Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - PRECIGEN, INC. | d319924d8k.htm |

Geno Germano, President JP Morgan Healthcare Conference – January 11th, 2017 Exhibit 99.1

Forward-Looking Statements Safe Harbor Statement Some of the statements made in this presentation are forward-looking statements that involve a number of risks and uncertainties and are made pursuant to the Safe harbor Provisions of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based upon Intrexon’s current expectations and projections about future events and generally relate to Intrexon’s plans, objectives and expectations for the development of Intrexon’s business. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties and actual future results may be materially different from the plans, objectives and expectations expressed in this presentation. These risks and uncertainties include, but are not limited to, (i) Intrexon’s current and future ECCs and joint ventures; (ii) Intrexon’s ability to successfully enter new markets or develop additional products, whether with its collaborators or independently; (iii) actual or anticipated variations in Intrexon’s operating results; (iv) actual or anticipated fluctuations in Intrexon’s competitors’ or its collaborators’ operating results or changes in their respective growth rates; (v) Intrexon’s cash position; (vi) market conditions in Intrexon’s industry; (vii) the volatility of Intrexon’s stock price; (viii) Intrexon’s ability, and the ability of its collaborators, to protect Intrexon’s intellectual property and other proprietary rights and technologies; (ix) Intrexon’s ability, and the ability of its collaborators, to adapt to changes in laws or regulations and policies; (x) the outcomes of pending and future litigation; (xi) the rate and degree of market acceptance of any products developed by a collaborator under an ECC or through a joint venture; (xii) Intrexon’s ability to retain and recruit key personnel; (xiii) Intrexon’s expectations related to the use of proceeds from its public offerings and other financing efforts; (xiv) Intrexon’s estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and (xv) Intrexon’s expectations relating to its subsidiaries and other affiliates. