Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a17-1608_18k.htm |
Exhibit 99.1
Innovative Therapies Novel Products Corporate Update January 2017

Forward looking statement This presentation contains "forward-looking" statements that involve risks and uncertainties. These statements typically may be identified by the use of forward-looking words or phrases such as "may," "believe," "expect," "forecast," "intend," "anticipate," "predict," "should," "planned," "likely," "opportunity," "estimated," and "potential," the negative use of these words or other similar words. All forward-looking statements included in this presentation are based on our current expectations, and we assume no obligation to update any such forward-looking statements. The Private Securities Litigation Reform Act of 1995 provides a "safe harbor" for such forward-looking statements. In order to comply with the terms of the safe harbor, we note that a variety of factors could cause actual results and experiences to differ materially from the anticipated results or other expectations expressed in such forward-looking statements. The risks and uncertainties that may affect the operations, performance, development, and results of our business include but are not limited to: the timing of initiation and completion of the post-approval clinical studies required as part of the approval of Qsymia by the U.S. Food and Drug Administration, or FDA; the response from the FDA to the data that we will submit relating to post-approval clinical studies; the impact of the indicated uses and contraindications contained in the Qsymia label and the Risk Evaluation and Mitigation Strategy requirements; that we may be required to provide further analysis of previously submitted clinical trial data; our ability to work with leading cardiovascular outcome trial experts in planning substantial revisions to the original design and execution of the clinical post-marketing cardiovascular outcomes trial, or CVOT, with the goal of reducing trial costs and obtaining FDA agreement that the revised CVOT would fulfill the requirement of demonstrating the long-term cardiovascular safety of Qsymia; our efforts with the European Medicines Agency, or EMA, relating to our CVOT, and the resubmission of an application for the grant of a marketing authorization to the EMA, the timing of such resubmission, if any, the results of the CVOT, assessment by the EMA of the application for marketing authorization, and their agreement with the data from the CVOT; our ability to successfully seek approval for Qsymia in other territories outside the U.S.; whether healthcare providers, payors and public policy makers will recognize the significance of the American Medical Association officially recognizing obesity as a disease, or the new American Association of Clinical Endocrinologists guidelines; our ability to successfully commercialize Qsymia including risks and uncertainties related to expansion to retail distribution, the broadening of payor reimbursement, the expansion of Qsymia’s primary care presence, and the outcomes of our discussions with pharmaceutical companies and our strategic and franchise-specific pathways for Qsymia; our ability to focus our promotional efforts on health-care providers and on patient education that, along with increased access to Qsymia and ongoing improvements in reimbursement, will result in the accelerated adoption of Qsymia; our ability to minimize expenses that are not essential to expanding the use of STENDRA and Qsymia or are related to product development; our ability to ensure that the entire supply chain for Qsymia efficiently and consistently delivers Qsymia to our customers; ©2017 VIVUS Inc. All rights reserved www.vivus.com 1

