shares registered hereunder will be at prevailing market prices or privately negotiated prices.
The Company is an emerging growth company under the Jumpstart Our Business Startups Act of 2012 (the “Jobs Act”) and as such, may elect to comply with certain reduced public company reporting requirements for future filings.
The purchase of the shares of common stock offered through this prospectus involves a high degree of risk. See the section of this prospectus entitled “Risk Factors” beginning at page 5.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
The date of this prospectus is ____________ __, 2017
TABLE OF CONTENTS
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission (the “SEC”) using the SEC’s registration rules for a delayed or continuous offering and sale of securities. Under the registration rules, using this prospectus and, if required, one or more prospectus supplements, the selling shareholders named herein may distribute the shares of common stock covered by this prospectus. This prospectus also covers any shares of common stock that may become issuable as a result of stock splits, stock dividends or similar transactions. A prospectus supplement may add, update or change information contained in this prospectus.
You should rely only on the information contained in this prospectus. We have not authorized any dealer, salesperson or other person to provide you with information concerning us, except for the information contained in this prospectus. The information contained in this prospectus is complete and accurate only as of the date on the front cover page of this prospectus, regardless when the time of delivery of this prospectus or the sale of any common stock. This prospectus is not an offer to sell, nor is it a solicitation of an offer to buy, our common stock in any jurisdiction in which the offer or sale is not permitted.
1
This summary provides an overview of all material information contained in this prospectus. It does not contain all the information you should consider before making a decision to purchase the shares our selling shareholders are offering. You should very carefully and thoroughly read the more detailed information in this prospectus and review our financial statements and all other information that is incorporated by reference in this prospectus.
Unless the context otherwise requires, references in this prospectus to “AMR,” “AVRA” “the Company,” “we,” “our” and “us” refers to Avra Medical Robotics, Inc.
Overview
AVRA was organized by a senior leadership team with broad and deep experience in medical research, innovation and development in the medical robotics field. The Company plans to exploit the growing demand for practical medical robotic devices by developing a platform-independent precision guidance system, applicable to a variety of minimally invasive and non-invasive procedures, with an initial focus on skin resurfacing. The Company believes that its team, which has been active in the medical robotics field for a number of years, brings the necessary skills and experience to develop and commercialize intelligent medical robotic systems, as well as in marketing, chain management, and the implementation of all other aspects of business operations. The Company believes that progress in mechanical and software engineering has made possible lightweight and relatively inexpensive robotic devices for difficult procedures in various medical fields.
Medical robots are already being successfully employed in several areas of surgery, including Urology (Prostate), Colo-Rectal, Gynecology, Thoracic, General Surgery, Neuro and Spine Surgery. Robots are also being used for Tele-medicine and assistive robotic methods are addressing the delivery of healthcare in inaccessible locations, ranging from rural areas lacking specialist expertise to post-disaster and battlefield areas. With the aging population dominating demographics in the U.S. across all spectrums of health care, robotic technologies are being developed toward promoting improved function, less morbidity and improved overall outcomes.
Today, the U.S. is the leader in robot-assisted surgery for the possibility of cure and improved quality of life. However, other countries are fast followers, having already recognized both the need and the promise of such technologies. The development of surgical robotics is motivated by the desire to enhance the effectiveness of a procedure by coupling information to action in the operating room or interventional suite, and transcend human physical limitations in performing surgery and other interventional procedures, while still affording human control over the procedure. Two decades after the first reported robotic surgical procedure, surgical robots are now being widely used in the operating room. Surgical robots are beginning to realize their potential in terms of improved accuracy and visualization1, as well as enabling new procedures.
Current robots used in surgery are under the direct control of a surgeon – the so-called “Master-slave system,” often in a teleoperation scenario in which a human operator manipulates a master input device and the patient-side robot follows the input. In contrast to commonly held beliefs where robots are autonomous, traditional minimally invasive surgical robots provide the surgeon with a higher degree of dexterity inside the body, eliminate operator tremor, scale down operator motions to a fraction of normal distances, and provide a very intuitive connection between the operator and the instrument tips. The surgeon can cut, cauterize, suture and reconstruct tissue with accuracy equal to or better than that of invasive open surgery. A surgical system contains both robotic devices and real-time imaging devices to visualize the operative field during the course of surgery.
The use of robotics in medicine inherently involves physical interaction between caregivers, patients, and robots – in all combinations. Developing user-friendly physical interfaces between humans and robots requires all the classic elements of a robotic system: sensing, perception, and action. A great variety of sensing and perception tasks are required, including recording the motions and forces of a surgeon to infer their intent, determining the mechanical parameters of human tissue, and estimating the forces between a rehabilitation robot and a moving stroke patient. The reciprocal nature of interaction means that the robot will also need to provide useful feedback to the human operator, whether that person is a caregiver or a patient. We need to consider systems that involve many human senses, the most common of which are vision, haptics (force and tactile), and sound. In management’s opinion, a major reason why systems involving physical collaboration between humans and robots are difficult to design is that, from the perspective of a robot, humans are extremely uncertain and dynamic.
1 http://uchealth.com/services/robotic-surgery/patient-information/benefits/
2
Unlike in a passive, static environment, humans dynamically change their motion, force, and immediate purpose throughout a procedure. These changes can be caused by something as simple as physiologic movement (e.g., a patient breathing during surgery), or as complex as the motions of a surgeon suturing during surgery. During physical interaction with a robot, the human is an integral part of a closed-loop feedback system, simultaneously exchanging information and energy with the robotic system, and thus cannot simply be thought of as an external system input. In addition, the loop is often closed with both human force and visual feedback, each with its own errors and delays that can potentially cause challenges in a human-robot system. Given these problems, how does one guarantee safe, collaborative and useful physical interaction between robots and humans? Management believes that to date, no existing system provides the user with an ideal experience of physically interacting with a robot.
AVRA’s overall design strategy, as opposed to that used in most non-autonomous systems, is to integrate image-guidance with navigation and organ-targeting to result in a medical robotic system that is truly diverse and multi-dimensional. Having identified limitations in the predominantly non-autonomous systems, AVRA proposes to employ a disruptive model in the design and development process, which considers design and development through a seamless collaboration of surgeons, engineers and scientists. Past efforts in medical robotics have generally been led by either the surgeons, the engineers, or the scientists, but rarely via a collaboration of all three. AVRA has been making a proactive effort to use this collaborative approach in all its development work.
For skin resurfacing, AVRA plans to incorporate recent technological improvements in motors, materials and high resolution imaging to develop robotic devices that will allow a surgeon to autonomously or semi-autonomously treat damaged skin. The basic principle behind the initial technology that AVRA is working on automating is the controlled delivery of micro skin injuries to stimulate remodeling of existing collagen and promote the formation of new collagen, elastin, and vascularization in the papillary dermis, which results in the reduction in appearance of fine lines and wrinkles, skin laxity, and scarring. Some of the advantages of using a robot for skin resurfacing can include greater precision, better visualization, quick automated adjustments during a procedure and shorter times. Presently, we are not aware of any commercially available robotic devices designed for this application.
AVRA is currently developing its initial intelligent medical robotic system for facial corrections (i.e., skin resurfacing) in partnership with the University of Central Florida (“UCF”), pursuant to a research agreement entered into with UCF effective as of May 1, 2016, (the “Research Agreement”). UCF is recognized particularly for its work in the area of medical robotic research and design, focusing on the guidance systems. We anticipate that application of this expertise will allow AVRA’s medical robotic system to handle a wide array of the currently available “tools” in the market. The Company plans to target a large market for its initial robotic system, which currently includes such solutions as Botox and CO2 lasers used for keratosis removal and treatment of scarring, discoloration and other skin problems that are often difficult to treat.
Moreover, while AVRA is in development of its robotic system and anticipates that it is ready to build a prototype, to date we have no products or training programs approved or ready for retail marketing and there can be no assurance as to when products will be ready to reach market. Unanticipated delays in market readiness will substantially harm the Company’s prospects.
To date, the Company has not generated revenues and has operated with limited capital. The Company will require significant capital to implement its business plan. There can be no assurance that the Company can raise the necessary funds, on favorable terms or otherwise. Failure to obtain sufficient capital will substantially harm the Company’s prospects.
1 Statista, http://www.statista.com/statistics/254612/global-skin-care-market-size/
3
Corporate Information
The Company was incorporated in the state of Florida on February 4, 2015 under the name “Avra Surgical Microsystems, Inc.” and changed its name to “Avra Medical Robotics Inc.”, on November 5, 2015.
Our principal executive offices are located at 3259 Progress Drive, Suite 126, Orlando, FL 32826. Our telephone number is 407.956.2250. Our corporate website is www.avramedical.com. Information appearing on our corporate website is not part of this prospectus.
Selling Shareholders
On February 4, 2015, the Company issued an aggregate of 13,100,400 shares to its founder at par value, for aggregate consideration of $1,310. The resale of 5,240,160 of these shares (including 1,497,880 shares, which were subsequently gifted by the founder to family members or sold to third parties in private sales) is covered by this prospectus.
Effective February 1, 2016, the Company issued an aggregate of 5,899,600 shares of common stock in a private offering to initial shareholders and investors (other than its founder) at par value, for aggregate consideration of $590. The resale of 3,372,240 of these shares is covered by this prospectus.
From April to August, 2016, the Company sold $480,000 in principal amount of 7.5% Convertible Promissory Notes due June 30, 2017 (the “Convertible Notes”) to 11 investors in a private offering. The Convertible Notes are convertible into shares of common stock at $0.50 per share at the option of the holder at any time prior to maturity and will automatically convert into shares of common stock upon completion of a private or public offering which generates at least $500,000 in proceeds to the Company at a conversion price equal to the lower of $0.50 per share or a 20% discount the per share price of shares sold such subsequent offering. The resale of 480,000 of these shares is covered by this prospectus.
In February 2017, we sold 135,000 shares of our common stock for an aggregate of $135,000, or $1.00 per share, to three investors in a private offering. The resale of 81,000 of these shares is covered by this prospectus.
The Offering
This prospectus relates to the resale from time to time by the selling shareholders identified in this prospectus of 10,802,714 shares of our common stock, par value $0.0001 per share. No shares are being offered for sale by the Company.
| Common stock offered by selling shareholders: | 10,802,714 shares of common stock. |
| Common stock outstanding as of the date of this prospectus: | 19,192,438 shares of common stock (1). |
| Terms of the Offering: | The selling shareholders will determine when and how they will sell the shares of common stock offered in this prospectus. |
| Use of proceeds: | We will not receive any proceeds from the sale of the shares of common stock offered by the selling shareholders under this prospectus. |
| Risk factors: | The common stock offered hereby involves a high degree of risk and should not be purchase by investors who cannot afford the loss of their entire investment. |
| (1) | Does not include (a) 3,000,000 shares of our common stock reserved for issuance under our 2016 Incentive Stock Plan and (b) 960,000 shares of our common stock issuable upon conversion of $480,000 in outstanding principal amount of the Convertible Notes. |
4
The following summary financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and the Financial Statements and Notes thereto, included elsewhere in this prospectus.
| For the Year | For the Period From Inception | |||||||
| Statement of Operations | Ended December 31, | (February 4, 2015) to December 31, | ||||||
| 2016 | 2015 | |||||||
| Revenues | $ | - | $ | - | ||||
| Cost of Sales | $ | - | $ | - | ||||
| Research and Development | $ | 138,377 | $ | - | ||||
| Other Selling, General and Interest Expenses | $ | 346,605 | $ | 66 | ||||
| Net Loss | $ | (484,982 | ) | $ | (66 | ) | ||
| Balance Sheet Data | As of December 31, | |||||||
| 2016 | 2015 | |||||||
| Cash | 248,219 | $ | 100 | |||||
| Total Assets | 292,232 | $ | 100 | |||||
| Total Liabilities | 647,292 | $ | 166 | |||||
| Total Stockholders' Deficiency | (355,060 | ) | $ | (66 | ) | |||
| Total Liabilities and Stockholders’ Deficit | (292,232 | ) | $ | 100 | ||||
5
The shares of our common stock being offered for resale by the selling shareholders are highly speculative in nature, involve a high degree of risk and should be purchased only by persons who can afford to lose the entire amount invested in the common stock. Before purchasing any of the shares of common stock, you should carefully consider the following factors relating to our business and prospects. If any of the following risks actually occurs, our business, financial condition or operating results could be materially adversely affected. In such case, you may lose all or part of your investment. You should carefully consider the risks described below and the other information in this process before investing in our common stock.
We are a development stage company with a limited operating history and our future profitability is uncertain.
We were incorporated in Florida on February 4, 2015 under the name "Avra Surgical Microsystems, Inc." We changed our name to “Avra Medical Robotics Inc.” on November 5, 2015. We are currently in the development stage with a limited operating history and no revenues from operations to date. We have not yet demonstrated our ability to generate revenue, and we may not be able to produce revenues or operate on a profitable basis. As a result, it is difficult to evaluate our performance and to forecast our future operating results. Our prospects must be considered in light of the risks, uncertainties, expenses and difficulties frequently encountered by an early development stage company in a relatively new, potentially highly competitive healthcare industry. Moreover, as an early stage company, we have no prior experience in implementing and managing our planned business in an operational setting. Accordingly, there can be no assurance that we will be able to successfully implement our business plans or strategies.
We cannot provide any assurance that we will be successful in addressing the risks which we may encounter, and our failure to do so could have a material adverse effect on our business, prospects, financial condition and results of operations.
AVRA is party to the Research Agreement with UCF, pursuant to which UCF is undertaking a significant portion of the development work for the prototype of our intelligent medical robot device for skin resurfacing. If UCF is unable to perform its obligations under this agreement, our business may be adversely affected and your investment may become worthless.
Effective May 1, 2016, AVRA entered into the Research Agreement with UCF, pursuant to which UCF is undertaking a significant portion of the development work for the prototype of our planned intelligent medical robotic device for minimally invasive surgical facial corrections (i.e., skin resurfacing). Our ability to develop and ultimately commercialize our initial planned product may be significantly adversely impacted if UCF is unable or otherwise fails to perform its obligations under the Research Agreement. In such case, we may be unable to develop our business as planned. In the event of such inabilities your investment in our common stock may become worthless.
As we develop our business we anticipate future losses and negative cash flow, which may limit or delay our ability to become profitable.
We have incurred losses since our inception and expect to experience operating losses and negative cash flow for the foreseeable future. For the year ended December 31, 2016, and for the period from inception to December 31, 2015, we had net losses of $484,982 and $66, respectively. We anticipate our losses will continue to increase from current levels because we expect to incur additional costs and expenses related to developing our business, including research and development costs, employee related costs, capital expenditures, the costs of complying with government regulations, sales force, manufacturing, distribution and training. We can provide no assurance as to when, or if, the products we intend to develop will become commercially viable, generate revenues, or become profitable. Even if we achieve profitability, we may not be able to sustain it.
6
We will require substantial additional financing to complete development of, obtain regulatory approvals and protections, manufacture and market our planned intelligent medical robotic system and to develop and commercialize other medical robotic systems in the future. A failure to obtain this necessary capital when needed on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our product development activities, other operations or commercialization efforts.
We have no products yet developed or approved for retail marketing, have generated no revenues from operations to date and may not be able to produce revenues in the foreseeable future. Our capital needs to date have been met by private offerings of our securities or loans. We believe that we will continue to expend substantial resources for the foreseeable future on the completion of development of our planned intelligent medical robotic system, securing necessary regulatory approvals, manufacture, marketing and other commercialization efforts for the AVRA medical robotic system and development and commercialization of additional medical robotic systems in the future.
Our future capital requirements depend on many factors, including:
| · | the scope, progress, results and costs of research and development of our planned medical robotic system and any future products we may seek to develop; |
| · | the cost, time and success of obtaining regulatory approval of our planned system; |
| · | The cost, time and success of obtaining patent and other intellectual property protections; |
| · | the cost of commercialization activities of our planned system and any future products which we may develop and seek to commercialize; |
| · | our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements, if we choose to utilize such arrangements in connection with the commercialization of our planned and future medical robotic systems; |
| · | the number and characteristics of any future product we may develop; |
| · | any product liability or other lawsuits related to our products or commenced against us; |
| · | the expenses needed to attract and retain skilled personnel; |
| · | the costs associated with being a public company; and |
| · | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims or other intellectual property rights, including litigation costs and the outcome of such litigation. |
Additional funds may not be available when we need them, on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to:
| · | delay, limit, reduce or terminate development activities for our current or future medical robotic systems, if any; |
| · | delay, limit, reduce or terminate our research and development activities; or |
| · | delay, limit, reduce or terminate our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize our current or future product candidates. |
7
Raising additional capital may cause dilution to our existing shareholders.
We expect to raise some or all of the funds we need to develop our products by selling our securities, including but not limited to sales of our common stock, in private or public offerings. Sales of our common stock will likely have a dilutive effect on our existing shareholders.
If our planned products do not achieve market acceptance, we will not be able to generate the revenue necessary to support our business.
We expect that our intelligent medical robotic system, once developed, will offer an important alternative device to perform certain skin resurfacing procedures. Achieving physician and patient acceptance of our system as a preferred device will be crucial to our success. If our products fail to achieve market acceptance, customers will not purchase our products and we will not be able to generate the revenue necessary to support our business. We believe that physicians’ acceptance of the benefits of procedures performed using our system will be essential for acceptance of our systems by patients. Our system will represent new technologies that will compete with established and emerging treatment options in the world of skin resurfacing procedures. Some of these procedures are widely accepted in the medical and skin resurfacing communities and in many cases have a long history of use. Physicians will not recommend the use of our system unless we can demonstrate that they produce results comparable or superior to existing techniques. Technological advances could also make such traditional treatments more effective or less expensive than using our planned system, which could render our system obsolete or unmarketable. Studies could also be published that show that other treatment options are more beneficial and/or cost-effective than our planned system. We cannot be certain that physicians will use our systems, if developed and approved, to replace or supplement established treatments or that our system will continue to be competitive with current or future technologies. If our medical robotic system, once developed, is not accepted and not competitive, our business and financial results will be materially adversely affected.
Even if we can prove the effectiveness of our planned intelligent medical robotic system through clinical trials, physicians may elect not to use our products. For example, physicians may continue to recommend existing techniques or current devices simply because such methods are already widely accepted. In addition, physicians may be slow to adopt our system because of the perceived liability risks arising from the use of a new system. If our system does not receive market acceptance, we will not be able to generate the revenue to support our business.
We do not have training experience or capabilities on this new system yet and we may encounter delays in developing such experience or capabilities or resistance from customers to the effort required to be trained, problems or delays that could result in lost revenue.
We anticipate that there will be a learning process involved for users of our intelligent medical robotic system to become proficient in its use. Broad use of our system will require training. Market acceptance could be delayed by the time required to complete this training. We may not be able to rapidly train users in numbers sufficient to generate adequate demand for our system.
Our industry is highly competitive and if we fail to effectively compete, our business and financial results would be significantly harmed.
We consider our primary initial competition will come from existing skin resurfacing procedures. Our success will depend in part on convincing hospitals, physician practices and patients to convert from conventional treatment methods to AVRA’s planned intelligent medical robotic system to perform these procedures.
In addition, a number of companies manufacture and market or are developing and planning to market various robotic systems that are designed to be used in performing various surgical procedures. Many of these companies have significantly greater experience and financial resources than does AVRA. While we currently do not know of any medical robotic companies currently targeting the skin resurfacing market, our revenues may be reduced or eliminated if our competitors do develop and market products that are more effective or less expensive than our products.
8
We believe that the primary competitive factors in the market we address will be procedural capability, cost of operations, efficacy, ease of use, quality, reliability, and effective sales support, training and service. If we fail to effectively compete, our business and financial results would be significantly harmed.
If defects are discovered in our products, we may incur additional unforeseen costs stemming from physicians, hospitals and other potential customers possibly not purchasing our products and our reputation may suffer. This would have a material adverse effect on our business.
Our products will incorporate mechanical parts, electrical components, optical components and computer software, any of which can contain errors or failures, especially when first introduced. In addition, new products such as ours or enhancements to them may contain undetected errors or performance problems that, despite testing, are discovered only after commercial shipment. Because our products will be designed to be used to perform complex surgical procedures, we expect that our customers will have an increased sensitivity to such defects. We cannot assure that, once developed and marketed, our products will not experience component aging, errors or performance problems. If we experience flaws or performance problems, any of the following could occur:
| ● | delays in product shipments; |
| ● | loss of revenue; |
| ● | delay in market acceptance; |
| ● | diversion of our resources; |
| ● | damage to our reputation; |
| ● | product recalls; |
| ● | regulatory actions; |
| ● | increased service or warranty costs; or |
| ● | product liability claims. |
The use of our products could result in product liability and negligence claims that could be expensive, divert management's attention and harm our business.
