Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Fortress Biotech, Inc. | v465839_8k.htm |
Exhibit 99.1

Proprietary Materials Corporate Presentation May 2017

Proprietary Materials Forward Looking Statements This presentation may contain “forward - looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such statements include, but are not limited to, any statements relating to our growth strategy and product development programs and any other statements that are not historical facts. Forward - looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock price. Factors that could cause actual results to differ materially from those currently anticipated include: risks related to our growth strategy; risks relating to the results of research and development activities; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; uncertainties relating to preclinical and clinical testing; our dependence on third party suppliers; our ability to attract, integrate, and retain key personnel; the early stage of products under development; our need for and continued access to additional funds; government regulation; patent and intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as may be required by law. Fortress Biotech 2

Proprietary Materials Fortress Biotech: Our Unique Approach What we do: Acquire, develop and commercialize novel biopharmaceutical products in all stages of development and across multiple therapeutic areas directly within Fortress Biotech and through our subsidiaries. Our business strategy: Build subsidiaries around marketed products and product candidates that create a pipeline providing our shareholders with a diversified long - term revenue stream. Product candidates 4 18 Marketed Clinical Development 11 Pre - clinical 3 Fortress Biotech
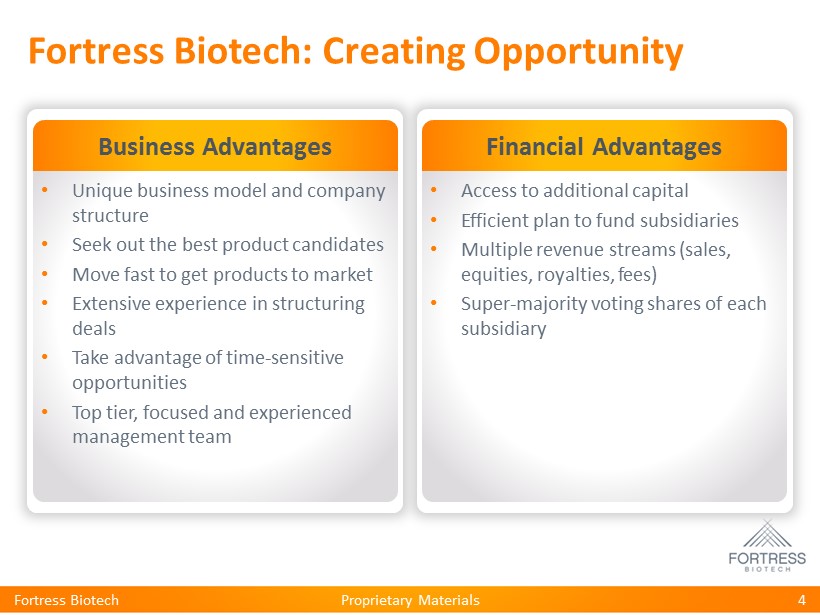
Proprietary Materials Fortress Biotech: Creating Opportunity Business Advantages • Unique business model and company structure • Seek out the best product candidates • Move fast to get products to market • Extensive experience in structuring deals • Take advantage of time - sensitive opportunities • Top tier, focused and experienced management team Financial Advantages • Access to additional capital • Efficient plan to fund subsidiaries • Multiple revenue streams (sales, equities, royalties, fees) • Super - majority voting shares of each subsidiary 4 Fortress Biotech
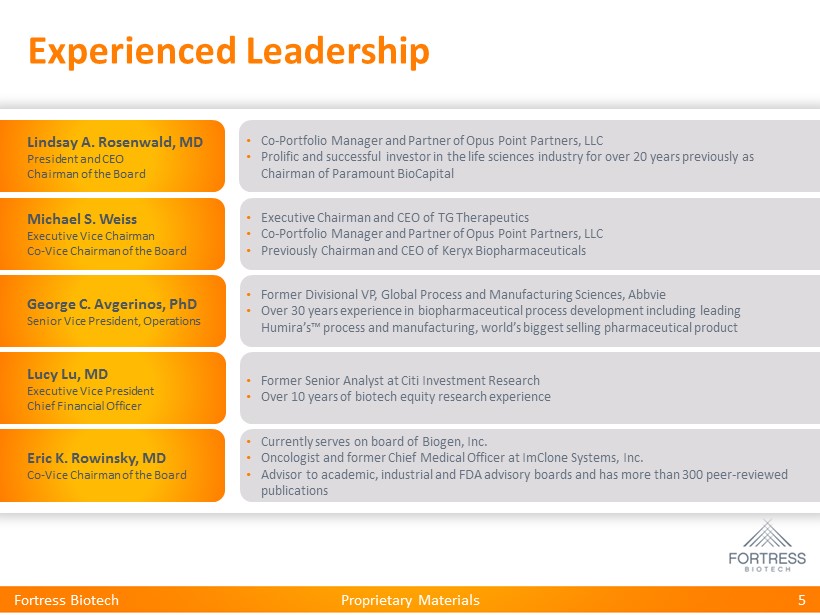