Forward looking statement risks and uncertainties related to the timing, strategy, tactics and success of the launches and commercialization of STENDRA® (avanafil) or SPEDRA™ (avanafil) by our sublicensees; our ability to successfully complete on acceptable terms, and on a timely basis, avanafil partnering discussions for territories under our license with Mitsubishi Tanabe Pharma Corporation in which we do not have a commercial collaboration, including Mexico and Central America; our ability to ensure that the entire supply chain for avanafil efficiently and consistently delivers avanafil to our sublicensees; the ability of our partners to maintain regulatory approvals to manufacture and adequately supply our products to meet demand; our ability to accurately forecast Qsymia demand; our ability to commercialize Qsymia efficiently; the number of Qsymia prescriptions dispensed through certified pharmacies; the impact of promotional programs for Qsymia on our net product revenue and net income (loss) in future periods; our history of losses and variable quarterly results; substantial competition; risks related to our ability to protect our intellectual property and litigation in which we are involved or may become involved; uncertainties of government or third-party payor reimbursement; our reliance on sole-source suppliers, third parties and our collaborative partners; our ability to continue to identify, acquire and develop innovative investigational drug candidates and drugs; risks related to the failure to obtain FDA or foreign authority clearances or approvals and noncompliance with FDA or foreign authority regulations; our ability to demonstrate through clinical testing the quality, safety, and efficacy of our investigational drug candidates; the timing of initiation and completion of clinical trials and submissions to foreign authorities; the results of post-marketing studies are not favorable; compliance with post-marketing regulatory standards, post-marketing obligations or pharmacovigilance rules is not maintained; the volatility and liquidity of the financial markets; our liquidity and capital resources; our expected future revenues, operations and expenditures; potential change in our business strategy to enhance long-term stockholder value; our ability to address or potentially reduce our outstanding debt balances; the impact, if any, of changes to our Board of Directors or management team; and other factors that are described from time to time in our periodic filings with the Securities and Exchange Commission. ©2017 VIVUS Inc. All rights reserved www.vivus.com 2

Vivus products and product candidate Qsymia (phentermine and topiramate extended-release) capsules CIV Treatment for chronic weight management for obese adult patients or overweight adult patients with one weight-related comorbidity Approved by the FDA in July 2012 Commercialized by VIVUS in the U.S. Avanafil Treatment of erectile dysfunction Approved by the FDA in April 2012 under the commercial name STENDRA, approved by the EC in June 2013 under the commercial name SPEDRA Commercialization rights licensed to third parties Tacrolimus Investigational product candidate for the treatment of pulmonary arterial hypertension In-licensed in January 2017 ©2017 VIVUS Inc. All rights reserved www.vivus.com 3
VIVUS recent Accomplishments Initiated business strategy review to reshape VIVUS’ business model Received $70 million for a license to commercialize STENDRA in the U.S., Canada, South America, and India In-licensed worldwide development and commercial rights for tacrolimus for the treatment of pulmonary arterial hypertension (PAH) and related vascular diseases Received favorable Markman ruling in Qsymia patent litigation Lawsuit against Hetero for infringement of STENDRA patents settled ©2017 VIVUS Inc. All rights reserved www.vivus.com 4