Our business will expose us to significant risks of product liability claims. The medical device industry has historically been litigious, and we will face financial exposure to product liability claims if the use of our products were to cause injury or death. There is also the possibility that defects in the design or manufacture of our products might necessitate a product recall. Any weaknesses in training and services associated with our products may also be subject to product liability lawsuits. Although we plan to obtain and maintain product liability insurance, the coverage limits of these policies may not be adequate to cover future claims. A product liability claim, regardless of its merit or eventual outcome, could result in significant legal defense costs. A product liability or negligence claim or any product recalls could also harm our reputation or result in a decline in revenues. Furthermore, if a patient is harmed by a surgical procedure in which our equipment is used, even in the absence of any alleged system malfunction or defect, we can be exposed to negligence claims based on alleged inadequacies in our training of physicians or our training of our personnel. A negligence claim, regardless of its merit or eventual outcome, could result in significant legal defense costs. Legal actions also divert management's attention from our business, resulting in a material adverse effect on our operations and financial results.
9
We do not have manufacturing experience or capabilities and we may encounter manufacturing problems or delays that could result in lost revenue.
We expect that manufacturing our planned medical robotic systems will be a complex process. As a result, we may encounter difficulties in scaling up production of our products, including:
| ● | problems involving production yields; |
| ● | quality control and assurance; |
| ● | component supply shortages; |
| ● | import or export restrictions on components, materials or technology; |
| ● | shortages of qualified personnel; and |
| ● | compliance with state, federal and foreign regulations. |
If demand for our products exceeds our manufacturing capacity, we could develop a substantial backlog of customer orders. If we are unable to maintain larger-scale manufacturing capabilities, our ability to generate revenues will be limited and our reputation in the marketplace could be damaged.
We do not have sales and marketing experience or capabilities, which could impair our ability to achieve profitability.
We do not have experience as a company in the sales and marketing of our planned products. As such, we may not be successful in marketing and selling those products that we develop and receive approval for in the U.S. or abroad. We intend to establish a sales and marketing organization supported by clinical and technical representatives who will provide training, clinical and technical support and other services to our customers before and during the surgery but may not be able to successfully create and sustain such an organization, which could delay the successful adoption of our products. Additionally, any sales and marketing organization that we develop may be competing against the experienced and well-funded sales and marketing organizations of some of our competitors. We will face significant challenges and risks in developing our sales and marketing organization, including, among others:
| ● | our ability to recruit, train and retain adequate numbers of qualified sales and marketing personnel; |
| ● | the ability of sales personnel to obtain access to leading surgeons and persuade adequate numbers of hospitals to purchase our products; |
| ● | costs associated with hiring, maintaining and expanding a sales and marketing organization; and |
| ● | government scrutiny with respect to promotional activities in the healthcare industry. |
If we are unable to develop and maintain these sales and marketing capabilities, we may be unable to generate revenue and may not become profitable.
10
If we are unable to protect the intellectual property to be contained in our products from use by third parties, our ability to compete in the market will be harmed.
Our commercial success will depend in part on patents we hope to receive and in obtaining additional patent and other intellectual property protection for the technologies to be contained in our products, and on successfully defending our patents and other intellectual property against third party challenges.
We may incur substantial costs in obtaining patents and, if necessary, defending our proprietary rights. The patent positions of medical device companies, including ours, can be highly uncertain and involve complex and evolving legal and factual questions. We cannot assure you that we will obtain the patent protection we seek or that the protection we receive will be found valid and enforceable if challenged. We also cannot assure you that we will be able to develop additional patentable proprietary technologies. If we fail to obtain adequate protection of our intellectual property, or if any protection we obtain is reduced or eliminated, others could use our intellectual property without compensating us, resulting in harm to our business. We may also determine that it is in our best interests to voluntarily challenge a third party's products or patents in litigation or administrative proceedings, including patent interferences or reexaminations. In addition, the laws of certain foreign countries do not protect intellectual property rights to the same extent as do the laws of the United States.
In addition to patents, we may rely on a combination of trade secret, copyright and trademark laws, nondisclosure agreements and other contractual provisions and technical security measures to protect our intellectual property rights. Nevertheless, these measures may not be adequate to safeguard the technology underlying our products. If these measures do not protect our rights adequately, third parties could use our technology, and our ability to compete in the market would be reduced. In addition, employees, consultants and others who participate in developing our products may breach their agreements with us regarding our intellectual property, and we may not have adequate remedies for the breach. We also may not be able to effectively protect our intellectual property rights in some foreign countries. For a variety of reasons, we may decide not to file for patent, copyright or trademark protection outside the United States. We also realize that our trade secrets may become known through other means not currently foreseen by us. Notwithstanding our efforts to protect our intellectual property, our competitors may independently develop similar or alternative technologies or products that are equal or superior to our technology and products without infringing any of our intellectual property rights, or may design around our proprietary technologies, which would harm our ability to compete in the market.
Others may assert that our products infringe on their intellectual property rights, which may cause us to engage in costly disputes and, if we are not successful in defending ourselves, could also require us to pay substantial damages, prohibiting us from selling our products.
We are aware of both United States and foreign patents issued to third parties that relate to computer-assisted surgery and minimally invasive surgery. We do not know whether any of these patents, if challenged, would be held valid, enforceable and infringed. The medical device industry has been characterized by extensive litigation and administrative proceedings regarding patents and other intellectual property rights, and companies have employed such actions to gain a competitive advantage. If third parties in any patent action are successful, our potential patent portfolio may be damaged, we may have to pay substantial damages, including treble damages, and we may be required to stop selling our products or obtain a license which, if available at all, may require us to pay substantial royalties. We cannot be certain that we will have the financial resources or the substantive arguments to defend our patents from infringement or claims of invalidity or unenforceability, or to defend against allegations of infringement of third-party patents. In addition, any public announcements related to litigation or administrative proceedings initiated by us, or initiated or threatened against us, could cause our stock price to decline.
11
The rights and measures we rely on to protect the intellectual property underlying our products may not be adequate to prevent third parties from using our technology, which could harm our ability to compete in the market.
In addition to patents, we plan to rely on a combination of trade secret, copyright and trademark laws, nondisclosure agreements and other contractual provisions and technical security measures to protect our intellectual property rights. Nevertheless, these measures may not be adequate to safeguard the technology underlying our products. If they do not protect our rights adequately, third parties could use our technology, and our ability to compete in the market would be reduced. In addition, employees, consultants and others who participate in developing our products may breach their agreements with us regarding our intellectual property, and we may not have adequate remedies for the breach. We also may not be able to effectively protect our intellectual property rights in some foreign countries. For a variety of reasons, we may decide not to file for patent, copyright or trademark protection outside the United States. We also realize that our trade secrets may become known through other means not currently foreseen by us. Notwithstanding our efforts to protect our intellectual property, our competitors may independently develop similar or alternative technologies or products that are equal or superior to our technology and products without infringing any of our intellectual property rights, or may design around our proprietary technologies.
We do not yet know what the consequences may be on our business of proposed significant changes in existing health care legislation.
President Trump, who took office on January 20, 2017, has pledged to repeal and replace the Patient Protection and Affordable Care Act (the "ACA"), which had been implemented by his predecessor, President Obama, in March 2010, in an effort to expand health insurance coverage to uninsured Americans. While the U.S. House of Representatives has passed a “repeal and replace bill," the Senate has not acted on such legislation and its future is uncertain. Accordingly, the timing and implementation of a repeal of the ACA and its substitution with a replacement plan and the effects thereof are uncertain. Accordingly, no assurance can be given that any legislation which is ultimately implemented will not have a material adverse effect on our planned business operations.
Our operations and our products will be subject to extensive domestic government regulation. If we fail to meet these compliance standards, our ability to market our products would be limited and our business and results of operations would be materially adversely affected.
Our products and operations will be subject to extensive and rigorous regulation in the United States by the United States Food and Drug Administration (the “FDA”) and by similar agencies in other countries or regions in which we may market our products. In addition, our products, when developed, must meet the requirements of a large and growing body of international standards which govern the design, manufacture, materials content and sourcing, testing, certification, packaging, installation, use and disposal of our products. We will be required to continually keep abreast of these standards and requirements and integrate compliance to these with the development and regulatory documentation for our products. Examples of groups of such standards are electrical safety standards such as those of the International Electrotechnical Commission (e.g. IEC 60601-ss series of standards), composition standards such as the Reduction of Hazardous Substances (RoHS) and Waste Electrical and Electronic Equipment (WEEE) Directives. Failure to comply with FDA or comparable foreign regulations or to meet these standards could materially adversely affect our business and results of operations.
We intend to utilize the services of third-party manufacturers and suppliers for our planned medical robotics system. While we have not as yet established relationships or entered into agreements with any such manufacturers or suppliers, if we do so, such third-party manufacturers or suppliers fail to comply with Federal, state or European manufacturing standards, our manufacturing operations could be interrupted and our product sales and operating results could suffer.
We intend to utilize the services of third-party manufacturers and suppliers for our planned medical robotics system. While we have not as yet established relationships or entered into agreements with any such manufacturers or suppliers, if we do so, such third-party manufacturers and suppliers will be required to comply with the FDA’s Good Manufacturing Practice requirements (“cGMP”) and requirements contained in the FDA’s Quality System Regulation (“QSR”), which covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our products. We and our manufacturers and suppliers will also be subject to compliance with International Organization for Standardization (“ISO”) quality system standards in order to produce products for sale in Europe, as well as the regulations of other foreign jurisdictions regarding the manufacturing process if we market our products elsewhere overseas. The FDA and the ISO enforce compliance with their respective systems through periodic and unannounced inspections of manufacturing facilities. To date, our facilities have not been subject to any inspections by regulatory authorities, but we anticipate that we and certain of our third-party manufacturers and suppliers will be subject to such inspections in the future. If our facilities or those of our manufacturers or suppliers fail to take satisfactory corrective action in response to an adverse inspection, the FDA, ISO, or other foreign governing agency could take enforcement action, including any of the following sanctions:
12
| ● | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| ● | customer notifications or repair, replacement, refunds, recall, detention or seizure of our products; |
| ● | operating restrictions or partial suspension or total shutdown of production; |
| ● | refusing or delaying requests for 510(k) clearance or PMA approvals of new products or modified products; |
| ● | withdrawing 510(k) clearances or PMA approvals that have already been granted; |
| ● | refusal to grant export approval for our products; or |
| ● | criminal prosecution. |
Compliance with complex foreign and U.S. laws and regulations that apply to our international operations increases our cost of doing business in international jurisdictions and could expose us or our employees to fines and penalties in the United States and/or abroad. These numerous and sometimes conflicting laws and regulations include U.S. laws such as the Foreign Corrupt Practices Act, and similar laws in foreign countries, such as the U.K. Bribery Act of 2010, which became effective on July 1, 2011. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers or our employees, prohibitions on the conduct of our business and damage to our reputation. Although we will implement policies and procedures designed to ensure compliance with these laws, there can be no assurance that our employees, contractors or agents will not violate our policies.
Complying with FDA regulations is a complex process, and our failure to obtain any necessary approvals could subject us to significant delay and could have an adverse effect on the Company
Our products and operations will be subject to extensive and rigorous regulation in the United States by the United States Food and Drug Administration (the “FDA”) and by similar agencies in other countries or regions in which we may market our products. Unless an exemption applies, each medical device that we intend to market in the U.S. must first receive either "510(k) clearance" or "Premarket (PMA) approval" from the FDA pursuant to the Federal Food, Drug, and Cosmetic Act. The FDA's 510(k) clearance process usually takes from four to 12 months, but it can last longer. The process of obtaining PMA approval is much more costly, lengthy and uncertain. It generally takes from one to three years or even longer. We cannot be sure that 510(k) clearance or PMA approval will be obtained in the future for any product we propose to market.
Complying with FDA regulations is a complex process, and our failure to comply fully could subject us to significant enforcement actions.
Because our products will be commercially distributed, numerous post-market regulatory requirements apply, including the following:
| ● | continued compliance with the QSR, which requires manufacturers to follow elaborate design, testing, control, documentation and other quality assurance procedures during the manufacturing process; |
| ● | labeling regulations; |
| ● | the FDA’s general prohibition against false or misleading statements in the labeling or promotion of products for unapproved or “off-label” uses; |
13
| ● | the Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; |
| ● | adequate use of the Corrective and Preventive Actions process to identify and correct or prevent significant systemic failures of products or processes or in trends which suggest same; and |
| ● | the reporting of Corrections and Removals, which requires that manufacturers report to the FDA recalls and field corrective actions taken to reduce a risk to health or to remedy a violation of the FFDCA that may pose a risk to health. |
We are subject to inspection and marketing surveillance by the FDA to determine our compliance with regulatory requirements. If the FDA finds that we have failed to comply, it can institute a wide variety of enforcement actions, ranging from a regulatory letter to a public Warning Letter to more severe civil and criminal sanctions including the seizure of our products and equipment or ban on the import or export of our products. Our failure to comply with applicable requirements could lead to an enforcement action that may have an adverse effect on our financial condition and results of operations.
To be able to market and sell our products in other countries, we must obtain regulatory approvals and comply with the regulations of those countries. These regulations, including the requirements for approvals, and the time required for regulatory review vary from country to country. Obtaining and maintaining foreign regulatory approvals is expensive, and we cannot be certain that we will receive regulatory approvals in any foreign country in which we plan to market our products. If we fail to obtain regulatory approval in any foreign country in which we plan to market our products, our ability to generate revenue will be harmed.
If we lose our key personnel or are unable to attract and retain additional personnel, our ability to compete will be harmed.
We are highly dependent on the principal members of our management and scientific staff. Our product development plans depend, in part, on our ability to attract and retain engineers with experience in mechanics, electronics and associated skills. Attracting and retaining qualified personnel will be critical to our success, and competition for qualified personnel is intense. We may not be able to attract and retain personnel on acceptable terms given the competition for such personnel among technology and healthcare companies and universities. The loss of any of these persons or our inability to attract and retain qualified personnel could harm our business and our ability to compete.
We currently rely on our CEO and the loss of his services could have an adverse effect on the Company.
Until we further build up our management infrastructure, our success depends in large part upon our CEO. The loss of his services would currently have a material adverse effect on AVRA. We are party to an employment agreement with our CEO but do not anticipate having key man insurance in place on him in the foreseeable future. Moreover, our CEO occupies similar executive and director positions in a number of companies, most of which are inactive. While we do not believe that such positions will materially interfere with his duties at AVRA or pose any conflict of interest, there can be no assurance given in this regard.
We intend to become subject to the periodic reporting requirements of the Securities Exchange Act of 1934 that will require us to incur audit fees and legal fees in connection with the preparation of such reports. These additional costs could reduce or eliminate our ability to earn a profit.
Following the effective date of our registration statement of which this prospectus is a part, we will be required to file periodic reports with the SEC pursuant to the Securities Exchange Act of 1934 (the “Exchange Act”) and the rules and regulations promulgated thereunder. In order to comply with these requirements, our independent registered public accounting firm will have to review our financial statements on a quarterly basis and audit our financial statements on an annual basis. Moreover, our legal counsel will have to review and assist in the preparation of such reports. The costs charged by these professionals for such services cannot be accurately predicted at this time because factors such as the number and type of transactions that we engage in and the complexity of our reports cannot be determined at this time and will have a major effect on the amount of time to be spent by our auditors and attorneys. However, the incurrence of such costs will obviously be an expense to our operations and thus have a negative effect on our ability to meet our overhead requirements and earn a profit. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock, if a market ever develops, could drop significantly.
14
Our internal controls may be inadequate, which could cause our financial reporting to be unreliable and lead to misinformation being disseminated to the public.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. As defined in Rule 13a-15(f) under the Exchange Act, internal control over financial reporting is a process designed by, or under the supervision of, the principal executive and principal financial officer and effected by the board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| ● | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and/or directors of the Company; and |
| ● | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. |
We will be required to include a report of management on the effectiveness of our internal control over financial reporting. We expect to incur additional expenses and diversion of management’s time as a result of performing the system and process evaluation, testing and remediation required in order to comply with the management certification requirements.
We do not have a sufficient number of employees to segregate responsibilities and may be unable to afford increasing our staff or engaging outside consultants or professionals to overcome our lack of employees. During the course of our testing, we may identify other deficiencies that we may not be able to timely remediate. Moreover, effective internal controls, particularly those related to revenue recognition, are necessary for us to produce reliable financial reports and are important to help prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock, if a market ever develops, could drop significantly.
The Jobs Act has reduced the information that the Company is required to disclose.
Under the Jobs Act, the information that the Company will be required to disclose has been reduced in a number of ways.
As a company that had gross revenues of less than $1 billion during the Company’s last fiscal year, the Company is an “emerging growth company,” as defined in the Jobs Act (an “EGC”). The Company will retain that status until the earliest of (a) the last day of the fiscal year which the Company has total annual gross revenues of $1,000,000,000 (as indexed for inflation in the manner set forth in the Jobs Act) or more; (b) the last day of the fiscal year of following the fifth anniversary of the date of the first sale of the common stock pursuant to an effective registration statement under the Securities Act; (c) the date on which the Company has, during the previous three year period, issued more than $1,000,000,000 in non-convertible debt; or (d) the date on which the Company is deemed to be a “large accelerated filer,” as defined in Rule 12b-2 under the Exchange Act or any successor thereto. As an EGC, the Company is relieved from the following:
15
| ● | The Company is excluded from Section 404(b) of Sarbanes-Oxley Act (“Sarbanes-Oxley”), which otherwise would have required the Company’s auditors to attest to and report on the Company’s internal control over financial reporting. The JOBS Act also amended Section 103(a)(3) of Sarbanes-Oxley to provide that (i) any new rules that may be adopted by the PCAOB requiring mandatory audit firm rotation or changes to the auditor’s report to include auditor discussion and analysis (each of which is currently under consideration by the PCAOB) shall not apply to an audit of an EGC; and (ii) any other future rules adopted by the PCAOB will not apply to the Company’s audits unless the SEC determines otherwise. |
| ● | The Jobs Act amended Section 7(a) of the Securities Act to provide that the Company need not present more than two years of audited financial statements in an initial public offering registration statement and in any other registration statement, need not present selected financial data pursuant to Item 301 of Regulation S-K for any period prior to the earliest audited period presented in connection with such initial public offering. In addition, the Company is not required to comply with any new or revised financial accounting standard until such date as a private company (i.e., a company that is not an “issuer” as defined by Section 2(a) of Sarbanes-Oxley) is required to comply with such new or revised accounting standard. Corresponding changes have been made to the Exchange Act, which relates to periodic reporting requirements, which would be applicable if the Company were required to comply with them. |
| ● | As long as the Company is an EGC, the Company may comply with Item 402 of Regulation S-K, which requires extensive quantitative and qualitative disclosure regarding executive compensation, by disclosing the more limited information required of a “smaller reporting company.” |
| ● | In the event that the Company registers the common stock under the Exchange Act as it intends to do, the Jobs Act will also exempt the Company from the following additional compensation-related disclosure provisions that were imposed on U.S. public companies pursuant to the Dodd-Frank Act: (i) the advisory vote on executive compensation required by Section 14A(a) of the Exchange Act; (ii) the requirements of Section 14A(b) of the Exchange Act relating to shareholder advisory votes on “golden parachute” compensation; (iii) the requirements of Section 14(i) of the Exchange Act as to disclosure relating to the relationship between executive compensation and our financial performance; and (iv) the requirement of Section 953(b)(1)of the Dodd-Frank Act, which requires disclosure as to the relationship between the compensation of the Company’s chief executive officer and median employee pay. |
The costs of being a public company could result in us being unable to continue as a going concern.
As a public company, we will have to comply with numerous financial reporting and legal requirements, including those pertaining to audits and internal control. The costs of this compliance could be significant. If our revenues do not increase and/or we cannot satisfy many of these costs through the issuance of our shares, we may be unable to satisfy these costs in the normal course of business which would result in our being unable to continue as a going concern.
Our status as an “emerging growth company” under the JOBS Act of 2012 may make it more difficult to raise capital as and when we need it.
Because of the exemptions from various reporting requirements provided to us as an “emerging growth company” and because we will have an extended transition period for complying with new or revised financial accounting standards, we may be less attractive to investors and it may be difficult for us to raise additional capital as and when we need it. Investors may be unable to compare our business with other companies in our industry if they believe that our financial accounting is not as transparent as other companies in our industry. If we are unable to raise additional capital as and when we need it, our financial condition and results of operations may be materially and adversely affected.
16
Our Articles of Incorporation, By Laws, employment agreements with our officers and appointment agreements with our directors, provide for indemnification of officers and directors at our expense and limit their liability that may result in a major cost to us and hurt the interests of our shareholders because corporate resources may be expended for the benefit of officers and/or directors.
Our Articles of Incorporation, Bylaws, employment agreements with our officers and appointment agreements with our directors, provide for the indemnification of our officers and directors. We have been advised that, in the opinion of the SEC, indemnification for liabilities arising under federal securities laws is against public policy as expressed in the Securities Act of 1933 (the “Securities Act”) and is, therefore, unenforceable.
The initial offering price of the shares has been arbitrarily determined by the Company.
The initial offering price of the shares has been arbitrarily determined by the Company and bears no relationship to the Company’s assets, book value, potential earnings or any other recognized criterion of value. The initial offering price of $2.00 per share will apply until our shares are quoted on the OTCQX or OTCQB tiers of the over-the-counter market operated by OTC Markets Group.
Currently, there is no public market for our securities, and we cannot assure you that any public market will ever develop and it is likely to be subject to significant price fluctuations.
Currently, there is no public market for our common stock and our common stock may never be traded on any exchange, or, if traded, a public market may not materialize. Even if we are successful in developing a public market, there may not be enough liquidity in such market to enable shareholders to sell their stock.