Proprietary Materials Experienced Leadership 5 • Co - Portfolio Manager and Partner of Opus Point Partners, LLC • Prolific and successful investor in the life sciences industry for over 20 years previously as Chairman of Paramount BioCapital Lindsay A. Rosenwald, MD President and CEO Chairman of the Board • Executive Chairman and CEO of TG Therapeutics • Co - Portfolio Manager and Partner of Opus Point Partners, LLC • Previously Chairman and CEO of Keryx Biopharmaceuticals Michael S. Weiss Executive Vice Chairman Co - Vice Chairman of the Board • Currently serves on board of Biogen, Inc. • Oncologist and former Chief Medical Officer at ImClone Systems, Inc. • Advisor to academic, industrial and FDA advisory boards and has more than 300 peer - reviewed publications Eric K. Rowinsky, MD Co - Vice Chairman of the Board • Former Senior Analyst at Citi Investment Research • Over 10 years of biotech equity research experience Lucy Lu, MD Executive Vice President Chief Financial Officer • Former Divisional VP, Global Process and Manufacturing Sciences, Abbvie • Over 30 years experience in biopharmaceutical process development including leading Humira’s™ process and manufacturing, world’s biggest selling pharmaceutical product George C. Avgerinos, PhD Senior Vice President, Operations Fortress Biotech
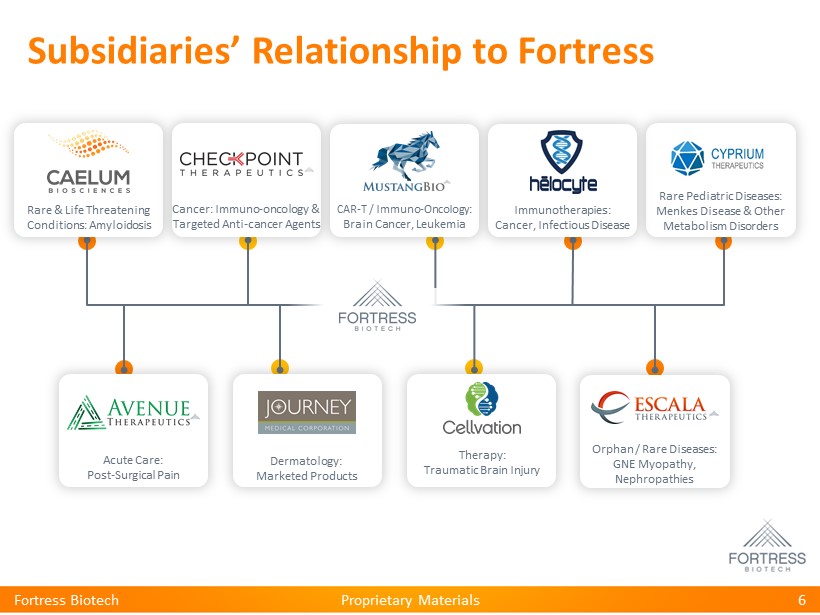
Proprietary Materials Subsidiaries’ Relationship to Fortress Fortress Biotech 6 Rare & Life Threatening Conditions: Amyloidosis Rare Pediatric Diseases: Menkes Disease & Other Metabolism Disorders Cancer: Immuno - oncology & Targeted Anti - cancer Agents CAR - T / Immuno - Oncology: Brain Cancer, Leukemia Acute Care: Post - Surgical Pain Immunotherapies: Cancer, Infectious Disease Orphan / Rare Diseases: GNE Myopathy, Nephropathies Dermatology : Marketed Products Therapy : Traumatic Brain Injury
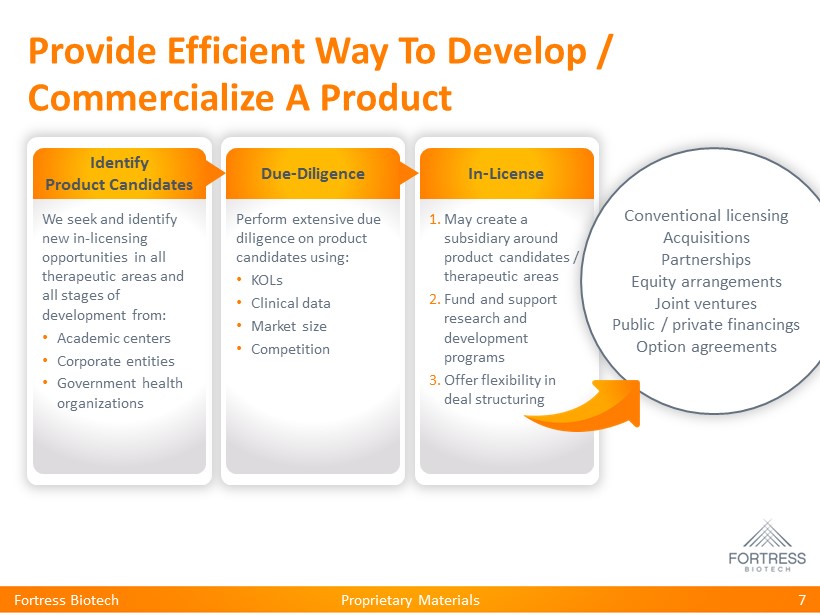
Proprietary Materials Provide Efficient Way To Develop / Commercialize A Product Fortress Biotech 7 Identify Product Candidates We seek and identify new in - licensing opportunities in all therapeutic areas and all stages of development from: • Academic centers • Corporate entities • Government health organizations Due - Diligence Perform extensive due diligence on product candidates using: • KOLs • Clinical data • Market size • Competition In - License 1. May create a subsidiary around product candidates / therapeutic areas 2. Fund and support research and development programs 3. Offer flexibility in deal structuring Conventional licensing Acquisitions Partnerships Equity arrangements Joint ventures Public / private financings Option agreements
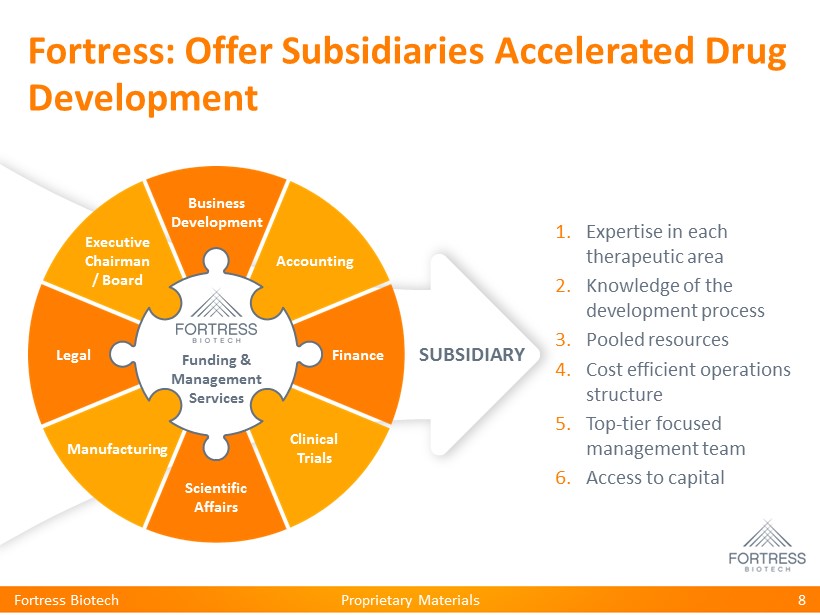
Proprietary Materials Fortress: Offer Subsidiaries Accelerated Drug Development Fortress Biotech 8 Scientific Affairs SUBSIDIARY 1. Expertise in each therapeutic area 2. Knowledge of the development process 3. Pooled resources 4. Cost efficient operations structure 5. Top - tier focused management team 6. Access to capital Funding & Management Services Business Development Finance Scientific Affairs Legal Accounting Clinical Trials Manufacturing Executive Chairman / Board
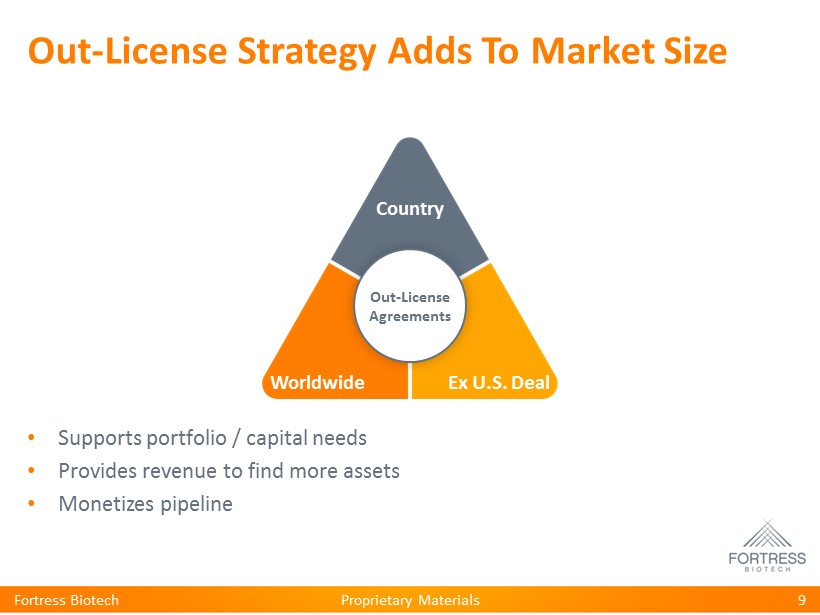