VIVUS Priorities –2017 Advance tacrolimus development, including the development of a proprietary formulation of tacrolimus Continue to efficiently monetize Qsymia in the U.S. and seek to monetize Qsymia and Avanafil outside of the U.S. Defend our Qsymia intellectual property rights for Qsymia Advance our efforts to address – in a cost-effective manner – the remaining Qsymia regulatory post-marketing requirements Evaluate additional potential in-licensing opportunities to build our portfolio of products and product candidates Address and potentially reduce our outstanding debt balances Effectively manage our cost structure ©2017 VIVUS Inc. All rights reserved www.vivus.com 5

Pulmonary Arterial Hypertension ©2017 VIVUS Inc. All rights reserved www.vivus.com 6

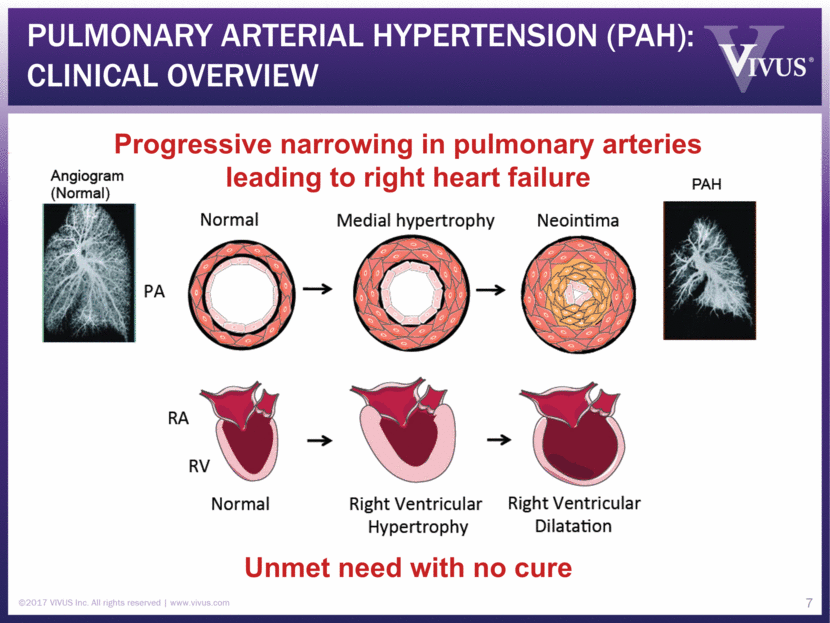
Pulmonary arterial hypertension (PAH): Clinical Overview ©2017 VIVUS Inc. All rights reserved www.vivus.com 7 Progressive narrowing in pulmonary arteries leading to right heart failure Unmet need with no cure
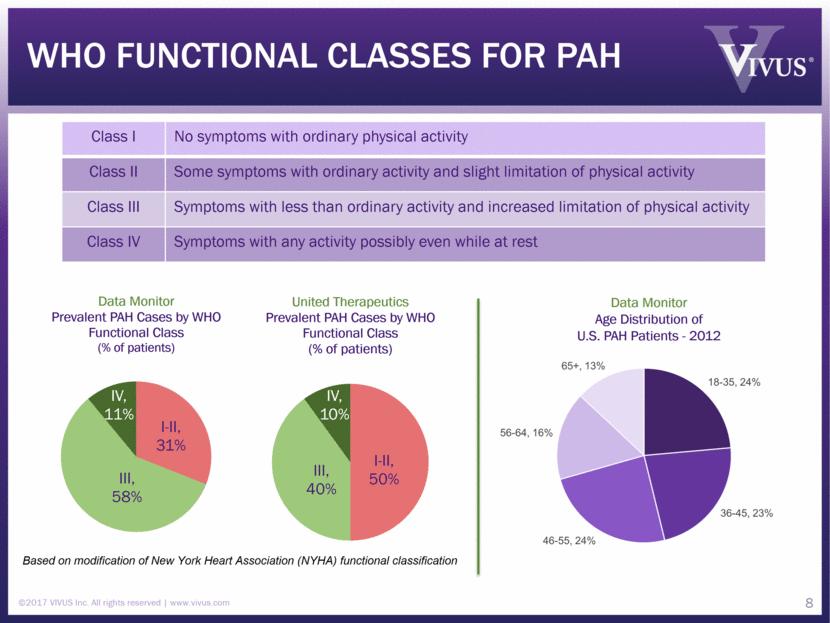
WHO functional classes For PAH ©2017 VIVUS Inc. All rights reserved www.vivus.com 8 Class I No symptoms with ordinary physical activity Class II Some symptoms with ordinary activity and slight limitation of physical activity Class III Symptoms with less than ordinary activity and increased limitation of physical activity Class IV Symptoms with any activity possibly even while at rest Based on modification of New York Heart Association (NYHA) functional classification Data Monitor Age Distribution of U.S. PAH Patients - 2012 I - II, 50% III, 40% IV, 10% United Therapeutics Prevalent PAH Cases by WHO Functional Class (% of patients) IV, 10% I-II, 50% III, 40% 65+, 13% 18-35, 24% 56-64, 16% 36-45, 23% 46-55, 24%
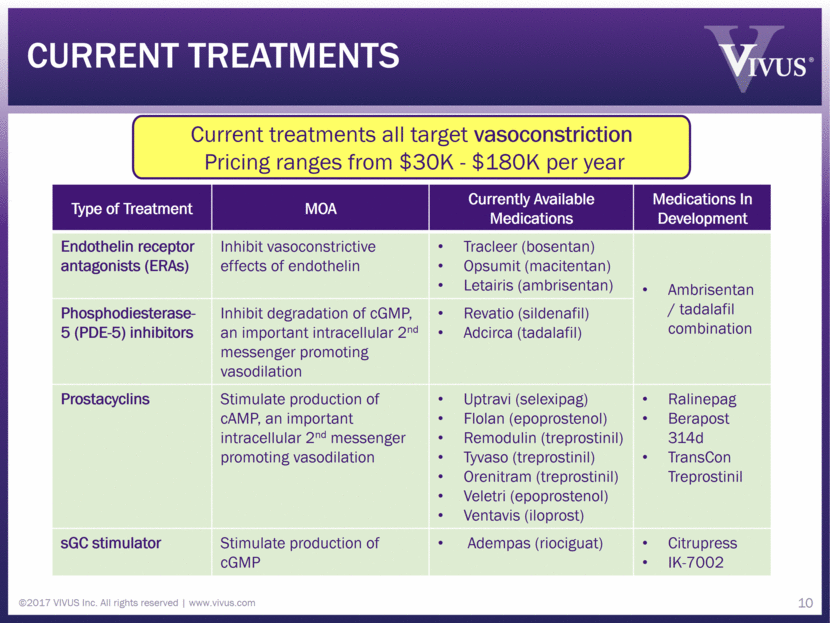
©2017 VIVUS Inc. All rights reserved www.vivus.com 9 Current treatments
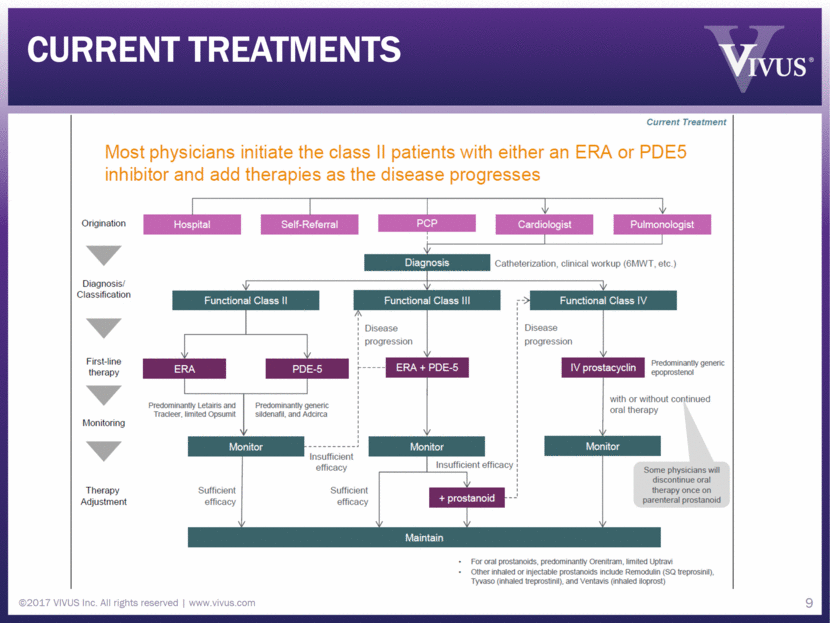
Current treatments Type of Treatment MOA Currently Available Medications Medications In Development Endothelin receptor antagonists (ERAs) Inhibit vasoconstrictive effects of endothelin Tracleer (bosentan) Opsumit (macitentan) Letairis (ambrisentan) Ambrisentan / tadalafil combination Phosphodiesterase-5 (PDE-5) inhibitors Inhibit degradation of cGMP, an important intracellular 2nd messenger promoting vasodilation Revatio (sildenafil) Adcirca (tadalafil) Prostacyclins Stimulate production of cAMP, an important intracellular 2nd messenger promoting vasodilation Uptravi (selexipag) Flolan (epoprostenol) Remodulin (treprostinil) Tyvaso (treprostinil) Orenitram (treprostinil) Veletri (epoprostenol) Ventavis (iloprost) Ralinepag Berapost 314d TransCon Treprostinil sGC stimulator Stimulate production of cGMP Adempas (riociguat) Citrupress IK-7002 10 Current treatments all target vasoconstriction Pricing ranges from $30K - $180K per year 10 ©2017 VIVUS Inc. All rights reserved www.vivus.com
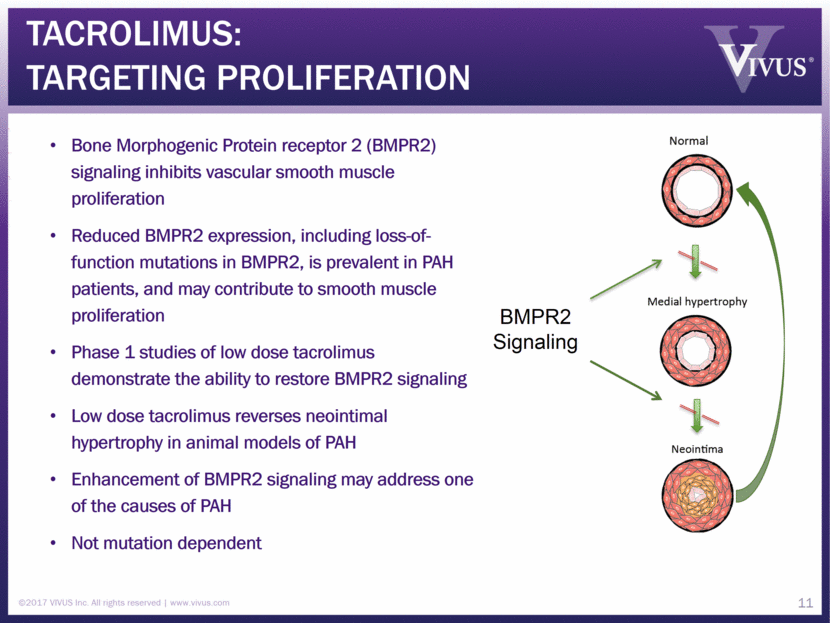
Tacrolimus: Targeting proliferation Bone Morphogenic Protein receptor 2 (BMPR2) signaling inhibits vascular smooth muscle proliferation Reduced BMPR2 expression, including loss-of-function mutations in BMPR2, is prevalent in PAH patients, and may contribute to smooth muscle proliferation Phase 1 studies of low dose tacrolimus demonstrate the ability to restore BMPR2 signaling Low dose tacrolimus reverses neointimal hypertrophy in animal models of PAH Enhancement of BMPR2 signaling may address one of the causes of PAH Not mutation dependent ©2017 VIVUS Inc. All rights reserved www.vivus.com 11 BMPR2 Signaling
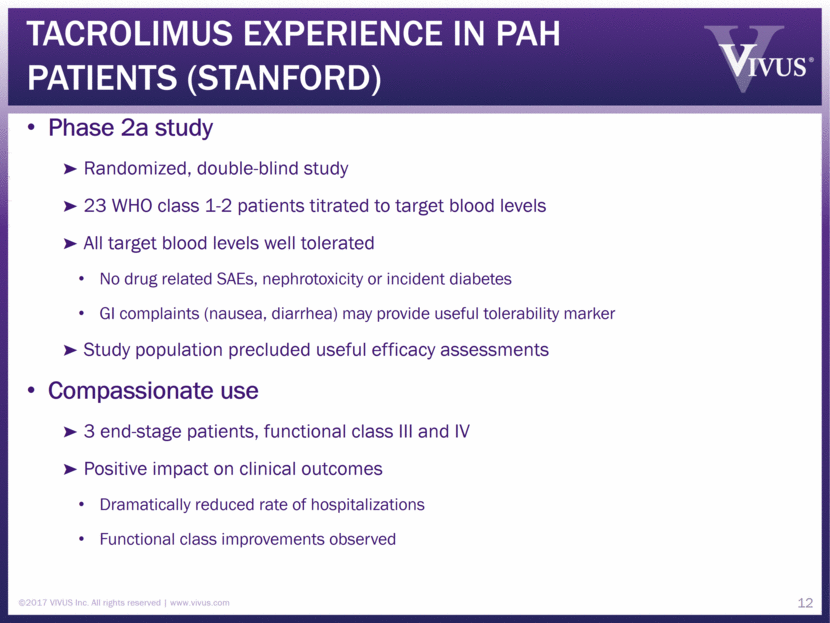
Tacrolimus Experience in PAH patients (StanFOrd) Phase 2a study Randomized, double-blind study 23 WHO class 1-2 patients titrated to target blood levels All target blood levels well tolerated No drug related SAEs, nephrotoxicity or incident diabetes GI complaints (nausea, diarrhea) may provide useful tolerability marker Study population precluded useful efficacy assessments Compassionate use 3 end-stage patients, functional class III and IV Positive impact on clinical outcomes Dramatically reduced rate of hospitalizations Functional class improvements observed ©2017 VIVUS Inc. All rights reserved www.vivus.com 12
