Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Zynerba Pharmaceuticals, Inc. | a17-9868_28k.htm |
Exhibit 99.1
Q4 2016 Earnings and Operating Results Conference Call March 27, 2017 A clinical-stage specialty pharmaceutical company dedicated to the development and commercialization of innovative transdermal synthetic cannabinoid treatments for patients with high unmet medical needs
Disclaimer The statements in this presentation may include forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements, among other things relate to the future operations, opportunities or financial performance of Zynerba Pharmaceuticals, Inc. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. These and other risks are described in our filings with the Securities and Exchange Commission, available at www.sec.gov. Any forward-looking statements that the Company makes in this presentation speak only as of the date of this PRESENTATION. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this presentation. 2 © 2017 Zynerba Pharmaceuticals, Inc. All rights reserved. Zynerba is a registered trademark of Zynerba Pharmaceuticals, Inc. All other trademarks and registered trademarks are property of their respective owners.
Agenda For Today’s Call Opening Remarks A. Anido Clinical Updates T. Sebree 2016 Financial Performance J. Fickenscher 2017 Milestones A. Anido Q&A 3

Company Highlights 4 A clinical-stage specialty pharmaceutical company pioneering the development and commercialization of patent-protected synthetic cannabinoid therapeutics for transdermal delivery Global ownership of two proprietary product candidates intended to treat diseases with significant unmet medical need and market potential ZYN002 – CBD Gel: epilepsy, osteoarthritis and fragile X syndrome ZYN001 – THC Pro-Drug Patch: fibromyalgia and peripheral neuropathic pain No royalty obligations owed on either product candidate Focused on valuable unmet medical need markets ~ 1.5 million adult epilepsy patients in the US with focal seizures; ~ 31 million OA patients; ~ 6 million Fibromyalgia patients; ~15 million PNP patients and 71,000 Fragile X Syndrome patients (Orphan Drug Designation)* Experienced team with deep expertise and proven track record of success in CNS and pain and patch and gel transdermal delivery, regulatory approval and commercialization * Data per Decision Resources, Data Monitor and The Fragile X Society
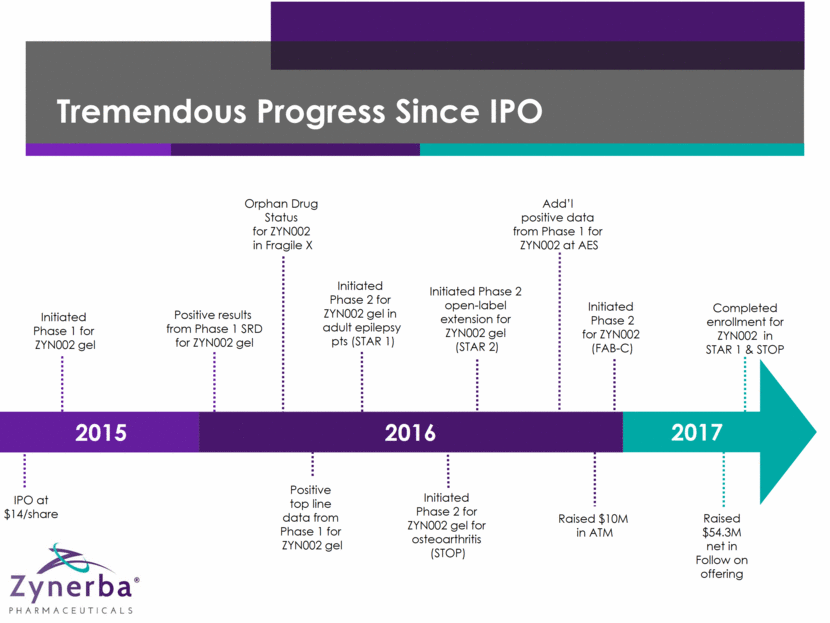
Tremendous Progress Since IPO 5 IPO at $14/share Initiated Phase 1 for ZYN002 gel Positive results from Phase 1 SRD for ZYN002 gel Orphan Drug Status for ZYN002 in Fragile X Positive top line data from Phase 1 for ZYN002 gel Initiated Phase 2 for ZYN002 gel in adult epilepsy pts (STAR 1) Initiated Phase 2 for ZYN002 gel for osteoarthritis (STOP) Initiated Phase 2 open-label extension for ZYN002 gel (STAR 2) Add’l positive data from Phase 1 for ZYN002 at AES Initiated Phase 2 for ZYN002 (FAB-C) Raised $54.3M net in Follow on offering 2015 2016 2017 Completed enrollment for ZYN002 in STAR 1 & STOP Raised $10M in ATM
