Attached files
| file | filename |
|---|---|
| EX-10.2 - Emergent BioSolutions Inc. | exhibit10_2.htm |
| EX-10.4 - SIXTH AMENDMENT TO CREDIT AGREEMENT - Emergent BioSolutions Inc. | exhibit10_4.htm |
| EX-32 - Emergent BioSolutions Inc. | exhibit32_2.htm |
| EX-32 - Emergent BioSolutions Inc. | exhibit32_1.htm |
| EX-31 - Emergent BioSolutions Inc. | exhibit31_2.htm |
| EX-31 - Emergent BioSolutions Inc. | exhibit31_1.htm |
| EX-12 - Emergent BioSolutions Inc. | ex12.htm |
| 10-Q - Emergent BioSolutions Inc. | form10-q063016.htm |
EXHIBIT 10.3
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. Double asterisks denote omissions.
|
AWARD/CONTRACT
|
1. THIS CONTRACT IS A RATED ORDER UNDER DPAS (15 CFR 700)
|
RATING
|
PAGE OF PAGES
1 42
|
|||||||||||||||||||||||||
|
2. CONTRACT (Proc. Inst. Indent.) NO.
HHSO100201700007C
|
3. EFFECTIVE DATE
See Block 20C
|
4. REQUISITION/PURCHASE REQUEST/PROJECT NO.
OS194860
|
||||||||||||||||||||||||||
|
5. ISSUED BY CODE
|
HHS/OS/ASPR/BARDA
|
6. ADMINISTERED BY (if other than Item 5)
|
CODE
|
ASPR-BARDA01
|
||||||||||||||||||||||||
|
HHS/OS/ASPR/BARDA
330 Independence Ave., SW
Room 640-G
Washington, DC 20201
|
ASPR-BARDA
330 Independence Ave, SW, Rm G644
Washington DC 20201
|
|||||||||||||||||||||||||||
|
7. NAME AND ADDRESS OF CONTRACTOR (No., street, county, State and ZIP Code)
EMERGENT BIODEFENSE OPERATIONS LANSING LLC 330303
EMERGENT BIODEFENSE OPERATIONS LANS
3500 N MARTIN LUTHER KING, JR BLVD #
LANSING, MI 489062933
|
8. DELIVERY
FOB ORIGIN Other (See below)
|
|||||||||||||||||||||||||||
|
9. DISCOUNT FOR PROMPT PAYMENT
|
||||||||||||||||||||||||||||
|
CODE 330303
|
FACILITY CODE
|
|||||||||||||||||||||||||||
|
11. SHIP TO/MARK FOR CODE
|
HHS/OS/ASPR
|
12. PAYMENT WILL BE MADE BY
|
CODE
|
PSC
|
||||||||||||||||||||||||
|
HHS/OS/ASPR
200 C St SW
WASHINGTON DC 20201
|
PSC
330 Independence Ave, SW, Rm G644
Washington DC 20201
|
|||||||||||||||||||||||||||
|
13. AUTHORITY FOR USING OTHER THAN FULL AND OPEN COMPETITION:
10 U.S.C. 2304(c) ( ) 41 U.S.C. 253(c) ( )
|
14. ACCOUNTING AND APPROPRIATION DATA
2017.1990008.26402
|
|||||||||||||||||||||||||||
|
15A. ITEM NO.
|
15B. SUPPLIES/SERVICES
|
15C. QUANTITY
|
15D. UNIT
|
15E. UNIT PRICE
|
15F. AMOUNT
|
|||||||||||||||||||||||
|
TITLE: AVA FOR THE SNS
Continued
|
||||||||||||||||||||||||||||
|
15G. TOTAL AMOUNT OF
|
$
|
99,941,719.80
|
||||||||||||||||||||||||||
|
16. TABLE OF CONTENTS
|
||||||||||||||||||||||||||||
|
(X)
|
SEC.
|
DESCRIPTION
|
PAGE(S)
|
(X)
|
SEC.
|
DESCRIPTION
|
PAGE(S)
|
|||||||||||||||||||||
|
PART I – THE SCHEDULE
|
PART II – CONTRACT CLAUSES
|
|||||||||||||||||||||||||||
|
X
|
A
|
SOLICITATION/CONTRACT FORM
|
1
|
X
|
I
|
CONTRACT CLAUSES
|
31
|
|||||||||||||||||||||
|
X
|
B
|
SUPPLIES OR SERVICES AND PRICES/COSTS
|
4
|
PART III – LIST OF DOCUMENTS, EXHIBITS AND OTHER ATTACH.
|
||||||||||||||||||||||||
|
X
|
C
|
DESCRIPTION/SPECS./WORK STATEMENT
|
7
|
X
|
J
|
LIST OF ATTACHMENTS
|
42
|
|||||||||||||||||||||
|
X
|
D
|
PACKAGING AND MARKING
|
15
|
PART IV – REPRESENTATIONS AND INSTRUCTIONS
|
||||||||||||||||||||||||
|
X
|
E
|
INSPECTION AND ACCEPTANCE
|
16
|
K
|
REPRESENTATIONS, CERTIFICATIONS AND
OTHER STATEMENTS OF OFFERORS
|
|||||||||||||||||||||||
|
X
|
F
|
DELIVERIES OR PERFORMANCE
|
17
|
|||||||||||||||||||||||||
|
X
|
G
|
CONTRACT ADMINISTRATION DATA
|
21
|
L
|
INSTRS, CONDS., AND NOTICES TO OFFERORS
|
|||||||||||||||||||||||
|
X
|
H
|
SPECIAL CONTRACT REQUIREMENTS
|
26
|
M
|
EVALUATION FACTORS FOR AWARD
|
|||||||||||||||||||||||
|
CONTRACTING OFFICER WILL COMPLETE ITEM 17 (SEALED-BID OR NEGOTIATED PROCUREMENT) OR 18 (SEALED-BID PROCUREMENT) AS APPLICABLE
|
||||||||||||||||||||||||||||
|
17. CONTRACTOR'S NEGOTIATED AGREEMENT (Contractor is required to sign this document and return __2__ copies to issuing office.) Contractor agrees to furnish and deliver all items or perform all the services set forth or otherwise identified above and on any continuation sheets for the consideration stated herein. The rights and obligations of the parties to this contract shall be subject to and governed by the following documents: (a) this award/contract, (b) the solicitation, if any, and (c) such provisions, representations, certifications, and specifications as are attached or incorporated by reference herein. (Attachments are listed herein.)
|
18. AWARD (Contractor is required to sign this document.) Your bid on Solicitation Number ________17-100-SOL-00010______________,
including the additions or changes made by you which additions or changes are set forth in full above, is hereby accepted as to the terms listed above and on any continuation sheets. This award consummates the contract which consists of the following documents: (a) the Government's soliciation and your bid, and (b) this award/contract. No further contractual document is necessary. (Block 18 should be checked only when awarding a sealed-bid contract.)
|
|||||||||||||||||||||||||||
|
19A. NAME AND TITLE OF SIGNER (Type or print)
|
20A. NAME OF CONTRACTING OFFICER
[**]
|
|||||||||||||||||||||||||||
|
19B. NAME OF CONTRACTOR
|
19C. DATE SIGNED
|
20B. UNITED STATES OF AMERICA
|
20C. DATE SIGNED
3/16/2017
|
|||||||||||||||||||||||||
|
BY
|
BY
|
|||||||||||||||||||||||||||
|
(Signature of person authorized to sign)
|
(Signature of Contracting Officer)
|
|||||||||||||||||||||||||||
|
AUTHORIZED FOR LOCAL REPRODUCTION
Previous edition is NOT usable
|
STANDARD FORM 26 (REV. 5/2011)
Prescribed by GSA – FAR (48 CFR) 53.214(a)
|
|||||||||||||||||||||||||||
|
CONTINUATION SHEET
|
REFERENCE NO. OF DOCUMENT BEING CONTINUED
HHSO100201700007C
|
PAGE OF
2 42
|
|||||
|
NAME OF OFFEROR OR CONTRACTOR
EMERGENT BIODEFENSE OPERATIONS LANSING LLC 330303
|
|||||||
|
ITEM NO.
(A)
|
SUPPLIES/SERVICES
(B)
|
QUANTITY
(C)
|
UNIT
(D)
|
UNIT PRICE
(E)
|
AMOUNT
(F)
|
||
|
Tax ID Number: [**]
DUNS Number: [**]
HHSO100201700007C Anthrax Vaccine for SNS
IGF::XX::IGF
Appr. Yr.: 2017 CAN: 1990008 Object Class: 26402
FOB: Destination
Period of Performance: 03/16/2017 to 03/15/2019
|
|||||||
|
1
|
ASPR-17-01726 -- Procurement of [**] does of
Bio Thrax (AVA) (CLIN0001)
Obligated Amount: $[**]
|
99,450,000.00
|
|||||
|
2
|
ASPR-17-01726 -- Logistics – Safe and secure
shipment of product to SNS (CLIN0002)
Obligated Amount: $[**]
|
491,719.80
|
|||||
|
AUTHORIZED FOR LOCAL REPRODUCTION
|
OPTIONAL FORM 336 (4-86)
Sponsored by GSA
FAR (48 CFR) 53.110
|
||||||
CONTRACT TABLE OF CONTENTS
|
B.1.
|
BRIEF DESCRIPTION OF SUPPLIES
|
Anthrax is an acute infectious disease caused by the spore-forming bacterium Bacillus anthracis, which can cause human disease via gastrointestinal, cutaneous, or inhalation (pulmonary) routes. The 2014 Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) Strategy and Implementation Plan identifies anthrax as a high-priority threat as determined by the Secretary of Homeland Security. Strategies to mitigate anthrax as a biothreat against civilian populations include anthrax vaccine suitable for Post-Exposure Prophylaxis (PEP) when given concomitantly with antibiotics. Currently, BioThrax® is the only licensed and FDA approved vaccine for both General- Use Prophylaxis (GUP) and PEP capable of eliciting a protective immune response when administered as a three-dose post-exposure prophylactic regimen. BioThrax® is currently procured and stockpiled by the USG in the Strategic National Stockpile.
|
B.2.
|
PRICES/COSTS
|
Below are the agreed upon price/cost provisions and Contract Line Item Numbers (CLINs) between the Government and the Contractor.
|
B.2.1.
|
BASE PERIOD OF PERFORMANCE: The base period is 24 months from date of award.
|
|
B.2.2.
|
FIRM FIXED PRICE
|
|
ITEM
|
SUPPLIES/SERVICES
|
QTY/UNIT
|
UNIT PRICE
|
Total Fixed Price
|
||||||||
|
0001
|
BioThrax®/AVA
BioThrax® [**] product [**] upon date of delivery [**] |
[**] Dose
|
$
|
[
|
**]
|
$
|
[
|
**]
|
||||
|
0002
|
Logistics – Safe and secure shipment of product to SNS
Delivery Address: TBD
|
[**] Trucks
|
$
|
[
|
**]
|
$
|
[
|
**]
|
||||
|
TOTAL
|
$
|
99,941,719.80
|
||||||||||
|
B.2.3.
|
OPTION PERIODS
|
This contract consists only of the base award period. No option CLINs are included in this award.
|
B.2.4.
|
RESERVED
|
|
B.2.5.
|
PRICE PROTECTIONS
|
Should the Contractor be unable to deliver product with [**] within [**] days of a scheduled delivery date the pricing for such lots shall be based upon the contract unit price for the number of delivered doses in the lot that have a [**] as of the actual delivery date. This does not apply if the shipment is rescheduled at the Government's request. Further, this does not apply if there are unresolved issues with the quality, safety, and/or efficacy of the delivered product.
|
B.2.6.
|
COSTS UNALLOWABLE UNLESS AUTHORIZED BY THE CONTRACTING OFFICER
|
This section prohibits or restricts the use of contract funds for the following items (costs unallowable unless otherwise approved by the Contracting Officer):
|
(a)
|
Acquisition, by purchase or lease, of any interest in real property;
|
|
(b)
|
Rearrangement or alteration of facilities;
|
|
(c)
|
Purchase of lease of any item of general purpose office furniture or office equipment regardless of dollar value;
|
|
(d)
|
Accountable Government Property;
|
|
(e)
|
Overtime;
|
|
(f)
|
General scientific meetings/conferences;
|
|
(g)
|
Travel costs including foreign travel;
|
|
(h)
|
Costs incurred in the performance of any cost-reimbursement type subcontract (including consulting agreements);
|
|
(i)
|
Costs to be paid for the performance of a fixed-price subcontract that exceeds $150,000.00;
|
|
(j)
|
Refreshments and Meal Expenditures;
|
|
(k)
|
Promotional Items
|
|
(l)
|
Printing
|
|
B.2.7.
|
SUBCONTRACTS AND CONSULTANTS
|
Award of any FFP subcontract or FFP consulting agreement in excess of $150,000 or any cost reimbursement subcontract or consulting agreement shall not proceed without the prior written consent of the Contracting Officer via a COA letter. COA letters will only be issued upon review of the supporting documentation required by FAR Clause 52.244-2, Subcontracts. After receiving written consent of the subcontract by the Contracting Officer, a copy of the signed, executed subcontract and consulting agreement will be provided to the Contracting Officer within [**] days.
This Advanced Understanding may be modified in subsequent task orders.
|
B.2.8.
|
CONFIDENTIAL TREATMENT OF SENSITIVE INFORMATION
|
The Contractor shall guarantee strict confidentiality of any information/data of a sensitive nature that is provided to the Contractor by the Government during the performance of the contract. The Government has determined that the information/data that the Contractor will be provided during the performance of the contract is of a sensitive nature.
Disclosure of information/data that is sensitive in nature, in whole or in part, by the Contractor can only be made after the Contractor receives prior written approval from the Contracting Officer. Whenever the Contractor is uncertain with regard to the proper handling of information/data under the contract, the Contractor shall obtain a written determination from the Contracting Officer. (See also HHSAR clause 352.224-71).
Notwithstanding the foregoing, such information/data shall not be deemed of a sensitive nature with respect to the Contractor for purposes of this contract if such information/data: (a) was already known to the Contractor; (b) was generally available or known, or was otherwise part of the public domain, at the time of its disclosure to the Contractor; (c) became generally available or known, or otherwise became part of the public domain, after its disclosure to, or, with respect to the information/data by, the Contractor through no fault of the Contractor; (d) was disclosed to the Contractor, other than under an obligation of confidentiality or non-use, by a third party who had no obligation to the Government that controls such information/data not to disclose such information/data to others; or (e) was independently discovered or developed by the Contractor, as evidenced by its written records, without the use of information/data belonging to the Government.
Contractor may disclose information/data of a sensitive nature provided by the Government to the extent that such disclosure is: (a) made in response to a valid order of a court of competent jurisdiction or other supra-national, federal, national, regional, state, provincial or local governmental or regulatory body of competent jurisdiction; provided, however, that the Contractor shall first have given notice to the Government and give the Government a reasonable opportunity to quash such order and to obtain a protective order requiring that the information/data of a sensitive nature that is the subject of such order be held in confidence by such court or agency or, if disclosed, be used only for the purposes for which the order was issued; and provided further that if a disclosure order is not quashed or a protective order is not obtained, the information/data disclosed in response to such court or governmental order shall be limited to that information which is legally required to be disclosed in response to such court or governmental order; (b) otherwise required by law, in the opinion of legal counsel to the Contractor as expressed in an opinion letter in form and substance reasonably satisfactory to the Government, which shall be provided to the Government at least two (2) business days prior to the Contractor's disclosure of the information/data; or (c) made by the Contractor to the Regulatory Authorities as required in connection with any filing, application or request for Regulatory Approval; provided, however, that reasonable measures shall be taken to assure confidential treatment of such information/data.
|
B.2.9.
|
SHARING OF CONTRACT DELIVERABLES WITHIN THE UNITED STATES GOVERNMENT
|
In an effort to build a robust medical countermeasure pipeline through increased collaboration, the Government may share technical deliverables with USG entities responsible for Medical Countermeasure Development. In accordance with recommendations from the Public Health Emergency Medical Countermeasure Enterprise Review, agreements established in the Portfolio Advisory Committee (PAC) Charter, Technology Transfer Agreements (TTA) between BARDA and the Defense Threat Reduction Agency and the National Institute of Allergies and Infectious Diseases (NIAID), the Government may share technical deliverables set forth in Section F with colleagues within the Integrated Portfolio. This advance understanding does not authorize the government to share financial information outside HHS. This clause does not limit the Government's ability to use or disclose data or information in accordance with the terms or conditions of this contract. The Contractor is advised to review the terms of FAR Clause 52.227-14 regarding the Government's rights to deliverables submitted during performance as well as the Government's rights in data.
|
B.2.10.
|
CONTRACT NUMBER DESIGNATION
|
On all correspondence submitted under this contract, the Contractor agrees to clearly identify the contract number on the face page of the contract.
|
B.2.11.
|
DEFINITION OF DAYS
|
In this contract, it is intended that the term "days" refers to calendar days. If this results in a deliverable becoming due on a weekend, the due date shall be assumed to be the soonest business day thereafter, inclusive of holidays.
|
C.1.
|
VACCINE PRODUCTION AND CGMP COMPLIANCE:
|
|
1.
|
The Contractor shall manufacture BioThrax® in accordance with current Good Manufacturing Practices (cGMP) guidelines
|
|
2.
|
BioThrax® must be delivered on any business day, except Federal holidays, within the scheduled month in accordance with the targeted delivery schedule. The Contractor shall notify the Government promptly upon becoming aware of any deviations from the targeted delivery schedule. All changes to the targeted delivery schedule must be approved by the Contracting Officer and/or the Contracting Officer's Representative (COR).
|
|
3.
|
Quantities for each scheduled delivery shall be of a specific quantity.
|
|
4.
|
The Contractor shall perform all requisite assays and release tests, including but not limited to potency, identity, and stability testing in accordance with the Food Drug Administration (FDA) approved Biologic License Application (BLA-License Number 1755, STN 103821, and any approved change).
|
|
5.
|
All BioThrax® delivered under this contract must be labeled with an expiration date consistent with its current product license at the time of manufacture.
|
|
6.
|
The Contractor shall provide primary and secondary points of contact that shall be available 24 hours per day, seven days per week to be notified in case of a public health emergency.
|
|
7.
|
The Contractor shall report to the Government material correspondence from the FDA regarding the quality, safety, or efficacy of BioThrax®.
|
|
8.
|
The Contractor shall provide the Government with access to and/or provide copies of the following documents: (1) Form 483s form FDA inspections of Contractor's Lansing facility, (2) Establishment Inspection Reports (EIRs) from FDA inspections of Contractor's Lansing facility; (3) W arning Letters relating to BioThrax®; and Contractor's Annual Safety Report to FDA regarding BioThrax®. These documents will be provided to the Contracting Officer within 2 business days of receipt.
|
|
9.
|
The Contractor shall notify the Government of any issues with the safety and efficacy of BioThrax® and/or manufacturing or quality of the FDA-licensed production lines at the Contractor's Lansing facility within [**] business days of the determination of potential to be reported to FDA.
|
|
10.
|
The Government will have the option to conduct site inspections of the Contractor's Lansing facility during the period of performance of the contract. Such inspections will be performed by the COR or the COR's designee(s).
|
|
11.
|
If the contractor should obtain FDA approval for the manufacture and production of BioThrax® having [**] while under this contract, the Government will accept delivery of those doses with the [**] in addition to doses with a [**]. The Contractor may invoice only for those doses actually delivered under this contract in accordance with Section B.
|
|
12.
|
The product must be delivered in accordance with cGMP guidelines.
|
|
13.
|
The Contractor shall notify BARDA at least [**] days' prior of estimated shipment of product. At least [**] business days prior to the product being ready for shipment to DSNS, the Contractor shall obtain delivery address from the Contracting Officer and provide to the Contracting Officer and COR the following:
|
|
(a)
|
The date the product will be ready for loading on the truck(s) and the intended delivery date of product to the DSNS
|
|
(b)
|
Number of pallets, vials, and doses to be loaded and delivered to DSNS
|
|
14.
|
At least [**] hours before each scheduled delivery, the Contractor shall provide the following to the Contracting Officer and COR:
|
|
(a)
|
Packing Slip
|
|
(b)
|
Certificate(s) of Analysis c. FDA Lot Release(s)
|
|
(c)
|
Actual number of pallets, vials and doses to be loaded
|
|
(d)
|
Diagram of product shipment pallet (how many vials per box, per pallet)
|
|
15.
|
TempTale monitors supplied with each delivery must be returned to Contractor per instructions provided with each shipment within [**] business days after product delivery. Within [**] hours of receipt of TempTale monitors, the Contractor shall provide to the Contracting Officer and COR a letter for each delivered lot from the Contractor's Quality Department containing the following information:
|
|
(a)
|
The remaining ambient exposure time letter disclosing accumulated ambient temperature exposure until the point that BARDA (or DSNS-designated personnel) assumed responsibility for temperature control, per Section F, for each lot from the Contractor's Quality Department. The letter must indicate that the product was manufactured and released in accordance with cGMP and has met all acceptance criteria to allow for Government distribution.
|
|
16.
|
Funds provided shall be paid on a price per doses basis only on those products delivered and accepted to DSNS under contract.
|
|
17.
|
Under CLIN 0001 of this contract the products shall have an [**] product. The Contractor shall target ≥[**] of the total [**] remaining when the Government takes delivery of the product.
|
|
C.2.
|
RESERVED
|
|
C.3.
|
MEETINGS/SITE VISITS/AUDITS
|
The Contractor and the Government shall participate in regular meetings to coordinate and oversee the work performed as requested by the Contracting Officer (CO) or Contracting Officer's Representative (COR). Such meetings may include, but are not limited to, a kickoff meeting to be held at a location determined by the COR, status update meeting or teleconferences, site visits to the Contractor's and/or subcontractor's facilities and meetings with individual Contractors and other HHS officials to discuss the technical, regulatory, and ethical aspects of the program. The Contractor shall provide data, reports, and presentations to groups of outside experts and USG personnel and USG-contracted subject matter experts as required by the CO/COR facilitating review of activities. The Government reserves the right to conduct a pre-award site visit of the manufacturing plant. Pre-award site visits may be made with short notice. Contractors are expected to guarantee the availability of key staff or other staff determined by the Government as essential for purposes of this site visit. The purpose of the kickoff meeting will be to orient the Contractor to HHS/BARDA and review contract requirements. This meeting usually occurs within a month after contract award. Bi-weekly or monthly status update meetings/teleconferences will be held throughout the life of the contract. The schedule for these meetings will be established by the CO and COR. The Government reserves the right to visit the contractor's site for purposes of assessing quality as deemed necessary by the Government throughout the period of performance of the contract. The Contractor shall provide data, reports and presentations to groups of outside experts and USG personnel and USG-contracted subject matter experts as required by the CO/COR facilitating review of activities. The contractor shall facilitate cGMP site-visit or inspection as requested by FDA/Center for Biologics Evaluation and Research (CBER) at the time of production of product lots destined for the SNS. Periodic site visits will occur on an ad hoc basis (at least twice a year).
Within [**] calendar days of an FDA audit of Contractor or subcontractor facilities, the Contractor shall provide copies of the audit findings, final report, and a plan for addressing areas of nonconformance to FDA regulations and guidance for GLP, GMP or GCP guidelines as identified in the final audit report.
Other U.S. Government Audits
The United States Government (USG) reserves the right to conduct unannounced audits of the Contractor without advance notice. The USG reserves the right to accompany the Contractor on routine and for-cause site-visits or audits of subcontractor(s). At the discretion of the USG and independent of testing conducted by the Contractor, BARDA reserves the right to conduct site visits/audits and collect samples of product held by the Contractor and subcontractors. Finally, BARDA reserves the right to conduct unannounced site visits with justification and critical need. All audits will be conducted between normal business hours, i.e. 8 a.m. through 6 p.m., Monday through Friday.
|
C.4.
|
PRODUCT DELIVERIES
|
|
C.4.1.
|
TEMPERATURE CONTROL AND MONITORING
|
FOB Destination Deliveries:
The Contractor shall be responsible for maintaining product temperature control until the product arrives at the DSNS and has completed product acceptance by the USG. The Contractor shall provide the Government with an ambient exposure letter that covers the time the product leaves the Contractor's validated [**]°C storage facility until arrival at DSNS. Upon Government acceptance of the product to the Government, the responsibility for temperature control shall transfer to the Government as well as the responsibility for logging ambient exposure time (temperatures between [**]°C). The Contractor will provide and place TempTale(s) on each pallet of product while the product is inside the Contractor's validated [**]°C storage facility prior to placing the product onto the truck(s) of the designated carrier. The Government's acceptance of the aforementioned responsibility applies only to temperature control and does not indicate its acceptance of the lot(s).
|
C.4.2.
|
DSNS QUALITY CONTROL UNIT (QCU) ACCEPTANCE PROCEDURE FOR BIOTHRAX (AVA)
|
At the time the product is delivered to a designated DSNS delivery location, all product will be placed into DSNS Quarantine pending receipt of the required lot distribution documentation and the remaining ambient exposure time letter from the Contractor. The Contractor shall supply the Government:
|
1.
|
Notification of practices that may impact DSNS shipping procedures, if applicable
|
|
2.
|
All items outlined for delivery of product.
|
|
C.4.3.
|
ACCEPTANCE PROCESS AND TIMEFRAME (FOB DESTINATION DELIVERY)
|
|
1.
|
Contractor shall deliver to the Government, via e-mail or facsimile:
|
|
(a)
|
All required documentation outlined for delivery of product
|
|
(b)
|
Notification of the date and time that the product was delivered.
|
|
2.
|
Acceptance Timeframe: The Government will have [**] full business days, after receipt of all documentation required to establish that the requirements have been satisfied and provide Contractor notice that DSNS accepts the lot(s).
|
|
(a)
|
For purposes of this acceptance timeframe, business days are defined as 9:00AM to 5:00PM Eastern Time, Monday through Friday, excluding U.S. Government Holidays.
|
|
(b)
|
For the avoidance of doubt, BARDA will provide the Contractor with a written acceptance or refusal of BioThrax® lot(s) no later than 5:00PM on the [**] business day after receipt of the documentation.
|
|
C.4.4.
|
BARDA RELEASE FOR BioThrax
|
The DSNS Quality Control Unit (QCU) recommends that the temperature acceptance range for BioThrax, using the temperature monitoring device accuracy of [**]°C, would be [**]°C to [**]°C. This temperature is consistent with licensed label specifications ([**]°C to [**]°C) and takes into account the contractor's rounding practices (memo dated 8/12/09), and the accuracy of the temperature monitoring device (TempTale Bio).