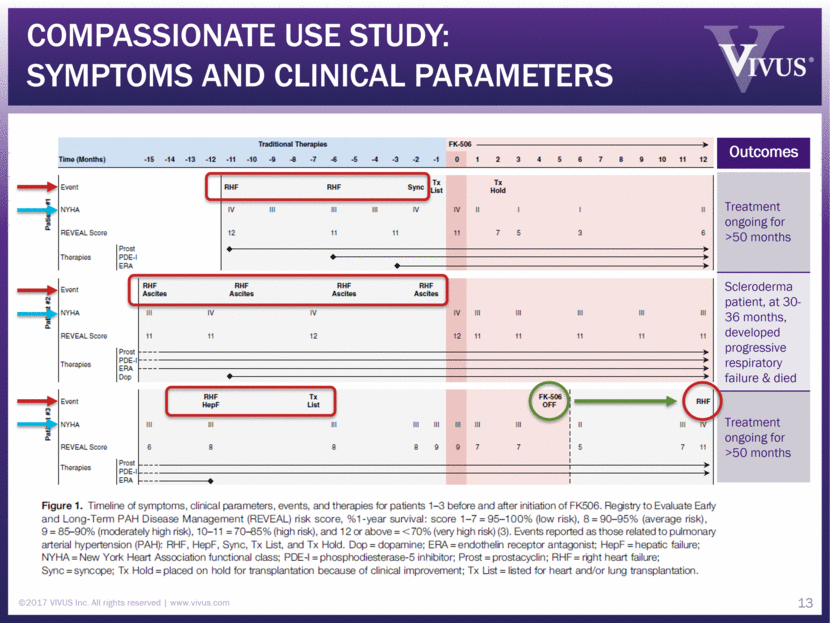
Compassionate Use Study: Symptoms and clinical parameters ©2017 VIVUS Inc. All rights reserved www.vivus.com 13 Outcomes Treatment ongoing for >50 months Scleroderma patient, at 30-36 months, developed progressive respiratory failure & died Treatment ongoing for >50 months
TACROLIMUS/PAH IP status US and foreign applications pending Filing date is April 30, 2012 so patent term to April 30, 2032 US patent allowed Issued as US9474745 on Oct. 25, 2016 Continuation filed 8 claims EP, AU, CA, and JP are filed 14 ©2017 VIVUS Inc. All rights reserved www.vivus.com
$4.5 Billion growth market - Worldwide (2015) 15 U.S. market estimated at $2.7B ©2017 VIVUS Inc. All rights reserved www.vivus.com 2015 ($M) 0.2 0.3 0.3 0.6 0.7 1.3 0.1 0.5 0.6 0.1 NA
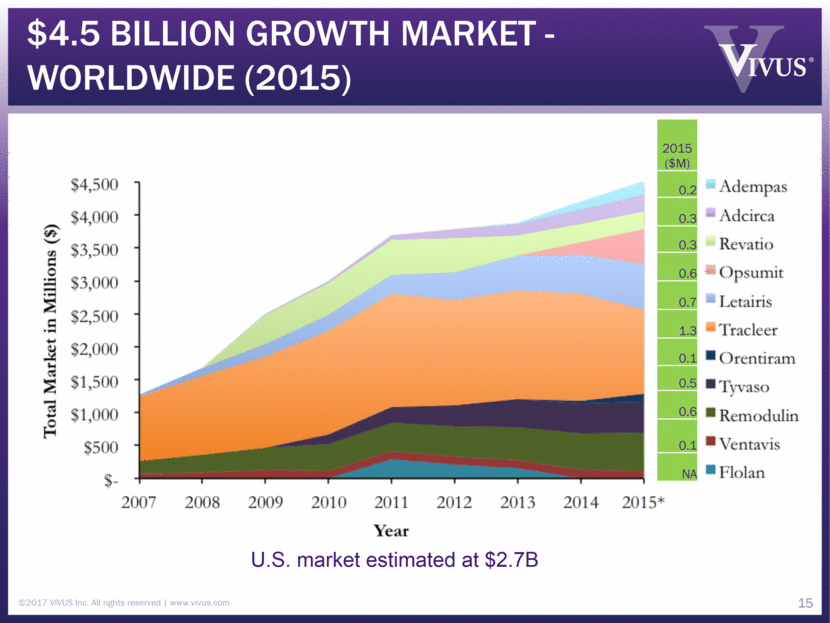
PAH Opportunity PAH is a serious, rare, and progressive disease Progressive narrowing in pulmonary arteries Resulting in right heart failure and ultimately death Median life expectancy: 5 years from diagnosis (~45 years old, class III/IV) Unmet need Existing drug therapies ONLY target symptoms and slow progression of disease Lung transplantation only option for advanced patients not responsive to drug therapies Large growth market: ~$4.5B worldwide, $2.7B U.S. in 2015 Treatment supports multiple "layering" therapies Orphan drug designation received Potential for “Breakthrough Therapy” designation Potentially class modifying, extending life expectancy Patent issued ©2017 VIVUS Inc. All rights reserved www.vivus.com 16

AVANAFIL 17 ©2017 VIVUS Inc. All rights reserved www.vivus.com
Stendra Prescribing information About STENDRA™ STENDRA is a prescription medicine used to treat erectile dysfunction (ED). STENDRA (avanafil) is licensed from Mitsubishi Tanabe Pharma Corporation. VIVUS has development and commercial rights to STENDRA for the treatment of sexual dysfunction worldwide with the exception of certain Asian Pacific Rim countries. Important Safety Information Do not take STENDRA if you take nitrates, often prescribed for chest pain, as this may cause a sudden, unsafe drop in blood pressure. Tell your healthcare provider about all the medicines you take and discuss your general health status to ensure that you are healthy enough to engage in sexual activity. If you experience chest pain, nausea, or any other discomforts during sex, seek immediate medical help. In the rare event of an erection lasting more than 4 hours, seek immediate medical help to avoid long-term injury. STENDRA in combination with other treatments for erectile dysfunction is not recommended. STENDRA does not protect against sexually transmitted diseases, including HIV. The most common side effects of STENDRA are headache, flushing, runny nose and congestion. ©2017 VIVUS Inc. All rights reserved www.vivus.com 18
Avanafil Only erectile dysfunction treatment with a clinically proven 15 minute onset-of-action Able to be taken with food and alcohol Strong safety profile ©2017 VIVUS Inc. All rights reserved www.vivus.com 19
Menarini partnership Signed in July 2013 40 Countries in Europe + AUS/NZ As of December 2016, SPEDRA available in 30 countries in the Menarini territories Additional launches expected in 2017 Upfront and potential milestone payments of 71M Euros 41M Euros earned to date Flat royalty on net sales ©2017 VIVUS Inc. All rights reserved www.vivus.com 20

Metuchen Partnership Signed in September 2016 United States, Canada, South America, and India Upfront payment of $70M Product sold through Mist Pharmaceuticals Metuchen is responsible for obtaining and maintaining regulatory approvals in its territory ©2017 VIVUS Inc. All rights reserved www.vivus.com 21

