Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - Marker Therapeutics, Inc. | v464986_ex32-2.htm |
| EX-32.1 - EXHIBIT 32.1 - Marker Therapeutics, Inc. | v464986_ex32-1.htm |
| EX-31.2 - EXHIBIT 31.2 - Marker Therapeutics, Inc. | v464986_ex31-2.htm |
| EX-31.1 - EXHIBIT 31.1 - Marker Therapeutics, Inc. | v464986_ex31-1.htm |
UNITED STATES SECURITIES AND EXCHANGE
COMMISSION
Washington, D.C. 20549
FORM 10-Q
x Quarterly Report Under Section 13 or 15(d) of the Securities Exchange Act of 1934 for the quarterly period ended March 31, 2017
¨ Transition Report Under Section 13 or 15(d) of the Securities Exchange Act of 1934 for the transition period from _____ to _____.
Commission File Number: 001-37939

TAPIMMUNE INC.
(Name of registrant in its charter)
| NEVADA | 45-4497941 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
| 50 N. Laura Street, Suite 2500 Jacksonville, FL |
32202 | |
| (Address of principal executive offices) | (Zip Code) | |
| 904-516-5436 | ||
| (Issuer's telephone number) |
Indicate by check mark whether the registrant (1) filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the past 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, non-accelerated filer, a smaller reporting company, or an emerging growth company. See definition of “accelerated filer”, “large accelerated filer”, “smaller reporting company”, and “emerging growth company” in Rule 12b-2 of the Exchange Act (check one):
| ¨ Large accelerated filer | ¨ Accelerated filer |
| ¨ Non-accelerated filer (Do not check | x Smaller reporting company |
| if smaller reporting company) | ¨ Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ¨ No x
As of May 2, 2017, the Company had 8,470,833 shares of common stock issued and outstanding.
NOTE REGARDING REVERSE STOCK SPLIT
On September 13, 2016, we filed a Certificate of Change pursuant to NRS 78.209 with the Secretary of State of the State of Nevada to effect a reverse split of our common stock at a ratio of one for twelve, effective on September 16, 2016. All historical share and per share amounts reflected in this report have been adjusted to reflect the reverse stock split.
CONDENSED CONSOLIDATED BALANCE SHEETS
(UNAUDITED)
| March 31, | December 31, | |||||||
| 2017 | 2016 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 5,927,565 | $ | 7,851,243 | ||||
| Prepaid expenses and deposits | 177,511 | 70,149 | ||||||
| Total current assets | 6,105,076 | 7,921,392 | ||||||
| Total assets | $ | 6,105,076 | $ | 7,921,392 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued liabilities | $ | 1,449,192 | $ | 1,224,940 | ||||
| Research agreement obligations | 492,365 | 492,365 | ||||||
| Warrant liability | 17,500 | 14,500 | ||||||
| Promissory note | 5,000 | 5,000 | ||||||
| Total current liabilities | 1,964,057 | 1,736,805 | ||||||
| Total liabilities | 1,964,057 | 1,736,805 | ||||||
| COMMITMENTS AND CONTINGENCIES | - | - | ||||||
| Stockholders' equity: | ||||||||
| Preferred stock - $0.001 par value, 5,000,000 shares authorized at March 31, 2017 and December 31, 2016, respectively | ||||||||
| Series A, $0.001 par value, 1,250,000 shares designated, 0 shares issued and outstanding as of March 31, 2017 and December 31, 2016, respectively | - | - | ||||||
| Series B, $0.001 par value, 1,500,000 shares designated, 0 shares issued and outstanding as of March 31, 2017 and December 31, 2016, respectively | - | - | ||||||
| Common stock, $0.001 par value, 41,666,667 shares authorized, 8,470,833 and 8,421,185 shares issued and outstanding as of March 31, 2017 and December 31, 2016, respectively | 8,471 | 8,421 | ||||||
| Additional paid-in capital | 152,368,241 | 151,991,974 | ||||||
| Accumulated deficit | (148,235,693 | ) | (145,815,808 | ) | ||||
| Total stockholders' equity | 4,141,019 | 6,184,587 | ||||||
| Total liabilities and stockholders' equity | $ | 6,105,076 | $ | 7,921,392 | ||||
See accompanying notes to these unaudited condensed consolidated financial statements.
| 1 |
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(UNAUDITED)
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2017 | 2016 | |||||||
| Operating expenses: | ||||||||
| Research and development | $ | 989,092 | $ | 985,751 | ||||
| General and administrative | 1,427,793 | 767,988 | ||||||
| Total operating expenses | 2,416,885 | 1,753,739 | ||||||
| Loss from operations | (2,416,885 | ) | (1,753,739 | ) | ||||
| Other expense: | ||||||||
| Change in fair value of warrant liability | (3,000 | ) | (2,996,000 | ) | ||||
| Net loss | $ | (2,419,885 | ) | $ | (4,749,739 | ) | ||
| Net loss per share, Basic and Diluted | $ | (0.29 | ) | $ | (0.81 | ) | ||
| Weighted average number of common shares outstanding | 8,429,595 | 5,882,770 | ||||||
See accompanying notes to these unaudited condensed consolidated financial statements.
| 2 |
CONDENSED CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
(UNAUDITED)
| Common Stock | Additional Paid- | Accumulated | Total Stockholders' | |||||||||||||||||
| Shares | Par value | in Capital | Deficit | Equity | ||||||||||||||||
| Balance January 1, 2017 | 8,421,185 | $ | 8,421 | $ | 151,991,974 | $ | (145,815,808 | ) | $ | 6,184,587 | ||||||||||
| Stock- based compensation | 49,648 | 50 | 376,267 | - | 376,317 | |||||||||||||||
| Net loss | - | - | - | (2,419,885 | ) | (2,419,885 | ) | |||||||||||||
| Balance at March 31, 2017 | 8,470,833 | $ | 8,471 | $ | 152,368,241 | $ | (148,235,693 | ) | $ | 4,141,019 | ||||||||||
See accompanying notes to these unaudited condensed consolidated financial statements.
| 3 |
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2017 | 2016 | |||||||
| Cash Flows from Operating Activities: | ||||||||
| Net Loss | $ | (2,419,885 | ) | $ | (4,749,739 | ) | ||
| Reconciliation of net loss to net cash used in operating activities: | ||||||||
| Changes in fair value of warrant liabilities | 3,000 | 2,996,000 | ||||||
| Stock-based compensation | 376,317 | 214,250 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and deposits | (107,362 | ) | 31,171 | |||||
| Accounts payable and accrued expenses | 224,252 | 678,555 | ||||||
| Net cash used in operating activities | (1,923,678 | ) | (829,763 | ) | ||||
| Cash Flows from Financing Activities: | ||||||||
| Repayment of promissory note | - | (25,000 | ) | |||||
| Net cash used in financing activities | - | (25,000 | ) | |||||
| Net decrease in cash | (1,923,678 | ) | (854,763 | ) | ||||
| Cash at beginning of period | 7,851,243 | 6,576,564 | ||||||
| Cash at end of period | $ | 5,927,565 | $ | 5,721,801 | ||||
See accompanying notes to these unaudited condensed consolidated financial statements.
