Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Evofem Biosciences, Inc. | neot-8k_20170403.htm |

Neothetics Corporate Overview April 2017 Exhibit 99.1

Safe Harbor Statement This presentation, including the accompanying oral presentation, may contain forward-looking statements concerning our business, operations and financial condition as well as our future plans, objectives and expectations. All forward-looking statements are subject to a number of risks, uncertainties and assumptions, and you should not rely upon forward-looking statements as predictions of future events. All forward-looking statements will be based upon current estimates and expectations about future events and financial and other trends. There is no guarantee that future results, performance or events reflected in the forward-looking statements will be achieved or occur. No person assumes responsibility for the accuracy and completeness of the forward-looking statements, and, except as required by law, no person undertakes any obligation to update any forward-looking statements for any reason after the date of this presentation. This presentation does not constitute an offer to sell or the solicitation of an offer to buy securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. Any offering of securities will only be made by means of a registration statement (including a prospectus) filed with the SEC and only after such registration statement becomes effective. No such registration statement has become effective as of the date of this presentation. Before you decide whether to buy securities, you should read when available the registration statement (including the prospectus and risk factors set forth therein), for more complete information about the company and any proposed offering. Please note that these materials contain proprietary and confidential information. By accepting these materials, you agree that no part of this document may be reproduced, disseminated, shared with other parties, transmitted, or used in any way except for the purpose of evaluation, without the express written permission of Neothetics, Inc.
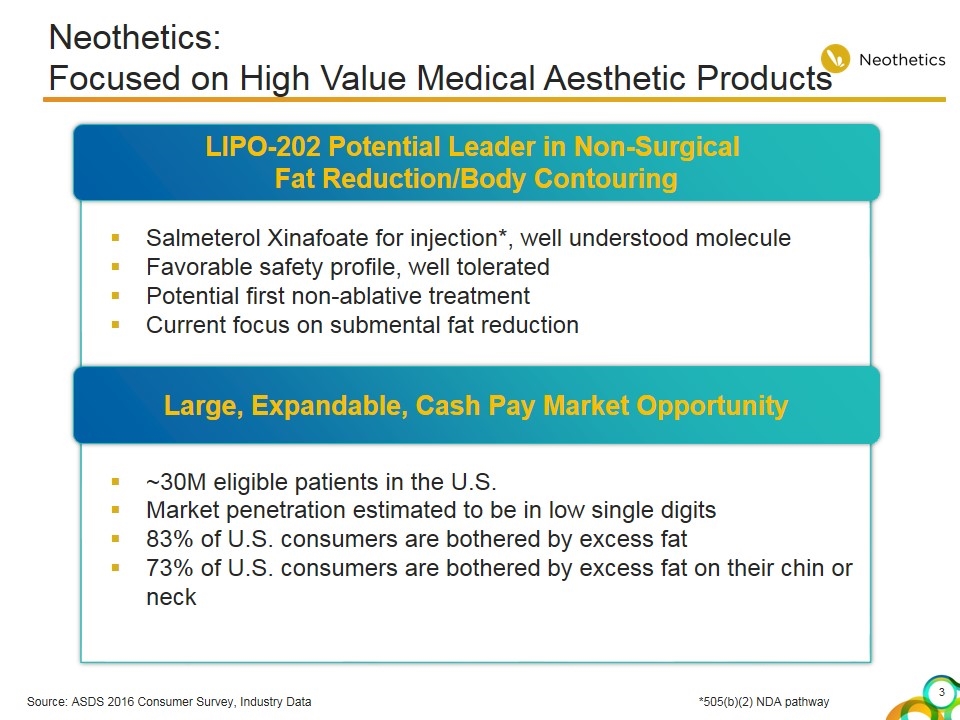
Neothetics: Focused on High Value Medical Aesthetic Products Salmeterol Xinafoate for injection*, well understood molecule Favorable safety profile, well tolerated Potential first non-ablative treatment Current focus on submental fat reduction ~30M eligible patients in the U.S. Market penetration estimated to be in low single digits 83% of U.S. consumers are bothered by excess fat 73% of U.S. consumers are bothered by excess fat on their chin or neck LIPO-202 Potential Leader in Non-Surgical Fat Reduction/Body Contouring Large, Expandable, Cash Pay Market Opportunity Source: ASDS 2016 Consumer Survey, Industry Data*505(b)(2) NDA pathway
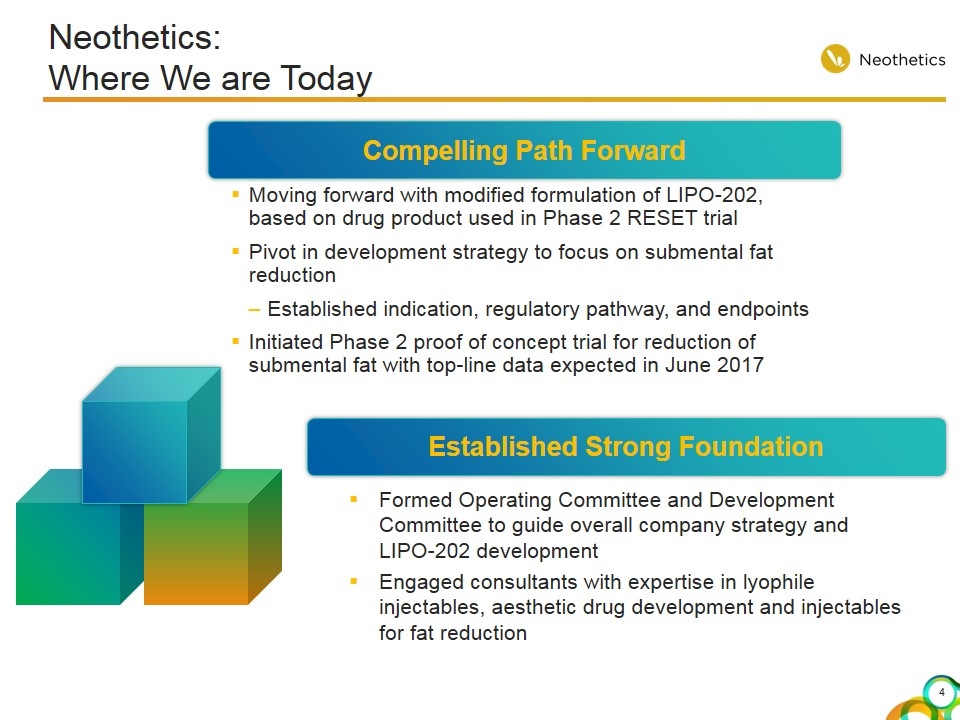
