Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AMICUS THERAPEUTICS, INC. | a17-12738_1ex99d1.htm |
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a17-12738_18k.htm |
Exhibit 99.2
1Q17 Financial Results & Corporate Updates Conference Call & Webcast May 9, 2017

Safe Harbor This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 relating to preclinical and clinical development of our product candidates, the timing and reporting of results from preclinical studies and clinical trials, the prospects and timing of the potential regulatory approval of our product candidates, commercialization plans, financing plans, and the projected cash position for the Company. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Any or all of the forward-looking statements in this presentation may turn out to be wrong and can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. For example, with respect to statements regarding the goals, progress, timing, and outcomes of discussions with regulatory authorities, and in particular the potential goals, progress, timing, and results of preclinical studies and clinical trials, actual results may differ materially from those set forth in this release due to the risks and uncertainties inherent in our business, including, without limitation: the potential that results of clinical or preclinical studies indicate that the product candidates are unsafe or ineffective; the potential that it may be difficult to enroll patients in our clinical trials; the potential that regulatory authorities, including the FDA, EMA, and PMDA, may not grant or may delay approval for our product candidates; the potential that we may not be successful in commercializing Galafold in Europe or our other product candidates if and when approved; the potential that preclinical and clinical studies could be delayed because we identify serious side effects or other safety issues; and the potential that we will need additional funding to complete all of our studies. Further, the results of earlier preclinical studies and/or clinical trials may not be predictive of future results for any of our product candidates. With respect to statements regarding projections of the Company's cash position, actual results may differ based on market factors and the Company's ability to execute its operational and budget plans. In addition, all forward-looking statements are subject to other risks detailed in our previous filings with the SEC and in our Annual Report on Form 10-K for the year ended December 31, 2016. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and we undertake no obligation to revise or update this news release to reflect events or circumstances after the date hereof. Introduction

2017 Key Strategic Priorities Introduction We Remain Sharply Focused on FIVE Key Strategic Priorities as We Continue to Build a Top Global Biotechnology Company Focused on Rare Devastating Diseases Advance International Galafold Launch Submit Japanese New Drug Application (J-NDA) for Migalastat Establish Definitive Proof of Concept for ATB200/AT2221 with Clear Path to Registration for Pompe Disease Successfully Complete Phase 3 EB Study Maintain Financial Strength
