Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - Moleculin Biotech, Inc. | v457040_ex23-1.htm |
| EX-4.1 - EXHIBIT 4.1 - Moleculin Biotech, Inc. | v457040_ex4-1.htm |
As filed with the Securities and Exchange Commission on January 18, 2017.
Registration No. 333-214898
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1 TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Moleculin Biotech, Inc.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | 2834 | 47-4671997 |
| (State or Other Jurisdiction of | (Primary Standard Industrial | (I.R.S. Employer |
| Incorporation or Organization) | Classification Code Number) | Identification Number) |
2575 West Bellfort, Suite 333
Houston, Texas 77054
(713) 300-5160
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Mr. Walter Klemp, Chief Executive Officer
2575 West Bellfort, Suite 333
Houston, Texas 77054
(713) 300-5160
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent For Service)
Copies to:
| Cavas S. Pavri | Robert F. Charron, Esq. |
| Schiff Hardin LLP | Ellenoff Grossman & Schole LLP |
| 100 N. 18th, Suite 300 | 1345 Avenue of the Americas |
| Philadelphia, PA 19103 | New York, NY 10105-0302 |
| Telephone: (202) 724-6847 | Telephone: (212) 370-1300 |
| Fax: (202) 778-6460 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Securities Exchange Act of 1934. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ |
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | x |
CALCULATION OF REGISTRATION FEE
Title of each class of securities to be registered | Proposed maximum aggregate offering price (1) | Amount of registration fee | ||||||
| Units, each consisting of one share of Common stock, par value $0.001 per share, and Warrants, to purchase shares of Common stock | 13,282,500.00 | 1,539.44 | ||||||
| Common stock included in the Units (3) | - | |||||||
| Warrants included in the Units (3) | - | |||||||
| Common Stock underlying the Warrants included in the Units (2) | 3,320,625.00 | 384.86 | ||||||
| Representatives’ warrant | - | |||||||
| Common stock issuable upon exercise of the Representatives’ warrant (2) | 929,775.00 | 107.76 | ||||||
| Total | $ | 17,532,900.00 | $ | 2,032.06 | (4) | |||
(1) Estimated solely for purposes of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933. The fee table assumes offering price of $2.31 per Unit, which is based on the closing price of the registrant’s common stock on January 13, 2017.
(2) Pursuant to Rule 416 under the Securities Act, the securities being registered hereunder include such indeterminate number of additional shares of common stock as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions.
(3) No fee is required pursuant to Rule 457(i) under the Securities Act of 1933, as amended.
(4) Of this amount, $1,738.50 was previously paid.
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement is filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell, nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to Completion Dated January 18, 2016
5,000,000 Units
Each Consisting of One Share of Common Stock and
Warrants to Purchase 0.25 Shares of Common Stock
Moleculin Biotech, Inc.
We are offering 5,000,000 units, with each unit consisting of one share of our common stock and warrants to purchase 0.25 of a share of our common stock (and the 1,250,000 shares of our common stock issuable from time to time upon exercise of the offered warrants). The purchase price for each unit is $ . Each warrant will have an exercise price of $ per share and will expire five years from the date of issuance. The units will not be issued or certificated. The shares of common stock and the warrants are immediately separable and will be issued separately, but will be purchased together in this offering. The shares of common stock, warrants and shares of common stock underlying the warrants are sometimes collectively referred to herein as the “securities.”
Our common stock is listed on the NASDAQ Capital Market under the symbol “MBRX.” On January 13, 2017, the last sale price for our common stock as reported on the NASDAQ Capital Market was $2.31 per share. There is no established public trading market for the warrants, and we do not expect a market to develop. In addition, we do not intend to apply for a listing of the warrants on any national securities exchange.
The offering is being underwritten on a firm commitment basis. We have granted the underwriters an option to buy up to an additional 750,000 shares and/or an additional 187,500 warrants, in any combinations thereof, from us to cover over-allotments. The underwriters may exercise this option at any time and from time to time during the 45-day period from the date of this prospectus.
We are an “emerging growth company” as defined in Section 2(a) of the Securities Act of 1933, as amended, and we have elected to comply with certain reduced public company reporting requirements.
| Per Unit(1) | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discounts and commissions (2) | $ | $ | ||||||
| Proceeds, before expenses, to us | $ | $ |
| (1) | The public offering price and underwriting discount correspond to an assumed public offering price per share of common stock of $____ and an assumed public offering price per warrant of $____. |
| (2) | We have also agreed to issue to the representative warrants to purchase shares of common stock in an amount equal to 7% of the aggregate number of shares sold in this offering, and to reimburse the representative for certain of its expenses. See “Underwriting” for a description of compensation payable to the underwriters. |
Investing in our securities involves a high degree of risk. See the section entitled “Risk Factors” appearing on page 12 of this prospectus for a discussion of information that should be considered in connection with an investment in our securities.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the common stock and warrants representing the units to purchasers on or about , 2017.
The date of this prospectus is , 2017
| Roth Capital Partners | National Securities Corporation |
Table of Contents
No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus. You must not rely on any unauthorized information or representations. This prospectus is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is current only as of its date.
Market data and certain industry data and forecasts used throughout this prospectus were obtained from internal company surveys, market research, consultant surveys, publicly available information, reports of governmental agencies and industry publications and surveys. Industry surveys, publications, consultant surveys and forecasts generally state that the information contained therein has been obtained from sources believed to be reliable, but that the accuracy and completeness of such information is not guaranteed. While we are not aware of any misstatements regarding the industry data presented in this prospectus, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” in this prospectus.
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information that you should consider before deciding to invest in our securities. You should read this entire prospectus carefully, including the “Risk Factors” section, our historical financial statements and the notes thereto, and unaudited pro forma financial information, each included elsewhere in this prospectus. The terms “MBI”, the “Company”, “our”, or “we” refer to Moleculin Biotech, Inc. and, unless the context otherwise requires, its predecessors.
Overview
We are a preclinical-stage pharmaceutical company focused on the development of anti-cancer drug candidates, some of which are based on license agreements with The University of Texas System on behalf of the M.D. Anderson Cancer Center, which we refer to as MD Anderson. Our lead drug candidate is liposomal Annamycin, which we refer to as Annamycin, an anthracycline intended for the treatment of relapsed or refractory acute myeloid leukemia, or AML. Annamycin has been in clinical trials pursuant to an Investigational New Drug application, or IND, that had been filed with the U.S. Food and Drug Administration, or FDA. Due to a lack of development activity by a prior drug developer, this IND was terminated. However, we intend to apply for a new IND based on the same data that supported the original IND, updated for subsequent clinical data, and to resume clinical trials for Annamycin. We have two other drug development projects in progress, one involving a portfolio of small molecules, which we refer to as the WP1066 Portfolio, focused on the modulation of key regulatory transcription factors involved in the progression of cancer, and the WP1122 Portfolio, a suite of molecules targeting the metabolic processes involved in cancer in general, and glioblastoma (the most common form of brain tumor) in particular.
We have been granted royalty-bearing, worldwide, exclusive licenses for the patent and technology rights related to our WP1066 Portfolio and WP1122 Portfolio drug technologies, as these patent rights are owned by MD Anderson. The Annamycin drug substance is no longer covered by any existing patent protection. We intend to submit patent applications for formulation, synthetic process and reconstitution related to our Annamycin drug product candidate, although there is no assurance that we will be successful in obtaining such patent protection. Independently from potential patent protection, we believe Annamycin will qualify for Orphan Drug status and have submitted our application to the FDA, which could entitle us to market exclusivity of up to 7 and 10 years from the date of approval of a New Drug Application (“NDA”) and Marketing Authorization (“MA”), in the US and the European Union (“EU”), respectively. However, there can be no assurance that such status will be granted. Separately, the FDA may also grant market exclusivity of up to five years for newly approved new chemical entities (of which Annamycin would be one), but there can be no assurance that such exclusivity will be granted or, if granted, for how long.
Our Drug Candidates
Annamycin
Our lead product candidate is Annamycin, an anthracycline intended to target the treatment of relapsed or refractory AML. Anthracyclines are a class of chemotherapy drugs designed to disrupt the DNA of, and eventually destroy, targeted cancer cells. They are considered to be among the most effective anticancer drugs developed and are used to treat a range of cancers, including leukemias, lymphomas, and breast, stomach, uterine, ovarian, bladder, and lung cancers. The effectiveness of currently approved anthracyclines is limited, however, by their propensity to cause heart damage (cardiotoxicity) and for cancer cells to become resistant by natural cell defense (multidrug resistance) mechanisms.
| 1 |
Leukemia is a cancer of the white blood cells and acute forms of leukemia can manifest quickly and leave patients with limited treatment options. AML is the most common type of acute leukemia in adults. It occurs when a clone of leukemic progenitor white blood cells proliferates in the bone marrow, suppressing the production of normal blood cells. Currently, the only viable option for acute leukemia patients is a bone marrow transplant, which is successful in a significant number of patients. However, in order to qualify for a bone marrow transplant, the patient’s leukemia cells must be decreased to a sufficiently low level. This begins with a therapy of combining two chemotherapeutic drugs, which always includes an anthracycline, in inducing remission (a complete response, or CR), which therapy has not improved since it was first used in the 1970s and we estimate that this induction therapy has the same success rate of about 20% as at that time. Unfortunately, the current clinically approved anthracyclines are cardiotoxic, which can result in damage to the heart and limit the dosage amount that may be administered to patients. Additionally, the tumor cells often present de novo or develop resistance to the first line anthracycline, often through what is called “multidrug resistance”, enabling them to purge themselves of the available anthracyclines. Consequently, there remains no effective therapy for these patients and most will succumb quickly to their leukemia. If a patient’s leukemia reappears before they can be prepared for a bone marrow transplant, they are considered to have “relapsed”. If a patient fails to achieve a sufficient response from the induction therapy to qualify for a bone marrow transplant, they are considered to be “refractory” (resistant to therapy). Together, this group of relapsed and refractory AML patients constitutes our primary focus for treatment with Annamycin and our intent is to pursue FDA approval for Annamycin as a second-line induction therapy for adult relapsed and refractory AML patients.
We believe that pursuing approval as a second line induction therapy for adult relapsed or refractory AML patients is the shortest path to regulatory approval, but we also believe that one of the most important potential uses of Annamycin is in the treatment of children with either AML or ALL (acute lymphoblastic leukemia, which is more common in children). Accordingly, we also intend to pursue approval for pediatric use when practicable.
Annamycin is a liposome formulated anthracycline (also referred to in literature as “L-Annamycin”). It has been tested in 6 clinical trials and 114 patients without any reporting of cardiotoxicity and, in the two of those clinical trials focused on leukemia, with fewer dose-limiting toxicities than are normally experienced with doxorubicin (one of the leading first-line anthracyclines used for induction therapy). Each of these trials was conducted by previous holders of the intellectual property surrounding Annamycin and not by our company. Annamycin demonstrated significant activity in 8 of 16 patients in a Phase I study in adult relapsed or refractory AML patients, with 6 of 14 patients completely clearing leukemic blasts. A 30 patient dose-ranging Phase I/II study in ALL demonstrated a similar level of activity, with 3 of 10 patients treated with the maximum tolerable dose clearing their leukemic blasts to a level sufficient to qualify for a bone marrow transplant. One of these patients went on to receive a successful curative bone marrow transplant. The other two of these three patients died of tumor lysis syndrome, a condition resulting from the overloading of their system with the debris from leukemic blast cells destroyed by the induction therapy. Armed with the knowledge of this potential, prophylactic pretreatment known to protect patients from the effects of tumor lysis syndrome will be deployed where appropriate in future trials. Based on the results of the above clinical trials, we believe Annamycin is different from currently approved induction therapy drugs in four key ways: (i) it has demonstrated clinical activity in a patient population for whom there are currently no effective therapies, (ii) it appears to be capable of avoiding the “multi-drug resistance” mechanisms that often limit the effectiveness of currently approved anthracyclines; (iii) it has been shown to be non-cardiotoxic in animal models and no events of cardiotoxicity have been reported from the use of Annamycin in 114 patients; and (iv) in AML cell lines, it has been shown to be more potent than one of the leading approved drugs.
Because the prior developer of Annamycin allowed their IND to lapse, we are required to submit a new IND for continued clinical trials with Annamycin. Toward this end, we recently submitted a request for a Pre-IND meeting with the FDA. Based on subsequent conversations with the FDA, we will be allowed to incorporate by reference the prior developer’s IND in submitting our new request for IND. We believe this will not only save time and expense with regard to our IND submission, but it should also reduce the risk that our IND submission is delayed due to a review of any of this prior data. Written communication from the FDA indicates they do not see a need for a Pre-IND meeting and plan to respond to questions in our Pre-IND briefing document in writing on December 6, 2016.
Based on initial conversations with the FDA, because of the serious unmet medical need, we believe Annamycin may qualify for accelerated approval based on our planned clinical trials. In order to facilitate our communication with the FDA, we requested access to and reviewed in detail the available data supporting the dose-ranging Phase I/II clinical trial discussed above, which was conducted by a previous developer of Annamycin. In October 2016, we announced that we had identified some positive findings from this review, which gave rise to a modification of our own clinical development plan. We had indicated that our plan was to conduct a detailed review of the clinical results generated by that prior developer, and then to use those results to reestablish an IND in order to continue clinical trials of Annamycin. However, in the course of our review, we identified that Annamycin may have greater potential for efficacy than we originally believed, based on an unexpected opportunity to increase the drug’s Maximum Tolerable Dose (“MTD”).
| 2 |
In particular, the Dose Limiting Toxicities (“DLTs”) reported in the previous trial that led to the establishment of the current MTD of 150 mg/m2 were all from patients who had an unusually high number of first-line induction therapy failures prior to being treated with Annamycin. Specifically, of the three patients in the last clinical trial who experienced these DLTs, one of them had failed nineteen prior induction therapy attempts, another had failed sixteen and the other had failed fifteen before being enrolled in the trial. We concluded from our review of this data that, if the heavily treated patients are excluded from the data set, the MTD could have been closer to 250 mg/m2, substantially higher than the level that was actually set by this previous trial.
We view this as an encouraging development because it means we may have an opportunity to increase the MTD for our next trial from 150 mg/m2 to 200 or even 250 mg/m2. If that turns out to be the case, we believe it could increase the chance for positive outcomes in our next trial.
With the discovery that we may be able to increase our MTD, we determined to adjust our clinical strategy by adding in a Phase I arm to our next Phase II trial, which will add expense to our development effort. We believe this change in strategy will add several months to the eventual final approval of the drug, if the drug is approved.
We have also applied for Orphan Drug status with the FDA for Annamycin. The FDA has responded to our request for Orphan Drug status by requesting additional information, which we have now provided. We expect a response to our revised application in the near future, but can provide no assurance as to when any response will be received.
The prevalence ceiling for qualifying rare diseases under the US Orphan Drug Act is 200,000 patients and proportionally similar guidelines exist in the EU. The most recently published prevalence statistics from the National Cancer Institute reported that an estimated 37,726 patients had acute myeloid leukemia in the United States as of January 1, 2011, and trend data since that publication would indicate that the prevalence today should still be well below the 200,000 patient limitation for Orphan Drugs, which would permit Annamycin for the treatment of acute myeloid leukemia to qualify for Orphan Drug status. Annamycin already qualified for Orphan Drug status with its prior developer and we intend to repeat that process. However, we can provide no assurance that we will be successful in obtaining Orphan Drug status for Annamycin.
The WP1066 Portfolio
We have a license agreement with MD Anderson pursuant to which we have been granted a royalty-bearing, worldwide, exclusive license for the patent and technology rights related to WP1066 and its close analogs, molecules targeting the modulation of key oncogenic transcription factors.
In vitro testing has shown a high level of activity for WP1066 against a wide range of solid tumors, and in vitro testing has shown significant activity against head and neck, pancreatic, stomach, and ovarian cancers, as well as metastatic melanoma and glioblastoma, among others. In vitro testing in mouse tumor models has shown that WP1066 inhibits tumor growth, blocks angiogenesis (a process that provides necessary blood supply to tumors) and increases survival.
With respect to our WP1066 Portfolio, we must complete pre-clinical toxicology testing, along with additional chemistry, manufacturing and control work to fully characterize the drug, establish the desired formulation and develop reference standards for future drug release, among others, prior to submitting an application for an IND. A clinician at MD Anderson has advised us that she is proceeding with a physician-sponsored IND for WP1066 treatment of brain tumors. We are not participating in this IND process. The clinician has submitted an IND to the FDA and has indicated that this IND is on hold until documentation of Good Manufacturing Process or GMP production of WP1066 can be presented to the FDA.
| 3 |
An analog of WP1066, referred to as WP1220, was previously the subject of an IND (WP1220 was referred to as MOL4239 for purposes of this IND) related to use of the molecule in the topical treatment of psoriasis. Clinical trials were commenced on WP1220 in the US, but were terminated early due to limited efficacy in the topical treatment of psoriatic plaques. Notwithstanding its limitations in treating psoriasis, our pre-clinical research has shown WP1220 to be effective in inhibiting cutaneous T-cell lymphoma (“CTCL”) in multiple CTCL cell lines. Based on this encouraging data, we are collaborating with a Polish drug development company, Dermin Sp. Zo. O. (“Dermin”), which has received a Polish government grant to begin a clinical trial in Poland for the topical treatment of early stage CTCL patients.
We also conducted a Phase II clinical trial for WP1066 for the topical treatment of psoriasis, however this trial was terminated early as a significant number of patients experienced a non-permanent worsening of their psoriatic plaques after extended use of the drug, suggesting that its use as a topical agent for non-life threatening diseases such as psoriasis will require further study to optimize dosing and scheduling regimens.
The WP1122 Portfolio
We have a license agreement with MD Anderson pursuant to which we have been granted a royalty-bearing, worldwide, exclusive license for the patent and technology rights related to our WP1122 Portfolio and similar molecules targeting the treatment of glioblastoma multiform, or GBM, and related central nervous system, or CNS, malignancies.
We believe this technology has the potential to target a wide variety of solid tumors, which eventually become resistant to all treatments, and thereby provide a large and important opportunity for novel drugs. Notwithstanding this potential, we are focused on the treatment of central nervous system malignancies and especially GBM. Although less prevalent than some larger categories of solid tumors, cancers of the central nervous system are particularly aggressive and resistant to treatment. The prognosis for such patients can be particularly grim and the treatment options available to their physicians are among the most limited of any cancer.
The American Cancer Society has estimated 23,770 new cases of brain and other nervous system cancers will occur in the United States in 2016, resulting in 16,050 deaths. Despite the severity and poor prognosis of these tumors, there are few FDA-approved drugs on the market.
We have proof of concept data for our WP1122 Portfolio, including data on survival of animals subjected to xenografts of human brain tumors, as well as biodistribution and pharmacokinetics. In non-optimal doses and treatment regimes, our WP1122 Portfolio performed equal to or better than the current market leader, temozolomide and provided for superior survival for animals treated in combination with temozolomide.
Risks Relating to Our Business
As a preclinical-stage, pharmaceutical company, our business and ability to execute our business strategy are subject to a number of risks of which you should be aware before you decide to buy our securities. In particular, you should consider the following risks, which are discussed more fully in the section entitled “Risk Factors”:
| • | we currently do not have regulatory approval for our lead drug candidate, Annamycin, or any other product candidates, in the United States or elsewhere, although we plan to conduct clinical trials in the United States for Annamycin and other drug candidates in the future, there is no assurance that we will be successful in our clinical trials or receive regulatory approval in a timely manner, or at all; |
| • | a portion of our business is dependent upon the intellectual property rights that we license from MD Anderson, and in the past we have been in breach of these license agreements. Although we are currently in compliance with our obligations under our license agreements, there is no assurance that in the future we will not again be in breach of these agreements. Additionally, the intellectual property that we have licensed from MD Anderson may have been developed under a funding agreement with the United States government. To the extent that is the case, our license agreements with and the intellectual property rights we have licensed from MD Anderson are subject to such a funding agreement and any superior rights that the U.S. government may have with respect to the licensed intellectual property; |
| 4 |
| • | our lead drug candidate, Annamycin, is not the subject of any patent protection, and, although we intend to apply for formulation and method-of-use patents for Annamycin, there is no assurance that we will be successful in obtaining such patents and, even if we are successful, such patents generally offer less protection than original composition of matter patents; |
| • | unforeseen side effects from any of our product candidates could arise during clinical development. For example, in the most recent Phase I/II dose-ranging clinical trial of Annamycin, two patients succumbed to tumor lysis syndrome (“TLS”) resulting from the debris created by Annamycin killing the targeted leukemic blasts more rapidly than anticipated. As another example, we intend to attempt to increase the maximum tolerable dose (“MTD”) for Annamycin by conducting another Phase I dose-ranging trial, however, unforeseen side effects could prevent us from increasing the MTD from the one established in the prior Phase I/II trial; |
| • | we do not currently carry product liability insurance covering any of our drug candidates and, although we intend to obtain product liability insurance for future clinical trial liability that we may incur, there can be no assurance that we will secure adequate coverage or that, even if we do so, any such coverage will be sufficient to prevent the exposure of our operations to significant potential liability in the future; |
| • | the patents we have licensed from MD Anderson may not be valid or enforceable and may not protect us against competitors who challenge those licensed patents, obtain their own patents that may have an adverse effect on our ability to conduct business, or are able to otherwise circumvent our patents. Additionally, our products and technologies are complex and one patent may not be sufficient to protect our products where a series of patents may be needed. Further, we may not have the necessary financial resources to enforce or defend our patents or patent applications. In addition, any patent applications we may have made or may make relating to inventions for our actual or potential products and technologies may not result in patents being issued or may result in patents that provide insufficient or incomplete coverage for our inventions; |
| • | third parties may claim that the manufacture, use or sale of our technologies infringe their intellectual property rights. As with any litigation where such claims may be asserted, we may have to seek licenses, defend infringement actions or challenge the validity of those patents in the patent office or the courts. If these are not resolved favorably, we may not be able to continue to develop and commercialize our product candidates. Even if we were able to obtain rights to a third party’s intellectual property, these rights may be non-exclusive, thereby giving our competitors potential access to the same intellectual property. If we are found liable for infringement or are not able to have these patents declared invalid or unenforceable, we may be liable for significant monetary damages, encounter significant delays in bringing products to market or be precluded from participating in the manufacture, use or sale of products or technologies by patents of others. We may not have identified, or be able to identify in the future, U.S. or foreign patents that pose a risk of potential infringement claims; |
| • | we have completed related party transactions that were not conducted on an arm’s length basis. We acquired the rights to the license agreement with MD Anderson covering our WP1122 Portfolio held by IntertechBio Corporation, a company affiliated with certain members of our management and board of directors. We acquired the rights to all data related to the development of Annamycin held by AnnaMed, Inc., a company affiliated with certain members of our management and board of directors. Prior to our IPO, Moleculin, LLC was merged with and into our company. Moleculin, LLC is affiliated with certain members of our management and board of directors. In addition, prior to our IPO, we entered into an agreement with Houston Pharmaceuticals, Inc., or HPI, whereby HPI agreed to terminate its option to sublicense certain rights to the WP1066 Portfolio and to enter into a co-development agreement with us. Our largest shareholder and a member of our management are shareholders of HPI. Since these transactions were not conducted on an arm’s length basis, it is possible that the terms were less favorable to us than in an arm’s length transaction. |
| 5 |
| • | we have never been profitable, have not generated significant revenue to date and we expect to incur significant additional losses to fund our clinical trials; |
| • | we will require substantial additional funding beyond the proceeds of the offering to which this prospectus relates to complete the development and commercialization of our drug candidates, and such funding may not be available on acceptable terms or at all; |
| • | our development work is dependent in part on collaborations with other drug development companies and funding from government and philanthropic funding sources and there can be no assurance that such collaborations and funding will continue in the future; |
| • | our short-to-medium term prospects depend largely on our ability to develop and commercialize one drug candidate, Annamycin, and our ability to generate revenues in the future will depend heavily on the successful development and commercialization of Annamycin; |
| • | we may be subject to delays in our clinical trials, which could result in increased costs and delays or limit our ability to obtain regulatory approval for Annamycin and/or any other potential drug candidates; |
| • | we have never commercialized any of our drug candidates, including Annamycin, and, even if approved, our drug candidates may not be accepted by healthcare providers or healthcare payors; and |
| • | we may be unable to maintain and protect our intellectual property assets, which could impair the advancement of our pipeline and commercial opportunities. |
Implications of Being an Emerging Growth Company
We qualify as an “emerging growth company” as the term is used in The Jumpstart Our Business Startups Act of 2012 (“JOBS Act”), and therefore, we may take advantage of certain exemptions from various public company reporting requirements, including:
| • | a requirement to only have two years of audited financial statements and only two years of related selected financial data and management’s discussion and analysis; |
| • | exemption from the auditor attestation requirement on the effectiveness of our internal controls over financial reporting; |
| • | reduced disclosure obligations regarding executive compensation; and |
| • | exemptions from the requirements of holding a nonbinding advisory stockholder vote on executive compensation and any golden parachute payments. |
We may take advantage of these provisions until December 31, 2021 or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.0 billion in annual revenues, have more than $700 million in market value of our capital stock held by non-affiliates or issue more than $1.0 billion of non-convertible debt over a three-year period. We may choose to take advantage of some, but not all, of the available benefits of the JOBS Act. We have taken advantage of some of the reduced reporting requirements in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock. In addition, the JOBS Act provides that an emerging growth company can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
| 6 |
Our principal executive offices are located at 2575 West Bellfort, Suite 333, Houston, Texas 77054. Our telephone number is (713) 300-5160. Our website address is www.moleculin.com. The information on or accessible through our website is not part of this prospectus.
| 7 |
The Offering
| Public offering price | $ per unit |
| Common stock we are offering | 5,000,000 shares, plus 1,250,000 shares of our common stock underlying the warrants offered in this offering. |
| Common stock outstanding immediately before this offering | 12,164,851 shares |
| Common stock outstanding immediately after this offering | 17,164,851 shares (assuming none of the warrants issued in this offering are exercised). |
| Warrants we are offering | Each warrant will have an initial exercise price of $ per share of common stock and will expire five years from the date of issuance. This prospectus also relates to the offering of shares of our common stock issuable upon exercise of the warrants. |
| Use of proceeds | We intend to use the proceeds from this offering for working capital and general corporate purposes. See “Use of Proceeds” for more information. |
| NASDAQ symbol | Our common stock is listed on the NASDAQ Capital Market under the symbol “MBRX”. There is no established public trading market for the warrants, and a market will likely never develop. The warrants are not and will not be listed for trading on the NASDAQ Capital Market, any other national securities exchange or other nationally recognized trading system. |
| Risk Factors | See “Risk Factors” and other information appearing elsewhere in this prospectus for a discussion of factors you should carefully consider before deciding whether to invest in our common stock. |
The number of shares of common stock to be outstanding after this offering is based on 12,164,851 shares of common stock outstanding as of January 17, 2017 and does not take into account, as of January 17, 2017:
• 510,000 shares of our common stock issuable upon exercise of outstanding stock options to purchase shares of our common stock at a weighted average exercise price of $5.28 per share;
• 107,802 shares of our common stock issuable upon the exercise of outstanding warrants with a weighted average exercise price of $7.50 per share;
• 1,990,000 shares of our common stock reserved for issuance under our 2015 Stock Plan;
• 1,818,861 shares upon the conversion of outstanding bridge notes. These notes were to be automatically converted according to their terms into our common stock upon the closing of our IPO to the extent and provided that no holder of these notes was or will be permitted to convert such notes to the extent that the holder or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such conversion. Due to this 4.99% limitation, certain of these notes remained outstanding and will be converted into shares of our common stock at such time as the 4.99% limitation continues to be met;
• 79,167 shares of our common stock issuable to a third-party in settlement of $237,500 in past due amounts;
• 1,250,000 shares of common stock issuable upon the exercise of the warrants included in the units being sold in this offering; and
| 8 |
• 350,000 shares of common stock issuable upon the exercise of the warrants issued to the representatives of the underwriters.
Unless otherwise indicated, all information in this prospectus assumes that no exercise of the outstanding options and warrants described above or the warrants offered hereby.
| 9 |
Summary Financial Data
The following table sets forth data derived from our balance sheet at December 31, 2015 and our statement of operations for the period from July 28, 2015 (Inception) to December 31, 2015:
| From July 28, 2015 (Inception) to December 31, 2015 | ||||
| Statement of Operations Data - MBI | ||||
| Revenue | $ | - | ||
| Research and development expense | 260,418 | |||
| General and administrative expense | 477,810 | |||
| Other expense | 10,132 | |||
| Net loss | $ | (748,360 | ) | |
| Net loss per common share | $ | (0.13 | ) | |
| December 31, 2015 | ||||
| Balance Sheet Data - MBI | ||||
| Cash and cash equivalents | $ | 28,091 | ||
| Working capital deficit | (744,699 | ) | ||
| Total assets | 28,091 | |||
| Accumulated deficit | (748,360 | ) | ||
| Total stockholders' deficit | (744,699 | ) | ||
The following table sets forth data derived from Moleculin, LLC’s balance sheets at December 31, 2015 and 2014 and the related statements of operations for each of the years then ended:
| Year Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Statements of Operations Data | ||||||||
| Revenue | $ | - | $ | - | ||||
| Research and development expense | 125,442 | 798,785 | ||||||
| General and administrative expense | 328,570 | 839,556 | ||||||
| Depreciation expense | 11,336 | 11,005 | ||||||
| Other (income) expense | (99,537 | ) | 159,740 | |||||
| Net loss | $ | (365,811 | ) | $ | (1,809,086 | ) | ||
| As of December 31, | ||||||||
| 2015 | 2014 | |||||||
| Statements of Financial Position Data | ||||||||
| Cash and cash equivalents | $ | 31,867 | $ | 524,477 | ||||
| Working capital deficit | (2,844,589 | ) | (1,095,276 | ) | ||||
| Total assets | 45,480 | 781,448 | ||||||
| Accumulated deficit | (14,203,516 | ) | (13,837,705 | ) | ||||
| Total members' deficit | (2,833,330 | ) | (2,467,519 | ) | ||||
| 10 |
The following table sets forth data derived from our balance sheet at September 30, 2016 and the related statement of operations for the nine months ended September 30, 2016 and the period from July 29, 2015 (Inception) to September 30, 2015. Moleculin, LLC was acquired by MBI on May 2, 2016 and ceased to exist after such acquisition by MBI.
| For the Nine Months Ended September 30, 2016 | From July 29, 2015 (Inception) to September 30, 2015 | |||||||
| (Unaudited) | (Unaudited) | |||||||
| Statement of Operations Data - MBI | ||||||||
| Revenue | $ | - | $ | - | ||||
| Research and development expense | 616,498 | 38,409 | ||||||
| General and administrative expense | 1,849,242 | 184,344 | ||||||
| Other expense | 37,307 | 1,562 | ||||||
| Net loss | $ | (2,503,047 | ) | $ | (224,315 | ) | ||
| Net loss per common share | $ | (0.28 | ) | $ | (0.05 | ) | ||
| September 30, 2016 | ||||
| (Unaudited) | ||||
| Balance Sheet Data - MBI | ||||
| Cash and cash equivalents | $ | 6,183,783 | ||
| Working capital deficit | 5,148,499 | |||
| Total assets | 17,573,788 | |||
| Accumulated deficit | (3,251,407 | ) | ||
| Total stockholders' equity | 16,243,635 | |||
| 11 |
Investing in our securities involves a high degree of risk. You should carefully consider each of the following risks, together with all other information set forth in this prospectus, including the financial statements and the related notes, before making a decision to buy our securities. If any of the following risks actually occurs, our business could be harmed. In that case, the trading price of our securities could decline, and you may lose all or part of your investment.
Risks Relating to Our Business
We will require substantial additional funding, which may not be available to us on acceptable terms, or at all, and, if not so available, may require us to delay, limit, reduce or cease our operations.
We intend to use the proceeds from our previous offering, as well as the proceeds from this offering, to, among other uses, advance Annamycin through clinical development. Developing pharmaceutical products, including conducting preclinical studies and clinical trials, is expensive. We will require substantial additional future capital in order to complete clinical development and commercialize Annamycin. If the FDA requires that we perform additional nonclinical studies or clinical trials, or if we determine, as we did in October 2016, that additional clinical trials are required for Annamycin, our expenses would further increase beyond what we currently expect and the anticipated timing of any potential approval of Annamycin would likely be delayed. Further, there can be no assurance that the costs we will need to incur to obtain regulatory approval of Annamycin will not increase.
We will continue to require substantial additional capital to continue our clinical development and commercialization activities. Because successful development of our product candidates is uncertain, we are unable to estimate the actual amount of funding we will require to complete research and development and commercialize our products under development.
The amount and timing of our future funding requirements will depend on many factors, including but not limited to:
| • | whether our updated plan for clinical trials will be completed on a timely basis and, if completed, will be successful in producing useful clinical data in 2017; |
| • | whether we are successful in obtaining an accelerated approval pathway with the FDA related to Annamycin; |
| • | the progress, costs, results of and timing of our clinical trials for Annamycin; |
| • | the outcome, costs and timing of seeking and obtaining FDA and any other regulatory approvals; |
| • | the continued progress of our collaborative drug development partner, which is dependent upon their continued access to grant funding; |
| • | the costs associated with securing and establishing commercialization and manufacturing capabilities; |
| • | market acceptance of our product candidates; |
| • | the costs of acquiring, licensing or investing in businesses, products, product candidates and technologies; |
| • | our ability to maintain, expand and enforce the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
| 12 |
| • | our need and ability to rely on data to be generated by our sublicensee partner, Dermin; |
| • | our need and ability to hire additional management and scientific and medical personnel; |
| • | the effect of competing drug candidates and new product approvals; |
| • | our need to implement additional internal systems and infrastructure, including financial and reporting systems; and |
| • | the economic and other terms, timing of and success of our existing licensing arrangements and any collaboration, licensing or other arrangements into which we may enter in the future. |
Some of these factors are outside of our control. Based upon our currently expected level of operating expenditures, we believe that we will be able to fund our operational plan through the third quarter of 2017. This period could be shortened if there are any significant increases in planned spending on development programs or more rapid progress of development programs than anticipated. We do not believe that our existing capital resources are sufficient to enable us to complete the development and commercialization of Annamycin, if approved, or to initiate any clinical trials or additional development work needed for any other drug candidates, other than as described above. Accordingly, we expect that we will need to raise additional funds in the future.
We may seek additional funding through a combination of equity offerings, debt financings, government or other third-party funding, commercialization, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders. In addition, the issuance of additional shares by us, or the possibility of such issuance, may cause the market price of our shares to decline.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail one or more of our research or development programs. We also could be required to seek funds through arrangements with collaborative partners or otherwise that may require us to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us.
We have in the past completed related party transactions that were not conducted on an arm’s length basis.
We acquired the rights to the license agreement with MD Anderson covering our WP1122 Portfolio held by IntertechBio Corporation, a company affiliated with certain members of our management and board of directors. We acquired the rights to all data related to the development of Annamycin held by AnnaMed, Inc., a company affiliated with certain members of our management and board of directors. Prior to our IPO, Moleculin, LLC merged with and into our company. Moleculin, LLC was affiliated with certain members of our management and board of directors. Prior to our IPO, we, on Moleculin, LLC’s behalf, entered into an agreement with HPI whereby HPI agreed to terminate its option to sublicense certain rights to the WP1066 Portfolio and entered into a co-development agreement with us. Our largest shareholder and a member of our management are shareholders of HPI.
None of the foregoing transactions were conducted on an arm’s length basis. As such, it is possible that the terms were less favorable to us than in an arm’s length transaction.
Our ability to retain the development rights to the WP1066 Portfolio will require us to make up to $1.75 million in future payments to HPI, in addition to payments of shares of our common stock and cash made in connection with our IPO, pursuant to the development agreement we entered into with HPI.
| 13 |
Our acquisition of Moleculin, LLC prior to our IPO provided us with the rights to the license agreement Moleculin, LLC had with MD Anderson covering the WP1066 Portfolio. However, Moleculin, LLC previously granted HPI an option to obtain an exclusive sub-license to develop the WP1066 Portfolio in all non-dermatological fields. Prior to our IPO, we, on Moleculin, LLC’s behalf, entered into two agreements with HPI. The first agreement terminated HPI’s option to obtain the aforementioned exclusive sublicense in exchange for a payment of $100,000 and the issuance of 629,000 shares of our common stock. The second agreement, the HPI Out-Licensing Agreement is a technology rights and development license agreement that provided HPI with a non-exclusive sublicense to develop the WP1066 Portfolio. Pursuant to this HPI Out-Licensing Agreement, we agreed to make payments to HPI of $750,000 over a three-year period commencing after our IPO in exchange for HPI allowing us to access any data, information or know-how resulting from the research and development conducted by HPI, which payments will be expensed when incurred. Notwithstanding our obligation to make the foregoing payments, the HPI Out-Licensing Agreement does not obligate HPI to conduct any specific research or to meet any milestones. Pursuant to the HPI Out-Licensing Agreement, we have the right within three years of the date we entered into the agreement to buy-out from HPI all rights granted to HPI under the agreement for a payment of $1.0 million. If we do not exercise the foregoing buy-out right within three years, the license granted to HPI shall convert into an exclusive license even as to our company. As such, if we do not exercise the buy-out right for any reason, we will no longer have access to the non-dermatology uses of the WP1066 Portfolio and all amounts paid to HPI prior to such date will have value only to the extent that the data, information and know-how may be applicable to dermatology applications of the WP1066 Portfolio. We do not expect to maintain a reserve of $1.0 million to exercise the buy-out payment and, as such, we will need to raise additional funds to make the buy-out payment. We cannot assure you that such additional funding, if required, will be available on satisfactory terms, or at all.
We have never been profitable, we have no products approved for commercial sale, and to date we have not generated any revenue from product sales. As a result, our ability to reduce our losses and reach profitability is unproven, and we may never achieve or sustain profitability.
We have never been profitable and do not expect to be profitable in the foreseeable future. We have not yet submitted any drug candidates for approval by regulatory authorities in the United States or elsewhere. For the nine-month period ended September 30, 2016, we incurred a net loss of $2,503,047. We had an accumulated deficit of $3,251,407 as of September 30, 2016.
To date, we have devoted most of our financial resources to research and development, including our drug discovery research, preclinical development activities and clinical trial preparation, as well as corporate overhead. We have not generated any revenues from product sales. We expect to continue to incur losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for Annamycin, prepare for and begin the commercialization of any approved products, and add infrastructure and personnel to support our continuing product development efforts. We anticipate that any such losses could be significant for the next several years. If Annamycin or any of our other drug candidates fail in clinical trials or does not gain regulatory approval, or if our drug candidates do not achieve market acceptance, we may never become profitable. As a result of the foregoing, we expect to continue to experience net losses and negative cash flows for the foreseeable future. These net losses and negative cash flows have had, and will continue to have, an adverse effect on our stockholders' equity and working capital.
Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. In addition, our expenses could increase if we are required by the FDA to perform studies or trials in addition to those currently expected, or if there are any delays in completing our clinical trials or the development of any of our drug candidates. The amount of future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues.
Our financial condition would be adversely impacted if our intangible assets become impaired.
As a result of the accounting for our acquisition of Moleculin, LLC and the agreement we, on Moleculin, LLC’s behalf, entered into with Houston Pharmaceuticals, Inc., we have carried on our balance sheet within intangible assets in-process research and development (“IPR&D”) of $11,128,790 as of September 30, 2016. Intangibles are evaluated quarterly and are tested for impairment at least annually or when events or changes in circumstances indicate the carrying value of each segment, and collectively our company taken as a whole, might exceed its fair value. We have retained a third party valuation firm to provide us with an initial valuation of these intangible assets; and we expect to receive their final report prior to the filing of our Form 10-K for the year ended December 31, 2016.
| 14 |
Intangible assets related to IPR&D are considered indefinite-lived intangible assets and are assessed for impairment annually or more frequently if impairment indicators exist. If the associated research and development effort is abandoned, the related assets will be written-off and the Company will record a noncash impairment loss on its statement of operations. For those compounds that reach commercialization, if any, the IPR&D assets will be amortized over their estimated useful lives.
If after receipt of the foregoing valuation report we determine that the value of our intangible assets is less than the amounts reflected on our balance sheet, we will be required to reflect an impairment of our intangible assets in the period in which such determination is made. An impairment of our intangible assets would result in our recognizing an expense in the amount of the impairment in the relevant period, which would also result in the reduction of our intangible assets and a corresponding reduction in our stockholders’ equity in the relevant period. As the transactions discussed above were related party transactions and were not conducted on an arm’s length basis, it is possible that the terms were less favorable to us than what we would have received in an arm’s length transaction. We can provide no assurance that the final valuation of our intangible assets will not result in an impairment charge.
We have a limited operating history and we expect a number of factors to cause our operating results to fluctuate on an annual basis, which may make it difficult to predict our future performance.
We are a preclinical pharmaceutical company with a limited operating history. Our operations to date have been limited to acquiring our technology portfolio. We have not yet commenced any clinical trials or obtained any regulatory approvals for any of our drug candidates. Consequently, any predictions made about our future success or viability may not be as accurate as they could be if we had a longer operating history or approved products on the market. Our operating results are expected to significantly fluctuate from quarter-to-quarter or year-to-year due to a variety of factors, many of which are beyond our control. Factors relating to our business that may contribute to these fluctuations include:
| · | any delays in regulatory review and approval of our product candidates in clinical development, including our ability to receive approval from the FDA for Annamycin; |
| · | delays in the commencement, enrollment and timing of clinical trials; |
| · | difficulties in identifying patients suffering from our target indications; |
| · | the success of our clinical trials through all phases of clinical development; |
| · | potential side effects of our product candidate that could delay or prevent approval or cause an approved drug to be taken off the market; |
| · | our ability to obtain additional funding to develop drug candidates; |
| · | our ability to identify and develop additional drug candidates beyond Annamycin and our WP1066 and WP1122 Portfolios; |
| · | competition from existing products or new products that continue to emerge; |
| · | the ability of patients or healthcare providers to obtain coverage or sufficient reimbursement for our products; |
| 15 |
| · | our ability to adhere to clinical trial requirements directly or with third parties such as contract research organizations (CROs); |
| · | our dependency on third-party manufacturers to manufacture our products and key ingredients; |
| · | our ability to establish or maintain collaborations, licensing or other arrangements, particularly with MD Anderson; |
| · | our ability to defend against any challenges to our intellectual property including, claims of patent infringement; |
| · | our ability to enforce our intellectual property rights against potential competitors; |
| · | our ability to secure additional intellectual property protection for our developing drug candidates and associated technologies; |
| · | our ability to attract and retain key personnel to manage our business effectively; and |
| · | potential product liability claims. |
Accordingly, the results of any historical quarterly or annual periods should not be relied upon as indications of future operating performance.
We cannot be certain that Annamycin will receive regulatory approval, and without regulatory approval we will not be able to market Annamycin.
Our business currently depends largely on the successful development and commercialization of Annamycin. Our ability to generate revenue related to product sales, if ever, will depend on the successful development and regulatory approval of Annamycin for the treatment of relapsed or refractory acute myeloid leukemia, or AML.
We currently have no products approved for sale and we cannot guarantee that we will ever have marketable products. The development of a product candidate and issues relating to its approval and marketing are subject to extensive regulation by the FDA in the United States and regulatory authorities in other countries, with regulations differing from country to country. We are not permitted to market our product candidates in the United States until we receive approval of a NDA from the FDA. We have not submitted any marketing applications for any of our product candidates.
NDAs must include extensive preclinical and clinical data and supporting information to establish the product candidate’s safety and effectiveness for each desired indication. NDAs must also include significant information regarding the chemistry, manufacturing and controls for the product. Obtaining approval of a NDA is a lengthy, expensive and uncertain process, and we may not be successful in obtaining approval. The FDA review processes can take years to complete and approval is never guaranteed. If we submit a NDA to the FDA, the FDA must decide whether to accept or reject the submission for filing. We cannot be certain that any submissions will be accepted for filing and review by the FDA. Regulators in other jurisdictions have their own procedures for approval of product candidates. Even if a product is approved, the FDA may limit the indications for which the product may be marketed, require extensive warnings on the product labeling or require expensive and time-consuming clinical trials or reporting as conditions of approval. Regulatory authorities in countries outside of the United States and Europe also have requirements for approval of drug candidates with which we must comply with prior to marketing in those countries. Obtaining regulatory approval for marketing of a product candidate in one country does not ensure that we will be able to obtain regulatory approval in any other country. In addition, delays in approvals or rejections of marketing applications in the United States, Europe or other countries may be based upon many factors, including regulatory requests for additional analyses, reports, data, preclinical studies and clinical trials, regulatory questions regarding different interpretations of data and results, changes in regulatory policy during the period of product development and the emergence of new information regarding our product candidates or other products. Also, regulatory approval for any of our product candidates may be withdrawn.
| 16 |
If we are unable to obtain approval from the FDA, or other regulatory agencies, for Annamycin and our other product candidates, or if, subsequent to approval, we are unable to successfully commercialize Annamycin or our other product candidates, we will not be able to generate sufficient revenue to become profitable or to continue our operations.