| 4 |
TAPIMMUNE INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2017 | 2016 | |||||||
| Supplemental schedule of non-cash activities: | ||||||||
| Reclassification of accrued liability upon issuance of common shares relating to Dr. Glynn Wilson’s compensation | $ | - | $ | 191,000 | ||||
See accompanying notes to these unaudited condensed consolidated financial statements.
| 5 |
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2017
(Unaudited)
| Note 1: | Nature of Operations |
TapImmune Inc. (the “Company” or “we”), a Nevada corporation incorporated in 1992, is a biotechnology company focusing on immunotherapy specializing in the development of innovative peptide and gene-based immunotherapeutics and vaccines for the treatment of oncology and infectious disease. Unlike other vaccine technologies that narrowly address the initiation of an immune response, TapImmune's approach broadly stimulates the cellular immune system by enhancing the function of killer T-cells and T-helper cells and by restoring antigen presentation in tumor cells allowing their recognition and killing by the immune system.
| NOTE 2: | Basis of Presentation |
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with the accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information and pursuant to the instructions to Form 10-Q and Article 8 of Regulation S-X of the Securities and Exchange Commission (“SEC”) and on the same basis as the Company prepares its annual audited consolidated financial statements. In the opinion of management, the accompanying unaudited condensed consolidated financial statements reflect all adjustments, consisting of normal recurring adjustments, considered necessary for a fair presentation of such interim results.
The results for the condensed consolidated statement of operations are not necessarily indicative of results to be expected for the year ending December 31, 2017 or for any future interim period. The condensed consolidated balance sheet at March 31, 2017 has been derived from unaudited financial statements; however, it does not include all of the information and notes required by U.S. GAAP for complete financial statements. The accompanying condensed consolidated financial statements should be read in conjunction with the consolidated financial statements for the year ended December 31, 2016, and notes thereto included in the Company’s annual report on Form 10-K filed on March 14, 2017.
| NOTE 3: | LIQUIDITY AND FINANCIAL CONDITION |
The Company’s activities since inception have consisted principally of acquiring product and technology rights, raising capital, and performing research and development. Successful completion of the Company’s development programs and, ultimately, the attainment of profitable operations are dependent on future events, including, among other things, its ability to access potential markets; secure financing, develop a customer base; attract, retain and motivate qualified personnel; and develop strategic alliances and collaborations. From inception, the Company has been funded by a combination of equity and debt financings.
The Company expects to continue to incur substantial losses over the next several years during its development phase. To fully execute its business plan, the Company will need to complete certain research and development activities and clinical studies. Further, the Company’s product candidates will require regulatory approval prior to commercialization. These activities may span many years and require substantial expenditures to complete and may ultimately be unsuccessful. Any delays in completing these activities could adversely impact the Company. The Company plans to meet its capital requirements primarily through issuances of debt and equity securities and, in the longer term, revenue from product sales.
As of March 31, 2017, the Company had cash of approximately $5,928,000. Historically, the Company had net losses and negative cash flows from operations. Management intends to continue its research efforts and to finance operations of the Company through debt and/or equity financings. Management plans to seek additional debt and/or equity financing through private or public offerings or through a business combination or strategic partnership. There can be no assurance that the Company will be successful in obtaining additional financing on favorable terms, or at all. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of these uncertainties.
| 6 |
| Note 4: | SIGNIFICANT ACCOUNTING POLICIES |
There have been no material changes in the Company’s significant accounting policies to those previously disclosed in the Company’s annual report on Form 10-K, which was filed with the SEC on March 14, 2017.
New Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies that we adopt as of the specified effective date. Unless otherwise discussed, we do not believe that the impact of recently issued standards that are not yet effective will have a material impact on our financial position or results of operations upon adoption.
Statement of Cash Flows
In August 2016, the FASB issued Accounting Standards Update (“ASU”) No. 2016-15, Statement of Cash Flows (Topic 230). This amendment provides guidance on the presentation and classification of specific cash flow items to improve consistency within the statement of cash flows. ASU 2016-15 is effective for fiscal years, and interim periods within those fiscal years beginning after December 15, 2017, with early adoption permitted. The Company is evaluating the effect that ASU 2016-15 will have on its financial statements and related disclosures.
Deferred Taxes
In November 2015, FASB issued ASU No. 2015-17, ”Balance Sheet Classification of Deferred Taxes”. ASU No. 2015-17 requires that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. ASU No. 2015-17 is effective for financial statements issued for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. The Company adopted ASU No. 2015-17 on January 1, 2017 and its adoption did not have a material impact on the Company’s financial position and results of operations.
Compensation-Stock Compensation
In March 2016, the FASB issued ASU No. 2016-09, Compensation-Stock Compensation (Topic 718), Improvements to Employee Share-Based Payment Accounting. Under ASU No. 2016-09, companies will no longer record excess tax benefits and certain tax deficiencies in additional paid-in capital (“APIC”). Instead, they will record all excess tax benefits and tax deficiencies as income tax expense or benefit in the income statement and the APIC pools will be eliminated. In addition, ASU No. 2016-09 eliminates the requirement that excess tax benefits be realized before companies can recognize them. ASU No. 2016-09 also requires companies to present excess tax benefits as an operating activity on the statement of cash flows rather than as a financing activity. Furthermore, ASU No. 2016-09 will increase the amount an employer can withhold to cover income taxes on awards and still qualify for the exception to liability classification for shares used to satisfy the employer’s statutory income tax withholding obligation. An employer with a statutory income tax withholding obligation will now be allowed to withhold shares with a fair value up to the amount of taxes owed using the maximum statutory tax rate in the employee’s applicable jurisdiction(s). ASU No. 2016-09 requires a company to classify the cash paid to a tax authority when shares are withheld to satisfy its statutory income tax withholding obligation as a financing activity on the statement of cash flows. Under current U.S. GAAP, it was not specified how these cash flows should be classified. In addition, companies will now have to elect whether to account for forfeitures on share-based payments by (1) recognizing forfeitures of awards as they occur or (2) estimating the number of awards expected to be forfeited and adjusting the estimate when it is likely to change, as is currently required. The amendments of this ASU are effective for reporting periods beginning after January 1, 2017, with early adoption permitted but all of the guidance must be adopted in the same period. The Company adopted this on January 1, 2017. The Company has evaluated the impact of ASU No. 2016-09 and has determined that the adoption of the impact of forfeitures, net of income taxes, did not have a material impact on the Company’s financial position and results of operations.
| Note 5: | NET LOSS PER SHARE APPLICABLE TO COMMON SHAREHOLDER |
Basic loss per common share is computed by dividing net loss by the weighted average number of common shares outstanding during the reporting period. Diluted loss per common share is computed similarly to basic loss per common share except that it reflects the potential dilution that could occur if dilutive securities or other obligations to issue common stock were exercised or converted into common stock.
| 7 |
Loss per-share amounts for all prior periods have been retroactively adjusted to reflect the Company’s 1-for-12 reverse stock split, which was effective September 16, 2016.