Our common stock is unlikely to be followed by any market analysts, and there may be few or no institutions acting as market makers for the common stock. Either of these factors could adversely affect the liquidity and trading price of our common stock. Until our common stock is fully distributed and an orderly market develops in our common stock, if ever, the price at which it trades is likely to fluctuate significantly. Prices for our common stock will be determined in the marketplace and may be influenced by many factors, including the depth and liquidity of the market for shares of our common stock, developments affecting our business, including the impact of the factors referred to elsewhere in these Risk Factors, investor perception of the Company, and general economic and market conditions. No assurances can be given that an orderly or liquid market will ever develop for the shares of our common stock.
If a trading market should develop for our common stock, it is likely that it will initially be deemed to be a “penny stock.” Therefore, trading of our common stock may be restricted by the SEC’s penny stock regulations and FINRA’s sales practice requirements, which may limit a shareholder’s ability to buy and sell our common stock.
In addition to the “penny stock” rules promulgated by the SEC, the Financial Industry Regulatory Authority (“FINRA”) has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, the FINRA believes that there is a high probability that speculative low-priced securities will not be suitable for at least some customers. FINRA’s requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock.
The market for penny stocks has experienced numerous frauds and abuses that could adversely impact investors in our stock.
Company management believes that the market for penny stocks has suffered from patterns of fraud and abuse. Such patterns include:
| ● | Control of the market for the security by one or a few broker-dealers that are often related to a promoter or issuer; |
| ● | Manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; |
17
| ● | “Boiler room” practices involving high pressure sales tactics and unrealistic price projections by sales persons; |
| ● | Excessive and undisclosed bid-ask differentials and markups by selling broker-dealers; and |
| ● | Wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the inevitable collapse of those prices with consequent investor losses. |
Any trading market that may develop for our common stock may be restricted by virtue of state securities “Blue Sky” laws that prohibit trading absent compliance with individual state laws. These restrictions may make it difficult or impossible to sell shares in those states.
There is currently no established public market for our common stock, and there can be no assurance that any established public market will develop in the foreseeable future. Transfer of our common stock may also be restricted under the securities laws and regulations promulgated by various states and foreign jurisdictions, commonly referred to as “blue sky” laws. Absent compliance with such individual state laws, our common stock may not be traded in such jurisdictions. Because the securities being registered hereunder have not been registered for resale under the blue sky laws of any state, the holders of such shares, and persons who desire to purchase them in any trading market that might develop in the future, should be aware that there may be significant state blue sky law restrictions upon the ability of investors to sell the securities and of purchasers to purchase the securities. These restrictions prohibit the secondary trading of our common stock. We currently do not intend to and may not be able to qualify securities for resale in a number of states that do not offer manual exemptions and require shares to be qualified before they can be resold by our shareholders. Accordingly, investors should consider the secondary market for our securities to be a limited one.
Our board of directors has the authority, without shareholder approval, to issue preferred stock with terms that may not be beneficial to common shareholders and with the ability to affect adversely shareholder voting power and perpetuate their control over us.
Our Amended and Restated Articles of Incorporation allows us to issue shares of preferred stock without any vote or further action by our shareholders. Our board of directors has the authority to fix and determine the relative rights and preferences of preferred stock. As a result, our board of directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock and the right to the redemption of the shares, together with a premium, prior to the redemption of our common stock.
The ability of our executive officers and directors, who are our principal shareholders, to control our business may limit or eliminate minority shareholders’ ability to influence corporate affairs.
Our executive officers and directors, who are our principal shareholders, own and, assuming the sale of the shares registered by them hereunder as selling shareholders, will continue to own a majority of our issued and outstanding common stock. Accordingly, they will be able to effectively control the election of directors, as well as all other matters requiring shareholder approval. The interests of our principal shareholders may differ from the interests of other shareholders with respect to the issuance of shares, business transactions with or sales to other companies, selection of other directors and other business decisions. The minority shareholders have no way of overriding decisions made by our principal shareholders. This level of control may also have an adverse impact on the market value of our shares because our principal shareholders may institute or undertake transactions, policies or programs that result in losses and may not take any steps to increase our visibility in the financial community and / or may sell sufficient numbers of shares to significantly decrease our price per share.
18
We do not expect to pay cash dividends in the foreseeable future.
We have never paid cash dividends on our common stock. We do not expect to pay cash dividends on our common stock at any time in the foreseeable future. The future payment of dividends directly depends upon our future earnings, capital requirements, financial requirements and other factors that our board of directors will consider. Since we do not anticipate paying cash dividends on our common stock, return on your investment, if any, will depend solely on an increase, if any, in the market value of our common stock.
Because we are not subject to compliance with rules requiring the adoption of certain corporate governance measures, our shareholders have limited protection against interested director transactions, conflicts of interest and similar matters.
Sarbanes-Oxley, as well as rule changes proposed and enacted by the SEC, the New York Stock Exchange/NYSE/AMEX and the Nasdaq Stock Market, as a result of Sarbanes-Oxley, require the implementation of various measures relating to corporate governance. These measures are designed to enhance the integrity of corporate management and the securities markets and apply to securities that are listed on those exchanges or the Nasdaq Stock Market. Because we are not presently required to comply with many of the corporate governance provisions and because we chose to avoid incurring the substantial additional costs associated with voluntary compliance, we have not yet adopted these measures.
We do not currently have independent audit or compensation committees. As a result, directors have the ability, among other things, to determine their own level of compensation. Until we comply with such corporate governance measures, regardless of whether such compliance is required, the absence of such standards of corporate governance may leave our shareholders without protections against interested director transactions, conflicts of interest, if any, and similar matters and investors may be reluctant to provide us with funds necessary to expand our operations as a result thereof.
We intend to comply with all corporate governance measures relating to director independence as and when required. However, we may find it very difficult or be unable to attract and retain qualified officers, directors and members of board committees required to provide for our effective management as a result of Sarbanes-Oxley and enactment of Sarbanes-Oxley has resulted in a series of rules and regulations by the SEC that increase responsibilities and liabilities of directors and executive officers. The perceived increased personal risk associated with these recent changes may make it costlier or deter qualified individuals from accepting these roles.
We will not receive any proceeds from the sale of the common stock offered through this prospectus by the selling shareholders. We have agreed to bear the expenses (other than any underwriting discounts or commissions or broker’s commissions) in connection with the registration of the common stock being offered hereby by the selling shareholders.
DETERMINATION OF OFFERING PRICE
Our common stock is presently not traded on any market or securities exchange. Given the foregoing, the selling shareholders will offer their shares at an initial offering price of $2.00 per share until the shares are quoted on the OTCQX or the OTCQB tiers of the over-the counter operated by OTC Markets Group. The initial offering price of the shares has been arbitrarily determined by the Company and bears no relationship to the Company’s assets, book value, potential earnings or any other recognized criterion of value. Although we intend to apply for quotation of our common stock on the OTCQX or the OTCQB tiers of the over-the counter market operated by OTC Markets Group, we may not secure this qualification. If we secure this qualification, the sale price to the public of the shares registered hereunder will be at prevailing market prices or privately negotiated prices. Accordingly, in such event the offering price will be determined by market factors and the independent decisions of the selling shareholders.
The common stock to be sold by the selling shareholders is common stock that is currently issued and outstanding. Accordingly, there will be no dilution to our existing shareholders as a result of this offering.
19
This prospectus covers the resale from time to time by the selling shareholders identified in the table below of up to 10,802,714 shares of our common stock, which were issued in various transactions exempt from registration under the Securities Act, as follows
| · | 5,240,160 of the shares registered hereby were issued to our founder in a private transaction upon incorporation of the Company on February 4, 2015, of which 1,497,880 of such shares were subsequently gifted to family members or sold to third parties in private sales; |
| · | 3,372,240 of the shares registered hereby were issued to initial shareholders and investors (other than our founder) in a private offering completed effective February 1, 2016; |
| · | 480,000 of the shares registered hereby are issuable upon conversion of the Convertible Notes, which we sold to 11 investors in a private offering consummated in various tranches from April to August 2016; and |
| · | 81,000 of the shares registered hereby were issued in a private offering to three investors consummated in February 2017. |
All of the foregoing shares were originally issued pursuant to the exemptions from registration afforded by Section 4(a)(2) under the Securities Act and Regulation D promulgated thereunder.
We are registering the shares to permit the selling shareholders and any of their pledgees, donees, transferees, assignees and successors-in-interest to, from time to time, sell any or all of their shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions when and as they deem appropriate in the manner described in “Plan of Distribution.” As of the date of this prospectus there are 19,192,438 shares of our common stock issued and outstanding.
The following table sets forth, as of the date of this prospectus, the name of each selling shareholder, the number and percentage of shares of our common stock beneficially owned by each selling shareholder prior to the offering for resale of the shares under this prospectus, the number of shares of our common stock beneficially owned by each selling shareholder that may be offered from time to time under this prospectus, and the number and percentage of shares of our common stock beneficially owned by the selling shareholder after the offering of the shares (assuming all of the offered shares are sold by the selling shareholder.
There are no agreements between the Company and any selling shareholder pursuant to which the shares subject to this registration statement were issued. The following selling shareholders have material relationships with the Company within the past three years:
| Name of Selling Shareholder | Nature of Relationship | |
| Barry F. Cohen | Founder, Chief Executive Officer and Director | |
| A. Christian Schauer | Chief Financial Officer | |
| Alexandre S. Clug | Vice President of Global Business Development | |
| Peter Carnegie | Director | |
| Thomas Grace | Senior Advisor | |
| Alen York | Senior Advisor | |
| Susan Friedlander Calzone | Senior Advisor | |
| Vipul Patel | Member, Medical Advisory Board | |
| Hiep T. Nguyen | Member, Medical Advisory Board | |
| Juan Jose Badimon | Member, Medical Advisory Board | |
| Yuman Fong | Member, Medical Advisory Board | |
| Heywood Y. Epstein | Member, Medical Advisory Board | |
| Farhan Taghizadeh | Member, Medical Advisory Board | |
| Ruth Pollak | Member, Medical Advisory Board | |
| Andrew Economos | Member, Medical Advisory Board | |
| Dale S. Bergman | Outside Corporate and Securities Counsel |
Beneficial ownership is determined in accordance with the rules of the SEC, and includes any shares of common stock as to which a person has sole or shared voting power or investment power and any shares of common stock which the person has the right to acquire within sixty (60) days through the exercise of any option, warrant or right, through conversion of any security or pursuant to the automatic termination of a power of attorney or revocation of a trust, discretionary account or similar arrangement.
20
| Name of Selling Shareholder | Total Shares
Owned by Selling Shareholder ** | Total Shares
to be Registered Pursuant to this Offering | Percentage of
Common Stock Before Offering ** | NOTE | Number of Shares
Owned by Selling Shareholder After Offering | Percentage of
Common Stock After Offering ** | ||||||||||||||||||
| Barry F. Cohen | 7,571,838 | 3,771,080 | 35.4 | % | 1 | 3,800,758 | 17.7 | % | ||||||||||||||||
| Avra Acquisitions, LLC | 3,672,700 | 1,469,080 | 17.1 | % | 1 | 2,203,620 | 10.3 | % | ||||||||||||||||
| The Mustang Trust | 1,000,000 | 500,000 | 4.7 | % | 2 | 500,000 | 2.3 | % | ||||||||||||||||
| Robert Beuret Revocable Trust | 900,000 | 450,000 | 4.2 | % | 3 | 450,000 | 2.1 | % | ||||||||||||||||
| The Fleisher Family Trust | 700,000 | 350,000 | 3.3 | % | 4 | 350,000 | 1.6 | % | ||||||||||||||||
| Sudhir Prem Srivastava | 320,000 | 160,000 | 1.5 | % | 160,000 | * | ||||||||||||||||||
| Andrew M. Economos | 312,500 | 187,500 | 1.5 | % | 125,000 | * | ||||||||||||||||||
| A. Christian Schauer | 296,000 | 148,000 | 1.4 | % | 148,000 | * | ||||||||||||||||||
| Thomas G. Grace | 241,417 | 144,850 | 1.1 | % | 19 | 96,567 | * | |||||||||||||||||
| Jacqueline Weisblum | 230,000 | 115,000 | 1.1 | % | 115,000 | * | ||||||||||||||||||
| Peter Carnegie | 224,167 | 89,667 | 1.0 | % | 134,500 | * | ||||||||||||||||||
| Robotic Minds, LLC | 213,500 | 128,100 | * | 5 | 85,400 | * | ||||||||||||||||||
| Davos Partners, LP | 210,000 | 126,000 | * | 6 | 84,000 | * | ||||||||||||||||||
| W Capital Management Limited | 200,000 | 120,000 | * | 7 | 80,000 | * | ||||||||||||||||||
| Pauline G. Runkle | 200,000 | 120,000 | * | 80,000 | * | |||||||||||||||||||
| Helayne R. Cohen | 200,000 | 100,000 | * | 100,000 | * | |||||||||||||||||||
| Sharon J. Doyle | 200,000 | 100,000 | * | 100,000 | * | |||||||||||||||||||
| Nicholas M. Cohen | 200,000 | 100,000 | * | 100,000 | * | |||||||||||||||||||
| Gary S. Cohen | 200,000 | 100,000 | * | 100,000 | * | |||||||||||||||||||
| Eardley Holding AG | 200,000 | 100,000 | * | 20, 22 | 100,000 | * | ||||||||||||||||||
| Alexander C. Spektor | 175,000 | 105,000 | * | 70,000 | * | |||||||||||||||||||
| Josephine Kling Trippe | 175,000 | 87,500 | * | 87,500 | * | |||||||||||||||||||
| Jack Pechter | 165,000 | 99,000 | * | 66,000 | * | |||||||||||||||||||
| Alen Sands York | 162,500 | 97,500 | * | 65,000 | * | |||||||||||||||||||
| Alexandre S. Clug | 150,000 | 75,000 | * | 2 | 75,000 | * | ||||||||||||||||||
| Robert Chanson & Katrin Chanson | 150,000 | 75,000 | * | 20 | 75,000 | * | ||||||||||||||||||
| Edward Andrew Kerbs | 130,000 | 78,000 | * | 52,000 | * | |||||||||||||||||||
| Paul M. Ross | 115,000 | 69,000 | * | 46,000 | * | |||||||||||||||||||
| Ruth Pollak | 106,250 | 63,750 | * | 42,500 | * | |||||||||||||||||||
| Barbara Present | 100,000 | 60,000 | * | 40,000 | * | |||||||||||||||||||
| Bruno Marcel Bernasconi | 100,000 | 50,000 | * | 20 | 50,000 | * | ||||||||||||||||||
| David P. Hanlon Living Trust | 100,000 | 50,000 | * | 20, 21 | 50,000 | * | ||||||||||||||||||
| Susan M. Ferris | 100,000 | 50,000 | * | 20 | 50,000 | * | ||||||||||||||||||
| Anthony J. Nicholson Trust TTEE | 90,000 | 36,000 | * | 8 | 54,000 | * | ||||||||||||||||||
| Susan Friedlander Calzone | 83,750 | 50,250 | * | 33,500 | * | |||||||||||||||||||
| Steven T. Calzone | 75,000 | 45,000 | * | 30,000 | * | |||||||||||||||||||
| Paula E. Grimm | 75,000 | 45,000 | * | 30,000 | * | |||||||||||||||||||
| Fire Coral Holdings, LLC | 71,000 | 42,600 | * | 14, 20 | 28,400 | * | ||||||||||||||||||
| Kerry Firstenberg | 65,000 | 39,000 | * | 26,000 | * | |||||||||||||||||||
| Robert Chanson | 64,000 | 38,400 | * | 25,600 | * | |||||||||||||||||||
| Daniel J. Gardella | 60,000 | 36,000 | * | 24,000 | * | |||||||||||||||||||
21
| Name of Selling Shareholder | Total Shares
Owned by Selling Shareholder ** | Total Shares
to be Registered Pursuant to this Offering | Percentage of
Common Stock Before Offering ** | NOTE | Number of Shares
Owned by Selling Shareholder After Offering | Percentage of
Common Stock After Offering ** | ||||||||||||||||||
| OM Capital LLC | 60,000 | 30,000 | * | 20, 24 | 30,000 | * | ||||||||||||||||||
| David P. Hanlon Living Trust | 58,000 | 34,800 | * | 9 | 23,200 | * | ||||||||||||||||||
| Nicole Bergman-Fong | 51,250 | 30,750 | * | 20,500 | * | |||||||||||||||||||
| William Lovelace | 50,000 | 30,000 | * | 20,000 | * | |||||||||||||||||||
| Pemon LLC | 50,000 | 30,000 | * | 10 | 20,000 | * | ||||||||||||||||||
| William R. Beuret | 50,000 | 30,000 | * | 20,000 | * | |||||||||||||||||||
| Bonny Beuret | 50,000 | 30,000 | * | 20,000 | * | |||||||||||||||||||
| Felix Gaehwiler | 50,000 | 25,000 | * | 20 | 25,000 | * | ||||||||||||||||||
| Green-A-Way Investments and Ventures Ltd. | 50,000 | 25,000 | * | 20, 23 | 25,000 | * | ||||||||||||||||||
| Inez E. D'Arcangelo | 50,000 | 25,000 | * | 20 | 25,000 | * | ||||||||||||||||||
| Robert Brown & Louise Loring Brown | 50,000 | 25,000 | * | 20 | 25,000 | * | ||||||||||||||||||
| Mary A. Perez | 45,000 | 27,000 | * | 18,000 | * | |||||||||||||||||||
| Yuman Fong | 43,500 | 26,100 | * | 17,400 | * | |||||||||||||||||||
| Ray Powers | 42,917 | 17,167 | * | 25,750 | * | |||||||||||||||||||
| Auris Surgical Robotics | 41,000 | 24,600 | * | 11 | 16,400 | * | ||||||||||||||||||
| Sandra R. Johnson | 37,500 | 22,500 | * | 15,000 | * | |||||||||||||||||||
| Conner S. Calzone | 36,000 | 21,600 | * | 14,400 | * | |||||||||||||||||||
| Hannah B. Calzone | 36,000 | 21,600 | * | 14,400 | * | |||||||||||||||||||
| Farhan Taghizadeh | 33,500 | 20,100 | * | 13,400 | * | |||||||||||||||||||
| Juan Jose Badimon | 33,500 | 20,100 | * | 13,400 | * | |||||||||||||||||||
| Christine A. Bayliss | 32,000 | 19,200 | * | 12,800 | * | |||||||||||||||||||
| OM Capital LLC | 31,000 | 18,600 | * | 12 | 12,400 | * | ||||||||||||||||||
| Anthony J. Nicholson | 30,000 | 18,000 | * | 12,000 | * | |||||||||||||||||||
| Chikkodi, LLC | 30,000 | 18,000 | * | 13 | 12,000 | * | ||||||||||||||||||
| Hiep T. Nguyen | 28,500 | 22,800 | * | 5,700 | * | |||||||||||||||||||
| Susan M. Ferris | 26,000 | 15,600 | * | 10,400 | * | |||||||||||||||||||
| Marcia Schotz | 25,000 | 15,000 | * | 10,000 | * | |||||||||||||||||||
| Stephanie Zacharisiewicz | 25,000 | 15,000 | * | 10,000 | * | |||||||||||||||||||
| Keith Kim | 25,000 | 15,000 | * | 10,000 | * | |||||||||||||||||||
| Michael J. Plotkowski | 20,000 | 12,000 | * | 8,000 | * | |||||||||||||||||||
| Nalin H. Tolia | 20,000 | 12,000 | * | 8,000 | * | |||||||||||||||||||
| Robert R. Santangelo | 20,000 | 12,000 | * | 8,000 | * | |||||||||||||||||||
| Robin Waite | 20,000 | 12,000 | * | 8,000 | * | |||||||||||||||||||
| Nick & Leena Shroff TTEES | 20,000 | 12,000 | * | 15 | 8,000 | * | ||||||||||||||||||
| Heywood Y. Epstein | 19,500 | 15,600 | * | 3,900 | * | |||||||||||||||||||
| Margaret A. Gilliam | 18,000 | 12,000 | * | 6,000 | * | |||||||||||||||||||
| Eric Goldman | 15,000 | 12,000 | * | 3,000 | * | |||||||||||||||||||
| Kathleene G. Chambers | 15,000 | 12,000 | * | 3,000 | * | |||||||||||||||||||
| Lois Ross | 15,000 | 12,000 | * | 3,000 | * | |||||||||||||||||||
| Phyllis J. Schauer | 15,000 | 12,000 | * | 3,000 | * | |||||||||||||||||||
| Catherine L. Douglas | 15,000 | 12,000 | * | 3,000 | * | |||||||||||||||||||
| Pamela Schlissel | 15,000 | 12,000 | * | 3,000 | * | |||||||||||||||||||
| Myra Cohen | 12,500 | 10,000 | * | 2,500 | * | |||||||||||||||||||
| Barbara Smith | 12,000 | 9,600 | * | 2,400 | * | |||||||||||||||||||
| Barbro C. Ehnbom | 12,000 | 9,600 | * | 2,400 | * | |||||||||||||||||||
| Bernard Thomas | 12,000 | 9,600 | * | 2,400 | * | |||||||||||||||||||
| Leonard Sandacata Trust | 11,000 | 8,800 | * | 16 | 2,200 | * | ||||||||||||||||||
| Grace Family Foundation | 10,000 | 6,000 | * | 19 | 4,000 | * | ||||||||||||||||||
| Arnold Ferolito | 10,000 | 8,000 | * | 2,000 | * | |||||||||||||||||||
| Dale Bergman | 50,000 | 8,000 | * | 42,000 | * | |||||||||||||||||||
| Gabriel Liscano | 10,000 | 8,000 | * | 2,000 | * | |||||||||||||||||||
| Kevin S. Forkey | 10,000 | 8,000 | * | 2,000 | * | |||||||||||||||||||
| Lance Coetzee | 10,000 | 8,000 | * | 2,000 | * | |||||||||||||||||||
| Lisa Gardella Bloom | 10,000 | 8,000 | * | 2,000 | * | |||||||||||||||||||
| Richard Roth | 10,000 | 8,000 | * | 2,000 | * | |||||||||||||||||||
| Robert S. & Janelle B. Scherban | 10,000 | 8,000 | * | 2,000 | * | |||||||||||||||||||
| Fanny Barret | 10,000 | 8,000 | * | 2,000 | * | |||||||||||||||||||
| Jo Anna Beazley | 10,000 | 8,000 | * | 2,000 | * | |||||||||||||||||||
| Marla S. Fleisher | 10,000 | 8,000 | * | 2,000 | * | |||||||||||||||||||
| Jeffery Berkley | 10,000 | 8,000 | * | 2,000 | * | |||||||||||||||||||
| Rufino Lopez | 9,000 | 7,200 | * | 1,800 | * | |||||||||||||||||||
| Vineet Shah | 9,000 | 7,200 | * | 1,800 | * | |||||||||||||||||||
| Michael E. Newman | 8,000 | 6,400 | * | 1,600 | * | |||||||||||||||||||
| Henry Gewanter | 7,500 | 6,000 | * | 1,500 | * | |||||||||||||||||||
| Ingrid Stadtaus | 7,500 | 6,000 | * | 1,500 | * | |||||||||||||||||||
| Marcia Ann Meacham | 7,500 | 6,000 | * | 1,500 | * | |||||||||||||||||||
| Mandy Dowd | 7,000 | 5,600 | * | 1,400 | * | |||||||||||||||||||
| Michael L. Plotkowski | 6,500 | 5,200 | * | 1,300 | * | |||||||||||||||||||
| Ettore Tomasetti | 6,000 | 4,800 | * | 1,200 | * | |||||||||||||||||||
| George Fertitta | 6,000 | 4,800 | * | 1,200 | * | |||||||||||||||||||
| Jesse York | 6,000 | 4,800 | * | 1,200 | * | |||||||||||||||||||
22
| Name of Selling Shareholder | Total Shares
Owned by Selling Shareholder ** | Total Shares
to be Registered Pursuant to this Offering | Percentage of
Common Stock Before Offering ** | NOTE | Number of Shares
Owned by Selling Shareholder After Offering | Percentage of
Common Stock After Offering ** | ||||||||||||||||||
| John-Henry Falk | 6,000 | 4,800 | * | 1,200 | * | |||||||||||||||||||
| Rafaela Echeverria | 6,000 | 4,800 | * | 1,200 | * | |||||||||||||||||||
| Aures Systems Ltd. | 5,000 | 4,000 | * | 17 | 1,000 | * | ||||||||||||||||||
| Eldorado Capital LLC | 5,000 | 4,000 | * | 18 | 1,000 | * | ||||||||||||||||||
| Elizabeth Ann Brown | 5,000 | 4,000 | * | 1,000 | * | |||||||||||||||||||
| James Dixon | 5,000 | 4,000 | * | 1,000 | * | |||||||||||||||||||
| Paul Howard Hollander | 5,000 | 4,000 | * | 1,000 | * | |||||||||||||||||||
| Vartan Mardirossian | 5,000 | 4,000 | * | 1,000 | * | |||||||||||||||||||
| Doug Holland | 5,000 | 4,000 | * | 1,000 | * | |||||||||||||||||||
| Frederick J. Yestadt | 4,650 | 3,720 | * | 930 | * | |||||||||||||||||||
| JoAnne R. Mulhall | 4,650 | 3,720 | * | 930 | * | |||||||||||||||||||
| Melvin Stier | 4,500 | 3,600 | * | 900 | * | |||||||||||||||||||
| Cheryl A. Cofrin | 4,000 | 3,200 | * | 800 | * | |||||||||||||||||||
| David Braden | 3,700 | 2,960 | * | 740 | * | |||||||||||||||||||
| Brian McLaughlin | 3,250 | 2,600 | * | 650 | * | |||||||||||||||||||
| David Franzen | 3,000 | 2,400 | * | 600 | * | |||||||||||||||||||
| Felipe Gonzalez-Gordon | 3,000 | 2,400 | * | 600 | * | |||||||||||||||||||
| Jonas Franzen | 3,000 | 2,400 | * | 600 | * | |||||||||||||||||||
| Karen A. Grimley | 2,500 | 2,000 | * | 500 | * | |||||||||||||||||||
| Lesley A. Healy-Curi | 2,500 | 2,000 | * | 500 | * | |||||||||||||||||||
| Lisa Jean LaChance | 2,500 | 2,000 | * | 500 | * | |||||||||||||||||||
| Morris Westman | 2,500 | 2,000 | * | 500 | * | |||||||||||||||||||
| Roger S. Edling | 2,500 | 2,000 | * | 500 | * | |||||||||||||||||||
| Stuart Howitt | 2,500 | 2,000 | * | 500 | * | |||||||||||||||||||
| Timothy Dowd | 2,250 | 1,800 | * | 450 | * | |||||||||||||||||||
| David R. Burch | 2,100 | 1,680 | * | 420 | * | |||||||||||||||||||
| Peter Acker | 1,800 | 1,440 | * | 360 | * | |||||||||||||||||||
| Catherine Cassat | 1,000 | 800 | * | 200 | * | |||||||||||||||||||
| Charles Dowd | 1,000 | 800 | * | 200 | * | |||||||||||||||||||
| James A. Stoller | 1,000 | 800 | * | 200 | * | |||||||||||||||||||
| Michael C. Dowd | 1,000 | 800 | * | 200 | * | |||||||||||||||||||
_________________________
* Less than 1%.