Transdermal ZYN002 vs. Oral CBD 6 Potential Benefits of Transdermal ZYN002 vs. Oral CBD More consistent, controlled and sustained plasma level Avoids first-pass metabolism in the liver Fewer drug-drug interactions Avoids the GI tract Very low incidence of gastrointestinal events Avoids degradation of CBD to THC; lower incidence of negative psychoactive effects
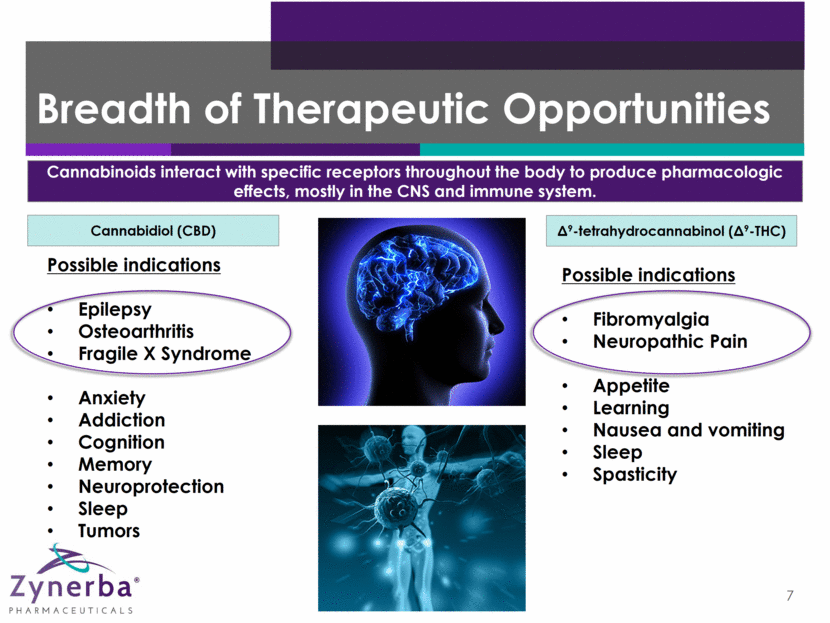
Breadth of Therapeutic Opportunities 7 Cannabinoids interact with specific receptors throughout the body to produce pharmacologic effects, mostly in the CNS and immune system. 9-tetrahydrocannabinol (9-THC) Cannabidiol (CBD) Possible indications Fibromyalgia Neuropathic Pain Appetite Learning Nausea and vomiting Sleep Spasticity Possible indications Epilepsy Osteoarthritis Fragile X Syndrome Anxiety Addiction Cognition Memory Neuroprotection Sleep Tumors
Focusing on Three Indications for ZYN002 8 Indication Rationale Epilepsy Highly predictive efficacy in animal models & robust success of oral CBD in third party trials suggests de-risked clinical program Osteoarthritis Preclinical proof of concept Significant market opportunity Fragile X Compelling case studies Mouse model demonstrating CBD metabolic inhibition of anandamide and 2-AG Orphan indication