Neothetics: Where We are Today Compelling Path Forward Established Strong Foundation Moving forward with modified formulation of LIPO-202, based on drug product used in Phase 2 RESET trial Pivot in development strategy to focus on submental fat reduction Established indication, regulatory pathway, and endpoints Initiated Phase 2 proof of concept trial for reduction of submental fat with top-line data expected in June 2017 Formed Operating Committee and Development Committee to guide overall company strategy and LIPO-202 development Engaged consultants with expertise in lyophile injectables, aesthetic drug development and injectables for fat reduction
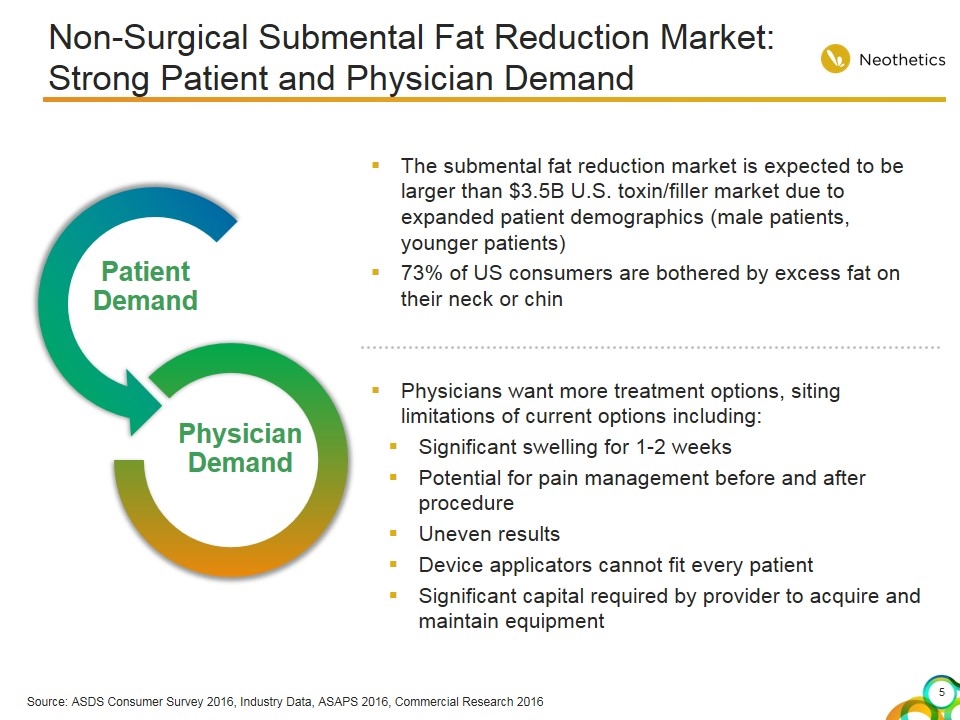
Source: ASDS Consumer Survey 2016, Industry Data, ASAPS 2016, Commercial Research 2016 Non-Surgical Submental Fat Reduction Market: Strong Patient and Physician Demand Patient Demand Physician Demand The submental fat reduction market is expected to be larger than $3.5B U.S. toxin/filler market due to expanded patient demographics (male patients, younger patients) 73% of US consumers are bothered by excess fat on their neck or chin Physicians want more treatment options, siting limitations of current options including: Significant swelling for 1-2 weeks Potential for pain management before and after procedure Uneven results Device applicators cannot fit every patient Significant capital required by provider to acquire and maintain equipment