Proprietary Materials Out - License Strategy Adds To Market Size • Supports portfolio / capital needs • Provides revenue to find more assets • Monetizes pipeline Fortress Biotech 9 Country Worldwide Ex U.S. Deal Out - License Agreements
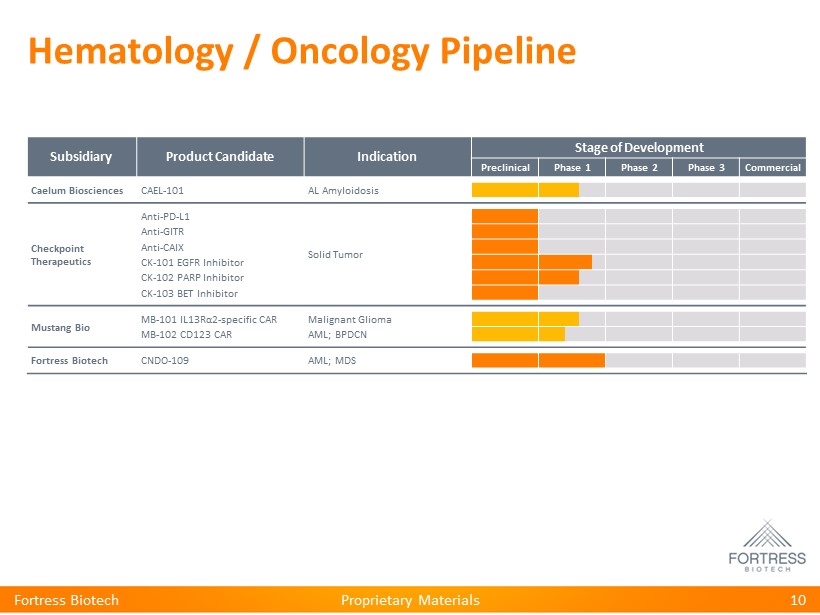
Proprietary Materials Hematology / Oncology Pipeline Subsidiary Product Candidate Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Commercial Caelum Biosciences CAEL - 101 AL Amyloidosis Checkpoint Therapeutics Anti - PD - L1 Solid Tumor Anti - GITR Anti - CAIX CK - 101 EGFR Inhibitor CK - 102 PARP Inhibitor CK - 103 BET Inhibitor Mustang Bio MB - 101 IL13R α 2 - specific CAR Malignant Glioma MB - 102 CD123 CAR AML; BPDCN Fortress Biotech CNDO - 109 AML; MDS 10 Fortress Biotech
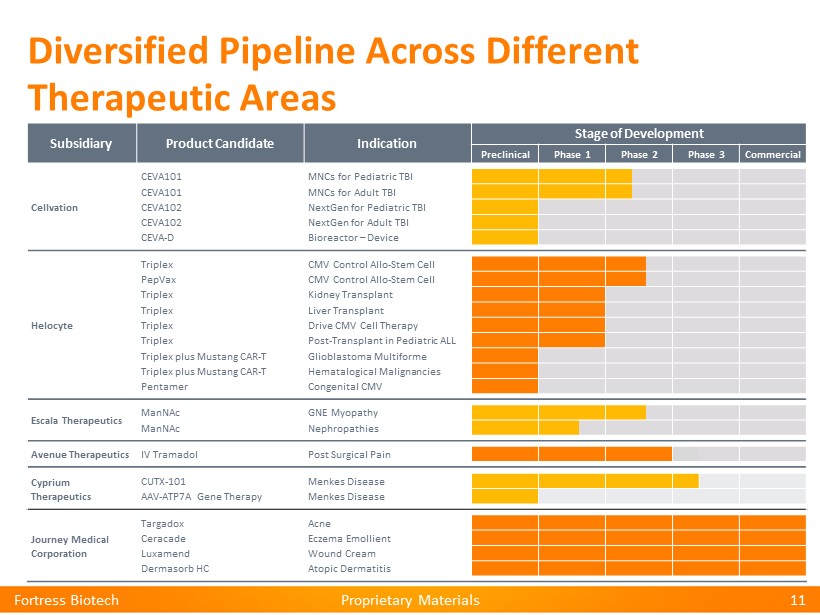
Proprietary Materials Proprietary Materials Diversified Pipeline Across Different Therapeutic Areas Fortress Biotech 11 Subsidiary Product Candidate Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Commercial Cellvation CEVA101 MNCs for Pediatric TBI CEVA101 MNCs for Adult TBI CEVA102 NextGen for Pediatric TBI CEVA102 NextGen for Adult TBI CEVA - D Bioreactor – Device Helocyte Triplex CMV Control Allo - Stem Cell PepVax CMV Control Allo - Stem Cell Triplex Kidney Transplant Triplex Liver Transplant Triplex Drive CMV Cell Therapy Triplex Post - Transplant in Pediatric ALL Triplex plus Mustang CAR - T Glioblastoma Multiforme Triplex plus Mustang CAR - T Hematalogical Malignancies Pentamer Congenital CMV Escala Therapeutics ManNAc GNE Myopathy ManNAc Nephropathies Avenue Therapeutics IV Tramadol Post Surgical Pain Cyprium Therapeutics CUTX - 101 Menkes Disease AAV - ATP7A Gene Therapy Menkes Disease Journey Medical Corporation Targadox Acne Ceracade Eczema Emollient Luxamend Wound Cream Dermasorb HC Atopic Dermatitis
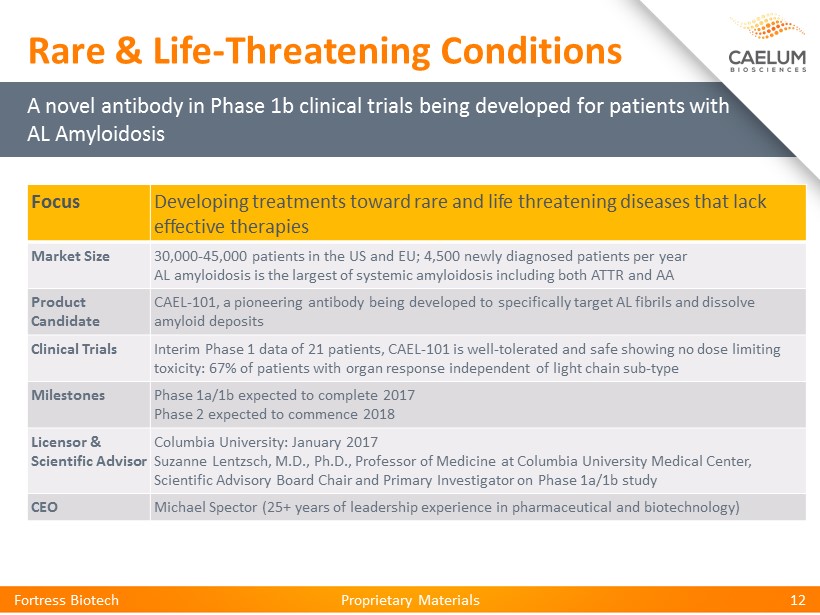
Proprietary Materials Proprietary Materials Fortress Biotech 12 Focus Developing treatments toward rare and life threatening diseases that lack effective therapies Market Size 30,000 - 45,000 patients in the US and EU; 4,500 newly diagnosed patients per year AL amyloidosis is the largest of systemic amyloidosis including both ATTR and AA Product Candidate CAEL - 101, a pioneering antibody being developed to specifically target AL fibrils and dissolve amyloid deposits Clinical Trials Interim Phase 1 data of 21 patients, CAEL - 101 is well - tolerated and safe showing no dose limiting toxicity: 67 % of patients with organ response independent of light chain sub - type Milestones Phase 1a/1b expected to complete 2017 Phase 2 expected to commence 2018 Licensor & Scientific Advisor Columbia University: January 2017 Suzanne Lentzsch, M.D., Ph.D., Professor of Medicine at Columbia University Medical Center, Scientific Advisory Board Chair and Primary Investigator on Phase 1a/1b study CEO Michael Spector (25+ years of leadership experience in pharmaceutical and biotechnology) A novel antibody in Phase 1b clinical trials being developed for patients with AL Amyloidosis Rare & Life - Threatening Conditions