TempTale device alarms will be set for shipments to alert for possible temperature deviations for further evaluation. BARDA will review the temperature data for DSNS internal processes. As a clarification and guide, the table below outlines temperature limits acceptable during the period that the Contractor is responsible for maintaining product temperature control and the resulting actions under each scenario. This table is based on the TempTale with an accuracy of [**]°C. If any of the following changes occur while the Government is responsible for temperature monitoring and control, DSNS QCU will seek guidance from the Contractor:
|
Temperature Range
|
Action
|
|
< [**]°C
|
☐ AVA Pallet will be placed into DSNS quarantine pending further disposition
|
|
[**]°C – [**]°C
|
☐ Acceptable for use by the SNS
|
|
≥[**]°C – [**]°C
|
☐ AVA Pallet will be placed into DSNS quarantine
☐ Release of product by BARDA will be pending quality disposition investigation, and remaining ambient exposure for the lot
|
|
>[**]°C
|
☐ AVA Pallet will be placed into DSNS quarantine pending further disposition
|
Temperature deviations during shipping are the responsibility of the Contractor for FOB Destination deliveries. Deviations during shipping shall not delay the Government's acceptance of the lots when deliveries are FOB Destination and shall be evaluated and fully documented by the Party responsible for temperature control and monitoring in accordance with standard procedures. The Contractor shall include an Event Description and Contractor's Product Impact Assessment in the Contractor's Certification Letter), for each lot impacted by such deviations. The Contractor shall state in the Certification Letter that, "A Full Deviation Report, including root cause analysis and corrective and preventative actions shall be submitted to the Government within [**] business days of report completion." The Party responsible for temperature control and monitoring is also responsible for root cause analysis and defining appropriate corrective and preventative actions.
|
C.5.
|
REPORTING REQUIREMENTS
|
See SECTION F.4 for specific reporting requirements. The Contractor shall submit to the CO and the COR technical progress reports as identified in any potential resultant contract. These reports shall be subject to the technical inspection and requests for clarification by the COR. These reports shall be brief, factual, and prepared in accordance with the following format:
|
A.
|
MONTHLY PROGRESS REPORT
|
This report shall include a description of the activities during the reporting period and the activities planned for the ensuing reporting period. The first reporting period consists of the first full month of performance plus any fractional part of the initial month. Thereafter, the reporting period shall consist of each calendar month.
The Contractor shall submit a Monthly Progress Report on or before the [**] calendar day following the last day of each reporting period and shall include the following:
Title Page: The title page for this report shall include the contract number and title; the type of report and period that it covers; the Contractor's name, address, telephone number, fax number, and e-mail address; and the date of submission.
Distribution List: A list of individuals receiving the Technical Progress report.
Progress:
SECTION I - An introduction covering the purpose and scope of the contract effort.
SECTION II Part A: SUMMARY - A description or table summarizing ongoing activities.
SECTION II Part B: MANAGEMENT AND ADMINISTRATIVE UPDATE – This section shall include a description of all meetings, conference calls, etc. that have taken place during the reporting period. Include progress on administration and management issues (e.g. evaluating and managing subcontractor performance and personnel changes). Please include all Quality Management System, Quality Control, and Quality Assurance Plans as part of this report or as requested by the COR.
SECTION II Part C: TECHNICAL PROGRESS – This section shall document the results of work completed and costs incurred during the period covered in relation to the proposed progress, effort, and budget. The report shall be in sufficient detail to explain comprehensively the results achieved.
SECTION II Part D: ISSUES – This section shall include a description of problems encountered and proposed corrective action; differences between planned and actual progress; why the differences have occurred and what corrective actions are planned; and if a project activity is delinquent, then what corrective action steps are planned. Revised timelines shall be provided.
SECTION II Part E: PROPOSED WORK – This section shall include a summary of work proposed as a rolling [**] month forecast for the next reporting period, by a certain date, and by whom.
SECTION II Part F: MANUFACTURING AND SUPPLY CHAIN MANAGEMENT – This section shall include a summary of the manufacturing and supply-chain related activities. Also include in this section updates to the production plan, capacity projections, stability results, inventory and shipment/distribution information.
Invoices: Summary of any invoices submitted during the reporting period. A Monthly Progress Report will not be required in the same month Annual Progress Reports or a Final Report are due.
|
B.
|
ANNUAL PROGRESS REPORT
|
This report shall include a summation of the activities during the reporting period, and the activities planned for the ensuing reporting period. The first reporting period consists of the first full year of performance plus any fractional part of the initial year. Thereafter, the reporting period shall consist of each calendar year.
The Contractor shall submit an Annual Progress Report on or before the [**] calendar day following the last day of each reporting period and shall include the following:
Title Page: The title page for this report shall include the contract number and title; the type of report and period that it covers; the Contractor's name, address, telephone number, fax number, and e-mail address; and the date of submission.
Distribution List: A list of individuals receiving the Technical Progress report.
Progress:
SECTION I - An introduction covering the purpose and scope of the contract effort.
SECTION II Part A: SUMMARY - A description or table summarizing ongoing activities.
SECTION II Part B: MANAGEMENT AND ADMINISTRATIVE UPDATE – This section shall include a description of all meetings, conference calls, etc. that have taken place during the reporting period. Include progress on administration and management issues (e.g. evaluating and managing subcontractor performance and personnel changes). Please include all Quality Management System, Quality Control, and Quality Assurance Plans as part of this report or as requested by the COR.
SECTION II Part C: TECHNICAL PROGRESS – This section shall document the results of work completed and costs incurred during the period covered in relation to proposed progress, effort, and budget. The report shall be in sufficient detail to explain comprehensively the results achieved.
SECTION II Part D: ISSUES – This section shall include a description of problems encountered and proposed corrective action; differences between planned and actual progress; why the differences have occurred and what corrective actions are planned; and if a project activity is delinquent, then what corrective action steps are planned. Revised timelines shall be provided.
SECTION II Part E: PROPOSED WORK – This section shall include a summary of work proposed as a rolling [**] month forecast for the next reporting period, by a certain date, and by whom.
SECTION II Part F: MANUFACTURING AND SUPPLY CHAIN MANAGEMENT – This section shall include a summary of the manufacturing and supply-chain related activities. Also include in this section updates to the production plan, capacity projections, stability results, inventory and shipment/distribution information.
Invoices: Summary of any invoices submitted during the reporting period. An Annual Progress Report will not be required for the period when the Final Technical Progress Report is due.
|
C.
|
DRAFT FINAL REPORT AND FINAL REPORT
|
These reports are to include a summation of the work performed and results obtained for execution of various studies or technical work packages during the entire contract period of performance. This report shall be in sufficient detail to describe comprehensively the results achieved. The Draft Final Progress Report shall be due [**] calendar days prior to the expiration date of the contract and the Final Progress Report is due on or before the expiration date of the contract. The USG reserves the right to comment on the draft report. Any comments on the draft report made by the USG must be included in the final report submitted by the Offeror. The report shall conform to the following format:
Title Page: The title for these reports shall include the contract number and title; the type of report and period that it covers; the Contractor's name, address, telephone number, fax number, and e-mail address; and the date of submission.
Distribution List: A list of individuals receiving the Technical Progress report.
Progress:
SECTION I: EXECUTIVE SUMMARY - Summarize the purpose and scope of the contract effort including a summary of the major accomplishments relative to the specific activities set forth in the Statement of Work.
SECTION II: RESULTS - A detailed description of the work performed and the results obtained including all expenses for the entire contract period of performance.
|
D.
|
FDA REGULATORY AGENCY CORRESPONDENCE, MEETING SUMMARIES, AND SUBMISSIONS
|
The Contractor shall provide a copy of any regulatory communications (e.g., meeting minutes, submissions, etc.) that occur during the contract period of performance pertaining to the product being procured (i.e. BioThrax). Any pertinent regulatory communications of issues that may impact the product or ability of the Contractor to complete delivery of the product on this particular contract shall be made known to ACMG/BARDA until delivery of all [**] doses are completed.
|
E.
|
OTHER REQUIREMENTS/DELIVERABLES
|
|
(a)
|
ANNUAL/FINAL INVENTION REPORT
All reports and documentation required by FAR Clause 52.227-11, Patent Rights- Ownership by the Contractor, including, but not limited to, the invention disclosure report, the confirmatory license, and the Government support certification. An Annual Invention Report shall be due on or before the [**] calendar day after the completion of each reporting period. A Final Invention Report (see FAR 27.303 (b)(2)(ii)) shall be due on or before the expiration date of the contract. If no invention is disclosed or no activity has occurred on a previously disclosed invention during the applicable reporting period, a negative report shall be submitted to the Contracting Officer. |
|
(b)
|
PRESS RELEASES
The Contractor agrees to accurately and factually represent the work conducted under this contract in all press releases. The Contractor shall ensure the Contracting Officer has received and approved an advanced copy of any press release not less than [**] business days prior to the issuance of any potential press release. |
|
(c)
|
SECURITY REPORT
The Contractor shall report to the government any activity; or incident that is in violation of established security standards; or indicates the loss or theft of government products. Reports shall be due within [**] hours after occurrence of an activity or incident. |
Packaging shall be consistent with the FDA approved labeling and packaging for this product at the time of manufacture. In addition, the product will be labeled and packaged with FDA approved components and in accordance with the criteria (e.g. Palletization) set forth by the CDC SNS. This configuration will include product label, product insert, individual vial carton, and other components deemed necessary to appropriately identify and control the product throughout the supply chain and eventual use.
|
D.1.
|
METHOD OF DELIVERY
|
Unless otherwise specified by the Contracting Officer, all deliverable items to be furnished to the Government under this contract (including invoices) shall be made by first class mail, overnight carrier, or email as described in SECTION F.
All deliverables required under this contract shall be packaged, marked and shipped in accordance with Government specifications. At a minimum, all deliverables shall be marked with the contract number and Contractor's name. The Contractor shall guarantee that all required materials shall be delivered in immediate usable and acceptable condition.
FAR 52.252-2 Clauses Incorporated by Reference (Feb 1998)
This contract incorporates one or more clauses by reference, with the same force and effect as if they were given in full text. Upon request, the Contracting Officer will make their full text available.
|
E.1.
|
FEDERAL ACQUISITION REGULATION (FAR) (48 CFR Chapter 1) CLAUSES
|
Full text of the FAR clauses may be accessed electronically at:
https://www.acquisition.gov/far/index.html
https://www.acquisition.gov/far/index.html
|
Reg
|
Clause
|
Date
|
Clause Title
|
|
FAR
|
52.246-2
|
Aug 1996
|
Inspection of Supplies - Fixed Price
|
|
FAR
|
52.246-16
|
Apr 1984
|
Responsibility for Supplies
|
|
E.2.
|
INSPECTION AND ACCEPTANCE (NON-PRODUCT DELIVERABLES)
|
Inspection and acceptance of materials, services, and documentation called for herein shall be accomplished by the Contracting Officer or a duly authorized representative. Technical inspection and acceptance will take place at:
Biomedical Advanced Research and Development Authority
Office of the Assistant Secretary for Preparedness and Response
200 C Street, S.W.
Washington, D.C. 20024
Office of the Assistant Secretary for Preparedness and Response
200 C Street, S.W.
Washington, D.C. 20024
Acceptance may be presumed unless otherwise indicated in writing by the Contracting Officer or the duty authorized representative within [**] days of receipt.
|
E.3.
|
INSPECTION AND ACCEPTANCE (PRODUCT DELIVERABLES)
|
Performance of the contract will be monitored by the CO/COR on a regular basis. The Contracting Officer will be responsible for inspection and acceptance of deliverables and services. Monitoring of the contract will be based on periodic reporting by the Contractor.
| FAR 52.252-2 |
Clauses Incorporated by Reference (Feb 1998)
|
This contract incorporates one or more clauses by reference, with the same force and effect as if they were given in full text. Upon request, the Contracting Officer will make their full text available.
|
F.1.
|
FEDERAL ACQUISITION REGULATION (FAR) (48 CFR Chapter 1) CLAUSES
|
Full text of the FAR clauses may be accessed electronically at:
https://www.acquisition.gov/far/index.html
https://www.acquisition.gov/far/index.html
|
Reg
|
Clause
|
Date
|
Clause Title
|
|
FAR
|
52.246-15
|
Aug 1989
|
Stop Work Order
|
|
FAR
|
52.246-15, Alternate 1
|
Apr 1984
|
Alternate 1
|
|
F.2.
|
PERIOD OF PERFORMANCE
|
The base period of performance under this contract shall be for twenty-four (24) months from date of award. There are no option periods under this contract and therefore no options will be exercised during the life of this contract.
|
F.3.
|
DELIVERIES
|
Successful performance of the final contract shall be deemed to occur upon performance of the work described in SECTION C of this contract and upon delivery and acceptance of the items by the Contracting Officer or their duly authorized representative as described in F.5.2.2.
|
F.4.
|
CONTRACT DELIVERABLES AND REPORTING REQUIREMENTS
|
|
F.4.1.
|
SUBMISSION OF CONTRACT DELIVERABLES (NON-PRODUCT DELIVERABLES)
|
Documents shall be delivered electronically via email to the Contracting Officer (CO) and the Contracting Officer's Representative (COR). When electronic deliverables are not preferable by the Contracting Officer, all deliverables and reports furnished to the Government under any potential resultant contract (including invoices) shall be addressed as follows:
|
UPS/FedEx/Courier
|
USPS Mail Packages
|
|
[**]
Contracting Officer HHS/ASPR/AMCG 200 C St. SW Washington, DC 20024 Email: [**] |
[**]
Contracting Officer HHS/ASPR/AMCG 200 C St. SW Washington, DC 20024 |
|
UPS/FedEx/Courier
|
USPS Mail Packages
|
|
[**]
Contracting Officer Representative HHS/ASPR/BARDA 200 C St. SW Washington, DC 20024 Email: [**] |
[**]
Contracting Officer Representative HHS/ASPR/BARDA 200 C St. SW Washington, DC 20024 |
|
F.4.2.
|
DELIVERABLE SCHEDULE
|
|
Item No.
|
Description
|
Addresses
|
Deliverable Schedule
|
|
1
|
Monthly Progress Report
|
CO: (1) electronic copy
COR: (1) electronic copy |
Reports are due on or before the [**] of each month following the end of each reporting period.
|
|
2
|
Annual Progress Report
|
CO: (1) electronic copy
COR: (1) electronic copy |
Reports are due on or before the [**] calendar day following the end of each reporting period.
|
|
3
|
Draft Final Progress Report
|
CO: (1) electronic copy
COR: (1) electronic copy |
Report is due [**] Calendar days prior to the expiration date of the contract.
|
|
4
|
Final Progress Report
|
CO: (1) electronic copy
COR: (1) electronic copy |
Report is due on or before the expiration date of the contract.
|
|
5
|
FDA/ Regulatory Agency Correspondence and Meeting Summaries
|
CO: (1) electronic copy
COR: (1) electronic copy |
Reports are due with the next applicable report from Items 1 through 4 above.
|
|
6
|
Annual/Final Invention Report
|
CO: (1) electronic copy
COR: (1) electronic copy |
An Annual Invention Report is due on or before the [**] calendar day after the completion of each reporting period. A Final Invention Report is due on or before the expiration date of the contract.
|
|
7
|
Press Releases
|
CO: (1) electronic copy COR: (1) electronic copy
|
Reports/Notices are due for approval to the CO not less than [**] business days prior to the issuance of any potential press release.
|
|
8
|
Security Report
|
CO: (1) electronic copy
COR: (1) electronic copy |
Reports are due within [**] hours after occurrence of an activity or incident.
|
|
F.5.
|
PRODUCT DELIVERIES
|
|
F.5.1.
|
USE OF PRODUCT BY THE U.S. GOVERNMENT
|
To the extent that third parties contact BARDA to obtain doses of BioThrax®, BARDA will notify such third parties that Emergent sells BioThrax commercially.
|
F.5.2.
|
CONTRACT DELIVERABLES AND REPORTING REQUIREMENTS
|
F.5.2.1. DELIVERY SCHEDULE
The offeror shall propose a delivery schedule of products to include number of doses and dates. Delivery is estimated to occur by [**]. The forecasted schedule, subject to revisions per Section F.5.2.3 below, is:
[**]
F.5.2.2. PRODUCT DELIVERY – PRODUCT DROP OFF BY CONTRACTOR (FOB DESTINATION DELIVERIES)
|
(a)
|
The delivery of BioThrax® product shall be F.O.B Destination at the USG designated drop off location.
|
|
(b)
|
At least [**] days prior to an estimated shipment vendor will provide the COR with the notices required under Section C.1.13 above and F.5.2.3 below.
|
|
(c)
|
The place of product drop off by the Contractor will be provided by the USG to the Contractor at least [**] business days prior to scheduled pick up by the designated carrier.
|
F.5.2.3. DELIVERY DOCUMENTATION
For product delivered FOB Destination, the Contractor shall deliver, within the specified timeframes, and submit the following documents to the Contracting Officer and COR:
|
(a)
|
At least [**] days prior to an estimated shipment the contractor shall notify the COR and receive the DSNS shipment delivery address from the COR.
|
|
(b)
|
At least [**] business days prior to each product shipment by the Contractor, the Contractor shall provide to the Contracting Officer and COR:
|
|
(i)
|
The delivery date: For FOB Destination deliveries, shall be the date the product will be scheduled for delivery by the Contractor
|
|
(ii)
|
Actual number of 40"x48" pallets, number of vials, and doses to be loaded
|
|
(c)
|
At least [**] hours before each scheduled shipment by the Contractor, the Contractor shall provide the following to the Contracting Officer and COR:
|
|
(i)
|
Packing Slip
|
|
(ii)
|
Certificate(s) of Analysis iii. FDA Lot Release(s)
|
|
(iii)
|
Confirm the number of pallets, vials and doses to be loaded
|
|
(iv)
|
Diagram of product shipment pallet (how many vials per box, per pallet)
|
|
(d)
|
On the shipment date, the Contractor shall provide a shipment confirmation including the TempTale ID#(s) associated with each pallet being delivered.
|
|
(e)
|
TempTale monitors supplied with each delivery must be returned to Contractor per instructions provided with each shipment within [**] business days upon product receipt. Within [**] hours of receipt of TempTale monitors, the Contractor shall provide to the Contracting Officer and COR a letter for each delivered lot from the Contractor's Quality Department containing the following information:
|
|
(i)
|
The remaining ambient temperature exposure time for the lot until the point that BARDA (or DSNS-designated personnel) assumed responsibility for temperature control, per Section C.4.1.
|
|
(ii)
|
This letter shall also indicate that the product was manufactured and released in accordance with cGMP and has met acceptance criteria to allow for Government distribution.
|
|
G.1.
|
CONTRACTING OFFICER (CO)
|
The following Contracting Officer (CO) will represent the Government for the purpose of this contract:
[**]
Contracting Officer
DHHS/OS/ASPR/AMCG
330 Independence Avenue, S.W., Room G640
Washington, D.C. 20201
E-mail: [**]
Contracting Officer
DHHS/OS/ASPR/AMCG
330 Independence Avenue, S.W., Room G640
Washington, D.C. 20201
E-mail: [**]
The following Contracting Specialist (CS) will represent the Government for the purpose of this contract:
[**]
Contracting Specialist
DHHS/OS/ASPR/AMCG
330 Independence Avenue, S.W., Room G640
Washington, D.C. 20201
E-mail: [**]
Contracting Specialist
DHHS/OS/ASPR/AMCG
330 Independence Avenue, S.W., Room G640
Washington, D.C. 20201
E-mail: [**]
The Contracting Officer is the only individual who can legally commit and bind the Government to the expenditure of public funds. No person other than the Contracting Officer can make any changes to the terms, conditions, general provisions or other stipulations of this contract. Any other commitment, either explicit or implied, is invalid.
The CO is the only person with authority to act as agent of the Government under this contract. Only the Contracting Officer has authority to:
|
1.
|
direct or negotiate any changes in the statement of objectives;
|
|
2.
|
modify or extend the period of performance;
|
|
3.
|
change the delivery schedule;
|
|
4.
|
authorize reimbursement to the Contractor for any costs incurred during the performance of this contract;
|
|
5.
|
obligate or de-obligate funds into the contract;
|
|
6.
|
sign written licensing agreements; or
|
|
7.
|
otherwise change any terms and conditions of this contract.
|
No information, other than that which may be contained in an authorized modification to this contract duly issued by the Contracting Officer, which may be received from any person employed by the United States Government, or otherwise, shall be considered grounds for deviation from any stipulation of this contract.
|
G.2.
|
CONTRACTING OFFICER'S REPRESENTATIVE (COR)
|
The Government's Contracting Officer's Representative (COR) is:
[**]
Contracting Officer's Representative (COR)
DHHS/OS/ASPR/BARDA
Independence Avenue, S.W., Room G640
Washington, D.C. 20201
E-mail: [**]
Contracting Officer's Representative (COR)
DHHS/OS/ASPR/BARDA
Independence Avenue, S.W., Room G640
Washington, D.C. 20201
E-mail: [**]
The Government's Alternate Contracting Officer's Representative (COR) is:
[**]
Contracting Officer's Representative (COR)
DHHS/OS/ASPR/BARDA
Independence Avenue, S.W., Room G640
Washington, D.C. 20201
E-mail: [**]
Contracting Officer's Representative (COR)
DHHS/OS/ASPR/BARDA
Independence Avenue, S.W., Room G640
Washington, D.C. 20201
E-mail: [**]
As delegated by the CO, the COR is responsible for:
|
1.
|
monitoring the Contractor's technical progress, including the surveillance and assessment of performance and recommending to the Contracting Officer changes in requirements;
|
|
2.
|
assisting the CO in interpreting the statement of work and any other technical performance requirements;
|
|
3.
|
performing technical evaluation as required;
|
|
4.
|
performing technical inspections required by this contract; and
|
|
5.
|
assisting in the resolution of technical problems encountered during performance.
|
|
G.3.
|
CONTRACTOR'S POINTS OF CONTACT
|
The Contractor shall provide primary and secondary points of contact that will be available 24 hours per day, 7 days per week, to be notified in case of a public health emergency. If there are any changes to these points of contact, the Contractor shall notify the CO and COR immediately.
Technical Contacts:
[**]
Emergent Biodefense Operations Lansing LLC
3500 N. Martin Luther King Jr. Blvd.
Lansing, MI 48906
Phone: [**]
Email: [**]
Emergent Biodefense Operations Lansing LLC
3500 N. Martin Luther King Jr. Blvd.
Lansing, MI 48906
Phone: [**]
Email: [**]
[**]
Emergent Biodefense Operations Lansing LLC
3500 N. Martin Luther King Jr. Blvd.
Lansing, MI 48906
Phone: [**]
Email: [**]
Emergent Biodefense Operations Lansing LLC
3500 N. Martin Luther King Jr. Blvd.
Lansing, MI 48906
Phone: [**]
Email: [**]
Administrative Business Contact:
[**]
Emergent BioSolutions, Inc.
400 Professional Dr, Suite 400
Gaithersburg, MD 20879
Phone: [**]
Email: [**]
Emergent BioSolutions, Inc.
400 Professional Dr, Suite 400
Gaithersburg, MD 20879
Phone: [**]
Email: [**]
|
G.4.
|
KEY PERSONNEL, HHSAR 352.237-75 (December 2015)
|
The key personnel specified in this contract are considered to be essential to work performance. At least [**] calendar days prior to the Contractor voluntarily diverting any of the specified individuals to other programs or contracts (or as soon as possible, if an individual must be replaced, for example, as a result of leaving the employ of the Contractor), the Contractor shall notify the Contracting Officer and shall submit comprehensive justification for the diversion or replacement request (including proposed substitutions for key personnel) to permit evaluation by the Government of the impact on performance under this contract. The Contractor shall not divert or otherwise replace any key personnel without the written consent of the Contracting Officer. The Government may modify the contract to add or delete key personnel at the request of the Contractor or Government.