The following table sets forth the computation of net loss per share:
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2017 | 2016 | |||||||
| Numerator: | ||||||||
| Net loss | $ | (2,419,885 | ) | $ | (4,749,739 | ) | ||
| Denominator: | ||||||||
| Weighted average common shares outstanding | 8,429,595 | 5,882,770 | ||||||
| Net loss per share data: | ||||||||
| Basic and Diluted | $ | (0.29 | ) | $ | (0.81 | ) | ||
The following securities, rounded to the thousand, were not included in the diluted net loss per share calculation because their effect was anti-dilutive for the periods presented:
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2017 | 2016 | |||||||
| Common stock options | 417,000 | 299,000 | ||||||
| Common stock warrants - equity treatment | 5,054,000 | 213,000 | ||||||
| Common stock warrants - liability treatment | 5,000 | 4,127,000 | ||||||
| Potentially dilutive securities | 5,476,000 | 4,639,000 | ||||||
| Note 6: | accounts payable and accrued liabilities |
Accounts payable and accrued liabilities consist of the following as of March 31, 2017 and December 31, 2016, respectively:
| March 31, | December 31, | |||||||
| 2017 | 2016 | |||||||
| Accounts payable | $ | 688,351 | $ | 680,181 | ||||
| Compensation and benefits | 161,439 | 217,622 | ||||||
| Professional fees | 188,828 | 53,428 | ||||||
| Consulting agreements | 82,119 | 94,576 | ||||||
| Technology license fees | 105,000 | 105,000 | ||||||
| Investor relations fees | 72,835 | - | ||||||
| Other (1) | 150,620 | 74,133 | ||||||
| Total accounts payable and accrued liabilities | $ | 1,449,192 | $ | 1,224,940 | ||||
(1) For the March 31, 2017 period, "Other" includes $76,360 of miscellaneous accounts payable invoices not yet received
| 8 |
| Note 7: | WARRANT LIABILITY AND FAIR VALUE MEASUREMENTS |
A summary of quantitative information with respect to valuation methodology and significant unobservable inputs used for the Company’s common stock purchase warrants that are categorized within Level 3 of the fair value hierarchy for the three months ended March 31, 2017 and 2016 is as follows:
| Three Months Ended | ||||||||
| March 31, | ||||||||
| 2017 | 2016 | |||||||
| Stock price | $ | 4.49 | $ | 8.16 | ||||
| Exercise price | $ | 1.20 | $1.20 - $300.00 | |||||
| Contractual term (years) | 0.12 - 1.28 | 0.53 - 4.45 | ||||||
| Volatility (annual) | 67% - 79% | 85% - 151% | ||||||
| Risk-free rate | 0.74% - 1.03% | 0.65% - 1.12% | ||||||
| Dividend yield (per share) | 0% | 0% | ||||||
The foregoing assumptions are reviewed quarterly and are subject to change based primarily on management’s assessment of the probability of the events described occurring. Accordingly, changes to these assessments could materially affect the valuations.
Financial Liabilities Measured at Fair Value on a Recurring Basis
Financial liabilities measured at fair value on a recurring basis are summarized below and disclosed on the balance sheet under Warrant liability:
| Fair value measured at March 31, 2017 | ||||||||||||||||
| Quoted prices in active | Significant other | Significant | ||||||||||||||
| markets | observable inputs | unobservable inputs | Fair value at | |||||||||||||
| (Level 1) | (Level 2) | (Level 3) | March 31, 2017 | |||||||||||||
| Warrant liability | $ | - | $ | - | $ | 17,500 | $ | 17,500 | ||||||||
| Fair value measured at December 31, 2016 | ||||||||||||||||
| Quoted prices in active | Significant other | Significant | ||||||||||||||
| markets | observable inputs | unobservable inputs | Fair value at | |||||||||||||
| (Level 1) | (Level 2) | (Level 3) | December 31, 2016 | |||||||||||||
| Warrant liability | $ | - | $ | - | $ | 14,500 | $ | 14,500 | ||||||||
The fair value accounting standards define fair value as the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is determined based upon assumptions that market participants would use in pricing an asset or liability. Fair value measurements are rated on a three-tier hierarchy as follows:
| · | Level 1 inputs: Quoted prices (unadjusted) for identical assets or liabilities in active markets; |
| · | Level 2 inputs: Inputs, other than quoted prices included in Level 1, that are observable either directly or indirectly; and |
| · | Level 3 inputs: Unobservable inputs for which there is little or no market data, which require the reporting entity to develop its own assumptions. |
There were no transfers between Level 1, 2 or 3 during the three months ended March 31, 2017.
| 9 |
The following table presents changes in Level 3 liabilities measured at fair value for the three months ended March 31, 2017:
| Warrant | ||||
| Liability | ||||
| Balance - December 31, 2016 | $ | 14,500 | ||
| Change in fair value of warrant liability | 3,000 | |||
| Balance – March 31, 2017 | $ | 17,500 | ||
| Note 8: | Promissory note |
At March 31, 2017 and December 31, 2016, the Company had an outstanding promissory note in the amount of $5,000. The promissory note outstanding and due at March 31, 2017 bears 10% annual interest.
| Note 9: | STOCKHOLDERS’ EQUITY |
Reverse Stock Split
On September 16, 2016, the Company effected a one for twelve reverse stock-split of our issued and outstanding common stock and has retroactively adjusted our common shares outstanding, options and warrants amounts outstanding. The Company has presented its share data for and as of all periods presented on this basis. The par value was not adjusted as a result of the one for twelve reverse stock split. All prior period share transactions included in the Company’s stock transactions and balances have been retroactively restated.
