Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Phio Pharmaceuticals Corp. | d386476dex991.htm |
| 8-K - 8-K - Phio Pharmaceuticals Corp. | d386476d8k.htm |
Exhibit 99.2
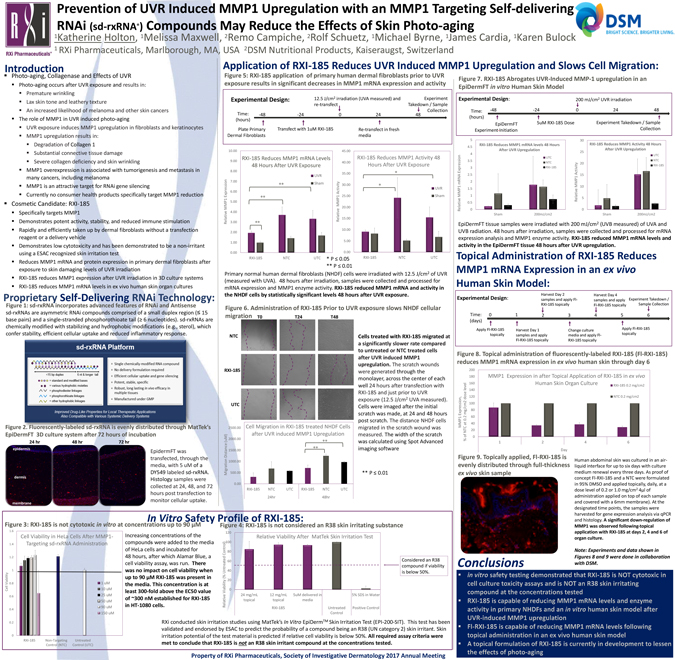
RXi Pharmaceuticals Prevention of UVR Induced MMP1 Upregulation with an MMP1 Targeting Self-delivering RNAi (sd-rxRNA®) Compounds May Reduce the Effects of Skin Photo-aging 1Katherine Holton, 1Melissa Maxwell, 2Remo Campiche, 2Rolf Schuetz, 1Michael Byrne, 1James Cardia, 1Karen Bulock 1 RXi Pharmaceuticals, Marlborough, MA, USA 2DSM Nutritional Products, Kaiseraugst, Switzerland DSM BRIGHT SCIENCE, BRIGHTER LIVING. Introduction Photo-aging, Collagenase and Effects of UVR Photo-aging occurs after UVR exposure and results in: Premature wrinkling Lax skin tone and leathery texture An increased likelihood of melanoma and other skin cancers The role of MMP1 in UVR induced photo-aging UVR exposure induces MMP1 upregulation in fibroblasts and keratinocytes MMP1 upregulation results in: Degradation of Collagen 1 Substantial connective tissue damage Severe collagen deficiency and skin wrinkling MMP1 overexpression is associated with tumorigenesis and metastasis in many cancers, including melanoma MMP1 is an attractive target for RNAi gene silencing Currently no consumer health products specifically target MMP1 reduction Cosmetic Candidate: RXI-185 Specifically targets MMP1 Demonstrates potent activity, stability, and reduced immune stimulation Rapidly and efficiently taken up by dermal fibroblasts without a transfection reagent or a delivery vehicle Demonstrates low cytotoxicity and has been demonstrated to be a non-irritant using a ESAC recognized skin irritation test Reduces MMP1 mRNA and protein expression in primary dermal fibroblasts after exposure to skin damaging levels of UVR irradiation RXI-185 reduces MMP1 expression after UVR irradiation in 3D culture systems RXI-185 reduces MMP1 mRNA levels in ex vivo human skin organ cultures Proprietary Self-Delivering RNAi Technology: Figure 1: sd-rxRNA incorporates advanced features of RNAi and Antisense sd-rxRNAs are asymmetric RNAi compounds comprised of a small duplex region (£ 15 base pairs) and a single-stranded phosphorothioate tail (³ 6 nucleotides). sd-rxRNAs are chemically modified with stabilizing and hydrophobic modifications (e.g., sterol), which confer stability, efficient cellular uptake and reduced inflammatory response. sd-rxRNA Platform 15 bp duplex 6 nt & longer tail = standard and modified bases = various hydrophobic moieties = phosphodiester linkages = phosphorothioate linkages = other hydrophobic linkages Single chemically-modified RNA compound No delivery formulation required Efficient cellular uptake and gene silencing Potent, stable, specific Robust, long lasting in vivo efficacy in multiple tissues Manufactured under GMP Improved Drug-Like Properties for Local Therapeutic Applications Also Compatible with Various Systemic Delivery Systems Figure 2. Fluorescently-labeled sd-rxRNA is evenly distributed through MatTek’s EpiDermFT 3D culture system after 72 hours of incubation epidermis dermis membrane EpidermFT was transfected, through the media, with 5 uM of a DY549 labeled sd-rxRNA. Histology samples were collected at 24, 48, and 72 hours post transfection to monitor cellular uptake. In Vitro Safety Profile of RXI-185: Figure 3: RXI-185 is not cytotoxic in vitro at concentrations up to 90 µM Cell Viability in HeLa Cells After MMP1- Targeting sd-rxRNA Administration Cell Viability 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 RXI-185 Non-Targeting Control (NTC) Untreated Control (UTC) 1 uM 10 uM 25 uM 50 uM 90 uM 150 uM Figure 4: RXI-185 is not considered an R38 skin irritating substance Relative Viability (% Untreated Control) 120 100 80 60 40 20 0 Relative Viability After MatTek Skin Irritation Test 24 mg/mL topical 12 mg/mL topical RXI-185 5uM delivered in Media Untreated Control 5% SDS in Water Positive Control Considered an R38 compound if viability is below 50%. Increasing concentrations of the compounds were added to the media of HeLa cells and incubated for 48 hours, after which Alamar Blue, a cell viability assay, was run. There was no impact on cell viability when up to 90 µM RXI-185 was present in the media. This concentration is at least 300-fold above the EC50 value of ~300 nM established for RXI-185 in HT-1080 cells. RXi conducted skin irritation studies using MatTek’s In Vitro EpiDermTM Skin Irritation Test (EPI-200-SIT). This test has been validated and endorsed by ESAC to predict the probability of a compound being an R38 (UN category 2) skin irritant. Skin irritation potential of the test material is predicted if relative cell viability is below 50%. All required assay criteria were met to conclude that RXI-185 is not an R38 skin irritant compound at the concentrations tested. Property of RXi Pharmaceuticals, Society of Investigative Dermatology 2017 Annual Meeting Application of RXI-185 Reduces UVR Induced MMP1 Upregulation and Slows Cell Migration: Figure 5: RXI-185 application of primary human dermal fibroblasts prior to UVR exposure results in significant decreases in MMP1 mRNA expression and activity Experimental Design: 12.5 J/cm2irradiation (UVA measured) and re-transfect Experiment Takedown / Sample Collection Time: (hours) -48 -24 0 24 48 Plate Primary Dermal Fibroblasts Transfect with 1uM RXI-185 Re-transfect in fresh media Relative MMP1 Expression 10.00 9.00 8.00 7.00 6.00 5.00 4.00 3.00 2.00 1.00 0.00 RXI-185 NTC UTC RXI-185 Reduces MMP1 mRNA Levels 48 Hours After UVR Exposure ** ** ** UVR Sham Relative MMP1 Activity 45.00 40.00 35.00 30.00 25.00 20.00 15.00 10.00 5.00 0.00 RXI-185 NTC UTC RXI-185 Reduces MMP1 Activity 48 Hours After UVR Exposure * * UVR Sham * P £ 0.05 ** P £ 0.01 Primary normal human dermal fibroblasts (NHDF) cells were irradiated with 12.5 J/cm2 of UVR (measured with UVA). 48 hours after irradiation, samples were collected and processed for mRNA expression and MMP1 enzyme activity. RXI-185 reduced MMP1 mRNA and activity in the NHDF cells by statistically significant levels 48 hours after UVR exposure. Figure 6. Administration of RXI-185 Prior to UVR exposure slows NHDF cellular migration T0 T24 T48 NTC RXI-185 UTC Migration Distance (uM) 2500.00 2000.00 1500.00 1000.00 500.00 0.00 Cell Migration in RXI-185 treated NHDF Cells after UVR induced MMP1 Upregulation ** ** RXI-185 NTC 24hr UTC RXI-185 NTC 48hr UTC ** P £ 0.01 Cells treated with RXI-185 migrated at a significantly slower rate compared to untreated or NTC treated cells after UVR induced MMP1 upregulation. The scratch wounds were generated through the monolayer, across the center of each well 24 