Any statements in this prospectus indicating that Annamycin has demonstrated preliminary evidence of efficacy are our own and are not based on the FDA’s or any other comparable governmental agency’s assessment of Annamycin and do not indicate that Annamycin will achieve favorable efficacy results in any later stage trials or that the FDA or any comparable agency will ultimately determine that Annamycin is effective for purposes of granting marketing approval.
Delays in the commencement, enrollment and completion of clinical trials could result in increased costs to us and delay or limit our ability to obtain regulatory approval for Annamycin and our other product candidates.
Delays in the commencement, enrollment and completion of clinical trials could increase our product development costs or limit the regulatory approval of our product candidates. We do not know whether any future trials or studies of our other product candidates will begin on time or will be completed on schedule, if at all. The start or end of a clinical study is often delayed or halted due to changing regulatory requirements, manufacturing challenges, including delays or shortages in available drug product, required clinical trial administrative actions, slower than anticipated patient enrollment, changing standards of care, availability or prevalence of use of a comparative drug or required prior therapy, clinical outcomes or financial constraints. For instance, delays or difficulties in patient enrollment or difficulties in retaining trial participants can result in increased costs, longer development times or termination of a clinical trial. Clinical trials of a new product candidate require the enrollment of a sufficient number of patients, including patients who are suffering from the disease the product candidate is intended to treat and who meet other eligibility criteria. Rates of patient enrollment are affected by many factors, including the size of the patient population, the eligibility criteria for the clinical trial, that include the age and condition of the patients and the stage and severity of disease, the nature of the protocol, the proximity of patients to clinical sites and the availability of effective treatments and/or availability of investigational treatment options for the relevant disease.
A product candidate can unexpectedly fail at any stage of preclinical and clinical development. The historical failure rate for product candidates is high due to scientific feasibility, safety, efficacy, changing standards of medical care and other variables. The results from preclinical testing or early clinical trials of a product candidate may not predict the results that will be obtained in later phase clinical trials of the product candidate. We, the FDA or other applicable regulatory authorities may suspend clinical trials of a product candidate at any time for various reasons, including, but not limited to, a belief that subjects participating in such trials are being exposed to unacceptable health risks or adverse side effects, or other adverse initial experiences or findings. We may not have the financial resources to continue development of, or to enter into collaborations for, a product candidate if we experience any problems or other unforeseen events that delay or prevent regulatory approval of, or our ability to commercialize, product candidates, including:
| · | inability to obtain sufficient funds required for a clinical trial; |
| · | inability to reach agreements on acceptable terms with prospective CROs and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| · | negative or inconclusive results from our clinical trials or the clinical trials of others for product candidates similar to ours, leading to a decision or requirement to conduct additional preclinical testing or clinical trials or abandon a program; |
| 17 |
| · | serious and unexpected drug-related side effects experienced by subjects in our clinical trials or by individuals using drugs similar to our product candidates; |
| · | conditions imposed by the FDA or comparable foreign authorities regarding the scope or design of our clinical trials; |
| · | delays in enrolling research subjects in clinical trials; |
| · | high drop-out rates and high fail rates of research subjects; |
| · | inadequate supply or quality of product candidate components or materials or other supplies necessary for the conduct of our clinical trials; |
| · | greater than anticipated clinical trial costs; |
| · | poor effectiveness of our product candidates during clinical trials; or |
| · | unfavorable FDA or other regulatory agency inspection and review of a clinical trial site or vendor. |
We have never conducted a clinical trial or submitted an NDA before, and any product candidate we advance through clinical trials may not have favorable results in later clinical trials or receive regulatory approval.
Clinical failure can occur at any stage of our clinical development. Clinical trials may produce negative or inconclusive results, and our collaborators or we may decide, or regulators may require us, to conduct additional clinical trials or nonclinical studies. In addition, data obtained from trials and studies are susceptible to varying interpretations, and regulators may not interpret our data as favorably as we do, which may delay, limit or prevent regulatory approval. Success in preclinical studies and early clinical trials does not ensure that subsequent clinical trials will generate the same or similar results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidate. A number of companies in the pharmaceutical industry, including those with greater resources and experience than us, have suffered significant setbacks in clinical trials, even after seeing promising results in earlier clinical trials.
In addition, the design of a clinical trial can determine whether its results will support approval of a product and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. We may be unable to design and execute a clinical trial to support regulatory approval. Further, clinical trials of potential products often reveal that it is not practical or feasible to continue development efforts.
If Annamycin is found to be unsafe or lack efficacy, we will not be able to obtain regulatory approval for it and our business would be harmed.
In some instances, there can be significant variability in safety and/or efficacy results between different trials of the same product candidate due to numerous factors, including changes in trial protocols, differences in composition of the patient populations, adherence to the dosing regimen and other trial protocols and the rate of dropout among clinical trial participants. We do not know whether any clinical trials we or any of our potential future collaborators may conduct will demonstrate the consistent or adequate efficacy and safety that would be required to obtain regulatory approval and market any products. If we are unable to bring Annamycin to market, or to acquire other products that are on the market or can be developed, our ability to create long-term stockholder value will be limited.
| 18 |
Our product candidates may have undesirable side effects that may delay or prevent marketing approval, or, if approval is received, require them to be taken off the market, require them to include safety warnings or otherwise limit their sales.
Unforeseen side effects from any of our product candidates could arise either during clinical development or, if Annamycin is approved, after the approved product has been marketed. For example, in the most recent Phase I/II dose-ranging clinical trial of Annamycin, two patients succumbed to tumor lysis syndrome (TLS) resulting from the debris created by Annamycin killing the targeted leukemic blasts more rapidly than anticipated. Now that this potential has been identified, prophylactic measures known to protect patients from TLS will be deployed in future clinical trials, but there can be no assurance that such measures will be effective or that other adverse events may not emerge related to our drug. As another example, we intend to attempt to increase the maximum tolerable dose (MTD) for Annamycin by conducting another Phase I dose-ranging trial, however, unforeseen side effects could prevent us from increasing the MTD from the one established in the prior Phase I/II trial. Additional or unforeseen side effects from Annamycin or any of our other product candidates could arise either during clinical development or, if approved, after the approved product has been marketed.
The range and potential severity of possible side effects from therapies such as Annamycin are significant. If Annamycin causes undesirable or unacceptable side effects in the future, this could interrupt, delay or halt clinical trials and result in the failure to obtain or suspension or termination of marketing approval from the FDA and other regulatory authorities, or result in marketing approval from the FDA and other regulatory authorities only with restrictive label warnings.
If any of our product candidates receives marketing approval and we or others later identify undesirable or unacceptable side effects caused by such products:
| · | regulatory authorities may require the addition of labeling statements, specific warnings, a contraindication or field alerts to physicians and pharmacies; |
| · | we may be required to change instructions regarding the way the product is administered, conduct additional clinical trials or change the labeling of the product; |
| · | we may be subject to limitations on how we may promote the product; |
| · | sales of the product may decrease significantly; |
| · | regulatory authorities may require us to take our approved product off the market; |
| · | we may be subject to litigation or product liability claims; and |
| · | our reputation may suffer. |
Any of these events could prevent us or our potential future collaborators from achieving or maintaining market acceptance of the affected product or could substantially increase commercialization costs and expenses, which in turn could delay or prevent us from generating significant revenues from the sale of our products.
| 19 |
If the FDA does not find the manufacturing facilities of our future contract manufacturers acceptable for commercial production, we may not be able to commercialize any of our product candidates.
We do not intend to manufacture the pharmaceutical products that we plan to sell. We are currently utilizing contract manufacturers for the production of the active pharmaceutical ingredients and the formulation of drug product for our trials of Annamycin that we will need to conduct prior to seeking regulatory approval. However, we do not have agreements for supplies of Annamycin or any of our other product candidates and we may not be able to reach agreements with these or other contract manufacturers for sufficient supplies to commercialize Annamycin if it is approved. Additionally, the facilities used by any contract manufacturer to manufacture Annamycin or any of our other product candidates must be the subject of a satisfactory inspection before the FDA approves the product candidate manufactured at that facility. We are completely dependent on these third-party manufacturers for compliance with the requirements of U.S. and non-U.S. regulators for the manufacture of our finished products. If our manufacturers cannot successfully manufacture material that conform to our specifications and the FDA’s current good manufacturing practice standards, or cGMP, and other requirements of any governmental agency whose jurisdiction to which we are subject, our product candidates will not be approved or, if already approved, may be subject to recalls. Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured our product candidates, including:
| · | the possibility that we are unable to enter into a manufacturing agreement with a third party to manufacture our product candidates; |
| · | the possible breach of the manufacturing agreements by the third parties because of factors beyond our control; and |
| · | the possibility of termination or nonrenewal of the agreements by the third parties before we are able to arrange for a qualified replacement third-party manufacturer. |
Any of these factors could cause the delay of approval or commercialization of our product candidates, cause us to incur higher costs or prevent us from commercializing our product candidates successfully. Furthermore, if any of our product candidates are approved and contract manufacturers fail to deliver the required commercial quantities of finished product on a timely basis at commercially reasonable prices and we are unable to find one or more replacement manufacturers capable of production at a substantially equivalent cost, in substantially equivalent volumes and quality and on a timely basis, we would likely be unable to meet demand for our products and could lose potential revenue. It may take several years to establish an alternative source of supply for our product candidates and to have any such new source approved by the government agencies that regulate our products.
We have no sales, marketing or distribution experience and we will have to invest significant resources to develop those capabilities or enter into acceptable third-party sales and marketing arrangements.
We have no sales, marketing or distribution experience. To develop sales, distribution and marketing capabilities, we will have to invest significant amounts of financial and management resources, some of which will need to be committed prior to any confirmation that Annamycin or any of our other product candidates will be approved by the FDA. For product candidates where we decide to perform sales, marketing and distribution functions ourselves or through third parties, we could face a number of additional risks, including that we or our third-party sales collaborators may not be able to build and maintain an effective marketing or sales force. If we use third parties to market and sell our products, we may have limited or no control over their sales, marketing and distribution activities on which our future revenues may depend.
We may not be successful in establishing and maintaining development and commercialization collaborations, which could adversely affect our ability to develop certain of our product candidates and our financial condition and operating results.
Because developing pharmaceutical products, conducting clinical trials, obtaining regulatory approval, establishing manufacturing capabilities and marketing approved products are expensive, we may seek to enter into collaborations with companies that have more experience. Additionally, if any of our product candidates receives marketing approval, we may enter into sales and marketing arrangements with third parties with respect to our unlicensed territories. If we are unable to enter into arrangements on acceptable terms, if at all, we may be unable to effectively market and sell our products in our target markets. We expect to face competition in seeking appropriate collaborators. Moreover, collaboration arrangements are complex and time consuming to negotiate, document and implement and they may require substantial resources to maintain. We may not be successful in our efforts to establish and implement collaborations or other alternative arrangements for the development of our product candidates.
| 20 |
When we collaborate with a third party for development and commercialization of a product candidate, we can expect to relinquish some or all of the control over the future success of that product candidate to the third party. For example, we have formed a collaboration with a Polish drug development company called Dermin, where we have provided them with sub-license rights to our technologies for use in limited territories in exchange for their use of Polish government grant funding to pay for development costs we would otherwise have to fund ourselves. With the exception of Annamycin, Dermin’s territories are primarily Poland and lesser surrounding countries, but not including any of the major European markets (UK, Germany, France, Spain and Italy). In the case of Annamycin, Dermin’s territories also include Germany, but we retain the right to repurchase that territory for $500,000 at any time in the future.
We announced in October 2016 that we had secured an agreement with Dermin to utilize Dermin’s supply of Annamycin for our upcoming clinical trial. If Dermin is unable to provide us with sufficient supply for clinical trials, or if the drug product supplied is unacceptable for use in our clinical trials, our clinical trials may be delayed or the outcomes may be adversely affected.
One or more of our collaboration partners may not devote sufficient resources to the commercialization of our product candidates or may otherwise fail in their commercialization. The terms of any collaboration or other arrangement that we establish may contain provisions that are not favorable to us. In addition, any collaboration that we enter into may be unsuccessful in the development and commercialization of our product candidates. In some cases, we may be responsible for continuing preclinical and initial clinical development of a product candidate or research program under a collaboration arrangement, and the payment we receive from our collaboration partner may be insufficient to cover the cost of this development. If we are unable to reach agreements with suitable collaborators for our product candidates, we would face increased costs, we may be forced to limit the number of our product candidates we can commercially develop or the territories in which we commercialize them. As a result, we might fail to commercialize products or programs for which a suitable collaborator cannot be found. If we fail to achieve successful collaborations, our operating results and financial condition could be materially and adversely affected.
Our success depends greatly on the success of Annamycin’s development for the treatment of relapsed or refractory AML, and our pipeline of product candidates beyond this lead indication is extremely early stage and limited.
Other than Annamycin, our other two drug candidates, our WP1066 Portfolio and our WP1122 Portfolio, are each in the early stages of development. As such, we are dependent on the success of Annamycin in the near term. We cannot provide you any assurance that we will be able to successfully advance any of these indications through the development process.
We face competition from other biotechnology and pharmaceutical companies and our operating results will suffer if we fail to compete effectively.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. We have competitors in the United States, Europe and other jurisdictions, including major multinational pharmaceutical companies, established biotechnology companies, specialty pharmaceutical and generic drug companies and universities and other research institutions. Many of our competitors have greater financial and other resources, such as larger research and development staff and more experienced marketing and manufacturing organizations than we do. Large pharmaceutical companies, in particular, have extensive experience in clinical testing, obtaining regulatory approvals, recruiting patients and manufacturing pharmaceutical products. These companies also have significantly greater research, sales and marketing capabilities and collaborative arrangements in our target markets with leading companies and research institutions. Established pharmaceutical companies may also invest heavily to accelerate discovery and development of novel compounds or to in-license novel compounds that could make the product candidates that we develop obsolete. As a result of all of these factors, our competitors may succeed in obtaining patent protection and/or FDA approval or discovering, developing and commercializing drugs for the diseases that we are targeting before we do or may develop drugs that are deemed to be more effective or gain greater market acceptance than ours. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. In addition, many universities and private and public research institutes may become active in our target disease areas. Our competitors may succeed in developing, acquiring or licensing on an exclusive basis, technologies and drug products that are more effective or less costly than any of our product candidates that we are currently developing or that we may develop, which could render our products obsolete or noncompetitive.
| 21 |
A number of attempts have been made or are under way to provide an improved treatment for AML. Drugs attempting to target a subset of AML patients who present with particular anomalies involving a gene referred to as FLT3 are currently in clinical trials. Other approaches to improve the effectiveness of induction therapy are in early stage clinical trials and, although they do not appear to address the underlying problems with anthracyclines, we can provide no assurance that such improvements, if achieved, would not adversely impact the need for improved anthracyclines. A modified version of doxorubicin designed to reduce cardiotoxicity is in clinical trials for the treatment of sarcoma and, although this drug does not appear to address multidrug resistance and is not currently intended for the treatment of acute leukemia, we can provide no assurance that it will not become a competitive alternative to Annamycin. Although we are not aware of any other single agent therapies in clinical trials that would directly compete against Annamycin in the treatment of relapsed and refractory AML, we can provide no assurance that such therapies are not in development, will not receive regulatory approval and will reach market before our drug candidate Annamycin. In addition, any such competing therapy may be more effective and / or cost-effective than ours.
If our competitors market products that are more effective, safer or less expensive or that reach the market sooner than our future products, if any, we may not achieve commercial success. In addition, because of our limited resources, it may be difficult for us to stay abreast of the rapid changes in each technology. If we fail to stay at the forefront of technological change, we may be unable to compete effectively. Technological advances or products developed by our competitors may render our technologies or product candidates obsolete, less competitive or not economical.
Annamycin does not have any patent protections, including composition of matter patent protection.
We intend to pursue patents with claims directed to Annamycin drug product formulations and the methods of use of Annamycin to treat relapsed or refractory AML and other conditions, and methods for its synthesis, as the composition of matter patent protection for Annamycin has expired. As a result, competitors may be able to offer and sell products so long as these competitors do not infringe any other patents that third parties or we hold, including formulation, synthesis and method of use patents. However, method of use patents, in particular, are more difficult to enforce than composition of matter patents because of the risk of off-label sale or use of the subject compounds. Physicians are permitted to prescribe an approved product for uses that are not described in the product's labeling. Although off-label prescriptions may infringe our method of use patents, the practice is common across medical specialties and such infringement is difficult to prevent or prosecute. Off-label sales would limit our ability to generate revenue from the sale of Annamycin, if approved for commercial sale.
The intellectual property rights we have licensed from MD Anderson are subject to the rights of the U.S. government.
We have obtained a royalty-bearing, worldwide, exclusive license to intellectual property rights, including patent rights related to our WP1066 Portfolio and WP1122 Portfolio drug product candidates from MD Anderson. Some of our licensed intellectual property rights from MD Anderson have been developed in the course of research funded by the U.S. government. As a result, the U.S. government may have certain rights to intellectual property embodied in our current or future products pursuant to the Bayh-Dole Act of 1980. Government rights in certain inventions developed under a government-funded program include a non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the U.S. government has the right to require us, or an assignee or exclusive licensee to such inventions, to grant licenses to any of these inventions to a third party if they determine that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; (iii) government action is necessary to meet requirements for public use under federal regulations; or (iv) the right to use or sell such inventions is exclusively licensed to an entity within the U.S. and substantially manufactured outside the U.S. without the U.S. government’s prior approval. Additionally, we may be restricted from granting exclusive licenses for the right to use or sell our inventions created pursuant to such agreements unless the licensee agrees to additional restrictions (e.g., manufacturing substantially all of the invention in the U.S.). The U.S. government also has the right to take title to these inventions if we fail to disclose the invention to the government and fail to file an application to register the intellectual property within specified time limits. In addition, the U.S. government may acquire title in any country in which a patent application is not filed within specified time limits. Additionally, certain inventions are subject to transfer restrictions during the term of these agreements and for a period thereafter, including sales of products or components, transfers to foreign subsidiaries for the purpose of the relevant agreements, and transfers to certain foreign third parties. If any of our intellectual property becomes subject to any of the rights or remedies available to the U.S. government or third parties pursuant to the Bayh-Dole Act of 1980, this could impair the value of our intellectual property and could adversely affect our business.
| 22 |
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights.
We may from time to time seek to enforce our intellectual property rights against infringers when we determine that a successful outcome is probable and may lead to an increase in the value of the intellectual property. If we choose to enforce our patent rights against a party, then that individual or company has the right to ask the court to rule that such patents are invalid or should not be enforced. Additionally, the validity of our patents and the patents we have licensed may be challenged if a petition for post grant proceedings such as inter-partes review and post grant review is filed within the statutorily applicable time with the U.S. Patent and Trademark Office (USPTO). These lawsuits and proceedings are expensive and would consume time and resources and divert the attention of managerial and scientific personnel even if we were successful in stopping the infringement of such patents. In addition, there is a risk that the court will decide that such patents are not valid and that we do not have the right to stop the other party from using the inventions. There is also the risk that, even if the validity of such patents is upheld, the court will refuse to stop the other party on the ground that such other party's activities do not infringe our intellectual property rights. In addition, in recent years the U.S. Supreme Court modified some tests used by the USPTO in granting patents over the past 20 years, which may decrease the likelihood that we will be able to obtain patents and increase the likelihood of a challenge of any patents we obtain or license.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in the biotechnology and pharmaceutical industries, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. We may be subject to claims that these employees, or we, have used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
If we are not able to adequately prevent disclosure of trade secrets and other proprietary information, the value of our technology and products could be significantly diminished.
We rely on trade secrets to protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
We will need to expand our operations and increase the size of our company, and we may experience difficulties in managing growth.
As of September 30, 2016, we had two full-time and four part-time employees. As we advance our product candidates through preclinical studies and clinical trials, we will need to increase our product development, scientific and administrative headcount to manage these programs. In addition, to meet our obligations as a public company, we may need to increase our general and administrative capabilities. Our management, personnel and systems currently in place may not be adequate to support this future growth. If we are unable to successfully manage this growth and increased complexity of operations, our business may be adversely affected.
| 23 |
We may not be able to manage our business effectively if we are unable to attract and retain key personnel and consultants.
We may not be able to attract or retain qualified management, finance, scientific and clinical personnel and consultants due to the intense competition for qualified personnel and consultants among biotechnology, pharmaceutical and other businesses. If we are not able to attract and retain necessary personnel and consultants to accomplish our business objectives, we may experience constraints that will significantly impede the achievement of our development objectives, our ability to raise additional capital and our ability to implement our business strategy.
We are highly dependent on the development, regulatory, commercialization and business development expertise of our management team, key employees and consultants. If we lose one or more of our executive officers or key employees or consultants, our ability to implement our business strategy successfully could be seriously harmed. Any of our executive officers or key employees or consultants may terminate their employment at any time. Replacing executive officers, key employees and consultants may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to develop, gain regulatory approval of and commercialize products successfully. Competition to hire and retain employees and consultants from this limited pool is intense, and we may be unable to hire, train, retain or motivate these additional key personnel and consultants. Our failure to retain key personnel or consultants could materially harm our business.
In addition, we have scientific and clinical advisors and consultants who assist us in formulating our research, development and clinical strategies. These advisors are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us and typically they will not enter into non-compete agreements with us. If a conflict of interest arises between their work for us and their work for another entity, we may lose their services. In addition, our advisors may have arrangements with other companies to assist those companies in developing products or technologies that may compete with ours.
Our chief executive officer and our chief operating officer are currently working for us on a part-time basis.
Two of our key employees are currently part-time and provide services for other biotechnology development efforts. Specifically, Walter Klemp, our chairman and chief executive officer is also the chief executive officer of Soliton, Inc., a medical device development company whose business operations we do not believe conflict with those of our company, and Donald Picker, our president and chief operating officer, is currently also serving as chief operating officer of two other biotechnology companies, whose business operations we do not believe conflict with those of our company. As we progress, if the full-time services of a CEO or COO are required and the current officers cannot provide that level of commitment, we will need to identify a suitable CEO or COO who can dedicate such time to our company. We can provide no assurance that we will be able to successfully identify and retain a qualified candidate for this position.
We do not expect that our insurance policies will cover all of our business exposures thus leaving us exposed to significant uninsured liabilities.
We do not carry insurance for all categories of risk that our business may encounter. In particular, we do not carry product liability insurance covering any clinical trials liability that we may incur. Although we intend to obtain such insurance before we commence any clinical trials, there can be no assurance that we will secure adequate insurance coverage or that any such insurance coverage will be sufficient to protect our operations to significant potential liability in the future. Any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our financial position and results of operations.
Risks Relating to this Offering of Our Units
Purchasers in this offering will experience immediate and substantial dilution in net tangible book value.
| 24 |
The public offering price in this offering is substantially higher than the net tangible book value of each outstanding share of our common stock. Purchasers of units in this offering will experience immediate and substantial dilution on a book value basis. The dilution per share in the net tangible book value per share of common stock (attributing no value to the warrants included in the units) will be $ per share. See “Dilution.”
Your ownership may be diluted if additional capital stock is issued to raise capital, to finance acquisitions or in connection with strategic transactions.
We intend to seek to raise additional funds, finance acquisitions or develop strategic relationships by issuing equity or convertible debt securities in addition to the shares issued in this offering, which would reduce the percentage ownership of our existing stockholders. Our board of directors has the authority, without action or vote of the stockholders, to issue all or any part of our authorized but unissued shares of common or preferred stock. Our certificate of incorporation authorizes us to issue up to 75,000,000 shares of common stock and 5,000,000 shares of preferred stock. Future issuances of common or preferred stock would reduce your influence over matters on which stockholders vote and would be dilutive to earnings per share. In addition, any newly issued preferred stock could have rights, preferences and privileges senior to those of the common stock. Those rights, preferences and privileges could include, among other things, the establishment of dividends that must be paid prior to declaring or paying dividends or other distributions to holders of our common stock or providing for preferential liquidation rights. These rights, preferences and privileges could negatively affect the rights of holders of our common stock, and the right to convert such preferred stock into shares of our common stock at a rate or price that would have a dilutive effect on the outstanding shares of our common stock.
The concentration of our common stock ownership by our current management will limit your ability to influence corporate matters.
Upon completion of this offering, our founders, directors and executive officers will beneficially own and will be able to vote in the aggregate approximately 41.3% of our outstanding common stock. As such, our founders, directors and executive officers, as stockholders, will continue to have the ability to exert significant influence over all corporate activities, including the election or removal of directors and the outcome of tender offers, mergers, proxy contests or other purchases of common stock that could give our stockholders the opportunity to realize a premium over the then-prevailing market price for their shares of common stock. This concentrated control will limit your ability to influence corporate matters and, as a result, we may take actions that purchasers in this offering do not view as beneficial. In addition, such concentrated control could discourage others from initiating changes of control. In such cases, the perception of our prospects in the market may be adversely affected and the market price of our common stock may decline.
Our stock price has been and may continue to be volatile, which could result in substantial losses for investors.
Since our IPO in June 2016, our stock price has ranged from a high of $9.58 to a low of $1.41, and the market price of our common stock is likely to continue to be highly volatile and could fluctuate widely in response to various factors, many of which are beyond our control. In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also significantly affect the market price of our common stock.
There is no public market for the warrants to purchase shares of our common stock being offered by us in this offering.
There is no established public trading market for the warrants being offered in this offering, and a public market may never develop. In addition, we do not intend to apply to list the warrants on any national securities exchange or other nationally recognized trading system, including the NASDAQ Capital Market. Without an active market, the liquidity of the warrants will be limited.
Holders of our warrants will have no rights as a common stockholder until such holders exercise their warrants and acquire our common stock.
Until holders of warrants acquire shares of our common stock upon exercise of the warrants, holders of warrants will have no rights with respect to the shares of our common stock underlying such warrants. Upon exercise of the warrants, the holders thereof will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
| 25 |
The warrants included in this offering may not have any value.
Each warrant has an initial exercise price of $ per share of common stock and will expire five years from the date of issuance. In the event our common stock price does not exceed the exercise price of the warrants during the period when the warrants are exercisable, the warrants may not have any value.
Significant holders or beneficial holders of our common stock may not be permitted to exercise warrants that they hold.
The warrant agreement governing the warrants being offered hereby will prohibit a holder from exercising its warrants if doing so would result in such holder (together with such holder’s affiliates and any other persons acting as a group together with such holder or any of such holder’s affiliates) beneficially owning more than 9.99% of our common stock. Furthermore, during any period in which a holder beneficially owns less than 5% of our common stock, the warrant agreement will limit the ability of such holder to exercise its warrants if doing so would result in such holder (together with such holder’s affiliates and any other persons acting as a group together with such holder or any of such holder’s affiliates) beneficially owning more than 4.99% of our common stock. As a result, you may not be able to exercise your warrants for shares of our common stock at a time when it would be financially beneficial for you to do so. In such circumstance you could seek to sell your warrants to realize value, but you may be unable to do so.
Certain provisions in our organizational documents could enable our board of directors to prevent or delay a change of control.
Our organizational documents contain provisions that may have the effect of discouraging, delaying or preventing a change of control of, or unsolicited acquisition proposals, that a stockholder might consider favorable. These include provisions:
| · | prohibiting the stockholders from acting by written consent; |
| · | requiring advance notice of director nominations and of business to be brought before a meeting of stockholders; |
| · | requiring a majority vote of the outstanding shares of common stock to amend the bylaws; and |
| · | limiting the persons who may call special stockholders’ meetings. |
Furthermore, our board of directors has the authority to issue shares of preferred stock in one or more series and to fix the rights and preferences of these shares without stockholder approval. Any series of preferred stock is likely to be senior to our common stock with respect to dividends, liquidation rights and, possibly, voting rights. The ability of our board of directors to issue preferred stock also could have the effect of discouraging unsolicited acquisition proposals, thus adversely affecting the market price of our common stock.
In addition, Delaware law makes it difficult for stockholders that recently have acquired a large interest in a corporation to cause the merger or acquisition of the corporation against the directors’ wishes. Under Section 203 of the Delaware General Corporation Law, a Delaware corporation may not engage in any merger or other business combination with an interested stockholder for a period of three years following the date that the stockholder became an interested stockholder except in limited circumstances, including by approval of the corporation’s board of directors.
We have no intention of declaring dividends in the foreseeable future.
The decision to pay cash dividends on our common stock rests with our board of directors and will depend on our earnings, unencumbered cash, capital requirements and financial condition. We do not anticipate declaring any dividends in the foreseeable future, as we intend to use any excess cash to fund our operations. Investors in our common stock should not expect to receive dividend income on their investment, and investors will be dependent on the appreciation of our common stock to earn a return on their investment.
| 26 |
Failure to maintain effective internal control over our financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could cause our financial reports to be inaccurate.
We are required pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, to maintain internal control over financial reporting and to assess and report on the effectiveness of those controls. This assessment includes disclosure of any material weaknesses identified by our management in our internal control over financial reporting. Our management concluded that our disclosure controls and procedures were, and continue to be ineffective, and as of June 30, 2016, identified a material weakness in our internal controls over the accounting and reporting for acquisitions. While management is working to remediate the material weakness, there is no assurance that the changes will remediate the identified material weakness or that the controls will prevent or detect future material weaknesses. If we are not able to maintain effective internal control over financial reporting, our financial statements, including related disclosures, may be inaccurate, which could have a material adverse effect on our business.
Failure to continue improving our accounting systems and controls could impair our ability to comply with the financial reporting and internal controls requirements for publicly traded companies.
As a public company, we operate in an increasingly demanding regulatory environment, which requires us to comply with the Sarbanes-Oxley Act of 2002, and the related rules and regulations of the SEC. Company responsibilities required by the Sarbanes-Oxley Act include establishing corporate oversight and adequate internal control over financial reporting and disclosure controls and procedures. Effective internal controls are necessary for us to produce reliable financial reports and are important to help prevent financial fraud.
Our management concluded that our disclosure controls and procedures were, and continue to be, ineffective and, as of June 30, 2016 identified a material weakness in our internal controls over the accounting and reporting for acquisitions. Section 404(a) of the Sarbanes-Oxley Act requires annual management assessments of the effectiveness of our internal control over financial reporting, starting with the second annual report that we would expect to file with the SEC. However, for as long as we remain an “emerging growth company” as defined in the JOBS Act, we have and intend to continue to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act. We may continue to take advantage of these reporting exemptions until we are no longer an “emerging growth company.” If we cannot provide reliable financial reports or prevent fraud, our business and results of operations could be harmed and investors could lose confidence in our reported financial information.
On November 14, 2016, our Audit Committee, after discussion with management and our independent registered public accountants, determined that our unaudited consolidated financial statements for the quarter ended June 30, 2016, as reported in our Quarterly Report on Form 10-Q filed on August 15, 2016 should no longer be relied upon due to an error identified therein, and that a restatement of these financial statements is required. We identified certain non-cash errors due to an error in the accounting for the business combination of Moleculin, LLC. We filed a Form 10-Q/A for the quarter ended June 30, 2016 on November 21, 2016 reflecting such corrections in errors.
As an “emerging growth company” under the Jumpstart Our Business Startups Act, or JOBS Act, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements.
As an “emerging growth company” under the JOBS Act, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements. We are an emerging growth company until the earliest of:
| · | the last day of the fiscal year during which we have total annual gross revenues of $1 billion or more; |
| · | the last day of the fiscal year following the fifth anniversary of our IPO, or December 31, 2021; |
| · | the date on which we have, during the previous 3-year period, issued more than $1 billion in non-convertible debt; or |
| · | the date on which we are deemed a “large accelerated issuer” as defined under the federal securities laws. |
| 27 |
For so long as we remain an emerging growth company, we will not be required to:
| · | have an auditor report on our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002; |
| · | comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis); |
| · | submit certain executive compensation matters to shareholders advisory votes pursuant to the “say on frequency” and “say on pay” provisions (requiring a non-binding shareholder vote to approve compensation of certain executive officers) and the “say on golden parachute” provisions (requiring a non-binding shareholder vote to approve golden parachute arrangements for certain executive officers in connection with mergers and certain other business combinations) of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010; |
| · | include detailed compensation discussion and analysis in our filings under the Securities Exchange Act of 1934, as amended, and instead may provide a reduced level of disclosure concerning executive compensation; and |
| · | may present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations, or MD&A. |
We intend to take advantage of all of these reduced reporting requirements and exemptions. Certain of these reduced reporting requirements and exemptions were already available to us due to the fact that we also qualify as a “smaller reporting company” under SEC rules. For instance, smaller reporting companies are not required to obtain an auditor attestation and report regarding management’s assessment of internal control over financial reporting; are not required to provide a compensation discussion and analysis; are not required to provide a pay-for-performance graph or CEO pay ratio disclosure; and may present only two years of audited financial statements and related MD&A disclosure.
Under the JOBS Act, we may take advantage of the above-described reduced reporting requirements and exemptions until December 31, 2021, or such earlier time that we no longer meet the definition of an emerging growth company. In this regard, the JOBS Act provides that we would cease to be an “emerging growth company” if we have more than $1.0 billion in annual revenues, have more than $700 million in market value of our common stock held by non-affiliates, or issue more than $1.0 billion in principal amount of non-convertible debt over a three-year period. Further, under current SEC rules, we will continue to qualify as a “smaller reporting company” for so long as we have a public float (i.e., the market value of common equity held by non-affiliates) of less than $75 million as of the last business day of our most recently completed second fiscal quarter.
We cannot predict if investors will find our securities less attractive due to our reliance on these exemptions. If investors were to find our common stock less attractive as a result of our election, we may have difficulty raising capital.
| 28 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
We make forward-looking statements under the “Summary,” “Risk Factors,” “Business,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and in other sections of this prospectus. In some cases, you can identify these statements by forward-looking words such as “may,” “might,” “should,” “would,” “could,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or “continue,” and the negative of these terms and other comparable terminology. These forward-looking statements, which are subject to known and unknown risks, uncertainties and assumptions about us, may include projections of our future financial performance based on our growth strategies and anticipated trends in our business. These statements are only predictions based on our current expectations and projections about future events. There are important factors that could cause our actual results, level of activity, performance or achievements to differ materially from the results, level of activity, performance or achievements expressed or implied by the forward-looking statements. In particular, you should consider the numerous risks and uncertainties described under “Risk Factors.”
While we believe we have identified material risks, these risks and uncertainties are not exhaustive. Other sections of this prospectus describe additional factors that could adversely impact our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risks and uncertainties emerge from time to time, and it is not possible to predict all risks and uncertainties, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
Although we believe the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, level of activity, performance or achievements. Moreover, neither we nor any other person assumes responsibility for the accuracy or completeness of any of these forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. We are under no duty to update any of these forward-looking statements after the date of this prospectus to conform our prior statements to actual results or revised expectations, and we do not intend to do so.
Forward-looking statements include, but are not limited to, statements about:
| · | our ability to obtain additional funding to develop our product candidates; |
| · | the need to obtain regulatory approval of our product candidates; |
| · | the success of our clinical trials through all phases of clinical development; |
| · | compliance with obligations under intellectual property licenses with third parties; |
| · | any delays in regulatory review and approval of product candidates in clinical development; |
| · | our ability to commercialize our product candidates; |
| · | market acceptance of our product candidates; |
| · | competition from existing products or new products that may emerge; |
| · | potential product liability claims; |
| · | our dependency on third-party manufacturers to supply or manufacture our products; |
| · | our ability to establish or maintain collaborations, licensing or other arrangements; |
| · | our ability and third parties’ abilities to protect intellectual property rights; |
| · | our ability to adequately support future growth; and |
| · | our ability to attract and retain key personnel to manage our business effectively. |
We caution you not to place undue reliance on the forward-looking statements, which speak only as of the date of this prospectus in the case of forward-looking statements contained in this prospectus.
| 29 |
The net proceeds from the sale of the units in this offering are estimated to be approximately $ million, or approximately $ million if the underwriters exercise in full their option to purchase additional units in this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
We currently intend to use the net proceeds from this offering for working capital and general corporate purposes.
The amounts and timing of our use of the net proceeds from this offering will depend on a number of factors, such as the timing and progress of our research and development efforts, the timing and progress of any partnering and commercialization efforts, and technological advances. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to us from this offering. Accordingly, our management will have broad discretion in the timing and application of these proceeds. Pending application of the net proceeds as described above, we intend to temporarily invest the proceeds in short-term, interest-bearing instruments. We reserve the right, at the sole discretion of our Board of Directors, to reallocate the proceeds of this offering in response to developments in our business and any other factors.
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain earnings, if any, to finance the growth and development of our business. We do not expect to pay any cash dividends on our common stock in the foreseeable future. Payment of future dividends, if any, will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements, restrictions contained in any financing instruments, provisions of applicable law and other factors the board deems relevant.
| 30 |
The following table sets forth our cash and cash equivalents and capitalization as of September 30, 2016 on:
| · | an actual basis; and |
| · | a pro forma as adjusted basis after giving effect to the sale of 5,000,000 shares of our common stock underlying the units in this offering at an assumed offering price of $ per share, and our receipt of the estimated $ million in net proceeds from this offering, after deducting underwriting commissions and estimated offering expenses payable by us. |
You should read this capitalization table together with “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes appearing elsewhere in this prospectus.
| At September 30, 2016 | ||||||||
| Actual | Pro Forma - As adjusted | |||||||
| Cash and cash equivalents | $ | 6,183,783 | ||||||
| Notes payable | $ | 297,656 | ||||||
| Stockholders' equity: | ||||||||
| Preferred stock, $0.001 par value: 5,000,000 shares authorized, no shares issued and outstanding | - | |||||||
| Common stock, $0.001 par value: 75,000,000 shares authorized; 12,054,813 shares issued and outstanding, actual; and shares issued and outstanding, pro forma as adjusted— minimum and pro forma as adjusted — maximum, respectively (1) | 12,055 | |||||||
| Additional paid-in capital | 19,485,987 | |||||||
| Subscription receivable | (3,000 | ) | ||||||
| Total stockholders' equity | 16,243,635 | |||||||
| Total capitalization | $ | 16,541,291 | ||||||
| 31 |
The number of shares of common stock to be outstanding after this offering is based on 12,164,851 shares of common stock outstanding as of January 17, 2017 and does not take into account, as of January 17, 2017:
· 510,000 shares of our common stock issuable upon exercise of outstanding stock options to purchase shares of our common stock at a weighted average exercise price of $5.28 per share;
· 107,802 shares of our common stock issuable upon the exercise of outstanding warrants with a weighted average exercise price of $7.50 per share;
· 1,990,000 shares of our common stock reserved for issuance under our 2015 Stock Plan;
· 1,818,861 shares upon the conversion of outstanding bridge notes. These notes were to be automatically converted according to their terms into our common stock upon the closing of our IPO to the extent and provided that no holder of these notes was or will be permitted to convert such notes to the extent that the holder or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such conversion. Due to this 4.99% limitation, certain of these notes remained outstanding and will be converted into shares of our common stock at such time as the 4.99% limitation continues to be met;
· 79,167 shares of our common stock issuable to a third-party in settlement of $237,500 in past due amounts;
· 1,250,000 shares of common stock issuable upon the exercise of the warrants included in the units being sold in this offering; and
· 350,000 shares of common stock issuable upon the exercise of the warrants issued to the representatives of the underwriters.
| 32 |
Purchasers of our units in this offering will experience an immediate dilution of net tangible book value per share from the public offering price. Dilution in net tangible book value per share represents the difference between the amount per share paid by the purchasers of shares of common stock (attributing no value to the warrants included in the units) and the net tangible book value per share immediately after this offering.
As of September 30, 2016, our net tangible book value was $5,114,845, or $0.42 per share of common stock. Net tangible book value per share represents our total tangible assets, less our total liabilities, divided by the number of outstanding shares of our common stock.
After giving effect to the issuance and sale by us of 5,000,000 units in this offering at an offering price of $ per unit, and after deducting underwriting discounts and commissions and estimated offering expense payable by us, our as adjusted net tangible book value as of September 30, 2016 would have been approximately $ million, or approximately $ per share. This amount represents an immediate increase in as adjusted net tangible book value of $ per share to our existing stockholders and an immediate dilution in the as adjusted net tangible book value of approximately $ per share to new investors purchasing shares of common stock in this offering at the offering price.
| Assumed public offering price per unit | ||||
| Net tangible book value per share at September 30, 2016 | $ | 0.42 | ||
| Increase in net tangible book value per share to the existing stockholders attributable to this offering | ||||
| Adjusted net tangible book value per share after this offering | ||||
| Dilution in net tangible book value per share to new investors |
The above discussion and table are based on 12,164,851 shares of common stock outstanding as of January 17, 2017, which does not include, as of January 17, 2017:
· 510,000 shares of our common stock issuable upon exercise of outstanding stock options to purchase shares of our common stock at a weighted average exercise price of $5.28 per share;
· 107,802 shares of our common stock issuable upon the exercise of outstanding warrants with a weighted average exercise price of $7.50 per share;
· 1,990,000 shares of our common stock reserved for issuance under our 2015 Stock Plan;
· 1,818,861 shares upon the conversion of outstanding bridge notes. These notes were to be automatically converted according to their terms into our common stock upon the closing of our IPO to the extent and provided that no holder of these notes was or will be permitted to convert such notes to the extent that the holder or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such conversion. Due to this 4.99% limitation, certain of these notes remained outstanding and will be converted into shares of our common stock at such time as the 4.99% limitation continues to be met;
· 79,167 shares of our common stock issuable to a third-party in settlement of $237,500 in past due amounts;
· 1,250,000 shares of common stock issuable upon the exercise of the warrants included in the units being sold in this offering; and
· 350,000 shares of common stock issuable upon the exercise of the warrants issued to the representatives of the underwriters.
| 33 |
UNAUDITED PRO FORMA COMBINED FINANCIAL INFORMATION
The following unaudited pro forma combined financial information is based on the historical financial statements of Moleculin Biotech, Inc. after giving effect to our acquisition of Moleculin, LLC described in Note 2 to our unaudited financial statements for the nine months ended September 30, 2016.
The unaudited pro forma combined statement of operations for the year ended December 31, 2015 and the nine months ended September 30, 2016 are presented as if our acquisition of Moleculin, LLC occurred on January 1, 2015 and were carried forward through to September 30, 2016.
We have based the pro forma adjustments upon available information and certain assumptions that we believe are reasonable under the circumstances. We describe in greater detail the assumptions underlying the pro forma combined financial statements in the notes to the unaudited pro forma combined financial statements. In many cases, we based these assumptions on preliminary information and estimates. The actual adjustments to our pro forma combined financial statements will depend upon a number of factors and additional information that will be available when we complete the valuation of Moleculin, LLC’s assets. Accordingly, the actual adjustments that will appear in our financial statements will differ from these pro forma adjustments, and those differences may be material.
We acquired Moleculin, LLC on May 2, 2016, and, going forward our financial statements include the operations of Moleculin, LLC. We account for acquired businesses using the acquisition method of accounting, which requires, among other things, that most assets acquired and liabilities assumed be recognized at their estimated fair values as of the acquisition date and that the fair value of acquired in-process research and development (“IPR&D”) be recorded on the balance sheet. Transaction costs are expensed as incurred. Any excess of the consideration transferred over the assigned values of the net assets acquired will be recorded as goodwill. The estimated fair values of assets acquired and liabilities assumed were determined based on management’s best estimates. Preliminary estimated fair values have been made by management and are subject to measurement period adjustments which represent updates made to the preliminary purchase price allocation based on revisions to valuation estimates in the interim period subsequent to the acquisition and initial accounting date up until the purchase price allocation is finalized which cannot be any later than one year from the acquisition date.
The unaudited pro forma combined financial information is not intended to represent or be indicative of our consolidated results of operations or financial position that we would have reported had the Moleculin, LLC acquisition been completed as of the date presented, and should not be taken as a representation of our future consolidated results of operations or financial position. The unaudited pro forma combined financial information does not reflect any operating efficiencies and/or cost savings that we may achieve with respect to combining the companies.
The following unaudited financial information should be read with the accompanying notes, the financial statements of Moleculin, LLC and the notes thereto included elsewhere in this prospectus and the financial statements of MBI and the notes thereto included elsewhere in this prospectus.
| 34 |
Moleculin Biotech, Inc.