ZYN002 – An Elegant Gel Formulation 9 Hydroalcoholic gel with the consistency and appearance of popular hand sanitizers Packaged in convenient single unit of use sachets Easy to apply – absorbs quickly
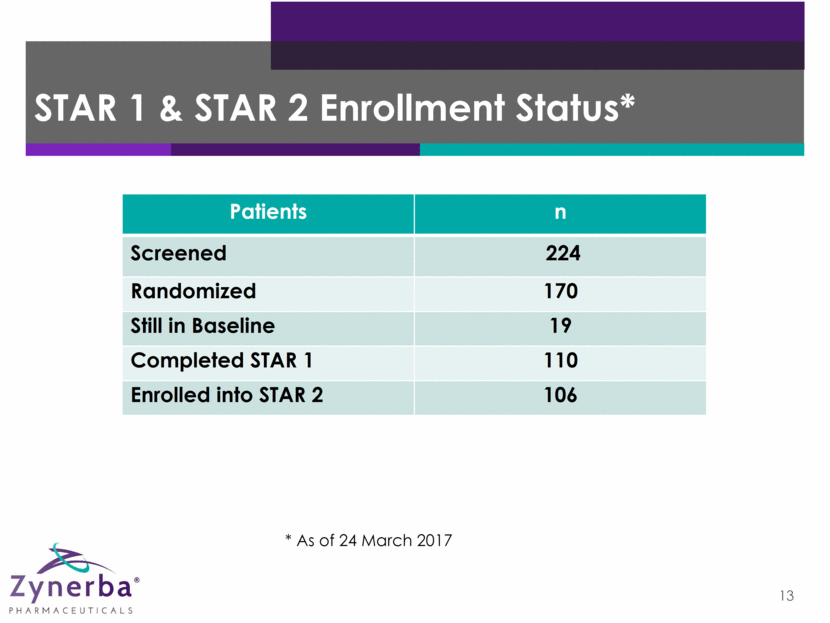
Epilepsy Phase 2 Clinical Trial 10 ZYN002 High-Dose CBD 195 mg* ZYN002 Low-Dose CBD 97.5 mg* Placebo Baseline Assess seizure frequency/type Open-Label Extension Synthetic Transdermal CAnnabidiol for the TReatment of Epilepsy *In 4.2% gel ZYN002 High-Dose CBD 195 mg* ZYN002 Low-Dose CBD 97.5 mg* STAR 2 Trial STAR 1 Trial As needed, dose reduced to: BID for 12 months BID for 12 weeks 8 weeks Randomized (1:1:1), double-blind, placebo-controlled - targeting 180 patients
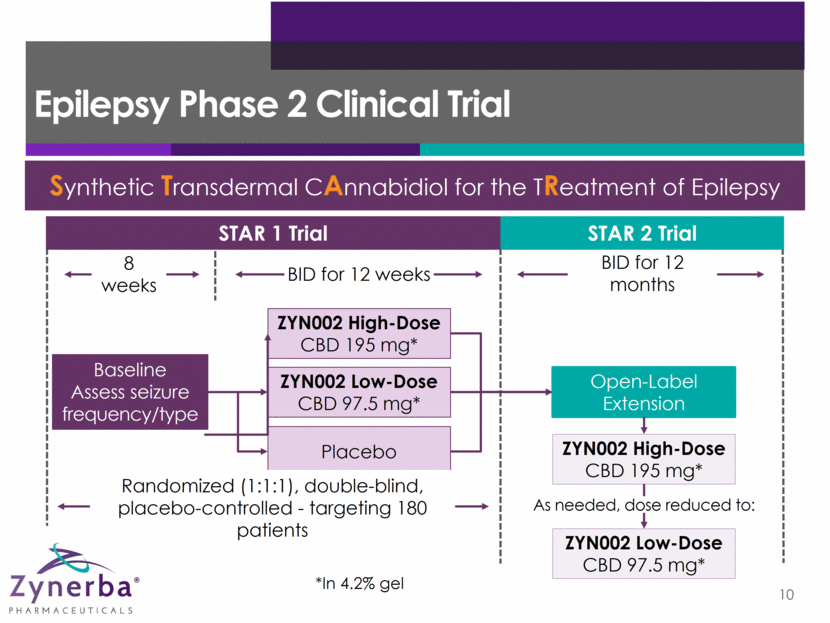
STAR 1 Efficacy Endpoints 11 Primary Endpoint: Median reduction in seizure frequency per 28-day period comparing baseline with the maintenance period via daily seizure diary Secondary Endpoints Proportion of patients with 50% reduction from baseline in seizure frequency Percent change from baseline in seizure frequency Change from baseline in seizure frequency Seizure-free days 100% seizure-free
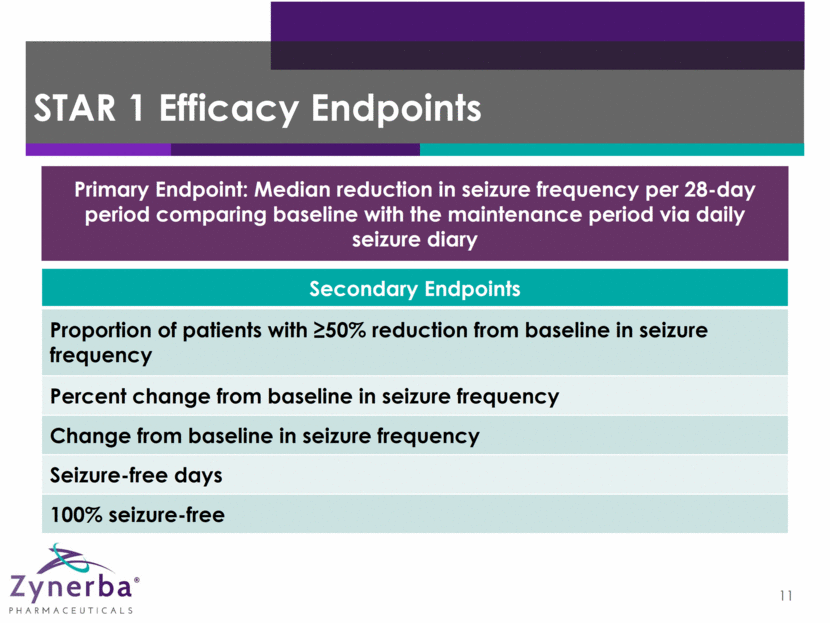
12 STAR 1 Baseline Information* Anti-Epileptic Drug Patients Anti-Epileptic Drug Patients Levetiracetam (Keppra) 72 Clonazepam 15 Carabamazepine 67 Zonisamide (Zonegran) 20 Lamotrigine 53 Oxcarbazepine 13 Perampanel (Fycompa) 16 Valproate 34 Lacosamide (Vimpat) 44 Phenytoin 12 Topiramate 24 Phenobarbitone 3 AED Use Average=2.5 AEDs Median=3.0 AEDs Median Seizure Frequency† 10.8 (3-335) †Median Monthly *As of 20 Mar 2017