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Intrexon’s actual results to differ from those contained in the forward-looking statements, see the section entitled ”Risk Factors“ in Intrexon’s Annual Report on Form 10-K, as well as discussions of potential risks, uncertainties, and other important factors in Intrexon’s subsequent filings with the Securities and Exchange Commission. All information in this presentation is as of the date of the release, and Intrexon undertakes no duty to update this information unless required by law. © 2017 Intrexon Corp. All rights reserved. Intrexon Corporation is sharing the following materials for informational purposes only. Such materials do not constitute an offer to sell or the solicitation of an offer to buy any securities of Intrexon. Any offer and sale of Intrexon’s securities will be made, if at all, only upon the registration and qualification of such securities under all applicable federal and state securities laws or pursuant to an exemption from such requirements. The attached information has been prepared in good faith by Intrexon. However, Intrexon makes no representations or warranties as to the completeness or accuracy of any such information. Any representations or warranties as to Intrexon shall be limited exclusively to any agreements that may be entered into by Intrexon and to such representations and warranties as may arise under law upon distribution of any prospectus or similar offering document by Intrexon.
Leadership in Engineering Biology FIRST-IN-CLASS ACHIEVEMENTS RheoSwitch®: 1st clinically validated transcriptional gene switch Sleeping Beauty: 1st in human CAR-T trials with this non-viral platform ExeGen® LDLR: 1st engineered miniswine research model cleared by FDA for commercial use AquAdvantage® Salmon: 1st FDA approved engineered food animal Arctic® Apples: 1st engineered non-browning apples approved by USDA Friendly™ Aedes: 1st bioengineered insect with commercial applications IEP: 1st natural gas bioconversion to isobutanol and other chemicals with engineered methanotrophs INTREXON APPLICATIONS Health Food Consumer Energy Environment

