Attached files
| file | filename |
|---|---|
| EX-10.15 - EXHIBIT 10.15 - DIRECTOR COMPENSATION POLICY - NxStage Medical, Inc. | nxtm20161231ex1015bodcomp.htm |
| EX-32.2 - EXHIBIT 32.2 - CERTIFICATION - NxStage Medical, Inc. | nxtm20161231ex322.htm |
| EX-32.1 - EXHIBIT 32.1 - CERTIFICATION - NxStage Medical, Inc. | nxtm20161231ex321.htm |
| EX-31.2 - EXHIBIT 31.2 - CERTIFICATION - NxStage Medical, Inc. | nxtm20161231ex312.htm |
| EX-31.1 - EXHIBIT 31.1 - CERTIFICATION - NxStage Medical, Inc. | nxtm20161231ex311.htm |
| EX-23 - EXHIBIT 23 - CONSENT - NxStage Medical, Inc. | nxtm20161231ex23.htm |
| EX-21 - EXHIBIT 21 - SUBSIDIARY LISTING - NxStage Medical, Inc. | nxtm20161231ex21.htm |
| EX-10.7 - EXHIBIT 10.7 - PERFORMANCE SHARE AWARD AGREEMENT - NxStage Medical, Inc. | nxtm20161231ex107psa.htm |
| EX-10.6 - EXHIBIT 10.6 - RESTRICTED STOCK UNIT AGREEMENT - NxStage Medical, Inc. | nxtm20161231ex106rsu.htm |
| EX-10.5 - EXHIBIT 10.5 - STOCK OPTION AGREEMENT - NxStage Medical, Inc. | nxtm20161231ex105option.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
(Mark One)
þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the fiscal year ended December 31, 2016 | ||
OR
¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the transition period from __________ to __________ | ||
Commission file number: 000-51567
NxStage Medical, Inc.
(Exact Name of Registrant as Specified in Its Charter)
Delaware | 04-3454702 | |
(State or Other Jurisdiction of Incorporation or Organization) | (I.R.S. Employer Identification No.) | |
350 Merrimack Street, Lawrence, MA | 01843 | |
(Address of Principal Executive Offices) | (Zip Code) | |
Registrant's Telephone Number, Including Area Code:
(978) 687-4700
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class | Name of Each Exchange on Which Registered | |
Common Stock, $0.001 par value | Nasdaq Global Select Market | |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. þ Yes o No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. o Yes þ No
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. þ Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). þ Yes o No
1
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act:
Large accelerated filer þ | Accelerated filer ¨ | Non-accelerated filer £ | Smaller reporting company £ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). o Yes þ No
The aggregate market value of common stock held by non-affiliates of the registrant was approximately $1.4 billion, as of June 30, 2016, based on the last reported sale price of the registrant's common stock on the NASDAQ Global Select Market on June 30, 2016.
There were 65,216,409 shares of the registrant's common stock outstanding as of the close of business on February 22, 2017.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant's definitive proxy statement for its 2017 Annual Meeting of Stockholders are incorporated by reference in response to Part III of this Annual Report.
2
NXSTAGE MEDICAL, INC.
2016 ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
Page | ||
Mine Safety Disclosures | ||
Item 10. | Directors, Executive Officers and Corporate Governance | |
Item 11. | Executive Compensation | |
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | |
Item 13. | Certain Relationships and Related Transactions, and Director Independence | |
Item 14. | Principal Accounting Fees and Services | |
3
CAUTIONARY NOTICE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report and certain information incorporated by reference herein contain forward-looking statements concerning our business, operations and financial condition, including statements with respect to:
•the growth of our business;
•our vision of enabling better clinical outcomes for patients at a lower cost to the healthcare system;
•the ability of our product pipeline to help us expand existing markets and enter new ones;
•achieving greater operating leverage and improved financial results in the future;
•expectations about achieving overall company profitability in 2017;
•our belief that the significant majority of our near-term revenues will come from sales of the System One in the U.S.;
•financial performance of our NxStage Kidney Care dialysis centers and our continued investments in them;
•estimates of the number of end-stage renal disease (ESRD) patients that could be treated at home with the System One;
•potential value and components of the global market for ESRD and critical care products;
• | our strategic initiatives to grow home hemodialysis adoption, expand globally, enhance our product offerings, expand into high growth adjacencies and enter peritoneal dialysis and their ability to unlock commercial opportunities and drive future growth; |
• | our belief that peritoneal dialysis is ripe for technology innovation and that we are well-positioned to launch into this market; |
• | access to home and more frequent hemodialysis; |
• | the potential for our nocturnal clearance and other initiatives to expand the home hemodialysis market and improve patient care; |
• | our plans for, and expected design and functionality of, our next-generation hemodialysis system, next-generation critical care system and peritoneal dialysis system; |
•the development and commercialization of new products and improvements to existing products;
•sales to our key customers, including DaVita HealthCare Partners and Fresenius Medical Care;
•the adequacy of our funding;
• | expectations with respect to future demand for our products and revenue growth, with components of such revenue growth including sales of disposable products; |
• | future financial results for our System One, In-Center and Services segments and total company; |
• | expectation of sustaining gross profit as a percentage of revenue in our System One segment above 50% and the underlying elements of such objective; |
• | future selling and marketing, research and development, distribution, and general and administrative expenses and the drivers for such expenses; |
• | our manufacturing operations and supply chain; |
•the scope and impact of our research and development efforts;
•availability of suitable facilities;
•expectations with respect to our working capital levels and requirements;
•availability of credit under our revolving credit facility;
•global economic conditions;
•expectations with respect to achieving positive operating margins and maintaining positive cash flows;
•volatility of our stock price;
•expectation to retain earnings and not issue dividends;
•legal proceedings;
•expectations with respect to product reliability;
•impact of the adoption of new accounting standards;
•future realization of our deferred tax assets;
• | anticipated benefits of manufacturing dialyzers for sale to Asahi Kasei Kuraray Medical Co. (Asahi) and future sales to Asahi; |
•our ability to withstand supply chain disruptions;
•the scope and adequacy of patent protection with respect to our products;
4
• | the availability of, and changes in, reimbursement for home and more frequent hemodialysis, including home nocturnal hemodialysis; and |
•the financial, commercial and operational impact of any of the above.
All statements other than statements of historical facts included in this Annual Report regarding our strategies, prospects, financial condition, costs, plans and objectives are forward-looking statements. When used in this Annual Report, the words “expect”, “anticipate”, “intend”, “plan”, “believe”, “seek”, “estimate”, “potential”, “continue”, “predict”, “may”, "will" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Because these forward-looking statements involve risks and uncertainties, actual results could differ materially from those expressed or implied by these forward-looking statements.
Readers should carefully review the Risk Factors and Management's Discussion and Analysis of Financial Condition and Results of Operations set forth in this Annual Report, as these sections describe important factors that could cause actual results to differ materially from those indicated by our forward-looking statements. We undertake no obligation to revise or update publicly any forward-looking statement.
NOTE REGARDING TRADEMARKS
NxStage®, Streamline®, ButtonHole®, MasterGuard®, Medisystems®, Nx2me Connected Health®, Nx2me®, LockSite® and OneSite® are registered trademarks of NxStage Medical, Inc. PureFlow SLTM and System OneTM are trademarks of NxStage Medical, Inc. iPad® is a registered trademark of Apple Inc.
5
PART I
For convenience, in this Annual Report “NxStage,” “we,” “us,” and “the Company” refer to NxStage Medical, Inc. and our consolidated subsidiaries, taken as a whole.
Item 1. | Business |
Our Company
NxStage Medical, Inc. is a medical technology company that develops, manufactures and markets innovative products and services for patients suffering from chronic or acute kidney failure. Since our initial public offering in 2005, we have built a strong business that we believe serves as a solid foundation for future growth. As a leader in home hemodialysis, we remain committed to not only growing this and our other existing areas, but also expanding to new markets where we believe our current and future technology has the ability to deliver value for both patients and our customers.
Our vision is to improve the standard of kidney care through technology leadership, therapy innovation and the efficient use of healthcare resources. By creating technology that is designed to have broad capability, be simple to use in a variety of settings, and incorporate greater automation with reduced equipment and supply costs, we believe we can ultimately enable better clinical outcomes for patients at a lower overall cost to the healthcare system. We work with our customers, patients, physicians, industry partners and government leaders to advance this vision.
Our primary product, the System One, was designed to satisfy an unmet clinical need for a system capable of delivering the therapeutic flexibility and clinical benefits of traditional dialysis machines in a smaller, portable, easy-to-use form that can be used by healthcare professionals and trained lay users alike in a variety of settings, including patient homes, as well as more traditional care settings such as hospitals and dialysis centers. Given its design, the System One is particularly well-suited for home hemodialysis and a range of dialysis therapies that are more practical to deliver in the home setting, including more frequent hemodialysis and nocturnal hemodialysis. Clinical literature suggests such therapies provide patients better clinical outcomes and improved quality of life.
We also operate a small number of NxStage Kidney Care dialysis centers, independently and in some instances as joint ventures, that treat end-stage renal disease patients directly. These centers provide us with valuable experience to better meet and anticipate the needs of both our customers and patients, while optimizing our product technology.
Our research and development efforts are important to our future success. Our product development organization is working to develop innovative technical approaches that address the limitations of current dialysis systems and disposable products. We are also working on enhancements to our product designs to improve ease of use, functionality, reliability and safety and to reduce product cost. We believe that our product pipeline will help us to both expand existing markets and enter new ones.
We report our operating results through three segments: System One, In-Center and Services. We sell our products in and provide our services in three markets: home, critical care and in-center. Our other business activities excluded from segment operating performance measures relate primarily to the manufacturing of dialyzers for sale to Asahi Kasei Kuraray Medical Co. (Asahi). Together with certain research and development and general and administrative expenses that are excluded from our business segment operating results, these business activities are reported in the Other category. The operating results of NxStage Kidney Care are included in our Services segment. For convenience, we use the term “products business” to refer collectively to our System One segment, In-Center segment, and Other category.
We are headquartered in Lawrence, Massachusetts, with manufacturing facilities in Mexico, Germany and Italy. Through our international network of affiliates and distribution partners, patients in over 21 countries have been treated with our products.
Our Financial Performance
The table below provides a three year history of revenues and income (loss) from operations summarized for the products business (which includes the results of our System One segment, In-Center segment and Other category), Services segment and in total. For detail below this summary level, please see further segment discussion under Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations and Item 8, Financial Statements and Supplementary Data.
6
(in thousands) | 2016 | 2015 | 2014 | ||||||||
Products Business (System One Segment, In-Center Segment & Other) | |||||||||||
Revenues | $ | 359,127 | $ | 332,845 | $ | 300,598 | |||||
Income (Loss) from operations | $ | 22,695 | $ | 9,197 | $ | (7,261 | ) | ||||
Services Segment | |||||||||||
Revenues | $ | 14,781 | $ | 6,412 | $ | 1,749 | |||||
Loss from operations | $ | (26,233 | ) | $ | (23,826 | ) | $ | (14,926 | ) | ||
Eliminations | |||||||||||
Elimination of intersegment revenues | $ | (7,530 | ) | $ | (3,134 | ) | $ | (846 | ) | ||
Elimination of intersegment gross profit | $ | (747 | ) | $ | — | $ | — | ||||
Total Company | |||||||||||
Revenues | $ | 366,378 | $ | 336,123 | $ | 301,501 | |||||
Loss from operations | $ | (4,285 | ) | $ | (14,629 | ) | $ | (22,187 | ) | ||
Since inception, we have focused on building a long-term sustainable business model based on innovative technologies and offerings. Although we have driven significant revenue growth, and have a robust product portfolio and product development pipeline, we have historically operated at a net loss. In recent years, we have started to achieve greater operating leverage and financial improvements, while still maintaining our focus on product development and topline growth. During 2016, we continued to generate operating income in our products business, and we expect to continue our focus on driving improvements in 2017 and beyond within this business, while we continue to invest in our existing NxStage Kidney Care dialysis centers. We expect to achieve positive net income for the full 2017 fiscal year as our products business continues to grow.
Our Market
Kidney Failure: Chronic and Acute
Chronic kidney disease is typically characterized by the progressive loss of kidney function due to damage caused by diabetes, high blood pressure and other causes. The final stage of chronic kidney disease is called end-stage renal disease (ESRD), which is an irreversible, life-threatening loss of kidney function that is treated predominantly with dialysis. Dialysis is a kidney replacement therapy that removes toxins and excess fluids from the bloodstream. Unless the patient receives a kidney transplant, dialysis is required for the remainder of the patient’s life. The primary types of dialysis are hemodialysis and peritoneal dialysis. Hemodialysis diverts the patient’s blood to an external dialyzer, where a filter and cleansing fluid (“dialysate”) remove toxins and excess fluids, and then returns the cleansed blood to the patient. Hemodialysis is traditionally performed by healthcare professionals in a dialysis clinic on a fixed schedule three times per week, referred to as “in-center” treatment, but can be performed by the patient, with the availability of a care partner, in the home three to seven times per week. Peritoneal dialysis is a home therapy in which toxins are removed through the peritoneum, a part of the patient’s abdomen, through multiple fluid exchanges each day.
Acute kidney failure happens suddenly, as a result of illness, injury or other conditions. Acute kidney failure is typically treated with renal replacement therapies, including hemodialysis, in a hospital or similar critical care setting.
Approximately 470,000 ESRD patients in the U.S. and 2.6 million ESRD patients worldwide rely on life-sustaining dialysis treatment.
7
Dialysis Modality Prevalence (U.S.)
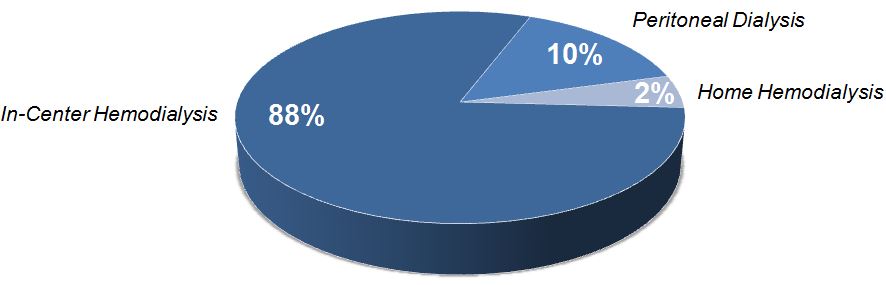
Although in-center hemodialysis is the most common ESRD therapy in the U.S., surveys of healthcare professionals suggest that a larger proportion of patients could take responsibility for their own care. In fact, more than 90% of surveyed U.S. nephrologists have said they would choose a home dialysis therapy for themselves if informed they needed renal replacement therapy, with home hemodialysis being the preferred option. With our current technology, we believe that approximately 10-15% of ESRD patients in the U.S. would be appropriate candidates for home hemodialysis with the System One.
Studies have consistently shown that home hemodialysis may be a viable alternative for ESRD patients to experience enhanced health, control and freedom. Home hemodialysis offers patients numerous benefits when compared with traditional in-center hemodialysis, including:
- improved survival;
- | independence and the ability to better understand and take control of one’s care; |
- | freedom from specific time constraints with greater ability to work and participate in “normal” life activities; |
- | greater freedom to travel; |
- | convenience of not driving to and from the dialysis center three times a week; and |
- | privacy and comfort of being at home. |
In addition, home hemodialysis presents the most practical setting for performing therapy at the frequency and duration that best suits a patient’s clinical needs. Traditional in-center hemodialysis treatment schedules are constrained by staffing and time slot availability, which presents practical and economic limitations on the ability to explore or implement innovative therapy delivery models that are more tailored to the unique clinical and lifestyle needs of patients, as well as responsive to the growing body of clinical literature reporting on the benefits of different therapy delivery models. Although the significant majority of ESRD patients are cared for in-center under a delivery model that provides for three treatments a week (Monday, Wednesday and Friday, or Tuesday, Thursday and Saturday), typically at 3 to 4 hours per session, there is mounting clinical evidence demonstrating the quality of life and clinical benefits of alternative therapy delivery schedules, including additional dialysis sessions (from four to seven sessions per week) as well as longer, nocturnal therapy sessions. More frequent therapy, in particular, has been shown to lead to better clinical outcomes such as:
- improved survival;
- | reduced risk of cardiovascular morbidity; |
- | improved regulation of blood pressure and phosphorus and reduced need for related medications; |
- | improved physical and mental quality of life; and |
- | better therapy tolerance. |
For more information about the clinical benefits of more frequent hemodialysis, please review the section below entitled “Clinical Evidence.”
Products Market
8
Based on the prevalence of kidney failure and related healthcare data, we estimate the potential value of the global market for ESRD and critical care products to be allocated among the following constituents:
Market Opportunity: Global ESRD & Critical Care Products
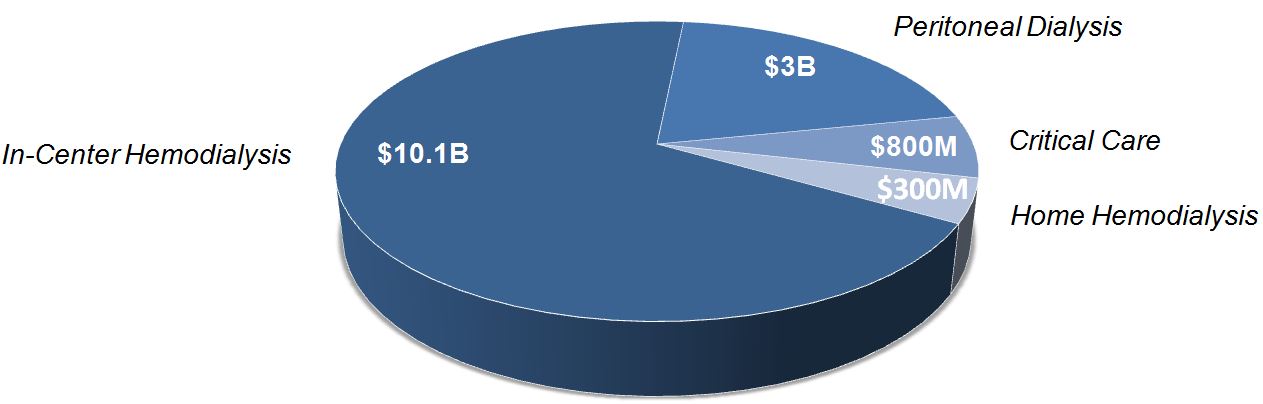
While we believe the significant majority of our near-term revenues will come from sales of the System One in the U.S., we are working on a number of strategic initiatives that we believe will unlock a greater market opportunity for us over the next few years.
Strategic Initiatives
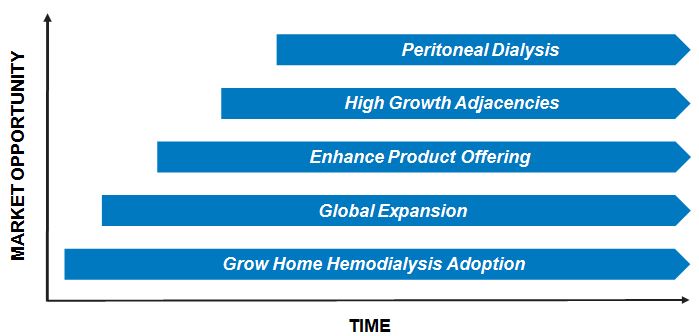
Grow Adoption of Home Hemodialysis with the System One
To increase patient awareness and demand for home and more frequent hemodialysis, we conduct and sponsor local and national patient outreach and awareness campaigns, participate in preeminent nephrology conferences, organize educational programs for patients and clinicians in collaboration with local service providers, collaborate with local and national kidney patient organizations, utilize direct mail and Internet campaigns to provide product literature and clinically relevant information, employ patient advocates, advertise in trade publications and broader media outlets and conduct numerous other educational and promotional activities. In addition, our NxStage Kidney Care dialysis centers are working to expand access to home therapies and are helping us to devise best practices for successful home dialysis programs.
We also advocate for increased access to the life changing benefits of home hemodialysis and more frequent hemodialysis for medically suitable patients. Even though healthcare professionals would choose home hemodialysis for themselves more than any other modality, many of their patients do not have adequate access to this option. According to data from the Centers for Medicare & Medicaid Services (CMS), only 18% of U.S. dialysis clinics actively offer home hemodialysis to their patients, and only 27% are certified to offer the therapy. Although access to home and more frequent hemodialysis continues to grow,
9
we believe that current Medicare reimbursement policies lead to adoption rates lower than rates commensurate with the percentage of patients experts believe can perform and medically benefit from this therapy. We believe further improving Medicare reimbursement for home hemodialysis training, as well as more predictable Medicare reimbursement for more frequent dialysis with less administrative burden and payment for medical oversight equal to that provided for overseeing in-center patients would allow U.S. adoption of home hemodialysis at rates more consistent with what is deemed to be appropriate by the medical community. We continue to engage in regular dialogue with CMS, Medicare contractors, policymakers and industry experts on such issues to encourage broader adoption of home and more frequent hemodialysis.
Drive Global Expansion
Historically, predominantly all of our revenues have come from sales of our products in the U.S. However, the U.S. represents a small portion of the global market for ESRD products. Accordingly, the international market represents a significant growth opportunity.
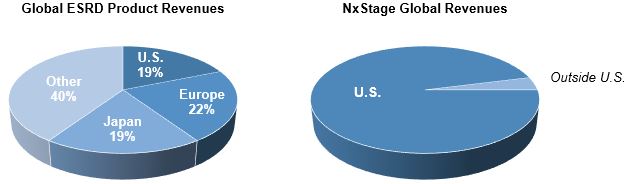
We commenced international sales in 2009 and have sold the System One in 21 countries. We seek to increase our product revenues by expanding our international presence within countries where we have an established footprint and entering new regions through third-party distributors who have an established presence in such regions. Furthermore, our product pipeline is expected to introduce features and enhancements that we believe will increase the appeal of our products to international customers.
Enhance the Ease of Use and Clinical Flexibility of our Product Offering
We promote our products’ ease of use and clinical flexibility. Accordingly, we continue to add new features and tools associated with our next-generation hemodialysis system to enhance usability and reduce the burden of therapy, and we continue work to expand the System One's applications.
In 2014, the System One became the first and only hemodialysis machine cleared in the U.S. to perform hemodialysis overnight while the patient and care partner sleep. The System One also is CE marked for home nocturnal hemodialysis in the EU. Home nocturnal hemodialysis is an important patient option associated with a number of lifestyle and clinical advantages. By doing therapy while sleeping, patients free up their day to pursue other activities thereby reducing the overall burden of therapy. A longer, overnight therapy also allows for greatly expanded flexibility in dialysis dose and schedule, better enabling physicians to match the dialysis prescription to individual patient needs. We believe that this nocturnal clearance will open home hemodialysis therapy to new segments of patients, and improve care for ESRD patients by expanding therapeutic options and flexibility.
We have also seen strong interest in Nx2me Connected Health, our telehealth platform for the collection and delivery of treatment and medical information for patients using the System One. By enhancing the ease of information collection and availability to the patient and care team, Nx2me may foster improved home patient retention and communication.
In addition, our research and development organization continues to work on enhancements to our product designs and is currently developing, among other things, our next-generation critical care system. Additional information about our innovative product pipeline is provided below in the sections entitled “Enter Peritoneal Dialysis,” “Next-Generation Hemodialysis System,” "Next-Generation Critical Care System" and “Product Development.”
In addition, our NxStage Kidney Care dialysis centers are gaining valuable clinical insights that will help us to optimize our product technology.
We believe that technological improvements which make the administration of home hemodialysis less burdensome can help to expand the commercial potential of home hemodialysis.
Expand into High Growth Adjacencies
10
We focus on expanding into adjacent markets, including skilled nursing facilities where we believe our product technology offers a differentiated and compelling value proposition.
Nursing home residents represent approximately 8% of all ESRD patients. Nursing home patients are generally transported off-site to dialysis clinics, which may introduce several risks to their health and compromise their care. The System One can be used at skilled nursing facilities to offer residents on-site hemodialysis, which may eliminate patient discomfort from traveling to an outside facility and the accompanying disruptions in rehabilitation, medication schedules and social activities. Offering on-site hemodialysis also eliminates the high costs of transporting patients to dialysis clinics, which is not always covered by reimbursement.
Enter Peritoneal Dialysis
The commercial opportunity with peritoneal dialysis is significantly larger than that for home hemodialysis and is well established as it is generally favored by healthcare programs and payors. We believe, however, that peritoneal dialysis is ripe for technology innovation and that we are well-positioned to launch into this market based on our capabilities and decade long history of technological innovation and leadership within home hemodialysis. We are developing our peritoneal dialysis system to offer a differentiated therapy solution with on-site dialysate generation from concentrate without the need for premixed bagged fluids, with pre-connected, ready to use disposables that require fewer touch contamination points, and automation to improve setup, training and use.
Future Peritoneal Dialysis System


Our Products and Services
We have over a decade long history of product innovation. Our products and services currently target the home, critical care and in-center markets. The following timeline highlights some of our key product introductions since 2003:
11
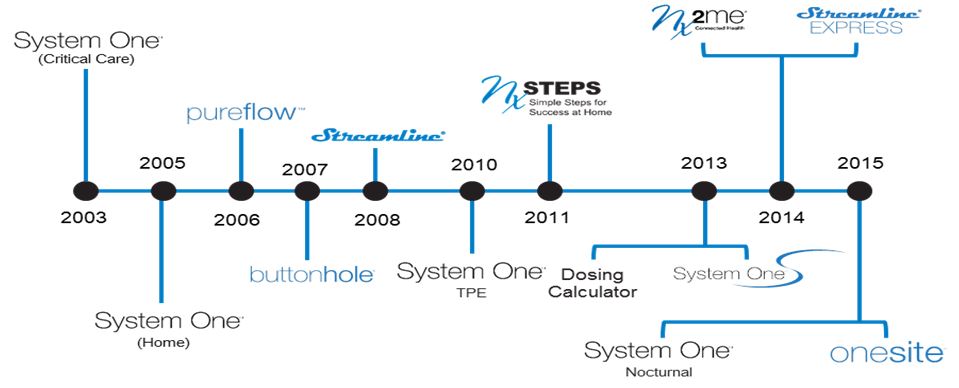
The following section describes our product offerings.
Home Dialysis
Our home product offerings currently target the home hemodialysis market. The NxStage System One is a small, portable, easy-to-use hemodialysis system that is used to perform treatments during the day or at night, while sleeping. The System One is the only portable hemodialysis system that is cleared by the U.S. Food and Drug Administration (FDA) for in-center and hospital use (2002), for home hemodialysis (2005) and home nocturnal hemodialysis (2014). Its simplicity and compact size are intended to allow for easy use in patients’ homes or other home-like settings and give patients the opportunity to travel with their therapy.
System One - Home

In addition to our machine, we provide patients with the following proprietary consumables and services which are used for each treatment with the System One:
- | The NxStage Cartridge. A disposable, integrated treatment cartridge that loads simply and easily into the System One. The cartridge incorporates a proprietary volumetric fluid management system and includes a pre-attached dialyzer. |
- | PureFlow SL and Premixed Dialysate. Our PureFlow SL accessory prepares on-site premixed dialysate fluid in batches before treatment in the patient’s home using ordinary tap water and dialysate concentrate. The volume of fluids used varies with treatment options, prescription and setting. To accommodate patient travel with the System |
12
One or in other circumstances in which the PureFlow SL is not available, we also supply premixed dialysate in sterile five liter bags.
- | Nx2me Connected Health. Our Nx2me Connected Health platform leverages cloud-based computing and wireless communications by using an application we developed for the iPad. The Nx2me Connected Health application collects important System One cycler data, as well as patient information such as blood pressure and weight. Patients can review, confirm, and transmit this data to their dialysis centers after each treatment and dialysis center staff can access the transmitted data with their own clinician portal. This gives the staff enhanced capabilities to review and follow treatment adherence and progress as well as the ability to transfer this data directly into their electronic health records system. |
Unlike traditional dialysis systems, the System One does not require any special disinfection and its operation does not require specialized electrical or plumbing infrastructure or modifications to the home. Trained patients can bring the System One home, plug it into a conventional electrical outlet and operate it, thereby eliminating what can be expensive plumbing and electrical household modifications required by traditional dialysis systems.
Next-Generation Hemodialysis System
In 2016, we commenced a phased launch of our next-generation hemodialysis system. The first phase included software changes improving ease of use and set up time. In 2017, we expect to launch a second phase of enhancements, including a touch screen user interface and integrated blood pressure cuff, upon regulatory clearance. The third phase of our next-generation system includes a new on-site on-demand bicarbonate-based dialysis generation system. We have elected to develop a second generation of this system before investing in the regulatory process for its market clearance. We expect this development cycle to further enhance its capabilities as well as further optimize and reduce the cost of the system.
Next-Generation Hemodialysis System

Critical Care
The System One also delivers a range of renal replacement therapies within the critical care market. The System One sold to hospitals for critical care is based on the same technology platform used in the home market but offers a wider range of therapies, including therapeutic plasma exchange. We configure our critical care system with an intuitive touch screen display that provides real-time treatment information as well as easy troubleshooting capabilities for hospital staff and an ergonomic mobile stand for exceptional portability.
System One - Critical Care
13
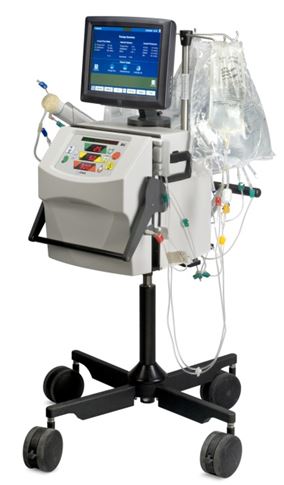
We also supply related disposable cartridges and treatment fluids necessary to perform dialysis treatment in the critical care market.
The clinical flexibility of the System One, coupled with its ease of use and portability, make our system well suited for hospital critical care environments.
Next-Generation Critical Care System
In June 2013, we entered into a research and development program sponsored by the Defense Advanced Research Projects Agency of the U.S. Department of Defense to develop an innovative device for use in military applications under a government sponsored program. This program aligns well with our technology and we expect that we will continue to leverage this product development work in the advancement of our next-generation critical care system. We expect that the design of our next-generation critical care system will incorporate a small footprint, intuitive touch screen interface, multi-stream fluid management system, and the option of on-demand fluid generation.
In-Center
We sell extracorporeal disposable products under our Medisystems brand that are primarily used for in-center hemodialysis treatments for ESRD patients. These products include hemodialysis blood tubing sets, arteriovenous (AV) fistula needles, apheresis needles, safety accessories and access management disposables.
Our Streamline blood tubing sets feature an efficient and airless design intended to enable providers to optimize dose delivery, and includes our patented LockSite needleless access sites, eliminating the need for sharp needles or costlier guarded needles to be used with the tubing set in connection with dialysis therapy. In addition, our Streamline Express dialyzer features a pre-attached blood tubing set, which is designed to reduce the number of touch point contamination sites. Our AV fistula and apheresis needles have been designed to achieve a smooth blood flow throughout the treatment, intended to result in less clotting, fewer pressure drops, and less stress on the patient’s blood. We also offer ButtonHole needles for hemodialysis therapies, which are used by patients that employ the constant-site technique, whereby a fistula needle is inserted in the same place each treatment.
NxStage Kidney Care
We operate a small number of NxStage Kidney Care dialysis centers, independently and in some instances as joint ventures, that treat ESRD patients directly. These centers provide us with valuable experience to better meet and anticipate the needs of both our customers and patients, while optimizing our product technology. In addition, these centers provide us with the opportunity to innovate and foster new care delivery models to advance the standard of renal care across other settings. More specifically, at NxStage Kidney Care we offer a range of treatment options, including home hemodialysis, peritoneal dialysis and flexible in-center hemodialysis. These centers also help us to devise best practices for successful home dialysis programs and provide sites for future clinical trials of our products.
Our Reporting Segments
We report our operating results through three segments: System One, In-Center and Services. Our other business activities relate primarily to the manufacturing of dialyzers for sale to Asahi Kasei Kuraray Medical Co. (Asahi). Together with certain
14
research and development and general and administrative expenses that are excluded from our business segment operating results, these business activities are reported in the Other category. We refer to our System One segment, In-Center segment and Other category as our products business. Additional financial information regarding our business segments and geographic data about our assets are contained in Note 9, Business Segment and Geographic Information to our consolidated financial statements and Management’s Discussion and Analysis of Financial Condition and Results of Operations included in this Annual Report. A discussion of the risks attendant to our operations is set forth in the Risk Factors section of this Annual Report.
System One
Our System One segment includes worldwide revenues from the sale and rental of the System One and PureFlow SL dialysate preparation equipment and the sale of disposable products in the home and critical care markets. Some of our largest customers in the home market provide outsourced renal dialysis services to some of our customers in critical care. Sales of products to both markets are made primarily through dedicated sales forces to dialysis centers and hospitals and delivered directly to the customer, or their patients, with certain products sold through distributors. In addition to specialized sales representatives, we also employ nurses in our sales force as clinical educators to support our sales efforts. We have a staff of customer support specialists to assist patients, clinics and hospitals with product orders and deliveries. We also provide technical support 24 hours a day, seven days a week through a dedicated staff of technical support representatives, to respond to questions raised by patients, clinics and hospitals concerning the System One.
Home. We market and sell the System One to dialysis clinics in the U.S. and other markets, which in turn provide the System One to their ESRD patients for chronic home hemodialysis treatment. In the U.S., Medicare regulations require that all chronic ESRD patients be under the care of a dialysis clinic, whether they are treated at home or in clinic. As a result, we do not sell the System One directly to Medicare patients. A significant majority of System One equipment in the home is purchased rather than rented by our customers. Purchased equipment pricing includes service for an initial contractual period after which the customer pays a standard service fee. We use a depot service model for equipment servicing and repair. If a device requires repair, we arrange for a replacement device to be shipped to the site of care, whether it is a patient’s home or a clinic, and for pick up and return to us of the system requiring service. This shipment is done by common carrier and, as there are no special installation requirements, the patient or clinic can quickly and easily set up the new machine.
After selling or renting a System One to a new clinic customer, our clinical educators train the clinic’s nurses and dialysis technicians on the proper use of the System One using our proprietary training materials. We then rely on our customers’ trained technicians and nurses to train home patients, their care partners and other technicians and nurses using the System One. In general, we are not responsible for, and do not provide, patient training except for NxStage Kidney Care patients. Patient training takes place at the clinic primarily during the patient’s prescribed treatment sessions. Training typically takes three to four weeks and consists of (1) basic education about ESRD, (2) training patients and care partners on inserting needles into the patient’s vascular access site and (3) instruction on the use and operation of the System One. Reimbursement for training sessions is provided by Medicare, at a fixed rate, and private insurance.
