Attached files
| file | filename |
|---|---|
| EX-99.1 - PRESS RELEASE - LUMOS PHARMA, INC. | nlnk-20170504x8kxex991.htm |
| 8-K - 8-K - LUMOS PHARMA, INC. | nlnk-20170504x8k.htm |

NewLink Genetics Corporation
Nasdaq: NLNK
May 4, 2017
First Quarter 2017 Results

Agenda
2
Introduction
Jack Henneman, Executive Vice President & CFO
IDO Pathway Program Developments
Charles J. Link, Jr., M.D., Chairman, CEO & CSO
Clinical Updates / Guidance on Timing of Data
Nicholas N. Vahanian, M.D., President & CMO
First Quarter 2017 Financial Results
Mr. Henneman

Cautionary Note Regarding Forward-Looking Statements
This presentation contains forward-looking statements of NewLink Genetics that involve substantial risks
and uncertainties. All statements, other than statements of historical facts, contained in this presentation
are forward-looking statements, within the meaning of The Private Securities Litigation Reform Act of 1995.
The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "target," "potential," "will,"
"could," "should," "seek" or the negative of these terms or other similar expressions are intended to identify
forward-looking statements, although not all forward-looking statements contain these identifying words.
These forward-looking statements include any statements other than statements of historical fact. Actual
results or events could differ materially from the plans, intentions and expectations disclosed in the forward-
looking statements that NewLink Genetics makes due to a number of important factors, including those
risks discussed in "Risk Factors" and elsewhere in NewLink Genetics' Annual Report on Form 10-K for the
year ended December 31, 2016 and other reports filed with the U.S. Securities and Exchange
Commission (SEC). The forward-looking statements in this presentation represent NewLink'
Genetics' views as of the date of this presentation. NewLink Genetics anticipates that subsequent events
and developments will cause its views to change. However, while it may elect to update these forward-
looking statements at some point in the future, it specifically disclaims any obligation to do so. You should,
therefore, not rely on these forward-looking statements as representing NewLink Genetics' views as of any
date subsequent to the date of this presentation.
3

Q1 Takeaways
Emerging clinical data are validating the IDO pathway as central to immunosuppression
IDO pathway inhibition has the potential to enhance patient outcomes when used in combination with other cancer
therapies
NewLink has two distinct types of IDO pathway inhibitors in the clinic
– Indoximod: wholly-owned by NewLink
– Navoximod (GDC0919): partnered with Genentech/Roche
– NLG802: NewLink’s next generation prodrug of indoximod to enter clinic later this year
Presented promising interim Phase 2 data of the IDO pathway inhibitor, indoximod, in combination with KEYTRUDA®
(pembrolizumab) for patients with advanced melanoma at the American Association of Cancer Research (AACR)
plenary session on April 4, 2017
Presented a poster on NLG802, “A novel prodrug of indoximod with enhanced pharmacokinetic properties,” at AACR
on April 4, 2017
Abstract accepted for presentation at the 2017 Annual Meeting of the American Society of Clinical Oncology (ASCO)
for a randomized double-blind, placebo-controlled Phase 2 study of indoximod in combination with the vaccine,
PROVENGE® (sipuleucel-T), for patients with metastatic castration resistant prostate cancer
Abstract accepted for presentation at the 2017 ASCO Annual Meeting submitted by our partner on a Phase 1b dose-
escalation study of navoximod (GDC-0919) in combination with TECENTRIQ® (atezolizumab) in multiple solid tumors
4
KEYTRUDA® is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
TECENTRIQ® is a registered trademark of Genentech, a member of the Roche Group.
PROVENGE® is a registered trademark of Dendreon/Valeant Pharmaceuticals International, Inc.

Zakharia Y, et al. Oral presentation at: 107th Annual Meeting of the American Association for Cancer Research (AACR); April 1-5, 2017; Washington, DC. Abstract CT117.
Best Response by Patient
5
Distinct Difference in Non-ocular Versus Ocular Patients
80%
100%
40%
60%
0%
20%
–40%
–20%
–60%
–80%
–100%
Threshold for progression
Threshold for partial response
Non-ocular
Ocular
*
*
P
e
rc
e
nt
c
h
a
n
g
e
i
n
t
u
m
or
v
o
lu
m
e
*Stable disease of primary lesion; new non-target lesions classified patients as progressive disease.
Note: 1 patient was unevaluable for response due to pleural effusion/collapsed left lung; the patient progressed based on several new non-target lesions at Week 13.