The following are considered key personnel for this contract:
|
·
|
[**]
|
|
G.5.
|
INVOICE SUBMISSION
|
| (a) |
The Contractor shall submit invoices electronically to the Contracting Officer (CO), the Contract Specialist (CS), the Contracting Officer's Representative (COR), and PSC (PSC_Invoices@psc.hhs.gov). The payment request shall be transmitted as an attachment via email. Invoice composition instructions are provided in Attachment #2 (Fixed Price Type Contracts). A sample invoice form is provided as Attachment #3.
|
| (b) |
The Contractor agrees to include (as a minimum) the following information on each invoice:
|
| 1. |
Contractor's Name & Address
|
| 2. |
Contractor's Tax Identification Number (TIN)
|
| 3. |
Contract Number
|
| 4. |
Invoice Number
|
| 5. |
Invoice Date
|
| 6. |
Contract Line Item Number
|
| 7. |
Quantity
|
| 8. |
Unit Price & Extended Amount for each line item
|
| 9. |
Total Amount of Invoice
|
| 10. |
Name, title and telephone number of person to be notified in the event of a defective invoice
|
| 11. |
Payment Address, if different from the information in (b)(1).
|
| (c) |
The invoice shall be signed by a person authorized to bind the Contractor.
|
| (d) |
The Contractor shall not submit an invoice prior to delivery of goods or services.
|
| (e) |
The Contractor shall include the following certification at the bottom of the payment request: "I hereby certify that the salaries billed in this payment request are in compliance with the current HHS Salary Rate Limitation Provisions in Section I of the contract."
|
|
G.6.
|
PROVIDING ACCELERATED PAYMENT TO SMALL BUSINESS SUBCONTRACTORS, FAR 52.232-40 (DEC 2013)
|
|
(a)
|
Upon receipt of accelerated payments from the Government, the Contractor shall make accelerated payments to its small business subcontractors under this contract, to the maximum extent practicable and prior to when such payment is otherwise required under the applicable contract or subcontract, after receipt of a proper invoice and all other required documentation from the small business subcontractor.
|
|
(b)
|
The acceleration of payments under this clause does not provide any new rights under the prompt Payment Act.
|
|
(c)
|
Include the substance of this clause, include this paragraph c, in all subcontracts with small business concerns, including subcontracts with small business concerns for the acquisition of commercial items.
|
|
G.7.
|
CONTRACT COMMUNICATIONS/CORRESPONDENCE
|
The Contractor shall identify all correspondence, reports, and other data pertinent to this contract by imprinting thereon the contract number from Page 1 of the contract.
|
G.8.
|
POST AWARD EVALUATION OF CONTRACTOR PERFORMANCE
|
|
(a)
|
Purpose: In accordance with FAR Subpart 42.15, the Contractor's performance will be periodically evaluated by the government in order to provide current information for source selection purposes. These evaluations will therefore be marked "Source Selection Information."
|
|
(b)
|
Performance Evaluation Period: The Contractor's performance will be evaluated at least [**].
|
|
(c)
|
Evaluators: The performance evaluation will be completed jointly by the Contracting Officer's Representative and the Contracting Officer.
|
|
(d)
|
Performance Evaluation Factors: The Contractor's performance will be evaluated in accordance with FAR Subpart 42.15.
|
|
(e)
|
Contractor Review: A copy of the evaluation will be provided to the Contractor as soon as practicable after completion of the evaluation. The Contractor shall submit comments, rebutting statements, or additional information to the Contracting Officer within [**] calendar days after receipt of the evaluation.
|
|
(f)
|
Resolving Disagreements between the Government and the Contractor: Disagreements between the parties regarding the evaluation will be reviewed at a level above the Contracting Officer. The ultimate conclusion on the performance evaluation is a decision of the contracting agency. Copies of the evaluation, Contractor's response, and review comments, if any, will be retained as part of the evaluation.
|
|
(g)
|
Release of Contractor Performance Evaluation Information: The completed evaluation will not be released to other than Government personnel and the Contractor whose performance is being evaluated. Disclosure of such information could cause harm both to the commercial interest of the Government and to the competitive position of the Contractor being evaluated, as well as impede the efficiency of Government operations.
|
|
(h)
|
Source Selection Information: Departments and agencies may share past performance information with other Government departments and agencies when requested to support future award decisions. The information may be provided through interview and/or by sending the evaluation and comment document to the requesting source selection official.
|
|
(i)
|
Retention Period: The agency will retain past performance information for a maximum period of [**] years after completion of contract performance for the purpose of providing source selection
|
|
H.1.
|
NEEDLE DISTRIBUTION
|
The Contractor shall not use contract funds to carry out any program of distributing sterile needles or syringes for the hypodermic injection of any illegal drug.
|
H.2.
|
ACKNOWLEDGEMENT OF FEDERAL FUNDING
|
Pursuant to Section 508 of Public Law 105-78, the contractor shall clearly state, when issuing statements, press releases, requests for proposals, bid solicitations and other documents describing projects or programs funded in whole or in part with Federal money that:
(1) the percentage of the total costs of the program or project which will be financed with Federal money;
(2) the dollar amount of Federal funds for the project or program; and
(3) the percentage and dollar amount of the total costs of the project or program that will be financed by nongovernmental sources.
This requirement is in addition to the continuing requirement to provide an acknowledgment of support and disclaimer on any publication reporting the results of a contract funded activity.
Subcontractors - Contractors shall require subcontractors to adhere to these requirements for HHS acknowledgement of support.
|
H.3.
|
RESTRICTIONS ON ABORTIONS
|
The Contractor shall not use funds for any abortion.
|
H.4.
|
GUN CONTROL
|
The Contractor shall not use contract funds in whole or in part, to advocate or promote gun control.
|
H.5.
|
DISSEMINATION OF FALSE OR DELIBERATELY MISLEADING INFORMATION
|
The Contractor shall not use contract funds to disseminate information that is deliberately false or misleading.
|
H.6.
|
CARE OF LIVE VERTEBRATE ANIMALS
|
|
(a)
|
Before undertaking performance of any contract involving animal-related activities where the species is regulated by the United Sates Department of Agriculture (USDA), the Contractor shall register with the Secretary of Agriculture of the United States in accordance with 7 U.S.C. 2136 and 9 CFR 2.25 through 2.28. The Contractor shall furnish evidence of the registration to the Contracting Officer.
|
|
(b)
|
The Contractor shall acquire vertebrate animals used in research from a dealer licensed by the Secretary of Agriculture under 7 U.S.C. 2133 and 9 CFR 2.1 2.11, or from a source that is exempt from licensing under those sections.
|
|
(c)
|
The Contractor agrees that the care, use, and intended use of any live vertebrate animals in the performance of this contract shall conform with the Public Health Service (PHS) Policy on Humane Care of Use of Laboratory Animals (PHS Policy), the current Animal Welfare Assurance (Assurance), the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC) and the pertinent laws and regulations of the United States Department of Agriculture (see 7 U.S.C. 2131 et seq. and 9 CFR subchapter A, Parts 1-4). In case of conflict between standards, the more stringent standard shall govern.
|
|
(d)
|
If at any time during performance of this contract, the Contracting Officer determines, in consultation with the Office of Laboratory Animal Welfare (OLAW), National Institutes of Health (NIH), that the Contractor is not in compliance with any of the requirements and standards stated in paragraphs (a) through (c) above, the Contracting Officer may immediately suspend, in whole or in part, work and further payments under this contract until the Contractor corrects the noncompliance. Notice of the suspension may be communicated by telephone and confirmed in writing. If the Contractor fails to complete corrective action within the period of time designated in the Contracting Officer's written notice of suspension, the Contracting Officer may, in consultation with OLAW, NIH, terminate this contract in whole or in part, and the Contractor's name may be removed from the list of those contractors with Animal Welfare Assurances.
|
Note: The Contractor may request registration of its facility and a current listing of licensed dealers from the Regional Office of the Animal and Plant Health Inspection Service (APHIS), USDA, for the region in which its research facility is located. The location of the appropriate APHIS Regional Office, as well as information concerning this program may be obtained by contacting the Animal Care Staff, USDA/APHIS, 4700 River Road, Riverdale, Maryland 20737 (Email: ace@aphis.usda.gov; Web site: (http://www.aphis.usda.gov/wps/portal/aphis/ourfocus/animalwelfare).
|
H.7.
|
ANIMAL WELFARE
|
All research involving live, vertebrate animals shall be conducted in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals (PHS Policy). The PHS Policy can be accessed at: http://grants1.nih.gov/grants/olaw/references/phspol.htm
|
H.8.
|
OMB CLEARANCE
|
In accordance with HHSAR 352.211-3, Paperwork Reduction Act of 1995 (44 U.S.C. section 3501), the Contractor shall not proceed with surveys or interviews until such time as Office of Management and Budget (OMB) Clearance for conducting interviews has been obtained by the Contracting Officer's Representative (COR) and the Contracting Officer has issued written approval to proceed.
|
H.9.
|
RESTRICTION ON PORNOGRAPHY ON COMPUTER NETWORKS
|
The Contractor shall not use contract funds to maintain or establish a computer network unless such network blocks the viewing, downloading, and exchanging of pornography.
|
H.10.
|
CERTIFICATION OF FILING AND PAYMENT OF TAXES
|
The Contractor must be in compliance with Section 518 of the Consolidated Appropriations Act of FY 2014.
|
H.11.
|
SUBCONTRACTING PROVISIONS
|
|
A.
|
SMALL BUSINESS SUBCONTRACTING PLAN
|
|
1.
|
The Small Business Subcontracting Plan, dated February 1, 2017 is attached hereto and made a part of this contract.
|
|
2.
|
The failure of any Contractor or subcontractor to comply in good faith with FAR Clause 52.219-8, entitled "Utilization of Small Business Concerns" incorporated in this contract and the attached Subcontracting Plan, will be a material breach of such contract or subcontract and subject to the remedies reserved to the Government under FAR Clause 52.219-16 entitled, "Liquidated Damages-Subcontracting Plan."
|
|
B.
|
SUBCONTRACTING REPORTS
|
The Contractor shall submit the following Subcontracting reports electronically via the "electronic Subcontracting Reporting System (eSRS) at http://www.esrs.gov.
|
1.
|
Individual Subcontract Reports (ISR)
|
Regardless of the effective date of this contract, the Report shall be due on the following dates for the entire life of this contract:
|
·
|
[**]
|
|
·
|
[**]
|
|
·
|
Expiration Date of Contract
|
|
2.
|
Summary Subcontract Report (SSR)
|
Regardless of the effective date of this contract, the Summary Subcontract Report shall be submitted annually on the following date for the entire life of this contract:
|
·
|
[**]
|
For both the Individual and Summary Subcontract Reports, the Contracting Officer shall be included as a contact for notification purposes at the following e-mail address defined in SECTION F.
|
H.12.
|
CONFIDENTIALITY OF INFORMATION
|
|
(a)
|
Confidential information, as used in this article, means information or data of a personal nature about individual, or proprietary information or data submitted by or pertaining to an institution or organization.
|
|
(b)
|
The Contracting Officer and the Contractor may, by mutual consent, identify elsewhere in this contract specific information and/or categories of information which the Government will furnish to the Contractor or that the Contractor is expected to generate which is confidential. Similarly, the Contracting Officer and the Contractor may, by mutual consent, identify such confidential information from time to time during the performance of the contract. Failure to agree will be settled pursuant to the "Disputes" clause.
|
|
(c)
|
If it is established elsewhere in this contract that information to be utilized under this contract, or a portion thereof, is subject to the Privacy Act, the Contractor will follow the rules and procedures of disclosure set forth in the Privacy Act of 1974, 5 U.S.C. 552a, and implementing regulations and policies, with respect to systems of records determined to be subject to the Privacy Act. (See HHSAR Clause 352.224-70).
|
|
(d)
|
Confidential information, as defined in paragraph (a) of this article, shall not be disclosed without the prior written consent of the individual, institution, or organization.
|
|
(e)
|
Whenever the Contractor is uncertain with regard to the proper handling of material under the contract, or if the material in question is subject to the Privacy Act or is confidential information subject to the provisions of this article, the Contractor shall obtain a written determination from the Contracting Officer prior to any release, disclosure, dissemination, or publication.
|
|
(f)
|
Contracting Officer determinations will reflect the result of internal coordination with appropriate program and legal officials.
|
|
(g)
|
The provisions of paragraph (d) of this article shall not apply to conflicting or overlapping provisions in other Federal, State or local laws.
|
|
H.13.
|
PUBLICATION AND PUBLICITY
|
The Contractor shall acknowledge the support of the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority whenever publicizing the work under this contract in any media by including an acknowledgment substantially as follows:
"This project has been funded in whole or in part with Federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under Contract No. HHSO100201700007C."
Press Releases:
Pursuant to Section 508 of Public Law 105-78, the Contractor shall clearly state, when issuing statements, press releases, requests for proposals, bid solicitations and other documents describing projects or programs funded in whole or in part with Federal money that: (1) the percentage of the total costs of the program or project which will be financed with Federal money; (2) the dollar amount of Federal funds for the project or program; and (3) the percentage and dollar amount of the total costs of the project or program that will be financed by non- Governmental sources.
|
H.14.
|
REPORTING MATTERS INVOLVING FRAUD, WASTE, AND ABUSE
|
Anyone who becomes aware of the existence or apparent existence of fraud, waste and abuse in BARDA funded programs is encouraged to report such matters to the HHS Inspector General's Office in writing or on the Inspector General's Hotline. The toll free number is 1-800-HHS-TIPS (1-800-447-8477). All telephone calls will be handled confidentially. The e-mail address is Htips@os.dhhs.gov and the mailing address is:
Office of Inspector General
Department of Health and Human Services
TIPS HOTLINE P.O. Box 23489
Washington, D.C. 20026
Department of Health and Human Services
TIPS HOTLINE P.O. Box 23489
Washington, D.C. 20026
|
H.15.
|
PROHIBITION ON CONTRACTOR INVOLVEMENT WITH TERRORIST ACTIVITIES
|
The Contractor acknowledges that U.S. Executive Orders and Laws, including but not limited to E.O. 13224 and Pub. L. 107-56, prohibit transactions with, and the provision of resources and support to, individuals and organizations associated with terrorism. It is the legal responsibility of the Contractor to ensure compliance with these Executive Orders and Laws. This clause must be included in all subcontracts issued under this contract.
|
H.16.
|
ACCESS TO DOCUMENTATION/DATA
|
The Government shall have physical and electronic access to all documentation and data generated under this contract, including: all data documenting Contractor performance, all data generated, all communications and correspondence with regulatory agencies and bodies to include all audit observations, inspection reports, milestone completion documents, and all Contractor commitments and responses. The Contractor shall provide the Government with an electronic copy of all correspondence with the FDA within [**] hours of receipt. The Government shall acquire unlimited rights to all data funded under this contract awarded in accordance with FAR Subpart 27.4 and FAR Clause 52.227-14.
|
H.17.
|
IDENTIFICATION AND DISPOSITION OF DATA
|
The Contractor will be required to provide certain data generated under this contract to the Department of Health and Human Services (HHS). HHS reserves the right to review any other data determined by HHS to be relevant to this contract. The Contractor shall keep copies of all data required by the Food and Drug Administration (FDA) relevant to this contract for the time specified by the FDA.
|
H.18.
|
DISSEMINATION OF INFORMATION
|
No information related to data obtained under this contract shall be released or publicized without the prior written consent of the COR, whose approval shall not be unreasonably withheld, conditioned, or delayed, provided that no such consent is required to comply with any law, rule, regulation, court ruling or similar order; for submission to any government entity' for submission to any securities exchange on which the Contractor's (or its parent corporation's) securities may be listed for trading; or to third parties relating to securing, seeking, establishing or maintaining regulatory or other legal approvals or compliance, financing and capital raising activities, or mergers, acquisitions, or other business transactions.
|
H.19.
|
DISSEMINATION OF FALSE OR DELIBERATELY MISLEADING INFORMATION
|
The Contractor shall not use contract funds to disseminate information that is deliberately false or misleading.
|
H.20.
|
INCORPORATION OF TECHNICAL PROPOSAL
|
The Contractor's Final Technical Proposal dated February 1, 2017 submitted in response to RFP-17-100-SOL-00010 is hereby incorporated into the Contract as Attachment #2 in Section J, List of Attachments. The Contractor shall perform the work substantially as set forth in the technical proposal. Any revisions to the technical proposal that would significantly alter the technical approach must be approved in writing by the Contracting Officer.
FAR 52.252-2 Clauses Incorporated by Reference (Feb 1998)
This contract incorporates one or more clauses by reference, with the same force and effect as if they were given in full text. Upon request, the Contracting Officer will make their full text available.
|
B.1.
|
FEDERAL ACQUISITION REGULATION (FAR) (48 CFR Chapter 1) CLAUSES
|
Full text of the FAR clauses may be accessed electronically at:
https://www.acquisition.gov/far/index.html
https://www.acquisition.gov/far/index.html
|
Reg
|
Clause
|
Date
|
Clause Title
|
|
FAR
|
52.202-1
|
Nov 2013
|
Definitions
|
|
FAR
|
52.203-3
|
Apr 1984
|
Gratuities
|
|
FAR
|
52.203-5
|
May 2014
|
Covenant Against Contingent Fees
|
|
FAR
|
52.203-7
|
May 2014
|
Anti-Kickback Procedures
|
|
FAR
|
52.203-8
|
May 2014
|
Cancellation, Recession, and Recovery of Funds for Illegal or Improper Activity
|
|
FAR
|
25.203-10
|
May 2014
|
Price or Fee Adjustment for Illegal or Improper Activity
|
|
FAR
|
52.203-12
|
Oct 2010
|
Limitation on Payments to Influence Certain Federal Transactions
|
|
FAR
|
52.203-14
|
Oct 2015
|
Display of Hotline Poster(s)
|
|
FAR
|
52.203-17
|
Apr 2014
|
Whistleblower Rights
|
|
FAR
|
52.204-4
|
May 2011
|
Printed or Copied Double-Sided on Postconsumer Fiber Content Paper
|
|
FAR
|
52.204-7
|
Jul 2013
|
System for Award Management
|
|
FAR
|
52.204-13
|
Jul 2013
|
System for Award Management Maintenance
|
|
FAR
|
52.209-10
|
Nov 2015
|
Prohibition on Contracting with Inverted Domestic Corporations
|
|
FAR
|
52.211-5
|
Aug 2000
|
Material Requirements
|
|
FAR
|
52.212-1
|
Oct 2016
|
Instructions to Offerors – Commercial Items
|
|
FAR
|
52.215-8
|
Oct 1997
|
Order of Precedence – Uniform Contract Format
|
|
FAR
|
52.218-23
|
Oct 2009
|
Limitations on Pass-Through Charges
|
|
FAR
|
52.219-8
|
Oct 2015
|
Utilization of Small Business Concerns
|
|
FAR
|
52.219-9
|
Oct 2015
|
Small Business Subcontracting Plan
|
|
FAR
|
52.222-1
|
Feb 1997
|
Notice to the Government of Labor Disputes
|
|
FAR
|
52.222-2
|
July 1990
|
Payment for Overtime Premiums
|
|
FAR
|
52.222-29
|
Apr 2015
|
Notification of Visa Denial
|
|
FAR
|
52.223-6
|
May 2001
|
Drug-Free Workplace
|
|
FAR
|
52.224-1
|
Apr 1984
|
Privacy Act Notification
|
|
FAR
|
52.224-2
|
Apr 1984
|
Privacy Act
|
|
FAR
|
52.225-13
|
Jun 2008
|
Restrictions on Certain Foreign Purchases
|
|
FAR
|
52.226-1
|
Jun 2000
|
Utilization of Indian Organizations and Indian-Owned Economic Enterprises
|
|
FAR
|
52.227-1
|
Dec 2007
|
Authorization and Consent
|
|
FAR
|
52.227-2
|
Dec 2007
|
Notice and Assistance Regarding Patent and Copyright Infringement
|
|
FAR
|
52.227-14
|
May 2014
|
Rights in Data – General
|
|
FAR
|
52.229-3
|
Feb 2013
|
Federal, State, and Local Taxes
|
|
FAR
|
52.232-8
|
Feb 2002
|
Discounts for Prompt Payment
|
|
FAR
|
52.232-9
|
Apr 1984
|
Limitation on Withholding of Payments
|
|
FAR
|
52.232-11
|
Apr 1984
|
Extras
|
|
FAR
|
52.232-17
|
May 2014
|
Interest
|
|
FAR
|
52.232-25
|
Jan 2017
|
Prompt Payment
|
|
FAR
|
52.232-39
|
June 2013
|
Unenforceability of Unauthorized Obligations
|
|
FAR
|
52.233-3
|
Aug 1996
|
Protest after Award
|
|
FAR
|
52.233-4
|
Oct 2004
|
Applicable Law for Breach of Contract Claim
|
|
FAR
|
52.242-13
|
Jul 1995
|
Bankruptcy
|
|
FAR
|
52.243-1
|
Aug 1987
|
Changes—Fixed Price
|
|
FAR
|
52.244-6
|
Jan 2017
|
Subcontracts for Commercial Items
|
|
FAR
|
52.246-23
|
Feb 1997
|
Limitation of Liability
|
|
FAR
|
52.248-1
|
Oct 2010
|
Value Engineering
|
|
B.2.
|
DEPARTMENT OF HEALTH AND HUMAN SERVICES ACQUISITION REGULATION (HHSAR) (48 CFR Chapter 3) CLAUSES
|
Full text of the HHSAR clauses can be found at
http://www.hhs.gov/grants/contracts/contract- policies-regulations/hhsar/index.html
http://www.hhs.gov/grants/contracts/contract- policies-regulations/hhsar/index.html
|
HHSAR
|
352.203-70
|
December 2015
|
Anti-Lobbying
|
|
HHSAR
|
352.208-70
|
December 2015
|
Printing and Duplication
|
|
HHSAR
|
352.215-70
|
December 2015
|
Late Proposals and Revisions
|
|
HHSAR
|
352.216-70
|
December 2015
|
Additional Cost Principles
|
|
HHSAR
|
352.222-70
|
December 2015
|
Contractor Cooperation in Equal Employment Opportunity Investigations
|
|
HHSAR
|
352.223-70
|
December 2015
|
Safety and Health
|
|
HHSAR
|
352.224-70
|
December 2015
|
Privacy Act
|
|
HHSAR
|
352.224-71
|
December 2015
|
Confidential Information
|
|
HHSAR
|
352.227-70
|
December 2015
|
Publications and Publicity
|
|
HHSAR
|
352.233-71
|
December 2015
|
Litigation and Claims
|
|
HHSAR
|
352-239.73
|
December 2015
|
Electronic Information and Technology Accessibility Notice
|
|
HHSAR
|
352.270-9
|
December 2015
|
Non-Discrimination for Conscience
|
|
B.3.
|
ADDITIONAL CONTRACT CLAUSES
|
|
B.3.1.
|
ADDITIONAL HHS ACQUISITION REGULATION (HHSAR) CLAUSES – IN FULL TEXT
|
HHSAR 352.211-3 Paperwork Reduction Act (Dec 2015)
|
(a)
|
This contract involves a requirement to collect or record information calling either for answers to identical questions from 10 or more persons other than Federal employees, or information from Federal employees which is outside the scope of their employment, for use by the Federal government or disclosure to third parties; therefore, the Paperwork Reduction Act of 1995 (44 U.S.C 3501 et seq.) shall apply to this contract. No plan, questionnaire, interview guide or other similar device for collecting information (whether repetitive or single time) may be used without the Office of Management and Budget (OMB) first providing clearance. Contractors and the Contracting Officer's Technical Representative shall be guided by the provisions of 5 CFR Part 1320, Controlling Paperwork Burdens on the Public, and seek the advice of the HHS operating division or Office of the Secretary Reports Clearance Officer to determine the procedures for acquiring OMB clearance.
|
|
(b)
|
The Contractor shall not expend any funds or begin any data collection until OMB Clearance is received. Once OMB Clearance is received from the Contracting Officer's Technical Representative, the Contracting Officer shall provide the Contractor with written notification authorizing the expenditure of funds and the collection of data. The Contractor shall allow at least 120 days for OMB clearance. The Contracting Officer will consider excessive delays caused by the Government which arise out of causes beyond the control and without the fault or negligence of the Contractor in accordance with the Excusable Delays or Default clause of this contract.
|
|
B.3.2.
|
ADDITIONAL FEDERAL ACQUISITION REGULATION (FAR) (48 CFR CHAPTER 1) CLAUSES – IN FULL TEXT
|
FAR 52.203-18, Prohibition on Contracting with Entities that Require Certain Internal Confidentiality Agreements – Representation (January 2017)
|
(a)
|
In accordance with section 743 of Division E, Title VII, of the Consolidated and Further Continuing Resolution Appropriations Act, 2015 (Pub. L. 113-235), Government agencies are not permitted to use funds appropriated (or otherwise made available) under that or any other Act for contracts with an entity that requires employees or subcontractors of such entity seeking to report fraud, waste, or abuse to sign internal confidentiality agreements or statements prohibiting or otherwise restricting such employees or subcontractors from lawfully reporting such waste, fraud, or abuse to a designated investigative or law enforcement representative of a Federal department or agency authorized to receive such information.
|
|
(b)
|
The prohibition in paragraph (a) of this provision does not contravene requirements applicable to Standard Form 312, Form 4414, or any other form issued by a Federal department or agency governing the nondisclosure of classified information.
|
|
(c)
|
Representation. By submission of its offer, the Contractor represents that it does not require employees or subcontractors of such entity seeking to report fraud, waste or abuse to sign internal confidentiality agreements or statements prohibiting or otherwise restricting such employees or subcontractors from lawfully reporting such waste, fraud, or abuse to a designated investigative or law enforcement representative of a Federal department or agency authorized to receive such information.
|
(End of provision)
FAR 52.212-4 -- Contract Terms and Conditions -- Commercial Items (January 2017)
(a) Inspection/Acceptance. See required procedures under FAR clause 52.246-2.
(b) Assignment. The Contractor or its assignee may assign its rights to receive payment due as a result of performance of this contract to a bank, trust company, or other financing institution, including any Federal lending agency in accordance with the Assignment of Claims Act (31 U.S.C.3727). However, when a third party makes payment (e.g., use of the Government-wide commercial purchase card), the Contractor may not assign its rights to receive payment under this contract.
(c) Changes. Changes in the terms and conditions of this contract may be made only by written agreement of the parties.
(d) Disputes. This contract is subject to 41 U.S.C. chapter 71, Contract Disputes. Failure of the parties to this contract to reach agreement on any request for equitable adjustment, claim, appeal or action arising under or relating to this contract shall be a dispute to be resolved in accordance with the clause at FAR 52.233-1, Disputes, which is incorporated herein by reference. The Contractor shall proceed diligently with performance of this contract, pending final resolution of any dispute arising under the contract.