For each month that a patient is treated with the System One, we bill the clinic for the purchase of the related disposable cartridges and treatment fluids necessary to perform treatment, and other related services, where applicable.
Our customers for products used in the home are highly consolidated. DaVita HealthCare Partners (DaVita) and Fresenius Medical Care (Fresenius) own and operate the two largest chains of dialysis clinics in the U.S. and are our two largest customers for products used in the home. Collectively, they provide treatment to more than two-thirds of U.S. dialysis patients and a similar portion of our home patients, and account for the majority of our System One segment revenues. Increased sales to DaVita and Fresenius have driven a large portion of our historical revenue growth and will be important to future growth. Our home agreements with DaVita and Fresenius are intended to support the continued expansion of patient access to home hemodialysis with the System One, but like all our agreements with home market customers, these agreements are not requirements contracts and contain no minimum purchase volumes. Our home agreement with DaVita extends through December 31, 2018, with monthly renewals thereafter unless terminated by either party with 30 days' prior notice. Our home agreement with Fresenius continues to renew on a monthly basis unless we and Fresenius choose to modify the terms with an amendment or new agreement.
Critical Care. We market the System One directly to hospitals for treatment of acute kidney failure and fluid overload in the U.S. and other geographies. Most of our customers in the critical care market use the System One to perform prolonged or continuous renal replacement therapy for their acute kidney failure or fluid overload patients. We position the System One as a differentiated platform for the delivery of renal care in acute settings because of its technologically innovative features, ease of use and portability. The System One’s continuous volumetric balancing offers a simple effluent drainage capacity. It also uses a disposable, integrated treatment cartridge to minimize maintenance and disinfection requirements, and pre-mixed dialysate to free it from cumbersome water processing systems.
15
A significant majority of System One equipment in the critical care market is purchased rather than rented by our customers. Purchased equipment pricing includes service during our standard one-year warranty period, and we sell one- and two-year service contracts for post-warranty periods. We also offer a bio-medical training program, whereby we train bio-medical engineers on how to service and repair certain aspects of the System One in the critical care setting. Unlike the home market, we do not use a depot service model for equipment servicing and repair, but instead generally service critical care equipment in the field. The nature of the hospital critical care setting, coupled with the practices of other intensive care unit dialysis equipment suppliers, requires that we offer on-site support for our systems in this environment, or for the use of a trained bio-medical engineer.
After selling or renting a System One to a new hospital customer, our clinical educators generally train the hospital’s intensive care unit and acute dialysis nurses on the proper use of the System One using our proprietary training materials. We then rely on the trained nurses to train other nurses. By adopting this “train the trainer” approach, our sales nurses do not need to return to the hospital each time a new nurse requires training.
We also supply hospitals using the System One with related disposable cartridges and treatment fluids necessary to perform treatment.
International. We sell the System One and certain other products internationally, through a combination of direct sales to dialysis clinics and hospitals in the United Kingdom and Canada, and sales through distributors in Europe and other select markets. Products sold to distributors are shipped directly to distributor warehouses and the distributors sell or rent our products to dialysis providers or hospitals and are responsible for marketing, clinic training and equipment servicing and repair.
We entered the international market in 2009 and to date our international sales have been limited and focused primarily on the home market. While we are still early in our international commercialization efforts, we believe that there is a large opportunity for us to expand outside the U.S. Several of our product development initiatives, including our peritoneal dialysis system and next-generation critical care system, will be important to these efforts.
In-Center
Our In-Center segment includes revenues from the sale of blood tubing sets and needles for hemodialysis, primarily for the treatment of ESRD patients at dialysis clinics, and needles for apheresis. In this market, our customers are independent dialysis clinics as well as dialysis clinics that are part of national or regional chains. Although in many instances we have direct contractual relationships with our customers, nearly all of our sales in this segment are made through national distributors in order to leverage national networks, shipping efficiencies and existing customer relationships. We plan our manufacturing and distribution activities based on distributor purchase orders. Finished goods are shipped directly to distributor warehouses. We support distributor selling and marketing efforts with brand marketing support and a team of clinical educators who assist with clinical in-service activities.
We market our extracorporeal disposable products under the Medisystems brand, which we acquired in 2007. Medisystems branded products have an established reputation for quality, ease of use and innovation, and have been in the in-center market since 1981. In our marketing efforts, we emphasize our Medisystems products’ strong clinical performance and cost-effectiveness.
Our In-Center segment revenues are highly concentrated in several significant purchasers. Henry Schein, Inc., accounted for 28% of our 2016 In-Center segment revenues. Gambro AB (a subsidiary of Baxter International, Inc.) accounted for 20% of our 2016 In-Center segment revenues, with all of Gambro's sales of our products being to DaVita.
Services
Our Services segment includes revenues from dialysis services provided to patients at our NxStage Kidney Care dialysis centers. As of February 3, 2017, we had 20 centers accepting patients in13 states, of which 19 have received Medicare certification. We continue to explore innovative service delivery models with our NxStage Kidney Care dialysis centers to advance the standard of renal care. Some of our service models vary on the number of treatment stations within a center, the configuration of the center and hours of operation. However, at all of our centers, we provide patients with a range of therapy options to address their clinical and lifestyle needs. For appropriate patients, such therapies may include home hemodialysis, flexible in-center hemodialysis and peritoneal dialysis.
Clinical Evidence
The vast majority of ESRD patients in the U.S. are prescribed traditional in-center hemodialysis, which consists of three dialysis sessions per week, typically at 3 to 4 hours per session, in a clinical setting. Despite falling mortality rates in hemodialysis patients during the past decade, rates remain much higher than in age-matched U.S. residents. Notably, among patients under age 50, mortality rates are 25 to 55 times higher than in general U.S. residents. Significant clinical literature strongly supports that home and more frequent hemodialysis therapy can lead to improved clinical outcomes.
16
Home Hemodialysis
Studies have consistently shown that home hemodialysis may offer enhanced health, control and freedom to ESRD patients. Several observational studies have found home hemodialysis to be associated with a lower mortality risk compared to in-center hemodialysis. Home therapy may also be an effective way by which to increase patient engagement in the delivery of dialysis, with accumulating health services research suggesting improved outcomes with higher patient activation. In addition, home patients are afforded a newfound independence and control over their care that can result in an improved quality of life.
More Frequent Hemodialysis
The traditional thrice weekly hemodialysis schedule is a clear departure from normal physiology in which the kidneys continuously filter blood. Accompanying each interval between consecutive dialysis sessions, changes in serum biochemistry and volume status may increase risks of both acute (e.g., cardiac arrhythmia) and chronic (e.g., end organ damage) complications. The roughly 72-hour interval between consecutive sessions on Friday and Monday or Saturday and Tuesday appears to be especially problematic. Multiple studies have suggested that this interval is associated with increased risks of mortality and morbidity.
More frequent hemodialysis, defined as the range of schedules that eliminates multiple-day intervals between consecutive sessions, mitigates the “unphysiology” of the usual hemodialysis schedule. Regimens range from every-other-day dialysis to daily dialysis. Accumulating evidence, including randomized clinical trials and large observational studies, indicates that more frequent hemodialysis confers multiple benefits, including reduced risk of cardiovascular morbidity, improved regulation of blood pressure and phosphorus and reduced need for related medications, better tolerability of hemodialysis and improved physical and mental quality of life.
Cardiovascular Disease. Cardiovascular disease is the leading cause of death in hemodialysis patients. An important predictor of cardiovascular mortality and morbidity in dialysis patients is left ventricular hypertrophy (LVH), a condition marked by enlargement and thickening of the walls of the left ventricle. LVH is an adaptive response to increased cardiac work, typically caused by combined pressure and volume overload. Multiple studies show that more frequent hemodialysis reduces left ventricular mass and is associated with significantly lower risk of cardiovascular mortality and morbidity, particularly due to heart failure and hypertensive disease.
Blood Pressure and Antihypertensive Medications. Hypertension is a cardinal feature of ESRD, with the prevalence of hypertension exceeding 85% in new ESRD patients. Highly elevated blood pressure is associated with poor outcomes in dialysis patients. With three hemodialysis sessions per week, blood pressure climbs during the interdialytic interval, in step with interdialytic weight gain, particularly among elderly patients and those with higher dry weight. Multiple randomized clinical trials show that frequent hemodialysis reduces blood pressure and the need for oral medications indicated for hypertension.
Serum Phosphorus and Phosphate Binders. ESRD patients commonly have elevated levels of serum phosphorus (“hyperphosphatemia”). Hyperphosphatemia is associated with higher risk of cardiovascular death. The treatment of hyperphosphatemia is burdensome. Dialysis patients consume on average 19 pills per day and 9 are phosphate binders. Moreover, Medicare Part D expenditures on binders for dialysis patients exceeded $840 million in 2014. Meanwhile, adherence to phosphate binders is poor, especially in younger patients and those with high pill burden. Multiple randomized clinical trials show that frequent hemodialysis reduces serum phosphorus. In observational research, frequent hemodialysis is also associated with a lower percentage of patients using binders.
Dialysis Tolerability. Dialysis treatment can be difficult to tolerate. Recurrent complications during and after the hemodialysis session may limit treatment persistence and engender withdrawal, which is the primary cause of death in 10% to 15% of patients. Long recovery time after treatment is common with the thrice-weekly hemodialysis schedule. In one study, recovery time was between two and six hours for 41% of hemodialysis patients and greater than six hours for 27%; recovery time greater than six hours was associated with substantially higher risks of death and hospitalization. In our prospective, observational FREEDOM (Following Rehabilitation, Economics, and Everyday Dialysis Outcome Measurements) study of daily home hemodialysis, recovery time was sharply reduced after 12 months of treatment, from roughly eight hours at baseline to merely one hour in per-protocol analysis. Meanwhile, recovery time after nocturnal hemodialysis may be only minutes in duration. In matched cohort studies, daily home hemodialysis was associated with almost 40% lower risk of death due to withdrawal or cachexia, relative to each of thrice-weekly in-center hemodialysis and peritoneal dialysis. By decreasing recovery time after treatment, frequent hemodialysis can improve the tolerability of dialysis treatment and may reduce the incidence of withdrawal from dialysis.
Quality of Life: Physical Health. Characteristics of poor physical health-related quality of life (HRQoL) include limitations in physical, self-care and social activities; severe bodily pain; frequent tiredness; and low self-rating of physical health. Mean physical HRQoL in hemodialysis patients is much lower than the U.S. general population norm. In both randomized clinical trials and prospective cohort studies, more frequent hemodialysis improves physical HRQoL. More
17
frequent hemodialysis is also associated with improvements in restless legs, especially in patients with severe symptoms, and sleep disturbances, including daytime somnolence.
Quality of Life: Mental Health. Characteristics of poor mental HRQoL include frequent psychological distress, social disability due to emotional problems and low self-rating of mental health. Poorer mental health, as measured by the Kidney Disease Quality of Life Short Form, has been associated with increased risks of both death and hospitalization in hemodialysis patients. Frequent hemodialysis can address depressive symptoms, as shown in our FREEDOM study. More frequent hemodialysis also has led to improvement in overall mental health, including large improvements in vitality and social functioning.
Potential Risks
In spite of the benefits of more frequent hemodialysis on cardiovascular function, health-related quality of life, and treatment tolerability, more frequent hemodialysis, including more frequent hemodialysis performed at home, may introduce specific risks pertaining to vascular access complications, infections, loss of residual renal function and increased burden on caregivers. All forms of hemodialysis, including treatments performed in-center and at home, involve some risks. In addition, there are certain risks unique to treatment in the home environment. Patients differ and not everyone will experience the reported benefits of home or more frequent hemodialysis. Certain risks associated with hemodialysis treatment are increased when performing nocturnal therapy due to the length of treatment time and because therapy is performed while the patient and care partner are sleeping.
Our Competition
The dialysis therapy market is mature, and we face competition from many sources. We believe that our competitive strengths include the quality and ease of use of our products and our history of leveraging innovative technology to deliver high value, clinically flexible solutions.
Home Hemodialysis
The System One is the first portable system indicated for home hemodialysis and is also indicated for home nocturnal hemodialysis in the U.S. We believe the System One's design is unique in terms of product quality and ease of use compared to other products cleared for home use in the U.S. because of its design, portability and avoidance of home modifications. Multiple competitors provide more traditional systems, against which the System One competes in the home hemodialysis market.
Critical Care
The System One is also used in the critical care market. Other providers of products for hemodialysis in the critical care market include Gambro (a subsidiary of Baxter), Fresenius, Nikkiso Co. Ltd., B. Braun Medical, Inc. (B. Braun) and several smaller companies. In addition, multiple competitors provide more traditional systems used in intensive care units.
We believe we compete favorably in terms of product quality and ease of use due to our System One design, portability, drop-in cartridge and use of premixed fluids.
In-Center
Our Medisystems branded bloodlines, needles and other consumables are sold to U.S. in-center providers and compete against products produced by Fresenius, Nipro Medical Corporation, JMS Co. Ltd. and others. Outside the U.S., we face competition from several firms, including B. Braun and Baxter, together with its subsidiary Gambro.
We believe that we compete favorably with a strong Medisystems brand and with respect to product quality, ease of use, cost effectiveness, clinical flexibility and performance.
Services
The U.S. dialysis services industry is highly competitive. A majority of U.S. dialysis centers are operated by the two largest dialysis organizations, DaVita and Fresenius, with the remaining facilities allocated among several mid-sized dialysis organizations and a number of small dialysis organizations and local hospitals.
Intellectual Property
We seek to protect our investment in the research, development, manufacturing and marketing of our products through the use of patent, trademark, copyright and trade secret law. We own rights to a number of patents, trademarks, copyrights, trade secrets and other intellectual property directly related and important to our business both in the U.S. and abroad. We also have domestic and foreign pending patent applications. Any of our trade secrets, know-how or other technology not protected by a patent could be misappropriated, or independently developed by, a competitor and could, under some circumstances, be used to
18
prevent us from further use of such information, know-how or technology. We require our employees, consultants and advisors to execute confidentiality agreements with us. We also require our employees to agree to disclose and assign to us inventions conceived by them during their employment. Similar obligations are imposed upon consultants and advisors performing work for us relating to the design or manufacture of our products. Despite efforts to protect our intellectual property, unauthorized parties may attempt to copy aspects of our products or obtain and use information that we regard as proprietary.
Patents. Our strategy is to develop patent portfolios for our research and development projects. Patents for individual products extend for varying periods of time according to the date a patent application is filed or a patent is granted and the term of the patent protection available in the jurisdiction granting the patent. The scope of protection provided by a patent can vary significantly from country to country.
As of December 31, 2016, we had 137 U.S. and foreign counterpart patents and 52 U.S. and foreign counterpart patents pending. The issued patents and pending patent applications cover, among other things:
•safety technology for automated blood treatments;
• | control and mechanical features aimed at set-up including priming of blood circuits and preparation of fluids including dialysate for hemodialysis and peritoneal dialysis; |
•blood circuit improvements aimed at safety, cost effectiveness and convenience;
•control technology aimed at reliability and safety of blood treatment machines;
•sensor technology including temperature and pressure sensors; and
•administration of peritoneal dialysis.
The approximate expiration periods for our issued patents are as follows:
Expiration | Portion of Patent Portfolio |
Within the next 5 years | 29% |
6-10 years | 26% |
11-15 years | 12% |
16-20 years | 33% |
Hemodialysis technologies have been in existence for over fifty years, and there are thousands of patents held by third parties that relate to dialytic technologies. Collectively, our patents and other intellectual property are important to us, although there is no single patent that is solely responsible for protecting our products. We believe that the duration of our patents is adequate relative to the expected lives of our products.
Product Development
Our product development organization is working to develop innovative technical approaches that address the limitations of current dialysis systems and disposable products. We focus our development innovation on new hardware and disposables that allow for lower cost and higher capability. This includes developing new sensors, software, disposable designs, pump designs, manufacturing techniques, assembly automation, plastic processing technologies, and user interfaces, to name a few of our important development areas. Our development team has skills across the range of technologies required to develop and maintain dialysis systems and products, including filters, tubing sets, mechanical systems, fluids, software and electronics.
We are continually working on enhancements to our product designs to improve ease of use, functionality, reliability and safety and to reduce product cost. We also seek to develop new products that supplement our existing product offerings, such as our peritoneal dialysis system and next-generation critical care system, and intend to continue to actively pursue research and development opportunities for complementary products.
We believe our research and development efforts are critical to our success and continue to make significant investments in our product pipeline. Our research and development expenses were $31.0 million (8% of total revenues), $26.2 million (8% of total revenues) and $22.6 million (8% of total revenues) during 2016, 2015 and 2014, respectively.
Manufacturing
We have significant manufacturing infrastructure dedicated to high-volume plastics disposables production. We have manufacturing facilities in Mexico, Germany and Italy. Manufacturing innovation of process and automation is a critical capability that has contributed to our ability to manufacture high quality, low-cost products. We have designed most of our production automation ourselves. At our facility in Tijuana, Mexico, we perform a number of manufacturing operations relating to our System One equipment and disposables and in-center bloodlines, and we service System One equipment. We manufacture our dialyzer filters at a facility in Germany owned by Asahi and operated by us, and we perform molding activities
19
at our facility in Italy. We complement our internal production capabilities by outsourcing the manufacture of premixed dialysate, needles and some components.
We depend upon a number of single-source suppliers for certain of our raw materials, components and finished goods, including the fiber used in our System One filters, our needles and sterile bags, as well as sterilization services. Finding alternative sources for these raw materials, components, finished goods and sterilization services would be difficult and in many cases entail a significant amount of time, disruption and cost. Where obtaining a second source is more difficult, we have tried to establish supply agreements that better protect our continuity of supply, although we do not have supply agreements with all of our single-source suppliers. Where we have no agreements in place, we work, to the extent economically feasible, to maintain enough inventory of the single-sourced component to allow us to, if needed, satisfy our requirements for the component while we secure an alternative source of supply.
Some of our most critical supply relationships are with Membrana GmbH and Laboratorios PiSA S.A. de C.V.
Membrana is our only supplier of the fiber used in our filters for System One products under an agreement that expires in December 2023, and contractually we cannot obtain an alternative source of fiber for our System One products. While our relationship with Asahi could afford us back-up supply in the event of supply disruptions at Membrana, we do not have the regulatory approvals necessary to use Asahi fiber in our System One cartridge.
Laboratorios PiSA supplies substantially all of our premixed dialysate. Our supply agreement with Laboratorios PiSA extends through December 2019. We have committed to purchase from Laboratorios PiSA a minimum quantity of premixed dialysate over the term of the agreement. While we purchase premixed dialysate from another qualified supplier, any significant disruption in Laboratorios PiSA’s ability to supply premixed dialysate to us would impair our business, at least in the near term.
Government Regulation
In the U.S., numerous laws and regulations govern all the processes by which medical devices are brought to market and, for our NxStage Kidney Care dialysis centers, the manner in which we administer and submit claims for patient care. In the foreign countries in which we market and sell our products, we are subject to local regulations affecting, among other things, design and product standards, packaging and labeling and promotion requirements.
Food and Drug Administration
In the U.S., our products are subject to regulation by the FDA as medical devices. The FDA regulates the design, development, clinical testing, manufacture, labeling, distribution, import and export, sale and promotion of medical devices. Noncompliance with applicable FDA requirements can result in, among other things:
- violation letters;
- fines, injunctions, and civil penalties;
- recall or seizure of products;
- | administrative detention, which is the detention by the FDA of medical devices believed to be adulterated or misbranded; |
- operating restrictions, partial suspension or total shutdown of production;
- failure of the government to grant pre-market clearance or pre-market approval for devices;
- withdrawal of marketing clearances or approvals; and
- criminal prosecution.
Unless an exemption applies, all medical devices must receive either 510(k) clearance or an approved pre-market application (PMA) from the FDA before they may be commercially distributed in the U.S. To obtain a 510(k) clearance for a device, a pre-market notification to the FDA must be submitted demonstrating that the device is substantially equivalent to a legally marketed predicate device. The FDA attempts to respond to a 510(k) pre-market notification within 90 days of submission, but as a practical matter, pre-market clearance can take significantly longer, potentially up to one year or more. The PMA process is much more demanding and uncertain than the 510(k) pre-market notification process and must be supported by extensive clinical, technical and other information, including at least one adequate and well-controlled clinical investigation. The FDA has 180 days from the date of filing to review an accepted PMA, although the review generally occurs over a significantly longer period of time, and can take up to several years.
FDA Regulatory Clearance Status
We currently have all of the regulatory clearances required to market the System One in the U.S. in both the home and critical care markets, all of which have thus far been granted as 510(k) clearances. The FDA has cleared the System One for the treatment, under a physician’s prescription, of renal failure or fluid overload using hemofiltration, hemodialysis and/or
20
ultrafiltration. The FDA has also specifically cleared the System One for both home hemodialysis and home nocturnal hemodialysis use under a physician’s prescription.
We continue to seek opportunities for product improvements and feature enhancements, which will, from time to time, require FDA clearance before market launch. In total, we have received from the FDA 41 product clearances for the System One and related products and 25 product clearances for our Medisystems branded extracorporeal disposable products.
Continuing FDA Regulation
After a device is placed on the market, numerous regulatory requirements apply, including:
- | Quality System Regulations, which require manufacturers to have a quality system for the design, testing, manufacture, packaging, labeling, storage, installation, and servicing of finished medical devices; |
- | labeling regulations, which govern product labels and labeling, prohibit the promotion of products for unapproved, or off-label, uses and impose other restrictions on labeling and promotional activities; |
- | clearance of product modifications that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared devices; |
- | medical device reporting (MDR) regulations, which require that manufacturers evaluate and investigate potential adverse events and malfunctions, and report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; |
- | the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; |
- | regulations pertaining to voluntary recalls; |
- | notices of corrections or removals; and |
- | product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action. |
We are registered with the FDA as a medical device manufacturer. The FDA seeks to ensure compliance with regulatory requirements through periodic facility inspections and these inspections may include the manufacturing facilities of our subcontractors.
Foreign Regulation of Medical Devices
We are also subject to regulations in the foreign countries in which we market and sell our products. We currently have limited sales outside of the U.S. Foreign regulations, which may vary substantially from country to country, relate to, among other things, product standards, packaging, labeling and promotion requirements, import restrictions, tariff regulations, duties and tax requirements.
Clearance or approval of our products by regulatory authorities comparable to the FDA, or in the case of the EU the affixing of the CE mark, may be necessary in foreign countries prior to marketing the product in those countries, whether or not FDA clearance has been obtained. The regulatory requirements for medical devices vary significantly from country to country. They can involve requirements for additional testing and may be time consuming and expensive. We cannot provide assurance that we will be able to obtain regulatory approvals in any other markets or be able to affix the CE mark to new products in the EU.
In the specific case of the EU, manufacturers of medical devices are required to conduct an assessment of the conformity of the devices with the Essential Requirements found in Annex I to Council Directive 93/42/EEC of 14 June 1993 concerning medical devices, commonly known as the Medical Devices Directive, and to affix a CE mark to these devices prior to marketing these within the EU. The Essential Requirements govern the quality, safety and performance of the medical devices. The classification of individual medical devices will determine whether the participation by a notified body in the conformity assessment process will be necessary. Notified bodies are private organizations that are licensed by the competent authorities of individual EU Member States to conduct conformity assessment procedures and to verify the conformity of manufacturers and their medical devices with the Essential Requirements. If, where the participation by a notified body is necessary, the outcome of the conformity assessment procedure is positive the notified body will issue a related CE Certificate of Conformity. The manufacturer of the device will then complete the technical file for the medical device and, after having prepared and signed a related Declaration of Conformity, affix the CE mark to the product.
CE Certificates of Conformity have been issued in relation to all of our products that require such certificates and we have affixed a CE mark to these products. However, if we introduce any substantial change to any of our CE marked medical devices in the EU this may require an additional conformity assessment process in relation to the substantial changes and modification or preparation of new CE Certificates of Conformity and Declarations of Conformity.
21
In the EU we must comply with a number of regulatory requirements for products that have been CE marked and placed on the market relating to:
- | registration of medical devices; |
- | pricing and reimbursement of medical devices; |
- | establishment of post-marketing surveillance and adverse event reporting procedures; |
- | field safety corrective actions, including product recalls and withdrawals; |
- | marketing and promotion of medical devices; and |
- | interactions with physicians. |
Failure to comply with these requirements may result in enforcement measures being taken by the competent authorities of the EU Member States. These can include fines, administrative penalties, compulsory product withdrawals, injunctions and criminal prosecution. Such enforcement measures would have an adverse effect on the marketing of our products in the EU and, consequently, on our business and financial position.
Licensure and Certification
Our NxStage Kidney Care dialysis centers must be certified by CMS to receive Medicare payments. In some states, these centers must also secure additional state licenses, permits or certificates of needs. Governmental authorities, primarily state departments of health, periodically inspect our centers to determine if we satisfy applicable federal and state standards and requirements, including the conditions of coverage by the Medicare ESRD program.
Fraud and Abuse Laws
U.S. federal healthcare laws apply when our customers and NxStage Kidney Care dialysis centers submit claims for items or services that are reimbursed under Medicare, Medicaid, or other federally-funded healthcare programs. The principal federal fraud and abuse laws include:
• | the Anti-Kickback Statute, which among other things prohibits the offer or payment of any remuneration for the purpose of inducing or rewarding patient referrals or the order, purchase or recommendation of items or services reimbursable by a federal healthcare program; |
• | the False Claims Act, which prohibits the submission of false or otherwise improper claims for payment to a federally-funded healthcare program or causing such claims to be submitted; and |
• | criminal healthcare fraud statutes that prohibit false statements and improper claims to any third-party payors. |
There are often similar state anti-kickback and false claims laws that apply to state-funded Medicaid and other healthcare programs, as well as to private third-party payors. In addition, the U.S. Foreign Corrupt Practices Act can be used to prosecute companies in the U.S. for arrangements with physicians or other parties outside the U.S. if the physician or party is a government official of another country and the arrangement violates the laws of that country. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products or of a provider’s services from reimbursement under government programs, criminal fines and imprisonment.
Similar laws are increasingly being introduced in the individual European states. The provision of benefits or advantages to physicians to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order or use of medical devices is prohibited. The provision of benefits or advantages to physicians is also governed by the national anti-bribery laws of European states. One such example is the UK Bribery Act. Payments made to physicians in certain EU Member States must also be publicly disclosed. Moreover, agreements with physicians must often be the subject of prior notification and approval by the physician’s employer or competent professional organization or the competent authorities of the individual European states. These requirements are provided in the national laws, industry codes or professional codes of conduct applicable in the European states. Failure to comply with these requirements could result in reputational harm, public reprimands, administrative penalties, fines or imprisonment.
Anti-Kickback and Related Statutes
The federal healthcare program Anti-Kickback Statute, and similar state laws, prohibit payments and other forms of remuneration that are intended to induce healthcare professionals or others either to refer patients or to purchase, lease, order or arrange for or recommend the purchase, lease or order of healthcare products or services. Other laws prohibit remuneration that the offeror knows or should know is likely to induce patients to select a particular provider of services, including for dialysis. A number of states have enacted laws that require pharmaceutical and medical device companies to monitor and report payments, gifts and other remuneration made to physicians and other healthcare professionals and healthcare organizations or marketing expenditures. In addition, some state statutes, most notably laws in Massachusetts and Vermont, impose outright bans on certain manufacturer gifts to physicians. Some of these laws, referred to as “aggregate spend” or “gift” laws, carry substantial
22
fines if they are violated. The federal Physician Payments Sunshine Act was enacted by Congress in 2010 as part of the comprehensive health care reform legislation, and the implementing regulations, released in February 2013, require us to collect and report certain data on payments and other transfers of value to physicians and teaching hospitals annually to CMS for public reporting. It is widely believed that public reporting under the Sunshine Act results in increased scrutiny of the financial relationships between industry, physicians and teaching hospitals.
The Anti-Kickback Statute is broad and potentially prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, the statute contains some exceptions and the Office of Inspector General of the Department of Health and Human Services (OIG) issued a series of regulations, known as the safe harbors, implementing these exceptions and establishing some additional protected areas of conduct. These safe harbors set forth conditions that, if met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within a safe harbor does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy an applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG. If scrutinized, arrangements that implicate the Anti-Kickback Statute, and that do not fall within a safe harbor, are analyzed by the OIG and other enforcement authorities on a case-by-case basis with review based on the totality of the facts and circumstances.
Government officials have focused recent enforcement efforts on, among other things, the sales and marketing activities of medical device manufacturers and other healthcare companies, and recently have brought cases against individuals or entities with personnel who allegedly offered unlawful inducements to potential or existing customers in an attempt to procure their business. Judgments and settlements of these cases by healthcare companies have involved significant fines and, in some instances, criminal pleas and convictions. Further, the 2010 Patient Protection and Affordable Care Act specified that any claims submitted as a result of a violation of the Anti-Kickback Statute are also false claims and subject to enforcement under the False Claims Act.
In addition to the federal Anti-Kickback Statute, many states have their own anti-kickback laws. Often, these laws closely follow the language of the federal law, although they do not always have the same exceptions or safe harbors. In some states, these anti-kickback laws apply with respect to all payors, including commercial health insurance companies.
The national legislation and industry codes of many foreign countries includes provisions equivalent in content and consequences to the federal Anti-Kickback Statute and the Sunshine Act.
False Claims Laws
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or knowingly making, or causing to be made, a false statement to get a false claim paid. Our NxStage Kidney Care dialysis centers are directly subject to these laws with respect to the reimbursement claims they file with government payors. In addition, medical device manufacturers can be held liable under false claims laws, even if they do not submit claims to the government, if they are found to have caused the submission of false claims, including through arrangements that violate the Anti-Kickback Statute. The federal False Claims Act also includes whistleblower provisions that allow private citizens to bring suit against an entity or individual on behalf of the U.S. and to recover a portion of any monetary recovery. Many of the recent highly publicized settlements in the healthcare industry related to the sales and marketing practices of pharmaceutical and medical device manufacturers have been cases brought under the False Claims Act. In addition, amendments to the False Claims Act included in the Affordable Care Act impose severe penalties for the knowing and improper retention of identified overpayments collected from governmental payors. Within 60 days of identifying an overpayment, a provider is required to notify CMS or its contractor of the overpayment and the reason for it and return the overpayment. These amendments could subject our procedures for identifying and processing overpayments to greater scrutiny.
Violations of The Ethics in Patient Referrals Act, commonly known as the Stark Act, can also form the basis for False Claims Act liability. The Stark Act generally prohibits a physician from referring a patient to an entity for certain designated health services payable by Medicare or Medicaid when the physician has a financial relationship with the entity, such as an investment interest or a medical directorship, unless an exception applies. While outpatient dialysis services and most drugs furnished by our NxStage Kidney Care dialysis centers are excluded from the Stark Act’s prohibitions on self-referrals, certain outpatient prescription drugs may be subject to the Stark Act.
The majority of states also have statutes or regulations similar to the federal false claims laws, which apply to items and services reimbursed under Medicaid and other state programs. Several states have false claims laws that apply regardless of the payor. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines and imprisonment.
The national legislation of many foreign countries includes provisions equivalent in content and consequences to the federal false claims laws.
23
Compliance Program
The healthcare laws and fraud and abuse laws applicable to our business are complex and subject to variable interpretations. We maintain certain compliance review, education and training and other programs to further our commitment to high standards of ethical and legal conduct and to endeavor to minimize the likelihood that we would engage in conduct or enter into arrangements in violation of applicable authorities. For example, we have (1) established a compliance team consisting of representatives from our Legal, Finance, Human Resources, Regulatory Affairs/Quality Assurance and Commercial departments that meets regularly; (2) established a compliance hotline that permits our employees to report anonymously any compliance issues that may arise; and (3) instituted other safeguards intended to help prevent any violations of the applicable fraud and abuse laws and healthcare laws, and to remediate any situations that could give rise to violations. We also review many of our transactions and agreements, both past and present, with qualified legal counsel to help ensure they are compliant.
Through our compliance efforts, we constantly strive to structure our business operations and relationships with our customers to comply with all applicable legal requirements. However, many of the laws and regulations applicable to us are broad in scope and may be interpreted or applied by prosecutorial, regulatory or judicial authorities in ways that we cannot predict. Thus, it is possible that governmental entities or other third parties could interpret these laws differently or assert non-compliance with respect to one or more of our business operations and relationships. Moreover, the standards of business conduct expected of healthcare companies under these laws and regulations have become more stringent in recent years, even in instances where there has been no change in statutory or regulatory language. If a claim were asserted against us for alleged non-compliance with fraud and abuse laws, false claims laws or other healthcare laws, and we were not to prevail, possible penalties and sanctions could have a material effect on our financial condition and ability to conduct our operations.
Privacy and Security
In the course of performing our business we obtain, from time to time, confidential patient health information. For example, we learn patient names and addresses when we ship our System One supplies and related products to home hemodialysis patients. We may learn patient names and be exposed to confidential patient health information when we provide training on our products to our customers’ staff. Our home hemodialysis patients call our customer service representatives directly and during the call may disclose confidential patient health information. We also receive and maintain confidential patient health information in connection with the operation of our NxStage Kidney Care dialysis centers. U.S. federal and state laws protect the confidentiality of certain patient health information, in particular individually identifiable information, and restrict the use and disclosure of that information. At the federal level, the Department of Health and Human Services promulgated health information privacy, security and breach notification regulations under the Health Insurance Portability and Accountability Act of 1996 and the Health Information Technology for Economic and Clinical Health Act (collectively, HIPAA). We are subject to HIPAA with regard to certain aspects of our business. In addition, many other state and federal laws regulate the use and disclosure of health information, including state medical privacy laws, state breach notification laws, and federal and state consumer protection laws. In many cases, these laws are not necessarily preempted by HIPAA, particularly if they afford greater protection to the individual than does HIPAA. These various laws may be subject to varying interpretations by courts and government agencies creating potentially complex compliance issues for our business. Violations of HIPAA may result in penalties and enforcement actions by the Department of Health and Human Services Office for Civil Rights (OCR). OCR has recently focused its enforcement efforts on compliance with the HIPAA security regulations (Security Rule), bringing actions against entities which have failed to implement security measures sufficient to reduce risks to electronic protected health information or to conduct an accurate and thorough risk analysis, among other violations. Enforcement actions may lead to monetary penalties and costly and restrictive corrective action plans.