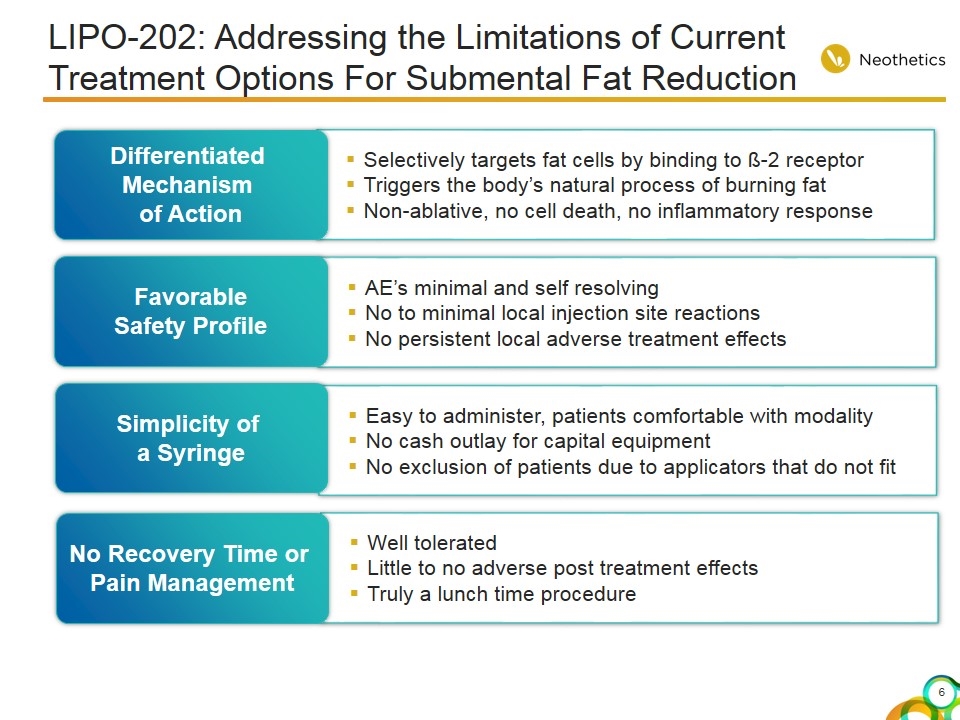
LIPO-202: Addressing the Limitations of Current Treatment Options For Submental Fat Reduction Selectively targets fat cells by binding to ß-2 receptor Triggers the body’s natural process of burning fat Non-ablative, no cell death, no inflammatory response Differentiated Mechanism of Action AE’s minimal and self resolving No to minimal local injection site reactions No persistent local adverse treatment effects Favorable Safety Profile Well tolerated Little to no adverse post treatment effects Truly a lunch time procedure No Recovery Time or Pain Management Easy to administer, patients comfortable with modality No cash outlay for capital equipment No exclusion of patients due to applicators that do not fit Simplicity of a Syringe
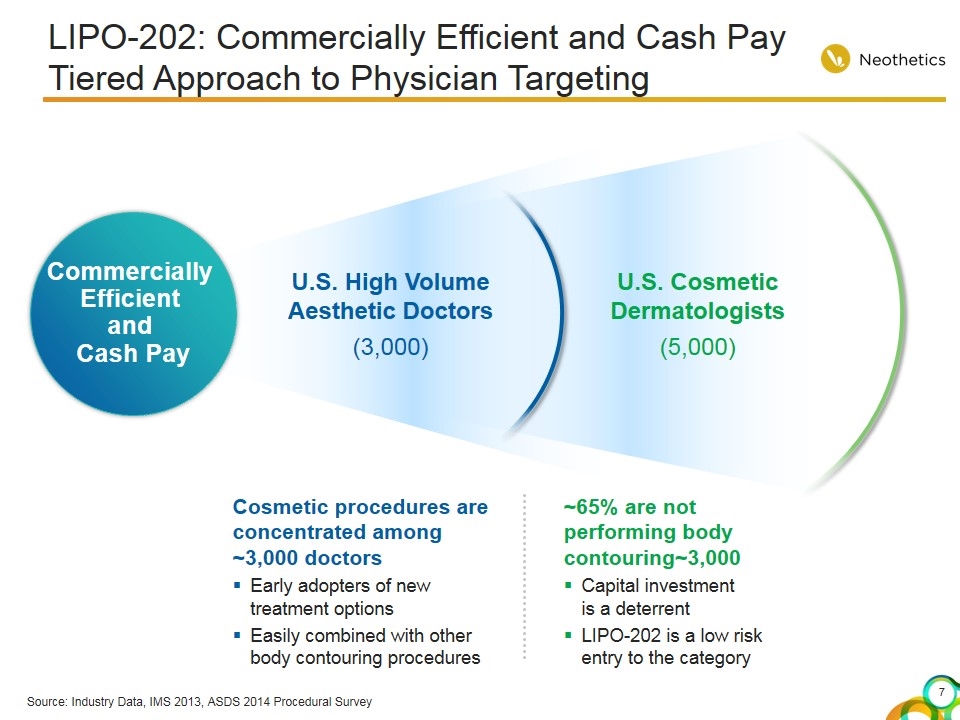
LIPO-202: Commercially Efficient and Cash Pay Tiered Approach to Physician Targeting Commercially Efficient and Cash Pay U.S. High Volume Aesthetic Doctors (3,000) U.S. Cosmetic Dermatologists (5,000) Cosmetic procedures are concentrated among ~3,000 doctors Early adopters of new treatment options Easily combined with other body contouring procedures ~65% are not performing body contouring~3,000 Capital investment is a deterrent LIPO-202 is a low risk entry to the category Source: Industry Data, IMS 2013, ASDS 2014 Procedural Survey
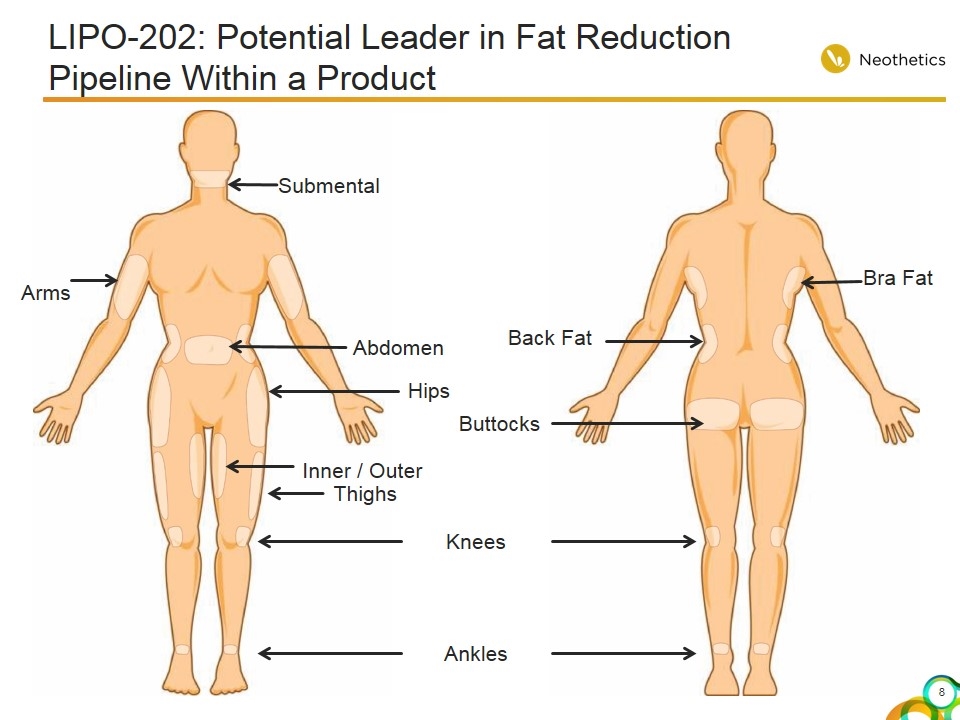