Unaudited Pro Forma Combined Statement of Operations
For the Year Ended December 31, 2015
| Pro Forma | Pro Forma | |||||||||||||||
| Moleculin, LLC | MBI | Adjustments | Combined | |||||||||||||
| Revenue | $ | - | $ | - | $ | - | $ | - | ||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 125,442 | 260,418 | - | 385,860 | ||||||||||||
| General and administrative | 328,570 | 477,810 | - | 806,380 | ||||||||||||
| Depreciation and amortization | 11,336 | - | - | (a) | 11,336 | |||||||||||
| Total operating expenses | 465,348 | 738,228 | - | 1,203,576 | ||||||||||||
| Net loss from operations | (465,348 | ) | (738,228 | ) | - | (1,203,576 | ) | |||||||||
| Other income (expense): | ||||||||||||||||
| Other income | 549,180 | - | - | 549,180 | ||||||||||||
| Interest expense | (449,643 | ) | (10,132 | ) | 422,874 | (b) | (36,901 | ) | ||||||||
| Total other income (expense) | 99,537 | (10,132 | ) | 422,874 | 512,279 | |||||||||||
| Net loss | $ | (365,811 | ) | $ | (748,360 | ) | $ | 422,874 | (c) | $ | (691,297 | ) | ||||
| Loss per common share - basic and diluted | $ | (0.13 | ) | $ | (0.10 | ) | ||||||||||
| Weighted average number of common shares outstanding - basic and diluted | 5,691,803 | 999,931 | (d) | 6,691,734 | ||||||||||||
| 35 |
Moleculin Biotech, Inc.
Unaudited Pro Forma Combined Statement of Operations
For the Nine Months Ended September 30, 2016
| Moleculin, LLC | (e) | MBI | Pro Forma Adjustments | Pro Forma Combined | ||||||||||||
| Revenue | $ | - | $ | - | $ | - | $ | - | ||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 47,964 | 616,498 | - | 664,462 | ||||||||||||
| General and administrative | 44,766 | 1,847,613 | - | 1,892,379 | ||||||||||||
| Depreciation and amortization | 3,438 | 1,629 | - | (a) | 5,067 | |||||||||||
| Total operating expenses | 96,168 | 2,465,740 | - | 2,561,908 | ||||||||||||
| Net loss from operations | (96,168 | ) | (2,465,740 | ) | - | (2,561,908 | ) | |||||||||
| Other income (expense): | ||||||||||||||||
| Other income | 69,569 | - | 69,569 | |||||||||||||
| Interest expense | (155,109 | ) | (37,307 | ) | 145,078 | (b) | (47,338 | ) | ||||||||
| Total other income (expense) | (85,540 | ) | (37,307 | ) | 145,078 | 22,231 | ||||||||||
| Net loss | $ | (181,708 | ) | $ | (2,503,047 | ) | $ | 145,078 | (c) | $ | (2,539,677 | ) | ||||
| Loss per common share - basic and diluted | $ | (0.28 | ) | $ | (0.27 | ) | ||||||||||
| Weighted average number of common shares outstanding - basic and diluted | 9,066,804 | 446,856 | (d) | 9,513,660 | ||||||||||||
| 36 |
Moleculin Biotech, Inc.
Notes to Unaudited Pro Forma Combined Financial Information
Note 1 – Basis of presentation
The unaudited pro forma combined statements of operations for the nine months ended September 30, 2016 and the year ended December 31, 2015 are based on the historical financial statements of Moleculin Biotech, Inc. and Moleculin, LLC after giving effect to our acquisition of Moleculin, LLC described in Note 2 to our financial statements for the nine months ended September 30, 2016.
We account for business combinations pursuant to Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (ASC) 805, Business Combinations. In accordance with ASC 805, MBI uses its best estimates and assumptions to assign fair value to the tangible and intangible assets acquired and liabilities assumed at the acquisition date. Goodwill as of the acquisition date is measured as the excess of purchase consideration over the fair value of net tangible and identifiable intangible assets acquired.
The fair values assigned to Moleculin, LLC’s tangible assets acquired and liabilities assumed are based on management’s estimates and assumptions. The estimated fair values of these assets acquired and liabilities assumed are considered preliminary and are based on the information and the account balances that were available. We believe that the information provides a reasonable basis for estimating the fair values of assets acquired and liabilities assumed. We expect to finalize the valuation of the net tangible and intangible assets prior to the filing of our Form 10-K for the year ended December 31, 2016, but not later than one year from the acquisition date.
The unaudited pro forma combined financial information is not intended to represent or be indicative of our results of operations or financial position that would have been reported had the acquisition been completed as of the date presented, and should not be taken as a representation of our future consolidated results of operations or financial position. The unaudited pro forma combined financial information does not reflect any operating efficiencies and/or cost savings that we may achieve with respect to the combined companies.
For purposes of these unaudited combined pro forma statements of operations, the acquisition of Moleculin, LLC is assumed to have occurred on January 1, 2015. The pro forma statement of operations for the year ended December 31, 2015 combined the results of Moleculin, LLC for the year ended December 31, 2015 and our results for the period from inception (July 28, 2015) through December 31, 2015.
Note 2 — Pro forma adjustments
The pro forma adjustments are based on our preliminary estimates and assumptions that are subject to change. The following adjustments have been reflected in the unaudited pro forma condensed combined financial information:
| (a) | The amount of the amortization expense related to the intangibles is uncertain at this time as the Company is still in the process of valuing the intangibles acquired, including IPR&D, as discussed in Note 1 above. |
| (b) | Reflects the removal of interest expense accrued and the amortization of deferred financing cost and debt discount related to Moleculin, LLC’s convertible notes payable to third parties and related parties. For the year ended December 31, 2015 and the nine months ended September 30, 2016, interest expense related to convertible notes payable to third parties and related parties was $422,874 and $145,078, respectively. |
| (c) | We have not reflected any pro forma adjustments to reflect the tax impact of the pro forma adjustments, as we believe that the tax impact would not be material. |
| (d) | Reflects the issuance of 999,931 common shares of our common stock to the holders of Moleculin, LLC equity interests and convertible notes and assumed these shares were issued on January 1, 2015. |
| (e) | Represents Moleculin, LLC’s results of operations for the period from January 1, 2016 to May 1, 2016 (prior to our acquisition on May 2, 2016). Moleculin, LLC ceased to exist after MBI’s acquisition on May 2, 2016. |
| 37 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This Management’s Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) contains certain forward-looking statements. Historical results may not indicate future performance. Our forward-looking statements reflect our current views about future events, are based on assumptions and are subject to known and unknown risks and uncertainties that could cause actual results to differ materially from those contemplated by these statements. Factors that may cause differences between actual results and those contemplated by forward-looking statements include, but are not limited to, those discussed in “Risk Factors.” We undertake no obligation to publicly update or revise any forward-looking statements, including any changes that might result from any facts, events, or circumstances after the date hereof that may bear upon forward-looking statements. Furthermore, we cannot guarantee future results, events, levels of activity, performance, or achievements.
Highlights
We are a preclinical-stage pharmaceutical development company organized as a Delaware corporation in July 2015 to focus on the development of anti-cancer drug candidates. We have three drug development projects. Our lead drug candidate is liposomal Annamycin, which is referred to as Annamycin, an anthracyline intended for the treatment of relapsed or refractory acute myeloid leukemia, or AML. Annamycin has been in clinical trials pursuant to an investigational new drug application, or IND, that had been filed with the U.S. Food and Drug Administration, or FDA. Due to a lack of development activity by a prior drug developer, this IND was terminated, however we intend to apply for a new IND based on the same data that supported the original IND, updated for subsequent clinical data, and to commence a clinical trial for Annamycin.
We have two other drug development projects in progress. One of them involves a collection of small molecules we refer to as the WP1066 Portfolio that was obtained via our acquisition of Moleculin, LLC and is focused on the modulation of key regulatory transcription factors involved in the progression of cancer. The other, which we call the WP1122 Portfolio, is a suite of molecules targeting the metabolic processes involved in cancer in general, and glioblastoma in particular that we acquired from IntertechBio Corporation. Both of these technologies are licensed on a worldwide exclusive basis from The University of Texas M.D. Anderson Cancer Center, or MD Anderson.
Overview
MBI was founded in 2015 in order to combine and consolidate the development efforts involving several anti-cancer technologies, some of which are based on license agreements with MD Anderson. This effort began with the acquisition of the Annamycin development project from AnnaMed, Inc., or AnnaMed, followed by the acquisition of the license rights to the WP1122 Portfolio from IntertechBio Corporation, or IntertechBio. Further, we, on Moleculin, LLC’s behalf, entered into a co-development agreement with Houston Pharmaceuticals, Inc., or HPI, which culminated with the acquisition of Moleculin, LLC and MBI coincident with our initial public offering allowing us to gain control of the WP1066 Portfolio.
AnnaMed was formed in 2012 to take over the development of Annamycin from a prior drug development company, Callisto Pharmaceuticals, Inc., or Callisto. Callisto ceased development work on Annamycin leading to the termination of its IND by the FDA. In order to satisfy unmet license obligations, Callisto agreed to transfer all available Annamycin data to AnnaMed, which data we intend to now use to apply for a new IND.
IntertechBio was formed in 2009 to license and begin development on the WP1122 Portfolio. In August 2015, IntertechBio agreed to assign all license rights to us in exchange for 630,000 shares of our common stock.
Moleculin, LLC was formed in 2006 and has been working to develop the WP1066 Portfolio it licensed from MD Anderson. On May 2, 2016, Moleculin, LLC was merged with and into MBI. As a result of the merger, we issued the holders of Moleculin, LLC equity interests and convertible notes an aggregate of approximately 999,931 shares of our common stock.
| 38 |
Since Moleculin, LLC commenced operations in 2006, substantially all of its efforts have been focused on research, development and the advancement of the WP1066 Portfolio. Moleculin, LLC did not generate any revenue from product sales and, as a result, incurred significant losses.
Neither Moleculin, LLC nor MBI has manufacturing facilities and all manufacturing activities are contracted out to third parties. Additionally, Moleculin, LLC utilized third-party clinical research organizations to carry out clinical trials. Neither Moleculin, LLC nor MBI have a sales organization.
Recent Business Developments
Accelerated Plan for Clinical Drug Production – The Company announced on October 7, 2016, that it had secured an agreement with Dermin Sp. Zo. O. (“Dermin”) to utilize Dermin’s supply of Annamycin for its upcoming clinical trial, substantially reducing the expenditures required for drug product and shortening the time required to produce clinical supplies. The Company believes that this is an important agreement and key milestone to be reached and that it reduces the potential for drug production to negatively impact its clinical timeline. With this agreement in place, the Company’s drug product expense for upcoming clinical trials should be below previous estimates.
Moleculin, LLC previously licensed Annamycin to Dermin within a limited region in Europe, enabling Dermin to deploy Polish grant funds toward producing Annamycin. The agreement reached between the two companies allows us to utilize this Annamycin in our upcoming clinical trials rather than having to produce new Annamycin for its own use. The Company believes Dermin benefits from a data sharing arrangement giving it access to our clinical data on a faster timeline than it would be able develop on its own.
Updated Plan for Clinical Trials for Annamycin – On October 20, 2016, the Company announced in a conference call that it had identified some significantly positive findings from its detailed review of the last clinical trial for Annamycin by a prior developer, which has given rise to a modification of its own clinical development plan.
As the Company previously disclosed, a prior developer had conducted a clinical trial with Annamycin, but then subsequently failed to maintain their IND with the FDA. The Company had previously indicated that its plan was to conduct a detailed review of the clinical results generated by that prior developer. The Company would then use those results to reestablish an IND in order to continue clinical trials of Annamycin. However, the Company announced recently that, in the course of its review, it identified that Annamycin may have greater potential for efficacy than the Company originally believed, based on an unexpected potential opportunity to increase the drug’s Maximum Tolerable Dose (“MTD”).
In particular, the Dose Limiting Toxicities (“DLTs”) reported in that previous trial that led to the establishment of the current MTD of 150 mg/m2 were all from patients who had an unusually high number of first-line induction therapy failures prior to being treated with Annamycin. Specifically, of the three patients in the last clinical trial who experienced these DLTs, one of them had failed nineteen prior induction therapy attempts, another had failed sixteen and the other had failed fifteen before being enrolled in the trial. The Company has concluded from its review of this data that, if the heavily treated patients are excluded from the data set, the MTD could have been closer to 250 mg/m2, substantially higher than the level that was actually set by this previous trial.
The Company views this as an encouraging development because it means it may have an opportunity to increase the MTD for its next trial from 150 mg/m2 to 200 or even 250 mg/m2. If that turns out to be the case, the Company believes it could increase the chance for positive outcomes in its next trial.
With the discovery that the Company may be able to increase its MTD, the Company determined to adjust its clinical strategy by adding in a Phase I arm to its next Phase II trial, which will add some expense to its development effort. However, the Company believes the money saved by utilizing the Dermin drug inventory (discussed above) will offset this added expense. The Company believes this change in strategy will add several months to the eventual final approval of the drug, however, the Company believes that it remains on track to generate useful Phase II data by the second half of 2017.
| 39 |
FDA Guidance Regarding Annamycin IND – On November 17, 2016, the Company announced it had received verbal positive guidance from the FDA regarding its planned IND submission indicating that the Company may incorporate by reference the IND established by a prior developer. The Company has indicated in previous disclosures that it expected to begin its next clinical trial by the first half of 2017, however this development may reduce that time frame by several months. The Company has submitted a pre-IND briefing document to the FDA along with key questions regarding its clinical development plan and a request for a meeting, if the FDA deems it necessary. The FDA recently indicated in writing that it intends to provide written responses to the Company by December 6, 2016 and that it does not believe a live meeting is necessary. Once those written responses are received, the Company will adjust its final IND submission document accordingly and submit for final FDA review. IND submissions are normally reviewed within 30 days of filing.
Update on WP1066 - A clinician at MD Anderson has advised the Company that she is proceeding with a physician-sponsored IND for WP1066 treatment of brain tumors. The Company is not participating nor has influence on this IND process. The clinician has submitted an IND to the FDA and has indicated that this IND is on hold until documentation of Good Manufacturing Process or GMP production of WP1066 can be presented to the FDA.
Advancement of Preclinical Testing for Brain Tumors with WP1122 – On October 25, 2016, the Company announced promising initial results of the preclinical toxicology work on WP1122, the Company’s unique inhibitor of glucose metabolism, which is an important driver of glycolytic brain tumor progression and survival. The Company views this as an important step toward future clinical trials for WP1122. A similar chemical structure to that which turns morphine into heroine has been used to allow WP1122 to successfully enter the brain and increase circulation time. The Company indicated that preliminary escalating single dose toxicity testing in mice (oral administration) was successfully completed and even at the highest possible dose, no toxic death was observed. In multiple therapeutic doses, WP1122 was well tolerated during intense twice-daily oral dosing.
The Company believes moving forward with preclinical toxicology is the key to its ability to generate proof of concept in humans. The Company had previously announced the presentation of promising preclinical data in July 2016, supporting the potential for using WP1122 as a treatment for glioblastoma.
Appointment of New Chief Financial Officer - On August 22, 2016, the Company announced the appointment, effective August 19, 2016, of Jonathan P. Foster as Executive Vice President and Chief Financial Officer. Effective on August 19, 2016, Mr. Foster assumed the duties of CFO from Louis Ploth, Jr., who had come out of retirement in 2015 to help guide the new company through its initial public offering.
Mr. Foster joined us from InfuSystem Holdings, Inc., an NYSE MKT listed company and a leading national provider of infusion pumps and related services to the healthcare industry in the United States and Canada, primarily related to the treatment of cancer, where he served as Executive Vice President and Chief Financial Officer, since 2012. He brings more than 30 years of financial experience holding a variety of executive and senior financial positions with public, private, and start-up to large corporate and international companies.
Settlement of Outstanding Amounts Due. In January 2017, we agreed to issue 79,167 shares of our common stock at a value of $3.00 per share to a third-party in settlement of $237,500 in past due amounts.
| 40 |
Results of Operations – MBI
The Company was formed on July 28, 2015; therefore, the financial information for 2015 is not comparable to the financial results of the three and nine months ended September 30, 2016. The following table sets forth, for the periods indicated, data derived from our statement of operations:
| Three Months Ended September 30, | From Inception through September 30 | Nine Months Ended September 30, | From Inception through September 30, | |||||||||||||
| 2016 | 2015 | 2016 | 2015 | |||||||||||||
| (Unaudited) | (Unaudited) | (Unaudited) | (Unaudited) | |||||||||||||
| Revenue | $ | - | $ | - | $ | - | $ | - | ||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 496,659 | 38,409 | 616,498 | 38,409 | ||||||||||||
| General and administrative | 924,041 | 184,344 | 1,847,613 | 184,344 | ||||||||||||
| Depreciation | 977 | - | 1,629 | - | ||||||||||||
| Total operating expense | 1,421,677 | 222,753 | 2,465,740 | 222,753 | ||||||||||||
| Loss from operations | (1,421,677 | ) | (222,753 | ) | (2,465,740 | ) | (222,753 | ) | ||||||||
| Other expense: | ||||||||||||||||
| Interest expense | (10,402 | ) | (1,562 | ) | (37,307 | ) | (1,562 | ) | ||||||||
| Net loss | $ | (1,432,079 | ) | $ | (224,315 | ) | $ | (2,503,047 | ) | $ | (224,315 | ) | ||||
Three Months Ended September 30, 2016 compared to the period from July 28, 2015 (Inception) to September 30, 2015
Research and Development Expense. Research and development expense was $496,659 and $38,409 for the three months ended September 30, 2016 and for the period from July 28, 2015 (inception) to September 30, 2015, respectively. The increase of approximately $458,000 mainly represents accrued license fees to MD Anderson for approximately $40,000, $37,500 for research performed by HPI, and approximately $228,000 related to MD Anderson sponsored research. We expect to incur increased research and development costs in the future as our product development activities expand.
General and Administrative Expense. General and administrative expense was $924,041 and $184,344 for the three months ended September 30, 2016 and for the period from July 28, 2015 (inception) to September 30, 2015, respectively. The expense increase of approximately $740,000 was mainly attributable to additional payroll and related expenses of approximately $459,000 related to a full three months of our Chief Financial Officer’s, Chief Operating Officer’s and Chief Executive Officer’s salaries and compensation for our Board of Directors. Also, included in this quarter’s expense was $118,000 related to the severance of the former Chief Financial Officer. The Company also incurred approximately $289,000 of expenses related to investor relations, audit and accounting, and insurance costs.
Interest Expense. Interest expense included expense accrued on our convertible promissory notes issued in 2015 and 2016 bearing interest at the rate of 8% per annum.
Net Loss. The net loss for the three months ended September 30, 2016 was $1,432,079 which included non-cash expenses of $48,420 ($977 for depreciation and $47,443 for stock based compensation) and a one-time expense of $118,000 related to the severance of the former Chief Financial Officer. This loss for the period is a significant increase from the loss for the period from July 28, 2015 (inception) to September 30, 2015 of $224,315 as the Company had, at that time, just begun operations.
| 41 |
Nine Months Ended September 30, 2016 compared to the period from July 28, 2015 (inception) through September 30, 2015
Research and Development Expense. Research and development expense was $616,498 and $38,409 for the nine months ended September 30, 2016 and for the period from July 28, 2015 (inception) to September 30, 2015, respectively. The increase of approximately $578,000 mainly represents accrued license fees to MD Anderson for approximately $92,000, $37,500 for research performed by HPI, and approximately $228,000 related to MD Anderson sponsored research. We expect to incur increased research and development costs in the future as our product development activities expand.
General and Administrative Expense. General and administrative expense was $1,847,613 and $184,344 for the nine months ended September 30, 2016 and for the period from July 28, 2015 (inception) to September 30, 2015, respectively. The expense increase of approximately $1,663,000 was mainly attributable to additional payroll and related expenses of approximately $551,000 related to compensation of our Chief Financial Officer’s, Chief Operating Officer’s and Chief Executive Officer’s salaries and compensation for our Board of Directors during the third quarter of 2016. The Company also incurred approximately $951,000 of expenses related to investor relations, legal, audit and accounting, and insurance costs.
Interest Expense. Interest expense included expense accrued on our convertible promissory notes issued in 2015 and 2016 bearing interest at the rate of 8% per annum.
Net Loss. The net loss for the nine months ended September 30, 2016 was $2,503,047 which included non-cash expenses of $210,568 ($1,629 for depreciation and $208,939 for stock based compensation and other stock based expenses) and a one-time expense of $118,000 related to the severance of the former Chief Financial Officer. This loss for the period is a significant increase from the loss for the period from July 28, 2015 (inception) to September 30, 2015 of $224,315 as the Company had, at that time, just begun operations.
The following table sets forth the components of MBI’s statement of operations for the period from July 28, 2015 (Inception) to December 31, 2015:
| From July 28, 2015 (Inception) to December 31, 2015 | ||||
| Revenue | $ | - | ||
| Operating expenses: | ||||
| Research and development | 260,418 | |||
| General and administrative | 477,810 | |||
| Total operating expense | 738,228 | |||
| Interest expense | (10,132 | ) | ||
| Net loss | $ | (748,360 | ) | |
Research and Development Expense. Research and development expense was $260,418 for the period from July 28, 2015 (Inception) to December 31, 2015. The expense is mainly related to patent acquisition cost and travel expense of our research and development personnel.
General and Administrative Expense. General and administrative expense was $477,810 for the period from July 28, 2015 (Inception) to December 31, 2015. The expense mainly included professional fees to our consultants, attorney, accountants and our previous investment banker and financial advisor for services related to our initial public offering.
| 42 |
Interest Expense. Interest expense included expense accrued on our convertible promissory notes issued in August, September and October 2015 bearing interest at the rate of 8% per annum.
Results of Operations – Moleculin
The following table sets forth the components of Moleculin, LLC’s audited statements of operations for the years ended December 31, 2015 and 2014:
| Year Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Revenue | $ | - | $ | - | ||||
| Operating expenses: | ||||||||
| Research and development | 125,442 | 798,785 | ||||||
| General and administrative | 328,570 | 839,556 | ||||||
| Depreciation | 11,336 | 11,005 | ||||||
| Total operating expense | 465,348 | 1,649,346 | ||||||
| Other income (expense) | 99,537 | (159,740 | ) | |||||
| Net loss | $ | (365,811 | ) | $ | (1,809,086 | ) | ||
Research and Development Expense. Research and development expense was approximately $125,000 for the year ended December 31, 2015 as compared to $799,000 for the prior year. This decrease of $673,000 was driven primarily by clinical trial costs reductions incurred during 2015 of approximately $250,000; a decrease of approximately $121,000 in manufacturing costs for clinical supplies; and a decrease in intellectual property costs of approximately $303,000 as compared to 2014.
General and Administrative Expense. General and administrative expense for the year ended December 31, 2015 was $329,000 as compared to $840,000 for the prior year. This $511,000 decrease in general and administrative expense related primarily to a reduction in payroll and related personnel costs of approximately $350,000, a reduction in professional services fees of $96,000, and a reduction of travel expenses of $35,000.
Depreciation Expense. Depreciation expense was $11,000 for each of the years ended December 31, 2015 and 2014. Depreciation is generated from our fixed assets.
Other Income (Expense). Other income for the year ended December 31, 2015 was approximately $100,000 as compared to other expense of $160,000 for the prior year. This decrease of $260,000 in expense related primarily to an increase of $289,000 in interest expense accrued on our convertible promissory notes issued late in 2014 and amortization of debt discount and deferred financing cost associated with those notes, offset by a gain on debt extinguishment of $549,000 recorded in fourth quarter of 2015 related to the forfeiture of units by MD Anderson Cancer Center in conjunction with the amendment of a license agreement.
Liquidity and Capital Resources
As of September 30, 2016, we had $6,183,783 in cash. During the period from January 1, 2016 through May 2, 2016, we sold 234,296 of common stock for $702,888. On May 31, 2016, we completed our initial public offering, pursuant to which we sold 1,540,026 shares of our common stock at $6.00 per share for net proceeds of $8,464,183 after deducting underwriting discounts and commissions and direct offering expenses payable by us. We believe that our existing cash and cash equivalents as of September 30, 2016 will be sufficient to fund our planned operations to the end of the third quarter of 2017, which includes our revised clinical trial plan for Annamycin.
| 43 |
We will not generate revenue from product sales unless and until we successfully complete development of, obtain regulatory approval for and begin to commercialize one or more of our product candidates, which we expect will take a number of years and is subject to significant uncertainty. Accordingly, we anticipate that we will need to raise additional capital to fund our future operations. Until such time that we can generate substantial revenue from product sales, if ever, we expect to finance our operating activities through a combination of equity offerings and debt financings and we may seek to raise additional capital through strategic collaborations. However, we may be unable to raise additional funds or enter into such arrangements when needed on favorable terms, or at all, which would have a negative impact on our financial condition and could force us to delay, limit, reduce or terminate our development programs or commercialization efforts or grant to others rights to develop or market product candidates that we would otherwise prefer to develop and market ourselves. Failure to receive additional funding could cause us to cease operations, in part or in full. Furthermore, even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital due to favorable market conditions or strategic considerations, which may cause dilution to our existing stockholders.
The following table sets forth the primary sources and uses of cash of MBI for the period indicated:
| For the Nine Months Ended September 30, 2016 | ||||
| (Unaudited) | ||||
| Net cash used in operating activities | $ | (2,596,647 | ) | |
| Net cash used in investing activities | (109,793 | ) | ||
| Net cash provided by financing activities | 8,862,132 | |||
| Net increase in cash and cash equivalents | $ | 6,155,692 | ||
Cash used in operating activities
Net cash used in operating activities was $2,596,647 for the nine months ended September 30, 2016 and mainly included payments made for payroll, travel, insurance and professional fees to our consultants, attorneys and accountants for services related to our becoming a publicly traded company and related filing fees, along with payments made to MD Anderson for license and maintenance fees. Additionally, prepayments were made for insurance.
| 44 |
Cash used in investing activities
Net cash used in investing activities was $109,793 for the nine months ended September 30, 2016 and primarily represents the cash paid to acquire Moleculin, LLC.
Cash provided by financing activities
Net cash provided by financing activities was $8,862,132 for the nine months ended September 30, 2016. We received $8,464,183 net proceeds from our IPO stock issuance, $702,888 from issuance of common shares at $3 per share, and $165,000 from issuance of convertible notes. Net cash used in financing activities included approximately $470,000 for payments of notes payable.
Since Moleculin, LLC’s inception and through December 31, 2015, Moleculin, LLC funded its operations primarily through the sale and issuance of convertible preferred units and convertible and non-convertible promissory notes. From May to November 2014, Moleculin, LLC issued various convertible notes to its creditors. The note proceeds were $1.5 million. These notes bear interest at 8% per annum and were due on the earlier of June 30, 2016 or the consummation of a liquidation event (which event includes the merger between MBI and Moleculin, LLC occurred on May 2, 2016), unless these notes are converted.
The following table sets forth the primary sources and uses of cash for the periods indicated for Moleculin, LLC:
| Year Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Net cash used in operating activities | $ | (724,910 | ) | $ | (1,354,145 | ) | ||
| Net cash provided by investing activities | 232,300 | - | ||||||
| Net cash provided by financing activities | - | 1,525,256 | ||||||
| Net increase (decrease) in cash and cash equivalents | $ | (492,610 | ) | $ | 171,111 | |||
Cash used in operating activities
Net cash used in operating activities was approximately $725,000 and $1,354,000 for the years ended December 31, 2015 and 2014, respectively. The $629,000 decrease in net cash used in operating activities was primarily due to a decrease in spending in research and development, specifically in sponsored research performed by MD Anderson, as well as decrease in payments made to professionals and employees.
Cash used in investing activities
Net cash provided by investing activities was approximately $232,000 and $0 for the years ended December 31, 2015 and 2014, respectively. The increase was due to a collection of long-term receivable from Dermin, Sp. Zo. O. to whom the Company had provided funding assistance for Dermin’s efforts in developing and commercializing certain pharmaceutical products.
Cash provided by financing activities
Net cash provided by financing activities was approximately $0 and $1,525,000 for the years ended December 31, 2015 and 2014, respectively. Cash was provided by issuance of convertible notes payable in 2014 of approximately $1,525,000.
JOBS Act and Recent Accounting Pronouncements
The recently enacted JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
| 45 |
We have implemented all new accounting pronouncements that are in effect and may impact our financial statements and we do not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on our financial position or results of operations.
Critical Accounting Policies and Significant Judgments and Estimates
The financial statements have been prepared in accordance with generally accepted accounting principles in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe that the following accounting policies are the most critical to aid in fully understanding and evaluating our reported financial results, and they require our most difficult, subjective or complex judgments, resulting from the need to make estimates about the effect of matters that are inherently uncertain.
Acquisition
We acquired Moleculin, LLC on May 2, 2016, and, going forward our financial statements will include the operations of Moleculin, LLC. We account for acquired businesses using the acquisition method of accounting, which requires, among other things, that most assets acquired and liabilities assumed be recognized at their estimated fair values as of the acquisition date and that the fair value of acquired in-process research and development (“IPR&D”) be recorded on the balance sheet. Transaction costs are expensed as incurred. Any excess of the consideration transferred over the assigned values of the net assets acquired will be recorded as goodwill. The estimated fair values of assets acquired and liabilities assumed were determined based on management’s best estimates. Preliminary estimated fair values are subject to measurement period adjustments which represent updates made to the preliminary purchase price allocation based on revisions to valuation estimates in the interim period subsequent to the acquisition and initial accounting date up until the purchase price allocation is finalized which cannot be any later than one year from the acquisition date.
Beneficial Conversion Feature
From time to time, we may issue convertible notes that have conversion prices that create an embedded beneficial conversion feature on the issuance date. A beneficial conversion feature exists on the date a convertible note is issued when the fair value of the underlying common stock to which the note is convertible into is in excess of the remaining unallocated proceeds of the note after first considering the allocation of a portion of the note proceeds to the fair value of any attached equity instruments, if any related equity instruments were granted with the debt. We estimate the fair value of our common stock using the most recent selling price available. The intrinsic value of the beneficial conversion feature is recorded as a debt discount with a corresponding amount to additional paid-in capital. The debt discount is amortized to interest expense over the life of the note using the effective interest method.
Research and Development Costs
We record accrued expenses for estimated costs of our research and development activities conducted by third-party service providers, which include the conduct of pre-clinical studies and clinical trials and contract manufacturing activities. We record the estimated costs of research and development activities based upon the estimated amount of services provided but not yet invoiced, and we include these costs in accrued liabilities in the balance sheets and within research and development expense in the statement of operations. These costs are a significant component of our research and development expenses. We record accrued expenses for these costs based on the estimated amount of work completed and in accordance with agreements established with these third parties.
| 46 |
We estimate the amount of work completed through discussions with internal personnel and external service providers as to the progress or stage of completion of the services and the agreed-upon fee to be paid for such services. We make significant judgments and estimates in determining the accrued balance in each reporting period. As actual costs become known, we adjust our accrued estimates. Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed, the number of patients enrolled and the rate of patient enrollment may vary from our estimates and could result in us reporting amounts that are too high or too low in any particular period. Our accrued expenses are dependent, in part, upon the receipt of timely and accurate reporting from clinical research organizations and other third-party service providers. To date, there have been no material differences from our accrued expenses to actual expenses.
Impairment of Long-Lived Assets
Management reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount may not be realizable or at a minimum annually during the fourth quarter of the year. If an evaluation is required, the estimated future undiscounted cash flows associated with the asset are compared to the asset’s carrying value to determine if an impairment of such asset is necessary. The effect of any impairment would be to expense the difference between the fair value of such asset and its carrying value.
Components of our Results of Operations and Financial Condition
Operating expenses
We classify our operating expenses into three categories: research and development, general and administrative and depreciation.
Research and development. Research and development expenses consist primarily of:
| · | costs incurred to conduct research, such as the discovery and development of our product candidates; |
| · | costs related to production of clinical supplies, including fees paid to contract manufacturers; |
| · | fees paid to clinical consultants, clinical trial sites and vendors, including clinical research organizations in conjunction with implementing and monitoring our clinical trials and acquiring and evaluating clinical trial data, including all related fees, such as patient screening fees, laboratory work and statistical compilation and analysis; and |
| · | costs related to compliance with drug development regulatory requirements. |
We recognize all research and development costs as they are incurred. Clinical trial costs, contract manufacturing and other development costs incurred by third parties are expensed as the contracted work is performed.
We expect our research and development expenses to increase in the future as we advance our product candidates into and through clinical trials and pursue regulatory approval of our product candidates in the United States and Europe. The process of conducting the necessary clinical research to obtain regulatory approval is costly and time-consuming. The actual probability of success for our product candidates may be affected by a variety of factors including: the quality of our product candidates, early clinical data, investment in our clinical program, competition, manufacturing capability and commercial viability. We may never succeed in achieving regulatory approval for any of our product candidates. As a result of the uncertainties discussed above, we are unable to determine the duration and completion costs of our research and development projects or when and to what extent, if any, we will generate revenue from the commercialization and sale of our product candidates.
| 47 |
General and administrative. General and administrative expense consists of personnel related costs, which include salaries, as well as the costs of professional services, such as accounting and legal, facilities, information technology and other administrative expenses. We expect our general and administrative expense to increase following the completion of this offering due to the anticipated growth of our business and related infrastructure as well as accounting, insurance, investor relations and other costs associated with becoming a public company.
Depreciation. Depreciation expense consists of depreciation on our property and equipment. We depreciate our assets over their estimated useful life. We estimate machinery and equipment to have a 5-year life and furniture and fixtures to have a 7-year life.
Other income (expense), net
Other income (expense), net consists of interest expense associated with our notes payable and interest income earned on our cash and investment balances.
| 48 |
Overview
We are a preclinical-stage, pharmaceutical company focused on the development of anti-cancer drug candidates, some of which are based on license agreements with The University of Texas System on behalf of the M.D. Anderson Cancer Center, which we refer to as MD Anderson. Our lead drug candidate is liposomal Annamycin, which we refer to as Annamycin, an anthracycline intended for the treatment of relapsed or refractory acute myeloid leukemia, or AML. Annamycin has been in clinical trials pursuant to an Investigational New Drug application, or IND, that had been filed with the FDA. Due to a lack of development activity by a prior drug developer, this IND was terminated. However we intend to apply for a new IND based on the same data that supported the original IND, updated for subsequent clinical data and to resume clinical trials for Annamycin. We have two other drug development projects in process, one involving a collection of small molecules, which we refer to as the WP1066 Portfolio, focused on the modulation of key regulatory transcription factors involved in the progression of cancer, and the WP1222 Portfolio, a suite of molecules targeting the metabolic processes involved in cancer in general and glioblastoma (the most common form of brain tumor) in particular. We also intend to sponsor ongoing research at MD Anderson in order to improve and expand our drug development pipeline.
We have been granted royalty-bearing, worldwide, exclusive licenses for the patent and technology rights related to our WP1066 Portfolio and WP1122 Portfolio drug technologies, as these patent rights are owned by MD Anderson. The Annamycin drug substance is no longer covered by any existing patent protection. We intend to submit patent applications for formulation, synthetic process and reconstitution related to our Annamycin drug product candidate, although there is no assurance that we will be successful in obtaining such patent protection. Independently from potential patent protection, we believe Annamycin will qualify for Orphan Drug status and we have applied for such status with the FDA, which would entitle us to market exclusivity of 7 and 10 years from the date of approval of a New Drug Application (NDA) and Marketing Authorization (MA), in the United States and the European Union (EU), respectively. Separately, the FDA may also grant market exclusivity of up to 5 years for newly approved new chemical entities (of which Annamycin would be one), but there can be no assurance that such exclusivity will be granted or, if granted, for how long.
Corporate History
We were founded in 2015 by Walter Klemp (our chairman and CEO), Dr. Don Picker (our President and Chief Operating Officer) and Dr. Waldemar Priebe of MD Anderson (Chairman of our Scientific Advisory Board) in order to combine and consolidate development efforts that include several MD Anderson anti-cancer technologies. This effort began with the acquisition of the Annamycin development project from AnnaMed, Inc., or AnnaMed, followed by the acquisition of the license rights to the WP1122 Portfolio from IntertechBio Corporation, or IntertechBio. Further, we undertook an effort to gain control of the WP1066 Portfolio, which culminated with the merger of Moleculin, LLC and MBI and the establishment of a co-development agreement with Houston Pharmaceuticals, Inc., or HPI, coincident with our IPO.
AnnaMed was formed in 2012 to take over the development of Annamycin from a prior drug development company, Callisto Pharmaceuticals, Inc., or Callisto. Callisto ceased development work on Annamycin, leading to the termination of its IND by the FDA. Callisto disclosed publicly in its Form 10-K filing for the year ended 2009 that the clinical data relating to acute leukemia patients “did not support further clinical evaluation of L-Annamycin as a single agent to treat relapsed or refractory adult acute leukemia patients.” In order to satisfy unmet license obligations, Callisto agreed to transfer all available Annamycin data to AnnaMed, which data we will use to apply for a new IND. As such, notwithstanding Callisto’s determination to terminate its development of Annamycin, we will be utilizing the clinical data from Callisto’s trials to apply for a new IND. The basis for our decision to proceed notwithstanding Callisto’s determination is that we believe the actual clinical data as reported by Dr. Robert Shepard, our Chief Medical Officer and who was Callisto’s Chief Medical Officer at the time of the clinical trials, to the 2009 Annual Meeting of the American Society of Clinical Oncology, and as further reported by the Principal Investigators of the clinical trials in a peer-reviewed journal article (Clin Lymphoma Myeloma Leuk. 2013 August; 13(4): 430–434. doi:10.1016/j.clml.2013.03.015.), makes clear that the data does support further clinical evaluation. In addition, the conclusion published in the 2013 Clinical Lymphoma, Myeloma & Leukemia journal article was that “Single agent nanomolecular liposomal annamycin appears to be well-tolerated and evidence of clinical activity as a single agent in refractory adult ALL.” As reported in both the ASCO presentation and the 2013 journal article referenced, the definition of efficacy is based on the following Response Criteria: “Response criteria were achievement of CR defined as ≤5% blasts, granulocyte count of ≥1×109/L, and a platelet count of ≥100×109/L. Partial remission was defined the same as CR, except for the presence of 6% to 25% blasts. Hematologic improvement was defined as for CR but platelet count <100×109/L.” The summary of patient response from the 2013 journal article reads: “After determining the MTD, a 10-patient phase IIA was conducted. Eight of the patients completed one cycle of the three days of treatment at the MTD. Of these, five (62%) demonstrated encouraging anti-leukemic activity with complete clearing of circulating peripheral blasts. Three of these subjects also cleared bone marrow blasts with one subsequently proceeding onto successful stem cell transplantation. The other two developed tumor lysis syndrome and unfortunately expired prior to response assessment.” In the Company’s review of these trials, it confirmed that the activity demonstrated in this summary corresponds with a “Partial remission” as described in the Response Criteria and that the three subjects who “cleared bone marrow blasts” correspond with “CR” (Complete Response).
| 49 |
In 2012, AnnaMed out-licensed development rights in a limited territory to a Polish special purpose drug development company called Dermin in exchange for Dermin’s development work based on its successful effort to obtain Polish government grant funding to assist in the development of Annamycin. Since that time, such grant funding has been used to produce Annamycin in preparation for future clinical trials. In August 2015, we entered into a rights transfer agreement with AnnaMed pursuant to which in exchange for 1,431,000 shares of our common stock AnnaMed agreed to transfer any and all data it had regarding the development of Annamycin and the Annamycin IND, including all trade secrets, know-how, confidential information and other intellectual property rights held by AnnaMed.
IntertechBio was formed in 2009 to license and begin development on the WP1122 Portfolio. The WP1122 Portfolio was also out-licensed to Dermin, which was awarded a Polish government grant to assist in drug development efforts. In August 2015, IntertechBio agreed to assign all license rights to us in exchange for our common stock.
Moleculin, LLC was formed in 2006 and has been working to develop the WP1066 Portfolio it licensed from MD Anderson. As a part of the formation of Moleculin, LLC, an agreement was reached with HPI to limit Moleculin, LLC’s development efforts to uses in dermatology only, leaving non-dermatology indications to HPI. From 2006 to 2014, Moleculin, LLC funded its operations through the sale of equity and debt securities and through fees received from an out-licensing agreement with a Japanese dermatology drug development company, Maruho, Ltd., or Maruho. Since 2012, Moleculin, LLC conducted a series of clinical trials focused on the topical treatment of psoriasis. Although those trials have demonstrated drug activity, the results to date are not conclusive enough to warrant full commercialization as a topical dermatology drug. Additional study is required to determine optimal dosing and scheduling regimens for the topical treatment of psoriatic plaques. As a result of this additional complexity with regard to the potential treatment of psoriasis, Maruho did not elect to continue its funding of the psoriasis drug development effort and, therefore, forfeited their license rights to the WP1066 Portfolio that had been granted to it by Moleculin, LLC. Dermin is planning to clinically evaluate a candidate from the WP1066 Portfolio for the topical treatment of cutaneous T-cell lymphoma, or CTCL. We do not have control of the clinical plan or timeline for Dermin’s development effort.
Prior to our IPO, Moleculin, LLC was merged with and into our company. The merger agreement contains mutual representations and warranties between the parties. Pursuant to the merger agreement, we agreed for a period of six years to indemnify and hold harmless each present and former director and/or officer of Moleculin, LLC whom Moleculin, LLC would have had the power to indemnify under Delaware law that is made a party or threatened to be made a party to any threatened, pending or completed proceeding or claim by reason of the fact that he or she was a director or officer of the Moleculin, LLC prior to the effective time of the merger and arising out of actions or omissions of the indemnified party in any such capacity occurring at or prior to the effective time of the merger against any losses or damages reasonably incurred in connection with any claim. To our knowledge, no such proceeding or claim exists or has been threatened on the date hereof and Moleculin, LLC made representations to this effect in the merger agreement. As additional consideration payable to the Moleculin, LLC unit holders, we agreed pursuant to the merger agreement that if drugs for dermatology indications are successfully developed by us (or our successors) using any of the Existing IP Assets, then the Moleculin, LLC unit holders, in the aggregate, will be entitled to receive a 2.5% royalty on the net revenues generated by such drugs. Any such net revenues would include a deduction for license fees or royalty obligations payable to MD Anderson for such Existing IP Assets. The merger agreement defined “Existing IP Assets” to mean all intellectual property, licensed by us and Moleculin, LLC as of the time of the merger, including, without limitation, the intellectual property licensed from MD Anderson under the Patent and Technology License Agreement entered into by and between IntertechBio Corporation and MD Anderson dated April 2, 2012, as amended, and the Patent and Technology License Agreement dated June 21, 2010, as amended, between MD Anderson and Moleculin, LLC, but excluding any intellectual property relating to Annamycin. The right to receive the contingent royalty payments described herein are for drugs developed only for dermatology indications, and do not include drugs developed for any other indications. We have no obligation of any nature to pursue the development of any drugs for dermatology indications.
| 50 |
From 2006 through 2015, HPI has been engaged in research related to the use of the WP1066 Portfolio for the treatment of non-dermatology cancers and has received grant awards totaling approximately $4.9 million toward this effort. Prior to our IPO, we entered into a co-development agreement with HPI whereby HPI is continuing its grant-funded research and making all resulting data available for our use in exchange for a development fee. We may buy HPI out of its co-development rights in the WP1066 Portfolio at our option. Please see the section “Business – License Agreements” for a description of our agreement with HPI.
Neither our founders nor MBI have or will have any ownership stake in Dermin, our Polish grant-funded licensee. No Dermin-related grant money is expected to be paid directly to us, but rather the sublicense agreements require Dermin to share resulting data, which we believe will reduce costs we might otherwise have to incur directly. There can be no assurance, however, that Dermin will continue to receive the funds they have been awarded or that such funds will be spent by Dermin in a manner that will benefit MBI. The sublicensed territories granted to Dermin for the WP1066 Portfolio are Poland, Ukraine, Czech Republic, Hungary, Romania, Slovakia, Belarus, Lithuania, Latvia and Estonia. For the WP1122 Portfolio, the territory expanded to include Russia and Kazakhstan. For Annamycin, the territory is further expanded to include Netherlands, Turkey, Belgium, Switzerland, Austria, Sweden, Greece, Portugal, Norway, Denmark, Ireland, Finland, Luxembourg, Iceland, Uzbekistan, Georgia, Armenia, Azerbaijan and Germany. However, in the case of Germany, this territory may be reclaimed by us for a payment of $500,000 to Dermin.