(e) Definitions. The clause at FAR 52.202-1, Definitions, is incorporated herein by reference.
(f) Excusable delays. The Contractor shall be liable for default unless nonperformance is caused by an occurrence beyond the reasonable control of the Contractor and without its fault or negligence such as, acts of God or the public enemy, acts of the Government in either its sovereign or contractual capacity, fires, floods, epidemics, quarantine restrictions, strikes, unusually severe weather, and delays of common carriers. The Contractor shall notify the Contracting Officer in writing as soon as it is reasonably possible after the commencement of any excusable delay, setting forth the full particulars in connection therewith, shall remedy such occurrence with all reasonable dispatch, and shall promptly give written notice to the Contracting Officer of the cessation of such occurrence.
(g) Invoice.
(1) The Contractor shall submit an original invoice and three copies (or electronic invoice, if authorized) to the address designated in the contract to receive invoices. An invoice must include
--
--
(i) Name and address of the Contractor;
(ii) Invoice date and number;
(iii) Contract number, contract line item number and, if applicable, the order number;
(iv) Description, quantity, unit of measure, unit price and extended price of the items delivered;
(v) Shipping number and date of shipment, including the bill of lading number and weight of shipment if shipped on Government bill of lading;
(vi) Terms of any discount for prompt payment offered;
(vii) Name and address of official to whom payment is to be sent;
(viii) Name, title, and phone number of person to notify in event of defective invoice; and
(ix) Taxpayer Identification Number (TIN). The Contractor shall include its TIN on the invoice only if required elsewhere in this contract. (x) Electronic funds transfer (EFT) banking information.
(ii) Invoice date and number;
(iii) Contract number, contract line item number and, if applicable, the order number;
(iv) Description, quantity, unit of measure, unit price and extended price of the items delivered;
(v) Shipping number and date of shipment, including the bill of lading number and weight of shipment if shipped on Government bill of lading;
(vi) Terms of any discount for prompt payment offered;
(vii) Name and address of official to whom payment is to be sent;
(viii) Name, title, and phone number of person to notify in event of defective invoice; and
(ix) Taxpayer Identification Number (TIN). The Contractor shall include its TIN on the invoice only if required elsewhere in this contract. (x) Electronic funds transfer (EFT) banking information.
(A) The Contractor shall include EFT banking information on the invoice only if required elsewhere in this contract.
(B) If EFT banking information is not required to be on the invoice, in order for the invoice to be a proper invoice, the Contractor shall have submitted correct EFT banking information in accordance with the applicable solicitation provision, contract clause (e.g., 52.232-33, Payment by Electronic Funds Transfer— System for Award Management, or 52.232-34, Payment by Electronic Funds Transfer—Other Than System for Award Management), or applicable agency procedures.
(C) EFT banking information is not required if the Government waived the requirement to pay by EFT.
(2) Invoices will be handled in accordance with the Prompt Payment Act (31 U.S.C. 3903) and Office of Management and Budget (OMB) prompt payment regulations at 5 CFR part 1315.
(h) Patent indemnity. The Contractor shall indemnify the Government and its officers, employees and agents against liability, including costs, for actual or alleged direct or contributory infringement of, or inducement to infringe, any United States or foreign patent, trademark or copyright, arising out of the performance of this contract, provided the Contractor is reasonably notified of such claims and proceedings.
(i) Payment.
(1) Items accepted. Payment shall be made for items accepted by the Government that have been delivered to the delivery destinations set forth in this contract.
(2) Prompt Payment. The Government will make payment in accordance with the Prompt Payment Act (31 U.S.C. 3903) and prompt payment regulations at 5 CFR Part 1315.
(3) Electronic Funds Transfer (EFT). If the Government makes payment by EFT, see 52.212-5(b) for the appropriate EFT clause.
(4) Discount. In connection with any discount offered for early payment, time shall be computed from the date of the invoice. For the purpose of computing the discount earned, payment shall be considered to have been made on the date which appears on the payment check or the specified payment date if an electronic funds transfer payment is made.
(5) Overpayments. If the Contractor becomes aware of a duplicate contract financing or invoice payment or that the Government has otherwise overpaid on a contract financing or invoice payment, the Contractor shall—
(i) Remit the overpayment amount to the payment office cited in the contract along with a description of the overpayment including the—
(A) Circumstances of the overpayment (e.g., duplicate payment, erroneous payment, liquidation errors, date(s) of overpayment);
(B) Affected contract number and delivery order number, if applicable;
(C) Affected contract line item or subline item, if applicable; and
(D) Contractor point of contact.
(ii) Provide a copy of the remittance and supporting documentation to the Contracting Officer.
(6) Interest.
(i) All amounts that become payable by the Contractor to the Government under this contract shall bear simple interest from the date due until paid unless paid within 30 days of becoming due. The interest rate shall be the interest rate established by the Secretary of the Treasury as provided in 41 U.S.C. 7109, which is applicable to the period in which the amount becomes due, as provided in (i)(6)(v) of this clause, and then at the rate applicable for each six-month period at fixed by the Secretary until the amount is paid.
(ii) The Government may issue a demand for payment to the Contractor upon finding a debt is due under the contract.
(iii) Final decisions. The Contracting Officer will issue a final decision as required by 33.211 if—
(A) The Contracting Officer and the Contractor are unable to reach agreement on the existence or amount of a debt within 30 days;
(B) The Contractor fails to liquidate a debt previously demanded by the Contracting Officer within the timeline specified in the demand for payment unless the amounts were not repaid because the Contractor has requested an installment payment agreement; or
(C) The Contractor requests a deferment of collection on a debt previously demanded by the Contracting Officer (see 32.607-2).
(iv) If a demand for payment was previously issued for the debt, the demand for payment included in the final decision shall identify the same due date as the original demand for payment.
(v) Amounts shall be due at the earliest of the following dates:
(A) The date fixed under this contract.
(B) The date of the first written demand for payment, including any demand for payment resulting from a default termination.
(vi) The interest charge shall be computed for the actual number of calendar days involved beginning on the due date and ending on—
(A) The date on which the designated office receives payment from the Contractor;
(B) The date of issuance of a Government check to the Contractor from which an amount otherwise payable has been withheld as a credit against the contract debt; or
(C) The date on which an amount withheld and applied to the contract debt would otherwise have become payable to the Contractor.
(vii) The interest charge made under this clause may be reduced under the procedures prescribed in 32.608-2 of the Federal Acquisition Regulation in effect on the date of this contract.
(j) Risk of loss. Unless the contract specifically provides otherwise, risk of loss or damage to the supplies provided under this contract shall remain with the Contractor until, and shall pass to the Government upon:
(1) Delivery of the supplies to a carrier, if transportation is f.o.b. origin; or
(k) Taxes. The contract price includes all applicable Federal, State, and local taxes and duties.
(l) Termination for the Government's convenience. The Government reserves the right to terminate this contract, or any part hereof, for its sole convenience. In the event of such termination, the Contractor shall immediately stop all work hereunder and shall immediately cause any and all of its suppliers and subcontractors to cease work. Subject to the terms of this contract, the Contractor shall be paid a percentage of the contract price reflecting the percentage of the work performed prior to the notice of termination, plus reasonable charges the Contractor can demonstrate to the satisfaction of the Government using its standard record keeping system, have resulted from the termination. The Contractor shall not be required to comply with the cost accounting standards or contract cost principles for this purpose. This paragraph does not give the Government any right to audit the Contractor's records. The Contractor shall not be paid for any work performed or costs incurred which reasonably could have been avoided.
(m) Termination for cause. The Government may terminate this contract, or any part hereof, for cause in the event of any default by the Contractor, or if the Contractor fails to comply with any contract terms and conditions, or fails to provide the Government, upon request, with adequate assurances of future performance. In the event of termination for cause, the Government shall not be liable to the Contractor for any amount for supplies or services not accepted, and the Contractor shall be liable to the Government for any and all rights and remedies provided by law. If it is determined that the Government improperly terminated this contract for default, such termination shall be deemed a termination for convenience.
(n) Title. Unless specified elsewhere in this contract, title to items furnished under this contract shall pass to the Government upon acceptance, regardless of when or where the Government takes physical possession.
(o) Warranty. The Contractor warrants and implies that the items delivered hereunder are merchantable and fit for use for the particular purpose described in this contract.
(p) Limitation of liability. Except as otherwise provided by an express warranty, the Contractor will not be liable to the Government for consequential damages resulting from any defect or deficiencies in accepted items.
(q) Other compliances. The Contractor shall comply with all applicable Federal, State and local laws, executive orders, rules and regulations applicable to its performance under this contract.
(r) Compliance with laws unique to Government contracts. The Contractor agrees to comply with 31 U.S.C. 1352 relating to limitations on the use of appropriated funds to influence certain Federal contracts; 18 U.S.C. 431 relating to officials not to benefit; 40 U.S.C. chapter 37, Contract Work Hours and Safety Standards; 41 U.S.C. chapter 87, Kickbacks; 41 U.S.C. 4712 and 10 U.S.C. 2409 relating to whistleblower protections; 49 U.S.C. 40118, Fly American; and 41 U.S.C. chapter 21 relating to procurement integrity.
(s) Order of precedence. Any inconsistencies in this contract shall be resolved by giving precedence in the following order:
(1) The schedule of supplies/services.
(2) The Assignments, Disputes, Payments, Invoice, Other Compliances, Compliance with Laws Unique to Government Contracts, and Unauthorized Obligations paragraphs of this clause.
(3) The clause at 52.212-5.
(4) Addenda to this solicitation or contract, including any license agreements for computer software.
(5) Solicitation provisions if this is a solicitation.
(6) Other paragraphs of this clause.
(7) The Standard Form 1449.
(8) Other documents, exhibits, and attachments.
(9) The specification.
(t) System for Award Management (SAM).
(1) Unless exempted by an addendum to this contract, the Contractor is responsible during performance and through final payment of any contract for the accuracy and completeness of the data within the SAM database, and for any liability resulting from the Government's reliance on inaccurate or incomplete data. To remain registered in the SAM database after the initial registration, the Contractor is required to review and update on an annual basis from the date of initial registration or subsequent updates its information in the SAM database to ensure it is current, accurate and complete. Updating information in the SAM does not alter the terms and conditions of this contract and is not a substitute for a properly executed contractual document.
(2)
(i) If a Contractor has legally changed its business name, "doing business as" name, or division name (whichever is shown on the contract), or has transferred the assets used in performing the contract, but has not completed the necessary requirements regarding novation and change-of-name agreements in Subpart 42.12, the Contractor shall provide the responsible Contracting Officer a minimum of one business day's written notification of its intention to:
(A) Change the name in the SAM database;
(B) Comply with the requirements of Subpart 42.12 of the FAR;
(C) Agree in writing to the timeline and procedures specified by the responsible Contracting Officer. The Contractor must provide with the notification sufficient documentation to support the legally changed name.
(ii) If the Contractor fails to comply with the requirements of paragraph (t)(2)(i) of this clause, or fails to perform the agreement at paragraph (t)(2)(i)(C) of this clause, and, in the absence of a properly executed novation or change-of-name agreement, the SAM information that shows the Contractor to be other than the Contractor indicated in the contract will be considered to be incorrect information within the meaning of the "Suspension of Payment" paragraph of the electronic funds transfer (EFT) clause of this contract.
(3) The Contractor shall not change the name or address for EFT payments or manual payments, as appropriate, in the SAM record to reflect an assignee for the purpose of assignment of claims (see FAR Subpart 32.8, Assignment of Claims). Assignees shall be separately registered in the SAM database. Information provided to the Contractor's SAM record that indicates payments, including those made by EFT, to an ultimate recipient other than that Contractor will be considered to be incorrect information within the meaning of the "Suspension of payment" paragraph of the EFT clause of this contract.
(4) Offerors and Contractors may obtain information on registration and annual confirmation requirements via SAM accessed through https://www.acquisition.gov.
(u) Unauthorized Obligations.
(1) Except as stated in paragraph (u)(2) of this clause, when any supply or service acquired under this contract is subject to any End Use License Agreement (EULA), Terms of Service (TOS), or similar legal instrument or agreement, that includes any clause requiring the Government to indemnify the Contractor or any person or entity for damages, costs, fees, or any other loss or liability that would create an Anti-Deficiency Act violation (31 U.S.C. 1341), the following shall govern:
(i) Any such clause is unenforceable against the Government.
(ii) Neither the Government nor any Government authorized end user shall be deemed to have agreed to such clause by virtue of it appearing in the EULA, TOS, or similar legal instrument or agreement. If the EULA, TOS, or similar legal instrument or agreement is invoked through an "I agree" click box or other comparable mechanism (e.g., "click-wrap" or "browse-wrap" agreements), execution does not bind the Government or any Government authorized end user to such clause.
(iii) Any such clause is deemed to be stricken from the EULA, TOS, or similar legal instrument or agreement.
(2) Paragraph (u)(1) of this clause does not apply to indemnification by the Government that is expressly authorized by statute and specifically authorized under applicable agency regulations and procedures.
(v) Incorporation by reference. The Contractor's representations and certifications, including those completed electronically via the System for Award Management (SAM), are incorporated by reference into the contract.
(End of Clause)
FAR 52.212-5 Contract Terms and Conditions Required to Implement Statutes or Executive Orders – Commercial Items (Deviation 2013-O0019) (January 2017)
a) Comptroller General Examination of Record. The Contractor shall comply with the provisions of this paragraph (a) if this contract was awarded using other than sealed bid, is in excess of the simplified acquisition threshold, and does not contain the clause at 52.215-2, Audit and Records -- Negotiation.
(1) The Comptroller General of the United States, or an authorized representative of the Comptroller General, shall have access to and right to examine any of the Contractor's directly pertinent records involving transactions related to this contract.
(2) The Contractor shall make available at its offices at all reasonable times the records, materials, and other evidence for examination, audit, or reproduction, until 3 years after final payment under this contract or for any shorter period specified in FAR Subpart 4.7, Contractor Records Retention, of the other clauses of this contract. If this contract is completely or partially terminated, the records relating to the work terminated shall be made available for 3 years after any resulting final termination settlement. Records relating to appeals under the disputes clause or to litigation or the settlement of claims arising under or relating to this contract shall be made available until such appeals, litigation, or claims are finally resolved.
(3) As used in this clause, records include books, documents, accounting procedures and practices, and other data, regardless of type and regardless of form. This does not require the Contractor to create or maintain any record that the Contractor does not maintain in the ordinary course of business or pursuant to a provision of law.
(b)
(1) Notwithstanding the requirements of any other clause in this contract, the Contractor is not required to flow down any FAR clause, other than those in this paragraph (b)(1) in a subcontract for commercial items. Unless otherwise indicated below, the extent of the flow down shall be as required by the clause—
(i) 52.203-13, Contractor Code of Business Ethics and Conduct (Oct 2015) (41 U.S.C. 3509).
(ii) 52.219-8, Utilization of Small Business Concerns (Oct 2014) (15 U.S.C. 637(d)(2) and (3)), in all subcontracts that offer further subcontracting opportunities. If the subcontract (except subcontracts to small business concerns) exceeds $650,000 ($1.5 million for construction of any public facility), the subcontractor must include 52.219-8 in lower tier subcontracts that offer subcontracting opportunities.
(iii) 52.222-17, Nondisplacement of Qualified Workers (May 2014) (E.O. 13495). Flow down required in accordance with paragraph (1) of FAR clause 52.222-17.
(iv) 52.222-21, Prohibition of Segregated Facilities (Apr 2015). (v) 52.222-26, Equal Opportunity (Sep 2016) (E.O. 11246).
(vi) 52.222-35, Equal Opportunity for Veterans (Oct 2015) (38 U.S.C. 4212).
(vii) 52.222-36, Equal Opportunity for Workers with Disabilities (Jul 2014) (29 U.S.C. 793).
(viii) 52.222-62 Paid Sick Leave Under Executive Order 13706 (JAN 2017) (E.O. 13706).
(ix) 52.222-37, Employment Reports on Veterans (Feb 2016) (38 U.S.C. 4212).
(x) 52.222-40, Notification of Employee Rights Under the National Labor Relations Act (Dec 2010) (E.O. 13496). Flow down required in accordance with paragraph (f) of FAR clause 52.222-40.
(xi) 52.222-41, Service Contract Labor Standards (May 2014), (41 U.S.C. chapter 67).
(xii) ______ (A) 52.222-50, Combating Trafficking in Persons (Mar 2015) (22 U.S.C. chapter 78 and E.O. 13627).
_______ (B) Alternate I (Mar 2015) of 52.222-50 (22 U.S.C. chapter 78 E.O. 13627).
(xiii) 52.222-51, Exemption from Application of the Service Contract Labor Standards to Contracts for Maintenance, Calibration, or Repair of Certain Equipment--Requirements (May 2014) (41 U.S.C. chapter 67.)
(xiv) 52.222-53, Exemption from Application of the Service Contract Labor Standards to Contracts for Certain Services--Requirements (May 2014) (41 U.S.C. chapter 67)
(xv) 52.222-54, Employment Eligibility Verification (Oct 2015).
(xvi) 52.222-55, Minimum Wages Under Executive Order 13658 (Dec 2015) (E.O. 13658).
(xvii) 52.222-59, Compliance with Labor Laws (Executive Order 13673) (Oct 2016) (Applies at $50 million for solicitations and resultant contracts issued from October 25, 2016 through April 24, 2017; applies at $500,000 for solicitations and resultant contracts issued after April 24, 2017).
Note to paragraph (b)(1)(xvi): By a court order issued on October 24, 2016, 52.222-59 is enjoined indefinitely as of the date of the order. The enjoined paragraph will become effective immediately if the court terminates the injunction. At that time, DoD, GSA, and NASA will publish a document in the Federal Register advising the public of the termination of the injunction.
(xviii) 52.222-60, Paycheck Transparency (Executive Order 13673) (Oct 2016). (xix) 52.225-26, Contractors Performing Private Security Functions Outside the United States (Jul 2013) (Section 862, as amended, of the National Defense Authorization Act for Fiscal Year 2008; 10 U.S.C. 2302 Note).
(xx) 52.226-6, Promoting Excess Food Donation to Nonprofit Organizations. (May 2014) (42 U.S.C. 1792). Flow down required in accordance with paragraph (e) of FAR clause 52.226-6.
(xxi) 52.247-64, Preference for Privately-Owned U.S. Flag Commercial Vessels (Feb 2006) (46 U.S.C. Appx 1241(b) and 10 U.S.C. 2631). Flow down required in accordance with paragraph (d) of FAR clause 52.247-64.
(2) While not required, the contractor may include in its subcontracts for commercial items a minimal number of additional clauses necessary to satisfy its contractual obligations.
(End of Clause)
|
1.
|
Security Plan
|
|
2.
|
Technical Proposal
|
|
3.
|
Invoice Instructions for Fixed Price Contracts
|
|
4.
|
Sample Invoice Form
|
|
5.
|
SF-LLL, Disclosure of Lobbying Activities
|
|
6.
|
ACH Vendor/ Miscellaneous Payment Enrollment Form
|
|
7.
|
Small Business Subcontracting Plan
|
|
8.
|
Responses to Negotiation Questions
|
F. Security
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of two pages were omitted. [**]
G. Quality Systems
Emergent has defined the Quality Management System (QMS) requirements for phase appropriate compliance from proof-of-concept and pre-clinical stages through commercial manufacturing.
Emergent's integrated QMS is regulated by our Quality Policy Manual. The SOP-supported manual describes QMS policies and activities performed at our Lansing facility. The Quality Policy Manual aligns with Emergent's strategic plan, corporate objectives, and cGMP regulations and expectations.
Each component of our QMS complies with applicable ICH and FDA regulatory standards and requirements applicable for each stage of the product lifecycle. Emergent's Quality Assurance Department will ensure internal compliance to these standards as well as compliance of subcontractors performing work on Emergent's behalf. Our QMS includes all of the following components:
|
·
|
management responsibility and review
|
|
·
|
personnel qualification and training
|
|
·
|
document and data control
|
|
·
|
validation
|
|
·
|
equipment and facilities control
|
|
·
|
supplier quality and materials management control
|
|
·
|
production and in-process controls
|
|
·
|
non-conformance handling
|
|
·
|
change control
|
|
·
|
corrective and preventive action
|
The senior leadership team at each site is ultimately responsible for ensuring an effective QMS is in place, demonstrating strong support of the QMS and continuous improvement efforts, and ensuring that roles, responsibilities and authorities are defined, communicated, and implemented at all levels in the organization.
Emergent will ensure employees involved with vaccine operations have the necessary education, training, and experience appropriate to their positions. Current job descriptions and curricula vitae, or similar documentation, are established and maintained for all personnel responsible for conducting or overseeing activities.
Training records are maintained for employees, and periodic and systematic reviews of personnel training records are conducted to ensure that employees are receiving the training demanded by their job or function.
Emergent will ensure established SOPs are in place to control all documents and processes required for phase appropriate compliance activities. The document management program prevents unapproved revisions to controlled documents that may in turn affect the safety, quality, identity, potency and purity of the Anthrax vaccine. Changes to documents are reviewed and approved by designated individuals and the changes are communicated through training on the revised procedure. Controlled documents are available to personnel conducting the work.
Data is fully traceable from the controlled record to the raw data and is maintained and stored in such a way as to remain legible, readily identifiable, and retrievable. Original records are archived in compliance with document control and records management procedures.
Materials and services shall only be purchased from suppliers that have been appropriately qualified. A material qualification process is in place to ensure that materials are fit for their intended use.
Materials are controlled from the time of receipt to maintain complete identification and traceability at all times in accordance with established SOPs and compliance regulations.
Emergent will ensure product release does not proceed until all activities have been completed, documentation is available, and disposition has been authorized by QA. The materials management program will ensure proper segregation of materials that are under quarantine, release, and reject.
|
Volume I – Technical Proposal Original
|
Offerer:
Emergent Biodefense Operations Lansing LLC
3500 N. Martin Luther King Jr. BLVD
Lansing, Ml 48906
DUNS: [**] CAGE CODE: 1HOB6
|
Administrative Business Contact:
[**]
Phone: [**]
E-Mail· [**]
|
Prepared for:
[**], Contracting Officer
Phone: [**]
HHS/OS/ASPR/AMCG
330 Independence AV, SW, RM G-460 Washington, DC 20024
Email: [**]
|
|
/s/ [**]
|
|
|
[**]
This proposal is predicated upon all the terms and conditions contained in the above referenced solicitation.
|
|
|
Technical Contacts:
[**]
Phone: [**]
E-Mail· [**]
[**]
Phone: [**]
E-Mail· [**]
|
Cognizant Audit Office:
The National Institutes of Health Office of Acquisition Mgmt. and Policy Division of Financial Advisory Services
6100 Executive Blvd., Room 6805
Rockville, MD 20892
|
Solicitation Amendments:
• N/A
By submitting this proposal, the Offeror, if selected for discussions, grants the Contracting Officer or an authorized representative the right to examine, at any time before an award, any of those books, records, documents, or other records directly pertinent to the information requested or submitted.
Unless disclosure is required by the Freedom of Information Act (5 U.S.C. 552, as amended) (the Act), and determined by Freedom of Information (FOI) officials of the Department of Health and Human Services (the Department) according to the Act, any data contained in the portions of this proposal which have been specifically identified by page number, paragraph, sheet, etc. by the Offeror as containing restricted information shall not be used or disclosed except for evaluation purposes. The Offeror acknowledges that the Department may not be able to withhold a record (data, document, etc.) or deny access to a record requested pursuant to the Act and that the Department's FOI officials must make that determination. The Offeror hereby agrees that the Government is not liable for disclosure if the Department has determined that disclosure is required by the Act. If a contract is awarded to the Offeror as a result of, or in connection with, this proposal submission, the Government shall have the right to use or disclose the data to the extent provided in the awarded contract. Proposals not resulting in a contract remain subject to the Act. The Offeror also agrees that the Government is not liable for disclosure or use of unmarked data and may use or disclose the data for any purpose, including the release of the information pursuant to requests under the Act. Data subject to this restriction are contained in all marked proposal pages.