LIPO-202: Potential Leader in Fat Reduction Pipeline Within a Product Inner / Outer Thighs Arms Hips Abdomen Submental Buttocks Bra Fat Knees Ankles Back Fat
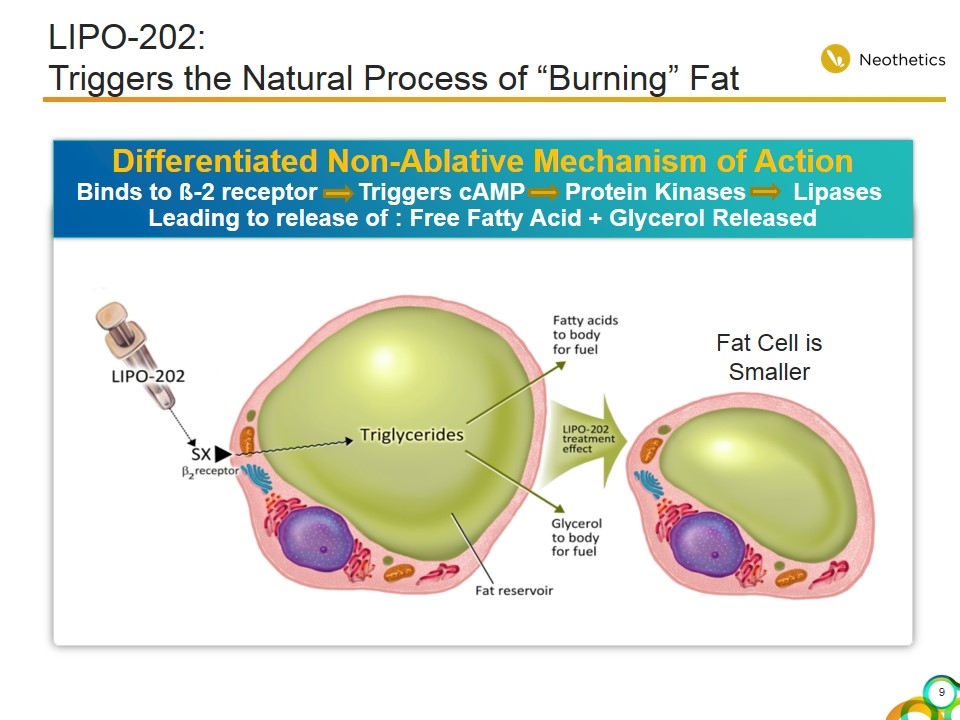
Differentiated Non-Ablative Mechanism of Action Binds to ß-2 receptor Triggers cAMP Protein Kinases Lipases Leading to release of : Free Fatty Acid + Glycerol Released Fat Cell is Smaller LIPO-202: Triggers the Natural Process of “Burning” Fat

LIPO-202-CL-31: Phase 2 Proof of Concept Trial For Reduction of Submental Fat Extensive clinical experience with LIPO-202 (~1,400 subjects treated) Feedback from key opinion leaders Experienced investigators Expert consultants Established indication, regulatory pathway, and endpoints Comprehensive Clinical Trial Design
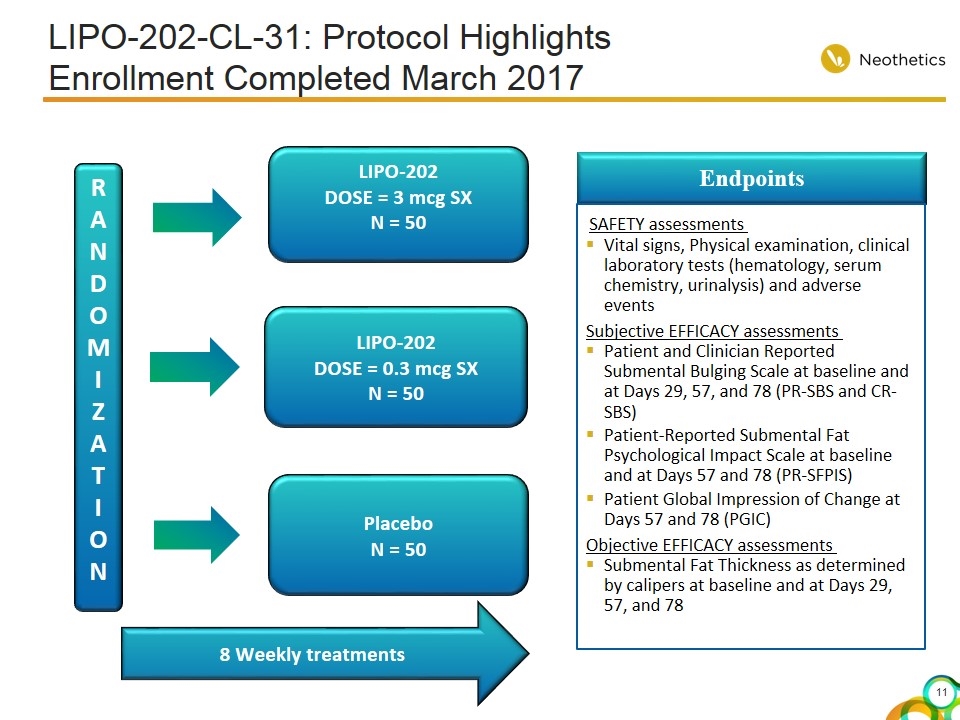
LIPO-202-CL-31: Protocol Highlights Enrollment Completed March 2017 RANDOMI ZAT I ON LIPO-202 DOSE = 3 mcg SX N = 50 Placebo N = 50 Endpoints SAFETY assessments Vital signs, Physical examination, clinical laboratory tests (hematology, serum chemistry, urinalysis) and adverse events Subjective EFFICACY assessments Patient and Clinician Reported Submental Bulging Scale at baseline and at Days 29, 57, and 78 (PR-SBS and CR-SBS) Patient-Reported Submental Fat Psychological Impact Scale at baseline and at Days 57 and 78 (PR-SFPIS) Patient Global Impression of Change at Days 57 and 78 (PGIC) Objective EFFICACY assessments Submental Fat Thickness as determined by calipers at baseline and at Days 29, 57, and 78 8 Weekly treatments LIPO-202 DOSE = 0.3 mcg SX N = 50