2017 Common Stock Transactions
2017 Management Compensation
On March 9, 2017, the Company issued 12,761 shares of stock in relation to the discretionary 2016 bonus awarded to Dr. Glynn Wilson. The fair value of the common stock of $55,000 was recognized as stock-based compensation in general and administrative expenses. The issuance was based on the closing price or our common stock of $4.31 per share, on the day immediately preceding the date the 2016 bonus award was approved by the Board of Directors.
On March 9, 2017, the Company issued 5,220 shares of stock in relation to the discretionary 2016 bonus awarded to Dr. John Bonfiglio. The fair value of the common stock of $22,500 was recognized as stock-based compensation in general and administrative expenses. The issuance was based on the closing price or our common stock of $4.31 per share, on the day immediately preceding the date the 2016 bonus award was approved by the Board of Directors.
Consulting Arrangements
During the three months ended March 31, 2017, the Company issued 31,667 shares of common stock as part of consulting agreements. The fair value of the common stock of approximately $133,000 was recognized as stock-based compensation in general and administrative expenses.
| Note 10: | STOCK-BASED COMPENSATION |
The Company recorded approximately $376,000 and $214,000 of stock-based compensation expense for the three months ended March 31, 2017 and 2016, respectively. At March 31, 2017, the total stock-based compensation cost related to unvested awards not yet recognized was $728,000. The expected weighted average period compensation costs to be recognized was 0.75 years.
Future option grants will impact the compensation expense recognized. Stock-based compensation expense is included in general and administrative expense on the condensed consolidated statements of operations.
| Note 11: | SUBSEQUENT EVENT |
On April 27, 2017, The Company and Dr. John Bonfiglio have entered into a Separation and Release agreement (the “Separation Agreement”) pursuant to which Dr. John Bonfiglio resigned as President, Chief Operating Officer and director of the Company to pursue other opportunities. Dr. Bonfiglio’s resignation was not due to any disagreement with the Company on any matter related to its operations, policies or practices.
The Separation Agreement provides for a customary release by Dr. Bonfiglio of claims against the Company and a mutual non-disparagement covenant. Dr. Bonfiglio is also obligated to comply with various restrictive covenants, including a non-compete, non-solicitation and protection of the Company’s confidential information. Any disputes arising under the Separation Agreement will be resolved by binding arbitration. The Separation Agreement is subject to revocation through May 4, 2017, and the Separation Agreement will not become effective and enforceable until the revocation period expires.
The Company’s Chief Executive Officer, Dr. Glynn Wilson, was appointed to serve as the President of the Company and the size of the Company’s board of directors was reduced from seven members to six members.
| 10 |
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This quarterly report on Form 10-Q contains forward-looking statements within the meaning of Section 21E of the Securities and Exchange Act of 1934, as amended, that involve risks and uncertainties. All statements other than statements relating to historical matters including statements to the effect that we “believe”, “expect”, “anticipate”, “plan”, “target”, “intend” and similar expressions should be considered forward-looking statements. Our actual results could differ materially from those discussed in the forward-looking statements as a result of a number of important factors, including factors discussed in this section and elsewhere in this quarterly report on Form 10-Q, and the risks discussed in our other filings with the Securities and Exchange Commission. Readers are cautioned not to place undue reliance on these forward-looking statements, which reflect management’s analysis, judgment, belief or expectation only as the date hereof. We assume no obligation to update these forward-looking statements to reflect events or circumstance that arise after the date hereof.
As used in this quarterly report: (i) the terms “we”, “us”, “our”, “TapImmune” and the “Company” mean TapImmune Inc. and its wholly owned subsidiary, GeneMax Pharmaceuticals Inc. which wholly owns GeneMax Pharmaceuticals Canada Inc., unless the context otherwise requires; (ii) “SEC” refers to the Securities and Exchange Commission; (iii) “Securities Act” refers to the Securities Act of 1933, as amended; (iv) “Exchange Act” refers to the Securities Exchange Act of 1934, as amended; and (v) all dollar amounts refer to United States dollars unless otherwise indicated.
The following should be read in conjunction with our unaudited condensed consolidated interim financial statements and related notes for the three months ended March 31, 2017 included in this quarterly report, as well as our Annual Report on Form 10-K for the year ended December 31, 2016 filed on March 14, 2017.
Company Overview
We are a clinical-stage immuno-oncology company specializing in the development of innovative peptide and gene-based immunotherapeutics and vaccines for the treatment of cancer and metastatic disease. We are also developing a proprietary technology to improve the ability of the cellular immune system to recognize and destroy diseased cells. This DNA expression technology named Polystart is in pre-clinical development.
Immuno-oncology has become the most rapidly growing sector in the pharmaceutical and biotech industry. The approval and success of checkpoint inhibitors Yervoy and Opdivo (Bristol Myers Squibb) and Keytruda (Merck & Co.) together with the development of CAR T-cell therapies (Juno Therapeutics, Kite Pharma) has provided much momentum in this sector. In addition, new evidence points to the increasing use of combination immunotherapies for the treatment of cancer. This has provided greater opportunities for the successful development of T-cell vaccines in combination with other approaches.
To enhance shareholder value and taking into account development timelines, we plan to focus on advancing our clinical programs including our Folate Receptor Alpha program for breast and ovarian cancers and our HER2/neu peptide antigen program into Phase II clinical trials. In parallel, we plan to complete the pre-clinical development of our Polystart technology as an integral component of our prime-and-boost vaccine methodology.
We believe, the strength of our science and development approaches is becoming more widely appreciated, particularly as our clinical program has now generated positive interim data on both clinical programs in breast and ovarian cancers.
We continue to be focused on our entry into Phase II Triple-Negative Breast Cancer Trials including application for Fast Track and Orphan Drug Status as well as planning for Phase II HER2/neu Breast Cancer Trials.
We expect to continue to prosecute our Polystart patent filings and develop new Polystart constructs to facilitate collaborative efforts in our current clinical indications. We will also evaluate those indications where others have already indicated interest in combination therapies.
We believe that these fundamental programs and corporate activities have positioned our company to capitalize on the acceptance of immunotherapy as a leading therapeutic strategy in cancer and infectious disease.
We are continuously working on improving our product formulation and supply. TPIV 200 and TPIV 110 are both off-the-shelf, lyophilized products that only require reconstitution at the clinical site before injection. We believe the investments we have made in the formulation work for both very stable products will result in commercially viable products consistent with typically high pharmaceutical profit margins.
| 11 |
We believe the Phase I data produced for both TPIV 200 and TPIV 100 in collaboration with the Mayo Clinic are the driving force behind the high-value collaborations we have established and maintained with organizations such as Mayo Clinic, AstraZeneca, Sloan Kettering and the U.S. Department of Defense. As we move forward into advancing the Phase II studies, some of which incorporate collaborations with prestigious third-party organizations, we believe this represents further independent validation of the potential of our technology.
