Attached files
| file | filename |
|---|---|
| EX-99.3 - EXHIBIT 99.3 - AMAG PHARMACEUTICALS, INC. | ex993ferahemedata.htm |
| EX-99.1 - EXHIBIT 99.1 - AMAG PHARMACEUTICALS, INC. | ex991q12017earningsrelease.htm |
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | amagq12017earningsrelease8.htm |

AMAG Pharmaceuticals
Q1-2017 Financial Results &
Corporate Update
May 2, 2017

Forward-Looking Statements
This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of
1995 (PSLRA) and other federal securities laws. Any statements contained herein which do not describe historical facts,
including, among others, Makena’s position in the market and future growth drivers for Makena, including its ability to
continue to gain share from compounders, grow the Makena @Home administration, expand use in the late preterm birth
segment and launch the Makena subcutaneous auto-injector; growth drivers for Cord Blood Registry (CBR), including plans to
differentiate CBR’s offerings, build the value proposition on storing newborn stem cells and leveraging advancements in stem
cell research; expectations regarding the commercial opportunity of Intrarosa, including the number of women who suffer
from dyspareunia in the U.S., and the expected Intrarosa launch timing of mid-2017; AMAG’s ability to leverage its existing
strengths, hire a new commercial team and initiate a Phase 3 female sexual dysfunction study in post-menopausal women in
the second half of 2017; growth drivers for Feraheme, including plans to optimize net revenue per gram, complete recent
group purchasing organization (GPO) sales, grow in key segments and expectations that the size of the addressable market, if
the broader indication is approved, would double and would require minimal expansion of sales force; expectations
regarding 2017 quarterly Makena ex-factory sales; AMAG’s 2017 financial guidance, including forecasted GAAP and non-
GAAP revenues, GAAP net income and operating income, and non-GAAP adjusted EBITDA; and expectations regarding
regulatory timelines for the Makena subcutaneous auto-injector, Feraheme broader label, Intrarosa, bremelanotide and Velo,
including anticipated FDA action and commercial launch for each product; are forward-looking statements which involve
risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking
statements.
Such risks and uncertainties include, among others, those risks identified in AMAG’s Securities and Exchange Commission
(“SEC”) filings, including its Annual Report on Form 10-K for the year ended December 31, 2016 and its Quarterly Report on
Form 10-Q for the quarter ended March 31, 2017 and subsequent filings with the SEC. AMAG cautions you not to place
undue reliance on any forward-looking statements, which speak only as of the date they are made. AMAG disclaims any
obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or
circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ
from those set forth in the forward-looking statements.
AMAG Pharmaceuticals® and Feraheme® are registered trademarks of AMAG Pharmaceuticals, Inc. MuGard® is a registered
trademark of Abeona Therapeutics, Inc. Makena® is a registered trademark of AMAG Pharmaceuticals IP, Ltd. Cord Blood
Registry® and CBR® are registered trademarks of CBR Systems, Inc. IntrarosaTM is a trademark of Endoceutics, Inc.
2
Q1-2017 Earnings Call Agenda
3
Q1-2017 and Recent Highlights 1
6 Q&A
2 Commercial Overview
5 2017 Key Priorities and Closing Remarks
4 Financial Results and Guidance
3 Feraheme Broad Label Study Results

Q1-2017 Highlights and Recent Events
4
Filed sNDA with the FDA for Makena subcutaneous (sub-q) auto-injector
Increased Makena market share by 2% over Q4-2016 to 44%
Reported positive Feraheme data from Phase 3 label expansion study
Closed licensing transactions in the women’s health space for rights to
IntrarosaTM and bremelanotide
Initiated the hiring of a new approximately 150-person women’s health
commercial team in preparation for mid-2017 launch of Intrarosa
Advanced ongoing clinical work with our partner Palatin for the planned
bremelanotide NDA submission in early 2018
Generated strong revenues in Q1-2017, including 33% growth in Makena
sales and 7% growth in Feraheme sales over Q1-2016
Ended Q1-2017 with $558 M of cash and investments
Q1-2017 Financial Highlights
5
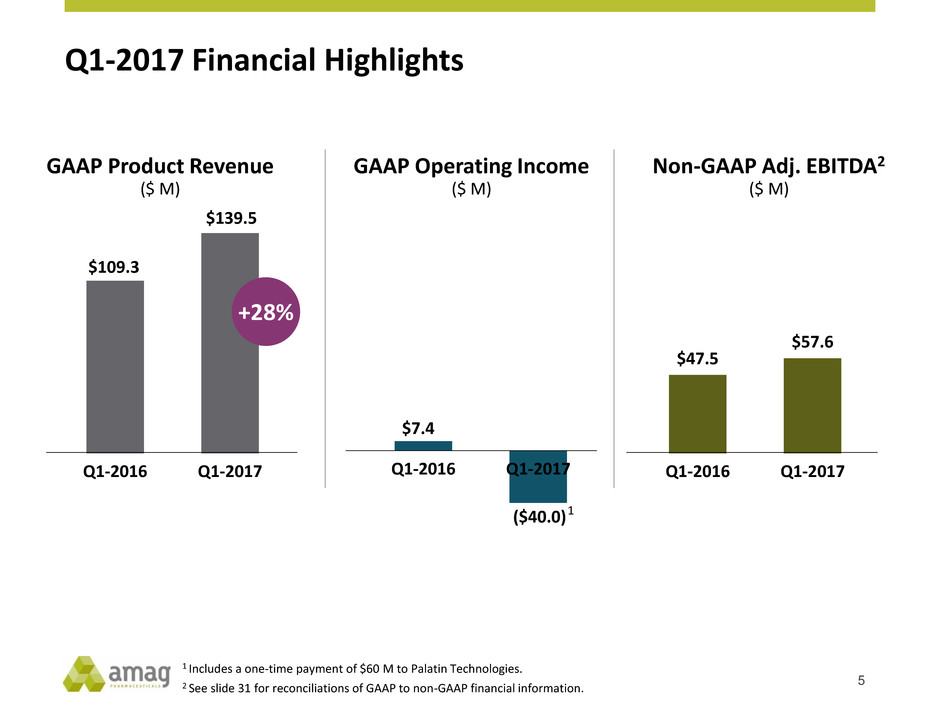
GAAP Product Revenue
($ M)
$109.3
$139.5
Q1-2016 Q1-2017
+28%
1 Includes a one-time payment of $60 M to Palatin Technologies.
2 See slide 31 for reconciliations of GAAP to non-GAAP financial information.
GAAP Operating Income
($ M)
Non-GAAP Adj. EBITDA2
($ M)
$47.5
$57.6
Q1-2016 Q1-2017
$7.4
($40.0)
Q1-2016 Q1-2017
1