** Includes shares issuable upon exercise of options within sixty (60) days of the date of this prospectus and upon conversion of the Convertible Notes.
| 1. | 7,571,838 shares owned by Mr. Barry F. Cohen directly and 3,672,700 shares held by Avra Acquisitions, LLC of which Mr. Cohen is managing member and over shares Mr. Cohen exercises voting and dispositive control. |
| 2. | 150,000 shares owned by Mr. Alexandre Clug directly and 1,000,000 shares held by The Mustang Trust of which Mr. Clug is Trustee and over shares Mr. Clug exercises voting and dispositive control. |
| 3. | Shares held by Robert Beuret Revocable Trust of which Mr. Robert Beuret is Trustee and over shares Mr. Beuret exercises voting and dispositive control. |
| 4. | Shares held by The Fleisher Family Trust of which Seymour & Libby Fleisher are Trustees and over shares Mr. and Mrs. Fleisher exercise voting and dispositive control. |
| 5. | Shares held by Robotic Minds, LLC of which Mr. Vipul Patel is managing member and over shares Mr. Patel exercises voting and dispositive control. |
| 6. | Shares held by Davos Partners, LP of which Mr. David Nolan is managing member and over shares Mr. Nolan exercises voting and dispositive control. |
| 7. | Shares held by W Capital Management Limited of which Jakob Hirschbaek and Tim Brockmann are Directors and over shares Mr. Hirschbaek and Mr. Brockmann exercise voting and dispositive control. |
| 8. | Shares held by Anthony J. Nicholson Trust TTEE of which Anthony J. Nicholson is Trustee and over shares Mr. Nicholson exercises voting and dispositive control. |
| 9. | Shares held by David P. Hanlon Living Trust of which David P. Hanlon is Trustee and over shares Mr. Hanlon exercises voting and dispositive control. |
| 10. | Shares held by Pemon LLC of which Eduardo Parra-Davila is managing member and over shares Mr. Davila exercises voting and dispositive control. |
| 11. | Shares held by Auris Surgical Robotics of which Frederic H. Moll is managing member and over shares Mr. Moll exercises voting and dispositive control. |
| 12. | Shares held by OM Capital LLC of which Mr. Bhambai is managing member and over shares Mr. Bhambai exercises voting and dispositive control. |
| 13. | Shares held by Chikkodi LLC of which Arathi Gadasalli is managing member and over shares Mr. Gadasalli exercises voting and dispositive control. |
| 14. | Shares held by Fire Coral Holdings LLC of which Jay E. Lasner is managing member and over shares Mr. Lasner exercises voting and dispositive control and of which 50,000 shares are as a result of the Note Conversion. |
| 15. | Shares held by Nick & Leena Shroff TTEES of which Nick Shroff is Trustee and over shares Mr. Shroff exercises voting and dispositive control. |
| 16. | Shares held by Leonard Sandacata Trust of which Leonard Sandacata is Trustee and over shares Mr. Sandacata exercises voting and dispositive control. |
| 17. | Shares held by Aures Systems Ltd. of which Michael Foudy is managing member and over shares Mr. Foudy exercises voting and dispositive control. |
| 18. | Shares held by Eldorado Capital LLC of which Jerry McRoberts is managing member and over shares Mr. McRoberts exercises voting and dispositive control. |
| 19. | Number includes 250,000 shares owned by Mr. Thomas G. Grace directly and 10,000 shares held by Thomas Grace Foundation of which Mr. Grace is managing member and over shares Mr. Grace exercises voting and dispositive control. |
| 20. | Shares issued as a result of the Conversion of Convertible Note |
| 21. | Shares held by David P. Hanlon Living Trust of which David P. Hanlon is Trustee and over shares Mr. Hanlon exercises voting and dispositive control. |
| 22. | Shares held by Eardley Holding AG of which Thomas Staehelin is Trustee and over shares Mr. Staehelin exercises voting and dispositive control. |
| 23. | Shares held by Green-A-Way Investments and Ventures Ltd. of which Daniel Amman is Trustee and over shares Mr. Ammanexercises voting and dispositive control. |
| 24. | Shares held by OM Capital LLC of which Niranjan Bhambhani is Trustee and over shares Mr. Bhambhani exercises voting and dispositive control. |
23
The selling shareholders and any of their pledgees, donees, transferees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. As there is currently no trading market for our shares, the selling shareholders will offer their shares at an initial offering price of $2.00 per share until the Company’s shares are quoted on the OTCQX or the OTCQB tiers of the over-the-counter market operated by the OTC Markets Group. Assuming we secure this qualification, thereafter the shares may be sold at fixed or negotiated prices. The selling shareholders may use any one or more of the following methods when selling shares:
| · | ordinary brokerage transactions and transactions in which the broker-dealer solicits investors; |
| · | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| · | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; | |
| · | an exchange distribution in accordance with the rules of the applicable exchange; |
| · | privately negotiated transactions; |
| · | to cover short sales made after the date that this registration statement is declared effective by the SEC; |
| · | broker-dealers may agree with the selling shareholders to sell a specified number of such shares at a stipulated price per share; |
| · | through the distribution of common stock by any selling shareholder to its partners, members or shareholders; |
| · | any other method permitted pursuant to applicable law; and |
| · | a combination of any such methods of sale. |
Broker-dealers engaged by the selling shareholders may arrange for broker-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling shareholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The selling shareholders do not expect these commissions and discounts to exceed what is customary in the types of transactions involved.
The selling shareholders may from time to time pledge or grant a security interest in some or all of the shares of common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell shares of common stock from time to time under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling shareholders to include the pledgee, transferee or other successors in interest as selling shareholders under this prospectus.
Upon a selling shareholder’s notification to us that any material arrangement has been entered into with a broker-dealer for the sale of such shareholder’s common stock through a block trade, special offering, exchange distribution or secondary distribution or a purchase by a broker or dealer, a supplement to this prospectus will be filed, if required, pursuant to Rule 424(b) under the Securities Act disclosing (i) the name of each such selling shareholder and of the participating broker-dealer(s), (ii) the number of shares involved, (iii) the price at which such shares of common stock were sold, (iv) the commissions paid or discounts or concessions allowed to such broker-dealer(s), where applicable, (v) that such broker-dealer(s) did not conduct any investigation to verify the information set out or incorporated by reference in this prospectus, and (vi) other facts material to the transaction. In addition, upon our being notified in writing by a selling shareholder that a donee or pledgee intends to sell more than 500 shares of common stock, a supplement to this prospectus will be filed if then required in accordance with applicable securities law.
24
The selling shareholders also may transfer the shares of common stock in other circumstances, in which case the donees, assignees, transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus and may sell the shares of common stock from time to time under this prospectus after we have filed any necessary supplements to this prospectus under Rule 424(b), or other applicable provisions of the Securities Act supplementing or amending the list of selling shareholders to include such donee, assignee, transferee, pledgee, or other successor-in-interest as a selling shareholder under this prospectus.
In the event that the selling shareholders are deemed to be “underwriters,” any broker-dealers or agents that are involved in selling the shares will be deemed to be “underwriters” within the meaning of the Securities Act, in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Discounts, concessions, commissions and similar selling expenses, if any, that can be attributed to the sale of the shares of common stock will be paid by the selling shareholder and/or the purchasers. Each selling shareholder has represented and warranted to us that it acquired the securities subject to this registration statement for his/her own account for investment and not for the benefit of any other person and not with a view to distribute or sell in violation of the Securities Act or any state securities laws or rules and regulations promulgated thereunder.
If a selling shareholder uses this prospectus for any sale of the common stock, it will be subject to the prospectus delivery requirements of the Securities Act. The selling shareholders will be responsible to comply with the applicable provisions of the Securities Act and the Exchange Act and the rules and regulations thereunder promulgated, including, without limitation, Regulation M, as applicable to such selling shareholders in connection with resales of their respective shares under this registration statement.
We are required to pay all fees and expenses incident to the registration of the shares, but we will not receive any proceeds from the sale of the common stock.
25
Overview
AVRA was organized by a senior leadership team with broad and deep experience in medical research, innovation and development in the medical robotics field. The Company plans to exploit the growing demand for practical medical robotic devices by developing a platform-independent precision guidance system, applicable to a variety of minimally and non-invasive procedures, with an initial focus on skin resurfacing. The Company believes that its team, which has been active in the medical robotics field for a number of years, brings the necessary skills and experience to develop and commercialize intelligent medical robotic systems, as well as in marketing, chain management, and the implementation of all other aspects of business operations. The Company believes that progress in mechanical and software engineering has made possible lightweight and relatively inexpensive robotic devices for difficult procedures in various medical fields.
Medical robots are already being successfully employed in several areas of surgery, including Urology (Prostate), Colo-Rectal, Gynecology, Thoracic, General Surgery, Neuro and Spine Surgery. Robots are also being used for Tele-medicine and assistive robotic methods are addressing the delivery of healthcare in inaccessible locations, ranging from rural areas lacking specialist expertise to post-disaster and battlefield areas. With the aging population dominating demographics in the U.S. across all spectrums of health care, robotic technologies are being developed toward promoting improved function, less morbidity and improved overall outcomes.
Today, the U.S. is the leader in robot-assisted surgery for the possibility of cure and improved quality of life. However, other countries are fast followers, having already recognized both the need and the promise of such technologies. The development of surgical robotics is motivated by the desire to enhance the effectiveness of a procedure by coupling information to action in the operating room or interventional suite, and transcend human physical limitations in performing surgery and other interventional procedures, while still affording human control over the procedure. Two decades after the first reported robotic surgical procedure, surgical robots are now being widely used in the operating room. Surgical robots are beginning to realize their potential in terms of improved accuracy and visualization, as well as enabling new procedures.
Current robots used in surgery are under the direct control of a surgeon – the so-called “Master-slave system,” often in a teleoperation scenario in which a human operator manipulates a master input device and the patient-side robot follows the input. In contrast to commonly held beliefs where robots are autonomous, traditional minimally invasive surgical robots provide the surgeon with a higher degree of dexterity inside the body, eliminate operator tremor, scale down operator motions to a fraction of normal distances, and provide a very intuitive connection between the operator and the instrument tips. The surgeon can cut, cauterize, suture and reconstruct tissue with accuracy equal to or better than that of invasive open surgery. A surgical system contains both robotic devices and real-time imaging devices to visualize the operative field during the course of surgery.
The use of robotics in medicine inherently involves physical interaction between caregivers, patients, and robots – in all combinations. Developing user-friendly physical interfaces between humans and robots requires all the classic elements of a robotic system: sensing, perception, and action. A great variety of sensing and perception tasks are required, including recording the motions and forces of a surgeon to infer their intent, determining the mechanical parameters of human tissue, and estimating the forces between a rehabilitation robot and a moving stroke patient. The reciprocal nature of interaction means that the robot will also need to provide useful feedback to the human operator, whether that person is a caregiver or a patient. We need to consider systems that involve many human senses, the most common of which are vision, haptics (force and tactile), and sound. In management’s opinion, a major reason why systems involving physical collaboration between humans and robots are so difficult to design well is that, from the perspective of a robot, humans are extremely uncertain and dynamic.
26
Unlike in a passive, static environment, humans dynamically change their motion, force, and immediate purpose throughout a procedure. These changes can be caused by something as simple as physiologic movement (e.g., a patient breathing during surgery), or as complex as the motions of a surgeon suturing during surgery. During physical interaction with a robot, the human is an integral part of a closed-loop feedback system, simultaneously exchanging information and energy with the robotic system, and thus cannot simply be thought of as an external system input. In addition, the loop is often closed with both human force and visual feedback, each with its own errors and delays that can potentially cause challenges in a human-robot system. Given these problems, how does one guarantee safe, collaborative and useful physical interaction between robots and humans? Management believes that to date, no existing system provides the user with an ideal experience of physically interacting with a robot.
AVRA’s overall design strategy, as opposed to that used in most non-autonomous systems, is to integrate image-guidance with navigation and organ-targeting to result in a medical robotic system that is truly diverse and multi-dimensional. Having identified limitations in the predominantly non-autonomous systems, AVRA proposes to employ a disruptive model in the design and development process, which considers design and development through a seamless collaboration of surgeons, engineers and scientists. Past efforts in medical robotics have generally been led by either the surgeons, the engineers, or the scientists, but rarely via a collaboration of all three. AVRA has been making a proactive effort to use this collaborative approach in all its development work.
For skin resurfacing, AVRA plans to incorporate recent technological improvements in motors, materials and high resolution imaging to develop robotic device that will allow a surgeon to autonomously or semi-autonomously treat damaged skin. The basic principle behind the initial technology that AVRA is working on automating is the controlled delivery of micro skin injuries to stimulate remodeling of existing collagen and promote the formation of new collagen, elastin, and vascularization in the papillary dermis, which results in the reduction in appearance of fine lines and wrinkles, skin laxity, and scarring. Some of the advantages of using a robot for skin resurfacing can include greater precision, better visualization, quick automated adjustments during a procedure and shorter times. Presently, we are not aware of any commercially available robotic devices designed for this application.
AVRA is currently developing its initial intelligent medical robotic system for facial corrections (i.e., skin resurfacing) in partnership with UCF, pursuant to the Research Agreement entered into with UCF effective as of May 1, 2016. UCF is recognized particularly for its work in the area of medical robotic research and design, focusing on the guidance systems. We anticipate that application of this expertise will allow AVRA’s medical robotic system to handle a wide array of the currently available “tools” in the market. The Company’s plans to target a large market for its initial robotic system, which currently includes such solutions as Botox and CO2 lasers used for keratosis removal and treatment of scarring, discoloration and other skin problems that are often difficult to treat.
Moreover, while AVRA is in development of its robotic system and anticipates that it is ready to build a prototype, to date we have no products or training programs approved or ready for retail marketing and there can be no assurance as to when products will be ready to reach market. Unanticipated delays in market readiness will substantially harm the Company’s prospects.
To date, the Company has not generated revenues and has operated with limited capital. The Company will require significant capital to implement its business plan. There can be no assurance that the Company can raise the necessary funds, on favorable terms or otherwise. Failure to obtain sufficient capital will substantially harm the Company’s prospects.
Current Market
The concept of using a robot in surgical procedures became a practical reality in 2000 when the FDA approved the da Vinci® robot, introduced to the market by Intuitive Surgical, Inc. (“ISRG”). For years, ISRG was, as essentially the only game in town, alone in enjoying the explosive growth in the rapidly emerging field of robotic-assisted, minimally invasive surgery (“MIS”).