We are also subject to laws and regulations in foreign countries covering data privacy and other protection of health and employee information that may be more onerous than corresponding U.S. laws. These regulations may require that we obtain individual consent before we collect or process any personal data, restrict our use or transfer of personal data, impose technical and organizational measures to ensure the security of personal data, and require that we notify regulatory agencies, individuals or the public about any data security breaches. As we expand our international operations, we may be required to expend significant time and resources to put in place additional mechanisms to ensure compliance with multiple data privacy laws. The regulatory framework for privacy and security issues worldwide is rapidly evolving and is likely to remain uncertain for the foreseeable future. For example, in October 2015, the European Court of Justice invalidated an international safe harbor agreement that provided a mechanism for transferring personal data between the U.S. and Europe. Although U.S. and European authorities have agreed to a replacement data-sharing framework called the Privacy Shield, some aspects of the new framework have been subject to challenges, whose outcomes remain uncertain. Failure to comply with these laws may result in significant fines and other administrative penalties and harm our business.
Reimbursement
Home and In-Center
24
Medicare regulations require that all Medicare ESRD patients requiring dialysis be under the care of a dialysis clinic, whether they are treated at home or in-clinic. We sell or rent our System One and sell our needles and blood tubing sets to dialysis clinics. These clinics, in turn, are reimbursed by Medicare, Medicaid and private insurers. According to the 2016 United States Renal Data Systems (USRDS) Annual Data Report, Medicare or Medicaid is the primary payor for approximately 83% of dialysis patients using hemodialysis and peritoneal dialysis. The report also indicates that approximately 5% of patients are covered by commercial insurance with Medicare as the secondary payor, with the remaining 12% of patients classified by the USRDS as “other” or “unknown.” Certain centers have indicated that more frequent home hemodialysis therapy with the System One attracts a higher percentage of commercial insurance patients than other forms of dialysis.
Medicare. Medicare generally provides health insurance coverage for persons who are age 65 or older and for persons who are completely disabled. For ESRD patients, however, Medicare coverage is not dependent on age or disability. Patients are eligible for Medicare based solely on ESRD. Coverage for patients eligible for Medicare based solely on ESRD begins on the first day of the fourth month after the patient begins dialysis treatments. During the three-month waiting period, Medicaid, private insurance or the patient is responsible for payment for dialysis services. Medicare waives this waiting period for individuals who participate in a home dialysis training program, or are hospitalized for a kidney transplant and the surgery occurs within a specified time period.
For ESRD patients under age 65 who have any employer group health insurance coverage, regardless of the size of the employer or the individual’s employment status, Medicare coverage is generally secondary to the employer coverage during the 30-month period that follows the establishment of Medicare eligibility or entitlement based on ESRD. During this period, the patient’s existing insurer is responsible for paying primary benefits at the rate specified in the applicable group health plan, which may be a negotiated rate or the healthcare provider’s usual and customary rate. As the secondary payor during this period, Medicare will make payments up to the applicable Medicare payment rate for dialysis services to supplement any primary payments by the employer group health plan if the plan covers the services but pays only a portion of the charge for the services.
Medicare generally is the primary payor for ESRD patients after the 30-month coordination period. Under current rules, Medicare is also the primary payor for ESRD patients during the 30-month period under certain circumstances. Medicare remains the primary payor when an individual becomes eligible for Medicare on the basis of ESRD if (1) the individual was already age 65 or over or was eligible for Medicare based on disability and (2) the individual’s private insurance coverage is not by reason of current employment or, if it is, the employer has fewer than 20 employees (in the case of eligibility by reason of age) or fewer than 100 employees (in the case of eligibility by reason of disability). The rules regarding entitlement to primary Medicare coverage when the patient is eligible for Medicare on the basis of both ESRD and age, or disability, have been, and may continue to be, the subject of frequent legislative and regulatory changes.
When Medicare is the primary payor for services furnished by dialysis clinics, it reimburses dialysis clinics for 80% of the allowable rate, leaving the secondary insurance or the patient responsible for the remaining 20%.
As a result of legislation passed by the U.S. Congress more than 30 years ago, Medicare provides broad and well established reimbursement in the U.S. for ESRD. Effective January 1, 2011, CMS implemented a new prospective payment system for dialysis treatment. Under the ESRD prospective payment system, CMS makes a single bundled payment to the dialysis facility for each dialysis treatment that covers all renal dialysis services and home dialysis and includes certain drugs (including erythropoiesis stimulating agents, iron and Vitamin D). The prospective payment system replaced the former system, which paid facilities a composite rate for a defined set of items and services, while paying separately for drugs, laboratory tests, and other services that were not included in the composite rate. With a vast majority of U.S. ESRD patients covered by Medicare, the Medicare reimbursement rate is an important factor in a potential customer’s decision to use the System One or our other products and limits the fees for which we can sell or rent our products. Additionally, current CMS rules limit the number of hemodialysis treatments paid for by Medicare to three times a week, unless there is medical justification provided by the dialysis facility based on information from the patient’s physician for additional treatments. Because the home setting provides the most practical and economic setting for the implementation of alternative therapy delivery models that have been well-documented to offer significant clinical and quality of life benefits, most patients using the System One in the home have been prescribed to receive more than three treatments per week. To the extent that Medicare contractors determine they will not pay for additional treatments, adoption of the System One would likely be impaired and revenues from our NxStage Kidney Care dialysis centers likely would be reduced.
Medicare has been consolidating the Medicare contractors that process and pay Medicare claims. Accordingly, there have been and may continue to be changes in the contractors that determine medical justification for dialysis treatments. This change in the reviewing entity for Medicare claims could lead to a change in whether a customer receives Medicare reimbursement for additional treatments. If an adverse change to historical payment practices occurs, adoption of our System One in the home market may be impaired and revenues from our NxStage Kidney Care dialysis centers likely would be reduced.
25
Based on a 2008 analysis of historical Medicare payment files by the University of Michigan Kidney Epidemiology and Cost Center, those delivering more frequent dialysis at home receive reimbursement, on average, for 1.5 times the number of treatments per month versus conventional dialysis, although this amount varies by jurisdiction. This variance arises from Medicare contractor policies, as well as from varying center billing practices. Currently, only four of the twelve Medicare contractor jurisdictions have issued formal local coverage determinations that describe medical justification for more frequent hemodialysis. In the remaining jurisdictions, medical justification is determined on a case by case basis. As there is no consistent national standard for what constitutes medical justification, a clinic’s decision as to how much it is willing to spend on home dialysis equipment and services will be at least partly dependent on the level of confidence the center has in the predictability of receiving reimbursement from Medicare for additional treatments per week based on submitted claims for medical justification.
A stated goal of the ESRD prospective payment system was to encourage home dialysis. To date, it has not had a positive impact on the adoption of home or more frequent hemodialysis or the price for which we can sell our products. However, the prospective payment system has had a significant positive impact on the adoption of peritoneal dialysis as evidenced by the significantly increased rates of training for peritoneal dialysis. We believe this increased focus on peritoneal dialysis growth and peritoneal dialysis training has been to the detriment of home hemodialysis training rates, as home training resources, including home training nurses in particular, have been more devoted to peritoneal dialysis training, leaving less time for home hemodialysis training.
CMS issued the 2017 final rule for the end-stage renal disease prospective payment system, which increases the base reimbursement rate less than 1% over 2016 rates. In addition, CMS increased the home and self-dialysis training add-on payment to $95.60 in 2017. The effect of these changes on the adoption of home and more frequent hemodialysis is not yet known.
Medicaid. Medicaid programs are state-administered programs partially funded by the federal government. These programs are intended to provide coverage for certain categories of patients whose income and assets fall below state defined levels and who are otherwise uninsured. For those who are eligible, the programs serve as supplemental insurance programs for the Medicare co-insurance portion and provide certain coverage, such as for self-administered outpatient prescription medications, that is not provided by Medicare. For ESRD treatment, state regulations generally follow Medicare coverage and reimbursement levels, but without any co-insurance amounts, which is pertinent mostly for the three-month waiting period for Medicare coverage. Certain states, however, require beneficiaries to pay a monthly share of the cost based upon levels of income or assets.
Private Insurers. Some ESRD patients have private insurance that covers dialysis services. Healthcare providers, including our NxStage Kidney Care dialysis centers, receive reimbursement for ESRD treatments from the patient or private insurance during a waiting period of up to three months before the patient becomes eligible for Medicare. In addition, if the private payor is an employer group health plan, it is generally required to continue to make primary payments for dialysis services during the 30-month period following eligibility or entitlement to Medicare. In general, employers may not reduce coverage or otherwise discriminate against ESRD patients by taking into account the patient’s eligibility or entitlement to Medicare benefits. On average, private insurance pays significantly more for dialysis services than Medicare and these patients with private insurance are generally viewed as more profitable to dialysis service providers.
Critical Care
For Medicare patients, both acute kidney failure and fluid overload therapies provided in an in-patient hospital setting are reimbursed under a traditional Medicare severity diagnosis related group system. Under this system, reimbursement is determined based on a patient’s primary diagnosis and is intended to cover all of the hospital’s costs of treating the patient. The presence of acute kidney failure or fluid overload increases the severity of the primary diagnosis and, accordingly, could increase the amount reimbursed. Longer hospitalization stays and higher labor needs, which are typical for patients with acute kidney failure and fluid overload, must be managed for care of these patients to be cost-effective. We believe that there is a significant incentive for hospitals to find a more cost-efficient way to treat these patients in order to improve hospital economics for these therapies.
Reimbursement Outside of the U.S.
The use of our products outside the U.S. is similarly affected by reimbursement policies adopted by foreign regulatory agencies, government managed health care systems and private insurance. Reimbursement for the treatment of patients using medical devices in the EU Member States is governed by complex mechanisms established on a national level in each country. These mechanisms vary widely among the EU Member States. Moreover, these mechanisms evolve constantly, reflecting the efforts of these countries to reduce public spending on healthcare.
The rules on the coverage and reimbursement of medical devices outside the U.S. and EU vary widely from country to country and often from hospital to hospital. In addition, healthcare reform proposals and medical cost containment measures in
26
many foreign countries could, among other things, limit the use of our products and treatments in those countries and further reduce reimbursement available for such use or eliminate coverage altogether. These reforms or cost containment measures, including the uncertainty in the medical community regarding their nature and effect, could have an adverse effect on our customers’ purchasing decisions regarding our products and treatments within these regions, as well as limit the prices we may charge for our products.
Our Employees
As of December 31, 2016, we had approximately 3,400 employees, including full-time, part-time and temporary employees. From time to time we also employ independent contractors to support our engineering, marketing, sales, clinical and administrative organizations. Most of our employees are involved in the manufacture of our products and are employed outside of the U.S., with the significant majority employed in Mexico.
Corporate Information
We were incorporated in Delaware in 1998 under the name QB Medical, Inc., and later changed our name to NxStage Medical, Inc. Our principal executive offices are located at 350 Merrimack Street, Lawrence, Massachusetts 01843.
Where You Can Find More Information
Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 are available free of charge through our website (www.nxstage.com) under the “Investor Relations” subsection of the “Our Company” section of the site as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission (SEC). We are not including the information contained on our website as part of, or incorporating it by reference into, this Annual Report.
Executive Officers
Our executive officers as of February 28, 2017 were as follows:
Jeffrey H. Burbank, 54, is our Chief Executive Officer and a member of our Board of Directors and has served in these positions since he founded the company in December 1998. Mr. Burbank has over 30 years of in-depth management experience with companies developing, marketing and manufacturing products for ESRD patients. He has led NxStage since its inception, guiding it through all of its developmental phases to the successful initiation and rapid growth of commercial activities, its initial public offering, the acquisition of Medisystems Corporation, the evolution of the product line, and into services. Prior to founding NxStage, Mr. Burbank co-founded Vasca, Inc., a company providing innovative implantable access devices, where he was the President and Chief Executive Officer, as well as Chairman of the Board. He gained significant renal industry experience during his nine years in the Renal Division at Gambro, Inc., a medical technology company, with his last position as Director of Marketing and Advanced Technologies. During his career he has been an inventor on over 50 U.S. patents for medical devices. Mr. Burbank received his BS in Industrial Engineering from Lehigh University.
Robert S. Brown, 58, is the President of NxStage Kidney Care and has served in this position since June 2013. Before that, he served as our Senior Vice President, Chief Financial Officer and Treasurer since 2006. Mr. Brown has more than 20 years of financial experience. Prior to joining NxStage, Mr. Brown held several leadership positions in the financial group of Boston Scientific Corporation, a medical device company, including Vice President, Corporate Analysis & Control, where he and his team were responsible for the company’s financial, compliance, and operational audits and reported directly to the audit committee of the board of directors. Mr. Brown also served as Vice President, International at Boston Scientific where he was responsible for the financial functions of the company's $2.5 billion international division in over 40 countries. Previous experience also includes financial reporting and special projects at United Technologies, a high technology manufacturer, and public accounting and consulting at Deloitte & Touche, an auditing firm. Mr. Brown received his BBA from The University of Toledo and his MBA from The University of Michigan. He is also a Certified Public Accountant.
Winifred L. Swan, 52, is our Senior Vice President and General Counsel and has served in these positions since January 2005. Before that, she served as our Vice President and General Counsel since November 2000. Ms. Swan has over 20 years of legal expertise in medical devices. Prior to joining NxStage, Ms. Swan was Senior Corporate Counsel at Boston Scientific Corporation, a medical device company, where she focused on mergers and acquisitions and business development transactions. Before that, she was an associate at Goodwin Procter LLP, a law firm, specializing in corporate and securities law. Prior to law school, Ms. Swan served as a financial analyst in the Capital Markets Group of Merrill Lynch, a financial management and advisory company. Ms. Swan received a BA in Economics and Public Policy from Duke University and her JD from the University of Pennsylvania.
27
Matthew W. Towse, 54, is our Senior Vice President, Chief Financial Officer and Treasurer and has served in these positions since July 2013. He has also served as our Chief Accounting Officer since January 2015. Mr. Towse has over 25 years of experience in global financial management with both public and private companies across multiple industries. Prior to joining NxStage, he served as Vice President and Chief Financial Officer of Vette Corp., a venture-capital backed global design, engineering and manufacturing technology company from 2006 until its acquisition by a strategic buyer in April 2012. From 2003 to 2005, he served as Senior Vice President and Chief Financial Officer of Fairchild Semiconductor International, Inc. Previously, Mr. Towse served as Fairchild’s Vice President and Corporate Treasurer from 1997 to 2003, and held various financial positions with National Semiconductor from 1991 to 1997 before becoming part of the team that spunoff Fairchild from National. Earlier in his career, Mr. Towse was an audit manager with Ernst & Young LLP, an auditing firm, and most recently, he served as an Interim Chief Financial Officer with Tatum, LLC, an executive services firm. Mr. Towse is a Certified Public Accountant and received his BBA from the University of Notre Dame.
Joseph E. Turk, Jr., 49, is our President and has served in this position since November 2013. Before that, Mr. Turk served as our President of North American Operations from December 2010 to November 2013, Senior Vice President, Commercial Operations from January 2005 to December 2010 and Vice President, Sales and Marketing from May 2000 to January 2005. Mr. Turk brings a strong strategy and market development background to NxStage. Prior to joining NxStage, he served as Director of New Business Development at Boston Scientific Corporation, a medical device company. Before that, he was one of the leaders of the Midwest Health Care and Biotechnology Practice of McKinsey & Company, a management consulting firm, as a Senior Engagement Manager in the firm's Chicago office. Mr. Turk received an AB in Economics from Wabash College and his MBA in Marketing and Finance from Northwestern University's J.L. Kellogg School.
28
Item 1A. | Risk Factors |
We face a number of risks and uncertainties that are difficult to predict and many of which are outside of our control. In this section, we describe what we believe are the material risks to our business and future development. This is not an exhaustive list of risks affecting our business. There may be other risks that are not currently known to us or that we currently believe are immaterial but turn out to be material in the future. If any of these risks were to materialize, it could adversely affect our business, financial condition, results of operation, reputation and growth prospects, and cause actual results to differ materially from those projected in any of our forward-looking statements. In that case, the value of our common stock could decline substantially.
Investors should carefully consider the risk factors described below together with the other cautionary statements included in Management’s Discussion and Analysis of Financial Condition and Results of Operations and elsewhere in this Annual Report.
Risks Related to our Business
The home hemodialysis market may not expand sufficiently to support our growth prospects.
While we believe our largest growth opportunity with our existing products is within the home hemodialysis market, home hemodialysis therapies have not been extensively adopted. With our current technology, we believe that approximately 10-15% of end-stage renal disease patients in the U.S. would be appropriate candidates for home hemodialysis. However, only 2% of U.S. chronic dialysis patients receive hemodialysis treatments at home.
Our growth requires that we continue to shift patients’ and the medical community’s understanding and view of home hemodialysis, and will require further increases in the number of patients who adopt home hemodialysis from current levels, physicians who are willing to prescribe home hemodialysis, and dialysis centers that are willing to support home hemodialysis growth. Most dialysis centers presently do not have the infrastructure to support a significant home hemodialysis patient population, including the availability of home hemodialysis training nurses, and may not be motivated to invest in home hemodialysis programs due, in part, to certain Medicare reimbursement policies. We will need to continue to devote significant resources to expanding the home hemodialysis market, but these efforts ultimately may not be successful.
Medicare reimbursement policies may limit patient access to our home hemodialysis products.
Medicare regulations that, directly or indirectly, have a disproportionate impact on home hemodialysis therapy may limit patient access to our home hemodialysis products. In 2011, the Centers for Medicare and Medicaid Services implemented a prospective payment system for dialysis treatment. Under this prospective payment system, the Centers for Medicare and Medicaid Services makes a single bundled payment to the dialysis center for each dialysis treatment that covers all renal dialysis services, inclusive of home dialysis and most drugs frequently administered to dialysis patients. This payment system replaced the former system which paid centers a composite rate for a defined set of items and services, while paying separately for drugs, laboratory tests, and other services that were not included in the composite rate. A stated goal of the new prospective payment system was to encourage home dialysis. To date, this reimbursement structure has not had a positive impact on the adoption of home or more frequent hemodialysis or the price of our products. However, the prospective payment system has had a significant positive impact on the adoption of peritoneal dialysis as evidenced by the significantly increased rates of training for peritoneal dialysis. We believe this increased focus on peritoneal dialysis growth and peritoneal dialysis training has been to the detriment of home hemodialysis training rates, as home training resources, including home training nurses in particular, have been more devoted to peritoneal dialysis training, leaving less time for home hemodialysis training.
Medicare provides broad and well-established reimbursement in the U.S. for treating end-stage renal disease patients with hemodialysis three times a week. Most patients using the System One in the home, however, have been prescribed to dialyze more than three times per week to attain the clinical benefits of more frequent dialysis. Given the increased provider costs associated with providing more frequent dialysis, access to our home hemodialysis products will be impacted by whether dialysis centers receive or pursue adequate reimbursement for the additional dialysis treatments. Reimbursement for more frequent hemodialysis requires medical justification provided by the dialysis center based on information from the patient’s physician, which increases the center’s administrative burden. In addition, there is no national standard for what constitutes medical justification, thus reimbursement for more frequent hemodialysis varies due to differing Medicare contractor policies and center billing practices. Dialysis centers may be unwilling to support more frequent home hemodialysis in the absence of predictable Medicare reimbursement for additional treatments per week based on submitted claims for medical justification.
Currently, only four of the twelve Medicare contractor jurisdictions have issued formal local coverage determinations that describe medical justification for more frequent hemodialysis. In the remaining jurisdictions, medical justification is determined on a case-by-case basis. One Medicare contractor without such a local coverage determination, Noridian Healthcare Solutions, has issued a Medicare coverage article discussing considerations for hemodialysis frequency. Certain language in the coverage article is unclear or inconsistent with long-standing Medicare policy, including that reiterated in recent
29
Medicare payment rules, but it may suggest a limitation to coverage of more frequent hemodialysis, even with medical justification. In partnership with other provider, patient, and professional organizations, we have actively engaged Noridian and the Centers for Medicare and Medicaid Services on this question. Many clinics have reported that they have continued to bill, and be paid for, additional treatments under this article; however, certain clinics have chosen at least at this time not to bill for more frequent dialysis sessions and this has impacted new patient access at those clinics. It remains unclear how this article will be implemented and what its impact will be in the long term.
In October 2016, the Centers for Medicare & Medicaid Services issued its final rule to update the payment policies and rates under the end-stage renal disease prospective payment system for 2017. Among other things, the Centers for Medicare & Medicaid Services increased the home and self-dialysis training add-on payment and reiterated its policy of paying for appropriately medically justified hemodialysis treatments provided in excess of three treatments per week.
Measures to reduce healthcare costs may hurt our business.
Our customers are healthcare providers who depend upon reimbursement by government and commercial insurance payors for dialysis treatments. With a vast majority of U.S. patients with end-stage renal disease covered by Medicare, the Medicare reimbursement rate is an important factor in a customer’s decision to use the System One or our other products and limits the prices we may charge for our products. The Centers for Medicare and Medicaid Services issued the 2017 final rule for the end-stage renal disease prospective payment system, which increases the base reimbursement rate less than 1% over 2016 rates. Commercial insurance payors may also exert downward pressure on payment rates for dialysis services. A reduction in reimbursement rates for dialysis treatments may adversely affect our customers’ businesses and cause them to enact cost reduction measures that may include reducing the scope of their home hemodialysis programs.
In recent years, there have been numerous initiatives on the federal and state levels for comprehensive reforms affecting the availability of and reimbursement for healthcare services. For example, in 2010, comprehensive U.S. health care reform legislation was passed that had imposed a 2.3% excise tax on domestic sales of certain medical devices, including our products, which reduced our profitability. In December 2015, this tax was suspended for two years, but will continue to have a negative financial impact when it is imposed again starting in 2018, unless permanently suspended or repealed. Rising healthcare costs have also led many European and other foreign countries to adopt healthcare reform proposals and medical cost containment measures, including government-imposed industry-wide price reductions, mandatory pricing systems, reference pricing systems, and payors limiting access to treatments based on cost-benefit analysis. Any of these measures, including the uncertainty in the medical community regarding their nature and effect, could have an adverse effect on our customers’ purchasing decisions regarding our products and treatments, as well as limit the prices we may charge for our products. During 2017, we face uncertainty regarding potential federal legislative and administrative efforts to repeal, substantially modify or invalidate some or all provisions of recent U.S. health care reforms. Such changes may negatively impact our prospects for revenue growth, increase our costs or benefit our competitors. Absent additional clarity on the terms of any such changes, the impact on our business is uncertain.
We sell a limited number of products.
We derive most of our revenues from the sale or rental of the System One and the related products used with the System One, with the remainder of our revenues largely coming from the sale of a few key disposable products, including blood tubing sets and needles. Although we are working on initiatives that should diversify our future revenues, including a system for peritoneal dialysis and our NxStage Kidney Care dialysis centers, our present business continues to be exposed to risks that are concentrated in a small number of products. As a result, any event that adversely affects these products or the markets for these products could have a significant adverse impact on our business.
Our relationships with DaVita and Fresenius are important to our business.
DaVita and Fresenius collectively provide treatment to over two-thirds of U.S. dialysis patients and are our two largest customers. Sales to them have driven a large portion of our historical revenue growth. Any adverse change in either customer's ordering or clinical practices, including in response to the establishment of our NxStage Kidney Care dialysis centers, would have an adverse impact on our revenues. In addition, these large dialysis providers have significant purchasing power, and we may be required to grant them favorable pricing and other terms for our products that reduce our gross margins and have an adverse effect on our operating results.
Our home market agreements with DaVita and Fresenius are intended to support the continued expansion of patient access to home hemodialysis with the System One, but like all our agreements with home customers, these agreements are not requirements contracts, and they contain no minimum purchase volumes. Our home market agreement with DaVita extends through December 31, 2018, with monthly renewals thereafter unless terminated by either party with 30 days' prior notice. Our home market agreement with Fresenius continues to renew on a monthly basis unless we and Fresenius choose to modify the terms with an amendment or new agreement.
We may be unable to achieve or sustain profitable operations.
30
Since inception, we have incurred negative operating margins and losses every quarter. Currently, we have a significant accumulated deficit. Although we are working towards achieving positive net income for the full 2017 fiscal year, we continue to invest in our operations, in particular with respect to our product pipeline and NxStage Kidney Care dialysis centers, to drive future growth. Accordingly, we cannot ensure the extent or sustainability of our future profitability.
Our NxStage Kidney Care dialysis centers introduce significant new risks to our business.
As health care providers and participants in federal health care programs, our NxStage Kidney Care dialysis centers must comply with complex regulations that are, in some instances, new to our business, including:
• | Medicare and Medicaid payment rules, including coverage rules that limit the clinical circumstances under which payment will be made for more frequent dialysis treatments; |
• | anti-kickback and related laws prohibiting payments and other remuneration intended to influence the referral of health care business or selection of a provider; |
• | prohibitions on submitting false claims for government or commercial insurance reimbursement; |
• | laws regulating the use and disclosure of patient health information; and |
• | laws regulating the storage and administration of pharmaceuticals and medical devices. |
If we violate such laws and regulations, we may face criminal and civil sanctions, including fines and civil monetary penalties and exclusion from participation in Medicare, Medicaid and other government programs. If we are found to have submitted improper claims for reimbursement to the government or commercial insurers, we may also have to repay amounts received from government or commercial payors and pay additional damages and interest.
Joint ventures have become common vehicles within the dialysis services industry and are designed to improve the quality of care while managing healthcare costs by sharing clinical expertise, management experience and industry knowledge in an efficient manner. A few of our NxStage Kidney Care dialysis centers are structured as joint ventures in which physicians hold an interest. These physician owners may also provide medical director services and refer patients to our dialysis centers. There has been growing governmental scrutiny of joint ventures and other financial arrangements with physicians or physician groups. Although we seek to structure our joint ventures in compliance with all regulatory requirements, the applicable laws are broadly written, and it is often difficult to determine precisely how these laws will be applied in specific circumstances. Regulatory authorities may challenge our joint ventures on the grounds that they are intended to induce patient referrals and, if successful, may require that we restructure or terminate our joint ventures, repay to Medicare amounts received by them pursuant to any prohibited referrals and incur the types of penalties described in the preceding paragraph.
Our NxStage Kidney Care dialysis centers must maintain enrollment in the Medicare program in order to bill and receive payment for dialysis services provided to patients covered by Medicare and certain private insurers. Medicare enrollment requires, among other things, that a center successfully complete a certification process conducted by individual state agencies on behalf of the Centers for Medicare and Medicaid Services and that certification requirements be met on an ongoing basis. Our NxStage Kidney Care dialysis centers may be unable to obtain Medicare certification in a timely manner, if at all, or could lose certification upon resurvey if they are found to not meet applicable requirements.
Our NxStage Kidney Care dialysis centers provide us with valuable experience to better meet and anticipate the needs of both our customers and patients, and optimize our product technology. Our customers may, however, perceive these centers to be directly competing with their business which could, and may have already, negatively impact product sales.
We face competition from many sources.
The dialysis therapy market is mature and we face competition from many sources, including those that are listed in the section of this report entitled “Business - Our Competition.” Our competitors may have significant competitive advantages by:
• | offering products and services that are more widely recognized by physicians, patients and providers; |
• | offering broader product lines which enable them to offer a broader bundle of products; |
• | having significantly more financial and human resources, more established service and customer support infrastructures and spending more on product development and marketing; |
• | having more established sales forces and distribution channels; and |
• | having more established relationships with the providers of dialysis therapy, including Fresenius which is the world’s largest provider of dialysis services and products and may at any time reduce its promotion of our dialysis products to its dialysis patients in favor of other, including its own, dialysis products. |
Further consolidation within the highly competitive dialysis industry may exacerbate these risks.
31
Our in-center business is increasingly subject to pricing and other competitive pressures within the highly consolidated U.S. dialysis services industry. A meaningful portion of that business was lost when our needle purchase agreement with DaVita expired in December 2014, and we experienced reduced demand for our blood tubing sets from Baxter during 2016. While we believe our in-center products offer benefits over competing products, our customers often regard blood tubing sets and needles as commodities, and we are vulnerable to large changes in purchasing patterns for these products. Unless we can successfully demonstrate to customers the differentiating features of our blood tubing sets and needles, we may continue to be susceptible to pressures to reduce our product pricing and more vulnerable to reduction in sales of our blood tubing sets and needles.
As we attain greater commercial success, our competitors are likely to develop products that offer features and functionality similar to the System One and our other products. Improvements in existing competitive products or the introduction of new competitive products may make it more difficult for us to compete for sales, particularly if those competitive products demonstrate better reliability, convenience or effectiveness or are offered at lower prices.
The development of viable medical, pharmacological and technological advances in treating or preventing kidney failure may also limit the market for our products and services. While kidney transplantation is the treatment of choice for most patients with end-stage renal disease, it is not currently a viable treatment for most patients due to the limited number of donor kidneys, the high incidence of kidney transplant rejection and the higher surgical risk associated with older patients. This may change, however, with the development of new medications designed to reduce the incidence of kidney transplant rejection, progress in using kidneys harvested from genetically engineered animals as a source of transplants and other advances in kidney transplantation.
We need to maintain strong product reliability to grow our business.
We need to maintain strong reliability for our existing products to achieve our growth and profitability objectives. Poor product reliability could lead to customer dissatisfaction, adversely affect our reputation and revenues, and increase our service and distribution costs and working capital requirements. We also need to establish strong product reliability for all new products we offer. With new products, we are more exposed to risks relating to product quality and reliability until the manufacturing processes for these new products mature. From time to time, we may transition the manufacturing and supply of products and components to different suppliers or locations. As we make these changes, we are more exposed to risks relating to product quality and reliability until the manufacturing processes mature. Like all transitions of this nature, they could increase our costs in the near term.
We need to develop and commercialize new products to grow our business.
Our future growth requires that we develop and commercialize new products in a timely manner to address changing market requirements, such as our peritoneal dialysis system and next-generation critical care system. Otherwise, we may lose revenues or market share to our competitors, which may be difficult to regain. Developing innovative products and bringing them to market is a highly costly, lengthy and uncertain process, and we may experience delays in commercializing new products. Our efforts may not produce commercially viable products due to the many technological, regulatory, operational and other risks associated with product development, including:
• | the new product may not perform as intended or may have safety concerns; |
• | the FDA and other regulatory authorities may not approve the new product or the facilities in which it is manufactured in a timely manner or at all; |
• | payors may not reimburse the new product sufficiently or at all; |
• | competing products may be safer, more effective or easier to use; |
• | we may be unable to manufacture sufficient quantities of the new product for development or commercialization activities in a timely and cost-effective manner; and |
• | market demand for the new product may fall below expectations. |
General economic and financial market conditions may exacerbate our business risks.
32
Global macro-economic conditions and the world's financial markets remain susceptible to significant stresses, resulting in reductions in available credit and government spending, economic downturn or stagnation, foreign currency fluctuations and volatility in the valuations of securities generally. Our customers and distributors may respond to such economic pressures by reducing or deferring their capital spending or reducing staff. As a result, they may choose to rent rather than purchase our equipment or enter into other less-capital intensive purchase structures with us, which may reduce our cash flows, and have fewer personnel available to train new patients for home hemodialysis. Our international business is particularly vulnerable to global macro economic conditions. Furthermore, unfavorable changes in foreign exchange rates versus the U.S. dollar could increase our product costs, reducing our gross profit, or render our products overly expensive, reducing our revenues.
We may not effectively manage our growth.
Our business growth will strain our administrative and operational infrastructure unless we:
• | increase our manufacturing capacity to meet customer demand; |
• | expand our sales and marketing and on-going development capabilities; |
• | improve our information technology infrastructure, operational, financial and management controls and reporting systems and procedures; and |
• | manage the increased complexity and scope of our relationships with various partners, distributors, suppliers, manufacturers and other organizations. |
We may be unable to implement such changes in an efficient and timely manner, and in the process of expansion may discover deficiencies in our existing systems and controls.
We need to effectively manage our field equipment.
Our home market relies upon an equipment service swap model and, for some of our customers, an equipment rental model that requires us to effectively manage our System One and PureFlow SL field equipment. While a majority of our home market customers currently purchase, rather than rent, our equipment, this may change due to pressures within the healthcare industry to reduce capital spending and other factors. Increases in our rental or service swap equipment would increase our ongoing cash requirements to fund working capital. In addition, our gross margins may be negatively impacted if we have excess equipment deployed and unused in the field. If we are unable to successfully track, service and redeploy equipment, we could incur increased costs, realize increased cash requirements and have material write-offs of equipment. This would negatively impact our working capital requirements and future profitability.
We may be subject to litigation claims from time to time.
From time to time, we are threatened with individual actions involving our business, including without limitation products liability, employment, intellectual property, commercial and tort claims. The manufacture and marketing of medical devices, in particular, has an attendant risk of product liability claims. If any of our employees or products is found to have caused or contributed to injuries or deaths, we could be held liable for substantial damages. Any claims made against us could adversely affect our reputation and damage our position in the market. Claims can also be time consuming, distracting and expensive to defend and could result in a diversion of management and financial resources away from our primary business, in which case our business may suffer. Any investigation into alleged unlawful conduct could increase our expenses, damage our reputation and divert management time and attention from operating our business. While we maintain insurance at levels deemed adequate by management, future claims may exceed our insurance coverage or may not be covered by any insurance.
Acquiring or developing businesses, technologies or products may present new challenges.
In the course of evaluating growth opportunities, we may acquire or develop businesses, technologies or products, as we did in 2007 with the acquisition of Medisystems and in 2013 with the introduction of our NxStage Kidney Care dialysis centers. We may also devote resources to potential acquisitions that are never completed or may fail to realize the anticipated benefits of such efforts. There are substantial risks and uncertainties associated with any growth or change in business lines or strategy that may prevent us from realizing the anticipated benefits of such opportunities or adversely affect our business, including:
• | need for significant investment without assurance of success; |
• | potential disruption of our ongoing business; |
• | need for involvement of senior management to develop the acquired businesses, technologies or products, which will take away from the time they ordinarily spend on the remainder of our business; |
• | entry into markets or types of businesses in which we have limited experience; |
• | impairment of relationships with key partners, customers or suppliers of ours or any acquired business; |
33
• | addition of new complex compliance obligations; |
• | difficulty in managing geographically remote units both in the United States and internationally; |
• | difficulty in successfully implementing, upgrading and deploying in a timely and effective manner new operational information systems and upgrades of our finance, accounting and product distribution systems; |
• | difficulty in incorporating acquired technology and rights into our product and service offerings; |
• | unanticipated expenses and delays in completing acquired development projects and technology integration; |
• | difficulty in transitioning and integrating the operations and personnel of an acquired businesses, including with respect to differing and complex accounting and financial reporting systems; |
• | customers delaying purchases of our products pending resolution of product integration between our existing and our newly acquired products; |
• | loss of key employees of an acquired company; and |
• | inaccurate assumptions of an acquired company's product or service quality. |
Further, any acquired technology or product may require additional development efforts prior to commercial sale, including clinical testing and approval by the FDA and applicable foreign regulatory authorities. All technology and product candidates are prone to risks of failure typical of medical device product development, including the possibility that a product candidate will not be shown to be sufficiently safe and effective for approval by regulatory authorities.