Focus on Healthcare Intrexon’s approach is to engineer cells and microbes with gene programs to enable targeted delivery of potent bio-therapeutics in a cost-effective and controlled manner Design – UltraVector® Platform Control – RheoSwitch® technology and other platforms for gene expression and regulation Delivery – Broad expertise in viral, non-viral DNA/mRNA, and cell-based delivery systems Construction – Multiple tools for Genome/DNA/RNA/Protein engineering including AttSite™ Recombinases Componentry – Natural, synthetic and hybrid parts utilized Complexity – Capable of complex multigene systems
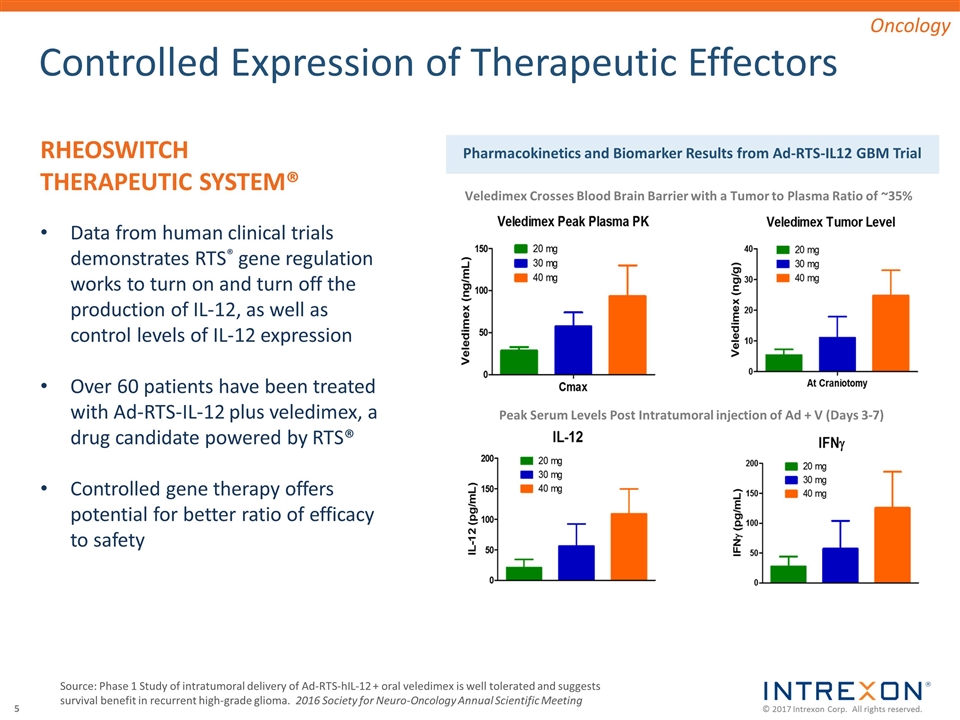
Controlled Expression of Therapeutic Effectors Source: Phase 1 Study of intratumoral delivery of Ad-RTS-hIL-12 + oral veledimex is well tolerated and suggests survival benefit in recurrent high-grade glioma. 2016 Society for Neuro-Oncology Annual Scientific Meeting RHEOSWITCH THERAPEUTIC SYSTEM® Data from human clinical trials demonstrates RTS® gene regulation works to turn on and turn off the production of IL-12, as well as control levels of IL-12 expression Over 60 patients have been treated with Ad-RTS-IL-12 plus veledimex, a drug candidate powered by RTS® Controlled gene therapy offers potential for better ratio of efficacy to safety Pharmacokinetics and Biomarker Results from Ad-RTS-IL12 GBM Trial Veledimex Crosses Blood Brain Barrier with a Tumor to Plasma Ratio of ~35% Peak Serum Levels Post Intratumoral injection of Ad + V (Days 3-7) Oncology
Promising Overall Survival Data in Recurrent GBM Phase I Trial with Ad-RTS-hIL-12 + veledimex in subjects with recurrent glioblastoma (GBM) Based on tolerability and survival benefit (median OS=12.7 months, n=15), 20 mg was selected for an expansion cohort and we are following patients’ overall survival data* Registration Pathway for Recurrent GBM: End-of-Phase 2 meeting with FDA in early Q1 2017 Upcoming trials in 1H’2017 using Ad-RTS-hIL-12: Pediatric brain tumors Combination trial with checkpoint inhibitor in GBM * As of January 6, 2017 Oncology 6