Galafold™ (Migalastat) Precision Medicine for Fabry Disease Continue Launch Execution and Geographic Expansion

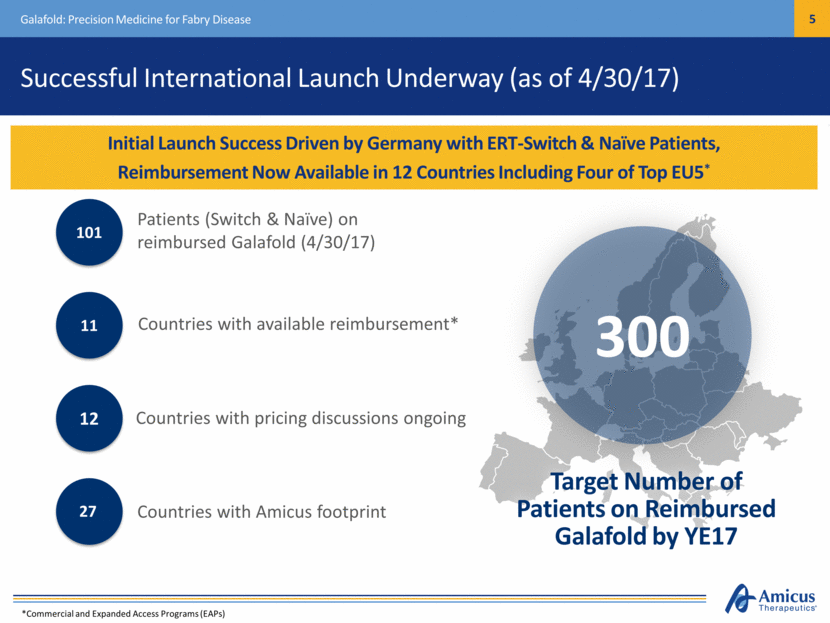
Successful International Launch Underway (as of 4/30/17) Initial Launch Success Driven by Germany with ERT-Switch & Naïve Patients, Reimbursement Now Available in 12 Countries Including Four of Top EU5* Galafold: Precision Medicine for Fabry Disease Patients (Switch & Naïve) on reimbursed Galafold (4/30/17) 101 Countries with pricing discussions ongoing 12 Countries with available reimbursement* 11 Countries with Amicus footprint 27 *Commercial and Expanded Access Programs (EAPs) Target Number of Patients on Reimbursed Galafold by YE17 300
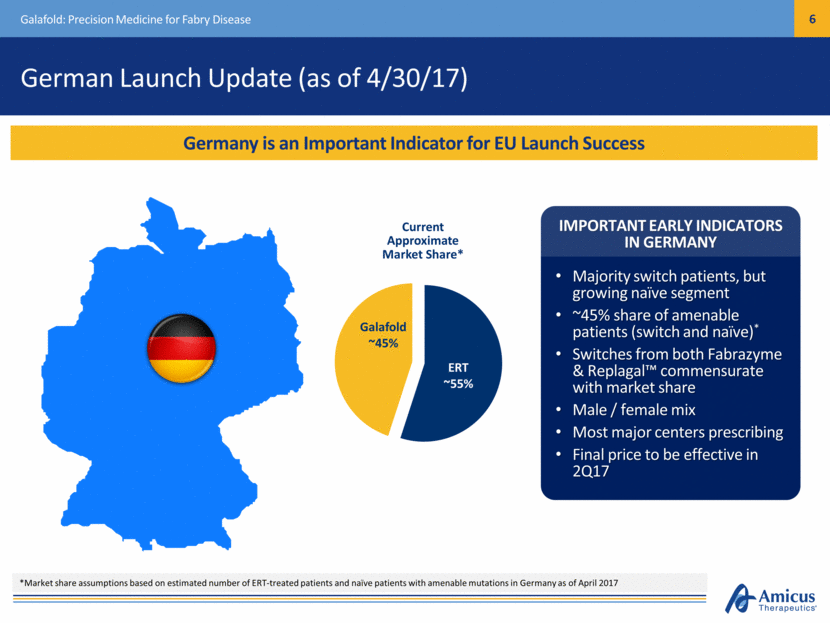
German Launch Update (as of 4/30/17) Galafold: Precision Medicine for Fabry Disease Germany is an Important Indicator for EU Launch Success *Market share assumptions based on estimated number of ERT-treated patients and naïve patients with amenable mutations in Germany as of April 2017 IMPORTANT EARLY INDICATORS IN GERMANY Majority switch patients, but growing naïve segment ~45% share of amenable patients (switch and naïve)* Switches from both Fabrazyme & Replagal™ commensurate with market share Male / female mix Most major centers prescribing Final price to be effective in 2Q17 Galafold ~45% ERT ~55% Current Approximate Market Share*