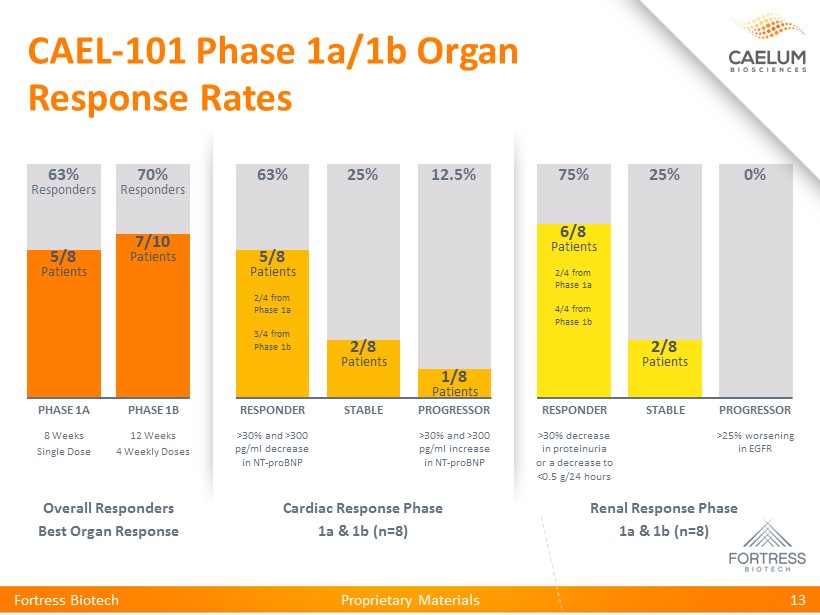
Proprietary Materials CAEL - 101 Phase 1a/1b Organ Response Rates Fortress Biotech 13 Renal Response Phase 1a & 1b (n=8) Cardiac Response Phase 1a & 1b (n=8) Overall Responders Best Organ Response 8 Weeks Single Dose PHASE 1A 63% Responders 12 Weeks 4 Weekly Doses PHASE 1B 70% Responders >30% and >300 pg/ml decrease in NT - proBNP RESPONDER 63% STABLE 25% >30% and >300 pg/ml increase in NT - proBNP PROGRESSOR 12.5% STABLE 25% RESPONDER 75% >30% decrease in proteinuria or a decrease to <0.5 g/24 hours PROGRESSOR 0% >25% worsening in EGFR 5/8 Patients 7/10 Patients 5/8 Patients 2/4 from Phase 1a 3/4 from Phase 1b 2/8 Patients 1/8 Patients 2/8 Patients 6/8 Patients 2/4 from Phase 1a 4/4 from Phase 1b

Proprietary Materials Proprietary Materials Fortress Biotech 14 Focus Acquire and develop novel immuno - oncology and targeted cancer agents alone and in combination to treat patients with solid tumors Market Size Anti - PD - (L)1 >$30B, Anti - GITR > $1B, CK - 101 EGFR > $3B, CK - 103 BET > $500M Product Candidates Two immuno - oncology “I/O” antibodies, licensed from Dana Farber Four targeted anti - cancer agents Clinical Trials CK - 101 (EGFR Inhibitor) Phase 1/2 study ongoing Milestones Mid - 2017: Anti - PD - L1 IND expected 2H 2017: CK - 101 (EGFR Inhibitor) Phase 2 expected initiation 2H 2017: CK - 103 (BET Inhibitor) target IND filing 2018 : Anti - GITR target IND expected TGTX Collaboration Joint development of anti - PD - L1 and anti - GITR mAbs , and BET inhibitor program with Checkpoint developing solid tumor indications and TG in liquid tumors Funding ~$35M (12/31/16 ) to support development programs through 2018 CEO James Oliviero (15+ years of leadership experience in pharmaceutical and biotechnology, previously senior management of Keryx , achieving a new drug approval) Building a platform to combine targeted agents with immuno - oncology agents to maximize anti - cancer effect Immuno - Oncology
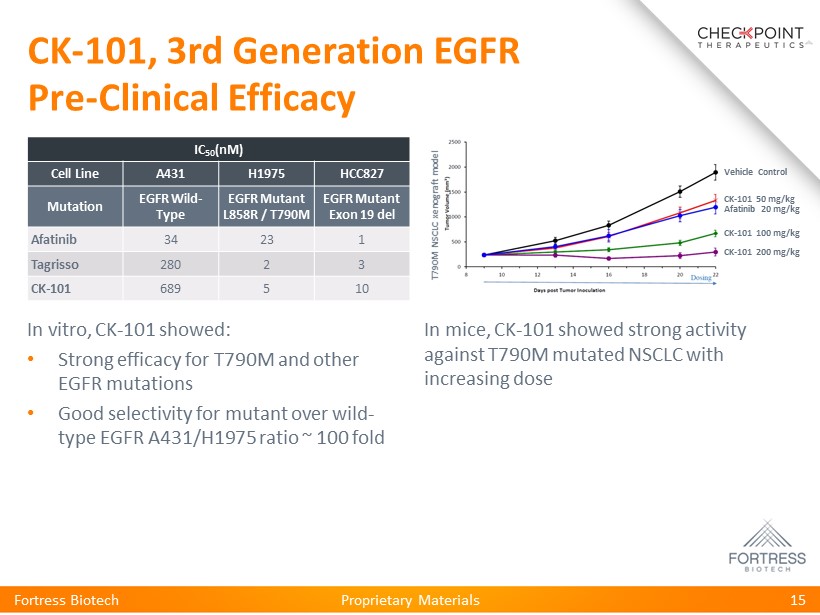
Proprietary Materials CK - 101, 3rd Generation EGFR Pre - Clinical Efficacy Fortress Biotech 15 Vehicle Control CK - 101 50 mg/kg CK - 101 100 mg/kg CK - 101 200 mg/kg Afatinib 20 mg/kg IC 50 ( nM ) Cell Line A431 H1975 HCC827 Mutation EGFR Wild - Type EGFR Mutant L858R / T790M EGFR Mutant Exon 19 del Afatinib 34 23 1 Tagrisso 280 2 3 CK - 101 689 5 10 In vitro, CK - 101 showed: • Strong efficacy for T790M and other EGFR mutations • Good selectivity for mutant over wild - type EGFR A431/H1975 ratio ~ 100 fold In mice, CK - 101 showed strong activity against T790M mutated NSCLC with increasing dose T790M NSCLC xenograft model
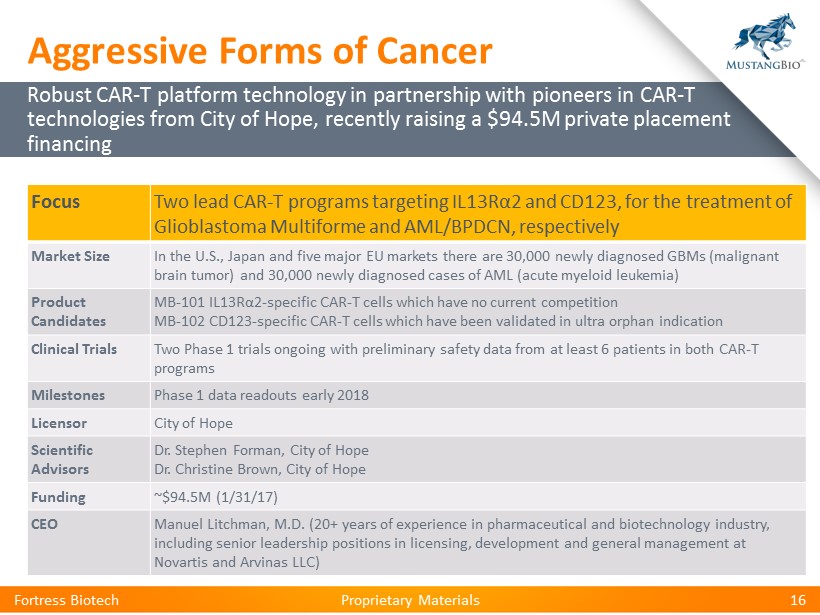