The following summarizes the transactional history and common relationships among HPI, IntertechBio, Moleculin, LLC, MBI, Callisto, AnnaMed, MD Anderson, and our officers and major shareholders:
| · | Moleculin, LLC: Prior to our IPO, Moleculin, LLC was merged with and into our company. Moleculin, LLC was the holder of the license agreement with MD Anderson covering our WP 1066 Portfolio. As a result of the merger, we issued the equity interests holders of Moleculin, LLC an aggregate of 999,931 shares of our common stock. Waldemar Priebe, chairman of our Scientific Advisory Board, Walter Klemp, our chairman and chief executive officer, and Don Picker, our president and chief operating officer, were members of Moleculin, LLC and received 6,046 shares, 22,795 shares and 6,046 shares, respectively, of our common stock as a result of the merger. In addition, Walter Klemp and Don Picker were members of the board of Moleculin, LLC. |
| · | Houston Pharmaceuticals, Inc.: Prior to our IPO, MBI on Moleculin, LLC’s behalf, entered into a co-development agreement with HPI whereby HPI is continuing its grant-funded research and making all resulting data available for our use in exchange for a development fee. Waldemar Priebe, chairman of our Scientific Advisory Board, and Don Picker, our president and chief operating officer, are shareholders of HPI, and Dr. Priebe has the voting and dispositive power over the shares held by HPI. |
| · | IntertechBio Corporation: In August 2015, in exchange for the issuance of 630,000 shares of common stock, we acquired the rights to the license agreement with MD Anderson covering our WP1122 Portfolio held by IntertechBio Corporation. Waldemar Priebe, chairman of our Scientific Advisory Board, and Don Picker, our president and chief operating officer, are shareholders of IntertechBio and control the voting and dispositive power over the shares held by IntertechBio. |
| 51 |
| · | AnnaMed, Inc.: In August 2015, in exchange for 1,431,000 shares of our common stock, we acquired the rights to the Annamycin data related to the original Annamycin IND and the development of Annamycin held by AnnaMed, Inc., a company controlled by Walter Klemp, our chairman and chief executive officer. |
| · | Callisto Pharmaceuticals, Inc.: Our president and chief operating officer, Don Picker and our chief medical officer, Robert Shepard, were chief operating officer and chief medical officer, respectively, of Callisto. Since 2009, neither individual has had any relationship or ownership with Callisto. |
| · | MD Anderson: Both Moleculin, LLC and IntertechBio entered into license agreements with MD Anderson. See “Business – Our Licenses Agreements” below. Waldemar Priebe, chairman of our Scientific Advisory Board, is a Professor of Medicinal Chemistry, Department of Experimental Therapeutics, Division of Cancer Medicine, at the University of Texas MD Anderson Cancer Center. |
Our Drug Candidates
Annamycin
Our lead product candidate is Annamycin. We intend to use a portion of the proceeds from this offering to conduct clinical trials for Annamycin as a monotherapy for the treatment of relapsed or refractory acute myeloid leukemia, or AML. If Annamycin is ultimately approved on this basis, it is possible, with the necessary subsequent testing and approval, that it could be used in the future in combination with other drugs.
Market for Annamycin
Leukemia is a cancer of the white blood cells and acute forms of leukemia can manifest quickly and leave patients with limited treatment options. AML is the most common type of acute leukemia in adults. It occurs when a clone of leukemic progenitor white blood cells proliferates in the bone marrow, suppressing the production of normal blood cells. Currently, the only viable option for acute leukemia patients is a bone marrow transplant, which is successful in a significant number of patients. However, in order to qualify for a bone marrow transplant, the patient’s leukemia cells must be decreased to a sufficiently low level. This begins with a therapy of combining two chemotherapeutic drugs, which always includes an anthracycline, in inducing remission (a complete response, or CR), which therapy has not improved since it was first used in the 1970s and we estimate that this induction therapy has the same success rate of about 20% as at that time. Unfortunately, the current clinically approved anthracyclines are cardiotoxic, which can result in damage to the heart and limit the dosage amount that may be administered to patients. Additionally, the tumor cells often present de novo or develop resistance to the first line anthracycline, often through what is called “multidrug resistance”, enabling them to purge themselves of the available anthracyclines. Consequently, there remains no effective therapy for these patients and unfortunately most will succumb quickly to their leukemia. If a patient’s leukemia reappears before they can be prepared for a bone marrow transplant, they are considered to have “relapsed”. If a patient fails to achieve a sufficient response from the induction therapy to qualify for a bone marrow transplant, they are considered to be “refractory” (resistant to therapy). Together, this group of relapsed and refractory AML patients constitutes our primary focus for treatment with Annamycin and our intent is to pursue FDA approval for Annamycin as a second-line induction therapy for adult relapsed and refractory AML patients.
We believe that pursuing approval as a second line induction therapy for adult relapsed or refractory AML patients is the shortest path to regulatory approval, but we also believe that one of the most important potential uses of Annamycin is in the treatment of children with either AML or ALL (acute lymphoblastic leukemia, which is more common in children). Accordingly, we also intend to pursue approval for pediatric use when practicable.
Of the estimated 18,860 U.S. cases of acute myeloid leukemia diagnosed in 2014, an estimated 97% were adult and although exact numbers are not available, we estimate that 70% to 80% (approximately 13,000 to 14,000 patients) were expected to relapse or be resistant to first-line therapy.
| 52 |
Prior Clinical Trials for Annamycin
Annamycin is a liposome formulated anthracycline (also referred to in literature as “L-Annamycin”). It has been tested in 6 clinical trials and 114 patients without any reported cardiotoxicity and, in the two clinical trials focused on leukemia, with fewer dose-limiting toxicities than are normally expected with doxorubicin (one of the leading first-line anthracyclines used for induction therapy). Each of these trials was conducted by Callisto Pharmaceuticals, Inc., the previous holder of the intellectual property surrounding Annamycin, and not by our company. Annamycin demonstrated significant activity in 8 of 16 patients in a Phase I study in adult relapsed or refractory AML patients, with 6 of 14 patients completely clearing leukemic blasts. A 30 patient dose-ranging Phase I/II study in ALL demonstrated a similar level of activity, with 3 of 10 patients treated with the maximum tolerable dose clearing their leukemic blasts to a level sufficient to qualify for a bone marrow transplant. One of these patients went on to receive a successful curative bone marrow transplant. The other two of these three patients died of tumor lysis syndrome, a condition resulting from the overloading of their system with the debris from leukemic blast cells destroyed by the induction therapy. Armed with the knowledge of this potential, prophylactic pretreatment known to protect patients from the effects of tumor lysis syndrome will be deployed in future trials. Based on the results of the above clinical trials, we believe Annamycin is different from currently approved induction therapy drugs in four key ways: (i) it has demonstrated clinical activity in a patient population for whom there are currently no effective therapies, (ii) it appears to be capable of avoiding the “multi-drug resistance” mechanisms that often limit the effectiveness of currently approved anthracyclines; (iii) it has been shown to be non-cardiotoxic in animal models and no events of cardiotoxicity have been reported from the use of Annamycin in 114 patients; and (iv) in AML cell lines, it has been shown to be more potent than one of the leading approved drugs.
Because the prior developer of Annamycin allowed their IND to lapse, we are required to submit a new IND for continued clinical trials with Annamycin. Toward this end, we recently submitted a request for a Pre-IND meeting with the FDA. Based on subsequent conversations with the FDA, we will be allowed to incorporate by reference the prior developer’s IND in submitting our new request for IND. We believe this will not only save time and expense with regard to our IND submission, but it should also reduce the risk that our IND submission is delayed due to a review of any of this prior data. Written communication from the FDA indicates they do not see a need for a Pre-IND meeting and plan to respond to questions in our Pre-IND briefing document in writing by December 6, 2016.
Based on initial conversations with the FDA, because of the serious unmet medical need, we believe Annamycin may qualify for accelerated approval based on our planned clinical trials. In order to facilitate our communication with the FDA, we requested access to and reviewed in detail the available data supporting the dose-ranging Phase I/II clinical trial discussed above, which was conducted by a previous developer of Annamycin. In October 2016, we announced that we had identified some positive findings from this review, which gave rise to a modification of our own clinical development plan. We had indicated that our plan was to conduct a detailed review of the clinical results generated by that prior developer, and then to use those results to reestablish an IND in order to continue clinical trials of Annamycin. However, in the course of our review, we identified that Annamycin may have greater potential for efficacy than we originally believed, based on an unexpected potential opportunity to increase the drug’s Maximum Tolerable Dose (“MTD”).
In particular, the Dose Limiting Toxicities (“DLTs”) reported in the previous trial that led to the establishment of the current MTD of 150 mg/m2 were all from patients who had an unusually high number of first-line induction therapy failures prior to being treated with Annamycin. Specifically, of the three patients in the last clinical trial who experienced these DLTs, one of them had failed nineteen prior induction therapy attempts, another had failed sixteen and the other had failed fifteen before being enrolled in the trial. We concluded from our review of this data that, if the heavily treated patients are excluded from the data set, the MTD could have been closer to 250 mg/m2, substantially higher than the level that was actually set by this previous trial.
We view this as an encouraging development because it means we may have an opportunity to increase the MTD for our next trial from 150 mg/m2 to 200 or even 250 mg/m2. If that turns out to be the case, we believe it could increase the chance for positive outcomes in our next trial.
| 53 |
With the discovery that we may be able to increase our MTD, we determined to adjust our clinical strategy by adding in a Phase I arm to our next Phase II trial, which will add expense to our development effort. We believe this change in strategy will add several months to the eventual final approval of the drug, if the drug is approved.
We have also applied for Orphan Drug status with the FDA for Annamycin. The FDA has responded to our request for Orphan Drug status by requesting additional information, which we have now provided. We expect a response to our revised application in the near future, but can provide no assurance as to when any response will be received.
The prevalence ceiling for qualifying rare diseases under the US Orphan Drug Act is 200,000 patients and proportionally similar guidelines exist in the EU. The most recently published prevalence statistics from the National Cancer Institute reported that an estimated 37,726 patients had acute myeloid leukemia in the United States as of January 1, 2011, and trend data since that publication would indicate that the prevalence today should still be well below the 200,000 patient limitation for Orphan Drugs, qualifying Annamycin for the treatment of acute myeloid leukemia to qualify for Orphan Drug status. Annamycin already qualified for Orphan Drug status with its prior developer and we intend to repeat that process. However, we can provide no assurance that we will be successful in obtaining Orphan Drug status for Annamycin.
Lack of Cardiotoxicity
One of the key dose-limiting toxicities associated with currently available anthracyclines like doxorubicin is their propensity to induce life-threatening heart damage. This is especially problematic for pediatric leukemia patients whose life spans can be severely shortened by the very induction therapy designed to cure them of acute leukemia. In the animal model relied upon by the FDA as an indicator of human cardiotoxicity, the non-liposomal (free) form of Annamycin has been shown to be significantly less likely than doxorubicin to create heart lesions in mice and the liposomal formulation (L-Annamycin) has been shown in animal models to have reduced cardiotoxicity to the point where it is unlikely to cause harm to human patients. This lack of cardiotoxicity means L-Annamycin may be able to be used more aggressively in helping patients achieve remission without the potentially deadly long-term side effects of cardiotoxicity. This would be especially valuable in the case of pediatric acute leukemia (both AML and ALL) where long-term survival can be greatly impacted by cardiotoxicity.
Circumventing Multidrug Resistance
In addition to cardiotoxicity, the effectiveness of currently approved anthracyclines is limited by their propensity for succumbing to “multidrug resistance”, whereby transporters (one type of which is referred to as a “P-glycoprotein pump”) develop on the outer surface of cells to expel drugs like doxorubicin as a natural defense mechanism. The dosing of current therapies cannot be increased in an attempt to overcome multidrug resistance because of the likelihood of cardiotoxicity and other serious side effects. This limitation prevents adequate dosing of current anthracyclines to produce lasting remission. Annamycin appears to resist being expelled by P-glycoprotein pumps and other similar tested multidrug resistance transporters and thereby would seem to circumvent multidrug resistance. This characteristic has been shown in pre-clinical testing to allow for higher drug uptake in diseased cells, which we believe should allow for more effective induction therapy with less risk to the patient.
| 54 |
In order for anthracyclines to provide effective induction therapy, they must be allowed to accumulate in leukemic cells sufficiently to enter the cell’s nucleus where they damage the cell’s DNA and induce apoptosis (cell death). As induction therapy progresses, however, the targeted cells can develop a natural defense mechanism to prevent the anthracycline activity. The graphic below provides a simplified depiction of the formation of a P-glycoprotein pump on the outer surface (membrane) of a leukemic cell. As typical anthracyclines enter the cell, they are attracted to such pumps and are expelled (referred to as “efflux”) before they can accumulate sufficiently to serve their purpose. In contrast, Annamycin avoids such pumps and is allowed to accumulate sufficiently to destroy the leukemia cell despite the presence of the multidrug resistance mechanisms.
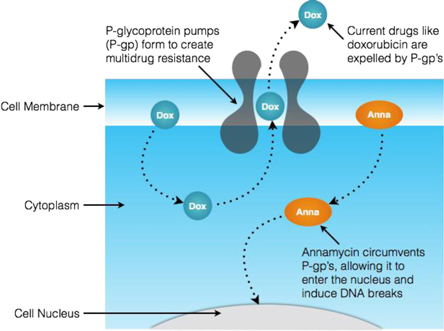
The WP1066 Portfolio
We have a license agreement with MD Anderson pursuant to which we have been granted a royalty-bearing, worldwide, exclusive license for the patent and technology rights related to our WP1066 Portfolio and its close analogs, molecules targeting the modulation of key oncogenic transcription factors.
Clinical Testing of WP1066 Portfolio
In vitro testing has shown a high level of activity for WP1066 against a wide range of solid tumors, and in vivo testing has shown significant activity against head and neck, pancreatic, stomach, and ovarian cancers, as well as metastatic melanoma and glioblastoma, among others. In vivo testing in mouse tumor models has shown that WP1066 inhibits tumor growth, blocks angiogenesis (a process that provides necessary blood supply to tumors) and increases survival.
With respect to our WP1066 Portfolio, we must complete pre-clinical toxicology testing, along with additional chemistry, manufacturing and control work to fully characterize the drug, establish the desired formulation and develop reference standards for future drug release, among others prior to submitting and application for IND. A clinician at MD Anderson has advised us that she is proceeding with a physician-sponsored IND for WP1066 treatment of brain tumors. We are not participating in this IND process. The clinician has submitted an IND to the FDA and has indicated that this IND is on hold until documentation of Good Manufacturing Process or GMP production of WP1066 can be presented to the FDA.
An analog of WP1066, referred to as WP1220, was previously the subject of an IND (WP1220 was referred to as MOL4239 for purposes of this IND) related to use of the molecule in the topical treatment of psoriasis. Clinical trials were commenced on WP1220 in the US, but were terminated early due to limited efficacy in the topical treatment of psoriatic plaques. Notwithstanding its limitations in treating psoriasis, our pre-clinical research has shown WP1220 to be effective in inhibiting cutaneous T-cell lymphoma (CTCL) in multiple CTCL cell lines. Based on this encouraging data, we are collaborating with a Polish drug development company, Dermin, which has received Polish government grant money to begin a clinical trial in Poland for the topical treatment of early stage CTCL patients. CTCL is a potentially deadly form of skin cancer for which there are limited treatment options.
| 55 |
We also conducted a Phase II clinical trial for WP1066 for the topical treatment of psoriasis, however this trial was terminated early as a significant number of patients experienced a non-permanent worsening of their psoriatic plaques after extended use of the drug, suggesting that its use as a topical agent for non-life threatening diseases such as psoriasis will require further study to optimize dosing and scheduling regimens.
Scientific Rationale for WP1066 Portfolio
Cellular biology depends upon signaling mechanisms to regulate functions such as cell growth, death and adaptation. Signal “transduction” is such a mechanism that converts an upstream stimulus to a cell into a specific cellular response. Signal transduction starts with a signal to a receptor or via a compound capable of passing through the cell membrane and ends with a change in cell function. The end result of this signal is often the activation of “transcription”, whereby genetic information is expressed and, in the case of oncogenic transcription, disease processes are initiated or maintained.
Receptors span the cell membrane, with part of the receptor outside and part inside the cell. When a chemical signal represented by a specific protein binds to the outer portion of the receptor, it conveys another signal inside the cell. Often there is a cascade of signals within the cell, wherein an upstream inducer starts a chain of events that resembles a domino effect. Collectively, this sequence is referred to as a “signaling pathway.” Eventually, the signal creates a change in the cell function by changing the expression of specific genes and production of specific proteins within the cell, and again, in the case of tumor development, such expression results in unwanted oncogenic processes.
Importantly, while normal healthy cell function relies on signaling mechanisms, diseases are capable of co-opting these mechanisms with negative consequences. Oncogenic processes (including inflammation and proliferation) depend upon signaling pathways that are responsible for coordinating functions such as cell growth, survival and cell differentiation. A particular class of proteins referred to as Signal Transducers and Activators of Transcription (such proteins are “STATs”) regulates the process of disease cell survival and proliferation, angiogenesis and immune system function and is persistently activated in a large number of human inflammatory processes and in hyper-proliferating diseases. Because certain of these proteins are known to be co-opted by tumor cells, we refer to them as “oncogenic transcription factors,” of which certain STATs are a subset.
Some STATs, such as STAT3, can be activated by any one of many different upstream inducers, making them very difficult to target by blocking just one or more of these upstream inducers. We believe that blocking a targeted STAT directly rather than via its multiple upstream inducers should result in greater efficacy with lower toxicity.
In the diagram shown below, any one of many different pathways (categorized as Growth Factor Receptors, Cytokine Receptors and Non-Receptor Tyrosine Kinases) triggers the activation of STAT3 proteins in a process called “phosphorylation”. In this process, phosphates attach to corresponding receptors on STAT3 and, eventually, two phosphorylated STAT3 proteins (“p-STAT3”) bind together in a pair referred to as a “dimer”. Once the dimer is formed, it enters the cell nucleus and triggers gene transcription. Conversely, if we reduce the presence of p-STAT3 before dimers can be formed, we can prevent the triggering of gene transcription and effectively inhibit the disease process.
| 56 |
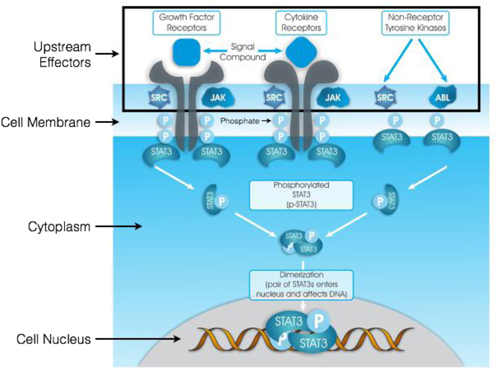
The upstream effectors shown in the above diagram (SRC, JAK and ABL) are just some of those capable of activating STAT3 once they themselves are activated by a variety of signal compounds. The complexity and diversity of pathways capable of activating STAT3 make it very difficult to develop effective drugs that attempt to target the upstream effectors. Furthermore, many of these upstream pathways are necessary for normal healthy cell function, so blocking them indiscriminately can lead to unwanted toxicities.
Published research has identified STAT3 as a master regulator of a wide range of tumors and clearly links STAT3 activation with the progression of these tumors. For this reason, it is believed that direct inhibition of p-STAT3 should be an effective way to reduce or eliminate the progression of these diseases.
Many research efforts have been directed toward development of specific methods to control activation of STAT3, but most have focused on targeting the upstream effectors of these pathways like growth factors, cytokines, and specific kinases including Janus kinases (JAKs). However, we believe that the multifactorial nature of the activation of STAT3 limits the effectiveness of such upstream approaches. Since the activity of p-STAT3 is a final and determinative step in triggering unwanted transcription, we believe it is preferable to inhibit p-STAT3 more directly and independently from upstream effectors.
We believe the WP1066 Portfolio represents a novel class of agents capable of hitting multiple targets, including p-STAT3, regardless of their upstream method of activation. By inhibiting the presence of p-STAT3, WP1066 directly attacks tumor cells, as has been demonstrated in numerous preclinical tests involving a wide range of tumor cells. We believe the effectiveness of WP1066 is not only the result of attacking tumors directly, but also indirectly by stimulating the immune system, increasing the patient’s natural ability to fight off tumor development. STAT1 is believed to stimulate T-cell activity and thereby the immune system responsible for fighting tumors. WP1066 has been shown to increase the activity of STAT1 at the same time it inhibits the activity of p-STAT3. We believe this dual activity makes WP1066 a uniquely promising anticancer drug candidate.
| 57 |
We believe the combination of the direct and indirect effects of WP1066 are to be credited with significant tumor suppression and increased survival in a number of in vitro cancer models. Below is one example showing a dramatic increase in survival by treating mice with metastatic melanoma with WP1066.
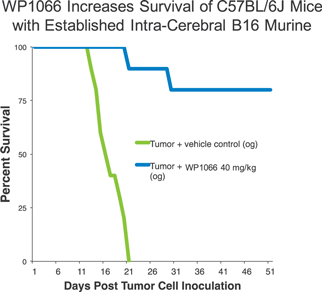
The WP1122 Portfolio
We have a license agreement with MD Anderson pursuant to which we have been granted a royalty-bearing, worldwide, exclusive license for the patent and technology rights related to our WP1122 Portfolio and similar molecules targeting the treatment of GBM and related CNS malignancies.
We believe this technology has the potential to target a wide variety of solid tumors, which eventually become resistant to all treatments, and thereby provide a large and important opportunity for novel drugs. Notwithstanding this potential, we are focused on the treatment of central nervous system malignancies and especially GBM. Although less prevalent than some larger categories of solid tumors, cancers of the central nervous system are particularly aggressive and resistant to treatment. The prognosis for such patients can be particularly grim and the treatment options available to their physicians are among the most limited of any cancer.
The American Cancer Society has estimated 23,770 new cases of brain and other nervous system cancers will occur in the United States in 2016, resulting in 16,050 deaths. Despite the severity and poor prognosis of these tumors, there are few FDA-approved drugs on the market.
We have proof of concept data for WP1122, including in vitro activity against cancer cell lines, as well as data on survival of animals subjected to xenografts of human brain tumors, including data regarding biodistribution and pharmacokinetics. In non-optimal doses and treatment regimes, WP1122 performed equal to or better than the current market leader, temozolomide and provided for superior survival for animals treated in combination with temozolomide.
Scientific Rationale for WP1122
Science has recognized that many cancer cells have a unique metabolism, distinct from that of normal cells. Dubbed the “Warburg Effect” by its discoverer, tumors rely preferentially on glycolysis for the metabolism of glucose, even in the presence of abundant oxygen, for energy (adenosine triphosphate (ATP)) production. This alternative form of energy production makes cancer cells as much as 17 times more dependent on glucose than normal cells.
| 58 |
The fundamental mechanism for imaging actively growing tumors using positron emission tomography (PET scans) is the Warburg Effect. A radiolabeled glucose decoy called F18DG accumulates disproportionately in tumors because of their dramatically increased rate of glucose uptake and accumulation.
Researchers have theorized that if a tumor’s access to glucose could be blocked the tumor could be starved out of existence. Previous attempts at targeting the metabolism of tumor cells have failed due to the rapid metabolism and short half-life (minutes) of the drugs being investigated. Efforts to target tumor metabolism in the brain were further thwarted by the inability to get glycolytic inhibitors into the brain in sufficient/therapeutic amounts due to the presence of what is called the “blood brain barrier”.
We believe WP1122 has the potential for developing a technology platform for enabling increased cellular uptake, increased drug half-life and, importantly, an increased ability to cross the blood brain barrier, enabling greater uptake in the brain. Our approach was inspired by the same principle that distinguishes morphine from heroin. Heroin is chemically the diacetyl ester of morphine. While morphine has a limited ability to cross the blood brain barrier (making it a good candidate for pain killing without impairing mental function), its diacetyl form, heroin, has the ability to accumulate in the brain by 10 to 100 times more than morphine. Once across the blood brain barrier, the acetyl groups are cleaved off by natural enzyme esterases, leaving pure morphine to accumulate in the brain. Similarly, we believe, based on pre-clinical testing, that WP1122, the diacetyl form of a glucose analog and decoy known as “2-DG”, crosses the blood brain barrier where its acetyl groups are cleaved off, allowing the resulting 2-DG to accumulate in the brain at a much higher rate than free 2-DG can do by itself.
Adding to the difficulty in getting free 2-DG across the blood brain barrier in therapeutic quantities is the relatively short half-life of 2-DG. The free form of 2-DG is rapidly metabolized and rendered ineffective within minutes of entering the body. In contrast, WP1122 has a half-life of approximately 6 hours, making it much more feasible to deliver quantities adequate for a therapeutic effect. In addition, we believe WP1122 may represent an improvement to current PET scan diagnostics techniques because of its unique ability to cross the blood brain barrier.
Overview of the market for our anti-cancer drugs
Cancer is the second leading cause of death in the United States behind heart disease. In 2014, an estimated 14.5 million people in the United States were living with a past or current diagnosis of cancer and, in 2016, the National Institutes of Health estimates that nearly 1.7 million new cases will be diagnosed and almost 600,000 Americans will die from cancer.
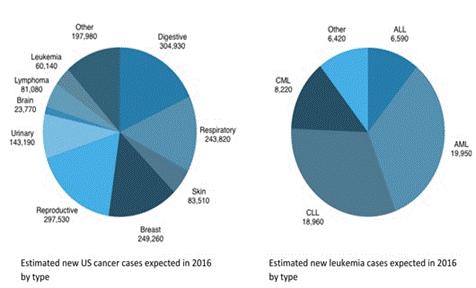
Source: American Cancer Society - Cancer Facts & Figures 2016
| 59 |
Digestive, reproductive, breast and respiratory cancers comprise 65% of expected cancer diagnoses in 2016, while cancers like leukemia and brain tumors are considered “rare diseases”. Leukemia in particular, can be divided into acute, chronic and other, with acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) comprising 26,540 of the estimated 60,140 new cases expected in the United States this year.
The worldwide cancer drug business has been estimated to represent approximately $100 billion in annual sales. Our lead drug, Annamycin, is in a class of drugs referred to as anthracyclines, which are chemotherapy drugs designed to destroy the DNA of targeted cancer cells. The most common approved anthracyclines are daunorubicin and doxorubicin and, prior to the expansion of their generic equivalents, annual revenues generated from anthracyclines have been estimated in the range of $600 million. Acute leukemia is one of a number of cancers that are treated with anthracyclines. One industry report estimates that annual drug revenues generated from the demand for AML-related therapies in the United States, United Kingdom, France, Germany, Italy and Spain were in the range of $151 million in 2012, and we believe that this number may increase if and when improved AML treatments are available.
Our other two active development projects have applications (among others) in the treatment of brain tumors, another rare disease for which there are few available treatments. The leading brain tumor drug is temozolomide, a drug introduced under the brand name Temodar. In 2012, one industry source reported annual revenues of approximately $882 million for Temodar before the expiration of its patent protection, at which point generic versions of the drug began to enter the market and reduce prices.
The Orphan Drug Act of 1983 and other legislative initiatives provide incentives and in some cases, accelerated approval pathways for companies that pursue the development of treatments for rare diseases and diseases for which there are few or no acceptable available treatment alternatives. Over the last 10 years, an increasing number of companies have begun using these designations to obtain new drug approvals for drugs where patent coverage has expired and/or where accelerated approval appears possible. An IMS Health report estimated that, in 2013, the sale of drugs with full or partial Orphan Drug status represented approximately $29 billion in revenue. We consider obtaining Orphan Drug status and accelerated approval pathways to be an important part of our development strategy for our drug candidates. Notwithstanding these potential opportunities, we can provide no assurance that our drugs will receive Orphan Drug status or any other special designation that could, among other things, provide for accelerated approval.
| 60 |
Our License Agreements
We acquired the rights and obligations under the Patent and Technology License Agreement entered into by and between IntertechBio and MD Anderson dated April 2, 2012 (the “IntertechBio Agreement”). Pursuant to that license agreement and an October 2015 amendment, IntertechBio obtained a royalty-bearing, worldwide, exclusive license to intellectual property including patent rights related to our WP1122 Portfolio and to our drug product candidate, WP1122. In consideration, IntertechBio agreed to make payments to MD Anderson, including an up-front payment, license documentation fee, annual maintenance fee, milestone payments and minimum annual royalty payments for sales of products developed under the license agreement. Specifically, under the IntertechBio Agreement, IntertechBio agreed to pay a nonrefundable upfront documentation fee in the amount of $80,000; annual maintenance fee in the amount of $10,000 on the first anniversary of the effective date of the IntertechBio Agreement, $20,000 on the second anniversary of the effective date of the agreement, $40,000 on the third anniversary of the effective date of the agreement, $60,000 on the fourth anniversary of the effective date of the agreement, $80,000 on the fifth anniversary of the effective date of the agreement and $100,000 on the sixth anniversary of the effective date of the agreement and every anniversary thereafter, except that such payments will no longer be due upon the first sale of a licensed product. Under the IntertechBio Agreement, IntertechBio also agreed to make a minimum annual royalty in the amount of $200,000 for the first anniversary following the first sale of a licensed product, $400,000 for the second anniversary following the first sale of a licensed product, and $600,000 for the third year following the first sale of a licensed product and every anniversary thereafter. IntertechBio also agreed to make one-time milestone payments in the amount of $200,000 upon commencement of a Phase II study for a licensed product, $250,000 upon commencement of a Phase III study for a licensed product, $400,000 upon filing of a New Drug Application (“NDA”) for a licensed product and $500,000 upon receipt of market approval for sale of a licensed product. MD Anderson has the right to terminate the agreement upon advance notice in the event of a default by IntertechBio. The agreement will also be terminated immediately upon IntertechBio’s insolvency. Additionally, MD Anderson has the right to terminate the license agreement if (i) a preclinical toxicology program for a licensed product is not initiated within one year of the of effective date of the amendment, (ii) an investigational new drug application (IND) is not filed with the Food and Drug Administration (FDA) for a Phase I study for a licensed product within three years of the effective date of the amendment, or (iii) a Phase I study for a licensed product is not commenced within five years of the effective date of the amendment. The IntertechBio Agreement will expire upon the expiration of the licensed intellectual property. In August 2015, the IntertechBio Agreement and amendments thereto were assigned to MBI. Under the assignment, MBI has assumed the rights and obligations of IntertechBio including, without limitation, the right to manufacture, have manufactured, use, import, offer to sell and/or sell products worldwide for any indication under the licensed intellectual property with the right to sublicense. However, the rights obtained pursuant to the assignment of the IntertechBio Agreement are made subject to the rights of the U.S. government to the extent that the technology covered by the licensed intellectual property was developed under a funding agreement between MD Anderson and the U.S. government. All out-of-pocket expenses incurred by MD Anderson in filing, prosecuting and maintaining the licensed patents have been and shall continue to be assumed by MBI. MBI is not required to issue MD Anderson any equity upon the completion of any milestones. On October 8, 2015, IntertechBio entered into a letter agreement with MD Anderson where MD Anderson agreed to receive past due maintenance fees and patent expenses of $98,108 owed by IntertechBio in four installments. The past due amount is related to certain metabolic inhibitor technology license that was assigned to MBI by IntertechBio and was owed by IntertechBio prior to MBI’s acquisition of the license. Pursuant to the letter, IntertechBio Corporation also agreed to pay $65,504 patent fees to a law firm. In order to have the license in good standing, MBI agreed to pay MD Anderson the $98,108 and the $65,504 to a law firm on behalf of IntertechBio Corporation; all such payments have been made in full by MBI.
Due to our acquisition of Moleculin, LLC, we obtained a royalty-bearing, worldwide, exclusive license to intellectual property rights, including patent rights related to our WP1066 drug product candidate from MD Anderson. Moleculin, LLC entered into a June 2010 Patent and Technology License Agreement with MD Anderson (the “Moleculin Agreement”). Under the Moleculin Agreement and an October 2015 amendment, Moleculin, LLC obtained the right to manufacture, have manufactured, use, import, offer to sell and/or sell products worldwide for any indication under the licensed intellectual property with the right to sublicense. In consideration, Moleculin, LLC agreed to make payments to MD Anderson including an up-front payment, milestone payments and minimum annual royalty payments for sales of products developed under the license agreement. Specifically, under the Moleculin Agreement, Moleculin, LLC agreed to pay a nonrefundable upfront documentation fee in the amount of $223,585; annual maintenance fee in the amount of $20,000 on the first anniversary of the effective date of the Moleculin Agreement, which shall increase in $10,000 increments on an annual basis thereafter up to a maximum of $100,000, except that such payments will no longer be due upon marketing approval in any country of a licensed product. Under the Moleculin Agreement, Moleculin, LLC also agreed to make a minimum annual royalty payment to MD Anderson in the amount of $200,000 after the first sale of a licensed product. Moleculin, LLC also agreed to make one-time milestone payments in the amount of $150,000 upon commencement of the first Phase III study for a licensed product within the United States, Europe, China or Japan; $500,000 upon submission of the first NDA for a licensed product in the United States; and $600,000 upon receipt of the first marketing approval for sale of a licensed product in the United States. MD Anderson has the right to terminate the Moleculin Agreement upon advanced notice in the event of a default by Moleculin, LLC. Moleculin, LLC has the right to terminate the Moleculin Agreement for any reason upon advance written notice to MD Anderson. The Moleculin Agreement will also be terminated immediately upon Moleculin, LLC’s insolvency. Per the October 2015 amendment to the Moleculin Agreement, MD Anderson relinquished any rights to any equity previously due MD Anderson in Moleculin, LLC. Upon completion of our acquisition of Moleculin, LLC, we assumed the rights and obligations of Moleculin, LLC including, without limitation, the right to manufacture, have manufactured, use, import, offer to sell and/or sell products worldwide for any indication under the licensed intellectual property with the right to sublicense. However, the rights we have obtained pursuant to the assignment of the Moleculin Agreement are made subject to the rights of the U.S. government to the extent that the technology covered by the licensed intellectual property was developed under a funding agreement between MD Anderson and the U.S. government. All out-of-pocket expenses incurred by MD Anderson in filing, prosecuting and maintaining the licensed patents have been and shall continue to be assumed by us. MBI is not required to issue MD Anderson any equity in our company upon the completion of any milestones.
| 61 |
On October 8, 2015, Moleculin, LLC entered into a letter agreement with MD Anderson for Moleculin, LLC’s past due fees to MD Anderson in the amount of $691,186 of which $300,000 had been paid prior to the letter agreement. Pursuant to the letter agreement, MD Anderson agreed to receive the remaining past due fee in three installments, which payments have been made in full.
Moleculin, LLC out-licensed certain intellectual property rights including rights covering the WP1066 drug product candidate to Dermin (“Moleculin Out-License Agreement”). The licensed intellectual property includes rights obtained by Moleculin, LLC pursuant to a license agreement with MD Anderson (“Moleculin-MD Anderson Agreement”). Under the Moleculin Out-License Agreement, Moleculin, LLC granted Dermin a royalty-bearing, exclusive license to manufacture, have manufactured, use, import, offer to sell and/or sell products in the field of human therapeutics under the licensed intellectual property in the countries of Belarus, Czech Republic, Estonia, Hungary, Latvia, Lithuania, Poland, Romani, Slovakia and Ukraine (“licensed territories”). Additionally, Moleculin, LLC agreed to develop and provide a dossier containing data related to the licensed subject matter to Dermin. In consideration, Dermin agreed to make payments to Moleculin, LLC, including upfront development fees, annual royalty payments, sublicense fee, and milestone payments. Specifically, under the Moleculin Out-License Agreement, Dermin agreed to make a nonrefundable upfront dossier development payment in the amount of $100,000; a service fee in the amount of $100,000 for assistance provided to Dermin in securing additional funding; and royalty payments on sales of any licensed product at a rate of no less than the royalty rate due to MD Anderson under the Moleculin-MD Anderson Agreement plus 1%. Dermin also agreed to provide a percentage of certain consideration Dermin receives pursuant to sublicense agreements in the amount of 25% prior to completion of a Phase IIb clinical study in a licensed territory and 10% on or after completion of a Phase IIb clinical study in a licensed territory, provided, however, if the sublicense fee is less than the sublicense fee due to MD Anderson under the Moleculin-MD Anderson Agreement, then Dermin shall be obligated to pay no less than the amount due to MD Anderson plus 5%. Also under the Moleculin Out-License Agreement, Dermin agreed to pay all out-of-pocket expenses incurred by Moleculin, LLC in filing, prosecuting and maintaining the licensed patents for which the license has been granted. The parties to the Moleculin Out-License Agreement each have the right to terminate the agreement upon advance notice in the event of a default by the other party. Dermin has the right to terminate the agreement if (i) Moleculin, LLC fails to timely provide the dossier to Dermin after Moleculin, LLC’s filing of an NDA for a licensed product in the United States; or (ii) Moleculin, LLC does not cooperate in assisting Dermin to secure funds to develop the licensed subject matter. Upon completion of our acquisition of Moleculin, LLC, we assumed the rights and obligations of Moleculin, LLC under the Moleculin Out-License Agreement.
We acquired the rights and obligations to the Patent and Technology License Agreement entered into by and between IntertechBio and Dermin dated April 15, 2011 (the “IntertechBio Out-License Agreement”). Pursuant to that license agreement, IntertechBio exclusively out-licensed intellectual property rights to Dermin, including rights covering the WP1122 drug product candidate obtained from MD Anderson pursuant to the IntertechBio Agreement. Under the IntertechBio Out-License Agreement, IntertechBio granted Dermin a royalty-bearing, exclusive license to manufacture, have manufactured, use, import, offer to sell and/or sell products in the field of human therapeutics under the licensed intellectual property in the countries of Belarus, Russia, Kazakhstan, Uzbekistan, Turkmenistan, Czech Republic, Estonia, Hungary, Latvia, Lithuania, Poland, Romania, Slovakia and Ukraine (“licensed territories”). Additionally, IntertechBio agreed to develop and provide a dossier containing data related to the licensed subject matter to Dermin. In consideration, Dermin agreed to make payments to IntertechBio, including upfront development fees, annual royalty payments, sublicense fees, and milestone payments. Specifically, under the IntertechBio Out-licensing Agreement, Dermin agreed to make a nonrefundable upfront dossier development fee in the amount of $35,000; a service fee in the amount of $40,000 for assistance provided to Dermin in securing additional funding; and royalty payments on sales of any licensed product at a rate of no less than the royalty rate due to MD Anderson under the IntertechBio Agreement plus 2%. Dermin also agreed to provide a percentage of certain consideration Dermin receives pursuant to sublicense agreements in the amount of 25% prior to completion of a Phase IIb clinical study in a licensed territory and 10% on or after completion of a Phase IIb clinical study in the licensed territories, provided, if the sublicense fee is less than the sublicense fee due to MD Anderson under the IntertechBio Agreement, then Dermin shall be obligated to pay not less than the amount due to MD Anderson plus 5%. Also under the IntertechBio Out-Licensing Agreement, Dermin agreed to pay all out-of-pocket expenses incurred by IntertechBio in filing, prosecution and maintaining the licensed patents for which the license has been granted. The parties to the IntertechBio Out-licensing Agreement each have the right to terminate the agreement upon advanced notice in the event of a default by the other party. Dermin has the right to terminate the agreement if (i) IntertechBio fails to timely provide the dossier to Dermin after IntertechBio’s filing of an NDA for a licensed product in the United States; or (ii) IntertechBio does not cooperate in assisting Dermin to secure funds to develop the licensed subject matter.
| 62 |
We entered into a May 2016 out-licensing agreement with HPI, pursuant to which we granted HPI certain intellectual property rights, including rights covering the potential drug candidate, WP1066 (“HPI Out-Licensing Agreement”). Under the HPI Out-Licensing Agreement we are required to make an upfront $100,000 payment and quarterly payments in the amount of $37,500 for the first four quarters following the effective date of the HPI Out-Licensing Agreement and $75,000 per quarter for the following eight quarters thereafter in consideration for the right to development data related to the development of licensed products. Notwithstanding our obligation to make the foregoing payments, the HPI Out-Licensing Agreement does not obligate HPI to conduct any specific research or to meet any milestones. Upon payment in the amount of $1,000,000 to HPI within three years of the effective date of the HPI Out-Licensing Agreement we will regain all rights to the licensed subject matter and rights to any and all development data and any regulatory submissions including any IND, NDA or ANDA related to the licensed subject matter. In the event that we do not exercise our right to regain our rights to the licensed subject matter within three years of the effective date of the HPI Out-Licensing Agreement the license granted to HPI shall convert to an exclusive license upon HPI’s written notice and we shall be obligated to transfer all existing data relating to licensed subject matter including any development data and any IND to HPI.
Annamed out-licensed certain intellectual property rights including rights covering the potential drug product, Annamycin to Dermin pursuant to a Patent and Technology Development and License Agreement dated June 28, 2012 (the “Annamed Agreement”). The licensed intellectual property includes rights obtained by Annamed pursuant to a license agreement with MD Anderson (“Annamed-MD Anderson Agreement”). Under the Annamed Agreement, Annamed granted Dermin a royalty-bearing, exclusive license to manufacture, have manufactured, use, import, offer to sell and/or sell products in the field of human therapeutics under the licensed intellectual property in the countries of Poland, Ukraine, Czech Republic, Hungary, Romania, Slovakia, Belarus, Lithuania, Latvia, Estonia, Netherlands, Turkey, Belgium, Switzerland, Austria, Sweden, Greece, Portugal, Norway, Denmark, Ireland, Finland, Luxembourg, Iceland, Kazachstan, Russian Federation, Uzbekistan, Georgia, Armenia, Azerbaijan and Germany (“Annamed licensed territories”). Additionally, Annamed agreed to develop and provide a dossier containing data related to the licensed subject matter to Dermin. In consideration, Annamed agreed to provide a running royalty at a rate of no less than the royalty due to MD Anderson under the Annamed-MD Anderson Agreement plus 2.5% for the sale of any licensed product in the Annamed licensed territories excluding Germany and at a rate of 15% for the sale of any licensed product in Germany. Dermin also agreed to provide a percentage of certain consideration Dermin receives pursuant to sublicense agreements in the amount of 25% prior to completion of a Phase IIb clinical study in an Annamed licensed territory and 10% on or after completion of a Phase IIb clinical study in an Annamed licensed territory, provided, however, if the sublicense fee is less than the sublicense fee due to MD Anderson under the Annamed-MD Anderson Agreement, then Dermin shall be obligated to pay no less than the amount due to MD Anderson plus 5%. Also under the Annamed Agreement, Dermin agreed to pay all out-of-pocket expenses incurred by Annamed in filing, prosecuting and maintaining the licensed patents for which the license has been granted. Annamed has the right to terminate the Annamed Agreement as to any country within the Annamed licensed territories if Dermin fails to provide evidence of its use of commercially reasonable efforts to commercialize a licensed product in such country within ninety days of Annamed’s written request. The Annamed Agreement may also be terminated by Annamed upon advanced written notice in the event that Dermin defaults. Dermin has the right to terminate the agreement if (i) Annamed fails to timely provide the documents and information required for Dermin to prepare a dossier within 30 days of Annamed’s filing of an NDA for a licensed product in the United States; or (ii) Annamed does not cooperate in assisting Dermin to secure funds to develop the licensed subject matter. As of August 2015, we have obtained the rights and obligations of Annamed under the Annamed Agreement.
Competition
We operate in a highly competitive segment of the pharmaceutical market, which market is highly competitive as a whole. We face competition from numerous sources including commercial pharmaceutical and biotechnology enterprises, academic institutions, government agencies, and private and public research institutions. Many of our competitors may have significantly greater financial, product development, manufacturing and marketing resources. Additionally, many universities and private and public research institutes are active in cancer research, and some may be in direct competition with us. We may also compete with these organizations to recruit scientists and clinical development personnel. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
| 63 |
The unmet medical need for more effective cancer therapies is such that anticancer drugs are, by far, the leading class of drugs in development. These include a wide array of products against cancer targeting many of the same indications as our drug candidates. While the introduction of newer targeted agents may result in extended overall survival, induction therapy regimens are likely to remain a cornerstone of cancer treatment in the foreseeable future.
There are a number of established therapies that may be considered competitive for the cancer indications for which we intend to develop our lead product, Annamycin. A key consideration when treating AML patients is whether the patient is suitable for intensive therapy. The standard of care for the treatment of newly diagnosed AML patients who can tolerate intensive therapy is cytarabine in combination with an anthracycline (i.e., doxorubicin or daunorubicin) – for some patients, primarily those less than 60 years of age, a stem cell transplant could also be considered if the induction regimen is effective in attaining a CR (Complete Response). The regimen of cytarabine in combination with an anthracycline has been the standard of care for decades. A patient not suitable for intensive therapy may be offered the option for low-intensity therapy such as low-dose cytarabine, azacitidine or decitabine. It should be noted that, in the U.S., these are not approved by the FDA for the treatment of AML patients and there remains no effective therapy for these patients or for relapsed or refractory AML. The initial focus for Annamycin development is in patients for whom the standard induction regimen has failed. Also, several major pharmaceutical companies and biotechnology companies are aggressively pursuing new cancer development programs for the treatment of AML.
A number of attempts have been made or are under way to provide an improved treatment for AML. Recently, Celator Pharmacetuicals, reported Phase III clinical trial results for a new combined formulation of cytarabine and daunorubicin (commonly used induction therapy drugs) they call Vyxeos. This new liposome formulation provides a 5:1 ratio of cytarabine and daunorubicin in each of three injections. When compared with patients receiving 7 injections of cytarabine and 3 injections of daunorubicin (traditional 7+3 induction therapy), patients receiving Vyxeos achieved an average increase in overall survival of approximately 3.5 months (9.5 months compared with 6 months). Despite this extension of overall survival, Vyxeos did not reduce the toxic side effects of daunorubicin (including cardiotoxicity) and it failed to qualify a significant majority of patients for curative bone marrow transplant.