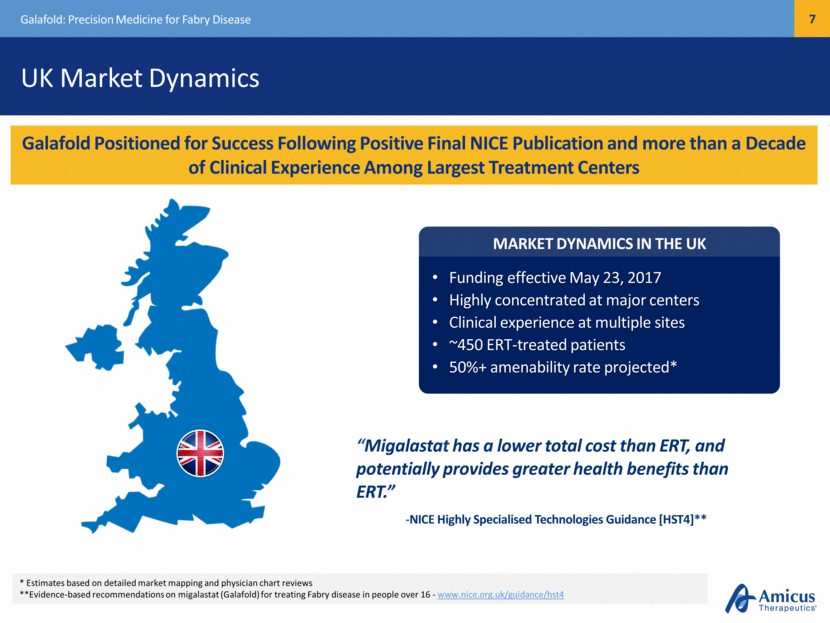
UK Market Dynamics Galafold: Precision Medicine for Fabry Disease Galafold Positioned for Success Following Positive Final NICE Publication and more than a Decade of Clinical Experience Among Largest Treatment Centers * Estimates based on detailed market mapping and physician chart reviews **Evidence-based recommendations on migalastat (Galafold) for treating Fabry disease in people over 16 - www.nice.org.uk/guidance/hst4 MARKET DYNAMICS IN THE UK Funding effective May 23, 2017 Highly concentrated at major centers Clinical experience at multiple sites ~450 ERT-treated patients 50%+ amenability rate projected* “Migalastat has a lower total cost than ERT, and potentially provides greater health benefits than ERT.” -NICE Highly Specialised Technologies Guidance [HST4]**
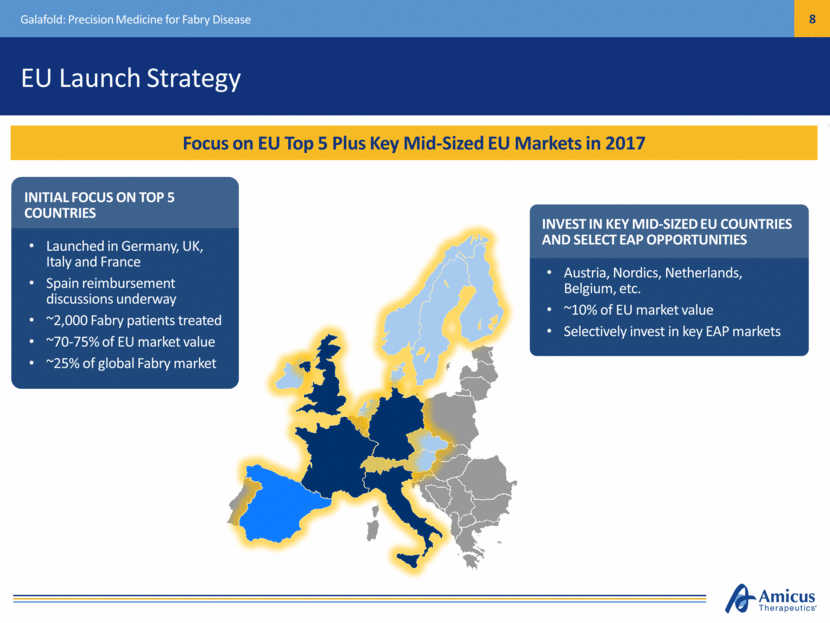
EU Launch Strategy INITIAL FOCUS ON TOP 5 COUNTRIES Launched in Germany, UK, Italy and France Spain reimbursement discussions underway ~2,000 Fabry patients treated ~70-75% of EU market value ~25% of global Fabry market Focus on EU Top 5 Plus Key Mid-Sized EU Markets in 2017 Galafold: Precision Medicine for Fabry Disease INVEST IN KEY MID-SIZED EU COUNTRIES AND SELECT EAP OPPORTUNITIES Austria, Nordics, Netherlands, Belgium, etc. ~10% of EU market value Selectively invest in key EAP markets
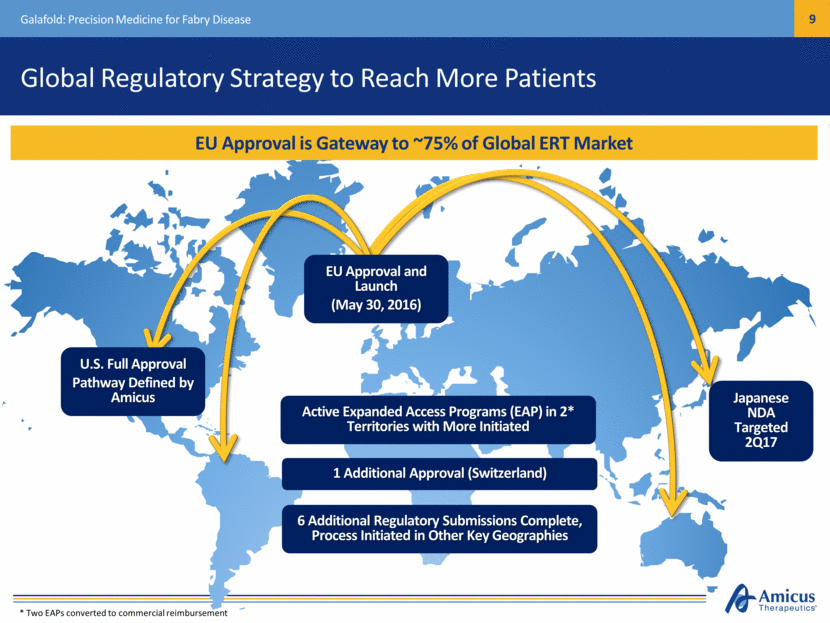