We have international operations that introduce a number of risks and uncertainties.
Substantially all of our manufacturing is done outside the United States. We operate manufacturing facilities in Germany, Italy and Mexico, and purchase components, products and supplies from foreign vendors. We also sell our products internationally. We are subject to a number of risks and challenges that specifically relate to these international operations, including:
• | foreign exchange risk, in particular with respect to the euro and peso, which could adversely affect our financial results and our ability to maintain mutually beneficial and profitable relationships with foreign vendors, distributors and customers, and increase our costs to attract and retain international personnel; |
• | expropriation and other restrictive government actions; |
• | changes in intellectual property legal protections and remedies; |
• | costs and challenges associated with sourcing and shipping goods internationally and importing and exporting goods; |
• | changes to U.S. and foreign trade policies, including enactment of tariffs or border-adjusted taxes on goods imported into the U.S.; |
• | difficulty managing operations in multiple locations; |
• | local regulations that may restrict or impair our ability to conduct our operations, increase compliance costs, and make it more expensive and complex to manage our workforce; |
• | fluctuations in local economic conditions; |
• | health issues, such as pandemic disease risk, and natural disasters, such as flooding, hurricanes and earthquakes, which could disrupt our manufacturing and logistical and import activities; and |
• | in certain locations, risks associated with local instability, including threats of violence, which could lead to disruptions in supply at our manufacturing facilities or key vendors. |
These risks and uncertainties may materially impact our growth strategy in these markets and overall operating profits. Risks associated with our international operations may increase where we sell our products and services directly rather than through distributors, as we do in the United Kingdom and Canada.
During June 2016, the referendum by UK voters to exit the European Union ("Brexit") adversely impacted global markets and resulted in a sharp decline of the British pound sterling against our reporting currency, the US dollar. Continued volatility in or devaluation of the British pound sterling may adversely affect our results of operations by reducing our reported international sales and earnings and causing our UK customers to reduce their investment in healthcare. The further impact of Brexit on our international business will depend on any agreements the UK makes to retain access to EU markets. Although it is unknown what the terms of the UK’s future relationship with the EU will be, the imposition of greater restrictions on imports
34
and exports between the UK and EU countries and an increase in regulatory complexity could adversely affect our relationships with our customers, suppliers and employees in the UK.
Our In-Center and international businesses rely heavily upon third-party distributors.
Substantially all of our blood tubing sets and needles are sold through distributors. We also use distributors to sell our products in most of our international markets. Relying on third-party distributors exposes us to many risks, including competitive pressure, compliance risks, credit risk and concentration. Distributors may sell products that compete with our products, and we may be unable to motivate them to focus their efforts on selling our products. The trend toward consolidation among distributors may yield greater purchasing leverage, which may increase the pricing pressures facing our business. If our distributors fail to comply with applicable laws in the sale and marketing of our products or fulfill any other responsibilities they may have, our revenues may decline and we may become involved in legal proceedings. Distributors may face financial difficulties, including bankruptcy, which could harm our collection of accounts receivable and financial results. Moving any of this business to other distributors would involve switching costs that may be material in the near term.
We rely on the expertise of a concentrated group of employees.
Our success depends upon the skills, experience and efforts of our senior executives and other key personnel, including our research and development and manufacturing executives and managers. Much of our expertise is concentrated in relatively few employees, the loss of whom for any reason could negatively affect our business. Competition for our highly skilled employees is intense, and we cannot prevent the future resignation of any employee.
Risks Related to the Regulatory Environment
Our products and business are subject to extensive regulation.
We need regulatory approvals to market new products and, in some cases, modifications to existing marketed products. Regulatory approval pathways for medical devices are complex, time consuming and difficult to define, and they may become more onerous through additional regulation. We may be unable to obtain the necessary approvals to market our new products and modifications to marketed products in a timely manner, if at all. Foreign markets are particularly challenging as the regulatory approval procedure varies from country to country and requires that we comply with numerous regulatory requirements that differ from the FDA approval process and are not superseded by obtaining approval from the FDA or another country's regulatory authority. As these regulatory requirements become increasingly more stringent, it may become more difficult and costly for us to expand into new markets. In certain foreign markets, some of our products are classified as drugs rather than medical devices, which require us to demonstrate compliance with separate regulations applicable to drug manufacturers and distributors. These complex regulations may impose additional approval, manufacturing, surveillance and reporting requirements. Compliance with these additional requirements may increase our costs of doing business in new foreign markets and delay or prevent our entry into such markets.
Following marketing approval, we must comply with numerous ongoing regulatory requirements and industry codes of conduct and consensus standards, including those described in the section of this report entitled “Business - Government Regulation.” Noncompliance with applicable regulations can result in, among other things:
• | violation letters; |
• | fines, injunctions, and civil penalties; |
• | recall or seizure of products; |
• | administrative detention, which is the detention by regulatory authorities of medical devices believed to be adulterated or misbranded; |
• | operating restrictions, partial suspension or total shutdown of production; |
• | failure of the government to grant pre-market clearance or pre-market approval for devices; |
• | withdrawal of marketing clearances or approvals; and |
• | criminal prosecution. |
Such enforcement measures would require unanticipated expenditures to address or defend such actions and may adversely affect our business.
New regulations, codes and standards are periodically adopted which may require us to change our existing product technologies, operating procedures or marketing practices in order to continue selling our products. In addition, regulatory authorities have been increasingly aggressive in their enforcement activities and scrutiny of medical device and healthcare companies. Any of these factors may expose us to increased compliance costs and the assessment of significant fines, as well
35
as risks that we may be unable to satisfy the new regulations, codes or standards, or more expansive interpretations of existing regulations and have to suspend, curtail or otherwise modify our selling and marketing efforts and other aspects of our operations.
Our products may be recalled from the market.
Medical devices can experience performance problems in the field that require review and possible corrective action. The occurrence of component failures, manufacturing errors, software errors, design defects or labeling inadequacies affecting a medical device could lead to a government-mandated or voluntary recall by the device manufacturer, in particular when such deficiencies may endanger health. From time to time we have chosen to voluntarily recall certain products that we believed were mislabeled or otherwise defective. Product recalls may materially divert management attention and financial resources, expose us to product liability or other claims, harm our reputation with customers and adversely impact our financial results.
We need to protect the privacy of patient health and other personal information.
In the course of performing our business we obtain, from time to time, confidential patient health information and other personal information. Federal and state laws, as well as the laws of foreign countries, protect the confidentiality of certain patient health information, in particular individually identifiable information and other personal information, and restrict the use and disclosure of that information. A description of these laws is included in the section of this report entitled “Business - Government Regulation - Privacy and Security.” Complying with the privacy and security requirements of such laws imposes compliance related costs, subjects us to potential regulatory audits and may restrict our business operations. These various laws may be subject to varying interpretations by courts and government agencies, creating potentially complex compliance issues for our business. If we were to violate any of our legal obligations to safeguard any confidential patient health or other personal information against improper use and disclosure, we could lose customers and be exposed to liability, including potential civil and criminal penalties and contractual liabilities, and our reputation and business could be harmed. Concerns or allegations about our practices with regard to the privacy or security of personal health information or other privacy-related matters, even if unfounded, could damage our reputation and harm our business.
We must comply with fraud and abuse laws.
Various federal and state laws, as well as the laws of foreign countries, prohibit payments to induce the referral of healthcare products or services and require medical device companies to monitor and report certain payments to health care professionals. These anti-kickback, public reporting and aggregate spend laws affect our sales, marketing and other promotional activities by limiting the kinds of financial arrangements, including sales programs, we may have with hospitals, physicians or other potential purchasers or users, including patients, of medical devices and services. They also impose additional administrative and compliance burdens on us. In particular, these laws influence, among other things, how we structure our sales and rental offerings, including discount practices, customer support, education and training programs and physician consulting and other service arrangements. For our NxStage Kidney Care dialysis centers, they also affect our arrangements with any joint venture partners in a position to refer patients, our medical directors and our patient billing and collection practices. If we were to offer or pay inappropriate inducements to purchase, order or use our products or services, or to refer patients to our NxStage Kidney Care dialysis centers, we could be subject to a claim under the federal healthcare program Anti-Kickback Statute, the federal patient inducement prohibition or similar state laws. If we fail to comply with particular reporting requirements, we could be subject to penalties under applicable federal or state laws. A shifting and diverse regulatory environment increases the associated compliance risks since different jurisdictions may have different reporting requirements.
Other federal and state laws, as well as the laws of foreign countries, generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payments to government or commercial payors that are false or fraudulent, or for items or services that were not provided as claimed. Medical device manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by providing inaccurate billing or coding information to customers, by providing improper financial inducements, or through certain other activities. In addition, our NxStage Kidney Care dialysis centers are directly subject to these laws with respect to the reimbursement claims they file with government payors. Potential false or fraudulent claim risk can arise from promoting and billing for services the government deems excessive or not medically necessary, as well as from other billing improprieties and from failure to timely return any identified overpayments. We attempt to ensure that billing by our NxStage Kidney Care dialysis centers is proper and that physicians who order NxStage Kidney Care dialysis services document medical need for patients for whom more frequent than thrice weekly therapy is ordered. Nevertheless, the government may not regard any billing errors that may be made as inadvertent and may examine our role in providing information to our customers, physicians and patients concerning the benefits and potential coverage of more frequent therapy. Likewise, our financial relationships with customers, physicians, patients or others in a position to influence the purchase or use of our products may be subject to government scrutiny or be alleged or found to violate applicable fraud and abuse laws. False claims laws prescribe civil, criminal and administrative penalties for noncompliance, which can be substantial, and raise the possibility of exclusion from participation in government
36
health care programs, potentially crippling to the line of business involved. Moreover, any investigation into our practices could cause adverse publicity and require a costly and time consuming response.
Foreign governments tend to impose strict price controls.
We market the System One and certain of our other products internationally. In some foreign countries, particularly in the European Union, the pricing of medical devices is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after a device has been CE marked. To obtain reimbursement or pricing approval in some countries, we may be required to supply data that compares the cost-effectiveness of our products to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, it may not be profitable to sell our products outside of the United States, which would negatively affect the long-term growth of our business. Furthermore, reimbursement provided for our products in other jurisdictions could change, positively or negatively. If reimbursements were to be negatively changed, such as in the United Kingdom or Canada where we sell our products directly, our ability to profitably sell our products could be impaired.
We must comply with import and export laws and regulations.
We import into the United States disposable medical supplies from our manufacturing facilities and vendors located outside the United States. We have manufacturing facilities in Mexico, Germany and Italy and export various components and assemblies related to those operations. To a lesser but increasing degree, we also export finished goods from the United States to foreign countries. The import and export of these items are subject to extensive and complex laws and regulations. If we fail to comply with these laws or regulations, or fail to interpret our obligations accurately, we may be subject to significant fines, liabilities, import holds and a disruption in our ability to deliver product. The U.S. federal government has called for substantial changes to trade, fiscal and tax policies which may include comprehensive tax reform and changes to existing trade agreements, including but not limited to the North American Free Trade Agreement. Changes to capital and exchange controls, expropriation or other restrictive government actions could adversely affect our business. We also are subject to changes in tax and tariff regulations, both domestically and abroad that could increase our costs and reduce our margins. If there are modifications to the Generalized System of Preferences or cancellation of the Nairobi Protocol tariff classifications that apply to our products such that our products would be subject to duties, or if the United States imposes tariffs or border-adjusted taxes on imports to the United States of goods manufactured outside the United States, our expenses could increase and our profitability may be negatively impacted.
We must comply with anti-bribery laws.
We are subject to the U.S. Foreign Corrupt Practices Act which generally prohibits U.S. companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business and requires companies to maintain accurate books and records and internal controls, including at foreign controlled subsidiaries. Through our international activities, we are also subject to the UK Anti-Bribery Act and other similar anti-bribery laws in other countries. While we have policies and procedures in place designed to promote compliance with such laws, our employees or other agents may nonetheless engage in prohibited conduct under these laws for which we or our executives might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
We must comply with environmental and occupational safety laws.
Our research and development programs as well as our manufacturing operations involve the controlled use of hazardous materials, and our NxStage Kidney Care dialysis centers produce medical waste in connection with providing dialysis services. Accordingly, we are subject to federal, state and local laws, as well as the laws of foreign countries, governing the use, handling and disposal of these materials. In the event of an accident or failure to comply with environmental or occupational safety laws, we could be held liable for resulting damages, and any such liability could exceed our insurance coverage.
Our business may be affected by U.S. government contracting risks.
We have agreements with Veterans Health Administration facilities and are one of the key subcontractors on a government contract to develop a portable medical device to treat sepsis. As a result, we must comply with and are affected by laws and regulations relating to the award, administration and performance of U.S. government contracts which, among other things, impose additional costs on our business. If we violate any of these laws or regulations, we may be liable for fines, penalties and any additional costs the government incurs in procuring replacement services, and we may be excluded from future U.S. government contracting.
Risks Related to Operations
We obtain some of our raw materials and production services from a single source.
37
We depend upon a number of single-source suppliers for certain of our raw materials, components and finished goods, including the fiber used in our System One filters, our needles and sterile bags, as well as sterilization services. Some of our most critical single-source supply relationships are with Membrana and Laboratorios PiSA.
Membrana is our only supplier of the fiber used in our filters for System One products under an agreement that expires in December 2023, and contractually we cannot obtain an alternative source of fiber for our System One products. While our relationship with Asahi could afford us back-up supply in the event of supply disruptions at Membrana, we do not have the regulatory approvals necessary to use Asahi fiber in our System One cartridge in the United States, and the performance of Asahi fiber in our System One has not yet been validated.
Laboratorios PiSA supplies substantially all of our premixed dialysate. Our supply agreement with Laboratorios PiSA extends through December 2019. We have committed to purchase from Laboratorios PiSA a minimum quantity of premixed dialysate over the term of the agreement. While we purchase premixed dialysate from another qualified supplier, any significant disruption in Laboratorios PiSA’s ability to supply premixed dialysate to us would impair our business, at least in the near term.
Our dependence upon these and other single-source suppliers of raw materials, components, finished goods and sterilization services exposes us to several risks, including disruptions in supply, price increases, late deliveries and an inability to meet customer demand. This could lead to customer dissatisfaction, damage to our reputation or customers switching to competitive products. Any interruption in supply could be particularly damaging to our customers using the System One to treat chronic end-stage renal disease who need access to the System One and related disposables to continue their therapy.
Finding alternative sources for these raw materials, components, finished goods and sterilization services would be difficult and in many cases entail a significant amount of time, disruption and cost. Although we believe our supply chain has sufficient inventory of raw materials, components and finished goods to withstand a temporary disruption in supply from any single source supplier, any permanent or long-term disruption in supply from any single source supplier could lead to supply delays or interruptions which would damage our business and impair our reputation, at least in the near term.
We do not have long-term supply contracts with many of our third-party suppliers.
We purchase raw materials and components from third-party suppliers, including some single-source suppliers, through purchase orders and do not have long-term supply contracts with many of our suppliers. Many of our suppliers are not obligated to perform services or supply products for any specific period, in any specific quantity or at any specific price, except as may be provided in a particular purchase order. We do not maintain large volumes of inventory from most of our suppliers. If we inaccurately forecast demand for finished goods, we may be unable to meet customer demand which could harm our competitive position and reputation. In addition, if we fail to effectively manage our relationships with our suppliers, we may be required to change suppliers, which may be time consuming and lead to disruptions in our product supply.
We may experience manufacturing disruptions.
We rely on our manufacturing facilities in Mexico, Italy and Germany for the production of our equipment and disposables. The loss of any of these facilities due to fire, natural disaster, war, power failure or other cause beyond our control could cause significant production delays, prevent us from meeting customer demand for our products, increase our product costs, impair our product quality or reliability and result in substantially decreased revenues.
While we have labor agreements with our production employees in Mexico and Italy, we may experience strikes, work stoppages, work slowdowns, grievances, complaints, claims of unfair labor practices, other collective bargaining disputes or other labor disputes at our manufacturing facilities. Some of our key single-source suppliers also have labor agreements in place but nonetheless may be subject to similar risks related to labor disputes. Any such activity likely would cause production delays and prevent us from delivering our production commitments to customers, which could adversely affect our reputation and cause our business and operating results to suffer.
Commodity price increases may adversely affect our financial results.
Resin is a key material in the manufacture of our products, including the System One cartridge. We currently source resin from a small number of suppliers. Periods of rising prices for crude oil, natural gas and other petrochemical intermediates from which resin is produced have resulted in significant price increases for this material, and similar periods of rising resin prices may occur in the future. Our contracts with customers restrict our ability to immediately pass on these price increases, and future pricing to customers may be insufficient to accommodate increasing resin costs. In addition, our overall cost reduction plans may not sufficiently offset the impact of increased resin costs, which could result in declining margins and operating results.
We currently incur significant inbound and outbound distribution costs, which are dependent upon fuel prices. Increases in fuel prices could lead to increases in our distribution costs, which could impair our ability to achieve profitability.
Our business is dependent upon the security and uninterrupted operation of our information technology infrastructure.
38
We rely on information technology and telephone networks and systems, including the Internet, to process and transmit sensitive electronic information, including confidential patient health information, and to manage or support a variety of business processes and activities, including sales, billing, customer service, procurement and supply chain, manufacturing and distribution. We use enterprise information technology systems to record, process, and summarize financial information and results of operations for internal reporting purposes and to comply with regulatory, financial reporting, legal and tax requirements. Our information technology systems, some of which are managed by third-parties and are highly interconnected, may be susceptible to damage, disruptions or shutdowns due to computer viruses, attacks by computer hackers, failures during the process of installing, upgrading or replacing software, databases or components thereof, power outages, hardware failures, telecommunication failures, user errors or catastrophic events. In addition, these systems can require significant resources to ensure their continuous operation. Despite the precautionary measures we have taken to prevent breakdowns in our information technology and telephone systems, if our systems suffer severe damage, disruption or shutdown and we are unable to effectively resolve the issues in a timely manner, we may be subject to material remediation expenses, reputational harm and litigation.
Risks Related to Intellectual Property
We have to protect our intellectual property.
We rely on patent protection, as well as a combination of copyright, trade secret and trademark laws, to protect our proprietary technology and prevent others from duplicating our products. However, these means may afford only limited protection and may not:
• | prevent our competitors from duplicating our products; |
• | prevent our competitors from gaining access to our proprietary information and technology; or |
• | permit us to gain or maintain a competitive advantage. |
These risks may increase in foreign countries whose laws do not protect intellectual property rights effectively or to the same extent as U.S. laws.
Any of our patents, including those we may license, may be challenged, invalidated, rendered unenforceable or circumvented. We may not prevail if our patents are challenged by competitors or other third parties. The U.S. federal courts or equivalent national courts or patent offices elsewhere may invalidate our patents, find them unenforceable or narrow their scope. Furthermore, competitors may be able to design around our patents, or obtain patent protection for more effective technologies, designs or methods for treating kidney failure. If these developments were to occur, our products may become less competitive, and sales of our products may decline.
We have filed numerous patent applications seeking protection of products and other inventions originating from our research and development. Our patent applications may not result in an issued patent, and any patents that are issued may not provide meaningful protection against competitors or competitive technologies.
Our products could infringe the intellectual property rights of others.
The medical device industry has been characterized by extensive litigation and administrative proceedings regarding patent infringement and intellectual property rights. Products to provide kidney replacement therapy have been available for more than 50 years, and our competitors hold a significant number of patents relating to kidney replacement devices, therapies, products and supplies. Competitors and other third parties may allege that our products or methods infringe their patents or other intellectual property rights, and the possibility of such infringement claims may increase as our business expands into new markets.
Infringement and other intellectual property claims and proceedings brought against us, whether successful or not, could result in substantial costs and harm to our reputation. Such claims and proceedings can also divert management and key personnel from other tasks important to the success of the business. In addition, intellectual property litigation or claims could require us to:
• | cease selling or using any of our products that incorporate the asserted intellectual property, which would adversely affect our revenues; |
• | pay substantial damages for past use of the asserted intellectual property; |
• | obtain a license from the holder of the asserted intellectual property, which license may not be available on reasonable terms, if at all, and which could reduce profitability; and |
• | redesign or rename, in the case of trademark claims, our products to avoid infringing the intellectual property rights of third parties, which may not be possible and could be costly and time consuming, if it is possible to do so. |
39
Disclosure of trade secrets and other proprietary information may harm our business.
In order to protect our proprietary technology and processes, we rely in part on confidentiality agreements with our corporate partners, employees, consultants, outside scientific collaborators and sponsored researchers, advisors and others. These agreements may not effectively prevent disclosure of confidential information and trade secrets and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover or reverse engineer trade secrets and proprietary information, and in such cases we may be unable to assert any trade secret rights against such party. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive position.
Many of our employees have worked at other medical device companies focused on the development of dialysis products, including our competitors. We may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Even if we are successful in defending against these claims, litigation could result in substantial costs and harm to our reputation and be a distraction to management.
Risks Related to our Common Stock
Our stock price may fluctuate significantly.
There may be periods of volatility in the market price of our common stock that delay or prevent you from selling your common stock at or above the price you paid for it. Some of the factors that may cause the market price of our common stock to fluctuate include:
• | timing of market launch and market acceptance of our products; |
• | timing of achieving profitability from operations; |
• | changes in estimates of our financial results or recommendations by securities analysts or the failure to meet or exceed securities analysts' expectations; |
• | actual or anticipated variations in our quarterly operating results; |
• | future debt or equity financings; |
• | developments or disputes with key vendors or customers, or adverse changes to the purchasing patterns of key customers and distributors; |
• | disruptions in product supply for any reason, our failure to appropriately forecast supply or demand, difficulties in moving products across international borders, or the failure of third party suppliers to produce needed products or components; |
• | reports by officials or health, medical or regulatory authorities or the general media regarding the potential benefits of the System One, similar dialysis products distributed by other companies or more frequent or home dialysis; |
• | delays or failures to obtain marketing approval for new products or modifications to marketed products; |
• | product recalls and withdrawals; |
• | defaults under our material contracts, including without limitation our credit agreement; |
• | regulatory developments in the United States and foreign countries; |
• | changes in third-party healthcare reimbursements, particularly a decline in the level of Medicare reimbursement for dialysis treatments, or the willingness of Medicare contractors to pay for more than three treatments a week where medically justified; |
• | regulatory changes that could affect our profitability, such as the imposition of import tariffs and border-adjusted taxes; |
• | litigation involving our company or our industry; |
• | announcements of technical innovations or new products by our competitors; |
• | developments or disputes concerning our patents or other proprietary rights; |
• | our ability to manufacture and supply our products to commercial standards; |
• | significant acquisitions, strategic partnerships, joint ventures or capital commitments by us or our competitors; |
40
• | departures of key personnel; |
• | investors' general perception of our company, our products, the economy and general market conditions; and |
• | the other risks and uncertainties described in these “Risk Factors.” |
The stock markets in general have experienced substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations may adversely affect the trading price of our common stock. Periods of volatility in the market price of our securities may engender class action securities litigation against us. Such litigation, if instituted, could result in substantial costs and diversion of management attention and resources, which could significantly harm our profitability and reputation.
Provisions in our governing documents and under Delaware law may discourage potential acquisition proposals and changes in management that stockholders may favor.
Provisions in our charter and bylaws and under the corporation law of Delaware, where we are incorporated, may delay or prevent a takeover attempt that could be viewed as beneficial to stockholders who wish to receive a premium for their shares from a potential bidder. These provisions may also discourage stockholders from attempting to replace or remove members of our board of directors, which in turn may delay or prevent changes in our current management team that stockholders may favor. These provisions include:
• | a prohibition on stockholder actions by written consent; |
• | the ability of our board of directors to issue preferred stock without stockholder approval, which could be used to institute a “shareholders rights plan” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; |
• | advance notice requirements for nominations of directors or stockholder proposals; |
• | the requirement that board vacancies be filled by a majority of our directors then in office; and |
• | the prohibition on a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner. |
If we obtain additional financing for acquisitions and other growth initiatives, it may reduce the market value of our common shares.
As part of our growth strategy, we may acquire other businesses and technologies and pursue additional business opportunities. To finance such activity, we may issue equity securities, which may dilute our existing stockholders, and incur debt, which may place restrictions on our business operations. Such financing activity may reduce the market value of our common shares and other securities, in particular if the initiatives being funded are not viewed favorably by our stockholders or are ultimately unsuccessful. Additional financing may not be available on terms favorable to us, or at all, particularly in light of the volatility in the financial markets and the valuations of securities generally.
Item 1B. | Unresolved Staff Comments |
Not applicable.
Item 2. | Properties |
Our corporate headquarters are located at a facility in Lawrence, Massachusetts under a lease expiring in 2023 that covers up to 141,000 square feet. The facility is also used for research and development, general and administrative support functions, customer service and IT support services.
We have a 238,161 square foot manufacturing facility in Tijuana, Mexico under leases that begin to expire in 2019, subject to renewal upon written notification. This facility supports both our System One and In-Center segments.
We have a 36,300 square foot manufacturing facility in Modena, Italy, a majority of which we own with the remainder subject to leases that begin to expire in 2018. This facility supports our System One and In-Center segments.
We have a 12,369 square foot manufacturing and research and development facility in Rosdorf, Germany under a lease that expires in December 2017 and is subject to annual renewals. This facility principally supports our System One segment.
We operate a 50,110 square foot manufacturing facility owned by Asahi in Goettingen, Germany where we manufacturer products for our System One segment and for sale to Asahi.
41
We lease clinical and office space for our NxStage Kidney Care business under leases that expire between 2018 and 2026. Our facilities range in size from approximately 1,300 to 8,000 square feet, with an average size of approximately 5,300 square feet.
We believe that our existing facilities are adequate for our current needs and that suitable additional or alternative space will be available on commercially reasonable terms at such time as it becomes needed.
Item 3. | Legal Proceedings |
Not applicable.
Item 4. | Mine Safety Disclosures |
Not applicable.
42
PART II
Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
Our common stock is quoted on the NASDAQ Global Select Market under the symbol “NXTM”. The following table sets forth, for the periods indicated, the high and low sales prices of our common stock.
High | Low | ||
2016 | |||
First Quarter | $21.79 | $13.49 | |
Second Quarter | $21.69 | $14.23 | |
Third Quarter | $26.07 | $21.35 | |
Fourth Quarter | $26.83 | $21.11 | |
2015 | |||
First Quarter | $19.19 | $16.14 | |
Second Quarter | $19.63 | $14.14 | |
Third Quarter | $18.50 | $13.47 | |
Fourth Quarter | $22.60 | $14.91 | |
Holders
As of February 22, 2017, there were approximately 44 holders of record of our common stock.
Dividends
We have never paid or declared any cash dividends on our common stock. We anticipate that we will retain our earnings for future growth and therefore do not anticipate paying cash dividends in the foreseeable future. Our revolving line of credit with Capital One Financial Corporation and Silicon Valley Bank restricts our ability to pay cash dividends while borrowings are outstanding. For more information about this revolving line of credit, please see Note 8, Debt and Capital Lease Obligations, to our consolidated financial statements included in this Annual Report.
43
Comparative Stock Performance Graph
The graph below compares the five-year cumulative total stockholder return on our common stock, the Total Return Index for the NASDAQ Stock Market (U.S. Companies), which we refer to as the NASDAQ Composite Index, and the NASDAQ Medical Equipment Index assuming the investment of $100.00 on December 31, 2011 with dividends being reinvested. Measurement points are the last trading days of each quarter during such five-year period.
The stock price performance included in this graph is not necessarily indicative of future stock price performance.
44
Item 6. | Selected Financial Data |
SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated financial data should be read together with the information under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the notes to those consolidated financial statements included elsewhere in this Annual Report. The selected statements of comprehensive loss data for the years ended December 31, 2016, 2015 and 2014 and balance sheet data as of December 31, 2016 and 2015 set forth below have been derived from our audited consolidated financial statements included elsewhere in this Annual Report. The selected statements of comprehensive loss data for the years ended December 31, 2013 and 2012 and balance sheet data as of December 31, 2014, 2013 and 2012 set forth below have been derived from the audited consolidated financial statements for such years included in prior Annual Reports on Form 10-K.
Years Ended December 31, | |||||||||||||||||||
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||||||
(In thousands, except per share data) | |||||||||||||||||||
Statement of Comprehensive Loss Data: | |||||||||||||||||||
Revenues | $ | 366,378 | $ | 336,123 | $ | 301,501 | $ | 263,429 | $ | 242,132 | |||||||||
Cost of revenues | 214,393 | 204,652 | 185,598 | 160,926 | 149,324 | ||||||||||||||
Gross profit | 151,985 | 131,471 | 115,903 | 102,503 | 92,808 | ||||||||||||||
Operating expenses: | |||||||||||||||||||
Selling and marketing | 63,878 | 58,528 | 55,385 | 47,842 | 40,485 | ||||||||||||||
Research and development | 31,032 | 26,237 | 22,635 | 18,887 | 17,111 | ||||||||||||||
Distribution | 28,579 | 26,211 | 26,001 | 21,246 | 18,888 | ||||||||||||||
General and administrative | 32,781 | 35,124 | 34,069 | 32,326 | 27,530 | ||||||||||||||
Total operating expenses | 156,270 | 146,100 | 138,090 | 120,301 | 104,014 | ||||||||||||||
Loss from operations | (4,285 | ) | (14,629 | ) | (22,187 | ) | (17,798 | ) | (11,206 | ) | |||||||||
Other expense, net | (1,930 | ) | (554 | ) | (873 | ) | (1,008 | ) | (2,914 | ) | |||||||||
Net loss before income taxes | (6,215 | ) | (15,183 | ) | (23,060 | ) | (18,806 | ) | (14,120 | ) | |||||||||
Provision for (benefit from) income taxes | 1,102 | 1,077 | 1,253 | (245 | ) | 1,033 | |||||||||||||
Net loss | (7,317 | ) | (16,260 | ) | (24,313 | ) | (18,561 | ) | (15,153 | ) | |||||||||
Less: Net loss attributable to noncontrolling interests | (2,546 | ) | (918 | ) | (367 | ) | — | — | |||||||||||
Net loss attributable to stockholders of NxStage Medical, Inc. | $ | (4,771 | ) | $ | (15,342 | ) | $ | (23,946 | ) | $ | (18,561 | ) | $ | (15,153 | ) | ||||
Net loss per share, basic and diluted | $ | (0.07 | ) | $ | (0.24 | ) | $ | (0.39 | ) | $ | (0.31 | ) | $ | (0.26 | ) | ||||
Weighted-average shares outstanding, basic and diluted | 64,520 | 63,384 | 61,700 | 60,261 | 57,890 | ||||||||||||||
December 31, | |||||||||||||||||||
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||||||
(In thousands) | |||||||||||||||||||
Balance Sheet Data: | |||||||||||||||||||
Cash and cash equivalents | $ | 59,632 | $ | 59,065 | $ | 52,884 | $ | 84,134 | $ | 106,439 | |||||||||
Working capital | 95,639 | 86,163 | 84,395 | 108,513 | 122,235 | ||||||||||||||
Total assets | 317,207 | 306,874 | 309,726 | 306,962 | 311,949 | ||||||||||||||
Long-term liabilities | 65,874 | 70,393 | 73,415 | 74,594 | 75,126 | ||||||||||||||
Accumulated deficit | (407,601 | ) | (402,830 | ) | (387,488 | ) | (363,542 | ) | (344,981 | ) | |||||||||
Total stockholders’ equity | $ | 202,023 | $ | 193,520 | $ | 191,555 | $ | 194,761 | $ | 197,591 | |||||||||
Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
Overview
45
The results of our operations are included in three separately reportable segments, System One, In-Center, and Services. Other business activities relates to the manufacturing of dialyzers for sale to Asahi, and research and development and general and administrative expenses that are excluded from the segment operating performance measures. We refer to our System One segment, In-Center segment, and Other category as our products business. In the System One segment we derive our revenues from the sale and rental of the System One and PureFlow SL dialysate preparation equipment and the sale of disposable products in the home and critical care markets. Home is devoted to the treatment of ESRD patients in the home or a home-like setting, while critical care is devoted to the treatment of hospital-based patients with acute kidney failure or fluid overload. In the In-Center segment, we derive our revenues from the sale of blood tubing sets and needles for hemodialysis primarily for the treatment of ESRD patients at dialysis centers, and needles for apheresis. Our Services segment includes revenues from dialysis services provided to patients at our NxStage Kidney Care dialysis centers.
Financial Performance
The following table summarizes our consolidated results (in thousands, except percentages):
Twelve Months Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
Products Business (System One Segment, In-Center Segment & Other) | |||||||||||
Revenues | $ | 359,127 | $ | 332,845 | $ | 300,598 | |||||
Gross profit | $ | 169,389 | $ | 146,188 | $ | 123,011 | |||||
Gross margin percentage | 47 | % | 44 | % | 41 | % | |||||
Income (Loss) from operations | $ | 22,695 | $ | 9,197 | $ | (7,261 | ) | ||||
Services Segment | |||||||||||
Revenues | $ | 14,781 | $ | 6,412 | $ | 1,749 | |||||
Gross profit | $ | (16,657 | ) | $ | (14,717 | ) | $ | (7,108 | ) | ||
Gross margin percentage | n/a | n/a | n/a | ||||||||
Loss from operations | $ | (26,233 | ) | $ | (23,826 | ) | $ | (14,926 | ) | ||
Eliminations | |||||||||||
Elimination of intersegment revenues | $ | (7,530 | ) | $ | (3,134 | ) | $ | (846 | ) | ||
Elimination of intersegment gross profit | $ | (747 | ) | $ | — | $ | — | ||||
Total Company | |||||||||||
Revenues | $ | 366,378 | $ | 336,123 | $ | 301,501 | |||||
Gross profit | $ | 151,985 | $ | 131,471 | $ | 115,903 | |||||
Gross margin percentage | 41 | % | 39 | % | 38 | % | |||||
Loss from operations | $ | (4,285 | ) | $ | (14,629 | ) | $ | (22,187 | ) | ||
For several years, we have focused on operating and financial improvements. During 2016 and 2015, these efforts resulted in revenues increasing by 9% to $366.4 million and by 11% to $336.1 million, respectively, versus prior years, with sales in the home and critical care markets principally driving the growth. Driving continued improvements will remain an area of focus in 2017 and beyond within our products business. At the same time, we expect to continue investing in our Services segment, which we expect will have a negative impact on our total operating performance in the near term and may offset performance improvements we expect in our products business.