Drugs attempting to target a subset of AML patients who present with particular anomalies involving a gene referred to as FLT3 are currently in clinical trials. Other approaches to improve the effectiveness of induction therapy are in early stage clinical trials and, although they do not appear to address the underlying problems with anthracyclines, we can provide no assurance that such improvements, if achieved, would not adversely impact the need for improved anthracyclines. A modified version of doxorubicin designed to reduce cardiotoxicity is in clinical trials for the treatment of sarcoma and, although this drug does not appear to address multidrug resistance and is not currently intended for the treatment of acute leukemia, we can provide no assurance that it will not become a competitive alternative to Annamycin. Although we are not aware of any other single agent therapies in clinical trials that would directly compete against Annamycin in the treatment of relapsed and refractory AML, we can provide no assurance that such therapies are not in development, will not receive regulatory approval and will reach market before our drug candidate Annamycin. In addition, any such competing therapy may be more effective and / or cost-effective than ours.
Government Regulation
Government authorities in the U.S., at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of products such as those we are developing. The pharmaceutical drug product candidates that we develop must be approved by the FDA before they may be legally marketed.
| 64 |
In the United States, the FDA regulates pharmaceutical products under the Federal Food, Drug and Cosmetic Act, and implementing regulations. Pharmaceutical products are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. FDA sanctions could include refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning letters, product recalls, product seizures, total or partial suspension of production or distribution injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us. The process required by the FDA before a non-biological pharmaceutical product may be marketed in the U.S. generally involves the following:
| · | Completion of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices or other applicable regulations; |
| · | Submission to the FDA of an Investigational New Drug, or IND, which must become effective before human clinical studies may begin; |
| · | Performance of adequate and well-controlled human clinical studies according to the FDA’s current good clinical practices (“GCP”), to establish the safety and efficacy of the proposed pharmaceutical product for its intended use; |
| · | Submission to the FDA of an NDA for a new pharmaceutical product; |
| · | Satisfactory completion of an FDA inspection of the manufacturing facility or facilities where the pharmaceutical product is produced to assess compliance with the cGMP, to assure that the facilities, methods and controls are adequate to preserve the pharmaceutical product’s identity, strength, quality and purity; |
| · | Potential FDA audit of the preclinical and clinical study sites that generated the data in support of the NDA; and |
| · | FDA review and approval of the NDA. |
The lengthy process of seeking required approvals and the continuing need for compliance with applicable statutes and regulations require the expenditure of substantial resources and approvals are inherently uncertain.
Before testing any compounds with potential therapeutic value in humans, the pharmaceutical product candidate enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies to assess the potential safety and activity of the pharmaceutical product candidate. These early proof-of-principle studies are done using sound scientific procedures and thorough documentation. The conduct of the single and repeat dose toxicology and toxicokinetic studies in animals must comply with federal regulations and requirements including good laboratory practices. The sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA has concerns and notifies the sponsor. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical study can begin. If resolution cannot be reached within the 30-day review period, either the FDA places the IND on clinical hold or the sponsor withdraws the application. The FDA may also impose clinical holds on a pharmaceutical product candidate at any time before or during clinical studies due to safety concerns or non-compliance. Accordingly, we cannot be sure that submission of an IND will result in the FDA allowing clinical studies to begin, or that, once begun, issues will not arise that suspend or terminate such clinical study.
| 65 |
Clinical studies involve the administration of the pharmaceutical product candidate to healthy volunteers or patients under the supervision of qualified investigators, generally physicians not employed by or under the clinical study sponsor’s control. Clinical studies are conducted under protocols detailing, among other things, the objectives of the clinical study, dosing procedures, subject selection and exclusion criteria, how the results will be analyzed and presented and the parameters to be used to monitor subject safety. Each protocol must be submitted to the FDA as part of the IND. Clinical studies must be conducted in accordance with GCP. Further, each clinical study must be reviewed and approved by an independent institutional review board (“IRB”) at, or servicing, each institution at which the clinical study will be conducted. An IRB is charged with protecting the welfare and rights of study participants and considers such items as whether the risks to individuals participating in the clinical studies are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical study subject or his or her legal representative and must monitor the clinical study until completed.
Human clinical studies are typically conducted in three sequential phases that may overlap or be combined:
| · | Phase 1: The pharmaceutical product is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases such as cancer, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients, with a goal of characterizing the safety profile of the drug and establishing a maximum tolerable dose (“MTD”). Our pharmaceutical products fall into this latter category because its products are intended to treat cancer and contain cytotoxic agents. Hence, our Phase 1 studies are conducted in late-stage cancer patients whose disease has progressed after treatment with other agents. |
| · | Phase 2: With the maximum tolerable dose established in a Phase 1 trial, the pharmaceutical product is evaluated in a limited patient population at the MTD to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases, to determine dosage tolerance, optimal dosage and dosing schedule and to identify patient populations with specific characteristics where the pharmaceutical product may be more effective. |
| · | Phase 3: Clinical studies are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These clinical studies are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product labeling. The studies must be well controlled and usually include a control arm for comparison. One or two Phase 3 studies are usually required by the FDA for an NDA approval, depending on the disease severity and other available treatment options. In some instances, where a Special Protocol Assessment is granted by the FDA, an NDA approval may be obtained based on Phase 2 clinical data with the understanding that the approved drug can be sold subject to a confirmatory Phase 3 trial to be conducted post-approval. |
Post-approval studies, or Phase 4 clinical studies, may be conducted after initial marketing approval. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication. The FDA also may require post-marketing testing, known as Phase 4 testing, risk minimization action plans and surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product.
Progress reports detailing the results of the clinical studies must be submitted at least annually to the FDA and written IND safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events or any finding from tests in laboratory animals that suggests a significant risk for human subjects. Phase 1, Phase 2 and Phase 3 clinical studies may not be completed successfully within any specified period, if at all. The FDA or the sponsor or its data safety monitoring board may suspend a clinical study at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical study at its institution if the clinical study is not being conducted in accordance with the IRB’s requirements or if the pharmaceutical product has been associated with unexpected serious harm to patients.
| 66 |
Concurrent with clinical studies, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the pharmaceutical product as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the pharmaceutical product candidate and, among other things, must develop methods for testing the identity, strength, quality and purity of the final pharmaceutical product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the pharmaceutical product candidate does not undergo unacceptable deterioration over its shelf life.
The results of product development, preclinical studies and clinical studies, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the pharmaceutical product, proposed labeling and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. The submission of an NDA is subject to the payment of substantial user fees. A waiver of such fees may be obtained under certain limited circumstances.
The FDA reviews all NDAs submitted before it accepts them for filing and may request additional information rather than accepting an NDA for filing. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. Under the goals and policies agreed to by the FDA under the Prescription Drug User Fee Act (“PDUFA”), the FDA has 10 months after the 60-day filing date in which to complete its initial review of a standard NDA and respond to the applicant, and six months after the 60-day filing date for a priority NDA. The FDA does not always meet its PDUFA goal dates for standard and priority NDAs.
After the NDA submission is accepted for filing, the FDA reviews the NDA application to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. The FDA may refer applications for novel pharmaceutical products or pharmaceutical products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. During the pharmaceutical product approval process, the FDA also will determine whether a risk evaluation and mitigation strategy (“REMS”) is necessary to assure the safe use of the pharmaceutical product. If the FDA concludes that a REMS is needed, the sponsor of the NDA must submit a proposed REMS; the FDA will not approve the NDA without a REMS, if required.
Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites as well as the site where the pharmaceutical product is manufactured to assure compliance with GCP and cGMP. If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. In addition, the FDA will require the review and approval of product labeling.
The NDA review and approval process is lengthy and difficult and the FDA may refuse to approve an NDA if the applicable regulatory criteria are not satisfied or may require additional clinical data or other data and information. Even if such data and information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data obtained from clinical studies are not always conclusive and the FDA may interpret data differently than we interpret the same data. The FDA will issue a complete response letter if the agency decides not to approve the NDA. The complete response letter usually describes all of the specific deficiencies in the NDA identified by the FDA. The deficiencies identified may be minor, for example, requiring labeling changes, or major, for example, requiring additional clinical studies. Additionally, the complete response letter may include recommended actions that the applicant might take to place the application in a condition for approval. If a complete response letter is issued, the applicant may either resubmit the NDA, addressing all of the deficiencies identified in the letter, or withdraw the application.
| 67 |
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling. In addition, the FDA may require Phase 4 testing which involves clinical studies designed to further assess pharmaceutical product safety and effectiveness and may require testing and surveillance programs to monitor the safety of approved products that have been commercialized.
Expedited Development and Review Programs
The FDA has a Fast Track program that is intended to expedite or facilitate the process for reviewing new pharmaceutical products that meet certain criteria. Specifically, new pharmaceutical products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. Unique to a Fast Track product, the FDA may consider for review sections of the NDA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA, if the FDA determines that the schedule is acceptable and if the sponsor pays any required user fees upon submission of the first section of the NDA.
Any product submitted to the FDA for market, including a Fast Track program, may also be eligible for other FDA programs intended to expedite development and review, such as priority review and accelerated approval. Any product is eligible for priority review if it has the potential to provide safe and effective therapy where no satisfactory alternative therapy exists or if it offers a significant improvement in the treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new pharmaceutical product designated for priority review in an effort to facilitate the review. Additionally, a product may be eligible for accelerated approval. Pharmaceutical products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, which means that the products may be approved on the basis of adequate and well-controlled clinical studies establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, the FDA may require that a sponsor of a pharmaceutical product receiving accelerated approval perform adequate and well-controlled post-marketing clinical studies. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could impact the timing of the commercial launch of the product. Fast Track designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process.
Post-Approval Requirements
Any pharmaceutical products for which the Company receives FDA approvals are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, complying with certain electronic records and signature requirements and complying with FDA promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, prohibitions on promoting pharmaceutical products for uses or in patient populations that are not described in the pharmaceutical product’s approved labeling (known as “off-label use”), industry-sponsored scientific and educational activities and promotional activities involving the internet. Failure to comply with FDA requirements can have negative consequences, including adverse publicity, enforcement letters from the FDA, actions by the U.S. Department of Justice and/or U.S. Department of Health and Human Services’ Office of Inspector General, mandated corrective advertising or communications with doctors, and civil or criminal penalties. Although physicians may prescribe legally available pharmaceutical products for off-label uses, manufacturers may not directly or indirectly market or promote such off-label uses.
| 68 |
We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our products. Manufacturers of our products are required to comply with applicable FDA manufacturing requirements contained in the FDA’s cGMP regulations. cGMP regulations require, among other things, quality control and quality assurance, as well as the corresponding maintenance of records and documentation. Pharmaceutical product manufacturers and other entities involved in the manufacture and distribution of approved pharmaceutical products are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance. Discovery of problems with a product after approval may result in restrictions on a product, manufacturer or holder of an approved NDA, including withdrawal of the product from the market. In addition, changes to the manufacturing process generally require prior FDA approval before being implemented and other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval.
Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of the FDA approval of the use of our pharmaceutical product candidates, some of our products covered by U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved pharmaceutical product is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent unless an extension is obtained. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and renders a decision on the application for any patent term extension or restoration. In the future, we may be able to apply for extension of patent term for one or more of our currently licensed patents or any future owned patents to add patent life beyond its current expiration date, depending upon the expected length of the clinical studies and other factors involved in the filing of the relevant NDA.
Market exclusivity provisions under the U.S. Food, Drug, and Cosmetic Act can also delay the submission or the approval of certain applications of other companies seeking to reference another company’s NDA. Currently seven years of reference product exclusivity are available to pharmaceutical products designated as Orphan Drugs, during which the FDA may not approve generic products relying upon the reference product’s data. Pediatric exclusivity is another type of regulatory market exclusivity in the U.S. Pediatric exclusivity, if granted, adds six months to existing exclusivity periods and patent terms. This six-month exclusivity, which runs from the end of other exclusivity protection or patent term, may be granted based on the voluntary completion of pediatric clinical studies in accordance with an FDA-issued “Written Request” for such a clinical study.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any pharmaceutical product candidates for which we may obtain regulatory approval. In the United States and in markets in other countries, sales of any products for which we receive regulatory approval for commercial sale will depend in part upon the availability of reimbursement from third-party payors. Third-party payors include government payors such as Medicare and Medicaid, managed care providers, private health insurers and other organizations. The process for determining whether a payor will provide coverage for a pharmaceutical product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the pharmaceutical product. Third-party payors may limit coverage to specific pharmaceutical products on an approved list, or formulary, which might not, and frequently does not, include all of the FDA-approved pharmaceutical products for a particular indication. Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. A payor’s decision to provide coverage for a pharmaceutical product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. In addition, in the United States there is a growing emphasis on comparative effectiveness research, both by private payors and by government agencies. We may need to conduct expensive pharmaco-economic studies in order to demonstrate the medical necessity and cost-effectiveness of our products, in addition to the costs required to obtain the FDA approvals. Our pharmaceutical product candidates may not be considered medically necessary or cost-effective. To the extent other drugs or therapies are found to be more effective than our products, payors may elect to cover such therapies in lieu of our products and/or reimburse our products at a lower rate.
| 69 |
Different pricing and reimbursement schemes exist in other countries. In the European Community, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national healthcare systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed upon. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical studies that compare the cost-effectiveness of a particular pharmaceutical product candidate to currently available therapies. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on healthcare costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert a commercial pressure on pricing within a country.
The marketability of any pharmaceutical product candidates for which we may receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, emphasis on managed care in the United States has increased and we expect this will continue to increase the pressure on pharmaceutical pricing. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we may receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
International Regulation
In addition to regulations in the United States, there are a variety of foreign regulations governing clinical studies and commercial sales and distribution of our current and future product candidates. Whether or not FDA approval is obtained for a product, approval of a product must be obtained by the comparable regulatory authorities of foreign countries, or jurisdictions such as the EU, before clinical studies or marketing of the product can commence in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical studies, product licensing, pricing and reimbursement vary greatly from country to country. In addition, certain regulatory authorities may require us to repeat previously conducted preclinical and/or clinical studies under specific criteria for approval in their respective country or jurisdictions, which may delay and/or increase the cost of approval in certain markets targeted for approval by us.
Employees
As of September 30, 2016, we had two full-time employees and four part-time employees, and accordingly, a high percentage of the work performed for our development projects is outsourced to qualified independent contractors.
Legal Proceedings
We are not subject to any litigation.
Properties
Our corporate and executive offices are in located in a leased facility in Houston, Texas. The current lease is month-to-month but is expected to be renegotiated in the next six months. We believe our facilities are sufficient to meet our current needs and that suitable space will be available as and when needed. We do not own any real property.
| 70 |
The following table sets forth certain information regarding our executive officers and directors as of November 28, 2016.
| Name | Age | Position | ||
| Walter V. Klemp | 56 | Chairman of the Board and Chief Executive Officer | ||
| Jonathan P. Foster | 53 | Chief Financial Officer and Executive Vice President | ||
| Donald Picker | 70 | President and Chief Operating Officer | ||
| Robert Shepard | 64 | Chief Medical Officer | ||
| Robert E. George | 66 | Director | ||
| Michael D. Cannon | 71 | Director | ||
| Jacqueline Northcut | 54 | Director |
Set forth below is biographical information about each of the individuals named in the tables above:
Walter V. Klemp - Chairman of the Board and Chief Executive Officer. Mr. Klemp is a co-founder of our company, and has served as our chairman of the board and chief executive officer since July 2015. Since 2006, Mr. Klemp has served as the chairman, co-founder and part-time chief executive officer of Moleculin, LLC. Since November 2011, Mr. Klemp has also served as chief executive officer of Soliton, Inc., a medical device company focused on developing new technology for use in aesthetics. Mr. Klemp served as president and chief executive officer of Zeno Corporation from 2004 to April 2011, where he developed and marketed dermatology devices and drugs from concept through FDA approval and market launch. From 1987 to 2000, Mr. Klemp served as chief executive officer and chairman of Drypers Corporation, a publicly traded multinational consumer products company that was listed as #1 on the INC 500 List of America’s Fastest Growing Companies. We believe that Mr. Klemp’s history with our company and background, coupled with his extensive experience in the medical field, provide him with the qualifications to serve as a Chairman of the Board and CEO.
Jonathan P. Foster - Chief Financial Officer and Executive Vice President. Mr. Foster has served as our chief financial officer and executive vice president since August 2016. Mr. Foster brings more than 30 years in financial experience holding a variety of executive and senior financial positions with public, private, start-up to large corporate and international companies. From February 2012 to August 2016, Mr. Foster served as Chief Financial Officer and Executive Vice President of InfuSystem Holdings, Inc., a national provider of infusion pumps and related services to the healthcare industry. From May 2011 to January 2012, Mr. Foster served as a consultant to the Chief Financial Officer of LSG Sky Chefs, USA, Inc., a subsidiary of Deutsche Lufthansa AG. Mr. Foster served on the Board of Financial Institutions for the State of South Carolina from 2006 to 2012. Mr. Foster is a Certified Public Accountant (South Carolina) and holds the designation of Chartered Global Management Accountant from the American Institute of Certified Public Accountants. He received his BS in Accounting from Clemson University in 1985.
Donald Picker, PhD - President and Chief Operating Officer. Dr. Picker joined Moleculin, LLC in 2007 as its chief executive officer and has served as our chief operating officer since July 2015 and as our president since January 2016. In 2007, Dr. Picker became the chief executive officer of IntertechBio. From 2006 through 2007, Dr. Picker was the President of Tapestry Pharmaceuticals. From 1998 to 2003, Dr. Picker was CEO of Synergy Pharmaceuticals. Synergy was merged into Callisto Pharmaceuticals where he was vice present of research and development until 2006. Dr. Picker led the development of carboplatin and cisplatin from concept to FDA approval. Dr. Picker received his BS degree from Brooklyn Polytechnic University and his PhD from SUNY Albany in 1975. Dr. Picker is currently devoting only part of his work time to us, and provide services as needed by us.
Robert Shepard, MD, FACP, Chief Medical Officer. Dr. Shepard has served as our chief medical officer since June 2016. From 2013 until 2014, Dr. Shepard served as vice president of scientific and medical affairs for Accelovance. Shepard has extensive research credentials in hematology and oncology and is board certified in oncology, hematology and internal medicine. He has a wide array of experience in translational medicine and clinical research and has been actively involved in oncology research since 1970, responsible for the clinical development of several drugs and immune therapies for biopharmaceutical companies, including serving as the consulting Chief Medical Officer for six companies. Dr. Shepard is a Magna Cum Laude graduate of Harvard University in biochemical sciences and molecular biophysics and studied in the Harvard-M.I.T. Health Sciences program. He held fellowships in hematology and oncology at the Tufts-New England Medical Center where he conducted laboratory research in leukemias, myeloma and myelodysplasia, as well as fellowship in pharmacology and molecular genetics at the Dana-Farber Cancer Center and Harvard Medical School. Dr. Shepard holds academic appointments at Harvard University, Tufts University and the University of Virginia.
| 71 |
Robert E. George - Director. Mr. George joined our board of directors upon our IPO. He was a partner with the international accounting firm of PricewaterhouseCoopers (PWC) for 27 years until 2010, where his client service sectors included healthcare, among others. Mr. George currently serves as Chairman of the Audit Committee for The University of Texas Health Science Center at Houston and, since June 2011, has been a member of The University of Texas at Austin, McCombs Graduate School of Business accounting faculty. Since January 2014, he also has been the Director of Energy Programs and Conferences for the Professional Development Institute. His past experience includes being an Editorial Advisor to the American Institute of Certified Public Accountants Journal of Accountancy and a contributing author for two accounting books. Mr. George graduated with accounting honors from the University of North Texas.
Michael D. Cannon - Director. Mr. Cannon joined our board of directors upon our IPO. Between 1997 and 2004, Mr. Cannon was the Chief Science Officer, EVP and a Director of SICOR, Inc., a U.S. public pharmaceutical company, until its acquisition by Teva Pharmaceutical Industries, Inc. SICOR focused on generic finished dosage injectable pharmaceuticals, active pharmaceutical ingredients and generic biopharmaceuticals. While at SICOR, he oversaw the acquisition and development of the biological business, including initiation and management of international partnerships, as well as on the design, construction, and licensure of protein manufacturing facilities. From July 2005 to December 2009, Mr. Cannon was a member of the scientific advisory board of Trevi Health Ventures LP, a New York investment fund specializing in health care investments. From May 2005 until December 2011, Mr. Cannon was a partner in a private partnership formed to evaluate and perform preliminary development of intellectual property in the healthcare sector. Since 2005, Mr. Cannon has served as a board member for several private companies. Mr. Cannon currently serves on the board of directors of 4 privately held biotech companies, including 2 focused on cancer-related treatments, and has been a partner and advisor in several biotech venture firms. Mr. Cannon has a degree in chemistry from Fordham College.
Jacqueline Northcut - Director. Ms. Northcut joined our board of directors upon our IPO. Ms. Northcut served as President and CEO of BioHouston from 2002 until 2015. BioHouston, Inc. is a non-profit organization founded by Houston, Texas area academic/research institutions to assist in the commercialization efforts of development stage life science and biotechnology companies. During this same period, Ms. Northcut became the founder and CEO of the Texas BioAlliance, an organization created to further assist with development efforts of biotechnology and medical device companies in the state of Texas. In these capacities she organized the Texas Life Science Forum and the Texas Life Science CEO Summit, helping connect biotech CEOs with funding, technical, regulatory and other resources. Ms. Northcut was previously a Partner in the international accounting firm of Arthur Andersen & Co., where she worked from 1984 to 2002. Ms. Northcut received her BBS from Harding University in 1984.
Director Independence
The rules of the Nasdaq Stock Market, or the Nasdaq Rules, require a majority of a listed company’s board of directors to be composed of independent directors within one year of listing. In addition, the Nasdaq Rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and governance committees be independent. Under the Nasdaq Rules, a director will only qualify as an independent director if, in the opinion of our board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. The Nasdaq Rules also require that audit committee members satisfy independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee, accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries or otherwise be an affiliated person of the listed company or any of its subsidiaries. In considering the independence of compensation committee members, the Nasdaq Rules require that our board of directors must consider additional factors relevant to the duties of a compensation committee member, including the source of any compensation we pay to the director and any affiliations with our company.
| 72 |
Our board of directors undertook a review of the composition of our board of directors and its committees and the independence of each director. Based upon information requested from and provided by each director concerning his background, employment and affiliations, including family relationships, our board of directors has determined that each of our directors, with the exception of Mr. Klemp are independent as defined under the Nasdaq Rules.
Compensation of Executive Officers
Summary Compensation Table
MBI was formed in July 2015. To provide you with a complete picture of the compensation paid to our executive officers for the fiscal years ended December 31, 2016 and 2015 when they acted in executive capacities for Moleculin, LLC and MBI, the following table and the related notes set forth information relating to the compensation paid to each of the named executive officers by Moleculin, LLC and us.
| Name and Principal Position | Year | Salary($) | Option awards ($) (1) | All other compensation ($) (2) | Total ($) | |||||||||||||||
| Moleculin, LLC: | ||||||||||||||||||||
| Walter V. Klemp, Chairman and Acting Chief Executive Officer | 2016 | ---- | ---- | ---- | ||||||||||||||||
| 2015 | 40,650 | ---- | 40,650 | |||||||||||||||||
| Don Picker, President and Chief Operating Officer | 2016 | ---- | ---- | ---- | ||||||||||||||||
| 2015 | 47,425 | ---- | 47,425 | |||||||||||||||||
| MBI: | ||||||||||||||||||||
| Walter V. Klemp, Chairman and Chief Executive Officer | 2016 | 175,000 | (3) | ---- | 175,000 | |||||||||||||||
| 2015 | ---- | ---- | ---- | |||||||||||||||||
| Don Picker, President and Chief Operating Officer | 2016 | 131,250 | ---- | 131,250 | ||||||||||||||||
| 2015 | ---- | ---- | ---- | |||||||||||||||||
| Jonathan P. Foster, Chief Financial Officer (4) | 2016 | 97,717 | 1,506,909 | 7,199 | 1,611,825 | |||||||||||||||
| Louis Ploth, Jr., Former Chief Financial Officer (5) | 2016 | 111,009 | 7,485 | 118,494 | ||||||||||||||||
| 2015 | 44,141 | (6) | 27,390 | 71,531 | ||||||||||||||||
(1) Represents the full grant date fair value of the option grant calculated in accordance with FASB ASC Topic 718. These amounts do not necessarily correspond to the actual value that may be realized by the named executive officer. For a summary of the assumptions made in the valuation of the awards made in 2015, please see Note 3 to our financial statements as of and for the period ended December 31, 2015 included elsewhere in this prospectus. For a summary of the assumptions made in the valuation of the awards made in 2016, please see Note 4 to our financial statements as of and for the period ended September 30, 2016 included elsewhere in this prospectus.
(2) Represents payments made for medical coverage.
(3) Mr. Klemp’s employment agreement provided that Mr. Klemp would defer 50% of his salary for 12 months, which deferred salary will be payable upon Mr. Klemp’s termination or on June 1, 2019. Of the foregoing $175,000 amount, $87,500 was paid during the year and $87,500 represents the deferred portion of Mr. Klemp’s salary.
(4) Mr. Foster became our chief financial officer in August 2016.
(5) Mr. Ploth resigned as our chief financial officer in August 2016.
(6) Includes $7,000 of consulting fees paid during the year.
Employment Agreements
Klemp Employment Agreement
On October 13, 2016, we entered into an employment agreement with Mr. Walter Klemp pursuant to which Mr. Klemp agreed to serve as our Chief Executive Officer commencing on such date. The agreement provides for an annual salary of $300,000, provided that Mr. Klemp has agreed to defer 50% of his salary for 12 months, which deferred salary will be payable upon Mr. Klemp’s termination or on June 1, 2019. If Mr. Klemp’s employment is terminated at our election without “cause” (as defined in the agreement), which requires 30 days advanced notice, or by Mr. Klemp for “good reason” (as defined in the agreement), Mr. Klemp shall be entitled to receive severance payments equal to the greater of 12 months of Mr. Klemp’s base salary or the base salary Mr. Klemp would have received had he remained employed through the third anniversary of the date of the agreement. Mr. Klemp has agreed not to compete with us for 12 months after the termination of his employment. If we determine to retain a new chief executive officer during the term of the agreement, we have the option, at our discretion, to convert Mr. Klemp into a consultant on the same terms as set forth above.
| 73 |
Foster Employment Agreement
On August 19, 2016, we entered into an employment agreement with Mr. Jonathan P. Foster pursuant to which Mr. Foster agreed to serve as our Chief Financial Officer and Executive Vice President commencing on such date for an initial term of three years, which will be automatically renewed for additional one-year terms unless either party chooses not to renew the agreement. The agreement provides for an annual salary of $250,000. Mr. Foster is entitled to receive an annual bonus payable. The final determination on the amount of the annual bonus will be made by the Compensation Committee of the Board of Directors, based on criteria established by the Compensation Committee.
Under the agreement, Mr. Foster was granted a ten-year option to purchase 400,000 shares at an exercise price per share equal to the closing price of our common stock on the date of execution of his employment agreement, which was $5.85. The option vests in four equal installments (or 100,000 shares each installment) on each of the succeeding four anniversary dates of the execution of the agreement, provided Mr. Foster is Chief Financial Officer on such vesting date. In the event of a “change of control” (as defined in the agreement) prior to the final vesting of all of the options, all of the unvested options shall immediately vest; provided, however, in the event the acquiring party desires to replace the unvested options with a substitute grant of equal or greater value, such proposed substitution shall be submitted to the Compensation Committee, and the Compensation Committee shall decide whether to allow the unvested options to vest or whether to cancel the unvested options and replace them with the substitute grant proposed by the acquiring party.
If Mr. Foster’s employment is terminated at our election without “cause” (as defined in the agreement), which requires 90 days advance notice, or by Mr. Foster for “good reason” (as defined in the agreement), Mr. Foster shall be entitled to receive severance payments equal to nine months of Mr. Foster’s base salary and a pro rata portion of the target bonus for the year in which such termination occurs. In addition, if Mr. Foster’s employment is terminated prior to the end of the term of the agreement by us without cause or by Mr. Foster for good reason, and such termination occurs within three months prior to a change in control, in contemplation of a change in control or within six months after a change in control, Mr. Foster shall be entitled to receive, in addition to the severance discussed above, an acceleration of the vesting of the option grant described in the prior paragraph. Mr. Foster agreed not to compete with us until nine months after the termination of his employment.
Equity Awards
The following table sets forth certain information concerning our outstanding options for our named executive officers at December 31, 2016.
Outstanding Equity Awards At Fiscal Year-End—2016
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price ($) | Option Expiration Date | ||||||||||
| Jonathan P. Foster | - | 400,000 | (1) | 5.85 | 8/19/2026 | |||||||||
| Louis Ploth (2) | - | 200,000 | (2) | 0.20 | 12/10/2025 | |||||||||
(1) The unvested shares underlying the option vest in equal annual installments over a four-year period commencing August 2017, provided Mr. Foster continues as our chief financial officer on each vesting date.
(2) The unvested shares underlying the option vested in equal annual installments over a four-year period commencing December 2017, provided Mr. Ploth was employed on each vesting date. Mr. Ploth resigned as our chief financial officer in August 2016. Pursuant to a separation agreement we entered into with Mr. Ploth, Mr. Ploth will be permitted to exercise 25% of the options to purchase common stock granted in December 2015, or 50,000 shares, until May 2020. The remaining options to purchase 150,000 shares were cancelled.
| 74 |
Director Compensation
On September 23, 2016, our board, after receiving the recommendation of the compensation committee, approved the following policy for compensating non-employee members of the board:
| · | Each non-employee director receives annual cash compensation of $30,000. In addition, the chairperson of the audit committee, compensation committee and nominating and governance committee receives an annual payment of $15,000, $10,000 and $7,500, respectively, and the other members of such committees shall receive an annual payment of $7,500, $5,000 and $3,750, respectively. |
| · | Upon the initial appointment (or election) of non-employee directors to the board, the director will be issued a 10-year option to purchase 20,000 shares of our common stock, under the Company's 2015 Stock Plan, with 3-year annual vesting and an exercise price equal the closing price of our common stock on the date of the appointment (or election). All non-employee directors serving as of September 23, 2016, the date of the initial adoption of the policy, received the foregoing initial stock option grant as of such date. |
| · | Annually, on the date of our annual meeting, each non-employee director will be issued a 10-year option to purchase 15,000 shares of our common stock, under the Company's 2015 Stock Plan, with 3-year annual vesting and an exercise price equal the closing price of our common stock on the date of the annual meeting. |
The following table sets forth the total compensation earned by our non-employee directors in 2016:
| Name | Fees earned or paid in cash ($) | Option awards ($) (1) | Total ($) | |||||||||
| Michael D. Cannon | 27,116 | 72,714 | 99,830 | |||||||||
| Robert E. George | 33,712 | 72,714 | 106,426 | |||||||||
| Jacqueline Northcut | 30,047 | 72,714 | 102,761 | |||||||||
(1) Represents the full grant date fair value of the 20,000 share option award our board approved and granted to each non-employee director on September 23, 2016, calculated in accordance with FASB ASC Topic 718. These amounts do not necessarily correspond to the actual value that may be realized by the director. For a summary of the assumptions made in the valuation of the awards made in 2016, please see Note 4 to our financial statements as of and for the period ended September 30, 2016 included elsewhere in this prospectus. As of December 31, 2016, the foregoing ten-year option to purchase 20,000 shares at an exercise price of $5.71 per share represented the sole option or stock award outstanding for each the non-employee directors.
Scientific Advisory Board
Our executive team is supported by our scientific advisory board, the members of which include scientists experienced in the fields in which we pursue.
Dr. Waldemar Priebe, Ph.D, Chair of Scientific Advisory Board (SAB). Waldemar Priebe is a founder of and one of the founding scientists at Moleculin, LLC and serves as Chairman of our Scientific Advisory Board. Dr. Priebe is also a Professor of Medicinal Chemistry at the Department of Experimental Therapeutics at The University of Texas MD Anderson Cancer Center. Dr. Priebe led the discovery of the molecules that form the basis for our lead drug candidates. As a founder or founding scientist at a number of successful biotechnology firms such as Aronex Pharmaceuticals, Houston Pharmaceuticals, Reata Pharmaceuticals, and IntertechBio, Dr. Priebe has been integral in advancing several drugs through the pipeline, three of which are currently in clinical development. He has also developed several new small molecule compounds that have been licensed as potential drugs.
Dr. Madeleine, Duvic, M.D., SAB Member. Dr. Duvic is Professor of Internal Medicine and Dermatology and Deputy Chairman of the Department of Dermatology at MD Anderson, a former board member of the American Academy of Dermatology and recent Vice President of the Society for Investigative Dermatology. Elected to the ADA, Dr. Duvic is also a Founder and board member of the United States Consortium for Cutaneous Lymphomas, performs translational research in CTCL, and has been engaged in the design and conduct of clinical trials that resulted in new therapies for CTCL. Dr. Duvic also serves on Medical and Scientific Boards of the National Alopecia Areata Foundation and Cutaneous Lymphoma Foundation. She is the Principal Investigator of the NAAF funded Alopecia Areata Registry.
| 75 |
Dr. John Paul Waymack, M.D., ScD, SAB Member. Dr. Waymack has served as CMO of Kitov Pharmaceuticals since July 2013. Dr. Waymack brings over 20 years' experience in the biopharma field. A former academic transplant surgeon and FDA medical officer, he has over 20 years' experience as a drug development consultant for major pharmaceutical companies, including Pfizer, Roche, Pharmacia, Warner Lambert and Searle. During his 10-year academic career, Dr. Waymack published over 100 scientific essays, mainly in the fields of prostaglandins and immunology. In addition, he was commissioned and served as a Major in the US Army Medical Corps in the position of Chief of Surgical Research at the Institute for Surgical Research. Dr. Waymack was also an Associate Professor of Surgery at the University of Texas Medical Branch and at the University of Medicine and Dentistry of New Jersey.
Dr. Sandra Silberman, M.D., Ph.D. Dr. Silberman’s career in clinical development began at Pfizer, Inc., where she oversaw the initiation of Tarceva (TM) clinical trials. Dr. Silberman then led the global development of Gleevec (TM) at Novartis. Dr. Silberman was a Vice President and Global Therapeutic Area Head in Oncology at Eisai. Subsequently, she served as a senior advisor to a number of biopharmaceutical companies, and numerous biotech companies as an independent industry consultant. She joined Quintiles in 2009 as the Vice President of Oncology and Global Head of Translational Medicine in the newly formed Innovation division, overseeing drug development and novel technologies for new partnerships with the pharmaceutical and biotechnology industries. Dr. Silberman earned her B.A., Sc.M. and Ph.D. from the Johns Hopkins University School of Arts and Sciences, School of Public Health and School of Medicine, respectively. Her major focus of investigation and doctoral thesis was in the burgeoning area of tumor immunology. Dr. Silberman received her M.D. from Cornell University Medical College, completing a postdoctoral training and her fellowship in hematology/oncology at the Brigham & Women's and the Dana Farber Cancer Institute in Boston. Dr. Silberman is currently an attending physician in the Hematology/Oncology clinic at the Duke VA in Durham, NC.
We intend to compensate the members of our Scientific Advisory Board, or SAB, based on our utilization of their time. As of the date hereof, we have entered into compensatory agreements with Drs. Waymack and Silberman pursuant to which we (i) agreed to pay the scientific advisors $4,000 per month, and (ii) made a one-time grant of a 10-year option to purchase 10,000 shares of our common stock, under the Company's 2015 Stock Plan, with 3-year annual vesting and an exercise price equal the closing price of our common stock on the date of the grant, which was $2.31 per share. We currently do not have any compensatory arrangements with Drs. Priebe or Duvic.
Other Executive Agreements
On August 21, 2016, we accepted the resignation of Mr. Louis Ploth from his position as our Chief Financial Officer effective immediately. On October 7, 2016, we entered into a separation agreement with Mr. Ploth, pursuant to which, among other items, Mr. Ploth generally released us from any claims he may have against us or our affiliates, and we agreed to pay Mr. Ploth a severance payment of $100,000 over a 12-month period and to pay Mr. Ploth’s medical insurance premiums until August 31, 2017. Pursuant to the separation agreement, Mr. Ploth will be permitted to exercise 25% of the options to purchase common stock granted to Mr. Ploth in December 2015, or 50,000 shares, until May 2020.
2015 Stock Plan
We have adopted a 2015 Stock Plan (the “Plan”). The Plan is a stock-based compensation plan that provides for discretionary grants of stock options, stock awards and stock unit awards to key employees and non-employee directors. The purpose of the Plan is to recognize contributions made to our company and its subsidiaries by key employees and non-employee directors and to provide them with additional incentive to achieve the objectives of our company. The following is a summary of the Plan.
Administration. The Plan will be administered by our Compensation Committee of the board of directors (we refer to body administering the Plan as the “Committee”). The Committee will have full authority to select the individuals who will receive awards under the Plan, determine the form and amount of each of the awards to be granted and establish the terms and conditions of awards.
Number of Shares of Common Stock. The number of shares of the common stock that may be issued under the Plan is 2,500,000. Shares issuable under the Plan may be authorized but unissued shares or treasury shares. If there is a lapse, forfeiture, expiration, termination or cancellation of any award made under the Plan for any reason, the shares subject to the award will again be available for issuance. Any shares subject to an award that are delivered to us by a participant, or withheld by us on behalf of a participant, as payment for an award or payment of withholding taxes due in connection with an award will not again be available for issuance, and all such shares will count toward the number of shares issued under the Plan. The number of shares of common stock issuable under the Plan is subject to adjustment, in the event of any reorganization, recapitalization, stock split, stock distribution, merger, consolidation, split-up, spin-off, combination, subdivision, consolidation or exchange of shares, any change in the capital structure of the company or any similar corporate transaction. In each case, the Committee has the discretion to make adjustments it deems necessary to preserve the intended benefits under the Plan. No award granted under the Plan may be transferred, except by will, the laws of descent and distribution.
Eligibility. All employees designated as key employees for purposes of the Plan and all non-employee directors are eligible to receive awards under the Plan. On November 1, 2015, six employees and all non-employee directors were eligible to participate in the Plan.
Awards to Participants. The Plan provides for discretionary awards of stock options, stock awards and stock unit awards to participants. Each award made under the Plan will be evidenced by a written award agreement specifying the terms and conditions of the award as determined by the Committee in its sole discretion, consistent with the terms of the Plan.
| 76 |
Stock Options. The Committee has the discretion to grant non-qualified stock options or incentive stock options to participants and to set the terms and conditions applicable to the options, including the type of option, the number of shares subject to the option and the vesting schedule; provided that the exercise price of each stock option will be the closing price of the common stock on the date on which the option is granted (“fair market value”), each option will expire ten years from the date of grant and no dividend equivalents may be paid with respect to stock options. It is intended that stock options qualify as “performance-based compensation” under Section 162(m) of the Code and thus be fully deductible by us for federal income tax purposes, to the extent permitted by law.
In addition, an incentive stock option granted to a key employee is subject to the following rules: (i) the aggregate fair market value (determined at the time the option is granted) of the shares of common stock with respect to which incentive stock options are exercisable for the first time by a key employee during any calendar year (under all incentive stock option plans of the company and its subsidiaries) cannot exceed $100,000, and if this limitation is exceeded, that portion of the incentive stock option that does not exceed the applicable dollar limit will be an incentive stock option and the remainder will be a non-qualified stock option; (ii) if an incentive stock option is granted to a key employee who owns stock possessing more than 10% of the total combined voting power of all class of stock of the company, the exercise price of the incentive stock option will be 110% of the closing price of the common stock on the date of grant and the incentive stock option will expire no later than five years from the date of grant; and (iii) no incentive stock option can be granted after ten years from the date the Plan was adopted.
Stock Awards. The Committee has the discretion to grant stock awards to participants. Stock awards will consist of shares of common stock granted without any consideration from the participant or shares sold to the participant for appropriate consideration as determined by the Board. The number of shares awarded to each participant, and the restrictions, terms and conditions of the award, will be at the discretion of the Committee. Subject to the restrictions, a participant will be a shareholder with respect to the shares awarded to him or her and will have the rights of a shareholder with respect to the shares, including the right to vote the shares and receive dividends on the shares; provided that dividends otherwise payable on any performance-based stock award will be held by us and will be paid to the holder of the stock award only to the extent the restrictions on such stock award lapse, and the Committee in its discretion can accumulate and hold such amounts payable on any other stock awards until the restrictions on the stock award lapse.
Stock Units. The Committee has the discretion to grant stock unit awards to participants. Each stock unit entitles the participant to receive, on a specified date or event set forth in the award agreement, one share of common stock or cash equal to the fair market value of one share on such date or event, as provided in the award agreement. The number of stock units awarded to each participant, and the terms and conditions of the award, will be at the discretion of the Committee. Unless otherwise specified in the award agreement, a participant will not be a shareholder with respect to the stock units awarded to him prior to the date they are settled in shares of common stock. The award agreement may provide that until the restrictions on the stock units lapse, the participant will be paid an amount equal to the dividends that would have been paid had the stock units been actual shares; provided that dividend equivalents otherwise payable on any performance-based stock units will be held by us and paid only to the extent the restrictions lapse, and the Committee in its discretion can accumulate and hold such amounts payable on any other stock units until the restrictions on the stock units lapse.
Performance-Based Awards. The Committee has the discretion to establish restrictions on the stock awards that qualify the awards as “performance-based compensation” under Section 162(m) of the Code so that they are fully deductible by us for federal income tax purposes. In such case, the Committee may establish performance goals for certain performance periods and targets for achievement of the performance goals, and the restrictions on the stock subject to the award will lapse if the performance goals and targets are achieved for the designated performance period. The performance goals will be based on one or more of the following criteria: (i) net earnings or net income (before or after taxes); (ii) earnings per share; (iii) net sales or revenue growth; (iv) net operating profit or income (including as a percentage of sales); (v) return measures (including, but not limited to, return on assets, capital, invested capital, equity, sales, or revenue); (vi) cash flow (including, but not limited to, operating cash flow, free cash flow, cash flow return on equity, and cash flow return on investment); (vii) earnings before or after taxes, interest, depreciation, and/or amortization; (viii) gross or operating margins; and (ix) share price (including, but not limited to, growth measures and total shareholder return). Performance goals may be absolute in their terms or measured against or in relationship to the performance of other companies or indices selected by the Committee. The performance goals may be particular to one or more lines of business or subsidiaries or may be based on our performance as a whole. The performance goals may be identical for all participants for a given performance period or, at the discretion of the Committee, may differ among participants. In addition, performance goals may be adjusted for any events or occurrences (including acquisition expenses, extraordinary charges, losses from discontinued operations, restatements and accounting charges and restructuring expenses), as may be determined by the Committee.
| 77 |
Payment for Stock Options and Withholding Taxes. The Committee may make one or more of the following methods available for payment of any award, including the exercise price of a stock option, and for payment of the minimum required tax obligation associated with an award: (i) cash; (ii) cash received from a broker-dealer to whom the holder has submitted an exercise notice together with irrevocable instructions to deliver promptly to us the amount of sales proceeds from the sale of the shares subject to the award to pay the exercise price or withholding tax; (iii) by directing us to withhold shares of common stock otherwise issuable in connection with the award having a fair market value equal to the amount required to be withheld; and (iv) by delivery of previously acquired shares of common stock that are acceptable to the Committee and that have an aggregate fair market value on the date of exercise equal to the exercise price or withholding tax, or certification of ownership by attestation of such previously acquired shares.
Provisions Relating to a “Change in Control” of the Company. Notwithstanding any other provision of the Plan or any award agreement, in the event of a “Change in Control” of the company, the Committee has the discretion to provide that all outstanding awards will become fully exercisable, all restrictions applicable to all awards will terminate or lapse, and performance goals applicable to any stock awards will be deemed satisfied at the highest target level. In addition, upon such Change in Control, the Committee has sole discretion to provide for the purchase of any outstanding stock option for cash equal to the difference between the exercise price and the then fair market value of the common stock subject to the option had the option been currently exercisable, make such adjustment to any award then outstanding as the Committee deems appropriate to reflect such Change in Control and cause any such award then outstanding to be assumed by the acquiring or surviving corporation after such Change in Control.
Amendment of Award Agreements; Amendment and Termination of the Plan; Term of the Plan. The Committee may amend any award agreement at any time, provided that no amendment may adversely affect the right of any participant under any agreement in any material way without the written consent of the participant, unless such amendment is required by applicable law, regulation or stock exchange rule.
The Board may terminate, suspend or amend the Plan, in whole or in part, from time to time, without the approval of the shareholders, unless such approval is required by applicable law, regulation or stock exchange rule, and provided that no amendment may adversely affect the right of any participant under any outstanding award in any material way without the written consent of the participant, unless such amendment is required by applicable law, regulation or rule of any stock exchange on which the shares are listed.
