Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AVADEL PHARMACEUTICALS PLC | avadel8k20170509slides.htm |

Q1 2017
Earnings Conference Call
May 9th, 2017

2
Safe Harbor
This presentation may include "forward-looking statements" within the meaning of the Private Securities Litigation
Reform Act of 1995. All statements herein that are not clearly historical in nature are forward-looking, and the words
"anticipate," "assume," "believe," "expect," "estimate," "plan," "will," "may," and the negative of these and similar
expressions generally identify forward-looking statements. All forward-looking statements involve risks, uncertainties
and contingencies, many of which are beyond Avadel’s control and could cause actual results to differ materially from
the results contemplated in such forward-looking statements. These risks, uncertainties and contingencies include the
risks relating to: our dependence on a small number of products and customers for the majority of our revenues; the
possibility that our Bloxiverz®, Vazculep® and Akovaz® products, which are not patent protected, could face substantial
competition resulting in a loss of market share or forcing us to reduce the prices we charge for those products; the
possibility that we could fail to successfully complete the research and development for the pipeline product we are
evaluating for potential application to the FDA pursuant to our "unapproved-to-approved" strategy, or that competitors
could complete the development of such product and apply for FDA approval of such product before us; our
dependence on the performance of third parties in partnerships or strategic alliances for the commercialization of some
of our products; the possibility that our products may not reach the commercial market or gain market acceptance; our
need to invest substantial sums in research and development in order to remain competitive; our dependence on
certain single providers for development of several of our drug delivery platforms and products; our dependence on a
limited number of suppliers to manufacture our products and to deliver certain raw materials used in our products; the
possibility that our competitors may develop and market technologies or products that are more effective or safer than
ours, or obtain regulatory approval and market such technologies or products before we do; the challenges in
protecting the intellectual property underlying our drug delivery platforms and other products; our dependence on key
personnel to execute our business plan; the amount of additional costs we will incur to comply with U.S. securities laws
as a result of our ceasing to qualify as a foreign private issuer; and the other risks, uncertainties and contingencies
described in the Company's filings with the U.S. Securities and Exchange Commission, including our annual report on
Form 10-K for the year ended December 31, 2016, all of which filings are also available on the Company's website.
Avadel undertakes no obligation to update its forward-looking statements as a result of new information, future events
or otherwise, except as required by law.

3
Strategy Execution: 1Q 2016 Overview
• Market Dynamics: Hospital Products
• Akovaz®
• Bloxiverz®
• Vazculep®
• REST-ON Phase III Trial Update
• Non-GAAP Financial Results
• GAAP Financial Results
• Product Sales
• Cash Flow
• 2017 Guidance

4
Market Dynamics: Akovaz®
Ephedrine Market Volume / Year
IMS Data Repackaging Market
• Three approved products in 1Q 2017
• Total vials per year ~7.5M
• ~5M vials captured by IMS data
• ~2.5M vials to private repackaging market
• Akovaz IMS share during 1Q 2017 was approximately 15% -
20%
• Total market share averaged approximately 40% for 1Q 2017

5
Market Dynamics: Bloxiverz®
0
200000
400000
600000
800000
1000000
1200000
1400000
1Q 2016 2Q 2016 3Q 2016 4Q 2016 1Q 2017
Neuromuscular Blockade Reversal Market Volume
Neostigmine Sugammadex
• IMS Data tracks neuromuscular blockade market at ~5M vials per year
• Neostigmine declined as a percent of the overall market on a y/y basis
• Bloxiverz has consistently held ~40% of total neostigmine volume
V
ials
/
Qu
arte
r

6
Market Dynamics: Vazculep®
0%
20%
40%
60%
80%
100%
1mL 5mL 10mL
Market Share by Vial Size
Avadel Other
• Vazculep held
approximately 40% of the
1mL market volume during
1Q 2017
• Vazculep 5mL & 10mL have
been sole source since
approval in 2014
• Relatively consistent share
and revenue over the last
year

7
Progress to Date
REST-ON Phase III Trial
• SPA in place with FDA
• Upfront agreement from FDA on powering and trial design
• Clinical sites actively recruiting patients
• USA
• Europe
• Canada
• Enrollment completion goal of year end 2017