27
The global medical robots market is expected to grow to $11.4 billion by 2020 from $4.2 billion in 2015 at a combined annual growth rate of 22.2%.[1]
As seen in the following charts, the non-surgical segment of the skin resurfacing market, the target for AVRA’s first planned medical robotic system, has enjoyed explosive growth and is foreseen to continue its fast growth in the near future:
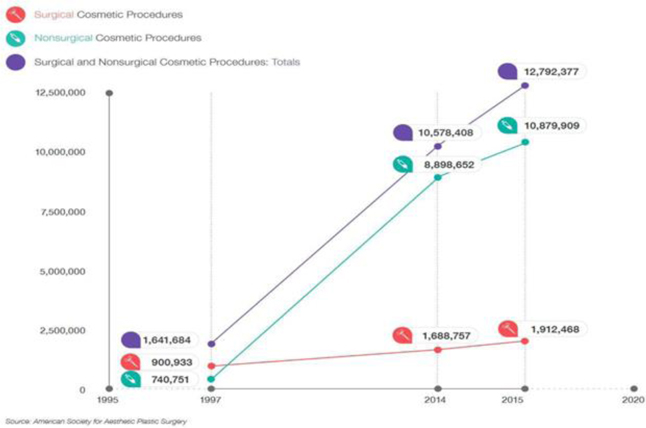
2 Markets And Markets research report, Nov 2015, Medical Robots Market by Product (Robotic systems (Surgical Robots, Rehabilitation Robots, Hospital Robots, Assistive Robots, Telemedicine Robots), Instruments & Accessories) & Application (Orthopedic, Laparoscopy, Neurology) - Global Forecasts to 2020
28
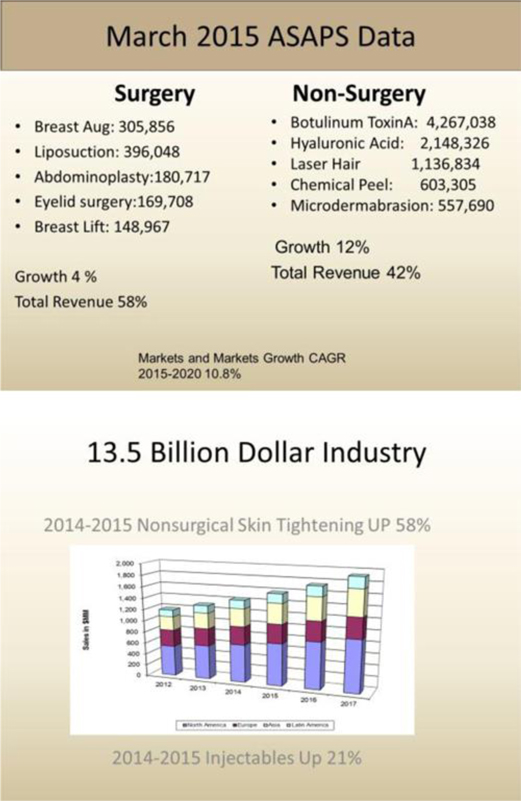
3 Statista, http://www.statista.com/statistics/254612/global-skin-care-market-size/
29
As of 2013, there were an estimated 9,600 dermatologists and 7,800 dermatology practices in the U.S. according to IMS Health; approximately, 34% of these are solo practices, while approximately 48% are multi-physician (three or more physicians) practices.
Most products designed to improve the appearance of the skin do not repair the skin itself; rather, they cover and hide scarring and blemishes temporarily. Wrinkles also are challenging as the skin ages and are hard to cover over. Some current products aim to slow or forestall the development of wrinkles, but with questionable effectiveness.
Botox injections and CO2 Lasers are the common procedures currently in use today for skin resurfacing. Other procedures include Needling, Radiofrequency, Ultrasound, and Cryolipolysis.
Botox injections have proved successful for cosmetic enhancement because it shrinks wrinkles dramatically. Botox has enjoyed rapid growth in sales and number of procedures (now about 7 million annually in the U.S. alone).[3] The downside of Botox is pain during the procedures and the need for repeated treatments given the temporary nature of the results achieved.
CO2 Laser resurfacing also can shrink wrinkles, even eliminating small wrinkles by removing the outer layer of skin, allowing new skin to form. While simple, this procedure can be painful. Laser resurfacing works by burning off skin; skin can reach 1500°F (800°C) in the process of being removed, and adjacent areas of skin can approach 400°F. (Unsurprisingly, general anesthesia is often required.) Open wounds are created and healing may take up to three weeks. Skin redness may persist for three months, during which the skin is particularly sensitive to UV light. Other risks of laser resurfacing include scarring, changes in skin pigmentation and bacterial infection.
Because wrinkles are a major cosmetic concern of both men and women alike, AVRA believes that a robotic system that actually reduces and eliminates wrinkles with minimal side effects has great market potential. Although no such product currently exists, AVRA intends to focus its initial efforts on addressing this void by being the first to develop, commercialize and market a medical robotic system to aid in skin resurfacing.
Efficiently to Market with a Superior Solution
AVRA believes that its resurfacing solution will be superior to Botox injections and CO2 Laser treatments, while being price competitive. Botox needs to be repeated every few months and the cost of acquiring a CO2 Laser, where a very basic model starts at approximately $50,000 but can be much higher than that, can be prohibitive.
AVRA plans to use a treatment delivered by a robotic device that improves the appearance of skin beyond wrinkle elimination. AVRA plans to integrate an existing procedure’s tool that has already received FDA approvals as a stand-alone procedure and adapt it to its robotic system. AVRA has researched many procedures and is currently finalizing its choice of the most appropriate procedure and tool for its robotic system. The basic principle behind the initial technology that AVRA is working on automating is the controlled delivery of micro skin injuries to stimulate remodeling of existing collagen and promote the formation of new collagen, elastin, and vascularization in the papillary dermis, which results in the reduction in appearance of fine lines and wrinkles, skin laxity, and scarring
The Company believes that the safety, efficacy and cost effectiveness of its planned technology, which underlying technology will have already received FDA 510(k) approvals by other users of the technology (the “Technology”), for the same application of skin resurfacing, but without any automation, will afford a significant medical and competitive advantage as employed by AVRA.
Upon the completion and testing of its prototype, AVRA intends to commence the FDA approval process for its initial application. Since the Company plans to use a device that has already received FDA 510(k) approval, the Company should be in a better position to advance the robotic control of this device. This process may take anywhere from six months to two years but the Company hopes to minimize this time by going through a pre-meeting with the FDA and thus reduce the list of issues it will have to respond to once studies commence. Costs for this process, in the Company’s estimation, can range anywhere from $200,000 to $500,000.
The use of a robotic system will ensure accuracy, speed and minimize human error while also allowing doctors to increase their productivity by performing more procedures daily.
4 2014 Allergan Presentation
30
The Company believes it can rapidly develop, commercialize and market its initial medical robotic system because of the following advantages:
| ● | AVRA has substantially completed the design phase and is ready to build a prototype. |
| ● | AVRA’s team is experienced in medical robotic engineering. |
| ● | The underlying Technology has already received FDA approvals for skin resurfacing which the Company anticipates will allow for a sharply reduced time to market when applying for approval of the Technology integrated with our planned medical robotic system. Numerous companies manufacture the underlying Technology and AVRA is working on selecting a manufacturer but as of this date has not entered into any agreements. |
| ● | AVRA is working in conjunction with preeminent physicians, engineers and scientific institutions. |
| ● | AVRA is prepared for ongoing research, development, commercialization, marketing, and manufacturing of products. |
| ● | AVRA believes that its modular approach will enable rapid entry into the skin resurfacing and other markets with new and improved devices. |
Product Elements
There are four key elements to the Company’s medical robotic devices, all of which can potentially generate revenues:
| ● | Robotic Systems: | Standardized arms, precision guidance system, and software controls designed to the needs of doctors and physicians. | |
| ● | Robotic Tools: | Standardized tools that can be modified to cover a wide range of medical procedures. | |
| ● | Maintenance: | Ongoing operation and service simplified so the medical user can perform much of the work in house. | |
| ● | Education, Training: | Remote and on-site programs for surgeons, hospitals and medical support staff. |
Marketing Strategy
AVRA and its management team have relationships with many top medical institutions as of the result of their combined years of experience and expertise in exploring new designs for medical robotic systems. Moreover, the Company’s Medical Advisory Board consists of some of the most experienced2 robotic surgeons in the world who are working to develop new, cutting edge procedures. As a result, AVRA believes that it will be able to capitalize on its experience and relationships to successfully develop, commercialize and launch a new system that it believes will be welcomed into hospital skin resurfacing departments, as well as into individual and skin resurfacing groups. The Company plans to handle initial sales on a direct basis to departments and practices through its extensive contacts in the medical industry.
AVRA plans to initially place the new AVRA Skin Resurfacing Robotic System into some of the major respected hospital names in the US. The Company already has relationships with many of these. AVRA will then leverage this into the larger skin resurfacing market with a direct cost effective marketing plan that includes well known social media outlets such as Facebook, Twitter, Instagram and Vimeo.
Due to the large market it addresses, the AVRA Skin Resurfacing Robotic System is expected to produce a much higher degree of interest and throng of inquiries than what the internal robotic surgical medical field would generate. The Company anticipates that this will help the company drive patient business directly to our AVRA Skin Resurfacing Robotic System’s doctors and centers.
AVRA will utilize the internet to answer the many expected inquiries and will upgrade its website(s) to automatically handle and direct these when appropriate.
Development Strategy
On May 1, 2016, the Company entered into the Research Agreement with the UCF for the development of a prototype surgical robotic device supporting minimal invasive surgical facial corrections. The Agreement provides that the University will provide personnel to accomplish the objectives as stated in the Statement of Work over a period extending to June 30, 2017. The Statement of Work includes the development of prototype navigation and control software for the robotic medical device and the integration of all the necessary subcomponents. The Company has agreed to provide funding of $163,307 for the project.
2 For example, AVRA Medical Advisory Board members Dr. Vipul Patel and Dr. Nikhil Shah have already performed more than 10,000 and 6,000 DaVinci robotic surgeries, respectively.
31
In addition, AVRA has paid $43,548 for outright ownership of the UCF’s Intellectual Property resulting from the collaboration, which amount is shown as Intellectual Property. Management has assessed the carrying value of the asset and believes there has been no diminution of its value and accordingly, no adjustment is necessary.
The total cost to the Company is:
| Research Expense - funded from existing funds | $ | 163,307 | ||
| Acquisition of Intellectual Property Rights | 43,548 | |||
| Total | $ | 206,855 |
As of December 31, 2016, $125,202 has been paid under the Research Agreement. The balance of the amount owing to the University was due November 1, 2016 ($40,827) and February 1, 2017 ($40,826). Both balances due have been fully paid on February 24, 2017 and on April 7, 2017, respectively. Additionally, a $68,952 matching funds grant from the Florida High Tech Corridor Council (FHTCC) was approved on July 16, 2016 which will provide UCF research funds in addition to the Company’s funding obligation to the University. The FHTCC research grant is subject to certain research obligations and action requirements which if not met may result in the loss of the FHTCC research funding which would require the Company to cover any resulting shortfall.
Intellectual Property
In connection with the design of its planned medical robotic systems, AVRA has applied for six provisional patents so far, will review existing patents in the United States and the European Union to reduce the risk of infringement claims and will file patent applications as its product development proceeds for elements of its products which qualify for patent protection.
The Company intends to file, as necessary, patent applications in the United States, as well as in other jurisdictions where it intends to distribute its products and where the dates of our initial patent applications will give us a right of priority.
Patents are granted for a fixed term and eventually expire. Upon expiration, the inventions claimed in a patent enter the public domain. There is no assurance that our patent applications will be granted or that patents granted will prevent others from developing competing products.
Government Regulation
Our products and operations will be subject to extensive and rigorous regulation in the United States by the United States Food and Drug Administration (the “FDA”) and by similar agencies in other countries or regions in which we may market our products.
Unless an exemption applies, each medical device that we intend to market in the U.S. must first receive either "510(k) clearance" or "Premarket (PMA) approval" from the FDA pursuant to the Federal Food, Drug, and Cosmetic Act. The FDA's 510(k) clearance process usually takes from four to 12 months, but it can last longer. The process of obtaining PMA approval is much more costly, lengthy and uncertain. It generally takes from one to three years or even longer. We cannot be sure that 510(k) clearance or PMA approval will be obtained in the future for any product we propose to market.
The FDA decides whether a device must undergo either the 510(k) clearance or PMA approval process based upon statutory criteria. These criteria include the level of risk that the agency perceives is associated with the device and a determination whether the product is similar to devices that are already legally marketed. Devices deemed to pose relatively less risk are placed in either class I or II, which requires the manufacturer to request 510(k) clearance, unless an exemption applies. The manufacturer must demonstrate that the proposed device is "substantially equivalent" in intended use, safety and effectiveness to a legally marketed "predicate device" that is either in class I, class II, or is a "preamendment" class III device, one that was in commercial distribution before May 28, 1976, for which the FDA has not yet called for submission of a PMA application. After a device receives 510(k) clearance, any modification to the device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could require a PMA approval.
32
Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a legally marketed predicate device, are placed in class III. Such devices are required to undergo the PMA approval process in which the manufacturer must prove the safety and effectiveness of the device to the FDA's satisfaction.
A PMA application must provide extensive preclinical and clinical trial data as well as information about the device and its components regarding, among other things, device design, manufacturing and labeling. As part of the PMA review, the FDA will inspect the manufacturer's facilities for compliance with CGMP and QSR requirements, which include elaborate testing, control, documentation and other quality assurance procedures. During the FDA's review, an FDA advisory committee, typically a panel of clinicians, likely will be convened to review the application and recommend to the FDA whether, or upon what conditions, the device should be approved. Although the FDA is not bound by the advisory panel decision, the panel's recommendation is important to the FDA's overall decision making process. If the FDA's evaluation of the PMA application is favorable, the FDA typically issues an "approvable letter" requiring the applicant's agreement to comply with specific conditions or to supply specific additional data or information in order to secure final PMA approval.
Once the approvable letter conditions are satisfied, the FDA will issue a PMA order for the approved indications, which can be more limited than those originally sought by the manufacturer. The PMA order can include post-approval conditions that the FDA believes necessary to ensure the safety and effectiveness of the device including, among other things, restrictions on labeling, promotion, sale and distribution. Failure to comply with the conditions of approval can result in an enforcement action, including withdrawal of the approval. The PMA process can be expensive and lengthy, and no assurance can be given that any PMA application will ever be approved for marketing. After approval of a PMA, a new PMA or PMA supplement may be required in the event of modifications to the device, its labeling or its manufacturing process.
A clinical trial may be required to support a 510(k) submission and generally is required for a PMA application. Such trials generally require an investigational device exemption application, or IDE, approved in advance by the FDA for a specified number of patients and study sites, unless the product is deemed an insignificant risk device eligible for more abbreviated IDE requirements. The IDE must be supported by appropriate data, such as animal and laboratory testing results. Clinical trials may begin if the FDA and the appropriate institutional review boards at the clinical trial sites approve the IDE. Trials must be conducted in conformance with FDA regulations and institutional review board requirements. The sponsor or the FDA may suspend these trials at any time if they are deemed to pose unacceptable health risks or if the FDA finds deficiencies in the way they are being conducted. Data from clinical trials are often subject to varying interpretations that could delay, limit or prevent FDA approval.
We will be subject to inspection and market surveillance by the FDA to determine compliance with regulatory requirements. If the FDA finds that we have failed to comply, the agency can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions. The FDA also has the authority to request repair, replacement or refund of the cost of any medical device manufactured or distributed by us. Our failure to comply with applicable requirements could lead to an enforcement action that may have an adverse effect on our financial condition and results of operations.
In order for us to market our products in other countries, we must obtain regulatory approvals and comply with extensive safety and quality regulations in other countries. These regulations, including the requirements for approvals or clearance and the time required for regulatory review, vary from country to country. Failure to obtain regulatory approval in any foreign country in which we plan to market our products may harm our ability to generate revenue and harm our business.
33
Employees
As of the date of this prospectus the Company employs six persons, including its executive officers. The Company also relies on independent third party consultants to perform additional services as needed. As we implement our business plan and subject to the availability of capital, additional employees will be hired in the future as our business expands.
Properties
The Company currently does not own any properties but leases an office from a UCF at 3259 Progress Drive, Orlando, FL 32826. The lease expires July 31, 2017 at a rental of $240 per month. Pursuant to the Research Agreement, research and development activities are conducted at UCF’s campus.
Legal Proceedings
Currently there are no legal proceedings pending or threatened against us. However, from time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in any such matter may harm our business.
MARKET FOR COMMON EQUITY AND RELATED SHAREHOLDER MATTERS
There is presently no public market for our common stock and there has never been a market for our common stock. We anticipate applying for quotation of our common stock on the OTCQX or the OTCQB tiers of the over-the- counter market operated by OTC Markets Group following the effectiveness of the registration statement of which this prospectus forms a part. However, we cannot assure you that our shares will be quoted on any tier of OTC Markets Group or, if quoted, that a public market will develop and if developed, be liquid and be sustained.
A market maker sponsoring a company’s securities is required to obtain a quotation of the securities on any of the public trading markets, including the OTCQX or OTCQB. If we are unable to obtain a market maker for our common stock, we will be unable to develop a trading market for our common stock. We may be unable to locate a market maker that will agree to sponsor our securities. Even if we do locate a market maker, there is no assurance that our securities will be able to meet the requirements for a quotation or that the securities will be accepted for quotation by OTC Markets Group on the OTCQX or OTCQB.
OTCQX and OTCQB securities are not quoted and traded on the floor of an organized national or regional stock exchange. Instead, securities transactions are conducted through a telephone and computer network connecting dealers in stocks. OTCQX and OTCQB stocks are traditionally smaller companies that do not meet the financial and other listing requirements of a regional or national stock exchange.
Holders of our Common Stock
As of the date of this prospectus, we had 19,192,438 shares of common stock issued and outstanding and 135 holders of record of our common stock.
Options, Warrants and Convertible Securities
In addition to our currently issued outstanding 19,192,438 shares of common stock, we have outstanding:
| ● | Options to purchase 2,598,000 shares of common stock pursuant to our 2016 Incentive Stock Plan; and |
| ● | The Convertible Notes, which are convertible into not less than 960,000 shares of our common stock. |
Transfer Agent
The Company has appointed VStock Transfer, LLC, Woodmere, New York, as transfer agent for its common stock.
Dividend Policy
The payment by us of dividends, if any, in the future rests within the discretion of our board of directors and will depend, among other things, upon our earnings, capital requirements and financial condition, as well as other relevant factors. We have not paid any dividends since our inception and we do not intend to pay any cash dividends in the foreseeable future, but intend to retain all earnings, if any, for use in our business.
34
Rule 144 Shares
Rule 144 under the Securities Act provides that a person who is not an affiliate and has held restricted securities for a prescribed period of at least six months (if the issuer is a reporting company) or 12 months (if the issuer is a non-reporting company, as is the case herein), may, under certain conditions, sell all or any of his shares without volume limitation. Affiliates, however, may not sell shares in excess of 1% of the Company’s outstanding common stock in any three-month period. There is no limit on the amount of restricted securities that may be sold by a non-affiliate (i.e., a shareholder who has not been an officer, director or control person for the three months prior to sale) after the restricted securities have been held by the owner for the aforementioned prescribed period of time. An aggregate of 8,389,724 of our outstanding shares of common stock not covered by this prospectus will be eligible for public sale pursuant to Rule 144, commencing 90 days after the date of this prospectus.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion contains forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those discussed in the forward-looking statements as a result of various factors, including, without limitation, those set forth in “Risk Factors,” “Special Note Regarding Forward-Looking Statements” and other matters included elsewhere in this prospectus. The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements and the notes thereto included elsewhere in this prospectus.
Introduction
AVRA is currently developing a prototype medical robotic system for skin resurfacing in collaboration with UCF, which is recognized particularly for its work in the area of medical robotics research and design, focusing on the guidance systems which will allow AVRA’s medical robotic system to handle any currently available “tool” in the market.
The financial statements appearing elsewhere in this prospectus have been prepared assuming the Company will continue as a going concern. The company was recently formed and has not established sufficient operations or revenues to sustain the company. These conditions raise substantial doubt about the company’s ability to continue as a going concern.
The following table provides selected financial data about our Company at December 31, 2016 and December 31, 2015:
| Balance Sheet Data | As of December 31, | |||||||
| 2016 | 2015 | |||||||
| Cash | $ | 248,219 | $ | 100 | ||||
| Total Assets | $ | 248,684 | $ | 100 | ||||
| Total Liabilities | $ | 647,292 | $ | 166 | ||||
| Total Stockholders' Deficiency | $ | (355,060 | ) | $ | (66 | ) | ||
| Total Liabilities and Stockholders’ Deficit | $ | 292,232 | $ | 100 | ||||
To date, the Company has relied on debt and equity raised in private offerings to finance operations and no other source of capital has been identified or sought. If we experience a shortfall in operating capital we could be faced with having to limit our research activities.
35
Results of Operations
Year ended December 31, 2016 as compared to period from Inception (February 4, 2015) to December 31, 2015
Revenues. We had no revenues during either the year ended December 31, 2016 or the period from Inception to December 31, 2015.
Research and Development Expenses. We incurred $138,377 in research and development expenses during the year ended December 31, 2016, as compared to $0 during the period from Inception (February 4, 2015) to December 31, 2015, reflecting the raising and deployment of initial capital and the commencement of product development.
General and Administrative Expenses. We incurred $324,116 in general and administrative expenses during the year ended December 31, 2016, as compared to $66 during the period from Inception (February 4, 2015) to December 31, 2015, reflecting the raising and deployment of initial capital and commencement of product development.