Notwithstanding the foregoing, neither the Plan nor any outstanding award agreement can be amended in a way that results in the repricing of a stock option. Repricing is broadly defined to include reducing the exercise price of a stock option or cancelling a stock option in exchange for cash, other stock options with a lower exercise price or other stock awards. (This prohibition on repricing without shareholder approval does not apply in case of an equitable adjustment to the awards to reflect changes in the capital structure of the company or similar events.)
No awards may be granted under the Plan on or after the tenth anniversary of the effective date of the Plan.
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
We were incorporated in July 2015. In August 2015, in exchange for the issuance of 630,000 shares of common stock, we acquired the rights to the license agreement with MD Anderson covering our WP1122 Portfolio held by IntertechBio Corporation. Waldemar Priebe, chairman of our Scientific Advisory Board, and Don Picker, our president and chief operating officer, are shareholders of IntertechBio and control the voting and dispositive power over the shares held by IntertechBio.
In August 2015, in exchange for the issuance of 1,431,000 shares of common stock, we acquired the rights to the Annamycin data related to the original Annamycin IND and the development of Annamycin held by AnnaMed, Inc., a company controlled by Walter Klemp, our Chairman and chief executive officer.
| 78 |
Prior to our IPO, in exchange for the issuance of 629,000 shares of common stock, we, on Moleculin, LLC’s behalf, entered into an agreement with HPI whereby HPI agreed to terminate its option to sublicense certain rights to the WP1066 Portfolio and to enter into a co-development agreement with us, allowing us to benefit from HPI’s grant-funded development efforts currently under way. Waldemar Priebe and Don Picker are shareholders of HPI, and Dr. Priebe have the voting and dispositive power over the shares held by HPI. For a detailed discussion of the HPI co-development agreement, see “Business – Our License Agreements.”
Prior to our IPO, we merged with Moleculin, LLC. Moleculin, LLC was the holder of the license agreement with MD Anderson covering our WP 1066 Portfolio. As a result of the merger, we issued the equity interests holders of Moleculin, LLC an aggregate of 999,931 shares of our common stock. Waldemar Priebe, Walter Klemp and Don Picker were members of Moleculin, LLC and received 6,046 shares, 22,795 shares and 6,046 shares, respectively, of our common stock as a result of the merger. In addition, Walter Klemp and Don Picker were members of the board of Moleculin, LLC.
In July and August 2015, in Messrs. Klemp, Picker and Priebe purchased 1,100,000 shares, 500,000 shares and 3,000,000 shares of our common stock, respectively, at a purchase price of $0.001 per share.
On July 1, 2013, Moleculin, LLC agreed to reimburse HPI for laboratory space used by Moleculin, LLC from January 2010 through December 2013 in the amount of $31,764. Moleculin, LLC stopped using the space in 2014. On September 1, 2013, Moleculin, LLC agreed to reimburse HPI for laboratory equipment used by Moleculin, LLC for the period from January 2009 through December 2013 in the amount of $35,000. Moleculin, LLC stopped using the equipment in 2014. During the year ended December 31, 2013, Moleculin, LLC paid $100,000 for laboratory services provided by HPI for the five years prior to August 1, 2013. We do not intend to utilize HPI for such services in the future.
We currently employ Lindsey Picker, the daughter of Don Picker, our president and chief operating officer, as a clinical research assistant on an at-will basis with an annual salary is $75,000.
Policies and Procedures for Related Party Transactions
Our audit committee charter provides that our audit committee is responsible for reviewing and approving in advance any related party transaction. This will cover, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, where the amount involved exceeds $120,000 and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. All of the transactions described in this section occurred prior to the creation of our audit committee and the adoption of this policy.
| 79 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information, as of January 17, 2017, regarding beneficial ownership of our common stock by:
| · | each of our directors; |
| · | each of our executive officers; |
| · | all directors and executive officers as a group; and |
| · | each person, or group of affiliated persons, known by us to beneficially own more than five percent of our shares of common stock. |
Beneficial ownership is determined according to the rules of the SEC, and generally means that person has beneficial ownership of a security if he or she possesses sole or shared voting or investment power of that security, and includes options that are currently exercisable or exercisable within 60 days. Each director or officer, as the case may be, has furnished us with information with respect to beneficial ownership. Except as otherwise indicated, we believe that the beneficial owners of common stock listed below, based on the information each of them has given to us, have sole investment and voting power with respect to their shares, except where community property laws may apply. Except as otherwise noted below, the address for each person or entity listed in the table is c/o Moleculin Biotech, Inc., 2575 West Bellfort, Suite 333, Houston, Texas 77054.
| Shares beneficially owned prior to Offering | Percentage beneficially owned prior to Offering (1) | Percentage beneficially owned after Offering | ||||||||||
| Name and Address of Beneficial Owner | ||||||||||||
| Walter V. Klemp | 2,480,724 | (2) | 20.4 | % | 14.5 | % | ||||||
| Donald Picker | 1,098,635 | (3) | 9.0 | % | 6.4 | % | ||||||
| Jonathan Foster | — | — | — | |||||||||
| Louis Ploth | 50,000 | (4) | * | * | ||||||||
| Robert Shepard | — | — | — | |||||||||
| Robert George | — | — | — | |||||||||
| Michael Cannon | — | — | — | |||||||||
| Jacqueline Northcut | — | — | — | |||||||||
| Directors and Named Executive Officers as a Group (7 persons) | 3,579,359 | (6) | 29.4 | % | 20.9 | % | ||||||
| 5% or greater shareholders | ||||||||||||
| Waldemar Priebe | 4,140,573 | (3)(5) | 34.0 | % | 24.1 | % | ||||||
| AnnaMed, Inc. | 1,425,000 | (2) | 11.7 | % | 8.3 | % | ||||||
| IntertechBio Corp. | 630,000 | (3) | 5.2 | % | 3.7 | % | ||||||
| Houston Pharmaceuticals, Inc. | 629,000 | (5) | 5.2 | % | 3.7 | % | ||||||
* Less than 1%.
(1) Based on 12,164,851 shares of common stock outstanding as of January 17, 2017.
(2) The 1,425,000 shares held by AnnaMed, Inc. have been included in the amount for Mr. Klemp. Mr. Klemp has voting and dispositive power over the shares held by AnnaMed, Inc.
(3) The 630,000 shares held by IntertechBio Corp. have been included in the amounts for Drs. Picker and Priebe. Drs. Picker and Priebe have voting and dispositive power over the shares held by IntertechBio Corp.
(4) Consists of shares underlying options.
(5) The 629,000 shares held by Houston Pharmaceuticals, Inc. have been included in the amount for Dr. Priebe. Dr. Priebe has voting and dispositive power over the shares held by Houston Pharmaceuticals, Inc.
(6) Does not include shares underlying options held by Mr. Ploth who resigned from the company in August 2016.
| 80 |
The following summary is a description of the material terms of our capital stock and is not complete. You should also refer to the Moleculin Biotech, Inc. certificate of incorporation and bylaws, which are included as exhibits to the registration statement of which this prospectus forms a part, and the applicable provisions of the Delaware General Corporation Law.
Our amended and restated certificate of incorporation authorizes us to issue up to 75,000,000 shares of common stock, par value $0.001 per share, and 5,000,000 shares of preferred stock, $0.001 par value per share. As of January 17, 2017, we have 12,164,851 shares of common stock outstanding.
Common Stock
Shares of our common stock have the following rights, preferences and privileges:
Voting
Each holder of common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of stockholders. Any action at a meeting at which a quorum is present will be decided by a majority of the voting power present in person or represented by proxy, except in the case of any election of directors, which will be decided by a plurality of votes cast. There is no cumulative voting.
Dividends
Holders of our common stock are entitled to receive dividends when, as and if declared by the our board of directors out of funds legally available for payment, subject to the rights of holders, if any, of any class of stock having preference over the common stock. Any decision to pay dividends on our common stock will be at the discretion of our board of directors. Our board of directors may or may not determine to declare dividends in the future. See “Dividend Policy.” The board’s determination to issue dividends will depend upon our profitability and financial condition any contractual restrictions, restrictions imposed by applicable law and the SEC, and other factors that our board of directors deems relevant.
Liquidation Rights
In the event of a voluntary or involuntary liquidation, dissolution or winding up of the company, the holders of our common stock will be entitled to share ratably on the basis of the number of shares held in any of the assets available for distribution after we have paid in full, or provided for payment of, all of our debts and after the holders of all outstanding series of any class of stock have preference over the common stock, if any, have received their liquidation preferences in full.
Other
Our issued and outstanding shares of common stock are fully paid and nonassessable. Holders of shares of our common stock are not entitled to preemptive rights. Shares of our common stock are not convertible into shares of any other class of capital stock, nor are they subject to any redemption or sinking fund provisions.
Preferred Stock
We are authorized to issue up to 5,000,000 shares of preferred stock. Our certificate of incorporation authorizes the board to issue these shares in one or more series, to determine the designations and the powers, preferences and relative, participating, optional or other special rights and the qualifications, limitations and restrictions thereof, including the dividend rights, conversion or exchange rights, voting rights (including the number of votes per share), redemption rights and terms, liquidation preferences, sinking fund provisions and the number of shares constituting the series. Our board of directors could, without stockholder approval, issue preferred stock with voting and other rights that could adversely affect the voting power and other rights of the holders of common stock and which could have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, a majority of our outstanding voting stock.
| 81 |
Convertible Notes
On various dates from August 31, 2015 through January 19, 2016, each as amended on March 10, 2016, we entered into unsecured promissory notes with third party investors. Each note bears interest at 8.0% per annum and was to mature on the earlier of June 30, 2016 or the completion of an IPO of our securities. Since the completion of the IPO occurred prior to June 30, 2016, these notes were to be automatically converted according to their terms into shares of our common stock at their applicable conversion prices upon the IPO to the extent and provided that no holder of these notes was or will be permitted to convert such notes to the extent that the holder or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such conversion. Due to this 4.99% limitation, a portion of these notes was not converted at the time of the IPO and the remaining unconverted principal and accrued interest amounts of the effected notes will remain outstanding and will be converted into shares of our common stock at such time as the 4.99% limitation continues to be met. Until such time as the notes are converted into shares of common stock, the maturity date of the notes will automatically be extended and we will not be required to repay the notes or the accrued interest relating to the notes in cash. The IPO was completed on May 31, 2016. On May 31, 2016, pursuant to the conversion feature of the foregoing notes and with restriction of the 4.99% beneficially owned condition limitation, discussed above, we issued 1,166,503 shares of common stock in total, reducing the convertible debt principal by $183,356 and accrued interest by $17,699. During the three months ended September 30, 2016, an additional 800,057 shares of common stock were issued due to the number of shares outstanding allowing for conversion of additional shares under the 4.99% beneficially owned condition limitation. This reduced the convertible debt principal by $133,988 and accrued interest by $6,742. The remaining convertible debt without consideration of accrued interest as of September 30, 2016, if converted on September 30, 2016, would result in an additional 1,928,899 common shares to be issued.
The holders of our convertible notes have agreed to the following lock-up of the common stock issuable upon conversion of the notes:
| · | beginning 90 days after the initial closing of our IPO and until the one-year anniversary of the initial closing of the IPO, (a) a holder of our convertible notes will be able to sell 1% of the number of shares of common stock into which such note is converted at the closing of the offering on a monthly basis, subject to a maximum sale on any trading day of 4% of the daily volume; (b) if our common stock price is over $7.00 per share for five consecutive trading days then the holder can sell up to 3% of the number of shares of common stock underlying the note on a monthly basis, subject to a maximum sale on any trading day of 4% of the daily volume; (c) if our common stock price is over $10.00 per share for five consecutive trading days then the holder can sell up to an additional 5% of the number of shares of common stock underlying the note on a monthly basis, subject to a maximum sale on any trading day of 7% of the daily volume; and (d) if our common stock price is over $14.00 per share then the holder is not restricted from making any sales until such time as our common stock price falls back below $14.00 per share; and |
| · | thereafter, until the two-year anniversary of the initial closing of this offering, the holder can sell on any trading day 10% of the daily volume; provided that if our common stock price is over $10.00 per share then the holder is not restricted from making any sales until such time as the common stock falls back below $10.00 per share. |
The foregoing lock-up restrictions relate to public sales and do not restrict the transfer of the shares privately, if permitted by applicable law, provided the acquirer of the shares agrees to comply with the above restrictions with respect to any public sales.
| 82 |
Certificate of Incorporation and Bylaw Provisions
Our certificate of incorporation and bylaws include a number of anti-takeover provisions that may have the effect of encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our board of directors rather than pursue non-negotiated takeover attempts. These provisions include:
Advance Notice Requirements. Our bylaws establish advance notice procedures with regard to stockholder proposals relating to the nomination of candidates for election as directors or new business to be brought before meetings of stockholders. These procedures provide that notice of stockholder proposals must be timely and given in writing to our corporate Secretary. Generally, to be timely, notice must be received at our principal executive offices not fewer than 120 calendar days prior to the first anniversary date on which our notice of meeting and related proxy statement were mailed to stockholders in connection with the previous year’s annual meeting of stockholders. The notice must contain the information required by the bylaws, including information regarding the proposal and the proponent.
Special Meetings of Stockholders. Our bylaws provides that special meetings of stockholders may be called at any time by only the Chairman of the Board, the Chief Executive Officer, the President or the board of directors, or in their absence or disability, by any vice president.
No Written Consent of Stockholders. Our certificate of incorporation and bylaws provide that any action required or permitted to be taken by stockholders must be effected at a duly called annual or special meeting of stockholders and may not be effected by any consent in writing by such stockholders.
Exclusive Forum Provision. Our certificate of incorporation provides that the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for (i) any derivative action or proceeding brought our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors or officers to us or our stockholders, (iii) any action asserting a claim against us arising pursuant to any provision of the Delaware General Corporation Law (the “DGCL”), or our certificate of incorporation or the bylaws, and (iv) any action asserting a claim against us governed by the internal affairs doctrine. This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and employees. Alternatively, a court could find these provisions of our certificate of incorporation to be inapplicable or unenforceable in respect of one or more of the specified types of actions or proceedings, which may require us to incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business and financial condition.
Amendment of Bylaws. Our stockholders may amend any provisions of our bylaws by obtaining the affirmative vote of the holders of a majority of each class of issued and outstanding shares of our voting securities, at a meeting called for the purpose of amending and/or restating our bylaws.
Preferred Stock. Our certificate of incorporation authorizes our board of directors to create and issue rights entitling our stockholders to purchase shares of our stock or other securities. The ability of our board to establish the rights and issue substantial amounts of preferred stock without the need for stockholder approval may delay or deter a change in control of us. See “Preferred Stock” above.
Delaware Takeover Statute
We are subject to Section 203 of the DGCL which, subject to certain exceptions, prohibits a Delaware corporation from engaging in any “business combination” (as defined below) with any interested stockholder for a period of three years following the date that such stockholder became an interested stockholder, unless: (1) prior to such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; (2) on consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding those shares owned (x) by persons who are directors and also officers and (y) by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to this plan will be tendered in a tender or exchange offer; or (3) on or subsequent to such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2⁄3% of the outstanding voting stock that is not owned by the interested stockholder.
| 83 |
Section 203 of the DGCL defines generally “business combination” to include: (1) any merger or consolidation involving the corporation and the interested stockholder; (2) any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; (3) subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; (4) any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; or (5) the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. In general, Section 203 defines an “interested stockholder” as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by such entity or person.
Limitations on Liability and Indemnification of Officers and Directors
Our certificate of incorporation and bylaws limit the liability of our officers and directors and provide that we will indemnify our officers and directors, in each case, to the fullest extent permitted by the Delaware General Corporation Law.
Listing
Our common stock is listed on the Nasdaq Capital Market under the symbol “MBRX”.
Transfer Agent
The transfer agent for our common stock is VStock Transfer, LLC.
| 84 |
In this offering, we are offering for sale 5,000,000 units, with each unit consisting of one share of our common stock and warrants to purchase 0.25 of a share of our common stock. The purchase price for each unit is $ . The units will not be issued or certificated. The shares of common stock and the warrants included in the units will be issued separately but can only be purchased together in the units in this offering.
Common Stock
The material terms and provisions of our common stock and each other class of our securities which qualifies or limits our common stock are described under the caption “Description of Capital Stock” in this prospectus.
Warrants
The following summary of certain terms and provisions of warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of warrant for a complete description of the terms and conditions of the warrants.
Duration and Exercise Price. Each warrant offered hereby will have an initial exercise price per share equal to $ . The warrants will be immediately exercisable and will expire on the fifth anniversary of the original issuance date. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price. The warrants will be issued separately from the common stock, and may be transferred separately immediately thereafter. A warrant to purchase 0.25 share of our common stock will be issued for every one share purchased in this offering.
Exercisability. The warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the warrant to the extent that the holder would own more than 4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants. No fractional shares of common stock will be issued in connection with the exercise of a warrant. In lieu of fractional shares, we will either pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next whole share.
Cashless Exercise. If, at the time a holder exercises its warrant, a registration statement registering the issuance of the shares of common stock underlying the warrants under the Securities Act is not then effective or available, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the warrant.
Exchange Listing. We do not intend to list the warrants on any securities exchange or nationally recognized trading system.
Right as a Stockholder. Except as otherwise provided in the warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their warrants.
| 85 |
We have entered into an underwriting agreement with the several underwriters listed in the table below. Roth Capital Partners, LLC is the representative of the underwriters. We refer to the several underwriters listed in the table below as the “underwriters.” Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and the underwriters have agreed to purchase from us, units. Our common stock trades on the NASDAQ Capital Market under the symbol “MBRX.”
Pursuant to the terms and subject to the conditions contained in the underwriting agreement, we have agreed to sell to the underwriters named below, and each underwriter severally has agreed to purchase from us, the respective number of units set forth opposite its name below:
| Underwriter | Number of Units | |||
| Roth Capital Partners, LLC | ||||
| National Securities Corporation | ||||
| Total | 5,000,000 | |||
The underwriting agreement provides that the obligation of the underwriters to purchase the units offered by this prospectus is subject to certain conditions. The underwriters are obligated to purchase all of the units offered hereby if any of the units are purchased.
We have granted the underwriters an option to buy up to an additional 750,000 shares of common stock and/or an additional 187,500 warrants at the public offering price per share and public offering price per warrant, respectively, less the underwriting discounts and commissions, in any combination thereof from us, to cover over-allotments, if any. The underwriters may exercise this option at any time, in whole or in part, during the 45-day period after the date of this prospectus.
Discounts, Commissions and Expenses
The underwriters propose to offer to the units purchased pursuant to the underwriting agreement to the public at the public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per unit. After this offering, the public offering price and concession may be changed by the underwriters. No such change shall change the amount of proceeds to be received by us as set forth on the cover page of this prospectus.
In connection with the sale of the units to be purchased by the underwriters, the underwriters will be deemed to have received compensation in the form of underwriting commissions and discounts. The underwriters' commissions and discounts will be % of the gross proceeds of this offering, or $ per unit, based on the public offering price per unit set forth on the cover page of this prospectus.
We have also agreed to reimburse Roth Capital Partners at closing for legal expenses incurred by it in connection with the offering up to a maximum of $75,000. In addition, we have agreed to issue to the representative warrants to purchase up to 7% of the aggregate number of shares of common stock sold in this offering. The representative warrants will have a term of no greater than 5 years from the effective date of this registration statement and an exercise price per share equal to the public offering price per share sold in this offering. Pursuant to FINRA Rule 5110(g), the representative warrants and any shares issued upon exercise of the representative warrants shall not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days immediately following the date of effectiveness or commencement of sales of this offering, except the transfer of any security:
| 86 |
| · | by operation of law or by reason of our reorganization; |
| · | to any FINRA member firm participating in the offering and the officers or partners thereof, if all securities so transferred remain subject to the lock-up restriction set forth above for the remainder of the time period; |
| · | if the aggregate amount of our securities held by the placement agent or related persons do not exceed 1% of the securities being offered; |
| · | that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund and the participating members in the aggregate do not own more than 10% of the equity in the fund; or |
| · | the exercise or conversion of any security, if all securities remain subject to the lock-up restriction set forth above for the remainder of the time period. |
Subject to the consummation of this offering, we have also agreed to give the representative a twelve-month right of first refusal to act as our lead underwriter or placement agent for any further capital raising transactions undertaken by us. We have also granted the representative a six-month tail fee equal to the cash and warrant compensation in this offering, if any investor with which we have had substantive discussions with respect to this offering, provides us with further capital during such six-month period following termination of our engagement of the representative.
The following table shows the underwriting discounts and commissions payable to the underwriters by us in connection with this offering (assuming both the exercise and non-exercise of the over-allotment option to purchase additional units we have granted to the underwriters):
| Per Unit | Total | |||||||||||||||
| Without Over- allotment | With Over- allotment | Without Over- allotment | With Over- allotment | |||||||||||||
| Public offering price (1) | $ | $ | ||||||||||||||
| Underwriting discounts and commissions paid by us | $ | $ | ||||||||||||||
| (1) | The public offering price and underwriting discount correspond to an assumed public offering price per share of common stock of $____ and an assumed public offering price per warrant or $____. |
Indemnification
Pursuant to the underwriting agreement, we have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments that the underwriters or such other indemnified parties may be required to make in respect of those liabilities.
Lock-Up Agreements
We have agreed not to (i) offer, pledge, issue, sell, contract to sell, purchase, contract to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock; (ii) enter into any swap or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of shares of common stock; or (iii) file any registration statement with the SEC relating to the offering of any shares of our common stock or any securities convertible into or exercisable or exchangeable for shares of our common stock, without the prior written consent the representative for a period of 90 days following the date of this prospectus, subject to an 18-day extension under certain circumstances (the "Lock-up Period"). This consent may be given at any time without public notice. These restrictions on future issuances are subject to exceptions for (i) the issuance of shares of our common stock sold in this offering, (ii) the issuance of shares of our common stock upon the exercise of outstanding options or warrants and the vesting of restricted stock awards or units, (iii) the issuance of employee stock options not exercisable during the Lock-up Period and the grant, redemption or forfeiture of restricted stock awards or restricted stock units pursuant to our equity incentive plans or as new employee inducement grants and (iv) the issuance of common stock or warrants to purchase common stock in connection with mergers or acquisitions of securities, businesses, property or other assets, joint ventures, strategic alliances, equipment leasing arrangements or debt financing.
| 87 |
In addition, each of our directors and executive officers has entered into a lock-up agreement with the underwriters. Under the lock-up agreements, the directors and executive officers may not, directly or indirectly, sell, offer to sell, contract to sell, or grant any option for the sale (including any short sale), grant any security interest in, pledge, hypothecate, hedge, establish an open "put equivalent position" (within the meaning of Rule 16a-1(h) under the Securities Exchange Act of 1934, as amended, or the Exchange Act), or otherwise dispose of, or enter into any transaction which is designed to or could be expected to result in the disposition of, any shares of our common stock or securities convertible into or exchangeable for shares of our common stock, or publicly announce any intention to do any of the foregoing, without the prior written consent of Roth Capital Partners, for a period of 90 days from the closing date of this offering, subject to an 18-day extension under certain circumstances. This consent may be given at any time without public notice. These restrictions on future dispositions by our directors and executive officers are subject to exceptions for (i) one or more bona fide gift transfers of securities to immediate family members who agree to be bound by these restrictions and (ii) transfers of securities to one or more trusts for bona fide estate planning purposes. Each officer and director shall be immediately and automatically released from all restrictions and obligations under the lock up agreement in the event that he or she ceases to be a director or officer of our company and has no further reporting obligations under Section 16 of the Exchange Act.
Electronic Distribution
This prospectus may be made available in electronic format on websites or through other online services maintained by the underwriters or by their affiliates. In those cases, prospective investors may view offering terms online and prospective investors may be allowed to place orders online. Other than this prospectus in electronic format, the information on the underwriters' websites or our website and any information contained in any other websites maintained by the underwriters or by us is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the underwriter in its capacity as underwriter, and should not be relied upon by investors.
Price Stabilization, Short Positions and Penalty Bids
In connection with the offering the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions and penalty bids in accordance with Regulation M under the Exchange Act:
| · | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. |
| · | Over-allotment involves sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares that they may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. The underwriters may close out any covered short position by either exercising their over-allotment option and/or purchasing shares in the open market. |
| · | Syndicate covering transactions involve purchases of the common stock in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. A naked short position occurs if the underwriters sell more shares than could be covered by the over-allotment option. This position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. |
| · | Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
| 88 |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. These transactions may be discontinued at any time.
Neither we nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our shares of common stock. In addition, neither we nor the underwriters make any representation that the underwriter will engage in these transactions or that any transaction, if commenced, will not be discontinued without notice.
Selling Restrictions
European Economic Area
This prospectus does not constitute an approved prospectus under Directive 2003/71/EC and no such prospectus is intended to be prepared and approved in connection with this offering. Accordingly, in relation to each Member State of the European Economic Area which has implemented Directive 2003/71/EC (each, a "Relevant Member State") an offer to the public of any shares of common stock which are the subject of the offering contemplated by this prospectus may not be made in that Relevant Member State except that an offer to the public in that Relevant Member State of any shares of common stock may be made at any time under the following exemptions under the Prospectus Directive, if and to the extent that they have been implemented in that Relevant Member State:
(a) to any legal entity which is a qualified investor as defined in the Prospectus Directive;
(b) to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), subject to obtaining the prior consent of the representatives of the underwriter for any such offer; or
(c) in any other circumstances which do not require any person to publish a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an "offer to the public" in relation to any shares of common stock in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares of common stock to be offered so as to enable an investor to decide to purchase any shares of common stock, as the expression may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State and the expression "Prospectus Directive" means Directive 2003/71/EC (and any amendments thereto including the 2010 PD Amending Directive to the extent implemented in each Relevant Member State) and includes any relevant implementing measure in each Relevant Member State and the expression "2010 PD Amending Directive" means Directive 2010/73/EU.
United Kingdom
This prospectus is not an approved prospectus for purposes of the UK Prospectus Rules, as implemented under the EU Prospectus Directive (2003/71/EC), and has not been approved under section 21 of the Financial Services and Markets Act 2000 (as amended) (the "FSMA") by a person authorized under FSMA. The financial promotions contained in this prospectus are directed at, and this prospectus is only being distributed to, (1) persons who receive this prospectus outside of the United Kingdom, and (2) persons in the United Kingdom who fall within the exemptions under articles 19 (investment professionals) and 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (all such persons together being referred to as "Relevant Persons"). This prospectus must not be acted upon or relied upon by any person who is not a Relevant Person. Any investment or investment activity to which this prospectus relates is available only to Relevant Persons and will be engaged in only with Relevant Persons. This prospectus and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other person that is not a Relevant Person.
The underwriter has represented, warranted and agreed that:
(a) it has only communicated or caused to be communicated and will only communicate or cause to be communicated any invitation or inducement to engage in investment activity (within the meaning of section 21 of the FSMA in connection with the issue or sale of any of the shares of common stock in circumstances in which section 21(1) of the FSMA does not apply to the issuer; and
(b) it has complied with and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares of common stock in, from or otherwise involving the United Kingdom.
| 89 |
The validity of the securities offered hereby will be passed upon for us by Schiff Hardin LLP, Washington, DC. Ellenoff Grossman & Schole LLP is acting as counsel to the underwriters in connection with this offering.
The financial statements of Moleculin Biotech, Inc. as of December 31, 2015 and for the period from July 28, 2015 (inception) to December 31, 2015 and the financial statements of Moleculin, LLC as of and for the years ended December 31, 2015 and 2014 included in this prospectus have been audited by GBH CPAs, PC, an independent registered public accounting firm, as stated in their reports appearing herein. Such financial statements have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act for the securities being offered by this prospectus. This prospectus, which is part of the registration statement, does not contain all of the information included in the registration statement and the exhibits. For further information about us and the securities offered by this prospectus, you should refer to the registration statement and its exhibits. References in this prospectus to any of our contracts or other documents are not necessarily complete, and you should refer to the exhibits attached to the registration statement for copies of the actual contract or document. You may read and copy any document that we file at the SEC’s public reference room located at 100 F Street, NE, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference rooms. SEC filings are also available to the public at the SEC’s website at www.sec.gov.
We are subject to the reporting and information requirements of the Exchange Act and, as a result, will file periodic and current reports, proxy statements and other information with the SEC. We expect to make our periodic reports and other information filed with or furnished to the SEC, available, free of charge, through our website as soon as reasonably practicable after those reports and other information are filed with or furnished to the SEC. Additionally, these periodic reports, proxy statements and other information will be available for inspection and copying at the public reference room and website of the SEC referred to above.
| 90 |
INDEX TO FINANCIAL STATEMENTS
F-1
Balance Sheets
| September 30, 2016 | December 31, 2015 | |||||||
| (Unaudited) | ||||||||
| Assets | ||||||||
| Current Assets: | ||||||||
| Cash and cash equivalents | $ | 6,183,783 | $ | 28,091 | ||||
| Prepaid expenses | 244,869 | – | ||||||
| Total current assets | 6,428,652 | 28,091 | ||||||
| Long-Term Assets: | ||||||||
| Furniture and equipment, net of accumulated depreciation | 16,346 | – | ||||||
| Intangible assets | 11,128,790 | – | ||||||
| Total Assets | $ | 17,573,788 | $ | 28,091 | ||||
| Liabilities and Stockholders' Equity (Deficit) | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable and accrued expenses | $ | 944,997 | $ | 322,790 | ||||
| Accounts payable and accrued expenses-related party | 37,500 | - | ||||||
| Convertible notes payable | 297,656 | 450,000 | ||||||
| Total current liabilities | 1,280,153 | 772,790 | ||||||
| Long-term payable-related party | 50,000 | – | ||||||
| Total Liabilities | 1,330,153 | 772,790 | ||||||
| Commitment and contingencies | ||||||||
| Stockholders' Equity (Deficit): | ||||||||
| Preferred stock, $0.001 par value; 5,000,000 authorized, no shares issued and outstanding | – | – | ||||||
| Common stock, $0.001 par value; 75,000,000 authorized, 12,054,813 and 6,661,000 shares issued and outstanding, respectively | 12,055 | 6,661 | ||||||
| Subscription receivable | (3,000 | ) | (3,000 | ) | ||||
| Additional paid-in capital | 19,485,987 | – | ||||||
| Accumulated deficit | (3,251,407 | ) | (748,360 | ) | ||||
| Total Stockholders' Equity (Deficit) | 16,243,635 | (744,699 | ) | |||||
| Total Liabilities and Stockholders' Equity (Deficit) | $ | 17,573,788 | $ | 28,091 | ||||
See accompanying notes to the unaudited financial statements.
F-2
Moleculin Biotech, Inc.
Statements of Operations
(Unaudited)
| Three Months Ended September 30, | From July 28, 2015 (Inception) Through September 30, | Nine Months Ended September 30, | From July 28, 2015 (Inception) Through September 30, | |||||||||||||
| 2016 | 2015 | 2016 | 2015 | |||||||||||||
| Revenue | $ | - | $ | - | $ | - | $ | - | ||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 496,659 | 38,409 | 616,498 | 38,409 | ||||||||||||
| General and administrative | 924,041 | 184,344 | 1,847,613 | 184,344 | ||||||||||||
| Depreciation | 977 | - | 1,629 | - | ||||||||||||
| Total operating expense | 1,421,677 | 222,753 | 2,465,740 | 222,753 | ||||||||||||
| Loss from operations | (1,421,677 | ) | (222,753 | ) | (2,465,740 | ) | (222,753 | ) | ||||||||
| Other expense: | ||||||||||||||||
| Interest expense | (10,402 | ) | (1,562 | ) | (37,307 | ) | (1,562 | ) | ||||||||
| Net loss | $ | (1,432,079 | ) | $ | (224,315 | ) | $ | (2,503,047 | ) | $ | (224,315 | ) | ||||
| Net loss per common share - basic and diluted | $ | (0.12 | ) | $ | (0.05 | ) | $ | (0.28 | ) | $ | (0.05 | ) | ||||
| Weighted average common shares outstanding - basic and diluted | 11,579,239 | 4,320,015 | 9,066,804 | 4,320,015 | ||||||||||||
See accompanying notes to the unaudited financial statements.
F-3
Moleculin Biotech, Inc.
Statements of Cash Flows
(Unaudited)
| Nine Months Ended September 30, | From July 28, 2015 (Inception) Through September 30, | |||||||
| 2016 | 2015 | |||||||
| Cash Flows from Operating Activities: | ||||||||
| Net loss | $ | (2,503,047 | ) | $ | (224,315 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 1,629 | - | ||||||
| Stock-based compensation | 208,939 | - | ||||||
| Shares issued for licenses used for research and development | - | 2,061 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses | (244,869 | ) | (23,000 | ) | ||||
| Accounts payable and accrued expenses | (146,799 | ) | 63,010 | |||||
| Accounts payable and accrued expenses – related parties | 87,500 | 19,584 | ||||||
| Net Cash Used in Operating Activities | (2,596,647 | ) | (162,660 | ) | ||||
| Cash Flows from Investing Activities: | ||||||||
| Cash paid for purchase of fixed assets | (10,155 | ) | - | |||||
| Cash paid for acquisition of Moleculin, LLC, net with cash acquired | (99,638 | ) | - | |||||
| Net Cash Used in Investing Activities | (109,793 | ) | - | |||||
| Cash Flows from Financing Activities: | ||||||||
| Proceeds from notes payable | 165,000 | 250,000 | ||||||
| Payments on notes payable | (469,939 | ) | - | |||||
| Proceeds from sale of common stock, net of direct offering costs | 9,167,071 | - | ||||||
| Net Cash Provided by Financing Activities | 8,862,132 | 250,000 | ||||||
| Net change in cash and cash equivalents | 6,155,692 | 87,340 | ||||||
| Cash and cash equivalents, at beginning of period | 28,091 | - | ||||||
| Cash and cash equivalents, at end of period | $ | 6,183,783 | $ | 87,340 | ||||
| Supplemental disclosures of cash flow information: | ||||||||
| Cash paid for interest | $ | 47,950 | $ | - | ||||
| Cash paid for income taxes | $ | - | $ | - | ||||
| Supplemental disclosure of non-cash investing and financing activities: | ||||||||
| Common stock issued to acquire Moleculin, LLC | $ | 9,773,586 | $ | - | ||||
| Common stock issued for conversion of debt | $ | 341,785 | $ | - | ||||
| Shares subscribed | $ | - | $ | 4,600 | ||||
See accompanying notes to the unaudited financial statements.
F-4
Notes to Financial Statements
(Unaudited)
Note 1 – Description of Business and Summary of Significant Accounting Policies
Nature of Business – The terms “MBI” or “the Company”, “we”, “our” and “us” are used herein to refer to Moleculin Biotech, Inc. MBI is a preclinical-stage pharmaceutical company organized as a Delaware corporation in July 2015 to focus on the development of anti-cancer drug candidates, some of which are based on license agreements with The University of Texas System on behalf of the M.D. Anderson Cancer Center, which we refer to as MD Anderson.
Our lead drug candidate is liposomal Annamycin, which we refer to as Annamycin, an anthracycline intended for the treatment of relapsed or refractory acute myeloid leukemia, or AML. In August 2015, the Company entered into a rights transfer agreement with AnnaMed, Inc. (“AnnaMed”), a company affiliated with certain members of the Company’s management and board of directors, pursuant to which, in exchange for 1,431,000 shares of the Company’s common stock, AnnaMed agreed to transfer any and all data it had regarding the development of Annamycin and the Annamycin IND, including all trade secrets, know-how, confidential information and other intellectual property rights held by AnnaMed. Annamycin has been in clinical trials pursuant to an investigational new drug application, or IND, that had been filed with the U.S. Food and Drug Administration, or FDA. This IND was terminated due to a lack of activity by a prior drug developer. The Company intends to apply for a new IND based on the same data that supported the original IND, updated for subsequent clinical data, and to commence clinical trials for Annamycin funded with the proceeds from our initial public offering which was completed on May 31, 2016.
The Annamycin drug substance is no longer covered by any existing patent protection. We intend to submit patent applications for formulation, synthetic process and reconstitution related to our Annamycin drug product candidate, although there is no assurance that we will be successful in obtaining such patent protection. Independently from potential patent protection, we believe Annamycin will qualify for Orphan Drug status, which could entitle us to market exclusivity of up to 7 and 10 years from the date of approval of a New Drug Application (“NDA”) and Marketing Authorization (“MA”), in the US and the European Union (“EU”), respectively. However, there can be no assurance that such status will be granted. Separately, the FDA may also grant market exclusivity of up to five years for newly approved new chemical entities (of which Annamycin would be one), but there can be no assurance that such exclusivity will be granted or, if granted, for how long.
We have two other drug development projects in progress, one involving a portfolio of small molecules, which we refer to as the WP1066 Portfolio, focused on the modulation of key oncogenic transcription factors involved in the progression of cancer, and the WP1122 Portfolio, a suite of molecules targeting the metabolic processes involved in cancer in general, and glioblastoma (the most common form of brain tumor) in particular. We have been granted royalty-bearing, worldwide, exclusive licenses for the patent and technology rights related to our WP1066 Portfolio and WP1122 Portfolio drug technologies, as these patent rights are owned by MD Anderson.
On August 11, 2015, the Company entered into a rights transfer agreement for WP1122 with IntertechBio Corporation (“IntertechBio”), a company affiliated with certain members of our management, whereby IntertechBio agreed to assign its license or sublicense its license to certain metabolic inhibitor technology owned by MD Anderson. In consideration, the Company issued 630,000 common shares to IntertechBio. IntertechBio agreed to make payments to MD Anderson including an up-front payment, license documentation fee, annual maintenance fee, milestone payments and minimum annual royalty payments for sales of products developed under the license agreement. The Company has assumed the rights and obligations of IntertechBio under the license agreement with MD Anderson. All out-of-pocket expenses incurred by MD Anderson in filing, prosecuting and maintaining the licensed patents have been and shall continue to be assumed by the Company.
The Company filed a registration statement on Form S-1 (which was declared effective on May 2, 2016) with respect to the Company’s initial public offering of shares of its common stock (“IPO”) to fund the development of its technologies. Prior to the declaration of effectiveness of the registration statement on Form S-1, we acquired Moleculin, LLC which was merged with and into MBI, which survived the merger. Moleculin, LLC was the holder of a license agreement with MD Anderson covering technology referred to as the WP1066 Portfolio, which is focused on the modulation of key oncogenic transcription factors.
Basis of Presentation - Unaudited Interim Financial Information – The accompanying unaudited interim financial statements and related notes have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information, and in accordance with the rules and regulations of the United States Securities and Exchange Commission (the “SEC”) with respect to Form 10-Q and Article 8 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by U.S. GAAP for complete financial statements. The unaudited interim financial statements furnished reflect all adjustments (consisting of normal recurring adjustments), which are, in the opinion of management, necessary for a fair statement of the results for the interim periods presented. Interim results are not necessarily indicative of the results for the full year. These interim unaudited financial statements should be read in conjunction with the audited financial statements of the Company as of December 31, 2015 and for the period from July 28, 2015 (inception) to December 31, 2015 and notes thereto contained in the Registration Statement on Form S-1 filed with the SEC on April 27, 2016.
F-5
Use of Estimates in Financial Statement Presentation - The preparation of these financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Acquisition – We acquired Moleculin, LLC (“Moleculin”) on May 2, 2016, and, going forward our financial statements include the operations of Moleculin, LLC. We account for acquired businesses using the acquisition method of accounting, which requires, among other things, that most assets acquired and liabilities assumed be recognized at their estimated fair values as of the acquisition date and that the fair value of acquired in-process research and development (“IPR&D”) be recorded on the balance sheet. Transaction costs are expensed as incurred. Any excess of the consideration transferred over the assigned values of the net assets acquired will be recorded as goodwill. The estimated fair values of assets acquired and liabilities assumed were determined based on management’s best estimates. Preliminary estimated fair values are subject to measurement period adjustments which represent updates made to the preliminary purchase price allocation based on revisions to valuation estimates in the interim period subsequent to the acquisition and initial accounting date up until the purchase price allocation is finalized which cannot be any later than one year from the acquisition date.
Going Concern - These financial statements have been prepared on a going concern basis, which assumes the Company will continue to realize its assets and discharge its liabilities in the normal course of business. The continuation of the Company as a going concern is dependent upon the continued financial support from its stockholders, the ability of the Company to obtain necessary equity financing to continue operations, and the attainment of profitable operations. As of September 30, 2016, the Company has incurred an accumulated deficit of $3,251,407 since inception, and had not yet generated any revenue from operations. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern. These financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern. The Company may seek additional funding through a combination of equity offerings, debt financings, government or other third-party funding, commercialization, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements.
Cash and Cash Equivalents - The Company considers all highly liquid accounts with original maturities of three months or less to be cash equivalents. At September 30, 2016, all of the Company’s cash was deposited in two banks and at December 31, 2015, all of the Company’s cash was deposited in one bank. Periodically, the Company may carry cash balances at financial institutions in excess of the federally insured limit of $250,000. The amount in excess of the FDIC insurance at September 30, 2016 was $5,693,442. On October 13, 2016, the Company consolidated all of its cash into one bank with $2 million and $3 million deposited into Certificate of Deposit Account Registry Service or CDARS accounts, which are all 100% federally insured, for 4-week and 13-week terms, respectively. The remaining cash is deposited into a checking and money market account.
Intangible assets - Intangible assets with finite lives are amortized using the straight-line method over their estimated period of benefit. If an intangible asset is identified as an in-process research & development asset then no amortization will occur until the development is complete. If the associated research and development effort is abandoned, the related assets will be written-off and the Company will record a noncash impairment loss on its statements of operations. For those compounds that reach commercialization, the IPR&D assets will be amortized over their estimated useful lives.
We evaluate the recoverability of intangible assets periodically and take into account events or circumstances that warrant revised estimates of useful lives or that indicate that impairment exists. No material impairments of intangible assets have been identified during any of the periods presented. Intangible assets are tested for impairment on an annual basis, and between annual tests if indicators of potential impairment exist, using a fair-value-based approach.
Beneficial Conversion Feature - From time to time, the Company may issue convertible notes that have conversion prices that create an embedded beneficial conversion feature on the issuance date. A beneficial conversion feature exists on the date a convertible note is issued when the fair value of the underlying common stock to which the note is convertible into is in excess of the remaining unallocated proceeds of the note after first considering the allocation of a portion of the note proceeds to the fair value of any attached equity instruments, if any related equity instruments were granted with the debt. Prior to the Company’s IPO, the Company estimates the fair value of its common stock using the most recent selling price available. The intrinsic value of the beneficial conversion feature is recorded as a debt discount with a corresponding amount to additional paid-in capital. The debt discount is amortized to interest expense over the life of the note using the effective interest method.
Income Taxes - The Company uses the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and the tax bases of reported assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company must then assess the likelihood that the resulting deferred tax assets will be realized. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
Stock-based Compensation - Stock-based compensation expense includes the estimated fair value of equity awards vested during the reporting period. The expense for equity awards vested during the reporting period is determined based upon the grant date fair value of the award and is recognized as expense over the applicable vesting period of the stock award using the straight-line method.
F-6
Earnings (Loss) Per Common Share - Basic net earnings (loss) per common share are computed by dividing net earnings (loss) available to common shareholders by the weighted-average number of common shares outstanding during the period. Diluted net earnings (loss) per common share is determined using the weighted-average number of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents. In periods when losses are reported, the weighted-average number of common shares outstanding excludes common stock equivalents, because their inclusion would have been anti-dilutive. As of September 30, 2016, the Company’s potentially dilutive shares included notes convertible to 1,928,899 common shares, options to purchase 510,000 common shares and warrants to purchase 107,802 common shares.
Research and Development Costs - Research and development costs are expensed as incurred.
Subsequent Events - The Company’s management reviewed all material events through the date these financial statements were issued for subsequent event disclosure consideration.
Recent Accounting Pronouncements
In May 2014, the FASB issued Accounting Standard Update ("ASU") 2014-09, Revenue from Contracts with Customers (Topic 606), which will replace numerous requirements in U.S. GAAP, including industry-specific requirements, and provide companies with a single revenue recognition model for recognizing revenue from contracts with customers. The core principle of the new standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. In August 2015, the FASB approved a proposal to defer the effective date of the guidance until annual and interim reporting periods beginning after December 15, 2017. The Company is currently evaluating the impact that this standard will have on its financial statements.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. Under the new guidance, management will be required to assess an entity’s ability to continue as a going concern, and to provide related footnote disclosures in certain circumstances. The provisions of this ASU are effective for annual periods beginning after December 15, 2016, and for annual and interim periods thereafter; early adoption is permitted. The Company is currently evaluating the impact that this standard will have on its financial statements.