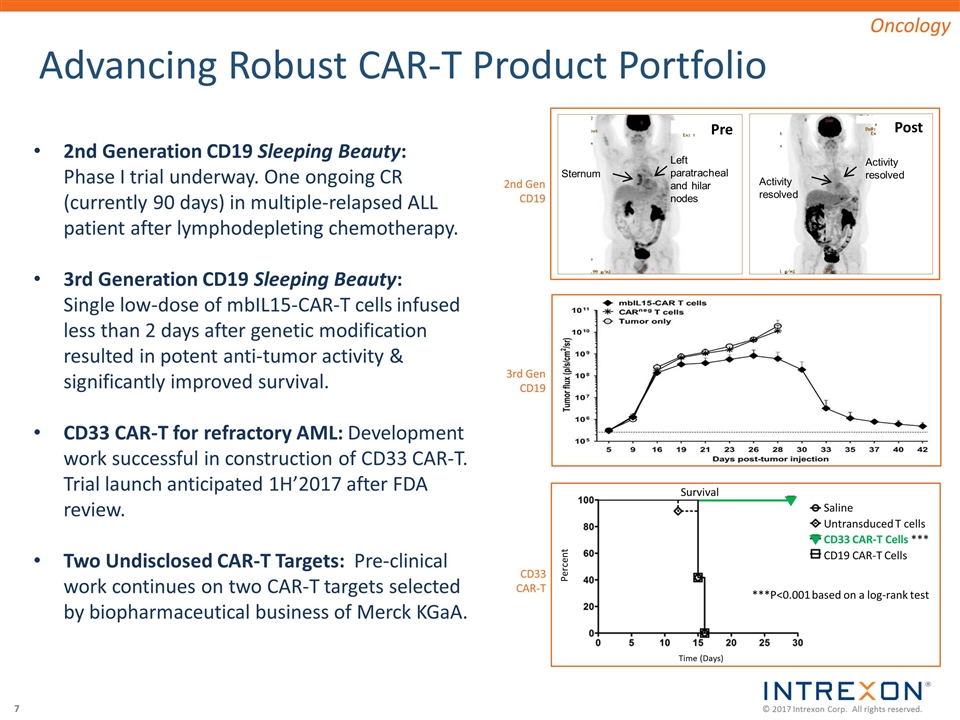
Advancing Robust CAR-T Product Portfolio 2nd Generation CD19 Sleeping Beauty: Phase I trial underway. One ongoing CR (currently 90 days) in multiple-relapsed ALL patient after lymphodepleting chemotherapy. 3rd Generation CD19 Sleeping Beauty: Single low-dose of mbIL15-CAR-T cells infused less than 2 days after genetic modification resulted in potent anti-tumor activity & significantly improved survival. CD33 CAR-T for refractory AML: Development work successful in construction of CD33 CAR-T. Trial launch anticipated 1H’2017 after FDA review. Two Undisclosed CAR-T Targets: Pre-clinical work continues on two CAR-T targets selected by biopharmaceutical business of Merck KGaA. Oncology Sternum Left paratracheal and hilar nodes Activity resolved Activity resolved Pre Post Saline Untransduced T cells CD33 CAR-T Cells *** CD19 CAR-T Cells ***P<0.001 based on a log-rank test Time (Days) Percent Survival 2nd Gen CD19 3rd Gen CD19 CD33 CAR-T
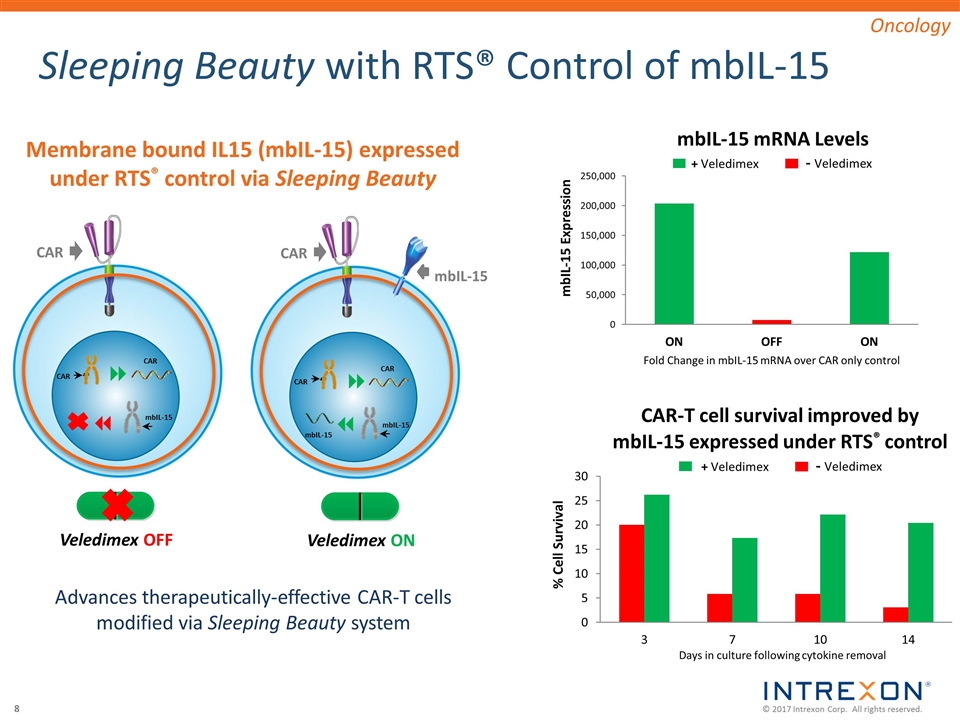
Sleeping Beauty with RTS® Control of mbIL-15 Membrane bound IL15 (mbIL-15) expressed under RTS® control via Sleeping Beauty Oncology Veledimex ON Veledimex OFF CAR mbIL-15 Fold Change in mbIL-15 mRNA over CAR only control - Veledimex + Veledimex - Veledimex + Veledimex CAR 8 7 CAR mbIL-15 CAR 7 mbIL-15 8 CAR mbIL-15 CAR Advances therapeutically-effective CAR-T cells modified via Sleeping Beauty system

Targeting Solid Tumors with Sleeping Beauty Oncology Intrexon and ZIOPHARM sign CRADA with the National Cancer Institute (NCI) Utilizing Sleeping Beauty System to Generate T cells Targeting Neoantigens – January 2017 Mol Ther. 2016 Jun;24(6):1078-89.
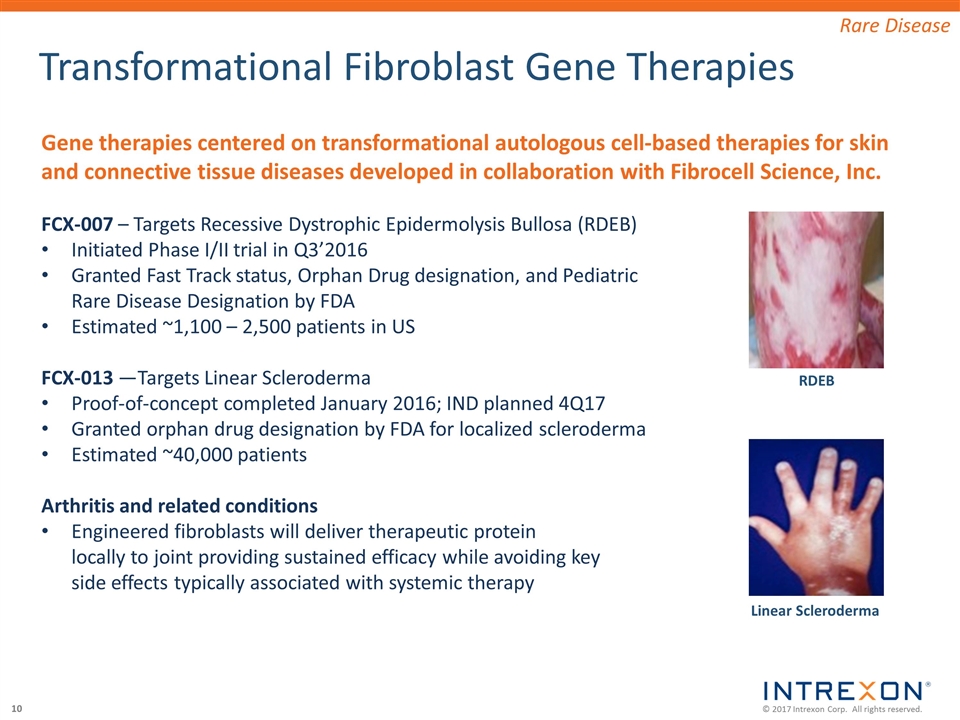
Transformational Fibroblast Gene Therapies Gene therapies centered on transformational autologous cell-based therapies for skin and connective tissue diseases developed in collaboration with Fibrocell Science, Inc. FCX-007 – Targets Recessive Dystrophic Epidermolysis Bullosa (RDEB) Initiated Phase I/II trial in Q3’2016 Granted Fast Track status, Orphan Drug designation, and Pediatric Rare Disease Designation by FDA Estimated ~1,100 – 2,500 patients in US FCX-013 —Targets Linear Scleroderma Proof-of-concept completed January 2016; IND planned 4Q17 Granted orphan drug designation by FDA for localized scleroderma Estimated ~40,000 patients Arthritis and related conditions Engineered fibroblasts will deliver therapeutic protein locally to joint providing sustained efficacy while avoiding key side effects typically associated with systemic therapy Rare Disease RDEB Linear Scleroderma
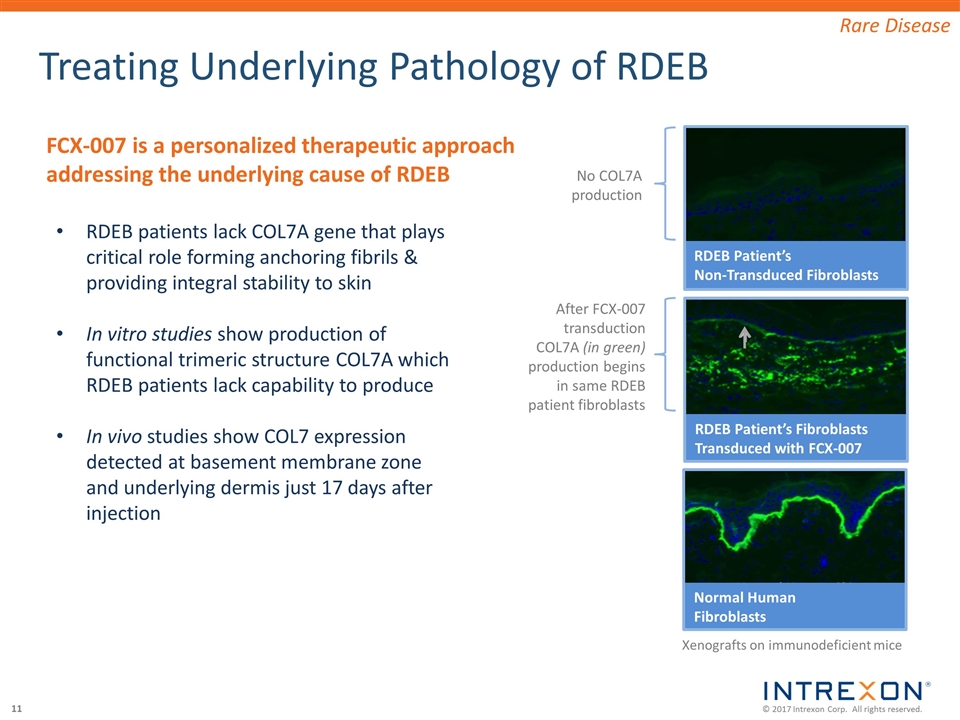
Treating Underlying Pathology of RDEB RDEB Patient’s Non-Transduced Fibroblasts RDEB patients lack COL7A gene that plays critical role forming anchoring fibrils & providing integral stability to skin In vitro studies show production of functional trimeric structure COL7A which RDEB patients lack capability to produce In vivo studies show COL7 expression detected at basement membrane zone and underlying dermis just 17 days after injection Rare Disease Xenografts on immunodeficient mice RDEB Patient’s Fibroblasts Transduced with FCX-007 Normal Human Fibroblasts FCX-007 is a personalized therapeutic approach addressing the underlying cause of RDEB No COL7A production After FCX-007 transduction COL7A (in green) production begins in same RDEB patient fibroblasts
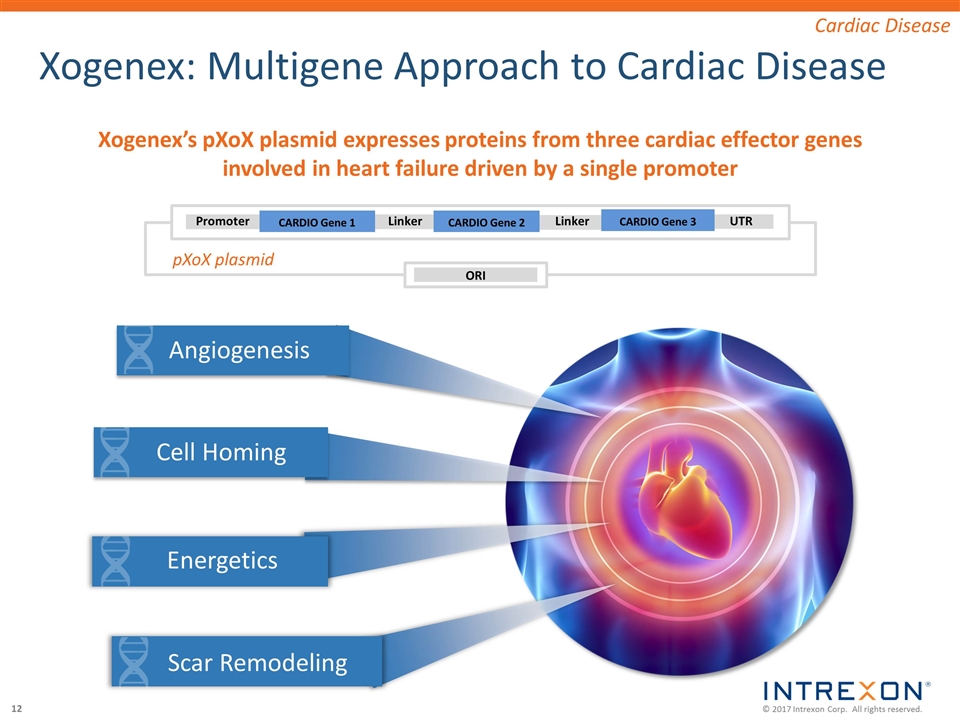
Xogenex: Multigene Approach to Cardiac Disease Promoter Linker ORI Linker UTR CARDIO Gene 1 CARDIO Gene 2 CARDIO Gene 3 pXoX plasmid Cardiac Disease Xogenex’s pXoX plasmid expresses proteins from three cardiac effector genes involved in heart failure driven by a single promoter Angiogenesis Cell Homing Energetics Scar Remodeling
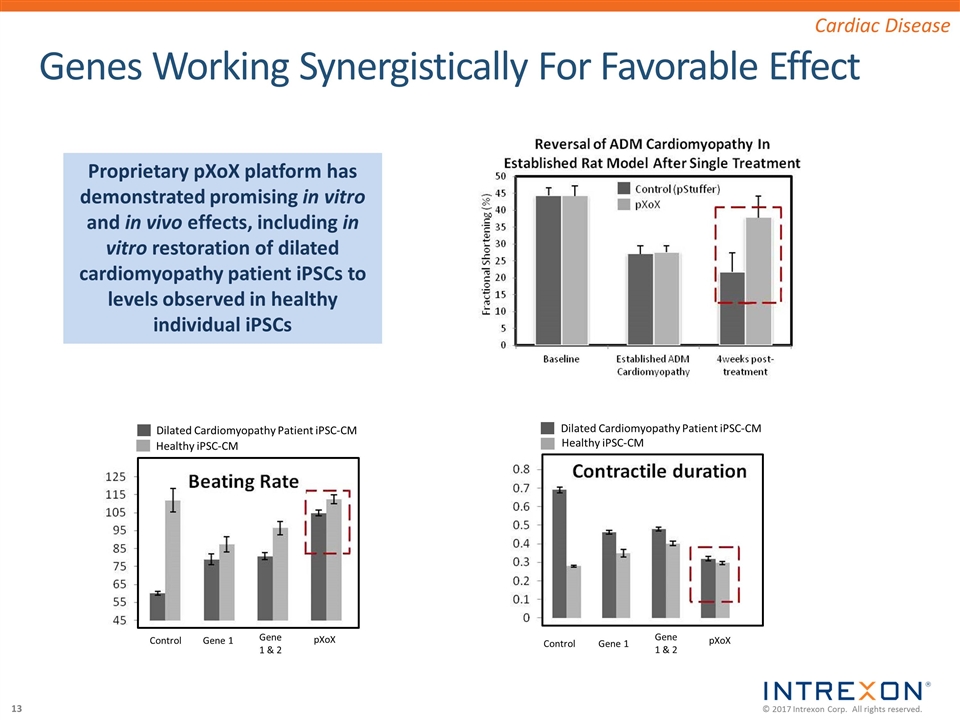
Genes Working Synergistically For Favorable Effect Cardiac Disease Dilated Cardiomyopathy Patient iPSC-CM Healthy iPSC-CM Dilated Cardiomyopathy Patient iPSC-CM Healthy iPSC-CM Control Gene 1 Control Gene 1 Gene 1 & 2 Gene 1 & 2 pXoX pXoX Proprietary pXoX platform has demonstrated promising in vitro and in vivo effects, including in vitro restoration of dilated cardiomyopathy patient iPSCs to levels observed in healthy individual iPSCs