Zakharia Y, et al. Oral presentation at: 107th Annual Meeting of the American Association for Cancer Research (AACR); April 1-5, 2017; Washington, DC. Abstract CT117.
Change in Tumor Volume Over Time
6
Durable and Ongoing Responses
PR, partial response.
Note: 1 patient was unevaluable for response due to pleural effusion/collapsed left lung; the patient progressed based on several new non-target lesions at Week 13.
–100
–80
–60
–40
–20
0
20
40
60
Baseline 84 78 72 66 60 54 48 42 36 30 24 18 12
Treatment duration in weeks
PR cut-off
P
e
rc
e
nt
c
h
a
n
g
e
i
n
t
u
m
or
v
o
lu
m
e

Anticipated Highlights for 2017 Clinical Programs
Metastatic castration resistant prostate cancer:
– Abstract 3066: Randomized placebo-controlled Phase 2 clinical trial data to be presented at ASCO on Monday,
June 5, 2017, 9:00 AM ET-12:30 PT ET
Metastatic pancreatic cancer:
– Indoximod in combination with gemcitabine + ABRAXANE® (nab-paclitaxel) Phase 2 trial data available at an
upcoming medical meeting in the second half of 2017
Acute Myeloid Leukemia (AML):
– Interim data from a Phase 1b dose-escalation study of indoximod in combination with standard of care
chemotherapy for patients with newly diagnosed AML available second half of 2017
Multiple solid tumors:
– Phase 1b dose-escalation trial data for navoximod (GDC-0919) plus TECENTRIQ® (atezolizumab) to be presented
at ASCO on Sunday, June 4, 2017 from 10:45 AM ET-12:15 PM ET by our partner (Abstract 105)
NLG802: next-generation, novel prodrug to enter clinic by end of Q3 2017
Advanced Melanoma:
– Initiate a pivotal trial of indoximod + anti-PD-1 inhibitors with dose confirmation stage in 2017 followed by a
randomized stage
7 ABRAXANE® is a registered trademark of Celgene Corporation
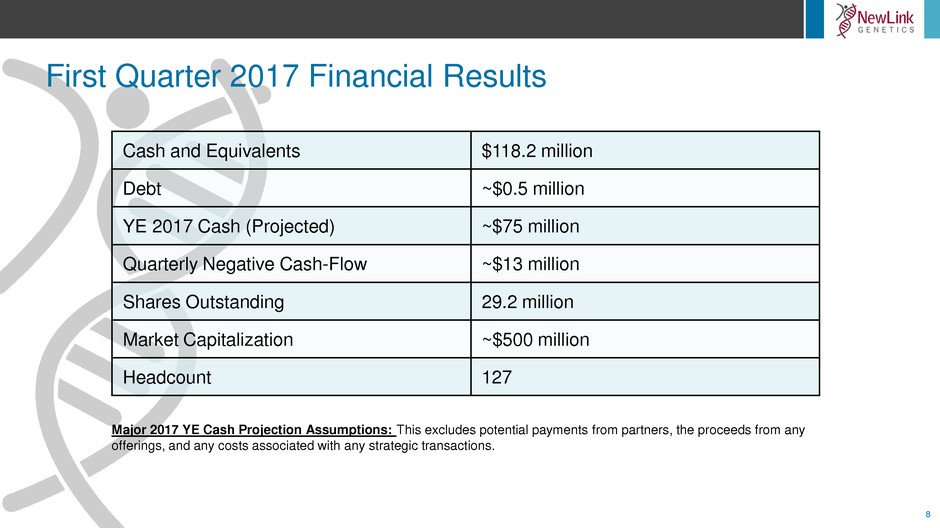
First Quarter 2017 Financial Results
Cash and Equivalents $118.2 million
Debt ~$0.5 million
YE 2017 Cash (Projected) ~$75 million
Quarterly Negative Cash-Flow ~$13 million
Shares Outstanding 29.2 million
Market Capitalization ~$500 million
Headcount 127
8
Major 2017 YE Cash Projection Assumptions: This excludes potential payments from partners, the proceeds from any
offerings, and any costs associated with any strategic transactions.

9
Q & A