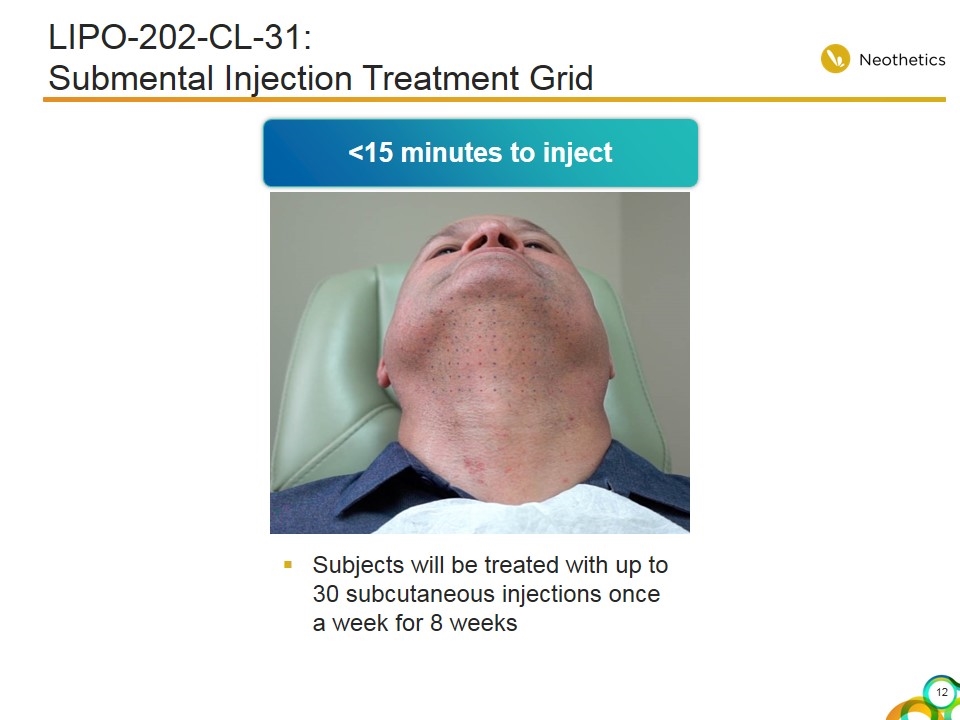
LIPO-202-CL-31: Submental Injection Treatment Grid Subjects will be treated with up to 30 subcutaneous injections once a week for 8 weeks <15 minutes to inject
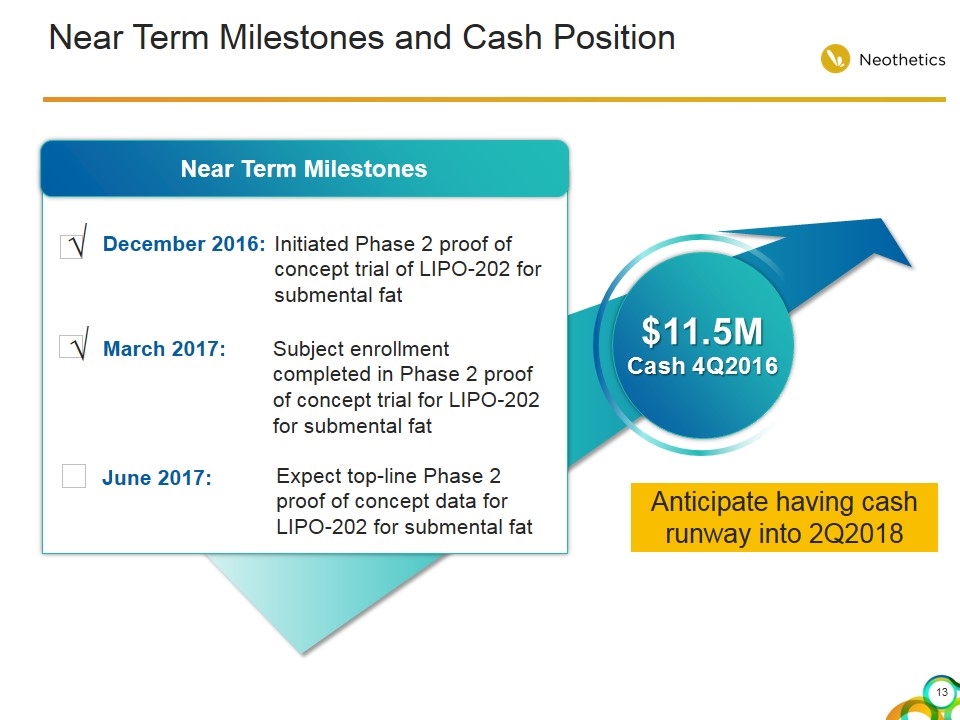
Near Term Milestones and Cash Position √ Near Term Milestones December 2016: $11.5M Cash 4Q2016 Anticipate having cash runway into 2Q2018 √ Initiated Phase 2 proof of concept trial of LIPO-202 for submental fat Subject enrollment completed in Phase 2 proof of concept trial for LIPO-202 for submental fat March 2017: Expect top-line Phase 2 proof of concept data for LIPO-202 for submental fat June 2017: √
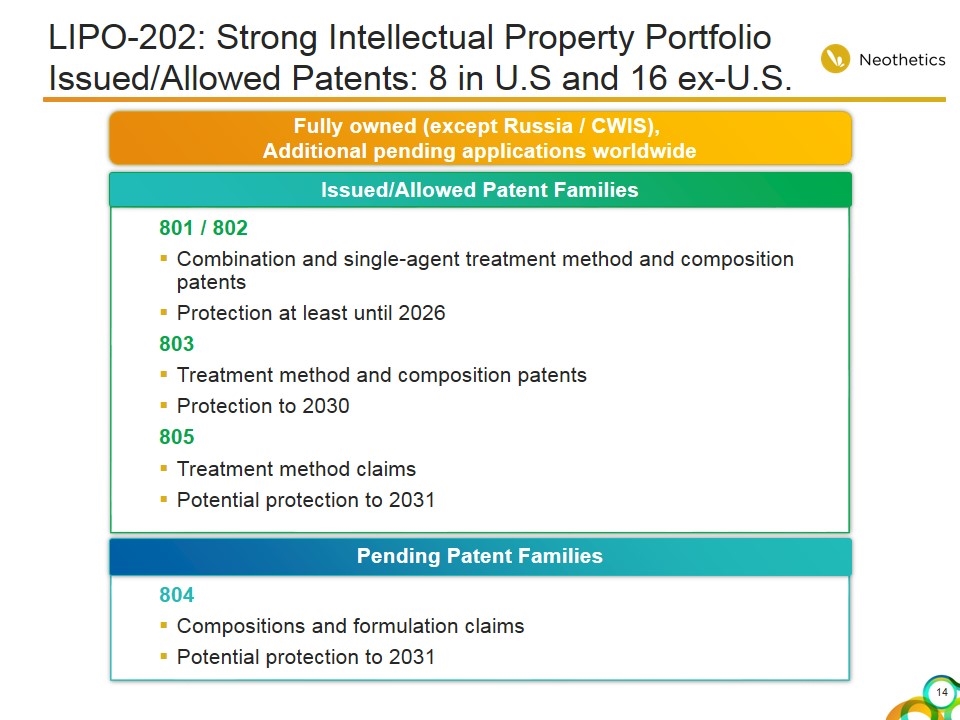
LIPO-202: Strong Intellectual Property Portfolio Issued/Allowed Patents: 8 in U.S and 16 ex-U.S. 801 / 802 Combination and single-agent treatment method and composition patents Protection at least until 2026 803 Treatment method and composition patents Protection to 2030 805 Treatment method claims Potential protection to 2031 Issued/Allowed Patent Families 804 Compositions and formulation claims Potential protection to 2031 Pending Patent Families Fully owned (except Russia / CWIS), Additional pending applications worldwide
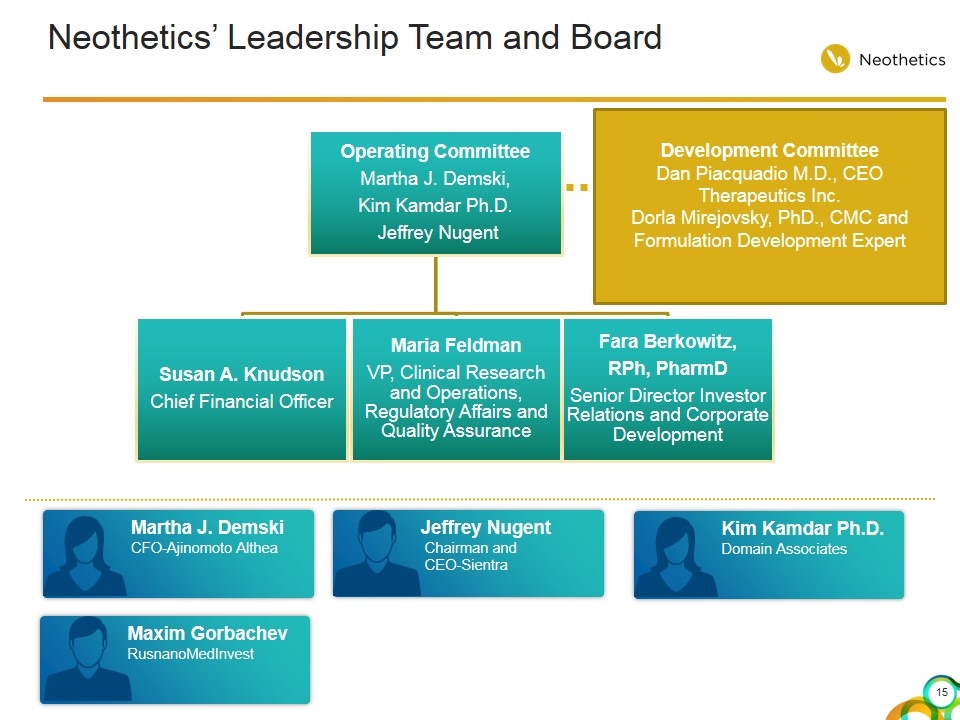