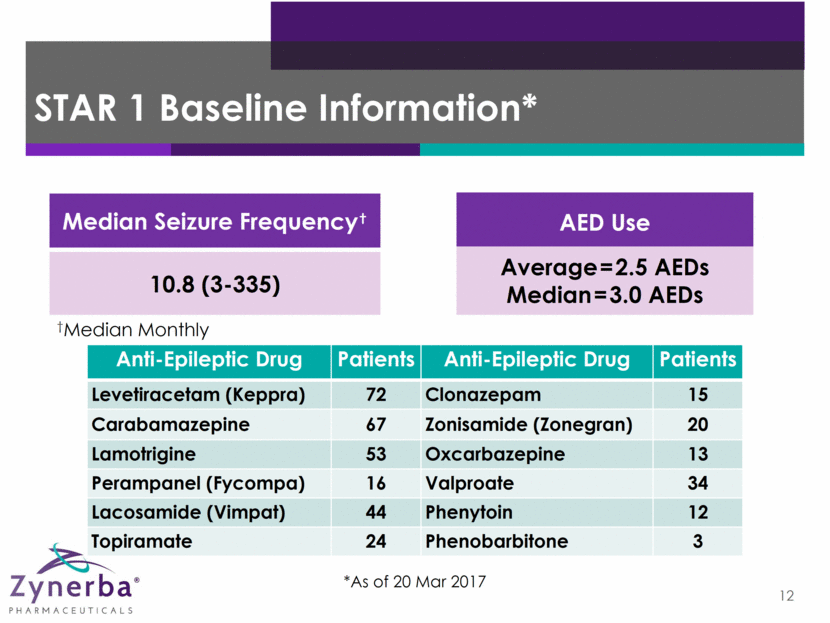
13 STAR 1 & STAR 2 Enrollment Status* Patients n Screened 224 Randomized 170 Still in Baseline 19 Completed STAR 1 110 Enrolled into STAR 2 106 * As of 24 March 2017
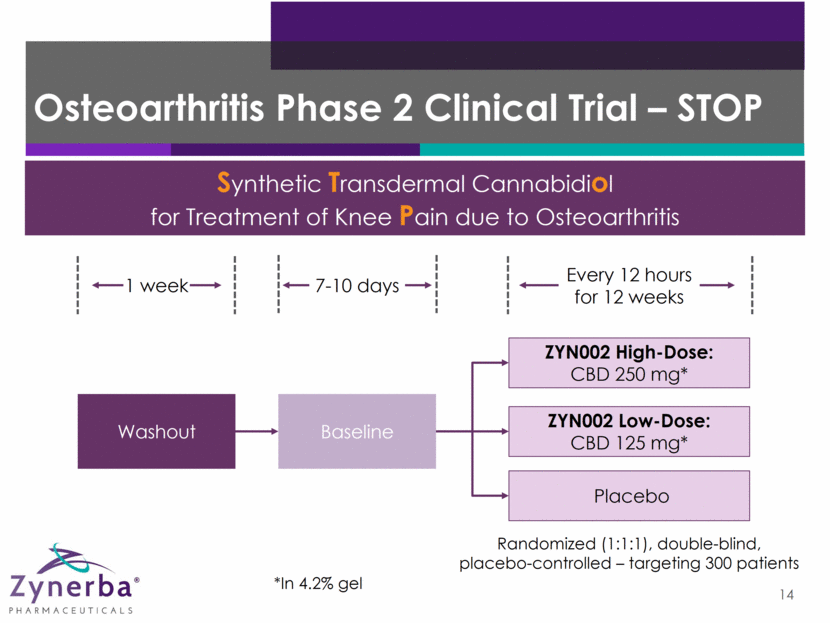
Osteoarthritis Phase 2 Clinical Trial – STOP 14 Synthetic Transdermal Cannabidiol for Treatment of Knee Pain due to Osteoarthritis ZYN002 High-Dose: CBD 250 mg* ZYN002 Low-Dose: CBD 125 mg* Placebo Washout Baseline 1 week Every 12 hours for 12 weeks 7-10 days *In 4.2% gel Randomized (1:1:1), double-blind, placebo-controlled – targeting 300 patients
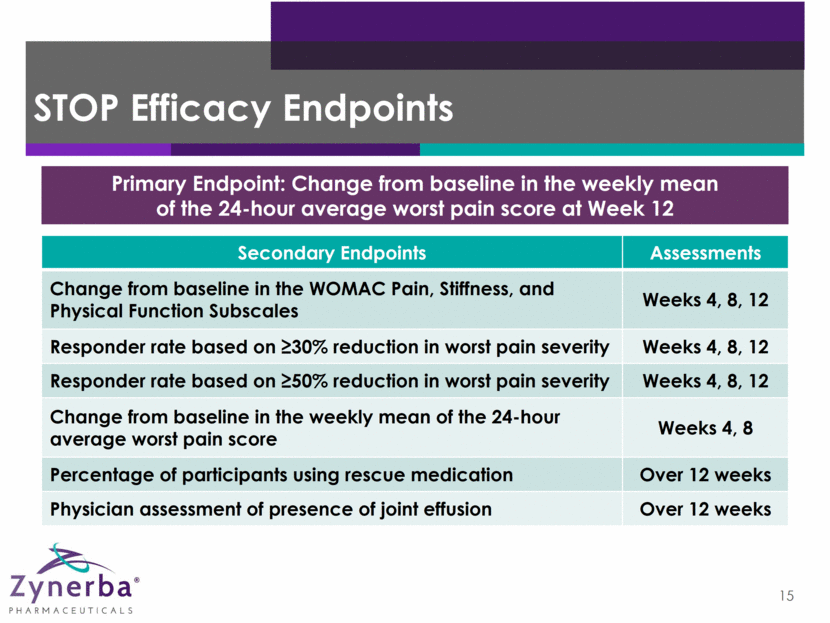
STOP Efficacy Endpoints 15 Primary Endpoint: Change from baseline in the weekly mean of the 24-hour average worst pain score at Week 12 Secondary Endpoints Assessments Change from baseline in the WOMAC Pain, Stiffness, and Physical Function Subscales Weeks 4, 8, 12 Responder rate based on 30% reduction in worst pain severity Weeks 4, 8, 12 Responder rate based on 50% reduction in worst pain severity Weeks 4, 8, 12 Change from baseline in the weekly mean of the 24-hour average worst pain score Weeks 4, 8 Percentage of participants using rescue medication Over 12 weeks Physician assessment of presence of joint effusion Over 12 weeks
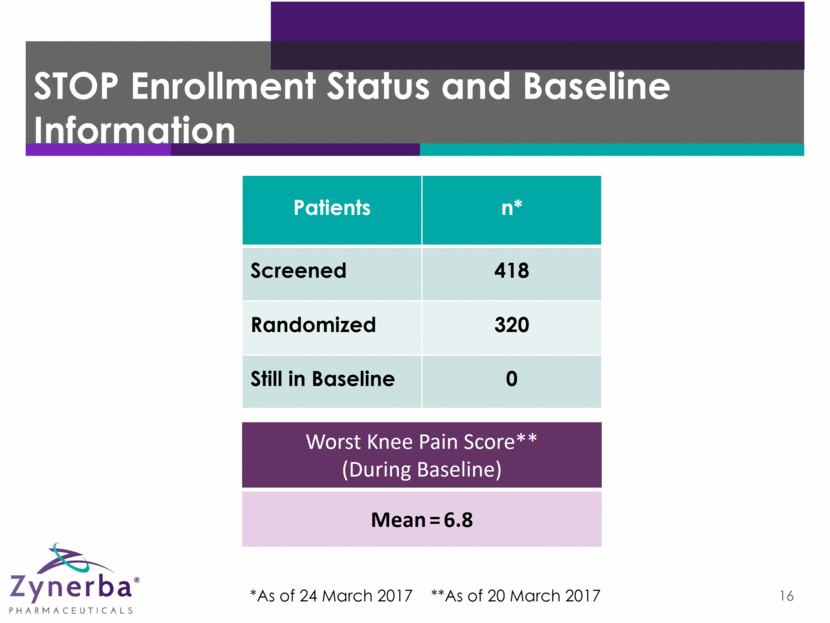