Further Expanding AMAG’s Product Portfolio
6
Pregnancy & Birth Wellness
Post-Menopausal
Health
Feraheme Makena
Velo Option
Cord Blood
Registry
Used for the treatment of iron
deficiency anemia (IDA) in adult
patients with chronic kidney disease
(CKD)
The only FDA-approved therapy
to reduce recurrent preterm
birth in certain at-risk women
World’s largest umbilical cord
stem cell collection and
storage company
At completion of a Phase 2b/3a
clinical trial, AMAG has the option
to acquire an orphan drug
candidate for the treatment of
severe preeclampsia
IntrarosaTM
FDA-approved non-
estrogen product to treat
moderate-to-severe
dyspareunia (a symptom of
VVA) and the only FDA
approved product without
a boxed warning
Successful completion of
two Phase 3 clinical trials
for on-demand treatment
of hypoactive sexual desire
disorder (HSDD)
Bremelanotide1
MuGard
Prescriptive mucoadhesive for the
management of oral mucositis, a
common side effect of radiation or
chemotherapy
Maternal and Women’s Health
Hematology/Oncology
1 Previously referred to as RekyndaTM

Product Portfolio
Commercial Overview
Nik Grund
Chief Commercial Officer
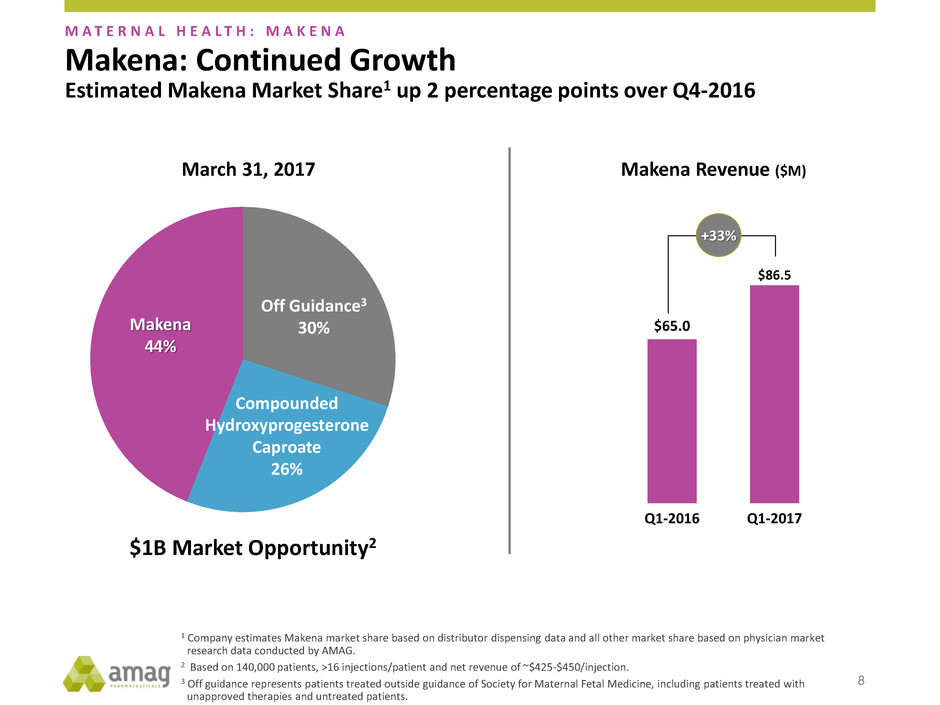
Makena: Continued Growth
8
$65.0
$86.5
Q1-2016 Q1-2017
Makena Revenue ($M)
M A T E R N A L H E A L T H : M A K E N A
+33%
1 Company estimates Makena market share based on distributor dispensing data and all other market share based on physician market
research data conducted by AMAG.
2 Based on 140,000 patients, >16 injections/patient and net revenue of ~$425-$450/injection.
3 Off guidance represents patients treated outside guidance of Society for Maternal Fetal Medicine, including patients treated with
unapproved therapies and untreated patients.
Off Guidance3
30%
March 31, 2017
Makena
44%
Compounded
Hydroxyprogesterone
Caproate
26%
$1B Market Opportunity2
Estimated Makena Market Share1 up 2 percentage points over Q4-2016
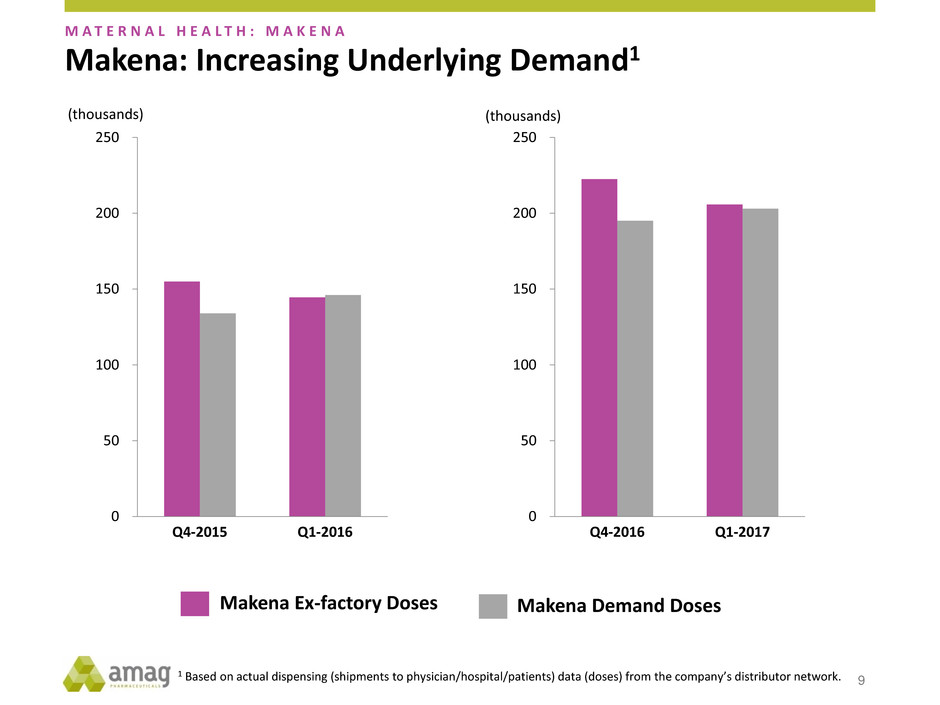
Makena: Increasing Underlying Demand1
9
M A T E R N A L H E A L T H : M A K E N A
0
50
100
150
200
250
Q4-2015 Q1-2016
Makena Ex-factory Doses Makena Demand Doses
0
50
100
150
200
250
Q4-2016 Q1-2017
(thousands) (thousands)
1 Based on actual dispensing (shipments to physician/hospital/patients) data (doses) from the company’s distributor network.