Proprietary Materials Proprietary Materials Focus Two lead CAR - T programs targeting IL13R α 2 and CD123, for the treatment of Glioblastoma Multiforme and AML/BPDCN, respectively Market Size In U.S., Japan and five major EU markets per year… ~30,000 newly diagnosed GBMs (malignant brain tumor) ~30,000 newly diagnosed cases of AML (acute myeloid leukemia) Product Candidates MB - 101 IL13R α 2 - specific CAR - T cells which have no current competition MB - 102 CD123 - specific CAR - T cells which have been validated in ultra orphan indication Clinical Trials Two Phase 1 trials ongoing with preliminary safety data from at least 6 patients in both CAR - T programs Milestones Phase 1 data readouts early 2018 NEJM case study demonstrates MB - 101 achieved complete remission in patient with recurrent GBM Licensor City of Hope Scientific Advisors Dr. Stephen Forman, City of Hope Dr. Christine Brown, City of Hope Focus Two lead CAR - T programs targeting IL13R α 2 and CD123, for the treatment of Glioblastoma Multiforme and AML/BPDCN, respectively Market Size In the U.S ., Japan and five major EU markets there are 30,000 newly diagnosed GBMs (malignant brain tumor ) and 30,000 newly diagnosed cases of AML (acute myeloid leukemia ) Product Candidates MB - 101 IL13R α 2 - specific CAR - T cells which have no current competition MB - 102 CD123 - specific CAR - T cells which have been validated in ultra orphan indication Clinical Trials Two Phase 1 trials ongoing with preliminary safety data from at least 6 patients in both CAR - T programs Milestones Phase 1 data readouts early 2018 Licensor City of Hope Scientific Advisors Dr. Stephen Forman, City of Hope Dr. Christine Brown, City of Hope Funding ~$94.5M (1/31/17) CEO Manuel Litchman , M.D. (20+ years of experience in pharmaceutical and biotechnology industry, including senior leadership positions in licensing, development and general management at Novartis and Arvinas LLC) Fortress Biotech 16 Robust CAR - T platform technology in partnership with pioneers in CAR - T technologies from City of Hope, recently raising a $94.5M private placement financing Aggressive Forms of Cancer
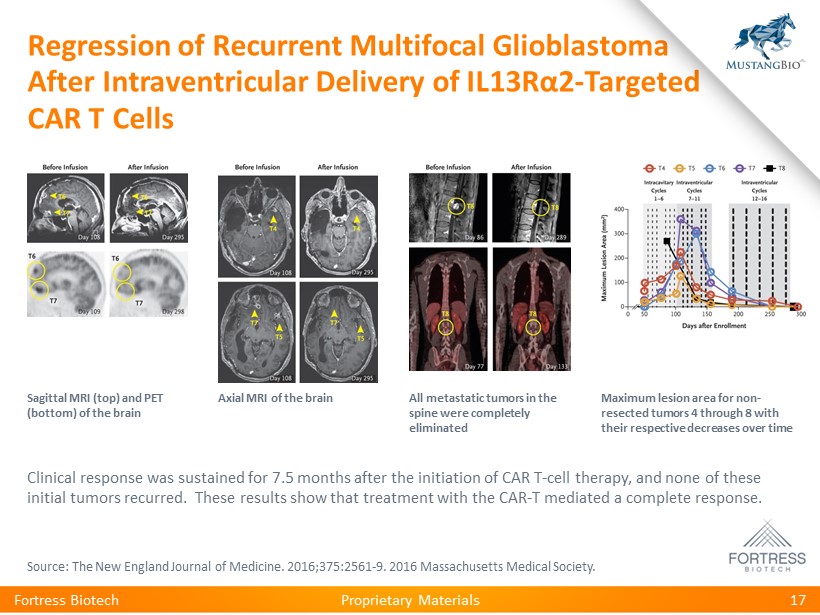
Proprietary Materials Regression of Recurrent Multifocal Glioblastoma After Intraventricular Delivery of IL13R α 2 - Targeted CAR T Cells Fortress Biotech 17 Sagittal MRI (top) and PET (bottom) of the brain Axial MRI of the brain All metastatic tumors in the spine were completely eliminated Maximum lesion area for non - resected tumors 4 through 8 with their respective decreases over time Clinical response was sustained for 7.5 months after the initiation of CAR T - cell therapy, and none of these initial tumors recurred. These results show that treatment with the CAR - T mediated a complete response. Source: The New England Journal of Medicine. 2016;375:2561 - 9. 2016 Massachusetts Medical Society.