Heart Failure Represents Large Unmet Clinical Need Cardiac Disease 825,000 new cases annually Disease costs US approximately $32 billion per year Experienced Scientific Advisory Board in retrograde biologics delivery Positive meetings to date with FDA Anticipate IND Filing in 2017
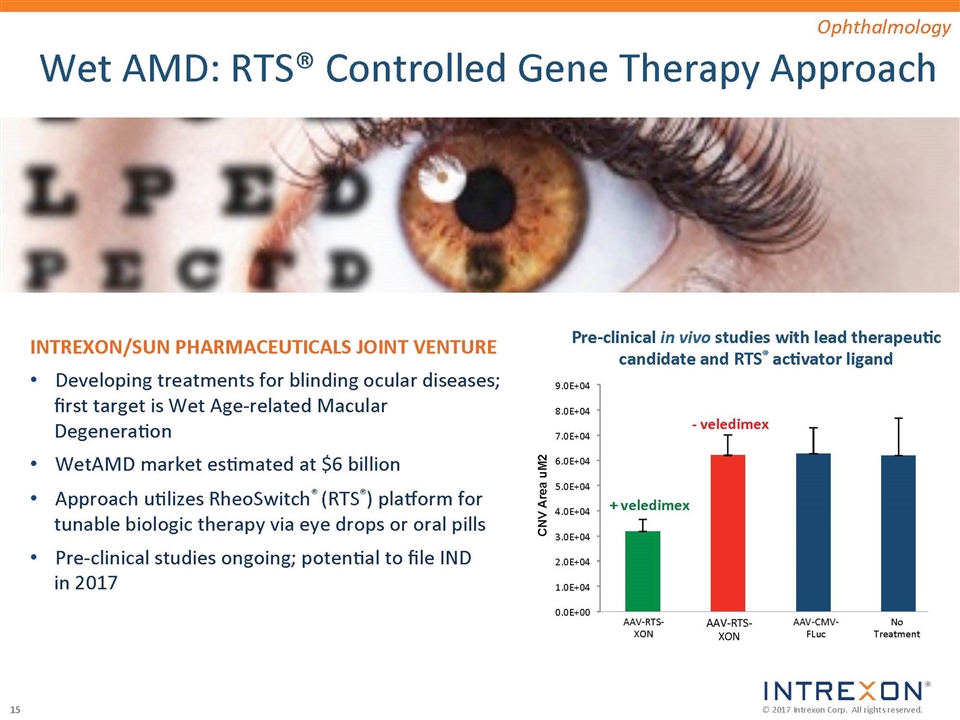
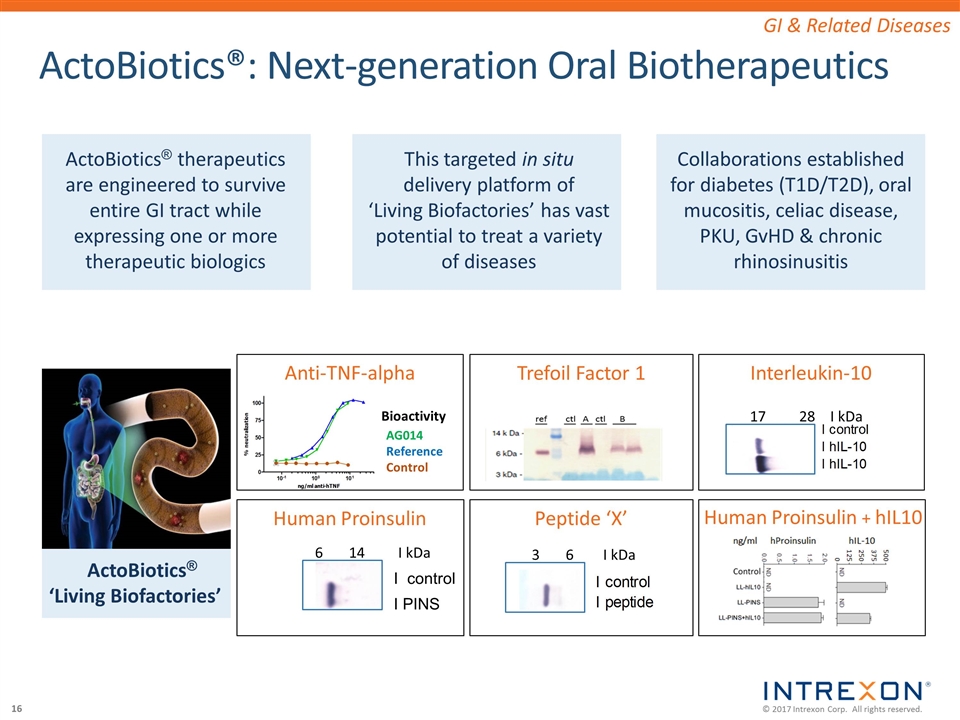
ActoBiotics®: Next-generation Oral Biotherapeutics 3 6 I kDa Trefoil Factor 1 Interleukin-10 Human Proinsulin Human Proinsulin + hIL10 Peptide ‘X’ AG014 Reference Control Bioactivity 6 14 I kDa 17 28 I kDa I control I PINS Anti-TNF-alpha Collaborations established for diabetes (T1D/T2D), oral mucositis, celiac disease, PKU, GvHD & chronic rhinosinusitis ActoBiotics® ‘Living Biofactories’ ActoBiotics® therapeutics are engineered to survive entire GI tract while expressing one or more therapeutic biologics This targeted in situ delivery platform of ‘Living Biofactories’ has vast potential to treat a variety of diseases GI & Related Diseases
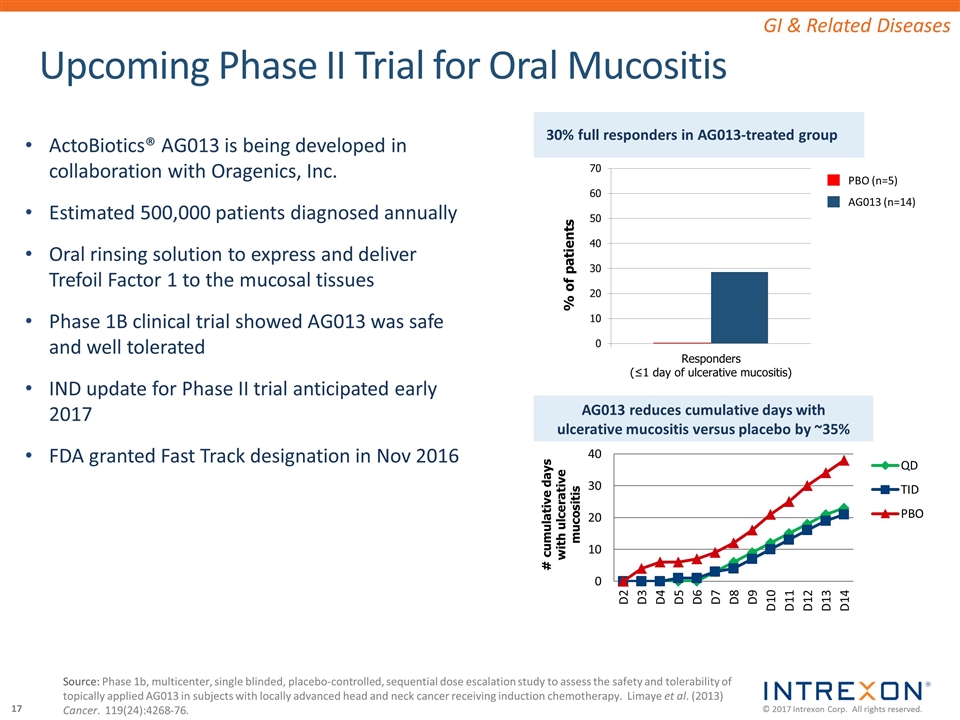
Upcoming Phase II Trial for Oral Mucositis 30% full responders in AG013-treated group AG013 reduces cumulative days with ulcerative mucositis versus placebo by ~35% Source: Phase 1b, multicenter, single blinded, placebo-controlled, sequential dose escalation study to assess the safety and tolerability of topically applied AG013 in subjects with locally advanced head and neck cancer receiving induction chemotherapy. Limaye et al. (2013) Cancer. 119(24):4268-76. GI & Related Diseases ActoBiotics® AG013 is being developed in collaboration with Oragenics, Inc. Estimated 500,000 patients diagnosed annually Oral rinsing solution to express and deliver Trefoil Factor 1 to the mucosal tissues Phase 1B clinical trial showed AG013 was safe and well tolerated IND update for Phase II trial anticipated early 2017 FDA granted Fast Track designation in Nov 2016 PBO (n=5) AG013 (n=14)
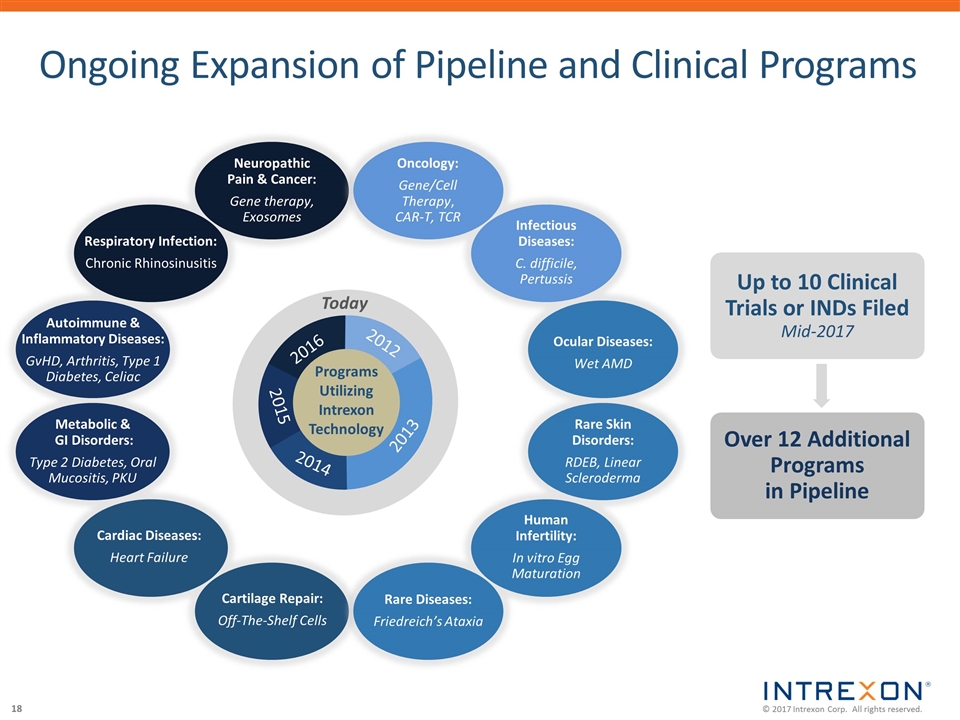