M A T E R N A L H E A L T H : M A K E N A
10
Continue share gains from compounders
and grow Makena @Home administration 1
Expand use in late preterm birth segment 2
Launch subcutaneous auto-injector1 3
Makena 2017 Growth Drivers
1 If regulatory approval is received.
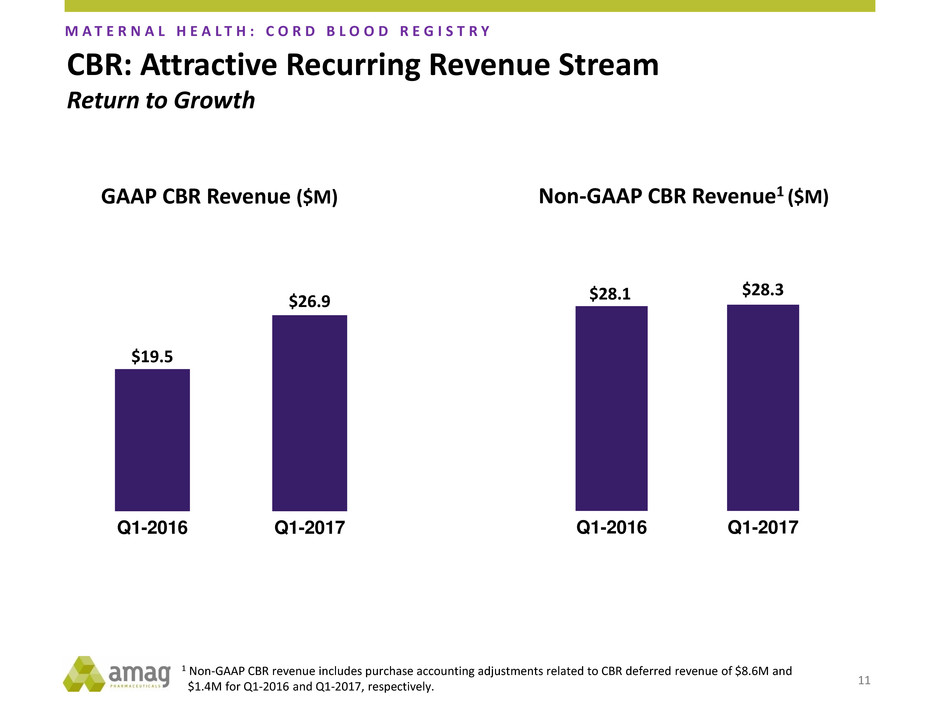
11
$19.5
$26.9
Q1-2016 Q1-2017
$28.1 $28.3
Q1-2016 Q1-2017
GAAP CBR Revenue ($M) Non-GAAP CBR Revenue1 ($M)
CBR: Attractive Recurring Revenue Stream
Return to Growth
M A T E R N A L H E A L T H : C O R D B L O O D R E G I S T R Y
1 Non-GAAP CBR revenue includes purchase accounting adjustments related to CBR deferred revenue of $8.6M and
$1.4M for Q1-2016 and Q1-2017, respectively.

CBR 2017 Growth Drivers
12
Differentiate CBR’s offerings
– Highlight cord tissue storage offering
– Enhance product offerings
1
Build value proposition on storing newborn
stem cells
– Harmonizing annual storage price across
client base
2
Leverage advancements in stem cell research
with OB/GYN’s and pregnant families 3
M A T E R N A L H E A L T H : C O R D B L O O D R E G I S T R Y

Advancements in Stem Cell Research
13
M A T E R N A L H E A L T H : C O R D B L O O D R E G I S T R Y

Affected, but not yet seeking treatment
Utilizing OTC treatments
Previous estrogen
therapy users
Dyspareunia: Sizable Untapped Treatment Market
14
1 Based on IMS SMART Tool NSP and NPA data.
2 IMS Health Plan Claims (April 2008-11).
3 AMAG estimates based on:
a) Wysocki et al. Management of Vaginal Atrophy: Implications from the REVIVE Survey. Clinical Medicine Insights: Reproductive Health 2014:8 23–30;
b) Kingsberg et al. Vulvar and Vaginal Atrophy in Postmenopausal Women: Findings from the REVIVE Survey. J Sex Med 2013;101790-1799;
c) F. Palma et al: Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study; and
d) Trinity Partners, Quantitative Market Assessment, December 2016 (n=100 PCPs, 100 OBGYNS).
4 Assumes parity pricing to current therapies (WAC ~$200/month).
Currently
on Rx estrogen
therapy
Local (intra-vaginal)
estrogen therapies = sales
of >$1B per year1
1.7M
women2
~6M
women3
~12M
women3
W O M E N ’ S H E A L T H : I N T R A R O S A
~20 M women in U.S. suffer from dyspareunia, a symptom of VVA
New potential
patients represent a
market opportunity
of ~$14 B/year4
Prasterone
Vaginal Inserts
Intent-to-prescribe market
research indicates sales potential:
>$500M/year

Leveraging existing strengths:
– Medical affairs
– Market access team
– Analytics team
– Digital consumer platform
Preparing for Launch
– In process of hiring a new approximately 150-person women’s health commercial team
– Conducting market research, including product positioning and concept testing
– Large presence at upcoming ACOG Annual Meeting (May 6-9)
Future Expansion
– Initiate Phase 3 female sexual dysfunction study in post-menopausal women in 2H-2017
W O M E N ’ S H E A L T H : I N T R A R O S A
15
Intrarosa: Investing Today for a Significant Tomorrow
Mid-2017 commercial launch
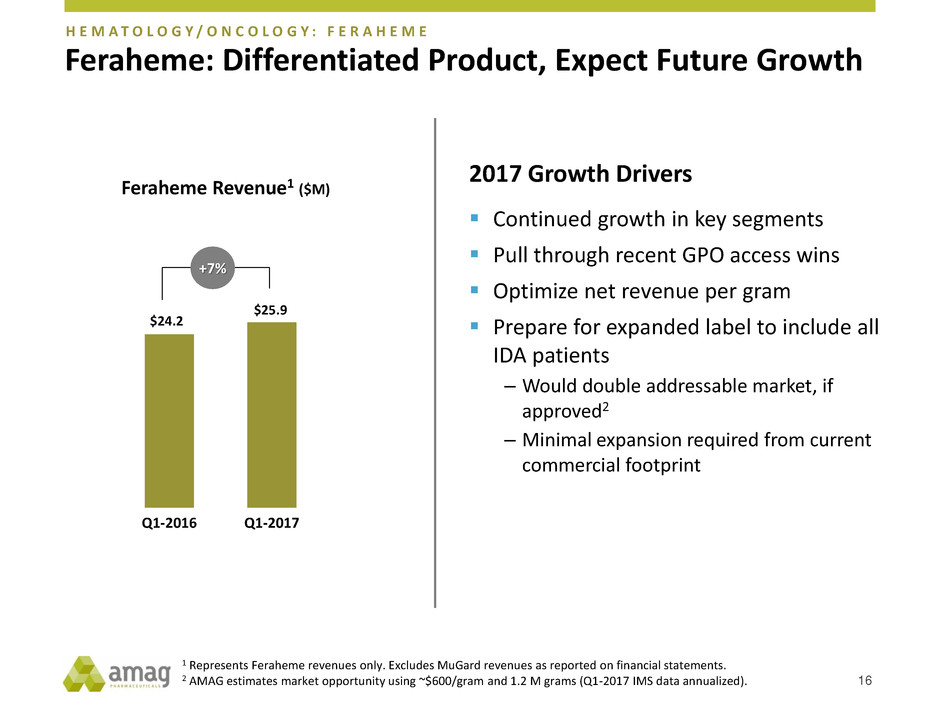
Feraheme: Differentiated Product, Expect Future Growth
16
$24.2
$25.9
Q1-2016 Q1-2017
Feraheme Revenue1 ($M)
+7%
H E M A T O L O G Y / O N C O L O G Y : F E R A H E M E
2017 Growth Drivers
Continued growth in key segments
Pull through recent GPO access wins
Optimize net revenue per gram
Prepare for expanded label to include all
IDA patients
– Would double addressable market, if
approved2
– Minimal expansion required from current
commercial footprint
1 Represents Feraheme revenues only. Excludes MuGard revenues as reported on financial statements.
2 AMAG estimates market opportunity using ~$600/gram and 1.2 M grams (Q1-2017 IMS data annualized).