Proprietary Materials Proprietary Materials Fortress Biotech 18 Focus Develop novel immunotherapies for the prevention and treatment of CMV that can cause life - threatening disease in those with weak immune systems Market Size CDC estimates 50 - 80% infected with Cytomegalovirus (CMV) by age of 40 CMV in Allogeneic Stem Cell Transplant: U.S. Incidence ~8,000 / EU Incidence ~15,000 CMV in Allogeneic Solid Organ Transplant: U.S. Incidence ~8,000 / EU Incidence ~15,000 Product Candidates PepVax : HLA - restricted, single antigen CMV vaccine Triplex: First universal, multi - antigen CMV vaccine Clinical Trials PepVax : Phase 2 ongoing, multi - center, double - blind trial for stem cell transplant (n=96) Phase 1b showed safe, effective and Published in Lancet Dec 2015 Triplex: Phase 2 ongoing, multi - center, double - blind trial for stem cell transplant (n=115) Phase 1 showed safe, immunogenic. Presented ASH 2015. Published in Blood Nov 2016 Upcoming Milestones Triplex: Phase 2 topline 100 day data by 2H2017 PepVax : Phase 2 topline data by 1H2018 Licensor City of Hope Funding Total budget (thru 1H2019): ~ $30M (Including $8M NCI grant funding) CEO Frank Taffy (15+ years of experience at Forest Labs and Life Tech in corporate development and operations) Three novel biologic immunotherapies (two in Phase 2) targeting billion dollar orphan market Cytomegalovirus (CMV): Common Virus

Proprietary Materials Phase 1 Studies: Journal Publications Phase 1b (Completed, Published in The Lancet ) Design Single - Center (City of Hope) Study in 36 Allogeneic HSCT CMV(+)Recipients Randomized (1:1) between Vaccine Arm (VA) and Observation Arm (OA) Dosing Schedule Two subcutaneous vaccinations after transplant • Day 28 • Day 56 1˚ Endpoint Overall safe and well - tolerated Published in The Lancet Haematology (12/28/2015) 2˚ Endpoint • Increase in CD8+ T - cells • Reduced CMV Reactivation, 6% vs.33%,p=0.044 • Reduced Relapse, 6% vs. 28%, p=0.015 • Reduced Death, 0vs. 39% Phase 1 (Completed, Published in Blood ) Fortress Biotech 19 Vaccine (n=18 ) Observation (n=18) Patients with serious adverse events 4 (22%) 9 (50%) Disease relapse 1 (6%) 5 (28%) Death 0 (0%) 7 (39%) CMV viraemia (≥500 gc/mL ) 1 (6%) 6 (33%) Design Single - Center (City of Hope) Dose Escalation (three levels) in 24 Healthy Volunteers (CMV +/ - ) Dosing Schedule Two IM injections four weeks apart Last patient dosed 4/2015 1˚ Endpoint Safe and well - tolerated in all dose cohorts Presented at ASH (December 2015) Published in Blood (November2016) 2˚ Endpoint ↑pp65 - , IE1 - , IE2 - specific CD8 and CD4 T - cells Particularly pronounced increase in T - cells in those with low baseline levels
Proprietary Materials Proprietary Materials Fortress Biotech 20 Focus Developing novel therapies for the treatment of rare, fatal pediatric diseases, with initial focus on Menkes disease and related copper metabolism disorders Market Size Menkes disease is a rare X - linked pediatric disease caused by gene mutations of copper transporter ATP7A, which affects approximately one in 100,000 newborns per year. Product Candidate CUTX - 101 (Copper Histidinate injection) is being developed to replenish copper levels in patients with Menkes disease. A preclinical AAV - based ATP7A gene therapy is being developed to deliver working copies of ATP7A to Menkes patients. Both programs have FDA Orphan Drug Designations. Clinical Trials In Phase 