Ongoing Expansion of Pipeline and Clinical Programs Neuropathic Pain & Cancer: Gene therapy, Exosomes Respiratory Infection: Chronic Rhinosinusitis Metabolic & GI Disorders: Type 2 Diabetes, Oral Mucositis, PKU Cartilage Repair: Off-The-Shelf Cells Cardiac Diseases: Heart Failure Autoimmune & Inflammatory Diseases: GvHD, Arthritis, Type 1 Diabetes, Celiac Oncology: Gene/Cell Therapy, CAR-T, TCR Infectious Diseases: C. difficile, Pertussis Rare Skin Disorders: RDEB, Linear Scleroderma Rare Diseases: Friedreich’s Ataxia Human Infertility: In vitro Egg Maturation Ocular Diseases: Wet AMD 2013 2012 2014 2016 2015 Programs Utilizing Intrexon Technology Today Up to 10 Clinical Trials or INDs Filed Mid-2017 Over 12 Additional Programs in Pipeline

Collaborative Business Model Primary objective is to leverage Intrexon technology to create a broad range of successful products which, in turn, will result in a vast portfolio of significant backend economics in commercialized goods across multiple industries Collaborator Exclusive Commercial Rights within a Field Right to Direct Work Toward Invention and Discovery Commercialize Valuable Products Intrexon Technology Access Fees Cost recovery (R&D reimbursement) Milestones Backend Economics
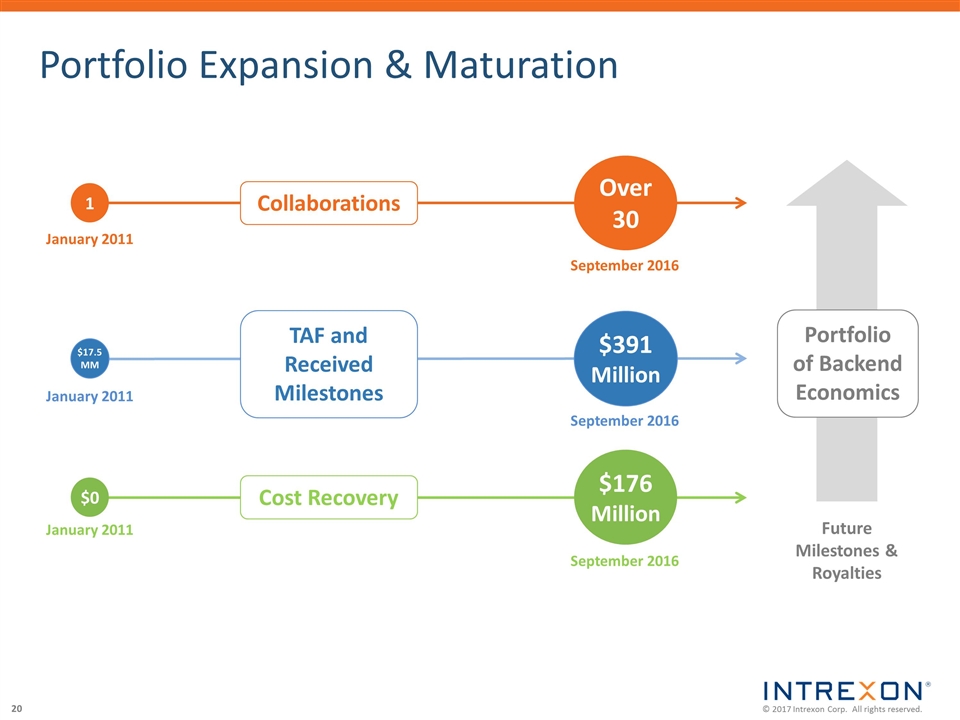
Portfolio Expansion & Maturation September 2016 Over 30 September 2016 $391 Million September 2016 $176 Million 1 January 2011 $17.5MM January 2011 $0 January 2011 Collaborations TAF and Received Milestones Cost Recovery Portfolio of Backend Economics Future Milestones & Royalties

Upcoming Events in Health Sector Oncology: Collaborator ZIOPHARM meeting with FDA in early Q1 regarding Ad-RTS-IL-12 Phase I trial for recurrent glioblastoma (GBM) ZIOPHARM anticipates multiple INDs and trials in 1H 2017: (1) CD33 CAR for refractory AML (2) off-the-shelf primary NK cells for AML (3) pediatric brain tumor with Ad-RTS-IL-12 (4) a combination approach in GBM Sleeping Beauty platform advancements in CAR & TCRs Ophthalmology: Pre-clinical studies ongoing; potential to file IND in 2017 Rare Diseases: First patient to be treated in Phase I/II trial for RDEB is expected to be dosed with FCX-007 in Q1 2017 Oral Mucositis: Collaborator Oragenics planning IND update in early 2017 for AG013 Oral Mucositis Phase II trial Infectious Disease: Oragenics anticipates IND filing for OG716, a lantibiotic for treatment of Clostridium difficile, in 2017 Cardiac Disease: Anticipate filing IND for multi-gene therapy in 2017