Other Expenses. We incurred $25,520 of interest expense related to our Convertible Notes during the year ended December 31, 2016 ,offset by interest income of $31, as compared to $0 during the period from Inception (February 4, 2015) to December 31, 2015.
Net Loss. We incurred a net loss of $484,982 during the year ended December 31, 2016, as compared to a net loss of $66 during the period from Inception (February 4, 2015) to December 31, 2015.
Liquidity and Capital Resources
The Company expects to require substantial funds for research and development, to continue to develop its initial proposed medical robotic system. The Company plans to meet its operating cash flow requirements by raising additional funds from the sale of our securities and, if possible on favorable terms, by entering into development partnerships to assist us with our technology development activities.
During the period from Inception (February 4, 2015) to December 31, 2016, the Company raised $1,900 from private offerings of its common stock and $480,000 from a private offering of its Convertible Notes in such principal amount. In addition, in February 2017, the Company raised an additional $135,000 from a private offering of 135,000 shares of common stock at a price of $1.00 per share made to three investors. While we have been successful in raising funds to fund our operations since inception and we believe that we will be successful in obtaining the necessary financing to fund our operations going forward, we do not have any committed sources of funding and there are no assurances that we will be able to secure additional funding. The accompanying financial statements have been prepared assuming that the Company will continue as a going concern; however, if the efforts noted above are not successful, it would raise substantial doubt about the Company’s ability to continue as a going concern. If we cannot obtain financing, then we may be forced to further curtail our operations or consider other strategic alternatives. Even if we are successful in raising the additional financing, there is no assurance regarding the terms of any additional investment and any such investment or other strategic alternative would likely substantially dilute our current shareholders.
36
Critical Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates included deferred revenue, costs incurred related to deferred revenue, the useful lives of property and equipment and the useful lives of intangible assets.
Income Taxes
The Company accounts for income taxes in accordance with ASC 740, Accounting for Income Taxes, as clarified by ASC 740-10, Accounting for Uncertainty in Income Taxes. Under this method, deferred income taxes are determined based on the estimated future tax effects of differences between the financial statement and tax basis of assets and liabilities given the provisions of enacted tax laws. Deferred income tax provisions and benefits are based on changes to the assets or liabilities from year to year. In providing for deferred taxes, the Company considers tax regulations of the jurisdictions in which the Company operates, estimates of future taxable income, and available tax planning strategies. If tax regulations, operating results or the ability to implement tax-planning strategies vary, adjustments to the carrying value of deferred tax assets and liabilities may be required. Valuation allowances are recorded related to deferred tax assets based on the “more likely than not” criteria of ASC 740.
ASC 740-10 requires that the Company recognize the financial statement benefit of a tax position only after determining that the relevant tax authority would more likely than not sustain the position following an audit. For tax positions meeting the “more-likely-than-not” threshold, the amount recognized in the financial statements is the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate settlement with the relevant tax authority.
Off-Balance Sheet Arrangements
There are no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
37
Directors and Executive Officers
Our directors and executive officers and their respective ages and titles are as follows:
| Name | Age | Position(s) and Office(s) Held | ||
| Barry F. Cohen | 76 | Chief Executive Officer and Director | ||
| A. Christian Schauer | 74 | Chief Financial Officer | ||
| Dr. Ray Powers | 67 | Chief Operating Officer | ||
| Alexandre S. Clug | 49 | Vice President of Global Business Development | ||
| Nicole Bergman-Fong | 55 | Corporate Counsel | ||
| Peter Carnegie | 47 | Director | ||
| Nikhil L. Shah, D.O. | 46 | Director |
Set forth below is a brief description of the background and business experience of our directors and executive officers.
Barry F. Cohen founded the Company and has served as its Chief Executive Officer and a director since February 4, 2015. Between 2006 and 2008, Mr. Cohen was a private investor and founded AVRA Surgical, Inc., a medical technology company. Prior to founding AVRA, Mr. Cohen was a director of Dualis Med-Tech from 2012 to 2014, and has been a director of AvraMiro since 2009 and Avra Surgical Robotics, Inc. since 2011, which companies are currently inactive. From approximately 1979 to 1983 he served as director of Synalloy Corp., a manufacturer of pipe, piping systems and specialty chemicals after which he was appointed to serve as President from 1984 to 1985. Mr. Cohen also served as Chairman of the Executive Board of Wolverine Technologies, Inc., a NYSE listed company from 1979 to 1983 and President of Barry F. Cohen & Co., an NASD member from 1983 to 1999. Mr. Cohen has over 50 years’ experience in managing private and public industrial companies, and 47 years’ experience as a securities executive. This significant experience qualifies Mr. Cohen to serve as a director.
A. Christian Schauer has served as the Company’s Chief Financial Officer since August 1, 2016. Since 2003, he has been serving as Chairman, President and Chief Executive Officer of PharmOptima LLC, a pre-clinical contract research company based in Portage, Michigan. Mr. Schauer only devotes a small portion of his working time to such positions. Mr. Schauer also served as Chief Financial Officer of Avra Surgical Robotics, Inc. from February 2013 to April 2014. From 1999 until it was acquired by Eimo of Finland in 2001, Mr. Schauer served as Chief Executive Officer of Triple S. Plastics. For 25 years prior thereto, he served in various senior management positions with Clausing Corporation, including as Chairman and Chief Executive Officer from 1984 until 1999. Before joining Clausing Corporation, Mr. Schauer was with Ernst & Ernst (now Ernst & Young) for approximately a decade. A certified public accountant, he holds a B.B.A. in accounting from Western Michigan University. Mr. Schauer has served as an executive and non-executive director for several companies, including First of America Bank, Durametallic Corporation, Triple S Plastics, Harter Corporation, The 600 Group Plc. (London), and Z Seven Fund. Mr. Schauer currently serves as a non-executive director for Griffith Foods, Inc., and as a Trustee of the Boards at Kalamazoo Valley Community College and Hope Network.
Dr. Ray Powers, who became our Chief Operating Officer on August 1. 2016, was an executive within the Bell System for 30 years prior to moving on to C-level positions in the technology sector serving in both private and public companies. He has served as Director of Standards for the Project Management Institute, and on their Board of Directors as well as on several non-profit boards. During the last 5 years, he has been a full-time professor and administrator in higher education. He holds a professional project manager credential (PMP); a bachelor of science degree in business from Arizona State University; master of arts degree in education; a master of arts degree in business (MBA); and a doctorate degree in leadership (EdD).
Alexandre S. Clug, who became our Vice President on August 1, 2016, was formerly CFO, and CEO of various businesses in the US, Latin America and Europe. His experience covers all aspects of building a business. He has taken companies public in both the US and Europe, developed a sales force from zero to 6,000 while at Etelix, and built a sales pipeline of over $100 million while at Secure Fortress which, as CEO, he took public in Europe. For the five years prior to joining the Company Mr. Clug was President of Dolphin Group LLC, a consultancy firm focused on early stage ventures and international opportunities. Recent projects included mining, farmland development, financial technologies, internet platforms, and were primarily focused on the Americas and mainland China. Mr. Clug graduated with honors from West Point receiving a BS in Electrical Engineering, served with distinction as a Captain in the Army Corps of Engineers, has a MBA from UCLA, and is fluent in English, French and Spanish.
38
Nicole Bergman-Fong, who became our Corporate Counsel on October 1, 2016, has been advising and supporting the development of a number of start-up businesses and small businesses in New York and across the globe since 2005. She previously was a finance partner in the law firm of Mayer Brown for 10 years. From 1984 through 1991 Ms. Bergman-Fong was a finance associate at Cravath, Swaine & Moore in the M&A to Structured Finance Area. She attended Brown University and she was a Harlan Fiske Stone Scholar at Columbia Law School where she graduated with honors. She is a member of the New York Bar.
Peter Carnegie, who became a director on August 15, 2016, is a leader in developing successful robotic surgery programs in storied institutions like the Cleveland Clinic, the University of Alabama, Birmingham, the University of Pittsburgh Medical Center and West Virginia University Hospitals. He also is an expert in developing robotic and medical device training programs, surgeon training programs, nurse training programs, and clinical and sales training programs. Since 2011, Mr. Carnegie has been the CEO of Minimally Invasive Solutions Consulting (MIS), a robotic surgery consulting firm, where he developed successful robotic programs with such clients as the University of Alabama, Birmingham; the University of Pittsburgh Medical Center Presbyterian in Pittsburgh, PA; East Alabama Medical Center in Opelika, AL; Bon Secours St. Mary's Hospital in Richmond, VA; Bay Medical Center in Panama City, Florida; SSM DePaul in St. Louis, MO; Christiana Care Hospital in Wilmington, DE; the University of Virginia Medical Center in Charlottesville, AV and Jack Ruby Memorial Hospital which is a part of West Virginia University Hospitals. He also developed clinical and sales mastery training programs for Mazor Robotics and ImaCor Monitoring. Prior to becoming the CEO of MIS, he was its Chief Operating Officer. Before, MIS, he worked at Intuitive Surgical, Inc. as U.S. Program Development Manager for Cardiothoracic, Head & Neck Surgery. Prior to working for Intuitive, he was at Ethicon Endo-Surgery of Johnson & Johnson. He has headed programs in robotic training, including the Symposium on Science, Technology and Advanced Robotics in partnership with the Links Inc. Professional Opportunities Program for Students of the University of Central Florida, the University of South Florida, and Valencia College. He is a decorated war veteran and recipient of the Douglas McArthur Leadership Award, and holds a BS degree in engineering from West Point. This significant experience qualifies Mr. Carnegie to serve as a director.
Nikhil L. Shah, D.O., who became a director on October 1, 2016, is one of the top global leaders in robotic surgery and has previously served as the AVRA Chief Medical Robotic Advisor. He is currently the Chief of Minimal Access & Robotic Surgery at Piedmont Healthcare in Atlanta, GA. He previously served as the Director of Urology & Urologic Oncology at Piedmont Atlanta Hospital from 2012 to 2016. He holds an Associate Professor (adjunct) at the Georgia Institute of Technology in the College of Computing – Robotics & Intelligent Machines. Prior positions also include the Section Chief of Urology, Department of Surgery, Saint Joseph’s Hospital of Atlanta, and the Director of Robotic Surgery, Saint Joseph’s Hospital of Atlanta. Dr. Shah is Founder and Board Member of the Men’s Health & Wellness Center in Atlanta. This is a 501-3c non-profit that works to educate Men on screening and prevention for all health issues affecting the aging Male as well as awareness of cancer conditions affecting Men and their Partners. Dr. Shah has a Masters in Health Management & Health Policy from the University of Michigan. Given his experience, he has been an invited Speaker and Advisor for organizations in the financial arena, academia and medical device Industry. This significant experience qualifies Dr. Shah to serve as a director.
Terms of Office
Our directors are appointed for a one-year term to hold office until the next annual meeting of our shareholders and until a successor is appointed and qualified, or until their removal, resignation, or death. Executive officers serve at the pleasure of the board of directors.
Director Independence
At present, we believe that our two non-employee directors (Mr. Carnegie and Dr. Shah) are “independent” as defined under Rule 10A-3(b)(1) under the Exchange Act.
Board Committees
Our board of directors does not currently have an audit committee, a compensation committee, or a corporate governance committee. We plan to establish such committees in the near future, all the members of which will be “independent” directors.
Code of Ethics
Effective August 15, 2016, we adopted a Code of Ethics that applies to employees, including our principal executive officer, principal financial officer, or persons performing similar functions.
39
Board of Directors Role in Risk Oversight
Members of the board of directors have periodic meetings with management and the Company’s independent auditors to perform risk oversight with respect to the Company’s internal control processes. The Company believes that the board’s role in risk oversight does not materially affect the leadership structure of the Company.
Medical Advisory Board
The Company has also established a medical advisory board, whose members meet periodically in person or by telephone with management and/or the board of directors to advise on scientific, product development and marketing matters. The current members of the medical advisory board are:
| ● | Dr. Juan Jose Badimon, PhD |
| ● | Dr. Heywood Y. Epstein |
| ● | Dr. Yuman Fong |
| ● | Dr. Hiep T. Nguyen |
| ● | Dr. Vipul Patel |
| ● | Dr. Farhan Taghizadeh |
Dr. Juan Jose Badimon, Ph.D., is a Professor of Medicine and Director of the Atherothrombosis Research Unit at the Cardiovascular Institute, Mount Sinai School of Medicine, New York. He graduated by the University of Barcelona in 1982. His academic appointments include Mayo Clinic, Massachusetts General Hospital, Harvard University, Boston (and Mount Sinai School of Medicine, New York. His major research interests are focused on pathogenesis and treatment of atherothrombosis and cardiovascular diseases. Dr. Badimon has published more than 370 peer-reviewed articles in athero-thrombosis, imaging and cardiovascular diseases. He serves as reviewer for 10 of the top journals in cardiovascular diseases.
Dr. Heywood Y. Epstein was Chief Resident in Radiation Therapy at Montefiore Hospital in the Bronx NY, Assistant Professor of Radiology at Columbia P&S, NYU, Mt. Sinai in NY, and Stoney Brook NY. While in the US Public Health Service he was both Director of Staten Island Radiology Residency Program, Director of their Radiology Technologist Training Program, and USPHS radiation safety officer for the Northeast United States. Dr. Epstein helped establish NYU’s first ultrasound section in their Radiology Department and has coauthored 25 articles for juried journals. Dr. Epstein has performed approximately 10,000 angiograms and interventional radiographic procedures, in addition to another 10,000 breast biopsies guided by ultrasound, and stereotactically.
Dr. Yuman Fong is an internationally recognized expert in cancer and is a sought-after consultant on a wide range of medical robotic research. Dr. Fong is currently Chair of the Department of Surgery for City of Hope and was at the renowned Memorial Sloan-Kettering Cancer Center in New York City for the prior two decades. Dr. Fong is also both author and innovator: he has developed many new surgical techniques and instruments and written and edited hundreds of scholarly articles as well as nearly a dozen textbooks.
Dr. Hiep Nguyen is a world renowned Pediatric Urologist specializing in robotic and minimally invasive surgery, ureter pelvic junction (UPJ) obstruction, vesicoureteral reflux and reconstructive surgery. Dr. Nguyen was Director of the Robotic Surgery, Research and Training Center Rose Zimmerman Mandell, Chair in Innovative Urological Technology; Associate Professor (Surgery), Harvard Medical School, and Boston Children's Hospital. He is currently at the Carson Children’s Medical Center in Mesa, Arizona.
Dr. Vipul Patel is Medical Director of the Global Robotics Institute at Florida Hospital. Founder of the Society of Robotic Surgery, Dr. Patel has personally performed the most robotic surgeries in the world, 10,000+ robotic prostatectomies. He is also a Professor at the University of Central Florida College of Medicine.
40
Dr. Farhan Taghizadeh has extensive experience as a board-certified ENT and facial plastic and reconstructive surgeon, Dr. Taghizadeh specializes in the use of the most advanced facial cosmetic procedures to help people who are dissatisfied with their appearance. Since 2005, Dr. Taghizadeh has performed more than 2,500 facelifts using the latest advanced surgical techniques that minimize recovery time and provide the most comfort for his clients. In addition, he also specializes in scar revision, revision facelifts, and revision rhinoplasty.
Members of the medical advisory board are compensated through the grant of a stock option awards under our 2016 Incentive Stock Plan. Current members each received a five-year option to purchase 36,000 shares at an exercise price of $0.15 per share, 6,000 shares of which vest upon grant and the balance of which vest in twelve quarterly installments of 2,500 shares each, subject to continued service.
41
Summary Compensation Table
The table below summarizes all compensation awarded to, earned by, or paid to our Chief Executive Officer, Chief Financial Officer and our other executive officers for the period from inception (February 4, 2015) through December 31, 2015 and for the year ended December 31, 2016.
SUMMARY COMPENSATION TABLE
| Nonqualified | ||||||||||||||||||||||||||||||||||||||||
| Non-Equity | Deferred | |||||||||||||||||||||||||||||||||||||||
| Name and | Stock | Option | Option | Incentive Plan | Compensation | All Other | ||||||||||||||||||||||||||||||||||
| Principal | Salary | Bonus | Awards | Awards | Awards | Compensation | Earnings | Compensation | Total | |||||||||||||||||||||||||||||||
| Position | Year | ($) | ($) | (#) | (#) | ($) | ($) | ($) | ($) | ($) | ||||||||||||||||||||||||||||||
| Barry F. Cohen, Chairman and Chief Executive Officer (1) | 2016 | 63,000 | 0 | 0 | 1,000,000 | 88,026 | 0 | 0 | 0 | 151,026 | ||||||||||||||||||||||||||||||
| 2015 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||||||
| Albert Christian Schauer, Chief Financial Officer (2) | 2016 | 45,000 | 0 | 0 | 210,000 | 18,485 | 0 | 0 | 0 | 63,485 | ||||||||||||||||||||||||||||||
| Ray Powers, Chief Operating Officer (3) | 2016 | 0 | 0 | 0 | 75,000 | 6,602 | 0 | 0 | 0 | 6,602 | ||||||||||||||||||||||||||||||
| Alexandre Clug, Vice-President of Global Business Development (4) | 2016 | 42,500 | 0 | 0 | 300,000 | 26,408 | 0 | 0 | 0 | 68,908 | ||||||||||||||||||||||||||||||
| Nicole Bergman-Fong, Corporate Counsel (5) | 2016 | 0 | 0 | 0 | 60,000 | 7,922 | 0 | 0 | 0 | 7,922 | ||||||||||||||||||||||||||||||
(1) Mr. Cohen was granted an option for 1,000,000 shares on August 15, 2016, all vested immediately. Includes $23,877 of accrued but unpaid salary.
(2) Mr. Schauer was granted an option for 210,000 shares on August 15, 2016, with 70,000 shares vesting on August 15, 2017, 70,000 on August 15, 2018 and 70,000 on August 15, 2019. Includes $45,000 of accrued but unpaid salary.
(3) Dr. Powers was granted an option for 75,000 shares on August 15, 2016 vesting in equal monthly installments over 36 months.
(4) Mr. Clug was granted an option for 300,000 shares on August 15, 2016, with 100,000 shares vesting August 15, 2017, 100,000 shares vesting on August 15, 2018 and 100,000 shares vesting on August 15, 2019. Includes $27,500 of accrued but unpaid salary.
(5) Ms. Bergman-Fong was granted an option for 60,000 shares on October 1, 2016, with 15,000 shares vesting immediately and the balance vesting in equal monthly installments over 36 months.
Employment Agreements
The Company is party to employment agreements with Barry F. Cohen, A. Christian Schauer and Alexandre S. Clug, its Chief Executive Officer, Chief Financial Officer and Vice President of Global Business Development, respectively.
Mr. Cohen’s employment agreement is for a term of four years commencing July 1, 2016, provides for an initial base salary of $10,000 per month increasing to $15,000 per month effective July 1, 2017, a $500 month travel stipend and the grant of a fully vested option under our 2016 Incentive Stock Plan to purchase 1,000,000 shares of our common stock at an exercise price of $0.10 per share.
42
Mr. Schauer’ s employment agreement is for a term of one year commencing August 1, 2016, provides for a base salary of $9,000 per month and the grant of an option under the 2016 Incentive Stock Plan to purchase 210,000 shares of our common stock at an exercise price of $0.10 per share, which vests in three equal annual installments on the first, second and third anniversaries of the date of the employment agreement.
Mr. Clug’s employment agreement is for a term of three years commencing August 1, 2016, provides for a base salary of $8,000 per month increasing to $12,000 per month effective July 1, 2017, a $500 month travel stipend and the grant of an option under the 2016 Incentive Stock Plan to purchase 300,000 shares of our common stock at an exercise price of $0.10 per share, which vests in three equal annual installments on the first, second and third anniversaries of the date of the employment agreement.
Each of the employment agreements provides for reimbursement of reasonable business expenses incurred by the executive officer in the performance of his duties and contains confidentiality and non-competition provisions.