Statement of Comprehensive Loss Components
Revenues
In the System One segment we derive our revenues from the sale and rental of equipment and the sale of disposable products in the home and critical care markets. In the home market, dialysis center customers purchase or rent the System One equipment, including cycler and PureFlow SL, and then purchase the related disposable products based on a specific patient prescription. In the critical care market, we sell or rent the System One and sell related disposables to hospital customers. In the In-Center segment, we derive our revenues from the sale of needles and blood tubing sets. Nearly all of our sales in the In-Center segment are through supply and distribution contracts with distributors. Our Services segment includes revenues from dialysis services provided to patients at our NxStage Kidney Care dialysis centers.
In the home market, the majority of our revenues are derived from recurring sales of disposable products. For customers that purchase the System One, we recognize revenues from the equipment sale ratably over the expected service obligation period which is typically five to seven years. In the case of certain skilled nursing facility and in-center settings, revenues may be recognized over a one year period. For customers that rent the System One, we recognize revenue on a monthly basis. We recognize revenues related to the disposable products upon delivery. Over time, as more home patients are treated with the
46
System One and more systems are placed in patient homes, we expect to derive a growing recurring revenue stream from the sale of related disposables.
Our contracts with dialysis centers in the home market for ESRD home dialysis patients generally include terms providing for the sale of disposable products to accommodate up to the number of prescribed treatments per month per patient and the purchase or monthly rental of System One cyclers and, in most instances, our PureFlow SL equipment. These contracts typically have a term of one to seven years, and may be renewed on a month-to-month basis thereafter, subject to a 30-day termination notice. Under these contracts, if home hemodialysis is prescribed, supplies are shipped directly to patient homes and paid for by the treating dialysis center. We also include vacation delivery terms, providing for the shipment of products to a designated vacation destination for a specified number of vacation days. We derive a small amount of revenues from the sale of supplementary products and services such as equipment maintenance and service fees, ancillaries, reserve inventory and special deliveries.
In the critical care market we recognize revenues upon delivery in accordance with contract terms. Our contracts with hospitals generally include terms providing for the sale of our System One equipment and disposables, although we also provide an equipment rental option. These contracts typically have a term of one year. We derive a small amount of revenues from the sale of one- and two-year service contracts following the expiration of our standard one-year warranty period for System One equipment. To further support service in the critical care market, we have a bio-medical training program, whereby we train bio-medical engineers on how to service and repair certain aspects of the System One in the critical care market. Bio-medical training is typically provided under a two-year contract following the expiration of our standard one-year warranty period for System One equipment. As more System One equipment is placed within hospitals, we expect to continue to derive a growing recurring revenue stream from the sale of disposable cartridges and fluids as well as, to a much lesser degree, from service and bio-medical training contracts.
In the In-Center segment nearly all sales to end users are structured through supply and distribution contracts with several significant distributors; however, in many instances we have direct contractual relationships with our end user customers. These contracts typically contain minimum volume commitments with negotiated pricing triggers at different volume tiers. Revenues are recognized upon delivery in accordance with contract terms.
The majority of our revenues have been generated from sales to customers in the U.S. We sell our System One and certain of our other products internationally through direct sales in the UK and Canada and through distributors in other countries. We recognize revenues from equipment sales to our international distributors at the time of shipment or, if applicable, delivery in accordance with contract terms. Disposable product revenues are recognized upon delivery. We also manufacture and sell dialyzers to Asahi and recognize revenues at the time of shipment in accordance with contract terms.
Revenues in our Services segment are derived from dialysis care services provided to patients at our NxStage Kidney Care dialysis centers.
We offer certain distributors rebates based on sales to specific end users. Our revenues are presented net of these rebates. For our System One segment, as of December 31, 2016, we had $5.4 million reserved against trade accounts receivable for future distributor rebates and recorded $14.0 million, $11.1 million and $8.5 million during 2016, 2015 and 2014, respectively, as a reduction of revenues in connection with distributor rebates. For our In-Center segment, as of December 31, 2016, we had $3.2 million reserved against trade accounts receivable for future estimated distributor rebates and recorded $7.4 million, $7.6 million, and $8.2 million during 2016, 2015 and 2014, respectively, as a reduction of revenues in connection with rebates.
Cost of Revenues
Cost of revenues consists primarily of direct product costs, material and labor required to manufacture our products, service of System One equipment that we sell or rent to customers and manufacturing overhead. It also includes the cost of servicing, repairing and inspecting System One equipment prior to sale or during the warranty period, patient care costs at our NxStage Kidney Care dialysis centers and stock-based compensation for certain personnel. The cost of our products depends on several factors, including the efficiency of our manufacturing operations, the cost at which we can obtain labor and products from third-party suppliers, product reliability and related servicing costs and the design of our products.
Operating Expenses
Selling and Marketing. Selling and marketing expenses consist primarily of salary, benefits and stock-based compensation for sales, marketing and business development personnel, travel, promotional and marketing materials and other expenses associated with providing clinical training to our customers. Included in selling and marketing are the costs of clinical educators, usually nurses, we employ to teach our customers about our products and prepare our customers to instruct their patients and their partners in the operation of our products, customer service and technical support personnel. Also included in this category are the personnel and other costs associated with our market development activities to establish, develop and operate our NxStage Kidney Care dialysis centers, including administrative support functions directly related to the startup and support of this initiative.
47
Research and Development. Research and development expenses consist primarily of salary, benefits and stock-based compensation for research and development personnel, supplies, materials and expenses associated with product design and development, clinical studies, regulatory submissions, reporting and compliance and expenses incurred for outside consultants or firms who furnish services related to these activities.
Distribution. Distribution expenses include the freight costs of delivering our products to our customers or our customers’ patients, depending on the market and the specific agreements with our customers, salary, benefits and stock-based compensation for distribution personnel and the cost of any equipment lost or damaged in the distribution process. We use common carriers and freight companies to deliver our products and do not operate our own delivery service. Also included in this category are the expenses of shipping products under warranty from customers back to our service center for repair and the related expense of shipping a replacement product to our customers or their patients.
General and Administrative. General and administrative expenses consist primarily of salary, benefits and stock-based compensation for our executive management, legal and finance and accounting staff, fees of outside legal counsel, fees for our annual audit and tax services and general expenses to operate the business, including insurance and other corporate-related expenses. Also included in general and administrative expenses, beginning in 2013, are tax expenses incurred related to the medical device excise tax.
Comparison of Years Ended December 31, 2016 and 2015
Revenues
Our revenues for 2016 and 2015 were as follows (in thousands, except as percentages of revenues):
Years Ended December 31, | |||||||||||||
2016 | 2015 | ||||||||||||
System One segment | |||||||||||||
Home | $ | 208,586 | 57 | % | $ | 182,572 | 54 | % | |||||
Critical Care | 75,337 | 21 | % | 65,203 | 20 | % | |||||||
Total System One segment | 283,923 | 78 | % | 247,775 | 74 | % | |||||||
In-Center segment | 63,038 | 17 | % | 74,768 | 22 | % | |||||||
Other | 12,166 | 3 | % | 10,302 | 3 | % | |||||||
Products subtotal | 359,127 | 98 | % | 332,845 | 99 | % | |||||||
Services segment | 14,781 | 4 | % | 6,412 | 2 | % | |||||||
Elimination of intersegment revenues | (7,530 | ) | (2 | )% | (3,134 | ) | (1 | )% | |||||
Total | $ | 366,378 | 100 | % | $ | 336,123 | 100 | % | |||||
Home product revenues increased $26.0 million, or 14%, for 2016 versus 2015, driven primarily by the increase in the number of patients prescribed to use the System One both in the U.S. and internationally, offset in part by unfavorable foreign currency fluctuations. We expect future demand for our products and revenue growth in the home market to be strong as we further penetrate this market, both in the U.S. and internationally, and leverage the annuity nature of our business. We further expect that our System One segment revenues will be susceptible to fluctuations in equipment sales, changes in inventory levels at our international distributors and changes in currency exchange rates.
Critical Care product revenues increased $10.1 million, or 16%, for 2016 versus 2015, driven by higher sales of System One disposables and equipment. We expect future demand for our products and revenue growth to be strong as we seek to further penetrate this market and leverage the annuity nature of our business. However, sales of our System One equipment in critical care may fluctuate due to timing of sales and the overall capital spending environment of our customers.
In-Center segment revenues decreased $11.7 million, or 16%, for 2016 versus 2015, due to lower demand for our blood tubing sets from Baxter offset in part by increased blood tubing set sales and needle sales to other customers, and variations in inventory management policies at both our distributors and end users. We anticipate In-Center segment revenues will remain consistent in 2017 compared to 2016.
Other revenues for 2016 and 2015 relate to dialyzers sold to Asahi. The increase in revenues was due to increased volume. Sales to Asahi may fluctuate due to timing of sales, inventory management policies at Asahi and changes in currency exchange rates.
48
Service segment revenues for 2016 and 2015 relate to dialysis services provided to patients at our NxStage Kidney Care dialysis centers. We expect future revenues to increase modestly, but may fluctuate in the near term based on payor mix and the timing of certain payments.
Gross Profit (Loss)
Our gross profit (loss) for 2016 and 2015 were as follows (in thousands, except as percentages of revenues):
Years Ended December 31, | |||||||||||
2016 | 2015 | ||||||||||
System One segment | $ | 149,770 | 53 | % | $ | 127,495 | 51 | % | |||
In-Center segment | 18,115 | 29 | % | 20,278 | 27 | % | |||||
Subtotal | 167,885 | 48 | % | 147,773 | 46 | % | |||||
Other | 1,504 | n/a | (1,585 | ) | n/a | ||||||
Products subtotal | $ | 169,389 | 47 | % | 146,188 | 44 | % | ||||
Services segment | (16,657 | ) | n/a | (14,717 | ) | n/a | |||||
Elimination of intersegment gross profit | (747 | ) | n/a | — | n/a | ||||||
Gross profit | $ | 151,985 | 41 | % | $ | 131,471 | 39 | % | |||
Gross profit as a percentage of revenues for the System One segment improved versus 2015 primarily driven by contractual price improvements, currency exchange rates and product mix, offset in part by increased service costs. We expect to sustain gross profit as a percentage of revenues in our System One segment above 50% as we continue to work to lower costs through process improvements, increase volume and improve our manufacturing operations.
Gross profit as a percentage of revenues for the In-Center segment increased versus 2015 driven primarily by favorable product mix and currency exchange rates. We expect gross profit as a percentage of revenues will decrease as a result of lower volumes and changes in product mix.
The Other category relates to costs associated with the manufacturing of dialyzers for sale to Asahi, which should provide us with long term cost efficiencies through increased dialyzer production volumes. In the first half of 2016, we received reimbursements from Asahi for $0.7 million related to additional startup costs incurred in 2015 with the build out of the manufacturing facility in Germany which was recorded as a reduction of cost of revenues.
The negative gross profit as a percentage of revenues incurred by our Services segment was driven by costs associated with the startup and support of our NxStage Kidney Care dialysis centers; however, the margin percentage improved versus 2015 due to continued revenue growth. We expect the Services segment gross margin will continue to be negatively impacted by costs associated with the development and operation of our NxStage Kidney Care dialysis centers, coupled with the impact of payor mix and the timing of certain payments.
In aggregate, total company gross profit as a percentage of revenues will be negatively impacted by costs associated with our continued investment in our Services segment.
Selling and Marketing
Our selling and marketing expenses and selling and marketing expenses as a percentage of revenues for 2016 and 2015 were as follows (in thousands, except percentages):
Years Ended December 31, | |||||||||||
2016 | 2015 | ||||||||||
System One segment | $ | 47,969 | 17 | % | $ | 43,607 | 18 | % | |||
In-Center segment | 6,333 | 10 | % | 5,812 | 8 | % | |||||
Products subtotal | 54,302 | 15 | % | 49,419 | 15 | % | |||||
Services segment | 9,576 | n/a | 9,109 | n/a | |||||||
Total Selling and marketing | $ | 63,878 | 17 | % | $ | 58,528 | 17 | % | |||
Selling and marketing expenses increased $5.4 million, or 9%, for 2016 versus 2015 but remained relatively consistent as a percentage of revenues.
49
Selling and marketing expenses for the System One segment increased due to increased personnel and personnel-related costs but decreased slightly as a percentage of revenues due to our ability to continue to leverage our infrastructure. Selling and marketing for the In-Center segment increased as a percentage of revenue primarily driven by lower revenues.
Selling and marketing expenses for our Services segment increased $0.5 million, for 2016 versus 2015 due to increased expenses for personnel and other costs associated with our market development activities to establish, develop and operate our NxStage Kidney Care dialysis centers, including administrative support functions directly related to the startup and support of this initiative.
We anticipate that selling and marketing expenses will continue to increase but remain relatively consistent as a percentage of revenues in the near term.
Research and Development
Our research and development expenses for 2016 and 2015 were as follows (in thousands, except percentages):
Years Ended December 31, | |||||||||||
2016 | 2015 | ||||||||||
Research and development | $ | 31,032 | 8 | % | $ | 26,237 | 8 | % | |||
Research and development expenses increased $4.8 million, or 18% for 2016 versus 2015. The increase was primarily due to increased personnel and personnel-related costs and increased project related spending.
For the near term, we expect research and development expenses will increase but remain consistent as a percentage of revenues as we seek to further develop and enhance the System One, invest in our peritoneal dialysis program and next-generation critical care system to expand our product portfolio.
Distribution
Our distribution expenses for 2016 and 2015 were as follows (in thousands, except percentages):
Years Ended December 31, | |||||||||||
2016 | 2015 | ||||||||||
System One segment | $ | 26,889 | 9 | % | $ | 24,140 | 10 | % | |||
In-Center segment | 1,690 | 3 | % | 2,071 | 3 | % | |||||
Total Distribution | $ | 28,579 | 8 | % | $ | 26,211 | 8 | % | |||
Distribution expenses increased $2.4 million, or 9%, for 2016 versus 2015 driven mainly by higher shipment volumes in the System One segment; however, it has remained relatively consistent as a percentage of revenues in both segments. We expect that distribution expenses will remain consistent as a percentage of revenues at least in the near term.
General and Administrative
Our general and administrative expenses for 2016 and 2015 were as follows (in thousands, except percentages):
Years Ended December 31, | |||||||||||
2016 | 2015 | ||||||||||
General and administrative | $ | 32,781 | 9 | % | $ | 35,124 | 10 | % | |||
General and administrative expenses decreased by $2.3 million, or 7%, for 2016 versus 2015. The decrease was primarily due to the suspension of the medical device tax, offset by increased personnel related costs, and professional service fees. We expect general and administrative expenses as a percentage of revenues to decline modestly compared to prior periods as we continue to leverage our infrastructure.
Other Expense
Interest expense decreased $0.1 million for 2016 versus 2015. Interest expense includes interest costs and other fees related to our debt obligations, including capital leases.
50
Other expense, net includes foreign currency gains and losses and during 2015 included $0.7 million in realized gains recognized from corporate equity instruments designated as short term trading securities.
Provision for Income Taxes
The provision for income taxes of $1.1 million during both 2016 and 2015 relates to the profitable operations of certain foreign subsidiaries.
Comparison of Years Ended December 31, 2015 and 2014
Revenues
Our revenues for 2015 and 2014 were as follows (in thousands, except percentages):
Years Ended December 31, | |||||||||||||
2015 | 2014 | ||||||||||||
System One segment | |||||||||||||
Home | $ | 182,572 | 54 | % | $ | 157,822 | 52 | % | |||||
Critical Care | 65,203 | 20 | % | 55,609 | 19 | % | |||||||
Total System One segment | 247,775 | 74 | % | 213,431 | 71 | % | |||||||
In-Center segment | 74,768 | 22 | % | 78,885 | 26 | % | |||||||
Other | 10,302 | 3 | % | 8,282 | 3 | % | |||||||
Products subtotal | 332,845 | 99 | % | 300,598 | 100 | % | |||||||
Services segment | 6,412 | 2 | % | 1,749 | — | % | |||||||
Elimination of intersegment revenues | (3,134 | ) | (1 | )% | (846 | ) | — | % | |||||
Total | $ | 336,123 | 100 | % | $ | 301,501 | 100 | % | |||||
Home product revenues increased $24.8 million, or 16%, during 2015 compared to 2014. This increase was driven primarily by the increase in the number of patients prescribed to use the System One both in the U.S. and internationally.
Critical Care product revenues increased $9.6 million, or 17%, during 2015 compared to 2014, driven by higher sales of System One disposables and equipment.
In-Center segment revenues decreased $4.1 million, or 5%, during 2015 compared to 2014, driven by lower needle sales consistent with the expiration of DaVita's needle purchase agreement in December 2014 offset in part by increased blood tubing set sales and needle sales to other customers.
Other revenues for 2015 and 2014 relate primarily to dialyzers sold to Asahi. The increase in revenues was due to increased volume, partially offset by unfavorable changes in currency exchange rates.
Service segment revenues for 2015 and 2014 relate to dialysis services provided to patients at our NxStage Kidney Care dialysis centers.
Gross Profit (Loss)
Our gross profit (loss) for 2015 and 2014 were as follows (in thousands, except percentages):
Years Ended December 31, | |||||||||||
2015 | 2014 | ||||||||||
System One segment | $ | 127,495 | 51 | % | $ | 102,420 | 48 | % | |||
In-Center segment | 20,278 | 27 | % | 20,765 | 26 | % | |||||
Subtotal | 147,773 | 46 | % | 123,185 | 42 | % | |||||
Other | (1,585 | ) | n/a | (174 | ) | n/a | |||||
Products subtotal | 146,188 | 44 | % | 123,011 | 41 | % | |||||
Services segment | (14,717 | ) | n/a | (7,108 | ) | n/a | |||||
Gross profit | $ | 131,471 | 39 | % | $ | 115,903 | 38 | % | |||
Gross profit as a percentage of revenues for the System One segment improved versus 2014 primarily driven by favorable product mix, favorable currency exchange rate changes versus the U.S. Dollar, decreased costs and contractual price improvements, offset in part by increased service costs.
51
Gross profit as a percentage of revenues for the In-Center segment increased versus 2014 driven primarily by unfavorable product mix, partially offset by favorable currency exchange rates and cost improvements.
The Other category relates to costs associated with the manufacturing of dialyzers for sale to Asahi, which should provide us with long term cost efficiencies through increased dialyzer production volumes.
The negative gross profit as a percentage of revenues incurred by our Services segment was driven by costs associated with starting up our NxStage Kidney Care dialysis centers.
Selling and Marketing
Our selling and marketing expenses for 2015 and 2014 were as follows (in thousands, except percentages):
Years Ended December 31, | |||||||||||
2015 | 2014 | ||||||||||
System One segment | $ | 43,607 | 18 | % | $ | 41,763 | 20 | % | |||
In-Center segment | 5,812 | 8 | % | 5,804 | 7 | % | |||||
Products subtotal | 49,419 | 15 | % | 47,567 | 16 | % | |||||
Services segment | 9,109 | n/a | 7,818 | n/a | |||||||
Total Selling and marketing | $ | 58,528 | 17 | % | $ | 55,385 | 18 | % | |||
Selling and marketing expenses increased $3.1 million, or 6%, during 2015 compared to 2014 but remained consistent as a percentage of revenues.
Selling and marketing expenses for the System One and In-Center segments increased due to increased personnel and personnel-related costs but decreased as a percentage of revenues in the System One segment due to our ability to continue to leverage our infrastructure.
Selling and marketing expenses for our Services segment increased $1.3 million versus 2014 due to increased expenses for personnel and other costs associated with our market development activities to establish, develop and operate NxStage Kidney Care dialysis centers, including administrative support functions directly related to the startup and support of this initiative.
Research and Development
Our research and development expenses for 2015 and 2014 were as follows (in thousands, except percentages):
Years Ended December 31, | |||||||||||
2015 | 2014 | ||||||||||
Research and development | $ | 26,237 | 8 | % | $ | 22,635 | 8 | % | |||
Research and development expenses increased $3.6 million, or 16%, but remained consistent as a percentage of revenues during 2015 compared to 2014. The increase was primarily due to increased personnel and personnel-related costs and increased project related spending.
Distribution
Our distribution expenses for 2015 and 2014 were as follows (in thousands, except percentages):
Years Ended December 31, | |||||||||||
2015 | 2014 | ||||||||||
System One segment | $ | 24,140 | 10 | % | $ | 23,855 | 11 | % | |||
In-Center segment | 2,071 | 3 | % | 2,146 | 3 | % | |||||
Total Distribution | $ | 26,211 | 8 | % | $ | 26,001 | 9 | % | |||
Distribution expenses increased $0.2 million, or 1%, during 2015 compared to 2014 driven mainly by higher shipment volumes; however, it remained relatively consistent as a percentage of revenues during 2015 compared to 2014 in both segments.
General and Administrative
Our general and administrative expenses for 2015 and 2014 were as follows (in thousands, except percentages):
52
Years Ended December 31, | |||||||||||
2015 | 2014 | ||||||||||
General and administrative | $ | 35,124 | 10 | % | $ | 34,069 | 11 | % | |||
General and administrative expenses increased $1.1 million, or 3%, but remained relatively consistent as a percentage of revenues during 2015 compared to 2014. The increase in general and administrative expenses was due to increased personnel and personnel-related costs and other related infrastructure costs.
Other Income (Expense)
Interest expense increased $0.3 million during 2015 compared to 2014. Interest expense includes interest costs and other fees related to our debt obligations, including our capital lease obligations.
Other income (expense), net includes foreign currency gains and losses and during 2015 includes $0.7 million realized gains recognized from corporate equity instruments designated as short term trading securities.
Provision for Foreign Income Taxes
The provision for income taxes of $1.1 million during 2015 and $1.3 million during 2014 relates to the profitable operations of certain foreign subsidiaries.
Liquidity and Capital Resources
We have operated at a loss since our inception in 1998. As of December 31, 2016, our accumulated deficit was $407.6 million and we had cash and cash equivalents of $59.6 million, with substantially all of that cash located in the U.S., and working capital of $95.6 million.
We expect to continue to generate positive cash flow. We believe, based on current projections and the current nature of our business, that we have the required resources to fund our ongoing operating requirements, which include selling and marketing activities to increase public awareness of the System One, our research and development activities to develop new products and enhance our existing products, and our continued investments in our existing NxStage Kidney Care dialysis centers. If we determine that additional investment in these or any other initiatives would be beneficial, we may choose to access the credit or capital markets to provide additional liquidity. However, we may not be successful in securing such additional financing.
Our ongoing cash requirements include funding normal working capital needs including inventory and field equipment assets as well as funding the losses from our NxStage Kidney Care dialysis centers. Field equipment assets include System One equipment rented to customers in the home market and our "service pool" of equipment, which is equipment owned and maintained by us that is swapped for equipment owned or rented by our customers that needs repair or maintenance. While a majority of our home market customers have committed to purchase, rather than rent, the significant majority of their future System One equipment requirements, any excess rental or service swap equipment would increase our working capital requirements.
We have a revolving credit facility with Capital One Financial Corporation and Silicon Valley Bank that allows for borrowing up to $35 million and expires in June 2019. Availability of credit is subject to a borrowing base that is calculated with reference to certain of our accounts receivable, inventory and equipment, and adjustments to such borrowing base are at the discretion of the lenders. The revolving credit facility requires that we comply with certain covenants while borrowings are outstanding, contains events of default customary for an agreement of this type and is secured by substantially all of our assets. As of December 31, 2016, there were no outstanding borrowings under the revolving credit facility, we were in compliance with all applicable covenants and, subject to the lenders’ adjustments described above, we had approximately $28 million of credit commitment available for borrowing.
We have several smaller loans with original principal amounts totaling $2.4 million with annual interest rates of approximately 2.0% to 6.0%, payable over a period of five to ten years secured by certain assets.
We maintain post-employment benefit plans for employees in certain foreign subsidiaries. The plans provide lump sum benefits, payable based on statutory regulations for voluntary or involuntary termination. Where required, we obtain an annual actuarial valuation of the benefit plans. We have recorded a liability of $1.7 million at December 31, 2016 and 2015, for costs associated with these plans. The expense recorded in connection with these plans was not significant during 2016, 2015 or 2014.
The following table sets forth the components of our cash flows for the periods indicated (in thousands):
53
Years Ended December 31, | |||||||
2016 | 2015 | ||||||
(In thousands) | |||||||
Net cash provided by operating activities | $ | 4,587 | $ | 10,542 | |||
Net cash used in investing activities | (9,361 | ) | (10,861 | ) | |||
Net cash provided by financing activities | 5,275 | 7,333 | |||||
Foreign exchange effect on cash and cash equivalents | 66 | (833 | ) | ||||
Net cash flow | $ | 567 | $ | 6,181 | |||
Net cash provided by operating activities. Net cash flows from operating activities decreased by $6.0 million during 2016, versus 2015 driven by higher working capital requirements including growth in inventory to support increased volume and timing of accounts receivable collections, offset by improved net loss after adjustments for non-cash items such as depreciation and amortization and stock-based compensation and timing of payments to our vendors. We expect working capital to fluctuate due to various factors including inventory requirements and the timing of certain payments from our customers and to our vendors.
Cash flow from deferred revenues improved by $0.1 million during 2016 as compared to 2015. Amortization of deferred revenues into revenues relating to sales of home equipment was $18.2 million during 2016 and $17.3 million during 2015.
Net cash used in investing activities. For each of the periods above, net cash used in investing activities reflected purchases of property and equipment, primarily for the build-out of NxStage Kidney Care dialysis centers coupled with expenditures for our manufacturing facilities as a result of our efforts to maintain and expand our manufacturing operations, along with purchases of equipment for research and development and information technology. For 2016, cash used in investing activities also includes $0.5 million related to our 2015 acquisition of controlling interest in a dialysis center.
The decrease of $2.7 million in purchases of property and equipment was driven primarily by decreased spending associated with our NxStage Kidney Care dialysis centers and our manufacturing facilities. Capital expenditures for our NxStage Kidney Care centers were $3.7 million, and $4.7 million during 2016 and 2015, respectively.
Net cash provided by financing activities. During 2016 and 2015, we received $6.0 million and $6.7 million, respectively, of net cash flows from stock plan activities. Proceeds from stock incentive plans are subject to fluctuation based primarily on the number of options exercised and, to a lesser extent, the weighted-average exercise price. During 2016 and 2015, we received $1.3 million and $0.9 million, respectively, in investments by noncontrolling interest holders. During 2015, we received $1.3 million in proceeds from a term loan. Cash provided by financing activities during both 2016 and 2015 was mainly reduced by cash used to pay our capital lease obligations.
Off-Balance Sheet Arrangements
We do not have any significant off-balance sheet arrangements.
Contractual Obligations
The following table summarizes our contractual commitments as of December 31, 2016 and the effect those commitments are expected to have on liquidity and cash flow in future periods (in thousands):
Payments Due By Period | |||||||||||||||||||
Total | Less Than One Year | 1-3 Years | 3-5 Years | More Than 5 Years | |||||||||||||||
Capital lease obligations, including interest | $ | 14,380 | $ | 1,872 | $ | 3,294 | $ | 2,544 | $ | 6,670 | |||||||||
Operating leases | 24,592 | 4,995 | 9,402 | 6,805 | 3,390 | ||||||||||||||
Debt, including interest | 1,833 | 407 | 815 | 444 | 167 | ||||||||||||||
Purchase obligations | 49,770 | 30,646 | 18,188 | 936 | — | ||||||||||||||
Total | $ | 90,575 | $ | 37,920 | $ | 31,699 | $ | 10,729 | $ | 10,227 | |||||||||
Our capital lease obligations include our capital lease obligation due to Asahi related to a manufacturing facility in Germany along with $5.9 million representing the estimated residual value of the manufacturing facility in Germany at the end of the estimated lease term which only becomes due and payable at Asahi's option if we terminate our agreement with Asahi.
Our purchase obligations include minimum purchase commitments under agreements with certain of our suppliers, primarily for the purchase of fluids for our System One segment and needles for our In-Center segment, and purchase orders to
54
purchase goods or services both of which are in the normal course of business. Certain of these commitments may be extended or canceled at our option.
The contractual commitments included in the table above do not include post-employment benefit obligations and unrecognized tax benefits. We maintain post-employment benefit plans for employees in certain foreign subsidiaries and may be required to make cash outlays related to the settlement of these obligations. However, the timing of such cash outlays is uncertain. Please see Note 13, Employee Benefit Plan to our consolidated financial statements included in this Annual Report for further details. We may be required to make cash outlays related to our unrecognized tax benefits. However, we are unable to make reasonably reliable estimates of the period of cash settlement, if any, with the respective taxing authorities. Please see Note 11, Income Taxes to our consolidated financial statements included in this Annual Report for further details.
Summary of Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these consolidated financial statements requires us to make significant estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. These items are regularly monitored and analyzed by management for changes in facts and circumstances, and material changes in these estimates could occur in the future. Changes in estimates are recorded in the period in which they become known. We base our estimates on historical experience and various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ substantially from our estimates.
Note 2, Summary of Significant Accounting Policies to our consolidated financial statements included in this report describes the significant accounting policies used in the preparation of our consolidated financial statements. A summary of those accounting policies and estimates that we believe are most critical to fully understanding and evaluating our financial results is set forth below. This summary should be read in conjunction with our consolidated financial statements and the related notes included elsewhere in this report.
Revenue Recognition
We recognize revenue from product sales and services when earned. Revenues are recognized when: (a) there is persuasive evidence of an arrangement, (b) the product has been shipped or services and supplies have been provided to the customer, (c) the sales price is fixed or determinable and (d) collection is reasonably assured.
Certain agreements with distributors allow for product returns and credits. For shipment of product sold to distributors, revenue is recognized at the time of sale if a reasonable estimate of future returns or credit can be made. If a reasonable estimate of future returns or credit cannot be made, we recognize revenue using the "sell-through" method. Under the "sell-through" method, revenue and related costs of revenue is deferred until the final resale of such products to end customers.
In addition to contractually determined volume discounts, in many agreements we offer rebates based on sales to specific end customers and discounts for early payment. Rebates and discounts are recorded as a reduction of sales and trade accounts receivable, based on our best estimate of the amount of probable future rebate or discount on current sales.
We enter into multiple-element arrangements that may include a combination of equipment, related disposables and services. Revenue arrangements with multiple elements are divided into separate units of accounting if specified criteria are met, including whether the delivered element has stand-alone value to the customer, the consideration received is allocated among the separate units based on their respective selling price, and the applicable revenue recognition criteria are applied to each of the separate units.
We determine selling price using vendor specific objective evidence ("VSOE"), if it exists, otherwise third-party evidence of selling price is used. If neither VSOE nor third-party evidence of selling price exists for a unit of accounting, we use best estimated selling price ("BESP"). We generally expect that we will not be able to establish third-party evidence due to the nature of our products and the markets in which we compete, and, as such, we typically will determine selling price using VSOE or BESP.
We determine BESP for an individual element based on consideration of both market and Company-specific factors, including the selling price and profit margin for similar products, the cost to produce the deliverable and the anticipated margin on that deliverable and the characteristics of the varying markets in which the deliverable is sold.
System One Segment
We derive revenue in the home market from the sales of hemodialysis therapy to customers in which the customer either purchases or rents the System One and/or PureFlow SL hardware and purchases a specified number of disposable products and service.
55
For customers that purchase the System One and PureFlow SL hardware, in the U.S. home market, due to the depot service model whereby equipment requiring service is picked up and a replacement device is shipped to the site of care, we recognize fees received from equipment sale as revenue on a straight-line basis over the expected term of our remaining service obligation and direct costs relating to the delivered equipment are deferred and amortized over the same expected period as the related revenue. Disposable products revenue is recognized on a monthly basis upon delivery.
Under the rental arrangements, revenue is recognized on a monthly basis in accordance with agreed upon contract terms and pursuant to binding customer purchase orders and fixed payment terms.
Our sales arrangements with our international distributors are structured as direct product sales and have no significant post-delivery obligations with the exception of standard warranty obligations. Revenue from direct product sales is recognized upon delivery in accordance with contract terms.
In the critical care market, we structure sales of the System One and disposable products as direct product sales and have no significant post-delivery obligations with the exception of standard warranty obligations. Revenue from direct product sales is recognized upon delivery in accordance with contract terms. Certain of these arrangements provide for training, technical support and extended warranty services to our customers. We recognize training and technical support revenue when the related services are performed. In the case of extended warranty, the service revenue is recognized ratably over the extended warranty period.
In-Center Segment
Our In-Center segment sales are structured as direct product sales primarily through distributors, and we have no significant post delivery obligations with the exception of standard warranty obligations. Revenue from direct product sales is recognized upon delivery in accordance with contract terms. Some of our distribution contracts for the In-Center segment contain minimum volume commitments with negotiated pricing discounts at different volume tiers. Each agreement may be canceled upon a material breach, subject to certain curing rights, and in many instances minimum volume commitments can be reduced or eliminated upon certain events.
Services Segment
Revenues in our Services segment are derived from dialysis care services provided to patients at our NxStage Kidney Care dialysis centers.
Revenues are recognized based on a customary fee schedule, net of estimated contractual allowances to reflect the estimated amounts to be received from the payor. Revenues are recognized in the period in which services are provided when we have the ability to reasonably estimate amounts ultimately collectible from the payor. In instances where we do not have the ability to reasonably estimate amounts ultimately collectible, as is often the case with non-contracted commercial health plans and amounts due from patients (including co-pay and deductible amounts), revenue is recognized in the period in which cash is received.
Inventory Valuation
Inventory is stated at the lower of cost, determined using the first-in first-out method (FIFO), or market (net realizable value). We write down the carrying value of inventory for estimated obsolescence when warranted by an amount equal to the difference between the cost of inventory and the estimated market value based on assumptions of future demand and remaining shelf-life. We also review our inventory value to determine if it reflects the lower of cost or market based on factors such as inventory items sold at negative gross margins and purchase commitments. The medical device industry is characterized by rapid development and technological advances as well as regulatory and quality manufacturing guidelines that could result in obsolescence of inventory. Additionally, our estimates of future product demand may prove to be inaccurate.
Field Equipment
Field equipment consists of equipment being utilized under disposable-based rental agreements as well as “service pool” equipment. Service pool equipment is equipment owned and maintained by us that are swapped for equipment that need repairs or maintenance by us while being rented or owned by a customer. We continually monitor the number of cyclers in the service pool, as well as cyclers that are in-transit or otherwise not being used by a patient, and assess whether there are any indicators of impairment for such equipment. We also review field equipment carrying value for reasonableness. We consider factors such as actual equipment disposals and our ability to verify the equipment’s existence in the field to identify lost equipment. We review the estimated useful life of our field equipment periodically for reasonableness and make changes when appropriate. Factors considered in determining the reasonableness of the useful life include expected future design improvements, equipment age and actual equipment disposals.
