Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - LA JOLLA PHARMACEUTICAL CO | pressreleaseexhibit.htm |
| 8-K - 8-K - LA JOLLA PHARMACEUTICAL CO | ljpc-5018xk.htm |

ATHOS-3 Phase 3 Study of LJPC-501
Positive Topline Results
February 27, 2017

Forward-Looking Statement
These slides contain forward-looking statements as that term is defined in the Private Securities
Litigation Reform Act of 1995. These statements relate to future events or the company’s future
results of operations. These statements are only predictions or statements of current expectations
and involve known and unknown risks, uncertainties and other factors, that may cause actual results
to be materially different from those anticipated by the forward-looking statements. The company
cautions readers not to place undue reliance on any such forward-looking statements, which speak
only as of the date they were made. Certain of these risks, uncertainties, and other factors are
described in greater detail in the company’s filings with the U.S. Securities and Exchange
Commission (SEC), all of which are available free of charge on the SEC’s web site www.sec.gov.
These risks include, but are not limited to, risks relating to: the timing of the NDA submission for
LJPC-501 and prospects for approval of the NDA; risks that the full data set from the ATHOS-3 study
will not be consistent with the top-line results of the study; risks relating to the scope of product labels
(if approved) and potential market sizes, as well as the broader commercial opportunity; the
anticipated timing for regulatory actions; the success of future development activities; potential
indications for which the company’s product candidates may be developed; and the expected
duration over which the company’s cash balances will fund its operations. Subsequent written and
oral forward-looking statements attributable to the company or to persons acting on its behalf are
expressly qualified in their entirety by the cautionary statements set forth in the company’s reports
filed with the SEC. The company expressly disclaims any intent to update any forward-looking
statements.
2

Mission Statement
La Jolla is dedicated to improving the lives of
patients suffering from life-threatening diseases
by discovering and developing innovative
therapies

Catecholamine Resistant Hypotension
(CRH) Remains a Major Unmet Medical Need

Shock
MORTALITY RATES COMPARED
Shock: Deadly, Costly and Prevalent
1. Vincent JL, De Backer D. N Engl J Med. 2013;369(18):1726-1734. 2. Sviri S, Hashoul J, Stav I, van Heerden PV. J Crit Care.
2014;29(1):157-160. 3. Readmissions and deaths-national. Data.Medicare.gov website. https://data.medicare.gov/Hospital-
Compare/Readmissions-and-Deaths-National/qqw3-t4ie/data. Accessed January 10, 2017
Abbreviations: AMI=acute myocardial infarction; CHF=congestive heart failure.
5
• A well-characterized syndrome1
§ Occurs when the organs and tissue of the body
do not receive an adequate flow of blood
(oxygen) due to a lack of blood pressure
(hypotension)
• Deadly
§ Mortality rate exceeds that of most acute
conditions requiring hospitalization2
§ Can kill old and young alike within hours2
• Costly
§ Estimated costs are 2-3 times greater
compared to other conditions
• Prevalent
§ Affects one-third of patients in the intensive
care unit1
30-day mortality rate3
14% 12%
16%
AMI CHF Pneumonia
≥50% mortality
in patients with
shock in the
ICU2

CMS Covered Charges for CRH Population Are Much
Greater Than for Other Acute Hospital Conditions
6
Source: CMS FY14 Inpatient Public Use File (IPUF)
$87,282
$42,243
$31,453 $30,702
CRH AMI CHF Pneumonia
Weighted Average CMS Covered Charges
Abbreviations: AMI=acute myocardial infarction; CHF=congestive heart failure.

U.S. Shock Patient Population and Treatment
Paradigm
7
1. 9.42MM annualized vials (785K vials sold in January 2017 X 12); Symphony Health Solutions, 2017. 97% of vials sold for hypotensive shock; estimate based on
medical literature. 13 vials used per patient; estimate based on Russell et al, N Engl J Med, 358:877-87, 2008 and Asfar et al, N Engl J Med, 370:1583-93, 2014
2. Wolters Kluwer PriceRx Pro, 2017
3. 3.01MM annualized vials (251K vials sold in January 2017 X 12); Symphony Health Solutions, 2017. 81% of vials sold for hypotensive shock; estimate based on
medical literature. 10 vials used per patient; estimate based on Dunser et al, Circulation, 107:2313-2319, 2003 and Gordon et al, Crit Care Med, 42(6):1325-1333,
2014
4. Decision Resources Group market research
First-Line
Standard-of-Care
Second-Line
Standard-of-Care
LJPC-501 Target
Patient Population
555,479
361,684
332,189Patients Who Do Not
Adequately Respond
to Norepinephrine
and Vasopressin
Vasopressin:
244,000 Patients per Year3
$1,385 per Patient2
$338MM Sales Run Rate
Norepinephrine:
703,000 Patients per Year1
$153 per Patient2
$108MM Sales Run Rate
196,000
Estimated
Patients4

Randomized Study of Vasopressin
8
VASST Overall Survival1
VASST=Vasopressin and Septic Shock Trial
1. Russell JA, Walley KR, Singer J, et al. for VASST Investigators. N Engl J Med. 2008;358(9):877-887
Day 28 HR=0.90
(95%CI: 0.75-1.08)
P=0.27
Day 90 HR=0.88
(95%CI: 0.76-1.03)
P=0.10

LJPC-501: The First Synthetic Human
Angiotensin II Tested in a Randomized
Phase 3 Study