Neothetics’ Leadership Team and Board Martha J. Demski CFO-Ajinomoto Althea Maxim Gorbachev RusnanoMedInvest Jeffrey Nugent Chairman and CEO-Sientra Kim Kamdar Ph.D. Domain Associates Development Committee Dan Piacquadio M.D., CEO Therapeutics Inc. Dorla Mirejovsky, PhD., CMC and Formulation Development Expert Operating Committee Martha J. Demski , Kim Kamdar Ph.D. Jeffrey Nugent Fara Berkowitz, RPh, PharmD Senior Director Investor Relations and Corporate Development Susan A. Knudson Chief Financial Officer Maria Feldman VP, Clinical Research and Operations, Regulatory Affairs and Quality Assurance

Neothetics: Focused on High Value Medical Aesthetic Products Well understood molecule with a de-risked safety profile Non-ablative, well tolerated, no-downtime Established indication, regulatory pathway, and endpoints (for submental fat reduction) ~30M eligible patients in the U.S. with low single digit penetration LIPO-202: Well Positioned to Be the Leading Next Generation, Non-surgical Product in Large, Cash Pay Market Source: Industry Data

Neothetics Corporate Overview April 2017 Appendix
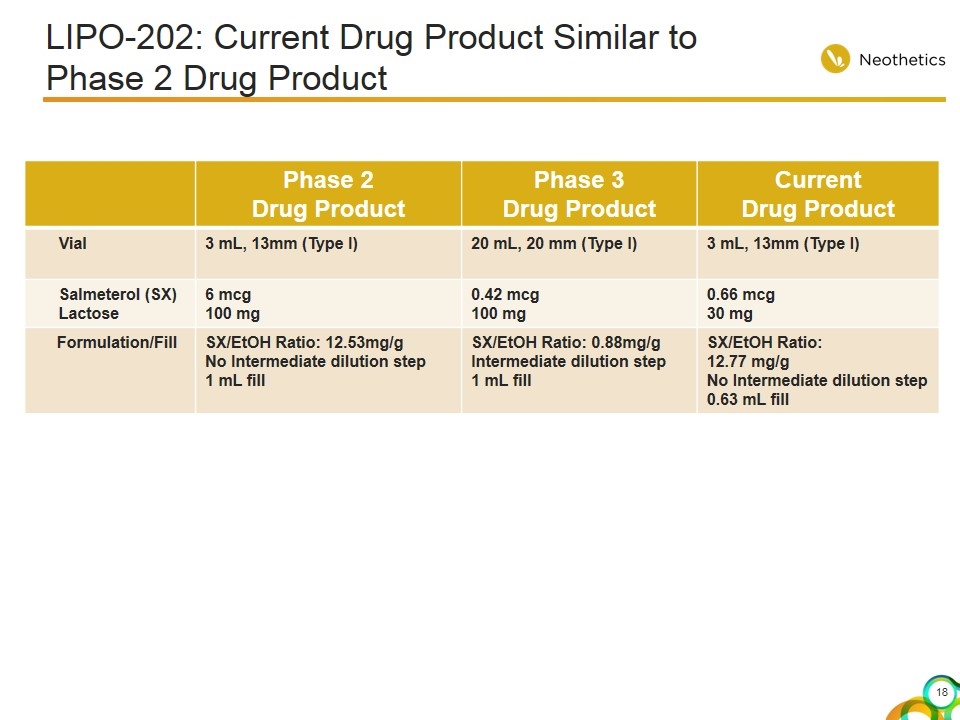
LIPO-202: Current Drug Product Similar to Phase 2 Drug Product Phase 2 Drug Product Phase 3 Drug Product Current Drug Product Vial 3 mL, 13mm (Type I) 20 mL, 20 mm (Type I) 3 mL, 13mm (Type I) Salmeterol (SX) Lactose 6 mcg 100 mg 0.42 mcg 100 mg 0.66 mcg 30 mg Formulation/Fill SX/EtOH Ratio: 12.53mg/g No Intermediate dilution step 1 mL fill SX/EtOH Ratio: 0.88mg/g Intermediate dilution step 1 mL fill SX/EtOH Ratio: 12.77 mg/g No Intermediate dilution step 0.63 mL fill