TABLE OF CONTENTS
PROPOSAL CROSS-REFERENCE TO SOLICITATION STATEMENT OF WORK & EVALUATION CRITERIA
|
C.1. VACCINE PRODUCTION AND CGMP COMPLIANCE
|
|
|
C.1.1 The Contractor shall manufacture BioThrax in accordance with current Good Manufacturing Practices (cGMP) guidelines
|
Sect IV.A
|
|
C.1.2 BioThrax® must be delivered on any business day, except Federal holidays, within the scheduled month in accordance with the targeted delivery schedule. The Contractor shall notify the Government promptly upon becoming aware of any deviations from the targeted delivery schedule. All changes to the targeted delivery schedule must be approved by the Contracting Officer and/or the Contracting Officer's Representative (COR).
|
Appendix B
|
|
C.1.3 Quantities for each scheduled delivery shall be of a specific quantity
|
Appendix B
|
|
C.1.4. The Contractor shall perform all requisite assays and release tests, including but not limited to potency, identity, and stability testing in accordance with the Food Drug Administration (FDA) approved Biologic License Application (BLA-License Number 1755, STN 103821, and any approved change).
|
Section IV.A
|
|
C.1.5. All BioThrax® delivered under this contract must be labeled with an expiration date consistent with its current product license at the time of manufacture.
|
Sections IV.A, V.E
|
|
C.1.6. The Contractor shall provide primary and secondary points of contact that shall be available 24 hours per day, seven days per week to be notified in case of a public health emergency.
|
Section VI.C
|
|
C.1.7. The Contractor shall report to the Government material correspondence from the FDA regarding the quality, safety, or efficacy of BioThrax®.
|
Section VI.A
|
|
C.1.8. The Contractor shall provide the Government with access to and/or provide copies of the following documents: (1) Form 483s form FDA inspections of Contractor's Lansing facility, (2) Establishment Inspection Reports (EIRs) from FDA inspections of Contractor's Lansing facility; (3) Warning Letters relating to BioThrax®; and Contractor's Annual Safety Report to FDA regarding BioThrax®. These documents will be provided to the Contracting Officer within 2 business days of receipt.
|
Section VI.A
|
|
C.1.9. The Contractor shall notify the Government of any issues with the safety and efficacy of BioThrax® and/or manufacturing or quality of the FDA-licensed production lines at the Contractor's Lansing facility within two business days of the determination of potential to be reported to FDA.
|
Section VI.A
|
|
C.1.10. The Government will have the option to conduct site inspections of the Contractor's Lansing facility during the period of performance of the contract. Such inspections will be performed by the COR or the COR's designee(s).
|
Section VI.A
|
|
C.1.11. If the contractor should obtain FDA approval for the manufacture and production of BioThrax® having [**] while under this contract, the Government will accept delivery of those doses with the [**] in addition to doses with a [**]. The Contractor may invoice only for those doses actually delivered under this contract in accordance with Section B.
|
Section IV.A
|
|
C.1.12. The product must be delivered in accordance with cGMP guidelines.
|
Section IV.B
|
|
C.1.13. The Contractor shall notify BARDA at least [**] days' prior of estimated shipment of product. At least [**] business days prior to the product being ready for shipment to DSNS, the Contractor shall obtain delivery address from the Contracting Officer and provide to the Contracting Officer and COR the following:
a. The date the product will be ready for loading on the truck(s) and the intended delivery date of product to the DSNS
b. Certificate(s) of Analysis d. FDA Lot Release(s)
c. Number of pallets, vials, and doses to be loaded and delivered to DSNS
|
Sections IV.B, VI.A
|
|
C.1.14. At least [**] hours before each scheduled delivery, the Contractor shall provide the following to the Contracting Officer and COR:
a. Packing Slip
b. Actual number of pallets, vials and doses to be loaded
c. Diagram of product shipment pallet (how many vials per box, per pallet)
|
Sections IV.B, VI.A
|
|
C.1.15. Within [**] hours after the product has been delivered to the DSNS, the Contractor shall provide to the Contracting Officer and COR:
a. The remaining ambient exposure time letter disclosing accumulated ambient temperature exposure until the point that BARDA (or DSNS-designated personnel) assumed responsibility for temperature control, per Section F, for each lot from the Contractor's Quality Department. The letter must indicate that the product was manufactured and released in accordance with cGMP and has met all acceptance criteria to allow for Government distribution.
|
Section VI.A
|
|
C.1.16. Funds provided shall be paid on a price per doses basis only on those products delivered and accepted to DSNS under contract.
|
See Business Vol. II
|
|
C.1.17. Under CLIN 0001 of this contract, the products shall have an [**] product. The Contractor shall target [**] of the [**] remaining when the Government takes delivery of the product.
|
Section IV.B
|
|
C.2. PROJECT MANAGEMENT & RISK MITIGATION OBJECTIVES
|
|
|
C.2.1 INTEGRATED MASTER PLAN AND EARNED VALUE MANAGEMENT
|
|
|
C.2.1.1 The Offeror shall provide an Integrated Master Project Plan (including tabular and Gantt forms) to BARDA that clearly indicates the critical path to support product approval. The Integrated Master Project Plan shall outline key, critical path milestones, with "Go/No Go" decision criteria and a contract Work Breakdown Structure (due within [**] days of contract award with updates as requested by the COR).
|
Section VII
|
|
C.2.1.2 The Offeror shall submit an updated Integrated Master Schedule in an approved format.
|
Section VII
|
|
C.2.1.3 The Offeror shall submit a plan for a Performance measurement Baseline Review (PMBR). Section VII
|
|
|
C.2.2 RISK MANAGEMENT OBJECTIVES
|
|
|
C.2.2.1 The Offeror shall develop and maintain an acceptable risk management plan. If changes are needed to the risk management plan during the execution of the contract, the Offeror shall submit draft changes to risk management plan to USG for approval prior to implementation.
|
Sections VI.D,VII
|
|
C.2.2.2 The Offeror shall participate in regular meetings to coordinate and oversee the work performed.
|
Section VI.A
|
|
C.2.2.3 The Offeror shall provide a list of individuals to serve as primary and secondary points of contact who will be available 24 hours a day, seven days a week, to be notified in case of a public health emergency.
|
Section VI.C
|
|
C.2.2.4 The Offeror shall provide a security plan, which is associated with all aspects of manufacture of product, process, storage, and inventory of the FDP including when procured for use under EUA and intended for delivery to the CDC/SNS.
|
Section VI.F
|
|
C.3. MEETING/SITE VISITS/AUDITS
|
Section VI.A
|
|
C.4. PRODUCT DELIVERIES
|
|
|
C.4.1 TEMPERATURE CONTROL AND MONITORING, FOB Destination Deliveries
|
Section IV.B
|
|
C.4.2 DSNS QUALITY CONTROL UNIT (QCU) ACCEPTANCE PROCEDURE FOR BIOTHRAX (AVA)
|
Section IV.B
|
|
C.4.3 ACCEPTANCE PROCESS AND TIMEFRAME (FOB DESTINATION DELIVERY)
|
Section IV.B
|
|
C.4.4 BARDA RELEASE FOR BioThrax
|
Section IV.B
|
|
C.5. REPORTING REQUIREMENTS
|
Section VI.A
|
|
M.1 FAR 52.212-2 EVALUATION – COMMERCIAL ITEMS (OCT 2014)
|
|
|
M.1.(a)1. The ability to manufacture BioThrax® vaccine in accordance with current Good Manufacturing Practices (cGMP) guidelines
|
Section IV.A
|
|
M.1.(a)2. The ability to manufacture and deliver [**] doses of Final Drug Product (FDP) with an acceptable delivery schedule.
|
Section V.E
|
|
M.1.(a)3. The ability to perform all requisite assays and release testing in accordance with the FDA approved Biologic License Application (BLA).
|
Section IV.A
|
|
M.1.(a)4. The ability to package doses according to FDA approved labeling and packaging requirements and ship doses to SNS facilities for USG acceptance
|
Section V.D
|
|
M.1(a)5. The product dose price shall be consistent with current dose prices under active USG procurement mechanisms
|
See Business Vol. II
|
(This page intentionally left blank.)
Emergent Biodefense Operations Lansing LLC1 is pleased to submit this proposal in response to Solicitation 17-100-SOL-00010 for procurement of [**] doses of anthrax vaccine for Biomedical Advanced Research and Development Agency (BARDA) to be delivered to the Strategic National Stockpile (SNS). Emergent is uniquely positioned as the manufacturer of the only FDA-licensed anthrax vaccine for human use, BioThrax® (Anthrax Vaccine Adsorbed). Emergent has:
|
·
|
A state-of-the-art current Good Manufacturing Practices (cGMP) high capacity manufacturing facility at its Lansing, Michigan campus
|
|
·
|
Available manufacturing capacity
|
|
·
|
Sufficient inventory and supply lines for required raw materials
|
|
·
|
Decades of experience and know-how in the manufacturing of BioThrax
|
|
·
|
Significant experience managing large and small business subcontractors currently utilized for fill-finish and logistics functions
|
This proposal outlines Emergent's unique product and capabilities to fulfill this stated need.
1 Emergent BioDefense Operations Lansing LLC is a subsidiary of Emergent BioSolutions Inc. (EBSI). Each EBSI company will be individually or collectively referred to as "Emergent" throughout this document.
(This page intentionally left blank.)
BioThrax is the only FDA-licensed vaccine for the prevention of disease caused by Bacillus anthracis in persons 18 through 65 years of age. BioThrax is approved for pre-exposure prophylaxis of disease in persons at high risk of exposure and for post-exposure prophylaxis of disease following suspected or confirmed Bacillus anthracis exposure, when administered in conjunction with recommended antibacterial drugs. The efficacy of BioThrax for post- exposure prophylaxis is based solely on studies in animal models of inhalational anthrax. BioThrax is manufactured from a sterile culture filtrate, made from a non-virulent strain of Bacillus anthracis, and contains no dead or live bacteria. BioThrax contains aluminum hydroxide as an adjuvant in a suspension for intramuscular or subcutaneous injection, administered in 0.5 mL doses from a 5 mL multidose vial. For pre- exposure prophylaxis, a six-month three-dose primary series is followed by booster doses at 6 and 12 months and annually thereafter. For post-exposure prophylaxis, BioThrax is administered at 0, 2, and 4 weeks post-exposure in conjunction with appropriate antimicrobial therapy.
The BioThrax manufacturing process was first licensed in the United States in the 1970 and was originally designed to produce several thousand doses of vaccine annually. Demand for the vaccine remained relatively low, confined largely to veterinarians and mill workers involved in the processing of animal hair and hides. In the 1990s, the threat of the use of Bacillus anthracis as a bioterrorism weapon became a major concern to the military, leading the U.S. Department of Defense (DoD) to vaccinate select troops during the Gulf War and to establish the Anthrax Vaccine Immunization Program (AVIP) in 1998. Increased demand for the vaccine has driven capacity expansion at the Lansing facility, most recently with the approval of our large scale, large volume facility that has almost tripled our annual production capacity. While numerous modifications and expansions have occurred over time, the manufacturing process has remained similar since its original licensure.
The U.S. National Institutes of Health (NIH) originally approved the manufacture and sale of BioThrax by the Michigan Department of Public Health in 1970. In 1972, responsibility for approving biological products transferred from the NIH to the U.S. Food and Drug Administration (FDA). The FDA set out to categorize the products according to evidence of safety and effectiveness and determine if the products should remain approved and on the market. In December 1985, the FDA issued a proposed rule containing a finding that BioThrax was safe and effective. However, the FDA did not finalize that proposed rule pursuant to applicable notice and comment requirements. In December 2005, based on a review of data from the study used to support the original marketing approval of BioThrax and other studies of the use of BioThrax in humans, including studies by the Centers for Disease Control (CDC) and the DoD, the FDA issued a final order regarding BioThrax. In the final order, the FDA affirmed its approval. In 2009, FDA approved intramuscular administration and reduced the primary pre-exposure vaccination series administrations from 6 doses to 5 doses over 18 months as well as 4-year expiry dating. In 2012, FDA approved a change to the pre-exposure primary series to a more manageable 3 doses over 6 months. Most recently, in 2015, FDA approved BioThrax for post-exposure prophylaxis with a 3-dose series of subcutaneous injections at 0, 2, and 4 weeks administered in conjunction with recommended antibacterial drugs. This marked the first FDA approval for a vaccine indication under the Animal Rule (21 CFR 601.90 through 601.95). Most recently, in 2016, FDA approved the 1,320 L scaled-up manufacture of BioThrax as an alternate for earlier manufacturing lines at the 110 L scale. This expands BioThrax manufacturing capacity up to approximately 25 million doses of BioThrax vaccine annually.
All of these regulatory milestones were pursued and achieved to fulfill U.S. Government needs for improving the efficacy and safety profiles of BioThrax, enhancing patient adherence with the vaccination regimen and reducing the number of doses required to protect each patient – while sustaining a manufacturing capacity commensurate with U.S. Government demand.
BioThrax is licensed for pre-exposure vaccination in the following countries:
|
·
|
Singapore - Health Sciences Authority (HSA) approved 2011
|
|
·
|
Germany – Paul-Ehrlich Institut (PEI) approved 2013
|
The most common (≥10%) local (injection-site) adverse reactions observed in clinical studies were tenderness, pain, erythema, edema, and arm motion limitation. The most common (≥5%) systemic adverse reactions were muscle aches, headache, and fatigue. Acute allergic reactions, including anaphylaxis, have occurred with BioThrax.
Vaccination with BioThrax should be avoided by individuals with a history of anaphylactic or anaphylactic-like reaction following a previous dose of BioThrax or any component of the vaccine. If BioThrax is used during pregnancy, or if the patient becomes pregnant during the immunization series, the patient should be apprised of the potential hazard to the fetus. Pregnant women should not be vaccinated unless the potential benefits of vaccination have been determined to outweigh the potential risk to the fetus. It is not known whether BioThrax is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when BioThrax is administered to a nursing woman.
The stopper of the vial contains natural rubber latex and may cause allergic reactions to patients with a possible history of latex sensitivity.
BioThrax may not protect all individuals vaccinated, particularly patients with impaired immune responses due to congenital or acquired immunodeficiency, or immunosuppressive therapy.
Individuals are not considered protected until they have completed the three-dose primary vaccination series.
The efficacy of BioThrax for post-exposure prophylaxis is based solely on studies in animal models of inhalational anthrax.
BioThrax is manufactured at Emergent's facility in Lansing, Michigan in accordance with cGMP. BioThrax to be provided in response to this RFP will be produced at Emergent's manufacturing line located on Emergent's Lansing, Michigan campus. All manufacturing processes have been validated and are approved by the FDA/CBER (BLA-License Number 1755, STN 103821). Emergent has extensive experience in all aspects of regulatory compliance with regard to vaccine manufacturing, and is compliant with Part 21 of the Code of Federal Regulations, which governs the manufacture of biological products. The FDA has repeatedly concluded that BioThrax is a safe and effective vaccine to protect against anthrax infection, including inhalation anthrax.
BioThrax is subject to the FDA's "lot release" program, which is used to verify that each lot of vaccine meets certain criteria, including sterility, potency and specified levels of certain ingredients (See FDA Final Order, 70 Fed. Reg. at 75,194). All lots of BioThrax released for administration to military personnel and other individuals meet these criteria. (Id.) In addition, FDA inspects all biological product license holders on a biennial basis and at additional times when FDA concludes that more regulatory oversight is warranted. (Id.) Emergent's most recent inspection by FDA for B12 was in April 2016. Emergent also hosted a pre-approval inspection (PAI) for Building 55 (B55) in June 2016. At the conclusion of this PAI, the company received a No Action Indicated decision and no Form 483 observations, followed by FDA approval in August 2016.
Emergent's FDA-approved testing program includes potency and stabilit y testing. BioThrax lot release testing is conducted on every vaccine lot prior to distribution and includes the following tests: [**]. Emergent's FDA-approved stability testing program is conducted on selected lots as described below and includes testing for [**].
The stability of BioThrax is continuously evaluated at ICH-defined intervals under long-term storage at [**]°C. Emergent places at least [**] into the stability program. BioThrax is currently approved for use up to [**] from the date of manufacture and all delivered product will be labeled with an expiration date in accordance with the current product license.
Emergent will coordinate deliveries of funded doses under this contract with the Contracting Officer and the Contracting Officer's Representative at least [**] days prior to delivery dates. SNS delivery destination will be provided by the Government at least [**] business days prior to the shipment date. All scheduled delivery dates will be on a business day, except Federal holidays. Any changes to the delivery schedule will require Government approval. Emergent will provide estimated number of doses and lots to be delivered at least [**] business days prior to the delivery date. The actual delivery date, dose totals, dose expiration dates, and FDA lot release letters will be prepared and supplied by Emergent at least [**] hours prior to each delivery date. Emergent will communicate with the Contracting Officer and Contracting Officer's Technical Representative (or their designees) regarding any potential changes from the delivery schedule.
Emergent will maintain product temperature control in our validated [**]º C storage facilities. Emergent will prepare orders for BioThrax per its Standard Operating Procedures (SOP). The material will be packed in shipping packages, up to [**] vials per shipping package. Multiple packages can be palletized using a 40" x 48" plastic pallet and will be secured for transit to the pallet utilizing plastic shrink-wrap material. One lot or Batch of BioThrax is packaged on one pallet.
Emergent will comply with Section C.4 of the Solicitation includes the parameters and requirements for each delivery and acceptance of goods delivered under this contract.
Section B. 2.2 requires delivered [**] product to have ≥[**] months remaining expiry dating upon date of delivery.
|
·
|
The requirement of ≥[**] months remaining expiry dating upon date of delivery was modified in the Scope of Work according to ACMG's response to Emergent's Question 3, received February 1, 2017. As a consequence of this Emergent will deliver product with ≥[**] months remaining expiry dating upon date of delivery.
|
Emergent will utilize temperature-monitoring devices (e.g. TempTale) packaged with the product during loading and shipment to the designated SNS facility. In-transit temperature data will be evaluated for delivery acceptance in accordance with our current license.
Emergent will facilitate and manage the shipment and cGMP delivery per current approved SOPs. [**].
Emergent takes great lengths in ensuring security of the payload. [**].
Emergent can [**].
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of five pages were omitted. [**].
Emergent will meet regularly with the Contracting Officer and/or Contracting Officer's Representative, including, but not limited to the following:
|
·
|
A Kickoff meeting
|
|
·
|
Status update meetings/teleconferences as required ([**] frequency is preferred given the scope of work)
|
|
·
|
Site visits and other meetings to discuss technical, regulatory and ethical aspects of performance of a contract awarded under this Solicitation
|
The Contracting Officer Representative and/or their designee(s) shall have the option to conduct site audits and inspections of Emergent's Lansing facility during the period of performance of this contract.
Regular performance reports will be provided according to the schedule in Solicitation section C. 5 unless otherwise specified by the Contracting Officer. Given the scope of this work, Emergent requests that:
|
·
|
The Monthly progress report frequency be revised to Quarterly
|
|
·
|
Submission of the Contract Final Report be tied to the last accepted delivery of goods under this contract, and formally mark the end of the period of performance under the contract
|
|
·
|
Earned Value Management (EVM) requirements were removed from the Scope of Work according to ACMG's response to Emergent's Question 1, received February 1, 2017. As a consequence of this Emergent will not submit an EVM plan or associated reports per section C.5.E.a in the Solicitation.
|
All of the communication requirements in Section C.1 are agreed to, subject to the following exceptions that Emergent requests that the Government consider revising in order to align with identical communication and documentation requirements already agreed under our Current BioThrax supply contract with the CDC. Emergent proposes these revisions to insure consistency in communication with the SNS:
Solicitation Section C.1.7 requires Emergent to report to the Government material correspondence with the FDA related to quality, safety or efficacy of BioThrax.
|
·
|
Emergent proposes sharing all such relevant material information as it does with all commercial customers related to FDA communications for BioThrax.
|
Solicitation Section C.1.8 requires specific regulatory documentation provided to/from FDA to be accessible and/or sent to the Government within [**] business days of sending or receipt.
|
·
|
Emergent proposes that this reporting requirement be covered by the modifications to Section C.1.7 above.
|
Solicitation Section C.1.9 requires Emergent to notify the Government of any issues with the safety and efficacy of BioThrax and/or manufacturing or quality of the FDA-licensed production lines within two business days of the determination of potential to be reported to FDA.
|
·
|
Emergent proposes notifying the Government within [**] days after a Biologic Process Deviation Report (BPDR) is submitted and including these notifications in periodic reports.
|
Section C.1.13 requires that Certificates of Analysis and FDA Lot Releases be provided to the Government at least [**] business days before the Delivery Date.
|
·
|
These communication requirements were modified in the Scope of Work according to ACMG's response to Emergent's Question 4, received February 1, 2017. As a consequence of this Emergent will provide Certificates of Analysis and FDA Lot Releases at least [**] prior to delivery.
|
Section C.5.D is related to FDA Regulatory correspondence, meeting summaries and submissions.
|
·
|
These Regulatory communication requirements were modified in the Scope of Work according to ACMG's response to Emergent's Question 2, received February 1, 2017. As a consequence of this Emergent shall provide a copy of any regulatory communications (e.g. meeting minutes, submissions, etc.) that occur during the contract period of performance pertaining to the product being procured (i.e. BioThrax). Any pertinent regulatory communications or issues that may impact the product or ability of Emergent to complete delivery of the product on this particular contract shall be made known to BARDA/AMCG until delivery of all [**] doses are completed.
|
Table 5-1 outlines audits and site visits conducted by the USG from 2011 to the present.
|
Year
|
Agency
|
Purpose
|
Outcome/Classification
|
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
Table 5-1. Government Audits and Site 2011-Present
Table 5-2 specifies Key Personnel and Emergent Points of Contact
The following key personnel will be available 24 hours a day, seven days a week to be notified in case of a public health emergency:
[**]
Their 24-hour contact information will be provided upon contract award
|
Name
|
Position
|
|
[**]
|
[**]
|
|
[**]
|
[**]
|
|
[**]
|
[**]
|
|
[**]
|
[**]
|
|
[**]
|
[**]
|
|
[**]
|
[**]
|
Table 5-2. Emergent Key Personnel
As a company, Emergent places great emphasis on identifying risks that potentially would impact our future success. Figure 5-1 depicts an overview of our risk management strategy.
We use a defined process for the identification, assessment, control, and review of risks within the program. During risk assessment activities, we log risks identified by pre-defined program triggers into a risk matrix and identify each by its Work Breakdown Structure task designation. We assess each risk for its Probability of Occurrence and its Severity of Impact to the project. Once we assign the initial risk level of high/medium/ low, we direct project resources toward the most value-added activities, addressing project risks in their order of importance.
Our mitigation strategies include:
|
·
|
Reduction: Reduction of risk wherever possible
|
|
·
|
Transfer: Determination that the risk is outside control of the functional team and the PD identifies the team responsible for mitigation
|
|
·
|
Retention: Determination that the risk to project cost, schedule, and performance can continue without action
|
|
·
|
Avoidance: Decision to avoid the risk by not implementing the task involving the risk
|
We implement risk mitigation strategies associated with the identified risks.

Figure 5-1. Emergent's risk management strategy uses a defined process for the identification, assessment, control, and review of risks within the program.
Our proposed subcontractors are experienced and have the requisite facilities necessary to perform their tasks under all applicable regulations and requirements. Subcontractors' quality systems and facilities are audited by Emergent prior to subcontract award and periodically thereafter to ensure compliance.
Subcontracts address the scope of work, schedule, price, risk, unique requirements such as the need for GMP/GCP/GLP/quality agreements/audits, technical and business assumptions, dispute resolution, etc. Subcontracts are managed in accordance with applicable Federal Acquisition Regulations.
Emergent's Contract Managers ensure that each subcontractor and Vendor is performing according to the requirements delineated in the subcontract. Contract Managers initiate and track subcontract modifications as required to adjust for changes in scope. We closely manage subcontractor performance, deliverables, and quality through regular communication, on site meetings, and schedule reviews. Noncompliance issues that cannot be resolved between Emergent and the subcontractor's management team are elevated to the subcontractor's senior management. Clauses are included in subcontracts to address noncompliance, contract termination, and dispute resolution.
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of two pages were omitted. [**].
Emergent has defined the Quality Management System (QMS) requirements for phase appropriate compliance from proof-of-concept and pre-clinical stages through commercial manufacturing.
Emergent's integrated QMS is regulated by our Quality Policy Manual. The SOP-supported manual describes QMS policies and activities performed at our Lansing facility. The Quality Policy Manual aligns with Emergent's strategic plan, corporate objectives, and cGMP regulations and expectations.
Each component of our QMS complies with applicable ICH and FDA regulatory standards and requirements applicable for each stage of the product lifecycle. Emergent's Quality Assurance Department will ensure internal compliance to these standards as well as compliance of subcontractors performing work on Emergent's behalf. Our QMS includes all of the following components:
|
·
|
management responsibility and review
|
|
·
|
personnel qualification and training
|
|
·
|
document and data control
|
|
·
|
validation
|
|
·
|
equipment and facilities control
|
|
·
|
supplier quality and materials management control
|
|
·
|
production and in-process controls
|
|
·
|
non-conformance handling
|
|
·
|
change control
|
|
·
|
corrective and preventive action
|
The senior leadership team at each site is ultimately responsible for ensuring an effective QMS is in place, demonstrating strong support of the QMS and continuous improvement efforts, and ensuring that roles, responsibilities and authorities are defined, communicated, and implemented at all levels in the organization.
Emergent will ensure employees involved with vaccine operations have the necessary education, training, and experience appropriate to their positions. Current job descriptions and curricula vitae, or similar documentation, are established and maintained for all personnel responsible for conducting or overseeing activities.
Training records are maintained for employees, and periodic and systematic reviews of personnel training records are conducted to ensure that employees are receiving the training demanded by their job or function.
Emergent will ensure established SOPs are in place to control all documents and processes required for phase appropriate compliance activities. The document management program prevents unapproved revisions to controlled documents that may in turn affect the safety, quality, identity, potency and purity of the Anthrax vaccine. Changes to documents are reviewed and approved by designated individuals and the changes are communicated through training on the revised procedure. Controlled documents are available to personnel conducting the work.
Data is fully traceable from the controlled record to the raw data and is maintained and stored in such a way as to remain legible, readily identifiable, and retrievable. Original records are archived in compliance with document control and records management procedures.
Materials and services shall only be purchased from suppliers that have been appropriately qualified. A material qualification process is in place to ensure that materials are fit for their intended use.
Materials are controlled from the time of receipt to maintain complete identification and traceability at all times in accordance with established SOPs and compliance regulations.
Emergent will ensure product release does not proceed until all activities have been completed, documentation is available, and disposition has been authorized by QA. The materials management program will ensure proper segregation of materials that are under quarantine, release, and reject.
The Emergent QMS extends to the oversight and review of all outsourced activities. Quality Agreements are implemented to delineate clear responsibilities between Emergent and the subcontractor to avoid conflicts. These agreements are reviewed and executed by both parties.
Emergent will maintain Quality Agreements with applicable material and service suppliers. The Quality Agreement shall define the respective responsibilities shared by Emergent and, suppliers, as they relate to the manufacturing, testing, storing, and shipping activities. Quality Agreements will be written to ensure that affected parties comply with phase appropriate compliance requirements.