Outstanding Equity Awards at Fiscal Year-End Table
The table below summarizes all unexercised options, stock that has not vested, and equity incentive plan awards for each of our executive officers outstanding as of December 31, 2016, the end of our last completed fiscal year.
| Number of Securities | Number of Securities | Market value | ||||||||||||||||||||||
| Underlying | Underlying | Number of | of shares of stock | |||||||||||||||||||||
| Unexercised Options Exercisable | Unexercised Options Unexercisable | Option Exercise Price | Option Expiration Date | Shares that have not vested | that have not vested* | |||||||||||||||||||
| Barry F. Cohen | 1,000,000 | 1,000,000 | $ | 0.10 | August 15, 2021 | 0 | ||||||||||||||||||
| A. Christian Schauer | 0 | 210,000 | $ | 0.10 | August 15, 2021 | 210,000 | $ | 24,514 | ||||||||||||||||
| Dr. Ray Powers | 10,417 | 75,000 | $ | 0.10 | August 15, 2021 | 64,583 | $ | 7,539 | ||||||||||||||||
| Alexandre S. Clug | 0 | 300,000 | $ | 0.10 | August 15, 2021 | 300,000 | $ | 35,020 | ||||||||||||||||
| Nicole Bergman-Fong | 18,750 | 60,000 | $ | 0.15 | October 1, 2021 | 41,250 | $ | 4,815 | ||||||||||||||||
| Peter Carnegie | 19,167 | 45,000 | $ | 0.10 | August 15, 2021 | 26,667 | $ | 3,113 | ||||||||||||||||
| Nikhil L. Shah, D.O. | 30,417 | 200,000 | $ | 0.15 | October 1, 2021 | 169,583 | $ | 19,796 | ||||||||||||||||
*Volume weighted average exercise/fair value price per share for all options awarded is $0.117
Compensation of Directors Table
The table below summarizes all compensation paid to our directors for the year ended December 31, 2016, our last completed fiscal year.
| DIRECTOR COMPENSATION | ||||||||||||||||||||||||||||
| Non-Qualified | ||||||||||||||||||||||||||||
| Fees Earned | Non-Equity | Deferred | ||||||||||||||||||||||||||
| or paid | Stock | Option | Incentive Plan | Compensation | All Other | |||||||||||||||||||||||
| in Cash | Awards | Awards | Compensation | Earnings | Compensation | Total | ||||||||||||||||||||||
| Name | ($) | ($) | ($) | ($) | ($) | ($) | ($) | |||||||||||||||||||||
| Barry F. Cohen | 0 | 0 | 88,026 | 0 | 0 | 63,000 | 151,026 | |||||||||||||||||||||
| Peter Carnegie | 0 | 0 | 3,961 | 0 | 0 | 0 | 3,961 | |||||||||||||||||||||
| Nikhil L. Shah, D.O. | 0 | 0 | 26,408 | 0 | 0 | 0 | 26,408 | |||||||||||||||||||||
43
Narrative Disclosure to the Director Compensation Table
Non-employee directors are currently compensated with a grant of options under the 2016 Incentive Stock Plan. One of the two current non-employee directors, Mr. Carnegie, was granted an option under the 2016 Incentive Stock Plan on August 15, 2016, to purchase 45,000 shares of our common stock at a purchase price of $0.10 per share. The options vested as to 15,000 shares on the date of grant, with the remaining 30,000 shares vesting monthly over a three-year period, subject to continued service. The other non-employee director, Dr. Shah, was granted an option under the Incentive Plan on October 1, 2016, to purchase 200,000 shares of our common stock at a purchase price of $0.15 per share. The options vested as to 15,000 shares on the date of grant, with the remaining 185,000 shares vesting monthly over a three-year period, subject to continued service. Non-employee directors are reimbursed for travel and lodging expenses in connection with their attendance at in-person meetings of the board. When the Company is sufficiently capitalized, the Company intends to institute payment of cash directors’ fees to its non-employee directors in amounts to be determined at that time.
2016 Incentive Stock Plan
Our 2016 Incentive Stock Plan provides for equity incentives to be granted to our employees, executive officers or directors or to key advisers or consultants. Equity incentives may be in the form of stock options with an exercise price not less than the fair market value of the underlying shares as determined pursuant to the 2016 Incentive Stock Plan, restricted stock awards, other stock based awards, or any combination of the foregoing. The 2016 Incentive Stock Plan is administered by the compensation committee, or alternatively, if there is no compensation committee, the board of directors. 3,000,000 shares of our common stock are reserved for issuance pursuant to the exercise of awards under the 2016 Incentive Stock Plan. The number of shares so reserved automatically adjusts upward on January 1 of each year, so that the number of shares covered by the 2016 Incentive Stock Plan is equal to 15% of our issued and outstanding common stock. As of the date of this prospectus, the Company has granted options to purchase 2,598,000 shares under the Incentive 2016 Incentive Stock Plan, exercisable at prices ranging from of $0.10 to $0.15 per share.
44
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of the date of this prospectus, the beneficial ownership of our common stock by each director and executive officer, by each person known by us to beneficially own 5% or more of our common stock and by directors and executive officers as a group. Unless otherwise stated, the address of the persons set forth in the table is c/o the Company, 3259 Progress Drive, Suite 126, Orlando, FL 32826.
| Names and addresses of
beneficial owners | Number of shares
of common stock* | Percentage of class (%)* | ||||||
| Barry F. Cohen (1) | 6,571,838 | 34.24 | % | |||||
| Avra Acquisitions, LLC(1) | 3,672,700 | 19.14 | % | |||||
| A. Christian Schauer | 296,000 | 1.54 | % | |||||
| Dr. Ray Powers | 20,000 | 0.10 | % | |||||
| Alexandre S. Clug(2) | 150,000 | 0.78 | % | |||||
| The Mustang Trust(2) | 1,000,000 | 5.21 | % | |||||
| Nicole Bergman-Fong | 25,000 | 0.13 | % | |||||
| Peter Carnegie | 200,000 | 1.04 | % | |||||
| Nikhil L. Shah, D. O. | - | 0.00 | % | |||||
| All directors and executive officers as a group (seven persons) | 11,878,100 | 61.89 | % | |||||
| * | Includes shares issuable upon the exercise of options within sixty (60) days of the date of this prospectus. |
| (1) | Includes 6,571,838 shares owned by Mr. Cohen directly and 3,672,700 shares held by Avra Acquisitions, LLC of which Mr. Cohen is managing member and over which shares Mr. Cohen exercises voting and dispositive control. |
| (2) | Includes 150,000 shares owned by Mr. Clug directly and 1,000,000 shares held by The Mustang Trust of which Mr. Clug is trustee and over which shares Mr. Clug exercises voting and dispositive control. |
The persons named above have full voting and investment power with respect to the shares indicated. Under the rules of the SEC, a person (or group of persons) is deemed to be a “beneficial owner” of a security if he or she, directly or indirectly, has or shares the power to vote or to direct the voting of such security, or the power to dispose of or to direct the disposition of such security. Accordingly, more than one person may be deemed to be a beneficial owner of the same security.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Related Party Transactions
From time to time since inception, Barry F. Cohen, our founder, Chief Executive Officer and a director advanced certain sums to us to fund our operations. At December 31, 2015 and December 31, 2016, the Company owed Mr. Cohen $21,877 and $166, respectively, for such advances. The amount is unsecured, non-interest bearing and due on demand.
Effective April 1, 2017, the Company entered into Conversion Agreements with its Chairman/CEO and the Chief Financial Officer whereby each agreed to convert the amounts owing to them as of March 31, 2017 as compensation into common stock of the Company at a price of $2.00 per share. Furthermore, the Chief Financial Officer has agreed to convert any future amounts due as compensation per his Employment Agreement effective through August 1, 2017, into shares of common stock at $2.00 per share as such amounts are earned, and the Chairman/CEO has agreed to convert any future amounts in excess of $2,500 per month due as compensation through July 1, 2017, per his Employment Agreement, into shares of common stock at $2.00 per share as such amounts are earned. The conversions will result in an additional 57,438 shares being issued and outstanding at April 1, 2017.
Review, Approval and Ratification of Related Party Transactions
Given our small size and limited financial resources, we had not adopted formal policies and procedures for the review, approval or ratification of transactions with our executive officers, directors and significant shareholders. However, we intend that such transactions will, on a going-forward basis, be subject to the review, approval or ratification of our board of directors, or an appropriate committee thereof.
45
Capital Stock
Our authorized capital stock consists of 100,000,000 shares of common stock, par value $0.0001 and 5,000,000 shares of preferred stock, par value $0.0001.
Common Stock
As of the date of this prospectus, 19,192,438 shares of common stock are outstanding. The shares of common stock presently outstanding are fully paid and non-assessable. Each holder of common stock is entitled to one vote for each share owned on all matters voted upon by shareholders, and a majority vote is required for all actions to be taken by shareholders. In the event we liquidate, dissolve or wind-up our operations, the holders of the common stock are entitled to share equally and ratably in our assets, if any, remaining after the payment of all our debts and liabilities and the liquidation preference of any shares of preferred stock that may then be outstanding. The common stock has no preemptive rights, no cumulative voting rights, and no redemption, sinking fund, or conversion provisions.
Holders of common stock are entitled to receive dividends, if and when declared by the board of directors, out of funds legally available for such purpose, subject to the dividend and liquidation rights of any preferred stock that may then be outstanding.
Preferred Stock
Our board of directors has the authority, without further action by the shareholders, to issue shares of preferred stock in one or more series and to fix the rights, preferences and the number of shares constituting any series or the designation of such series. While our Amended and Restated Articles of Incorporation and bylaws do not contain any provisions that may delay, defer or prevent a change in control, the issuance of preferred stock may have the effect of delaying or preventing a change in control or make removal of our management more difficult. No shares of preferred stock are outstanding as of the date of this prospectus.
The validity of the common stock being offered hereby has been passed upon by Gutiérrez Bergman Boulris, PLLC, Coral Gables, Florida. A member of such law firm beneficially owns 10,000 shares of our common stock, whose resale is covered by the prospectus forming a part of this registration statement and holds an option granted under the Incentive Plan to purchase an additional 40,000 shares of our common stock.
The audited financial statements included in this prospectus and elsewhere in the registration have so been included in reliance upon the report of De Leon & Company, P. A., independent registered public accountants, upon the authority of said firm as experts in accounting and auditing in giving said report.
We have filed a registration statement on Form S-1 under the Securities Act with the SEC with respect to the shares of our common stock offered through this prospectus. This prospectus is filed as a part of that registration statement, but does not contain all of the information contained in the registration statement and exhibits. Statements made in the registration statement are summaries of the material terms of the referenced contracts, agreements or documents of the company. We refer you to our registration statement and each exhibit attached to it for a more detailed description of matters involving the company. You may inspect the registration statement, exhibits and schedules filed with the SEC at the SEC’s principal office in Washington, D.C. Copies of all or any part of the registration statement may be obtained from the Public Reference Section of the SEC, 100 F Street, N.E. Washington, D.C. 20549. Please call the Commission at 1-800-SEC-0330 for further information on the operation of the public reference rooms. The SEC also maintains a web site at http://www.sec.gov that contains reports, proxy Statements and information regarding registrants that file electronically with the SEC. Our registration statement and the referenced exhibits can also be found on this site.
46
DISCLOSURE OF SEC POSITION ON INDEMNIFICATION
FOR SECURITIES ACT LIABILITIES
In accordance with the provisions in our Amended and Restated Articles of Incorporation, by laws, employment and non-employee director appointment letters we will indemnify an officer, director, or former officer or director, to the full extent permitted by law.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
47
INDEX TO FINANCIAL STATEMENTS
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors of AVRA Medical Robotics, Inc.
We have audited the accompanying balance sheets of AVRA Medical Robotics, Inc.as of December 31, 2016 and 2015 and the related statements of operations, shareholders’ equity and cash flows for the two years then ended. AVRA Medical Robotics Inc.’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audit. We were not engaged to examine management’s assertion about the effectiveness of AVRA Medical Robotics, Inc’s internal control over financial reporting as of December 31, 2016 and 2015.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of AVRA Medical Robotics, Inc. as of December 31, 2016 and 2015, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company’s ability to raise additional capital through debt and/or equity financing to fund its operating costs is unknown, which raises substantial doubt about its ability to continue as a going concern. Management’s plan in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| De Leon & Company, P.A. | |
| Pembroke Pines, Florida | |
| May 2, 2017 |
| F-2 |
BALANCE SHEETS
DECEMBER 31, 2016 and DECEMBER 31, 2015
| December 31, | December 31, | |||||||
| 2016 | 2015 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 248,219 | $ | 100 | ||||
| Other prepaid expenses and deposit | 465 | |||||||
| Total Current Assets | 248,684 | 100 | ||||||
| OTHER ASSETS | ||||||||
| Intellectual Property | 43,548 | |||||||
| TOTAL ASSETS | $ | 292,232 | $ | 100 | ||||
| LIABILITIES AND SHAREHOLDERS' EQUITY (DEFICIT) | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts Payable | $ | 54,827 | ||||||
| Accrued expenses | 90,588 | |||||||
| Due to majority shareholder | 21,877 | 166 | ||||||
| Promissory Notes | 480,000 | |||||||
| Total Current Liabilities | 647,292 | 166 | ||||||
| SHAREHOLDERS' DEFICIT | ||||||||
| Common stock, 100,000,000 shares authorized, $.0001 par value, 19,000,000 and 13,100,400 issued and outstanding, respectively | 1,900 | 1,310 | ||||||
| Subscriptions receivable | (1,310 | ) | ||||||
| 1,900 | 0 | |||||||
| Preferred stock, 5,000,000 shares authorized, $.0001 par value, none issued or outstanding | 0 | 0 | ||||||
| Additional paid-in capital | 128,088 | 0 | ||||||
| Accumulated Deficit | (485,048 | ) | (66 | ) | ||||
| Total Shareholders' Deficit | (355,060 | ) | (66 | ) | ||||
| TOTAL LIABILITIES AND SHAREHOLDERS' DEFICIT | $ | 292,232 | $ | 100 | ||||
See accompanying Notes to Financial Statements
| F-3 |
STATEMENTS OF OPERATIONS
FOR YEAR ENDED DECEMBER 31, 2016 AND THE PERIOD
FROM INCEPTION (FEBRUARY 4, 2015) TO DECEMBER 31, 2015
| Year | Period from | |||||||
| Ended | Inception (February 4, 2015) | |||||||
| December 31, 2016 | to December 31, 2015 | |||||||
| REVENUES | $ | - | $ | - | ||||
| OPERATING EXPENSES | ||||||||
| Research and Development | 138,377 | |||||||
| General and Administrative | 324,116 | 66 | ||||||
| Total Operating Expenses | 462,493 | 66 | ||||||
| OTHER (INCOME) AND EXPENSE | ||||||||
| Interest earned | (31 | ) | ||||||
| Interest Expense | 22,520 | |||||||
| Total Other Income and Expense | 22,489 | 0 | ||||||
| LOSS BEFORE INCOME TAXES | (484,982 | ) | (66 | ) | ||||
| PROVISION FOR INCOME TAXES | 0 | 0 | ||||||
| NET LOSS | $ | (484,982 | ) | $ | (66 | ) | ||
| BASIC AND DILUTED LOSS PER SHARE | $ | 0.03 | - | |||||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING BASIC | 18,508,367 | 13,100,400 | ||||||
See accompanying Notes to Financial Statements
| F-4 |
STATEMENT OF SHAREHOLDERS' EQUITY (DEFICIT)
PERIOD FROM INCEPTION (FEBRUARY 4, 2015) TO DECEMBER 31, 2016
| Common Stock | Subscriptions | Additional | Accumulated | Total Shareholders' | ||||||||||||||||||||||||
| Number | Amount | Receivable | Paid-in capital | Deficit | Deficit | |||||||||||||||||||||||
| BALANCE AT INCEPTION (FEBRUARY 4, 2015) | ||||||||||||||||||||||||||||
| Common stock issued | 13,100,400 | $ | 1,310 | $ | 1,310 | 0 | ||||||||||||||||||||||
| Net loss for the period February 4, 2015 to December 31, 2015 | (66 | ) | (66 | ) | ||||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2015 | 13,100,400 | 1,310 | 1,310 | (66 | ) | (66 | ) | |||||||||||||||||||||
| Stock based compensation expense | 128,088 | 128,088 | ||||||||||||||||||||||||||
| Payment of subscription receivable | (1,900 | ) | 1,900 | |||||||||||||||||||||||||
| Subscriptions for purchase of Common Stock Issued | 5,899,600 | 590 | 590 | 0 | ||||||||||||||||||||||||
| Net loss for the period January 1, 2016 to December 31 ,2016 | (484,982 | ) | (484,982 | ) | ||||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2016 | 19,000,000 | $ | 1,900 | $ | $ | 128,088 | $ | (485,048 | ) | $ | (355,060 | ) | ||||||||||||||||
See accompanying Notes to Financial Statements
| F-5 |
STATEMENT OF CASH FLOWS
FOR YEAR ENDED DECEMBER 31, 2016 AND
FROM INCEPTION (FEBRUARY 4, 2015) TO DECEMBER 31, 2015
| From Inception (February 4, 2015) to | ||||||||
| Year Ended December 31, 2016, | December 31, 2015 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net Loss | $ | (484,482 | ) | $ | (66 | ) | ||
| Adjustments to reconcile net loss to net cash (used) provided in operating activities: | ||||||||
| Stock compensation expense | 128,088 | |||||||
| Changes in operating assets and liabilities: | ||||||||
| Increase in prepaid expenses | (465 | ) | ||||||
| Increase in due to majority shareholder | 21,377 | 166 | ||||||
| Increase in accounts payable and accrued expenses | 145,249 | |||||||
| Net Cash (Used) Provided in Operating Activities | (211,610 | ) | 100 | |||||
| INVESTING ACTIVITIES | ||||||||
| Acquisition of intellectual property | (43,548 | ) | 0 | |||||
| Net Cash Used in Investing Activities | (43,548 | ) | 0 | |||||
| FINANCING ACTIVITIES | ||||||||
| Proceeds from issuance of Promissory Notes | 580,000 | |||||||
| Repayment of Promissory Note | (100,000 | ) | ||||||
| Issuance of common stock for cash | 1,900 | |||||||
| Net Cash Provided by Financing Activities | 481,900 | 0 | ||||||
| INCREASE IN CASH AND CASH EQUIVALENTS | 248,119 | 100 | ||||||
| CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 100 | 0 | ||||||
| CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 248,219 | $ | 100 | ||||
| SUPPLEMENTAL INFORMATION OF NON-CASH INVESTING AND FINANCING ACTIVITIES | ||||||||
| Cash paid for interest | $ | 1,932 | 0 | |||||
See accompanying Notes to Financial Statements
| F-6 |
NOTES TO FINANCIAL STATEMENTS
YEAR ENDED DECEMBER 31, 2016
NOTE 1 – ORGANIZATION AND BASIS OF ACCOUNTING
AVRA Medical Robotics, Inc. (the “Company” or “AVRA”) was incorporated as AVRA Surgical Microsystems, Inc. in the State of Florida on February 4, 2015. Effective November 5, 2015, the Company’s corporate name was changed to AVRA Medical Robotics, Inc. The Company was established to develop advanced medical surgical devices. The Company is structured to invest in four principal areas –surgical robotic systems, surgical tools, implantable devices and surgical robotic training.
The accompanying financial statements are prepared on the basis of accounting principles generally accepted in the United States of America (“GAAP”). The Company is a development-stage enterprise devoting substantial efforts to establishing a new business, financial planning, raising capital, and research into products which may become part of the Company’s product portfolio. The Company has not realized significant sales through December 31, 2016. A development stage company is defined as one in which all efforts are devoted substantially to establishing a new business and, even if planned principal operations have commenced, revenues are insignificant.
The accompanying financial statements have been prepared assuming the continuation of the Company as a going concern. The Company has not yet established an ongoing source of revenues sufficient to cover its operating costs and is dependent on debt and equity financing to fund its operations. Management of the Company is making efforts to raise additional funding until a registration statement relating to an equity funding facility is in effect. While management of the Company believes that it will be successful in its capital formation and planned operating activities, there can be no assurance that the Company will be able to raise additional equity capital, or be successful in the development and commercialization of the products it develops or initiates collaboration agreements thereon. The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the possible inability of the Company to continue as a going concern.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Cash and Cash Equivalents
The Company considers all cash on hand, cash accounts not subject to withdrawal restrictions or penalties, and all highly liquid debt instruments purchased with a maturity of three months or less to be cash and cash equivalents
Stock Compensation Expense
The Company accounts for equity instruments issued in exchange for the receipt of goods or services from other than employees in accordance with Accounting Standards Codification ("ASC") Topic 505, "Equity." Costs are measured at the estimated fair market value of the consideration received or the estimated fair value of the equity instruments issued, whichever is more reliably measurable. The value of equity instruments issued for consideration other than employee services is determined on the earlier of a performance commitment or completion of performance by the provider of goods or services as defined by ASC Topic 505. See Note 5.
| F-7 |
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Income Taxes
The Company accounts for income taxes pursuant to ASC Topic 740 "Income Taxes." Under ASC Topic 740, deferred tax assets and liabilities are determined based on temporary differences between the bases of certain assets and liabilities for income tax and financial reporting purposes. The deferred tax assets and liabilities are classified according to the consolidated financial statement classification of the assets and liabilities generating the differences. A valuation allowance is recorded when it is more likely than not that some or all of the deferred tax assets will not be realized.
The Company applies the provisions of ASC Topic 740-10-05 "Accounting for Uncertainty in Income Taxes." The ASC clarifies the accounting for uncertainty in income taxes recognized in an enterprise's financial statements. The ASC prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The ASC provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities and expenses. The Company regularly evaluates estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates made by management.
Concentration of Credit Risk
The Company maintains its cash and cash equivalents in financial institutions in the United States which at times may exceed insurance limits. The Company has not experienced any losses on such accounts.
Basic and Diluted Loss per Share
In accordance with ASC Topic 260 "Earnings Per Share," basic loss per common share is computed by dividing net loss available to common stockholders by the weighted average number of common shares outstanding during the period. Diluted loss per common share gives effect to dilutive convertible securities, options, warrants and other potential common stock outstanding during the period, only in periods in which such effect is dilutive. The Company only has stock options that may be converted to outstanding potential common shares.
| F-8 |
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Revenue Recognition
The Company recognizes revenue when all of the following criteria are met: (1) the Company has evidence of an arrangement with a customer; (2) the Company delivers the specified services; (3) terms are fixed or determinable; and (4) collection is probable.