Global Regulatory Strategy to Reach More Patients Galafold: Precision Medicine for Fabry Disease EU Approval and Launch (May 30, 2016) Active Expanded Access Programs (EAP) in 2* Territories with More Initiated EU Approval is Gateway to ~75% of Global ERT Market 6 Additional Regulatory Submissions Complete, Process Initiated in Other Key Geographies U.S. Full Approval Pathway Defined by Amicus Japanese NDA Targeted 2Q17 1 Additional Approval (Switzerland) * Two EAPs converted to commercial reimbursement
ATB200 Novel ERT for Pompe Disease Establishing Human Proof of Concept and Validating Biologics Platform in 2017

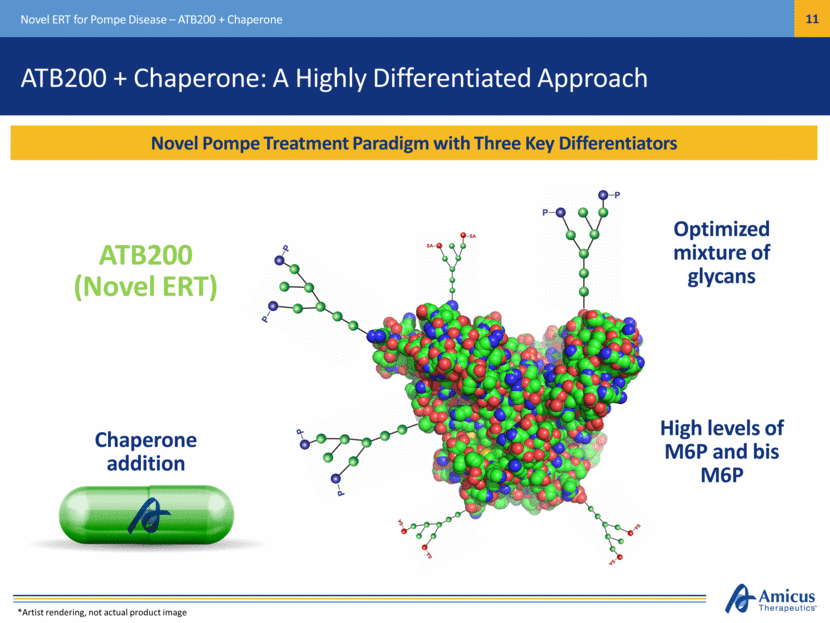
Novel Pompe Treatment Paradigm with Three Key Differentiators Novel ERT for Pompe Disease – ATB200 + Chaperone Chaperone addition Optimized mixture of glycans ATB200 + Chaperone: A Highly Differentiated Approach ATB200 (Novel ERT) High levels of M6P and bis M6P *Artist rendering, not actual product image
Phase 1/2 ATB200-02 Study Design Pompe Phase 1/2 Study ATB200-02 Preliminary Data Phase 1/2 Clinical Study to Evaluate Safety, Tolerability, Pharmacokinetics (PK), and Pharmacodynamics (PD) of ATB200 + Chaperone (ATB200/AT2221) 18-Week Primary Treatment Period with Long-Term Extension (n=20) ATB200 5mg/kg (wk 2) 10mg/kg (wk 4) 20mg/kg (wk 6) ATB200 20mg/kg + AT2221 (Low Dose) wks 8,10,12 ATB200 20mg/kg + AT2221 (High Dose) wk 14+ Cohort 1 (Ambulatory ERT-Switch, n=11) Cohort 2 (Non-Ambulatory ERT-Switch, n=4) & Cohort 3 (ERT-Naïve, n=5) ATB200 20mg/kg + AT2221 (High Dose) wk 2+ Plasma PK Safety/Tolerability Infusion-Associated Reactions Antibody & Cytokine Levels Pharmacodynamics Efficacy (Long-Term Extension) Assessments:

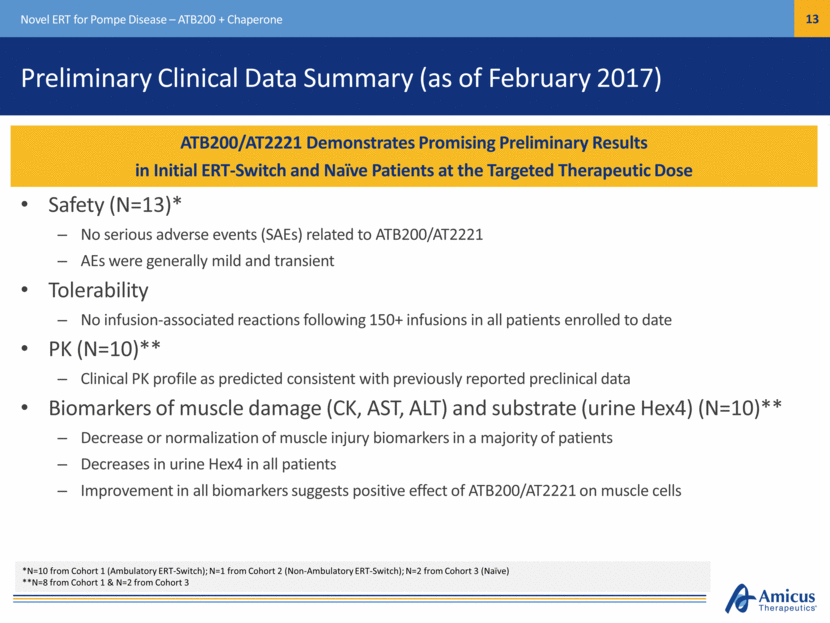
Preliminary Clinical Data Summary (as of February 2017) Safety (N=13)* No serious adverse events (SAEs) related to ATB200/AT2221 AEs were generally mild and transient Tolerability No infusion-associated reactions following 150+ infusions in all patients enrolled to date PK (N=10)** Clinical PK profile as predicted consistent with previously reported preclinical data Biomarkers of muscle damage (CK, AST, ALT) and substrate (urine Hex4) (N=10)** Decrease or normalization of muscle injury biomarkers in a majority of patients Decreases in urine Hex4 in all patients Improvement in all biomarkers suggests positive effect of ATB200/AT2221 on muscle cells Novel ERT for Pompe Disease – ATB200 + Chaperone ATB200/AT2221 Demonstrates Promising Preliminary Results in Initial ERT-Switch and Naïve Patients at the Targeted Therapeutic Dose *N=10 from Cohort 1 (Ambulatory ERT-Switch); N=1 from Cohort 2 (Non-Ambulatory ERT-Switch); N=2 from Cohort 3 (Naïve) **N=8 from Cohort 1 & N=2 from Cohort 3
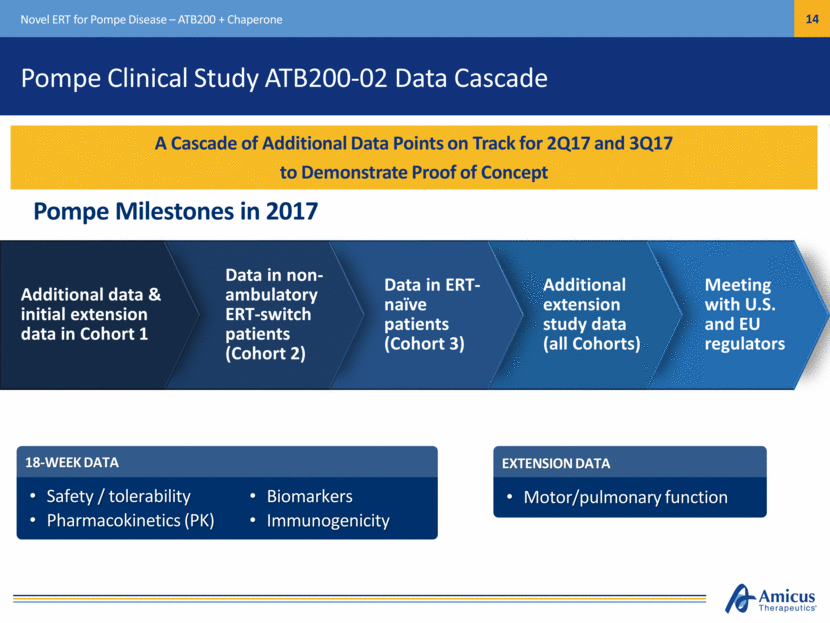