hours after transfection with RXI-185 and just prior to UVR exposure (12.5 J/cm2 UVA measured). Cells were imaged after the initial scratch was made, at 24 and 48 hours post scratch. The distance NHDF cells migrated in the scratch wound was measured. The width of the scratch was calculated using Spot Advanced imaging software Figure 7. RXI-185 Abrogates UVR-Induced MMP-1 upregulation in an EpiDermFT in vitro Human Skin Model Experimental Design: 200 mJ/cm2UVR irradiation Time: (hours) -48 -24 0 24 48 EpiDermFT Experiment Initiation 5uM RXI-185 Dose ExperimentTakedown / Sample Collection Relative MMP1 mRNA Expression 5 4.5 4 3.5 3 2.5 2 1.5 1 0.5 0 RXI-185 Reduces MMP1 mRNA levels 48 Hours After UVR Upregulation UTC NTC RXI-185 Sham 200mJ/cm2 Relative MMP1 Activity 30 25 20 15 10 5 0 RXI-185 Reduces MMP1 Activity 48 Hours After UVR Upregulation UTC NTC RXI-185 Sham 200mJ/cm2 EpiDermFT tissue samples were irradiated with 200 mJ/cm2 (UVB measured) of UVA and UVB radiation. 48 hours after irradiation, samples were collected and processed for mRNA expression analysis and MMP1 enzyme activity. RXI-185 reduced MMP1 mRNA levels and activity in the EpiDermFT tissue 48 hours after UVR upregulation. Topical Administration of RXI-185 Reduces MMP1 mRNA Expression in an ex vivo Human Skin Model: Experimental Design: Harvest Day 2 samples and apply Fl- RXI-185 topically Harvest Day 4 samples and apply Fl-RXI-185 topically Experiment Takedown /Sample Collection Time: (days) 0 1 2 3 4 5 6 Apply Fl-RXI-185 topically Harvest Day 1 samples and apply Fl-RXI-185 topically Change culture media and apply Fl-RXI-185 topically Apply Fl-RXI-185 topically Figure 8. Topical administration of fluorescently-labeled RXI-185 (Fl-RXI-185) reduces MMP1 mRNA expression in ex vivo human skin through day 6 MMP1 Expression, of NTC at 0.2 mg/cm2 dose level 200 180 160 140 120 100 80 60 40 20 0 MMP1 Expression in after Topical Application of RXI-185 in ex vivo Human Skin Organ Culture RXI-185 0.2 mg/cm2 NTC 0.2 mg/cm2 1 2 4 6 Figure 9. Topically applied, Fl-RXI-185 is evenly distributed through full-thickness ex vivo skin sample Human abdominal skin was cultured in an air-liquid interface for up to six days with culture medium renewal every three days. As proof of concept Fl-RXI-185 and a NTC were formulated in 95% DMSO and applied topically, daily, at a dose level of 0.2 or 1.0 mg/cm2 4µl of administration applied on top of each sample and covered with a 6mm membrane). At the designated time points, the samples were harvested for gene expression analysis via qPCR and histology. A significant down-regulation of MMP1 was observed following topical application with RXI-185 at days 2, 4 and 6 of organ culture. Note: Experiments and data shown in Figures 8 and 9 were done in collaboration with DSM. Conclusions in vitro safety testing demonstrated that RXI-185 is NOT cytotoxic in cell culture toxicity assays and is NOT an R38 skin irritating compound at the concentration tested RXI-185 is capable of reducing MMP1 mRNA levels and enzyme activity in primary NHDFs and an in vitro human skin model after UVR-induced MMP1 upregulation FI-RXI-185 is capable of reducing MMP1 mRNA levels following topical administration in an ex vivo human skin model A topical formulation of RXI-185 is currently in development to lessen the effects of photo-aging