8
Pediatric Products & Business Development
• Karbinal ER
• TRx up 26% quarter / quarter
• TRx up 67% year / year
• Launched Flexichamber® for use with
metered dose inhalers for the treatment
of asthma launched
$179.2M in Cash & Marketable Securities - Actively looking for acquisitions to
add new revenue streams and products for sales reps to leverage

9
Non-GAAP Financial Results
*Reconciliations from GAAP to Non-GAAP can be found in the appendix
(in 000s)
03/31/17 12/31/16 03/31/16
Sales 52,507$ 43,085$ 36,216$
Cost of products and services sold 3,856 3,610 3,143
Research and development expenses 7,206 13,476 5,388
Selling, general and admin expenses 11,812 10,688 9,461
Intangible asset amortization - - -
Restructuring costs - - -
Operating expenses 22,874 27,774 17,992
Contingent consideration payments and accruals 9,616 7,645 6,445
Operating income (loss) 20,017 7,666 11,779
Interest and other expense (net) 266 294 25
Other Expense - changes in fair value of related party payable (1,299) (1,018) (892)
Income (loss) before income taxes 18,984 6,942 10,912
Income tax provision 7,692 6,875 9,078
Net income (loss) 11,292$ 67$ 1,834$
Diluted earnings (loss) per share 0.26$ -$ 0.04$
Three Months Ended

10
GAAP Financial Results
(in 000s)
03/31/17 12/31/16 03/31/16
Sales 52,507$ 43,085$ 36,216$
Cost of products and services sold 3,902 2,591 3,906
Research and development expenses 7,206 13,476 5,388
Selling, general and admin expenses 11,812 10,688 9,461
Intangible asset amortization 564 2,970 3,514
Restructuring costs 2,653 - -
Operating expenses 26,137 29,725 22,269
Fair value adjustments of contingent consideration (6,971) (3,704) 8,243
Operating income (loss) 33,341 17,064 5,704
Interest and other expense (net) 35 1,429 (2,916)
Other Expense - changes in fair value of related party payable 550 (413) (1,534)
Income (loss) before income taxes 33,926 18,080 1,254
Income tax provision 8,525 13,346 7,312
Net income (loss) 25,401$ 4,734$ (6,058)$
Diluted earnings (loss) per share 0.59$ 0.11$ (0.15)$
Three Months Ended

11
Product Sales
in $000's Q1 2016 Q4 2016 Q1 2016
Q1 2017
vs.
Q4 2016
Q1 2017
vs.
Q1 2016
Bloxiverz 13,902$ 16,938$ 24,747$ (3,036)$ (10,845)$
Vazculep 10,179 10,629 9,406 (450) 773
Akovaz 25,638 11,263 - 14,375 25,638
Other 2,038 3,534 1,200 (1,496) 838
Total product sales and services 51,757$ 42,364$ 35,353$ 9,393$ 16,404$
License and research revenue 750$ 721$ 863$ 29$ (113)$
Total revenues 52,507$ 43,085$ 36,216$ 9,422$ 16,291$
12
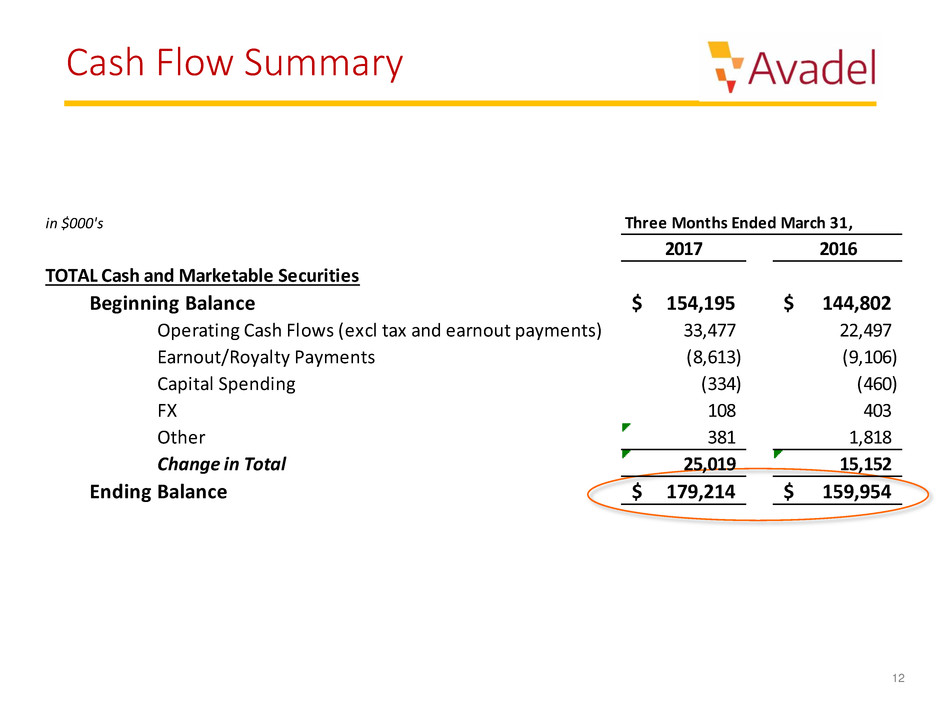
Cash Flow Summary
in $000's
2017 2016
TOTAL Cash and Marketable Securities
Beginning Balance 154,195$ 144,802$
Operating Cash Flows (excl tax and earnout payments) 33,477 22,497
Earnout/Royalty Payments (8,613) (9,106)
Capital Spending (334) (460)
FX 108 403
Other 381 1,818
Change in Total 25,019 15,152
Ending Balance 179,214$ 159,954$
Three Months Ended March 31,