Sanofi partnership Signed in December 2013 Africa, Middle East, Turkey, CIS countries Upfront and potential milestone payments of $61M $5M earned to date Tiered royalty on net sales Sanofi is responsible for obtaining and maintaining regulatory approvals in its territory ©2017 VIVUS Inc. All rights reserved www.vivus.com 22

ANDA/paragraph iv certification ANDA Filer: Hetero USA, Inc. Paragraph IV certifications for Orange Book listed patents Notice received on June 20, 2016 VIVUS filed a patent infringement lawsuit on July 27, 2016 on the basis of Hetero’s ANDA submission Lawsuit settled in January 2017 – Hetero granted license to enter market no earlier than six months prior to expiration of last to expire patent ©2017 VIVUS Inc. All rights reserved www.vivus.com 23
Qsymia 24 ©2017 VIVUS Inc. All rights reserved www.vivus.com
About Qsymia Qsymia is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults with an initial body mass index (BMI) of 30 kg/m2 or greater (obese) or 27 kg/m2 or greater (overweight) in the presence of at least one weight-related medical condition such as high blood pressure, type 2 diabetes, or high cholesterol. Important Safety Information Qsymia (phentermine and topiramate extended-release) capsules CIV is contraindicated in pregnancy; in patients with glaucoma; in hyperthyroidism; in patients receiving treatment or within 14 days following treatment with monoamine oxidase inhibitors (MAOIs); or in patients with hypersensitivity to sympathomimetic amines, topiramate, or any of the inactive ingredients in Qsymia. Qsymia can cause fetal harm. Females of reproductive potential should have a negative pregnancy test before treatment and monthly thereafter and use effective contraception consistently during Qsymia therapy. If a patient becomes pregnant while taking Qsymia, treatment should be discontinued immediately, and the patient should be informed of the potential hazard to the fetus. The most commonly observed side effects in controlled clinical studies, 5% or greater and at least 1.5 times placebo, include paraesthesia, dizziness, dysgeusia, insomnia, constipation, and dry mouth. Qsymia Prescribing information ©2017 VIVUS Inc. All rights reserved www.vivus.com 25
QSYMIA Summary Qsymia is a safe and effective therapy for chronic weight management Proprietary extended-release formulation combining low doses of active ingredients from two previously approved compounds, phentermine and topiramate Marketed in the U.S. utilizing a dedicated in-house sales force covering the most productive prescribers of anti-obesity medications Available in over 40,000 retail pharmacies nationwide or via a certified mail order pharmacy network Opportunity for growth and expansion outside the U.S. VIVUS holds global rights to Qsymia High potential geographies include the EU, CIS, Japan/China/Korea and the Middle East/North Africa ©2017 VIVUS Inc. All rights reserved www.vivus.com 26
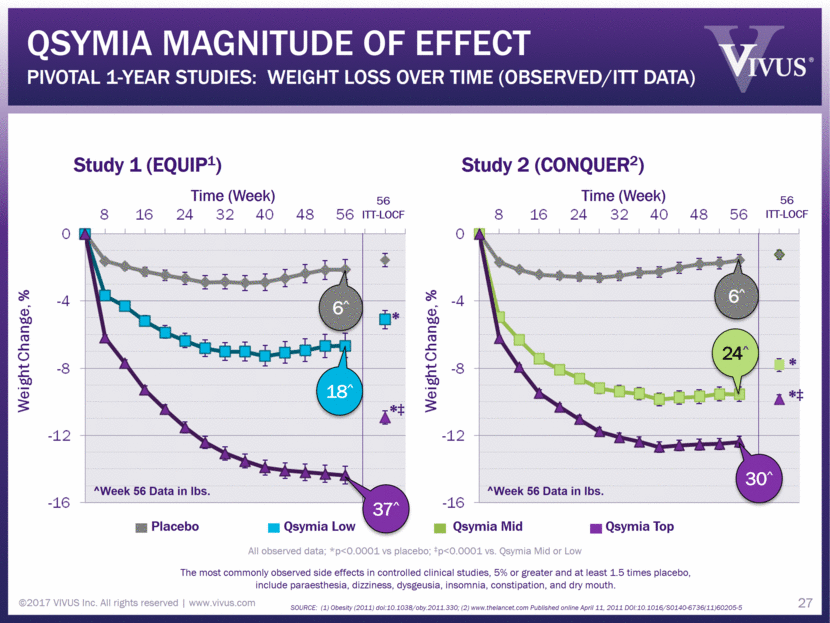
Qsymia magnitude of effect Pivotal 1-Year Studies: Weight Loss Over Time (Observed/ITT Data) ©2017 VIVUS Inc. All rights reserved www.vivus.com 27 Study 1 (EQUIP1) Study 2 (CONQUER2) Time (Week) Time (Week) 56 ITT-LOCF 56 ITT-LOCF All observed data; *p<0.0001 vs placebo; ‡p<0.0001 vs. Qsymia Mid or Low The most commonly observed side effects in controlled clinical studies, 5% or greater and at least 1.5 times placebo, include paraesthesia, dizziness, dysgeusia, insomnia, constipation, and dry mouth. SOURCE: (1) Obesity (2011) doi:10.1038/oby.2011.330; (2) www.thelancet.com Published online April 11, 2011 DOI:10.1016/S0140-6736(11)60205-5 Placebo Qsymia Low Qsymia Mid Qsymia Top * *‡ * *‡ 27 6^ 24^ 30^ 37^ 18^ 6^ ^Week 56 Data in lbs. ^Week 56 Data in lbs.