Accounting for Stock-Based Awards
56
Stock-based compensation expense is estimated as of the grant date based on the fair value of the award and is recognized as expense over the requisite service period, which generally equals the vesting period, based on the number of awards that are expected to vest. Estimating the fair value for stock options requires judgment, including the expected term of our stock options, volatility of our stock, expected dividends, risk-free interest rates over the expected term of the options and the expected forfeiture rate. In connection with our performance based programs, we make assumptions principally related to the number of awards that are expected to vest after assessing the probability that certain performance criteria will be met.
Valuation of Intangibles and Other Long-Lived Assets
Long-lived assets, including intangible assets, are evaluated for recoverability whenever events or circumstances indicate that their carrying value may not be recoverable. Factors we consider important include, but are not limited to, significant underperformance relative to historical or projected future results, significant negative industry factors and significant changes in strategy or operations that negatively affect the utilization of our long-lived assets. The evaluation of the impairment of long-lived assets, other than goodwill, is based on expectations of non-discounted future cash flows compared to the carrying value of the long-lived asset groups. If the sum of the expected non-discounted future cash flows is less than the carrying amount of the long-lived assets, we would recognize an impairment loss if the carrying amount of the asset group exceeds its fair value. Our expected non-discounted future cash flows are based upon cash flow projections and, if appropriate, include assumed proceeds upon sale of the asset group at the end of the cash flow period. We believe our procedures for developing cash flow projections, including the estimated sales proceeds, are reasonable and consistent with current market conditions for each of the dates when impairment testing has been performed. The cash flow projections that are used contain our best estimates, using appropriate and customary assumptions and projections at the time. If the cash flow projections or the significant operating assumptions upon which they are based change in the future, we may be required to record additional impairment charges.
Goodwill
We assess goodwill for impairment annually in the fourth quarter and whenever events or circumstances indicate impairment may exist. This test includes first a qualitative assessment and then, if necessary, a quantitative assessment to determine if the fair value of a reporting unit is less than its carrying amount. Our System One, In-center and Services reporting units contain goodwill of $41.1 million, $0.5 million and $1.0 million, respectively. Factors considered in the qualitative assessment include, but are not limited to, both macroeconomic conditions and entity-specific conditions. For the quantitative assessment the reporting unit's fair value is estimated using a discounted cash flow or other fair value measurement. Assessing the impairment of goodwill requires us to make assumptions and judgments including the identification of reporting units and determination of the fair value of our reporting units based on estimates of future cash flows and the selection of discount rates. Changes in these estimates and assumptions could materially affect the estimated fair values of reporting units and could result in a goodwill impairment charge in a future period. However, our annual impairment testing indicated no impairment.
Accounting for Income Taxes
We periodically assess our income tax positions and record tax benefits for all years subject to examination based upon our evaluation of the facts, circumstances and information available at the reporting date. If our judgment as to the likely resolution of the position changes, if the matter is ultimately settled or if the statute of limitation expires, the effects of the change would be recognized in the period in which the change, resolution or expiration occurs.
We conduct business globally and file income tax returns in the U.S. federal jurisdiction, various states and foreign jurisdictions. We estimate our income taxes in each of the jurisdictions in which we operate. This process involves estimating our current tax expense, including assessing the risks associated with tax positions, together with assessing temporary and permanent differences resulting from differing treatment of items for tax and financial reporting purposes. We evaluate the need for valuation allowances on our deferred tax assets based on positive and negative evidence about our ability to realize deferred tax attributes. Our estimates can vary due to the profitability mix of jurisdictions, foreign exchange movements, changes in tax law, regulations or accounting principles, as well as certain discrete items. In the event that actual results differ from our estimates or we adjust our estimates in the future, we may need to increase or decrease income tax expense, which could have a material impact on our financial position and results of operations.
Recent Accounting Pronouncements
A discussion of recent accounting pronouncements is included in Note 2, Summary of Significant Accounting Policies to our consolidated financial statements in this Annual Report.
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
Interest Rate Risk
Our interest rate risk is currently limited to our investments. However, this risk is mitigated given our investment portfolio currently consists of treasury obligations money market funds. We manage our investment portfolio in accordance with our
57
investment policy. The primary objectives of our investment policy are to preserve principal, maintain a high degree of liquidity to meet operating needs and obtain competitive returns subject to prevailing market conditions. Our investment policy specifies the credit quality standards for our investments and limits the amount of exposure from any single issue, issuer or type of investment.
Foreign Currency Exchange Risk
Our functional currency is the U.S. dollar and a majority of our revenues and expenses are denominated in U.S. dollars. However, we have manufacturing, service and distribution operations in foreign locations. In addition, we purchase products for resale in the U.S. from foreign companies and have agreed to pay them in currencies other than the U.S. dollar, including the euro. Furthermore, certain revenues are denominated in other currencies, primarily the British pound, Canadian dollar and euro. As a result, we are potentially exposed to adverse as well as beneficial movements in foreign currency exchange rates. For example, a hypothetical 10% adverse change in exchange rates could have the net effect of reducing our operating profit by approximately $0.7 million.
We utilize natural hedges to minimize our transaction exposures. Also, certain of our long-term supply agreements include foreign exchange risk sharing at different exchange rate levels limiting our exposure to fluctuations in foreign exchange rates for our purchase commitment exposures. Finally, we enter into foreign exchange forward contracts on peso and euro denominated expenses to further reduce our exposure to foreign currency exchange rate fluctuations from our foreign manufacturing and service operations located in Mexico and Europe.
Our foreign exchange forward contracts are entered into with large financial institutions and have durations of up to twelve months. These contracts are designated as cash flow hedges intended to offset the effect of exchange rate fluctuations on forecasted manufacturing and service costs. The effective portion of changes in the fair value of derivatives designated and qualifying as cash flow hedges is recorded in accumulated other comprehensive loss and is subsequently reclassified into earnings in the period in which the hedged forecasted transaction affects earnings. As of December 31, 2016, the notional amount of our outstanding contracts that are designated as cash flow hedges was approximately $18.6 million. Based on our analysis, a hypothetical adverse foreign exchange rate movement of 10% against our contracts would have resulted in a net loss in fair value of these contracts of approximately $2.0 million.
58
Item 8. | Financial Statements and Supplementary Data |
NXSTAGE MEDICAL, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
59
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Shareholders of NxStage Medical, Inc.
We have audited the accompanying consolidated balance sheets of NxStage Medical, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of comprehensive loss, changes in stockholders' equity and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of NxStage Medical, Inc. at December 31, 2016 and 2015, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2016, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), NxStage Medical, Inc.'s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 28, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Boston, Massachusetts
February 28, 2017
60
NXSTAGE MEDICAL, INC.
CONSOLIDATED BALANCE SHEETS
December 31, | |||||||
2016 | 2015 | ||||||
(In thousands, except share data) | |||||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 59,632 | $ | 59,065 | |||
Accounts receivable, net | 32,286 | 25,195 | |||||
Inventory | 46,845 | 38,391 | |||||
Prepaid expenses and other current assets | 6,136 | 6,254 | |||||
Total current assets | 144,899 | 128,905 | |||||
Property and equipment, net | 61,561 | 66,711 | |||||
Field equipment, net | 22,309 | 20,744 | |||||
Deferred cost of revenues | 33,165 | 33,068 | |||||
Intangible assets, net | 9,688 | 11,744 | |||||
Goodwill | 42,648 | 42,710 | |||||
Other assets | 2,937 | 2,992 | |||||
Total assets | $ | 317,207 | $ | 306,874 | |||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 14,177 | $ | 10,767 | |||
Accrued expenses | 30,985 | 27,266 | |||||
Current portion of long-term debt | 328 | 315 | |||||
Other current liabilities | 3,770 | 4,394 | |||||
Total current liabilities | 49,260 | 42,742 | |||||
Deferred revenues | 49,001 | 51,362 | |||||
Long-term debt | 1,305 | 1,664 | |||||
Other long-term liabilities | 15,568 | 17,367 | |||||
Total liabilities | 115,134 | 113,135 | |||||
Commitments and contingencies (Note 10) | |||||||
Noncontrolling interests subject to put provisions | 50 | 219 | |||||
Stockholders’ equity: | |||||||
Undesignated preferred stock: par value $0.001 per share, 5,000,000 shares authorized; no shares issued and outstanding as of December 31, 2016 and 2015 | — | — | |||||
Common stock: par value $0.001 per share, 100,000,000 shares authorized; 65,883,026 and 64,873,038 shares issued as of December 31, 2016 and 2015, respectively | 65 | 64 | |||||
Additional paid-in capital | 631,219 | 612,487 | |||||
Accumulated deficit | (407,601 | ) | (402,830 | ) | |||
Accumulated other comprehensive loss | (6,101 | ) | (4,031 | ) | |||
Treasury stock, at cost: 936,360 and 822,059 shares as of December 31, 2016 and 2015, respectively | (16,184 | ) | (13,864 | ) | |||
Total NxStage Medical, Inc. stockholders’ equity | 201,398 | 191,826 | |||||
Noncontrolling interests not subject to put provisions | 625 | 1,694 | |||||
Total stockholders’ equity | 202,023 | 193,520 | |||||
Total liabilities and stockholders’ equity | $ | 317,207 | $ | 306,874 | |||
See accompanying notes to these consolidated financial statements.
61
NXSTAGE MEDICAL, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
Years Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
(In thousands, except per share data) | |||||||||||
Revenues | $ | 366,378 | $ | 336,123 | $ | 301,501 | |||||
Cost of revenues | 214,393 | 204,652 | 185,598 | ||||||||
Gross profit | 151,985 | 131,471 | 115,903 | ||||||||
Operating expenses: | |||||||||||
Selling and marketing | 63,878 | 58,528 | 55,385 | ||||||||
Research and development | 31,032 | 26,237 | 22,635 | ||||||||
Distribution | 28,579 | 26,211 | 26,001 | ||||||||
General and administrative | 32,781 | 35,124 | 34,069 | ||||||||
Total operating expenses | 156,270 | 146,100 | 138,090 | ||||||||
Loss from operations | (4,285 | ) | (14,629 | ) | (22,187 | ) | |||||
Other (expense) income: | |||||||||||
Interest expense, net | (1,000 | ) | (1,115 | ) | (863 | ) | |||||
Other (expense) income, net | (930 | ) | 561 | (10 | ) | ||||||
(1,930 | ) | (554 | ) | (873 | ) | ||||||
Net loss before income taxes | (6,215 | ) | (15,183 | ) | (23,060 | ) | |||||
Provision for income taxes | 1,102 | 1,077 | 1,253 | ||||||||
Net loss | (7,317 | ) | (16,260 | ) | (24,313 | ) | |||||
Less: Net loss attributable to noncontrolling interests | (2,546 | ) | (918 | ) | (367 | ) | |||||
Net loss attributable to stockholders of NxStage Medical, Inc. | $ | (4,771 | ) | $ | (15,342 | ) | $ | (23,946 | ) | ||
Net loss per share, basic and diluted | $ | (0.07 | ) | $ | (0.24 | ) | $ | (0.39 | ) | ||
Weighted-average shares outstanding, basic and diluted | 64,520 | 63,384 | 61,700 | ||||||||
Other comprehensive loss, net of tax | (2,070 | ) | (1,839 | ) | (2,404 | ) | |||||
Total comprehensive loss | (9,387 | ) | (18,099 | ) | (26,717 | ) | |||||
Less: Comprehensive loss attributable to noncontrolling interests | (2,546 | ) | (918 | ) | (367 | ) | |||||
Total comprehensive loss attributable to stockholders of NxStage Medical, Inc. | $ | (6,841 | ) | $ | (17,181 | ) | $ | (26,350 | ) | ||
See accompanying notes to these consolidated financial statements.
62
NXSTAGE MEDICAL, INC
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
Accumulated | ||||||||||||||||||||||||||||||||||
Other | Total NxStage | Noncontrolling | ||||||||||||||||||||||||||||||||
Additional | Comprehensive | Medical, Inc. | Interest | Total | ||||||||||||||||||||||||||||||
Common Stock | Paid-In | Accumulated | Income | Treasury | Stockholders' | Not Subject to | Stockholders’ | |||||||||||||||||||||||||||
Shares | Amount | Capital | Deficit | (Loss) | Stock | Equity | Put Provisions | Equity | ||||||||||||||||||||||||||
(In thousands, except share data) | ||||||||||||||||||||||||||||||||||
Balance at December 31, 2013 | 61,666,048 | $ | 61 | $ | 567,468 | $ | (363,542 | ) | $ | 212 | $ | (9,963 | ) | $ | 194,236 | $ | 525 | $ | 194,761 | |||||||||||||||
Net loss | — | — | — | (23,946 | ) | — | — | (23,946 | ) | (367 | ) | (24,313 | ) | |||||||||||||||||||||
Adjustments to other comprehensive loss, net of tax | — | — | — | — | (2,404 | ) | — | (2,404 | ) | — | (2,404 | ) | ||||||||||||||||||||||
Exercise of stock options | 1,524,601 | 2 | 13,775 | — | — | (3,026 | ) | 10,751 | — | 10,751 | ||||||||||||||||||||||||
Shares issued under employee restricted stock plans | 166,127 | — | (422 | ) | — | — | — | (422 | ) | — | (422 | ) | ||||||||||||||||||||||
Shares issued under employee stock purchase plan | 70,899 | — | 804 | — | — | — | 804 | — | 804 | |||||||||||||||||||||||||
Shares issued to Directors in lieu of cash | 1,330 | — | 18 | — | — | — | 18 | — | 18 | |||||||||||||||||||||||||
Stock-based compensation expense | — | — | 11,430 | — | — | — | 11,430 | — | 11,430 | |||||||||||||||||||||||||
Changes in noncontrolling interest | — | — | — | — | — | — | — | 930 | 930 | |||||||||||||||||||||||||
Balance at December 31, 2014 | 63,429,005 | 63 | 593,073 | (387,488 | ) | (2,192 | ) | (12,989 | ) | 190,467 | 1,088 | 191,555 | ||||||||||||||||||||||
Net loss | — | — | — | (15,342 | ) | — | — | (15,342 | ) | (918 | ) | (16,260 | ) | |||||||||||||||||||||
Adjustments to other comprehensive loss, net of tax | — | — | — | — | (1,839 | ) | — | (1,839 | ) | — | (1,839 | ) | ||||||||||||||||||||||
Exercise of stock options | 1,020,552 | 1 | 7,790 | — | — | (875 | ) | 6,916 | — | 6,916 | ||||||||||||||||||||||||
Shares issued under employee restricted stock plans | 306,908 | — | (943 | ) | — | — | — | (943 | ) | — | (943 | ) | ||||||||||||||||||||||
Shares issued under employee bonus plans | 66,230 | — | 1,103 | — | — | — | 1,103 | — | 1,103 | |||||||||||||||||||||||||
Shares issued under employee stock purchase plan | 50,343 | — | 690 | — | — | — | 690 | — | 690 | |||||||||||||||||||||||||
Stock-based compensation expense | — | — | 10,774 | — | — | — | 10,774 | — | 10,774 | |||||||||||||||||||||||||
Changes in noncontrolling interest | — | — | — | — | — | — | — | 1,524 | 1,524 | |||||||||||||||||||||||||
Balance at December 31, 2015 | 64,873,038 | $ | 64 | $ | 612,487 | $ | (402,830 | ) | $ | (4,031 | ) | $ | (13,864 | ) | $ | 191,826 | $ | 1,694 | $ | 193,520 | ||||||||||||||
See accompanying notes to these consolidated financial statements.
63
NXSTAGE MEDICAL, INC
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
Accumulated | ||||||||||||||||||||||||||||||||||
Other | Total NxStage | Noncontrolling | ||||||||||||||||||||||||||||||||
Additional | Comprehensive | Medical, Inc. | Interest | Total | ||||||||||||||||||||||||||||||
Common Stock | Paid-In | Accumulated | Income | Treasury | Stockholders' | Not Subject to | Stockholders’ | |||||||||||||||||||||||||||
Shares | Amount | Capital | Deficit | (Loss) | Stock | Equity | Put Provisions | Equity | ||||||||||||||||||||||||||
(In thousands, except share data) | ||||||||||||||||||||||||||||||||||
Balance at December 31, 2015 | 64,873,038 | $ | 64 | $ | 612,487 | $ | (402,830 | ) | $ | (4,031 | ) | $ | (13,864 | ) | $ | 191,826 | $ | 1,694 | $ | 193,520 | ||||||||||||||
Net loss | — | — | — | (4,771 | ) | — | — | (4,771 | ) | (2,378 | ) | (7,149 | ) | |||||||||||||||||||||
Adjustments to other comprehensive loss, net of tax | — | — | — | — | (2,070 | ) | — | (2,070 | ) | — | (2,070 | ) | ||||||||||||||||||||||
Exercise of stock options | 658,444 | 1 | 8,233 | — | — | (2,320 | ) | 5,914 | — | 5,914 | ||||||||||||||||||||||||
Shares issued under employee restricted stock plans | 314,013 | — | (687 | ) | — | — | — | (687 | ) | — | (687 | ) | ||||||||||||||||||||||
Shares issued under employee bonus plans | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||
Shares issued under employee stock purchase plan | 37,531 | — | 767 | — | — | — | 767 | — | 767 | |||||||||||||||||||||||||
Stock-based compensation expense | — | — | 10,419 | — | — | — | 10,419 | — | 10,419 | |||||||||||||||||||||||||
Changes in noncontrolling interest | — | — | — | — | — | — | — | 1,309 | 1,309 | |||||||||||||||||||||||||
Balance at December 31, 2016 | 65,883,026 | $ | 65 | $ | 631,219 | $ | (407,601 | ) | $ | (6,101 | ) | $ | (16,184 | ) | $ | 201,398 | $ | 625 | $ | 202,023 | ||||||||||||||
See accompanying notes to these consolidated financial statements.
64
NXSTAGE MEDICAL, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
Years Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
(In thousands) | |||||||||||
Cash flows from operating activities: | |||||||||||
Net loss | $ | (7,317 | ) | $ | (16,260 | ) | $ | (24,313 | ) | ||
Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | |||||||||||
Depreciation and amortization | 32,637 | 30,715 | 27,697 | ||||||||
Stock-based compensation | 10,117 | 12,598 | 12,881 | ||||||||
Other | 2,445 | 1,302 | 1,877 | ||||||||
Changes in operating assets and liabilities: | |||||||||||
Accounts receivable | (7,388 | ) | (1,130 | ) | (4,188 | ) | |||||
Inventory | (31,069 | ) | (12,113 | ) | (35,063 | ) | |||||
Prepaid expenses and other assets | (68 | ) | (77 | ) | (2,214 | ) | |||||
Accounts payable | 3,532 | (2,775 | ) | (212 | ) | ||||||
Accrued expenses and other liabilities | 4,141 | 635 | 4,235 | ||||||||
Deferred revenues | (2,443 | ) | (2,353 | ) | (973 | ) | |||||
Net cash provided by (used in) operating activities | 4,587 | 10,542 | (20,273 | ) | |||||||
Cash flows from investing activities: | |||||||||||
Cash paid for acquisitions, net of cash acquired | (513 | ) | — | — | |||||||
Proceeds from sales of marketable securities | — | 676 | — | ||||||||
Purchases of property and equipment | (8,848 | ) | (11,537 | ) | (20,097 | ) | |||||
Net cash used in investing activities | (9,361 | ) | (10,861 | ) | (20,097 | ) | |||||
Cash flows from financing activities: | |||||||||||
Proceeds from exercise of stock options and warrants and employee stock purchase plans | 5,994 | 6,663 | 11,133 | ||||||||
Investment by noncontrolling interest holder | 1,255 | 929 | 930 | ||||||||
Proceeds from loans and lines of credit | — | 1,275 | — | ||||||||
Repayments on loans and lines of credit | (384 | ) | (143 | ) | (98 | ) | |||||
Payments on capital leases | (1,590 | ) | (1,391 | ) | (1,448 | ) | |||||
Debt issuance costs | — | — | (1,056 | ) | |||||||
Net cash provided by financing activities | 5,275 | 7,333 | 9,461 | ||||||||
Foreign exchange effect on cash and cash equivalents | 66 | (833 | ) | (341 | ) | ||||||
Increase (decrease) in cash and cash equivalents | 567 | 6,181 | (31,250 | ) | |||||||
Cash and cash equivalents, beginning of year | 59,065 | 52,884 | 84,134 | ||||||||
Cash and cash equivalents, end of year | $ | 59,632 | $ | 59,065 | $ | 52,884 | |||||
See accompanying notes to these consolidated financial statements.
65
NXSTAGE MEDICAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. | Nature of Operations, Basis of Presentation and Principles of Consolidation |
Nature of Operations
We are a medical technology company that develops, manufactures and markets innovative products and services for patients suffering from chronic or acute kidney failure. Our primary product, the System One, was designed to satisfy an unmet clinical need for a system capable of delivering the therapeutic flexibility and clinical benefits of traditional dialysis machines in a smaller, portable, easy-to-use form that can be used by healthcare professionals and trained lay users alike in a variety of settings, including patient homes, as well as more traditional care settings such as hospitals and dialysis centers. Given its design, the System One is particularly well-suited for home hemodialysis and a range of dialysis therapies that are more practical to deliver in the home setting, including more frequent hemodialysis and nocturnal hemodialysis. Clinical literature suggests such therapies provide patients better clinical outcomes and improved quality of life. We also operate a small number of NxStage Kidney Care dialysis centers, independently and in some instances as joint ventures, that treat end-stage renal disease (ESRD) patients directly. These centers provide us with valuable experience to better meet and anticipate the needs of both our customers and patients, while optimizing our product technology. In addition, these centers provide us with the opportunity to innovate and foster new care delivery models to advance the standard of renal care across other markets. More specifically, at NxStage Kidney Care we offer a range of treatment options, including home hemodialysis, peritoneal dialysis and flexible in-center hemodialysis. These centers also help us to devise best practices for successful home dialysis programs and provide sites for future clinical trials of our products. We are headquartered in Lawrence, Massachusetts, with manufacturing facilities in Mexico, Germany and Italy. Through our international network of affiliates and distribution partners, patients in over 21 countries have been treated with our products.
Basis of Presentation
The accompanying consolidated financial statements of NxStage Medical, Inc. have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and with the requirements of Regulation S-X.
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Such financial statements reflect all adjustments that, in the opinion of management, are necessary for a fair presentation of the results of the periods presented. All such adjustments are of a normal recurring nature.
Principles of Consolidation
Our consolidated financial statements include the accounts of NxStage Medical, Inc. and our wholly-owned subsidiaries and other entities in which we maintain a majority voting interests or for which we maintain effective control including variable interest entities ("VIEs") for which we are deemed the primary beneficiary. All significant intercompany balances and transactions have been eliminated. Noncontrolling interests represent the proportionate equity interests in the consolidated entities that are not wholly owned by us. Noncontrolling interests of acquired entities are recognized at their initial fair value.
2. | Summary of Significant Accounting Policies |
Revenue Recognition
We recognize revenue from product sales and services when earned. Revenues are recognized when: (a) there is persuasive evidence of an arrangement, (b) the product has been shipped or services and supplies have been provided to the customer, (c) the sales price is fixed or determinable and (d) collection is reasonably assured.
Certain agreements with distributors allow for product returns and credits. For shipment of product sold to distributors, revenue is recognized at the time of sale if a reasonable estimate of future returns or credit can be made. If a reasonable estimate of future returns or credit cannot be made, we recognize revenue using the "sell-through" method. Under the "sell-through" method, revenue and related costs of revenue is deferred until the final resale of such products to end customers.
In addition to contractually determined volume discounts, in many agreements we offer rebates based on sales to specific end customers and discounts for early payment. Rebates and discounts are recorded as a reduction of sales and trade accounts receivable, based on our best estimate of the amount of probable future rebate or discount on current sales.
66
We enter into multiple-element arrangements that may include a combination of equipment, related disposables and services. Revenue arrangements with multiple elements are divided into separate units of accounting if specified criteria are met, including whether the delivered element has stand-alone value to the customer, the consideration received is allocated among the separate units based on their respective selling price, and the applicable revenue recognition criteria are applied to each of the separate units.
We determine selling price using vendor specific objective evidence ("VSOE"), if it exists, otherwise third-party evidence of selling price is used. If neither VSOE nor third-party evidence of selling price exists for a unit of accounting, we use best estimated selling price ("BESP"). We generally expect that we will not be able to establish third-party evidence due to the nature of our products and the markets in which we compete, and, as such, we typically will determine selling price using VSOE or BESP.
We determine BESP for an individual element based on consideration of both market and Company-specific factors, including the selling price and profit margin for similar products, the cost to produce the deliverable and the anticipated margin on that deliverable and the characteristics of the varying markets in which the deliverable is sold.
Any tax assessed by a governmental authority that is incurred as a result of a revenue transactions (e.g. sales tax) is excluded from revenues and reported on a net basis
System One Segment
We derive revenue in the home market from the sales of hemodialysis therapy to customers in which the customer either purchases or rents the System One and/or PureFlow SL hardware and purchases a specified number of disposable products and service.
For customers that purchase the System One and PureFlow SL hardware, in the U.S. home market, due to the depot service model whereby equipment requiring service is picked up and a replacement device is shipped to the site of care, we recognize fees received from equipment sale as revenue on a straight-line basis over the expected term of our remaining service obligation and direct costs relating to the delivered equipment are deferred and amortized over the same expected period as the related revenue. Disposable products revenue is recognized on a monthly basis upon delivery.
Under the rental arrangements, revenue is recognized on a monthly basis in accordance with agreed upon contract terms and pursuant to binding customer purchase orders and fixed payment terms.
Our sales arrangements with our international distributors are structured as direct product sales and have no significant post-delivery obligations with the exception of standard warranty obligations. Revenue from direct product sales is recognized upon delivery in accordance with contract terms.
In the critical care market, we structure sales of the System One and disposable products as direct product sales and have no significant post-delivery obligations with the exception of standard warranty obligations. Revenue from direct product sales is recognized upon delivery in accordance with contract terms. Certain of these arrangements provide for training, technical support and extended warranty services to our customers. We recognize training and technical support revenue when the related services are performed. In the case of extended warranty, the service revenue is recognized ratably over the extended warranty period.
In-Center Segment
Our In-Center segment sales are structured as direct product sales primarily through distributors, and we have no significant post delivery obligations with the exception of standard warranty obligations. Revenue from direct product sales is recognized upon delivery in accordance with contract terms. Some of our distribution contracts for the In-Center segment contain minimum volume commitments with negotiated pricing discounts at different volume tiers. Each agreement may be canceled upon a material breach, subject to certain curing rights, and in many instances minimum volume commitments can be reduced or eliminated upon certain events.
Services Segment
Revenues in our Services segment are derived from dialysis care services provided to patients at our NxStage Kidney Care dialysis centers.
Revenues are recognized based on a customary fee schedule, net of estimated contractual allowances to reflect the estimated amounts to be received from the payor. Revenues are recognized in the period in which services are provided when we have the ability to reasonably estimate amounts ultimately collectible from the payor. In instances where we do not have the ability to reasonably estimate amounts ultimately collectible, as is often the case with non-contracted commercial health plans and amounts due from patients (including co-pay and deductible amounts), revenue is recognized in the period in which cash is received.
67
Foreign Currency Translation and Transactions
Assets and liabilities of our foreign operations are translated into U.S. dollars at current exchange rates, and income and expense items are translated at average rates of exchange prevailing during the year. Foreign exchange gains and (losses) on intercompany loans considered permanent investments are recorded in other comprehensive loss. Gains and (losses) realized from transactions denominated in foreign currencies, including intercompany balances not considered permanent investments, are included in the consolidated statements of comprehensive loss within other income (expense), net.
Cash and Cash Equivalents
We consider all highly-liquid investments purchased with original maturities of 90 days or less to be cash equivalents. Cash equivalents include amounts invested in treasury obligation money market funds. Cash equivalents are stated at cost plus accrued interest, which approximates fair value.
Concentration of Credit Risk
Financial instruments that potentially subject us to concentrations of credit risk consist principally of cash and cash equivalents, derivatives and accounts receivable. To mitigate such risk, with respect to cash and cash equivalents, we place our cash in bank deposit accounts with financial institutions that have investment grade ratings and capital ratios exceeding minimum Federal Reserve Adequacy Guidelines and in treasury obligation money market funds. To mitigate concentration of credit risk with respect to derivatives we enter into transactions with highly-rated financial institutions and frequently monitor the credit worthiness of our counterparties.
Concentration of credit risk with respect to accounts receivable is primarily limited to certain customers to whom we make substantial sales. Two customers represented 12% and 10% of accounts receivable at December 31, 2016 and one customer represented 12% of accounts receivable at December 31, 2015. To reduce risk, we routinely assess the financial strength of our customers and closely monitor their amounts due and, as a result of our assessment, we believe that our accounts receivable credit risk exposure is limited. Historically, we have not experienced any significant credit losses related to an individual customer or group of customers in any particular market or geographic area. We maintain an allowance for doubtful accounts based on an analysis of historical losses from uncollectible accounts, aging of unpaid accounts receivable balances and risks identified for specific customers who may not be able to make required payments. Provisions for the allowance for doubtful accounts are recorded in general and administrative expenses in the accompanying consolidated statements of comprehensive loss.
Activity related to allowance for doubtful accounts consisted of the following (in thousands):
Year Ended | Balance at Beginning of Year | Provision | Write-offs | Balance at End of Year | ||||||||||||
December 31, 2016 | $ | 417 | $ | 179 | $ | (61 | ) | $ | 535 | |||||||
December 31, 2015 | $ | 425 | $ | 20 | $ | (28 | ) | $ | 417 | |||||||
December 31, 2014 | $ | 321 | $ | 219 | $ | (115 | ) | $ | 425 | |||||||
We use and are dependent upon a number of single source suppliers of raw materials, components, finished goods and sterilization services. We are dependent on the ability of our suppliers to provide products on a timely basis and on favorable pricing terms. The loss of certain principal suppliers or a significant reduction in product availability from principal suppliers would have a material adverse effect on us, at least in the near term. We believe that our relationships with our suppliers are satisfactory.
Fair Value Measurements
U.S. GAAP defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. U.S. GAAP requires an entity to maximize the use of observable inputs, where available, and minimize the use of unobservable inputs when measuring fair value.
The Company has certain financial assets and liabilities that are measured at fair value on a recurring basis, certain nonfinancial assets that may be measured at fair value on a nonrecurring basis. The fair value disclosures of these assets and liabilities are based on a three-level hierarchy, which is defined as follows:
Level 1 | Quoted prices in active markets for identical assets or liabilities that the entity can access at the measurement date. |
Level 2 | Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
68
Level 3 | Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. Our assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment and considers factors specific to the asset or liability.
Derivative Instruments and Hedging
Derivative instruments, namely our foreign exchange forward contracts, are recognized on the balance sheet at fair value at the balance sheet date. Changes in the fair value of derivatives that are designated and highly effective as cash flow hedges are deferred in accumulated other comprehensive income (loss) and subsequently recognized in cost of revenues in the same period the hedged items are recognized. The ineffective portion of derivative instruments designated as cash flow hedges, are recorded in other income (expense), net. If the underlying forecasted transaction does not occur, or it becomes probable that it will not occur, the gains and losses on the related derivative instrument are recognized in earnings and any related gains and losses recorded in other comprehensive income (loss) are reclassified into earnings.
Inventory
Inventory is stated at the lower of cost, determined using the first-in first-out method (FIFO), or market (net realizable value). We write down the carrying value of inventory for estimated obsolescence when warranted by an amount equal to the difference between the cost of inventory and the estimated market value based on assumptions of future demand and remaining shelf-life. We also review our inventory value to determine if it reflects the lower of cost or market based on factors such as inventory items sold at negative gross margins and purchase commitments.
Property and Equipment and Field Equipment
Property and equipment and field equipment are recorded at cost less accumulated depreciation. Depreciation is provided over the estimated useful lives of the related assets using the straight-line method for financial statement purposes. The estimated useful lives of our assets are periodically reviewed for reasonableness. Changes in useful lives are accounted for prospectively. Repairs and maintenance are expensed as incurred. When property and equipment are retired, sold or otherwise disposed of, the asset's carrying amount and related accumulated depreciation are removed from the accounts and any gain or loss is included in operations. When field equipment is sold, the asset's carrying amount and related accumulated depreciation is removed from the accounts and any gain or loss is deferred and recognized in operations on a straight-line basis over the same period as the related revenues.
We capitalize certain costs, including internal payroll and external direct project costs, incurred in connection with developing or obtaining software designated for internal use. These costs are included in property and equipment and are amortized over the estimated useful lives of the related software.
Construction-in-process is stated at cost, which includes the cost of construction and other direct costs attributable to the construction. No provision for depreciation is made on construction-in-process until such time as the relevant assets are completed and put into use. Construction-in-process at December 31, 2016 and 2015 primarily represents the costs of building, machinery and equipment under installation.
Field equipment consists of equipment being utilized under disposable-based rental agreements as well as “service pool” equipment. Service pool equipment is equipment owned and maintained by us that are swapped for equipment that need repairs or maintenance by us while being rented or owned by a customer. We record a provision for any excess, lost or damaged equipment when warranted based on an assessment of the equipment in the service pool. Write-downs for equipment are included in distribution expenses.
The estimated useful lives of property and equipment and field equipment are as follows:
Estimated Useful Life | |
Buildings | 30 years |
Equipment and tooling | 5 to 12 years |
Leasehold improvements | Lesser of the lease term (including any renewal periods if appropriate) or estimated useful life of the asset |
Computer and office equipment | 3 to 5 years |
Molds | 5 to 7 years |
Furniture | 5 to 7 years |
Field equipment | 5 to 7 years |
69
Intangibles and Other Long-Lived Assets
Intangible assets are carried at cost less accumulated amortization. For assets with determinable useful lives, amortization is recognized using the straight-line method over the estimated economic lives of the respective intangible assets. Long-lived assets, including intangible assets, are evaluated for recoverability whenever events or circumstances indicate that their carrying value may not be recoverable.
In 2016 and 2015, events and circumstances have indicated that certain long-lived tangible assets in the Services segment may not be recoverable. Therefore, a recoverability test, as required, was performed at the center level by comparing the centers' estimated future undiscounted cash flows, within the initial lease term (which is the equivalent to the depreciable life of the centers' most significant asset, its leasehold improvements), to the carrying value of the centers. As of December 31, 2016, our estimate of future undiscounted net cash flows for the majority of our centers indicated such carrying amounts were expected to be recovered. We recognized in cost of revenues an impairment charge of $0.5 million and $0.2 million in 2016 and 2015, respectively. During 2014, no impairment was recognized.