Intellectual Property Strategies
A key component to success is having a comprehensive patent strategy that continually updates and extends patent coverage for key products. It is highly unlikely that early patents will extend through ultimate product marketing, so extending patent life is an important strategy for ensuring product protection.
We have three active patent families that we are supporting:
1. Filed patents on Polystart expression vector (owned by TapImmune and filed in 2014: this IP covers the use with TAP). We announced the allowance of this patent in February 2016.
2. Filed patents on HER2/neu Class II and Class I antigens: exclusive license from Mayo Foundation; and
3. Filed patents on Folate Receptor Alpha antigens: exclusive license from Mayo Foundation
While the pathway to successful product development takes time, we believe we have put in place significant for success. The strength of our product pipeline and access to leading scientists and institutions gives us a unique opportunity to make a major contribution to global health care.
With respect to the broader market, a major driver and positive influence on our activities has been the emergence and general acceptance of the potential of a new generation of immunotherapies that promise to change the standard of care for cancer. The immunotherapy sector has been greatly stimulated by the approval of Provenge® for prostate cancer and Yervoy™ for metastatic melanoma, progression of the areas of checkpoint inhibitors and adoptive T-cell therapy and multiple approaches reaching Phase II and Phase III status.
We believe that through our combination of technologies, we are well positioned to be a leading player in this emerging market. It is important to note that many of the late-stage immunotherapies currently in development do not represent competition to our programs, but instead offer synergistic opportunities to partner our antigen based immunotherapeutics, and Polystart expression system. Thus, the use of naturally processed T-cell antigens discovered using samples derived from cancer patients plus our Polystart expression technology to improve antigen presentation to T-cells could not only produce an effective cancer vaccine in its own right but also to enhance the efficacy of other immunotherapy approaches such as CAR-T and PD1 inhibitors for example.
Products and Technology in Development-Clinical
TPIV 200
Phase I Human Clinical Trials – Folate Alpha Breast and Ovarian Cancers – Mayo Clinic
Folate Receptor Alpha is expressed in over 80% of triple-negative breast cancers and in addition, over 90% of ovarian cancers, for which the only treatment options are surgery, radiation therapy and chemotherapy, leaving a very important and urgent clinical need for a new therapeutic. Time to recurrence is relatively short for these types of cancer and survival prognosis is extremely poor after recurrence. In the United States alone, there are approximately 30,000 ovarian cancer patients and 40,000 triple-negative breast cancer patients newly diagnosed every year.
A 24-patient Phase I clinical trial using TPIV 200 was completed in 2015. The vaccine is well tolerated and safe and 20 out of 21 evaluable patients showed positive immune responses which provided a strong rationale for progressing to Phase II trials. Good Manufacturing Practice (“GMP”) for Phase II trials resulted in a commercially viable formulation. On July 27, 2015, we exercised our option agreement with Mayo Clinic with the signing of a worldwide exclusive license agreement to commercialize a proprietary Folate Receptor Alpha Vaccine technology for all cancer indications. As part of this Agreement, the investigational new drug (“IND”) for the Folate Receptor Alpha (TPIV 200) Phase I trial was transferred from Mayo Clinic to the Company for amendment to our Phase II Clinical Trials on our lead product.
| 12 |
On September 15, 2015, we announced that our collaborators at the Mayo Clinic had been awarded a grant of $13.3 million from the U.S. Department of Defense. This grant, commencing September 15, 2015, will cover the costs for a 280-patient Phase II Clinical Trial of Folate Receptor Alpha Vaccine in patients with triple-negative breast cancer. We are working closely with Mayo Clinic on this clinical trial by providing clinical and manufacturing expertise as well as providing GMP vaccines to supply the study. The vaccine formulation is suitable for multiple Phase II clinical programs in triple-negative breast and ovarian cancers in combination with other immunotherapeutics.
On December 9, 2015, we announced that we received Orphan Drug Designation from the U.S. Food & Drug Administration’s Office of Orphan Products Development (“OOPD”) for our cancer vaccine TPIV 200 in the treatment of ovarian cancer. The TPIV 200 ovarian cancer clinical program will now receive benefits including tax credits on clinical research and seven-year market exclusivity upon receiving marketing approval. TPIV 200 is a multi-epitope peptide vaccine that targets Folate Receptor Alpha which is overexpressed in multiple cancers.
On February 3, 2016, we announced that the U.S. Food & Drug Administration (“FDA”) has designated the investigation of multiple-epitope Folate Receptor Alpha Peptide Vaccine (TPIV 200) with GM-CSF adjuvant for maintenance therapy in subjects with platinum-sensitive advanced ovarian cancer who achieved stable disease or partial response following completion of standard of care chemotherapy, as a Fast Track Development Program. A Phase II study in this indication was initiated at the end of 2016.
We are currently enrolling a Company-sponsored triple-negative breast cancer study at twelve clinical sites nation-wide. The study will enroll a total of 80 patients. It is open-label and designed to look at dosing regimens, immune responses and efficacy.
An ovarian cancer study sponsored by Memorial Sloan Kettering Cancer Center in New York City in collaboration with AstraZeneca Pharmaceuticals was initiated in 2016. This study is currently enrolling platinum resistant ovarian cancer patients and is designed to look at the effects of combination therapy with AstraZeneca’s checkpoint inhibitor “durvalumab”. The study will enroll a total of 40 patients and is open label. We have no business relationship with AstraZeneca and we are paying for half of the clinical study plus providing our TPIV 200 for the study.
TPIV 100/110
Phase I Human Clinical Trials – HER2/neu+ Breast Cancer – Mayo Clinic
A Phase I study using TPIV 100 (four peptide product) was completed in 2015. Final safety analysis on all the patients treated is complete and shown to be safe. In addition, 19 out of 20 evaluable patients showed robust T-cell immune responses to the antigens in the vaccine composition providing a solid case for advancement to Phase II in 2017. An additional secondary endpoint incorporated into this Phase I Trial will be a two-year follow on recording time to disease recurrence in the participating breast cancer patients.
For Phase I(b)/II studies, we plan to add a Class I peptide, licensed from the Mayo Clinic (April 16, 2012), to the four Class II peptides, producing TPIV 110 (five peptide product). Management believes that the combination of Class I and Class II HER2/neu antigens, gives us the leading HER2/neu vaccine platform. We plan to amend the IND to incorporate the fifth peptide in the Phase I(b)/II study. Discussions with the FDA have resulted in a pre-clinical development project that should allow us to file the amended IND in 2017.