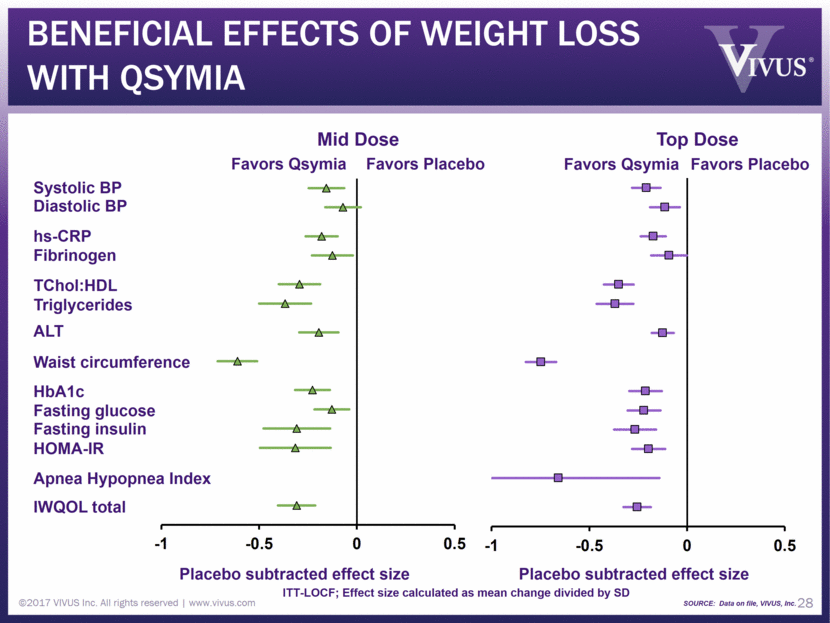
BENEFICIAL EFFECTS of WEIGHT LOSS WITH Qsymia ©2017 VIVUS Inc. All rights reserved www.vivus.com 28 Diastolic BP Systolic BP Fibrinogen Triglycerides TChol:HDL IWQOL total Fasting glucose HOMA-IR Fasting insulin HbA1c hs-CRP ALT Apnea Hypopnea Index Placebo subtracted effect size Placebo subtracted effect size Favors Qsymia Favors Placebo Favors Qsymia Favors Placebo Waist circumference ITT-LOCF; Effect size calculated as mean change divided by SD SOURCE: Data on file, VIVUS, Inc. Mid Dose Top Dose -1 -0.5 0 0.5 -1 -0.5 0 0.5
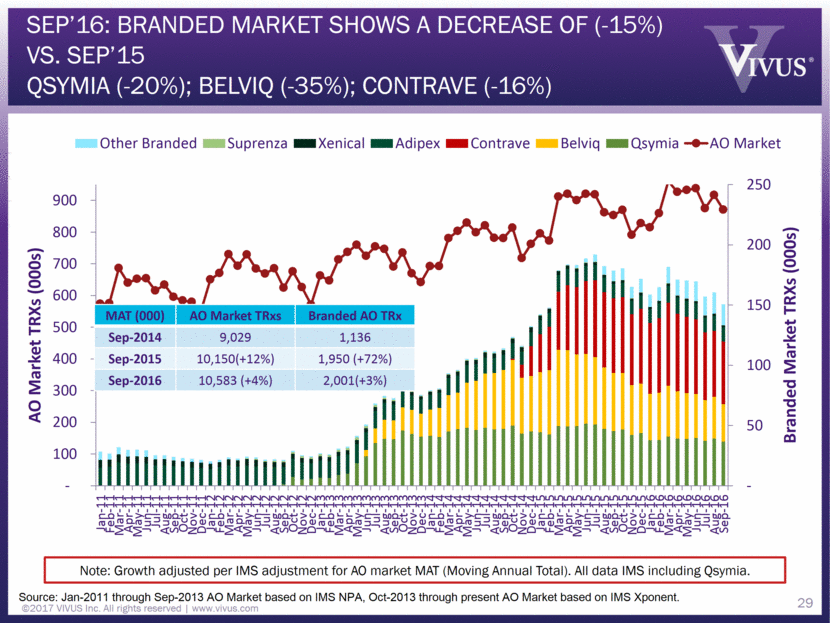
Sep’16: Branded Market shows a decrease of (-15%) vs. Sep’15 Qsymia (-20%); Belviq (-35%); Contrave (-16%) Source: Jan-2011 through Sep-2013 AO Market based on IMS NPA, Oct-2013 through present AO Market based on IMS Xponent. MAT (000) AO Market TRxs Branded AO TRx Sep-2014 9,029 1,136 Sep-2015 10,150(+12%) 1,950 (+72%) Sep-2016 10,583 (+4%) 2,001(+3%) Note: Growth adjusted per IMS adjustment for AO market MAT (Moving Annual Total). All data IMS including Qsymia. ©2017 VIVUS Inc. All rights reserved www.vivus.com 29 - 50 100 150 200 250 - 100 200 300 400 500 600 700 800 900 Jan-11 Feb-11 Mar-11 Apr-11 May-11 Jun-11 Jul-11 Aug-11 Sep-11 Oct-11 Nov-11 Dec-11 Jan-12 Feb-12 Mar-12 Apr-12 May-12 Jun-12 Jul-12 Aug-12 Sep-12 Oct-12 Nov-12 Dec-12 Jan-13 Feb-13 Mar-13 Apr-13 May-13 Jun-13 Jul-13 Aug-13 Sep-13 Oct-13 Nov-13 Dec-13 Jan-14 Feb-14 Mar-14 Apr-14 May-14 Jun-14 Jul-14 Aug-14 Sep-14 Oct-14 Nov-14 Dec-14 Jan-15 Feb-15 Mar-15 Apr-15 May-15 Jun-15 Jul-15 Aug-15 Sep-15 Oct-15 Nov-15 Dec-15 Jan-16 Feb-16 Mar-16 Apr-16 May-16 Jun-16 Jul-16 Aug-16 Sep-16 Branded Market TRXs (000s) AO Market TRXs (000s) Other Branded Suprenza Xenical Adipex Contrave Belviq Qsymia AO Market