The evaluation of the impairment of long-lived assets, other than goodwill, is based on expectations of non-discounted future cash flows compared to the carrying value of the long-lived asset groups. If the sum of the expected non-discounted future cash flows is less than the carrying amount of the long-lived assets, we would recognize an impairment loss if the carrying amount of the asset group exceeds its fair value. Our expected non-discounted future cash flows are based upon cash flow projections and, if appropriate, include assumed proceeds upon sale of the asset group at the end of the cash flow period. We believe our procedures for developing cash flow projections, including the estimated sales proceeds, are reasonable and consistent with current market conditions for each of the dates when impairment testing has been performed.
As of December 31, 2016, our expected non-discounted future cash flows for the majority of our centers indicated no impairment. Developing cash flow projections used in our impairment test requires significant estimates and judgment. Among other things, slower than expected patient ramp or lower than expected reimbursement rates would negatively impact our cash flow projections in the near term, which could result in the need to recognize an impairment for at least some portion of these centers' assets in the future.
For certain of our centers, we recorded an impairment of $0.5 million in cost of revenues during the fourth quarter of 2016 to write-down the asset group’s carrying value to its fair value. Fair value of the asset group was estimated using a discounted cash flow approach. Estimating fair value requires significant judgment in the selection of the valuation technique and assumptions used in developing cash flow projections, growth rates and discount rates. Our assumptions are based on our best estimates, using appropriate and customary market participant assumptions. Any adverse changes in certain valuation assumptions or future cash flow projections could result in the need to record additional impairment to write down all or a portion the centers’ remaining asset carrying value of $2.4 million at December 31, 2016.
Assets to be disposed of are reported at the lower of the carrying amount or fair value less selling costs.
Goodwill
Goodwill represents the excess of the cost of an acquired business over the acquisition value of the related net assets at the date of acquisition. We test goodwill at least annually for impairment, or more frequently when events or changes in circumstances indicate that the goodwill might be impaired. This impairment test is performed annually during the fourth quarter. This test includes first a qualitative assessment and then, if necessary, a quantitative assessment to determine if the fair value of a reporting unit is less than its carrying amount. Our System One, In-center and Services reporting units contain goodwill of $41.1 million, $0.5 million and $1.0 million, respectively. Factors considered in the qualitative assessment include, but are not limited to, both macroeconomic conditions and entity-specific conditions. For the quantitative assessment the reporting unit's fair value is estimated using a discounted cash flow or other fair value measurement.
During 2016, 2015 and 2014 we utilized the qualitative assessment to assess the fair value of our System One and In-center reporting units and concluded that it was more likely than not that the fair value of our reporting units was greater than their carrying value.
During 2016, for our Services reporting unit, we utilized the quantitative assessment noting that the fair value of the reporting unit exceeds its carrying value, indicating that goodwill was not impaired. We estimated the fair value of our Services reporting unit using a discounted cash flow approach. Estimating the fair value of our Services reporting unit requires significant judgment in the selection of the valuation technique and assumptions used in cash flow projections, growth rates and discount rates. Our assumptions are based on our best estimates, using appropriate and customary market participant assumptions. Developing cash flow projections involves significant judgment with respect to patient ramp and reimbursement rates, operating income, capital expenditures and changes in working capital. Reductions in our cash flow projections due to slower than expected patient ramp or lower than expected reimbursement rates, among other things, or adverse changes in certain valuation assumptions could result in a goodwill impairment charge of up to $1.0 million in the future.
70
Stock-Based Compensation
Stock-based compensation expense is estimated as of the grant date based on the fair value of the award. We use the Black-Scholes option pricing model to estimate the fair value of stock options and quoted market prices of our common stock to estimate fair value of restricted stock. The expected term is estimated based on the contractual term of each grant and takes into account the historical experience and relevant factors concerning expected exercise and termination behavior of participants. The risk free interest rate for each grant is equal to the U.S. Treasury rate in effect at the time of grant for instruments with an expected life similar to the expected term. The stock volatility assumption is based solely on our historical volatility over the expected term of the award. The dividend yield of zero is based upon the fact that we have not historically granted cash dividends, and do not expect to issue dividends in the foreseeable future.
We recognize stock-based compensation expense over the requisite service period, which equals the vesting period, net of forfeitures. Forfeiture rates are estimated based on historical pre-vesting forfeiture history and are updated on a quarterly basis to reflect actual forfeitures of unvested awards and other known events. For awards that vest based on employment, we recognize the associated compensation expense on a straight-line basis. For performance based awards, we recognize expense using the graded vesting methodology based on the number of shares expected to vest. Compensation expense associated with these performance based awards is adjusted quarterly to reflect subsequent changes in the estimated outcome of performance-related conditions until the date the results are determined.
Warranty Costs
We accrue estimated costs that we may incur under our product warranty programs at the time the product revenue is recognized, based on contractual rights and historical experience. Warranty expense is included in cost of revenues in the consolidated statements of comprehensive loss. The following is a rollforward of our warranty accrual (in thousands):
Year Ended | Balance at Beginning of Year | Provision | Usage | Balance at End of Year | ||||||||||||
December 31, 2016 | $ | 389 | $ | 457 | $ | (566 | ) | $ | 280 | |||||||
December 31, 2015 | $ | 287 | $ | 526 | $ | (424 | ) | $ | 389 | |||||||
December 31, 2014 | $ | 297 | $ | 441 | $ | (451 | ) | $ | 287 | |||||||
Distribution Expenses
Distribution expenses are charged to operations as incurred and consist of costs incurred in shipping product to and from customers and the cost of any equipment lost or damaged in the distribution process. Shipping and handling costs billed to customers are included in revenues.
Research and Development Costs
Research and development costs are charged to operations as incurred.
Income Taxes
We record the tax effect of transactions when such transactions are recorded in our consolidated statement of comprehensive loss. We record a deferred tax asset or liability based on the difference between the financial statement and tax basis of assets and liabilities, as measured by the enacted tax rates. Our provision for income taxes represents the amount of taxes currently payable, if any, plus the change in the amount of net deferred tax assets or liabilities. A valuation allowance is provided against net deferred tax assets if recoverability is uncertain on a more likely than not basis.
We periodically assess our exposures related to our provisions for income taxes and accrue for contingencies that may result in potential tax obligations. For those positions where it is more likely than not that a tax benefit will be sustained, we record the largest amount of tax benefit with a greater than 50 percent likelihood of being realized upon ultimate settlement with a taxing authority that has full knowledge of all relevant information. For those income tax positions where it is not more likely than not that a tax benefit will be sustained, no tax benefit is recognized in the financial statements. We recognize interest and penalties for uncertain tax positions in income tax expense.
We conduct business globally and file income tax returns in the U.S. federal jurisdiction, various states and foreign jurisdictions. We estimate our income taxes in each of the jurisdictions in which we operate. We have accumulated significant losses since our inception in 1998. The utilization of these losses may be limited in future years based on the profitability of certain entities.
Subsequent Events
71
Events occurring subsequent to December 31, 2016 have been evaluated for potential recognition or disclosure in the consolidated financial statements.
Recent Accounting Pronouncements
Recently Implemented Accounting Pronouncements
In April 2015, the FASB issued ASU No. 2015-5, Intangibles - Goodwill and Other - Internal-Use Software (Subtopic 350-40): "Customer's Accounting for Fees Paid in a Cloud Computing Arrangement." Under this standard, if a cloud computing arrangement includes a software license, the software license element of the arrangement should be accounted for consistent with the acquisition of other software licenses. If a cloud computing arrangement does not include a software license, the arrangement should be accounted for as a service contract. The update was effective for us beginning January 1, 2016. The adoption of this standard did not have a material impact on our financial statements.
In September 2015, the FASB issued ASU No. 2015-16, Business Combinations (Topic 805): "Simplifying the Accounting for Measurement-Period Adjustments". The new standard requires that an acquirer recognize adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined and sets forth new disclosure requirements related to the adjustments. The new standard was effective for us beginning January 1, 2016. The adoption of this standard did not have a material impact on our financial statements.
Recent Accounting Pronouncements Not Yet Adopted
In May 2014, the FASB issued ASU No. 2014-9, “Revenue from Contracts with Customers.” The standard provides that revenue should be recognized when an entity transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. In addition, the standard requires disclosure of the nature, amount, timing and uncertainty of revenues and cash flow arising from contracts with customers. The FASB has issued several amendments and updates to the new revenue standard, including how an entity should identify performance obligations. As amended, the new guidance is effective for us beginning January 1, 2018. The new guidance allows for full retrospective adoption applied to all periods presented or a modified retrospective adoption with the cumulative effect of initially applying the new guidance recognized at the date of initial application. In 2016, we commenced our evaluation of the impact of the standard, by establishing a cross functional team and evaluating the anticipated impact on significant contracts from each of our business segments. We are evaluating the potential impact and method of adoption of on our consolidated financial statements and related disclosures and are comparing our current policies and practices to the requirements of the standard. We have developed a project plan to develop processes and tools and to assess the internal control structure in order to adopt the standard on January 1, 2018. We believe, at a minimum, that the standard will impact the timing of revenue recognition for our Services segment as the standard eliminates cash basis revenue recognition and instead requires revenues to be estimated and recognized upon transfer of the promised goods and services. We have periodically briefed our Audit Committee on our progress made towards adoption. Based on our evaluation to date, we anticipate being able to estimate the impacts of adopting the ASU in the second half of 2017.
In July 2015, the FASB issued ASU No. 2015-11, Inventory (Topic 330): "Simplifying the Measurement of Inventory." The update requires that an entity should measure in scope inventory at the lower of cost and net realizable value. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The amendments will be effective for us beginning January 1, 2017. The adoption of this update is not expected to have a material impact on our financial statements.
In February 2016, the FASB issued ASU No. 2016-02: "Accounting for Leases" which amends the existing accounting standards for leases. The new standard requires lessees to record a right-of-use asset and a corresponding lease liability on the balance sheet for all leases with terms longer than twelve months. For lessees, leases will continue to be classified as either operating or financing in the income statement. This ASU is required to be applied with a modified retrospective approach and requires application of the new standard at the beginning of the earliest comparative period presented. The new guidance is effective for us beginning January 1, 2019 and early adoption is permitted. We intend to adopt this standard as of January 1, 2019. We are currently evaluating the potential impact this standard will have on our financial statements.
In March 2016, the FASB issued ASU No. 2016-09: “Improvements to Employee Share-Based Payment Accounting,” which simplifies several aspects of the accounting for employee share-based payment transactions for both public and nonpublic entities, including the accounting for income taxes, forfeitures, and statutory tax withholding requirements, as well as classification in the statement of cash flows. The new guidance is effective for us beginning January 1, 2017 and early adoption is permitted. We intend to adopt this standard as of January 1, 2017. We have elected to continue to estimate forfeiture rates. This new standard will impact our income tax footnote disclosures. Upon adoption the cumulative excess tax deductions related to stock-based compensation that were previously unrecognized and instead tracked and disclosed separately, will be recognized as a deferred tax asset with an offsetting valuation allowance. Other than this change in footnote disclosure, the adoption will not have a material impact on our financial statements and related disclosure.
72
In January 2017, the FASB issued ASU No. 2017-01: "Business Combinations (Topic 805): Clarifying the Definition of a Business" which changes the definition of a business to assist entities with evaluating when a set of transferred assets and activities is a business. The update is effective for us beginning January 1, 2018. Early adoption is permitted, including for interim or annual periods in which the financial statements have not been issued or made available for issuance. The adoption of this update is not expected to have a material impact on our financial statements.
In January 2017, the FASB issued ASU No. 2017-04: "Intangibles - Goodwill and Other Topics (Topic 350): Simplifying the Test for Goodwill Impairment". The purpose of this update is to reduce the cost and complexity of evaluating goodwill for impairment. It eliminates the need for entities to calculate the impaired fair value of goodwill by assigning the fair value of a reporting unit to all of its assets and liabilities as if that reporting unit had been acquired in a business combination. Under this update, an entity will perform its goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount. An impairment charge is recognized for the amount by which the carrying value exceeds the reporting unit's fair value. The update is effective for us beginning January 1, 2020. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. We anticipate early adopting the update in 2017 on a prospective basis.
3. | Inventory |
Inventory includes material, labor and overhead. The components of inventory are as follows (in thousands):
December 31, | |||||||
2016 | 2015 | ||||||
Purchased components | $ | 14,967 | $ | 15,294 | |||
Work in process | 13,939 | 10,080 | |||||
Finished goods | 17,939 | 13,017 | |||||
$ | 46,845 | $ | 38,391 | ||||
4. | Property and Equipment, Field Equipment and Deferred Cost of Revenues |
Property and Equipment, net
The components of property and equipment, net are as follows (in thousands):
December 31, | |||||||
2016 | 2015 | ||||||
Equipment and tooling | $ | 39,921 | $ | 38,084 | |||
Leasehold improvements | 35,146 | 29,927 | |||||
Computer and office equipment | 10,035 | 9,632 | |||||
Molds | 6,805 | 6,580 | |||||
Furniture | 3,561 | 2,930 | |||||
Building | 8,057 | 8,122 | |||||
Land | 956 | 999 | |||||
Construction-in-process | 4,237 | 7,298 | |||||
108,718 | 103,572 | ||||||
Less accumulated depreciation | (47,157 | ) | (36,861 | ) | |||
Property and equipment, net | $ | 61,561 | $ | 66,711 | |||
Depreciation expense, including amortization of capital leases, for property and equipment was $11.6 million, $10.4 million and $7.1 million during 2016, 2015 and 2014, respectively. Capitalized computer development costs, net were $1.8 million and $2.7 million at December 31, 2016 and December 31, 2015 respectively, and we recognized amortization expense of $0.9 million during each of 2016, 2015 and 2014.
Our property and equipment includes the following amounts for assets subject to capital leases (amounts in thousands):
73
December 31, | |||||||
2016 | 2015 | ||||||
Manufacturing facility in Germany | $ | 21,007 | $ | 21,762 | |||
Other assets subject to capital lease | 3,024 | $ | 2,889 | ||||
Less accumulated depreciation | (6,402 | ) | (4,699 | ) | |||
Assets subject to capital leases, net | $ | 17,629 | $ | 19,952 | |||
Manufacturing Facility in Germany
Pursuant to our Dialyzer Production Agreement entered into in May 2009 with Asahi we agreed to oversee construction of a new manufacturing facility in Germany which was completed in December 2012 and operate the facility and manufacture dialyzers for our own use and for sale to Asahi under a manufacturing agreement during the initial term of the agreement through June 2021 and thereafter, unless either party provides notice of its intent not to renew. Asahi funded construction costs of the facility, including land, building and equipment. Given our involvement in the facility during construction and our continued involvement in its operation we have recorded the cost of the new facility, including building and equipment, within property and equipment, net on our consolidated balance sheet, as required, along with a corresponding liability which has been divided into two separate components, namely a capital lease obligation and deferred revenue, based on their relative fair values. The capital lease obligation is decreased by payments made to Asahi for dialyzers manufactured for our own use and increased by interest expense. The deferred revenue is recognized in revenues on a straight-line basis over the expected term of the Dialyzer Production Agreement.
During 2015, additional assets were placed into service at a total cost of $1.3 million. The corresponding liability was divided into two separate components, including a capital lease obligation and deferred revenue, based on their relative fair values of $0.7 million and $0.7 million, respectively.
The fair value of the capital lease obligation was determined based on the present value of the financing payments due plus the residual value guarantee. The key assumptions used to determine the fair value of this liability included our incremental borrowing rate, the fixed amount per dialyzer payment due to Asahi totaling fifty percent of the cost of the facility paid by Asahi, and the estimated residual value of the facility assets at the end of the estimated lease term all of which we determined to be Level 3 inputs within the fair value hierarchy.
The fair value of the deferred revenue was determined using cost plus a reasonable margin for contract manufacturing in Germany, Level 3 inputs within the fair value hierarchy.
The capital lease obligation and deferred revenue balances are $11.0 million and $6.2 million, respectively, at December 31, 2016.
Field Equipment, net
The components of field equipment, net are as follows (in thousands):
December 31, | |||||||
2016 | 2015 | ||||||
Field equipment | $ | 70,743 | $ | 64,796 | |||
Less accumulated depreciation | (48,434 | ) | (44,052 | ) | |||
Field equipment, net | $ | 22,309 | $ | 20,744 | |||
Depreciation expense for field equipment, which is recorded in costs of revenues in the consolidated statements of comprehensive loss, was $5.5 million, $5.0 million and $4.2 million during 2016, 2015 and 2014, respectively.
Deferred Costs of Revenues
Amortization expense of direct costs relating to deferred equipment revenues was $13.3 million, $12.6 million and $13.5 million during 2016, 2015 and 2014, respectively.
5. | Intangible Assets |
The components of intangible assets, net are as follows (in thousands):
74
December 31, 2016 | December 31, 2015 | Estimated | |||||||||||||||
Cost | Accumulated Amortization | Cost | Accumulated Amortization | Useful Life | |||||||||||||
Bloodline, needle and other patented and unpatented technology | $ | 6,200 | $ | (6,200 | ) | $ | 6,200 | $ | (6,200 | ) | 8 years | ||||||
Trade names | 2,300 | (1,520 | ) | 2,300 | (1,355 | ) | 14 years | ||||||||||
Customer relationships | 26,138 | (17,230 | ) | 26,166 | (15,367 | ) | 10/14 years | ||||||||||
Intangible assets, net | $ | 34,638 | $ | (24,950 | ) | $ | 34,666 | $ | (22,922 | ) | |||||||
We recognized amortization expense of $2.0 million, $2.6 million and $2.8 million during 2016, 2015 and 2014, respectively.
The estimated future aggregated amortization expense for intangible assets as of December 31, 2016 is as follows (in thousands):
2017 | $ | 2,035 | |
2018 | 2,035 | ||
2019 | 2,035 | ||
2020 | 2,035 | ||
2021 | 1,548 | ||
$ | 9,688 | ||
6. | Net Loss per Share |
Basic net loss per share is computed by dividing loss attributable to NxStage Medical, Inc. common stockholders (the numerator) by the weighted-average number of common shares outstanding (the denominator) for the period. The computation of diluted loss per share is similar to basic loss per share, except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potentially dilutive common shares had been issued.
The following potential common stock equivalents, as calculated using the treasury stock method, were not included in the computation of diluted net loss per share as their effect would have been anti-dilutive due to the net loss incurred (in thousands):
Years Ended December 31, | ||||||||
2016 | 2015 | 2014 | ||||||
Options to purchase common stock | 785 | 732 | 876 | |||||
Unvested restricted stock | 361 | 350 | 236 | |||||
Total | 1,146 | 1,082 | 1,112 | |||||
7. | Accrued Expenses, Other Current Liabilities, and Other Long Term Liabilities |
The components of accrued expenses are as follows (in thousands):
December 31, | |||||||
2016 | 2015 | ||||||
Payroll, compensation and related benefits | $ | 14,086 | $ | 14,061 | |||
Distribution expenses | 4,804 | 3,465 | |||||
General and administrative expenses | 4,415 | 2,309 | |||||
Other | 7,680 | 7,431 | |||||
Total | $ | 30,985 | $ | 27,266 | |||
The components of other current liabilities are as follows (in thousands):
75
December 31, | |||||||
2016 | 2015 | ||||||
Deferred revenue, current portion | $ | 1,035 | $ | 1,363 | |||
Capital lease obligations | 1,840 | 2,184 | |||||
Other | 895 | 847 | |||||
Total | $ | 3,770 | $ | 4,394 | |||
The components of other long term liabilities are as follows (in thousands):
December 31, | |||||||
2016 | 2015 | ||||||
Capital lease obligations | $ | 9,991 | $ | 10,815 | |||
Lease incentive obligations | 3,059 | 3,743 | |||||
Benefit plan obligations | 1,678 | 1,681 | |||||
Other | 840 | 1,128 | |||||
Total | $ | 15,568 | $ | 17,367 | |||
8. | Debt and Capital Lease Obligations |
Revolving Line of Credit
We have a revolving line of credit, currently with Capital One Financial Corporation and SVB, that allows for borrowings up to $35 million. Availability of credit is subject to a borrowing base that is calculated with reference to certain of our accounts receivable, inventory and equipment, and adjustments to such borrowing base are at the discretion of the lenders. The revolving line of credit is secured by substantially all of our assets and expires in June 2019. Borrowings bear interest at an annual rate equal to (1) a LIBOR rate plus 2.5% or (2) a base rate plus 1.5%, where the base rate is the highest of (a) the Prime Rate, (b) the Federal Funds Rate plus 0.5% and (c) a LIBOR rate plus 1%, at our election. The revolving credit facility requires us to comply with certain covenants while borrowings are outstanding and contains events of default customary for a transaction of this type.
Other Debt
We have several smaller loans with original principal amounts totaling $2.4 million with annual interest rates of approximately 2.0% to 6.0%, payable over a period of five to ten years secured by certain assets.
Capital Lease Obligations
Our capital lease obligations consist of certain property and equipment financed through capital leases and our capital lease obligation due to Asahi related to the manufacturing facility in Germany. Approximate future minimum payments under our capital leases as of December 31, 2016 are as follows (in thousands):
2017 | $ | 1,872 | |
2018 | 1,688 | ||
2019 | 1,606 | ||
2020 | 1,517 | ||
2021 | 1,027 | ||
Thereafter (1) | 6,670 | ||
Total minimum lease payments | 14,380 | ||
Less: Amount representing interest | (2,549 | ) | |
Present value of future minimum lease payments | $ | 11,831 | |
_________________________________
(1) Amount includes $5.9 million representing the estimated residual value of the manufacturing facility in Germany at the end of the estimated lease term, which only becomes due and payable at Asahi's option if we terminate our agreement with Asahi.
9. | Business Segment and Geographic Information |
76
We have three reportable business segments: System One, In-Center, and Services. The operating results of NxStage Kidney Care are included in our Services segment. We refer to our System One segment, In-Center segment, and Other category as our products business.
Our System One segment includes revenues from the sale and rental of the System One and PureFlow SL dialysate preparation equipment and the sale of disposable products to customers in the home market, including through our NxStage Kidney Care dialysis centers, and critical care market. The home market is devoted to the treatment of ESRD patients in the home or a home-like setting, while the critical care market is devoted to the treatment of hospital-based patients with acute kidney failure or fluid overload. Some of our largest customers in the home market provide outsourced renal dialysis services to some of our customers in the critical care market. Sales of product to both markets are made primarily through dedicated sales forces and distributed directly to the customer, or the patient, with certain products sold through distributors.
Our In-Center segment includes revenues from the sale of blood tubing sets and needles for hemodialysis primarily for the treatment of ESRD patients at dialysis centers and needles for apheresis. Nearly all In-Center products are sold through national distributors.
The remainder of our products business, which is included within the Other category, relates to the manufacturing of dialyzers for sale to Asahi Kasei Kuraray Medical Co., Ltd. (Asahi) and research and development and general and administrative expenses that are excluded from the segment operating performance measures.
Our Services segment includes revenues from dialysis services provided to patients at our NxStage Kidney Care dialysis centers. Sales of the System One and related products to our NxStage Kidney Care dialysis centers are included in System One segment revenues, which are then eliminated upon consolidation.
The accounting policies of our reportable segments are the same as those described in Note 2, Summary of Significant Accounting Policies. Our chief operating decision maker allocates resources to our business segments and assesses segment performance based on segment profit (loss), which consists of revenues less cost of revenues, selling and marketing and distribution expenses.
The following summarizes the operating performance of our reportable segments (in thousands):
System One | In-Center | Other | Services | Intersegment Elimination | Total | ||||||||||||||||||
Year Ended December 31, 2016 | |||||||||||||||||||||||
Revenues from external customers | $ | 276,393 | $ | 63,038 | $ | 12,166 | $ | 14,781 | $ | — | $ | 366,378 | |||||||||||
Intersegment Revenues | 7,530 | — | — | — | (7,530 | ) | — | ||||||||||||||||
Revenues | 283,923 | 63,038 | 12,166 | 14,781 | (7,530 | ) | 366,378 | ||||||||||||||||
Segment profit (loss) | 74,912 | 10,092 | (62,309 | ) | (26,233 | ) | (747 | ) | (4,285 | ) | |||||||||||||
Depreciation and amortization | 23,671 | 2,003 | 4,427 | 4,781 | (2,245 | ) | 32,637 | ||||||||||||||||
Segment assets | 135,038 | 21,246 | 138,273 | 23,620 | (970 | ) | 317,207 | ||||||||||||||||
Year Ended December 31, 2015 | |||||||||||||||||||||||
Revenues from external customers | $ | 244,641 | $ | 74,768 | $ | 10,302 | $ | 6,412 | $ | — | $ | 336,123 | |||||||||||
Intersegment Revenues | 3,134 | — | — | — | (3,134 | ) | — | ||||||||||||||||
Revenues | 247,775 | 74,768 | 10,302 | 6,412 | (3,134 | ) | 336,123 | ||||||||||||||||
Segment profit (loss) | 59,748 | 12,395 | (62,946 | ) | (23,826 | ) | — | (14,629 | ) | ||||||||||||||
Depreciation and amortization | 20,554 | 1,835 | 5,004 | 3,322 | — | 30,715 | |||||||||||||||||
Segment assets | 120,253 | 24,232 | 142,842 | 19,547 | — | 306,874 | |||||||||||||||||
Year Ended December 31, 2014 | |||||||||||||||||||||||
Revenues from external customers | $ | 212,585 | $ | 78,885 | $ | 8,282 | $ | 1,749 | $ | — | $ | 301,501 | |||||||||||
Intersegment Revenues | 846 | — | — | — | (846 | ) | — | ||||||||||||||||
Revenues | 213,431 | 78,885 | 8,282 | 1,749 | (846 | ) | 301,501 | ||||||||||||||||
Segment profit (loss) | 36,802 | 12,815 | (56,878 | ) | (14,926 | ) | — | (22,187 | ) | ||||||||||||||
Depreciation and amortization | 20,205 | 1,608 | 4,829 | 1,055 | — | 27,697 | |||||||||||||||||
Segment assets | 127,212 | 29,613 | 136,041 | 16,860 | — | 309,726 | |||||||||||||||||
The following table presents a reconciliation of the total segment assets to total assets (in thousands):
77
December 31, | |||||||
2016 | 2015 | ||||||
Total segment assets | $ | 179,904 | $ | 164,032 | |||
Intersegment elimination | (970 | ) | — | ||||
Corporate assets: | |||||||
Cash and cash equivalents | 59,632 | 59,065 | |||||
Accounts Receivable, net | 632 | 846 | |||||
Property and equipment, net | 16,600 | 19,232 | |||||
Intangible assets, net | 9,688 | 11,744 | |||||
Goodwill | 42,648 | 42,710 | |||||
Prepaid and other assets | 9,073 | 9,245 | |||||
Total assets | $ | 317,207 | $ | 306,874 | |||
Long-lived tangible assets consist of property and equipment, net and field equipment, net. The following table presents total long-lived tangible assets by geographic area (in thousands):
December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
United States | $ | 46,724 | $ | 46,551 | $ | 45,501 | |||||
Mexico | 13,431 | 14,233 | 12,689 | ||||||||
Other Americas | 571 | 825 | 482 | ||||||||
Total Americas | 60,726 | 61,609 | 58,672 | ||||||||
Germany | 17,574 | 19,053 | 21,246 | ||||||||
Other Europe | 5,754 | 6,793 | 7,774 | ||||||||
Total Europe | 23,328 | 25,846 | 29,020 | ||||||||
Total | $ | 84,054 | $ | 87,455 | $ | 87,692 | |||||
Substantially all of our revenues are derived from the sale of the System One and related products, which cannot be used with any other dialysis system, and from needles and blood tubing sets in the U.S.
The following table summarizes the number of customers who individually make up greater than ten percent of total revenues:
Years Ended December 31, | ||||||||
2016 | 2015 | 2014 | ||||||
DaVita | 20 | % | 20 | % | 21 | % | ||
Fresenius | 18 | % | 17 | % | 16 | % | ||
Sales to DaVita HealthCare Partners Inc. (DaVita) and Fresenius Medical Care (Fresenius) are in the System One segment.
10. | Commitments and Contingencies |
Minimum Purchase Commitments
We have agreements with certain of our suppliers, primarily for the purchase of fluids for our System One segment and needles for our In-Center segment, that include minimum purchase commitments. As of December 31, 2016, we had a total of $28.9 million in minimum purchase commitments under these agreements, $9.8 million over the next year, $18.2 million over the next one to two years and $0.9 million over the next four to five years.
Operating Leases
Our operating leases relate to our corporate headquarters and NxStage Kidney Care facilities, as well as our foreign leased manufacturing facilities in Mexico, Germany and Italy.
Our corporate headquarters lease has an initial term of eleven years through mid-2023 with an early termination provision after seven years, subject to certain terms and conditions, with two 5-year options to extend beyond the initial term on
78
substantially the same terms and at rent equal to ninety-five percent of the then fair market value. The lease included a tenant improvement allowance of $4.3 million.
Our NxStage Kidney Care facility lease agreements have initial lease terms expiring from 2018 to 2026, and contain renewal options ranging from five to ten years at the fair rental value at the time of renewal. The leases are generally subject to periodic consumer price index increases or contain fixed escalation clauses and include tenant improvement allowances ranging from $0.1 million to $0.3 million per facility.
Our foreign leased manufacturing facilities are subject to lease agreements with termination dates through December 2017 to 2021 and contain renewal options at the fair rental value at the time of renewal.
Our lease agreements contain certain provisions that require us to pay executor costs such as real estate taxes, operating expenses and common utilities. Rent expense is recorded on a straight-line basis over the lease term. Tenant improvement allowances are recorded as a deferred rent obligation and amortized to rent expense over the term of the lease.
Rent expense under our operating leases was $5.0 million, $4.5 million and $3.8 million during 2016, 2015 and 2014, respectively.
The future minimum rental payments as of December 31, 2016 under our operating leases are as follows (in thousands):
2017 | $ | 4,995 | |
2018 | 5,115 | ||
2019 | 4,287 | ||
2020 | 3,710 | ||
2021 | 3,095 | ||
Thereafter | 3,390 | ||
$ | 24,592 | ||
Legal Contingencies
From time to time, during the ordinary course of operations, we are party to litigation and arbitration and are subject to investigations relating to various aspects of our business. We regularly analyze current information about such claims for probable losses and provide accruals for such matters, including the estimated legal expenses and consulting services in connection with these matters, as appropriate. We utilize our internal legal department as well as external resources for these assessments. In making the decision regarding the need for loss accrual, we consider the degree of probability of an unfavorable outcome and our ability to make a reasonable estimate of the amount of loss.
In management's opinion, we are not currently involved in any legal proceedings which, individually or in the aggregate, are reasonably likely to have a material adverse effect on our financial condition or results of operations.
11. | Income Taxes |
The following is a summary of income (loss) before income taxes by geography (in thousands):
Years Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
U.S. | $ | (9,265 | ) | $ | (17,672 | ) | $ | (24,315 | ) | ||
Foreign | 3,050 | 2,489 | 1,255 | ||||||||
Total | $ | (6,215 | ) | $ | (15,183 | ) | $ | (23,060 | ) | ||
The components of the provision for income taxes are as follows (in thousands):
79
Years Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
Current: | |||||||||||
U.S. - State | $ | 73 | $ | 86 | $ | 57 | |||||
Foreign | 1,200 | 1,225 | 1,278 | ||||||||
Total Current | 1,273 | 1,311 | 1,335 | ||||||||
Deferred: | |||||||||||
Foreign | (171 | ) | (234 | ) | (82 | ) | |||||
Total Deferred | (171 | ) | (234 | ) | (82 | ) | |||||
Total Provision | $ | 1,102 | $ | 1,077 | $ | 1,253 | |||||
We recorded income tax expense during 2016, 2015 and 2014 for certain profitable foreign subsidiaries.
A reconciliation of the U.S. federal statutory tax rate to the effective tax rate is as follows:
Years Ended December 31, | ||||||||
2016 | 2015 | 2014 | ||||||
Federal statutory rate | 34.0 | % | 34.0 | % | 34.0 | % | ||
Stock compensation | (1.5 | )% | (2.5 | )% | 2.0 | % | ||
State income tax, net of federal tax benefit | (2.3 | )% | (2.8 | )% | 1.9 | % | ||
U.S. research credits | 18.9 | % | (0.3 | )% | 4.1 | % | ||
Foreign income inclusion in the U.S. | (10.4 | )% | (5.0 | )% | (2.8 | )% | ||
Valuation allowance | (47.4 | )% | (25.8 | )% | (39.7 | )% | ||
Change in tax reserves | (0.5 | )% | (0.5 | )% | — | % | ||
Minority Interest | (15.9 | )% | (0.9 | )% | (0.5 | )% | ||
Other, net | 7.4 | % | (3.3 | )% | (4.4 | )% | ||
Effective tax rate | (17.7 | )% | (7.1 | )% | (5.4 | )% | ||
Deferred income tax assets and liabilities reflect the tax effects of differences in the recognition of income and expense items for tax and financial reporting purposes. During our fiscal year ended December 31, 2015 we began presenting all deferred tax assets and liabilities as noncurrent on our Consolidated Balance Sheets prospectively. Deferred tax assets (liabilities) are made up of the following (in thousands):
80
December 31, | |||||||
2016 | 2015 | ||||||
Deferred tax assets: | |||||||
Net operating loss carryforwards | $ | 115,151 | $ | 115,851 | |||
Tax credits | 11,180 | 10,052 | |||||
Stock-based compensation | 7,339 | 6,684 | |||||
Capitalized research and development | 2,167 | 2,598 | |||||
Financing liabilities | 5,161 | 6,217 | |||||
Other | 8,349 | 7,625 | |||||
Total deferred tax assets | 149,347 | 149,027 | |||||
Deferred tax liabilities: | |||||||
Fixed assets | (5,754 | ) | (7,442 | ) | |||
Intangible assets | (3,606 | ) | (4,282 | ) | |||
Other | (187 | ) | (524 | ) | |||
Total deferred tax liabilities | (9,547 | ) | (12,248 | ) | |||
Net deferred tax assets before valuation allowance | 139,800 | 136,779 | |||||
Less valuation allowance | (138,795 | ) | (135,817 | ) | |||
Net deferred tax assets | $ | 1,005 | $ | 962 | |||
As of December 31, 2016, we had U.S. federal and state net operating loss carryforwards of approximately $371 million and $177 million, respectively, available to offset future taxable income. A portion of the federal net operating loss, $52.9 million, and state net operating losses, $34.7 million, is attributable to excess tax deductions related to stock-based compensation. We will record the benefit of these excess tax deductions beginning with the year-ended December 31, 2017, offset by a full valuation allowance, in accordance with ASU No. 2016-09: “Improvements to Employee Share-Based Payment Accounting”. The federal and state net operating loss carryforwards will expire between 2017 and 2036 if not utilized. We also had federal and state research and development credit carryforwards of $7.8 million and $2.3 million ($3.4 million gross), respectively, which begin to expire in 2020 if not utilized. We also had foreign tax credits of approximately $1.0 million that will expire between 2018 and 2021 if not utilized. All years remain open for examination by the United States Internal Revenue Service ("IRS") due to the losses incurred and years 2011 through 2016 remain open for examination in the various states and non-US tax jurisdictions in which we files tax returns.