13
Full Year 2017 Guidance - Updated
UPDATED PREVIOUS
Sales $170M - $185M $170M - $200M
R&D Expense $40M - $50M $40M - $50M
SG&A Expense ~$40M ~$40M
Income Tax Rate 60% - 70% 70% - 80%
Diluted EPS (Adjusted) $0.30 - $0.45 $0.20 - $0.35
2017 Guidance

14
Question & Answer

15
APPENDIX

16
GAAP to NON-GAAP Reconciliations
Three Months Ended March 31, 2017:
(in thousands - USD$) Include
GAAP
Intangible asset
amortization
Foreign
exchange
(gain)/loss
Restructuring
impacts
Purchase
accounting
adjustments -
FSC
Contingent
related party
payable
fair value
remeasurements
Contingent
related party
payable
paid/accrued
Total
Adjustments NON-GAAP
Product sales and services 51,757$ -$ -$ -$ -$ -$ -$ -$ 51,757$
License and research revenue 750 - - - - - - - 750
Total revenue 52,507 - - - - - - - 52,507
Cost of products and services sold 3,902 - - - (46) - - (46) 3,856
Research and development expenses 7,206 - - - - - - - 7,206
Selling, general and administrative expenses 11,812 - - - - - - - 11,812
Intangible asset amortization 564 (564) - - - - - (564) -
Changes in fair value of related party contingent
consideration (6,971) - - - - 6,971 9,616 16,587 9,616
Restructuring costs 2,653 - - (2,653) - - - (2,653) -
Total operating expenses 19,166 (564) - (2,653) (46) 6,971 9,616 13,324 32,490
Operating income (loss) 33,341 564 - 2,653 46 (6,971) (9,616) (13,324) 20,017
Investment Income 529 - - - - - - - 529
Interest Expense (263) - - - - - - - (263)
Other Expense - changes in fair value of related party
payable 550 - - - - (550) (1,299) (1,849) (1,299)
Foreign exchange gain (loss) (231) - 231 - - - - 231 -
Income (loss) before income taxes 33,926 564 231 2,653 46 (7,521) (10,915) (14,942) 18,984
Income tax provision 8,525 201 - - 17 (360) (691) (833) 7,692
Income Tax Rate 25% 36% - - 37% 5% 6% 6% 41%
Net Loss 25,401$ 363$ 231$ 2,653$ 29$ (7,161)$ (10,224)$ (14,109)$ 11,292$
Net loss per share - Diluted 0.59$ 0.01$ 0.01$ 0.06$ -$ (0.17)$ (0.24)$ (0.33)$ 0.26$
Weighted average number of shares outstanding - Diluted 42,810 42,810 42,810 42,810 42,810 42,810 42,810 42,810 42,810
Adjustments
Exclude