1/2 clinical studies conducted at NICHD, early treatment of Menkes patients with CUTX - 101 led to an improvement in neurodevelopmental outcomes and survival. Milestones Natural History Study of untreated Menkes patients in 1H2017 FDA meeting to confirm regulatory pathway in 2017 Licensor & Scientific Advisor Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), part of the National Institutes of Health (NIH): March 2017 (CRADA & Exclusive License Agreement) Stephen G. Kaler, M.D., Senior Investigator and Head, Section on Translational Neuroscience, Molecular Medicine Branch, NICHD Principal Investigator for Menkes disease clinical studies CEO Lung S. Yam, M.D., Ph.D. (Senior Analyst, Opus Point Partners; BD Consultant involved in identifying and in - licensing of multiple assets to Fortress and affiliated companies) A novel therapy in Phase 3 clinical trial being developed for patients with AL Menkes Disease Rare & Fatal Pediatric Diseases
Proprietary Materials Proprietary Materials Fortress Biotech 21 IV Tramadol For Acute Post Surgical Pain IV Tramadol, if approved, would be the only Schedule IV intravenous opioid in the U.S. Focus IV tramadol for the treatment of post - surgical pain Market Size IV analgesics sells ~$ 1bn per year in the U.S. IV acetaminophen sells >$250MM with ~3 to 4% of the unit volume Product Candidate Intravenous (IV) Tramadol, an opioid without the typical side effects of narcotics , for the treatment of moderate to moderately severe pain Regulatory Path 505b(2) Status Phase 3 ready Funding ~$30M to complete Phase 3 CEO Lucy Lu, M.D. (CFO, Fortress Biotech and previously Citi biotechnology equity research analyst)
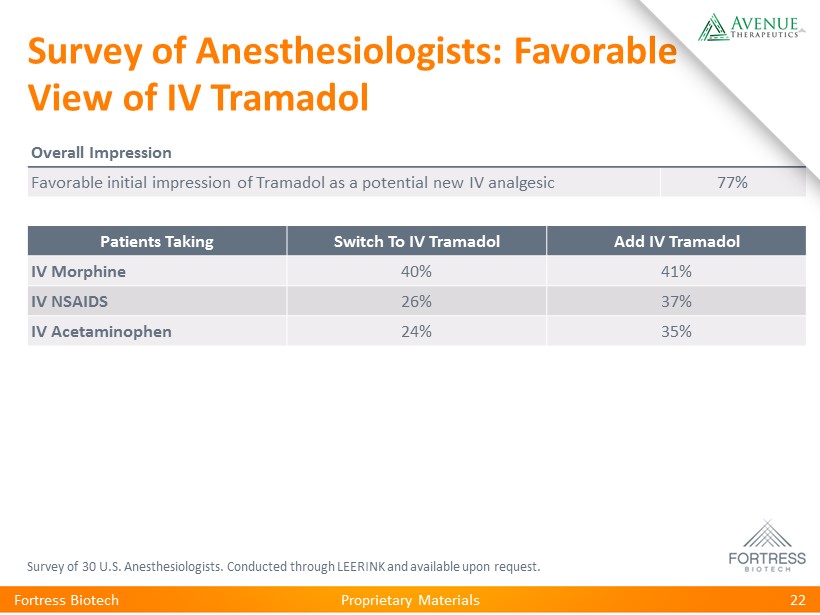
Proprietary Materials Survey of Anesthesiologists: Favorable View of IV Tramadol Survey of 30 U.S. Anesthesiologists. Conducted through LEERINK and available upon request. Fortress Biotech 22 Patients Taking Switch To IV Tramadol Add IV Tramadol IV Morphine 40% 41% IV NSAIDS 26% 37% IV Acetaminophen 24% 35% Overall Impression Favorable initial impression of Tramadol as a potential new IV analgesic 77%

Proprietary Materials Fortress Biotech 23 Focus Identify and commercialize innovative, differentiated prescription dermatology products through efficient and potent sales and marketing model Product Candidates Targadox ( doxycyline tablets ) : Sever e acne Ceracade (skin emulsion) : Atopic and various types of dermatitis Luxamend (wound cream) : Wounds from superficial to full thickness and 1 st and 2 nd degree burns Dermasorb HC (hydrocortisone lotion) Kit: Seborrheic dermatitis Market 5 ,000 top prescribing dermatologists CEO Claude Maraoui (25+ years commercializing dermatology products; previously Vice President of Sales at Medicis ) Team of industry experts successfully launched four dermatology products in 12 months Innovative Dermatology Products
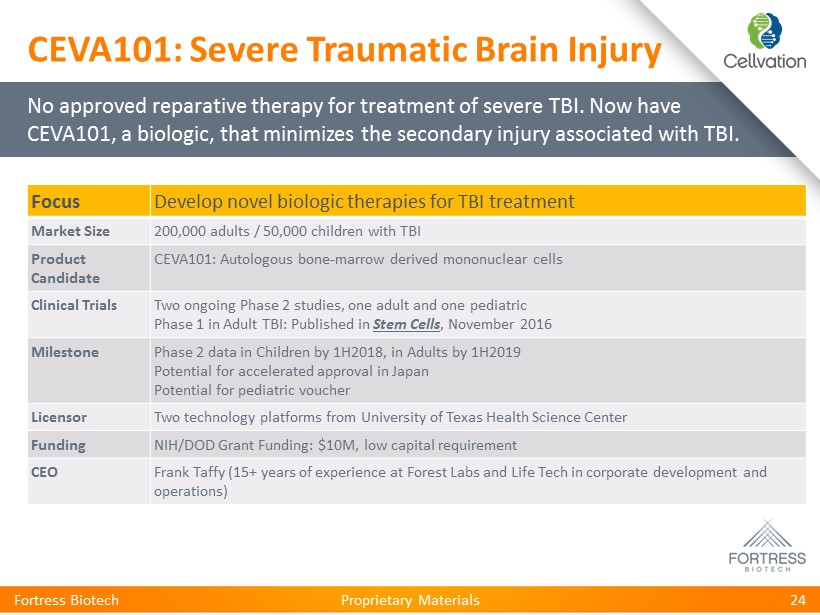
Proprietary Materials Fortress Biotech 24 Focus Develop novel biologic therapies for TBI treatment Market Size 200,000 adults / 50,000 children with TBI Product Candidate CEVA101: Autologous bone - marrow derived mononuclear cells Clinical Trials Two ongoing Phase 2 studies, one adult and one pediatric Phase 1 in Adult TBI: Published in Stem Cells , November 2016 Milestone Phase 2 data in Children by 1H2018, in Adults by 1H2019 Potential for accelerated approval in Japan Potential for pediatric voucher Licensor Two technology platforms from University of Texas Health Science Center Funding NIH/DOD Grant Funding: $10M, low capital requirement CEO Frank Taffy ( 15+ years of experience at Forest Labs and Life Tech in corporate development and operations) No approved reparative therapy for treatment of severe TBI. Now have CEVA101, a biologic, that minimizes the secondary injury associated with TBI. CEVA101: Severe Traumatic Brain Injury