In January 2016, the FASB issued ASU No. 2016-01, Financial Instruments – Overall: Recognition and Measurement of Financial Assets and Financial Liabilities (“ASU 2016-01”). ASU 2016-01 affects the accounting for equity investments, financial liabilities under the fair value option and the presentation and disclosure requirements of financial instruments. ASU 2016-01 is effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. The Company is currently evaluating the impact that this standard will have on its financial statements.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842) (“ASU 2016-02”). Under ASU 2016-02, an entity will be required to recognize right-of-use assets and lease liabilities on its balance sheet and disclose key information about leasing arrangements. ASU 2016-02 offers specific accounting guidance for a lessee, a lessor and sale and leaseback transactions. Lessees and lessors are required to disclose qualitative and quantitative information about leasing arrangements to enable a user of the financial statements to assess the amount, timing and uncertainty of cash flows arising from leases. For public companies, ASU 2016-02 is effective for annual reporting periods beginning after December 15, 2018, including interim periods within that reporting period, and requires a modified retrospective adoption, with early adoption permitted. The Company is currently evaluating the impact that this standard will have on its financial statements.
In March 2016, the FASB issued ASU 2016-09, Compensation-Stock Compensation (Topic 718). The new guidance changes the accounting and simplifies various aspects of the accounting for share-based payments to employees. The guidance allows for a policy election to account for forfeitures as they occur or based on an estimated number of awards that are expected to vest. ASU 2016-09 is effective for annual periods beginning after December 15, 2016, with early adoption permitted. The Company is currently evaluating the impact that this standard will have on its financial statements.
The Company does not believe that any other recently issued effective pronouncements, or pronouncements issued but not yet effective, if adopted, would have a material effect on the accompanying financial statements.
F-7
Note 2 – Intangible Assets
The Acquisition of Moleculin, LLC
On May 2, 2016, Moleculin, LLC, a Texas limited liability company, was merged with and into the Company. As a result of the merger, the Company issued to the holders of Moleculin equity interests an aggregate of 999,931 shares of the Company’s common stock valued at $5,999,586 based on the estimated acquisition-date fair value of our common stock of $6.00 per share, equal to the IPO price announced in our prospectus filed on that date. These shares contain certain trading restrictions. Prior to the Company’s acquisition of Moleculin, the Company had loaned $57,822 to Moleculin which was treated as part of the consideration paid to acquire Moleculin.
As additional consideration payable to the Moleculin unit holders, we agreed pursuant to the merger agreement that if drugs for dermatology indications are successfully developed by us using any of the Existing IP Assets, then the Moleculin, LLC unit holders, in the aggregate, will be entitled to receive a 2.5% royalty on the net revenues generated by such drugs. Any such net revenues would include a deduction for license fees or royalty obligations payable to MD Anderson for such Existing IP Assets. The merger agreement defined “Existing IP Assets” to mean all intellectual property, licensed by us and Moleculin as of the time of the merger, including, without limitation, the intellectual property licensed from MD Anderson under the Patent and Technology License Agreement entered into by and between IntertechBio Corporation and MD Anderson dated April 2, 2012, as amended, and the Patent and Technology License Agreement dated June 21, 2010, as amended, between MD Anderson and Moleculin, LLC, but excluding any intellectual property relating to Annamycin. The right to receive the contingent royalty payments described herein is limited to drugs developed only for dermatology indications, and does not include drugs developed for any other indications. We have no obligation of any nature to pursue the development of any drugs for dermatology indications.
Our acquisition of Moleculin, LLC, occurring prior to our IPO offering, provided us with the rights to the license agreement that Moleculin, LLC had with MD Anderson covering the WP1066 Portfolio. However, Moleculin, LLC had previously granted Houston Pharmaceuticals, Inc. (“HPI”), a related party, an option, which could be exercised at any time, to obtain an exclusive sub-license to develop the WP1066 Portfolio in all non-dermatological fields. Moleculin, LLC had previously pursued development of the WP1066 Portfolio for treatment of psoriasis, however, psoriasis related clinical trials had been terminated. Because WP1066 has shown significant activity against a wide range of tumors, Moleculin, LLC focus prior to the acquisition included the development of drugs for cancer treatment. However, the exclusive sub-license option held by HPI precluded Moleculin, LLC from pursuing drug development related to non-skin cancers, in addition to potentially creating significant intellectual property, clinical and commercialization risks associated with drug development for skin cancers. Re-acquisition of the HPI option was therefore essential for the values of both the WP1066 Portfolio and Moleculin, LLC.
In connection with the acquisition of Moleculin, LLC, we also negotiated on behalf of Moleculin, LLC two agreements with HPI. Under the first agreement, the HPI’s option to obtain the aforementioned exclusive sublicense was terminated in exchange for a payment of $100,000 and the issuance of 629,000 shares of our common stock. Under the second agreement (HPI Out-Licensing Agreement), HPI has received a non-exclusive technology rights and development sublicense under which it may continue its ongoing work to develop the WP1066 Portfolio related to treatment of non-skin cancer. Pursuant to this HPI Out-Licensing Agreement, we agreed to make payments to HPI totaling $750,000 over a three-year period commencing after the IPO offering in exchange for HPI allowing us to access any data, information or know-how resulting from the research and development conducted by HPI which will be expensed, as incurred, as research and development expense. Notwithstanding our obligation to make the foregoing payments, the HPI Out-Licensing Agreement does not obligate HPI to conduct any specific research or to meet any milestones. Pursuant to the HPI Out-Licensing Agreement, we have the right within three years of the effective date to buy-out from HPI all rights granted to HPI under the agreement for a payment of $1.0 million. Upon our exercise of the buy-out we will no longer be obligated to make any payments to HPI remaining from the $750,000 obligation discussed above. If we do not exercise the foregoing buy-out right within three years, the license granted to HPI shall convert into an exclusive license. As such, if we do not exercise the buy-out right for any reason, we will no longer have access to the non-skin cancer uses of the WP1066 Portfolio. As noted above, this will also potentially create risks for the development of skin cancer drugs. We do not intend to set aside and designate cash and cash equivalents in the amount of $1.0 million to make the buy-out payment. If we ultimately decide to exercise the buy-out right from HPI all rights granted the HPI under the agreement, we will need to raise additional funds to make the buy-out payment. We cannot assure that such additional funding will be available on satisfactory terms, or at all.
The agreements with HPI were executed on May 2, 2016, simultaneously with the closing of the Moleculin, LLC acquisition, and were non-cancelable but contingent on the Company’s ability to complete the IPO by June 30, 2016. They became effective on May 31, 2016.
F-8
The termination of the HPI option was completed on behalf of Moleculin, LLC, was required to enable the sale of Moleculin, LLC by materializing the value of its most significant asset, and was non-cancelable by either party. Further, the HPI option termination price was determined simultaneously with the acquisition on May 2, 2016 as our IPO price was established at that time. Accordingly, we concluded that this transaction was primarily for the benefit of Moleculin, LLC and its former owners, resulting in control of the underlying intellectual property and thereby increasing the value of Moleculin, LLC intangible assets immediately prior to the closing of its acquisition by us.
The HPI option termination price amounted to $3,874,000, consisting of 629,000 shares of our common stock valued at the IPO price of $6.00 per share, and $100,000 paid in cash in July 2016, and was included in acquisition-date liabilities assumed.
Purchase Price Allocation
The acquisition price was allocated on a preliminary basis, which is subject to change, to the assets acquired and liabilities assumed based upon their estimated fair values and the information available to management. The following table summarizes the estimated fair values of the assets acquired and liabilities assumed as of the acquisition date.
| Cash | $ | 362 | ||
| Property and equipment | 7,820 | |||
| Intangibles | 11,128,790 | |||
| Total assets acquired | $ | 11,136,972 | ||
| Liability assumed (HPI) | (3,874,000 | ) | ||
| Liabilities assumed | (1,205,564 | ) | ||
| Net assets acquired/total consideration transferred | $ | 6,057,408 |
The Company is in the process of obtaining input from third-parties regarding its tangible and intangible assets and other information necessary to measure the fair value of the assets acquired and liabilities assumed in connection with the acquisition of Moleculin, LLC; thus the provisional measurements of current assets, property and equipment, intangibles, and liabilities assumed are subject to change, which could be significant. We will finalize the amounts recognized as we obtain the information necessary to complete our analysis. As of this date, management believes all or most of the intangible assets are IPR&D related to the WP1066 Portfolio, and, as such, no amortization has been recorded to date. Any changes to the provisional measurements will be recognized in the period in which they are determined. We expect to finalize these amounts as soon as possible but no later than one year from the acquisition date.
Intangible assets consisted of the following at September 30, 2016 and December 31, 2015:
| September 30, 2016 | December 31, 2015 | |||||||
| Intangibles acquired from Moleculin, LLC | $ | 11,128,790 | $ | – | ||||
F-9
Unaudited Pro Forma Results of Operations
The following comparative table presents the unaudited condensed pro forma results of operations that reflect the acquisition of Moleculin as if the acquisition had occurred as of the first day of each period presented, adjusted for items that are directly attributable to the acquisition. This information has been compiled from historical financial statements and is not necessarily indicative of the results that actually would have been achieved had the transaction already occurred or that may be achieved in the future.
| For the Three Months Ended September 30, 2016 | Pro Forma For the Nine Months Ended September 30, 2016 | Pro Forma For the Three Months Ended September 30, 2015 | Pro Forma For the Nine Months Ended September 30, 2015 | |||||||||||||
| Total operating expenses | $ | (1,421,677 | ) | $ | (2,561,908 | ) | $ | (295,422 | ) | $ | (769,549 | ) | ||||
| Net loss | $ | (1,432,079 | ) | $ | (2,539,677 | ) | $ | (297,129 | ) | $ | (779,779 | ) | ||||
| Net loss per common share – basic and diluted | $ | (0.12 | ) | $ | (0.27 | ) | $ | (0.07 | ) | $ | (0.38 | ) | ||||
| Weighted average outstanding common shares – basic and diluted | 11,579,239 | 9,513,660 | 4,052,116 | 2,028,506 | ||||||||||||
The nine months ended September 30, 2016 are adjusted on a pro forma basis to exclude $145,078 in net interest expense related to the amortization of deferred financing costs and debt discount amortization for Moleculin, LLC’s convertible notes. The holders of the convertible notes were issued the Company’s common shares upon the Company’s acquisition of Moleculin, LLC.
The three months ended September 30, 2015 are adjusted on a pro forma basis to exclude $112,846 in net interest expense related to the interest expense, the amortization of deferred financing costs and debt discount amortization for Moleculin, LLC’s convertible notes.
The nine months ended September 30, 2015 are adjusted on a pro forma basis to exclude $322,547 in net interest expense related to the interest expense, the amortization of deferred financing costs and debt discount amortization for Moleculin, LLC’s convertible notes.
Note 3 – Convertible Notes Payable
On various dates from August 31, 2015 through January 19, 2016, each as amended on March 10, 2016, the Company entered into seven unsecured promissory notes with three separate third party investors. Each note bears interest at 8.0% per annum and was to mature on the earlier of June 30, 2016 or the completion of an IPO of the Company’s securities.
Since the completion of the IPO occurred prior to June 30, 2016, these notes were to be automatically converted according to their terms into shares of the Company’s common stock at applicable conversion price upon the Company’s IPO to the extent and provided that no holder of these notes was or will be permitted to convert such notes to the extent that the holder or any of its affiliates would beneficially own in excess of 4.99% of our common stock after such conversion. Due to this 4.99% limitation, a portion of these notes was not converted at the time of the IPO and the remaining unconverted principal and accrued interest amounts of the effected notes will remain outstanding and will be converted into shares of our common stock at such time as the 4.99% limitation continues to be met. Until such time as the notes are converted into shares of common stock, the maturity date of the notes will automatically be extended and we will not be required to repay the notes or the accrued interest relating to the notes in cash.
F-10
The IPO was completed on May 31, 2016. On May 31, 2016, pursuant to the conversion feature of the foregoing notes and with restriction of the 4.99% beneficially owned condition limitation, discussed above, the Company issued 1,166,503 common shares in total, reducing convertible debt principal by $183,356 and accrued interest by $17,699. During the three months ended September 30, 2016, an additional 800,057 common shares were issued due to the number of common shares outstanding allowing for conversion of additional shares under the 4.99% beneficially owned condition limitation. This reduced the convertible debt principal by $133,988 and accrued interest by $6,742. The remaining convertible debt without consideration of accrued interest as of September 30, 2016, if converted on September 30, 2016, would result in an additional 1,928,899 common shares to be issued.
The convertible notes were analyzed for a beneficial conversion feature on various issuance dates, at which time it was concluded that a beneficial conversion feature did not exist.
The table below represents the shares that are convertible at September 30, 2016 relating to the principal amounts of these convertible notes payable and excludes any shares that are convertible relating to the associated accrued interest:
| Issuance Date | September 30, 2016 | December 31, 2015 | Conversion Rate | Shares Convertible at September 30, 2016 | ||||||||||||
| August 31, 2015 (a) | $ | 38,299 | $ | 125,000 | $ | 0.1299 | 294,832 | |||||||||
| September 3, 2015 | 125,000 | 125,000 | 0.1299 | 962,279 | ||||||||||||
| October 4, 2015(a)(c) | 30,280 | 147,000 | 0.20 | 151,402 | ||||||||||||
| October 4, 2015(b) | – | 3,000 | 0.20 | – | ||||||||||||
| October 28, 2015(b) | – | 50,000 | 0.20 | – | ||||||||||||
| January 14, 2016(c) | 21,577 | – | 0.20 | 107,886 | ||||||||||||
| January 19, 2016 | 82,500 | – | 0.20 | 412,500 | ||||||||||||
| Total | $ | 297,656 | $ | 450,000 | 1,928,899 | |||||||||||
| (a) | Debt partially converted on May 31, 2016 and on August 19, 2016. | |
| (b) | Debt fully converted to common shares on May 31, 2016. | |
| (c) | Debt partially converted on September 1, 2016. |
The common shares relating to the above mentioned convertible notes payable contain the following trading restrictions: (a) beginning 90 days after the initial closing of our IPO and until the one-year anniversary of the initial closing of the IPO, the holder of the note will be able to sell 1% of the number of shares of common stock underlying the note on a monthly basis, subject to a maximum sale on any trading day of 4% of the daily volume; (b) if the common stock price is over $7.00 per share for five consecutive trading days then the holder of the note can sell up to 3% of the number of shares of common stock underlying the note on a monthly basis, subject to a maximum sale on any trading day of 4% of the daily volume; (c) if the common stock price is over $10.00 per share for five consecutive trading days then the holder of the note can sell up to an additional 5% of the number of shares of common stock underlying the note on a monthly basis, subject to a maximum sale on any trading day of 7% of the daily volume; and (d) if the common stock price is over $14.00 per share then the holder of the note is not restricted from making any sales until such time as the common stock price falls back below $14.00 per share; and (b) thereafter, until the two-year anniversary of the initial closing of IPO, the holder of the note can sell on any trading day 10% of the daily volume; provided that if the common stock price is over $10.00 per share then the holder of the note is not restricted from making any sales until such time as the common stock falls back below $10.00 per share. The foregoing lock-up restrictions relate to public sales and do not restrict the transfer of the shares privately, if permitted by applicable law, provided the acquirer of the shares agrees to comply with the above restrictions with respect to any public sales.
F-11
Note 4 – Equity
On May 2, 2016, the Company amended and restated its certificate of incorporation to increase the number of shares authorized to 80,000,000 of which 5,000,000 shares of preferred stock are authorized and 75,000,000 shares of common stock are authorized.
Preferred Stock
We are authorized to issue up to 5,000,000 shares of preferred stock. Our certificate of incorporation authorizes the board to issue these shares in one or more series, to determine the designations and the powers, preferences and relative, participating, optional or other special rights and the qualifications, limitations and restrictions thereof, including the dividend rights, conversion or exchange rights, voting rights (including the number of votes per share), redemption rights and terms, liquidation preferences, sinking fund provisions and the number of shares constituting the series. As of September 30, 2016, there was no designated preferred stock.
Common Stock
On May 31, 2016, the Company completed its IPO and sold 1,540,026 shares of the Company’s common stock. The IPO price per share was $6.00. The Company received net proceeds of $8,464,183 after deducting underwriting discounts, commissions and direct offering expenses payable by us. Pursuant to our agreement with our underwriters, as additional compensation, we issued the underwriters warrants to purchase 107,802 shares of common stock exercisable for a period of 5 years from date of issuance at an exercise price of $7.50 per share. The relative fair value of these warrants was $374,763 calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model include: (1) discount rate of 1.39% (2) expected life of 5 years, (3) expected volatility of 80.61%, and (4) zero expected dividends.
In August 2015, the Company agreed to issue 4,600,000 shares of common stock to its director, officers and founders for subscriptions of $4,600 cash to be received. As of September 30, 2016, the Company had not collected the proceeds for $3,000 of the subscriptions.
During the period from January 1, 2016 through May 2, 2016, the Company sold 234,296 common shares for $702,888. These shares are subject to the following lock-up agreement, from and after the later of six months after issuance or 90 days from the effective date of our IPO registration statement until the one-year anniversary thereof, (a) the holder of the shares can sell up to 10% of the purchased shares per month, subject to a maximum sale on any trading day of 8% of the daily volume of the common stock; (b) if the common stock price is over $7.00 per share for five consecutive trading days then the holder of the shares can sell up to 20% of the purchased shares per month, subject to a maximum sale on any trading day of 10% of the daily volume of the common stock; and (c) if the common stock price is over $12.00 per share then the holder of the shares is not restricted from making any sales until such time as the common stock price falls back below $12.00 per share.
On June 20, 2016, the Company agreed to issue 24,000 shares of common stock to PCG Advisory Group, the Company’s investor relations firm, for services provided. The fair value of these shares was $157,688 based on the market price on the grant date.
Adoption of 2015 Stock Plan
On December 5, 2015, the Board of Directors of the Company approved the Company’s 2015 Stock Plan, which was amended on April 22, 2016. The expiration date of the plan is December 5, 2025 and the total number of underlying shares of the Company’s common stock available for grant to employees, directors and consultants under the plan is 2,500,000 shares. The awards under the 2015 Stock Plan can be in the form of stock options, stock awards or stock unit awards. The following is a summary of option activities for the nine months ended September 30, 2016:
| Number of Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value | |||||||||||||
| Outstanding, December 31, 2015 | 200,000 | $ | 0.20 | |||||||||||||
| Granted | 460,000 | 5.83 | ||||||||||||||
| Cancelled | (150,000 | ) | 0.20 | |||||||||||||
| Outstanding, September 30, 2016 | 510,000 | $ | 5.28 | 9.29 | $ | 275,500 | ||||||||||
| Exercisable, September 30, 2016 | 50,000 | $ | 0.20 | 3.67 | $ | 275,500 | ||||||||||
During the nine months ended September 30, 2016, the Company granted an employee and its board of directors options, in the aggregate, to purchase 460,000 shares of the Company’s common stock with an exercise price ranging from $5.71 per share to $5.85 per share, a term of 10 years, and a vesting period of 3 to 4 years. The exercise price was based upon the closing price of the stock on the day of the grant. The options have an aggregated fair value of $1,725,052 that was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model include: (1) discount of 1.30% (2) expected lives of 6 to 6.25 years, (3) expected volatility of 70.18% to 70.44%, and (4) zero expected dividends. During the nine months ended September 30, 2016, the Company recorded $51,251 in stock-based compensation in relation to these options . The Company entered into a separation agreement with its former Chief Financial Officer in October 2016 and as part of the agreement, options to purchase 150,000 shares of common stock issued to the former Chief Financial Officer were cancelled and the vesting was accelerated on the remaining options to purchase 50,000 shares of common stock.
F-12
Note 5 – Income Taxes
As of September 30, 2016, the Company had an operating loss carry forward of approximately $3,343,000 which expires commencing in 2035. The value of these carryforwards depends on the Company’s ability to generate taxable income. A change in ownership, as defined by federal income tax regulations, could significantly limit the Company’s ability to utilize our net operating loss carryforwards. Additionally, because federal tax laws limit the time during which the net operating loss carryforwards may be applied against future taxes, if the Company fails to generate taxable income prior to the expiration dates the Company may not be able to fully utilize the net operating loss carryforwards to reduce future income taxes. The Company has cumulative losses and there is no assurance of future taxable income, therefore, valuation allowances have been recorded to fully offset the deferred tax asset at September 30, 2016.
Note 6 – Commitments and Contingencies
MD Anderson – IntertechBio Agreement
On August 11, 2015, the Company acquired the rights and obligations under the Patent and Technology License Agreement entered into between IntertechBio and MD Anderson dated April 2, 2012. Pursuant to the agreement, IntertechBio obtained a royalty-bearing, worldwide, exclusive license to intellectual property including patent rights related to the Company’s drug product candidate, WP1122. Under the agreement, IntertechBio agreed to pay annual maintenance fee in the amount of $10,000 on the first anniversary of the effective date of the agreement, $20,000 on the second anniversary of the effective date of the agreement, $40,000 on the third anniversary of the effective date of the agreement, $60,000 on the fourth anniversary of the effective date of the agreement, $80,000 on the fifth anniversary of the effective date of the agreement and $100,000 on the sixth anniversary of the effective date of the agreement, except that such payments will no longer be due upon the first sale of a licensed product. Under the agreement, IntertechBio also agreed to make a minimum annual royalty payment in the amount of $200,000 for the first anniversary following the first sale of a licensed product, $400,000 for the second anniversary following the first sale of a licensed product, and $600,000 for the third year following the first sale of a licensed product. IntertechBio also agreed to make certain milestone payments. Pursuant to an amendment on October 19, 2015, the Company will pay milestone payments as follows:
| Phase | Amount | |||
| Commencement of Phase II Study for a licensed product | $ | 200,000 | ||
| Commencement of Phase III Study for a licensed product | $ | 250,000 | ||
| Filing of a New Drug Application for a licensed product | $ | 400,000 | ||
| Receipt of market approval for a licensed product | $ | 500,000 | ||
Per the October 2015 amendment to the agreement, MD Anderson has the right to terminate the license agreement if (i) a preclinical toxicology program for a licensed product is not initiated within one year of the effective date of the amendment (which has occurred), (ii) an investigational new drug application is not filed with the Food and Drug Administration for a Phase I study for a licensed product within three years of the effective date of the amendment, or (iii) a Phase I study for a licensed product is not commenced within five years of the effective date of the amendment. The agreement will expire upon the expiration of the licensed intellectual property. The rights obtained by the Company pursuant to the agreement are made subject to the rights of the U.S. government to the extent that the technology covered by the licensed intellectual property was developed under a funding agreement between MD Anderson and the U.S. government. All out-of-pocket expenses incurred by MD Anderson in filing, prosecuting and maintaining the licensed patents have been and shall continue to be assumed by the Company.
On October 8, 2015, IntertechBio Corporation entered into a letter agreement with MD Anderson wherein MD Anderson agreed to receive past due maintenance fees and patent expenses of $98,108 owed by IntertechBio Corporation in four installments. The past due amount is related to certain metabolic inhibitor technology license that was assigned to the Company by IntertechBio Corporation and was owed by IntertechBio Corporation prior to the Company’s acquisition of the license. Pursuant to the letter, IntertechBio Corporation also agreed to pay $65,504 in patent fees to a law firm. In order to have the license in good standing, the Company agreed to pay MD Anderson the $98,108 and the $65,504 in patent fees to a patent law firm on behalf of IntertechBio Corporation. As of December 31, 2015, $45,000 of the past due amount to MD Anderson and $42,504 in patent fees to a patent law firm were still outstanding and were included in accounts payable and accrued liabilities. On April 15, 2016, the Company entered into a letter agreement with MD Anderson where MD Anderson agreed to receive the remaining outstanding amount on or before the earlier of a) May 31, 2016 or b) four days after the Company’s completion of the IPO. These amounts were paid prior to or on May 31, 2016.
MD Anderson – Patent & Technology License Agreement
Upon the Company’s acquisition of Moleculin, LLC on May 2, 2016, we obtained a royalty-bearing, worldwide, exclusive license to intellectual property rights, including patent rights related to our WP1066 drug product candidate from MD Anderson through a Patent and Technology License Agreement Moleculin, LLC entered with MD Anderson on June 21, 2010 (the “Moleculin License Agreement”). Under the Moleculin License Agreement, Moleculin, LLC obtained the right to manufacture, have manufactured, use, import, offer to sell or sell products worldwide for any indication under the licensed intellectual property with the right to sublicense. In consideration, Moleculin, LLC agreed to make payments to MD Anderson including an up-front payment, milestone payments and minimum annual royalty payments for sales of products developed under the license agreement. Specifically, under the Moleculin License Agreement, Moleculin, LLC agreed to pay a nonrefundable upfront documentation fee and an annual maintenance fee in the amount of $20,000 on June 21, 2011, which has and shall increase in $10,000 increments on an annual basis thereafter up to a maximum of $100,000, except that such payments will no longer be due upon marketing approval in any country of a licensed product. Under the Moleculin License Agreement, Moleculin, LLC also agreed to make a minimum annual royalty payment to MD Anderson in the amount of $200,000 after the first sale of a licensed product.
F-13
Upon completion of our acquisition of Moleculin, LLC, we assumed the rights and obligations of Moleculin, LLC. However, the rights we have obtained pursuant to the assignment of the Moleculin License Agreement are made subject to the rights of the U.S. government to the extent that the technology covered by the licensed intellectual property was developed under a funding agreement between MD Anderson and the U.S. government. All out-of-pocket expenses incurred by MD Anderson in filing, prosecuting and maintaining the licensed patents have been and shall continue to be assumed by us.
On October 8, 2015, Moleculin, LLC entered into a letter agreement with MD Anderson for Moleculin, LLC’s past due fees to MD Anderson in the amount of $691,186 of which $300,000 had been paid prior to the letter agreement. Pursuant to the letter agreement, MD Anderson agreed to receive the remaining past due fee in three installments: a) $125,000 on October 31, 2015; b) $175,000 on January 31, 2016; and c) $91,186 on April 30, 2016. Moleculin, LLC paid $125,000 to MD Anderson on November 2, 2015.
On October 19, 2015, the agreement was amended for the milestone payments. The amended milestone payments are as follows: (i) commencement of Phase III Study for first licensed drug/product within the United States, Europe, China or Japan - $150,000; (ii) submission of the first NDA within the United States - $500,000; and (iii) receipt of first marketing approval for sale of a license product in the United States $600,000.
On January 28, 2016, the Company and Moleculin, LLC entered into a letter agreement with MD Anderson where MD Anderson agreed to receive the remaining outstanding amount on or before the earlier of April 30, 2016 or four days after our IPO. This date was amended and per the amended agreement, the Company paid the outstanding Moleculin, LLC fees on May 31, 2016 in the amount of $306,186.
Bonwick Capital Partners LLC
On January 22, 2016, as amended on February 15, 2016, the Company entered into a letter agreement with Bonwick Capital Partners LLC. (“Bonwick”) to engage Bonwick as an exclusive financial advisor of the Company. Pursuant to the agreement, the Company agreed to: a) pay success fees equal to 7% of the gross proceeds from any form of financing; and b) issue five-year warrants to purchase 7% of the Company’s equity securities sold with a cashless exercise provision, exercisable at 125% of the price per share of the Company’s common stock paid by investors in the transaction. In addition, the Company agreed to reimburse Bonwick for all of its out-of-pocket expenses incurred in connection with the offering, not to exceed $25,000, and fees and expenses of their counsel not to exceed $100,000. Upon completion of the Company’s IPO, the Company paid Bonwick a $50,000 advisory fee.
Bonwick is also entitled to a success fee as set forth above if the Company completes a financing with parties introduced by Bonwick prior to the termination agreement or during the 6-month period following the termination of the agreement, which occurred on August 2, 2016. In connection with the Company’s IPO, Bonwick received a success fee of $646,872, warrants to purchase 107,802 shares of common stock at an exercise price of $7.50 per share, and $6,266 for reimbursement of expenses.
Houston Pharmaceuticals, Inc.
Our acquisition of Moleculin, LLC, occurring prior to our IPO offering, provided us with the rights of the license agreement that Moleculin, LLC had with MD Anderson covering the WP1066 Portfolio. As discussed in Note 2, we are obligated to make payments to HPI totaling $750,000 over a three-year period commencing after the IPO offering in exchange for HPI allowing us to access any data, information or know-how resulting from the research and development conducted by HPI. Pursuant to the HPI Out-Licensing Agreement, we have the right within three years of the date we enter into the agreement to buy-out from HPI all rights granted to HPI under the agreement for a payment of $1.0 million. Upon our exercise of the buy-out we will no longer be obligated to make any payments to HPI remaining from the $750,000 obligation discussed above. We will need to raise additional funds to make the buy-out payment. We cannot assure that such additional funding will be available on satisfactory terms, or at all.
Employment Agreement
On October 13, 2016, the Company and its Chief Executive Officer entered into an employment agreement, pursuant to which, during the period commencing June 1, 2016 and ending June 1, 2017, $12,500 per month of the compensation is deferred. The deferred compensation shall be payable in a lump sum on the earlier of the termination of the employment agreement or June 1, 2019. As of September 30, 2016, deferred compensation of $50,000 was included in long-term payable-related party.
Note 7 – Subsequent Events
In December 2016, the Company issued 110,038 shares of its common stock for conversion of convertible notes previously issued and agreed to issue 79,167 shares of its common stock to a third-party in settlement of $237,500 in past due amounts.
F-14
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Moleculin Biotech, Inc.
Houston, Texas
We have audited the accompanying balance sheet of Moleculin Biotech, Inc. as of December 31, 2015 and the related statements of operations, changes in stockholders’ deficit, and cash flows for the period from July 28, 2015 (Inception) to December 31, 2015. Moleculin Biotech, Inc.’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Moleculin Biotech, Inc. as of December 31, 2015 and the results of its operations and cash flows for the period from July 28, 2015 (Inception) to December 31, 2015 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that Moleculin Biotech, Inc. will continue as a going concern. As discussed in Note 1 to the financial statements, Moleculin Biotech, Inc. incurred an accumulated loss and has not yet generated any revenue from operations that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ GBH CPAs, PC | |
| GBH CPAs, PC | |
| www.gbhcpas.com | |
| Houston, Texas | |
| March 21, 2016 |
F-15
Balance Sheet
| December 31, 2015 | ||||
| ASSETS | ||||
| Current Assets: | ||||
| Cash and cash equivalents | $ | 28,091 | ||
| Total Assets | $ | 28,091 | ||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||
| Current Liabilities: | ||||
| Accounts payable and accrued liabilities | $ | 322,790 | ||
| Notes payable | 450,000 | |||
| Total Liabilities | 772,790 | |||
| Commitments and contingencies | ||||
| Stockholders’ Deficit: | ||||
| Common stock, $0.001 par value; 20,000,000 shares authorized, 6,661,000 shares issued and outstanding | 6,661 | |||
| Subscription receivable | (3,000 | ) | ||
| Accumulated deficit | (748,360 | ) | ||
| Total Stockholders’ Deficit | (744,699 | ) | ||
| Total Liabilities and Stockholders’ Deficit | $ | 28,091 | ||
See accompanying notes to financial statements.
F-16
Statement of Operations
| From July 28, 2015 (Inception) to December 31, 2015 | ||||
| Revenue | $ | - | ||
| Operating expenses: | ||||
| Research and development | 260,418 | |||
| General and administrative | 477,810 | |||
| Total operating expenses | 738,228 | |||
| Loss from operations | (738,228 | ) | ||
| Other expense: | ||||
| Interest expense | (10,132 | ) | ||
| Total other expense | (10,132 | ) | ||
| Net loss | $ | (748,360 | ) | |
| Loss per common share - basic and diluted | $ | (0.13 | ) | |
| Weighted average number of common shares outstanding - basic and diluted | 5,691,803 | |||
See accompanying notes to financial statements.
F-17
Statement of Changes in Stockholders’ Deficit
| Common Stock | Subscription | Accumulated | ||||||||||||||||||
| Shares | Amount | Receivable | Deficit | Total | ||||||||||||||||
| Balance at July 28, 2015 (Inception) | - | $ | - | $ | - | $ | - | $ | - | |||||||||||
| Issuance of shares for cash | 4,600,000 | 4,600 | (3,000 | ) | - | 1,600 | ||||||||||||||
| Shares issued for licenses used for research and development | 2,061,000 | 2,061 | - | - | 2,061 | |||||||||||||||
| Net loss | - | - | - | (748,360 | ) | (748,360 | ) | |||||||||||||
| Balance at December 31, 2015 | 6,661,000 | $ | 6,661 | $ | (3,000 | ) | $ | (748,360 | ) | $ | (744,699 | ) | ||||||||
See accompanying notes to financial statements.
F-18
Statement of Cash Flows
| For the period from July 28, 2015 (Inception) to December 31, 2015 | ||||
| Cash Flows From Operating Activities: | ||||
| Net loss | $ | (748,360 | ) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||
| Shares issued for licenses used for research and development | 2,061 | |||
| Changes in operating assets and liabilities: | ||||
| Accounts payable and accrued liabilities | 322,790 | |||
| Net Cash Used In Operating Activities | (423,509 | ) | ||
| Cash Flows From Financing Activities: | ||||
| Proceeds from stock issuance | 1,600 | |||
| Proceeds from notes payable | 450,000 | |||
| Net Cash Provided By Financing Activities | 451,600 | |||
| Net change in cash and cash equivalents | 28,091 | |||
| Cash and cash equivalent at beginning of period | - | |||
| Cash and cash equivalent at end of period | $ | 28,091 | ||
| Supplemental cash flows disclosures: | ||||
| Cash paid for interest | $ | - | ||
| Cash paid for income taxes | $ | - | ||
| Non-cash investing and financing activities: | ||||
| Shares subscribed | $ | 3,000 | ||
See accompanying notes to financial statements.
F-19
Notes to Financial Statements
Note 1 - Description of Business and Summary of Significant Accounting Policies
Nature of Business – Moleculin Biotech, Inc. (“MBI”, the “Company”) is a preclinical-stage pharmaceutical company organized as a Delaware corporation in July 2015 to focus on the development of anti-cancer drug candidates. The Company has two in-licensed drug development technologies.The lead drug candidate is Lipsosomal Annamycin, which is referred to as Annamycin, an anthracyline that targets the treatment of relapsed or refractory acute myeloid leukemia, or AML. In August 2015, the Company entered into a rights transfer agreement with AnnaMed, Inc. (“AnnaMed”), a company affiliated with certain members of our management and board of directors, pursuant to which, in exchange for 1,431,000 shares of our common stock, AnnaMed agreed to transfer any and all data it had regarding the development of Annamycin and the Annamycin IND, including all trade secrets, know-how, confidential information and other intellectual property rights held by AnnaMed. Annamycin has been in clinical trials pursuant to an investigational new drug application, or IND, that had been filed with the U.S. Food and Drug Administration, or FDA. This IND was terminated due to a lack of activity by a prior drug developer who was developing the drug for a different indication. We intend to apply for a new IND based on the same data that supported the original IND, updated for subsequent clinical data, and to commence a Phase II clinical trial for Annamycin.
The Company also has a second drug development technology, WP1122, a molecule targeting the treatment of glioblastoma through metabolic inhibition, which was developed at The University of Texas M.D. Anderson Cancer Center (“MD Anderson”).
On August 11, 2015, the Company entered into a rights transfer agreement with IntertechBio Corporation (“IntertechBio”), a company affiliated with certain members of our management and board of directors, whereby IntertechBio agreed to assign its license or sublicense its license to certain metabolic inhibitor technology owned by MD Anderson. In consideration, the Company issued 630,000 common shares to Intertech Bio. IntertechBio agreed to make payments to MD Anderson including an up-front payment, license documentation fee, annual maintenance fee, milestone payments and minimum annual royalty payments for sales of products developed under the license agreement. The Company has assumed the rights and obligations of IntertechBio under the license agreement with MD Anderson. All out-of-pocket expenses incurred by MD Anderson in filing, prosecuting and maintaining the licensed patents have been and shall continue to be assumed by the Company.
The Company intends to file a registration statement on Form S-1 with respect to the Company’s initial public offering of shares of its common stock (“IPO”) to fund the development of its technologies and also intends to acquire Moleculin, LLC, a Texas limited liability company. Moleculin, LLC is the holder of a license agreement with MD Anderson covering technology referred to as WP1066 Portfolio, which is focused on the modulation of key oncogenic transcription factors. Immediately prior to the declaration of effectiveness of the registration statement on Form S-1, Moleculin, LLC will be merged with and into MBI, which will survive the merger.
Use of Estimates in Financial Statement Presentation - The preparation of these financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Going Concern - These financial statements have been prepared on a going concern basis, which assumes the Company will continue to realize its assets and discharge its liabilities in the normal course of business. The continuation of the Company as a going concern is dependent upon the continued financial support from its stockholders, the ability of the Company to obtain necessary equity financing to continue operations, and the attainment of profitable operations. As of December 31, 2015, the Company has incurred an accumulated loss of $748,360 since inception, had a working capital deficit of $744,699, and had not yet generated any revenue from operations. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern. These financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern. The Company intends to fund its future operations through equity offerings.
Cash and Cash Equivalents - The Company considers all highly liquid accounts with original maturities of three months or less to be cash equivalents. At December 31, 2015, all of the Company’s cash was deposited in one bank.
Beneficial Conversion Feature - From time to time, the Company may issue convertible notes that have conversion prices that create an embedded beneficial conversion feature on the issuance date. A beneficial conversion feature exists on the date a convertible note is issued when the fair value of the underlying common stock to which the note is convertible into is in excess of the remaining unallocated proceeds of the note after first considering the allocation of a portion of the note proceeds to the fair value of any attached equity instruments, if any related equity instruments were granted with the debt. The Company estimates the fair value of its common stock using the most recent selling price available. The intrinsic value of the beneficial conversion feature is recorded as a debt discount with a corresponding amount to additional paid-in capital. The debt discount is amortized to interest expense over the life of the note using the effective interest method.
Income Taxes - The Company uses the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and the tax bases of reported assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company must then assess the likelihood that the resulting deferred tax assets will be realized. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
F-20
Stock-based Compensation - Stock-based compensation expense includes the estimated fair value of equity awards vested during the reporting period. The expense for equity awards vested during the reporting period is determined based upon the grant date fair value of the award and is recognized as expense over the applicable vesting period of the stock award using the straight-line method.
Earnings Per Share - Basic net earnings (loss) per common share are computed by dividing net earnings (loss) available to common shareholders by the weighted-average number of common shares outstanding during the period. Diluted net earnings (loss) per common share is determined using the weighted-average number of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents. In periods when losses are reported, the weighted-average number of common shares outstanding excludes common stock equivalents, because their inclusion would be anti-dilutive.
Research and Development Costs - Research and development costs are expensed as incurred. Research and development reimbursements and grants are recorded by the Company as a reduction of research and development costs.
Subsequent Events - The Company’s management reviewed all material events through the date these financial statements were issued for subsequent event disclosure consideration.
Recent Accounting Pronouncements - The Company does not believe that any recently issued effective pronouncements, or pronouncements issued but not yet effective, if adopted, would have a material effect on the accompanying financial statements.
Note 2 - Notes Payable
On August 31 and September 3, 2015, as amended on March 10, 2016, the Company entered into two unsecured convertible promissory notes with a third party, in the amount totaling $250,000. These notes bear interest at 8.0% per annum and mature on the earlier of June 30, 2016 or the completion of an IPO of the Company’s securities. However, if the completion of the IPO occurs prior to June 30, 2016, these notes shall be automatically converted according to its terms into shares of the Company’s common stock at a conversion price of $0.1299 per share upon the Company’s IPO.
On October 6, 2015, as amended on March 10, 2016, the Company entered into an unsecured promissory note with a third party in the amount of $150,000. The note bears interest at 8.0% per annum and matures on the earlier of June 30, 2016 or the completion of an IPO of the Company’s securities. However, if the completion of the IPO occurs prior to June 30, 2016, the note shall be automatically converted according to its terms into shares of the Company common stock at a conversion price equal to $0.20 per share upon the Company’s IPO.
On October 28, 2015, as amended on March 10, 2016, the Company entered into an unsecured promissory note with a third party in the amount of $50,000. The note bears interest at 8.0% per annum and matures on the earlier of June 30, 2016 or the completion of an IPO of the Company’s securities. However, if the completion of the IPO occurs prior to June 30, 2016, the note shall be automatically converted according to its terms into shares of the Company common stock at a conversion price equal to $0.20 per share upon the Company’s IPO.
Pursuant to the note agreements dated October 6, 2015 and October 28, 2015, the Company agreed, during the term of the notes, not to have greater than 8,171,000 shares of its common stock outstanding, excluding any shares underlying convertible notes that will convert into 3,750,000 shares of the Company’s common stock, and excluding options to purchase up to 300,000 shares of the Company’s common stock at an exercise price of no less than $0.75 per share that may be issued. The Company is currently in compliance with this covenant.
The convertible notes were analyzed for a beneficial conversion feature at which time it was concluded that a beneficial conversion feature did not exist.
Note 3 - Equity
Common Stock
In August 2015, the Company entered into an agreement to issue 4,600,000 shares of common stock to its director and officers for subscriptions of $4,600 cash to be received. As of December 31, 2015, the Company had not collected the proceeds for $3,000 of the subscriptions.
On August 11, 2015, the Company was granted an assignment of a license agreement between MD Anderson and IntertechBio in exchange for 630,000 shares of the Company’s common stock. The sublicense gives the Company rights to access certain metabolic inhibitor technology owned by MD Anderson that had been licensed to IntertechBio. The shares were valued at a total of $630 and the related expense included in research and development costs.
On August 21, 2015, the Company acquired the right to the intellectual property of AnnaMed in exchange for 1,431,000 shares of the Company’s common stock. The license gives the Company full ownership rights to the data package supporting the FDA IND Number 46869, allowing the Company to resubmit a request for IND to the FDA to begin development work on Annamycin. The shares were valued at a total of $1,431 and the related expense included in research and development costs.
Adoption of 2015 Stock Plan
On December 5, 2015, the Board of Directors of the Company approved the Company’s 2015 Stock Plan. The expiration date of the plan is December 5, 2025 and the total number of underlying shares of the Company’s common stock available for grant to employees, directors and consultants under the plan is 1,500,000 shares.
Stock Options
On December 10, 2015, the Board of Directors of the Company granted it Chief Financial Officer, Louis Ploth, options to purchase 200,000 shares of the Company’s common stock with exercise price of $0.20 per share and a term of 10 years. These options vest in four years. The grant date fair value of $27,390 was calculated using the Black-Scholes model. Variables used in the Black-Scholes model include: (1) discount rate of 2.24% (2) expected life of 6.25 years, (3) expected volatility of 74.2%, and (4) zero expected dividends. As of December 31, 2015, no option is exercisable.
F-21
Note 4 - Income Taxes
The Company is subject to the United States federal income taxes at an approximate rate of 35%. The reconciliation of the provision for income taxes at the United States federal statutory rate compared to the Company’s income tax expense as reported is as follows:
| From July 28, 2015 (Inception) to December 31, 2015 | ||||
| Income tax benefit computed at the statutory rate | $ | 261,926 | ||
| Change in valuation allowance | (261,926 | ) | ||
| Provision for income taxes | $ | - | ||
Significant components of the Company’s deferred tax assets after applying enacted corporate income tax rates are as follows:
| As of December 31, 2015 | ||||
| Deferred income tax assets | ||||
| Net operating losses | $ | 261,926 | ||
| Less: valuation allowance | (261,926 | ) | ||
| Net deferred income tax assets | $ | - | ||
The Company has an operating loss carry forward of approximately $748,000, which expires commencing in 2035.
Note 5 – Commitments and contingencies
Burnham Securities Inc.
On August 5, 2015, the Company entered into a letter agreement with Burnham Securities Inc. (“Burnham”) to engage Burnham as an exclusive financial advisor of the Company for 12 months. Pursuant to the agreement, the Company agreed to: a) pay success fees equal to 7% of the gross proceeds from any form of financing; b) issue warrants to purchase 7% of the Company’s equity securities sold with a cashless exercise provision, exercisable at a price per share of the equity securities paid by investors in the transaction. In addition, the Company agreed to reimburse Burnham for all of its reasonable out-of-pocket expenses. Upon execution of this agreement, the Company also paid Burnham a $50,000 advisory fee and agreed to pay an additional advisory fee of $50,000 upon completion of the Company’s IPO. For the period from inception to December 31, 2015, the Company incurred $31,500 success fees related to the notes issued to in August, September and October 2015. See Note 3. This agreement was terminated by mutual consent on January 22, 2016.