LJPC-501: The First Synthetic Human
Angiotensin II Tested in a Randomized Phase 3 Study
10
• LJPC-501 is a proprietary formulation of synthetic human angiotensin II,
a naturally occurring regulator of blood pressure
• Angiotensin II has been shown to raise blood pressure in a pilot,
randomized, placebo-controlled, pilot study in CRH1, as well as in animal
models of hypotension
• Special Protocol Assessment (SPA) agreement reached with FDA for
Phase 3 study design
§ Agreement reached that blood pressure can be the primary endpoint for approval
• ATHOS-3 enrollment completed in Q4 2016
1. Chawla et al. Critical Care 2014, 18:534

Three Systems Work in Harmony to Regulate Blood
Pressure
Existing Treatments for Shock Only Utilize Two Systems
THERAPIES AND ASSOCIATED
ADVERSE EVENTS
SYMPATHETIC
NERVOUS
ARGININE-
VASOPRESSIN
RENIN
ANGIOTENSIN-
ALDOSTERONE
11
CATECHOLAMINES1: SYMPATHETIC NERVOUS
Prolonged elevated heart rate, tachyarrhythmia, acute
cardiac arrest or death, pulmonary hypertension
VASOPRESSIN: ARGININE-VASOPRESSIN
Myocardial ischemia, decreases gut blood flow
RENIN ANGIOTENSIN-ALDOSTERONE
No current therapies
1. Catecholamines include: norepinephrine, epinephrine, dopamine, phenylephrine, ephedrine

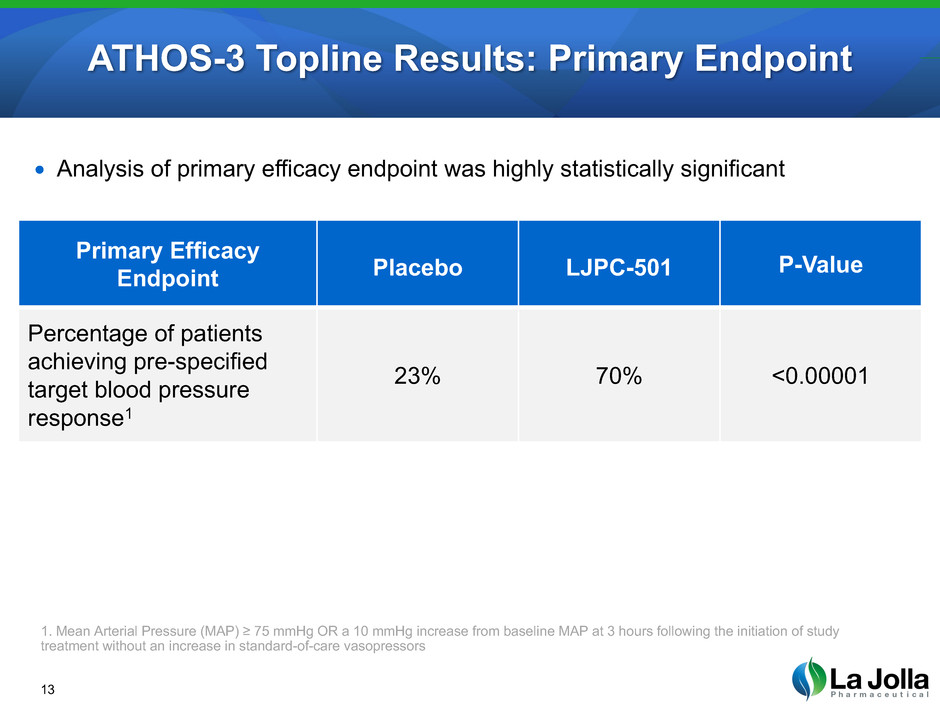
Primary endpoint
Percentage of patients
achieving pre-specified
target blood pressure
response1
ATHOS-3 (Angiotensin II for the Treatment of
High-Output Shock) Study Design
12
1. Mean Arterial Pressure (MAP) ≥ 75 mmHg OR a 10 mmHg increase from baseline MAP at 3 hours following the initiation of study
treatment without an increase in standard-of-care vasopressors
Patient
population:
• Adult patients
with CRH
• N=344 enrolled
• N=321 treated
Study Conducted In 74 Centers Across 9 Countries
1:1
double-blind
randomization
Placebo
N=158
LJPC-501
N=163
ATHOS-3 Topline Results: Primary Endpoint
Primary Efficacy
Endpoint Placebo LJPC-501 P-Value
Percentage of patients
achieving pre-specified
target blood pressure
response1
23% 70% <0.00001
13
1. Mean Arterial Pressure (MAP) ≥ 75 mmHg OR a 10 mmHg increase from baseline MAP at 3 hours following the initiation of study
treatment without an increase in standard-of-care vasopressors
• Analysis of primary efficacy endpoint was highly statistically significant

ATHOS-3 Topline Results: Mortality
Estimated
Risk Reduction Hazard Ratio
1
95%
Confidence
Interval
P-Value
22% 0.78 0.57-1.07 0.12
1. Proportional hazards estimate (unadjusted) of mortality to Day 28 comparing placebo-treated patients to LJPC-501-treated patients
14
• Trend toward longer survival observed

ATHOS-3 Topline Results: Safety
Placebo LJPC-501
Percentage of patients experiencing
at least one adverse event 92% 87%
Percentage of patients discontinuing
treatment due to an adverse event 22% 14%
15
• Throughout the study, safety outcomes were followed by an independent Data
Safety Monitoring Board (DSMB)
§ The DSMB recommended that the study continue as originally planned

We plan to present and publish detailed results
from ATHOS-3 later this year
Thank You