16 STOP Enrollment Status and Baseline Information Worst Knee Pain Score** (During Baseline) Mean=6.8 *As of 24 March 2017 **As of 20 March 2017 Patients n* Screened 418 Randomized 320 Still in Baseline 0
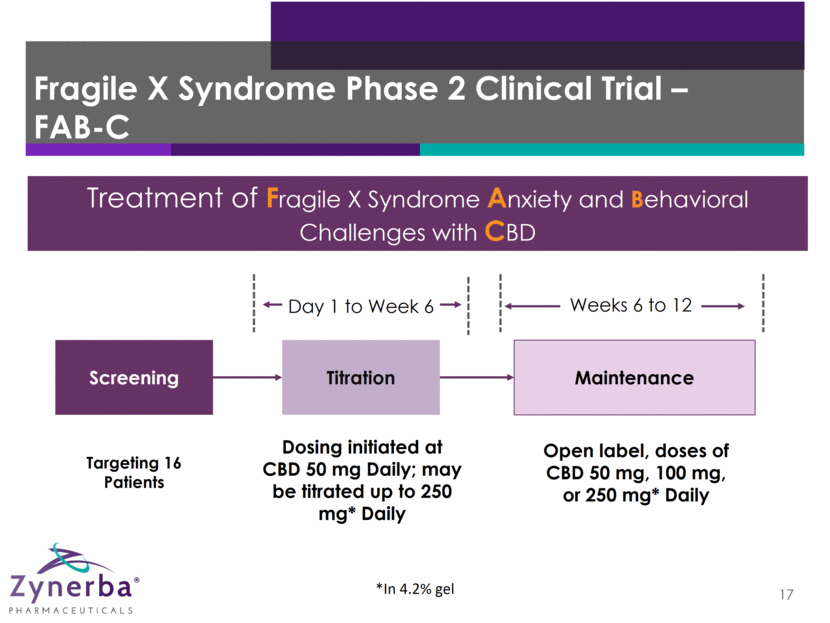
Fragile X Syndrome Phase 2 Clinical Trial – FAB-C 17 Maintenance Dosing initiated at CBD 50 mg Daily; may be titrated up to 250 mg* Daily Screening Titration Weeks 6 to 12 Day 1 to Week 6 Open label, doses of CBD 50 mg, 100 mg, or 250 mg* Daily *In 4.2% gel Treatment of Fragile X Syndrome Anxiety and Behavioral Challenges with CBD Targeting 16 Patients
FAB – C Outcome Measures 18 Changes in Anxiety, Depression and Mood vs. Baseline as Measured by the ADAMS Scale Secondary Outcome Measures Aberrant Behavior Checklist Visual Analog Scale (VAS) to Assess Hyperactivity / Impulsivity Quality of Sleep- Sleep onset, sleep time, nighttime awakenings Primary Outcome Measure
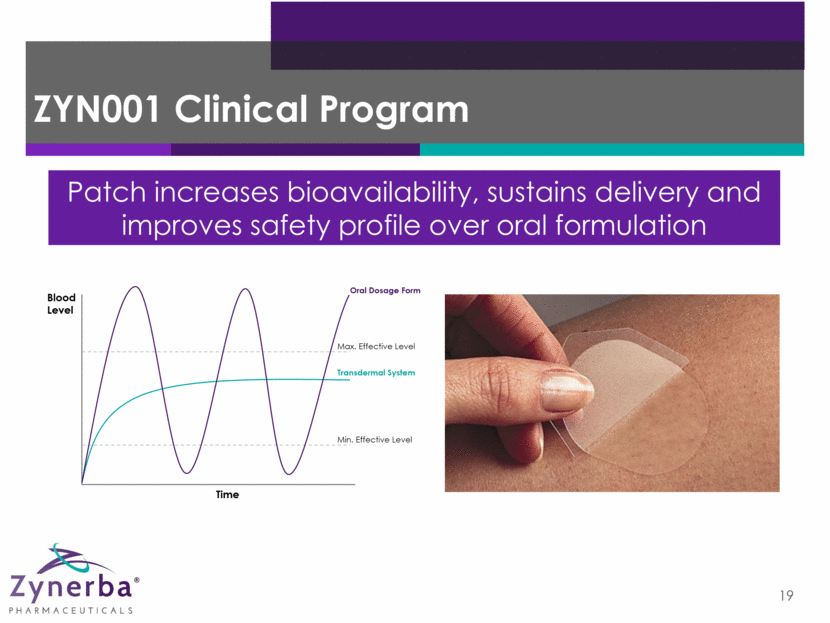
ZYN001 Clinical Program 19 Patch increases bioavailability, sustains delivery and improves safety profile over oral formulation Blood Level Time Max. Effective Level Min. Effective Level Oral Dosage Form Transdermal System
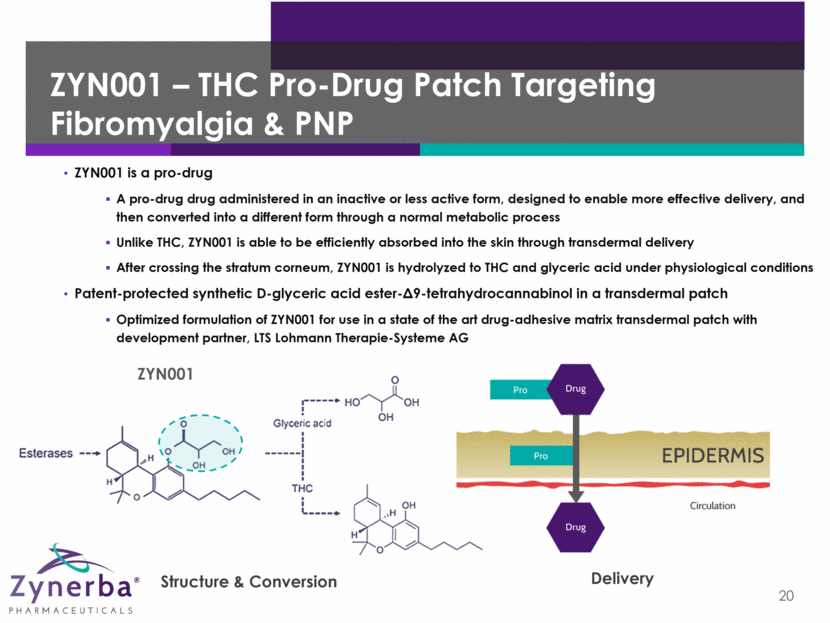
ZYN001 – THC Pro-Drug Patch Targeting Fibromyalgia & PNP 20 ZYN001 is a pro-drug A pro-drug drug administered in an inactive or less active form, designed to enable more effective delivery, and then converted into a different