Over 30 collaborations in place capitalizing on our leadership position in the engineering of biology Strong cash position with $280.7 million in cash, cash equivalents, and short and long-term investments, and equity securities and preferred stock valued at $163 million Developing high value bio-solutions with our collaborators in large established markets Positioned to deliver meaningful returns to our shareholders through our scalable, capital efficient model and successful execution of current & future opportunities Summary
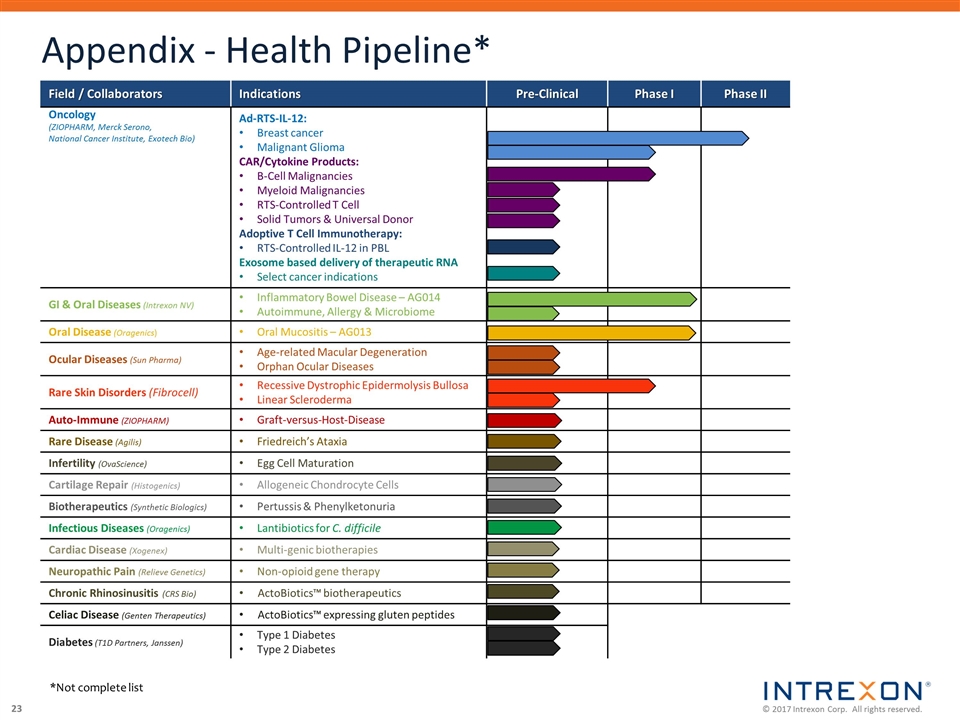
Appendix - Health Pipeline* Field / Collaborators Indications Pre-Clinical Phase I Phase II Oncology (ZIOPHARM, Merck Serono, National Cancer Institute, Exotech Bio) Ad-RTS-IL-12: Breast cancer Malignant Glioma CAR/Cytokine Products: B-Cell Malignancies Myeloid Malignancies RTS-Controlled T Cell Solid Tumors & Universal Donor Adoptive T Cell Immunotherapy: RTS-Controlled IL-12 in PBL Exosome based delivery of therapeutic RNA Select cancer indications GI & Oral Diseases (Intrexon NV) Inflammatory Bowel Disease – AG014 Autoimmune, Allergy & Microbiome Oral Disease (Oragenics) Oral Mucositis – AG013 Ocular Diseases (Sun Pharma) Age-related Macular Degeneration Orphan Ocular Diseases Rare Skin Disorders (Fibrocell) Recessive Dystrophic Epidermolysis Bullosa Linear Scleroderma Auto-Immune (ZIOPHARM) Graft-versus-Host-Disease Rare Disease (Agilis) Friedreich’s Ataxia Infertility (OvaScience) Egg Cell Maturation Cartilage Repair (Histogenics) Allogeneic Chondrocyte Cells Biotherapeutics (Synthetic Biologics) Pertussis & Phenylketonuria Infectious Diseases (Oragenics) Lantibiotics for C. difficile Cardiac Disease (Xogenex) Multi-genic biotherapies Neuropathic Pain (Relieve Genetics) Non-opioid gene therapy Chronic Rhinosinusitis (CRS Bio) ActoBiotics™ biotherapeutics Celiac Disease (Genten Therapeutics) ActoBiotics™ expressing gluten peptides Diabetes (T1D Partners, Janssen) Type 1 Diabetes Type 2 Diabetes *Not complete list