Emergent will ensure non-conforming events (e.g., deviations, Out of Specification, or Out of Trend results) are identified and investigated to correct the issue, protect end users from harm, and eliminate the root cause. Investigations are thorough in gathering all relevant facts, performing risk assessments, and determining a root cause whenever possible. The scope of the investigation is all inclusive of any product or processes that may have been impacted. Systemic problems are recognized and resolved.
Emergent will ensure any changes are documented, controlled and approved to prevent undesired events that could adversely affect the Safety, Quality, Identity, Potency, or Purity (SQIPP) of BioThrax, and will collect information about the actual impact of the change. Risk assessment is incorporated into the change control process based on the scope and potential impact of the change.
Emergent will ensure facilities and equipment are adequately designed, qualified, and maintained. Emergent will ensure all buildings used in the manufacturing, testing, storing, or shipping of the cell banks, bulk drug substance, and final drug product are maintained in a good state of repair, cleanliness, and sanitary condition. The operational areas and equipment used in the manufacturing, testing, storing, or shipping of the cell banks, bulk drug substance, and final drug product must be of appropriate design, adequate size, and suitably located to facilitate operation for its intended use and for cleaning and maintenance. Equipment is constructed so that surfaces that contact components are not reactive, additive, or absorptive to make sure the SQIPP of BioThrax is not adversely affected.
Calibration and Preventative Maintenance Program Critical and non-critical equipment and utilities supporting manufacturing, testing, storing or shipping are included in the calibration and preventative maintenance programs. Instruments on equipment and utilities are calibrated and the systems themselves routinely maintained based on established schedules or history of performance. Out-of-tolerance conditions are documented, reviewed, and their impact on product and process are assessed. Appropriate corrective action is taken.
Emergent will ensure analytical and/or microbial quality control testing is performed according to established specifications, sampling plans, test procedures, and laboratory control mechanisms required by the FDA regulations and guidance documents. Any deviations from the written specifications, standards, sampling plans, test procedures, or other laboratory control mechanisms are recorded and investigated.
All raw data generated in the course of testing raw materials, packaging materials, in process, and/or finished products shall be retained according to Emergent's corporate records retention schedule. Under no circumstance shall raw data be discarded, obliterated, or otherwise rendered unreadable or irretrievable during its established retention period.
Through oversight of the above listed requirements and process controls, Emergent QA will ensure that the cell banks, bulk drug substance, and final drug product is produced in compliance with Emergent's appropriate cGMP compliance requirements.
Earned Value Management (EVM) requirements were removed from the Scope of Work according to ACMG's response to Emergent's Question 1, received February 1, 2017. As a consequence of this Emergent will not submit an EVM plan.
Emergent is uniquely qualified to supply BioThrax to fulfill the specified requirement in this Solicitation. Not only do we offer BioThrax, the only FDA-licensed anthrax vaccine indicated for both pre-exposure and post-exposure prophylaxis, but we also have more than two decades of experience supplying BioThrax to multiple governments around the world for their anthrax medical countermeasure preparedness needs. Emergent has the manufacturing expertise, plus certified high-capacity cGMP manufacturing operations and all required support systems in place to confidently supply BioThrax as required under this Solicitation.
The following Appendices are attached to this proposal:
(This page intentionally left blank.)
SECTION B
(http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/UCM074923.pdf)

FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
BioThrax is a vaccine indicated for the active immunization for the prevention of disease caused by Bacillus anthracis in persons 18 through 65 years of age.
|
1.1
|
BioThrax is approved for pre-exposure prophylaxis of disease in persons whose occupation or other activities place them at high risk of exposure.
|
|
1.2
|
BioThrax is approved for post-exposure prophylaxis of disease following suspected or confirmed Bacillus anthracis exposure, when administered in conjunction with recommended antibacterial drugs.
|
The efficacy of BioThrax for post-exposure prophylaxis is based solely on studies in animal models of inhalation anthrax.
2 DOSAGE AND ADMINISTRATION
For intramuscular or subcutaneous injection only.
|
2.1
|
Dose
|
Each dose is 0.5 mL.
Pre-Exposure Prophylaxis:
|
Schedule
|
Route of Administration
|
Dosing Schedule
|
|
Primary Series
|
Intramuscular
|
0, 1, and 6 months
|
|
Booster Series
|
Intramuscular
|
6 and 12 months after completion of the primary series and at 12-month intervals thereafter
|
In persons who are at risk for hematoma formation following intramuscular injection, BioThrax may be administered by the subcutaneous route. The pre-exposure prophylaxis schedule for BioThrax administered subcutaneously is 0, 2, 4 weeks, and 6 months with booster doses at 6 and 12 months after completion of the primary series and at 12-month intervals thereafter.
The optimal schedule for catch up of missed or delayed booster doses is unknown. [See Clinical Studies (14)]
|
Post-Exposure Prophylaxis:
|
||
|
Schedule
|
Route of Administration
|
Dosing Schedule
|
|
Primary Series
|
Subcutaneous
|
0, 2, and 4 weeks post-exposure combined with antimicrobial therapy
|
|
2.2
|
Administration
|
Shake the vial thoroughly to ensure that the suspension is homogeneous during withdrawal. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, do not administer the vaccine.
Administer pre-exposure prophylaxis vaccinations intramuscularly into the deltoid muscle. If pre-exposure prophylaxis requires subcutaneous administration, administer over the deltoid muscle. Administer post-exposure prophylaxis vaccinations subcutaneously over the deltoid muscle.
Do not mix with any other product in the syringe.
3 DOSAGE FORMS AND STRENGTHS
BioThrax is a suspension for injection (0.5 mL dose) in 5 mL multidose vials. See Description (11) for the complete listing of ingredients.
4 CONTRAINDICATIONS
Do not administer BioThrax to individuals with a history of anaphylactic or anaphylactic-like reaction following a previous dose of BioThrax or any component of the vaccine, including aluminum, benzethonium chloride, and formaldehyde. [See Description (11)]
5 WARNINGS AND PRECAUTIONS
|
5.1
|
Hypersensitivity Reactions
|
Acute allergic reactions, including anaphylaxis, have occurred with BioThrax. Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine. [See Contraindications (4)]
|
5.2
|
Latex
|
The stopper of the vial contains natural rubber latex and may cause allergic reactions to patients with a possible history of latex sensitivity. [See How Supplied/Storage and Handling (16)]
|
5.3
|
Pregnancy
|
BioThrax can cause fetal harm when administered to a pregnant woman. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus. Weigh the potential benefits of vaccination against the potential risk to the fetus. [See Use in Specific Populations (8.1)]
Pregnant women should not be vaccinated against anthrax unless the potential benefits of vaccination have been determined to outweigh the potential risk to the fetus. Results of a large observational study that examined the rate of birth defects among 37,140 infants born to U.S. military service women who received anthrax vaccine in pregnancy between 1998 and 2004 showed that birth defects were slightly more common in first trimester-exposed infants (odds ratio = 1.18, 95% confidence interval: 0.997, 1.41) when compared with infants of women vaccinated outside of the first trimester and compared to unvaccinated women.1 While the increased birth defect rates were not statistically significant when compared with infants born to women vaccinated outside of pregnancy, pregnant women should not be vaccinated against anthrax unless the potential benefits of vaccination have been determined to outweigh the potential risk to the fetus.
|
5.4
|
History of Anthrax Disease
|
History of anthrax disease may increase the potential for severe local adverse reactions.
|
5.5
|
Altered Immunocompetence
|
If BioThrax is administered to immunocompromised persons, including those receiving immunosuppressive therapy, the immune response may be diminished.
|
5.6
|
Limitations of Vaccine Effectiveness
|
Vaccination with BioThrax may not protect all individuals.
6 ADVERSE REACTIONS
The most common (≥10%) local (injection-site) adverse reactions observed in clinical studies were tenderness, pain, erythema, edema, and arm motion limitation. The most common (≥5%) systemic adverse reactions were muscle aches, headache, and fatigue.
|
6.1
|
Clinical Trials Experience
|
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a product cannot be directly compared to rates in the clinical trials of another product and may not reflect the rates observed in clinical practice.
Pre-Exposure Prophylaxis
In an open-label safety study of 15,907 doses of BioThrax administered by the subcutaneous route to approximately 7,000 textile employees, laboratory workers and other at risk individuals, local and systemic reactions were monitored. Over the course of the 5-year study the following local adverse reactions were reported: 24 (0.15% of doses administered) severe local adverse reactions (defined as edema or induration measuring greater than 120 mm in diameter or accompanied by marked limitation of arm motion or marked axillary node tenderness), 150 (0.94% of doses administered) moderate local adverse reactions (edema or induration greater than 30 mm but less than 120 mm in diameter), and 1,373 (8.63% of doses administered) mild local adverse reactions (erythema only or induration measuring less than 30 mm in diameter). Four cases of systemic adverse reactions were reported during the 5-year reporting period (<0.06% of doses administered). These reactions, which were reported to have been transient, included fever, chills, nausea, and general body aches.
In a randomized, double-blinded, placebo-controlled, and active-controlled multi-center clinical study, 1,564 healthy subjects were enrolled. The objective of this study was to evaluate the effect of (1) changing the route of vaccine administration from subcutaneous (SC) to intramuscular (IM), and (2) of reducing the number of doses on the safety and immunogenicity of BioThrax. The dosing schedules and routes studied are provided in Table 1. [See Clinical Studies (14)]
Group A (8SC) (N=259) received BioThrax via the SC route of administration at Weeks 0, 2, 4, and Months 6, 12, 18 followed by 2 annual boosters (original U S. licensed route/schedule). Group A served as the active control in this study.
Group B (8IM) (N=262) received BioThrax via the IM route of administration at Weeks 0, 2, 4, and Months 6, 12, 18 followed by 2 annual boosters.
Group C (COM) (N=782) received BioThrax via the IM route of administration at Weeks 0, 4 (no Week 2 dose), and Month 6 with various schedules thereafter. (Group C represents data from 3 randomized groups [Groups D, E, and F] combined for the analysis, through Month 7 because the schedules are identical through the Month 6 dose.)
Group D (7IM) (N=256) received BioThrax via the IM route of administration at Weeks 0, 4 (no Week 2 dose), and Months 6, 12, 18 followed by 2 annual boosters.
Group E (5IM) (N=258) received BioThrax via the IM route of administration at Weeks 0, 4 (no Week 2 dose), and Months 6, 18 followed by 1 booster dose at Month 42 (2 year interval).
Group F (4IM) (N=268) received BioThrax via the IM route of administration at Weeks 0, 4 (no Week 2 dose), and Month 6 followed by 1 booster dose at Month 42 (3 year interval).
|
Table 1: Vaccination Schedules and Routes Evaluated
|
||||||||
|
Group/Route
|
Weeks
|
Months
|
||||||
|
0
|
2
|
4
|
6
|
12
|
18
|
30
|
42
|
|
|
Group A (8SC)a
|
V
|
V
|
V
|
V
|
V
|
V
|
V
|
V
|
|
Group B (8IM)
|
V
|
V
|
V
|
V
|
V
|
V
|
V
|
V
|
|
Group D (7IM)
|
V
|
S
|
V
|
V
|
V
|
V
|
V
|
V
|
|
Group E (5IM)
|
V
|
S
|
V
|
V
|
S
|
V
|
S
|
V
|
|
Group F (4IM)
|
V
|
S
|
V
|
V
|
S
|
S
|
S
|
V
|
|
Placebob
|
S
|
S
|
S
|
S
|
S
|
S
|
S
|
S
|
|
SC: subcutaneous; IM: intramuscular; V: vaccine; S: Saline
a Active Control
b Subjects randomized to the control group were then re-randomized (1:1) to receive saline by the IM or SC route. The IM and SC placebo groups are combined in analyses.
|
||||||||
Subjects were instructed to complete a 14-day post-vaccination diary card after the first 2 doses and a 28 day diary card after the subsequent doses to capture solicited and unsolicited adverse reactions. Adverse reaction data were also collected from in-clinic exams, which were performed prior to, and 15 to 60 minutes after each injection, at 1 to 3 days after each injection for the first two injections, and at 28 days after injections 3 through 8. The mean age, gender ratio, and race distribution were not significantly different across treatment groups among the vaccinated cohort (N=1563). The mean age was 39 years (range 18 to 62 years). Fifty-one percent of participants were female and 49% were male. Seventy-four percent were white, 21% were black and 5% were categorized as "other".
Shown in Table 2 are the rates (percentage) of prospectively defined local and systemic solicited adverse reactions observed in the in-clinic exams for doses 1-4 as well as the rates (percentage) of local and systemic solicited adverse reactions observed in the in-clinic exams for doses 5-8.
Analysis of injection site (local) adverse reactions by study group was performed after each dose. It was observed that groups receiving BioThrax by the IM route had a statistically significant lower incidence (p ≤ 0.05) of any (one or more) local adverse reactions compared to the BioThrax SC route, by dose in the in-clinic data set, in 23 out of 24 analyses. (This excludes doses where IM groups received a placebo.) Individual injection site adverse reactions occurring at statistically significantly lower frequencies (p ≤ 0.05) in participants given BioThrax by the IM route included warmth (in all analyses), tenderness (in 19 out of 24 analyses), itching (in 22 out of 24 analyses), erythema (in all analyses), induration (in all analyses), edema (in 20 out of 24 analyses), and nodule (in all analyses). However, by dose, the incidences of arm motion limitation were comparable or higher in each BioThrax IM group compared to the 8SC group, with statistically significantly higher incidences (p ≤ 0.05) observed in 10 out of 24 analyses. The incidence of any moderate or severe local adverse reactions was lower in BioThrax IM groups, compared to the 8SC group after each dose. Route of administration did not affect the occurrence of systemic adverse reactions, with the exception of muscle ache (increased incidence in the BioThrax IM groups after most doses). There was no pattern for differences in the incidence of any moderate or severe systemic adverse reactions for BioThrax IM groups compared to the 8SC group after each dose. The proportion of participants with severe local or systemic adverse reactions reported by adverse reaction category after each dose was very low (generally <1%).
Overall, women had a higher incidence of any local adverse reaction than did men, by dose, within BioThrax groups, regardless of the route of administration. Overall, women also had a higher incidence of any systemic adverse reaction than men, within BioThrax groups, regardless of the route of administration. A brief pain or burning sensation, felt immediately after vaccine injection, and distinct from injection site pain, was reported by 45 - 97% of all study participants receiving BioThrax. Reporting frequency and event intensity varied with route of administration and vaccine dose. Up to 11% of subjects rated the brief pain or burning they experienced immediately after vaccine injection as 8 out of 10 or greater. Female participants generally experienced a higher pain scale rating than male participants.
Eight serious adverse events (SAEs) were reported with 6 subjects and determined to be possibly related to the administration of BioThrax: (1) a case of generalized allergic reaction, (2) a case of ANA positive autoimmune disorder manifesting as a moderate bilateral arthralgia of the metacarpophalangeal (MCP) joints, (3) a right shoulder supraspinatus tendon tear, (4) a case of bilateral pseudotumor cerebri with bilateral disc edema, (5) a case of generalized seizure and hospitalization for evaluation of hydrocephalus and endoscopic fluid ventriculostomy, (6) a case of bilateral ductal carcinoma of the breast. No SAEs were determined by the investigator to be probably or definitely related to administration of BioThrax. The percent of serious adverse events was similar between the BioThrax combined groups (193/1303 or 15%) and the placebo group (38/260 or 15%).
Fifty-one pregnancies were reported in this study, 33 of which occurred in women who received BioThrax as their last dose prior to conception and 18 in women who received placebo as their last dose prior to conception. Pregnancy outcomes where BioThrax was given within 30 days prior to conception (n=5) were 3 full-term live births (including 1 healthy term infant with a mild right clubbed foot abnormality), 1 spontaneous abortion, and 1 first trimester intra-utero fetal death. Pregnancy outcomes in the placebo group (n=5) were 4 full-term live births (including one with bilateral congenital hip dysplasia) and 1 elective abortion.

Solicited and unsolicited adverse reactions observed from Day 0 through month 43 at a higher frequency (by at least 5%) in the BioThrax groups (IM and SC) as compared to the placebo (P) group were: headache (70.4% IM, 78.4% SC, 68.1% P); myalgia (72% IM, 76.1% SC, 50% P); and fatigue (70.1% IM, 76.8% SC, 60.8% P).
Post-Exposure Prophylaxis
A phase 3, open-label, uncontrolled, multi-center study evaluated the three-dose post-exposure prophylaxis BioThrax schedule (Week 0, 2 and 4) in 200 healthy adult subjects. The most common solicited adverse reactions reported 7 days after each vaccination comprised local reactions, including symptoms of lump, tenderness, and erythema. The most common solicited systemic reactions comprised fatigue, headache, and myalgia. Of the subjects that reported local and systemic solicited reactions, ≥ 98% required minimal or no treatment and resulted in little to no interference with subjects' daily activity. The most common (> 2.0%) unsolicited related adverse reactions reported following at least one dose up to 100 days after the third dose were: headache (4.0%), fatigue (3.5%), skin hyperpigmentation (3.5%), decreased joint range of motion (2.5%), myalgia (2.5%). No deaths were reported and neither of the two SAEs reported were considered to be related to vaccination. There were no pregnancies reported or subject withdrawals from the study due to adverse events.
|
6.2
|
Postmarketing Experience
|
The following adverse events have been reported spontaneously. Since these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The reports included below are listed due to one or more of the following factors: (1) seriousness of the event, (2) number of reports, or (3) strength of causal relationship to the drug.
|
·
|
Blood and lymphatic system disorders
|
Lymphadenopathy
|
·
|
Gastrointestinal Disorders
|
Nausea
|
·
|
Immune system disorders
|
Allergic reactions (including anaphylaxis, angioedema, rash, urticaria, pruritus, erythema multiforme, anaphylactoid reaction, and Stevens Johnson syndrome)
|
·
|
Nervous system disorders
|
Paresthesia syncope, dizziness, tremor, ulnar nerve neuropathy
|
·
|
Musculoskeletal, connective tissue, and bone disorders
|
Arthralgia, arthropathy, myalgia, rhabdomyolysis, alopecia
|
·
|
General disorders and administration site conditions
|
Malaise, pain, cellulitis, flu-like symptoms
|
·
|
Psychiatric disorders
|
Insomnia
|
·
|
Skin and Subcutaneous disorders
|
Pruritis, rash, urticaria
|
·
|
Vascular disorders
|
Flushing
Infrequent reports were also received of multisystem disorders defined as chronic symptoms involving at least two of the following three categories: fatigue, mood-cognition, and musculoskeletal system.
7 DRUG INTERACTIONS
|
7.1
|
Ciprofloxacin
|
Co-administration of 0.5 mL BioThrax SC with oral ciprofloxacin in human subjects did not alter the pharmacokinetics of ciprofloxacin or the immunogenicity of BioThrax as measured by the anthrax lethal toxin neutralization assay. [See Clinical Studies (14-3)]
|
7.2
|
Concomitant Administration with Other Vaccines
|
The safety and efficacy of concomitant administration of BioThrax with other licensed vaccines has not been evaluated.
BioThrax should not be mixed with any other vaccine in the same syringe or vial. If BioThrax is to be given at the same time as another injectable vaccine(s), the vaccine(s) should be administered at different injection sites.
|
7.3
|
Immunosuppressive Therapies
|
Immunosuppressive therapies, including chemotherapy, corticosteroids (used in high-doses longer than 2 weeks), and radiation therapy may reduce the response of BioThrax.
8 USE IN SPECIFIC POPULATIONS
|
8.1
|
Pregnancy
|
Pregnancy Category D. [See Warnings and Precautions (5.3)]
Healthcare practitioners are encouraged to register women who receive BioThrax during pregnancy in Emergent's vaccination pregnancy registry by calling 1-619-553-9255.
Male Fertility: A retrospective study was performed at an in-vitro fertilization clinic to evaluate whether BioThrax may impact reproductive function in men.. This study compared semen parameters, embryo quality, and pregnancy outcomes in 254 male clients who stated that they had received BioThrax, with those of 791 male clients who did not.2 Prior receipt of BioThrax did not influence semen parameters (including concentration, motility, and morphology), fertilization rate, embryo quality or clinical pregnancy rates.
8.3 Nursing Mothers
It is not known whether BioThrax is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when BioThrax is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established for BioThrax.
8.5 Geriatric Use
BioThrax has not been approved for use in patients greater than 65 years of age.
11 DESCRIPTION
BioThrax® (Anthrax Vaccine Adsorbed) is a sterile, milky-white suspension for intramuscular or subcutaneous injections made from cell-free filtrates of microaerophilic cultures of an avirulent, nonencapsulated strain of Bacillus anthracis. The production cultures are grown in a chemically defined protein-free medium consisting of a mixture of amino acids, vitamins, inorganic salts, and sugars. The final product, prepared from the sterile filtrate culture fluid contains proteins, including the 83kDa protective antigen (PA) protein, released during the growth period and contains no dead or live bacteria. The final product is formulated to contain 1.2 mg/mL aluminum, added as aluminum hydroxide in 0.85% sodium chloride. The final product is formulated to contain 25 mcg/mL benzethonium chloride and 100 mcg/mL formaldehyde, added as preservatives.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Anthrax is a zoonotic disease caused by the Gram-positive spore-forming bacterium Bacillus anthracis. BioThrax induces antibodies raised against PA that may contribute to protection by neutralizing the activities of the cytotoxic lethal toxin and edema toxin of Bacillus anthracis.3 Bacillus anthracis proteins other than PA may be present in BioThrax, but their contribution to protection has not been determined.
13 NONCLINICAL TOXICOLOGY
The effect of BioThrax on embryo-fetal and pre-weaning development was evaluated in a developmental toxicity study using pregnant rabbits. One group of rabbits was administered BioThrax twice prior to gestation and during the period of organogenesis (gestation day 7). A second group of rabbits was administered BioThrax twice prior to gestation and on gestation day 17. BioThrax was administered at 0.5 ml/rabbit/occasion, by intramuscular injection. No adverse effects on mating, fertility, pregnancy, parturition, lactation, embryo-fetal or pre-weaning development were observed. There were no vaccine-related fetal malformations or other evidence of teratogenesis noted in this study.
13.2 Animal Pharmacology
Since it is not feasible or ethical to conduct controlled clinical trials with anthrax, the efficacy of BioThrax in a post-exposure setting is based on studies in animals. Pre-exposure prophylaxis animal models were used to derive protective antibody thresholds to bridge animal efficacy and human immunogenicity data and predict efficacy in humans.
Pivotal efficacy animal studies were conducted in rabbits and nonhuman primates (NHPs). Animals received two IM vaccinations four weeks apart with serial dilutions of BioThrax and were subjected to lethal challenge on study day 70 with aerosolized B. anthracis spores at a target dose exceeding the 50% lethal dose by 200-fold. Serum samples were collected at various time points prior to challenge for immune response analysis via anthrax lethal toxin neutralizing antibody (TNA) assay. The relationship between pre-challenge serum TNA levels and survival was evaluated. Logistic regression analysis demonstrated that a 70% probability of survival was associated with a TNA NF50 (50% neutralization factor) level of 0.56 in rabbits and 0.29 in NHPs.
The ability of BioThrax to increase survival after the cessation of the post-exposure antimicrobial treatment, as compared with antimicrobial treatment alone, was investigated in two post-exposure animal model studies. In these studies, rabbits were challenged via inhalation with aerosolized B. anthracis spores and subsequently treated with levofloxacin administered via oral gavage once daily for 7 days starting at 6-12 hours post-exposure, with or without two intramuscular injections of BioThrax one week apart. Survival among animals that received both antimicrobial treatment and vaccination was between 70 – 100% and increased in a vaccine dose-dependent manner. In contrast, only 44% and 23% survival was observed among animals that received antimicrobial treatment only in the first and the second study, respectively (p < 0.0006 and p < 0.004, respectively). [See Clinical Studies (14.2)]
14 CLINICAL STUDIES
14.1 Pre-Exposure Prophylaxis
A controlled field study using an earlier version of a protective antigen-based anthrax vaccine developed in the 1950's and supplied by G. G. Wright and associates of the U.S. Army Chemical Corps, Fort Detrick, Frederick, MD, that consisted of an aluminum potassium sulfate-precipitated cell-free filtrate from an aerobic culture, was conducted from 1955-1959.4 This study included 1,249 workers [379 received anthrax vaccine, 414 received placebo, 116 received incomplete inoculations (with either vaccine or placebo) and 340 were in the observational group (no treatment)] in four mills in the northeastern United States that processed imported animal hides. The anthrax vaccine was administered subcutaneously at 0, 2, 4 weeks, 6, 12, 18 months. Prior to vaccination, the yearly average number of human anthrax cases (both cutaneous and inhalational) was 1.2 cases per 100 employees in these mills. During the trial, 26 cases of anthrax were reported across the four mills – 5 inhalation and 21 cutaneous. Of the five inhalation cases (four of which were fatal), two received placebo and three were in the observational group. Of the 21 cutaneous cases, 15 received placebo, three were in the observational group, and three received anthrax vaccine. Of those three cases in the vaccine group, one case occurred just prior to administration of the scheduled third dose, one case occurred 13 months after an individual received the third of the scheduled 6 doses (but no subsequent doses), and one case occurred prior to receiving the scheduled fourth dose of vaccine. The calculated efficacy of the vaccine to prevent all types of anthrax disease, regardless of the route of exposure or clinical manifestations, was 92.5% (lower 95% Confidence Interval (CI) = 65%).
Between 1962 and 1974, the Centers for Disease Control and Prevention (CDC) collected surveillance data on the occurrence of anthrax disease in mill workers or those living near mills in the United States.5, 6 In that time period, individuals received either BioThrax or the earlier protective antigen-based anthrax vaccine used in the field trial described above. Of the 27 reported cases of anthrax, 24 cases occurred in unvaccinated individuals. In vaccinated individuals one case occurred after the person had been given one dose of anthrax vaccine and two cases occurred after individuals had been given two doses of anthrax vaccine. No documented cases of anthrax were reported for individuals who had received at least three doses of the originally licensed six-dose series of anthrax vaccine.