Products and Technology-Pre-clinical
Polystart
On February 7, 2017, we announced that we received a Notice of Allowance from the U.S. Patent and Trademark Office of our patent application titled, “Chimeric nucleic acid molecules with non-AUG initiation sequences and uses thereof,” which represents our first patent on our Polystart program. We anticipate additional patent filings in connection with our research and development in this area. We plan to develop Polystart as both a stand-alone therapy and as a ‘boost strategy’ to be used synergistically with our peptide-based vaccines for breast and ovarian cancers.
| 13 |
TapImmune’s Clinical Program Pipeline
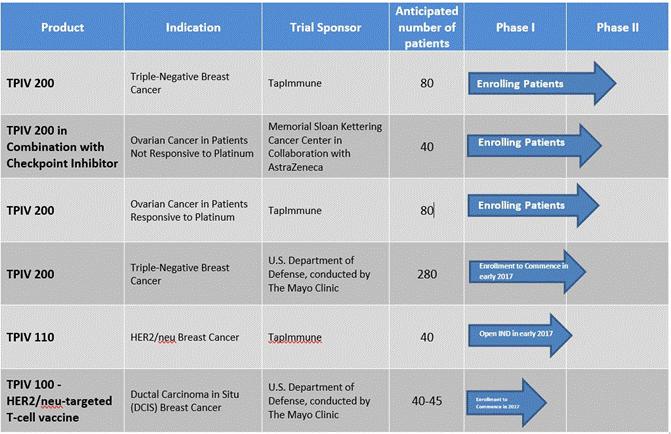
Refer to the “Clinical Program Pipeline Status Updates” section below for latest updates on above clinical pipeline chart.
In addition to the exciting clinical developments, our peptide vaccine technology may be coupled with our recently developed in-house Polystart nucleic acid-based technology designed to make vaccines significantly more effective by producing four times the required peptides for the immune systems to recognize and act on. Our nucleic acid-based systems can also incorporate “TAP” which stands for Transporter associated with Antigen Presentation.
Recent Developments and Updates
Completed GMP Manufacturing Scale Up and Second Clinical Lot of TPIV 200; to Supply Additional Phase II Clinical Trials
We successfully completed a multi-gram production scale-up as well as GMP manufacturing of a second clinical lot of TPIV 200. The vaccine supply will be used in the company’s ongoing Phase II study in platinum-sensitive ovarian cancer, as well as the planned 280-patient Phase II study sponsored by the Mayo Clinic and funded by the U.S. Department of Defense for treating triple-negative breast cancer. We also made various improvements to the vaccine manufacturing process, resulting in, what we believe to be, a superior formulation of the vaccine that is more amenable to large scale manufacturing and commercialization.
| 14 |
Clinical Program Pipeline Status Updates
Enrolling Patients: Phase II TPIV 200 Trial in Triple-Negative Breast Cancer
We have opened twelve clinical sites and have begun treating patients in a Phase II trial of our Folate Receptor Alpha cancer vaccine, TPIV 200, in the treatment of triple-negative breast cancer, one of the most difficult cancers to treat, representing a clear unmet medical need. The open-label, 80-patient clinical trial is designed to evaluate dosing regimens, adjuvants, efficacy, and immune responses in women with triple-negative breast cancer. Key data from the trial is expected to be included in a future New Drug Application submission to the FDA for marketing clearance. This trial is sponsored and conducted by TapImmune.
An independent data safety monitoring board (DSMB) reviewed the safety in this ongoing Phase 2 currently enrolling women with stage IIb-III triple-negative breast cancer who have completed initial surgery and chemo/radiation therapy. The randomized four-arm study is evaluating two doses of TPIV 200 (a high dose and a low dose), each of which will be tested both with and without immune priming with cyclophosphamide prior to vaccination. The planned safety review was performed when enrollment reached the 25% benchmark (20 out of 80 total patients), and showing no safety issues, the study has continued to enroll patients at multiple clinical sites. The study is expected to complete enrollment by year end 2017, with top-line data expected in early 2018. Details regarding this trial can be found at www.clinicaltrials.gov under identifier numbers NCT02593227 and FRV-002.
Enrolling Patients: Phase II Trial at Memorial Sloan Kettering of TPIV 200 in Platinum-Resistant Ovarian Cancer
A Phase II study of TPIV 200 in ovarian cancer patients who are not responsive to platinum, a commonly used chemotherapy for ovarian cancer, sponsored by Memorial Sloan Kettering Cancer Center (“MSKCC”), and in collaboration with AstraZeneca and TapImmune, has begun enrollment for a 40-patient study. The open-label study is designed to evaluate a combination therapy which includes our TPIV 200 T-cell vaccine and AstraZeneca’s checkpoint inhibitor, durvalumab. Because they are unresponsive to platinum, these patients have no real remaining options. If the combination therapy proves effective, we believe it would address a critical unmet need. TPIV 200 has received Orphan Drug designation for use in the treatment of ovarian cancer. We successfully completed the first safety cohort. This enabled MSKCC to increase the number of patients that can be enrolled and will subsequently increase the study’s enrollment rate. Currently more than 50% of patients have been enrolled. An interim analysis is planned in the fourth quarter of 2017. Details regarding this trial can be found at www.clinicaltrials.gov under identifier numbers NCT02764333.
Enrolling Patients: Phase II TPIV 200 Trial in Platinum-Sensitive Ovarian Cancer
We have opened one clinical site (with at least another 10 sites anticipated to open during 2017) in a Phase II trial of TPIV 200 for an 80-patient study on ovarian cancer patients who are responsive to platinum. We have received the FDA’s Fast Track designation to develop TPIV 200 as a maintenance therapy in combination with platinum, in platinum responsive ovarian cancer patients. This multi-center, double-blind efficacy study is sponsored and conducted by TapImmune. We expect to complete enrollment mid-2019. An interim analysis is planned based upon 50% patient enrollment, which the company anticipates completing in the second half of 2018. Details regarding this trial can be found at www.clinicaltrials.gov under identifier numbers NCT02978222 and FRV-004.
Patient Enrollment to Commence in 2017: Phase II Mayo Clinic-U.S. DOD Trial of TPIV 200 in Triple-Negative Breast Cancer
We anticipate this Phase II study of TPIV 200 in the treatment of triple-negative breast cancer, conducted by the Mayo Clinic and sponsored by the U.S. Department of Defense (“DOD”), will begin to enroll patients in 2017. The anticipated 280-patient study will be led by Dr. Keith Knutson of the Mayo Clinic in Jacksonville, Florida. Dr. Knutson is the inventor of the technology and in the Scientific Advisory Board at TapImmune. While TapImmune is supplying doses of TPIV 200 for the trial, and being reimbursed for the costs associated with manufacturing, the remaining costs associated with conducting this study will be funded by a $13.3 million grant made by the DOD to the Mayo Clinic.