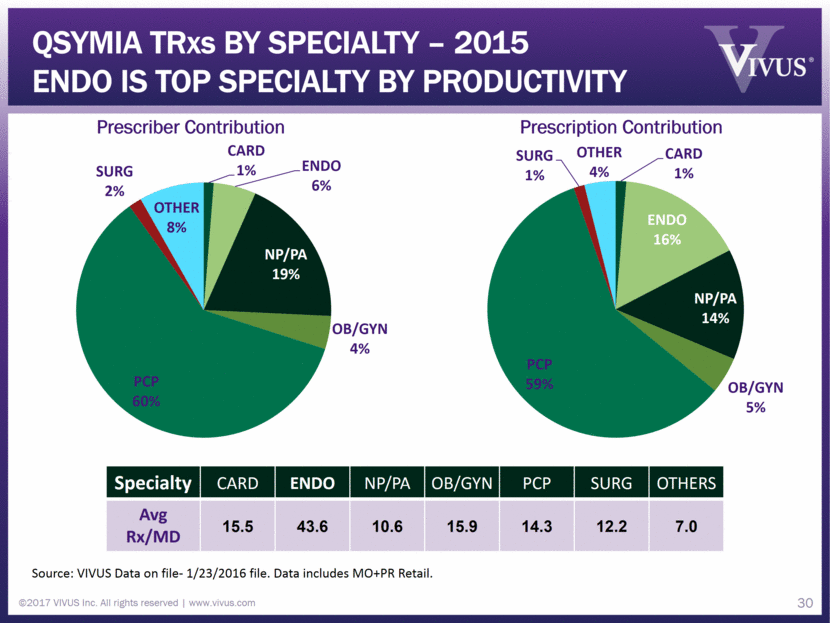
Qsymia trxs by Specialty – 2015 Endo is top specialty by productivity Source: VIVUS Data on file- 1/23/2016 file. Data includes MO+PR Retail. 30 Specialty CARD ENDO NP/PA OB/GYN PCP SURG OTHERS Avg Rx/MD 15.5 43.6 10.6 15.9 14.3 12.2 7.0 ©2017 VIVUS Inc. All rights reserved www.vivus.com CARD 1% ENDO 6% NP/PA 19% OB/GYN 4% PCP 60% SURG 2% OTHER 8% Prescriber Contribution CARD 1% ENDO 16% NP/PA 14% OB/GYN 5% PCP 59% SURG 1% OTHER 4% Prescription Contribution
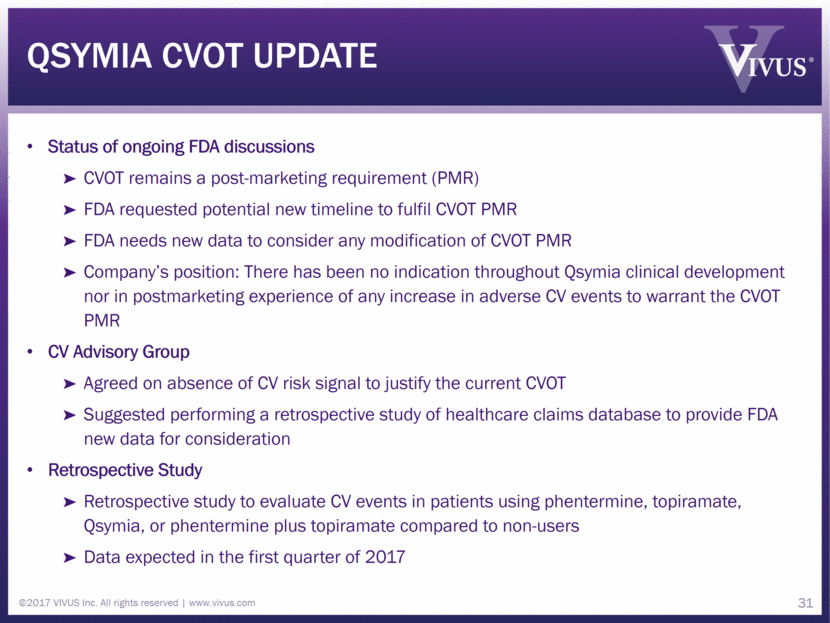
Qsymia CVOT update Status of ongoing FDA discussions CVOT remains a post-marketing requirement (PMR) FDA requested potential new timeline to fulfil CVOT PMR FDA needs new data to consider any modification of CVOT PMR Company’s position: There has been no indication throughout Qsymia clinical development nor in postmarketing experience of any increase in adverse CV events to warrant the CVOT PMR CV Advisory Group Agreed on absence of CV risk signal to justify the current CVOT Suggested performing a retrospective study of healthcare claims database to provide FDA new data for consideration Retrospective Study Retrospective study to evaluate CV events in patients using phentermine, topiramate, Qsymia, or phentermine plus topiramate compared to non-users Data expected in the first quarter of 2017 ©2017 VIVUS Inc. All rights reserved www.vivus.com 31
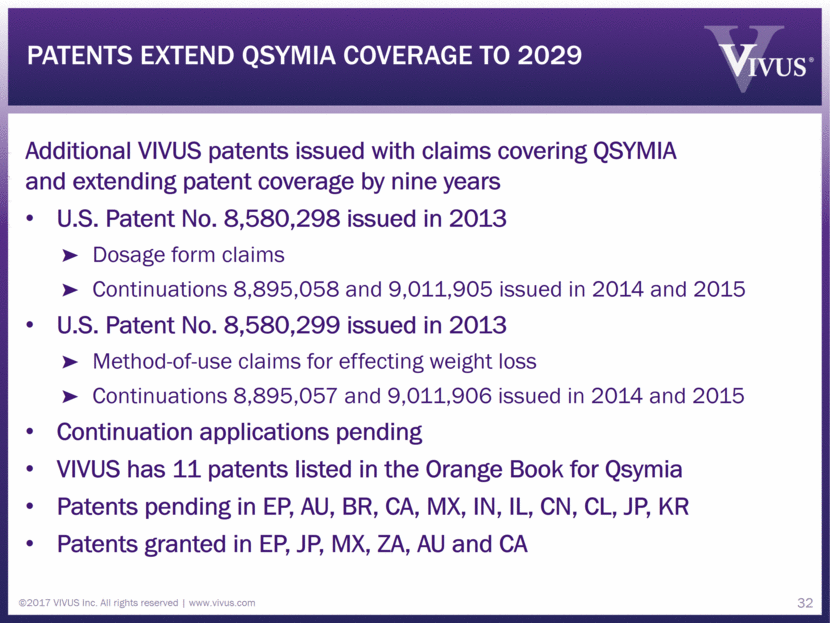