Feraheme Broad Label Study
Results
Julie Krop, MD
Chief Medical Officer

Feraheme Phase 3 Label Expansion Study Overview
Sample size • 1,997 patients randomized in a 1:1 ratio
Dosing Regimen • 2 doses of Feraheme (1.02g)* or Injectafer (1.5g)*, dosed 7 days apart
Key entry criteria
• Subjects with IDA and in whom intravenous iron treatment is indicated
and have failed previous course of oral iron
Primary endpoint
• Incidence of moderate-to-severe hypersensitivity reactions (including
anaphylaxis) and/or moderate-to-severe hypotension
Secondary endpoints
• Incidence of any of the following: moderate-to-severe hypersensitivity
reactions (including anaphylaxis), serious cardiovascular events, death
• Mean change in hemoglobin/g of iron delivered
• Mean change in hemoglobin from baseline to week 5
# of sites/Region • ~130 sites, global (U.S., Canada, Europe)
18
H E M A T O L O G Y / O N C O L O G Y : F E R A H E M E
* FDA approved dose
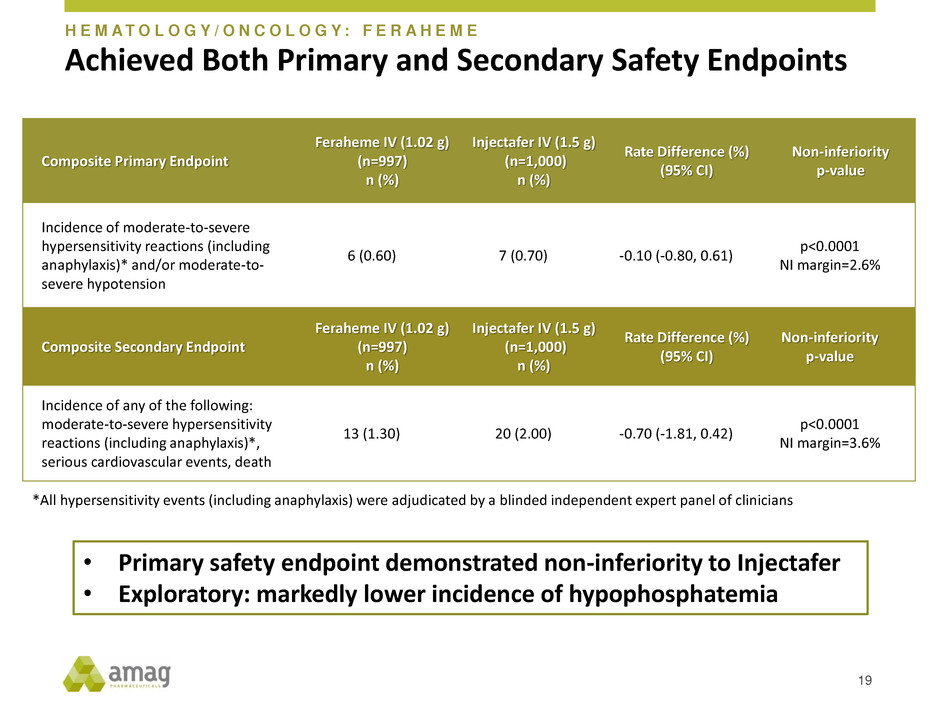
Achieved Both Primary and Secondary Safety Endpoints
Composite Primary Endpoint
Feraheme IV (1.02 g)
(n=997)
n (%)
Injectafer IV (1.5 g)
(n=1,000)
n (%)
Rate Difference (%)
(95% CI)
Non-inferiority
p-value
Incidence of moderate-to-severe
hypersensitivity reactions (including
anaphylaxis)* and/or moderate-to-
severe hypotension
6 (0.60) 7 (0.70) -0.10 (-0.80, 0.61)
p<0.0001
NI margin=2.6%
Composite Secondary Endpoint
Feraheme IV (1.02 g)
(n=997)
n (%)
Injectafer IV (1.5 g)
(n=1,000)
n (%)
Rate Difference (%)
(95% CI)
Non-inferiority
p-value
Incidence of any of the following:
moderate-to-severe hypersensitivity
reactions (including anaphylaxis)*,
serious cardiovascular events, death
13 (1.30) 20 (2.00) -0.70 (-1.81, 0.42)
p<0.0001
NI margin=3.6%
19
• Primary safety endpoint demonstrated non-inferiority to Injectafer
• Exploratory: markedly lower incidence of hypophosphatemia
H E M A T O L O G Y / O N C O L O G Y : F E R A H E M E
*All hypersensitivity events (including anaphylaxis) were adjudicated by a blinded independent expert panel of clinicians
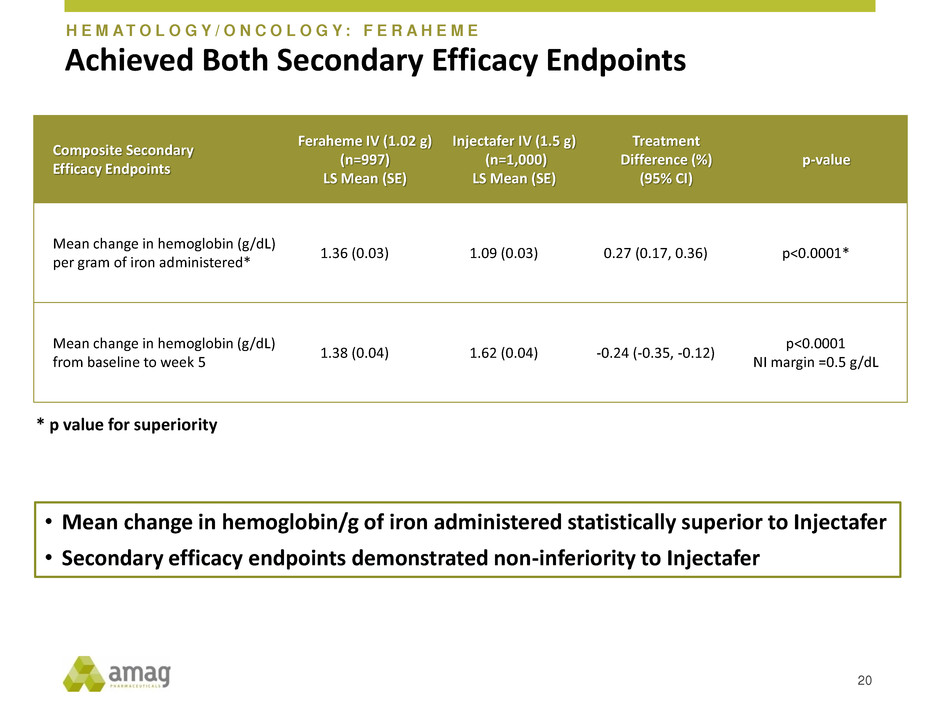
Achieved Both Secondary Efficacy Endpoints
Composite Secondary
Efficacy Endpoints
Feraheme IV (1.02 g)
(n=997)
LS Mean (SE)
Injectafer IV (1.5 g)
(n=1,000)
LS Mean (SE)
Treatment
Difference (%)
(95% CI)
p-value
Mean change in hemoglobin (g/dL)
per gram of iron administered*
1.36 (0.03) 1.09 (0.03) 0.27 (0.17, 0.36) p<0.0001*
Mean change in hemoglobin (g/dL)
from baseline to week 5
1.38 (0.04) 1.62 (0.04) -0.24 (-0.35, -0.12)
p<0.0001
NI margin =0.5 g/dL
20
* p value for superiority
• Mean change in hemoglobin/g of iron administered statistically superior to Injectafer
• Secondary efficacy endpoints demonstrated non-inferiority to Injectafer
H E M A T O L O G Y / O N C O L O G Y : F E R A H E M E
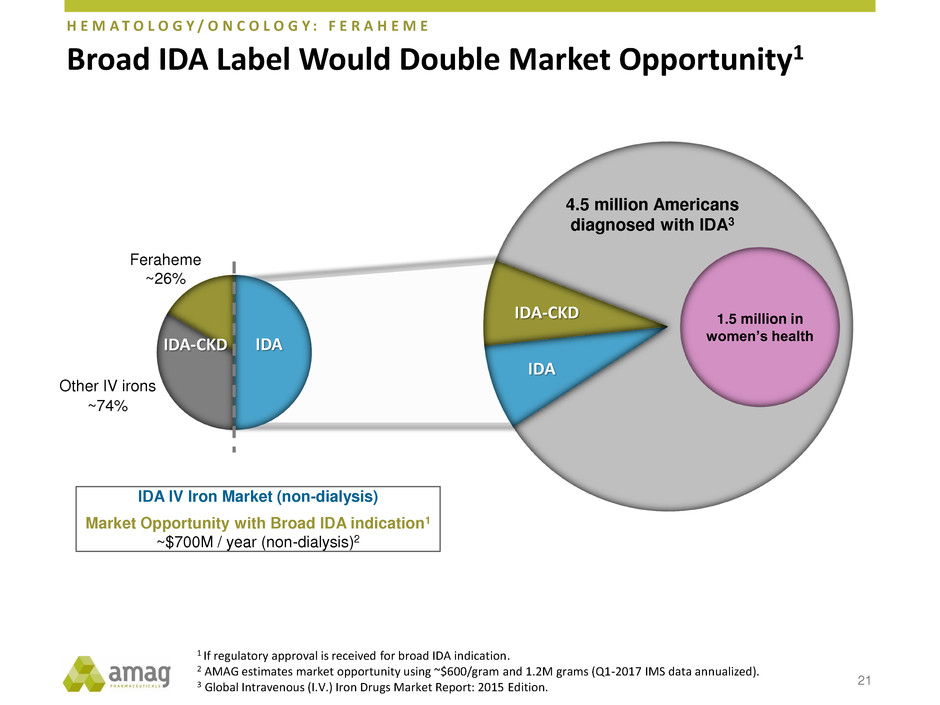
Broad IDA Label Would Double Market Opportunity1
21
Feraheme
~26%
Other IV irons
~74%
IDA IV Iron Market (non-dialysis)
Market Opportunity with Broad IDA indication1
~$700M / year (non-dialysis)2
IDA IDA-CKD
IDA-CKD
IDA
4.5 million Americans
diagnosed with IDA3
1.5 million in
women’s health
1 If regulatory approval is received for broad IDA indication.
2 AMAG estimates market opportunity using ~$600/gram and 1.2M grams (Q1-2017 IMS data annualized).
3 Global Intravenous (I.V.) Iron Drugs Market Report: 2015 Edition.
H E M A T O L O G Y / O N C O L O G Y : F E R A H E M E