Pompe Clinical Study ATB200-02 Data Cascade Novel ERT for Pompe Disease – ATB200 + Chaperone A Cascade of Additional Data Points on Track for 2Q17 and 3Q17 to Demonstrate Proof of Concept Pompe Milestones in 2017 Meeting with U.S. and EU regulators 18-WEEK DATA Safety / tolerability Pharmacokinetics (PK) EXTENSION DATA Motor/pulmonary function Additional extension study data (all Cohorts) Data in ERT-naïve patients (Cohort 3) Data in non-ambulatory ERT-switch patients (Cohort 2) Additional data & initial extension data in Cohort 1 Biomarkers Immunogenicity
SD-101 for Epidermolysis Bullosa Potential First-in-Class Treatment with Phase 3 Data Anticipated 3Q17

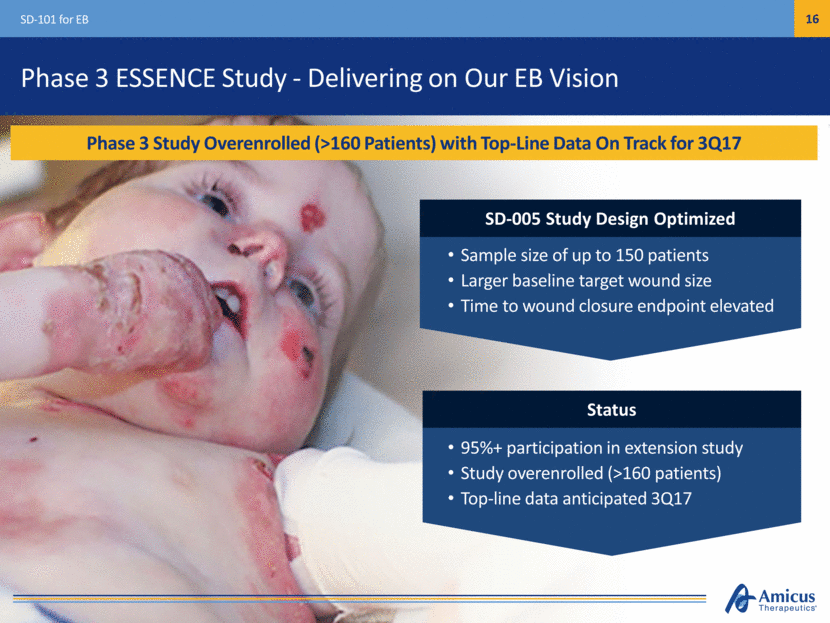
Phase 3 ESSENCE Study - Delivering on Our EB Vision Phase 3 Study Overenrolled (>160 Patients) with Top-Line Data On Track for 3Q17 SD-101 for EB SD-005 Study Design Optimized 95%+ participation in extension study Study overenrolled (>160 patients) Top-line data anticipated 3Q17 Status Sample size of up to 150 patients Larger baseline target wound size Time to wound closure endpoint elevated
Financial Summary

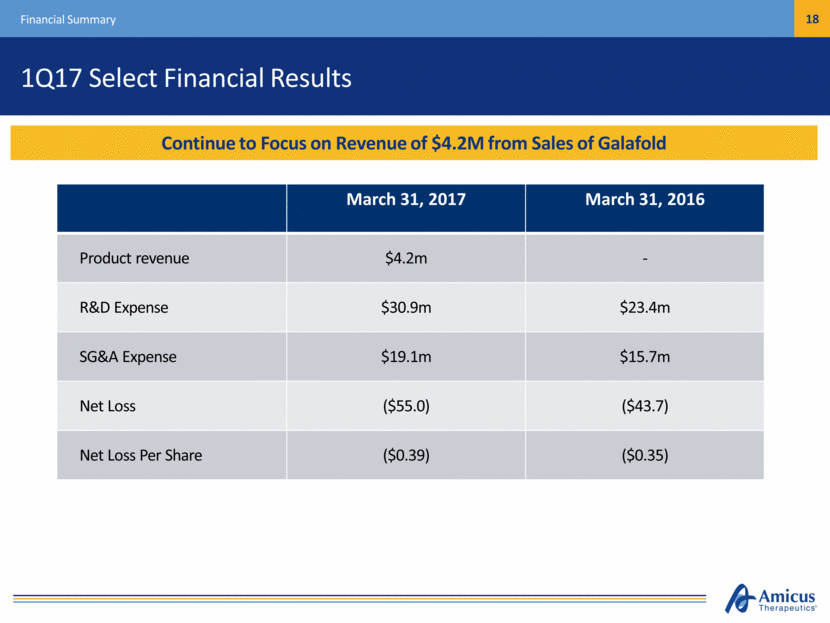
1Q17 Select Financial Results Financial Summary March 31, 2017 March 31, 2016 Product revenue $4.2m - R&D Expense $30.9m $23.4m SG&A Expense $19.1m $15.7m Net Loss ($55.0) ($43.7) Net Loss Per Share ($0.39) ($0.35) Continue to Focus on Revenue of $4.2M from Sales of Galafold
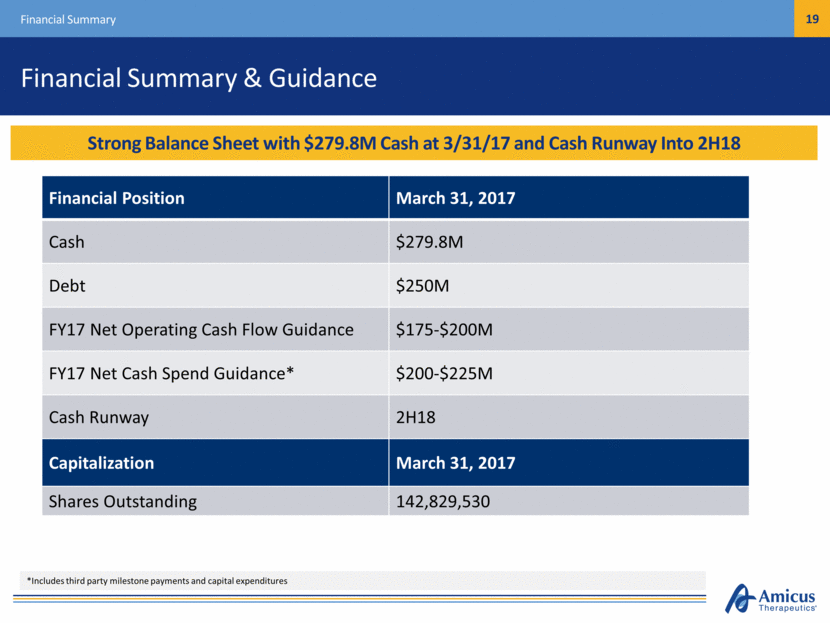