Open IND with FDA for TPIV 100 in 2017: Phase II Protocol Now in Preparation
We have enhanced the reformulation of our second cancer vaccine product, TPIV 110 (five peptide product), following very strong safety and immune responses from a Phase I Mayo Clinic study using TPIV 100 (four peptide product). TPIV 110 targets HER2/neu, which makes it applicable to breast, ovarian and colorectal cancers. The enhanced TPIV product adds a fifth antigen which should produce an even more robust immune response activating both CD4+ and CD8+ T-cells. We have participated in a pre-Investigational New Drug (pre”IND”) meeting with the FDA and are now in discussions with the FDA as to requirements for filing the amended IND containing the fifth peptide. We expect to file the amended IND later this year. Although we cannot know for sure, we anticipate having an open IND sometime in the third or fourth quarter of 2017 pending comments from the FDA. The protocol for a Phase II trial of TPIV 110 in the treatment of HER2/neu positive breast cancer patients is currently under review by our Scientific Advisory Board and collaborators. Our collaborators at Mayo Clinic recently announced a $3.8 million grant which will fully fund a Phase II trial in DCIS that we had planned for our HER2/neu vaccine.
| 15 |
Mayo Clinic to Vaccinate Women With Ductal Carcinoma In Situ (DCIS) Using TapImmune TPIV 110 HER2-targeted T-Cell Vaccine
Recently, we announced that our partners at the Mayo Clinic received a grant from the U.S. Department of Defense to conduct a Phase II study of our HER2-targeted vaccine candidate in an early form of breast cancer called DCIS. This is the second TapImmune vaccine to be tested in a fully funded Phase II study sponsored by the Mayo Clinic. If successful, TapImmune’s vaccine may replace standard surgery and chemotherapy, and potentially could become part of a routine immunization schedule for preventing breast cancer in healthy women. The study is expected to enroll 40-45 women with DCIS and commence in 2017.
Results of Operations
In this discussion of the Company’s results of operations and financial condition, amounts, other than per-share amounts, have been rounded to the nearest thousand dollars.
Three Months Ended March 31, 2017 Compared to Three Months Ended March 31, 2016
We recorded a net loss of $2,420,000 or ($0.29) basic and diluted per share during the three months ended March 31, 2017 compared to a net loss of $4,750,000 or ($0.81) basic and diluted per share during the three months ended March 31, 2016. The change in net loss period over period was due to the following changes in operating expenses and other expense:
Operating Expenses
Operating expenses incurred during the three months ended March 31, 2017 were $2,417,000 compared to $1,754,000 in the prior period. Significant changes in operating expenses are outlined as follows:
| · | Research and development costs during the three months ended March 31, 2017 were $989,000 compared to $986,000 during the prior year period. This increase is mainly due to the Company’s planned increased expenses during the three months of 2017 relating to the Company’s clinical trials. |
| · | General and administrative expenses increased to $1,428,000 during the three months ended March 31, 2017 from $768,000 during the prior year period. This was due to increased expenses relating to: |
| o | stock-based compensation for employees and outside consultants, |
| o | compensation expenses resulting from increased headcount, |
| o | investor relations expenses, |
| o | NASDAQ and other public-related expenses, and |
| o | increased legal, audit and other professional fees. |
Other Expense
The change in fair value of warrant liabilities for the three months ended March 31, 2017 was ($3,000) as compared to ($2,996,000) for the three months ended March 31, 2016. On August 10, 2016, we amended the Series A and A-1, Series C and C-1, Series D and D-1 and Series E and E-1 warrants agreements issued by us in January and March 2015 to remove the clause that caused the warrants to be classified as warrant liabilities, and the variance period over period is due to that reason, We revalue the remaining warrant liabilities at each balance sheet date to fair value. The fair value is determined using Black-Scholes valuation model using various assumptions. The most significant changes in the assumptions is the strike price used at March 31, 2017 of $4.49. As such, the fair value of the warrant liabilities increased by $3,000 for the three months ended March 31, 2017 and is reflected by a corresponding loss in the condensed consolidated statement of operations.
| 16 |
Liquidity and Capital Resources
We have not generated any revenues since inception. We have financed our operations primarily through public and private offerings of our stock and debt including warrants and the exercises thereof.
The following table sets forth our cash and working capital as of March 31, 2017 and December 31, 2016:
| March 31, 2017 | December 31, 2016 | |||||||
| Cash | $ | 5,928,000 | $ | 7,851,000 | ||||
| Working capital | $ | 4,141,000 | $ | 6,185,000 | ||||
Cash Flows
The following table summarizes our cash flows for the three months ended March 31, 2017 and 2016:
| Three Months Ended March 31, | ||||||||
| 2017 | 2016 | |||||||
| Net cash used in: | ||||||||
| Operating activities | $ | (1,924,000 | ) | $ | (830,000 | ) | ||
| Financing activities | - | (25,000 | ) | |||||
| Net decrease in cash | $ | (1,924,000 | ) | $ | (855,000 | ) | ||
Financings
Our previous funding has come from financings that we conducted in January and March of 2015, from the exercises of stock warrants, and from our August 2016 private placement.
Future Capital Requirements
As of March 31, 2017, we had working capital of $4,141,000, compared to working capital of $6,185,000 as of December 31, 2016. We expect our expenses to continue at a similar pace during the remainder of 2017 and into 2018 primarily to fund our Phase II clinical trials. Our collaborators at Mayo Clinic recently announced a $3.8 million grant which will fully fund a Phase II trial in DCIS that we had planned for our HER2/neu vaccine.
Our capital requirements through and beyond 2017 will depend on numerous factors, including the success of our research and development, the resources we devote to develop and support our technologies and our success in pursuing strategic licensing and funded product development collaborations with external partners. Subject to our ability to raise additional capital, we expect to incur substantial expenditures to further develop our technologies including continued increases in costs related to research, nonclinical testing and clinical studies and trials, as well as costs associated with our capital raising efforts and being a public company.
We believe our existing cash could fund our operations through 2017. We will require additional substantial capital to conduct research and development, to fund nonclinical testing and Phase II clinical trials of our licensed, patented technologies, and to begin cultivating collaborative relationships for the Phase II and future Phase III clinical testing. Our plans could include seeking both equity and debt financing, alliances or other partnership agreements with entities interested in our technologies, or other business transactions that would generate sufficient resources to ensure continuation of our operations and research and development programs.