MD Anderson – IntertechBio Agreement
On August 11, 2015, the Company acquired the rights and obligations under the Patent and Technology License Agreement entered into between IntertechBio and MD Anderson dated April 2, 2012. Pursuant to the agreement, IntertechBio obtained a royalty-bearing, worldwide, exclusive license to intellectual property including patent rights related to the Company’s drug product candidate, WP1122. Under the agreement, IntertechBio agreed to pay annual maintenance fee in the amount of $10,000 on the first anniversary of the effective date of the agreement, $20,000 on the second anniversary of the effective date of the agreement, $40,000 on the third anniversary of the effective date of the agreement, $60,000 on the fourth anniversary of the effective date of the agreement (this payment was not made and the parties expect to enter into an amendment to defer this payment until the earlier of May 31, 2016 or four days after the IPO), $80,000 on the fifth anniversary of the effective date of the agreement and $100,000 on the sixth anniversary of the effective date of the agreement, except that such payments will no longer be due upon the first sale of a licensed product. Under the agreement, IntertechBio also agreed to make a minimum annual royalty in the amount of $200,000 for the first anniversary following the first sale of a licensed product, $400,000 for the second anniversary following the first sale of a licensed product, and $600,000 for the third year following the first sale of a licensed product. IntertechBio also agreed to make certain milestone payments. On October 19, 2015, pursuant to an amendment, the Company will pay milestone payments as follows:
| Phase | Amount | |||
| Commencement of Phase II Study for a licensed product | $ | 200,000 | ||
| Commencement of Phase III Study for a licensed product | $ | 250,000 | ||
| Filing of a New Drug Application for a licensed product | $ | 400,000 | ||
| Receipt of market approval for a licensed product | $ | 500,000 | ||
F-22
MD Anderson has the right to terminate the agreement upon advanced notice in the event of a default by IntertechBio. The agreement will also be terminated immediately upon IntertechBio’s insolvency. Additionally, per the October 2015 amendment to the agreement, MD Anderson has the right to terminate the license agreement if (i) a preclinical toxicology program for a licensed product is not initiated within one year of the effective date of the amendment, (ii) an investigational new drug application is not filed with the Food and Drug Administration for a Phase I study for a licensed product within three years of the effective date of the amendment, or (iii) a Phase I study for a licensed product is not commenced within five years of the effective date of the amendment. The agreement will expire upon the expiration of the licensed intellectual property. The rights obtained by the Company pursuant to the agreement are made subject to the rights of the U.S. government to the extent that the technology covered by the licensed intellectual property was developed under a funding agreement between MD Anderson and the U.S. government. All out-of-pocket expenses incurred by MD Anderson in filing, prosecuting and maintaining the licensed patents have been and shall continue to be assumed by the Company.
On October 8, 2015, IntertechBio Corporation entered into a letter agreement with MD Anderson where MD Anderson agreed to receive past due maintenance fees and patent expenses of $98,108 owed by IntertechBio Corporation in four installments. The past due amount is related to certain metabolic inhibitor technology license that was assigned to the Company by IntertechBio Corporation and was owed by IntertechBio Corporation prior to the Company’s acquisition of the license. Pursuant to the letter, IntertechBio Corporation also agreed to pay $65,504 in patent fees to a law firm. In order to have the license in good standing, the Company agreed to pay MD Anderson the $98,108 and the $65,504 in patent fees to a patent law firm on behalf of IntertechBio Corporation. As of December 31, 2015, $45,000 of the past due amount to MD Anderson and $42,504 in patent fees to a patent law firm were still outstanding and were included in accounts payable and accrued liabilities. On January 28, 2016, the Company entered into a letter agreement with MD Anderson where MD Anderson agreed to receive the remaining outstanding amount on or before the earlier of a) April 30, 2016 or b) four days after the Company’s IPO.
MD Anderson – Option Agreement
On October 15, 2015, the Company entered into an exclusive option agreement with MD Anderson granting the Company a period of time to evaluate the potential of acquiring and to negotiate the purchase of an exclusive license in the field of cancer therapeutics. The option period extends from October 15, 2015 through May 11, 2016. MD Anderson shall not offer the license to any other third party during the option period. In consideration of the option granted, the Company paid $5,000 to MD Anderson.
Note 6 - Subsequent Events
On January 14, 2016, as amended March 10, 2016, the Company entered into an unsecured convertible promissory note with a third party in the amount of $82,500. The note bears interest at 8.0% per annum and matures on the earlier of June 30, 2016 or the completion of an IPO of the Company’s securities. However, if the completion of the IPO occurs prior to June 30, 2016, the note shall be converted according to its terms into shares of the Company common stock at a conversion price equal to $0.20 per share upon the Company’s IPO.
On January 19, 2016, as amended March 10, 2016, the Company entered into an unsecured convertible promissory note with a third party in the amount of $82,500. The note bears interest at 8.0% per annum and matures on the earlier of June 30, 2016 or the completion of an IPO of the Company’s securities. However, if the completion of the IPO occurs prior to June 30, 2016, the note shall be converted according to its terms into shares of the Company common stock at a conversion price equal to $0.20 per share upon the Company’s IPO.
On January 22, 2016, as amended on February 15, 2016, the Company entered into a letter agreement with Bonwick Capital Partners LLC. (“Bonwick”) to engage Bonwick as an exclusive financial advisor of the Company. Pursuant to the agreement, the Company agreed to: a) pay success fees equal to 7% of the gross proceeds from any form of financing; b) issue warrants to purchase 7% of the Company’s equity securities sold with a cashless exercise provision, exercisable at 125% of the price per share of the Company’s common stock paid by investors in the transaction. The warrants should have a term of 5 years. In addition, the Company agreed to reimburse Burnham for all of its out-of-pocket expenses incurred in connection with this offering, which shall not exceed $25,000, and fees and expenses of their counsel not to exceed $100,000. Upon completion of the Company’s IPO, the Company shall pay Bonwick a $50,000 advisory fee. The agreement will expire on the earlier of August 6, 2016 or the mutual written agreement of the Company and Bonwick. Bonwick shall be entitled to a success fee as set forth above if the Company complete a financing with parties introduced by Bonwick prior to the termination agreement during the 6 months period following the termination of the agreement.
On January 8, 2016, Moleculin, LLC issued a revolving line of credit promissory note to the Company where the Company agreed to loan up to $50,000 to Moleculin LLC. The note bears annual interest rate at 8% and has a late charge of 5% for amount overdue. Interest is payable monthly to the Company commencing on July 8, 2016 and all outstanding balance is payable on January 8, 2017. The Company has funded $25,000 to Moleculin, LLC subsequent to January 8, 2016.
Subsequent to December 31, 2015 through March 21, 2016, the Company sold 128,833 common shares for $386,499.
Note 7 - Additional Subsequent Events (Unaudited)
During April 2016, the Company sold a total of 97,130 common shares for $291,390 to several investors.
F-23
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Moleculin, LLC
Houston, Texas
We have audited the accompanying statements of financial position of Moleculin, LLC as of December 31, 2015 and 2014 and the related statements of operations, changes in members’ deficit, and cash flows for the years then ended. Moleculin, LLC’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Moleculin, LLC as of December 31, 2015 and 2014 and the results of its operations and cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that Moleculin, LLC will continue as a going concern. As discussed in Note 1 to the financial statements, Moleculin, LLC has suffered recurring losses from operations and has insufficient working capital that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ GBH CPAs, PC
GBH CPAs, PC
www.gbhcpas.com
Houston, Texas
March 21, 2016
F-24
Statements of Financial Position
As of December 31, 2015 and 2014
| December 31, | December 31, | |||||||
| 2015 | 2014 | |||||||
| ASSETS | ||||||||
| Current Assets: | ||||||||
| Cash and cash equivalents | $ | 31,867 | $ | 524,477 | ||||
| Prepaid expenses and other current assets | 2,354 | 2,076 | ||||||
| Total Current Assets | 34,221 | 526,553 | ||||||
| Other receivables | - | 232,300 | ||||||
| Property and equipment, net of accumulated depreciation | 11,259 | 22,595 | ||||||
| Total Assets | $ | 45,480 | $ | 781,448 | ||||
| LIABILITIES AND MEMBERS’ DEFICIT | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable and accrued liabilities | $ | 838,072 | $ | 1,047,455 | ||||
| Accounts payable and accrued liabilities - related parties | 66,871 | 25,744 | ||||||
| Common units payable | - | 548,630 | ||||||
| Note payable | 55,774 | - | ||||||
| Current portion of long-term note payable | 414,165 | - | ||||||
| Convertible notes payable, net of $79,076 discount and $35,787 deferred financing costs | 1,020,064 | - | ||||||
| Convertible notes payable- related parties, net of $30,215 discount | 483,864 | - | ||||||
| Total Current Liabilities | 2,878,810 | 1,621,829 | ||||||
| Long-term note payable | - | 414,165 | ||||||
| Convertible notes payable, net of $237,664 discount and $107,558 deferred financing costs | - | 789,705 | ||||||
| Convertible notes payable - related parties, net of $90,811 discount | - | 423,268 | ||||||
| Total Liabilities | 2,878,810 | 3,248,967 | ||||||
| Commitments and contingencies | ||||||||
| Members’ Deficit: | ||||||||
| Preferred units, 16,321,558 units authorized, 13,515,820 units issued and outstanding | 10,620,186 | 10,620,186 | ||||||
| Common units, 4,727,074 authorized, 4,178,444 units issued and outstanding | 750,000 | 750,000 | ||||||
| Accumulated deficit | (14,203,516 | ) | (13,837,705 | ) | ||||
| Total Members’ Deficit | (2,833,330 | ) | (2,467,519 | ) | ||||
| Total Liabilities and Members’ Deficit | $ | 45,480 | $ | 781,448 | ||||
See accompanying notes to the financial statements.
F-25
Statements of Operations
For the Years Ended December 31, 2015 and 2014
| 2015 | 2014 | |||||||
| Revenue | $ | - | $ | - | ||||
| Operating expenses: | ||||||||
| Research and development | 125,442 | 798,785 | ||||||
| General and administrative | 328,570 | 839,556 | ||||||
| Depreciation | 11,336 | 11,005 | ||||||
| Total operating expenses | 465,348 | 1,649,346 | ||||||
| Loss from operations | (465,348 | ) | (1,649,346 | ) | ||||
| Other income (expense): | ||||||||
| Gain on extinguishment of liability | 548,630 | - | ||||||
| Other income | 550 | 800 | ||||||
| Interest expense | (449,643 | ) | (160,540 | ) | ||||
| Total other income (expense) | 99,537 | (159,740 | ) | |||||
| Net loss | $ | (365,811 | ) | $ | (1,809,086 | ) | ||
See accompanying notes to the financial statements.
F-26
Statements of Changes in Members’ Deficit
For the Years Ended December 31, 2015 and 2014
| Preferred Units | Common Units | Accumulated | ||||||||||||||||||||||
| Units | Amount | Units | Amount | Deficit | Total | |||||||||||||||||||
| Balance at December 31, 2013 | 13,515,820 | $ | 10,207,934 | 4,178,444 | $ | 750,000 | $ | (12,028,619 | ) | $ | (1,070,685 | ) | ||||||||||||
| Beneficial conversion feature of convertible notes | - | 412,252 | - | - | - | 412,252 | ||||||||||||||||||
| Net loss | - | - | - | - | (1,809,086 | ) | (1,809,086 | ) | ||||||||||||||||
| Balance at December 31, 2014 | 13,515,820 | $ | 10,620,186 | 4,178,444 | $ | 750,000 | $ | (13,837,705 | ) | $ | (2,467,519 | ) | ||||||||||||
| Net loss | - | - | - | - | (365,811 | ) | (365,811 | ) | ||||||||||||||||
| Balance at December 31, 2015 | 13,515,820 | $ | 10,620,186 | 4,178,444 | $ | 750,000 | $ | (14,203,516 | ) | $ | (2,833,330 | ) | ||||||||||||
See accompanying notes to the financial statements.
F-27
Statements of Cash Flows
For the Years Ended December 31, 2015 and 2014
| 2015 | 2014 | |||||||
| Cash Flows From Operating Activities: | ||||||||
| Net loss | $ | (365,811 | ) | $ | (1,809,086 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation expense | 11,336 | 11,005 | ||||||
| Amortization of debt discount | 219,184 | 83,777 | ||||||
| Amortization of deferred financing costs | 71,771 | 16,192 | ||||||
| Gain on extinguishment of liabilities | (548,630 | ) | - | |||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | (278 | ) | 6,386 | |||||
| Accounts payable and accrued liabilities | (153,609 | ) | 317,673 | |||||
| Accounts payable and accrued liabilities - related parties | 41,127 | 19,908 | ||||||
| Net Cash Used In Operating Activities | (724,910 | ) | (1,354,145 | ) | ||||
| Cash Flows From Investing Activities: | ||||||||
| Collection from other receivables | 232,300 | - | ||||||
| Net Cash Provided By Investing Activities | 232,300 | - | ||||||
| Cash Flows From Financing Activities: | ||||||||
| Proceeds from issuances of convertible notes payable | - | 1,011,177 | ||||||
| Proceeds from issuances of convertible notes payable - related parties | - | 514,079 | ||||||
| Net Cash Provided By Financing Activities | - | 1,525,256 | ||||||
| Net change in cash and cash equivalents | (492,610 | ) | 171,111 | |||||
| Cash and cash equivalents at beginning of year | 524,477 | 353,366 | ||||||
| Cash and cash equivalents at end of year | $ | 31,867 | $ | 524,477 | ||||
| Supplemental cash flow disclosures: | ||||||||
| Cash paid for interest | $ | - | $ | - | ||||
| Cash paid for income taxes | $ | - | $ | - | ||||
| Non-cash investing and financing activities: | ||||||||
| Long-term note payable issued to settle accounts payable | $ | - | $ | 414,165 | ||||
| Note payable issued to settle accounts payable | $ | 55,774 | $ | - | ||||
| Beneficial conversion feature of convertible notes | $ | - | $ | 412,252 | ||||
See accompanying notes to the financial statements.
F-28
Notes to Financial Statements
Note 1 - Description of Business and Summary of Significant Accounting Policies
Nature of Business - Moleculin, LLC, a Texas limited liability company, (the “Company” or “Moleculin”) is an early-stage Houston-based pharmaceutical company formed in December 2006 to create novel therapies for skin disorders and the development of cancer related technology. Although the Company previously focused on the development of treatments for skin disorders, the Company’s current focus is on the development of drugs for cancer treatment.
Moleculin intends to be acquired by Moleculin Biotech, Inc. (“MBI”) by merging into MBI. The intended transaction is scheduled to occur immediately prior to the declaration of effectiveness of MBI’s registration statement on Form S-1 with respect to MBI’s initial public offering of its common stock.
Use of Estimates in Financial Statement Presentation - The preparation of these financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of these financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Going Concern - These financial statements have been prepared on a going concern basis, which assumes the Company will continue to realize its assets and discharge its liabilities in the normal course of business. The continuation of the Company as a going concern is dependent upon the continued financial support from its members, the ability of the Company to obtain necessary equity financing to continue operations, and the attainment of profitable operations. The Company has suffered recurring losses from operating and has insufficient working capital. As of December 31, 2015, the Company has incurred accumulated deficit of $14,203,516 since inception, had a working capital deficit of $2,844,589, and has not yet generated any significant revenue from operations. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern. These financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern. The Company intends to be acquired by MBI and to fund the operations through MBI’s equity offerings.
Cash and Cash Equivalents - The Company considers all highly liquid accounts with original maturities of three months or less to be cash equivalents. Balances held by the Company are typically in excess of Federal Deposit Insurance Corporation’s insured limits ($250,000 per depositor per bank). At December 31, 2015 and 2014, all of the Company’s cash was deposited in one bank.
Property and Equipment - Property and equipment are recorded at cost and depreciated on a straight-line basis over estimated useful lives. Leasehold improvements are recorded at cost and depreciated on a straight-line basis over the shorter of the estimated useful life or the lease term. When assets are retired or sold, the cost and related accumulated depreciation are removed from the accounts, and any related gain or loss is reflected in income (loss) for the period. Expenditures for major additions which extend the useful lives of assets are capitalized. Minor replacements, maintenance and repairs, which do not improve or extend the life of such assets are charged to expense as incurred.
Impairment of Long-Lived Assets - Management reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount may not be realizable or at a minimum annually during the fourth quarter of the year. If an evaluation is required, the estimated future undiscounted cash flows associated with the asset are compared to the asset’s carrying value to determine if an impairment of such asset is necessary. The effect of any impairment would be to expense the difference between the fair value of such asset and its carrying value. For the years ended December 31, 2015 and 2014, there were no impairments recorded.
Fair Value of Financial Instruments - Fair value is defined as the price that would be received to sell an asset, or paid to transfer a liability, in an orderly transaction between market participants. A fair value hierarchy has been established for valuation inputs that give the highest priority to quoted prices in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. The fair value hierarchy is as follows:
Level 1 Inputs - Unadjusted quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date.
Level 2 Inputs - Inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly. These might include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability (such as interest rates, volatilities, prepayment speeds, credit risks, etc.) or inputs that are derived principally from or corroborated by market data by correlation or other means.
Level 3 Inputs - Unobservable inputs for determining the fair values of assets or liabilities that reflect an entity’s own assumptions about the assumptions that market participants would use in pricing the assets or liabilities.
F-29
Income Taxes - The Company elected to be taxed as a partnership under the Internal Revenue Code. The Company pays no U.S. taxes on its earnings. The Company’s net earnings/losses are passed through to the Company’s members and, as such, reports no income tax expense or liability.
Revenue Recognition - The Company recognizes revenues when persuasive evidence of an arrangement exists, delivery has occurred or services have been provided, the purchase price is fixed or determinable and collectability is reasonably assured. The Company may receive cost reimbursement-based grants. For these grants, revenues are based on costs incurred that are specifically covered under reimbursement arrangements. These revenues are recognized as grant-related expenses are incurred by the Company or its subcontractors.
Research and Development Costs - Research and development costs are expensed as incurred.
Subsequent Events - The Company’s management reviewed all material events through the date these financial statements were issued for subsequent event disclosure consideration.
Recent Accounting Pronouncements - In June 2014, the FASB issued ASU 2014-10, Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements. ASU 2014-10 eliminates the distinction of a development stage entity and certain related disclosure requirements, including the elimination of inception-to-date information on the statements of operations, cash flows and stockholders’ equity. The amendments in ASU 2014-10 will be effective prospectively for annual reporting periods beginning after December 15, 2014, and interim periods within those annual periods, however early adoption is permitted. The Company evaluated and adopted ASU 2014-10 as of December 31, 2014.
In April 2015, the FASB issued ASU 2015-03, Interest – Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs. The amended guidance requires that debt issuance costs be presented in the balance sheet as a direct reduction from the carrying amount of the recognized debt liability, consistent with the treatment of debt discounts. Amortization of debt issuance costs is to be reported as interest expense. The updated guidance is effective for reporting periods beginning after December 15, 2015, and interim periods within those annual periods, however early adoption is permitted. The Company evaluated and adopted ASU 2015-13 as of December 31, 2014.
The Company does not believe that any other recently issued effective pronouncements, or pronouncements issued but not yet effective, if adopted, would have a material effect on the accompanying financial statements.
Note 2 – Other Receivables
On October 27, 2010, the Company entered into a Patent and Technology Development and License Agreement with Dermin, Sp. Zo. O. (“Dermin”), a Polish limited liability company, to assist in the development and commercialization of certain pharmaceutical products within a described licensed territory in exchange for royalty payments. On December 19, 2011, the Company advanced $200,000 to Dermin providing funding assistance to Dermin’s efforts in developing and commercializing these pharmaceutical products in and around the Poland European region. Further, throughout the year 2012, the Company paid $32,300 of expenses on behalf of Dermin. As of December 31, 2014, the Company had $232,300 long-term note receivable from Dermin which was collected in August 2015.
Note 3 - Property and Equipment
Property and equipment consisted of the following at December 31, 2015 and 2014:
| Lives | December 31, 2015 | December 31, 2014 | ||||||||||
| Leasehold improvements | 1 year | $ | 81,602 | $ | 81,602 | |||||||
| Machinery and equipment | 5 years | 57,327 | 57,327 | |||||||||
| Furniture and fixtures | 7 years | 7,955 | 7,955 | |||||||||
| Less: accumulated depreciation | (135,625 | ) | (124,289 | ) | ||||||||
| Property and equipment, net | $ | 11,259 | $ | 22,595 | ||||||||
Depreciation expense for the years ended December 31, 2015 and 2014 were $11,336 and $11,005, respectively.
F-30
Note 4 – Notes Payable
On September 8, 2015, the Company issued an unsecured promissory note to Hilltop Research in the amount of $55,774 to settle an outstanding payable. The note matures on June 30, 2016 and accrues interest at 6.0% per annum. In the event that the Company or a significant portion of the Company’s assets are acquired by MBI prior to the maturity date, the loan becomes due and payable upon closing of MBI’s initial public offering.
On September 16, 2014, the Company issued a promissory note in the amount of $414,165 to Davos Chemical Corporation to settle an outstanding accounts payable balance. The note bears interest at an annual rate of 6.0% and matures on the earlier of December 31, 2016 or the completion of one of the following prior to December 31, 2016:
| a) | The Company (i) is awarded a grant from the Cancer Prevention and Research Institute of Texas (“CPRIT”) of at least $5,000,000, and (ii) is deemed by CPRIT to have received the 50% matching funds required by the CPRIT grant; or | |
| b) | The Company concludes a privately placed sale of the Company’s Series A Preferred Units of at least $3,000,000. |
At December 31, 2015 and 2014, accrued interest related these notes were $34,275 and $7,217, respectively.
Note 5 – Convertible Notes Payable
From May to November 2014, the Company issued various convertible notes to its creditors. Gross proceeds from third and related parties were $1,134,927 and $514,079, respectively. These notes bear interest at 8% per annum and are due on the earlier of June 30, 2016 or the consummation of a liquidation event, unless these notes are converted, pursuant to the following:
| a) | Automatic conversion upon CPRIT qualified financing- When the Company is awarded a grant from the CPRIT of at least $3,500,000 and is deemed by CPRIT to have received the 50% matching funds required by the CPRIT grant. The conversion price is $0.80 per unit in the event of a CPRIT qualified financing. | |
| b) | First tier automatic conversion - When the Company concludes a privately placed sale of the Company’s Series A Preferred Units of at least $3,000,000 and is at a pre-money valuation of not less than $20,000,000. The conversion price is 80% of the lowest per unit purchase price in the privately placed sale in the event of a first tier automatic conversion. | |
| c) | Second tier automatic conversion - When the Company concludes a privately placed sale of the Company’s Series A Preferred Units of at least $2,000,000. The conversion price is 70% of the lowest per unit purchase price in the privately placed sale in the event of a second tier automatic conversion. | |
| d) | Voluntary conversion - At any time after the earlier of June 30, 2016 or a liquidation event, the note holders can elect to convert the notes into the Company’s Preferred Units at $0.80 per unit. |
A liquidation event means: a) the acquisition of the Company by another entity; b) a sale, exclusive license, lease or other conveyance of all or substantially all of the Company’s assets; c) any liquidation, dissolution of the Company; d) the acquisition of an aggregate of 50% or more of the Company’s voting power by a party; e) in a bankruptcy proceeding. Upon a liquidation event, the note holders is entitle to 150% of the then outstanding principal and unpaid interest.
These convertible notes were analyzed for a beneficial conversion feature at which time it was concluded that a beneficial conversion feature existed. The beneficial conversion feature was measured using the commitment-date stock price and was determined to be $283,732 for third party notes and $128,520 for related party notes. This amount was recorded as a debt discount and is amortized to interest expense over the term of these notes. For the years ended December 31, 2015, amortization of debt discount for third and related parties were $158,588 and $60,596, respectively. For the years ended December 31, 2014, amortization of debt discount for third and related parties were $46,068 and $37,709, respectively.
On September 30, 2014, the Company entered into an agreement with Brompton Group NA, LLC (“Brompton”) for a non-exclusive arrangement to identify investors or lenders interested in providing financing for the Company. In exchange for their services, the Company agreed to pay Brompton 15% of the proceeds received from any financing provided by the investors introduced by Brompton. Brompton agreed to receive two-thirds of the fee, at their option, in either cash or in the form of the same securities offered in the financing being offered to the identified investors or lenders. The remaining fee should be paid in the form of the same securities offered in the financing. Of the $1,254,874 debt raised from third party investors during the fourth quarter of 2014, $825,000 was raised through investors introduced by Brompton. As such, $123,750 of finder’s fee was recorded as debt discount and amortized over the life of the notes. For the years ended December 31, 2015 and 2014, interest expense associated with the amortization of the finder’s fee was $71,771 and $16,192, respectively.
As of December 31, 2015, accrued interest for third party and related party notes were $118,400 and $66,871, respectively. As of December 31, 2014, accrued interest for third party and related party notes were $27,605 and $25,744, respectively.
F-31
Note 6 - Commitments and Contingencies
MD Anderson Agreements
On June 21, 2010, the Company entered into a patent and technology license agreement with the University of Texas M.D. Anderson Cancer Center (“MD Anderson”) under which the Company is obligated to make upfront and periodic progress payments. The agreement allows the Company to obtain from MD Anderson an exclusive, worldwide royalty-bearing license to research, develop, manufacture, have manufactured, use, import, offer to sell or sell products comprising or made through the use of certain patent and technology rights owned by MD Anderson. In consideration, the Company agreed to pay for all out-of-pocket expenses incurred by MD Anderson in filing, prosecuting and maintaining patent rights. Pursuant to the agreement, the Company also agreed to pay: a) an annual maintenance fee starting at $20,000 per year and increase by $10,000 each year up to a maximum of $100,000; b) royalties equal to 2.5% of net sales for licensed products approved for dermatological use; and c) royalties equal to 4.0% of net sales for licensed products approved for all other uses. The 2.5% and 4.0% royalties could be further reduced if certain criteria are met. Annual minimum royalties are $200,000 following the first sale after marketing approval is obtained from MD Anderson by the Company for any licensed products. In addition, the Company will be required to issue 3.5% of the total issued and outstanding common units of the Company on a fully diluted basis to MD Anderson at the time the Company commences its first phase II trial of a drug covered by the agreement. The Company is also required to pay certain milestone payments.
On October 8, 2015, the Company entered into a letter agreement with MD Anderson for the Company’s past due fees to MD Anderson in the amount of $691,186 of which $300,000 has been paid prior to the letter agreement. Pursuant to the letter agreement, MD Anderson agreed to receive the remaining past due fee in three installments: a) $125,000 due on October 31, 2015; b) $175,000 due on January 31, 2016; and c) $91,186 due on April 30, 2016. The Company paid $125,000 to MD Anderson on November 2, 2015.
On October 19, 2015, the agreement was amended for the milestone payments. The amended milestone payments are as follows:
| Phase | Amount | |||
| Commencement of Phase III Study for first licensed drug/product within the United States, Europe, China or Japan | $ | 150,000 | ||
| Submission of the first NDA within the United States | $ | 500,000 | ||
| Receipt of first marketing approval for sale of a license product in the United States | $ | 600,000 | ||
The amendment also removed the term regarding the issuance of 3.5% of the Company outstanding units.
On January 28, 2016, the Company entered into a letter agreement with MD Anderson where MD Anderson agreed to receive the remaining outstanding amount on or before the earlier of a) April 30, 2016 or b) four days after the Company’s IPO.
On October 15, 2015, the Company entered into a $5,000 exclusive option agreement with MD Anderson allowing the Company a period of time to evaluate the potential of acquiring and to negotiate the purchase of an exclusive license in the field of cancer therapeutics. The option period extends from October 15, 2015 through August 31, 2016. MD Anderson shall not offer a license to any third party during the option period. The $5,000 option fee was paid by MBI.
Dermin Agreement
On October 27, 2010, the Company entered into a Patent and Technology Development and License Agreement with Dermin. The agreement provided Dermin with sub-license rights to the Company’s technologies for use in limited territories in exchange for Dermin’s use of Polish government grant funding to pay for development costs the Company would otherwise have been required to fund. Dermin’s territories are primarily Poland and surrounding countries, but not including any of the major European markets (UK, Germany, France, Spain and Italy). Pursuant to the agreement, Dermin has to pay the Company a running royalty on sales of license products. As of December 31, 2015, no sales have occurred and no royalty paid to the Company by Dermin.
Lease Agreements
On July 31, 2007, the Company entered into a rental agreement for office space at 2575 West Belfort, Houston, Texas. The agreement was renewed on July 26, 2010 for one year. After July 2011, the lease continues on a month-to-month basis. Therefore, there are no future minimum obligations on the lease. Rent expense for the year ended December 31, 2015 and 2014 were $29,765 and $26,600, respectively, and are included in general and administrative expenses on the statements of operations.
F-32
Note 7 - Equity
In March 2011, the Company amended its articles of incorporation to create two classes of interests referred to as ‘Series A Preferred Membership Interests’ and ‘Common Membership Interests’, with each interest issued being measured as Units (Series A Preferred Units and Common Units, respectively). The Series A Preferred Units are further broken down into 4 series including Series A-1, Series A-2, Series A-3 and Series A-4.
Pursuant to Moleculin, LLC's Company Agreement, the Board shall distribute net cash, as defined in the Company Agreement, to the members at such times and in such amounts as the Board may determine in the following order and priority:
| a) | First, to the Series A Preferred members in proportion to each such members’ original capital contribution until such members receive net cash equal to 150% of their original capital contribution. | |
| b) | Second, to the members in proportion to their percentage interests until Series A Preferred members have received net cash equal to 300% of their original capital contribution. | |
| c) | Third, to the common members in proportion to each common member’s common units until the common members have receive aggregate distributions of net cash equal to the then total amount of distributions to all members for all company fiscal years multiplied by the common members’ aggregate percentage interests; and | |
| d) | Fourth, to the members in accordance with their percentage interests. |
As of December 31, 2015 and 2014, the Preferred Units are summarized as follows:
| Units Outstanding | ||||||||||||
| Series | December 31, 2015 | December 31, 2014 | Contribution | |||||||||
| Series A-1 | 283,604 | 283,604 | $ | 150,000 | ||||||||
| Series A-2 | 1,595,552 | 1,595,552 | 600,000 | |||||||||
| Series A-3 | 1,942,400 | 1,942,400 | 513,672 | |||||||||
| Series A-4 | 9,694,264 | 9,694,264 | 9,356,514 | |||||||||
| Total | 13,515,820 | 13,515,820 | $ | 10,620,186 | ||||||||
During the first quarter of 2013, the Company commenced the first Phase II Study of a licensed product in the patent and technology license agreement between the Company and MD Anderson. Pursuant to the agreement, the Company was obligated to issue MD Anderson 548,630 common units, representing 3.5% of its issued and outstanding equity units. These common units were valued at their estimated fair value of $548,630, which was recorded as a research and development expense. In October 2015, MD Anderson agreed to forfeit its right to these units. The Company recorded a gain on liability extinguishment of $548,630 upon the forfeiture.
Note 8 - Subsequent Event
On January 2, 2016, the Company purchased 189,070 common units from one of the Company's unit holders for $100.
On January 8, 2016, the Company issued a revolving line of credit promissory note to MBI where MBI agreed to loan up to $50,000 to the Company. The note bears annual interest rate at 8% and has a late charge of 5% for amount overdue. Interest is payable monthly commencing on July 8, 2016 and all outstanding balance is payable on January 8, 2017. $25,000 under this note has been received subsequent to January 8, 2016.
F-33
______________ Units
Each Consisting of One Share of Common Stock and
One Warrant to Purchase ______________ Shares of Common Stock
Moleculin Biotech, Inc.
| Roth Capital Partners | National Securities Corporation |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the estimated costs and expenses to be incurred in connection with the issuance and distribution of the securities of Moleculin Biotech, Inc. (the “Registrant”) which are registered under this Registration Statement on Form S-1 (this “Registration Statement”), other than underwriting discounts and commissions. All amounts are estimates except the Securities and Exchange Commission registration fee and the Financial Industry Regulatory Authority, Inc. filing fee.
The following expenses will be borne solely by the Registrant:
| Amount to be | ||||
| Paid | ||||
| SEC Registration fee | $ | 1,738.50 | ||
| Financial Industry Regulatory Authority, Inc. filing fee | $ | * | ||
| Legal fees and expenses | $ | * | ||
| Accounting fees and expenses | $ | * | ||
| Transfer Agent’s fees | $ | * | ||
| Miscellaneous fees and expenses | $ | * | ||
| Total | * | |||
* To be provided by amendment.
Item 14. Indemnification of Directors and Officers.
Pursuant to Section 145 of the Delaware General Corporation Law (the “DGCL”), a corporation shall have the power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than a derivative action by or in the right of such corporation) by reason of the fact that such person is or was a director, officer, employee or agent of such corporation, or serving at the request of such corporation in such capacity for another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, if such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of such corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
The DGCL also permits indemnification by a corporation under similar circumstances for expenses (including attorneys’ fees) actually and reasonably incurred by such persons in connection with the defense or settlement of a derivative action or suit, except that no indemnification shall be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to such corporation unless the Delaware Court of Chancery or the court in which such action or suit was brought shall determine upon application that such person is fairly and reasonably entitled to indemnity for such expenses which such court shall deem proper.
To the extent a present or former director or officer is successful in the defense of such an action, suit or proceeding referenced above, or in defense of any claim, issue or matter therein, a corporation is required by the DGCL to indemnify such person for actual and reasonable expenses incurred in connection therewith. Expenses (including attorneys’ fees) incurred by such persons in defending any action, suit or proceeding may be paid in advance of the final disposition of such action, suit or proceeding upon in the case of a current officer or director, receipt of an undertaking by or on behalf of such person to repay such amount if it is ultimately determined that such person is not entitled to be so indemnified.
| II-1 |
The DGCL provides that the indemnification described above shall not be deemed exclusive of other indemnification that may be granted by a corporation pursuant to its bylaws, disinterested directors’ vote, stockholders’ vote and agreement or otherwise.
Section 102(b)(7) of the DGCL enables a corporation, in its certificate of incorporation or an amendment thereto, to eliminate or limit the personal liability of a director to the corporation or its stockholders for monetary damages for violations of the directors’ fiduciary duty, except (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL (providing for liability of directors for unlawful payment of dividends or unlawful stock purchases or redemptions) or (iv) for any transaction from which a director derived an improper personal benefit. The Registrant’s certificate of incorporation provides for such limitations on liability for its directors.
The DGCL also provides corporations with the power to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of such corporation, or is or was serving at the request of such corporation in a similar capacity for another corporation, partnership, joint venture, trust or other enterprise, against any liability asserted against him or her in any such capacity or arising out of his or her status as such, whether or not the corporation would have the power to indemnify him or her against such liability as described above. In connection with this offering, the Registrant will obtain liability insurance for its directors and officers. Such insurance would be available to its directors and officers in accordance with its terms.
The Registrant’s certificate of incorporation in requires the Registrant to indemnify and hold harmless, to the fullest extent permitted by applicable law as it presently exists or may hereafter be amended, any person (a “covered person”) who was or is made or is threatened to be made a party or is otherwise involved in any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (a “proceeding”) by reason of the fact that he or she is or was a director, officer or member of a committee of the Registrant, or, while a director or officer of the Registrant, is or was serving at the request of the Registrant as a director or officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise or non-profit entity, including service with respect to employee benefit plans, against all liability and loss suffered and expenses (including attorneys’ fees), judgment, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with a proceeding.
In addition, under the Registrant’s certificate of incorporation, in certain circumstances, the Registrant shall pay the expenses (including attorneys’ fees) incurred by a covered person in defending a proceeding in advance of the final disposition of such proceeding; provided, however, that the Registrant shall not be required to advance any expenses to a person against whom the Registrant directly brings an action, suit or proceeding alleging that such person (1) committed an act or omission not in good faith or (2) committed an act of intentional misconduct or a knowing violation of law. Additionally, an advancement of expenses incurred by a covered person shall be made only upon delivery to the Registrant of an undertaking, by or on behalf of such covered person, to repay all amounts so advanced if it shall ultimately be determined by final judicial decision from which there is no further right to appeal or otherwise in accordance with Delaware law that such covered person is not entitled to be indemnified for such expenses.
The foregoing statements are subject to the detailed provisions of Section 145 of the DGCL and the full text of the Registrant’s certificate of incorporation, which is filed as Exhibit 3.1 hereto. Reference is made to the form of underwriting agreement to be filed as Exhibit 1.1 hereto for provisions providing that the underwriters are obligated under certain circumstances, to indemnify our directors, officers and controlling persons against certain liabilities under the Securities Act of 1933, as amended.
| II-2 |
Item 15. Recent Sales of Unregistered Securities.
Except as set forth below, in the three years preceding the filing of this Registration Statement, the Registrant has not issued any securities that were not registered under the Securities Act:
In August 2015, Messrs. Klemp, Picker and Priebe purchased 1,100,000 shares, 500,000 shares and 3,000,000 shares of our common stock, respectively, at a purchase price of $0.001 per share.
In August 2015, in exchange for the issuance of 630,000 shares of common stock, we acquired the rights to the license agreement with MD Anderson covering our WP1122 Portfolio held by IntertechBio Corporation, a company affiliated with Messrs. Priebe and Picker. In August 2015, in exchange for the issuance of 1,431,000 shares of common stock, we acquired the rights to the Annamycin IND and all data related to the Annamycin IND or the development of Annamycin held by AnnaMed, Inc., a company affiliated with Mr. Klemp.
In May 2016, Moleculin, LLC, a Texas limited liability company, was merged with and into MBI, which survived the merger. As a result of the merger, we issued the equity interests holders of Moleculin, LLC (including the convertible noteholders of Moleculin, LLC) an aggregate of 999,931 shares of our common stock. Messrs. Klemp, Picker and Priebe are members of Moleculin, LLC and received shares of our common stock as a result of the merger.
In August and September 2015, the Registrant issued two 8% convertible notes in an aggregate of $250,000 in principal amount of convertible notes to one investor, which principal and accrued interest automatically converted into shares of common stock upon the closing of the Company’s initial public offering at a conversion rate of $0.1299 per share. In October 2015, the Registrant issued two 8% convertible notes in an aggregate of $200,000 in principal amount of convertible notes to two investors, which principal and accrued interest automatically converted into shares of common stock upon the closing of the Company’s initial public offering at a conversion rate of $0.20 per share. At the time of such purchase in October 2015, the convertible note investors and the Registrant agreed that the investors would fund the Registrant up to the filing of its initial non-confidential registration statement an additional $165,000 on the same terms as in the October financing. Pursuant to such agreement, such amounts were received in January 2016.
Between January and May 2016, the Registrant sold 225,963 shares of common stock for a total purchase price of $677,889 to 19 investors.
On June 20, 2016, the Registrant agreed to issue 24,000 shares of common stock to an investor relations firm for services provided.
In January 2017, the Registrant agreed to issue 79,167 shares of common stock to a third-party in settlement of $237,500 in past due amounts.
None of these transactions involved a public offering. We believe that each of the above issuances was exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act regarding transactions not involving a public offering.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits: Reference is made to the Exhibit Index following the signature pages hereto, which Exhibit Index is hereby incorporated into this Item.
(b) Financial Statement Schedules: All schedules are omitted because the required information is inapplicable or the information is presented in the financial statements and the related notes.
Item 17. Undertakings
The undersigned hereby undertakes:
(a) The undersigned Registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
| II-3 |
(b) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions referenced in Item 14 of this Registration Statement, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered hereunder, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(c) The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new Registration Statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| II-4 |
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Houston, Texas, on January 18, 2017.
| MOLECULIN BIOTECH, INC. | ||
| (Registrant) | ||
| By: | /s/ Walter V. Klemp | |
| Walter V. Klemp | ||
| Director and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed below by the following persons in the capacities and on the dates indicated:
| SIGNATURE | TITLE | DATE | ||
| /s/ Walter V. Klemp | ||||
| Walter V. Klemp | Chief Executive Officer and Sole Director | January 18, 2017 | ||
| (Principal Executive Officer) | ||||
| /s/ Jonathan P. Foster | ||||
| Jonathan P. Foster | Chief Financial Officer | January 18, 2017 | ||
| (Principal Financial Officer and Principal Accounting Officer) | ||||
| * | ||||
| Donald Picker | President and Chief Operating Officer | January 18, 2017 | ||
| * | ||||
| Robert George | Director | January 18, 2017 | ||
| * | ||||
| Michael Cannon | Director | January 18, 2017 | ||
| * | ||||
| Jacqueline Northcut | Director | January 18, 2017 |
| * By: | /s/ Walter V. Klemp | |
| Walter V. Klemp | ||
| Attorney-in-Fact |
EXHIBIT INDEX
| Exhibit Number |
Description |
| 1.1 | Form of Underwriting Agreement (1) |
| 3.1 | Amended and Restated Certificate of Incorporation of Moleculin Biotech, Inc. (incorporated by reference to exhibit 3.1 of the Form S-1/A filed March 21, 2016) |
| 3.2 | Amended and Restated Bylaws of Moleculin Biotech, Inc. (incorporated by reference to exhibit 3.2 of the Form S-1/A filed March 21, 2016) |
| 4.1* | Warrant Agent Agreement between Moleculin Biotech, Inc. and VStock Transfer LLC |
| 5 | Opinion of Schiff Hardin LLP as to legality of the securities being registered (1) |
| 10.1 | Moleculin Biotech, Inc. 2015 Incentive Plan (incorporated by reference to exhibit 10.1 of the Form S-1/A filed March 21, 2016) |
| 10.2 | Rights Transfer Agreement between Moleculin Biotech, Inc. and AnnaMed, Inc. (incorporated by reference to exhibit 10.2 of the Form S-1/A filed March 21, 2016) |
| 10.3 | Patent and Technology License Agreement dated June 21, 2010 by and between The Board of Regents of the University of Texas System and Moleculin, LLC (incorporated by reference to exhibit 10.3 of the Form S-1/A filed March 21, 2016) |
| 10.4 | Amendment No. 1 to the Patent and Technology License Agreement dated June 21, 2010 by and between The Board of Regents of the University of Texas System and Moleculin, LLC (incorporated by reference to exhibit 10.4 of the Form S-1/A filed March 21, 2016) |
| 10.5 | Patent and Technology License Agreement dated April 2, 2012 by and between The Board of Regents of the University of Texas System and IntertechBio Corporation (incorporated by reference to exhibit 10.5 of the Form S-1/A filed March 21, 2016) |
| 10.6 | Amendment No. 1 to the Patent and Technology License Agreement dated June 21, 2010 by and between The Board of Regents of the University of Texas System and IntertechBio Corporation (incorporated by reference to exhibit 10.6 of the Form S-1/A filed March 21, 2016) |
| 10.7 | Patent and Technology Development and License Agreement June 28, 2012 by and between Annamed, Inc. and Dermin Sp. z.o.o (incorporated by reference to exhibit 10.7 of the Form S-1/A filed April 15, 2016) |
| 10.8 | Patent and Technology Development and License Agreement dated April 15, 2011 by and between IntertechBio Corporation and Dermin Sp. z.o.o (incorporated by reference to exhibit 10.8 of the Form S-1/A filed March 21, 2016) |
| 10.9 | Patent and Technology Development and License Agreement dated October 27, 2010 by and between Moleculin, LLC and Dermin Sp. z.o.o (incorporated by reference to exhibit 10.9 of the Form S-1/A filed March 21, 2016) |
| 10.10 | Rights Transfer Agreement dated between Moleculin Biotech, Inc. and IntertechBio Corporation dated August 11, 2015 (incorporated by reference to exhibit 10.10 of the Form S-1/A filed March 21, 2016) |
| 10.11 | Agreement and Plan of Merger between Moleculin Biotech, Inc. and Moleculin, LLC (incorporated by reference to exhibit 10.11 of the Form S-1/A filed March 21, 2016) |
| 10.12 | Technology Rights and Development License Agreement to be entered into by Moleculin Biotech, Inc. and Houston Pharmaceuticals, Inc. (incorporated by reference to exhibit 10.13 of the Form S-1/A filed April 15, 2016) |
| 10.13 | Employment Agreement between Moleculin Biotech, Inc. and Jonathan P. Foster dated August 19, 2016 (incorporated by reference to Exhibit 10.1 of the Form 8-K filed August 25, 2016) |
| 10.14 | Executive Employment Agreement between Moleculin Biotech, Inc. and Walter Klemp dated October 13, 2016 (incorporated by reference to Exhibit 10.1 of the Form 8-K filed October 13, 2016) |
| 10.15 | General Release and Separation Agreement between Moleculin Biotech, Inc. and Louis Ploth dated October 7, 2016 (incorporated by reference to Exhibit 10.2 of the Form 8-K filed October 13, 2016) |
| 10.16 | Development Collaboration Agreement between Moleculin Biotech, Inc. and Dermin Sp. Z o. o. dated September 30, 2016 (incorporated by reference to Exhibit 10.4 of the Form 10-Q filed November 21, 2016) |
| 21 | Subsidiaries of the Registrant (incorporated by reference to exhibit 21 of the Form S-1/A filed April 15, 2016) |
| 23.1* | Consent of GBH CPAs, PC |
| 23.2 | Consent of Schiff Hardin LLP (included in Exhibit 5) (1) |
| 101.INS | XBRL Instance Document (1) |
| 101.SCH | XBRL Taxonomy Extension Schema Document (1) |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document (1) |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document (1) |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document (1) |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document (1) |
* Filed herewith.
(1) To be filed by amendment.