Financial Overview
Ted Myles
Chief Financial Officer
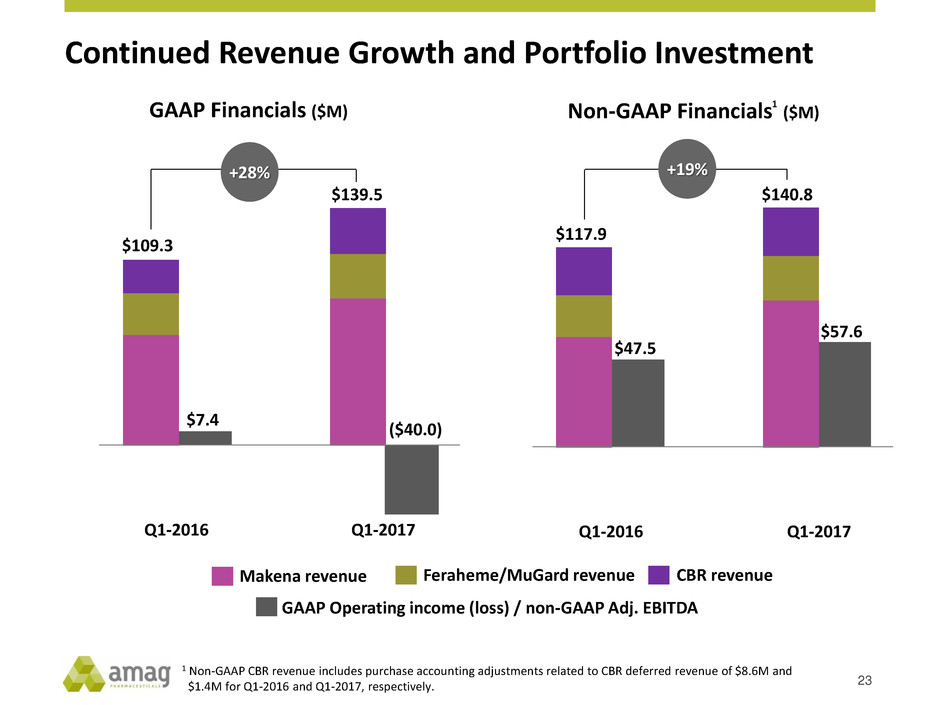
23
$109.3
$139.5
($40.0)
$7.4
Q1-2016 Q1-2017
+28%
Makena revenue CBR revenue Feraheme/MuGard revenue
GAAP Operating income (loss) / non-GAAP Adj. EBITDA
GAAP Financials ($M)
$117.9
$140.8
$47.5
$57.6
Q1-2016 Q1-2017
+19%
Non-GAAP Financials ($M)
1 Non-GAAP CBR revenue includes purchase accounting adjustments related to CBR deferred revenue of $8.6M and
$1.4M for Q1-2016 and Q1-2017, respectively.
Continued Revenue Growth and Portfolio Investment
1
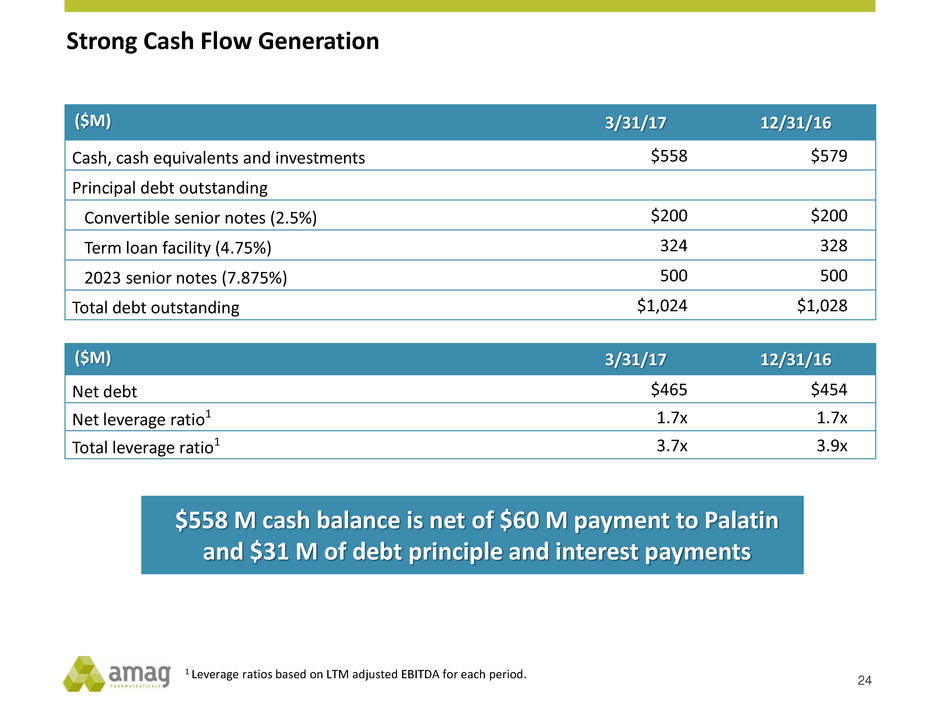
Strong Cash Flow Generation
($M) 3/31/17 12/31/16
Cash, cash equivalents and investments $558 $579
Principal debt outstanding
Convertible senior notes (2.5%) $200 $200
Term loan facility (4.75%) 324 328
2023 senior notes (7.875%) 500 500
Total debt outstanding $1,024 $1,028
($M) 3/31/17 12/31/16
Net debt $465 $454
Net leverage ratio1 1.7x 1.7x
Total leverage ratio1 3.7x 3.9x
$558 M cash balance is net of $60 M payment to Palatin
and $31 M of debt principle and interest payments
24
1 Leverage ratios based on LTM adjusted EBITDA for each period.
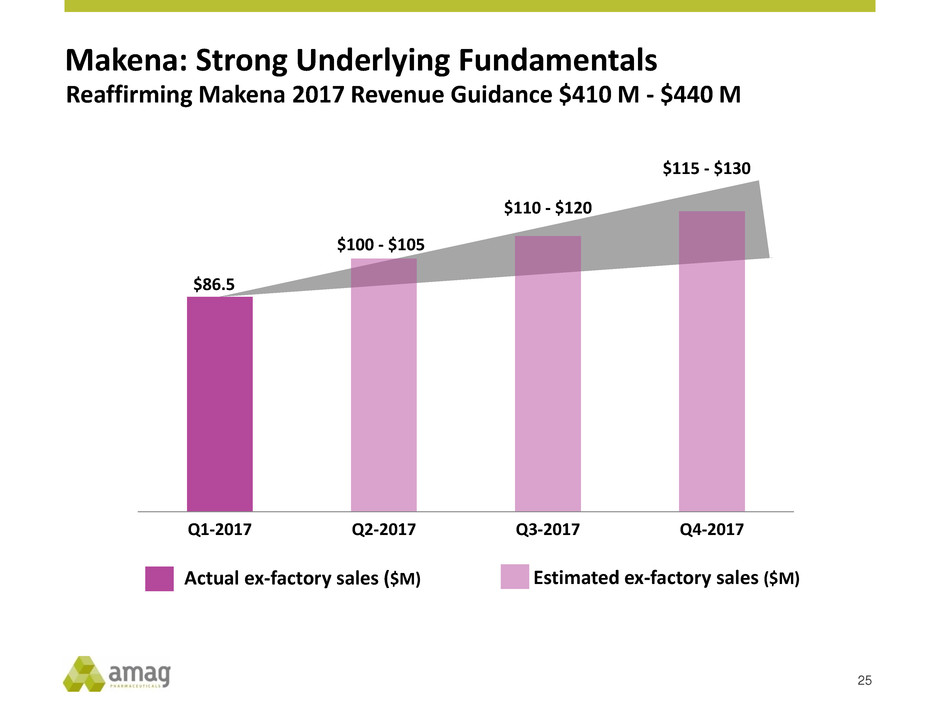