Financial Summary & Guidance Financial Summary Financial Position March 31, 2017 Cash $279.8M Debt $250M FY17 Net Operating Cash Flow Guidance $175-$200M FY17 Net Cash Spend Guidance* $200-$225M Cash Runway 2H18 Capitalization March 31, 2017 Shares Outstanding 142,829,530 Cash Position Provides Runway Under Current Operating Plan into mid-2017 Strong Balance Sheet with $279.8M Cash at 3/31/17 and Cash Runway Into 2H18 *Includes third party milestone payments and capital expenditures
Closing Remarks

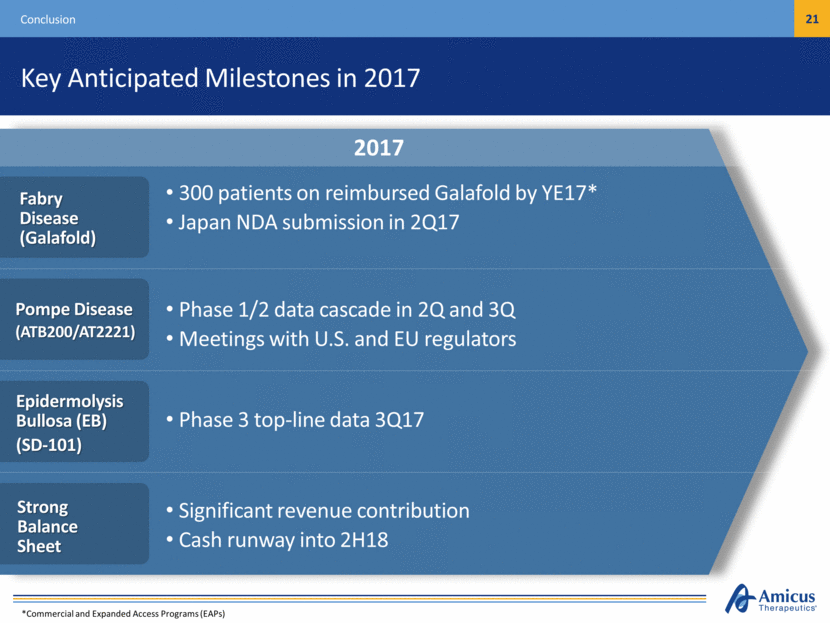
Pompe Phase 1/2 data cascade Pompe end of Phase 2 meeting Runway into 2H18 Meaningful revenue contribution from Galafold EB Phase 3 data Galafold international launch continues Japan NDA submission Fabry GI study initiation 2017 Key Anticipated Milestones in 2017 Conclusion Phase 1/2 data cascade in 2Q and 3Q Meetings with U.S. and EU regulators Significant revenue contribution Cash runway into 2H18 Phase 3 top-line data 3Q17 300 patients on reimbursed Galafold by YE17* Japan NDA submission in 2Q17 Pompe Disease (ATB200/AT2221) Strong Balance Sheet Epidermolysis Bullosa (EB) (SD-101) Fabry Disease (Galafold) *Commercial and Expanded Access Programs (EAPs)
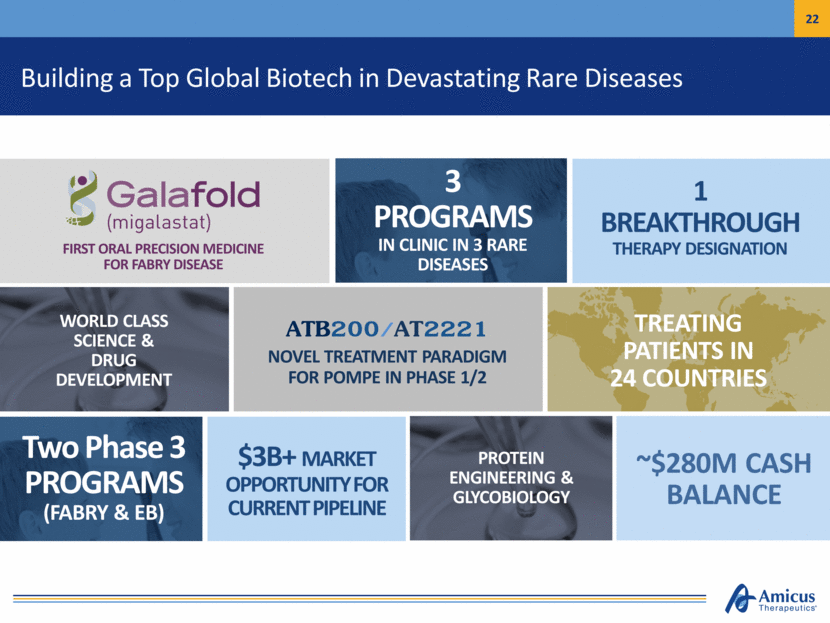
Building a Top Global Biotech in Devastating Rare Diseases FIRST ORAL PRECISION MEDICINE FOR FABRY DISEASE WORLD CLASS SCIENCE & DRUG DEVELOPMENT ATB200/AT2221 NOVEL TREATMENT PARADIGM FOR POMPE IN PHASE 1/2 ~$280M CASH BALANCE Two Phase 3 PROGRAMS (FABRY & EB) 1 BREAKTHROUGH THERAPY DESIGNATION TREATING PATIENTS IN 24 COUNTRIES 3 PROGRAMS IN CLINIC IN 3 RARE DISEASES $3B+ MARKET OPPORTUNITY FOR CURRENT PIPELINE PROTEIN ENGINEERING & GLYCOBIOLOGY
Thank You