17
GAAP to NON-GAAP Reconciliations
Three Months Ended December 31, 2016:
(in thousands - USD$) Include
GAAP
Intangible asset
amortization
Foreign
exchange
(gain)/loss
Cross-border
merger impacts
Purchase
accounting
adjustments -
FSC
Contingent
related party
payable
fair value
remeasurements
Contingent
related party
payable
paid/accrued
Total
Adjustments NON-GAAP
Product sales and services 42,364$ -$ -$ -$ -$ -$ -$ -$ 42,364$
License and research revenue 721 - - - - - - - 721
Total revenue 43,085 - - - - - - - 43,085
Cost of products and services sold 2,591 - - - 1,019 - - 1,019 3,610
Research and development expenses 13,476 - - - - - - - 13,476
Selling, general and administrative expenses 10,688 - - - - - - - 10,688
Intangible asset amortization 2,970 (2,970) - - - - - (2,970) -
Changes in fair value of related party contingent
consideration (3,704) - - - - 3,704 7,645 11,349 7,645
Restructuring costs - - - - - - - - -
Total operating expenses 26,021 (2,970) - - 1,019 3,704 7,645 9,398 35,419
Operating income (loss) 17,064 2,970 - - (1,019) (3,704) (7,645) (9,398) 7,666
Investment Income 555 - - - - - - - 555
Interest Expense (261) - - - - - - - (261)
Other Expense - changes in fair value of related party
payable (413) - - - - 413 (1,018) (605) (1,018)
Foreign exchange gain (loss) 1,135 - (1,135) - - - - (1,135) -
Income (loss) before income taxes 18,080 2,970 (1,135) - (1,019) (3,291) (8,663) (11,138) 6,942
Income tax provision 13,346 1,066 - (6,754) (366) 82 (499) (6,471) 6,875
Income Tax Rate 74% 36% - - 36% (2%) 6% 58% 99%
Net Loss 4,734$ 1,904$ (1,135)$ 6,754$ (653)$ (3,373)$ (8,164)$ (4,667)$ 67$
Net loss per share - Diluted 0.11$ 0.04$ (0.03)$ 0.16$ (0.02)$ (0.08)$ (0.19)$ (0.11)$ -$
Weighted average number of shares outstanding - Diluted 42,808 42,808 42,808 42,808 42,808 42,808 42,808 42,808 42,808
Adjustments
Exclude

18
GAAP to NON-GAAP Reconciliations
Three Months Ended March 31, 2016:
(in thousands - USD$) Include
GAAP
Intangible asset
amortization
Foreign
exchange
(gain)/loss
Purchase
accounting
adjustments -
FSC
Contingent
related party
payable
fair value
remeasurements
Contingent
related party
payable
paid/accrued
Total
Adjustments NON-GAAP
Product sales and services 35,353$ -$ -$ -$ -$ -$ -$ 35,353$
License and research revenue 863 - - - - - - 863
Total revenue 36,216 - - - - - - 36,216
Cost of products and services sold 3,906 - - (763) - - (763) 3,143
Research and development expenses 5,388 - - - - - - 5,388
Selling, general and administrative expenses 9,461 - - - - - - 9,461
Intangible asset amortization 3,514 (3,514) - - - - (3,514) -
Changes in fair value of related party contingent
consideration 8,243 - - - (8,243) 6,445 (1,798) 6,445
Restructuring costs - - - - - - - -
Total operating expenses 30,512 (3,514) - (763) (8,243) 6,445 (6,075) 24,437
Operating income (loss) 5,704 3,514 - 763 8,243 (6,445) 6,075 11,779
Investment Income 200 - - - - - - 200
Interest Expense (175) - - - - - - (175)
Other Expense - changes in fair value of related party
payable (1,534) - - - 1,534 (892) 642 (892)
Foreign exchange gain (loss) (2,941) - 2,941 - - - 2,941 -
Income (loss) before income taxes 1,254 3,514 2,941 763 9,777 (7,337) 9,658 10,912
Income tax provision 7,312 1,262 - 274 551 (321) 1,766 9,078
Income Tax Rate 583% 36% - 36% 6% 4% 18% 83%
Net Loss (6,058)$ 2,252$ 2,941$ 489$ 9,226$ (7,016)$ 7,892$ 1,834$
Net loss per share - Diluted (0.15)$ 0.05$ 0.07$ 0.01$ 0.22$ (0.17)$ 0.19$ 0.04$
Weighted average number of shares outstanding - Diluted 41,241 41,241 41,241 41,241 41,241 41,241 41,241 41,241
Adjustments
Exclude