Actual ex-factory sales ($M) Estimated ex-factory sales ($M)
$86.5
$100 - $105
$110 - $120
$115 - $130
Q1-2017 Q2-2017 Q3-2017 Q4-2017
Makena: Strong Underlying Fundamentals
Reaffirming Makena 2017 Revenue Guidance $410 M - $440 M
25
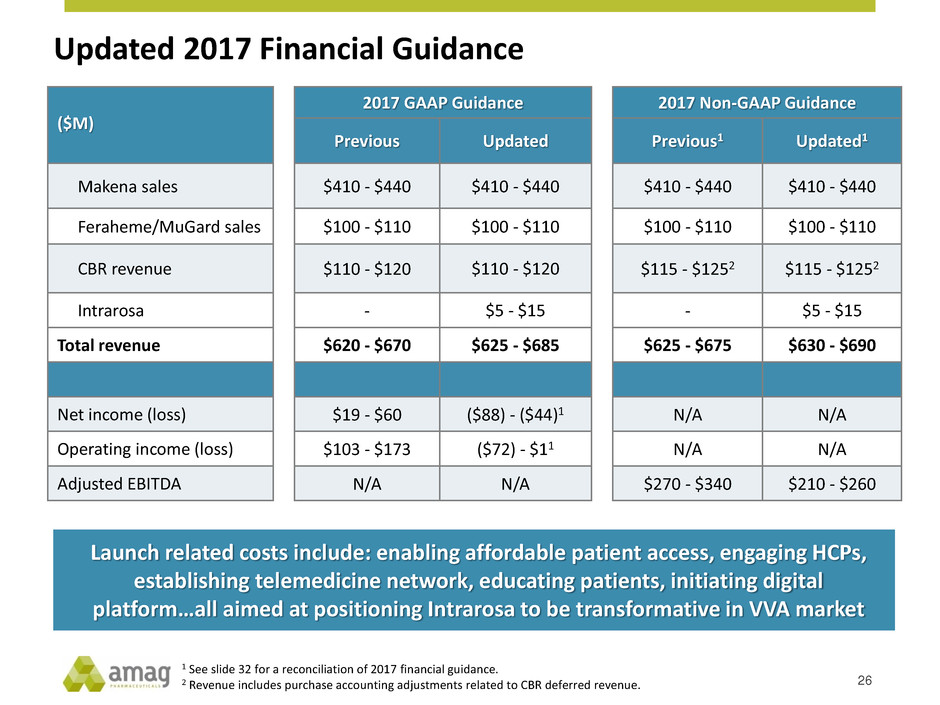
Updated 2017 Financial Guidance
26
($M)
2017 GAAP Guidance 2017 Non-GAAP Guidance
Previous Updated Previous1 Updated1
Makena sales $410 - $440 $410 - $440 $410 - $440 $410 - $440
Feraheme/MuGard sales $100 - $110 $100 - $110 $100 - $110 $100 - $110
CBR revenue $110 - $120 $110 - $120 $115 - $1252 $115 - $1252
Intrarosa - $5 - $15 - $5 - $15
Total revenue $620 - $670 $625 - $685 $625 - $675 $630 - $690
Net income (loss) $19 - $60 ($88) - ($44)1 N/A N/A
Operating income (loss) $103 - $173 ($72) - $11 N/A N/A
Adjusted EBITDA N/A N/A $270 - $340 $210 - $260
1 See slide 32 for a reconciliation of 2017 financial guidance.
2 Revenue includes purchase accounting adjustments related to CBR deferred revenue.
Launch related costs include: enabling affordable patient access, engaging HCPs,
establishing telemedicine network, educating patients, initiating digital
platform…all aimed at positioning Intrarosa to be transformative in VVA market