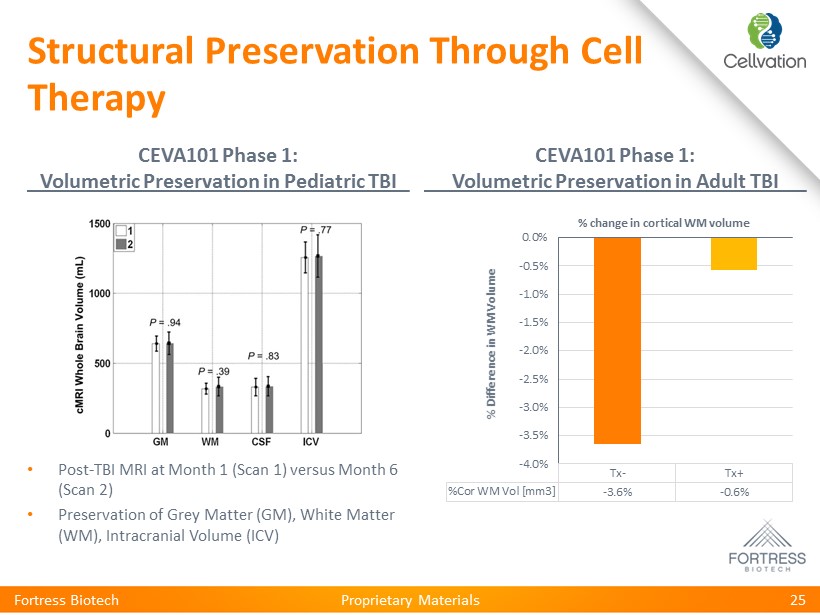
Proprietary Materials Structural Preservation Through Cell Therapy CEVA101 Phase 1: Volumetric Preservation in Pediatric TBI CEVA101 Phase 1: Volumetric Preservation in Adult TBI Fortress Biotech 25 • Post - TBI MRI at Month 1 (Scan 1) versus Month 6 (Scan 2) • Preservation of Grey Matter (GM), White Matter (WM), Intracranial Volume (ICV) Tx- Tx+ %Cor WM Vol [mm3] -3.6% -0.6% -4.0% -3.5% -3.0% -2.5% -2.0% -1.5% -1.0% -0.5% 0.0% % Difference in WM Volume % change in cortical WM volume

Proprietary Materials Proprietary Materials Fortress Biotech 26 Focus Develop and commercialize b.i.d oral treatment for GNE Myopathy and primary podocyte nephropathies. No other company has this focus. Market Size GNE Myopathy : U.S. – 400 and WW – 2000 diagnosed Nephropathy (including diabetic): ~220,000 WW Product Candidate ManNAc =N - Acetyl - D - Mannosamine , a naturally - occurring monosaccharide and precusor to sialic acid Clinical Trials In collaboration with NIH on 3 clinical studies GNE Myopathy : Natural History study ongoing ,Phase 2 open label ongoing, Phase 1 completed Primary Podocyte Nephropathies: Phase 1 trial in progress (recruiting) Upcoming Milestone Phase 2 GNE Myopathy trial ongoing and Phase 3 planned for 2017 Licensor Acquired from New Zealand Pharmaceuticals Ltd which is the exclusive global supplier of ManNAc CEO Hootan Khatami, MD (12+ years of pharmaceutical and biotechnology experience at Genzyme/Sanofi, Roche/Genentech, Merck & Daiichi Sankyo) GNE Myopathy has no approved therapies. FDA granted ManNAc orphan designation. Rare & Orphan Diseases
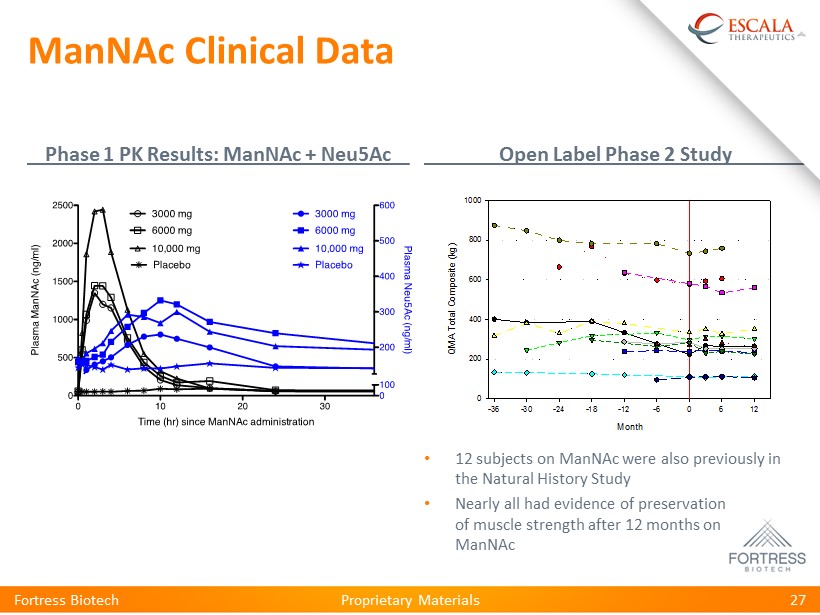
Proprietary Materials ManNAc Clinical Data Phase 1 PK Results: ManNAc + Neu5Ac Open Label Phase 2 Study Fortress Biotech 27 • 12 subjects on ManNAc were also previously in the Natural History Study • Nearly all had evidence of preservation of muscle strength after 12 months on ManNAc Proprietary Materials Month -36 -30 -24 -18 -12 -6 0 6 12 Q M A T o t a l C o m p o s i t e ( k g ) 0 200 400 600 800 1000 ManNAc Clinical Data Phase 1 PK Results: ManNAc + Neu5Ac Open Label Phase 2 Study Fortress Biotech 24 • 12 subjects on ManNAc were also previously in the Natural History Study • Nearly all had evidence of preservation of muscle strength after 12 months on ManNAc
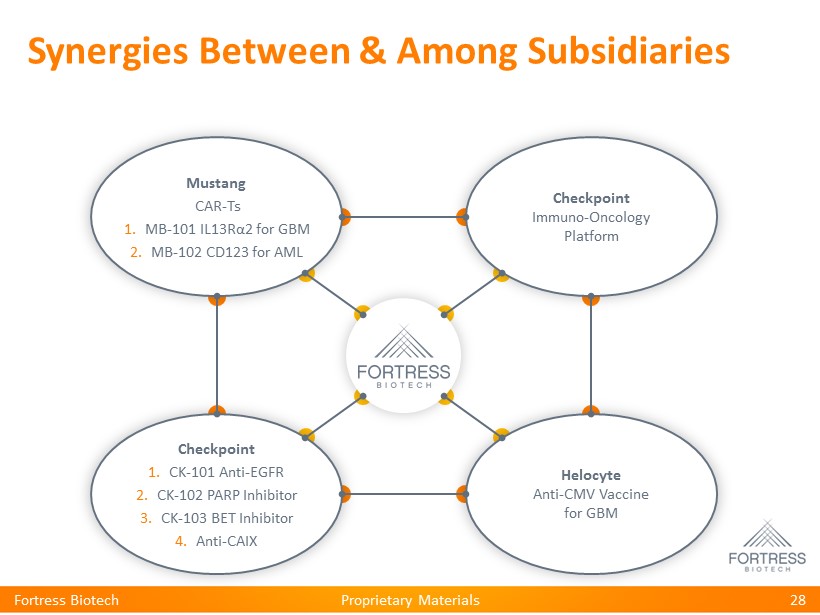
Proprietary Materials Checkpoint Immuno - Oncology Platform Helocyte Anti - CMV Vaccine for GBM Checkpoint 1. CK - 101 Anti - EGFR 2. CK - 102 PARP Inhibitor 3. CK - 103 BET Inhibitor 4. Anti - CAIX Mustang CAR - Ts 1. MB - 101 IL13R α 2 for GBM 2. MB - 102 CD123 for AML Synergies Between & Among Subsidiaries 28 Fortress Biotech
Proprietary Materials Accelerated Drug Development Model Fortress Subsidiaries Are Creating A Pipeline of Therapies For Life - Threatening Diseases Fortress Biotech 29 Diversified Pipeline Experienced, Proven Leadership

Proprietary Materials Corporate Presentation May 2017