form through a normal metabolic process Unlike THC, ZYN001 is able to be efficiently absorbed into the skin through transdermal delivery After crossing the stratum corneum, ZYN001 is hydrolyzed to THC and glyceric acid under physiological conditions Patent-protected synthetic D-glyceric acid ester-9-tetrahydrocannabinol in a transdermal patch Optimized formulation of ZYN001 for use in a state of the art drug-adhesive matrix transdermal patch with development partner, LTS Lohmann Therapie-Systeme AG Structure & Conversion Delivery ZYN001
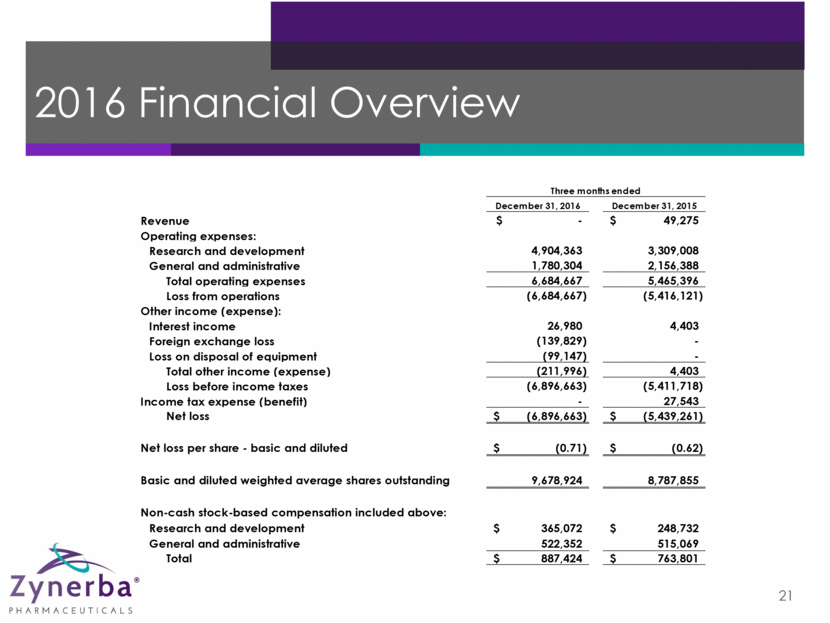
2016 Financial Overview 21
22 Cash & cash equivalents at 12/31/2016 was $31.0 million Raised $54.3 million in net proceeds from the sale and issuance of 3.2 million common shares through secondary offering at $18.00 per share in 1Q17 Cash at 12/31/2016 plus net proceeds from Q1 raise totals $85.3 million We believe that cash is sufficient to develop five Phase 3 ready programs and, assuming feedback from the FDA supports a decision to move forward, initiate at least one Phase 3 program and fund operations and capital requirements into 2019 Total shares outstanding after giving effect to Q1 offering was 13,214,825 shares Strong Balance Sheet Provides Expected Runway into 2019
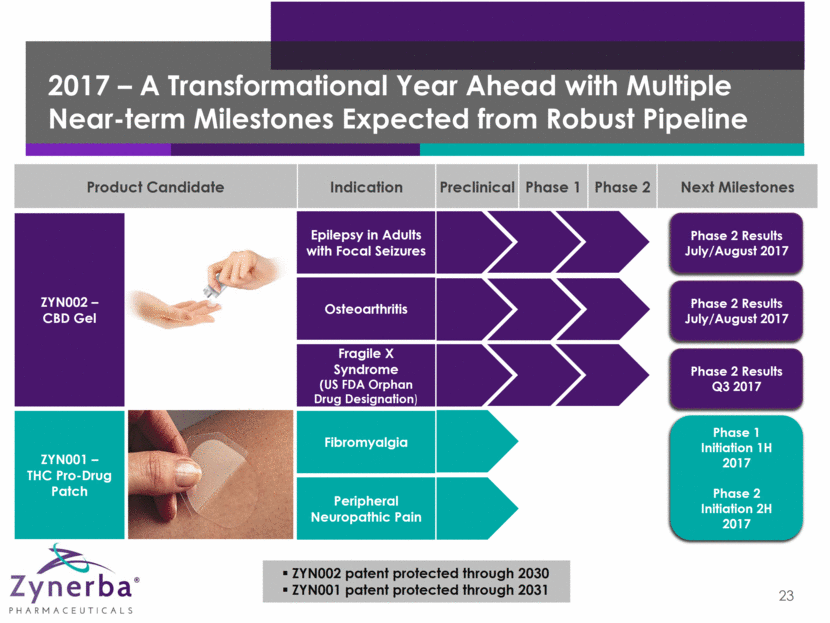