During 2016, the deferred tax valuation allowance increased by approximately $3.0 million, primarily as the result of increases to federal and state tax credits and changes to deferred temporary tax differences. A full valuation allowance has been recorded in the accompanying consolidated financial statements to offset our U.S. and specific foreign deferred tax assets because the future realizability of such assets is uncertain. In assessing the realizability of deferred tax assets we consider, among other things, the expected reversal of deferred tax liabilities and projected future taxable income. Valuation allowances are reversed only when we have adequate history of taxable income and projections for future taxable income. We believe that the future realization of these assets is not more likely than not given the expected future tax losses in these jurisdictions.
Under the provisions of the Internal Revenue Code, the net operating loss and tax credit carryforwards are subject to review and possible adjustment by the Internal Revenue Service and state tax authorities. Net operating loss and tax credit carryforwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant shareholders over a three-year period in excess of 50 percent, as defined under Sections 382 and 383 of the Internal Revenue Code, respectively, as well as similar state provisions. This could limit the amount of tax attributes that can be utilized annually to offset future taxable income or tax liabilities. The amount of the annual limitation is determined based on the value of the Company immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. The Company has completed several financings since its inception which it believes has resulted in a change in control as defined by Sections 382 and 383 of the Internal Revenue Code. We believe $1.5 million of our net operating losses will expire unused due to limitations.
For applicable years, the Company generated research credits but has not conducted a study to document its qualified activities. This study may result in an adjustment to the Company’s research and development credit carryforwards; however, until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position. A full valuation allowance has been provided against the Company’s research and development credits and, if an adjustment is
81
required, this adjustment would be offset by an adjustment to the deferred tax asset established for the research and development credit carry-forwards and the valuation allowance.
The below table details the changes in unrecognized tax benefits, which if recognized would favorably impact our effective tax rate (in thousands):
Years Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
Balance at beginning of the year | $ | 379 | $ | 347 | $ | 411 | |||||
Increase in unrecognized tax benefits as a result of tax positions taken during the current year | 89 | 66 | 88 | ||||||||
Increase (decrease) in unrecognized tax benefits as a result of tax positions taken during a prior year | 115 | (34 | ) | (110 | ) | ||||||
Reductions due to lapse of applicable statute of limitations | (66 | ) | — | — | |||||||
Reductions due to audit settlement | — | — | (42 | ) | |||||||
Balance at end of year | $ | 517 | $ | 379 | $ | 347 | |||||
As of December 31, 2016 we had a liability for unrecognized tax benefits included in the balance sheet of approximately $0.5 million, all of which would impact our effective tax rate if recognized. We had less than $0.1 million accrued as of December 31, 2016, 2015 and 2014 for interest and penalties related to unrecognized tax benefits.
We monitor the undistributed earnings of foreign subsidiaries and, as necessary, provide for income taxes on those earnings that are not deemed permanently invested. As of December 31, 2016, there were no significant undistributed earnings of foreign subsidiaries that were deemed permanently invested.
12. | Stock Plans and Stock-Based Compensation |
Stock Incentive Plans
We maintain the 2014 Omnibus Incentive Plan (2014 Plan) that governs awards to both employees and non-employees. The 2014 Plan replaced and superseded our 2005 Stock Incentive Plan (2005 Plan) except that awards granted under the 2005 Plan remain in effect pursuant to their original terms. While the 2014 Plan authorizes a variety of equity-based and cash awards, we generally offer equity incentives under the 2014 Plan in the form of restricted stock units, stock options and performance shares. Restricted stock units customarily vest over a period of three or four years. Stock options expire ten years from the date of grant and customarily vest over a period of four years for employees and one year for members of our Board of Directors. Performance shares are issued to certain employees and executive officers and entitle recipients to earn restricted stock units based on the achievement of annual corporate financial performance metrics; any earned restricted stock units customarily vest over a period of three years. We settle stock option exercises and the vesting of restricted stock units with newly issued common shares. Pursuant to the 2014 Plan, each share award other than options or stock appreciation rights will reduce the number of total shares available for grant by 1.62 shares. A total of 5.6 million shares, together with any unissued shares that may carry over from the 2005 Plan, have been authorized for grant under the 2014 Plan and at December 31, 2016, 2.0 million shares remained available for future grant.
We also maintain the 2005 Employee Stock Purchase Plan (2005 Purchase Plan), which authorizes the issuance of up to 0.9 million shares of common stock to participating employees through a series of periodic offerings. Each six-month offering period begins in January and July. An employee becomes eligible to participate in the 2005 Purchase Plan once he or she has been employed for at least three months and is regularly employed for at least 20 hours per week for more than three months in a calendar year. The price at which employees can purchase common stock in an offering is 95 percent of the closing price of our common stock on the NASDAQ Global Select Market on the lower of the first or last day of the offering period, unless otherwise determined by the Board of Directors or Compensation Committee of the Board. As of December 31, 2016, 0.2 million shares were available for future issuance under the 2005 Purchase Plan.
Stock Options
A summary of the status of stock options granted under all of our plans at December 31, 2016, and changes during the year then ended, is as follows:
82
Stock Options | Shares | Weighted Average Exercise Price | Aggregate Intrinsic Value | Average Remaining Contractual Life | |||||||||
(In thousands) | (In years) | ||||||||||||
Outstanding at beginning of year | 4,084,256 | $ | 14.29 | ||||||||||
Granted | 1,334,351 | $ | 15.94 | ||||||||||
Exercised | (658,444 | ) | $ | 12.50 | |||||||||
Forfeited or expired | (103,758 | ) | $ | 14.02 | |||||||||
Outstanding at end of year | 4,656,405 | $ | 15.02 | $ | 52,094 | 6.71 | |||||||
Fully vested and exercisable | 2,818,091 | $ | 14.54 | $ | 32,896 | 5.58 | |||||||
Fully vested, exercisable and expected to vest | 4,511,178 | $ | 15.00 | $ | 50,577 | 6.66 | |||||||
The aggregate intrinsic value for stock options is calculated based on the market price of our common stock as of December 31, 2016, less the exercise price of the underlying awards, excluding out-of-the-money awards. The total fair value of options that vested during 2016, 2015 and 2014 was $6.1 million, $4.8 million and $4.9 million, respectively. The aggregate intrinsic value of options exercised during 2016, 2015 and 2014 was $5.6 million, $10.4 million and $9.2 million, respectively. The aggregate intrinsic value of options exercised is calculated based on the market price of our common stock on the exercise date, less the exercise price of underlying award.
The weighted-average fair value of options granted during 2016, 2015 and 2014 was $5.89, $6.16 and $5.80 per option, respectively. The fair value of options at date of grant was estimated using the Black-Scholes option-pricing model with the following assumptions:
Years Ended December 31, | |||||
2016 | 2015 | 2014 | |||
Expected life (in years) | 5.00 to 9.92 | 4.80 to 10.00 | 4.40 to 5.00 | ||
Risk-free interest rate | 1.20% to 2.45% | 1.34% to 2.27% | 1.56% to 1.69% | ||
Expected stock price volatility | 38% to 43.05% | 38% to 42.52% | 43% to 45% | ||
Expected dividend yield | — | — | — | ||
Restricted Stock
The total fair value of restricted stock that vested was $5.1 million, $5.5 million and $3.2 million during 2016, 2015 and 2014, respectively. The weighted-average fair value of restricted stock granted during 2016, 2015 and 2014 was $16.33, $16.71 and $14.35 per unit, respectively. The following table summarizes the status of the unvested restricted stock:
Shares | Weighted Average Grant-date Fair Value | Aggregate Intrinsic Value | Weighted Average Remaining Contractual Life | |||||||||
(In thousands) | (In years) | |||||||||||
Unvested at December 31, 2015 | 1,251,079 | $ | 15.02 | |||||||||
Granted | 668,846 | $ | 16.33 | |||||||||
Vested | (357,461 | ) | $ | 14.22 | ||||||||
Forfeited | (418,664 | ) | $ | 16.28 | ||||||||
Unvested at December 31, 2016 | 1,143,800 | $ | 15.57 | $ | 29,979 | 1.88 | ||||||
The aggregate intrinsic value for restricted stock is calculated based on the market price of our common stock as of December 31, 2016.
Employee Stock Purchase Plan
The weighted-average fair value of stock purchase rights granted as part of the 2005 Purchase Plan during 2016, 2015 and 2014 was $4.07, $1.74 and $1.46 per share, respectively. The fair value of the employees' stock purchase rights was estimated using the Black-Scholes option-pricing model with the following assumptions:
83
Years Ended December 31, | |||||
2016 | 2015 | 2014 | |||
Expected life (in months) | 6 | 6 | 6 | ||
Risk-free interest rate | 0.37% to 0.49% | 0.11% to 0.13% | 0.07% to 0.10% | ||
Expected stock price volatility | 39.6% to 48.2% | 18.4% to 22.4% | 26.3% to 27.4% | ||
Expected dividend yield | — | — | — | ||
There were 37,531, 50,343 and 70,899 shares issued under the 2005 Purchase Plan during 2016, 2015 and 2014, respectively, which resulted in share-based compensation expense of $0.2 million in 2016 and $0.1 million in each of 2015 and 2014.
Stock-based Compensation Expense
The following table presents stock-based compensation expense included in the consolidated statements of comprehensive loss (in thousands):
Years Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
Cost of revenues | $ | 1,371 | $ | 1,068 | $ | 1,212 | |||||
Selling and marketing | 3,304 | 4,458 | 4,442 | ||||||||
Research and development | 1,536 | 1,979 | 1,726 | ||||||||
General and administrative | 3,906 | 5,093 | 5,501 | ||||||||
Total stock-based compensation expense | $ | 10,117 | $ | 12,598 | $ | 12,881 | |||||
As of December 31, 2016, approximately $16.7 million of unrecognized stock compensation cost related to nonvested stock options and restricted stock (net of estimated forfeitures) is expected to be recognized over a weighted-average period of 2.6 years.
13. | Employee Benefit Plans |
401(k) Retirement Plan
We have a 401(k) retirement plan (401(k) Plan) for the benefit of eligible employees, as defined. Each participant may elect to contribute up to 75% of his or her compensation to the 401(k) Plan each year, subject to certain IRS limitations. We contribute 100% of the first 3% of the employee's contribution and 50% of the next 2% of the employee's contribution. We contributed $2.5 million, $1.8 million and $1.7 million to the 401(k) Plan during 2016, 2015 and 2014, respectively.
Other Compensation Plans
We maintain postemployment benefit plans for employees in certain foreign subsidiaries. These unfunded plans provide lump sum benefits, payable based on statutory regulations for voluntary or involuntary termination. We have recorded a liability of $1.7 million, as of December 31, 2016, 2015 and 2014, respectively, as other long-term liabilities for costs associated with these plans. The expense recorded in connection with these plans was not significant during 2016, 2015 and 2014.
14. | Stockholders' Equity |
We received 114,301, 49,786 and 196,378 shares of common stock that were surrendered in payment for the exercise of stock options during 2016, 2015 and 2014, respectively.
15. Business Combination
During 2015, as part of our NxStage Kidney Care market development activities, we acquired a fifty-one percent controlling interest in a privately held dialysis clinic that offers home hemodialysis and peritoneal dialysis services for $0.6 million in cash, paid in 2016.
16. Noncontrolling Interests
Noncontrolling interests represent the third-party equity ownership interests in consolidated entities for which we have effective control, including variable interest entities (VIE) for which we are deemed the primary beneficiary.
84
We assess the terms of our investment interests to determine if any of our investees meet the definition of a VIE. For any VIEs, we perform an analysis to determine whether our variable interests give us a controlling financial interest in a VIE. The analysis identifies the primary beneficiary of a VIE as the enterprise that has both 1) the power to direct activities of a VIE that most significantly impact the entity’s economic performance and 2) the obligation to absorb losses of the entity or the right to receive benefits from the entity.
As of December 31, 2016, we have 7 VIEs included in our consolidated financial statements all of which are NxStage Kidney Care dialysis centers. We are the managing member or we have a majority seat on the entity’s board of managers, manage these entities through a management services agreement, and provide operating and capital funding as necessary for the entities to accomplish their operational and strategic objectives which transfer substantial power over and economic responsibility for the entities to us.
The analysis upon which these consolidation determinations rest are complex, involve uncertainties, and require significant judgment on various matters. At December 31, 2016 and 2015, total assets of our VIEs were $8.0 million and $11.0 million, and total liabilities and noncontrolling interests of our VIEs were $7.2 million and $10.2 million, respectively.
We have potential obligations to purchase the noncontrolling interests held by third parties in certain of our consolidated subsidiaries. These obligations are in the form of put provisions and are exercisable at the third-party owners' discretion given specific facts and circumstances as outlined in each specific put provision. If these put provisions were exercised, we would be required to purchase all the third-party owners' noncontrolling interests at a fair value at the time of exercise pursuant to the terms of the agreement. At December 31, 2016 the Company's noncontrolling interests subject to put provisions were less than $0.1 million and none of the rights were exercisable.
The following table sets forth the changes in noncontrolling interest not subject to put provisions for the periods indicated (in thousands):
Years Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
Balance at beginning of period | $ | 1,694 | $ | 1,088 | $ | 525 | |||||
Capital contributions by noncontrolling interest | 630 | 1,316 | 930 | ||||||||
Sales of noncontrolling interests | 679 | 208 | — | ||||||||
Net loss attributable to noncontrolling interest in consolidated subsidiary | (2,378 | ) | (918 | ) | (367 | ) | |||||
Balance at end of period | $ | 625 | $ | 1,694 | $ | 1,088 | |||||
17. Derivative Instruments and Hedging
We operate manufacturing and service facilities in Mexico, Germany, and Italy and we purchase materials and pay our employees at those facilities in pesos and euros, and as such, we are potentially exposed to adverse as well as beneficial movements in currency exchange rates. We enter into foreign exchange forward contracts to minimize the impact of currency exchange rate fluctuations on these peso and euro denominated expenses. These contracts have a duration of up to twelve months and are designated as cash flow hedges. The counterparties to these foreign exchange forward contracts are creditworthy financial institutions; therefore, we do not consider the risk of counterparty nonperformance to be material. The notional amount of our outstanding contracts that are designated as cash flow hedges was $18.6 million and $22.1 million at December 31, 2016 and December 31, 2015, respectively. The fair value of these contracts is recorded on the balance sheet within prepaid expenses and other current assets or accrued expenses depending on the gain (loss) position. The fair value of these contracts was a liability of $1.8 million and $1.1 million at December 31, 2016 and December 31, 2015, respectively. The cash flows related to our currency exchange contracts are classified as operating cash flows, which is consistent with the cash flow treatment of the underlying items being hedged.
Gains or losses related to hedge ineffectiveness recognized in earnings were not material during 2016, 2015 or 2014. Given the short-term nature of our contracts, any gains or losses recorded within accumulated other comprehensive income (loss) will be recognized in earnings within the next twelve months.
The following table presents the effect of these contracts designated as cash flow hedges on our consolidated financial statements (in thousands):
Gain (Loss) Recognized in OCI (Effective Portion) | Gain (Loss) Reclassified from OCI into Income (Effective Portion) | Classification within the Condensed Consolidated Statement of Comprehensive Loss | ||||||||
Year Ended December 31, 2016 | ||||||||||
Foreign exchange forward contracts | $ | (2,712 | ) | $ | (1,910 | ) | Cost of revenues | |||
Year Ended December 31, 2015 | ||||||||||
Foreign exchange forward contracts | $ | (2,140 | ) | $ | (1,745 | ) | Cost of revenues | |||
Year Ended December 31, 2014 | ||||||||||
Foreign exchange forward contracts | $ | (1,223 | ) | $ | (258 | ) | Cost of revenues | |||
18. Accumulated Other Comprehensive (Loss) Income
The following additional information is provided with respect to the accumulated other comprehensive (loss) income as presented on the condensed consolidated balance sheets (in thousands):
Unrealized gain (loss) on derivative instruments | Other (2) | Total | ||||||||||
Balance, net of tax, as of December 31, 2013 | $ | (123 | ) | $ | 335 | $ | 212 | |||||
Other comprehensive income before reclassifications | (1,223 | ) | (1,439 | ) | (2,662 | ) | ||||||
Loss reclassified to earnings (1) | 258 | — | 258 | |||||||||
Total other comprehensive income (loss) | (965 | ) | (1,439 | ) | (2,404 | ) | ||||||
Balance, net of tax, as of December 31, 2014 | $ | (1,088 | ) | $ | (1,104 | ) | $ | (2,192 | ) | |||
Other comprehensive income before reclassifications | (2,140 | ) | (1,444 | ) | (3,584 | ) | ||||||
Gain reclassified to earnings (1) | 1,745 | — | 1,745 | |||||||||
Total other comprehensive income (loss) | (395 | ) | (1,444 | ) | (1,839 | ) | ||||||
Balance, net of tax, as of December 31, 2015 | $ | (1,483 | ) | $ | (2,548 | ) | $ | (4,031 | ) | |||
Other comprehensive income before reclassifications | (2,712 | ) | (1,268 | ) | (3,980 | ) | ||||||
Gain/Loss reclassified to earnings (1) | 1,910 | — | 1,910 | |||||||||
Total other comprehensive income (loss) | (802 | ) | (1,268 | ) | (2,070 | ) | ||||||
Balance, net of tax, as of December 31, 2016 | $ | (2,285 | ) | $ | (3,816 | ) | $ | (6,101 | ) | |||
(1) Reclassifications of gains (losses) on derivative instruments are included in cost of revenues on the consolidated statement of comprehensive loss. See Note 17, Derivative Instruments and Hedging for further information.
(2) Other includes cumulative translation adjustments and, to a lesser extent, pension benefits.
19. Fair Value Measurements
We measure the fair value of our foreign exchange forward contracts classified as derivative instruments using an income approach, based on prevailing market forward rates less the contract rate multiplied by the notional amount. The product of this calculation is then adjusted for counterparty risk.
We did not have any transfers between Level 1 and Level 2 and Level 3 during the twelve months ended December 31, 2016.
The following tables present assets and liabilities measured at fair value on a recurring basis and their level within the value hierarchy (in thousands):
86
December 31, 2016 | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | Total Fair Value | ||||||||||||
Assets | ||||||||||||||||
Money market funds (1) | $ | 34,804 | $ | — | $ | — | $ | 34,804 | ||||||||
Liabilities | ||||||||||||||||
Foreign exchange forward contracts (2) | $ | — | $ | 1,771 | $ | — | $ | 1,771 | ||||||||
December 31, 2015 | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | Total Fair Value | ||||||||||||
Assets | ||||||||||||||||
Money market funds (1) | $ | 34,776 | $ | — | $ | — | $ | 34,776 | ||||||||
Foreign exchange forward contracts (2) | — | 51 | — | 51 | ||||||||||||
Liabilities | ||||||||||||||||
Foreign exchange forward contracts (2) | $ | — | $ | 1,192 | $ | — | $ | 1,192 | ||||||||
(1) Money market funds are included within cash and cash equivalents.
(2) | Foreign exchange forward contracts are included within prepaid expenses and other current assets or accrued expenses depending on the gain (loss) position. |
The carrying amount of our long-term debt approximates fair value at December 31, 2016. The fair value of our long-term debt was estimated using inputs derived principally from market observable data, including current rates offered to us for debt of the same or similar remaining maturities. Within the hierarchy of fair value measurements, these are Level 2 inputs.
The carrying amounts reflected in the consolidated balance sheets for cash and cash equivalents (including money market funds), accounts receivable, prepaid expenses and other current and non-current assets, accounts payable and accrued expenses approximate fair value due to their short-term nature.
20. Supplemental Cash Flow Information
The following additional information is provided with respect to the consolidated statements of cash flows (in thousands):
Years Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
Supplemental Disclosures: | |||||||||||
Cash paid for interest | $ | 639 | $ | 703 | $ | 671 | |||||
Cash paid for taxes | 983 | 919 | 903 | ||||||||
Noncash Investing and Financing Activities: | |||||||||||
Transfers from inventory to field equipment | $ | 20,920 | $ | 16,675 | $ | 25,370 | |||||
Transfers from field equipment to deferred cost of revenues | 13,400 | 11,638 | 13,110 | ||||||||
Payment of corporate bonus plan in common stock | — | 1,103 | — | ||||||||
Market value of shares received in payment for exercise of stock options | 2,320 | 875 | 3,026 | ||||||||
Construction-in-process financed by construction liability | 187 | 1,332 | 4,898 | ||||||||
Property and equipment acquired under capital lease | 127 | 354 | 76 | ||||||||
Acquisition of business | — | 513 | — | ||||||||
Increase in deferred revenues related to the new manufacturing facility in Germany | — | 663 | 3,608 | ||||||||
Capital contributions by noncontrolling interest | 54 | 325 | — | ||||||||
21. Quarterly Financial Data (Unaudited)
The following table sets forth selected quarterly information (unaudited) (in thousands, except per share data):
87
Three Months Ended | |||||||||||||||
March 31, 2016 | June 30, 2016 | September 30, 2016 | December 31, 2016 | ||||||||||||
Revenues | $ | 89,207 | $ | 92,207 | $ | 91,951 | $ | 93,013 | |||||||
Gross profit | 36,517 | 38,423 | 39,181 | 37,864 | |||||||||||
Loss from operations | (975 | ) | (1,180 | ) | 24 | (2,154 | ) | ||||||||
Net loss | (1,783 | ) | (2,225 | ) | (890 | ) | (2,419 | ) | |||||||
Net loss attributable to stockholders of NxStage Medical, Inc. | (1,276 | ) | (1,732 | ) | (166 | ) | (1,597 | ) | |||||||
Net loss per share, basic and diluted | $ | (0.02 | ) | $ | (0.03 | ) | $ | (0.00 | ) | $ | (0.02 | ) | |||
Three Months Ended | |||||||||||||||
March 31, 2015 | June 30, 2015 | September 30, 2015 | December 31, 2015 | ||||||||||||
Revenues | $ | 79,482 | $ | 80,316 | $ | 86,521 | $ | 89,804 | |||||||
Gross profit | 30,126 | 30,864 | 34,718 | 35,763 | |||||||||||
Loss from operations | (5,579 | ) | (5,116 | ) | (1,635 | ) | (2,299 | ) | |||||||
Net loss | (5,863 | ) | (5,594 | ) | (2,002 | ) | (2,801 | ) | |||||||
Net loss attributable to stockholders of NxStage Medical, Inc. | (5,658 | ) | (5,327 | ) | (1,687 | ) | (2,670 | ) | |||||||
Net loss per share, basic and diluted | $ | (0.09 | ) | $ | (0.08 | ) | $ | (0.03 | ) | $ | (0.04 | ) | |||
Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
Not applicable.
Item 9A. | Controls and Procedures |
Our management, with the participation of our chief executive officer and chief financial officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2016. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities and Exchange Act of 1934, or the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC's rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company's management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Our management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2016, our chief executive officer and chief financial officer concluded that, as of such date, our disclosure controls and procedures were effective to achieve their stated purpose.
No change in our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act) occurred during the three months ended December 31, 2016 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Management's Report on Internal Control over Financial Reporting
We, as management of NxStage Medical, Inc., are responsible for establishing and maintaining adequate internal control over financial reporting. Pursuant to the rules and regulations of the Securities and Exchange Commission, internal control over financial reporting is a process designed by, or under the supervision of, the company's principal executive and principal financial officer, or persons performing similar functions, and effected by the company's board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
• | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
• | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
88
• | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management has evaluated the effectiveness of its internal control over financial reporting as of December 31, 2016, based on the control criteria established in a report entitled Internal Control - Integrated Framework, issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework).
Based on such evaluation, we have concluded that NxStage's internal control over financial reporting is effective as of December 31, 2016.
The independent registered public accounting firm of Ernst & Young LLP, as auditors of NxStage's consolidated financial statements, has issued an attestation report on its assessment of NxStage's internal control over financial reporting.
89
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Shareholders of NxStage Medical, Inc.
We have audited NxStage Medical, Inc.'s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework) (the COSO criteria). NxStage Medical, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, NxStage Medical, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of NxStage Medical, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of comprehensive loss, changes in stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2016 of NxStage Medical, Inc., and our report dated February 28, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Boston, Massachusetts
February 28, 2017
90
Item 9B. | Other Information |
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
We have included information about our executive officers in Part I of this Annual Report under the caption “Executive Officers”. Certain documents relating to our corporate governance, including our Code of Business Conduct and Ethics, which is applicable to our directors, officers and employees, and the charters of the Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee of our Board of Directors, are available on our website at www.nxstage.com. We intend to make all required disclosures regarding any amendments to, or waivers from, provisions of our Code of Business Conduct and Ethics on our website. We include our website address in this Annual Report only as an inactive textual reference and do not intend it to be an active link to our website.
The response to the remainder of this item is incorporated by reference from the discussion responsive thereto in the sections entitled “Election of Directors,” “Corporate Governance,” and “Other Matters - Section 16(a) Beneficial Ownership Reporting Compliance” contained in the proxy statement for our 2017 annual meeting of stockholders.
Item 11. Executive Compensation
The response to this item is incorporated by reference from the discussion responsive thereto in the sections entitled “Information About Executive and Director Compensation” and “Corporate Governance” contained in the proxy statement for our 2017 annual meeting of stockholders.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The response to this item is incorporated by reference from the discussion responsive thereto in the sections entitled “Stock Ownership of Certain Beneficial Owners and Management” and “Information About Executive and Director Compensation” contained in the proxy statement for our 2017 annual meeting of stockholders.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The response to this item is incorporated by reference from the discussion responsive thereto in the sections entitled “Certain Relationships and Related Transactions” and “Corporate Governance” contained in the proxy statement for our 2017 annual meeting of stockholders.
Item 14. Principal Accountant Fees and Services
The response to this item is incorporated by reference from the discussion responsive thereto in the section entitled “Corporate Governance” contained in the proxy statement for our 2017 annual meeting of stockholders.
91
PART IV
Item 15. | Exhibits and Financial Statement Schedules |
(a) Financial Statements
The following consolidated financial statements are filed as part of this Annual Report under “Item 8 — Financial Statements and Supplementary Data”:
(b) Exhibits
The exhibits listed in the Exhibit Index immediately preceding the exhibits are incorporated herein by reference and are filed as part of this Annual Report.
(c) Financial Statement Schedules
None. No financial statement schedules have been filed as part of this Annual Report because they are either not applicable or the required information has been included in the accompanying notes to the consolidated financial statements.
Item 16. | Form 10-K Summary |
Not applicable.
92
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
NXSTAGE MEDICAL, INC. | ||
By: | /s/ Jeffrey H. Burbank | |
Jeffrey H. Burbank | ||
Chief Executive Officer | ||
February 28, 2017 | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ Jeffrey H. Burbank | Chief Executive Officer and Director | February 28, 2017 | ||
Jeffrey H. Burbank | (Principal Executive Officer) | |||
/s/ Matthew W. Towse | Chief Financial Officer and Senior Vice President | February 28, 2017 | ||
Matthew W. Towse | (Principal Financial and Accounting Officer) | |||
/s/ Robert G. Funari | Chairman of the Board of Directors | February 28, 2017 | ||
Robert G. Funari | ||||
/s/ Heyward R. Donigan | Director | February 28, 2017 | ||
Heyward R. Donigan | ||||
/s/ Daniel A. Giannini | Director | February 28, 2017 | ||
Daniel A. Giannini | ||||
/s/ Earl R. Lewis | Director | February 28, 2017 | ||
Earl R. Lewis | ||||
/s/ Jean K. Mixer | Director | February 28, 2017 | ||
Jean K. Mixer | ||||
/s/ Craig W. Moore | Director | February 28, 2017 | ||
Craig W. Moore | ||||
/s/ Reid S. Perper | Director | February 28, 2017 | ||
Reid S. Perper | ||||
/s/ James J. Peters | Director | February 28, 2017 | ||
James J. Peters | ||||
93
EXHIBIT INDEX
Incorporated by Reference to^ | |||||||||
Exhibit Number | Description | Form or Schedule | Exhibit No. | Filing Date with SEC | |||||
3.1 | Restated Certificate of Incorporation | S-1/A | 3.4 | 10/7/2005 | |||||
3.2 | Amended and Restated By-Laws | S-1/A | 3.5 | 10/7/2005 | |||||
4.1 | Specimen certificate evidencing shares of common stock | S-1/A | 4.1 | 10/7/2005 | |||||
10.1# | 2005 Stock Incentive Plan, as amended | DEF 14A | Appendix B | 4/28/2011 | |||||
10.2# | Forms of Incentive Stock Option Agreement and Nonstatutory Stock Option Agreement under the 2005 Stock Incentive Plan | S-1/A | 10.22 | 10/20/2005 | |||||
10.3# | Form of Restricted Stock Unit Agreement under the 2005 Stock Incentive Plan | 10-Q | 10.3 | 5/8/2014 | |||||
10.4# | 2014 Omnibus Incentive Plan | DEF 14A | Appendix B | 4/24/2014 | |||||
*10.5# | Form of Stock Option Agreement under the 2014 Omnibus Incentive Plan | ||||||||
*10.6# | Form of Restricted Stock Unit Agreement under the 2014 Omnibus Incentive Plan | ||||||||
*10.7# | Form of Performance Share Award Agreement under the 2014 Omnibus Incentive Plan | ||||||||
10.8# | 2005 Employee Stock Purchase Plan, as amended | DEF 14A | Appendix A | 4/23/2015 | |||||
10.9# | Employment Agreement dated October 19, 2005 between the Registrant and Jeffrey H. Burbank | S-1/A | 10.12 | 10/20/2005 | |||||
10.10# | Employment Agreement dated October 18, 2005 between the Registrant and Joseph E. Turk, Jr. | S-1/A | 10.15 | 10/20/2005 | |||||
10.11# | Employment Agreement dated October 18, 2005 between the Registrant and Winifred L. Swan | S-1/A | 10.16 | 10/20/2005 | |||||
10.12# | Employment Agreement dated November 27, 2006 between the Registrant and Robert S. Brown | 10-K | 10.10 | 3/16/2007 | |||||
10.13# | Employment Agreement dated as of July 15, 2013 between the Registrant and Matthew W. Towse | 10-Q | 10.2 | 11/7/2013 | |||||
10.14# | Form of Indemnification Agreement entered into between the Registrant and each of its directors and executive officers | S-1/A | 10.21 | 9/21/2005 | |||||
*10.15# | Director Compensation Policy | ||||||||
10.16 | Credit Agreement dated as of June 9, 2014 among the Registrant and certain of its subsidiaries, Capital One Financial Corporation and Silicon Valley Bank | 10-Q | 10.1 | 8/7/2014 | |||||
10.17† | Second Amended and Restated National Service Provider Agreement dated as of March 1, 2013 between the Registrant and DaVita Healthcare Partners Inc. | 10-Q | 10.1 | 7/25/2013 | |||||
10.18† | Amendment to Second Amended and Restated National Service Provider Agreement dated as of June 13, 2016 between the Registrant and DaVita Healthcare Partners, Inc. | 10-Q | 10.1 | 8/4/2016 | |||||
10.19† | Chronic Outpatient Therapy Agreement, as amended through January 10, 2014, between the Registrant and Fresenius USA Marketing, Inc. | 10-Q/A | 10.1 | 10/1/2014 | |||||
10.20† | Supply Agreement dated as of January 5, 2007 between the Registrant and Membrana GmbH | 10-K | 10.27 | 3/16/2007 | |||||
10.21† | Supply Agreement dated April 10, 2009 between the Registrant and Laboratorios PiSA SA de C.V. | 10-Q/A | 10.45 | 10/19/2009 | |||||
10.22† | Amendment to Supply Agreement dated as of July 22, 2013 between Medisystems Corporation and Laboratorios PiSA SA de C.V. | 10-Q | 10.1 | 11/7/2013 | |||||
10.23† | Technology and Trademark License Agreement effective June 15, 2009 between the Registrant and Asahi Kasei Kuraray Medical Co., Ltd. | 10-Q | 10.48 | 8/7/2009 | |||||
10.24† | Lease dated as of June 22, 2011 between Registrant and 350 Riverwalk, LLC | 10-Q/A | 10.33 | 10/7/2011 | |||||
*21 | List of Subsidiaries | ||||||||
*23 | Consent of Ernst & Young LLP | ||||||||
*31.1 | Certification of Chief Executive Officer pursuant to Exchange Act Rules 13a-14a or 15d-14a, as adopted pursuant to Section 302 of Sarbanes-Oxley Act of 2002 | ||||||||
*31.2 | Certification of Chief Financial Officer pursuant to Exchange Act Rules 13a-14a or 15d-14a, as adopted pursuant to Section 302 of Sarbanes-Oxley Act of 2002 | ||||||||
94
**32.1 | Certification of Chief Executive Officer pursuant to Exchange Act Rules 13a-14(b) or 15d-14(b) and 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of Sarbanes-Oxley Act of 2002 | ||||||||
**32.2 | Certification of Chief Financial Officer pursuant to Exchange Act Rules 13a-14(b) or 15d-14(b) and 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of Sarbanes-Oxley Act of 2002 | ||||||||
*101.INS | XBRL Instance Document | ||||||||
*101.SCH | XBRL Taxonomy Extension Schema | ||||||||
*101.CAL | XBRL Taxonomy Extension Calculation Linkbase | ||||||||
*101.DEF | XBRL Taxonomy Extension Definition Linkbase | ||||||||
*101.LAB | XBRL Taxonomy Extension Label Linkbase | ||||||||
*101.PRE | XBRL Taxonomy Extension Presentation Linkbase | ||||||||
_______________________________________
^ | Exhibits previously filed with the Securities and Exchange Commission as exhibits to Form S-1 or S-1/A were filed under Commission File Number 333-126711. All other previously filed exhibits were filed under Commission File Number 0-51567. | |
* | Filed herewith. | |
** | Furnished herewith. | |
† | Confidential treatment has been granted or requested with respect to portions of this exhibit. Confidential portions are omitted and filed separately with the Securities and Exchange Commission. | |
# | Management contract or compensatory plan or arrangement. | |
95