patents extend qsymia coverage to 2029 Additional VIVUS patents issued with claims covering QSYMIA and extending patent coverage by nine years U.S. Patent No. 8,580,298 issued in 2013 Dosage form claims Continuations 8,895,058 and 9,011,905 issued in 2014 and 2015 U.S. Patent No. 8,580,299 issued in 2013 Method-of-use claims for effecting weight loss Continuations 8,895,057 and 9,011,906 issued in 2014 and 2015 Continuation applications pending VIVUS has 11 patents listed in the Orange Book for Qsymia Patents pending in EP, AU, BR, CA, MX, IN, IL, CN, CL, JP, KR Patents granted in EP, JP, MX, ZA, AU and CA ©2017 VIVUS Inc. All rights reserved www.vivus.com 32
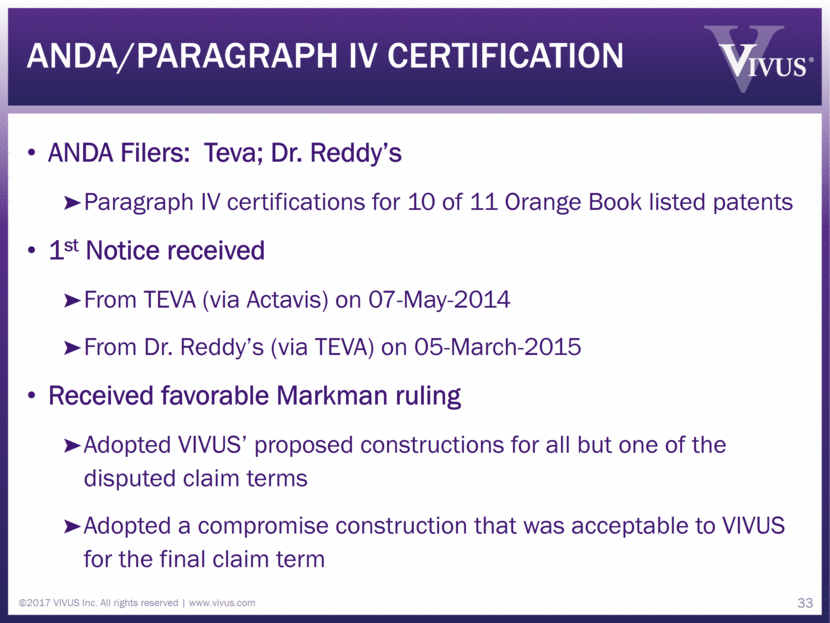
ANDA/paragraph iv certification ANDA Filers: Teva; Dr. Reddy’s Paragraph IV certifications for 10 of 11 Orange Book listed patents 1st Notice received From TEVA (via Actavis) on 07-May-2014 From Dr. Reddy’s (via TEVA) on 05-March-2015 Received favorable Markman ruling Adopted VIVUS’ proposed constructions for all but one of the disputed claim terms Adopted a compromise construction that was acceptable to VIVUS for the final claim term ©2017 VIVUS Inc. All rights reserved www.vivus.com 33
Financial Summary 34 ©2017 VIVUS Inc. All rights reserved www.vivus.com

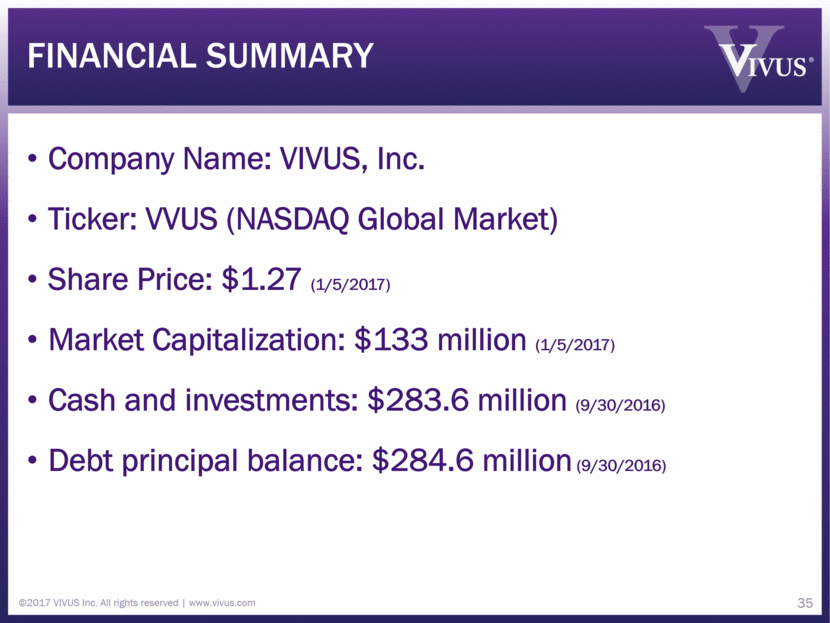
Financial Summary Company Name: VIVUS, Inc. Ticker: VVUS (NASDAQ Global Market) Share Price: $1.27 (1/5/2017) Market Capitalization: $133 million (1/5/2017) Cash and investments: $283.6 million (9/30/2016) Debt principal balance: $284.6 million (9/30/2016) ©2017 VIVUS Inc. All rights reserved www.vivus.com 35
Innovative Therapies Novel Products Corporate Update January 2017