Property and Equipment
Property and equipment are recorded at cost and depreciated using the straight-line method at rates determined to estimate the useful lives of the assets. The annual rates used in calculating depreciation is as follows:
Equipment -5 years straight-line
Long-lived Assets
In accordance with ASC 360, “Property Plant and Equipment”, the Company tests long-lived assets or asset groups for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not limited to : significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset and current expectation that the asset will more than likely not be sold or disposed significantly before the end of its estimated useful life. Recoverability is assessed based on the carrying amount of the asset and its fair value which is generally determined based on the sum of the discounted cash flows expected to result from the use and the eventual disposal of the asset, as well as specific appraisal in certain circumstances. An impairment loss is recognized when the carrying amount is not recoverable and exceeds fair value.
Fair Value of Financial Instruments
Our financial instruments consist principally of accounts receivable, amounts due to related parties and promissory notes payable.
ASC 820 Fair Value Measurements and Disclosures and ASC 825, Financial Instruments establish a framework for measuring fair value, establishes a fair value hierarchy based on the quality of the inputs used to measure fair value, and enhances disclosure requirements for fair value measurements.
| F-9 |
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Fair Value Hierarchy
The Company has categorized its financial statements, based on the priority of inputs to the valuation technique, into a three-tier fair value hierarchy. The fair value hierarchy gives the highest priority to quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest level priority to unobservable inputs (Level 3).
Financial assets and liabilities recorded on the balance sheet are categorized based on inputs to the valuation techniques as follows:
| Level 1 | Financial assets and liabilities for which values are based on unadjusted quoted prices for identical assets or liabilities in an active market that management has the ability to access. |
| Level 2 | Financial assets and liabilities for which values are based on quoted prices in markets that are not active or model inputs that are observable either directly or indirectly for substantially the full term of the asset or liability (commodity derivatives and interest rate swaps). |
| Level 3 | Financial assets and liabilities for which values are based on prices or valuation techniques that require inputs that are both unobservable and significant to the overall fair value measurement. These inputs reflect management’s own assumptions about the assumptions a market participant would use in pricing the asset or liability. |
When the inputs used to measure fair value fall within different levels of the hierarchy, the level within which the fair value measurement is categorized is based on the lowest level input that is significant to the fair value measurement in its entirety.
The carrying amounts of cash and cash equivalents and promissory notes approximate fair value because of the short-term nature of these items.
NOTE 3– Research collaboration agreement
Effective May 1, 2016, the Company entered into a Research Agreement with the University of Central Florida for the development of a prototype surgical robotic device supporting minimal invasive surgical facial corrections.
The Agreement provides that the University will provide personnel to accomplish the objectives as stated in the Statement of Work over a period extending to June 30, 2017. The Company has agreed to extend funding of $163,307 from AVRA’s existing funds.
The amount of $138,777 shown as Research & Development expense represents three billings from The University of Central Florida comprising $40,827 invoiced at time of signing the Agreement, $40,827 invoiced August 1, 2016 and $40,827 invoiced November 1, 2016, and various billings adding up to $15,896.
In addition, AVRA has paid $43,548 for outright ownership of the University’s Intellectual Property resulting from the collaboration, which amount is shown as Intellectual Property. Management has assessed the carrying value of the asset and believes there has been no diminution of its value and accordingly, no adjustment is necessary.
| F-10 |
NOTE 3– Research collaboration agreement (continued)
The total cost to the Company is:
| Research Expense -funded from existing funds | $ | 163,307 | ||
| Acquisition of Intellectual Property Rights | 43,548 | |||
| Total | $ | 206,855 |
As of December 31, 2016, $125,202 has been paid under the Agreement. The balance of the amount owing to the University was due November 1, 2016 ($40,827) and February 1, 2017 ($40, 826). Both balances due have been fully paid on February 24, 2017 and on April 7, 2017, respectively. Additionally, a $68,952 matching funds grant from the Florida High Tech Corridor Council (FHTCC) was approved on July 16, 2016 which will provide the University research funds in addition to the Company’s funding obligation to the University. The FHTCC research grant is subject to certain research obligations and action requirements which if not met may result in the loss of the FHTCC research funding which would require the Company to cover any resulting shortfall.
The agreement further provides for the payment of a 1% royalty to the University in any year when the sales of products using the intellectual property exceeds $20,000,000.
NOTE 4 – PROMISSORY NOTES
During the year ended December 31, 2016, the Company borrowed $480,000 under 7.5% Convertible Promissory Note Agreements. The Notes are due June 30, 2017 and bear interest at 7.5%. The notes are convertible into common stock of the Company at $0.50 per share in the event of a voluntary conversion on or before an optional prepayment or the maturity date, or (1) the lower of $0.50 or (2) a 20% discount to the effective price per share offering price in the event of a mandatory conversion upon consummation of a “Qualified Financing”, as defined. The Company has pledged all assets as security for the notes. In the event of default, the notes will bear interest at 12% per annum.
Further, the Company borrowed $100,000 from an individual on May 16, 2016 under a note bearing interest at 5%. The note, along with accrued interest, was repaid on September 30, 2016.
NOTE 5– INCOME TAXES
The Company’s deferred tax assets at December 31, 2016 and December 31, 2015 consist of net operating loss carry forwards of $484,982 and $66, respectively. Using a federal statutory tax rate of 35%, the valuation allowance balance as of December 31, 2016 and December 31, 2015 total $169,743 and $23, respectively. The increase in the valuation allowance balance for the year ended December 31, 2016 of $169,743 is entirely attributable to the net operating loss.
Due to the uncertainty of their realization, no income tax benefits have been recorded by the Company for these loss carry forwards as valuation allowances have been established for any such benefits. The increase in the valuation allowance was the result of increases in the net operating losses discussed above. Therefore, the Company’s provision for income taxes is $0 for the year ended December 31, 2016 and the period ended December 31, 2015.
| F-11 |
At December 31, 2016, the Company had no material unrecognized tax benefits and no adjustments to liabilities or operations were required. The Company does not expect that its unrecognized tax benefits will materially increase within the next twelve months. The Company recognizes interest and penalties related to uncertain tax positions in general and administrative expense. At December 31, 2016, the Company has not recorded any provisions for accrued interest and penalties related to uncertain tax positions.
The Company files U.S. federal and state income tax returns in jurisdictions with varying statutes of limitations.
NOTE 6– SHAREHOLDERS’ DEFICIT
The Company is authorized to issue up to 100,000,000 shares of common stock, $0.0001 par value per share plus 5,000,000 shares of preferred stock, par value $0.0001. For the period ended December 31, 2015, 13,100,400 shares were issued to the majority shareholder at a price of $0.0001 per share (total $1,310) and was paid in 2016. On February 1, 2016 subscriptions were issued for an additional 5,899,600 shares of common stock at $0.0001 per share (total $590). Holders are entitled to one vote for each share of common stock. No preferred stock has been issued.
NOTE 7 –EQUITY INCENTIVE PLAN
On August 1, 2016, the Company approved the AVRA 2016 Omnibus Incentive Plan (the “Plan”). The Plan provides for the granting of options to employees, directors, consultants and advisors to purchase up to 3,000,000 shares of the Company’s common stock. The Board is responsible for administration of the Plan. The Board determines the term of each option, the option exercise price, the number of shares for which each option is granted and the rate at which each option is exercisable. Incentive stock options may be granted to any officer or employee at an exercise price per share of not less than the fair market value per common share on the date of the grant.
On August 15, 2016, five-year options were granted to certain participants for the purchase of 1,702,000 shares at an exercise price of $0.10 per share. 1,000,000 shares vest immediately and the balance vest over three years.
On October 1, 2016, five-year options vesting over three years were granted to certain participants for the purchase of 856,000 shares at an exercise price of $0.15 per share.
At December 31, 2016 options representing 1,176,000 were vested or exercisable.
No options were exercised or forfeited during the year ended December 31, 2016.
Stock options are accounted for in accordance with FASB ASC Topic 718, Compensation –Stock Compensation, with option expense amortized over the vesting period based on the Black-Scholes option-pricing model fair value on the grant date, which includes a number of estimates that affect the amount of expense. During the year ended December 31, 2016, $128,088 has been recorded as stock-based compensation and classified in General and Administrative expense in the Income Statement. The total amount of unrecognized compensation cost related to non-vested options was $62,070 as of December 31, 2016. This amount will be recognized over a weighted average period of 2.7 years.
| F-12 |
The grant date fair value of options granted during the year of 2016 were estimated on the grant date using the Black-Scholes model with the following assumptions: expected volatility of 181%, expected term of 2.9 years, risk-free interest rate of 2.00% and expected dividend yield of 0% for the options granted on August 15, 2016 with an exercise price of $0.10 per share and; expected volatility of 73.64%, expected term of 2.9 years, risk-free interest rate of 2.00% and expected dividend yield of 0% for the options granted on October 1, 2016 with an exercise price of $0.15 per share.
Expected volatility is based on the average of the historical volatility of the stock prices of a blend of five publicly traded companies operating in a similar industry as that of the Company. The risk-free rate is based on the rate of U.S Treasury zero-coupon issues with a remaining term equal to the expected life of the options. The Company uses historical data to estimate pre-vesting for feature rates.
NOTE 8- EMPLOYMENT AGREEMENTS
On July 1, 2016, the Company entered into a four-year Employment Agreement with its Chairman and Chief Executive Officer. The agreement provides for an annual salary of $120,000 per year, increasing to $180,000 per year beginning July 2017. The employee agrees to not receive the compensation in cash until the Board of Directors deems it prudent to pay some or all of his salary. Further the Agreement provides that the employee will receive a three-year option to purchase 1,000,000 shares of the Company’s common stock at an exercise price of $0.10 per share, and becoming fully vested on August 15, 2016.
On August 1, 2016, the Company entered into a one-year Employment Agreement with its Chief Financial Officer. The agreement provides for an annual salary of $108,000 per year. The employee agrees to not receive the compensation in cash until the Board of Directors deems it prudent to pay some or all of his salary. Further the Agreement provides that the employee will receive a three-year option to purchase 210,000 shares of the Company’s common stock at an exercise price of $0.10 per share, with 70,000 shares becoming fully vested upon each yearly anniversary. The options are to be surrendered and cancelled if the Agreement is terminated.
On August 1, 2016, the Company entered into a three-year Employment Agreement with its Vice President of Global Business Development. The agreement provides for an annual salary of $96,000 per year, increasing to $144,000 per year beginning July 2017. The employee agrees to not receive the compensation in cash until the Chief Executive Officer deems it prudent to pay some or all of his salary. Further the Agreement provides that the employee will receive a three-year option to purchase 300,000 shares of the Company’s common stock at an exercise price of $0.10 per share, with 100,000 shares vested on each yearly anniversary.
Further, on July 1, 2016, the Company entered into Indemnification Agreements with the Chairman and Chief Executive Officer, and on August 1, 2016, the Chief Financial Officer and the Vice-President of Global Business Development providing for the Company to indemnify the individuals for all expenses judgments, etc. incurred while serving in various capacities with the Company.
NOTE 9 – EARNINGS PER SHARE
Basic earnings per share (“basic EPS”) is computed by dividing the net income or loss by the weighted average number of common shares outstanding for the reporting period. Diluted earnings per share (“diluted EPS”) gives effect to all dilutive potential shares outstanding. For the year ended December 31, 2016 the potential conversion of the Promissory Notes into common stock was excluded from the computation of fully-diluted loss per share as the effect was anti-dilutive. Further, the potential exercise of stock options has been excluded from the computation of loss per share has been excluded as the effect was anti-dilutive.
| F-13 |
NOTE 10– LEASE COMMITMENT
The Company occupies office and laboratory space in Orlando, Florida under a lease agreement expiring July 31, 2017. The agreement provides that the Company pay insurance, maintenance and taxes. Monthly lease expense is $240.
NOTE 11– SUBSEQUENT EVENTS
The Company has evaluated subsequent events through the date that the financial statements were issued. All the options issued to-date expire after five years from the issue date. Except for the options for one million shares issued to the CEO and the options for 40,000 shares issued to legal counsel that all vested immediately, all the options issued to-date vest over three years.
Effective April 1, 2017, the Company entered into Conversion Agreements with its Chairman/CEO and the Chief Financial Officer whereby each agreed to convert the amounts owing to them as of March 31, 2017 as compensation into common stock of the Company at a price of $2.00 per share. Furthermore, the Chief Financial Officer has agreed to convert any future amounts due as compensation per his Employment Agreement effective through August 1, 2017, into shares of common stock at $2.00 per share as such amounts are earned, and the Chairman/CEO has agreed to convert any future amounts in excess of $2,500 per month due as compensation through July 1, 2017, per his Employment Agreement, into shares of common stock at $2.00 per share as such amounts are earned. The conversions will result in an additional 57,438 shares being issued and outstanding at April 1, 2017.
The Company has an agreement with legal counsel whereby counsel will receive payment for up to 50% of fees (up to $100,000) in the form of a grant of options. On January 1, 2017, the Company issued to legal counsel options for 40,000 shares with an exercise price of $0.15 per share that all vested immediately.
Effective May 1, 2017, the research agreement with the University of Central Florida has been extended to June 30, 2019.
| F-14 |
Until ___________ __, 2017 (90 days from the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this Offering, may be required to deliver a prospectus. This is in addition to the dealer’s obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
You should rely only on the information contained in this prospectus. We have not authorized any dealer, salesperson or other person to give you different information. This prospectus does not constitute an offer to sell nor are they seeking an offer to buy the securities referred to in this prospectus in any jurisdiction where the offer or sale is not permitted. The information contained in this prospectus and the documents incorporated by reference are correct only as of the date shown on the cover page of these documents, regardless of the time of the delivery of these documents or any sale of the securities referred to in this prospectus.
AVRA MEDICAL ROBOTICS, INC.
10,802,714 Shares of Common Stock
PROSPECTUS
________ __, 2017
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
| Registration Fees | $ | 2,504.07 | ||
| Transfer Agent Fees | $ | * | ||
| Accounting Fees and Expenses | $ | * | ||
| Legal Fees and Expenses | $ | * | ||
| Miscellaneous Fees and Expenses | $ | * | ||
| Total | $ | * |
*To be filed by amendment.
All amounts are estimates other than the SEC’s registration fee. We are paying all expenses of the offering listed above. No portion of these expenses will be borne by the selling shareholders. The selling shareholders, however, will pay any other expenses incurred in selling their common stock, including any brokerage commissions or costs of sale.
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS
Our Amended and Restated Articles of Incorporation, bylaws, employment agreements and non-employee director appointment letters provide for indemnification of our officers and directors to the fullest extent permitted by the Florida Business Corporation Act.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES
During the past two years, we effected the following transactions in reliance upon exemptions from registration under the Securities Act, as amended:
(a) On February 4, 2015, we issued 13,100,400 shares of our common stock to our founder and Chief Executive Officer for par value, a purchase price of $1,310.
(b) Effective February 1, 2016, we sold an aggregate of 5,899,600 shares to our founders, initial shareholders and initial investors in a private offering for aggregate consideration of $590.
(c) From April to August, 2016, we sold $480,000 in principal amount of our 7.5% Convertible Promissory Notes due June 30, 2017 (the “Convertible Notes”) to 12 investors in a private offering. The Convertible Notes are convertible into shares of our common stock at $0.50 per share at the option of the holder at any time prior to maturity and will automatically convert into shares of common stock upon completion of a private or public offering which generates at least $500,000 in proceeds to the Company at a conversion price equal to the lower of $0.50 per share or a 20% discount the per share price of shares sold such subsequent offering.
(d) In February 2017, we sold 135,000 shares of our common stock for an aggregate of $135,000 or $1.00 per share to three investors in a private offering.
(e) Between July 1, 2016 and through the date of this registration statement, we have issued options to purchase 2,598,000 shares under our 2016 Incentive Stock Plan to 23 employees and outside consultants, including the following directors and executive officers:
| Name | Title | Number of Shares | ||
| Barry F. Cohen | Chairman/Chief Executive Officer | 1,000,000 | ||
| A. Christian Schauer | Chief Financial Officer | 210,000 | ||
| Dr. Ray Powers | Chief Operating Officer | 75,000 | ||
| Alexandre S. Clug | Vice President of Global Business Development | 300,000 | ||
| Nicole Bergman-Fong | General Counsel | 60,000 | ||
| Peter Carnegie | Director | 45,000 | ||
| Nikhil L. Shah, D.O. | Director | 200,000 |
All of the foregoing securities were issued in accordance with the exemption from registration afforded by Section 4(a) (2) of and Regulation D or Rule 701 promulgated under the Securities Act, as amended, as the persons receiving such shares having provided the Company with appropriate representations as to their investment intent and their status as “accredited investors” as defined in Rule 501(a) of Regulation D promulgated under the Securities Act.
(f) Effective March 31, 2017, we converted $42,876 and $72,000 in accrued compensation due to our Chairman/CEO and our chief financial officer into 21,438 shares and 36,000 shares of our common stock, respectively, at a conversion price of $2.00 per share.
| II-1 |
ITEM 16. EXHIBITS
| Exhibit Number |
Description | |
| 3.1(i) | Amended and Restated Articles of Incorporation* | |
| 3.2 | By-Laws* | |
| 5.1 | Opinion of Gutierrez Bergman Boulris, PLLC** | |
| 10.1 | 2016 Incentive Stock Plan*(1) | |
| 10.2 | Research Agreement with the University of Central Florida* | |
| 10.3 | Employment Agreement with Barry F. Cohen*(1) | |
| 10.4 | Employment Agreement with A. Christian Schauer*(1) | |
| 10.5 | Employment Agreement with Alexandre S. Clug*(1) | |
| 10.6 | Form of Director Appointment Agreement*(1) | |
| 10.7 | Code of Ethical Conduct * | |
| 10.8 | Form of Indemnification Agreement***(1) | |
| 10.9 | Form of 7.5% Convertible Promissory Note due June 30, 2017*** | |
| 23.1 | Consent of De Leon & Company, P.A.*** | |
| 23.2 | Consent of Gutierrez Bergman Boulris, PLLC (Included in Exhibit 5.1)** | |
| 24 | Power of Attorney (included in signature page to this registration statement) |
*Previously filed.
**To be filed by amendment.
***Filed herewith.
| (1) | Management compensation plan or arrangement. |
ITEM 17. UNDERTAKINGS
The undersigned registrant hereby undertakes:
1. To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement;
(a) to include any prospectus required by Section 10(a) (3) of the Securities Act of 1933;
(b) to reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information in the registration statement; and notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospects filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in the volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.; and
(c) to include any material information with respect to the plan of distribution not previously disclosed in this registration statement or any material change to such information in the registration statement.
| II-2 |
2. That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered herein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
3. To remove from registration by means of a post-effective amendment any of the securities being registered hereby which remain unsold at the termination of the offering.
4. That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities:, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(a) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424 of this chapter);
(b) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(c) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(d) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons pursuant to the provisions above, or otherwise, we been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities, other than the payment by us of expenses incurred or paid by one of our directors, officers, or controlling persons in the successful defense of any action, suit or proceeding, is asserted by one of our directors, officers, or controlling persons in connection with the securities being registered, we will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification is against public policy as expressed in the Securities Act of 1933, and we will be governed by the final adjudication of such issue.
Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B of the Securities Act or other than prospectuses filed in reliance on Rule 430A of the Securities Act, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness, provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
| II-3 |
SIGNATURES
In accordance with the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-1 and authorized this Amendment No. 1 to the Registration Statement to be signed on its behalf by the undersigned, in Orlando, Florida, on May 11, 2017.
| AVRA MEDICAL ROBOTICS, INC. | ||
| By: | /s/ Barry F. Cohen | |
| Barry F. Cohen, Chief Executive Officer | ||
| (Principal Executive Officer) | ||
| By: | /s/ A. Christian Schauer | |
| A. Christian Schauer, Chief Financial Officer | ||
| (Principal Financial and Accounting Officer) | ||
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Barry F. Cohen and A. Christian Schauer and each of them, as a true and lawful attorney-in-fact and agent, with full power of substitution and re-substitution, for each of them and in each name, place and stead, in any and all capacities, to sign any and all pre- or post-effective amendments to this registration statement, and to file the same with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all intents and purposes as each might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent or his substitute, may lawfully do or cause to be done by virtue hereof. In accordance with the requirements of the Securities Act of 1933, as amended, this registration statement was signed by the following person in the capacities and on the dates stated.
| Signatures | Title(s) | Date | |||
| By: | /s/ Barry F. Cohen | Chief Executive Officer and Director | May 11, 2017 | ||
| Barry F. Cohen | (Principal Executive Officer) | ||||
| By: | /s/ A. Christian Schauer | Chief Financial Officer | May 11, 2017 | ||
| A. Christian Schauer | (Principal Financial and Accounting Officer) | ||||
| By: | /s/ Peter Carnegie | Director | May 11, 2017 | ||
| Peter Carnegie | |||||
| By: | /s/ Nikhil L. Shah | Director | May 11, 2017 | ||
| Nikhil L. Shah, D.O. | |||||
| II-4 |