We expect to continue to seek additional funding for our operations. Any such required additional capital may not be available on reasonable terms, if at all. If we were unable to obtain additional financing, we may be required to reduce the scope of, delay or eliminate some or all of our planned clinical testing and research and development activities, which could harm our business. The sale of additional equity or debt securities may result in additional dilution to our shareholders. If we raise additional funds through the issuance of debt securities or preferred stock, these securities could have rights senior to those holders of our common stock and could contain covenants that would restrict our operations. We also will require additional capital beyond our currently forecasted amounts.
| 17 |
Because of the numerous risks and uncertainties associated with research, development and commercialization of our product candidates, we are unable to estimate the exact amounts of our future working capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
| · | the number and characteristics of the product candidates we pursue; |
| · | the scope, progress, results and costs of researching and developing our product candidates, and conducting pre-clinical and clinical trials including the research and development expenditures we expect to make in connection with our license agreements with Mayo Foundation; |
| · | the timing of, and the costs involved in, obtaining regulatory approvals for our product candidates; |
| · | our ability to maintain current research and development licensing agreements and to establish new strategic partnerships and collaborations, licensing or other arrangements and the financial terms of such agreements; |
| · | our ability to achieve our milestones under our licensing arrangements and the payment obligations we may have; |
| · | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; and |
| · | the timing, receipt and amount of sales of, or royalties on, our future products, if any. |
We have based our estimates on assumptions that may prove to be wrong. We may need to obtain additional funds sooner or in greater amounts than we currently anticipate.
Various conditions outside of our control may detract from our ability to raise additional capital needed to execute our plan of operations, including overall market conditions in the international and local economies. We recognize that the United States economy has suffered through a period of uncertainty during which the capital markets have been impacted, and that there is no certainty that these levels will stabilize or reverse despite the optics of an improving economy. Any of these factors could have a material impact upon our ability to raise financing and, as a result, upon our short-term or long-term liquidity.
Critical Accounting Policies
The condensed consolidated financial statements are prepared in conformity with U.S. GAAP, which require the use of estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities at the date of the financial statements, and the reported amounts of expenses in the periods presented. We believe that the accounting estimates employed are appropriate and resulting balances are reasonable; however, due to inherent uncertainties in making estimates, actual results could differ from the original estimates, requiring adjustments to these balances in future periods. The critical accounting estimates that affect the consolidated financial statements and the judgments and assumptions used are consistent with those described under Part II, Item 7 of our Annual Report on Form 10-K for the year ended December 31, 2016.
Going Concern
We have no sources of revenue to provide incoming cash flows to sustain our future operations. As outlined above, our ability to pursue our planned business activities is dependent upon our successful efforts to raise additional capital.
These factors raise substantial doubt regarding our ability to continue as a going concern. Our condensed consolidated financial statements have been prepared on a going concern basis, which implies that we will continue to realize our assets and discharge our liabilities in the normal course of business. Our financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes of financial condition, revenues, expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
| 18 |
Item 4. Controls and Procedures
| (a) | Evaluation of Disclosure Controls and Procedures |
Our management, with the participation of our Chief Executive Officer and our Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this report. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in the reports we file or submit under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. Based on such evaluation, our Chief Executive Officer and our Chief Financial Officer have concluded that, as of the end of the period covered by this report, our disclosure controls and procedures are not effective in recording, processing, summarizing and reporting, on a timely basis, information required to be disclosed by us in the reports that we file or submit under the Exchange Act.
It should be noted that any system of controls is based in part upon certain assumptions designed to obtain reasonable (and not absolute) assurance as to its effectiveness, and there can be no assurance that any design will succeed in achieving its stated goals.
| (b) | Changes in Internal Control Over Financial Reporting |
There have been no changes in our internal control over financial reporting during the three months ended March 31, 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management is not aware of any material legal proceedings and there are no pending material procedures that would affect the property of the Company. Management is not aware of any legal proceedings contemplated by any government authority or any other party involving the Company. As of the date of this Quarterly Report, no director, officer or affiliate is (i) a party adverse to us in any legal proceeding, or (ii) has an adverse interest to us in any legal proceeding.
For risk factors, see Item 1.A.-“Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2016 filed on March 14, 2017.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
| (a) | We issued the following unrestricted securities during the period covered by this report to the named individual pursuant to exemptions under the Securities Act of 1933 including Section 4(2): |
| 19 |
On March 9, 2017, we issued 12,761 shares of restricted, immediately vested, common stock in relation to the discretionary 2016 bonus awarded to Dr. Glynn Wilson under the Company’s 2014 Omnibus Stock Ownership Plan, based on the closing price or our common stock of $4.31 per share, on the day immediately preceding the date the 2016 bonus award was approved.
On March 9, 2017, we issued 5,220 shares of restricted, immediately vested, common stock in relation to the discretionary 2016 bonus awarded to Dr. John Bonfiglio under the Company’s 2014 Omnibus Stock Ownership Plan, based on the closing price or our common stock of $4.31 per share, on the day immediately preceding the date the 2016 bonus award was approved.
On March 15, 2017, we issued 16,667 shares of common stock to Collision Capital, LLC pursuant to a vendor agreement.
On March 28, 2017, we issued 15,000 shares of common stock to Omnicor Media, LLC pursuant to a vendor agreement.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Mine Safety Disclosure
Not applicable.
Not applicable.
| 20 |
The following exhibits are included with this Quarterly Report on Form 10-Q:
| Incorporated by Reference | ||||||||||||
| Exhibit number |
Exhibit description | Form | File no. | Exhibit | Filing date |
Filed herewith | ||||||
| 31.1 | Certification of Chief Executive Officer Pursuant to Rule 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1933, as amended. | X | ||||||||||
| 31.2 | Certification of Chief Financial Officer and Chief Accounting Officer Pursuant to Rule 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1933, as amended | X | ||||||||||
| 32.1 | Certification of Chief Executive Officer Pursuant to Rule 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1933, as amended. | X | ||||||||||
| 32.2 | Certification of Chief Financial Officer and Chief Principal Accounting Officer pursuant to 18 U.S.C. 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | X | ||||||||||
The interactive data files on Exhibit 101 hereto are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, are deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise are not subject to liability under those sections.
Exhibit 101
101.INS - XBRL Instance Document
101.SCH - XBRL Taxonomy Extension Schema Document
101.CAL - XBRL Taxonomy Extension Calculation Linkbase Document
101.DEF - XBRL Taxonomy Extension Definition Linkbase Document
101.LAB - XBRL Taxonomy Extension Label Linkbase Document
101.PRE - XBRL Taxonomy Extension Presentation Linkbase Document
| 21 |
In accordance with the requirements of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
TAPIMMUNE INC.
| /s/ Glynn Wilson | ||
| Glynn Wilson | ||
| Chairman, Chief Executive Officer | ||
| Date: May 5, 2017 |
| 22 |