AMAG Portfolio: Multiple Value Drivers
27
Milestone 2017 2018
MAKENA AUTO-INJECTOR PROGRAM Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4
Topline PK data
sNDA submission
Expected FDA action and commercial launch
FERAHEME IDA LABEL EXPANSION Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4
Enrollment completed
Topline data
sNDA submission
Expected FDA action and commercial launch
INTRAROSA Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4
Commercial launch in dyspareunia
Initiate Phase 3 female sexual dysfunction study
BREMELANOTIDE Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4
NDA submission
Expected FDA action and commercial launch
VELO – SEVERE PREECLAMPSIA
Initiate Phase 2b/3a study
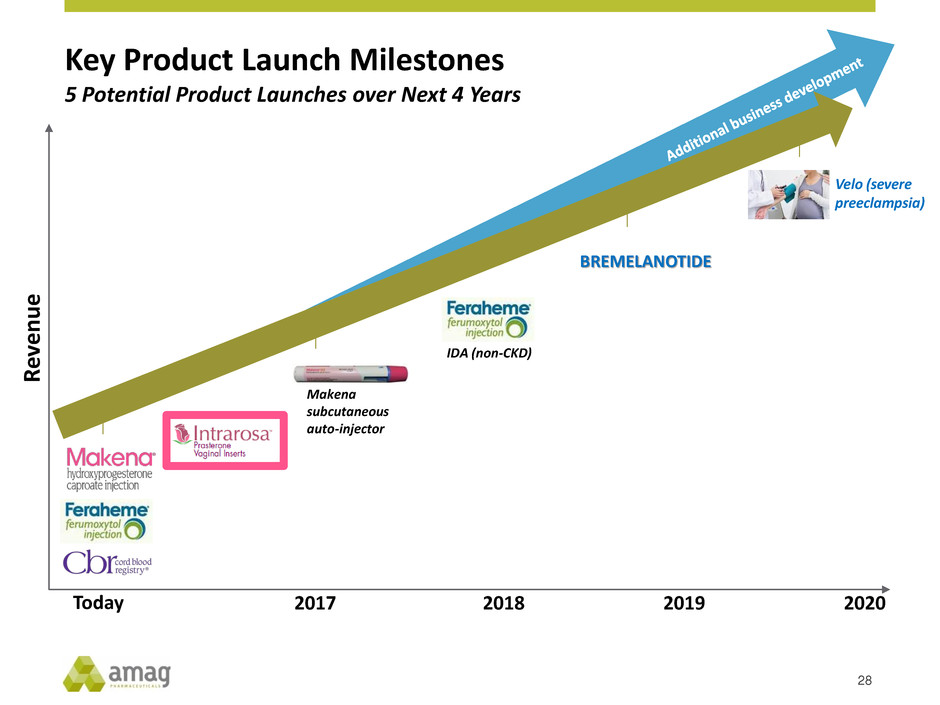
Key Product Launch Milestones
28
5 Potential Product Launches over Next 4 Years
Velo (severe
preeclampsia)
R
ev
e
nu
e
Today 2017 2018 2019 2020
Makena
subcutaneous
auto-injector
IDA (non-CKD)
BREMELANOTIDE