Between 2002 and 2007, a prospective double-blinded, randomized, placebo-controlled and active-controlled study was conducted to evaluate the impact on safety and immunogenicity on changing the administration route from SC to IM, and reducing the number of doses. This study enrolled 1,564 healthy civilian men and women between the ages of 18 and 61. A total of 1,563 subjects received at least one dose (one subject withdrew consent prior to the first injection). Subjects were randomized to one of six groups. See Table 1.
Using an Enzyme-Linked Immunosorbent Assay (ELISA), Immunoglobulin G (IgG) antibodies directed against anthrax protective antigen (PA) were measured at the Week 8 and Months 7, 13, 19, 31, and 43 time points. The three primary immunogenicity endpoints were: (1) Geometric Mean Concentration (GMC) (mcg/mL), (2) Geometric Mean Titer (GMT) and (3) percentage with 4-fold rise in anti-PA antibody titer from baseline.
The criteria for non-inferiority of comparisons based on ratios of GMCs and GMTs and differences in the rates of 4-fold rise in antibody titer were defined as follows:
Mean antibody concentration ratio: non-inferiority was achieved when the upper bound of the 95% confidence limit was < 1.5
Mean antibody titer ratio: non-inferiority was achieved when the upper bound of the 95% confidence limit was < 1.5
4-fold rise in antibody titer: non-inferiority was achieved when the upper bound of the 95% confidence limit was < 0.10
To compare the originally licensed 6-dose SC schedule (0, 2, 4 weeks and 6, 12, and 18 months) versus a 3-dose IM primary series (at 0, 1, and 6 months) non-inferiority analyses were performed for all three primary immunogenicity endpoints. This evaluation compared the immune response at Month 7 for Group C (COM, where COM is Combined, as described in 6.1) to Month 19 for Group A (TRT-8SC, where TRT is Treatment) and Group B (TRT-8IM). Non-inferiority was demonstrated for all analyses (See Table 3). These results support a 3 dose primary series of BioThrax administered IM at 0, 1 and 6 months, followed by booster doses at 12 and 18 months and at 1-year intervals thereafter to maintain protective immunity.
The Month 7 antibody levels of Group A (TRT-8SC) were non-inferior to Month 13 and 19 antibody levels after a 0, 2, 4 week and 6 month primary SC series followed by SC booster injections at 12 and 18 months (see Table 3). These results support a 4 dose SC primary series of BioThrax administered at weeks 0, 2, 4, and at 6 months followed by booster doses at 12 and 18 months after initiation of the series, and at 1-year intervals thereafter to maintain protective immunity.
Catch-Up Administration for Delayed or Missed Doses
In subjects who did not receive booster doses at 12, 18, and 30 months, PA antibody levels decline over time following the third dose of BioThrax administered intramuscularly at 6 months. (Group F; 4IM; 0, 1, 6, and 42 months). In the absence of booster doses it is not known whether these individuals are adequately protected between 12 months and receipt of a booster dose at 42 months. One month following a dose of BioThrax at 42 months the immune response for Group F met the criteria for non-inferiority relative to Group A (8SC) for all three primary immunogenicity endpoints (see Table 3). The optimal schedule for further intramuscular booster doses among persons administered a single booster dose at 42 months following completion of a three-dose primary series at 0, 1, and 6 months is not known.

14.2 Post-Exposure Prophylaxis
Based on the rabbit model-derived TNA threshold [See Nonclinical Toxicology (13.2)], a pivotal clinical study was conducted to evaluate the immunogenicity and safety of a post-exposure SC administration schedule of BioThrax in healthy adults following 3 doses at 0, 2, and 4 weeks. Two hundred subjects were enrolled and followed for 128 days. The primary objective was to assess immunogenicity using TNA following the completion of three SC doses of BioThrax. The primary immunogenicity endpoint was the proportion of subjects achieving a threshold TNA NF50 value ≥0.56 at Day 63, 5 weeks after the third vaccination. Success was concluded if the lower bound of the 2-sided 95% CI of the proportion of human subjects achieving the TNA NF50 threshold was ≥ 40%.
Overall, 71.2% of subjects achieved an NF50 value ≥0.56 on Day 63 in the pivotal study. The lower bound of the 95% CI was 94.1%. (See Table 4.)
In a separate analysis of the pivotal clinical study using the threshold associated with a 70% probability of survival in NHPs, 93.5% of subjects achieved an NF50 value ≥0.29 on Day 63 (Table 4). The lower bound of the 95% CI was 88.9% (Table 4). The bridging of human immunogenicity data to the NHP study was supportive of the primary analysis comparing human threshold data with rabbit survival. [See Nonclinical Toxicology (13.2)]
|
Table 4: Proportion of Subjects Achieving TNA NF50 Thresholda in the Pivotal Clinical Study
(PP Populationb)
|
||||||||
|
Animal Model
|
Time Point Human/Animal
|
n
|
Human GMT TNA
NF50
(SD)
|
Animal TNA NF50
Threshold
|
Number of Subjects Meeting Threshold
|
Proportion of Subjects
Meeting Threshold (%)d |
||
|
Point
Est. (%) |
95% CI (%)
|
|||||||
|
Lower Bound
|
Upper Bound
|
|||||||
|
Rabbite
|
Day 63/Day 69
|
184
|
0.86
(2.09)
|
0.56
|
131
|
71.2
|
64.1
|
77.6
|
|
Non-human Primatef
|
Day 63/Day 70
|
184
|
0.86
(2.09)
|
0.29
|
172
|
93.5
|
88.9
|
96.6
|
|
CI = confidence interval; NF50 = 50% neutralization factor; PP = per protocol; SD = standard deviation; TNA = toxin neutralizing antibody.
Note: sample size (N) and denominators used for percentages are based on the number of subjects meeting the PP criteria at specified day(s).
a TNA NF50 threshold is defined as the TNA NF50 value associated with 70% survival in the animal challenge studies.
b Human data are from the pivotal clinical study (NCT01491607).
c A logistic regression model with log10-transformed TNA NF50 values as the predictor and survival as the response is used to derive the TNA NF50 threshold associated with 70% probability of survival in rabbis and non-human primates, respectively.
d 95% CI is calculated with the exact (Clopper-Pearson) method.
e The proportion of subjects achieving a TNA NF50 response at Day 63 that met or exceeded the TNA NF50 threshold in the rabbit model at Day 69 comprised the primary immunogenicity endpoint.
f Comparison of the human TNA NF50 response at Day 63 with the NHP TNA NF50 threshold at Day 70 was defined as an immunogenicity endpoint and was supportive of the bridging of human immunogenicity data to rabbit survival.
|
||||||||
| 14.3 |
Non-Interference of Post-Exposure Prophylaxis Vaccination and Antimicrobials When Used Concurrently
|
An open-label study was conducted to evaluate the potential impact 0.5mL BioThrax administered SC at 0, 2 and 4 weeks had on the pharmacokinetics of ciprofloxacin in healthy adult male and female subjects (N=154). It also evaluated the potential impact of ciprofloxacin on immunogenicity of BioThrax two weeks following the last BioThrax dose.
Co-administration of 0.5 mL BioThrax SC with oral ciprofloxacin in human subjects did not alter the pharmacokinetics of ciprofloxacin or the immunogenicity of BioThrax as measured by the anthrax lethal toxin neutralization assay.
15 REFERENCES
| 1. |
Ryan MA, Smith TC, Sevick CJ, Honner WK, Loach RA, Moore CA, Erickson JD. 2008. Birth defects among infants born to women who received anthrax vaccine in pregnancy. Am J Epidemiol, 168:434-442.
|
| 2. |
Catherino WH, Levi A, Kao TC, Leondires MP, McKeeby J, Segars JH. 2005. The anthrax vaccine does not affect semen parameters, embryo quality, or pregnancy outcome in couples with a vaccinated male military service member. Fertility and Sterility, 83:480-483
|
| 3. |
Brachman P, Friedlander A, Grabenstein J. 2008. Anthrax Vaccine. In: Vaccines, Fifth Edition, Plotkin, S.A.: Orenstein W.A. and Offit P.A. (eds.), 111-126
|
| 4. |
Brachman, PS, Gold H, Plotkin SA, Fekety FR, Werrin M, Ingraham NR. 1962. Field evaluation of a human anthrax vaccine. Amer. J. Public Health, 52:632-645.
|
| 5. |
Food and Drug Administration, 2005, Biological Products; Bacterial Vaccines and Toxoids; Implementation of Efficacy Review; Anthrax Vaccine Adsorbed; Final Order. FDA Federal Register 2005; 70(242): 75180-75198.
|
| 6. |
Food and Drug Administration. Biological Products; Bacterial vaccines and toxoids; Implementation of efficacy review. Federal Register (December 13, 1985), 50(240):51002-51117.
|
16 HOW SUPPLIED/STORAGE AND HANDLING
BioThrax is supplied in 5 mL multidose vials containing ten 0.5 mL doses.
NDC 64678-211-05 (vial), 64678-211-01 (carton)
Store at 2 °C to 8 °C (36 °F to 46 °F). Do not freeze. Do not use BioThrax after the expiration date printed on the label.
The stopper of the vial contains natural rubber latex and may cause allergic reactions in latex sensitive individuals.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
Advise women of the potential risk to the fetus. Encourage women who are exposed to BioThrax during pregnancy to inform their healthcare provider and enroll in the BioThrax (Anthrax) Vaccine in Pregnancy Registry (Phone: 1-619-553-9255). [See Warnings and Precautions (5.3) and Use in Specific Populations (8.1)]
Inform patients of the benefits and risks of immunization with BioThrax.
Instruct patients to report any serious adverse reaction to their health care provider.
Manufactured by:
Emergent BioDefense Operations Lansing LLC
Lansing, MI 48906
US License No. 1755
BioThrax® is a registered trademark of Emergent BioDefense Operations Lansing LLC
Information for Patients
BioThrax® (Anthrax Vaccine Adsorbed)
BioThrax® (Anthrax Vaccine Adsorbed)
Please read this Patient Information summary carefully before you get this shot. This summary does not take the place of talking with your healthcare provider about BioThrax. If you have questions or would like more information, please talk with your healthcare provider.
What is BioThrax?
|
·
|
BioThrax is a vaccine licensed by the FDA to protect against anthrax disease in persons 18 through 65 years of age:
|
|
o
|
It can be used before exposure to anthrax to protect people at high risk of getting the disease.
|
|
o
|
It can be used after exposure to anthrax, along with antibiotics, to protect people from getting the disease.
|
|
·
|
BioThrax may not protect all people who get the vaccine.
|
|
·
|
How well BioThrax works when given after exposure to anthrax has been studied only in animals. It has not been studied in humans because there are not enough people who get the disease naturally, and it is not ethical to expose people to anthrax on purpose to find out how well BioThrax works.
|
|
·
|
The safety of BioThrax was studied in healthy adults.
|
What is BioThrax?
You should not get BioThrax if you have a history of severe allergic reaction to any ingredient of the vaccine, including aluminum hydroxide, benzethonium chloride, and formaldehyde or had a serious reaction after getting BioThrax previously.
What should I tell my healthcare provider before getting BioThrax?
|
·
|
If you may be pregnant, plan to get pregnant soon, or are nursing a baby.
|
|
·
|
About medicines that you take, including over-the-counter medicines and supplements.
|
|
·
|
About immune problems you have, including steroid treatments and cancer treatments.
|
|
·
|
About blood clotting problems or if you have "blood thinners."
|
|
·
|
If you are allergic to latex.
|
What if I discover I was pregnant at the time I got BioThrax?
|
·
|
Inform your healthcare provider
|
|
·
|
You can enroll in the BioThrax (Anthrax) Vaccine in Pregnancy Registry (Phone: 1-619-553-9255), if eligible
|
How is BioThrax given?
BioThrax is given as a shot in your arm.
After getting the first shot, you should come back for the next shots on the schedule given to you by your health care provider. It is important that you get all your shots to get the best protection.
If you get BioThrax because you may have been exposed to anthrax, it is important that you also take antibiotics for 60 days.
What are the possible or reasonably likely side effects of BioThrax?
The most common side effects of BioThrax are:
|
·
|
Pain, tenderness, redness, bruising, or problems moving the arm in which you got the shot
|
|
·
|
Muscle aches
|
|
·
|
Headaches
|
|
·
|
Fatigue
|
|
·
|
Fainting
|
Tell your healthcare provider about any side effects that concern you. Your healthcare provider can give you a complete list of side effects available to healthcare professionals.
You may report side effects to FDA by calling 1-800-822-7967 or to the website www.vaers.hhs.gov. You may also report side effects directly to Emergent BioSolutions at 1-877-246-8472 or at productsafety@ebsi.com.
What are the ingredients in BioThrax?
BioThrax does not contain live bacteria. BioThrax contains non-infectious proteins, aluminum hydroxide, benzothonium chloride and formaldehyde (as preservatives).
The vial stopper contains natural rubber latex.
Manufactured by Emergent BioDefense Operations Lansing LLC
Lansing, MI 48906, US
License No. 1755
Lansing, MI 48906, US
License No. 1755
|
Lot
|
CLIN
|
Delivery #
|
Shipment Date
|
Delivery Date
|
Expiry Date*
|
Remaining Expiry (Months)*
|
Expected Doses
|
Cum Expected Doses
|
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|||
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
Notes:
|
·
|
Delivery Date is dependent upon delivery destination provided by Government at least [**] business days prior to Shipment Date
|
|
·
|
For planning purposes, typical transit time between Shipment Date and Delivery Date is [**] days
|
|
·
|
Actual delivery dates and quantities subject to available FDA-released lots of BioThrax on hand for shipment
|
ATTACHMENT #3
INVOICE/FINANCING REQUEST INSTRUCTIONS FOR FIXED PRICE TYPE
CONTRACTS
INVOICE/FINANCING REQUEST INSTRUCTIONS FOR FIXED PRICE TYPE
CONTRACTS
General The Offeror shall submit vouchers or invoices as prescribed herein.
Format Standard Form l034, Public Voucher for Purchases and Services Other Than Personal, and Standard Form l035, Public Voucher for Purchases and Services Other than Personal--Continuation Sheet, and the payee's letterhead or self-designed form should be used to submit claims for reimbursement.
Number of Copies: As indicated in the contract.
Frequency Invoices submitted in accordance with the Payment Clause shall be submitted monthly upon delivery of goods or services unless otherwise authorized by the Contracting Officer.
Preparation and Itemization of the Invoice The invoice shall be prepared as follows:
(a) Designated Billing Office and address:
HHS/ASPR/BARDA
330 Independence Ave, Room G640
Washington DC 20201
ATTN: Contracting Officer
330 Independence Ave, Room G640
Washington DC 20201
ATTN: Contracting Officer
(b) Invoice Number
(c) Date of Invoice
(d) Contract number and date
(e) Payee's name and address. Show the Offeror's name (as it appears in the contract), correct address, and the title and phone number of the responsible official to whom payment is to be sent. When an approved assignment has been made by the Offeror, or a different payee has been designated, then insert the name and address of the payee instead of the Offeror.
(f) Description of goods or services, quantity, unit price, (where appropriate), and total amount.
(g) Charges for freight or express shipments other than F.O.B. destination. (If shipped by freight or express and charges are more than $25, attach prepaid bill.)
(h) Equipment - If there is a contract clause authorizing the purchase of any item of equipment, the final invoice must contain a statement indicating that no item of equipment was purchased or include a completed form HHS-565, Report of Capitalized Nonexpendable Equipment.
Currency: Where payments are made in a currency other than United States dollars, billings on the contract shall be expressed, and payment by the United States Government shall be made, in that other currency at amounts coincident with actual costs incurred. Currency fluctuations may not be a basis of gain or loss to the Offeror. Notwithstanding the above, the total of all invoices paid under this contract may not exceed the United States dollars authorized.
ATTACHMENT #4
SAMPLE INVOICE FORM
SAMPLE INVOICE FORM
|
Company Name
|
||||
|
Designated Billing Office Name and Address:
DHHS/OS/ASPR/AMCG
Attn: Contracting Officer 330 Independence Avenue., S.W. Room G640 Washington, D.C. 20201 Contractor's Address and Contact Information:
|
Invoice/Finance Number:
|
|||
|
Date Invoice Prepared:
|
||||
|
Contract No.
|
||||
|
Effective Date:
|
||||
|
Total Estimated Cost of Order:
|
||||
|
Office of Acquisitions:
Contracting Officer (insert name here) Office of Acquisitions Management, Contracts, and Grants (AMCG) Central Point of Distribution:
|
||||
|
POC: Name of accountant or COO or signatory authority for invoice
Title:
Phone: E‐Mail: |
||||
|
TIN:
DUNS #: |
||||
This invoice represents reimbursable costs for the period from
|
Amount Billed
|
|||
|
Expenditure Category
|
Current
|
Cumulative
|
Contract Value
|
|
Direct Costs:
|
|||
|
Direct Labor
|
|||
|
Fringe Benefits 0.00%
|
|||
|
Total Labor Costs:
|
|||
|
Overhead 0.00%
|
|||
|
Travel
|
|||
|
Subcontracts
|
|||
|
Consultant Fees
|
|||
|
Materials and Supplies
|
|||
|
Other
|
|||
|
Total Direct Costs
|
|||
|
G&A Rate 0.00%
|
|||
|
Subtotal:
|
|||
|
Fixed Fee 0.0%
|
|||
|
Total Amount Claimed
|
|||
|
Adjustments
|
|||
|
Grand Total
|
$ ‐
|
||
I certify that all payments requested are for appropriate purposes and in accordance with the contract.
Name/signature of signatory authority for invoicing
D. Attachment D- Disclosure of Lobbying Activities
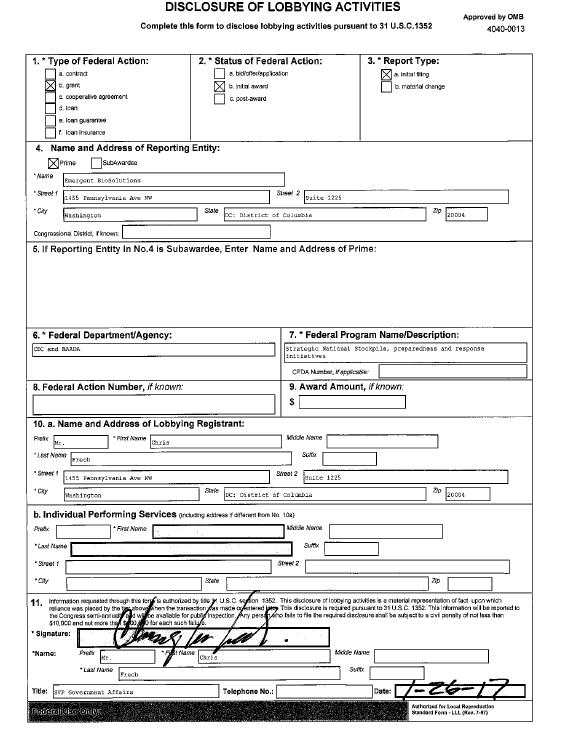
G. Attachment G – ACH Vendor / Miscellaneous Payment Enrollment Form
ACH VENDOR / MISCELLANEOUS PAYMENT ENROLLMENT FORM
Payment Information Form
The information requested on this form concerns your financial institution, your account at that institution, and personal information which needs to verified and completed.
Privacy Act Statement
The following information is provided to comply with the Privacy Act of 1974 (P.L. 93-579). All information collected on this form is required under the provisions of 31 USC 3322 and 31 CFR 210. This information will be used by the Treasury Department to transmit payment data, by electronic means to your financial institution. Failure to provide the requested information may delay or prevent the receipt of payments through the Automated Clearing House Payment System
Check one of the following:
|
X
|
|||||
|
Federal Employee:
|
Contractor:
|
Vendor:
|
|||
|
Name:
|
Emergent BioSolutions, Inc.
|
|
|
Business
Address: |
400 Professional Drive, Suite 400
|
|
|
Gaithersburg, MD 20879
|
||
|
Remit To
|
||
|
(If same as above, leave blank. Must match address on invoice for internal control purposes.)
|
||
|
Address:
|
||
Taxpayer Identification # (TIN): 14-1902018
(If you are an individual, this may be your Social Security number)
1. Payee's Telephone Number: (240) 631-3200
The following information must be completed by your financial institution representative:
| 2. |
Name of Financial Institution:
|
[**]
| 3. |
Address of Financial Institution:
|
[**]
4. Financial Institution's 9-digit ABA Routing Number for
Transfer of Funds: [**]
Transfer of Funds: [**]
| 5. |
Depositor Account Title: Emergent BioSolutions, Inc.
|
|
6. Depositor Account Number:
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|
X
|
||||
|
7. Type of Account
|
Checking
|
Savings
|
||
| 8. |
Signature and Title of Authorized Official of Financial Institution:
|
Please refer to attached letter from our bank.
Telephone Number: (____) ________________ Date: ______________
|
************The following must be signed by the payee************
|
|
|
I have verified the information on this form.
|
|
|
Signature
|
Date
|
E. Attachment E – Small Business Subcontracting Plan
OFFICE OF SMALL AND DISADVANTAGED BUSINESS UTILIZATION
SMALL BUSINESS SUBCONTRACTING PLAN
SMALL BUSINESS SUBCONTRACTING PLAN
The following outline meets the minimum requirements of section 8(d) of the Small Business Act, as amended, and implemented by the Federal Acquisition Regulations (FAR) Subpart 19.7. The U.S. Department of Health and Human Services (HHS), Office of Small and Disadvantaged Business Utilization (OSDBU) recommend offerors use the following format to submit proposed Individual Subcontracting Plans, including modifications. It is not intended to replace any existing Corporate/Commercial Plan that is more extensive. A subcontracting Plan is required if the estimated cost of the contract may exceed $650,000 ($1,500,000 for construction) Small businesses are excluded. Questions should be forwarded to the Contracting Officer or Operating Division (OPDIV) Small Business Specialist.
Operating Division (OPDIV):
SOLICITATION OR CONTRACT NUMBER: 17-100-SOL-00010
DATE OF PLAN: February 1, 2017
CONTRACTOR: Emergent Biodefense Operations Lansing LLC
ADDRESS: 3500 N. Martin Luther King Jr. Blvd
STATE/ZIP CODE Lansing, Michigan 48906
DUNN & BRADSTREET NUMBER: [**]
ITEM/SERVICE (Description): Manufacturing and Delivery of BioThrax® to SNS
NEW/INITIAL CONTRACT
PERIOD OF CONTRACT PERFORMANCE: 04/01/2017 – 03/31/2019
Base (plus options) $99,941,720 Performance Period: 04/01/17 – 03/31/19
$99,941,720 Total Contract Cost
CONTRACT MODIFICATION (if applicable)
NEW PERIOD OF CONTRACT PERFORMANCE (MM/DD/YYYY –
MM/DD/YYYY): ______N/A_______
MM/DD/YYYY): ______N/A_______
Original/Base $______N/A________________________ Performance Period/Quantity ______
Modification $______N/A________________________ Performance Period/Quantity ______
Task Order $______N/A________________________ Performance Period/Quantity ______
$______N/A________________________ Modified Total Contract Cost
Failure to include the essential information of FAR Subpart 19.7 may be cause for either a delay in acceptance or the rejection of a bid or offer when a subcontracting plan is required. "SUBCONTRACT," as used in this clause, means any agreement (other than one involving an employer-employee relationship) entered into by a Federal Government prime contractor or subcontractor requesting supplies or services required for performance of the contract or subcontract.
If assistance is needed to locate small business sources, contact the Small Business Specialist (SBS) supporting the OPDIV. SBS contact information is located on the OSDBU website (http://www.hhs.gov/about/smallbusiness/osdbustaff.html) or you may contact the OSDBU headquarters at (202) 690-7300.
HHS current subcontracting goal is 33.0% for Small Business (hereafter referred to as SB), 5.00% for Small Disadvantaged Business, including 8(a) Program Participants, Alaska Native Corporations (ANC) and Indian Tribes (hereafter referred to as SDB), 5.00% for Women-Owned Small Business and Economically Disadvantaged Women-Owned Small Business (hereafter referred to as WOSB), 3.00% HubZone business (hereafter referred to as HUBZone), 3.00% Veteran Owned Small Business (hereafter referred to as VOSB) and 3.00% Service Disabled Veteran-Owned Small Business (hereafter referred to as SDVOSB) concerns for Fiscal Year (FY) 2017. For this procurement, HHS expects all proposed subcontracting plans to contain at a minimum the aforementioned percentages. These percentages shall be expressed as percentages of the total estimated subcontracting dollars.
| 1. |
Type of Plan (check one)
|
X Individual plan (all elements developed specifically for this contract and applicable for the full term of this contract).
Master plan (goals developed for this contract) all other elements standardized and approved by a lead agency Federal Official; must be renewed every three years and contractor must provide copy of lead agency approval.