2017 – A Transformational Year Ahead with Multiple Near-term Milestones Expected from Robust Pipeline 23 ZYN002 patent protected through 2030 ZYN001 patent protected through 2031 Product Candidate Indication Preclinical Phase 1 Phase 2 Next Milestones ZYN001 – THC Pro-Drug Patch ZYN002 – CBD Gel Epilepsy in Adults with Focal Seizures Osteoarthritis Fragile X Syndrome (US FDA Orphan Drug Designation) Fibromyalgia Peripheral Neuropathic Pain Phase 2 Results July/August 2017 Phase 2 Results July/August 2017 Phase 2 Results Q3 2017 Phase 1 Initiation 1H 2017 Phase 2 Initiation 2H 2017
Why Zynerba is a Compelling Investment Opportunity in the High-Growth Cannabinoid Space 24 We believe CBD gel for focal seizures is a significantly de-risked lead candidate Reliable animal models show efficacy of CBD in epilepsy Third party studies have shown activity of CBD in reducing focal seizures STAR 1 trial is well powered to show clinical benefit versus placebo Zynerba’s transdermal approach has significant benefits Patent protection Synthetic manufacturing process can be scaled for large volume markets Attractive COGS and no royalty obligations should result in high gross margins Consistent, controlled blood levels of drug and potentially a better safety profile than oral formulations Focused on valuable unmet medical need markets ~ 1.5 million adult epilepsy patients in the US with focal seizures; ~ 31 million OA patients; ~ 5.6 million Fibromyalgia patients; ~14 million PNP patients and Orphan designation for Fragile X Syndrome Significant clinical progress anticipated in the near-term