AMAG Pharmaceuticals
Q1-2017 Financial Results &
Corporate Update
May 2, 2017

Appendix
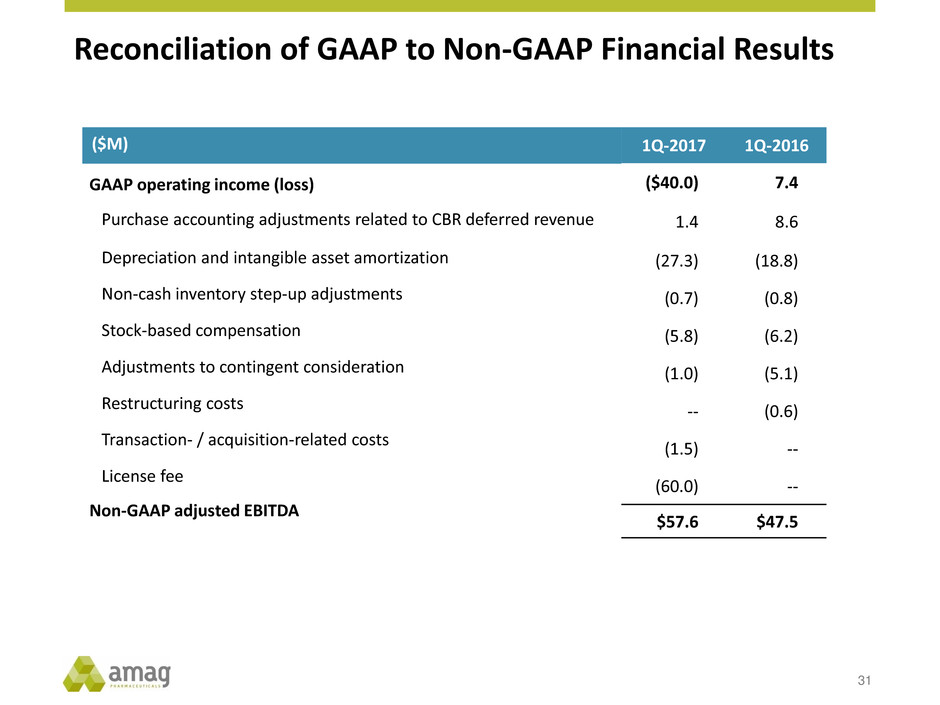
Reconciliation of GAAP to Non-GAAP Financial Results
31
($M)
GAAP operating income (loss)
Purchase accounting adjustments related to CBR deferred revenue
Depreciation and intangible asset amortization
Non-cash inventory step-up adjustments
Stock-based compensation
Adjustments to contingent consideration
Restructuring costs
Transaction- / acquisition-related costs
License fee
Non-GAAP adjusted EBITDA
1Q-2017 1Q-2016
($40.0) 7.4
1.4 8.6
(27.3) (18.8)
(0.7) (0.8)
(5.8) (6.2)
(1.0) (5.1)
-- (0.6)
(1.5) --
(60.0) --
$57.6 $47.5
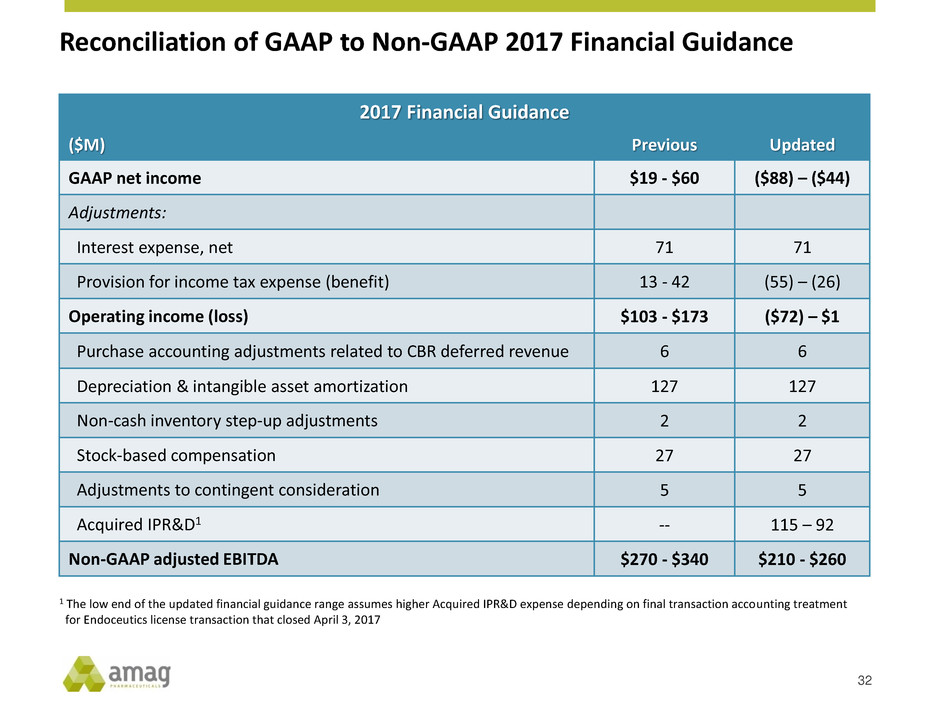
Reconciliation of GAAP to Non-GAAP 2017 Financial Guidance
2017 Financial Guidance
($M) Previous Updated
GAAP net income $19 - $60 ($88) – ($44)
Adjustments:
Interest expense, net 71 71
Provision for income tax expense (benefit) 13 - 42 (55) – (26)
Operating income (loss) $103 - $173 ($72) – $1
Purchase accounting adjustments related to CBR deferred revenue 6 6
Depreciation & intangible asset amortization 127 127
Non-cash inventory step-up adjustments 2 2
Stock-based compensation 27 27
Adjustments to contingent consideration 5 5
Acquired IPR&D1 -- 115 – 92
Non-GAAP adjusted EBITDA $270 - $340 $210 - $260
1 The low end of the updated financial guidance range assumes higher Acquired IPR&D expense depending on final transaction accounting treatment
for Endoceutics license transaction that closed April 3, 2017
32
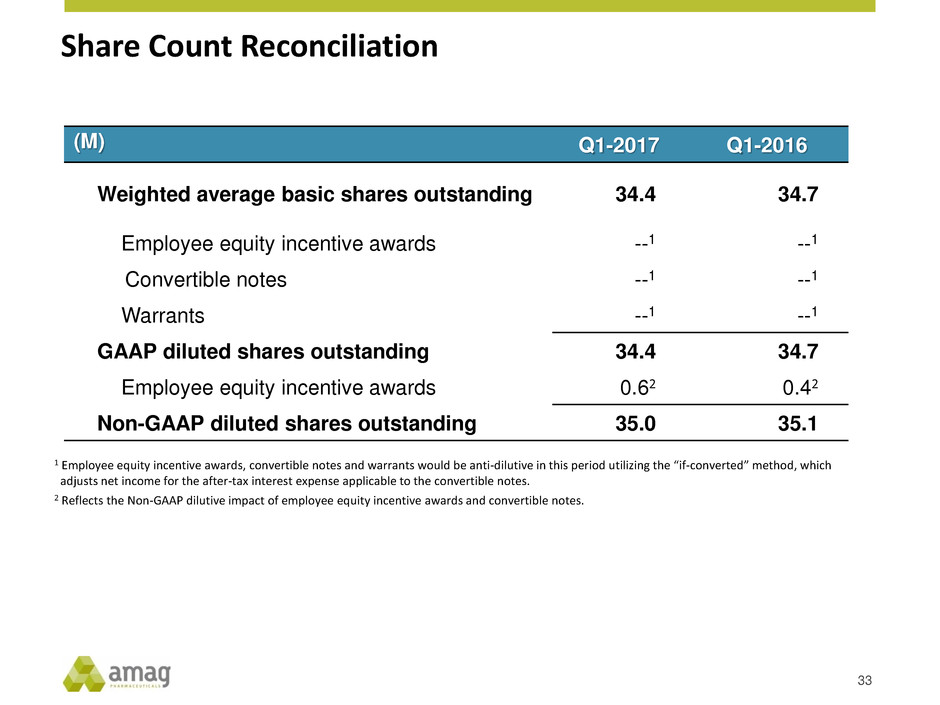
Share Count Reconciliation
1 Employee equity incentive awards, convertible notes and warrants would be anti-dilutive in this period utilizing the “if-converted” method, which
adjusts net income for the after-tax interest expense applicable to the convertible notes.
2 Reflects the Non-GAAP dilutive impact of employee equity incentive awards and convertible notes.
(M) Q1-2017 Q1-2016
Weighted average basic shares outstanding 34.4 34.7
Employee equity incentive awards --1 --1
Convertible notes --1 --1
Warrants --1 --1
GAAP diluted shares outstanding 34.4 34.7
Employee equity incentive awards 0.62 0.42
Non-GAAP diluted shares outstanding 35.0 35.1
33

AMAG Pharmaceuticals
Q1-2017 Financial Results &
Corporate Update
May 2, 2017