Commercial products/service plan (goals are negotiated with the initial agency on a company-wide basis rather than for individual contracts) this plan applies to the entire production of commercial service or items or a portion thereof. The contractor sells commercial products and services customarily used for non- government purposes. The plan is effective during the offeror's fiscal year (attach a copy). The Summary Subcontracting Report (SSR) must include a breakout of subcontracting prorated for HHS and other Federal agencies.
| 2. |
Goals
|
Below indicate the dollar and percentage goals for Small Business (SB), Small Disadvantaged (SDB) including Alaska Native Corporations and Indian Tribes, Women-owned and Economically Disadvantaged Women-Owned (WOSB), Historically Underutilized Business Zone (HUBZone), Veteran Owned Small Business (VOSB), Service-Disabled Veteran-Owned (SDVOSB) Small Businesses and "Other than Small Business" (Other) as subcontractors. Indicate the base year and each option year, as specified in FAR 19.704 or project annual subcontracting base and goals under commercial plans. If any contract has more four options, please attach additional sheets which illustrate dollar amounts and percentages. PLEASE NOTE: Zero dollars is not an acceptable goal for the SB, SDB, WOSB, HUBZone, VOSB or SDVOSB categories since this does not demonstrate a good faith effort throughout the period of performance of the contract. Formula for below: 2.b. + 2.h. = 2.a.
a. Total estimated dollar value of ALL planned subcontracting, i.e., with ALL types of concerns under this contract is $[**] (Base Period – plus options).
b. Total estimated dollar value and percent of planned subcontracting with SMALL BUSINESSES (including SDB, WOSB, HUBZone, VOSB and SDVOSB): (% of "a") $[**] and [**]% (Base Period – plus options)
c. Total estimated dollar value and percent of planned subcontracting with SMALL DISADVANTAGED BUSINESSES: (% of "a") $[**] and [**]% (Base Period – plus options)
d. Total estimated dollar value and percent of planned subcontracting with WOMEN-OWNED SMALL BUSINESSES: (% of "a") $[**] and [**]% (Base Period – plus options)
e. Total estimated dollar and percent of planned subcontracting with HUBZone SMALL BUSINESSES:
(% of "a") $[**] and [**]% (Base Period – plus options)
f. Total estimated dollar and percent of planned subcontracting with VETERAN-OWNED SMALL BUSINESSES: (% of "a") $[**] and [**]% (Base Period – plus options)
g. Total estimated dollar and percent of planned subcontracting with SERVICE- DISABLED VETERAN-OWNED SMALL BUSINESSES: (% of "a")
$[**] and [**]% (Base Period – plus options)
$[**] and [**]% (Base Period – plus options)
h. Total estimated dollar and percent of planned subcontracting with "OTHER THAN SMALL BUSINESSES" (As defined by the Small Business Administration as "any entity that is not classified as a small business. This includes large businesses, state and local governments, non-profit organizations, public utilities, educational institutions and foreign-owned firms.) (% of "a")$[**] and [**]% (Base Period – plus options)
i. Provide a description of ALL the products and/or services to be subcontracted under this contract, and indicate the size and type of business supplying them (check all that apply):
|
Products and/or
Services
|
Other
|
Small Business
|
SDB
|
WOSB
|
Hubz
|
VOSB
|
SDVOSB
|
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
[**]
|
j. Provide a description of the method used to develop the subcontracting goals for SB, SDB, WOSB, HUBZone and SDVOSB concerns. Address efforts made to ensure that maximum practicable subcontracting opportunities have been made available for those concerns and explain the method used to identify potential sources for solicitation purposes. Explain the method and state the quantitative basis (in dollars) used to establish the percentage goals. Also, explain how the areas to be subcontracted to SB, WOSB, HUBZone, VOSB and SDVOSB concerns were determined, how the capabilities of these concerns were considered contract opportunities and how such data comports with the cost proposal. Identify any source lists or other resources used in the determination process. (Attach additional sheets, if necessary.)
Emergent Biodefense Operations Lansing LLC (Emergent) solicited proposals from sources with the qualifications required to perform commercial item manufacturing and delivery services, which are the same as those required under this contract. Those contractors that best meet the business specifications, capability, performance expectations, cost competitiveness and other relevant criteria have been considered for this effort. Emergent plans to utilize small business concerns to the maximum extent practical and regularly surveys the healthcare community to identify small businesses with the skills and experience to provide either validated cGMP manufacturing services or to provide validated controlled-temperature shipping services to destinations in the continental United States. BioThrax anthrax vaccine is a FDA-licensed product and manufacturing and shipment must conform to FDA regulations. Subcontractors such as our fill finish facilities must also be FDA-licensed to manufacture BioThrax. When we initially licensed our fill finish subcontractors, they were small businesses. However, subsequently they were purchased by large businesses. The process to identify, qualify and then, when required, FDA-license a new subcontractor is comparatively long and expensive. In addition, delivery under this contract requires a niche skill set and due to the complexity and very specialized nature of the program, there is a very small pool of qualified small businesses from which to make a selection and switching from our currently established subcontractors may introduce significant delivery risk. With these caveats, Emergent will use small businesses to perform work under this contract whenever practicable.
k. Indirect costs have _____ have not _X been included in the dollar and percentage subcontracting goals above (check one).
l. If indirect costs have been included, explain the method used to determine the proportionate share of such costs to be allocated as subcontracts to SB, SDB, WOSB, HUBZone, VOSB and SDVOSB concerns:
| 3. |
Program Administrator:
|
NAME: [**]
TITLE: [**]
ADDRESS: 400 Professional Drive, Suite 500
Gaithersburg, Maryland 20879
TELEPHONE: [**]
E-MAIL: [**]
Duties: Does the individual named above have general overall responsibility for the company's subcontracting program, i.e., developing, preparing, and executing subcontracting plans and monitoring performance relative to the requirements of those subcontracting plans and perform the following duties? (If NO is checked, please who in the company performs those duties, or indicate why the duties are not performed in your company on a separate sheet of paper and submit with the proposed subcontracting plan.)
| a. |
Developing and promoting company-wide policy initiatives that demonstrate the company's support for awarding contracts and subcontracts to SB, SDB, WOSB, HUBZone, VOSB and SDVOSB concerns; and for assuring that these concerns are included on the source lists for solicitations for products and services they are capable of providing; X yes __ no
|
| b. |
Developing and maintaining bidder source lists of SB, SDB, WOSB, HUBZone, VOSB and SDVOSB concerns from all possible sources; X yes __ no
|
| c. |
Ensuring periodic rotation of potential subcontractors on bidder's lists; X yes __ no
|
| d. |
Assuring that SB, SDB, WOSB, HUBZone, VOSB and SDVOSB businesses are included on the bidders' list for every subcontract solicitation for products and services that they are capable of providing. X yes __ no
|
| e. |
Ensuring that Requests for Proposals (RFPs) are designed to permit the maximum practicable participation of SB, SDB, WOSB, HUBZone, VOSB and SDVOSB concerns. X yes __ no
|
| f. |
Reviewing subcontract solicitations to remove statements, clauses, etc., which might tend to restrict or prohibit small, 8(a), SDB, WOSB, HUBZone, VOSB and SDVOSB small business participation. X yes __ no
|
| g. |
Accessing various sources for the identification of SB, SDB, WOSB, HUBZone, VOSB and SDVOSB concerns to include the Central Contractor Registration (http://www.ccr.gov/), local small business and minority associations, local chambers of commerce and Federal Agencies' Small Business Offices; X yes __ no
|
| h. |
Establishing and maintaining contract and subcontract award records; X yes __ no
|
| i. |
Participating in Business Opportunity Workshops, Minority Business Enterprise Seminars, Trade Fairs, Procurement Conferences, etc.; X yes __ no
|
| j. |
Ensuring that SB, SDB, WOSB, HUBZone, VOSB and SDVOSB concerns are made aware of subcontracting opportunities and assisting concerns in preparing responsive bids to the company; X yes __ no
|
| k. |
Conducting or arranging for the conduct of training for purchasing personnel regarding the intent and impact of Section 8(d) of the Small Business Act, as amended; X yes __ no
|
| l. |
Monitoring the company's subcontracting program performance and making any adjustments necessary to achieve the subcontract plan goals; X yes __ no
|
| m. |
Preparing and submitting timely, required subcontract reports; X yes __ no
|
| n. |
Conducting or arranging training for purchasing personnel regarding the intent and impact of 8(d) of the Small Business Act on purchasing procedures; X yes __ no
|
| o. |
Coordinating the company's activities during the conduct of compliance reviews by Federal agencies; and X yes __ no
|
| p. | Other duties: |
| 4. |
Equitable Opportunity
|
Describe efforts the offeror will undertake to ensure that SB, SDB, WOSB, HUBZone, VOSB and SDVOSB concerns will have an equitable opportunity to compete for subcontracts. These efforts include, but are not limited to, the following activities:
a. Outreach efforts to obtain sources:
| 1. |
Contact minority and small business trade associations; 2) contact business development organizations and local chambers of commerce; 3) attend SB, SDB, WOSB, HUBZone, VOSB and SDVOSB procurement conferences and trade fairs; 4) review sources from the Central Contractor Registration (http://www.ccr.gov/); 5) review sources from the Small Business Administration (SBA), Central Contractor Registration (CCR); 6) Consider using other sources such as the National Institutes of Health (NIH) e-Portals in Commerce, (e-PIC), (http://epic.od.nih.gov/). The NIH e-PIC is not a mandatory source; however, it may be used at the offeror's discretion; and 7) Utilize newspaper and magazine ads to encourage new sources.
|
b. Internal efforts to guide and encourage purchasing personnel:
| 1. |
Conduct workshops, seminars and training programs;
|
| 2. |
Establish, maintain, and utilize SB, SDB, WOSB, HUBZone, VOSB and SDVOSB source lists, guides, and other data for soliciting subcontractors; and
|
| 3. |
Monitor activities to evaluate compliance with the subcontracting plan.
|
Additional efforts:
| 5. |
Flow Down Clause
|
The contractor agrees to include the provisions under FAR 52.219-8, "Utilization of Small Business Concerns," in all acquisitions exceeding the simplified acquisition threshold that offers further subcontracting opportunities. All subcontractors, except small business concerns, that receive subcontracts in excess of $650,000 ($1,500,000 for construction) must adopt and comply with a plan similar to the plan required by FAR 52.219-9, "Small Business Subcontracting Plan." Note: In accordance with FAR 52.212-5(e) and 52.244-6(c) the contractor is not required to include flow-down clause FAR 52.219.-9 if it is subcontracting commercial items.
| 6. |
Reporting and Cooperation
|
The contractor gives assurance of 1) cooperation in any studies or surveys that may be required; 2) submission of periodic reports which illustrate compliance with the subcontracting plan; 3) submission of its Individual Subcontracting Report (ISR) and Summary Subcontract Report (SSR); and 4) subcontractors' submission of ISRs and SSRs. ISRs and SSRs shall be submitted via the Electronic Subcontracting Reporting System (eSRS) website https://esrs.symplicity.com/index?_tab=signin&cck=1
|
Reporting Period
|
Report Due
|
Due Date
|
|
Oct 1 - Mar 31
|
ISR
|
4/30
|
|
Apr 1 - Sept 30
|
ISR
|
10/30
|
|
Oct 1 - Sept 30
|
SSR
|
10/30
|
|
Contract Completion
|
Year End SDB Report
|
30 days after completion
|
Please refer to FAR Part 19.7 for instruction concerning the submission of a Commercial Plan: SSR is due on 10/30 each year for the previous fiscal year ending 9/30.
| a. |
Submit ISR (bi-annually) for the awarding Contracting Officer's review and acceptance via the eSRS website.
|
| b. |
Currently, SSR (annually) must be submitted for the HHS eSRS Agency Coordinator review and acceptance via the eSRS website. (Note: Log onto the OSDBU website to view the HHS Agency Coordinator contact information (http://www.hhs.gov/about/smallbusiness/osdbustaff.html).
|
Note: The Request for Proposal (RFP) will indicate whether a subcontracting plan is required. Due to the nature and complexity of many HHS contracts, particularly the Centers for Medicare and Medicaid (CMS), the contractor may not be required to submit its subcontracting reports through the eSRS. The Contracting Officer will confirm reporting requirements prior to the issuance of an award. For more information, contact Courtney Carter, Agency Coordinator-eSRS (Courtney.Carter@hhs.gov).
| 7. |
Record keeping
|
FAR 19.704(a) (11) requires a list of the types of records your company will maintain to demonstrate the procedures adopted to comply with the requirements and goals in the subcontracting plan. The following is a recitation of the types of records the contractor will maintain to demonstrate the procedures adopted to comply with the requirements and goals in the subcontracting plan. These records will include, but not be limited to, the following:
| a. |
SB, SDB, WOSB, HUBZone, VOSB and SDVOSB source lists, guides and other data identifying such vendors;
|
| b. |
Organizations contacted in an attempt to locate SB, SDB, WOSB, HUBZone, VOSB and SDVOSB sources;
|
| c. |
On a contract-by-contract basis, records on all subcontract solicitations over $100,000, which indicate for each solicitation (1) whether SB, SDB, WOSB, HUBZone, VOSB and/or SDVOSB concerns were solicited, if not, why not and the reasons solicited concerns did not receive subcontract awards;
|
| d. |
Records to support other outreach efforts, e.g., contacts with minority and small business trade associations, attendance at small and minority business procurement conferences and trade fairs;
|
| e. |
Records to support internal guidance and encouragement provided to buyers through (1) workshops, seminars, training programs, incentive awards; and (2) monitoring performance to evaluate compliance with the program and requirements; and
|
| f. |
On a contract-by-contract basis, records to support subcontract award data including the name, address, and business type and size of each subcontractor. (This is not required on a contract–by–contract basis for commercial plans.)
|
| g. |
Other records to support your compliance with the subcontracting plan: (Please describe)
|
| 8. |
Timely Payments to Subcontractors
|
FAR 19.702 requires your company to establish and use procedures to ensure the timely payment of amounts due pursuant to the terms of your subcontracts with SB concerns, SOB, WOSB, HUBZone, VOSB and SDVOSB concerns.
Your company has established and used such procedures: X yes __ no
| 9. |
Description of Good Faith Effort
|
Maximum practicable utilization of SB, SOB, WOSB, HUBZone, VOSB and SDVOSB concerns as subcontractors in Government contracts is a matter of national interest with both social and economic benefits. When a contractor fails to make a good faith effort to comply with a subcontracting plan, these objectives are not achieved, and 15 U.S.C. 637(d) (4) (F) directs that liquidated damages shall be paid by the contractor. In order to demonstrate your compliance with a good faith effort to achieve the SB, SOB, WOSB, HUBZone, VOSB and SDVOSB small business subcontracting goals, outline the steps your company plans to take. These steps will be negotiated with the contracting official prior to approval of the plan.
Implement a supplier diversity program, 2) upgrade current vendor system software to allow for enhanced measurement of Small/Minority Business activities, 3) attend Small Business seminars to identify qualified candidates, 4) review all contract forms to ensure terms support Small Business subcontracting goals.
SIGNATURE PAGE
Signatures Required
This subcontracting plan was submitted by:
Signature: [**]
Typed/Print Name: [**]
Title: [**]
Date: February 1, 2017
This plan was reviewed by:
Signature:
Typed/Print Name:
Title: Contracting OfficerDate:
This plan was reviewed by:
Signature:
Typed/Print Name:
Title: HHS Small Business SpecialistDate:
This plan was reviewed by:
Signature:
Typed/Print Name:
Title: Small Business Administration Procurement Center Representative
Date:
This plan was approved by:
Signature:
Typed/Print Name:
Title: Contracting OfficerDate:
HHS SUBCONTRACTING PLAN REVIEW FORM
OSDBU Control No.: Multiple Awards?Yes:No:(If yes, attach a copy of each subcontracting plan)
|
PROJECT INFORMATION
|
||||||||||
|
Solicitation/Contract No.: RFP-17-100-SOL-00010
|
MOD No. (If applicable):
|
|||||||||
|
Title of Acquisition: Anthrax Vaccine for the Strategic National Stockpile (SNS)
|
||||||||||
|
Contractor's Name: Emergent BioSolutions
|
||||||||||
|
Period of Performance: 04/01/17-03/31/19
|
Total Contract Amount (including options): $ 99,941,720
|
|||||||||
|
Total Modification Amount: (if applicable) $
|
Base Period (if there are options): $
|
|||||||||
|
Option 1 (if applicable): $
|
Option 2 (if applicable): $
|
|||||||||
|
Option 3 (if applicable): $
|
Option 4 (if applicable): $
|
|||||||||
|
Contracting Officer/Specialist Name: [**]
|
Tei&Fax: [**]
|
|||||||||
|
OPDIV/Division/Branch (including location): HHS/ASPR/AMCG O'Neil 11B16
|
Email: [**]
|
|||||||||
|
SUBCONTRACT PLAN REQUIREMENTS
|
||||||||||
|
1. Type of Plan (check one):
|
Individual X MasterCommercial
|
|||||||||
|
(A=Acceptable; U=Unacceptable)
|
C.O.
|
SBS
|
SBA/PCR
|
|||||||
|
2. Subcontracting Goal Data
|
A
|
U
|
A
|
U
|
A
|
U
|
||||
|
a. Total Subcontracting Dollars [(2b+2h=2a), except when subcontract baseline equals contract value]:
$[**]
|
X
|
X
|
||||||||
|
b. Total Subcontracting Dollars & Percentage with Small Businesses (incl. SDB, WOSB, HUBZone, SDVOSB)- [Percentage of 2a.]:
$ [**] and[**] %
|
X
|
X
|
||||||||
|
c. Total Subcontracting Dollars & Percentage with Small Disadvantaged Businesses - [Percentage of 2.a.)
$ [**] and[**] %
|
X
|
X
|
||||||||
|
d. Total Subcontracting Dollars & Percentage with Woman-owned Small Businesses - [Percentage of 2.a.]
$[**] and[**] %
|
X
|
X
|
||||||||
|
e. Total Subcontracting Dollars & Percentage with HUBZone Small Businesses - [Percentage of 2.a.]
$[**] and[**] %
|
X
|
X
|
||||||||
|
f. Total Subcontracting Dollars & Percentage with'Veteran Owned Small Businesses - [Percentage of 2.a.)
$[**] and[**] %
|
X
|
X
|
||||||||
|
g. Total Subcontracting Dollars & Percentage with Service-Disabled Veteran Small Businesses - [Percentage of 2.a.]
$[**] and[**] %
|
X
|
X
|
||||||||
|
h. Total Subcontracting Dollars & Percentage with "Other" than Small Businesses (i.e., large companies, non profits, etc.) [Percentage of 2.a.]
$[**] and [**] %
|
X
|
X
|
||||||||
|
i. Subcontracting Opportunities (description of all principal products/services to be subcontracted to all types of concerns)
• Raw Materials/Supplies: other, SB, WOSB
• Fill/Finish: other
• Shipping: other, SB
|
X
|
X
|
||||||||
|
j.k.l. Methodology used to develop goals & identify potential sources (e.g. historical trends, information on technical and competitive bidding, formula for calculating goals, etc.)
|
X
|
|||||||||
HHS SUBCONTRACTING PLAN REVIEW FORM – Page 2
|
SUBCONTRACT PLAN REQUIREMENTS (con't)
|
C.O.
|
SBS
|
SBA/PCR
|
|||
|
(A=Acceptable; U=Unacceptable)
|
A
|
U
|
A
|
U
|
A
|
U
|
|
3. Subcontracting Plan Administrator's Name (Contractor): [**]
|
X
|
X
|
||||
|
4. Description of efforts to ensure the Small Businesses (incl. SDB, WOSB, HUBZone, SDVOSB) entities have equitable opportunity to compete for subcontracts.
|
X
|
X
|
||||
|
5. Required flow-down clause(s) to be included in prime contractor's subcontracts (i.e., FAR Clause 52.219-8, 52-219-9, 52.212-5(e)):
|
X
|
X
|
||||
|
6. Reporting and Cooperation:
|
||||||
|
a. Agreement to submit required reports
|
X
|
X
|
||||
|
b. Agreement to cooperate in studies and surveys
|
X
|
X
|
||||
|
7. Record keeping
|
X
|
X
|
||||
|
8. Timely Payment to Subcontractos
|
X
|
X
|
||||
|
9. Description of Good Faith Effort
|
X
|
X
|
||||
|
CONTRACTING OFFICER DETERMINATION, OSDBU SMALL BUSINESS SPECIALIST AND SBA PCR RECOMMENDATION
|
C.O.
|
SBS
|
SBA/PCR
|
|||
|
Yes
|
No
|
Yes
|
No
|
Yes
|
No
|
|
|
1. The proposed plan meets the requirements of FAR 19.704.
|
X
|
X
|
X
|
|||
|
2. In accordance with 19.705-4, past performance has been considered when determining acceptability of this plan.
|
X
|
X
|
||||
|
3. The proposed plan requires an additional pre-award review.
|
X
|
X
|
X
|
|||
|
HHS OPDIV Contracting Officer Signature
|
||||||
|
Contracting Officer: /s/ [**] Date: 2/21/17
Additional Comments:
Though the contractor did not meet most of HHS's SB subcontracting goals, per the justification from the contractor as to the reason why, the proposed SB plan is deemed acceptable.
|
||||||
|
HHS OSDBU SBS Signature
|
||||||
 Small Business Specialist: Date: 2/23/17
COMMENTS: If any elements are determined to be unacceptable, summarize below:
|
||||||
|
SBA PCR Signature
|
||||||
|
SBA PCR:
 Date: 2/23/2017 Date: 2/23/2017 COMMENTS: If any elements are determined to be unacceptable, summarize below:
Justification accepted for goals below agency-established minimums.
|
||||||
|
HHS SUBCONTRACTING PLAN REVIEW FORM INSTRUCTIONS
|
|
|
OSDBU Control No.: Insert the control number from the HHS 653 which was assigned by the SBS
Multiple Awards: indicate as appropriate. If yes, attach a copy of each subcontracting plan
PROJECT INFORMATION:
· Solicitation/Contract No.: Enter the assigned RFP or Contract Number
· Modification: Identify the modification number for the contract if applicable.
· Title of Acquisition: Enter the item/service description or project title
· Contractor's Name: Enter Successful Offeror/Contractor's name
· Period of Performance: Enter the estimated performance period, including all options, in the following format (mm/dd/yy - mm/dd/yy)
· Total Contract Amount: Enter the total estimated dollar value of the contract, including all options & modifications.
· Total Modification Amount: (if applicable)
· Base Period & Options 1 through Option 4: Complete these boxes if options are part of the contract.
· CO/CS Contact Information: Enter Contracting Officer/Specialist's Name, OPDIV, Building, Room, Telephone, and Fax and e-mail.
SUBCONTRACTING PLAN REQUIREMENTS (ITEMS 1 - 9)
1. Enter type of plan: individual, master or commercial
2. For each of the sub-items 2.a. through 2.1., the C.O. must review the plan & determine if the requirement is acceptable or unacceptable.
For each of the sub-items 2.b. through 2.h., the C.O. shall include the subcontracting dollars & percentage for the category. In calculating percentage, use the subcontracting dollars for the sub-item as the numerator & the total subcontracting dollars for the contract as the denominator. (i.e. 2.b. divided by 2.a. =%for 2.b.)
3. through 9: the C.O. must review the plan & determine if the requirements are acceptable or unacceptable.
CONTRACTING OFFICER DETERMINATION, OSDBU SMALL BUSINESS SPECIALIST & SBA PCR RECOMMENDATION (ITEMS 1- 3)
1. The CO must review the plan to determine if it meets the requirements in FAR 19.7.
2. Indicate whether past performance had been considered when determining acceptability of plan
3. Indicate whether plan requires further pre-award review.
|
SIGNATURES
· The CO who has the authority to bind the government will make a determination, sign and date.
· The HHS SBS will sign and date the review form and the subcontracting plan signature page if acceptable. During the plan review, the SBS may require additional input from the CO and/or contractor. The SBS may also include comments regarding the plan as necessary
· The SBA PCR shall sign and date the review form & the subcontracting plan signature page if acceptable. Concurrence or non-concurrence of the acquisition method determined by the CO The SBA PCR may also include comments regarding the plan as necessary.
NOTE: In order for the HHS Small Business Specialist to conduct a comprehensive review of each plan, at a minimum, the documentation forwarded by the CO should include:
1. A completed HHS Subcontracting Plan Review Form signed by the Contracting Official
2. A completed Subcontracting Plan, using the HHS Subcontracting Plan template, signed by the offeror and the CO.
3. The Summary of Proposed Costs from the offeror's Final Proposal Revision.
4. Any narrative, attachment or supplemental documentation to the offeror's plan describing efforts to locate small business subcontractors, rationale for using other than small businesses as subcontractors, etc.
|
February 1, 2017
| Attn: |
[**]
|
Emergent Bio Solutions
400 Professional Drive, Suite 400
Gaithersburg, MD 20879
400 Professional Drive, Suite 400
Gaithersburg, MD 20879
Subject: Solicitation # 17-100-SOL-00010 Responses to Questions
Dear [**],
We received several questions regarding solicitation # 17-100-SOL-00010 and would like to provide responses to those questions.
Please let us know if you have any further questions.
Thanks,
[**]
[**]
Contracting Officer, Section Chief
Office of Acquisitions Management, Contracts & Grants (AMCG)
Assistant Secretary for Preparedness & Response (ASPR)
U.S. Department of Health & Human Services
Contracting Officer, Section Chief
Office of Acquisitions Management, Contracts & Grants (AMCG)
Assistant Secretary for Preparedness & Response (ASPR)
U.S. Department of Health & Human Services
Encl.
Solicitation # 17-100-SOL-00010: USG's Responses to
Emergent's January 27, 2016 Questions
Emergent's January 27, 2016 Questions
Question 1 Response:
Earned Value Management (EVM) deliverables will not be required for this procurement and will be removed from Section C.
Question 2 Response:
Section C.5.D. will be altered to state that the Offeror shall provide a copy of any regulatory communications (e.g. meeting minutes, submissions, etc.) that occur during the contract period of performance pertaining to the product being procured (i.e. BioThrax). Any pertinent regulatory communications or issues that may impact the product or ability of the Offeror to complete delivery of the product on this particular contract shall be made known to BARDA/AMCG until delivery of all [**] doses are completed.
Question 3 Response:
Yes, the lower limit will be aligned with the new CDC [**] contract at [**] months.
Question 4 Response:
Yes, the items (Certificate(s) of Analysis and FDA Lot Release(s)) can be provided at least [**] before each scheduled shipment.