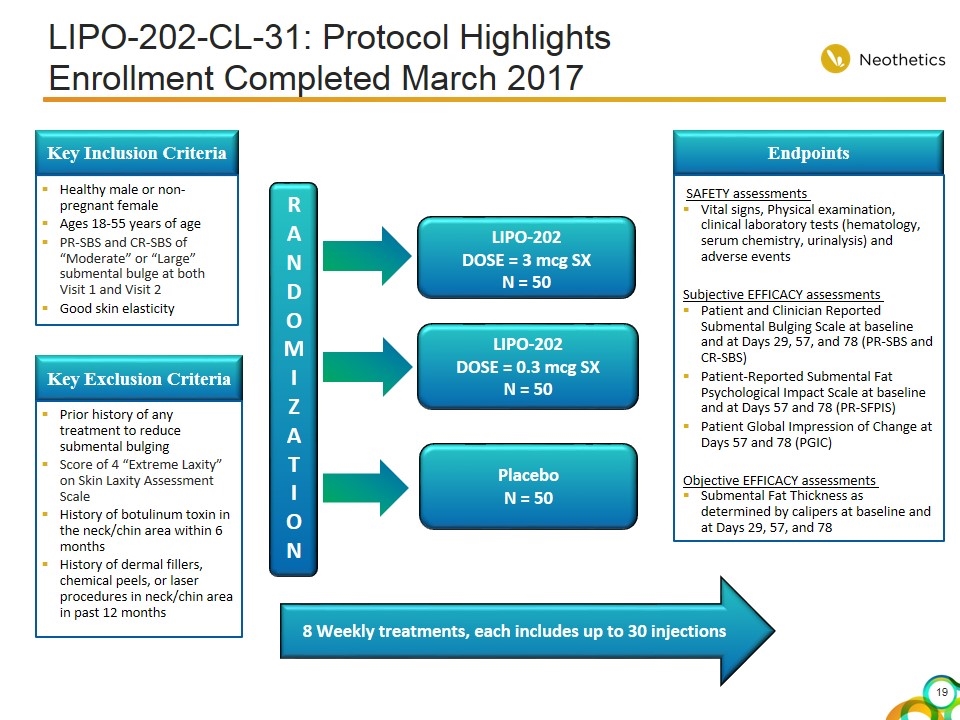
LIPO-202-CL-31: Protocol Highlights Enrollment Completed March 2017 Key Inclusion Criteria Healthy male or non-pregnant female Ages 18-55 years of age PR-SBS and CR-SBS of “Moderate” or “Large” submental bulge at both Visit 1 and Visit 2 Good skin elasticity Key Exclusion Criteria Prior history of any treatment to reduce submental bulging Score of 4 “Extreme Laxity” on Skin Laxity Assessment Scale History of botulinum toxin in the neck/chin area within 6 months History of dermal fillers, chemical peels, or laser procedures in neck/chin area in past 12 months RANDOMI ZAT I ON LIPO-202 DOSE = 3 mcg SX N = 50 Placebo N = 50 Endpoints SAFETY assessments Vital signs, Physical examination, clinical laboratory tests (hematology, serum chemistry, urinalysis) and adverse events Subjective EFFICACY assessments Patient and Clinician Reported Submental Bulging Scale at baseline and at Days 29, 57, and 78 (PR-SBS and CR-SBS) Patient-Reported Submental Fat Psychological Impact Scale at baseline and at Days 57 and 78 (PR-SFPIS) Patient Global Impression of Change at Days 57 and 78 (PGIC) Objective EFFICACY assessments Submental Fat Thickness as determined by calipers at baseline and at Days 29, 57, and 78 8 Weekly treatments, each includes up to 30 injections LIPO-202 DOSE = 0.3 mcg SX N = 50

